Study of Reaction Temperature and Sulfur Dioxide Feed Rate in the Sulfur Iodine Production Cycle Reactions
|
|
|
- Barnaby Foster
- 6 years ago
- Views:
Transcription
1 IMECS 215, March 18-2, 215, Hong Kong Study of Reaction Temperature and Sulfur Dioxide Feed Rate in the Sulfur Iodine Production Cycle Reactions Warunee Chankerd, Paisan Kittisupakorn and Iqbal Mohammed Mujtaba Abstract The Bunsen reaction is one of the sulfur iodine production cycle (SI or IS) reactions in the thermochemical process that involves in the production of HI which is one of the most important intermediate chemicals in the hydrogen energy. In this work, the Bunsen reaction in a designed plug flow reactor (1 inch diameter x 12 inches length) equipped with a cooling jacket for the production of hydroiodicacid and sulfuric acid is studied. The Bunsen reaction is simulated by commercial simulation software based on the kinetic data from literatures with the temperature of 33 K, excess iodine, water feed rate and the feed rate of sulfur dioxide of 5 kg/hr. In order to validate the model, the amount of iodine, sulfur dioxide, water, sulfuric acid and hydroiodic acid are compared with experimental data; the relative discrepancies are.22 %, 1.43%,.17%, 2.85% and 2.88%, respectively. These simulation results guarantee that the model is reliable to be used to study the effects of reaction temperature and sulfur dioxide feed rate on the amounts of products. In the effect of reaction temperature, the amounts of products (hydroiodic acid and sulfuric acid) increase when the reaction temperature is raised from 293 to 373 K but they decrease when the temperature is higher than 373 K. These results indicate that the reaction temperature of higher than 373 K promotes the side reaction and inverse the Bunsen reaction. Moreover, the amounts of products increase when the sulfur dioxide feed rate increase. These results show that the amount of products are directly depended on the sulfur dioxide feed rate. In contrast, the amounts of iodine and water decrease when the feed rate of sulfur dioxide increases. Keywords simulation, Bunsen reaction, plug flow reactor, hydroiodic acid, sulfur iodine D I. INTRODUCTION uring the last decade, the demand of the energy has been suddenly increased while the rate of energy production from fossil fuel to supply that demand are still the same. To avoid the lack of energy supply in the future, there are many researches about the alternative energy resources which have the properties as good as to fossil fuel such as hydrogen and sulfur iodine [1]. Manuscript received January 1, 215; revised February 5, 215. Warunee Chankerd Department of Chemical Engineering, Faculty of Engineering, Chulalongkorn University, Bangkok 133, Thailand. (corresponding author to provide warunee.cha@student.chula.ac.th). Paisan Kittisupakorn Department of Chemical Engineering, Faculty of Engineering, Chulalongkorn University, Bangkok 133, Thailand. (corresponding author to provide paisan.k@chula.ac.th). Iqbal Mohammed Mujtaba School of Engineering, Design & Technology, University of Bradford, Bradford BD7 1DP, UK. (corresponding author to provide I.M.Mujtaba@bradford.ac.uk). The sulfur iodine (SI or IS) is the thermochemical process that increases the potential of overall efficiency and the operating cost is lower than the fossil fuel production process [2]. The cycle of sulfur iodine production consists of three chemical reactions. A scheme of a reference process is proposed by General Atomics (GA) [3]. Bunsen reaction: I 2 + SO 2 + 2H 2 O 2HI + H 2 SO 4 (1) Decomposition reaction of hydroiodic acid: 2HI I 2 + H 2 (2) Decomposition reaction of Sulfuric acid: H 2 SO 4 SO 2 + H 2 O +1/2O 2 (3) The Bunsen reaction (Eq.1) is an exothermic reaction at the temperature K [1]. The reaction of sulfur dioxide (SO 2 ), the excess of water (H 2 O) and iodine (I 2 ) generates hydroiodic acid (HI) and sulfuric acid (H 2 SO 4 ) as the products [3]. The excess of iodine and water provide for avoiding the reversible Bunsen reaction. The products in this process always separate into two liquid phases because hydroiodic acid and sulfuric acid are immiscible liquids. The separation between two phases takes place under gravity. The upper phase consists of sulfuric acid, sulfur dioxide as well as water and the lower phase consists of hydroiodic acid, iodine, sulfur dioxide and water. After separation section, hydroiodic acid decomposes into iodine and hydrogen. The decomposition of hydroiodic acid (Eq.2) is an endothermic reaction at the temperature K [1]. In addition, Sulfuric acid is decomposed to sulfur dioxide, water and oxygen. The decomposition of sulfuric acid (Eq.3) is an endothermic reaction at the temperature K [4]. The net of reactions is a dissociation of water into hydrogen and oxygen. The other chemicals are recycled in the closed cycle process. ISSN: (Print); ISSN: (Online) IMECS 215
2 IMECS 215, March 18-2, 215, Hong Kong H 2 SO 4 1/2O 2 + SO 2 + H 2 O 1/2O 2 H 2 S O H 2 SO 4 + 2HI I 2 + SO 2 +2H 2 O SO 2, H 2 O 2HI I 2 HI I 2 + H 2 H 2 Fig. 1 Schematic diagram of the sulfur-iodine thermochemical cycle for hydrogen production. Establishing the closed-cycle operation technology and improving the thermal efficiency of the process are both highly sensitive to several factors involved in the Bunsen reaction [4] such as reaction temperature, the amount of sulfur dioxide in the feed and the composition of process solution. In this work, the Bunsen reaction is simulated by using commercial simulation software. In addition, the effect of reaction temperature is investigated by changing the reaction temperature in range of K and the effect of sulfur dioxide feed rate is investigated by varying the sulfur dioxide feed rate from 4 to 6 kg/hr. II. EXPERIMENTAL METHODS A. Plug flow reactor simulation A plug flow reactor is selected to use in this work because it is a better choice over a CSTR [5].This process based on the low feed rate of sulfur dioxide (SO 2 ) to the Bunsen reactor that is designed by Matt Channon. The reactor s specification for this work is shown in Fig. 2. The simulation flow model in commercial simulation software is shown in Fig. 3. SO 2, I 2, H 2O, H 2SO 4, HI Fig. 3.The simulation model of Bunsen reaction in plug flow reactor. This reactor is produced from a glass lined pipe and equipped with a cooling jacket. The materials for producing the cooling jacket depend on the section of coolant [5]. For this work, the cooling jacket is made by glass and water use as a coolant. In the simulation section, the temperature in a Bunsen reaction operates at 33 K with excess iodine and water feed rate [7]. The feed rate of sulfur dioxide to the Bunsen reactor is 5 kg/hr. TABLE I FEED RATE AND VALUES RECOMMENDED BY Lee et al. (28) Species Feed in to reactor Iodine Sulfur dioxide 5 Water In this work, the simulation results of Bunsen reaction by commercial simulation software are compared with experimental data [5]. In addition, the effects of reaction temperature and sulfur dioxide feed rate are studied. Coolant out Cooling jacket Cooling in Reactor s specification Inner diameter 1 inch Length 12 inches III. RESULTS AND DISCUSSION In order to validate the model, the amount of iodine, sulfur dioxide, water, sulfuric acid and hydroiodic acid are compared with experimental data; the relative discrepancies are.22 %, 1.43%,.17%, 2.85% and 2.88%, respectively. The simulation results are slightly different from the experimental data as presented in Table II. Therefore, the model is reliable to be used to study the effects of reaction temperature and sulfur dioxide feed rate. SO 2, I 2, H 2O Fig. 2.The design of plug flow reactor. ISSN: (Print); ISSN: (Online) IMECS 215
3 IMECS 215, March 18-2, 215, Hong Kong Species Feed in to reactor TABLE II SIMULATION RESULTS Output from reactor experimental Simulation data Relative discrepancy (%) I SO H 2O H 2SO HI Iodine A. Effect of reaction temperature on the Bunsen reaction To investigate the influence of reaction temperature, the reactants such as iodine (I 2 ), sulfur dioxide (SO 2 ) and water (H 2 O) are fed into the reactor with flow rate equal to 991.4, 5 and kg/hr, respectively. The reaction temperature that use in this work is in range of 293 K to 393 K sulfuric acid Hydroiodic acid Fig. 4.The effect of temperature on the amount of products (sulfuric acid and hydroiodic acid) Sulfur dioxide Fig. 5a.The effect of temperature on the amount of sulfur dioxide. Fig.5b.The effect of temperature on the amount of iodine Fig.5c.The effect of temperature on the amount of water. From Fig.4., the trends of the amounts of sulfuric acid (H 2 SO 4 ) and hydroiodic acid (HI) are increased when the reaction temperature is increased from 293 K to 373 K. However, the amounts of products start to decrease when the reaction temperature is higher than 373 K. These results indicate that the reaction temperature higher than 373 K promotes the side reaction and inverse Bunsen reaction. In the other hand, the amount of reactants (sulfur dioxide, iodine and water) are decreased when the reaction temperature is increased from 293 K to 393 K but it increases when the reaction temperature is higher than 373 K as shown in Fig. 5a-5c. These phenomena can be explained by the endothermic property of the Bunsen reaction and the active kinetic of the compositions in both of two acid phases at the higher reaction temperature [1, 8, and 1]. B. Effect of the sulfur dioxide feed rate on the Bunsen reaction Water To study the effect of the sulfur dioxide feed rate on the Bunsen reaction, the experiment are performed by varying the sulfur dioxide feed rate from 4 to 6 kg/hr and operated at 293 K. The water and iodine feed rate are set as constant at 182.8, kg/hr, respectively. ISSN: (Print); ISSN: (Online) IMECS 215
4 IMECS 215, March 18-2, 215, Hong Kong Fig.6.The effect of sulfur dioxidefeed rate on the amount of products and reactants. Output of hydroiodic acid from reactor Fig.7a.The effect of sulfur dioxidefeed rate on the amount of hydroiodic acid. Output of sulfuric acid from reactor Iodine Sulfur dioxide Water feed rate 4 kg/hr feed rate 45 kg/hr feed rate 5 kg/hr feed rate 55 kg/hr feed rate 6 kg/hr Sulfuric acid Hydroiodic acid Fig.7b.The effect of sulfur dioxidefeed rate on the amount of sulfuric acid. Output of water from reactor Output of iodine from reactor Fig.7c.The effect of sulfur dioxidefeed rate on the amount of iodine Fig.7d.The effect of sulfur dioxidefeed rate on the amount of water. From Fig. 7a and 7b., the amounts of sulfuric acid (H 2 SO 4 ) and hydroiodic acid (HI) are increased when the sulfur dioxide feed rate is increased. These results show that the amount of products are directly depended on sulfur dioxide feed rate. In contrast, the amount of iodine and water are decreased when increasing the feed rate of sulfur dioxide as shown in Fig. 7c-7d. IV. CONCLUSION The Bunsen reaction in a plug flow reactor is simulated by commercial simulation software. In order to validate the model, the amount of iodine, sulfur dioxide, water, sulfuric acid and hydroiodic acid are compared with experimental data. The simulation results show a slightly different trend from the experimental data and there are guarantee that this model is reliable to study the effects of reaction temperature and sulfur dioxide feed rate in the sulfur iodine production cycle (IS) reactions. In the effect of reaction temperature, the amounts of products (hydroiodic acid and sulfuric acid) increase when the reaction temperature is raised from 293 to 373 K but they decrease when the temperature is higher than 373 K. These results indicate that the reaction temperature of higher than 373 K produce elemental sulfur or hydrogen sulfide (H 2 S) [8] and inverse the Bunsen reaction. Moreover, the amounts of products (hydroiodic acid and sulfuric acid) are directly increased when the feed rate of sulfur dioxide increase. ISSN: (Print); ISSN: (Online) IMECS 215
5 IMECS 215, March 18-2, 215, Hong Kong ACKNOWLEDGMENT The authors would like to acknowledge financial support of the Research Grant No. PHD/335/2552 from the Royal Golden Jubilee Ph.D.Program and Chulalongkornuniversity. REFERENCES [1] Q. Zhu, Y. Zhang, Z. Ying, J. Zhou, Z. Wang and K. Cen, Occurrence of the Bunsen side reaction in the sulfur-iodine thermochemical cycle for hydrogen production, Journal of Zhejiang University-SCIENCE A (Applied Physics & Engineering), vol.14(4), pp. 3-36, 213. [2] M. E. Davis and W. L. Conger, An entropy production and efficiency analysis of the Bunsen reaction in the general atomic sulfur-iodine thermochemical hydrogen production cycle, International Journal of Hydrogen Energy, vol.5, pp , 198. [3] Norman, J., Besenbruch, G., Brown, L., O Keefe, D. and Allen, C., Thermochemical Water-Spitting Cycle, Bench-Scale Investigations, and Process Engineering. Final Report, February 1977 December 31, 1981 General Atomics-Report GA-A16713, DOE Report DOE/ET/ [4] Q. Zhu, Y. Zhang, C. Zhao, Z. Wang, J. Zhouand K. Cen Optimization of Liquid-Liquid Phase Separation Characteristics in the Bunsen Section of the Sulfur-Iodine Hydrogen Production Process, International Journal of Hydrogen Energy, vol. 37, pp , 212. [5] R. C. Moore, Design options for a Bunsen reactor, Sandia National Laboratories, October 212. [6] Y. Zhang, P. Peng, Z. Ying, Q. Zhu, J. Zhou, Z. Wang, J. Liu and K. Cen, Experimental investigation on multiphase Bunsen reaction in the thermochemical Sulfur-iodine cycle Industrial & Engineering Chemistry Research, vol.53, pp , February 214. [7] B. J. Lee, H. C. NO, H. J. Yoon, S. J. Kim and E. S. Kim, An optimal operating window for the Bunsen process in the I-S thermochemical cycle, International Journal of Hydrogen Energy, vol.33, pp , 28. [8] M. Sakurai, H. Nakajima, R. Amir, K. Onuki and S. Shimizu, Experimental study on side-reaction occurrence condition in the iodine-sulfur thermochemical hydrogen production process,international Journal of Hydrogen Energy, vol.25, pp , 2. [9] H. S. Kim, Y. H. Kim, S. J. Han, C. S. Park, K. K. Bae and J. G. Lee, Continuous Bunsen reaction and simultaneous separation using a counter-current flow reactor for the sulfur-iodine hydrogen production process, International Journal of Hydrogen Energy, vol.38, pp , 213. [1] M. Parisi, A. Giaconia, S. Sau, A. Spadoni, G. Caputo and P.Tarquini, Bunsen reaction and hydriodic phase purification in the sulfur-iodine process: An experimental investigation, International Journal of Hydrogen Energy, vol.36, pp , 211. ISSN: (Print); ISSN: (Online) IMECS 215
Occurrence of the Bunsen side reaction in the sulfur-iodine thermochemical cycle for hydrogen production *
 300 Journal of Zhejiang University-SCIENCE A (Applied Physics & Engineering) ISSN 1673-565X (Print); ISSN 1862-1775 (Online) www.zju.edu.cn/jzus; www.springerlink.com E-mail: jzus@zju.edu.cn Occurrence
300 Journal of Zhejiang University-SCIENCE A (Applied Physics & Engineering) ISSN 1673-565X (Print); ISSN 1862-1775 (Online) www.zju.edu.cn/jzus; www.springerlink.com E-mail: jzus@zju.edu.cn Occurrence
Effects of Excess Iodine and Water on Bunsen Reaction for Over-Azeotropic Limit
 Effects of Excess Iodine and Water on Bunsen Reaction for Over-Azeotropic Limit Sachidanand R. Satpute 1, Jun Kyu Park 2, Shripad T. Revankar *3 1 Department of Chemical Engineering, College of Engineering,
Effects of Excess Iodine and Water on Bunsen Reaction for Over-Azeotropic Limit Sachidanand R. Satpute 1, Jun Kyu Park 2, Shripad T. Revankar *3 1 Department of Chemical Engineering, College of Engineering,
Membrane distillation of H 2 O-HI mixtures for the S-I thermochemical water splitting process
 Membrane distillation of H 2 O-HI mixtures for the S-I thermochemical water splitting process Caputo G., Giaconia A., Felici C., Lanchi M., Sau S. ENEA Casaccia Research Centre, Via Anguillarese 301, 00123
Membrane distillation of H 2 O-HI mixtures for the S-I thermochemical water splitting process Caputo G., Giaconia A., Felici C., Lanchi M., Sau S. ENEA Casaccia Research Centre, Via Anguillarese 301, 00123
"Thermodynamic Analysis of Processes for Hydrogen Generation by Decomposition of Water"
 "Thermodynamic Analysis of Processes for Hydrogen Generation by Decomposition of Water" by John P. O'Connell Department of Chemical Engineering University of Virginia Charlottesville, VA 22904-4741 A Set
"Thermodynamic Analysis of Processes for Hydrogen Generation by Decomposition of Water" by John P. O'Connell Department of Chemical Engineering University of Virginia Charlottesville, VA 22904-4741 A Set
The catalytic decomposition of hydrogen iodide in the IS thermochemical cycle
 The catalytic decomposition of hydrogen iodide in the IS thermochemical cycle Chu-Sik Park, Jung-Min KIM, Kyoung-Soo KANG, Gab-Jin Hwang, Ki-Kwang Bae Hydrogen Energy Research Group, Korea Institute of
The catalytic decomposition of hydrogen iodide in the IS thermochemical cycle Chu-Sik Park, Jung-Min KIM, Kyoung-Soo KANG, Gab-Jin Hwang, Ki-Kwang Bae Hydrogen Energy Research Group, Korea Institute of
Optimization of Batch Distillation Involving Hydrolysis System
 273 Optimization of Batch Distillation Involving Hydrolysis System Elmahboub A. Edreder 1, Iqbal M. Mujtaba 2, Mansour Emtir 3* 1 Libyan Petroleum Institute, P.O. Box 6431, Tripoli, Libya 2 School of Engineering
273 Optimization of Batch Distillation Involving Hydrolysis System Elmahboub A. Edreder 1, Iqbal M. Mujtaba 2, Mansour Emtir 3* 1 Libyan Petroleum Institute, P.O. Box 6431, Tripoli, Libya 2 School of Engineering
VAPOUR REACTIVE DISTILLATION PROCESS FOR HYDROGEN
 VAPOUR REACTIVE DISTILLATION PROCESS FOR HYDROGEN PRODUCTION BY HI DECOMPOSITION FROM HI- -H 2 O SOLUTIONS B. BELAISSAOUI, R.THERY, X.M. MEYER 1, M. MEYER, V. GERBAUD and X. JOULIA LGC-Laboratoire de Génie
VAPOUR REACTIVE DISTILLATION PROCESS FOR HYDROGEN PRODUCTION BY HI DECOMPOSITION FROM HI- -H 2 O SOLUTIONS B. BELAISSAOUI, R.THERY, X.M. MEYER 1, M. MEYER, V. GERBAUD and X. JOULIA LGC-Laboratoire de Génie
R&D Program on Thermochemical Water-Splitting Iodine-Sulfur Process at JAERI
 GENES4/ANP2003, Sep. 15-19, 2003, Kyoto, JAPAN Paper 1072 R&D Program on Thermochemical Water-Splitting Iodine-Sulfur Process at JAERI Shinji KUBO, Hayato NAKAJIMA, Shunichi HIGASHI, Tomoo MASAKI, Seiji
GENES4/ANP2003, Sep. 15-19, 2003, Kyoto, JAPAN Paper 1072 R&D Program on Thermochemical Water-Splitting Iodine-Sulfur Process at JAERI Shinji KUBO, Hayato NAKAJIMA, Shunichi HIGASHI, Tomoo MASAKI, Seiji
Chemistry Chapter 16. Reaction Energy
 Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Chemistry Stoichiometry and Heat Exam (ver.1) Mr. Thaler. Please do not write on this exam. Mark your answers on the scantron only.
 1. Identify from the unbalanced equations below the one that does not represent a redox reaction. a. H 2O 2(aq) + MnO 4 - (aq) O 2(g) + Mn 2+ (aq) b. H 2(g) + N 2(g) NH 3(g) c. NaCl (aq) + AgNO 3(aq) NaNO
1. Identify from the unbalanced equations below the one that does not represent a redox reaction. a. H 2O 2(aq) + MnO 4 - (aq) O 2(g) + Mn 2+ (aq) b. H 2(g) + N 2(g) NH 3(g) c. NaCl (aq) + AgNO 3(aq) NaNO
EQUILIBRIUM CONSTANT, K eq or K. The Law of Chemical Equilibrium: (Guldberg & Waage, 1864)
 1 EQUILIBRIUM CONSTANT, K eq or K The Law of Chemical Equilibrium: (Guldberg & Waage, 1864) States that: At equilibrium, there is a constant ratio between the concentration of the products and the concentration
1 EQUILIBRIUM CONSTANT, K eq or K The Law of Chemical Equilibrium: (Guldberg & Waage, 1864) States that: At equilibrium, there is a constant ratio between the concentration of the products and the concentration
Chemical Reactions. Chemical changes are occurring around us all the time
 Chemical changes are occurring around us all the time Food cooking Fuel being burned in a car s engine Oxygen being used in the human body The starting materials are called reactants The ending materials
Chemical changes are occurring around us all the time Food cooking Fuel being burned in a car s engine Oxygen being used in the human body The starting materials are called reactants The ending materials
Fundamentals of Combustion
 Fundamentals of Combustion Lec 3: Chemical Thermodynamics Dr. Zayed Al-Hamamre Content Process Heat Transfer 1-3 Process Heat Transfer 1-4 Process Heat Transfer 1-5 Theoretical and Excess Air Combustion
Fundamentals of Combustion Lec 3: Chemical Thermodynamics Dr. Zayed Al-Hamamre Content Process Heat Transfer 1-3 Process Heat Transfer 1-4 Process Heat Transfer 1-5 Theoretical and Excess Air Combustion
Integrated Knowledge Based System for Process Synthesis
 17 th European Symposium on Computer Aided Process Engineering ESCAPE17 V. Plesu and P.S. Agachi (Editors) 2007 Elsevier B.V. All rights reserved. 1 Integrated Knowledge Based System for Process Synthesis
17 th European Symposium on Computer Aided Process Engineering ESCAPE17 V. Plesu and P.S. Agachi (Editors) 2007 Elsevier B.V. All rights reserved. 1 Integrated Knowledge Based System for Process Synthesis
Name: Unit 9- Stoichiometry Day Page # Description IC/HW
 Name: Unit 9- Stoichiometry Day Page # Description IC/HW Due Date Completed ALL 2 Warm-up IC 1 3 Stoichiometry Notes IC 1 4 Mole Map IC X 1 5 Mole to Mole Practice IC 1 6 Mass to Mole Practice IC 1/2 X
Name: Unit 9- Stoichiometry Day Page # Description IC/HW Due Date Completed ALL 2 Warm-up IC 1 3 Stoichiometry Notes IC 1 4 Mole Map IC X 1 5 Mole to Mole Practice IC 1 6 Mass to Mole Practice IC 1/2 X
Hydrogen Sulfide Removal from Waste Air by Oxidation reaction with Sodium Hypochlorite in CSTR
 he9 The Forth PSU Engineering onference 8-9 December 5 ydrogen Sulfide Removal from Waste Air by Oxidation reaction with Sodium ypochlorite in STR harun Bunyakan* Juntima hungsiriporn Junya Intamanee azardous
he9 The Forth PSU Engineering onference 8-9 December 5 ydrogen Sulfide Removal from Waste Air by Oxidation reaction with Sodium ypochlorite in STR harun Bunyakan* Juntima hungsiriporn Junya Intamanee azardous
Lecture (9) Reactor Sizing. Figure (1). Information needed to predict what a reactor can do.
 Lecture (9) Reactor Sizing 1.Introduction Chemical kinetics is the study of chemical reaction rates and reaction mechanisms. The study of chemical reaction engineering (CRE) combines the study of chemical
Lecture (9) Reactor Sizing 1.Introduction Chemical kinetics is the study of chemical reaction rates and reaction mechanisms. The study of chemical reaction engineering (CRE) combines the study of chemical
Section 1 - Thermochemistry
 Reaction Energy Section 1 - Thermochemistry Virtually every chemical reaction is accompanied by a change in energy. Chemical reactions usually absorb or release energy as heat. You learned in Chapter 12
Reaction Energy Section 1 - Thermochemistry Virtually every chemical reaction is accompanied by a change in energy. Chemical reactions usually absorb or release energy as heat. You learned in Chapter 12
Student Achievement. Chemistry 12
 Student Achievement Chemistry 12 Key Elements: Reaction Kinetics Estimated Time: 14 16 hours By the end of this course, students will be able to explain the significance of reaction rates, demonstrate
Student Achievement Chemistry 12 Key Elements: Reaction Kinetics Estimated Time: 14 16 hours By the end of this course, students will be able to explain the significance of reaction rates, demonstrate
Quantitative Relationships in Chemical Reactions Chapter 7
 Quantitative Relationships in Chemical Reactions Chapter 7 The burning of charcoal releases heat (thermal energy) that grills our food. But the combustion of charcoal and fossil fuels also releases CO
Quantitative Relationships in Chemical Reactions Chapter 7 The burning of charcoal releases heat (thermal energy) that grills our food. But the combustion of charcoal and fossil fuels also releases CO
Additional Calculations: 10. How many joules are required to change the temperature of 80.0 g of water from 23.3 C to 38.8 C?
 Additional Calculations: 10. How many joules are required to change the temperature of 80.0 g of water from 23.3 C to 38.8 C? q = m C T 80 g (4.18 J/gC)(38.8-23.3C) = 5183 J 11. A piece of metal weighing
Additional Calculations: 10. How many joules are required to change the temperature of 80.0 g of water from 23.3 C to 38.8 C? q = m C T 80 g (4.18 J/gC)(38.8-23.3C) = 5183 J 11. A piece of metal weighing
Accelerated Chemistry Study Guide Chapter 12, sections 1 and 2: Heat in Chemical Reactions
 Accelerated Chemistry Study Guide Chapter 12, sections 1 and 2: Heat in Chemical Reactions Terms, definitions, topics Joule, calorie (Re-read p 57-58) Thermochemistry Exothermic reaction Endothermic reaction
Accelerated Chemistry Study Guide Chapter 12, sections 1 and 2: Heat in Chemical Reactions Terms, definitions, topics Joule, calorie (Re-read p 57-58) Thermochemistry Exothermic reaction Endothermic reaction
Ni catalyst deactivation in the reaction of hydrogen iodide
 Ni catalyst deactivation in the reaction of hydrogen iodide Favuzza P. a, Felici C. a, Mazzocchia C. b, Spadoni A. a, Tarquini P. a, Tito A.C. b a. Enea Casaccia, 31 Via Anguillarese, 3 S.Maria di Galeria,
Ni catalyst deactivation in the reaction of hydrogen iodide Favuzza P. a, Felici C. a, Mazzocchia C. b, Spadoni A. a, Tarquini P. a, Tito A.C. b a. Enea Casaccia, 31 Via Anguillarese, 3 S.Maria di Galeria,
The SI cycle was first developed by General Atomics (GA) in the early 70s. The cycle is characterised by the following reactions: Equation 3: 2HI H
 Novel organic solvents for the Bunsen Reaction Marie L. Taylor 1, Peter Styring 1 and Ray W. K. Allen 1 1 Department of Chemical and Process Engineering, University of Sheffield, Mappin Street, Sheffield,
Novel organic solvents for the Bunsen Reaction Marie L. Taylor 1, Peter Styring 1 and Ray W. K. Allen 1 1 Department of Chemical and Process Engineering, University of Sheffield, Mappin Street, Sheffield,
Persulfate salt as an oxidizer for biocidal energetic nano-thermites
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supplemental information Persulfate salt as an oxidizer for biocidal energetic
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supplemental information Persulfate salt as an oxidizer for biocidal energetic
Chemistry 101 Chapter 10 Energy
 Chemistry 101 Chapter 10 Energy Energy: the ability to do work or produce heat. Kinetic energy (KE): is the energy of motion. Any object that is moving has kinetic energy. Several forms of kinetic energy
Chemistry 101 Chapter 10 Energy Energy: the ability to do work or produce heat. Kinetic energy (KE): is the energy of motion. Any object that is moving has kinetic energy. Several forms of kinetic energy
Chemical Reaction Engineering. Multiple Reactions. Dr.-Eng. Zayed Al-Hamamre
 Chemical Reaction Engineering Multiple Reactions Dr.-Eng. Zayed Al-Hamamre 1 Content Types of Reactions Selectivity Reaction Yield Parallel Reactions Series Reactions Net Rates of Reaction Complex Reactions
Chemical Reaction Engineering Multiple Reactions Dr.-Eng. Zayed Al-Hamamre 1 Content Types of Reactions Selectivity Reaction Yield Parallel Reactions Series Reactions Net Rates of Reaction Complex Reactions
Stoichiometric Reactor Module
 Reactor Analysis Types of Reactors Stoichiometric Reactor Module Stoichiometric Reactors (1) Stoichiometric Reactors (2) Stoichiometric Reactors (3) Equilibrium Reactors Gibbs Reactor RGibbs Module Equilibrium
Reactor Analysis Types of Reactors Stoichiometric Reactor Module Stoichiometric Reactors (1) Stoichiometric Reactors (2) Stoichiometric Reactors (3) Equilibrium Reactors Gibbs Reactor RGibbs Module Equilibrium
Chemistry: The Central Science. Chapter 5: Thermochemistry
 Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
(b) Increase in pressure. (1)
 1 This question is about the equilibrium reaction between hydrogen and carbon dioxide. H 2 (g) + O 2 (g) H 2 O(g) + O(g) H = +40 kj mol 1 What effect would the following changes have on the rate of reaction
1 This question is about the equilibrium reaction between hydrogen and carbon dioxide. H 2 (g) + O 2 (g) H 2 O(g) + O(g) H = +40 kj mol 1 What effect would the following changes have on the rate of reaction
Integral-based Algorithm for Parameter Identification of the Heat Exchanger
 Proceedings of the International MultiConference of Engineers and Computer Scientists 218 Vol II IMECS 218, March 14-16, 218, Hong Kong Integral-based Algorithm for Parameter Identification of the Heat
Proceedings of the International MultiConference of Engineers and Computer Scientists 218 Vol II IMECS 218, March 14-16, 218, Hong Kong Integral-based Algorithm for Parameter Identification of the Heat
Process design decisions and project economics Dr. V. S. Moholkar Department of chemical engineering Indian Institute of Technology, Guwahati
 Process design decisions and project economics Dr. V. S. Moholkar Department of chemical engineering Indian Institute of Technology, Guwahati Module - 02 Flowsheet Synthesis (Conceptual Design of a Chemical
Process design decisions and project economics Dr. V. S. Moholkar Department of chemical engineering Indian Institute of Technology, Guwahati Module - 02 Flowsheet Synthesis (Conceptual Design of a Chemical
INTEGRATED PROCESS FOR γ-butyrolactone PRODUCTION
 U.P.B. Sci. Bull., Series B, Vol. 76, Iss. 3, 214 ISSN 1454 2331 INTEGRATED PROCESS FOR γ-butyrolactone PRODUCTION Ahtesham JAVAID 1, Costin Sorin BILDEA 2 An integrated process for the production of γ-butyrolactone
U.P.B. Sci. Bull., Series B, Vol. 76, Iss. 3, 214 ISSN 1454 2331 INTEGRATED PROCESS FOR γ-butyrolactone PRODUCTION Ahtesham JAVAID 1, Costin Sorin BILDEA 2 An integrated process for the production of γ-butyrolactone
11B, 11E Temperature and heat are related but not identical.
 Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Data requirements for reactor selection. Professor John H Atherton
 Data requirements for reactor selection Professor John H Atherton What is my best option for manufacturing scale? Microreactor Mesoscale tubular reactor Tubular reactor OBR (oscillatory baffled flow reactor)
Data requirements for reactor selection Professor John H Atherton What is my best option for manufacturing scale? Microreactor Mesoscale tubular reactor Tubular reactor OBR (oscillatory baffled flow reactor)
NN-Based MPC for Heat Exchanger System in Hard Chrome Electroplating
 P. Kittisupakorn, E. Nueaklong, and W. Daosud 21 NN-Based MPC for Heat Exchanger System in Hard Chrome Electroplating P. Kittisupakorn 1, E. Nueaklong 1, and W. Daosud 2, ABSTRACT To develop an accurate
P. Kittisupakorn, E. Nueaklong, and W. Daosud 21 NN-Based MPC for Heat Exchanger System in Hard Chrome Electroplating P. Kittisupakorn 1, E. Nueaklong 1, and W. Daosud 2, ABSTRACT To develop an accurate
Interactions between oxygen permeation and homogeneous-phase fuel conversion on the sweep side of an ion transport membrane
 Interactions between oxygen permeation and homogeneous-phase fuel conversion on the sweep side of an ion transport membrane The MIT Faculty has made this article openly available. Please share how this
Interactions between oxygen permeation and homogeneous-phase fuel conversion on the sweep side of an ion transport membrane The MIT Faculty has made this article openly available. Please share how this
Energy and Chemical Change
 Energy and Chemical Change Section 16.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Energy and Chemical Change Section 16.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
QUESTIONS: Equilibria AS & AS
 QUESTION (2012:2) Phosphorus pentachloride gas, PCl 5 (g), decomposes to form phosphorus trichloride gas, PCl 3 (g), and chlorine gas, Cl 2 (g). The equilibrium can be represented as: PCl 5 (g) Ý PCl 3
QUESTION (2012:2) Phosphorus pentachloride gas, PCl 5 (g), decomposes to form phosphorus trichloride gas, PCl 3 (g), and chlorine gas, Cl 2 (g). The equilibrium can be represented as: PCl 5 (g) Ý PCl 3
Chapter 6. Thermochemistry
 Chapter 6 Thermochemistry Section 5.6 The Kinetic Molecular Theory of Gases http://www.scuc.txed.net/webpages/dmackey/files /chap06notes.pdf ..\..\..\..\..\..\Videos\AP Videos\Thermochemistry\AP
Chapter 6 Thermochemistry Section 5.6 The Kinetic Molecular Theory of Gases http://www.scuc.txed.net/webpages/dmackey/files /chap06notes.pdf ..\..\..\..\..\..\Videos\AP Videos\Thermochemistry\AP
17.2 Thermochemical Equations
 17.2. Thermochemical Equations www.ck12.org 17.2 Thermochemical Equations Lesson Objectives Define enthalpy, and know the conditions under which the enthalpy change in a reaction is equal to the heat absorbed
17.2. Thermochemical Equations www.ck12.org 17.2 Thermochemical Equations Lesson Objectives Define enthalpy, and know the conditions under which the enthalpy change in a reaction is equal to the heat absorbed
The reactions we have dealt with so far in chemistry are considered irreversible.
 1. Equilibrium Students: model static and dynamic equilibrium and analyse the differences between open and closed systems investigate the relationship between collision theory and reaction rate in order
1. Equilibrium Students: model static and dynamic equilibrium and analyse the differences between open and closed systems investigate the relationship between collision theory and reaction rate in order
SIR MICHELANGELO REFALO
 SIR MICELANGELO REFALO SIXT FORM alf-yearly Exam 2014 Name: CEMISTRY ADV 1 ST 3 hrs ANSWER ANY 7 QUESTIONS. All questions carry equal marks. You are reminded of the importance of clear presentation in
SIR MICELANGELO REFALO SIXT FORM alf-yearly Exam 2014 Name: CEMISTRY ADV 1 ST 3 hrs ANSWER ANY 7 QUESTIONS. All questions carry equal marks. You are reminded of the importance of clear presentation in
Burgoyne Consultants Ltd., Burgoyne House, Chantry Drive, Ilkley, West Yorkshire, U.K.
 A SIMPLE METHOD OF ESTIMATING EXOTHERMICITY BY AVERAGE BOND ENERGY SUMMATION ARTHUR D. CRAVEN A simple method is described whereby the approximate exothermicity of a chemical reaction or decomposition
A SIMPLE METHOD OF ESTIMATING EXOTHERMICITY BY AVERAGE BOND ENERGY SUMMATION ARTHUR D. CRAVEN A simple method is described whereby the approximate exothermicity of a chemical reaction or decomposition
CHAPTER 2 CONTINUOUS STIRRED TANK REACTOR PROCESS DESCRIPTION
 11 CHAPTER 2 CONTINUOUS STIRRED TANK REACTOR PROCESS DESCRIPTION 2.1 INTRODUCTION This chapter deals with the process description and analysis of CSTR. The process inputs, states and outputs are identified
11 CHAPTER 2 CONTINUOUS STIRRED TANK REACTOR PROCESS DESCRIPTION 2.1 INTRODUCTION This chapter deals with the process description and analysis of CSTR. The process inputs, states and outputs are identified
Allotropes (Diamond and Graphite) Revision Pack (C3)
 Allotropes: Allotropes are different forms of the same element in the same physical state; the atoms are bonded differently. Carbon has allotropes: - Diamond - Graphite - Buckminsterfullerene Diamond Properties
Allotropes: Allotropes are different forms of the same element in the same physical state; the atoms are bonded differently. Carbon has allotropes: - Diamond - Graphite - Buckminsterfullerene Diamond Properties
CLIFFSIDE PARK HIGH SCHOOL CURRICULUM MAPPING IN CHEMISTRY Month September October November December Essential
 Month September October November December Essential What are the physical and Question(s) chemical properties of matter? Skills/ Objectives How do scientists use tools of measurements in scientific investigations?
Month September October November December Essential What are the physical and Question(s) chemical properties of matter? Skills/ Objectives How do scientists use tools of measurements in scientific investigations?
MSWI Flue Gas Two-Stage Dry Treatment: Modeling and Simulation
 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 26, 2012 Guest Editors: Valerio Cozzani, Eddy De Rademaeker Copyright 2012, AIDIC Servizi S.r.l., ISBN 978-88-95608-17-4; ISSN 1974-9791 The Italian
A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 26, 2012 Guest Editors: Valerio Cozzani, Eddy De Rademaeker Copyright 2012, AIDIC Servizi S.r.l., ISBN 978-88-95608-17-4; ISSN 1974-9791 The Italian
Thermodynamics. Thermodynamics of Chemical Reactions. Enthalpy change
 Thermodynamics 1 st law (Cons of Energy) Deals with changes in energy Energy in chemical systems Total energy of an isolated system is constant Total energy = Potential energy + kinetic energy E p mgh
Thermodynamics 1 st law (Cons of Energy) Deals with changes in energy Energy in chemical systems Total energy of an isolated system is constant Total energy = Potential energy + kinetic energy E p mgh
CHEMICAL EQUILIBRIA: GENERAL CONCEPTS
 CHEMICAL EQUILIBRIA: GENERAL CONCEPTS THE NATURE OF THE EQUILIBRIUM STATE: Equilibrium is the state where the concentrations of all reactants and products remain constant with time. (in stoichiometry,
CHEMICAL EQUILIBRIA: GENERAL CONCEPTS THE NATURE OF THE EQUILIBRIUM STATE: Equilibrium is the state where the concentrations of all reactants and products remain constant with time. (in stoichiometry,
Gravity is a force which keeps us stuck to the earth. The Electrostatic force attracts electrons to protons in an atom.
 Energy Relations in Chemistry: Thermochemistry The Nature of Energy Sugar you eat is "combusted" by your body to produce CO 2 and H 2 O. During this process energy is also released. This energy is used
Energy Relations in Chemistry: Thermochemistry The Nature of Energy Sugar you eat is "combusted" by your body to produce CO 2 and H 2 O. During this process energy is also released. This energy is used
Energy and Chemical Change
 Energy and Chemical Change Section 15.1 Energy Section 15.2 Heat Section 15.3 Thermochemical Equations Section 15.4 Calculating Enthalpy Change Section 15.5 Reaction Spontaneity Click a hyperlink or folder
Energy and Chemical Change Section 15.1 Energy Section 15.2 Heat Section 15.3 Thermochemical Equations Section 15.4 Calculating Enthalpy Change Section 15.5 Reaction Spontaneity Click a hyperlink or folder
(g) + 3 H 2. (g) 2 NH 3. 1) Only gases and dissolved species appear in an equilibrium expression. 4 NH 3. O(g) K c = (s) + 2N 2.
 Chapter 16: Chemical Equilibrium What is meant by an equilibrium system? What is an equilibrium expression? N 2 +3 H 2 2 NH 3 1) Only gases and dissolved species appear in an equilibrium expression. 4
Chapter 16: Chemical Equilibrium What is meant by an equilibrium system? What is an equilibrium expression? N 2 +3 H 2 2 NH 3 1) Only gases and dissolved species appear in an equilibrium expression. 4
IMPROVED ADIABATIC CALORIMETRY IN THE PHI-TEC APPARATUS USING AUTOMATED ON-LINE HEAT LOSS COMPENSATION
 # 27 IChemE IMPROVED ADIABATIC CALORIMETRY IN THE PHI-TEC APPARATUS USING AUTOMATED ON-LINE HEAT LOSS COMPENSATION B Kubascikova, D.G. Tee and S.P. Waldram HEL Ltd, 5 Moxon Street, Barnet, Hertfordshire,
# 27 IChemE IMPROVED ADIABATIC CALORIMETRY IN THE PHI-TEC APPARATUS USING AUTOMATED ON-LINE HEAT LOSS COMPENSATION B Kubascikova, D.G. Tee and S.P. Waldram HEL Ltd, 5 Moxon Street, Barnet, Hertfordshire,
Types of Chemical Reactors. Nasir Hussain Production and Operations Engineer PARCO Oil Refinery
 Types of Chemical Reactors Nasir Hussain Production and Operations Engineer PARCO Oil Refinery Introduction Reactor is the heart of Chemical Process. A vessel designed to contain chemical reactions is
Types of Chemical Reactors Nasir Hussain Production and Operations Engineer PARCO Oil Refinery Introduction Reactor is the heart of Chemical Process. A vessel designed to contain chemical reactions is
Name: Thermochemistry. Practice Test C. General Chemistry Honors Chemistry
 Name: Thermochemistry C Practice Test C General Chemistry Honors Chemistry 1 Objective 1: Use the relationship between mass, specific heat, and temperature change to calculate the heat flow during a chemical
Name: Thermochemistry C Practice Test C General Chemistry Honors Chemistry 1 Objective 1: Use the relationship between mass, specific heat, and temperature change to calculate the heat flow during a chemical
CBE 142: Chemical Kinetics & Reaction Engineering
 CBE 142: Chemical Kinetics & Reaction Engineering Midterm #2 November 6 th 2014 This exam is worth 100 points and 20% of your course grade. Please read through the questions carefully before giving your
CBE 142: Chemical Kinetics & Reaction Engineering Midterm #2 November 6 th 2014 This exam is worth 100 points and 20% of your course grade. Please read through the questions carefully before giving your
Name Date Class THE FLOW OF ENERGY HEAT AND WORK
 17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
Be prepared to discuss the quantitative comparison method in the oral exam.
 Subject: Ring Experiment III 8 Shell and Tube Heat Exchanger Control The shell and Tube Heat Exchanger has two control valves: one on the process fluid flowing to the tubes and one on the cooling water
Subject: Ring Experiment III 8 Shell and Tube Heat Exchanger Control The shell and Tube Heat Exchanger has two control valves: one on the process fluid flowing to the tubes and one on the cooling water
Acetone Process Energy Recovery by Means of Energy Analysis
 Chemical Engineering Letters: Modeling, Simulation and Control 3 (2018) 16 21 Chemical Engineering Letters: Modeling, Simulation and Control Journal Homepage: www.sciera.org/index.php/celmsc Acetone Process
Chemical Engineering Letters: Modeling, Simulation and Control 3 (2018) 16 21 Chemical Engineering Letters: Modeling, Simulation and Control Journal Homepage: www.sciera.org/index.php/celmsc Acetone Process
12.3 Heats of Reaction
 12.3 Heats of Reaction All chemical reactions involve energy changes. Some reactions like combustion (burning) are obviously exothermic. You can feel the heat and see the light emitted from a burning campfire
12.3 Heats of Reaction All chemical reactions involve energy changes. Some reactions like combustion (burning) are obviously exothermic. You can feel the heat and see the light emitted from a burning campfire
CHEMICAL REACTORS - PROBLEMS OF REACTOR ASSOCIATION 47-60
 2011-2012 Course CHEMICL RECTORS - PROBLEMS OF RECTOR SSOCITION 47-60 47.- (exam jan 09) The elementary chemical reaction in liquid phase + B C is carried out in two equal sized CSTR connected in series.
2011-2012 Course CHEMICL RECTORS - PROBLEMS OF RECTOR SSOCITION 47-60 47.- (exam jan 09) The elementary chemical reaction in liquid phase + B C is carried out in two equal sized CSTR connected in series.
Unit 9a: Kinetics and Energy Changes
 Unit 9a: Kinetics and Energy Changes Student Name: Key Class Period: Website upload 2015 Page 1 of 43 Unit 9a (Kinetics & Energy Changes) Key Page intentionally blank Website upload 2015 Page 2 of 43 Unit
Unit 9a: Kinetics and Energy Changes Student Name: Key Class Period: Website upload 2015 Page 1 of 43 Unit 9a (Kinetics & Energy Changes) Key Page intentionally blank Website upload 2015 Page 2 of 43 Unit
Chemical Engineering
 Chemical Engineering Basic Principles: Energy and material balances Transport Processes Momentum Transfer: Fluid Flow Energy Transfer: Heat Mass Transfer: mixing and separation processes Physical and Chemical
Chemical Engineering Basic Principles: Energy and material balances Transport Processes Momentum Transfer: Fluid Flow Energy Transfer: Heat Mass Transfer: mixing and separation processes Physical and Chemical
6. Which expression correctly describes the equilibrium constant for the following reaction? 4NH 3 (g) + 5O 2 (g) 4NO(g) + 6H 2 O(g)
 1. Which of the following can we predict from an equilibrium constant for a reaction? 1. The extent of a reaction 2. Whether the reaction is fast or slow 3. Whether a reaction is exothermic or endothermic
1. Which of the following can we predict from an equilibrium constant for a reaction? 1. The extent of a reaction 2. Whether the reaction is fast or slow 3. Whether a reaction is exothermic or endothermic
AN ABSTRACT OF THE DISSERTATION OF. Kevin R. Caple for the degree of Doctor of Philosophy in Chemical Engineering presented on January 24, 2014.
 1 AN ABSTRACT OF THE DISSERTATION OF Kevin R. Caple for the degree of Doctor of Philosophy in Chemical Engineering presented on January 24, 2014. Title: Detailed Analysis of the Hydrogen Sulfide Production
1 AN ABSTRACT OF THE DISSERTATION OF Kevin R. Caple for the degree of Doctor of Philosophy in Chemical Engineering presented on January 24, 2014. Title: Detailed Analysis of the Hydrogen Sulfide Production
Observations of Container. Hot Same Size. Hot Same Size. Hot Same Size. Observations of Container. Cold Expanded. Cold Expanded.
 Chapter 9: Phenomena Phenomena: Below is data from three different reactions carried out with three different amounts of reactants. All reactants were carried out in expandable/contractable containers
Chapter 9: Phenomena Phenomena: Below is data from three different reactions carried out with three different amounts of reactants. All reactants were carried out in expandable/contractable containers
Chapter Objectives. Chapter 9 Energy and Chemistry. Chapter Objectives. Energy Use and the World Economy. Energy Use and the World Economy
 Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
Chemical Reaction Engineering - Part 12 - multiple reactions Richard K. Herz,
 Chemical Reaction Engineering - Part 12 - multiple reactions Richard K. Herz, rherz@ucsd.edu, www.reactorlab.net Multiple reactions are usually present So far we have considered reactors in which only
Chemical Reaction Engineering - Part 12 - multiple reactions Richard K. Herz, rherz@ucsd.edu, www.reactorlab.net Multiple reactions are usually present So far we have considered reactors in which only
Direct measurement of giant electrocaloric effect in BaTiO 3 multilayer thick film structure beyond theoretical prediction
 Direct measurement of giant electrocaloric effect in BaTiO 3 multilayer thick film structure beyond theoretical prediction Yang Bai 1,2, Guangping Zheng 1 and Sanqiang Shi 1 1 Department of Mechanical
Direct measurement of giant electrocaloric effect in BaTiO 3 multilayer thick film structure beyond theoretical prediction Yang Bai 1,2, Guangping Zheng 1 and Sanqiang Shi 1 1 Department of Mechanical
This reaction is ENDOTHERMIC. Energy is being transferred from the room/flask/etc. (the SURROUNDINGS) to the reaction itself (the SYSTEM).
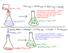 151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
s Traditionally, we use the calorie as a unit of energy. The nutritional Calorie, Cal = 1000 cal. Kinetic Energy and Potential Energy
 AP Chemistry: Thermochemistry Lecture Outline 5.1 The Nature of Energy Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationships between chemical
AP Chemistry: Thermochemistry Lecture Outline 5.1 The Nature of Energy Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationships between chemical
CHAPTER 16 REVIEW. Reaction Energy. SHORT ANSWER Answer the following questions in the space provided.
 CHAPTER 16 REVIEW Reaction Energy SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. For elements in their standard state, the value of H 0 f is 0. 2. The formation and decomposition
CHAPTER 16 REVIEW Reaction Energy SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. For elements in their standard state, the value of H 0 f is 0. 2. The formation and decomposition
Experimental Assessment of Unbalanced Magnetic Force according to Rotor Eccentricity in Permanent Magnet Machine
 Journal of Magnetics 23(1), 68-73 (218) ISSN (Print) 1226-175 ISSN (Online) 2233-6656 https://doi.org/1.4283/jmag.218.23.1.68 Experimental Assessment of Unbalanced Magnetic Force according to Rotor Eccentricity
Journal of Magnetics 23(1), 68-73 (218) ISSN (Print) 1226-175 ISSN (Online) 2233-6656 https://doi.org/1.4283/jmag.218.23.1.68 Experimental Assessment of Unbalanced Magnetic Force according to Rotor Eccentricity
Developing Carbon Tolerance Catalyst for Dry Methane Reforming
 745 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 32, 2013 Chief Editors: Sauro Pierucci, Jiří J. Klemeš Copyright 2013, AIDIC Servizi S.r.l., ISBN 978-88-95608-23-5; ISSN 1974-9791 The Italian
745 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 32, 2013 Chief Editors: Sauro Pierucci, Jiří J. Klemeš Copyright 2013, AIDIC Servizi S.r.l., ISBN 978-88-95608-23-5; ISSN 1974-9791 The Italian
Matter and Energy. Section 2.1 Chapter 2. Representations of Matter: Models and Symbols. Goal 1. Goal 2
 Section 2.1 Chapter 2 Matter and Energy Representations of Matter: Models and Symbols Goal 1 Goal 2 Identify and explain the difference among observations of matter at the macroscopic, microscopic, and
Section 2.1 Chapter 2 Matter and Energy Representations of Matter: Models and Symbols Goal 1 Goal 2 Identify and explain the difference among observations of matter at the macroscopic, microscopic, and
C. S. R. Prasad, H. Z. Fani, S. B. Menon
 Heterogeneous Bunsen Reaction nalysis & Experimental study of Chemical absorption of Sulfur dioxide and dissolution of Iodine into aqueous reacting system C. S. R. Prasad, H. Z. Fani, S. B. Menon Chemical
Heterogeneous Bunsen Reaction nalysis & Experimental study of Chemical absorption of Sulfur dioxide and dissolution of Iodine into aqueous reacting system C. S. R. Prasad, H. Z. Fani, S. B. Menon Chemical
Carbon dioxide reforming of methane under periodic operation
 SHORT COMMUNICATION Carbon dioxide reforming of methane under periodic operation Eakkapon Promaros, Suttichai Assabumrungrat, Navadol Laosiripojana*, Piyasan Praserthdam, Tomohiko Tagawa** and Shigeo Goto**
SHORT COMMUNICATION Carbon dioxide reforming of methane under periodic operation Eakkapon Promaros, Suttichai Assabumrungrat, Navadol Laosiripojana*, Piyasan Praserthdam, Tomohiko Tagawa** and Shigeo Goto**
Chemical Kinetics and Reaction Engineering
 Chemical Kinetics and Reaction Engineering MIDTERM EXAMINATION II Friday, April 9, 2010 The exam is 100 points total and 20% of the course grade. Please read through the questions carefully before giving
Chemical Kinetics and Reaction Engineering MIDTERM EXAMINATION II Friday, April 9, 2010 The exam is 100 points total and 20% of the course grade. Please read through the questions carefully before giving
Chapter 2 Basic Chemistry Outline
 Chapter 2 Basic Chemistry Outline 1.0 COMPOSITION OF MATTER 1.1 Atom 1.2 Elements 1.21 Isotopes 1.22 Radioisotopes 1.3 Compounds 1.31 Compounds Formed by Ionic Bonding 1.32 Compounds Formed by Covalent
Chapter 2 Basic Chemistry Outline 1.0 COMPOSITION OF MATTER 1.1 Atom 1.2 Elements 1.21 Isotopes 1.22 Radioisotopes 1.3 Compounds 1.31 Compounds Formed by Ionic Bonding 1.32 Compounds Formed by Covalent
Reaction Rates & Equilibrium. What determines how fast a reaction takes place? What determines the extent of a reaction?
 Reaction Rates & Equilibrium What determines how fast a reaction takes place? What determines the extent of a reaction? Reactants Products 1 Reaction Rates Vary TNT exploding. A car rusting. Dead plants
Reaction Rates & Equilibrium What determines how fast a reaction takes place? What determines the extent of a reaction? Reactants Products 1 Reaction Rates Vary TNT exploding. A car rusting. Dead plants
Name AP CHEM / / Collected AP Exam Essay Answers for Chapter 16
 Name AP CHEM / / Collected AP Exam Essay Answers for Chapter 16 1980 - #7 (a) State the physical significance of entropy. Entropy (S) is a measure of randomness or disorder in a system. (b) From each of
Name AP CHEM / / Collected AP Exam Essay Answers for Chapter 16 1980 - #7 (a) State the physical significance of entropy. Entropy (S) is a measure of randomness or disorder in a system. (b) From each of
Suggested Answers to Chemical Kinetics Revision Exercise. The results of some investigations of the rate of this reaction are shown below.
 Suggested Answers to Chemical Kinetics Revision Exercise 1 At 973K, nitrogen monoxide and hydrogen react as follows: 2NO(g) + 2H 2 (g) N 2 (g) + 2H 2 O(g) The results of some investigations of the rate
Suggested Answers to Chemical Kinetics Revision Exercise 1 At 973K, nitrogen monoxide and hydrogen react as follows: 2NO(g) + 2H 2 (g) N 2 (g) + 2H 2 O(g) The results of some investigations of the rate
Quantities in Chemical Reactions
 Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Reaction Energy. Thermochemistry
 Reaction Energy Thermochemistry Thermochemistry The study of the transfers of energy as heat that accompany chemical reactions & physical changes Thermochemistry -In studying heat changes, think of defining
Reaction Energy Thermochemistry Thermochemistry The study of the transfers of energy as heat that accompany chemical reactions & physical changes Thermochemistry -In studying heat changes, think of defining
Microwave catalytic reduction of nitric oxide in activated carbon bed with a new microwave catalytic reactor system
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2014, 6(6):1412-1417 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Microwave catalytic reduction of nitric oxide
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2014, 6(6):1412-1417 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Microwave catalytic reduction of nitric oxide
"Thermodynamic Analysis of Processes for Hydrogen Generation by Decomposition of Water"
 "Thermodynamic Analysis of Processes for Hydrogen Generation by Decomposition of Water" by John P. O'Connell Department of Chemical Engineering University of Virginia Charlottesville, VA 22904-4741 A Set
"Thermodynamic Analysis of Processes for Hydrogen Generation by Decomposition of Water" by John P. O'Connell Department of Chemical Engineering University of Virginia Charlottesville, VA 22904-4741 A Set
Calculating Rates with Stoichiometry
 Calculating Rates with Stoichiometry 1. If NOCl(g) is decomposing at a rate of 1.1 x 10 8 mol/l/min in the following reaction: 2 NOCl(g) 2 NO(g) + Cl 2 (g) a) What is the rate of formation of NO(g)? b)
Calculating Rates with Stoichiometry 1. If NOCl(g) is decomposing at a rate of 1.1 x 10 8 mol/l/min in the following reaction: 2 NOCl(g) 2 NO(g) + Cl 2 (g) a) What is the rate of formation of NO(g)? b)
Nonlinear Behaviour of a Low-Density Polyethylene Tubular Reactor-Separator-Recycle System
 European Symposium on Computer Arded Aided Process Engineering 15 L. Puigjaner and A. Espuña (Editors) 2005 Elsevier Science B.V. All rights reserved. Nonlinear Behaviour of a Low-Density Polyethylene
European Symposium on Computer Arded Aided Process Engineering 15 L. Puigjaner and A. Espuña (Editors) 2005 Elsevier Science B.V. All rights reserved. Nonlinear Behaviour of a Low-Density Polyethylene
Elucidation of the Influence of Ni-Co Catalytic Properties on Dry Methane Reforming Performance
 925 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 43, 2015 Chief Editors: Sauro Pierucci, Jiří J. Klemeš Copyright 2015, AIDIC Servizi S.r.l., ISBN 978-88-95608-34-1; ISSN 2283-9216 The Italian
925 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 43, 2015 Chief Editors: Sauro Pierucci, Jiří J. Klemeš Copyright 2015, AIDIC Servizi S.r.l., ISBN 978-88-95608-34-1; ISSN 2283-9216 The Italian
Reaction Rates & Equilibrium. What determines how fast a reaction takes place? What determines the extent of a reaction?
 Reaction Rates & Equilibrium What determines how fast a reaction takes place? What determines the extent of a reaction? Reactants Products 1 Reaction Rates Vary TNT exploding. A car rusting. Dead plants
Reaction Rates & Equilibrium What determines how fast a reaction takes place? What determines the extent of a reaction? Reactants Products 1 Reaction Rates Vary TNT exploding. A car rusting. Dead plants
Properties of Matter
 Properties of Matter Chapter 4 Hein and Arena Version 1.0 Eugene Passer Chemistry Department Bronx Community 1 College John Wiley and Sons, Inc Properties of Substances 2 Properties of a Substance A property
Properties of Matter Chapter 4 Hein and Arena Version 1.0 Eugene Passer Chemistry Department Bronx Community 1 College John Wiley and Sons, Inc Properties of Substances 2 Properties of a Substance A property
Enthalpy Chapter 5.3-4,7
 Enthalpy Chapter 5.3-4,7 heat transfer in (endothermic), +q heat transfer out (exothermic), -q SYSTEM E = q + w w transfer in (+w) w transfer out (-w) Internal Energy at Constant Volume E = E K + E P ΔE
Enthalpy Chapter 5.3-4,7 heat transfer in (endothermic), +q heat transfer out (exothermic), -q SYSTEM E = q + w w transfer in (+w) w transfer out (-w) Internal Energy at Constant Volume E = E K + E P ΔE
Skills/Activities Resources Assessments
 Month September October Content/Essential Quest lab equipment lab safety states of matter physical vs. chemical change element names and symbols graph construction and interpretation classifying matter
Month September October Content/Essential Quest lab equipment lab safety states of matter physical vs. chemical change element names and symbols graph construction and interpretation classifying matter
Name SCS- Date. Experiment 5: Hydrogen Formation and Reaction with Oxygen
 Name SCS- Date Experiment 5: Hydrogen Formation and Reaction with Oxygen Aims: What are the energy changes associated with chemical bond formation and breakage? How are changes in potential energy related
Name SCS- Date Experiment 5: Hydrogen Formation and Reaction with Oxygen Aims: What are the energy changes associated with chemical bond formation and breakage? How are changes in potential energy related
Experimental Study on NO Removal Using Non-thermal Plasma Oxidation-alkali Absorption
 Experimental Study on NO Removal Using Non-thermal Plasma Oxidation-alkali Absorption Shuilan Ding 1, Qi Yu, Yingzhou Zhang 1, Yuan Liu 1, Chunxue Xie 1, Gang Yu*, 1, 3 1 School of Environment Engineering,
Experimental Study on NO Removal Using Non-thermal Plasma Oxidation-alkali Absorption Shuilan Ding 1, Qi Yu, Yingzhou Zhang 1, Yuan Liu 1, Chunxue Xie 1, Gang Yu*, 1, 3 1 School of Environment Engineering,
Supplementary Text and Figures
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Text and Figures NaCl Induced Nickel-Cobalt Inverse Spinel
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Text and Figures NaCl Induced Nickel-Cobalt Inverse Spinel
Thermochemistry. Energy (and Thermochemistry) World of Chemistry Chapter 10. Energy. Energy
 Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
CHEMISTRY LEVEL 4C (CHM415115)
 CHEMISTRY LEVEL 4C (CHM415115) THERMOCHEMISTRY & ENERGY CHANGES THEORY SUMMARY & REVISION QUESTIONS Tasmanian TCE Chemistry Revision Guides by Jak Denny are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives
CHEMISTRY LEVEL 4C (CHM415115) THERMOCHEMISTRY & ENERGY CHANGES THEORY SUMMARY & REVISION QUESTIONS Tasmanian TCE Chemistry Revision Guides by Jak Denny are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives
COMBUSTION OF FUEL 12:57:42
 COMBUSTION OF FUEL The burning of fuel in presence of air is known as combustion. It is a chemical reaction taking place between fuel and oxygen at temperature above ignition temperature. Heat is released
COMBUSTION OF FUEL The burning of fuel in presence of air is known as combustion. It is a chemical reaction taking place between fuel and oxygen at temperature above ignition temperature. Heat is released
