Light scattering by fine particles during the Pittsburgh Air Quality Study: Measurements and modeling
|
|
|
- Lesley Jefferson
- 6 years ago
- Views:
Transcription
1 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 109,, doi: /2003jd004155, 2004 Light scattering by fine particles during the Pittsburgh Air Quality Study: Measurements and modeling Juan C. Cabada, Andrey Khlystov, and Ann E. Wittig Department of Chemical Engineering, Carnegie Mellon University, Pittsburgh, Pennsylvania, USA Christodoulos Pilinis Department of Environmental Studies, University of Aegean, Mytilene, Greece Spyros N. Pandis Departments of Chemical Engineering and Engineering and Public Policy, Carnegie Mellon University, Pittsburgh, Pennsylvania, USA Received 16 September 2003; revised 3 May 2004; accepted 10 May 2004; published 17 July [1] Light scattering by fine particulate matter was measured during the Pittsburgh Air Quality Study (PAQS) as close to ambient conditions as possible. Several approaches are used for the theoretical calculation of the scattering coefficient and the results are compared to the direct measurements. The first approach uses ambient high time and daily resolved PM 2.5 composition concentrations to estimate the scattering coefficient assuming that the aerosol is an external mixture. The second approach uses a thermodynamic model and Mie theory to predict the scattering coefficient of aerosols from daily size composition distributions. The third approach introduces high time and daily resolved ambient aerosol water concentrations and concentrations of sulfate, nitrate, organic material, and soil with fixed scattering efficiencies. During the summer the first two approaches underestimate the measured scattering coefficient by around 20%. Agreement within experimental error is obtained between the measured scattering coefficient and the model, incorporating measured water aerosol concentrations. During the winter the first two approaches tend to overpredict the measured scattering by around 15%. This overprediction is weakly correlated to the organic mass. The modeling approaches suggest that sulfate and the associated water contribute 65 73% to the scattering coefficient during the summer, with organic material contributing 25 30%. During the winter, sulfate accounts for 35 43%, nitrate accounts for 24 32%, and organic material accounts for 30 40% of the scattering coefficient. INDEX TERMS: 0305 Atmospheric Composition and Structure: Aerosols and particles (0345, 4801); 0345 Atmospheric Composition and Structure: Pollution urban and regional (0305); 0365 Atmospheric Composition and Structure: Troposphere composition and chemistry; KEYWORDS: atmospheric aerosols, optical properties, Pittsburgh Air Quality Study Citation: Cabada, J. C., A. Khlystov, A. E. Wittig, C. Pilinis, and S. N. Pandis (2004), Light scattering by fine particles during the Pittsburgh Air Quality Study: Measurements and modeling, J. Geophys. Res., 109,, doi: /2003jd Introduction [2] Visibility degradation is probably the most readily perceived impact of atmospheric pollution. The protection of good visibility conditions for scenic views and the reduction of haze in rural and urban areas is one of the main goals of the Clean Air Act (U.S. Environmental Protection Agency, 1999). Figure 1 shows the effect of fine particle mass concentrations on visibility impairment for the Pittsburgh metropolitan area. High fine particulate concentrations can deteriorate visibility reducing the visual range to a few hundred meters. Copyright 2004 by the American Geophysical Union /04/2003JD004155$09.00 [3] Fine particulate matter (below 2.5 microns in aerodynamic size) is efficient in scattering light in the atmosphere and is the main cause of visibility degradation over urban and remote areas. Light scattering from fine particles depends on various factors such as chemical composition, ambient relative humidity, and size distribution of the aerosol [Ouimette and Flagan, 1982; Sloane, 1984; Seinfeld and Pandis, 1998; Watson, 2002]. Sulfate and organic material are the most common components of the fine particulate matter (below 2.5 mm) for the eastern United States [Malm, 2000; U.S. Environmental Protection Agency, 2002; NARSTO, 2003]. The mass of these particles tends to accumulate between 0.1 and 1 mm. Scattering by particles of sizes comparable to the wavelength of visible light ( mm) is mostly responsible for visibility reduction in the atmosphere. 1of13
2 a) b) Figure 1. Comparison of visual characteristics during PAQS. View of downtown Pittsburgh from the PAQS main site at Schenley Park. (a) Day with low aerosol concentrations: PM 2.5 =6mg/m 3,b sp = 5Mm 1, RH = 60%. (b) Polluted day: PM 2.5 =70mg/m 3,b sp = 255 Mm 1, RH = 74%. See color version of this figure at back of this issue. [4] The presence of water in the aerosol phase increases the ability of these particles to scatter light. Hygroscopic growth of inorganic salts as a function of relative humidity has been thoroughly studied by several authors [Tang et al., 1981; Tang, 1996; Cruz and Pandis, 1998, 2000; Hand et al., 2000]. Thermodynamic models that calculate the equilibrium of the inorganic aerosol with the gas phase have been developed, and the estimates of these models are usually in agreement with the laboratory measurements [Pilinis and Seinfeld, 1987; Wexler and Seinfeld, 1990, 1991; Nenes et al., 1998; Ansari and Pandis, 1999]. [5] The role of organic material in the aerosol water uptake is not well understood. In some cases an increase in aerosol water is observed, while in others a decrease in aerosol water content is reported when organics are added to atmospheric inorganic aerosols [Cruz and Pandis, 1998, 2000; Saxena et al., 1995]. Relatively little theoretical work has been done trying to explain the water uptake from organic aerosol, mainly because of the difficulties in estimating the physical properties of organic aerosols, which are composed of hundreds of different compounds [Saxena and Hildemann, 1997; Jang and Kamens, 1998; Ansari and Pandis, 2000; Hemming and Seinfeld, 2001; Koo et al., 2003]. However, Ansari and Pandis [2000] reported that the secondary organic aerosol (SOA) fraction of the aerosol could account for around 20% of the aerosol water. Koo et al. [2003] reported that SOA increased the water uptake from aerosols by 2 15%, depending on the conditions. [6] Several authors have used approaches of different complexity to estimate the visibility degradation due to aerosols in the atmosphere [Watson, 2002; Molenar et al., 1994; White, 1986]. The simpler models are based on bulk concentrations of fine particulate matter (PM) [Trijonis et al., 1988; Charlson et al., 1992; Gebhart et al., 1994; Malm et al., 1994, 2000a]. These models assume an external mixture of the aerosol components and average scattering efficiencies for each individual compound to determine the increase in the scattering efficiency as a function of relative humidity. In more complex models, additional characteristics of the aerosol (i.e., mixing state, size distributions of the different compounds, and the physical shape of the particles) are taken into account, and Mie theory is used to calculate the optical properties [Heintzenberg, 1980; Jaggard et al., 1981; Ouimiette and Flagan, 1982; Sloane, 1984; Wu et al., 1996; Hegg et al., 1993; Zhang et al., 1994; Lowenthal et al., 1995; Tang, 1996]. In the most advanced models the aerosol water 2of13
3 content is calculated by thermodynamic models or estimated from semiempirical relationships [Sloane and Wolff, 1985; Sloane, 1983, 1986; Larson et al., 1988; Pilinis, 1989; Nemesure et al., 1995; Pilinis et al., 1995; Quinn et al., 1995; McMurry et al., 1996; West et al., 1998; Koloutsou-Vakakis et al., 1998; Malm et al., 2000a, 2000b]. [7] Most previous studies found that measured scattering was generally consistent with the aerosol composition measurements [White et al., 1994; Pilinis et al., 1995; Malm et al., 1996], assuming that the inorganic aerosol components were responsible for the PM water uptake [Malm et al., 2003]. Discrepancies between predictions and observations have been attributed to PM composition measurement errors or lack of high temporal resolution composition measurements, lack of information about the aerosol size distribution, lack of understanding of the particle hygroscopic growth, and uncertainties in the scattering efficiencies of the different components. [8] Despite the advances, there are a number of remaining issues. The ability of the more advanced theoretical tools to simulate visibility reduction has rarely been tested in the eastern United States. The few available studies [Sloane and Wolff, 1985; Lowenthal et al., 1995; Malm et al., 2000a; Kreisberg et al., 2001; Malm and Day, 2000] have relied on daily or semidaily average composition measurements and not on higher-resolution information. The causes of the discrepancies between models and measurements are not well understood because the aerosol water concentration has not been measured in any of the previous field campaigns. Finally, the role of organics in the water uptake by aerosols and therefore their contribution to scattering is yet to be elucidated. [9] In this work, several modeling approaches of varying complexity are used to predict the scattering coefficient of aerosol particles using composition and size distribution measurements during the Pittsburgh Air Quality Study (PAQS) during the summer of 2001 (July August) and during the winter of 2002 (January). The results of each approach are compared to the measured scattering coefficient from nephelometry. High time resolution (1 hour) and daily measurements of the principal components of the fine particulate matter are used for the estimations of the scattering coefficient for the simplest approach proposed. Daily concentrations and size distributions are used for a thermodynamic model applying Mie theory to estimate the scattering coefficient. Finally, for the first time we use continuous measurements of the aerosol water concentration to directly calculate its contribution to light scattering. We conclude by addressing the role of aerosol water and organics in the optical properties of the aerosol measured at PAQS. 2. Experiment 2.1. Scattering Coefficient Measurements [10] An integrating nephelometer (NGN-3, Optec, Inc., Lowell, Michigan) was used to measure the scattering coefficient of PM 2.5 continuously during the summer of 2001 (July August) and during the winter of 2002 (January) at PAQS. The main sampling site was located on the top of a hill just outside the Carnegie Mellon University campus, around 5 km from downtown Pittsburgh. [11] Scatter of light inside the instrument chamber is measured over a 170 angle, from 5 to 175 at a range of wavelengths, peaking near 550 nm for typical atmospheric aerosols [Molenar, 1997]. A cyclone is used in the sampling inlet for the collection of PM 2.5 from ambient air. Raleigh scattering (clean air) calibrations were performed automatically by the instrument approximately every 12 hours. The nephelometer was operated as close to ambient conditions as possible, without using the heater or any treatment of the incoming sample air. However, small differences in the ambient air temperature and the chamber were still observed. Air temperature inside the chamber was on average 3 C higher than the ambient air temperature for the length of the project. Differences in the chamber and the ambient temperature affect the relative humidity of the sampled air. The chamber relative humidity was estimated on the basis of the Clausius-Clapeyron equation [Gebhart et al., 1994]: T amb T cham RH cham ¼ RH amb exp 5210:5 ; ð1þ T amb T cham where RH cham is the relative humidity inside the nephelometer measuring chamber, RH amb is the ambient relative humidity, T amb is the ambient temperature ( K), and T cham is the nephelometer chamber temperature ( K). Relative humidity inside the chamber of the nephelometer ranged from 15 to 92% during the measurements. [12] Ambient relative humidity (RH) was measured by the meteorological station installed at the main site of the PAQS with a Campbell Scientific HMP45C probe. The probe contained a Vaisala HUMICAP capacitive relative humidity sensor (expected accuracy at 20 C is ±2% from 0 to 90% RH and ±3% from 90 to 100% RH) Aerosol Sampling [13] Several samplers were operated during the Pittsburgh Supersite project for the determination and speciation of PM 2.5 aerosol mass. Continuous and semicontinuous instruments were used for the determination of total PM 2.5 mass, organic and elemental carbon, sulfate, nitrate, and ammonium aerosol concentrations from July 2001 to July 2002 [Wittig et al., 2004a]. The uncertainties in the measurements of the major ions are described by Wittig et al. [2004a, 2004b] and the merging of the different data sets for the same parameter to one self-consistent data set in the work of Takahama et al. [2004]. [14] A tamper element oscillating microscale (TEOM, Series 1400a, Rupprecht and Patashnick Co., Inc., Albany, New York) was operated continuously during PAQS, reporting PM 2.5 mass concentrations. The TEOM operated at 30 C and was equipped with a Nafion diffusion dryer sample equilibration system (SES, Rupprecht and Patashnick Co., Inc.). Atmospheric aerosol was collected on quartz fiber filters using several sampler configurations for the determination of the carbonaceous material concentrations [Cabada et al., 2004a; Subramanian et al., 2004]. High time resolution samples were collected with a denuded in situ semicontinuous sampler (Carbon aerosol analysis field instrument, Sunset Lab, Tigard, Oregon), 3of13
4 with 2 4 hour time resolution. The denuder was used to remove gas-phase organics and to minimize the positive organic aerosol sampling artifact. Daily samples were collected using an undenuded sampler with the use of backup filters for the correction of positive and negative artifacts [Subramanian et al., 2004]. The ambient organic and elemental carbon concentrations were determined following the National Institute for Occupational Safety and Health protocol with the thermal/optical transmittance method and a flame ionozation detector (FID) [Cabada et al., 2004a]). Concentrations of secondary organic aerosol (SOA) were estimated using the elemental carbon (EC) tracer method [Cabada et al., 2004a]. Aerosol concentrations of sulfate and nitrate were measured on a 10-min basis using Rupprecht and Patashnick (R&P) instrument models 8400N and 8400S, respectively [Wittig et al., 2004b]. High time resolution total (gas + aerosol) ammonium, nitrate, and chloride concentrations were measured using a steam sampler [Khlystov et al., 1995]. The crustal material concentrations were less than 5% of the PM 2.5 concentration [Rees et al., 2004]. The comparison of the aerosol mass concentration (measured using the federal reference method) and the sum of the component concentrations during PAQS are discussed by Rees et al. [2004]. After accounting for the water retained on the filters when the aerosol was acidic, mass balance was achieved within 10% for all days, well within experimental error. The aerosol was neutral during the winter but was often acidic during the daytime of the summer days [Rees et al., 2004]. [15] Size distributions of the major inorganic and carbonaceous material were measured using a micro-orifice uniform deposit impactor (MOUDI). Daily samples were collected for 15 days during the summer (July 2001) and for 8 days during the winter intensive (January 2002) [Cabada et al., 2004b]. Samples were collected on aluminum foils for analysis of carbonaceous material and on Teflon filters for analysis of particle mass and inorganic compounds. Carbonaceous concentrations from the MOUDI samples were determined by the thermal/optical method (same as the filter-based samplers), and the inorganic compound concentrations were determined by ion chromatography. During the summer, around 50% of the organic material was lost from the aluminum foils as compared to a filterbased sampler. The organic aerosol losses were negligible during the winter. The corrected MOUDI values (uniform correction for all stages), based on the artifact corrected filter measurements, were used in the present study. Cabada et al. [2004b] showed that these corrections satisfy the mass balance of all MOUDI stages. [16] The comparison of the inorganic aerosol measurements is discussed by Wittig et al. [2004a], the comparison of the organic aerosol measurements is discussed by Subramanian et al. [2004] and Cabada et al. [2004a], and the comparison of the MOUDI results with the bulk measurements is discussed by Cabada et al. [2004b]. The results of all of these approaches with different time and size resolutions were merged into one self-consistent data set [Takahama et al., 2004; Cabada et al., 2004b] using the artifact-corrected traditional filter measurements as the basis. When more than one continuous method was used, the average of the corrected values was used for each hour. In the present study the daily average values of the continuous measurements are the same as these of the daily filter measurements, and the sum of the MOUDI concentrations are equal to the artifact corrected bulk measurements Aerosol Water Content [17] A dry-ambient aerosol size spectrometer (DAASS) system [Stanier et al., 2004] operated during PAQS reporting number, surface area, and volume distributions of aerosols. The system consists of two scanning mobility particle sizers (SMPS, TSI 3936N25 and TSI 3936L10) and an aerodynamic particle sizer (APS, TSI APS 3320) that measure the aerosol size distribution between 3 nm and 10 mm in diameter. The inlets of the instruments and their sheath air lines were equipped with computer controlled valves that direct air through Nafion dryers or bypass them. The Nafion dryers reduce the RH to below 30%, at which ambient particles are expected to lose most or all of the water. The instrument cycles between dried and the ambient conditions every 7 min and is synchronized with the scan times of the aerosol spectrometers. The aerosol water concentrations measurements are described in detail by A. Khlystov et al. (Water content of ambient aerosol during the Pittsburgh Air Quality Study, submitted to Journal of Geophysical Research, 2004, hereinafter referred to as Khlystov et al., submitted manuscript, 2004). The amount of aerosol water can be found from the difference of the integrated dry and wet aerosol volumes. Owing to malfunctions in the APS system, aerosol water concentrations are only calculated from this approach for July and August Scattering Coefficient Calculation 3.1. Approach 1a: Use of Bulk PM 2.5 Composition (High Time Resolution) [18] The scattering coefficient was calculated assuming external mixing of the aerosol components and utilizing bulk PM 2.5 concentrations of the different compounds with the ability to scatter light, using the relationship proposed by Malm et al. [2000a]: Bsp ¼ 4:125 SO 2 4 fðrhþsalt þ 3:87 NO 3 þ 4 ½OMŠfðRHÞ org þ 1 SOIL fðrhþsalt ½ Š; ð2þ where [SO 4 2 ] is the sulfate concentration (mg/m 3 ), [NO 3 ]is the nitrate concentration (mg/m 3 ), [OM] is the organic mass concentration (1.8 [OC]) (mg/m 3 ) and [SOIL] is the crustal material ambient concentration, estimated here to be 4% of the TEOM fine mass (mg/m 3 ). The dry scatter efficiency of sulfates and nitrates is assumed to be 3 m 2 /g, 4m 2 /g corresponds to the organic material dry scattering efficiency, and 1 m 2 /g corresponds to the crustal material scattering efficiency [Trijonis et al., 1988; Gebhart et al., 1994]. The above parameters were estimated from measurements mainly in the western United States. Sulfate was assumed to be fully neutralized (i.e., ammonium sulfate) for both summer and winter, and the factor in equation (2) accounts for both the scatter efficiency and the ammonium corresponding to the sulfate. The increase in scattering efficiency as a function of relative humidity for the inorganic hygroscopic aerosols, f(rh) salt, was calculated 4of13
5 Table 1. Average Conditions Used to Calculate the Scattering Efficiency Curves During Summer and Winter Summer Winter Temperature, K Total NH 3, a mg/m Total HNO 3, a mg/m Total HCl, a mg/m Size range, mm Sulfate, mg/m 3 Sulfate, mg/m from Mie theory and the average size distribution of sulfate (Table 1) in PAQS using the model described by Pilinis [1989] (see also the description of approach 2). For the summer the aerosol was assumed to be always liquid based on the PM water concentration measurements of Khlystov et al. (submitted manuscript, 2004). For the winter the particles were assumed to be solid below approximately 60% RH and liquid for higher RH based on the same measurements. The same f(rh) salt was used both for sulfate and nitrate since the scattering efficiency curves are very similar for these two compounds [Tang, 1996]. Figure 2 shows the calculated scattering efficiencies for the two seasons. A higher scattering efficiency at high RH is calculated for the winter. During the winter the geometric mean diameter of the aerosol is smaller than during the summer [Cabada et al., 2004b]. For this approach the contribution of the organic matter to aerosol water is considered to be negligible; therefore f (RH) org is set to 1. [19] Most mass balance studies use a value of 1.4 for the organic carbon (OC) multiplier. Recent work by Turpin and Lim [2001] examines this factor, recommending values ranging from 1.1 for fresh emissions to for an aged aerosol. Comparison of PAQS main site data with satellite sites indicates that the air quality in Pittsburgh is dominated by regional transport [Tang et al., 2004]. We therefore used a multiplication factor of 1.8, which is representative of an aged, regional aerosol to estimate total organic mass from OC measurements. Our choice of the 1.8 for the conversion of the OC to organic aerosol concentration is supported by the mass closure study of Rees et al. [2004] Approach 1b: Use of Bulk PM 2.5 Composition (Daily Resolution) [20] For this approach the scattering from particles was also calculated from equation (2), but daily averaged concentrations were used. Since the effect of relative humidity on the scattering from particles is nonlinear, daily averaged values need to account for this to avoid biasing the calculations of the model low. To estimate the effect, the daily average of f (RH) is calculated from hourly resolved data (from approach 1a). From the daily average value of f (RH), the effective value of relative humidity is estimated. On average, this effective relative humidity value is 4% higher than the arithmetic average of relative humidity for each day. Therefore the f(rh) salt is calculated at the daily averaged RH plus 4% for all days Approach 2: Use of Mie Theory, Size Composition Distributions, and a Thermodynamic Model [21] In this approach the scattering coefficient was calculated using a thermodynamic model and Mie theory. The model is described in detail by Pilinis [1989]. Briefly, the thermodynamic model uses as inputs the measured size distributions of sulfate, sodium, organic material, elemental carbon, crustal material (Tables 2 and 3), and total concentrations (gas + aerosol) of ammonium, nitrate, and chloride together with the ambient temperature and RH. The model finds the appropriate form of the sulfate in the particulate phase (sulfate, bisulfate, or sulfuric acid), partitions the total inorganic semivolatile species (nitrate, ammonium, chloride) between the gas and aerosol phases, calculates their size distribution, and also calculates the aerosol water size distribution. Therefore the thermodynamic model provides the full size composition distribution of the particles including water. After the thermodynamic calculation, Mie theory is used to estimate the scattering coefficient of the aerosol. The relative humidity used is the effective relative humidity used in approach 1b. The performance of one size-resolved equilibrium calculation per day reduces significantly the computational cost and the potential numerical problems of this method. For the days where no size/composition measurements were available (e.g., August 2001, second half of January 2002), the average distribution for the month was used together with the bulk concentrations measured during that day Approach 3a: Use of Measured Aerosol Water Concentrations (High Time Resolution) [22] Since ambient aerosol water concentrations are available from the DAASS system, the contribution of water to the scattering coefficient can be directly estimated. For this analysis, equation (2) is modified to account for the water aerosol as an ambient concentration as follows: Bsp ¼ 4:125 SO 2 4 þ 3:87 NO 3 þ 4 ½OMŠþ 1 ½SOILŠþ3 ½WATERŠ: ð3þ Figure 2. Scattering efficiency curve, f (RH), as a function of relative humidity for the summer (solid line) and winter (dashed line) during PAQS. 5of13
6 Table 2. Summer Average Size Distributions (mg/m 3 ) of Compounds Used and Predicted by the Thermodynamic Model Size Range, mm Sodium Sulfate OM EC Dust Nitrate Ammonium The terms used in this equation are the same as in equation (2), with the introduction of the water aerosol concentration [WATER] (mg/m 3 ). Note that for equation (3) the dependence of the scattering efficiency as a function of relative humidity, f (RH), is not needed. The aerosol water scattering coefficient is assumed to be 3 m 2 /g. The choice of the water mass scattering efficiency is based on the similarity of the optical properties and size distribution of water with that of the sulfates and nitrates [Seinfeld and Pandis, 1998]. The use of the same scattering efficiency for the inorganic salts and water is implicitly consistent with the previous studies of aerosol optical properties that calculated f (RH) on the basis of the measured PM hygroscopic growth Approach 3b: Use of Measured Aerosol Water Concentrations (Daily Resolution) [23] For this approach, daily averaged concentrations of sulfate, nitrate, organic mass, and water were used to estimate the scattering coefficient from equation (3). 4. Results and Discussion 4.1. Approach 1a: Use of Bulk PM 2.5 Composition (High Time Resolution) [24] The time series of measured and estimated scattering coefficient using this approach is shown in Figure 3. For both seasons the model predictions track the actual measurements with the exception of localized episodes mostly during the summer. Figure 4 depicts the comparison between the measured scattering coefficient from the nephelometer and the estimated value from equation (2) for the high time resolution measurements. Good correlation between the measured and the reconstructed scatter is shown during the summer, and a weaker correlation is observed during the winter due to lower values (Table 4). The uncertainty of the predicted values because of the PM composition measurement uncertainty is estimated to be around 15% and can explain most but not all the discrepancies between predictions and measurements. [25] During the daytime of the summer months, Pittsburgh aerosol is often acidic, whereas for the other seasons the aerosol is mostly neutralized [Rees et al., 2004]. During periods when the aerosol is acidic, the assumption of neutralized particles may introduce errors. Malm et al. [2000a] showed that errors in the assumed degree of neutralization can explain some of the discrepancies between predictions and observations. However, for our case, these errors are expected to be less than 5% on a daily average basis because of the small difference in the mass between ammonium sulfate and bisulfate and because aerosol was acidic only during the daytime when the RH was low. This is consistent with the weak correlation (R 2 = 0.08) of the model error for scattering to the sulfate concentrations. The error was also not correlated with the nitrate (R 2 = 0.12) and the organic aerosol (R 2 = 0.14) concentrations. However, a relatively high and statistically significant correlation (at the 99.5% level) was found between this error and the measured concentration of ambient aerosol water (R 2 = 0.53). The link of the error to the aerosol water indicates a potential limitation of the model used to deal with the effect of hygroscopic growth on the scattering from particles. [26] In relative terms, greater discrepancies between the model and the measurements are observed for the winter (Table 4). There is practically no correlation between the ambient concentrations of the different compounds and the error although there is a downward trend in all of them. The organic mass seems to inversely correlate weakly (R 2 0.2) with the error. This could be due to an overestimation of the organic mass since the mass conversion factor used (1.8 [OC]) might be too high for the winter carbonaceous aerosol. The hydrophobic organics could also have a negative effect on the ability of the inorganics to absorb water. [27] The assumption of an externally mixed aerosol is not physically realistic but provides a good approximation of the scattering properties of atmospheric aerosol. The error in the scattering coefficient introduced by neglecting the Table 3. Winter Average Size Distributions (mg/m 3 ) of Compounds Used by the Thermodynamic Model Size Range, mm Sodium Sulfate OM EC Dust Nitrate Ammonium of13
7 Figure 3. Time series of measured scattering coefficient (line) and estimated scattering coefficient (symbols) from approach 1a. Each point corresponds to 1-hour average. interactions among the different aerosol species coexisting in the same particle is only a few percent [Seinfeld and Pandis, 1998]. One of the advantages of this approach is the direct apportionment of the scattering to the aerosol components [White, 1986]. On average, for the summer, 70% of the estimated scattering is due to sulfate, followed by the organic material with 25% and the remaining fraction is due to nitrates and soil. During the winter, sulfate accounts for 43% of the scattering, organics account for 31%, and nitrates contribute 24% on average. Changes in one of the aerosol components may result in changes in the other components (e.g., an increase in nitrate may accompany a decrease in sulfate), so these results should be used with caution [Seinfeld and Pandis, 1998]. The reported contributions are based on the external mixture assumption neglecting these interactions Approach 1b: Use of Bulk PM 2.5 Composition (Daily Resolution) [28] Figure 5 shows time series of measured and estimated scattering coefficient applying equation (2) with daily averaged measurements. Model predictions for scattering track the measurements both for summer and winter. The scatterplot of measurements versus predictions for the two seasons analyzed is shown in Figure 6. The effect of averaging the concentrations over 24-hour periods reduces Figure 4. Comparison of measured scattering coefficient versus the calculated value approach 1a for two seasons during PAQS. 7of13
8 Table 4. Model Performance Statistics for the Different Approaches Used to Estimate Scattering Summer N Average, Mm 1 R 2 Error, Mm 1 Bias Measured Approach 1a Approach 1b Approach Approach 3a Approach 3b Winter N Average, Mm 1 R 2 Error, Mm 1 Bias Measured 39 Approach 1a Approach 1b Approach the random errors of the measurements used as input by the model and therefore reduces model errors in both summer and winter (Table 4). McMurry et al. [1996] reported that significant uncertainty is introduced by this type of models when the scattering efficiencies are assumed constant for long periods of analysis since the characteristics of the aerosol populations are likely to vary within hours. This uncertainty is present in all approaches using daily average values Approach 2: Use of Mie Theory, Size Composition Distributions, and a Thermodynamic Model [29] The measured and calculated scattering coefficients for the summer and winter seasons during PAQS are shown in Figure 7. The model predictions and the measurements are in good agreement with the exception of the period of 4 9 August. Figure 8 shows the scatterplot of measurements versus predictions from the model for the two different seasons analyzed. Relatively good correlation is shown between the measurements and the predictions for the summer and the winter (Table 4). During the summer the model tends to under-predict the measured scattering coefficient, although this model shows better agreement between the measurements and the predictions. The errors once more is only weakly correlated to sulfate (R 2 = 0.17), nitrate (R 2 = 0.06), and organics (R 2 = 0.10). The error of this model shows a weak correlation with ambient aerosol water (R 2 = 0.22) and PM 2.5 mass concentrations (R 2 = 0.37). The weak correlation of the model and PM 2.5 from the TEOM, which also includes some water, suggests that at least part of the error may be related to the hygroscopic properties of the aerosol. Water aerosol concentrations are calculated from the model assuming that only the inorganic material uptakes water at high relative humidity. In the case Figure 5. Time series of measured scattering coefficient (line) and estimated scattering coefficient (symbols) from approach 1b. Each point corresponds to 24-hour averages. 8of13
9 Figure 6. Comparison of measured scattering coefficient versus the calculated value from approach 1b for two seasons during PAQS. that carbonaceous material contributes to the water uptake into the aerosol phase, this could not be predicted from the model, and the actual scattering would be underestimated. Khlystov et al. (submitted manuscript, 2004) also reported that during certain periods in the summer the measured aerosol water was underestimated from theoretical thermodynamic calculations. The effect of using averaged size distributions during the summer does not seem to affect significantly the overall performance of the thermodynamic model. The corresponding scattering efficiencies for the major aerosol components were approximately the same as those used in approach 1. [30] Winter estimates from this model tend to overpredict the measured scattering coefficient. The model shows a weaker correlation with the measurements as compared to the other approaches used, but the overall error is smaller (Table 4). No correlation was observed between the aerosol components and the error. The organic mass showed a weak anticorrelation (R 2 0.2), which indicates either an overestimation of the organic mass from the organic carbon measurements or a negative effect of the organics on the water absorption by the inorganics. [31] Summer contribution of sulfates and nitrates to the scattering coefficient from this model is 67%, followed by Figure 7. Time series of measured scattering coefficient (line) and estimated scattering coefficient from approach 2. Each point corresponds to 24-hour averages. Open circles point correspond to model calculations using actual size distributions. Open triangles were calculated used the average size distributions. 9of13
10 Figure 8. Comparison of measured scattering coefficient versus the calculated value from a thermodynamic model using Mie theory for two different seasons during PAQS. Each point corresponds to a 24-hour sampling period. the organic material with 30%. Negligible contributions are estimated from the other components. These contributions are estimated comparing the base case with the case where the component is not present. For winter, sulfates and nitrates account for 56% of the estimated scattering followed by 39% from the organic material. Other inorganic material and elemental carbon contribute 4% of the estimated scattering for the winter Approach 3a: Use of Measured Aerosol Water Concentrations (High Time Resolution) [32] Figure 9 shows the time series of measured scattering coefficient and the estimated scattering coefficient for equation (3). The model does a good job in reproducing the observations including the higher peaks. The scatterplot of the measurements and the estimates for the scattering coefficient is shown in Figure 10. Good correlation (R 2 = 0.89) is observed between the model predictions and the measurements (Table 4). This model has the lowest absolute error (less than 10%) and a low absolute bias (5%). The consistency of the results is encouraging and indicates that the aerosol water content measurements by the DAASS are quite accurate. The improved agreement also suggests that a large fraction of the error of the previous approaches is indeed due to the estimation of the water uptake of the particles. [33] A small source of error in this approach is that the water aerosol is measured with the DAASS system at slightly different conditions of relative humidity than the nephelometer. The measurements of aerosol size distributions and scattering coefficient are done at slightly lower than ambient relative humidity because of heating of the sample in both instruments. This difference in RH is a source of discrepancy between the model predictions and the corresponding scattering coefficient measurements. The magnitude of the discrepancy depends on the ambient RH and is on average around 10%. The error introduced by this RH difference is similar in magnitude to the model error. [34] The scattering budget estimated from this model indicates that, for the summer, sulfates account for around 38% of the estimated scattering coefficient, water aerosol accounts for 35%, and organic material contributes with 24%. Negligible contributions from nitrate and soil are estimated. In this approach the sulfates, organics, and the corresponding water are decoupled Approach 3b: Use of Measured Aerosol Water Concentrations (Daily Resolution) [35] Time series of predictions from the model and daily measurements are shown in Figure 11. The model predicts values closely to the measurements (Figure 12). A high correlation (R 2 = 0.95) exists between the model and the Figure 9. Time series of measured (line) and estimated scattering coefficient (symbols) from approach 3a. Aerosol water concentrations are only available for July and August 2001 during PAQS. Each point corresponds to 1-hour average sampling times. 10 of 13
11 Figure 10. Scatterplot of measured scattering coefficient versus predicted values from approach 3. measured scattering (Table 4). The absolute error of the model is less than 10% for the summer and can be easily explained by the experimental errors. The model slightly overpredicts the scattering coefficient by around 5% (Table 4). The good agreement and performance of this model indicates that the other approaches do not describe accurately the water uptake by fine particles in Pittsburgh. 5. Conclusions [36] The three modeling approaches used for the calculation of the scattering coefficients (bulk composition and average scattering coefficients, size-resolved composition with Mie theory and a thermodynamic model, and use of the measured water) were able to reproduce the hourly observations with an absolute error less than 25% and a bias of less than 15%. The approach using the measured aerosol water concentration reduces the error to 15% and the bias to 1%. This error is similar to the uncertainty of the predictions due to the PM composition measurement uncertainty. These results suggest that while our water measurements are consistent with the observed optical properties, the current models have still some difficulties predicting the aerosol Figure 12. Scatterplot of measured scattering coefficient versus predicted values from approach 3. water content. The fact that the thermodynamic model is underestimating the scattering coefficient during the summer is an indication that organics may be contributing to water uptake into the aerosol phase. However, the weak correlation between the error and the organic PM mass suggests that the effect is complex and is probably related not to the total organic matter (OM) but to the hydrophilic fraction of the organic component. [37] The simplest of the approaches used, bulk PM 2.5 composition and average scattering efficiencies, was relatively successful in reproducing the observations even if its parameters were derived from measurements in other parts of the United States [Malm et al., 2000a], and the aerosol acidity during the summer was neglected. This indicates that the model is relatively robust in describing aerosol scattering over not only the western but also the northeastern United States. The use of high time resolution measurements did not result in a significant improvement of the scattering coefficient prediction over using daily resolved concentrations. However, a correction has to be made to the daily relative humidity to account for the nonlinear effect in the scattering coefficient. Also, the relative success (and sometimes failure) of the approach to reproduce hour by Figure 11. Time series of measured (line) and estimated scattering coefficient (symbols) from approach 3b. Aerosol water concentrations are only available for July and August 2001 during PAQS. Each point corresponds to 1-hour average sampling times. 11 of 13
12 hour variations of the scattering coefficient provides an excellent test for our ability to perform accurate semicontinuous measurements of PM 2.5 composition and of our understanding of the PM 2.5 properties. [38] A size-resolved thermodynamic model, using daily averaged concentrations, has practically the same performance during the summer as the simpler bulk compositionbased model of approach 1. The fact that the growth curves, f (RH), in the bulk model were calculated from Mie theory and a thermodynamic model for the average conditions over the two seasons explains part of its success. During the winter the use of size-resolved thermodynamic model marginally improves the estimates of the scattering coefficient. [39] The summer scattering budget indicates that sulfates and the associated water contribute around 70% and organic material contributes with around 30%. Negligible contributions are estimated for nitrate and soils. During the winter, sulfates are responsible for around 40%, organics for 30%, and nitrates for 25% of the scattering. Negligible contributions to scattering are estimated from the soil ambient concentrations. The budget estimates of the different modeling approaches are within a few percent of each other. [40] Acknowledgments. This research was conducted as part of the Pittsburgh Air Quality Study that was supported by U.S. Environmental Protection Agency under contract R and the U.S. Department of Energy National Energy Technology Laboratory under contract DE-FC26-01NT This paper has not been subject to EPA s required peer and policy review and therefore does not necessarily reflect the views of the Agency. No official endorsement should be inferred. References Ansari, A. S., and S. N. Pandis (1999), Prediction of multicomponent inorganic atmospheric aerosol behavior, Atmos. Environ., 33, Ansari, A. S., and S. N. Pandis (2000), Water absorption by secondary organic aerosol and its effect on inorganic aerosol behavior, Environ. Sci. Technol., 34, Cabada, J. C., S. N. Pandis, R. Subramanian, A. L. Robinson, A. Polidori, and B. J. Turpin (2004a), Estimating the secondary organic aerosol contribution to PM 2.5 using the OC to EC tracer method, Aerosol Sci. Technol., 38, suppl. 1, Cabada, J. C., S. Rees, S. Takahama, A. Khlystov, S. N. Pandis, C. I. Davidson, and A. L. Robinson (2004b), Mass size distributions and size resolved chemical composition of fine particulate matter at the Pittsburgh supersite, Atmos. Environ., 38, Charlson, R. J., S. E. Schwartz, J. M. Hales, R. D. Cess, J. A. Coakley, J. E. Hansen, and D. J. Hofmann (1992), Climate forcing by anthropogenic aerosols, Science, 255, Cruz, C., and S. N. Pandis (1998), The effect of organic coatings on the cloud condensation nuclei activation of inorganic atmospheric aerosol, J. Geophys. Res., 103, 13,111 13,123. Cruz, C., and S. N. Pandis (2000), Deliquescence and hygroscopic growth of mixed inorganic-organic atmospheric aerosol, Environ. Sci. Technol., 34, Gebhart, K. A., W. C. Malm, and D. Day (1994), Examination of the effects of sulfate acidity and relative humidity on light scattering at Shenandoah national park, Atmos. Environ., 28, Hand, J. L., R. B. Ames, S. M. Kreidenweis, D. E. Day, and W. C. Malm (2000), Estimates of particle hygroscopicity during the southern aerosol and visibility study, J. Air Waste Manage. Assoc., 50, Hegg, D., T. Larson, and P. F. Yuen (1993), A theoretical study on the effect of relative humidity on light scattering by tropospheric aerosols, J. Geophys. Res., 98, 18,435 18,439. Heintzenberg, J. (1980), Particle size distribution and optical properties in arctic haze, Tellus, 32, Hemming, B. L., and J. H. Seinfeld (2001), On the hygroscopic behavior of atmospheric organic aerosols, Indust. Eng. Chem. Res., 40, Jaggard, D. L., C. Hill, R. W. Shorthill, D. Stuart, M. Glantz, F. Rosswog, B. Taggart, and S. Hammond (1981), Light scattering from particles of regular and irregular shape, Atmos. Environ., 15, Jang, M., and R. M. Kamens (1998), A thermodynamic approach for modeling partitioning of semivolatile organic compounds on atmospheric particulate matter: Humidity effects, Environ. Sci. Technol., 32, Khlystov, A., G. P. Wyers, and J. Slanina (1995), The Steam-Jet Aerosol Collector, Atmos. Environ., 29, Koloutsou-Vakakis, S., M. J. Rood, A. Nenes, and C. Pilinis (1998), Modeling of aerosol properties related to radiative forcing, J. Geophys. Res., 103, 17,009 17,032. Koo, B., A. Ansari, and S. N. Pandis (2003), Integrated approaches to modeling the organic and inorganic atmospheric aerosol components, Atmos. Environ., 37, Kreisberg, N. M., M. R. Stolzenburg, S. V. Hering, W. D. Dick, and P. H. McMurry (2001), A new method for measuring the dependence of particle size distributions on relative humidity, with application to the Southeastern Aerosol and Visibility Study, J. Geophys. Res., 106, 14,935 14,949. Larson, S. M., G. R. Cass, K. J. Hussey, and F. Luce (1988), Verification of image processing based visibility models, Environ. Sci. Technol., 22, Lowenthal, D. H., C. F. Rogers, P. Saxena, J. Watson, and J. C. Chow (1995), Sensitivity of estimated light extinction coefficients to model assumptions and measurement errors, Atmos. Environ., 29, Malm, W. C. (2000), Spatial and seasonal patterns and temporal variability of haze and its constituents in the United States, Rep. III, Coop. Inst. for Res. in the Atmos., Fort Collins, Colo. Malm, W. C., and D. E. Day (2000), Optical properties of aerosols at Grand Canyon National Park, Atmos. Environ., 34, Malm, W. C., J. F. Sisler, D. Huffman, R. A. Eldred, and T. A. Cahill (1994), Spatial and seasonal trends in particle concentrations and optical extinction in the United States, J. Geophys. Res., 99, Malm, W. C., J. V. Molenar, R. A. Eldred, and J. F. Sisler (1996), Examining the relationship among atmospheric aerosols and light scattering and extinction in the Grand Canyon area, J. Geophys. Res., 101, 19,251 19,265. Malm, W. C., D. E. Day, and S. M. Kreidenweis (2000a), Light scattering characteristics of aerosols as a function of relative humidity: Part I. A comparison of measured scattering and aerosol concentrations using theoretical models, J. Air Waste Manage. Assoc., 50, Malm, W. C., D. E. Day, and S. M. Kreidenweis (2000b), Light scattering characteristics of aerosols as a function of relative humidity: Part II. A comparison of measured scattering and aerosol concentrations using statistical models, J. Air Waste Manage. Assoc., 50, Malm, W. C., D. E. Day, S. M. Kreidenweis, J. L. Collett, and T. Lee (2003), Humidity-dependent optical properties of fine particles during the Big Bend Regional Aerosol and Visibility Observational Study, J. Geophys. Res., 108(D9), 4279, doi: /2002jd McMurry, P. H., X. Zhang, and C.-T. Lee (1996), Issues in aerosol measurement for optics assessments, J. Geophys. Res., 101, 19,189 19,197. Molenar, J. V. (1997), Theoretical and experimental analysis of the Optec NGN nephelometer, Air Resour. Spec. Inc., Fort Collins, Colo. Molenar, J. V., W. C. Malm, and C. E. Johnson (1994), Visual air quality simulation techniques, Atmos. Environ., 28, NARSTO (2003), Particulate matter science for policy makers: A NARSTO assessment, NARSTO Manage. Coord. Off., Eagle Reach, Pasco, Wash. Nemesure, S., R. Wagener, and S. E. Schwartz (1995), Direct shortwave forcing of climate by the anthropogenic sulfate aerosol: Sensitivity to particle size, composition, and relative humidity, J. Geophys. Res., 100, 26,105 26,116. Nenes, A., S. N. Pandis, and C. Pilinis (1998), ISORROPIA: A new thermodynamic equilibrium model for multiphase multicomponent inorganic aerosols, Aquat. Geochem., 4, Ouimette, J., and R. Flagan (1982), The extinction coefficient of multicomponent aerosols, Atmos. Environ., 16, Pilinis, C. (1989), Numerical simulation of visibility degradation due to particulate matter: Model development and evaluation, J. Geophys. Res., 94, Pilinis, C., and J. H. Seinfeld (1987), Continued development of a general equilibrium model for inorganic multicomponent atmospheric aerosols, Atmos. Environ., 21, Pilinis, C., S. N. Pandis, and J. H. Seinfeld (1995), Sensitivity of direct climate forcing by atmospheric aerosols to aerosol size and composition, J. Geophys. Res., 100, 18,739 18,754. Quinn, P. K., S. F. Marshall, T. S. Bates, D. S. Covert, and V. N. Kapustin (1995), Comparison of measured and calculated aerosol properties relevant to the direct radiative forcing of tropospheric sulfate aerosol on climate, J. Geophys. Res., 100, Rees, S. L., A. L. Robinson, A. Khylstov, C. O. Stanier, and S. N. Pandis (2004), Mass balance closure and the Federal Reference Method for PM 2.5 in Pittsburgh, Pennsylvania, Atmos. Environ., 38, of 13
13 Saxena, P., and L. M. Hildemann (1997), Water absorption by organics: Survey of laboratory evidence and evaluation of UNIFAC for estimating water activity, Environ. Sci. Technol., 31, Saxena, P., L. Hildemann, P. H. McMurry, and J. H. Seinfeld (1995), Organics alter hygroscopic behavior of atmospheric particles, J. Geophys. Res., 100, 18,755 18,770. Seinfeld, J. H., and S. N. Pandis (1998), Atmospheric Chemistry and Physics: From Air Pollution to Global Change, John Wiley, Hoboken, N. J. Sloane, C. S. (1983), Optical properties of aerosols: Comparisons of measurements with model calculations, Atmos. Environ., 17, Sloane, C. S. (1984), Optical properties of aerosols of mixed compositions, Atmos. Environ., 18, Sloane, C. S. (1986), Effect of compositions on aerosol light scattering efficiencies, Atmos. Environ., 20, Sloane, C. S., and G. Wolff (1985), Prediction of ambient light scattering using a physical model responsive to relative humidity: Validation with measurements from Detroit, Atmos. Environ., 19, Stanier, C. O., A. Y. Khlystov, W. R. Chan, M. Mandiro, and S. N. Pandis (2004), A method for the in situ measurement of fine aerosol water content of ambient aerosols: The Dry-Ambient Aerosol Size Spectrometer (DAASS), Aerosol Sci. Technol., 38, suppl. 1, Subramanian, R., A. Y. Khlystov, J. C. Cabada, and A. L. Robinson (2004), Positive and negative artifacts in particulate organic carbon measurements with denuded and undenuded sampler configurations, Aerosol Sci. Technol., 38, suppl. 1, Takahama, S., B. Wittig, D. V. Vayenas, C. I. Davidson, and S. N. Pandis (2004), Modeling the diurnal variation of nitrate during the Pittsburgh Air Quality Study, J. Geophys. Res., 109, doi: /2003jd004149, in press. Tang, I. N. (1996), Chemical and size effects of hygroscopic aerosols on light scattering coefficients, J. Geophys. Res., 101, 19,245 19,250. Tang, I. N., W. T. Wong, and H. R. Mulkelwitz (1981), The relative importance of atmospheric sulfates and nitrates in visibility reduction, Atmos. Environ., 15, Tang, W., T. Raymond, B. Wittig, C. I. Davidson, S. N. Pandis, A. L. Robinson, and K. Crist (2004), Spatial variations in PM 2.5 during the Pittsburgh Air Quality Study, Aerosol Sci. Technol., in press. Trijonis, J., et al., (1988), RESOLVE final project report: Visibility conditions and causes of visibility degradation in the Mojave Desert of California, Rep. NWC TP 6869, Nav. Weapons Cent., China Lake, Calif. Turpin, B. J., and H. J. Lim (2001), Species contributions to PM 2.5 mass concentrations: Revisiting common assumptions for estimating organic mass, Aerosol Sci. Technol., 35, U.S. Environmental Protection Agency (2002), Air quality criteria for particulate matter, Rep. EPA/600/P-99/002aC, Washington, D. C. Watson, J. G. (2002), Visibility: Science and regulation, J. Air Waste Manage. Assoc., 52, West, J. J., C. Pilinis, A. Nenes, and S. N. Pandis (1998), Marginal direct climate forcing by atmospheric aerosols, Atmos. Environ., 32, Wexler, A. S., and J. H. Seinfeld (1990), The distribution of ammonium salts among a size and composition dispersed aerosol, Atmos. Environ., 24, Wexler, A. S., and J. H. Seinfeld (1991), Second-generation inorganic aerosol model, Atmos. Environ., 12, White, W. H. (1986), On the theoretical and empirical basis for apportioning extinction by aerosols: A critical review, Atmos. Environ., 20, White, W. H., E. S. Macias, R. C. Ninger, and S. Schoran (1994), Sizeresolved measurements of light scattering by ambient particles in the southwestern USA, Atmos. Environ., 28, Wittig,A.E.,S.Takahama,A.Y.Khylstov,S.N.Pandis,S.Hering, B. Kirby, and C. Davidson (2004a), Semi-continuous PM 2.5 inorganic composition measurements during the Pittsburgh Air Quality Study, Atmos. Environ., 38, Wittig, A. E., N. Anderson, A. Y. Khlystov, S. N. Pandis, C. Davidson, and A. L. Robinson (2004b), Pittsburgh Air Quality Study overview, Atmos. Environ., 38, Wu, X. A., C. Seigneur, and R. Bergstrom (1996), Evaluation of a sectional representation of size distributions for calculating aerosol optical properties, J. Geophys. Res., 101, 19,277 19,283. Zhang, X., B. J. Turpin, P. H. McMurry, S. V. Hering, and M. R. Stolzenburg (1994), Mie theory evaluation of species contributions to 1990 wintertime visibility reduction in the Grand Canyon, J. Air Waste Manage. Assoc., 44, J. C. Cabada, A. Khlystov, and A. E. Wittig, Department of Chemical Engineering, Carnegie Mellon University, Pittsburgh, PA 15213, USA. S. N. Pandis, Departments of Chemical Engineering and Engineering and Public Policy, Carnegie Mellon University, Pittsburgh, PA 15213, USA. (spyros@andrew.cmu.edu) C. Pilinis, Department of Environmental Studies, University of Aegean, 17 Karadoni St., Mytilene GR-81100, Greece. 13 of 13
14 Figure 1. Comparison of visual characteristics during PAQS. View of downtown Pittsburgh from the PAQS main site at Schenley Park. (a) Day with low aerosol concentrations: PM 2.5 =6mg/m 3,b sp = 5Mm 1, RH = 60%. (b) Polluted day: PM 2.5 =70mg/m 3,b sp = 255 Mm 1, RH = 74%. 2of13
Water content of ambient aerosol during the Pittsburgh Air Quality Study
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110,, doi:10.1029/2004jd004651, 2005 Water content of ambient aerosol during the Pittsburgh Air Quality Study Andrey Khlystov Department of Civil and Environmental
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110,, doi:10.1029/2004jd004651, 2005 Water content of ambient aerosol during the Pittsburgh Air Quality Study Andrey Khlystov Department of Civil and Environmental
Mass balance closure and the Federal Reference Method for PM 2.5 in Pittsburgh, Pennsylvania
 ARTICLE IN PRESS Atmospheric Environment 38 (2004) 3305 3318 Mass balance closure and the Federal Reference Method for PM 2.5 in Pittsburgh, Pennsylvania Sarah L. Rees a,b, Allen L. Robinson b,c, *, Andrey
ARTICLE IN PRESS Atmospheric Environment 38 (2004) 3305 3318 Mass balance closure and the Federal Reference Method for PM 2.5 in Pittsburgh, Pennsylvania Sarah L. Rees a,b, Allen L. Robinson b,c, *, Andrey
3) Big Bend s Aerosol and Extinction Budgets during BRAVO
 3) Big Bend s Aerosol and Extinction Budgets during BRAVO 3.1 Introduction The primary goal of the BRAVO study was to apportion the major aerosol species to their emission sources, with the secondary goals
3) Big Bend s Aerosol and Extinction Budgets during BRAVO 3.1 Introduction The primary goal of the BRAVO study was to apportion the major aerosol species to their emission sources, with the secondary goals
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110, D07S14, doi: /2004jd005038, 2005
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110,, doi:10.1029/2004jd005038, 2005 Simulation of the thermodynamics and removal processes in the sulfate-ammonia-nitric acid system during winter: Implications for
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110,, doi:10.1029/2004jd005038, 2005 Simulation of the thermodynamics and removal processes in the sulfate-ammonia-nitric acid system during winter: Implications for
Review of the IMPROVE Equation for Estimating Ambient Light Extinction
 Review of the IMPROVE Equation for Estimating Ambient Light Extinction Jenny Hand 1 Bill Malm 2 1 CIRA, Colorado State University 2 National Park Service OUTLINE Introduction Sampling Biases Chemical forms
Review of the IMPROVE Equation for Estimating Ambient Light Extinction Jenny Hand 1 Bill Malm 2 1 CIRA, Colorado State University 2 National Park Service OUTLINE Introduction Sampling Biases Chemical forms
Haze Communication using the CAMNET and IMPROVE Archives: Case Study at Acadia National Park
 Haze Communication using the CAMNET and IMPROVE Archives: Case Study at Acadia National Park Light absorption by fine Light scattering by fine Clear line of sight in absence of fine Light extinction is
Haze Communication using the CAMNET and IMPROVE Archives: Case Study at Acadia National Park Light absorption by fine Light scattering by fine Clear line of sight in absence of fine Light extinction is
5. Light Extinction In The Desert Southwest
 5. Light Extinction In The Desert Southwest This chapter describes the spatial and temporal variations of light extinction and its components over the study area. 5.1 Principles of Light Extinction Perception
5. Light Extinction In The Desert Southwest This chapter describes the spatial and temporal variations of light extinction and its components over the study area. 5.1 Principles of Light Extinction Perception
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D7, 8414, doi: /2001jd001592, 2003
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D7, 8414, doi:10.1029/2001jd001592, 2003 An evaluation of the thermodynamic equilibrium assumption for fine particulate composition: Nitrate and ammonium
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D7, 8414, doi:10.1029/2001jd001592, 2003 An evaluation of the thermodynamic equilibrium assumption for fine particulate composition: Nitrate and ammonium
CHAPTER 8. AEROSOLS 8.1 SOURCES AND SINKS OF AEROSOLS
 1 CHAPTER 8 AEROSOLS Aerosols in the atmosphere have several important environmental effects They are a respiratory health hazard at the high concentrations found in urban environments They scatter and
1 CHAPTER 8 AEROSOLS Aerosols in the atmosphere have several important environmental effects They are a respiratory health hazard at the high concentrations found in urban environments They scatter and
A Method for the In Situ Measurement of Fine Aerosol Water Content of Ambient Aerosols: The Dry-Ambient Aerosol Size Spectrometer (DAASS)
 Aerosol Science and Technology, 38(S1):215 228, 2004 Copyright c American Association for Aerosol Research ISSN: 0278-6826 print / 1521-7388 online DOI: 10.1080/02786820390229525 A Method for the In Situ
Aerosol Science and Technology, 38(S1):215 228, 2004 Copyright c American Association for Aerosol Research ISSN: 0278-6826 print / 1521-7388 online DOI: 10.1080/02786820390229525 A Method for the In Situ
Aerosol Optical Properties
 ATM 507 Lecture 25 Text reading Chapter 15 Paper Due Dec. 9 Review Session Dec. 9 Final Dec. 12 (10:30 AM-12:30 PM) Today s topic Aerosol Optical Properties 1 Aerosol Optical Properties There are a number
ATM 507 Lecture 25 Text reading Chapter 15 Paper Due Dec. 9 Review Session Dec. 9 Final Dec. 12 (10:30 AM-12:30 PM) Today s topic Aerosol Optical Properties 1 Aerosol Optical Properties There are a number
Comparing Modal and Sectional Approaches in Modeling Particulate Matter in Northern California
 Comparing Modal and Sectional Approaches in Modeling Particulate Matter in Northern California K. Max Zhang* [1], Jinyou Liang [2], Anthony S. Wexler [1], and Ajith Kaduwela [1,2] 1. University of California,
Comparing Modal and Sectional Approaches in Modeling Particulate Matter in Northern California K. Max Zhang* [1], Jinyou Liang [2], Anthony S. Wexler [1], and Ajith Kaduwela [1,2] 1. University of California,
Chapter 5 Conclusions and Future Studies
 177 Chapter 5 Conclusions and Future Studies 178 Conclusions and Future Studies The results of the studies presented in this thesis confirm that ambient and laboratory-generated aerosols exhibit complex
177 Chapter 5 Conclusions and Future Studies 178 Conclusions and Future Studies The results of the studies presented in this thesis confirm that ambient and laboratory-generated aerosols exhibit complex
Semi-volatile aerosols in Beijing (R.P. China): Characterization and influence on various PM 2.5 measurements
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2006jd007448, 2007 Semi-volatile aerosols in Beijing (R.P. China): Characterization and influence on various PM 2.5 measurements J. Sciare, 1 H.
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2006jd007448, 2007 Semi-volatile aerosols in Beijing (R.P. China): Characterization and influence on various PM 2.5 measurements J. Sciare, 1 H.
Size Distribution and Hygroscopic Properties of Agricultural Aerosols
 Size Distribution and Hygroscopic Properties of Agricultural Aerosols Naruki Hiranuma 1, Sarah D. Brooks 1, Brent W. Auvermann 2, Rick Littleton 3 1 Department of Atmospheric Sciences, Texas A&M University.
Size Distribution and Hygroscopic Properties of Agricultural Aerosols Naruki Hiranuma 1, Sarah D. Brooks 1, Brent W. Auvermann 2, Rick Littleton 3 1 Department of Atmospheric Sciences, Texas A&M University.
Chasing Aerosol Particles Down to Nano Sizes
 Chasing Aerosol Particles Down to Nano Sizes ERC Seminar 13 June 2013 Armin Sorooshian Chemical & Environmental Engineering Atmospheric Sciences University of Arizona Outline of Talk 1. What are aerosol
Chasing Aerosol Particles Down to Nano Sizes ERC Seminar 13 June 2013 Armin Sorooshian Chemical & Environmental Engineering Atmospheric Sciences University of Arizona Outline of Talk 1. What are aerosol
6.5 Interrelationships of Fine Mass, Sulfur and Absorption Daily Scatter Plots Shenandoah National Park
 TABLE OF CONTENTS Chapter Page OVERVIEW AND SUMMARY S-1 S.1 Optical and Aerosol Data S-1 S.2 Spatial and Seasonal Distribution of Aerosol Concentration and S-4 Chemical Composition S.3 Light Extinction
TABLE OF CONTENTS Chapter Page OVERVIEW AND SUMMARY S-1 S.1 Optical and Aerosol Data S-1 S.2 Spatial and Seasonal Distribution of Aerosol Concentration and S-4 Chemical Composition S.3 Light Extinction
2.444 A FEASIBILTY STUDY OF THE MARGA TOOL AS AN AEROSOL ANALYZER
 2.444 A FEASIBILTY STUDY OF THE MARGA TOOL AS AN AEROSOL ANALYZER Rufus Ty White ac, Dr. Vernon Morris abc Department of Chemistry a, Program in Atmospheric Science b, NOAA Center for Atmospheric Science
2.444 A FEASIBILTY STUDY OF THE MARGA TOOL AS AN AEROSOL ANALYZER Rufus Ty White ac, Dr. Vernon Morris abc Department of Chemistry a, Program in Atmospheric Science b, NOAA Center for Atmospheric Science
An assessment of the ability of three-dimensional air quality models with current thermodynamic equilibrium models to predict aerosol NO 3
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110,, doi:10.1029/2004jd004718, 2005 An assessment of the ability of three-dimensional air quality models with current thermodynamic equilibrium models to predict
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110,, doi:10.1029/2004jd004718, 2005 An assessment of the ability of three-dimensional air quality models with current thermodynamic equilibrium models to predict
CHAPTER 7 AEROSOL ACIDITY
 CHAPTER 7 AEROSOL ACIDITY It has been established by a number of investigators, that especially during the summer, aerosols along the coast of Washington are commonly acidic. 1-2 Although the measurements
CHAPTER 7 AEROSOL ACIDITY It has been established by a number of investigators, that especially during the summer, aerosols along the coast of Washington are commonly acidic. 1-2 Although the measurements
Big Bend Regional Aerosol & Visibility Observational Study
 Big Bend Regional Aerosol & Visibility Observational Study BRAVO - Results Bret Schichtel National Park Service, Schichtel@cira.colostate.edu Presented at the BRAVO Public Meeting Alpine, Texas September
Big Bend Regional Aerosol & Visibility Observational Study BRAVO - Results Bret Schichtel National Park Service, Schichtel@cira.colostate.edu Presented at the BRAVO Public Meeting Alpine, Texas September
Spatial and Seasonal Patterns and Temporal Variability of Haze and its Constituents in the United States
 IIMP MPR RO V E Interagency Monitoring of Protected Visual Environments Spatial and Seasonal Patterns and Temporal Variability of Haze and its Constituents in the United States Acadia NP PM2.5 (µg/m3)
IIMP MPR RO V E Interagency Monitoring of Protected Visual Environments Spatial and Seasonal Patterns and Temporal Variability of Haze and its Constituents in the United States Acadia NP PM2.5 (µg/m3)
Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables
 Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2008jd010527, 2008 Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables Fangqun Yu 1 Received 2 June
Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2008jd010527, 2008 Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables Fangqun Yu 1 Received 2 June
Comparison of AERONET inverted size distributions to measured distributions from the Aerodyne Aerosol Mass Spectrometer
 Comparison of inverted size distributions to measured distributions from the Aerodyne Aerosol Mass Spectrometer Peter DeCarlo Remote Sensing Project April 28, 23 Introduction The comparison of direct in-situ
Comparison of inverted size distributions to measured distributions from the Aerodyne Aerosol Mass Spectrometer Peter DeCarlo Remote Sensing Project April 28, 23 Introduction The comparison of direct in-situ
Continuous measurement of airborne particles and gases
 Continuous measurement of airborne particles and gases Jeff Collett and Taehyoung Lee Atmospheric Science Department Colorado State University Funding: USDA/AES and NPS Outline Why measure particles and
Continuous measurement of airborne particles and gases Jeff Collett and Taehyoung Lee Atmospheric Science Department Colorado State University Funding: USDA/AES and NPS Outline Why measure particles and
The Atmospheric Chemistry and Physics of Ammonia
 The Atmospheric Chemistry and Physics of Ammonia Russell Dickerson Dept. Meteorology, The University of Maryland Presented at the National Atmospheric Deposition Program Ammonia Workshop October 23, 2003
The Atmospheric Chemistry and Physics of Ammonia Russell Dickerson Dept. Meteorology, The University of Maryland Presented at the National Atmospheric Deposition Program Ammonia Workshop October 23, 2003
Aerosol time-of-flight mass spectrometry during the Atlanta Supersite Experiment: 2. Scaling procedures
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D7, 8427, doi:10.1029/2001jd001563, 2003 Aerosol time-of-flight mass spectrometry during the Atlanta Supersite Experiment: 2. Scaling procedures Ryan J. Wenzel
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D7, 8427, doi:10.1029/2001jd001563, 2003 Aerosol time-of-flight mass spectrometry during the Atlanta Supersite Experiment: 2. Scaling procedures Ryan J. Wenzel
Different Methods of Monitoring PM
 Different Methods of Monitoring PM Melita Keywood Improving PM10 Monitoring in NZ 10 October 2005 CSIRO Marine and Atmospheric Research www.csiro.au Methods Integrated filter sampling Impactor or cyclone
Different Methods of Monitoring PM Melita Keywood Improving PM10 Monitoring in NZ 10 October 2005 CSIRO Marine and Atmospheric Research www.csiro.au Methods Integrated filter sampling Impactor or cyclone
IMPROVE Sampling & Analysis: Evaluation & Development
 IMPROVE Sampling & Analysis: Evaluation & Development Chuck McDade Crocker Nuclear Laboratory University of California, Davis Okefenokee, Georgia October 2008 AEROSOL GENERATION CHAMBER Selected Species
IMPROVE Sampling & Analysis: Evaluation & Development Chuck McDade Crocker Nuclear Laboratory University of California, Davis Okefenokee, Georgia October 2008 AEROSOL GENERATION CHAMBER Selected Species
Jianfei Peng et al. Correspondence to: Jianfei Peng Min Hu and Renyi Zhang
 Supplement of Atmos. Chem. Phys., 17, 10333 10348, 2017 https://doi.org/10.5194/acp-17-10333-2017-supplement Author(s) 2017. This work is distributed under the Creative Commons Attribution 3.0 License.
Supplement of Atmos. Chem. Phys., 17, 10333 10348, 2017 https://doi.org/10.5194/acp-17-10333-2017-supplement Author(s) 2017. This work is distributed under the Creative Commons Attribution 3.0 License.
Source apportionment of fine particulate matter over the Eastern U.S. Part I. Source sensitivity simulations using CMAQ with the Brute Force method
 S1 SUPPORTING MATERIAL Source apportionment of fine particulate matter over the Eastern U.S. Part I. Source sensitivity simulations using CMAQ with the Brute Force method Michael Burr and Yang Zhang Department
S1 SUPPORTING MATERIAL Source apportionment of fine particulate matter over the Eastern U.S. Part I. Source sensitivity simulations using CMAQ with the Brute Force method Michael Burr and Yang Zhang Department
Response to Referee 2
 Response to Referee 2 S. Metzger et al. 10 August 2018 We thank the referee for the manuscript review. Please find our pointby-point reply below. Accordingly, the revised MS will include clarifications.
Response to Referee 2 S. Metzger et al. 10 August 2018 We thank the referee for the manuscript review. Please find our pointby-point reply below. Accordingly, the revised MS will include clarifications.
Fine Particles: Why We Care
 Fine Particles: Why We Care Visibility/Radiative Forcing Health Effects A function of chemical composition PM2.5 Mostly 1) Sulfate 2) Carbonaceous - Organic - Elemental (Soot) 3) Metals, minerals, Metals,
Fine Particles: Why We Care Visibility/Radiative Forcing Health Effects A function of chemical composition PM2.5 Mostly 1) Sulfate 2) Carbonaceous - Organic - Elemental (Soot) 3) Metals, minerals, Metals,
2 April 2004 Copenhagen, Denmark
 Seminar: Sampling, Real-time Monitoring and Conditioning of Air Samples for Determination of Particle Mass R&P Technology Roadmap PM Measurement Issues FDMS System 2 April 2004 Copenhagen, Denmark Michael
Seminar: Sampling, Real-time Monitoring and Conditioning of Air Samples for Determination of Particle Mass R&P Technology Roadmap PM Measurement Issues FDMS System 2 April 2004 Copenhagen, Denmark Michael
Field Evaluation of the Differential TEOM Monitor for Continuous PM 2.5 Mass Concentrations
 Aerosol Science and Technology, 38(S1):49 59, 2004 Copyright c American Association for Aerosol Research ISSN: 0278-6826 print / 1521-7388 online DOI: 10.1080/02786820390229435 Field Evaluation of the
Aerosol Science and Technology, 38(S1):49 59, 2004 Copyright c American Association for Aerosol Research ISSN: 0278-6826 print / 1521-7388 online DOI: 10.1080/02786820390229435 Field Evaluation of the
Resolving Autocorrelation Bias in the Determination of the Extinction Efficiency of Fugitive Dust from Cattle Feedyards
 Resolving Autocorrelation Bias in the Determination of the Extinction Efficiency of Fugitive Dust from Cattle Feedyards Extended Abstract # 8 Jeetendra K. Upadhyay, Brent W. Auvermann, and K. Jack Bush
Resolving Autocorrelation Bias in the Determination of the Extinction Efficiency of Fugitive Dust from Cattle Feedyards Extended Abstract # 8 Jeetendra K. Upadhyay, Brent W. Auvermann, and K. Jack Bush
The Effect of Future Climate Change on Aerosols: Biogenic SOA and Inorganics
 The Effect of Future Climate Change on Aerosols: Biogenic SOA and Inorganics GCAP Phase 2 Science Team Meeting October 12, 2007 Havala O. T. Pye 1, Hong Liao 2, John Seinfeld 1, Shiliang Wu 3, Loretta
The Effect of Future Climate Change on Aerosols: Biogenic SOA and Inorganics GCAP Phase 2 Science Team Meeting October 12, 2007 Havala O. T. Pye 1, Hong Liao 2, John Seinfeld 1, Shiliang Wu 3, Loretta
Hygroscopic Growth of Aerosols and their Optical Properties
 Hygroscopic Growth of Aerosols and their Optical Properties Cynthia Randles Atmospheric & Oceanic Sciences Princeton University V. Ramaswamy and L. M. Russell ! Introduction Presentation Overview! Aerosol
Hygroscopic Growth of Aerosols and their Optical Properties Cynthia Randles Atmospheric & Oceanic Sciences Princeton University V. Ramaswamy and L. M. Russell ! Introduction Presentation Overview! Aerosol
Mass size distributions and size resolved chemical composition offine particulate matter at the Pittsburgh supersite
 Atmospheric Environment 8 (4) 17 141 Mass size distributions and size resolved chemical composition offine particulate matter at the Pittsburgh supersite Juan C. Cabada a, Sarah Rees b,d, Satoshi Takahama
Atmospheric Environment 8 (4) 17 141 Mass size distributions and size resolved chemical composition offine particulate matter at the Pittsburgh supersite Juan C. Cabada a, Sarah Rees b,d, Satoshi Takahama
Parameterization of the nitric acid effect on CCN activation
 Atmos. Chem. Phys., 5, 879 885, 25 SRef-ID: 168-7324/acp/25-5-879 European Geosciences Union Atmospheric Chemistry and Physics Parameterization of the nitric acid effect on CCN activation S. Romakkaniemi,
Atmos. Chem. Phys., 5, 879 885, 25 SRef-ID: 168-7324/acp/25-5-879 European Geosciences Union Atmospheric Chemistry and Physics Parameterization of the nitric acid effect on CCN activation S. Romakkaniemi,
PARTICULATE MATTER SIZE DISTRIBUTION MEASUREMENTS AT AKROTIRI STATION, CRETE, GREECE
 PARTICULATE MATTER SIZE DISTRIBUTION MEASUREMENTS AT AKROTIRI STATION, CRETE, GREECE I. Kopanakis, M. Lazaridis Department of Environmental Engineering, Technical University of Crete, Chania, Greece N.
PARTICULATE MATTER SIZE DISTRIBUTION MEASUREMENTS AT AKROTIRI STATION, CRETE, GREECE I. Kopanakis, M. Lazaridis Department of Environmental Engineering, Technical University of Crete, Chania, Greece N.
Atmospheric Environment
 Atmospheric Environment 43 (2009) 1932 1939 Contents lists available at ScienceDirect Atmospheric Environment journal homepage: www.elsevier.com/locate/atmosenv Aerosol physical, chemical and optical properties
Atmospheric Environment 43 (2009) 1932 1939 Contents lists available at ScienceDirect Atmospheric Environment journal homepage: www.elsevier.com/locate/atmosenv Aerosol physical, chemical and optical properties
Uncertainties in PM2.5 Gravimetric and Speciation Measurements and What Can We Learn from Them
 Uncertainties in PM2.5 Gravimetric and Speciation Measurements and What Can We Learn from Them William Malm Cooperative Institute for Research in the Atmosphere, Colorado State University, Fort Collins,
Uncertainties in PM2.5 Gravimetric and Speciation Measurements and What Can We Learn from Them William Malm Cooperative Institute for Research in the Atmosphere, Colorado State University, Fort Collins,
Lab 4 Major Anions In Atmospheric Aerosol Particles
 Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 4 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 4: This experiment will involve determining
Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 4 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 4: This experiment will involve determining
Chemical mass closure of atmospheric aerosol collected over Athens, Greece.
 Chemical mass closure of atmospheric aerosol collected over Athens, Greece. Paraskevopoulou D. 1, 2, Liakakou E. 1, Theodosi C. 2, Zarmpas P. 2, Gerasopoulos E. 1 1, 2*, Mihalopoulos N. 1 Institute for
Chemical mass closure of atmospheric aerosol collected over Athens, Greece. Paraskevopoulou D. 1, 2, Liakakou E. 1, Theodosi C. 2, Zarmpas P. 2, Gerasopoulos E. 1 1, 2*, Mihalopoulos N. 1 Institute for
Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes
 DISTRIBUTION STATEMENT A. Approved for public release; distribution is unlimited. Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes John H. Seinfeld California Institute of Technology,
DISTRIBUTION STATEMENT A. Approved for public release; distribution is unlimited. Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes John H. Seinfeld California Institute of Technology,
Aerosol Composition and Radiative Properties
 Aerosol Composition and Radiative Properties Urs Baltensperger Laboratory of Atmospheric Chemistry Paul Scherrer Institut, 5232 Villigen PSI, Switzerland WMO-BIPM Workshop Geneva, 30 March 1 April 2010
Aerosol Composition and Radiative Properties Urs Baltensperger Laboratory of Atmospheric Chemistry Paul Scherrer Institut, 5232 Villigen PSI, Switzerland WMO-BIPM Workshop Geneva, 30 March 1 April 2010
Analysis of Data from the 2009 SOOT Experiment
 Analysis of Data from the 2009 SOOT Experiment Renyi Zhang Department of Atmospheric Sciences and Department of Chemistry Center for Atmospheric Chemistry and the Environment Texas A&M University College
Analysis of Data from the 2009 SOOT Experiment Renyi Zhang Department of Atmospheric Sciences and Department of Chemistry Center for Atmospheric Chemistry and the Environment Texas A&M University College
Mixtures of pollution, dust, sea salt, and volcanic aerosol during ACE-Asia: Radiative properties as a function of relative humidity
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D23, 8650, doi:10.1029/2003jd003405, 2003 Mixtures of pollution, dust, sea salt, and volcanic aerosol during ACE-Asia: Radiative properties as a function
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D23, 8650, doi:10.1029/2003jd003405, 2003 Mixtures of pollution, dust, sea salt, and volcanic aerosol during ACE-Asia: Radiative properties as a function
Title of file for HTML: Supplementary Information Description: Supplementary Figures, Supplementary Tables and Supplementary References
 Title of file for HTML: Supplementary Information Description: Supplementary Figures, Supplementary Tables and Supplementary References Title of file for HTML: Peer Review File Description: g e (RH) (bulk)
Title of file for HTML: Supplementary Information Description: Supplementary Figures, Supplementary Tables and Supplementary References Title of file for HTML: Peer Review File Description: g e (RH) (bulk)
Lecture 26. Regional radiative effects due to anthropogenic aerosols. Part 2. Haze and visibility.
 Lecture 26. Regional radiative effects due to anthropogenic aerosols. Part 2. Haze and visibility. Objectives: 1. Attenuation of atmospheric radiation by particulates. 2. Haze and Visibility. Readings:
Lecture 26. Regional radiative effects due to anthropogenic aerosols. Part 2. Haze and visibility. Objectives: 1. Attenuation of atmospheric radiation by particulates. 2. Haze and Visibility. Readings:
Aerosol-Cloud-Radiation Interactions in Atmospheric Forecast Models
 Aerosol-Cloud-Radiation Interactions in Atmospheric Forecast Models John H. Seinfeld, Principal Investigator California Institute of Technology 1200 E. California Blvd., M/C 210-41 Pasadena, CA 91125 (626)
Aerosol-Cloud-Radiation Interactions in Atmospheric Forecast Models John H. Seinfeld, Principal Investigator California Institute of Technology 1200 E. California Blvd., M/C 210-41 Pasadena, CA 91125 (626)
Particle scattering, backscattering, and absorption coefficients: An in situ closure and sensitivity study
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D21, 8122, doi:10.1029/2000jd000234, 2002 Particle scattering, backscattering, and absorption coefficients: An in situ closure and sensitivity study Heike
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D21, 8122, doi:10.1029/2000jd000234, 2002 Particle scattering, backscattering, and absorption coefficients: An in situ closure and sensitivity study Heike
INSITU project within AeroCom Phase III: Description and Model Output Request
 INSITU project within AeroCom Phase III: Description and Model Output Request Primary Contact: Secondary Contacts: Betsy Andrews (betsy.andrews@noaa.gov) Lauren Schmeisser (lauren.schmeisser@noaa.gov),
INSITU project within AeroCom Phase III: Description and Model Output Request Primary Contact: Secondary Contacts: Betsy Andrews (betsy.andrews@noaa.gov) Lauren Schmeisser (lauren.schmeisser@noaa.gov),
HIPS data are now reported with a consistent calibration (2003 present) what do they say?
 HIPS data are now reported with a consistent calibration (2003 present) So what do they say? Well, Fabs correlates well with EC (!!), as seen below with site-year means. Each point shows the arithmetic
HIPS data are now reported with a consistent calibration (2003 present) So what do they say? Well, Fabs correlates well with EC (!!), as seen below with site-year means. Each point shows the arithmetic
Reactive Nitrogen Monitoring
 Reactive Nitrogen Monitoring Some definitions NOy NO + NO 2 + NO 3 + 2xN2 2 O 5 + HNO 3 + HONO + HO 2 NO 2 + RONO 2 (organic nitrates such as PAN and alkyl nitrates) + RONO (organic nitrites) + NO 3 -
Reactive Nitrogen Monitoring Some definitions NOy NO + NO 2 + NO 3 + 2xN2 2 O 5 + HNO 3 + HONO + HO 2 NO 2 + RONO 2 (organic nitrates such as PAN and alkyl nitrates) + RONO (organic nitrites) + NO 3 -
ARTICLE IN PRESS. Journal of Aerosol Science
 Journal of Aerosol Science 41 (2010) 99 107 Contents lists available at ScienceDirect Journal of Aerosol Science journal homepage: www.elsevier.com/locate/jaerosci Use of proton backscattering to determine
Journal of Aerosol Science 41 (2010) 99 107 Contents lists available at ScienceDirect Journal of Aerosol Science journal homepage: www.elsevier.com/locate/jaerosci Use of proton backscattering to determine
4 th AMS Users Meeting Brainstorming on (1) Experiments to Nail our Absolute Quantification (2) Best Operating Procedures
 th AMS Users Meeting Brainstorming on (1) Experiments to Nail our Absolute Quantification () Best Operating Procedures Jose-Luis Jimenez Caltech Pasadena, CA Oct. -7, Reminder of the Quantification Issues.
th AMS Users Meeting Brainstorming on (1) Experiments to Nail our Absolute Quantification () Best Operating Procedures Jose-Luis Jimenez Caltech Pasadena, CA Oct. -7, Reminder of the Quantification Issues.
6. Chemical Contributions to Extinction
 6. Chemical Contributions to Extinction This section estimates the contribution from the major chemical components of light scattering and absorption and examines how these contributions vary from site
6. Chemical Contributions to Extinction This section estimates the contribution from the major chemical components of light scattering and absorption and examines how these contributions vary from site
Update on IMPROVE Light Extinction Equation and Natural Conditions Estimates. Tom Moore, WRAP Technical Coordinator. May 23, 2006
 Update on IMPROVE Light Extinction Equation and Natural Conditions Estimates Tom Moore, WRAP Technical Coordinator May 23, 2006 OR. Defining Regional Haze Impacts with Aerosol Sampling Data AND. Knowing
Update on IMPROVE Light Extinction Equation and Natural Conditions Estimates Tom Moore, WRAP Technical Coordinator May 23, 2006 OR. Defining Regional Haze Impacts with Aerosol Sampling Data AND. Knowing
What are Aerosols? Suspension of very small solid particles or liquid droplets Radii typically in the range of 10nm to
 What are Aerosols? Suspension of very small solid particles or liquid droplets Radii typically in the range of 10nm to 10µm Concentrations decrease exponentially with height N(z) = N(0)exp(-z/H) Long-lived
What are Aerosols? Suspension of very small solid particles or liquid droplets Radii typically in the range of 10nm to 10µm Concentrations decrease exponentially with height N(z) = N(0)exp(-z/H) Long-lived
Physio-chemical and Optical Characterization of Anthropogenic and Natural Aerosol: Implications for Assessing Global Effects
 Physio-chemical and Optical Characterization of Anthropogenic and Natural Aerosol: Implications for Assessing Global Effects GLOBE Pollution Southern Japan TRACE-P, 2001 Dust Antony Clarke, University
Physio-chemical and Optical Characterization of Anthropogenic and Natural Aerosol: Implications for Assessing Global Effects GLOBE Pollution Southern Japan TRACE-P, 2001 Dust Antony Clarke, University
Helsinki, Finland. 1 Aerodyne Research Inc., Billerica, MA, USA. 2 Chemistry Department, Boston College, Chestnut Hill, MA, USA
 Supplementary material to article: Relationship between aerosol oxidation level and hygroscopic properties of laboratory generated secondary organic aerosol (SOA) particles (paper #0GL0 to Geophysical
Supplementary material to article: Relationship between aerosol oxidation level and hygroscopic properties of laboratory generated secondary organic aerosol (SOA) particles (paper #0GL0 to Geophysical
Highly Biased Hygroscopicity Derived from Size-Resolved Cloud Condensation Nuclei Activation Ratios without Data Inversion
 ATMOSPHERIC AND OCEANIC SCIENCE LETTERS, 2014, VOL. 7, NO. 3, 254 259 Highly Biased Hygroscopicity Derived from Size-Resolved Cloud Condensation Nuclei Activation Ratios without Data Inversion DENG Zhao-Ze
ATMOSPHERIC AND OCEANIC SCIENCE LETTERS, 2014, VOL. 7, NO. 3, 254 259 Highly Biased Hygroscopicity Derived from Size-Resolved Cloud Condensation Nuclei Activation Ratios without Data Inversion DENG Zhao-Ze
Gas/aerosol partitioning: 1. A computationally efficient model
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D16, 4312, 10.1029/2001JD001102, 2002 Gas/aerosol partitioning: 1. A computationally efficient model Swen Metzger, 1 Frank Dentener, 2 Spyros Pandis, 3 and
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D16, 4312, 10.1029/2001JD001102, 2002 Gas/aerosol partitioning: 1. A computationally efficient model Swen Metzger, 1 Frank Dentener, 2 Spyros Pandis, 3 and
THESIS THE ROLE OF AEROSOLS IN VISIBILITY DEGRADATION DURING TWO FIELD CAMPAIGNS. Submitted by. Ezra J.T. Levin. Department of Atmospheric Science
 THESIS THE ROLE OF AEROSOLS IN VISIBILITY DEGRADATION DURING TWO FIELD CAMPAIGNS Submitted by Ezra J.T. Levin Department of Atmospheric Science In partial fulfillment of the requirements For the Degree
THESIS THE ROLE OF AEROSOLS IN VISIBILITY DEGRADATION DURING TWO FIELD CAMPAIGNS Submitted by Ezra J.T. Levin Department of Atmospheric Science In partial fulfillment of the requirements For the Degree
Figure 1. A terrain map of Texas and Mexico as well as some major cites and points of interest to the BRAVO study.
 Big Bend Regional Aerosol and Visibility Observational (BRAVO) Study Results: Air Quality Data and Source Attribution Analyses Results from the National Park Service / Cooperative Institute for Research
Big Bend Regional Aerosol and Visibility Observational (BRAVO) Study Results: Air Quality Data and Source Attribution Analyses Results from the National Park Service / Cooperative Institute for Research
Effect of aging on cloud nucleating properties of atmospheric aerosols
 Effect of aging on cloud nucleating properties of atmospheric aerosols Yan Ma Nanjing University of Information Science & Technology The 1st Regional GEOS-Chem Asia Meeting 2015 年 5 月 8 日 Indirect effect
Effect of aging on cloud nucleating properties of atmospheric aerosols Yan Ma Nanjing University of Information Science & Technology The 1st Regional GEOS-Chem Asia Meeting 2015 年 5 月 8 日 Indirect effect
Comprehensive Measurement of Atmospheric Aerosols with a Wide Range Aerosol Spectrometer
 Comprehensive Measurement of Atmospheric Aerosols with a Wide Range Aerosol Spectrometer Juergen Spielvogel, Lothar Keck, Xiaoai Guo, Markus Pesch Email: jsp@grimm-aerosol.com GRIMM Aerosol Technik GmbH
Comprehensive Measurement of Atmospheric Aerosols with a Wide Range Aerosol Spectrometer Juergen Spielvogel, Lothar Keck, Xiaoai Guo, Markus Pesch Email: jsp@grimm-aerosol.com GRIMM Aerosol Technik GmbH
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D23, 4688, doi: /2002jd002465, 2002
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D23, 4688, doi:10.1029/2002jd002465, 2002 Clear-column radiative closure during ACE-Asia: Comparison of multiwavelength extinction derived from particle size
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D23, 4688, doi:10.1029/2002jd002465, 2002 Clear-column radiative closure during ACE-Asia: Comparison of multiwavelength extinction derived from particle size
OPTICAL PROPERTIES OF AEROSOLS AND THEIR SENSITIVITY TO RELATIVE HUMIDITY AND SIZE DISTRIBUTION IN THE NEW YORK CITY URBAN-COASTAL AREA
 EARSeL eproceedings 11, 1/2012 52 OPTICAL PROPERTIES OF AEROSOLS AND THEIR SENSITIVITY TO RELATIVE HUMIDITY AND SIZE DISTRIBUTION IN THE NEW YORK CITY URBAN-COASTAL AREA Daniela Viviana Vladutescu 1, Yonghua
EARSeL eproceedings 11, 1/2012 52 OPTICAL PROPERTIES OF AEROSOLS AND THEIR SENSITIVITY TO RELATIVE HUMIDITY AND SIZE DISTRIBUTION IN THE NEW YORK CITY URBAN-COASTAL AREA Daniela Viviana Vladutescu 1, Yonghua
Direct radiative forcing due to aerosols in Asia during March 2002
 Direct radiative forcing due to aerosols in Asia during March 2002 Soon-Ung Park, Jae-In Jeong* Center for Atmospheric and Environmental Modeling *School of Earth and Environmental Sciences, Seoul National
Direct radiative forcing due to aerosols in Asia during March 2002 Soon-Ung Park, Jae-In Jeong* Center for Atmospheric and Environmental Modeling *School of Earth and Environmental Sciences, Seoul National
Hygroscopic growth of ammonium sulfate/dicarboxylic acids
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D20, 4638, doi:10.1029/2003jd003775, 2003 Hygroscopic growth of ammonium sulfate/dicarboxylic acids Matthew E. Wise, Jason D. Surratt, Daniel B. Curtis, John
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D20, 4638, doi:10.1029/2003jd003775, 2003 Hygroscopic growth of ammonium sulfate/dicarboxylic acids Matthew E. Wise, Jason D. Surratt, Daniel B. Curtis, John
Particuology 9 (2011) Contents lists available at SciVerse ScienceDirect. Particuology. j o ur nal homep ag e:
 Particuology 9 (2011) 606 610 Contents lists available at SciVerse ScienceDirect Particuology j o ur nal homep ag e: www.elsevier.com/locate/partic Evaluation of particle growth systems for sampling and
Particuology 9 (2011) 606 610 Contents lists available at SciVerse ScienceDirect Particuology j o ur nal homep ag e: www.elsevier.com/locate/partic Evaluation of particle growth systems for sampling and
Aerosol characterization studies at Great Smoky Mountains National Park, summer 2006
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 114,, doi:10.1029/2008jd011274, 2009 Aerosol characterization studies at Great Smoky Mountains National Park, summer 2006 Douglas Lowenthal, 1 Barbara Zielinska, 1
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 114,, doi:10.1029/2008jd011274, 2009 Aerosol characterization studies at Great Smoky Mountains National Park, summer 2006 Douglas Lowenthal, 1 Barbara Zielinska, 1
Chapter Eight: Conclusions and Future Work
 2004 PhD Thesis 202 Chapter Eight: Conclusions and Future Work 8.1 Conclusions The Aerodyne aerosol mass spectrometer is capable of providing quantitative information on the chemical composition of the
2004 PhD Thesis 202 Chapter Eight: Conclusions and Future Work 8.1 Conclusions The Aerodyne aerosol mass spectrometer is capable of providing quantitative information on the chemical composition of the
Modeling of in situ ultrafine atmospheric particle formation in the eastern United States
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110,, doi:10.1029/2004jd004683, 2005 Modeling of in situ ultrafine atmospheric particle formation in the eastern United States Timothy M. Gaydos and Charles O. Stanier
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110,, doi:10.1029/2004jd004683, 2005 Modeling of in situ ultrafine atmospheric particle formation in the eastern United States Timothy M. Gaydos and Charles O. Stanier
Who is polluting the Columbia River Gorge?
 Who is polluting the Columbia River Gorge? Final report to the Yakima Nation Prepared by: Dan Jaffe, Ph.D Northwest Air Quality, Inc. 7746 Ravenna Avenue NE Seattle WA 98115 NW_airquality@hotmail.com December
Who is polluting the Columbia River Gorge? Final report to the Yakima Nation Prepared by: Dan Jaffe, Ph.D Northwest Air Quality, Inc. 7746 Ravenna Avenue NE Seattle WA 98115 NW_airquality@hotmail.com December
Visibility and Air Quality Measurement Methods Used by the National Park Service
 Visibility and Air Quality Measurement Methods Used by the National Park Service U.S. Department of the Interior National Park Service Air Quality Division February 1990 Visibility and Air Quality Measurement
Visibility and Air Quality Measurement Methods Used by the National Park Service U.S. Department of the Interior National Park Service Air Quality Division February 1990 Visibility and Air Quality Measurement
Response to Reviewer s comments
 Response to Reviewer s comments (MS Ref. No.: acp-2010-98): Long-term record of aerosol optical properties and chemical composition from a high-altitude site (Manora Peak) in Central Himalaya by K. Ram
Response to Reviewer s comments (MS Ref. No.: acp-2010-98): Long-term record of aerosol optical properties and chemical composition from a high-altitude site (Manora Peak) in Central Himalaya by K. Ram
Physicochemical and Optical Properties of Aerosols in South Korea
 Physicochemical and Optical Properties of Aerosols in South Korea Seungbum Kim, Sang-Sam Lee, Jeong-Eun Kim, Ju-Wan Cha, Beom-Cheol Shin, Eun-Ha Lim, Jae-Cheol Nam Asian Dust Research Division NIMR/KMA
Physicochemical and Optical Properties of Aerosols in South Korea Seungbum Kim, Sang-Sam Lee, Jeong-Eun Kim, Ju-Wan Cha, Beom-Cheol Shin, Eun-Ha Lim, Jae-Cheol Nam Asian Dust Research Division NIMR/KMA
PROPOSED FLAG LEVEL II AND III VISIBILITY ASSESSMENT
 PROPOSED FLAG LEVEL II AND III VISIBILITY ASSESSMENT Bret A. Schichtel National Park Service, CSU/CIRA, Foothills Campus, Fort Collins CO 80523 John Molenar Air Resource Specialists, 1901 Sharp Point Drive,
PROPOSED FLAG LEVEL II AND III VISIBILITY ASSESSMENT Bret A. Schichtel National Park Service, CSU/CIRA, Foothills Campus, Fort Collins CO 80523 John Molenar Air Resource Specialists, 1901 Sharp Point Drive,
Arctic Chemistry And Climate
 21 July 2016 Connaught Summer Institute 1 Arctic Chemistry And Climate Connaught Summer Institute 2016 William (Bill) Simpson Geophysical Institute and Department of Chemistry, University of Alaska Fairbanks
21 July 2016 Connaught Summer Institute 1 Arctic Chemistry And Climate Connaught Summer Institute 2016 William (Bill) Simpson Geophysical Institute and Department of Chemistry, University of Alaska Fairbanks
Thermodynamic characterization of Mexico City aerosol during MILAGRO 2006
 Atmos. Chem. Phys., 9, 2141 2156, 2009 Author(s) 2009. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics Thermodynamic characterization of Mexico
Atmos. Chem. Phys., 9, 2141 2156, 2009 Author(s) 2009. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics Thermodynamic characterization of Mexico
ATOC 3500/CHEM 3152 Week 9, March 8, 2016
 ATOC 3500/CHEM 3152 Week 9, March 8, 2016 Hand back Midterm Exams (average = 84) Interaction of atmospheric constituents with light Haze and Visibility Aerosol formation processes (more detail) Haze and
ATOC 3500/CHEM 3152 Week 9, March 8, 2016 Hand back Midterm Exams (average = 84) Interaction of atmospheric constituents with light Haze and Visibility Aerosol formation processes (more detail) Haze and
Aerosol Basics: Definitions, size distributions, structure
 Aerosol Basics: Definitions, size distributions, structure Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of
Aerosol Basics: Definitions, size distributions, structure Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of
Comparison of measured and calculated scattering from surface aerosols with an average, a sizedependent, and a time-dependent refractive index
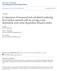 University of Wyoming Wyoming Scholars Repository Atmospheric Science Faculty Publications Atmospheric Science 1-20-2011 Comparison of measured and calculated scattering from surface aerosols with an average,
University of Wyoming Wyoming Scholars Repository Atmospheric Science Faculty Publications Atmospheric Science 1-20-2011 Comparison of measured and calculated scattering from surface aerosols with an average,
Sensitivity of cloud condensation nuclei activation processes to kinetic parameters
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 111,, doi:10.1029/2005jd006529, 2006 Sensitivity of cloud condensation nuclei activation processes to kinetic parameters P. Y. Chuang 1 Received 25 July 2005; revised
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 111,, doi:10.1029/2005jd006529, 2006 Sensitivity of cloud condensation nuclei activation processes to kinetic parameters P. Y. Chuang 1 Received 25 July 2005; revised
Environmental impact of atmospheric NH 3 emissions under present and future conditions in the eastern United States
 Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 35, L12808, doi:10.1029/2008gl033732, 2008 Environmental impact of atmospheric NH 3 emissions under present and future conditions in the eastern
Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 35, L12808, doi:10.1029/2008gl033732, 2008 Environmental impact of atmospheric NH 3 emissions under present and future conditions in the eastern
EVALUATION OF ORIGINAL AND IMPROVED VERSIONS OF CALPUFF USING THE 1995 SWWYTAF DATA BASE. Technical Report. Prepared by
 EVALUATION OF ORIGINAL AND IMPROVED VERSIONS OF CALPUFF USING THE 1995 SWWYTAF DATA BASE Technical Report Prepared by Prakash Karamchandani, Shu-Yun Chen and Rochelle Balmori Atmospheric and Environmental
EVALUATION OF ORIGINAL AND IMPROVED VERSIONS OF CALPUFF USING THE 1995 SWWYTAF DATA BASE Technical Report Prepared by Prakash Karamchandani, Shu-Yun Chen and Rochelle Balmori Atmospheric and Environmental
Intercomparison of standard and capture vaporizer in aerosol mass spectrometer (AMS)
 Intercomparison of standard and capture vaporizer in aerosol mass spectrometer (AMS) Weiwei Hu 1, Pedro Campuzano-Jost 1, Douglas A. Day 1, Philip Croteau 2, Manjula R. Canagaratna 2, John T. Jayne 2,
Intercomparison of standard and capture vaporizer in aerosol mass spectrometer (AMS) Weiwei Hu 1, Pedro Campuzano-Jost 1, Douglas A. Day 1, Philip Croteau 2, Manjula R. Canagaratna 2, John T. Jayne 2,
Remote sensing of aerosol water uptake
 GEOPHYSICAL RESEARCH LETTERS, VOL. 36,, doi:10.109/008gl036576, 009 Remote sensing of aerosol water uptake Gregory L. Schuster, 1 Bing Lin, 1 and Oleg Dubovik Received 5 November 008; revised 10 December
GEOPHYSICAL RESEARCH LETTERS, VOL. 36,, doi:10.109/008gl036576, 009 Remote sensing of aerosol water uptake Gregory L. Schuster, 1 Bing Lin, 1 and Oleg Dubovik Received 5 November 008; revised 10 December
An assessment of time changes of the health risk of PM10 based on GRIMM analyzer data and respiratory deposition model
 J. Keder / Landbauforschung Völkenrode Special Issue 38 57 An assessment of time changes of the health risk of PM based on GRIMM analyzer data and respiratory deposition model J. Keder Abstract PM particles
J. Keder / Landbauforschung Völkenrode Special Issue 38 57 An assessment of time changes of the health risk of PM based on GRIMM analyzer data and respiratory deposition model J. Keder Abstract PM particles
Inconsistency of ammonium-sulfate aerosol ratios with thermodynamic models in the eastern US: a possible role of organic aerosol
 Inconsistency of ammonium-sulfate aerosol ratios with thermodynamic models in the eastern US: a possible role of organic aerosol Rachel Silvern AGU Fall Meeting December 15, 2016 with Daniel Jacob 1, Patrick
Inconsistency of ammonium-sulfate aerosol ratios with thermodynamic models in the eastern US: a possible role of organic aerosol Rachel Silvern AGU Fall Meeting December 15, 2016 with Daniel Jacob 1, Patrick
Collection Efficiency: A summary of what we know so far.
 Collection Efficiency: A summary of what we know so far. AMS Users Highlighted work Eben Cross, Tim Onasch Ann Middlebrook, Roya Bahreini Brendan Matthews Collection Efficiency Definition Why is it important?
Collection Efficiency: A summary of what we know so far. AMS Users Highlighted work Eben Cross, Tim Onasch Ann Middlebrook, Roya Bahreini Brendan Matthews Collection Efficiency Definition Why is it important?
Measuring Total Reactive N and its Composition
 Measuring Total Reactive N and its Composition Bret A. Schichtel 1, Katie Benedict 2, Christian M. Carrico 2, Anthony Prenni 2, Jr. 2, Ezra Levin 2, Derek Day 3, Doris Chen 2, John Ray 1, William C. Malm
Measuring Total Reactive N and its Composition Bret A. Schichtel 1, Katie Benedict 2, Christian M. Carrico 2, Anthony Prenni 2, Jr. 2, Ezra Levin 2, Derek Day 3, Doris Chen 2, John Ray 1, William C. Malm
UNCERTAINTY ASSOCIATED WITH ESTIMATING A SHORT-TERM (1 3 HR) PARTICULATE MATTER CONCENTRATION FROM A HUMAN-SIGHTED VISUAL RANGE
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln JFSP Research Project Reports U.S. Joint Fire Science Program 2013 UNCERTAINTY ASSOCIATED WITH ESTIMATING A SHORT-TERM (1
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln JFSP Research Project Reports U.S. Joint Fire Science Program 2013 UNCERTAINTY ASSOCIATED WITH ESTIMATING A SHORT-TERM (1
AMS CE for Chamber SOA
 AMS CE for Chamber SOA Ken Docherty et al. Alion Science & Technology and NERL, EPA 1 Alion Science and Technology, P.O. Box 12313, Research Triangle Park, NC 27713 2 Cooperative Institute for Research
AMS CE for Chamber SOA Ken Docherty et al. Alion Science & Technology and NERL, EPA 1 Alion Science and Technology, P.O. Box 12313, Research Triangle Park, NC 27713 2 Cooperative Institute for Research
Measurements of the Aerosol Light-Scattering Coefficient at Ambient and 85% Relative Humidity on the ONR Pelican During ACE-2
 Measurements of the Aerosol Light-Scattering Coefficient at Ambient and 85% Relative Humidity on the ONR Pelican During ACE-2 Dean A. Hegg University of Washington Department of Atmospheric Sciences Box
Measurements of the Aerosol Light-Scattering Coefficient at Ambient and 85% Relative Humidity on the ONR Pelican During ACE-2 Dean A. Hegg University of Washington Department of Atmospheric Sciences Box
Kirk R. Baker University of Illinois at Chicago, Chicago, Illinois, USA Lake Michigan Air Directors Consortium, Des Plaines, Illinois, USA
 Photochemical Model Performance for PM2.5 Sulfate, Nitrate, Ammonium, and pre-cursor species SO2, HNO3, and NH3 at Background Monitor Locations in the Central and Eastern United States Kirk R. Baker University
Photochemical Model Performance for PM2.5 Sulfate, Nitrate, Ammonium, and pre-cursor species SO2, HNO3, and NH3 at Background Monitor Locations in the Central and Eastern United States Kirk R. Baker University
