Cross-Linking Amine-Rich Compounds into High Performing Selective CO 2 Absorbents
|
|
|
- Leslie Marshall
- 6 years ago
- Views:
Transcription
1 Supplementary Information for Cross-Linking Amine-Rich Compounds into High Performing Selective CO 2 Absorbents Enrico Andreoli, Eoghan P. Dillon, Laurie Cullum, Lawrence B. Alemany, Andrew R. Barron* Correspondence to: arb@rice.edu This Supplementary Information includes: PEI-C Characterization CO 2 Absorption/Desorption at Atmospheric Pressure Effect of the Molecular Weight of PEI Identification of the Chemical Species Involved in the Absorption of CO 2 Figures S1 to S12 Tables S1 to S3 PEI-C characterization Thermogravimetric analysis: The results of the thermogravimetric analysis (TGA) of PEI-C are given in Figure S1. The absorbent was heated up from room temperature to 0 C at a rate of 2 C/min. During the scan the temperature was held at the two fixed values of and 3 C for 1 h and 12 h, respectively. This was done to allow the full completion of their corresponding processes which are the initial conditioning of the adsorbent in Ar and the decomposition of PEI in air, respectively. The weight decrease with time recorded during the second stage is given in the inset. 100 Weight (%) 74.0 % Weight (%) 35 3 o C / 12 h in Air Time (h).7 % 10 Ar Air 1.1 % Temperature ( C) Fig. S1. TGA characterization of PEI-C heated up from room temperature to 0 C at a scanning rate of 2 C/min. The temperature increase was halted and held at C for 1h and 3 C for 12 h. The inset shows the weight decrease associated with the second stage. After conditioning in Ar, the absorbent weight decreased to 74.0% of its initial value. At this point, air was allowed to flow in place of Ar. The weight of the absorbent dropped then to.7% after the PEI was decomposed at 3 C. A further drop to 1.1% was recorded on going from 3 to 6 C, corresponding to the decomposition of C. From these results, the portion of PEI is = 53.3% and that of C is = 19.6%. The PEI/C weight ratio can be thus calculated as 53.3/19.6. Of the 53.3 parts of PEI, 29.7 are of C and 17.3 of N, which, together with the C of C, give an overall C/N weight ratio of about 74/26. X-ray photoelectron spectroscopy: The X-ray photoelectron spectroscopy (XPS) survey and high resolution spectra of PEI-C conditioned in N 2 are presented in Figure S2. The two main elements detected are C and N, at about 285 and 0 ev, respectively. A third small signal at about 532 ev is related to the presence of a residual
2 amount of O. The C/N/O atomic concentration ratio of the three elements is 71.8/27.1/1.1, corresponding to a C/N weight ratio of about /. This value compares reasonably well with that obtained earlier for the bulk material with TGA, 74/26, the difference might be due to the fact that the XPS analysis is confined to a ~ 1 µm 3 volume portion on the surface of the absorbent. Fig. S2. XPS characterization of PEI-C after conditioning in N 2. The three peaks C1s, N1s and O1s of the survey spectrum are shown in high resolution in their respective insets. Surface area measurement: The surface area of PEI-C was characterized using N 2 adsorbate. In the relative pressure range of 5 to 0., two portions of the gas adsorption isotherm could be linearized using BET and Langmuir equations, as reported in Figure S3. The surface area of the absorbent is on the order of about m 2 /g. Fig. S3. Linearizations of the N 2 adsorption isotherm of PEI-C. The two linear fittings correspond to the BET and Langmuir models, respectively. Scanning electron microscopy: The scanning electron microscopy (SEM) images of as prepared PEI-C are given in Figure S4. The microporosity of the absorbent is evident at low magnification, Figure S4 (a), where free volume is present between small droplets and larger clots. The porosity is heterogeneously distributed with some regions characterized by pores smaller than a micrometer and others completely compact, as shown in the two images collected at higher magnification, Figures S4 (b) and (c). A close up of one of these last regions, Figure S4 (d), shows that at a scale below 1 µm the material is completely compact excluding the presence of mesopores. Fig. S4. SEM images of as prepared PEI-C showing the presence of non-homogeneously distributed microporosity (a, b and c). At the nanoscale level (d) the absorbent appears compact.
3 Atomic force microscopy: The results of the atomic force microscopy (AFM) characterization of PEI-C are shown in Figure S5. In this case the absorbent was probe sonicated in water and drop cast on a cleaved mica substrate as a suspension, due to the insolubility of PEI-C in solvents. As can be seen, the core of a large aggregate in the center of the image is composed of several small particles that are 1 nm in height, indicating that the C has been internalized and the PEI externalized in a micellar-like structure. The PEI spreads out on the surface due to the hydrophilicity of mica. Fig. S5. AFM image and sectional analysis of PEI-C showing internalized C and indicating a height for the central cluster of about 2 nm. Further evidence for the externalization of PEI comes from the SEM images of PEI-C in Figure S4. The SEM images reveal a highly interconnected network of polymer nodules and given that we used branched PEI with many primary amines, one can expect that one PEI chain would react with several nanocarbon molecules resulting in a highly interconnected network. CO 2 absorption/desorption at atmospheric pressure Thermogravimetric analysis: The CO 2 absorption performance of PEI-C at atmospheric pressure is shown in Figure S6. In this plot there are three gravimetric curves (lower) associated with the same temperature program (upper) used for each of the gravimetric curves. The gases used during the measurements are also indicated above the plots. Gas Ar 0.6 II CO 2 or N 2 wet CO 2 III Ar Temperature ( ο C) 0.5 Gas uptake (g/g) I dry CO wet N Fig. S6. Thermogravimetric characterization of the CO 2 capture performance of PEI-C. Initially, the weight of the absorbent falls with time while the temperature is increased in Ar. Then, the weight increases on exposure to wet or dry CO 2, or wet N 2. The absorption of CO 2 as itself, stage I, or with H 2 O, as the temperature is decreased, stage II, is followed by a last stage, III, relative to the regeneration of the absorbent in Ar. Three absorption conditions were investigated: wet CO 2 (red line), dry CO 2 (blue line), and wet N 2 (green line). Wet or dry gases were prepared at room temperature using bubblers filled either with deionized water or dry molecular sieves, respectively. Freshly prepared samples lost about g/g of their weight upon heating to C in Ar due to the release of moisture and adsorbed gases, i.e., CO 2. The carrier gas was then switched to either wet or dry CO 2 or wet N 2 and the weight increases determined while the temperature was lowered (Figure S6). Both wet and dry
4 CO 2 showed immediate absorption of 0.16 g/g and 0.14 g/g at C for wet and dry CO 2, respectively. In contrast, the sample exposed to wet N 2 showed no appreciable mass change until the temperature decreased below 75 C, at which point a maximum weight increase of 9 g/g was observed once the sample reached room temperature. The dry CO 2 sample shows no additional absorption with lowering of the temperature, but the wet CO 2 sample shows a second increase below 75 C to a maximum of about 0. g/g. It is easy to recognize, therefore, that the absorption of CO 2 went through two stages, I and II, in Figure S6. During stage I (between and 75 C) CO 2 is captured mainly in dry form (ca g/g), although a smaller portion associated with H 2 O (ca. 2 g/g) is observed. In stage II (75 C to room temperature) CO 2 is captured in combination with H 2 O and the total amount collectively absorbed during stage II can be calculated from the difference between wet and dry CO 2, i.e., = 0.46 g/g. If we consider a H 2 O uptake of 9 g/g, as from the wet N 2 absorption, the amount of CO 2 absorbed during stage II would be = 0.37 g/g. However, this value may be incorrect since the amount of H 2 O absorbed with N 2 or CO 2 could be different. This is evident in stage I where no H 2 O is absorbed with wet N 2 while it is with wet CO 2. A rationale for this difference could be that the driving force of H 2 O absorption for bicarbonate formation is greater than that of amine protonation. The exact amounts of absorbed CO 2 and H 2 O can be obtained from the elemental analysis of PEI-C conditioned in dry Ar and wet CO 2, as reported in Section 2.2. The regenerative performance of PEI-C is also presented in Figure S6. The portion of the wet CO 2 curve labeled as stage III shows that upon switching CO 2 to Ar the sample lost at room temperature % of the captured mass. Another % was lost below C. Furthermore, PEI-C maintained more than % of its starting absorption capacity after 100 absorption/desorption cycles at C, as shown in Figure S7. CO 2 uptake (g/g) CO 2 Abs. Perform. (%) Cycle Number Fig. S7. Thermogravimetric characterization of the CO 2 absorption/desorption performance of PEI-C at C. The first and last ten of a total of 100 cycles are presented. In the inset, the CO 2 absorption performance of the absorbent is shown as a function of the number of cycles. Elemental analysis: The total amounts of absorbed CO 2 and H 2 O can be calculated from the comparison of the elemental analyses of PEI-C conditioned in dry Ar and wet CO 2. The elemental analyses indicate that the chemical formula of PEI-C conditioned in dry Ar is CH 1.73 N 0.33, while that in wet CO 2 is CH 2. N 0.31 O The stoichiometric balance for the molecular components (C 2 H 5 N) x (C ) y (CO 2 ) z (H 2 O) w can be then calculated as x = 0.33 and y = 053 (z = w = 0), in dry Ar, and as x = 0.31, y = 0, z = 8 and w = 0.32, in wet CO 2. Details of the experimental results and calculations are given as follows. The PEI-C samples were conditioned in two environments: 1) dry Ar and 2) wet CO 2. The obtained conditioned samples were kept in containers filled with the same gas used for the conditioning and analyzed. The time per analysis was about min. Three measurements in sequence were performed per sample. The thermogravimetric profiles for the conditioning of PEI-C in dry Ar and wet CO 2 are reported in Figure S Ar 0.5 Gas uptake (g/g) dry Ar Temperature ( o C) Gas uptake (g/g) wet CO 2 Temperature ( o C) Fig. S8. Thermogravimetric profiles for the conditioning of PEI-C in dry Ar or wet CO 2.
5 The sample in dry Ar lost about % of its weight at the end of the conditioning, while that in wet CO 2 captured an overall amount of CO 2 +H 2 O of 0.55 g/g. The calibration lines for carbon, nitrogen and hydrogen obtained with acetanilide standard are presented in Figure S9. Response (mvs) k R 2 = k k 15k 10k 5k Response (mvs) 2.0k R 2 = k 1.0k Response (mvs) 10k R 2 = k 6k 4k 2k 0 0 Y = 74.95*X Y = 33.64*X Amount of C (mg) Amount of N (mg) Fig. S9. Calibration lines for carbon, nitrogen and hydrogen (R 2 > 0.999). Y = *X Amount of H (mg) The responses for the elemental analysis of PEI-C are given in Table S1. Sample weight (mg) Table S1. Responses for the elemental analyses of PEI-C conditioned in dry Ar and wet CO 2. dry Ar wet CO 2 Sample 1 Sample 2 Sample 3 Sample 1 Sample 2 Sample Element Response (mvs) Response (mvs) Response (mvs) Response (mvs) Response (mvs) Response (mvs) C N H These responses are converted to weights using the calibration lines. The weights are then converted into moles from which the stoichiometric ratios can be calculated as presented in Tables S2 and S3. From Table S2, the N/C stoichiometric ratio is reproducible among all samples, about The amount of H increases with time, most likely because of absorption of moisture since the samples were run in chronological order. For the same reason the difference between weighted and calculated weight (Diff%) also increases due to the oxygen of moisture. Table S2. Results from the elemental analysis of C -PEI conditioned in dry Ar. PEI-C dry Ar Sample mg C E H E N E mg Diff% 0.58 % Sample mg C E H E N E mg Diff% 4.41 % Sample mg C E H E N E mg Diff% 7.74 %
6 The sample most representative of PEI-C conditioned in dry Ar is the first less exposed to the air. The formula obtained for PEI-C conditioned in dry Ar is then C 1 H 1.73 N From this, it is possible to find the PEI/C weight ratio as follows: PEI = (aziridine) X = (C 2 H 5 N) X PEI-C = (C 2 H 5 N) X (C ) Y C 1 H 1.73 N 0.33 = (C 2 H 5 N) X (C ) Y = C 2X+Y H 5X N X 1 = 2X + Y ; 1.73 = 5X ; 0.33 = X X = 0.33, Y = 053 MW(C 2 H 5 N) = g/mol PEI = MW(C 2 H 5 N)*0.33 = g MW(C ) = 7.64 g/mol C = MW(C )*053 = 3.82 g PEI/C weight ratio = / 3.82 = 78.8 / From Table S3, the N/C stoichiometric ratio is reproducible among all samples, about 0.. The amount of H decreases with time, most likely because of desorption of captured H 2 O since the samples were run in chronological order. For the same reason the difference between weighted and calculated weight (Diff%) also decreases due to loss of captured gases. Note that these differences are due to the presence of oxygen. The amount of oxygen can be estimated as the difference between amount of sample taken and total amount of CHN measured. Table S3. Results from the elemental analysis of PEI-C conditioned in wet CO 2. PEI-C wet CO 2 Sample mg C E H E N E O = E mg Diff% % Sample mg C E H E N E O = E mg Diff% % % Sample mg C E H E N E O = E mg Diff% % The sample most representative of PEI-C conditioned in wet CO 2 is the first less exposed to the air. The formula obtained for C -PEI conditioned in wet CO 2 is then C 1 H 2. N 0.31 O From this, it is possible to find the absorbed amounts of CO 2 and H 2 O as follows: PEI = (aziridine) X = (C 2 H 5 N) X PEI-C + CO 2 + H 2 O = (C 2 H 5 N) X (C ) Y (CO 2 ) Z (H 2 O) W C 1 H 2. N 0.31 O 0.48 = (C 2 H 5 N) X (C ) Y (CO 2 ) Z (H 2 O) W = C 2X+Y+Z H 5X+2W N X O 2Z+W 1 = 2X + Y + Z ; 2. = 5X +2W ; 0.31 = X ; 0.48 = 2Z + W X = 0.31, W = (2.-5*0.31)/2 = 0.32, Z = ( )/2 = 8, Y = (1-2*0.31-8)/ = 0 X = 0.31, Y = 05, Z = 8, W = 0.32
7 MW(C 2 H 5 N) = g/mol PEI = MW(C 2 H 5 N)*0.31 = g MW(C ) = 7.64 g/mol C = MW(C )*0 = 3. g MW(CO 2 ) = g/mol CO 2 = MW(CO 2 )*8 = 3.52 g MW(H 2 O) = g/mol H 2 O = MW(H 2 O)*0.32 = 5.76 g PEI/C weight ratio = / 3. = 78.8 / The PEI/C weight ratio is exactly as that previously calculated for PEI-C conditioned in dry Ar. The CO 2 and H 2 O uptakes can be calculated as follows: Captured CO 2 = 3.52 / ( ) = 0.21 g/g Captured H 2 O = 5.76 / ( ) = 0.34 g/g The total captured mass is 0.55 g/g, the same as the amount measured with the TGA (Figure S8). The overall CO 2 :H 2 O stoichiometric ratio is 8:0.32 = 1:4 (as that for the CO 2 :N ratio). The N:H 2 O (aziridine:h 2 O) stoichiometric ratio is 0.31:0.32 = 1:1. The total CO 2 capture capacity of C -PEI is then 0.21 g/g corresponding to 4.8 mmol/g. It is interesting to note that PEI-C absorbs mainly H 2 O during stage II (Figure S6), but this does not affect the total amount of CO 2 captured in the presence of moisture in the gas feed. Also, the overall CO 2 :H 2 O molar ratio of 1:4 and a N:H 2 O one of 1:1. It seems that PEI-C absorbs H 2 O until each amine group in the absorbent is saturated with one molecule of H 2 O. Effect of the molecular weight of PEI We have previously shown with PEI-SWNT conjugates that the higher the PEI molecular weight the greater the uptake of CO 2 (19). Figure S10 shows the thermogravimetric curves for the absorption of wet CO 2 of PEI-C prepared with different PEIs, but using the same synthetic procedure and PEI/C weight ratio. It is clear that the overall absorption of CO 2 +H 2 O improves with increasing molecular weight. It is important to note that in the case of PEI(0)-C the adduct melts at the C. The best absorption performance is obtained with branched PEI 25,000 Da reaching 0.15 g/g uptake in min. at C and a total of CO 2 +H 2 O of 0.45 g/g going to 25 C in other 1 min. T ( o C) wet CO 2 Ar PEI0 PEI1,0 PEI10,000 PEI25,000 Gas uptake ( g/g) Fig. S10. Thermogravimetric characterization of the CO 2 absorption/desorption performance of PEI-C at C prepared from PEIs of different molecular weights. Identification of the chemical species involved in the absorption of CO 2 Nuclear magnetic resonance: The solid state 13 C and 15 N NMR spectra of the PEI-C conditioned in wet CO 2, dry CO 2, and wet N 2 are shown in Figure S11 (note that this figure is the same as Figure 4 of the main manuscript and it is reported here again for convenience). The 15 N spectra demonstrate the presence of carbamate. The 13 C spectra do not allow a secure differentiation of the carbamate carbonyl signal from the bicarbonate carbonyl signal that may also be present, as will now be discussed.
8 Fig. S11. Solid state (a) 1 H- 13 C and (b) 1 H- 15 N CP-MAS NMR characterization of PEI-C after conditioning in wet CO 2, dry CO 2 or N 2. Two distinctive bands are present in all 13 C CP-MAS NMR spectra, Figure S11 (a): a band with a peak maximum at about ppm from the sp 3 carbons of PEI and a weaker band with a peak maximum at about 1 ppm from the sp 2 carbons of functionalized C. A third sharper signal at 164 ppm is also evident in spectra for samples exposed to wet or dry CO 2 (but not in the spectrum exposed to wet N 2 ). Since this signal can be attributed to carbonate and/or carbamate species, we cannot readily determine the relative contributions of these two species in a MAS 13 C NMR spectrum of PEI-C conditioned in CO 2 ; this is not surprising in light of prior work. Ammonium bicarbonate gives a single, very sharp MAS 13 C NMR signal at ppm in agreement with a previous report [1]; the carbonyl peak position of bicarbonate is commonly observed at about 162 ppm in CO 2 -amine-h 2 O solutions [2]. In contrast, ammonium carbamate (H 2 NCO 2 NH 4 ) gives a pair of 13 C signals differing in peak height and linewidth in an MAS NMR spectrum obtained at a low magnetic field strength [1] because the 14 N quadrupole prevents complete averaging of the 13 C- 14 N dipolar coupling [3-5]. The effect becomes greater as the field strength decreases, and the separation of the two signals is field dependent, as demonstrated by the carbonyl lineshape of ammonium carbamate at 25 MHz 13 C [1] and at MHz 13 C (our work). These aspects significantly complicate analyzing the carbonyl region in an MAS spectrum if both bicarbonate and carbamate are present, as the bicarbonate signal may overlap part of the complex carbamate signal [1] since their chemical shifts are very similar (an aqueous solution of ammonium carbamate gives a signal at ppm [6-7], while solutions of anionic N-alkyl carbamates give a signal at ppm [8-12]). Two other factors cause additional difficulties in interpreting the signal at 164 ppm in the MAS 13 C NMR spectrum of PEI-C conditioned with CO 2. First, the distinctive, very sharp signal of NH 4 HCO 3 is significantly broadened when mixed with H 2 NCO 2 NH 4 [1] and thus is no longer as easily recognizable; the same effect would be expected if bicarbonate is present in our sample. Second, the carbamate carbonyl signal may be just a single peak, even at MHz 13 C, as shown by the MAS 13 C NMR spectrum of CO 2 adsorbed on PEI immobilized on a mesoporous silica support [13]. The half-height linewidth of this signal [13] is similar to that of the carbonyl signals in Figure S11 (a) with the same field strength and same line broadening used. Fortunately, CP-MAS 15 N NMR presents a much more secure way to determine the presence of carbamate in the presence of bicarbonate, and, so, the 15 N CP-MAS NMR spectra of the PEI-C conditioned in N 2 and dry CO 2 were also collected, Figure S11 (b). In the 15 N spectrum recorded after conditioning in dry CO 2, the band at about -347 ppm can reasonably be assigned to PEI amine nitrogen environments, while the signal at about -297 ppm can reasonably be assigned to PEI-NH-COO - carbamate species. In particular, the carbamate assignment is consistent with the 15 N chemical shifts reported for a CO 2 -treated, amine-modified, nanoporous clay [14] and with the 15 N chemical shifts of ammonium carbamate and other simple carbamates [15-18]. In the sample conditioned in N 2, the only appreciable signal, after more than,000 scans, was that of the PEI amine nitrogens. X-ray photoelectron spectroscopy: The XPS characterization of PEI-C conditioned in wet CO 2 (Figure S12) also supports the formation of bicarbonate and/or carbamate species. The C1s high resolution spectrum shows a peak at ev consistent with the presence of the sp 2 C=C bonds of C, while the two peaks at and ev are related to the sp 3 C-C and C-N bonds in PEI. A fourth smaller peak is also present at ev in agreement with the formation of bicarbonate and/or carbamate species both found in this binding energy region [19-]. The intensity of this peak cannot be related to the effective amount of CO 2 captured by PEI-C because most of the CO 2 is likely desorbed in the UHV chamber. The contributions at and ev in the O1s spectrum are consistent with the presence of two different CO 2 absorption species such as those of bicarbonate and carbamate. The other peak at ev is due to a residual trace of H 2 O present in the absorbent.
9 Fig. S12. High resolution (a) C1s and (b) O1s XPS characterization of PEI-C after conditioning in wet CO 2. References 1. X. Li, E. Hagaman, C. Tsouris, J. W. Lee, Energy Fuels 17, (03). 2. J. Y. Park, S. J. Yoon, H. Lee, Environ. Sci. Technol. 37, (03). 3. J. G. Hexem, M. H. Frey, S. J. Opella, J. Am. Chem. Soc. 103, (1981). 4. A. Naito, S. Ganapathy, C. A. McDowell, J. Chem. Phys. 74, (1981). 5. E. M. Menger, W. S. Veeman, J. Magn. Reson. 46, (1982). 6. N. Wen, M. H. Brooker, J. Phys. Chem. 99, (1995). 7. F. Mani, M. Peruzzini,; P. Stoppioni, Green Chem. 8, (06). 8. R. J. Hook, Ind. Eng. Chem. Res. 36, (1997). 9. A. Dibenedetto, M. Aresta, C. Fragale, M. Narracci, Green. Chem. 4, (02). 10. W. Böttinger, M. Maiwald, H. Hasse, Fluid Phase Equilib. 263, (08). 11. F. Barzagli, F. Mani, M. Peruzzini, Energy Environ. Sci. 2, (09). 12. G-j. Fan, A. G. H. Wee, R. Idem, P. Tontiwachwuthikul, Ind. Eng. Chem. Res. 48, (09). 13. T. C. Drage, A. Arenillas, K. M. Smith, C. E. Snape, Microporous Mesoporous Mater. 116, (08). 14. M. L. Pinto, L. Mafra, J. M. Guil, J. Pires, J. Rocha, Chem. Mater. 23, (11). 15. J. P. Warren, J. D. Roberts, J. Phys. Chem. 78, (1974). 16. J. M. Burns, M. E. Ashley, G. C. Crockett, T. H. Koch, J. Am. Chem. Soc. 99, (1977). 17. H. R. Kricheldorf, Org. Magn. Reson. 14, (19). 18. W. McGhee, D. Riley, K. Christ, Y. Pan, B. Parnas, J. Org. Chem., (1995). 19. I. George, P. Viel, C. Bureau, J. Suski, G. Lecayon, Surf. Interf. Anal. 24, (1996).. B. Luo, J. E. Rossini, W. L. Gladfelter, Langmuir 25, (09).
Amine-impregnated silica monolith with a hierarchical pore structure: enhancement of CO 2 capture capacity
 1 Electronic Supplementary Information (ESI) Amine-impregnated silica monolith with a hierarchical pore structure: enhancement of CO 2 capture capacity for Chao Chen, Seung-Tae Yang, Wha-Seung Ahn* and
1 Electronic Supplementary Information (ESI) Amine-impregnated silica monolith with a hierarchical pore structure: enhancement of CO 2 capture capacity for Chao Chen, Seung-Tae Yang, Wha-Seung Ahn* and
1. Materials All chemicals and solvents were purchased from Sigma Aldrich or SAMCHUN and used without further purification.
 1. Materials All chemicals and solvents were purchased from Sigma Aldrich or SAMCHUN and used without further purification. 2. Experimental procedures Benzo[1,2-c:3,4-c':5,6-c'']trifuran, 1: The synthesis
1. Materials All chemicals and solvents were purchased from Sigma Aldrich or SAMCHUN and used without further purification. 2. Experimental procedures Benzo[1,2-c:3,4-c':5,6-c'']trifuran, 1: The synthesis
Supplementary Information for
 Supplementary Information for Facile transformation of low cost thiourea into nitrogen-rich graphitic carbon nitride nanocatalyst with high visible light photocatalytic performance Fan Dong *a, Yanjuan
Supplementary Information for Facile transformation of low cost thiourea into nitrogen-rich graphitic carbon nitride nanocatalyst with high visible light photocatalytic performance Fan Dong *a, Yanjuan
Supporting Information. for. Angew. Chem. Int. Ed. Z Wiley-VCH 2003
 Supporting Information for Angew. Chem. Int. Ed. Z52074 Wiley-VCH 2003 69451 Weinheim, Germany Kinetic and Thermodynamic Control via Chemical Bond Rearrangement on Si(001) Surface Chiho Hamai, Akihiko
Supporting Information for Angew. Chem. Int. Ed. Z52074 Wiley-VCH 2003 69451 Weinheim, Germany Kinetic and Thermodynamic Control via Chemical Bond Rearrangement on Si(001) Surface Chiho Hamai, Akihiko
The Effect of Water and Confinement on Self-Assembly of
 Supporting Information: The Effect of Water and Confinement on Self-Assembly of Imidazolium Based Ionic Liquids at Mica Interface H.-W. Cheng, J.-N. Dienemann, P. Stock, C. Merola, Y.-J. Chen and M. Valtiner*
Supporting Information: The Effect of Water and Confinement on Self-Assembly of Imidazolium Based Ionic Liquids at Mica Interface H.-W. Cheng, J.-N. Dienemann, P. Stock, C. Merola, Y.-J. Chen and M. Valtiner*
Magnetite decorated graphite nanoplatelets as cost effective CO 2 adsorbent
 Supplementary Information Magnetite decorated graphite nanoplatelets as cost effective CO 2 adsorbent Ashish Kumar Mishra and Sundara Ramaprabhu * Alternative Energy and Nanotechnology Laboratory (AENL),
Supplementary Information Magnetite decorated graphite nanoplatelets as cost effective CO 2 adsorbent Ashish Kumar Mishra and Sundara Ramaprabhu * Alternative Energy and Nanotechnology Laboratory (AENL),
Synthesis of nano-sized anatase TiO 2 with reactive {001} facets using lamellar protonated titanate as precursor
 Supporting Information Synthesis of nano-sized anatase TiO 2 with reactive {001} facets using lamellar protonated titanate as precursor Liuan Gu, Jingyu Wang *, Hao Cheng, Yunchen Du and Xijiang Han* Department
Supporting Information Synthesis of nano-sized anatase TiO 2 with reactive {001} facets using lamellar protonated titanate as precursor Liuan Gu, Jingyu Wang *, Hao Cheng, Yunchen Du and Xijiang Han* Department
Covalent-Organic Frameworks: Potential Host Materials for Sulfur Impregnation in Lithium-Sulfur Batteries
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2014 Covalent-Organic Frameworks: Potential Host Materials for Sulfur Impregnation
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2014 Covalent-Organic Frameworks: Potential Host Materials for Sulfur Impregnation
Supplementary Figure S1. AFM image and height profile of GO. (a) AFM image
 Supplementary Figure S1. AFM image and height profile of GO. (a) AFM image and (b) height profile of GO obtained by spin-coating on silicon wafer, showing a typical thickness of ~1 nm. 1 Supplementary
Supplementary Figure S1. AFM image and height profile of GO. (a) AFM image and (b) height profile of GO obtained by spin-coating on silicon wafer, showing a typical thickness of ~1 nm. 1 Supplementary
Ethers in a Porous Metal-Organic Framework
 Supporting Information Enhanced Isosteric Heat of H 2 Adsorption by Inclusion of Crown Ethers in a Porous Metal-Organic Framework Hye Jeong Park and Myunghyun Paik Suh* Department of Chemistry, Seoul National
Supporting Information Enhanced Isosteric Heat of H 2 Adsorption by Inclusion of Crown Ethers in a Porous Metal-Organic Framework Hye Jeong Park and Myunghyun Paik Suh* Department of Chemistry, Seoul National
Functionalized Tertiary Amines as SO2 Absorbents
 Proceedings of the World Congress on Civil, Structural, and Environmental Engineering (CSEE 16) Prague, Czech Republic March 30 31, 2016 Paper No. ICESDP 112 DOI: 10.11159/icesdp16.112 Functionalized Tertiary
Proceedings of the World Congress on Civil, Structural, and Environmental Engineering (CSEE 16) Prague, Czech Republic March 30 31, 2016 Paper No. ICESDP 112 DOI: 10.11159/icesdp16.112 Functionalized Tertiary
Acidic Water Monolayer on Ruthenium(0001)
 Acidic Water Monolayer on Ruthenium(0001) Youngsoon Kim, Eui-seong Moon, Sunghwan Shin, and Heon Kang Department of Chemistry, Seoul National University, 1 Gwanak-ro, Seoul 151-747, Republic of Korea.
Acidic Water Monolayer on Ruthenium(0001) Youngsoon Kim, Eui-seong Moon, Sunghwan Shin, and Heon Kang Department of Chemistry, Seoul National University, 1 Gwanak-ro, Seoul 151-747, Republic of Korea.
Xiaoping Wangab. Electronic Supplementary Material (ESI) for Journal of Materials Chemistry This journal is The Royal Society of Chemistry 2012
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry Supplementary Information of Highly Efficient Dye Adsorption and Removal: Functional Hybrid of Reduced Graphene Oxide Fe 3 O 4
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry Supplementary Information of Highly Efficient Dye Adsorption and Removal: Functional Hybrid of Reduced Graphene Oxide Fe 3 O 4
Electronic Supplementary Information
 Electronic Supplementary Information Pyrene-Directed Growth of Nanoporous Benzimidazole-Linked Nanofibers and their Application to Selective Capture and Separation Mohammad Gulam Rabbani, Ali Kemal Sekizkardes,
Electronic Supplementary Information Pyrene-Directed Growth of Nanoporous Benzimidazole-Linked Nanofibers and their Application to Selective Capture and Separation Mohammad Gulam Rabbani, Ali Kemal Sekizkardes,
DMOF-1 as a Representative MOF for SO 2 Adsorption in both Humid and Dry Conditions
 DMOF-1 as a Representative MOF for SO 2 Adsorption in both Humid and Dry Conditions Julian Hungerford, Souryadeep Bhattacharyya, Uma Tumuluri, Sankar Nair, Zili Wu, and Krista S. Walton* School of Chemical
DMOF-1 as a Representative MOF for SO 2 Adsorption in both Humid and Dry Conditions Julian Hungerford, Souryadeep Bhattacharyya, Uma Tumuluri, Sankar Nair, Zili Wu, and Krista S. Walton* School of Chemical
Supplementary information
 Supplementary information Supplementary Information for Exceptional Ammonia Uptake by a Covalent Organic Framework Christian J. Doonan, David J. Tranchemontagne,T. Grant Glover, Joseph R. Hunt, Omar M.
Supplementary information Supplementary Information for Exceptional Ammonia Uptake by a Covalent Organic Framework Christian J. Doonan, David J. Tranchemontagne,T. Grant Glover, Joseph R. Hunt, Omar M.
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Micro- and mesoporous poly(schiff-base)s
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Micro- and mesoporous poly(schiff-base)s
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Cyclic Molecule Aerogels: A Robust Cyclodextrin
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Cyclic Molecule Aerogels: A Robust Cyclodextrin
Surface Chemical Modification of Nanosized Oxide Particles with a Titanate Coupling Reagent in Isopropanol
 Ind. Eng. Chem. Res. 2008, 47, 1513-1517 1513 Surface Chemical Modification of Nanosized Oxide Particles with a Titanate Coupling Reagent in Isopropanol Xiao-Yong Fang, Ting-Jie Wang,* Hai-Xia Wu, and
Ind. Eng. Chem. Res. 2008, 47, 1513-1517 1513 Surface Chemical Modification of Nanosized Oxide Particles with a Titanate Coupling Reagent in Isopropanol Xiao-Yong Fang, Ting-Jie Wang,* Hai-Xia Wu, and
Surface Chemistry of Alanine on Ni{111} Supporting Information
 S1 Surface Chemistry of Alanine on Ni{111} Richard E. J. Nicklin 1, Alix Cornish 1, Andrey Shavorskiy 2, Silvia Baldanza 1, Karina Schulte 3, Zhi Liu 2, Roger A. Bennett 1, Georg Held 1,4 1 Department
S1 Surface Chemistry of Alanine on Ni{111} Richard E. J. Nicklin 1, Alix Cornish 1, Andrey Shavorskiy 2, Silvia Baldanza 1, Karina Schulte 3, Zhi Liu 2, Roger A. Bennett 1, Georg Held 1,4 1 Department
Adsorption Processes. Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad
 Adsorption Processes Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad Contents Introduction Principles of adsorption Types of adsorption Definitions Brief history Adsorption isotherms Mechanism
Adsorption Processes Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad Contents Introduction Principles of adsorption Types of adsorption Definitions Brief history Adsorption isotherms Mechanism
Metal-Organic Frameworks and Porous Polymer Networks for Carbon Capture
 Carbon Capture Workshop, Tuesday, April 3 rd, Texas A&M, Qatar Metal-Organic Frameworks and Porous Polymer Networks for Carbon Capture J. P. Sculley, J.-R. Li, J. Park, W. Lu, and H.-C. Zhou Texas A&M
Carbon Capture Workshop, Tuesday, April 3 rd, Texas A&M, Qatar Metal-Organic Frameworks and Porous Polymer Networks for Carbon Capture J. P. Sculley, J.-R. Li, J. Park, W. Lu, and H.-C. Zhou Texas A&M
Supplementary Information
 Supplementary Information Supplementary Figure S1: Structure and composition of Teflon tape. (a) XRD spectra of original Teflon tape and Teflon tape subjected to annealing at 150 o C under Ar atmosphere.
Supplementary Information Supplementary Figure S1: Structure and composition of Teflon tape. (a) XRD spectra of original Teflon tape and Teflon tape subjected to annealing at 150 o C under Ar atmosphere.
Supplementary figure 1 Molecular electrostatic potential surface of CF 3 X. Reprinted with permission from Supplementary Reference #4.
 Supplementary figure 1 Molecular electrostatic potential surface of CF 3 X. Reprinted with permission from Supplementary Reference #4. Copyright 2007 Springer. S1 Supplementary figure 2 Stability tests
Supplementary figure 1 Molecular electrostatic potential surface of CF 3 X. Reprinted with permission from Supplementary Reference #4. Copyright 2007 Springer. S1 Supplementary figure 2 Stability tests
A high-efficient monoclinic BiVO 4 adsorbent for selective capture toxic selenite
 Supporting Online Materials for A high-efficient monoclinic BiVO 4 adsorbent for selective capture toxic selenite Huan Ouyang, Yuanyuan Sun*, and Jianqiang Yu* Collaborative Innovation Center for Marine
Supporting Online Materials for A high-efficient monoclinic BiVO 4 adsorbent for selective capture toxic selenite Huan Ouyang, Yuanyuan Sun*, and Jianqiang Yu* Collaborative Innovation Center for Marine
Supplementary Information
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2016 Supplementary Information Cross-linker Mediated Formation of Sulfur-functionalized V 2 O 5 /Graphene
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2016 Supplementary Information Cross-linker Mediated Formation of Sulfur-functionalized V 2 O 5 /Graphene
Kinetic enhancement of adsorbent for CO2 capture from atmosphere by porous material
 Engineering Conferences International ECI Digital Archives CO2 Summit II: Technologies and Opportunities Proceedings Spring 4-13-2016 Kinetic enhancement of adsorbent for CO2 capture from atmosphere by
Engineering Conferences International ECI Digital Archives CO2 Summit II: Technologies and Opportunities Proceedings Spring 4-13-2016 Kinetic enhancement of adsorbent for CO2 capture from atmosphere by
Doctor of Philosophy
 STUDIES ON THE CORROSION INHIBITION BEHAVIOUR OF SOME AMINO ACID SURFACTANT ADDITIVES ABSTRACT SUBMITTED FOR THE AWARD OF THE DEGREE OF Doctor of Philosophy IN APPLIED CHEMISTRY By MOSARRAT PARVEEN UNDER
STUDIES ON THE CORROSION INHIBITION BEHAVIOUR OF SOME AMINO ACID SURFACTANT ADDITIVES ABSTRACT SUBMITTED FOR THE AWARD OF THE DEGREE OF Doctor of Philosophy IN APPLIED CHEMISTRY By MOSARRAT PARVEEN UNDER
Infrared Spectroscopy
 Infrared Spectroscopy Introduction Spectroscopy is an analytical technique which helps determine structure. It destroys little or no sample. The amount of light absorbed by the sample is measured as wavelength
Infrared Spectroscopy Introduction Spectroscopy is an analytical technique which helps determine structure. It destroys little or no sample. The amount of light absorbed by the sample is measured as wavelength
ORGANIC - BROWN 8E CH INFRARED SPECTROSCOPY.
 !! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
!! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
Adsorption of Methylene Blue on Mesoporous SBA 15 in Ethanol water Solution with Different Proportions
 2015 2 nd International Conference on Material Engineering and Application (ICMEA 2015) ISBN: 978-1-60595-323-6 Adsorption of Methylene Blue on Mesoporous SBA 15 in Ethanol water Solution with Different
2015 2 nd International Conference on Material Engineering and Application (ICMEA 2015) ISBN: 978-1-60595-323-6 Adsorption of Methylene Blue on Mesoporous SBA 15 in Ethanol water Solution with Different
Measurement techniques
 Measurement techniques 1 GPC GPC = gel permeation chromatography GPC a type of size exclusion chromatography (SEC), that separates analytes on the basis of size. The column used for GPC is filled with
Measurement techniques 1 GPC GPC = gel permeation chromatography GPC a type of size exclusion chromatography (SEC), that separates analytes on the basis of size. The column used for GPC is filled with
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) A photoluminescent covalent
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) A photoluminescent covalent
Supporting Information
 Supporting Information Photocatalytic Suzuki Coupling Reaction using Conjugated Microporous Polymer with Immobilized Palladium Nanoparticles under Visible Light Zi Jun Wang, Saman Ghasimi, Katharina Landfester
Supporting Information Photocatalytic Suzuki Coupling Reaction using Conjugated Microporous Polymer with Immobilized Palladium Nanoparticles under Visible Light Zi Jun Wang, Saman Ghasimi, Katharina Landfester
Supplementary Figure 1. SEM images of (a) 1, (b) 1 PSt/PMMA, and (c) polymer blend isolated from 1 PSt/PMMA. The size and morphology of the host
 Supplementary Figure 1. SEM images of (a) 1, (b) 1 PSt/PMMA, and (c) polymer blend isolated from 1 PSt/PMMA. The size and morphology of the host crystals were the almost same to those of the blend polymer
Supplementary Figure 1. SEM images of (a) 1, (b) 1 PSt/PMMA, and (c) polymer blend isolated from 1 PSt/PMMA. The size and morphology of the host crystals were the almost same to those of the blend polymer
ESI. Core-Shell Polymer Nanoparticles for Prevention of GSH Drug Detoxification and Cisplatin Delivery to Breast Cancer Cells
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2015 ESI Core-Shell Polymer Nanoparticles for Prevention of GSH Drug Detoxification and Cisplatin Delivery
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2015 ESI Core-Shell Polymer Nanoparticles for Prevention of GSH Drug Detoxification and Cisplatin Delivery
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 218 Rel. intensity Rel. intensity Electronic Supplementary Information Under-cover stabilization
Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 218 Rel. intensity Rel. intensity Electronic Supplementary Information Under-cover stabilization
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature14327 Supplementary Text Structure solution and Rietveld refinement of mmen-mn 2 (dobpdc) Initially, the previously reported crystal structure of the isostructural Zn 2 (dobpdc) 13, with
doi:10.1038/nature14327 Supplementary Text Structure solution and Rietveld refinement of mmen-mn 2 (dobpdc) Initially, the previously reported crystal structure of the isostructural Zn 2 (dobpdc) 13, with
Supplementary Figure 1 Morpholigical properties of TiO 2-x SCs. The statistical particle size distribution (a) of the defective {001}-TiO 2-x SCs and
 Supplementary Figure 1 Morpholigical properties of TiO 2-x s. The statistical particle size distribution (a) of the defective {1}-TiO 2-x s and their typical TEM images (b, c). Quantity Adsorbed (cm 3
Supplementary Figure 1 Morpholigical properties of TiO 2-x s. The statistical particle size distribution (a) of the defective {1}-TiO 2-x s and their typical TEM images (b, c). Quantity Adsorbed (cm 3
Supporting Information
 Supporting Information Yao et al. 10.1073/pnas.1416368111 Fig. S1. In situ LEEM imaging of graphene growth via chemical vapor deposition (CVD) on Pt(111). The growth of graphene on Pt(111) via a CVD process
Supporting Information Yao et al. 10.1073/pnas.1416368111 Fig. S1. In situ LEEM imaging of graphene growth via chemical vapor deposition (CVD) on Pt(111). The growth of graphene on Pt(111) via a CVD process
Cobalt-Porphyrin /Dansyl Piperazine Complex Coated Filter. Paper for Turn on Fluorescence Sensing of Ammonia Gas
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 215 Electronic Supplementary Information Cobalt-Porphyrin /Dansyl Piperazine Complex Coated Filter
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 215 Electronic Supplementary Information Cobalt-Porphyrin /Dansyl Piperazine Complex Coated Filter
Cu 2 O/g-C 3 N 4 nanocomposites: An insight into the band structure tuning and catalytic efficiencies
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 216 Cu 2 O/g-C 3 N 4 nanocomposites: An insight into the band structure tuning and catalytic efficiencies
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 216 Cu 2 O/g-C 3 N 4 nanocomposites: An insight into the band structure tuning and catalytic efficiencies
Experimental study into carbon dioxide solubility and species distribution in aqueous alkanolamine solutions
 Air Pollution XX 515 Experimental study into carbon dioxide solubility and species distribution in aqueous alkanolamine solutions H. Yamada, T. Higashii, F. A. Chowdhury, K. Goto S. Kazama Research Institute
Air Pollution XX 515 Experimental study into carbon dioxide solubility and species distribution in aqueous alkanolamine solutions H. Yamada, T. Higashii, F. A. Chowdhury, K. Goto S. Kazama Research Institute
Supporting information A Porous Zr-cluster-based Cationic Metal-Organic Framework for Highly Efficient Cr 2 O 7
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Supporting information A Porous Zr-cluster-based Cationic Metal-Organic Framework for Highly Efficient
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Supporting information A Porous Zr-cluster-based Cationic Metal-Organic Framework for Highly Efficient
ph-depending Enhancement of Electron Transfer by {001} Facet-Dominating TiO 2 Nanoparticles for Photocatalytic H 2 Evolution under Visible Irradiation
 S1 ph-depending Enhancement of Electron Transfer by {001} Facet-Dominating TiO 2 Nanoparticles for Photocatalytic H 2 Evolution under Visible Irradiation Masato M. Maitani a *, Zhan Conghong a,b, Dai Mochizuki
S1 ph-depending Enhancement of Electron Transfer by {001} Facet-Dominating TiO 2 Nanoparticles for Photocatalytic H 2 Evolution under Visible Irradiation Masato M. Maitani a *, Zhan Conghong a,b, Dai Mochizuki
Supporting Information. Effect of Backbone Chemistry on the Structure of Polyurea Films Deposited by Molecular Layer Deposition
 Supporting Information Effect of Backbone Chemistry on the Structure of Polyurea Films Deposited by Molecular Layer Deposition David S. Bergsman, Richard G. Closser, Christopher J. Tassone, Bruce M. Clemens
Supporting Information Effect of Backbone Chemistry on the Structure of Polyurea Films Deposited by Molecular Layer Deposition David S. Bergsman, Richard G. Closser, Christopher J. Tassone, Bruce M. Clemens
PHYSICAL AND CHEMICAL PROPERTIES OF ATMOSPHERIC PRESSURE PLASMA POLYMER FILMS
 PHYSICAL AND CHEMICAL PROPERTIES OF ATMOSPHERIC PRESSURE PLASMA POLYMER FILMS O. Goossens, D. Vangeneugden, S. Paulussen and E. Dekempeneer VITO Flemish Institute for Technological Research, Boeretang
PHYSICAL AND CHEMICAL PROPERTIES OF ATMOSPHERIC PRESSURE PLASMA POLYMER FILMS O. Goossens, D. Vangeneugden, S. Paulussen and E. Dekempeneer VITO Flemish Institute for Technological Research, Boeretang
Dry-gel conversion synthesis of Cr-MIL-101 aided by grinding: High surface area high yield synthesis with minimum purification
 Electronic Supporting Informations (ESI): Dry-gel conversion synthesis of Cr-MIL-101 aided by grinding: High surface area high yield synthesis with minimum purification Jun Kim, Yu-Ri Lee and Wha-Seung
Electronic Supporting Informations (ESI): Dry-gel conversion synthesis of Cr-MIL-101 aided by grinding: High surface area high yield synthesis with minimum purification Jun Kim, Yu-Ri Lee and Wha-Seung
What type of samples are common? Time spent on different operations during LC analyses. Number of samples? Aims. Sources of error. Sample preparation
 What type of samples are common? Sample preparation 1 2 Number of samples? Time spent on different operations during LC analyses 3 4 Sources of error Aims Sample has to be representative Sample has to
What type of samples are common? Sample preparation 1 2 Number of samples? Time spent on different operations during LC analyses 3 4 Sources of error Aims Sample has to be representative Sample has to
Properties of Compounds
 Chapter 6. Properties of Compounds Comparing properties of elements and compounds Compounds are formed when elements combine together in fixed proportions. The compound formed will often have properties
Chapter 6. Properties of Compounds Comparing properties of elements and compounds Compounds are formed when elements combine together in fixed proportions. The compound formed will often have properties
High H2 Adsorption by Coordination Framework Materials
 Arianna Marchioro Florian Degueldre High H2 Adsorption by Coordination Framework Materials Xiang Lin, Junhua Jia, Xuebo Zhao, K. Mark Thomas, Alexender J. Black, Gavin S. Walker, Neil R. Champness, Peter
Arianna Marchioro Florian Degueldre High H2 Adsorption by Coordination Framework Materials Xiang Lin, Junhua Jia, Xuebo Zhao, K. Mark Thomas, Alexender J. Black, Gavin S. Walker, Neil R. Champness, Peter
NanoEngineering of Hybrid Carbon Nanotube Metal Composite Materials for Hydrogen Storage Anders Nilsson
 NanoEngineering of Hybrid Carbon Nanotube Metal Composite Materials for Hydrogen Storage Anders Nilsson Stanford Synchrotron Radiation Laboratory (SSRL) and Stockholm University Coworkers and Ackowledgement
NanoEngineering of Hybrid Carbon Nanotube Metal Composite Materials for Hydrogen Storage Anders Nilsson Stanford Synchrotron Radiation Laboratory (SSRL) and Stockholm University Coworkers and Ackowledgement
Storage of Hydrogen, Methane and Carbon Dioxide in Highly Porous Covalent Organic Frameworks for Clean Energy Applications
 Storage of Hydrogen, Methane and Carbon Dioxide in Highly Porous Covalent Organic Frameworks for Clean Energy Applications (Supporting Information: 33 pages) Hiroyasu Furukawa and Omar M. Yaghi Center
Storage of Hydrogen, Methane and Carbon Dioxide in Highly Porous Covalent Organic Frameworks for Clean Energy Applications (Supporting Information: 33 pages) Hiroyasu Furukawa and Omar M. Yaghi Center
SUPPORTING INFORMATION. and Mark E. Davis*
 SUPPORTING INFORMATION Active Sites in Sn-Beta for Glucose Isomerization to Fructose and Epimerization to Mannose Ricardo Bermejo-Deval, Marat Orazov, Rajamani Gounder** Son-Jong Hwang and Mark E. Davis*
SUPPORTING INFORMATION Active Sites in Sn-Beta for Glucose Isomerization to Fructose and Epimerization to Mannose Ricardo Bermejo-Deval, Marat Orazov, Rajamani Gounder** Son-Jong Hwang and Mark E. Davis*
Supplementary Information
 Supplementary Information Supplementary Figure 1. Photographs show the titration experiments by dropwise adding ~5 times number of moles of (a) LiOH and LiOH+H 2 O, (b) H 2 O 2 and H 2 O 2 +LiOH, (c) Li
Supplementary Information Supplementary Figure 1. Photographs show the titration experiments by dropwise adding ~5 times number of moles of (a) LiOH and LiOH+H 2 O, (b) H 2 O 2 and H 2 O 2 +LiOH, (c) Li
STUDY ON THE IMPROVEMENT OF THE REDUCTION CAPACITY OF ACTIVATED CARBON FIBER
 STUDY ON THE IMPROVEMENT OF THE REDUCTION CAPACITY OF ACTIVATED CARBON FIBER Chen Shuixia, Zeng Hanmin Materials Science Institute, Zhongshan University, Guangzhou 51275, China Key Laboratory for Polymeric
STUDY ON THE IMPROVEMENT OF THE REDUCTION CAPACITY OF ACTIVATED CARBON FIBER Chen Shuixia, Zeng Hanmin Materials Science Institute, Zhongshan University, Guangzhou 51275, China Key Laboratory for Polymeric
Electronic Supplementary Information. Noninvasive Functionalization of Polymers of Intrinsic Microporosity for Enhanced CO 2 Capture
 Electronic Supplementary Information Noninvasive Functionalization of Polymers of Intrinsic Microporosity for Enhanced CO 2 Capture Hasmukh A. Patel and Cafer T. Yavuz* Oxide and Organic Nanomaterials
Electronic Supplementary Information Noninvasive Functionalization of Polymers of Intrinsic Microporosity for Enhanced CO 2 Capture Hasmukh A. Patel and Cafer T. Yavuz* Oxide and Organic Nanomaterials
Supporting Information
 Supporting Information For the manuscript Catalyst-free Preparation of Melamine-based Microporous Polymer Networks through Schiff Base Chemistry by Matthias Georg Schwab, + Birgit Faßbender, + Hans Wolfgang
Supporting Information For the manuscript Catalyst-free Preparation of Melamine-based Microporous Polymer Networks through Schiff Base Chemistry by Matthias Georg Schwab, + Birgit Faßbender, + Hans Wolfgang
Production of Mesoporous Carbon from Waste Tire
 Production of Mesoporous Carbon from Waste Tire E.L.K. Mui and G. M c Kay Department of Chemical Engineering Hong Kong University of Science and Technology Clear Water Bay, Kowloon, Hong Kong Corresponding
Production of Mesoporous Carbon from Waste Tire E.L.K. Mui and G. M c Kay Department of Chemical Engineering Hong Kong University of Science and Technology Clear Water Bay, Kowloon, Hong Kong Corresponding
Amphiphilic diselenide-containing supramolecular polymers
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2014 Amphiphilic diselenide-containing supramolecular polymers Xinxin Tan, Liulin Yang, Zehuan
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2014 Amphiphilic diselenide-containing supramolecular polymers Xinxin Tan, Liulin Yang, Zehuan
SUPPORTING INFORMATION
 SUPPORTING INFORMATION Table of Contents S1 1. General materials and methods S2 2. Syntheses of {Pd 84 } and {Pd 17 } S3-S4 3. MS studies of {Pd 84 }, {Pd 17 } and the two-component reactions S5-S6 4.
SUPPORTING INFORMATION Table of Contents S1 1. General materials and methods S2 2. Syntheses of {Pd 84 } and {Pd 17 } S3-S4 3. MS studies of {Pd 84 }, {Pd 17 } and the two-component reactions S5-S6 4.
Experimental Section Chemicals. Tetraethyl orthosilicate (TEOS), ammonia aqueous solution (NH 4 OH, 28 wt.%), and dopamine hydrochloride (DA) were
 Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 Experimental Section Chemicals. Tetraethyl orthosilicate (TEOS), ammonia aqueous
Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 Experimental Section Chemicals. Tetraethyl orthosilicate (TEOS), ammonia aqueous
Self-Growth-Templating Synthesis of 3D N,P,Co-Doped. Mesoporous Carbon Frameworks for Efficient Bifunctional
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information Self-Growth-Templating Synthesis of
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information Self-Growth-Templating Synthesis of
Electronic Supporting Information
 Electronic Supporting Information Fluorescent Microporous Polyimides based on Perylene and Triazine for Highly CO 2 -Selective Carbon Materials Yaozu Liao, a Jens Weber b and Charl F.J. Faul a* a School
Electronic Supporting Information Fluorescent Microporous Polyimides based on Perylene and Triazine for Highly CO 2 -Selective Carbon Materials Yaozu Liao, a Jens Weber b and Charl F.J. Faul a* a School
KEY: FREE RESPONSE. Question Ammonium carbamate, NH 4CO 2NH 2, reversibly decomposes into CO 2 and NH 3 as shown by the balanced equation below.
 KEY: FREE RESPONSE Question 1 1. Ammonium carbamate, NH 4CO 2NH 2, reversibly decomposes into CO 2 and NH 3 as shown by the balanced equation below. NH 4CO 2NH 2(s) 2 NH 3(g) + CO 2(g) K p =? A student
KEY: FREE RESPONSE Question 1 1. Ammonium carbamate, NH 4CO 2NH 2, reversibly decomposes into CO 2 and NH 3 as shown by the balanced equation below. NH 4CO 2NH 2(s) 2 NH 3(g) + CO 2(g) K p =? A student
10.1 Acids and Bases in Aqueous Solution
 10.1 Acids and Bases in Aqueous Solution Arrhenius Definition of Acids and Bases An acid is a substance that gives hydrogen ions, H +, when dissolved in water. In fact, H + reacts with water and produces
10.1 Acids and Bases in Aqueous Solution Arrhenius Definition of Acids and Bases An acid is a substance that gives hydrogen ions, H +, when dissolved in water. In fact, H + reacts with water and produces
Yujuan Zhou, Kecheng Jie and Feihe Huang*
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 A redox-responsive selenium-containing pillar[5]arene-based macrocyclic amphiphile: synthesis,
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 A redox-responsive selenium-containing pillar[5]arene-based macrocyclic amphiphile: synthesis,
Miho Nitta a, Masaki Hirose a, Toru Abe a, Yukio Furukawa a, *, Hiroshi Sato b, Yasuro Yamanaka c
 Available online at www.sciencedirect.com Energy Procedia 37 (013 ) 869 876 GHGT-11 13 C-NMR Spectroscopic Study on Chemical Species in H O System before and after Heating Miho Nitta a, Masaki Hirose a,
Available online at www.sciencedirect.com Energy Procedia 37 (013 ) 869 876 GHGT-11 13 C-NMR Spectroscopic Study on Chemical Species in H O System before and after Heating Miho Nitta a, Masaki Hirose a,
Supporting Information
 A Calcium Coordination Framework Having Permanent Porosity and High CO 2 /N 2 Selectivity Debasis Banerjee, a, * Zhijuan Zhang, b Anna M. Plonka, c Jing Li, b, * and John B. Parise a, c, d, * (a) Department
A Calcium Coordination Framework Having Permanent Porosity and High CO 2 /N 2 Selectivity Debasis Banerjee, a, * Zhijuan Zhang, b Anna M. Plonka, c Jing Li, b, * and John B. Parise a, c, d, * (a) Department
SUPPLEMENTARY INFORMATION
 Supporting Online Material for Lead-Free Solid State Organic-Inorganic Halide Perovskite Solar Cells Feng Hao, 1 Constantinos C. Stoumpos, 1 Hanh Cao, 1 Robert P. H. Chang, 2 Mercouri G. Kanatzidis 1*
Supporting Online Material for Lead-Free Solid State Organic-Inorganic Halide Perovskite Solar Cells Feng Hao, 1 Constantinos C. Stoumpos, 1 Hanh Cao, 1 Robert P. H. Chang, 2 Mercouri G. Kanatzidis 1*
Bistriazole-p-benzoquinone and its alkali salts: electrochemical behaviour in aqueous alkaline solutions
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Bistriazole-p-benzoquinone and its alkali salts: electrochemical behaviour in aqueous
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Bistriazole-p-benzoquinone and its alkali salts: electrochemical behaviour in aqueous
Cu 2 graphene oxide composite for removal of contaminants from water and supercapacitor
 Electronic Supplementary Information (ESI) for Cu 2 O@reduced graphene oxide composite for removal of contaminants from water and supercapacitor Baojun Li, a Huaqiang Cao,* a Gui Yin, b Yuexiang Lu, a
Electronic Supplementary Information (ESI) for Cu 2 O@reduced graphene oxide composite for removal of contaminants from water and supercapacitor Baojun Li, a Huaqiang Cao,* a Gui Yin, b Yuexiang Lu, a
Supporting Information
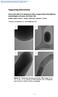 Supporting Information Interaction effects of mesoporous silica nanoparticles with different morphologies on human red blood cells Madhura Joglekar, Robert A. Roggers, Yannan Zhao, and Brian G. Trewyn
Supporting Information Interaction effects of mesoporous silica nanoparticles with different morphologies on human red blood cells Madhura Joglekar, Robert A. Roggers, Yannan Zhao, and Brian G. Trewyn
Fabrication of graphene quantum dot-decorated graphene sheets via. chemical surface modification
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supplementary Information for: Fabrication of graphene quantum dot-decorated graphene sheets via
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supplementary Information for: Fabrication of graphene quantum dot-decorated graphene sheets via
PRODUCING ACTIVED CARBONS FROM PINECONES VIA CHEMICAL ACTIVATION. Abstract. Introduction. Experimental
 PRODUCING ACTIVED CARBONS FROM PINECONES VIA CHEMICAL ACTIVATION Esin Apaydın Varol, Dept. of Chemical Engineering, Anadolu University, Eskisehir, Turkey Ersan Pütün, Dept. of Material Science and Engineering,
PRODUCING ACTIVED CARBONS FROM PINECONES VIA CHEMICAL ACTIVATION Esin Apaydın Varol, Dept. of Chemical Engineering, Anadolu University, Eskisehir, Turkey Ersan Pütün, Dept. of Material Science and Engineering,
Fabrication of a One-dimensional Tube-in-tube Polypyrrole/Tin oxide Structure for Highly Sensitive DMMP Sensor Applications
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information (ESI) for Fabrication of a One-dimensional
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information (ESI) for Fabrication of a One-dimensional
Local Deprotonation Enables Cation Exchange, Porosity. Modulation and Tunable Adsorption Selectivity in a. Metal-Organic Framework
 Supporting Information for Local Deprotonation Enables Cation Exchange, Porosity Modulation and Tunable Adsorption Selectivity in a Metal-Organic Framework Jun-Hao Wang,, Dong Luo, Mian Li, and Dan Li
Supporting Information for Local Deprotonation Enables Cation Exchange, Porosity Modulation and Tunable Adsorption Selectivity in a Metal-Organic Framework Jun-Hao Wang,, Dong Luo, Mian Li, and Dan Li
Supplementary Information for. Silver Nanoparticles Embedded Anti-microbial Paints Based on Vegetable Oil
 Supplementary Information for Silver Nanoparticles Embedded Anti-microbial Paints Based on Vegetable Oil Ashavani Kumar #, Praveen Kumar Vemula #, Pulickel M. Ajayan, George John * Department of Chemistry,
Supplementary Information for Silver Nanoparticles Embedded Anti-microbial Paints Based on Vegetable Oil Ashavani Kumar #, Praveen Kumar Vemula #, Pulickel M. Ajayan, George John * Department of Chemistry,
SEPARATION BY BARRIER
 SEPARATION BY BARRIER SEPARATION BY BARRIER Phase 1 Feed Barrier Phase 2 Separation by barrier uses a barrier which restricts and/or enhances the movement of certain chemical species with respect to other
SEPARATION BY BARRIER SEPARATION BY BARRIER Phase 1 Feed Barrier Phase 2 Separation by barrier uses a barrier which restricts and/or enhances the movement of certain chemical species with respect to other
Electrically pulsatile responsive drug delivery platform for treatment of Alzheimer s disease
 Electronic Supplementary Material Electrically pulsatile responsive drug delivery platform for treatment of Alzheimer s disease Li Wu 1,2, Jiasi Wang 1,2, Nan Gao 1, Jinsong Ren 1, Andong Zhao 1,2, and
Electronic Supplementary Material Electrically pulsatile responsive drug delivery platform for treatment of Alzheimer s disease Li Wu 1,2, Jiasi Wang 1,2, Nan Gao 1, Jinsong Ren 1, Andong Zhao 1,2, and
Understanding the function of cetyltrimethyl ammonium bromide in Lithium/Sulfur Cells
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Understanding the function of cetyltrimethyl ammonium
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Understanding the function of cetyltrimethyl ammonium
Influence of Amine Grafting on Carbon Dioxide Adsorption Behaviors of Activated Carbons
 Influence of Amine Grafting on Carbon Dioxide Adsorption Behaviors Bull. Korean Chem. Soc. 2011, Vol. 32, No. 9 3377 http://dx.doi.org/10.5012/bkcs.2011.32.9.3377 Influence of Amine Grafting on Carbon
Influence of Amine Grafting on Carbon Dioxide Adsorption Behaviors Bull. Korean Chem. Soc. 2011, Vol. 32, No. 9 3377 http://dx.doi.org/10.5012/bkcs.2011.32.9.3377 Influence of Amine Grafting on Carbon
22 and Applications of 13 C NMR
 Subject Chemistry Paper No and Title Module No and Title Module Tag 12 and rganic Spectroscopy 22 and Applications of 13 C NMR CHE_P12_M22 TABLE F CNTENTS 1. Learning utcomes 2. Introduction 3. Structural
Subject Chemistry Paper No and Title Module No and Title Module Tag 12 and rganic Spectroscopy 22 and Applications of 13 C NMR CHE_P12_M22 TABLE F CNTENTS 1. Learning utcomes 2. Introduction 3. Structural
Extracting organic molecules out of water using the metal-organic framework
 Extracting organic molecules out of water using the metal-organic framework Cr III (OH).{O 2 C-C 6 H 4 -CO 2 } Michael Maes a, Stijn Schouteden a, Luc Alaerts a, Diederik Depla b, Dirk E. De Vos a* a Centre
Extracting organic molecules out of water using the metal-organic framework Cr III (OH).{O 2 C-C 6 H 4 -CO 2 } Michael Maes a, Stijn Schouteden a, Luc Alaerts a, Diederik Depla b, Dirk E. De Vos a* a Centre
Supporting Information High Activity and Selectivity of Ag/SiO 2 Catalyst for Hydrogenation of Dimethyloxalate
 Supporting Information High Activity and Selectivity of Ag/SiO 2 Catalyst for Hydrogenation of Dimethyloxalate An-Yuan Yin, Xiao-Yang Guo, Wei-Lin Dai*, Kang-Nian Fan Shanghai Key Laboratory of Molecular
Supporting Information High Activity and Selectivity of Ag/SiO 2 Catalyst for Hydrogenation of Dimethyloxalate An-Yuan Yin, Xiao-Yang Guo, Wei-Lin Dai*, Kang-Nian Fan Shanghai Key Laboratory of Molecular
Design and Synthesis of Nitrogen-Doped Porous Carbon Materials for CO 2 Capture and Investigation of CO 2 Sorption Kinetics
 Design and Synthesis of Nitrogen-Doped Porous Carbon Materials for CO 2 Capture and Investigation of CO 2 Sorption Kinetics Investigators Jennifer Wilcox, Assistant Professor, Energy Resources Engineering;
Design and Synthesis of Nitrogen-Doped Porous Carbon Materials for CO 2 Capture and Investigation of CO 2 Sorption Kinetics Investigators Jennifer Wilcox, Assistant Professor, Energy Resources Engineering;
e 2m e c I, (7.1) = g e β B I(I +1), (7.2) = erg/gauss. (7.3)
 Chemistry 126 Molecular Spectra & Molecular Structure Week # 7 Electron Spin Resonance Spectroscopy, Supplement Like the hydrogen nucleus, an unpaired electron in a sample has a spin of I=1/2. The magnetic
Chemistry 126 Molecular Spectra & Molecular Structure Week # 7 Electron Spin Resonance Spectroscopy, Supplement Like the hydrogen nucleus, an unpaired electron in a sample has a spin of I=1/2. The magnetic
S BET vs. S DFT. Supporting Information
 Supporting Information Naturally Nitrogen and Calcium-doped Nanoporous Carbon Derived from Pine Cone with Superior CO 2 Capture Capacities Bingjun Zhu, Congxiao Shang and Zhengxiao Guo S BET vs. S DFT
Supporting Information Naturally Nitrogen and Calcium-doped Nanoporous Carbon Derived from Pine Cone with Superior CO 2 Capture Capacities Bingjun Zhu, Congxiao Shang and Zhengxiao Guo S BET vs. S DFT
AP Chem Chapter 14 Study Questions
 Class: Date: AP Chem Chapter 14 Study Questions 1. A burning splint will burn more vigorously in pure oxygen than in air because a. oxygen is a reactant in combustion and concentration of oxygen is higher
Class: Date: AP Chem Chapter 14 Study Questions 1. A burning splint will burn more vigorously in pure oxygen than in air because a. oxygen is a reactant in combustion and concentration of oxygen is higher
Urchin-like Ni-P microstructures: A facile synthesis, properties. and application in the fast removal of heavy-metal ions
 SUPPORTING INFORMATION Urchin-like Ni-P microstructures: A facile synthesis, properties and application in the fast removal of heavy-metal ions Yonghong Ni *a, Kai Mi a, Chao Cheng a, Jun Xia a, Xiang
SUPPORTING INFORMATION Urchin-like Ni-P microstructures: A facile synthesis, properties and application in the fast removal of heavy-metal ions Yonghong Ni *a, Kai Mi a, Chao Cheng a, Jun Xia a, Xiang
Supporting Information Water-soluble 1,2,4-Triazole with Diethylene Glycol Monoethyl Ether
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is The Royal Society of Chemistry 2014 Supporting Information Water-soluble 1,2,4-Triazole with Diethylene
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is The Royal Society of Chemistry 2014 Supporting Information Water-soluble 1,2,4-Triazole with Diethylene
Silver Loading Effect for the Activated Carbon Fibers Pre-treated with Acid
 Silver Loading Effect for the Acid-activated Carbon Fibers Bull. Korean Chem. Soc. 2004, Vol. 25, No. 8 1189 Silver Loading Effect for the Activated Carbon Fibers Pre-treated with Acid Won-Chun Oh * and
Silver Loading Effect for the Acid-activated Carbon Fibers Bull. Korean Chem. Soc. 2004, Vol. 25, No. 8 1189 Silver Loading Effect for the Activated Carbon Fibers Pre-treated with Acid Won-Chun Oh * and
Interfacial Chemistry and Adhesion Phenomena: How to Analyse and How to Optimise
 Interfacial Chemistry and Adhesion Phenomena: How to Analyse and How to Optimise John F Watts Department of Mechanical Engineering Sciences The Role of Surface Analysis in Adhesion Studies Assessing surface
Interfacial Chemistry and Adhesion Phenomena: How to Analyse and How to Optimise John F Watts Department of Mechanical Engineering Sciences The Role of Surface Analysis in Adhesion Studies Assessing surface
Electronic supplementary information
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Electronic supplementary information Heterogeneous nucleation and growth of highly crystalline
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Electronic supplementary information Heterogeneous nucleation and growth of highly crystalline
Supporting Information
 Supporting Information Cotton Fabric Functionalized with a β-cyclodextrin Polymer Captures Organic Pollutants from Contaminated Air and Water Diego M. Alzate-Sánchez, 1,3 Brian J. Smith, 2 Alaaeddin Alsbaiee,
Supporting Information Cotton Fabric Functionalized with a β-cyclodextrin Polymer Captures Organic Pollutants from Contaminated Air and Water Diego M. Alzate-Sánchez, 1,3 Brian J. Smith, 2 Alaaeddin Alsbaiee,
Carbon Dioxide Capture by Basic Dry Water
 Electronic Supplementary Material (ESI) for Energy. This journal is The Royal Society of Chemistry 2014 Carbon Dioxide Capture by Basic Dry Water Robert Dawson, a Lee Stevens, b Weixing Wang, c Benjamin
Electronic Supplementary Material (ESI) for Energy. This journal is The Royal Society of Chemistry 2014 Carbon Dioxide Capture by Basic Dry Water Robert Dawson, a Lee Stevens, b Weixing Wang, c Benjamin
Supporting Information for. A Fluorescence Ratiometric Sensor for Trace Vapor Detection of. Hydrogen Peroxide
 Supporting Information for A Fluorescence Ratiometric Sensor for Trace Vapor Detection of Hydrogen Peroxide Miao Xu 1,, Ji-Min Han 1,, Chen Wang 1, Xiaomei Yang 1, Jian Pei 2 and Ling Zang 1, * 1 Department
Supporting Information for A Fluorescence Ratiometric Sensor for Trace Vapor Detection of Hydrogen Peroxide Miao Xu 1,, Ji-Min Han 1,, Chen Wang 1, Xiaomei Yang 1, Jian Pei 2 and Ling Zang 1, * 1 Department
PAPER No.12 :Organic Spectroscopy MODULE No.29: Combined problem on UV, IR, 1 H NMR, 13 C NMR and Mass - Part I
 Subject Chemistry Paper No and Title Module No and Title Module Tag 12: rganic Spectroscopy 29: Combined problem on UV, IR, 1 H NMR, 13 C NMR and Mass - Part I CHE_P12_M29 TABLE F CNTENTS 1. Learning utcomes
Subject Chemistry Paper No and Title Module No and Title Module Tag 12: rganic Spectroscopy 29: Combined problem on UV, IR, 1 H NMR, 13 C NMR and Mass - Part I CHE_P12_M29 TABLE F CNTENTS 1. Learning utcomes
Adsorption of Methyl mercaptan on Surface Modified Activated Carbon
 Adsorption of Methyl mercaptan on Surface Modified Activated Carbon Hisashi. Tamai *, Hisato Nagoya, Takeshi. Shiono Department of Applied Chemistry Graduate School of Engineering, Hiroshima University
Adsorption of Methyl mercaptan on Surface Modified Activated Carbon Hisashi. Tamai *, Hisato Nagoya, Takeshi. Shiono Department of Applied Chemistry Graduate School of Engineering, Hiroshima University
SYNTHESIS AND CHARACTERIZATION OF CARBON NANOTUBES USING EGG ALBUMIN BY CHEMICAL METHOD
 International Journal of Metallurgical & Materials Science and Engineering (IJMMSE) ISSN 2278-2516 Vol. 3, Issue 2, Jun 2013, 13-20 TJPRC Pvt. Ltd. SYNTHESIS AND CHARACTERIZATION OF CARBON NANOTUBES USING
International Journal of Metallurgical & Materials Science and Engineering (IJMMSE) ISSN 2278-2516 Vol. 3, Issue 2, Jun 2013, 13-20 TJPRC Pvt. Ltd. SYNTHESIS AND CHARACTERIZATION OF CARBON NANOTUBES USING
