Experiment 6, page 1 Version of March 17, 2015
|
|
|
- Maximillian Farmer
- 6 years ago
- Views:
Transcription
1 Exprimnt 6, pag 1 Vrsion of March 17, 015 Exprimnt 6 VIBRATION-ROTATION SPECTROSCOPY OF DIATOMIC MOLECULES IMPORTANT: Th cll should b stord in th dssicator whn not in us to prvnt moistur in th atmosphr (Ys, this is Dlawar!!!) from injuring th cll windows. Thory Figur 6.1. Th ffctiv lctronmdiatd intrnuclar potntial as a function of intrnuclar sparation. For a diatomic molcul, th stat of th nucli may b considrd to b th rsult of an ffctiv lctron-mdiatd potntial nrgy that dpnds on nuclar sparation, E(R). [Figur 6.1.] R is th intrnuclar sparation and R th quilibrium sparation. D is th quilibrium lctronic nrgy rlativ to th vacuum, or th wll dpth. In quantum mchanics, on solvs Schrodingr s quation for th nuclar motion to giv th ignstats and thir nrgis as a function of th quantum numbrs: E = D + Evib + Erot (6.1) Th nrgy consists of an lctronic contribution, D, a vibrational contribution, E vib, and a rotational contribution, E rot, ach with its own quantum numbrs. In th harmonic approximation, th vibrational nrgy dpnds on a quantum numbr, n, which can b any non-ngativ intgr: 1 Evib, n = n + hcω, (6.) in which c is th spd of light in fr spac, and ω is th fundamntal vibrational frquncy dtrmind by th forc constant, K, and th rducd mass, µ. 1 1 Various units ar usd to xprss nrgy. If h has units of nrgy tim, ω has units of lngth -1. In this rgion of th spctrum, nrgis ar oftn quotd in cm -1, th official IUPAC/IUPAP nam for which is th kaysr. (It is namd for Hinrich Gustav Johanns Kaysr, a Grman physicist who mad studis of th spctroscopy of chmical lmnts in th lat 19 th and arly 0 th cnturis.) Th nrgis in this rgion ar thrfor givn by E ( cm 1) = E / vib hc. In this xprimnt, w writ all nrgis in units of cm -1. Convrsion to othr units is possibl by multiplication by th appropriat factor.
2 Exprimnt 6, pag Vrsion of March 17, 015 ω = 1 K πc µ. (6.3) Th nrgy in quation (6.) is corrct only if th molcul is subjct to a harmonic K potntial [i.., V = ( x x q ) ]. On may rlas this assumption through inclusion of anharmonic trms to giv a mor complt dscription of th vibrational nrgy. Th rsulting xprssion for th vibrational nrgy is a sum of contributions: En = n + ω n + xω + n + yω +... (6.4) Th additional trms contain nrgy paramtrs x ω, y ω,... to account for th anharmonicity of th potntial function. Usually only th first corrction trm is of significanc, th sum bing truncatd at th harmonic and first anharmonic trms. Typical data for svral diatomic molculs ar givn in Tabl 6.1. Tabl 6.1. Ground-stat Spctroscopic Constants of Slctd Diatomic Molculs a Molcul ω /cm -1 x ω /cm -1 B /cm -1 α /cm -1 1 H C 16 O H 79 Br H 19 F H 19 F a Noggl, J. H. Physical Chmistry, 3 rd Edition; Harpr-Collins: Nw York, Vibrational Spctroscopy. Whn a sampl is in contact with a radiation fild, it may tak up or mit nrgy by having molculs chang stat. Th nrgy xchang only occurs at rsonanc, i.. if th nrgy spacing in th molcul is qual to th quantum of nrgy of th fild, ω. E final Einitial = ω (6.5) For th momnt, considr only changs in vibrational nrgy of a diatomic molcul such as HCl or CO. For most molculs, hcω >>> k b T nar room tmpratur. 3 Thrfor, most molculs in a sampl ar in th lowst-nrgy (or ground) vibrational stat (n = 0). Undr ths conditions, on only considrs transitions from this stat to xplain absorption spctra, i.. E initial = E vib,0. Whthr a transition occurs dpnds not only on nrgy matching at rsonanc, but also on quantum mchanical slction ruls. Thr ar two important slction ruls. For infrard spctroscopy, th molcul must hav a dipol momnt and transitions ar allowd btwn all stats: 4 n = ± 1, ±, ±, (6.6) Sinc th ground stat is so havily populatd undr typical conditions, th dominant fatur of vibrational spctroscopy is th transition from n = 0 to n = 1, calld th fundamntal. To within th first anharmonic corrction, th nrgy of th photon ncssary to xcit this transition is: Latr w add th possibility of rotational-nrgy transitions. 3 k b is Boltzmann s constant, J K For a pur harmonic oscillator, only th n = ± 1 transition is allowd.
3 Exprimnt 6, pag 3 Vrsion of March 17, 015 Evib = ω xω. (6.7) For transitions in which th nrgy changs by mor than 1 quantum, on may driv formulas for th xpctd wavnumbr of th transition, as wll. For xampl, th n = 0 n = transition is known as th first ovrton and occurs at an nrgy: Eovrton = ω 6xω (6.8) to th sam lvl of approximation. Singl-quantum ( n = ± 1) transitions from stats with n > 0 ar allowd, but th smallr populations of ths stats at thrmal quilibrium at room tmpratur mak such transitions lss intns and mor difficult to obsrv at room tmpratur. For xampl, th transition from th stat with n = 1 to th stat with n = occurs at Ehot = ω 4xω (6.9) This is clos to th fundamntal. Such transitions ar calld hot bands bcaus thir intnsitis incras (rlativ to th fundamntal) as on incrass th tmpratur. Rotational Transitions. To analyz infrard spctra proprly, on must includ th ffcts of rotational-nrgy changs, as wll as vibrational-nrgy changs. Ths may b tratd, to a first approximation, as nrgtically indpndnt of th vibrational stat. In th rigid-rotor approximation, th rotational nrgy of a diatomic molcul dpnds on th rotational quantum numbr, J. 1 E rot, Jm = m R J ( J + 1), (6.10) whr th nrgy, in this quation, is in rgs or jouls. <.> indicats an avrag ovr th vibrational wav function. As a first approximation, on trats th molcul as if it xists only at th quilibrium bond distanc, R, and 1 1 = R R. Th quilibrium momnt of inrtia I (= µr ) and th quilibrium rotational constant, B, ar dfind in trms of this quantity. B = 4πIc (6.11) and Erot, Jm = B J ( J + 1), (6.1) whr th nrgy and B ar xprssd in cm Th quilibrium rotational constant dpnds on th momnt of inrtia, which in turn dpnds on th rducd mass and th quilibrium bond lngth. Th rducd mass dpnds on th mass of ach isotop. In Tabl 6. ar th xact masss of svral atoms (xprssd on a molar basis). Tabl 6.. Exact Masss of Svral Atoms Atom Mass (g/mol) Atom Mass (g/mol) 1 H O H (D) O C 1 (xactly) 35 Cl C Cl In ths quations, th units of B and E rot ar cm -1, providd that is in rg s, c is in cm s -1, and I is in g cm.
4 Exprimnt 6, pag 4 Vrsion of March 17, 015 For a ral diatomic molcul, th avrag ovr th vibrational stat dos not xactly giv th rotational constant B. Instad, it is found that th rotational constant dpnds on vibrational stat. Th rotational constant including th corrction for vibrational avraging is givn th symbol B n, whr th xplicit dpndnc on th vibrational stat is indicatd by th subscript. In this way, th rotational nrgy of a diatomic molcul in a stat with quantum numbrs, n, J and m is givn by: EnJm = Bn J ( J +1) (6.13) Prturbation thory givs th vibration-dpndnt rotation constant, in a first approximation, as: 1 Bn = B α n +. (6.14) α is th vibration-rotation constant. It dscribs how th vibrational stat affcts th rotational nrgy. In analyzing spctra, it is tratd as a paramtr to b dtrmind, just lik B. In addition to this corrction, thr is anothr purly rotational prturbation that changs th rotational nrgy of a stat - th cntrifugal distortion. This ffct is takn into account by an additional trm in th nrgy dtrmind by th cntrifugal distortion cofficint, D c. Vibration-Rotation Enrgy. With all ths dfinitions, th total vibration-plus-rotation nrgy of a diatomic molcul in a stat with quantum numbrs n, J and m J is (again in units of cm -1 ) 1 1 En, J, m = D + n + ω n + xω + BnJ ( J + 1) DcJ ( J + 1). (6.15) J Bcaus th nrgy sparations of th rotational stats (ach having a diffrnt valu of J) may not b much gratr than k b T, a sampl may contain apprciabl numbrs of molculs in xcitd rotational stats, vn at room tmpratur. Whn rotational nrgis ar considrd, transitions from stats with various valus of J ar sn in a spctrum. Vibration-Rotation Spctroscopy. Infrard spctroscopy concrns changs of vibrational and rotational stat, without chang of lctronic stat. Hnc, th infrard spctrum is a vibrationrotation spctrum. Th usual slction ruls ar: n = ± 1 (Transitions of highr ordr ar known as ovrton bands and occur at highr frquncis; w shall not considr thm furthr hr, xcpt in th Discussion Qustions.) J = ± 1 (for th htronuclar diatomic molculs xamind hr) Th molcul must hav a prmannt lctric dipol momnt. Considring only transitions that involv th ground vibrational stat, th nrgy chang is: α E = ω xω + B [ J '( J ' + 1) J ( J + 1) ] [ 3J '( J ' + 1) J ( J + 1) ] (6.16) c[ J ' ( J ' + 1) J ( J + 1) ] with th quantum numbr of th initial rotational stat bing J and that of th final rotational stat bing J. Whn J = J + 1, thr is a nt absorption of rotational nrgy; whn J = J 1, thr is a nt mission of rotational nrgy (although ovrall thr is absorption of nrgy). Transitions of th formr kind form th R branch and thos of th lattr form th P branch of
5 Exprimnt 6, pag 5 Vrsion of March 17, 015 th spctrum. 6 Using quation (6.16), th approximat nrgis of transition dpnd on th quantum numbr J for th initial stat. Nglcting th cntrifugal distortion trms, this givs: E = ω (1 x ) + ( B a )( J + 1) a ( J + 1) R branch E = ω (1 x ) ( B + a ) J a J P branch Th infrard spctrum consists of a sris of transitions du to th diffrnt rotational stats involvs. (6.17) Intnsitis in th Infrard Spctrum. On apparnt quality of such a spctrum is th variation in intnsity of ths transition lins. Th intnsity distribution givs th rlativ probabilitis of occupation of th initial rotational stats (thos with quantum numbr J). From ths intnsitis, on can dfin a rotational tmpratur, T r, for th distribution, assuming it to b Boltzmann in form. Th intnsity of a lin that ariss from th stat with quantum numbr J (compard to that for th lin that coms from a stat with J = 0) is givn by th following quation that accounts for th Boltzmann factor and th dgnracy of th rotational lvl on m. I J N J g J = = xp[ ( EJ E0) / ktr ] = (J + 1) xp[ BnhcJ ( J + 1) / ktr ] (6.18) I 0 N 0 go whr g J is th dgnracy of th rotational lvl with quantum numbr, J, and k is Boltzmann s constant. A comparison of intnsitis allows on to stimat T r ; or, knowing T r, on may stimat B n. In som xprimnts in which systms ar prturbd by lasr xcitation, on can chang th distribution of molculs in th rotational stats (at last for a sufficintly long tim to mak a masurmnt) such that th rotational tmpratur is diffrnt from th laboratory tmpratur. Undr th conditions of this xprimnt, th rotational dgrs of frdom ar in quilibrium with th translational dgrs of frdom and th rotational tmpratur is th sam as th laboratory tmpratur. Quantum Calculations. Th quantum thory discussd abov assums that th intrnuclar potntial nrgy function is known or can b approximatd. In actuality, this function is an intgral ovr th instantanous lctronic wav function of th molcul. Empirical paramtrs that dscrib th function lik th forc constants ar givn in a complt thory by intgrals ovr th lctronic stat of th molcul. To calculat ths, on must hav knowldg of th lctronic stat of th systm. With prsnt-day computrs, on may do numrical stimations of th lctronic wav functions rathr asily and with quit good prcision. 7 Onc known, th wav functions can b numrically intgratd to giv stimats of paramtrs such as th forc constant, K, or th fundamntal frquncy, ω. Many complx oprations involvd in such calculations hav bn collctd into cannd programs such as GAUSSIAN09 8 or SPARTAN, so that th chmist may us th computr without daling with problms of computr programming or numrical analysis. 6 In spctra of diatomic molculs, th transition for which J = 0 is not allowd. This transition is calld th Q branch of th spctrum. For mor complx molculs, this transition may b allowd, and a Q branch may b part of th spctrum, showing as an intns transition btwn th R and P branchs. 7 Only a fw yars ago, such calculations wr only don by a rathr small numbr of xprts on rathr larg computrs. Today thy may b don with th aid of a prsonal computr at on s dsk. 8 John Popl was awardd th 1998 Nobl Priz in Chmistry principally for th dvlopmnt of GAUSSIAN.
6 Exprimnt 6, pag 6 Vrsion of March 17, 015 Howvr, thr is a grat dal of chmical knowldg on must still bring to bar on th procss to obtain rliabl rsults. Th principal problm on must cop with in carrying out ab initio quantum calculations is that any procdur uss som approximation to th molcular lctronic wav function(s). Th quality of th approximation dtrmins how good calculatd proprtis ar. A commonly usd mthod is linar combination of atomic orbitals (LCAO), in which on xprsss th molcular lctronic wav function (or molcular orbital [MO]) in trms of atomic orbitals of th constitunt atoms. Sinc th forms of atomic orbitals ar not wll known xcpt for hydrogn, vn th choic of functional forms of atomic orbitals is an approximation whos quality affcts th rsults. Th st of functions usd is calld th basis. A commonly usd basis is th Slatrtyp orbitals (STO), but othr bass ar somtims usd, for xampl Gaussian-typ orbitals (GTO). Ths basis sts hav nams that dnot crtain faturs of th st of orbitals, such as 3-1G or 6-31G or G(d,p). In principl, an infinitly larg basis st allows on to solv th lctronic stat xactly. Howvr, that would tak a grat amount of tim, so that calculations ar always don with a truncatd basis; again, th quality of th rsults dpnds on how wll th truncatd st approximats th ral wav function. Onc chosn, th basis is usd to dtrmin th bst lctronic wav function by som critrion, such as minimization of nrgy. A common mthod is th Hartr-Fock slfconsistnt-fild (HF-SCF) mthod, which mphasizs th avrag ffcts of intrlctronic intractions, rathr than instantanous intractions. This itrativ mthod finds paramtrs of th xpansion of th molcular orbital that minimiz th variational nrgy intgral. Anothr common tchniqu is known as dnsity functional thory (DFT). This mthod combins a rasonably low computational cost (i.., th calculations ar quick) with an accuracy that is sufficint to obtain rliabl chmical rsults. Th ssnc of this tchniqu lis in th ability to rduc th 3N spatial coordinats that dscrib th intracting lctrons of a systm into a thrdimnsional function dscribing th lctron dnsity. Convnintly, computr programs lik GAUSSIAN do all of th tdious work, onc on dtrmins th dsird basis for th situation appropriatly, rturning usabl information on th stat in th form of paramtrs. Procdur CAUTION: HCl and DCl ar corrosiv gass. ALWAYS handl th matrials xtrmly carfully. Obtaining th Infrard Spctra. Th instrumnt for masuring th spctra is a Nicolt Magna IR 550 FTIR spctromtr. If you hav not alrady larnd how to us this instrumnt, your laboratory instructor can hlp you with th oprating paramtrs for th instrumnt. If you hav takn instrumntal analysis, this instrumnt should b familiar to you. It has a liquid-nitrogncoold cadmium tllurid dtctor. Bfor bginning any spctroscopy, obtain liquid nitrogn and fill th rsrvoir until liquid nitrogn ovrflows th fill hol. An important part of gtting rliabl data is positioning th sampl. Th cll holdr must b positiond accuratly to allow th full IR intnsity to b dtctd. Mak sur th bam passs through th cntr of th cll. Tak grat car in insrting th sampl into th sampl compartmnt. Do not forc th sampl to go in, as that may brak th sampl. Wait at last two minuts to allow th systm to b purgd of air aftr closing th compartmnt lid.
7 Exprimnt 6, pag 7 Vrsion of March 17, 015 Whn obtaining th spctrum of th mixtur of HCl and DCl, rcord all prtinnt data so you hav thm whn doing th analysis. 1. You should bgin by obtaining a background spctrum of th instrumnt without a sampl prsnt. Follow th procdur for collcting a background spctrum which is ultimatly subtractd from th spctrum of ach sampl.. For th sampls of gass, w hav a woodn cradl into which th cll fits. Install th cradl carfully. If it dos not fit proprly, do not forc it. Hav th laboratory instructor hlp you with this stp if you ncountr any problms. 3. Bfor taking th spctrum of a gas sampl, tak a background spctrum of th mpty compartmnt, using th sam paramtrs as you intnd to us in th xprimntal masurmnt. This is don with th command Collct Sampl, whr it prompts you to tak a background spctrum, which it automatically subtracts from your nxt spctrum. (If you hav problms, ask th laboratory instructor for hlp.) 4. Carfully insrt th gas cll into th cradl. Again, if it dos not fit asily into th spac, s th laboratory instructor bfor procding. 5. A window appars prompting you to tak th xprimntal spctrum. Install th sampl, and wait for th purg for a fw minuts. Click on th button to tak th spctrum. HCl is an impurity in a sampl of DCl that can b idntifid bcaus of its lowr signals in th spctrum. 6. Look at th spctrum to b crtain you s both th bands of th HCl and DCl. Thr may b othr bands from a bit of watr vapor in th compartmnt, and thr ar som bands from th poxy that holds th windows on som clls. Th important point is that th spctrum contains bands from HCl and DCl that you can asily discrn. 7. Whn you hav rcordd th spctrum, ach studnt should sav th data on hr/his flash driv so that th data may b latr analyzd. Us th Sav As command, bing sur th fil typ is CSV. A fil of this typ can b importd into a program lik EXCEL on your computr for crating a graph and prcisly rading th pak positions. 8. Obtain a spctrum of th CO sampl in th sam mannr. 9. Sign th logbook. Calculational Chmistry. Th calculations ar don on MACs in th laboratory. Thr ar two oprations: (1) stting up th calculation with GaussViw, and () running a Gaussian calculation with Gaussian09. Subsquntly, on can viw th rsults with GaussViw. Start th MAC, using th password providd. 1. Click on th GaussViw icon. This action should display th main mnu of GaussViw, as shown on th nxt pag.. To build th HCl molcul. Click on th atom icon of th main display; this action gts you to th priodic tabl. Thn click on th Cl on this display. Clos th Slct Elmnt display. You should s an HCl molcul in th Currnt Fragmnt display. 3. Click on th Nw fil window and this should put a copy of th HCl molcul into your nw fil. 4. Click on th bond lngth icon on th main mnu. This action causs th Currnt Fragmnt display to bcom blu. Go to th HCl window. 5. Click first on ithr th H or Cl atom and thn on th othr. A window should opn with a slidr to st th lngth of th bond. St it at som distanc (in Angstrøm units) such as 1.35.
8 Exprimnt 6, pag 8 Vrsion of March 17, 015 Atom Icon Bond Lngth Inquir Currnt Fragmnt Nw fil Window 6. Go to th Calculat mnu and slct Gaussian. This action brings up th Calculat mnu in a window with various tabs. Mak sur that th Calculation Typ is Enrgy undr th Job Typ tab. In th Mthod tab, slct ground-stat DFT with unrstrictd spin. Slct th functional B3LYP and us th basis st G(3df,3dp). 7. Submit th job. Th program asks you to sav an input fil. Us a nam lik HCl135. Mak crtain it is savd as a Gaussian job fil (.gjf) in th pchmusr foldr. Th calculation should start at this point. 8. Whn th calculation is finishd (in a fw sconds), th computr opns a window tlling you th job is finishd. You may click Ys to clos th Gaussian window. From GaussViw a box pops up asking if you want to opn th fil, click Ys to opn th fil. You must indicat that th output fil nds with th xtnsion.log to gt output. You can rad th fil from GaussViw using th Rsults mnu. You may click on Summary, which causs a box with prtinnt rsults to b shown. You may viw th ntir fil as wll, if you wish. 9. If you ar looking at th full fil, th output bgins with a lot of prliminary stuff. Sarch th fil to find th lin that bgins with SCF Don. Th quantity calld E(UB3LYP) is th calculatd nrgy of th molcul, in atomic units, a. u., or hartrs. This rportd nrgy is th total nrgy, that is, th nrgy rquird to
9 Exprimnt 6, pag 9 Vrsion of March 17, 015 sparat all of th lctrons and nucli to infinity. Rcord this numbr along with th intrnuclar sparation in your notbook. 10. Rpat this calculation for a sris of diffrnt bond lngths. Each bond lngth that you dfin yilds a diffrnt quantity for E(UB3LYP). It is asist to call back th first fil you mad, click on th two atoms aftr clicking on th bond lngth icon and stting th nw lngth. Sav th fil with a uniqu nam that hlps to idntify which fil corrsponds to which bond lngth upon starting th Gaussian calculation. Us a st of bond lngths that run from about 1. Å to 1.5 Å. B sur to gt sufficint points that you can dfin th potntial nrgy function wll nough to dtrmin th point at which it is a minimum. (It hlps to plot ths as you ar calculating, to s what is going on.) 11. Onc you hav found th lowst nrgy, crat a fil having th bond lngth giving th lowst nrgy abov. St up a nw calculation, choosing Opt+Frq undr th Job Typ tab. Undr th Mthod tab, choos to run a ground-stat DFT calculation using rstrictd spin. Slct th functional B3LYP and th basis st G(3df,3dp). This action dos an optimization and a frquncy calculation. Whn this calculation is finishd, b sur to rcord th optimizd bond lngth (click on th Inquir button, thn on th two atoms) and th dipol momnt from th Summary. This calculation rports th vibrational frquncy, ω, of HCl in wavnumbrs and th rotational constant, B. B sur to rcord ths numbrs from th rsults, as thy must b rportd. Th vibrational frquncy can b found in th Rsults mnu undr Vibrations. To find th rotational constant you hav to viw th whol log fil. Undr th Rsults mnu, click Viw Fil. This opns th log fil and you must scroll down towards th nd and look for Thrmochmistry. Locat th zro-point nrgy and rcord it in your notbook as wll. 1. To calculat th hmolytic dissociation nrgy of HCl, calculat th ground-stat nrgis of isolatd chlorin and hydrogn atoms. Ths ar two sparat calculations. Th sum of ths two nrgis rprsnts th nrgy whn th atoms ar at an infinit distanc from ach othr. Whn you plot th potntial wll as a function of th distanc sparating th hydrogn and chlorin atoms, this valu should b tratd as a rfrnc (i.., this valu should b plottd as zro and all points calculatd at a finit distanc hav som ngativ valu rlativ to this valu). To prform ths calculations, plac a singl atom on your window and calculat th nrgy using dnsity functional thory using unrstrictd spin. Slct th functional B3LYP and th basis st G(3df,3dp). Rcord th nrgis of ths atoms in your notbook whn th calculations finish.
10 Exprimnt 6, pag 10 Vrsion of March 17, 015 Calculations Data Analysis of th Spctroscopic Rsults 1. Analysis of HCl Spctra a. Mak sparat tabls for 1 H 35 Cl and 1 H 37 Cl of th positions of th lins (in cm -1 ) and th corrsponding valus of J. 9 [On must sarch through th EXCEL fil for th paks in ach spctrum. It is asist to rcord ths as spradshts in EXCEL. You hav to dcid which paks to associat with ach molcul and which valu of J.] b. For ach typ of molcul, on on graph, plot th lin positions of transitions in th R branch vrsus J + 1 and th lin positions of transitions in th P branch vrsus J. [Us a dummy indx, m, for J + 1 or J to mak ths plots.] c. By multipl-rgrssion analysis of th plots for both spcis, dtrmin ω -x ω, B, and α for ach matrial. (You should do th analysis for both th R and P branchs and assum D c is zro.) 10. Mak a tabl of lin positions for ach DCl spcis and analyz ths data by th sam mthod as in stp Analyz th data for CO by th sam mthod as in stp Summariz all drivd rsults on all gass in a singl tabl. Thortical Chmistry 5. From th rsults of th Gaussian09 calculations, mak a tabl of bond lngth and nrgy. Rfrnc your nrgy scal such that th sum of th nrgis of th isolatd hydrogn and chlorin atoms is zro in whatvr unit you us. Convnint units for this purpos ar kj/mol. 6. Mak a plot of th calculatd nrgy vrsus bond lngth. 7. Compar th optimal bond lngth dtrmind in th optimization calculation with th valu, R, from your analysis of th plot mad in stp 6, and with th litratur valu. 8. Rport th dipol momnt at th optimal bond lngth prdictd by this calculation. Compar ths xprimntal and thortical paramtrs with litratur valus. [B sur to quot your litratur sourc.] 9. Compar th calculatd (with GAUSSIAN09) valus of ω for HCl to your xprimntal valus of ω x ω and with th litratur valu. Assuming your xprimntal valu is corrct and that th litratur valu of ω is corrct, find x ω. Discussion Qustions 1. Driv quations (6.7) and (6.8).. Why can on distinguish th spctroscopic transitions of 1 H 35 Cl and 1 H 37 Cl (or H 35 Cl and H 37 Cl)? Suppos that th rsolution was 5 cm -1. Could on distinguish ths diffrnt spcis undr thos xprimntal conditions? 9 J is th rotational quantum numbr of th initial rotational stat in ach cas. B carful. 10 B sur to includ stimats of uncrtainty in your valus for ths paramtrs.
11 Exprimnt 6, pag 11 Vrsion of March 17, Calculat th momnts of inrtia of ach of th four possibl spcis in th HCl/DCl sampl from th rotational constants, B, you dtrmind. From ach momnt of inrtia, calculat R for ach of th four isotopomrs. Within xprimntal rror, ar ths bond distancs diffrnt from ach othr? Explain your answr. 4. Th xpctd position of th Q branch of HCl is diffrnt from that of DCl. What ar your xprimntal valus? Assuming that th anharmonic trm is zro, calculat th ratio of th rducd masss of H 35 Cl and D 35 Cl from you data for th xpctd positions of th Q branch. How dos this agr with litratur data for this quantity? 5. Explain why th IR cll is constructd th way it is. In particular, why is it not mad compltly out of glass?
22/ Breakdown of the Born-Oppenheimer approximation. Selection rules for rotational-vibrational transitions. P, R branches.
 Subjct Chmistry Papr No and Titl Modul No and Titl Modul Tag 8/ Physical Spctroscopy / Brakdown of th Born-Oppnhimr approximation. Slction ruls for rotational-vibrational transitions. P, R branchs. CHE_P8_M
Subjct Chmistry Papr No and Titl Modul No and Titl Modul Tag 8/ Physical Spctroscopy / Brakdown of th Born-Oppnhimr approximation. Slction ruls for rotational-vibrational transitions. P, R branchs. CHE_P8_M
Lecture 28 Title: Diatomic Molecule : Vibrational and Rotational spectra
 Lctur 8 Titl: Diatomic Molcul : Vibrational and otational spctra Pag- In this lctur w will undrstand th molcular vibrational and rotational spctra of diatomic molcul W will start with th Hamiltonian for
Lctur 8 Titl: Diatomic Molcul : Vibrational and otational spctra Pag- In this lctur w will undrstand th molcular vibrational and rotational spctra of diatomic molcul W will start with th Hamiltonian for
5.80 Small-Molecule Spectroscopy and Dynamics
 MIT OpnCoursWar http://ocw.mit.du 5.80 Small-Molcul Spctroscopy and Dynamics Fall 008 For information about citing ths matrials or our Trms of Us, visit: http://ocw.mit.du/trms. Lctur # 3 Supplmnt Contnts
MIT OpnCoursWar http://ocw.mit.du 5.80 Small-Molcul Spctroscopy and Dynamics Fall 008 For information about citing ths matrials or our Trms of Us, visit: http://ocw.mit.du/trms. Lctur # 3 Supplmnt Contnts
Title: Vibrational structure of electronic transition
 Titl: Vibrational structur of lctronic transition Pag- Th band spctrum sn in th Ultra-Violt (UV) and visibl (VIS) rgions of th lctromagntic spctrum can not intrprtd as vibrational and rotational spctrum
Titl: Vibrational structur of lctronic transition Pag- Th band spctrum sn in th Ultra-Violt (UV) and visibl (VIS) rgions of th lctromagntic spctrum can not intrprtd as vibrational and rotational spctrum
Atomic and Laser Spectroscopy
 L-E B, OL, MOV 83 Atomic and Lasr Spctroscopy Th aim of this xrcis is to giv an ovrviw of th fild of lasr spctroscopy and to show modrn spctroscopic mthods usd in atomic, molcular and chmical physics.
L-E B, OL, MOV 83 Atomic and Lasr Spctroscopy Th aim of this xrcis is to giv an ovrviw of th fild of lasr spctroscopy and to show modrn spctroscopic mthods usd in atomic, molcular and chmical physics.
Hydrogen Atom and One Electron Ions
 Hydrogn Atom and On Elctron Ions Th Schrödingr quation for this two-body problm starts out th sam as th gnral two-body Schrödingr quation. First w sparat out th motion of th cntr of mass. Th intrnal potntial
Hydrogn Atom and On Elctron Ions Th Schrödingr quation for this two-body problm starts out th sam as th gnral two-body Schrödingr quation. First w sparat out th motion of th cntr of mass. Th intrnal potntial
Determination of Vibrational and Electronic Parameters From an Electronic Spectrum of I 2 and a Birge-Sponer Plot
 5 J. Phys. Chm G Dtrmination of Vibrational and Elctronic Paramtrs From an Elctronic Spctrum of I 2 and a Birg-Sponr Plot 1 15 2 25 3 35 4 45 Dpartmnt of Chmistry, Gustavus Adolphus Collg. 8 Wst Collg
5 J. Phys. Chm G Dtrmination of Vibrational and Elctronic Paramtrs From an Elctronic Spctrum of I 2 and a Birg-Sponr Plot 1 15 2 25 3 35 4 45 Dpartmnt of Chmistry, Gustavus Adolphus Collg. 8 Wst Collg
Ch. 24 Molecular Reaction Dynamics 1. Collision Theory
 Ch. 4 Molcular Raction Dynamics 1. Collision Thory Lctur 16. Diffusion-Controlld Raction 3. Th Matrial Balanc Equation 4. Transition Stat Thory: Th Eyring Equation 5. Transition Stat Thory: Thrmodynamic
Ch. 4 Molcular Raction Dynamics 1. Collision Thory Lctur 16. Diffusion-Controlld Raction 3. Th Matrial Balanc Equation 4. Transition Stat Thory: Th Eyring Equation 5. Transition Stat Thory: Thrmodynamic
The van der Waals interaction 1 D. E. Soper 2 University of Oregon 20 April 2012
 Th van dr Waals intraction D. E. Sopr 2 Univrsity of Orgon 20 pril 202 Th van dr Waals intraction is discussd in Chaptr 5 of J. J. Sakurai, Modrn Quantum Mchanics. Hr I tak a look at it in a littl mor
Th van dr Waals intraction D. E. Sopr 2 Univrsity of Orgon 20 pril 202 Th van dr Waals intraction is discussd in Chaptr 5 of J. J. Sakurai, Modrn Quantum Mchanics. Hr I tak a look at it in a littl mor
Constants and Conversions:
 EXAM INFORMATION Radial Distribution Function: P 2 ( r) RDF( r) Br R( r ) 2, B is th normalization constant. Ordr of Orbital Enrgis: Homonuclar Diatomic Molculs * * * * g1s u1s g 2s u 2s u 2 p g 2 p g
EXAM INFORMATION Radial Distribution Function: P 2 ( r) RDF( r) Br R( r ) 2, B is th normalization constant. Ordr of Orbital Enrgis: Homonuclar Diatomic Molculs * * * * g1s u1s g 2s u 2s u 2 p g 2 p g
Brief Introduction to Statistical Mechanics
 Brif Introduction to Statistical Mchanics. Purpos: Ths nots ar intndd to provid a vry quick introduction to Statistical Mchanics. Th fild is of cours far mor vast than could b containd in ths fw pags.
Brif Introduction to Statistical Mchanics. Purpos: Ths nots ar intndd to provid a vry quick introduction to Statistical Mchanics. Th fild is of cours far mor vast than could b containd in ths fw pags.
Elements of Statistical Thermodynamics
 24 Elmnts of Statistical Thrmodynamics Statistical thrmodynamics is a branch of knowldg that has its own postulats and tchniqus. W do not attmpt to giv hr vn an introduction to th fild. In this chaptr,
24 Elmnts of Statistical Thrmodynamics Statistical thrmodynamics is a branch of knowldg that has its own postulats and tchniqus. W do not attmpt to giv hr vn an introduction to th fild. In this chaptr,
A Propagating Wave Packet Group Velocity Dispersion
 Lctur 8 Phys 375 A Propagating Wav Packt Group Vlocity Disprsion Ovrviw and Motivation: In th last lctur w lookd at a localizd solution t) to th 1D fr-particl Schrödingr quation (SE) that corrsponds to
Lctur 8 Phys 375 A Propagating Wav Packt Group Vlocity Disprsion Ovrviw and Motivation: In th last lctur w lookd at a localizd solution t) to th 1D fr-particl Schrödingr quation (SE) that corrsponds to
Why is a E&M nature of light not sufficient to explain experiments?
 1 Th wird world of photons Why is a E&M natur of light not sufficint to xplain xprimnts? Do photons xist? Som quantum proprtis of photons 2 Black body radiation Stfan s law: Enrgy/ ara/ tim = Win s displacmnt
1 Th wird world of photons Why is a E&M natur of light not sufficint to xplain xprimnts? Do photons xist? Som quantum proprtis of photons 2 Black body radiation Stfan s law: Enrgy/ ara/ tim = Win s displacmnt
Principles of Humidity Dalton s law
 Principls of Humidity Dalton s law Air is a mixtur of diffrnt gass. Th main gas componnts ar: Gas componnt volum [%] wight [%] Nitrogn N 2 78,03 75,47 Oxygn O 2 20,99 23,20 Argon Ar 0,93 1,28 Carbon dioxid
Principls of Humidity Dalton s law Air is a mixtur of diffrnt gass. Th main gas componnts ar: Gas componnt volum [%] wight [%] Nitrogn N 2 78,03 75,47 Oxygn O 2 20,99 23,20 Argon Ar 0,93 1,28 Carbon dioxid
Higher order derivatives
 Robrto s Nots on Diffrntial Calculus Chaptr 4: Basic diffrntiation ruls Sction 7 Highr ordr drivativs What you nd to know alrady: Basic diffrntiation ruls. What you can larn hr: How to rpat th procss of
Robrto s Nots on Diffrntial Calculus Chaptr 4: Basic diffrntiation ruls Sction 7 Highr ordr drivativs What you nd to know alrady: Basic diffrntiation ruls. What you can larn hr: How to rpat th procss of
The pn junction: 2 Current vs Voltage (IV) characteristics
 Th pn junction: Currnt vs Voltag (V) charactristics Considr a pn junction in quilibrium with no applid xtrnal voltag: o th V E F E F V p-typ Dpltion rgion n-typ Elctron movmnt across th junction: 1. n
Th pn junction: Currnt vs Voltag (V) charactristics Considr a pn junction in quilibrium with no applid xtrnal voltag: o th V E F E F V p-typ Dpltion rgion n-typ Elctron movmnt across th junction: 1. n
Statistical Thermodynamics: Sublimation of Solid Iodine
 c:374-7-ivap-statmch.docx mar7 Statistical Thrmodynamics: Sublimation of Solid Iodin Chm 374 For March 3, 7 Prof. Patrik Callis Purpos:. To rviw basic fundamntals idas of Statistical Mchanics as applid
c:374-7-ivap-statmch.docx mar7 Statistical Thrmodynamics: Sublimation of Solid Iodin Chm 374 For March 3, 7 Prof. Patrik Callis Purpos:. To rviw basic fundamntals idas of Statistical Mchanics as applid
Coupled Pendulums. Two normal modes.
 Tim Dpndnt Two Stat Problm Coupld Pndulums Wak spring Two normal mods. No friction. No air rsistanc. Prfct Spring Start Swinging Som tim latr - swings with full amplitud. stationary M +n L M +m Elctron
Tim Dpndnt Two Stat Problm Coupld Pndulums Wak spring Two normal mods. No friction. No air rsistanc. Prfct Spring Start Swinging Som tim latr - swings with full amplitud. stationary M +n L M +m Elctron
Numerical Problem Set for Atomic and Molecular Spectroscopy. Yr 2 HT SRM
 Numrical Problm St for Atomic and Molcular Spctroscopy Yr HT SRM Sction 1: Atomic Spctra 1. For ach of th atomic trm symbols 1 S, P, 3 P, 3 D, 4 D, writ down: a) Th associatd valus of th total spin and
Numrical Problm St for Atomic and Molcular Spctroscopy Yr HT SRM Sction 1: Atomic Spctra 1. For ach of th atomic trm symbols 1 S, P, 3 P, 3 D, 4 D, writ down: a) Th associatd valus of th total spin and
Background: We have discussed the PIB, HO, and the energy of the RR model. In this chapter, the H-atom, and atomic orbitals.
 Chaptr 7 Th Hydrogn Atom Background: W hav discussd th PIB HO and th nrgy of th RR modl. In this chaptr th H-atom and atomic orbitals. * A singl particl moving undr a cntral forc adoptd from Scott Kirby
Chaptr 7 Th Hydrogn Atom Background: W hav discussd th PIB HO and th nrgy of th RR modl. In this chaptr th H-atom and atomic orbitals. * A singl particl moving undr a cntral forc adoptd from Scott Kirby
Classical Magnetic Dipole
 Lctur 18 1 Classical Magntic Dipol In gnral, a particl of mass m and charg q (not ncssarily a point charg), w hav q g L m whr g is calld th gyromagntic ratio, which accounts for th ffcts of non-point charg
Lctur 18 1 Classical Magntic Dipol In gnral, a particl of mass m and charg q (not ncssarily a point charg), w hav q g L m whr g is calld th gyromagntic ratio, which accounts for th ffcts of non-point charg
A central nucleus. Protons have a positive charge Electrons have a negative charge
 Atomic Structur Lss than ninty yars ago scintists blivd that atoms wr tiny solid sphrs lik minut snookr balls. Sinc thn it has bn discovrd that atoms ar not compltly solid but hav innr and outr parts.
Atomic Structur Lss than ninty yars ago scintists blivd that atoms wr tiny solid sphrs lik minut snookr balls. Sinc thn it has bn discovrd that atoms ar not compltly solid but hav innr and outr parts.
2. Laser physics - basics
 . Lasr physics - basics Spontanous and stimulatd procsss Einstin A and B cofficints Rat quation analysis Gain saturation What is a lasr? LASER: Light Amplification by Stimulatd Emission of Radiation "light"
. Lasr physics - basics Spontanous and stimulatd procsss Einstin A and B cofficints Rat quation analysis Gain saturation What is a lasr? LASER: Light Amplification by Stimulatd Emission of Radiation "light"
E hf. hf c. 2 2 h 2 2 m v f ' f 2f ' f cos c
 EXPERIMENT 9: COMPTON EFFECT Rlatd Topics Intractions of photons with lctrons, consrvation of momntum and nrgy, inlastic and lastic scattring, intraction cross sction, Compton wavlngth. Principl Whn photons
EXPERIMENT 9: COMPTON EFFECT Rlatd Topics Intractions of photons with lctrons, consrvation of momntum and nrgy, inlastic and lastic scattring, intraction cross sction, Compton wavlngth. Principl Whn photons
Give the letter that represents an atom (6) (b) Atoms of A and D combine to form a compound containing covalent bonds.
 1 Th diagram shows th lctronic configurations of six diffrnt atoms. A B C D E F (a) You may us th Priodic Tabl on pag 2 to hlp you answr this qustion. Answr ach part by writing on of th lttrs A, B, C,
1 Th diagram shows th lctronic configurations of six diffrnt atoms. A B C D E F (a) You may us th Priodic Tabl on pag 2 to hlp you answr this qustion. Answr ach part by writing on of th lttrs A, B, C,
Collisions between electrons and ions
 DRAFT 1 Collisions btwn lctrons and ions Flix I. Parra Rudolf Pirls Cntr for Thortical Physics, Unirsity of Oxford, Oxford OX1 NP, UK This rsion is of 8 May 217 1. Introduction Th Fokkr-Planck collision
DRAFT 1 Collisions btwn lctrons and ions Flix I. Parra Rudolf Pirls Cntr for Thortical Physics, Unirsity of Oxford, Oxford OX1 NP, UK This rsion is of 8 May 217 1. Introduction Th Fokkr-Planck collision
Addition of angular momentum
 Addition of angular momntum April, 0 Oftn w nd to combin diffrnt sourcs of angular momntum to charactriz th total angular momntum of a systm, or to divid th total angular momntum into parts to valuat th
Addition of angular momntum April, 0 Oftn w nd to combin diffrnt sourcs of angular momntum to charactriz th total angular momntum of a systm, or to divid th total angular momntum into parts to valuat th
Quasi-Classical States of the Simple Harmonic Oscillator
 Quasi-Classical Stats of th Simpl Harmonic Oscillator (Draft Vrsion) Introduction: Why Look for Eignstats of th Annihilation Oprator? Excpt for th ground stat, th corrspondnc btwn th quantum nrgy ignstats
Quasi-Classical Stats of th Simpl Harmonic Oscillator (Draft Vrsion) Introduction: Why Look for Eignstats of th Annihilation Oprator? Excpt for th ground stat, th corrspondnc btwn th quantum nrgy ignstats
Chemical Physics II. More Stat. Thermo Kinetics Protein Folding...
 Chmical Physics II Mor Stat. Thrmo Kintics Protin Folding... http://www.nmc.ctc.com/imags/projct/proj15thumb.jpg http://nuclarwaponarchiv.org/usa/tsts/ukgrabl2.jpg http://www.photolib.noaa.gov/corps/imags/big/corp1417.jpg
Chmical Physics II Mor Stat. Thrmo Kintics Protin Folding... http://www.nmc.ctc.com/imags/projct/proj15thumb.jpg http://nuclarwaponarchiv.org/usa/tsts/ukgrabl2.jpg http://www.photolib.noaa.gov/corps/imags/big/corp1417.jpg
Brief Notes on the Fermi-Dirac and Bose-Einstein Distributions, Bose-Einstein Condensates and Degenerate Fermi Gases Last Update: 28 th December 2008
 Brif ots on th Frmi-Dirac and Bos-Einstin Distributions, Bos-Einstin Condnsats and Dgnrat Frmi Gass Last Updat: 8 th Dcmbr 8 (A)Basics of Statistical Thrmodynamics Th Gibbs Factor A systm is assumd to
Brif ots on th Frmi-Dirac and Bos-Einstin Distributions, Bos-Einstin Condnsats and Dgnrat Frmi Gass Last Updat: 8 th Dcmbr 8 (A)Basics of Statistical Thrmodynamics Th Gibbs Factor A systm is assumd to
u x v x dx u x v x v x u x dx d u x v x u x v x dx u x v x dx Integration by Parts Formula
 7. Intgration by Parts Each drivativ formula givs ris to a corrsponding intgral formula, as w v sn many tims. Th drivativ product rul yilds a vry usful intgration tchniqu calld intgration by parts. Starting
7. Intgration by Parts Each drivativ formula givs ris to a corrsponding intgral formula, as w v sn many tims. Th drivativ product rul yilds a vry usful intgration tchniqu calld intgration by parts. Starting
orbiting electron turns out to be wrong even though it Unfortunately, the classical visualization of the
 Lctur 22-1 Byond Bohr Modl Unfortunatly, th classical visualization of th orbiting lctron turns out to b wrong vn though it still givs us a simpl way to think of th atom. Quantum Mchanics is ndd to truly
Lctur 22-1 Byond Bohr Modl Unfortunatly, th classical visualization of th orbiting lctron turns out to b wrong vn though it still givs us a simpl way to think of th atom. Quantum Mchanics is ndd to truly
Chapter 8: Electron Configurations and Periodicity
 Elctron Spin & th Pauli Exclusion Principl Chaptr 8: Elctron Configurations and Priodicity 3 quantum numbrs (n, l, ml) dfin th nrgy, siz, shap, and spatial orintation of ach atomic orbital. To xplain how
Elctron Spin & th Pauli Exclusion Principl Chaptr 8: Elctron Configurations and Priodicity 3 quantum numbrs (n, l, ml) dfin th nrgy, siz, shap, and spatial orintation of ach atomic orbital. To xplain how
Molecular Orbitals in Inorganic Chemistry
 Outlin olcular Orbitals in Inorganic Chmistry Dr. P. Hunt p.hunt@imprial.ac.uk Rm 167 (Chmistry) http://www.ch.ic.ac.uk/hunt/ octahdral complxs forming th O diagram for Oh colour, slction ruls Δoct, spctrochmical
Outlin olcular Orbitals in Inorganic Chmistry Dr. P. Hunt p.hunt@imprial.ac.uk Rm 167 (Chmistry) http://www.ch.ic.ac.uk/hunt/ octahdral complxs forming th O diagram for Oh colour, slction ruls Δoct, spctrochmical
EXST Regression Techniques Page 1
 EXST704 - Rgrssion Tchniqus Pag 1 Masurmnt rrors in X W hav assumd that all variation is in Y. Masurmnt rror in this variabl will not ffct th rsults, as long as thy ar uncorrlatd and unbiasd, sinc thy
EXST704 - Rgrssion Tchniqus Pag 1 Masurmnt rrors in X W hav assumd that all variation is in Y. Masurmnt rror in this variabl will not ffct th rsults, as long as thy ar uncorrlatd and unbiasd, sinc thy
Exam 1. It is important that you clearly show your work and mark the final answer clearly, closed book, closed notes, no calculator.
 Exam N a m : _ S O L U T I O N P U I D : I n s t r u c t i o n s : It is important that you clarly show your work and mark th final answr clarly, closd book, closd nots, no calculator. T i m : h o u r
Exam N a m : _ S O L U T I O N P U I D : I n s t r u c t i o n s : It is important that you clarly show your work and mark th final answr clarly, closd book, closd nots, no calculator. T i m : h o u r
Addition of angular momentum
 Addition of angular momntum April, 07 Oftn w nd to combin diffrnt sourcs of angular momntum to charactriz th total angular momntum of a systm, or to divid th total angular momntum into parts to valuat
Addition of angular momntum April, 07 Oftn w nd to combin diffrnt sourcs of angular momntum to charactriz th total angular momntum of a systm, or to divid th total angular momntum into parts to valuat
The failure of the classical mechanics
 h failur of th classical mchanics W rviw som xprimntal vidncs showing that svral concpts of classical mchanics cannot b applid. - h blac-body radiation. - Atomic and molcular spctra. - h particl-li charactr
h failur of th classical mchanics W rviw som xprimntal vidncs showing that svral concpts of classical mchanics cannot b applid. - h blac-body radiation. - Atomic and molcular spctra. - h particl-li charactr
Search sequence databases 3 10/25/2016
 Sarch squnc databass 3 10/25/2016 Etrm valu distribution Ø Suppos X is a random variabl with probability dnsity function p(, w sampl a larg numbr S of indpndnt valus of X from this distribution for an
Sarch squnc databass 3 10/25/2016 Etrm valu distribution Ø Suppos X is a random variabl with probability dnsity function p(, w sampl a larg numbr S of indpndnt valus of X from this distribution for an
On the Hamiltonian of a Multi-Electron Atom
 On th Hamiltonian of a Multi-Elctron Atom Austn Gronr Drxl Univrsity Philadlphia, PA Octobr 29, 2010 1 Introduction In this papr, w will xhibit th procss of achiving th Hamiltonian for an lctron gas. Making
On th Hamiltonian of a Multi-Elctron Atom Austn Gronr Drxl Univrsity Philadlphia, PA Octobr 29, 2010 1 Introduction In this papr, w will xhibit th procss of achiving th Hamiltonian for an lctron gas. Making
Fourier Transforms and the Wave Equation. Key Mathematics: More Fourier transform theory, especially as applied to solving the wave equation.
 Lur 7 Fourir Transforms and th Wav Euation Ovrviw and Motivation: W first discuss a fw faturs of th Fourir transform (FT), and thn w solv th initial-valu problm for th wav uation using th Fourir transform
Lur 7 Fourir Transforms and th Wav Euation Ovrviw and Motivation: W first discuss a fw faturs of th Fourir transform (FT), and thn w solv th initial-valu problm for th wav uation using th Fourir transform
Schrodinger Equation in 3-d
 Schrodingr Equation in 3-d ψ( xyz,, ) ψ( xyz,, ) ψ( xyz,, ) + + + Vxyz (,, ) ψ( xyz,, ) = Eψ( xyz,, ) m x y z p p p x y + + z m m m + V = E p m + V = E E + k V = E Infinit Wll in 3-d V = x > L, y > L,
Schrodingr Equation in 3-d ψ( xyz,, ) ψ( xyz,, ) ψ( xyz,, ) + + + Vxyz (,, ) ψ( xyz,, ) = Eψ( xyz,, ) m x y z p p p x y + + z m m m + V = E p m + V = E E + k V = E Infinit Wll in 3-d V = x > L, y > L,
Contemporary, atomic, nuclear, and particle physics
 Contmporary, atomic, nuclar, and particl physics 1 Blackbody radiation as a thrmal quilibrium condition (in vacuum this is th only hat loss) Exampl-1 black plan surfac at a constant high tmpratur T h is
Contmporary, atomic, nuclar, and particl physics 1 Blackbody radiation as a thrmal quilibrium condition (in vacuum this is th only hat loss) Exampl-1 black plan surfac at a constant high tmpratur T h is
Exam 2 Thursday (7:30-9pm) It will cover material through HW 7, but no material that was on the 1 st exam.
 Exam 2 Thursday (7:30-9pm) It will covr matrial through HW 7, but no matrial that was on th 1 st xam. What happns if w bash atoms with lctrons? In atomic discharg lamps, lots of lctrons ar givn kintic
Exam 2 Thursday (7:30-9pm) It will covr matrial through HW 7, but no matrial that was on th 1 st xam. What happns if w bash atoms with lctrons? In atomic discharg lamps, lots of lctrons ar givn kintic
Chapter 7b Electron Spin and Spin- Orbit Coupling
 Wintr 3 Chm 356: Introductory Quantum Mchanics Chaptr 7b Elctron Spin and Spin- Orbit Coupling... 96 H- atom in a Magntic Fild: Elctron Spin... 96 Total Angular Momntum... 3 Chaptr 7b Elctron Spin and
Wintr 3 Chm 356: Introductory Quantum Mchanics Chaptr 7b Elctron Spin and Spin- Orbit Coupling... 96 H- atom in a Magntic Fild: Elctron Spin... 96 Total Angular Momntum... 3 Chaptr 7b Elctron Spin and
MA 262, Spring 2018, Final exam Version 01 (Green)
 MA 262, Spring 218, Final xam Vrsion 1 (Grn) INSTRUCTIONS 1. Switch off your phon upon ntring th xam room. 2. Do not opn th xam booklt until you ar instructd to do so. 3. Bfor you opn th booklt, fill in
MA 262, Spring 218, Final xam Vrsion 1 (Grn) INSTRUCTIONS 1. Switch off your phon upon ntring th xam room. 2. Do not opn th xam booklt until you ar instructd to do so. 3. Bfor you opn th booklt, fill in
MCB137: Physical Biology of the Cell Spring 2017 Homework 6: Ligand binding and the MWC model of allostery (Due 3/23/17)
 MCB37: Physical Biology of th Cll Spring 207 Homwork 6: Ligand binding and th MWC modl of allostry (Du 3/23/7) Hrnan G. Garcia March 2, 207 Simpl rprssion In class, w drivd a mathmatical modl of how simpl
MCB37: Physical Biology of th Cll Spring 207 Homwork 6: Ligand binding and th MWC modl of allostry (Du 3/23/7) Hrnan G. Garcia March 2, 207 Simpl rprssion In class, w drivd a mathmatical modl of how simpl
PHYS-333: Problem set #2 Solutions
 PHYS-333: Problm st #2 Solutions Vrsion of March 5, 2016. 1. Visual binary 15 points): Ovr a priod of 10 yars, two stars sparatd by an angl of 1 arcsc ar obsrvd to mov through a full circl about a point
PHYS-333: Problm st #2 Solutions Vrsion of March 5, 2016. 1. Visual binary 15 points): Ovr a priod of 10 yars, two stars sparatd by an angl of 1 arcsc ar obsrvd to mov through a full circl about a point
COMPUTER GENERATED HOLOGRAMS Optical Sciences 627 W.J. Dallas (Monday, April 04, 2005, 8:35 AM) PART I: CHAPTER TWO COMB MATH.
 C:\Dallas\0_Courss\03A_OpSci_67\0 Cgh_Book\0_athmaticalPrliminaris\0_0 Combath.doc of 8 COPUTER GENERATED HOLOGRAS Optical Scincs 67 W.J. Dallas (onday, April 04, 005, 8:35 A) PART I: CHAPTER TWO COB ATH
C:\Dallas\0_Courss\03A_OpSci_67\0 Cgh_Book\0_athmaticalPrliminaris\0_0 Combath.doc of 8 COPUTER GENERATED HOLOGRAS Optical Scincs 67 W.J. Dallas (onday, April 04, 005, 8:35 A) PART I: CHAPTER TWO COB ATH
Physical Chemistry Spring 2018 TR 5:00-6:15 pm, 207 BrL Quiz #1
 Physical Chmistry 444-0 Spring 08 TR 5:00-6:5 pm, 07 BrL Quiz # Nam KEY Problm (3 points). Ammonia gas is vry hygroscopic (asily racts with watr), so it is packagd for shipping in a small dry gas cylindr
Physical Chmistry 444-0 Spring 08 TR 5:00-6:5 pm, 07 BrL Quiz # Nam KEY Problm (3 points). Ammonia gas is vry hygroscopic (asily racts with watr), so it is packagd for shipping in a small dry gas cylindr
4. Money cannot be neutral in the short-run the neutrality of money is exclusively a medium run phenomenon.
 PART I TRUE/FALSE/UNCERTAIN (5 points ach) 1. Lik xpansionary montary policy, xpansionary fiscal policy rturns output in th mdium run to its natural lvl, and incrass prics. Thrfor, fiscal policy is also
PART I TRUE/FALSE/UNCERTAIN (5 points ach) 1. Lik xpansionary montary policy, xpansionary fiscal policy rturns output in th mdium run to its natural lvl, and incrass prics. Thrfor, fiscal policy is also
Optics and Non-Linear Optics I Non-linear Optics Tutorial Sheet November 2007
 Optics and Non-Linar Optics I - 007 Non-linar Optics Tutorial Sht Novmbr 007 1. An altrnativ xponntial notion somtims usd in NLO is to writ Acos (") # 1 ( Ai" + A * $i" ). By using this notation and substituting
Optics and Non-Linar Optics I - 007 Non-linar Optics Tutorial Sht Novmbr 007 1. An altrnativ xponntial notion somtims usd in NLO is to writ Acos (") # 1 ( Ai" + A * $i" ). By using this notation and substituting
Lecture 37 (Schrödinger Equation) Physics Spring 2018 Douglas Fields
 Lctur 37 (Schrödingr Equation) Physics 6-01 Spring 018 Douglas Filds Rducd Mass OK, so th Bohr modl of th atom givs nrgy lvls: E n 1 k m n 4 But, this has on problm it was dvlopd assuming th acclration
Lctur 37 (Schrödingr Equation) Physics 6-01 Spring 018 Douglas Filds Rducd Mass OK, so th Bohr modl of th atom givs nrgy lvls: E n 1 k m n 4 But, this has on problm it was dvlopd assuming th acclration
Math 34A. Final Review
 Math A Final Rviw 1) Us th graph of y10 to find approimat valus: a) 50 0. b) y (0.65) solution for part a) first writ an quation: 50 0. now tak th logarithm of both sids: log() log(50 0. ) pand th right
Math A Final Rviw 1) Us th graph of y10 to find approimat valus: a) 50 0. b) y (0.65) solution for part a) first writ an quation: 50 0. now tak th logarithm of both sids: log() log(50 0. ) pand th right
Alpha and beta decay equation practice
 Alpha and bta dcay quation practic Introduction Alpha and bta particls may b rprsntd in quations in svral diffrnt ways. Diffrnt xam boards hav thir own prfrnc. For xampl: Alpha Bta α β alpha bta Dspit
Alpha and bta dcay quation practic Introduction Alpha and bta particls may b rprsntd in quations in svral diffrnt ways. Diffrnt xam boards hav thir own prfrnc. For xampl: Alpha Bta α β alpha bta Dspit
Atomic energy levels. Announcements:
 Atomic nrgy lvls Announcmnts: Exam solutions ar postd. Problm solving sssions ar M3-5 and Tusday 1-3 in G-140. Will nd arly and hand back your Midtrm Exam at nd of class. http://www.colorado.du/physics/phys2170/
Atomic nrgy lvls Announcmnts: Exam solutions ar postd. Problm solving sssions ar M3-5 and Tusday 1-3 in G-140. Will nd arly and hand back your Midtrm Exam at nd of class. http://www.colorado.du/physics/phys2170/
The Matrix Exponential
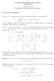 Th Matrix Exponntial (with xrciss) by D. Klain Vrsion 207.0.05 Corrctions and commnts ar wlcom. Th Matrix Exponntial For ach n n complx matrix A, dfin th xponntial of A to b th matrix A A k I + A + k!
Th Matrix Exponntial (with xrciss) by D. Klain Vrsion 207.0.05 Corrctions and commnts ar wlcom. Th Matrix Exponntial For ach n n complx matrix A, dfin th xponntial of A to b th matrix A A k I + A + k!
Forces. Quantum ElectroDynamics. α = = We have now:
 W hav now: Forcs Considrd th gnral proprtis of forcs mdiatd by xchang (Yukawa potntial); Examind consrvation laws which ar obyd by (som) forcs. W will nxt look at thr forcs in mor dtail: Elctromagntic
W hav now: Forcs Considrd th gnral proprtis of forcs mdiatd by xchang (Yukawa potntial); Examind consrvation laws which ar obyd by (som) forcs. W will nxt look at thr forcs in mor dtail: Elctromagntic
Lecture 2: Rotational and Vibrational Spectra
 Lctur : Rotational and Vibrational Spctra 1. Light-mattr intraction. Rigid-rotor modl for diatomic molcul 3. Non-rigid rotation 4. Vibration-rotation for diatomics H O 1. Light-mattr intraction Possibilitis
Lctur : Rotational and Vibrational Spctra 1. Light-mattr intraction. Rigid-rotor modl for diatomic molcul 3. Non-rigid rotation 4. Vibration-rotation for diatomics H O 1. Light-mattr intraction Possibilitis
The Matrix Exponential
 Th Matrix Exponntial (with xrciss) by Dan Klain Vrsion 28928 Corrctions and commnts ar wlcom Th Matrix Exponntial For ach n n complx matrix A, dfin th xponntial of A to b th matrix () A A k I + A + k!
Th Matrix Exponntial (with xrciss) by Dan Klain Vrsion 28928 Corrctions and commnts ar wlcom Th Matrix Exponntial For ach n n complx matrix A, dfin th xponntial of A to b th matrix () A A k I + A + k!
The Compton Effect. c 2 E 1. m e 1 E 1 = 2. c (2) + m e. E e. c 2 E (3)
 PHY 19 Compton Effct 1 Th Compton Effct Introduction In this xprimnt w will study two aspcts of th intraction of photons with lctrons. Th first of ths is th Compton ffct namd aftr Arthur Holly Compton
PHY 19 Compton Effct 1 Th Compton Effct Introduction In this xprimnt w will study two aspcts of th intraction of photons with lctrons. Th first of ths is th Compton ffct namd aftr Arthur Holly Compton
That is, we start with a general matrix: And end with a simpler matrix:
 DIAGON ALIZATION OF THE STR ESS TEN SOR INTRO DUCTIO N By th us of Cauchy s thorm w ar abl to rduc th numbr of strss componnts in th strss tnsor to only nin valus. An additional simplification of th strss
DIAGON ALIZATION OF THE STR ESS TEN SOR INTRO DUCTIO N By th us of Cauchy s thorm w ar abl to rduc th numbr of strss componnts in th strss tnsor to only nin valus. An additional simplification of th strss
ELECTRON-MUON SCATTERING
 ELECTRON-MUON SCATTERING ABSTRACT Th lctron charg is considrd to b distributd or xtndd in spac. Th diffrntial of th lctron charg is st qual to a function of lctron charg coordinats multiplid by a four-dimnsional
ELECTRON-MUON SCATTERING ABSTRACT Th lctron charg is considrd to b distributd or xtndd in spac. Th diffrntial of th lctron charg is st qual to a function of lctron charg coordinats multiplid by a four-dimnsional
University of Illinois at Chicago Department of Physics. Thermodynamics & Statistical Mechanics Qualifying Examination
 Univrsity of Illinois at Chicago Dpartmnt of hysics hrmodynamics & tatistical Mchanics Qualifying Eamination January 9, 009 9.00 am 1:00 pm Full crdit can b achivd from compltly corrct answrs to 4 qustions.
Univrsity of Illinois at Chicago Dpartmnt of hysics hrmodynamics & tatistical Mchanics Qualifying Eamination January 9, 009 9.00 am 1:00 pm Full crdit can b achivd from compltly corrct answrs to 4 qustions.
1.2 Faraday s law A changing magnetic field induces an electric field. Their relation is given by:
 Elctromagntic Induction. Lorntz forc on moving charg Point charg moving at vlocity v, F qv B () For a sction of lctric currnt I in a thin wir dl is Idl, th forc is df Idl B () Elctromotiv forc f s any
Elctromagntic Induction. Lorntz forc on moving charg Point charg moving at vlocity v, F qv B () For a sction of lctric currnt I in a thin wir dl is Idl, th forc is df Idl B () Elctromotiv forc f s any
A=P=E M-A=N Alpha particle Beta Particle. Periodic table
 Nam Pr. Atomic Structur/Nuclar Chmistry (Ch. 3 & 21) OTHS Acadmic Chmistry Objctivs: Undrstand th xprimntal dsign and conclusions usd in th dvlopmnt of modrn atomic thory, including Dalton's Postulats,
Nam Pr. Atomic Structur/Nuclar Chmistry (Ch. 3 & 21) OTHS Acadmic Chmistry Objctivs: Undrstand th xprimntal dsign and conclusions usd in th dvlopmnt of modrn atomic thory, including Dalton's Postulats,
ph People Grade Level: basic Duration: minutes Setting: classroom or field site
 ph Popl Adaptd from: Whr Ar th Frogs? in Projct WET: Curriculum & Activity Guid. Bozman: Th Watrcours and th Council for Environmntal Education, 1995. ph Grad Lvl: basic Duration: 10 15 minuts Stting:
ph Popl Adaptd from: Whr Ar th Frogs? in Projct WET: Curriculum & Activity Guid. Bozman: Th Watrcours and th Council for Environmntal Education, 1995. ph Grad Lvl: basic Duration: 10 15 minuts Stting:
General Notes About 2007 AP Physics Scoring Guidelines
 AP PHYSICS C: ELECTRICITY AND MAGNETISM 2007 SCORING GUIDELINES Gnral Nots About 2007 AP Physics Scoring Guidlins 1. Th solutions contain th most common mthod of solving th fr-rspons qustions and th allocation
AP PHYSICS C: ELECTRICITY AND MAGNETISM 2007 SCORING GUIDELINES Gnral Nots About 2007 AP Physics Scoring Guidlins 1. Th solutions contain th most common mthod of solving th fr-rspons qustions and th allocation
Introduction to Condensed Matter Physics
 Introduction to Condnsd Mattr Physics pcific hat M.P. Vaughan Ovrviw Ovrviw of spcific hat Hat capacity Dulong-Ptit Law Einstin modl Dby modl h Hat Capacity Hat capacity h hat capacity of a systm hld at
Introduction to Condnsd Mattr Physics pcific hat M.P. Vaughan Ovrviw Ovrviw of spcific hat Hat capacity Dulong-Ptit Law Einstin modl Dby modl h Hat Capacity Hat capacity h hat capacity of a systm hld at
Radiation Physics Laboratory - Complementary Exercise Set MeBiom 2016/2017
 Th following qustions ar to b answrd individually. Usful information such as tabls with dtctor charactristics and graphs with th proprtis of matrials ar availabl in th cours wb sit: http://www.lip.pt/~patricia/fisicadaradiacao.
Th following qustions ar to b answrd individually. Usful information such as tabls with dtctor charactristics and graphs with th proprtis of matrials ar availabl in th cours wb sit: http://www.lip.pt/~patricia/fisicadaradiacao.
SECTION where P (cos θ, sin θ) and Q(cos θ, sin θ) are polynomials in cos θ and sin θ, provided Q is never equal to zero.
 SETION 6. 57 6. Evaluation of Dfinit Intgrals Exampl 6.6 W hav usd dfinit intgrals to valuat contour intgrals. It may com as a surpris to larn that contour intgrals and rsidus can b usd to valuat crtain
SETION 6. 57 6. Evaluation of Dfinit Intgrals Exampl 6.6 W hav usd dfinit intgrals to valuat contour intgrals. It may com as a surpris to larn that contour intgrals and rsidus can b usd to valuat crtain
EEO 401 Digital Signal Processing Prof. Mark Fowler
 EEO 401 Digital Signal Procssing Prof. Mark Fowlr ot St #18 Introduction to DFT (via th DTFT) Rading Assignmnt: Sct. 7.1 of Proakis & Manolakis 1/24 Discrt Fourir Transform (DFT) W v sn that th DTFT is
EEO 401 Digital Signal Procssing Prof. Mark Fowlr ot St #18 Introduction to DFT (via th DTFT) Rading Assignmnt: Sct. 7.1 of Proakis & Manolakis 1/24 Discrt Fourir Transform (DFT) W v sn that th DTFT is
Derivation of Electron-Electron Interaction Terms in the Multi-Electron Hamiltonian
 Drivation of Elctron-Elctron Intraction Trms in th Multi-Elctron Hamiltonian Erica Smith Octobr 1, 010 1 Introduction Th Hamiltonian for a multi-lctron atom with n lctrons is drivd by Itoh (1965) by accounting
Drivation of Elctron-Elctron Intraction Trms in th Multi-Elctron Hamiltonian Erica Smith Octobr 1, 010 1 Introduction Th Hamiltonian for a multi-lctron atom with n lctrons is drivd by Itoh (1965) by accounting
The following information relates to Questions 1 to 4:
 Th following information rlats to Qustions 1 to 4: QUESTIN 1 Th lctrolyt usd in this ful cll is D watr carbonat ions hydrogn ions hydroxid ions QUESTIN 2 Th product formd in th ful cll is D hydrogn gas
Th following information rlats to Qustions 1 to 4: QUESTIN 1 Th lctrolyt usd in this ful cll is D watr carbonat ions hydrogn ions hydroxid ions QUESTIN 2 Th product formd in th ful cll is D hydrogn gas
High Energy Physics. Lecture 5 The Passage of Particles through Matter
 High Enrgy Physics Lctur 5 Th Passag of Particls through Mattr 1 Introduction In prvious lcturs w hav sn xampls of tracks lft by chargd particls in passing through mattr. Such tracks provid som of th most
High Enrgy Physics Lctur 5 Th Passag of Particls through Mattr 1 Introduction In prvious lcturs w hav sn xampls of tracks lft by chargd particls in passing through mattr. Such tracks provid som of th most
REGISTER!!! The Farmer and the Seeds (a parable of scientific reasoning) Class Updates. The Farmer and the Seeds. The Farmer and the Seeds
 How dos light intract with mattr? And what dos (this say about) mattr? REGISTER!!! If Schrödingr s Cat walks into a forst, and no on is around to obsrv it, is h rally in th forst? sourc unknown Phys 1010
How dos light intract with mattr? And what dos (this say about) mattr? REGISTER!!! If Schrödingr s Cat walks into a forst, and no on is around to obsrv it, is h rally in th forst? sourc unknown Phys 1010
Einstein Equations for Tetrad Fields
 Apiron, Vol 13, No, Octobr 006 6 Einstin Equations for Ttrad Filds Ali Rıza ŞAHİN, R T L Istanbul (Turky) Evry mtric tnsor can b xprssd by th innr product of ttrad filds W prov that Einstin quations for
Apiron, Vol 13, No, Octobr 006 6 Einstin Equations for Ttrad Filds Ali Rıza ŞAHİN, R T L Istanbul (Turky) Evry mtric tnsor can b xprssd by th innr product of ttrad filds W prov that Einstin quations for
Dealing with quantitative data and problem solving life is a story problem! Attacking Quantitative Problems
 Daling with quantitati data and problm soling lif is a story problm! A larg portion of scinc inols quantitati data that has both alu and units. Units can sa your butt! Nd handl on mtric prfixs Dimnsional
Daling with quantitati data and problm soling lif is a story problm! A larg portion of scinc inols quantitati data that has both alu and units. Units can sa your butt! Nd handl on mtric prfixs Dimnsional
Data Assimilation 1. Alan O Neill National Centre for Earth Observation UK
 Data Assimilation 1 Alan O Nill National Cntr for Earth Obsrvation UK Plan Motivation & basic idas Univariat (scalar) data assimilation Multivariat (vctor) data assimilation 3d-Variational Mthod (& optimal
Data Assimilation 1 Alan O Nill National Cntr for Earth Obsrvation UK Plan Motivation & basic idas Univariat (scalar) data assimilation Multivariat (vctor) data assimilation 3d-Variational Mthod (& optimal
ECE507 - Plasma Physics and Applications
 ECE507 - Plasma Physics and Applications Lctur 7 Prof. Jorg Rocca and Dr. Frnando Tomasl Dpartmnt of Elctrical and Computr Enginring Collisional and radiativ procsss All particls in a plasma intract with
ECE507 - Plasma Physics and Applications Lctur 7 Prof. Jorg Rocca and Dr. Frnando Tomasl Dpartmnt of Elctrical and Computr Enginring Collisional and radiativ procsss All particls in a plasma intract with
 Mor Tutorial at www.dumblittldoctor.com Work th problms without a calculator, but us a calculator to chck rsults. And try diffrntiating your answrs in part III as a usful chck. I. Applications of Intgration
Mor Tutorial at www.dumblittldoctor.com Work th problms without a calculator, but us a calculator to chck rsults. And try diffrntiating your answrs in part III as a usful chck. I. Applications of Intgration
ME 321 Kinematics and Dynamics of Machines S. Lambert Winter 2002
 3.4 Forc Analysis of Linkas An undrstandin of forc analysis of linkas is rquird to: Dtrmin th raction forcs on pins, tc. as a consqunc of a spcifid motion (don t undrstimat th sinificanc of dynamic or
3.4 Forc Analysis of Linkas An undrstandin of forc analysis of linkas is rquird to: Dtrmin th raction forcs on pins, tc. as a consqunc of a spcifid motion (don t undrstimat th sinificanc of dynamic or
PH300 Modern Physics SP11 Final Essay. Up Next: Periodic Table Molecular Bonding
 PH Modrn Physics SP11 Final Essay Thr will b an ssay portion on th xam, but you don t nd to answr thos qustions if you submit a final ssay by th day of th final: Sat. 5/7 It dosnʼt mattr how smart you
PH Modrn Physics SP11 Final Essay Thr will b an ssay portion on th xam, but you don t nd to answr thos qustions if you submit a final ssay by th day of th final: Sat. 5/7 It dosnʼt mattr how smart you
Application of Vague Soft Sets in students evaluation
 Availabl onlin at www.plagiarsarchlibrary.com Advancs in Applid Scinc Rsarch, 0, (6):48-43 ISSN: 0976-860 CODEN (USA): AASRFC Application of Vagu Soft Sts in studnts valuation B. Chtia*and P. K. Das Dpartmnt
Availabl onlin at www.plagiarsarchlibrary.com Advancs in Applid Scinc Rsarch, 0, (6):48-43 ISSN: 0976-860 CODEN (USA): AASRFC Application of Vagu Soft Sts in studnts valuation B. Chtia*and P. K. Das Dpartmnt
ECE602 Exam 1 April 5, You must show ALL of your work for full credit.
 ECE62 Exam April 5, 27 Nam: Solution Scor: / This xam is closd-book. You must show ALL of your work for full crdit. Plas rad th qustions carfully. Plas chck your answrs carfully. Calculators may NOT b
ECE62 Exam April 5, 27 Nam: Solution Scor: / This xam is closd-book. You must show ALL of your work for full crdit. Plas rad th qustions carfully. Plas chck your answrs carfully. Calculators may NOT b
Intro to Nuclear and Particle Physics (5110)
 Intro to Nuclar and Particl Physics (5110) March 09, 009 Frmi s Thory of Bta Dcay (continud) Parity Violation, Nutrino Mass 3/9/009 1 Final Stat Phas Spac (Rviw) Th Final Stat lctron and nutrino wav functions
Intro to Nuclar and Particl Physics (5110) March 09, 009 Frmi s Thory of Bta Dcay (continud) Parity Violation, Nutrino Mass 3/9/009 1 Final Stat Phas Spac (Rviw) Th Final Stat lctron and nutrino wav functions
CS 361 Meeting 12 10/3/18
 CS 36 Mting 2 /3/8 Announcmnts. Homwork 4 is du Friday. If Friday is Mountain Day, homwork should b turnd in at my offic or th dpartmnt offic bfor 4. 2. Homwork 5 will b availabl ovr th wknd. 3. Our midtrm
CS 36 Mting 2 /3/8 Announcmnts. Homwork 4 is du Friday. If Friday is Mountain Day, homwork should b turnd in at my offic or th dpartmnt offic bfor 4. 2. Homwork 5 will b availabl ovr th wknd. 3. Our midtrm
PHY 192 Compton Effect 1
 PHY 19 Compton Effct 1 Th Compton Effct Introduction In this xprimnt w will study two aspcts of th intraction of photons with lctrons. Th first of ths is th Compton ffct namd aftr Arthur Holly Compton
PHY 19 Compton Effct 1 Th Compton Effct Introduction In this xprimnt w will study two aspcts of th intraction of photons with lctrons. Th first of ths is th Compton ffct namd aftr Arthur Holly Compton
Thermodynamical insight on the role of additives in shifting the equilibrium between white and grey tin
 hrmodynamical insight on th rol of additivs in shifting th quilibrium btwn whit and gry tin Nikolay Dmntv Dpartmnt of Chmistry, mpl Univrsity, Philadlphia, PA 19122 Abstract In this study mthods of statistical
hrmodynamical insight on th rol of additivs in shifting th quilibrium btwn whit and gry tin Nikolay Dmntv Dpartmnt of Chmistry, mpl Univrsity, Philadlphia, PA 19122 Abstract In this study mthods of statistical
Part 7: Capacitance And Capacitors
 Part 7: apacitanc And apacitors 7. Elctric harg And Elctric Filds onsidr a pair of flat, conducting plats, arrangd paralll to ach othr (as in figur 7.) and sparatd by an insulator, which may simply b air.
Part 7: apacitanc And apacitors 7. Elctric harg And Elctric Filds onsidr a pair of flat, conducting plats, arrangd paralll to ach othr (as in figur 7.) and sparatd by an insulator, which may simply b air.
Chapter 1 Late 1800 s Several failures of classical (Newtonian) physics discovered
 Chaptr 1 Lat 1800 s Svral failurs of classical (Nwtonian) physics discovrd 1905 195 Dvlopmnt of QM rsolvd discrpancis btwn xpt. and classical thory QM Essntial for undrstanding many phnomna in Chmistry,
Chaptr 1 Lat 1800 s Svral failurs of classical (Nwtonian) physics discovrd 1905 195 Dvlopmnt of QM rsolvd discrpancis btwn xpt. and classical thory QM Essntial for undrstanding many phnomna in Chmistry,
Electrochemistry L E O
 Rmmbr from CHM151 A rdox raction in on in which lctrons ar transfrrd lctrochmistry L O Rduction os lctrons xidation G R ain lctrons duction W can dtrmin which lmnt is oxidizd or rducd by assigning oxidation
Rmmbr from CHM151 A rdox raction in on in which lctrons ar transfrrd lctrochmistry L O Rduction os lctrons xidation G R ain lctrons duction W can dtrmin which lmnt is oxidizd or rducd by assigning oxidation
CE 530 Molecular Simulation
 CE 53 Molcular Simulation Lctur 8 Fr-nrgy calculations David A. Kofk Dpartmnt of Chmical Enginring SUNY Buffalo kofk@ng.buffalo.du 2 Fr-Enrgy Calculations Uss of fr nrgy Phas quilibria Raction quilibria
CE 53 Molcular Simulation Lctur 8 Fr-nrgy calculations David A. Kofk Dpartmnt of Chmical Enginring SUNY Buffalo kofk@ng.buffalo.du 2 Fr-Enrgy Calculations Uss of fr nrgy Phas quilibria Raction quilibria
Answer Homework 5 PHA5127 Fall 1999 Jeff Stark
 Answr omwork 5 PA527 Fall 999 Jff Stark A patint is bing tratd with Drug X in a clinical stting. Upon admiion, an IV bolus dos of 000mg was givn which yildd an initial concntration of 5.56 µg/ml. A fw
Answr omwork 5 PA527 Fall 999 Jff Stark A patint is bing tratd with Drug X in a clinical stting. Upon admiion, an IV bolus dos of 000mg was givn which yildd an initial concntration of 5.56 µg/ml. A fw
GEOMETRICAL PHENOMENA IN THE PHYSICS OF SUBATOMIC PARTICLES. Eduard N. Klenov* Rostov-on-Don, Russia
 GEOMETRICAL PHENOMENA IN THE PHYSICS OF SUBATOMIC PARTICLES Eduard N. Klnov* Rostov-on-Don, Russia Th articl considrs phnomnal gomtry figurs bing th carrirs of valu spctra for th pairs of th rmaining additiv
GEOMETRICAL PHENOMENA IN THE PHYSICS OF SUBATOMIC PARTICLES Eduard N. Klnov* Rostov-on-Don, Russia Th articl considrs phnomnal gomtry figurs bing th carrirs of valu spctra for th pairs of th rmaining additiv
Extraction of Doping Density Distributions from C-V Curves
 Extraction of Doping Dnsity Distributions from C-V Curvs Hartmut F.-W. Sadrozinski SCIPP, Univ. California Santa Cruz, Santa Cruz, CA 9564 USA 1. Connction btwn C, N, V Start with Poisson quation d V =
Extraction of Doping Dnsity Distributions from C-V Curvs Hartmut F.-W. Sadrozinski SCIPP, Univ. California Santa Cruz, Santa Cruz, CA 9564 USA 1. Connction btwn C, N, V Start with Poisson quation d V =
Outline. Thanks to Ian Blockland and Randy Sobie for these slides Lifetimes of Decaying Particles Scattering Cross Sections Fermi s Golden Rule
 Outlin Thanks to Ian Blockland and andy obi for ths slids Liftims of Dcaying Particls cattring Cross ctions Frmi s Goldn ul Physics 424 Lctur 12 Pag 1 Obsrvabls want to rlat xprimntal masurmnts to thortical
Outlin Thanks to Ian Blockland and andy obi for ths slids Liftims of Dcaying Particls cattring Cross ctions Frmi s Goldn ul Physics 424 Lctur 12 Pag 1 Obsrvabls want to rlat xprimntal masurmnts to thortical
September 23, Honors Chem Atomic structure.notebook. Atomic Structure
 Atomic Structur Topics covrd Atomic structur Subatomic particls Atomic numbr Mass numbr Charg Cations Anions Isotops Avrag atomic mass Practic qustions atomic structur Sp 27 8:16 PM 1 Powr Standards/ Larning
Atomic Structur Topics covrd Atomic structur Subatomic particls Atomic numbr Mass numbr Charg Cations Anions Isotops Avrag atomic mass Practic qustions atomic structur Sp 27 8:16 PM 1 Powr Standards/ Larning
Precise Masses of particles
 /1/15 Physics 1 April 1, 15 Ovrviw of topic Th constitunts and structur of nucli Radioactivity Half-lif and Radioactiv dating Nuclar Binding Enrgy Nuclar Fission Nuclar Fusion Practical Applications of
/1/15 Physics 1 April 1, 15 Ovrviw of topic Th constitunts and structur of nucli Radioactivity Half-lif and Radioactiv dating Nuclar Binding Enrgy Nuclar Fission Nuclar Fusion Practical Applications of
