Crystal Structure and Elementary Properties of Na x CoO 2 (x = 0.32, 0.5, 0.6, 0.75, and 0.92) in the Three-Layer NaCoO 2 Family
|
|
|
- Alexis Davis
- 5 years ago
- Views:
Transcription
1 Crystal Structure and Elementary Properties of Na x CoO 2 (x = 0.32, 0.5, 0.6, 0.75, and 0.92) in the Three-Layer NaCoO 2 Family L. Viciu, 1* J.W.G. Bos 1, H.W. Zandbergen, 2 Q. Huang, 3 M. L. Foo 1, S. Ishiwata, 1,4 A. P. Ramirez, 5 M. Lee, 6 N.P. Ong 6 and R.J. Cava 1 1 Department of Chemistry, Princeton University, Princeton NJ National Centre for HREM, Department of Nanoscience, Delft Institute of Technology, Al Delft, The Netherlands 3 NIST Center for Neutron Research, NIST, Gaithersburg, MD Department of Applied Physics, Waseda Univ., Ookubo, Shinjuku, Tokyo , Japan 5 Bell Laboratories, Lucent Technologies, Murray Hill NJ Department of Physics, Princeton University, Princeton, NJ Abstract The crystal structures of the Na x CoO 2 phases based on three-layer NaCoO 2, with x=0.32, x=0.51, x=0.60, x=0.75 and x=0.92, determined by powder neutron diffraction, are reported. The structures have triangular CoO 2 layers interleaved by sodium ions, and evolve with variation in Na content in a more complex way than has been observed in the two-layer Na x CoO 2 system. The highest and lowest Na containing phases studied (x=0.92 and x=0.32) are trigonal, with three CoO 2 layers per cell and octahedral Na ion coordination. The intermediate compositions have monoclinic structures. The x=0.75 compound has one CoO 2 layer per cell, with Na in octahedral coordination and an 1
2 incommensurate superlattice. The x=0.6 and x=0.5 phases are also single-layer, but the Na is found in trigonal prismatic coordination. The magnetic behavior of the phases is similar to that observed in the two-layer system. Both the susceptibility and the electronic contribution to the specific heat are largest for x=0.6. Introduction Na x CoO 2 has been widely studied as solid-state cathode in Na batteries. 1 The discovery of superconductivity (T C =4.5K) in two-layer Na 0.3 CoO 2 intercalated with water in 2003, 2 made the study of the Na x CoO 2 system an active area of research. The degree of filling of the Na layer controls the charge in the CoO 2 planes, giving rise to different properties as a function of x. The two-layer form of Na x CoO 2 has been of significant recent interest, as it displays a variety of interesting properties. In addition to the superconductivity in the hydrated phase, a large thermopower (100µV/K at 300K) has been found for x~0.7 3 and attributed to the spin entropy carried by strongly correlated electrons hopping on a triangular lattice. 4 A transition to an insulating state takes place in Na 0.5 CoO 2 at low temperatures that has been attributed to charge ordering. 5 Four different phases have been previously reported in the thermodynamic Na x CoO 2 chemical system. 6 In all the phases, sheets of edge-sharing CoO 6 octahedra are interleaved by sodium ions. The stacking sequence of the oxygen layers gives the number of sheets within a unit cell. Either two or three CoO 2 sheets per unit cell are found. Three of the four phases are reported to be three-layer structures, delineated as (1) the α- phase for 0.9 x 1; (2) the α -phase for x=0.75 and (3) the β-phase for 0.55 x 0.6. Only one 2
3 thermodynamic phase has a two-layer structure, known as the γ phase, for x ~0.7. The coordination of sodium ions in these structures is either octahedral or trigonal prismatic. These four thermodynamically stable phases in the Na x CoO 2 system can be obtained by classic solid state reactions. Topochemical methods can be used to tune the sodium composition within these structures. Thus, two layer Na 0.5 CoO 2 and Na 0.3 CoO 2 have been obtained by chemical deintercalation of the higher x counterparts. 7 In addition, chemical intercalation can be used to increase the sodium content of the γ phase from x=0.7 up to x=1. 7 The crystal structure of two-layer Na x CoO 2 with 0.3 x 1 has been extensively studied by Rietveld refinement using neutron diffraction data. 7 In the threelayer structure, however, crystallographic studies are reported only for x=1 (single crystals), 8 and for x=0.67 (polycrystalline powder). 9 At x=1 the reported structure is trigonal (R-3m with a=2.889(2) and c=15.609(3)å) while at x=0.67 the crystal structure is single-layer monoclinic (C2/m, with a=4.9023(4), b=2.8280(2), c=5.7198(6) and β= ). Early on, the powder patterns for x=0.5 and x=0.6 were indexed with a monoclinic cell but the structure was not determined. 10 Here we report a structural study, by neutron powder diffraction analysis, of the Na x CoO 2 phases derived from three-layer NaCoO 2, for x=0.92, 0.75, 0.60, 0.51, and It is found that the crystal structure changes from one sodium composition to another in an unexpected way. For example, Na 0.92 CoO 2 is trigonal, with Na in octahedral coordination. Deintercalation of this compound using Br 2 results in the formation of Na 0.3 CoO 2, which has the same crystal structure. Deintercalation of trigonal Na 0.92 CoO 2 with I 2 forms Na 0.5 CoO 2, which has a monoclinically distorted single-layer structure. Na 0.6 CoO 2 has also a single-layer unit cell and is found to be isostructural with the 3
4 previously reported Na 0.67 CoO 2. 9 Na 0.75 CoO 2 has a complex crystal structure. An average structure for this phase is reported based on the main reflections in the neutron diffraction data, indexed with a monoclinic cell. Susceptibility and heat capacity measurements are also reported for these phases. Although different crystal structures are found for the three-layer as opposed to the two-layer structures, the basic electronic properties of these materials are similar, supporting the general understanding that the electronic systems, dominated by the in-plane character of the CoO 2 layers, are highly two dimensional in character. Experimental Samples of Na x CoO 2 with x=0.92, 0.75, and x=0.60, were obtained as previously described. 6 Stoichiometric amounts of Na 2 O 2 (Alfa, 93% min) and Co 3 O 4 (Alfa, 99.7%) were mixed together in an argon filled glove box. The powders were then quickly removed from the glove box and placed in a tube furnace to prevent the hydration of sodium peroxide by air exposure. The temperature was slowly (5 C/min) increased to 550 C, held constant for 16 hours and then slowly (5 C/min) cooled to room temperature under flowing oxygen. Na 0.5 CoO 2 and Na 0.3 CoO 2 were synthesized by chemical deintercalation of Na 0.92 CoO 2. Na 0.5 CoO 2 was prepared by mixing NaCoO 2 with excess I 2 (10x) dissolved in acetonitrile. After 5 days of stirring, the product was washed with acetonitrile, dried and stored under argon. Single crystals of Na 0.5 CoO 2 were obtained by chemical deintercalation of NaCoO 2 single crystals stirred with H 2 O 2 at room temperature for 5days. Na 0.3 CoO 2 was obtained by mixing Na 0.92 CoO 2 with a molar excess of 40x bromine dissolved in acetonitrile. The reaction time was 5 days, after which the product 4
5 was washed with acetonitrile and stored under argon. Minimum exposure to atmospheric conditions is required to prevent water intercalation. All samples were analyzed by powder X-ray diffraction using Cu Kα radiation and a diffracted beam monochromator. Neutron diffraction data were collected on each sample at the NIST Center for Neutron Research on the high resolution powder neutron diffractometer with monochromatic neutrons of wavelength Å produced by a Cu(311) monochromator. Collimators with horizontal divergences of 15', 20' and 7' of arc were used before and after the monochromator and after the sample, respectively. Data were collected in the 2-theta range of 3º to 168º with a step size of The structural parameters were refined using the program GSAS. 11 The neutron scattering amplitudes used in the refinements were 0.363, 0.253, and ( cm) for Na, Co, and O, respectively. All sodium contents for the phases were determined by the structure refinements, and were in good agreement with those expected from nominal compositions. Electron microscopy analysis was performed with Philips CM300UT electron microscopes having a field emission gun and operated at 300kV. Electron transparent areas of specimens were obtained by crushing them slightly under ethanol to form a suspension and then dripping a droplet of this suspension on a carbon-coated holey film on a Cu grid or Au grid. The magnetic susceptibilities were measured with a Quantum Design MPMS SQUID system. Zero field cooled (ZFC) magnetic data were taken between 2K and 300K in an applied field of 5kOe. The specific heat samples were prepared by cold-sintering the sample powder with Ag powder. Measurements were made in a commercial cryostat 5
6 using the relaxation method. A four-probe method using a Quantum Design PPMS system was used to measure the resistivity of x=0.5 phase in the 5-300K temperature range. 12 Results The purity of the Na x CoO 2 compounds was confirmed by X-ray diffraction analysis, while structural investigations were performed by neutron diffraction analysis. Only Na 0.92 CoO 2 and Na 0.32 CoO 2 had powder diffraction patterns that could be indexed with a hexagonal cell. Their neutron powder patterns were indexed within the trigonal space group R-3m (No. 166), with the cell parameters given in table 1. None of the intermediate sodium compositions maintained the hexagonal structure. The neutron diffraction patterns of Na 0.5 CoO 2 and Na 0.6 CoO 2 were indexed based on centered monoclinic cells in the space group C 2/m (No. 12). The cell constants are presented in table 1. The monoclinic cells arise through shifts of the CoO 2 planes of approximately an angstrom relative to each other to accommodate changes in the local Na-O coordination. The shifts are in one direction, parallel to the crystallographic a axis. The X-ray diffraction pattern of Na 0.75 CoO 2 could be well indexed based on a monoclinic cell (space group C 2/m), but the neutron diffraction pattern showed the presence of relatively strong incommensurate superstructure reflections also seen in electron diffraction (described below). The main reflections of the neutron diffraction pattern fit the cell parameters presented in table 1. In the following, the nomenclature introduced earlier to describe layered structures of this type 13 is employed to most easily distinguish the phases: O and P designations refer to octahedral or prismatic coordination, respectively, of Na in the phase, and the numerical designation 1, 2, or 3 refers to the number of CoO 2 layers in a 6
7 unit cell repeat. In this system of nomenclature, the two layer phases commonly studied are P2 compounds, whereas the parent compound for the current studies, NaCoO 2, has the O3 type. Structural Characterization. x=0.92 and x=0.32. [O3 structure type] The Na 0.92 CoO 2 and Na 0.3 CoO 2 phases have been found to be isostructural. Their structural analysis by the Rietveld method was carried out in the space group R-3m. The sodium ions are on the (0, 0, ½) site, and are coordinated octahedrally to the oxygens from the CoO 2 layers. The refined structural parameters for both phases are presented in tables 2 and 3. Sodium contents of 0.921(7) and 0.32(1) were determined by refinement for these two phases. As an example, the structural model for Na 0.92 CoO 2 is shown in figure 1. x=0.6 and x=0.51. [P1 structure type] Na 0.6 CoO 2 and Na 0.5 CoO 2 have similar crystal structures and are found to be isostructural with Na 0.67 CoO 2. 9 This is a single-layer structure (space group C 2/m) where the sodium ions are in trigonal prismatic coordination. The structural parameters for Na 0.6 CoO 2 and Na 0.5 CoO 2 are presented in table 4 and table 5 respectively. Whereas in Na 0.5 CoO 2 the sodium ions are found on the 4i site (x,0,z), in Na 0.6 CoO 2 the sodium ions are displaced from the 4i site to a more general position 8j (x, y, z). No such displacement of the sodium ions was detected for Na 0.5 CoO 2 within the standard deviations of the positional parameters. In these structures, each sodium layer of Na 0.5 CoO 2 has only ~25% of a honeycomb-geometry sodium lattice occupied, while in Na 0.6 CoO 2 the sodium ions form a distorted honeycomb lattice only 30% filled. No information about any possible ordering of the Na ions within the partially occupied sites was obtained in the present study. A very good agreement of the sodium 7
8 content with the nominal compositions is found for both phases by Rietveld refinement: x=0.596(3) and x=0.512(4). As an example, the observed, calculated and the difference plots of the refinement for the x=0.51 phase are presented in figure 2. The idealized crystal structure is shown in figure 1. x=0.75. [O1 structure type] A different crystal structure from the ones described above is found for Na 0.75 CoO 2. The Rietveld refinement was performed in the C 2/m space group based on a single-layer structure. The crystal structure of the x=0.92 phase was used as a starting model with the atom coordinates in the three-layer structure transformed into the single-layer monoclinic cell. This model gave a very good fit to both the powder X-ray diffraction data (χ 2 =1.26, wrp=7.86%, Rp=6.22%) and the main reflections in the neutron diffraction pattern. The refined structural parameters based on the neutron diffraction data are presented in table 6. Figure 3 shows the observed, calculated and difference plots for the refinements based on this model for both X-ray data and neutron data (inset in fig 3). The structural model is presented in figure 1. Electron diffraction studies were performed to characterize the superstructure observed in the neutron diffraction pattern of Na 0.75 CoO 2. Figure 4 shows an [010] electron diffraction pattern of Na 0.75 CoO 2 taken in nano-diffraction mode with a spot size of about 10 nm. A quite dominant superstructure can be distinguished. The superstructure reflections can be indexed best using four dimensions, resulting in four Miller indices, hklm, in which m indicates the order of the satellite measured from the nearby main reflection. The hklm indexing of two superreflections is shown in figure 4. The superreflections in the electron diffraction patterns are much more visible than in the neutron powder diffraction pattern (they are not seen in the X-ray powder pattern). This 8
9 is due to the strong dynamic scattering in electron diffraction, which results in an enhancement of the weak reflections compared to the strong ones. The observed superstructure for x=0.75 is relatively strong, since quite a number of 1 st order superreflections can be seen in the neutron diffraction pattern (see figure 3). The indexing of the superreflections in the neutron diffraction pattern is based on the a* c* modulation vector determined from electron diffraction data. Determination of the incommensurately modulated structure of Na 0.75 CoO 2 from the powder diffraction data is beyond the scope of the present study. Magnetic Characterization. The variation of molar susceptibility with x is presented in figure 5. At temperatures higher than 50K, the temperature dependence of the susceptibility (χ vs. T) for x 0.6 follows the Curie-Weiss law, χ=χ 0 +(C/T-θ), with the Curie constant (C), Weiss constant (θ) and temperature independent term (χ 0 ) presented in table 7. The negative Weiss constants indicate antiferromagnetically interacting spins. Given a simple localized picture where only Co 4+ ions carry the spin moment S=1/2, the effective moments for the 0.92, 0.75 and 0.6 compositions are expected to be 0.49, 0.87 and 1.09 µ B /(formula unit), respectively. The observed moments are 0.32, 0.88 and 0.82 µ B /(formula unit) respectively. Although the differences between the calculated values assuming a simple model and the observed ones are not large, developing experiments in the two-layer phase indicate that a more complex electronic and magnetic system is at play (see e.g. ref. 14). It can be inferred, however, that low spin configurations for Co 3+ and Co 4+ ions are found in these phases. Small deviations from the Curie law are noted for x=0.92 and x=0.6 below 30K, and a magnetic transition at ~30K is seen for x=0.75, at a similar temperature to that observed for the two-layer x=0.75 phase. 14 For x=0.5, χ vs. 9
10 T has a different shape and no Curie Weiss behavior is found. Two cusps are observed in the χ vs T data: one at ~88K and another one at ~52K. These two transitions are observed at nearly the same temperatures where transitions are observed for two-layer Na 0.5 CoO 2. 5 In the two-layer structure the two anomalies signal the onset of an insulating state that has been attributed to a charge ordered phase. For x=0.3 in the three-layer structure, χ is independent of temperature above 75K. A Curie contribution is seen at lower temperatures. The origin of this behavior is not known. Resistivity Measurements. Resistivity measurements were performed on x=0.5 single crystals (figure 6). A transition to insulating state is observed. The primary resistive transition is observed at 50K, but the derivative of the resistivity data (inset, fig 6) shows that the transition observed in the susceptibility near 88K also impacts the resistivity. The behavior of the resistivity in the three-layer phase is very similar to that of two-layer Na 0.5 CoO 2, though in the latter case the resistive transition often shows an initial increase near 50K with the main transition at lower temperatures. Heat Capacity. The heat capacity data as a function of x for the three-layer derived Na x CoO 2 phases are presented in figure 7. The specific heat for all compositions decreases as the temperature approaches 2K. For x=0.75, a small transition is seen around 25K, associated with the magnetic ordering observed in the χ vs T data. The electronic contribution to the specific heat (γ) was extracted from the Debye formula at temperatures lower than 10K. The inset of the figure shows γ for each composition. As expected, the lowest γ is found for x=0.5 where a transition from metallic to insulator takes place. Below and above this composition, the larger carrier concentrations are expected to result in larger values for γ. 10
11 Discussion In contrast to the relatively straightforward structural behavior of the frequently studied two layer Na x CoO 2 system, significant structural changes take place when the sodium composition is varied in three-layer Na x CoO 2. In the two layer system, P2 type phases exist over a large composition range, dominating the phase diagram from x=0.3 to x=1. In that two layer system, x=0.5 is a special composition structurally, and there are small two-phase regions between different P2 type structures in the high sodium content region. The Na is in trigonal prismatic coordination across the whole two-layer series, resulting in the fact that neighboring CoO 2 layers are stacked in the same position relative to each other in all compounds. The same is not the case for the three-layer family. Our studies show that octahedral coordination for Na is apparently destabilized near half filling (i.e. x ~ 0.5) of the Na planes. The energetic reason for this is not known. The appearance of P1 phases in the middle of the three-layer phase diagram due to this octahedral site destabilization is the major factor in complicating the structural phase diagram. Thus, unlike the two-layer system, the regions of solid solution in the threelayer Na x CoO 2 are relatively narrow and compositions intermediate to those described here are classic two-phase mixtures. The changes in Na coordination across the series cause the CoO 2 layers to shift relative to each other to create the appropriate shape coordination polyhedra for Na and the right position of the adjacent O layers. The Na coordination polyhedra observed are presented in figure 8. Both the bond lengths and shapes of the octahedra and trigonal prisms are consistent with expectations for Na - O polyhedra and very similar to what is seen in the two-layer series. 7 As in the two-layer series, the NaO 2 plane layer expands as 11
12 Na is removed: when the sodium site occupancy within the layers decreases, the repulsion between the CoO 2 sheets is enhanced, and also, the coulombic forces holding the layers together decrease. Figure 9 shows that in spite of the changes in coordination of the Na across the series, the thickness of the NaO 2 layers changes continuously with Na content. A characterization of the relation of the CoO 2 planes relative to one another across the series is presented in figure 10. In the upper panel, the distances between the CoO 2 planes (from Co plane to Co plane) are shown for all compounds. There is a uniform change to smaller separation with increasing Na concentration, due primarily to the changing concentration of Na in the NaO 2 plane, as described above. The structures change symmetry across the series and the Na coordination changes from octahedral to trigonal prismatic and back again. A uniform description of the family can be made, however, by defining a pseudomonoclinic cell for all cases, with a= a hex and b=a hex 3 for the in-plane cell parameters, and c the distance from one Co to its equivalent Co one layer away. A pseudomonoclinic β angle gives the angle between the c and a pseudomonoclinic axes, and is a good measure of the relative positions of neighboring layers. As an example, figure 11 shows the pseudomonoclinic cell derived from the hexagonal cell for x=0.92 and x=0.75. To characterize how the planes shift relative to one another across the series, the pseudomonoclinc angle and the actual distance of the plane shift on going from one compound to the next are shown in the bottom two panels of figure 10. The middle panel suggests that the x=0.75 compound has an unexpectedly large shift of the layers relative to the behavior of the rest of the series, where the angles change continuously with Na 12
13 concentration. This unusual character for the x=0.75 phase is also suggested by the bottom panel: though the coordination of the Na remains octahedral when decreasing the Na content from x=0.92 to x=0.75, the layer has shifted by 0.6 Å. Interestingly, this shift is of the same magnitude, though in the opposite sense, as occurs when the layers shift between x=0.75 and x=0.6 to accommodate a change in the Na coordination from octahedral to prismatic. A somewhat smaller shift is observed on going from the trigonal prismatic to octahedral coordination between x=0.51 and It is interesting that in all cases the magnitudes of the shifts are relatively small, 0.6 Å in the largest case. As sodium content is varied in Na x CoO 2, the formal oxidation state of Co must change. For ideal NaCoO 2, Na 0.5 CoO 2 and CoO 2, for example, the Co formal oxidation states will be 3+, 3.5+, and 4+, respectively. Thus, like in the copper oxide superconductors where the charge in the CuO 2 planes can be deduced from the oxygen content or electropositive non transition metal ratios (e.g. in La 2-x Ba x CuO 4 ), the sodium content can be used as a measure of the charge state of the electronic system in the CoO 2 planes in Na x CoO 2. There are two caveats: the strict use of Na content for this purpose in two layer Na x CoO 2 has been called into question for compositions where x is less than 0.5 by titration measurements, 15 and, secondly, like in other highly oxidized transition metal compounds, the formal charge value says nothing about how the excess positive charge, on going from Co 3+ to Co 4+, is distributed among the Co or its coordinating oxygens. Plotting of structural parameters relevant to the electronic system as a function of Na content is straightforward, however, and can be used to infer general characteristics of the electronic system. 13
14 The structural characterization of the CoO 2 plane as a function of Na content in three-layer Na x CoO 2 is presented in figure 12. As the sodium composition decreases, the in-plane Co-Co distance decreases, as seen in the upper panel. In the simplest picture, this is a result of increasing the formal oxidation state of cobalt from mainly Co 3+ in Na 0.92 CoO 2 to Co in Na 0.32 CoO 2 : the in-plane size of the CoO 6 octahedra is expected to shrink as the Co 4+ /Co 3+ ratio increases. It is of interest that the size change, though monotonic, is not linear across the series, but changes most in the mid composition regions. Structural study of the two-layer Na x CoO 2 system has lead to the suggestion that the thickness of the CoO 2 layers may be a good reflection of redistribution of charge among different electronic orbitals with variation of electron count in that system. 7 The thickness of the CoO 2 layer as a function of sodium content in the three-layer system is shown in the middle panel of figure 12. As expected, for x=0.92 where the cobalt ions are mainly Co 3+, the CoO 2 layer thickness is largest, because the octahedra are largest, while for x=0.32, where the formal oxidation state is Co 3.68+, the CoO 2 layer thickness is smallest. The figure shows (middle panel), however, that the variation in layer thickness, in this series is not monotonic, suggesting that there is a significant redistribution of charge among different electronic orbitals across the three-layer series as a function of Na content. Significantly, the Co-O bond length varies continuously across the series, reflecting a systematic, continuous change in the formal Co oxidation state. The data presented in this figure suggest that in three-layer Na x CoO 2, the electronic system changes, and the crystal structure responds, over the whole composition region, from x=0.32 to x=
15 Finally, figures 13 and 14 present a general comparison of the electronic characteristics of the CoO 2 planes as a function of Na concentration in the two-layer and three-layer systems. Figure 13 shows that the variation in Co-O bond length across the two series, are very similar, and follow the expected trend toward larger size with decreased Co oxidation state. There do appear to be subtle differences observed, but more detailed study would be required to clarify them. This type of continuous bond length change with x would not be expected if oxygen vacancies occurred in significant numbers for x<0.5, halting the possible oxidation state of Co at an upper limit of Figure 14 shows the thickness of the CoO 2 layer relative to that expected if the CoO 6 octahedra have an ideal shape, as a function of composition. This quantity is determined from the length of the edges of the in-plane face of the CoO 6 octahedra and the ideal geometric relationship between the edge length and the diagonal height of an octahedron. The CoO 2 layer is highly compressed from what is expected for ideal octahedra (only 85% of the ideal value) in both phase families, suggesting that the structural distortion should be large enough to strongly influence the relative energies of different Co 3d suborbitals. This thickness varies across the series, reflecting a redistribution of charge within the CoO 2 layers as a function of composition and comparison of the two-layer and three-layer phases suggests that there are subtle differences in the electronic systems. In particular, the figure suggests that the three-layer Na 0.75 CoO 2 phase has a different kind of electronic structure than is seen in the two-layer variant at the same composition. Also shown in figure 14 are the single phase and multiple regions in the two families of compounds. This indicates how strongly the type of stacking influences the crystal chemistry of these systems. In addition, the figure 15
16 illustrates a comparison of the Co positions in the two-layer and three-layer structure types. In the former, the Co planes are eclipsed while in the later the Co planes are staggered. Conclusions The crystal structures of the Na x CoO 2 phases derived from three-layer NaCoO 2 are more complex than the much studied two-layer structures. This is due in large part to the fact that unlike the two-layer system, in the three-layer derived system the Na coordination changes in the structural series. In the two-layer family, the sodium forms many ordered phases, both commensurate and incommensurate with the underlying CoO 2 lattice. In the present system, due to the different types of sites encountered (i.e. octahedral rather than prismatic) the Na ordering may be expected to be different. No information on that ordering is provided in the average structure determinations presented here, and would be of interest in future studies. The structural complexity of the system is reflected in the crystal structure of the x=0.75 composition, where unlike the case of the analogous two-layer variant, the high intensities of the incommensurate superlattice reflections suggest that the underlying CoO 2 lattice experiences some kind of structural modulation. The determination of this structure will be of interest, as well as modeling to determine whether the structural modulation is electronically driven. Magnetic susceptibility data in the current family are consistent with Co 3+ and Co 4+ ions being in low spin configuration, and the magnetic behavior is similar to that observed in the two-layer system, though some differences are seen; particularly the presence of a Curie-Weiss susceptibility in the x=0.3 sample. The three-layer derived x=0.5 phase, in its initial characterization reported here, appears to be analogous to the 16
17 two-layer variant, suggesting that the electronic instability that gives rise to the insulating behavior is strongly two-dimensional in character, due to the fact that the stacking of the CoO 2 layers differs in the two systems. Finally, the composition dependence of the electronic contribution to the specific heat in the three-layer system appears to be substantially different from what has been reported in the two-layer system; 16, 17,18 in the present case showing the largest γ value at x=0.6. This may be due to the special character of the x=0.75 composition in the three-layer system, which may suppress γ. These similarities and differences suggest that further work on the three-layer derived phases and comparison to the two-layer phases will be of interest. Acknowledgements The work at Princeton was supported by the Department of Energy, grant DOE-FG , and by the National Science Foundation, grant DMR
18 Table 1. The Cell Parameters for Na x CoO 2 (x=0.92, 0.6, 0.48 and 0.32). Compound Space Group Na 0.92 CoO 2 R -3 m (No 166) Na 0.75 CoO 2 C 2/m (No.12) Na 0.60 CoO 2 Na 0.51 CoO 2 Na 0.32 CoO 2 C2/ m (No 12) C2/ m (No 12) R -3 m (No 166) Cell Constants (Å) a = (5) c = (3) a = (5) b = (3) c = (7) β = (7) a = (2) b = (1) c = (3) β = (3) a = (1) b = (9) c = (2) β = (2) a = (7) c = (1) Volume (Å 3 ) Volume/F.U. (Å 3 ) (5) (1) (6) (4) (1)
19 Table 2. Crystallographic data for Na 0.92 CoO 2 in the space group R -3 m (No 166) Atom Wyckoff x y z Uiso*100 Occ position Co 3a (3) 1 Na 3b (4) 0.921(7) O 6c (3) 0.91(1) 1 χ 2 =1.39, wr p =4.95%; R p =4.26% Table 3. Crystallographic data for Na 0.32 CoO 2 in the space group R -3 m (No 166) Atom Wyckoff x y z Uiso*100 Occ position Co 3a (6) 1 Na 3b (2) 0.32(1) O 6c (1) 1.07(4) 1 χ 2 =1.19, wr p =5.26%; R p =4.33% Table 4. Crystallographic data for Na 0.5 CoO 2 in the space group C2/m (No 12) Atom Wyckoff position x y z Uiso*100 Occ Co 2a (5) 1 Na 4i 0.806(2) (2) 2.1(2) 0.256(7) O 4i (3) (4) 0.92(3) 1 χ 2 =1.48, wr p =4.51%; R p =3.74% Table 5: Crystallographic data for Na 0.6 CoO 2 in the space group C 2/m (No 12) Atom Wyckoff position x y z Uiso*100 Occ Co(1) 2a (5) 1 Na(1) 8j 0.812(2) 0.049(6) 0.493(1) 1.6(3) 0.149(3) O(1) 4i ( (3) 0.94(2) 1 χ 2 =2.25, wr p =5.84%; R p =4.72% 19
20 Table 6: Crystallographic data for Na 0.75 CoO 2 in the space group C 2/m (No 12) Atom Wyckoff position x y z Uiso*100 Occ Co(1) 2a (2) 1 Na(1) 2d (3) 0.75 O(1) 4i (7) (6) 2.16(8) 1 Table 7. Summary of Magnetic Data for three-layer Na x CoO 2. Na x CoO 2 C (cm 3 K/mole) µ eff (µ B ) θ (K) χ 0 (emu/mole Co Oe) x= (5) (±3) (1) x= (9) (±1) (1) x= (3) ±3) (8) 20
21 References 1 M.G.S.R. Thomas, P.G. Bruce, J.B. Goodenough, Solid State Ionics, 17, 13, (1985). 2 K. Takada, H. Sakurai, E. Takayama-Muromachi, F. Izumi, R.A. Dilanian, and T. Sasaki, Nature, 422, 53 (2003) 3 I. Terasaki, Y.Sasago, K.Uchinokura, Phys.Rev. B56, 12685, (1997) 4 Y.Wang, N.S.Rogado, R.J.Cava and N.P.Ong, Nature, 423, 425 (2003) 5 M.L.Foo, Y.Wang, S.Watauchi, H.W. Zandbergen, T. He, R.J. Cava, N.P.Ong, Phys. Rev. Lett. 92, (2004). 6 Claude Fouassier, Guy Matejka, Jean-Maurice Reau, Paul Hagenmuller, J. Solid State Chem. 6, 532 (1973). 7 Q. Huang, M.L.Foo, R.A. Pascal, Jr., J.W.Lynn, B.H.Toby, T. He, H.W. Zandbergen, R.J.Cava, Phys. Rev B 70, (2004). 8 Y. Takahashi, Y.Gotoh, J. Akimoto, J. Solid State Chem. 172, 22 (2003). 9 Y. Ono, R. Ishikawa, Y. Miyazaki, Y. Ishii, Y. Morii, T. Kajitani, J. Solid State Chem. 166, 177 (2002) 10 S. Kikkawa, S. Miyazaki, M. Koizumi, J. Solid State Chem, 62, 35, (1986). 11 Larson, A.; Von Dreele, R. B. GSAS: Generalized Structure Analysis System; Los Alamos National Laboratory: Los Alamos, NM (1994) 12 Certain commercial product or equipment, is identified in this report to describe the subject adequately. Such identification does not imply recommendation or endorsement by the NIST, nor does it imply that the equipment identified is necessarily the best available for the purpose. 21
22 13 C. Delmas, J.J. Braconnier, C. Fouassier, P. Hagenmuller, Solid State Ionics, 3/4, 165 (1981). 14 J. L. Gavilano, D. Rao, B. Pedrini, J. Hinderer, H.R. Ott, S.M. Kazakov, J. Karpinski, Phys. Rev. B 69, (2004) 15 M. Karppinen, I. Asako, T. Motohashi, H. Yamauchi, Phys. Rev. B 71, (2005) 16 M. Yokoi, T. Moyoshi, Y. Kobayashi, M. Soda, Y. Yasui, M. Sato, K. Kakumai, Condmat/ R. Jin, B.C. Sales, S.Li and D. Mandrus, Phys Rev B 72, (R) (2005). 18 M. Brühwiler, B. Batlogg, S. M. Kazakov, J. Karpinski, cond-mat/
23 Figure Captions Figure 1. The crystal structures of Na x CoO 2 phases (x=0.92, 0.75, 0.6, 0.48, 0.32) derived from three-layer NaCoO 2. Smaller and bigger black spheres represent Co and sodium ions respectively while the grey spheres are the oxygen ions. Figure 2. Observed (crosses) and calculated (solid line) neutron diffraction intensities for single-layer Na 0.51 CoO 2 at 295K. Vertical bars show the Bragg peak positions. The difference plot is shown at the bottom. Figure 3. Observed (crosses) and calculated (solid line) X-ray diffraction intensities for Na 0.75 CoO 2. Vertical bars show the Bragg peak positions. The inset shows the superreflection peaks (marked with arrows) in the neutron diffraction pattern indexed in a 4D cell. Figure 4. [010] diffraction pattern of Na 0.75 CoO 2. Strong superreflections are present. The diffraction pattern was taken with the beam partly on a relatively thick area and partly over the adjacent hole, which configuration resulted in a tail of the reflections to the upper left corner. The indexing of some of the reflections is given. The vector q, describing the satellites, is 0.330a* c*. Figure 5. Temperature dependence of the magnetic susceptibility for Na x CoO 2 with x=0.92, 0.75, 0.6, 0.51, and 0.3. Figure 6. The temperature dependence of the resistivity in the plane parallel to the CoO 2 layers in a single crystal of x=0.5. The inset shows the derivative curve. Figure 7. Temperature dependence of the specific heat for Na x CoO 2 with x=0.92, 0.75, 0.6, 0.51, and Figure 8. The NaO 6 coordination polyhedra found in the Na x CoO 2 structures. 23
24 Figure 9. Top: The thickness of the NaO 2 layer as a function of x. Figure 10. Top: The distance between neighboring CoO 2 layers (from Co plane to Co plane); Middle: Pseudo-monoclinic cell angle; and Bottom: The layer shift in Na x CoO 2 phases; as a function of x in three-layer Na x CoO 2. Figure 11. The pseudomonoclinic cell derived from the three-layer hexagonal structure for x=0.92 and x=0.75. The actual unit cell for both compositions is shown with thick lines. Figure 12. Top: The in-plane Co-Co separation; Middle: the thickness of the CoO 2 layers; and Bottom: The variation of the Co-O bond length; as a function of x in threelayer Na x CoO 2. Figure 13. Comparison of the Co-O bond lengths as a function of x in Na x CoO 2 in the two-layer and three-layer series. The line drawn is a guide to the eye. Figure 14. Upper panel: the thickness of the CoO 2 plane relative to the thickness expected for an ideal CoO 6 octahedron (ideal thickness = 1); as a function of x in threelayer Na x CoO 2. Lower panel: the same characteristic for the two-layer phase. Shaded regions are two phases regions. The nomenclature P2, O3, O1 and P1 are used to describe the Na ion coordination and the number of CoO 2 layers per cell (1, 2 or 3). H1, H2 and H3 refer to subtle differences in crystal structure within the P2 phase. The illustration on the top show a comparison of the Co position in the two-layer and three-layer variants. In the two layer phases the Co planes are the same in all layers. In the three-layer phases the triangular Co planes are staggered: black balls represent layer 1; dark grey balls represent layer 2 and light grey balls represent layer 3. 24
25 B O3 A P1 A B C B B A 0.6 and 0.5 A C A C O1 B B A A 0.92 and Figure 1 25
26 Figure 2 26
27 Figure 3 27
28 0010 c* a* Figure 4 28
29 χ (10 3 emu/mole Co Oe) Na x CoO 2 x=0.5 x=0.32 χ 10K *10 3 (emu/mole Co Oe) x in Na x CoO 2 x=0.6 x=0.75 x=0.92 Figure T(K) 29
30 Na 0.5 CoO 2 ρ (mω cm) dρ/dt (mω cm/k) T (K) T (K) Figure 6 30
31 C/T (J/moleK 2 ) γ(mj/molek 2 ) X 0.04 Na x CoO 2 x=0.92 x= x=0.6 x=0.5 x= Temperature (K) Figure 7 31
32 Figure 8 32
33 Figure 9 33
34 Figure 10 34
35 x=0.92 x=0.75 Figure 11 35
36 Figure 12 36
37 three-layer derived structure two-layer structure Co-O bond distance (Å) x in Na x CoO 2 Figure 13 37
38 Co planes in two-layer Co planes in three-layer CoO 2 thickness (T obs /T calc ) Three-layer O3 P1 O1 O3 Two-layer H1 H2 H all are P2 phases x in Na x CoO 2 Figure 14 38
Superconductivity in three-layer Na 0.3 CoO 2 1.3H 2 O
 Superconductivity in three-layer Na 0.3 CoO 2 1.3H 2 O M.L. Foo 1, T. Klimczuk 1,3, Lu Li 2, N.P. Ong 2, and R.J. Cava 1 1 Department of Chemistry, Princeton University, Princeton NJ 08544, 2 Department
Superconductivity in three-layer Na 0.3 CoO 2 1.3H 2 O M.L. Foo 1, T. Klimczuk 1,3, Lu Li 2, N.P. Ong 2, and R.J. Cava 1 1 Department of Chemistry, Princeton University, Princeton NJ 08544, 2 Department
Superconductivity, charge ordering and structural properties of
 Superconductivity, charge ordering and structural properties of α, β-na x CoO 2 y(h 2 O, D 2 O) Y. G. Shi, H. X. Yang, H. Huang, X. Liu, and J.Q. Li* Beijing National Laboratory for Condensed Matter Physics,
Superconductivity, charge ordering and structural properties of α, β-na x CoO 2 y(h 2 O, D 2 O) Y. G. Shi, H. X. Yang, H. Huang, X. Liu, and J.Q. Li* Beijing National Laboratory for Condensed Matter Physics,
Phases of Na x CoO 2
 Phases of Na x CoO 2 by Aakash Pushp (pushp@uiuc.edu) Abstract This paper deals with the various phases of Na x CoO 2 ranging from charge ordered insulator to Curie-Weiss metal to superconductor as the
Phases of Na x CoO 2 by Aakash Pushp (pushp@uiuc.edu) Abstract This paper deals with the various phases of Na x CoO 2 ranging from charge ordered insulator to Curie-Weiss metal to superconductor as the
Electrochemical synthesis and properties of CoO 2, the x = 0 phase of the A x CoO 2 systems (A = Li, Na)
 Journal of Applied Physics 103, 07C902 (2008) Proceedings of 52nd Magnetism and Magnetic Materials Conference Electrochemical synthesis and properties of CoO 2, the x = 0 phase of the A x CoO 2 systems
Journal of Applied Physics 103, 07C902 (2008) Proceedings of 52nd Magnetism and Magnetic Materials Conference Electrochemical synthesis and properties of CoO 2, the x = 0 phase of the A x CoO 2 systems
Structure and Dynamics of Superconducting Na x CoO 2 Hydrate and Its Unhydrated Analog
 Structure and Dynamics of Superconducting Na x CoO 2 Hydrate and Its Unhydrated Analog J. W. Lynn 1, Q. Huang 1, C. M. Brown 1,2, V. L. Miller 3, M.L. Foo 3, R.E. Schaak 3, C. Y. Jones, 1 E. A. Mackey
Structure and Dynamics of Superconducting Na x CoO 2 Hydrate and Its Unhydrated Analog J. W. Lynn 1, Q. Huang 1, C. M. Brown 1,2, V. L. Miller 3, M.L. Foo 3, R.E. Schaak 3, C. Y. Jones, 1 E. A. Mackey
Structural and magnetic characterization of the new GdMn 1-x. O 3 perovskite material
 Journal of Physics: Conference Series PAPER OPEN ACCESS Structural and magnetic characterization of the new GdMn 1-x Fe x O 3 perovskite material To cite this article: J A Cardona Vasquez et al 2016 J.
Journal of Physics: Conference Series PAPER OPEN ACCESS Structural and magnetic characterization of the new GdMn 1-x Fe x O 3 perovskite material To cite this article: J A Cardona Vasquez et al 2016 J.
A Hydrated Superconductor
 A Hydrated Superconductor Karmela Padavic, Bikash Padhi, Akshat Puri A brief discussion of Superconductivity in 2D CoO 2 Layers Kazunori Takada, Hiroya Sakurai, Eiji Takayama Muromachi, Fujio Izumi, Ruben
A Hydrated Superconductor Karmela Padavic, Bikash Padhi, Akshat Puri A brief discussion of Superconductivity in 2D CoO 2 Layers Kazunori Takada, Hiroya Sakurai, Eiji Takayama Muromachi, Fujio Izumi, Ruben
First Experimental Evidence of Potassium Ordering in Layered K 4 Co 7 O 14
 Inorg. Chem. 2005, 44, 9299 9304 First Experimental Evidence of Potassium Ordering in Layered K 4 Co 7 O 14 Maxime Blangero, Rodolphe Decourt, Dany Carlier, Gerbrand Ceder, Michael Pollet, Jean-Pierre
Inorg. Chem. 2005, 44, 9299 9304 First Experimental Evidence of Potassium Ordering in Layered K 4 Co 7 O 14 Maxime Blangero, Rodolphe Decourt, Dany Carlier, Gerbrand Ceder, Michael Pollet, Jean-Pierre
Keywords: superconductivity, Fe-based superconductivity, FeTe, alcohol, wine
 Superconductivity in FeTe1-xSx induced by alcohol Keita Deguchi 1,2,3, Yoshikazu Mizuguchi 1,2,3, Toshinori Ozaki 1,3, Shunsuke Tsuda 1,3, Takahide Yamaguchi 1,3 and Yoshihiko Takano 1,2,3 1. National
Superconductivity in FeTe1-xSx induced by alcohol Keita Deguchi 1,2,3, Yoshikazu Mizuguchi 1,2,3, Toshinori Ozaki 1,3, Shunsuke Tsuda 1,3, Takahide Yamaguchi 1,3 and Yoshihiko Takano 1,2,3 1. National
Magnetic Order versus superconductivity in the Iron-based
 Magnetic Order versus superconductivity in the Iron-based layered La(O 1-x F x )FeAs systems Clarina de la Cruz 1,2, Q. Huang 3, J. W. Lynn 3, Jiying Li 3,4, W. Ratcliff II 3, J. L. Zarestky 5, H. A. Mook
Magnetic Order versus superconductivity in the Iron-based layered La(O 1-x F x )FeAs systems Clarina de la Cruz 1,2, Q. Huang 3, J. W. Lynn 3, Jiying Li 3,4, W. Ratcliff II 3, J. L. Zarestky 5, H. A. Mook
T. Hatakeda, T. Noji, S. Hosono, T. Kawamata, M. Kato, and Y. Koike
 Resistive superconducting transition and effects of atmospheric exposure in the intercalation superconductor A x (A = Li, Na) T. Hatakeda, T. Noji, S. Hosono, T. Kawamata, M. Kato, and Y. Koike Department
Resistive superconducting transition and effects of atmospheric exposure in the intercalation superconductor A x (A = Li, Na) T. Hatakeda, T. Noji, S. Hosono, T. Kawamata, M. Kato, and Y. Koike Department
Iridium Containing Honeycomb Delafossites by Topotactic Cation Exchange. Supplemental Material
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2016 Iridium Containing Honeycomb Delafossites by Topotactic Cation Exchange Supplemental
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2016 Iridium Containing Honeycomb Delafossites by Topotactic Cation Exchange Supplemental
Tb 2 Hf 2 O 7 R 2 B 2 7 R B R 3+ T N
 Tb Hf O 7 7 χ ac(t ) χ(t ) M(H) C p(t ) µ χ ac(t ) µ 7 7 7 R B 7 R B R 3+ 111 7 7 7 7 111 θ p = 19 7 7 111 7 15 7 7 7 7 7 7 7 7 T N.55 3+ 7 µ µ B 7 7 7 3+ 4f 8 S = 3 L = 3 J = 6 J + 1 = 13 7 F 6 3+ 7 7
Tb Hf O 7 7 χ ac(t ) χ(t ) M(H) C p(t ) µ χ ac(t ) µ 7 7 7 R B 7 R B R 3+ 111 7 7 7 7 111 θ p = 19 7 7 111 7 15 7 7 7 7 7 7 7 7 T N.55 3+ 7 µ µ B 7 7 7 3+ 4f 8 S = 3 L = 3 J = 6 J + 1 = 13 7 F 6 3+ 7 7
Chemical control of colossal magnetoresistance in manganites
 Materials Chemistry and Physics 72 (2001) 281 285 Chemical control of colossal magnetoresistance in manganites Chih-Hung Shen a, Ru-Shi Liu a,, Ravi Gundakaram a, Shu-Fen Hu b, Jauyn Grace Lin c, Chao-Yuan
Materials Chemistry and Physics 72 (2001) 281 285 Chemical control of colossal magnetoresistance in manganites Chih-Hung Shen a, Ru-Shi Liu a,, Ravi Gundakaram a, Shu-Fen Hu b, Jauyn Grace Lin c, Chao-Yuan
Shigeki Yonezawa*, Yuji Muraoka and Zenji Hiroi Institute for Solid State Physics, University of Tokyo, Kashiwa, Chiba
 New β-pyrochlore Oxide Superconductor CsOs 2 O 6 Shigeki Yonezawa*, Yuji Muraoka and Zenji Hiroi Institute for Solid State Physics, University of Tokyo, Kashiwa, Chiba 277-8581 Abstract The discovery of
New β-pyrochlore Oxide Superconductor CsOs 2 O 6 Shigeki Yonezawa*, Yuji Muraoka and Zenji Hiroi Institute for Solid State Physics, University of Tokyo, Kashiwa, Chiba 277-8581 Abstract The discovery of
YBCO. CuO 2. the CuO 2. planes is controlled. from deviation from. neutron. , blue star for. Hg12011 (this work) for T c = 72
 Supplementary Figure 1 Crystal structures and joint phase diagram of Hg1201 and YBCO. (a) Hg1201 features tetragonal symmetry and one CuO 2 plane per primitive cell. In the superconducting (SC) doping
Supplementary Figure 1 Crystal structures and joint phase diagram of Hg1201 and YBCO. (a) Hg1201 features tetragonal symmetry and one CuO 2 plane per primitive cell. In the superconducting (SC) doping
Ohoyama., TEM, neutron diffraction, ordered perovskite, CE structure. Abstract An A-site ordered manganese perovskite YBaMn 2
 New Stacking Variations of the CE-type Structure in the Metal-Ordered Manganite YBaMn 2 1 H. Kageyama, 1 T. Nakajima, 1 M. Ichihara, 1 Y. Ueda, and 2 H. Yoshizawa, 3 K. Ohoyama 1 Material Design and Characterization
New Stacking Variations of the CE-type Structure in the Metal-Ordered Manganite YBaMn 2 1 H. Kageyama, 1 T. Nakajima, 1 M. Ichihara, 1 Y. Ueda, and 2 H. Yoshizawa, 3 K. Ohoyama 1 Material Design and Characterization
Low temperature synthesis of layered Na x CoO 2 and K x CoO 2 from NaOH/KOH fluxes and their ion exchange properties
 Proc. Indian Acad. Sci. (Chem. Sci.), Vol. 115, Nos 5 & 6, October December 2003, pp 447 457 Indian Academy of Sciences Low temperature synthesis of layered Na x CoO 2 and K x CoO 2 from NaOH/KOH fluxes
Proc. Indian Acad. Sci. (Chem. Sci.), Vol. 115, Nos 5 & 6, October December 2003, pp 447 457 Indian Academy of Sciences Low temperature synthesis of layered Na x CoO 2 and K x CoO 2 from NaOH/KOH fluxes
Superconductor made by electrolyzed and oxidized water
 1 Superconductor made by electrolyzed and oxidized water Chia-Jyi Liu 1, Tsung-Hsien Wu 1, Lin-Li Hsu 1, Jung-Sheng Wang 1, Shu-Yo Chen 1, Wei Jen Chang 2, & Jiunn-Yuan Lin 3 1 Department of Physics, National
1 Superconductor made by electrolyzed and oxidized water Chia-Jyi Liu 1, Tsung-Hsien Wu 1, Lin-Li Hsu 1, Jung-Sheng Wang 1, Shu-Yo Chen 1, Wei Jen Chang 2, & Jiunn-Yuan Lin 3 1 Department of Physics, National
New Li-Ethylenediamine-Intercalated Superconductor Li x (C 2 H 8 N 2 ) y Fe 2-z Se 2 with T c = 45 K
 New Li-Ethylenediamine-Intercalated Superconductor Li x (C 2 H 8 N 2 ) y Fe 2-z Se 2 with T c = 45 K Takehiro Hatakeda, Takashi Noji, Takayuki Kawamata, Masatsune Kato, and Yoji Koike Department of Applied
New Li-Ethylenediamine-Intercalated Superconductor Li x (C 2 H 8 N 2 ) y Fe 2-z Se 2 with T c = 45 K Takehiro Hatakeda, Takashi Noji, Takayuki Kawamata, Masatsune Kato, and Yoji Koike Department of Applied
Sodium Trimer Ordering on Na x CoO 2 Surface. C. Chou 1,4. Taiwan, R.O.C. R.O.C. FL 32611, U.S.A.
 Sodium Trimer Ordering on Na x CoO 2 Surface Woei Wu Pai 1,*, S. H. Huang 1,2, Ying S. Meng 3, Y. C. Chao 1, C. H. Lin 1, H. L. Liu 2, F. C. Chou 1,4 1 Center for Condensed Matter Sciences, National Taiwan
Sodium Trimer Ordering on Na x CoO 2 Surface Woei Wu Pai 1,*, S. H. Huang 1,2, Ying S. Meng 3, Y. C. Chao 1, C. H. Lin 1, H. L. Liu 2, F. C. Chou 1,4 1 Center for Condensed Matter Sciences, National Taiwan
Imaging Self-Organized Domains at the Micron Scale in Antiferromagnetic Elemental Cr Using Magnetic X-ray Microscopy
 Mat. Res. Soc. Symp. Proc. Vol. 690 2002 Materials Research Society Imaging Self-Organized Domains at the Micron Scale in Antiferromagnetic Elemental Cr Using Magnetic X-ray Microscopy P. G. Evans, 1 E.
Mat. Res. Soc. Symp. Proc. Vol. 690 2002 Materials Research Society Imaging Self-Organized Domains at the Micron Scale in Antiferromagnetic Elemental Cr Using Magnetic X-ray Microscopy P. G. Evans, 1 E.
Structure and Magnetism of the mono-layer hydrate Na 0.3 NiO 2 0.7H 2 O
 Structure and Magnetism of the mono-layer hydrate Na 0.3 NiO 2 0.7H 2 O S. Park a, W.-S. Yoon b, T. Vogt a, * a USC NanoCenter & Department of Chemistry and Biochemistry, University of South Carolina,
Structure and Magnetism of the mono-layer hydrate Na 0.3 NiO 2 0.7H 2 O S. Park a, W.-S. Yoon b, T. Vogt a, * a USC NanoCenter & Department of Chemistry and Biochemistry, University of South Carolina,
Chemical Differences between K and Na in Alkali Cobaltates
 Chemical Differences between K and Na in Alkali Cobaltates K.-W. Lee and W. E. Pickett Department of Physics, University of California, Davis, California 95616 (Dated: May 22, 27) K xcoo 2 shares many
Chemical Differences between K and Na in Alkali Cobaltates K.-W. Lee and W. E. Pickett Department of Physics, University of California, Davis, California 95616 (Dated: May 22, 27) K xcoo 2 shares many
Magnetic Transition in the Kondo Lattice System CeRhSn 2. Z. Hossain 1, L.C. Gupta 2 and C. Geibel 1. Germany.
 Magnetic Transition in the Kondo Lattice System CeRhSn 2 Z. Hossain 1, L.C. Gupta 2 and C. Geibel 1 1 Max-Planck Institute for Chemical Physics of Solids, Nöthnitzer Str. 40, 01187 Dresden, Germany. 2
Magnetic Transition in the Kondo Lattice System CeRhSn 2 Z. Hossain 1, L.C. Gupta 2 and C. Geibel 1 1 Max-Planck Institute for Chemical Physics of Solids, Nöthnitzer Str. 40, 01187 Dresden, Germany. 2
Neutron Powder Diffraction Theory and Instrumentation
 NTC, Taiwen Aug. 31, 212 Neutron Powder Diffraction Theory and Instrumentation Qingzhen Huang (qing.huang@nist.gov) NIST Center for Neutron Research (www.ncnr.nist.gov) Definitions E: energy; k: wave vector;
NTC, Taiwen Aug. 31, 212 Neutron Powder Diffraction Theory and Instrumentation Qingzhen Huang (qing.huang@nist.gov) NIST Center for Neutron Research (www.ncnr.nist.gov) Definitions E: energy; k: wave vector;
Magnetic Properties and Scaling Behavior in Perovskite like La 0.7 (Ba 1-x Pb x ) 0.3 CoO 3 System
 Mat. Res. Soc. Symp. Proc. Vol. 674 21 Materials Research Society Magnetic Properties and Scaling Behavior in Perovskite like La.7 (Ba 1-x Pb x ).3 CoO 3 System Chiung-Hsiung Chen, Ting-Sheng Huang and
Mat. Res. Soc. Symp. Proc. Vol. 674 21 Materials Research Society Magnetic Properties and Scaling Behavior in Perovskite like La.7 (Ba 1-x Pb x ).3 CoO 3 System Chiung-Hsiung Chen, Ting-Sheng Huang and
Isothermal section of the Na 0.3 CoO 2 H 2 O phase diagram at 22 C from 11 to 100% relative humidity
 Solid State Sciences 7 (2005) 1312 1316 www.elsevier.com/locate/ssscie Isothermal section of the Na 0.3 CoO 2 H 2 O phase diagram at 22 C from 11 to 100% relative humidity Viktor V. Poltavets a, Konstantin
Solid State Sciences 7 (2005) 1312 1316 www.elsevier.com/locate/ssscie Isothermal section of the Na 0.3 CoO 2 H 2 O phase diagram at 22 C from 11 to 100% relative humidity Viktor V. Poltavets a, Konstantin
Topotactic Reduction and Reoxidation of Hexagonal RCu 0.5 Ti 0.5 O 3 (R =Y, Eu-Lu) Phases
 Topotactic Reduction and Reoxidation of Hexagonal RCu 0.5 Ti 0.5 O 3 (R =Y, Eu-Lu) Phases 1 Peng Jiang, Romian Berthelot, Jun Li, A.W. Sleight, and M.A. Subramanian* Department of Chemistry, Oregon State
Topotactic Reduction and Reoxidation of Hexagonal RCu 0.5 Ti 0.5 O 3 (R =Y, Eu-Lu) Phases 1 Peng Jiang, Romian Berthelot, Jun Li, A.W. Sleight, and M.A. Subramanian* Department of Chemistry, Oregon State
Supporting Information. Ze-Min Zhang, Lu-Yi Pan, Wei-Quan Lin, Ji-Dong Leng, Fu-Sheng Guo, Yan-Cong Chen, Jun-Liang Liu, and Ming-Liang Tong*
 Supporting Information Wheel-shaped nanoscale 3d-4f {Co II 16Ln III 24} clusters (Ln = Dy and Gd) Ze-Min Zhang, Lu-Yi Pan, Wei-Quan Lin, Ji-Dong Leng, Fu-Sheng Guo, Yan-Cong Chen, Jun-Liang Liu, and Ming-Liang
Supporting Information Wheel-shaped nanoscale 3d-4f {Co II 16Ln III 24} clusters (Ln = Dy and Gd) Ze-Min Zhang, Lu-Yi Pan, Wei-Quan Lin, Ji-Dong Leng, Fu-Sheng Guo, Yan-Cong Chen, Jun-Liang Liu, and Ming-Liang
alkaline-metal-ethylenediamine-intercalated superconductors A x (C 2 H 8 N 2 ) y Fe 2-z Se 2 (A = Li, Na) with T c = 45 K
 Synthesis and post-annealing effects of alkaline-metal-ethylenediamine-intercalated superconductors A x (C N Fe -z Se (A = Li, Na) with T c = K Takashi Noji, Takehiro Hatakeda, Shohei Hosono, Takayuki
Synthesis and post-annealing effects of alkaline-metal-ethylenediamine-intercalated superconductors A x (C N Fe -z Se (A = Li, Na) with T c = K Takashi Noji, Takehiro Hatakeda, Shohei Hosono, Takayuki
Chemical differences between K and Na in alkali cobaltates
 Chemical differences between K and Na in alkali cobaltates K.-W. Lee* and W. E. Pickett Department of Physics, University of California, Davis, California 95616, USA Received 22 May 27; published 19 October
Chemical differences between K and Na in alkali cobaltates K.-W. Lee* and W. E. Pickett Department of Physics, University of California, Davis, California 95616, USA Received 22 May 27; published 19 October
Study on Magnetic Properties of Vermiculite Intercalation compounds
 Study on Magnetic Properties of Vermiculite Intercalation compounds M. Suzuki and I.S. Suzuki Department of Physics, State University of New York at Binghamton (October, ) I. INTRODUCTION In recent years
Study on Magnetic Properties of Vermiculite Intercalation compounds M. Suzuki and I.S. Suzuki Department of Physics, State University of New York at Binghamton (October, ) I. INTRODUCTION In recent years
Materials 218/UCSB: Superconductivity and High T C copper oxide superconductors:
 Materials 218/UCSB: Superconductivity and High T C copper oxide superconductors: Ram Seshadri (seshadri@mrl.ucsb.edu) The Ruddlesden-Popper phases: Ruddlesden-Popper phases are intergrowths of perovskite
Materials 218/UCSB: Superconductivity and High T C copper oxide superconductors: Ram Seshadri (seshadri@mrl.ucsb.edu) The Ruddlesden-Popper phases: Ruddlesden-Popper phases are intergrowths of perovskite
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information "Redox Properties of Alluaudite Sodium Cobalt Manganese Sulfates
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information "Redox Properties of Alluaudite Sodium Cobalt Manganese Sulfates
Superconductivity at 41.0 K in the F-doped LaFeAsO 1-x F x
 Superconductivity at 41.0 K in the F-doped LaFeAsO 1-x F x Wei Lu, Xiao-Li Shen, Jie Yang, Zheng-Cai Li, Wei Yi, Zhi-An Ren*, Xiao-Li Dong, Guang-Can Che, Li-Ling Sun, Fang Zhou, Zhong-Xian Zhao* National
Superconductivity at 41.0 K in the F-doped LaFeAsO 1-x F x Wei Lu, Xiao-Li Shen, Jie Yang, Zheng-Cai Li, Wei Yi, Zhi-An Ren*, Xiao-Li Dong, Guang-Can Che, Li-Ling Sun, Fang Zhou, Zhong-Xian Zhao* National
Superconductivity and Cobalt Oxidation State in Metastable Na x CoO 2- yh 2 O (x 1 / 3 ; y 4x)
 Superconductivity and Cobalt Oxidation State in Metastable Na x CoO 2- yh 2 O (x 1 / 3 ; y 4x) P. W. Barnes, M. Avdeev*, J. D. Jorgensen, D. G. Hinks, H. Claus, and S. Short Materials Science Division,
Superconductivity and Cobalt Oxidation State in Metastable Na x CoO 2- yh 2 O (x 1 / 3 ; y 4x) P. W. Barnes, M. Avdeev*, J. D. Jorgensen, D. G. Hinks, H. Claus, and S. Short Materials Science Division,
Phthalocyanine-Based Single-Component
 Phthalocyanine-Based Single-Component Molecular Conductor [Mn Ⅲ (Pc)(CN)] 2 O Mitsuo Ikeda, Hiroshi Murakawa, Masaki Matsuda, and Noriaki Hanasaki *, Department of Physics, Graduate School of Science,
Phthalocyanine-Based Single-Component Molecular Conductor [Mn Ⅲ (Pc)(CN)] 2 O Mitsuo Ikeda, Hiroshi Murakawa, Masaki Matsuda, and Noriaki Hanasaki *, Department of Physics, Graduate School of Science,
Structural and magnetic phase diagram of CeFeAsO 1-x F x and. its relationship to high-temperature superconductivity
 Structural and magnetic phase diagram of CeFeAsO 1-x F x and its relationship to high-temperature superconductivity Jun Zhao 1, Q. Huang 2, Clarina de la Cruz 1,3, Shiliang Li 1, J. W. Lynn 2, Y. Chen
Structural and magnetic phase diagram of CeFeAsO 1-x F x and its relationship to high-temperature superconductivity Jun Zhao 1, Q. Huang 2, Clarina de la Cruz 1,3, Shiliang Li 1, J. W. Lynn 2, Y. Chen
Synthetizing and Measuring Superconducting Sodium Cobalt Oxide (Na x CoO 2 ) Crystals
 Synthetizing and Measuring Superconducting Sodium Cobalt Oxide (Na x CoO 2 ) Crystals Rados law Tomasz Chrapkiewicz under the direction of Prof. Young S. Lee Massachusetts Institute of Technology Research
Synthetizing and Measuring Superconducting Sodium Cobalt Oxide (Na x CoO 2 ) Crystals Rados law Tomasz Chrapkiewicz under the direction of Prof. Young S. Lee Massachusetts Institute of Technology Research
SAMANTHA GORHAM FRANK H. MORRELL CAMPUS ADVISOR: Prof. TREVOR A. TYSON (NJIT)
 SAMANTHA GORHAM FRANK H. MORRELL CAMPUS ADVISOR: Prof. TREVOR A. TYSON (NJIT) I WANT TO THANK PROFESSOR TREVOR A. TYSON FOR HIS HELP IN ASSISTING ME THROUGHOUT THE COURSE OF THIS PRJECT AND RESEARCH. I
SAMANTHA GORHAM FRANK H. MORRELL CAMPUS ADVISOR: Prof. TREVOR A. TYSON (NJIT) I WANT TO THANK PROFESSOR TREVOR A. TYSON FOR HIS HELP IN ASSISTING ME THROUGHOUT THE COURSE OF THIS PRJECT AND RESEARCH. I
arxiv: v1 [cond-mat.supr-con] 9 May 2013
![arxiv: v1 [cond-mat.supr-con] 9 May 2013 arxiv: v1 [cond-mat.supr-con] 9 May 2013](/thumbs/92/109271866.jpg) Journal of the Physical Society of Japan LETTERS Superconductivity in Tetragonal LaPt 2 x Ge 2+x arxiv:135.224v1 [cond-mat.supr-con] 9 May 213 Satoki Maeda 1, Kazuaki Matano 1, Hiroki Sawaoka 1, Yoshihiko
Journal of the Physical Society of Japan LETTERS Superconductivity in Tetragonal LaPt 2 x Ge 2+x arxiv:135.224v1 [cond-mat.supr-con] 9 May 213 Satoki Maeda 1, Kazuaki Matano 1, Hiroki Sawaoka 1, Yoshihiko
Synthesis and characterization of Ruddlesden Popper (RP) type phase LaSr 2 MnCrO 7
 J. Chem. Sci., Vol. 122, No. 6, November 2010, pp. 807 811. Indian Academy of Sciences. Synthesis and characterization of Ruddlesden Popper (RP) type phase LaSr 2 MnCrO 7 DEVINDER SINGH* and RAJINDER SINGH
J. Chem. Sci., Vol. 122, No. 6, November 2010, pp. 807 811. Indian Academy of Sciences. Synthesis and characterization of Ruddlesden Popper (RP) type phase LaSr 2 MnCrO 7 DEVINDER SINGH* and RAJINDER SINGH
Direct visualization of the Jahn Teller effect coupled to Na ordering in Na 5/8 MnO 2
 Direct visualization of the Jahn Teller effect coupled to Na ordering in Na 5/8 MnO 2 Xin Li 1, Xiaohua Ma 1, Dong Su 2, Lei Liu 1, Robin Chisnell 3, Shyue Ping Ong 1, Hailong Chen 1, Alexandra Toumar
Direct visualization of the Jahn Teller effect coupled to Na ordering in Na 5/8 MnO 2 Xin Li 1, Xiaohua Ma 1, Dong Su 2, Lei Liu 1, Robin Chisnell 3, Shyue Ping Ong 1, Hailong Chen 1, Alexandra Toumar
Superconductivity in the Mn 5 Si 3 -type Zr 5 Sb 3 system
 Superconductivity in the Mn 5 Si 3 -type Zr 5 Sb 3 system B. Lv, 1 * X. Y. Zhu, 1 B. Lorenz, 1 F. Y. Wei, 1 Y. Y. Xue, 1 Z. P. Yin, 2 G. Kotliar 2 and C. W. Chu 1,3 * 1 Department of Physics and Texas
Superconductivity in the Mn 5 Si 3 -type Zr 5 Sb 3 system B. Lv, 1 * X. Y. Zhu, 1 B. Lorenz, 1 F. Y. Wei, 1 Y. Y. Xue, 1 Z. P. Yin, 2 G. Kotliar 2 and C. W. Chu 1,3 * 1 Department of Physics and Texas
SECOND PUBLIC EXAMINATION. Honour School of Physics Part C: 4 Year Course. Honour School of Physics and Philosophy Part C C3: CONDENSED MATTER PHYSICS
 2753 SECOND PUBLIC EXAMINATION Honour School of Physics Part C: 4 Year Course Honour School of Physics and Philosophy Part C C3: CONDENSED MATTER PHYSICS TRINITY TERM 2011 Wednesday, 22 June, 9.30 am 12.30
2753 SECOND PUBLIC EXAMINATION Honour School of Physics Part C: 4 Year Course Honour School of Physics and Philosophy Part C C3: CONDENSED MATTER PHYSICS TRINITY TERM 2011 Wednesday, 22 June, 9.30 am 12.30
From Double-Shelled Grids to Supramolecular Frameworks
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 From Double-Shelled Grids to Supramolecular Frameworks Jianfeng Wu, Mei Guo, Xiao-Lei Li, Lang
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 From Double-Shelled Grids to Supramolecular Frameworks Jianfeng Wu, Mei Guo, Xiao-Lei Li, Lang
Superconductivity in oxygen-annealed FeTe 1-x S x single crystal
 Superconductivity in oxygen-annealed FeTe 1-x S x single crystal Yoshikazu Mizuguchi 1,2,3, Keita Deguchi 1,2,3, Yasuna Kawasaki 1,2,3, Toshinori Ozaki 1,2, Masanori Nagao 4, Shunsuke Tsuda 1,2, Takahide
Superconductivity in oxygen-annealed FeTe 1-x S x single crystal Yoshikazu Mizuguchi 1,2,3, Keita Deguchi 1,2,3, Yasuna Kawasaki 1,2,3, Toshinori Ozaki 1,2, Masanori Nagao 4, Shunsuke Tsuda 1,2, Takahide
2 ( º ) Intensity (a.u.) Supplementary Figure 1. Crystal structure for composition Bi0.75Pb0.25Fe0.7Mn0.05Ti0.25O3. Highresolution
 Intensity (a.u.) Y Obs Y Cal Y Obs - Y Cal Bragg position Cc 20 40 60 80 100 2 ( º ) Supplementary Figure 1. Crystal structure for composition Bi0.75Pb0.25Fe0.7Mn0.05Ti0.25O3. Highresolution X-ray diffraction
Intensity (a.u.) Y Obs Y Cal Y Obs - Y Cal Bragg position Cc 20 40 60 80 100 2 ( º ) Supplementary Figure 1. Crystal structure for composition Bi0.75Pb0.25Fe0.7Mn0.05Ti0.25O3. Highresolution X-ray diffraction
The Chemical Control of Superconductivity in Bi 2 Sr 2 (Ca 1 x Y x )Cu 2 O 8+±
 CHINESE JOURNAL OF PHYSICS VOL. 38, NO. 2-II APRIL 2000 The Chemical Control of Superconductivity in Bi 2 Sr 2 (Ca 1 x Y x )Cu 2 O 8+± R. S. Liu 1, I. J. Hsu 1, J. M. Chen 2, and R. G. Liu 2 1 Department
CHINESE JOURNAL OF PHYSICS VOL. 38, NO. 2-II APRIL 2000 The Chemical Control of Superconductivity in Bi 2 Sr 2 (Ca 1 x Y x )Cu 2 O 8+± R. S. Liu 1, I. J. Hsu 1, J. M. Chen 2, and R. G. Liu 2 1 Department
Ferromagnetism in the Mott insulator Ba 2 NaOsO 6
 Ferromagnetism in the Mott insulator Ba 2 NaOsO 6 SLAC-PUB-39 cond-mat/6385 A. S. Erickson, S. Misra, 2 G. J. Miller, 2 R. R. Gupta, 3 Z. Schlesinger, 3 W. A. Harrison, J. M. Kim, I. R. Fisher Department
Ferromagnetism in the Mott insulator Ba 2 NaOsO 6 SLAC-PUB-39 cond-mat/6385 A. S. Erickson, S. Misra, 2 G. J. Miller, 2 R. R. Gupta, 3 Z. Schlesinger, 3 W. A. Harrison, J. M. Kim, I. R. Fisher Department
Supporting Information. for. Angew. Chem. Int. Ed Wiley-VCH 2004
 Supporting Information for Angew. Chem. Int. Ed. 246736 Wiley-VCH 24 69451 Weinheim, Germany 1 Challenges in Engineering Spin Crossover. Structures and Magnetic Properties of six Alcohol Solvates of Iron(II)
Supporting Information for Angew. Chem. Int. Ed. 246736 Wiley-VCH 24 69451 Weinheim, Germany 1 Challenges in Engineering Spin Crossover. Structures and Magnetic Properties of six Alcohol Solvates of Iron(II)
1 Crystal Structures. of three-dimensional crystals. Here we use two-dimensional examples to illustrate the concepts.
 3 1 Crystal Structures A crystal is a periodic array of atoms. Many elements and quite a few compounds are crystalline at low enough temperatures, and many of the solid materials in our everyday life (like
3 1 Crystal Structures A crystal is a periodic array of atoms. Many elements and quite a few compounds are crystalline at low enough temperatures, and many of the solid materials in our everyday life (like
Phase identification and structure determination from multiphasic crystalline powder samples by rotation electron diffraction
 Supporting information Phase identification and structure determination from multiphasic crystalline powder samples by rotation electron diffraction Yifeng Yun ab, Wei Wan ab, Faiz Rabbani b, Jie Su ab,
Supporting information Phase identification and structure determination from multiphasic crystalline powder samples by rotation electron diffraction Yifeng Yun ab, Wei Wan ab, Faiz Rabbani b, Jie Su ab,
Supplementary Information for
 Supplementary Information for Chiral discotic derivatives of 1,3,5-triphenyl-6-oxoverdazyl radical Aleksandra Jankowiak, a Damian Pociecha, b Jacek Szczytko, c and Piotr Kaszynski* a,d a Department of
Supplementary Information for Chiral discotic derivatives of 1,3,5-triphenyl-6-oxoverdazyl radical Aleksandra Jankowiak, a Damian Pociecha, b Jacek Szczytko, c and Piotr Kaszynski* a,d a Department of
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. Crystal structure of 1T -MoTe 2. (a) HAADF-STEM image of 1T -MoTe 2, looking down the [001] zone (scale bar, 0.5 nm). The area indicated by the red rectangle
Supplementary Figures Supplementary Figure 1. Crystal structure of 1T -MoTe 2. (a) HAADF-STEM image of 1T -MoTe 2, looking down the [001] zone (scale bar, 0.5 nm). The area indicated by the red rectangle
arxiv: v1 [cond-mat.supr-con] 4 Jan 2014
![arxiv: v1 [cond-mat.supr-con] 4 Jan 2014 arxiv: v1 [cond-mat.supr-con] 4 Jan 2014](/thumbs/88/115192775.jpg) Conventional Superconductivity properties of the ternary boron-nitride Nb 2 BN O. V. Cigarroa 1, S. T. Renosto 1, A. J. S. Machado 1 1 Escola de Engenharia de Lorena, Universidade de São Paulo, P.O. Box
Conventional Superconductivity properties of the ternary boron-nitride Nb 2 BN O. V. Cigarroa 1, S. T. Renosto 1, A. J. S. Machado 1 1 Escola de Engenharia de Lorena, Universidade de São Paulo, P.O. Box
Geometrical frustration, phase transitions and dynamical order
 Geometrical frustration, phase transitions and dynamical order The Tb 2 M 2 O 7 compounds (M = Ti, Sn) Yann Chapuis PhD supervisor: Alain Yaouanc September 2009 ann Chapuis (CEA/Grenoble - Inac/SPSMS)
Geometrical frustration, phase transitions and dynamical order The Tb 2 M 2 O 7 compounds (M = Ti, Sn) Yann Chapuis PhD supervisor: Alain Yaouanc September 2009 ann Chapuis (CEA/Grenoble - Inac/SPSMS)
Anisotropic magnetic properties of TbNi 2 B 2 C single crystals
 PHYSICAL REVIEW B VOLUME 53, NUMBER 1 1 JANUARY 1996-I Anisotropic magnetic properties of TbNi 2 B 2 C single crystals C. V. Tomy, L. A. Afalfiz, M. R. Lees, J. M. Martin, and D. McK. Paul Department of
PHYSICAL REVIEW B VOLUME 53, NUMBER 1 1 JANUARY 1996-I Anisotropic magnetic properties of TbNi 2 B 2 C single crystals C. V. Tomy, L. A. Afalfiz, M. R. Lees, J. M. Martin, and D. McK. Paul Department of
The oxygen deficient Ruddlesden Popper La 3 Ni 2 O 7 d (d = 0.65) phase: Structure and properties
 Materials Research Bulletin 41 (2006) 955 960 www.elsevier.com/locate/matresbu The oxygen deficient Ruddlesden Popper La 3 Ni 2 O 7 d (d = 0.65) phase: Structure and properties Viktor V. Poltavets a, Konstantin
Materials Research Bulletin 41 (2006) 955 960 www.elsevier.com/locate/matresbu The oxygen deficient Ruddlesden Popper La 3 Ni 2 O 7 d (d = 0.65) phase: Structure and properties Viktor V. Poltavets a, Konstantin
Roger Johnson Structure and Dynamics: Displacive phase transition Lecture 9
 9.1. Summary In this Lecture we will consider structural phase transitions characterised by atomic displacements, which result in a low temperature structure that is distorted compared to a higher temperature,
9.1. Summary In this Lecture we will consider structural phase transitions characterised by atomic displacements, which result in a low temperature structure that is distorted compared to a higher temperature,
Novel Synthesis Method of Nonstoichiometric Na 2-x IrO 3 Crystal Structure, Transport and Magnetic Properties
 J. Chem. Chem. Eng. 10 (2016) 153-160 doi: 10.17265/1934-7375/2016.04.001 D DAVID PUBLISHING Novel Synthesis Method of Nonstoichiometric Na 2-x IrO 3 Crystal Structure, Katharina Rolfs* 1, Ekaterina Pomjakushina
J. Chem. Chem. Eng. 10 (2016) 153-160 doi: 10.17265/1934-7375/2016.04.001 D DAVID PUBLISHING Novel Synthesis Method of Nonstoichiometric Na 2-x IrO 3 Crystal Structure, Katharina Rolfs* 1, Ekaterina Pomjakushina
Synchrotron powder X-ray diffraction and structural analysis of Eu0.5La0.5FBiS2-xSex
 Synchrotron powder X-ray diffraction and structural analysis of Eu0.5La0.5FBiS2-xSex K. Nagasaka 1, G. Jinno 1, O. Miura 1, A. Miura 2, C. Moriyoshi 3, Y. Kuroiwa 3, Y. Mizuguchi 1 * 1. Department of Electrical
Synchrotron powder X-ray diffraction and structural analysis of Eu0.5La0.5FBiS2-xSex K. Nagasaka 1, G. Jinno 1, O. Miura 1, A. Miura 2, C. Moriyoshi 3, Y. Kuroiwa 3, Y. Mizuguchi 1 * 1. Department of Electrical
3.012 Quiz Fall points total
 3.012 Quiz 1 3.012 09.23.04 Fall 2004 100 points total Structure (50 points total): 1.. (16 points). Let s consider an ionic compound of composition A 2 B 3 in which the coordination number C NA of every
3.012 Quiz 1 3.012 09.23.04 Fall 2004 100 points total Structure (50 points total): 1.. (16 points). Let s consider an ionic compound of composition A 2 B 3 in which the coordination number C NA of every
Chemical pressure effect on superconductivity of BiS 2 -based Ce 1-x Nd x O 1-y F y BiS 2 and Nd 1-z Sm z O 1-y F y BiS 2
 Chemical pressure effect on superconductivity of BiS 2 -based Ce 1-x Nd x O 1-y F y BiS 2 and Nd 1-z Sm z O 1-y F y BiS 2 Joe Kajitani*, Takafumi Hiroi, Atsushi Omachi, Osuke Miura, and Yoshikazu Mizuguchi
Chemical pressure effect on superconductivity of BiS 2 -based Ce 1-x Nd x O 1-y F y BiS 2 and Nd 1-z Sm z O 1-y F y BiS 2 Joe Kajitani*, Takafumi Hiroi, Atsushi Omachi, Osuke Miura, and Yoshikazu Mizuguchi
Field-Temperature Evolution of Antiferromagnetic Phases in Ludvigites Ni 3-x Mn x BO 5
 Field-Temperature Evolution of Antiferromagnetic Phases in Ludvigites Ni 3-x Mn x BO 5 L. N. Bezmaternykh, E. M. Kolesnikova*, E. V. Eremin, S. N. Sofronova, N. V. Volkov, and M. S. Molokeev Kirensky Institute
Field-Temperature Evolution of Antiferromagnetic Phases in Ludvigites Ni 3-x Mn x BO 5 L. N. Bezmaternykh, E. M. Kolesnikova*, E. V. Eremin, S. N. Sofronova, N. V. Volkov, and M. S. Molokeev Kirensky Institute
Superconductivity and non-metallicity induced by doping the. topological insulators Bi 2 Se 3 and Bi 2 Te 3
 Superconductivity and non-metallicity induced by doping the topological insulators Bi 2 Se 3 and Bi 2 Te 3 Y. S. Hor 1, J. G. Checkelsky 2, D. Qu 2, N. P. Ong 2, and R. J. Cava 1 1 Department of Chemistry,
Superconductivity and non-metallicity induced by doping the topological insulators Bi 2 Se 3 and Bi 2 Te 3 Y. S. Hor 1, J. G. Checkelsky 2, D. Qu 2, N. P. Ong 2, and R. J. Cava 1 1 Department of Chemistry,
High T C copper oxide superconductors and CMR:
 High T C copper oxide superconductors and CMR: Ram Seshadri (seshadri@mrl.ucsb.edu) The Ruddlesden-Popper phases: Ruddlesden-Popper phases are intergrowths of perovskite slabs with rock salt slabs. First
High T C copper oxide superconductors and CMR: Ram Seshadri (seshadri@mrl.ucsb.edu) The Ruddlesden-Popper phases: Ruddlesden-Popper phases are intergrowths of perovskite slabs with rock salt slabs. First
X-ray Diffraction. Diffraction. X-ray Generation. X-ray Generation. X-ray Generation. X-ray Spectrum from Tube
 X-ray Diffraction Mineral identification Mode analysis Structure Studies X-ray Generation X-ray tube (sealed) Pure metal target (Cu) Electrons remover inner-shell electrons from target. Other electrons
X-ray Diffraction Mineral identification Mode analysis Structure Studies X-ray Generation X-ray tube (sealed) Pure metal target (Cu) Electrons remover inner-shell electrons from target. Other electrons
Tuning order in cuprate superconductors
 Tuning order in cuprate superconductors arxiv:cond-mat/0201401 v1 23 Jan 2002 Subir Sachdev 1 and Shou-Cheng Zhang 2 1 Department of Physics, Yale University, P.O. Box 208120, New Haven, CT 06520-8120,
Tuning order in cuprate superconductors arxiv:cond-mat/0201401 v1 23 Jan 2002 Subir Sachdev 1 and Shou-Cheng Zhang 2 1 Department of Physics, Yale University, P.O. Box 208120, New Haven, CT 06520-8120,
Ferromagnetic and spin-glass properties of single-crystalline U 2 NiSi 3
 Materials Science-Poland, Vol. 25, No. 4, 2007 Ferromagnetic and spin-glass properties of single-crystalline U 2 NiSi 3 * M. SZLAWSKA **, A. PIKUL, D. KACZOROWSKI Institute of Low Temperature and Structure
Materials Science-Poland, Vol. 25, No. 4, 2007 Ferromagnetic and spin-glass properties of single-crystalline U 2 NiSi 3 * M. SZLAWSKA **, A. PIKUL, D. KACZOROWSKI Institute of Low Temperature and Structure
University of Chinese Academy of Sciences, Beijing , China. To whom correspondence should be addressed :
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2018 NaBaM III Q 3 (M III = Al, Ga; Q = S, Se): First quaternary chalcogenides with the isolated
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2018 NaBaM III Q 3 (M III = Al, Ga; Q = S, Se): First quaternary chalcogenides with the isolated
SUPPLEMENTARY INFORMATION
 Materials and Methods Single crystals of Pr 2 Ir 2 O 7 were grown by a flux method [S1]. Energy dispersive x-ray analysis found no impurity phases, no inhomogeneities and a ratio between Pr and Ir of 1:1.03(3).
Materials and Methods Single crystals of Pr 2 Ir 2 O 7 were grown by a flux method [S1]. Energy dispersive x-ray analysis found no impurity phases, no inhomogeneities and a ratio between Pr and Ir of 1:1.03(3).
arxiv:cond-mat/ v1 [cond-mat.supr-con] 23 Feb 1999
![arxiv:cond-mat/ v1 [cond-mat.supr-con] 23 Feb 1999 arxiv:cond-mat/ v1 [cond-mat.supr-con] 23 Feb 1999](/thumbs/93/112651486.jpg) NEUTRON SCATTERING STUDY OF ELASTIC MAGNETIC SIGNALS IN SUPERCONDUCTING La 1.94 Sr 0.06 CuO 4 arxiv:cond-mat/9902319v1 [cond-mat.supr-con] 23 Feb 1999 S. Wakimoto, K. Yamada, S. Ueki, G. Shirane, Y. S.
NEUTRON SCATTERING STUDY OF ELASTIC MAGNETIC SIGNALS IN SUPERCONDUCTING La 1.94 Sr 0.06 CuO 4 arxiv:cond-mat/9902319v1 [cond-mat.supr-con] 23 Feb 1999 S. Wakimoto, K. Yamada, S. Ueki, G. Shirane, Y. S.
Keble College - Hilary 2012 Section VI: Condensed matter physics Tutorial 2 - Lattices and scattering
 Tomi Johnson Keble College - Hilary 2012 Section VI: Condensed matter physics Tutorial 2 - Lattices and scattering Please leave your work in the Clarendon laboratory s J pigeon hole by 5pm on Monday of
Tomi Johnson Keble College - Hilary 2012 Section VI: Condensed matter physics Tutorial 2 - Lattices and scattering Please leave your work in the Clarendon laboratory s J pigeon hole by 5pm on Monday of
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information (ESI) A Large Spin, Magnetically Anisotropic, Octanuclear
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information (ESI) A Large Spin, Magnetically Anisotropic, Octanuclear
Fe Co Si. Fe Co Si. Ref. p. 59] d elements and C, Si, Ge, Sn or Pb Alloys and compounds with Ge
![Fe Co Si. Fe Co Si. Ref. p. 59] d elements and C, Si, Ge, Sn or Pb Alloys and compounds with Ge Fe Co Si. Fe Co Si. Ref. p. 59] d elements and C, Si, Ge, Sn or Pb Alloys and compounds with Ge](/thumbs/72/66917236.jpg) Ref. p. 59] 1.5. 3d elements and C, Si, Ge, Sn or Pb 7 1.75 1.50 Co Si 0.8 0. 3.50 3.5 Co Si 0.8 0. H cr Magnetic field H [koe] 1.5 1.00 0.75 0.50 0.5 C C IF "A" P Frequency ωγ / e [koe] 3.00.75.50.5.00
Ref. p. 59] 1.5. 3d elements and C, Si, Ge, Sn or Pb 7 1.75 1.50 Co Si 0.8 0. 3.50 3.5 Co Si 0.8 0. H cr Magnetic field H [koe] 1.5 1.00 0.75 0.50 0.5 C C IF "A" P Frequency ωγ / e [koe] 3.00.75.50.5.00
Superconducting properties of FeSe 0.5 Te 0.5
 Superconducting properties of FeSe 0.5 Te 0.5 C.V. Tomy G. Balakrishnan and M.R. Lees Dept. of Physics, University of Warwick, UK Pradip Das and A.K. Grover Department of CMP&MS, TIFR Ravi P. Singh, Anil
Superconducting properties of FeSe 0.5 Te 0.5 C.V. Tomy G. Balakrishnan and M.R. Lees Dept. of Physics, University of Warwick, UK Pradip Das and A.K. Grover Department of CMP&MS, TIFR Ravi P. Singh, Anil
Comparison of the Crystal Structure of the Heavy-Fermion Materials
 Proceedings ICNS2001, supplement of Applied Physics A. Material Science & Processing Comparison of the Crystal Structure of the Heavy-Fermion Materials CeCoIn5,, CeRhIn5 and CeIrIn5 E. G. Moshopoulou 1*,
Proceedings ICNS2001, supplement of Applied Physics A. Material Science & Processing Comparison of the Crystal Structure of the Heavy-Fermion Materials CeCoIn5,, CeRhIn5 and CeIrIn5 E. G. Moshopoulou 1*,
Electronic states of a strongly correlated two-dimensional system, Pd(dmit) 2 salts, controlled by uni-axial strain and counter cations
 J. Phys. IV France 114 (2004) 411-417 EDP Sciences, Les Ulis DOI: 10.1051/jp4:2004114099 411 Electronic states of a strongly correlated two-dimensional system, Pd(dmit) 2 salts, controlled by uni-axial
J. Phys. IV France 114 (2004) 411-417 EDP Sciences, Les Ulis DOI: 10.1051/jp4:2004114099 411 Electronic states of a strongly correlated two-dimensional system, Pd(dmit) 2 salts, controlled by uni-axial
Structure Report for J. Reibenspies
 X-ray Diffraction Laboratory Center for Chemical Characterization and Analysis Department of Chemistry Texas A & M University Structure Report for J. Reibenspies Project Name: Sucrose Date: January 29,
X-ray Diffraction Laboratory Center for Chemical Characterization and Analysis Department of Chemistry Texas A & M University Structure Report for J. Reibenspies Project Name: Sucrose Date: January 29,
SUPPLEMENTARY INFORMATION
 Coexistence of superconductivity and antiferromagnetism in (Li 0.8 Fe 0.2 )OHFeSe superconductor X. F. Lu 1,2, N. Z. Wang 1,2, H. Wu 3,7, Y. P. Wu 1,2, D. Zhao 1,2, X. Z. Zeng 1,2, X. G. Luo 1,2,8, T.
Coexistence of superconductivity and antiferromagnetism in (Li 0.8 Fe 0.2 )OHFeSe superconductor X. F. Lu 1,2, N. Z. Wang 1,2, H. Wu 3,7, Y. P. Wu 1,2, D. Zhao 1,2, X. Z. Zeng 1,2, X. G. Luo 1,2,8, T.
New High Temperature Superconductor Phase of Y-Ba-Cu-O System
 International Journal of Advanced Research in Physical Science (IJARPS) Volume 2, Issue 7, July 2015, PP 33-39 ISSN 2349-7874 (Print) & ISSN 2349-7882 (Online) www.arcjournals.org New High Temperature
International Journal of Advanced Research in Physical Science (IJARPS) Volume 2, Issue 7, July 2015, PP 33-39 ISSN 2349-7874 (Print) & ISSN 2349-7882 (Online) www.arcjournals.org New High Temperature
High Pressure Effects on Superconductivity in the β-pyrochlore Oxides AOs 2 O 6 (A=K, Rb, Cs)
 High Pressure Effects on Superconductivity in the β-pyrochlore Oxides AOs 2 O 6 (A=K, Rb, Cs) Takaki MURAMATSU, Shigeki YONEZAWA, Yuji MURAOKA and Zenji HIROI Institute for Solid States Physics, University
High Pressure Effects on Superconductivity in the β-pyrochlore Oxides AOs 2 O 6 (A=K, Rb, Cs) Takaki MURAMATSU, Shigeki YONEZAWA, Yuji MURAOKA and Zenji HIROI Institute for Solid States Physics, University
Rare double spin canting antiferromagnetic behaviours in a. [Co 24 ] cluster
![Rare double spin canting antiferromagnetic behaviours in a. [Co 24 ] cluster Rare double spin canting antiferromagnetic behaviours in a. [Co 24 ] cluster](/thumbs/76/73822148.jpg) Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2016 Rare double spin canting antiferromagnetic behaviours in a [Co 24 ] cluster Guang-Ming Liang, Qing-Ling
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2016 Rare double spin canting antiferromagnetic behaviours in a [Co 24 ] cluster Guang-Ming Liang, Qing-Ling
The Solid State. Phase diagrams Crystals and symmetry Unit cells and packing Types of solid
 The Solid State Phase diagrams Crystals and symmetry Unit cells and packing Types of solid Learning objectives Apply phase diagrams to prediction of phase behaviour Describe distinguishing features of
The Solid State Phase diagrams Crystals and symmetry Unit cells and packing Types of solid Learning objectives Apply phase diagrams to prediction of phase behaviour Describe distinguishing features of
Supporting Information:
 Supporting Information: In Situ Synthesis of Magnetically Recyclable Graphene Supported Pd@Co Core-Shell Nanoparticles as Efficient Catalysts for Hydrolytic Dehydrogenation of Ammonia Borane Jun Wang,
Supporting Information: In Situ Synthesis of Magnetically Recyclable Graphene Supported Pd@Co Core-Shell Nanoparticles as Efficient Catalysts for Hydrolytic Dehydrogenation of Ammonia Borane Jun Wang,
M2 TP. Low-Energy Electron Diffraction (LEED)
 M2 TP Low-Energy Electron Diffraction (LEED) Guide for report preparation I. Introduction: Elastic scattering or diffraction of electrons is the standard technique in surface science for obtaining structural
M2 TP Low-Energy Electron Diffraction (LEED) Guide for report preparation I. Introduction: Elastic scattering or diffraction of electrons is the standard technique in surface science for obtaining structural
Observation of slow relaxation of the magnetization and hysteresis. loop in antiferromagnetic ordered phase of a 2D framework based on
 Observation of slow relaxation of the magnetization and hysteresis loop in antiferromagnetic ordered phase of a 2D framework based on Co II magnetic chains Shi-Yuan Zhang, a Wei Shi,* a Yanhua Lan, b Na
Observation of slow relaxation of the magnetization and hysteresis loop in antiferromagnetic ordered phase of a 2D framework based on Co II magnetic chains Shi-Yuan Zhang, a Wei Shi,* a Yanhua Lan, b Na
Bipartite magnetic parent phases in the iron oxypnictide superconductor
 M. Hiraishi 1, S. Iimura 2, K. M. Kojima 1,3*, J. Yamaura 4, H. Hiraka 1, K. Ikeda 1, P. Miao 1,3, Y. Ishikawa 1, S. Torii 1, M. Miyazaki 1, I. Yamauchi 1, A. Koda 1,3, K. Ishii 5, M. Yoshida 5,6, J. Mizuki
M. Hiraishi 1, S. Iimura 2, K. M. Kojima 1,3*, J. Yamaura 4, H. Hiraka 1, K. Ikeda 1, P. Miao 1,3, Y. Ishikawa 1, S. Torii 1, M. Miyazaki 1, I. Yamauchi 1, A. Koda 1,3, K. Ishii 5, M. Yoshida 5,6, J. Mizuki
Chemistry Institute B6, SUPRATECS, University of Liège, Sart-Tilman, B-4000 Liège, Belgium b
 Synthesis and characterization of Bi 2 Sr 2 CaCu 2 O 8 ceramics prepared in presence of sodium S. Rahier a*, S. Stassen a, R. Cloots a and M. Ausloos b a Chemistry Institute B6, SUPRATECS, University of
Synthesis and characterization of Bi 2 Sr 2 CaCu 2 O 8 ceramics prepared in presence of sodium S. Rahier a*, S. Stassen a, R. Cloots a and M. Ausloos b a Chemistry Institute B6, SUPRATECS, University of
Evolution of superconductivity in LaO 1-x F x BiS 2 prepared by high pressure technique
 Evolution of superconductivity in LaO 1-x F x BiS 2 prepared by high pressure technique K. Deguchi 1,2,3, Y. Mizuguchi 1,2,4, S. Demura 1,2,3, H. Hara 1,2,3, T. Watanabe 1,2,3, S. J. Denholme 1,2, M. Fujioka
Evolution of superconductivity in LaO 1-x F x BiS 2 prepared by high pressure technique K. Deguchi 1,2,3, Y. Mizuguchi 1,2,4, S. Demura 1,2,3, H. Hara 1,2,3, T. Watanabe 1,2,3, S. J. Denholme 1,2, M. Fujioka
Structure and Dynamics : An Atomic View of Materials
 Structure and Dynamics : An Atomic View of Materials MARTIN T. DOVE Department ofearth Sciences University of Cambridge OXFORD UNIVERSITY PRESS Contents 1 Introduction 1 1.1 Observations 1 1.1.1 Microscopic
Structure and Dynamics : An Atomic View of Materials MARTIN T. DOVE Department ofearth Sciences University of Cambridge OXFORD UNIVERSITY PRESS Contents 1 Introduction 1 1.1 Observations 1 1.1.1 Microscopic
Heterometallic M II Ln III (M = Co / Zn; Ln = Dy / Y) complexes with pentagonal bipyramidal 3d centres: syntheses, structures, and magnetic properties
 Supporting Information for Heterometallic M II Ln III (M = Co / Zn; Ln = Dy / Y) complexes with pentagonal bipyramidal 3d centres: syntheses, structures, and magnetic properties Fu-Xing Shen, Hong-Qing
Supporting Information for Heterometallic M II Ln III (M = Co / Zn; Ln = Dy / Y) complexes with pentagonal bipyramidal 3d centres: syntheses, structures, and magnetic properties Fu-Xing Shen, Hong-Qing
Specific Heat and Electrical Transport Properties of Sn 0.8 Ag 0.2 Te Superconductor
 Specific Heat and Electrical Transport Properties of Sn 0.8 Ag 0.2 Te Superconductor Yoshikazu Mizuguchi 1 *, Akira Yamada 2, Ryuji Higashinaka 2, Tatsuma D. Matsuda 2, Yuji Aoki 2, Osuke Miura 1, and
Specific Heat and Electrical Transport Properties of Sn 0.8 Ag 0.2 Te Superconductor Yoshikazu Mizuguchi 1 *, Akira Yamada 2, Ryuji Higashinaka 2, Tatsuma D. Matsuda 2, Yuji Aoki 2, Osuke Miura 1, and
*Corresponding author: Yoshikazu Mizuguchi
 Stabilization of high-tc phase of BiS2-based superconductor LaO0.5F0.5BiS2 using high-pressure synthesis Yoshikazu Mizuguchi 1*, Takafumi Hiroi 1, Joe Kajitani 1, Hiroshi Takatsu 2, Hiroaki Kadowaki 2
Stabilization of high-tc phase of BiS2-based superconductor LaO0.5F0.5BiS2 using high-pressure synthesis Yoshikazu Mizuguchi 1*, Takafumi Hiroi 1, Joe Kajitani 1, Hiroshi Takatsu 2, Hiroaki Kadowaki 2
Impeller-like dodecameric water clusters in metal organic nanotubes
 Electronic Supplementary Material (ESI) for CrystEngComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Impeller-like dodecameric water clusters in metal organic
Electronic Supplementary Material (ESI) for CrystEngComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Impeller-like dodecameric water clusters in metal organic
Structural Chemistry and Magnetic Properties of Nd 18 Li 8 Fe 5 O 39 and Nd 18 Li 8 Co 4 O 39 : the Interplay of Cation and Spin Ordering
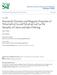 Missouri University of Science and Technology Scholars' Mine Chemistry Faculty Research & Creative Works Chemistry 12-1-2008 Structural Chemistry and Magnetic Properties of Nd 18 Li 8 Fe 5 O 39 and Nd
Missouri University of Science and Technology Scholars' Mine Chemistry Faculty Research & Creative Works Chemistry 12-1-2008 Structural Chemistry and Magnetic Properties of Nd 18 Li 8 Fe 5 O 39 and Nd
Structural Study of [Nd 0.5 (Ca 0.25 Ba 0.25 ) MnO 3 ] and [Nd 0.5 (Ca 0.25 Sr 0.25 )MnO 3 ] Perovskites at Room Temperature
![Structural Study of [Nd 0.5 (Ca 0.25 Ba 0.25 ) MnO 3 ] and [Nd 0.5 (Ca 0.25 Sr 0.25 )MnO 3 ] Perovskites at Room Temperature Structural Study of [Nd 0.5 (Ca 0.25 Ba 0.25 ) MnO 3 ] and [Nd 0.5 (Ca 0.25 Sr 0.25 )MnO 3 ] Perovskites at Room Temperature](/thumbs/82/86626971.jpg) Egypt. J. Sol., Vol. (24), No. (1), (2001) 33 Structural Study of [Nd 0.5 (Ca 0.25 Ba 0.25 ) MnO 3 ] and [Nd 0.5 (Ca 0.25 Sr 0.25 )MnO 3 ] Perovskites at Room Temperature F. F. Hanna Faculty of Petroleum
Egypt. J. Sol., Vol. (24), No. (1), (2001) 33 Structural Study of [Nd 0.5 (Ca 0.25 Ba 0.25 ) MnO 3 ] and [Nd 0.5 (Ca 0.25 Sr 0.25 )MnO 3 ] Perovskites at Room Temperature F. F. Hanna Faculty of Petroleum
BiS 2 - based superconductivity in F-substituted NdOBiS 2
 BiS 2 - based superconductivity in F-substituted NdOBiS 2 Satoshi Demura, 1,2 Yoshikazu Mizuguchi, 1,3 Keita Deguchi, 1,2 Hiroyuki Okazaki, 1 Hiroshi Hara, 1,2 Tohru Watanabe, 1,2 Saleem James Denholme,
BiS 2 - based superconductivity in F-substituted NdOBiS 2 Satoshi Demura, 1,2 Yoshikazu Mizuguchi, 1,3 Keita Deguchi, 1,2 Hiroyuki Okazaki, 1 Hiroshi Hara, 1,2 Tohru Watanabe, 1,2 Saleem James Denholme,
