Supporting Information. Jasisoquinolines A and B, Architecturally New Isoquinolines, from. a Marine Sponge Jaspis sp.
|
|
|
- Lorraine Strickland
- 5 years ago
- Views:
Transcription
1 Supporting Information Jasisoquinolines A and B, Architecturally ew Isoquinolines, from a Marine Sponge Jaspis sp. Yasufumi Imae, Kentaro Takada, Shuhei Murayama, Shigeru kada, Yuji Ise, and Shigeki Matsunaga Table of Contents I. Experimental Procedures... 3 General Procedures... 3 Animal Material... 3 Extraction and Isolation... 3 Chemical Degradation of Jasisoquinoline A (1)... 4 Preparation of (R)-MTPA Ester of 3-Methyl-1-decanol Cathepsin B Inhibitory Assay... 5 II. Tables... 6 Table S1. 1 and 13 C MR data for jasisoquinoline A (1) in CD3D Table S2. 1 and 13 C MR data for jasisoquinoline A (1) in DMS-d Table S3. 1 and 13 C MR data for jasisoquinoline B (2) in CD3D III. Figures... 9 Figure S1. 1 MR spectrum for jasisoquinoline A (1) in CD3D Figure S2. CSY spectrum for jasisoquinoline A (1) in CD3D Figure S3. TCSY spectrum for jasisoquinoline A (1) in CD3D Figure S4. SQC spectrum for jasisoquinoline A (1) in CD3D Figure S5. MBC spectrum for jasisoquinoline A (1) in CD3D Figure S6. 1 spectrum for jasisoquinoline A (1) in DMS-d Figure S7. SQC spectrum for jasisoquinoline A (1) in DMS-d Figure S8. MBC spectrum for jasisoquinoline A (1) in DMS-d Figure S9. RESY spectrum for jasisoquinoline A (1) in DMS-d Figure S10. 1 MR spectrum for jasisoquinoline B (2) in CD3D Figure S11. CSY spectrum for jasisoquinoline B (2) in CD3D Figure S12. SQC spectrum for jasisoquinoline B (2) in CD3D Figure S13. MBC spectrum for jasisoquinoline B (2) in CD3D Figure S14. 1 MR spectrum for schulzeine A (3) in CD3D S1-
2 Figure S15. 1 MR spectrum for schulzeine C (4) in CD3D Figure S16. 1 MR spectrum for MTPA ester Figure S17. Tandem FABMS fragment ions for Figure S18. Tandem FABMS analysis of 5 (m/z 0-772) Figure S19. Tandem FABMS analysis of 5 (m/z ) Figure S20. Tandem FABMS analysis of 5 (m/z ) Figure S21. Tandem FABMS analysis of 5 (m/z ) Figure S22. Tandem FABMS analysis of 5 (m/z ) Figure S23. CD spectra for schulzeines S2-
3 I. Experimental Procedures General Procedures MR spectra were recorded on a JEL delta 600 MR spectrometer at 600 Mz. 1 and 13 C MR chemical shifts were referenced to the solvent cross peak on SQC spectra at δ 3.31 and δ for CD 3 D and at δ 2.50 and δ for DMS-d 6, respectively. ESIMS spectra were measured on a JEL JMS-T100LC time-of-flight mass spectrometer. FABMS spectra were measured on a JEL JMS-700T MStation. Fluorescence for enzyme inhibition assay was detected with a Molecular Devices SPECTRA MAX fluorescence spectrometer. UV spectra were measured in Me using a Shimadzu BioSpec-1600 spectrophotometer. ptical rotations were measured on a JASC DIP-1000 degital polarimeter. CD spectra were recorded in Me on a JASC J-720 spectrapolarimeter. Animal Material The sponge sample was collected by dredging at a depth of 150 m at shima-shinsone (28 80 ; E) in The specimen was frozen after collection and kept at -20 C until extraction. Extraction and Isolation The sponge (400 g, wet weight) was extracted with Me (500 ml) and Et (500 ml). The combined extracts were concentrated and partitioned between 2 and CCl 3, and the aqueous layer was further extracted with n-bu. The CCl 3 layer was separated between 90% Me and n-hexane, and the 90% Me layer was partitioned between 60% Me and CCl 3. The n-bu layer and the 60% Me layer were combined and separated by silica gel column chromatography using a stepwise gradient (CCl 3 /Me/ 2 10/0/0 5/5/2). Fractions eluted with CCl 3 /Me/ 2 6/4/1 and 5/5/2 were combined and separated by DS flash chromatography using a stepwise gradient of aqueous Me (0 -S3-
4 100%) and aqueous MeC (70 and 80%). The 40% Me elute was chromatographed by RP-PLC (Cosmosil AR-II; mm) with 50% MeC containing 1.0 M acl 4. Active fractions were further purified by RP-PLC (Develosil C 30 -UG-5; mm) with 50% MeC containing 0.7M acl 4 to afford jasisoquinolines A (2.0 mg), B (0.7 mg), schulzeines A (4.0 mg), and C (1.5 mg). Jasisoquinoline A (1): white powder; [α] 24 D -1.1 (c 0.05, Me); UV (Me) λ max 211 nm (ε 17,000), 278 nm (1760); CD Δε (Me) (242 nm), +(284 nm); 1 and 13 C MR data, see Table S1 and S2; RFABMS m/z (M a) - [C S 3 a 2 (Δ 0.4 mmu)]. Jasisoquinoline B (2): white powder; [α] 24 D +7.6 (c 0.03, Me); UV (Me) λ max 215 nm (ε 16,200), 282 nm (2720); CD Δε (Me) (242 nm), +(284 nm); 1 and 13 C MR data, see Table S3; RFABMS m/z (M a) - [C S 3 a 2 (Δ +2.8 mmu)]. Chemical Degradation of Jasisoquinoline A (1) 1 (1 mg) was diluted with Me (1 ml) and desulfated with p-ts (catalytic amount) at room temperature for 2 h. The reaction mixture was neutralized with 10% ac 3 followed by desalting with Inertsep RP-1. The resulting triol 5 was cleaved by the treatment with 2eq. of ai 4 in 75% Me at room temperature followed by reduction with 50 mg of ab 4. The reaction mixture was quenched with Ac and extracted with C 2 Cl 2 to give diol 6 and primary alcohol 7. The presence of 6 was verified by FABMS analysis (m/z 601 [M + ] + ). Preparation of (R)-MTPA Ester of 3-Methyl-1-decanol mg portion of 7 was treated with (S)-MTPACl in C 2 Cl 2 /pyridine (1:1, 100μL). The reaction mixture was partitioned between 2 and CCl 3, and the organic layer was -S4-
5 purified by RP-PLC (Develosil C 30 -UG-5; mm) with linear gradient of % aqueous Me to give (R)-MTPA ester 8. 8: MR (CD 3 D) δ 7.72, 7.63, 4.22 (1), 3.64 (Me), 1.69 (3), 1.44 (2), (4 11). Cathepsin B Inhibitory Assay Cathepsin B inhibitory assay was carried out according to the modified method of iwasa et al. 1 Stock solution of bovine catheosin B (Sigma Chemical Company; 1unit/mL in 0.05 M MES p 6.0 and 0.1% Brij-35) was diluted by 100 times with the buffer. 50 μl of 25μM substrate solution (Z-Arg-Arg-AMC; Peptide Institute,Inc. in DMS) was added to the mixture of 100 μl cathepsin B solution and 4 μl of test sample solution. The mixture was incubated at 37 C for 30 min. The fluorescence of the reaction mixture was measured with an excitation at 345 nm and an emission at 440 nm. 1 T. iwasa, J. Fujita-Yoshigaki, M. Shirouzu,. Koide, T. Sawada, S. Sakiyama, S. Yokokawa, Cancer Lett. 1993, 69, S5-
6 II. Tables Table S1. 1 and 13 C MR data for jasisoquinoline A (1) in CD 3 D. jasisoquinoline A (1) in CD 3 D 1 13 C MBC 1 b (brs), 3.03 a a, 2.76 (brd, 13.3) a 4a (brs) , 6, 7, 8a (brs) , 6, a (m), 2.28 (m) (m) (m) (m) (quin, 5.9) (m) , (m) (m) c , 26, , 1.41 (m) (m) , 1.18 (m) (m) (m) , (t, 7.0) , (d, 6.4) , 27, 28 1' b 2' 3' 3.51, 3.43 (dt, 6.9, 13.3) ', 5' 4' 2.64 (t, 6.8) ', 5', 6', 10' 5' ' 6.10 (brd, 2.1) ', 8', 10' 7' ' 6.12 (brd, 2.1) ', 9' 9' ' 6.10 (brd, 2.1) ', 8', 9' a Signals overlapped. b Signals were undetectable in CD 3 D. c This signal overlapped with D signal. -S6-
7 Table S2. 1 and 13 C MR data for jasisoquinoline A (1) in DMS-d 6. a jasisoquinoline A (1) in DMS-d C MBC (m), 2.93 (brd, 7.75) , 4a (brs), 2.38 (brs) a 4a (d, 2.4) , 6, 7, 8a (brs) (d, 2.4) , 8, 8a (brs) 8a (m), 1.8 (m) , 8a, 10, 1' (m) (m) (m) (m) (quin, 5.4) (m) , (m) (dt, 8.3, 4.2) (dt, 9.5, 3.6) (m), 1.24 (m) (brs) (m), 1.06 (m) (m) (m) , (t, 7.2) , (d, 6.0) ' ' 8.78 (brd, 2.4) 1', 3' 3' 3.30 b ', 4', 5' 4' 2.55 (t, 7.2) ', 6', 10' 5' ' 6.05 (d, 1.8) ', 7', 8', 9', 10' 7' ' (brs) 8' 6.04 (t, 1.8) ', 9' 9' ' (brs) 10' 6.05 (d, 1.8) ', 6', 7', 8', 9' a Contaminated Ac signals were detected: 1 MR δ 1.88 (2); 13 C MR δ (C1), 21.6 (C2). b This signal overlapped with D signal. -S7-
8 Table S3. 1 and 13 C MR data for jasisoquinoline B (2) in CD 3 D. jasisoquinoline B (2) in CD 3 D , , a a a , ' a 2' 3' 3.49, ' ' ' ' ' ' ' a Signals were undetectable in CD 3 D. a 13 C -S8-
9 III. Figures Figure S1. 1 MR spectrum for jasisoquinoline A (1) in CD 3 D. 8 S 3 a a 3 S a 3 S Jasisoquinoline A (1) -S9-
10 Figure S2. CSY spectrum for jasisoquinoline A (1) in CD 3 D. 8 S 3 a a 3 S a 3 S Jasisoquinoline A (1) -S10-
11 Figure S3. TCSY spectrum for jasisoquinoline A (1) in CD 3 D. 8 S 3 a a 3 S a 3 S Jasisoquinoline A (1) -S11-
12 Figure S4. SQC spectrum for jasisoquinoline A (1) in CD 3 D. 8 S 3 a a 3 S a 3 S Jasisoquinoline A (1) -S12-
13 Figure S5. MBC spectrum for jasisoquinoline A (1) in CD 3 D. 8 S 3 a a 3 S a 3 S Jasisoquinoline A (1) -S13-
14 Figure S6. 1 spectrum for jasisoquinoline A (1) in DMS-d 6. 8 S 3 a a 3 S a 3 S Jasisoquinoline A (1) -S14-
15 Figure S7. SQC spectrum for jasisoquinoline A (1) in DMS-d 6. 8 S 3 a a 3 S a 3 S Jasisoquinoline A (1) -S15-
16 Figure S8. MBC spectrum for jasisoquinoline A (1) in DMS-d 6. 8 S 3 a a 3 S a 3 S Jasisoquinoline A (1) -S16-
17 Figure S9. RESY spectrum for jasisoquinoline A (1) in DMS-d 6 8 S 3 a a 3 S a 3 S Jasisoquinoline A (1) -S17-
18 Figure S10. 1 MR spectrum for jasisoquinoline B (2) in CD 3 D. 8 S 3 a a 3 S a 3 S Jasisoquinoline B (2) -S18-
19 Figure S11. CSY spectrum for jasisoquinoline B (2) in CD 3 D. 8 S 3 a a 3 S a 3 S Jasisoquinoline B (2) -S19-
20 Figure S12. SQC spectrum for jasisoquinoline B (2) in CD 3 D. 8 S 3 a a 3 S a 3 S Jasisoquinoline B (2) -S20-
21 Figure S13. MBC spectrum for jasisoquinoline B (2) in CD 3 D. 8 S 3 a a 3 S a 3 S Jasisoquinoline B (2) -S21-
22 Figure S14. 1 MR spectrum for schulzeine A (3) in CD 3 D. 9 S 3 a a 3 S a 3 S Schulzeine A (3) -S22-
23 Figure S15. 1 MR spectrum for schulzeine C (4) in CD 3 D. 9 S 3 a a 3 S a 3 S Schulzeine C (4) -S23-
24 Figure S16. 1 MR spectrum for MTPA ester 8. -S24-
25 Figure S17. Tandem FABMS fragment ions for (C 2 ) S25-
26 Figure S18. Tandem FABMS analysis of 5 (m/z 0-772). -S26-
27 Figure S19. Tandem FABMS analysis of 5 (m/z ). -S27-
28 Figure S20. Tandem FABMS analysis of 5 (m/z ). -S28-
29 Figure S21. Tandem FABMS analysis of 5 (m/z ). -S29-
30 Figure S22. Tandem FABMS analysis of 5 (m/z ). -S30-
31 Figure S23. CD spectra for schulzeines 9 S 3 a a 3 S a 3 S RESY P-helicity 9 S 3 a a 3 S a 3 S RESY M-helicity -S31-
Supporting Information for
 Supporting Information for Schilancitrilactones A C: Three Unique Nortriterpenoids from Schisandra lancifolia Xiao Luo,, Yi-Ming Shi,, Rong-ua Luo, Shi-ong Luo, Xiao-Nian Li, Rui-Rui Wang, Sheng-ong Li,
Supporting Information for Schilancitrilactones A C: Three Unique Nortriterpenoids from Schisandra lancifolia Xiao Luo,, Yi-Ming Shi,, Rong-ua Luo, Shi-ong Luo, Xiao-Nian Li, Rui-Rui Wang, Sheng-ong Li,
Supporting Information
 Supporting Information The Bulky Side Chain of Antillatoxin is Important for Potent Toxicity: Rational Design of Photoresponsive Cytotoxins Based on SAR Study Ken kura, Shigeru Matsuoka and Masayuki Inoue*
Supporting Information The Bulky Side Chain of Antillatoxin is Important for Potent Toxicity: Rational Design of Photoresponsive Cytotoxins Based on SAR Study Ken kura, Shigeru Matsuoka and Masayuki Inoue*
Photooxidations of 2-(γ,ε-dihydroxyalkyl) furans in Water: Synthesis of DE-Bicycles of the Pectenotoxins
 S1 Photooxidations of 2-(γ,ε-dihydroxyalkyl) furans in Water: Synthesis of DE-Bicycles of the Pectenotoxins Antonia Kouridaki, Tamsyn Montagnon, Maria Tofi and Georgios Vassilikogiannakis* Department of
S1 Photooxidations of 2-(γ,ε-dihydroxyalkyl) furans in Water: Synthesis of DE-Bicycles of the Pectenotoxins Antonia Kouridaki, Tamsyn Montagnon, Maria Tofi and Georgios Vassilikogiannakis* Department of
Supporting Information for. Singlet oxygen initiated cascade transformation of a simple difuran into the key ABC motif of the pectenotoxins
 Supporting Information for Singlet oxygen initiated cascade transformation of a simple difuran into the key ABC motif of the pectenotoxins Georgios Vassilikogiannakis, * Ioanna Alexopoulou, Maria Tofi
Supporting Information for Singlet oxygen initiated cascade transformation of a simple difuran into the key ABC motif of the pectenotoxins Georgios Vassilikogiannakis, * Ioanna Alexopoulou, Maria Tofi
Supporting Information
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 214 Supporting Information Rapid and sensitive detection of acrylic acid using a novel fluorescence
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 214 Supporting Information Rapid and sensitive detection of acrylic acid using a novel fluorescence
Supplementary Information. Novel Stereocontrolled Amidoglycosylation of Alcohols with Acetylated Glycals and Sulfamate Ester
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supplementary Information Novel Stereocontrolled Amidoglycosylation of Alcohols with Acetylated
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supplementary Information Novel Stereocontrolled Amidoglycosylation of Alcohols with Acetylated
Novel Water-Soluble Near-Infrared Cyanine Dyes: Synthesis, Spectral Properties, and Use in the Preparation of Internally Quenched Fluorescent Probes
 ovel Water-Soluble ear-infrared Cyanine Dyes: Synthesis, Spectral Properties, and Use in the Preparation of Internally Quenched Fluorescent Probes Cédric Bouteiller,,, Guillaume Clavé,,, Aude Bernardin,,
ovel Water-Soluble ear-infrared Cyanine Dyes: Synthesis, Spectral Properties, and Use in the Preparation of Internally Quenched Fluorescent Probes Cédric Bouteiller,,, Guillaume Clavé,,, Aude Bernardin,,
Department of Chemistry, Colorado State University, Fort Collins, Colorado University of Colorado Cancer Center, Aurora, Colorado 80045
 Improved Biomimetic Total Synthesis of d,l-stephacidin A Thomas J. Greshock 1 and Robert M. Williams 1,2 * 1 Department of Chemistry, Colorado State University, Fort Collins, Colorado 80523 2 University
Improved Biomimetic Total Synthesis of d,l-stephacidin A Thomas J. Greshock 1 and Robert M. Williams 1,2 * 1 Department of Chemistry, Colorado State University, Fort Collins, Colorado 80523 2 University
Corygaline A, Hexahydrobenzophenanthridine Alkaloid with. Unusual Carbon Skeleton from Corydalis bungeana Turcz.
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2018 Supporting Information for Corygaline A, Hexahydrobenzophenanthridine Alkaloid
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2018 Supporting Information for Corygaline A, Hexahydrobenzophenanthridine Alkaloid
Supporting Information For:
 Supporting Information For: Peptidic α-ketocarboxylic Acids and Sulfonamides as Inhibitors of Protein Tyrosine Phosphatases Yen Ting Chen, Jian Xie, and Christopher T. Seto* Department of Chemistry, Brown
Supporting Information For: Peptidic α-ketocarboxylic Acids and Sulfonamides as Inhibitors of Protein Tyrosine Phosphatases Yen Ting Chen, Jian Xie, and Christopher T. Seto* Department of Chemistry, Brown
Ratiometric and intensity-based zinc sensors built on rhodol and rhodamine platforms
 Supporting Information Ratiometric and intensity-based zinc sensors built on rhodol and rhodamine platforms Elisa Tomat and Stephen J. Lippard* Department of Chemistry, Massachusetts Institute of Technology,
Supporting Information Ratiometric and intensity-based zinc sensors built on rhodol and rhodamine platforms Elisa Tomat and Stephen J. Lippard* Department of Chemistry, Massachusetts Institute of Technology,
Supporting Information
 Supporting Information Carboxylate Anions Accelerate Pyrrolidinopyridine (PPy)-Catalyzed Acylation: Catalytic Site-Selective Acylation of a Carbohydrate by In Situ Counter Anion Exchange Masanori Yanagi,
Supporting Information Carboxylate Anions Accelerate Pyrrolidinopyridine (PPy)-Catalyzed Acylation: Catalytic Site-Selective Acylation of a Carbohydrate by In Situ Counter Anion Exchange Masanori Yanagi,
The First Asymmetric Total Syntheses and. Determination of Absolute Configurations of. Xestodecalactones B and C
 Supporting Information The First Asymmetric Total Syntheses and Determination of Absolute Configurations of Xestodecalactones B and C Qiren Liang, Jiyong Zhang, Weiguo Quan, Yongquan Sun, Xuegong She*,,
Supporting Information The First Asymmetric Total Syntheses and Determination of Absolute Configurations of Xestodecalactones B and C Qiren Liang, Jiyong Zhang, Weiguo Quan, Yongquan Sun, Xuegong She*,,
Solid-Supported DNA for Asymmetric Synthesis: a Stepping Stone toward Practical Applications
 Solid-Supported DA for Asymmetric Synthesis: a Stepping Stone toward Practical Applications Soyoung Park, * a Keiichi Ikehata, a and iroshi Sugiyama*,a,b,c a Department of Chemistry, Graduate School of
Solid-Supported DA for Asymmetric Synthesis: a Stepping Stone toward Practical Applications Soyoung Park, * a Keiichi Ikehata, a and iroshi Sugiyama*,a,b,c a Department of Chemistry, Graduate School of
Supporting Information
 Supporting Information Efficient Short Step Synthesis of Corey s Tamiflu Intermediate Nsiama Tienabe Kipassa, Hiroaki kamura, * Kengo Kina, Tetsuo Iwagawa, and Toshiyuki Hamada Department of Chemistry
Supporting Information Efficient Short Step Synthesis of Corey s Tamiflu Intermediate Nsiama Tienabe Kipassa, Hiroaki kamura, * Kengo Kina, Tetsuo Iwagawa, and Toshiyuki Hamada Department of Chemistry
Curtius-Like Rearrangement of Iron-Nitrenoid Complex and. Application in Biomimetic Synthesis of Bisindolylmethanes
 Supporting Information Curtius-Like Rearrangement of Iron-itrenoid Complex and Application in Biomimetic Synthesis of Bisindolylmethanes Dashan Li,, Ting Wu,, Kangjiang Liang,, and Chengfeng Xia*,, State
Supporting Information Curtius-Like Rearrangement of Iron-itrenoid Complex and Application in Biomimetic Synthesis of Bisindolylmethanes Dashan Li,, Ting Wu,, Kangjiang Liang,, and Chengfeng Xia*,, State
Multistep Electron Transfer Systems Based. on Silicon Phthalocyanine, [60]Fullerene and. Trinitrofluorenone
![Multistep Electron Transfer Systems Based. on Silicon Phthalocyanine, [60]Fullerene and. Trinitrofluorenone Multistep Electron Transfer Systems Based. on Silicon Phthalocyanine, [60]Fullerene and. Trinitrofluorenone](/thumbs/91/105731933.jpg) Supporting Information Multistep Electron Transfer Systems Based on Silicon Phthalocyanine, [60]Fullerene and Trinitrofluorenone Luis Martín-Gomis, a Kei hkubo, b Fernando Fernández-Lázaro, a Shunichi
Supporting Information Multistep Electron Transfer Systems Based on Silicon Phthalocyanine, [60]Fullerene and Trinitrofluorenone Luis Martín-Gomis, a Kei hkubo, b Fernando Fernández-Lázaro, a Shunichi
Poly(4-vinylimidazolium)s: A Highly Recyclable Organocatalyst Precursor for. Benzoin Condensation Reaction
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 24 Supporting Information Poly(4-vinylimidazolium)s: A Highly Recyclable rganocatalyst Precursor
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 24 Supporting Information Poly(4-vinylimidazolium)s: A Highly Recyclable rganocatalyst Precursor
Phil S. Baran*, Jeremy M. Richter and David W. Lin SUPPORTING INFORMATION
 Direct Coupling of Pyrroles with Carbonyl Compounds: Short, Enantioselective Synthesis of (S)-Ketorolac Phil S. Baran*, Jeremy M. Richter and David W. Lin SUPPRTIG IFRMATI General Procedures. All reactions
Direct Coupling of Pyrroles with Carbonyl Compounds: Short, Enantioselective Synthesis of (S)-Ketorolac Phil S. Baran*, Jeremy M. Richter and David W. Lin SUPPRTIG IFRMATI General Procedures. All reactions
Supporting Information
 Supporting Information Responsive Prodrug Self-Assembled Vesicles for Targeted Chemotherapy in Combination with Intracellular Imaging Hongzhong Chen, Huijun Phoebe Tham,, Chung Yen Ang, Qiuyu Qu, Lingzhi
Supporting Information Responsive Prodrug Self-Assembled Vesicles for Targeted Chemotherapy in Combination with Intracellular Imaging Hongzhong Chen, Huijun Phoebe Tham,, Chung Yen Ang, Qiuyu Qu, Lingzhi
A "turn-on" coumarin-based fluorescent sensor with high selectivity for mercury ions in aqueous media
 A "turn-on" coumarin-based fluorescent sensor with high selectivity for mercury ions in aqueous media Styliani Voutsadaki, George K. Tsikalas, Emmanuel Klontzas, George E. Froudakis, and Haralambos E.
A "turn-on" coumarin-based fluorescent sensor with high selectivity for mercury ions in aqueous media Styliani Voutsadaki, George K. Tsikalas, Emmanuel Klontzas, George E. Froudakis, and Haralambos E.
Supporting Information for DOI: /s Georg Thieme Verlag KG Stuttgart New York Thieme
 Supporting Information for DI: 10.1055/s-0036-1588866 Georg Thieme Verlag KG Stuttgart ew York 2017 Thieme Base catalyzed transcarbamoylation Benoît Rhoné a,b,c Vincent Semetey a,b a PSL Research University,
Supporting Information for DI: 10.1055/s-0036-1588866 Georg Thieme Verlag KG Stuttgart ew York 2017 Thieme Base catalyzed transcarbamoylation Benoît Rhoné a,b,c Vincent Semetey a,b a PSL Research University,
Rational design of a ratiometric fluorescent probe with a large emission shift for the facile detection of Hg 2+
 Rational design of a ratiometric fluorescent probe with a large emission shift for the facile detection of Hg 2+ Weimin Xuan, a Chen Chen, b Yanting Cao, a Wenhan He, a Wei Jiang, a Kejian Li, b* and Wei
Rational design of a ratiometric fluorescent probe with a large emission shift for the facile detection of Hg 2+ Weimin Xuan, a Chen Chen, b Yanting Cao, a Wenhan He, a Wei Jiang, a Kejian Li, b* and Wei
Supplementary Figure 2. Full power on times. Histogram showing on times of bursts with 100 pm 1, 100 pm 2 and 1 nm Et 3 N at full laser power.
 S1 Supplementary Figures Supplementary Figure 1. Time-correlated still frame images. Expanded still frames images from TIRFM video of CuAAC of 1 and 2 and corresponding intensity trajectory of a single
S1 Supplementary Figures Supplementary Figure 1. Time-correlated still frame images. Expanded still frames images from TIRFM video of CuAAC of 1 and 2 and corresponding intensity trajectory of a single
Supporting Information for Synthesis of C(3) Benzofuran Derived Bis-Aryl Quaternary Centers: Approaches to Diazonamide A
 Fuerst et al. Synthesis of C(3) Benzofuran Derived Bis-Aryl Quaternary Centers: Approaches to Diazonamide A S1 Supporting Information for Synthesis of C(3) Benzofuran Derived Bis-Aryl Quaternary Centers:
Fuerst et al. Synthesis of C(3) Benzofuran Derived Bis-Aryl Quaternary Centers: Approaches to Diazonamide A S1 Supporting Information for Synthesis of C(3) Benzofuran Derived Bis-Aryl Quaternary Centers:
Supporting Information
 An Improved ynthesis of the Pyridine-Thiazole Cores of Thiopeptide Antibiotics Virender. Aulakh, Marco A. Ciufolini* Department of Chemistry, University of British Columbia 2036 Main Mall, Vancouver, BC
An Improved ynthesis of the Pyridine-Thiazole Cores of Thiopeptide Antibiotics Virender. Aulakh, Marco A. Ciufolini* Department of Chemistry, University of British Columbia 2036 Main Mall, Vancouver, BC
Supporting Information. Light-Induced Hydrogen Sulfide release from Caged gem-dithiols
 Supporting Information Light-Induced Hydrogen Sulfide release from Caged gem-dithiols elmi O. Devarie-Baez, Powell E. Bagdon, Bo Peng, Yu Zhao, Chung-Min Park and Ming Xian* Department of Chemistry, Washington
Supporting Information Light-Induced Hydrogen Sulfide release from Caged gem-dithiols elmi O. Devarie-Baez, Powell E. Bagdon, Bo Peng, Yu Zhao, Chung-Min Park and Ming Xian* Department of Chemistry, Washington
Electronic Supplementary Information. Discovery of 7-hydroxyaporphines as conformational restricted
 Electronic Supplementary Material (ESI) for MedChemComm. This journal is The Royal Society of Chemistry 208 Electronic Supplementary Information Discovery of 7-hydroxyaporphines as conformational restricted
Electronic Supplementary Material (ESI) for MedChemComm. This journal is The Royal Society of Chemistry 208 Electronic Supplementary Information Discovery of 7-hydroxyaporphines as conformational restricted
Formal Total Synthesis of Optically Active Ingenol via Ring-Closing Olefin Metathesis
 Formal Total Synthesis of Optically Active Ingenol via Ring-Closing Olefin Metathesis Kazushi Watanabe, Yuto Suzuki, Kenta Aoki, Akira Sakakura, Kiyotake Suenaga, and Hideo Kigoshi* Department of Chemistry,
Formal Total Synthesis of Optically Active Ingenol via Ring-Closing Olefin Metathesis Kazushi Watanabe, Yuto Suzuki, Kenta Aoki, Akira Sakakura, Kiyotake Suenaga, and Hideo Kigoshi* Department of Chemistry,
SUPPLEMENTARY INFORMATION
 Supplementary Method Synthesis of 2-alkyl-MPT(R) General information (R) enantiomer of 2-alkyl (18:1) MPT (hereafter designated as 2-alkyl- MPT(R)), was synthesized as previously described 1, with some
Supplementary Method Synthesis of 2-alkyl-MPT(R) General information (R) enantiomer of 2-alkyl (18:1) MPT (hereafter designated as 2-alkyl- MPT(R)), was synthesized as previously described 1, with some
SUPPORTING INFORMATION
 SUPPORTING INFORMATION Synthesis of Functionalized Thia Analogues of Phlorins and Covalently Linked Phlorin-Porphyrin Dyads Iti Gupta a, Roland Fröhlich b and Mangalampalli Ravikanth *a a Department of
SUPPORTING INFORMATION Synthesis of Functionalized Thia Analogues of Phlorins and Covalently Linked Phlorin-Porphyrin Dyads Iti Gupta a, Roland Fröhlich b and Mangalampalli Ravikanth *a a Department of
Parallel sheet structure in cyclopropane γ-peptides stabilized by C-H O hydrogen bonds
 Parallel sheet structure in cyclopropane γ-peptides stabilized by C- hydrogen bonds M. Khurram N. Qureshi and Martin D. Smith* Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge
Parallel sheet structure in cyclopropane γ-peptides stabilized by C- hydrogen bonds M. Khurram N. Qureshi and Martin D. Smith* Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge
Indium Triflate-Assisted Nucleophilic Aromatic Substitution Reactions of. Nitrosobezene-Derived Cycloadducts with Alcohols
 Supporting Information Indium Triflate-Assisted ucleophilic Aromatic Substitution Reactions of itrosobezene-derived Cycloadducts with Alcohols Baiyuan Yang and Marvin J. Miller* Department of Chemistry
Supporting Information Indium Triflate-Assisted ucleophilic Aromatic Substitution Reactions of itrosobezene-derived Cycloadducts with Alcohols Baiyuan Yang and Marvin J. Miller* Department of Chemistry
Synthetic Studies on Norissolide; Enantioselective Synthesis of the Norrisane Side Chain
 rganic Lett. (Supporting Information) 1 Synthetic Studies on Norissolide; Enantioselective Synthesis of the Norrisane Side Chain Charles Kim, Richard Hoang and Emmanuel A. Theodorakis* Department of Chemistry
rganic Lett. (Supporting Information) 1 Synthetic Studies on Norissolide; Enantioselective Synthesis of the Norrisane Side Chain Charles Kim, Richard Hoang and Emmanuel A. Theodorakis* Department of Chemistry
Electronic Supplementary Information for Catalytic Asymmetric Hydrophosphonylation of Ynones
 Electronic Supplementary Information for Catalytic Asymmetric Hydrophosphonylation of Ynones Daisuke Uraguchi, Takaki Ito, Shinji Nakamura, and Takashi oi* Department of Applied Chemistry, Graduate School
Electronic Supplementary Information for Catalytic Asymmetric Hydrophosphonylation of Ynones Daisuke Uraguchi, Takaki Ito, Shinji Nakamura, and Takashi oi* Department of Applied Chemistry, Graduate School
Simplified platensimycin analogues as antibacterial agents
 Simplified platensimycin analogues as antibacterial agents Dragan Krsta, a Caron Ka, a Ian T. Crosby, a Ben Capuano a and David T. Manallack a * a Medicinal Chemistry and Drug Action, Monash Institute
Simplified platensimycin analogues as antibacterial agents Dragan Krsta, a Caron Ka, a Ian T. Crosby, a Ben Capuano a and David T. Manallack a * a Medicinal Chemistry and Drug Action, Monash Institute
Electronic Supplementary Material (ESI) for Medicinal Chemistry Communications This journal is The Royal Society of Chemistry 2012
 Supporting Information. Experimental Section: Summary scheme H 8 H H H 9 a H C 3 1 C 3 A H H b c C 3 2 3 C 3 H H d e C 3 4 5 C 3 H f g C 2 6 7 C 2 H a C 3 B H c C 3 General experimental details: All solvents
Supporting Information. Experimental Section: Summary scheme H 8 H H H 9 a H C 3 1 C 3 A H H b c C 3 2 3 C 3 H H d e C 3 4 5 C 3 H f g C 2 6 7 C 2 H a C 3 B H c C 3 General experimental details: All solvents
hydroxyanthraquinones related to proisocrinins
 Supporting Information for Regiodefined synthesis of brominated hydroxyanthraquinones related to proisocrinins Joyeeta Roy, Tanushree Mal, Supriti Jana and Dipakranjan Mal* Address: Department of Chemistry,
Supporting Information for Regiodefined synthesis of brominated hydroxyanthraquinones related to proisocrinins Joyeeta Roy, Tanushree Mal, Supriti Jana and Dipakranjan Mal* Address: Department of Chemistry,
Supporting Information
 Supporting Information Syntheses and characterizations: Compound 1 was synthesized according to Scheme S-1. Scheme S-1 2 N N 5 i N 4 P Et Et iii N 6 ii P Et Et iv v, vi N N i) Fmoc-Su, DIPEA, Acetone;
Supporting Information Syntheses and characterizations: Compound 1 was synthesized according to Scheme S-1. Scheme S-1 2 N N 5 i N 4 P Et Et iii N 6 ii P Et Et iv v, vi N N i) Fmoc-Su, DIPEA, Acetone;
Supporting Information. Table of Contents. 1. General Notes Experimental Details 3-12
 Supporting Information Table of Contents page 1. General Notes 2 2. Experimental Details 3-12 3. NMR Support for Timing of Claisen/Diels-Alder/Claisen 13 4. 1 H and 13 C NMR 14-37 General Notes All reagents
Supporting Information Table of Contents page 1. General Notes 2 2. Experimental Details 3-12 3. NMR Support for Timing of Claisen/Diels-Alder/Claisen 13 4. 1 H and 13 C NMR 14-37 General Notes All reagents
Figure S1 - Enzymatic titration of HNE and GS-HNE.
 Figure S1 - Enzymatic titration of HNE and GS-HNE. Solutions of HNE and GS-HNE were titrated through their reduction to the corresponding alchools catalyzed by AR, monitoring the decrease in absorbance
Figure S1 - Enzymatic titration of HNE and GS-HNE. Solutions of HNE and GS-HNE were titrated through their reduction to the corresponding alchools catalyzed by AR, monitoring the decrease in absorbance
VEBSA, RRR-VEPSA, RRR-VEBPA, RRR-VEPPA) was obtained from the hydrolysis of the
 Supplemental Material and thods Synthesis of α-vitamin E analogs All racemic α-tocopherol (synthetic compound) was purchased from Acros (Morris Plains, NJ) and was used to synthesize the all-racemic α-tocopherol
Supplemental Material and thods Synthesis of α-vitamin E analogs All racemic α-tocopherol (synthetic compound) was purchased from Acros (Morris Plains, NJ) and was used to synthesize the all-racemic α-tocopherol
Electronic Supplementary Information for
 Electronic Supplementary Information for Sequence Selective Dual-Emission Detection of (i, i+1) Bis-Phosphorylated Peptide Using Diazastilbene-Type Zn(II)-Dpa Chemosensor Yoshiyuki Ishida, Masa-aki Inoue,
Electronic Supplementary Information for Sequence Selective Dual-Emission Detection of (i, i+1) Bis-Phosphorylated Peptide Using Diazastilbene-Type Zn(II)-Dpa Chemosensor Yoshiyuki Ishida, Masa-aki Inoue,
Molecular Imaging of Labile Iron(II) Pools in Living Cells with a Turn-on Fluorescent Probe
 Supporting Information for Molecular Imaging of Labile Iron(II) Pools in Living Cells with a Turn-on Fluorescent Probe Ho Yu Au-Yeung, Jefferson Chan, Teera Chantarojsiri and Christopher J. Chang* Departments
Supporting Information for Molecular Imaging of Labile Iron(II) Pools in Living Cells with a Turn-on Fluorescent Probe Ho Yu Au-Yeung, Jefferson Chan, Teera Chantarojsiri and Christopher J. Chang* Departments
Synthesis of fluorophosphonylated acyclic nucleotide analogues via Copper (I)- catalyzed Huisgen 1-3 dipolar cycloaddition
 Synthesis of fluorophosphonylated acyclic nucleotide analogues via Copper (I)- catalyzed Huisgen 1-3 dipolar cycloaddition Sonia Amel Diab, Antje Hienzch, Cyril Lebargy, Stéphante Guillarme, Emmanuel fund
Synthesis of fluorophosphonylated acyclic nucleotide analogues via Copper (I)- catalyzed Huisgen 1-3 dipolar cycloaddition Sonia Amel Diab, Antje Hienzch, Cyril Lebargy, Stéphante Guillarme, Emmanuel fund
ARTEMETHER AND LUMEFANTRINE TABLETS: Final text for addition to The International Pharmacopoeia (July 2008)
 July 2008 ARTEMETER AND LUMEFANTRINE TABLETS: Final text for addition to The International Pharmacopoeia (July 2008) This monograph was adopted at the Forty-second W Expert Committee on Specifications
July 2008 ARTEMETER AND LUMEFANTRINE TABLETS: Final text for addition to The International Pharmacopoeia (July 2008) This monograph was adopted at the Forty-second W Expert Committee on Specifications
Supporting Information Solid Phase Synthesis of Ultra-Photostable Cyanine NIR dye library
 Supporting Information Solid Phase Synthesis of Ultra-Photostable Cyanine IR dye library Raj Kumar Das, a Animesh Samanta, a Hyung-Ho Ha, b and Young-Tae Chang a,c * a Department of Chemistry & MedChem
Supporting Information Solid Phase Synthesis of Ultra-Photostable Cyanine IR dye library Raj Kumar Das, a Animesh Samanta, a Hyung-Ho Ha, b and Young-Tae Chang a,c * a Department of Chemistry & MedChem
Synthesis of Dihydroquinoline Based Merocyanines as Naked Eye and Fluorogenic sensors for Hydrazine Hydrate in Aqueous Medium
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Synthesis of Dihydroquinoline Based Merocyanines as Naked Eye and Fluorogenic sensors for Hydrazine
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Synthesis of Dihydroquinoline Based Merocyanines as Naked Eye and Fluorogenic sensors for Hydrazine
Photostability of a dyad of magnesium porphyrin and fullerene and its application to photocurrent conversion
 Supplementary Information Photostability of a dyad of magnesium porphyrin and fullerene and its application to photocurrent conversion Takahiko Ichiki, Yutaka Matsuo,* and Eiichi akamura* Department of
Supplementary Information Photostability of a dyad of magnesium porphyrin and fullerene and its application to photocurrent conversion Takahiko Ichiki, Yutaka Matsuo,* and Eiichi akamura* Department of
Supporting Information for
 Supporting Information for Room Temperature Palladium-Catalyzed Arylation of Indoles icholas R. Deprez, Dipannita Kalyani, Andrew Krause, and Melanie S. Sanford* University of Michigan Department of Chemistry,
Supporting Information for Room Temperature Palladium-Catalyzed Arylation of Indoles icholas R. Deprez, Dipannita Kalyani, Andrew Krause, and Melanie S. Sanford* University of Michigan Department of Chemistry,
Supporting Information
 Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2012 Subcellular Localization and Activity of Gambogic Acid Gianni Guizzunti,* [b] Ayse Batova, [a] Oraphin Chantarasriwong,
Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2012 Subcellular Localization and Activity of Gambogic Acid Gianni Guizzunti,* [b] Ayse Batova, [a] Oraphin Chantarasriwong,
Electronic Supplementary Information (ESI)
 Electronic Supplementary Information (ESI) A thin-layered chromatography plate prepared from naphthalimide-based receptor immobilized SiO 2 nanoparticles as a portable chemosensor and adsorbent for Pb
Electronic Supplementary Information (ESI) A thin-layered chromatography plate prepared from naphthalimide-based receptor immobilized SiO 2 nanoparticles as a portable chemosensor and adsorbent for Pb
Development of Fluorescein Analogue, TokyoMagenta, as a Novel Scaffold. for Fluorescence Probes in Red Region
 Supplementary Material (ESI) for Chemical Communications Supporting Information for: Development of Fluorescein Analogue, TokyoMagenta, as a Novel Scaffold for Fluorescence Probes in Red Region Takahiro
Supplementary Material (ESI) for Chemical Communications Supporting Information for: Development of Fluorescein Analogue, TokyoMagenta, as a Novel Scaffold for Fluorescence Probes in Red Region Takahiro
SYNTHESIS OF A 3-THIOMANNOSIDE
 Supporting Information SYNTHESIS OF A 3-THIOMANNOSIDE María B Comba, Alejandra G Suárez, Ariel M Sarotti, María I Mangione* and Rolando A Spanevello and Enrique D V Giordano Instituto de Química Rosario,
Supporting Information SYNTHESIS OF A 3-THIOMANNOSIDE María B Comba, Alejandra G Suárez, Ariel M Sarotti, María I Mangione* and Rolando A Spanevello and Enrique D V Giordano Instituto de Química Rosario,
Responsive supramolecular polymer formed by orthogonal metal-coordination and cryptand-based host guest interaction
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Responsive supramolecular polymer formed by orthogonal metal-coordination and cryptand-based host
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Responsive supramolecular polymer formed by orthogonal metal-coordination and cryptand-based host
A Combination of Visible-light Photoredox and Metal Catalysis for the Mannich-type Reaction of N-Aryl Glycine Esters
 A Combination of Visible-light Photoredox and Metal Catalysis for the Mannich-type Reaction of -Aryl Glycine Esters Izumi kamura, 1 Soyoung Park,* 1 Ji Hoon Han, 1 Shunta otsu, 3 and Hiroshi Sugiyama*
A Combination of Visible-light Photoredox and Metal Catalysis for the Mannich-type Reaction of -Aryl Glycine Esters Izumi kamura, 1 Soyoung Park,* 1 Ji Hoon Han, 1 Shunta otsu, 3 and Hiroshi Sugiyama*
Post-Synthetic Approach for the Synthesis of 2 -O-Methyldithiomethyl-Modified Oligonucleotides Responsive to Reducing Environment
 Supplementary Information Post-Synthetic Approach for the Synthesis of 2 -O-Methyldithiomethyl-Modified Oligonucleotides Responsive to Reducing Environment Yosuke Ochi, Osamu Nakagawa, Katsunori Sakaguchi,
Supplementary Information Post-Synthetic Approach for the Synthesis of 2 -O-Methyldithiomethyl-Modified Oligonucleotides Responsive to Reducing Environment Yosuke Ochi, Osamu Nakagawa, Katsunori Sakaguchi,
Supporting Information for Unique X-ray Sheet Structure of 1,8- Bis(imidazolium) Anthracene and Its Application to Fluorescent Probe for DNA and DNase
 Supporting Information for Unique X-ray Sheet Structure of 1,8- Bis(imidazolium) Anthracene and Its Application to Fluorescent Probe for DA and Dase Ha a Kim, a Jisoo Lim, b Han a Lee, a Ju-Woo Ryu, c
Supporting Information for Unique X-ray Sheet Structure of 1,8- Bis(imidazolium) Anthracene and Its Application to Fluorescent Probe for DA and Dase Ha a Kim, a Jisoo Lim, b Han a Lee, a Ju-Woo Ryu, c
Heterogeneously catalyzed selective aerobic oxidative cross-coupling of terminal alkynes and amides with simple copper(ii) hydroxide
 Electronic Supplementary Information (ESI) for Heterogeneously catalyzed selective aerobic oxidative cross-coupling of terminal alkynes and amides with simple copper(ii) hydroxide Xiongjie Jin, Kazuya
Electronic Supplementary Information (ESI) for Heterogeneously catalyzed selective aerobic oxidative cross-coupling of terminal alkynes and amides with simple copper(ii) hydroxide Xiongjie Jin, Kazuya
Supporting Information. Asperones A-E, Five Dimeric Polyketides with New Carbon Skeletons from the Fungus Aspergillus sp. AWG 1-15
 Electronic Supplementary Material (ESI) for rganic Chemistry Frontiers. This journal is the Partner rganisations 2018 1 Supporting Information Asperones A-E, Five Dimeric Polyketides with New Carbon Skeletons
Electronic Supplementary Material (ESI) for rganic Chemistry Frontiers. This journal is the Partner rganisations 2018 1 Supporting Information Asperones A-E, Five Dimeric Polyketides with New Carbon Skeletons
Supporting Information
 Supporting Information Cobalt(II)-Catalyzed Acyloxylation of C- Bonds in Aromatic Amides with Carboxylic Acids Rina Ueno, Satoko atsui, and aoto Chatani* Department of Applied Chemistry, Faculty of Engineering,
Supporting Information Cobalt(II)-Catalyzed Acyloxylation of C- Bonds in Aromatic Amides with Carboxylic Acids Rina Ueno, Satoko atsui, and aoto Chatani* Department of Applied Chemistry, Faculty of Engineering,
Organocatalytic Enantioselective (3+2) Cycloaddition using Stable Azomethine Ylides
 Organocatalytic Enantioselective (3+2) Cycloaddition using Stable Azomethine Ylides aiara Fernández, Luisa Carrillo*, Jose L. Vicario,* Dolores Badía and Efraím Reyes. Department of Organic Chemistry II,
Organocatalytic Enantioselective (3+2) Cycloaddition using Stable Azomethine Ylides aiara Fernández, Luisa Carrillo*, Jose L. Vicario,* Dolores Badía and Efraím Reyes. Department of Organic Chemistry II,
A Mild, Catalytic and Highly Selective Method for the Oxidation of α,β- Enones to 1,4-Enediones. Jin-Quan Yu, a and E. J.
 A Mild, Catalytic and Highly Selective Method for the Oxidation of α,β- Enones to 1,4-Enediones Jin-Quan Yu, a and E. J. Corey b * a Department of Chemistry, Cambridge University, Cambridge CB2 1EW, United
A Mild, Catalytic and Highly Selective Method for the Oxidation of α,β- Enones to 1,4-Enediones Jin-Quan Yu, a and E. J. Corey b * a Department of Chemistry, Cambridge University, Cambridge CB2 1EW, United
Ansalactams B-D Illustrate Further Biosynthetic Plasticity within the Ansamycin Pathway
 Ansalactams B-D Illustrate Further Biosynthetic Plasticity within the Ansamycin Pathway Tu Cam Le, Inho Yang, Yeo Joon Yoon, Sang-Jip Nam,*, and William Fenical *, Department of Chemistry and Nano Science,
Ansalactams B-D Illustrate Further Biosynthetic Plasticity within the Ansamycin Pathway Tu Cam Le, Inho Yang, Yeo Joon Yoon, Sang-Jip Nam,*, and William Fenical *, Department of Chemistry and Nano Science,
Supporting Information. for. Angew. Chem. Int. Ed. Z Wiley-VCH 2003
 Supporting Information for Angew. Chem. Int. Ed. Z53001 Wiley-VCH 2003 69451 Weinheim, Germany 1 Ordered Self-Assembly and Electronic Behavior of C 60 -Anthrylphenylacetylene Hybrid ** Seok Ho Kang 1,
Supporting Information for Angew. Chem. Int. Ed. Z53001 Wiley-VCH 2003 69451 Weinheim, Germany 1 Ordered Self-Assembly and Electronic Behavior of C 60 -Anthrylphenylacetylene Hybrid ** Seok Ho Kang 1,
Supporting Information
 Supporting Information Selective allosteric antagonists for the G protein-coupled receptor GPRC6A based on the 2-phenylindole privileged structure scaffold enrik Johansson,, Michael Worch Boesgaard, Lenea
Supporting Information Selective allosteric antagonists for the G protein-coupled receptor GPRC6A based on the 2-phenylindole privileged structure scaffold enrik Johansson,, Michael Worch Boesgaard, Lenea
Cascade enzymatic cleavage of the β-o-4 linkage in a lignin model compound
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2015 Cascade enzymatic cleavage of the β--4 linkage in a lignin model compound
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2015 Cascade enzymatic cleavage of the β--4 linkage in a lignin model compound
Supporting Information. 2-(4-Tolylsulfonyl)ethoxymethyl(TEM) - A New 2 -OH. Protecting Group For Solid Support RNA Synthesis
 Supporting Information 2-(4-Tolylsulfonyl)ethoxymethyl(TEM) - A ew 2 -H Protecting Group For Solid Support RA Synthesis Chuanzheng Zhou, Dmytro Honcharenko and Jyoti Chattopadhyaya* Department of Bioorganic
Supporting Information 2-(4-Tolylsulfonyl)ethoxymethyl(TEM) - A ew 2 -H Protecting Group For Solid Support RA Synthesis Chuanzheng Zhou, Dmytro Honcharenko and Jyoti Chattopadhyaya* Department of Bioorganic
Supporting Information
 Supporting Information Wiley-VC 2005 69451 Weinheim, Germany Stereoselective Lewis Acid-Mediated [1,3] Ring Contraction of 2,5-Dihydrooxepins as a Route to Polysubstituted Cyclopentanes Supplementary Material
Supporting Information Wiley-VC 2005 69451 Weinheim, Germany Stereoselective Lewis Acid-Mediated [1,3] Ring Contraction of 2,5-Dihydrooxepins as a Route to Polysubstituted Cyclopentanes Supplementary Material
Oxidative Pd(II)-Catalyzed C-H Bond Amination to Carbazoles at Ambient Temperature
 xidative Pd(II)-Catalyzed C- Bond Amination to Carbazoles at Ambient Temperature Supplementary Information ( Pages) James A. Jordan-ore, Carin C. C. Johansson, Moises Guilias Costa, Elizabeth M. Beck and
xidative Pd(II)-Catalyzed C- Bond Amination to Carbazoles at Ambient Temperature Supplementary Information ( Pages) James A. Jordan-ore, Carin C. C. Johansson, Moises Guilias Costa, Elizabeth M. Beck and
Supporting Information. Copyright Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2006
 Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Supplementary Material Rational Design of Aziridine containing Cysteine Protease Inhibitors with improved Potency
Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Supplementary Material Rational Design of Aziridine containing Cysteine Protease Inhibitors with improved Potency
J. Am. Chem. Soc. Supporting Information Page 1
 J. Am. Chem. Soc. Supporting Information Page 1 Short Total Synthesis of (±)-Sceptrin Phil S. Baran*, Alexandros L. Zografos, and Daniel P. Malley Contribution from the Department of Chemistry, The Scripps
J. Am. Chem. Soc. Supporting Information Page 1 Short Total Synthesis of (±)-Sceptrin Phil S. Baran*, Alexandros L. Zografos, and Daniel P. Malley Contribution from the Department of Chemistry, The Scripps
Ammonium-Bearing Dinuclear Copper(II) Complex: A Highly Selective and Sensitive Colorimetric Probe for Pyrophosphate
 Ammonium-Bearing Dinuclear Copper(II) Complex: A Highly Selective and Sensitive Colorimetric Probe for Pyrophosphate Wenxiang Yu, Jian Qiang, Jun Yin, Srinivasulu Kambam, Fang Wang, Yong Wang, and Xiaoqiang
Ammonium-Bearing Dinuclear Copper(II) Complex: A Highly Selective and Sensitive Colorimetric Probe for Pyrophosphate Wenxiang Yu, Jian Qiang, Jun Yin, Srinivasulu Kambam, Fang Wang, Yong Wang, and Xiaoqiang
SUPPLEMENTARY INFORMATION
 Synthetic chemistry ML5 and ML4 were identified as K P.(TREK-) activators using a combination of fluorescence-based thallium flux and automated patch-clamp assays. ML5, ML4, and ML5a were synthesized using
Synthetic chemistry ML5 and ML4 were identified as K P.(TREK-) activators using a combination of fluorescence-based thallium flux and automated patch-clamp assays. ML5, ML4, and ML5a were synthesized using
An Efficient Total Synthesis and Absolute Configuration. Determination of Varitriol
 An Efficient Total Synthesis and Absolute Configuration Determination of Varitriol Ryan T. Clemens and Michael P. Jennings * Department of Chemistry, University of Alabama, 500 Campus Dr. Tuscaloosa, AL
An Efficient Total Synthesis and Absolute Configuration Determination of Varitriol Ryan T. Clemens and Michael P. Jennings * Department of Chemistry, University of Alabama, 500 Campus Dr. Tuscaloosa, AL
All solvents and reagents were used as obtained. 1H NMR spectra were recorded with a Varian
 SUPPLEMETARY OTE Chemistry All solvents and reagents were used as obtained. 1H MR spectra were recorded with a Varian Inova 600 MR spectrometer and referenced to dimethylsulfoxide. Chemical shifts are
SUPPLEMETARY OTE Chemistry All solvents and reagents were used as obtained. 1H MR spectra were recorded with a Varian Inova 600 MR spectrometer and referenced to dimethylsulfoxide. Chemical shifts are
Novel fluorescent cationic benzothiazole dye response to G-quadruplex aptamer as a novel K + sensor
 Electronic Supplementary Material (ESI) for Analyst. This journal is The Royal Society of Chemistry 2017 Novel fluorescent cationic benzothiazole dye response to G-quadruplex aptamer as a novel K + sensor
Electronic Supplementary Material (ESI) for Analyst. This journal is The Royal Society of Chemistry 2017 Novel fluorescent cationic benzothiazole dye response to G-quadruplex aptamer as a novel K + sensor
Fast and Flexible Synthesis of Pantothenic Acid and CJ-15,801.
 Fast and Flexible Synthesis of Pantothenic Acid and CJ-15,801. Alan L. Sewell a, Mathew V. J. Villa a, Mhairi Matheson a, William G. Whittingham b, Rodolfo Marquez a*. a) WestCHEM, School of Chemistry,
Fast and Flexible Synthesis of Pantothenic Acid and CJ-15,801. Alan L. Sewell a, Mathew V. J. Villa a, Mhairi Matheson a, William G. Whittingham b, Rodolfo Marquez a*. a) WestCHEM, School of Chemistry,
Protein and antibody functionalization using continuous flow microreactor technology. Table of Contents
 1 Protein and antibody functionalization using continuous flow microreactor technology Meaghan M. Sebeika, Nicholas G. Gedeon, Sara Sadler, Nicholas L. Kern, Devan J. Wilkins, David E. Bell and Graham
1 Protein and antibody functionalization using continuous flow microreactor technology Meaghan M. Sebeika, Nicholas G. Gedeon, Sara Sadler, Nicholas L. Kern, Devan J. Wilkins, David E. Bell and Graham
Palladium-Catalyzed Benzene Arylation: Incorporation of Catalytic Pivalic Acid as a Proton Shuttle and a Key Element in Catalytic Design
 S1 Palladium-Catalyzed Benzene Arylation: Incorporation of Catalytic Pivalic Acid as a Proton Shuttle and a Key Element in Catalytic esign Marc Lafrance and Keith Fagnou* Center for Catalysis Research
S1 Palladium-Catalyzed Benzene Arylation: Incorporation of Catalytic Pivalic Acid as a Proton Shuttle and a Key Element in Catalytic esign Marc Lafrance and Keith Fagnou* Center for Catalysis Research
Backbone modification of a parathyroid hormone receptor-1 antagonist/inverse agonist
 SUPPORTING INFORMATION Backbone modification of a parathyroid hormone receptor-1 antagonist/inverse agonist Ross W. Cheloha, Tomoyuki Watanabe, Thomas Dean, Samuel H. Gellman*, Thomas J. Gardella* *Corresponding
SUPPORTING INFORMATION Backbone modification of a parathyroid hormone receptor-1 antagonist/inverse agonist Ross W. Cheloha, Tomoyuki Watanabe, Thomas Dean, Samuel H. Gellman*, Thomas J. Gardella* *Corresponding
Supporting Information
 Inhibitory Efficiencies for Mechanism-Based Inactivators of Sialidases Kobra Khazaei, 1 Juliana H. F. Yeung, 2 Margo M. Moore 2 and Andrew J. Bennet 1,* Departments of Chemistry 1 and Biological Sciences,
Inhibitory Efficiencies for Mechanism-Based Inactivators of Sialidases Kobra Khazaei, 1 Juliana H. F. Yeung, 2 Margo M. Moore 2 and Andrew J. Bennet 1,* Departments of Chemistry 1 and Biological Sciences,
Synthesis of Levulinic Acid based Poly(amine-co-ester)s
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2018 Synthesis of Levulinic Acid based Poly(amine-co-ester)s Yann Bernhard, Lucas Pagies, Sylvain
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2018 Synthesis of Levulinic Acid based Poly(amine-co-ester)s Yann Bernhard, Lucas Pagies, Sylvain
Metal-free general procedure for oxidation of secondary amines to nitrones
 S1 Supporting information Metal-free general procedure for oxidation of secondary amines to nitrones Carolina Gella, Èric Ferrer, Ramon Alibés, Félix Busqué,* Pedro de March, Marta Figueredo,* and Josep
S1 Supporting information Metal-free general procedure for oxidation of secondary amines to nitrones Carolina Gella, Èric Ferrer, Ramon Alibés, Félix Busqué,* Pedro de March, Marta Figueredo,* and Josep
An insulin-sensing sugar-based fluorescent hydrogel
 Supplementary Information An insulin-sensing sugar-based fluorescent hydrogel Sankarprasad Bhuniya and Byeang yean Kim* ational Research Laboratory, Department of Chemistry, Division of Molecular and Life
Supplementary Information An insulin-sensing sugar-based fluorescent hydrogel Sankarprasad Bhuniya and Byeang yean Kim* ational Research Laboratory, Department of Chemistry, Division of Molecular and Life
Supporting information
 Supporting information Structure-dependent binding of hnrp A1 to telomere RA Xiao Liu 1,Takumi Ishizuka 1, Hong-Liang Bao 1, Kei Wada 2, Yuuma Takada 1, Keisuke lida 3, Kazuo agasawa 3, Danzhou Yang 4,
Supporting information Structure-dependent binding of hnrp A1 to telomere RA Xiao Liu 1,Takumi Ishizuka 1, Hong-Liang Bao 1, Kei Wada 2, Yuuma Takada 1, Keisuke lida 3, Kazuo agasawa 3, Danzhou Yang 4,
Supplementary Figure 1. Structures of substrates tested with 1. Only one enantiomer is shown.
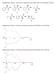 Supplementary Figure 1. Structures of substrates tested with 1. Only one enantiomer is shown. Supplementary Figure 2. CD spectra obtained using 1 and (R)-3 (blue) and (S)-3 (red) Supplementary Figure 3.
Supplementary Figure 1. Structures of substrates tested with 1. Only one enantiomer is shown. Supplementary Figure 2. CD spectra obtained using 1 and (R)-3 (blue) and (S)-3 (red) Supplementary Figure 3.
Facile Synthesis of Flavonoid 7-O-Glycosides
 Facile Synthesis of Flavonoid 7-O-Glycosides Ming Li, a Xiuwen Han, a Biao Yu b * a State Key Laboratory of Catalyst, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China
Facile Synthesis of Flavonoid 7-O-Glycosides Ming Li, a Xiuwen Han, a Biao Yu b * a State Key Laboratory of Catalyst, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China
Supporting Information
 Supporting Information Synthesis of the Tetracyclic Structure of Batrachotoxin Enabled by Bridgehead Radical Coupling and Pd/Ni-Promoted Ullmann Reaction Komei Sakata, Yinghua Wang, Daisuke Urabe, and
Supporting Information Synthesis of the Tetracyclic Structure of Batrachotoxin Enabled by Bridgehead Radical Coupling and Pd/Ni-Promoted Ullmann Reaction Komei Sakata, Yinghua Wang, Daisuke Urabe, and
ARTEMETHER AND LUMEFANTRINE ORAL SUSPENSION:Final text for addition to The International Pharmacopoeia (November 2008)
 November 2008 ` ARTEMETER AND LUMEFANTRINE RAL SUSPENSIN:Final text for addition to The International Pharmacopoeia (November 2008) Category. Antimalarial. Storage. Artemether and lumefantrine oral suspension
November 2008 ` ARTEMETER AND LUMEFANTRINE RAL SUSPENSIN:Final text for addition to The International Pharmacopoeia (November 2008) Category. Antimalarial. Storage. Artemether and lumefantrine oral suspension
A fluorinated dendritic TsDPEN-Ru(II) catalyst for asymmetric transfer hydrogenation of prochiral ketones in aqueous media
 Supplementary Information A fluorinated dendritic TsDPEN-Ru(II) catalyst for asymmetric transfer hydrogenation of prochiral ketones in aqueous media Weiwei Wang and Quanrui Wang* Department of Chemistry,
Supplementary Information A fluorinated dendritic TsDPEN-Ru(II) catalyst for asymmetric transfer hydrogenation of prochiral ketones in aqueous media Weiwei Wang and Quanrui Wang* Department of Chemistry,
Pyridine-Containing m-phenylene Ethynylene Oligomers Having Tunable Basicities
 Supporting nformation Pyridine-Containing m-phenylene Ethynylene ligomers Having Tunable Basicities Jennifer M. Heemstra and Jeffrey S. Moore* Departments of Chemistry and Materials Science & Engineering,
Supporting nformation Pyridine-Containing m-phenylene Ethynylene ligomers Having Tunable Basicities Jennifer M. Heemstra and Jeffrey S. Moore* Departments of Chemistry and Materials Science & Engineering,
Coupling of 6 with 8a to give 4,6-Di-O-acetyl-2-amino-2-N,3-O-carbonyl-2-deoxy-α-Dglucopyranosyl-(1 3)-1,2:5,6-di-O-isopropylidene-α-D-glucofuranose.
 General Experimental Procedures. NMR experiments were conducted on a Varian Unity/Inova 400-MHz Fourier Transform NMR Spectrometer. Chemical shifts are downfield from tetramethylsilane in CDCl 3 unless
General Experimental Procedures. NMR experiments were conducted on a Varian Unity/Inova 400-MHz Fourier Transform NMR Spectrometer. Chemical shifts are downfield from tetramethylsilane in CDCl 3 unless
Supporting Information for. A New Method for the Cleavage of Nitrobenzyl Amides and Ethers
 SI- 1 Supporting Information for A ew Method for the Cleavage of itrobenzyl Amides and Ethers Seo-Jung Han, Gabriel Fernando de Melo, and Brian M. Stoltz* The Warren and Katharine Schlinger Laboratory
SI- 1 Supporting Information for A ew Method for the Cleavage of itrobenzyl Amides and Ethers Seo-Jung Han, Gabriel Fernando de Melo, and Brian M. Stoltz* The Warren and Katharine Schlinger Laboratory
LUMEFANTRINUM LUMEFANTRINE
 July 2008 LUMEFANTRINE: Final text for addition to The International Pharmacopoeia (July 2008) This monograph was adopted at the Forty-second WHO Expert Committee on Specifications for Pharmaceutical Preparations
July 2008 LUMEFANTRINE: Final text for addition to The International Pharmacopoeia (July 2008) This monograph was adopted at the Forty-second WHO Expert Committee on Specifications for Pharmaceutical Preparations
Supporting Information. A fluorogenic assay for screening Sirt6 modulators
 This journal is The Royal Society of Chemistry 213 Supporting Information A fluorogenic assay for screening Sirt6 modulators Jing Hu, Bin He, Shiva Bhargava, Hening Lin* Department of Chemistry and Chemical
This journal is The Royal Society of Chemistry 213 Supporting Information A fluorogenic assay for screening Sirt6 modulators Jing Hu, Bin He, Shiva Bhargava, Hening Lin* Department of Chemistry and Chemical
Structure-activity effects in peptide self-assembly and gelation Dendritic versus linear architectures
 Structure-activity effects in peptide self-assembly and gelation Dendritic versus linear architectures Cecile A. Lagadec a and David K. Smith*,a SUPPLEMETARY IFRMATI Contents 1 General Experimental Methods
Structure-activity effects in peptide self-assembly and gelation Dendritic versus linear architectures Cecile A. Lagadec a and David K. Smith*,a SUPPLEMETARY IFRMATI Contents 1 General Experimental Methods
Stereoselective synthesis of ( )-lepadins A C. Mercedes Amat,* Alexandre Pinto, Rosa Griera, and Joan Bosch
 Stereoselective synthesis of ( )-lepadins A C Mercedes Amat,* Alexandre Pinto, Rosa Griera, and Joan Bosch Laboratory of Organic Chemistry, Faculty of Pharmacy and Institute of Biomedicine (IBUB), University
Stereoselective synthesis of ( )-lepadins A C Mercedes Amat,* Alexandre Pinto, Rosa Griera, and Joan Bosch Laboratory of Organic Chemistry, Faculty of Pharmacy and Institute of Biomedicine (IBUB), University
Synthesis of Trifluoromethylated Naphthoquinones via Copper-Catalyzed. Cascade Trifluoromethylation/Cyclization of. 2-(3-Arylpropioloyl)benzaldehydes
 Supporting Information to Synthesis of Trifluoromethylated Naphthoquinones via Copper-Catalyzed Cascade Trifluoromethylation/Cyclization of 2-(3-Arylpropioloyl)benzaldehydes Yan Zhang*, Dongmei Guo, Shangyi
Supporting Information to Synthesis of Trifluoromethylated Naphthoquinones via Copper-Catalyzed Cascade Trifluoromethylation/Cyclization of 2-(3-Arylpropioloyl)benzaldehydes Yan Zhang*, Dongmei Guo, Shangyi
Development of a Reversible Fluorescent Gold Sensor with High. Selectivity
 Supporting Information for Development of a Reversible Fluorescent Gold Sensor with igh Selectivity Jiaoliang Wang, Weiying Lin*, Lin Yuan, Jizeng Song, and Wensha Gao State Key Laboratory of Chemo/Biosensing
Supporting Information for Development of a Reversible Fluorescent Gold Sensor with igh Selectivity Jiaoliang Wang, Weiying Lin*, Lin Yuan, Jizeng Song, and Wensha Gao State Key Laboratory of Chemo/Biosensing
