CHEMISTRY PRESENTATION SYSTEM
|
|
|
- Laurence Cross
- 5 years ago
- Views:
Transcription
1 CHEMISTRY PRESENTATION SYSTEM FOR FLEXIBLE DEMONSTRATION EXPERIMENTS CHEMISTRY
2 EXPERIMENTATION WITH A CLEAR OVERVIEW THE CHEMISTRY PRESENTATION SYSTEM The electrochemistry demonstration system is ideal for demonstration experiments, as the experiments can also be seen from the back row. 2 ADVANTAGES AT A GLANCE rapid set-up of your own apparatus through defined distances simple corrections with magnetic holders clear experimental set-ups against a single-colour background no inconvenient stand material glass connectors with silicone seals replace tubing pre-assembled modules for complex experiment set-ups special equipment for all areas of chemistry
3 Instead of conventional materials on a stand - various modules suspended in a frame 1.2 DEMONSTRATION EXPERIMENTS CHEMISTRY The sensor-cassy can also be suspended in the frame NEW CPSflex Good visibility from a distance through singlecolour background Glassware is fixed to the panels with magnetic spring clips Magnetic holders enable individual items of the apparatus to be set up on the adhesive panels Adhesive panel can also be written on with whiteboard markers, thus replacing the board drawing Can be combined with conventional laboratory equipment 3
4 SAVE TIME ON PREPARATION WITH CPSflex RAPID SET-UP OF INDIVIDUAL EQUIPMENT HOLDER SIZE AND DISTANCE ARE DESIGNED SUCH THAT THE EXPERI- MENTAL SET-UP IS AUTOMATICALLY ARRANGED IN ONE PLANE Insert adhesive magnetic board into the CPS frame. Place magnetic holder on the panel Insert the glassware into the spring clips of the holders. Adjust the glassware position with the magnetic holders Join the glass connectors together and tighten the GL screw fitting. The experiment can start. 4
5 In the LEYBOLD YouTube channel you will find a video on experimentation with the Chemistry Presentation System. For disassembly, remove all apparatus. To do this, just tilt the magnets. Alternatively, first remove the glassware, then the magnetic holders. MAGNETIC HOLDERS NEW For setting up the apparatus five different sizes of holders are available. With these clips it is possible to attach glass equipment from 9 mm to 32 mm in diameter. Each magnet can hold 0.6 kg - sufficient for most applications. Large items of equipment are attached in individual CPS modules (see pages 8 to 13). EASY CORRECTION WITH MAGNETIC HOLDERS To attach... You need... Item Article No. Article description Glass tube, tubing Ground glass NS14 Screw fitting GL14 Screw fitting GL18 Ground glass NS19 Screw fitting GL25 Ground glass NS29 Screw fitting GL32 Chromatography column Screw fitting GL45 Gas syringe mm Magnetic holder, Size 1, mm mm Magnetic holder, Size 2, mm mm Magnetic holder, Size 3, mm mm Magnetic holder, Size 4, mm mm Magnetic holder, Size 5, mm 5
6 INDIVIDUAL AND FLEXIBLE MODULAR SYSTEM Glass connectors provide gas-tight connections between glassware Simple set-up and break-down with GL screw fittings Experiment set-ups without baked-on tubing. GLASS CONNECTORS WITH SILICONE SEALS REPLACE TUBING Screw up tight = glassware is fixed, gas-tight connection Unscrew = glassware can be removed 6
7 CPS FRAME accepts all types of experiment panels fits into any fume cupboard 50 cm wide for narrow experiments (C50) 97 cm wide for wide experiments (C100) Profile frame C50, two rows, for CPS ADHESIVE MAGNETIC BOARDS Ferromagnetic board, painted, with printed cross-grid. Can be written on with washable pens (whiteboard markers). Profile frame C100, three rows, for CPS Profile frame C100, two rows, for CPS NEW Adhesive magnetic board, 500 mm Adhesive magnetic board, 300 mm MAGNETIC HOLDERS Spring clips with defined distance connected to adhesive magnets. For setting up chemical apparatus on the adhesive magnetic board mm 300 mm NEW Holder, magnetic, Size 1, mm Holder, magnetic, Size 2, mm Holder, magnetic, Size 3, mm Holder, magnetic, Size 4, mm Holder, magnetic, Size 5, mm GLASS CONNECTORS Three different shapes are available for connecting glassware together. Glass connector, angled Glass connector, 2 x GL Glass connector, 1 x GL 18 with glass olive CPSflex STARTER PACKS NEW Contents: Adhesive magnetic boards and suitable magnetic holders in a frame. narrow, c. 56 cm wide ( P) wide, c. 97 cm wide ( P) CPSflex Starter Pack, C50 CPSflex Starter Pack, C P P 7
8 SOLUTIONS FOR LARGE EQUIPMENT HOOK-IN EQUIPMENT PLATFORMS AND HOLDERS Large and heavy items of equipment, such as measuring instruments and power supplies, can be placed on the equipment platforms. 8
9 NO INCONVENIENT STAND MATERIAL EXPERIMENT SET-UPS AGAINST A SINGLE-COLOUR BACKGROUND YOU HAVE GLASSWARE THAT DOESN'T FIT IN THE MAGNETIC HOLDERS? WITH THE UNIVERSAL HOLDER CPS MODULES YOU CAN USE THESE TOO Universal glassware holder, CPS Console Equipment platform 500 mm Equipment platform 300 mm Pedestal, CPS Blank panel 200 mm, CPS Blank panel 100 mm, CPS Blank panel 300 mm, CPS Holder with bush, height adjustable, CPS Metal labelling plates, CPS, set of
10 COMPLEX CPS MODULES FIXED DIRECTLY FOR SECURE SUPPORT Drawing as set-up aid and "board drawing" Heavy equipment is firmly screwed on Safety is paramount - large glass equipment is supplied preassembled. PRE-ASSEMBLED MODULES FOR COMPLEX EXPERIMENT SET-UPS measurement and power supply equipment - inconspicuous integration in the apparatus heavy equipment - safe and secure experiment set-up 10
11 Woulff s bottle with manometer, CPS Holder for Minican canisters Combustion chamber with incandescent wire, CPS Voltage supply, switchable, CPS Aeration pump, controllable, CPS Digital thermometer, CPS Sensor-CASSY 2 CASSY-Display INTEGRATED IN DESIGN Complex equipment is pre-assembled and wired up. Electrical, measurement and power supply equipment can be hooked into the CPS frame. Woulff s bottle with manometer, CPS Holder for pressurised gas canisters Combustion chamber with incandescent wire, CPS Voltage supply, switchable, CPS Aeration pump, controllable, CPS Digital thermometer, CPS Sensor-CASSY CASSY-Display USB 11
12 SPECIAL EQUIPMENT CPS MODULES FOR ALL AREAS OF CHEMISTRY Catalogue No. Description Inorganic Chemistry Organic Chemistry Analytical Chemistry Physical Chemistry Technical Chemistry Biochemistry Example experiments Experiment No. in Experiment Catalogue Chemistry Electrolysis apparatus, CPS x x Electrolytic water decomposition Determination of the Faraday constant Mineral oil distillation, bubble tray column, CPS Calorimeter, for solids and liquids, CPS x x x x x x x Mineral oil distillation C Determination of enthalpy of combustion and calorific value Combustion chamber, water synthesis, CPS x x x Quantitative synthesis of water Bioreactor, basic configuation, CPS x Metering unit for bioreactor, CPS x Electrochemistry demonstration unit, CPS with Gas chromatograph LD1 with base panel, CPS Fermentation experiments based on the batch method Fermentation experiments based on the batch method Conductivity of materials x x x Electrochemical series of metals Galvanic elements Corrosion and corrosion protection Gas chromatographic investigation of lighter x x x fuel C C C C C C C PEM fuel cell stack, CPS x x Investigations using the fuel cell stack C C HydroStik PRO, CPS x x x x Fuel cell stack Haber-Bosch method Determination of molecular mass C C C Bubble counter, CPS x x x x Investigations using the fuel cell stack C C Electrical load, CPS x x Investigations using the fuel cell stack C C
13 Gas chromatographical analysis of cigarette lighter gas (butane gas) (C ) Cat.-Nr. Name Gas chromatograph LD Hydrocarbon sensor Separation column silicone OV Pocket-CASSY 2 Bluetooth Rechargeable battery for Pocket-CASSY 2 Bluetooth 1* Bluetooth dongle 1* CASSY Lab UIP sensor S Aquarium pump, 100 l/h Bubble counter, with flash back valve Disposable syringe, 1 ml, with Luer fitting Cannula, 0.45 diam., 10 pcs., with Luer fitting Connecting leads, 19 A, 50 cm, red/blue, pair Base plate for bunsen stand, 130 x 210 mm Stand rod, 450 x 12 mm diam., M10 thread Universal clamp, mm Bosshead S Septa, silicone, 13 mm diam., 10 pcs Silicone tubing, 4 mm diam., 1 m Fine regulating valve for minican gas canisters Minican pressurised gas canister, ethane Minican pressurised gas canister, n-butane 1 additionally required: cigarette lighter(s) * additionally recommended 1 Chromatogram of the analysis of lighter gases CPS SET-UPS FOR EXPERIMENTS IN ALL AREAS OF CHEMISTRY Technical Chemistry, e.g. experiment C Electrochemistry, e.g. experiment C YOU WILL FIND THESE AND OTHER EXPERIMENTS IN THE CHEMISTRY EX- PERIMENTS CATALOGUE Molecular mass determination, e.g. experiment C Distillation, e.g. experiment C The new Leybold catalogue has arrived with more than 100 experiments for schools and universities! The collection of experiments covers all relevant topics in chemistry education. Furthermore, we also offer special systems in the fields of fuel cell technology, electrochemistry and spectrometry. Active catalogue on our Internet site at ANALYTICAL CHEMISTRY CHROMATOGRAPHY C3.2.1 GAS CHROMATOGRAPHY C Gas chromatographical analysis of cigarette lighter gas (butane gas) C In use all around the world today, gas chromatography is a method for analyzing chemical substances and mixtures. Especially useful for identifying the components of gaseous hydrocarbons, e.g. natural gas, it can also be used to study volatile substances such as fragrances or alcohols. Substances are separated in a two-phase system comprising a stationary phase - the separation column with column material - and a mobile phase - the carrier gas. Samples are introduced into the carrier gas stream and travel along the column at different speeds depending on polarity, which makes it possible to separate them. Cigarette lighter gas is a mixture of different gaseous hydrocarbons. They can be easily separated by gas chromatography techniques. The stationary phase is silicone oil OV-101 on silica gel. Air is used as the mobile phase. The proportions of the individual hydrocarbons in the gas mixture is different in every cigarette lighter - depending on the source of the natural gas. This is studied in experiment C
14 EXPERIMENTS WITH HYDROGEN IT WAS NEVER SO SIMPLE Produce hydrogen for experiments with the Hydrofill PRO. 14 In the LD DIDACTIC YouTube channel we show you how easy it is to produce H 2 using the Hydrofill PRO. PRODUCE YOUR H 2 REQUIREMENT SIMPLY FROM THE MAINS SOCKET AND ALSO SAVE SPACE!
15 NEW CHARGE IT UP - AS EASY AS A MOBILE PHONE: 1. Place it in the HydroFill PRO charging station 2. Charge for 4 to 6 hours 3. Use the hydrogen for experiments H 2 charging process: HydroFill PRO fills the screwed-in HydroStik PRO. HYDROFILL PRO HydroStik PRO =HydroFill PRO 10 l H 2 The HydroFill PRO supplies hydrogen through the electrolysis of distilled water. Only a mains socket is required. The hydrogen is stored directly in the HydroStik PRO in the form of a metal hydride. In this way, experiments can be performed with hydrogen without the use of gas bottles, e.g. for experiments with fuels cells. A DEMONSTRATION OF HYDROGEN TECHNOLOGY PEM fuel cell stack consisting of four individual cells which can be quickly connected in series or in parallel Clear layout that is easily visible from a distance: Ideally suited to demonstrations or project work In combination with the electrical load module: Simple recording of characteristic curves and measurement of efficiency factors Hydrogen from the HydroStik PRO, no gas bottle needed HydroStik PRO, CPS Bubble counter, CPS PEM Fuel Cell Stack, CPS Electrical load, CPS HydroFill PRO Experiment set-up (C ) for investigations with PEM fuel cells from the Chemistry Experiments catalogue. 15
16 CONTACT LD Technical details subject to change without notice GERMANY: LD DIDACTIC GmbH Leyboldstr. 1 D Hürth Tel.: Fax: info@ld didactic.de didactic.com UK: Feedback Instruments Limited 5 & 6 Warren Court Park Road, Crowborough East Sussex TN6 2QX Tel.: +44 (0) Fax: +44 (0) sales@feedback-instruments.com USA: Feedback Incorporated 437 Dimmocks Mill Road Suite 27 Hillsborough NC Tel.: +1 (919) Fax: +1 (919) sales@feedback-instruments.com BRANDS OF THE LD DIDACTIC GROUP
 P6.5.5.4 Atomic and nuclear physics Nuclear physics γ spectroscopy Identifying and determining the activity of radioactive samples Description from CASSY Lab 2 For loading examples and settings, please
P6.5.5.4 Atomic and nuclear physics Nuclear physics γ spectroscopy Identifying and determining the activity of radioactive samples Description from CASSY Lab 2 For loading examples and settings, please
CHEMISTRY EXPERIMENTS
 CHEMISTRY EXPERIMENTS CHEMISTRY FOR DEMONSTRATION IN SCHOOLS AND UNIVERSITIES CHEMISTRY WITH LEYBOLD LEYBOLD products bring classroom instructions to life and help teachers prepare and present course content.
CHEMISTRY EXPERIMENTS CHEMISTRY FOR DEMONSTRATION IN SCHOOLS AND UNIVERSITIES CHEMISTRY WITH LEYBOLD LEYBOLD products bring classroom instructions to life and help teachers prepare and present course content.
p tot Mechanics LD Physics Leaflets
 GENZ 2014-12 Mechanics Aerodynamics and hydrodynamics Measuring air resistance LD Physics Leaflets Measuring the air resistance as a function of the wind speed Measuring the wind speed with a pressure
GENZ 2014-12 Mechanics Aerodynamics and hydrodynamics Measuring air resistance LD Physics Leaflets Measuring the air resistance as a function of the wind speed Measuring the wind speed with a pressure
Physical Chemistry. LD Chemistry Leaflets. Reaction of malachite green with hydroxide ions: Influence of the concentration C4.1.3.
 SW-2014-06 Physical Chemistry Reaction kinetics Influencing the reaction rate LD Chemistry Leaflets Reaction of malachite green with hydroxide ions: Influence of the concentration Aims of the experiment
SW-2014-06 Physical Chemistry Reaction kinetics Influencing the reaction rate LD Chemistry Leaflets Reaction of malachite green with hydroxide ions: Influence of the concentration Aims of the experiment
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *8769716985* PHYSICS 0625/21 Paper 2 Core May/June 2013 1 hour 15 minutes Candidates answer on
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *8769716985* PHYSICS 0625/21 Paper 2 Core May/June 2013 1 hour 15 minutes Candidates answer on
Determination of Volatile Substances Proof of Food Adulteration
 ANALYSIS OF FOOD AND NATURAL PRODUCTS LABORATORY EXERCISE Determination of Volatile Substances Proof of Food Adulteration (method: gas chromatography with mass spectrometric detection) Exercise guarantor:
ANALYSIS OF FOOD AND NATURAL PRODUCTS LABORATORY EXERCISE Determination of Volatile Substances Proof of Food Adulteration (method: gas chromatography with mass spectrometric detection) Exercise guarantor:
Thermochemistry/Calorimetry. Determination of the enthalpy of vaporization of liquids LEC 02. What you need: What you can learn about
 LEC 02 Thermochemistry/Calorimetry Determination of the enthalpy of vaporization of liquids What you can learn about Enthalpy of vaporisation Entropy of vaporisation Trouton s rule Calorimetry Heat capacity
LEC 02 Thermochemistry/Calorimetry Determination of the enthalpy of vaporization of liquids What you can learn about Enthalpy of vaporisation Entropy of vaporisation Trouton s rule Calorimetry Heat capacity
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education CHEMISTRY
 Centre Number Candidate Number Name UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education CHEMISTRY 06/06 Paper 6 Alternative to Practical Candidates
Centre Number Candidate Number Name UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education CHEMISTRY 06/06 Paper 6 Alternative to Practical Candidates
Electrolysis SCIENCE TOPICS PROCESS SKILLS VOCABULARY
 SIDE DISPLAY Electrolysis Visitors observe two glass tubes containing water. Electrodes are passing an electric current through the water. Oxygen gas is formed in the tube connected to the positive electrode.
SIDE DISPLAY Electrolysis Visitors observe two glass tubes containing water. Electrodes are passing an electric current through the water. Oxygen gas is formed in the tube connected to the positive electrode.
Electricity. Electrolysis. Current and the transport of charge DETERMINATION OF THE FARADAY CONSTANT BASIC PRINCIPLES
 Electricity Current and the transport of charge Electrolysis DETERMINATION OF THE FARADAY CONSTANT Production of hydrogen by means of electrolysis and determining the volume of the hydrogen V. Determining
Electricity Current and the transport of charge Electrolysis DETERMINATION OF THE FARADAY CONSTANT Production of hydrogen by means of electrolysis and determining the volume of the hydrogen V. Determining
Determination of freezing points of pure substances with Cobra4 TEC
 Determination of freezing points of pure substances TEC Related concept Crystallization point, Gibbs free energy, enthalpy, entropy, heat of fusion, freezing point depression. Principle When a pure substance
Determination of freezing points of pure substances TEC Related concept Crystallization point, Gibbs free energy, enthalpy, entropy, heat of fusion, freezing point depression. Principle When a pure substance
Ballistic pendulum Operating Instructions Fig. 1: Ballistic pendulum SAFETY PRECAUTIONS
 R Ballistic pendulum 11229.00 PHYWE Systeme GmbH & Co. KG Robert-Bosch-Breite 10 D-37079 Göttingen 6 3.8 3.7 3.6 3.5 Phone +49 (0) 551 604-0 Fax +49 (0) 551 604-107 E-mail info@phywe.de Internet www.phywe.de
R Ballistic pendulum 11229.00 PHYWE Systeme GmbH & Co. KG Robert-Bosch-Breite 10 D-37079 Göttingen 6 3.8 3.7 3.6 3.5 Phone +49 (0) 551 604-0 Fax +49 (0) 551 604-107 E-mail info@phywe.de Internet www.phywe.de
Common Acids and Bases
 Common Acids and Bases Objective To determine the ph values of common household substances. Modules and Sensors Computer with NeuLog TM Software Data Cable, 10cm S98242-48ND USB Module - S98242-45ND ph
Common Acids and Bases Objective To determine the ph values of common household substances. Modules and Sensors Computer with NeuLog TM Software Data Cable, 10cm S98242-48ND USB Module - S98242-45ND ph
Name SCS- Date. Experiment 5: Hydrogen Formation and Reaction with Oxygen
 Name SCS- Date Experiment 5: Hydrogen Formation and Reaction with Oxygen Aims: What are the energy changes associated with chemical bond formation and breakage? How are changes in potential energy related
Name SCS- Date Experiment 5: Hydrogen Formation and Reaction with Oxygen Aims: What are the energy changes associated with chemical bond formation and breakage? How are changes in potential energy related
MIXTURES, COMPOUNDS, & SOLUTIONS
 MIXTURES, COMPOUNDS, & SOLUTIONS As with elements, few compounds are found pure in nature and usually found as mixtures with other compounds. A mixture is a combination of two or more substances that are
MIXTURES, COMPOUNDS, & SOLUTIONS As with elements, few compounds are found pure in nature and usually found as mixtures with other compounds. A mixture is a combination of two or more substances that are
3B SCIENTIFIC PHYSICS
 3B SCIENTIFIC PHYSICS Torsion Apparatus 1018550 Torsion Apparatus Supplement Set 1018787 Instruction manual 11/15 TL/UD 1. Description The torsion equipment is designed for determining the torsion coefficient
3B SCIENTIFIC PHYSICS Torsion Apparatus 1018550 Torsion Apparatus Supplement Set 1018787 Instruction manual 11/15 TL/UD 1. Description The torsion equipment is designed for determining the torsion coefficient
Institute for Chemical Education, Fun With Chemistry; Vol. 1, Sarquis, Mickey and Sarquis, Gerry, Ed.; University of Wisconsin Madison, 1991,
 EXPERIIMENT #7 LIIQUIID CHROMATOGRAPHY References: Bidlingmeyer, B. A.; Warren Jr., F. V. An Inexpensive Experiment for the Introduction of High Performance Liquid Chromatography J. Chem. Educ. 1984, 61,
EXPERIIMENT #7 LIIQUIID CHROMATOGRAPHY References: Bidlingmeyer, B. A.; Warren Jr., F. V. An Inexpensive Experiment for the Introduction of High Performance Liquid Chromatography J. Chem. Educ. 1984, 61,
Freezing point depression (Item No.: P )
 Freezing point depression (Item No.: P3021101) Curricular Relevance Area of Expertise: Chemistry Education Level: University Topic: General Chemistry Subtopic: Solutions and Mixtures Experiment: Freezing
Freezing point depression (Item No.: P3021101) Curricular Relevance Area of Expertise: Chemistry Education Level: University Topic: General Chemistry Subtopic: Solutions and Mixtures Experiment: Freezing
Electrochemistry. Conductivity of strong and weak electrolytes LEC 06. What you need: What you can learn about. Principle and tasks
 LEC 06 Electrochemistry What you can learn about Kohlrausch s law Equivalent conductivity Temperature-dependence of conductivity Ostwald s dilution law Principle and tasks It is possible to differentiate
LEC 06 Electrochemistry What you can learn about Kohlrausch s law Equivalent conductivity Temperature-dependence of conductivity Ostwald s dilution law Principle and tasks It is possible to differentiate
NORMACLAMP Hose Clamps
 TORRO Worm Drive Hose Clips to DIN 3017 Short description of technical features TORRO hose clips are specially suitable for applications under high mechanical loads. Since we are continuously working to
TORRO Worm Drive Hose Clips to DIN 3017 Short description of technical features TORRO hose clips are specially suitable for applications under high mechanical loads. Since we are continuously working to
Electricity. Measuring the force on current-carrying conductors in a homogeneous magnetic field. LEYBOLD Physics Leaflets P
 Electricity Magnetostatics The effects of force in a magnetic field LEYBOLD Physics Leaflets Measuring the force on current-carrying conductors in a homogeneous magnetic field Recording with CASSY Objects
Electricity Magnetostatics The effects of force in a magnetic field LEYBOLD Physics Leaflets Measuring the force on current-carrying conductors in a homogeneous magnetic field Recording with CASSY Objects
Stoichiometry Rockets
 Stoichiometry Rockets The objective of this lab is to to: calculate the needed volume of fuel to react with a given volume of gas and result in a productive explosion determine the heat of the reaction
Stoichiometry Rockets The objective of this lab is to to: calculate the needed volume of fuel to react with a given volume of gas and result in a productive explosion determine the heat of the reaction
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education * 5978594 060* PHYSICS 0625/32 Paper 3 Extended May/June 2010 1 hour 15 minutes Candidates answer
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education * 5978594 060* PHYSICS 0625/32 Paper 3 Extended May/June 2010 1 hour 15 minutes Candidates answer
PRINCIPLES AND APPLICATION OF CHROMATOGRAPHY. Dr. P. Jayachandra Reddy Mpharm PhD Principal & professor KTPC
 PRINCIPLES AND APPLICATION OF CHROMATOGRAPHY Dr. P. Jayachandra Reddy Mpharm PhD Principal & professor KTPC CHROMATOGRAPHY Laboratory technique for the Separation of mixtures Chroma -"color" and graphein
PRINCIPLES AND APPLICATION OF CHROMATOGRAPHY Dr. P. Jayachandra Reddy Mpharm PhD Principal & professor KTPC CHROMATOGRAPHY Laboratory technique for the Separation of mixtures Chroma -"color" and graphein
Chromatography Lab # 4
 Chromatography Lab # 4 Chromatography is a method for separating mixtures based on differences in the speed at which they migrate over or through a stationary phase which means that a complex mixture will
Chromatography Lab # 4 Chromatography is a method for separating mixtures based on differences in the speed at which they migrate over or through a stationary phase which means that a complex mixture will
Thermochemistry/Calorimetry. Determination of the enthalpy of combustion with a calorimetric bomb LEC 02. What you need:
 LEC 02 Thermochemistry/Calorimetry with a calorimetric bomb What you can learn about 1st law of thermodynamics Hess law Enthalpy of combustion Enthalpy of formation Heat capacity Principle and tasks The
LEC 02 Thermochemistry/Calorimetry with a calorimetric bomb What you can learn about 1st law of thermodynamics Hess law Enthalpy of combustion Enthalpy of formation Heat capacity Principle and tasks The
Petrochemical. Transformer Oil Gas Analysis - TOGA
 Petrochemical Transformer Oil Gas Analysis - TOGA www.dps-instruments.com The DPS TOGA GC Systems are designed to analyze oil from electrical insulation materials that may have decomposed under thermal,
Petrochemical Transformer Oil Gas Analysis - TOGA www.dps-instruments.com The DPS TOGA GC Systems are designed to analyze oil from electrical insulation materials that may have decomposed under thermal,
Safety in the Chemistry Laboratory
 Safety in the Chemistry Laboratory CHAPTER1 Safety must be everyone s primary concern in the chemistry lab. Understanding and following all safety rules in the organic chemistry lab is critical to your
Safety in the Chemistry Laboratory CHAPTER1 Safety must be everyone s primary concern in the chemistry lab. Understanding and following all safety rules in the organic chemistry lab is critical to your
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *6384565021* PHYSICS 0625/33 Paper 3 Extended October/November 2013 1 hour 15 minutes Candidates
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *6384565021* PHYSICS 0625/33 Paper 3 Extended October/November 2013 1 hour 15 minutes Candidates
NORMACLAMP Hose Clamps
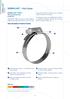 NORMACLAMP NORMACLAMP NORMACLAMP TORRO Worm Drive Hose Clips to DIN 3017 Short description of technical features NORMACLAMP TORRO hose clips are specially suitable for applications under high mechanical
NORMACLAMP NORMACLAMP NORMACLAMP TORRO Worm Drive Hose Clips to DIN 3017 Short description of technical features NORMACLAMP TORRO hose clips are specially suitable for applications under high mechanical
Mechanics. Surface tension by the ring method (Du Nouy method) Mechanics of Liquids and Gaseous Bodies. What you need:
 Mechanics of Liquids and Gaseous Bodies Mechanics Surface tension by the ring method (Du Nouy method) What you can learn about Surface energy Interface Surface tension Adhesion Critical point Eötvös equation
Mechanics of Liquids and Gaseous Bodies Mechanics Surface tension by the ring method (Du Nouy method) What you can learn about Surface energy Interface Surface tension Adhesion Critical point Eötvös equation
Gas Chromatography (GC)
 Gas Chromatography (GC) Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11541 Saudi Arabia Office: AA53
Gas Chromatography (GC) Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11541 Saudi Arabia Office: AA53
6.5. RESEARCH ON THE CONDUCTIVITY OF VARIOUS WATER SAMPLES
 6.5. RESEARCH ON THE CONDUCTIVITY OF VARIOUS WATER SAMPLES Purpose of experiment Determine the conductivity of various water samples. Tasks of experiment: Determine the average conductivity of tap water,
6.5. RESEARCH ON THE CONDUCTIVITY OF VARIOUS WATER SAMPLES Purpose of experiment Determine the conductivity of various water samples. Tasks of experiment: Determine the average conductivity of tap water,
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *6993431972* PHYSICS 0625/21 Paper 2 Core May/June 2011 1 hour 15 minutes Candidates answer on
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *6993431972* PHYSICS 0625/21 Paper 2 Core May/June 2011 1 hour 15 minutes Candidates answer on
COC Biotechnology Program
 COC Biotechnology Program High Performance Liquid Chromatography (HPLC) Version B Chromatography is used by scientists to separate one substance from another in companies such as: food and beverage, pharmaceutical,
COC Biotechnology Program High Performance Liquid Chromatography (HPLC) Version B Chromatography is used by scientists to separate one substance from another in companies such as: food and beverage, pharmaceutical,
Instruction for Use /01/08
 THE WORLD OF WEATHER DATA - THE WORLD OF WEATHER DATA - THE WORLD OF WEATHER DATA Mercury- Station Barometer Mounting Board Instruction for Use 020624/01/08 Mercury Station Barometer 3.1550.17.0xx ADOLF
THE WORLD OF WEATHER DATA - THE WORLD OF WEATHER DATA - THE WORLD OF WEATHER DATA Mercury- Station Barometer Mounting Board Instruction for Use 020624/01/08 Mercury Station Barometer 3.1550.17.0xx ADOLF
PROPULSION LAB MANUAL
 PROPULSION LAB MANUAL Measurement of Calorific Value of a Solid Fuel Sample using a Bomb Calorimeter DEPARTMENT OF AEROSPACE ENGINEERING Indian Institute of Technology Kharagpur CONTENTS 1. Introduction
PROPULSION LAB MANUAL Measurement of Calorific Value of a Solid Fuel Sample using a Bomb Calorimeter DEPARTMENT OF AEROSPACE ENGINEERING Indian Institute of Technology Kharagpur CONTENTS 1. Introduction
CHEMICAL SEPARATION EXPERIMENT 2
 CHEMICAL SEPARATION EXPERIMENT 2 INTRODUCTION The term analysis in chemistry usually refer to the quantitative and qualitative determination of the components of a sample. Qualitative refering to the identity
CHEMICAL SEPARATION EXPERIMENT 2 INTRODUCTION The term analysis in chemistry usually refer to the quantitative and qualitative determination of the components of a sample. Qualitative refering to the identity
Experiment C-15 Distillation - part 1
 1 Experiment C-15 Distillation - part 1 Objectives To learn about the three classical phases of matter, phase changes, and heating and cooling curves. To investigate the technique of distillation and to
1 Experiment C-15 Distillation - part 1 Objectives To learn about the three classical phases of matter, phase changes, and heating and cooling curves. To investigate the technique of distillation and to
How do you explain HPLC? 2 AZURA Educational System for tomorrow s HPLC professionals
 How do you explain HPLC? 2 AZURA Educational System for tomorrow s HPLC professionals AZURA Compact HPLC Analytical HPLC for the training of future HPLC professionals We believe that a lively and entertaining
How do you explain HPLC? 2 AZURA Educational System for tomorrow s HPLC professionals AZURA Compact HPLC Analytical HPLC for the training of future HPLC professionals We believe that a lively and entertaining
Lab 12 Pressure-Temperature Relationship in Gases
 Lab 12 Pressure-Temperature Relationship in Gases INTRODUCTION /PURPOSE/PLE LAB QUESTION Gases are made up of molecules that are in constant motion and exert pressure when they collide with the walls of
Lab 12 Pressure-Temperature Relationship in Gases INTRODUCTION /PURPOSE/PLE LAB QUESTION Gases are made up of molecules that are in constant motion and exert pressure when they collide with the walls of
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certifi cate of Secondary Education
 *8106479956* UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certifi cate of Secondary Education CHEMISTRY 0620/61 Paper 6 Alternative to Practical October/November 2013 1 hour
*8106479956* UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certifi cate of Secondary Education CHEMISTRY 0620/61 Paper 6 Alternative to Practical October/November 2013 1 hour
Basic Analytical Techniques, Calorimeter, and Conductivity Meter
 Basic Analytical Techniques, Calorimeter, and Conductivity Meter Santosh Vijapur ABC s of Electrochemistry 01/12/2012 Outline Calorimeter Conductivity meter ph meter Analytical balance Glassware cleaning
Basic Analytical Techniques, Calorimeter, and Conductivity Meter Santosh Vijapur ABC s of Electrochemistry 01/12/2012 Outline Calorimeter Conductivity meter ph meter Analytical balance Glassware cleaning
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *8792218070* PHYSICS 0625/23 Paper 2 Core October/November 2010 1 hour 15 minutes Candidates
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *8792218070* PHYSICS 0625/23 Paper 2 Core October/November 2010 1 hour 15 minutes Candidates
user manual blotting Hoefer PR648 Slot blot manifold um PR648-IM/Rev.F0/08-12
 user manual blotting Hoefer PR648 Slot blot manifold um PR648-IM/Rev.F0/08-12 Page finder Introduction...1 Specifications...2 Procedure for standard use of the Hoefer PR648...3 Setting up the Hoefer PR648...3
user manual blotting Hoefer PR648 Slot blot manifold um PR648-IM/Rev.F0/08-12 Page finder Introduction...1 Specifications...2 Procedure for standard use of the Hoefer PR648...3 Setting up the Hoefer PR648...3
Equation of state of ideal gases Students worksheet
 3.2.1 Tasks For a constant amount of gas (in our case air) investigate the correlation between 1. Volume and pressure at constant temperature (Boyle-Marriotte s law) 2. Temperature and volume at constant
3.2.1 Tasks For a constant amount of gas (in our case air) investigate the correlation between 1. Volume and pressure at constant temperature (Boyle-Marriotte s law) 2. Temperature and volume at constant
Experiment 2 - Using Physical Properties to Identify an Unknown Liquid
 Experiment 2 - Using Physical Properties to Identify an Unknown Liquid We usually think of chemists as scientists who do things with chemicals. We can picture a chemist's laboratory with rows of bottles
Experiment 2 - Using Physical Properties to Identify an Unknown Liquid We usually think of chemists as scientists who do things with chemicals. We can picture a chemist's laboratory with rows of bottles
DEHYDRATION OF ALCOHOLS-GAS CHROMATOGRAPHY
 DEHYDRATION OF ALCOHOLS-GAS CHROMATOGRAPHY OBJECTIVE In this lab, one will examine the phosphoric acid catalyzed dehydration of 2-methylcyclohexanol. Gas chromatography will be used to monitor the outcome
DEHYDRATION OF ALCOHOLS-GAS CHROMATOGRAPHY OBJECTIVE In this lab, one will examine the phosphoric acid catalyzed dehydration of 2-methylcyclohexanol. Gas chromatography will be used to monitor the outcome
SECTION 1 - WHAT IS A BTU METER? BTU's = Flow x ΔT Any ISTEC BTU Meter System consists of the following main components:
 SECTION 1 - WHAT IS A BTU METER? ISTEC BTU Meters measure energy usage by multiplying flow rate and temperature difference. As the water (or other liquid) passes through these lines, the multi-wing turbine
SECTION 1 - WHAT IS A BTU METER? ISTEC BTU Meters measure energy usage by multiplying flow rate and temperature difference. As the water (or other liquid) passes through these lines, the multi-wing turbine
Chromatography & instrumentation in Organic Chemistry
 Chromatography & instrumentation in Organic Chemistry What is Chromatography? Chromatography is a technique for separating mixtures into their components in order to analyze, identify, purify, and/or quantify
Chromatography & instrumentation in Organic Chemistry What is Chromatography? Chromatography is a technique for separating mixtures into their components in order to analyze, identify, purify, and/or quantify
Experiment 28 DIRECT METHANOL FUEL CELL
 Experiment 28 Direct methanol fuel cell 1 Experiment 28 DIRECT METHANOL FUEL CELL Objective The purpose of this experiment is to learn the principle of direct methanol fuel cell (DMFC) and set up a simple
Experiment 28 Direct methanol fuel cell 1 Experiment 28 DIRECT METHANOL FUEL CELL Objective The purpose of this experiment is to learn the principle of direct methanol fuel cell (DMFC) and set up a simple
Calorimetery and Hess s Law
 Calorimetery and Hess s Law Overview: Calorimetry is the technique used to measure the heat required or evolved during a chemical reaction. Heat has units of joules, so one might expect to be using a joule
Calorimetery and Hess s Law Overview: Calorimetry is the technique used to measure the heat required or evolved during a chemical reaction. Heat has units of joules, so one might expect to be using a joule
Circular Motion and Centripetal Force
 [For International Campus Lab ONLY] Objective Measure the centripetal force with the radius, mass, and speed of a particle in uniform circular motion. Theory ----------------------------- Reference --------------------------
[For International Campus Lab ONLY] Objective Measure the centripetal force with the radius, mass, and speed of a particle in uniform circular motion. Theory ----------------------------- Reference --------------------------
High-energy Hydrogen III Teacher Page
 High-energy Hydrogen III Teacher Page Student Objective The student: will be able to explain how hydrogen can be extracted from water will be able to design and conduct an experiment demonstrating how
High-energy Hydrogen III Teacher Page Student Objective The student: will be able to explain how hydrogen can be extracted from water will be able to design and conduct an experiment demonstrating how
Acid-Base Titration. Evaluation copy
 Acid-Base Titration Computer 7 A titration is a process used to determine the volume of a solution that is needed to react with a given amount of another substance. In this experiment, your goal is to
Acid-Base Titration Computer 7 A titration is a process used to determine the volume of a solution that is needed to react with a given amount of another substance. In this experiment, your goal is to
Titration of a strong acid with a strong base with Cobra4
 Titration of a strong acid with a strong base with Cobra4 TEC Related topics Strong and weak acids and bases, ph value, titration curves, equivalence point, potentiometry. Principle Hydrochloric acid is
Titration of a strong acid with a strong base with Cobra4 TEC Related topics Strong and weak acids and bases, ph value, titration curves, equivalence point, potentiometry. Principle Hydrochloric acid is
Method for the determination of 1,3-butadiene
 Federation of the Employment Accidents Insurance Institutions of Germany (Hauptverband der Berufsgenossenschaften) Centre for Accident Prevention and Occupational Medicine Alte Heerstraße 111, 53757 Sankt
Federation of the Employment Accidents Insurance Institutions of Germany (Hauptverband der Berufsgenossenschaften) Centre for Accident Prevention and Occupational Medicine Alte Heerstraße 111, 53757 Sankt
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *1264537134* PHYSICS 0625/21 Paper 2 Core October/November 2011 1 hour 15 minutes Candidates
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *1264537134* PHYSICS 0625/21 Paper 2 Core October/November 2011 1 hour 15 minutes Candidates
Cambridge International Examinations Cambridge International General Certifi cate of Secondary Education
 *0474927560* Cambridge International Examinations Cambridge International General Certifi cate of Secondary Education CHEMISTRY 06/63 Paper 6 Alternative to Practical May/June 15 1 hour Candidates answer
*0474927560* Cambridge International Examinations Cambridge International General Certifi cate of Secondary Education CHEMISTRY 06/63 Paper 6 Alternative to Practical May/June 15 1 hour Candidates answer
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *0342021651* PHYSICS 0625/42 Paper 4 Theory (Extended) February/March 2017 1 hour 15 minutes Candidates
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *0342021651* PHYSICS 0625/42 Paper 4 Theory (Extended) February/March 2017 1 hour 15 minutes Candidates
Cambridge International Examinations Cambridge Ordinary Level
 Cambridge International Examinations Cambridge Ordinary Level *4817101212* PHYSICS 5054/21 Paper 2 Theory May/June 2016 1 hour 45 minutes Candidates answer on the Question Paper. No Additional Materials
Cambridge International Examinations Cambridge Ordinary Level *4817101212* PHYSICS 5054/21 Paper 2 Theory May/June 2016 1 hour 45 minutes Candidates answer on the Question Paper. No Additional Materials
Our Drop Counter sensor now features housing for two electrode sensors, an anti-twist mechanism, an indicator LED and two cable guides.
 The Drop Counter sensor is an optical sensor that accurately records the number of drops of titrant added during a titration. The Drop Counter sensor software can automatically convert the number of drops
The Drop Counter sensor is an optical sensor that accurately records the number of drops of titrant added during a titration. The Drop Counter sensor software can automatically convert the number of drops
 Cambridge International Examinations Cambridge Ordinary Level *1298459531* PHYSICS 5054/21 Paper 2 Theory May/June 2014 1 hour 45 minutes Candidates answer on the Question Paper. No Additional Materials
Cambridge International Examinations Cambridge Ordinary Level *1298459531* PHYSICS 5054/21 Paper 2 Theory May/June 2014 1 hour 45 minutes Candidates answer on the Question Paper. No Additional Materials
Optics. Measuring the line spectra of inert gases and metal vapors using a prism spectrometer. LD Physics Leaflets P
 Optics Spectrometer Prism spectrometer LD Physics Leaflets P5.7.1.1 Measuring the line spectra of inert gases and metal vapors using a prism spectrometer Objects of the experiment Adjusting the prism spectrometer.
Optics Spectrometer Prism spectrometer LD Physics Leaflets P5.7.1.1 Measuring the line spectra of inert gases and metal vapors using a prism spectrometer Objects of the experiment Adjusting the prism spectrometer.
Partial molar volumes (Item No.: P )
 Partial molar volumes (Item No.: P3020501) Curricular Relevance Area of Expertise: Chemistry Education Level: University Topic: Physical Chemistry Subtopic: Thermochemistry, Calorimetry Experiment: Partial
Partial molar volumes (Item No.: P3020501) Curricular Relevance Area of Expertise: Chemistry Education Level: University Topic: Physical Chemistry Subtopic: Thermochemistry, Calorimetry Experiment: Partial
Experiment P-9 An Inclined Plane
 1 Experiment P-9 An Inclined Plane Objectives To understand the principles of forces on an inclined plane. To measure the parallel component of the gravitational force and compare it to the calculated
1 Experiment P-9 An Inclined Plane Objectives To understand the principles of forces on an inclined plane. To measure the parallel component of the gravitational force and compare it to the calculated
ANALYTICAL METHOD DETERMINATION OF VOLATILE ALDEHYDES IN AMBIENT AIR Page 1 of 11 Air sampling and analysis
 DETERMINATION OF VOLATILE ALDEHYDES IN AMBIENT AIR Page 1 of 11 Replaces: Dated: Author: Date: AM-No.: New New Nils Arne Jentoft 18.06.2014 0 CHANGES This procedure is new. 1 SCOPE This document describes
DETERMINATION OF VOLATILE ALDEHYDES IN AMBIENT AIR Page 1 of 11 Replaces: Dated: Author: Date: AM-No.: New New Nils Arne Jentoft 18.06.2014 0 CHANGES This procedure is new. 1 SCOPE This document describes
Cambridge International Examinations Cambridge International General Certifi cate of Secondary Education
 *74678444* Cambridge International Examinations Cambridge International General Certifi cate of Secondary Education CHEMISTRY 62/61 Paper 6 Alternative to Practical October/November 215 1 hour Candidates
*74678444* Cambridge International Examinations Cambridge International General Certifi cate of Secondary Education CHEMISTRY 62/61 Paper 6 Alternative to Practical October/November 215 1 hour Candidates
Gas Chromatography. Presented By Mr. Venkateswarlu Mpharm KTPC
 Gas Chromatography Gas Chromatography Presented By Mr. Venkateswarlu Mpharm KTPC What is Gas Chromatography? It is also known as Gas-Liquid Chromatography (GLC) GAS CHROMATOGRAPHY Separation of gaseous
Gas Chromatography Gas Chromatography Presented By Mr. Venkateswarlu Mpharm KTPC What is Gas Chromatography? It is also known as Gas-Liquid Chromatography (GLC) GAS CHROMATOGRAPHY Separation of gaseous
Mixtures 1 of 38 Boardworks Ltd 2016
 Mixtures 1 of 38 Boardworks Ltd 2016 Mixtures 2 of 38 Boardworks Ltd 2016 Pure and impure substances 3 of 38 Boardworks Ltd 2016 All materials can be classified as either a pure substance or an impure
Mixtures 1 of 38 Boardworks Ltd 2016 Mixtures 2 of 38 Boardworks Ltd 2016 Pure and impure substances 3 of 38 Boardworks Ltd 2016 All materials can be classified as either a pure substance or an impure
LABOTRON EXTRACTION & SYNTHESIS 2450 MHz
 LABOTRON EXTRACTION & SYNTHESIS 2450 MHz The LABOTRON TM series is the generic name for a ground breaking range of integrated reactor and microwave transmission systems especially designed to carry out
LABOTRON EXTRACTION & SYNTHESIS 2450 MHz The LABOTRON TM series is the generic name for a ground breaking range of integrated reactor and microwave transmission systems especially designed to carry out
Chemistry: The Central Science. Chapter 20: Electrochemistry
 Chemistry: The Central Science Chapter 20: Electrochemistry Redox reaction power batteries Electrochemistry is the study of the relationships between electricity and chemical reactions o It includes the
Chemistry: The Central Science Chapter 20: Electrochemistry Redox reaction power batteries Electrochemistry is the study of the relationships between electricity and chemical reactions o It includes the
Air Monitoring. Semi-continuous determination of ambient air quality
 Air Monitoring Semi-continuous determination of ambient air quality The Particle Into Liquid Sampler a simple solution for the determination of ions in aerosol particles 02 Combustion of fossil fuels for
Air Monitoring Semi-continuous determination of ambient air quality The Particle Into Liquid Sampler a simple solution for the determination of ions in aerosol particles 02 Combustion of fossil fuels for
Atomic and nuclear physics
 Atomic and nuclear physics X-ray physics Physics of the atomic shell LEYBOLD Physics Leaflets Moseley s law and determination of the Rydberg constant P6.3.3.6 Objects of the experiment Measuring the K-absorption
Atomic and nuclear physics X-ray physics Physics of the atomic shell LEYBOLD Physics Leaflets Moseley s law and determination of the Rydberg constant P6.3.3.6 Objects of the experiment Measuring the K-absorption
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *5638748962* PHYSICS 0625/32 Paper 3 Extended May/June 2013 1 hour 15 minutes Candidates answer
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *5638748962* PHYSICS 0625/32 Paper 3 Extended May/June 2013 1 hour 15 minutes Candidates answer
Based on the work you have completed in S1 to S3, complete Prior Learning 3.1.
 3.2: Metals In this chapter you will learn about the structure of pure metals, alloys and how they react with other substances. You will develop skills in the correct use of the SQA data booklet to create
3.2: Metals In this chapter you will learn about the structure of pure metals, alloys and how they react with other substances. You will develop skills in the correct use of the SQA data booklet to create
DATES: LAB: Liquid Chromatography Separation of Grape Kool-Aid
 NAME: AP CHEMISTRY DATES: LAB: Liquid Chromatography Separation of Grape Kool-Aid PURPOSE There are a number of analytical techniques used to separate components of a mixture, or solution. They include
NAME: AP CHEMISTRY DATES: LAB: Liquid Chromatography Separation of Grape Kool-Aid PURPOSE There are a number of analytical techniques used to separate components of a mixture, or solution. They include
Acid-Base Titration. Sample
 Acid-Base Titration Computer 7 A titration is a process used to determine the volume of a solution that is needed to react with a given amount of another substance. In this experiment, your goal is to
Acid-Base Titration Computer 7 A titration is a process used to determine the volume of a solution that is needed to react with a given amount of another substance. In this experiment, your goal is to
Even wall thickness to with stand maximum G force.
 0 3 0 CENTRIFUGE TUBES EASY TO OPEN & EASY TO CLOSE THE UNIQUE GRIP SCREW CAP PROVIDES A SECURE GRIP IN WET CONDITIONS CORROSIVE CHEMICAL PROOF PRINTING NABL APPROVED ACCURATE GRADUATION CENTRIFUGATION
0 3 0 CENTRIFUGE TUBES EASY TO OPEN & EASY TO CLOSE THE UNIQUE GRIP SCREW CAP PROVIDES A SECURE GRIP IN WET CONDITIONS CORROSIVE CHEMICAL PROOF PRINTING NABL APPROVED ACCURATE GRADUATION CENTRIFUGATION
3B SCIENTIFIC PHYSICS
 3B SCIENTIFIC PHYSICS 30 V, 50/60 Hz: 1018884 / U07001-30 115 V, 50/60 Hz: 101888 / U07001-115 Millikan s Apparatus Instruction sheet 07/16 UD/ALF Safety instructions Millikan s apparatus conforms to the
3B SCIENTIFIC PHYSICS 30 V, 50/60 Hz: 1018884 / U07001-30 115 V, 50/60 Hz: 101888 / U07001-115 Millikan s Apparatus Instruction sheet 07/16 UD/ALF Safety instructions Millikan s apparatus conforms to the
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *8627088378* PHYSICS 0625/32 Paper 3 Extended October/November 2014 1 hour 15 minutes Candidates
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *8627088378* PHYSICS 0625/32 Paper 3 Extended October/November 2014 1 hour 15 minutes Candidates
Physical Separations and Chromatography
 Lab #5A & B: Physical Separations and Chromatography Individual Objectives: At the end of these experiments you should be able to: Ø Distinguish between Rf and tr; chromatograph and chromatogram; adsorption
Lab #5A & B: Physical Separations and Chromatography Individual Objectives: At the end of these experiments you should be able to: Ø Distinguish between Rf and tr; chromatograph and chromatogram; adsorption
Inserting grids into capsule Removing capsules from grid box Immunolabeling using template guide Preparing for TEM insertion
 Protocol Immunolabeling protocols are easily adapted to mprep/g processing. This document illustrates a typical protocol using a primary antibody and a secondary antibody conjugated to colloidal gold,
Protocol Immunolabeling protocols are easily adapted to mprep/g processing. This document illustrates a typical protocol using a primary antibody and a secondary antibody conjugated to colloidal gold,
Compact ph Measuring Instruments With Pro Engineering
 testo 205, 206, 230 Compact ph Measuring Instruments With Pro Engineering With interchangeable measurement electrode ph C The new ph instruments with innovative probe engineering Measurement of the ph
testo 205, 206, 230 Compact ph Measuring Instruments With Pro Engineering With interchangeable measurement electrode ph C The new ph instruments with innovative probe engineering Measurement of the ph
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *3241510999* PHYSICS 0625/23 Paper 2 Core May/June 2014 1 hour 15 minutes Candidates answer on the
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *3241510999* PHYSICS 0625/23 Paper 2 Core May/June 2014 1 hour 15 minutes Candidates answer on the
CHROMATOGRAPHY AND MASS SPECTROMETER
 22 CHROMATOGRAPHY AND MASS SPECTROMETER 22.1 INTRODUCTION We know that the biochemistry or biological chemistry deals with the study of molecules present in organisms. These molecules are called as biomolecules
22 CHROMATOGRAPHY AND MASS SPECTROMETER 22.1 INTRODUCTION We know that the biochemistry or biological chemistry deals with the study of molecules present in organisms. These molecules are called as biomolecules
Chemistry Instrumental Analysis Lecture 31. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 31 High Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) Solvent Delivery
Chemistry 4631 Instrumental Analysis Lecture 31 High Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) Solvent Delivery
Prelab Reading Assignment: Laboratory Techniques in Organic Chemistry, 4 th Ed. Chapter 19
 CHEM 213 Technique Experiments Experiment 5: Column Chromatography Number of labs - one Reactions performed None Chemicals used: Fluorene-fluorenone mixture, hexanes, methylene chloride, silica gel Supplies
CHEM 213 Technique Experiments Experiment 5: Column Chromatography Number of labs - one Reactions performed None Chemicals used: Fluorene-fluorenone mixture, hexanes, methylene chloride, silica gel Supplies
Sulfuric acid is hazardous: Safety glasses are REQUIRED during this experiment.
 CHEMICAL COMPOSITION Life exists on Earth because of the abundant presence of liquid water. While other planets have water, it may be primarily found as either a gas, as on Venus, or as a solid, such as
CHEMICAL COMPOSITION Life exists on Earth because of the abundant presence of liquid water. While other planets have water, it may be primarily found as either a gas, as on Venus, or as a solid, such as
TRANSPIRATION With LabQuest INTRODUCTION
 TRANSPIRATION With LabQuest LAB 10 From Biology with Vernier Westminster College INTRODUCTION Water is transported in plants from the roots to the leaves, following a decreasing water potential gradient.
TRANSPIRATION With LabQuest LAB 10 From Biology with Vernier Westminster College INTRODUCTION Water is transported in plants from the roots to the leaves, following a decreasing water potential gradient.
Thermal Energy and Temperature Lab. Experiment Question: How can the difference between thermal energy and temperature be experimentally observed?
 Thermal Energy and Temperature Lab Name 7 th Grade PSI Grade / 20 Experiment Question: How can the difference between thermal energy and temperature be experimentally observed? Hypothesis Starters: 1.
Thermal Energy and Temperature Lab Name 7 th Grade PSI Grade / 20 Experiment Question: How can the difference between thermal energy and temperature be experimentally observed? Hypothesis Starters: 1.
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level
 www.xtremepapers.com UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level * 7072495009* SCIENCE 5124/02 Paper 2 Physics October/November 2009 1 hour 15 minutes
www.xtremepapers.com UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level * 7072495009* SCIENCE 5124/02 Paper 2 Physics October/November 2009 1 hour 15 minutes
IMPORTANT SAFETY INFORMATION
 CHEM 51LB EXPERIMENT 5 DEHYDRATION OF 1- AND 2-BUTANOL AND DEHYDROBROMINATION OF 1- AND 2-BROMOBUTANE: ANALYSIS OF GASEOUS PRODUCTS BY GAS CHROMATOGRAPHY 1 REACTIONS: Elimination TECHNIQUES: Gas Chromatography
CHEM 51LB EXPERIMENT 5 DEHYDRATION OF 1- AND 2-BUTANOL AND DEHYDROBROMINATION OF 1- AND 2-BROMOBUTANE: ANALYSIS OF GASEOUS PRODUCTS BY GAS CHROMATOGRAPHY 1 REACTIONS: Elimination TECHNIQUES: Gas Chromatography
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level *4711189762* CEMISTRY 5070/41 Paper 4 Alternative to Practical October/November 2013 1 hour Candidates
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level *4711189762* CEMISTRY 5070/41 Paper 4 Alternative to Practical October/November 2013 1 hour Candidates
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level *6644304748* PHYSICS 5054/21 Paper 2 Theory May/June 2013 1 hour 45 minutes Candidates answer on the Question
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level *6644304748* PHYSICS 5054/21 Paper 2 Theory May/June 2013 1 hour 45 minutes Candidates answer on the Question
Chapter 27: Gas Chromatography
 Chapter 27: Gas Chromatography Gas Chromatography Mobile phase (carrier gas): gas (He, N 2, H 2 ) - do not interact with analytes - only transport the analyte through the column Analyte: volatile liquid
Chapter 27: Gas Chromatography Gas Chromatography Mobile phase (carrier gas): gas (He, N 2, H 2 ) - do not interact with analytes - only transport the analyte through the column Analyte: volatile liquid
o Test tube In this experiment, you ll be observing the signs of chemical reactions. These include the following:
 Experiment: Chemical Reactions & Chemical s Objective In this experiment, students perform a variety of chemical reactions. For each reaction, student identify the signs that a reaction has occurred, write
Experiment: Chemical Reactions & Chemical s Objective In this experiment, students perform a variety of chemical reactions. For each reaction, student identify the signs that a reaction has occurred, write
NAME:.ADM NO: SCHOOL..CANDIDATE S SIGNATURE 232/1 PHYSICS PAPER 1 TIME 2 HOURS
 NAME:.ADM NO: SCHOOL..CANDIDATE S SIGNATURE DATE: EMBU NORTH COMMON EVALUATION EXAMINATION 2 ND TERM YEAR 2018 232/1 PHYSICS PAPER 1 TIME 2 HOURS INSTRUCTIONS TO CANDIDATE: (a) Write your name and admission
NAME:.ADM NO: SCHOOL..CANDIDATE S SIGNATURE DATE: EMBU NORTH COMMON EVALUATION EXAMINATION 2 ND TERM YEAR 2018 232/1 PHYSICS PAPER 1 TIME 2 HOURS INSTRUCTIONS TO CANDIDATE: (a) Write your name and admission
Thermochemistry/Calorimetry LEC Heat capacity of gases. What you need: What you can learn about. Principle and tasks
 Thermochemistry/Calorimetry LEC 02 What you can learn about 1st law of thermodynamics Universal gas constant Isobars Isotherms Isochors and adiabatic changes of state Principle and tasks Heat is added
Thermochemistry/Calorimetry LEC 02 What you can learn about 1st law of thermodynamics Universal gas constant Isobars Isotherms Isochors and adiabatic changes of state Principle and tasks Heat is added
Wöhler DP 700 Leakage Tester
 LEAKAGE CLASS ACHIEVED? Wöhler DP 700 Leakage Tester The Measure of Technology Made in Germany COLOR DISPLAY EINFACHE BEDIENUNG Wöhler DP 700 Leakage Tester Just 3 entries to classify an air ductwork system
LEAKAGE CLASS ACHIEVED? Wöhler DP 700 Leakage Tester The Measure of Technology Made in Germany COLOR DISPLAY EINFACHE BEDIENUNG Wöhler DP 700 Leakage Tester Just 3 entries to classify an air ductwork system
