Diffusion pathways of hydrogen across the steps of a vicinal Si(001) surface
|
|
|
- Clifford Stevens
- 5 years ago
- Views:
Transcription
1 Diffusion pathways of hydrogen across the steps of a vicinal Si(001) surface M. Lawrenz, 1 P. Kratzer, 2,3 C. H. Schwalb, 1 M. Dürr, 1,4 and U. Höfer 1 1 Fachbereich Physik und Zentrum für Materialwissenschaften, Philipps-Universität, D Marburg, Germany 2 Fritz-Haber-Institut der Max-Planck-Gesellschaft, Faradayweg 4-6, D Berlin, Germany 3 Fachbereich Physik, Universität Duisburg-Essen, D Duisburg, Germany 4 Fakultät Angewandte Naturwissenschaften, Hochschule Esslingen, D Esslingen, Germany Received 5 January 2007; published 26 March 2007 Hydrogen diffusion across D B steps on Si 001 surfaces is investigated by means of variable-temperature scanning tunneling microscopy and first-principles calculations. Experimentally, the hopping rate for diffusion from the step sites to the Si dimers of the upper terrace was found to be more than one order of magnitude higher than that for diffusion to the lower terrace. This clear preference, opposite to the trend for the respective binding energies, is explained by first-principles calculations that identify a metastable intermediate to be responsible for the unexpected lowering of the energy barrier for upward diffusion. DOI: /PhysRevB PACS number s : Jk, Ef, Mb, r I. INTRODUCTION Surface diffusion is an important elementary step in many technological processes, such as crystal growth or reactive surface etching. With the possibility to follow single atomic events by microscopy, much insight has been gained into the complex microscopic mechanisms governing these processes. In particular, scanning tunneling microscopy STM studies have contributed much to the atomistic understanding of diffusion processes on semiconductor surfaces. However, the complexity of real surfaces due to steps, kinks, etc. and the possibly important role of rare events leaves ample room for further exploration. This is exemplified by state-of-the-art simulations of crystal growth, where for most materials the simple solid-on-solid model 1 still prevails due to the lack of more detailed microscopic information that could be input to the simulations. This widely used approach relies on the assumption that the activation energy for a process depends only on its initial state, or, more generally, on a linear combination of the initial and final state energies. Only in a few cases, e.g., self-diffusion on metal surfaces, 2 this assumption has been tested theoretically. In situations where steps play an important role, e.g., for the step-flow growth mode of silicon, it is common practice to add the relevant processes in a phenomenological way to the growth model. 3 Alternatively, calculated barrier heights from first-principles calculations for Si diffusion across steps could be used. 4 In the present paper, we show that the assumed proportionality of energy barriers and binding energies cannot be generalized to step diffusion at semiconductor surfaces. Real-space, real-time measurements of hydrogen diffusion at step sites of Si 001 show a clear preference of diffusion from the steps to the upper terrace although the binding energy on the upper terrace is lower than on the lower terrace. This observation is in contradiction to common assumptions about the scaling of diffusion barriers. However, it can be explained by calculations using density-functional theory: It is found that the geometric and electronic configurations near the steps support a metastable intermediate state along the diffusion path from the step to the upper terrace, which reduces the diffusion barrier. Such an intermediate state is absent for diffusion to the lower terrace. Hydrogen diffusion on the flat Si 001 surface has been in the focus of previous STM studies. 5 7 Activation energies for hopping between the two dangling bonds of one dimer 1.0 ev Ref. 7 and along the dimer rows 1.7 ev Ref. 5 were determined and found to be in qualitative agreement with theoretical results Diffusion from one dimer row to the next is not observed experimentally due to the highenergy barrier for this process Steps perpendicular to the dimer rows are expected to hinder the quasi-one-dimensional diffusive motion along the rows, i.e., hopping across steps is a rare event. While STM studies of diffusion were so far limited to relatively frequent events, we succeeded to extend this technique to the rare hopping events across steps by exploiting the adsorption properties of the H/Si 001 system. Dissociative adsorption of molecular H 2 has a much lower activation barrier at double-height D B step sites than at terrace sites As a consequence, at room temperature H 2 adsorption is highly site selective for double-height steps and it is possible to fully decorate the steps with atomic hydrogen while keeping the terraces free. 15,16 Hydrogen diffusion away from the step sites can then be imaged in real time with a variable-temperature STM at surface temperatures of 550 K and higher. In this way, the various single atomic hopping processes that occur at step sites can be identified and the corresponding microscopic diffusion rates can be determined. II. EXPERIMENT The measurements presented here were performed using a commercial variable-temperature STM Omicron VT-STM. The experimental setup is enclosed in an ultrahigh vacuum chamber with a base pressure of about mbar. All experiments were performed with n-doped vicinal Si 001 samples cut under an angle of 5.5 ±0.5 toward the 110 direction. The Si 001 surfaces were prepared by resistive current heating. 16 After the preparation, the surface shows a 2 1 structure and consists of terraces separated by straight steps two atomic layers in height, known as D B steps. 17 The surface temperature was calibrated against the heating current by using a K-type thermocouple, which was glued onto the sample after a complete cycle of experiments. Hydrogen gas % purity was dosed via a gas inlet system /2007/75 12 / The American Physical Society
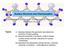
2 LAWRENZ et al. were analyzed. It is obvious that most of the step sites remain saturated black step sites. Information on the diffusion processes was obtained from the sites where the brightness changes between two subsequent images. III. THEORY FIG. 1. Color online Upper row: STM-images of the Si 001 surface with clean left and hydrogen-saturated D B steps right. Hydrogen adsorption is seen to suppress the bright features associated with the dangling bonds of the step sites. The black bar in the left and the white bar in the right image indicate the upper edge of a terrace; the step saturation is 98%. Second row: subsequently acquired STM images of the same area Å 2 at T s =568 K. Hydrogen diffusion away from the H-saturated, dark step sites in the left image to the first dimer on the terrace can be seen as a newly appearing bright spot in the right image and is marked by red dark gray circles. Hopping from this configuration back to the step site is identified by the disappearance of these features marked by blue light gray circles. equipped with a liquid nitrogen trap to freeze out residual H 2 O. The quality of the surface preparation was checked by scanning areas of the clean Si 001 surface as large as Å 2. A tunneling voltage U= 2.1 V and tunneling currents from I = 0.21 na to I = 0.44 na were employed to acquire filled-state images. Empty-state images were measured under conditions of U= +0.8 V and similar tunneling currents; the tunneling conditions were carefully adjusted not to induce movements of the adsorbates by the scanning process itself. An example is shown in Fig. 1, where the D B steps can be easily identified. With a hydrogen exposure of L 1 L= mbar s, the selective adsorption at the D B sites leads to hydrogen saturated step edges but otherwise clean Si 001. A maximum step saturation of 99% was obtained. This saturation of the step sites is observed both in the filled-state images negative sample bias and unfilled-state images positive sample bias by the disappearance of the bright features at the step sites. 16 Since different configurations of the hydrogen atoms at the step sites can be distinguished more easily in empty-state images, these images are used exclusively in the following discussion. For the real-time diffusion measurements, successive scans of the same area of the surface after definite time intervals of 92 s were performed at 568 K. As shown in Fig. 1 lower panels, representative regions of the Si 001 surface could be relocated and the configurations near the step edge In order to understand the microscopic mechanism of hydrogen diffusion across Si step edges, especially the experimentally observed asymmetry between step-up and stepdown diffusion see below, we performed first-principles total-energy calculations for various atomic configurations. We used density-functional theory DFT in conjunction with pseudopotentials and a plane-wave basis set. 18 The exchange-correlation functional was treated by the generalized-gradient approximation GGA. 19 For silicon, we generated an ab initio, norm-conserving pseudopotential, 20 while the bare Coulomb potential was used for hydrogen. We performed transition state searches for H diffusion in the configuration space of the H atoms and the Si atoms of the four topmost layers using the ridge method. 21 In addition, we checked that the found transition state indeed connects the intended potential minima by performing unconstrained relaxations of the atomic positions for starting configurations slightly displaced to either side of the transition state. All calculations, including geometry optimizations, were performed with a basis set of plane waves up to a cutoff energy of 30 Ry. For the barrier heights reported below, the calculations were repeated with the same geometries, but with a cutoff of 40 Ry. As geometrical model for the D B step, vicinal surfaces with Miller indices 1 17 or were employed by using a monoclinic supercell for the calculations. In this geometry, periodically repeated rebonded D B steps are separated by terraces with two or four Si dimers, respectively. Four k points in the irreducible part of the Brillouin zone were used for k-space integration. In order to support the assignment of observed STM images to atomic configurations, the output of the DFT calculations was used to generate calculated STM images, using the Tersoff-Hamann approximation. 22 For simulating the empty-state images, the unoccupied electronic density of states was integrated in an energy interval between the top of the valence band and 0.7 ev above it. Subsequently, the isocontour surface of this integrated density of states was plotted at a value of bohr 3 above the surface and compared to the experimental STM images. This calculational procedure corresponds to an infinitely sharp, -functionlike tip. IV. EXPERIMENTAL RESULTS The first step in the analysis of the changes between successive STM images is the identification of the relevant configurations. In addition to a comparison with previous experimental results for H adsorption on Si 001, 16,23,24 relevant configurations were identified by comparing to simulated empty-state STM images, as shown in Fig. 2. Note that the simulated STM images were calculated for supercells with a period of four lattice constants two dimer rows in the di
3 DIFFUSION PATHWAYS OF HYDROGEN ACROSS THE FIG. 2. Measured left panels and calculated right panels STM images for different configurations of hydrogen at the step edge. In the middle, the situation is sketched with occupied Si atoms dark gray and bare Si atoms light gray and white. The upper terrace is in the left part of the images. The saturated step B can clearly be distinguished from a pair of unsaturated step sites A. One hydrogen atom has moved to the adjacent Si dimer of the upper terrace in C and to the lower Si dimer in D. rection parallel to the step, whereas the experimental images show single, isolated features. In the experimental images, a saturated step site as in B can be clearly distinguished from the completely H-free step site A. The former appears dark, while the latter shows a very bright feature due to two dangling bonds. The calculated images support this interpretation, demonstrating that the STM is mainly sensitive to the electronic density of states in the band gap introduced by the dangling bonds. While saturating the dangling bond of a Si atom in a Si dimer by a hydrogen atom quenches its density of states, the persisting dangling bond at the other Si atom of the dimer appears brighter than usual. This dangling bond is shifted up in energy and partly depleted of charge when the H atom adsorbs on the neighboring Si atom and therefore becomes more pronounced in an empty-state STM image. This effect allows us to identify motions of the H atoms, both in the experimental and in the simulated STM images. Since the simulated STM images correspond to an unrealistically sharp tip, more detailed features are visible in the simulations, but a comparison of the brightest features only allows for a consistent interpretation. In C, a configuration is shown where one hydrogen atom is bound to the step edge and another one to the adjacent dimer of the upper terrace. We note that C is the configuration which is expected after a hydrogen hopping event to the upper terrace, i.e., step-up diffusion. It is similar to the configuration after a hopping event from the step site onto the lower terrace, i.e., stepdown diffusion, shown in D. However, for the latter one the hydrogen atom on the lower terrace induces another bright spot on the respective dimer row on the lower terrace. This allows for a clear distinction between step-up and step-down diffusion. Analyzing images like those in Fig. 1, the preferred diffusion pathway for atomic hydrogen away from the D B step sites can be identified: Hopping from the steps onto the upper terrace is strongly preferred. Additionally, we observe a high probability for the hydrogen atom to diffuse back to the step sites: In the STM images, back diffusion from the upper terrace can be identified by the disappearance of feature C between two successive images see Fig. 1. A first estimate for the hopping rates e.g., for the step-up diffusion rate can be obtained by counting the number of all identified events, e.g., where a step site changes from type B to C, and by dividing by the number of initially occupied sites and the elapsed time t=92 s. With 193 events out of 1912 initial configurations for diffusion from the steps to the upper terraces, we calculated a diffusion rate of s 1 for this process. Likewise, this simple analysis leads to diffusion rates of s 1 for the diffusion back from the upper terrace onto the steps and s 1 for diffusion along the dimer rows. Diffusion of H atoms from the step sites to the lower terraces is found to have the smallest rate: Only eight of such events could be observed for this diffusion process leading to a rate of s 1. For the given diffusion rates associated with diffusion onto the upper terrace and back to the step edge, there exists a non-negligible probability that the H atoms perform multiple hops between two 92 s scans. For a more accurate treatment of the step-up diffusion rate, we therefore consider a system of three coupled rate equations describing the population of the step site P s, of the upper terrace edge site P te, and of an adjacent regular terrace site P tr. Diffusion from the step to the lower terrace can safely be neglected in this analysis because of its very low diffusion rate, dp s /dt = R step up P s 1 P te + R terrace step P te 1 P s, dp te /dt = R step up P s + R terrace P tr 1 P te R terrace step 1 P s + R terrace 1 P tr P te, dp tr /dt = R terrace P te 1 P tr P tr / 1+R terrace t. We solve the coupled rate equations for two different initial conditions, i.e., initial occupation of the step site alone, P s =1, P te =0, and P tr =0, and initial occupation of only the upper terrace edge site, P te =1, P s =0, and P tr =0. For the rate constant for terrace diffusion R terrace, we used the value as determined by direct counting these events, i.e., s 1 at T=568 K. This rate might be a slight underestimation due to multiple hopping events as well. However, the difference between this value and the diffusion rates reported for the flat Si 001 surface s 1 Ref. 5 might be rather attributed to the differences in the barrier heights on the six-dimer-wide terraces of the vicinal surface and on flat Si 001. The hitherto unknown rate constants for hopping from the step to the upper terrace R step up and for the reverse process R terrace step are then obtained by fitting the appropriate solution of the rate equations over the time interval of the measurement, t=92 s, to the measured prob
4 LAWRENZ et al. TABLE I. Experimentally derived rates at 568 K and estimated barriers, as well as energy barriers from first-principles calculations for elementary hops. The error bars in the experimental values describe the statistical error as estimated from the square root of the counts. The values for R step up and R terrace step were derived by analysis via the coupled rate equations. T=568 K Rate exp. R 10 3 s 1 Estimated barrier E A ev Barrier DFT E B ev R step up 1.71± R step down 0.05± R terrace step 4.41± R terrace 2.45± abilities. In this way, the microscopic diffusion rate R step up = s 1 at 568 K is obtained. It is more than 30 times higher than R step down = s 1,asobtained by directly analyzing the same STM images. In other words, more than 95% of all events were recognized as step-up diffusion. Analogously, the rate for hopping from the upper terrace edge site back to the step site back diffusion is obtained as R terrace step = s 1. Independent of the details of the analysis, it is found to be larger than hopping away from the step by a constant factor of 3. All the measured rates are summarized in Table I. For easier comparison with calculated energy barriers see below, the experimental rates have been converted to barrier heights under the assumption of a pre-exponential factor =10 13 s 1 middle column in Table I. At this point, it should be noted that it is difficult to distinguish between sites with the H atom on the first dimer and sites with the H atom on the second dimer of the upper terrace. We therefore combined these two sites in the evaluation of the STM images and refer to them jointly as terrace edge sites whose population is denoted by P te. Depending on the actual potential-energy surface, this might cause, e.g., slight under- and overestimation of the barriers for step-up and intrarow diffusion, respectively. However, when considering the two sites separately in a more complex scenario, we did not find any noteworthy changes. The hopping rate R step up = s 1 is nearly one order of magnitude larger than the step depletion rate as determined by means of second-harmonic generation SHG measurements. 15 However, for a comparison one has to keep in mind that, due to back diffusion, this depletion rate must be lower than the microscopic hopping rate R step up,asdetermined by means of STM. In order to check the consistency between both experimental methods, we repeated our measurements at a lower temperature, aiming at the determination of the overall step depletion rate. A series of images was acquired at 550 K, and the step depletion as a function of time was recorded. Starting with a step coverage of 95% at t=0, fitting an exponential decay to the step coverage yields an overall step depletion rate of s 1. This result is in good agreement with the value of s 1 extrapolated from the Arrhenius behavior previously found. 15 We note that the present experimental setup is unsuitable to test the Arrhenius law, since working at higher temperatures would destroy the special initial state step saturation that is crucial for our approach. V. ANALYSIS OF THE DIFFUSION MECHANISM The experimental results on the diffusion rates from the step sites to the lower and upper terraces are surprising when compared to the respective binding energies. The H-Si bond is strongest at the step sites. 15 On the terraces, the hydrogen is found to be weaker bound on the sites above the step edge when compared to the sites below the step edge. 25 As a consequence, one might expect a higher diffusion barrier and therefore lower diffusion rate for diffusion from the steps to the upper terrace when compared to diffusion from the steps to the lower terrace, in contrast to our experimental observations. In order to gain an atomistic understanding of this unexpected behavior and its underlying mechanisms, we performed first-principles total-energy calculations for various atomic configurations. As energy reference for our calculations, we chose the situation where the rebonded Si atoms at the step are completely hydrogen saturated, while all surface Si atoms on the terraces are free of hydrogen. As known from previous work, step sites bind hydrogen more strongly than any terrace site. 15,25 Hence, this initial configuration is the thermodynamic ground state at low coverage and low temperature. To investigate the binding energies of an H atom at other surface sites, we move one H atom from the rebonded Si step atom to a different surface Si atom of our choice, keeping the H coverage constant. In all calculations, attaching a H atom to a buckled Si dimer reduces the buckling angle to a few degrees. We find a slight energetic preference of the H atom binding to the lower Si atom of the dimer. Moreover, the binding energy of the H atom is found to decrease monotonously from the lower end to the higher end of the terrace. These findings are in agreement with earlier calculations for pairs of H atoms, both adsorbed at the same Si dimer on a vicinal surface. 25 The transition state searches identify a concerted process for H diffusion across the step, involving rebonding and relaxation of the surface Si atoms cf. Fig. 3. For diffusion of H from a step site to the upper terrace, the first stage is the insertion of the H atom into the Si-Si bond of the rebonded Si atom at the step edge, associated with a barrier at T u1 of 1.36 ev. Two further, larger energy barriers, 1.78 and 1.96 ev relative to the ground state, occur at T u2 and T u3, respectively, when the H atom climbs up to the first Si dimer on the upper terrace. Between T u2 and T u3, establishing a weak bond to the Si atom at the upper ledge introduces an intermediate state. Therefore, the barrier is lower than expected for a hypothetical one-step process. We note that a similar lowering of diffusion barriers by an intermediate has been found in calculations addressing H diffusion away from a Si-ad-dimer on Si The potential energy for the diffusion pathway away from the step site D B is displayed in Fig. 4. The weak local minimum associated with the intermediate state for step-up diffusion is illustrated by the ball-and-stick model at the upper right of Fig. 4. The calculations show that the binding energy
5 DIFFUSION PATHWAYS OF HYDROGEN ACROSS THE FIG. 3. a Calculated atomic configurations during H diffusion, seen in top view. Small balls are H atoms and large balls are Si atoms; multiple images of the atoms, appearing in different shading, indicate concerted motions of the atoms along the diffusion pathway. The lower right part of the figure illustrates step-up diffusion and the upper left part illustrates step-down diffusion. The atoms shown in a are highlighted in the perspective view of the step shown in b. of the H atom at the first Si dimer of the upper terrace minimum D 1 in Fig. 4 is significantly reduced by 0.6 ev compared to the step site, whereas the reduction is only 0.3 ev for the adjacent Si dimer on the lower terrace minimum D 1 in Fig. 4. This can be understood from the fact that the H atom bound to the upper terrace edge breaks a chain of bonds between the dangling bond orbitals of the Si atoms along the upper edge. Only after overcoming the energy barrier of 2.17 ev at T t, the H atom reaches a more stable position at the second Si dimer on the upper terrace minimum D 2 in Fig. 4. Therefore, we use the value of 2.17 ev when comparing to the experimental apparent activation energy for step-up diffusion in Table I. Similarly, the rate-limiting step for diffusion from the upper terrace to the step is the overcoming of the energy barrier T t coming from the right in Fig. 4, which amounts to 1.84 ev. Since the two Si atoms of the Si dimer on the upper terrace edge are geometrically not equivalent, there are, strictly speaking, two different diffusion pathways for hydrogen atoms from the step to the upper terrace, one leading to the lower Si atom of the Si terrace edge dimer and one leading to its upper Si atom. By calculating the diffusion pathway and transition states for both cases, we find that the potentialenergy surface is very similar, and energy barriers agree within 0.1 ev. In particular, the intermediate state responsible for the lowering of barriers is found to be present in both pathways. Diffusion from the step to the lower terrace step-down diffusion is calculated to be associated with an energy barrier of 2.81 ev at transition state T d. Again, diffusion occurs as a concerted process that also involves considerable motion of the Si atoms. In order to overcome the large distance between initial and final states, the rebonded Si atom at the step partially follows the motion of the H atom away from the step. Therefore, its Si-Si bond to the ledge is broken at the transition state T d inset at the upper left of Fig. 4, and only restored after the H atom has moved to the Si dimer on the lower terrace lower left of Fig. 4. In contrast to step-up diffusion, no intermediate stabilization by weak bonds to Si atoms is possible along this pathway. Thus, the one-step hopping process for step-down diffusion results in a much higher barrier compared to step-up diffusion. To put the barrier heights into perspective, we also calculate the barrier for H diffusion along the Si dimer rows on the terrace. The obtained value of 1.89 ev is larger than the estimate of 1.76 ev derived from the experiments. This is in line with the general trend observed in this study. We therefore conclude that the GGA functional 19 generally overestimates hydrogen diffusion barriers, while it correctly predicts the relation between barrier heights of different processes. They are summarized in Table I and follow the same order as the experimentally derived activation energies. VI. SUMMARY FIG. 4. Calculated potential energy surface for diffusion of one H atom from a step site lowest minimum D B to the Si dimers on the upper terrace minima D 1 and D 2 in the right part of the plot or to the Si dimer on the lower terrace minimum D 1 to the left of the plot. The insets below show the geometry of the local minima and the insets above show the transition state for step-down diffusion T d and the metastable intermediate state for step-up diffusion. All pictures are in side view, small balls are H atoms, and large balls are Si atoms. In summary, we find that H atoms bound to step sites on vicinal Si 001 have a strong preference to diffuse to the upper terrace, which is unexpected when judging on the basis of the binding energies with the binding energy for H at the upper terrace sites being lower than on the lower terrace sites. Density-functional theory calculations show that this is due to a reduction of the energy barrier for diffusion to the upper ledge by stabilization of an intermediate state. We believe that such stabilization is of relevance also for other atomic species diffusing across steps on Si 001. More importantly, this study shows that simple rules relating diffusion barriers to initial or final state energies are not generally applicable to semiconductor surfaces
6 LAWRENZ et al. 1 G. H. Gilmer and P. Bennema, J. Appl. Phys. 43, K. A. Fichthorn and M. Scheffler, Phys. Rev. Lett. 84, B. Voigtländer, Th. Weber, P. Šmilauer, and D. E. Wolf, Phys. Rev. Lett. 78, E. Kim, C. W. Oh, and Y. H. Lee, Phys. Rev. Lett. 79, J. H. G. Owen, D. R. Bowler, C. M. Goringe, K. Miki, and G. A. D. Briggs, Phys. Rev. B 54, M. McEllistrem, M. Allgeier, and J. J. Boland, Science 279, E. Hill, B. Freelon, and E. Ganz, Phys. Rev. B 60, A. Vittadini, A. Selloni, and M. Casarin, Surf. Sci. 289, L C. J. Wu, I. V. Ionova, and E. A. Carter, Phys. Rev. B 49, P. Nachtigall and K. D. Jordan, J. Chem. Phys. 102, P. Kratzer, E. Pehlke, M. Scheffler, M. B. Raschke, and U. Höfer, Phys. Rev. Lett. 81, M. B. Raschke and U. Höfer, Appl. Phys. B: Lasers Opt. 68, M. Dürr, M. B. Raschke, E. Pehlke, and U. Höfer, Phys. Rev. Lett. 86, M. Dürr and U. Höfer, Surf. Sci. Rep. 61, M. B. Raschke and U. Höfer, Phys. Rev. B 59, M. Dürr, Z. Hu, A. Biedermann, U. Höfer, and T. F. Heinz, Phys. Rev. B 63, R D. J. Chadi, Phys. Rev. Lett. 59, M. Bockstedte, A. Kley, J. Neugebauer, and M. Scheffler, Comput. Phys. Commun. 107, J. P. Perdew, J. A. Chevary, S. H. Vosko, K. A. Jackson, M. R. Pederson, D. J. Singh, and C. Fiolhais, Phys. Rev. B 46, M. Fuchs and M. Scheffler, Comput. Phys. Commun. 116, I. V. Ionova and E. A. Carter, J. Chem. Phys. 98, J. Tersoff and D. R. Hamann, Phys. Rev. B 31, M. Dürr, Z. Hu, A. Biedermann, U. Höfer, and T. F. Heinz, Phys. Rev. Lett. 88, M. Dürr, A. Biedermann, Z. Hu, U. Höfer, and T. F. Heinz, Science 296, E. Pehlke and P. Kratzer, Phys. Rev. B 59, D. R. Bowler, Phys. Rev. B 67,
Chemical Physics Letters
 Chemical Physics Letters 483 (2009) 209 213 Contents lists available at ScienceDirect Chemical Physics Letters journal homepage: www.elsevier.com/locate/cplett Site-selective reactivity of ethylene on
Chemical Physics Letters 483 (2009) 209 213 Contents lists available at ScienceDirect Chemical Physics Letters journal homepage: www.elsevier.com/locate/cplett Site-selective reactivity of ethylene on
arxiv:mtrl-th/ v2 5 Mar 1997
 Ab initio molecular dynamics study of the desorption of from Si(100) Axel Gross, Michel Bockstedte, and Matthias Scheffler Fritz-Haber-Institut der Max-Planck-Gesellschaft, Faradayweg 4-6, D-14195 Berlin-Dahlem,
Ab initio molecular dynamics study of the desorption of from Si(100) Axel Gross, Michel Bockstedte, and Matthias Scheffler Fritz-Haber-Institut der Max-Planck-Gesellschaft, Faradayweg 4-6, D-14195 Berlin-Dahlem,
Effect of the cluster size in modeling the H 2 desorption and dissociative adsorption on Si 001
 JOURNAL OF CHEMICAL PHYSICS VOLUME 110, NUMBER 8 22 FEBRUARY 1999 Effect of the cluster size in modeling the H 2 desorption and dissociative adsorption on Si 001 E. Penev, P. Kratzer, and M. Scheffler
JOURNAL OF CHEMICAL PHYSICS VOLUME 110, NUMBER 8 22 FEBRUARY 1999 Effect of the cluster size in modeling the H 2 desorption and dissociative adsorption on Si 001 E. Penev, P. Kratzer, and M. Scheffler
Influence of steps and defects on the dissociative adsorption of molecular hydrogen on silicon surfaces
 Appl. Phys. B 68, 649 655 (1999) Applied Physics B Lasers and Optics Springer-Verlag 1999 Influence of steps and defects on the dissociative adsorption of molecular hydrogen on silicon surfaces M.B. Raschke,
Appl. Phys. B 68, 649 655 (1999) Applied Physics B Lasers and Optics Springer-Verlag 1999 Influence of steps and defects on the dissociative adsorption of molecular hydrogen on silicon surfaces M.B. Raschke,
Scanning Tunneling Microscopy. how does STM work? the quantum mechanical picture example of images how can we understand what we see?
 Scanning Tunneling Microscopy how does STM work? the quantum mechanical picture example of images how can we understand what we see? Observation of adatom diffusion with a field ion microscope Scanning
Scanning Tunneling Microscopy how does STM work? the quantum mechanical picture example of images how can we understand what we see? Observation of adatom diffusion with a field ion microscope Scanning
Prerequisites for reliable modeling with first-principles methods. P. Kratzer Fritz-Haber-Institut der MPG D Berlin-Dahlem, Germany
 Prerequisites for reliable modeling with first-principles methods P. Kratzer Fritz-Haber-Institut der MPG D-14195 Berlin-Dahlem, Germany Prerequisites for modeling (I) Issues to consider when applying
Prerequisites for reliable modeling with first-principles methods P. Kratzer Fritz-Haber-Institut der MPG D-14195 Berlin-Dahlem, Germany Prerequisites for modeling (I) Issues to consider when applying
High resolution STM imaging with oriented single crystalline tips
 High resolution STM imaging with oriented single crystalline tips A. N. Chaika a, *, S. S. Nazin a, V. N. Semenov a, N. N Orlova a, S. I. Bozhko a,b, O. Lübben b, S. A. Krasnikov b, K. Radican b, and I.
High resolution STM imaging with oriented single crystalline tips A. N. Chaika a, *, S. S. Nazin a, V. N. Semenov a, N. N Orlova a, S. I. Bozhko a,b, O. Lübben b, S. A. Krasnikov b, K. Radican b, and I.
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/315/5819/1692/dc1 Supporting Online Material for Enhanced Bonding of Gold Nanoparticles on Oxidized TiO 2 (110) D. Matthey, J. G. Wang, S. Wendt, J. Matthiesen, R. Schaub,
www.sciencemag.org/cgi/content/full/315/5819/1692/dc1 Supporting Online Material for Enhanced Bonding of Gold Nanoparticles on Oxidized TiO 2 (110) D. Matthey, J. G. Wang, S. Wendt, J. Matthiesen, R. Schaub,
Scanning tunneling microscopy of monoatomic gold chains on vicinal Si(335) surface: experimental and theoretical study
 phys. stat. sol. (b) 4, No., 33 336 (005) / DOI 10.100/pssb.00460056 Scanning tunneling microscopy of monoatomic gold chains on vicinal Si(335) surface: experimental and theoretical study M. Krawiec *,
phys. stat. sol. (b) 4, No., 33 336 (005) / DOI 10.100/pssb.00460056 Scanning tunneling microscopy of monoatomic gold chains on vicinal Si(335) surface: experimental and theoretical study M. Krawiec *,
Model for nucleation in GaAs homoepitaxy derived from first principles
 PHYSICAL REVIEW B VOLUME 59, NUMBER 23 15 JUNE 1999-I Model for nucleation in homoepitaxy derived from first principles P. Kratzer Fritz-Haber-Institut der Max-Planck-Gesellschaft, Faradayweg 4-6, D-14195
PHYSICAL REVIEW B VOLUME 59, NUMBER 23 15 JUNE 1999-I Model for nucleation in homoepitaxy derived from first principles P. Kratzer Fritz-Haber-Institut der Max-Planck-Gesellschaft, Faradayweg 4-6, D-14195
Supporting Online Material (1)
 Supporting Online Material The density functional theory (DFT) calculations were carried out using the dacapo code (http://www.fysik.dtu.dk/campos), and the RPBE (1) generalized gradient correction (GGA)
Supporting Online Material The density functional theory (DFT) calculations were carried out using the dacapo code (http://www.fysik.dtu.dk/campos), and the RPBE (1) generalized gradient correction (GGA)
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/NCHEM.2491 Experimental Realization of Two-dimensional Boron Sheets Baojie Feng 1, Jin Zhang 1, Qing Zhong 1, Wenbin Li 1, Shuai Li 1, Hui Li 1, Peng Cheng 1, Sheng Meng 1,2, Lan Chen 1 and
DOI: 10.1038/NCHEM.2491 Experimental Realization of Two-dimensional Boron Sheets Baojie Feng 1, Jin Zhang 1, Qing Zhong 1, Wenbin Li 1, Shuai Li 1, Hui Li 1, Peng Cheng 1, Sheng Meng 1,2, Lan Chen 1 and
Breakdown of cation vacancies into anion vacancy-antisite complexes on III-V semiconductor surfaces
 Breakdown of cation vacancies into anion vacancy-antisite complexes on III-V semiconductor surfaces A. Höglund and S. Mirbt Department of Physics, Uppsala University, Box 530, SE-75121 Uppsala, Sweden
Breakdown of cation vacancies into anion vacancy-antisite complexes on III-V semiconductor surfaces A. Höglund and S. Mirbt Department of Physics, Uppsala University, Box 530, SE-75121 Uppsala, Sweden
Scanning tunneling microscopy on unpinned GaN(11 00) surfaces: Invisibility of valence-band states
 Scanning tunneling microscopy on unpinned GaN(11 00) surfaces: Invisibility of valence-band states Ph. Ebert, 1, * L. Ivanova, 2 and H. Eisele 2 1 Institut für Festkörperforschung, Forschungszentrum Jülich
Scanning tunneling microscopy on unpinned GaN(11 00) surfaces: Invisibility of valence-band states Ph. Ebert, 1, * L. Ivanova, 2 and H. Eisele 2 1 Institut für Festkörperforschung, Forschungszentrum Jülich
CITY UNIVERSITY OF HONG KONG. Theoretical Study of Electronic and Electrical Properties of Silicon Nanowires
 CITY UNIVERSITY OF HONG KONG Ë Theoretical Study of Electronic and Electrical Properties of Silicon Nanowires u Ä öä ªqk u{ Submitted to Department of Physics and Materials Science gkö y in Partial Fulfillment
CITY UNIVERSITY OF HONG KONG Ë Theoretical Study of Electronic and Electrical Properties of Silicon Nanowires u Ä öä ªqk u{ Submitted to Department of Physics and Materials Science gkö y in Partial Fulfillment
O 2 -coverage-dependent CO oxidation on reduced TiO : A first principles study
 THE JOURNAL OF CHEMICAL PHYSICS 125, 144706 2006 O 2 -coverage-dependent CO oxidation on reduced TiO 2 110 : A first principles study Devina Pillay and Gyeong S. Hwang a Department of Chemical Engineering,
THE JOURNAL OF CHEMICAL PHYSICS 125, 144706 2006 O 2 -coverage-dependent CO oxidation on reduced TiO 2 110 : A first principles study Devina Pillay and Gyeong S. Hwang a Department of Chemical Engineering,
Dynamics of step fluctuations on a chemically heterogeneous surface of AlÕSi )Ã)
 Dynamics of step fluctuations on a chemically heterogeneous surface of AlÕSi 111 - )Ã) I. Lyubinetsky, D. B. Dougherty, T. L. Einstein, and E. D. Williams* University of Maryland, Department of Physics
Dynamics of step fluctuations on a chemically heterogeneous surface of AlÕSi 111 - )Ã) I. Lyubinetsky, D. B. Dougherty, T. L. Einstein, and E. D. Williams* University of Maryland, Department of Physics
Reaction dynamics of molecular hydrogen on silicon surfaces
 PHYSICAL REVIEW B VOLUME 54, NUMBER 8 Reaction dynamics of molecular hydrogen on silicon surfaces P. Bratu Max-Planck-Institut für Quantenoptik, D-85740 Garching, Germany W. Brenig Physik-Department, Technische
PHYSICAL REVIEW B VOLUME 54, NUMBER 8 Reaction dynamics of molecular hydrogen on silicon surfaces P. Bratu Max-Planck-Institut für Quantenoptik, D-85740 Garching, Germany W. Brenig Physik-Department, Technische
A general rule for surface reconstructions of III V semiconductors
 Surface Science 422 (1999) L177 L182 Surface Science Letters A general rule for surface reconstructions of III V semiconductors S. Mirbt a,*, N. Moll b, A. Kley b, J.D. Joannopoulos a a Department of Physics,
Surface Science 422 (1999) L177 L182 Surface Science Letters A general rule for surface reconstructions of III V semiconductors S. Mirbt a,*, N. Moll b, A. Kley b, J.D. Joannopoulos a a Department of Physics,
Elementary Steps of the Catalytic NO x Reduction with NH 3 : Cluster Studies on Reactant Adsorption at Vanadium Oxide Substrate
 Elementary Steps of the Catalytic NO x Reduction with NH 3 : Cluster Studies on Reactant Adsorption at Vanadium Oxide Substrate M. Gruber and K. Hermann Inorg. Chem. Dept., Fritz-Haber-Institut der Max-Planck-Gesellschaft,
Elementary Steps of the Catalytic NO x Reduction with NH 3 : Cluster Studies on Reactant Adsorption at Vanadium Oxide Substrate M. Gruber and K. Hermann Inorg. Chem. Dept., Fritz-Haber-Institut der Max-Planck-Gesellschaft,
Hydrogen-induced instability on the flat Si 001 surface via steric repulsion
 PHYSICAL REVIEW B, VOLUME 63, 125316 Hydrogen-induced instability on the flat Si 001 surface via steric repulsion F. A. Reboredo,* S. B. Zhang, and Alex Zunger National Renewable Energy Laboratory, Golden,
PHYSICAL REVIEW B, VOLUME 63, 125316 Hydrogen-induced instability on the flat Si 001 surface via steric repulsion F. A. Reboredo,* S. B. Zhang, and Alex Zunger National Renewable Energy Laboratory, Golden,
Application of single crystalline tungsten for fabrication of high resolution STM probes with controlled structure 1
 Application of single crystalline tungsten for fabrication of high resolution STM probes with controlled structure 1 A. N. Chaika a, S. S. Nazin a, V. N. Semenov a, V. G. Glebovskiy a, S. I. Bozhko a,b,
Application of single crystalline tungsten for fabrication of high resolution STM probes with controlled structure 1 A. N. Chaika a, S. S. Nazin a, V. N. Semenov a, V. G. Glebovskiy a, S. I. Bozhko a,b,
1 Adsorption of NO 2 on Pd(100) Juan M. Lorenzi, Sebastian Matera, and Karsten Reuter,
 Supporting information: Synergistic inhibition of oxide formation in oxidation catalysis: A first-principles kinetic Monte Carlo study of NO+CO oxidation at Pd(100) Juan M. Lorenzi, Sebastian Matera, and
Supporting information: Synergistic inhibition of oxide formation in oxidation catalysis: A first-principles kinetic Monte Carlo study of NO+CO oxidation at Pd(100) Juan M. Lorenzi, Sebastian Matera, and
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Towards wafer-size graphene layers by atmospheric pressure graphitization of silicon carbide Supporting online material Konstantin V. Emtsev 1, Aaron Bostwick 2, Karsten Horn
SUPPLEMENTARY INFORMATION Towards wafer-size graphene layers by atmospheric pressure graphitization of silicon carbide Supporting online material Konstantin V. Emtsev 1, Aaron Bostwick 2, Karsten Horn
1 IMEM-CNR, U.O.S. Genova, Via Dodecaneso 33, Genova, IT. 2 Dipartimento di Fisica, Università di Genova, Via Dodecaneso 33, Genova, IT
 Spontaneous Oxidation of Ni Nanoclusters on MgO Monolayers Induced by Segregation of Interfacial Oxygen. M. Smerieri 1, J. Pal 1,2, L. Savio 1*, L. Vattuone 1,2, R. Ferrando 1,3, S. Tosoni 4, L. Giordano
Spontaneous Oxidation of Ni Nanoclusters on MgO Monolayers Induced by Segregation of Interfacial Oxygen. M. Smerieri 1, J. Pal 1,2, L. Savio 1*, L. Vattuone 1,2, R. Ferrando 1,3, S. Tosoni 4, L. Giordano
Spatially resolving density-dependent screening around a single charged atom in graphene
 Supplementary Information for Spatially resolving density-dependent screening around a single charged atom in graphene Dillon Wong, Fabiano Corsetti, Yang Wang, Victor W. Brar, Hsin-Zon Tsai, Qiong Wu,
Supplementary Information for Spatially resolving density-dependent screening around a single charged atom in graphene Dillon Wong, Fabiano Corsetti, Yang Wang, Victor W. Brar, Hsin-Zon Tsai, Qiong Wu,
Supplementary Figure 1: Change of scanning tunneling microscopy (STM) tip state. a, STM tip transited from blurred (the top dark zone) to orbital
 Supplementary Figure 1: Change of scanning tunneling microscopy (STM) tip state. a, STM tip transited from blurred (the top dark zone) to orbital resolvable (the bright zone). b, Zoomedin tip-state changing
Supplementary Figure 1: Change of scanning tunneling microscopy (STM) tip state. a, STM tip transited from blurred (the top dark zone) to orbital resolvable (the bright zone). b, Zoomedin tip-state changing
Correlations in coverage-dependent atomic adsorption energies on Pd(111)
 Correlations in coverage-dependent atomic adsorption energies on Pd(111) John R. Kitchin* Department of Chemical Engineering, Carnegie Mellon University, Pittsburgh, Pennsylvania 15213, USA Received 23
Correlations in coverage-dependent atomic adsorption energies on Pd(111) John R. Kitchin* Department of Chemical Engineering, Carnegie Mellon University, Pittsburgh, Pennsylvania 15213, USA Received 23
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Method: Epitaxial graphene was prepared by heating an Ir(111) crystal to 550 K for 100 s under 2 x 10-5 Pa partial pressure of ethylene, followed by a flash anneal to 1420 K 1.
SUPPLEMENTARY INFORMATION Method: Epitaxial graphene was prepared by heating an Ir(111) crystal to 550 K for 100 s under 2 x 10-5 Pa partial pressure of ethylene, followed by a flash anneal to 1420 K 1.
Construction of Two Dimensional Chiral Networks
 Supporting Information Construction of Two Dimensional Chiral Networks through Atomic Bromine on Surfaces Jianchen Lu, De-Liang Bao, Huanli Dong, Kai Qian, Shuai Zhang, Jie Liu, Yanfang Zhang, Xiao Lin
Supporting Information Construction of Two Dimensional Chiral Networks through Atomic Bromine on Surfaces Jianchen Lu, De-Liang Bao, Huanli Dong, Kai Qian, Shuai Zhang, Jie Liu, Yanfang Zhang, Xiao Lin
Protection of excited spin states by a superconducting energy gap
 Protection of excited spin states by a superconducting energy gap B. W. Heinrich, 1 L. Braun, 1, J. I. Pascual, 1, 2, 3 and K. J. Franke 1 1 Institut für Experimentalphysik, Freie Universität Berlin, Arnimallee
Protection of excited spin states by a superconducting energy gap B. W. Heinrich, 1 L. Braun, 1, J. I. Pascual, 1, 2, 3 and K. J. Franke 1 1 Institut für Experimentalphysik, Freie Universität Berlin, Arnimallee
Reconstruction and intermixing in thin Ge layers on Si 001
 Reconstruction and intermixing in thin Ge layers on Si 001 L. Nurminen, 1 F. Tavazza, 2 D. P. Landau, 1,2 A. Kuronen, 1 and K. Kaski 1 1 Laboratory of Computational Engineering, Helsinki University of
Reconstruction and intermixing in thin Ge layers on Si 001 L. Nurminen, 1 F. Tavazza, 2 D. P. Landau, 1,2 A. Kuronen, 1 and K. Kaski 1 1 Laboratory of Computational Engineering, Helsinki University of
arxiv: v1 [cond-mat.mes-hall] 15 Aug 2014
![arxiv: v1 [cond-mat.mes-hall] 15 Aug 2014 arxiv: v1 [cond-mat.mes-hall] 15 Aug 2014](/thumbs/89/97831186.jpg) The potential applications of phosphorene as anode arxiv:1408.3488v1 [cond-mat.mes-hall] 15 Aug 2014 materials in Li-ion batteries Shijun Zhao,, and Wei Kang, HEDPS, Center for Applied Physics and Technology,
The potential applications of phosphorene as anode arxiv:1408.3488v1 [cond-mat.mes-hall] 15 Aug 2014 materials in Li-ion batteries Shijun Zhao,, and Wei Kang, HEDPS, Center for Applied Physics and Technology,
Structure and dynamics of the diarsenic complex in crystalline silicon
 Structure and dynamics of the diarsenic complex in crystalline silicon Scott A. Harrison, Thomas F. Edgar, and Gyeong S. Hwang* Department of Chemical Engineering, University of Texas, Austin, Texas 78713,
Structure and dynamics of the diarsenic complex in crystalline silicon Scott A. Harrison, Thomas F. Edgar, and Gyeong S. Hwang* Department of Chemical Engineering, University of Texas, Austin, Texas 78713,
2) Atom manipulation. Xe / Ni(110) Model: Experiment:
 2) Atom manipulation D. Eigler & E. Schweizer, Nature 344, 524 (1990) Xe / Ni(110) Model: Experiment: G.Meyer, et al. Applied Physics A 68, 125 (1999) First the tip is approached close to the adsorbate
2) Atom manipulation D. Eigler & E. Schweizer, Nature 344, 524 (1990) Xe / Ni(110) Model: Experiment: G.Meyer, et al. Applied Physics A 68, 125 (1999) First the tip is approached close to the adsorbate
Atomic processes during Cl supersaturation etching of Si(100)-(2Ã 1)
 Atomic processes during Cl supersaturation etching of Si(100)-(2Ã 1) C. M. Aldao,* Abhishek Agrawal, R. E. Butera, and J. H. Weaver Department of Materials Science and Engineering, University of Illinois
Atomic processes during Cl supersaturation etching of Si(100)-(2Ã 1) C. M. Aldao,* Abhishek Agrawal, R. E. Butera, and J. H. Weaver Department of Materials Science and Engineering, University of Illinois
Modeling STM tips by single absorbed atoms on W(100) films: 5d transition metal atoms.
 Modeling STM tips by single absorbed atoms on W(100) films: 5d transition metal atoms. W. A. Hofer, 1 J. Redinger Institut für Technische Elektrochemie und Festkörperchemie and Center for Computational
Modeling STM tips by single absorbed atoms on W(100) films: 5d transition metal atoms. W. A. Hofer, 1 J. Redinger Institut für Technische Elektrochemie und Festkörperchemie and Center for Computational
SUPPLEMENTARY FIGURES
 1 SUPPLEMENTARY FIGURES Supplementary Figure 1: Initial stage showing monolayer MoS 2 islands formation on Au (111) surface. a, Scanning tunneling microscopy (STM) image of molybdenum (Mo) clusters deposited
1 SUPPLEMENTARY FIGURES Supplementary Figure 1: Initial stage showing monolayer MoS 2 islands formation on Au (111) surface. a, Scanning tunneling microscopy (STM) image of molybdenum (Mo) clusters deposited
Supporting information for: Coverage-driven. Electronic Decoupling of Fe-Phthalocyanine from a. Ag(111) Substrate
 Supporting information for: Coverage-driven Electronic Decoupling of Fe-Phthalocyanine from a Ag() Substrate T. G. Gopakumar,, T. Brumme, J. Kröger, C. Toher, G. Cuniberti, and R. Berndt Institut für Experimentelle
Supporting information for: Coverage-driven Electronic Decoupling of Fe-Phthalocyanine from a Ag() Substrate T. G. Gopakumar,, T. Brumme, J. Kröger, C. Toher, G. Cuniberti, and R. Berndt Institut für Experimentelle
Supplementary Fig. 1. Progress of the surface mediated Ullmann coupling reaction using STM at 5 K. Precursor molecules
 Supplementary Fig. 1. Progress of the surface mediated Ullmann coupling reaction using STM at 5 K. Precursor molecules (4-bromo-1-ethyl-2-fluorobenzene) are dosed on a Cu(111) surface and annealed to 80
Supplementary Fig. 1. Progress of the surface mediated Ullmann coupling reaction using STM at 5 K. Precursor molecules (4-bromo-1-ethyl-2-fluorobenzene) are dosed on a Cu(111) surface and annealed to 80
Basics of DFT applications to solids and surfaces
 Basics of DFT applications to solids and surfaces Peter Kratzer Physics Department, University Duisburg-Essen, Duisburg, Germany E-mail: Peter.Kratzer@uni-duisburg-essen.de Periodicity in real space and
Basics of DFT applications to solids and surfaces Peter Kratzer Physics Department, University Duisburg-Essen, Duisburg, Germany E-mail: Peter.Kratzer@uni-duisburg-essen.de Periodicity in real space and
Supplementary Figure 1 Experimental setup for crystal growth. Schematic drawing of the experimental setup for C 8 -BTBT crystal growth.
 Supplementary Figure 1 Experimental setup for crystal growth. Schematic drawing of the experimental setup for C 8 -BTBT crystal growth. Supplementary Figure 2 AFM study of the C 8 -BTBT crystal growth
Supplementary Figure 1 Experimental setup for crystal growth. Schematic drawing of the experimental setup for C 8 -BTBT crystal growth. Supplementary Figure 2 AFM study of the C 8 -BTBT crystal growth
EFFECTS OF STOICHIOMETRY ON POINT DEFECTS AND IMPURITIES IN GALLIUM NITRIDE
 EFFECTS OF STOICHIOMETRY ON POINT DEFECTS AND IMPURITIES IN GALLIUM NITRIDE C. G. VAN DE WALLE AND J. E. NORTHRUP Palo Alto Research Center, 3333 Coyote Hill Road, Palo Alto, CA 930, USA E-mail: vandewalle@parc.com
EFFECTS OF STOICHIOMETRY ON POINT DEFECTS AND IMPURITIES IN GALLIUM NITRIDE C. G. VAN DE WALLE AND J. E. NORTHRUP Palo Alto Research Center, 3333 Coyote Hill Road, Palo Alto, CA 930, USA E-mail: vandewalle@parc.com
Scanning Tunneling Microscopy (STM)
 Page 1 of 8 Scanning Tunneling Microscopy (STM) This is the fastest growing surface analytical technique, which is replacing LEED as the surface imaging tool (certainly in UHV, air and liquid). STM has
Page 1 of 8 Scanning Tunneling Microscopy (STM) This is the fastest growing surface analytical technique, which is replacing LEED as the surface imaging tool (certainly in UHV, air and liquid). STM has
Supplementary Materials for Oxygen-induced self-assembly of quaterphenyl molecule on metal surfaces
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supplementary Materials for Oxygen-induced self-assembly of quaterphenyl molecule on metal surfaces
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supplementary Materials for Oxygen-induced self-assembly of quaterphenyl molecule on metal surfaces
STM spectroscopy (STS)
 STM spectroscopy (STS) di dv 4 e ( E ev, r) ( E ) M S F T F Basic concepts of STS. With the feedback circuit open the variation of the tunneling current due to the application of a small oscillating voltage
STM spectroscopy (STS) di dv 4 e ( E ev, r) ( E ) M S F T F Basic concepts of STS. With the feedback circuit open the variation of the tunneling current due to the application of a small oscillating voltage
b imaging by a double tip potential
 Supplementary Figure Measurement of the sheet conductance. Resistance as a function of probe spacing including D and 3D fits. The distance is plotted on a logarithmic scale. The inset shows corresponding
Supplementary Figure Measurement of the sheet conductance. Resistance as a function of probe spacing including D and 3D fits. The distance is plotted on a logarithmic scale. The inset shows corresponding
SUPPLEMENTARY INFORMATION
 Simultaneous and coordinated rotational switching of all molecular rotors in a network Y. Zhang, H. Kersell, R. Stefak, J. Echeverria, V. Iancu, U. G. E. Perera, Y. Li, A. Deshpande, K.-F. Braun, C. Joachim,
Simultaneous and coordinated rotational switching of all molecular rotors in a network Y. Zhang, H. Kersell, R. Stefak, J. Echeverria, V. Iancu, U. G. E. Perera, Y. Li, A. Deshpande, K.-F. Braun, C. Joachim,
Vacancy migration, adatom motion, a.nd atomic bistability on the GaAs(110) surface studied by scanning tunneling microscopy
 acancy migration, adatom motion, a.nd atomic bistability on the GaAs(110) surface studied by scanning tunneling microscopy s. Gwo, A. R. Smith, and C. K. Shih Department of Physics, The University of Texas
acancy migration, adatom motion, a.nd atomic bistability on the GaAs(110) surface studied by scanning tunneling microscopy s. Gwo, A. R. Smith, and C. K. Shih Department of Physics, The University of Texas
Oxidation of Germanium and Silicon surfaces (100): a comparative study through DFT methodology
 IOP Conference Series: Materials Science and Engineering Oxidation of Germanium and Silicon surfaces (100): a comparative study through DFT methodology To cite this article: C Mastail et al 2012 IOP Conf.
IOP Conference Series: Materials Science and Engineering Oxidation of Germanium and Silicon surfaces (100): a comparative study through DFT methodology To cite this article: C Mastail et al 2012 IOP Conf.
STRUCTURAL AND MECHANICAL PROPERTIES OF AMORPHOUS SILICON: AB-INITIO AND CLASSICAL MOLECULAR DYNAMICS STUDY
 STRUCTURAL AND MECHANICAL PROPERTIES OF AMORPHOUS SILICON: AB-INITIO AND CLASSICAL MOLECULAR DYNAMICS STUDY S. Hara, T. Kumagai, S. Izumi and S. Sakai Department of mechanical engineering, University of
STRUCTURAL AND MECHANICAL PROPERTIES OF AMORPHOUS SILICON: AB-INITIO AND CLASSICAL MOLECULAR DYNAMICS STUDY S. Hara, T. Kumagai, S. Izumi and S. Sakai Department of mechanical engineering, University of
Energy band of manipulated atomic structures on an insulator substrate
 Energy band of manipulated atomic structures on an insulator substrate Toshishige Yamada and Yoshihisa Yamamoto ERATO Quantum Fluctuation Project, Edward L. Ginzton Laboratory, Stanford University, Stanford,
Energy band of manipulated atomic structures on an insulator substrate Toshishige Yamada and Yoshihisa Yamamoto ERATO Quantum Fluctuation Project, Edward L. Ginzton Laboratory, Stanford University, Stanford,
Xiang-Kui Gu,, Botao Qiao,,, Chuan-Qi Huang, Wu-Chen Ding, Keju Sun, Ensheng Zhan,, Tao Zhang, Jingyue Liu*,,, and Wei-Xue Li*,
 Supported Single Pt 1 /Au 1 Atoms for Methanol Steam Reforming Xiang-Kui Gu,, Botao Qiao,,, Chuan-Qi Huang, Wu-Chen Ding, Keju Sun, Ensheng Zhan,, Tao Zhang, Jingyue Liu*,,, and Wei-Xue Li*, State Key
Supported Single Pt 1 /Au 1 Atoms for Methanol Steam Reforming Xiang-Kui Gu,, Botao Qiao,,, Chuan-Qi Huang, Wu-Chen Ding, Keju Sun, Ensheng Zhan,, Tao Zhang, Jingyue Liu*,,, and Wei-Xue Li*, State Key
Supporting Information for. Structural and Chemical Dynamics of Pyridinic Nitrogen. Defects in Graphene
 Supporting Information for Structural and Chemical Dynamics of Pyridinic Nitrogen Defects in Graphene Yung-Chang Lin, 1* Po-Yuan Teng, 2 Chao-Hui Yeh, 2 Masanori Koshino, 1 Po-Wen Chiu, 2 Kazu Suenaga
Supporting Information for Structural and Chemical Dynamics of Pyridinic Nitrogen Defects in Graphene Yung-Chang Lin, 1* Po-Yuan Teng, 2 Chao-Hui Yeh, 2 Masanori Koshino, 1 Po-Wen Chiu, 2 Kazu Suenaga
Morphology and surface reconstructions of m-plane GaN
 Morphology and surface reconstructions of m-plane GaN C. D. Lee, 1 R. M. Feenstra, 1 J. E. Northrup, 2 L. Lymperakis, 3 J. Neugebauer 3 1 Department of Physics, Carnegie Mellon University, Pittsburgh,
Morphology and surface reconstructions of m-plane GaN C. D. Lee, 1 R. M. Feenstra, 1 J. E. Northrup, 2 L. Lymperakis, 3 J. Neugebauer 3 1 Department of Physics, Carnegie Mellon University, Pittsburgh,
Site seectivity in the initial oxidation of the Si 111-7=7 surface
 Applied Surface Science 126 1998 317 322 ž / Site seectivity in the initial oxidation of the Si 111-7=7 surface Jeong Sook Ha a,), Kang-Ho Park a, El-Hang Lee a, Seong-Ju Park b a Research Department,
Applied Surface Science 126 1998 317 322 ž / Site seectivity in the initial oxidation of the Si 111-7=7 surface Jeong Sook Ha a,), Kang-Ho Park a, El-Hang Lee a, Seong-Ju Park b a Research Department,
Surface physics, Bravais lattice
 Surface physics, Bravais lattice 1. Structure of the solid surface characterized by the (Bravais) lattice + space + point group lattice describes also the symmetry of the solid material vector directions
Surface physics, Bravais lattice 1. Structure of the solid surface characterized by the (Bravais) lattice + space + point group lattice describes also the symmetry of the solid material vector directions
Surface Physics Surface Diffusion. Assistant: Dr. Enrico Gnecco NCCR Nanoscale Science
 Surface Physics 008 8. Surface Diffusion Assistant: Dr. Enrico Gnecco NCCR Nanoscale Science Random-Walk Motion Thermal motion of an adatom on an ideal crystal surface: - Thermal excitation the adatom
Surface Physics 008 8. Surface Diffusion Assistant: Dr. Enrico Gnecco NCCR Nanoscale Science Random-Walk Motion Thermal motion of an adatom on an ideal crystal surface: - Thermal excitation the adatom
Structure, energetics, and vibrational properties of Si-H bond dissociation in silicon
 PHYSICAL REVIEW B VOLUME 59, NUMBER 20 15 MAY 1999-II Structure, energetics, and vibrational properties of Si-H bond dissociation in silicon Blair Tuttle Department of Physics, University of Illinois,
PHYSICAL REVIEW B VOLUME 59, NUMBER 20 15 MAY 1999-II Structure, energetics, and vibrational properties of Si-H bond dissociation in silicon Blair Tuttle Department of Physics, University of Illinois,
Formation of clean dimers during gas-source growth of Si(001)
 Formation of clean dimers during gas-source growth of Si(001) D.R.Bowler Department of Physics and Astronomy, University College London, Gower Street, London WC1E 6BT, UK (Dated: October 18, 2002) Elevated
Formation of clean dimers during gas-source growth of Si(001) D.R.Bowler Department of Physics and Astronomy, University College London, Gower Street, London WC1E 6BT, UK (Dated: October 18, 2002) Elevated
Scanning Tunneling Microscopy Studies of the Ge(111) Surface
 VC Scanning Tunneling Microscopy Studies of the Ge(111) Surface Anna Rosen University of California, Berkeley Advisor: Dr. Shirley Chiang University of California, Davis August 24, 2007 Abstract: This
VC Scanning Tunneling Microscopy Studies of the Ge(111) Surface Anna Rosen University of California, Berkeley Advisor: Dr. Shirley Chiang University of California, Davis August 24, 2007 Abstract: This
Surface Structure and Chemisorption
 Surface Structure and Chemisorption Eckhard Pehlke, Institut für Laser- und Plasmaphysik, Universität Essen, 457 Essen, Germany. Topics: (i) interplay between the geometric and electronic structure of
Surface Structure and Chemisorption Eckhard Pehlke, Institut für Laser- und Plasmaphysik, Universität Essen, 457 Essen, Germany. Topics: (i) interplay between the geometric and electronic structure of
Comparisons of DFT-MD, TB- MD and classical MD calculations of radiation damage and plasmawallinteractions
 CMS Comparisons of DFT-MD, TB- MD and classical MD calculations of radiation damage and plasmawallinteractions Kai Nordlund Department of Physics and Helsinki Institute of Physics University of Helsinki,
CMS Comparisons of DFT-MD, TB- MD and classical MD calculations of radiation damage and plasmawallinteractions Kai Nordlund Department of Physics and Helsinki Institute of Physics University of Helsinki,
Experiment Section Fig. S1 Fig. S2
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Supplementary Materials Experiment Section The STM experiments were carried out in an ultrahigh
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Supplementary Materials Experiment Section The STM experiments were carried out in an ultrahigh
Cover Page. The handle holds various files of this Leiden University dissertation.
 Cover Page The handle http://hdl.handle.net/1887/44295 holds various files of this Leiden University dissertation. Author: Badan, C. Title: Surface-structure dependence of water-related adsorbates on platinum
Cover Page The handle http://hdl.handle.net/1887/44295 holds various files of this Leiden University dissertation. Author: Badan, C. Title: Surface-structure dependence of water-related adsorbates on platinum
Hydrogenated Graphene
 Hydrogenated Graphene Stefan Heun NEST, Istituto Nanoscienze-CNR and Scuola Normale Superiore Pisa, Italy Outline Epitaxial Graphene Hydrogen Chemisorbed on Graphene Hydrogen-Intercalated Graphene Outline
Hydrogenated Graphene Stefan Heun NEST, Istituto Nanoscienze-CNR and Scuola Normale Superiore Pisa, Italy Outline Epitaxial Graphene Hydrogen Chemisorbed on Graphene Hydrogen-Intercalated Graphene Outline
Theoretical Studies of Self-Diffusion and Dopant Clustering in Semiconductors
 phys. stat. sol. (b) zzz, No. z, zzz zzz (2002) Theoretical Studies of Self-Diffusion and Dopant Clustering in Semiconductors B.P. Uberuaga )(a), G. Henkelman (b), H. Jónsson (b) (c), S.T. Dunham (d),
phys. stat. sol. (b) zzz, No. z, zzz zzz (2002) Theoretical Studies of Self-Diffusion and Dopant Clustering in Semiconductors B.P. Uberuaga )(a), G. Henkelman (b), H. Jónsson (b) (c), S.T. Dunham (d),
Diffusion of oxygen atom in the topmost layer of the Si(100) surface: Structures and oxidation kinetics
 Surface Science 601 (2007) 2339 2343 www.elsevier.com/locate/susc Diffusion of oxygen atom in the topmost layer of the Si(100) surface: Structures and oxidation kinetics A. Hemeryck a, *, N. Richard b,
Surface Science 601 (2007) 2339 2343 www.elsevier.com/locate/susc Diffusion of oxygen atom in the topmost layer of the Si(100) surface: Structures and oxidation kinetics A. Hemeryck a, *, N. Richard b,
Supplementary information: Topological Properties Determined by Atomic Buckling in Self-Assembled Ultrathin Bi (110)
 Supplementary information: Topological Properties Determined by Atomic Buckling in Self-Assembled Ultrathin Bi (110) Yunhao Lu, *,, Wentao Xu, Mingang Zeng, Guanggeng Yao, Lei Shen, Ming Yang, Ziyu Luo,
Supplementary information: Topological Properties Determined by Atomic Buckling in Self-Assembled Ultrathin Bi (110) Yunhao Lu, *,, Wentao Xu, Mingang Zeng, Guanggeng Yao, Lei Shen, Ming Yang, Ziyu Luo,
Oxygen adsorption on Ag 111 : A density-functional theory investigation
 PHYSICAL REVIEW B, VOLUME 65, 075407 Oxygen adsorption on Ag 111 : A density-functional theory investigation Wei-Xue Li, 1 Catherine Stampfl, 1,2 and Matthias Scheffler 1 1 Fritz-Haber-Institut der Max-Planck-Gesellschaft,
PHYSICAL REVIEW B, VOLUME 65, 075407 Oxygen adsorption on Ag 111 : A density-functional theory investigation Wei-Xue Li, 1 Catherine Stampfl, 1,2 and Matthias Scheffler 1 1 Fritz-Haber-Institut der Max-Planck-Gesellschaft,
Electronic Properties of Ultimate Nanowires. F. J. Himpsel, S. C. Erwin, I. Barke,
 Electronic Properties of Ultimate Nanowires F. J. Himpsel, S. C. Erwin, I. Barke, Nanostructures with Atomic Precision Single-Atom Wire, Single Wave Function Ultimate Limits of Electronics, Data Storage
Electronic Properties of Ultimate Nanowires F. J. Himpsel, S. C. Erwin, I. Barke, Nanostructures with Atomic Precision Single-Atom Wire, Single Wave Function Ultimate Limits of Electronics, Data Storage
Equilibrium morphologies for Cl-roughened Si 100 at K: Dependence on Cl concentration
 Equilibrium morphologies for Cl-roughened Si 100 at 700 750 K: Dependence on Cl concentration G. J. Xu, Koji S. Nakayama, B. R. Trenhaile, C. M. Aldao,* and J. H. Weaver Department of Materials Science
Equilibrium morphologies for Cl-roughened Si 100 at 700 750 K: Dependence on Cl concentration G. J. Xu, Koji S. Nakayama, B. R. Trenhaile, C. M. Aldao,* and J. H. Weaver Department of Materials Science
Evidence for partial dissociation of water on flat MgO(1 0 0) surfaces
 6 February 2002 Chemical Physics Letters 352 (2002) 318 322 www.elsevier.com/locate/cplett Evidence for partial dissociation of water on flat MgO(1 0 0) surfaces Y.D. Kim a, R.M. Lynden-Bell b, *, A. Alavi
6 February 2002 Chemical Physics Letters 352 (2002) 318 322 www.elsevier.com/locate/cplett Evidence for partial dissociation of water on flat MgO(1 0 0) surfaces Y.D. Kim a, R.M. Lynden-Bell b, *, A. Alavi
Defects in Semiconductors
 Defects in Semiconductors Mater. Res. Soc. Symp. Proc. Vol. 1370 2011 Materials Research Society DOI: 10.1557/opl.2011. 771 Electronic Structure of O-vacancy in High-k Dielectrics and Oxide Semiconductors
Defects in Semiconductors Mater. Res. Soc. Symp. Proc. Vol. 1370 2011 Materials Research Society DOI: 10.1557/opl.2011. 771 Electronic Structure of O-vacancy in High-k Dielectrics and Oxide Semiconductors
Au-C Au-Au. g(r) r/a. Supplementary Figures
 g(r) Supplementary Figures 60 50 40 30 20 10 0 Au-C Au-Au 2 4 r/a 6 8 Supplementary Figure 1 Radial bond distributions for Au-C and Au-Au bond. The zero density regime between the first two peaks in g
g(r) Supplementary Figures 60 50 40 30 20 10 0 Au-C Au-Au 2 4 r/a 6 8 Supplementary Figure 1 Radial bond distributions for Au-C and Au-Au bond. The zero density regime between the first two peaks in g
Theoretical Studies of Self-Diffusion and Dopant Clustering in Semiconductors
 phys. stat. sol. (b) 233, No., 24 30 (2002) Theoretical Studies of Self-Diffusion and Dopant Clustering in Semiconductors B. P. Uberuaga )(a), G. Henkelman (b), H. Jónsson (b, c), S. T. Dunham (d), W.
phys. stat. sol. (b) 233, No., 24 30 (2002) Theoretical Studies of Self-Diffusion and Dopant Clustering in Semiconductors B. P. Uberuaga )(a), G. Henkelman (b), H. Jónsson (b, c), S. T. Dunham (d), W.
C. D. Lee and R. M. Feenstra Dept. Physics, Carnegie Mellon University, Pittsburgh, PA 15213
 Morphology and surface reconstructions of GaN(1 1 00) surfaces C. D. Lee and R. M. Feenstra Dept. Physics, Carnegie Mellon University, Pittsburgh, PA 15213 J. E. Northrup Palo Alto Research Center, 3333
Morphology and surface reconstructions of GaN(1 1 00) surfaces C. D. Lee and R. M. Feenstra Dept. Physics, Carnegie Mellon University, Pittsburgh, PA 15213 J. E. Northrup Palo Alto Research Center, 3333
Cross-Section Scanning Tunneling Microscopy of InAs/GaSb Superlattices
 Cross-Section Scanning Tunneling Microscopy of InAs/GaSb Superlattices Cecile Saguy A. Raanan, E. Alagem and R. Brener Solid State Institute. Technion, Israel Institute of Technology, Haifa 32000.Israel
Cross-Section Scanning Tunneling Microscopy of InAs/GaSb Superlattices Cecile Saguy A. Raanan, E. Alagem and R. Brener Solid State Institute. Technion, Israel Institute of Technology, Haifa 32000.Israel
A dynamically and kinetically consistent mechanism for H 2 adsorption/desorption from Si
 PHYSICAL REVIEW B VOLUME 54, NUMBER 16 15 OCTOBER 1996-II A dynamically and kinetically consistent mechanism for H 2 adsorption/desorption from Si 100-2 1 Michelle R. Radeke and Emily A. Carter* Department
PHYSICAL REVIEW B VOLUME 54, NUMBER 16 15 OCTOBER 1996-II A dynamically and kinetically consistent mechanism for H 2 adsorption/desorption from Si 100-2 1 Michelle R. Radeke and Emily A. Carter* Department
Supplementary Information:
 Supplementary Figures Supplementary Information: a b 1 2 3 0 ΔZ (pm) 66 Supplementary Figure 1. Xe adsorbed on a Cu(111) surface. (a) Scanning tunnelling microscopy (STM) topography of Xe layer adsorbed
Supplementary Figures Supplementary Information: a b 1 2 3 0 ΔZ (pm) 66 Supplementary Figure 1. Xe adsorbed on a Cu(111) surface. (a) Scanning tunnelling microscopy (STM) topography of Xe layer adsorbed
Ab initio-based Approach to N pair Formation on GaAs(001)-(2 4) Surfaces
 e-journal of Surface Science and Nanotechnology 31 January 2014 e-j. Surf. Sci. Nanotech. Vol. 12 (2014) 6-10 Conference - ACSIN-12&ICSPM21 - Ab initio-based Approach to N pair Formation on GaAs(001)-(2
e-journal of Surface Science and Nanotechnology 31 January 2014 e-j. Surf. Sci. Nanotech. Vol. 12 (2014) 6-10 Conference - ACSIN-12&ICSPM21 - Ab initio-based Approach to N pair Formation on GaAs(001)-(2
Adsorption, desorption, and diffusion on surfaces. Joachim Schnadt Divsion of Synchrotron Radiation Research Department of Physics
 Adsorption, desorption, and diffusion on surfaces Joachim Schnadt Divsion of Synchrotron Radiation Research Department of Physics Adsorption and desorption Adsorption Desorption Chemisorption: formation
Adsorption, desorption, and diffusion on surfaces Joachim Schnadt Divsion of Synchrotron Radiation Research Department of Physics Adsorption and desorption Adsorption Desorption Chemisorption: formation
Charge Carriers in Semiconductor
 Charge Carriers in Semiconductor To understand PN junction s IV characteristics, it is important to understand charge carriers behavior in solids, how to modify carrier densities, and different mechanisms
Charge Carriers in Semiconductor To understand PN junction s IV characteristics, it is important to understand charge carriers behavior in solids, how to modify carrier densities, and different mechanisms
Hydrogenation of Penta-Graphene Leads to Unexpected Large. Improvement in Thermal Conductivity
 Supplementary information for Hydrogenation of Penta-Graphene Leads to Unexpected Large Improvement in Thermal Conductivity Xufei Wu, a Vikas Varshney, b,c Jonghoon Lee, b,c Teng Zhang, a Jennifer L. Wohlwend,
Supplementary information for Hydrogenation of Penta-Graphene Leads to Unexpected Large Improvement in Thermal Conductivity Xufei Wu, a Vikas Varshney, b,c Jonghoon Lee, b,c Teng Zhang, a Jennifer L. Wohlwend,
Pt-induced nanowires on Ge(001): A density functional theory study
 PHYSICAL REVIEW B 81, 085410 2010 Pt-induced nanowires on Ge(001): A density functional theory study Danny E. P. Vanpoucke and Geert Brocks Computational Materials Science, Faculty of Science and Technology
PHYSICAL REVIEW B 81, 085410 2010 Pt-induced nanowires on Ge(001): A density functional theory study Danny E. P. Vanpoucke and Geert Brocks Computational Materials Science, Faculty of Science and Technology
Atomic and electronic structures of Si(111)-( 3x 3)R30 o -Au and (6x6)-Au surfaces
 Atomic and electronic structures of Si(111)-( 3x 3)R30 o -Au and (6x6)-Au surfaces C. H. Patterson School of Physics, Trinity College Dublin, Dublin 2, Ireland (Dated: June 13, 2015) Si(111)-Au surfaces
Atomic and electronic structures of Si(111)-( 3x 3)R30 o -Au and (6x6)-Au surfaces C. H. Patterson School of Physics, Trinity College Dublin, Dublin 2, Ireland (Dated: June 13, 2015) Si(111)-Au surfaces
Supporting Information for Ultra-narrow metallic armchair graphene nanoribbons
 Supporting Information for Ultra-narrow metallic armchair graphene nanoribbons Supplementary Figure 1 Ribbon length statistics. Distribution of the ribbon lengths and the fraction of kinked ribbons for
Supporting Information for Ultra-narrow metallic armchair graphene nanoribbons Supplementary Figure 1 Ribbon length statistics. Distribution of the ribbon lengths and the fraction of kinked ribbons for
DFT EXERCISES. FELIPE CERVANTES SODI January 2006
 DFT EXERCISES FELIPE CERVANTES SODI January 2006 http://www.csanyi.net/wiki/space/dftexercises Dr. Gábor Csányi 1 Hydrogen atom Place a single H atom in the middle of a largish unit cell (start with a
DFT EXERCISES FELIPE CERVANTES SODI January 2006 http://www.csanyi.net/wiki/space/dftexercises Dr. Gábor Csányi 1 Hydrogen atom Place a single H atom in the middle of a largish unit cell (start with a
Author(s) Okuyama, H; Aruga, T; Nishijima, M. Citation PHYSICAL REVIEW LETTERS (2003), 91(
 Title Vibrational characterization of the Si(111)-(7x7) Author(s) Okuyama, H; Aruga, T; Nishijima, M Citation PHYSICAL REVIEW LETTERS (2003), 91( Issue Date 2003-12-19 URL http://hdl.handle.net/2433/49840
Title Vibrational characterization of the Si(111)-(7x7) Author(s) Okuyama, H; Aruga, T; Nishijima, M Citation PHYSICAL REVIEW LETTERS (2003), 91( Issue Date 2003-12-19 URL http://hdl.handle.net/2433/49840
Adsorption±Desorption of H 2 /Si: A 5-D Dynamical Model
 W. Brenig et al.: Adsorption±Desorption of H 2 /Si: A 5-D Dynamical Model 75 phys. stat. sol. (a) 159, 75 (1997) Subject classification: 68.35.Ja; 68.45.Kg; S5.11 Adsorption±Desorption of H 2 /Si: A 5-D
W. Brenig et al.: Adsorption±Desorption of H 2 /Si: A 5-D Dynamical Model 75 phys. stat. sol. (a) 159, 75 (1997) Subject classification: 68.35.Ja; 68.45.Kg; S5.11 Adsorption±Desorption of H 2 /Si: A 5-D
Step-induced electronic resonance at vicinal Si(001) observed by spectroscopic SHG and RAS
 Step-induced electronic resonance at vicinal Si(001) observed by spectroscopic SHG and RAS Robert Ehlert, Jinhee Kwon and Michael C. Downer Department of Physics, The University of Texas at Austin, Austin
Step-induced electronic resonance at vicinal Si(001) observed by spectroscopic SHG and RAS Robert Ehlert, Jinhee Kwon and Michael C. Downer Department of Physics, The University of Texas at Austin, Austin
Supporting Information
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2015 Supporting Information Pyrite FeS 2 for High-rate and Long-life Rechargeable
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2015 Supporting Information Pyrite FeS 2 for High-rate and Long-life Rechargeable
Cleavage Planes of Icosahedral Quasicrystals: A Molecular Dynamics Study
 Cleavage Planes of Icosahedral Quasicrystals: A Molecular Dynamics Study Frohmut Rösch 1, Christoph Rudhart 1, Peter Gumbsch 2,3, and Hans-Rainer Trebin 1 1 Universität Stuttgart, Institut für Theoretische
Cleavage Planes of Icosahedral Quasicrystals: A Molecular Dynamics Study Frohmut Rösch 1, Christoph Rudhart 1, Peter Gumbsch 2,3, and Hans-Rainer Trebin 1 1 Universität Stuttgart, Institut für Theoretische
1 Corresponding author:
 Scanning Tunneling Microscopy Study of Cr-doped GaN Surface Grown by RF Plasma Molecular Beam Epitaxy Muhammad B. Haider, Rong Yang, Hamad Al-Brithen, Costel Constantin, Arthur R. Smith 1, Gabriel Caruntu
Scanning Tunneling Microscopy Study of Cr-doped GaN Surface Grown by RF Plasma Molecular Beam Epitaxy Muhammad B. Haider, Rong Yang, Hamad Al-Brithen, Costel Constantin, Arthur R. Smith 1, Gabriel Caruntu
Supplementary Information for Solution-Synthesized Chevron Graphene Nanoribbons Exfoliated onto H:Si(100)
 Supplementary Information for Solution-Synthesized Chevron Graphene Nanoribbons Exfoliated onto H:Si(100) Adrian Radocea,, Tao Sun,, Timothy H. Vo, Alexander Sinitskii,,# Narayana R. Aluru,, and Joseph
Supplementary Information for Solution-Synthesized Chevron Graphene Nanoribbons Exfoliated onto H:Si(100) Adrian Radocea,, Tao Sun,, Timothy H. Vo, Alexander Sinitskii,,# Narayana R. Aluru,, and Joseph
Graphene Annealing: How Clean Can It Be?
 Supporting Information for Graphene Annealing: How Clean Can It Be? Yung-Chang Lin, 1 Chun-Chieh Lu, 1 Chao-Huei Yeh, 1 Chuanhong Jin, 2 Kazu Suenaga, 2 Po-Wen Chiu 1 * 1 Department of Electrical Engineering,
Supporting Information for Graphene Annealing: How Clean Can It Be? Yung-Chang Lin, 1 Chun-Chieh Lu, 1 Chao-Huei Yeh, 1 Chuanhong Jin, 2 Kazu Suenaga, 2 Po-Wen Chiu 1 * 1 Department of Electrical Engineering,
Design of Efficient Catalysts with Double Transition Metal. Atoms on C 2 N Layer
 Supporting Information Design of Efficient Catalysts with Double Transition Metal Atoms on C 2 N Layer Xiyu Li, 1, Wenhui Zhong, 2, Peng Cui, 1 Jun Li, 1 Jun Jiang 1, * 1 Hefei National Laboratory for
Supporting Information Design of Efficient Catalysts with Double Transition Metal Atoms on C 2 N Layer Xiyu Li, 1, Wenhui Zhong, 2, Peng Cui, 1 Jun Li, 1 Jun Jiang 1, * 1 Hefei National Laboratory for
Special Properties of Au Nanoparticles
 Special Properties of Au Nanoparticles Maryam Ebrahimi Chem 7500/750 March 28 th, 2007 1 Outline Introduction The importance of unexpected electronic, geometric, and chemical properties of nanoparticles
Special Properties of Au Nanoparticles Maryam Ebrahimi Chem 7500/750 March 28 th, 2007 1 Outline Introduction The importance of unexpected electronic, geometric, and chemical properties of nanoparticles
Structure and Energetics of P-rich GaP(001) Surfaces
 phys. stat. sol. (a) 184, No. 1, 105 110 (2001) Structure and Energetics of P-rich GaP(001) Surfaces O. Pulci 1 ), W. G. Schmidt, and F. Bechstedt Institut für Festkörpertheorie und Theoretische Optik,
phys. stat. sol. (a) 184, No. 1, 105 110 (2001) Structure and Energetics of P-rich GaP(001) Surfaces O. Pulci 1 ), W. G. Schmidt, and F. Bechstedt Institut für Festkörpertheorie und Theoretische Optik,
Direct Measurement of Electron Transfer through a Hydrogen Bond
 Supporting Information Direct Measurement of Electron Transfer through a Hydrogen Bond between Single Molecules Tomoaki Nishino,*, Nobuhiko Hayashi, and Phuc T. Bui Nanoscience and Nanotechnology Research
Supporting Information Direct Measurement of Electron Transfer through a Hydrogen Bond between Single Molecules Tomoaki Nishino,*, Nobuhiko Hayashi, and Phuc T. Bui Nanoscience and Nanotechnology Research
