On the Ontogeny of Leptodora kindtii (Crustacea, Branchiopoda, Cladocera), with Notes on the Phylogeny of the Cladocera
|
|
|
- Miles Reynolds
- 6 years ago
- Views:
Transcription
1 JOURNAL OF MORPHOLOGY 256: (2003) On the Ontogeny of Leptodora kindtii (Crustacea, Branchiopoda, Cladocera), with Notes on the Phylogeny of the Cladocera Jørgen Olesen, 1,2 * Stefan Richter, 1 and Gerhard Scholtz 1 1 Humboldt-Universität zu Berlin, Institut für Biologie/Vergleichende Zoologie, D Berlin, Germany 2 Zoological Museum, University of Copenhagen, DK-2100 Copenhagen OE, Denmark ABSTRACT Leptodora kindtii, a large predaceous cladoceran, is among the most deviant species of the Cladocera. Therefore, its phylogenetic position has traditionally proven difficult to determine. Its many peculiar features include, among others, long, stenopodous, forwardly directed trunk limbs, a posteriorly placed dorsal brood pouch, a tri-lobed lower lip, and a long, segmented abdomen. This study describes the ontogeny of L. kindtii (Haplopoda), including general body proportions, appendages, the carapace, and other external structures in an attempt to facilitate the comparison of its aberrant morphology to that of other branchiopods. In general, the early embryos are similar to the early embryos of other cladoceran taxa with respect to body shape and size and position and orientation of the early limb buds. Many of the unusual features of L. kindtii appear late in ontogeny. The carapace appears at an early stage as a pair of dorsolateral swellings in a position corresponding to the gap between the mandibles and the first pair of trunk limbs; it later becomes posteriorly transposed by a gradual fusion of its more anterior parts to the dorsal side of the thorax. The tri-lobed lower lip, under the labrum of the late embryo and the adult, develops as a fusion of the first maxillae (lateral lobes) to an elevated sternal region behind the mouth (median lobe). The stenopodous, segmented trunk limbs in the adult develop from embryonic, elongate, subdivided limb buds, similar to those seen in early stages of other branchiopods. Two conflicting possibilities for the phylogeny of the Cladocera, involving two different positions of L. kindtii (Haplopoda), are discussed. Several characters support a sister-group relationship between the Haplopoda and Onychopoda. However, some characters support the Anomopoda and Onychopoda as sister groups, leaving the Haplopoda outside this clade. In contrast to recent suggestions, we prefer to retain the term Cladocera in its original sense as comprising the Haplopoda, Ctenopoda, Anomopoda, and Onychopoda. J. Morphol. 256: , Wiley-Liss, Inc. KEY WORDS: Diplostraca; Haplopoda; SEM; ultrastructure; limb development; carapace; Cyclestheria; Onychopoda; Distal-less; Hoechst nuclear stain Cladocera ( water fleas ) is the most diverse and species-rich group within the Branchiopoda. Whereas the other branchiopods (traditionally grouped as Anostraca, Notostraca, and Conchostraca) mainly live in temporary freshwater bodies, cladoceran representatives inhabit all kinds of freshwater and even the marine environment. The evolutionary success of the Cladocera is also reflected in a great variety of lifestyles and morphologies, ranging from filter-feeders with plesiomorphically phyllopodous limbs to raptorial species with stenopodous limbs and a reduced carapace. The phylogenetic relationships within the Cladocera are still incompletely resolved, and even cladoceran monophyly has been questioned. In particular, Leptodora kindtii (Haplopoda) has been problematic to place phylogenetically because of its unusual adult morphology and lifestyle as a large, hyaline, planktonic predator. Some authors have placed it as the sister group to the remaining cladocerans, or have given it a separate status within the Cladocera, mainly because it is the only cladoceran that has a free-living larva as part of its life cycle (Eriksson, 1934; Wingstrand, 1978; Negrea et al., 1999). Others have placed it as a sister group to the Onychopoda due to the presence of stenopodous trunk limbs, a reported similarity in the fusion of some of the anterior ganglia, and a reduction of the carapace to a dorsal brood pouch (e.g., Martin and Cash-Clark, 1995; Olesen, 1998). The most significant contributions to our knowledge about the morphology of the adult Leptodora kindtii are those of Lilljeborg (1860, 1901), Müller (1868), Wagner, (1868), Weismann (1874), Gerschler (1911), Sebestyén (1931), and Sars (1861 [1993]). From these studies it is known that Leptodora Contract grant sponsor: Deutsche Forschungsgemeinschaft; Contract grant numbers: 436 RUS 18/15/98 (to SR), Scho 442/6-1 (to GS); Contract grant sponsors: the Australian Museum (to SR), the Environmental Research Institute of Supervising Scientists (ERISS) and Chris Humphrey (to SR), the Danish Natural Science Research Council (to JO). *Corresondence to: Jørgen Olesen, Zoological Museum, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen OE, Denmark. j1olesen@zmuc.ku.dk DOI: /jmor WILEY-LISS, INC.
2 236 J. OLESEN ET AL. kindtii, among other peculiarities, possesses a large, lobe-like lower lip, but no recognizable maxillae 1 and 2. There are a number of investigations dealing with various aspects of development of Leptodora kindtii (Müller, 1868; Wagner, 1868; Sars, 1873; Weismann, 1876; Samter, 1895, 1900; Warren, 1901; Andrews, 1948; Gerberding, 1997). Müller (1868) provided the first detailed description of development in the parthenogenetic part of the life cycle (summer eggs) in Leptodora kindtii, from early oogenesis to the release of juveniles from the brood pouch (some of Müller s drawings were published in more detail by Røen, 1994). Oogenesis was intensely studied by Weismann (1876). Most of our knowledge about development of the germ band until limb formation was provided by Samter (1900). Gerberding (1997) added further details about early development, such as the presence of lateral neuroblasts. Leptodora kindtii also produces resting eggs (winter eggs), from which free-living larvae hatch. This was first noted by Sars (1873), who produced some illustrations of various larval stages. A striking feature of these larvae is the long, uniramous, and unsegmented mandibular palp, which is unique among cladocerans. Sars (1873) also noted that all individuals of the first generation, including the adults, have a naupliar eye, whereas all later generations lack it. Apart from these features, the freeswimming stages of L. kindtii look much like the early stages in the parthenogenetic part of the life cycle. The development of various anatomical features of the free-living larvae is described by Warren (1901). These developmental studies led to somewhat contradictory interpretations concerning several aspects of morphogenesis and adult morphology of Leptodora kindtii. For instance, Wagner (1868) suggested that the maxillae are involved in the formation of the lower lip, while Weismann (1874) stressed that this is not the case, a view also held by Warren (1901) based on a study of the development of the resting eggs. The present investigation of the development of Leptodora kindtii was undertaken for two reasons. One was the application of new techniques such as scanning electron microscopy (SEM), fluorescence microscopy, and molecular markers to address the contradictory results concerning several aspects of development, such as the anlagen of maxillae 1 and 2, the nature and composition of the lower lip, limb development, and the formation of the carapace. The other was phylogenetic, as ontogeny provides an additional set of characters that often make it possible to resolve phylogenetic relationships in cases where the analysis of adult morphology leads to ambiguous conclusions. Considering the unusual morphology of the adult of Leptodora kindtii, we found it potentially promising to focus on earlier developmental stages in order to facilitate comparison with other cladocerans and branchiopods. The study concentrates on the period in development where the anlagen of the appendages have appeared until the morphology of the juvenile is approached, immediately prior to the release from the brood chamber. The ontogeny of the trunk limbs was described in more detail in Olesen et al. (2001). MATERIALS AND METHODS All material of Leptodora kindtii* (Focke, 1844) for this study was collected in the Tegeler See, Berlin, Germany, in August/ September, Specimens were sorted in the laboratory. Between 70 and 100 female brood pouches were inspected and used for the study. Material for SEM was fixed variously in Bouin s fluid, 3.7% formaldehyde, or Karnovski fixative in Sörensen phosphate buffer (Romeis, 1989). Bouin s fluid (following Bouin, 1897, cited in Romeis, 1989) proved most useful for SEM since it caused the least shrinkage. Some parent animals were fixed with embryos still situated in the brood pouch; some individual embryos were dissected out and fixed separately. The material was dehydrated in an ethanol series (80%, 90%, 100%), critical-point-dried, mounted, and gold-coated, following standard procedures. Observations were made with a JEOL JSM-840 SEM at the Zoological Museum, University of Copenhagen (ZMUC). The material used for SEM is stored in the ZMUC collection (ZMUC CRU3691, 3692). Immunostaining followed the description of Panganiban (pers. commun.). The embryos were transferred to the PEM-FA fixative (0.1 M PIPES (ph 6.95), 2.0 mm EGTA, 0.1 mm MgSO 4, 3.7% formaldehyde) for min. After fixation the embryos were washed two times for 5 min in PBS, two times for 5 min in PBT (PBS, 2% BSA, 0.1% Triton X-100) and then kept for 30 min in PBT. After that the embryos were incubated in PBT and polyclonal anti-dll (dilution 100:1) overnight at 4 C. After incubation they were washed three times for 5 min and four times for 30 min in PBT and again incubated overnight at 4 C in PBT and goat antirabbit IgG (Jackson Immunoresearch, West Grove, PA), which was added at a dilution of about 800:1. After incubation the embryos were washed three times for 5 min and four times for 30 min in PBT and then transferred to a solution of 1 mg/ml DAB (diaminobenzidine) in PBT (dilution 1:2) for 10 min. H 2 O 2 (3%) was added to a dilution of 100:1 and the reaction was allowed to proceed for about 10 min. The stained embryos were transferred to PBS and counterstained with fluorescent dye (0.1% solution of bisbenzimid H33258) for 10 min. Then they were washed in PBS for 10 min and mounted in glycerol on a slide. Further analysis and photography were done with brightfield, differential interference contrast (Nomarski optics) and fluorescence microscopy. The embryos used for histology for light microscopy were fixed in Bouin s fluid, embedded in Technovit, and sectioned at a thickness of 1.5 m. Additional scanning electron micrographs of other cladocerans are included for comparative purposes in the discussion: Bytho- *Leptodora kindtii was originally described as Polyphemus kindtii in 1844 by Focke, who found the species in the Bremer Stadtgraben. Lilljeborg (1860), not aware of Focke s record, described it as Leptodora hyalina from lakes in Sweden. Wagner (1868) found it in a lake close to Kasan and unaware of the two other descriptions described it under the name Hyalosoma dux. Poppe (1889) was probably the first to use the now well-accepted combination Leptodora kindtii. Some more recent authors use kindti instead of kindtii (e.g., Flössner, 1972, 2000); however, according to rule IRZN kindtii and kindti are deemed to be homonyms and according to rule 33.4 IRZN kindti is deemed to be an incorrect subsequent spelling of the species name. Therefore, the correct name is Leptodora kindtii (Focke, 1844).
3 trephes longimanus (Leydig, 1860) (Onychopoda) was collected at the same time and place as L. kindtii; Polyphemus pediculus Linnaeus, 1761 (Onychopoda), collected in Mølleåen, Denmark, in 1993; Eurycercus glacialis Lilljeborg, 1887 (Anomopoda), collected at Disko Island, Greenland, 1992; Macrothrix laticornis (Jurine, 1820) (Anomopoda), collected in Denmark, 1994; Cercopagis pengoi (Ostroumov, 1892) (Onychopoda), collected in the northern part of the Caspian Sea, off Zhemtschuznyi Island, 1998; and Cyclestheria hislopi (Baird, 1859) ( Conchostraca ), collected in Colombia, 1995 (Fig. 9D,E) and in the Northern Territory, Australia, 1999 (Fig. 10C,D). RESULTS General The various embryos of Leptodora kindtii examined in this study could conveniently be grouped into four stages (in the following referred to as Stages 1 4). The four stages are defined by a similar degree of limb development, although the total body length may sometimes be different. The number of (true) stages, or instars, separated by molts was not examined in this work. That the sizes of the embryos and the degree of limb development do not always correspond must be explained either as occurring naturally or as an artifact of preparation. It is beyond the scope of the present work to examine this in detail. Stage 1 (Figs. 1A,B, 7E G, 8A). Embryos are m long, globular or slightly elongate, with a large amount of yolk. The anlagen to the following body parts are demarcated externally: labrum, antennae 1, antennae 2, mandibles, and six pairs of trunk limbs. The labrum is large and rounded and is located anteromedially. Antennae 1 are undifferentiated, slightly elongated, distally expanded limb buds, located along the sides of the labrum. Antennae 2 are large and biramous, attached laterally at the embryo, reaching posteriorly to trunk limbs 3 4; the ventral margin of the protopod is subdivided into two parts, probably reflecting a coxa and a basis; the endopod (the ventral ramus) is superficially subdivided into 3 4 subunits; both rami are slightly curved. The mandibles are triangular limb buds along the laterodistal margin of the labrum, proximally touching the distal part of antennae 1; the distance between the tips of the mandibles is large compared to later stages. Between the labrum and the first pair of trunk limbs, located under the tips of the mandibles, are two small spots of Dll expression (first maxillae) on the same line as the proximal Dll expression in trunk limbs 1 5 (Fig. 7E G). Trunk limbs 1 6 are two widely separated rows of elongate limb buds; trunk limbs 1 are largest, with more posterior limbs gradually decreasing in size; subdivision of the limb buds is not clearly present externally, but expression of Dll shows that five distinct portions can be recognized in trunk limbs 1 5, while trunk limb 6 appears not to be subdivided; the Dll expression in the proximal portions of trunk limbs 1 5 are weaker than in the more distal portions; in trunk limb 1 the proximal portion of Dll is closer to the midline of the embryo than in ONTOGENY OF LEPTODORA KINDTII 237 the posterior limbs. The posterior limbless part of the body (the future abdomen) is relatively short and constitutes only approximately 15% of the total body length. Stage 2 (Figs. 1C G, 2, 6A,B, 7A D, 8B). Embryos are m long, elongate, almost twice as long as wide, with a large yolk portion. The anterior parts of the head section and the abdomen are enlarged compared to the previous stage. The carapace is visible as a pair of dorsolateral swellings in the region corresponding to the gap between the mandibles and the first pair of trunk limbs. The following body parts are demarcated externally: labrum, antennae 1, antennae 2, mandibles, and six pairs of trunk limbs. The labrum, antennae 1, antennae 2, and the mandibles have detached partly from the main body (at least in the later phases in this stage), whereas the trunk limbs are still attached in their full length. Dorsally at the head section is a large, kidney-shaped dorsal organ. The labrum is rounded, located at a greater distance from the anteriormost margin than in stage 1, which is caused by the enlarged head section. The labrum is set off from the remaining head by a transverse furrow (in most specimens), anterior to which is a pair of lateral buds. In more advanced embryos of Stage 2, a tri-lobed lower lip is present (Fig. 7C,D), composed of a wide median lobe (same width as labrum) and two smaller lateral lobes (maxillae 1, see below); early in Stage 2 the lateral lobes are only incipiently developed (Fig. 7A,B). Antennae 1 are slender, elongate, far apart, located lateroventrally at the head section, and flat against the sides of the head and the labrum, reaching the mandibles. Antennae 2 are large and biramous, reaching posteriorly to the abdomen; the protopod is divided into a small proximal portion, which will become the basal folding area in the adult, and a large portion which is weakly divided into two portions of similar size (coxa and basis); the proximal small portion has a small dorsal seta; the endopod is four-segmented, with setae developed at the ventral margin of segments 2 4; the exopod is four-segmented, the proximal segment being small, with setae incipiently developed at the ventral margins of segments 2 4. The mandibles insert posterior to antennae 2 and lateral to the labrum; they are widest basally, taper distally, and are curved with the convex sides facing the labrum. Between the mandibles and the first trunk limbs is a gap without limb anlagen. Trunk limbs 1 6 are, as in Stage 1, developed as two widely separated rows of elongate, subdivided limb buds, attached to the trunk in their full length; trunk limbs 1 are the largest and the limbs gradually decrease in size posteriorly; each limb slightly overlaps the limb immediately posterior to it; trunk limbs 2 5 are each subdivided into five portions, not all of which are visible externally on the various SEMs but are revealed by Hoechst fluorescent stain (Fig. 7A); trunk limb 1 is also divided into five por-
4 238 J. OLESEN ET AL. tions but the proximal portion is less distinct than on the more posterior limbs. Under the proximal part of the first pair of trunk limbs is a structure of uncertain origin, apparently connected to the limb (marked by? in Fig. 2F). Medial to the bases of trunk limbs 1 is a pair of small processes that we interpret as a part of the proximal limb portion (the so-called maxillary processes, see below). At the posterior margin of all limbs, two rows of setae are present. Between the trunk limbs of each side is a wide, median area, which is slightly elevated and distinctly separated from the limbs and the abdomen. The abdomen is elongate, narrowing distally, and constitutes 25 30% of the total body length; three segments and a telson are present dorsally with the anlagen to the paired telsonal setae; the abdomen terminates in two rounded lobes, each with an incipiently developed furcal ramus. Stage 3 (Fig. 3). Embryos are approximately 570 m long, with a relatively small yolk portion, visible externally as an enlarged, curved dorsal mound. The general body appearance is elongate, with a curved dorsal side and a part of the abdomen ventrally flexed. The body is dorsally divided into a head section and a trunk section. The head is elongate, with a large, kidney-shaped dorsal organ approximately at the level of the labrum or antennae 2. Behind the dorsal organ is a median pointed process. The anlage of the carapace is a transverse rim at the dorsoanterior part of the trunk. The labrum is wide and flattened. Antennae 1 are small, tubular, arising lateroanteriorly at the head, some distance from the labrum. Antennae 2 are large, extending almost to the caudal end of the body, and arising laterally at the head, approximately at the level of the labrum. The mandibles arise laterally at the head section, slightly posterior to antennae 2 and are large and U-shaped with acute tips. Under the labrum, between the mandibles, is a rounded, elevated area, which is the median lobe of the lower lip (see Stage 4); lateral lobes in the lower lip are probably covered by a membrane but are assumed also to be present in this stage since they are present in the most advanced embryos of Stage 2. Posterior to the bases of the mandibles (laterally on the body) is a pair of relatively large lateral buds and posterior to these a smaller pair, both of uncertain status (see below). Trunk limbs 1 6 are directed posterolaterally attached to the main body in more or less their full length; trunk limbs 1 4 have an S-shaped appearance; the basal, short part of the limbs is in an upright position relative to the main body, the longer middle part is oriented dorsoventrally along the side of the body, and the short distal part is directed lateroposteriorly; trunk limbs 5 and 6 are straight and directed posteriorly, the latter very short; the segmentation is not clear on trunk limbs 1 4 (covering membrane), trunk limbs 5 are 4 5 segmented, and trunk limbs 6 are unsegmented. The abdomen is elongate, ventrally flexed, terminating in two rounded furcal lobes with furcal rami incipiently developed; the length is 35 40% of the full body length. The anlagen to the paired telsonal setae are present dorsally. Stage 4 (Figs. 4, 5, 6C F, 8C). The general body morphology in Stage 4 is much like that of the adult, with a length of m. All appendages have attained much of the same orientation and segmentation as in the adult. A major difference from the adult is that all setation is only incipiently developed. In the least-advanced embryos of this stage, the remains of the yolk are left as a dorsal thickening of the trunk. A division into a head and a trunk can be seen dorsally between the proximal parts of the mandibles and the carapace. Nine trunk segments can be identified, including six thoracic segments (inferred to be present because of the presence of six pairs of trunk limbs), three abdominal segments, and the telson. The head is anteriorly rounded (following the shape of the compound eye). The dorsal organ is large, oval, and swollen, situated as a saddle dorsally between the compound eye and the region of antennae 2. Early in this stage the carapace is developed dorsally as a short, posteriorly directed flap in the region corresponding approximately to trunk limbs 1. Immediately dorsal to the bases of the first trunk limbs is a pair of small buds on each side of the trunk, which are tentatively interpreted as the maxillae 2 limb buds (marked by? in Fig. 4C). In the most advanced embryos of Stage 4, the incipient carapace extends in the full length of the thorax (Fig. 4E,F). The labrum has a flattened, spade-like, wide distal margin with a pair of papillae at the inner surface. Under the labrum is a large, elevated, tri-lobed lower lip, composed of a large median lobe and two smaller lateral lobes, each with a medial seta. Antennae 1 are small, tubular, slightly swollen in the distal one-third, with setae at the tip, located at the ventrolateral edge of the head between the labrum and the distal end of the head. Antennae 2 are large, extending almost to the telson; they arise laterally from the head at the same level as the labrum; the protopod is divided into two sections, one very short with a small dorsal seta (the basal folding area), and one very large (probably the fused coxa and basis, see below); the exopod has one small proximal segment lacking se- Fig. 1. Leptodora kindtii, early embryonic stages. A: Stage 1, ventral view. B: Stage 1, lateral view showing appendages of right side; arrow indicates separation between assumed coxa and basis. C: Early Stage 2, ventral view, outer membrane removed to show limb portions. D: Early Stage 2, lateral view; same as in C. E: Late Stage 2, ventral view. F: Late Stage 2, ventrolateral view. G: Late Stage 2, lateral view; arrow indicates dorsal transverse furrow between head and trunk regions. A1, antenna 1; a2, antenna 2; ca, carapace; do, dorsal organ; en, endopod; ex, exopod; la, labrum; md, mandible; te, telson; tl1, trunk limb 1; tl6, trunk limb 6; ts7, trunk segment 7; ts8, trunk segment 8, ts9, trunk segment 9.
5 ONTOGENY OF LEPTODORA KINDTII 239 Figure 1
6 240 J. OLESEN ET AL. Figure 2
7 tae, followed by three larger segments with setae along the ventral margin; the endopod has four segments with setae along the ventral margin. The mandibles arise laterally from the head, behind antennae 2; they are S-shaped with swollen basal parts distinctly set off from a longer distal, curved part, tapering distally and pointing between the labrum and the lower lip. Trunk limbs 1 6 have attained an upright, vertical position and are no longer attached to the trunk in their full length; in the leastadvanced embryos of Stage 4 the trunk limbs arise from the thorax along a straight line at the same level in lateral view (Fig. 4A,C), whereas in moreadvanced embryos of Stage 4, the first pair of trunk limbs arises dorsal to this line (Fig. 4E); trunk limbs 1 5 have four segments: one large proximal one and three smaller ones distally; trunk limbs 6 have two segments. The abdomen is large, 3 4 times the length of the thorax; it consists of three segments and the telson, but the segmentation of the first two abdominal segments is obscured in the leastadvanced embryos of Stage 4 because of the yolk; the first and third abdominal segments (ts7 and ts9) are large each about the size of the thoracic region while the second abdominal segment (ts8) is small; the telson is also large and continues into long caudal spines, without articulation to the remaining part of the telson; a pair of telsonal setae are present dorsally at the telson, close to its anterior margin. Morphogenesis of Appendages and Other Structures Body shape and proportions. Leptodora kindtii undergoes an extreme morphological change from the early differentiation of the germ band of the embryo to the final release of juveniles with an appearance as miniature adults from the brood pouch of the mother. Most significant is the profound elongation of the body caused mainly by growth of the head and the abdomen. From the early Fig. 2. Leptodora kindtii, Stage 2. A: Early Stage 2. Frontal view showing first antennae, mandibles, labrum and pore leading to compound eyes. B: Late Stage 2. Dorsal view showing left side carapace anlagen and antennae 2; arrow indicates boundary between coxa and basis. C: Late Stage 2. Dorsolateral view of anterior part showing dorsal organ and proximal segment of antenna with small seta (arrow) (becomes folding portion in adult). D: Late Stage 2. Dorsal organ. E: Late Stage 2. Left side showing primordial segmentation of antenna 2 and left side anlagen to carapace. Arrows indicate dorsal transverse furrow between head and trunk regions and incipient separation of antennal protopod into basis, coxa, and future folding region. F: Early Stage 2. Early segmentation of left-side trunk limbs; arrow points at so-called maxillary process. G: Late Stage 2. Dorsal view of caudal region showing anlagen of paired telson setae and caudal spines (arrows). A1, antenna 1; a2, antenna 2; ca, carapace; ce pore, pore leading to compound eye; do, dorsal organ; en, endopod; ex, exopod; la, labrum; md, mandible; tl1, trunk limb 1; numbers 1 5, trunk limb portions 1 5. ONTOGENY OF LEPTODORA KINDTII 241 Stage 2, a dorsal, transverse suture between the dorsal organ and the paired primordial carapace swellings separates the body into an anterior head region and a posterior trunk region (Figs. 1G, 2E) (please note that the terms head and trunk are used to describe the anterior and posterior regions of the embryos and that these do not correspond necessarily to the true head or trunk in other Crustacea). Antennae 1 and 2 belong to the head region, while the mandibles and the remaining appendages belong to the trunk region. This separation into two regions is continued into Stage 3, and is even more significant here (Fig. 3A,C,D), but the mandibles are now located between the two regions. In Stage 4 this suture is apparently gone, but there is another more posterior dorsal suture behind the mandibles, indicating that these functionally have become a part of the head region. The undeveloped globular embryo is about 300 m in diameter and it grows to a length of approximately 1 mm immediately before its release from the brood pouch. Details of the ontogeny are described in the following sections. Dorsal organ and carapace. The dorsal organ of Leptodora kindtii is first visible in Stage 2 as a large kidney-shaped structure occupying the major part of the head dorsally (Fig. 2D). In the following stages it maintains its shape but becomes more swollen, especially in Stage 4 (Figs. 4C,D, 5E). In the adult it has an oval shape and occupies a relatively smaller part of the head compared to the earlier stages (adult: e.g., Sars, 1861 [1993], pls. 110 and 111). In some older literature the dorsal organ is referred to as the head shield (e.g., Müller, 1868). The carapace is first visible as two dorsolateral swellings in Stage 2 (Figs. 1G, 2B,E). The position of these in relation to the ventral appendages appears to be immediately behind the mandibles, approximately corresponding to the gaps between the mandibles and the first pair of trunk limbs. The position cannot be specified precisely in relation to the position of the first and second maxillae, since these are much reduced in this stage. In Stage 3, the two swellings have fused and form a continuous dorsal rim anteriorly at the trunk immediately behind the head (Fig. 3B D), but the paired origin is still recognizable as the rim is thickest at the two sides. The position of the carapace in relation to the ventral appendages still appears to be immediately behind the mandibular region (Fig. 3D). In the leastadvanced embryos of Stage 4 the carapace is a little dorsal flap attached to the trunk in approximately the region of trunk limbs 1 or slightly behind this (Fig. 4A,C,D), and has hence moved slightly posteriorly compared to the earlier stages. In the more advanced embryos of Stage 4, the carapace is attached to the thorax approximately along its entire length (Fig. 4E,F). In the adult, the free valves of the carapace are attached posteriorly at the thorax, immediately anterior to the first abdominal segment (e.g., Sars 1861 [1993]: pl. 110). We interpret the
8 Figure 3
9 information summarized above as indicating that the carapace in Leptodora kindtii moves posteriorly by a gradual fusion of the more anterior part to the dorsal side of the thorax. Antennae 1 and 2. In Stage 1, antennae 1 are a pair of elongate buds positioned between the labrum and antennae 2, still attached along their full length to the main body (Fig. 1A,B). In Stage 2, they have become slightly more elongate; the distal ends are swollen and have detached from the main body in the most advanced embryos of Stage 2 (Fig. 1E G) and have developed some weak distal setation. In Stage 4, the morphology of the adult antennae has been approached, which is a relatively small clubshaped limb with only distal setation. The distance to the labrum increases during development due the elongation of the head section, whereas the distance between the limbs is maintained. There is no change in position of the limbs towards the midaxis of the animal, as in certain other cladocerans. Antennae 2 in Stage 1 are large compared to all other appendages and reach approximately to the buds of trunk limbs 4 (Fig. 1A,B). They insert laterally at the head, but are still attached to the main body along their full length. The protopod is subdivided into two portions at its anterior margin (Fig. 1B), probably a coxa and a basis. The endopod is divided into four segments as is the exopod, the proximal segment being quite small. Both the endopod and the exopod are slightly bent dorsally. During Stage 2, antennae 2 have become free from the main body and are only attached proximally (Fig. 1E). The appendages nearly reach the posterior end of the body and especially the protopodal part has elongated. The protopod is now subdivided into three portions: a short proximal portion, which will become the basal flexing area in the adult, followed by a larger portion indistinctly divided into two portions (basis and coxa) (Fig. 2E). A small seta is present dorsally at the short proximal portion near the margin (Fig. 2C). The rami have the same segmentation as earlier, with setation indicated along the ventral margins (Fig. 2E). In Stage 4, the full development of antenna 2 is approached (Fig. 4D), but the size of the protopod in the adult will become even longer relative to the length of the complete limb and the setation will develop further. Fig. 3. Leptodora kindtii, Stage 3. A: Ventral view. Question mark refers to two lateral buds of uncertain status. B: Lateral view. C: Dorsal view of head and thorax. D: Lateral view of head and anterior thorax. Question mark refers to two lateral buds of uncertain status. E: Right-side. Labrum, first antennae, mandible, trunk limb 1 and two buds of uncertain significance (marked by question marks). F: Left side. Trunk limbs. G: Left side. Trunk limbs; same specimen as in F. Question mark refers to basalmost part of limb which showed no expression of Dll. A1, antenna 1; a2, antenna 2; ca, carapace; do, dorsal organ; la, labrum; md, mandible; tl1, trunk limb 1; tl6, trunk limb 6; numbers 1 4, trunk limb portions 1 4. ONTOGENY OF LEPTODORA KINDTII 243 Mandible and labrum. The mandibles are visible in Stage 1 as triangular limb buds on each side of the labrum (Fig. 1A,B). In Stage 2, the limbs insert laterally at the head and the tip has detached from the main body and curves with the convex side facing the labrum (Fig. 1C G). In Stage 3, the mandibles have become larger, the curvature more pronounced and the tips acute (Fig. 3A,E). This development continues into Stage 4, where the limb has a wide proximal part distinctly set off from a slender, curved, distal part with an acute tip (Figs. 4B,C, 5D). The proximal portions of the mandibles are close together dorsally. Some incipient denticles are present near the tip. The labrum in Stage 1 is large and rounded (Fig. 1A,B). In Stage 2, it has elongated and the tip has detached from the main body (Fig. 1E G). In Stage 3, it has attained a more upright orientation and has a wider and more flattened distal part (Fig. 3A,D,E). The tip is wide and flattened in Stage 4 (Figs. 4B, 5A C). A pair of papillae is present on the inner surface of the labrum (Fig. 5C). Maxilla 1, maxilla 2, and the lower lip. The first unambiguous sign of maxillae is in Stage 1, where two groups of cells located under the tips of the mandibles, close to the margin of the labrum, show expression of the Dll gene (Fig. 7E G). These two Dll spots are serially aligned with the proximal portions of the trunk limbs, which also show Dll expression. No more than one pair of expression areas of the Dll gene has been found in this region in any stage examined. We interpret these expressions as the anlagen of maxillae 1, based on the fact that they are closer to the mandibles than to the first pair of trunk limbs, which would leave room for the lacking maxillae 2, but also because maxillae 2 can be accounted for in another way (see below). Slightly later in development, the characteristic tri-lobed lower lip starts to develop. The median lobe of the lower lip becomes distinguishable first as an elevated plate directly under the labrum. During Stage 2, a pair of very small lateral lobes appears exactly in the position of the paired Dll expression (first maxillae) present slightly earlier in development (Fig. 7B,D; two lateral lobes marked by white arrowheads). Hence, during ontogeny the early anlagen of the first maxillae end up representing the lateral lobes of the tri-lobed lower lip as found in intermediate embryos (Fig. 7C,D), late embryos/juveniles (Fig. 5A D), and in the adults (e.g., Lilljeborg, 1860, pl. 8, fig. 17; Wagner, 1868, pl. 3, fig. 1; Sebestyén, 1931, fig. 6a,b). The ontogeny of maxillae 1 is summarized in Figure 8. The median lobe of the threelobed lower lip is formed by an elevation of the sternal region under the labrum. We found no direct evidence concerning the position of maxillae 2 in the earliest stages of Leptodora kindtii. None of the techniques used by us (Dll expression, fluorescence microscopy, or SEM) have revealed any structures that convincingly could be
10 Fig. 4. Leptodora kindtii, Stage 4. A: Early Stage 4. Lateral view. B: Early Stage 4. Cephalic region, left side. Same specimen as A. C: Intermediate Stage 4. Cephalic and thorax regions. Left side. Question mark refers to small bud tentatively interpreted as the vestiges of the second maxilla. D: Intermediate Stage 4. Dorsal view; arrows point at telsonal setae. E: Late Stage 4. Lateral view of thorax region showing carapace and trunk limbs. F: Late Stage 4. Dorsal view of thorax region showing carapace; same specimen as in E. A1, antenna 1; a2, antenna 2; cau spi, caudal spine; ca, carapace; do, dorsal organ; la, labrum; md, mandible; te, telson, tl1, trunk limb 1; tl6, trunk limb 6; ts8, trunk segment 8; ts9, trunk segment 9.
11 ONTOGENY OF LEPTODORA KINDTII 245 Fig. 5. Leptodora kindtii, Stage 4. A: Early Stage 4. Ventral view of cephalon; same specimen as in Figure 4A. B: Late Stage 4. Ventral view of lower lip, labrum and mandibles; same specimen as Figure 4E. C: Early Stage 4. Cavity between lower lip and labrum; same specimen as A. Note pair of papillae under labrum. D: Late Stage 4. Lower lip, labrum and mandibles. E: Late Stage 4. Dorsal organ; same specimen as Figure 4A. F: Intermediate Stage 4. Pair of dorsal telsonal setae. A1, antenna 1; a2, antenna 2; ce pore, pore leading to compound eye; la, labrum; lat lobe, lateral lobe of lower lip ; md, mandible; med lobe, median lobe of lower lip ; tl1, trunk limb 1. identified as the early anlagen of maxillae 2. Samter (1900) identified the anlagen of both maxillae 1 and 2 between the anlagen of the mandibles and the first trunk limbs, situated very close together and close to the midline in the germ band of a very early stage. In late stages of L. kindtii, the openings of the max-
12 246 J. OLESEN ET AL. Fig. 6. Histology of embryos of Leptodora kindtii. Light microscopy. A: Late Stage 2. Sagittal section approximately along midaxis; dark grey arrow points at median lobe of primordial lower lip. B: Late Stage 2 (same specimen as in A). Dark grey arrow points at lateral lobe of primordial lower lip, black arrows indicate beginning segmentation of abdomen. C: Early Stage 4. Sagittal section approximately along midaxis. D: Early Stage 4. Horizontal section of anterior region. E: Close-up of D, showing early invagination of duct to maxillary gland. F: Close-up of C showing the early carapace fold. A1, antenna 1; ca, carapace; ce, compound eye; do, dorsal organ; la, labrum; md, mandible; mx op, maxillary gland opening; tl1, trunk limb 1. illary glands are found laterally at the anterior part of the thorax, immediately dorsal to the bases of the first trunk limbs (based on histology, Fig. 6D,E). This is approximately the same position reported for the adult by Weismann (1874). A pair of small buds is found in a similar position, which we tentatively interpret as vestigial remains of the maxillae 2 that have moved to an extreme lateral position slightly
13 ONTOGENY OF LEPTODORA KINDTII 247 Fig. 7. Dll expression and fluorescence microscopy in embryos of Leptodora kindtii. A: Intermediate Stage 2. Hoechst nuclear stain. B: Intermediate Stage 2. Hoechst nuclear stain; close-up of A. Upper arrows point at weakly developed lateral lobes of lower lip, lower arrows point at anlagen of so-called maxillary processes. C: Late Stage 2. Hoechst nuclear stain. D: Late Stage 2. Close-up of C. Arrows point at two lateral lobes of lower lip, while median lobe is situated in between. E: Late Stage 1. Dll expression. Arrowheads point to the anlagen of the first maxillae expressing Dll under the tip of the mandibles. F: Late Stage 1. Dll expression. Arrowheads point at anlagen of first maxillae expressing Dll. G: Late Stage 1, Dll expression, close-up of E. Arrowheads point to anlagen of the first maxillae expressing Dll under the tip of mandibles. A1, antenna 1; a2, antenna 2; md, mandible; tl1, trunk limb 1.
14 248 J. OLESEN ET AL. Fig. 8. Schematic representation of the ontogeny of maxillae 1 in Leptodora kindtii, showing how these appendages form the lateral lobes of a lower lip. Maxillae 1 are indicated by red; the removed limbs are indicated by grey areas. A: Stage 1, based on expression of Dll as shown in Figure 7E G. B: Intermediate Stage 2, based on Hoechst nuclear stain, as shown in Figure 7A,B. C: Stage 4, based on SEM as shown in Figure 5. dorsal to the bases of the first pair of trunk limbs (Fig. 4C,? ). A pair of relatively large buds is seen posterior to the bases of the mandibles in developmental Stage 3 and posterior to these buds another smaller pair (Fig. 3A,D,E,? ). They are apparently only present during this single stage of development. At first sight it is tempting to interpret these as the limb buds to the first and second maxillae, but for several reasons we have come to another conclusion. The anteriormost and largest pair of buds are too large and in the wrong position to be the first maxillae. The anlagen to the first maxillae have been shown in earlier stages to be closer to the midline of the body (Dll expression, Fig. 7E G; Samter, 1900) than these buds. Also, at this stage of development the vestiges of first maxillae have already been combined with the elevated sternal region (median lobe) to form the primordial tri-lobed lower lip (see earlier; Fig. 7C,D). Hence, the large anterior buds in Stage 3 must be something else and are structures of uncertain affinities. We interpret the smaller lateral buds, located directly under the bases of the first pair of trunk limbs, as the future basal part of these limbs. Trunk limbs. The trunk limbs in Leptodora kindtii are formed as two rows of ventral, elongated, subdivided limb buds. The separate portions of the early limb bud develop into the segments seen in the limbs of the adults. The details are presented below. The elongate limb buds 1 5 of early embryos consist of five portions, as revealed by expression of the Dll gene (Fig. 7E), and in part by SEM (Fig. 2F) and the fluorescent nuclear dye (Fig. 7A). In contrast, trunk limbs 1 5 in the adult of Leptodora kindtii comprise only four segments, one long proximal segment and three smaller ones distally (e.g., see Sars, 1861 [1993], pl. 112). It is clear that the three distal (or lateral) portions of the early limb bud will end up constituting the three distal short segments present in trunk limbs 1 5 in the adult, since the fate of these limb portions can be followed unambiguously during ontogeny. The fate of the two proximal portions seen in the early embryo, revealed by Dll expression, is more difficult to follow and, furthermore, the development of trunk limb 1 is apparently slightly different from that of trunk limbs 2 5. For a detailed description and discussion of trunk limb development, see Olesen et al. (2001). During most of the development of Leptodora kindtii the trunk limbs are aligned ventrally along an anterior posterior axis in lateral view (e.g., Fig. 1C G). In the later stages, the axis has started to shift so that the anterior trunk limbs are directed slightly more anteriorly than the posterior limbs (Fig. 4C,E). After the embryos are released from the brood pouch, the axis of the limbs shifts dramatically to a dorsoventral orientation. In particular, the posterior limbs are moved in an anterior direction, probably caused by growth in the ventral posterior part of the thorax. Abdomen and telson. The abdomen is here referred to as the part of the trunk posterior to the limb-bearing thorax. In the earliest stages examined
15 the abdomen only constitutes a very small part of the body (Fig. 1A,B). In Stage 2, the abdomen is about the same length as the thorax (Fig. 1E,F), becoming narrower distally, while in Stage 4 it is about three times as long (Fig. 4A,D). As a part of the elongation process, the abdomen undertakes a characteristic ventral bend in Stage 3, but it is straightened out again in Stage 4. Abdominal segmentation is visible already in early Stage 2 (Fig. 1C,D). In Stage 4, two abdominal segments (ts8 and ts9) plus a large telson are clearly visible (Fig. 4A,D), while the first abdominal segment (ts7) is obscured because of the dorsally situated yolk, which is still present at this stage. The second abdominal segment is the smallest (ts8). In Stage 2, the posterior part of the trunk terminates in a pair of rounded lobes, each with anlagen to the spine-like caudal furcae (Fig. 2G). It is not before Stage 4 that a distinct telson is visible externally. In this stage the caudal furcae have become long and acute (Fig. 4A,D) and are attached directly to the telson without articulation. The anlagen of the telsonal setae ( postabdominal setae ) are present in early Stage 2 (before the telson is distinctly delineated externally) (Fig. 2G). More developed telsonal setae are present dorsally in Stage 4, close to the anterior margin of the telson (Figs. 4D, 5F). DISCUSSION General The development of Leptodora kindtii will be compared to that of the conchostracan Cyclestheria hislopi and certain other branchiopods. The reasons for especially focusing on C. hislopi for comparison are discussed below. There is now considerable morphological and molecular evidence that Cyclestheria hislopi is the sister group to a monophyletic Cladocera (e.g., Olesen, 1998; Braband et al., 2000). It is therefore reasonable to assume that direct development at least in the parthenogenetic part of the life cycle evolved only once during evolution of the diplostracan branchiopods, namely, in the ancestral lineage leading to C. hislopi and the Cladocera (Cladoceromorpha, after Ax, 1999). The ontogenies in C. hislopi and in various cladocerans are therefore most likely modifications of direct development in such a common ancestor, which makes a detailed comparison between the taxa highly appropriate and promising for phylogenetic discussions. Furthermore, it appears likely that the type of ontogeny in C. hislopi closely approaches that of the common ancestor to these taxa, an assumption we partly base on C. hislopi s sister-group relationship to the Cladocera and on the fact that C. hislopi in general exhibits so many similarities to other conchostracans. However, it is important to be aware that C. hislopi is Recent, and as such, it may have achieved a number of its own ONTOGENY OF LEPTODORA KINDTII 249 specializations since the split from the line giving rise to the Recent cladocerans. Hence, we cannot be sure that the ontogeny of C. hislopi (and other aspects of its morphology) and the common ancestor to the Cladoceromorpha corresponds exactly. Embryology of Leptodora kindtii and Cyclestheria hislopi General Aspects The early embryos of Leptodora kindtii have a general morphology similar to that of the early embryos of other cladocerans and Cyclestheria hislopi. The overall body shapes are largely identical (ovoid), as are the position, orientation, and relative size of the early appendages and labrum, which furthermore develop approximately in the same order. In both L. kindtii and C. hislopi, at least in some stages, the anterior part of the body bearing the labrum, antennae 1 and 2, the mandible, and the dorsal organ is set off from the rest of the body in a distinct head region (compare Figs. 1G, 2E, 3D, and Olesen, 1999: fig. 3b), something which is seen also in free-living spinicaudate conchostracan larvae (see Sars, 1896a: pl. 2, fig. 3). Further similarities are that the first antennae and the mandibles are small limb buds and the second antennae are large and biramous, with dorsally bent rami. The three mentioned limb pairs are situated along the sides of the labrum or almost surrounding it, as also seen in a number of other cladocerans where the early embryos have been examined in a similar way (e.g., Diaphanosoma brachyurum [Liévin, 1848], Polyphemus pediculus, Eurycercus glacialis, Macrothrix laticornis, Acantholeberis curvirostris [Müller, 1776] and Simocephalus vetulus [Müller, 1776]; see Olesen [1998]). Therefore, this is a possible synapomorphy further substantiating the monophyly of the Cladoceromorpha. In the Cladocera, the second antennae tend to be situated more laterally on the head than in Cyclestheria hislopi. This could be a synapomorphy for the Cladocera, but needs more investigation. In the later part of development, the embryos of Leptodora kindtii and Cyclestheria hislopi become very different and begin approaching the morphology of the adults. L. kindtii begins elongating the whole body, especially the head region and the abdomen, and develops numerous other unique structures, such as the tri-lobed lower lip, the styliform mandibles, the forwardly orientated, segmented trunk limbs, and the posterior brood pouch. It has been possible to account for many of the unusual structures in the adult of L. kindtii by comparing this species ontogeny with that of other cladocerans and branchiopods. Dorsal Organ Some onychopods like Polyphemus pediculus, Bythotrephes longimanus, and Cercopagis spp. also
16 250 J. OLESEN ET AL. have a very large dorsal organ occupying a major part of the neck region of the adults (Rivier, 1998). We hesitate using this as a synapormorphy for the Haplopoda and Onychopoda since species of the Podonidae (Onychopoda) have a smaller, circular dorsal organ, similar in shape to what is seen in a number of other cladocerans. Carapace We interpret the data about carapace ontogeny in Leptodora kindtii as indicating that it moves posteriorly by a gradual fusion of the more anterior part to the dorsal side of the thorax. The same interpretation was put forward in the detailed accounts of Müller (1868) and Samter (1895). Müller (1868) stated that the carapace develops as a cuticular duplicature growing out from the mandibular segment (he did not account for the position of the two pairs of maxillae), fusing with the dorsal side of the thorax. Samter (1895) recorded the maxillar region as the point of origin of the carapace in very early juveniles. He described a movement of the carapace from anterior to posterior during development. Both descriptions are congruent with our observations, even though we found the point of origin to be immediately behind the mandibular level. It is likely that the posterior displacement of the carapace in ontogeny reflects what took place in the evolution of L. kindtii. Probably, the anterior part of the carapace has gradually fused to the dorsal part of the thorax during evolution, leaving only the posterior parts of the valves free. If this suggested evolution of the brood pouch in L. kindtii is correct, then, strictly speaking, it is only homologous to a part of the carapace of other branchiopods, since the anterior part in L. kindtii was fused to the thorax. A comparable ontogeny has been described for certain malacostracans where the carapace also fuses with the thoracomeres. The interpretation of this phenomenon within malacostracans, however, is still under debate (e.g., Casanova, 1991; Dahl, 1991; Scholtz, 1998; Richter and Scholtz, 2001). Along with the posterior displacement of the carapace, the orientation of the trunk limbs changes from an anterior posterior alignment to a dorsoventral alignment during ontogeny so that the limbs become directed forward. Together, these changes give the thorax a rotated appearance, as suggested to have taken place in the evolution of Leptodora kindtii by Fryer (1996). We explain these changes are more plausibly explained by a fusion of the anterior part of the carapace to the thorax and by a growth of the thorax posteroventrally, effectively pushing the posterior trunk limbs anteriorly. Maxillae 1 and the Lower Lip The described ontogeny of the first maxillae into the lateral lobes of the lower lip must be an indication of what took place during the evolution of this structure in Leptodora kindtii. The lateral lobes of the lower lip in L. kindtii are therefore considered homologous to the first maxillae of other branchiopods. Interestingly enough, this was already inferred in an early publication by Wagner (1868). He reported that what he called the lower jaws meaning maxillae, but without distinguishing between maxillae 1 and 2 and the lower lip (what we refer to as the median lobe) were immovably attached to each other. However, Wagner s suggestion that the maxillae participate in the formation of the lower lip was not accepted by Weismann (1874), and since then, Wagner s contribution, written in Russian, has been largely forgotten. Wagner also labeled a structure as mx in an embryo (his plate IV, fig. 5). However, other investigations indicate the anlagen to maxillae 1 or 2 to be much smaller and situated much closer to the midline of the animal than Wagner has shown them (Samter, 1900: fig. 18 and Dll expression herein, Fig. 7E G). The structures referred to by Wagner (1868) as mx probably are the same as the lobate lateral structures we found in Stage 3 (Fig. 3) and of which we had some difficulty interpreting the significance. Since the publications of Wagner (1868), Weismann (1874), and Samter (1900), it has repeatedly been stated that L. kindtii lacks maxillae (e.g., Fryer, 1987; Rivier, 1998), and on one occasion it has even been said that the maxillae do not enter into the formation of the lower lip in free-living larvae of L. kindtii (Warren 1901). We have not examined free-living larvae of L. kindtii, but we find it unlikely that the ontogeny of the lower lip should be different in free-living larvae and parthenogenetic embryos, since they are very similar in most respects. In his otherwise fine account, Warren (1901) probably followed some structures that were either not the maxillae or were only a part of the maxillae. The median lobe in Leptodora kindtii approximately corresponds to the sternal region of the mandible and first maxilla in other branchiopods. A groove similar to the one in the midline of the median lobe of L. kindtii can be seen in a very early stage of Artemia franciscana (Walossek 1993: fig. 53D) and in intermediate stages of the earliest known branchiopod, Rehbachiella kinnekullensis (Walossek, 1993: plate 11:4). It is interesting that certain early stages of R. kinnekullensis, similar to L. kindtii, have an elevated sternal region, but it is shorter than in L. kindtii, involving mainly the mandibular sternite. We have found no paragnaths in L. kindtii like those present in Recent branchiopods (Cannon and Leak, 1933) and in R. kinnekullensis (see Walossek, 1993). One could interpret the lateral lobes of the lower lip as modified paragnaths. However, this is very unlikely since there are no examples of crustacean paragnaths expressing Dll (Scholtz et al., 1998). The setose appearance of the lateral lobes of the lower lip in the adult suggests
17 ONTOGENY OF LEPTODORA KINDTII 251 Fig. 9. Maxillae 1 and 2 of two other branchiopod species. A: Cercopagis pengoi (Onychopoda), early embryo. View from posterior of mouth region showing first maxillae limb buds, as well as labrum, mandibles, and anterior trunk limbs. B: Cercopagis pengoi, late embryo. Lateral view of thorax region showing opening of maxillary gland with an associated bud, interpreted as the rudiment of the second maxillae (arrowhead). Also visible are antenna 2, mandible, trunk limbs, penial structure with presumed opening pore of vas deferens. C: Cercopagis pengoi, enlargement of B. D: Cyclestheria hislopi ( Conchostraca ), early embryo. Ventral view of anterior region showing labrum, first antennae, second antennae, first and second maxillae. E: Cyclestheria hislopi, late embryo. Ventral view showing first and second maxillae. A1, antenna 1; a2, antenna 2; la. labrum; md, mandible; mx1, maxilla 1; mx2, maxilla 2; pen, penial structure; tl1, trunk limb 1; tl4, trunk limb 4. them to be vestigial first maxillae (Sebestyén, 1931: fig. 6a,b), since their morphology is quite similar to that of the first maxillae in a number of other branchiopods. The adult has two rows of small setae at the outer margin of each of the lateral lobes and one additional larger posterior seta. This arrangement can conveniently be derived from the small lobate maxillae 1 with a row of curved or straight setae seen in so many other branchiopods (e.g., Cannon and Leak, 1933) and the homology of the lateral lobes to the first maxillae of other branchiopods is therefore further substantiated. Considering the reduced appearance of the first maxillae in many branchiopods as a pair of small setose limbs closely associated with the paragnaths and the mandibles (e.g., see Fig. 9E for a late embryo of Cyclestheria hislopi and Cannon and Clark, 1933: fig. 29, for an adult of Sida crystallina) it is not difficult to imagine how they could have become fused with the elevated sternites and together form a lower lip in the evolutionary line leading to L. kindtii. This cannot be a primary argument for the hypothesis, but at least it shows that the homologization of the lateral lobes of the lower lip in L. kindtii with the first maxillae of other branchiopods provides the basis for a reasonable evolutionary scenario. Sebestyén (1931) notes that L. kindtii sucks out its prey and that the outer lobes of both lips (i.e., labrum and lower lip ) protect the food already in the mouth from being washed out. Probably the maxillae 1 of L. kindtii has become modified to take part in the ventral and lateral sealing of the atrium oris to facilitate the sucking out of the bodily fluids from the prey. We are not aware of any other crustaceans where the first maxillae are incorporated in a lower lip during development, and this arrangement should probably be considered unique for Leptodora kindtii. For comparative purposes we examined embryos of various taxa of the Onychopoda, another taxon of
18 252 J. OLESEN ET AL. macrophagous cladocerans and possibly closely related to L. kindtii. In Cercopagis pengoi and Bythotrephes longimanus, maxillae 1 are formed as small limb buds of the common branchiopod type (Figs. 9A, 10A,B). We are sure that these limb buds represent maxillae 1, since in certain later embryos of Cercopagis pengoi we have been able to identify the rudimentary maxillae 2 in a more posterior position (Fig. 9B,C), as indicated by the opening of the maxillary gland (see below). Maxillae 1 are shown in an adult of Bythotrephes cederstroemi in SEM by Martin and Cash-Clark (1995). Another example of a lower lip formed partly by the first maxillae is found outside the Crustacea in the myriapod groups Diplopoda and Pauropoda, where the first pair of maxillae together with the intervening sternite form a lower lip (gnathochilarium) during ontogeny (Dohle, 1964, 1980, 1998), exactly as is the case in Leptodora kindtii. Maxillae 2 and Maxillary Glands The ducts to the maxillary glands open in association with the second maxillae in branchiopods. In embryos, the future maxillary glands open directly into the early limb buds of the second maxilla of Cyclestheria hislopi ( Conchostraca ) and certain cladocerans representing the Ctenopoda and Anomopoda (Olesen, 1998, 1999). In late juveniles of Leptodora kindtii, in contrast, the openings of the maxillary glands are situated laterally on the thorax, immediately dorsal to where the first pair of trunk limbs emerges (Fig. 6D,E), which is also approximately the position mentioned for the adult by Weismann (1874). With this in mind, we tentatively interpret the buds dorsal to the trunk limbs in late juvenile stages as vestigial second maxillae (Fig. 4C,? ). In late embryos of Cercopagis pengoi, we have found the openings of the maxillary glands to be in a similar position as in Leptodora kindtii, which is at the lateral side of the trunk, dorsal to the first pair of trunk limbs, behind the mandibles (Fig. 9B,C). The openings are in a pair of small buds, which almost certainly are vestiges of the second maxillae. This similarity in the position of the opening of the maxillary glands, possibly associated with the vestiges of the second maxillae, is a putative synapomorphy for the Haplopoda and Onychopoda (used in the Phylogenetic section, below). However, it is not so unusual to have the openings of the maxillary glands displaced slightly laterally relative to the remainder of the maxilla 2 (e.g., Triops cancriformis (Bosc, 1801), see Claus 1873: pl. 7, fig. 6 and Lynceus brachyurus Müller, 1776, see Sars, 1896b: pl. 19, fig. 4; see also Cannon and Leak, 1933), but we are not aware of other cases where this has been taken to such an extreme as in L. kindtii and C. pengoi (Onychopoda). Cannon and Leak (1933) suggest that the lateral displacement of the maxillary gland openings in, for example, Triops spp., is possibly connected with an apparent lack of a forward current along the midventral line. There is therefore no continuous stream of water to sweep away the excretion as it leaves the duct, and so the openings are located at some distance from the mouth. A similar explanation could be suggested for the extreme lateral displacement of the maxillary gland openings in L. kindtii and C. pengoi, where there certainly is no forward current along the midventral line of the body produced by trunk limbs. Such a functional explanation does not negate the shared position of the gland openings as a putative synapomorphy for L. kindtii and the Onychopoda. Abdomen and Telson and Associated Structures In Cyclestheria hislopi there is a simple and direct correlation between the length of the trunk and the number of appendages under development. Appendages are in all stages present in a gradually decreasing degree of development almost to the terminal end of the trunk (Olesen, 1999). This is similar to the situation in Leptodora kindtii only in the short, early stages (Fig. 1A). Quite soon L. kindtii develops a three-segmented abdomen plus an elongate telson, together constituting about the same length as the limb-bearing region (Stage 2) (e.g., Fig. 1E) ( abdomen is, by definition, used for the trunk region posterior to the limbs). Posteriorly is a pair of caudal lobes and the embryos have at Stage 2 the same shape and general appearance as Stage VI of C. hislopi (compare Fig. 2G with Olesen 1999: fig. 6a), only the limbs posterior to the sixth pair are lacking in L. kindtii. Further development in L. kindtii is very different from that of C. hislopi. At a certain stage (Fig. 3) the abdomen makes a ventral bend before it stretches out and begins an extreme growth. In late juvenile stages the abdomen is about twice the length of the limb-bearing region (Fig. 4A); in the adult it is about three times as long. The number of segments is still the same (three plus telson). One could interpret the high total number of Fig. 10. Dll expression and fluorescence microscopy in embryos of Bythotrephes longimanus (Onychopoda) and Cyclestheria hislopi ( Conchostraca ), showing maxillae 1 and 2 (if present). A: Bythotrephes longimanus (Onychopoda), intermediate embryo. Ventral view, Hoechst nuclear stain. The small maxillae 1 limb buds are seen posterior to the large mandible limb buds. B: Bythotrephes longimanus, intermediate embryo. Ventral view of anterior region showing labrum and limb buds of antennae 1, mandibles, and maxillae 1. C: Cyclestheria hislopi, early embryo. Ventral view, Hoechst nuclear stain. Anterior region showing antennae, labrum maxillae, and first pair of trunk limbs. D: Cyclestheria hislopi, intermediate embryo. Ventral view, Dll expression. Anterior region showing same appendages as in C. A1, antenna 1; a2, antenna 2; la, labrum; md, mandible; mx1, maxilla 1; mx2, maxilla 2; tl1, trunk limb 1.
19 ONTOGENY OF LEPTODORA KINDTII 253 Figure 10
20 254 J. OLESEN ET AL. trunk segments (nine plus telson) as a retained plesiomorphy for the Cladocera, but the extremely large size of the three abdominal segments and the telson as an autapomorphy of L. kindtii. The reason for interpreting the high number of segments as probably retained is the similarity of the posterior trunk region of certain embryos of C. hislopi and L. kindtii (compare Figs. 1E, 2G with figs. 5c, 6a in Olesen, 1999). The appearance of the posterior trunk region of L. kindtii is similar to the posterior trunk region of C. hislopi, with a pair of caudal lobes of the same shape and the anlagen to the telson filaments in the same position. The main difference between the embryos of the two species is the missing limbs in the posterior trunk region of L. kindtii. Based on this similarity it seems plausible that the limbless region of L. kindtii has appeared through the loss of limbs in this region. Another possibility would be that the long, limbless posterior trunk region in L. kindtii appeared secondarily from a shorter-bodied ancestor. In any case, because of the paraphyletic status of the Conchostraca, the limbless posterior trunk region of L. kindtii has evolved from an ancestor without an abdomen (like in the Conchostraca ), and the abdomen in L. kindtii is therefore not homologous with that of the Anostraca. Leptodora kindtii is not the only cladoceran to have abdominal segmentation. Some other cladocerans of the order Onychopoda are also known to have a long segmented abdomen, such as Bythotrephes longimanus with two segments (plus telson) and Cercopagis spp. with one segment (plus telson) (Rivier, 1998). The telson bears a pair of small setae dorsally, close to the anterior border. This pair of setae is most certainly homologous to the so-called postabdominal setae found, to our knowledge, in all other cladocerans, and to the similarly situated pair of setae in conchostracans and notostracans. The anlagen to these setae in early stages of Leptodora kindtii and Cyclestheria hislopi are located in identical positions on the dorsal side of the telson in relation to the caudal lobes (compare Fig. 2G and Olesen, 1999: fig. 5c), which is a strong indication of homology. Hence, apparently all species of the Phyllopoda (sensu Preuss, 1951) have such a pair of homologous setae, which is therefore a convincing synapomorphy for this taxon (see Martin and Cash- Clark, 1995). In early stages of Leptodora kindtii, the terminal part of the telson ends in a pair of caudal lobes which are very similar to those of early stages of Cyclestheria hislopi. From these lobes in C. hislopi a pair of immovable furcal claws develops, which in the adults becomes articulated to the telson, whereas in L. kindtii a pair of long spine-like furcae develop which are not articulated to the telson in the adult. Based on the similar ontogeny, homology of these structures seems to be reasonable. It is interesting to note that nonarticulated furcal claws are also known from the adults of onychopods (Rivier, 1998), which is in contrast to adults of spinicaudate conchostracans and ctenopod and anomopod cladocerans. Note on the Phylogenetic Position of Leptodora Because of the unusual morphology of the adult of Leptodora kindtii, its exact phylogenetic position has traditionally proved difficult to establish. Assuming that the Cladocera is a monophyletic assemblage, we discuss two conflicting sets of characters for higher-level grouping within the Cladocera. Some characters support the Haplopoda (L. kindtii) and Onychopoda as sister taxa, some characters support the Anomopoda and Onychopoda as sister taxa, and then possibly with the Haplopoda as the sister group to the remaining cladocerans. These two conflicting possibilities are depicted as cladograms in Figure 13 and as a taxonomical hierarchy with two possible groupings indicated in Figure 14. The Haplopoda and Onychopoda are supported as putative sister taxa (Fig. 13A) by the following characters (see also Martin and Cash-Clark, 1995): 1. Trunk limbs stenopodous and segmented (four segments). In Leptodora kindtii and most onychopods, the trunk limbs are composed of a large basal segment and three smaller distal segments. Some additional supporting characters are possibly correlated with the segmented and stenopodous morphology of the trunk limbs and they are therefore treated here. One of these characters is the loss of a food groove, although remnants of this groove have been detected in Bythotrephes cederstroemi (see Martin and Cash-Clark, 1995). Another of these characters is the absence of epipods in both the Haplopoda and Onychopoda. This character could appear to be correlated with the presence of stenopodous limbs, but since many eumalacostracans possess both stenopodous limbs and epipods, it seems justified to treat this as a separate character. Lack of exopods is not a valid character (mentioned and critically discussed by Martin and Cash-Clark, 1995), since exopods are found in both the Polyphemidae and Podonidae. 2. Maxillary gland openings located laterally at the trunk. (This is discussed above.) 3. Carapace reduced in size, constituting a dorsal brood pouch. In all other cladocerans, the carapace covers the trunk limbs, which is also the case in all conchostracans. 4. Caudal furcae immovable (at least not articulated) in their connection to the telson. This is probably a neotenic character. 5. Morphology of the compound eye. Haplopoda and Onychopoda possess a particular kind of optical
21 ONTOGENY OF LEPTODORA KINDTII 255 Fig. 11. Illustration of putative synapomorphy for the Onychopoda and Anomopoda. The first antennae have a similar ontogeny in Polyphemus pediculus (Onychopoda) and a number of anomopod species. Early in ontogeny the first antennae are widely separated, lateral to the labrum, but during ontogeny they move closer together and form a V in certain stages (see text for details). A: Polyphemus pediculus (Onychopoda). Lateral view of early stage, antennae 1 far apart. B: Polyphemus pediculus, ventral view of intermediate stage. Antennae 1 have moved closer together and form a V. C: Eurycercus glacialis (Anomopoda). Ventral view of early stage. Antennae 1 placed widely apart. D: Eurycercus glacialis. Ventral view of intermediate stage. Antennae 1 closer together forming a V. A1, antenna; a2, antenna 2; la, labrum; md, mandible; mx1, maxilla 1; mx2, maxilla 2; tl1, trunk limb 1; tl5, trunk limb 5.
22 256 J. OLESEN ET AL. Fig. 12. Illustration of putative synapomorphy for the Onychopoda and Anomopoda. Two species, one of each group, have a similar morphology of their trunk limbs in certain embryonic stages. A: Polyphemus pediculus (Onychopoda). Ventral view of intermediate stage. B: Macrothrix laticornis (Anomopoda). Ventral view of intermediate stage. Arabic number 1 refers to the gnathobasic portion of the three anterior trunk limbs in Polyphemus and Macrothrix. design, the transparent apposition eye. In all other Branchiopoda the apposition eye is of the simple kind. Outside the Branchiopoda a transparent eye is known from some amphipods (see Nilsson, 1989). 6. Loss of the nauplius eye. This character was put forward by Martin and Cash-Clark (1995). The validity of this character can be questioned because of the recorded presence of a nauplius eye in the first generation of Leptodora kindtii hatched from resting eggs (Sars, 1873; Weismann, 1874; Samter, 1895). However, in the other adult stages of L. kindtii as well as in onychopods the nauplius eye is absent. Despite its use by Olesen (1998), the concentration of the thoracic ganglia might not be a valid character. In the adult of L. kindtii the six pairs of ganglia are fused longitudinally as well as transversely into a single mass. This single ganglion mass therefore represents all 12 original thoracic ganglia (Weismann, 1874). Claus (1877) described, also for the Onychopoda, a concentration of the thoracic ganglia, but the condition is different from that of L. kindtii. Besides the presence of only four pairs of ganglia, left and right ganglia are clearly separate and the four pairs of ganglia are not completely fused longitudinally and can still be distinguished. Moreover, this condition is not really different from what is found, for example, in the anomopod Simocephalus sp. (see Cunnington, 1903). Another possibility for which supporting characters can be found is the Onychopoda and Anomopoda as sister taxa (Fig. 13B). 1. First antennae move during ontogeny from a lateral, widely separated position to a median position, sometimes forming a v (Fig. 11). This special ontogeny of the first antennae has so far been found in Polyphemus pediculus (Onychopoda) (Fig. 11A,B), Eurycercus glacialis (Fig. 11C,D), and in Simocephalus vetulus, Moina sp., Daphnia pulex, Macrothrix laticornis (all anomopods, unpublished information by JO). In Leptodora kindtii (Haplopoda), the first antennae remain in a separate position during ontogeny (documented in this article), which is also the case for Diaphanosoma brachyurum (see a relatively late stage in Olesen, 1998: fig. 8A) and for Cyclestheria hislopi (outgroup) (see Olesen, 1999). 2. Similar ontogeny of trunk limbs (Fig. 12). In Polyphemus pediculus (Onychopoda) and Macrothrix hirsuticornis (Anomopoda) the trunk limbs are very similar morphologically in certain intermediate ontogenetic stages. The similarities include similar position and orientation on the
Jùrgen Olesen. Acta Zoologica (Stockholm) 80: 163±184 (April 1999)
 Acta Zoologica (Stockholm) 80: 163±184 (April 1999) Larval and post-larval development of the branchiopod clam shrimp Cyclestheria hislopi (Baird, 1859) (Crustacea, Branchiopoda, Conchostraca, Spinicaudata)
Acta Zoologica (Stockholm) 80: 163±184 (April 1999) Larval and post-larval development of the branchiopod clam shrimp Cyclestheria hislopi (Baird, 1859) (Crustacea, Branchiopoda, Conchostraca, Spinicaudata)
Phylogeny of Branchiopoda (Crustacea) Character Evolution and Contribution of Uniquely Preserved Fossils
 Arthropod Systematics & Phylogeny 3 67 (1) 3 39 Museum für Tierkunde Dresden, eissn 1864-8312, 17.6.2009 Phylogeny of Branchiopoda (Crustacea) Character Evolution and Contribution of Uniquely Preserved
Arthropod Systematics & Phylogeny 3 67 (1) 3 39 Museum für Tierkunde Dresden, eissn 1864-8312, 17.6.2009 Phylogeny of Branchiopoda (Crustacea) Character Evolution and Contribution of Uniquely Preserved
JØRGEN OLESEN*, STEFAN RICHTER and GERHARD SCHOLTZ. Humboldt-Universität zu Berlin, Institut für Biologie, Vergleichende Zoologie, Berlin, Germany
 Int. J. Dev. Biol. 45: 869-876 (2001) Original Article The evolutionary transformation of phyllopodous to stenopodous limbs in the Branchiopoda (Crustacea) - Is there a common mechanism for early limb
Int. J. Dev. Biol. 45: 869-876 (2001) Original Article The evolutionary transformation of phyllopodous to stenopodous limbs in the Branchiopoda (Crustacea) - Is there a common mechanism for early limb
Københavns Universitet
 university of copenhagen Københavns Universitet The unique dorsal brood pouch of Thermosbaenacea (Crustacea, Malacostraca) and description of an advanced developmental stage of Tulumella unidens from the
university of copenhagen Københavns Universitet The unique dorsal brood pouch of Thermosbaenacea (Crustacea, Malacostraca) and description of an advanced developmental stage of Tulumella unidens from the
Axis Specification in Drosophila
 Developmental Biology Biology 4361 Axis Specification in Drosophila November 2, 2006 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Developmental Biology Biology 4361 Axis Specification in Drosophila November 2, 2006 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
^ ^ LIBRARY Division of Crustace;
 /re/// ^ ^ LIBRARY Division of Crustace; Larval Development of Helice tridens wuana Rathbun ^Y RTEBRAT and H. tridens tridens de Haan (Crustacea, Brachyura) ZOOLOGY Reared in the Laboratory Crustacea By
/re/// ^ ^ LIBRARY Division of Crustace; Larval Development of Helice tridens wuana Rathbun ^Y RTEBRAT and H. tridens tridens de Haan (Crustacea, Brachyura) ZOOLOGY Reared in the Laboratory Crustacea By
Chapter 12: Aquatic Mandibulates
 Chapter 12: Aquatic Mandibulates Phylum Arthropoda Subphylum: Crustacea (Latin crusta = shell) Class: Malacostraca Order: Decapoda Order: Euphausiacea Order: Amphipoda Order: Isopoda Class: Maxillopoda
Chapter 12: Aquatic Mandibulates Phylum Arthropoda Subphylum: Crustacea (Latin crusta = shell) Class: Malacostraca Order: Decapoda Order: Euphausiacea Order: Amphipoda Order: Isopoda Class: Maxillopoda
Axis Specification in Drosophila
 Developmental Biology Biology 4361 Axis Specification in Drosophila November 6, 2007 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Developmental Biology Biology 4361 Axis Specification in Drosophila November 6, 2007 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Bulletin Zoölogisch Museum
 Bulletin Zoölogisch Museum UNIVERSITEIT VAN AMSTERDAM Vol.11 No. 15 1988 Redescription of johanna Monod, 1926 Virgin Isls (Isopoda) from St. John, Hans Georg Müller Summary Based on the type material,
Bulletin Zoölogisch Museum UNIVERSITEIT VAN AMSTERDAM Vol.11 No. 15 1988 Redescription of johanna Monod, 1926 Virgin Isls (Isopoda) from St. John, Hans Georg Müller Summary Based on the type material,
Phylogeny of Branchiopoda (Crustacea) based on a combined analysis of morphological data and six molecular loci
 Cladistics Cladistics 23 (2007) 301 336 10.1111/j.1096-0031.2007.00148.x Phylogeny of Branchiopoda (Crustacea) based on a combined analysis of morphological data and six molecular loci Stefan Richter 1,3
Cladistics Cladistics 23 (2007) 301 336 10.1111/j.1096-0031.2007.00148.x Phylogeny of Branchiopoda (Crustacea) based on a combined analysis of morphological data and six molecular loci Stefan Richter 1,3
Comparative Mouthpart Morphology and Evolution of the Carnivorous Heptageniidae (Ephemeroptera) 1
 l Aquatic Insects, Vol. 8 (1986). No. 2, pp. 83-89 0165-0424/86/0802-0083 $3.00 Swets & Zeitlinger Comparative Mouthpart Morphology and Evolution of the Carnivorous Heptageniidae (Ephemeroptera) 1 by W.
l Aquatic Insects, Vol. 8 (1986). No. 2, pp. 83-89 0165-0424/86/0802-0083 $3.00 Swets & Zeitlinger Comparative Mouthpart Morphology and Evolution of the Carnivorous Heptageniidae (Ephemeroptera) 1 by W.
Introduction to Embryology. He who sees things grow from the beginning will have the finest view of them.
 He who sees things grow from the beginning will have the finest view of them. Aristotle 384 322 B.C. Introduction to Embryology This lecture will introduce you to the science of developmental biology or
He who sees things grow from the beginning will have the finest view of them. Aristotle 384 322 B.C. Introduction to Embryology This lecture will introduce you to the science of developmental biology or
/. W. Martin and C. E. Cash-Clark
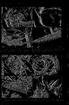 78 /. W. Martin and C. E. Cash-Clark 7 ig. 16. A. Outer view of spine-like exopods of thoracopods 1-3. Exopod of t4 also visible (although not spine-like). B. Exopod of thoracopod wo. Note small seta at
78 /. W. Martin and C. E. Cash-Clark 7 ig. 16. A. Outer view of spine-like exopods of thoracopods 1-3. Exopod of t4 also visible (although not spine-like). B. Exopod of thoracopod wo. Note small seta at
18.4 Embryonic development involves cell division, cell differentiation, and morphogenesis
 18.4 Embryonic development involves cell division, cell differentiation, and morphogenesis An organism arises from a fertilized egg cell as the result of three interrelated processes: cell division, cell
18.4 Embryonic development involves cell division, cell differentiation, and morphogenesis An organism arises from a fertilized egg cell as the result of three interrelated processes: cell division, cell
v Scientists have identified 1.3 million living species of animals v The definition of an animal
 Biosc 41 9/10 Announcements BIOSC 041 v Genetics review: group problem sets Groups of 3-4 Correct answer presented to class = 2 pts extra credit Incorrect attempt = 1 pt extra credit v Lecture: Animal
Biosc 41 9/10 Announcements BIOSC 041 v Genetics review: group problem sets Groups of 3-4 Correct answer presented to class = 2 pts extra credit Incorrect attempt = 1 pt extra credit v Lecture: Animal
Biosc 41 9/10 Announcements
 Biosc 41 9/10 Announcements v Genetics review: group problem sets Groups of 3-4 Correct answer presented to class = 2 pts extra credit Incorrect attempt = 1 pt extra credit v Lecture: Animal Body Plans
Biosc 41 9/10 Announcements v Genetics review: group problem sets Groups of 3-4 Correct answer presented to class = 2 pts extra credit Incorrect attempt = 1 pt extra credit v Lecture: Animal Body Plans
Outline. v Definition and major characteristics of animals v Dividing animals into groups based on: v Animal Phylogeny
 BIOSC 041 Overview of Animal Diversity: Animal Body Plans Reference: Chapter 32 Outline v Definition and major characteristics of animals v Dividing animals into groups based on: Body symmetry Tissues
BIOSC 041 Overview of Animal Diversity: Animal Body Plans Reference: Chapter 32 Outline v Definition and major characteristics of animals v Dividing animals into groups based on: Body symmetry Tissues
Larval development PARAPENAEOPSIS STYLIFERA (H. MILNE EDWARDS)
 IX Larval development PARAPENAEOPSIS STYLIFERA (H. MILNE EDWARDS) M.S. Muthu N. N. Pillai K- V. George Parapenaeopsis stylifera was reared from the egg to the postlarval stage in the Narakkal laboratory.
IX Larval development PARAPENAEOPSIS STYLIFERA (H. MILNE EDWARDS) M.S. Muthu N. N. Pillai K- V. George Parapenaeopsis stylifera was reared from the egg to the postlarval stage in the Narakkal laboratory.
Post-embryonic development of remipede crustaceans
 EVOLUTION & DEVELOPMENT 9:2, 117 121 (2007) Post-embryonic development of remipede crustaceans Stefan Koenemann, a, Frederick R. Schram, b Armin Bloechl, a Thomas M. Iliffe, c Mario Hoenemann, a and Christoph
EVOLUTION & DEVELOPMENT 9:2, 117 121 (2007) Post-embryonic development of remipede crustaceans Stefan Koenemann, a, Frederick R. Schram, b Armin Bloechl, a Thomas M. Iliffe, c Mario Hoenemann, a and Christoph
A new asellote isopod of the genus Microjanira Schiecke & Fresi, 1970 (Crustacea: Isopoda: Asellota: Janiridae) from Japan
 Bull. Kitakyushu Mus. Nat. Hist. Hum. Hist., Ser. A, 6: 13-18, March 31, 2008 A new asellote isopod of the genus Microjanira Schiecke & Fresi, 1970 (Crustacea: Isopoda: Asellota: Janiridae) from Japan
Bull. Kitakyushu Mus. Nat. Hist. Hum. Hist., Ser. A, 6: 13-18, March 31, 2008 A new asellote isopod of the genus Microjanira Schiecke & Fresi, 1970 (Crustacea: Isopoda: Asellota: Janiridae) from Japan
Helicopsyche agnetae, new species (Trichoptera, Helicopsychidae) described from Hong Kong
 Zootaxa 1854: 63 68 (2008) www.mapress.com/zootaxa/ Copyright 2008 Magnolia Press ISSN 1175-5326 (print edition) ZOOTAXA ISSN 1175-5334 (online edition) Helicopsyche agnetae, new species (Trichoptera,
Zootaxa 1854: 63 68 (2008) www.mapress.com/zootaxa/ Copyright 2008 Magnolia Press ISSN 1175-5326 (print edition) ZOOTAXA ISSN 1175-5334 (online edition) Helicopsyche agnetae, new species (Trichoptera,
Arthropoda ARTHRO JOINTED PODA FEET
 Arthropoda ARTHRO JOINTED PODA FEET The arthropods are a group of animals which has attained the greatest biological success largest number of species and individuals and occupy the greatest number of
Arthropoda ARTHRO JOINTED PODA FEET The arthropods are a group of animals which has attained the greatest biological success largest number of species and individuals and occupy the greatest number of
Axis Specification in Drosophila
 Developmental Biology Biology 4361 Axis Specification in Drosophila July 9, 2008 Drosophila Development Overview Fertilization Cleavage Gastrulation Drosophila body plan Oocyte formation Genetic control
Developmental Biology Biology 4361 Axis Specification in Drosophila July 9, 2008 Drosophila Development Overview Fertilization Cleavage Gastrulation Drosophila body plan Oocyte formation Genetic control
GHABBOUR, M.W. Plant Protection Research Institute, Agricultural Research Center, Ministry of Agriculture, Dokki, Egypt.
 Entomologica, Bari, 33, (1999): 73-83 GHABBOUR, M.W. Plant Protection Research Institute, Agricultural Research Center, Ministry of Agriculture, Dokki, Egypt. DESCRIPTIONS OF THE FIRST-INSTAR NYMPHS OF
Entomologica, Bari, 33, (1999): 73-83 GHABBOUR, M.W. Plant Protection Research Institute, Agricultural Research Center, Ministry of Agriculture, Dokki, Egypt. DESCRIPTIONS OF THE FIRST-INSTAR NYMPHS OF
Biology 340 Comparative Embryology Lecture 4 Dr. Stuart Sumida. Overview of Pre-Metazoan. and Protostome Development (Insects)
 Biology 340 Comparative Embryology Lecture 4 Dr. Stuart Sumida Overview of Pre-Metazoan and Protostome Development (Insects) Plants Fungi Animals In1998 fossilized animal embryos were reported from the
Biology 340 Comparative Embryology Lecture 4 Dr. Stuart Sumida Overview of Pre-Metazoan and Protostome Development (Insects) Plants Fungi Animals In1998 fossilized animal embryos were reported from the
Kingdom Animalia. Zoology the study of animals
 Kingdom Animalia Zoology the study of animals Summary Animals are multicellular and eukaryotic. consume and digest organic materials thereby being heterotrophs. Most are motile at some time in their lives.
Kingdom Animalia Zoology the study of animals Summary Animals are multicellular and eukaryotic. consume and digest organic materials thereby being heterotrophs. Most are motile at some time in their lives.
Mosquito Systematics Vol. 6(Z) June 1974
 Mosquito Systematics Vol. 6(Z) June 1974 93 Research on the Mosquitoes of Angola. VII - Redescription of the Larva of Aedes durbanensis durbanensis (Theo., 1903) and Description of Aedes durbanensis angozae
Mosquito Systematics Vol. 6(Z) June 1974 93 Research on the Mosquitoes of Angola. VII - Redescription of the Larva of Aedes durbanensis durbanensis (Theo., 1903) and Description of Aedes durbanensis angozae
Contumacious Beasts: A Story of Two Diastylidae (Cumacea) from Arctic Waters
 The University of Maine DigitalCommons@UMaine Marine Sciences Faculty Scholarship School of Marine Sciences 2-1-2000 Contumacious Beasts: A Story of Two Diastylidae (Cumacea) from Arctic Waters S. Gerken
The University of Maine DigitalCommons@UMaine Marine Sciences Faculty Scholarship School of Marine Sciences 2-1-2000 Contumacious Beasts: A Story of Two Diastylidae (Cumacea) from Arctic Waters S. Gerken
Biology 211 (2) Week 1 KEY!
 Biology 211 (2) Week 1 KEY Chapter 1 KEY FIGURES: 1.2, 1.3, 1.4, 1.5, 1.6, 1.7 VOCABULARY: Adaptation: a trait that increases the fitness Cells: a developed, system bound with a thin outer layer made of
Biology 211 (2) Week 1 KEY Chapter 1 KEY FIGURES: 1.2, 1.3, 1.4, 1.5, 1.6, 1.7 VOCABULARY: Adaptation: a trait that increases the fitness Cells: a developed, system bound with a thin outer layer made of
Patterns of Evolution
 Patterns of Evolution A tree that represents an estimate (hypothesis) of evolutionary relatedness is a phylogeny Classifications can be based on groupings within a phylogeny Groupings can be categorized
Patterns of Evolution A tree that represents an estimate (hypothesis) of evolutionary relatedness is a phylogeny Classifications can be based on groupings within a phylogeny Groupings can be categorized
Classifications can be based on groupings g within a phylogeny
 Patterns of Evolution A tree that represents an estimate (hypothesis) of evolutionary relatedness is a phylogeny Classifications can be based on groupings g within a phylogeny y Groupings can be categorized
Patterns of Evolution A tree that represents an estimate (hypothesis) of evolutionary relatedness is a phylogeny Classifications can be based on groupings g within a phylogeny y Groupings can be categorized
8/23/2014. Introduction to Animal Diversity
 Introduction to Animal Diversity Chapter 32 Objectives List the characteristics that combine to define animals Summarize key events of the Paleozoic, Mesozoic, and Cenozoic eras Distinguish between the
Introduction to Animal Diversity Chapter 32 Objectives List the characteristics that combine to define animals Summarize key events of the Paleozoic, Mesozoic, and Cenozoic eras Distinguish between the
Drosophila Life Cycle
 Drosophila Life Cycle 1 Early Drosophila Cleavage Nuclei migrate to periphery after 10 nuclear divisions. Cellularization occurs when plasma membrane folds in to divide nuclei into cells. Drosophila Superficial
Drosophila Life Cycle 1 Early Drosophila Cleavage Nuclei migrate to periphery after 10 nuclear divisions. Cellularization occurs when plasma membrane folds in to divide nuclei into cells. Drosophila Superficial
LARVAL DEVELOPMENT OF THE CRUSTACEAN THOR - j ^-J- \ ^L, BY A. C. BROAD 4 ^ M" 0 ^ Duke University Marine Laboratory, Beaufort, North Carolina
 OL.C. IUDIL&LAU&I!***XP&T**/. LARVAL DEVELOPMENT OF THE CRUSTACEAN THOR - j ^-J- \ ^L, FLORIDANjm. KINGSLEY BY A. C. BROAD 4 ^ M" 0 ^ Duke University Marine Laboratory, Beaufort, North Carolina OIVISlOiM
OL.C. IUDIL&LAU&I!***XP&T**/. LARVAL DEVELOPMENT OF THE CRUSTACEAN THOR - j ^-J- \ ^L, FLORIDANjm. KINGSLEY BY A. C. BROAD 4 ^ M" 0 ^ Duke University Marine Laboratory, Beaufort, North Carolina OIVISlOiM
Introduction to Animals
 Introduction to Animals Characteristics of Animals multicellular Except for sponges, animal cells are arranged into tissues. Tissues are necessary to produce organs and organ systems. Tissues, organs,
Introduction to Animals Characteristics of Animals multicellular Except for sponges, animal cells are arranged into tissues. Tissues are necessary to produce organs and organ systems. Tissues, organs,
OF THE LEMNA FROND MORPHOLOGY
 MORPHOLOGY OF THE LEMNA FROND FREDERICK H. BLODGETT (WITH PLATE XIV AND ONE FIGURE) In the case of structure simplified by reduction, it is sometimes necessary to trace the development of the parts through
MORPHOLOGY OF THE LEMNA FROND FREDERICK H. BLODGETT (WITH PLATE XIV AND ONE FIGURE) In the case of structure simplified by reduction, it is sometimes necessary to trace the development of the parts through
AN UPDATED PHYLOGENY OF THE CONCHOSTRACA CLADOCERA CLADE (BRANCHIOPODA, DIPLOSTRACA)
 AN UPDATED PHYLOGENY OF THE CONCHOSTRACA CLADOCERA CLADE (BRANCHIOPODA, DIPLOSTRACA) BY JØRGEN OLESEN 1 ) Humboldt Universität zu Berlin, Institut für Biologie, Vergleichende Zoologie, Philippstrasse 13,
AN UPDATED PHYLOGENY OF THE CONCHOSTRACA CLADOCERA CLADE (BRANCHIOPODA, DIPLOSTRACA) BY JØRGEN OLESEN 1 ) Humboldt Universität zu Berlin, Institut für Biologie, Vergleichende Zoologie, Philippstrasse 13,
Workshop: The Evolution of Animalia body symmetry embryonic germ layers ontogenetic origins I. What is an Animal? II. Germ Layers
 Workshop: The Evolution of Animalia by Dana Krempels Perhaps even more than the other Eukarya, Animalia is characterized by a distinct progression of complexity in form and function as one moves from the
Workshop: The Evolution of Animalia by Dana Krempels Perhaps even more than the other Eukarya, Animalia is characterized by a distinct progression of complexity in form and function as one moves from the
Chapter 18 Lecture. Concepts of Genetics. Tenth Edition. Developmental Genetics
 Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
Ceratophysella michalinae, a new species from Poland (Collembola: Hypogastruridae)
 Genus Vol. 16 (1): 1-5 Wroc³aw, 31 III 2005 Ceratophysella michalinae, a new species from Poland (Collembola: Hypogastruridae) DARIUSZ SKAR YÑSKI Zoological Institute, Wroc³aw University, Przybyszewskiego
Genus Vol. 16 (1): 1-5 Wroc³aw, 31 III 2005 Ceratophysella michalinae, a new species from Poland (Collembola: Hypogastruridae) DARIUSZ SKAR YÑSKI Zoological Institute, Wroc³aw University, Przybyszewskiego
MORPHOLOGICAL STUDIES OF PLEIDS (HEMIPTERA: PLEIDAE) USING SEM
 28 Journal of Management and Science ISSN: 2249-1260 e-issn: 2250-1819 Vol.7. No.4 December-2017 MORPHOLOGICAL STUDIES OF PLEIDS (HEMIPTERA: PLEIDAE) USING SEM Miriam Cecilia Vassou PG and Research Department
28 Journal of Management and Science ISSN: 2249-1260 e-issn: 2250-1819 Vol.7. No.4 December-2017 MORPHOLOGICAL STUDIES OF PLEIDS (HEMIPTERA: PLEIDAE) USING SEM Miriam Cecilia Vassou PG and Research Department
Parasitology Research
 Parasitology Research Electronic Supplementary Material for: Sarcophaga (Liosarcophaga) tibialis Macquart 1851 (Diptera: Sarcophagidae): micromorphology of preimaginal stages of a fly of medical and veterinary
Parasitology Research Electronic Supplementary Material for: Sarcophaga (Liosarcophaga) tibialis Macquart 1851 (Diptera: Sarcophagidae): micromorphology of preimaginal stages of a fly of medical and veterinary
A NEW SPECIES OF OPHIOMEGISTUS (ACARI: PARAMEGISTIDAE) FROM A MALAYSIAN KUKRI SNAKE 1
 Pacific Insects Vol. 22, no. 3-4: 380-384 17 December 1980 1980 by the Bishop Museum A NEW SPECIES OF OPHIOMEGISTUS (ACARI: PARAMEGISTIDAE) FROM A MALAYSIAN KUKRI SNAKE 1 By M. Lee Goff 2 Abstract. Ophiomegistus
Pacific Insects Vol. 22, no. 3-4: 380-384 17 December 1980 1980 by the Bishop Museum A NEW SPECIES OF OPHIOMEGISTUS (ACARI: PARAMEGISTIDAE) FROM A MALAYSIAN KUKRI SNAKE 1 By M. Lee Goff 2 Abstract. Ophiomegistus
Functional morphology of giant mole crab larvae: a possible case of defensive enrollment
 Rudolf et al. Zoological Letters (2016) 2:17 DOI 10.1186/s40851-016-0052-5 RESEARCH ARTICLE Open Access Functional morphology of giant mole crab larvae: a possible case of defensive enrollment Nicole R.
Rudolf et al. Zoological Letters (2016) 2:17 DOI 10.1186/s40851-016-0052-5 RESEARCH ARTICLE Open Access Functional morphology of giant mole crab larvae: a possible case of defensive enrollment Nicole R.
SCIENTIFIC EVIDENCE TO SUPPORT THE THEORY OF EVOLUTION. Using Anatomy, Embryology, Biochemistry, and Paleontology
 SCIENTIFIC EVIDENCE TO SUPPORT THE THEORY OF EVOLUTION Using Anatomy, Embryology, Biochemistry, and Paleontology Scientific Fields Different fields of science have contributed evidence for the theory of
SCIENTIFIC EVIDENCE TO SUPPORT THE THEORY OF EVOLUTION Using Anatomy, Embryology, Biochemistry, and Paleontology Scientific Fields Different fields of science have contributed evidence for the theory of
Primary Plant Body: Embryogenesis and the Seedling
 BIOL 221 Concepts of Botany Primary Plant Body: Embryogenesis and the Seedling (Photo Atlas: Figures 1.29, 9.147, 9.148, 9.149, 9.150, 9.1, 9.2) A. Introduction Plants are composed of fewer cell types,
BIOL 221 Concepts of Botany Primary Plant Body: Embryogenesis and the Seedling (Photo Atlas: Figures 1.29, 9.147, 9.148, 9.149, 9.150, 9.1, 9.2) A. Introduction Plants are composed of fewer cell types,
Chapter 32, 10 th edition Q1.Which characteristic below is shared by plants, fungi, and animals? ( Concept 32.1)
 Chapter 32, 10 th edition Q1.Which characteristic below is shared by plants, fungi, and animals? ( Concept 32.1) A) They are multicellular eukaryotes. B) They are heterotrophs. C) Their cells are supported
Chapter 32, 10 th edition Q1.Which characteristic below is shared by plants, fungi, and animals? ( Concept 32.1) A) They are multicellular eukaryotes. B) They are heterotrophs. C) Their cells are supported
Georg Brenneis 1,2* and Claudia P. Arango 3
 Brenneis and Arango Zoological Letters (2019) 5:4 https://doi.org/10.1186/s40851-018-0118-7 RESEARCH ARTICLE Open Access First description of epimorphic development in Antarctic Pallenopsidae (Arthropoda,
Brenneis and Arango Zoological Letters (2019) 5:4 https://doi.org/10.1186/s40851-018-0118-7 RESEARCH ARTICLE Open Access First description of epimorphic development in Antarctic Pallenopsidae (Arthropoda,
DESCRIPTIONS OF TEN XANTHOIDEAN (CRUSTACEA: DECAPODA: BRACHYURA) FIRST STAGE ZOEAS FROM INHACA ISLAND, MOZAMBIQUE
 THE RAFFLES BULLETIN OF ZOOLOGY 2003 THE RAFFLES BULLETIN OF ZOOLOGY 2003 51(2): 323-378 DESCRIPTIONS OF TEN XANTHOIDEAN (CRUSTACEA: DECAPODA: BRACHYURA) FIRST STAGE ZOEAS FROM INHACA ISLAND, MOZAMBIQUE
THE RAFFLES BULLETIN OF ZOOLOGY 2003 THE RAFFLES BULLETIN OF ZOOLOGY 2003 51(2): 323-378 DESCRIPTIONS OF TEN XANTHOIDEAN (CRUSTACEA: DECAPODA: BRACHYURA) FIRST STAGE ZOEAS FROM INHACA ISLAND, MOZAMBIQUE
PR1VATE LIBRARY OE WILLIAM L P.EIER_.S
 PR1VATE LIBRARY OE WILLIAM L P.EIER_.S PENTAGENllDAE: A NEW FAMILY OF EPHEMEROIDEA (EPHEMEROPTERA) 1 2 W. P. McCAFFERTY 3 Department of Entomology University of Georgia Athens, Georgia 30601 ABSTRACT On
PR1VATE LIBRARY OE WILLIAM L P.EIER_.S PENTAGENllDAE: A NEW FAMILY OF EPHEMEROIDEA (EPHEMEROPTERA) 1 2 W. P. McCAFFERTY 3 Department of Entomology University of Georgia Athens, Georgia 30601 ABSTRACT On
Frank D. Ferrari, Viatcheslav N. Ivanenko, and Hans-Uwe Dahms
 BODY ARCHITECTURE AND RELATIONSHIPS AMONG BASAL COPEPODS Frank D. Ferrari, Viatcheslav N. Ivanenko, and Hans-Uwe Dahms (FDF, correspondence, ferrarif@si.edu) IZ/MSC, MRC-534, National Museum of Natural
BODY ARCHITECTURE AND RELATIONSHIPS AMONG BASAL COPEPODS Frank D. Ferrari, Viatcheslav N. Ivanenko, and Hans-Uwe Dahms (FDF, correspondence, ferrarif@si.edu) IZ/MSC, MRC-534, National Museum of Natural
ESS 345 Ichthyology. Systematic Ichthyology Part II Not in Book
 ESS 345 Ichthyology Systematic Ichthyology Part II Not in Book Thought for today: Now, here, you see, it takes all the running you can do, to keep in the same place. If you want to get somewhere else,
ESS 345 Ichthyology Systematic Ichthyology Part II Not in Book Thought for today: Now, here, you see, it takes all the running you can do, to keep in the same place. If you want to get somewhere else,
ZOOLOGISCHE MEDEDELINGEN
 ZOOLOGISCHE MEDEDELINGEN UITGEGEVEN DOOR HET RIJKSMUSEUM VAN NATUURLIJKE HISTORIE TE LEIDEN (MINISTERIE VAN CULTUUR, RECREATIE EN MAATSCHAPPELIJK WERK) Deel 43 no. 11 20 december 1968 PARABOMOLOCHUS GLOBICEPS
ZOOLOGISCHE MEDEDELINGEN UITGEGEVEN DOOR HET RIJKSMUSEUM VAN NATUURLIJKE HISTORIE TE LEIDEN (MINISTERIE VAN CULTUUR, RECREATIE EN MAATSCHAPPELIJK WERK) Deel 43 no. 11 20 december 1968 PARABOMOLOCHUS GLOBICEPS
A NEW GENUS, SPECIES, AND SUBTRIBE OF TERMITOPHILOUS STAPHYLINIDAE FROM AUSTRALIA (Coleoptera)
 Pacific Insects 12 (3): 499-596 30 October 1970 A NEW GENUS, SPECIES, AND SUBTRIBE OF TERMITOPHILOUS STAPHYLINIDAE FROM AUSTRALIA (Coleoptera) With a Description of its Glands 1 By David H. Kistner 2 Abstract:
Pacific Insects 12 (3): 499-596 30 October 1970 A NEW GENUS, SPECIES, AND SUBTRIBE OF TERMITOPHILOUS STAPHYLINIDAE FROM AUSTRALIA (Coleoptera) With a Description of its Glands 1 By David H. Kistner 2 Abstract:
Cap n collar differentiates the mandible from the maxilla in the beetle Tribolium castaneum
 Coulcher and Telford EvoDevo 2012, 3:25 RESEARCH Open Access Cap n collar differentiates the mandible from the maxilla in the beetle Tribolium castaneum Joshua F Coulcher and Maximilian J Telford * Abstract
Coulcher and Telford EvoDevo 2012, 3:25 RESEARCH Open Access Cap n collar differentiates the mandible from the maxilla in the beetle Tribolium castaneum Joshua F Coulcher and Maximilian J Telford * Abstract
Description of Haliplus larvae from Lebanon (Coleoptera: Haliplidae)
 Koleopterologische Rundschau 81 41 54 Wien, September 2011 Description of Haliplus larvae from Lebanon (Coleoptera: Haliplidae) B.J. van VONDEL Abstract The third instar larvae of Haliplus kulleri VONDEL
Koleopterologische Rundschau 81 41 54 Wien, September 2011 Description of Haliplus larvae from Lebanon (Coleoptera: Haliplidae) B.J. van VONDEL Abstract The third instar larvae of Haliplus kulleri VONDEL
Embryogenesis of an insect nervous system II: A second class of neuron precursor cells and the origin of the intersegmental connectives
 J. Embryol. exp. Morph. Vol. 61,pp. 317-330, 1981 3J7 Printed in Great Britain @ Company of Biologists Limited 1981 Embryogenesis of an insect nervous system II: A second class of neuron precursor cells
J. Embryol. exp. Morph. Vol. 61,pp. 317-330, 1981 3J7 Printed in Great Britain @ Company of Biologists Limited 1981 Embryogenesis of an insect nervous system II: A second class of neuron precursor cells
Analysis of the Wnt gene repertoire in an onychophoran provides new insights into the evolution of segmentation
 Hogvall et al. EvoDevo 2014, 5:14 RESEARCH Analysis of the Wnt gene repertoire in an onychophoran provides new insights into the evolution of segmentation Open Access Mattias Hogvall 1, Anna Schönauer
Hogvall et al. EvoDevo 2014, 5:14 RESEARCH Analysis of the Wnt gene repertoire in an onychophoran provides new insights into the evolution of segmentation Open Access Mattias Hogvall 1, Anna Schönauer
Axis determination in flies. Sem 9.3.B.5 Animal Science
 Axis determination in flies Sem 9.3.B.5 Animal Science All embryos are in lateral view (anterior to the left). Endoderm, midgut; mesoderm; central nervous system; foregut, hindgut and pole cells in yellow.
Axis determination in flies Sem 9.3.B.5 Animal Science All embryos are in lateral view (anterior to the left). Endoderm, midgut; mesoderm; central nervous system; foregut, hindgut and pole cells in yellow.
Midterm 1. Average score: 74.4 Median score: 77
 Midterm 1 Average score: 74.4 Median score: 77 NAME: TA (circle one) Jody Westbrook or Jessica Piel Section (circle one) Tue Wed Thur MCB 141 First Midterm Feb. 21, 2008 Only answer 4 of these 5 problems.
Midterm 1 Average score: 74.4 Median score: 77 NAME: TA (circle one) Jody Westbrook or Jessica Piel Section (circle one) Tue Wed Thur MCB 141 First Midterm Feb. 21, 2008 Only answer 4 of these 5 problems.
Classification, Phylogeny yand Evolutionary History
 Classification, Phylogeny yand Evolutionary History The diversity of life is great. To communicate about it, there must be a scheme for organization. There are many species that would be difficult to organize
Classification, Phylogeny yand Evolutionary History The diversity of life is great. To communicate about it, there must be a scheme for organization. There are many species that would be difficult to organize
Phytogeny, evolution and classification of the Branchiopoda (Crustacea)
 l - I y d w b U M w 412: 101-2 12, i m (f) 1999 K hm er Academie Publisher.';. Printed in the Netherlands. 191 Phytogeny, evolution and classification of the Branchiopoda (Crustacea) Stefan Negrea1, Nicolae
l - I y d w b U M w 412: 101-2 12, i m (f) 1999 K hm er Academie Publisher.';. Printed in the Netherlands. 191 Phytogeny, evolution and classification of the Branchiopoda (Crustacea) Stefan Negrea1, Nicolae
Amphigomphus somnuki n. sp. from North Thailand (Odonata: Gomphidae) MATTI HAMALAINEN
 Entomol. Z., 106(5), 1996 177 Amphigomphus somnuki n. sp. from North Thailand (Odonata: Gomphidae) MATTI HAMALAINEN With 8 figures Abstract: A new dragonfly species, Amphigomphus somnuki n. sp. (holotype
Entomol. Z., 106(5), 1996 177 Amphigomphus somnuki n. sp. from North Thailand (Odonata: Gomphidae) MATTI HAMALAINEN With 8 figures Abstract: A new dragonfly species, Amphigomphus somnuki n. sp. (holotype
Unicellular: Cells change function in response to a temporal plan, such as the cell cycle.
 Spatial organization is a key difference between unicellular organisms and metazoans Unicellular: Cells change function in response to a temporal plan, such as the cell cycle. Cells differentiate as a
Spatial organization is a key difference between unicellular organisms and metazoans Unicellular: Cells change function in response to a temporal plan, such as the cell cycle. Cells differentiate as a
H. MILNE EDWARDS (Crustacea, Brachyura) Reared in the Laboratory
 Larval Development of Sesarma (Holometopus) dehaani H. MILNE EDWARDS (Crustacea, Brachyura) Reared in the Laboratory By Keiji BABA and Keisuke MIYATA Reprinted from the Memoirs of the Faculty of Education,
Larval Development of Sesarma (Holometopus) dehaani H. MILNE EDWARDS (Crustacea, Brachyura) Reared in the Laboratory By Keiji BABA and Keisuke MIYATA Reprinted from the Memoirs of the Faculty of Education,
Male reproductive system. Spicule
 Lecture 06 - Male Reproductive System The production of sperms takes place in testis. In nematodes, whenever the number of testis is one, it is known as monarchic conditions and when they are tow in number,
Lecture 06 - Male Reproductive System The production of sperms takes place in testis. In nematodes, whenever the number of testis is one, it is known as monarchic conditions and when they are tow in number,
Chapter 11. Development: Differentiation and Determination
 KAP Biology Dept Kenyon College Differential gene expression and development Mechanisms of cellular determination Induction Pattern formation Chapter 11. Development: Differentiation and Determination
KAP Biology Dept Kenyon College Differential gene expression and development Mechanisms of cellular determination Induction Pattern formation Chapter 11. Development: Differentiation and Determination
Chapter 1- An Orientation to the Human Body NOTES
 Chapter 1- An Orientation to the Human Body NOTES Overview of Anatomy and Physiology: -Anatomy- of body parts and their relationships to one another. -Gross or Macroscopic= large and easily observable
Chapter 1- An Orientation to the Human Body NOTES Overview of Anatomy and Physiology: -Anatomy- of body parts and their relationships to one another. -Gross or Macroscopic= large and easily observable
The First Instar Larva of Hydrobius pauper SHARP (Coleoptera, Hydrophilidae)
 Elytra, Tokyo, New Series, 2 (2): 279 284 December 31, 2012 title 279 The First Instar Larva of Hydrobius pauper SHARP (Coleoptera, Hydrophilidae) Yûsuke MINOSHIMA 1) & Masakazu HAYASHI 2) 1) Systematic
Elytra, Tokyo, New Series, 2 (2): 279 284 December 31, 2012 title 279 The First Instar Larva of Hydrobius pauper SHARP (Coleoptera, Hydrophilidae) Yûsuke MINOSHIMA 1) & Masakazu HAYASHI 2) 1) Systematic
The Swimming Setae of Daphnia carinata By W. E. AGAR
 353 The Swimming Setae of Daphnia carinata By W. E. AGAR {From the Zoology Department, The University of Melbourne) SUMMARY I. The structure, renewal, embryonic development, and regeneration of the swimming
353 The Swimming Setae of Daphnia carinata By W. E. AGAR {From the Zoology Department, The University of Melbourne) SUMMARY I. The structure, renewal, embryonic development, and regeneration of the swimming
Description of the Immature Stages of Trioza uniqua (Caldwell) (Homoptera: Psyllidae)1
 Vol. 31, December 31,1992 219 Description of the Immature Stages of Trioza uniqua (Caldwell) (Homoptera: Psyllidae)1 GRANT K. UCHIDA2 and JOHN W. BEARDSLEY3 ABSTRACT. The laxonomic position of Triout uniqua
Vol. 31, December 31,1992 219 Description of the Immature Stages of Trioza uniqua (Caldwell) (Homoptera: Psyllidae)1 GRANT K. UCHIDA2 and JOHN W. BEARDSLEY3 ABSTRACT. The laxonomic position of Triout uniqua
Chapter 16: Reconstructing and Using Phylogenies
 Chapter Review 1. Use the phylogenetic tree shown at the right to complete the following. a. Explain how many clades are indicated: Three: (1) chimpanzee/human, (2) chimpanzee/ human/gorilla, and (3)chimpanzee/human/
Chapter Review 1. Use the phylogenetic tree shown at the right to complete the following. a. Explain how many clades are indicated: Three: (1) chimpanzee/human, (2) chimpanzee/ human/gorilla, and (3)chimpanzee/human/
Biologists have used many approaches to estimating the evolutionary history of organisms and using that history to construct classifications.
 Phylogenetic Inference Biologists have used many approaches to estimating the evolutionary history of organisms and using that history to construct classifications. Willi Hennig developed d the techniques
Phylogenetic Inference Biologists have used many approaches to estimating the evolutionary history of organisms and using that history to construct classifications. Willi Hennig developed d the techniques
Is there a ventral neural ridge in chick embryos? Implications for the origin of adenohypophyseal and other APUD cells
 /. Embryol. exp. Morph. Vol. 57, pp. 71-78, 1980 7 \ Printed in Great Britain Company of Biologists Limited 1980 Is there a ventral neural ridge in chick embryos? Implications for the origin of adenohypophyseal
/. Embryol. exp. Morph. Vol. 57, pp. 71-78, 1980 7 \ Printed in Great Britain Company of Biologists Limited 1980 Is there a ventral neural ridge in chick embryos? Implications for the origin of adenohypophyseal
Classification and Phylogeny
 Classification and Phylogeny The diversity it of life is great. To communicate about it, there must be a scheme for organization. There are many species that would be difficult to organize without a scheme
Classification and Phylogeny The diversity it of life is great. To communicate about it, there must be a scheme for organization. There are many species that would be difficult to organize without a scheme
Chapter 32. Objectives. Table of Contents. Characteristics. Characteristics, continued. Section 1 The Nature of Animals
 Introduction to Animals Table of Contents Objectives Identify four important characteristics of animals. List two kinds of tissues found only in animals. Explain how the first animals may have evolved
Introduction to Animals Table of Contents Objectives Identify four important characteristics of animals. List two kinds of tissues found only in animals. Explain how the first animals may have evolved
The post-embryonic development of Remipedia (Crustacea) additional results and new insights
 Dev Genes Evol (2009) 219:131 145 DOI 10.1007/s00427-009-0273-0 ORIGINAL ARTICLE The post-embryonic development of Remipedia (Crustacea) additional results and new insights Stefan Koenemann & Jørgen Olesen
Dev Genes Evol (2009) 219:131 145 DOI 10.1007/s00427-009-0273-0 ORIGINAL ARTICLE The post-embryonic development of Remipedia (Crustacea) additional results and new insights Stefan Koenemann & Jørgen Olesen
Observing Daphnia. Student Resources 1.4 Observing Daphnia, Pages 1 and Counting Daphnia Populations Inquiry Focus Observe
 Observing Daphnia Observing Daphnia, Page 1 30 minutes Pairs Observe the daphnia in your cup. List two ways you can tell the adults from the babies: 1 Babies are smaller. 2 Babies are brownish. How do
Observing Daphnia Observing Daphnia, Page 1 30 minutes Pairs Observe the daphnia in your cup. List two ways you can tell the adults from the babies: 1 Babies are smaller. 2 Babies are brownish. How do
Comparative morphology of the first zoea of twelve brachyuran species (Crustacea: Decapoda) from the Amazon region
 http://dx.doi.org/10.1590/s1984-46702013000300004 Comparative morphology of the first zoea of twelve brachyuran species (Crustacea: Decapoda) from the Amazon region Adelson S. de Souza 1, Rauquírio M.
http://dx.doi.org/10.1590/s1984-46702013000300004 Comparative morphology of the first zoea of twelve brachyuran species (Crustacea: Decapoda) from the Amazon region Adelson S. de Souza 1, Rauquírio M.
EXTERNAL ANATOMY OF INSECTS
 External Anatomy of Insects 1 The insect s exoskeleton is made up of a series of plates EXTERNAL ANATOMY OF INSECTS These plates make up the insect s exoskeleton. These plates are connected by joints or
External Anatomy of Insects 1 The insect s exoskeleton is made up of a series of plates EXTERNAL ANATOMY OF INSECTS These plates make up the insect s exoskeleton. These plates are connected by joints or
A complete three-dimensional reconstruction of the myoanatomy of Loricifera: comparative morphology of an adult and a Higgins larva stage
 Neves et al. Frontiers in Zoology 2013, 10:19 RESEARCH Open Access A complete three-dimensional reconstruction of the myoanatomy of Loricifera: comparative morphology of an adult and a Higgins larva stage
Neves et al. Frontiers in Zoology 2013, 10:19 RESEARCH Open Access A complete three-dimensional reconstruction of the myoanatomy of Loricifera: comparative morphology of an adult and a Higgins larva stage
Classification and Phylogeny
 Classification and Phylogeny The diversity of life is great. To communicate about it, there must be a scheme for organization. There are many species that would be difficult to organize without a scheme
Classification and Phylogeny The diversity of life is great. To communicate about it, there must be a scheme for organization. There are many species that would be difficult to organize without a scheme
SPECIES FROM THAILAND, MEXICO AND BRAZIL (Diptera: Nycteribiidae)!
 Pacific Insects 10 (1): 25-32 10 May 1968 NEW BASILIA SPECIES FROM THAILAND, MEXICO AND BRAZIL (Diptera: Nycteribiidae)! By T. C. Maa 2 Abstract: Females of Basilia benkingi, B. traubi and B. producta
Pacific Insects 10 (1): 25-32 10 May 1968 NEW BASILIA SPECIES FROM THAILAND, MEXICO AND BRAZIL (Diptera: Nycteribiidae)! By T. C. Maa 2 Abstract: Females of Basilia benkingi, B. traubi and B. producta
Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo
 Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo (magenta), Fas2 (blue) and GFP (green) in overview (A) and
Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo (magenta), Fas2 (blue) and GFP (green) in overview (A) and
Paramecium. Sub-Order Peniculina. Genus Paramecium
 Paramecium Kingdom Animalia Phylum Protozoa Sub-Phylum Ciliophora Class Ciliata or Infusoria Sub-Class Holotricha Order Hymenostomatida Sub-Order Peniculina Family Paramecidae Genus Paramecium Introduction:
Paramecium Kingdom Animalia Phylum Protozoa Sub-Phylum Ciliophora Class Ciliata or Infusoria Sub-Class Holotricha Order Hymenostomatida Sub-Order Peniculina Family Paramecidae Genus Paramecium Introduction:
Principles of Experimental Embryology
 Biology 4361 Developmental Biology Principles of Experimental Embryology September 19, 2006 Major Research Questions How do forces outside the embryo affect its development? (Environmental Developmental
Biology 4361 Developmental Biology Principles of Experimental Embryology September 19, 2006 Major Research Questions How do forces outside the embryo affect its development? (Environmental Developmental
Fig. 16. Majority rule consensus tree depicting phylogenetic relationships inferred among 74 species of heterokont algae. Note that A.
 Plate 1 Figs. 1-8. Light microscopic images of Anthophysa vegetans colonies and individual motile cells. Figs 1-5. Stalked (arrow; Figs 1,2) or unstalked (Figs 3-5) colonies consisting of ca. 10-20 spherical
Plate 1 Figs. 1-8. Light microscopic images of Anthophysa vegetans colonies and individual motile cells. Figs 1-5. Stalked (arrow; Figs 1,2) or unstalked (Figs 3-5) colonies consisting of ca. 10-20 spherical
On the systematic of the water mite Piona annulata (Thor, 1900) (Acari, Hydrachnidia: Pionidae)
 ISSN 2336-9744 (online) ISSN 2337-0173 (print) The journal is available on line at www.biotaxa.org/em Correspondence On the systematic of the water mite Piona annulata (Thor, 1900) (Acari, Hydrachnidia:
ISSN 2336-9744 (online) ISSN 2337-0173 (print) The journal is available on line at www.biotaxa.org/em Correspondence On the systematic of the water mite Piona annulata (Thor, 1900) (Acari, Hydrachnidia:
Department of Entomology University of New Hampshire Durham, New Hampshire 03824
 LARVAE OF WRACK COLEOPTERA IN THE FAMILIES CORYLOPHIDAE, RHIZOPHAGIDAE, AND LATHRIDIIDAE* BY DONALD S. CHANDLER Department of Entomology University of New Hampshire Durham, New Hampshire 03824 Wrack studies
LARVAE OF WRACK COLEOPTERA IN THE FAMILIES CORYLOPHIDAE, RHIZOPHAGIDAE, AND LATHRIDIIDAE* BY DONALD S. CHANDLER Department of Entomology University of New Hampshire Durham, New Hampshire 03824 Wrack studies
Biology 1B Evolution Lecture 2 (February 26, 2010) Natural Selection, Phylogenies
 1 Natural Selection (Darwin-Wallace): There are three conditions for natural selection: 1. Variation: Individuals within a population have different characteristics/traits (or phenotypes). 2. Inheritance:
1 Natural Selection (Darwin-Wallace): There are three conditions for natural selection: 1. Variation: Individuals within a population have different characteristics/traits (or phenotypes). 2. Inheritance:
Introduction to Lepidoptera
 Introduction to Lepidoptera Taxonomic Workshop for Early Detection of Important Tortricidae and Other Lepidopteran Agricultural and Silvicultural Pests UMass Amherst 15-17 July 2013 Todd M. Gilligan, Ph.D.
Introduction to Lepidoptera Taxonomic Workshop for Early Detection of Important Tortricidae and Other Lepidopteran Agricultural and Silvicultural Pests UMass Amherst 15-17 July 2013 Todd M. Gilligan, Ph.D.
Fertilization of sperm and egg produces offspring
 In sexual reproduction Fertilization of sperm and egg produces offspring In asexual reproduction Offspring are produced by a single parent, without the participation of sperm and egg CONNECTIONS BETWEEN
In sexual reproduction Fertilization of sperm and egg produces offspring In asexual reproduction Offspring are produced by a single parent, without the participation of sperm and egg CONNECTIONS BETWEEN
Homeotic genes in flies. Sem 9.3.B.6 Animal Science
 Homeotic genes in flies Sem 9.3.B.6 Animal Science So far We have seen that identities of each segment is determined by various regulators of segment polarity genes In arthopods, and in flies, each segment
Homeotic genes in flies Sem 9.3.B.6 Animal Science So far We have seen that identities of each segment is determined by various regulators of segment polarity genes In arthopods, and in flies, each segment
Acrobotrys tritubus Riedel
 151 Acrobotrys tritubus Riedel Acrobotrys tritubus Riedel, 1957, p.80, pl.1, fig.5 DESCRIPTION Cephalis trilobate, with large subglobular [antecephalic] lobe, smaller globular [cephalic] lobe, and inflated-conical
151 Acrobotrys tritubus Riedel Acrobotrys tritubus Riedel, 1957, p.80, pl.1, fig.5 DESCRIPTION Cephalis trilobate, with large subglobular [antecephalic] lobe, smaller globular [cephalic] lobe, and inflated-conical
Systematics Lecture 3 Characters: Homology, Morphology
 Systematics Lecture 3 Characters: Homology, Morphology I. Introduction Nearly all methods of phylogenetic analysis rely on characters as the source of data. A. Character variation is coded into a character-by-taxon
Systematics Lecture 3 Characters: Homology, Morphology I. Introduction Nearly all methods of phylogenetic analysis rely on characters as the source of data. A. Character variation is coded into a character-by-taxon
A CONTRIBUTION TO THE STUDY OF RELATIVE GROWTH OF PARTS IN INACHUS DORSETTENSIS
 145 A CONTRIBUTION TO THE STUDY OF RELATIVE GROWTH OF PARTS IN INACHUS DORSETTENSIS BY M. E. SHAW, M.Sc. (From the Zoological Laboratory, King's College, University of London.) (With Eleven Text-figures.)
145 A CONTRIBUTION TO THE STUDY OF RELATIVE GROWTH OF PARTS IN INACHUS DORSETTENSIS BY M. E. SHAW, M.Sc. (From the Zoological Laboratory, King's College, University of London.) (With Eleven Text-figures.)
1/30/2009. Copyright The McGraw Hill Companies, Inc. Permission required for reproduction or display.
 CHAPTER 9 Architectural Pattern of an Animal New Designs for Living Zoologists recognize 34 major phyla of living multicellular animals Survivors of around 100 phyla that appeared 600 million years ago
CHAPTER 9 Architectural Pattern of an Animal New Designs for Living Zoologists recognize 34 major phyla of living multicellular animals Survivors of around 100 phyla that appeared 600 million years ago
Carolin Haug 1*, Wafaa S Sallam 2, Andreas Maas 3, Dieter Waloszek 3, Verena Kutschera 3 and Joachim T Haug 1
 Haug et al. Frontiers in Zoology 2012, 9:31 RESEARCH Open Access Tagmatization in Stomatopoda reconsidering functional units of modern-day mantis shrimps (Verunipeltata, Hoplocarida) and implications for
Haug et al. Frontiers in Zoology 2012, 9:31 RESEARCH Open Access Tagmatization in Stomatopoda reconsidering functional units of modern-day mantis shrimps (Verunipeltata, Hoplocarida) and implications for
Biology 224 Human Anatomy and Physiology - II Week 1; Lecture 1; Monday Dr. Stuart S. Sumida. Review of Early Development of Humans.
 Biology 224 Human Anatomy and Physiology - II Week 1; Lecture 1; Monday Dr. Stuart S. Sumida Review of Early Development of Humans Special Senses Review: Historical and Developmental Perspectives Ontogeny
Biology 224 Human Anatomy and Physiology - II Week 1; Lecture 1; Monday Dr. Stuart S. Sumida Review of Early Development of Humans Special Senses Review: Historical and Developmental Perspectives Ontogeny
Amira A. AL-Hosary PhD of infectious diseases Department of Animal Medicine (Infectious Diseases) Faculty of Veterinary Medicine Assiut
 Amira A. AL-Hosary PhD of infectious diseases Department of Animal Medicine (Infectious Diseases) Faculty of Veterinary Medicine Assiut University-Egypt Phylogenetic analysis Phylogenetic Basics: Biological
Amira A. AL-Hosary PhD of infectious diseases Department of Animal Medicine (Infectious Diseases) Faculty of Veterinary Medicine Assiut University-Egypt Phylogenetic analysis Phylogenetic Basics: Biological
