Blackbody Radiation OBJECTIVES
|
|
|
- Annis Cecily Austin
- 6 years ago
- Views:
Transcription
1 Name Class Date Skills Practice Lab Blackbody Radiation A perfect absorber of radiation also happens to be a perfect radiator of that radiation as well. Such objects are called blackbodies, because darker objects tend to absorb (and therefore radiate) energy better than light objects, and the best absorber/radiator is a perfectly black object. In the late 19th century, blackbodies were studied to gain better understanding of the properties of electromagnetic radiation, which includes radio waves, infrared radiation, ultraviolet radiation, and x-rays, as well as visible light. As a blackbody is heated, it emits radiation that is both brighter and of shorter wavelength (that is, more toward the blue end of the spectrum). Although not blackbodies in the strictest sense, stars and other incandescent objects simulate blackbody behavior. Therefore, the hotter these objects are, the brighter and bluer is the light that they emit. In this lab, you will observe the blackbody behavior of two incandescent light bulbs, and how the light from them changes as the electric current increases and the filament in the bulb becomes hotter. You will then apply this information to make predictions about the temperature and brightness of stars. OBJECTIVES Using Scientific Methods Model blackbody behavior using an incandescent bulb. Using Scientific Methods Observe changes in the light as electric current is varied. Apply the results of the lab to the colors and brightness of stars. Draw conclusions about the nature of those stars. MATERIALS electric lamp with threaded socket (can accept light bulbs up to 100 W) incandescent light bulb, 50 W, 1 incandescent light bulb, 100 W, 1 variable resistor, alternating current, 110 V SAFETY PROCEDURE 1. Insert the 50 W light bulb into the socket of the lamp, and plug the lamp into the outlet of the variable resistor. CAUTION: Handle light bulbs with care. They can break easily and cut skin. Touching a hot light bulb may cause serious burns. Holt Earth Science 49 Stars, Galaxies and the Universe
2 Name Class Date 2. Check to be sure the variable resistor unit is turned off, and that the dial is set to zero. Plug the resistor into a ground fault circuit interruptor (GFCI) protected circuit. 3. Turn the variable resistor on, then switch the lamp on. Gradually turn the dial of the resistor until the lamp begins to glow. 4. Note the color and brightness of the filament of the lamp. Carefully place your hand near the bulb without touching the glass surface. Note whether the glass is warm or cool. Write down your observations in Table 1, below. 5. Increase the setting of the variable resistor to the low position. Again note the color and brightness of the lamp s filament. Carefully place your hand near the bulb without touching it, and note whether it is cool, warm, or hot. Write down your observations in Table Repeat Step 5, increasing the setting of the resistor first to medium, and then to medium-high. The bulb should appear very bright. CAUTION: Do not turn the setting all the way up to high. TABLE 1 50 W LIGHT BULB Variable Resistor Setting Color of Filament Relative Brightness of Filament Relative Temperature of Bulb 7. Turn the variable resistor dial back to zero, and switch off both the lamp and the resistor. Wait until the bulb has cooled sufficiently, then remove it from the lamp and replace it with the 100 W light bulb. Holt Earth Science 50 Stars, Galaxies and the Universe
3 Name Class Date 8. Repeat Steps 1 through 6 using the 100 W light bulb. Record your observations in Table 2. TABLE W LIGHT BULB Variable Resistor Setting Color of Filament Relative Brightness of Filament Relative Temperature of Bulb 9. Turn the variable resistor dial back to zero, and switch off both the lamp and the resistor. Unplug the resistor from the outlet and the lamp from the resistor. CAUTION: The bulb will probably be very hot; therefore, do not remove the bulb from the lamp. ANALYSIS AND CONCLUSION 1. Evaluating Methods Using only your eyes, you have been able to differentiate between the colors and brightness of the light bulbs at the different variable resistor settings. What other methods could be used to make these observations? 2. Evaluating Results What differences did you observe in the light from the two different light bulbs? What conclusions can you draw from your observations? Holt Earth Science 51 Stars, Galaxies and the Universe
4 Name Class Date 3. Making Predictions Certain stars in the night sky appear red or orange, while others appear yellow, white, or blue-white. From your observations, what can you state about these stars? 4. Making Predictions Certain red stars are as bright or brighter than certain blue stars. Explain why this does not contradict your observations. EXTENSION 1. Applying Conclusions If the equipment is available, obtain a prism or grating spectroscope and repeat the lab. For each setting of the variable resistor, observe the spectrum of the light, and notice if it is brighter in the red, yellow, or blue part of the spectrum. Record your observations below, and note whether the added information helps you to draw more exact conclusions. Holt Earth Science 52 Stars, Galaxies and the Universe
5 Skills Practice Lab Blackbody Radiation Teacher s Notes TIME REQUIRED one 45-minute class period LAB RATINGS Teacher Prep 2 Student Set-Up 2 Concept Level 3 Clean Up 1 Easy Hard Gabrielle Schavran Western Rockingham Syosset High School Syosset, New York SKILLS ACQUIRED Constructing Models Experimenting Inferring Predicting THE SCIENTIFIC METHOD In this lab, students will Make Observations Draw Conclusions MATERIALS Depending on how many variable resistors are available, each lab station may have between two and four students. CAUTION: Use ground fault circuit interruptor (GFCI) protected circuits when performing this lab, and make sure students do not work on the lab near water. Students should exercise caution when operating electrical equipment, as well as when handling the bulbs to prevent breakage. Be sure students do not touch the bulbs with bare hands while performing this lab. Holt Earth Science 75 Stars, Galaxies and the Universe
6 TIPS AND TRICKS Students should gradually increase the resistance until the dial is only about halfway to its maximum setting. If too much current is passed through the bulb, the bulb will burn out. Be sure to have extra bulbs on hand in case of burnouts or breakage. If you have several hand-held diffraction gratings available, you may wish to distribute these to students and have them try to determine whether the spectrum of the light for a given setting is brighter in the red or blue regions. Students may also be able to determine whether brighter settings are much brighter than dimmer settings in the blue portion of the spectrum. Holt Earth Science 76 Stars, Galaxies and the Universe
7 Name Class Date Skills Practice Lab Blackbody Radiation A perfect absorber of radiation also happens to be a perfect radiator of that radiation as well. Such objects are called blackbodies, because darker objects tend to absorb (and therefore radiate) energy better than light objects, and the best absorber/radiator is a perfectly black object. In the late 19th century, blackbodies were studied to gain better understanding of the properties of electromagnetic radiation, which includes radio waves, infrared radiation, ultraviolet radiation, and x-rays, as well as visible light. As a blackbody is heated, it emits radiation that is both brighter and of shorter wavelength (that is, more toward the blue end of the spectrum). Although not blackbodies in the strictest sense, stars and other incandescent objects simulate blackbody behavior. Therefore, the hotter these objects are, the brighter and bluer is the light that they emit. In this lab, you will observe the blackbody behavior of two incandescent light bulbs, and how the light from them changes as the electric current increases and the filament in the bulb becomes hotter. You will then apply this information to make predictions about the temperature and brightness of stars. OBJECTIVES Using Scientific Methods Model blackbody behavior using an incandescent bulb. Using Scientific Methods Observe changes in the light as electric current is varied. Apply the results of the lab to the colors and brightness of stars. Draw conclusions about the nature of those stars. MATERIALS electric lamp with threaded socket (can accept light bulbs up to 100 W) incandescent light bulb, 50 W, 1 incandescent light bulb, 100 W, 1 variable resistor, alternating current, 110 V SAFETY PROCEDURE 1. Insert the 50 W light bulb into the socket of the lamp, and plug the lamp into the outlet of the variable resistor. CAUTION: Handle light bulbs with care. They can break easily and cut skin. Touching a hot light bulb may cause serious burns. Holt Earth Science 49 Stars, Galaxies and the Universe 77
8 Name Class Date 2. Check to be sure the variable resistor unit is turned off, and that the dial is set to zero. Plug the resistor into a ground fault circuit interruptor (GFCI) protected circuit. 3. Turn the variable resistor on, then switch the lamp on. Gradually turn the dial of the resistor until the lamp begins to glow. 4. Note the color and brightness of the filament of the lamp. Carefully place your hand near the bulb without touching the glass surface. Note whether the glass is warm or cool. Write down your observations in Table 1, below. 5. Increase the setting of the variable resistor to the low position. Again note the color and brightness of the lamp s filament. Carefully place your hand near the bulb without touching it, and note whether it is cool, warm, or hot. Write down your observations in Table Repeat Step 5, increasing the setting of the resistor first to medium, and then to medium-high. The bulb should appear very bright. CAUTION: Do not turn the setting all the way up to high. TABLE 1 50 W LIGHT BULB Variable Resistor Setting Color of Filament Relative Brightness of Filament Relative Temperature of Bulb 7. Turn the variable resistor dial back to zero, and switch off both the lamp and the resistor. Wait until the bulb has cooled sufficiently, then remove it from the lamp and replace it with the 100 W light bulb. Holt Earth Science 50 Stars, Galaxies and the Universe 78
9 Name Class Date 8. Repeat Steps 1 through 6 using the 100 W light bulb. Record your observations in Table 2. TABLE W LIGHT BULB Variable Resistor Setting Color of Filament Relative Brightness of Filament Relative Temperature of Bulb 9. Turn the variable resistor dial back to zero, and switch off both the lamp and the resistor. Unplug the resistor from the outlet and the lamp from the resistor. CAUTION: The bulb will probably be very hot; therefore, do not remove the bulb from the lamp. ANALYSIS AND CONCLUSION 1. Evaluating Methods Using only your eyes, you have been able to differentiate between the colors and brightness of the light bulbs at the different variable resistor settings. What other methods could be used to make these observations? Answers may vary. Sample answer: These observations could be also be made by using instruments, such as spectroscopes or photometers, to measure differences in the bulbs color and brightness. 2. Evaluating Results What differences did you observe in the light from the two different light bulbs? What conclusions can you draw from your observations? Answers may vary. Sample answer: The more current that passes through the filament, the hotter it becomes, and the brighter and bluer the light it emits becomes. Holt Earth Science Stars, Galaxies and the Universe
10 Name Class Date 3. Making Predictions Certain stars in the night sky appear red or orange, while others appear yellow, white, or blue-white. From your observations, what can you state about these stars? Answers may vary. Sample answer: The redder stars are cooler than the whiter and bluer stars. However, the brightness of the stars depends on factors other than the star s temperature. 4. Making Predictions Certain red stars are as bright or brighter than certain blue stars. Explain why this does not contradict your observations. Answers may vary. Sample answer: The redder stars may be closer than the blue stars, and therefore they appear brighter. The red stars may also be red giants or red supergiants, which are brighter because they are much larger than the other stars. EXTENSION 1. Applying Conclusions If the equipment is available, obtain a prism or grating spectroscope and repeat the lab. For each setting of the variable resistor, observe the spectrum of the light, and notice if it is brighter in the red, yellow, or blue part of the spectrum. Record your observations below, and note whether the added information helps you to draw more exact conclusions. Answer may vary. Holt Earth Science 52 Stars, Galaxies and the Universe 80
high energy state for the electron in the atom low energy state for the electron in the atom
 Atomic Spectra Objectives The objectives of this experiment are to: 1) Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. 2) Measure several wavelengths of light
Atomic Spectra Objectives The objectives of this experiment are to: 1) Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. 2) Measure several wavelengths of light
Atomic Emission Spectra
 Atomic Emission Spectra Objectives The objectives of this laboratory are as follows: To build and calibrate a simple meter-stick spectroscope that is capable of measuring wavelengths of visible light.
Atomic Emission Spectra Objectives The objectives of this laboratory are as follows: To build and calibrate a simple meter-stick spectroscope that is capable of measuring wavelengths of visible light.
Experiment 24: Spectroscopy
 Experiment 24: Spectroscopy Figure 24.1: Spectroscopy EQUIPMENT High Voltage Power Supply Incandescent Light Source (3) Gas Discharge Tubes: 1. Helium 2. Hydrogen 3. Unknown Element Spectrometer Felt (1)
Experiment 24: Spectroscopy Figure 24.1: Spectroscopy EQUIPMENT High Voltage Power Supply Incandescent Light Source (3) Gas Discharge Tubes: 1. Helium 2. Hydrogen 3. Unknown Element Spectrometer Felt (1)
Astron 104 Laboratory #5 Colors of Stars
 Name: Date: Section: Astron 104 Laboratory #5 Colors of Stars Section 11.1 Introduction The night sky in a dark location is full of stars tiny pinpoints of light. It is pretty obvious from even a casual
Name: Date: Section: Astron 104 Laboratory #5 Colors of Stars Section 11.1 Introduction The night sky in a dark location is full of stars tiny pinpoints of light. It is pretty obvious from even a casual
ASTRO Fall 2012 LAB #7: The Electromagnetic Spectrum
 ASTRO 1050 - Fall 2012 LAB #7: The Electromagnetic Spectrum ABSTRACT Astronomers rely on light to convey almost all of the information we have on distant astronomical objects. In addition to measuring
ASTRO 1050 - Fall 2012 LAB #7: The Electromagnetic Spectrum ABSTRACT Astronomers rely on light to convey almost all of the information we have on distant astronomical objects. In addition to measuring
Laboratory Exercise. Atomic Spectra A Kirchoff Potpourri
 1 Name: Laboratory Exercise Atomic Spectra A Kirchoff Potpourri Purpose: To examine the atomic spectra from several gas filled tubes and understand the importance of spectroscopy to astronomy. Introduction
1 Name: Laboratory Exercise Atomic Spectra A Kirchoff Potpourri Purpose: To examine the atomic spectra from several gas filled tubes and understand the importance of spectroscopy to astronomy. Introduction
CPO Science Foundations of Physics. Unit 8, Chapter 26
 CPO Science Foundations of Physics Unit 8, Chapter 26 Unit 8: Matter and Energy Chapter 26 Heat Transfer 26.1 Heat Conduction 26.2 Convection 26.3 Radiation Chapter 26 Objectives 1. Explain the relationship
CPO Science Foundations of Physics Unit 8, Chapter 26 Unit 8: Matter and Energy Chapter 26 Heat Transfer 26.1 Heat Conduction 26.2 Convection 26.3 Radiation Chapter 26 Objectives 1. Explain the relationship
Properties of Electromagnetic Radiation Chapter 5. What is light? What is a wave? Radiation carries information
 Concepts: Properties of Electromagnetic Radiation Chapter 5 Electromagnetic waves Types of spectra Temperature Blackbody radiation Dual nature of radiation Atomic structure Interaction of light and matter
Concepts: Properties of Electromagnetic Radiation Chapter 5 Electromagnetic waves Types of spectra Temperature Blackbody radiation Dual nature of radiation Atomic structure Interaction of light and matter
SPECTROSCOPY PRELAB. 2) Name the 3 types of spectra and, in 1 sentence each, describe them.
 NAME: SPECTROSCOPY PRELAB 1) What is a spectrum? 2) Name the 3 types of spectra and, in 1 sentence each, describe them. a. b. c. 3) Use Wien s law to calculate the surface temperature of the star Alnilam
NAME: SPECTROSCOPY PRELAB 1) What is a spectrum? 2) Name the 3 types of spectra and, in 1 sentence each, describe them. a. b. c. 3) Use Wien s law to calculate the surface temperature of the star Alnilam
ACTIVITY 2 Exploring Light Patterns
 Name: Class: SOLIDS & Visual Quantum Mechanics LIGHT ACTIVITY 2 Exploring Light Patterns Goal We will continue to investigate the properties of LEDs and the incandescent lamp by observing and exploring
Name: Class: SOLIDS & Visual Quantum Mechanics LIGHT ACTIVITY 2 Exploring Light Patterns Goal We will continue to investigate the properties of LEDs and the incandescent lamp by observing and exploring
Atomic Theory: Spectroscopy and Flame Tests
 Atomic Theory: Spectroscopy and Flame Tests Introduction Light energy is also known as electromagnetic (EM) radiation. The light that we observe with our eyes, visible light, is just a small portion of
Atomic Theory: Spectroscopy and Flame Tests Introduction Light energy is also known as electromagnetic (EM) radiation. The light that we observe with our eyes, visible light, is just a small portion of
Atomic Theory: Spectroscopy and Flame Tests
 Atomic Theory: Spectroscopy and Flame Tests Introduction Light energy is also known as electromagnetic (EM) radiation. The light that we observe with our eyes, visible light, is just a small portion of
Atomic Theory: Spectroscopy and Flame Tests Introduction Light energy is also known as electromagnetic (EM) radiation. The light that we observe with our eyes, visible light, is just a small portion of
AS 101: Day Lab #2 Summer Spectroscopy
 Spectroscopy Goals To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are related To see spectral lines from different elements in emission and
Spectroscopy Goals To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are related To see spectral lines from different elements in emission and
Atomic Theory: Spectroscopy and Flame Tests
 Atomic Theory: Spectroscopy and Flame Tests Introduction Light energy is also known as electromagnetic (EM) radiation. The light that we observe with our eyes, visible light, is just a small portion of
Atomic Theory: Spectroscopy and Flame Tests Introduction Light energy is also known as electromagnetic (EM) radiation. The light that we observe with our eyes, visible light, is just a small portion of
EXPERIMENT 17: Atomic Emission
 EXPERIMENT 17: Atomic Emission PURPOSE: To construct an energy level diagram of the hydrogen atom To identify an element from its line spectrum. PRINCIPLES: White light, such as emitted by the sun or an
EXPERIMENT 17: Atomic Emission PURPOSE: To construct an energy level diagram of the hydrogen atom To identify an element from its line spectrum. PRINCIPLES: White light, such as emitted by the sun or an
Lab: Excited Electrons
 Part A: EMISSION SPECTROSCOPY Lab: Excited Electrons According to the Bohr atomic model, electrons orbit the nucleus within specific energy levels. These levels are defined by unique amounts of energy.
Part A: EMISSION SPECTROSCOPY Lab: Excited Electrons According to the Bohr atomic model, electrons orbit the nucleus within specific energy levels. These levels are defined by unique amounts of energy.
SKINAKAS OBSERVATORY Astronomy Projects for University Students COLOUR IN ASTRONOMY
 P R O J E C T 3 COLOUR IN ASTRONOMY Objective: Explain what colour means in an astronomical context and its relationship with the temperature of a star. Learn how to create colour-colour diagrams and how
P R O J E C T 3 COLOUR IN ASTRONOMY Objective: Explain what colour means in an astronomical context and its relationship with the temperature of a star. Learn how to create colour-colour diagrams and how
Name Class Date. Chapter 18 The Electromagnetic Spectrum and Light Investigation 18A
 Chapter 18 The Electromagnetic Spectrum and Light Investigation 18A Predicting Spectra Background Information Some materials are transparent to nearly all frequencies of visible light. This means that
Chapter 18 The Electromagnetic Spectrum and Light Investigation 18A Predicting Spectra Background Information Some materials are transparent to nearly all frequencies of visible light. This means that
Modern Astronomy Review #1
 Modern Astronomy Review #1 1. The red-shift of light from distant galaxies provides evidence that the universe is (1) shrinking, only (3) shrinking and expanding in a cyclic pattern (2) expanding, only
Modern Astronomy Review #1 1. The red-shift of light from distant galaxies provides evidence that the universe is (1) shrinking, only (3) shrinking and expanding in a cyclic pattern (2) expanding, only
Physics LESSON PLAN PERFORMANCE OBJECTIVE(S): STUDENTS WILL BE ABLE TO:
 Physics LESSON PLAN Subject: Physics Grade: 7 12 Date: 6/21/2012 Concept: The wave nature of light, Estimated Time of Lesson: 100 Minutes PERFORMANCE OBJECTIVE(S): STUDENTS WILL BE ABLE TO: (1) Understand
Physics LESSON PLAN Subject: Physics Grade: 7 12 Date: 6/21/2012 Concept: The wave nature of light, Estimated Time of Lesson: 100 Minutes PERFORMANCE OBJECTIVE(S): STUDENTS WILL BE ABLE TO: (1) Understand
Name Date Time to Complete. NOTE: The multimeter s 10 AMP range, instead of the 300 ma range, should be used for all current measurements.
 Name Date Time to Complete h m Partner Course/ Section / Grade Complex Circuits In this laboratory you will continue your exploration of dc electric circuits with a steady current. The circuits will be
Name Date Time to Complete h m Partner Course/ Section / Grade Complex Circuits In this laboratory you will continue your exploration of dc electric circuits with a steady current. The circuits will be
Activity 4: The Electric-Circuit Interaction
 RECORD SHEET Activity 4: The Electric-Circuit Interaction Name Date Class Key Questions 1. 2. Explore Your Ideas Experiment 1: When does an electric-circuit interaction occur? 1. Draw a picture of the
RECORD SHEET Activity 4: The Electric-Circuit Interaction Name Date Class Key Questions 1. 2. Explore Your Ideas Experiment 1: When does an electric-circuit interaction occur? 1. Draw a picture of the
 Pre-Lab Reading Questions GS106 Lab 3 Answer Key - How We Use Light in Astronomy Life Cycle of a Wave: 1) initialized as oscillating vibrations ("disturbances"), 2) wave travel from origin to destination,
Pre-Lab Reading Questions GS106 Lab 3 Answer Key - How We Use Light in Astronomy Life Cycle of a Wave: 1) initialized as oscillating vibrations ("disturbances"), 2) wave travel from origin to destination,
The Spectroscopy of Stars
 The Spectroscopy of Stars In this activity you will use a hand held spectroscope to investigate a number of known and unknown light sources. A spectroscope is an instrument that helps to observe the spectrum
The Spectroscopy of Stars In this activity you will use a hand held spectroscope to investigate a number of known and unknown light sources. A spectroscope is an instrument that helps to observe the spectrum
Name: Partner(s): 1102 or 3311: Desk # Date: Spectroscopy Part I
 Name: Partner(s): 1102 or 3311: Desk # Date: Spectroscopy Part I Purpose Investigate Kirchhoff s Laws for continuous, emission and absorption spectra Analyze the solar spectrum and identify unknown lines
Name: Partner(s): 1102 or 3311: Desk # Date: Spectroscopy Part I Purpose Investigate Kirchhoff s Laws for continuous, emission and absorption spectra Analyze the solar spectrum and identify unknown lines
2. Discrete means unique, that other states don t overlap it. 3. Electrons in the outer electron shells have greater potential energy.
 30 Light Emission Answers and Solutions for Chapter 30 Reading Check Questions 1. At these high frequencies, ultraviolet light is emitted. 2. Discrete means unique, that other states don t overlap it.
30 Light Emission Answers and Solutions for Chapter 30 Reading Check Questions 1. At these high frequencies, ultraviolet light is emitted. 2. Discrete means unique, that other states don t overlap it.
Activity: Cosmic Colors and Spectroscopy
 1 Activity: Cosmic Colors and Spectroscopy Background: The energy of a photon of light depends on the wavelength (frequency) of the light higher frequency f = higher energy E = shorter wavelength λ) red
1 Activity: Cosmic Colors and Spectroscopy Background: The energy of a photon of light depends on the wavelength (frequency) of the light higher frequency f = higher energy E = shorter wavelength λ) red
APAS Laboratory { PAGE } Spectroscopy SPECTROSCOPY
 SPECTROSCOPY SYNOPSIS: In this lab you will eplore different types of emission spectra, calibrate a spectrometer using the spectrum of a known element, and use your calibration to identify an unknown element.
SPECTROSCOPY SYNOPSIS: In this lab you will eplore different types of emission spectra, calibrate a spectrometer using the spectrum of a known element, and use your calibration to identify an unknown element.
Name Date Time to Complete
 Name Date Time to Complete h m Partner Course/ Section / Grade Complex Circuits In this laboratory you will connect electric lamps together in a variety of circuits. The purpose of these exercises is to
Name Date Time to Complete h m Partner Course/ Section / Grade Complex Circuits In this laboratory you will connect electric lamps together in a variety of circuits. The purpose of these exercises is to
Types of Spectra. How do spectrum lines form? 3/30/09. Electron cloud. Atom. Nucleus
 The electron should be thought of as a distribution or cloud of probability around the nucleus that on average behave like a point particle on a fixed circular path Types of Spectra How do spectrum lines
The electron should be thought of as a distribution or cloud of probability around the nucleus that on average behave like a point particle on a fixed circular path Types of Spectra How do spectrum lines
Atomic Theory C &03
 Atomic Theory Part One: Flame Tests Part Two: Atomic Spectra Part Three: Applications of Spectra (optional) C12-2-02 &03 This activity will focus on the visible portion of the electromagnetic spectrum.
Atomic Theory Part One: Flame Tests Part Two: Atomic Spectra Part Three: Applications of Spectra (optional) C12-2-02 &03 This activity will focus on the visible portion of the electromagnetic spectrum.
Experiment #4 Nature of Light: Telescope and Microscope and Spectroscope
 Experiment #4 Nature of Light: Telescope and Microscope and Spectroscope In this experiment, we are going to learn the basic principles of the telescope and the microscope that make it possible for us
Experiment #4 Nature of Light: Telescope and Microscope and Spectroscope In this experiment, we are going to learn the basic principles of the telescope and the microscope that make it possible for us
Producing and Harnessing Light
 Chemical Dominoes Activity 5 Producing and Harnessing Light GOALS In this activity you will: Describe the relationship between energy, frequency, and wavelength of electromagnetic radiation. Explain how
Chemical Dominoes Activity 5 Producing and Harnessing Light GOALS In this activity you will: Describe the relationship between energy, frequency, and wavelength of electromagnetic radiation. Explain how
Focusing on Light What is light? Is it a particle or a wave? An age-old debate that has persisted among scientists is related to the question, "Is
 Focusing on Light What is light? Is it a particle or a wave? An age-old debate that has persisted among scientists is related to the question, "Is light a wave or a stream of particles?" Very noteworthy
Focusing on Light What is light? Is it a particle or a wave? An age-old debate that has persisted among scientists is related to the question, "Is light a wave or a stream of particles?" Very noteworthy
Light Part II (and review) Lecture 8 2/11/2014
 Light Part II (and review) Lecture 8 2/11/2014 Announcements Celebration of Knowledge (aka Exam 1) will be February 13, will include all information covered including today closed note bring a calculator
Light Part II (and review) Lecture 8 2/11/2014 Announcements Celebration of Knowledge (aka Exam 1) will be February 13, will include all information covered including today closed note bring a calculator
Astronomy-part 3 notes Properties of Stars
 Astronomy-part 3 notes Properties of Stars What are Stars? Hot balls of that shine because nuclear fusion (hydrogen to helium) is happening at their cores. They create their own. Have different which allow
Astronomy-part 3 notes Properties of Stars What are Stars? Hot balls of that shine because nuclear fusion (hydrogen to helium) is happening at their cores. They create their own. Have different which allow
Reading for Meaning and the Electromagnetic Spectrum!
 Earth Science Zimmerman Name: Period: Reading for Meaning and the Electromagnetic Spectrum! HOOK: An astronomer discovers a new galaxy. How can the Doppler Effect be applied to determine if that galaxy
Earth Science Zimmerman Name: Period: Reading for Meaning and the Electromagnetic Spectrum! HOOK: An astronomer discovers a new galaxy. How can the Doppler Effect be applied to determine if that galaxy
Using the spectrometer
 MATERIALS LIST Investigation 13.1 Stars and Spectroscopy 4 Spectrometer (also known as a spectroscope) 4 Colored pencils 4 Incandescent light source ChAPTER 13 The Universe How can we use a spectrometer
MATERIALS LIST Investigation 13.1 Stars and Spectroscopy 4 Spectrometer (also known as a spectroscope) 4 Colored pencils 4 Incandescent light source ChAPTER 13 The Universe How can we use a spectrometer
Astronomy 210 Spring 2017: Quiz 5 Question Packet 1. can: 2. An electron moving between energy levels
 Permitted energy levels Astronomy 210 Spring 2017: Quiz 5 Question Packet 1 1. An electron in energy level 1 2 can: (A) only emit a photon. (B) only absorb a photon. (C) either emit, or absorb a photon.
Permitted energy levels Astronomy 210 Spring 2017: Quiz 5 Question Packet 1 1. An electron in energy level 1 2 can: (A) only emit a photon. (B) only absorb a photon. (C) either emit, or absorb a photon.
Chemistry 212 ATOMIC SPECTROSCOPY
 Chemistry 212 ATOMIC SPECTROSCOPY The emission and absorption of light energy of particular wavelengths by atoms and molecules is a common phenomenon. The emissions/absorptions are characteristic for each
Chemistry 212 ATOMIC SPECTROSCOPY The emission and absorption of light energy of particular wavelengths by atoms and molecules is a common phenomenon. The emissions/absorptions are characteristic for each
Experiment#1 Beer s Law: Absorption Spectroscopy of Cobalt(II)
 : Absorption Spectroscopy of Cobalt(II) OBJECTIVES In successfully completing this lab you will: prepare a stock solution using a volumetric flask; use a UV/Visible spectrometer to measure an absorption
: Absorption Spectroscopy of Cobalt(II) OBJECTIVES In successfully completing this lab you will: prepare a stock solution using a volumetric flask; use a UV/Visible spectrometer to measure an absorption
In class quiz - nature of light. Moonbow with Sailboats (Matt BenDaniel)
 In class quiz - nature of light Moonbow with Sailboats (Matt BenDaniel) Nature of light - review Light travels at very high but finite speed. Light is electromagnetic wave characterized by wavelength (or
In class quiz - nature of light Moonbow with Sailboats (Matt BenDaniel) Nature of light - review Light travels at very high but finite speed. Light is electromagnetic wave characterized by wavelength (or
Energy and the Electron: Atomic View and Argumentation. b. Draw what you think an atom looks like. Label the different parts of the atom.
 Name Energy and the Electron: Atomic View and Argumentation Part I: Warm Up 1. Consider the following questions individually: a. What do you know about the structure of the atom? b. Draw what you think
Name Energy and the Electron: Atomic View and Argumentation Part I: Warm Up 1. Consider the following questions individually: a. What do you know about the structure of the atom? b. Draw what you think
The Nature of Light I: Electromagnetic Waves Spectra Kirchoff s Laws Temperature Blackbody radiation
 The Nature of Light I: Electromagnetic Waves Spectra Kirchoff s Laws Temperature Blackbody radiation Electromagnetic Radiation (How we get most of our information about the cosmos) Examples of electromagnetic
The Nature of Light I: Electromagnetic Waves Spectra Kirchoff s Laws Temperature Blackbody radiation Electromagnetic Radiation (How we get most of our information about the cosmos) Examples of electromagnetic
Gas Tube Spectroscopy
 Gas Tube Spectroscopy - 1 of 12 Gas Tube Spectroscopy Brief Summary This is an exploration station where visitors can view glowing gases through diffraction grating glasses and see the gases' spectral
Gas Tube Spectroscopy - 1 of 12 Gas Tube Spectroscopy Brief Summary This is an exploration station where visitors can view glowing gases through diffraction grating glasses and see the gases' spectral
Light! Lecture 3, Oct. 8! Astronomy 102, Autumn 2009! Oct. 8, 2009 #1. Astronomy 102, Autumn 2009, E. Agol & J. Dalcanton U.W.
 Light! Lecture 3, Oct. 8! Astronomy 102, Autumn 2009! Oct. 8, 2009 #1 Questions of the Day! I. What is light?! II. What are the wave/particle properties of light?! III. How do energy and wavelength vary
Light! Lecture 3, Oct. 8! Astronomy 102, Autumn 2009! Oct. 8, 2009 #1 Questions of the Day! I. What is light?! II. What are the wave/particle properties of light?! III. How do energy and wavelength vary
Introduction to light Light is a form of energy called electromagnetic radiation. A chart of the electromagnetic spectrum is shown below.
 Experiment: Spectroscopy Introduction to light Light is a form of energy called electromagnetic radiation. A chart of the electromagnetic spectrum is shown below. Radiowave Microwave Infrared Visible Ultraviolet
Experiment: Spectroscopy Introduction to light Light is a form of energy called electromagnetic radiation. A chart of the electromagnetic spectrum is shown below. Radiowave Microwave Infrared Visible Ultraviolet
Experiment 9. Emission Spectra. measure the emission spectrum of a source of light using the digital spectrometer.
 Experiment 9 Emission Spectra 9.1 Objectives By the end of this experiment, you will be able to: measure the emission spectrum of a source of light using the digital spectrometer. find the wavelength of
Experiment 9 Emission Spectra 9.1 Objectives By the end of this experiment, you will be able to: measure the emission spectrum of a source of light using the digital spectrometer. find the wavelength of
Lightbulbs. Lecture 18 : Blackbody spectrum Improving lightbulb efficiency
 Lightbulbs Lecture 18 : Blackbody spectrum Improving lightbulb efficiency Reminders: HW 7 due Monday at 10pm Simulations available in G116 Reading quiz on Tuesday, 10.1 EM radiation so far EM radiation
Lightbulbs Lecture 18 : Blackbody spectrum Improving lightbulb efficiency Reminders: HW 7 due Monday at 10pm Simulations available in G116 Reading quiz on Tuesday, 10.1 EM radiation so far EM radiation
The Spectrophotometer and Atomic Spectra of Hydrogen Physics 246
 The Spectrophotometer and Atomic Spectra of Hydrogen Physics 46 Introduction: When heated sufficiently, most elements emit light. With a spectrometer, the emitted light can be broken down into its various
The Spectrophotometer and Atomic Spectra of Hydrogen Physics 46 Introduction: When heated sufficiently, most elements emit light. With a spectrometer, the emitted light can be broken down into its various
10/21/2015. Lightbulbs. Blackbody spectrum. Temperature and total emitted power (brightness) Blackbody spectrum and temperature
 Lightbulbs EM radiation so far EM radiation is a periodic modulation of the electric field: travels as a wave Wavelength (or frequency) determines: - type of EM radiation - if in visible range, wavelength
Lightbulbs EM radiation so far EM radiation is a periodic modulation of the electric field: travels as a wave Wavelength (or frequency) determines: - type of EM radiation - if in visible range, wavelength
Buy-back points tallied and added: 750 points bought-back. Last Withdrawal date: this friday, Oct 31st.
 Announcements HW #3: Available online now. Due in 1 week, Nov 3rd, 11pm. Buy-back points tallied and added: 750 points bought-back. Last Withdrawal date: this friday, Oct 31st. Evening Observing: next
Announcements HW #3: Available online now. Due in 1 week, Nov 3rd, 11pm. Buy-back points tallied and added: 750 points bought-back. Last Withdrawal date: this friday, Oct 31st. Evening Observing: next
Ohm's Law 04/20/2006. Lecture 7 1
 Review: What makes a bulb light up? On the rightness of ulbs Resistance lackbody Radiation Ohm s s Law The critical ingredient is closing a circuit so that current is forced through the bulb filament more
Review: What makes a bulb light up? On the rightness of ulbs Resistance lackbody Radiation Ohm s s Law The critical ingredient is closing a circuit so that current is forced through the bulb filament more
L 18 Thermodynamics [3] Heat flow. Conduction. Convection. Thermal Conductivity. heat conduction. Heat transfer
![L 18 Thermodynamics [3] Heat flow. Conduction. Convection. Thermal Conductivity. heat conduction. Heat transfer L 18 Thermodynamics [3] Heat flow. Conduction. Convection. Thermal Conductivity. heat conduction. Heat transfer](/thumbs/78/76842080.jpg) L 18 Thermodynamics [3] Heat transfer convection conduction emitters of seeing behind closed doors Greenhouse effect Heat Capacity How to boil water Heat flow HEAT the energy that flows from one system
L 18 Thermodynamics [3] Heat transfer convection conduction emitters of seeing behind closed doors Greenhouse effect Heat Capacity How to boil water Heat flow HEAT the energy that flows from one system
ACTIVITY 1. Exploring Light from Gases
 Name: WAVES of matter Class: Visual Quantum Mechanics ACTIVITY 1 Exploring Light from Gases Goal We will view the colors of light which are emitted by different gases. From these patterns of light we gain
Name: WAVES of matter Class: Visual Quantum Mechanics ACTIVITY 1 Exploring Light from Gases Goal We will view the colors of light which are emitted by different gases. From these patterns of light we gain
Astronomy 122. Lunar Eclipse. Make sure to pick up a grating from Emily! You need to give them back after class.
 Astronomy 122 Make sure to pick up a grating from Emily! You need to give them back after class. This Class (Lecture 11): Twinkle, Twinkle, Little Star Next Class: Stellar Evolution: The Main Sequence
Astronomy 122 Make sure to pick up a grating from Emily! You need to give them back after class. This Class (Lecture 11): Twinkle, Twinkle, Little Star Next Class: Stellar Evolution: The Main Sequence
LED s and R e sist or s
 Provided by TryEngineering - L e s s o n F o c u s Expanding on understanding of simple circuits, this lesson explores LEDs and resistors and reviews the differences between parallel and series circuit
Provided by TryEngineering - L e s s o n F o c u s Expanding on understanding of simple circuits, this lesson explores LEDs and resistors and reviews the differences between parallel and series circuit
INSIDE LAB 5: Spectroscopic Identification of Gaseous Elements
 INSIDE LAB 5: Spectroscopic Identification of Gaseous Elements OBJECTIVE: To examine the light emitted by glowing gases in order to identify the elements that compose the gases. DISCUSSION: If a gas is
INSIDE LAB 5: Spectroscopic Identification of Gaseous Elements OBJECTIVE: To examine the light emitted by glowing gases in order to identify the elements that compose the gases. DISCUSSION: If a gas is
AS 102 Lab The Luminosity of the Sun
 AS 102 Lab The Luminosity of the Sun The Problem SOHO Image of the Sun The luminosity of a light source whether it is a star or the Sun or a light bulb is a measure of the actual light output of the source.
AS 102 Lab The Luminosity of the Sun The Problem SOHO Image of the Sun The luminosity of a light source whether it is a star or the Sun or a light bulb is a measure of the actual light output of the source.
DAY LABORATORY EXERCISE: SPECTROSCOPY
 AS101 - Day Laboratory: Spectroscopy Page 1 DAY LABORATORY EXERCISE: SPECTROSCOPY Goals: To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are
AS101 - Day Laboratory: Spectroscopy Page 1 DAY LABORATORY EXERCISE: SPECTROSCOPY Goals: To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are
Homework 9. Quiz Instruc!ons. Question 1. Question 2. 1 pts. 1 pts. ! This is a preview of the draft version of the quiz. Started: Mar 29 at 9:24pm
 Homework 9! This is a preview of the draft version of the quiz Started: Mar 29 at 9:24pm Quiz Instruc!ons Question 1 The Sun's spectrum peaks in the yellow-green region of optical light. The Main Sequence
Homework 9! This is a preview of the draft version of the quiz Started: Mar 29 at 9:24pm Quiz Instruc!ons Question 1 The Sun's spectrum peaks in the yellow-green region of optical light. The Main Sequence
Spectroscopy Lesson Outline
 Spectroscopy Lesson Outline Syllabus References 9.7.3.1 Spectroscopy is a vital tool for astronomers and provides a wealth of information Pre-video Activity: Spectroscopy Worksheet Resources Video: Spectra
Spectroscopy Lesson Outline Syllabus References 9.7.3.1 Spectroscopy is a vital tool for astronomers and provides a wealth of information Pre-video Activity: Spectroscopy Worksheet Resources Video: Spectra
Discussion Review Test #2. Units 12-19: (1) (2) (3) (4) (5) (6)
 Discussion Review Test #2 Units 12-19: (1) (2) (3) (4) (5) (6) (7) (8) (9) Galileo used his observations of the changing phases of Venus to demonstrate that a. the sun moves around the Earth b. the universe
Discussion Review Test #2 Units 12-19: (1) (2) (3) (4) (5) (6) (7) (8) (9) Galileo used his observations of the changing phases of Venus to demonstrate that a. the sun moves around the Earth b. the universe
PHYS 160 Astronomy Test #2 Fall 2017 Version A
 PHYS 160 Astronomy Test #2 Fall 2017 Version A I. True/False (1 point each) Circle the T if the statement is true, or F if the statement is false on your answer sheet. 1. A blackbody emits all of its radiation
PHYS 160 Astronomy Test #2 Fall 2017 Version A I. True/False (1 point each) Circle the T if the statement is true, or F if the statement is false on your answer sheet. 1. A blackbody emits all of its radiation
Atomic Spectra & Electron Energy Levels
 CHM151LL: ATOMIC SPECTRA & ELECTRON ENERGY LEVELS 1 Atomic Spectra & Electron Energy Levels OBJECTIVES: To measure the wavelength of visible light emitted by excited atoms to calculate the energy of that
CHM151LL: ATOMIC SPECTRA & ELECTRON ENERGY LEVELS 1 Atomic Spectra & Electron Energy Levels OBJECTIVES: To measure the wavelength of visible light emitted by excited atoms to calculate the energy of that
Black Body Radiation and Planck's Quantum Hypothesis
 Section 3: Black Body Radiation and Planck's Quantum Hypothesis Definitions Opaque materials: materials in which no light is allowed to pass through; all light is either absorbed or reflected. Radiation:
Section 3: Black Body Radiation and Planck's Quantum Hypothesis Definitions Opaque materials: materials in which no light is allowed to pass through; all light is either absorbed or reflected. Radiation:
Activity #5 How Do Atmospheres Change Over Time? The Greenhouse Effect [Cadette]
![Activity #5 How Do Atmospheres Change Over Time? The Greenhouse Effect [Cadette] Activity #5 How Do Atmospheres Change Over Time? The Greenhouse Effect [Cadette]](/thumbs/83/88151439.jpg) Activity #5 How Do Atmospheres Change Over Time? The Greenhouse Effect [Cadette] Adapted from: Global Warming & The Greenhouse Effect, Great Explorations in Math and Science (GEMS) Lawrence Hall of Science,
Activity #5 How Do Atmospheres Change Over Time? The Greenhouse Effect [Cadette] Adapted from: Global Warming & The Greenhouse Effect, Great Explorations in Math and Science (GEMS) Lawrence Hall of Science,
Electromagnetic radiation simply a stream of photons (a bundle of energy) What are photons???
 Electromagnetic radiation simply a stream of photons (a bundle of energy) What are photons??? no mass travel in a wave like pattern move at the speed of light contain a certain amount (or bundle) of energy
Electromagnetic radiation simply a stream of photons (a bundle of energy) What are photons??? no mass travel in a wave like pattern move at the speed of light contain a certain amount (or bundle) of energy
The Universe. 3. Base your answer to the following question on The diagram below represents the bright-line spectrum for an element.
 A) B) The Universe 1. According to the Big Bang theory, which graph hest represents the relationship between time and the size of the universe from the beginning of the universe to the present? C) D) 2.
A) B) The Universe 1. According to the Big Bang theory, which graph hest represents the relationship between time and the size of the universe from the beginning of the universe to the present? C) D) 2.
λ is a distance, so its units are m, cm, or mm, etc.
 Electromagnetic Radiation (How we get most of our information about the cosmos) Radiation travels as waves. Waves carry information and energy. Properties of a wave Examples of electromagnetic radiation:
Electromagnetic Radiation (How we get most of our information about the cosmos) Radiation travels as waves. Waves carry information and energy. Properties of a wave Examples of electromagnetic radiation:
Instructor Resources
 SPECTROSCOPY Quantitative Analysis with Light Instructor Resources Learning Objectives The objectives of this experiment are to: identify band and line spectra, and relate the physical state of a light-emitting
SPECTROSCOPY Quantitative Analysis with Light Instructor Resources Learning Objectives The objectives of this experiment are to: identify band and line spectra, and relate the physical state of a light-emitting
Atomic Spectra Introduction
 Atomic Spectra Introduction: Light and all other electromagnetic radiation is energy that is emitted in the form of waves. Thus light behaves like a wave, and the energy of light varies with the wavelength
Atomic Spectra Introduction: Light and all other electromagnetic radiation is energy that is emitted in the form of waves. Thus light behaves like a wave, and the energy of light varies with the wavelength
Planetary Science: Investigations 9-10 I-Check Quiz STUDY GUIDE Name HR Date
 1. How are different types of radiation arranged along the electromagnetic spectrum? A. By how fast they travel incorrect answer B. By their sources incorrect answer C. By the amount of energy they carry
1. How are different types of radiation arranged along the electromagnetic spectrum? A. By how fast they travel incorrect answer B. By their sources incorrect answer C. By the amount of energy they carry
chapter 31 Stars and Galaxies
 chapter 31 Stars and Galaxies Day 1:Technology and the Big Bang Studying the Stars A. Telescopes - Electromagnetic radiation emitted by stars and other objects include light, radio, and X-ray Space telescopes
chapter 31 Stars and Galaxies Day 1:Technology and the Big Bang Studying the Stars A. Telescopes - Electromagnetic radiation emitted by stars and other objects include light, radio, and X-ray Space telescopes
Chapter 17, Electromagnetic Waves Physical Science, McDougal-Littell, 2008
 SECTION 1 (PP. 553-558): ELECTROMAGNETIC WAVES HAVE UNIQUE TRAITS. Georgia Standards: S8P4a Identify the characteristics of electromagnetic and mechanical waves; S8P4d Describe how the behavior of waves
SECTION 1 (PP. 553-558): ELECTROMAGNETIC WAVES HAVE UNIQUE TRAITS. Georgia Standards: S8P4a Identify the characteristics of electromagnetic and mechanical waves; S8P4d Describe how the behavior of waves
SNC2D PHYSICS 4/27/2013. LIGHT & GEOMETRIC OPTICS L What Is Light? (P ) What Is Light? What Is Light?
 SNC2D PHYSICS LIGHT & GEOMETRIC OPTICS L What Is Light? (P.380-391) What Is Light? For centuries, scientists have tried to understand the nature of light and its properties. Some of these properties are
SNC2D PHYSICS LIGHT & GEOMETRIC OPTICS L What Is Light? (P.380-391) What Is Light? For centuries, scientists have tried to understand the nature of light and its properties. Some of these properties are
Physics Unit Review. 3. The electric field between a positive point charge and a negative point charge is represented by
 Physics Unit Review 1. What is the gravitational field strength on the surface of a planetoid with a mass of 7.4 x 10 22 kg and a radius of 1.7 x 10 6 m? a. 0.69 N/kg b. 1. 7 N/kg c. 9.8 N/kg d. 2.9 x
Physics Unit Review 1. What is the gravitational field strength on the surface of a planetoid with a mass of 7.4 x 10 22 kg and a radius of 1.7 x 10 6 m? a. 0.69 N/kg b. 1. 7 N/kg c. 9.8 N/kg d. 2.9 x
Susan Cartwright Our Evolving Universe 1
 Atoms and Starlight Why do the stars shine? planets shine by reflected sunlight but what generates the Sun s light? What does starlight tell us about the stars? their temperature their chemical composition
Atoms and Starlight Why do the stars shine? planets shine by reflected sunlight but what generates the Sun s light? What does starlight tell us about the stars? their temperature their chemical composition
Laboratory Atomic Emission Spectrum
 Laboratory Atomic Emission Spectrum Pre-Lab Questions: Answer the following questions in complete sentences by reading through the Overview and Background sections below. 1. What is the purpose of the
Laboratory Atomic Emission Spectrum Pre-Lab Questions: Answer the following questions in complete sentences by reading through the Overview and Background sections below. 1. What is the purpose of the
PLANCK S CONSTANT IN THE LIGHT OF AN INCANDESCENT LAMP
 PLANCK S CONSTANT IN THE LIGHT OF AN INCANDESCENT LAMP In 1900 Planck introduced the hypothesis that light is emitted by matter in the form of quanta of energy hν. In 1905 Einstein extended this idea proposing
PLANCK S CONSTANT IN THE LIGHT OF AN INCANDESCENT LAMP In 1900 Planck introduced the hypothesis that light is emitted by matter in the form of quanta of energy hν. In 1905 Einstein extended this idea proposing
Chapter 5 Light and Matter
 Chapter 5 Light and Matter Stars and galaxies are too far for us to send a spacecraft or to visit (in our lifetimes). All we can receive from them is light But there is much we can learn (composition,
Chapter 5 Light and Matter Stars and galaxies are too far for us to send a spacecraft or to visit (in our lifetimes). All we can receive from them is light But there is much we can learn (composition,
hf = E 1 - E 2 hc = E 1 - E 2 λ FXA 2008 Candidates should be able to : EMISSION LINE SPECTRA
 1 Candidates should be able to : EMISSION LINE SPECTRA Explain how spectral lines are evidence for the existence of discrete energy levels in isolated atoms (i.e. in a gas discharge lamp). Describe the
1 Candidates should be able to : EMISSION LINE SPECTRA Explain how spectral lines are evidence for the existence of discrete energy levels in isolated atoms (i.e. in a gas discharge lamp). Describe the
Laboratory Exercise. Quantum Mechanics
 Laboratory Exercise Quantum Mechanics Exercise 1 Atomic Spectrum of Hydrogen INTRODUCTION You have no doubt been exposed many times to the Bohr model of the atom. You may have even learned of the connection
Laboratory Exercise Quantum Mechanics Exercise 1 Atomic Spectrum of Hydrogen INTRODUCTION You have no doubt been exposed many times to the Bohr model of the atom. You may have even learned of the connection
Astronomy 101 Lab: Spectra
 Name: Astronomy 101 Lab: Spectra You will access your textbook in this lab. Pre-Lab Assignment: In class, we've talked about different kinds of spectra and what kind of object produces each kind of spectrum.
Name: Astronomy 101 Lab: Spectra You will access your textbook in this lab. Pre-Lab Assignment: In class, we've talked about different kinds of spectra and what kind of object produces each kind of spectrum.
Where do they come from?
 Exploring Meteorite Mysteries Lesson 5 Looking at Asteroids Objectives Students will: gather data by observing, measuring, and manipulating objects. record observations, analyze data and draw analogies.
Exploring Meteorite Mysteries Lesson 5 Looking at Asteroids Objectives Students will: gather data by observing, measuring, and manipulating objects. record observations, analyze data and draw analogies.
Light: Transverse WAVE
 Light Longitudinal WAVES Light: Transverse WAVE Light: Particle or wave Photon The Wave Nature of Light 1. Unlike other branches of science, astronomers cannot touch or do field work on their samples.
Light Longitudinal WAVES Light: Transverse WAVE Light: Particle or wave Photon The Wave Nature of Light 1. Unlike other branches of science, astronomers cannot touch or do field work on their samples.
CHAPTER 28 STARS AND GALAXIES
 CHAPTER 28 STARS AND GALAXIES 28.1 A CLOSER LOOK AT LIGHT Light is a form of electromagnetic radiation, which is energy that travels in waves. Waves of energy travel at 300,000 km/sec (speed of light Ex:
CHAPTER 28 STARS AND GALAXIES 28.1 A CLOSER LOOK AT LIGHT Light is a form of electromagnetic radiation, which is energy that travels in waves. Waves of energy travel at 300,000 km/sec (speed of light Ex:
Light is an important form of energy for all of us
 What is Light? Light is an important form of energy for all of us it allows us to see plants rely on light for photosynthesis many chemical reactions produce light life on Earth would not exist without
What is Light? Light is an important form of energy for all of us it allows us to see plants rely on light for photosynthesis many chemical reactions produce light life on Earth would not exist without
Astronomical Observations: Distance & Light 7/2/09. Astronomy 101
 Astronomical Observations: Distance & Light 7/2/09 Astronomy 101 Astronomy Picture of the Day Astronomy 101 Something Cool: Lasers on the Moon Astronomy 101 Outline for Today Astronomy Picture of the Day
Astronomical Observations: Distance & Light 7/2/09 Astronomy 101 Astronomy Picture of the Day Astronomy 101 Something Cool: Lasers on the Moon Astronomy 101 Outline for Today Astronomy Picture of the Day
Experiment #9. Atomic Emission Spectroscopy
 Introduction Experiment #9. Atomic Emission Spectroscopy Spectroscopy is the study of the interaction of light with matter. This interaction can be in the form of the absorption or the emission of electromagnetic
Introduction Experiment #9. Atomic Emission Spectroscopy Spectroscopy is the study of the interaction of light with matter. This interaction can be in the form of the absorption or the emission of electromagnetic
In this lab you will measure and quantify emission spectra from several different visible light sources.
 Lab 2 Spectroscopy In this lab you will measure and quantify emission spectra from several different visible light sources. 2.1 Spectral Lines In physics, we typically use the word spectrum to refer to
Lab 2 Spectroscopy In this lab you will measure and quantify emission spectra from several different visible light sources. 2.1 Spectral Lines In physics, we typically use the word spectrum to refer to
ASTRONOMY 161. Introduction to Solar System Astronomy. Class 9
 ASTRONOMY 161 Introduction to Solar System Astronomy Class 9 Light Monday, January 29 Look, but don t touch. - Astronomers Motto Light: Key Concepts (1) Visible light is just one form of electromagnetic
ASTRONOMY 161 Introduction to Solar System Astronomy Class 9 Light Monday, January 29 Look, but don t touch. - Astronomers Motto Light: Key Concepts (1) Visible light is just one form of electromagnetic
Spectroscopy. Materials Diffraction grating Grating tube Spectrometer Incandescent light source
 Name: Date: Spectroscopy Hazards: The power supply used to run the lights is HIGH VOLTAGE. You should not need to change any tubes, but if you do please call the instructor over for assistance, and turn
Name: Date: Spectroscopy Hazards: The power supply used to run the lights is HIGH VOLTAGE. You should not need to change any tubes, but if you do please call the instructor over for assistance, and turn
Lights. And God said, "Let there be light"; and there was light. And God saw that the light was good; (Bible: Genesis I)
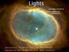 Lights Astronomy is based on observing lights from celestial bodies. And God said, "Let there be light"; and there was light. And God saw that the light was good; (Bible: Genesis I) Cat's Eye planetary
Lights Astronomy is based on observing lights from celestial bodies. And God said, "Let there be light"; and there was light. And God saw that the light was good; (Bible: Genesis I) Cat's Eye planetary
STUDENT NAME. Science Grade 4. Read each question and choose the best answer. Be sure to mark all of your answers. Switch Lightbulb Battery
 FORMATIVE MINI ASSESSMENTS Third Grading Period 2009-10 February 1-5 STUDENT NAME DATE Science Grade 4 Read each question and choose the best answer. Be sure to mark all of your answers. + Switch Lightbulb
FORMATIVE MINI ASSESSMENTS Third Grading Period 2009-10 February 1-5 STUDENT NAME DATE Science Grade 4 Read each question and choose the best answer. Be sure to mark all of your answers. + Switch Lightbulb
Chemical Bonds. MATERIALS 24-well microplate calcium chloride candle citric acid conductivity tester ethanol gloves iron ring lab apron
 Microscale Chemical Bonds Chemical compounds are combinations of atoms held together by chemical bonds. These chemical bonds are of two basic types ionic and covalent. Ionic bonds result when one or more
Microscale Chemical Bonds Chemical compounds are combinations of atoms held together by chemical bonds. These chemical bonds are of two basic types ionic and covalent. Ionic bonds result when one or more
Spectroscopy of Various Light Sources: The Interactions between Light and Matter ASTR 170B1, Spring 2017, Lab #2. 1 Introduction.
 Spectroscopy of Various Light Sources: The Interactions between Light and Matter ASTR 170B1, Spring 2017, Lab #2 DUE IN CLASS ON Thursday Sept 28! You CAN work in a group of 2, but you need only turn in
Spectroscopy of Various Light Sources: The Interactions between Light and Matter ASTR 170B1, Spring 2017, Lab #2 DUE IN CLASS ON Thursday Sept 28! You CAN work in a group of 2, but you need only turn in
Light and Atoms. ASTR 1120 General Astronomy: Stars & Galaxies. ASTR 1120 General Astronomy: Stars & Galaxies !ATH REVIEW: #AST CLASS: "OMEWORK #1
 ASTR 1120 General Astronomy: Stars & Galaxies!ATH REVIEW: Tonight, 5-6pm, in RAMY N1B23 "OMEWORK #1 -Due THU, Sept. 10, by 5pm, on Mastering Astronomy CLASS RECORDED STARTED - INFO WILL BE POSTED on CULEARN
ASTR 1120 General Astronomy: Stars & Galaxies!ATH REVIEW: Tonight, 5-6pm, in RAMY N1B23 "OMEWORK #1 -Due THU, Sept. 10, by 5pm, on Mastering Astronomy CLASS RECORDED STARTED - INFO WILL BE POSTED on CULEARN
ESAC VOSPEC SCIENCE TUTORIAL
 SCIENCE ARCHIVES AND VO TEAM ESAC VOSPEC SCIENCE TUTORIAL COMPARING SPECTRA OF THE SUN AND SIMILAR STARS THEORY SECTION Tutorial created by Luis Sánchez, ESAC SOHO Archive scientist, adapted from the Tracking
SCIENCE ARCHIVES AND VO TEAM ESAC VOSPEC SCIENCE TUTORIAL COMPARING SPECTRA OF THE SUN AND SIMILAR STARS THEORY SECTION Tutorial created by Luis Sánchez, ESAC SOHO Archive scientist, adapted from the Tracking
Physics 1C OPTICAL SPECTROSCOPY Rev. 2-AH. Introduction
 Introduction In this lab you will use a diffraction grating to split up light into its various colors (like a rainbow). You will assemble a spectrometer, incorporating the diffraction grating. A spectrometer
Introduction In this lab you will use a diffraction grating to split up light into its various colors (like a rainbow). You will assemble a spectrometer, incorporating the diffraction grating. A spectrometer
