Image Classification of Temperature
|
|
|
- Osborn Quinn
- 5 years ago
- Views:
Transcription
1 Image Classification of Temperature Distribution Using Fourier Series Strategy Image Classification of Temperature Distribution Using Fourier Series Strategy M.A.Salim 1, A. R. M. Rosdzimin 2, K.Osman 3, A.Noordin 4, M.A.Mohd Rosli 5 1, 5 Faculty of Mechanical Engineering, Universiti Teknikal Malaysia Melaka, Karung Berkunci 1752, Pejabat Pos Durian Tunggal, 7619 Durian Tunggal, Melaka, Malaysia. 2 Department of Mechanical, Faculty of Engineering, Universiti Pertahanan Nasional Malaysia, Kem Sungai Besi, 57 Kuala Lumpur, Malaysia. 3 Faculty of Mechanical Engineering, Universiti Teknologi Malaysia, 8131 Skudai, Johor Bahru, Johor, Malaysia. 4 Faculty of Electrical Engineering, Universiti Teknikal Malaysia Melaka, Karung Berkunci 1752, Pejabat Pos Durian Tunggal, 7619 Durian Tunggal, Melaka, Malaysia. 1 azli@utem.edu.my, 2 rosdzimin@upnm.edu.my, 3 kahar@fkm.utm.my, 4 aminurrashid@ utem.edu.my, 5 afzanizam@utem.edu.my ABSTRACT This paper presents the analysis of temperature distribution by using Fourier series strategy. Using this strategy, two analyses have been made where an even and odd number is applied in the series. In even number, the trivial solution occur where there are no solution been made, but in odd number the solution is succeed. By using the equation made by odd number, the equation has been analysis using MatLab-Programming. In this analysis, the contour mapping of temperature distribution is shown clearly. Based on the mapping, it can be concluded that the temperature distribution happens because of the adiabatic phenomenon of the material properties itself. KEYWORDS: Fourier series, Isotherms, Adiabatic, Temperature distribution. 1. INTRODUCTION It is not easy to give an exact definition of temperature because 11
2 Journal of Mechanical Engineering and Technology majority of human around the world classify temperature to only familiar with hotness or coldness. In physiological ambiance, the level of temperature can be measured as a cold, freezing cold, warm, hot and red hot. For the basic example, metal will feel much colder than the wood when both materials are at the same temperature and same place. According to this example, several materials properties can be changed regarding the changes of the temperature around the material itself. The material properties can be changed with temperature in a repeatable and predictable way according the basis of accurate temperature measurement. In a common example, a cup of hot tea present on the table ultimately cools off and cold drink sooner or later warms up. In this phenomenon, a body of hot tea and cold drink are brought into contact with another body with different temperature and heat transfer phenomenon is happen where the hot temperature transfer it temperature to the low temperature areas. This phenomenon will continue until both area reach with same temperature. When both areas are in a same temperature, these two areas are said in a thermal equilibrium condition. Zeroth Law of Thermodynamics explains that if two bodies in thermal equilibrium with a third body, they are also in thermal equilibrium for each others. This law had been formulated by R.H.Fowler in In this study, the authors have studied the mediums properties according to the heat transfer effect in several materials. Therefore, the knowledge on medium s value of temperature at all point is necessary. Heat transfer analysis basically plays a central role in the design of chemical processes and in the development of process system. In order to make accurate analysis to the heat transfer problem, parameters such as the roll speed, thermal conductivity, rate of cold air, thickness and temperature surface is needed. Heat transfer problem can occur by three mechanisms and there are conduction, convection and radiation. Conduction mechanism is a collision of molecules causes the thermal energy to be transferred from one molecule to other one molecule. In this heat transfer process, the very energetic molecules will lose their energy while the lower energy molecules will get the more energy. In convection mechanism, it only occurs when the energy in macroscopic flow in fluid was associated with a parcel. Then, the fluid was converted to another region of space. In this case, it also can be called as an unsteady state behaviour. The radiation mechanism happens when the molecular vibrate and give an electromagnet radiation which certain amount to the other molecular. The radiation behaviour transmits the energy throughout the space 12
3 Image Classification of Temperature Distribution Using Fourier Series Strategy and vacuum containing. These three mechanisms have a potential to generate the instantaneous values of temperature at all points of the medium of interest and also called as a temperature distribution or field. The unsteady temperature distribution appears when medium s temperature not only varies from point to point, but also depends on time. In time domain, the temperature at a various points in a medium can be changed and then the internal energy of the molecular also changed. A steady state temperature distribution occurs when a temperature at a given point never varies with time and this type is called as a space coordinates only. The temperature distribution by governing equations also called a three dimensional. This equation hree of dimensional. temperature is describes as a function of three space coordinates,.. Therefore, if the points of a medium with equal temperatures are connected, the resulting surfaces are called isothermal surfaces. This intersection of isothermal surfaces with a plane yields a family of isotherms on the place surface. Important to note that the two isothermal surfaces never cut each other since there are no part of the medium can have two different temperatures at the same time, respectively (Kreysig, 26), (Howard et al., 23). Fourier series of all dimension is a general type of summation process under which the convergence or non-convergence of the corresponding partial sums at a given point depend only on the behaviour of the function at given point, and that continuity of the function at the point is sufficient for convergence (Bochner, 1935). Fourier series has been used for solving many heat transfer problems. Maria and Power (2) was developed an efficient BEM scheme for the numerical solution of two-dimensional heat problems. The double Fourier series was rewritten using Green function that obtained by the images method. The double Fourier series is use in the domain integral of the integral representation formula to transform such integral into equivalent surface integrals. Maksimovich and Tsybul skii (24) determined nonstationary nonaxisymmetric temperature fields in bodies of revolution appearing on heating by internal heat sources through and due to convective heat exchange with an external medium. The solution of the problem is represented in the form of a Fourier series in an angular coordinate with coefficients being determined by a method of boundary elements. 13
4 Journal of Mechanical Engineering and Technology 2. FOURIER SERIES STRATEGY Fourier series is a one method to analyze the periodic phenomenon and they occur quite frequently in engineering and elsewhere such as in rotating of machines, alternating electric currents or the motions of planets. Fourier series is also called as a Trigonometric series and it represent in periodic functions. The function on trigonometric can be etric series and i, and has a period of if defined from for all values of x. According to the definition of the periodic, most practical situations such as a function can be expressed as a complex Fourier series like (Kreysig, 26), (Black, 28): The number is called as complex coefficients periodic functions. It can be compute by the integration as: This Fourier series also can be written as sine and cosine function. The new equation of this Fourier series represents as a: By the denoting the equation: Yields: This series is called a Fourier sine-cosine expansion. For this case,, for all values of j, which it is, implies that must be real and then: 14
5 Image Classification of Temperature Distribution Using Fourier Series Strategy Suppose that, Fourier series expansion for a more general function of and having a period of p instead of. Then, the new function can be introduced by: This has a period of. This function of can be rewrite to a new equation and representing as: Where as: Therefore the equation can be express into: Sometimes, there occur functions that can be expanding as a series of a sine term only or as a series of cosine terms only. In a case, if the function is originally defined for Therefore, it can be making as a This is a series where it involves only a sine terms. Similarly that, if 15
6 Journal of Mechanical Engineering and Technology This term consists only cosine term and finally, the equation can be represented as: Whereas, this equation can be modifying as: Fourier series is a function of approximation using a finite number of terms and then, the resulting function may oscillate in regions where the actual function is discontinues or in other hand it is called as changes rapidly. This undesirable behaviour can be reduced by using a smoothing procedure described by Lanezos where it use this Fourier series and close it related to a function of and it is defined by a local averaging process according to Equation 16 below. The averaging interval of should be a small fraction of the period of. Then, rewrite the equation of with. The functions of and are identical as. Hence, also match exactly at any point of x, where varies linearly between Importantly, this agreed closely with for a small value of but at the same time in a Fourier series which converges more rapidly than the series for. Last but not least, it can be defined as: 16
7 Image Classification of Temperature Distribution Using Fourier Series Strategy Where and for. Then, evidently this Fourier series of the coefficients of are easily obtained from the function of. When this series for converges slowly, using the same number of the terms in the series for often gives an approximation preferable to that provided by the series of. Finally this process is called as smoothing. 3. FOURIER SERIES ANALYSIS OF THIN SQUARE METAL PLATE y = π T x = y = FIGURE 1 Plate x = π A thin square metal plate has three sides that are held at temperature. The other side (the top) is fixed at a temperature above. Using the boundary conditions: According to the Laplace Equation, it can be derived as a: 17
8 Journal of Mechanical Engineering and Technology Let: Since, Where, From Equation 2, it can be rewritten as: Re-arrange the equation and substitute Equation 21 and 22 into. The new equation can be written as: From Equation 23, it needs to be divided with FG. Therefore, the equation can be expressed as: Based on Equation 24, equating the equation with where it is a constant value. Re-written of Equation 24 is: 18
9 Image Classification of Temperature Distribution Using Fourier Series Strategy Hence, And So, The left and the right side of the boundary conditions can be implied as: It can give as a: Hence that, The Ordinary Differential Equations (ODEs) for G with represent as a: can 19
10 Journal of Mechanical Engineering and Technology Then, From Equation 28, differentiate respect to y by putting boundary condition Obtained A = B. Therefore from Equation 28: From Equation 29, using Euler s formula in trigonometry, the equation can be expressed as: Multiply both F and G, represent the equation as a: The boundary condition of then is applied at the top side of the thin plate which subsequently the Equation 31 can be rewritten again as: The final equation can represent as a: 2
11 Image Classification of Temperature Distribution Using Fourier Series Strategy Then, If n is even number, n = 2, 4, 6, and 8 Equation 36 will become as: If n is odd number, n = 1, 3, 5, 7 Therefore, From Equation 37 and above, the general equation of the Fourier series of this problem can be representing as a: When n = 1 21
12 Journal of Mechanical Engineering and Technology 4. RESULT AND DISCUSSION Un(x,y)=-2T/pi * sum [ (cos nx-1)sin nx sinh ny / n sinh npi ] y x FIGURE 2 Temperature contour of thin square metal plate y x FIGURE 3 Isotherms line of thin square metal plate y x FIGURE 4 Two dimensional of thin square metal plate 22
13 Image Classification of Temperature Distribution Using Fourier Series Strategy.9 u(x,y) y 1 1 x FIGURE 5 Three dimensional of thin square metal plate Figure 2 shows the temperature contour of thin square metal plate. It can be seen that heat is transfer from high temperature to low temperature. The direction of heat transfer is due to temperature setting at the boundary condition. Left and right edges are set as cold (T=) and the bottom edge set as an adiabatic boundary condition and the top edge is set at temperature T. Isotherm lines was plotted in Figure 3, it shows isotherm move out from the bottom edge. This is due the adiabatic boundary condition. At the left and right edges of thin plate, the isotherm lines keep parallel with these edges. This occurs due to left and right edges of thin plate was set as a cold (T=). Figure 4 and Figure 5 show the two and three dimensional of temperature distribution of thin square metal plate. Figure 5 clearly shows the steady state heat transfer conditions. At steady state condition, heat transfer from the top edge of thin square metal plate is equal to heat transfer out from bottom edge of thin square metal plate. 5. CONCLUSION Fourier series analysis is also known as a trigonometric series analysis. This series has a potential to solve the analytical solution on temperature distribution of plate. Temperature distribution can be distributed independently according to the adiabatic phenomenon. This phenomenon is based on the time domain. 23
14 Journal of Mechanical Engineering and Technology 6. ACKNOWLEDGEMENT The author would like to express appreciation and gratitude to Faculty of Mechanical Engineering, Universiti Teknikal Malaysia Melaka and Universiti Teknologi Malaysia for the technical supports. 7. REFERENCES Erwin Kreysig. 26. Advanced Engineering Mathematics. 9th Edition. Wiley International Edition. Howard B. Wilson, Louis H. Turcotte and David Halpen. 23. Advanced Mathematics and Mechanics and Application Using MATLAB. 3rd Edition. Chapman and Hall. Maksimovich, V. N and Tsybul skii, O. A. 24. Application of fourierseries methods and integral equations for solving nonstationary nonaxisymmetric heat conduction problems for bodies of revolution Journal of Engineering Physics and Thermophysics Volume 68, Number 6 / November, 1995 pp Maria T. Ibanez and H. Power, 2. An efficient direct BEM numerical scheme for heat transfer problems using Fourier series, International Journal of Numerical Methods for Heat & Fluid Flow Vol. 1/7 ISSN pp S. Bochner, Summation of Derived Fourier Series. An application to Fourier expansions on compact Lie groups. Annals of Mathematics. Vol 37, No. 2 Taddeus H. Black. 28. Derivation of Applied Mathematics. Revised. Debian Project. 24
SIMULATION OF MIXED CONVECTIVE HEAT TRANSFER USING LATTICE BOLTZMANN METHOD
 International Journal of Automotive and Mechanical Engineering (IJAME) ISSN: 2229-8649 (Print); ISSN: 2180-1606 (Online); Volume 2, pp. 130-143, July-December 2010 Universiti Malaysia Pahang DOI: http://dx.doi.org/10.15282/ijame.2.2010.3.0011
International Journal of Automotive and Mechanical Engineering (IJAME) ISSN: 2229-8649 (Print); ISSN: 2180-1606 (Online); Volume 2, pp. 130-143, July-December 2010 Universiti Malaysia Pahang DOI: http://dx.doi.org/10.15282/ijame.2.2010.3.0011
TEMPERATURE. 8. Temperature and Heat 1
 TEMPERATURE Heat is the energy that is transferred between objects because of a temperature difference Terms such as transfer of heat or heat flow from object A to object B simply means that the total
TEMPERATURE Heat is the energy that is transferred between objects because of a temperature difference Terms such as transfer of heat or heat flow from object A to object B simply means that the total
First Law of Thermodynamics Second Law of Thermodynamics Mechanical Equivalent of Heat Zeroth Law of Thermodynamics Thermal Expansion of Solids
 Slide 1 / 66 1 What is the name of the following statement: "When two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium with each other"? A B C D E First Law
Slide 1 / 66 1 What is the name of the following statement: "When two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium with each other"? A B C D E First Law
Chapters 16 Temperature and Heat
 Chapters 16 Temperature and Heat 1 Overview of Chapter 16 Temperature and the Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion Heat and Mechanical Work Specific Heat Conduction, Convection,
Chapters 16 Temperature and Heat 1 Overview of Chapter 16 Temperature and the Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion Heat and Mechanical Work Specific Heat Conduction, Convection,
Chapter 14 Temperature and Heat
 Nicholas J. Giordano www.cengage.com/physics/giordano Chapter 14 Temperature and Heat Thermodynamics Starting a different area of physics called thermodynamics Thermodynamics focuses on energy rather than
Nicholas J. Giordano www.cengage.com/physics/giordano Chapter 14 Temperature and Heat Thermodynamics Starting a different area of physics called thermodynamics Thermodynamics focuses on energy rather than
18.13 Review & Summary
 5/2/10 10:04 PM Print this page 18.13 Review & Summary Temperature; Thermometers Temperature is an SI base quantity related to our sense of hot and cold. It is measured with a thermometer, which contains
5/2/10 10:04 PM Print this page 18.13 Review & Summary Temperature; Thermometers Temperature is an SI base quantity related to our sense of hot and cold. It is measured with a thermometer, which contains
DEPARTMENT OF MATHEMATICS FACULTY OF ENGINERING AND TECHNOLOGY SRM UNIVERSITY MA0211- MATHEMATICS III SEMESTER III ACADEMIC YEAR:
 DEPARTMENT OF MATHEMATICS FACULTY OF ENGINERING AND TECHNOLOGY SRM UNIVERSITY MA0211- MATHEMATICS III SEMESTER III ACADEMIC YEAR: 2011 2012 LECTURE SCHEME / PLAN The objective is to equip the students
DEPARTMENT OF MATHEMATICS FACULTY OF ENGINERING AND TECHNOLOGY SRM UNIVERSITY MA0211- MATHEMATICS III SEMESTER III ACADEMIC YEAR: 2011 2012 LECTURE SCHEME / PLAN The objective is to equip the students
Conduction is the transfer of heat by the direct contact of particles of matter.
 Matter and Energy Chapter 9 energy flows from a material at a higher temperature to a material at a lower temperature. This process is called heat transfer. How is heat transferred from material to material,
Matter and Energy Chapter 9 energy flows from a material at a higher temperature to a material at a lower temperature. This process is called heat transfer. How is heat transferred from material to material,
Chapter 18 Temperature, Heat, and the First Law of Thermodynamics. Thermodynamics and Statistical Physics
 Chapter 18 Temperature, Heat, and the First Law of Thermodynamics Thermodynamics and Statistical Physics Key contents: Temperature scales Thermal expansion Temperature and heat, specific heat Heat and
Chapter 18 Temperature, Heat, and the First Law of Thermodynamics Thermodynamics and Statistical Physics Key contents: Temperature scales Thermal expansion Temperature and heat, specific heat Heat and
Thermodynamics - Heat Transfer June 04, 2013
 THERMODYNAMICS - Heat and Heat Transfer: Heat (Q) is a form of Energy that is transferred between an object and another object or its surrounding environment due to a difference in Temperature. Heat is
THERMODYNAMICS - Heat and Heat Transfer: Heat (Q) is a form of Energy that is transferred between an object and another object or its surrounding environment due to a difference in Temperature. Heat is
What Is Air Temperature?
 2.2 Read What Is Air Temperature? In Learning Set 1, you used a thermometer to measure air temperature. But what exactly was the thermometer measuring? What is different about cold air and warm air that
2.2 Read What Is Air Temperature? In Learning Set 1, you used a thermometer to measure air temperature. But what exactly was the thermometer measuring? What is different about cold air and warm air that
3 rd class Mech. Eng. Dept. hamdiahmed.weebly.com Fourier Series
 Definition 1 Fourier Series A function f is said to be piecewise continuous on [a, b] if there exists finitely many points a = x 1 < x 2
Definition 1 Fourier Series A function f is said to be piecewise continuous on [a, b] if there exists finitely many points a = x 1 < x 2
HEAT TRANSFER 1 INTRODUCTION AND BASIC CONCEPTS 5 2 CONDUCTION
 HEAT TRANSFER 1 INTRODUCTION AND BASIC CONCEPTS 5 2 CONDUCTION 11 Fourier s Law of Heat Conduction, General Conduction Equation Based on Cartesian Coordinates, Heat Transfer Through a Wall, Composite Wall
HEAT TRANSFER 1 INTRODUCTION AND BASIC CONCEPTS 5 2 CONDUCTION 11 Fourier s Law of Heat Conduction, General Conduction Equation Based on Cartesian Coordinates, Heat Transfer Through a Wall, Composite Wall
VISUAL PHYSICS ONLINE THERMODYNAMICS THERMAL ENERGY
 VISUAL PHYSICS ONLINE THERMODYNAMICS THERMAL ENERGY INTERNAL ENERGY A thermodynamic System is composed of molecules in a solid state and/or a liquid and/or a gas state. The molecules always have some random
VISUAL PHYSICS ONLINE THERMODYNAMICS THERMAL ENERGY INTERNAL ENERGY A thermodynamic System is composed of molecules in a solid state and/or a liquid and/or a gas state. The molecules always have some random
Physics 121, April 24. Heat and the First Law of Thermodynamics. Department of Physics and Astronomy, University of Rochester
 Physics 121, April 24. Heat and the First Law of Thermodynamics. Physics 121. April 24, 2008. Course Information Topics to be discussed today: Heat First law of thermodynamics Second law of thermodynamics
Physics 121, April 24. Heat and the First Law of Thermodynamics. Physics 121. April 24, 2008. Course Information Topics to be discussed today: Heat First law of thermodynamics Second law of thermodynamics
Physics 121, April 24. Heat and the First Law of Thermodynamics. Physics 121. April 24, Physics 121. April 24, Course Information
 Physics 121, April 24. Heat and the First Law of Thermodynamics. Physics 121. April 24, 2008. Course Information Topics to be discussed today: Heat First law of thermodynamics Second law of thermodynamics
Physics 121, April 24. Heat and the First Law of Thermodynamics. Physics 121. April 24, 2008. Course Information Topics to be discussed today: Heat First law of thermodynamics Second law of thermodynamics
ANALYSIS OF TRANSIENT HEAT CONDUCTION IN DIFFERENT GEOMETRIES BY POLYNOMIAL APPROXIMATION METHOD
 Int. J. Mech. Eng. & Rob. Res. Devanshu Prasad, Research Paper ISSN 78 9 www.ijmerr.com Vol., No., April IJMERR. All Rights Reserved ANALYSIS OF TRANSIENT HEAT CONDUCTION IN DIFFERENT GEOMETRIES Y POLYNOMIAL
Int. J. Mech. Eng. & Rob. Res. Devanshu Prasad, Research Paper ISSN 78 9 www.ijmerr.com Vol., No., April IJMERR. All Rights Reserved ANALYSIS OF TRANSIENT HEAT CONDUCTION IN DIFFERENT GEOMETRIES Y POLYNOMIAL
Unit 11: Temperature and heat
 Unit 11: Temperature and heat 1. Thermal energy 2. Temperature 3. Heat and thermal equlibrium 4. Effects of heat 5. Transference of heat 6. Conductors and insulators Think and answer a. Is it the same
Unit 11: Temperature and heat 1. Thermal energy 2. Temperature 3. Heat and thermal equlibrium 4. Effects of heat 5. Transference of heat 6. Conductors and insulators Think and answer a. Is it the same
Chapter 18. Temperature, Heat, and the First Law of Thermodynamics Temperature
 Chapter 18 Temperature, Heat, and the First Law of Thermodynamics 18.2 Temperature 18.3: The Zeroth aw of Thermodynamics If bodies A and B are each in thermal equilibrium with a third body T, then A and
Chapter 18 Temperature, Heat, and the First Law of Thermodynamics 18.2 Temperature 18.3: The Zeroth aw of Thermodynamics If bodies A and B are each in thermal equilibrium with a third body T, then A and
Unit B-4: List of Subjects
 ES312 Energy Transfer Fundamentals Unit B: First Law of Thermodynamics ROAD MAP... B-1: The Concept of Energy B-2: Work Interactions B-3: First Law of Thermodynamics B-4: Heat Transfer Fundamentals Unit
ES312 Energy Transfer Fundamentals Unit B: First Law of Thermodynamics ROAD MAP... B-1: The Concept of Energy B-2: Work Interactions B-3: First Law of Thermodynamics B-4: Heat Transfer Fundamentals Unit
Temperature and Its Measurement
 Temperature and Its Measurement When the physical properties are no longer changing, the objects are said to be in thermal equilibrium. Two or more objects in thermal equilibrium have the same temperature.
Temperature and Its Measurement When the physical properties are no longer changing, the objects are said to be in thermal equilibrium. Two or more objects in thermal equilibrium have the same temperature.
Chapter 12 Thermodynamics
 Chapter 12 Thermodynamics 12.1 Thermodynamic Systems, States, and Processes System: definite quantity of matter with real or imaginary boundaries If heat transfer is impossible, the system is thermally
Chapter 12 Thermodynamics 12.1 Thermodynamic Systems, States, and Processes System: definite quantity of matter with real or imaginary boundaries If heat transfer is impossible, the system is thermally
Applied Thermodynamics HEAT TRANSFER. Introduction What and How?
 LANDMARK UNIVERSITY, OMU-ARAN LECTURE NOTE: 3 COLLEGE: COLLEGE OF SCIENCE AND ENGINEERING DEPARTMENT: MECHANICAL ENGINEERING PROGRAMME: ENGR. ALIYU, S.J Course code: MCE 311 Course title: Applied Thermodynamics
LANDMARK UNIVERSITY, OMU-ARAN LECTURE NOTE: 3 COLLEGE: COLLEGE OF SCIENCE AND ENGINEERING DEPARTMENT: MECHANICAL ENGINEERING PROGRAMME: ENGR. ALIYU, S.J Course code: MCE 311 Course title: Applied Thermodynamics
Bernoulli s Principle. Application: Lift. Bernoulli s Principle. Main Points 3/13/15. Demo: Blowing on a sheet of paper
 Bernoulli s Principle Demo: Blowing on a sheet of paper Where the speed of a fluid increases, internal pressure in the fluid decreases. Due to continuous flow of a fluid: what goes in must come out! Fluid
Bernoulli s Principle Demo: Blowing on a sheet of paper Where the speed of a fluid increases, internal pressure in the fluid decreases. Due to continuous flow of a fluid: what goes in must come out! Fluid
Chapter 16 Temperature and Heat
 Chapter 16 Temperature and Heat Temperature and the Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion Heat and Mechanical Work Specific Heats Conduction, Convection, and Radiation 16-1
Chapter 16 Temperature and Heat Temperature and the Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion Heat and Mechanical Work Specific Heats Conduction, Convection, and Radiation 16-1
Chapter 21: Temperature, Heat and Expansion
 Chapter 21: Temperature, Heat and Expansion All matter solid, liquid and gas is made of atoms or molecules, which are continually jiggling. As this jiggling is a movement, all these particles must have
Chapter 21: Temperature, Heat and Expansion All matter solid, liquid and gas is made of atoms or molecules, which are continually jiggling. As this jiggling is a movement, all these particles must have
Introduction to thermodynamics
 Chapter 6 Introduction to thermodynamics Topics First law of thermodynamics Definitions of internal energy and work done, leading to du = dq + dw Heat capacities, C p = C V + R Reversible and irreversible
Chapter 6 Introduction to thermodynamics Topics First law of thermodynamics Definitions of internal energy and work done, leading to du = dq + dw Heat capacities, C p = C V + R Reversible and irreversible
Lecture 2: Zero law of thermodynamics
 Lecture 2: Zero law of thermodynamics 1. Thermometers and temperature scales 2. Thermal contact and thermal equilibrium 3. Zeroth law of thermodynamics 1. Thermometers and Temperature scales We often associate
Lecture 2: Zero law of thermodynamics 1. Thermometers and temperature scales 2. Thermal contact and thermal equilibrium 3. Zeroth law of thermodynamics 1. Thermometers and Temperature scales We often associate
Law of Heat Transfer
 Law of Heat Transfer The Fundamental Laws which are used in broad area of applications are: 1. The law of conversion of mass 2. Newton s second law of motion 3. First and second laws of thermodynamics
Law of Heat Transfer The Fundamental Laws which are used in broad area of applications are: 1. The law of conversion of mass 2. Newton s second law of motion 3. First and second laws of thermodynamics
Lecture Outlines Chapter 16. Physics, 3 rd Edition James S. Walker
 Lecture Outlines Chapter 16 Physics, 3 rd Edition James S. Walker 2007 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the use of instructors in
Lecture Outlines Chapter 16 Physics, 3 rd Edition James S. Walker 2007 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the use of instructors in
2,000-gram mass of water compared to a 1,000-gram mass.
 11.2 Heat To change the temperature, you usually need to add or subtract energy. For example, when it s cold outside, you turn up the heat in your house or apartment and the temperature goes up. You know
11.2 Heat To change the temperature, you usually need to add or subtract energy. For example, when it s cold outside, you turn up the heat in your house or apartment and the temperature goes up. You know
HEAT AND TEMPERATURE Vikasana-Bridge Course 2012
 HEAT AND TEMPERATURE TOPICS Introduction Effects of heat Specific heat Basics of thermodynamics Introduction Heat may be defined as energy in transit from a high temperature region to a lower temperature
HEAT AND TEMPERATURE TOPICS Introduction Effects of heat Specific heat Basics of thermodynamics Introduction Heat may be defined as energy in transit from a high temperature region to a lower temperature
Thermal Energy. Thermal Energy Transfers
 Thermal Energy Thermal Energy Transfers Key Concepts What is the effect of having a small specific heat? What happens to a material when it is heated? In what ways can thermal energy be transferred? What
Thermal Energy Thermal Energy Transfers Key Concepts What is the effect of having a small specific heat? What happens to a material when it is heated? In what ways can thermal energy be transferred? What
Conduction. Heat Transfer Methods. Conduction. Conduction
 Heat Transfer Methods Conduction: Thermal kinetic energy passed from particle-to-particle along a length of material. Convection: Thermal energy carried by moving fluid. Radiation: Thermal energy carried
Heat Transfer Methods Conduction: Thermal kinetic energy passed from particle-to-particle along a length of material. Convection: Thermal energy carried by moving fluid. Radiation: Thermal energy carried
Lecture 3: Light and Temperature
 Lecture 3: Light and Temperature terrestrial radiative cooling Solar radiative warming (Light) Global Temperature atmosphere ocean land Light Temperature Different forms of energy Energy conservation energy,
Lecture 3: Light and Temperature terrestrial radiative cooling Solar radiative warming (Light) Global Temperature atmosphere ocean land Light Temperature Different forms of energy Energy conservation energy,
[Engineering Mathematics]
![[Engineering Mathematics] [Engineering Mathematics]](/thumbs/82/85860352.jpg) [MATHS IV] [Engineering Mathematics] [Partial Differential Equations] [Partial Differentiation and formation of Partial Differential Equations has already been covered in Maths II syllabus. Present chapter
[MATHS IV] [Engineering Mathematics] [Partial Differential Equations] [Partial Differentiation and formation of Partial Differential Equations has already been covered in Maths II syllabus. Present chapter
Lecture 22. Temperature and Heat
 Lecture 22 Temperature and Heat Today s Topics: 0 th Law of Thermodynamics Temperature Scales Thermometers Thermal Expansion Heat, Internal Energy and Work Heat Transfer Temperature and the Zeroth Law
Lecture 22 Temperature and Heat Today s Topics: 0 th Law of Thermodynamics Temperature Scales Thermometers Thermal Expansion Heat, Internal Energy and Work Heat Transfer Temperature and the Zeroth Law
Physical Science. Thermal Energy & Heat
 Physical Science Thermal Energy & Heat Sometimes called internal energy Depends on the object's mass, temperature, and phase (solid, liquid, gas) TOTAL potential and kinetic energy of all the particles
Physical Science Thermal Energy & Heat Sometimes called internal energy Depends on the object's mass, temperature, and phase (solid, liquid, gas) TOTAL potential and kinetic energy of all the particles
A thermodynamic system is taken from an initial state X along the path XYZX as shown in the PV-diagram.
 AP Physics Multiple Choice Practice Thermodynamics 1. The maximum efficiency of a heat engine that operates between temperatures of 1500 K in the firing chamber and 600 K in the exhaust chamber is most
AP Physics Multiple Choice Practice Thermodynamics 1. The maximum efficiency of a heat engine that operates between temperatures of 1500 K in the firing chamber and 600 K in the exhaust chamber is most
Heat Transfer. Conduction Radiation Convection
 Heat Transfer Conduction Radiation Convection Real World Experience We are going outside to experiences heat transfer. Instructions: while outside place hand on the concrete. Note whether it feels cold
Heat Transfer Conduction Radiation Convection Real World Experience We are going outside to experiences heat transfer. Instructions: while outside place hand on the concrete. Note whether it feels cold
Heat What is heat? Work = 2. PdV 1
 eat What is heat? eat (Q) is the flow or transfer of energy from one system to another Often referred to as heat flow or heat transfer Requires that one system must be at a higher temperature than the
eat What is heat? eat (Q) is the flow or transfer of energy from one system to another Often referred to as heat flow or heat transfer Requires that one system must be at a higher temperature than the
FOURIER SERIES. Chapter Introduction
 Chapter 1 FOURIER SERIES 1.1 Introduction Fourier series introduced by a French physicist Joseph Fourier (1768-1830), is a mathematical tool that converts some specific periodic signals into everlasting
Chapter 1 FOURIER SERIES 1.1 Introduction Fourier series introduced by a French physicist Joseph Fourier (1768-1830), is a mathematical tool that converts some specific periodic signals into everlasting
Question. Woodstoves. Thermal Energy. Heat. Burning Wood. Chemical Forces. Which is more effective at heating a room:
 Question Which is more effective at heating a room: 1. a black woodstove 2. a white woodstove Thermal Energy is disordered energy is the kinetic and potential energies of atoms gives rise to temperature
Question Which is more effective at heating a room: 1. a black woodstove 2. a white woodstove Thermal Energy is disordered energy is the kinetic and potential energies of atoms gives rise to temperature
SKMM 2413 Thermodynamics
 SKMM 2413 Thermodynamics Md. Mizanur Rahman, PhD Department of Thermo-Fluids Faculty of Mechanical Engineering Universiti Teknologi Malaysia UTM Office: C23-228 mizanur@fkm.utm.my Semester I, 2016-2017
SKMM 2413 Thermodynamics Md. Mizanur Rahman, PhD Department of Thermo-Fluids Faculty of Mechanical Engineering Universiti Teknologi Malaysia UTM Office: C23-228 mizanur@fkm.utm.my Semester I, 2016-2017
T s change via collisions at boundary (not mechanical interaction)
 Lecture 14 Interaction of 2 systems at different temperatures Irreversible processes: 2nd Law of Thermodynamics Chapter 19: Heat Engines and Refrigerators Thermal interactions T s change via collisions
Lecture 14 Interaction of 2 systems at different temperatures Irreversible processes: 2nd Law of Thermodynamics Chapter 19: Heat Engines and Refrigerators Thermal interactions T s change via collisions
- Apply closed system energy balances, observe sign convention for work and heat transfer.
 CHAPTER : ENERGY AND THE FIRST LAW OF THERMODYNAMICS Objectives: - In this chapter we discuss energy and develop equations for applying the principle of conservation of energy. Learning Outcomes: - Demonstrate
CHAPTER : ENERGY AND THE FIRST LAW OF THERMODYNAMICS Objectives: - In this chapter we discuss energy and develop equations for applying the principle of conservation of energy. Learning Outcomes: - Demonstrate
Lecture Outline Chapter 18. Physics, 4 th Edition James S. Walker. Copyright 2010 Pearson Education, Inc.
 Lecture Outline Chapter 18 Physics, 4 th Edition James S. Walker Chapter 18 The Laws of Thermodynamics Units of Chapter 18 The Zeroth Law of Thermodynamics The First Law of Thermodynamics Thermal Processes
Lecture Outline Chapter 18 Physics, 4 th Edition James S. Walker Chapter 18 The Laws of Thermodynamics Units of Chapter 18 The Zeroth Law of Thermodynamics The First Law of Thermodynamics Thermal Processes
INSTRUCTOR: PM DR MAZLAN ABDUL WAHID
 SMJ 4463: HEAT TRANSFER INSTRUCTOR: PM DR MAZLAN ABDUL WAHID http://www.fkm.utm.my/~mazlan TEXT: Introduction to Heat Transfer by Incropera, DeWitt, Bergman, Lavine 5 th Edition, John Wiley and Sons DR
SMJ 4463: HEAT TRANSFER INSTRUCTOR: PM DR MAZLAN ABDUL WAHID http://www.fkm.utm.my/~mazlan TEXT: Introduction to Heat Transfer by Incropera, DeWitt, Bergman, Lavine 5 th Edition, John Wiley and Sons DR
Chapter 12. The Laws of Thermodynamics. First Law of Thermodynamics
 Chapter 12 The Laws of Thermodynamics First Law of Thermodynamics The First Law of Thermodynamics tells us that the internal energy of a system can be increased by Adding energy to the system Doing work
Chapter 12 The Laws of Thermodynamics First Law of Thermodynamics The First Law of Thermodynamics tells us that the internal energy of a system can be increased by Adding energy to the system Doing work
Physics 111. Thursday, Dec. 9, 3-5pm and 7-9pm. Announcements. Thursday, December 9, 2004
 ics day, ember 9, 2004 Ch 18: diagrams isobaric process isochoric process isothermal process adiabatic process 2nd Law of Thermodynamics Class Reviews/Evaluations For the rest of the semester day,. 9,
ics day, ember 9, 2004 Ch 18: diagrams isobaric process isochoric process isothermal process adiabatic process 2nd Law of Thermodynamics Class Reviews/Evaluations For the rest of the semester day,. 9,
Preview. Heat Section 1. Section 1 Temperature and Thermal Equilibrium. Section 2 Defining Heat. Section 3 Changes in Temperature and Phase
 Heat Section 1 Preview Section 1 Temperature and Thermal Equilibrium Section 2 Defining Heat Section 3 Changes in Temperature and Phase Heat Section 1 TEKS The student is expected to: 6E describe how the
Heat Section 1 Preview Section 1 Temperature and Thermal Equilibrium Section 2 Defining Heat Section 3 Changes in Temperature and Phase Heat Section 1 TEKS The student is expected to: 6E describe how the
Chapter 12. The Laws of Thermodynamics
 Chapter 12 The Laws of Thermodynamics First Law of Thermodynamics The First Law of Thermodynamics tells us that the internal energy of a system can be increased by Adding energy to the system Doing work
Chapter 12 The Laws of Thermodynamics First Law of Thermodynamics The First Law of Thermodynamics tells us that the internal energy of a system can be increased by Adding energy to the system Doing work
Partial Differential Equations Summary
 Partial Differential Equations Summary 1. The heat equation Many physical processes are governed by partial differential equations. temperature of a rod. In this chapter, we will examine exactly that.
Partial Differential Equations Summary 1. The heat equation Many physical processes are governed by partial differential equations. temperature of a rod. In this chapter, we will examine exactly that.
CERT Educational Series Heat Transfer
 Student Lab Sheet Answer Key CERT Educational Series Heat Transfer Name Date: Are HEAT and TEMPERATURE the same thing? YES NO Heat and Temperature are not the same thing. They have different units. Heat
Student Lab Sheet Answer Key CERT Educational Series Heat Transfer Name Date: Are HEAT and TEMPERATURE the same thing? YES NO Heat and Temperature are not the same thing. They have different units. Heat
Kinetic Theory of Matter. Matter & Energy
 Kinetic Theory of Matter Matter & Energy 1 Kinetic Theory of Matter All matter is made up of atoms and molecules that act as tiny particles. 2 Kinetic Theory of Matter These tiny particles are always in
Kinetic Theory of Matter Matter & Energy 1 Kinetic Theory of Matter All matter is made up of atoms and molecules that act as tiny particles. 2 Kinetic Theory of Matter These tiny particles are always in
Conduction, Convection, and Radiation
 Conduction Thermal energy is transferred from place to place by conduction, convection, and radiation. Conduction is the transfer of thermal energy by collisions between particles in matter. Conduction
Conduction Thermal energy is transferred from place to place by conduction, convection, and radiation. Conduction is the transfer of thermal energy by collisions between particles in matter. Conduction
Honors Physics. Notes Nov 16, 20 Heat. Persans 1
 Honors Physics Notes Nov 16, 20 Heat Persans 1 Properties of solids Persans 2 Persans 3 Vibrations of atoms in crystalline solids Assuming only nearest neighbor interactions (+Hooke's law) F = C( u! u
Honors Physics Notes Nov 16, 20 Heat Persans 1 Properties of solids Persans 2 Persans 3 Vibrations of atoms in crystalline solids Assuming only nearest neighbor interactions (+Hooke's law) F = C( u! u
Chapter 16 Thermodynamics
 Nicholas J. Giordano www.cengage.com/physics/giordano Chapter 16 Thermodynamics Thermodynamics Introduction Another area of physics is thermodynamics Continues with the principle of conservation of energy
Nicholas J. Giordano www.cengage.com/physics/giordano Chapter 16 Thermodynamics Thermodynamics Introduction Another area of physics is thermodynamics Continues with the principle of conservation of energy
Assess why particular characteristics are necessary for effective conduction KEY POINTS
 Conduction LEARNING OBJECTIVES Assess why particular characteristics are necessary for effective conduction KEY POINTS On a microscopic scale, conduction occurs as rapidly moving or vibrating atoms and
Conduction LEARNING OBJECTIVES Assess why particular characteristics are necessary for effective conduction KEY POINTS On a microscopic scale, conduction occurs as rapidly moving or vibrating atoms and
Chapter: Heat and States
 Table of Contents Chapter: Heat and States of Matter Section 1: Temperature and Thermal Energy Section 2: States of Matter Section 3: Transferring Thermal Energy Section 4: Using Thermal Energy 1 Temperature
Table of Contents Chapter: Heat and States of Matter Section 1: Temperature and Thermal Energy Section 2: States of Matter Section 3: Transferring Thermal Energy Section 4: Using Thermal Energy 1 Temperature
The Kinetic Theory of Matter. Temperature. Temperature. Temperature. Temperature. Chapter 6 HEAT
 The Kinetic Theory of Matter Hewitt/Lyons/Suchocki/Yeh Conceptual Integrated Science Chapter 6 HEAT Kinetic Theory of Matter: Matter is made up of tiny particles (atoms or molecules) that are always in
The Kinetic Theory of Matter Hewitt/Lyons/Suchocki/Yeh Conceptual Integrated Science Chapter 6 HEAT Kinetic Theory of Matter: Matter is made up of tiny particles (atoms or molecules) that are always in
P5 Heat and Particles Revision Kinetic Model of Matter: States of matter
 P5 Heat and Particles Revision Kinetic Model of Matter: States of matter State Size Shape Solid occupies a fixed volume has a fixed shape Liquid occupies a fixed volume takes the shape of its container
P5 Heat and Particles Revision Kinetic Model of Matter: States of matter State Size Shape Solid occupies a fixed volume has a fixed shape Liquid occupies a fixed volume takes the shape of its container
CHAPTER 4. Basics of Fluid Dynamics
 CHAPTER 4 Basics of Fluid Dynamics What is a fluid? A fluid is a substance that can flow, has no fixed shape, and offers little resistance to an external stress In a fluid the constituent particles (atoms,
CHAPTER 4 Basics of Fluid Dynamics What is a fluid? A fluid is a substance that can flow, has no fixed shape, and offers little resistance to an external stress In a fluid the constituent particles (atoms,
1 Solutions in cylindrical coordinates: Bessel functions
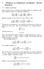 1 Solutions in cylindrical coordinates: Bessel functions 1.1 Bessel functions Bessel functions arise as solutions of potential problems in cylindrical coordinates. Laplace s equation in cylindrical coordinates
1 Solutions in cylindrical coordinates: Bessel functions 1.1 Bessel functions Bessel functions arise as solutions of potential problems in cylindrical coordinates. Laplace s equation in cylindrical coordinates
Thermochemistry. The study of energy transfers and chemical reactions
 Thermochemistry The study of energy transfers and chemical reactions Energy Energy is the ability to do work Work = Force x distance SI unit is the Joule (J) 1000 J = 1 kj other unit: calorie (cal) 1000
Thermochemistry The study of energy transfers and chemical reactions Energy Energy is the ability to do work Work = Force x distance SI unit is the Joule (J) 1000 J = 1 kj other unit: calorie (cal) 1000
Non-Continuum Energy Transfer: Overview
 Non-Continuum Energy Transfer: Overview D. B. Go Slide 1 Topics Covered To Date Conduction - transport of thermal energy through a medium (solid/ liquid/gas) due to the random motion of the energy carriers
Non-Continuum Energy Transfer: Overview D. B. Go Slide 1 Topics Covered To Date Conduction - transport of thermal energy through a medium (solid/ liquid/gas) due to the random motion of the energy carriers
Heat Tracing Basics. By: Homi R. Mullan 1
 Heat Tracing Basics By: Homi R. Mullan 1 Heat Tracing Basics Topics of Discussion What is Heat Tracing? Why Heat Tracing? Fundamentals of Heat Loss and Heat Replenishment Rules to Remember in the Heat
Heat Tracing Basics By: Homi R. Mullan 1 Heat Tracing Basics Topics of Discussion What is Heat Tracing? Why Heat Tracing? Fundamentals of Heat Loss and Heat Replenishment Rules to Remember in the Heat
Thermodynamic Systems, States, and Processes
 Thermodynamics Thermodynamic Systems, States, and Processes A thermodynamic system is described by an equation of state, such as the ideal gas law. The location of the state can be plotted on a p V diagram,
Thermodynamics Thermodynamic Systems, States, and Processes A thermodynamic system is described by an equation of state, such as the ideal gas law. The location of the state can be plotted on a p V diagram,
Chapter 10 Temperature and Heat
 Chapter 10 Temperature and Heat Thermodynamics deals with 1. Temperature. 2. The transfer and transformation of energy. 3. The relationship between macroscopic properties and microscopic dynamics. Temperature
Chapter 10 Temperature and Heat Thermodynamics deals with 1. Temperature. 2. The transfer and transformation of energy. 3. The relationship between macroscopic properties and microscopic dynamics. Temperature
Chapter 9 NATURAL CONVECTION
 Heat and Mass Transfer: Fundamentals & Applications Fourth Edition in SI Units Yunus A. Cengel, Afshin J. Ghajar McGraw-Hill, 2011 Chapter 9 NATURAL CONVECTION PM Dr Mazlan Abdul Wahid Universiti Teknologi
Heat and Mass Transfer: Fundamentals & Applications Fourth Edition in SI Units Yunus A. Cengel, Afshin J. Ghajar McGraw-Hill, 2011 Chapter 9 NATURAL CONVECTION PM Dr Mazlan Abdul Wahid Universiti Teknologi
Chapter 1 Heating Processes
 Chapter 1 Heating Processes Section 1.1 Heat and temperature Worked example: Try yourself 1.1.1 CALCULATING THE CHANGE IN INTERNAL ENERGY A student places a heating element and a paddle wheel apparatus
Chapter 1 Heating Processes Section 1.1 Heat and temperature Worked example: Try yourself 1.1.1 CALCULATING THE CHANGE IN INTERNAL ENERGY A student places a heating element and a paddle wheel apparatus
A, B, and C, in the P V plane. i C. D: none of the above
 pressure A gas is in a container with a piston lid and is taken from the state, i, to a state, f, by several different paths, A, B, and C, in the P V plane. A B f D: none of the above i C volume The work
pressure A gas is in a container with a piston lid and is taken from the state, i, to a state, f, by several different paths, A, B, and C, in the P V plane. A B f D: none of the above i C volume The work
Knight: Chapter 18. The Micro/Macro Connection. (Thermal Interactions and Heat & Irreversible Processes and the 2 nd Law of Thermodynamics)
 Knight: Chapter 18 The Micro/Macro Connection (Thermal Interactions and Heat & Irreversible Processes and the 2 nd Law of Thermodynamics) Last time p Thermal energy of a Monatomic gas.. E th = 3 2 NK BT
Knight: Chapter 18 The Micro/Macro Connection (Thermal Interactions and Heat & Irreversible Processes and the 2 nd Law of Thermodynamics) Last time p Thermal energy of a Monatomic gas.. E th = 3 2 NK BT
ANSWERS 403 INDEX. Bulk modulus 238 Buoyant force 251
 ANSWERS 403 INDEX A Absolute scale temperature 276 Absolute zero 276 Acceleration (linear) 45 Acceleration due to gravity 49,189 Accuracy 22 Action-reaction 97 Addition of vectors 67 Adiabatic process
ANSWERS 403 INDEX A Absolute scale temperature 276 Absolute zero 276 Acceleration (linear) 45 Acceleration due to gravity 49,189 Accuracy 22 Action-reaction 97 Addition of vectors 67 Adiabatic process
Question 1: For the positions labeled on the image, list in order from highest to lowest potential energy of the roller coaster. How is the kinetic
 Question 1: For the positions labeled on the image, list in order from highest to lowest potential energy of the roller coaster. How is the kinetic energy of the roller coaster related to these values?
Question 1: For the positions labeled on the image, list in order from highest to lowest potential energy of the roller coaster. How is the kinetic energy of the roller coaster related to these values?
THERMODYNAMICS SSC-JE STAFF SELECTION COMMISSION MECHANICAL ENGINEERING STUDY MATERIAL THERMODYNAMICS THERMODYNAMICS THERMODYNAMICS
 1 SSC-JE STAFF SELECTION COMMISSION MECHANICAL ENGINEERING STUDY MATERIAL 2 Syllabus: Thermal Engineering (Thermodynamics) Properties of Pure Substances : p-v & P-T diagrams of pure substance like H 2
1 SSC-JE STAFF SELECTION COMMISSION MECHANICAL ENGINEERING STUDY MATERIAL 2 Syllabus: Thermal Engineering (Thermodynamics) Properties of Pure Substances : p-v & P-T diagrams of pure substance like H 2
Time-Frequency Analysis
 Time-Frequency Analysis Basics of Fourier Series Philippe B. aval KSU Fall 015 Philippe B. aval (KSU) Fourier Series Fall 015 1 / 0 Introduction We first review how to derive the Fourier series of a function.
Time-Frequency Analysis Basics of Fourier Series Philippe B. aval KSU Fall 015 Philippe B. aval (KSU) Fourier Series Fall 015 1 / 0 Introduction We first review how to derive the Fourier series of a function.
A). Yes. B). No. Q15 Is it possible for a solid metal ball to float in mercury?
 Q15 Is it possible for a solid metal ball to float in mercury? A). Yes. B). No. The upward force is the weight of liquid displaced and the downward force is the weight of the ball. If the density of the
Q15 Is it possible for a solid metal ball to float in mercury? A). Yes. B). No. The upward force is the weight of liquid displaced and the downward force is the weight of the ball. If the density of the
Heat Transfer. Heat always moves from a warmer place to a cooler place. Hot objects in a cooler room will cool to room temperature.
 Heat Transfer Heat always moves from a warmer place to a cooler place. Hot objects in a cooler room will cool to room temperature. Cold objects in a warmer room will heat up to room temperature. Question
Heat Transfer Heat always moves from a warmer place to a cooler place. Hot objects in a cooler room will cool to room temperature. Cold objects in a warmer room will heat up to room temperature. Question
 Academic Year 2016-2017 First Term Science Revision sheets PHYSICS ( Answer key ) Name: Grade: 10 Date: Section: (A) Science Practice : Q1: Choose the letter of the choice that best answer the questions:
Academic Year 2016-2017 First Term Science Revision sheets PHYSICS ( Answer key ) Name: Grade: 10 Date: Section: (A) Science Practice : Q1: Choose the letter of the choice that best answer the questions:
Energy in Thermal Processes. Heat and Internal Energy
 Energy in Thermal Processes Heat and Internal Energy Internal energy U: associated with the microscopic components of a system: kinetic and potential energies. The larger the number of internal degrees
Energy in Thermal Processes Heat and Internal Energy Internal energy U: associated with the microscopic components of a system: kinetic and potential energies. The larger the number of internal degrees
Physics Thermodynamics. Science and Mathematics Education Research Group
 F FA ACULTY C U L T Y OF O F EDUCATION E D U C A T I O N Department of Curriculum and Pedagogy Physics Thermodynamics Science and Mathematics Education Research Group Supported by UBC Teaching and Learning
F FA ACULTY C U L T Y OF O F EDUCATION E D U C A T I O N Department of Curriculum and Pedagogy Physics Thermodynamics Science and Mathematics Education Research Group Supported by UBC Teaching and Learning
Chapter 16 Temperature and Heat
 Chapter 16 Temperature and Heat 16-1 Temperature and the Zeroth Law of Thermodynamics Definition of heat: Heat is the energy transferred between objects because of a temperature difference. Objects are
Chapter 16 Temperature and Heat 16-1 Temperature and the Zeroth Law of Thermodynamics Definition of heat: Heat is the energy transferred between objects because of a temperature difference. Objects are
CHAPTER 4. Introduction to the. Heat Conduction Model
 A SERIES OF CLASS NOTES FOR 005-006 TO INTRODUCE LINEAR AND NONLINEAR PROBLEMS TO ENGINEERS, SCIENTISTS, AND APPLIED MATHEMATICIANS DE CLASS NOTES 4 A COLLECTION OF HANDOUTS ON PARTIAL DIFFERENTIAL EQUATIONS
A SERIES OF CLASS NOTES FOR 005-006 TO INTRODUCE LINEAR AND NONLINEAR PROBLEMS TO ENGINEERS, SCIENTISTS, AND APPLIED MATHEMATICIANS DE CLASS NOTES 4 A COLLECTION OF HANDOUTS ON PARTIAL DIFFERENTIAL EQUATIONS
Thermal Systems. What and How? Physical Mechanisms and Rate Equations Conservation of Energy Requirement Control Volume Surface Energy Balance
 Introduction to Heat Transfer What and How? Physical Mechanisms and Rate Equations Conservation of Energy Requirement Control Volume Surface Energy Balance Thermal Resistance Thermal Capacitance Thermal
Introduction to Heat Transfer What and How? Physical Mechanisms and Rate Equations Conservation of Energy Requirement Control Volume Surface Energy Balance Thermal Resistance Thermal Capacitance Thermal
Energy Transfer Subtitle
 Energy Transfer Subtitle Objectives Review Earth System Review the Water cycle Go over heat transfer through conduction, convection, and radiation Review Greenhouse Effect 2 July 22, 2012 Footer text here
Energy Transfer Subtitle Objectives Review Earth System Review the Water cycle Go over heat transfer through conduction, convection, and radiation Review Greenhouse Effect 2 July 22, 2012 Footer text here
Energy, Temperature, & Heat. Energy, Temperature, & Heat. Temperature Scales 1/17/11
 Energy, Temperature, & Heat Energy is the ability to do work (push, pull, lift) on some form of matter. Chapter 2 Potential energy is the potential for work (mass x gravity x height) Kinetic energy is
Energy, Temperature, & Heat Energy is the ability to do work (push, pull, lift) on some form of matter. Chapter 2 Potential energy is the potential for work (mass x gravity x height) Kinetic energy is
THE UNSTEADY FREE CONVECTION FLOW OF ROTATING MHD SECOND GRADE FLUID IN POROUS MEDIUM WITH EFFECT OF RAMPED WALL TEMPERATURE
 THE UNSTEADY FREE CONVECTION FLOW OF ROTATING MHD SECOND GRADE FLUID IN POROUS MEDIUM WITH EFFECT OF RAMPED WALL TEMPERATURE 1 AHMAD QUSHAIRI MOHAMAD, ILYAS KHAN, 3 ZULKHIBRI ISMAIL AND 4* SHARIDAN SHAFIE
THE UNSTEADY FREE CONVECTION FLOW OF ROTATING MHD SECOND GRADE FLUID IN POROUS MEDIUM WITH EFFECT OF RAMPED WALL TEMPERATURE 1 AHMAD QUSHAIRI MOHAMAD, ILYAS KHAN, 3 ZULKHIBRI ISMAIL AND 4* SHARIDAN SHAFIE
WELCOME TO PERIOD 5: THERMAL ENERGY, THE MICROSCOPIC PICTURE. Homework #4 is due today at the beginning of class.
 WELCOME TO PERIOD 5: THERMAL ENERGY, THE MICROSCOPIC PICTURE Homework #4 is due today at the beginning of class. PHYSICS 1104 PERIOD 5 How are temperatures measured? How do atoms and molecules act at different
WELCOME TO PERIOD 5: THERMAL ENERGY, THE MICROSCOPIC PICTURE Homework #4 is due today at the beginning of class. PHYSICS 1104 PERIOD 5 How are temperatures measured? How do atoms and molecules act at different
Chemical Reaction Engineering Prof. JayantModak Department of Chemical Engineering Indian Institute of Science, Bangalore
 Chemical Reaction Engineering Prof. JayantModak Department of Chemical Engineering Indian Institute of Science, Bangalore Module No. #05 Lecture No. #29 Non Isothermal Reactor Operation Let us continue
Chemical Reaction Engineering Prof. JayantModak Department of Chemical Engineering Indian Institute of Science, Bangalore Module No. #05 Lecture No. #29 Non Isothermal Reactor Operation Let us continue
CHAPTER 17 WORK, HEAT, & FIRST LAW OF THERMODYNAMICS
 CHAPTER 17 WORK, HEAT, and the FIRST LAW OF THERMODYNAMICS In this chapter, we will examine various thermal properties of matter, as well as several mechanisms by which energy can be transferred to and
CHAPTER 17 WORK, HEAT, and the FIRST LAW OF THERMODYNAMICS In this chapter, we will examine various thermal properties of matter, as well as several mechanisms by which energy can be transferred to and
Module 3 - Thermodynamics. Thermodynamics. Measuring Temperatures. Temperature and Thermal Equilibrium
 Thermodynamics From the Greek thermos meaning heat and dynamis meaning power is a branch of physics that studies the effects of changes in temperature, pressure, and volume on physical systems at the macroscopic
Thermodynamics From the Greek thermos meaning heat and dynamis meaning power is a branch of physics that studies the effects of changes in temperature, pressure, and volume on physical systems at the macroscopic
Chapter 1 INTRODUCTION AND BASIC CONCEPTS
 Heat and Mass Transfer: Fundamentals & Applications 5th Edition in SI Units Yunus A. Çengel, Afshin J. Ghajar McGraw-Hill, 2015 Chapter 1 INTRODUCTION AND BASIC CONCEPTS Mehmet Kanoglu University of Gaziantep
Heat and Mass Transfer: Fundamentals & Applications 5th Edition in SI Units Yunus A. Çengel, Afshin J. Ghajar McGraw-Hill, 2015 Chapter 1 INTRODUCTION AND BASIC CONCEPTS Mehmet Kanoglu University of Gaziantep
Lesson 12. Luis Anchordoqui. Physics 168. Tuesday, November 28, 17
 Lesson 12 Physics 168 1 Temperature and Kinetic Theory of Gases 2 Atomic Theory of Matter On microscopic scale, arrangements of molecules in solids, liquids, and gases are quite different 3 Temperature
Lesson 12 Physics 168 1 Temperature and Kinetic Theory of Gases 2 Atomic Theory of Matter On microscopic scale, arrangements of molecules in solids, liquids, and gases are quite different 3 Temperature
Physics 9e/Cutnell. correlated to the. College Board AP Physics 2 Course Objectives
 correlated to the College Board AP Physics 2 Course Objectives Big Idea 1: Objects and systems have properties such as mass and charge. Systems may have internal structure. Enduring Understanding 1.A:
correlated to the College Board AP Physics 2 Course Objectives Big Idea 1: Objects and systems have properties such as mass and charge. Systems may have internal structure. Enduring Understanding 1.A:
Chapter 5. Formulation of FEM for Unsteady Problems
 Chapter 5 Formulation of FEM for Unsteady Problems Two alternatives for formulating time dependent problems are called coupled space-time formulation and semi-discrete formulation. The first one treats
Chapter 5 Formulation of FEM for Unsteady Problems Two alternatives for formulating time dependent problems are called coupled space-time formulation and semi-discrete formulation. The first one treats
Thermal Physics. Topics to be covered. Slide 2 / 105. Slide 1 / 105. Slide 3 / 105. Slide 4 / 105. Slide 5 / 105. Slide 6 / 105.
 Slide 1 / 105 Slide 2 / 105 Topics to be covered Thermal Physics Temperature and Thermal quilibrium Gas Laws Internal nergy Heat Work Laws of Thermodynamics Heat ngines Slide 3 / 105 Thermodynamics System
Slide 1 / 105 Slide 2 / 105 Topics to be covered Thermal Physics Temperature and Thermal quilibrium Gas Laws Internal nergy Heat Work Laws of Thermodynamics Heat ngines Slide 3 / 105 Thermodynamics System
Work by Friction. A box slides 10 m across a surface. A frictional force of 20 N is acting on the box.
 Work by Friction A box slides 10 m across a surface. A frictional force of 20 N is acting on the box. What is the work done by friction? What happened to this energy? Work by Friction A box slides 10 m
Work by Friction A box slides 10 m across a surface. A frictional force of 20 N is acting on the box. What is the work done by friction? What happened to this energy? Work by Friction A box slides 10 m
5.2. November 30, 2012 Mrs. Poland. Verifying Trigonometric Identities
 5.2 Verifying Trigonometric Identities Verifying Identities by Working With One Side Verifying Identities by Working With Both Sides November 30, 2012 Mrs. Poland Objective #4: Students will be able to
5.2 Verifying Trigonometric Identities Verifying Identities by Working With One Side Verifying Identities by Working With Both Sides November 30, 2012 Mrs. Poland Objective #4: Students will be able to
x = B sin ( t ) HARMONIC MOTIONS SINE WAVES AND SIMPLE HARMONIC MOTION Here s a nice simple fraction: y = sin (x) Differentiate = cos (x)
 SINE WAVES AND SIMPLE HARMONIC MOTION Here s a nice simple fraction: y = sin (x) HARMONIC MOTIONS dy Differentiate = cos (x) dx So sin (x) has a stationary value whenever cos (x) = 0. 3 5 7 That s when
SINE WAVES AND SIMPLE HARMONIC MOTION Here s a nice simple fraction: y = sin (x) HARMONIC MOTIONS dy Differentiate = cos (x) dx So sin (x) has a stationary value whenever cos (x) = 0. 3 5 7 That s when
