Physics is time symmetric Nature is not
|
|
|
- Lora Nelson
- 6 years ago
- Views:
Transcription
1 Fundamental theories of physics don t depend on the direction of time Newtonian Physics Electromagnetism Relativity Quantum Mechanics Physics is time symmetric Nature is not II law of thermodynamics - only law of nature that exhibits direction of time!
2 The First Law of Thermodynamics: the only allowed changes for a system are those that do not change the total energy of the Universe. ΔU = ΔQ + ΔW ΔU system + ΔU surrounding = 0 Energy conservation is not a criterion to decide if a process will occur or not!!!! ΔU = 0 This rxn occurs in one direction and not in the opposite.
3 Spontaneous process v A spontaneous process can occur in a system left to itself; no outside action is necessary v A non-spontaneous process cannot take place in a system left to itself If a process is spontaneous, the reverse process is non-spontaneous and vice versa. Spontaneous says nothing about how fast a process occurs Diamond is thermodynamically favored to convert to graphite, but not kinetically favored.
4 Irreversible Processes a change in the thermodynamic state of a system and all of its surroundings cannot be precisely restored to its initial state by infinitesimal changes in some property of the system without expenditure of energy. All Real processes are irreversible. Each state reached during the transition is far more probable than that which preceded it. P >> P i + 1 i 1
5 Reversible proces The system changes in such a way that the system and surroundings can be put back in their original states by exactly reversing the process. The occurrence of each such step is infinitesimally more probable than its non-occurrence. In a reversible process changes are infinitesimally small. P+ 1 P i i 1
6 Efficiency Free energy transduction is least efficient when it proceeds by the uncontrolled release of a big constraint. Free energy transduction is most efficient when it proceeds by the incremental, controlled release of many small constraints.
7 Enthalpy H = U + PV & ΔH = ΔU + Δ(PV) For a system at constant pressure: ΔH = ΔU + PΔV and w p = -PΔV ΔH = H final H initial State function (path independent) Enthalpy is an extensive property ΔH total = (moles reacted) x ΔH rxn
8 Standard Enthalpy Change The standard enthalpy change, ΔH o, of a reaction is the enthalpy change when reactants and products are in their standard states. ΔH o rxn = n ΔH o products m ΔH o reac tants (n & m are stechiometric coefficients) The change of enthalpy of a reaction is fairly independent of temperature.
9 Second law of Thermodynamics
10 Entropy Rudolf CLAUSIUS ( ) 1865: Definition of entropy for a process occurring at constant temperature is: ds I = dq rev T Q rev = the heat that is transferred when the process is carried out reversibly at a constant temperature. T = temperature in Kelvin. For irreversible processes in a nonisolated system. For thermally isolated systems ΔS 0. ds I > Entropy is an extensive property 2 dqrev ΔS total = (ΔS system ) x (moles reacted) ΔS = = S(2) S(1) T 1 dq T
11 Thermodynamics and atomic hypothesis Ludwig BOLTZMAN 1872: Kinetic theory 1880: Statistical interpretation of entropy: disorder in energy space The Boltzmann (physical) Entropy. It is a thermodynamic state function that measure how the dispersal of energy alters when a system changes from one state to another. There are W(U) states available to a physical system with energy U. Once the system has come to equilibrium, its entropy is defined as: S( U) = k ln[ W( U)] B k B = J/K
12 Entropy is a state function describes the direction of a process due to changes in heat input or output and the associated molecular rearrangements. PROBABILITY suggests that a spontaneous reaction will result in entropy created by the dispersal * of energy ( S surround ) * of matter ( S system ) Higher entropy is often associated with greater disorder, but really measures how energy is spread out in a system.
13 Global States vs. Microstates out of equilibrium unstable metastable F = 0 stable global state Energy Reaction coordinate microstates
14 /8 Multiplicity p(k) /4 1/ /16 1/ k outcome sequence composition For 4 trials, P HHHH = P HTHH = (0.5) 4, All sequences are equally probable But not all compositions W ( n, N) = N! n!*( N n)! HHHH 0T4H THHH HTHH HHTH HHHT 1T3H W is the Wahrscheinlichkeit, the frequency of occurrence of a macrostate or the number of possible microstates corresponding to the macroscopic state of a system
15 Multiplicity W ( n, N) = N! n!*( N n)! N = 4 n W lnw W 4! = 1 0!4! 4! = 4 1!3! 4! = 6 2!2! 4! = 4 31!! 4! = 1 4!0! Total W = n HHHH 0T4H THHH HTHH HHTH HHHT 1T3H
16 Multiplicity N = 4 N = 10 N = W W W n n n In any number of trials the composition has a tendency to 50%H50%T For any system, if W(n) is known, n* where dw(n)/dn = 0 is the most likely number to observe The same applies to lnw(n)
17 Barometric formula a particle above the ground h E pot = m gh potential energy = mgh = work against the gravity
18 A column of compressible gas in the gravity field: T is constant, but density depends on height dp = ρ( h) gdh dh p p dp density ideal gas equation nm ρ = pv = nkt V n number of particles m - mass substituting: dp = mg kt pdh p po dp p = mg kt h 0 dh Barometric formula p o ln p po = mgh kt p p e mgh/ kt = o
19 Barometric formula p mgh / kt p o = e because pressure is proportional to the number of particles p ~ n n mgh / kt n o = e n = number of particles per unit volume c E / kt c o = e pot normalizing to the volume c = n/v c = concentration (which is probability) c ΔH / kt c o = e H = U + E pot in our case U is constant because T is constant
20 Boltzmann equation uses probabilities the relative populations of particles in states i and j separated by an energy gap p i ( Ei E j ) / kt p j = e the fraction of particles in each state: 2 3 ΔE 3-2 p j = t e j= 1 E e j E / kt j / kt 1 ΔE 2-1 t j= 1 e E j / kt - partition function
21 The energy difference here represents enthalpy H = U + W (internal energy +work) S = k lnw p i W - the number of micro-states p j ΔH ΔH p i ΔH / kt p j = e p i / p j e -1 = 0.37 e -2 = e -3 = 0.05 e -4 = e -5 = Free energy difference ΔG = ΔH - TΔS entropic advantage For two global states which can be ensembles of microstates: p i ΔG / kt p j = e
22 Multiplicity can be connected to the macroscopic world through definition of Entropy ΔS = dq dt There are W(U) states available to a physical system with energy U. Once the system has come to equilibrium, its entropy is defined as: S( U) = k ln[ W( U)] B k B = J/K!low of the heat into the system allows this system to occupy more states
23 Example of enthropy estimation An isothermal (du = 0) expansion. Initial state of a reversible expansion through absorption of heat, dq. dw = -dq 0, and ds rev = dq/t; Initial state for irreversible expansion caused by removal of a partition dividing the chamber dw = dq = du = 0, and ds irr > 0; The identical final state that is reached, with the final volume = twice initial volume.
24 The first experiment - reversible The change in piston position and molecular distribution is a result of the absorption of a heat at constant temperature. dq dw ds = = = T T pdv T For constant temperature (du(t) = 0) dq = dw For 1 mole of a dilute 2 molecular population, dv V ΔS = R = R ln = R ln pv=rt V V ds = RT T dv V and = R 1 1 dv V 2 2 V 2 = 2V 1
25 The second experiment irreversible For a large number of particles (1 mole), this redistribution is irreversable. If there are N identical molecules, and N j and N k in each compartment. The relative probability of having a given number N j and N k on each side of the partition N! is proportional to the number of different W j, k = ways, W j,k, in which this may occur. N j! Nk! The initial state all N particles N! on one side. W = W = = W 1 j, k N,0 1 N!0!
26 After the partition is removed, there are N /2 particles on each side. W j, k = W N / 2, N / 2 N! = 2 N! 2 N! = k ln N! 2 W From the Boltzmann expression for S, 1 ΔS = k ln 2 W2 since W 1 = 1 "Stirling s approximation" (large N) Substituting in the expression for ΔS ln N!= N ln N N N N ΔS = k N ln N N 2 ln = kn ln = kn N / 2 N ln2 N the gas constant R kn A ΔS = Rln2 The entropy change is the same when calculated from particle in the box experiments based on the view of Clausius or that of Boltzmann (entropy is a state function).
27 Entropy changes in the surroundings are determined by the heat flow. The size of S surr depends on temperature. An exothermic process is favored because by giving up heat, the entropy of the surroundings increases. ΔS surr = - ΔH T sys P, V The number of microstates and, therefore, the entropy tends to increase with increases in v Temperature. v Volume (gases) v The number of independently moving molecules. v The freedom of motion of molecules. v The more atoms in its molecules, the greater is the entropy of a substance.
28 Microstates and Macrostates Example 1: Gas in a Box Described by microstate {p i, q i }. The position and momentum of all particles. 3 positions, q i and 3 momentums, p i per particle. So in all (3+3) x10 23 microvariables. Macrostate described by 3 quantities T, V, N.
29 Microstates and Macrostates Example 2: Polymer Chain The microstate described a particular configuration of the chain on the lattice. If there are N links on the chain, there are N variables. Macrostate described by just the thermodynamic radius R of the chain.
30 Microstates and Macrostates Example 3: Gas Adsorption The gas binds on the binding sites on the substrate with favorable energy. The microstate described by a particular binding configuration of the gas on the substrate. Macrostate described by just the average number <N> of particles bound to the substrate.
31 Boltzmann Distribution Law Predicts How the System Be Distributed Among Available Microstates n i n 3 n 2 n 1 i ε i ε 3 ε 2 ε 1 p * i p p = ε i exp kbt ε i exp kbt i ( εi = exp kbt * ε i j ) * j ε i exp kbt = Z Boltzmann distribution law de2ines probability to 2ind a particle in microstate i Exponential distribution law de2ines relative occupancy of microstates i and j p ( 10kBT) 4.5*10-5 p ( 1kBT) 0.37 P 0.6 ε enrgy of a particle, k B T
32 Standard Molar Entropy Change for the System Standard molar entropy, S, is the entropy of one mole of a substance at standard state (usually at 25 C). Standard State - solids or liquids: pure element or compound at 1 atm and temperature of interest. - gases: pure gas behaving as an ideal gas at 1 atm and temperature of interest. Standard molar entropies of selected substances at 298 K. Standard entropy changes for chemical reactions: ΔS = n S (products) - m S (reactants) n,m - stoichiometric coefficients Standard entropies tend to increase with increasing molar mass.
33 Entropy of a substance increases with temperature. ΔS = ΔH T transition transition
34 The Second Law of Thermodynamics Once disorder is created, it cannot be destroyed. ΔS Universe = System + Surroundings universe = ΔS system + ΔS surroundin g 0 An irreversible (spontaneous) process, in the absence of external forces, a system moves toward a state of greater probability or greater disorder. The entropy of the Universe must increase. ΔS sys + ΔS surr > 0 In reversible processes, the system remains in, or very close to, a state of maximum probability (at equilibrium). The entropy of the Universe does not change. ΔS sys + ΔS surr = 0 ΔS sys = ΔS created + ΔS transferred
35 The meaning of 2nd law Energy is conserved in all processes, but not all energy conserving processes can happen! 100% heat work (process A) cannot occur, but 100% work heat (time reversal of A) can occur 2nd law refers to macroscopic processes only. Some macroscopic processes are not reversible!
36 ENTROPY & INFORMATION Second Law of Thermodynamics The entropy can only increase Over time, the information contained in an isolated system can only be destroyed
37 A physical system of particle reaches equilibrium when all information but the one that must be conserved (mass, momentum, energy) have vanished. A living system may be characterized as an ordered and information processing macroscopic system. A system can pass to the state with smaller entropy, ΔS syst < 0, if ΔS surr > 0. The existence of all living beings is based on entropy export. - The evolution is a process of such successive selforganizational steps. - Each step is driven by interactions, self-reproduction, mutation and selection.
38 Transmitting information Coding theory uses redundancy to transmit binary bits of information coding Transmission 010 Reconstruction errors (2nd Principle) 110 at reception (correction) 111
39 A cell is a big factory designed to duplicate the information contained in the DNA The cell divides before the information contained in the DNA fades away In this way, cell division and DNA duplication at fast pace, conserve the genetic information for millions of years.
40 Entropy & Information summary The Second Law of Thermodynamics leads to global loss of information. Systems out of equilibrium produce information to the cost of the environment. Information can be coded, transmitted, memorized, hidden, treated. Life is a way of producing information: genetic code, proteins, chemical signals, pattern formation, neurons, brain.
The Second Law of Thermodynamics (Chapter 4)
 The Second Law of Thermodynamics (Chapter 4) First Law: Energy of universe is constant: ΔE system = - ΔE surroundings Second Law: New variable, S, entropy. Changes in S, ΔS, tell us which processes made
The Second Law of Thermodynamics (Chapter 4) First Law: Energy of universe is constant: ΔE system = - ΔE surroundings Second Law: New variable, S, entropy. Changes in S, ΔS, tell us which processes made
Chapter 19 Chemical Thermodynamics
 Chapter 19 Chemical Thermodynamics Spontaneous Processes Entropy and the Second Law of Thermodynamics The Molecular Interpretation of Entropy Entropy Changes in Chemical Reactions Gibbs Free Energy Free
Chapter 19 Chemical Thermodynamics Spontaneous Processes Entropy and the Second Law of Thermodynamics The Molecular Interpretation of Entropy Entropy Changes in Chemical Reactions Gibbs Free Energy Free
Chapter 19 Chemical Thermodynamics Entropy and free energy
 Chapter 19 Chemical Thermodynamics Entropy and free energy Learning goals and key skills: Explain and apply the terms spontaneous process, reversible process, irreversible process, and isothermal process.
Chapter 19 Chemical Thermodynamics Entropy and free energy Learning goals and key skills: Explain and apply the terms spontaneous process, reversible process, irreversible process, and isothermal process.
Chapter 19 Chemical Thermodynamics
 Chapter 19 Chemical Thermodynamics Kinetics How fast a rxn. proceeds Equilibrium How far a rxn proceeds towards completion Thermodynamics Study of energy relationships & changes which occur during chemical
Chapter 19 Chemical Thermodynamics Kinetics How fast a rxn. proceeds Equilibrium How far a rxn proceeds towards completion Thermodynamics Study of energy relationships & changes which occur during chemical
MME 2010 METALLURGICAL THERMODYNAMICS II. Fundamentals of Thermodynamics for Systems of Constant Composition
 MME 2010 METALLURGICAL THERMODYNAMICS II Fundamentals of Thermodynamics for Systems of Constant Composition Thermodynamics addresses two types of problems: 1- Computation of energy difference between two
MME 2010 METALLURGICAL THERMODYNAMICS II Fundamentals of Thermodynamics for Systems of Constant Composition Thermodynamics addresses two types of problems: 1- Computation of energy difference between two
Chemical thermodynamics the area of chemistry that deals with energy relationships
 Chemistry: The Central Science Chapter 19: Chemical Thermodynamics Chemical thermodynamics the area of chemistry that deals with energy relationships 19.1: Spontaneous Processes First law of thermodynamics
Chemistry: The Central Science Chapter 19: Chemical Thermodynamics Chemical thermodynamics the area of chemistry that deals with energy relationships 19.1: Spontaneous Processes First law of thermodynamics
Thermodynamics: Free Energy and Entropy. Suggested Reading: Chapter 19
 Thermodynamics: Free Energy and Entropy Suggested Reading: Chapter 19 System and Surroundings System: An object or collection of objects being studied. Surroundings: Everything outside of the system. the
Thermodynamics: Free Energy and Entropy Suggested Reading: Chapter 19 System and Surroundings System: An object or collection of objects being studied. Surroundings: Everything outside of the system. the
Entropy A measure of molecular disorder
 Entropy A measure of molecular disorder Second Law uses Entropy, S, to identify spontaneous change. Restatement of Second Law: The entropy of the universe tends always towards a maximum (S universe > 0
Entropy A measure of molecular disorder Second Law uses Entropy, S, to identify spontaneous change. Restatement of Second Law: The entropy of the universe tends always towards a maximum (S universe > 0
Chemical Thermodynamics
 Chemical Thermodynamics David A. Katz Department of Chemistry Pima Community College Tucson, AZ 85709, USA First Law of Thermodynamics The First Law of Thermodynamics was expressed in the study of thermochemistry.
Chemical Thermodynamics David A. Katz Department of Chemistry Pima Community College Tucson, AZ 85709, USA First Law of Thermodynamics The First Law of Thermodynamics was expressed in the study of thermochemistry.
Chapter 3. The Second Law Fall Semester Physical Chemistry 1 (CHM2201)
 Chapter 3. The Second Law 2011 Fall Semester Physical Chemistry 1 (CHM2201) Contents The direction of spontaneous change 3.1 The dispersal of energy 3.2 The entropy 3.3 Entropy changes accompanying specific
Chapter 3. The Second Law 2011 Fall Semester Physical Chemistry 1 (CHM2201) Contents The direction of spontaneous change 3.1 The dispersal of energy 3.2 The entropy 3.3 Entropy changes accompanying specific
Chapter 19 Chemical Thermodynamics
 Chapter 19 Chemical Thermodynamics Kinetics How fast a rxn. proceeds Equilibrium How far a rxn proceeds towards completion Thermodynamics Study of energy relationships & changes which occur during chemical
Chapter 19 Chemical Thermodynamics Kinetics How fast a rxn. proceeds Equilibrium How far a rxn proceeds towards completion Thermodynamics Study of energy relationships & changes which occur during chemical
Practice Examinations Chem 393 Fall 2005 Time 1 hr 15 min for each set.
 Practice Examinations Chem 393 Fall 2005 Time 1 hr 15 min for each set. The symbols used here are as discussed in the class. Use scratch paper as needed. Do not give more than one answer for any question.
Practice Examinations Chem 393 Fall 2005 Time 1 hr 15 min for each set. The symbols used here are as discussed in the class. Use scratch paper as needed. Do not give more than one answer for any question.
Chapter 19. Chemical Thermodynamics
 Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 19 John D. Bookstaver St. Charles Community College Cottleville, MO First Law of You will
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 19 John D. Bookstaver St. Charles Community College Cottleville, MO First Law of You will
Second law of thermodynamics
 Second law of thermodynamics It is known from everyday life that nature does the most probable thing when nothing prevents that For example it rains at cool weather because the liquid phase has less energy
Second law of thermodynamics It is known from everyday life that nature does the most probable thing when nothing prevents that For example it rains at cool weather because the liquid phase has less energy
CHEMICAL ENGINEERING THERMODYNAMICS. Andrew S. Rosen
 CHEMICAL ENGINEERING THERMODYNAMICS Andrew S. Rosen SYMBOL DICTIONARY 1 TABLE OF CONTENTS Symbol Dictionary... 3 1. Measured Thermodynamic Properties and Other Basic Concepts... 5 1.1 Preliminary Concepts
CHEMICAL ENGINEERING THERMODYNAMICS Andrew S. Rosen SYMBOL DICTIONARY 1 TABLE OF CONTENTS Symbol Dictionary... 3 1. Measured Thermodynamic Properties and Other Basic Concepts... 5 1.1 Preliminary Concepts
Chapter 19. Chemical Thermodynamics. Chemical Thermodynamics
 Chapter 19 Enthalpy A thermodynamic quantity that equal to the internal energy of a system plus the product of its volume and pressure exerted on it by its surroundings; Enthalpy is the amount of energy
Chapter 19 Enthalpy A thermodynamic quantity that equal to the internal energy of a system plus the product of its volume and pressure exerted on it by its surroundings; Enthalpy is the amount of energy
Chapter Seventeen Thermodynamics: Spontaneity, Entropy, and Free Energy
 1 Thermodynamics: Spontaneity, Entropy, and Free Energy 2 Introductory Concepts Thermodynamics examines the relationship between heat (q) and work (w) Spontaneity is the notion of whether or not a process
1 Thermodynamics: Spontaneity, Entropy, and Free Energy 2 Introductory Concepts Thermodynamics examines the relationship between heat (q) and work (w) Spontaneity is the notion of whether or not a process
First Law Limitations
 First Law Limitations First Law: During any process, the energy of the universe is constant. du du du ZERO!!! universe sys surroundings Any energy transfer between system and surroundings is accomplished
First Law Limitations First Law: During any process, the energy of the universe is constant. du du du ZERO!!! universe sys surroundings Any energy transfer between system and surroundings is accomplished
Chapter 19 Chemical Thermodynamics Entropy and free energy
 Chapter 19 Chemical Thermodynamics Entropy and free energy Learning goals and key skills: Understand the meaning of spontaneous process, reversible process, irreversible process, and isothermal process.
Chapter 19 Chemical Thermodynamics Entropy and free energy Learning goals and key skills: Understand the meaning of spontaneous process, reversible process, irreversible process, and isothermal process.
Chemical Thermodynamics
 Page III-16-1 / Chapter Sixteen Lecture Notes Chemical Thermodynamics Thermodynamics and Kinetics Chapter 16 Chemistry 223 Professor Michael Russell How to predict if a reaction can occur, given enough
Page III-16-1 / Chapter Sixteen Lecture Notes Chemical Thermodynamics Thermodynamics and Kinetics Chapter 16 Chemistry 223 Professor Michael Russell How to predict if a reaction can occur, given enough
Chapter Eighteen. Thermodynamics
 Chapter Eighteen Thermodynamics 1 Thermodynamics Study of energy changes during observed processes Purpose: To predict spontaneity of a process Spontaneity: Will process go without assistance? Depends
Chapter Eighteen Thermodynamics 1 Thermodynamics Study of energy changes during observed processes Purpose: To predict spontaneity of a process Spontaneity: Will process go without assistance? Depends
Entropy, free energy and equilibrium. Spontaneity Entropy Free energy and equilibrium
 Entropy, free energy and equilibrium Spontaneity Entropy Free energy and equilibrium Learning objectives Discuss what is meant by spontaneity Discuss energy dispersal and its relevance to spontaneity Describe
Entropy, free energy and equilibrium Spontaneity Entropy Free energy and equilibrium Learning objectives Discuss what is meant by spontaneity Discuss energy dispersal and its relevance to spontaneity Describe
Chemistry 123: Physical and Organic Chemistry Topic 2: Thermochemistry
 Recall the equation. w = -PΔV = -(1.20 atm)(1.02 L)( = -1.24 10 2 J -101 J 1 L atm Where did the conversion factor come from? Compare two versions of the gas constant and calculate. 8.3145 J/mol K 0.082057
Recall the equation. w = -PΔV = -(1.20 atm)(1.02 L)( = -1.24 10 2 J -101 J 1 L atm Where did the conversion factor come from? Compare two versions of the gas constant and calculate. 8.3145 J/mol K 0.082057
Chapter 17. Free Energy and Thermodynamics. Chapter 17 Lecture Lecture Presentation. Sherril Soman Grand Valley State University
 Chapter 17 Lecture Lecture Presentation Chapter 17 Free Energy and Thermodynamics Sherril Soman Grand Valley State University First Law of Thermodynamics You can t win! The first law of thermodynamics
Chapter 17 Lecture Lecture Presentation Chapter 17 Free Energy and Thermodynamics Sherril Soman Grand Valley State University First Law of Thermodynamics You can t win! The first law of thermodynamics
Ch. 19 Entropy and Free Energy: Spontaneous Change
 Ch. 19 Entropy and Free Energy: Spontaneous Change 19-1 Spontaneity: The Meaning of Spontaneous Change 19-2 The Concept of Entropy 19-3 Evaluating Entropy and Entropy Changes 19-4 Criteria for Spontaneous
Ch. 19 Entropy and Free Energy: Spontaneous Change 19-1 Spontaneity: The Meaning of Spontaneous Change 19-2 The Concept of Entropy 19-3 Evaluating Entropy and Entropy Changes 19-4 Criteria for Spontaneous
Disorder and Entropy. Disorder and Entropy
 Disorder and Entropy Suppose I have 10 particles that can be in one of two states either the blue state or the red state. How many different ways can we arrange those particles among the states? All particles
Disorder and Entropy Suppose I have 10 particles that can be in one of two states either the blue state or the red state. How many different ways can we arrange those particles among the states? All particles
4/19/2016. Chapter 17 Free Energy and Thermodynamics. First Law of Thermodynamics. First Law of Thermodynamics. The Energy Tax.
 Chemistry: A Molecular Approach, 2nd Ed. Nivaldo Tro First Law of Thermodynamics Chapter 17 Free Energy and Thermodynamics You can t win! First Law of Thermodynamics: Energy cannot be created or destroyed
Chemistry: A Molecular Approach, 2nd Ed. Nivaldo Tro First Law of Thermodynamics Chapter 17 Free Energy and Thermodynamics You can t win! First Law of Thermodynamics: Energy cannot be created or destroyed
THE SECOND LAW OF THERMODYNAMICS. Professor Benjamin G. Levine CEM 182H Lecture 5
 THE SECOND LAW OF THERMODYNAMICS Professor Benjamin G. Levine CEM 182H Lecture 5 Chemical Equilibrium N 2 + 3 H 2 2 NH 3 Chemical reactions go in both directions Systems started from any initial state
THE SECOND LAW OF THERMODYNAMICS Professor Benjamin G. Levine CEM 182H Lecture 5 Chemical Equilibrium N 2 + 3 H 2 2 NH 3 Chemical reactions go in both directions Systems started from any initial state
OCN 623: Thermodynamic Laws & Gibbs Free Energy. or how to predict chemical reactions without doing experiments
 OCN 623: Thermodynamic Laws & Gibbs Free Energy or how to predict chemical reactions without doing experiments Definitions Extensive properties Depend on the amount of material e.g. # of moles, mass or
OCN 623: Thermodynamic Laws & Gibbs Free Energy or how to predict chemical reactions without doing experiments Definitions Extensive properties Depend on the amount of material e.g. # of moles, mass or
Thermodynamic Third class Dr. Arkan J. Hadi
 5.5 ENTROPY CHANGES OF AN IDEAL GAS For one mole or a unit mass of fluid undergoing a mechanically reversible process in a closed system, the first law, Eq. (2.8), becomes: Differentiation of the defining
5.5 ENTROPY CHANGES OF AN IDEAL GAS For one mole or a unit mass of fluid undergoing a mechanically reversible process in a closed system, the first law, Eq. (2.8), becomes: Differentiation of the defining
CHEMICAL THERMODYNAMICS. Nature of Energy. ΔE = q + w. w = PΔV
 CHEMICAL HERMODYNAMICS Nature of Energy hermodynamics hermochemistry Energy (E) Work (w) Heat (q) Some Definitions Study the transformation of energy from one form to another during physical and chemical
CHEMICAL HERMODYNAMICS Nature of Energy hermodynamics hermochemistry Energy (E) Work (w) Heat (q) Some Definitions Study the transformation of energy from one form to another during physical and chemical
ENTROPY HEAT HEAT FLOW. Enthalpy 3/24/16. Chemical Thermodynamics. Thermodynamics vs. Kinetics
 Chemical Thermodynamics The chemistry that deals with energy exchange, entropy, and the spontaneity of a chemical process. HEAT The energy that flows into or out of system because of a difference in temperature
Chemical Thermodynamics The chemistry that deals with energy exchange, entropy, and the spontaneity of a chemical process. HEAT The energy that flows into or out of system because of a difference in temperature
Irreversible Processes
 Irreversible Processes Examples: Block sliding on table comes to rest due to friction: KE converted to heat. Heat flows from hot object to cold object. Air flows into an evacuated chamber. Reverse process
Irreversible Processes Examples: Block sliding on table comes to rest due to friction: KE converted to heat. Heat flows from hot object to cold object. Air flows into an evacuated chamber. Reverse process
8 A Microscopic Approach to Entropy
 8 A Microscopic Approach to Entropy The thermodynamic approach www.xtremepapers.com Internal energy and enthalpy When energy is added to a body, its internal energy U increases by an amount ΔU. The energy
8 A Microscopic Approach to Entropy The thermodynamic approach www.xtremepapers.com Internal energy and enthalpy When energy is added to a body, its internal energy U increases by an amount ΔU. The energy
Thermodynamic Laws, Gibbs Free Energy & pe/ph
 Thermodynamic Laws, Gibbs Free Energy & pe/ph or how to predict chemical reactions without doing experiments OCN 623 Chemical Oceanography Definitions Extensive properties Depend on the amount of material
Thermodynamic Laws, Gibbs Free Energy & pe/ph or how to predict chemical reactions without doing experiments OCN 623 Chemical Oceanography Definitions Extensive properties Depend on the amount of material
Chem Lecture Notes 6 Fall 2013 Second law
 Chem 340 - Lecture Notes 6 Fall 2013 Second law In the first law, we determined energies, enthalpies heat and work for any process from an initial to final state. We could know if the system did work or
Chem 340 - Lecture Notes 6 Fall 2013 Second law In the first law, we determined energies, enthalpies heat and work for any process from an initial to final state. We could know if the system did work or
UNIT 15: THERMODYNAMICS
 UNIT 15: THERMODYNAMICS ENTHALPY, DH ENTROPY, DS GIBBS FREE ENERGY, DG ENTHALPY, DH Energy Changes in Reactions Heat is the transfer of thermal energy between two bodies that are at different temperatures.
UNIT 15: THERMODYNAMICS ENTHALPY, DH ENTROPY, DS GIBBS FREE ENERGY, DG ENTHALPY, DH Energy Changes in Reactions Heat is the transfer of thermal energy between two bodies that are at different temperatures.
Chapter 19. Chemical Thermodynamics
 Chapter 19. Chemical Thermodynamics 19.1 Spontaneous Processes Chemical thermodynamics is concerned with energy relationships in chemical reactions. We consider enthalpy and we also consider entropy in
Chapter 19. Chemical Thermodynamics 19.1 Spontaneous Processes Chemical thermodynamics is concerned with energy relationships in chemical reactions. We consider enthalpy and we also consider entropy in
Chemical Thermodynamics. Chapter 18
 Chemical Thermodynamics Chapter 18 Thermodynamics Spontaneous Processes Entropy and Second Law of Thermodynamics Entropy Changes Gibbs Free Energy Free Energy and Temperature Free Energy and Equilibrium
Chemical Thermodynamics Chapter 18 Thermodynamics Spontaneous Processes Entropy and Second Law of Thermodynamics Entropy Changes Gibbs Free Energy Free Energy and Temperature Free Energy and Equilibrium
THERMODYNAMICS. Dr. Sapna Gupta
 THERMODYNAMICS Dr. Sapna Gupta FIRST LAW OF THERMODYNAMICS Thermodynamics is the study of heat and other forms of energy involved in chemical or physical processes. First Law of Thermodynamics Energy cannot
THERMODYNAMICS Dr. Sapna Gupta FIRST LAW OF THERMODYNAMICS Thermodynamics is the study of heat and other forms of energy involved in chemical or physical processes. First Law of Thermodynamics Energy cannot
3/30/2017. Section 17.1 Spontaneous Processes and Entropy Thermodynamics vs. Kinetics. Chapter 17. Spontaneity, Entropy, and Free Energy
 Chapter 17 Spontaneity, Entropy, and Thermodynamics vs. Kinetics Domain of Kinetics Rate of a reaction depends on the pathway from reactants to products. Thermodynamics tells us whether a reaction is spontaneous
Chapter 17 Spontaneity, Entropy, and Thermodynamics vs. Kinetics Domain of Kinetics Rate of a reaction depends on the pathway from reactants to products. Thermodynamics tells us whether a reaction is spontaneous
10, Physical Chemistry- III (Classical Thermodynamics, Non-Equilibrium Thermodynamics, Surface chemistry, Fast kinetics)
 Subect Chemistry Paper No and Title Module No and Title Module Tag 0, Physical Chemistry- III (Classical Thermodynamics, Non-Equilibrium Thermodynamics, Surface chemistry, Fast kinetics) 0, Free energy
Subect Chemistry Paper No and Title Module No and Title Module Tag 0, Physical Chemistry- III (Classical Thermodynamics, Non-Equilibrium Thermodynamics, Surface chemistry, Fast kinetics) 0, Free energy
So far changes in the state of systems that occur within the restrictions of the first law of thermodynamics were considered:
 Entropy So far changes in the state of systems that occur within the restrictions of the first law of thermodynamics were considered: Energy is transferred from one state to another by any possible forms,
Entropy So far changes in the state of systems that occur within the restrictions of the first law of thermodynamics were considered: Energy is transferred from one state to another by any possible forms,
Chpt 19: Chemical. Thermodynamics. Thermodynamics
 CEM 152 1 Reaction Spontaneity Can we learn anything about the probability of a reaction occurring based on reaction enthaplies? in general, a large, negative reaction enthalpy is indicative of a spontaneous
CEM 152 1 Reaction Spontaneity Can we learn anything about the probability of a reaction occurring based on reaction enthaplies? in general, a large, negative reaction enthalpy is indicative of a spontaneous
Solutions to Problem Set 6
 Solutions to Problem Set 6 1. non- ideal gas, 1 mol 20.0 L 300 K 40.0 L 300 K isothermal, reversible Equation of state: (a)b is a constant independent of T Given du = ( U/ T) V dt + ( U/ V) T dv U = U(T,V)
Solutions to Problem Set 6 1. non- ideal gas, 1 mol 20.0 L 300 K 40.0 L 300 K isothermal, reversible Equation of state: (a)b is a constant independent of T Given du = ( U/ T) V dt + ( U/ V) T dv U = U(T,V)
CHEM Thermodynamics. Entropy, S
 hermodynamics Change in Change in Entropy, S Entropy, S Entropy is the measure of dispersal. he natural spontaneous direction of any process is toward greater dispersal of matter and of energy. Dispersal
hermodynamics Change in Change in Entropy, S Entropy, S Entropy is the measure of dispersal. he natural spontaneous direction of any process is toward greater dispersal of matter and of energy. Dispersal
 The underlying prerequisite to the application of thermodynamic principles to natural systems is that the system under consideration should be at equilibrium. http://eps.mcgill.ca/~courses/c220/ Reversible
The underlying prerequisite to the application of thermodynamic principles to natural systems is that the system under consideration should be at equilibrium. http://eps.mcgill.ca/~courses/c220/ Reversible
CHM 112 Chapter 16 Thermodynamics Study Guide
 CHM 112 Chapter 16 Thermodynamics Study Guide Remember from Chapter 5: Thermodynamics deals with energy relationships in chemical reactions Know the definitions of system, surroundings, exothermic process,
CHM 112 Chapter 16 Thermodynamics Study Guide Remember from Chapter 5: Thermodynamics deals with energy relationships in chemical reactions Know the definitions of system, surroundings, exothermic process,
Physical Biochemistry. Kwan Hee Lee, Ph.D. Handong Global University
 Physical Biochemistry Kwan Hee Lee, Ph.D. Handong Global University Week 3 CHAPTER 2 The Second Law: Entropy of the Universe increases What is entropy Definition: measure of disorder The greater the disorder,
Physical Biochemistry Kwan Hee Lee, Ph.D. Handong Global University Week 3 CHAPTER 2 The Second Law: Entropy of the Universe increases What is entropy Definition: measure of disorder The greater the disorder,
Thermodynamics. Chem 36 Spring The study of energy changes which accompany physical and chemical processes
 Thermodynamics Chem 36 Spring 2002 Thermodynamics The study of energy changes which accompany physical and chemical processes Why do we care? -will a reaction proceed spontaneously? -if so, to what extent?
Thermodynamics Chem 36 Spring 2002 Thermodynamics The study of energy changes which accompany physical and chemical processes Why do we care? -will a reaction proceed spontaneously? -if so, to what extent?
Physics 408 Final Exam
 Physics 408 Final Exam Name You are graded on your work (with partial credit where it is deserved) so please do not just write down answers with no explanation (or skip important steps)! Please give clear,
Physics 408 Final Exam Name You are graded on your work (with partial credit where it is deserved) so please do not just write down answers with no explanation (or skip important steps)! Please give clear,
Classical Thermodynamics. Dr. Massimo Mella School of Chemistry Cardiff University
 Classical Thermodynamics Dr. Massimo Mella School of Chemistry Cardiff University E-mail:MellaM@cardiff.ac.uk The background The field of Thermodynamics emerged as a consequence of the necessity to understand
Classical Thermodynamics Dr. Massimo Mella School of Chemistry Cardiff University E-mail:MellaM@cardiff.ac.uk The background The field of Thermodynamics emerged as a consequence of the necessity to understand
Lecture 14. Entropy relationship to heat
 Lecture 14 Entropy relationship to heat Reading: Lecture 14, today: Chapter 7: 7.20 end Lecture 15, Wednesday: Ref. (2) 2/29/16 1 Hemoglobin and probability Oxygen binding molecule. Its quaternary structure
Lecture 14 Entropy relationship to heat Reading: Lecture 14, today: Chapter 7: 7.20 end Lecture 15, Wednesday: Ref. (2) 2/29/16 1 Hemoglobin and probability Oxygen binding molecule. Its quaternary structure
Problem: Calculate the entropy change that results from mixing 54.0 g of water at 280 K with 27.0 g of water at 360 K in a vessel whose walls are
 Problem: Calculate the entropy change that results from mixing 54.0 g of water at 280 K with 27.0 g of water at 360 K in a vessel whose walls are perfectly insulated from the surroundings. Is this a spontaneous
Problem: Calculate the entropy change that results from mixing 54.0 g of water at 280 K with 27.0 g of water at 360 K in a vessel whose walls are perfectly insulated from the surroundings. Is this a spontaneous
where R = universal gas constant R = PV/nT R = atm L mol R = atm dm 3 mol 1 K 1 R = J mol 1 K 1 (SI unit)
 Ideal Gas Law PV = nrt where R = universal gas constant R = PV/nT R = 0.0821 atm L mol 1 K 1 R = 0.0821 atm dm 3 mol 1 K 1 R = 8.314 J mol 1 K 1 (SI unit) Standard molar volume = 22.4 L mol 1 at 0 C and
Ideal Gas Law PV = nrt where R = universal gas constant R = PV/nT R = 0.0821 atm L mol 1 K 1 R = 0.0821 atm dm 3 mol 1 K 1 R = 8.314 J mol 1 K 1 (SI unit) Standard molar volume = 22.4 L mol 1 at 0 C and
Chapter 27. Energy and Disorder
 Chapter 27 Energy and Disorder Why Reactions Occur Exothermic Rxns - Take place spontaneously Go from high energy to low energy Downhill Endothermic Rxns. - Not usually spontaneous Go from low energy to
Chapter 27 Energy and Disorder Why Reactions Occur Exothermic Rxns - Take place spontaneously Go from high energy to low energy Downhill Endothermic Rxns. - Not usually spontaneous Go from low energy to
Hence. The second law describes the direction of energy transfer in spontaneous processes
 * Heat and Work The first law of thermodynamics states that: Although energy has many forms, the total quantity of energy is constant. When energy disappears in one form, it appears simultaneously in other
* Heat and Work The first law of thermodynamics states that: Although energy has many forms, the total quantity of energy is constant. When energy disappears in one form, it appears simultaneously in other
Chapter 19. Chemical Thermodynamics
 Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 19 John D. Bookstaver St. Charles Community College Cottleville, MO First Law of You will
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 19 John D. Bookstaver St. Charles Community College Cottleville, MO First Law of You will
Physics 132- Fundamentals of Physics for Biologists II. Statistical Physics and Thermodynamics
 Physics 132- Fundamentals of Physics for Biologists II Statistical Physics and Thermodynamics QUIZ 2 25 Quiz 2 20 Number of Students 15 10 5 AVG: STDEV: 5.15 2.17 0 0 2 4 6 8 10 Score 1. (4 pts) A 200
Physics 132- Fundamentals of Physics for Biologists II Statistical Physics and Thermodynamics QUIZ 2 25 Quiz 2 20 Number of Students 15 10 5 AVG: STDEV: 5.15 2.17 0 0 2 4 6 8 10 Score 1. (4 pts) A 200
Chapter 17.3 Entropy and Spontaneity Objectives Define entropy and examine its statistical nature Predict the sign of entropy changes for phase
 Chapter 17.3 Entropy and Spontaneity Objectives Define entropy and examine its statistical nature Predict the sign of entropy changes for phase changes Apply the second law of thermodynamics to chemical
Chapter 17.3 Entropy and Spontaneity Objectives Define entropy and examine its statistical nature Predict the sign of entropy changes for phase changes Apply the second law of thermodynamics to chemical
Module 5 : Electrochemistry Lecture 21 : Review Of Thermodynamics
 Module 5 : Electrochemistry Lecture 21 : Review Of Thermodynamics Objectives In this Lecture you will learn the following The need for studying thermodynamics to understand chemical and biological processes.
Module 5 : Electrochemistry Lecture 21 : Review Of Thermodynamics Objectives In this Lecture you will learn the following The need for studying thermodynamics to understand chemical and biological processes.
Gibb s Free Energy. This value represents the maximum amount of useful work (non PV-work) that can be obtained by a system.
 Gibb s Free Energy 1. What is Gibb s free energy? What is its symbol? This value represents the maximum amount of useful work (non PV-work) that can be obtained by a system. It is symbolized by G. We only
Gibb s Free Energy 1. What is Gibb s free energy? What is its symbol? This value represents the maximum amount of useful work (non PV-work) that can be obtained by a system. It is symbolized by G. We only
Physics 4311 ANSWERS: Sample Problems for Exam #2. (1)Short answer questions:
 (1)Short answer questions: Physics 4311 ANSWERS: Sample Problems for Exam #2 (a) Consider an isolated system that consists of several subsystems interacting thermally and mechanically with each other.
(1)Short answer questions: Physics 4311 ANSWERS: Sample Problems for Exam #2 (a) Consider an isolated system that consists of several subsystems interacting thermally and mechanically with each other.
Thermodynamics II. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Thermodynamics II Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Spontaneous Physical and Chemical Processes A waterfall runs downhill A lump of sugar dissolves
Thermodynamics II Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Spontaneous Physical and Chemical Processes A waterfall runs downhill A lump of sugar dissolves
Adiabatic Expansion (DQ = 0)
 Adiabatic Expansion (DQ = 0) Occurs if: change is made sufficiently quickly and/or with good thermal isolation. Governing formula: PV g = constant where g = C P /C V Adiabat P Isotherms V Because PV/T
Adiabatic Expansion (DQ = 0) Occurs if: change is made sufficiently quickly and/or with good thermal isolation. Governing formula: PV g = constant where g = C P /C V Adiabat P Isotherms V Because PV/T
In previous chapters we have studied: Why does a change occur in the first place? Methane burns but not the reverse CH 4 + 2O 2 CO 2 + 2H 2 O
 Chapter 19. Spontaneous Change: Entropy and Free Energy In previous chapters we have studied: How fast does the change occur How is rate affected by concentration and temperature How much product will
Chapter 19. Spontaneous Change: Entropy and Free Energy In previous chapters we have studied: How fast does the change occur How is rate affected by concentration and temperature How much product will
SCORING. The exam consists of 5 questions totaling 100 points as broken down in this table:
 UNIVERSITY OF CALIFORNIA, BERKELEY CHEM C130/MCB C100A MIDTERM EXAMINATION #2 OCTOBER 20, 2016 INSTRUCTORS: John Kuriyan and David Savage THE TIME LIMIT FOR THIS EXAMINATION: 1 HOUR 50 MINUTES SIGNATURE:
UNIVERSITY OF CALIFORNIA, BERKELEY CHEM C130/MCB C100A MIDTERM EXAMINATION #2 OCTOBER 20, 2016 INSTRUCTORS: John Kuriyan and David Savage THE TIME LIMIT FOR THIS EXAMINATION: 1 HOUR 50 MINUTES SIGNATURE:
Entropy, Free Energy, and Equilibrium
 Entropy, Free Energy, and Equilibrium Chapter 17 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Spontaneous Physical and Chemical Processes A waterfall runs
Entropy, Free Energy, and Equilibrium Chapter 17 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Spontaneous Physical and Chemical Processes A waterfall runs
Concentrating on the system
 Concentrating on the system Entropy is the basic concept for discussing the direction of natural change, but to use it we have to analyze changes in both the system and its surroundings. We have seen that
Concentrating on the system Entropy is the basic concept for discussing the direction of natural change, but to use it we have to analyze changes in both the system and its surroundings. We have seen that
Chemistry 2000 Lecture 9: Entropy and the second law of thermodynamics
 Chemistry 2000 Lecture 9: Entropy and the second law of thermodynamics Marc R. Roussel January 23, 2018 Marc R. Roussel Entropy and the second law January 23, 2018 1 / 29 States in thermodynamics The thermodynamic
Chemistry 2000 Lecture 9: Entropy and the second law of thermodynamics Marc R. Roussel January 23, 2018 Marc R. Roussel Entropy and the second law January 23, 2018 1 / 29 States in thermodynamics The thermodynamic
S = k log W CHEM Thermodynamics. Change in Entropy, S. Entropy, S. Entropy, S S = S 2 -S 1. Entropy is the measure of dispersal.
 , S is the measure of dispersal. The natural spontaneous direction of any process is toward greater dispersal of matter and of energy. Dispersal of matter: Thermodynamics We analyze the constraints on
, S is the measure of dispersal. The natural spontaneous direction of any process is toward greater dispersal of matter and of energy. Dispersal of matter: Thermodynamics We analyze the constraints on
Theme Music: Duke Ellington Take the A Train Cartoon: Lynn Johnson For Better or for Worse
 March 4, 2016 Prof. E. F. Redish Theme Music: Duke Ellington Take the A Train Cartoon: Lynn Johnson For Better or for Worse 1 Foothold principles: Newton s Laws Newton 0: An object responds only to the
March 4, 2016 Prof. E. F. Redish Theme Music: Duke Ellington Take the A Train Cartoon: Lynn Johnson For Better or for Worse 1 Foothold principles: Newton s Laws Newton 0: An object responds only to the
1. Thermodynamics 1.1. A macroscopic view of matter
 1. Thermodynamics 1.1. A macroscopic view of matter Intensive: independent of the amount of substance, e.g. temperature,pressure. Extensive: depends on the amount of substance, e.g. internal energy, enthalpy.
1. Thermodynamics 1.1. A macroscopic view of matter Intensive: independent of the amount of substance, e.g. temperature,pressure. Extensive: depends on the amount of substance, e.g. internal energy, enthalpy.
THERMODYNAMICS. Extensive properties Intensive properties
 Thermodynamics The branch of chemistry deals with the energy change associated with chemical reactions is called chemical thermodynamics. System and surrounding A system may be defined as the specified
Thermodynamics The branch of chemistry deals with the energy change associated with chemical reactions is called chemical thermodynamics. System and surrounding A system may be defined as the specified
Chapter 5. Simple Mixtures Fall Semester Physical Chemistry 1 (CHM2201)
 Chapter 5. Simple Mixtures 2011 Fall Semester Physical Chemistry 1 (CHM2201) Contents The thermodynamic description of mixtures 5.1 Partial molar quantities 5.2 The thermodynamic of Mixing 5.3 The chemical
Chapter 5. Simple Mixtures 2011 Fall Semester Physical Chemistry 1 (CHM2201) Contents The thermodynamic description of mixtures 5.1 Partial molar quantities 5.2 The thermodynamic of Mixing 5.3 The chemical
Entropy and Free Energy
 Page 1 Entropy and Free Energy How to predict if a reaction can occur at a reasonable rate? KINEICS Chapter 17 How to predict if a reaction can occur, given enough time? HERMODYNAMICS 1 Objectives Spontaneity
Page 1 Entropy and Free Energy How to predict if a reaction can occur at a reasonable rate? KINEICS Chapter 17 How to predict if a reaction can occur, given enough time? HERMODYNAMICS 1 Objectives Spontaneity
Spontaneity, Entropy, and Free Energy
 Spontaneity, Entropy, and Free Energy A ball rolls spontaneously down a hill but not up. Spontaneous Processes A reaction that will occur without outside intervention; product favored Most reactants are
Spontaneity, Entropy, and Free Energy A ball rolls spontaneously down a hill but not up. Spontaneous Processes A reaction that will occur without outside intervention; product favored Most reactants are
S = k log W 11/8/2016 CHEM Thermodynamics. Change in Entropy, S. Entropy, S. Entropy, S S = S 2 -S 1. Entropy is the measure of dispersal.
 Entropy is the measure of dispersal. The natural spontaneous direction of any process is toward greater dispersal of matter and of energy. Dispersal of matter: Thermodynamics We analyze the constraints
Entropy is the measure of dispersal. The natural spontaneous direction of any process is toward greater dispersal of matter and of energy. Dispersal of matter: Thermodynamics We analyze the constraints
Exergy. What s it all about? Thermodynamics and Exergy
 Exergy What s it all about? Thermodynamics and Exergy Quality of Energy General recognition that some forms of energy are more useful than others Electricity can be used for light, heat, cooling, mechanical
Exergy What s it all about? Thermodynamics and Exergy Quality of Energy General recognition that some forms of energy are more useful than others Electricity can be used for light, heat, cooling, mechanical
du = δq + δw = δq rev + δw rev = δq rev + 0
 Chem 4501 Introduction to hermodynamics, 3 Credits Kinetics, and Statistical Mechanics Module Number 6 Active Learning Answers and Optional Problems/Solutions 1. McQuarrie and Simon, 6-6. Paraphrase: Compute
Chem 4501 Introduction to hermodynamics, 3 Credits Kinetics, and Statistical Mechanics Module Number 6 Active Learning Answers and Optional Problems/Solutions 1. McQuarrie and Simon, 6-6. Paraphrase: Compute
Thermodynamic Processes and Thermochemistry
 General Chemistry Thermodynamic Processes and Thermochemistry 박준원교수 ( 포항공과대학교화학과 ) 이번시간에는! Systems, states, and processes The first law of thermodynamics: internal energy, work, and heat Heat capacity,
General Chemistry Thermodynamic Processes and Thermochemistry 박준원교수 ( 포항공과대학교화학과 ) 이번시간에는! Systems, states, and processes The first law of thermodynamics: internal energy, work, and heat Heat capacity,
THERMODYNAMICS I. TERMS AND DEFINITIONS A. Review of Definitions 1. Thermodynamics = Study of the exchange of heat, energy and work between a system
 THERMODYNAMICS I. TERMS AND DEFINITIONS A. Review of Definitions 1. Thermodynamics = Study of the exchange of heat, energy and work between a system and its surroundings. a. System = That part of universe
THERMODYNAMICS I. TERMS AND DEFINITIONS A. Review of Definitions 1. Thermodynamics = Study of the exchange of heat, energy and work between a system and its surroundings. a. System = That part of universe
Thermodynamics and Phase Transitions in Minerals
 Studiengang Geowissenschaften M.Sc. Wintersemester 2004/05 Thermodynamics and Phase Transitions in Minerals Victor Vinograd & Andrew Putnis Basic thermodynamic concepts One of the central themes in Mineralogy
Studiengang Geowissenschaften M.Sc. Wintersemester 2004/05 Thermodynamics and Phase Transitions in Minerals Victor Vinograd & Andrew Putnis Basic thermodynamic concepts One of the central themes in Mineralogy
Chapter Notes Subject: Chemistry Class: XI Chapter: Thermodynamics Top concepts
 Chapter Notes Subject: Chemistry Class: XI Chapter: Thermodynamics Top concepts 1. The branch of science which deals with study of different forms of energy and their interconversion is called thermodynamics.
Chapter Notes Subject: Chemistry Class: XI Chapter: Thermodynamics Top concepts 1. The branch of science which deals with study of different forms of energy and their interconversion is called thermodynamics.
Entropy and the Second and Third Laws of Thermodynamics
 CHAPTER 5 Entropy and the Second and Third Laws of Thermodynamics Key Points Entropy, S, is a state function that predicts the direction of natural, or spontaneous, change. Entropy increases for a spontaneous
CHAPTER 5 Entropy and the Second and Third Laws of Thermodynamics Key Points Entropy, S, is a state function that predicts the direction of natural, or spontaneous, change. Entropy increases for a spontaneous
For more info visit
 Basic Terminology: Terms System Open System Closed System Isolated system Surroundings Boundary State variables State Functions Intensive properties Extensive properties Process Isothermal process Isobaric
Basic Terminology: Terms System Open System Closed System Isolated system Surroundings Boundary State variables State Functions Intensive properties Extensive properties Process Isothermal process Isobaric
Unit 12. Thermochemistry
 Unit 12 Thermochemistry A reaction is spontaneous if it will occur without a continuous input of energy However, it may require an initial input of energy to get it started (activation energy) For Thermochemistry
Unit 12 Thermochemistry A reaction is spontaneous if it will occur without a continuous input of energy However, it may require an initial input of energy to get it started (activation energy) For Thermochemistry
To what extent a reaction will achieve completion?
 Reactants Products To what extent a reaction will achieve completion? - The foundation of the concept of equilibrium relies on THERMODYNAMICS. - How fast will it achieve equilibrium? This question requires
Reactants Products To what extent a reaction will achieve completion? - The foundation of the concept of equilibrium relies on THERMODYNAMICS. - How fast will it achieve equilibrium? This question requires
Ch 17 Free Energy and Thermodynamics - Spontaneity of Reaction
 Ch 17 Free Energy and Thermodynamics - Spontaneity of Reaction Modified by Dr. Cheng-Yu Lai spontaneous nonspontaneous Spontaneous Processes Processes that are spontaneous in one direction are nonspontaneous
Ch 17 Free Energy and Thermodynamics - Spontaneity of Reaction Modified by Dr. Cheng-Yu Lai spontaneous nonspontaneous Spontaneous Processes Processes that are spontaneous in one direction are nonspontaneous
Stuff 1st Law of Thermodynamics First Law Differential Form Total Differential Total Differential
 Stuff ---onight: Lecture 4 July ---Assignment has been posted. ---Presentation Assignment posted. --Some more thermodynamics and then problem solving in class for Assignment #. --Next week: Free Energy
Stuff ---onight: Lecture 4 July ---Assignment has been posted. ---Presentation Assignment posted. --Some more thermodynamics and then problem solving in class for Assignment #. --Next week: Free Energy
Thermodynamics. Thermodynamics of Chemical Reactions. Enthalpy change
 Thermodynamics 1 st law (Cons of Energy) Deals with changes in energy Energy in chemical systems Total energy of an isolated system is constant Total energy = Potential energy + kinetic energy E p mgh
Thermodynamics 1 st law (Cons of Energy) Deals with changes in energy Energy in chemical systems Total energy of an isolated system is constant Total energy = Potential energy + kinetic energy E p mgh
a. 4.2x10-4 m 3 b. 5.5x10-4 m 3 c. 1.2x10-4 m 3 d. 1.4x10-5 m 3 e. 8.8x10-5 m 3
 The following two problems refer to this situation: #1 A cylindrical chamber containing an ideal diatomic gas is sealed by a movable piston with cross-sectional area A = 0.0015 m 2. The volume of the chamber
The following two problems refer to this situation: #1 A cylindrical chamber containing an ideal diatomic gas is sealed by a movable piston with cross-sectional area A = 0.0015 m 2. The volume of the chamber
A Chemist s View of the Universe. System what we care about Surroundings everything else Boundary separates system from surroundings
 A Chemist s View of the Universe System what we care about Surroundings everything else Boundary separates system from surroundings Some basic definitions Work motion against an opposing (external) force
A Chemist s View of the Universe System what we care about Surroundings everything else Boundary separates system from surroundings Some basic definitions Work motion against an opposing (external) force
Chapter 16. Thermodynamics. Thermochemistry Review. Calculating H o rxn. Predicting sign for H o rxn. Creative Commons License
 Chapter 16 Thermodynamics GCC CHM152 Creative Commons License Images and tables in this file have been used from the following sources: OpenStax: Creative Commons Attribution License 4.0. ChemWiki (CC
Chapter 16 Thermodynamics GCC CHM152 Creative Commons License Images and tables in this file have been used from the following sources: OpenStax: Creative Commons Attribution License 4.0. ChemWiki (CC
Chapter 16: Spontaneity, Entropy, and Free Energy Spontaneous Processes and Entropy
 Chapter 16: Spontaneity, Entropy, and Free Energy 16.1 Spontaneous Processes and Entropy 1 3 The first law of thermodynamics the law of conservation of energy: Energy can be neither created nor destroyed
Chapter 16: Spontaneity, Entropy, and Free Energy 16.1 Spontaneous Processes and Entropy 1 3 The first law of thermodynamics the law of conservation of energy: Energy can be neither created nor destroyed
Chemical thermodynamics and bioenergetics
 Chemical thermodynamics and bioenergetics Thermodynamics is a branch of physics that studies energy, the forms of its transformation, and the laws controlling its properties. Basic Concepts and Definitions.
Chemical thermodynamics and bioenergetics Thermodynamics is a branch of physics that studies energy, the forms of its transformation, and the laws controlling its properties. Basic Concepts and Definitions.
Chapter 19. Chemical Thermodynamics
 Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 19 John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice Hall,
Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 19 John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice Hall,
OCR Chemistry A H432
 All the energy changes we have considered so far have been in terms of enthalpy, and we have been able to predict whether a reaction is likely to occur on the basis of the enthalpy change associated with
All the energy changes we have considered so far have been in terms of enthalpy, and we have been able to predict whether a reaction is likely to occur on the basis of the enthalpy change associated with
First Law of Thermodynamics
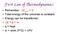 First Law of Thermodynamics Remember: ΔE univ = 0 Total energy of the universe is constant. Energy can be transferred: ΔE = q + w q = heat w = work (F*D) = ΔPV 1 st Law, review For constant volume process:
First Law of Thermodynamics Remember: ΔE univ = 0 Total energy of the universe is constant. Energy can be transferred: ΔE = q + w q = heat w = work (F*D) = ΔPV 1 st Law, review For constant volume process:
Energy is the capacity to do work
 1 of 10 After completing this chapter, you should, at a minimum, be able to do the following. This information can be found in my lecture notes for this and other chapters and also in your text. Correctly
1 of 10 After completing this chapter, you should, at a minimum, be able to do the following. This information can be found in my lecture notes for this and other chapters and also in your text. Correctly
