Lecture 12: Review of Structure & Decay
|
|
|
- Diana Alexander
- 6 years ago
- Views:
Transcription
1 Lecture 12: Review of Structure & Decay Highlights from Lectures 1-10 of PHYS7501 This doesn t necessarily cover everything that will be asked on the midterm, but it encompasses most of the main take-aways PHYS7501 Greatest Hits VOLUME I Lecture 12: Ohio University PHYS7501, Fall 2017, Z. Meisel (meisel@ohio.edu)
2 How big is a nucleus? Phenomenological estimates: A nucleus s mass is roughly: M(Z,A) = A*amu 1amu = atomic mass unit = u = MeV/c x10-24 g The amu is defined such that M( 12 C) 12u A nucleus s (charge) radius is roughly: R(Z,A) = (1.2fm)*A 1/3 fm = femtometer (a.k.a. fermi) = m The radius of a nucleon is often referred to as r 0 =1.2fm For the RMS radius, multiply by 3/5 Therefore, an estimate for the nuclear density is: ρρ = MM VV = MM 4 = 1uu AA 3 ππrr3 4 3 ππ(1.2ffff)3 AA 0.14 nnnnnnnnnnnnnnnn/ffff3 For fun, in terms of mass-density: ρ = gg AA 4 3 ππ(1.2ffff)3 AA which doesn t sound like much, but this is 2x10 14 g/cm 3 The definition of u being based on 12 C means it is a valuable tool for high-precision mass measurements, e.g. C.Scheidenberger et al., Nuc. Phys. A 2002 Since A cancels in the ρ expression, the nuclear density is independent of the nuclear size, much as a liquid s density is independent of the size of the drop. Partly inspired by this property, some basic nuclear calculations are based on this liquid drop analogy. (G. Gamow, Proc. Roy. Soc. A 1929,1930) gg ffff 3 (the Great Pyramid of Giza is only ~10 12 grams) 2
3 Nuclear Transmutation Rules for converting one nuclide (or nuclides) to another (or others) Charge conservation: qq bbbbbbbbbbbb = qq aaaaaaaaaa (q from protons + positrons + electrons) Baryon conservation: AA bbbbbbbbbbbb = AA aaaaaaaaaa (A from neutrons + protons) Lepton number conservation: [NN llllllllllllll NN aaaaaaaa llllllllllllll ] bbbbbbbbbbbb = [NN llllllllllllll NN aaaaaaaa llllllllllllll ] aaaaaaaaaa * Transmutation likelihoods are impacted by energetics and spin/parity selection rules The first nuclear reaction intentionally made in the laboratory was 14 N(α,p) in (E. Rutherford, Nature 1935). Two Types: Reactions Multiple reactants create one or more products Notation: A+b c+d is written as A(b,c)D, where M(b)<M(A) and M(c)<M(D) The first measured radioactive decay was α decay from uranium. (H. Becquerel, Comptes Rendus 1896). E.g. 12 C+α 16 O+γ is 12 C(α,γ) 16 O or even just 12 C(α,γ) and is called carbon-twelve alpha gamma Decays α, β +, β -, e - -capture, β-delayed γ/p/α/n emission, fission, cluster emission, prompt γ Lose nucleons for all above except β decay, e - -capture, and prompt γ (following a reaction) 3
4 The Semi-Empirical Mass Formula BE(Z,A) = Volume - Surface - Coulomb - Asymmetry ± Pairing One mathematical parameterization* (of many!): BBBB ZZ, AA = aa vv ff vv AA aa ss ff ss AA aa cc ff cc ZZ, AA aa aa ff aa ZZ, AA + iiaa pp ff pp (AA) Volume: Nucleons cohesively bind, so: ff vv AA = AA Surface: Since radius goes as RR AA 1 3 and surface area goes as SSSS RR 2, ff ss AA = AA Coulomb: Energy for a charged sphere goes as qq2 RR and RR AA 1 3, so ff cc ZZ, AA = ZZ(ZZ 11) Asymmetry: Z=N favored (want Z=A/2) but lesser problem for large A, so ff aa ZZ, AA = ZZ AA 22 Pairing: Favor spin-0 nucleon pairs & disfavor unpaired nucleons, empirically ff pp AA = AA) -1 Even-Z, Even-N: ii = +11 Odd-Z, Odd-N: ii = 11 Even-Odd: ii = 00 aa ii are fit to data *from B. Martin, Nuclear and Particle Physics (2009) A mnemonic for remembering SEMF contributions is VSCAP. AA ff pp AA = AA 22 AA 11 4
5 Nuclear Mass Differences The energy released in a nuclear reaction is the Q-value QQ = rreeeeeeeeeeeeeeee MMMM ZZ, AA pprrrrrrrrrrrrrr MMMM(ZZ, AA), For example, QQ68 SSSS(pp,γγ) 69 BBBB = MMMM 68 SSSS + MMMM pp MMMM 69 BBBB = MMMMMM + (7.288MMMMMM) ( MMMMMM) = 0.641MMMMMM Considering the case above, we calculated the energy released by adding one proton to 68 Se, which corresponds to the energy it takes to remove one proton from 69 Br, a.k.a. the proton separation energy, S p Similarly, can calculate the energy to remove 1-neutron S n, two-protons S 2p, or two-neutrons S 2n SS pp ZZ, NN = MMMM ZZ 1, NN + MMMM pp MMMM(ZZ, NN) SS nn ZZ, NN = MMMM ZZ, NN 1 + MMMM nn MMMM(ZZ, NN) SS 2pp ZZ, NN = MMMM ZZ 2, NN + 2 MMMM pp MMMM(ZZ, NN) SS 2nn ZZ, NN = MMMM ZZ, NN MMMM nn MMMM(ZZ, NN) 5
6 Nuclear charge distribution Review: R. Hofstadter, Rev. Mod. Phys. (1956) Comparing measured σ scatt (θ ) to calculations w/ various F (q ) reveals a Fermi-like distribution 125MeV e - on Be 125MeV e - on Au (R. Woods & D. Saxon, Phys. Rev. (1954)) R. Hofstadter, H. Fechter, & J. McIntyre, Phys. Rev. (1953) Measured ρ ch examples B.A. Brown, Lecture Notes in Nuclear Structure Physics,
7 Electric & Magnetic Moments In general, a moment is a distance multiplied by a physical quantity. For distributions you integrate the quantity s distribution with respect to distance. The nuclear magnetic moments describe the distribution of electric currents in the nucleus Causes nuclei to align along an external magnetic field, which can be exploited using NMR, MRI, etc. The nuclear electric moments describe the distribution of electric charges in the nucleus Used as a measure of the nuclear shape 7
8 First stab at the potential, VV: The Harmonic Oscillator Based on some evidence (and logic) that nuclei aren t perfectly constant in density, Heisenberg (Z. Phys. 1935) posited that a parabolic potential could be assumed, conveniently allowing the adoption of the harmonic oscillator solutions (one of the few analytically solved systems!) This provides evenly spaced energy levels nn, with EE nn = nn hυ. The corresponding angular momenta are ll = nn 1, nn 3, 0. i.e. only odd or even functions are allowed for each oscillator shell The number of particles per angular momentum is 2(2ll + 1) for 2ll + 1 projections & 2 spins So, the number of particles per level is: Loveland, Morrissey, & Seaborg, Modern Nuclear Chemistry (2006) Could the HO potential still be useful for some cases? can get the job done for light nuclei (e.g. H. Guo et al. PRC 2017) but need to be careful, because can impact results (B.Kay et al arxiv 2017) 8
9 Move to an empirical potential: Woods-Saxon Since the nuclear interaction is short-range, a natural improvement would be to adopt a central potential mimicking the empirical density distribution This is basically a square well with soft edges, as described by the Woods-Saxon potential: R. Woods & D. Saxon, Phys. Rev. (1954) 22 MeV protons on Pt Using the Woods-Saxon is a good idea because of commitment to reality but we re no wiser as to the origin of the magic numbers Cumulative Was this step completely useless? No! It broke the degeneracy in l Loveland, Morrissey, & Seaborg, Modern Nuclear Chemistry (2006) 2 9
10 The missing link: the spin-orbit interaction LS Due to desperation or genius (or both) Maria Göppert-Mayer [Phys. Rev. February 1949] (and nearly simultaneously Haxel, Jensen, & Suess [Phys. Rev. April 1949]) posited that nucleon spin and orbital angular momentum interacted strongly, making j the good quantum number for a nucleon: ȷȷ = ll + ss Prior to this approach, angular momentum was coupled as is typically done for atoms, where JJ = LL + SS, LL = nnuuuuuuuuuuuuuu ll, and SS = nnuuuuuuuuuuuuuu ss This is LS coupling Positing that the spin-orbit interaction is stronger than spin-spin or orbit-orbit means that instead, JJ = nnuuuuuuuuuuuuuu ȷȷ and ȷȷ = ll + ss This is jj coupling A. Kastberg, Lecture Notes Physique Atomique jj 10
11 Filling the shells We can construct a nucleus using our shell model : A nucleon will go in the lowest-energy level which isn t already filled, i.e. the largest angular momentum, jj for the lowest orbital angular momentum, ll for the lowest oscillator shell, n 2jj + 1 protons or neutrons are allowed per level Each level is referred to by its nnnnnn nn by the # for the oscillator shell (convention either starts with 0 or 1) ll by spectroscopic notation (s=0,p=1,d=2,f=3, ) jj by the half-integer corresponding to the spin Loveland, Morrissey, & Seaborg, Modern Nuclear Chemistry (2006) For example: 7 Li (Z=3, N=4) 11
12 Isomers on the Nuclear Chart L. van Dommelen, Quantum Mechanics for Engineers (2012) Even-Z, Odd N Odd-Z, Even N Odd-Z, Odd N Special cases exist (mostly for higher-a nuclides) where even-even nuclei have isomers (e.g. M. Müller-Veggian et al., Z.Phys.A (1979)) 12
13 Collective Model There are compelling reasons to think that our nucleus isn t a rigid sphere The liquid drop model gives a pretty successful description of some nuclear properties. can t liquids slosh around? Many nuclei have non-zero electric quadrupole moments (charge distributions) this means there s a non-spherical shape. can t non-spherical things rotate? Then, we expect nuclei to be able to be excited rotationally & vibrationally We should (and do) see the signature in the nuclear excited states The relative energetics of rotation vs vibration can be inferred from geometry The rotational frequency should go as ωω rr 1 RR 2 (because II LL ωω and II MMRR2 ) The vibrational frequency should go as ωω vv 1 RR 2 (because it s like an oscillator) So ωω rr ωω vv 13
14 Predicted regions of deformation B. Harvey, Introduction to Nuclear Physics and Chemistry (1962) P. Möller et al. ADNDT (2016) b ββ 2 αα 2,0 = 4 3 ππ 5 aa bb RR sssss Loveland, Morrissey, Seaborg, Modern Nuclear Chemistry (2006) 14
15 Rotational bands: sequences of excited states EE rrrrrr = ћ2 jj(jj+1), so for a given II, EE jj(jj + 1) 2II Note that parity needs to be maintained because rotation is symmetric upon reflection and so 0 + ground-states can only have j=0,2,4, (because ππ = ( 1) JJ ) Without observing the decay scheme, picking-out associated rotational states could be pretty difficult Experimentally, coincidence measurements allow schemes to be mapped B. Harvey, Introduction to Nuclear Physics and Chemistry (1962) 158 Er L. van Dommelen, Quantum Mechanics for Engineers (2012) T.Dinokoet al. EPJ Web Conf. (2013) 15
16 Vibrational energy levels Just as the quantum harmonic oscillator eigenvalues are quantized, so too will the energy levels for different quanta (phonons) of a vibrational mode. Similarly, the energy levels have an even spacing, EE nn = (nn )ћωω Even-even nuclides have 0+ ground states, and thus, for a λ = 2 vibration, nn = 2 excitations will maintain the symmetry of the wave-function (i.e. nn = 1 excitations would violate parity) Therefore, the 1 st vibrational state will be 2 + Loveland, Morrissey, Seaborg, Modern Nuclear Chemistry (2006) We can excite an independent quadrupole vibration by adding a second phonon The second phonon will build excitations on the first, coupling to either 0 +,2 +, or 4 + Employing a nuclear potential instead winds up breaking the degeneracy for states associated with a given number of phonons 16
17 Nilsson model: single-particle level splitting Consider the options for our nucleon s orbit around the nucleus Orbits with the same principle quantum number will have the same radius Notice that the orbit with the smaller projection of jj (KK 1 ) sticks closer to the bulk of the nucleus during its orbit Since the nuclear force is attractive, the KK 1 orbit will be more bound (i.e. lower energy) than the KK 2 orbit The opposite would be true if the nucleus in our picture was oblate, squishing out toward the KK 2 orbit Therefore, for prolate nuclei, lower KK single-particle levels will be more bound (lower-energy), whereas larger KK states will be more bound for oblate nuclei K jj R.Casten, Nuclear Structure from a Simple Perspective (1990) K Loveland, Morrissey, Seaborg, Modern Nuclear Chemistry (2006) 17
18 Nilsson Model: Example Consider 25 Al, for which we expect ββ 2 0.2, like 27 Al There are 13 protons and 12 neutrons, so the unpaired proton will be responsible for JJ ππ Filling the single-particle levels, We place two protons in the 1s 1/2 level, which isn t shown Then two more in 1/2 -, two more in 3/2 -, two more in 1/2 -, two more in the 1/2 +, two more in the 3/2 + And the last one winds up in the 5/2 + level So, we predict JJ ππ gg.ss = For the first excited sate, it seems likely the proton will hop up to the nearby 1/2 + level Agrees with data Since 25 Al is deformed, we should see rotational bands with states that have (integer)+jj and jj(jj + 1) spacing 5 2+ B. Harvey, Introduction to Nuclear Physics and Chemistry (1962) 18
19 Connection to thermodynamics Now we can solve for our entropy: SS EE = dddd TT(EE ) + cccccccccccccccc kk BB Since a zero-temperature system has zero entropy, SS EE kk BB 2 aaee Recall from the microscopic picture, SS = kk BB ln(gg) aa EE dddd + cccccccccccccccc So, the number of accessible configurations (a.k.a. nuclear states) for our system is gg exp 2 aaee The density of states is going to be proportional to the total number of states So, the state density ρρ EE = CC exp 2 aaee, where CC is a constant A more careful treatment using partition functions and other statistical mechanics tools yields: ρρ EE ππ = 5/4 exp 2 aaee 12aa 1/4 EE Going back to our estimate for 238 U and using aa = 1/dd and EE = SS nn, we get ρρ MMMMMM 1 In practice, CC and aa are usually fit to data H. Bethe, Phys. Rev. (1936) CC in particular isn t so relevant, since we can normalize ρρ EE to the region at low excitation energy where individual levels can be counted and ideally also to ρρ EE = SS nn 19
20 Experimental results confirm the exponential behavior of ρρ(ee ) One challenge in comparing to counts of discrete states is knowing if your measurement missed any levels T. Ericson, Nuc. Phys. (1959) Techniques which are sensitive to the integrated number of levels can overcome this though with assistance from models M. Guttormsen et al. Euro.Phys.J.A (2015) 20
21 σσ 2 and the spin distribution Having found σσ 2 = IIkk BBTT, now we need to estimate ћ the moment of inertia 2 II Since we assumed a spherical nucleus earlier to justify the degeneracy of EE in MM, we ll double-down and use II for a rigid sphere: II = 2 5 MMRR2 Using this and the previously derived formula for the nuclear temperature TT 1 EE /aa, and the standard estimate kk BB for the nuclear radius RR = rr 0 AA 1 3: σσ 2 = 2MMrr 0 2 AA 2 3 EE Using MM = AAmm uu MMMMMM cc 2 A, 5ћ 2 ћcc 197MMMMMM ffff, rr 0 1.2ffff: σσ AA 5 3 It turns out, fits to neutron resonances yield aa AA 8 MMMMMM 1 Therefore, σσ AA 7 6 EE 21 aa EE aa IAEA, RIPL-2 Handbook Implied spin distributions
22 Decay Equations for One Nuclear Species Solving for NN(tt) from the seemingly innocuous equation dddd = λnn(tt), is actually pretty hard, dddd unless one employs the Laplace transform, which turns our differential equation into an algebraic one For the LHS, we assume NN(tt) to be an exponential function (empirically a safe bet) and use the derivative property of Laplace transform: dddd dddd ssss ss NN(0) For the RHS, simply swap-in ss for time: λnn tt λnn(ss) Therefore, ssss(ss) NN(0) = λλnn(ss) Which is re-written as: NN ss = NN(0) (ss+λ) Using one of several different methods (See E.g. D.Pressyanov, Am.J.Phys. 2002, or a Math Methods book), the inverse Laplace transform can be employed, yielding the familiar relation: NN tt = NN(0)ee λλtt, the number of nuclei existing at time tt Since the Activity (decays/second) AA = λλnn, AA tt = AA(0)ee λtt 22
23 Common Descriptors for Decay Aside from the decay constant λ, other more intuitive quantities are often used It is common to state the time at which half of the nuclei in a radioactive sample will have undergone decay, a.k.a. the half-life: tt ½ By definition: NN(tt ½) = 1 so, 1 = NN(0) 2 2 ee λtt ½ Re-write as 2 = ee λtt ½, which makes it apparent that tt ½ = ln(2) An alternative piece of trivia is the mean lifetime for the nuclei in the sample, ττ By definition: ττ = 0 tttt tt dddd = 0 tttt tt dddd 0 NN tt dddd NN(0)/λ Therefore tt ½ = ln(2)ττ 0.693ττ = NN(0)/λ2 NN(0)/λ = 1 λ We can re-state the lifetime in terms of the equivalent energy width using the Heisenberg uncertainty principle: EE tt ћ Taking the mean lifetime as the time uncertainty, EE = ћ ττ MMMMMM ττ (iiii ssssssssssssss) λλ 23
24 Secular Equilibrium & Radioactivity from Nuclear Reactions If a radioactive nucleus is made in a nuclear reaction (e.g. in a star, from cosmic rays, or using an accelerator), the constant replenishment of the daughter is similar to the case of a very long-lived parent In particular this is true of the reaction rate is much slower than the daughter decay which is pretty much always the case Starting with NN 2 0 = 0 and recasting decay of 1 2 as a reaction, RR 12 = λ 1 NN 1 0 = AA 1 (0), AA 2 tt = λ 2 AA λ 2 λ 1 0 ee λ1tt ee λ 2tt + AA 2 0 ee λ2tt becomes AA 2 tt = λ 2 RR 1 λ 2 λ 12 ee λ1tt ee λ 2tt 1 Since λ 1 0, AA 2 tt RR 12 1 ee λ 2tt, which is known as the activation equation Linear growth of the daughter at short times, but have diminishing returns as time increases If you re making a custom radioactive source on-site, this lets you know how long to bother performing the production reaction A. Bielajew, Intro. to Special Relativity, Quantum Mechanics, and Nuclear Physics For Nuclear Engineers (2014) This relation allows nuclear reaction cross sections to be measured using the daughter decay, and it lets you know how long to perform the reaction 24
25 α energy from Q α When an α is emitted, it will share some energy with the heavy recoil, so KKKK αα isn t quite equal to QQ αα We just need to employ conservation of momentum and energy pp pppppppppppp = pp ddddddddddddddd + pp αα Conveniently pp pppppppppppp = 0, so the daughter and α will move in opposite directions and pp ddddddddddddddd = pp αα 2 pp pppppppppppp 2AA pppppppppppp + QQ αα = pp ddddddddddddddd 2 + pp αα = 2AA ddddddddddddddd 2AA αα QQ αα = AA αα+aa ddddddddddddddd AA ddddddddddddddd KKKK αα = AA pppppppppppp AA ddddddddddddddd KKKK αα 2 pp αα 2 + pp αα = 2AA ddddddddddddddd 2AA αα KKKK αα = AA ddddddddddddddd QQ AA αα pppppppppppp So it s a pretty small effect (though not so for β-delayed particle emission in lighter nuclei) Conveniently, α sources typically have several E α from the decay chain, and so they provide several energy calibration points 2 AA αα AA ddddddddddddddd KKKK αα + KKKK αα 226 Ra decay sequence Spectrum from M.Mroz, K. Brandenburg, A. Mamum, & A. Pun 25
26 Geiger-Nuttall relation Geiger & Nuttall, Philisoph. Mag. (1912) In an early effort to characterize α-decay, Geiger & Nuttall (H. Geiger & J.M. Nuttall, Philisoph. Mag. (1911, 1912)) compared the range of α particles in a material vs t ½ of the α-source and found a linear relationship in log-log space In modern terms, using QQ αα instead of range, we get the Geiger-Nuttall relation: log 10 tt ½ = aa + bbbbqq αα ½ Obviously the αα energy somehow impacts tt ½ incredibly strongly For ~ 2 increase in QQ αα, nearly 20 orders of magnitude decrease in tt ½!! Loveland, Morrissey, & Seaborg, Modern Nuclear Chemistry (2006) What does this imply about useful α sources? There s a relatively limited range of E α available. Large E α sources aren t active for long enough, while low E α sources require huge amounts to have an appreciable activity (A=λN). 26
27 Tunneling through a thick barrier TT = ee 2GG, where 2GG = 2 ee2 ћcc ZZ ααzz ddddddddddddddd 2μμcc 2 QQ αα cccccc 1 RR bb RR bb 1 RR bb Conveniently, for most cases bb RR, so the Gamow factor 2GG ππ 2 ee 2 ћcc ZZ ααzz ddddddddddddddd 2μμcc 2 Since the decay half-life will be inversely proportional to the tunneling probability, tt ½ ee 2GG ee ZZ/ QQ αα You may notice this is what Geiger & Nuttall told us all along ½ log 10 tt ½ = aa + bbbbqq αα QQ αα is pretty ugly B.A. Brown, Lecture Notes in Nuclear Structure Physics (2005) 238 U Aside: Gamow realized this formalism would work just as well RR bb for a charged-particle tunneling in (i.e. for nuclear fusion). For nuclear fusion, 2G is often written instead as 2πη, where η is the Sommerfeld parameter. In nuclear astrophysics, T=P=exp(-2πη) is multiplied by the Maxwell-Boltzmann distribution to get the Gamow window. 27
28 Why is it α particles that are being emitted? So far we ve been smugly pleased with ourselves about our ability to describe α decay but why α decay? Why not proton decay, or 3 He decay, or 12 C decay? The short answer is Q-values, Coulomb barriers, and clustering probabilities Q-value: The cluster decay must be energetically favorable Coulomb barrier: Higher-Z particles will have a larger barrier to tunnel through Clustering probability: It s less likely for more nucleons to congregate within a nucleus 28
29 β decay spectrum, spin conservation, and the neutrino Early experiments investigating the β ray showed that it was not emitted with a singular energy, like the α ray, but rather in a continuum of energies Though the maximum energy is equal to the decay Q-value Furthermore, the reaction nn pp + ee doesn t conserve spin! JJ nn = JJ pp = JJ ee = 1 so 0 JJ 2 pp + JJ ee To remedy this issue, Pauli proposed the involvement of a 3 rd hypothetical particle, the neutrino ν Given the above considerations, it was postulated that ν is a spin-½ particle ( fermion ) that it is massless* and electrically neutral (of course this isn t quite true, but true enough for our purposes) R. Evans, The Atomic Nucleus (1955) Like a proper old-timey physicist, he made this proposal not in a paper, but in a letter to physicist Lisa Meitner In one of his last works before switching to primarily performing experimental work, Fermi postulated (E. Fermi, Z. Phys. 1934) that nucleons could act as sources & sinks of electrons and neutrinos, in analogy to charged particles acting as sources and sinks of photons in quantum electrodynamics (the only successful theory of interactions between quantum particles at that point) For what it s worth, Nature rejected Fermi s paper for being too remote from physical reality 29
30 β decay phase space factor & the β energy spectrum Considering the β decay rate for an electron momentum within pp ee + ddpp ee, λ(pp ee )dddd ee = 2ππ Ψ ћ ffffffffff HH 2 Ψ iiiiiiiiiiiiii ρρ(ee) The matrix element is just some number, so the functional form is from ρρ(ee) 2 Therefore, we expect λ pp ee dddd ee QQ KKKK 2 pp ppee = QQ KKKK 2 ee 2mm ee KKKK ee = QQ pp ee 2 2mm ee 2 pp ee 2 k Wu & Albert, Phys. Rev (1949) Not too bad, but what effect are we forgetting that will cause positrons and electrons to behave differently? Coulomb repulsion! 30
31 Decay selection rules and forbidden decays λλ = 2ππ ћ ΨΨ ffffffffffhh ΨΨ iiiiiiiiiiiiii ddττ 2 ρρ(ee ff ) As was just alluded to, ignoring higher-order terms of the ee ii( pp ν+ pp ee ) rr ћ Taylor expansion omits the possibility for angular momentum transfer If angular momentum transfer is to occur, higher-order terms need to be included and it will no longer be the case that MM ffff 2 is independent of ppee In fact, for these cases JJ > 1 and/or ππ = yyyyyy, the leading-order overlap MM ffff 2 = 0 and so a higher-order term will be necessary The order that s required will correspond to the angular momentum transfer of the decay JJ This combined with whether or not parity is changed is referred to as how forbidden a transition is even though it s just a hindrance "ssssssssss aaaaaaaaaaaaaa" oooo JJ = 0 oooo 1 aaaaaa ππ = nnnn "aaaaaaaaaaaaaaa JJ = 0 oooo 1, ππ = yyyyyy "ffffffffff ffffffffffffffffff" JJ = 2, ππ = nnnn "ssssssssssss fffffffffffffffffff For a given transition type, ffff will typically be within an order of magnitude of some value 31
32 λ for Electron capture Rather than a nucleon undergoing transmutation by its lonesome, instead e - -capture can occur This is either due to a capture of a low-lying (usually the K-shell ) electron or due to the electron Fermi energy in an electron-degenerate environment being high enough to overcome the electron-capture Q-value The decay constant for electron-capture decay is a bit different than for β decay, because the final state only consists of a nucleon and a neutrino i.e. KKKK ee = 0 and Ψ ff = ψ dd,kk φφ ν The decay constant is then: λλ = GG FF 2 MM 2 2ππ 3 ћ 3 cc 3 ffff TTν 2 φφ KK (0) 2, where φφ KK (0) is the wave-function for the inner-most atomic electron (the one in the K-shell ) You may recall from your Quantum class, φφ KK 0 = 1 ππ ZZmm ee ee 2 4ππεε 0 ћ 2 As such, the ratio of electron-capture to β + decay for a nucleus goes as λ KK λ β + ZZ3 (of course, QQ ββ > 2mm ee is a requirement for β + decay to be possible in the first place) Since EC decay only emits a neutrino, which will be almost impossible for us to detect, how do you figure EC decay is usually detected? X-ray and Auger electron emission due to atomic electrons filling the vacated orbital 3/2 λλ = 2ππ ћ ΨΨ ffffffffffhh ΨΨ iiiiiiiiiiiiii ddττ 2 ρρ(ee ff ) 32
33 γ decay basics γ decay is a de-excitation from an excited bound state to a lower energy state, preceded by some decay or reaction [Just to be clear] Z & A are unchanged γ ray energies can span anywhere from several kev to several MeV γ decay lifetimes are typically extremely short (ττ femtoseconds) [with the exception of isomeric states] INEL γ Spectrum Catalog 33
34 γ decay types Parity and angular momentum are conserved during γ decay Photons carry some integer angular momentum with a minimum ll = 1, where ll is referred to by the multipole 2 ll ll = 1: dddddddddddd, ll = 2 qqqqqqqqqqqqqqqqqqqq, A photon s parity depends not only on ll, but also on the decay type A photon decay corresponds to shift in the nucleus s charge and matter distribution Shift in the charge distribution = change in electric field = Electric Shift in the current distribution [i.e. orbitals of protons]= change in magnetic field = Magnetic The selection rules corresponding to a particular decay type are: 34
35 How does happen? Internal conversion Since ll mmmmmm = 1 for a photon, de-excitation by photon emission isn t possible Instead the process of internal conversion can happen, whereby a nucleus interacts electromagnetically with an orbital electron and de-excites by ejecting that orbital electron This process operates in competition with γ decay for any transition, not just The energy of the emitted electron is: EE IIII = EE xxxx EE BBBB,ee, where EE xxxx is the decay transition energy, and EE BBBB,ee is the electron binding energy hyperphysics Ahmad & Wagner, Nucl. Instrum. Meth. (1974) A similar, but different phenomenon is Internal Pair Conversion, where a photon with E γ >2m e c 2 interacts with the coulomb field of the nucleus to create an e + -e - pair. See e.g. A. Wuosmaa et al. Phys. Rev. C Rapid Comm
36 Weisskopf (a.k.a. single-particle) estimates for λ λ ll γγ, JJ ii, ππ JJ ff, ππ = 8ππ ll γγ+1 ll γγ 2ll γγ +1!! 2 EEγγ ћcc 2llγγ+1 ћ BB(ll γγ, JJ ii, ππ JJ ff, ππ), Reduced transition probabilities assuming the initial to final state transition is due to a single nucleon re-orienting itself within a nucleus of uniform density with RR = rr 0 AA 1/3 are: BB ss.pp. EE, ll γγ = 1 4ππ BB ss.pp. MM, ll γγ = 10 ππ 3 ll γγ +3 3 ll γγ rr 0 2ll γγ AA 2ll γγ/3 ee 2 (ffff) 2ll γγ rr 0 (2ll γγ 2)/2 μμnn 2 (ffff) 2ll γγ 2, where the nuclear magneton μμ nn = Note: There is a steep dependency of λ on ll, so only one multipole of a decay type will matter The equations above are still a huge pain to work with and more noble souls have worked-out the decay constant for various situations. Using QQ in MeV, λ γγ in ss 1 for a nucleus with mass number AA is given by: The units for B change with l γ! Weisskopf estimates are generally within an order of magnitude of the real answer, so γ decay constants are often quoted as the ratio to this estimate in Weisskopf Units [w.u.] eeћ 2mm pp cc L. van Dommelen, Quantum Mechanics for Engineers (2012) 36
37 Weisskopf (a.k.a. single-particle) estimates for tt ½ tt ½ (EE γγ ) for Electric Transitions (from Weisskopf) tt ½ (EE γγ ) for Magnetic Transitions (from Moszkowski) L. van Dommelen, Quantum Mechanics for Engineers (2012) Note that E transitions of a given multipole and E γ are ~100X faster than M transitions with the same E γ,l Now we see how it is that low-energy high-spin states exist as isomeric states. 37
38 Internal conversion coefficient, α Don t forget about our old friend, internal conversion, which competes with γ decay Competition between the two is described by the internal conversion coefficient αα = λ IIII λ γγ, so λ = λ IIII + λ γ = λ γ (1 + αα) αα depends on the density of electrons near the nucleus, and so some friendly atomic physicists have done the dirty work of calculating the following approximate formulas: αα EEEE = ZZ3 ll αα 4 2mm ee cc 2 ll+ 5 2 ZZ nn 3 ll+1 ff.ss. ; αα MMll = 3 αα 4 2mm ee cc 2 ll+ 3 2 These rely on the Born QQ nn 3 ff.ss., approximation, so QQ where αα ff.ss. 1 Z<<137 ought to apply, QQ is the transition Q-value, 137 and nn is the principal quantum number of the orbital electron being ejected The atomic orbitals KK, LL, MM, NN, OO, correspond to nn = 1, 2, 3, 4, 5, Clearly this process is favored for high-zz nuclei, but also for QQ < 1.022MMMMMM ll = 0 transitions For transitions, λ EEE = 3.8 ZZ 3 AA 4/3 QQ 1/2, with Q in MeV and λ in s -1 38
39 γ angular correlations, general case Generally speaking, WW(θθ) for any γ-γ coincidence is defined by a sum of Legendre polynomials: WW θθ = ii=ll ii=0 aa 2ii PP 2ii (cosθθ) i.e. WW θθ = 1 + aa 2 cos 2 θθ + aa 4 cos 4 (θθ)+ aa 2ll cos 2ll (θθ), where the normalization is such that WW 90 = 1 The coefficients aa ii are fit to data and the results are checked against the expected results for particular combinations of JJ ii, JJ ii, JJ ff, ll 1, ll 2 R.Evans, The Atomic Nucleus (1955) For common cases, pre-tabulated values are available to compare to Arfken, Klema, & McGowan, Phys. Rev. Lett. (1952) 106 Pd 39
40 Steps of fission 1. A nucleus becomes deformed either due to an external perturbation that brings in energy or an internal cluster rattling around within the potential well 2. The energy is absorbed as a collective excitation that manifests itself as a drastic shape change, elongating the nucleus into a peanut shape 3. The separation of the two lobes of the peanut becomes great enough that the two repel each other, splitting apart at the scission point 4. The coulomb repulsion accelerates the two fragments apart 5. The two fragments are each highly excited and de-excite initially via neutron emission, followed by γ emission (meaning prompt neutrons will be emitted along the direction of the fragments) 6. The neutron-rich fragments will then β decay back to stability, possibly emitting delayed neutrons via β-delayed neutron emission Why do you figure there is neutron emission at first and finally only γ emission? A massive particle is better suited to remove the large angular momentum present in high-lying excited states. At lower excitation energies, a particle has to tunnel out of the nucleus, while the γ doesn t. Loveland, Morrissey, & Seaborg, Modern Nuclear Chemistry (2006) 40
41 Energetics of shape change: Liquid drop picture For the deformed shape, which is an ellipsoid in this picture, the nuclear radius can be parameterized as an expansion in terms of Legendre polynomials [which for axial symmetry will only keep the ll = 2 term] B.R. Martin, Nuclear and Particle Physics (2009) RR θθ = RR 0 [1 + αα 2 PP 2 (cosθθ)] αα 2 is the quadrupole distortion parameter, which is related to the quadrupole deformation by αα 2 = 5 ππββ 1 4 2, and the ellipsoid axes by: aa = RR 0 (1 + αα 2 ), bb = RR 0 1+αα 2 It turns out, expanding the Coulomb and surface energy terms as a power series in αα 2 yields EE ZZ cc aa 2 cc 1 1 αα AA 1/ and EE ss aa ss AA 2/ αα Meaning the energy cost for deformation is EE = αα aa ss AA 2/3 aa cc ZZ 2 So, when the non-deformed Coulomb energy is twice the non-deformed surface energy or greater, there is zero energetic cost (or even an energetic gain) to deform (and ultimately fission!) The fissionability parameter xx = EE cc 2EE ss = aa cc 2aa ss ZZ 2 AA ZZ 2 AA AA 1/3 ZZ 2 AA cccccccccccccccc is a measure of fission favorability 41
42 Spontaneous fission rate Spontaneous fission is akin to α decay (or proton or cluster decay, for that matter), where a barrier is assaulted at some rate and there is probability for tunneling through the barrier Loveland, Morrissey, & Seaborg, Modern Nuclear Chemistry (2006) Here, the difference is that the potential is not from the nucleus, but rather the potential energy surface for the landscape of possible shapes. The nucleus itself is tunneling through the barrier. tt ½ = ln(2), where ff is the assault frequency and PP is the tunneling probability ffff ff corresponds to the rate at which the nuclear shape is changing, i.e. the frequency of surface oscillations, which is ~10 20 ss 1 (Hill & Wheeler, Phys. Rev. 1953) For the simplest case of a one-humped barrier, approximated as a inverted parabola, (due to the liquid-drop +single particle potential for deformation, See Lec. 4), the tunneling probability is (Hill & Wheeler, Phys. Rev. 1953) PP = 1 1+exp 2ππEE ff /ћωω So, tt ½ exp(2ππee ff ћωω) Extremely sensitive to predictions of E f 42
43 Spontaneous fission rate The height of the fission barrier is related to the fissionability parameter xx (recall ~48 means fission immediately) So without resorting to fancy calculations of EE ff, the half-life for spontaneous fission can be ball-parked But this method is extremely rough Loveland, Morrissey, & Seaborg, Modern Nuclear Chemistry (2006).using shell-corrected masses gives some improvement: Vandenbosch & Huizenga, Nuclear Fission (1973) Hill & Wheeler, Phys. Rev Even-Odd Even-Even 43
44 Fission fragment mass distribution The culprit for the asymmetric mass distribution are the ZZ = 50, NN = 82 shell closures, which favors nuclei in this range for one of the fission fragments. The other fragment has the remainder of most of the rest of the nucleons K. Flynn et al. PRC Vol
45 Fission fragments: kinetic energy The total kinetic energy of fission products will roughly be the coulomb repulsion energy of the two main fission fragments, TTTTTT = E.g. 240 Pu ZZ 1ZZ 2 ααћc 1.8 AA 1 1/3 +AA2 1/3 where r 0 =1.8fm is used instead of 1.2 because of the strong deformation at scission AA/ZZ If ZZ hiiiii = 50, NN hiiiii = 82, then ZZ llllll = = 44, NN llllll = = 64 (50)(44)ααћc TTTTTT = 178MMMMMM / /3 240 Pu Schunk & Robledo, Rep. Prog. Phys. (2016) 45
Lecture 10: Fission Conceptual process Fissionability Decay rate Decay branching Mass distribution Kinetic energy Neutrons
 Lecture 10: Fission Conceptual process Fissionability Decay rate Decay branching Mass distribution Kinetic energy Neutrons Lecture 10: Ohio University PHYS7501, Fall 2017, Z. Meisel (meisel@ohio.edu) Steps
Lecture 10: Fission Conceptual process Fissionability Decay rate Decay branching Mass distribution Kinetic energy Neutrons Lecture 10: Ohio University PHYS7501, Fall 2017, Z. Meisel (meisel@ohio.edu) Steps
Lecture 8: β Decay Basic process & energetics Fermi theory ft-values Electron-capture Parity violation Special cases
 Lecture 8: β Decay Basic process & energetics Fermi theory ft-values Electron-capture Parity violation Special cases Lecture 8: Ohio University PHYS7501, Fall 017, Z. Meisel (meisel@ohio.edu) What is β
Lecture 8: β Decay Basic process & energetics Fermi theory ft-values Electron-capture Parity violation Special cases Lecture 8: Ohio University PHYS7501, Fall 017, Z. Meisel (meisel@ohio.edu) What is β
PHL424: Nuclear Shell Model. Indian Institute of Technology Ropar
 PHL424: Nuclear Shell Model Themes and challenges in modern science Complexity out of simplicity Microscopic How the world, with all its apparent complexity and diversity can be constructed out of a few
PHL424: Nuclear Shell Model Themes and challenges in modern science Complexity out of simplicity Microscopic How the world, with all its apparent complexity and diversity can be constructed out of a few
Lecture 13: The Actual Shell Model Recalling schematic SM Single-particle wavefunctions Basis states Truncation Sketch view of a calculation
 Lecture 13: The Actual Shell Model Recalling schematic SM Single-particle wavefunctions Basis states Truncation Sketch view of a calculation Lecture 13: Ohio University PHYS7501, Fall 2017, Z. Meisel (meisel@ohio.edu)
Lecture 13: The Actual Shell Model Recalling schematic SM Single-particle wavefunctions Basis states Truncation Sketch view of a calculation Lecture 13: Ohio University PHYS7501, Fall 2017, Z. Meisel (meisel@ohio.edu)
Lecture 5: Nuclear Structure 3 Fermi Gas Model Microscopic approach Thermodynamic approach Nuclear level density
 Lecture 5: Nuclear Structure 3 Fermi Gas Model Microscopic approach Thermodynamic approach Nuclear level density Lecture 5: Ohio University PHYS7501, Fall 2017, Z. Meisel (meisel@ohio.edu) The nucleus
Lecture 5: Nuclear Structure 3 Fermi Gas Model Microscopic approach Thermodynamic approach Nuclear level density Lecture 5: Ohio University PHYS7501, Fall 2017, Z. Meisel (meisel@ohio.edu) The nucleus
Lecture 3: Nuclear Structure 1 Why structure? The nuclear potential Schematic shell model
 Lecture 3: Nuclear Structure 1 Why structure? The nuclear potential Schematic shell model Lecture 3: Ohio University PHYS7501, Fall 2017, Z. Meisel (meisel@ohio.edu) Empirically, several striking trends
Lecture 3: Nuclear Structure 1 Why structure? The nuclear potential Schematic shell model Lecture 3: Ohio University PHYS7501, Fall 2017, Z. Meisel (meisel@ohio.edu) Empirically, several striking trends
Lecture 4: Nuclear Structure 2 Independent vs collective models Collective Model Rotation Vibration Coupled excitations Nilsson model
 Lecture 4: Nuclear Structure 2 Independent vs collective models Collective Model Rotation Vibration Coupled excitations Nilsson model Lecture 4: Ohio University PHYS7501, Fall 2017, Z. Meisel (meisel@ohio.edu)
Lecture 4: Nuclear Structure 2 Independent vs collective models Collective Model Rotation Vibration Coupled excitations Nilsson model Lecture 4: Ohio University PHYS7501, Fall 2017, Z. Meisel (meisel@ohio.edu)
The IC electrons are mono-energetic. Their kinetic energy is equal to the energy of the transition minus the binding energy of the electron.
 1 Lecture 3 Nuclear Decay modes, Nuclear Sizes, shapes, and the Liquid drop model Introduction to Decay modes (continued) Gamma Decay Electromagnetic radiation corresponding to transition of nucleus from
1 Lecture 3 Nuclear Decay modes, Nuclear Sizes, shapes, and the Liquid drop model Introduction to Decay modes (continued) Gamma Decay Electromagnetic radiation corresponding to transition of nucleus from
Chapter 44. Nuclear Structure
 Chapter 44 Nuclear Structure Milestones in the Development of Nuclear Physics 1896: the birth of nuclear physics Becquerel discovered radioactivity in uranium compounds Rutherford showed the radiation
Chapter 44 Nuclear Structure Milestones in the Development of Nuclear Physics 1896: the birth of nuclear physics Becquerel discovered radioactivity in uranium compounds Rutherford showed the radiation
14. Structure of Nuclei
 14. Structure of Nuclei Particle and Nuclear Physics Dr. Tina Potter Dr. Tina Potter 14. Structure of Nuclei 1 In this section... Magic Numbers The Nuclear Shell Model Excited States Dr. Tina Potter 14.
14. Structure of Nuclei Particle and Nuclear Physics Dr. Tina Potter Dr. Tina Potter 14. Structure of Nuclei 1 In this section... Magic Numbers The Nuclear Shell Model Excited States Dr. Tina Potter 14.
PHL424: Nuclear fusion
 PHL424: Nuclear fusion Hot Fusion 5 10 15 5 10 8 projectiles on target compound nuclei 1 atom Hot fusion (1961 1974) successful up to element 106 (Seaborgium) Coulomb barrier V C between projectile and
PHL424: Nuclear fusion Hot Fusion 5 10 15 5 10 8 projectiles on target compound nuclei 1 atom Hot fusion (1961 1974) successful up to element 106 (Seaborgium) Coulomb barrier V C between projectile and
Chapter VIII: Nuclear fission
 Chapter VIII: Nuclear fission 1 Summary 1. General remarks 2. Spontaneous and induced fissions 3. Nucleus deformation 4. Mass distribution of fragments 5. Number of emitted electrons 6. Radioactive decay
Chapter VIII: Nuclear fission 1 Summary 1. General remarks 2. Spontaneous and induced fissions 3. Nucleus deformation 4. Mass distribution of fragments 5. Number of emitted electrons 6. Radioactive decay
α particles, β particles, and γ rays. Measurements of the energy of the nuclear
 .101 Applied Nuclear Physics (Fall 004) Lecture (1/1/04) Nuclear ecays References: W. E. Meyerhof, Elements of Nuclear Physics (McGraw-Hill, New York, 1967), Chap 4. A nucleus in an excited state is unstable
.101 Applied Nuclear Physics (Fall 004) Lecture (1/1/04) Nuclear ecays References: W. E. Meyerhof, Elements of Nuclear Physics (McGraw-Hill, New York, 1967), Chap 4. A nucleus in an excited state is unstable
13. Basic Nuclear Properties
 13. Basic Nuclear Properties Particle and Nuclear Physics Dr. Tina Potter Dr. Tina Potter 13. Basic Nuclear Properties 1 In this section... Motivation for study The strong nuclear force Stable nuclei Binding
13. Basic Nuclear Properties Particle and Nuclear Physics Dr. Tina Potter Dr. Tina Potter 13. Basic Nuclear Properties 1 In this section... Motivation for study The strong nuclear force Stable nuclei Binding
Lecture 11: Nucleon-Nucleon Interaction Basic properties The deuteron NN scattering Meson exchange model
 Lecture 11: Nucleon-Nucleon Interaction Basic properties The deuteron NN scattering Meson exchange model Lecture 11: Ohio University PHYS7501, Fall 2017, Z. Meisel (meisel@ohio.edu) Zach Weinersmith (SMBC)
Lecture 11: Nucleon-Nucleon Interaction Basic properties The deuteron NN scattering Meson exchange model Lecture 11: Ohio University PHYS7501, Fall 2017, Z. Meisel (meisel@ohio.edu) Zach Weinersmith (SMBC)
Alpha decay. Introduction to Nuclear Science. Simon Fraser University Spring NUCS 342 February 21, 2011
 Alpha decay Introduction to Nuclear Science Simon Fraser University Spring 2011 NUCS 342 February 21, 2011 NUCS 342 (Lecture 13) February 21, 2011 1 / 27 Outline 1 The Geiger-Nuttall law NUCS 342 (Lecture
Alpha decay Introduction to Nuclear Science Simon Fraser University Spring 2011 NUCS 342 February 21, 2011 NUCS 342 (Lecture 13) February 21, 2011 1 / 27 Outline 1 The Geiger-Nuttall law NUCS 342 (Lecture
CHAPTER 4 Structure of the Atom
 CHAPTER 4 Structure of the Atom Fall 2018 Prof. Sergio B. Mendes 1 Topics 4.1 The Atomic Models of Thomson and Rutherford 4.2 Rutherford Scattering 4.3 The Classic Atomic Model 4.4 The Bohr Model of the
CHAPTER 4 Structure of the Atom Fall 2018 Prof. Sergio B. Mendes 1 Topics 4.1 The Atomic Models of Thomson and Rutherford 4.2 Rutherford Scattering 4.3 The Classic Atomic Model 4.4 The Bohr Model of the
Nuclear Fission Fission discovered by Otto Hahn and Fritz Strassman, Lisa Meitner in 1938
 Fission Readings: Modern Nuclear Chemistry, Chapter 11; Nuclear and Radiochemistry, Chapter 3 General Overview of Fission Energetics The Probability of Fission Fission Product Distributions Total Kinetic
Fission Readings: Modern Nuclear Chemistry, Chapter 11; Nuclear and Radiochemistry, Chapter 3 General Overview of Fission Energetics The Probability of Fission Fission Product Distributions Total Kinetic
RFSS: Lecture 6 Gamma Decay
 RFSS: Lecture 6 Gamma Decay Readings: Modern Nuclear Chemistry, Chap. 9; Nuclear and Radiochemistry, Chapter 3 Energetics Decay Types Transition Probabilities Internal Conversion Angular Correlations Moessbauer
RFSS: Lecture 6 Gamma Decay Readings: Modern Nuclear Chemistry, Chap. 9; Nuclear and Radiochemistry, Chapter 3 Energetics Decay Types Transition Probabilities Internal Conversion Angular Correlations Moessbauer
c E If photon Mass particle 8-1
 Nuclear Force, Structure and Models Readings: Nuclear and Radiochemistry: Chapter 10 (Nuclear Models) Modern Nuclear Chemistry: Chapter 5 (Nuclear Forces) and Chapter 6 (Nuclear Structure) Characterization
Nuclear Force, Structure and Models Readings: Nuclear and Radiochemistry: Chapter 10 (Nuclear Models) Modern Nuclear Chemistry: Chapter 5 (Nuclear Forces) and Chapter 6 (Nuclear Structure) Characterization
Basic science. Atomic structure. Electrons. The Rutherford-Bohr model of an atom. Electron shells. Types of Electrons. Describing an Atom
 Basic science A knowledge of basic physics is essential to understanding how radiation originates and behaves. This chapter works through what an atom is; what keeps it stable vs. radioactive and unstable;
Basic science A knowledge of basic physics is essential to understanding how radiation originates and behaves. This chapter works through what an atom is; what keeps it stable vs. radioactive and unstable;
CHEM 312 Lecture 7: Fission
 CHEM 312 Lecture 7: Fission Readings: Modern Nuclear Chemistry, Chapter 11; Nuclear and Radiochemistry, Chapter 3 General Overview of Fission Energetics The Probability of Fission Fission Product Distributions
CHEM 312 Lecture 7: Fission Readings: Modern Nuclear Chemistry, Chapter 11; Nuclear and Radiochemistry, Chapter 3 General Overview of Fission Energetics The Probability of Fission Fission Product Distributions
Lecture 22 Highlights Phys 402
 Lecture 22 Highlights Phys 402 Scattering experiments are one of the most important ways to gain an understanding of the microscopic world that is described by quantum mechanics. The idea is to take a
Lecture 22 Highlights Phys 402 Scattering experiments are one of the most important ways to gain an understanding of the microscopic world that is described by quantum mechanics. The idea is to take a
α particles, β particles, and γ rays. Measurements of the energy of the nuclear
 .101 Applied Nuclear Physics (Fall 006) Lecture (1/4/06) Nuclear Decays References: W. E. Meyerhof, Elements of Nuclear Physics (McGraw-Hill, New York, 1967), Chap 4. A nucleus in an excited state is unstable
.101 Applied Nuclear Physics (Fall 006) Lecture (1/4/06) Nuclear Decays References: W. E. Meyerhof, Elements of Nuclear Physics (McGraw-Hill, New York, 1967), Chap 4. A nucleus in an excited state is unstable
2007 Fall Nuc Med Physics Lectures
 2007 Fall Nuc Med Physics Lectures Tuesdays, 9:30am, NN203 Date Title Lecturer 9/4/07 Introduction to Nuclear Physics RS 9/11/07 Decay of radioactivity RS 9/18/07 Interactions with matter RM 9/25/07 Radiation
2007 Fall Nuc Med Physics Lectures Tuesdays, 9:30am, NN203 Date Title Lecturer 9/4/07 Introduction to Nuclear Physics RS 9/11/07 Decay of radioactivity RS 9/18/07 Interactions with matter RM 9/25/07 Radiation
Chemical Engineering 412
 Chemical Engineering 412 Introductory Nuclear Engineering Lecture 12 Radiation/Matter Interactions II 1 Neutron Flux The collisions of neutrons of all energies is given by FF = ΣΣ ii 0 EE φφ EE dddd All
Chemical Engineering 412 Introductory Nuclear Engineering Lecture 12 Radiation/Matter Interactions II 1 Neutron Flux The collisions of neutrons of all energies is given by FF = ΣΣ ii 0 EE φφ EE dddd All
General Physics (PHY 2140)
 General Physics (PHY 140) Lecture 18 Modern Physics Nuclear Physics Nuclear properties Binding energy Radioactivity The Decay Process Natural Radioactivity Last lecture: 1. Quantum physics Electron Clouds
General Physics (PHY 140) Lecture 18 Modern Physics Nuclear Physics Nuclear properties Binding energy Radioactivity The Decay Process Natural Radioactivity Last lecture: 1. Quantum physics Electron Clouds
Part II Particle and Nuclear Physics Examples Sheet 4
 Part II Particle and Nuclear Physics Examples Sheet 4 T. Potter Lent/Easter Terms 018 Basic Nuclear Properties 8. (B) The Semi-Empirical mass formula (SEMF) for nuclear masses may be written in the form
Part II Particle and Nuclear Physics Examples Sheet 4 T. Potter Lent/Easter Terms 018 Basic Nuclear Properties 8. (B) The Semi-Empirical mass formula (SEMF) for nuclear masses may be written in the form
Beta and gamma decays
 Beta and gamma decays April 9, 2002 Simple Fermi theory of beta decay ² Beta decay is one of the most easily found kinds of radioactivity. As we have seen, this result of the weak interaction leads to
Beta and gamma decays April 9, 2002 Simple Fermi theory of beta decay ² Beta decay is one of the most easily found kinds of radioactivity. As we have seen, this result of the weak interaction leads to
Lecture 15: Scattering Rutherford scattering Nuclear elastic scattering Nuclear inelastic scattering Quantum description The optical model
 Lecture 15: Scattering Rutherford scattering Nuclear elastic scattering Nuclear inelastic scattering Quantum description The optical model Lecture 15: Ohio University PHYS7501, Fall 017, Z. Meisel (meisel@ohio.edu)
Lecture 15: Scattering Rutherford scattering Nuclear elastic scattering Nuclear inelastic scattering Quantum description The optical model Lecture 15: Ohio University PHYS7501, Fall 017, Z. Meisel (meisel@ohio.edu)
8 Nuclei. introduc)on to Astrophysics, C. Bertulani, Texas A&M-Commerce 1
 8 Nuclei introduc)on to Astrophysics, C. Bertulani, Texas A&M-Commerce 1 8.1 - The nucleus The atomic nucleus consists of protons and neutrons. Protons and neutrons are called nucleons. A nucleus is characterized
8 Nuclei introduc)on to Astrophysics, C. Bertulani, Texas A&M-Commerce 1 8.1 - The nucleus The atomic nucleus consists of protons and neutrons. Protons and neutrons are called nucleons. A nucleus is characterized
RFSS: Lecture 8 Nuclear Force, Structure and Models Part 1 Readings: Nuclear Force Nuclear and Radiochemistry:
 RFSS: Lecture 8 Nuclear Force, Structure and Models Part 1 Readings: Nuclear and Radiochemistry: Chapter 10 (Nuclear Models) Modern Nuclear Chemistry: Chapter 5 (Nuclear Forces) and Chapter 6 (Nuclear
RFSS: Lecture 8 Nuclear Force, Structure and Models Part 1 Readings: Nuclear and Radiochemistry: Chapter 10 (Nuclear Models) Modern Nuclear Chemistry: Chapter 5 (Nuclear Forces) and Chapter 6 (Nuclear
Nuclear Decays. Alpha Decay
 Nuclear Decays The first evidence of radioactivity was a photographic plate, wrapped in black paper and placed under a piece of uranium salt by Henri Becquerel on February 26, 1896. Like many events in
Nuclear Decays The first evidence of radioactivity was a photographic plate, wrapped in black paper and placed under a piece of uranium salt by Henri Becquerel on February 26, 1896. Like many events in
Interaction with matter
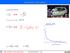 Interaction with matter accelerated motion: ss = bb 2 tt2 tt = 2 ss bb vv = vv 0 bb tt = vv 0 2 ss bb EE = 1 2 mmvv2 dddd dddd = mm vv 0 2 ss bb 1 bb eeeeeeeeeeee llllllll bbbbbbbbbbbbbb dddddddddddddddd
Interaction with matter accelerated motion: ss = bb 2 tt2 tt = 2 ss bb vv = vv 0 bb tt = vv 0 2 ss bb EE = 1 2 mmvv2 dddd dddd = mm vv 0 2 ss bb 1 bb eeeeeeeeeeee llllllll bbbbbbbbbbbbbb dddddddddddddddd
Alpha Decay. Decay alpha particles are monoenergetic. Nuclides with A>150 are unstable against alpha decay. E α = Q (1-4/A)
 Alpha Decay Because the binding energy of the alpha particle is so large (28.3 MeV), it is often energetically favorable for a heavy nucleus to emit an alpha particle Nuclides with A>150 are unstable against
Alpha Decay Because the binding energy of the alpha particle is so large (28.3 MeV), it is often energetically favorable for a heavy nucleus to emit an alpha particle Nuclides with A>150 are unstable against
Lecture 2: Nuclear Phenomenology Mass models & systematics Charge & matter distributions Moments & deformation Spin, parity, & isospin
 Lecture 2: Nuclear Phenomenology Mass models & systematics Charge & matter distributions Moments & deformation Spin, parity, & isospin Empirically-motivated & focused theory Lecture 2: Ohio University
Lecture 2: Nuclear Phenomenology Mass models & systematics Charge & matter distributions Moments & deformation Spin, parity, & isospin Empirically-motivated & focused theory Lecture 2: Ohio University
FACTS WHY? C. Alpha Decay Probability 1. Energetics: Q α positive for all A>140 nuclei
 C. Alpha Decay Probability 1. Energetics: Q α positive for all A>140 nuclei 2. Range of Measured Half-Lives (~10 44 ) 10 16 y > t 1/2 > 10 21 s 3. Why α? a. Proton & Neutron Emission: Q p, Q n are negative
C. Alpha Decay Probability 1. Energetics: Q α positive for all A>140 nuclei 2. Range of Measured Half-Lives (~10 44 ) 10 16 y > t 1/2 > 10 21 s 3. Why α? a. Proton & Neutron Emission: Q p, Q n are negative
The nucleus and its structure
 The nucleus and its structure Presently no complete theory to fully describe structure and behavior of nuclei based solely on knowledge of force between nucleons (although tremendous progress for A < 12
The nucleus and its structure Presently no complete theory to fully describe structure and behavior of nuclei based solely on knowledge of force between nucleons (although tremendous progress for A < 12
PHY492: Nuclear & Particle Physics. Lecture 6 Models of the Nucleus Liquid Drop, Fermi Gas, Shell
 PHY492: Nuclear & Particle Physics Lecture 6 Models of the Nucleus Liquid Drop, Fermi Gas, Shell Liquid drop model Five terms (+ means weaker binding) in a prediction of the B.E. r ~A 1/3, Binding is short
PHY492: Nuclear & Particle Physics Lecture 6 Models of the Nucleus Liquid Drop, Fermi Gas, Shell Liquid drop model Five terms (+ means weaker binding) in a prediction of the B.E. r ~A 1/3, Binding is short
Thursday, April 23, 15. Nuclear Physics
 Nuclear Physics Some Properties of Nuclei! All nuclei are composed of protons and neutrons! Exception is ordinary hydrogen with just a proton! The atomic number, Z, equals the number of protons in the
Nuclear Physics Some Properties of Nuclei! All nuclei are composed of protons and neutrons! Exception is ordinary hydrogen with just a proton! The atomic number, Z, equals the number of protons in the
Chemical Engineering 412
 Chemical Engineering 412 Introductory Nuclear Engineering Lecture 7 Nuclear Decay Behaviors Spiritual Thought Sooner or later, I believe that all of us experience times when the very fabric of our world
Chemical Engineering 412 Introductory Nuclear Engineering Lecture 7 Nuclear Decay Behaviors Spiritual Thought Sooner or later, I believe that all of us experience times when the very fabric of our world
Nuclear vibrations and rotations
 Nuclear vibrations and rotations Introduction to Nuclear Science Simon Fraser University Spring 2011 NUCS 342 February 2, 2011 NUCS 342 (Lecture 9) February 2, 2011 1 / 29 Outline 1 Significance of collective
Nuclear vibrations and rotations Introduction to Nuclear Science Simon Fraser University Spring 2011 NUCS 342 February 2, 2011 NUCS 342 (Lecture 9) February 2, 2011 1 / 29 Outline 1 Significance of collective
Lecture 11 Krane Enge Cohen Williams. Beta decay` Ch 9 Ch 11 Ch /4
 Lecture 11 Krane Enge Cohen Williams Isospin 11.3 6.7 6.3 8.10 Beta decay` Ch 9 Ch 11 Ch 11 5.3/4 Problems Lecture 11 1 Discuss the experimental evidence for the existence of the neutrino. 2 The nuclide
Lecture 11 Krane Enge Cohen Williams Isospin 11.3 6.7 6.3 8.10 Beta decay` Ch 9 Ch 11 Ch 11 5.3/4 Problems Lecture 11 1 Discuss the experimental evidence for the existence of the neutrino. 2 The nuclide
Physics 228 Today: April 22, 2012 Ch. 43 Nuclear Physics. Website: Sakai 01:750:228 or
 Physics 228 Today: April 22, 2012 Ch. 43 Nuclear Physics Website: Sakai 01:750:228 or www.physics.rutgers.edu/ugrad/228 Nuclear Sizes Nuclei occupy the center of the atom. We can view them as being more
Physics 228 Today: April 22, 2012 Ch. 43 Nuclear Physics Website: Sakai 01:750:228 or www.physics.rutgers.edu/ugrad/228 Nuclear Sizes Nuclei occupy the center of the atom. We can view them as being more
Quantum Theory of Many-Particle Systems, Phys. 540
 Quantum Theory of Many-Particle Systems, Phys. 540 Questions about organization Second quantization Questions about last class? Comments? Similar strategy N-particles Consider Two-body operators in Fock
Quantum Theory of Many-Particle Systems, Phys. 540 Questions about organization Second quantization Questions about last class? Comments? Similar strategy N-particles Consider Two-body operators in Fock
PHL424: Feynman diagrams
 PHL424: Feynman diagrams In 1940s, R. Feynman developed a diagram technique to describe particle interactions in space-time. Feynman diagram example Richard Feynman time Particles are represented by lines
PHL424: Feynman diagrams In 1940s, R. Feynman developed a diagram technique to describe particle interactions in space-time. Feynman diagram example Richard Feynman time Particles are represented by lines
Introduction to Nuclear Physics and Nuclear Decay
 Introduction to Nuclear Physics and Nuclear Decay Larry MacDonald macdon@uw.edu Nuclear Medicine Basic Science Lectures September 6, 2011 toms Nucleus: ~10-14 m diameter ~10 17 kg/m 3 Electron clouds:
Introduction to Nuclear Physics and Nuclear Decay Larry MacDonald macdon@uw.edu Nuclear Medicine Basic Science Lectures September 6, 2011 toms Nucleus: ~10-14 m diameter ~10 17 kg/m 3 Electron clouds:
Chapter Three (Nuclear Radiation)
 Al-Mustansiriyah University College of Science Physics Department Fourth Grade Nuclear Physics Dr. Ali A. Ridha Chapter Three (Nuclear Radiation) (3-1) Nuclear Radiation Whenever a nucleus can attain a
Al-Mustansiriyah University College of Science Physics Department Fourth Grade Nuclear Physics Dr. Ali A. Ridha Chapter Three (Nuclear Radiation) (3-1) Nuclear Radiation Whenever a nucleus can attain a
Nice Try. Introduction: Development of Nuclear Physics 20/08/2010. Nuclear Binding, Radioactivity. SPH4UI Physics
 SPH4UI Physics Modern understanding: the ``onion picture Nuclear Binding, Radioactivity Nucleus Protons tom and neutrons Let s see what s inside! 3 Nice Try Introduction: Development of Nuclear Physics
SPH4UI Physics Modern understanding: the ``onion picture Nuclear Binding, Radioactivity Nucleus Protons tom and neutrons Let s see what s inside! 3 Nice Try Introduction: Development of Nuclear Physics
Chapter 22 - Nuclear Chemistry
 Chapter - Nuclear Chemistry - The Nucleus I. Introduction A. Nucleons. Neutrons and protons B. Nuclides. Atoms identified by the number of protons and neutrons in the nucleus 8 a. radium-8 or 88 Ra II.
Chapter - Nuclear Chemistry - The Nucleus I. Introduction A. Nucleons. Neutrons and protons B. Nuclides. Atoms identified by the number of protons and neutrons in the nucleus 8 a. radium-8 or 88 Ra II.
Applied Nuclear Physics (Fall 2006) Lecture 12 (10/25/06) Empirical Binding Energy Formula and Mass Parabolas
 22.101 Applied Nuclear Physics (Fall 2006) Lecture 12 (10/25/06) Empirical Binding Energy Formula and Mass Parabolas References: W. E. Meyerhof, Elements of Nuclear Physics (McGraw-Hill, New York, 1967),
22.101 Applied Nuclear Physics (Fall 2006) Lecture 12 (10/25/06) Empirical Binding Energy Formula and Mass Parabolas References: W. E. Meyerhof, Elements of Nuclear Physics (McGraw-Hill, New York, 1967),
Nuclear Physics. PHY232 Remco Zegers Room W109 cyclotron building.
 Nuclear Physics PHY232 Remco Zegers zegers@nscl.msu.edu Room W109 cyclotron building http://www.nscl.msu.edu/~zegers/phy232.html Periodic table of elements We saw that the periodic table of elements can
Nuclear Physics PHY232 Remco Zegers zegers@nscl.msu.edu Room W109 cyclotron building http://www.nscl.msu.edu/~zegers/phy232.html Periodic table of elements We saw that the periodic table of elements can
Nuclear Properties. Thornton and Rex, Ch. 12
 Nuclear Properties Thornton and Rex, Ch. 12 A pre-history 1896 Radioactivity discovered - Becquerel a rays + (Helium) b rays - (electrons) g rays 0 (EM waves) 1902 Transmutation observed - Rutherford and
Nuclear Properties Thornton and Rex, Ch. 12 A pre-history 1896 Radioactivity discovered - Becquerel a rays + (Helium) b rays - (electrons) g rays 0 (EM waves) 1902 Transmutation observed - Rutherford and
Angular Momentum, Electromagnetic Waves
 Angular Momentum, Electromagnetic Waves Lecture33: Electromagnetic Theory Professor D. K. Ghosh, Physics Department, I.I.T., Bombay As before, we keep in view the four Maxwell s equations for all our discussions.
Angular Momentum, Electromagnetic Waves Lecture33: Electromagnetic Theory Professor D. K. Ghosh, Physics Department, I.I.T., Bombay As before, we keep in view the four Maxwell s equations for all our discussions.
 More Energetics of Alpha Decay The energy released in decay, Q, is determined by the difference in mass of the parent nucleus and the decay products, which include the daughter nucleus and the particle.
More Energetics of Alpha Decay The energy released in decay, Q, is determined by the difference in mass of the parent nucleus and the decay products, which include the daughter nucleus and the particle.
Nuclear Properties. Thornton and Rex, Ch. 12
 Nuclear Properties Thornton and Rex, Ch. 12 A pre-history 1896 Radioactivity discovered - Becquerel a rays + (Helium) b rays - (electrons) g rays 0 (EM waves) 1902 Transmutation observed - Rutherford and
Nuclear Properties Thornton and Rex, Ch. 12 A pre-history 1896 Radioactivity discovered - Becquerel a rays + (Helium) b rays - (electrons) g rays 0 (EM waves) 1902 Transmutation observed - Rutherford and
NERS 311 Current Old notes notes Chapter Chapter 1: Introduction to the course 1 - Chapter 1.1: About the course 2 - Chapter 1.
 NERS311/Fall 2014 Revision: August 27, 2014 Index to the Lecture notes Alex Bielajew, 2927 Cooley, bielajew@umich.edu NERS 311 Current Old notes notes Chapter 1 1 1 Chapter 1: Introduction to the course
NERS311/Fall 2014 Revision: August 27, 2014 Index to the Lecture notes Alex Bielajew, 2927 Cooley, bielajew@umich.edu NERS 311 Current Old notes notes Chapter 1 1 1 Chapter 1: Introduction to the course
CHAPTER 12 The Atomic Nucleus
 CHAPTER 12 The Atomic Nucleus 12.1 Discovery of the Neutron 12.2 Nuclear Properties 12.3 The Deuteron 12.4 Nuclear Forces 12.5 Nuclear Stability 12.6 Radioactive Decay 12.7 Alpha, Beta, and Gamma Decay
CHAPTER 12 The Atomic Nucleus 12.1 Discovery of the Neutron 12.2 Nuclear Properties 12.3 The Deuteron 12.4 Nuclear Forces 12.5 Nuclear Stability 12.6 Radioactive Decay 12.7 Alpha, Beta, and Gamma Decay
(1) Correspondence of the density matrix to traditional method
 (1) Correspondence of the density matrix to traditional method New method (with the density matrix) Traditional method (from thermal physics courses) ZZ = TTTT ρρ = EE ρρ EE = dddd xx ρρ xx ii FF = UU
(1) Correspondence of the density matrix to traditional method New method (with the density matrix) Traditional method (from thermal physics courses) ZZ = TTTT ρρ = EE ρρ EE = dddd xx ρρ xx ii FF = UU
Nuclear Physics for Applications
 Stanley C. Pruss'm Nuclear Physics for Applications A Model Approach BICENTENNIAL WILEY-VCH Verlag GmbH & Co. KGaA VII Table of Contents Preface XIII 1 Introduction 1 1.1 Low-Energy Nuclear Physics for
Stanley C. Pruss'm Nuclear Physics for Applications A Model Approach BICENTENNIAL WILEY-VCH Verlag GmbH & Co. KGaA VII Table of Contents Preface XIII 1 Introduction 1 1.1 Low-Energy Nuclear Physics for
Strand J. Atomic Structure. Unit 2. Radioactivity. Text
 Strand J. Atomic Structure Unit 2. Radioactivity Contents Page Unstable Nuclei 2 Alpha, Beta and Gamma Radiation 5 Balancing Equations for Radioactive Decay 10 Half Life 12 J.2.1. Unstable Nuclei. The
Strand J. Atomic Structure Unit 2. Radioactivity Contents Page Unstable Nuclei 2 Alpha, Beta and Gamma Radiation 5 Balancing Equations for Radioactive Decay 10 Half Life 12 J.2.1. Unstable Nuclei. The
General Physics (PHY 2140)
 General Physics (PHY 2140) Lecture 37 Modern Physics Nuclear Physics Radioactivity Nuclear reactions http://www.physics.wayne.edu/~apetrov/phy2140/ Chapter 29 1 Lightning Review Last lecture: 1. Nuclear
General Physics (PHY 2140) Lecture 37 Modern Physics Nuclear Physics Radioactivity Nuclear reactions http://www.physics.wayne.edu/~apetrov/phy2140/ Chapter 29 1 Lightning Review Last lecture: 1. Nuclear
RFSS: Lecture 2 Nuclear Properties
 RFSS: Lecture 2 Nuclear Properties Readings: Modern Nuclear Chemistry: Chapter 2 Nuclear Properties Nuclear and Radiochemistry: Chapter 1 Introduction, Chapter 2 Atomic Nuclei Nuclear properties Masses
RFSS: Lecture 2 Nuclear Properties Readings: Modern Nuclear Chemistry: Chapter 2 Nuclear Properties Nuclear and Radiochemistry: Chapter 1 Introduction, Chapter 2 Atomic Nuclei Nuclear properties Masses
Alpha decay, ssion, and nuclear reactions
 Alpha decay, ssion, and nuclear reactions March 11, 2002 1 Energy release in alpha-decay ² Consider a nucleus which is stable against decay by proton or neutron emission { the least bound nucleon still
Alpha decay, ssion, and nuclear reactions March 11, 2002 1 Energy release in alpha-decay ² Consider a nucleus which is stable against decay by proton or neutron emission { the least bound nucleon still
Decay Mechanisms. The laws of conservation of charge and of nucleons require that for alpha decay, He + Q 3.1
 Decay Mechanisms 1. Alpha Decay An alpha particle is a helium-4 nucleus. This is a very stable entity and alpha emission was, historically, the first decay process to be studied in detail. Almost all naturally
Decay Mechanisms 1. Alpha Decay An alpha particle is a helium-4 nucleus. This is a very stable entity and alpha emission was, historically, the first decay process to be studied in detail. Almost all naturally
Nuclear Properties. Thornton and Rex, Ch. 12
 Nuclear Properties Thornton and Rex, Ch. 12 A pre-history 1896 Radioactivity discovered - Becquerel a rays + (Helium) b rays - (electrons) g rays 0 (EM waves) 1902 Transmutation observed - Rutherford and
Nuclear Properties Thornton and Rex, Ch. 12 A pre-history 1896 Radioactivity discovered - Becquerel a rays + (Helium) b rays - (electrons) g rays 0 (EM waves) 1902 Transmutation observed - Rutherford and
15. Nuclear Decay. Particle and Nuclear Physics. Dr. Tina Potter. Dr. Tina Potter 15. Nuclear Decay 1
 15. Nuclear Decay Particle and Nuclear Physics Dr. Tina Potter Dr. Tina Potter 15. Nuclear Decay 1 In this section... Radioactive decays Radioactive dating α decay β decay γ decay Dr. Tina Potter 15. Nuclear
15. Nuclear Decay Particle and Nuclear Physics Dr. Tina Potter Dr. Tina Potter 15. Nuclear Decay 1 In this section... Radioactive decays Radioactive dating α decay β decay γ decay Dr. Tina Potter 15. Nuclear
Lecture 4: Nuclear Energy Generation
 Lecture 4: Nuclear Energy Generation Literature: Prialnik chapter 4.1 & 4.2!" 1 a) Some properties of atomic nuclei Let: Z = atomic number = # of protons in nucleus A = atomic mass number = # of nucleons
Lecture 4: Nuclear Energy Generation Literature: Prialnik chapter 4.1 & 4.2!" 1 a) Some properties of atomic nuclei Let: Z = atomic number = # of protons in nucleus A = atomic mass number = # of nucleons
Photons in the universe. Indian Institute of Technology Ropar
 Photons in the universe Photons in the universe Element production on the sun Spectral lines of hydrogen absorption spectrum absorption hydrogen gas Hydrogen emission spectrum Element production on the
Photons in the universe Photons in the universe Element production on the sun Spectral lines of hydrogen absorption spectrum absorption hydrogen gas Hydrogen emission spectrum Element production on the
Big Bang Planck Era. This theory: cosmological model of the universe that is best supported by several aspects of scientific evidence and observation
 Big Bang Planck Era Source: http://www.crystalinks.com/bigbang.html Source: http://www.odec.ca/index.htm This theory: cosmological model of the universe that is best supported by several aspects of scientific
Big Bang Planck Era Source: http://www.crystalinks.com/bigbang.html Source: http://www.odec.ca/index.htm This theory: cosmological model of the universe that is best supported by several aspects of scientific
Stability of heavy elements against alpha and cluster radioactivity
 CHAPTER III Stability of heavy elements against alpha and cluster radioactivity The stability of heavy and super heavy elements via alpha and cluster decay for the isotopes in the heavy region is discussed
CHAPTER III Stability of heavy elements against alpha and cluster radioactivity The stability of heavy and super heavy elements via alpha and cluster decay for the isotopes in the heavy region is discussed
Alpha decay. Introduction to Nuclear Science. Simon Fraser University Spring NUCS 342 February 21, 2011
 Alpha decay Introduction to Nuclear Science Simon Fraser University Spring 2011 NUCS 342 February 21, 2011 NUCS 342 (Lecture 13) February 21, 2011 1 / 29 Outline 1 The decay processes NUCS 342 (Lecture
Alpha decay Introduction to Nuclear Science Simon Fraser University Spring 2011 NUCS 342 February 21, 2011 NUCS 342 (Lecture 13) February 21, 2011 1 / 29 Outline 1 The decay processes NUCS 342 (Lecture
Fundamental Forces. Range Carrier Observed? Strength. Gravity Infinite Graviton No. Weak 10-6 Nuclear W+ W- Z Yes (1983)
 Fundamental Forces Force Relative Strength Range Carrier Observed? Gravity 10-39 Infinite Graviton No Weak 10-6 Nuclear W+ W- Z Yes (1983) Electromagnetic 10-2 Infinite Photon Yes (1923) Strong 1 Nuclear
Fundamental Forces Force Relative Strength Range Carrier Observed? Gravity 10-39 Infinite Graviton No Weak 10-6 Nuclear W+ W- Z Yes (1983) Electromagnetic 10-2 Infinite Photon Yes (1923) Strong 1 Nuclear
A is called the mass number gives, roughly, the mass of the nucleus or atom in atomic mass units = amu = u
 5/5 A is called the mass number gives, roughly, the mass of the nucleus or atom in atomic mass units = amu = u The number of neutrons in the nucleus is given by the symbol N. Clearly, N = A Z. Isotope:
5/5 A is called the mass number gives, roughly, the mass of the nucleus or atom in atomic mass units = amu = u The number of neutrons in the nucleus is given by the symbol N. Clearly, N = A Z. Isotope:
Chapter 10 - Nuclear Physics
 The release of atomic energy has not created a new problem. It has merely made more urgent the necessity of solving an existing one. -Albert Einstein David J. Starling Penn State Hazleton PHYS 214 Ernest
The release of atomic energy has not created a new problem. It has merely made more urgent the necessity of solving an existing one. -Albert Einstein David J. Starling Penn State Hazleton PHYS 214 Ernest
What did you learn in the last lecture?
 What did you learn in the last lecture? Charge density distribution of a nucleus from electron scattering SLAC: 21 GeV e s ; λ ~ 0.1 fm (to first order assume that this is also the matter distribution
What did you learn in the last lecture? Charge density distribution of a nucleus from electron scattering SLAC: 21 GeV e s ; λ ~ 0.1 fm (to first order assume that this is also the matter distribution
Introductory Nuclear Physics. Glatzmaier and Krumholz 7 Prialnik 4 Pols 6 Clayton 4.1, 4.4
 Introductory Nuclear Physics Glatzmaier and Krumholz 7 Prialnik 4 Pols 6 Clayton 4.1, 4.4 Each nucleus is a bound collection of N neutrons and Z protons. The mass number is A = N + Z, the atomic number
Introductory Nuclear Physics Glatzmaier and Krumholz 7 Prialnik 4 Pols 6 Clayton 4.1, 4.4 Each nucleus is a bound collection of N neutrons and Z protons. The mass number is A = N + Z, the atomic number
1 Introduction. 2 The hadronic many body problem
 Models Lecture 18 1 Introduction In the next series of lectures we discuss various models, in particluar models that are used to describe strong interaction problems. We introduce this by discussing the
Models Lecture 18 1 Introduction In the next series of lectures we discuss various models, in particluar models that are used to describe strong interaction problems. We introduce this by discussing the
Applied Nuclear Physics (Fall 2004) Lecture 11 (10/20/04) Nuclear Binding Energy and Stability
 22.101 Applied Nuclear Physics (Fall 2004) Lecture 11 (10/20/04) Nuclear Binding Energy and Stability References: W. E. Meyerhof, Elements of Nuclear Physics (McGraw-Hill, New York, 1967), Chap.2. The
22.101 Applied Nuclear Physics (Fall 2004) Lecture 11 (10/20/04) Nuclear Binding Energy and Stability References: W. E. Meyerhof, Elements of Nuclear Physics (McGraw-Hill, New York, 1967), Chap.2. The
Introduction to Nuclear Science
 Introduction to Nuclear Science PAN Summer Science Program University of Notre Dame June, 2014 Tony Hyder Professor of Physics Topics we will discuss Ground-state properties of the nucleus size, shape,
Introduction to Nuclear Science PAN Summer Science Program University of Notre Dame June, 2014 Tony Hyder Professor of Physics Topics we will discuss Ground-state properties of the nucleus size, shape,
Quantum Mechanics. An essential theory to understand properties of matter and light. Chemical Electronic Magnetic Thermal Optical Etc.
 Quantum Mechanics An essential theory to understand properties of matter and light. Chemical Electronic Magnetic Thermal Optical Etc. Fall 2018 Prof. Sergio B. Mendes 1 CHAPTER 3 Experimental Basis of
Quantum Mechanics An essential theory to understand properties of matter and light. Chemical Electronic Magnetic Thermal Optical Etc. Fall 2018 Prof. Sergio B. Mendes 1 CHAPTER 3 Experimental Basis of
Lecture Outlines Chapter 32. Physics, 3 rd Edition James S. Walker
 Lecture Outlines Chapter 32 Physics, 3 rd Edition James S. Walker 2007 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the use of instructors in
Lecture Outlines Chapter 32 Physics, 3 rd Edition James S. Walker 2007 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the use of instructors in
Describe the structure of the nucleus Calculate nuclear binding energies Identify factors affecting nuclear stability
 Atomic and Nuclear Structure George Starkschall, Ph.D. Lecture Objectives Describe the atom using the Bohr model Identify the various electronic shells and their quantum numbers Recall the relationship
Atomic and Nuclear Structure George Starkschall, Ph.D. Lecture Objectives Describe the atom using the Bohr model Identify the various electronic shells and their quantum numbers Recall the relationship
Physics 142 Modern Physics 2 Page 1. Nuclear Physics
 Physics 142 Modern Physics 2 Page 1 Nuclear Physics The Creation of the Universe was made possible by a grant from Texas Instruments. Credit on a PBS Program Overview: the elements are not elementary The
Physics 142 Modern Physics 2 Page 1 Nuclear Physics The Creation of the Universe was made possible by a grant from Texas Instruments. Credit on a PBS Program Overview: the elements are not elementary The
Photon Interactions in Matter
 Radiation Dosimetry Attix 7 Photon Interactions in Matter Ho Kyung Kim hokyung@pusan.ac.kr Pusan National University References F. H. Attix, Introduction to Radiological Physics and Radiation Dosimetry,
Radiation Dosimetry Attix 7 Photon Interactions in Matter Ho Kyung Kim hokyung@pusan.ac.kr Pusan National University References F. H. Attix, Introduction to Radiological Physics and Radiation Dosimetry,
Chapter 30 Nuclear Physics and Radioactivity
 Chapter 30 Nuclear Physics and Radioactivity 30.1 Structure and Properties of the Nucleus Nucleus is made of protons and neutrons Proton has positive charge: Neutron is electrically neutral: 30.1 Structure
Chapter 30 Nuclear Physics and Radioactivity 30.1 Structure and Properties of the Nucleus Nucleus is made of protons and neutrons Proton has positive charge: Neutron is electrically neutral: 30.1 Structure
12.744/ The Basic Rules, Nuclear Stability, Radioactive Decay and Radioactive Dating
 12.744/12.754 The Basic Rules, Nuclear Stability, Radioactive Decay and Radioactive Dating What we see in the earth and oceans is the product of the "cosmic" abundance (i.e. the original) pattern of elements,
12.744/12.754 The Basic Rules, Nuclear Stability, Radioactive Decay and Radioactive Dating What we see in the earth and oceans is the product of the "cosmic" abundance (i.e. the original) pattern of elements,
Lesson 1. Introduction to Nuclear Science
 Lesson 1 Introduction to Nuclear Science Introduction to Nuclear Chemistry What is nuclear chemistry? What is the relation of nuclear chemistry to other parts of chemistry? Nuclear chemistry vs nuclear
Lesson 1 Introduction to Nuclear Science Introduction to Nuclear Chemistry What is nuclear chemistry? What is the relation of nuclear chemistry to other parts of chemistry? Nuclear chemistry vs nuclear
THE NUCLEUS: A CHEMIST S VIEW Chapter 20
 THE NUCLEUS: A CHEMIST S VIEW Chapter 20 "For a long time I have considered even the craziest ideas about [the] atom[ic] nucleus... and suddenly discovered the truth." [shell model of the nucleus]. Maria
THE NUCLEUS: A CHEMIST S VIEW Chapter 20 "For a long time I have considered even the craziest ideas about [the] atom[ic] nucleus... and suddenly discovered the truth." [shell model of the nucleus]. Maria
Chapter IV: Radioactive decay
 Chapter IV: Radioactive decay 1 Summary 1. Law of radioactive decay 2. Decay chain/radioactive filiation 3. Quantum description 4. Types of radioactive decay 2 History Radioactivity was discover in 1896
Chapter IV: Radioactive decay 1 Summary 1. Law of radioactive decay 2. Decay chain/radioactive filiation 3. Quantum description 4. Types of radioactive decay 2 History Radioactivity was discover in 1896
Introduction to Nuclear Science
 Introduction to Nuclear Science PIXIE-PAN Summer Science Program University of Notre Dame 2006 Tony Hyder, Professor of Physics Topics we will discuss Ground-state properties of the nucleus Radioactivity
Introduction to Nuclear Science PIXIE-PAN Summer Science Program University of Notre Dame 2006 Tony Hyder, Professor of Physics Topics we will discuss Ground-state properties of the nucleus Radioactivity
Physics 107 Final Exam May 6, Your Name: 1. Questions
 Physics 107 Final Exam May 6, 1996 Your Name: 1. Questions 1. 9. 17. 5.. 10. 18. 6. 3. 11. 19. 7. 4. 1. 0. 8. 5. 13. 1. 9. 6. 14.. 30. 7. 15. 3. 8. 16. 4.. Problems 1. 4. 7. 10. 13.. 5. 8. 11. 14. 3. 6.
Physics 107 Final Exam May 6, 1996 Your Name: 1. Questions 1. 9. 17. 5.. 10. 18. 6. 3. 11. 19. 7. 4. 1. 0. 8. 5. 13. 1. 9. 6. 14.. 30. 7. 15. 3. 8. 16. 4.. Problems 1. 4. 7. 10. 13.. 5. 8. 11. 14. 3. 6.
(1) Introduction: a new basis set
 () Introduction: a new basis set In scattering, we are solving the S eq. for arbitrary VV in integral form We look for solutions to unbound states: certain boundary conditions (EE > 0, plane and spherical
() Introduction: a new basis set In scattering, we are solving the S eq. for arbitrary VV in integral form We look for solutions to unbound states: certain boundary conditions (EE > 0, plane and spherical
Chapter VI: Beta decay
 Chapter VI: Beta decay 1 Summary 1. General principles 2. Energy release in decay 3. Fermi theory of decay 4. Selections rules 5. Electron capture decay 6. Other decays 2 General principles (1) The decay
Chapter VI: Beta decay 1 Summary 1. General principles 2. Energy release in decay 3. Fermi theory of decay 4. Selections rules 5. Electron capture decay 6. Other decays 2 General principles (1) The decay
SECTION C: NUCLEAR RADIATION AND NUCLEAR ENERGY LOSS PROCESSES. " N & = '!t and so N = N 0. implying ln! N $
 SECTO C: UCLEAR RADATO AD UCLEAR EERGY LOSS PROCESSES n this section we discuss decay and transmutation processes in nuclei (including α, β, and γ decay, as well as fission and fusion processes), using
SECTO C: UCLEAR RADATO AD UCLEAR EERGY LOSS PROCESSES n this section we discuss decay and transmutation processes in nuclei (including α, β, and γ decay, as well as fission and fusion processes), using
Chem 481 Lecture Material 1/30/09
 Chem 481 Lecture Material 1/30/09 Nature of Radioactive Decay The Standard Model in physics postulates that all particles in nature are composed of quarks and leptons and that they interact by exchange
Chem 481 Lecture Material 1/30/09 Nature of Radioactive Decay The Standard Model in physics postulates that all particles in nature are composed of quarks and leptons and that they interact by exchange
Nuclear Physics and Nuclear Reactions
 Slide 1 / 33 Nuclear Physics and Nuclear Reactions The Nucleus Slide 2 / 33 Proton: The charge on a proton is +1.6x10-19 C. The mass of a proton is 1.6726x10-27 kg. Neutron: The neutron is neutral. The
Slide 1 / 33 Nuclear Physics and Nuclear Reactions The Nucleus Slide 2 / 33 Proton: The charge on a proton is +1.6x10-19 C. The mass of a proton is 1.6726x10-27 kg. Neutron: The neutron is neutral. The
The Proper)es of Nuclei. Nucleons
 The Proper)es of Nuclei Z N Nucleons The nucleus is made of neutrons and protons. The nucleons have spin ½ and (individually) obey the Pauli exclusion principle. Protons p 938.3 MeV 2.79µ N Neutrons n
The Proper)es of Nuclei Z N Nucleons The nucleus is made of neutrons and protons. The nucleons have spin ½ and (individually) obey the Pauli exclusion principle. Protons p 938.3 MeV 2.79µ N Neutrons n
Atomic fluorescence. The intensity of a transition line can be described with a transition probability inversely
 Atomic fluorescence 1. Introduction Transitions in multi-electron atoms Energy levels of the single-electron hydrogen atom are well-described by EE nn = RR nn2, where RR = 13.6 eeee is the Rydberg constant.
Atomic fluorescence 1. Introduction Transitions in multi-electron atoms Energy levels of the single-electron hydrogen atom are well-described by EE nn = RR nn2, where RR = 13.6 eeee is the Rydberg constant.
Fundamental Stellar Parameters. Radiative Transfer. Stellar Atmospheres. Equations of Stellar Structure
 Fundamental Stellar Parameters Radiative Transfer Stellar Atmospheres Equations of Stellar Structure Nuclear Reactions in Stellar Interiors Binding Energy Coulomb Barrier Penetration Hydrogen Burning Reactions
Fundamental Stellar Parameters Radiative Transfer Stellar Atmospheres Equations of Stellar Structure Nuclear Reactions in Stellar Interiors Binding Energy Coulomb Barrier Penetration Hydrogen Burning Reactions
