Early in the last century, Robert Millikan developed
|
|
|
- Megan Holland
- 6 years ago
- Views:
Transcription
1 Millikan s Oil-Drop Experiment: A Centennial Setup Revisited in the Virtual World Michel Gagnon, Université de Saint-Boniface, Winnipeg, MB Canada Early in the last century, Robert Millikan developed a precise method of determining the electric charge carried by oil droplets. 1-3 Using a microscope and a small incandescent lamp, he observed the fall of charged droplets under the influence of an electric field inside a small observation chamber. In so doing, Millikan demonstrated the existence of a fundamental unit of electric charge, and established its quantization. Now renowned as one of the most famous experiments of 20th-century physics, Millikan s oil-drop experiment has been reproduced with more or less success in most, if not all, high school and university physics classes. This has encouraged many improvements of the apparatus, now making this experiment much more accurate and easier to realize for advanced students. However, the required apparatus remains rather expensive, and for introductory college or high school students the experiment is still quite difficult to conduct. As an alternative to the traditional setup, a realistic computer-based simulator to replicate the Millikan oil-drop experiment has been developed. Using this software, students are able to undertake a complete experiment, obtain an accurate set of results, and thus gain a better understanding of the original experiment and its historical importance. Historical and beautiful, yet tedious and delicate The heart of the instrument consists of the chamber with a parallel pair of horizontal metal plates with a separation of 4 mm. By applying a potential difference to the two plates, a uniform electric field is created in the space between. Originally, a fine mist of oil droplets with very low vapor pressure was sprayed into the chamber. Today, the droplets are often replaced by tiny plastic beads. As they are sprayed, the beads acquire electric charges through friction with the nozzle; changing the voltage across the plates can make them rise or fall. A microscope and a small lamp allow observation of what is happening inside the chamber, as well as the graduation engraved on the reticle. Basically, to conduct the experiment, one has to spot a bead at the center of the viewing area and evaluate its velocity using a stopwatch and the graduation on the reticle. Of course, this process has to be repeated many times. Alternatively, one can try to immobilize a bead by varying the voltage to the terminals of the plates; however, in reality, this is close to impossible to achieve. Using variations of the original setup, generations of students have spent hours of observation and tricky fine-tuning to achieve final results that may have up to a 20% margin of error. 4 Difficulties associated with the experiment have led many laboratory instructors to attempt to render the oil-drop experiment more student friendly 4-6 or simply to replace it with something more accessible. 7 Moreover, recent innovations to available apparatus on the market have considerably decreased the inherent difficulties of the experiment. These successes have to be celebrated as the original setup has now evolved into an excellent training tool for advanced students. Indeed, they should now be ready to pay more attention to the details and be more careful when dealing with an experiment precise enough to measure a quantity as small as the fundamental unit of electric charge. Unfortunately, despite its beauty and historical importance, this delicate experiment remains costly and difficult to conduct at the introductory level. To overcome these problems, other instructors have developed computer-based simulations to mimic the Millikan apparatus. 8 However, these appear to be either more appropriate for a class demonstration, or almost as difficult to use as the real setup. Therefore, our software has been developed in order to greatly attenuate the many difficulties (fine-tuning, reading, etc.) associated with this particular experiment. This allows students to concentrate more on the scientific concepts than the difficulties associated with data acquisition. Of course, students using the software still have to perform measurements of times, distances, or voltages, giving rise to an uncertainty of about 1-2 % with no need to introduce an arbitrary uncertainty using the random number generator. Two methods of conducting the experiment Prior to discussing how to conduct the experiment using the software, it is useful to review its theoretical background. 9 There exist some variations of Millikan s original experiment using oil droplets; however, whatever the available apparatus, modeling the experiment requires the following four forces to be acting on free-falling particles in an electric field: 1. the weight of droplets: where r and r stand for radius and density; 2. the up-thrust due to the weight of air displaced by the droplet:, with r a as the density of the air; 3. the drag force described by Stokes law: F d = 6phrv, where h is the viscosity of the air and v the terminal velocity of the falling droplet; and 4. the electric force: F E = qe, where q is the charge on the droplet and E the electric field. 98 The Physics Teacher Vol. 50, February 2012 DOI: /
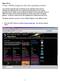
2 With this equation, it is possible to determine the radius of the droplet if one knows the viscosity of the air. The terminal velocity of the droplet is easily calculated using a stopwatch and the lines on the reticle. This is the usual path to follow when using actual oil-droplets, and it must be repeated in order for each droplet to be included in the data set. Of course, with modern apparatus, which uses plastic beads of known radius, this step became unnecessary. However, for pedagogical purposes, one can assume the viscosity of the air as being unknown; this can be easily calculated as: (2) Fig. 1. The main window of the Millikan oil-drop experiment software shows the observation area in black surrounded by the Millikan apparatus on the left, the power supply on the bottom, and virtual tools on the right. Next, the electric field is turned on. This introduces the fourth force, F E, which is proportional to the electric charge we want to discover. If the field force is upward, and sufficient to make the bead rise, then the drag force shifts downward, and the Newton law can be written: r e (3) where v e is the terminal velocity in the presence of the field, and has to be calculated. Using the expression found above for h, we get: g with the electric field given as E = V d where V is the potential difference across the apparatus chamber. The velocity v g has to be calculated only once. However, the velocity v e of the charged bead when the electric field is turned on has to be calculated for each individual bead in order to acquire an adequate data set. (4) Fig. 2. The heart of the software is the Millikan apparatus with its observation chamber, microscope, sprayer, lamp, and bubble-level. b) The Second Method An alternative approach is to adjust the electric field until the droplet remains steady. In that case, the null velocity disappears from the equations and with it the viscosity of the air. Consequently, there is no need to calculate r, h, nor v g, and thus we find: (5) Fig. 3. The power supply has two sliders for adjusting the voltage and a switch to reverse the polarity. a) The First Method In the absence of an electric field, the droplet will rapidly reach a constant velocity v g. When this is achieved, the total force acting on it must be zero, which means the three acting forces cancel out. This implies: (1) This steady state is very difficult to reach in practice with the real apparatus. However, using the magnifier to zoom in on a bead while it is rising, the simulator makes it possible to fine-tune the process and to achieve the determination of the stopping potential. This provides an alternate, simplified method to calculate the electric charge. The software As mentioned earlier, the software has been developed to alleviate the practical experimental difficulties, and to overcome the cost barrier of the equipment. The Physics Teacher Vol. 50, February
3 Table I. Raising velocities of beads under the influence of an electric field and the corresponding computed electric charge they carried. Data have been reorganized in ascending values of the electric charges. Prior to these measurements, the falling velocity of beads in the absence of electric field has been measured as m/s, and after this the voltage has been set to 40 V for the remaining experiment. Table I. First Method Velocity (10-4 m/s) Charge (10-19 C) Table II. Voltages used to immobilize beads and the corresponding electric charge carried by the beads. Data are reorganized in ascending values of the electric charges. Table II. Second Method Voltage (V) Charge (10-19 C) Fig. 4. The central part of the screen replicates what can be seen in the microscope. Tools are available to accurately measure the flight time and the distance traveled , The main screen of the software is divided into six sections (Fig. 1). The left one (in brown, Fig. 2) is meant to reproduce the apparatus itself, with the observation chamber (in green) surrounded by a droplet sprayer, a variable collimated lamp, and a small microscope with a slider to control the magnification (up to 32 ). The lamp and bubble level must be set correctly to enable spraying and zooming. By clicking on the sprayer, a single droplet with random speed and random electric charge is injected into the chamber. An option can be used to keep these parameters unchanged for subsequent droplets to be injected for as long as it is necessary for the student to complete the measurements. The power supply (in blue, Fig. 3) is on the bottom right. Apart from the main switch, there is a lever to allow reversing the polarity, and two sliders for coarse- and fine-tuning of the voltage. The central part of the screen is the observation area (black, Fig. 4) which reproduces what can be seen through Fig. 5. Data can be entered on a grid and saved in a file. the microscope. On the right side of the screen, a ruler (in millimeters) measures the distance covered by the droplet. Of course, zooming with the magnification ring resets the graduation accordingly. However, to make the required measurements (time and distance), it is better to use the provided tools: the chronometer (in gray) and the measuring tape (in yellow), on the right side of the screen. The measurements must be taken at constant speed. Therefore, one simply actuates the chronometer when the droplet s path is vertical, and stops it before the droplet reaches the bottom (or the top) of the observation area. This way, one obtains a mark on both ends of the path and may calculate the corresponding flight time. After that, it is sufficient to use the measuring tape. One click on both ending marks gives the distance traveled and makes it possible to compute the droplet s velocity. The grid s control center (Fig. 5) is below the stopwatch. The first button (grid) makes the grid appear or disappear over the observation area. The second button (pencil) is there to enter the voltage, the distance, and the duration of the last observed droplets on the grid. The last button (eraser) is used to erase the grid content. The buttons beside the grid allow for the erasing of single lines of data. 100 The Physics Teacher Vol. 50, February 2012
4 To conduct a complete experiment using the first method, students must follow these steps: 1. Prepare the apparatus (level, align the reticle, open the lamp). 2. Spray a bead. 3. Determine its velocity v g in the absence of an electric field using the virtual tools. 4. Turn on the virtual power supply and set to 40 V to actuate the electric field. 5. Spray beads until they get a rising one. If necessary, reverse the polarity. 6. Determine the velocity v e of the selected bead. 7. Repeat steps 5 and 6 until they have sufficient data. 8. Using the appropriate equation, compute the corresponding set of charges. 9. Organize the charges in ascending order of the computed electric charge. 10. Trace a graph. The second method does not require the calculation of velocities. Instead, students must adjust the voltage until the charged bead remains steady. The necessary steps are as follow: 1. Prepare the apparatus (level, align the reticle, open the lamp). 2. Spray a bead. 3. Adjust the voltage to stop the bead. 4. Zoom in using the magnifying slider to check if the bead is really immobile. 5. If not, repeat steps 3 and 4. The magnifying powers are 1, 2, 4, 8, 16, and Repeat steps 2 to 5 until they have sufficient data. 7. Using the appropriate equation, compute the corresponding set of charges. 8. Organize the charges in ascending order of the computed electric charge. 9. Trace a graph. Table I shows an example of a small set of data obtained with the first method: data have been arranged in ascending order of the computed electric charge using Eq. (4). The matching raising velocities v e are given in the first column and the falling velocity v g in the absence of an electric field has been computed to be m/s. On the corresponding chart (Fig. 6), one can clearly see that the electric charges carried by beads can be organized to exhibit a stair pattern with regular step height, or integer multiples of this height. Table II shows another set of data now obtained with the second method. This time, students have to determine the voltage to apply in order to stop the falling beads. Once again, data have been arranged in ascending order of the computed electric charge [Eq. (5)] and reported on a stair pattern chart (Fig. 7). Fig. 6. Data set obtained with the first method, organized in ascending order and showing a stair pattern. Fig. 7. Data set obtained with the second method and organized in ascending order. It still exhibits a stair pattern and moreover the lower value equates the fundamental unit of electric charge. If we analyze both sets of data, we find comparable uncertainties of less than 2% and 1%, respectively. The first method has the benefit of being closer to the real experiment. However, if we want to be able to make measurements with beads carrying only one unit of charge, we need a voltage of 120 V to get an electric force strong enough to raise beads. Unfortunately, such a voltage makes beads with more than three units of charge too fast to be measured. On the other hand, comparing Figs. 6 and 7, we realize that the second method is more suitable to observe a wider spectrum of carried charges. In particular, in the second example, the smallest value is equal to the step height and can be interpreted as the smallest indivisible electric charge. Moreover, we believe it may be of interest to students to realize that the same result can be obtained with two different kinds of data: time and distance for the first method and voltage for the second one. Conclusion By using a computer simulation to replicate circuit laboratory in a large-scale introductory physics course, we have demonstrated 10 that the use of computers appears to enhance learning for science students. After using the Millikan apparatus simulator in our introductory laboratories for two years, we too have noticed a significant improvement in students ability to deal with the Millikan experiment and related physical concepts. In particular, students obtain more accurate results, which, in turn, make analysis easier and more enlighten- The Physics Teacher Vol. 50, February
5 ing. Based upon our experience, this appears to be an effective way to make physics more accessible to all students, increase their interest, and improve their learning. A Windows compatible (98 and later) copy of the software (in English and French) is available as a free download at cusb/physique/nos_simulateurs-maison/telechargement/ or upon request from the author. Acknowledgments I would like to express my appreciation to my colleagues, Petra Franzen and Bryan Rivers, for having kindly helped me to improve the quality of this text. References 1. R. A. Millikan, The isolation of an ion, a precision measurement of its charge, and the correction of Stokes s law, Phys. Rev. (Series 1) 32, (April 1911). 2. R. A. Millikan, On the elementary electric charge and the Avogadro constant, Phys. Rev. (Series II) 2, (Aug. 1913). 3. A. Franklin, Millikan s oil-drop experiments, The Chem. Educ. 2 (1), 1 14 (1997). 4. Anthony Papirio, Jr., Claude M. Penchina, and Hajime Sakai, Novel approach to the oil-drop experiment, Phys. Teach. 38, (Jan. 2000). 5. Steve Brehmer, Millikan without the eyestrain, Phys. Teach. 29, 310 (May 1991). 6. Ray C. Jones, The Millikan oil-drop experiment: Making it worthwhile, Am. J. Phys. 63, (Nov. 1995). 7. Lowell I. McCann and Earl D. Blodgett, The nut-drop experiment Bringing Millikan s challenge to introductory students, Phys. Teach. 47, (Sept. 2009). 8. Dan MacIsaac, Websites for teaching high school and introductory college modern physics topics, Phys. Teach. 45, 124 (Feb. 2007). 9. R. A. Millikan, The Electron: Its Isolation and Measurement and the Determination of Some of its Properties (University of Chicago Press, 1917). 10. N. D. Finkelstein et al., When learning about the real world is better done virtually: A study of substituting computer simulations for laboratory equipment, Phys. Rev. ST - Phys. Educ. Res. 1, (2005). Physics teachers... get your students registered for the preliminary exam in the U.S. Physics Team selection process. All physics students are encouraged to participate in the American Association of Physics Teachers Fnet=ma Contest! The Fnet=ma Contest is the United States Physics Team selection process that leads to participation in the 43rd Annual International Physics Olympiad (IPhO) in Tallinn/Tartu, Estonia, July 15-24, The U.S. Physics Team Program provides a oncein-a-lifetime opportunity for students to enhance their physics knowledge as well as their creativity, leadership, and commitment to a goal. For program information and registration visit: (Registration is open now through January 3, 2012) Michel Gagnon joined the Université de Saint-Boniface in 1996, where he is an associate professor of physics. He received a PhD in theoretical highenergy physics in 1994 from Université Laval in Quebec City, following this with postdoctoral research and a science teaching certificate. His current interests include the design and development of computer simulations of scientific experiments, as well as the history of single lens reflex photography through its technical evolution. MiGagnon@ustboniface.mb.ca American Astronomical Society 102 The Physics Teacher Vol. 50, February 2012
PHYS-102 LAB 2. Millikan Oil Drop Experiment
 PHYS-102 LAB 2 Millikan Oil Drop Experiment 1. Objective The objectives of this lab are to: 2. Theory Verify the atomic nature of electricity. Determine the size of the charge on an electron. In today
PHYS-102 LAB 2 Millikan Oil Drop Experiment 1. Objective The objectives of this lab are to: 2. Theory Verify the atomic nature of electricity. Determine the size of the charge on an electron. In today
PHYS 3324 Lab Millikan Oil Drop Experiment: Demonstration of the Quantization of Charge
 PHYS 3324 Lab Millikan Oil Drop Experiment: Demonstration of the Quantization of Charge Background reading Read the introduction below before answering the Prelab Questions Prelab Questions 1. A typical
PHYS 3324 Lab Millikan Oil Drop Experiment: Demonstration of the Quantization of Charge Background reading Read the introduction below before answering the Prelab Questions Prelab Questions 1. A typical
Evidence for the Quantization of Electric Charge and Measurement of the Fundamental Unit of Electric Charge
 PHYSICS 286: Modern Physics Laboratory SPRING 2009 (K. Kumar and A. Dinsmore, March 2009) Experiment 7: The Millikan Oil Drop Experiment: INTRODUCTION Evidence for the Quantization of Electric Charge and
PHYSICS 286: Modern Physics Laboratory SPRING 2009 (K. Kumar and A. Dinsmore, March 2009) Experiment 7: The Millikan Oil Drop Experiment: INTRODUCTION Evidence for the Quantization of Electric Charge and
Lab in a Box Millikan s oil drop experiment
 Safety Precautions The mains voltage in the mains powered equipment is dangerous but is screened in normal use. Introduction At the start of the 20 th century scientists knew about the existence of electrons
Safety Precautions The mains voltage in the mains powered equipment is dangerous but is screened in normal use. Introduction At the start of the 20 th century scientists knew about the existence of electrons
Elementary charge and Millikan experiment Students worksheet
 Tasks This experiment deals with the observation of charged oil droplets, which are accelerated between two capacitor plates.. Measure some rise and fall times of oil droplets at different voltages. Determine
Tasks This experiment deals with the observation of charged oil droplets, which are accelerated between two capacitor plates.. Measure some rise and fall times of oil droplets at different voltages. Determine
Determining the smallest quantum of electric charge
 Determining the smallest quantum of electric charge S. A. Lucas Department of Physics and Astronomy University College London 15 th December 2009 Abstract: Using a modern adaptation of Millikan s oil drop
Determining the smallest quantum of electric charge S. A. Lucas Department of Physics and Astronomy University College London 15 th December 2009 Abstract: Using a modern adaptation of Millikan s oil drop
Millikan Oil Drop Experiment
 Millikan Oil Drop Experiment Introduction The electronic charge, or electrical charge carried by an electron, is a fundamental constant in physics. During the years 1909 to 1913, R.A. Millikan used the
Millikan Oil Drop Experiment Introduction The electronic charge, or electrical charge carried by an electron, is a fundamental constant in physics. During the years 1909 to 1913, R.A. Millikan used the
The Millikan Oil Drop Experiment: A Simulation Suitable For Classroom Use
 Georgia Journal of Science Volume 74 No. 2 Scholarly Contributions from the Membership and Others Article 7 2016 The Millikan Oil Drop Experiment: A Simulation Suitable For Classroom Use Benjamin Hogan
Georgia Journal of Science Volume 74 No. 2 Scholarly Contributions from the Membership and Others Article 7 2016 The Millikan Oil Drop Experiment: A Simulation Suitable For Classroom Use Benjamin Hogan
PhysicsAndMathsTutor.com 1
 PhysicsAndMathsTutor.com 1 1. Millikan determined the charge on individual oil droplets using an arrangement as represented in the diagram. The plate voltage necessary to hold a charged droplet stationary
PhysicsAndMathsTutor.com 1 1. Millikan determined the charge on individual oil droplets using an arrangement as represented in the diagram. The plate voltage necessary to hold a charged droplet stationary
BRITISH PHYSICS OLYMPIAD BPhO Round 1 Section 2 18 th November 2016
 BRITISH PHYSICS OLYMPIAD 2016-17 BPhO Round 1 Section 2 18 th November 2016 Instructions This question paper must not be taken out of the exam room. Time: 1 hour 20 minutes on this section. Questions:
BRITISH PHYSICS OLYMPIAD 2016-17 BPhO Round 1 Section 2 18 th November 2016 Instructions This question paper must not be taken out of the exam room. Time: 1 hour 20 minutes on this section. Questions:
EXPERIMENT 18. Millikan Oil-Drop. Introduction. Description of the apparatus
 EXPERIMENT 18 Millikan Oil-Drop Introduction With the apparatus supplied it is possible to repeat Millikan s very important experiment of 1909 in which he established the discreteness of the electronic
EXPERIMENT 18 Millikan Oil-Drop Introduction With the apparatus supplied it is possible to repeat Millikan s very important experiment of 1909 in which he established the discreteness of the electronic
Chapter 1 The discovery of the electron 1.1 Thermionic emission of electrons
 Chapter 1 The discovery of the electron 1.1 Thermionic emission of electrons Learning objectives: What are cathode rays and how were they discovered? Why does the gas in a discharge tube emit light of
Chapter 1 The discovery of the electron 1.1 Thermionic emission of electrons Learning objectives: What are cathode rays and how were they discovered? Why does the gas in a discharge tube emit light of
Millikan oil drop experiment: Determination of elementary charge of electrons
 Millikan oil drop experiment: Determination of elementary charge of electrons Mahesh Gandikota Y1011025 Semester IV Integrated MSc National Institute of Science Education and Research Experiment completed:
Millikan oil drop experiment: Determination of elementary charge of electrons Mahesh Gandikota Y1011025 Semester IV Integrated MSc National Institute of Science Education and Research Experiment completed:
ACTIVITY 5. Figure 5-1: Simulated electron interference pattern with your prediction for the next electron s position.
 Name: WAVES of matter Class: Visual Quantum Mechanics ACTIVITY 5 Interpr preting Wave Functions Goal We will return to the two- slit experiment for electrons. Using this experiment we will see how matter
Name: WAVES of matter Class: Visual Quantum Mechanics ACTIVITY 5 Interpr preting Wave Functions Goal We will return to the two- slit experiment for electrons. Using this experiment we will see how matter
You should be able to demonstrate and show your understanding of:
 OCR B Physics H557 Module 6: Field and Particle Physics You should be able to demonstrate and show your understanding of: 6.1: Fields (Charge and Field) Field: A potential gradient Field Strength: Indicates
OCR B Physics H557 Module 6: Field and Particle Physics You should be able to demonstrate and show your understanding of: 6.1: Fields (Charge and Field) Field: A potential gradient Field Strength: Indicates
Lab 6 - Electron Charge-To-Mass Ratio
 Lab 6 Electron Charge-To-Mass Ratio L6-1 Name Date Partners Lab 6 - Electron Charge-To-Mass Ratio OBJECTIVES To understand how electric and magnetic fields impact an electron beam To experimentally determine
Lab 6 Electron Charge-To-Mass Ratio L6-1 Name Date Partners Lab 6 - Electron Charge-To-Mass Ratio OBJECTIVES To understand how electric and magnetic fields impact an electron beam To experimentally determine
LAB 2 - ONE DIMENSIONAL MOTION
 Name Date Partners L02-1 LAB 2 - ONE DIMENSIONAL MOTION OBJECTIVES Slow and steady wins the race. Aesop s fable: The Hare and the Tortoise To learn how to use a motion detector and gain more familiarity
Name Date Partners L02-1 LAB 2 - ONE DIMENSIONAL MOTION OBJECTIVES Slow and steady wins the race. Aesop s fable: The Hare and the Tortoise To learn how to use a motion detector and gain more familiarity
MILLIKAN OIL DROP EXPERIMENT
 1 Sep 07 Millikan.1 MILLIKAN OIL DROP EXPERIMENT This experimt is designed to show the quantization of electric charge and allow determination of the elemtary charge, e. As in Millikan s original experimt,
1 Sep 07 Millikan.1 MILLIKAN OIL DROP EXPERIMENT This experimt is designed to show the quantization of electric charge and allow determination of the elemtary charge, e. As in Millikan s original experimt,
Lab 5 - ELECTRON CHARGE-TO-MASS RATIO
 81 Name Date Partners Lab 5 - ELECTRON CHARGE-TO-MASS RATIO OBJECTIVES To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass ratio
81 Name Date Partners Lab 5 - ELECTRON CHARGE-TO-MASS RATIO OBJECTIVES To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass ratio
Friday 20 January 2012 Morning
 Friday 20 January 2012 Morning AS GCE PHYSICS B (ADVANCING PHYSICS) G492 Understanding Processes/Experimentation and Data Handling *G411640112* Candidates answer on the Question Paper. OCR supplied materials:
Friday 20 January 2012 Morning AS GCE PHYSICS B (ADVANCING PHYSICS) G492 Understanding Processes/Experimentation and Data Handling *G411640112* Candidates answer on the Question Paper. OCR supplied materials:
Students will explore Stellarium, an open-source planetarium and astronomical visualization software.
 page 22 STELLARIUM* OBJECTIVE: Students will explore, an open-source planetarium and astronomical visualization software. BACKGROUND & ACKNOWLEDGEMENTS This lab was generously provided by the Red Rocks
page 22 STELLARIUM* OBJECTIVE: Students will explore, an open-source planetarium and astronomical visualization software. BACKGROUND & ACKNOWLEDGEMENTS This lab was generously provided by the Red Rocks
Is there life outside of Earth? Activity 2: Moving Stars and Their Planets
 Is there life outside of Earth? Activity 2: Moving Stars and Their Planets Overview In this activity, students are introduced to the wobble-method (officially known as the radial velocity method) of detecting
Is there life outside of Earth? Activity 2: Moving Stars and Their Planets Overview In this activity, students are introduced to the wobble-method (officially known as the radial velocity method) of detecting
Point values are given for each problem. Within a problem, the points are not necessarily evenly divided between the parts. The total is 50 points.
 Physics 103 Hour Exam #3 Solution Point values are given for each problem. Within a problem, the points are not necessarily evenly divided between the parts. The total is 50 points. 1. [15 points] (a)
Physics 103 Hour Exam #3 Solution Point values are given for each problem. Within a problem, the points are not necessarily evenly divided between the parts. The total is 50 points. 1. [15 points] (a)
Experiment #4: Optical Spectrometer and the Prism Deviation
 Experiment #4: Optical Spectrometer and the Prism Deviation Carl Adams October 2, 2011 1 Purpose In the first part of this lab you will set up and become familiar with an optical spectrometer. In the second
Experiment #4: Optical Spectrometer and the Prism Deviation Carl Adams October 2, 2011 1 Purpose In the first part of this lab you will set up and become familiar with an optical spectrometer. In the second
Circular Motion. I. Centripetal Impulse. The centripetal impulse was Sir Isaac Newton s favorite force.
 Circular Motion I. Centripetal Impulse The centripetal impulse was Sir Isaac Newton s favorite force. The Polygon Approximation. Newton made a business of analyzing the motion of bodies in circular orbits,
Circular Motion I. Centripetal Impulse The centripetal impulse was Sir Isaac Newton s favorite force. The Polygon Approximation. Newton made a business of analyzing the motion of bodies in circular orbits,
3B SCIENTIFIC PHYSICS
 3B SCIENTIFIC PHYSICS 30 V, 50/60 Hz: 1018884 / U07001-30 115 V, 50/60 Hz: 101888 / U07001-115 Millikan s Apparatus Instruction sheet 07/16 UD/ALF Safety instructions Millikan s apparatus conforms to the
3B SCIENTIFIC PHYSICS 30 V, 50/60 Hz: 1018884 / U07001-30 115 V, 50/60 Hz: 101888 / U07001-115 Millikan s Apparatus Instruction sheet 07/16 UD/ALF Safety instructions Millikan s apparatus conforms to the
MILLlKAN S OIL DROP EXPERIMENT
 MILLlKAN S OIL DROP EXPERIMENT Measurement of the Charge of the Electron by the Millikan Method Introduction Robert Andrews Millikan was an important person in the development of physics. Best known for
MILLlKAN S OIL DROP EXPERIMENT Measurement of the Charge of the Electron by the Millikan Method Introduction Robert Andrews Millikan was an important person in the development of physics. Best known for
Lab 5 - ELECTRON CHARGE-TO-MASS RATIO
 79 Name Date Partners OBJECTIVES OVERVIEW Lab 5 - ELECTRON CHARGE-TO-MASS RATIO To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass
79 Name Date Partners OBJECTIVES OVERVIEW Lab 5 - ELECTRON CHARGE-TO-MASS RATIO To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass
Atomic Spectra HISTORY AND THEORY
 Atomic Spectra HISTORY AND THEORY When atoms of a gas are excited (by high voltage, for instance) they will give off light. Each element (in fact, each isotope) gives off a characteristic atomic spectrum,
Atomic Spectra HISTORY AND THEORY When atoms of a gas are excited (by high voltage, for instance) they will give off light. Each element (in fact, each isotope) gives off a characteristic atomic spectrum,
THE STANDARD MODEL IN A NUTSHELL BY DAVE GOLDBERG DOWNLOAD EBOOK : THE STANDARD MODEL IN A NUTSHELL BY DAVE GOLDBERG PDF
 Read Online and Download Ebook THE STANDARD MODEL IN A NUTSHELL BY DAVE GOLDBERG DOWNLOAD EBOOK : THE STANDARD MODEL IN A NUTSHELL BY DAVE Click link bellow and free register to download ebook: THE STANDARD
Read Online and Download Ebook THE STANDARD MODEL IN A NUTSHELL BY DAVE GOLDBERG DOWNLOAD EBOOK : THE STANDARD MODEL IN A NUTSHELL BY DAVE Click link bellow and free register to download ebook: THE STANDARD
Introduction to Computer Tools and Uncertainties
 Experiment 1 Introduction to Computer Tools and Uncertainties 1.1 Objectives To become familiar with the computer programs and utilities that will be used throughout the semester. To become familiar with
Experiment 1 Introduction to Computer Tools and Uncertainties 1.1 Objectives To become familiar with the computer programs and utilities that will be used throughout the semester. To become familiar with
Motion on a linear air track
 Motion on a linear air track Introduction During the early part of the 17 th century, Galileo experimentally examined the concept of acceleration. One of his goals was to learn more about freely falling
Motion on a linear air track Introduction During the early part of the 17 th century, Galileo experimentally examined the concept of acceleration. One of his goals was to learn more about freely falling
; F- Faraday constant, N A - Avagadro constant. Best. uncertainty ~1.6 ppm. 4. From Josephson (K J = 2e. constants. ) and von Klitzing R h
 1. Measuring of the charge of electron. 2. Robert Millikan and his oil drop experiment 3. Theory of the experiment 4. Laboratory setup 5. Data analysis 9/25/2017 2 1. Oil drop experiment. Robert A. Millikan..
1. Measuring of the charge of electron. 2. Robert Millikan and his oil drop experiment 3. Theory of the experiment 4. Laboratory setup 5. Data analysis 9/25/2017 2 1. Oil drop experiment. Robert A. Millikan..
Rutgers-Newark PHYSICS RUTGERS THE STATE UNIVERSITY OF NEW JERSEY NEWARK
 THE STATE UNIVERSITY OF NEW JERSEY RUTGERS NEWARK Rutgers-Newark PHYSICS Number of programs offered.............. 4 Number of students in program........... 10 Average size of upper-level classes.............
THE STATE UNIVERSITY OF NEW JERSEY RUTGERS NEWARK Rutgers-Newark PHYSICS Number of programs offered.............. 4 Number of students in program........... 10 Average size of upper-level classes.............
Circular Motion and Centripetal Force
 [For International Campus Lab ONLY] Objective Measure the centripetal force with the radius, mass, and speed of a particle in uniform circular motion. Theory ----------------------------- Reference --------------------------
[For International Campus Lab ONLY] Objective Measure the centripetal force with the radius, mass, and speed of a particle in uniform circular motion. Theory ----------------------------- Reference --------------------------
Investigating Factors that Influence Climate
 Investigating Factors that Influence Climate Description In this lesson* students investigate the climate of a particular latitude and longitude in North America by collecting real data from My NASA Data
Investigating Factors that Influence Climate Description In this lesson* students investigate the climate of a particular latitude and longitude in North America by collecting real data from My NASA Data
Electric Field Mapping (approx. 2 h 15 min.) (8/8/2018)
 Electric Field Mapping (approx. 2 h 15 min.) (8/8/2018) Equipment shallow glass pan pitcher for water masking tape graph paper (8.5 x14 ) colored pencils metal shapes sand paper paper towels DC power supply
Electric Field Mapping (approx. 2 h 15 min.) (8/8/2018) Equipment shallow glass pan pitcher for water masking tape graph paper (8.5 x14 ) colored pencils metal shapes sand paper paper towels DC power supply
Lab 1 Uniform Motion - Graphing and Analyzing Motion
 Lab 1 Uniform Motion - Graphing and Analyzing Motion Objectives: < To observe the distance-time relation for motion at constant velocity. < To make a straight line fit to the distance-time data. < To interpret
Lab 1 Uniform Motion - Graphing and Analyzing Motion Objectives: < To observe the distance-time relation for motion at constant velocity. < To make a straight line fit to the distance-time data. < To interpret
Inverted Pendulum System
 Introduction Inverted Pendulum System This lab experiment consists of two experimental procedures, each with sub parts. Experiment 1 is used to determine the system parameters needed to implement a controller.
Introduction Inverted Pendulum System This lab experiment consists of two experimental procedures, each with sub parts. Experiment 1 is used to determine the system parameters needed to implement a controller.
Electric Fields and Equipotentials
 OBJECTIVE Electric Fields and Equipotentials To study and describe the two-dimensional electric field. To map the location of the equipotential surfaces around charged electrodes. To study the relationship
OBJECTIVE Electric Fields and Equipotentials To study and describe the two-dimensional electric field. To map the location of the equipotential surfaces around charged electrodes. To study the relationship
Simple Harmonic Motion
 Introduction Simple Harmonic Motion The simple harmonic oscillator (a mass oscillating on a spring) is the most important system in physics. There are several reasons behind this remarkable claim: Any
Introduction Simple Harmonic Motion The simple harmonic oscillator (a mass oscillating on a spring) is the most important system in physics. There are several reasons behind this remarkable claim: Any
Ph 3455/MSE 3255 Experiment 2: Atomic Spectra
 Ph 3455/MSE 3255 Experiment 2: Atomic Spectra Background Reading: Tipler, Llewellyn pp. 163-165 Apparatus: Spectrometer, sodium lamp, hydrogen lamp, mercury lamp, diffraction grating, watchmaker eyeglass,
Ph 3455/MSE 3255 Experiment 2: Atomic Spectra Background Reading: Tipler, Llewellyn pp. 163-165 Apparatus: Spectrometer, sodium lamp, hydrogen lamp, mercury lamp, diffraction grating, watchmaker eyeglass,
Technologies for Learning - Velocity and Acceleration
 Technologies for Learning - Velocity and Acceleration The goal of this unit is to understand how different technologies and approaches can contribute to the understanding of velocity and acceleration.
Technologies for Learning - Velocity and Acceleration The goal of this unit is to understand how different technologies and approaches can contribute to the understanding of velocity and acceleration.
Distance From the Sun
 Distance From the Sun Computer 32 Have you ever thought about what it would be like if you were on another planet looking back at the sun? In this activity, you will use the Light Probe to get an idea
Distance From the Sun Computer 32 Have you ever thought about what it would be like if you were on another planet looking back at the sun? In this activity, you will use the Light Probe to get an idea
Lab 5 RC Circuits. What You Need To Know: Physics 212 Lab
 Lab 5 R ircuits What You Need To Know: The Physics In the previous two labs you ve dealt strictly with resistors. In today s lab you ll be using a new circuit element called a capacitor. A capacitor consists
Lab 5 R ircuits What You Need To Know: The Physics In the previous two labs you ve dealt strictly with resistors. In today s lab you ll be using a new circuit element called a capacitor. A capacitor consists
Motion II. Goals and Introduction
 Motion II Goals and Introduction As you have probably already seen in lecture or homework, and if you ve performed the experiment Motion I, it is important to develop a strong understanding of how to model
Motion II Goals and Introduction As you have probably already seen in lecture or homework, and if you ve performed the experiment Motion I, it is important to develop a strong understanding of how to model
I. Pre-Lab Introduction
 I. Pre-Lab Introduction Please complete the following pages before the lab by filling in the requested items. A. Atomic notation: Atoms are composed of a nucleus containing neutrons and protons surrounded
I. Pre-Lab Introduction Please complete the following pages before the lab by filling in the requested items. A. Atomic notation: Atoms are composed of a nucleus containing neutrons and protons surrounded
Exp. #1-1 : Measurement of the Characteristics of the Centripetal Force by Using Springs and a Computer Interface
 PAGE 1/13 Exp. #1-1 : Measurement of the Characteristics of the Centripetal Force by Using Springs and a Computer Interface Student ID Major Name Team No. Experiment Lecturer Student's Mentioned Items
PAGE 1/13 Exp. #1-1 : Measurement of the Characteristics of the Centripetal Force by Using Springs and a Computer Interface Student ID Major Name Team No. Experiment Lecturer Student's Mentioned Items
Lab 6 - ELECTRON CHARGE-TO-MASS RATIO
 101 Name Date Partners OBJECTIVES OVERVIEW Lab 6 - ELECTRON CHARGE-TO-MASS RATIO To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass
101 Name Date Partners OBJECTIVES OVERVIEW Lab 6 - ELECTRON CHARGE-TO-MASS RATIO To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass
Assignment #0 Using Stellarium
 Name: Class: Date: Assignment #0 Using Stellarium The purpose of this exercise is to familiarize yourself with the Stellarium program and its many capabilities and features. Stellarium is a visually beautiful
Name: Class: Date: Assignment #0 Using Stellarium The purpose of this exercise is to familiarize yourself with the Stellarium program and its many capabilities and features. Stellarium is a visually beautiful
Simple circuits - 3 hr
 Simple circuits - 3 hr Resistances in circuits Analogy of water flow and electric current An electrical circuit consists of a closed loop with a number of different elements through which electric current
Simple circuits - 3 hr Resistances in circuits Analogy of water flow and electric current An electrical circuit consists of a closed loop with a number of different elements through which electric current
Experiment: Oscillations of a Mass on a Spring
 Physics NYC F17 Objective: Theory: Experiment: Oscillations of a Mass on a Spring A: to verify Hooke s law for a spring and measure its elasticity constant. B: to check the relationship between the period
Physics NYC F17 Objective: Theory: Experiment: Oscillations of a Mass on a Spring A: to verify Hooke s law for a spring and measure its elasticity constant. B: to check the relationship between the period
Derivatives at Several Points: An Important Step between Derivative at a Point and Derivative Function
 Derivatives at Several Points: An Important Step between Derivative at a Point and Derivative Function Abstract We present activities, with an interactive computer program, that serve to bridge two related
Derivatives at Several Points: An Important Step between Derivative at a Point and Derivative Function Abstract We present activities, with an interactive computer program, that serve to bridge two related
LAB 3: VELOCITY AND ACCELERATION
 Lab 3 - Velocity & Acceleration 25 Name Date Partners LAB 3: VELOCITY AND ACCELERATION A cheetah can accelerate from to 5 miles per hour in 6.4 seconds. A Jaguar can accelerate from to 5 miles per hour
Lab 3 - Velocity & Acceleration 25 Name Date Partners LAB 3: VELOCITY AND ACCELERATION A cheetah can accelerate from to 5 miles per hour in 6.4 seconds. A Jaguar can accelerate from to 5 miles per hour
AC : SOFTLAB VIRTUAL LABORATORY ENVIRONMENT. THERMODYNAMICS EXAMPLES
 AC 2007-1912: SOFTLAB VIRTUAL LABORATORY ENVIRONMENT. THERMODYNAMICS EXAMPLES Gerald Rothberg, Stevens Institute of Technology Gerald Rothberg is a professor of physics and a professor of materials engineering
AC 2007-1912: SOFTLAB VIRTUAL LABORATORY ENVIRONMENT. THERMODYNAMICS EXAMPLES Gerald Rothberg, Stevens Institute of Technology Gerald Rothberg is a professor of physics and a professor of materials engineering
Determination of Density 1
 Introduction Determination of Density 1 Authors: B. D. Lamp, D. L. McCurdy, V. M. Pultz and J. M. McCormick* Last Update: February 1, 2013 Not so long ago a statistical data analysis of any data set larger
Introduction Determination of Density 1 Authors: B. D. Lamp, D. L. McCurdy, V. M. Pultz and J. M. McCormick* Last Update: February 1, 2013 Not so long ago a statistical data analysis of any data set larger
Physics E-1ax, Fall 2014 Experiment 3. Experiment 3: Force. 2. Find your center of mass by balancing yourself on two force plates.
 Learning Goals Experiment 3: Force After you finish this lab, you will be able to: 1. Use Logger Pro to analyze video and calculate position, velocity, and acceleration. 2. Find your center of mass by
Learning Goals Experiment 3: Force After you finish this lab, you will be able to: 1. Use Logger Pro to analyze video and calculate position, velocity, and acceleration. 2. Find your center of mass by
PHY 111L Activity 2 Introduction to Kinematics
 PHY 111L Activity 2 Introduction to Kinematics Name: Section: ID #: Date: Lab Partners: TA initials: Objectives 1. Introduce the relationship between position, velocity, and acceleration 2. Investigate
PHY 111L Activity 2 Introduction to Kinematics Name: Section: ID #: Date: Lab Partners: TA initials: Objectives 1. Introduce the relationship between position, velocity, and acceleration 2. Investigate
Curvature of the Universe from Cosmic Microwave Background Fluctuations
 Curvature of the Universe from Cosmic Microwave Background Fluctuations Introduction Daniel M. Smith, Jr., South Carolina State University, dsmith@scsu.edu The Big Bang Theory that explains the creation,
Curvature of the Universe from Cosmic Microwave Background Fluctuations Introduction Daniel M. Smith, Jr., South Carolina State University, dsmith@scsu.edu The Big Bang Theory that explains the creation,
PHY222 Lab 2 - Electric Fields Mapping the Potential Curves and Field Lines of an Electric Dipole
 Print Your Name PHY222 Lab 2 - Electric Fields Mapping the Potential Curves and Field Lines of an Electric Dipole Print Your Partners' Names Instructions January 23, 2015 Before lab, read the Introduction,
Print Your Name PHY222 Lab 2 - Electric Fields Mapping the Potential Curves and Field Lines of an Electric Dipole Print Your Partners' Names Instructions January 23, 2015 Before lab, read the Introduction,
Module 2 : Electrostatics Lecture 6 : Quantization Of Charge
 Module 2 : Electrostatics Lecture 6 : Quantization Of Charge Objectives In this lecture you will learn the following Quantization Of Charge and its measunement Coulomb's Law of force between electric charge
Module 2 : Electrostatics Lecture 6 : Quantization Of Charge Objectives In this lecture you will learn the following Quantization Of Charge and its measunement Coulomb's Law of force between electric charge
Transpiration. Evaluation copy
 Transpiration Computer 9 Water is transported in plants, from the roots to the leaves, following a decreasing water potential gradient. Transpiration, or loss of water from the leaves, helps to create
Transpiration Computer 9 Water is transported in plants, from the roots to the leaves, following a decreasing water potential gradient. Transpiration, or loss of water from the leaves, helps to create
Exp. #2-4 : Measurement of Characteristics of Magnetic Fields by Using Single Coils and a Computer Interface
 PAGE 1/17 Exp. #2-4 : Measurement of Characteristics of Magnetic Fields by Using Single Coils and a Computer Interface Student ID Major Name Team No. Experiment Lecturer Student's Mentioned Items Experiment
PAGE 1/17 Exp. #2-4 : Measurement of Characteristics of Magnetic Fields by Using Single Coils and a Computer Interface Student ID Major Name Team No. Experiment Lecturer Student's Mentioned Items Experiment
; F- Faraday constant, N A - Avagadro constant. Best. uncertainty ~1.6 ppm. 4. From Josephson (K J = 2e. constants. ) and von Klitzing R h
 1. Measuring of the charge of electron. 2. Robert Millikan and his oil drop experiment 3. Theory of the experiment 4. Laboratory setup 5. Data analysis 2/15/2016 2 1. Oil drop experiment. Robert A. Millikan..
1. Measuring of the charge of electron. 2. Robert Millikan and his oil drop experiment 3. Theory of the experiment 4. Laboratory setup 5. Data analysis 2/15/2016 2 1. Oil drop experiment. Robert A. Millikan..
2 Electric Field Mapping Rev1/05
 2 Electric Field Mapping Rev1/05 Theory: An electric field is a vector field that is produced by an electric charge. The source of the field may be a single charge or many charges. To visualize an electric
2 Electric Field Mapping Rev1/05 Theory: An electric field is a vector field that is produced by an electric charge. The source of the field may be a single charge or many charges. To visualize an electric
Conventional versus electron flow
 Conventional versus electron flow "The nice thing about standards is that there are so many of them to choose from." Andrew S. Tanenbaum, computer science professor When Benjamin Franklin made his conjecture
Conventional versus electron flow "The nice thing about standards is that there are so many of them to choose from." Andrew S. Tanenbaum, computer science professor When Benjamin Franklin made his conjecture
Simple Harmonic Motion
 Physics Topics Simple Harmonic Motion If necessary, review the following topics and relevant textbook sections from Serway / Jewett Physics for Scientists and Engineers, 9th Ed. Hooke s Law (Serway, Sec.
Physics Topics Simple Harmonic Motion If necessary, review the following topics and relevant textbook sections from Serway / Jewett Physics for Scientists and Engineers, 9th Ed. Hooke s Law (Serway, Sec.
AP Physics C 1976 Free Response Questions
 AP Physics C 1976 Free Response Questions The materials included in these files are intended for use by AP teachers for course and exam preparation in the classroom; permission for any other use must be
AP Physics C 1976 Free Response Questions The materials included in these files are intended for use by AP teachers for course and exam preparation in the classroom; permission for any other use must be
Lab 12.1 Calorimetry. Name School Date
 Name School Date Lab 12.1 Calorimetry Purpose To investigate the flow of heat between two bodies due to a difference in their temperatures. To investigate the flow of heat involved in changes in phase.
Name School Date Lab 12.1 Calorimetry Purpose To investigate the flow of heat between two bodies due to a difference in their temperatures. To investigate the flow of heat involved in changes in phase.
Center of Mass. Evaluation copy
 Center of Mass Experiment 19 INTRODUCTION In the most of the previous experiments you have examined the motion of a single object as it underwent a variety of motions. You learned that an object subject
Center of Mass Experiment 19 INTRODUCTION In the most of the previous experiments you have examined the motion of a single object as it underwent a variety of motions. You learned that an object subject
Parts II-V Sabbatical Leave Report
 Parts II-V Sabbatical Leave Report II. III. Re-statement of Sabbatical Leave Application I propose to spend my sabbatical updating all of the lab manuals for the five physics courses. The lab manuals are
Parts II-V Sabbatical Leave Report II. III. Re-statement of Sabbatical Leave Application I propose to spend my sabbatical updating all of the lab manuals for the five physics courses. The lab manuals are
Zeeman Effect Physics 481
 Zeeman Effect Introduction You are familiar with Atomic Spectra, especially the H- atom energy spectrum. Atoms emit or absorb energies in packets, or quanta which are photons. The orbital motion of electrons
Zeeman Effect Introduction You are familiar with Atomic Spectra, especially the H- atom energy spectrum. Atoms emit or absorb energies in packets, or quanta which are photons. The orbital motion of electrons
Pick-up Calibration in CESR Beam Position Monitors
 Pick-up Calibration in CESR Beam Position Monitors Beau Meredith Department of Physics, Greenville College, Greenville, IL, 62246 (Dated: August 21, 2003) The beam position algorithm presently used in
Pick-up Calibration in CESR Beam Position Monitors Beau Meredith Department of Physics, Greenville College, Greenville, IL, 62246 (Dated: August 21, 2003) The beam position algorithm presently used in
Acs Exam Study Guide Organic Chemistry
 ACS EXAM STUDY GUIDE ORGANIC CHEMISTRY PDF - Are you looking for acs exam study guide organic chemistry Books? Now, you will be happy that at this time acs exam study guide organic chemistry PDF is available
ACS EXAM STUDY GUIDE ORGANIC CHEMISTRY PDF - Are you looking for acs exam study guide organic chemistry Books? Now, you will be happy that at this time acs exam study guide organic chemistry PDF is available
AP Physics B 1979 Free Response Questions
 AP Physics B 1979 Free Response Questions The materials included in these files are intended for use by AP teachers for course and exam preparation in the classroom; permission for any other use must be
AP Physics B 1979 Free Response Questions The materials included in these files are intended for use by AP teachers for course and exam preparation in the classroom; permission for any other use must be
How Do I Create a Hubble Diagram to show the expanding universe?
 How Do I Create a Hubble Diagram to show the expanding universe? An extremely important topic in astronomy is the expansion of the universe. Although the expanding universe is nearly always discussed in
How Do I Create a Hubble Diagram to show the expanding universe? An extremely important topic in astronomy is the expansion of the universe. Although the expanding universe is nearly always discussed in
Mathematics 1104B. Systems of Equations and Inequalities, and Matrices. Study Guide. Text: Mathematics 11. Alexander and Kelly; Addison-Wesley, 1998.
 Adult Basic Education Mathematics Systems of Equations and Inequalities, and Matrices Prerequisites: Mathematics 1104A, Mathematics 1104B Credit Value: 1 Text: Mathematics 11. Alexander and Kelly; Addison-Wesley,
Adult Basic Education Mathematics Systems of Equations and Inequalities, and Matrices Prerequisites: Mathematics 1104A, Mathematics 1104B Credit Value: 1 Text: Mathematics 11. Alexander and Kelly; Addison-Wesley,
Lab 4: Gauss Gun Conservation of Energy
 Lab 4: Gauss Gun Conservation of Energy Before coming to Lab Read the lab handout Complete the pre-lab assignment and hand in at the beginning of your lab section. The pre-lab is written into this weeks
Lab 4: Gauss Gun Conservation of Energy Before coming to Lab Read the lab handout Complete the pre-lab assignment and hand in at the beginning of your lab section. The pre-lab is written into this weeks
SPECIMEN. Date Morning/Afternoon Time allowed: 1 hour 30 minutes. AS Level Physics A H156/01 Breadth in physics Sample Question Paper PMT
 AS Level Physics A H156/01 Breadth in physics Sample Question Paper Date Morning/Afternoon Time allowed: 1 hour 30 minutes You must have: the Data, Formulae and Relationships Booklet You may use: a scientific
AS Level Physics A H156/01 Breadth in physics Sample Question Paper Date Morning/Afternoon Time allowed: 1 hour 30 minutes You must have: the Data, Formulae and Relationships Booklet You may use: a scientific
MASSACHUSETTS INSTITUTE OF TECHNOLOGY Physics Department
 MASSACHUSETTS INSTITUTE OF TECHNOLOGY Physics Department Physics 8.01T Fall Term 2004 Experiment 06: Work, Energy and the Harmonic Oscillator Purpose of the Experiment: In this experiment you allow a cart
MASSACHUSETTS INSTITUTE OF TECHNOLOGY Physics Department Physics 8.01T Fall Term 2004 Experiment 06: Work, Energy and the Harmonic Oscillator Purpose of the Experiment: In this experiment you allow a cart
EXPERIMENT 7: ANGULAR KINEMATICS AND TORQUE (V_3)
 TA name Lab section Date TA Initials (on completion) Name UW Student ID # Lab Partner(s) EXPERIMENT 7: ANGULAR KINEMATICS AND TORQUE (V_3) 121 Textbook Reference: Knight, Chapter 13.1-3, 6. SYNOPSIS In
TA name Lab section Date TA Initials (on completion) Name UW Student ID # Lab Partner(s) EXPERIMENT 7: ANGULAR KINEMATICS AND TORQUE (V_3) 121 Textbook Reference: Knight, Chapter 13.1-3, 6. SYNOPSIS In
A SHORT INTRODUCTION TO ADAMS
 A. AHADI, P. LIDSTRÖM, K. NILSSON A SHORT INTRODUCTION TO ADAMS FOR ENGINEERING PHYSICS DIVISION OF MECHANICS DEPARTMENT OF MECHANICAL ENGINEERING LUND INSTITUTE OF TECHNOLOGY 2017 FOREWORD THESE EXERCISES
A. AHADI, P. LIDSTRÖM, K. NILSSON A SHORT INTRODUCTION TO ADAMS FOR ENGINEERING PHYSICS DIVISION OF MECHANICS DEPARTMENT OF MECHANICAL ENGINEERING LUND INSTITUTE OF TECHNOLOGY 2017 FOREWORD THESE EXERCISES
Lab 5: Projectile Motion
 Lab 5 Projectile Motion 47 Name Date Partners Lab 5: Projectile Motion OVERVIEW We learn in our study of kinematics that two-dimensional motion is a straightforward application of onedimensional motion.
Lab 5 Projectile Motion 47 Name Date Partners Lab 5: Projectile Motion OVERVIEW We learn in our study of kinematics that two-dimensional motion is a straightforward application of onedimensional motion.
Microscale Chemistry Technology Exchange at Argonne National Laboratory - East
 Microscale Chemistry Technology Exchange at Argonne National Laboratory - East Rose Pausma (rpausma@depanlgov) and Deon Ettinger (dettinger@dep anlgov) Division of Educational Programs + Argonne National
Microscale Chemistry Technology Exchange at Argonne National Laboratory - East Rose Pausma (rpausma@depanlgov) and Deon Ettinger (dettinger@dep anlgov) Division of Educational Programs + Argonne National
Physics Attitudes, Skills, & Knowledge Survey (PASKS) Form 3
 Physics Attitudes, Skills, & Knowledge Survey (PASKS) Form 3 Directions to Students: Do not open this booklet until you are told to do so. Please respond to the following items by marking the best answer
Physics Attitudes, Skills, & Knowledge Survey (PASKS) Form 3 Directions to Students: Do not open this booklet until you are told to do so. Please respond to the following items by marking the best answer
PEP530 Fundamental Principles of Physical Science 1. Stevens Institute of Technology
 SEF 530 Fundamentals Principles of Physical Science Stevens Institute of Technology School: Course Title: Program(s): Engineering and Science Fundamental Principles of Physical Science Science & Engineering
SEF 530 Fundamentals Principles of Physical Science Stevens Institute of Technology School: Course Title: Program(s): Engineering and Science Fundamental Principles of Physical Science Science & Engineering
Teacher s notes 15 Temperature volume relationship in a gas (Charles law, Gay-Lussac s law)
 Sensors: Loggers: Temperature, Rotary Motion Any EASYSENSE Physics Logging time: EasyLog Teacher s notes 15 Temperature volume relationship in a gas (Charles law, Gay-Lussac s law) Read The relationship
Sensors: Loggers: Temperature, Rotary Motion Any EASYSENSE Physics Logging time: EasyLog Teacher s notes 15 Temperature volume relationship in a gas (Charles law, Gay-Lussac s law) Read The relationship
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education
 www.xtremepapers.com UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *4808110537* PHYSICS 0625/21 Paper 2 Core May/June 2012 1 hour 15 minutes
www.xtremepapers.com UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *4808110537* PHYSICS 0625/21 Paper 2 Core May/June 2012 1 hour 15 minutes
Thermal Convection of a Fluid
 C04 Thermal Convection of a Fluid http://web.ics.purdue.edu/~braile/edumod/convect/convect.htm Focus on Inquiry The students will calculate the velocity of convection currents using vegetable oil and thyme
C04 Thermal Convection of a Fluid http://web.ics.purdue.edu/~braile/edumod/convect/convect.htm Focus on Inquiry The students will calculate the velocity of convection currents using vegetable oil and thyme
Experiment 3 1. The Michelson Interferometer and the He- Ne Laser Physics 2150 Experiment No. 3 University of Colorado
 Experiment 3 1 Introduction The Michelson Interferometer and the He- Ne Laser Physics 2150 Experiment No. 3 University of Colorado The Michelson interferometer is one example of an optical interferometer.
Experiment 3 1 Introduction The Michelson Interferometer and the He- Ne Laser Physics 2150 Experiment No. 3 University of Colorado The Michelson interferometer is one example of an optical interferometer.
through any three given points if and only if these points are not collinear.
 Discover Parabola Time required 45 minutes Teaching Goals: 1. Students verify that a unique parabola with the equation y = ax + bx+ c, a 0, exists through any three given points if and only if these points
Discover Parabola Time required 45 minutes Teaching Goals: 1. Students verify that a unique parabola with the equation y = ax + bx+ c, a 0, exists through any three given points if and only if these points
Please bring the task to your first physics lesson and hand it to the teacher.
 Pre-enrolment task for 2014 entry Physics Why do I need to complete a pre-enrolment task? This bridging pack serves a number of purposes. It gives you practice in some of the important skills you will
Pre-enrolment task for 2014 entry Physics Why do I need to complete a pre-enrolment task? This bridging pack serves a number of purposes. It gives you practice in some of the important skills you will
Chemical Engineering: 4C3/6C3 Statistics for Engineering McMaster University: Final examination
 Chemical Engineering: 4C3/6C3 Statistics for Engineering McMaster University: Final examination Duration of exam: 3 hours Instructor: Kevin Dunn 07 April 2012 dunnkg@mcmaster.ca This exam paper has 8 pages
Chemical Engineering: 4C3/6C3 Statistics for Engineering McMaster University: Final examination Duration of exam: 3 hours Instructor: Kevin Dunn 07 April 2012 dunnkg@mcmaster.ca This exam paper has 8 pages
Lab 11 Simple Harmonic Motion A study of the kind of motion that results from the force applied to an object by a spring
 Lab 11 Simple Harmonic Motion A study of the kind of motion that results from the force applied to an object by a spring Print Your Name Print Your Partners' Names Instructions April 20, 2016 Before lab,
Lab 11 Simple Harmonic Motion A study of the kind of motion that results from the force applied to an object by a spring Print Your Name Print Your Partners' Names Instructions April 20, 2016 Before lab,
PAPER 2 Theory MAY/JUNE SESSION hour 45 minutes
 Centre Number Candidate Number Candidate Name CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level PHYSICS 5054/2 PAPER 2 Theory MAY/JUNE SESSION 2002 1 hour 45 minutes
Centre Number Candidate Number Candidate Name CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level PHYSICS 5054/2 PAPER 2 Theory MAY/JUNE SESSION 2002 1 hour 45 minutes
Effective January 2008 All indicators in Standard / 14
 Scientific Inquiry 7-1 The student will demonstrate an understanding of technological design and scientific inquiry, including the process skills, mathematical thinking, controlled investigative design
Scientific Inquiry 7-1 The student will demonstrate an understanding of technological design and scientific inquiry, including the process skills, mathematical thinking, controlled investigative design
Demonstrating the Quantization of Electrical Charge Millikan s Experiment
 Demonstrating the Quantization o Electrical Charge Millikan s Experiment Objecties o the experiment To demonstrate that electrical charge is quantized, and to determine the elementary electron charge by
Demonstrating the Quantization o Electrical Charge Millikan s Experiment Objecties o the experiment To demonstrate that electrical charge is quantized, and to determine the elementary electron charge by
Asimple spring-loaded toy that jumps up off
 Springbok: The Physics of Jumping Robert J. Dufresne, William J. Gerace, and William J. Leonard Asimple spring-loaded toy that jumps up off the table when compressed and released offers the physics teacher
Springbok: The Physics of Jumping Robert J. Dufresne, William J. Gerace, and William J. Leonard Asimple spring-loaded toy that jumps up off the table when compressed and released offers the physics teacher
Dynamics. Newton s First Two Laws of Motion. A Core Learning Goals Activity for Science and Mathematics
 CoreModels Dynamics Newton s First Two Laws of Motion A Core Learning Goals Activity for Science and Mathematics Summary: Students will investigate the first and second laws of motion in laboratory activities.
CoreModels Dynamics Newton s First Two Laws of Motion A Core Learning Goals Activity for Science and Mathematics Summary: Students will investigate the first and second laws of motion in laboratory activities.
Lab 4: Projectile Motion
 59 Name Date Partners OVEVIEW Lab 4: Projectile Motion We learn in our study of kinematics that two-dimensional motion is a straightforward extension of one-dimensional motion. Projectile motion under
59 Name Date Partners OVEVIEW Lab 4: Projectile Motion We learn in our study of kinematics that two-dimensional motion is a straightforward extension of one-dimensional motion. Projectile motion under
