Module 4 : THERMOELECTRICITY Lecture 21 : Seebeck Effect
|
|
|
- Wesley Walton
- 6 years ago
- Views:
Transcription
1 Module 4 : THERMOELECTRICITY Lecture 21 : Seebeck Effect Objectives In this lecture you will learn the following Seebeck effect and thermo-emf. Thermoelectric series of metals which can be used to form thermocouples. Peltier effect, i.e. absorption or evolution of heat at junctions of dissimilar metals if a current exists in the circuit. Thomoson effect of heat exchange in a circuit of a single metal in which a temperature gradient exists. Seebeck Effect : In 1821 Thomas Seebeck, a German physicist discovered that when two dissimilar metal ( Seebeck used copper and bismuth) wires are joined at two ends to form a loop, a voltage is developed in the circuit if the two junctions are kept at different temperatures. The pair of metals forming the circuit is called a thermocouple. The effect is due to conversion of thermal energy to electrical energy. The existence of current in the closed circuit may be confirmed by the deflection of a magnetic needle caused by the magnetic field of the current Joule heating produced in the wires closing the circuit with a capacitor to accumulate measurable charge placing a sensitive ammeter or a galvanometer in the circuit measuring the amount of chemiucal deposit at the electrodes of an electrochemical cell.
2 Click here for animations Thermoelectric Power Click here for animations The open circuit potential difference in the circuit whose junctions are maintained at temperatures and is given by where the coefficient of proportionality is known as the thermoelectric power or the Seebeck coefficient. The term thermoelectric power is a misnomer because it does not measure any power and is measured in volt/ K. By convention, Seebeck coefficient's sign is the sign of the potential of the cold end with respect to the hot end. Thus if is positive, convertional current flows from A to B at the hot junction. Seeback coefficient is not a constant but is dependant on temperature. The temperature dependence of a commercial thermocouple is usually expressed as a polynomial expansion in powers of temperature. For instance, for a thermocouple with Platinum as one of the metals and an alloy of Pt-Rh (90:10) the open circuit voltage is given approximately by a quadratic so that the thermoelectric power is given by The relationship between and is a parabola. The temperature at which the thermoelectric power is maximum is called the neutral temperature. The temperatures and at which a small change in the difference of the junction temperatures leads to a change in the sign of emf is called the inversion temperature.
3 A complete understanding of Seebeck effect requires knowledge of behaviour of electron in a metal which is rather complicated. The Seebeck coefficient depends on factors like work functions of the two metals, electron densities of the two components, scattering mechanism within each solid etc. However, a simple minded picture is as follows. Seebeck effect is a manifestation of the fact that if two points in a conductor (or a semiconductor) are maintained at different temperatures, the charged carriers (electorns or holes) in the hotter region, being more energetic (and, therefore, having higher velocities) will diffuse towards region of lower temperature. The diffusion stops when the electric field generated because of movement of charges has established a strong enough field to stop further movement of charges. For a metal, carriers being negatively charged electrons, the colder end would become negative so that Seebeck cofficient is negative. For a p-type semiconductor on the other hand, holes diffuse towards the lower temperature resulting in a positive Seebeck coefficient. Performance of a thermocouple is determined by the Seebeck coefficient of the pair of metals forming the thermocouple. As it is impracticable to list the coefficient of all possible pairs, the Seebeck coefficients of metals are usually given with respect to Platinum as standard whose Seebeck coefficient is taken as zero. The following table gives the Seebeck coefficient (in ) of some standard thermocouple material at 0 C. Material Seebeck Material Seeback Material Seebeck coefficient coefficient coefficient Bismuth Lead 4 Iron 19 Constantan Tantalum 4.5 Nichrome 25 Nickel Rhodium 6 Antimony 47
4 Potassium Gold 6.5 Germanium 300 Sodium Silver 6.5 Silicon 440 Mercury 0.6 Copper 6.5 Tellurium 500 Carbon 3 Cadmium 7.5 Selenium 900 Aluminium 3.5 Tungsten 7.5 An alloy of copper (60%) and nickel (40%) An alloy of Ni-Cr-Fe-Si Example -1 Seebeck voltage for Copper- Constantan (Cn) thermocouple is given by the linear relation, where is the absolute temperature of the hot junction and and are constants given by Cu : mv mv/k Cn : mv mv/k Calculate the thermoelectric power when the hot junction is at 100 C. Solution The thermoelectric power Thermoelectric Series A thermoelectric series is an ordering of thermocouple elements in such a way that when any pair of materials in the series is used to form a thermocouple, Seebeck current at the cold junction flows from the member occuring earlier in the series to that occuring later. One should be careful in using the series as the opposite convention is sometimes used. As one uses pairs of members which are closer in the series, the thermoelectric yield goes down when one reduces the temperature difference. The following table gives the thermal emf (in ) with respect to Platinum when the cold junction is at C while the hot junction is at C. Material thermo-emf Material thermo-emf
5 Chromel 2810 Rh 700 Fe 1980 Pb 440 W 1120 Al 420 Cd 900 Ta 330 Au 780 Alumel 1290 Cu 760 Ni 1480 Zn 760 Constantan 3510 Ag 740 Bi 7340 An alloy of Ni-Cr-Mn An alloy of Ni-Al-Mn If the temperatures of the two junctions are kept fixed at and, then the emf of a circuit consisting of a pair of metals A and B is equal to the difference between the emfs of two circuits, one consisting of the pair A-C and the other of the pair B-C, i.e. In view of this, it is convenient to measure the thermo-emf with respect to a reference metal C. Platinum is taken to be such a reference. Example-2 Compute the thermo-emf of a copper-constantan thermocouple with its junctions at C and C. Solution From the table above we have and. Thus Thus the emf yield of a copper-constantan thermocouple is 4.27 mv. Exercise The thermoelectric power of a thermocouple whose cold junction is maintained at C and the hot junction at C is given by the following graph. (1) Sketch the thermo-emf with temperature. (2) Determine the neutral temperature and the temperature of inversion. How will these two temperatures change if the cold junction were maintained at C?
6 (Ans. (ii) C, C; No change, C) Exercise A chromel-constantan thermocouple with the cold junction at C has a linear variation of the open circuit voltage for temperatures of the hot junction between C and C. Calculate the thermoemf when the hot junction is maintained at the same temperature] (Ans. 9.5 mv) Peltier Effect C. [ Hint : Emf is zero when both the junctions are at In 1834 Jean Peltier, a french watch maker, discovered a second thermoelectric effect. If a current flows through a circuit containing junction of two dis-similar metals, it leads to an absorption or liberation of heat at the junctions. Heat is given out or absorbed depending on the pairs of metals and the direction of the current. The phenomenon of heat evolution is different from the Joule heat as Peltier effect is a reversible process while Joule loss is irreversible. If the direction of the current at the junction is same as the direction of the Seebeck current, heat is liberated if the Seebeck junction is a hot junction or is absorbed if the junction is cold. Thus for a copper - constantan thermocouple, if the current flow at the junction is from copper (+) to constantan (-), heat is absorbed. On changing the direction of the current, heat will be liberated at the same junction, showing that the phenomenon is reversible. The amount of heat liberated to (or absorbed from) the surroundings in order that the junction may be kept at the same temperature is proportional to the current passing through the junction where the constant is called the Peltier coefficient. The Peltier coefficient depends on the pair
7 of materials A and B of the junction and also on the junction temperature. Thomson Effect William Thomson (later well known as Lord Kelvin) discovered a third thermoelectric effect which provides a link between Seebeck effect and Peltier effect. Thomson found that when a current is passed through an wire of single homogeneous material along which a temperature gradient exists, heat must be exchanged with the surrounding in order that the original temperature gradient may be maintained along the wire. (The exchange of heat is required at all places of the circuit where a temperature gradient exists.) Thomson effect may be understood by a simple picture. A conductor has free charge carriers, which are, electrons in metals, electrons and holes in semiconductors and ions in case of ionic conductors. Consider a section of such a conductor whose one end is hotter than the other end. Charge carriers at the hot end, being more energetic, will diffuse towards the colder end. The charge separation sets up an electric field. Diffusion of carriers would stop when the attractive force on the carriers due to this field is strong enough to retard the motion of the carriers due to thermal effect. We can represent the effect of the thermal gradient responsible for the diffusive motion of the carriers by an effective field as. This effective field is proportional to the thermal gradient and can be written where is known as the Thomson coefficient for the material of the conductor. The Thomson electromotive force is given by where and are the temperatures at the two ends of the rod. Thomson effect is a manifestation of the Thomson emf described above. Clearly, one cannot demonstrate the existence of the emf by using it to drive a current in a close circuit. This is because if one uses a single metal with a temperature gradient, the integral around a close loop is zero. For dis-similar metals, Peltier effect dominates over Thomson effect. When a current is passed through a homogeneous conductor with a temperature gradient, the rate of heat production per unit volume is given by where is the resistivity of the sample. The first term is the irreversible Joule heat. The second term is
8 due to Thomson emf. In metals such as copper and zinc, the hotter end is at a higher potential (as shown in the figure above). In such a situation if the current due to an external supply is in the same direction as the direction of decreasing potential, there is additional evolution of heat due to Thomson effect and the net heat produced is more than the Joule heat. If the direction of the current is reversed, heat energy is converted to electrical energy due to Thomson effect and the rate production of heat is reduced. This is known as positive Thomson effect. An anomalous situation occurs in metals such as cobalt and iron. In these metals the hotter end is at a lower potential so that charge carriers move against the thermal gradient. The effect is opposite of what happens in case of positive Thomson effect. Such anomalous effect is known as negative thomson effect. Lead shows zero Thomson effect. The simple physical picture given above cannot explain the strange behaviour. Recap In this course you have learnt the following A current flows in a circuit of two dissimilar metals, when the two junctions are kept at different temperatures. The junction circuit is called thermocouple. The magnitude and sign of the thermo-emf developed depends on the material of the two metals and thetemperatures of the two junctions. The metals can be arranged in an order called the thermoelectric series such that in a thermocouple made with two metals in the series, the current in the hot junction is from the metal preceding in the series to the one occuring later. The inverse effect in which heat is evolved or absorbed at the junctions of two dissimilar conductors if a current is flowing in the circuit is known as Peltier effect. Pelter effect and Seebeck effect are linked by Thompson effect according to which when a current is passed through a single wire in which temperature gradient exists, heat exchange with the surround must take plac if the temperature gradient is to be maintained.
Sensing, Computing, Actuating
 Sensing, Computing, ctuating Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC EFFECT (Chapter 5.11) 3 Thermocouple cylinder head temperature (thermocouple)
Sensing, Computing, ctuating Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC EFFECT (Chapter 5.11) 3 Thermocouple cylinder head temperature (thermocouple)
Energy Conversion in the Peltier Device
 Laboratory exercise 4 Energy Conversion in the Peltier Device Preface The purpose of this exercise is to become familiar with the Peltier effect. Students will observe Peltier device working as a heat
Laboratory exercise 4 Energy Conversion in the Peltier Device Preface The purpose of this exercise is to become familiar with the Peltier effect. Students will observe Peltier device working as a heat
Thermoelectric effect
 Hiroyuki KOIZUMI 1. Principle Thermoelectric effect Seebeck effect Temperature difference ΔT Voltage difference ΔV Peltier effect I Q Thomson effect I Current Q Heat transfer Thermoelectric effect Seebeck
Hiroyuki KOIZUMI 1. Principle Thermoelectric effect Seebeck effect Temperature difference ΔT Voltage difference ΔV Peltier effect I Q Thomson effect I Current Q Heat transfer Thermoelectric effect Seebeck
Sensors and Actuators Sensors Physics
 Sensors and ctuators Sensors Physics Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC SENSORS (Chapter 3.9, 16.4) 3 Thermoelectric effect thermoelectric
Sensors and ctuators Sensors Physics Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC SENSORS (Chapter 3.9, 16.4) 3 Thermoelectric effect thermoelectric
Lecture 36: Temperatue Measurements
 Lecture 36: Temperatue Measurements Contents Principle of thermocouples Materials for themocouples Cold junction compensation Compensating wires Selection of thermocouples Illustration of gas temperature
Lecture 36: Temperatue Measurements Contents Principle of thermocouples Materials for themocouples Cold junction compensation Compensating wires Selection of thermocouples Illustration of gas temperature
EXPERIMENT VARIATION OF THERMO-EMF WITH TEMPERATURE. Structure. 7.1 Introduction Objectives
 EXPERIMENT 7 VARIATION OF THERMO-EMF WITH TEMPERATURE Thermo-EMF Structure 7.1 Introduction Objectives 7.2 Working Principle of a Potentiometer 7.3 Measurement of Thermo-EMF and its Variation with Temperature
EXPERIMENT 7 VARIATION OF THERMO-EMF WITH TEMPERATURE Thermo-EMF Structure 7.1 Introduction Objectives 7.2 Working Principle of a Potentiometer 7.3 Measurement of Thermo-EMF and its Variation with Temperature
Section 7. Temperature Measurement
 Section 7 Temperature Measurement 7/25/2017 Engineering Measurements 7 1 Working Definition Temperature is a measure of the average kinetic energy of the molecules that make of a substance. After time,
Section 7 Temperature Measurement 7/25/2017 Engineering Measurements 7 1 Working Definition Temperature is a measure of the average kinetic energy of the molecules that make of a substance. After time,
Temperature. Sensors. Measuring technique. Eugene V. Colla. 10/25/2017 Physics 403 1
 Temperature. Sensors. Measuring technique. Eugene V. Colla 10/25/2017 Physics 403 1 Outline Temperature Sensors Measuring equipment and ideas Sensor calibration Temperature scales 10/25/2017 Physics 403
Temperature. Sensors. Measuring technique. Eugene V. Colla 10/25/2017 Physics 403 1 Outline Temperature Sensors Measuring equipment and ideas Sensor calibration Temperature scales 10/25/2017 Physics 403
15 - THERMAL AND CHEMICAL EFFECTS OF CURRENTS Page 1 ( Answers at the end of all questions )
 5 - THERMAL AND CHEMICAL EFFECTS OF CURRENTS Page A heater coil is cut into two equal parts and only one part is now used in the heater. The heat generated will now be four times doubled halved ( d one-fourth
5 - THERMAL AND CHEMICAL EFFECTS OF CURRENTS Page A heater coil is cut into two equal parts and only one part is now used in the heater. The heat generated will now be four times doubled halved ( d one-fourth
Thermoelectric effect
 Thermoelectric effect See Mizutani the temperature gradient can also induce an electrical current. linearized Boltzmann transport equation in combination with the relaxation time approximation. Relaxation
Thermoelectric effect See Mizutani the temperature gradient can also induce an electrical current. linearized Boltzmann transport equation in combination with the relaxation time approximation. Relaxation
Lecture 9: Metal-semiconductor junctions
 Lecture 9: Metal-semiconductor junctions Contents 1 Introduction 1 2 Metal-metal junction 1 2.1 Thermocouples.......................... 2 3 Schottky junctions 4 3.1 Forward bias............................
Lecture 9: Metal-semiconductor junctions Contents 1 Introduction 1 2 Metal-metal junction 1 2.1 Thermocouples.......................... 2 3 Schottky junctions 4 3.1 Forward bias............................
Lecture 11 Temperature Sensing. ECE 5900/6900 Fundamentals of Sensor Design
 EE 4900: Fundamentals of Sensor Design Lecture 11 Temperature Sensing 1 Temperature Sensing Q: What are we measuring? A: Temperature 2 SI Units: Celcius ( C), Kelvin (K) British Units: Fahrenheit ( F)
EE 4900: Fundamentals of Sensor Design Lecture 11 Temperature Sensing 1 Temperature Sensing Q: What are we measuring? A: Temperature 2 SI Units: Celcius ( C), Kelvin (K) British Units: Fahrenheit ( F)
Using a Mercury itc with thermocouples
 Technical Note Mercury Support Using a Mercury itc with thermocouples Abstract and content description This technical note describes how to make accurate and reliable temperature measurements using an
Technical Note Mercury Support Using a Mercury itc with thermocouples Abstract and content description This technical note describes how to make accurate and reliable temperature measurements using an
Temperature Scales. Temperature, and Temperature Dependent on Physical Properties. Temperature. Temperature Scale
 Temperature Scales The Celsius, Fahrenheit, and Kelvin Temperature Scales: Temperature, and Temperature Dependent on Physical Properties Physics Enhancement Programme Dr. M.H. CHAN, HKBU 9 T F T 5 T T
Temperature Scales The Celsius, Fahrenheit, and Kelvin Temperature Scales: Temperature, and Temperature Dependent on Physical Properties Physics Enhancement Programme Dr. M.H. CHAN, HKBU 9 T F T 5 T T
(b) The diagram given below has T2>T1. Explain. Ans.: We know that V IR, T indicates the temperature I 1. (Lower temperature) (Higher Temperature)
 BHSEC: 2009 (a) How can a galvanometer be converted into an ammeter of desired range? Explain with the help of diagram. By connecting a low resistance (shunt) in parallel to the galvanometer. As per ohm
BHSEC: 2009 (a) How can a galvanometer be converted into an ammeter of desired range? Explain with the help of diagram. By connecting a low resistance (shunt) in parallel to the galvanometer. As per ohm
Temperature Measurement
 MECE 3320 Measurements & Instrumentation Temperature Measurement Dr. Isaac Choutapalli Department of Mechanical Engineering University of Texas Pan American Introduction Temperature is one of the most
MECE 3320 Measurements & Instrumentation Temperature Measurement Dr. Isaac Choutapalli Department of Mechanical Engineering University of Texas Pan American Introduction Temperature is one of the most
Resistivity and Temperature Coefficients (at 20 C)
 Homework # 4 Resistivity and Temperature Coefficients (at 0 C) Substance Resistivity, Temperature ( m) Coefficient, (C ) - Conductors Silver.59 x 0-0.006 Copper.6 x 0-0.006 Aluminum.65 x 0-0.0049 Tungsten
Homework # 4 Resistivity and Temperature Coefficients (at 0 C) Substance Resistivity, Temperature ( m) Coefficient, (C ) - Conductors Silver.59 x 0-0.006 Copper.6 x 0-0.006 Aluminum.65 x 0-0.0049 Tungsten
SENSORS and TRANSDUCERS
 SENSORS and TRANSDUCERS Tadeusz Stepinski, Signaler och system The Thermal Energy Domain Physics» Seebeck effect» Peltier effect» Thomson effect Thermal effects in semiconductors Thermoelectric sensors
SENSORS and TRANSDUCERS Tadeusz Stepinski, Signaler och system The Thermal Energy Domain Physics» Seebeck effect» Peltier effect» Thomson effect Thermal effects in semiconductors Thermoelectric sensors
Influence of electric field dynamics on the generation of thermo emf by some advanced thermocouples in the high temperature range
 Volume 11 Issue 11 ISSN : 0974-7486 Influence of electric field dynamics on the generation of thermo emf by some advanced thermocouples in the high temperature range Jaspal Singh, S.S.Verma* Department
Volume 11 Issue 11 ISSN : 0974-7486 Influence of electric field dynamics on the generation of thermo emf by some advanced thermocouples in the high temperature range Jaspal Singh, S.S.Verma* Department
ELECTRICITY & CIRCUITS
 ELECTRICITY & CIRCUITS Reason and justice tell me there s more love for humanity in electricity and steam than in chastity and vegetarianism. Anton Chekhov LIGHTNING, PART 2 Electricity is really just
ELECTRICITY & CIRCUITS Reason and justice tell me there s more love for humanity in electricity and steam than in chastity and vegetarianism. Anton Chekhov LIGHTNING, PART 2 Electricity is really just
UNIT II CURRENT ELECTRICITY
 UNIT II CUENT ELECTICITY Weightage : 07 Marks Electric current; flow of electric charges in a metllic conductor, drift velocity, mobility and their relation with electric current. Ohm s law electrical
UNIT II CUENT ELECTICITY Weightage : 07 Marks Electric current; flow of electric charges in a metllic conductor, drift velocity, mobility and their relation with electric current. Ohm s law electrical
V, I, R measurements: how to generate and measure quantities and then how to get data (resistivity, magnetoresistance, Hall). Makariy A.
 V, I, R measurements: how to generate and measure quantities and then how to get data (resistivity, magnetoresistance, Hall). 590B Maariy A. Tanatar September 28, 2009 Thermo- galvano-magnetic effects
V, I, R measurements: how to generate and measure quantities and then how to get data (resistivity, magnetoresistance, Hall). 590B Maariy A. Tanatar September 28, 2009 Thermo- galvano-magnetic effects
I m. R s. Digital. R x. OhmmetersxSeries Shunt Digital. R m
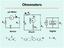 µa Meter I I s I m s E Digital x I Voltmeter x x E µa Meter m Is OhmmetersxSeries Shunt Digital EIx= = ()E sm x mxvi= x Shunt Ohmmeter Shunt s x E µa Meter I m I m V m E ) ( v I E ) ( E v E v E I When
µa Meter I I s I m s E Digital x I Voltmeter x x E µa Meter m Is OhmmetersxSeries Shunt Digital EIx= = ()E sm x mxvi= x Shunt Ohmmeter Shunt s x E µa Meter I m I m V m E ) ( v I E ) ( E v E v E I When
The Underground Experimental Investigation of Thermocouples
 The Underground Experimental Investigation of Thermocouples April 14, 21 Abstract This experiment investigates a K-type thermocouple in order to demonstrate the Seebeck and Peltier coefficients. Temperature
The Underground Experimental Investigation of Thermocouples April 14, 21 Abstract This experiment investigates a K-type thermocouple in order to demonstrate the Seebeck and Peltier coefficients. Temperature
Tridib s Physics Tutorials visit NCERT-XII / Unit- 03 Current Electricity
 CURRENT ELECTRICITY OHM S LAW:- Let us consider a conductor through which a current I is flowing and V be the potential difference between its ends,then Ohm s law states that V I or, V = R I..(1) where
CURRENT ELECTRICITY OHM S LAW:- Let us consider a conductor through which a current I is flowing and V be the potential difference between its ends,then Ohm s law states that V I or, V = R I..(1) where
Question 3: How is the electric potential difference between the two points defined? State its S.I. unit.
 EXERCISE (8 A) Question : Define the term current and state its S.I unit. Solution : Current is defined as the rate of flow of charge. I = Q/t Its S.I. unit is Ampere. Question 2: Define the term electric
EXERCISE (8 A) Question : Define the term current and state its S.I unit. Solution : Current is defined as the rate of flow of charge. I = Q/t Its S.I. unit is Ampere. Question 2: Define the term electric
18.2 Voltaic Cell. Generating Voltage (Potential) Dr. Fred Omega Garces. Chemistry 201. Miramar College. 1 Voltaic Cell.
 18.2 Voltaic Cell Generating Voltage (Potential) Dr. Fred Omega Garces Chemistry 201 Miramar College 1 Voltaic Cell Redox Between If Zn (s) and Cu 2+ (aq) is in the same solution, then the electrons transfer
18.2 Voltaic Cell Generating Voltage (Potential) Dr. Fred Omega Garces Chemistry 201 Miramar College 1 Voltaic Cell Redox Between If Zn (s) and Cu 2+ (aq) is in the same solution, then the electrons transfer
Electric current is a flow of electrons in a conductor. The SI unit of electric current is ampere.
 C h a p t e r at G l a n c e 4. Electric Current : Electric current is a flow of electrons in a conductor. The SI unit of electric current is ampere. Current = Charge time i.e, I = Q t The SI unit of charge
C h a p t e r at G l a n c e 4. Electric Current : Electric current is a flow of electrons in a conductor. The SI unit of electric current is ampere. Current = Charge time i.e, I = Q t The SI unit of charge
Peltier Application Note
 Peltier Application Note Early 19th century scientists, Thomas Seebeck and Jean Peltier, first discovered the phenomena that are the basis for today s thermoelectric industry. Seebeck found that if you
Peltier Application Note Early 19th century scientists, Thomas Seebeck and Jean Peltier, first discovered the phenomena that are the basis for today s thermoelectric industry. Seebeck found that if you
1 Written and composed by: Prof. Muhammad Ali Malik (M. Phil. Physics), Govt. Degree College, Naushera
 CURRENT ELECTRICITY Q # 1. What do you know about electric current? Ans. Electric Current The amount of electric charge that flows through a cross section of a conductor per unit time is known as electric
CURRENT ELECTRICITY Q # 1. What do you know about electric current? Ans. Electric Current The amount of electric charge that flows through a cross section of a conductor per unit time is known as electric
The following table of work functions for metals may be helpful for some of the problems in this homework:
 Photoelectric Effect Homework Activity Learning Goals: To be able to explain how the photoelectric effect experiment works and why a photon model of light is necessary to explain the results. To be determine
Photoelectric Effect Homework Activity Learning Goals: To be able to explain how the photoelectric effect experiment works and why a photon model of light is necessary to explain the results. To be determine
Module-1: Electrode Potential And Cells 2015
 Lecture-2 Standard Electrode potential Standard electrode potential is the electrode potential when the metal is in contact with a solution of its own ions of unit concentration (1M) at 298K. If the electrode
Lecture-2 Standard Electrode potential Standard electrode potential is the electrode potential when the metal is in contact with a solution of its own ions of unit concentration (1M) at 298K. If the electrode
Lecture 30 Chapter 19, Sections 3-4 Galvanic Cells Electrochemical Potential
 Lecture 30 Chapter 19, Sections 3-4 Galvanic Cells Electrochemical Potential Galvanic Cells Defined Standard Hydrogen Electrode Standard Reduction Potentials Redox Balancing One More Example OK, then here
Lecture 30 Chapter 19, Sections 3-4 Galvanic Cells Electrochemical Potential Galvanic Cells Defined Standard Hydrogen Electrode Standard Reduction Potentials Redox Balancing One More Example OK, then here
V, I, R measurements: how to generate and measure quantities and then how to get data (resistivity, magnetoresistance, Hall). Makariy A.
 V, I, R measurements: how to generate and measure quantities and then how to get data (resistivity, magnetoresistance, Hall). 590B Maariy A. Tanatar November 14, 2008 Thermo- galvano-magnetic effects Seebec
V, I, R measurements: how to generate and measure quantities and then how to get data (resistivity, magnetoresistance, Hall). 590B Maariy A. Tanatar November 14, 2008 Thermo- galvano-magnetic effects Seebec
Temperature Measurements
 Engineering 80 Spring 2015 Temperature Measurements SOURCE: http://www.eng.hmc.edu/newe80/pdfs/vishaythermdatasheet.pdf SOURCE: http://elcodis.com/photos/19/51/195143/to-92-3_standardbody to-226_straightlead.jpg
Engineering 80 Spring 2015 Temperature Measurements SOURCE: http://www.eng.hmc.edu/newe80/pdfs/vishaythermdatasheet.pdf SOURCE: http://elcodis.com/photos/19/51/195143/to-92-3_standardbody to-226_straightlead.jpg
CHEMISTRY 13 Electrochemistry Supplementary Problems
 1. When the redox equation CHEMISTRY 13 Electrochemistry Supplementary Problems MnO 4 (aq) + H + (aq) + H 3 AsO 3 (aq) Mn 2+ (aq) + H 3 AsO 4 (aq) + H 2 O(l) is properly balanced, the coefficients will
1. When the redox equation CHEMISTRY 13 Electrochemistry Supplementary Problems MnO 4 (aq) + H + (aq) + H 3 AsO 3 (aq) Mn 2+ (aq) + H 3 AsO 4 (aq) + H 2 O(l) is properly balanced, the coefficients will
Semiconductor thermogenerator
 Semiconductor thermogenerator LEP 4.1.07 Related topics Seebeck effect (thermoelectric effect), thermoelectric e.m.f., efficiency, Peltier coefficient, Thomson coefficient, Seebeck coefficient, direct
Semiconductor thermogenerator LEP 4.1.07 Related topics Seebeck effect (thermoelectric effect), thermoelectric e.m.f., efficiency, Peltier coefficient, Thomson coefficient, Seebeck coefficient, direct
Electrochemistry 1 1
 Electrochemistry 1 1 Half-Reactions 1. Balancing Oxidation Reduction Reactions in Acidic and Basic Solutions Voltaic Cells 2. Construction of Voltaic Cells 3. Notation for Voltaic Cells 4. Cell Potential
Electrochemistry 1 1 Half-Reactions 1. Balancing Oxidation Reduction Reactions in Acidic and Basic Solutions Voltaic Cells 2. Construction of Voltaic Cells 3. Notation for Voltaic Cells 4. Cell Potential
ICP/MS Multi-Element Standards
 Standards Ultra Pure Matrix Special Packaging Traceability to National Reference Materials AccuStandard s ICP/MS Standards are formulated to meet the needs of this very special instrument. As matrix effect
Standards Ultra Pure Matrix Special Packaging Traceability to National Reference Materials AccuStandard s ICP/MS Standards are formulated to meet the needs of this very special instrument. As matrix effect
Chapter 2 Atoms and the Periodic Table
 Chapter 2 1 Chapter 2 Atoms and the Periodic Table Solutions to In-Chapter Problems 2.1 Each element is identified by a one- or two-letter symbol. Use the periodic table to find the symbol for each element.
Chapter 2 1 Chapter 2 Atoms and the Periodic Table Solutions to In-Chapter Problems 2.1 Each element is identified by a one- or two-letter symbol. Use the periodic table to find the symbol for each element.
Unit 6 Current Electricity and Circuits
 Unit 6 Current Electricity and Circuits 2 Types of Electricity Electricity that in motion. Electricity that in motion. Occurs whenever an moves through a. 2 Types of Current Electricity Electricity that
Unit 6 Current Electricity and Circuits 2 Types of Electricity Electricity that in motion. Electricity that in motion. Occurs whenever an moves through a. 2 Types of Current Electricity Electricity that
Chapter 18. Electrochemistry
 Chapter 18 Electrochemistry Section 17.1 Spontaneous Processes and Entropy Section 17.1 http://www.bozemanscience.com/ap-chemistry/ Spontaneous Processes and Entropy Section 17.1 Spontaneous Processes
Chapter 18 Electrochemistry Section 17.1 Spontaneous Processes and Entropy Section 17.1 http://www.bozemanscience.com/ap-chemistry/ Spontaneous Processes and Entropy Section 17.1 Spontaneous Processes
Chemistry 213. Electrochemistry I
 1 Chemistry 213 Electrochemistry I Electrochemical Cells Objective Oxidation/reduction reactions find their most important use in the construction of voltaic cells (chemical batteries). In this experiment,
1 Chemistry 213 Electrochemistry I Electrochemical Cells Objective Oxidation/reduction reactions find their most important use in the construction of voltaic cells (chemical batteries). In this experiment,
Electricity CHAPTER ELECTRIC CURRENT AND CIRCUIT
 CHAPTER 12 Electricity Electricity has an important place in modern society. It is a controllable and convenient form of energy for a variety of uses in homes, schools, hospitals, industries and so on.
CHAPTER 12 Electricity Electricity has an important place in modern society. It is a controllable and convenient form of energy for a variety of uses in homes, schools, hospitals, industries and so on.
Unit #8, Chapter 10 Outline Electrochemistry and Redox Reactions
 Unit #8, Chapter 10 Outline Electrochemistry and Redox Reactions Lesson Topics Covered Homework Questions and Assignments 1 Introduction to Electrochemistry definitions 1. Read pages 462 467 2. On page
Unit #8, Chapter 10 Outline Electrochemistry and Redox Reactions Lesson Topics Covered Homework Questions and Assignments 1 Introduction to Electrochemistry definitions 1. Read pages 462 467 2. On page
Note that the protons and neutrons are each almost 2,000 times more massive than an electron; What is the approximate diameter of an atom?
 Atomic Structure and the Periodic Table Evolution of Atomic Theory The ancient Greek scientist Democritus is often credited with developing the idea of the atom Democritus proposed that matter was, on
Atomic Structure and the Periodic Table Evolution of Atomic Theory The ancient Greek scientist Democritus is often credited with developing the idea of the atom Democritus proposed that matter was, on
Thermoelectric Study of Peltier Effect Using Cu-Fe, Pb-Fe and Cu-Constantan Couples
 International Journal of Innovative Scientific & Engineering Technologies Research 4(4):1-12, Oct-Dec. 2016 SEAHI PUBLICATIONS, 2016 www.seahipaj.org ISSN: 2360-896X Thermoelectric Study of Peltier Effect
International Journal of Innovative Scientific & Engineering Technologies Research 4(4):1-12, Oct-Dec. 2016 SEAHI PUBLICATIONS, 2016 www.seahipaj.org ISSN: 2360-896X Thermoelectric Study of Peltier Effect
1.11 Electrochemistry
 1.11 Electrochemistry Recap from 1.7: Oxidation and Reduction: Oxidation and Reduction: Oxidation and reduction reactions can be identified by looking at the reaction in terms of electron transfer: Definitions:
1.11 Electrochemistry Recap from 1.7: Oxidation and Reduction: Oxidation and Reduction: Oxidation and reduction reactions can be identified by looking at the reaction in terms of electron transfer: Definitions:
Electric Current & DC Circuits
 Electric Current & DC Circuits Circuits Click on the topic to go to that section Conductors Resistivity and Resistance Circuit Diagrams Measurement EMF & Terminal Voltage Kirchhoff's Rules Capacitors*
Electric Current & DC Circuits Circuits Click on the topic to go to that section Conductors Resistivity and Resistance Circuit Diagrams Measurement EMF & Terminal Voltage Kirchhoff's Rules Capacitors*
Lecture 27 Chapter 19, Sections 3-4 Galvanic Cells Electrochemical Potential
 Lecture 27 Chapter 19, Sections 3-4 Galvanic Cells Electrochemical Potential Galvanic Cells Defined Standard Hydrogen Electrode Standard Reduction Potentials Redox Balancing One More Example This time
Lecture 27 Chapter 19, Sections 3-4 Galvanic Cells Electrochemical Potential Galvanic Cells Defined Standard Hydrogen Electrode Standard Reduction Potentials Redox Balancing One More Example This time
Writing Chemical formula with polyatomic groups
 Writing Chemical formula with polyatomic groups 1. Use the Periodic table to determine the combining powers of single elements. Eg. Magnesium is in Group 2 and has a combining power of 2. 2. Use Table
Writing Chemical formula with polyatomic groups 1. Use the Periodic table to determine the combining powers of single elements. Eg. Magnesium is in Group 2 and has a combining power of 2. 2. Use Table
Slide 1. Temperatures Light (Optoelectronics) Magnetic Fields Strain Pressure Displacement and Rotation Acceleration Electronic Sensors
 Slide 1 Electronic Sensors Electronic sensors can be designed to detect a variety of quantitative aspects of a given physical system. Such quantities include: Temperatures Light (Optoelectronics) Magnetic
Slide 1 Electronic Sensors Electronic sensors can be designed to detect a variety of quantitative aspects of a given physical system. Such quantities include: Temperatures Light (Optoelectronics) Magnetic
CHAPTER 1 ELECTRICITY
 CHAPTER 1 ELECTRICITY Electric Current: The amount of charge flowing through a particular area in unit time. In other words, it is the rate of flow of electric charges. Electric Circuit: Electric circuit
CHAPTER 1 ELECTRICITY Electric Current: The amount of charge flowing through a particular area in unit time. In other words, it is the rate of flow of electric charges. Electric Circuit: Electric circuit
Professional Article. Fire and Ice
 Professional Article Fire and Ice Professional Article Spectrumanalysis Fire and Ice Temperature measurement is important in the industry. In the chemical industry as well as for environmental protection
Professional Article Fire and Ice Professional Article Spectrumanalysis Fire and Ice Temperature measurement is important in the industry. In the chemical industry as well as for environmental protection
Single-Element Standards for AAS
 Single-Element Standards for AAS for AAS Flame Silver Ag in 2-5% HNO 3 Aluminium Al in 2-5% HCl Aluminium Al in 2-5% HNO 3 Arsenic As in 2-5% HCl Arsenic As in 2-5% HNO 3 Gold Au in 2-5% HCl Boron B in
Single-Element Standards for AAS for AAS Flame Silver Ag in 2-5% HNO 3 Aluminium Al in 2-5% HCl Aluminium Al in 2-5% HNO 3 Arsenic As in 2-5% HCl Arsenic As in 2-5% HNO 3 Gold Au in 2-5% HCl Boron B in
Full file at
 16 Chapter 2: Atoms and the Periodic Table Solutions to In-Chapter Problems 2.1 Each element is identified by a one- or two-letter symbol. Use the periodic table to find the symbol for each element. a.
16 Chapter 2: Atoms and the Periodic Table Solutions to In-Chapter Problems 2.1 Each element is identified by a one- or two-letter symbol. Use the periodic table to find the symbol for each element. a.
Electric Charge. Electric Charge ( q ) unbalanced charges positive and negative charges. n Units Coulombs (C)
 Electric Charge Electric Charge ( q ) unbalanced charges positive and negative charges n Units Coulombs (C) Electric Charge How do objects become charged? Types of materials Conductors materials in which
Electric Charge Electric Charge ( q ) unbalanced charges positive and negative charges n Units Coulombs (C) Electric Charge How do objects become charged? Types of materials Conductors materials in which
Chapter 19: Redox & Electrochemistry
 Chapter 19: Redox & Electrochemistry 1. Oxidation-Reduction Reactions Definitions Oxidation - refers to the of electrons by a molecule, atom or ion Reduction - refers to the of electrons by an molecule,
Chapter 19: Redox & Electrochemistry 1. Oxidation-Reduction Reactions Definitions Oxidation - refers to the of electrons by a molecule, atom or ion Reduction - refers to the of electrons by an molecule,
MAGNETISM. Magnetism. Magnetism is a result of electrons spinning on their own axis around the nucleus (Figure 18). Basic Electrical Theory
 Basic Electrical Theory Certain metals and metallic oxides have the ability to attract other metals. This property is called magnetism, and the materials which have this property are called magnets. Some
Basic Electrical Theory Certain metals and metallic oxides have the ability to attract other metals. This property is called magnetism, and the materials which have this property are called magnets. Some
materials and their properties
 materials and their properties macroscopic properties phase state strength / stiffness electrical conductivity chemical properties color / transparence spectroscopical properties surface properties density
materials and their properties macroscopic properties phase state strength / stiffness electrical conductivity chemical properties color / transparence spectroscopical properties surface properties density
Measurement of Temperature in the Plastics Industry
 Sawi Mess- und Regeltechnik AG CH 8405 Winterthur-Gotzenwil, Switzerland Telephone +41 52 320 50 50, Fax +41 52 320 50 55 www.sawi.ch Measurement of Temperature in the Plastics Industry Johannes Wild,
Sawi Mess- und Regeltechnik AG CH 8405 Winterthur-Gotzenwil, Switzerland Telephone +41 52 320 50 50, Fax +41 52 320 50 55 www.sawi.ch Measurement of Temperature in the Plastics Industry Johannes Wild,
UNIT 3 ELECTRIC CHARGE ELECTRIC CIRCUITS: ELECTRIC CHARGE:
 AS Physics 9702 unit 3: Electric Charge 1 UNIT 3 ELECTRIC CHARGE ELECTRIC CIRCUITS: For the current to exist it must have a complete path of conductors. This complete path of conductors is called electric
AS Physics 9702 unit 3: Electric Charge 1 UNIT 3 ELECTRIC CHARGE ELECTRIC CIRCUITS: For the current to exist it must have a complete path of conductors. This complete path of conductors is called electric
Measurements & Instrumentation. Module 3: Temperature Sensors
 Measurements & Instrumentation PREPARED BY Academic Services Unit August 2013 Institute of Applied Technology, 2013 Module Objectives Upon successful completion of this module, students should be able
Measurements & Instrumentation PREPARED BY Academic Services Unit August 2013 Institute of Applied Technology, 2013 Module Objectives Upon successful completion of this module, students should be able
Chapter 20 Electrochemistry
 Chapter 20 Electrochemistry Learning goals and key skills: Identify oxidation, reduction, oxidizing agent, and reducing agent in a chemical equation Complete and balance redox equations using the method
Chapter 20 Electrochemistry Learning goals and key skills: Identify oxidation, reduction, oxidizing agent, and reducing agent in a chemical equation Complete and balance redox equations using the method
A Review of Circuitry
 1 A Review of Circuitry There is an attractive force between a positive and a negative charge. In order to separate these charges, a force at least equal to the attractive force must be applied to one
1 A Review of Circuitry There is an attractive force between a positive and a negative charge. In order to separate these charges, a force at least equal to the attractive force must be applied to one
4. Thermometry. Temperature and Heat Flow Temperature Scales Thermometers
 4. Thermometry Measuring temperature by sensation is very imprecise. That is why we need a temperature scale and a thermometer to measure temperature more accurately. Temperature and Heat Flow Temperature
4. Thermometry Measuring temperature by sensation is very imprecise. That is why we need a temperature scale and a thermometer to measure temperature more accurately. Temperature and Heat Flow Temperature
A Comparison of Figureof Merit for Some Common ThermocouplesintheHighTemperatureRange
 Global Journal of Researches in Engineering Electrical and Electronics Engineering Volume 13 Issue 11 Version 1.0 Year 2013 Type: Double Blind Peer Reviewed International Research Journal Publisher: Global
Global Journal of Researches in Engineering Electrical and Electronics Engineering Volume 13 Issue 11 Version 1.0 Year 2013 Type: Double Blind Peer Reviewed International Research Journal Publisher: Global
Measurement in Engineering
 Measurement in Engineering Responsible person for the course: Ing. Martin Novak, Ph.D. Report on the laboratory experiment Measurement of temperature of the 12.10.10 - made by Sebastian Kößler Report on
Measurement in Engineering Responsible person for the course: Ing. Martin Novak, Ph.D. Report on the laboratory experiment Measurement of temperature of the 12.10.10 - made by Sebastian Kößler Report on
Electro - Principles I
 Electro - Principles I Page 10-1 Atomic Theory It is necessary to know what goes on at the atomic level of a semiconductor so the characteristics of the semiconductor can be understood. In many cases a
Electro - Principles I Page 10-1 Atomic Theory It is necessary to know what goes on at the atomic level of a semiconductor so the characteristics of the semiconductor can be understood. In many cases a
Chapter 18 Electrochemistry. Electrochemical Cells
 Chapter 18 Electrochemistry Chapter 18 1 Electrochemical Cells Electrochemical Cells are of two basic types: Galvanic Cells a spontaneous chemical reaction generates an electric current Electrolytic Cells
Chapter 18 Electrochemistry Chapter 18 1 Electrochemical Cells Electrochemical Cells are of two basic types: Galvanic Cells a spontaneous chemical reaction generates an electric current Electrolytic Cells
Electrochemistry. A. Na B. Ba C. S D. N E. Al. 2. What is the oxidation state of Xe in XeO 4? A +8 B +6 C +4 D +2 E 0
 Electrochemistry 1. Element M reacts with oxygen to from an oxide with the formula MO. When MO is dissolved in water, the resulting solution is basic. Element M is most likely: A. Na B. Ba C. S D. N E.
Electrochemistry 1. Element M reacts with oxygen to from an oxide with the formula MO. When MO is dissolved in water, the resulting solution is basic. Element M is most likely: A. Na B. Ba C. S D. N E.
APPENDIX ELEVEN Open Fire Temperature Measurements
 APPENDIX ELEVEN Open Fire Temperature Measurements Temperatures are usually recorded with alcohol or mercury thermometers, with thermistors or resistance temperature detectors (platinum resistance thermometers),
APPENDIX ELEVEN Open Fire Temperature Measurements Temperatures are usually recorded with alcohol or mercury thermometers, with thermistors or resistance temperature detectors (platinum resistance thermometers),
(18) WMP/Jun10/CHEM5
 Electrochemistry 18 7 The electrons transferred in redox reactions can be used by electrochemical cells to provide energy. Some electrode half-equations and their standard electrode potentials are shown
Electrochemistry 18 7 The electrons transferred in redox reactions can be used by electrochemical cells to provide energy. Some electrode half-equations and their standard electrode potentials are shown
Temperature measurement
 emperature measurement BASICS Interest in emperature measurements appeared definitively later as respect to other quantities. emperature is an intensive quantity (a quantity strictly correlated to temperature
emperature measurement BASICS Interest in emperature measurements appeared definitively later as respect to other quantities. emperature is an intensive quantity (a quantity strictly correlated to temperature
OPPA European Social Fund Prague & EU: We invest in your future.
 OPPA European Social Fund Prague & EU: We invest in your future. PELTIER CELL OBJECT Your task is to get acquainted with the Peltier cell behavior in the ThermoElectric Generator mode (TEG) and in the
OPPA European Social Fund Prague & EU: We invest in your future. PELTIER CELL OBJECT Your task is to get acquainted with the Peltier cell behavior in the ThermoElectric Generator mode (TEG) and in the
Chapter 1 Overview of Semiconductor Materials and Physics
 Chapter 1 Overview of Semiconductor Materials and Physics Professor Paul K. Chu Conductivity / Resistivity of Insulators, Semiconductors, and Conductors Semiconductor Elements Period II III IV V VI 2 B
Chapter 1 Overview of Semiconductor Materials and Physics Professor Paul K. Chu Conductivity / Resistivity of Insulators, Semiconductors, and Conductors Semiconductor Elements Period II III IV V VI 2 B
Temperature Measurement
 Temperature Measurement Temperature is one of the most common measurements What is Temperature? Intuitively understood as sensation of hot/cold Early Researchers: Galileo (1564-1642) Newton (1642-1727)
Temperature Measurement Temperature is one of the most common measurements What is Temperature? Intuitively understood as sensation of hot/cold Early Researchers: Galileo (1564-1642) Newton (1642-1727)
HEAT AND TEMPERATURE Vikasana-Bridge Course 2012
 HEAT AND TEMPERATURE TOPICS Introduction Effects of heat Specific heat Basics of thermodynamics Introduction Heat may be defined as energy in transit from a high temperature region to a lower temperature
HEAT AND TEMPERATURE TOPICS Introduction Effects of heat Specific heat Basics of thermodynamics Introduction Heat may be defined as energy in transit from a high temperature region to a lower temperature
MEASURING INSTRUMENTS
 Albaha University Faculty of Engineering Mechanical Engineering g Department MEASURING INSTRUMENTS AND CALIBRATION Lecture (7) Temperature measurement By: Ossama Abouelatta o_abouelatta@yahoo.com Mechanical
Albaha University Faculty of Engineering Mechanical Engineering g Department MEASURING INSTRUMENTS AND CALIBRATION Lecture (7) Temperature measurement By: Ossama Abouelatta o_abouelatta@yahoo.com Mechanical
CHAPTER ELECTROCHEMISTRY
 149 CHAPTER ELECTROCHEMISTRY 1. On electrolysis of dilute sulphuric acid using platinum electrodes, the product obtained at the anode will be hydrogen oxygen hydrogen sulphide sulphur dioxide 2. Which
149 CHAPTER ELECTROCHEMISTRY 1. On electrolysis of dilute sulphuric acid using platinum electrodes, the product obtained at the anode will be hydrogen oxygen hydrogen sulphide sulphur dioxide 2. Which
What are the two types of current? The two types of current are direct current and alternating current.
 Electric Current What are the two types of current? The two types of current are direct current and alternating current. Electric Current The continuous flow of electric charge is an electric current.
Electric Current What are the two types of current? The two types of current are direct current and alternating current. Electric Current The continuous flow of electric charge is an electric current.
Electrochemical Reactions
 1 of 20 4/11/2016 1:00 PM Electrochemical Reactions Electrochemical Reactions Electrical Work From Spontaneous Oxidation- Reduction Reactions Predicting Spontaneous Redox Reactions from the Sign of E Line
1 of 20 4/11/2016 1:00 PM Electrochemical Reactions Electrochemical Reactions Electrical Work From Spontaneous Oxidation- Reduction Reactions Predicting Spontaneous Redox Reactions from the Sign of E Line
AP Physics C - E & M
 AP Physics C - E & M Current and Circuits 2017-07-12 www.njctl.org Electric Current Resistance and Resistivity Electromotive Force (EMF) Energy and Power Resistors in Series and in Parallel Kirchoff's
AP Physics C - E & M Current and Circuits 2017-07-12 www.njctl.org Electric Current Resistance and Resistivity Electromotive Force (EMF) Energy and Power Resistors in Series and in Parallel Kirchoff's
Chapter 29. Simple chemical cells
 Chapter 29 Simple chemical cells 29.1 Simple chemical cells consisting of two metal electrodes and an electrolyte 29.2 The Electrochemical Series of metals 29.3 Simple chemical cells consisting of metal-metal
Chapter 29 Simple chemical cells 29.1 Simple chemical cells consisting of two metal electrodes and an electrolyte 29.2 The Electrochemical Series of metals 29.3 Simple chemical cells consisting of metal-metal
Physics Module Form 5 Chapter 2- Electricity GCKL 2011 CHARGE AND ELECTRIC CURRENT
 2.1 CHARGE AND ELECTRIC CURRENT Van de Graaf 1. What is a Van de Graaff generator? Fill in each of the boxes the name of the part shown. A device that produces and store electric charges at high voltage
2.1 CHARGE AND ELECTRIC CURRENT Van de Graaf 1. What is a Van de Graaff generator? Fill in each of the boxes the name of the part shown. A device that produces and store electric charges at high voltage
Unit 11 Reactivity of metals
 Unit 11 Reactivity of metals Comparing the reactivity of metals In unit 4, you learned that different extraction methods are used in the extractions of metals. Some metals, like silver and gold can exist
Unit 11 Reactivity of metals Comparing the reactivity of metals In unit 4, you learned that different extraction methods are used in the extractions of metals. Some metals, like silver and gold can exist
Electricity and magnetism Solid state physics
 Physics: Physics: Electricity and magnetism Solid state physics Thermoelectricity he direct conversion of heat into electrical energy, or the reverse, in solid or liquid conductors by means of three interrelated
Physics: Physics: Electricity and magnetism Solid state physics Thermoelectricity he direct conversion of heat into electrical energy, or the reverse, in solid or liquid conductors by means of three interrelated
Chemistry 132 NT. Electrochemistry. Oxidation-Reduction Reactions
 Chemistry 132 NT If you ever catch on fire, try to avoid seeing yourself in the mirror, because I bet that s what really throws you into a panic. Jack Handey 1 Chem 132 NT Electrochemistry Module 1 HalfReactions
Chemistry 132 NT If you ever catch on fire, try to avoid seeing yourself in the mirror, because I bet that s what really throws you into a panic. Jack Handey 1 Chem 132 NT Electrochemistry Module 1 HalfReactions
CHAPTER 12. Practice exercises
 CHAPTER 12 Practice exercises 12.1 2Al(s) + 3Cl 2 (g) 2AlCl 3 (aq) Aluminium is oxidised and is therefore the reducing agent. Chlorine is reduced and is therefore the oxidising agent. 12.3 First the oxidation
CHAPTER 12 Practice exercises 12.1 2Al(s) + 3Cl 2 (g) 2AlCl 3 (aq) Aluminium is oxidised and is therefore the reducing agent. Chlorine is reduced and is therefore the oxidising agent. 12.3 First the oxidation
5. ELECTRIC CURRENTS
 5. ELECTRIC CURRENTS TOPIC OUTLINE Section Recommended Time Giancoli Section 5.1 Potential Difference, Current, Resistance 5.2 Electric Circuits 3h 19.1, 19.2 6.2 Electric Field and Force 6.3 Magnetic
5. ELECTRIC CURRENTS TOPIC OUTLINE Section Recommended Time Giancoli Section 5.1 Potential Difference, Current, Resistance 5.2 Electric Circuits 3h 19.1, 19.2 6.2 Electric Field and Force 6.3 Magnetic
 CHAPTER 10 ELECTROCHEMISTRY TEXT BOOK EXERCISE Q1. Multiple choice questions. (i) The cathode reaction in the electrolysis of dill. H2SO4 with Pt electrode is (a) Reduction (b) Oxidation (c) Both oxidation
CHAPTER 10 ELECTROCHEMISTRY TEXT BOOK EXERCISE Q1. Multiple choice questions. (i) The cathode reaction in the electrolysis of dill. H2SO4 with Pt electrode is (a) Reduction (b) Oxidation (c) Both oxidation
CHAPTER 4 DC Electricity
 CHAPTER 4 DC Electricity Benjamin Franklin proved an important scientific point, which is that electricity originates inside clouds. There, it forms into lightning, which is attracted to the earth by golfers.
CHAPTER 4 DC Electricity Benjamin Franklin proved an important scientific point, which is that electricity originates inside clouds. There, it forms into lightning, which is attracted to the earth by golfers.
THERMOCOUPLE CHARACTERISTICS TRAINER
 THERMOCOUPLE CHARACTERISTICS TRAINER (Model No : ) User Manual Version 2.0 Technical Clarification /Suggestion : / Technical Support Division, Vi Microsystems Pvt. Ltd., Plot No :75,Electronics Estate,
THERMOCOUPLE CHARACTERISTICS TRAINER (Model No : ) User Manual Version 2.0 Technical Clarification /Suggestion : / Technical Support Division, Vi Microsystems Pvt. Ltd., Plot No :75,Electronics Estate,
Chapter 17. Electrochemistry
 Chapter 17 Electrochemistry Contents Galvanic cells Standard reduction potentials Cell potential, electrical work, and free energy Dependence of cell potential on concentration Batteries Corrosion Electrolysis
Chapter 17 Electrochemistry Contents Galvanic cells Standard reduction potentials Cell potential, electrical work, and free energy Dependence of cell potential on concentration Batteries Corrosion Electrolysis
Chapter 20. Electrochemistry
 Chapter 20. Electrochemistry 20.1 Oxidation-Reduction Reactions Oxidation-reduction reactions = chemical reactions in which the oxidation state of one or more substance changes (redox reactions). Recall:
Chapter 20. Electrochemistry 20.1 Oxidation-Reduction Reactions Oxidation-reduction reactions = chemical reactions in which the oxidation state of one or more substance changes (redox reactions). Recall:
High Temperature Measurement System Design for Thermoelectric Materials In Power Generation Application
 Mat. Res. Soc. Symp. Proc. Vol. 793 24 Materials Research Society S9.4.1 High Temperature Measurement System Design for Thermoelectric Materials In Power Generation Application Sim Loo, Jarrod Short, Kuei
Mat. Res. Soc. Symp. Proc. Vol. 793 24 Materials Research Society S9.4.1 High Temperature Measurement System Design for Thermoelectric Materials In Power Generation Application Sim Loo, Jarrod Short, Kuei
100% ionic compounds do not exist but predominantly ionic compounds are formed when metals combine with non-metals.
 2.21 Ionic Bonding 100% ionic compounds do not exist but predominantly ionic compounds are formed when metals combine with non-metals. Forming ions Metal atoms lose electrons to form +ve ions. Non-metal
2.21 Ionic Bonding 100% ionic compounds do not exist but predominantly ionic compounds are formed when metals combine with non-metals. Forming ions Metal atoms lose electrons to form +ve ions. Non-metal
Objective of Lecture Discuss resistivity and the three categories of materials Chapter 2.1 Show the mathematical relationships between charge,
 Objective of Lecture Discuss resistivity and the three categories of materials Chapter 2.1 Show the mathematical relationships between charge, current, voltage, and energy. Chapter 2.2-2.4 Define resistance
Objective of Lecture Discuss resistivity and the three categories of materials Chapter 2.1 Show the mathematical relationships between charge, current, voltage, and energy. Chapter 2.2-2.4 Define resistance
Oxidation-Reduction Reactions and Introduction to Electrochemistry
 ADVANCED PLACEMENT CHEMISTRY Oxidation-Reduction Reactions and Introduction to Electrochemistry Students will be able to: identify oxidation and reduction of chemical species; identify oxidants and reductants
ADVANCED PLACEMENT CHEMISTRY Oxidation-Reduction Reactions and Introduction to Electrochemistry Students will be able to: identify oxidation and reduction of chemical species; identify oxidants and reductants
Properties of Materials
 Tao Deng, dengtao@sjtu.edu.cn 1 1896 1920 1987 2006 Properties of Materials Chapter 3 Electrical Properties of Materials Tao Deng 3.1.4.4 The superconducting tunneling effect (Josephson effect) Tao Deng,
Tao Deng, dengtao@sjtu.edu.cn 1 1896 1920 1987 2006 Properties of Materials Chapter 3 Electrical Properties of Materials Tao Deng 3.1.4.4 The superconducting tunneling effect (Josephson effect) Tao Deng,
