THERMODYNAMICS. Temperature
|
|
|
- Joleen Reeves
- 5 years ago
- Views:
Transcription
1 HERMODYNMICS hermodynamcs s the henomenologcal scence whch descrbes the behavor of macroscoc objects n terms of a small number of macroscoc arameters. s an examle, to descrbe a gas n terms of volume ressure temerature, number of artcles, and ther tye. he nterestng asect of ths s that s ossble at all. fter all, a macroscoc object contans ~10 5 artcles or more. Each would aear to need at least 6 numbers to descrbe t 3 oston and 3 momentum or velocty. So why are only a few (~ 10) needed n ractce? he subject s aroached n two very dfferent ways. he frst s the henomenologcal method stated above. he second s from the mcroscoc laws of Newton or quantum mechancs (f needed). hs second aroach s called statstcal mechancs. We wll use both, alternatng between them. emerature he concets of volume and ressure are already famlar, but temerature (n the scentfc sense) s not. We begn wth an objectve defnton of temerature we show how to measure t. Suose we have macroscoc objects, and B. Now suose we lace them n thermal contact so that t s ossble for energy to flow between them. In ractce, t s nearly mossble to revent such flow by radaton f no other way. If energy tends to flow from to B we say that the temerature of s hgher than that of B. If energy goes from B then B If there s no tendency for energy to flow we say that B Now add a thrd object C. hen the 0 th Law of hermodynamcs states that f B and
2 B C hen C hs seems obvous, but t contans a subtle assumton that only temerature affects the drecton of heat flow. hs turns out to be true exermentally but t dd not have to be that way. hus t s generally stated as an ndeendent law. emerature Scale Havng thus defned temerature t s a smle matter to obtan a scale and a method of assgnng a numercal temerature to an object. We observe that a very large number of hyscal roertes deend on a temerature (length, volume, color, resstance, etc.). We need merely ck a roerty and two fxed onts and we have a scale and a thermometer. For examle, a very convenent choce s an deal gas whch we wll dscuss n a moment. Frst we consder the famlar mercury n a tube thermometer. Here the roerty s the heght of the column of mercury. he two fnal onts are the meltng and bolng onts of water (under certan condtons). We lace the tube of mercury n the ce water mxture and mark ts heght. We then lace t n the bolng water and mark the new heght. We then dvde the sace between the marks nto equal ntervals. We now have a thermometer. If we take meltng ce to be 0 and bolng water to be 100, we have the famlar centgrade scale. If we take them to be 3 ad 1, we have the even more famlar Fahrenhet scale. If we take them to be 73 and 373, we have the Kelvn scale. Note that nether Centgrade nor Fahrenhet s ntrnscally better than the other. Kelvn on the other hand s fundamentally sueror as we wll see. Ideal Gas o get an dea of the fundamental mcroscoc meanng of temerature we consder an deal gas, defned to be a gas n whch there s no nteracton between the molecules excet at the nstant of collson. hs s never comletely accurate. It becomes arbtrarly close to realty as the densty of the gas 0. (Infntely far aart, they can t see each other.) Consder a cubcal box of sde L contanng N molecules. By the sotroy of sace we wll have equal numbers movng n each drecton. More accurately, the comonents of velocty n each drecton wll be the same. Furthermore, the molecules wll have some average magntude of velocty v. hen n one second each molecule movng n the x drecton wll make
3 v L collsons wth the wall at x = 0. When t does so ts momentum wll change by mv (mv n mv out). hus the total change n momentum n the x-drecton/sec s N v mv 3 L However, by Newton s nd law ths must be the force exerted on the gas by the wall. Hence F Nmv N KE 3 av 3L 3 V Where V = L 3 s the volume of the gas. By Newton s law however, ths s also the F/ exerted on the wall by the gas or the ressure. hus We now defne the deal gas temerature to be PV NKEav 3 KEav 3 k where k = Boltzman s constant = J/ K. Note that the lowest ossble temerature s the state of lowest ossble KE. In classcal hyscs ths would be KE = 0. Clearly ths works snce f KE = 0 so does dvm/dt and hence, P. he Kelvn scale smly starts wth 0 at the lowest ossble temerature whch haens to be -73 C. hs s why the Kelvn scale s ntrnscally sueror to the other two. hus we have an dea about the fundamental meanng of temerature at the mcroscoc level t s a measure of the random KE of the molecules makng u the materal. Next we wll turn to a statstcal mechancs descrton of an deal gas where we do not assume an average velocty at the outset. Statstcal Mechancs We need a way to characterze the mcrostate of a system. Begn by consderng a monatomc deal gas. hen each molecule can be located by gvng ts oston and momentum. hs wll requre 6 numbers 3 oston and 3 momentum. We thus magne a 6-dmenstonal sace. We slt ths sace nto nfntesmal boxes of volume
4 We now defne a dstrbuton functon, f, by dxdydzdxdyd z # of molecules n box x, y,z,,, dx d x y z z macroscoc state s then gven by secfyng the occuaton of each box. For the case of a monatomc deal gas or any other for that matter we must recognze three dstnct ossbltes. Case 1 Classcal In classcal mechancs each artcle s dstngushable from all the others we can kee track of whch s whch. Case QM Ferm Drac In quantum mechancs dentcal artcles are not dstngushable we can t kee track of whch s whch. here turn out to be two dfferent classes of artcles and hence two dfferent statstcs. One s the Ferm-Drac artcles and the other Bose-Ensten. Case 3 QM Bose-Ensten We wll, for now, only consder the classcal case. We now make the fundamental assumton of statstcal mechancs that all ossble mcrostates are equally lkely and therefore that the most robable macrostate wll be that one whch arses from the largest number of mcrostates. Hence we need to know how many mcrostates corresond to the same macrostate. macrostate s secfed by gvng the number of artcles n each nfntesmal box. Let n be the number of artcles n the th box, and N be the total number of artcles. How many ways can we have n artcles n the 1 st box? We care whch artcles they are, but not he order n whch they were chosen. he 1 st artcle can be chosen n N way, the second n N-1 ways, etc. Hence there are N N 1... N n 1 ways to do ths. However, there are n 1! orders n whch they can have been chosen. Hence the number of dstnct mcrostates s N N 1... N n1 n! 1
5 Now take the next box. he number of ways s Hence the total number of ways s N n... Nn n n! N N1 1 N! n!n 1! n! n! hus the robablty of the macrostate secfed by the n s roortonal to or N! n! P q N! n! We now assume that all the n and N >> 1 (smly choose large enough boxes). hs wll not work for quantum statstcs, but s fne here. hen t s easest to use lnp nstead of P. hus We now use Strng s roxmsaton hus np nq nn! nn! nn! N nn N np nq N n N N n n n n nq N n N n n n 1 Next we must mose constrants on the system the total number of artcles s fxed and the total energy fxed. In other words, we are dealng wth an solated system. here are other ossbltes, but ths wll do for now. Here N n E n
6 where s the energy of artcles n the th box. We then fnd the macrostate as the one for whch lnp s greatest consstent wth the constrants. n np0nnn n nn 1n nnn n snce n 0and We use the method of Lagrange Multlers dd these equatons together to get n, n 0 n 0, n 0 nn n 0 nn n 0, Snce ths must be true for any choce of n we must have nn, n e e, subject to the condtons, e e N, e, e E In classcal mechancs we relace the sum by an ntegral snce the boxes can be made nfntesmally small (ths s not true n QM systems). hus N e dxdz e, x1z
7 or e N, xz dxdz e hus far we have not used the fact that we have a monatomc deal gas. For that case the only energy s knetc hus e 3, x y z m N x y z m m m x y z dxdydz d e d e d e N V e md he Gaussan ntegral n [ ] can be easly evaluated as follows: Hence x y e dx e dx e dy e dxdy 1/ x x y / r 1/ 1/ 1/ r r rdrde re dr e 0 1/
8 N e 3/ m V o determne β we calculate the ressure ths system would exert on a wall of the contaner. We do t as before, excet now the artcles n box have the velocty /m rather than the average velocty. hus the ressure exerted by the artcles n box s n Now we must add ths u for all the boxes. y and z can be anythng, but x must be > 0 (else the artcles won t ht the wall. Further, the box must be wthn x /m of the wall or the artcles can t reach the wall n a second. Hence 0 y z x x0 y z x m x0 x x m x x x m x y z P e e dxd N m 3/ m V x m d e dx x N 1 m N x d 1/ x e d x e m d m 1/ m m V d 0 mv m 0 1/ 1/ N d N 1 N m N 1/ 1/ 3/ m m V V mv d m m mv m But we know that the deal gas temerature s gven by Hence PV Nk Nk N 1 V V k z
9 hus we arrve at the famous Boltzmann Dstrbuton. f e k wth k e dx d N z Degrees of Freedom Next we consder what haens when we do not have a monatomc deal gas.
6. Hamilton s Equations
 6. Hamlton s Equatons Mchael Fowler A Dynamcal System s Path n Confguraton Sace and n State Sace The story so far: For a mechancal system wth n degrees of freedom, the satal confguraton at some nstant
6. Hamlton s Equatons Mchael Fowler A Dynamcal System s Path n Confguraton Sace and n State Sace The story so far: For a mechancal system wth n degrees of freedom, the satal confguraton at some nstant
Dr. Shalabh Department of Mathematics and Statistics Indian Institute of Technology Kanpur
 Analyss of Varance and Desgn of Exerments-I MODULE III LECTURE - 2 EXPERIMENTAL DESIGN MODELS Dr. Shalabh Deartment of Mathematcs and Statstcs Indan Insttute of Technology Kanur 2 We consder the models
Analyss of Varance and Desgn of Exerments-I MODULE III LECTURE - 2 EXPERIMENTAL DESIGN MODELS Dr. Shalabh Deartment of Mathematcs and Statstcs Indan Insttute of Technology Kanur 2 We consder the models
1.72, Groundwater Hydrology Prof. Charles Harvey Lecture Packet #3: Hydraulic Head and Fluid Potential. p o. p o + p
 1.7, Groundwater Hydrology Prof. Charles Harvey Lecture Packet #3: Hydraulc Head and Flud Potental What makes water flow? Consder ressure Water Level o A Water Level C o o + B Pressure at A atmosherc (
1.7, Groundwater Hydrology Prof. Charles Harvey Lecture Packet #3: Hydraulc Head and Flud Potental What makes water flow? Consder ressure Water Level o A Water Level C o o + B Pressure at A atmosherc (
The Dirac Equation for a One-electron atom. In this section we will derive the Dirac equation for a one-electron atom.
 The Drac Equaton for a One-electron atom In ths secton we wll derve the Drac equaton for a one-electron atom. Accordng to Ensten the energy of a artcle wth rest mass m movng wth a velocty V s gven by E
The Drac Equaton for a One-electron atom In ths secton we wll derve the Drac equaton for a one-electron atom. Accordng to Ensten the energy of a artcle wth rest mass m movng wth a velocty V s gven by E
Mechanics Physics 151
 Mechancs hyscs 151 Lecture Canoncal Transformatons (Chater 9) What We Dd Last Tme Drect Condtons Q j Q j = = j, Q, j, Q, Necessary and suffcent j j for Canoncal Transf. = = j Q, Q, j Q, Q, Infntesmal CT
Mechancs hyscs 151 Lecture Canoncal Transformatons (Chater 9) What We Dd Last Tme Drect Condtons Q j Q j = = j, Q, j, Q, Necessary and suffcent j j for Canoncal Transf. = = j Q, Q, j Q, Q, Infntesmal CT
STATISTICAL MECHANICS
 STATISTICAL MECHANICS Thermal Energy Recall that KE can always be separated nto 2 terms: KE system = 1 2 M 2 total v CM KE nternal Rgd-body rotaton and elastc / sound waves Use smplfyng assumptons KE of
STATISTICAL MECHANICS Thermal Energy Recall that KE can always be separated nto 2 terms: KE system = 1 2 M 2 total v CM KE nternal Rgd-body rotaton and elastc / sound waves Use smplfyng assumptons KE of
Lecture 3 Examples and Problems
 Lecture 3 Examles and Problems Mechancs & thermodynamcs Equartton Frst Law of Thermodynamcs Ideal gases Isothermal and adabatc rocesses Readng: Elements Ch. 1-3 Lecture 3, 1 Wllam Thomson (1824 1907) a.k.a.
Lecture 3 Examles and Problems Mechancs & thermodynamcs Equartton Frst Law of Thermodynamcs Ideal gases Isothermal and adabatc rocesses Readng: Elements Ch. 1-3 Lecture 3, 1 Wllam Thomson (1824 1907) a.k.a.
Lecture 4. Macrostates and Microstates (Ch. 2 )
 Lecture 4. Macrostates and Mcrostates (Ch. ) The past three lectures: we have learned about thermal energy, how t s stored at the mcroscopc level, and how t can be transferred from one system to another.
Lecture 4. Macrostates and Mcrostates (Ch. ) The past three lectures: we have learned about thermal energy, how t s stored at the mcroscopc level, and how t can be transferred from one system to another.
Translational Equations of Motion for A Body Translational equations of motion (centroidal) for a body are m r = f.
 Lesson 12: Equatons o Moton Newton s Laws Frst Law: A artcle remans at rest or contnues to move n a straght lne wth constant seed there s no orce actng on t Second Law: The acceleraton o a artcle s roortonal
Lesson 12: Equatons o Moton Newton s Laws Frst Law: A artcle remans at rest or contnues to move n a straght lne wth constant seed there s no orce actng on t Second Law: The acceleraton o a artcle s roortonal
Lecture 10. Reading: Notes and Brennan Chapter 5
 Lecture tatstcal Mechancs and Densty of tates Concepts Readng: otes and Brennan Chapter 5 Georga Tech C 645 - Dr. Alan Doolttle C 645 - Dr. Alan Doolttle Georga Tech How do electrons and holes populate
Lecture tatstcal Mechancs and Densty of tates Concepts Readng: otes and Brennan Chapter 5 Georga Tech C 645 - Dr. Alan Doolttle C 645 - Dr. Alan Doolttle Georga Tech How do electrons and holes populate
Physics 207 Lecture 27
 hyscs 07 Lecture 7 hyscs 07, Lecture 7, Dec. 6 Agenda: h. 0, st Law o Thermodynamcs, h. st Law o thermodynamcs ( U Q + W du dq + dw ) Work done by an deal gas n a ston Introducton to thermodynamc cycles
hyscs 07 Lecture 7 hyscs 07, Lecture 7, Dec. 6 Agenda: h. 0, st Law o Thermodynamcs, h. st Law o thermodynamcs ( U Q + W du dq + dw ) Work done by an deal gas n a ston Introducton to thermodynamc cycles
Evaluating Thermodynamic Properties in LAMMPS
 D. Keffer ME 64 Det. of Materals cence & Engneerng Unversty of ennessee Knoxvlle Evaluatng hermodynamc Proertes n LAMMP Davd Keffer Deartment of Materals cence & Engneerng Unversty of ennessee Knoxvlle
D. Keffer ME 64 Det. of Materals cence & Engneerng Unversty of ennessee Knoxvlle Evaluatng hermodynamc Proertes n LAMMP Davd Keffer Deartment of Materals cence & Engneerng Unversty of ennessee Knoxvlle
Linear Momentum. Center of Mass.
 Lecture 6 Chapter 9 Physcs I 03.3.04 Lnear omentum. Center of ass. Course webste: http://faculty.uml.edu/ndry_danylov/teachng/physcsi Lecture Capture: http://echo360.uml.edu/danylov03/physcssprng.html
Lecture 6 Chapter 9 Physcs I 03.3.04 Lnear omentum. Center of ass. Course webste: http://faculty.uml.edu/ndry_danylov/teachng/physcsi Lecture Capture: http://echo360.uml.edu/danylov03/physcssprng.html
Physics 5153 Classical Mechanics. Principle of Virtual Work-1
 P. Guterrez 1 Introducton Physcs 5153 Classcal Mechancs Prncple of Vrtual Work The frst varatonal prncple we encounter n mechancs s the prncple of vrtual work. It establshes the equlbrum condton of a mechancal
P. Guterrez 1 Introducton Physcs 5153 Classcal Mechancs Prncple of Vrtual Work The frst varatonal prncple we encounter n mechancs s the prncple of vrtual work. It establshes the equlbrum condton of a mechancal
PHY688, Statistical Mechanics
 Department of Physcs & Astronomy 449 ESS Bldg. Stony Brook Unversty January 31, 2017 Nuclear Astrophyscs James.Lattmer@Stonybrook.edu Thermodynamcs Internal Energy Densty and Frst Law: ε = E V = Ts P +
Department of Physcs & Astronomy 449 ESS Bldg. Stony Brook Unversty January 31, 2017 Nuclear Astrophyscs James.Lattmer@Stonybrook.edu Thermodynamcs Internal Energy Densty and Frst Law: ε = E V = Ts P +
π e ax2 dx = x 2 e ax2 dx or x 3 e ax2 dx = 1 x 4 e ax2 dx = 3 π 8a 5/2 (a) We are considering the Maxwell velocity distribution function: 2πτ/m
 Homework Solutons Problem In solvng ths problem, we wll need to calculate some moments of the Gaussan dstrbuton. The brute-force method s to ntegrate by parts but there s a nce trck. The followng ntegrals
Homework Solutons Problem In solvng ths problem, we wll need to calculate some moments of the Gaussan dstrbuton. The brute-force method s to ntegrate by parts but there s a nce trck. The followng ntegrals
Mechanics Physics 151
 Mechancs Physcs 151 Lecture 22 Canoncal Transformatons (Chater 9) What We Dd Last Tme Drect Condtons Q j Q j = = j P, Q, P j, P Q, P Necessary and suffcent P j P j for Canoncal Transf. = = j Q, Q, P j
Mechancs Physcs 151 Lecture 22 Canoncal Transformatons (Chater 9) What We Dd Last Tme Drect Condtons Q j Q j = = j P, Q, P j, P Q, P Necessary and suffcent P j P j for Canoncal Transf. = = j Q, Q, P j
Physics 607 Exam 1. ( ) = 1, Γ( z +1) = zγ( z) x n e x2 dx = 1. e x2
 Physcs 607 Exam 1 Please be well-organzed, and show all sgnfcant steps clearly n all problems. You are graded on your wor, so please do not just wrte down answers wth no explanaton! Do all your wor on
Physcs 607 Exam 1 Please be well-organzed, and show all sgnfcant steps clearly n all problems. You are graded on your wor, so please do not just wrte down answers wth no explanaton! Do all your wor on
PHYS 705: Classical Mechanics. Calculus of Variations II
 1 PHYS 705: Classcal Mechancs Calculus of Varatons II 2 Calculus of Varatons: Generalzaton (no constrant yet) Suppose now that F depends on several dependent varables : We need to fnd such that has a statonary
1 PHYS 705: Classcal Mechancs Calculus of Varatons II 2 Calculus of Varatons: Generalzaton (no constrant yet) Suppose now that F depends on several dependent varables : We need to fnd such that has a statonary
Non-Ideality Through Fugacity and Activity
 Non-Idealty Through Fugacty and Actvty S. Patel Deartment of Chemstry and Bochemstry, Unversty of Delaware, Newark, Delaware 19716, USA Corresondng author. E-mal: saatel@udel.edu 1 I. FUGACITY In ths dscusson,
Non-Idealty Through Fugacty and Actvty S. Patel Deartment of Chemstry and Bochemstry, Unversty of Delaware, Newark, Delaware 19716, USA Corresondng author. E-mal: saatel@udel.edu 1 I. FUGACITY In ths dscusson,
Chapter 8 Balances on Nonreactive Processes 8.1 Elements of Energy Balances Calculations 8.1a Reference States A Review
 Chater 8 Balances on Nonreactve Processes 8.1 Elements of Energy Balances Calculatons 8.1a Reference States A Revew We can never know the absolute values of U and H for a seces at a gven state. t Fortunately,
Chater 8 Balances on Nonreactve Processes 8.1 Elements of Energy Balances Calculatons 8.1a Reference States A Revew We can never know the absolute values of U and H for a seces at a gven state. t Fortunately,
At zero K: All atoms frozen at fixed positions on a periodic lattice.
 September, 00 Readng: Chapter Four Homework: None Entropy and The Degree of Dsorder: Consder a sold crystallne materal: At zero K: All atoms frozen at fxed postons on a perodc lattce. Add heat to a fnte
September, 00 Readng: Chapter Four Homework: None Entropy and The Degree of Dsorder: Consder a sold crystallne materal: At zero K: All atoms frozen at fxed postons on a perodc lattce. Add heat to a fnte
Introduction to Vapor/Liquid Equilibrium, part 2. Raoult s Law:
 CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
EP523 Introduction to QFT I
 EP523 Introducton to QFT I Toc 0 INTRODUCTION TO COURSE Deartment of Engneerng Physcs Unversty of Gazante Setember 2011 Sayfa 1 Content Introducton Revew of SR, QM, RQM and EMT Lagrangan Feld Theory An
EP523 Introducton to QFT I Toc 0 INTRODUCTION TO COURSE Deartment of Engneerng Physcs Unversty of Gazante Setember 2011 Sayfa 1 Content Introducton Revew of SR, QM, RQM and EMT Lagrangan Feld Theory An
Adsorption: A gas or gases from a mixture of gases or a liquid (or liquids) from a mixture of liquids is bound physically to the surface of a solid.
 Searatons n Chemcal Engneerng Searatons (gas from a mxture of gases, lquds from a mxture of lquds, solds from a soluton of solds n lquds, dssolved gases from lquds, solvents from gases artally/comletely
Searatons n Chemcal Engneerng Searatons (gas from a mxture of gases, lquds from a mxture of lquds, solds from a soluton of solds n lquds, dssolved gases from lquds, solvents from gases artally/comletely
Thermodynamics and Kinetics of Solids 33. III. Statistical Thermodynamics. Â N i = N (5.3) N i. i =0. Â e i = E (5.4) has a maximum.
 hermodynamcs and Knetcs of Solds 33 III. Statstcal hermodynamcs 5. Statstcal reatment of hermodynamcs 5.1. Statstcs and Phenomenologcal hermodynamcs. Calculaton of the energetc state of each atomc or molecular
hermodynamcs and Knetcs of Solds 33 III. Statstcal hermodynamcs 5. Statstcal reatment of hermodynamcs 5.1. Statstcs and Phenomenologcal hermodynamcs. Calculaton of the energetc state of each atomc or molecular
Fermi-Dirac statistics
 UCC/Physcs/MK/EM/October 8, 205 Fer-Drac statstcs Fer-Drac dstrbuton Matter partcles that are eleentary ostly have a type of angular oentu called spn. hese partcles are known to have a agnetc oent whch
UCC/Physcs/MK/EM/October 8, 205 Fer-Drac statstcs Fer-Drac dstrbuton Matter partcles that are eleentary ostly have a type of angular oentu called spn. hese partcles are known to have a agnetc oent whch
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2015
 Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
Rate of Absorption and Stimulated Emission
 MIT Department of Chemstry 5.74, Sprng 005: Introductory Quantum Mechancs II Instructor: Professor Andre Tokmakoff p. 81 Rate of Absorpton and Stmulated Emsson The rate of absorpton nduced by the feld
MIT Department of Chemstry 5.74, Sprng 005: Introductory Quantum Mechancs II Instructor: Professor Andre Tokmakoff p. 81 Rate of Absorpton and Stmulated Emsson The rate of absorpton nduced by the feld
Physics 53. Rotational Motion 3. Sir, I have found you an argument, but I am not obliged to find you an understanding.
 Physcs 53 Rotatonal Moton 3 Sr, I have found you an argument, but I am not oblged to fnd you an understandng. Samuel Johnson Angular momentum Wth respect to rotatonal moton of a body, moment of nerta plays
Physcs 53 Rotatonal Moton 3 Sr, I have found you an argument, but I am not oblged to fnd you an understandng. Samuel Johnson Angular momentum Wth respect to rotatonal moton of a body, moment of nerta plays
Dr. Shalabh Department of Mathematics and Statistics Indian Institute of Technology Kanpur
 Analyss of Varance and Desgn of Exerments-I MODULE II LECTURE - GENERAL LINEAR HYPOTHESIS AND ANALYSIS OF VARIANCE Dr. Shalabh Deartment of Mathematcs and Statstcs Indan Insttute of Technology Kanur 3.
Analyss of Varance and Desgn of Exerments-I MODULE II LECTURE - GENERAL LINEAR HYPOTHESIS AND ANALYSIS OF VARIANCE Dr. Shalabh Deartment of Mathematcs and Statstcs Indan Insttute of Technology Kanur 3.
EPR Paradox and the Physical Meaning of an Experiment in Quantum Mechanics. Vesselin C. Noninski
 EPR Paradox and the Physcal Meanng of an Experment n Quantum Mechancs Vesseln C Nonnsk vesselnnonnsk@verzonnet Abstract It s shown that there s one purely determnstc outcome when measurement s made on
EPR Paradox and the Physcal Meanng of an Experment n Quantum Mechancs Vesseln C Nonnsk vesselnnonnsk@verzonnet Abstract It s shown that there s one purely determnstc outcome when measurement s made on
Chapter 3 The Kinetic Theory of Gases 3.1. Ideal Gases Experimental Laws and the Equation of State
 Chater 3 The Knetc Theory of Gases 3.1. Ideal Gases 3.1.1. Exermental Laws and the Equaton of State 3.1.2. Molecular Model of an Ideal Gas 3.3. Mean Free Path 3.4. The Boltzmann Dstrbuton Law and The Dstrbuton
Chater 3 The Knetc Theory of Gases 3.1. Ideal Gases 3.1.1. Exermental Laws and the Equaton of State 3.1.2. Molecular Model of an Ideal Gas 3.3. Mean Free Path 3.4. The Boltzmann Dstrbuton Law and The Dstrbuton
LECTURE 5: FIBRATIONS AND HOMOTOPY FIBERS
 LECTURE 5: FIBRATIONS AND HOMOTOPY FIBERS In ts lecture we wll ntroduce two mortant classes of mas of saces, namely te Hurewcz fbratons and te more general Serre fbratons, wc are bot obtaned by mosng certan
LECTURE 5: FIBRATIONS AND HOMOTOPY FIBERS In ts lecture we wll ntroduce two mortant classes of mas of saces, namely te Hurewcz fbratons and te more general Serre fbratons, wc are bot obtaned by mosng certan
n α j x j = 0 j=1 has a nontrivial solution. Here A is the n k matrix whose jth column is the vector for all t j=0
 MODULE 2 Topcs: Lnear ndependence, bass and dmenson We have seen that f n a set of vectors one vector s a lnear combnaton of the remanng vectors n the set then the span of the set s unchanged f that vector
MODULE 2 Topcs: Lnear ndependence, bass and dmenson We have seen that f n a set of vectors one vector s a lnear combnaton of the remanng vectors n the set then the span of the set s unchanged f that vector
Review of Classical Thermodynamics
 Revew of Classcal hermodynamcs Physcs 4362, Lecture #1, 2 Syllabus What s hermodynamcs? 1 [A law] s more mpressve the greater the smplcty of ts premses, the more dfferent are the knds of thngs t relates,
Revew of Classcal hermodynamcs Physcs 4362, Lecture #1, 2 Syllabus What s hermodynamcs? 1 [A law] s more mpressve the greater the smplcty of ts premses, the more dfferent are the knds of thngs t relates,
Mathematical Preparations
 1 Introducton Mathematcal Preparatons The theory of relatvty was developed to explan experments whch studed the propagaton of electromagnetc radaton n movng coordnate systems. Wthn expermental error the
1 Introducton Mathematcal Preparatons The theory of relatvty was developed to explan experments whch studed the propagaton of electromagnetc radaton n movng coordnate systems. Wthn expermental error the
STATISTICAL MECHANICAL ENSEMBLES 1 MICROSCOPIC AND MACROSCOPIC VARIABLES PHASE SPACE ENSEMBLES. CHE 524 A. Panagiotopoulos 1
 CHE 54 A. Panagotopoulos STATSTCAL MECHACAL ESEMBLES MCROSCOPC AD MACROSCOPC ARABLES The central queston n Statstcal Mechancs can be phrased as follows: f partcles (atoms, molecules, electrons, nucle,
CHE 54 A. Panagotopoulos STATSTCAL MECHACAL ESEMBLES MCROSCOPC AD MACROSCOPC ARABLES The central queston n Statstcal Mechancs can be phrased as follows: f partcles (atoms, molecules, electrons, nucle,
Week 9 Chapter 10 Section 1-5
 Week 9 Chapter 10 Secton 1-5 Rotaton Rgd Object A rgd object s one that s nondeformable The relatve locatons of all partcles makng up the object reman constant All real objects are deformable to some extent,
Week 9 Chapter 10 Secton 1-5 Rotaton Rgd Object A rgd object s one that s nondeformable The relatve locatons of all partcles makng up the object reman constant All real objects are deformable to some extent,
Temperature. Chapter Heat Engine
 Chapter 3 Temperature In prevous chapters of these notes we ntroduced the Prncple of Maxmum ntropy as a technque for estmatng probablty dstrbutons consstent wth constrants. In Chapter 9 we dscussed the
Chapter 3 Temperature In prevous chapters of these notes we ntroduced the Prncple of Maxmum ntropy as a technque for estmatng probablty dstrbutons consstent wth constrants. In Chapter 9 we dscussed the
Lecture 16. Chapter 11. Energy Dissipation Linear Momentum. Physics I. Department of Physics and Applied Physics
 Lecture 16 Chapter 11 Physcs I Energy Dsspaton Lnear Momentum Course webste: http://aculty.uml.edu/andry_danylov/teachng/physcsi Department o Physcs and Appled Physcs IN IN THIS CHAPTER, you wll learn
Lecture 16 Chapter 11 Physcs I Energy Dsspaton Lnear Momentum Course webste: http://aculty.uml.edu/andry_danylov/teachng/physcsi Department o Physcs and Appled Physcs IN IN THIS CHAPTER, you wll learn
On New Selection Procedures for Unequal Probability Sampling
 Int. J. Oen Problems Comt. Math., Vol. 4, o. 1, March 011 ISS 1998-66; Coyrght ICSRS Publcaton, 011 www.-csrs.org On ew Selecton Procedures for Unequal Probablty Samlng Muhammad Qaser Shahbaz, Saman Shahbaz
Int. J. Oen Problems Comt. Math., Vol. 4, o. 1, March 011 ISS 1998-66; Coyrght ICSRS Publcaton, 011 www.-csrs.org On ew Selecton Procedures for Unequal Probablty Samlng Muhammad Qaser Shahbaz, Saman Shahbaz
Linear Momentum and Collisions
 Lnear Momentum and Collsons Chater 9 Lnear Momentum [kg m/s] x y mv x mv y Newton s nd Law n terms o momentum: Imulse I - [kg m/s] I t t Fdt I = area under curve bounded by t axs Imulse-Momentum Theorem
Lnear Momentum and Collsons Chater 9 Lnear Momentum [kg m/s] x y mv x mv y Newton s nd Law n terms o momentum: Imulse I - [kg m/s] I t t Fdt I = area under curve bounded by t axs Imulse-Momentum Theorem
Naïve Bayes Classifier
 9/8/07 MIST.6060 Busness Intellgence and Data Mnng Naïve Bayes Classfer Termnology Predctors: the attrbutes (varables) whose values are used for redcton and classfcaton. Predctors are also called nut varables,
9/8/07 MIST.6060 Busness Intellgence and Data Mnng Naïve Bayes Classfer Termnology Predctors: the attrbutes (varables) whose values are used for redcton and classfcaton. Predctors are also called nut varables,
Physics 5153 Classical Mechanics. D Alembert s Principle and The Lagrangian-1
 P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
Managing Capacity Through Reward Programs. on-line companion page. Byung-Do Kim Seoul National University College of Business Administration
 Managng Caacty Through eward Programs on-lne comanon age Byung-Do Km Seoul Natonal Unversty College of Busness Admnstraton Mengze Sh Unversty of Toronto otman School of Management Toronto ON M5S E6 Canada
Managng Caacty Through eward Programs on-lne comanon age Byung-Do Km Seoul Natonal Unversty College of Busness Admnstraton Mengze Sh Unversty of Toronto otman School of Management Toronto ON M5S E6 Canada
From Newton s 2 nd Law: v v. The time rate of change of the linear momentum of a particle is equal to the net force acting on the particle.
 From Newton s 2 nd Law: F ma d dm ( ) m dt dt F d dt The tme rate of change of the lnear momentum of a artcle s equal to the net force actng on the artcle. Conseraton of Momentum +x The toy rocket n dee
From Newton s 2 nd Law: F ma d dm ( ) m dt dt F d dt The tme rate of change of the lnear momentum of a artcle s equal to the net force actng on the artcle. Conseraton of Momentum +x The toy rocket n dee
Hidden Markov Model Cheat Sheet
 Hdden Markov Model Cheat Sheet (GIT ID: dc2f391536d67ed5847290d5250d4baae103487e) Ths document s a cheat sheet on Hdden Markov Models (HMMs). It resembles lecture notes, excet that t cuts to the chase
Hdden Markov Model Cheat Sheet (GIT ID: dc2f391536d67ed5847290d5250d4baae103487e) Ths document s a cheat sheet on Hdden Markov Models (HMMs). It resembles lecture notes, excet that t cuts to the chase
Algorithms for factoring
 CSA E0 235: Crytograhy Arl 9,2015 Instructor: Arta Patra Algorthms for factorng Submtted by: Jay Oza, Nranjan Sngh Introducton Factorsaton of large ntegers has been a wdely studed toc manly because of
CSA E0 235: Crytograhy Arl 9,2015 Instructor: Arta Patra Algorthms for factorng Submtted by: Jay Oza, Nranjan Sngh Introducton Factorsaton of large ntegers has been a wdely studed toc manly because of
Review of Thermodynamics
 Revew of hermodynamcs from Statstcal Physcs usng Mathematca James J. Kelly, 1996-2002 We revew the laws of thermodynamcs and some of the technques for dervaton of thermodynamc relatonshs. Introducton Equlbrum
Revew of hermodynamcs from Statstcal Physcs usng Mathematca James J. Kelly, 1996-2002 We revew the laws of thermodynamcs and some of the technques for dervaton of thermodynamc relatonshs. Introducton Equlbrum
Poisson brackets and canonical transformations
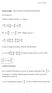 rof O B Wrght Mechancs Notes osson brackets and canoncal transformatons osson Brackets Consder an arbtrary functon f f ( qp t) df f f f q p q p t But q p p where ( qp ) pq q df f f f p q q p t In order
rof O B Wrght Mechancs Notes osson brackets and canoncal transformatons osson Brackets Consder an arbtrary functon f f ( qp t) df f f f q p q p t But q p p where ( qp ) pq q df f f f p q q p t In order
Statistical Inference. 2.3 Summary Statistics Measures of Center and Spread. parameters ( population characteristics )
 Ismor Fscher, 8//008 Stat 54 / -8.3 Summary Statstcs Measures of Center and Spread Dstrbuton of dscrete contnuous POPULATION Random Varable, numercal True center =??? True spread =???? parameters ( populaton
Ismor Fscher, 8//008 Stat 54 / -8.3 Summary Statstcs Measures of Center and Spread Dstrbuton of dscrete contnuous POPULATION Random Varable, numercal True center =??? True spread =???? parameters ( populaton
Physics 240: Worksheet 30 Name:
 (1) One mole of an deal monatomc gas doubles ts temperature and doubles ts volume. What s the change n entropy of the gas? () 1 kg of ce at 0 0 C melts to become water at 0 0 C. What s the change n entropy
(1) One mole of an deal monatomc gas doubles ts temperature and doubles ts volume. What s the change n entropy of the gas? () 1 kg of ce at 0 0 C melts to become water at 0 0 C. What s the change n entropy
The Feynman path integral
 The Feynman path ntegral Aprl 3, 205 Hesenberg and Schrödnger pctures The Schrödnger wave functon places the tme dependence of a physcal system n the state, ψ, t, where the state s a vector n Hlbert space
The Feynman path ntegral Aprl 3, 205 Hesenberg and Schrödnger pctures The Schrödnger wave functon places the tme dependence of a physcal system n the state, ψ, t, where the state s a vector n Hlbert space
Physics 181. Particle Systems
 Physcs 181 Partcle Systems Overvew In these notes we dscuss the varables approprate to the descrpton of systems of partcles, ther defntons, ther relatons, and ther conservatons laws. We consder a system
Physcs 181 Partcle Systems Overvew In these notes we dscuss the varables approprate to the descrpton of systems of partcles, ther defntons, ther relatons, and ther conservatons laws. We consder a system
Moments of Inertia. and reminds us of the analogous equation for linear momentum p= mv, which is of the form. The kinetic energy of the body is.
 Moments of Inerta Suppose a body s movng on a crcular path wth constant speed Let s consder two quanttes: the body s angular momentum L about the center of the crcle, and ts knetc energy T How are these
Moments of Inerta Suppose a body s movng on a crcular path wth constant speed Let s consder two quanttes: the body s angular momentum L about the center of the crcle, and ts knetc energy T How are these
Physics 207: Lecture 20. Today s Agenda Homework for Monday
 Physcs 207: Lecture 20 Today s Agenda Homework for Monday Recap: Systems of Partcles Center of mass Velocty and acceleraton of the center of mass Dynamcs of the center of mass Lnear Momentum Example problems
Physcs 207: Lecture 20 Today s Agenda Homework for Monday Recap: Systems of Partcles Center of mass Velocty and acceleraton of the center of mass Dynamcs of the center of mass Lnear Momentum Example problems
The Dirac Equation. Elementary Particle Physics Strong Interaction Fenomenology. Diego Bettoni Academic year
 The Drac Equaton Eleentary artcle hyscs Strong Interacton Fenoenology Dego Betton Acadec year - D Betton Fenoenologa Interazon Fort elatvstc equaton to descrbe the electron (ncludng ts sn) Conservaton
The Drac Equaton Eleentary artcle hyscs Strong Interacton Fenoenology Dego Betton Acadec year - D Betton Fenoenologa Interazon Fort elatvstc equaton to descrbe the electron (ncludng ts sn) Conservaton
Open Systems: Chemical Potential and Partial Molar Quantities Chemical Potential
 Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Robert Eisberg Second edition CH 09 Multielectron atoms ground states and x-ray excitations
 Quantum Physcs 量 理 Robert Esberg Second edton CH 09 Multelectron atoms ground states and x-ray exctatons 9-01 By gong through the procedure ndcated n the text, develop the tme-ndependent Schroednger equaton
Quantum Physcs 量 理 Robert Esberg Second edton CH 09 Multelectron atoms ground states and x-ray exctatons 9-01 By gong through the procedure ndcated n the text, develop the tme-ndependent Schroednger equaton
= z 20 z n. (k 20) + 4 z k = 4
 Problem Set #7 solutons 7.2.. (a Fnd the coeffcent of z k n (z + z 5 + z 6 + z 7 + 5, k 20. We use the known seres expanson ( n+l ( z l l z n below: (z + z 5 + z 6 + z 7 + 5 (z 5 ( + z + z 2 + z + 5 5
Problem Set #7 solutons 7.2.. (a Fnd the coeffcent of z k n (z + z 5 + z 6 + z 7 + 5, k 20. We use the known seres expanson ( n+l ( z l l z n below: (z + z 5 + z 6 + z 7 + 5 (z 5 ( + z + z 2 + z + 5 5
Statistics Chapter 4
 Statstcs Chapter 4 "There are three knds of les: les, damned les, and statstcs." Benjamn Dsrael, 1895 (Brtsh statesman) Gaussan Dstrbuton, 4-1 If a measurement s repeated many tmes a statstcal treatment
Statstcs Chapter 4 "There are three knds of les: les, damned les, and statstcs." Benjamn Dsrael, 1895 (Brtsh statesman) Gaussan Dstrbuton, 4-1 If a measurement s repeated many tmes a statstcal treatment
Physics for Scientists and Engineers. Chapter 9 Impulse and Momentum
 Physcs or Scentsts and Engneers Chapter 9 Impulse and Momentum Sprng, 008 Ho Jung Pak Lnear Momentum Lnear momentum o an object o mass m movng wth a velocty v s dened to be p mv Momentum and lnear momentum
Physcs or Scentsts and Engneers Chapter 9 Impulse and Momentum Sprng, 008 Ho Jung Pak Lnear Momentum Lnear momentum o an object o mass m movng wth a velocty v s dened to be p mv Momentum and lnear momentum
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM
 ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
A particle in a state of uniform motion remain in that state of motion unless acted upon by external force.
 The fundamental prncples of classcal mechancs were lad down by Galleo and Newton n the 16th and 17th centures. In 1686, Newton wrote the Prncpa where he gave us three laws of moton, one law of gravty,
The fundamental prncples of classcal mechancs were lad down by Galleo and Newton n the 16th and 17th centures. In 1686, Newton wrote the Prncpa where he gave us three laws of moton, one law of gravty,
and Statistical Mechanics Material Properties
 Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
Parton Model. 2 q Q, 1
 Parton Model How do we exect the structure functons w and w to behave? It s nstructve to consder some secal cases: ) e e elastc scatterng from a ont-lke roton w, q q q w, q q m m m Defnng and notng that
Parton Model How do we exect the structure functons w and w to behave? It s nstructve to consder some secal cases: ) e e elastc scatterng from a ont-lke roton w, q q q w, q q m m m Defnng and notng that
Errors for Linear Systems
 Errors for Lnear Systems When we solve a lnear system Ax b we often do not know A and b exactly, but have only approxmatons  and ˆb avalable. Then the best thng we can do s to solve ˆx ˆb exactly whch
Errors for Lnear Systems When we solve a lnear system Ax b we often do not know A and b exactly, but have only approxmatons  and ˆb avalable. Then the best thng we can do s to solve ˆx ˆb exactly whch
Q e E i /k B. i i i i
 Water and Aqueous Solutons 3. Lattce Model of a Flud Lattce Models Lattce models provde a mnmalst, or coarse-graned, framework for descrbng the translatonal, rotatonal, and conformatonal degrees of freedom
Water and Aqueous Solutons 3. Lattce Model of a Flud Lattce Models Lattce models provde a mnmalst, or coarse-graned, framework for descrbng the translatonal, rotatonal, and conformatonal degrees of freedom
Frequency dependence of the permittivity
 Frequency dependence of the permttvty February 7, 016 In materals, the delectrc constant and permeablty are actually frequency dependent. Ths does not affect our results for sngle frequency modes, but
Frequency dependence of the permttvty February 7, 016 In materals, the delectrc constant and permeablty are actually frequency dependent. Ths does not affect our results for sngle frequency modes, but
PY2101 Classical Mechanics Dr. Síle Nic Chormaic, Room 215 D Kane Bldg
 PY2101 Classcal Mechancs Dr. Síle Nc Chormac, Room 215 D Kane Bldg s.ncchormac@ucc.e Lectures stll some ssues to resolve. Slots shared between PY2101 and PY2104. Hope to have t fnalsed by tomorrow. Mondays
PY2101 Classcal Mechancs Dr. Síle Nc Chormac, Room 215 D Kane Bldg s.ncchormac@ucc.e Lectures stll some ssues to resolve. Slots shared between PY2101 and PY2104. Hope to have t fnalsed by tomorrow. Mondays
( ) 2 ( ) ( ) Problem Set 4 Suggested Solutions. Problem 1
 Problem Set 4 Suggested Solutons Problem (A) The market demand functon s the soluton to the followng utlty-maxmzaton roblem (UMP): The Lagrangean: ( x, x, x ) = + max U x, x, x x x x st.. x + x + x y x,
Problem Set 4 Suggested Solutons Problem (A) The market demand functon s the soluton to the followng utlty-maxmzaton roblem (UMP): The Lagrangean: ( x, x, x ) = + max U x, x, x x x x st.. x + x + x y x,
Lecture # 02: Pressure measurements and Measurement Uncertainties
 AerE 3L & AerE343L Lecture Notes Lecture # 0: Pressure measurements and Measurement Uncertantes Dr. Hu H Hu Deartment of Aerosace Engneerng Iowa State Unversty Ames, Iowa 500, U.S.A Mechancal Pressure
AerE 3L & AerE343L Lecture Notes Lecture # 0: Pressure measurements and Measurement Uncertantes Dr. Hu H Hu Deartment of Aerosace Engneerng Iowa State Unversty Ames, Iowa 500, U.S.A Mechancal Pressure
Introduction to Statistical Methods
 Introducton to Statstcal Methods Physcs 4362, Lecture #3 hermodynamcs Classcal Statstcal Knetc heory Classcal hermodynamcs Macroscopc approach General propertes of the system Macroscopc varables 1 hermodynamc
Introducton to Statstcal Methods Physcs 4362, Lecture #3 hermodynamcs Classcal Statstcal Knetc heory Classcal hermodynamcs Macroscopc approach General propertes of the system Macroscopc varables 1 hermodynamc
Problem Set 9 Solutions
 Desgn and Analyss of Algorthms May 4, 2015 Massachusetts Insttute of Technology 6.046J/18.410J Profs. Erk Demane, Srn Devadas, and Nancy Lynch Problem Set 9 Solutons Problem Set 9 Solutons Ths problem
Desgn and Analyss of Algorthms May 4, 2015 Massachusetts Insttute of Technology 6.046J/18.410J Profs. Erk Demane, Srn Devadas, and Nancy Lynch Problem Set 9 Solutons Problem Set 9 Solutons Ths problem
The Multiple Classical Linear Regression Model (CLRM): Specification and Assumptions. 1. Introduction
 ECONOMICS 5* -- NOTE (Summary) ECON 5* -- NOTE The Multple Classcal Lnear Regresson Model (CLRM): Specfcaton and Assumptons. Introducton CLRM stands for the Classcal Lnear Regresson Model. The CLRM s also
ECONOMICS 5* -- NOTE (Summary) ECON 5* -- NOTE The Multple Classcal Lnear Regresson Model (CLRM): Specfcaton and Assumptons. Introducton CLRM stands for the Classcal Lnear Regresson Model. The CLRM s also
5.03, Inorganic Chemistry Prof. Daniel G. Nocera Lecture 2 May 11: Ligand Field Theory
 5.03, Inorganc Chemstry Prof. Danel G. Nocera Lecture May : Lgand Feld Theory The lgand feld problem s defned by the followng Hamltonan, h p Η = wth E n = KE = where = m m x y z h m Ze r hydrogen atom
5.03, Inorganc Chemstry Prof. Danel G. Nocera Lecture May : Lgand Feld Theory The lgand feld problem s defned by the followng Hamltonan, h p Η = wth E n = KE = where = m m x y z h m Ze r hydrogen atom
Title: Radiative transitions and spectral broadening
 Lecture 6 Ttle: Radatve transtons and spectral broadenng Objectves The spectral lnes emtted by atomc vapors at moderate temperature and pressure show the wavelength spread around the central frequency.
Lecture 6 Ttle: Radatve transtons and spectral broadenng Objectves The spectral lnes emtted by atomc vapors at moderate temperature and pressure show the wavelength spread around the central frequency.
THERMAL DISTRIBUTION IN THE HCL SPECTRUM OBJECTIVE
 ame: THERMAL DISTRIBUTIO I THE HCL SPECTRUM OBJECTIVE To nvestgate a system s thermal dstrbuton n dscrete states; specfcally, determne HCl gas temperature from the relatve occupatons of ts rotatonal states.
ame: THERMAL DISTRIBUTIO I THE HCL SPECTRUM OBJECTIVE To nvestgate a system s thermal dstrbuton n dscrete states; specfcally, determne HCl gas temperature from the relatve occupatons of ts rotatonal states.
5.62 Physical Chemistry II Spring 2008
 MIT OpenCourseWare http://ocw.mt.edu 5.62 Physcal Chemstry II Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.62 Lecture #8: Boltzmann, Ferm-Drac,
MIT OpenCourseWare http://ocw.mt.edu 5.62 Physcal Chemstry II Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.62 Lecture #8: Boltzmann, Ferm-Drac,
Statistical mechanics handout 4
 Statstcal mechancs handout 4 Explan dfference between phase space and an. Ensembles As dscussed n handout three atoms n any physcal system can adopt any one of a large number of mcorstates. For a quantum
Statstcal mechancs handout 4 Explan dfference between phase space and an. Ensembles As dscussed n handout three atoms n any physcal system can adopt any one of a large number of mcorstates. For a quantum
Page 1. Physics 131: Lecture 14. Today s Agenda. Things that stay the same. Impulse and Momentum Non-constant forces
 Physcs 131: Lecture 14 Today s Agenda Imulse and Momentum Non-constant forces Imulse-momentum momentum thm Conservaton of Lnear momentum Eternal/Internal forces Eamles Physcs 201: Lecture 1, Pg 1 Physcs
Physcs 131: Lecture 14 Today s Agenda Imulse and Momentum Non-constant forces Imulse-momentum momentum thm Conservaton of Lnear momentum Eternal/Internal forces Eamles Physcs 201: Lecture 1, Pg 1 Physcs
Chapter 3. r r. Position, Velocity, and Acceleration Revisited
 Chapter 3 Poston, Velocty, and Acceleraton Revsted The poston vector of a partcle s a vector drawn from the orgn to the locaton of the partcle. In two dmensons: r = x ˆ+ yj ˆ (1) The dsplacement vector
Chapter 3 Poston, Velocty, and Acceleraton Revsted The poston vector of a partcle s a vector drawn from the orgn to the locaton of the partcle. In two dmensons: r = x ˆ+ yj ˆ (1) The dsplacement vector
First Law: A body at rest remains at rest, a body in motion continues to move at constant velocity, unless acted upon by an external force.
 Secton 1. Dynamcs (Newton s Laws of Moton) Two approaches: 1) Gven all the forces actng on a body, predct the subsequent (changes n) moton. 2) Gven the (changes n) moton of a body, nfer what forces act
Secton 1. Dynamcs (Newton s Laws of Moton) Two approaches: 1) Gven all the forces actng on a body, predct the subsequent (changes n) moton. 2) Gven the (changes n) moton of a body, nfer what forces act
Supplemental Material: Causal Entropic Forces
 Supplemental Materal: Causal Entropc Forces A. D. Wssner-Gross 1, 2, and C. E. Freer 3 1 Insttute for Appled Computatonal Scence, Harvard Unversty, Cambrdge, Massachusetts 02138, USA 2 The Meda Laboratory,
Supplemental Materal: Causal Entropc Forces A. D. Wssner-Gross 1, 2, and C. E. Freer 3 1 Insttute for Appled Computatonal Scence, Harvard Unversty, Cambrdge, Massachusetts 02138, USA 2 The Meda Laboratory,
ACTM State Calculus Competition Saturday April 30, 2011
 ACTM State Calculus Competton Saturday Aprl 30, 2011 ACTM State Calculus Competton Sprng 2011 Page 1 Instructons: For questons 1 through 25, mark the best answer choce on the answer sheet provde Afterward
ACTM State Calculus Competton Saturday Aprl 30, 2011 ACTM State Calculus Competton Sprng 2011 Page 1 Instructons: For questons 1 through 25, mark the best answer choce on the answer sheet provde Afterward
A total variation approach
 Denosng n dgtal radograhy: A total varaton aroach I. Froso M. Lucchese. A. Borghese htt://as-lab.ds.unm.t / 46 I. Froso, M. Lucchese,. A. Borghese Images are corruted by nose ) When measurement of some
Denosng n dgtal radograhy: A total varaton aroach I. Froso M. Lucchese. A. Borghese htt://as-lab.ds.unm.t / 46 I. Froso, M. Lucchese,. A. Borghese Images are corruted by nose ) When measurement of some
Lecture 12: Discrete Laplacian
 Lecture 12: Dscrete Laplacan Scrbe: Tanye Lu Our goal s to come up wth a dscrete verson of Laplacan operator for trangulated surfaces, so that we can use t n practce to solve related problems We are mostly
Lecture 12: Dscrete Laplacan Scrbe: Tanye Lu Our goal s to come up wth a dscrete verson of Laplacan operator for trangulated surfaces, so that we can use t n practce to solve related problems We are mostly
Lecture 7: Boltzmann distribution & Thermodynamics of mixing
 Prof. Tbbtt Lecture 7 etworks & Gels Lecture 7: Boltzmann dstrbuton & Thermodynamcs of mxng 1 Suggested readng Prof. Mark W. Tbbtt ETH Zürch 13 März 018 Molecular Drvng Forces Dll and Bromberg: Chapters
Prof. Tbbtt Lecture 7 etworks & Gels Lecture 7: Boltzmann dstrbuton & Thermodynamcs of mxng 1 Suggested readng Prof. Mark W. Tbbtt ETH Zürch 13 März 018 Molecular Drvng Forces Dll and Bromberg: Chapters
Module 3 LOSSY IMAGE COMPRESSION SYSTEMS. Version 2 ECE IIT, Kharagpur
 Module 3 LOSSY IMAGE COMPRESSION SYSTEMS Verson ECE IIT, Kharagpur Lesson 6 Theory of Quantzaton Verson ECE IIT, Kharagpur Instructonal Objectves At the end of ths lesson, the students should be able to:
Module 3 LOSSY IMAGE COMPRESSION SYSTEMS Verson ECE IIT, Kharagpur Lesson 6 Theory of Quantzaton Verson ECE IIT, Kharagpur Instructonal Objectves At the end of ths lesson, the students should be able to:
Module 14: THE INTEGRAL Exploring Calculus
 Module 14: THE INTEGRAL Explorng Calculus Part I Approxmatons and the Defnte Integral It was known n the 1600s before the calculus was developed that the area of an rregularly shaped regon could be approxmated
Module 14: THE INTEGRAL Explorng Calculus Part I Approxmatons and the Defnte Integral It was known n the 1600s before the calculus was developed that the area of an rregularly shaped regon could be approxmated
Rigid body simulation
 Rgd bod smulaton Rgd bod smulaton Once we consder an object wth spacal etent, partcle sstem smulaton s no longer suffcent Problems Problems Unconstraned sstem rotatonal moton torques and angular momentum
Rgd bod smulaton Rgd bod smulaton Once we consder an object wth spacal etent, partcle sstem smulaton s no longer suffcent Problems Problems Unconstraned sstem rotatonal moton torques and angular momentum
Conservation of Angular Momentum = "Spin"
 Page 1 of 6 Conservaton of Angular Momentum = "Spn" We can assgn a drecton to the angular velocty: drecton of = drecton of axs + rght hand rule (wth rght hand, curl fngers n drecton of rotaton, thumb ponts
Page 1 of 6 Conservaton of Angular Momentum = "Spn" We can assgn a drecton to the angular velocty: drecton of = drecton of axs + rght hand rule (wth rght hand, curl fngers n drecton of rotaton, thumb ponts
CHAPTER 14 GENERAL PERTURBATION THEORY
 CHAPTER 4 GENERAL PERTURBATION THEORY 4 Introducton A partcle n orbt around a pont mass or a sphercally symmetrc mass dstrbuton s movng n a gravtatonal potental of the form GM / r In ths potental t moves
CHAPTER 4 GENERAL PERTURBATION THEORY 4 Introducton A partcle n orbt around a pont mass or a sphercally symmetrc mass dstrbuton s movng n a gravtatonal potental of the form GM / r In ths potental t moves
From Biot-Savart Law to Divergence of B (1)
 From Bot-Savart Law to Dvergence of B (1) Let s prove that Bot-Savart gves us B (r ) = 0 for an arbtrary current densty. Frst take the dvergence of both sdes of Bot-Savart. The dervatve s wth respect to
From Bot-Savart Law to Dvergence of B (1) Let s prove that Bot-Savart gves us B (r ) = 0 for an arbtrary current densty. Frst take the dvergence of both sdes of Bot-Savart. The dervatve s wth respect to
Confidence intervals for weighted polynomial calibrations
 Confdence ntervals for weghted olynomal calbratons Sergey Maltsev, Amersand Ltd., Moscow, Russa; ur Kalambet, Amersand Internatonal, Inc., Beachwood, OH e-mal: kalambet@amersand-ntl.com htt://www.chromandsec.com
Confdence ntervals for weghted olynomal calbratons Sergey Maltsev, Amersand Ltd., Moscow, Russa; ur Kalambet, Amersand Internatonal, Inc., Beachwood, OH e-mal: kalambet@amersand-ntl.com htt://www.chromandsec.com
G4023 Mid-Term Exam #1 Solutions
 Exam1Solutons.nb 1 G03 Md-Term Exam #1 Solutons 1-Oct-0, 1:10 p.m to :5 p.m n 1 Pupn Ths exam s open-book, open-notes. You may also use prnt-outs of the homework solutons and a calculator. 1 (30 ponts,
Exam1Solutons.nb 1 G03 Md-Term Exam #1 Solutons 1-Oct-0, 1:10 p.m to :5 p.m n 1 Pupn Ths exam s open-book, open-notes. You may also use prnt-outs of the homework solutons and a calculator. 1 (30 ponts,
10.40 Appendix Connection to Thermodynamics and Derivation of Boltzmann Distribution
 10.40 Appendx Connecton to Thermodynamcs Dervaton of Boltzmann Dstrbuton Bernhardt L. Trout Outlne Cannoncal ensemble Maxmumtermmethod Most probable dstrbuton Ensembles contnued: Canoncal, Mcrocanoncal,
10.40 Appendx Connecton to Thermodynamcs Dervaton of Boltzmann Dstrbuton Bernhardt L. Trout Outlne Cannoncal ensemble Maxmumtermmethod Most probable dstrbuton Ensembles contnued: Canoncal, Mcrocanoncal,
ESCI 341 Atmospheric Thermodynamics Lesson 10 The Physical Meaning of Entropy
 ESCI 341 Atmospherc Thermodynamcs Lesson 10 The Physcal Meanng of Entropy References: An Introducton to Statstcal Thermodynamcs, T.L. Hll An Introducton to Thermodynamcs and Thermostatstcs, H.B. Callen
ESCI 341 Atmospherc Thermodynamcs Lesson 10 The Physcal Meanng of Entropy References: An Introducton to Statstcal Thermodynamcs, T.L. Hll An Introducton to Thermodynamcs and Thermostatstcs, H.B. Callen
A Quadratic Cumulative Production Model for the Material Balance of Abnormally-Pressured Gas Reservoirs F.E. Gonzalez M.S.
 Natural as Engneerng A Quadratc Cumulatve Producton Model for the Materal Balance of Abnormally-Pressured as Reservors F.E. onale M.S. Thess (2003) T.A. Blasngame, Texas A&M U. Deartment of Petroleum Engneerng
Natural as Engneerng A Quadratc Cumulatve Producton Model for the Materal Balance of Abnormally-Pressured as Reservors F.E. onale M.S. Thess (2003) T.A. Blasngame, Texas A&M U. Deartment of Petroleum Engneerng
