Chapter 11 Structure of Matter 133 Answers to the Conceptual Questions
|
|
|
- Earl Rice
- 5 years ago
- Views:
Transcription
1 hapter 11 Structure of Matter 1 Answers to the onceptual Questons 1. These models gave detaled explanatons for events that had already occurred but lacked any predctve power.. Many examples apply.. Although cruel, one mght magne sealng off the machne and thus takng away essentals such as food and water for a perod of tme. 4. If the Sun s movng, we must be usng the geocentrc model.. Addtonal examples n whch expermental results agree wth a model strengthen our belef n t. However, a model can never be proven true. The next brown can he tres mght snk. 6. The new model mght be that only det drnks float. The data s consstent wth ths new model but the model remans tentatve untl all possble cans are tested. 7. The atomstc nature of matter s not mportant for most day-to-day actvtes n whch macroscopc propertes are all that are mportant. 8. The alchemsts contrbuted to a new world vew n whch external appearances were not the essence of matter. In addton, they developed a wde varety of technques for manpulatng materals.. Water, salt and grante are not elements as they can be separated nto ther consttuent parts. 10. arbon monoxde s a compound as t can be separated nto ts consttuent parts, carbon and oxygen. 11. The powder s a compound, because the mercury combnes wth somethng n the ar. 1. Many examples apply. The most common response from students s combnng hydrogen and oxygen to form water. 1. ompounds are new substances wth ther own propertes resultng from substances combnng accordng to the law of defnte proportons, whereas mxtures retan the propertes of the orgnal consttuent parts. 14. Water and salt form a mxture that retans the propertes of the orgnal consttuent parts amu 16 amu 6 amu 16. The atomc mass of the two ron atoms s 160 amu (16 amu) 11 amu. The atomc mass of one ron atom must be half ths, whch s 6 amu. 17. An atom of slver s 108 tmes as heavy as an atom of hydrogen. It would therefore take 108 tmes fewer slver atoms to make a gram than t would hydrogen atoms. 18. A mole always contans an Avogadro s number of partcles. 1. They have the same number of molecules. 0. Both contaners hold the same number of molecules. arbon doxde molecules comprse atoms each whereas oxygen molecules comprse only atoms each. Therefore the carbon doxde contaner wll contan 0% more atoms. 1. An atomc mass number of grams of any substance contans an Avogadro's number of unts. Therefore, you would need grams of sulfur.. An atomc mass number of grams of any substance contans an Avogadro's number of unts. The atomc mass of water s 18 amu. Therefore, you would need 18 grams of water.. The deal gas model cannot be used wth lquds as the dstances between the partcles are no longer large enough to neglect nter-partcle nteractons. However, t does tell us that the partcles are much closer together. 4. As the pressure ncreases, the average dstance between partcles decreases. Ths ncreases the lkelhood of non-contact nteractons, whch volates the prmary assumpton of the deal gas law.. The ball exerts the larger pressure because t has a smaller surface area touchng the floor.
2 14 hapter 11 Structure of Matter 6. When you push on a thumbtack you dstrbute the force over a relatvely large area, whch results n a low pressure. Applyng the same force to the much smaller head of a needle produces a huge pressure, whch causes the skn to puncture. 7. The surroundng ar pressure ncreases as you descend nto the valley. Because the walls are flexble, the pressure of the gas also ncreases and ts volume must decrease. 8. The tre must support the same weght. Wth half the pressure t must have double the contact area, whch s 400 square centmeters.. The ncrease n the number of molecules means that more molecules wll be httng the walls per unt tme. 0. As they heat up, the ar molecules gan knetc energy. Therefore, they move faster and strke the walls more frequently wth more momentum. 1. The perfume molecules travel very crooked paths.. The added energy causes the average speed to ncrease.. The large volume of alcohol causes a larger change n the heght of the column, thereby amplfyng the effect due to a gven change n temperature. 4. hanges n the atmospherc pressure wll also affect the heght of the alcohol column.. ondtons such as atmospherc pressure and the purty of the water must be mantaned to guarantee a repeatable, fxed temperature. 6. Body temperature vares among people and wth tme for a gven person. 7. Although both students have temperatures of degrees, the student wth the o temperature has the hgher fever because the elsus degree s larger than the ahrenhet degree. 8. Reference to g shows that 0 o s the hotter of the two temperatures.. Reference to g shows that the temperature s o. 40. Reference to g shows that the correspondng temperature s o. 41. The freezng pont of water s 0 o + 7 K 7 K. 4. Lqud ntrogen bols at 77 K 7 K 16 o. 4. The average knetc energy of the molecules doubles. 44. The absolute temperature would have to quadruple, because knetc energy depends on the square of the speed. 4. They would have the same average knetc energes as they have the same temperature. 46. Both gases have the same average knetc energes, so the helum atoms would have the greater average speed. 47. The partcles have more knetc energy. Therefore, they are movng faster and strke the walls more frequently wth more momentum. 48. The pressure drops to one-half ts orgnal value. 4. The volume doubles. 0. As the temperature of the gas decreases, ts volume decreases. We assume that the pressure stays the same because the walls are flexble. 1. The temperature drops to one-half on the Kelvn scale.. The flexng of the sdewalls causes the tre to heat up. Therefore the pressure rses (assumng that the volume stays fxed).. The partcles strke the walls more frequently producng a larger average mpulse wth the walls of the contaner. 4. The volume drops to one-thrd of ts orgnal value.. The more energetc molecules leave va evaporaton lowerng the average knetc energy and, therefore, the temperature of the remanng water. 6. The more energetc alcohol molecules leave va evaporaton lowerng the average knetc energy of the
3 hapter 11 Structure of Matter 1 remanng alcohol. Ths causes a flow of energy from your body to the remanng alcohol. 7. rom the coolng of ther bodes due to the evaporaton of the water on them. 8. Bolng removes the hgher knetc energy molecules. As more heat s added, the rate of the molecules leavng the surface ncreases, leavng the average knetc energy of the remanng molecules (and thus the temperature of the water) fxed. Answers to the Exercses 1. Mh m h 1g Mo 4 g m o 8g g m n 14 g. Mn Mh 18 g 84 g m h g. Oxygen s the lmtng amount. Therefore, 16 g + (1 g) 18 g. 4. Oxygen s the lmtng amount. Therefore, 48 g + (1 g) 84 g.. Bread s the lmtng amount g bread s 0 g bread 0 sandwches sandwch m 60 g 100 g s s m 000 g 0 10 g 800 g of ham h 6. Oxygen s the lmtng amount 1000 g oxygen water 16 g oxygen 6. moles of water mole of water m 18 g 11 g of water water water m m m 000 g 11 g 87 g of hydrogen h tot water 1g 1amu # amu g 1g 1amu # amu g 1kg 1amu 18 amu kg 10 atoms 10 atoms #. 10 molecules g 1 amu 10. # molecules 4 amu g 11. The number of lters tells us how many molecules of the orgnal combne to form ths number of molecules of the product. Usng these numbers as multplers n a chemcal equaton gves us 1 + H 1x H y Makng sure that we have the same number of atoms of each knd on each sde, we get x 1 and y. 1. See the soluton to Exercse 11 for the procedure. 1O + 1H 1HO
4 16 hapter 11 Structure of Matter cm 10 m # 10 A atom 10 ( 10 m) 0.01 m m 0 P atm A π 0.00 m m 16 ( 0.)( 1600 kg)( 10 m s ) A m P. 10 Pa P P 1 f L P f P f Tf T T T 7 K + 7 K 600 K 18. P 100 atm 1. f L 160L 160balloons P f 1. atm Tf 1 K 0. Pf P ( ps + 14 ps) 4.1 ps T K Therefore, the gauge pressure s.1 ps. Tf 10 cm 1 K 1. Pf P 1atm 1.6 atm f T 100 cm K f Pf 1000 cm 1 atm. Tf T 7 K 4 K 4 P 00 cm atm Answers to the Problems n Problem Solvng 1. Because 1 L of ntrogen produces L of ammona, 40 L of ntrogen wll produce 80 L of ammona.. Because L of carbon monoxde produce L of carbon doxde, 0 L of carbon monoxde wll produce 0 L of carbon doxde.. Because 1 molecule of methane produces molecules of hydrogen, 100 L of methane wll produce 00 L of hydrogen. 4. Because molecules of acetylene combne wth molecules of oxygen, t wll requre. tanks of oxygen to burn each tank of acetylene cm 1 m t A 10 m 100 cm 10 cm 0 L 1000 cm 1 m t A 400 m 1 L 100 cm cm
5 hapter 11 Structure of Matter cm 7 1 m 4 A 10 cm 1000 m ; less than one-half t 10 cm 100 cm 4 8. At L 00m 10 m 0L 10 m A 0 cm atoms 8 A atom 10 cm M 0.01 kg m. 10 atoms 1 ( mv ) Pa.7 10 kg/atom ( 8 amu) ( 40 m s ) cm kg P 10 cm 1m 1amu 1 1. P ( mv ) ( 44 amu) kg 100 cm 400 m s 400cm 1amu 1m Pa ( m ) 7 ( kg)( 8 10 m ) P v 44 m s m Average velocty only depends on the temperature of the gas. Assumng that the gas on the nsde of the tre s at the same temperature as the gas outsde, they wll have the same average velocty. P m 1000cm 1m cm kg 1600 m s 10 mv T ( T ) ( 0 ) 17.8 T ( T ) ( 68 ) 0 T T T Tc T T c T ( T )
6 18 hapter 11 Structure of Matter T Tc T Tc TX T Xf 0 T Tf + Tb Tf TXb T Xf TX T Xf 0 T Tf + ( Tb Tf ) + ( 180 ) 140 TXb T Xf 0 T T 7 + 0X + 0X 17X Tcb T cf 100 c cf 0. TX TXf ( TXb TXf ) T T f TR TRf + ( TRb TRf ) 4 R + ( 180 R) Tb T f 180. The Rankne degree s the same sze as the ahrenhet degree. You would stll be "runnng a three degree fever.". T T 7 K 0 K 7 K 7 K T TK 7 K 10 K 7 K 7 4. T T 7 K 17 K 7 K T v n K T mo 1 vo 11 m s 4 m s m 0.88 vh mc v m c h n Tf 7 K f L.L T K P P f P f P f Tf T K 86 K 1 Pf P atm 8 atm 1 f 4 The rms speed of the gas molecules stays the same. It depends only on the temperature of the gas. f Pf 100 cm atm Tf T 400K 00K 7 P 400 cm 1 atm
7 hapter 11 Structure of Matter Tf 00 cm K Pf P atm 1.4atm f T 800 cm K P 80 atm f 4L 1L P f 1.1 atm Because there wll be 4 L left n the tank, we can only blow up 87 balloons at 1 L each. P 100 atm f 0.6 m 48 m P f 1. atm Because there wll be 0.6 m left n the tank, we only have 47.4 m to blow up the balloons. f 47.4 m 100 cm balloon 000 cm 1 m 480
( ) 1/ 2. ( P SO2 )( P O2 ) 1/ 2.
 Chemstry 360 Dr. Jean M. Standard Problem Set 9 Solutons. The followng chemcal reacton converts sulfur doxde to sulfur troxde. SO ( g) + O ( g) SO 3 ( l). (a.) Wrte the expresson for K eq for ths reacton.
Chemstry 360 Dr. Jean M. Standard Problem Set 9 Solutons. The followng chemcal reacton converts sulfur doxde to sulfur troxde. SO ( g) + O ( g) SO 3 ( l). (a.) Wrte the expresson for K eq for ths reacton.
Thermodynamics General
 Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
Homework Chapter 21 Solutions!!
 Homework Chapter 1 Solutons 1.7 1.13 1.17 1.19 1.6 1.33 1.45 1.51 1.71 page 1 Problem 1.7 A mole sample of oxygen gas s confned to a 5 lter vessel at a pressure of 8 atm. Fnd the average translatonal knetc
Homework Chapter 1 Solutons 1.7 1.13 1.17 1.19 1.6 1.33 1.45 1.51 1.71 page 1 Problem 1.7 A mole sample of oxygen gas s confned to a 5 lter vessel at a pressure of 8 atm. Fnd the average translatonal knetc
Introduction to Vapor/Liquid Equilibrium, part 2. Raoult s Law:
 CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
PART I: MULTIPLE CHOICE (32 questions, each multiple choice question has a 2-point value, 64 points total).
 CHEMISTRY 123-07 Mdterm #2 answer key November 04, 2010 Statstcs: Average: 68 p (68%); Hghest: 91 p (91%); Lowest: 37 p (37%) Number of students performng at or above average: 58 (53%) Number of students
CHEMISTRY 123-07 Mdterm #2 answer key November 04, 2010 Statstcs: Average: 68 p (68%); Hghest: 91 p (91%); Lowest: 37 p (37%) Number of students performng at or above average: 58 (53%) Number of students
Physics 115. Molecular motion and temperature Phase equilibrium, evaporation
 Physcs 115 General Physcs II Sesson 9 Molecular moton and temperature Phase equlbrum, evaporaton R. J. Wlkes Emal: phy115a@u.washngton.edu Home page: http://courses.washngton.edu/phy115a/ 4/14/14 Physcs
Physcs 115 General Physcs II Sesson 9 Molecular moton and temperature Phase equlbrum, evaporaton R. J. Wlkes Emal: phy115a@u.washngton.edu Home page: http://courses.washngton.edu/phy115a/ 4/14/14 Physcs
Physics for Scientists and Engineers. Chapter 9 Impulse and Momentum
 Physcs or Scentsts and Engneers Chapter 9 Impulse and Momentum Sprng, 008 Ho Jung Pak Lnear Momentum Lnear momentum o an object o mass m movng wth a velocty v s dened to be p mv Momentum and lnear momentum
Physcs or Scentsts and Engneers Chapter 9 Impulse and Momentum Sprng, 008 Ho Jung Pak Lnear Momentum Lnear momentum o an object o mass m movng wth a velocty v s dened to be p mv Momentum and lnear momentum
Why? Chemistry Crunch #4.1 : Name: KEY Phase Changes. Success Criteria: Prerequisites: Vocabulary:
 Chemstry Crunch #4.1 : Name: KEY Phase Changes Why? Most substances wll eventually go through a phase change when heated or cooled (sometmes they chemcally react nstead). Molecules of a substance are held
Chemstry Crunch #4.1 : Name: KEY Phase Changes Why? Most substances wll eventually go through a phase change when heated or cooled (sometmes they chemcally react nstead). Molecules of a substance are held
= r. / cisely It was not isothermal, nor exactly adia- ! If / l/l /! i i \ i LjSj?
 376 Lea & Burke Physcs; The Nature of Thngs 19.44 J»(Pa) At constant V A \ LjSj?! '/! If / l/l /!! j j J [ ^ 1 I X j j> 1 ' : / J! 60 100 T('K) 200 Constant V lnes are mapped onto straght lnes on the P-T
376 Lea & Burke Physcs; The Nature of Thngs 19.44 J»(Pa) At constant V A \ LjSj?! '/! If / l/l /!! j j J [ ^ 1 I X j j> 1 ' : / J! 60 100 T('K) 200 Constant V lnes are mapped onto straght lnes on the P-T
CHEMICAL ENGINEERING
 Postal Correspondence GATE & PSUs -MT To Buy Postal Correspondence Packages call at 0-9990657855 1 TABLE OF CONTENT S. No. Ttle Page no. 1. Introducton 3 2. Dffuson 10 3. Dryng and Humdfcaton 24 4. Absorpton
Postal Correspondence GATE & PSUs -MT To Buy Postal Correspondence Packages call at 0-9990657855 1 TABLE OF CONTENT S. No. Ttle Page no. 1. Introducton 3 2. Dffuson 10 3. Dryng and Humdfcaton 24 4. Absorpton
and Statistical Mechanics Material Properties
 Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
At zero K: All atoms frozen at fixed positions on a periodic lattice.
 September, 00 Readng: Chapter Four Homework: None Entropy and The Degree of Dsorder: Consder a sold crystallne materal: At zero K: All atoms frozen at fxed postons on a perodc lattce. Add heat to a fnte
September, 00 Readng: Chapter Four Homework: None Entropy and The Degree of Dsorder: Consder a sold crystallne materal: At zero K: All atoms frozen at fxed postons on a perodc lattce. Add heat to a fnte
A Tale of Friction Basic Rollercoaster Physics. Fahrenheit Rollercoaster, Hershey, PA max height = 121 ft max speed = 58 mph
 A Tale o Frcton Basc Rollercoaster Physcs Fahrenhet Rollercoaster, Hershey, PA max heght = 11 t max speed = 58 mph PLAY PLAY PLAY PLAY Rotatonal Movement Knematcs Smlar to how lnear velocty s dened, angular
A Tale o Frcton Basc Rollercoaster Physcs Fahrenhet Rollercoaster, Hershey, PA max heght = 11 t max speed = 58 mph PLAY PLAY PLAY PLAY Rotatonal Movement Knematcs Smlar to how lnear velocty s dened, angular
Gasometric Determination of NaHCO 3 in a Mixture
 60 50 40 0 0 5 15 25 35 40 Temperature ( o C) 9/28/16 Gasometrc Determnaton of NaHCO 3 n a Mxture apor Pressure (mm Hg) apor Pressure of Water 1 NaHCO 3 (s) + H + (aq) Na + (aq) + H 2 O (l) + CO 2 (g)
60 50 40 0 0 5 15 25 35 40 Temperature ( o C) 9/28/16 Gasometrc Determnaton of NaHCO 3 n a Mxture apor Pressure (mm Hg) apor Pressure of Water 1 NaHCO 3 (s) + H + (aq) Na + (aq) + H 2 O (l) + CO 2 (g)
Be true to your work, your word, and your friend.
 Chemstry 13 NT Be true to your work, your word, and your frend. Henry Davd Thoreau 1 Chem 13 NT Chemcal Equlbrum Module Usng the Equlbrum Constant Interpretng the Equlbrum Constant Predctng the Drecton
Chemstry 13 NT Be true to your work, your word, and your frend. Henry Davd Thoreau 1 Chem 13 NT Chemcal Equlbrum Module Usng the Equlbrum Constant Interpretng the Equlbrum Constant Predctng the Drecton
Momentum. Momentum. Impulse. Momentum and Collisions
 Momentum Momentum and Collsons From Newton s laws: orce must be present to change an object s elocty (speed and/or drecton) Wsh to consder eects o collsons and correspondng change n elocty Gol ball ntally
Momentum Momentum and Collsons From Newton s laws: orce must be present to change an object s elocty (speed and/or drecton) Wsh to consder eects o collsons and correspondng change n elocty Gol ball ntally
Assignment 4. Adsorption Isotherms
 Insttute of Process Engneerng Assgnment 4. Adsorpton Isotherms Part A: Compettve adsorpton of methane and ethane In large scale adsorpton processes, more than one compound from a mxture of gases get adsorbed,
Insttute of Process Engneerng Assgnment 4. Adsorpton Isotherms Part A: Compettve adsorpton of methane and ethane In large scale adsorpton processes, more than one compound from a mxture of gases get adsorbed,
Chapter 21 - The Kinetic Theory of Gases
 hapter 1 - he Knetc heory o Gases 1. Δv 8.sn 4. 8.sn 4. m s F Nm. 1 kg.94 N Δ t. s F A 1.7 N m 1.7 a N mv 1.6 Use the equaton descrbng the knetc-theory account or pressure:. hen mv Kav where N nna NA N
hapter 1 - he Knetc heory o Gases 1. Δv 8.sn 4. 8.sn 4. m s F Nm. 1 kg.94 N Δ t. s F A 1.7 N m 1.7 a N mv 1.6 Use the equaton descrbng the knetc-theory account or pressure:. hen mv Kav where N nna NA N
Physics 2A Chapter 3 HW Solutions
 Phscs A Chapter 3 HW Solutons Chapter 3 Conceptual Queston: 4, 6, 8, Problems: 5,, 8, 7, 3, 44, 46, 69, 70, 73 Q3.4. Reason: (a) C = A+ B onl A and B are n the same drecton. Sze does not matter. (b) C
Phscs A Chapter 3 HW Solutons Chapter 3 Conceptual Queston: 4, 6, 8, Problems: 5,, 8, 7, 3, 44, 46, 69, 70, 73 Q3.4. Reason: (a) C = A+ B onl A and B are n the same drecton. Sze does not matter. (b) C
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2015
 Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
Physics 207 Lecture 6
 Physcs 207 Lecture 6 Agenda: Physcs 207, Lecture 6, Sept. 25 Chapter 4 Frames of reference Chapter 5 ewton s Law Mass Inerta s (contact and non-contact) Frcton (a external force that opposes moton) Free
Physcs 207 Lecture 6 Agenda: Physcs 207, Lecture 6, Sept. 25 Chapter 4 Frames of reference Chapter 5 ewton s Law Mass Inerta s (contact and non-contact) Frcton (a external force that opposes moton) Free
First Law: A body at rest remains at rest, a body in motion continues to move at constant velocity, unless acted upon by an external force.
 Secton 1. Dynamcs (Newton s Laws of Moton) Two approaches: 1) Gven all the forces actng on a body, predct the subsequent (changes n) moton. 2) Gven the (changes n) moton of a body, nfer what forces act
Secton 1. Dynamcs (Newton s Laws of Moton) Two approaches: 1) Gven all the forces actng on a body, predct the subsequent (changes n) moton. 2) Gven the (changes n) moton of a body, nfer what forces act
Week3, Chapter 4. Position and Displacement. Motion in Two Dimensions. Instantaneous Velocity. Average Velocity
 Week3, Chapter 4 Moton n Two Dmensons Lecture Quz A partcle confned to moton along the x axs moves wth constant acceleraton from x =.0 m to x = 8.0 m durng a 1-s tme nterval. The velocty of the partcle
Week3, Chapter 4 Moton n Two Dmensons Lecture Quz A partcle confned to moton along the x axs moves wth constant acceleraton from x =.0 m to x = 8.0 m durng a 1-s tme nterval. The velocty of the partcle
Number Average Molar Mass. Mass Average Molar Mass. Z-Average Molar Mass
 17 Molar mass: There are dfferent ways to report a molar mass lke (a) Number average molar mass, (b) mass average molar mass, (c) Vscosty average molar mass, (d) Z- Average molar mass Number Average Molar
17 Molar mass: There are dfferent ways to report a molar mass lke (a) Number average molar mass, (b) mass average molar mass, (c) Vscosty average molar mass, (d) Z- Average molar mass Number Average Molar
Chapters 18 & 19: Themodynamics review. All macroscopic (i.e., human scale) quantities must ultimately be explained on the microscopic scale.
 Chapters 18 & 19: Themodynamcs revew ll macroscopc (.e., human scale) quanttes must ultmately be explaned on the mcroscopc scale. Chapter 18: Thermodynamcs Thermodynamcs s the study o the thermal energy
Chapters 18 & 19: Themodynamcs revew ll macroscopc (.e., human scale) quanttes must ultmately be explaned on the mcroscopc scale. Chapter 18: Thermodynamcs Thermodynamcs s the study o the thermal energy
STATISTICAL MECHANICS
 STATISTICAL MECHANICS Thermal Energy Recall that KE can always be separated nto 2 terms: KE system = 1 2 M 2 total v CM KE nternal Rgd-body rotaton and elastc / sound waves Use smplfyng assumptons KE of
STATISTICAL MECHANICS Thermal Energy Recall that KE can always be separated nto 2 terms: KE system = 1 2 M 2 total v CM KE nternal Rgd-body rotaton and elastc / sound waves Use smplfyng assumptons KE of
v c motion is neither created nor destroyed, but transferred via interactions. Fri. Wed (.18,.19) Introducing Potential Energy RE 6.
 r. 6.5-.7 (.) Rest Mass,ork by Changng orces Columba Rep 3pm, here RE 6.b (last day to drop) ed. 6.8-.9(.8,.9) Introducng Potental Energy RE 6.c Tues. H6: Ch 6 Pr s 58,59, 99(a-c), 05(a-c) moton s nether
r. 6.5-.7 (.) Rest Mass,ork by Changng orces Columba Rep 3pm, here RE 6.b (last day to drop) ed. 6.8-.9(.8,.9) Introducng Potental Energy RE 6.c Tues. H6: Ch 6 Pr s 58,59, 99(a-c), 05(a-c) moton s nether
Page 1. Physics 131: Lecture 14. Today s Agenda. Things that stay the same. Impulse and Momentum Non-constant forces
 Physcs 131: Lecture 14 Today s Agenda Imulse and Momentum Non-constant forces Imulse-momentum momentum thm Conservaton of Lnear momentum Eternal/Internal forces Eamles Physcs 201: Lecture 1, Pg 1 Physcs
Physcs 131: Lecture 14 Today s Agenda Imulse and Momentum Non-constant forces Imulse-momentum momentum thm Conservaton of Lnear momentum Eternal/Internal forces Eamles Physcs 201: Lecture 1, Pg 1 Physcs
Exercises of Fundamentals of Chemical Processes
 Department of Energ Poltecnco d Mlano a Lambruschn 4 2056 MILANO Exercses of undamentals of Chemcal Processes Prof. Ganpero Gropp Exercse 7 ) Estmaton of the composton of the streams at the ext of an sothermal
Department of Energ Poltecnco d Mlano a Lambruschn 4 2056 MILANO Exercses of undamentals of Chemcal Processes Prof. Ganpero Gropp Exercse 7 ) Estmaton of the composton of the streams at the ext of an sothermal
Physics 114 Exam 2 Fall 2014 Solutions. Name:
 Physcs 114 Exam Fall 014 Name: For gradng purposes (do not wrte here): Queston 1. 1... 3. 3. Problem Answer each of the followng questons. Ponts for each queston are ndcated n red. Unless otherwse ndcated,
Physcs 114 Exam Fall 014 Name: For gradng purposes (do not wrte here): Queston 1. 1... 3. 3. Problem Answer each of the followng questons. Ponts for each queston are ndcated n red. Unless otherwse ndcated,
Physics 111: Mechanics Lecture 11
 Physcs 111: Mechancs Lecture 11 Bn Chen NJIT Physcs Department Textbook Chapter 10: Dynamcs of Rotatonal Moton q 10.1 Torque q 10. Torque and Angular Acceleraton for a Rgd Body q 10.3 Rgd-Body Rotaton
Physcs 111: Mechancs Lecture 11 Bn Chen NJIT Physcs Department Textbook Chapter 10: Dynamcs of Rotatonal Moton q 10.1 Torque q 10. Torque and Angular Acceleraton for a Rgd Body q 10.3 Rgd-Body Rotaton
Chem 2A Exam 1. First letter of your last name
 Frst letter of your last name NAME: PERM# INSTRUCTIONS: Fll n your name, perm number and frst ntal of your last name above. Be sure to show all of your work for full credt. Use the back of the page f necessary.
Frst letter of your last name NAME: PERM# INSTRUCTIONS: Fll n your name, perm number and frst ntal of your last name above. Be sure to show all of your work for full credt. Use the back of the page f necessary.
Name ID # For relatively dilute aqueous solutions the molality and molarity are approximately equal.
 Name ID # 1 CHEMISTRY 212, Lect. Sect. 002 Dr. G. L. Roberts Exam #1/Sprng 2000 Thursday, February 24, 2000 CLOSED BOOK EXM No notes or books allowed. Calculators may be used. tomc masses of nterest are
Name ID # 1 CHEMISTRY 212, Lect. Sect. 002 Dr. G. L. Roberts Exam #1/Sprng 2000 Thursday, February 24, 2000 CLOSED BOOK EXM No notes or books allowed. Calculators may be used. tomc masses of nterest are
TIME OF COMPLETION NAME SOLUTION DEPARTMENT OF NATURAL SCIENCES. PHYS 2211, Exam 2 Section 1 Version 1 October 18, 2013 Total Weight: 100 points
 TIME OF COMPLETION NAME SOLUTION DEPARTMENT OF NATURAL SCIENCES PHYS, Exam Secton Verson October 8, 03 Total Weght: 00 ponts. Check your examnaton or completeness pror to startng. There are a total o nne
TIME OF COMPLETION NAME SOLUTION DEPARTMENT OF NATURAL SCIENCES PHYS, Exam Secton Verson October 8, 03 Total Weght: 00 ponts. Check your examnaton or completeness pror to startng. There are a total o nne
Chapter 3 and Chapter 4
 Chapter 3 and Chapter 4 Chapter 3 Energy 3. Introducton:Work Work W s energy transerred to or rom an object by means o a orce actng on the object. Energy transerred to the object s postve work, and energy
Chapter 3 and Chapter 4 Chapter 3 Energy 3. Introducton:Work Work W s energy transerred to or rom an object by means o a orce actng on the object. Energy transerred to the object s postve work, and energy
Chemical Engineering Department University of Washington
 Chemcal Engneerng Department Unversty of Washngton ChemE 60 - Exam I July 4, 003 - Mass Flow Rate of Steam Through a Turbne (5 onts) Steam enters a turbne at 70 o C and.8 Ma and leaves at 00 ka wth a qualty
Chemcal Engneerng Department Unversty of Washngton ChemE 60 - Exam I July 4, 003 - Mass Flow Rate of Steam Through a Turbne (5 onts) Steam enters a turbne at 70 o C and.8 Ma and leaves at 00 ka wth a qualty
Energy, Entropy, and Availability Balances Phase Equilibria. Nonideal Thermodynamic Property Models. Selecting an Appropriate Model
 Lecture 4. Thermodynamcs [Ch. 2] Energy, Entropy, and Avalablty Balances Phase Equlbra - Fugactes and actvty coeffcents -K-values Nondeal Thermodynamc Property Models - P-v-T equaton-of-state models -
Lecture 4. Thermodynamcs [Ch. 2] Energy, Entropy, and Avalablty Balances Phase Equlbra - Fugactes and actvty coeffcents -K-values Nondeal Thermodynamc Property Models - P-v-T equaton-of-state models -
ME 300 Exam 2 November 18, :30 p.m. to 7:30 p.m.
 CICLE YOU LECTUE BELOW: Frst Name Last Name 1:3 a.m. 1:3 p.m. Nak Gore ME 3 Exam November 18, 14 6:3 p.m. to 7:3 p.m. INSTUCTIONS 1. Ths s a closed book and closed notes examnaton. You are provded wth
CICLE YOU LECTUE BELOW: Frst Name Last Name 1:3 a.m. 1:3 p.m. Nak Gore ME 3 Exam November 18, 14 6:3 p.m. to 7:3 p.m. INSTUCTIONS 1. Ths s a closed book and closed notes examnaton. You are provded wth
Physics 240: Worksheet 30 Name:
 (1) One mole of an deal monatomc gas doubles ts temperature and doubles ts volume. What s the change n entropy of the gas? () 1 kg of ce at 0 0 C melts to become water at 0 0 C. What s the change n entropy
(1) One mole of an deal monatomc gas doubles ts temperature and doubles ts volume. What s the change n entropy of the gas? () 1 kg of ce at 0 0 C melts to become water at 0 0 C. What s the change n entropy
AS-Level Maths: Statistics 1 for Edexcel
 1 of 6 AS-Level Maths: Statstcs 1 for Edecel S1. Calculatng means and standard devatons Ths con ndcates the slde contans actvtes created n Flash. These actvtes are not edtable. For more detaled nstructons,
1 of 6 AS-Level Maths: Statstcs 1 for Edecel S1. Calculatng means and standard devatons Ths con ndcates the slde contans actvtes created n Flash. These actvtes are not edtable. For more detaled nstructons,
Open Systems: Chemical Potential and Partial Molar Quantities Chemical Potential
 Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Lecture 22: Potential Energy
 Lecture : Potental Energy We have already studed the work-energy theorem, whch relates the total work done on an object to the change n knetc energy: Wtot = KE For a conservatve orce, the work done by
Lecture : Potental Energy We have already studed the work-energy theorem, whch relates the total work done on an object to the change n knetc energy: Wtot = KE For a conservatve orce, the work done by
Conservation of Angular Momentum = "Spin"
 Page 1 of 6 Conservaton of Angular Momentum = "Spn" We can assgn a drecton to the angular velocty: drecton of = drecton of axs + rght hand rule (wth rght hand, curl fngers n drecton of rotaton, thumb ponts
Page 1 of 6 Conservaton of Angular Momentum = "Spn" We can assgn a drecton to the angular velocty: drecton of = drecton of axs + rght hand rule (wth rght hand, curl fngers n drecton of rotaton, thumb ponts
Period & Frequency. Work and Energy. Methods of Energy Transfer: Energy. Work-KE Theorem 3/4/16. Ranking: Which has the greatest kinetic energy?
 Perod & Frequency Perod (T): Tme to complete one ull rotaton Frequency (): Number o rotatons completed per second. = 1/T, T = 1/ v = πr/t Work and Energy Work: W = F!d (pcks out parallel components) F
Perod & Frequency Perod (T): Tme to complete one ull rotaton Frequency (): Number o rotatons completed per second. = 1/T, T = 1/ v = πr/t Work and Energy Work: W = F!d (pcks out parallel components) F
Robert Eisberg Second edition CH 09 Multielectron atoms ground states and x-ray excitations
 Quantum Physcs 量 理 Robert Esberg Second edton CH 09 Multelectron atoms ground states and x-ray exctatons 9-01 By gong through the procedure ndcated n the text, develop the tme-ndependent Schroednger equaton
Quantum Physcs 量 理 Robert Esberg Second edton CH 09 Multelectron atoms ground states and x-ray exctatons 9-01 By gong through the procedure ndcated n the text, develop the tme-ndependent Schroednger equaton
CHEMISTRY Midterm #2 answer key October 25, 2005
 CHEMISTRY 123-01 Mdterm #2 answer key October 25, 2005 Statstcs: Average: 70 pts (70%); Hghest: 97 pts (97%); Lowest: 33 pts (33%) Number of students performng at or above average: 62 (63%) Number of students
CHEMISTRY 123-01 Mdterm #2 answer key October 25, 2005 Statstcs: Average: 70 pts (70%); Hghest: 97 pts (97%); Lowest: 33 pts (33%) Number of students performng at or above average: 62 (63%) Number of students
Temperature. Chapter Heat Engine
 Chapter 3 Temperature In prevous chapters of these notes we ntroduced the Prncple of Maxmum ntropy as a technque for estmatng probablty dstrbutons consstent wth constrants. In Chapter 9 we dscussed the
Chapter 3 Temperature In prevous chapters of these notes we ntroduced the Prncple of Maxmum ntropy as a technque for estmatng probablty dstrbutons consstent wth constrants. In Chapter 9 we dscussed the
Lecture 4. Macrostates and Microstates (Ch. 2 )
 Lecture 4. Macrostates and Mcrostates (Ch. ) The past three lectures: we have learned about thermal energy, how t s stored at the mcroscopc level, and how t can be transferred from one system to another.
Lecture 4. Macrostates and Mcrostates (Ch. ) The past three lectures: we have learned about thermal energy, how t s stored at the mcroscopc level, and how t can be transferred from one system to another.
13. One way of expressing the power dissipated by a resistor is P = ( V)
 Current and esstance 9. One way of expressng the power dsspated by a resstor s ( ). Thus, f the potental dfference across the resstor s doubled, the power wll be ncreased by a factor of 4, to a value of
Current and esstance 9. One way of expressng the power dsspated by a resstor s ( ). Thus, f the potental dfference across the resstor s doubled, the power wll be ncreased by a factor of 4, to a value of
Design Equations. ν ij r i V R. ν ij r i. Q n components. = Q f c jf Qc j + Continuous Stirred Tank Reactor (steady-state and constant phase)
 Desgn Equatons Batch Reactor d(v R c j ) dt = ν j r V R n dt dt = UA(T a T) r H R V R ncomponents V R c j C pj j Plug Flow Reactor d(qc j ) dv = ν j r 2 dt dv = R U(T a T) n r H R Q n components j c j
Desgn Equatons Batch Reactor d(v R c j ) dt = ν j r V R n dt dt = UA(T a T) r H R V R ncomponents V R c j C pj j Plug Flow Reactor d(qc j ) dv = ν j r 2 dt dv = R U(T a T) n r H R Q n components j c j
THE VIBRATIONS OF MOLECULES II THE CARBON DIOXIDE MOLECULE Student Instructions
 THE VIBRATIONS OF MOLECULES II THE CARBON DIOXIDE MOLECULE Student Instructons by George Hardgrove Chemstry Department St. Olaf College Northfeld, MN 55057 hardgrov@lars.acc.stolaf.edu Copyrght George
THE VIBRATIONS OF MOLECULES II THE CARBON DIOXIDE MOLECULE Student Instructons by George Hardgrove Chemstry Department St. Olaf College Northfeld, MN 55057 hardgrov@lars.acc.stolaf.edu Copyrght George
Estimation of the composition of the liquid and vapor streams exiting a flash unit with a supercritical component
 Department of Energ oltecnco d Mlano Va Lambruschn - 05 MILANO Eercses of Fundamentals of Chemcal rocesses rof. Ganpero Gropp Eercse 8 Estmaton of the composton of the lqud and vapor streams etng a unt
Department of Energ oltecnco d Mlano Va Lambruschn - 05 MILANO Eercses of Fundamentals of Chemcal rocesses rof. Ganpero Gropp Eercse 8 Estmaton of the composton of the lqud and vapor streams etng a unt
Econ107 Applied Econometrics Topic 3: Classical Model (Studenmund, Chapter 4)
 I. Classcal Assumptons Econ7 Appled Econometrcs Topc 3: Classcal Model (Studenmund, Chapter 4) We have defned OLS and studed some algebrac propertes of OLS. In ths topc we wll study statstcal propertes
I. Classcal Assumptons Econ7 Appled Econometrcs Topc 3: Classcal Model (Studenmund, Chapter 4) We have defned OLS and studed some algebrac propertes of OLS. In ths topc we wll study statstcal propertes
LNG CARGO TRANSFER CALCULATION METHODS AND ROUNDING-OFFS
 CARGO TRANSFER CALCULATION METHODS AND ROUNDING-OFFS CONTENTS 1. Method for determnng transferred energy durng cargo transfer. Calculatng the transferred energy.1 Calculatng the gross transferred energy.1.1
CARGO TRANSFER CALCULATION METHODS AND ROUNDING-OFFS CONTENTS 1. Method for determnng transferred energy durng cargo transfer. Calculatng the transferred energy.1 Calculatng the gross transferred energy.1.1
Lecture 4. Instructor: Haipeng Luo
 Lecture 4 Instructor: Hapeng Luo In the followng lectures, we focus on the expert problem and study more adaptve algorthms. Although Hedge s proven to be worst-case optmal, one may wonder how well t would
Lecture 4 Instructor: Hapeng Luo In the followng lectures, we focus on the expert problem and study more adaptve algorthms. Although Hedge s proven to be worst-case optmal, one may wonder how well t would
Solution Thermodynamics
 Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
GROSS VAPORIZATION. Definitions
 GROSS VAPORIZATION Defntons Vaporzaton: Vaporzaton s the process by whch molecules leave ther prevous lqud or sold phase or attachment to a substrate and become gas molecules. In some papers, the term
GROSS VAPORIZATION Defntons Vaporzaton: Vaporzaton s the process by whch molecules leave ther prevous lqud or sold phase or attachment to a substrate and become gas molecules. In some papers, the term
ERROR RESEARCH ON A HEPA FILTER MEDIA TESTING SYSTEM OF MPPS(MOST PENETRATION PARTICLE SIZE) EFFICIENCY
 Proceedngs: Indoor Ar 2005 ERROR RESEARCH ON A HEPA FILTER MEDIA TESTING SYSTEM OF MPPS(MOST PENETRATION PARTICLE SIZE) EFFICIENCY S Lu, J Lu *, N Zhu School of Envronmental Scence and Technology, Tanjn
Proceedngs: Indoor Ar 2005 ERROR RESEARCH ON A HEPA FILTER MEDIA TESTING SYSTEM OF MPPS(MOST PENETRATION PARTICLE SIZE) EFFICIENCY S Lu, J Lu *, N Zhu School of Envronmental Scence and Technology, Tanjn
Lecture 16. Chapter 11. Energy Dissipation Linear Momentum. Physics I. Department of Physics and Applied Physics
 Lecture 16 Chapter 11 Physcs I Energy Dsspaton Lnear Momentum Course webste: http://aculty.uml.edu/andry_danylov/teachng/physcsi Department o Physcs and Appled Physcs IN IN THIS CHAPTER, you wll learn
Lecture 16 Chapter 11 Physcs I Energy Dsspaton Lnear Momentum Course webste: http://aculty.uml.edu/andry_danylov/teachng/physcsi Department o Physcs and Appled Physcs IN IN THIS CHAPTER, you wll learn
Moments of Inertia. and reminds us of the analogous equation for linear momentum p= mv, which is of the form. The kinetic energy of the body is.
 Moments of Inerta Suppose a body s movng on a crcular path wth constant speed Let s consder two quanttes: the body s angular momentum L about the center of the crcle, and ts knetc energy T How are these
Moments of Inerta Suppose a body s movng on a crcular path wth constant speed Let s consder two quanttes: the body s angular momentum L about the center of the crcle, and ts knetc energy T How are these
Atomic Mass and Atomic Mass Number. Moles and Molar Mass. Moles and Molar Mass
 Atomic Mass and Atomic Mass Number The mass of an atom is determined primarily by its most massive constituents: protons and neutrons in its nucleus. The sum of the number of protons and neutrons is called
Atomic Mass and Atomic Mass Number The mass of an atom is determined primarily by its most massive constituents: protons and neutrons in its nucleus. The sum of the number of protons and neutrons is called
Chapter 07: Kinetic Energy and Work
 Chapter 07: Knetc Energy and Work Conservaton o Energy s one o Nature s undamental laws that s not volated. Energy can take on derent orms n a gven system. Ths chapter we wll dscuss work and knetc energy.
Chapter 07: Knetc Energy and Work Conservaton o Energy s one o Nature s undamental laws that s not volated. Energy can take on derent orms n a gven system. Ths chapter we wll dscuss work and knetc energy.
CinChE Problem-Solving Strategy Chapter 4 Development of a Mathematical Model. formulation. procedure
 nhe roblem-solvng Strategy hapter 4 Transformaton rocess onceptual Model formulaton procedure Mathematcal Model The mathematcal model s an abstracton that represents the engneerng phenomena occurrng n
nhe roblem-solvng Strategy hapter 4 Transformaton rocess onceptual Model formulaton procedure Mathematcal Model The mathematcal model s an abstracton that represents the engneerng phenomena occurrng n
Physics 2A Chapter 9 HW Solutions
 Phscs A Chapter 9 HW Solutons Chapter 9 Conceptual Queston:, 4, 8, 13 Problems: 3, 8, 1, 15, 3, 40, 51, 6 Q9.. Reason: We can nd the change n momentum o the objects b computng the mpulse on them and usng
Phscs A Chapter 9 HW Solutons Chapter 9 Conceptual Queston:, 4, 8, 13 Problems: 3, 8, 1, 15, 3, 40, 51, 6 Q9.. Reason: We can nd the change n momentum o the objects b computng the mpulse on them and usng
Part C Dynamics and Statics of Rigid Body. Chapter 5 Rotation of a Rigid Body About a Fixed Axis
 Part C Dynamcs and Statcs of Rgd Body Chapter 5 Rotaton of a Rgd Body About a Fxed Axs 5.. Rotatonal Varables 5.. Rotaton wth Constant Angular Acceleraton 5.3. Knetc Energy of Rotaton, Rotatonal Inerta
Part C Dynamcs and Statcs of Rgd Body Chapter 5 Rotaton of a Rgd Body About a Fxed Axs 5.. Rotatonal Varables 5.. Rotaton wth Constant Angular Acceleraton 5.3. Knetc Energy of Rotaton, Rotatonal Inerta
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM
 ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
Section 8.1 Exercises
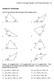 Secton 8.1 Non-rght Trangles: Law of Snes and Cosnes 519 Secton 8.1 Exercses Solve for the unknown sdes and angles of the trangles shown. 10 70 50 1.. 18 40 110 45 5 6 3. 10 4. 75 15 5 6 90 70 65 5. 6.
Secton 8.1 Non-rght Trangles: Law of Snes and Cosnes 519 Secton 8.1 Exercses Solve for the unknown sdes and angles of the trangles shown. 10 70 50 1.. 18 40 110 45 5 6 3. 10 4. 75 15 5 6 90 70 65 5. 6.
Physics 2A Chapters 6 - Work & Energy Fall 2017
 Physcs A Chapters 6 - Work & Energy Fall 017 These notes are eght pages. A quck summary: The work-energy theorem s a combnaton o Chap and Chap 4 equatons. Work s dened as the product o the orce actng on
Physcs A Chapters 6 - Work & Energy Fall 017 These notes are eght pages. A quck summary: The work-energy theorem s a combnaton o Chap and Chap 4 equatons. Work s dened as the product o the orce actng on
Thermal-Fluids I. Chapter 18 Transient heat conduction. Dr. Primal Fernando Ph: (850)
 hermal-fluds I Chapter 18 ransent heat conducton Dr. Prmal Fernando prmal@eng.fsu.edu Ph: (850) 410-6323 1 ransent heat conducton In general, he temperature of a body vares wth tme as well as poston. In
hermal-fluds I Chapter 18 ransent heat conducton Dr. Prmal Fernando prmal@eng.fsu.edu Ph: (850) 410-6323 1 ransent heat conducton In general, he temperature of a body vares wth tme as well as poston. In
Principles of Food and Bioprocess Engineering (FS 231) Solutions to Example Problems on Heat Transfer
 Prncples of Food and Boprocess Engneerng (FS 31) Solutons to Example Problems on Heat Transfer 1. We start wth Fourer s law of heat conducton: Q = k A ( T/ x) Rearrangng, we get: Q/A = k ( T/ x) Here,
Prncples of Food and Boprocess Engneerng (FS 31) Solutons to Example Problems on Heat Transfer 1. We start wth Fourer s law of heat conducton: Q = k A ( T/ x) Rearrangng, we get: Q/A = k ( T/ x) Here,
Lab 2e Thermal System Response and Effective Heat Transfer Coefficient
 58:080 Expermental Engneerng 1 OBJECTIVE Lab 2e Thermal System Response and Effectve Heat Transfer Coeffcent Warnng: though the experment has educatonal objectves (to learn about bolng heat transfer, etc.),
58:080 Expermental Engneerng 1 OBJECTIVE Lab 2e Thermal System Response and Effectve Heat Transfer Coeffcent Warnng: though the experment has educatonal objectves (to learn about bolng heat transfer, etc.),
Problem Set #6 solution, Chem 340, Fall 2013 Due Friday, Oct 11, 2013 Please show all work for credit
 Problem Set #6 soluton, Chem 340, Fall 2013 Due Frday, Oct 11, 2013 Please show all work for credt To hand n: Atkns Chap 3 Exercses: 3.3(b), 3.8(b), 3.13(b), 3.15(b) Problems: 3.1, 3.12, 3.36, 3.43 Engel
Problem Set #6 soluton, Chem 340, Fall 2013 Due Frday, Oct 11, 2013 Please show all work for credt To hand n: Atkns Chap 3 Exercses: 3.3(b), 3.8(b), 3.13(b), 3.15(b) Problems: 3.1, 3.12, 3.36, 3.43 Engel
THE MIND & THE SCIENCE OF SUCCESS - All rights reserved - - APPENDIX -
 - APPENDIX - MAYARD S PROBLEM SOLVING METOD A sample o word problems rom hgh school (nd cycle) to college leel s used to llustrate ths smple method that can also be used n all grade leels. 4 smple steps
- APPENDIX - MAYARD S PROBLEM SOLVING METOD A sample o word problems rom hgh school (nd cycle) to college leel s used to llustrate ths smple method that can also be used n all grade leels. 4 smple steps
University of Washington Department of Chemistry Chemistry 452/456 Summer Quarter 2014
 Lecture 16 8/4/14 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 214. Real Vapors and Fugacty Henry s Law accounts or the propertes o extremely dlute soluton. s shown n Fgure
Lecture 16 8/4/14 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 214. Real Vapors and Fugacty Henry s Law accounts or the propertes o extremely dlute soluton. s shown n Fgure
Week 8: Chapter 9. Linear Momentum. Newton Law and Momentum. Linear Momentum, cont. Conservation of Linear Momentum. Conservation of Momentum, 2
 Lnear omentum Week 8: Chapter 9 Lnear omentum and Collsons The lnear momentum of a partcle, or an object that can be modeled as a partcle, of mass m movng wth a velocty v s defned to be the product of
Lnear omentum Week 8: Chapter 9 Lnear omentum and Collsons The lnear momentum of a partcle, or an object that can be modeled as a partcle, of mass m movng wth a velocty v s defned to be the product of
PHYS 1101 Practice problem set 12, Chapter 32: 21, 22, 24, 57, 61, 83 Chapter 33: 7, 12, 32, 38, 44, 49, 76
 PHYS 1101 Practce problem set 1, Chapter 3: 1,, 4, 57, 61, 83 Chapter 33: 7, 1, 3, 38, 44, 49, 76 3.1. Vsualze: Please reer to Fgure Ex3.1. Solve: Because B s n the same drecton as the ntegraton path s
PHYS 1101 Practce problem set 1, Chapter 3: 1,, 4, 57, 61, 83 Chapter 33: 7, 1, 3, 38, 44, 49, 76 3.1. Vsualze: Please reer to Fgure Ex3.1. Solve: Because B s n the same drecton as the ntegraton path s
Physics 181. Particle Systems
 Physcs 181 Partcle Systems Overvew In these notes we dscuss the varables approprate to the descrpton of systems of partcles, ther defntons, ther relatons, and ther conservatons laws. We consder a system
Physcs 181 Partcle Systems Overvew In these notes we dscuss the varables approprate to the descrpton of systems of partcles, ther defntons, ther relatons, and ther conservatons laws. We consder a system
G4023 Mid-Term Exam #1 Solutions
 Exam1Solutons.nb 1 G03 Md-Term Exam #1 Solutons 1-Oct-0, 1:10 p.m to :5 p.m n 1 Pupn Ths exam s open-book, open-notes. You may also use prnt-outs of the homework solutons and a calculator. 1 (30 ponts,
Exam1Solutons.nb 1 G03 Md-Term Exam #1 Solutons 1-Oct-0, 1:10 p.m to :5 p.m n 1 Pupn Ths exam s open-book, open-notes. You may also use prnt-outs of the homework solutons and a calculator. 1 (30 ponts,
Week 9 Chapter 10 Section 1-5
 Week 9 Chapter 10 Secton 1-5 Rotaton Rgd Object A rgd object s one that s nondeformable The relatve locatons of all partcles makng up the object reman constant All real objects are deformable to some extent,
Week 9 Chapter 10 Secton 1-5 Rotaton Rgd Object A rgd object s one that s nondeformable The relatve locatons of all partcles makng up the object reman constant All real objects are deformable to some extent,
Week 11: Chapter 11. The Vector Product. The Vector Product Defined. The Vector Product and Torque. More About the Vector Product
 The Vector Product Week 11: Chapter 11 Angular Momentum There are nstances where the product of two vectors s another vector Earler we saw where the product of two vectors was a scalar Ths was called the
The Vector Product Week 11: Chapter 11 Angular Momentum There are nstances where the product of two vectors s another vector Earler we saw where the product of two vectors was a scalar Ths was called the
A particle in a state of uniform motion remain in that state of motion unless acted upon by external force.
 The fundamental prncples of classcal mechancs were lad down by Galleo and Newton n the 16th and 17th centures. In 1686, Newton wrote the Prncpa where he gave us three laws of moton, one law of gravty,
The fundamental prncples of classcal mechancs were lad down by Galleo and Newton n the 16th and 17th centures. In 1686, Newton wrote the Prncpa where he gave us three laws of moton, one law of gravty,
Outline. Unit Eight Calculations with Entropy. The Second Law. Second Law Notes. Uses of Entropy. Entropy is a Property.
 Unt Eght Calculatons wth Entropy Mechancal Engneerng 370 Thermodynamcs Larry Caretto October 6, 010 Outlne Quz Seven Solutons Second law revew Goals for unt eght Usng entropy to calculate the maxmum work
Unt Eght Calculatons wth Entropy Mechancal Engneerng 370 Thermodynamcs Larry Caretto October 6, 010 Outlne Quz Seven Solutons Second law revew Goals for unt eght Usng entropy to calculate the maxmum work
Chapter 18, Part 1. Fundamentals of Atmospheric Modeling
 Overhead Sldes for Chapter 18, Part 1 of Fundamentals of Atmospherc Modelng by Mark Z. Jacobson Department of Cvl & Envronmental Engneerng Stanford Unversty Stanford, CA 94305-4020 January 30, 2002 Types
Overhead Sldes for Chapter 18, Part 1 of Fundamentals of Atmospherc Modelng by Mark Z. Jacobson Department of Cvl & Envronmental Engneerng Stanford Unversty Stanford, CA 94305-4020 January 30, 2002 Types
Chapter 3 The Kinetic Theory of Gases 3.1. Ideal Gases Experimental Laws and the Equation of State
 Chater 3 The Knetc Theory of Gases 3.1. Ideal Gases 3.1.1. Exermental Laws and the Equaton of State 3.1.2. Molecular Model of an Ideal Gas 3.3. Mean Free Path 3.4. The Boltzmann Dstrbuton Law and The Dstrbuton
Chater 3 The Knetc Theory of Gases 3.1. Ideal Gases 3.1.1. Exermental Laws and the Equaton of State 3.1.2. Molecular Model of an Ideal Gas 3.3. Mean Free Path 3.4. The Boltzmann Dstrbuton Law and The Dstrbuton
Physics 53. Rotational Motion 3. Sir, I have found you an argument, but I am not obliged to find you an understanding.
 Physcs 53 Rotatonal Moton 3 Sr, I have found you an argument, but I am not oblged to fnd you an understandng. Samuel Johnson Angular momentum Wth respect to rotatonal moton of a body, moment of nerta plays
Physcs 53 Rotatonal Moton 3 Sr, I have found you an argument, but I am not oblged to fnd you an understandng. Samuel Johnson Angular momentum Wth respect to rotatonal moton of a body, moment of nerta plays
LAB # 4 - Torque. d (1)
 LAB # 4 - Torque. Introducton Through the use of Newton's three laws of moton, t s possble (n prncple, f not n fact) to predct the moton of any set of partcles. That s, n order to descrbe the moton of
LAB # 4 - Torque. Introducton Through the use of Newton's three laws of moton, t s possble (n prncple, f not n fact) to predct the moton of any set of partcles. That s, n order to descrbe the moton of
Physics 207: Lecture 20. Today s Agenda Homework for Monday
 Physcs 207: Lecture 20 Today s Agenda Homework for Monday Recap: Systems of Partcles Center of mass Velocty and acceleraton of the center of mass Dynamcs of the center of mass Lnear Momentum Example problems
Physcs 207: Lecture 20 Today s Agenda Homework for Monday Recap: Systems of Partcles Center of mass Velocty and acceleraton of the center of mass Dynamcs of the center of mass Lnear Momentum Example problems
PY2101 Classical Mechanics Dr. Síle Nic Chormaic, Room 215 D Kane Bldg
 PY2101 Classcal Mechancs Dr. Síle Nc Chormac, Room 215 D Kane Bldg s.ncchormac@ucc.e Lectures stll some ssues to resolve. Slots shared between PY2101 and PY2104. Hope to have t fnalsed by tomorrow. Mondays
PY2101 Classcal Mechancs Dr. Síle Nc Chormac, Room 215 D Kane Bldg s.ncchormac@ucc.e Lectures stll some ssues to resolve. Slots shared between PY2101 and PY2104. Hope to have t fnalsed by tomorrow. Mondays
Chemical Equilibrium. Chapter 6 Spontaneity of Reactive Mixtures (gases) Taking into account there are many types of work that a sysem can perform
 Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
Please review the following statement: I certify that I have not given unauthorized aid nor have I received aid in the completion of this exam.
 NME (Last, Frst): Please revew the followng statement: I certfy that I have not gven unauthorzed ad nor have I receved ad n the completon of ths exam. Sgnature: INSTRUCTIONS Begn each problem n the space
NME (Last, Frst): Please revew the followng statement: I certfy that I have not gven unauthorzed ad nor have I receved ad n the completon of ths exam. Sgnature: INSTRUCTIONS Begn each problem n the space
EN40: Dynamics and Vibrations. Homework 4: Work, Energy and Linear Momentum Due Friday March 1 st
 EN40: Dynamcs and bratons Homework 4: Work, Energy and Lnear Momentum Due Frday March 1 st School of Engneerng Brown Unversty 1. The fgure (from ths publcaton) shows the energy per unt area requred to
EN40: Dynamcs and bratons Homework 4: Work, Energy and Lnear Momentum Due Frday March 1 st School of Engneerng Brown Unversty 1. The fgure (from ths publcaton) shows the energy per unt area requred to
If two volatile and miscible liquids are combined to form a solution, Raoult s law is not obeyed. Use the experimental data in Table 9.
 9.9 Real Solutons Exhbt Devatons from Raoult s Law If two volatle and mscble lquds are combned to form a soluton, Raoult s law s not obeyed. Use the expermental data n Table 9.3: Physcal Chemstry 00 Pearson
9.9 Real Solutons Exhbt Devatons from Raoult s Law If two volatle and mscble lquds are combned to form a soluton, Raoult s law s not obeyed. Use the expermental data n Table 9.3: Physcal Chemstry 00 Pearson
Review of Classical Thermodynamics
 Revew of Classcal hermodynamcs Physcs 4362, Lecture #1, 2 Syllabus What s hermodynamcs? 1 [A law] s more mpressve the greater the smplcty of ts premses, the more dfferent are the knds of thngs t relates,
Revew of Classcal hermodynamcs Physcs 4362, Lecture #1, 2 Syllabus What s hermodynamcs? 1 [A law] s more mpressve the greater the smplcty of ts premses, the more dfferent are the knds of thngs t relates,
THERMAL DISTRIBUTION IN THE HCL SPECTRUM OBJECTIVE
 ame: THERMAL DISTRIBUTIO I THE HCL SPECTRUM OBJECTIVE To nvestgate a system s thermal dstrbuton n dscrete states; specfcally, determne HCl gas temperature from the relatve occupatons of ts rotatonal states.
ame: THERMAL DISTRIBUTIO I THE HCL SPECTRUM OBJECTIVE To nvestgate a system s thermal dstrbuton n dscrete states; specfcally, determne HCl gas temperature from the relatve occupatons of ts rotatonal states.
CHAPTER 8 Potential Energy and Conservation of Energy
 CHAPTER 8 Potental Energy and Conservaton o Energy One orm o energy can be converted nto another orm o energy. Conservatve and non-conservatve orces Physcs 1 Knetc energy: Potental energy: Energy assocated
CHAPTER 8 Potental Energy and Conservaton o Energy One orm o energy can be converted nto another orm o energy. Conservatve and non-conservatve orces Physcs 1 Knetc energy: Potental energy: Energy assocated
Dr. Shalabh Department of Mathematics and Statistics Indian Institute of Technology Kanpur
 Analyss of Varance and Desgn of Exerments-I MODULE III LECTURE - 2 EXPERIMENTAL DESIGN MODELS Dr. Shalabh Deartment of Mathematcs and Statstcs Indan Insttute of Technology Kanur 2 We consder the models
Analyss of Varance and Desgn of Exerments-I MODULE III LECTURE - 2 EXPERIMENTAL DESIGN MODELS Dr. Shalabh Deartment of Mathematcs and Statstcs Indan Insttute of Technology Kanur 2 We consder the models
ESCI 341 Atmospheric Thermodynamics Lesson 6 Thermodynamic Processes
 ESCI 341 Atmosherc Thermodynamcs Lesson 6 Thermodynamc Processes Reerences: An Introducton to Atmosherc Thermodynamcs, Tsons Introducton to Theoretcal Meteorology, Hess Physcal Chemstry (4 th edton), Lene
ESCI 341 Atmosherc Thermodynamcs Lesson 6 Thermodynamc Processes Reerences: An Introducton to Atmosherc Thermodynamcs, Tsons Introducton to Theoretcal Meteorology, Hess Physcal Chemstry (4 th edton), Lene
Physics 3 (PHYF144) Chap 2: Heat and the First Law of Thermodynamics System. Quantity Positive Negative
 Physcs (PHYF hap : Heat and the Frst aw of hermodynamcs -. Work and Heat n hermodynamc Processes A thermodynamc system s a system that may exchange energy wth ts surroundngs by means of heat and work.
Physcs (PHYF hap : Heat and the Frst aw of hermodynamcs -. Work and Heat n hermodynamc Processes A thermodynamc system s a system that may exchange energy wth ts surroundngs by means of heat and work.
4 Analysis of Variance (ANOVA) 5 ANOVA. 5.1 Introduction. 5.2 Fixed Effects ANOVA
 4 Analyss of Varance (ANOVA) 5 ANOVA 51 Introducton ANOVA ANOVA s a way to estmate and test the means of multple populatons We wll start wth one-way ANOVA If the populatons ncluded n the study are selected
4 Analyss of Varance (ANOVA) 5 ANOVA 51 Introducton ANOVA ANOVA s a way to estmate and test the means of multple populatons We wll start wth one-way ANOVA If the populatons ncluded n the study are selected
Introduction to Statistical Methods
 Introducton to Statstcal Methods Physcs 4362, Lecture #3 hermodynamcs Classcal Statstcal Knetc heory Classcal hermodynamcs Macroscopc approach General propertes of the system Macroscopc varables 1 hermodynamc
Introducton to Statstcal Methods Physcs 4362, Lecture #3 hermodynamcs Classcal Statstcal Knetc heory Classcal hermodynamcs Macroscopc approach General propertes of the system Macroscopc varables 1 hermodynamc
