Supplementary Materials for
|
|
|
- Elwin Goodman
- 5 years ago
- Views:
Transcription
1 advances.sciencemag.org/cgi/content/full/2/4/e /dc1 Supplementary Materials for Electrolytes induce long-range orientational order and free energy changes in the H-bond network of bulk water Yixing Chen, Halil I. Okur, Nikolaos Gomopoulos, Carlos Macias-Romero, Paul S. Cremer, Poul B. Petersen, Gabriele Tocci, David M. Wilkins, Chungwen Liang, Michele Ceriotti, Sylvie Roke Published 8 April 2016, Sci. Adv. 2, e (2016) DOI: /sciadv The PDF file includes: S1. Constants and properties of H2O and D2O S2. Femtosecond elastic second harmonic scattering S3. Phenomenological discussion of the response of spherical domains S4. Spherical domain size dependence of the fs-eshs signal S5. Molecular dynamics simulations S6. fs-eshs from pure H2O and D2O S7. Mean-field models S8. Surface tension measurements and interpretation Table S1. Constants and properties of H2O and D2O. Table S2. Domain radius, corresponding number of hydration shells, and anisotropy in case the domain size is only determined by the average ion separation. Fig. S1. Elastic and inelastic second harmonic scattering. Fig. S2. Sketch of the relevant parameters needed in the phenomenological discussion. Fig. S3. I as a function of domain size and relative structural anisotropy. Fig. S4. fs-eshs patterns for pure H2O and pure D2O. Fig. S5. Mean-field models and temperature dependence. References (61 87)
2 Supplementary Text S1. Constants and properties of H 2O and D 2O table S1: Constants and properties of H2O and D2O Property H2O D2O Refractive index (61), 298 K 514 nm nm Dipole moment (D) gas phase (62) liquid phase (63), 300K 2.95 Permittivity at infinite frequency (64) 4.49 Static dielectric constant (65), 298 K xxz zxx in gas phase (66-70) ( x10-53 C 3 m 3 J -2 yyz ) zyy zzz zxx 5.7 in liquid phase (71) ( x10-53 C 3 m 3 J -2 zyy 10.9 ) zzz 31.6 (3) xxxx 768 (3) yyyy 4936 (3) in liquid phase (71) (3) zzzz 2504 ( x10-65 C 4 m 4 J -3 ) (3) xxyy 902 (3) zzyy 1197 (3) zzxx 490
3 S2. Femtosecond elastic second harmonic scattering Even though the fundamental and second harmonic (SH) beams are not resonant with any transitions of the water molecules, it is important to verify that the measurements are purely elastic and thus purely second-order in nature. Here we record the scattered SH intensity as well as the spectrum of the scattered light as a function of the incident laser power (P). If perturbing effects (for example realignment of water molecules by the laser field (38)) occur, the SH intensity divided by the square of the incident power (I SH/P 2 ) will no longer be power independent. If energy transfer between the pulses and the water occurs, then inelastic scattering will lead to changes in the spectral shape. Figure S1A shows a measurement of the emitted SH intensity from pure H 2O, plotted as I SH/P 2 versus the incident laser power P. Figure S1B shows SH spectra recorded for different incident laser powers. Both recordings were done in S outs ins in polarization (where the electronic single molecule response is maximum) and at a scattering angle of 90 o. The data shows that the SH scattering depends quadratically on the incident power up to 80 mw, and that up to this incident power the spectral shape does not display any changes. At higher powers it can be seen that the power dependence of the SH intensity starts to deviate from quadratic behavior, and that the SH spectra become blue shifted and broadened with increasing intensity on the wings. fs-eshs measurements of electrolyte solutions are performed at 60 mw. fig. S1. Elastic and inelastic second harmonic scattering. (A) Plots of the SH intensity divided by the square of the incident power as a function of the incident power (bottom axis) and fluence (top axis). The red line indicates the quadratic dependence of the SH intensity on the incident power. (B) Normalized SH spectra of pure H2O at different incident powers.
4 S3. Phenomenological discussion of the response of spherical domains Following the observed polarization dependence of the fs-eshs intensity, we consider two contributions that can be used to explain the change in fs-eshs intensity observed in Figs. 1C and 2: 1. The short-lived structural perturbations as arising from an unknown structural correlation, which we represented by spherical shells with a certain adjustable anisotropy ( ) that surround domains with a radius R around a central ion. These shells with structurally correlated water molecules generate second harmonic photons. 2. The single molecule electronic response of water as described by the third-order hyperpolarizability elements β (3), that couples to the electromagnetic fields of the incoming laser pulses and the screened DC electric field of the ions in solution. We use a nonlinear Rayleigh-Gans-Debye light scattering model, which assumes that the linear refractive index is perturbed minimally by the presence of ions (72, 73) and is valid for domain sizes R < 250 nm (74). In this model we make use of the following coordinate systems (from large to small length scale): The right-handed Cartesian coordinate system of the laboratory frame (x,y,z). Here, x,z determines the horizontal plane of scattering, with z being directed along the second harmonic 2k 1 vector, and y indicates the vertical direction out of the scattering plane. The polarization of light is indicated with P or S, and labeled with u i (as well as the electric field components). In this frame q is defined as the scattering wave vector, such that q k 0-2k 1, and the scattering angle is defined as the angle between the scattered wave and the incoming beam. The right-handed Cartesian coordinate system of the effective susceptibility, which is needed to calculate scattering integrals analytically and allows easy predictions of symmetry selection rules (75). This frame is rotated with respect to the lab frame (and depends on the scattering geometry), with the principal axis lying along the scattering vector q. In coordinate transformations we label these indices with a i. The spherical coordinate system of the spherical domain around the ions, which is indicated by (r, ', '). In coordinate transformations we label these indices with b i. The Cartesian molecular coordinates, designated by (a,b,c), where c represents the direction of the dipole moment of water. In coordinate transformations we label these indices with c i.
5 These coordinate systems are illustrated in Fig. S2. fig. S2. Sketch of the relevant parameters needed in the phenomenological discussion. The illustration shows an ion and its extended hydration shell that are illuminated by fundamental laser pulses with a wave vector k1. The fs-eshs intensity is measured at a scattering angle (θ) of 90. The scattering vector q is defined as the difference k0-2k1. The scattering vector defines the direction of the orthonormal coordinate system used for calculating the effective susceptibility (Γ ) elements. The inset shows a close-up of the water shell, relative to which the χ elements are defined. The principal coordinate system of the water molecule is (a,b,c). The molecular hyperpolarizability β is defined with respect to that system. The response from structural correlations The total SH polarization of a single spherical domain originating from structural perturbations of water is given by (76) P u0 u 1 u 2 2 ~ a 0,a 1,a 2 Γ a0 a 1 a 2 i=0(q ai e ui ) E u1 E u2 (1) where E ui is the amplitude of the incoming electric field (the subscript u i refers to its polarization and here u 1 = u 2 for fs-eshs), Γ a0 a 1 a 2 is an effective susceptibility element (which is further described below), q ai denotes the scattering vector (q = k 0 2k 1 ), and e ui denotes the polarization state unit vector of the electrical field. The effective susceptibility Γ a0 a 1 a 2 elements can be calculated using (73, 75) Γ a0 a 1 a 2 = b χ b0 b 1 b 2 (r )f(r )e iq r i=0(q ai e bi ) d 3 0,b 1,b 2 r V 0 2
6 where V 0 denotes the volume of the domain. The structural correlations of water molecules are represented by χ b0 b 1 b 2, which is the angle averaged susceptibility of correlated water molecules at each point on the shell (r = Rr ), and e iq r, which is the phase factor. For the purpose of elucidating the contributions from different length scales, we will here assume that scattering occurs over a monomolecular shell (Fig. S3) taking f(r ) = δ( r Rr ). The results of the integrals in Eq. 2, as reported in Ref. (73), can be expanded as a power series of the radius R of the shell. The power series is given by Γ ~χ {R 2 (g 1 (qr) + g 3 (qr) 3 + O[qR] 7 )} (3) with g i the expansion coefficients. For the present case, qr 1, so the effective susceptibility Γ is mainly determined by the first term in the above expansion. Therefore, the total SH polarization P u0 u 1 u 2 has a cubic dependence on the radius of the shell (i.e., P u0 u 1 u 2 ~R 3 ) for nanometer length scales. The relation between χ b0 b 1 b 2 coordinate transform and the second-order molecular hyperpolarizability β c0 c 1 c 2 is given by a χ b0 b 1 b 2 (r ) = N m T b0 c 0 (r )T b1 c 1 (r )T b2 c 2 (r ) β c0 c 1 c 2 (4) where N m is the number density of water molecules and T b0 c 0 (r )T b1 c 1 (r )T b2 c 2 (r ) is the averaged transformation from the molecular coordinate system (c 0, c 1, c 2 ) to the domain boundary or water shell surface coordinate system (b 0, b 1, b 2 ). The averaged transformation is determined by the orientational distribution function (ODF) of water molecules, and reflects the local structural correlation of molecules. If the water molecules exhibit no structural correlation and thus are completely isotropic, the summation of Eq. 4 equals to zero and no coherent fs-eshs signal is generated. Note that in order to introduce the effective susceptibility we had to assume that the orientation of molecules is correlated with the position of the central ion, but not with the orientation of neighboring molecules. The extent of the structural correlation is described by an average angle between the dipole of a water molecule and the radial direction (assuming azimuthal symmetry). The product T b0 c 0 (r )T b1 c 1 (r )T b2 c 2 (r ) can be calculated as a function of this average angle. The solutions for the expression T b0 c 0 (r )T b1 c 1 (r )T b2 c 2 (r ) can be found in Ref. (77). Changes of the water structure, which
7 result from all possible interactions in the system, are included in this relation. Since we do not have any information on the precise molecular structures, we introduce a relative structural anisotropy parameter η to capture the ion induced structural changes in the nonlinear susceptibility. Our definition of the relative structural anisotropy is η = χ r r r (N m β ccc), where r is the shell normal / radial direction, and c is the direction of the dipole moment of water. Since a nonzero χ b0 b 1 b 2 results from changes in the structural correlation between molecules, η reports on the ion induced water structuring that results in changes in the fs-eshs intensity. The response from third-order single molecule interactions In addition to structural correlations between water molecules, there is also a contribution to the fs-eshs response from a pure third-order electronic single-molecule interaction. Here, the intensity of the scattered light is determined by the third-order hyperpolarizability β (3). Following Ref. (76), the induced SH polarization of a single water molecule at position r is given by p (3) i (r )~β ijkr (r )E DC (r )E j (r )E k (r ) (5) Since E DC (r ) is directed in the radial direction, the rotational average of this induced SH polarization has (3) the same symmetry properties as χ b0 b 1 b 2. A similar quantity χ b0 b 1 b 2 r (3) (arising from β ijkr ) can thus be (3) calculated in the same way as Eq. 4. An effective susceptibility Γ a0 a 1 a 2 r, can be calculated as well if we include the electrostatic field E DC (r ) in the integral of Eq. 2. The β (3) related total SH polarization of water molecules within an extended water domain around an ion is then given by (3) P u0 u 1 u 2 (3) 2 ~ a 0,a 1,a 2 Γ a0 a 1 a 2 r i=0(q ai e ui ) E u1 E u2 (6) which is a function of the volume in which water molecules are contributing to the SH polarization. The effective size of the volume is determined by the strength of the electrostatic field E DC. Combining both second- and third-order effects, the intensity of the scattered SH light of structurally correlated water molecules in a single domain is now given by I corr,u 0 u 1 u 2 = C 2 k 0 4 r2 E 2 u1 E u2 0 (3) 2 a 0,a 1,a 2 (Γ a0 a 1 a 2 + Γ a0 a 1 a 2 r) i=0(q ai e ui ) 2 (7) 2
8 where C is a constant (which depends on the chosen system of units), r 0 is the detector position (in the far field), k 0 is the magnitude of the scattered second harmonic wave vector. The fs-eshs intensity from uncorrelated water molecules in an isotropic liquid, is given by (78) I uncorr,u0 u 1 u 2 = C 2 k 0 4 r2 E 2 u1 E u β u0 u 1 u 2 N m d 3 r (8) V 0 where N m is again the number density of water molecules and the water volume that is associated with one ion. V 0 d 3 r denotes the volume integral over
9 S4. Spherical domain size dependence of the fs-eshs signal To estimate the length scale that contributes most to the relative change in fs-eshs intensity (as plotted in Figs. 1C and 2), we calculate the response of all extended hydration shells in the solution by dividing the intensity of a single ion domain (arising from single molecule responses (Eq. 8) as well as structural correlations and third-order interactions (Eq. 7)) by the intensity from uncorrelated water molecules in the same volume (Eq. 8), i.e. I = I corr + I uncorr. We use the values of Table S1 as input. The domain size (R) I uncorr and the relative structural anisotropy ( ) are adjustable parameters. By adjusting these we can find a (R, ) parameter space which is compatible with the measured intensity change. At very low electrolyte concentrations, we can assume the position of the ions to be largely uncorrelated, so the intensity will increase linearly with the concentration of ions. The change in the total fs-eshs signal can then be taken as the linear sum of the intensities from all ions. At higher electrolyte concentrations (when the intensity curve in Fig. 1C is no longer linear), ions or their domains become correlated. We will discuss the implications of ion correlations in the next section. Figure S3 shows the computational results for dilute electrolyte solutions for four different fs-eshs intensity changes that correspond to four different electrolyte concentrations. We assume for simplicity that only a mono-molecular shell of radius R contributes to the signal, so that the intensity that arises from a single ion can be obtained from the absolute square of Eq. 1. This treatment differs from the description of bulk water in Ref. (44) where entire bulk domains are used. Both methods, however, do not provide any information about the structure of water and serve to assess length scales only. We can now investigate which combinations of domain size and relative anisotropy are compatible with the measurements. The red curve represents the parameter space of (R, ) for which an intensity change of I = 1.05 is calculated, which corresponds to the measured value at a NaCl concentration of 10 M. Such a response can be achieved with a monomolecular shell with a domain radius of 21.8 nm and a relative structural anisotropy of In this case, each water molecule has an additional radial alignment that corresponds to 2.45 % of the electronic anisotropy of a single water molecule. Smaller domain radii but with larger relative anisotropies that lie on the red curve can also lead to I = The orange, green and blue curves represent I for different values that correspond to different NaCl concentrations (50, 100, and 500 M). The horizontal dashed lines indicate the domain radius that corresponds to half the average distance between the ions, here calculated from simple cubic packing of
10 ions. Table S2 summarizes the relevant domain sizes, corresponding number of hydration shells, and anisotropy in case that the domain size is only determined by the average ion separation. fig. S3. I as a function of domain size and relative structural anisotropy. The solid curves represent calculated relative intensities that match the relative intensities for 10, 50, 100 and 500 M NaCl solutions, respectively, as a function of the amount of structural anisotropy (, plotted on the X axis) and domain size (R, Y axis). The dashed lines indicate half of the average separation of ions for each electrolyte concentration. The corresponding relative structural anisotropy that is needed to obtain the measured I is also indicated. Figure S3 shows that if the structural anisotropy matches the electronic anisotropy in magnitude ( = 1), a minimum domain radius of ~5 nm is needed to reach the observed increase of the fs-eshs intensity. Alternatively, from Fig. 1C we see that the ion positions become correlated at ~55 M, which requires a domain radius of nm and a relative anisotropy of , which corresponds to the range of values that are permissible by taking either the domain radius as being determined by the average separation or the relative anisotropy ( = 1) as limiting parameters. Furthermore, the domains become structurally intertwined when the data in Fig. 1C levels off at ~500 M, which requires a maximum radius of 5.9 nm and a structural anisotropy of If the observed effect would be short-range and the increase of signal of electrolyte solutions from 0 to 10 M would originate from a monomolecular shell of ~1 nm size, then a relative anisotropy of ~8.4 x 10 6 would be needed, which is physically unreasonable.
11 The importance of the single molecule third-order contribution can also be estimated. Using the average separation of ions as an indicator for the effective size of the volume for the calculation of the β (3) contribution to the SH polarization, we see that this contribution to the signal is minor: At an electrolyte concentration of 50 M, the pure β (3) contribution to the relative increase of the SH intensity is only %, which is negligible compared to the total increase of 15 %. Table S2: Domain radius, corresponding number of hydration shells, and anisotropy in case the domain size is only determined by the average ion separation. c ( M) R (nm) number of water layers (hydration shells)
12 S5. Ion-induced H-bond correlations at the atomic scale To capture the essentials of the observed behavior and to test the principal hypothesis of this work, namely that collective H-bond interactions are needed to account for the perturbation induced by ions, we explored the orientational correlations obtained from the analysis of force-field molecular dynamics (MD) simulations of aqueous solutions at an electrolyte concentration of 8 mm. Fully atomistic MD simulations were performed for both pure water and an 8 mm aqueous NaCl solution for systems containing ~264,000 water molecules, using the GROMACS package (58) and making extensive use of GPU acceleration. The TIP4P-2005 model (59) was chosen to model water, and the CHARMM36 (60) parameters for the Na + and Cl - ions. The SETTLE algorithm (79) was applied to constrain the bond lengths and angles of the water molecules, allowing an integration time step of 2 fs. Long-range electrostatic interactions were calculated by the Particle-Mesh-Ewald (PME) method (80) with a grid spacing of 0.12 nm. Short-range repulsive and attractive dispersion interactions were described with the Lennard-Jones potentials, using 0.9 nm for the cutoff length. The simulation systems were constructed in the following steps. First, a box was filled with pre-equilibrium water molecules, and several ion pairs were randomly added to the systems to reach the desired concentrations. Second, after steepest descent energy minimization, the systems were equilibrated for 2 ns in the NPT ensemble simulations using the Berendsen thermostat and barostat (49) to reach a target temperature and pressure of 298 K and 1 bar, respectively. The ion concentration was 8 mm (40 ion pairs), the number of water molecules was (0 mm) and (8 mm), and the length of the cubic simulation box was nm. After equilibration, 50 ns long MD simulations were performed in the NVT ensemble. The target temperature of 298 K was kept constant using the canonical velocity rescaling thermostat (81), with a coupling time of 1 ps. The analysis of the water-water correlations (Fig. 2) has been performed over the full duration of the simulations, sampling every 2 ps. The water-water correlations between all pairs of water molecules as a function of distance in pure water (Fig. 2C) was computed by calculating the orientational correlation of each water molecule with all other molecules. The ion-induced changes in the water-water orientational correlations for a NaCl solution with a concentration of 8 mm (Fig. 2D) were computed by calculating the difference in orientational correlations between the ionic solution and the pure water. This can be related to the fs-eshs response as follows: The coherent contribution to the fs-eshs response involves a quadratic distance dependent term, which is written for a pair of molecules as 0 dr sin(qr) qr R 2 dω 1 dω 2 g(r, Ω 1, Ω 2 ) β ijk (Ω1 )β ijk (Ω2 ) (9)
13 see Bersohn et al. (32), Eq. 55. Here, R is the separation between two water molecules (labeled 1 and 2), with orientational angles The orientational correlations that enter the definition of g(r, Ω 1, Ω 2) are contained in the MD simulations. To express clearly the effect of ions on such correlations, we focus only on the angle between the dipoles of a pair of water molecules. Considering that for the experiment sin(qr) qr 1, the contribution to the fs-eshs response (Eq. 9) can be represented by dr R 2 < cos φ > 0 (10) Figure 2D shows the distance dependence of the ion induced change in the fs-eshs response, i.e. it shows the difference in the average orientational distribution with and without 8 mm NaCl, ( cos φ c cos φ 0 ) multiplied by R 2. The MD simulations of Fig. 2 shows that very strong short-range (up to ~1 nm) intrinsic correlations are present in neat liquid water. These correlations become much weaker at longer distances. The effect of the ions (Fig. 2D) is to induce weak but very long-range perturbations in the water-water correlations. It is clear from the figure that the perturbation induced by the presence of the ions extends much further than the first two to three hydration shells, and does not decay to zero within the size of the simulation cell, which is much larger than what is typically employed in similar simulations. The difference in waterwater orientational correlations is representative of the increase in the fs-eshs intensity.
14 S6. fs-eshs from pure H 2O and D 2O Figure S4 shows fs-eshs scattering patterns of bulk H 2O and D 2O. It can be seen that the scattering patterns are isotropic for the S outs ins in polarization combination and peaked towards the forward direction in the P outp inp in polarization combination. The straight lines represent a calculation of the fs-eshs response assuming that only the incoherent electronic single molecule response gives rise to fs-eshs signal. According to this assumption, the curves should obey the following equations (Ref. (32), Eq. 50, only the first term) 2 I SSS = A [β YYY] (11) 2 I PPP = A [β XXX] cos 2 2 θ + A [β ZXX] sin 2 θ (12) in which A is the scaling constant, θ is the scattering angle as defined in Fig. 1B, and β IJK is the effective hyperpolarizability in the laboratory frame coordinates (Fig. S2). represents an orientational and 2 temporal average over a large number of molecules. [β YYY] 2 = [βxxx] = [x (3.20 x C 3 m 3 J -2 ) 2 2 ] and [β ZXX] = 33.2 [x (3.20 x C 3 m 3 J -2 ) 2 ], for isolated water molecules in an isotropic environment. These values are calculated based on the hyperpolarizability values given in Table S1 and in combination with the response for molecules of the C 2v point group (Appendix B of Ref. (32)). Based on this ideal gas model we should not expect a difference between H 2O and D 2O. Furthermore, the shape of the data would follow the calculated lines. The S outs ins in data can be seen to follow the trend of the predicted line shape by Eq. 11, while the P outp inp in data does not follow the predicted line shape of Eq. 12. Additionally, the D 2O response is significantly larger than the H 2O response (e.g. 40 % bigger for P outp inp in at = 90 ; or 20 % for S outs ins in). In addition, the intensity ratio of S outs ins in / P outp inp in at the same scattering angle is 7.8 ± 0.3 (H 2O), and 6.9 ± 0.3 (D 2O), whereas a purely incoherent ideal gas response would have a ratio of 8.5 for both H 2O and D 2O (combining the values and formulas of Refs. (32, 71)). The above deviations (magnitude of the polarization ratios, deviations in the scattering pattern and the isotope effect) suggest that structural correlations/collective H-bond interactions play a significant role here. Only by including these interactions we can account for the differences between the data and the ideal gas model. Incorporating collective H-bond interactions in the explanation, a direct relationship between H-bond properties and fs-eshs response is expected: As D 2O has stronger H-bonds than H 2O
15 there is more orientational order, which translates into an increasing fs-eshs response. In addition, in agreement with the observation, the polarization ratios for D 2O are expected to deviate further from ideal gas behavior than those of H 2O. fig. S4. fs-eshs patterns for pure H2O and pure D2O. Both the SoutSinSin (circles, red for H2O and blue for D2O) and the PoutPinPin fs-eshs patterns (squares, red for H2O and blue for D2O) are plotted. The straight lines correspond to an incoherent averaged single molecule response. It can be seen that such a response does not fully capture the shape of the data. Note that the computed single molecule responses are here scaled to coincide with the data at a scattering angle of = 90.
16 S7. Mean-field models Here we obtain an analytical expression for the intensity of scattered SH light using mean-field models that describe water as a dipolar gas that interacts with ions. Water-water correlations are here neglected, and the electrolyte solution is modeled as a gas of non-interacting dipoles, for which the statistical mechanics is well known. The structural correlations of water molecules are here solely determined by the interaction potential that each water molecule has with a single ion, and are described by an ionic form factor (F), while the ion-ion correlations are later included by means of a structure factor (S). The derivation borrows concepts from different studies (76, 82-85). From the analysis we find that a meanfield model does not capture the essence of the experimental observations. As can be seen from the below analysis (S7.2 S7.4, Fig. S5) we find that: (1). The outcome of a mean field model critically depends on the shape of the chosen interaction potential and specifically on how it is decaying at nanoscopic distances. Values for the saturated intensity deviate by a factor of Models can either under or over represent the found intensities, and the half saturation concentration and the saturated intensity cannot be predicted simultaneously for either H 2O or D 2O solutions. (3). Differences between H 2O and D 2O electrolyte solutions cannot be accounted for. (4). Temperature dependent measurements do not agree to the predicted (1/T) trend by the mean field theory. S7.1. Ordering of dipoles around a single central ion Considering first the water molecules surrounding a single monovalent ion, and referring to the P u 0 u 1 u 2 term, inserting Eq. 2 and 4 into Eq. 1 and using the fact that T bi c i = (e bi e ci ) gives the average polarization P u 0 u 1 u 2 = N m (e b0 e c0 (r ))(e b1 e c1 (r ))(e b2 e c2 (r )) T β c 0 c 1 c 2 e iq r d 3 r (13)
17 where the average is weighted by a Boltzmann factor. Next, we take a Boltzmann average of the nonobservable quantity P u 0 u 1 u 2 because we are assuming that water molecules, while correlated by the ion, are not orientationally correlated with each other. The energy of a dipole at r is μ E(r ) = μe(r ) cos ψ, where ψ is the angle between the dipole and the electric field E(r ), and μ is the static dipole moment. The ensemble-average can be written as an average over all orientations (e b0 e c0 (r )) (e b1 e c1 (r )) (e b2 e c2 (r )) T = (e b0 e c0 (r ))(e b1 e c1 (r ))(e b2 e c2 (r ))e +μ E(r )/k B T (14) which, expanded to first order, is (with the constant term giving an average over three direction cosines and thus zero average) μe(r ) k B T (e b 0 e c0 (r ))(e b1 e c1 (r ))(e b2 e c2 (r )) cos ψ (15) Carrying out the averages, and taking the sum in Eq. 13 gives P u 0 u 1 u 2 = N m μβ 3k B T E(r ) r e u 0 r e iq r d 3 r (16) with β = δ u0 u 1 β + (1 δu0 u 1 )β (17) where β and β are defined by Ref. (32), and c is the molecular dipole axis. A similar analysis can be carried out for the third-order hyperpolarizability β (3) to give P (3) u 0 u 1 u 2 = N m β (3) E(r ) r e u 0 e iq r d 3 r (18) r with β (3) = δ u0 u 1 β (3) + (1 δu0 u 1 )β (3). The final expression for the intensity of second harmonic generated light due to ion-dipole correlations around a single ion is thus
18 I corr,u0 u 1 u 2 = C 2 k 0 4 E r 2 u 2 0 E 2 u1 N 2 2 m μβ + 0 3k B T β(3) E(r ) r e u 0 r e iq r d 3 2 r (19) where E(r ) stands for the radial electric field originating from the ion. We will consider various possible expressions for E(r ) at a later stage. In the experiment the relative change in fs-eshs intensity, ΔI = I solution I solvent, is reported. Note that this I is the same quantity as in S4 and can be found from Eq. 19. For a solution with ionic number density ρ, it becomes ΔI(q, ρ) = Kρ F(q, ρ) 2 S(q, ρ) (20) In this expression, K is a constant depending on the experimental setup and the molecular properties of pure water. For the P outp inp in polarization combination it reads K = ( μβ 3k B T )2 ρ S f(0) 2 Z 2 e 2 ε 2 β2 ZXX (21) where μ is the dipole moment of a water molecule in gas phase, ρ S is the solvent number density, f(0) is the Lorentz field factor, and β 2 β2 ZXX = 2.8 (see Ref.(71)). F(q, ρ), is the ionic form factor, i.e., the amplitude scattered by each ion that can be written as the Fourier transform of the electric field. Contrary to what is usually seen in other scattering experiments, F(q, ρ) can have a pronounced concentration dependence, which is related to the decay of E(r ) as we will discuss in the following paragraphs. Finally, S(q, ρ) is the structure factor, which accounts for the correlations between the ions, and for liquids can be written in terms of the Fourier transform of the radial distribution function. S7.2. Different mean field models Next we describe the scattered intensity I (Eq. 20) as a function of for different scenarios involving different choices for the form and structure factors. For the form factor we have compared three possibilities with three slightly different but very similar electrostatic potentials.
19 1. A dielectrically but otherwise unscreened Coulomb potential V(r), and corresponding form factor F c V(r) = Ze 4πε 0 εr (22) F C = 1/q (23) 2. The same Coulombic potential that is truncated at the position where the next ionic domain starts (estimating this mean domain radius from a simple-cubic packing of ions). We obtain for the form factor (F TC) F TC (q, ρ) = sin(qr) qr q 2 R, R(ρ) = 1 3 (24) 2 2ρ 3. An exponentially-screened potential as obtained from Debye-Hückel theory and the corresponding form factor F D-H V(r) = e κ D (ρ)r Ze 4πε 0 εr F DH (q, ρ) = (25) q q 2 +κ D (ρ) 2 (26) where κ D (ρ) 2 = 2Ι(ρ)e2 stands for the Debye screening parameter and Ι(ρ) is the ionic strength. ε 0 εk B T These form factors can be compared with different structure factors. At very low concentrations (when the ions are not correlated in position), the contributions from individual ions can reasonably be represented by summing them incoherently. The structure factor takes the form: S(q, ρ) 1. We refer to this structure factor as S 1. I at different concentrations would then be determined solely by the ionic form factor. At intermediate and larger concentrations, the distribution of ions will not anymore be random, and ion-ion correlations exist. Both positively and negatively charged ions contribute to the signal, and so one has to combine the structure factors of same-sign and opposite-sign species. If one calculates the appropriate combination within Debye-Hückel theory, one obtains for the structure factor (86) S DH (q, ρ) = q 2 q 2 +κ D (ρ) 2 (27)
20 Monovalent electrolytes are used in the calculation as a representative, for which Ι(ρ) = ρz 2. S7.3. Experimental data vs mean field models: dependence on ionic strength and temperature Figure S5A shows incoherent combinations (S(q, ρ) = S I = 1) of the different form factors F C (green curve) and F DH (red curve). The computed incoherent response for a truncated potential (F T) does not generate a I bigger than 10-3 and is omitted from the graph. If neither screening nor ion correlations are included (F C+S 1, green curve), the intensity increases linearly with concentration. Clearly this overshoots the experimental data (black lines). On the other hand, if one considers the Coulomb field to be abruptly truncated at half the mean ion separation (F TC+S 1, not shown) the predicted signal is orders of magnitude smaller than what is observed. A Debye-Hückel screened potential agrees somewhat with an increasing signal at low concentration (F DH+S 1, red curve), and has a maximum that agrees with the 50 % saturation point of the H 2O data (indicated by the black vertical line), but not that of D 2O. At higher ionic strengths, I returns to the level of the neat water intensity without saturating. Thus, even though the electrostatic potential of the ions (V(r)) are almost indistinguishable up to several tens of nanometers away from the ion, the three implemented potentials result in curves for I that differ by orders of magnitude. Already for the very low ionic strengths, where ion-ion correlations are absent, the curves are remarkably different. Thus, due to the long-range nature of fs-eshs experiments, minute changes in the long-range screening of the potential result in dramatic changes of the predicted signal. Including ion-ion correlations, by combining F DH with S DH (grey curve) or F C with S DH (blue curve) we find that big differences between those two models remain: The grey curve is seen to reach a maximum, while the blue curve saturates. Interestingly the blue curve, a combination of an unscreened Coulombic potential and a Debye-Hückel structure factor, captures the shape of the experimental data. This combination, which corresponds to the linear superposition of the effect of ions on dipolar water molecules with correlated (ionic) positions, is arguably the most consistent application of Debye-Hückel theory, and we will focus on it in what follows. The 50 % saturation point (45 M) is close to that found for H 2O, but not for the one found for D 2O (310 M). The saturated value for I is reached at 2.8, which is an over representation of the experimental data (1.3 / 1.09 for H 2O / D 2O, respectively). The ionic strength at the point of 50 % saturation ( 50) and the saturation intensity ( I s) are given by the following expressions
21 ρ 50 = q2 ε 0 εk B T 2(Ze) 2 (28) I s = ( μβ 3k B T )2 ρ S f(0) 2 k B TZ 2 e 2 2ε 0 ε β2 ZXX (29) It can be seen that 50 does not contain any parameters that would generate a different curve for H 2O and D 2O. Similarly I s does not contain parameters that allow for a downward adjustment by a factor of 6 (H 2O) or 20 (D 2O), in order for it to agree to the experimental result. fig. S5. Mean-field models and temperature dependence. (A) fs-eshs response relative to that of pure water computed for a solution of monovalent ions in water. Plotted are the combination of a form factor arising from a Coulombic potential (FC) with a structure factor of uncorrelated ions (S1, green line), a form factor arising from a Debye-Hückel potential (FDH) with a structure factor of uncorrelated ions (S1, red line), a form factor arising from a Debye-Hückel potential (FDH) with a Debye-Hückel structure factor of correlated ions (SDH, grey line), and a form factor arising from a Coulombic potential (FC) with a Debye- Hückel structure factor of correlated ions (SDH, blue line). The black lines indicate the data for NaCl in H2O and D2O, and the vertical lines mark the ionic strengths of half saturation. (B) Temperature dependence of the saturated fs-eshs intensity. fs- ESHS intensities were recorded for a 10 mm NaCl solution in H2O at different temperatures (red markers, left axis). The computed saturation value obtained from the combination of FC and SDH is plotted as a black line (right axis). The inset shows both curves on the same scale. Both expressions do allow for a further check in terms of the broader validity of the model. By performing temperature dependent measurements, we can check the validity of Eq. 29, and hence determine how well this particular mean field model describes the experimental findings. Figure S5B shows the measured intensity of saturation I s for a 10 mm NaCl solution in H 2O (red data points, left axis). The computed value for I s is plotted as well (black line, right axis). The inset shows both traces on
22 the same scale. It can be seen that the experimental data does not change much in magnitude in the temperature range from 4 C to 50 C. There is a slight reduction in I s at both high and low temperatures. The mean-field model predicts a decrease in I s going from low to high temperatures. It therefore seems that the combination of an F C form factor and an S DH structure factor are not well suited to predict the trends that are experimentally observed. Thus, several approaches for mean-field models that treat the water as an ideal gas that interacts with a cloud of ions using electrostatic potentials that differ only marginally have been considered. We observe that the computed fs-eshs signal of a monovalent electrolyte solution relative to that of pure water varies from a negligible contribution to the fs-eshs response to a fs-eshs response twice that of pure water. The models do not allow for the prediction of a difference between H 2O and D 2O, nor can they capture the temperature dependence. Since small differences in the interaction potentials lead to large differences in the fs-eshs response, it is necessary to consider the fs-eshs response using a molecular dynamics simulation, which contains not only the ion-dipole interactions, but also other water-water interactions. These interactions may modify the interaction potentials and lead to additional cooperative effects that are not included in any of the mean field models considered here. To capture also the observed difference between light water and heavy water, more sophisticated models (that are still to be developed) are needed.
23 S8. Surface tension measurements and interpretation Measurement. The Wilhelmy plate method (55) uses a vertically suspended Pt plate with a perimeter l, which is pulled out of the electrolyte solution towards air. The force (F) acting on this plate is correlated to the surface tension by the following equation γ = F l cos (φ) (30) where φ is the contact angle between Pt and water, which has a value φ = 0 (87). The measurement accuracy of the method is 0.1 % (55). Interpretation. The surface tension ( ) can be defined as the change in the free energy when the surface area is increased by a unit area (55). For an air (a) / water (w) interface it can be expressed by γ = 1 2 W w W a W wa (31) where W w and W a are the reversible work needed to separate two unit areas of two surfaces from contact with each other to infinite spacing in vacuum. W wa refers to the work of adhesion due to the interfacial interaction energy per unit area of the air and water. Since molecules in an ideal gas are non-interacting, W a 0. W wa 0 as well because of the low concentration of gas molecules compared to the concentration of water molecules (~a factor of 1000 lower). In Fig. 3C we measured the change in the surface tension ( = solution - water) as a function of electrolyte concentration, which can be expressed as γ c = 1 2 W w c + W a c W wa c = 1 2 W w c (33) since W a 0 and W wa 0, W a c = W wa c = 0.
Generalized expressions for hyper-rayleigh scattering from isotropic liquids
 Generalized expressions for hyper-rayleigh scattering from isotropic liquids Yixing Chen Sylvie Roke # Laboratory for fundamental BioPhotonics (LBP) Institute of Bio-engineering (IBI) and Institute of
Generalized expressions for hyper-rayleigh scattering from isotropic liquids Yixing Chen Sylvie Roke # Laboratory for fundamental BioPhotonics (LBP) Institute of Bio-engineering (IBI) and Institute of
Mean-Field Theory of Water-Water Correlations in Electrolyte Solutions
 Mean-Field Theory of Water-Water Correlations in Electrolyte Solutions David M. Wilkins, 1, 2, a) David E. Manolopoulos, 3 Sylvie Roke, 2 and Michele Ceriotti 1 1) Laboratory of Computational Science and
Mean-Field Theory of Water-Water Correlations in Electrolyte Solutions David M. Wilkins, 1, 2, a) David E. Manolopoulos, 3 Sylvie Roke, 2 and Michele Ceriotti 1 1) Laboratory of Computational Science and
The change in free energy on transferring an ion from a medium of low dielectric constantε1 to one of high dielectric constant ε2:
 The Born Energy of an Ion The free energy density of an electric field E arising from a charge is ½(ε 0 ε E 2 ) per unit volume Integrating the energy density of an ion over all of space = Born energy:
The Born Energy of an Ion The free energy density of an electric field E arising from a charge is ½(ε 0 ε E 2 ) per unit volume Integrating the energy density of an ion over all of space = Born energy:
2.1 Experimental and theoretical studies
 Chapter 2 NiO As stated before, the first-row transition-metal oxides are among the most interesting series of materials, exhibiting wide variations in physical properties related to electronic structure.
Chapter 2 NiO As stated before, the first-row transition-metal oxides are among the most interesting series of materials, exhibiting wide variations in physical properties related to electronic structure.
Supplementary Figure 1. Additional SFG data. Comparison of an SFS spectrum of water droplets in a hydrophobic liquid (black line, 1%v.
 Supplementary Figure 1. Additional SFG data. Comparison of an SFS spectrum of water droplets in a hydrophobic liquid (black line, 1%v. D 2O in 5 mm Span80 in d 34-hexadecane) with SFG reflection spectra
Supplementary Figure 1. Additional SFG data. Comparison of an SFS spectrum of water droplets in a hydrophobic liquid (black line, 1%v. D 2O in 5 mm Span80 in d 34-hexadecane) with SFG reflection spectra
1. Electricity and Magnetism (Fall 1995, Part 1) A metal sphere has a radius R and a charge Q.
 1. Electricity and Magnetism (Fall 1995, Part 1) A metal sphere has a radius R and a charge Q. (a) Compute the electric part of the Maxwell stress tensor T ij (r) = 1 {E i E j 12 } 4π E2 δ ij both inside
1. Electricity and Magnetism (Fall 1995, Part 1) A metal sphere has a radius R and a charge Q. (a) Compute the electric part of the Maxwell stress tensor T ij (r) = 1 {E i E j 12 } 4π E2 δ ij both inside
1. Poisson-Boltzmann 1.1. Poisson equation. We consider the Laplacian. which is given in spherical coordinates by (2)
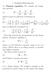 1. Poisson-Boltzmann 1.1. Poisson equation. We consider the Laplacian operator (1) 2 = 2 x + 2 2 y + 2 2 z 2 which is given in spherical coordinates by (2) 2 = 1 ( r 2 ) + 1 r 2 r r r 2 sin θ θ and in
1. Poisson-Boltzmann 1.1. Poisson equation. We consider the Laplacian operator (1) 2 = 2 x + 2 2 y + 2 2 z 2 which is given in spherical coordinates by (2) 2 = 1 ( r 2 ) + 1 r 2 r r r 2 sin θ θ and in
Supplementary Information for: Controlling Cellular Uptake of Nanoparticles with ph-sensitive Polymers
 Supplementary Information for: Controlling Cellular Uptake of Nanoparticles with ph-sensitive Polymers Hong-ming Ding 1 & Yu-qiang Ma 1,2, 1 National Laboratory of Solid State Microstructures and Department
Supplementary Information for: Controlling Cellular Uptake of Nanoparticles with ph-sensitive Polymers Hong-ming Ding 1 & Yu-qiang Ma 1,2, 1 National Laboratory of Solid State Microstructures and Department
Computer simulation methods (2) Dr. Vania Calandrini
 Computer simulation methods (2) Dr. Vania Calandrini in the previous lecture: time average versus ensemble average MC versus MD simulations equipartition theorem (=> computing T) virial theorem (=> computing
Computer simulation methods (2) Dr. Vania Calandrini in the previous lecture: time average versus ensemble average MC versus MD simulations equipartition theorem (=> computing T) virial theorem (=> computing
Module 4 : Third order nonlinear optical processes. Lecture 28 : Inelastic Scattering Processes. Objectives
 Module 4 : Third order nonlinear optical processes Lecture 28 : Inelastic Scattering Processes Objectives In this lecture you will learn the following Light scattering- elastic and inelastic-processes,
Module 4 : Third order nonlinear optical processes Lecture 28 : Inelastic Scattering Processes Objectives In this lecture you will learn the following Light scattering- elastic and inelastic-processes,
The micro-properties of [hmpy+] [Tf 2 N-] Ionic liquid: a simulation. study. 1. Introduction
![The micro-properties of [hmpy+] [Tf 2 N-] Ionic liquid: a simulation. study. 1. Introduction The micro-properties of [hmpy+] [Tf 2 N-] Ionic liquid: a simulation. study. 1. Introduction](/thumbs/72/66941683.jpg) ISBN 978-1-84626-081-0 Proceedings of the 2010 International Conference on Application of Mathematics and Physics Volume 1: Advances on Space Weather, Meteorology and Applied Physics Nanjing, P. R. China,
ISBN 978-1-84626-081-0 Proceedings of the 2010 International Conference on Application of Mathematics and Physics Volume 1: Advances on Space Weather, Meteorology and Applied Physics Nanjing, P. R. China,
Optical Imaging Chapter 5 Light Scattering
 Optical Imaging Chapter 5 Light Scattering Gabriel Popescu University of Illinois at Urbana-Champaign Beckman Institute Quantitative Light Imaging Laboratory http://light.ece.uiuc.edu Principles of Optical
Optical Imaging Chapter 5 Light Scattering Gabriel Popescu University of Illinois at Urbana-Champaign Beckman Institute Quantitative Light Imaging Laboratory http://light.ece.uiuc.edu Principles of Optical
Lecture 3. E α (ω) exp( iωt) dω = F 1 [E α ](t), (1 )
, (1 ) Lecture 3. E α (ω) exp( iωt) dω = F 1 [E α ](t), (1 )](/thumbs/88/116554615.jpg) Nonlinear Optics 5A551 (200) Lecture notes Lecture Susceptibility tensors in the frequency domain The susceptibility tensors in the frequency domain arise when the electric field E α (t) of the light is
Nonlinear Optics 5A551 (200) Lecture notes Lecture Susceptibility tensors in the frequency domain The susceptibility tensors in the frequency domain arise when the electric field E α (t) of the light is
Notes: Most of the material presented in this chapter is taken from Jackson, Chap. 2, 3, and 4, and Di Bartolo, Chap. 2. 2π nx i a. ( ) = G n.
 Chapter. Electrostatic II Notes: Most of the material presented in this chapter is taken from Jackson, Chap.,, and 4, and Di Bartolo, Chap... Mathematical Considerations.. The Fourier series and the Fourier
Chapter. Electrostatic II Notes: Most of the material presented in this chapter is taken from Jackson, Chap.,, and 4, and Di Bartolo, Chap... Mathematical Considerations.. The Fourier series and the Fourier
Peptide folding in non-aqueous environments investigated with molecular dynamics simulations Soto Becerra, Patricia
 University of Groningen Peptide folding in non-aqueous environments investigated with molecular dynamics simulations Soto Becerra, Patricia IMPORTANT NOTE: You are advised to consult the publisher's version
University of Groningen Peptide folding in non-aqueous environments investigated with molecular dynamics simulations Soto Becerra, Patricia IMPORTANT NOTE: You are advised to consult the publisher's version
Coulombs Law and Electric Field Intensity
 Coulombs Law and Electric Field Intensity 2.1 Coulomb s law Coulomb stated that the force between two very small objects separated in a vacuum or free space by a distance, which is large compared to their
Coulombs Law and Electric Field Intensity 2.1 Coulomb s law Coulomb stated that the force between two very small objects separated in a vacuum or free space by a distance, which is large compared to their
Microscopic Structure and Dynamics of Air/Water Interface by Computer Simulations and Comparison with Sum-Frequency Generation Experiments
 107 Chapter 5 Microscopic Structure and Dynamics of Air/Water Interface by Computer Simulations and Comparison with Sum-Frequency Generation Experiments The hydrogen (H) bonded structure of water at the
107 Chapter 5 Microscopic Structure and Dynamics of Air/Water Interface by Computer Simulations and Comparison with Sum-Frequency Generation Experiments The hydrogen (H) bonded structure of water at the
SUPPLEMENTARY INFORMATION
 doi:1.138/nature1878 I. Experimental setup OPA, DFG Ti:Sa Oscillator, Amplifier PD U DC U Analyzer HV Energy analyzer MCP PS CCD Polarizer UHV Figure S1: Experimental setup used in mid infrared photoemission
doi:1.138/nature1878 I. Experimental setup OPA, DFG Ti:Sa Oscillator, Amplifier PD U DC U Analyzer HV Energy analyzer MCP PS CCD Polarizer UHV Figure S1: Experimental setup used in mid infrared photoemission
Introduction to molecular dynamics
 1 Introduction to molecular dynamics Yves Lansac Université François Rabelais, Tours, France Visiting MSE, GIST for the summer Molecular Simulation 2 Molecular simulation is a computational experiment.
1 Introduction to molecular dynamics Yves Lansac Université François Rabelais, Tours, France Visiting MSE, GIST for the summer Molecular Simulation 2 Molecular simulation is a computational experiment.
Electromagnetic Field Theory (EMT)
 Electromagnetic Field Theory (EMT) Lecture # 9 1) Coulomb s Law and Field Intensity 2) Electric Fields Due to Continuous Charge Distributions Line Charge Surface Charge Volume Charge Coulomb's Law Coulomb's
Electromagnetic Field Theory (EMT) Lecture # 9 1) Coulomb s Law and Field Intensity 2) Electric Fields Due to Continuous Charge Distributions Line Charge Surface Charge Volume Charge Coulomb's Law Coulomb's
Theoretische Physik 2: Elektrodynamik (Prof. A-S. Smith) Home assignment 9
 WiSe 202 20.2.202 Prof. Dr. A-S. Smith Dipl.-Phys. Ellen Fischermeier Dipl.-Phys. Matthias Saba am Lehrstuhl für Theoretische Physik I Department für Physik Friedrich-Alexander-Universität Erlangen-Nürnberg
WiSe 202 20.2.202 Prof. Dr. A-S. Smith Dipl.-Phys. Ellen Fischermeier Dipl.-Phys. Matthias Saba am Lehrstuhl für Theoretische Physik I Department für Physik Friedrich-Alexander-Universität Erlangen-Nürnberg
Why Proteins Fold? (Parts of this presentation are based on work of Ashok Kolaskar) CS490B: Introduction to Bioinformatics Mar.
 Why Proteins Fold? (Parts of this presentation are based on work of Ashok Kolaskar) CS490B: Introduction to Bioinformatics Mar. 25, 2002 Molecular Dynamics: Introduction At physiological conditions, the
Why Proteins Fold? (Parts of this presentation are based on work of Ashok Kolaskar) CS490B: Introduction to Bioinformatics Mar. 25, 2002 Molecular Dynamics: Introduction At physiological conditions, the
Notes on x-ray scattering - M. Le Tacon, B. Keimer (06/2015)
 Notes on x-ray scattering - M. Le Tacon, B. Keimer (06/2015) Interaction of x-ray with matter: - Photoelectric absorption - Elastic (coherent) scattering (Thomson Scattering) - Inelastic (incoherent) scattering
Notes on x-ray scattering - M. Le Tacon, B. Keimer (06/2015) Interaction of x-ray with matter: - Photoelectric absorption - Elastic (coherent) scattering (Thomson Scattering) - Inelastic (incoherent) scattering
J10M.1 - Rod on a Rail (M93M.2)
 Part I - Mechanics J10M.1 - Rod on a Rail (M93M.2) J10M.1 - Rod on a Rail (M93M.2) s α l θ g z x A uniform rod of length l and mass m moves in the x-z plane. One end of the rod is suspended from a straight
Part I - Mechanics J10M.1 - Rod on a Rail (M93M.2) J10M.1 - Rod on a Rail (M93M.2) s α l θ g z x A uniform rod of length l and mass m moves in the x-z plane. One end of the rod is suspended from a straight
Non-bonded interactions
 speeding up the number-crunching Marcus Elstner and Tomáš Kubař May 8, 2015 why care? key to understand biomolecular structure and function binding of a ligand efficiency of a reaction color of a chromophore
speeding up the number-crunching Marcus Elstner and Tomáš Kubař May 8, 2015 why care? key to understand biomolecular structure and function binding of a ligand efficiency of a reaction color of a chromophore
Chap. 7. Dielectric Materials and Insulation
 Chap. 7. Dielectric Materials and Insulation - The parallel plate capacitor with free space as an insulator: - The electric dipole moment for a pair of opposite changes +Q and -Q separated by a finite
Chap. 7. Dielectric Materials and Insulation - The parallel plate capacitor with free space as an insulator: - The electric dipole moment for a pair of opposite changes +Q and -Q separated by a finite
Supplementary Figure S1 Definition of the wave vector components: Parallel and perpendicular wave vector of the exciton and of the emitted photons.
 Supplementary Figure S1 Definition of the wave vector components: Parallel and perpendicular wave vector of the exciton and of the emitted photons. Supplementary Figure S2 The calculated temperature dependence
Supplementary Figure S1 Definition of the wave vector components: Parallel and perpendicular wave vector of the exciton and of the emitted photons. Supplementary Figure S2 The calculated temperature dependence
The Scattering of Light by Small Particles. Advanced Laboratory, Physics 407 University of Wisconsin Madison, Wisconsin 53706
 (4/28/09) The Scattering of Light by Small Particles Advanced Laboratory, Physics 407 University of Wisconsin Madison, Wisconsin 53706 Abstract In this experiment we study the scattering of light from
(4/28/09) The Scattering of Light by Small Particles Advanced Laboratory, Physics 407 University of Wisconsin Madison, Wisconsin 53706 Abstract In this experiment we study the scattering of light from
Electromagnetic Theory I
 Electromagnetic Theory I Final Examination 18 December 2009, 12:30-2:30 pm Instructions: Answer the following 10 questions, each of which is worth 10 points. Explain your reasoning in each case. Use SI
Electromagnetic Theory I Final Examination 18 December 2009, 12:30-2:30 pm Instructions: Answer the following 10 questions, each of which is worth 10 points. Explain your reasoning in each case. Use SI
Supporting Information for the manuscript "The. interfacial tension of nanoscopic oil droplets in. water is hardly affected by SDS surfactant"
 for the manuscript "The interfacial tension of nanoscopic oil droplets in water is hardly affected by SDS surfactant" Hilton B. de Aguiar, Alex G. F. de Beer, Matthew L. Strader, and Sylvie Roke Max-Planck
for the manuscript "The interfacial tension of nanoscopic oil droplets in water is hardly affected by SDS surfactant" Hilton B. de Aguiar, Alex G. F. de Beer, Matthew L. Strader, and Sylvie Roke Max-Planck
Department of Physics and Astronomy PhD Qualifying Examination (Part I) Spring 2015
 University of Louisville College of Arts and Sciences Department of Physics and Astronomy PhD Qualifying Examination (Part I) Spring 215 Paper E Contemporary Physics Time allowed 4 minutes each section
University of Louisville College of Arts and Sciences Department of Physics and Astronomy PhD Qualifying Examination (Part I) Spring 215 Paper E Contemporary Physics Time allowed 4 minutes each section
What is Classical Molecular Dynamics?
 What is Classical Molecular Dynamics? Simulation of explicit particles (atoms, ions,... ) Particles interact via relatively simple analytical potential functions Newton s equations of motion are integrated
What is Classical Molecular Dynamics? Simulation of explicit particles (atoms, ions,... ) Particles interact via relatively simple analytical potential functions Newton s equations of motion are integrated
Downloaded from UvA-DARE, the institutional repository of the University of Amsterdam (UvA)
 Downloaded from UvA-DARE, the institutional repository of the University of Amsterdam (UvA) http://dare.uva.nl/document/351205 File ID 351205 Filename 5: Vibrational dynamics of the bending mode of water
Downloaded from UvA-DARE, the institutional repository of the University of Amsterdam (UvA) http://dare.uva.nl/document/351205 File ID 351205 Filename 5: Vibrational dynamics of the bending mode of water
Non-bonded interactions
 speeding up the number-crunching continued Marcus Elstner and Tomáš Kubař December 3, 2013 why care? number of individual pair-wise interactions bonded interactions proportional to N: O(N) non-bonded interactions
speeding up the number-crunching continued Marcus Elstner and Tomáš Kubař December 3, 2013 why care? number of individual pair-wise interactions bonded interactions proportional to N: O(N) non-bonded interactions
Analysis of second-harmonic generation microscopy under refractive index mismatch
 Vol 16 No 11, November 27 c 27 Chin. Phys. Soc. 19-1963/27/16(11/3285-5 Chinese Physics and IOP Publishing Ltd Analysis of second-harmonic generation microscopy under refractive index mismatch Wang Xiang-Hui(
Vol 16 No 11, November 27 c 27 Chin. Phys. Soc. 19-1963/27/16(11/3285-5 Chinese Physics and IOP Publishing Ltd Analysis of second-harmonic generation microscopy under refractive index mismatch Wang Xiang-Hui(
Neighbor Tables Long-Range Potentials
 Neighbor Tables Long-Range Potentials Today we learn how we can handle long range potentials. Neighbor tables Long-range potential Ewald sums MSE485/PHY466/CSE485 1 Periodic distances Minimum Image Convention:
Neighbor Tables Long-Range Potentials Today we learn how we can handle long range potentials. Neighbor tables Long-range potential Ewald sums MSE485/PHY466/CSE485 1 Periodic distances Minimum Image Convention:
6. LIGHT SCATTERING 6.1 The first Born approximation
 6. LIGHT SCATTERING 6.1 The first Born approximation In many situations, light interacts with inhomogeneous systems, in which case the generic light-matter interaction process is referred to as scattering
6. LIGHT SCATTERING 6.1 The first Born approximation In many situations, light interacts with inhomogeneous systems, in which case the generic light-matter interaction process is referred to as scattering
Advanced Molecular Molecular Dynamics
 Advanced Molecular Molecular Dynamics Technical details May 12, 2014 Integration of harmonic oscillator r m period = 2 k k and the temperature T determine the sampling of x (here T is related with v 0
Advanced Molecular Molecular Dynamics Technical details May 12, 2014 Integration of harmonic oscillator r m period = 2 k k and the temperature T determine the sampling of x (here T is related with v 0
lim = F F = F x x + F y y + F z
 Physics 361 Summary of Results from Lecture Physics 361 Derivatives of Scalar and Vector Fields The gradient of a scalar field f( r) is given by g = f. coordinates f g = ê x x + ê f y y + ê f z z Expressed
Physics 361 Summary of Results from Lecture Physics 361 Derivatives of Scalar and Vector Fields The gradient of a scalar field f( r) is given by g = f. coordinates f g = ê x x + ê f y y + ê f z z Expressed
Supplementary Figure 1 Schematics of an optical pulse in a nonlinear medium. A Gaussian optical pulse propagates along z-axis in a nonlinear medium
 Supplementary Figure 1 Schematics of an optical pulse in a nonlinear medium. A Gaussian optical pulse propagates along z-axis in a nonlinear medium with thickness L. Supplementary Figure Measurement of
Supplementary Figure 1 Schematics of an optical pulse in a nonlinear medium. A Gaussian optical pulse propagates along z-axis in a nonlinear medium with thickness L. Supplementary Figure Measurement of
Elastic Scattering. R = m 1r 1 + m 2 r 2 m 1 + m 2. is the center of mass which is known to move with a constant velocity (see previous lectures):
 Elastic Scattering In this section we will consider a problem of scattering of two particles obeying Newtonian mechanics. The problem of scattering can be viewed as a truncated version of dynamic problem
Elastic Scattering In this section we will consider a problem of scattering of two particles obeying Newtonian mechanics. The problem of scattering can be viewed as a truncated version of dynamic problem
Gas-liquid phase separation in oppositely charged colloids: stability and interfacial tension
 7 Gas-liquid phase separation in oppositely charged colloids: stability and interfacial tension We study the phase behaviour and the interfacial tension of the screened Coulomb (Yukawa) restricted primitive
7 Gas-liquid phase separation in oppositely charged colloids: stability and interfacial tension We study the phase behaviour and the interfacial tension of the screened Coulomb (Yukawa) restricted primitive
MANIPAL INSTITUTE OF TECHNOLOGY
 SCHEME OF EVAUATION MANIPA INSTITUTE OF TECHNOOGY MANIPA UNIVERSITY, MANIPA SECOND SEMESTER B.Tech. END-SEMESTER EXAMINATION - MAY SUBJECT: ENGINEERING PHYSICS (PHY/) Time: 3 Hrs. Max. Marks: 5 Note: Answer
SCHEME OF EVAUATION MANIPA INSTITUTE OF TECHNOOGY MANIPA UNIVERSITY, MANIPA SECOND SEMESTER B.Tech. END-SEMESTER EXAMINATION - MAY SUBJECT: ENGINEERING PHYSICS (PHY/) Time: 3 Hrs. Max. Marks: 5 Note: Answer
CE 530 Molecular Simulation
 1 CE 530 Molecular Simulation Lecture 14 Molecular Models David A. Kofke Department of Chemical Engineering SUNY Buffalo kofke@eng.buffalo.edu 2 Review Monte Carlo ensemble averaging, no dynamics easy
1 CE 530 Molecular Simulation Lecture 14 Molecular Models David A. Kofke Department of Chemical Engineering SUNY Buffalo kofke@eng.buffalo.edu 2 Review Monte Carlo ensemble averaging, no dynamics easy
Lecture 8 Notes, Electromagnetic Theory II Dr. Christopher S. Baird, faculty.uml.edu/cbaird University of Massachusetts Lowell
 Lecture 8 Notes, Electromagnetic Theory II Dr. Christopher S. Baird, faculty.uml.edu/cbaird University of Massachusetts Lowell 1. Scattering Introduction - Consider a localized object that contains charges
Lecture 8 Notes, Electromagnetic Theory II Dr. Christopher S. Baird, faculty.uml.edu/cbaird University of Massachusetts Lowell 1. Scattering Introduction - Consider a localized object that contains charges
The effect of surface dipoles and of the field generated by a polarization gradient on the repulsive force
 Journal of Colloid and Interface Science 263 (2003) 156 161 www.elsevier.com/locate/jcis The effect of surface dipoles and of the field generated by a polarization gradient on the repulsive force Haohao
Journal of Colloid and Interface Science 263 (2003) 156 161 www.elsevier.com/locate/jcis The effect of surface dipoles and of the field generated by a polarization gradient on the repulsive force Haohao
Molecular alignment, wavepacket interference and Isotope separation
 Molecular alignment, wavepacket interference and Isotope separation Sharly Fleischer, Ilya Averbukh and Yehiam Prior Chemical Physics, Weizmann Institute Yehiam.prior@weizmann.ac.il Frisno-8, Ein Bokek,
Molecular alignment, wavepacket interference and Isotope separation Sharly Fleischer, Ilya Averbukh and Yehiam Prior Chemical Physics, Weizmann Institute Yehiam.prior@weizmann.ac.il Frisno-8, Ein Bokek,
Supporting information
 Electronic Supplementary Material ESI for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2018 Supporting information Understanding three-body contributions to coarse-grained force
Electronic Supplementary Material ESI for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2018 Supporting information Understanding three-body contributions to coarse-grained force
Supplementary Figure S1: Numerical PSD simulation. Example numerical simulation of the power spectral density, S(f) from a trapped particle
 Supplementary Figure S1: Numerical PSD simulation. Example numerical simulation of the power spectral density, S(f) from a trapped particle oscillating at Ω 0 /(2π) = f xy = 600Hz and subject to a periodic
Supplementary Figure S1: Numerical PSD simulation. Example numerical simulation of the power spectral density, S(f) from a trapped particle oscillating at Ω 0 /(2π) = f xy = 600Hz and subject to a periodic
Review. Spring Semester /21/14. Physics for Scientists & Engineers 2 1
 Review Spring Semester 2014 Physics for Scientists & Engineers 2 1 Notes! Homework set 13 extended to Tuesday, 4/22! Remember to fill out SIRS form: https://sirsonline.msu.edu Physics for Scientists &
Review Spring Semester 2014 Physics for Scientists & Engineers 2 1 Notes! Homework set 13 extended to Tuesday, 4/22! Remember to fill out SIRS form: https://sirsonline.msu.edu Physics for Scientists &
Phys 122 Lecture 3 G. Rybka
 Phys 122 Lecture 3 G. Rybka A few more Demos Electric Field Lines Example Calculations: Discrete: Electric Dipole Overview Continuous: Infinite Line of Charge Next week Labs and Tutorials begin Electric
Phys 122 Lecture 3 G. Rybka A few more Demos Electric Field Lines Example Calculations: Discrete: Electric Dipole Overview Continuous: Infinite Line of Charge Next week Labs and Tutorials begin Electric
Lecture 6 Scattering theory Partial Wave Analysis. SS2011: Introduction to Nuclear and Particle Physics, Part 2 2
 Lecture 6 Scattering theory Partial Wave Analysis SS2011: Introduction to Nuclear and Particle Physics, Part 2 2 1 The Born approximation for the differential cross section is valid if the interaction
Lecture 6 Scattering theory Partial Wave Analysis SS2011: Introduction to Nuclear and Particle Physics, Part 2 2 1 The Born approximation for the differential cross section is valid if the interaction
Simulations of the Infrared, Raman, and 2D-IR Photon Echo Spectra of Water in Nanoscale Silica Pores Paul C. Burris, 1 Damien Laage, 2, a) 1, b)
 Simulations of the Infrared, Raman, and 2D-IR Photon Echo Spectra of Water in Nanoscale Silica Pores Paul C. Burris, 1 Damien Laage, 2, a) 1, b) and Ward H. Thompson 1) Department of Chemistry, University
Simulations of the Infrared, Raman, and 2D-IR Photon Echo Spectra of Water in Nanoscale Silica Pores Paul C. Burris, 1 Damien Laage, 2, a) 1, b) and Ward H. Thompson 1) Department of Chemistry, University
Supplementary Information to. Longitudinal domain wall formation in elongated assemblies of ferromagnetic nanoparticles.
 Supplementary Information to Longitudinal domain wall formation in elongated assemblies of ferromagnetic nanoparticles authored by Miriam Varón, Marco Beleggia, Jelena Jordanovic, Jakob Schiøtz, Takeshi
Supplementary Information to Longitudinal domain wall formation in elongated assemblies of ferromagnetic nanoparticles authored by Miriam Varón, Marco Beleggia, Jelena Jordanovic, Jakob Schiøtz, Takeshi
Molecular Dynamics Simulations. Dr. Noelia Faginas Lago Dipartimento di Chimica,Biologia e Biotecnologie Università di Perugia
 Molecular Dynamics Simulations Dr. Noelia Faginas Lago Dipartimento di Chimica,Biologia e Biotecnologie Università di Perugia 1 An Introduction to Molecular Dynamics Simulations Macroscopic properties
Molecular Dynamics Simulations Dr. Noelia Faginas Lago Dipartimento di Chimica,Biologia e Biotecnologie Università di Perugia 1 An Introduction to Molecular Dynamics Simulations Macroscopic properties
 A Nobel Prize for Molecular Dynamics and QM/MM What is Classical Molecular Dynamics? Simulation of explicit particles (atoms, ions,... ) Particles interact via relatively simple analytical potential
A Nobel Prize for Molecular Dynamics and QM/MM What is Classical Molecular Dynamics? Simulation of explicit particles (atoms, ions,... ) Particles interact via relatively simple analytical potential
Supporting information for Metal-semiconductor. nanoparticle hybrids formed by self-organization: a platform to address exciton-plasmon coupling
 Supporting information for Metal-semiconductor nanoparticle hybrids formed by self-organization: a platform to address exciton-plasmon coupling Christian Strelow, T. Sverre Theuerholz, Christian Schmidtke,
Supporting information for Metal-semiconductor nanoparticle hybrids formed by self-organization: a platform to address exciton-plasmon coupling Christian Strelow, T. Sverre Theuerholz, Christian Schmidtke,
Far-field radiation pattern in Coherent Anti-stokes Raman Scattering (CARS) Microscopy.
 Far-field radiation pattern in Coherent Anti-stokes Raman Scattering (CARS) Microscopy. David Gachet, Nicolas Sandeau, Hervé Rigneault * Institut Fresnel, Mosaic team, Domaine Univ. St Jérôme, 13397 Marseille
Far-field radiation pattern in Coherent Anti-stokes Raman Scattering (CARS) Microscopy. David Gachet, Nicolas Sandeau, Hervé Rigneault * Institut Fresnel, Mosaic team, Domaine Univ. St Jérôme, 13397 Marseille
 Title Super- and subcritical hydration of Thermodynamics of hydration Author(s) Matubayasi, N; Nakahara, M Citation JOURNAL OF CHEMICAL PHYSICS (2000), 8109 Issue Date 2000-05-08 URL http://hdl.handle.net/2433/50350
Title Super- and subcritical hydration of Thermodynamics of hydration Author(s) Matubayasi, N; Nakahara, M Citation JOURNAL OF CHEMICAL PHYSICS (2000), 8109 Issue Date 2000-05-08 URL http://hdl.handle.net/2433/50350
Supplementary Figure 1: ADF images and profiles for several types of atomic chains encapsulated in DWNTs. (a d) ADF images of NaI, CsF, CsCl, and CsI
 Supplementary Figure 1: ADF images and profiles for several types of atomic chains encapsulated in DWNTs. (a d) ADF images of NaI, CsF, CsCl, and CsI atomic chains encapsulated in DWNTs, respectively.
Supplementary Figure 1: ADF images and profiles for several types of atomic chains encapsulated in DWNTs. (a d) ADF images of NaI, CsF, CsCl, and CsI atomic chains encapsulated in DWNTs, respectively.
Supporting Information: Ultraintense. femtosecond magnetic nanoprobes induced by. azimuthally polarized laser beams
 Supporting Information: Ultraintense femtosecond magnetic nanoprobes induced by azimuthally polarized laser beams Manuel Blanco, Ferran Cambronero, M. Teresa Flores-Arias, Enrique Conejero Jarque, Luis
Supporting Information: Ultraintense femtosecond magnetic nanoprobes induced by azimuthally polarized laser beams Manuel Blanco, Ferran Cambronero, M. Teresa Flores-Arias, Enrique Conejero Jarque, Luis
Research Statement. Shenggao Zhou. November 3, 2014
 Shenggao Zhou November 3, My research focuses on: () Scientific computing and numerical analysis (numerical PDEs, numerical optimization, computational fluid dynamics, and level-set method for interface
Shenggao Zhou November 3, My research focuses on: () Scientific computing and numerical analysis (numerical PDEs, numerical optimization, computational fluid dynamics, and level-set method for interface
Electrophoretic Light Scattering Overview
 Electrophoretic Light Scattering Overview When an electric field is applied across an electrolytic solution, charged particles suspended in the electrolyte are attracted towards the electrode of opposite
Electrophoretic Light Scattering Overview When an electric field is applied across an electrolytic solution, charged particles suspended in the electrolyte are attracted towards the electrode of opposite
In Situ Imaging of Cold Atomic Gases
 In Situ Imaging of Cold Atomic Gases J. D. Crossno Abstract: In general, the complex atomic susceptibility, that dictates both the amplitude and phase modulation imparted by an atom on a probing monochromatic
In Situ Imaging of Cold Atomic Gases J. D. Crossno Abstract: In general, the complex atomic susceptibility, that dictates both the amplitude and phase modulation imparted by an atom on a probing monochromatic
Interaction X-rays - Matter
 Interaction X-rays - Matter Pair production hν > M ev Photoelectric absorption hν MATTER hν Transmission X-rays hν' < hν Scattering hν Decay processes hν f Compton Thomson Fluorescence Auger electrons
Interaction X-rays - Matter Pair production hν > M ev Photoelectric absorption hν MATTER hν Transmission X-rays hν' < hν Scattering hν Decay processes hν f Compton Thomson Fluorescence Auger electrons
B 2 P 2, which implies that g B should be
 Enhanced Summary of G.P. Agrawal Nonlinear Fiber Optics (3rd ed) Chapter 9 on SBS Stimulated Brillouin scattering is a nonlinear three-wave interaction between a forward-going laser pump beam P, a forward-going
Enhanced Summary of G.P. Agrawal Nonlinear Fiber Optics (3rd ed) Chapter 9 on SBS Stimulated Brillouin scattering is a nonlinear three-wave interaction between a forward-going laser pump beam P, a forward-going
Phys 622 Problems Chapter 6
 1 Problem 1 Elastic scattering Phys 622 Problems Chapter 6 A heavy scatterer interacts with a fast electron with a potential V (r) = V e r/r. (a) Find the differential cross section dσ dω = f(θ) 2 in the
1 Problem 1 Elastic scattering Phys 622 Problems Chapter 6 A heavy scatterer interacts with a fast electron with a potential V (r) = V e r/r. (a) Find the differential cross section dσ dω = f(θ) 2 in the
PHYSICS PART II SECTION- I. Straight objective Type
 PHYSICS PAT II SECTION- I Straight objective Tpe This section contains 9 multiple choice questions numbered to 1. Each question has choices,, (C) and, out of which ONLY ONE is correct.. A parallel plate
PHYSICS PAT II SECTION- I Straight objective Tpe This section contains 9 multiple choice questions numbered to 1. Each question has choices,, (C) and, out of which ONLY ONE is correct.. A parallel plate
Unit Cell-Level Thickness Control of Single-Crystalline Zinc Oxide Nanosheets Enabled by Electrical Double Layer Confinement
 Unit Cell-Level Thickness Control of Single-Crystalline Zinc Oxide Nanosheets Enabled by Electrical Double Layer Confinement Xin Yin, Yeqi Shi, Yanbing Wei, Yongho Joo, Padma Gopalan, Izabela Szlufarska,
Unit Cell-Level Thickness Control of Single-Crystalline Zinc Oxide Nanosheets Enabled by Electrical Double Layer Confinement Xin Yin, Yeqi Shi, Yanbing Wei, Yongho Joo, Padma Gopalan, Izabela Szlufarska,
M04M.1 Particles on a Line
 Part I Mechanics M04M.1 Particles on a Line M04M.1 Particles on a Line Two elastic spherical particles with masses m and M (m M) are constrained to move along a straight line with an elastically reflecting
Part I Mechanics M04M.1 Particles on a Line M04M.1 Particles on a Line Two elastic spherical particles with masses m and M (m M) are constrained to move along a straight line with an elastically reflecting
2 Structure. 2.1 Coulomb interactions
 2 Structure 2.1 Coulomb interactions While the information needed for reproduction of living systems is chiefly maintained in the sequence of macromolecules, any practical use of this information must
2 Structure 2.1 Coulomb interactions While the information needed for reproduction of living systems is chiefly maintained in the sequence of macromolecules, any practical use of this information must
Dielectric Polarization, Bound Charges, and the Electric Displacement Field
 Dielectric Polarization, Bound Charges, and the Electric Displacement Field Any kind of matter is full of positive and negative electric charges. In a, these charges are bound they cannot move separately
Dielectric Polarization, Bound Charges, and the Electric Displacement Field Any kind of matter is full of positive and negative electric charges. In a, these charges are bound they cannot move separately
Interaction of particles with matter - 2. Silvia Masciocchi, GSI and University of Heidelberg SS2017, Heidelberg May 3, 2017
 Interaction of particles with matter - 2 Silvia Masciocchi, GSI and University of Heidelberg SS2017, Heidelberg May 3, 2017 Energy loss by ionization (by heavy particles) Interaction of electrons with
Interaction of particles with matter - 2 Silvia Masciocchi, GSI and University of Heidelberg SS2017, Heidelberg May 3, 2017 Energy loss by ionization (by heavy particles) Interaction of electrons with
Structural characterization. Part 1
 Structural characterization Part 1 Experimental methods X-ray diffraction Electron diffraction Neutron diffraction Light diffraction EXAFS-Extended X- ray absorption fine structure XANES-X-ray absorption
Structural characterization Part 1 Experimental methods X-ray diffraction Electron diffraction Neutron diffraction Light diffraction EXAFS-Extended X- ray absorption fine structure XANES-X-ray absorption
Scattering of Electromagnetic Radiation. References:
 Scattering of Electromagnetic Radiation References: Plasma Diagnostics: Chapter by Kunze Methods of experimental physics, 9a, chapter by Alan Desilva and George Goldenbaum, Edited by Loveberg and Griem.
Scattering of Electromagnetic Radiation References: Plasma Diagnostics: Chapter by Kunze Methods of experimental physics, 9a, chapter by Alan Desilva and George Goldenbaum, Edited by Loveberg and Griem.
OPTI 511L Fall A. Demonstrate frequency doubling of a YAG laser (1064 nm -> 532 nm).
 R.J. Jones Optical Sciences OPTI 511L Fall 2017 Experiment 3: Second Harmonic Generation (SHG) (1 week lab) In this experiment we produce 0.53 µm (green) light by frequency doubling of a 1.06 µm (infrared)
R.J. Jones Optical Sciences OPTI 511L Fall 2017 Experiment 3: Second Harmonic Generation (SHG) (1 week lab) In this experiment we produce 0.53 µm (green) light by frequency doubling of a 1.06 µm (infrared)
Measurement of electric potential fields
 Measurement of electric potential fields Matthew Krupcale, Oliver Ernst Department of Physics, Case Western Reserve University, Cleveland Ohio, 44106-7079 18 November 2012 Abstract In electrostatics, Laplace
Measurement of electric potential fields Matthew Krupcale, Oliver Ernst Department of Physics, Case Western Reserve University, Cleveland Ohio, 44106-7079 18 November 2012 Abstract In electrostatics, Laplace
Lecture 4. Diffusing photons and superradiance in cold gases
 Lecture 4 Diffusing photons and superradiance in cold gases Model of disorder-elastic mean free path and group velocity. Dicke states- Super- and sub-radiance. Scattering properties of Dicke states. Multiple
Lecture 4 Diffusing photons and superradiance in cold gases Model of disorder-elastic mean free path and group velocity. Dicke states- Super- and sub-radiance. Scattering properties of Dicke states. Multiple
(a) Write down the total Hamiltonian of this system, including the spin degree of freedom of the electron, but neglecting spin-orbit interactions.
 1. Quantum Mechanics (Spring 2007) Consider a hydrogen atom in a weak uniform magnetic field B = Bê z. (a) Write down the total Hamiltonian of this system, including the spin degree of freedom of the electron,
1. Quantum Mechanics (Spring 2007) Consider a hydrogen atom in a weak uniform magnetic field B = Bê z. (a) Write down the total Hamiltonian of this system, including the spin degree of freedom of the electron,
Scientific Computing II
 Scientific Computing II Molecular Dynamics Simulation Michael Bader SCCS Summer Term 2015 Molecular Dynamics Simulation, Summer Term 2015 1 Continuum Mechanics for Fluid Mechanics? Molecular Dynamics the
Scientific Computing II Molecular Dynamics Simulation Michael Bader SCCS Summer Term 2015 Molecular Dynamics Simulation, Summer Term 2015 1 Continuum Mechanics for Fluid Mechanics? Molecular Dynamics the
Physics 208 Exam 1 Oct. 3, 2007
 1 Name: Student ID: Section #: Physics 208 Exam 1 Oct. 3, 2007 Print your name and section clearly above. If you do not know your section number, write your TA s name. Your final answer must be placed
1 Name: Student ID: Section #: Physics 208 Exam 1 Oct. 3, 2007 Print your name and section clearly above. If you do not know your section number, write your TA s name. Your final answer must be placed
All-optical generation of surface plasmons in graphene
 All-optical generation of surface plasmons in graphene T. J. Constant, 1, S. M. Hornett, 1 D. E. Chang, 2, and E. Hendry 1 1 Electromagnetic Materials Group, Department of Physics, College of Engineering,
All-optical generation of surface plasmons in graphene T. J. Constant, 1, S. M. Hornett, 1 D. E. Chang, 2, and E. Hendry 1 1 Electromagnetic Materials Group, Department of Physics, College of Engineering,
Main Notation Used in This Book
 Main Notation Used in This Book z Direction normal to the surface x,y Directions in the plane of the surface Used to describe a component parallel to the interface plane xoz Plane of incidence j Label
Main Notation Used in This Book z Direction normal to the surface x,y Directions in the plane of the surface Used to describe a component parallel to the interface plane xoz Plane of incidence j Label
Problem 1: Spin 1 2. particles (10 points)
 Problem 1: Spin 1 particles 1 points 1 Consider a system made up of spin 1/ particles. If one measures the spin of the particles, one can only measure spin up or spin down. The general spin state of a
Problem 1: Spin 1 particles 1 points 1 Consider a system made up of spin 1/ particles. If one measures the spin of the particles, one can only measure spin up or spin down. The general spin state of a
Hands-on : Model Potential Molecular Dynamics
 Hands-on : Model Potential Molecular Dynamics OUTLINE 0. DL_POLY code introduction 0.a Input files 1. THF solvent molecule 1.a Geometry optimization 1.b NVE/NVT dynamics 2. Liquid THF 2.a Equilibration
Hands-on : Model Potential Molecular Dynamics OUTLINE 0. DL_POLY code introduction 0.a Input files 1. THF solvent molecule 1.a Geometry optimization 1.b NVE/NVT dynamics 2. Liquid THF 2.a Equilibration
Advanced Optical Communications Prof. R. K. Shevgaonkar Department of Electrical Engineering Indian Institute of Technology, Bombay
 Advanced Optical Communications Prof. R. K. Shevgaonkar Department of Electrical Engineering Indian Institute of Technology, Bombay Lecture No. # 15 Laser - I In the last lecture, we discussed various
Advanced Optical Communications Prof. R. K. Shevgaonkar Department of Electrical Engineering Indian Institute of Technology, Bombay Lecture No. # 15 Laser - I In the last lecture, we discussed various
Dielectrics - III. Lecture 22: Electromagnetic Theory. Professor D. K. Ghosh, Physics Department, I.I.T., Bombay
 Dielectrics - III Lecture 22: Electromagnetic Theory Professor D. K. Ghosh, Physics Department, I.I.T., Bombay We continue with our discussion of dielectric medium. Example : Dielectric Sphere in a uniform
Dielectrics - III Lecture 22: Electromagnetic Theory Professor D. K. Ghosh, Physics Department, I.I.T., Bombay We continue with our discussion of dielectric medium. Example : Dielectric Sphere in a uniform
Notes on excitation of an atom or molecule by an electromagnetic wave field. F. Lanni / 11feb'12 / rev9sept'14
 Notes on excitation of an atom or molecule by an electromagnetic wave field. F. Lanni / 11feb'12 / rev9sept'14 Because the wavelength of light (400-700nm) is much greater than the diameter of an atom (0.07-0.35
Notes on excitation of an atom or molecule by an electromagnetic wave field. F. Lanni / 11feb'12 / rev9sept'14 Because the wavelength of light (400-700nm) is much greater than the diameter of an atom (0.07-0.35
Molecular Dynamics Simulation of a Nanoconfined Water Film
 Molecular Dynamics Simulation of a Nanoconfined Water Film Kyle Lindquist, Shu-Han Chao May 7, 2013 1 Introduction The behavior of water confined in nano-scale environment is of interest in many applications.
Molecular Dynamics Simulation of a Nanoconfined Water Film Kyle Lindquist, Shu-Han Chao May 7, 2013 1 Introduction The behavior of water confined in nano-scale environment is of interest in many applications.
Dave S. Walker and Geraldine L. Richmond*
 J. Phys. Chem. C 2007, 111, 8321-8330 8321 Understanding the Effects of Hydrogen Bonding at the Vapor-Water Interface: Vibrational Sum Frequency Spectroscopy of H 2 O/HOD/D 2 O Mixtures Studied Using Molecular
J. Phys. Chem. C 2007, 111, 8321-8330 8321 Understanding the Effects of Hydrogen Bonding at the Vapor-Water Interface: Vibrational Sum Frequency Spectroscopy of H 2 O/HOD/D 2 O Mixtures Studied Using Molecular
MARTINI simulation details
 S1 Appendix MARTINI simulation details MARTINI simulation initialization and equilibration In this section, we describe the initialization of simulations from Main Text section Residue-based coarsegrained
S1 Appendix MARTINI simulation details MARTINI simulation initialization and equilibration In this section, we describe the initialization of simulations from Main Text section Residue-based coarsegrained
Electricity & Magnetism Study Questions for the Spring 2018 Department Exam December 4, 2017
 Electricity & Magnetism Study Questions for the Spring 2018 Department Exam December 4, 2017 1. a. Find the capacitance of a spherical capacitor with inner radius l i and outer radius l 0 filled with dielectric
Electricity & Magnetism Study Questions for the Spring 2018 Department Exam December 4, 2017 1. a. Find the capacitance of a spherical capacitor with inner radius l i and outer radius l 0 filled with dielectric
Supplementary Materials
 Supplementary Materials Sample characterization The presence of Si-QDs is established by Transmission Electron Microscopy (TEM), by which the average QD diameter of d QD 2.2 ± 0.5 nm has been determined
Supplementary Materials Sample characterization The presence of Si-QDs is established by Transmission Electron Microscopy (TEM), by which the average QD diameter of d QD 2.2 ± 0.5 nm has been determined
24/ Rayleigh and Raman scattering. Stokes and anti-stokes lines. Rotational Raman spectroscopy. Polarizability ellipsoid. Selection rules.
 Subject Chemistry Paper No and Title Module No and Title Module Tag 8/ Physical Spectroscopy 24/ Rayleigh and Raman scattering. Stokes and anti-stokes lines. Rotational Raman spectroscopy. Polarizability
Subject Chemistry Paper No and Title Module No and Title Module Tag 8/ Physical Spectroscopy 24/ Rayleigh and Raman scattering. Stokes and anti-stokes lines. Rotational Raman spectroscopy. Polarizability
MOLECULAR SPECTROSCOPY
 MOLECULAR SPECTROSCOPY First Edition Jeanne L. McHale University of Idaho PRENTICE HALL, Upper Saddle River, New Jersey 07458 CONTENTS PREFACE xiii 1 INTRODUCTION AND REVIEW 1 1.1 Historical Perspective
MOLECULAR SPECTROSCOPY First Edition Jeanne L. McHale University of Idaho PRENTICE HALL, Upper Saddle River, New Jersey 07458 CONTENTS PREFACE xiii 1 INTRODUCTION AND REVIEW 1 1.1 Historical Perspective
Supplementary Figure 1. Schematics of light transmission and reflection from a slab confined between
 Supplementary Figures: Supplementary Figure. Schematics of light transmission and reflection from a slab confined between two infinite media. Supplementary Figure. Reflectivity of a magneto-electric slab
Supplementary Figures: Supplementary Figure. Schematics of light transmission and reflection from a slab confined between two infinite media. Supplementary Figure. Reflectivity of a magneto-electric slab
Supporting Information
 Supporting Information Improved Working Model for Interpreting the Excitation Wavelength- and Fluence-Dependent Response in Pulsed aser-induced Size Reduction of Aqueous Gold Nanoparticles Daniel Werner
Supporting Information Improved Working Model for Interpreting the Excitation Wavelength- and Fluence-Dependent Response in Pulsed aser-induced Size Reduction of Aqueous Gold Nanoparticles Daniel Werner
Dielectrics. Lecture 20: Electromagnetic Theory. Professor D. K. Ghosh, Physics Department, I.I.T., Bombay
 What are dielectrics? Dielectrics Lecture 20: Electromagnetic Theory Professor D. K. Ghosh, Physics Department, I.I.T., Bombay So far we have been discussing electrostatics in either vacuum or in a conductor.
What are dielectrics? Dielectrics Lecture 20: Electromagnetic Theory Professor D. K. Ghosh, Physics Department, I.I.T., Bombay So far we have been discussing electrostatics in either vacuum or in a conductor.
Lecture 06. Fundamentals of Lidar Remote Sensing (4) Physical Processes in Lidar
 Lecture 06. Fundamentals of Lidar Remote Sensing (4) Physical Processes in Lidar Light interactions with objects (continued) Resonance fluorescence Laser induced fluorescence Doppler effect (Doppler shift
Lecture 06. Fundamentals of Lidar Remote Sensing (4) Physical Processes in Lidar Light interactions with objects (continued) Resonance fluorescence Laser induced fluorescence Doppler effect (Doppler shift
Fundamentals on light scattering, absorption and thermal radiation, and its relation to the vector radiative transfer equation
 Fundamentals on light scattering, absorption and thermal radiation, and its relation to the vector radiative transfer equation Klaus Jockers November 11, 2014 Max-Planck-Institut für Sonnensystemforschung
Fundamentals on light scattering, absorption and thermal radiation, and its relation to the vector radiative transfer equation Klaus Jockers November 11, 2014 Max-Planck-Institut für Sonnensystemforschung
