12052 Pigeonite Basalt 1866 grams
|
|
|
- Martina Malone
- 5 years ago
- Views:
Transcription
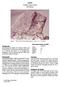
1 12052 Pigeonite Basalt 1866 grams Revised Figure 1: Photo of broken side of showing vugs. Sample is 6.5 cm high. NASA # S Summary of Age Data for Ar/Ar Rb/Sr Nyquist 1977 (recalculated) Murthy et al ± 0.18 b.y. Compston et al ± 0.19 (3.22 ± 0.19)
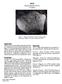
2 Figure 2a: Reflected light photo of thin section 12052,6. Scale about 3 cm. NASA #S Introduction is a porphyritic pigeonite basalt about 3.2 b.y old. It was rounded and covered with zap pits on all sides, before it was broken (figures 1 and 9). Petrography Bence et al. (1970) reported that is composed of euhedral and skeletal phenocrysts of olivine and clinopyroxene (pigeonite cores with augite rims) surrounded by a groundmass including high-iron clinopyroxene, chrome spinel, ilmenite, pyroxferroite and anorthite. Bence et al. (1971) described the rock as porphyritic with variolitic groundmass (figures 2 Mineralogical Mode for Neal et Papike et Champness al al et al Olivine Pyroxene Plagioclase Opaques Ilmenite 3.5 Chromite +Usp 2.5 mesostasis 3 silica trace Figure 2b: Transmitted light photo of thin section 12052,6. NASA #S and 3). Champness et al. (1971) explain that this rock consists of elongated phenocrysts of pyroxene (3-4 mm) in random orientation, together with skeletal or rounded olivine crystals (1 mm) in a finer-grained variolitic groundmass of pyroxene-plagioclase and opaques minerals, chiefly ilmenites, which are parallel to the pyroxene laths. Mineralogy Olivine: Champness et al. (1971) report olivine is Fo Pyroxene: Bence et al. (1970, 1971) studied the complex zoning patterns in the large pyroxenes in Pigeonite cores have sharp boundaries with augite rims (figure 4). Pyroxene Figure 3,a,b: Photomicrographs of thin section 12052,7 showing zoned pyroxene phenocrysts in cross section and variolitic groundmass. Scale is about 2.5 mm across. NASA #S
3
4 Bence et al Di Hd sample/ chondrite 1 En Fs Fo compiled by C Meyer Figure 4: Bence et al. (1970, 1971) determined the composition of pyroxene in rim and groundmass pyroxene is increasingly Fe-rich. Papike et al. (1971) and Takeda (1971) reported crystallographic data and discussed epitaxy and exsolution of pyroxenes in Champness et al. (1971) used high-voltage TEM to study fine structure (exsolution). Fa 0.1 TiO La Pr Sm Gd Dy Er Yb Ce Nd Eu Tb Ho Tm Lu Figure 5: Normalized rare-earth-element diagram for (data from Wanke et al. connected). Lunar Basalts A11 A17 Plagioclase: Plagioclase crystals in are narrow (55 microns) (Baldridge et al. 1979). 4 2 A A12 Ilmenite: Ilmenite in occurs as lath-shaped crystals up to 400 micron long and 50 micron wide, typically found intergrown with plumose plagioclasepyroxene aggregates of the groundmass (Gibb et al. 1970) MgO Figure 6: Composition of compared with that of other lunar basalts. Figure 7: Rb/Sr isochron diagram for (from Compston et al. 1971). Figure 8: Rb/Sr isochron for (from Murthy et al 1971).
5 Table 1a. Chemical composition of reference Maxwell71 Kushiro71 LSPET70 Murthy71 O Kelly71 Wanke71 Schnetzler71 Compston71 weight 1 g 201 g 204 mg SiO2 % (f) 42 (c ) 47.3 (a) (g) TiO (a) 3.35 (g) Al2O (a) 9.95 (g) FeO (a) 20.7 (g) MnO (a) 0.28 (g) MgO (a) 8.07 (g) CaO (a) (g) Na2O (a) 0.26 (g) K2O (d) (e) (a) (d) (g) P2O (g) S % 0.07 (g) sum Sc ppm 52.3 (b) (a) V 160 (b) (g) Cr 3675 (b) (a) 3140 (g) Co 42 (b) (a) 34 (g) Ni 23 (b) (a) 6 (g) Cu 16 (b) 39 (a) 8 (g) Zn 9 (g) Ga 3.9 (a) 2.1 (g) Ge ppb 60 (a) As (a) Se Rb (d) 1.26 (d) 1.22 (g) 1.26 (d) Sr 85 (b) (d) 110 (a) 116 (d) (g) (d) Y 48 (b) (g) Zr 150 (b) (g) Nb 7 (g) Mo Ru Rh Pd ppb Ag ppb Cd ppb In ppb 7.8 (a) Sn ppb Sb ppb Te ppb Cs ppm Ba (d) 70 (a) 75 (d) 70 (g) La 7.4 (a) 6.52 (a) 5 (g) Ce 20 (a) 21 (a) 18.8 (d) 12 (g) Pr Nd 19.5 (a) 14.7 (d) Sm 5.8 (a) 4.5 (a) 4.91 (d) Eu 1.01 (a) 1.08 (a) 1.04 (d) Gd 7.1 (a) 6.87 (d) Tb 1.62 (a) 1.35 (a) Dy 5.3 (a) 7.44 (a) 7.74 (d) Ho 1.34 (a) Er 4.55 (d) Tm 0.72 (a) Yb 4.5 (a) 3.7 (a) 4.32 (d) Lu 0.64 (a) 0.58 (a) (d) Hf 3.1 (a) 3.8 (a) Ta 0.77 (a) 0.44 (a) W ppb 150 (a) Re ppb Os ppb Ir ppb 3.6 (a) Pt ppb Au ppb 0.9 (a) Th ppm 1.03 (e) 1.28 (a) U ppm 0.27 (e) (a) technique: (a) INAA, (b) OES, (c ), (d) IDMS, (e) radiation couting, (f) conventional wet, (g) XRF, (h) SSMS
6 Table 1b. Chemical composition of reference Taylor71 Tatsumoto71 weight SiO2 % TiO2 Al2O3 FeO MnO MgO CaO Na2O K2O P2O5 S % sum Sc ppm 54 (h) V 160 (h) Cr 3300 (h) Co 42 (h) Ni 21 (h) Cu 5 (h) Zn Ga Ge ppb As Se Rb Sr 110 (h) Y 48 (h) Zr 130 (h) Nb 6 (h) Mo 0.03 (h) Ru Rh Pd ppb Ag ppb Cd ppb In ppb Sn ppb 0.16 (h) Sb ppb Te ppb Cs ppm 0.03 (h) Ba 65 (h) La 6.3 (h) Ce 21 (h) Pr 2.6 (h) Nd 17 (h) Sm 6.3 (h) Eu 0.9 (h) Gd 9 (h) Tb 2 (h) Dy 9.6 (h) Ho 2.7 (h) Er 7.2 (h) Tm 1.1 (h) Yb 5.3 (h) Lu Hf 4.6 (h) Ta W ppb Re ppb Os ppb Ir ppb Pt ppb Au ppb Th ppm 1.15 (h) (d) U ppm 0.3 (h) (d) technique: (a) INAA, (b) OES, (c ), (d) IDMS, (e) radiation couting, (f) conventional wet, (g) XRF, (h) SSMS Chromite-ulvöspinel: Gibb et al. (1970) determined the composition of Al-chromite cores and Ti-ulvöspinel rims. Iron: Champness et al. (1971) report about 2 % Ni and 1.5 % Co in metallic iron grains. Chemistry The major element composition was determined by Maxwell and Wiik (1971), Kushiro et al. (1971) and Compston et al. (1971). Trace elements were determined by Taylor et al. (1971), Wanke et al. (1971) and Schnetzler et al. (1971)(figure 5). Radiogenic age dating Murthy et al. (1971) determined a Rb-Sr mineral isochron with age 2.92 ± 0.18 b.y. (figure 8) and Compston et al. (1971) determined 3.28 ± 0.19 b.y. (figure 7). Cosmogenic isotopes and exposure ages Burnett et al. (1975) determined an exposure age of 230 ± 40 m.y. by 81 Kr/ 83 Kr and Marti and Lugmair (1971) determined 129 ± 7 m.y by 81 Kr/ 83 Kr. Hintenberger et al. (1971) also determined exposure ages for using 3 He (120 m.y.), 21 Ne (140 m.y.) and 38 Ar (130 m.y.). Other Studies Bogard et al. (1971) and Hintenberger et al. (1971) reported the content and isotopic composition of rare gases in Processing A large amount of was used for the biopool sample. List of Photo #s for S S S ,1 S ,2 S TS color S TS color S TS color S mug color S sawing S saw cut
7 Figure 9: Photo of broken surface of 12052,1 showing pyroxene crystals in vugs. NASA #S Scale 1 cm. C Meyer g,1,3 PB,2,11 C,12 B,13 A1,14 A2,15,19 12 g,36 E,29 D,26 10 g,18,4,9 TS, g Bio pool, g W1 End, g
8
9
10
11
12
13 References for Baldridge W.S., Beaty D.W., Hill S.M.R. and Albee A.L. (1979) The petrology of the Apollo 12 pigeonite basalt suite. Proc. 10 th Lunar Planet. Sci. Conf Bence A.E., Papike J.J. and Prewitt C.T. (1970) Apollo 12 clinopyroxene chemical trends. Earth Planet. Sci. Lett. 8, Bence A.E., Papike J.J. and Lindsley D.H. (1971) Crystallization histories of clinopyroxenes in two porphyritic rocks from Oceanus Procellarum. Proc. 2 nd Lunar Sci. Conf Bence A.E. and Papike J.J. (1972) Pyroxenes as recorders of lunar basalt petrogenesis: Chemical trends due to crystalliquid interaction. Proc. 3 rd Lunar Sci. Conf Bence A.E. and Autier B. (1972) Secondary ion analysis of pyroxenes from two porphritic lunar basalts. In The Apollo 15 Lunar Samples, The Lunar Science Institute, Houston. Bogard D.D., Funkhouser J.G., Schaeffer O.A. and Zahringer J. (1971) Noble gas abundances in lunar material-cosmic ray spallation products and radiation ages from the Sea of Tranquility and the Ocean of Storms. J. Geophys. Res. 76, Compston W., Berry H., Vernon M.J., Chappell B.W. and Kay M.J. (1971) Rubidium-strontium chronology and chemistry of lunar material from the Ocean of Storms. Proc. 2 nd Lunar Sci. Conf Gibb F.G.F., Stumpfl E.F. and Zussman J. (1970) Opaque minerals in an Apollo 12 rock. Earth Planet. Sci. Lett. 9, Gibb F.G.F. and Zussman J. (1971) Zoned olivine in four Apollo 12 samples. Earth Planet. Sci. Lett. 11, Hintenberger H., Weber H.W. and Takaoka N. (1971) Concentrations and isotopic abundances of the rare gases in lunar matter. Proc. 2 nd Lunar Sci. Conf Kushiro I., Nakamura Y., Kitayama K. and Akimoto S-I. (1971) Petrology of some Apollo 12 crystalline rocks. Proc. 2 nd Lunar Sci. Conf Marti K. and Lugmair G.W. (1971) Kr81-Kr and Kr-Ar40 ages, cosmic-ray spallation products and neutron effects in lunar samples from Oceanus Procellarum. Proc. 2 nd Lunar Sci. Conf Maxwell J.A. and Wiik H.B. (1971) Chemical composition of Apollo 12 lunar samples 12004, 12033, 12051, and Earth Planet. Sci. Lett. 10, Murthy V.R., Evensen N.M., Jahn B.-M. and Coscio M.R. (1971) Rb-Sr ages and elemental abundances of K, Rb, Sr and Ba in samples from the Ocean of Storms. Geochim. Cosmochim. Acta 35, Neal C.R., Hacker M.D., Snyder G.A., Taylor L.A., Liu Y.- G. and Schmitt R.A. (1994a) Basalt generation at the Apollo 12 site, Part 1: New data, classification and re-evaluation. Meteoritics 29, Neal C.R., Hacker M.D., Snyder G.A., Taylor L.A., Liu Y.- G. and Schmitt R.A. (1994b) Basalt generation at the Apollo 12 site, Part 2: Source heterogeneity, multiple melts and crustal contamination. Meteoritics 29, Nyquist L.E., Bansal B.M., Wooden J. and Wiesmann H. (1977) Sr-isotopic constraints on the peterogenesis of Apollo 12 mare basalts. Proc. 8 th Lunar Sci. Conf O Kelley G.D., Eldridge J.S., Schonfeld E. and Bell P.R. (1971a) Abundances of the primordial radionuclides K, Th, and U in Apollo 12 luanr samples by nondestructive gammaray spectroscopy: implications for the origin of lunar soils. Proc. Second Lunar Sci. Conf O Kelley G.D., Eldridge J.S., Schonfeld E. and Bell P.R. (1971b) Cosmogenic radionuclide concentrations and exposure ages of lunar samples from Apollo 12. Proc. Second Lunar Sci. Conf Papike J.J., Bence A.E., Brown G.E., Prewitt C.T. and Wu C.H. (1971) Apollo 12 clinopyroxenes: Exsolution and epitaxy. Earth Planet. Sci. Lett. 10, Papike J.J., Hodges F.N., Bence A.E., Cameron M. and Rhodes J.M. (1976) Mare basalts: Crystal chemistry, mineralogy and petrology. Rev. Geophys. Space Phys. 14, Schnetzler C.C. and Philpotts J.A. (1971) Alkali, alkaline earth, and rare earth element concentrations in some Apollo 12 soils, rocks, and separated phases. Proc. 2 nd Lunar Sci. Conf Takeda H. (1972) Structural studies of rim augite and core pigeonite from lunar rock Earth Planet. Sci. Lett. 15, Tatsumoto M., Knight R.J. and Doe B.R. (1971) U-Th-Pb systematic of Apollo lunar samples. Proc. 2 nd Lunar Sci. Conf
14 Taylor S.R., Rudowski R., Muir P., Graham A. and Kaye M. (1971) Trace element chemistry of lunar samples from the Ocean of Storms. Proc. 2 nd Lunar Sci. Conf Wänke H., Wlotzka F., M. and Rieder R. (1971) Apollo 12 samples: Chemical composition and its relation to sample locations and exposure ages, the two component origin of the various soil samples and studies on lunar metallic particles. Proc. 2 nd Lunar Sci. Conf
IV. Governador Valadares clinopyroxenite, 158 grams find
 IV. Governador Valadares clinopyroxenite, 158 grams find Figure IV-1. Photograph of Governador Valadares (158 grams) from Dr. Fernanda Ferrucci via Dr. Giuseppe Cavarretta. Photo taken by L. Spinozzi.
IV. Governador Valadares clinopyroxenite, 158 grams find Figure IV-1. Photograph of Governador Valadares (158 grams) from Dr. Fernanda Ferrucci via Dr. Giuseppe Cavarretta. Photo taken by L. Spinozzi.
,1,2,3,4,9, Impact Melt Breccia 65.4 grams
 63335 Impact Melt Breccia 65.4 grams,1,2,3,4,9,10,8 Figure 2: 63335,6. Cube is 1 cm. S75-33389.,7 Introduction 63335 is a sample chipped off of Shadow Rock (Ulrich 1973). It was collected as several fragments
63335 Impact Melt Breccia 65.4 grams,1,2,3,4,9,10,8 Figure 2: 63335,6. Cube is 1 cm. S75-33389.,7 Introduction 63335 is a sample chipped off of Shadow Rock (Ulrich 1973). It was collected as several fragments
65055 Basaltic Impact Melt grams
 65055 Basaltic Impact Melt 500.8 grams Figure 1: Photo of 65055 showing large zap pit. Cube is 1 cm. S72-43869. Introduction According to the Apollo 16 Catalog by Ryder and Norman, 65055 is an aluminous,
65055 Basaltic Impact Melt 500.8 grams Figure 1: Photo of 65055 showing large zap pit. Cube is 1 cm. S72-43869. Introduction According to the Apollo 16 Catalog by Ryder and Norman, 65055 is an aluminous,
DRAFT Coarse-fines 569 grams
 10085 Coarse-fines 569 grams DRAFT Figure 1: Selected coarse fines from 10085. Scale is in mm. Photo from Wood et al. 1969. Introduction!0085 and 10084 were created during the Apollo 11 preliminary examination
10085 Coarse-fines 569 grams DRAFT Figure 1: Selected coarse fines from 10085. Scale is in mm. Photo from Wood et al. 1969. Introduction!0085 and 10084 were created during the Apollo 11 preliminary examination
74235 Vitrophric Basalt 59 grams
 74235 Vitrophric Basalt 59 grams Figure 1: Photo of 74235. Cube is 1 cm. S73-16017 Figure 2: Map of station 4, Apollo 17. Transcript (while the LMP was discussing the core with CC, the CDR made a discovery)
74235 Vitrophric Basalt 59 grams Figure 1: Photo of 74235. Cube is 1 cm. S73-16017 Figure 2: Map of station 4, Apollo 17. Transcript (while the LMP was discussing the core with CC, the CDR made a discovery)
72320 Partially Shadowed Soil (portion frozen) grams. boulder # 2 station 2 South Massif
 Partially Shadowed Soil (portion ) 106.31 grams Nansen Crater boulder # 2 station 2 South Massif Figure 1: Location of soil sample in shadow of boulder 2, at station 2, Apollo 17. NASA photo #AS17-137-20925.
Partially Shadowed Soil (portion ) 106.31 grams Nansen Crater boulder # 2 station 2 South Massif Figure 1: Location of soil sample in shadow of boulder 2, at station 2, Apollo 17. NASA photo #AS17-137-20925.
15595 and Vuggy Vitrophyric Pigeonite Basalt and grams
 and 15596 Vuggy Vitrophyric Pigeonite Basalt 237.6 and 224.8 grams Figure 1: Photo of. Sample is about 9 cm across. NASA S71-44491. Figure 2: Photo of 15596 showing surface that was exposed to micrometeorites.
and 15596 Vuggy Vitrophyric Pigeonite Basalt 237.6 and 224.8 grams Figure 1: Photo of. Sample is about 9 cm across. NASA S71-44491. Figure 2: Photo of 15596 showing surface that was exposed to micrometeorites.
grams grams Poikilitic Impact Melt Breccia
 64567 13.8 grams 64569-14.3 grams Poikilitic Impact Melt Breccia Figure 1: Photo of 64567. Scale in mm. S72-55386 Mineralogical Mode by Simonds et al. (1973) 64567 64569 Plagioclase 69% 57 Pyroxene 10
64567 13.8 grams 64569-14.3 grams Poikilitic Impact Melt Breccia Figure 1: Photo of 64567. Scale in mm. S72-55386 Mineralogical Mode by Simonds et al. (1973) 64567 64569 Plagioclase 69% 57 Pyroxene 10
12013 Breccia with granite 82.3 grams
 12013 Breccia with granite 82.3 grams Figure 1: Close-up photo of 12013,11. Field of view is 4.5 cm. NASA# S70-43636 Introduction 12013 is a unique lunar sample, but it did not appear to be remarkable
12013 Breccia with granite 82.3 grams Figure 1: Close-up photo of 12013,11. Field of view is 4.5 cm. NASA# S70-43636 Introduction 12013 is a unique lunar sample, but it did not appear to be remarkable
grams grams Regolith Breccia
 10019 297 grams 10066 60 grams Regolith Breccia Figure 1: Photo of 10019,1. Cube is 1 inch and scale is in cm. NASA S76-23354. Introduction Kramer et al. (1077) reported that 10019 and 10066 appeared to
10019 297 grams 10066 60 grams Regolith Breccia Figure 1: Photo of 10019,1. Cube is 1 inch and scale is in cm. NASA S76-23354. Introduction Kramer et al. (1077) reported that 10019 and 10066 appeared to
10003 Ilmenite Basalt (low K) 213 grams
 10003 Ilmenite Basalt (low K) 213 grams Figure 1: Photo of 10003,25. Sample is 5 cm across. NASA S76-25545. Introduction Lunar sample 10003 is a low-k, high-ti basalt (figure 1). It is about 3.9 b.y. old,
10003 Ilmenite Basalt (low K) 213 grams Figure 1: Photo of 10003,25. Sample is 5 cm across. NASA S76-25545. Introduction Lunar sample 10003 is a low-k, high-ti basalt (figure 1). It is about 3.9 b.y. old,
10020 Ilmenite Basalt (low K) 425 grams
 10020 Ilmenite Basalt (low K) 425 grams Figure 1: Photo of 10020 taken in the F-201 during initial processing. Sample is 6 cm across. NASA S-69-45261. Introduction 10020 is a low-k variety of fine-grained
10020 Ilmenite Basalt (low K) 425 grams Figure 1: Photo of 10020 taken in the F-201 during initial processing. Sample is 6 cm across. NASA S-69-45261. Introduction 10020 is a low-k variety of fine-grained
DRAFT Cataclastic Dunite grams
 72415-72418 Cataclastic Dunite 58.74 grams DRAFT Figure 1: Boulder 3 at Station 2, with large dunite clast (72415-72418). The bright spot is the location of samples. A sample of the boulder matrix (72435)
72415-72418 Cataclastic Dunite 58.74 grams DRAFT Figure 1: Boulder 3 at Station 2, with large dunite clast (72415-72418). The bright spot is the location of samples. A sample of the boulder matrix (72435)
72335 Impact melt Breccia grams
 Impact melt Breccia 108.9 grams Figure 1: Location of on boulder #2 on landslide off of South Massif. Boulder is ~ 2-3 meters high. AS17-137-20918. Transcript Hey, that s a different rock, Gene (station
Impact melt Breccia 108.9 grams Figure 1: Location of on boulder #2 on landslide off of South Massif. Boulder is ~ 2-3 meters high. AS17-137-20918. Transcript Hey, that s a different rock, Gene (station
Ilmenite Basalt grams
 Ilmenite Basalt 737.6 grams Figure 1: Picute of 5 meter boulder on rim of Shorty Crater showing where was collected. Trench in forground is location of orange soil sample 74220. Photo number AS17-137-20990
Ilmenite Basalt 737.6 grams Figure 1: Picute of 5 meter boulder on rim of Shorty Crater showing where was collected. Trench in forground is location of orange soil sample 74220. Photo number AS17-137-20990
70035 Ilmenite Basalt 5765 grams
 Ilmenite Basalt 5765 grams Figure 1: Photograph of top surface of illustrating vugs and vesicles. Note the smooth, rounded surface shaped by micrometeorite bombardment. Small cube is 1 cm. NASA photo #
Ilmenite Basalt 5765 grams Figure 1: Photograph of top surface of illustrating vugs and vesicles. Note the smooth, rounded surface shaped by micrometeorite bombardment. Small cube is 1 cm. NASA photo #
Element Cube Project (x2)
 Element Cube Project (x2) Background: As a class, we will construct a three dimensional periodic table by each student selecting two elements in which you will need to create an element cube. Helpful Links
Element Cube Project (x2) Background: As a class, we will construct a three dimensional periodic table by each student selecting two elements in which you will need to create an element cube. Helpful Links
61015 Dimict Breccia 1789 grams
 61015 Dimict Breccia 1789 grams Figure 1: Photo of 61015. NASA # S72-37758. Scale and cube in cm. Note the zap pits on the T1 surface and the residual black glass coating on the sides. Introduction Breccia
61015 Dimict Breccia 1789 grams Figure 1: Photo of 61015. NASA # S72-37758. Scale and cube in cm. Note the zap pits on the T1 surface and the residual black glass coating on the sides. Introduction Breccia
Radiometric Dating (tap anywhere)
 Radiometric Dating (tap anywhere) Protons Neutrons Electrons Elements on the periodic table are STABLE Elements can have radioactive versions of itself called ISOTOPES!! Page 1 in your ESRT has your list!
Radiometric Dating (tap anywhere) Protons Neutrons Electrons Elements on the periodic table are STABLE Elements can have radioactive versions of itself called ISOTOPES!! Page 1 in your ESRT has your list!
Atoms and the Periodic Table
 Atoms and the Periodic Table Parts of the Atom Proton Found in the nucleus Number of protons defines the element Charge +1, mass 1 Parts of the Atom Neutron Found in the nucleus Stabilizes the nucleus
Atoms and the Periodic Table Parts of the Atom Proton Found in the nucleus Number of protons defines the element Charge +1, mass 1 Parts of the Atom Neutron Found in the nucleus Stabilizes the nucleus
grams grams Double Drive tube 61 cm
 68002 583.5 grams 68001 840.7 grams Double Drive tube 61 cm Figure 1a: Surface photo for double drive tube 68001-2. AS16-108-17684. Introduction Station 8 was the closest to South Ray Crater so premission
68002 583.5 grams 68001 840.7 grams Double Drive tube 61 cm Figure 1a: Surface photo for double drive tube 68001-2. AS16-108-17684. Introduction Station 8 was the closest to South Ray Crater so premission
15556 Vesicular Olivine-normative Basalt grams
 15556 Vesicular Olivine-normative Basalt 1542.3 grams Figure 1: Photo of 15556,0. Largest vesicles are about 6 mm. NASA S87-48187. Introduction 15556 was collected about 60 meters from the edge of Hadley
15556 Vesicular Olivine-normative Basalt 1542.3 grams Figure 1: Photo of 15556,0. Largest vesicles are about 6 mm. NASA S87-48187. Introduction 15556 was collected about 60 meters from the edge of Hadley
The Periodic Table. Periodic Properties. Can you explain this graph? Valence Electrons. Valence Electrons. Paramagnetism
 Periodic Properties Atomic & Ionic Radius Energy Electron Affinity We want to understand the variations in these properties in terms of electron configurations. The Periodic Table Elements in a column
Periodic Properties Atomic & Ionic Radius Energy Electron Affinity We want to understand the variations in these properties in terms of electron configurations. The Periodic Table Elements in a column
Solutions and Ions. Pure Substances
 Class #4 Solutions and Ions CHEM 107 L.S. Brown Texas A&M University Pure Substances Pure substance: described completely by a single chemical formula Fixed composition 1 Mixtures Combination of 2 or more
Class #4 Solutions and Ions CHEM 107 L.S. Brown Texas A&M University Pure Substances Pure substance: described completely by a single chemical formula Fixed composition 1 Mixtures Combination of 2 or more
The Periodic Table of Elements
 The Periodic Table of Elements 8 Uuo Uus Uuh (9) Uup (88) Uuq (89) Uut (8) Uub (8) Rg () 0 Ds (9) 09 Mt (8) 08 Hs (9) 0 h () 0 Sg () 0 Db () 0 Rf () 0 Lr () 88 Ra () 8 Fr () 8 Rn () 8 At (0) 8 Po (09)
The Periodic Table of Elements 8 Uuo Uus Uuh (9) Uup (88) Uuq (89) Uut (8) Uub (8) Rg () 0 Ds (9) 09 Mt (8) 08 Hs (9) 0 h () 0 Sg () 0 Db () 0 Rf () 0 Lr () 88 Ra () 8 Fr () 8 Rn () 8 At (0) 8 Po (09)
grams g g g g Regolith Breccia
 15306 134.2 grams 15315 35.6 g 15324 32.3 g 15325 57.8 g 15330 57.8 g Regolith Breccia Figure 1a,b: Photo of dust-covered 15306. Sample is 7 cm across. S71-43064 and 067. Introduction 15306 is a regolith
15306 134.2 grams 15315 35.6 g 15324 32.3 g 15325 57.8 g 15330 57.8 g Regolith Breccia Figure 1a,b: Photo of dust-covered 15306. Sample is 7 cm across. S71-43064 and 067. Introduction 15306 is a regolith
The Periodic Table of the Elements
 The Periodic Table of the Elements All matter is composed of elements. All of the elements are composed of atoms. An atom is the smallest part of an element which still retains the properties of that element.
The Periodic Table of the Elements All matter is composed of elements. All of the elements are composed of atoms. An atom is the smallest part of an element which still retains the properties of that element.
7) Applications of Nuclear Radiation in Science and Technique (1) Analytical applications (Radiometric titration)
 7) Applications of Nuclear Radiation in Science and Technique (1) (Radiometric titration) The radioactive material is indicator Precipitation reactions Complex formation reactions Principle of a precipitation
7) Applications of Nuclear Radiation in Science and Technique (1) (Radiometric titration) The radioactive material is indicator Precipitation reactions Complex formation reactions Principle of a precipitation
15016 Vesicular Olivine-normative Basalt grams
 15016 Vesicular Olivine-normative Basalt 923.7 grams Figure 1: Photograph of vesicular basalt 15016. NASA# S71-46632. Cube is 1 inch. Introduction Lunar Sample 15016 is a highly-vesicular, olivinenormative,
15016 Vesicular Olivine-normative Basalt 923.7 grams Figure 1: Photograph of vesicular basalt 15016. NASA# S71-46632. Cube is 1 inch. Introduction Lunar Sample 15016 is a highly-vesicular, olivinenormative,
Nucleus. Electron Cloud
 Atomic Structure I. Picture of an Atom Nucleus Electron Cloud II. Subatomic particles Particle Symbol Charge Relative Mass (amu) protons p + +1 1.0073 neutrons n 0 1.0087 electrons e - -1 0.00054858 Compare
Atomic Structure I. Picture of an Atom Nucleus Electron Cloud II. Subatomic particles Particle Symbol Charge Relative Mass (amu) protons p + +1 1.0073 neutrons n 0 1.0087 electrons e - -1 0.00054858 Compare
Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
10017 Ilmenite Basalt (high K) 973 grams
 10017 Ilmenite Basalt (high K) 973 grams Figure 1: Close-up photo of dusted surface of 10017,81 N1 face. Sample is 4 cm across. NASA #76-25451. Introduction 10017 is the largest rock sample returned by
10017 Ilmenite Basalt (high K) 973 grams Figure 1: Close-up photo of dusted surface of 10017,81 N1 face. Sample is 4 cm across. NASA #76-25451. Introduction 10017 is the largest rock sample returned by
Using the Periodic Table
 MATH SKILLS TRANSPARENCY WORKSHEET Using the Periodic Table 6 Use with Chapter 6, Section 6.2 1. Identify the number of valence electrons in each of the following elements. a. Ne e. O b. K f. Cl c. B g.
MATH SKILLS TRANSPARENCY WORKSHEET Using the Periodic Table 6 Use with Chapter 6, Section 6.2 1. Identify the number of valence electrons in each of the following elements. a. Ne e. O b. K f. Cl c. B g.
DO NOW: Retrieve your projects. We will be reviewing them again today. Textbook pg 23, answer questions 1-3. Use the section 1.2 to help you.
 DO NOW: Retrieve your projects. We will be reviewing them again today. Textbook pg, answer questions. Use the section. to help you. Chapter test is FRIDAY. The Periodic Table of Elements 8 Uuo Uus Uuh
DO NOW: Retrieve your projects. We will be reviewing them again today. Textbook pg, answer questions. Use the section. to help you. Chapter test is FRIDAY. The Periodic Table of Elements 8 Uuo Uus Uuh
Last 4 Digits of USC ID:
 Chemistry 05 B Practice Exam Dr. Jessica Parr First Letter of last Name PLEASE PRINT YOUR NAME IN BLOCK LETTERS Name: Last 4 Digits of USC ID: Lab TA s Name: Question Points Score Grader 8 2 4 3 9 4 0
Chemistry 05 B Practice Exam Dr. Jessica Parr First Letter of last Name PLEASE PRINT YOUR NAME IN BLOCK LETTERS Name: Last 4 Digits of USC ID: Lab TA s Name: Question Points Score Grader 8 2 4 3 9 4 0
SCIENCE 1206 UNIT 2 CHEMISTRY. September 2017 November 2017
 SCIENCE 1206 UNIT 2 CHEMISTRY September 2017 November 2017 UNIT OUTLINE 1. Review of Grade 9 Terms & the Periodic Table Bohr diagrams Evidence for chemical reactions Chemical Tests 2. Naming & writing
SCIENCE 1206 UNIT 2 CHEMISTRY September 2017 November 2017 UNIT OUTLINE 1. Review of Grade 9 Terms & the Periodic Table Bohr diagrams Evidence for chemical reactions Chemical Tests 2. Naming & writing
PERIODIC TABLE OF THE ELEMENTS
 Useful Constants and equations: K = o C + 273 Avogadro's number = 6.022 x 10 23 d = density = mass/volume R H = 2.178 x 10-18 J c = E = h = hc/ h = 6.626 x 10-34 J s c = 2.998 x 10 8 m/s E n = -R H Z 2
Useful Constants and equations: K = o C + 273 Avogadro's number = 6.022 x 10 23 d = density = mass/volume R H = 2.178 x 10-18 J c = E = h = hc/ h = 6.626 x 10-34 J s c = 2.998 x 10 8 m/s E n = -R H Z 2
Chem Exam 1. September 26, Dr. Susan E. Bates. Name 9:00 OR 10:00
 Chem 1711 Exam 1 September 26, 2013 Dr. Susan E. Bates Name 9:00 OR 10:00 N A = 6.022 x 10 23 mol 1 I A II A III B IV B V B VI B VII B VIII I B II B III A IV A V A VI A VII A inert gases 1 H 1.008 3 Li
Chem 1711 Exam 1 September 26, 2013 Dr. Susan E. Bates Name 9:00 OR 10:00 N A = 6.022 x 10 23 mol 1 I A II A III B IV B V B VI B VII B VIII I B II B III A IV A V A VI A VII A inert gases 1 H 1.008 3 Li
Summary of test results for Daya Bay rock samples. by Patrick Dobson Celia Tiemi Onishi Seiji Nakagawa
 Summary of test results for Daya Bay rock samples by Patrick Dobson Celia Tiemi Onishi Seiji Nakagawa October 2004 Summary A series of analytical tests were conducted on a suite of granitic rock samples
Summary of test results for Daya Bay rock samples by Patrick Dobson Celia Tiemi Onishi Seiji Nakagawa October 2004 Summary A series of analytical tests were conducted on a suite of granitic rock samples
CHEM 10113, Quiz 5 October 26, 2011
 CHEM 10113, Quiz 5 October 26, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
CHEM 10113, Quiz 5 October 26, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
Part 2. Multiple choice (use answer card). 90 pts. total. 3 pts. each.
 1 Exam I CHEM 1303.001 Name (print legibly) Seat no. On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Part 1. Nomenclature. 10 pts. total. 2 pts. each. Fill in
1 Exam I CHEM 1303.001 Name (print legibly) Seat no. On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Part 1. Nomenclature. 10 pts. total. 2 pts. each. Fill in
Made the FIRST periodic table
 Made the FIRST periodic table 1869 Mendeleev organized the periodic table based on the similar properties and relativities of certain elements Later, Henri Moseley organized the elements by increasing
Made the FIRST periodic table 1869 Mendeleev organized the periodic table based on the similar properties and relativities of certain elements Later, Henri Moseley organized the elements by increasing
Ch. 9 NOTES ~ Chemical Bonding NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics.
 Ch. 9 NOTES ~ Chemical Bonding NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Review: Comparison of ionic and molecular compounds Molecular compounds Ionic
Ch. 9 NOTES ~ Chemical Bonding NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Review: Comparison of ionic and molecular compounds Molecular compounds Ionic
Chemistry 431 Practice Final Exam Fall Hours
 Chemistry 431 Practice Final Exam Fall 2018 3 Hours R =8.3144 J mol 1 K 1 R=.0821 L atm mol 1 K 1 R=.08314 L bar mol 1 K 1 k=1.381 10 23 J molecule 1 K 1 h=6.626 10 34 Js N A = 6.022 10 23 molecules mol
Chemistry 431 Practice Final Exam Fall 2018 3 Hours R =8.3144 J mol 1 K 1 R=.0821 L atm mol 1 K 1 R=.08314 L bar mol 1 K 1 k=1.381 10 23 J molecule 1 K 1 h=6.626 10 34 Js N A = 6.022 10 23 molecules mol
14041, and Regolith Breccia 166.3, and 65.2 grams
 14041, and 14045 Regolith Breccia 166.3, 103.2 and 65.2 grams Figure 1a: Sample 14041-14045 on lunar surface. MET and LM in distance. AS14-68-9409. Introduction Samples 14041 14046 are fragments from a
14041, and 14045 Regolith Breccia 166.3, 103.2 and 65.2 grams Figure 1a: Sample 14041-14045 on lunar surface. MET and LM in distance. AS14-68-9409. Introduction Samples 14041 14046 are fragments from a
Worked Example of Batch Melting: Rb and Sr
 Worked Example of Batch Melting: Rb and Sr Basalt with the mode: Table 9.2. Conversion from mode to weight percent Mineral Mode Density Wt prop Wt% ol 15 3.6 54 0.18 cpx 33 3.4 112.2 0.37 plag 51 2.7 137.7
Worked Example of Batch Melting: Rb and Sr Basalt with the mode: Table 9.2. Conversion from mode to weight percent Mineral Mode Density Wt prop Wt% ol 15 3.6 54 0.18 cpx 33 3.4 112.2 0.37 plag 51 2.7 137.7
Chapter 12 The Atom & Periodic Table- part 2
 Chapter 12 The Atom & Periodic Table- part 2 Electrons found outside the nucleus; negatively charged Protons found in the nucleus; positive charge equal in magnitude to the electron s negative charge Neutrons
Chapter 12 The Atom & Periodic Table- part 2 Electrons found outside the nucleus; negatively charged Protons found in the nucleus; positive charge equal in magnitude to the electron s negative charge Neutrons
62255 Anorthosite with melt 1239 grams
 62255 Anorthosite with melt 1239 grams Figure 1: Photo of 62255 showing glass splash on anorthosite. Cube is 1 cm. NASA S # S72-38309. Introduction Lunar sample 62255 is significant because it is largely
62255 Anorthosite with melt 1239 grams Figure 1: Photo of 62255 showing glass splash on anorthosite. Cube is 1 cm. NASA S # S72-38309. Introduction Lunar sample 62255 is significant because it is largely
DRAFT Regolith Breccia 155 grams
 12034 Regolith Breccia 155 grams DRAFT Figure 1: Lunar sample 12034 after first saw cut in 1970. NASA S75-34235. Cube is 1 cm. Introduction 12034 was collected from the bottom of the same trench as soil
12034 Regolith Breccia 155 grams DRAFT Figure 1: Lunar sample 12034 after first saw cut in 1970. NASA S75-34235. Cube is 1 cm. Introduction 12034 was collected from the bottom of the same trench as soil
Spin Cut-off Parameter of Nuclear Level Density and Effective Moment of Inertia
 Commun. Theor. Phys. (Beijing, China) 43 (005) pp. 709 718 c International Academic Publishers Vol. 43, No. 4, April 15, 005 Spin Cut-off Parameter of Nuclear Level Density and Effective Moment of Inertia
Commun. Theor. Phys. (Beijing, China) 43 (005) pp. 709 718 c International Academic Publishers Vol. 43, No. 4, April 15, 005 Spin Cut-off Parameter of Nuclear Level Density and Effective Moment of Inertia
Atomic Structure & Interatomic Bonding
 Atomic Structure & Interatomic Bonding Chapter Outline Review of Atomic Structure Atomic Bonding Atomic Structure Atoms are the smallest structural units of all solids, liquids & gases. Atom: The smallest
Atomic Structure & Interatomic Bonding Chapter Outline Review of Atomic Structure Atomic Bonding Atomic Structure Atoms are the smallest structural units of all solids, liquids & gases. Atom: The smallest
9/20/2017. Elements are Pure Substances that cannot be broken down into simpler substances by chemical change (contain Only One Type of Atom)
 CAPTER 6: TE PERIODIC TABLE Elements are Pure Substances that cannot be broken down into simpler substances by chemical change (contain Only One Type of Atom) The Periodic Table (Mendeleev) In 1872, Dmitri
CAPTER 6: TE PERIODIC TABLE Elements are Pure Substances that cannot be broken down into simpler substances by chemical change (contain Only One Type of Atom) The Periodic Table (Mendeleev) In 1872, Dmitri
(C) Pavel Sedach and Prep101 1
 (C) Pavel Sedach and Prep101 1 (C) Pavel Sedach and Prep101 1 (C) Pavel Sedach and Prep101 2 (C) Pavel Sedach and Prep101 2 (C) Pavel Sedach and Prep101 3 (C) Pavel Sedach and Prep101 3 (C) Pavel Sedach
(C) Pavel Sedach and Prep101 1 (C) Pavel Sedach and Prep101 1 (C) Pavel Sedach and Prep101 2 (C) Pavel Sedach and Prep101 2 (C) Pavel Sedach and Prep101 3 (C) Pavel Sedach and Prep101 3 (C) Pavel Sedach
Earth Materials I Crystal Structures
 Earth Materials I Crystal Structures Isotopes same atomic number, different numbers of neutrons, different atomic mass. Ta ble 1-1. Su mmar y of quantu m num bers Name Symbol Values Principal n 1, 2,
Earth Materials I Crystal Structures Isotopes same atomic number, different numbers of neutrons, different atomic mass. Ta ble 1-1. Su mmar y of quantu m num bers Name Symbol Values Principal n 1, 2,
CLASS TEST GRADE 11. PHYSICAL SCIENCES: CHEMISTRY Test 4: Matter and materials 1
 CLASS TEST GRADE PHYSICAL SCIENCES: CHEMISTRY Test 4: Matter and materials MARKS: 45 TIME: hour INSTRUCTIONS AND INFORMATION. Answer ALL the questions. 2. You may use non-programmable calculators. 3. You
CLASS TEST GRADE PHYSICAL SCIENCES: CHEMISTRY Test 4: Matter and materials MARKS: 45 TIME: hour INSTRUCTIONS AND INFORMATION. Answer ALL the questions. 2. You may use non-programmable calculators. 3. You
CHM 101 PRACTICE TEST 1 Page 1 of 4
 CHM 101 PRACTICE TEST 1 Page 1 of 4 Please show calculations (stuffed equations) on all mathematical problems!! On the actual test, "naked answers, with no work shown, will receive no credit even if correct.
CHM 101 PRACTICE TEST 1 Page 1 of 4 Please show calculations (stuffed equations) on all mathematical problems!! On the actual test, "naked answers, with no work shown, will receive no credit even if correct.
Why all the repeating Why all the repeating Why all the repeating Why all the repeating
 Why all the repeating Why all the repeating Why all the repeating Why all the repeating Patterns What Patterns have you observed in your life? Where to Get Help If you don t understand concepts in chapter
Why all the repeating Why all the repeating Why all the repeating Why all the repeating Patterns What Patterns have you observed in your life? Where to Get Help If you don t understand concepts in chapter
Guide to the Extended Step-Pyramid Periodic Table
 Guide to the Extended Step-Pyramid Periodic Table William B. Jensen Department of Chemistry University of Cincinnati Cincinnati, OH 452201-0172 The extended step-pyramid table recognizes that elements
Guide to the Extended Step-Pyramid Periodic Table William B. Jensen Department of Chemistry University of Cincinnati Cincinnati, OH 452201-0172 The extended step-pyramid table recognizes that elements
If anything confuses you or is not clear, raise your hand and ask!
 CHM 1045 Dr. Light s Section December 10, 2002 FINAL EXAM Name (please print) Recitation Section Meeting Time This exam consists of six pages. Make sure you have one of each. Print your name at the top
CHM 1045 Dr. Light s Section December 10, 2002 FINAL EXAM Name (please print) Recitation Section Meeting Time This exam consists of six pages. Make sure you have one of each. Print your name at the top
6.3 Classifying Elements with the Periodic Table
 6.3 Classifying Elements with the Periodic Table The Periodic Table was developed by scientists to organize elements in such a way as to make sense of the growing information about their properties. The
6.3 Classifying Elements with the Periodic Table The Periodic Table was developed by scientists to organize elements in such a way as to make sense of the growing information about their properties. The
M10/4/CHEMI/SPM/ENG/TZ2/XX+ CHEMISTRY. Wednesday 12 May 2010 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES
 M10/4/CHEMI/SPM/ENG/TZ/XX+ 106116 CHEMISTRY standard level Paper 1 Wednesday 1 May 010 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
M10/4/CHEMI/SPM/ENG/TZ/XX+ 106116 CHEMISTRY standard level Paper 1 Wednesday 1 May 010 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
Chapter 3: Stoichiometry
 Chapter 3: Stoichiometry Chem 6A Michael J. Sailor, UC San Diego 1 Announcements: Thursday (Sep 29) quiz: Bring student ID or we cannot accept your quiz! No notes, no calculators Covers chapters 1 and
Chapter 3: Stoichiometry Chem 6A Michael J. Sailor, UC San Diego 1 Announcements: Thursday (Sep 29) quiz: Bring student ID or we cannot accept your quiz! No notes, no calculators Covers chapters 1 and
PART 1 Introduction to Theory of Solids
 Elsevier UK Job code: MIOC Ch01-I044647 9-3-2007 3:03p.m. Page:1 Trim:165 240MM TS: Integra, India PART 1 Introduction to Theory of Solids Elsevier UK Job code: MIOC Ch01-I044647 9-3-2007 3:03p.m. Page:2
Elsevier UK Job code: MIOC Ch01-I044647 9-3-2007 3:03p.m. Page:1 Trim:165 240MM TS: Integra, India PART 1 Introduction to Theory of Solids Elsevier UK Job code: MIOC Ch01-I044647 9-3-2007 3:03p.m. Page:2
1 of 5 14/10/ :21
 X-ray absorption s, characteristic X-ray lines... 4.2.1 Home About Table of Contents Advanced Search Copyright Feedback Privacy You are here: Chapter: 4 Atomic and nuclear physics Section: 4.2 Absorption
X-ray absorption s, characteristic X-ray lines... 4.2.1 Home About Table of Contents Advanced Search Copyright Feedback Privacy You are here: Chapter: 4 Atomic and nuclear physics Section: 4.2 Absorption
5 questions, 3 points each, 15 points total possible. 26 Fe Cu Ni Co Pd Ag Ru 101.
 Physical Chemistry II Lab CHEM 4644 spring 2017 final exam KEY 5 questions, 3 points each, 15 points total possible h = 6.626 10-34 J s c = 3.00 10 8 m/s 1 GHz = 10 9 s -1. B= h 8π 2 I ν= 1 2 π k μ 6 P
Physical Chemistry II Lab CHEM 4644 spring 2017 final exam KEY 5 questions, 3 points each, 15 points total possible h = 6.626 10-34 J s c = 3.00 10 8 m/s 1 GHz = 10 9 s -1. B= h 8π 2 I ν= 1 2 π k μ 6 P
[ ]:543.4(075.8) 35.20: ,..,..,.., : /... ;. 2-. ISBN , - [ ]:543.4(075.8) 35.20:34.
![[ ]:543.4(075.8) 35.20: ,..,..,.., : /... ;. 2-. ISBN , - [ ]:543.4(075.8) 35.20:34. [ ]:543.4(075.8) 35.20: ,..,..,.., : /... ;. 2-. ISBN , - [ ]:543.4(075.8) 35.20:34.](/thumbs/74/69911105.jpg) .. - 2-2009 [661.87.+661.88]:543.4(075.8) 35.20:34.2373-60..,..,..,..,.. -60 : /... ;. 2-. : -, 2008. 134. ISBN 5-98298-299-7 -., -,,. - «,, -, -», - 550800,, 240600 «-», -. [661.87.+661.88]:543.4(075.8)
.. - 2-2009 [661.87.+661.88]:543.4(075.8) 35.20:34.2373-60..,..,..,..,.. -60 : /... ;. 2-. : -, 2008. 134. ISBN 5-98298-299-7 -., -,,. - «,, -, -», - 550800,, 240600 «-», -. [661.87.+661.88]:543.4(075.8)
Circle the letters only. NO ANSWERS in the Columns!
 Chemistry 1304.001 Name (please print) Exam 5 (100 points) April 18, 2018 On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Circle the letters only. NO ANSWERS in
Chemistry 1304.001 Name (please print) Exam 5 (100 points) April 18, 2018 On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Circle the letters only. NO ANSWERS in
Essential Chemistry for Biology
 1 Chapter 2 Essential Chemistry for Biology Biology and Society: More Precious than Gold A drought is a period of abnormally dry weather that changes the environment and one of the most devastating disasters.
1 Chapter 2 Essential Chemistry for Biology Biology and Society: More Precious than Gold A drought is a period of abnormally dry weather that changes the environment and one of the most devastating disasters.
MANY ELECTRON ATOMS Chapter 15
 MANY ELECTRON ATOMS Chapter 15 Electron-Electron Repulsions (15.5-15.9) The hydrogen atom Schrödinger equation is exactly solvable yielding the wavefunctions and orbitals of chemistry. Howev er, the Schrödinger
MANY ELECTRON ATOMS Chapter 15 Electron-Electron Repulsions (15.5-15.9) The hydrogen atom Schrödinger equation is exactly solvable yielding the wavefunctions and orbitals of chemistry. Howev er, the Schrödinger
610B Final Exam Cover Page
 1 st Letter of Last Name NAME: 610B Final Exam Cover Page No notes or calculators of any sort allowed. You have 3 hours to complete the exam. CHEM 610B, 50995 Final Exam Fall 2003 Instructor: Dr. Brian
1 st Letter of Last Name NAME: 610B Final Exam Cover Page No notes or calculators of any sort allowed. You have 3 hours to complete the exam. CHEM 610B, 50995 Final Exam Fall 2003 Instructor: Dr. Brian
Metallurgical Chemistry. An Audio Course for Students
 Laval University From the SelectedWorks of Fathi Habashi February, 1987 Metallurgical Chemistry. An Audio Course for Students Fathi Habashi Available at: https://works.bepress.com/fathi_habashi/27/ METALLURGICAL
Laval University From the SelectedWorks of Fathi Habashi February, 1987 Metallurgical Chemistry. An Audio Course for Students Fathi Habashi Available at: https://works.bepress.com/fathi_habashi/27/ METALLURGICAL
(please print) (1) (18) H IIA IIIA IVA VA VIA VIIA He (2) (13) (14) (15) (16) (17)
 CHEM 10113, Quiz 3 September 28, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
CHEM 10113, Quiz 3 September 28, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
8. Relax and do well.
 CHEM 1515 Exam II John II. Gelder October 14, 1993 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last two pages include a periodic table, a
CHEM 1515 Exam II John II. Gelder October 14, 1993 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last two pages include a periodic table, a
K. 27 Co. 28 Ni. 29 Cu Rb. 46 Pd. 45 Rh. 47 Ag Cs Ir. 78 Pt.
 1 IA 1 H Hydrogen 1.01 Atomic number Element symbol Element name Atomic mass VIIIA 1 H 1.01 IIA IIIA IVA VA VIA VIIA 2 He 4.00 Metalloids 3 Li 6.94 4 Be 9.01 5 B 10.81 6 C 12.01 7 N 14.01 8 O 16.00 9 F
1 IA 1 H Hydrogen 1.01 Atomic number Element symbol Element name Atomic mass VIIIA 1 H 1.01 IIA IIIA IVA VA VIA VIIA 2 He 4.00 Metalloids 3 Li 6.94 4 Be 9.01 5 B 10.81 6 C 12.01 7 N 14.01 8 O 16.00 9 F
Circle the letters only. NO ANSWERS in the Columns! (3 points each)
 Chemistry 1304.001 Name (please print) Exam 4 (100 points) April 12, 2017 On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Circle the letters only. NO ANSWERS in
Chemistry 1304.001 Name (please print) Exam 4 (100 points) April 12, 2017 On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Circle the letters only. NO ANSWERS in
Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Advanced Chemistry. Mrs. Klingaman. Chapter 5: Name:
 Advanced Chemistry Mrs. Klingaman Chapter 5: The Periodic Law Name: _ Mods: Chapter 5: The Periodic Law Reading Guide 5.1 History of the Periodic Table (pgs. 125-129) 1) What did Dimitri Mendeleev notice
Advanced Chemistry Mrs. Klingaman Chapter 5: The Periodic Law Name: _ Mods: Chapter 5: The Periodic Law Reading Guide 5.1 History of the Periodic Table (pgs. 125-129) 1) What did Dimitri Mendeleev notice
NAME: SECOND EXAMINATION
 1 Chemistry 64 Winter 1994 NAME: SECOND EXAMINATION THIS EXAMINATION IS WORTH 100 POINTS AND CONTAINS 4 (FOUR) QUESTIONS THEY ARE NOT EQUALLY WEIGHTED! YOU SHOULD ATTEMPT ALL QUESTIONS AND ALLOCATE YOUR
1 Chemistry 64 Winter 1994 NAME: SECOND EXAMINATION THIS EXAMINATION IS WORTH 100 POINTS AND CONTAINS 4 (FOUR) QUESTIONS THEY ARE NOT EQUALLY WEIGHTED! YOU SHOULD ATTEMPT ALL QUESTIONS AND ALLOCATE YOUR
PHYSICAL SCIENCES GRADE : 10
 PHYSICAL SCIENCES GRADE : 0 TIME : hour TOTAL : 75 INSTRUCTIONS AND INFORMATION. Write your full name on your answer book in the appropriate place. 2. The question paper consists of SEVEN questions. Answer
PHYSICAL SCIENCES GRADE : 0 TIME : hour TOTAL : 75 INSTRUCTIONS AND INFORMATION. Write your full name on your answer book in the appropriate place. 2. The question paper consists of SEVEN questions. Answer
NAME: FIRST EXAMINATION
 1 Chemistry 64 Winter 1994 NAME: FIRST EXAMINATION THIS EXAMINATION IS WORTH 100 POINTS AND CONTAINS 4 (FOUR) QUESTIONS THEY ARE NOT EQUALLY WEIGHTED! YOU SHOULD ATTEMPT ALL QUESTIONS AND ALLOCATE YOUR
1 Chemistry 64 Winter 1994 NAME: FIRST EXAMINATION THIS EXAMINATION IS WORTH 100 POINTS AND CONTAINS 4 (FOUR) QUESTIONS THEY ARE NOT EQUALLY WEIGHTED! YOU SHOULD ATTEMPT ALL QUESTIONS AND ALLOCATE YOUR
Secondary Support Pack. be introduced to some of the different elements within the periodic table;
 Secondary Support Pack INTRODUCTION The periodic table of the elements is central to chemistry as we know it today and the study of it is a key part of every student s chemical education. By playing the
Secondary Support Pack INTRODUCTION The periodic table of the elements is central to chemistry as we know it today and the study of it is a key part of every student s chemical education. By playing the
Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Grade 11 Science Practice Test
 Grade 11 Science Practice Test Nebraska Department of Education 2012 Directions: On the following pages of your test booklet are multiple-choice questions for Session 1 of the Grade 11 Nebraska State Accountability
Grade 11 Science Practice Test Nebraska Department of Education 2012 Directions: On the following pages of your test booklet are multiple-choice questions for Session 1 of the Grade 11 Nebraska State Accountability
Atomic Emission Spectra. and. Flame Tests. Burlingame High School Chemistry
 Atomic Structure Atomic Emission Spectra and Flame Tests Flame Tests Sodium potassium lithium When electrons are excited they bump up to a higher energy level. As they bounce back down they release energy
Atomic Structure Atomic Emission Spectra and Flame Tests Flame Tests Sodium potassium lithium When electrons are excited they bump up to a higher energy level. As they bounce back down they release energy
HANDOUT SET GENERAL CHEMISTRY II
 HANDOUT SET GENERAL CHEMISTRY II Periodic Table of the Elements 1 2 3 4 5 6 7 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 IA VIIIA 1 2 H He 1.00794 IIA IIIA IVA VA VIA VIIA 4.00262 3 Li 6.941 11 Na 22.9898
HANDOUT SET GENERAL CHEMISTRY II Periodic Table of the Elements 1 2 3 4 5 6 7 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 IA VIIIA 1 2 H He 1.00794 IIA IIIA IVA VA VIA VIIA 4.00262 3 Li 6.941 11 Na 22.9898
Faculty of Natural and Agricultural Sciences Chemistry Department. Semester Test 1. Analytical Chemistry CMY 283. Time: 120 min Marks: 100 Pages: 6
 Faculty of Natural and Agricultural Sciences Chemistry Department Semester Test 1 Analytical Chemistry CMY 283 Date: 5 September 2016 Lecturers : Prof P Forbes, Dr Laurens, Mr SA Nsibande Time: 120 min
Faculty of Natural and Agricultural Sciences Chemistry Department Semester Test 1 Analytical Chemistry CMY 283 Date: 5 September 2016 Lecturers : Prof P Forbes, Dr Laurens, Mr SA Nsibande Time: 120 min
Chemistry 1 First Lecture Exam Fall Abbasi Khajo Levine Mathias Mathias/Ortiz Metlitsky Rahi Sanchez-Delgado Vasserman
 Chemistry 1 First Lecture Exam Fall 2011 Page 1 of 9 NAME Circle the name of your recitation/lab instructor(s) Abbasi Khajo Levine Mathias Mathias/Ortiz Metlitsky Rahi Sanchez-Delgado Vasserman Before
Chemistry 1 First Lecture Exam Fall 2011 Page 1 of 9 NAME Circle the name of your recitation/lab instructor(s) Abbasi Khajo Levine Mathias Mathias/Ortiz Metlitsky Rahi Sanchez-Delgado Vasserman Before
What monitoring techniques are appropriate and effective for detecting CO2 migration in groundwater: isotope-based monitoring Philippe Négrel
 What monitoring techniques are appropriate and effective for detecting CO2 migration in groundwater: isotope-based monitoring Philippe Négrel Acting in complicity with Pauline Humez. Results from Pauline
What monitoring techniques are appropriate and effective for detecting CO2 migration in groundwater: isotope-based monitoring Philippe Négrel Acting in complicity with Pauline Humez. Results from Pauline
CHEM 167 FINAL EXAM MONDAY, MAY 2 9:45 11:45 A.M GILMAN HALL
 PROF. JOHN VERKADE SPRING 2005 THIS EXAM CONSISTS OF 12 QUESTIONS ON 9 PAGES CHEM 167 HOUR EXAM IV APRIL 20, 2005 SEAT NO. NAME RECIT. INSTR. RECIT. SECT. GRADING PAGE Page 2 Page 3 Page 4 Page 5 Page
PROF. JOHN VERKADE SPRING 2005 THIS EXAM CONSISTS OF 12 QUESTIONS ON 9 PAGES CHEM 167 HOUR EXAM IV APRIL 20, 2005 SEAT NO. NAME RECIT. INSTR. RECIT. SECT. GRADING PAGE Page 2 Page 3 Page 4 Page 5 Page
BROOKLYN COLLEGE Department of Chemistry. Chemistry 1 Second Lecture Exam Nov. 27, Name Page 1 of 5
 BROOKLYN COLLEGE Department of Chemistry Chemistry 1 Second Lecture Exam Nov. 27, 2002 Name Page 1 of 5 Circle the name of your lab instructor Kobrak, Zhou, Girotto, Hussey, Du Before you begin the exam,
BROOKLYN COLLEGE Department of Chemistry Chemistry 1 Second Lecture Exam Nov. 27, 2002 Name Page 1 of 5 Circle the name of your lab instructor Kobrak, Zhou, Girotto, Hussey, Du Before you begin the exam,
8. Relax and do well.
 CHEM 1215 Exam III John III. Gelder November 11, 1998 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and
CHEM 1215 Exam III John III. Gelder November 11, 1998 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and
CHEM 130 Exp. 8: Molecular Models
 CHEM 130 Exp. 8: Molecular Models In this lab, we will learn and practice predicting molecular structures from molecular formulas. The Periodic Table of the Elements IA 1 H IIA IIIA IVA VA VIA VIIA 3 5
CHEM 130 Exp. 8: Molecular Models In this lab, we will learn and practice predicting molecular structures from molecular formulas. The Periodic Table of the Elements IA 1 H IIA IIIA IVA VA VIA VIIA 3 5
Chemistry Standard level Paper 1
 M15/4/CHEMI/SPM/ENG/TZ1/XX Chemistry Standard level Paper 1 Thursday 14 May 2015 (afternoon) 45 minutes Instructions to candidates Do not open this examination paper until instructed to do so. Answer all
M15/4/CHEMI/SPM/ENG/TZ1/XX Chemistry Standard level Paper 1 Thursday 14 May 2015 (afternoon) 45 minutes Instructions to candidates Do not open this examination paper until instructed to do so. Answer all
single-layer transition metal dichalcogenides MC2
 single-layer transition metal dichalcogenides MC2 Period 1 1 H 18 He 2 Group 1 2 Li Be Group 13 14 15 16 17 18 B C N O F Ne 3 4 Na K Mg Ca Group 3 4 5 6 7 8 9 10 11 12 Sc Ti V Cr Mn Fe Co Ni Cu Zn Al Ga
single-layer transition metal dichalcogenides MC2 Period 1 1 H 18 He 2 Group 1 2 Li Be Group 13 14 15 16 17 18 B C N O F Ne 3 4 Na K Mg Ca Group 3 4 5 6 7 8 9 10 11 12 Sc Ti V Cr Mn Fe Co Ni Cu Zn Al Ga
02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr
 Chemistry 05 B First Letter of PLEASE PRINT YOUR NAME IN BLOCK LETTERS Exam last Name Name: 02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr Lab TA s Name: Question Points Score Grader 2 2 9 3 9 4 2
Chemistry 05 B First Letter of PLEASE PRINT YOUR NAME IN BLOCK LETTERS Exam last Name Name: 02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr Lab TA s Name: Question Points Score Grader 2 2 9 3 9 4 2
Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Figure of rare earth elemental abundances removed due to copyright restrictions.
 Figure of rare earth elemental abundances removed due to copyright restrictions. See figure 3.1 on page 26 of Tolstikhin, Igor and Jan Kramers. The Evolution of Matter: From the Big Bang to the Present
Figure of rare earth elemental abundances removed due to copyright restrictions. See figure 3.1 on page 26 of Tolstikhin, Igor and Jan Kramers. The Evolution of Matter: From the Big Bang to the Present
1 Genesis 1:1. Chapter 10 Matter. Lesson. Genesis 1:1 In the beginning God created the heavens and the earth. (NKJV)
 1 Genesis 1:1 Genesis 1:1 In the beginning God created the heavens and the earth. (NKJV) 1 Vocabulary Saturated having all the solute that can be dissolved at that temperature Neutron a particle with no
1 Genesis 1:1 Genesis 1:1 In the beginning God created the heavens and the earth. (NKJV) 1 Vocabulary Saturated having all the solute that can be dissolved at that temperature Neutron a particle with no
CHEM 108 (Spring-2008) Exam. 3 (105 pts)
 CHEM 08 (Spring-008) Exam. (05 pts) Name: --------------------------------------------------------------------------, CLID # -------------------------------- LAST NAME, First (Circle the alphabet segment
CHEM 08 (Spring-008) Exam. (05 pts) Name: --------------------------------------------------------------------------, CLID # -------------------------------- LAST NAME, First (Circle the alphabet segment
XM1/331 XM1/331 BLFX-3 XM1/331
 a b AkC AkC strontian fluoro-apatite clinopyroxene phlogopite K-richterite XM1/331 clinopyroxene XM1/331 Fe-Ti ox c d clinopyroxene kric AkC ilmenite Sr-barite AkC XM1/331 BLFX-3 Supplementary Figure 1.
a b AkC AkC strontian fluoro-apatite clinopyroxene phlogopite K-richterite XM1/331 clinopyroxene XM1/331 Fe-Ti ox c d clinopyroxene kric AkC ilmenite Sr-barite AkC XM1/331 BLFX-3 Supplementary Figure 1.
