Introduction to the Problem-Solving Methodology (PSM)
|
|
|
- Millicent Wells
- 5 years ago
- Views:
Transcription
1 Problem Name: Problem Description: Date: P01 Simple Reactor Introduction to the Problem-Solving Methodology (PSM) Your Name: Problem Session Objectives To learn the five stages of the problem solving methodology. To learn about the material balances for a continuous reactor. To complete a conceptual model using the problem statement. To write a mathematical model using the conceptual model. To construct a mathematical algorithm using the mathematical model. To create the numerical solution using the mathematical algorithm. To generate heuristic observations about the numerical solution, mathematical algorithm, mathematical model, and conceptual model. Reference Readings Felder and Rousseau, 3 rd Edition, Section 4.1, Process Classification. Felder and Rousseau, 3 rd Edition, Section 4.2, Balances. Felder and Rousseau, 3 rd Edition, Section 4.3, Material Balance Calculations. Felder and Rousseau, 3 rd Edition, Section 4.6, Chemical Reaction Stoichiometry. Felder and Rousseau, 3 rd Edition, Section 4.7, Balances on Reactive Processes. Felder and Rousseau, 3 rd Edition, Chapter 3, Processes and Process Variables. Review Materials Hanyak s Development of a Conceptual Model, CinChE Manual, Chapter 4. Hanyak s Development of a Mathematical Model, CinChE Manual, Chapter 4. Hanyak s Development of a Mathematical Algorithm, CinChE Manual, Chapter 4. Hanyak s Development of a Numerical Solution, CinChE Manual, Chapter 4. Hanyak s Development of the Heuristic Observations, CinChE Manual, Chapter 4. Hanyak s Anatomy of a Math Model and Algorithm, CinChE Manual, Chapter 4. Interaction 1: Topic: Simple Continuous Reactor Background: Welcome to the eleaps problem session about a chemical reactor. Save this script document to the desktop. Click here to open and save the solution template also to the desktop. Close all internet browser windows. Open the two saved documents with Adobe Reader. In the solution template document, right click and select Print, choose Document and Markups under Comments and Forms, and print it to get a PAPER COPY. Print to a color printer for the best effect. You will fill-in this paper copy as you do the problem session. Close the template document and then delete it, since it is no longer needed. v , Michael E. Hanyak, Jr., All Rights Reserved Page 1 of 22
2 P01 eleaps Problem Solution Template Coaching Script and Solution Template This coaching script contains two kinds of pages script and template. They are arranged similar to the left and right pages in a book. The left page is an interaction in the coaching script. The right page is the current focus in the solution template that is associated with the left coaching script page. How you navigate through the coaching script depends up the type of computer that you are using a personal computer with a mouse or an Apple ipad with a stylus pen. In either case, you have opened this coaching script using the Acrobat Reader program that is installed on your computer and not the Acrobat Reader plug-in found in a web browser. Please complete the first interaction in the first coaching script page. Then, proceed to navigate through the coaching script based upon your computer type, as describe below. Personal Computer with a Mouse The Acrobat Reader program should have displayed this coaching script in its two-page view mode. If not, then select the View/Page Display/Two Page Scrolling option from the menu bar. In the two-page view mode, the left column of pages will be the coaching script, while the right column of pages will be the current focus in the solution template. You can magnify the view (i.e., zoom in) so that the coaching script page is readable. Then, you can use the horizontal scroll bar to move between the left page (the coaching script) and its right page (the template solution). After you manually complete a portion of your PAPER COPY of the problem solution template (as directed by its associated coaching script interaction), you can then delete the boxes in the right page to view the correct answers. You can also view the popup notes found in the right page. You proceed to the next script Interaction by scrolling down to the next set of two pages in the Acrobat Reader program. Apple ipad with a Rubber-Domed Stylus Pen The Acrobat Reader app for the ipad (downloaded from the App Store) does not support the two-page view mode. To simulate this viewing mode, select the Single Page option under Document Modes in the menu bar. In the Single Page mode, you will be able to horizontally swipe between the left page (the coaching script) and its right page (the template solution). After you manually complete a portion of your PAPER COPY of the problem solution template (as directed by its associated coaching script interaction), you can then delete the boxes in the right page to view the correct answers. You can also view the popup notes found in the right page. You proceed to the next script Interaction by swiping pass the current right page in the Acrobat Reader app. If you quickly tap the Home button on the ipad twice, you can conveniently switch between the Adobe Reader and any other apps.
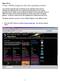
3 Interaction 2: Topic: Simple Reactor Background: A simple reactor problem is used to introduce you to the Problem- Solving Methodology that we will use in this course. Conceptual Model Question: The first step in the problem-solving methodology (PSM) is to analyze the problem statement and create a conceptual model composed of a labeled Diagram, Other Givens, Finds, and initial Assumptions. Chapter 4 in the white CinChE manual presents the guidelines for the development of a conceptual model. This development contains five sub-parts. First, you identify the process operation and represent it as a diagram. View the problem statement and possible diagrams to the right of this script page. Which process diagram applies to this problem? Option 1: Diagram (a). Option 2: Diagram (b). Option 3: Diagram (c). Option 4: Diagram (d). Feedback 1: Incorrect! Diagram (a) is for a batch process, where no material flows in or out. Feedback 2: Incorrect! Diagram (b) is for a continuous process, with one income stream and two out going streams. Feedback 3: Correct! Diagram (c) is for a continuous process with a feed stream entering and a product stream exiting. Feedback 4: Incorrect! Diagram (d) is for a semi-batch process, where some material flows out. Leave the development of a conceptual model browser window open until further notice. v , Michael E. Hanyak, Jr., All Rights Reserved Page 2 of 22
4 Problem Statement Chemists in our Research and Development Department have discovered a catalyst, which will produce styrene monomer from toluene and methanol in one step by the reaction pathway: C 7 H 8 + CH 3 OH C 8 H 8 + H 2 O + H 2 toluene methanol styrene water hydrogen Based on their experimental reactor studies, our chemists report that 80 mole % of the toluene is converted to styrene monomer when an equimolar mixture of methanol and toluene are fed to a small-scale reactor at 520ºC and 300 kpa. For a feed rate of L/s, our company needs to know what the molar flow rate and composition would be for the product stream, which exits the adiabatic reactor as a gas at 337ºC and 270 kpa. The mass flow of this gas stream is also needed in kilograms per hour. As a sophomore chemical engineer in our company, please provide a welldocument solution to this problem using our standard problem-solving methodology. Diagram (a) (b) (c) (d)
5 Interaction 3: Topic: Simple Reactor Background: Second, you label the process state of each material stream in the sketched diagram by analyzing the problem statement. The process state is a stream's temperature, pressure, flow rate, and composition. You also label the phase state of the stream; that is, whether it is a gas, a liquid, a solid, vapor-liquid, etc. When a property is not given, you label it with a question mark. Conceptual Model Question: View the problem statement with its conceptual diagram to the right of this script page. As indicated by the yellow and green highlights, complete labeling the process state of each material stream on Page 1 in your PAPER COPY of the template document. Since the phase of the feed stream is not given, you must determine it or assume it. Currently, you do not have the knowledge to determine it; but, you can use your engineering judgment to assume it. What is the phase state of Stream F? Option 1: liquid. Option 2: gas. Option 3: solid. Option 4: I don t know! Feedback 1: Incorrect! The temperature of the feed stream is well above the normal boiling temperatures for toluene and methanol ( C and 64.7 C at 1 atm, see Table B.1 in F&R, 3rd Edition). Feedback 2: Correct! Since the product stream is a gas, the feed stream must also be a gas because its temperature is higher. Also, the feed stream is an ideal gas, because its pressure is less then or equal to 3 atm (303 kpa or 3.03 bar). Feedback 3: Incorrect! See the explanation under Feedback 1. Feedback 4: Incorrect! The phase state is one of the other three. Try again! v , Michael E. Hanyak, Jr., All Rights Reserved Page 3 of 22
6 Problem Statement Chemists in our Research and Development Department have discovered a catalyst, which will produce styrene monomer from toluene and methanol in one step by the reaction pathway: C7Hlo + CH30H --+ CsHs + Hz0 + H2 toluene methanol Styrene water hydrogen Based on their experimental reactor studies, our chemists report that 80 mole % of the toluene is converted to styrene monomer when an equimolar mixture of methanol and toluene are fed to a small-scale reactor at 520 C and 300 kpa. For a feed rate of Lls, our company needs to know what the molar flow rate and composition would be for the product stream, which exits the adiabatic reactor as a gas at 337OC and 270 kpa. The mass flow of this gas stream is also needed in kilograms per hour. As a sophomore chemical engineer in our company, please provide a welldocument solution to this problem using our standard problem-solving methodology. other Givens:
7 Interaction 4: Topic: Simple Reactor Background: View the problem statement with its labeled diagram to the right of this script page. As shown in the diagram, molar variables were chosen for the flow rates and compositions. The flow rate variables associated with streams in the diagram could be mass or molar quantities, but NOT volumetric flow rates. Also, the composition variables could be mass or mole fractions, but NOT volume fractions or concentrations. After reading the problem statement, you must decide which to use mass or molar quantities. Conceptual Model Which reasons below suggest that molar quantities should be chosen Question: for the diagram? Option 1: Most given and find quantities are in mass units. Option 2: Reaction material balances based on moles are simpler to write. Option 3: Most given and find quantities are in molar units. Option 4: Reaction material balances based on mass are easier to write. Feedback 1: Incorrect! Only one mass quantity is to be found. Feedback 2: Correct! Mole balances with chemical reactions are easier to solve. Feedback 3: Correct! One molar quantity is given, and two others are to be found. Feedback 4: Incorrect! Mass balances with chemical reactions are harder to write and solve. v , Michael E. Hanyak, Jr., All Rights Reserved Page 4 of 22
8 Problem Statement Chemists in our Research and Development Department have discovered a catalyst, which will produce styrene monomer from toluene and methanol in one step by the reaction pathway: C7Hlo + CH30H --+ CsHs + Hz0 + H2 toluene methanol Styrene water hydrogen Based on their experimental reactor studies, our chemists report that 80 mole % of the toluene is converted to styrene monomer when an equimolar mixture of methanol and toluene are fed to a small-scale reactor at 520 C and 300 kpa. For a feed rate of Lls, our company needs to know what the molar flow rate and composition would be for the product stream, which exits the adiabatic reactor as a gas at 337OC and 270 kpa. The mass flow of this gas stream is also needed in kilograms per hour. As a sophomore chemical engineer in our company, please provide a welldocument solution to this problem using our standard problem-solving methodology. other Givens:
9 Interaction 5: Topic: Simple Reactor Background: As shown in the diagram to the right of this script page and also in your PAPER COPY of the template document, the product stream was labeled to contain toluene (TL), methanol (ME), styrene monomer (SM), water (WA), and hydrogen (H2). Why? Conceptual Model Question: Since only partial conversion of the toluene occurs, left over toluene and methanol must appear in the product. Furthermore, the toluene that reacts with methanol, based on the chemical reaction, produces styrene monomer, water, and hydrogen. These chemical components (or compounds) must appear in the product stream. What percent conversion of toluene would lead to NO toluene and methanol appearing in the product stream? Option 1: 0%. Option 2: 35%. Option 3: 70%. Option 4: 100%. Feedback 1: Incorrect! A molar conversion of 0% for toluene would mean that no reactions take place. Thus, all of the toluene and methanol that enters the reactor would leave in the product stream. Feedback 2: Incorrect! A molar conversion of 35% for toluene would mean that some of the toluene reacts with the methanol (on a 1:1 molar basis). The unreacted toluene and methanol that entered the reactor would leave in the product stream. Feedback 3: Incorrect! A molar conversion of 70% for toluene would mean that some of the toluene reacts with the methanol (on a 1:1 molar basis). The unreacted toluene and methanol that entered the reactor would leave in the product stream. Feedback 4: Correct! A molar conversion of 100% for toluene would mean that all of the toluene reacts entirely with methanol (on a 1:1 molar basis). Thus, no toluene and methanol would leave in the product stream. As another example, let's say the feed to the reactor was NOT equimolar but 40% toluene and 60% methanol on a molar basis. For 100% conversion, no toluene would appear in the product stream, but some methanol would, because toluene is the limiting reactant. v , Michael E. Hanyak, Jr., All Rights Reserved Page 5 of 22
10 Problem Statement Chemists in our Research and Development Department have discovered a catalyst, which will produce styrene monomer from toluene and methanol in one step by the reaction pathway: C7Hlo + CH30H --+ CsHs + Hz0 + H2 toluene methanol Styrene water hydrogen Based on their experimental reactor studies, our chemists report that 80 mole % of the toluene is converted to styrene monomer when an equimolar mixture of methanol and toluene are fed to a small-scale reactor at 520 C and 300 kpa. For a feed rate of Lls, our company needs to know what the molar flow rate and composition would be for the product stream, which exits the adiabatic reactor as a gas at 337OC and 270 kpa. The mass flow of this gas stream is also needed in kilograms per hour. As a sophomore chemical engineer in our company, please provide a welldocument solution to this problem using our standard problem-solving methodology. other Givens:
11 Interaction 6: Topic: Simple Reactor Background: Third, you list other given information that appears directly and/or is implied in the problem statement. For example, the chemical reaction was written and then balanced. Conceptual Model Question: Fourth, you list the quantities with units that are to be found. For example, the total molar flow rate of Stream P was written. View the problem statement with its conceptual diagram to the right of this script page. As indicated by the yellow and green highlights, complete labeling the rest of the Givens and Finds on Page 1 in your PAPER COPY of the template document. Note that for the "Finds," you choose the units for the important variables to be calculated. These units are either given in the problem or selected by you. Why was kgmol/h picked for the molar flow rate of Stream P? Option 1: It does not matter what you pick! Option 2: It does matter what you pick! Feedback 1: Incorrect! See the explanation under Feedback 2. Feedback 2: Correct! Because you are asked to find the mass flow rate of the product in kg/h, anything other than kgmol/h, such as g-mol/h or lbmol/h, will probably require more unit conversions when doing the calculations. v , Michael E. Hanyak, Jr., All Rights Reserved Page 6 of 22
12 Problem Statement Chemists in our Research and Development Department have discovered a catalyst, which will produce styrene monomer from toluene and methanol in one step by the reaction pathway: C7Hlo + CH30H --+ CsHs + Hz0 + H2 toluene methanol Styrene water hydrogen Based on their experimental reactor studies, our chemists report that 80 mole % of the toluene is converted to styrene monomer when an equimolar mixture of methanol and toluene are fed to a small-scale reactor at 520 C and 300 kpa. For a feed rate of Lls, our company needs to know what the molar flow rate and composition would be for the product stream, which exits the adiabatic reactor as a gas at 337OC and 270 kpa. The mass flow of this gas stream is also needed in kilograms per hour. As a sophomore chemical engineer in our company, please provide a welldocument solution to this problem using our standard problem-solving methodology. other Givens:
13 Interaction 7: Topic: Simple Reactor Background: Fifth and final sub-part in the development of a conceptual model, you begin listing the initial assumptions, in order to complete the conceptual model. For Assumption 1, identify the process type, either continuous, batch, semi-batch, or semi-continuous. For Assumption 2, identify the operation state, either steady state or unsteady state for the material within the imaginary system boundary. As indicated by the yellow highlight to the right of this script page, complete labeling the first two assumptions on Page 1 in your PAPER COPY of the template document. Conceptual Model What would be the third assumption? Question: Option 1: Stream P is a non-ideal gas. Option 2: Stream F is an ideal gas. Feedback 1: Incorrect! Because the pressure of Stream P is less then or equal to 3 atm (303 kpa or 3.03 bar), it can be treated as an ideal gas. Feedback 2: Correct! Because the pressure of Stream F is less then or equal to 3 atm (303 kpa or 3.03 bar), it can be treated as an ideal gas. Before continuing, close the development of a conceptual model browser window. v , Michael E. Hanyak, Jr., All Rights Reserved Page 7 of 22
14 Continuous Reactor Problem Statement Chemists in our Research and Development Department have discovered a catalyst, which will produce styrene monomer from toluene and methanol in one step by the reaction pathway: C7Hlo + CH30H --+ CsHs + Hz0 + H2 toluene methanol Styrene water hydrogen Based on their experimental reactor studies, our chemists report that 80 mole % of the toluene is converted to styrene monomer when an equimolar mixture of methanol and toluene are fed to a small-scale reactor at 520 C and 300 kpa. For a feed rate of Lls, our company needs to know what the molar flow rate and composition would be for the product stream, which exits the adiabatic reactor as a gas at 337OC and 270 kpa. The mass flow of this gas stream is also needed in kilograms per hour. As a sophomore chemical engineer in our company, please provide a welldocument solution to this problem using our standard problem-solving methodology. other Givens: v , Michael E. Hanyak, Jr., All Rights Reserved 1 of 6
15 Interaction 8: Topic: Simple Reactor Background: The second step in the problem-solving methodology (PSM) is to review the conceptual model and create a mathematical model composed of first principle equations, additional equations, and a degrees-of-freedom analysis. Mathematical Model Question: Chapter 4 in the white CinChE manual presents the guidelines for the development of a mathematical model. Using Guidelines 1 to 5, you are to finish writing the first-principle equations in your PAPER COPY of the template document in the first six equations, as indicated by the yellow highlights to the right of this script page. In the total balance, the reaction term is a number times the extent of reaction. The number is found by summing the product coefficients in the BALANCED chemical reaction and then subtracting the sum of the reactant coefficients in that same reaction. In a component balance, the number in a reaction term is the component's coefficient from the chemical reaction with a negative sign for a reactant or a positive sign for a product. If the component does not participate in the chemical reaction, then its number is zero; that is, the reaction term is not written. Writing material balances is an accounting technique on the chemical substances that are associated with the system boundary. You can count atoms, molecules, or mass. Why was the accounting done the way it is shown in the first six equations of the mathematical model? Option 1: because most information in the conceptual model is given in terms of atoms. Option 2: because most information in the conceptual model is given in terms of mass. Option 3: because most information in the conceptual model is given in terms of moles. Feedback 1: Incorrect! The conceptual model contains mostly molar quantities. You use atom balances only when you know that chemical reactions are occurring, but you have NOT be given what those reactions are. If you do not know the reactions, you can not write the extent-of-reaction terms [ R s or ξ s ( Xi) ] that appear in the mole balances. Feedback 2: Incorrect! The conceptual model contains mostly molar quantities. You rarely use mass balances when chemical reactions are occurring, because the resulting equations tend to be more complex than the mole balances. Feedback 3: Correct! Since the conceptual model contains mostly molar quantities, you use mole balances when chemical reactions are occurring, and you know the chemical reactions. The mole balance equations tend not to be as complex as the mass balance equations v , Michael E. Hanyak, Jr., All Rights Reserved Page 8 of 22
16 Continuous Reactor v , Michael E. Hanyak, Jr., All Rights Reserved 2 of 6
17 Interaction 9: Topic: Simple Reactor Background: Guidelines 1 to 4 produced the six material balance equations shown in your PAPER COPY of the template document for the mathematical model. Guideline 5 adds any additional assumptions to the conceptual model. For this example, no additional assumptions where added. Mathematical Model Question: At this point in the development of the mathematical model, you should inspect the material balances for correctness. For example, the reaction terms in the component balances should sum to the same reaction term in the total balance. Component flow rate terms with mole fractions should sum to a term in the total balance. Component flow rate terms without mole fractions should have a proper sign, positive for an incoming flow and negative for an outgoing flow. Using Guideline 6, you are to finish writing the mixture equation for Stream P in the check equation on your PAPER COPY of the template document, as indicated by the yellow highlight to the right of this script page. Guideline 7 was used to select the CHECK equation, and the application of Guideline 8 resulted in numbering the linear independent equations in the mathematical model. Applying Guideline 9, what are the degrees of freedom for the first six linear independent equations? Option 1: zero. Option 2: one. Option 3: two. Feedback 1: Incorrect! A DOF = 0 means that you have as many unknown variables as equations. Since a flow rate is known in the problem and we have not used it yet in the mathematical model, the DOF cannot be zero. Try counting again. Feedback 2: Incorrect! A DOF = 1 means that you have one more unknown variable as you do equations. However, the degrees of freedom are greater than one for the first six equations. Try counting again. Feedback 3: Correct! The DOF for the first six equations is two. Since a flow rate is known in the problem, we would like to have the DOF be one. We need to add one or more equations to the math model, in order to get the DOF to become one. v , Michael E. Hanyak, Jr., All Rights Reserved Page 9 of 22
18 Continuous Reactor v , Michael E. Hanyak, Jr., All Rights Reserved 2 of 6
19 Interaction 10: Topic: Simple Reactor Background: Guidelines 6 and 7 produced the mixture equation and identified it as the CHECK. Guideline 9 lead to the degrees of freedom (DOF) equal to two. Mathematical Model Question: Using Guideline 10, you should inspect the conceptual model to see if any information has not been used yet in the mathematical model. Based on that inspection, you are to finish writing the conversion equation (Eq. 7) and re-calculating the DOF on your PAPER COPY of the template document, as indicated by the yellow highlights to the right of this script page. The conversion equation increased the equation count by one, but it did not increase the variable count. Since the DOF now equals 1, is a molar flow rate given in the conceptual model? Option 1: yes. Option 2: no. Feedback 1: Incorrect! A molar flow rate is NOT given in the conceptual model; however, a volumetric flow rate for Stream F is known. Feedback 2: Correct! A volumetric flow rate for Stream F is given. You must find a way to relate the volumetric flow rate to the molar flow rate that appears in the material balances. v , Michael E. Hanyak, Jr., All Rights Reserved Page 10 of 22
20 Continuous Reactor v , Michael E. Hanyak, Jr., All Rights Reserved 2 of 6
21 Interaction 11: Topic: Simple Reactor Background: Using Guide 11, you are to write an equation in the mathematical model that relates the volumetric and molar flow rates of Stream F in Eq. 8 on your PAPER COPY of the template document, as indicated by the yellow highlight to the right of this script page. The third assumption in the conceptual model provides a clue for you. Mathematical Model Question: Also, you are to re-calculate the degrees of freedom. What variables in the conceptual model do you know that would satisfy the DOF in the mathematical model? Option 1: the T and P of Stream F and the T and P of Stream P. Option 2: the T and P of Stream F, as well as its two mole fractions. Option 3: the T and P of Stream F, its volumetric flow rate, and the gas constant. Feedback 1: Incorrect! See the explanation under Feedback 3. Feedback 2: Incorrect! See the explanation under Feedback 3. Feedback 3: Correct! The re-calculate DOF is four. Since the ideal gas law was applied to Stream F, its temperature, pressure, and volumetric flow rate are known. You can find values for the gas constant in the back cover of the Felder and Rousseau textbook. v , Michael E. Hanyak, Jr., All Rights Reserved Page 11 of 22
22 Continuous Reactor v , Michael E. Hanyak, Jr., All Rights Reserved 2 of 6
23 Interaction 12: Topic: Simple Reactor Background: Using Guide 12, you are to write the additional equations need to complete the mathematical model (i.e., Eqs. 9 to 15) on your PAPER COPY of the template document, as indicated by the yellow highlights to the right of this script page. Mathematical Model Question: Using Guide 13, you are to show the degrees of freedom (DOF) for the completed math model. After reviewing Guide 14, what are the five additional variables needed to satisfy the DOF of nine? Option 1: the T and P of Stream P, the two mole fractions of Stream F, and the molar conversion appearing in the conceptual model. Option 2: the molecular weights of the five chemical components (i.e., compounds) appearing in the mathematical model. Feedback 1: Incorrect! The T and P of Stream P are not required to solve the problem. The two mole fractions and molar conversion already appear in the mathematical model as numbers. Feedback 2: Correct! The five molecular weights are considered constants in the mathematical model, since you can look them up in Table B.1 of the Felder and Rousseau textbook. To simplify the counting process in future problems that we will solve, we will establish the convention NOT to count constants, like the gas constant and molecular weight, as variables. If we had followed this convention for this problem, the DOF would be three and not nine. Before continuing, close the development of a mathematical model browser window. v , Michael E. Hanyak, Jr., All Rights Reserved Page 12 of 22
24 Continuous Reactor v , Michael E. Hanyak, Jr., All Rights Reserved 2 of 6
25 Interaction 13: Topic: Simple Reactor Background: The third step in the problem-solving methodology (PSM) is to transform the mathematical model into a mathematical algorithm. A mathematical algorithm does not tell you how to solve, but it identifies the order in which the equations are to be solved. Mathematical Algorithm Question: Click here to see the guidelines for the development of a mathematical algorithm. Using Guideline 1, you are to finish writing the functional form for the "reactor" mathematical algorithm on Page 3 in your PAPER COPY of the template document, as indicated by the yellow highlights to the right of this script page. For this problem only, we are counting constants as being variables. Thus, we must list those constants as independent variables in the functional form for the mathematical algorithm. In future problems, we will not list constants because we will not count them as variables. Could a mathematical model have more than one mathematical algorithm? Option 1: yes. Option 2: no. Feedback 1: Correct! A problem could have two parts that use the same mathematical model. One part might ask to solve the mathematical model for a specific set of known variables. The other part could be for another set of known variables. The two mathematical algorithms would be different, but they both would have the same mathematical model. Feedback 2: Incorrect! See the explanation under Feedback 1. v , Michael E. Hanyak, Jr., All Rights Reserved Page 13 of 22
26 Continuous Reactor v , Michael E. Hanyak, Jr., All Rights Reserved 3 of 6
27 Interaction 14: Topic: Simple Reactor Background: Using Guidelines 2 to 3, identify the first equation and write it as the first step in the mathematical algorithm on Page 3 in your PAPER COPY of the template document, as indicated by the yellow highlights to the right of this script page. Using Steps 3 to 6 of the Partitioning Procedure, you are to complete the development of the mathematical algorithm on your PAPER COPY of the template document, as indicated by the yellow highlights to the right of this script page. v , Michael E. Hanyak, Jr., All Rights Reserved Page 14 of 22
28 Continuous Reactor v , Michael E. Hanyak, Jr., All Rights Reserved 3 of 6
29 Interaction 15: Topic: Simple Reactor Background: Inspect the complete mathematical algorithm to the right of this script page for correctness. Start with the first assignment; look at each variable in the expression on the right side of the arrow. Ask yourself if this variable is specified or a constant; that is, appears in the right side of the functional form for the mathematical algorithm. If yes, move on to the next variable in the expression. If it is not a specified variable or constant, then it must be a calculated variable. Ask yourself, has it been calculated by a previous step in the mathematical algorithm. If yes, then move on to the next variable in the expression checking it for correctness. If all variables in the expression have been checked, then move on to the next assignment and check it for correctness. If no, then the assignment is not in the correct position of the mathematical algorithm. Fix it by repositioning it to a correct place. Mathematical Algorithm The mathematical algorithm to the right of this script page has eleven Question: steps, all of which are assignment statements. Why is that (select one or more that apply)? Option 1: because Step 6 in the Partitioning Procedure did not apply. Option 2: because Step 4 in the Partitioning Procedure did apply. Option 3: because Step 3 in the Partitioning Procedure did apply. Option 4: because Step 5 in the Partitioning Procedure did apply. Feedback 1: Correct! Step 6 of the Partitioning Procedure was never used. If it was used, the mathematical algorithm would have fewer steps, because a nonlinear set of equations would need to be solved simultaneously. Thus, this NSOLVE construct would be counted as one step, but it would contain more than one equation. Feedback 2: Incorrect! Step 4 of the Partitioning Procedure was never used. If it was, it would have created an assignment for an equation. Feedback 3: Correct! Step 3 of the Partitioning Procedure was the only one needed to transform the mathematical model into the mathematical algorithm. Steps 4 to 6 were not used, because all of the equations could be solved one by one. Feedback 4: Incorrect! Step 5 of the Partitioning Procedure was never used. If it was used, the mathematical algorithm would have fewer steps, because a linear set of equations would need to be solved simultaneously. Thus, this SOLVE construct would be counted as one step, but it would contain more than one equation. v , Michael E. Hanyak, Jr., All Rights Reserved Page 15 of 22
30
31 Interaction 16: Topic: Simple Reactor Background: After completing the Partitioning Procedure, some equations in the mathematical algorithm must be analyzed for consistency of units. Click here to view the guidelines for the Dimensional Consistency Analysis, and then click here to view the results of its application to the mathematical algorithm on the right. Mathematical Algorithm Question: Two forms of the Check equation exist -- a standard form and its normalized form. The standard form says the total flow rate is equal to the sum of its component flow rates. The normalized form says the sum of the composition quantities must equal one. The normalized form is gotten by dividing the total flow rate through both sides of the standard form equation. These two forms are shown after the mathematical algorithm on Page 3 in your PAPER COPY of the template document and also as Page 3 to the right of this script page, only to make a point. We will not write the check equation as being part of the mathematical algorithm. You must choose which form to use when doing your check on the Numerical Solution under the Heuristic Observation part of the Problem-Solving Methodology. Which CHECK equation should be chosen, because it will check more steps in the mathematical algorithm? Option 1: standard form; that is, total = sum of the components. Option 2: normalized form; that is, component composition must sum to one. Feedback 1: Incorrect! The standard form would only check the first eight steps in the mathematical algorithm. Feedback 2: Correct! The normalized form would check the first thirteen steps in the mathematical algorithm. The last two steps would be checked using the total mass balance. Before continuing, close the development of a mathematical algorithm browser window. v , Michael E. Hanyak, Jr., All Rights Reserved Page 16 of 22
32 Continuous Reactor v , Michael E. Hanyak, Jr., All Rights Reserved 3 of 6
33 Interaction 17: Topic: Simple Reactor Background: The fourth step in the problem-solving methodology (PSM) is to generate the Numerical Solution using the mathematical algorithm as a blueprint or guide. Click here to see the guidelines for the development of a numerical solution. Using Guidelines 1 and 2, you are to finish writing the basis and the givens on Page 4 in your PAPER COPY of the template document, as indicated by the yellow highlights to the right of this script page. Also, you are to finish the calculations on Page 4, using Guideline 3. Numerical Solution Because a chemical reaction is occurring, the extent of reaction Question: [ R or ξ ( Xi) ] appears in the total and component mole balances. What are the proper units for the extent of reaction? Option 1: kg-mol/h. Option 2: g-rxns/h. Option 3: g-mol/h. Option 4: kg-rxns/h. Feedback 1: Incorrect! The units of kg-mol/h represent the number of molecules flowing per unit of time. Feedback 2: Incorrect! The units of g-rxns/h represent the number of chemical reactions occurring per unit of time, when the molar flow rate has units of g-mol/h. Feedback 3: Incorrect! The units of g-mol/h represent the number of molecules flowing per unit of time. Feedback 4: Correct! The units of kg-rxns/h represent the number of chemical reactions occurring per unit of time [ R or ξ ( Xi) ], when the molar flow rate has units of kg-mol/h. v , Michael E. Hanyak, Jr., All Rights Reserved Page 17 of 22
34 Continuous Reactor v , Michael E. Hanyak, Jr., All Rights Reserved 4 of 6
35 Interaction 18: Topic: Simple Reactor Background: Using Guidelines 5 to 6, you are to account for precision and box-in the answers on Page 4 in your PAPER COPY of the template document, as indicated by the yellow highlights to the right of this script page. Numerical Solution What is the precision for this problem? Question: Option 1: two digits. Option 2: three digits. Option 3: four digits. Feedback 1: Correct! The "Finds" quantities are to be reported to 2-digits of precision. The least significant digits in the problem statement are two digits. Feedback 2: Incorrect! See the explanation under Feedback 1. Feedback 3: Incorrect! See the explanation under Feedback 1. Before continuing, close the development of a numerical solution browser window.. v , Michael E. Hanyak, Jr., All Rights Reserved Page 18 of 22
36 Continuous Reactor v , Michael E. Hanyak, Jr., All Rights Reserved 4 of 6
37 Interaction 19: Topic: Simple Reactor Background: The fifth step in the problem-solving methodology (PSM) is to generate the Heuristic Observations about the numerical solution, the mathematical algorithm, the mathematical model, and the conceptual model. Click here to see the guidelines for the development of the heuristic observation. Using Guideline 1, you are to finish writing the heuristic observations for the numerical solution on Page 5 in your PAPER COPY of the template document, as indicated by the yellow highlights to the right of this script page. Heuristic Observations What equation checks the last two steps in the numerical solution? Question: Option 1: the sum of the mole fractions equals one. Option 2: total mass flow in equals total mass flow out. Feedback 1: Incorrect! The normalized mixture equation for Stream P checks only the first thirteen equations in the numerical solution. Feedback 2: Correct! The total mass balance checks the last two steps in the numerical solution. Since total mass is always conserved during chemical reactions, using the total mass balance is a very good check equation, whenever the mathematical model contains molar flow rate variables. v , Michael E. Hanyak, Jr., All Rights Reserved Page 19 of 22
38 Continuous Reactor v , Michael E. Hanyak, Jr., All Rights Reserved 5 of 6
39 Interaction 20: Topic: Simple Reactor Background: Guideline 2 was applied to generate a heuristic observation. Specifically, how would the mathematical algorithm change if the total molar flow rate of Stream P where known and you wanted to calculate the volumetric flow rate of Stream F? Heuristic Observations Question: The answer to this what if question is shown as the mathematical algorithm on Page 5 in your PAPER COPY of the template document and also as Page 5 to the right of this script page. Compared to the original mathematical algorithm, how complex is the new algorithm? Option 1: less complex. Option 2: about the same. Option 3: more complex. Feedback 1: Incorrect! See the explanation under Feedback 3. Feedback 2: Incorrect! See the explanation under Feedback 3. Feedback 3: Correct! The new algorithm is more complex, because it contains a SOLVE construct. The original algorithm has no such construct. v , Michael E. Hanyak, Jr., All Rights Reserved Page 20 of 22
40 Continuous Reactor v , Michael E. Hanyak, Jr., All Rights Reserved 5 of 6
41 Interaction 21: Topic: Simple Reactor Background: Guidelines 3 and 4 were applied to give the heuristic observations that answer the following "what if" questions: How would the mathematical model change if the total mole balance was the CHECK equation or if the IDEAL GAS assumption was dropped? How would the conceptual model and the mathematical model change if a second reaction were occurring? The answers to these questions are shown on Page 6 in your PAPER COPY of the template document and also as Page 6 to the right of this script page. Heuristic Observations The function eos[... ] is for an equation of state. Which ONES of the Question: following ways could be used to represent the function "eos"? Option 1: table. Option 2: graph. Option 3: equations. Option 4: computer program. Feedback 1: Correct! Any function can be represented by a table, a graph, a set of equations, or a computer program like HYSYS or Excel Solver. Feedback 2: Correct! See the explanation under Feedback 1. Feedback 3: Correct! See the explanation under Feedback 1. Feedback 4: Correct! See the explanation under Feedback 1. Before continuing, close the development of heuristic observations browser window.. v , Michael E. Hanyak, Jr., All Rights Reserved Page 21 of 22
42 Continuous Reactor v , Michael E. Hanyak, Jr., All Rights Reserved 6 of 6
43 Interaction 22: Topic: Simple Reactor Background: Thank you for completing this problem session. Please place your filled-in PAPER COPY of the template document in your technical journal. Click here for the correct solution to the template document. If you so desire, you could print this eleaps script (two pages per sheet and on both side of a sheet) and place it also in your technical journal. If you have any questions or concerns about the problem session, please contact your instructor. Read the Important Observations below and consult the Reference Readings and Review Materials. Problem Session Observations The problem solving methodology is a multi-step procedure to solve the problem. The differential material balances are used for a continuous process. The conceptual model concisely presents the problem in terms of variables. The mathematical model presents the first principles to model the problem. The mathematical algorithm presents the order in which to solve the equations. The numerical solution identifies the basis and presents the final answers. The heuristic observations provide important reflections about the solution. Reference Readings Felder and Rousseau, 3 rd Edition, Section 4.1, Process Classification. Felder and Rousseau, 3 rd Edition, Section 4.2, Balances. Felder and Rousseau, 3 rd Edition, Section 4.3, Material Balance Calculations. Felder and Rousseau, 3 rd Edition, Section 4.6, Chemical Reaction Stoichiometry. Felder and Rousseau, 3 rd Edition, Section 4.7, Balances on Reactive Processes. Felder and Rousseau, 3 rd Edition, Chapter 3, Processes and Process Variables. Review Materials Hanyak s Development of a Conceptual Model, CinChE Manual, Chapter 4. Hanyak s Development of a Mathematical Model, CinChE Manual, Chapter 4. Hanyak s Development of a Mathematical Algorithm, CinChE Manual, Chapter 4. Hanyak s Development of a Numerical Solution, CinChE Manual, Chapter 4. Hanyak s Development of the Heuristic Observations, CinChE Manual, Chapter 4. Hanyak s Anatomy of a Math Model and Algorithm, CinChE Manual, Chapter 4. v , Michael E. Hanyak, Jr., All Rights Reserved Page 22 of 22
Chapter 4. Problem SM.7 Ethylbenzene/Styrene Column
 Background Chapter 4. Problem SM.7 Ethylbenzene/Styrene Column In Problem SM.6 of the HYSYS manual, a modified form of successive substitution, called the Wegstein method, was used to close the material
Background Chapter 4. Problem SM.7 Ethylbenzene/Styrene Column In Problem SM.6 of the HYSYS manual, a modified form of successive substitution, called the Wegstein method, was used to close the material
1. (25 points) C 6 H O 2 6CO 2 + 7H 2 O C 6 H O 2 6CO + 7H 2 O
 MEEBAL Exam 2 November 2013 Show all work in your blue book. Points will be deducted if steps leading to answers are not shown. No work outside blue books (such as writing on the flow sheets) will be considered.
MEEBAL Exam 2 November 2013 Show all work in your blue book. Points will be deducted if steps leading to answers are not shown. No work outside blue books (such as writing on the flow sheets) will be considered.
Stoichiometric Reactor Simulation Robert P. Hesketh and Concetta LaMarca Chemical Engineering, Rowan University (Revised 4/8/09)
 Stoichiometric Reactor Simulation Robert P. Hesketh and Concetta LaMarca Chemical Engineering, Rowan University (Revised 4/8/09) In this session you will learn how to create a stoichiometric reactor model
Stoichiometric Reactor Simulation Robert P. Hesketh and Concetta LaMarca Chemical Engineering, Rowan University (Revised 4/8/09) In this session you will learn how to create a stoichiometric reactor model
Chapter 4. Fundamentals of Material Balance
 Chapter 4 Fundamentals of Material Balance Introduction to Chapter 4 1) In chapter 4 we will present methods for organizing known information about process variables, setting up martial balance equations,
Chapter 4 Fundamentals of Material Balance Introduction to Chapter 4 1) In chapter 4 we will present methods for organizing known information about process variables, setting up martial balance equations,
LAB 2 - ONE DIMENSIONAL MOTION
 Name Date Partners L02-1 LAB 2 - ONE DIMENSIONAL MOTION OBJECTIVES Slow and steady wins the race. Aesop s fable: The Hare and the Tortoise To learn how to use a motion detector and gain more familiarity
Name Date Partners L02-1 LAB 2 - ONE DIMENSIONAL MOTION OBJECTIVES Slow and steady wins the race. Aesop s fable: The Hare and the Tortoise To learn how to use a motion detector and gain more familiarity
Process Classification
 Process Classification Before writing a material balance (MB) you must first identify the type of process in question. Batch no material (mass) is transferred into or out of the system over the time period
Process Classification Before writing a material balance (MB) you must first identify the type of process in question. Batch no material (mass) is transferred into or out of the system over the time period
Using Microsoft Excel
 Using Microsoft Excel Objective: Students will gain familiarity with using Excel to record data, display data properly, use built-in formulae to do calculations, and plot and fit data with linear functions.
Using Microsoft Excel Objective: Students will gain familiarity with using Excel to record data, display data properly, use built-in formulae to do calculations, and plot and fit data with linear functions.
Comprehend and execute the 10 elements of effective problem
 Lecture 8, 3/9/2012 Chapter 7: A GENERAL Strategy for Solving Material Balance Problems Objectives: Comprehend and execute the 10 elements of effective problem Drive a flow chart and Place labels on the
Lecture 8, 3/9/2012 Chapter 7: A GENERAL Strategy for Solving Material Balance Problems Objectives: Comprehend and execute the 10 elements of effective problem Drive a flow chart and Place labels on the
 MEEBAL Exam 1 October 2012 Show all work in your blue book. Points will be deducted if steps leading to answers are not shown. No work outside blue books (such as writing on the flow sheets) will be considered.
MEEBAL Exam 1 October 2012 Show all work in your blue book. Points will be deducted if steps leading to answers are not shown. No work outside blue books (such as writing on the flow sheets) will be considered.
Objective: To Evaluate Predictions of Alternative Models
 www.optience.com Methanol Synthesis Objective: To Evaluate Predictions of Alternative Models In this example, we propose and evaluate Mass Action and Langmuir Hinshelwood (LHHW) models for methanol synthesis
www.optience.com Methanol Synthesis Objective: To Evaluate Predictions of Alternative Models In this example, we propose and evaluate Mass Action and Langmuir Hinshelwood (LHHW) models for methanol synthesis
Shifting Reactions B
 Shifting Reactions B Name Lab Section Log on to the Internet. Type the following address into the location-input line of your browser: http://introchem.chem.okstate.edu/dcicla/ergbn.htm This will load
Shifting Reactions B Name Lab Section Log on to the Internet. Type the following address into the location-input line of your browser: http://introchem.chem.okstate.edu/dcicla/ergbn.htm This will load
Computational Chemistry Lab Module: Conformational Analysis of Alkanes
 Introduction Computational Chemistry Lab Module: Conformational Analysis of Alkanes In this experiment, we will use CAChe software package to model the conformations of butane, 2-methylbutane, and substituted
Introduction Computational Chemistry Lab Module: Conformational Analysis of Alkanes In this experiment, we will use CAChe software package to model the conformations of butane, 2-methylbutane, and substituted
(1) This reaction mechanism includes several undesired side reactions that produce toluene and benzene:
 HYSYS Multiple Reactions - Styrene Prepared by Robert P. Hesketh Spring 005 Styrene Reactor System You have been studying how to use HYSYS using the example of a Styrene reactor system. In this session
HYSYS Multiple Reactions - Styrene Prepared by Robert P. Hesketh Spring 005 Styrene Reactor System You have been studying how to use HYSYS using the example of a Styrene reactor system. In this session
C 6 H H 2 C 6 H 12. n C6H12 n hydrogen n benzene. n C6H6 n H2 100 C 6 H 6 n 2 n C6H H 2. n 1
 1. Cyclohexane (C 6 H 12 ) can be made by the reaction of benzene (C 6 H 6 ) and hydrogen gas. The products from the reactor are sent to a separator where the cyclohexane and some of the unreacted hydrogen
1. Cyclohexane (C 6 H 12 ) can be made by the reaction of benzene (C 6 H 6 ) and hydrogen gas. The products from the reactor are sent to a separator where the cyclohexane and some of the unreacted hydrogen
A GENERAL Strategy for Solving Material Balance Problems. Comprehend and execute the 10 elements of effective problem
 Chapter 7: A GENERAL Strategy for Solving Material Balance Problems Objectives: Comprehend and execute the 10 elements of effective problem Drive a flow chart and Place labels on the diagram. Choose a
Chapter 7: A GENERAL Strategy for Solving Material Balance Problems Objectives: Comprehend and execute the 10 elements of effective problem Drive a flow chart and Place labels on the diagram. Choose a
EXPERIMENT 15. USING CONDUCTIVITY TO LOOK AT SOLUTIONS: DO WE HAVE CHARGED IONS OR NEUTRAL MOLECULES? rev 7/09
 EXPERIMENT 15 USING CONDUCTIVITY TO LOOK AT SOLUTIONS: DO WE AVE CARGED IONS OR NEUTRAL MOLECULES? rev 7/09 GOAL After you complete this experiment, you should have a better understanding of aqueous solutions
EXPERIMENT 15 USING CONDUCTIVITY TO LOOK AT SOLUTIONS: DO WE AVE CARGED IONS OR NEUTRAL MOLECULES? rev 7/09 GOAL After you complete this experiment, you should have a better understanding of aqueous solutions
ChE 344 Winter 2013 Mid Term Exam I Tuesday, February 26, Closed Book, Web, and Notes. Honor Code
 ChE 344 Winter 2013 Mid Term Exam I Tuesday, February 26, 2013 Closed Book, Web, and Notes Name Honor Code (Sign at the end of exam period) 1) / 5 pts 2) / 5 pts 3) / 5 pts 4) / 5 pts 5) / 5 pts 6) / 5
ChE 344 Winter 2013 Mid Term Exam I Tuesday, February 26, 2013 Closed Book, Web, and Notes Name Honor Code (Sign at the end of exam period) 1) / 5 pts 2) / 5 pts 3) / 5 pts 4) / 5 pts 5) / 5 pts 6) / 5
Reactors. Reaction Classifications
 Reactors Reactions are usually the heart of the chemical processes in which relatively cheap raw materials are converted to more economically favorable products. In other cases, reactions play essential
Reactors Reactions are usually the heart of the chemical processes in which relatively cheap raw materials are converted to more economically favorable products. In other cases, reactions play essential
Exercises for Windows
 Exercises for Windows CAChe User Interface for Windows Select tool Application window Document window (workspace) Style bar Tool palette Select entire molecule Select Similar Group Select Atom tool Rotate
Exercises for Windows CAChe User Interface for Windows Select tool Application window Document window (workspace) Style bar Tool palette Select entire molecule Select Similar Group Select Atom tool Rotate
POC via CHEMnetBASE for Identifying Unknowns
 Table of Contents A red arrow was used to identify where buttons and functions are located in CHEMnetBASE. Figure Description Page Entering the Properties of Organic Compounds (POC) Database 1 Swain Home
Table of Contents A red arrow was used to identify where buttons and functions are located in CHEMnetBASE. Figure Description Page Entering the Properties of Organic Compounds (POC) Database 1 Swain Home
Chemistry 112 Laboratory Experiment 7: Determination of Reaction Stoichiometry and Chemical Equilibrium
 Chemistry 112 Laboratory Experiment 7: Determination of Reaction Stoichiometry and Chemical Equilibrium Introduction The word equilibrium suggests balance or stability. The fact that a chemical reaction
Chemistry 112 Laboratory Experiment 7: Determination of Reaction Stoichiometry and Chemical Equilibrium Introduction The word equilibrium suggests balance or stability. The fact that a chemical reaction
Shifting Reactions A
 Shifting Reactions A Name Lab Section Log on to the Internet. Type the following address into the location-input line of your browser: http://introchem.chem.okstate.edu/dcicla/ergbm.htm This will load
Shifting Reactions A Name Lab Section Log on to the Internet. Type the following address into the location-input line of your browser: http://introchem.chem.okstate.edu/dcicla/ergbm.htm This will load
Getting started with BatchReactor Example : Simulation of the Chlorotoluene chlorination
 Getting started with BatchReactor Example : Simulation of the Chlorotoluene chlorination 2011 ProSim S.A. All rights reserved. Introduction This document presents the different steps to follow in order
Getting started with BatchReactor Example : Simulation of the Chlorotoluene chlorination 2011 ProSim S.A. All rights reserved. Introduction This document presents the different steps to follow in order
Experiment 0 ~ Introduction to Statistics and Excel Tutorial. Introduction to Statistics, Error and Measurement
 Experiment 0 ~ Introduction to Statistics and Excel Tutorial Many of you already went through the introduction to laboratory practice and excel tutorial in Physics 1011. For that reason, we aren t going
Experiment 0 ~ Introduction to Statistics and Excel Tutorial Many of you already went through the introduction to laboratory practice and excel tutorial in Physics 1011. For that reason, we aren t going
FDE 211-MATERIAL AND ENERGY BALANCES: MATERIAL BALANCES ON REACTIVE SYSTEMS. Dr. Ilgın PakerYıkıcı Fall 2015
 FDE 211-MATERIAL AND ENERGY BALANCES: MATERIAL BALANCES ON REACTIVE SYSTEMS 1 Dr. Ilgın PakerYıkıcı Fall 2015 Learning Objectives Write a balanced chemical reaction and use stoichiometry to determine the
FDE 211-MATERIAL AND ENERGY BALANCES: MATERIAL BALANCES ON REACTIVE SYSTEMS 1 Dr. Ilgın PakerYıkıcı Fall 2015 Learning Objectives Write a balanced chemical reaction and use stoichiometry to determine the
Chemical Reaction Engineering - Part 12 - multiple reactions Richard K. Herz,
 Chemical Reaction Engineering - Part 12 - multiple reactions Richard K. Herz, rherz@ucsd.edu, www.reactorlab.net Multiple reactions are usually present So far we have considered reactors in which only
Chemical Reaction Engineering - Part 12 - multiple reactions Richard K. Herz, rherz@ucsd.edu, www.reactorlab.net Multiple reactions are usually present So far we have considered reactors in which only
Process Design Decisions and Project Economics Prof. Dr. V. S. Moholkar Department of Chemical Engineering Indian Institute of Technology, Guwahati
 Process Design Decisions and Project Economics Prof. Dr. V. S. Moholkar Department of Chemical Engineering Indian Institute of Technology, Guwahati Module - 2 Flowsheet Synthesis (Conceptual Design of
Process Design Decisions and Project Economics Prof. Dr. V. S. Moholkar Department of Chemical Engineering Indian Institute of Technology, Guwahati Module - 2 Flowsheet Synthesis (Conceptual Design of
MoLE Gas Laws Activities *
 MoLE Gas Laws Activities * To begin this assignment you must be able to log on to the Internet using Internet Explorer (Microsoft) 4.5 or higher. If you do not have the current version of the browser,
MoLE Gas Laws Activities * To begin this assignment you must be able to log on to the Internet using Internet Explorer (Microsoft) 4.5 or higher. If you do not have the current version of the browser,
CinChE Problem-Solving Strategy Chapter 4 Development of a Mathematical Algorithm. partitioning. procedure
 Development o a Mathematical Algorithm Transormation Process Mathematical Model partitioning procedure Mathematical Algorithm The mathematical algorithm is a plan (or blueprint) that identiies the independent
Development o a Mathematical Algorithm Transormation Process Mathematical Model partitioning procedure Mathematical Algorithm The mathematical algorithm is a plan (or blueprint) that identiies the independent
Determination of the Equivalent Weight and the K a or K b for a Weak Acid or Base
 INTRODUCTION Determination of the Equivalent Weight and the K a or K b for a Weak Acid or Base Chemists frequently make use of the equivalent weight (eq. wt.) as the basis for volumetric calculations.
INTRODUCTION Determination of the Equivalent Weight and the K a or K b for a Weak Acid or Base Chemists frequently make use of the equivalent weight (eq. wt.) as the basis for volumetric calculations.
Designing a Quilt with GIMP 2011
 Planning your quilt and want to see what it will look like in the fabric you just got from your LQS? You don t need to purchase a super expensive program. Try this and the best part it s FREE!!! *** Please
Planning your quilt and want to see what it will look like in the fabric you just got from your LQS? You don t need to purchase a super expensive program. Try this and the best part it s FREE!!! *** Please
Introduction to Hartree-Fock calculations in Spartan
 EE5 in 2008 Hannes Jónsson Introduction to Hartree-Fock calculations in Spartan In this exercise, you will get to use state of the art software for carrying out calculations of wavefunctions for molecues,
EE5 in 2008 Hannes Jónsson Introduction to Hartree-Fock calculations in Spartan In this exercise, you will get to use state of the art software for carrying out calculations of wavefunctions for molecues,
Fundamentals of Material Balances
 Chapter 4 Fundamentals of Material Balances Material Balance-Part 1 Process Classifications 3 type of chemical processes: - Concept of boundary of the process 1. Batch process Feed is charge to the process
Chapter 4 Fundamentals of Material Balances Material Balance-Part 1 Process Classifications 3 type of chemical processes: - Concept of boundary of the process 1. Batch process Feed is charge to the process
Determining the Conductivity of Standard Solutions
 Determining the Conductivity of Standard Solutions by Anna Cole and Shannon Clement Louisiana Curriculum Framework Content Strand: Science as Inquiry, Physical Science Grade Level 11-12 Objectives: 1.
Determining the Conductivity of Standard Solutions by Anna Cole and Shannon Clement Louisiana Curriculum Framework Content Strand: Science as Inquiry, Physical Science Grade Level 11-12 Objectives: 1.
Experiment 1 Scientific Writing Tools
 Experiment 1 Scientific Writing Tools OUTCOMES After completing this experiment, the student should be able to: insert a variety of mathematical equations into a Word document. draw line structures of
Experiment 1 Scientific Writing Tools OUTCOMES After completing this experiment, the student should be able to: insert a variety of mathematical equations into a Word document. draw line structures of
Safety: BE SURE TO KEEP YOUR SMART CART UPSIDE-DOWN WHEN YOU RE NOT ACTIVELY USING IT TO RECORD DATA.
 Why do people always ignore Objective: 1. Determine how an object s mass affects the friction it experiences. 2. Compare the coefficient of static friction to the coefficient of kinetic friction for each
Why do people always ignore Objective: 1. Determine how an object s mass affects the friction it experiences. 2. Compare the coefficient of static friction to the coefficient of kinetic friction for each
Introduction to MoLEs Activities Computer Simulations *
 Introduction to MoLEs Activities Computer Simulations * Molecular Level Experiments (MoLEs) are chemistry laboratory experiments based on computer simulations. There are two types of simulation programs
Introduction to MoLEs Activities Computer Simulations * Molecular Level Experiments (MoLEs) are chemistry laboratory experiments based on computer simulations. There are two types of simulation programs
let s examine pupation rates. With the conclusion of that data collection, we will go on to explore the rate at which new adults appear, a process
 Population Dynamics and Initial Population Size (Module website: http://web.as.uky.edu/biology/faculty/cooper/population%20dynamics%20examples%20 with%20fruit%20flies/theamericanbiologyteacher-populationdynamicswebpage.html
Population Dynamics and Initial Population Size (Module website: http://web.as.uky.edu/biology/faculty/cooper/population%20dynamics%20examples%20 with%20fruit%20flies/theamericanbiologyteacher-populationdynamicswebpage.html
MoLE Gas Laws Activities
 MoLE Gas Laws Activities To begin this assignment you must be able to log on to the Internet using Internet Explorer (Microsoft) 4.5 or higher. If you do not have the current version of the browser, go
MoLE Gas Laws Activities To begin this assignment you must be able to log on to the Internet using Internet Explorer (Microsoft) 4.5 or higher. If you do not have the current version of the browser, go
Methodology for Analysis of Metallurgical Processes
 Methodology for Analysis of Metallurgical Processes Metallurgical and chemical processes are classified as batch, continuous and semibatch 1. Batch processes The feed is charged into a vessel at the beginning
Methodology for Analysis of Metallurgical Processes Metallurgical and chemical processes are classified as batch, continuous and semibatch 1. Batch processes The feed is charged into a vessel at the beginning
How to Create a Substance Answer Set
 How to Create a Substance Answer Set Select among five search techniques to find substances Since substances can be described by multiple names or other characteristics, SciFinder gives you the flexibility
How to Create a Substance Answer Set Select among five search techniques to find substances Since substances can be described by multiple names or other characteristics, SciFinder gives you the flexibility
Mass and Particle Relationships
 Mass and Particle Relationships Name Lab Section Log on to the Internet. Type the following address into the location-input line of your browser: http://cheminfo.chem.ou.edu/~mra/ccli2004/srgm1.htm This
Mass and Particle Relationships Name Lab Section Log on to the Internet. Type the following address into the location-input line of your browser: http://cheminfo.chem.ou.edu/~mra/ccli2004/srgm1.htm This
Empirical Gas Laws (Parts 1 and 2) Pressure-volume and pressure-temperature relationships in gases
 Empirical Gas Laws (Parts 1 and 2) Pressure-volume and pressure-temperature relationships in gases Some of the earliest experiments in chemistry and physics involved the study of gases. The invention of
Empirical Gas Laws (Parts 1 and 2) Pressure-volume and pressure-temperature relationships in gases Some of the earliest experiments in chemistry and physics involved the study of gases. The invention of
module, with the exception that the vials are larger and you only use one initial population size.
 Population Dynamics and Space Availability (http://web.as.uky.edu/biology/faculty/cooper/population%20dynamics%20examples%2 0with%20fruit%20flies/TheAmericanBiologyTeacher- PopulationDynamicsWebpage.html
Population Dynamics and Space Availability (http://web.as.uky.edu/biology/faculty/cooper/population%20dynamics%20examples%2 0with%20fruit%20flies/TheAmericanBiologyTeacher- PopulationDynamicsWebpage.html
REPLACE DAMAGED OR MISSING TEXTBOOK BARCODE LABEL
 Destiny Textbook Manager allows users to create and print replacement barcode labels for textbooks. In this tutorial you will learn how to: Replace damaged textbook barcode label(s) Replace missing textbook
Destiny Textbook Manager allows users to create and print replacement barcode labels for textbooks. In this tutorial you will learn how to: Replace damaged textbook barcode label(s) Replace missing textbook
Lab 7 Energy. What You Need To Know: Physics 225 Lab
 b Lab 7 Energy What You Need To Know: The Physics This lab is going to cover all of the different types of energy that you should be discussing in your lecture. Those energy types are kinetic energy, gravitational
b Lab 7 Energy What You Need To Know: The Physics This lab is going to cover all of the different types of energy that you should be discussing in your lecture. Those energy types are kinetic energy, gravitational
Computer simulation of radioactive decay
 Computer simulation of radioactive decay y now you should have worked your way through the introduction to Maple, as well as the introduction to data analysis using Excel Now we will explore radioactive
Computer simulation of radioactive decay y now you should have worked your way through the introduction to Maple, as well as the introduction to data analysis using Excel Now we will explore radioactive
Shown below is a sample titration curve for a diprotic acid. Note the two equivalence points.
 EXPERIMENT 9 Titration Curve for a Polyprotic Acid INTRODUCTION Other than by strength and concentration, another way of classifying acids involves the number of H + ions an acid can donate. A monoprotic
EXPERIMENT 9 Titration Curve for a Polyprotic Acid INTRODUCTION Other than by strength and concentration, another way of classifying acids involves the number of H + ions an acid can donate. A monoprotic
How Do I Create a Hubble Diagram to show the expanding universe?
 How Do I Create a Hubble Diagram to show the expanding universe? An extremely important topic in astronomy is the expansion of the universe. Although the expanding universe is nearly always discussed in
How Do I Create a Hubble Diagram to show the expanding universe? An extremely important topic in astronomy is the expansion of the universe. Although the expanding universe is nearly always discussed in
The coefficients of a balanced chemical equation tell us how many of each species are involved in the reaction.
 Stoichiometry Chemical Equations Reactants are written on the left side of the arrow and products are written on the right side of the arrow. The Law of Conservation of Mass tells us that the number of
Stoichiometry Chemical Equations Reactants are written on the left side of the arrow and products are written on the right side of the arrow. The Law of Conservation of Mass tells us that the number of
Using Tables and Graphing Calculators in Math 11
 Using Tables and Graphing Calculators in Math 11 Graphing calculators are not required for Math 11, but they are likely to be helpful, primarily because they allow you to avoid the use of tables in some
Using Tables and Graphing Calculators in Math 11 Graphing calculators are not required for Math 11, but they are likely to be helpful, primarily because they allow you to avoid the use of tables in some
2. Review on Material Balances
 2. Review on Material Balances Objectives After completing this chapter, students should be able to recall the principle of the conservation of mass recall the principle of the stoichiometry of chemical
2. Review on Material Balances Objectives After completing this chapter, students should be able to recall the principle of the conservation of mass recall the principle of the stoichiometry of chemical
Chemwatch How To. Create Labels for Chemicals, Products & Mixtures.
 Chemwatch How To. Create Labels for Chemicals, Products & Mixtures. Dr Ian Lane Radiation and Chemical Manager Faculty of Science Version 1.0, April 2017 Outline: (i) Important Note! Part A: Creating a
Chemwatch How To. Create Labels for Chemicals, Products & Mixtures. Dr Ian Lane Radiation and Chemical Manager Faculty of Science Version 1.0, April 2017 Outline: (i) Important Note! Part A: Creating a
Fundamentals of Combustion
 Fundamentals of Combustion Lec 3: Chemical Thermodynamics Dr. Zayed Al-Hamamre Content Process Heat Transfer 1-3 Process Heat Transfer 1-4 Process Heat Transfer 1-5 Theoretical and Excess Air Combustion
Fundamentals of Combustion Lec 3: Chemical Thermodynamics Dr. Zayed Al-Hamamre Content Process Heat Transfer 1-3 Process Heat Transfer 1-4 Process Heat Transfer 1-5 Theoretical and Excess Air Combustion
THE CHEMICAL REACTION EQUATION AND STOICHIOMETRY
 9.1 Stoichiometry Stoichiometry provides a quantitative means of relating the amount of products produced by chemical reaction(s) to the amount of reactants. You should take the following steps in solving
9.1 Stoichiometry Stoichiometry provides a quantitative means of relating the amount of products produced by chemical reaction(s) to the amount of reactants. You should take the following steps in solving
41. Sim Reactions Example
 HSC Chemistry 7.0 41-1(6) 41. Sim Reactions Example Figure 1: Sim Reactions Example, Run mode view after calculations. General This example contains instruction how to create a simple model. The example
HSC Chemistry 7.0 41-1(6) 41. Sim Reactions Example Figure 1: Sim Reactions Example, Run mode view after calculations. General This example contains instruction how to create a simple model. The example
Computer Aided Process Plant Design
 Computer Aided rocess lant Design Material Balance S. Balasubramanian Department of Chemical Engineering SRM University, Kattankulathur-603203 1 OUTLINE 1 Material Balance 2 Classification 3 Algorithm
Computer Aided rocess lant Design Material Balance S. Balasubramanian Department of Chemical Engineering SRM University, Kattankulathur-603203 1 OUTLINE 1 Material Balance 2 Classification 3 Algorithm
Introduction to Computer Tools and Uncertainties
 Experiment 1 Introduction to Computer Tools and Uncertainties 1.1 Objectives To become familiar with the computer programs and utilities that will be used throughout the semester. To become familiar with
Experiment 1 Introduction to Computer Tools and Uncertainties 1.1 Objectives To become familiar with the computer programs and utilities that will be used throughout the semester. To become familiar with
ISIS/Draw "Quick Start"
 ISIS/Draw "Quick Start" Click to print, or click Drawing Molecules * Basic Strategy 5.1 * Drawing Structures with Template tools and template pages 5.2 * Drawing bonds and chains 5.3 * Drawing atoms 5.4
ISIS/Draw "Quick Start" Click to print, or click Drawing Molecules * Basic Strategy 5.1 * Drawing Structures with Template tools and template pages 5.2 * Drawing bonds and chains 5.3 * Drawing atoms 5.4
Chemical Reaction Engineering. Multiple Reactions. Dr.-Eng. Zayed Al-Hamamre
 Chemical Reaction Engineering Multiple Reactions Dr.-Eng. Zayed Al-Hamamre 1 Content Types of Reactions Selectivity Reaction Yield Parallel Reactions Series Reactions Net Rates of Reaction Complex Reactions
Chemical Reaction Engineering Multiple Reactions Dr.-Eng. Zayed Al-Hamamre 1 Content Types of Reactions Selectivity Reaction Yield Parallel Reactions Series Reactions Net Rates of Reaction Complex Reactions
Lightcloud Application
 Controlling Your Lightcloud System Lightcloud Application Lightcloud Application Navigating the Application Devices Device Settings Organize Control Energy Scenes Schedules Demand Response Power Up State
Controlling Your Lightcloud System Lightcloud Application Lightcloud Application Navigating the Application Devices Device Settings Organize Control Energy Scenes Schedules Demand Response Power Up State
Lab 1 Uniform Motion - Graphing and Analyzing Motion
 Lab 1 Uniform Motion - Graphing and Analyzing Motion Objectives: < To observe the distance-time relation for motion at constant velocity. < To make a straight line fit to the distance-time data. < To interpret
Lab 1 Uniform Motion - Graphing and Analyzing Motion Objectives: < To observe the distance-time relation for motion at constant velocity. < To make a straight line fit to the distance-time data. < To interpret
Kinetics CHAPTER IN THIS CHAPTER
 CHAPTER 14 Kinetics IN THIS CHAPTER Summary: Thermodynamics often can be used to predict whether a reaction will occur spontaneously, but it gives very little information about the speed at which a reaction
CHAPTER 14 Kinetics IN THIS CHAPTER Summary: Thermodynamics often can be used to predict whether a reaction will occur spontaneously, but it gives very little information about the speed at which a reaction
Chemical Reactions. Chapter 17
 Chemical Reactions Chapter 17 Chemical Equations C+O 2 CO 2 C (s) +O 2 (g) CO 2 (g) Reactants on left, products on right Each are balanced because same number of atoms of reactants as products Some equations
Chemical Reactions Chapter 17 Chemical Equations C+O 2 CO 2 C (s) +O 2 (g) CO 2 (g) Reactants on left, products on right Each are balanced because same number of atoms of reactants as products Some equations
Pressure Swing Distillation with Aspen Plus V8.0
 Pressure Swing Distillation with Aspen Plus V8.0 1. Lesson Objectives Aspen Plus property analysis RadFrac distillation modeling Design Specs NQ Curves Tear streams Understand and overcome azeotrope Select
Pressure Swing Distillation with Aspen Plus V8.0 1. Lesson Objectives Aspen Plus property analysis RadFrac distillation modeling Design Specs NQ Curves Tear streams Understand and overcome azeotrope Select
Gas Pressure and Temperature Relationships *
 Gas Pressure and Temperature Relationships * MoLE Activities To begin this assignment you must be able to log on to the Internet (the software requires OSX for mac users). Type the following address into
Gas Pressure and Temperature Relationships * MoLE Activities To begin this assignment you must be able to log on to the Internet (the software requires OSX for mac users). Type the following address into
Electric Fields and Equipotentials
 OBJECTIVE Electric Fields and Equipotentials To study and describe the two-dimensional electric field. To map the location of the equipotential surfaces around charged electrodes. To study the relationship
OBJECTIVE Electric Fields and Equipotentials To study and describe the two-dimensional electric field. To map the location of the equipotential surfaces around charged electrodes. To study the relationship
AE 205 Materials and Energy Balances Asst. Prof. Dr. Tippabust Eksangsri. Chapter 4 Stoichiometry and MB with Reactions
 AE 205 Materials and Energy Balances Asst. Prof. Dr. Tippabust Eksangsri Chapter 4 Stoichiometry and MB with Reactions Stoichiometry Stoichiometry provides a quantitative means of relating the amount of
AE 205 Materials and Energy Balances Asst. Prof. Dr. Tippabust Eksangsri Chapter 4 Stoichiometry and MB with Reactions Stoichiometry Stoichiometry provides a quantitative means of relating the amount of
ON SITE SYSTEMS Chemical Safety Assistant
 ON SITE SYSTEMS Chemical Safety Assistant CS ASSISTANT WEB USERS MANUAL On Site Systems 23 N. Gore Ave. Suite 200 St. Louis, MO 63119 Phone 314-963-9934 Fax 314-963-9281 Table of Contents INTRODUCTION
ON SITE SYSTEMS Chemical Safety Assistant CS ASSISTANT WEB USERS MANUAL On Site Systems 23 N. Gore Ave. Suite 200 St. Louis, MO 63119 Phone 314-963-9934 Fax 314-963-9281 Table of Contents INTRODUCTION
Kinematics Lab. 1 Introduction. 2 Equipment. 3 Procedures
 Kinematics Lab 1 Introduction An object moving in one dimension and undergoing constant or uniform acceleration has a position given by: x(t) =x 0 +v o t +1/2at 2 where x o is its initial position (its
Kinematics Lab 1 Introduction An object moving in one dimension and undergoing constant or uniform acceleration has a position given by: x(t) =x 0 +v o t +1/2at 2 where x o is its initial position (its
STUDY GUIDE Math 20. To accompany Intermediate Algebra for College Students By Robert Blitzer, Third Edition
 STUDY GUIDE Math 0 To the students: To accompany Intermediate Algebra for College Students By Robert Blitzer, Third Edition When you study Algebra, the material is presented to you in a logical sequence.
STUDY GUIDE Math 0 To the students: To accompany Intermediate Algebra for College Students By Robert Blitzer, Third Edition When you study Algebra, the material is presented to you in a logical sequence.
A (Mostly) Correctly Formatted Sample Lab Report. Brett A. McGuire Lab Partner: Microsoft Windows Section AB2
 A (Mostly) Correctly Formatted Sample Lab Report Brett A. McGuire Lab Partner: Microsoft Windows Section AB2 August 26, 2008 Abstract Your abstract should not be indented and be single-spaced. Abstracts
A (Mostly) Correctly Formatted Sample Lab Report Brett A. McGuire Lab Partner: Microsoft Windows Section AB2 August 26, 2008 Abstract Your abstract should not be indented and be single-spaced. Abstracts
WISE Regression/Correlation Interactive Lab. Introduction to the WISE Correlation/Regression Applet
 WISE Regression/Correlation Interactive Lab Introduction to the WISE Correlation/Regression Applet This tutorial focuses on the logic of regression analysis with special attention given to variance components.
WISE Regression/Correlation Interactive Lab Introduction to the WISE Correlation/Regression Applet This tutorial focuses on the logic of regression analysis with special attention given to variance components.
Chapter 6 Chemical Reactions Equations Worksheet Answers
 Chapter 6 Chemical Reactions Equations Worksheet Answers We have made it easy for you to find a PDF Ebooks without any digging. And by having access to our ebooks online or by storing it on your computer,
Chapter 6 Chemical Reactions Equations Worksheet Answers We have made it easy for you to find a PDF Ebooks without any digging. And by having access to our ebooks online or by storing it on your computer,
Calculating NMR Chemical Shifts for beta-ionone O
 Calculating NMR Chemical Shifts for beta-ionone O Molecular orbital calculations can be used to get good estimates for chemical shifts. In this exercise we will calculate the chemical shifts for beta-ionone.
Calculating NMR Chemical Shifts for beta-ionone O Molecular orbital calculations can be used to get good estimates for chemical shifts. In this exercise we will calculate the chemical shifts for beta-ionone.
Acid/Base Interactions
 Acid/Base Interactions Name Lab Section Log on to the Internet. Type the following address into the location-input line of your browser: http://cheminfo.chem.ou.edu/~mra/ccli2004/acids+bm.htm This will
Acid/Base Interactions Name Lab Section Log on to the Internet. Type the following address into the location-input line of your browser: http://cheminfo.chem.ou.edu/~mra/ccli2004/acids+bm.htm This will
Aspen Dr. Ziad Abuelrub
 Aspen Plus Lab Pharmaceutical Plant Design Aspen Dr. Ziad Abuelrub OUTLINE 1. Introduction 2. Getting Started 3. Thermodynamic Models & Physical Properties 4. Pressure Changers 5. Heat Exchangers 6. Flowsheet
Aspen Plus Lab Pharmaceutical Plant Design Aspen Dr. Ziad Abuelrub OUTLINE 1. Introduction 2. Getting Started 3. Thermodynamic Models & Physical Properties 4. Pressure Changers 5. Heat Exchangers 6. Flowsheet
mylab: Chemical Safety Module Last Updated: January 19, 2018
 : Chemical Safety Module Contents Introduction... 1 Getting started... 1 Login... 1 Receiving Items from MMP Order... 3 Inventory... 4 Show me Chemicals where... 4 Items Received on... 5 All Items... 5
: Chemical Safety Module Contents Introduction... 1 Getting started... 1 Login... 1 Receiving Items from MMP Order... 3 Inventory... 4 Show me Chemicals where... 4 Items Received on... 5 All Items... 5
Esterification in a PFR with Aspen Plus V8.0
 Esterification in a PFR with Aspen Plus V8.0 1. Lesson Objectives Use Aspen Plus to determine whether a given reaction is technically feasible using a plug flow reactor. 2. Prerequisites Aspen Plus V8.0
Esterification in a PFR with Aspen Plus V8.0 1. Lesson Objectives Use Aspen Plus to determine whether a given reaction is technically feasible using a plug flow reactor. 2. Prerequisites Aspen Plus V8.0
SAVE THIS SYLLABUS FOR REFERENCE DURING THE SEMESTER.
 SYLLABUS Course: General Chemistry I: CHEM-1030-001 (call #11403) Lecture: 8:30-9:55 AM Tue.-Thur.; Room 6006 Recitation: 1 hour per week: Thur.; 12:00-1:00 Room 3066 Laboratory: 3 hours per week: Thur.;
SYLLABUS Course: General Chemistry I: CHEM-1030-001 (call #11403) Lecture: 8:30-9:55 AM Tue.-Thur.; Room 6006 Recitation: 1 hour per week: Thur.; 12:00-1:00 Room 3066 Laboratory: 3 hours per week: Thur.;
Chemistry Midterm Review. Topics:
 Chemistry Midterm Review Unit 1: laboratory equipment and safety rules accuracy vs precision scientific method: observation, hypothesis. experimental design: independent vs dependent variables, control
Chemistry Midterm Review Unit 1: laboratory equipment and safety rules accuracy vs precision scientific method: observation, hypothesis. experimental design: independent vs dependent variables, control
Please be sure to save a copy of this activity to your computer!
 Thank you for your purchase Please be sure to save a copy of this activity to your computer! This activity is copyrighted by AIMS Education Foundation. All rights reserved. No part of this work may be
Thank you for your purchase Please be sure to save a copy of this activity to your computer! This activity is copyrighted by AIMS Education Foundation. All rights reserved. No part of this work may be
Assignment 70 LE CHATELIER'S PRINCIPLE AND EQUILIBRIUM CONCENTRATIONS
 BACKGROUND Assignment 70 LE CHATELIER'S PRINCIPLE AND EQUILIBRIUM CONCENTRATIONS The theoretical yield calculations of prior assignments are made on the assumption that the reaction goes to completion
BACKGROUND Assignment 70 LE CHATELIER'S PRINCIPLE AND EQUILIBRIUM CONCENTRATIONS The theoretical yield calculations of prior assignments are made on the assumption that the reaction goes to completion
Simple circuits - 3 hr
 Simple circuits - 3 hr Resistances in circuits Analogy of water flow and electric current An electrical circuit consists of a closed loop with a number of different elements through which electric current
Simple circuits - 3 hr Resistances in circuits Analogy of water flow and electric current An electrical circuit consists of a closed loop with a number of different elements through which electric current
E-BOOK // OF CHEMICAL FORMULAS OWNERS MANUAL EBOOK
 02 January, 2018 E-BOOK // OF CHEMICAL FORMULAS OWNERS MANUAL EBOOK Document Filetype: PDF 389.32 KB 0 E-BOOK // OF CHEMICAL FORMULAS OWNERS MANUAL EBOOK This is a list of common chemical compounds with
02 January, 2018 E-BOOK // OF CHEMICAL FORMULAS OWNERS MANUAL EBOOK Document Filetype: PDF 389.32 KB 0 E-BOOK // OF CHEMICAL FORMULAS OWNERS MANUAL EBOOK This is a list of common chemical compounds with
Project 3: Molecular Orbital Calculations of Diatomic Molecules. This project is worth 30 points and is due on Wednesday, May 2, 2018.
 Chemistry 362 Spring 2018 Dr. Jean M. Standard April 20, 2018 Project 3: Molecular Orbital Calculations of Diatomic Molecules In this project, you will investigate the molecular orbitals and molecular
Chemistry 362 Spring 2018 Dr. Jean M. Standard April 20, 2018 Project 3: Molecular Orbital Calculations of Diatomic Molecules In this project, you will investigate the molecular orbitals and molecular
General Chemistry Lab Molecular Modeling
 PURPOSE The objectives of this experiment are PROCEDURE General Chemistry Lab Molecular Modeling To learn how to use molecular modeling software, a commonly used tool in chemical research and industry.
PURPOSE The objectives of this experiment are PROCEDURE General Chemistry Lab Molecular Modeling To learn how to use molecular modeling software, a commonly used tool in chemical research and industry.
Chapter 3 Chemical Reactions and Reaction Stoichiometry
 Chapter 3 Chemical Reactions and Reaction Stoichiometry 2015 Pearson Education, Inc. Chemical Reactions and Reaction Stoichiometry 3.1 Chemical Equations 3.2 Simple Patterns of Chemical Reactivity 3.3
Chapter 3 Chemical Reactions and Reaction Stoichiometry 2015 Pearson Education, Inc. Chemical Reactions and Reaction Stoichiometry 3.1 Chemical Equations 3.2 Simple Patterns of Chemical Reactivity 3.3
Unit B Analysis Questions
 Unit B Analysis Questions ACTIVITY 12 1. What two types of information do you think are the most important in deciding which material to use to make drink containers? Explain. 2. What additional information
Unit B Analysis Questions ACTIVITY 12 1. What two types of information do you think are the most important in deciding which material to use to make drink containers? Explain. 2. What additional information
Standards-Based Quantification in DTSA-II Part II
 Standards-Based Quantification in DTSA-II Part II Nicholas W.M. Ritchie National Institute of Standards and Technology, Gaithersburg, MD 20899-8371 nicholas.ritchie@nist.gov Introduction This article is
Standards-Based Quantification in DTSA-II Part II Nicholas W.M. Ritchie National Institute of Standards and Technology, Gaithersburg, MD 20899-8371 nicholas.ritchie@nist.gov Introduction This article is
Lab 5 RC Circuits. What You Need To Know: Physics 212 Lab
 Lab 5 R ircuits What You Need To Know: The Physics In the previous two labs you ve dealt strictly with resistors. In today s lab you ll be using a new circuit element called a capacitor. A capacitor consists
Lab 5 R ircuits What You Need To Know: The Physics In the previous two labs you ve dealt strictly with resistors. In today s lab you ll be using a new circuit element called a capacitor. A capacitor consists
Remember that C is a constant and ë and n are variables. This equation now fits the template of a straight line:
 CONVERTING NON-LINEAR GRAPHS INTO LINEAR GRAPHS Linear graphs have several important attributes. First, it is easy to recognize a graph that is linear. It is much more difficult to identify if a curved
CONVERTING NON-LINEAR GRAPHS INTO LINEAR GRAPHS Linear graphs have several important attributes. First, it is easy to recognize a graph that is linear. It is much more difficult to identify if a curved
M E R C E R W I N WA L K T H R O U G H
 H E A L T H W E A L T H C A R E E R WA L K T H R O U G H C L I E N T S O L U T I O N S T E A M T A B L E O F C O N T E N T 1. Login to the Tool 2 2. Published reports... 7 3. Select Results Criteria...
H E A L T H W E A L T H C A R E E R WA L K T H R O U G H C L I E N T S O L U T I O N S T E A M T A B L E O F C O N T E N T 1. Login to the Tool 2 2. Published reports... 7 3. Select Results Criteria...
Titrator 3.0 Tutorial: Calcite precipitation
 Titrator 3.0 Tutorial: Calcite precipitation November 2008 Steve Cabaniss A. Introduction This brief tutorial is intended to acquaint you with some of the features of the program Titrator. It assumes that
Titrator 3.0 Tutorial: Calcite precipitation November 2008 Steve Cabaniss A. Introduction This brief tutorial is intended to acquaint you with some of the features of the program Titrator. It assumes that
Introduction to Coastal GIS
 Introduction to Coastal GIS Event was held on Tues, 1/8/13 - Thurs, 1/10/13 Time: 9:00 am to 5:00 pm Location: Roger Williams University, Bristol, RI Audience: The intended audiences for this course are
Introduction to Coastal GIS Event was held on Tues, 1/8/13 - Thurs, 1/10/13 Time: 9:00 am to 5:00 pm Location: Roger Williams University, Bristol, RI Audience: The intended audiences for this course are
Star Cluster Photometry and the H-R Diagram
 Star Cluster Photometry and the H-R Diagram Contents Introduction Star Cluster Photometry... 1 Downloads... 1 Part 1: Measuring Star Magnitudes... 2 Part 2: Plotting the Stars on a Colour-Magnitude (H-R)
Star Cluster Photometry and the H-R Diagram Contents Introduction Star Cluster Photometry... 1 Downloads... 1 Part 1: Measuring Star Magnitudes... 2 Part 2: Plotting the Stars on a Colour-Magnitude (H-R)
SAVE THIS SYLLABUS FOR REFERENCE DURING THE SEMESTER.
 SYLLABUS Course: General Chemistry I: (call #31437) Lecture: 8:30-10:00AM Mon.-Wed.; Room 6006 Recitation: 1 hour per week: Mon.; 12:00-1:00 Room 3066 Laboratory: 3 hours per week: Mon;1:00-4:00 Room 3066
SYLLABUS Course: General Chemistry I: (call #31437) Lecture: 8:30-10:00AM Mon.-Wed.; Room 6006 Recitation: 1 hour per week: Mon.; 12:00-1:00 Room 3066 Laboratory: 3 hours per week: Mon;1:00-4:00 Room 3066
Amount of Substance and Its Unit Mole- Connecting the Invisible Micro World to the Observable Macro World Part 2 (English, mp4)
 Amount of Substance and Its Unit Mole- Connecting the Invisible Micro World to the Observable Macro World Part 2 (English, mp4) [MUSIC PLAYING] Instructor: Hi, everyone. Welcome back. I hope you had some
Amount of Substance and Its Unit Mole- Connecting the Invisible Micro World to the Observable Macro World Part 2 (English, mp4) [MUSIC PLAYING] Instructor: Hi, everyone. Welcome back. I hope you had some
Propylene Hydroformylation
 www.optience.com Propylene Hydroformylation Objective: Building Fedbatch reactors with Pressure Control In this example, we simulate a multiphase batch reactor for Propylene Hydroformylation and also explain
www.optience.com Propylene Hydroformylation Objective: Building Fedbatch reactors with Pressure Control In this example, we simulate a multiphase batch reactor for Propylene Hydroformylation and also explain
Experiment 1: The Same or Not The Same?
 Experiment 1: The Same or Not The Same? Learning Goals After you finish this lab, you will be able to: 1. Use Logger Pro to collect data and calculate statistics (mean and standard deviation). 2. Explain
Experiment 1: The Same or Not The Same? Learning Goals After you finish this lab, you will be able to: 1. Use Logger Pro to collect data and calculate statistics (mean and standard deviation). 2. Explain
