Chemical Composition Monitoring Using An In-Situ Infrared Probe
|
|
|
- Reynard Clarke
- 5 years ago
- Views:
Transcription
1 Technical Note AN 904 Rev. E Chemical Composition Monitoring Using An In-Situ Infrared Probe Walter M. Doyle This paper describes a new approach to process analysis utilizing an infrared probe which can be immersed within a volume of liquid chemical. Based on the internal reflectance principle, the immersion probe can be coupled to a process FTIR spectrometer to provide essentially real-time analysis of either batch or continuous reactions. After describing the design of the probe, the paper presents the results of a series of laboratory scale experiments in which a small version of the immersion probe was used to monitor various polymerization reactions. 1. Introduction Over the past several years, infrared spectroscopy has emerged from the laboratory to become a powerful new tool for online chemical process monitoring. Several developments have come together to make this important advance possible. These include high performance FTIR spectrometers exhibiting the stability and ruggedness demanded by the manufacturing environment, software capable of converting the wealth of spectral information available into readouts of the desired process variable, and highly reliable sampling equipment to couple the IR instrumentation to the process line. As of this writing, over one hundred dedicated process FTIR systems have been placed in operation with applications ranging from process gas analysis to monitoring the composition of hot polymer melts. These spectrometers operate in either the near-ir or mid-ir regions of the spectrum. In the near- IR, FTIR technology provides the high stability needed to separate the contributions from chemical species with broadly overlapping absorption bands. When used in mid-ir, FTIR provides an additional important benefit - the ability to directly monitor the sharp spectral bands which characterize functional groups. Since it is the functional groups that control chemical reactivity, mid-infrared FTIR can be said to provide a window into the chemical process. Most of the present on-line FTIR systems are being used to The author is with Hellma Axiom, 1451-A Edinger Ave, Tustin, California W.M. Doyle s address is michael. doyle@hellma.com. monitor continuous processes. In these, systems, a portion of the process stream flows through a sampling device employing either the transmission or the attenuated total reflectance (ATR) principle. The present paper reports on a new sampling capability which has made it possible to extend the power of process FTIR to batch processes while, at the same time, promising to provide enhanced performance for many continuous processes (1). This capability involves the use of a family of infrared immersion probes which can be inserted into a batch process kettle or large diameter process line to provide true in-situ chemical analysis during the manufacturing process. This makes it possible, for example, to monitor the concentrations of individual constituents in a batch reaction so as to terminate the process when the optimum concentrations have been reached. The new Deep Immersion Probes have been developed within the past two and one-half years. Within ten months of the first commercial application of the principle on a laboratory scale, the immersion probes had made the transition to full scale on-line application. This rapid conversion was made possible by the generally high level FTIR technology available and by the skills of key scientists within the process industry. The use of the infrared immersion technique is already starting to have significant impact on quality, efficiency, and yields in a variety of chemical processes. 2. Deep Immersion Probe Design The optical design of the immersion probes is illustrated in AN-904 / Hellma Axiom, Inc. 1
2 Figure 1. As this figure suggests, the design is conceptually quite simple, involving the use of the hollow metallic light guides to transmit IR radiation to and from an ATR element at the lower end of the probe. This apparent simplicity prompts the obvious question as to why this technology wasn t available sooner. The answer is two-fold. First, the potential utility of an in-situ IR probe for process use wasn t recognized until FTIR hardware had reached the level of performance required for process application. Second, the development of the probe did require technical advances - the design of sufficiently rugged and compact sensing head optics and an efficient means of coupling the IR radiation between the spectrometer and the probe. The use of metallic light guides for this application may at first seem obvious. However, this approach had been generally rejected in the past due to the inherent divergence of incoherent IR radiation coupled with the fact that the reflectance of most materials is poor for angles far from normal incidence. The present system overcomes these obstacles by using radiation which is very nearly collimated and a light guide coating which exhibits especially high reflectance at angles very close to grazing. An alternative approach to transmitting the radiation to and from the probe might be the use of infrared fiber optic s2. However, this approach has two fundamental problems. First, suitable mid-ir fibers have yet to be developed. Second, the relatively low energy of thermal radiation sources in the mid- IR necessitates the use of optical systems with high geometric thoughput. In the case of fiber optics, this would require either the use of individual fibers with diameters of the order of a millimeter or equally large fiber bundles with a very high collection efficiency. The operation of the probe sensing head is based on the attenuated total reflectance (ATR) method 3 In this method, the IR radiation is transmitted through an optical element having a high index of refraction in such a way that it alternatively reflects from opposite sides of the element at an incidence angle of approximately 458. Due to the high refractive index, the radiation is totally reflected at this angle when the element is surrounded by air. However, when the element is immersed in an absorbing medium, a portion of the radiation is absorbed at each reflection, making it possible to obtain an absorption spectrum of the surrounding medium. Since the effective penetration depth on each reflection is rather small, this technique is especially useful for the very strong absorption bands encountered in the mid-ir. The design of the sensing head had been discussed in detail previously (1)(4). Thus I will only outline it briefly here. The heart of the probe is a cylindrical rod of an infrared transmitting material with a relatively high index of refraction. The Figure 1 A - Process scale deep immersion probe mounted in a batch reaction vessel B - Probe Sensing head construction 2 Hellma Axiom, Inc.. / AN-904
3 ends of the rod are in the form of 908 cones. As illustrated in Figure 1, the collimated radiation traveling down through one of the internal light guides first strikes a reflecting cone the sides of which are at an angle of to the light guide axis. The reflected radiation then enters the rod approximately normal to the conical end surface, after which it travels through the rod at an angle of 458 to its axis, alternately being reflected from one side and then the other. After leaving the lower end of the rod, the radiation is recollimated by reflection from a second cone. Optical stops at each end eliminate radiation which would otherwise travel through the rod without striking the reflecting cones. A large reflecting cone at the bottom of the probe redirects the radiation up through a second light guide. The design outlined above was initially developed to meet the size constraints imposed by the ports available on typical reaction vessels. However, it also provides several additional benefits. For example, the optical characteristics are essentially machined into the structure. Since there are no adjustments, nothing can change. The result is a very high degree of performance stability and repeatability, even from probe to probe. In addition, the design concentrates the radiation at the optimum angle of incidence. The result is enhanced sensitivity compared to other ATR devices and a very high degree of absorbance linearity. Along with these benefits, goes the ruggedness which results both from the lack of adjustable parts and the solid stainless steel construction. 2. Scaling Considerations An important consideration in designing any process monitoring system is the need to employ the system on a small scale to model the process and then to scale it up for full scale online operation - with minimal change in performance. In our program, this need was met by developing a series of probes having essentially identical spectroscopic characteristics but designed to mate to reaction vessels ranging in capacity from a few hundred milliliters to thousands of gallons. To date, we have constructed probes with diameters of 0.98, 2, and 3.5 inches and lengths ranging from 9 inches to 8 feet. The diameters of the ATR elements corresponding to the three probe diameters are 0.125, 01.25, and 0.5 inches respectively. However, in each case the length to diameter ratio of the element was kept constant so as to provide approximately the same number of reflections at the interface between the ATR rod and the surrounding sample. For a given combination of ATR element material and sample species, the measured infrared band strength will depend on the angle of incidence of the radiation and the number of reflections. Thus, all of our probes produce essentially the same measurements, independent of overall size. The structural designs of all the probes are quite similar, the main difference being the method of attachment to the reaction vessel. In the case of the laboratory scale (0.98 diameter) probes, the interface is in the form of a sliding tapered joined to mate to a joint on a typical glass reaction vessel. For Figure 2: Experimental arrangement using a laboratory scale Deep Immersion Probe. AN-904 / Hellma Axiom, Inc. 3
4 Figure 3: A - Spectrum of a 20% solution of styrene monomer in toluene. T indicates the band used for toluene quantization. B - Spectrum of toluene. C - Spectrum A minus spectrum B. This is the spectrum of styrene. Figure 4: A - Acid catalyzed reaction mixture after 25 minutes. B - Spectrum A less toluene. C - Spectrum B less styrene (spectrum 3,C). This is a spectrum of polystyrene. 4 Hellma Axiom, Inc.. / AN-904
5 larger probes, we use standard industrial flanges, gusseted when necessary to withstand the torques encountered in the typical stirred process kettle. 3. Laboratory Scale Experimental Configuration The experimental arrangement used for the measurements to be discussed below is shown in Figure 2. The collimated radiation obtained from the side port of an FTIR spectrometer (in this case, Laser Precision Analytical s Model RFX-40) is routed to be the experiment by a series of light guides and 90 degree rotary mirror assemblies. Any number of these can be used as long as the total length is less than roughly fifty feet. A 4x beam condenser is used to reduce the diameter of the collimated beam from 1.25 to so as to match the small light guides used within the probe. After passing down through the probe, interacting with the liquid chemicals, and returning back to the top of the probe, the beam is expanded back to 1.25 in diameter so as to match the detector assembly optics. For our experiments, we used a wide-band HgCdTe detector operating at liquid nitrogen temperature. The round bottom flask shown in Figure 2 was used for our heated free radical polymerization experiments discussed below. In this case, the probe was inserted through the central 29/42 ground glass joint of the flask. The probe is equipped with a sliding tapered joint which was lowered and clamped in place. A reflux condenser was mounted on a second ground glass joint, and a thermocouple temperature sensor (not shown) was inserted into the solution through the reflux condenser. Since the experiments were carried out primarily under reflux conditions, no mechanical stirring was used. For the room temperature acid catalyzed experiments, the round bottom flask was replaced by an Erlenmeyer flask equipped with a magnetic stirring rod. 4. Process FTIR Software A wealth of quantitative software is available for use in converting the large amounts of data obtained in FTIR spectroscopy into numeric readout of process variables 5. All of these rely on the fact that, in most situations, infrared absorbance at a given spectral frequency will be linearly dependent on the concentrations of the various chemical species present in the solution. The work discussed below employed the P-matrix approach, in which the concentrations of the various species present are considered as functions of the absorbencies, ie: C = PA. Here, C represents the set of concentrations of the various species present, A is the set of IR absorbencies at selected frequencies, and P is the matrix of coefficients which relates concentration to absorbance. In calibrating the system to monitor a given process, one normally prepares a set of samples containing measured quantities of each constituent of the process, with concentrations covering the full range of values to be encountered. The computer then performs a least squares fit to determine the set of matrix coefficients which best characterizes the calibration Figure 5: Concentration versus time of (1) styrene, (2) Polystyrene, and (3) toluene in the acid-catalyzed polymerization process. AN-904 / Hellma Axiom, Inc. 5
6 data. In doing this, the program will automatically compensate for any impurities that are present, as long as a full range of possible impurity values are included in the calibration set. This feature proved useful in the work discussed below by enabling us to compensate for various temperature dependent effects. 5. Monitoring of Selected Polymerization Reactions To illustrate the capabilities of the FTIR immersion approach to process monitoring, we carried out a series of laboratory scale polymerization experiments. These included the acid-catalyzed polymerization of styrene, the free radical polymerization of both styrene and methyl methacrylate, and the formation of a copolymer of styrene and methyl methacrylate. The acid-catalyzed process was carried out at room temperature while the free radical experiments were carried out under reflex conditions using the apparatus illustrated in Figure 2. Spectra were obtained using the spectrometer s automatic collection mode at a rate of about one spectrum every 30 seconds during each of the polymerization runs. For our experiments, we saved all of the spectra and replayed them later using multiple component analysis software to obtain plots of the concentration of each species versus time. In a practical situation, a laboratory experiment such as this would be used to select the correct parameters and methods for data analysis. On-line, the data analysis would be run simultaneously with the data collection so as to provide an essentially real time read-out of the concentrations. 6. Acid Catalyzed Polymerization In developing a methodology for monitoring a specific process using FTIR, the first step is to select a set of spectral bands that are representative of the various chemical species present. This can be done automatically by using chemometric software. However, for the work reported here, we simply examined the spectra and selected bands having minimum overlap with those of the other species. In Figure 3, the upper trace (A) is spectrum of a 20% solution of the styrene monomer in toluene, 3B is toluene alone, and 3C is the spectrum of styrene obtained by subtracting the toluene spectrum from the mixture. This figure illustrates one of the powers of mid-ir spectroscopy - the ability to positively identify an unknown component of a mixture by sequentially stripping away the spectra of the know components. This is a result of the sharpness and stability of the mid-ir spectral bands and of the high degree of linearity and precision of the FTIR spectrometers. Figure 4A is a spectrum of the reaction mixture about 25 minutes after adding the catalyst. Figures 4B and 4C are the results of sequentially subtracting the toluene spectrum (3A) and the styrene solution spectrum (3B) from this spectrum. The final result can be easily identified as polystyrene. By examining the various spectra of figures 3 and 4, we were Figure 6: Spectra of toluene at (A) 258C and (B) 1008C, and the difference between these two spectra (C). 6 Hellma Axiom, Inc.. / AN-904
7 Figure 7: Indicated concentration versus time of (1) styrene, (2) methyl methacrylate, (3) polystyrene, and (4) PMMA during the free radical polystyrene polymerization process. Figure 8: A - Spectrum of methyl methacrylate after subtracting toluene. B - The final reaction product (PMMA) after subtracting spectra of toluene and methyl Methacrylate. AN-904 / Hellma Axiom, Inc. 7
8 able to select a convenient set of spectral bands for use in the quantitative analysis program. These are indicated by the letters T (toluene), S (styrene), and P (polystyrene) in the two figures. It is not necessary for the bands of the various species to be completely non-overlapping. The computer program solves the simultaneous equations to obtain the concentrations of the individual constituents. The spectra of Figures 3B, 3C, and 4C were used as standards in setting up the analysis program. Figure 5 is a plot of concentration versus time for the three constituents of the acid catalyzed reaction. As the figure shows, this process has a long initiation period followed by a short reaction time. This type of behavior illustrates the utility of the immersion probe technique in accurately determining when a reaction is complete so that the process can be terminated at the optimum point. The dip in the toluene concentration may be due to a temporary inhomogeneity caused by preferential polymerization in the vicinity of the probe. 7. Free Radical Polymerization The free radical polymerization runs were initiated at room temperature and then brought up to the reflux temperature of approximately 1098C. This introduced two new factors into the analysis, the temperature dependence of the spectral bands of the various process constituents and that of the Chemraz O rings used in the probe. These effects are illustrated in Figure 6, which consists of toluene spectra at 258C and 1008C and the difference. The broad features in the difference spectrum are due to the temperature dependence of the O ring spectrum. The sharp negative peaks are due to the fact that the toluene bands become weaker and broader at elevated temperature. In the case of the process constituents, the temperature artifacts were eliminated by obtaining high temperature calibration spectra of toluene and the two monomer solutions and by using the final reaction product spectra (after the appropriate subtractions) as the polymer standards. In addition, toluene spectra taken at various temperatures were included in the calibration set to minimize the dependence of the analysis on the characteristics of both toluene and Chemraz. In this set of experiments, although toluene and Chemraz were included implicitly in the calibration, their concentrations were not displayed. Figure 7 is a time history of the free radical styrene polymerization run, revealing a much different time dependence than the acid catalyzed run of Figure 5. The process is generally much more gradual, taking place under diffusion controlled conditions. Note that Figure 7 includes curves for all four of the variables, styrene, polystyrene, MMA, and PMMA even though only two of these constituents were present for each run. This was done to validate the method for later use in the copolymerization experiment. Figure 9: Indicated concentration versus time of (1) styrene, (2) methyl methacrylate, (3) polystyrene, and (4) PMMA during the free radical copolymerization process. 8 Hellma Axiom, Inc.. / AN-904
9 A free radical polymerization run of methyl methacrylate gave results similar to those of Figure 7, but with an even slower reaction rate. The time required for consumption of one half of the monomer was about 66 minutes as opposed to 33 minutes for styrene polymerization. The bands used for this run and for copolymerization run discussed below are indicated in Figure 8. This includes the spectra of dissolved methyl methacrylate (A) and of the final product, polymethyl methacrylate (B). In both cases, we decided to use the strong carbonyl band in the vicinity of 1730 cm -1 for the analysis since it does not overlap any strong bands of toluene, styrene, or polystyrene. Of course, the carbonyl band is present in both the monomer (MMA) and the polymer (PMMA), but it is sufficiently shifted to higher frequency in the polymer to provide adequate separation. At this point, a comment is in order about the methods used in doing the calibration for the quantitative analyses. In the case of the monomers, we had initial spectra of solutions with known concentrations. However, in the case of the polymers, we used the final spectra of the reaction mixtures for calibration. Although, in principle, the concentrations of the polymers can be inferred from the change in monomer concentrations, we did not take this step. Thus, the polymer concentration scale is arbitrary. 8. Copolymerization of Styrene and Methyl Methacrylate The free radical copolymerization was carried out starting with a solution of ten percent styrene and ten percent MMA in toluene. In this case, the P-matrix calculation involved a total of four explicit variable constituents, the two monomers and two polymers, plus three implicit variable, toluene, Chemraz, and temperature. The concentrations of the four components of interest are shown in Figure 9. In interpreting the data of Figure 9, we can interpret the vertical scale between the lower control limit (LCL) and the upper control limit (UCL) as running from 0 to 100% concentration. This is meaningful in the case of monomers, suggesting the process consumed about twice as much styrene monomer as MMA. Since the polymer scale is arbitrary, we can not draw such an overall conclusion about the final polymer values reached. However, it is apparent that polystyrene was being formed preferentially during the early stages of the process, with PMMA formation accelerating after 15 or 20 minutes. As Figure 9 illustrates, the use of the IR immersion probe makes it possible to precisely monitor the degree of polymerization at any point during a reaction. This capability could prove very useful in cases such as the production of glues and resins in which the correct degree of partial polymerization is Figure 10: Comparison of the final reaction products deposited as thin films on the immersion probe sensing element. A - Product of the copolymerization process. B - Polystyrene. C - Spectrum A minus spectrum B. D - PMMA. AN-904 / Hellma Axiom, Inc. 9
10 crucial to the quality of the product. In addition, the ability to closely monitor the balance between the various constituents in a reaction creates the opportunity not only to optimize reaction conditions but also to tailor the properties of the final product to meet a specific need. Since free radical polymerization takes place under diffusion controlled conditions, one would expect the final product to exhibit a random sequence of styrene and MMA units. Its characteristics should thus approximate those of an ideal copolymer rather than a mixture of two individual polymers. This assumption can be tested by doing an infrared analysis of the final product. To obtain samples of the final products of our experiments, we simply removed the probe from the reaction vessel while the mixture was still hot. When this is done, the solvent immediately evaporates, leaving a uniform polymer film on the probe structure and ATR element. The results are given in Figure 10. It is clear that, while the copolymer spectrum has some features in common with both polystyrene and PMMA, there are also significant difference. On subtracting the polystyrene spectrum from the copolymer, we found that the bands of the former were present but were shifted to higher frequency. Comparing this result with PMMA, we find that the strong carbonyl band has been relatively unaffected by copolymerization but that the bending modes between 1100 and 1300 wavenumbers had been substantially altered. The degree of alteration suggests that the great majority of MMA units are bordered by at least one styrene unit. This final step is not only useful in testing the degree of copolymerization but can be used to validate the choice of bands used for P-matrix analysis. Clearly, the choice of the carbonyl band as an indicator of PMMA concentration is probably not as accurate due to the band shifts. However, the accuracy could be improved by averaging over a range of frequencies encompassing the extent of the shift. to thank Bruce McIntosh for providing the FTIR hardware and software and for assisting me in their operation, Warren Vidrine for carrying out the P-matrix multicomponent analysis and for his help in interpreting the data, and Jerry Kacsir and Hue Phan for sample preparation and experimental assistance. References 1. Doyle, W.M. and Jennings, N.A., Fourier Transform-In frared Chemical Reaction Monitoring Using an In Situ Deep Immersion Probe, Spectroscopy, Vol. 5, No. 1, Jan. 1990, p Archibald, D.D., Lin, L.T., and Honigs, D.E., Remote Near-IR Spectroscopy Over an Optical Fiber with a Modified FT Spectrometer, Applied Spectroscopy, Vol. 42, No. 3, 1988, p Harrick, N.J., Internal Reflection Spectroscopy, John Wiley, New York, Doyle, W.M., Absorbance Linearity and Repeatability in Cylindrical Internal Reflectance FT-IR Spectroscopy of Liquids, Applied Spectroscopy, Vol. 44, No. 1, 1990, p Crocombe, R.A., Olsen, M.L., and Hill, S.L., Quantita tive Fourier Transform Infrared Methods for Real Com plex Samples, Computerized Quantitative Infrared Analysis, ASTM STP 934, G.L. McClure, Ed., Ameri can Society for Testing and Materials, Philadelphia, 1987,p Conclusion The experiments reported above illustrate the fact that the use of an IR immersion probe with a modern FTIR spectrometer can yield an intimate view of chemical reaction, making it possible to optimize all of the parameters and to terminate the reaction at any desired degree of completion. Experiments carried out on a laboratory scale can be used to fine-tune the parameters of a process scale reaction. In addition, the monitoring technique itself can be scaled up, making it possible to obtain continuous on-line data which is fully consistent with the data obtained in the lab. Acknowledgements In carrying out the work reported in this paper, I have had the able assistance of several members of the staff of Laser Precision Analytical. In particular, I would like 10 Hellma Axiom, Inc.. / AN-904
Principles and Applications of Fourier Transform Infrared (FTIR) Process Analysis
 Technical Note AN 906 Rev. C Principles and Applications of Fourier Transform Infrared (FTIR) Process Analysis Walter M. Doyle The past few years have seen rapid growth in the use of infrared spectroscopy
Technical Note AN 906 Rev. C Principles and Applications of Fourier Transform Infrared (FTIR) Process Analysis Walter M. Doyle The past few years have seen rapid growth in the use of infrared spectroscopy
Monitoring Emulsion Polymerization by Raman Spectroscopy
 An Executive Summary Monitoring Emulsion Polymerization by Raman Spectroscopy Why process analytical matters to process development R&D. Serena Stephenson, PhD Senior R&D Analytical Manager Kishori Deshpande,
An Executive Summary Monitoring Emulsion Polymerization by Raman Spectroscopy Why process analytical matters to process development R&D. Serena Stephenson, PhD Senior R&D Analytical Manager Kishori Deshpande,
Signal Level Considerations For Remote Diffuse Reflectance Analysis
 TECHNICAL NOTE: AN-916 (Rev. B) (Feb. 28, 2012) Signal Level Considerations For Remote Diffuse Reflectance Analysis W. M. Doyle Axiom Analytical, Inc INTRODUCTION: Near infrared (NIR) spectroscopy has
TECHNICAL NOTE: AN-916 (Rev. B) (Feb. 28, 2012) Signal Level Considerations For Remote Diffuse Reflectance Analysis W. M. Doyle Axiom Analytical, Inc INTRODUCTION: Near infrared (NIR) spectroscopy has
Absorbance Linearity and Repeatability in Cylindrical Internal Reflectance FT-IR Spectroscopy of Liquids
 Technical Note AN 902 Rev. C Published in: Applied Spectroscopy, 41, 50 (1990) Absorbance Linearity and Repeatability in Cylindrical Internal Reflectance FT-IR Spectroscopy of Liquids Walter M. Doyle This
Technical Note AN 902 Rev. C Published in: Applied Spectroscopy, 41, 50 (1990) Absorbance Linearity and Repeatability in Cylindrical Internal Reflectance FT-IR Spectroscopy of Liquids Walter M. Doyle This
POLYMERIZATION REACTION MONITORING FOR PSA PRODUCTION USING AN ATR-FTIR PROBE
 POLYMERIZATION REACTION MONITORING FOR PSA PRODUCTION USING AN ATR-FTIR PROBE Renata Jovanović, Doctoral student, Department of Chemical Engineering, University of Ottawa, Ottawa, Canada, (jovanovi@genie.uottawa.ca)
POLYMERIZATION REACTION MONITORING FOR PSA PRODUCTION USING AN ATR-FTIR PROBE Renata Jovanović, Doctoral student, Department of Chemical Engineering, University of Ottawa, Ottawa, Canada, (jovanovi@genie.uottawa.ca)
Chemistry Instrumental Analysis Lecture 15. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 15 IR Instruments Types of Instrumentation Dispersive Spectrophotometers (gratings) Fourier transform spectrometers (interferometer) Single beam Double beam
Chemistry 4631 Instrumental Analysis Lecture 15 IR Instruments Types of Instrumentation Dispersive Spectrophotometers (gratings) Fourier transform spectrometers (interferometer) Single beam Double beam
Introduction to Fourier Transform Infrared Spectroscopy
 molecular spectroscopy Introduction to Fourier Transform Infrared Spectroscopy Part of Thermo Fisher Scientific Introduction What is FT-IR? FT-IR stands for Fourier Transform InfraRed, the preferred method
molecular spectroscopy Introduction to Fourier Transform Infrared Spectroscopy Part of Thermo Fisher Scientific Introduction What is FT-IR? FT-IR stands for Fourier Transform InfraRed, the preferred method
ANALYSIS OF LOW DENSITY PARTICLES USING DIFFERENTIAL CENTRIFUGAL SEDIMENTATION
 ANALYSIS OF LOW DENSITY PARTICLES USING DIFFERENTIAL CENTRIFUGAL SEDIMENTATION Conventional Centrifugal Methods Centrifugal sedimentation of particles suspended in a fluid is a well known method (1, 2)
ANALYSIS OF LOW DENSITY PARTICLES USING DIFFERENTIAL CENTRIFUGAL SEDIMENTATION Conventional Centrifugal Methods Centrifugal sedimentation of particles suspended in a fluid is a well known method (1, 2)
Fourier transform infrared spectroscopy (FTIR) is a method used to obtain an infrared
 Fourier Transform Infrared Spectroscopy: Low Density Polyethylene, High Density Polyethylene, Polypropylene and Polystyrene Eman Mousa Alhajji North Carolina State University Department of Materials Science
Fourier Transform Infrared Spectroscopy: Low Density Polyethylene, High Density Polyethylene, Polypropylene and Polystyrene Eman Mousa Alhajji North Carolina State University Department of Materials Science
Mid-IR Sampling Techniques for Biological Molecules
 Mid-IR Sampling Techniques for Biological Molecules Mid-IR Sampling Techniques LIQUIDS Transmission ATR (Attenuated Total Reflectance) Solids Transmission (KBr pellets, Mulls) ATR Diffuse Reflectance Sampling
Mid-IR Sampling Techniques for Biological Molecules Mid-IR Sampling Techniques LIQUIDS Transmission ATR (Attenuated Total Reflectance) Solids Transmission (KBr pellets, Mulls) ATR Diffuse Reflectance Sampling
VASCO Flex TM Remote Particle size Analyzer
 Technical note NT01-VASCO Flex_EN_V01 VASCO Flex TM Remote Particle size Analyzer Nano-particle Size measurements brought into your process! 1 VASCO Flex TM Analyzer: «in situ» Nano Particle size measurement
Technical note NT01-VASCO Flex_EN_V01 VASCO Flex TM Remote Particle size Analyzer Nano-particle Size measurements brought into your process! 1 VASCO Flex TM Analyzer: «in situ» Nano Particle size measurement
Introduction to Fourier Transform Infrared Spectroscopy
 Introduction to Fourier Transform Infrared Spectroscopy Introduction What is FTIR? FTIR stands for Fourier transform infrared, the preferred method of infrared spectroscopy. In infrared spectroscopy, IR
Introduction to Fourier Transform Infrared Spectroscopy Introduction What is FTIR? FTIR stands for Fourier transform infrared, the preferred method of infrared spectroscopy. In infrared spectroscopy, IR
FTIR Spectrometer. Basic Theory of Infrared Spectrometer. FTIR Spectrometer. FTIR Accessories
 FTIR Spectrometer Basic Theory of Infrared Spectrometer FTIR Spectrometer FTIR Accessories What is Infrared? Infrared radiation lies between the visible and microwave portions of the electromagnetic spectrum.
FTIR Spectrometer Basic Theory of Infrared Spectrometer FTIR Spectrometer FTIR Accessories What is Infrared? Infrared radiation lies between the visible and microwave portions of the electromagnetic spectrum.
DAY LABORATORY EXERCISE: SPECTROSCOPY
 AS101 - Day Laboratory: Spectroscopy Page 1 DAY LABORATORY EXERCISE: SPECTROSCOPY Goals: To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are
AS101 - Day Laboratory: Spectroscopy Page 1 DAY LABORATORY EXERCISE: SPECTROSCOPY Goals: To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are
Fourier Transform Infrared. Spectrometry
 Fourier Transform Infrared. Spectrometry Second Editio n PETER R. GRIFFITH S JAMES A. de HASETH PREFACE x v CHAPTER 1 INTRODUCTION TO VIBRATIONAL SPECTROSCOPY 1 1.1. Introduction 1 1.2. Molecular Vibrations
Fourier Transform Infrared. Spectrometry Second Editio n PETER R. GRIFFITH S JAMES A. de HASETH PREFACE x v CHAPTER 1 INTRODUCTION TO VIBRATIONAL SPECTROSCOPY 1 1.1. Introduction 1 1.2. Molecular Vibrations
Supporting Information
 1 Supporting Information 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 Materials and Methods Experiment In this study, an alloy IR probe which allowed us to get access to spectral
1 Supporting Information 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 Materials and Methods Experiment In this study, an alloy IR probe which allowed us to get access to spectral
ONYX -MCE MULTI-CHANNEL OPTICAL FIBER PYROMETERS WITH ACTIVE EMISSIVITY COMPENSATION PRECISION TEMPERATURE MEASUREMENT FOR DEMANDING INDUSTRIAL
 ONYX -MCE MULTI-CHANNEL OPTICAL FIBER PYROMETERS WITH ACTIVE EMISSIVITY COMPENSATION PRECISION TEMPERATURE MEASUREMENT FOR DEMANDING INDUSTRIAL APPLICATIONS Accurate, repeatable, and reliable temperature
ONYX -MCE MULTI-CHANNEL OPTICAL FIBER PYROMETERS WITH ACTIVE EMISSIVITY COMPENSATION PRECISION TEMPERATURE MEASUREMENT FOR DEMANDING INDUSTRIAL APPLICATIONS Accurate, repeatable, and reliable temperature
Glossary. Analyte - the molecule of interest when performing a quantitative analysis.
 Glossary This glossary contains definitions of many important FTIR terms. Many of the terms listed here appeared in italics in the body of the book. Words that appear in italics in the glossary are defined
Glossary This glossary contains definitions of many important FTIR terms. Many of the terms listed here appeared in italics in the body of the book. Words that appear in italics in the glossary are defined
Marine bio-inspired underwater contact adhesion
 Marine bio-inspired underwater contact adhesion Sean K. Clancy, Antonio Sodano, Dylan J. Cunningham, Sharon S. Huang, Piotr J. Zalicki, Seunghan Shin, * and B. Kollbe Ahn * Marine Science Institute, University
Marine bio-inspired underwater contact adhesion Sean K. Clancy, Antonio Sodano, Dylan J. Cunningham, Sharon S. Huang, Piotr J. Zalicki, Seunghan Shin, * and B. Kollbe Ahn * Marine Science Institute, University
Analysis of Polymers and Plastics. Innovation with Integrity. Quality Control & Failure Analysis FTIR
 Analysis of Polymers and Plastics Quality Control & Failure Analysis Innovation with Integrity FTIR Quality Control for Cost-Efficiency Plastics are used in countless products such as automotive parts,
Analysis of Polymers and Plastics Quality Control & Failure Analysis Innovation with Integrity FTIR Quality Control for Cost-Efficiency Plastics are used in countless products such as automotive parts,
Spectroscopy tools for PAT applications in the Pharmaceutical Industry
 Spectroscopy tools for PAT applications in the Pharmaceutical Industry Claude Didierjean Sr. Technology and Applications Consultant Real Time Analytics Mettler Toledo AutoChem, Inc. claude.didierjean@mt.com
Spectroscopy tools for PAT applications in the Pharmaceutical Industry Claude Didierjean Sr. Technology and Applications Consultant Real Time Analytics Mettler Toledo AutoChem, Inc. claude.didierjean@mt.com
Modern Techniques in Applied Molecular Spectroscopy
 Modern Techniques in Applied Molecular Spectroscopy Edited by FRANCIS M. MIRABELLA Equistar Chemicals, LP A Wiley-Interscience Publication JOHN WILEY & SONS, INC. New York Chichester Weinheim Brisbane
Modern Techniques in Applied Molecular Spectroscopy Edited by FRANCIS M. MIRABELLA Equistar Chemicals, LP A Wiley-Interscience Publication JOHN WILEY & SONS, INC. New York Chichester Weinheim Brisbane
Analysis of Polymers and Plastics. Innovation with Integrity. Quality Control & Failure Analysis FT-IR
 Analysis of Polymers and Plastics Quality Control & Failure Analysis Innovation with Integrity FT-IR Reliable quality control is essential to achieve a cost-saving production of high quality plastic products.
Analysis of Polymers and Plastics Quality Control & Failure Analysis Innovation with Integrity FT-IR Reliable quality control is essential to achieve a cost-saving production of high quality plastic products.
AS 101: Day Lab #2 Summer Spectroscopy
 Spectroscopy Goals To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are related To see spectral lines from different elements in emission and
Spectroscopy Goals To see light dispersed into its constituent colors To study how temperature, light intensity, and light color are related To see spectral lines from different elements in emission and
Chemistry 524--Final Exam--Keiderling Dec. 12, pm SES
 Chemistry 524--Final Exam--Keiderling Dec. 12, 2002 --4-8 pm -- 238 SES Please answer all questions in the answer book provided. Calculators, rulers, pens and pencils are permitted plus one 8.5 x 11 sheet
Chemistry 524--Final Exam--Keiderling Dec. 12, 2002 --4-8 pm -- 238 SES Please answer all questions in the answer book provided. Calculators, rulers, pens and pencils are permitted plus one 8.5 x 11 sheet
Spectroscopy in Transmission
 Spectroscopy in Transmission + Reflectance UV/VIS - NIR Absorption spectra of solids and liquids can be measured with the desktop spectrograph Lambda 9. Extinctions up to in a wavelength range from UV
Spectroscopy in Transmission + Reflectance UV/VIS - NIR Absorption spectra of solids and liquids can be measured with the desktop spectrograph Lambda 9. Extinctions up to in a wavelength range from UV
Mid-IR Methods. Matti Hotokka
 Mid-IR Methods Matti Hotokka Traditional methods KBr disk Liquid cell Gas cell Films, fibres, latexes Oil suspensions GC-IR PAS KBr Disk 1 mg sample, 200 mg KBr May be used for quantitative analysis Mix
Mid-IR Methods Matti Hotokka Traditional methods KBr disk Liquid cell Gas cell Films, fibres, latexes Oil suspensions GC-IR PAS KBr Disk 1 mg sample, 200 mg KBr May be used for quantitative analysis Mix
Calibrating the Thermal Camera
 1 of 5 4/19/2012 5:33 AM from photonics.com: 12/01/2009 http://www.photonics.com/article.aspx?aid=40679 Calibrating the Thermal Camera As thermal cameras gain ground in the commercial market, testing becomes
1 of 5 4/19/2012 5:33 AM from photonics.com: 12/01/2009 http://www.photonics.com/article.aspx?aid=40679 Calibrating the Thermal Camera As thermal cameras gain ground in the commercial market, testing becomes
Agilent Cary 630 FTIR Spectrometer Supporting Organic Synthesis in Academic Teaching Labs
 Agilent Cary 630 FTIR Spectrometer Supporting Organic Synthesis in Academic Teaching Labs Application note Academic Author Frank Higgins and Alan Rein Agilent Technologies Danbury, CT, USA Introduction
Agilent Cary 630 FTIR Spectrometer Supporting Organic Synthesis in Academic Teaching Labs Application note Academic Author Frank Higgins and Alan Rein Agilent Technologies Danbury, CT, USA Introduction
HOW ADVANCED PYROMETERS INCREASE THERMAL PROCESS REPEATABILITY AND PRODUCT QUALITY
 HOW ADVANCED PYROMETERS INCREASE THERMAL PROCESS REPEATABILITY AND PRODUCT QUALITY Accurate temperature measurement is key for controlling the stability and repeatability of many temperature-critical processes.
HOW ADVANCED PYROMETERS INCREASE THERMAL PROCESS REPEATABILITY AND PRODUCT QUALITY Accurate temperature measurement is key for controlling the stability and repeatability of many temperature-critical processes.
Speed of Light in Air
 Speed of Light in Air Electromagnetic waves represent energy in the form of oscillating electric and magnetic fields which propagate through vacuum with a speed c = 2.9979246x10 8 m/s. Electromagnetic
Speed of Light in Air Electromagnetic waves represent energy in the form of oscillating electric and magnetic fields which propagate through vacuum with a speed c = 2.9979246x10 8 m/s. Electromagnetic
A faster, more accurate way of characterizing cube beamsplitters using the Agilent Cary 7000 Universal Measurement Spectrophotometer (UMS)
 A faster, more accurate way of characterizing cube beamsplitters using the Agilent Cary 7000 Universal Measurement Spectrophotometer (UMS) Application note Materials Authors Travis Burt, Chris Colley,
A faster, more accurate way of characterizing cube beamsplitters using the Agilent Cary 7000 Universal Measurement Spectrophotometer (UMS) Application note Materials Authors Travis Burt, Chris Colley,
Use of High Speed/High Resolution Size-Based Chromatographic Separation of Polymeric Mixtures with Offline Infrared Detection
 Use of High Speed/High Resolution Size-Based Chromatographic Separation of Polymeric Mixtures with Michael O Leary 1, Jennifer Gough 1, Damian Morrison 1, Alain Creissen 2 1 Waters Corporation, Milford,
Use of High Speed/High Resolution Size-Based Chromatographic Separation of Polymeric Mixtures with Michael O Leary 1, Jennifer Gough 1, Damian Morrison 1, Alain Creissen 2 1 Waters Corporation, Milford,
Experiment 9 Dehydration of Methylcyclohexanol Friday/Monday 1
 Experiment 9 Dehydration of Methylcyclohexanol Friday/Monday 1 There are three distinct steps in most organic preparative reactions: 1) the reaction itself, 2) isolation of the crude product, and 3) final
Experiment 9 Dehydration of Methylcyclohexanol Friday/Monday 1 There are three distinct steps in most organic preparative reactions: 1) the reaction itself, 2) isolation of the crude product, and 3) final
Reference Standards Page 156 For calibrating your spectrometer. PIKECalc Page 159 For FTIR sampling computations
 Standards, Software, Databases We strive to provide you with useful sampling tools for spectroscopy and offer these additional products and information to serve your laboratory requirements. If you have
Standards, Software, Databases We strive to provide you with useful sampling tools for spectroscopy and offer these additional products and information to serve your laboratory requirements. If you have
Preview from Notesale.co.uk Page 1 of 38
 F UNDAMENTALS OF PHOTONICS Module 1.1 Nature and Properties of Light Linda J. Vandergriff Director of Photonics System Engineering Science Applications International Corporation McLean, Virginia Light
F UNDAMENTALS OF PHOTONICS Module 1.1 Nature and Properties of Light Linda J. Vandergriff Director of Photonics System Engineering Science Applications International Corporation McLean, Virginia Light
Detection of trace contamination on metal surfaces using the handheld Agilent 4100 ExoScan FTIR
 Detection of trace contamination on metal surfaces using the handheld Agilent 4100 ExoScan FTIR Ensuring ultimate cleanliness for maximum adhesion Application Note Author John Seelenbinder Agilent Technologies,
Detection of trace contamination on metal surfaces using the handheld Agilent 4100 ExoScan FTIR Ensuring ultimate cleanliness for maximum adhesion Application Note Author John Seelenbinder Agilent Technologies,
Chapter 24 Photonics Question 1 Question 2 Question 3 Question 4 Question 5
 Chapter 24 Photonics Data throughout this chapter: e = 1.6 10 19 C; h = 6.63 10 34 Js (or 4.14 10 15 ev s); m e = 9.1 10 31 kg; c = 3.0 10 8 m s 1 Question 1 Visible light has a range of photons with wavelengths
Chapter 24 Photonics Data throughout this chapter: e = 1.6 10 19 C; h = 6.63 10 34 Js (or 4.14 10 15 ev s); m e = 9.1 10 31 kg; c = 3.0 10 8 m s 1 Question 1 Visible light has a range of photons with wavelengths
Reprinted from February Hydrocarbon
 February2012 When speed matters Loek van Eijck, Yokogawa, The Netherlands, questions whether rapid analysis of gases and liquids can be better achieved through use of a gas chromatograph or near infrared
February2012 When speed matters Loek van Eijck, Yokogawa, The Netherlands, questions whether rapid analysis of gases and liquids can be better achieved through use of a gas chromatograph or near infrared
Infrared Spectroscopy: Identification of Unknown Substances
 Infrared Spectroscopy: Identification of Unknown Substances Suppose a white powder is one of the four following molecules. How can they be differentiated? H N N H H H H Na H H H H H A technique that is
Infrared Spectroscopy: Identification of Unknown Substances Suppose a white powder is one of the four following molecules. How can they be differentiated? H N N H H H H Na H H H H H A technique that is
CHAPTER 6 FAULT DIAGNOSIS OF UNBALANCED CNC MACHINE SPINDLE USING VIBRATION SIGNATURES-A CASE STUDY
 81 CHAPTER 6 FAULT DIAGNOSIS OF UNBALANCED CNC MACHINE SPINDLE USING VIBRATION SIGNATURES-A CASE STUDY 6.1 INTRODUCTION For obtaining products of good quality in the manufacturing industry, it is absolutely
81 CHAPTER 6 FAULT DIAGNOSIS OF UNBALANCED CNC MACHINE SPINDLE USING VIBRATION SIGNATURES-A CASE STUDY 6.1 INTRODUCTION For obtaining products of good quality in the manufacturing industry, it is absolutely
Our Drop Counter sensor now features housing for two electrode sensors, an anti-twist mechanism, an indicator LED and two cable guides.
 The Drop Counter sensor is an optical sensor that accurately records the number of drops of titrant added during a titration. The Drop Counter sensor software can automatically convert the number of drops
The Drop Counter sensor is an optical sensor that accurately records the number of drops of titrant added during a titration. The Drop Counter sensor software can automatically convert the number of drops
Measuring Laser Diode Optical Power with an Integrating Sphere
 Measuring Laser Diode Optical Power with an Integrating Sphere Introduction Characterizing radiant sources like laser diodes accurately depends on the ability to measure their optical power output accurately.
Measuring Laser Diode Optical Power with an Integrating Sphere Introduction Characterizing radiant sources like laser diodes accurately depends on the ability to measure their optical power output accurately.
Introduction to FT-IR Spectroscopy
 Introduction to FT-IR Spectroscopy An FT-IR Spectrometer is an instrument which acquires broadband NIR to FIR spectra. Unlike a dispersive instrument, i.e. grating monochromator or spectrograph, an FT-IR
Introduction to FT-IR Spectroscopy An FT-IR Spectrometer is an instrument which acquires broadband NIR to FIR spectra. Unlike a dispersive instrument, i.e. grating monochromator or spectrograph, an FT-IR
1 WHAT IS SPECTROSCOPY?
 1 WHAT IS SPECTROSCOPY? 1.1 The Nature Of Electromagnetic Radiation Anyone who has been sunburnt will know that light packs a punch: in scientific terms, it contains considerable amounts of energy. All
1 WHAT IS SPECTROSCOPY? 1.1 The Nature Of Electromagnetic Radiation Anyone who has been sunburnt will know that light packs a punch: in scientific terms, it contains considerable amounts of energy. All
Design Considerations for a Variable Angle Absolute Reflectance Accessory For the LAMBDA 950/850/650 UV/Vis/NIR and UV/Vis Spectrophotometers
 Design Considerations for a Variable Angle Absolute Reflectance Accessory For the LAMBDA 950/850/650 UV/Vis/NIR and UV/Vis Spectrophotometers UV/VIS AND UV/VIS/NIR SPECTROSCOPY A P P L I C A T I O N N
Design Considerations for a Variable Angle Absolute Reflectance Accessory For the LAMBDA 950/850/650 UV/Vis/NIR and UV/Vis Spectrophotometers UV/VIS AND UV/VIS/NIR SPECTROSCOPY A P P L I C A T I O N N
= 6 (1/ nm) So what is probability of finding electron tunneled into a barrier 3 ev high?
 STM STM With a scanning tunneling microscope, images of surfaces with atomic resolution can be readily obtained. An STM uses quantum tunneling of electrons to map the density of electrons on the surface
STM STM With a scanning tunneling microscope, images of surfaces with atomic resolution can be readily obtained. An STM uses quantum tunneling of electrons to map the density of electrons on the surface
Supplementary Material (ESI) for Chemical Communications This journal is (c) The Royal Society of Chemistry 2009
 1. Materials: Styrene (St), methyl methacrylate (MMA) and acrylic acid (AA) (Lingfeng Chemical reagent Co. Ltd, Shanghai, China) were distilled and stored at 4 ºC if not used immediately, ammonium persulfate
1. Materials: Styrene (St), methyl methacrylate (MMA) and acrylic acid (AA) (Lingfeng Chemical reagent Co. Ltd, Shanghai, China) were distilled and stored at 4 ºC if not used immediately, ammonium persulfate
2001 Spectrometers. Instrument Machinery. Movies from this presentation can be access at
 2001 Spectrometers Instrument Machinery Movies from this presentation can be access at http://www.shsu.edu/~chm_tgc/sounds/sound.html Chp20: 1 Optical Instruments Instrument Components Components of various
2001 Spectrometers Instrument Machinery Movies from this presentation can be access at http://www.shsu.edu/~chm_tgc/sounds/sound.html Chp20: 1 Optical Instruments Instrument Components Components of various
Fourier Transform Infrared Spectroscopy (Perkin Elmer - Spectrum One)
 Fourier Transform Infrared Spectroscopy (Perkin Elmer - Spectrum One) This operating procedure intends to provide guidance for transmission/absorbance measurements with the FTIR. For additional modes of
Fourier Transform Infrared Spectroscopy (Perkin Elmer - Spectrum One) This operating procedure intends to provide guidance for transmission/absorbance measurements with the FTIR. For additional modes of
Experiment 2: THE DENSITY OF A SOLID UNKNOWN AND CALIBRATION WITH DATASTUDIO SOFTWARE
 Experiment 2: THE DENSITY OF A SOLID UNKNOWN AND CALIBRATION WITH DATASTUDIO SOFTWARE Concepts: Density Equipment Calibration Approximate time required: 90 minutes for density 90 minutes for two thermometers
Experiment 2: THE DENSITY OF A SOLID UNKNOWN AND CALIBRATION WITH DATASTUDIO SOFTWARE Concepts: Density Equipment Calibration Approximate time required: 90 minutes for density 90 minutes for two thermometers
Characterisation of vibrational modes of adsorbed species
 17.7.5 Characterisation of vibrational modes of adsorbed species Infrared spectroscopy (IR) See Ch.10. Infrared vibrational spectra originate in transitions between discrete vibrational energy levels of
17.7.5 Characterisation of vibrational modes of adsorbed species Infrared spectroscopy (IR) See Ch.10. Infrared vibrational spectra originate in transitions between discrete vibrational energy levels of
Qualitative analysis of aramide polymers by FT-IR spectroscopy
 International Journal of Engineering Science Invention ISSN (Online): 2319 6734, ISSN (Print): 2319 6726 Volume 3 Issue 2 ǁ February 2014 ǁ PP.01-07 Qualitative analysis of aramide polymers by FT-IR spectroscopy
International Journal of Engineering Science Invention ISSN (Online): 2319 6734, ISSN (Print): 2319 6726 Volume 3 Issue 2 ǁ February 2014 ǁ PP.01-07 Qualitative analysis of aramide polymers by FT-IR spectroscopy
Lab #13: Polarization
 Lab #13: Polarization Introduction In this experiment we will investigate various properties associated with polarized light. We will study both its generation and application. Real world applications
Lab #13: Polarization Introduction In this experiment we will investigate various properties associated with polarized light. We will study both its generation and application. Real world applications
Aziridine in Polymers: A Strategy to Functionalize Polymers by Ring- Opening Reaction of Aziridine
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) Aziridine in Polymers: A Strategy to Functionalize
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) Aziridine in Polymers: A Strategy to Functionalize
Agilent Cary 630 FTIR sample interfaces combining high performance and versatility
 Agilent Cary 630 FTIR sample interfaces combining high performance and versatility Technical Overview Introduction The Agilent Cary 630 is a robust, easy to use, superior performing FTIR in a compact package.
Agilent Cary 630 FTIR sample interfaces combining high performance and versatility Technical Overview Introduction The Agilent Cary 630 is a robust, easy to use, superior performing FTIR in a compact package.
Supporting Information s for
 Supporting Information s for # Self-assembling of DNA-templated Au Nanoparticles into Nanowires and their enhanced SERS and Catalytic Applications Subrata Kundu* and M. Jayachandran Electrochemical Materials
Supporting Information s for # Self-assembling of DNA-templated Au Nanoparticles into Nanowires and their enhanced SERS and Catalytic Applications Subrata Kundu* and M. Jayachandran Electrochemical Materials
Advanced Pharmaceutical Analysis
 Lecture 2 Advanced Pharmaceutical Analysis IR spectroscopy Dr. Baraa Ramzi Infrared Spectroscopy It is a powerful tool for identifying pure organic and inorganic compounds. Every molecular compound has
Lecture 2 Advanced Pharmaceutical Analysis IR spectroscopy Dr. Baraa Ramzi Infrared Spectroscopy It is a powerful tool for identifying pure organic and inorganic compounds. Every molecular compound has
CRYOGENIC CONDUCTION COOLING TEST OF REMOVABLE PANEL MOCK-UP FOR ITER CRYOSTAT THERMAL SHIELD
 CRYOGENIC CONDUCTION COOLING TEST OF REMOVABLE PANEL MOCK-UP FOR ITER CRYOSTAT THERMAL SHIELD K. Nam, a D. K. Kang, a W. Chung, a C. H. Noh, a J. Yu, b N. I. Her, b C. Hamlyn-Harris, b Y. Utin, b and K.
CRYOGENIC CONDUCTION COOLING TEST OF REMOVABLE PANEL MOCK-UP FOR ITER CRYOSTAT THERMAL SHIELD K. Nam, a D. K. Kang, a W. Chung, a C. H. Noh, a J. Yu, b N. I. Her, b C. Hamlyn-Harris, b Y. Utin, b and K.
Infra Red Spectroscopy
 CH 2252 Instrumental Methods of Analysis Unit I Infra Red Spectroscopy M. Subramanian Assistant Professor Department of Chemical Engineering Sri Sivasubramaniya Nadar College of Engineering Kalavakkam
CH 2252 Instrumental Methods of Analysis Unit I Infra Red Spectroscopy M. Subramanian Assistant Professor Department of Chemical Engineering Sri Sivasubramaniya Nadar College of Engineering Kalavakkam
Importance of in situ Monitoring in MOCVD Process and Future Prospects
 G u e s t F o r u m Guest Forum Series of Lectures by Screening Committees of the Second Masao Horiba Awards Importance of in situ Monitoring in MOCVD Process and Future Prospects Hiroshi Funakubo Tokyo
G u e s t F o r u m Guest Forum Series of Lectures by Screening Committees of the Second Masao Horiba Awards Importance of in situ Monitoring in MOCVD Process and Future Prospects Hiroshi Funakubo Tokyo
Fig. 8.1 illustrates the three measurements. air medium A. ray 1. air medium A. ray 2. air medium A. ray 3. Fig For Examiner s Use
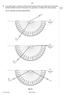 9 9 9 14 8 In an optics lesson, a Physics student traces the paths of three s of light near the boundary between medium A and. The student uses a protractor to measure the various angles. Fig. 8.1 illustrates
9 9 9 14 8 In an optics lesson, a Physics student traces the paths of three s of light near the boundary between medium A and. The student uses a protractor to measure the various angles. Fig. 8.1 illustrates
Using Calibrated Specular Reflectance Standards for Absolute and Relative Reflectance Measurements
 Using Calibrated Specular Reflectance Standards for Absolute and Relative Reflectance Measurements Applications Overview here are two fundamental techniques for measuring specular reflectance with a UV/VIS/NIR
Using Calibrated Specular Reflectance Standards for Absolute and Relative Reflectance Measurements Applications Overview here are two fundamental techniques for measuring specular reflectance with a UV/VIS/NIR
Chemistry 524--Final Exam--Keiderling May 4, :30 -?? pm SES
 Chemistry 524--Final Exam--Keiderling May 4, 2011 3:30 -?? pm -- 4286 SES Please answer all questions in the answer book provided. Calculators, rulers, pens and pencils are permitted. No open books or
Chemistry 524--Final Exam--Keiderling May 4, 2011 3:30 -?? pm -- 4286 SES Please answer all questions in the answer book provided. Calculators, rulers, pens and pencils are permitted. No open books or
SUPPORTING INFORMATION
 SUPPORTING INFORMATION Rapid, puncture-initiated healing via oxygen-mediated polymerization Scott R. Zavada, Nicholas R. McHardy, Keith L. Gordon, and Timothy F. Scott* Experimental Section Materials:
SUPPORTING INFORMATION Rapid, puncture-initiated healing via oxygen-mediated polymerization Scott R. Zavada, Nicholas R. McHardy, Keith L. Gordon, and Timothy F. Scott* Experimental Section Materials:
Atomic Spectra HISTORY AND THEORY
 Atomic Spectra HISTORY AND THEORY When atoms of a gas are excited (by high voltage, for instance) they will give off light. Each element (in fact, each isotope) gives off a characteristic atomic spectrum,
Atomic Spectra HISTORY AND THEORY When atoms of a gas are excited (by high voltage, for instance) they will give off light. Each element (in fact, each isotope) gives off a characteristic atomic spectrum,
LAB 10: OPTICAL MATERIALS AND DISPERSION I
 OPTI 202L - Geometrical and Instrumental Optics Lab LAB 10: OPTICAL MATERIALS AND DISPERSION I 10-1 Measuring the refractive index of a material is one of the most fundamental optical measurements, and
OPTI 202L - Geometrical and Instrumental Optics Lab LAB 10: OPTICAL MATERIALS AND DISPERSION I 10-1 Measuring the refractive index of a material is one of the most fundamental optical measurements, and
Ultraviolet-Visible and Infrared Spectrophotometry
 Ultraviolet-Visible and Infrared Spectrophotometry Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451
Ultraviolet-Visible and Infrared Spectrophotometry Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451
ABB temperature measurement Radiation thermometry. Measurement made easy. Process temperature measurement practice--non-contacting
 Whitepaper_ WP_T_Non-Contacting_Temperature_Measurement ABB temperature measurement Radiation thermometry Measurement made easy Process temperature measurement practice--non-contacting By Gary Freeman,
Whitepaper_ WP_T_Non-Contacting_Temperature_Measurement ABB temperature measurement Radiation thermometry Measurement made easy Process temperature measurement practice--non-contacting By Gary Freeman,
Surface plasmon resonance based refractive index sensor for liquids
 Indian Journal of Pure & Applied Physics Vol. 43, November 005, pp. 854-858 Surface plasmon resonance based refractive index sensor for liquids Navina Mehan, Vinay Gupta, K Sreenivas & Abhai Mansingh Department
Indian Journal of Pure & Applied Physics Vol. 43, November 005, pp. 854-858 Surface plasmon resonance based refractive index sensor for liquids Navina Mehan, Vinay Gupta, K Sreenivas & Abhai Mansingh Department
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/NCHEM.2633 Mechanically controlled radical polymerization initiated by ultrasound Hemakesh Mohapatra, Maya Kleiman, Aaron P. Esser-Kahn Contents 1. Materials and methods 2 2. Procedure for
DOI: 10.1038/NCHEM.2633 Mechanically controlled radical polymerization initiated by ultrasound Hemakesh Mohapatra, Maya Kleiman, Aaron P. Esser-Kahn Contents 1. Materials and methods 2 2. Procedure for
Design and Development of a Smartphone Based Visible Spectrophotometer for Analytical Applications
 Design and Development of a Smartphone Based Visible Spectrophotometer for Analytical Applications Bedanta Kr. Deka, D. Thakuria, H. Bora and S. Banerjee # Department of Physicis, B. Borooah College, Ulubari,
Design and Development of a Smartphone Based Visible Spectrophotometer for Analytical Applications Bedanta Kr. Deka, D. Thakuria, H. Bora and S. Banerjee # Department of Physicis, B. Borooah College, Ulubari,
The Claviature of Gas Analysis
 The Claviature of Gas Analysis Mass spectrometric gas analysis for quality assurance of gases and gas mixtures High purity and special gases are becoming increasingly important in the development of modern
The Claviature of Gas Analysis Mass spectrometric gas analysis for quality assurance of gases and gas mixtures High purity and special gases are becoming increasingly important in the development of modern
OPSE FINAL EXAM Fall 2016 YOU MUST SHOW YOUR WORK. ANSWERS THAT ARE NOT JUSTIFIED WILL BE GIVEN ZERO CREDIT.
 CLOSED BOOK. Equation Sheet is provided. YOU MUST SHOW YOUR WORK. ANSWERS THAT ARE NOT JUSTIFIED WILL BE GIVEN ZERO CREDIT. ALL NUMERICAL ANSERS MUST HAVE UNITS INDICATED. (Except dimensionless units like
CLOSED BOOK. Equation Sheet is provided. YOU MUST SHOW YOUR WORK. ANSWERS THAT ARE NOT JUSTIFIED WILL BE GIVEN ZERO CREDIT. ALL NUMERICAL ANSERS MUST HAVE UNITS INDICATED. (Except dimensionless units like
Sodium Chloride - Analytical Standard
 Sodium Chloride - Analytical Standard Determination of Total Mercury Former numbering: ECSS/CN 312-1982 & ESPA/CN-E-106-1994 1. SCOPE AND FIELD OF APPLICATION The present EuSalt Analytical Standard describes
Sodium Chloride - Analytical Standard Determination of Total Mercury Former numbering: ECSS/CN 312-1982 & ESPA/CN-E-106-1994 1. SCOPE AND FIELD OF APPLICATION The present EuSalt Analytical Standard describes
Chapter 4 Scintillation Detectors
 Med Phys 4RA3, 4RB3/6R03 Radioisotopes and Radiation Methodology 4-1 4.1. Basic principle of the scintillator Chapter 4 Scintillation Detectors Scintillator Light sensor Ionizing radiation Light (visible,
Med Phys 4RA3, 4RB3/6R03 Radioisotopes and Radiation Methodology 4-1 4.1. Basic principle of the scintillator Chapter 4 Scintillation Detectors Scintillator Light sensor Ionizing radiation Light (visible,
Experiment 3 1. The Michelson Interferometer and the He- Ne Laser Physics 2150 Experiment No. 3 University of Colorado
 Experiment 3 1 Introduction The Michelson Interferometer and the He- Ne Laser Physics 2150 Experiment No. 3 University of Colorado The Michelson interferometer is one example of an optical interferometer.
Experiment 3 1 Introduction The Michelson Interferometer and the He- Ne Laser Physics 2150 Experiment No. 3 University of Colorado The Michelson interferometer is one example of an optical interferometer.
Fourier Transform Infrared Spectrometry
 Fourier Transform Infrared Spectrometry \ ' PETER R. GRIFFITHS Department of Chemistry University of California Riverside, California JAMES A. de HASETH Department of Chemistry University of Georgia Athens,
Fourier Transform Infrared Spectrometry \ ' PETER R. GRIFFITHS Department of Chemistry University of California Riverside, California JAMES A. de HASETH Department of Chemistry University of Georgia Athens,
Vibrational Spectroscopies. C-874 University of Delaware
 Vibrational Spectroscopies C-874 University of Delaware Vibrational Spectroscopies..everything that living things do can be understood in terms of the jigglings and wigglings of atoms.. R. P. Feymann Vibrational
Vibrational Spectroscopies C-874 University of Delaware Vibrational Spectroscopies..everything that living things do can be understood in terms of the jigglings and wigglings of atoms.. R. P. Feymann Vibrational
(Refer Slide Time: 00:00:43 min) Welcome back in the last few lectures we discussed compression refrigeration systems.
 Refrigeration and Air Conditioning Prof. M. Ramgopal Department of Mechanical Engineering Indian Institute of Technology, Kharagpur Lecture No. # 14 Vapour Absorption Refrigeration Systems (Refer Slide
Refrigeration and Air Conditioning Prof. M. Ramgopal Department of Mechanical Engineering Indian Institute of Technology, Kharagpur Lecture No. # 14 Vapour Absorption Refrigeration Systems (Refer Slide
Water Hardness and Softening (Bring a water sample from home) Minneapolis Community and Technical College Principles of Chemistry II, C1152 v.2.
 Water Hardness and Softening (Bring a water sample from home) Minneapolis Community and Technical College Principles of Chemistry II, C1152 v.2.16 I. Introduction Hard Water and Water Softening Water that
Water Hardness and Softening (Bring a water sample from home) Minneapolis Community and Technical College Principles of Chemistry II, C1152 v.2.16 I. Introduction Hard Water and Water Softening Water that
Handheld FTIR spectroscopy applications using the Agilent ExoScan 4100 FTIR with diffuse sample interface
 Handheld FTIR spectroscopy applications using the Agilent ExoScan 4100 FTIR with diffuse sample interface Onsite, non-destructive analysis for geological, fabric, paint and plastic applications Application
Handheld FTIR spectroscopy applications using the Agilent ExoScan 4100 FTIR with diffuse sample interface Onsite, non-destructive analysis for geological, fabric, paint and plastic applications Application
Chapter 2 Controlling Residence Time
 Chapter 2 Controlling Residence Time Abstract For a batch reaction, the conditions within a reactor (the concentrations of materials and products) change with time. In contrast, for a flow reaction, the
Chapter 2 Controlling Residence Time Abstract For a batch reaction, the conditions within a reactor (the concentrations of materials and products) change with time. In contrast, for a flow reaction, the
HFM 100 Series. Thermal Conductivity Meter for measurement of insulation and construction materials.
 HFM 100 Series Conforms to International Standards ASTM C518, ISO 8301, and EN 12667 Thermal Conductivity Meter for measurement of insulation and construction materials. Hot Disk TPS -160 to 1000 C HFM
HFM 100 Series Conforms to International Standards ASTM C518, ISO 8301, and EN 12667 Thermal Conductivity Meter for measurement of insulation and construction materials. Hot Disk TPS -160 to 1000 C HFM
The design and operational theory of ph electrodes is a very complex subject, explored only briefly here. What is important to understand is that thes
 ph measurement A very important measurement in many liquid chemical processes (industrial, pharmaceutical, manufacturing, food production, etc.) is that of ph: the measurement of hydrogen ion concentration
ph measurement A very important measurement in many liquid chemical processes (industrial, pharmaceutical, manufacturing, food production, etc.) is that of ph: the measurement of hydrogen ion concentration
Engineering Physics 1 Prof. G.D. Vermaa Department of Physics Indian Institute of Technology-Roorkee
 Engineering Physics 1 Prof. G.D. Vermaa Department of Physics Indian Institute of Technology-Roorkee Module-04 Lecture-02 Diffraction Part - 02 In the previous lecture I discussed single slit and double
Engineering Physics 1 Prof. G.D. Vermaa Department of Physics Indian Institute of Technology-Roorkee Module-04 Lecture-02 Diffraction Part - 02 In the previous lecture I discussed single slit and double
Ultra-narrow-band tunable laserline notch filter
 Appl Phys B (2009) 95: 597 601 DOI 10.1007/s00340-009-3447-6 Ultra-narrow-band tunable laserline notch filter C. Moser F. Havermeyer Received: 5 December 2008 / Revised version: 2 February 2009 / Published
Appl Phys B (2009) 95: 597 601 DOI 10.1007/s00340-009-3447-6 Ultra-narrow-band tunable laserline notch filter C. Moser F. Havermeyer Received: 5 December 2008 / Revised version: 2 February 2009 / Published
Chapter 28 Atomic Physics
 Chapter 28 Atomic Physics GOALS After you have mastered the contents of this chapter, you will be able to achieve the following goals: Definitions Define each of the following terms and use it in an operational
Chapter 28 Atomic Physics GOALS After you have mastered the contents of this chapter, you will be able to achieve the following goals: Definitions Define each of the following terms and use it in an operational
Introduction. Molecules, Light and Natural Dyes. Experiment
 Experiment Molecules, Light and Natural Dyes 11 Introduction Chemistry of Color The production of dyes was the basis for the creation of modern chemical industry. During the mid-19 th century all dyes
Experiment Molecules, Light and Natural Dyes 11 Introduction Chemistry of Color The production of dyes was the basis for the creation of modern chemical industry. During the mid-19 th century all dyes
Instruction Manual. HI Refractometer for Sucrose in Wine and Grape Products Measurements
 Instruction Manual HI 96811 Refractometer for Sucrose in Wine and Grape Products Measurements TABLE OF CONTENTS PRELIMINARY EXAMINATION... 2 GENERAL DESCRIPTION... 3 SPECIFICATIONS... 3 PRINCIPLE OF OPERATION...
Instruction Manual HI 96811 Refractometer for Sucrose in Wine and Grape Products Measurements TABLE OF CONTENTS PRELIMINARY EXAMINATION... 2 GENERAL DESCRIPTION... 3 SPECIFICATIONS... 3 PRINCIPLE OF OPERATION...
Achieve a deeper understanding of polymeric systems
 The nanoscale spectroscopy company The world leader in nanoscale IR spectroscopy Achieve a deeper understanding of polymeric systems nanoir spectroscopy uniquely and unambiguously identifies the chemical
The nanoscale spectroscopy company The world leader in nanoscale IR spectroscopy Achieve a deeper understanding of polymeric systems nanoir spectroscopy uniquely and unambiguously identifies the chemical
Determination of Optical Constants of Polystyrene Films from IR Reflection-Absorption Spectra
 ANALELE UNIVERSITĂłII EFTIMIE MURGU REŞIłA ANUL XVIII, NR. 1, 2011, ISSN 1453-7397 Simion Jitian Determination of Optical Constants of Polystyrene Films from IR Reflection-Absorption Spectra Determination
ANALELE UNIVERSITĂłII EFTIMIE MURGU REŞIłA ANUL XVIII, NR. 1, 2011, ISSN 1453-7397 Simion Jitian Determination of Optical Constants of Polystyrene Films from IR Reflection-Absorption Spectra Determination
OPTICAL PROPERTIES OF THE DIRC FUSED SILICA CHERENKOV RADIATOR
 OPTICAL PROPERTIES OF THE DIRC FUSED SILICA CHERENKOV RADIATOR J. Cohen-Tanugi, M. Convery, B. Ratcliff, X. Sarazin, J. Schwiening, and J. Va'vra * Stanford Linear Accelerator Center, Stanford University,
OPTICAL PROPERTIES OF THE DIRC FUSED SILICA CHERENKOV RADIATOR J. Cohen-Tanugi, M. Convery, B. Ratcliff, X. Sarazin, J. Schwiening, and J. Va'vra * Stanford Linear Accelerator Center, Stanford University,
How to use GPC/SEC for compositional analysis
 How to use GPC/SEC for compositional analysis Determining the relative concentration of two components in a polymer sample MOLECULAR SIZE MOLECULAR STRUCTURE MOLECULAR WEIGHT Introduction Over the last
How to use GPC/SEC for compositional analysis Determining the relative concentration of two components in a polymer sample MOLECULAR SIZE MOLECULAR STRUCTURE MOLECULAR WEIGHT Introduction Over the last
ECEN 4606, UNDERGRADUATE OPTICS LAB
 ECEN 4606, UNDERGRADUATE OPTICS LAB Lab 6: Polarization Original: Professor McLeod SUMMARY: In this lab you will become familiar with the basics of polarization and learn to use common optical elements
ECEN 4606, UNDERGRADUATE OPTICS LAB Lab 6: Polarization Original: Professor McLeod SUMMARY: In this lab you will become familiar with the basics of polarization and learn to use common optical elements
DOWNLOAD OR READ : INFRARED AND RAMAN SPECTROSCOPY CONCEPTS AND APPLICATIONS PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : INFRARED AND RAMAN SPECTROSCOPY CONCEPTS AND APPLICATIONS PDF EBOOK EPUB MOBI Page 1 Page 2 infrared and raman spectroscopy concepts and applications infrared and raman spectroscopy
DOWNLOAD OR READ : INFRARED AND RAMAN SPECTROSCOPY CONCEPTS AND APPLICATIONS PDF EBOOK EPUB MOBI Page 1 Page 2 infrared and raman spectroscopy concepts and applications infrared and raman spectroscopy
CONFOCHECK. Innovation with Integrity. Infrared Protein Analysis FT-IR
 CONFOCHECK Infrared Protein Analysis Innovation with Integrity FT-IR CONFOCHECK: FT-IR System for Protein Analytics FT-IR Protein Analysis Infrared spectroscopy measures molecular vibrations due to the
CONFOCHECK Infrared Protein Analysis Innovation with Integrity FT-IR CONFOCHECK: FT-IR System for Protein Analytics FT-IR Protein Analysis Infrared spectroscopy measures molecular vibrations due to the
IR SPECTROSCOPY FOR BONDING SURFACE CONTAMINATION CHARACTERIZATION
 IR SPECTROSCOPY FOR BONDING SURFACE CONTAMINATION CHARACTERIZATION INTRODUCTION Lee H. Pearson Thiokol Corporation Advanced Technology Brigham City, Utah 8432-77 Organic contaminants such as hydrocarbons
IR SPECTROSCOPY FOR BONDING SURFACE CONTAMINATION CHARACTERIZATION INTRODUCTION Lee H. Pearson Thiokol Corporation Advanced Technology Brigham City, Utah 8432-77 Organic contaminants such as hydrocarbons
INTRODUCTION Strained Silicon Monochromator Magnesium Housing Windows for Monochromator Shutter and Collimator Fission Detector HOPG Monochromator
 Design for a Four-Blade Neutron Interferometer INTRODUCTION Strained Silicon Monochromator The neutron beam used for this interferometer is separated from the NIST reactor's main beam using a strained
Design for a Four-Blade Neutron Interferometer INTRODUCTION Strained Silicon Monochromator The neutron beam used for this interferometer is separated from the NIST reactor's main beam using a strained
ASSET INTEGRITY INTELLIGENCE. Featured Article. ACHIEVING A COMPREHENSIVE FIRED HEATER HEALTH MONITORING PROGRAM By Tim Hill, Quest Integrity Group
 ASSET INTEGRITY INTELLIGENCE Featured Article ACHIEVING A COMPREHENSIVE FIRED HEATER HEALTH MONITORING PROGRAM By Tim Hill, Quest Integrity Group VOLUME 20, ISSUE 5 SEPTEMBER OCTOBER 2014 ACHIEVING A COMPREHENSIVE
ASSET INTEGRITY INTELLIGENCE Featured Article ACHIEVING A COMPREHENSIVE FIRED HEATER HEALTH MONITORING PROGRAM By Tim Hill, Quest Integrity Group VOLUME 20, ISSUE 5 SEPTEMBER OCTOBER 2014 ACHIEVING A COMPREHENSIVE
