Supporting Information
|
|
|
- Hugo Harrington
- 5 years ago
- Views:
Transcription
1 Supporting Information Hydrolyzable Polyureas earing Hindered Urea onds Hanze Ying and Jianjun heng* epartment of Materials Science and ngineering, University of Illinois at Urbana-hampaign, 1304 West Green Street, Urbana, Illinois, 61801, USA *orresponding author: S1
2 Table of ontents General Materials Instrumentation eterminations of binding constants of hindered urea bonds etermination of binding constant of 2 Figure S1. 1 H NMR spectrum of the mixture of compound S1 and S2 Figure S2. 1 H NMR spectrum of the mixture of compound S1, S2, and S4 Figure S3. 1 H NMR spectrum of the mixture of compound S1, S4, and S6 etermination of binding constant of 3 Figure S4. 1 H NMR spectrum of the mixture of compound S7 and S2 Figure S5. 1 H NMR spectrum of the mixture of compound S7, S2, and S4 Figure S6. 1 H NMR spectrum of the mixture of compound S7, S4, and S6 etermination of binding constant of 4 Figure S7. 1 H NMR spectrum of the mixture of compound S10 and S6 Figure S8. 1 H NMR spectrum of the mixture of compound S10, S6, and S12 Figure S9. 1 H NMR spectrum of the mixture of compound S10, S12, and S14 etermination of binding constant of 5 Figure S10. 1 H NMR spectrum of the mixture of compound S1, S6, and S12 Figure S11. 1 H NMR spectrum of the mixture of compound S1, S12, and S14 eterminations of dissociation rates of hindered urea bonds Figure S12. issociation rate of urea 2 Figure S13. issociation rate of urea 3 Figure S14. issociation rate of urea 4 S4 S4 S4 S5 S6 S6 S8 S10 S12 S12 S13 S15 S17 S17 S19 S21 S23 S24 S25 S27 S28 S30 S32 S2
3 Figure S15. issociation rate of urea 5 eterminations of hydrolysis kinetics of hindered urea bonds Figure S16. hydrolysis of urea 1 Figure S17. hydrolysis of urea 2 Figure S18. hydrolysis of urea 3 Figure S19. hydrolysis of urea 4 Figure S20. hydrolysis of urea 5 Synthesis of hindered polyurea Figure S21. 1 H NMR and 13 NMR spectra of poly(6/9) Figure S22. 1 H NMR and 13 NMR spectra of poly(7/9) Figure S23. 1 H NMR and 13 NMR spectra of poly(8/10) Figure S24. 1 H NMR and 13 NMR spectra of poly(6/10) Figure S25. Thermo gravimetric analysis (TGA) of linear polymers. Figure S26. ifferential scanning calorimetry (S) of linear polymers. Water degradation of linear hindered polyurea Figure S27. egradation of poly(6/9) by water Figure S28. egradation of poly(7/9) by water Figure S29. egradation of poly(8/10) by water Figure S30. egradation of poly(6/10) by water egradation of cross-linked hindered polyurea by water egradation of hydrophobic cross-linked polyurea by water egradation of hydrophilic cross-linked polyurea by water S33 S35 S36 S37 S38 S39 S40 S41 S43 S44 S45 S46 S47 S48 S49 S50 S51 S52 S53 S54 S54 S54 S3
4 General Materials. Anhydrous dimethylformamide (MF) was dried by a column packed with 4Å molecular sieves. m-xylylene diisocyanate was purchased from TI America (Portland, OR, USA) and used as received. Tri-functional homopolymer of hexamethylene diisocyanate (HI) (esmodur N3900, ayer MaterialsScience) was obtained from Innovadex. All deuterated solvents were purchased from ambridge Isotope Laboratories, Inc. and used as received. All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used as received unless otherwise specified. Instrumentation. NMR spectra were recorded on Varian U400 (400 MHz), U500 (500 MHz), VXR-500 (500 MHz), UI500N (500 MHz) spectrometer. Gel permeation chromatography (GP) experiments were performed on a system uipped with an isocratic pump (Model 1100, Agilent Technology, Santa lara, A, USA), a AWN HLOS multi-angle laser light scattering detector (MALLS detector, Wyatt Technology, Santa arbara, A, USA) and an Optilab rx refractive index detector (Wyatt Technology, Santa arbara, A, USA). The detection wavelength of TROS was set at 658 nm. Separations were performed using serially connected size exclusion columns (10 2 Å, 10 3 Å, 10 4 Å, 10 5 Å and 10 6 Å Phenogel columns, 5 µm, mm, Phenomenex, Torrance, A, USA) using MF as the mobile phase. S4
5 etermination of binding constants of hindered urea bonds If the binding constant of urea is large, it is difficult to determine the binding constant K directly through the uilibrium concentrations of isocyanate, amine and urea species. The K of the hindered urea bond increases with the decrease of the substituents bulkiness. To accurately determine the binding constants, we used an indirect method through uilibrium reactions between different urea species. (All used l3 as the solvent) K1~K5 are mostly in the range that can all be accurately determined by 1 H NMR, we could determine K by: K K K K K K1 K2 K3 (when R3=t-u, R4=t) or K K (when R3=i-Pr, R4=t) S5
6 etermination of binding constant of urea 2 (etermination of binding constant of urea 1 with similar methods has been reported in ref. 19a in text) yclohexylmethyl isocyanante (S1, 11.1 mg, mmol) and 2,2,6,6-tetramethylpiperidine (S2, 8.0 mg, mmol) were dissolved in l3 (0.55 ml). 1 H NMR spectra were collected 0.5 h after S1 and S2 were mixed at room temperature when uilibrium was reached/peaks integral stopped changing (Figure S1). oncentration of each species was calculated based on the integral ratios of the 1 H NMR signals and the initial concentrations of S1 and S2. The uilibrium constants were calculated as K S3] / ([ S1] S ] ). 1 [ 2 A A A A Figure S1. 1 H NMR spectrum of the mixture of compound S1 and S2. Peaks are assigned to each compound except for the protons in cyclohexyl group. The spectrum was taken 30 min after S6
7 S1 and S2 were mixed. inding constant K1 was determined as K -1 [ S3] / ([ S1] S ] ) /( ) M 24 M S7
8 yclohexylmethyl isocyanante (S1, 10.0 mg, mmol), 2,2,6,6-tetramethylpiperidine (S2, 9.5 mg, mmol) and N-tertbutyl-N-isopropyl amine (S4, 6.8 mg, mmol) were dissolved in l3 (0.55 ml) and added to the NMR tubes. 1 H NMR spectra were collected 12 h after mixing at room temperature after uilibrium was reached/peaks integral stopped changing (Figure S2). The uilibrium constant of the reaction K2 was calculated according to the concentration ratio of each species: K S5] S2] /([ S3] S ] ). 2 ([ 4 A G F A G F G G A A F F F G A A F G Figure S2. 1 H NMR spectrum of the mixture of compound S1, S2, and S4 (and the produced compound S3, S5). Peaks are assigned to each compound except for the protons in S8
9 cyclohexyl group. The spectrum was taken 12 h after mixing. The region containing peaks,, F and F for the calculation of the concentration of each species is zoomed in. K S5] S2] /([ S3] S ] ) = ([ 4 S9
10 yclohexylmethyl isocyanante (S1, 10.2 mg, mmol), N-tertbutyl-N-isopropyl amine (S4, 7.2 mg, mmol) and N-tertbutyl-N-ethyl amine (S6, 6.7 mg, mmol) were dissolved in l3 (0.55 ml) and added to the NMR tubes. 1 H NMR spectra were collected 12 h after mixing at room temperature after uilibrium was reached/peaks integral stopped changing (Figure S3). The uilibrium constant of the reaction K2 was calculated according to the concentration ratio of each species: K 2] S4] /([ S5] S ] ). 3 ([ 6 A G H A G H F G F A A F H F G G A A G Figure S3. 1 H NMR spectrum of the mixture of compound S1, S4, and S6 (and the produced compound S5, 2). Peaks are assigned to each compound except for the protons in cyclohexyl group. The spectrum was taken 12 h after mixing. The region containing peaks,, S10
11 G and G for the calculation of the concentration of each species is zoomed in. K 2] S4] /([ S5] S ] ) = ([ 6 4 So, for urea 2, K K K 7. 1 M -1,2 1 2 K3 10 S11
12 etermination of binding constant of urea 3 enzyl isocyanante (S7, 11.1 mg, mmol) and 2,2,6,6-tetramethylpiperidine (S2, 9.4 mg, mmol) were dissolved in l3 (0.55 ml). 1 H NMR spectra were collected 0.5 h after S7 and S2 were mixed at room temperature when uilibrium was reached/peaks integral stopped changing (Figure S4). oncentration of each species was calculated based on the integral ratios of the 1 H NMR signals and the initial concentrations of S7 and S2. The uilibrium constants were calculated as K S8] / ([ S7] S ] ). 1 [ 2 A A F A A F Figure S4. 1 H NMR spectrum of the mixture of compound S7 and S2. Peaks are assigned to each compound. The spectrum was taken 30 min after S7 and S2 were mixed. inding constant -1 K1 was determined as K S8] / ([ S7] S ] ) /( ) M 92 M 1 [ 2-1 S12
13 enzyl isocyanante (S7, 11.9 mg, mmol), 2,2,6,6-tetramethylpiperidine (S2, 8.5 mg, mmol) and N-tertbutyl-N-isopropyl amine (S4, 6.0 mg, mmol) were dissolved in l3 (0.55 ml) and added to the NMR tubes. 1 H NMR spectra were collected 12 h after mixing at room temperature after uilibrium was reached/peaks integral stopped changing (Figure S5). The uilibrium constant of the reaction K2 was calculated according to the concentration ratio of each species: K S9] S2] /([ S8] S ] ). 2 ([ 4 A F G I H A F I H G G G H A A F H I I I F F H H G I S13
14 Figure S5. 1 H NMR spectrum of the mixture of compound S7, S2, and S4 (and the produced compound S8, S9). Peaks are assigned to each compound. The spectrum was taken 12 h after mixing. The region containing peaks, H, H and for the calculation of the concentration of each species is zoomed in. K S9] S2] /([ S8] S ] ) = ([ 4 S14
15 enzyl isocyanante (S7, 10.7 mg, mmol), N-tertbutyl-N-isopropyl amine (S4, 7.5 mg, mmol) and N-tertbutyl-N-ethyl amine (S6, 7.9 mg, mmol) were dissolved in l3 (0.55 ml) and added to the NMR tubes. 1 H NMR spectra were collected 12 h after mixing at room temperature after uilibrium was reached/peaks integral stopped changing (Figure S6). The uilibrium constant of the reaction K3 was calculated according to the concentration ratio of each species: K 3] S4] /([ S9] S ] ). 3 ([ 6 A F G H I A F G H I G H H A A F F H H I I Figure S6. 1 H NMR spectrum of the mixture of compound S7, S4, and S6 (and the produced compound S9, 3). Peaks are assigned to each compound. The spectrum was taken 12 S15
16 h after mixing. The region containing peaks,, H and H for the calculation of the concentration of each species is zoomed in. K 3] S4] /([ S9] S ] ) = ([ 6 5 So, for urea 3, K K K 5. 5 M -1,3 1 2 K3 10 S16
17 etermination of binding constant of urea 4 3-Isopropenyl-α,α-dimethybenzyl isocyanate (S10, 14.0 mg, mmol) and N-tertbutyl-Nethyl amine (S6, 7.8 mg, mmol) were dissolved in l3 (0.55 ml). 1 H NMR spectra were collected 0.5 h after S10 and S6 were mixed at room temperature when uilibrium was reached/peaks integral stopped changing (Figure S7). oncentration of each species was calculated based on the integral ratios of the 1 H NMR signals and the initial concentrations of S10 and S6. The uilibrium constants were calculated as K S11] / ([ S10] [ S ] ). 1* 2* 3 [ 6 A Ar-H G A Ar-H H F G Ar-H A F A H F G G Figure S7. 1 H NMR spectrum of the mixture of compound S10 and S6. Peaks are assigned to each compound. The spectrum was taken 0.5 h after S10 and S6 were mixed. inding constant S17
18 K1*2*3 was determined as K S11] / ([ S10] [ S ] ) 1* 2* 3 [ / ( ) M M -1 S18
19 3-Isopropenyl-α,α-dimethybenzyl isocyanate (S10, 14.1 mg, mmol), N-tertbutyl-N-ethyl amine (S6, 14.3 mg, mmol) and N-tertbutyl-N-methyl amine (S12, 8.9 mg, mmol) were dissolved in l3 (0.55 ml) and added to the NMR tubes. 1 H NMR spectra were collected 15 d after mixing at room temperature after uilibrium was reached/peaks integral stopped changing (Figure S8). The uilibrium constant of the reaction K4 was calculated according to the concentration ratio of each species: K 4 S13] S6] /([ S11] S12] ). ([ A J Ar-H G H I F A Ar-H I J F G H F J G J G I I H Ar-H A A G J J G F H S19
20 Figure S8. 1 H NMR spectrum of the mixture of compound S10, S6, and S12 (and the produced compound S11, S13). Peaks are assigned to each compound. The spectrum was taken 15 d after mixing. The region containing peaks G, G, J and J for the calculation of the concentration of each species is zoomed in. K 4 S13] S6] /([ S11] S12] ) = 6.8. ([ S20
21 3-Isopropenyl-α,α-dimethybenzyl isocyanate (S10, 10.1 mg, mmol), N-tertbutyl-N-methyl amine (S12, 8.0 mg, mmol) and N-isopropyl-N-ethyl amine (S14, 8.3 mg, mmol) were dissolved in l3 (0.55 ml) and added to the NMR tubes. 1 H NMR spectra were collected 15 d after mixing at room temperature after uilibrium was reached/peaks integral stopped changing (Figure S9). The uilibrium constant of the reaction K5 was calculated according to the concentration ratio of each species: K 5 4] S12] /([ S13] S14] ). ([ A Ar-H F G H I J K A Ar-H H I J K F G F G H Ar-H K H A J I I J K S21
22 Figure S9. 1 H NMR spectrum of the mixture of compound S10, S12, and S14 (and the produced compound S13, 4). Peaks are assigned to each compound. The spectrum was taken 15 d after mixing. Here, no peaks for S13 were observed. y integrating area of 2.83 ppm ~ 2.89 ppm where peak G for S13 should be, we have K 5 4] S12] /([ S13] S14] ) > 840. ([ 6 So, for urea 4, K K K 1. 0 M -1,4 1* 2* 3 4 K5 10 S22
23 etermination of binding constant of urea 5 4 We already have the binding constant for 2: K K K 7. 1 M -1,2 1 2 K3 10 yclohexylmethyl isocyanante (S1, 14.1 mg, mmol), N-tertbutyl-N-ethyl amine (S6, 11.3 mg, mmol) and N-tertbutyl-N-methyl amine (S12, 8.1 mg, mmol) were dissolved in l3 (0.55 ml) and added to the NMR tubes. 1 H NMR spectra were collected 15 d after mixing at room temperature after uilibrium was reached/peaks integral stopped changing (Figure S10). The uilibrium constant of the reaction K4 was calculated according to the concentration ratio of each species: K 4 S15] S6] /([ 2] S12] ). ([ S23
24 A G A G F F G F G G F A A G Figure S10. 1 H NMR spectrum of the mixture of compound S1, S6, and S12 (and the produced compound 2, S15). Peaks are assigned to each compound except for the protons in cyclohexyl group. The spectrum was taken 15 d after mixing. The region containing peaks,, G and G for the calculation of the concentration of each species is zoomed in. K 4 S15] S6] /([ 2] S12] ) = 5.7. ([ S24
25 yclohexylmethyl isocyanante (S1, 9.4 mg, mmol), N-tertbutyl-N-methyl amine (S12, 7.2 mg, mmol) and N-isopropyl-N-ethyl amine (S14, 8.1 mg, mmol) were dissolved in l3 (0.55 ml) and added to the NMR tubes. 1 H NMR spectra were collected 41 d after mixing at room temperature after uilibrium was reached/peaks integral stopped changing (Figure S11). The uilibrium constant of the reaction K5 was calculated according to the concentration ratio of each species: K 5 5] S12] /([ S15] S14] ). ([ A G H A G H F F F F G A F G H H Figure S11. 1 H NMR spectrum of the mixture of compound S1, S12, and S14 (and the produced compound S15, 5). Peaks are assigned to each compound except for the protons in cyclohexyl group. The spectrum was taken 41 d after mixing. Here, no peaks for S15 were S25
26 observed except for a very weak peak as shown in zoomed in picture. K 5 5] S12] /([ S15] S14] ) > ([ 8 So, for urea 5, K K K 1. 0 M -1,5,2 4 K5 10 S26
27 eterminations of dissociation rates of hindered urea bonds (etermination of dissociation rate of urea 1 with similar methods has been reported in ref. 18 in text) The dissociation kinetics (k-1) of hindered urea compounds 2~5 were determined (Figure S12-15, respectively). To urea compounds were added butyl isocyanate to capture the released free amine. The rates of consumption of urea compounds were monitored by 1 H NMR at 37 o and dissociation rates were calculated based on those data. (All used l3 as the solvent) S27
28 i) A F H G I A F H G I H H F F I I A A G G ii) ackground noise 2 0 min S17 30 min Figure S12 issociation rate of urea 2. i) 1 H NMR spectrum of the mixture of compound 2 (6.2 mg, mmol) and S16 (10.1 mg, mmol, and the produced compound S1 and S17) in l3 (550 μl). The spectrum was taken 30 min after 2 and S16 were mixed at 37 o. Peaks are assigned to each compound except for the protons in cyclohexyl group. ii) 1 H NMR spectra showing exchange reaction between 2 and S16 at 37 o. The rate of consumption of 2 was used to S28
29 calculate the dissociation rate with the following uation: [ 2] ln [ 2] 0 ln 0.91 k -1 = 0.19 h -1 T 0.5 h (T: reaction time, peak A used for the calculation overlapped with background noise in the same range, which has been deducted in the calculation) S29
30 i) A F A I G J H J I G H F J J A A F F G H H I I G ii) S min 30 min Figure S13. issociation rate of urea 3. i) 1 H NMR spectrum of the mixture of compound 3 (7.4 mg, mmol) and S16 (11.9 mg, mmol, and the produced compound S7 and S17) in l3 (550 μl). The spectrum was taken 30 min after 3 and S16 were mixed at 37 o. Peaks are assigned to each compound. ii) 1 H NMR spectra showing exchange reaction between 3 and S30
31 S16 at 37 o. The rate of consumption of 3 was used to calculate the dissociation rate with the following uation: k [ 3] ln [ 3] 0 T -1 ln 0.91 = 0.19 h -1 (T: reaction time) 0.5 h S31
32 i) A Ar-H F H K M A I Ar-H K M J L J L G F G H I F F M K K L L I I J J Ar-H A A G G H H M ii) 0 h S10 4 S h Figure S14. issociation rate of urea 4. i) 1 H NMR spectrum of the mixture of compound 4 (8.3 mg, mmol) and S16 (9.6 mg, mmol, and the produced compound S10 and S17) in l3 (550 μl). The spectrum was taken 16 h after 4 and S16 were mixed at 37 o. Peaks are assigned to each compound. ii) 1 H NMR spectra showing exchange reaction between 4 and S16 at 37 o. The rate of consumption of 4 was used to calculate the dissociation rate with the following uation: [ 4] ln [ 4] 0 ln 0.88 k -1 = h -1 (T: reaction time) T 16 h S32
33 i) A F H G I J A H J F G I J A A I I F F H H G G J ii) S16 S17 5+S17 16 h 250 h Figure S15. issociation rate of urea 5. i) 1 H NMR spectrum of the mixture of compound 5 (6.4 mg, mmol) and S16 (8.3 mg, mmol, and the produced compound S1 and S17) in S33
34 l3 (550 μl). The spectrum was taken 250 h after 5 and S16 were mixed at 37 o. Peaks are assigned to each compound except for the protons in cyclohexyl group. ii) 1 H NMR spectra showing exchange reaction between 5 and S16 at 37 o. The rate of consumption of 5 was used to calculate the dissociation rate with the following uation: [ 5] ln [5] 0 ln 0.79 k -1 = h -1 T 250 h (T: reaction time) S34
35 etermination of hydrolysis kinetics of hindered urea bonds We compared the hydrolysis kinetics of urea 1~5 by comparing the percentage of hydrolysis after 24 h at 37 o environment. Urea 1~5 were dissolved in mixture of d6-mso and 2O (v(d6-mso):v(2o)=5:1) with concentration of 0.1 M. After incubation at 37 o for 24 h, 1 H NMR spectra were collected to characterize the percentage of hydrolysis (through the ratio of produced amine and original urea). S35
36 A F G H A F G H 1 S6 G G F F A A G G H H Figure S16. Hydrolysis of urea 1. Peaks are assigned to each compound. The spectrum was taken 24 h after dissolving in mixture of d6-mso and 2O (v(d6-mso):v(2o)=5:1) at 37 o. The region containing peaks G, G for the calculation of the percentage of hydrolysis is zoomed in. The percentage of hydrolysis was determined as 58%. S36
37 A A 2 S6 A A Figure S17. Hydrolysis of urea 2. Peaks are assigned to each compound except for the protons in cyclohexyl group. The spectrum was taken 24 h after dissolving in mixture of d6-mso and 2O (v(d6-mso):v(2o)=5:1) at 37 o. The region containing peaks, for the calculation of the percentage of hydrolysis is zoomed in. The percentage of hydrolysis was determined as 85%. S37
38 A A 3 S6 A A Figure S18. Hydrolysis of urea 3. Peaks are assigned to each compound. The spectrum was taken 24 h after dissolving in mixture of d6-mso and 2O (v(d6-mso):v(2o)=5:1) at 37 o. The region containing peaks, for the calculation of the percentage of hydrolysis is zoomed in. The percentage of hydrolysis was determined as 55%. S38
39 A Ar-H G H A Ar-H G H F F F 4 S14 F H H Ar-H A A F G F Figure S19. Hydrolysis of urea 4. Peaks are assigned to each compound. The spectrum was taken 24 h after dissolving in mixture of d6-mso and 2O (v(d6-mso):v(2o)=5:1) at 37 o. The region containing peaks F, F for the calculation of the percentage of hydrolysis is zoomed in. The percentage of hydrolysis was determined as 10%. S39
40 A A Figure S20. Hydrolysis of urea 5. Peaks are assigned to each compound except for the protons in cyclohexyl group. The spectrum was taken 24 h after dissolving in mixture of d6-mso and 2O (v(d6-mso):v(2o)=5:1) at 37 o. No detectable hydrolysis was observed. S40
41 Synthesis of hindered polyurea Synthesis of polymer (poly(6/9)): qual molar 1,3-bis(isocyanatomethyl)cyclohexane (6, 1.94 g, 10.0 mmol) and N,N'-di-tert-butylethylene-diamine (9, 1.72 g, 10.0 mmol) were dissolved in MF (10 g). The mixture was stirred at room temperature overnight vigorously. The polymer solution was directly used for GP characterization and degradation study. qual molar of 6 (0.194 g, 1.0 mmol) and 9 (0.172 g, 1.0 mmol) were mixed in l3 (1.0g). The solution was stirred at room temperature overnight vigorously, diluted by a factor of 10, and directly characterized by NMR without purification. Polymer solution in MF was precipitated by ether (100 ml 3). The white solid was collected by centrifuge and dried by vacuum oven overnight (yield: 90%, Mn = 22K, PI = 1.55). The obtained solid was used for TGA and S characterizations. Synthesis of polymer (poly(7/9)): qual molar m-xylylene diisocyanate (7, 1.88 g, 10.0 mmol) and N,N'-di-tert-butylethylene-diamine (9, 1.72 g, 10.0 mmol) were dissolved in MF (10 g). The mixture was stirred at room temperature overnight vigorously. The polymer solution was directly used for GP characterization and degradation study. qual molar of 7 (0.188 g, 1.0 mmol) and 9 (0.172 g, 1.0 mmol) were mixed in l3 (1.0g). The solution was stirred at room temperature overnight vigorously, diluted by a factor of 10, and directly characterized by NMR without purification. Polymer solution in MF was precipitated by ether (100 ml 3). The white solid was collected by centrifuge and dried by vacuum oven overnight (yield: 92%, Mn = 22K, PI = 1.33). The obtained solid was used for TGA and S characterizations. S41
42 Synthesis of polymer (poly(8/10)): qual molar 1,3-is(1-isocyanato-1-methylethyl)benzene (8, 2.44 g, 10.0 mmol) and N,N'-di-iso-propylethylene-diamine (10, 1.44 g, 10.0 mmol) were dissolved in MF (10 g). The mixture was stirred at room temperature overnight vigorously. The polymer solution was directly used for GP characterization and degradation study. qual molar of 8 (0.244 g, 1.0 mmol) and 10 (0.144 g, 1.0 mmol) were mixed in l3 (1.0g). The solution was stirred at room temperature overnight vigorously, diluted by a factor of 10, and directly characterized by NMR without purification. Polymer solution in MF was precipitated by ether (100 ml 3). The white solid was collected by centrifuge and dried by vacuum oven overnight (yield: 87%, Mn = 44K, PI = 1.45). The obtained solid was used for TGA and S characterizations. Synthesis of polymer (poly(6/10)): qual molar 1,3-bis(isocyanatomethyl)cyclohexane (6, 1.94 g, 10.0 mmol) and N,N'-di-iso-propylethylene-diamine (10, 1.44 g, 10.0 mmol) were dissolved in MF (10 g). The mixture was stirred at room temperature overnight vigorously. The polymer solution was directly used for GP characterization and degradation study. qual molar of 6 (0.194 g, 1.0 mmol) and 10 (0.144 g, 1.0 mmol) were mixed in l3 (1.0g). The solution was stirred at room temperature overnight vigorously, diluted by a factor of 10, and directly characterized by NMR without purification. Polymer solution in MF was precipitated by ether (100 ml 3). The white solid was collected by centrifuge and dried by vacuum oven overnight (yield: 95%, Mn = 120K, PI = 1.72). The obtained solid was used for TGA and S characterizations. S42
43 A A Figure S21. 1 H NMR and 13 NMR spectra of poly(6/9) S43
44 A A Figure S22. 1 H NMR and 13 NMR spectra of poly(7/9) S44
45 A A Figure S23. 1 H NMR and 13 NMR spectra of poly(8/10) S45
46 A A Figure S24. 1 H NMR and 13 NMR spectra of poly(6/10) S46
47 Weight ratio (%) poly(6/9) poly(7/9) poly(8/10) poly(6/10) Temperature ( o ) Figure S25. Thermo gravimetric analysis (TGA) of linear polymers. S47
48 1.0 Heat flow (mw/mg) poly(6/9) poly(7/9) poly(8/10) poly(6/10) Temperature ( o ) Figure S26. ifferential scanning calorimetry (S) of linear polymers. Second heating curve from -50 o to 175 o were shown. S48
49 Water degradation of linear hindered polyurea To 500 µl as prepared MF solution of each polymer (see page S38-39), 25 µl water was added. The mixture was incubated at 37 o with vigorous stirring. Samples were taken out at different time intervals for monitoring of molecular weight change by GP. S49
50 0 h 12 h 24 h48 h72 h 96 h 0 h 12 h 24 h 48 h 72 h 96 h lution Time (min) Figure S27. Water degradation of poly(6/9). GP curves from light scattering detector showing water degradation of poly(6/9) after incubation in MF (containing 5% water) at 37 o for variant time intervals. S50
51 0 h 12 h 48 h 24 h 72 h 96 h 0 h 12 h 24 h 48 h 72 h 96 h lution Time (min) Figure S28. Water degradation of poly(7/9). GP curves from light scattering detector showing water degradation of poly(7/9) after incubation in MF (containing 5% water) at 37 o for variant time intervals. S51
52 0 h h 0 h 12 h 24 h 48 h 72 h 96 h lution Time (min) Figure S29. Water degradation of poly(8/10). GP curves from light scattering detector showing water degradation of poly(8/10) after incubation in MF (containing 5% water) at 37 o for variant time intervals. S52
53 0 h h 0 h 12 h 24 h 48 h 72 h 96 h lution Time (min) Figure S30. Water degradation of poly(6/10). GP curves from light scattering detector showing water degradation of poly(6/10) after incubation in MF (containing 5% water) at 37 o for variant time intervals. S53
54 Water degradation of cross-linked hindered polyurea Water degradation of hydrophobic cross-linked hindered polyurea. Tri-functional homopolymer of hexamethylene diisocyanate (11, 100 mg, mmol) was dissolved in MF (650 µl). A solution of N,N'-di-tert-butylethylene-diamine (9, 51.4 mg, mmol) in MF (205 µl) and water (50 µl) was added. The mixture was homogenized for 5 s and let sit for 1 min at room temperature for gelation to happen. After that, the gel was incubated at 37 o for degradation study. Water degradation of hydrophilic cross-linked hindered polyurea. Poly(ethylene glycol) methyl ether methacrylate (12, 4.13 g, 8.26 mmol), 2-Isocyanatoethyl methacrylate (S23, 128 mg, mmol), N,N'-di-tert-butylethylene-diamine (9, 71.2 mg, mmol) and 2-Hydroxy-4 -(2- hydroxyethoxy)-2-methylpropiophenone (S24, 40 mg, dissolved in 40 µl MSO) were mixed and irradiated by UV (365 nm, 40 mw/cm 2 ) for 15 min to yield cross-linked polymer G1. G1 was divided and transferred into 15 ml centrifuge tubes with each one containing 300 mg polymer. Polymer was first immersed in deionized water for 12 h at 37 o to remove all the unreacted monomers, solvent and photo initiator. After that the tubes were filled with PS and incubated at 37 o to start water degradation study. At different time point, samples were taken out and washed with deionized water for 3 times and weighed after drying by lyophilization. egree of weight loss was used to characterize the degradation kinetics. The experiments were repeated in triplicate. As negative control, N,N'-di-iso-propylethylene-diamine (10, 59.5 mg, mmol) instead of N,N'-di-tert-butylethylene-diamine were used to synthesize cross-linked polymer G2. Water degradations of G2 were characterized with the same procedures. S54
Supporting Information
 Electronic Supplementary Material (ESI) for Biomaterials Science. This journal is The Royal Society of Chemistry 2017 Supporting Information Degradable and Biocompatible Hydrogels Bearing Hindered Urea
Electronic Supplementary Material (ESI) for Biomaterials Science. This journal is The Royal Society of Chemistry 2017 Supporting Information Degradable and Biocompatible Hydrogels Bearing Hindered Urea
F G C G H H C B B A A DD E E
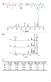 a D E F G H D E I F G H F F I DD E E G H H b c [1] 0 (M) [2a] 0 (M) [1] (M) [2a] (M) [3a] (M) K (M -1 ) a) 0.160 0.139 0.049 0.028 0.111 81 b) 0.147 0.180 0.025 0.058 0.122 84 c) 0.157 0.270 0.013 0.126
a D E F G H D E I F G H F F I DD E E G H H b c [1] 0 (M) [2a] 0 (M) [1] (M) [2a] (M) [3a] (M) K (M -1 ) a) 0.160 0.139 0.049 0.028 0.111 81 b) 0.147 0.180 0.025 0.058 0.122 84 c) 0.157 0.270 0.013 0.126
Supporting Information
 Supporting Information Controlled Ring-Opening Polymerization of O-Carboxyanhydrides Using a b-diiminate Zinc Catalyst Ruibo Wang, Jiawei Zhang, Qian Yin, Yunxiang Xu, Jianjun Cheng,* and Rong Tong* anie_201605508_sm_miscellaneous_information.pdf
Supporting Information Controlled Ring-Opening Polymerization of O-Carboxyanhydrides Using a b-diiminate Zinc Catalyst Ruibo Wang, Jiawei Zhang, Qian Yin, Yunxiang Xu, Jianjun Cheng,* and Rong Tong* anie_201605508_sm_miscellaneous_information.pdf
Supporting information
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Supporting information From competition to cooperation: a highly efficient strategy towards well-defined
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Supporting information From competition to cooperation: a highly efficient strategy towards well-defined
Ring-Opening Polymerization of N-Carboxyanhydrides Initiated by a Hydroxyl Group
 SUPPRTING INFRMATIN Ring-pening Polymerization of N-Carboxyanhydrides Initiated by a Hydroxyl Group Špela Gradišar, Ema Žagar, and David Pahovnik* National Institute of Chemistry, Department of Polymer
SUPPRTING INFRMATIN Ring-pening Polymerization of N-Carboxyanhydrides Initiated by a Hydroxyl Group Špela Gradišar, Ema Žagar, and David Pahovnik* National Institute of Chemistry, Department of Polymer
Supporting Information. One-Pot Synthesis of Brush-Like Polymers via Integrated Ring-Opening Metathesis
 Supporting Information One-Pot Synthesis of Brush-Like Polymers via Integrated Ring-Opening Metathesis Polymerization and Polymerization of Amino Acid N-Carboxyanhydrides Hua Lu, Jing Wang, Yao Lin, *
Supporting Information One-Pot Synthesis of Brush-Like Polymers via Integrated Ring-Opening Metathesis Polymerization and Polymerization of Amino Acid N-Carboxyanhydrides Hua Lu, Jing Wang, Yao Lin, *
Supporting Information
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supporting Information Synthesis of Poly(dihydroxystyrene-block-styrene) (PDHSt-b-PSt) by the RAFT
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supporting Information Synthesis of Poly(dihydroxystyrene-block-styrene) (PDHSt-b-PSt) by the RAFT
One-pot polymer brush synthesis via simultaneous isocyanate coupling chemistry and grafting from RAFT polymerization
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2014 One-pot polymer brush synthesis via simultaneous isocyanate coupling chemistry and grafting
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2014 One-pot polymer brush synthesis via simultaneous isocyanate coupling chemistry and grafting
SUPPORTING INFORMATION
 SUPPORTING INFORMATION Cis-Selective Ring-Opening Metathesis Polymerization with Ruthenium Catalysts Benjamin K. Keitz, Alexey Fedorov, Robert H. Grubbs* Arnold and Mabel Beckman Laboratories of Chemical
SUPPORTING INFORMATION Cis-Selective Ring-Opening Metathesis Polymerization with Ruthenium Catalysts Benjamin K. Keitz, Alexey Fedorov, Robert H. Grubbs* Arnold and Mabel Beckman Laboratories of Chemical
Supporting Information
 Supporting Information Azo Polymer Janus Particles and Their Photoinduced Symmetry-Breaking Deformation Xinran Zhou, Yi Du, Xiaogong Wang* Department of Chemical Engineering, Laboratory of Advanced Materials
Supporting Information Azo Polymer Janus Particles and Their Photoinduced Symmetry-Breaking Deformation Xinran Zhou, Yi Du, Xiaogong Wang* Department of Chemical Engineering, Laboratory of Advanced Materials
Aziridine in Polymers: A Strategy to Functionalize Polymers by Ring- Opening Reaction of Aziridine
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) Aziridine in Polymers: A Strategy to Functionalize
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) Aziridine in Polymers: A Strategy to Functionalize
Hyperbranched Poly(N-(2-Hydroxypropyl) Methacrylamide) via RAFT Self- Condensing Vinyl Polymerization
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2016 Hyperbranched Poly(N-(2-Hydroxypropyl) Methacrylamide) via RAFT Self- Condensing Vinyl
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2016 Hyperbranched Poly(N-(2-Hydroxypropyl) Methacrylamide) via RAFT Self- Condensing Vinyl
Chemically recyclable alternating copolymers with low polydispersity from
 Electronic Supplementary Information Chemically recyclable alternating copolymers with low polydispersity from conjugated/aromatic aldehydes with vinyl ethers: selective degradation to another monomer
Electronic Supplementary Information Chemically recyclable alternating copolymers with low polydispersity from conjugated/aromatic aldehydes with vinyl ethers: selective degradation to another monomer
Supporting information
 Supporting information Temperature and ph-dual Responsive AIE-Active Core Crosslinked Polyethylene Poly(methacrylic acid) Multimiktoarm Star Copolymers ` Zhen Zhang,*,, and Nikos Hadjichristidis*, School
Supporting information Temperature and ph-dual Responsive AIE-Active Core Crosslinked Polyethylene Poly(methacrylic acid) Multimiktoarm Star Copolymers ` Zhen Zhang,*,, and Nikos Hadjichristidis*, School
Scheme 1: Reaction scheme for the synthesis of p(an-co-mma) copolymer
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Design and Development of Poly (acrylonitrile-co-methyl methacrylate) Copolymer to Improve
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Design and Development of Poly (acrylonitrile-co-methyl methacrylate) Copolymer to Improve
Supporting Information. Reduction- and Thermo-Sensitive Star Polypeptide Micelles. and Hydrogels for On-Demand Drug Delivery
 Supporting Information Reduction- and Thermo-Sensitive Star Polypeptide Micelles and ydrogels for n-demand Drug Delivery Dong-Lin Liu, Xiao Chang, Chang-Ming Dong* Department of Polymer Science & Engineering,
Supporting Information Reduction- and Thermo-Sensitive Star Polypeptide Micelles and ydrogels for n-demand Drug Delivery Dong-Lin Liu, Xiao Chang, Chang-Ming Dong* Department of Polymer Science & Engineering,
Supporting Information
 Supporting Information Controlled Radical Polymerization and Quantification of Solid State Electrical Conductivities of Macromolecules Bearing Pendant Stable Radical Groups Lizbeth Rostro, Aditya G. Baradwaj,
Supporting Information Controlled Radical Polymerization and Quantification of Solid State Electrical Conductivities of Macromolecules Bearing Pendant Stable Radical Groups Lizbeth Rostro, Aditya G. Baradwaj,
SUPPLEMENTARY INFORMATION
 Methods: Poly(zwitterionic)protein conjugates offer increased stability without sacrificing binding affinity or bioactivity Andrew J. Keefe and Shaoyi Jiang Materials: α-chymotrypsin from bovine pancreas
Methods: Poly(zwitterionic)protein conjugates offer increased stability without sacrificing binding affinity or bioactivity Andrew J. Keefe and Shaoyi Jiang Materials: α-chymotrypsin from bovine pancreas
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/NCHEM.2633 Mechanically controlled radical polymerization initiated by ultrasound Hemakesh Mohapatra, Maya Kleiman, Aaron P. Esser-Kahn Contents 1. Materials and methods 2 2. Procedure for
DOI: 10.1038/NCHEM.2633 Mechanically controlled radical polymerization initiated by ultrasound Hemakesh Mohapatra, Maya Kleiman, Aaron P. Esser-Kahn Contents 1. Materials and methods 2 2. Procedure for
Supporting information
 Supporting information Imidazolium end-functionalized poly(l-lactide) for Efficient Carbon Nanotube Dispersion. Franck Meyer, a Jean-Marie Raquez, a Olivier Coulembier, a Julien De Winter, b Pascal Gerbaux,
Supporting information Imidazolium end-functionalized poly(l-lactide) for Efficient Carbon Nanotube Dispersion. Franck Meyer, a Jean-Marie Raquez, a Olivier Coulembier, a Julien De Winter, b Pascal Gerbaux,
Cationic Alkylaluminum-Complexed Zirconocene Hydrides as Participants in Olefin-Polymerization Catalysis. Supporting Information
 Cationic Alkylaluminum-Complexed Zirconocene Hydrides as Participants in Olefin-Polymerization Catalysis Steven M. Baldwin, John E. Bercaw, *, and Hans H. Brintzinger*, Contribution from the Arnold and
Cationic Alkylaluminum-Complexed Zirconocene Hydrides as Participants in Olefin-Polymerization Catalysis Steven M. Baldwin, John E. Bercaw, *, and Hans H. Brintzinger*, Contribution from the Arnold and
Optimizing Ion Transport in Polyether-based Electrolytes for Lithium Batteries
 Supporting Information Optimizing Ion Transport in Polyether-based Electrolytes for Lithium Batteries Qi Zheng, 1 Danielle M. Pesko, 1 Brett M. Savoie, Ksenia Timachova, Alexandra L. Hasan, Mackensie C.
Supporting Information Optimizing Ion Transport in Polyether-based Electrolytes for Lithium Batteries Qi Zheng, 1 Danielle M. Pesko, 1 Brett M. Savoie, Ksenia Timachova, Alexandra L. Hasan, Mackensie C.
Supporting Information
 Supporting Information One Pot Synthesis of 1,3- Bis(phosphinomethyl)arene PCP/PNP Pincer Ligands and Their Nickel Complexes Wei-Chun Shih and Oleg V. Ozerov* Department of Chemistry, Texas A&M University,
Supporting Information One Pot Synthesis of 1,3- Bis(phosphinomethyl)arene PCP/PNP Pincer Ligands and Their Nickel Complexes Wei-Chun Shih and Oleg V. Ozerov* Department of Chemistry, Texas A&M University,
Self-Healing Polymers with PEG Oligomer Side Chains. Based on Multiple H-Bonding and Adhesion Properties
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2015 Supporting Information Self-Healing Polymers with PEG Oligomer Side Chains Based on Multiple
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2015 Supporting Information Self-Healing Polymers with PEG Oligomer Side Chains Based on Multiple
1,1,3,3-Tetramethylguanidine-Promoted Ring-Opening Polymerization of N-Butyl N-Carboxyanhydride Using Alcohol Initiators
 Supporting Information 1,1,3,3-Tetramethylguanidine-Promoted Ring-Opening Polymerization of N-Butyl N-Carboxyanhydride Using Alcohol Initiators Brandon A. Chan, Sunting Xuan, Matthew Horton, and Donghui
Supporting Information 1,1,3,3-Tetramethylguanidine-Promoted Ring-Opening Polymerization of N-Butyl N-Carboxyanhydride Using Alcohol Initiators Brandon A. Chan, Sunting Xuan, Matthew Horton, and Donghui
Supporting Information
 Supporting Information UCST or LCST? Composition-Dependent Thermoresponsive Behavior of Poly(N-Acryloylglycinamide-co-Diacetone Acrylamide) Wenhui Sun, Zesheng An*, Peiyi Wu * Experimental Materials Glycinamide
Supporting Information UCST or LCST? Composition-Dependent Thermoresponsive Behavior of Poly(N-Acryloylglycinamide-co-Diacetone Acrylamide) Wenhui Sun, Zesheng An*, Peiyi Wu * Experimental Materials Glycinamide
Magnetic Iron Oxide Nanoparticles as Long Wavelength Photoinitiators for Free Radical Polymerization
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2015 SUPPORTING INFORMATION Magnetic Iron Oxide Nanoparticles as Long Wavelength Photoinitiators
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2015 SUPPORTING INFORMATION Magnetic Iron Oxide Nanoparticles as Long Wavelength Photoinitiators
Facile Polymerization of Water and Triple-bond
 Supporting Information Facile Polymerization of Water and Triple-bond ased Monomers towards Functional Polyamides Jie Zhang, Wenjie Wang, Yong Liu, Jing Zhi Sun, Anjun Qin,,, and en Zhong Tang,,,, MOE
Supporting Information Facile Polymerization of Water and Triple-bond ased Monomers towards Functional Polyamides Jie Zhang, Wenjie Wang, Yong Liu, Jing Zhi Sun, Anjun Qin,,, and en Zhong Tang,,,, MOE
Supporting Information
 Supporting Information Nonhemolytic and Antibacterial Amphiphilic Acrylic Copolymers with Hexamethyleneamine and Poly(ethylene glycol) Side Chains Ashish Punia, Andrew Mancuso, Probal Banerjee, and Nan-Loh
Supporting Information Nonhemolytic and Antibacterial Amphiphilic Acrylic Copolymers with Hexamethyleneamine and Poly(ethylene glycol) Side Chains Ashish Punia, Andrew Mancuso, Probal Banerjee, and Nan-Loh
Supporting Information
 Supporting Information Facile polyisobutylene functionalization via thiol-ene Click chemistry Andrew J. D. Magenau, Justin W. Chan, Charles E. Hoyle, and Robson F. Storey School of Polymers and High Performance
Supporting Information Facile polyisobutylene functionalization via thiol-ene Click chemistry Andrew J. D. Magenau, Justin W. Chan, Charles E. Hoyle, and Robson F. Storey School of Polymers and High Performance
Supporting Information
 Supporting Information Solid Polymer Electrolytes Based on Functionalized Tannic Acids from Natural Resources for All-Solid-State Lithium- Ion Batteries Jimin Shim, [a] Ki Yoon Bae, [b] Hee Joong Kim,
Supporting Information Solid Polymer Electrolytes Based on Functionalized Tannic Acids from Natural Resources for All-Solid-State Lithium- Ion Batteries Jimin Shim, [a] Ki Yoon Bae, [b] Hee Joong Kim,
Supporting Information
 Supporting Information for A Cucurbit[8]uril Sponge Vijayakumar Ramalingam, Sharon K. Kwee, Lisa M. Ryno, and Adam R. Urbach* Department of Chemistry, Trinity University, San Antonio, TX, 78212 * to whom
Supporting Information for A Cucurbit[8]uril Sponge Vijayakumar Ramalingam, Sharon K. Kwee, Lisa M. Ryno, and Adam R. Urbach* Department of Chemistry, Trinity University, San Antonio, TX, 78212 * to whom
A novel smart polymer responsive to CO 2
 A novel smart polymer responsive to CO 2 Zanru Guo, a,b Yujun Feng,* a Yu Wang, a Jiyu Wang, a,b Yufeng Wu, a,b and Yongmin Zhang a,b a Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences,
A novel smart polymer responsive to CO 2 Zanru Guo, a,b Yujun Feng,* a Yu Wang, a Jiyu Wang, a,b Yufeng Wu, a,b and Yongmin Zhang a,b a Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences,
Photo-Cleavage of Cobalt-Carbon Bond: Visible. Light-Induced Living Radical Polymerization Mediated by. Organo-Cobalt Porphyrins
 Photo-Cleavage of Cobalt-Carbon Bond: Visible Light-Induced Living Radical Polymerization Mediated by Organo-Cobalt Porphyrins Yaguang Zhao, Mengmeng Yu, and Xuefeng Fu* Beijing National Laboratory for
Photo-Cleavage of Cobalt-Carbon Bond: Visible Light-Induced Living Radical Polymerization Mediated by Organo-Cobalt Porphyrins Yaguang Zhao, Mengmeng Yu, and Xuefeng Fu* Beijing National Laboratory for
Supplementary Information. Rational Design of Soluble and Clickable Polymers Prepared by. Conventional Free Radical Polymerization of
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2017 Supplementary Information Rational Design of Soluble and Clickable Polymers Prepared by
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2017 Supplementary Information Rational Design of Soluble and Clickable Polymers Prepared by
Supporting Information
 Supporting Information Molecular Weight Dependence of Zero-Shear Viscosity in Atactic Polypropylene Bottlebrush Polymers Samuel J. Dalsin, Marc A. Hillmyer,*, and Frank S. Bates*, Department of Chemical
Supporting Information Molecular Weight Dependence of Zero-Shear Viscosity in Atactic Polypropylene Bottlebrush Polymers Samuel J. Dalsin, Marc A. Hillmyer,*, and Frank S. Bates*, Department of Chemical
SUPPORTING INFORMATION
 A Novel Copper Containing Photoinitiator, Copper (II) Acylphosphinate, and Its Application in Both the Photomediated CuAAC Reaction and in Atom Transfer Radical Polymerization Tao Gong, Brian J. Adzima
A Novel Copper Containing Photoinitiator, Copper (II) Acylphosphinate, and Its Application in Both the Photomediated CuAAC Reaction and in Atom Transfer Radical Polymerization Tao Gong, Brian J. Adzima
Supporting Information
 Supporting Information A Rational Design of Highly Controlled Suzuki-Miyaura Catalyst-Transfer Polycondensation for Precision Synthesis of Polythiophenes and their Block Copolymers: Marriage of Palladacycle
Supporting Information A Rational Design of Highly Controlled Suzuki-Miyaura Catalyst-Transfer Polycondensation for Precision Synthesis of Polythiophenes and their Block Copolymers: Marriage of Palladacycle
Supporting Information
 Supporting Information Kamaly et al. 10.1073/pnas.1303377110 SI Materials and Methods Materials. Ac2-26 peptide (AMVSEFLKQAWFIENEEQEYV- QTVK) was purchased from Tocris Biosciences. Zymosan A was purchased
Supporting Information Kamaly et al. 10.1073/pnas.1303377110 SI Materials and Methods Materials. Ac2-26 peptide (AMVSEFLKQAWFIENEEQEYV- QTVK) was purchased from Tocris Biosciences. Zymosan A was purchased
Supporting Information
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2014 Supporting Information A synthetic strategy for the preparation of sub-100nm functional
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2014 Supporting Information A synthetic strategy for the preparation of sub-100nm functional
Supporting Information. Sequence-Regulated Copolymers via Tandem Catalysis of Living Radical Polymerization and In Situ Transesterification
 Supporting Information Sequence-Regulated Copolymers via Tandem Catalysis of Living Radical Polymerization and In Situ Transesterification Kazuhiro Nakatani, Yusuke Ogura, Yuta Koda, Takaya Terashima*,
Supporting Information Sequence-Regulated Copolymers via Tandem Catalysis of Living Radical Polymerization and In Situ Transesterification Kazuhiro Nakatani, Yusuke Ogura, Yuta Koda, Takaya Terashima*,
Supporting Information
 Supporting Information Efficient Temperature Sensing Platform Based on Fluorescent Block Copolymer Functionalized Graphene Oxide Hyunseung Yang, Kwanyeol Paek, and Bumjoon J. Kim * : These authors contributed
Supporting Information Efficient Temperature Sensing Platform Based on Fluorescent Block Copolymer Functionalized Graphene Oxide Hyunseung Yang, Kwanyeol Paek, and Bumjoon J. Kim * : These authors contributed
Reduction-free synthesis of stable acetylide cobalamins. Table of Contents. General information. Preparation of compound 1
 Electronic Supporting Information Reduction-free synthesis of stable acetylide cobalamins Mikołaj Chromiński, a Agnieszka Lewalska a and Dorota Gryko* a Table of Contents General information Numbering
Electronic Supporting Information Reduction-free synthesis of stable acetylide cobalamins Mikołaj Chromiński, a Agnieszka Lewalska a and Dorota Gryko* a Table of Contents General information Numbering
Convenient Synthesis of Nucleoside 5 -Triphosphates for RNA Transcription. Supplemental Materials
 Supplementary Material (ESI) for Chemical Communications This journal is The Royal Society of Chemistry 2010 Convenient Synthesis of ucleoside 5 -Triphosphates for RA Transcription Julianne Caton-Williams,
Supplementary Material (ESI) for Chemical Communications This journal is The Royal Society of Chemistry 2010 Convenient Synthesis of ucleoside 5 -Triphosphates for RA Transcription Julianne Caton-Williams,
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. Photonic sensing via SRS method. Reflection spectra of a) a dried SiO 2 opal and b-d) the SiO 2 opal infiltrated with different organic solvents, whose refractive
Supplementary Figures Supplementary Figure 1. Photonic sensing via SRS method. Reflection spectra of a) a dried SiO 2 opal and b-d) the SiO 2 opal infiltrated with different organic solvents, whose refractive
Well-defined Click-able Copolymers in One-Pot Synthesis
 Electronic Supplementary Material (ESI) for hemomm. This journal is The Royal Society of hemistry 2014 Well-defined lick-able opolymers in ne-pot Synthesis egar Ghasdian, Mark A. Ward and Theoni K. Georgiou*
Electronic Supplementary Material (ESI) for hemomm. This journal is The Royal Society of hemistry 2014 Well-defined lick-able opolymers in ne-pot Synthesis egar Ghasdian, Mark A. Ward and Theoni K. Georgiou*
Supporting Information
 Supporting Information Syntheses and characterizations: Compound 1 was synthesized according to Scheme S-1. Scheme S-1 2 N N 5 i N 4 P Et Et iii N 6 ii P Et Et iv v, vi N N i) Fmoc-Su, DIPEA, Acetone;
Supporting Information Syntheses and characterizations: Compound 1 was synthesized according to Scheme S-1. Scheme S-1 2 N N 5 i N 4 P Et Et iii N 6 ii P Et Et iv v, vi N N i) Fmoc-Su, DIPEA, Acetone;
Silver-catalyzed decarboxylative acylfluorination of styrenes in aqueous media
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supporting Information Silver-catalyzed decarboxylative acylfluorination of styrenes in aqueous
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supporting Information Silver-catalyzed decarboxylative acylfluorination of styrenes in aqueous
Sequence-Defined Polymers via Orthogonal Allyl Acrylamide Building Blocks
 Sequence-Defined Polymers via Orthogonal Allyl Acrylamide Building Blocks Mintu Porel and Christopher A. Alabi* School of Chemical and Biomolecular Engineering, Cornell University, Ithaca, New York 14853,
Sequence-Defined Polymers via Orthogonal Allyl Acrylamide Building Blocks Mintu Porel and Christopher A. Alabi* School of Chemical and Biomolecular Engineering, Cornell University, Ithaca, New York 14853,
ELECTRONIC SUPPORTING INFORMATION Pentablock star shaped polymers in less than 90 minutes via
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2015 ELECTRONIC SUPPORTING INFORMATION Pentablock star shaped polymers in less than 90 minutes
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2015 ELECTRONIC SUPPORTING INFORMATION Pentablock star shaped polymers in less than 90 minutes
Supplementary Information
 Facile Preparation of Fluorovinylene Aryl Ether Telechelic Polymers with Dual Functionality for Thermal Chain Extension and Tandem Crosslinking Scott T. Iacono, Stephen M. Budy, Dirk Ewald, and Dennis
Facile Preparation of Fluorovinylene Aryl Ether Telechelic Polymers with Dual Functionality for Thermal Chain Extension and Tandem Crosslinking Scott T. Iacono, Stephen M. Budy, Dirk Ewald, and Dennis
Dual Use of a Chemical Auxiliary: Molecularly Imprinted Polymers for the Selective Recovery of Products from Biocatalytic Reaction Mixtures
 SUPPORTING INFORMATION Dual Use of a Chemical Auxiliary: Molecularly Imprinted Polymers for the Selective Recovery of Products from Biocatalytic Reaction Mixtures Aaron T. Larsen, Tiffany Lai, Vanja Polic,
SUPPORTING INFORMATION Dual Use of a Chemical Auxiliary: Molecularly Imprinted Polymers for the Selective Recovery of Products from Biocatalytic Reaction Mixtures Aaron T. Larsen, Tiffany Lai, Vanja Polic,
RAFT /MADIX polymerization of N-vinylcaprolactam in water-ethanol solvent mixtures
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2017 Supporting information for RAFT /MADIX polymerization of N-vinylcaprolactam in water-ethanol
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2017 Supporting information for RAFT /MADIX polymerization of N-vinylcaprolactam in water-ethanol
Supplementary Figure 1. Temperature profile of self-seeding method for polymer single crystal preparation in dilute solution.
 Supplementary Figure 1. Temperature profile of self-seeding method for polymer single crystal preparation in dilute solution. Supplementary Figure 2. 1 H nuclear magnetic resonance (NMR) spectra (a) and
Supplementary Figure 1. Temperature profile of self-seeding method for polymer single crystal preparation in dilute solution. Supplementary Figure 2. 1 H nuclear magnetic resonance (NMR) spectra (a) and
Supporting Information
 Supporting Information Branched polyethylene mimicry by metathesis copolymerization of fatty acid-based α,ω-dienes. Thomas Lebarbé, a,b,d Mehdi Neqal, a,b Etienne Grau, a,b Carine Alfos, c and Henri Cramail
Supporting Information Branched polyethylene mimicry by metathesis copolymerization of fatty acid-based α,ω-dienes. Thomas Lebarbé, a,b,d Mehdi Neqal, a,b Etienne Grau, a,b Carine Alfos, c and Henri Cramail
Stoichiometric Reductions of Alkyl-Substituted Ketones and Aldehydes to Borinic Esters Lauren E. Longobardi, Connie Tang, and Douglas W.
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2014 Supplementary Data for: Stoichiometric Reductions of Alkyl-Substituted Ketones and Aldehydes
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2014 Supplementary Data for: Stoichiometric Reductions of Alkyl-Substituted Ketones and Aldehydes
Supplementary Material (ESI) for Chemical Communications This journal is (c) The Royal Society of Chemistry 2008
 Supplementary Information for: Scrambling Reaction between Polymers Prepared by Step-growth and Chain-growth Polymerizations: Macromolecular Cross-metathesis between 1,4-Polybutadiene and Olefin-containing
Supplementary Information for: Scrambling Reaction between Polymers Prepared by Step-growth and Chain-growth Polymerizations: Macromolecular Cross-metathesis between 1,4-Polybutadiene and Olefin-containing
Supporting Material. 2-Oxo-tetrahydro-1,8-naphthyridine-Based Protein Farnesyltransferase Inhibitors as Antimalarials
 Supporting Material 2-Oxo-tetrahydro-1,8-naphthyridine-Based Protein Farnesyltransferase Inhibitors as Antimalarials Srinivas Olepu a, Praveen Kumar Suryadevara a, Kasey Rivas b, Christophe L. M. J. Verlinde
Supporting Material 2-Oxo-tetrahydro-1,8-naphthyridine-Based Protein Farnesyltransferase Inhibitors as Antimalarials Srinivas Olepu a, Praveen Kumar Suryadevara a, Kasey Rivas b, Christophe L. M. J. Verlinde
Supplementary Figure 1. Structures of substrates tested with 1. Only one enantiomer is shown.
 Supplementary Figure 1. Structures of substrates tested with 1. Only one enantiomer is shown. Supplementary Figure 2. CD spectra obtained using 1 and (R)-3 (blue) and (S)-3 (red) Supplementary Figure 3.
Supplementary Figure 1. Structures of substrates tested with 1. Only one enantiomer is shown. Supplementary Figure 2. CD spectra obtained using 1 and (R)-3 (blue) and (S)-3 (red) Supplementary Figure 3.
*Correspondence to:
 Supporting Information for Carbonate-promoted hydrogenation of carbon dioxide to multi-carbon carboxylates Aanindeeta Banerjee 1 and Matthew W. Kanan 1 * 1 Department of Chemistry, Stanford University,
Supporting Information for Carbonate-promoted hydrogenation of carbon dioxide to multi-carbon carboxylates Aanindeeta Banerjee 1 and Matthew W. Kanan 1 * 1 Department of Chemistry, Stanford University,
O-Allylation of phenols with allylic acetates in aqueous medium using a magnetically separable catalytic system
 Supporting information for -Allylation of phenols with allylic acetates in aqueous medium using a magnetically separable catalytic system Amit Saha, John Leazer* and Rajender S. Varma* Sustainable Technology
Supporting information for -Allylation of phenols with allylic acetates in aqueous medium using a magnetically separable catalytic system Amit Saha, John Leazer* and Rajender S. Varma* Sustainable Technology
Supporting Information
 Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2012 Subcellular Localization and Activity of Gambogic Acid Gianni Guizzunti,* [b] Ayse Batova, [a] Oraphin Chantarasriwong,
Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2012 Subcellular Localization and Activity of Gambogic Acid Gianni Guizzunti,* [b] Ayse Batova, [a] Oraphin Chantarasriwong,
Supporting Information
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 214 Supporting Information Rapid and sensitive detection of acrylic acid using a novel fluorescence
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 214 Supporting Information Rapid and sensitive detection of acrylic acid using a novel fluorescence
High Frequency sonoatrp of 2-Hydroxyethyl Acrylate in an Aqueous Medium
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2018 High Frequency sonoatrp of 2-Hydroxyethyl Acrylate in an Aqueous Medium Joe Collins, Thomas
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2018 High Frequency sonoatrp of 2-Hydroxyethyl Acrylate in an Aqueous Medium Joe Collins, Thomas
Tunable thermo-responsive water-dispersed multi walled. carbon nanotubes
 Tunable thermo-responsive water-dispersed multi walled carbon nanotubes Gaojian Chen, Peter M. Wright, Jin Geng, Giuseppe Mantovani, and David M. Haddleton* Department of Chemistry, University of Warwick,
Tunable thermo-responsive water-dispersed multi walled carbon nanotubes Gaojian Chen, Peter M. Wright, Jin Geng, Giuseppe Mantovani, and David M. Haddleton* Department of Chemistry, University of Warwick,
Photocontrolled RAFT Polymerization Mediated by a
 Supporting Information Photocontrolled RAFT Polymerization Mediated by a Supramolecular Catalyst Liangliang Shen, Qunzan Lu, Anqi Zhu, Xiaoqing Lv, and Zesheng An* Institute of Nanochemistry and Nanobiology,
Supporting Information Photocontrolled RAFT Polymerization Mediated by a Supramolecular Catalyst Liangliang Shen, Qunzan Lu, Anqi Zhu, Xiaoqing Lv, and Zesheng An* Institute of Nanochemistry and Nanobiology,
Photolabile Protecting Groups: A Strategy for Making
 Electronic Supplementary aterial (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 217 Supporting Information: Photolabile Protecting Groups: A Strategy for aking Primary Amine
Electronic Supplementary aterial (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 217 Supporting Information: Photolabile Protecting Groups: A Strategy for aking Primary Amine
Supporting information. Cooperatively Enhanced Ion Pair Binding with a Hybrid Receptor
 Supporting information Cooperatively Enhanced Ion Pair Binding with a Hybrid Receptor Toni Mäkelä, a Elina Kalenius a and Kari Rissanen a* a University of Jyvaskyla, Department of Chemistry, Nanoscience
Supporting information Cooperatively Enhanced Ion Pair Binding with a Hybrid Receptor Toni Mäkelä, a Elina Kalenius a and Kari Rissanen a* a University of Jyvaskyla, Department of Chemistry, Nanoscience
Supporting Information for Polybenzimidazolium Salts: A New Class of. Anion-Conducting Polymer
 Supporting Information for Polybenzimidazolium Salts: A ew Class of Anion-Conducting Polymer Owen D. Thomas, Kristen J. W. Y. Soo, Timothy J. Peckham, Mahesh P. Kulkarni and Steven Holdcroft* Department
Supporting Information for Polybenzimidazolium Salts: A ew Class of Anion-Conducting Polymer Owen D. Thomas, Kristen J. W. Y. Soo, Timothy J. Peckham, Mahesh P. Kulkarni and Steven Holdcroft* Department
SUPPLEMENTARY INFORMATION
 Synthetic chemistry ML5 and ML4 were identified as K P.(TREK-) activators using a combination of fluorescence-based thallium flux and automated patch-clamp assays. ML5, ML4, and ML5a were synthesized using
Synthetic chemistry ML5 and ML4 were identified as K P.(TREK-) activators using a combination of fluorescence-based thallium flux and automated patch-clamp assays. ML5, ML4, and ML5a were synthesized using
CHAPTER 3. FABRICATION TECHNOLOGIES OF CdSe/ZnS / Au NANOPARTICLES AND NANODEVICES. 3.1 THE SYNTHESIS OF Citrate-Capped Au NANOPARTICLES
 CHAPTER 3 FABRICATION TECHNOLOGIES OF CdSe/ZnS / Au NANOPARTICLES AND NANODEVICES 3.1 THE SYNTHESIS OF Citrate-Capped Au NANOPARTICLES Au NPs with ~ 15 nm were prepared by citrate reduction of HAuCl 4
CHAPTER 3 FABRICATION TECHNOLOGIES OF CdSe/ZnS / Au NANOPARTICLES AND NANODEVICES 3.1 THE SYNTHESIS OF Citrate-Capped Au NANOPARTICLES Au NPs with ~ 15 nm were prepared by citrate reduction of HAuCl 4
Tuning Porosity and Activity of Microporous Polymer Network Organocatalysts by Co-Polymerisation
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supporting Information Tuning Porosity and Activity of Microporous Polymer Network Organocatalysts
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supporting Information Tuning Porosity and Activity of Microporous Polymer Network Organocatalysts
Supporting Information
 Electronic Supplementary Material (ESI) for CrystEngComm. This journal is The Royal Society of Chemistry 2015 A rare case of a dye co-crystal showing better dyeing performance Hui-Fen Qian, Yin-Ge Wang,
Electronic Supplementary Material (ESI) for CrystEngComm. This journal is The Royal Society of Chemistry 2015 A rare case of a dye co-crystal showing better dyeing performance Hui-Fen Qian, Yin-Ge Wang,
applied as UV protective films
 Nanocomposite gels via in-situ photoinitiation and disassembly of TiO 2 -Clay composites with polymers applied as UV protective films Chuanan Liao, Qing Wu, Teng Su, Da Zhang, Qingsheng Wu and Qigang Wang*
Nanocomposite gels via in-situ photoinitiation and disassembly of TiO 2 -Clay composites with polymers applied as UV protective films Chuanan Liao, Qing Wu, Teng Su, Da Zhang, Qingsheng Wu and Qigang Wang*
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES Supplementary Figure 1. Gel permeation chromatograms of a cleavable diblock copolymer before and after UV irradiation. Multi-peak-fitting analysis was performed to estimate the percentage
SUPPLEMENTARY FIGURES Supplementary Figure 1. Gel permeation chromatograms of a cleavable diblock copolymer before and after UV irradiation. Multi-peak-fitting analysis was performed to estimate the percentage
Supplementary Material (ESI) for Chemical Communication
 Supplementary Material (ESI) for Chemical Communication Syntheses and Characterization of Polymer-Supported Organotrifluoroborates: Applications in Radioiodination Reactions Li Yong; Min-Liang Yao; James
Supplementary Material (ESI) for Chemical Communication Syntheses and Characterization of Polymer-Supported Organotrifluoroborates: Applications in Radioiodination Reactions Li Yong; Min-Liang Yao; James
Supporting information
 Supporting information ontrollable and stable deformation of a self-healing photo-responsive supramolecular assembly for an optically actuated manipulator arm Qianyu Si, a Yiyu Feng, a,c,d * Weixiang Yang,
Supporting information ontrollable and stable deformation of a self-healing photo-responsive supramolecular assembly for an optically actuated manipulator arm Qianyu Si, a Yiyu Feng, a,c,d * Weixiang Yang,
Supplementary Material for
 www.sciencemag.org/content/343/6173/873/suppl/dc1 Supplementary Material for Nonswellable Hydrogel Without Mechanical Hysteresis Hiroyuki Kamata, Yuki Akagi, Yuko Kayasuga-Kariya, Ung-il Chung, Takamasa
www.sciencemag.org/content/343/6173/873/suppl/dc1 Supplementary Material for Nonswellable Hydrogel Without Mechanical Hysteresis Hiroyuki Kamata, Yuki Akagi, Yuko Kayasuga-Kariya, Ung-il Chung, Takamasa
Bulk ring-opening transesterification polymerization of the renewable δ-decalactone using
 Bulk ring-opening transesterification polymerization of the renewable δ-decalactone using an organocatalyst Mark T. Martello, Adam Burns, and Marc Hillmyer* *Department of Chemistry, University of Minnesota,
Bulk ring-opening transesterification polymerization of the renewable δ-decalactone using an organocatalyst Mark T. Martello, Adam Burns, and Marc Hillmyer* *Department of Chemistry, University of Minnesota,
Supporting Information for
 Supporting Information for Visible-Light Induced Thiol Ene Reaction on Natural Lignin Hailing Liu and Hoyong Chung * Department of Chemical and Biomedical Engineering, Florida State University, Tallahassee,
Supporting Information for Visible-Light Induced Thiol Ene Reaction on Natural Lignin Hailing Liu and Hoyong Chung * Department of Chemical and Biomedical Engineering, Florida State University, Tallahassee,
Helix Formation of Poly(phenylacetylene)s Bearing Azide Groups through Click Polymer Reaction with Optically Active Acetylenes
 Supporting Information Helix Formation of Poly(phenylacetylene)s earing Azide Groups through Click Polymer Reaction with Optically Active Acetylenes Ken Itomi, Shinzo Kobayashi, Kazuhide Morino, Hiroki
Supporting Information Helix Formation of Poly(phenylacetylene)s earing Azide Groups through Click Polymer Reaction with Optically Active Acetylenes Ken Itomi, Shinzo Kobayashi, Kazuhide Morino, Hiroki
High-Performance Semiconducting Polythiophenes for Organic Thin Film. Transistors by Beng S. Ong,* Yiliang Wu, Ping Liu and Sandra Gardner
 Supplementary Materials for: High-Performance Semiconducting Polythiophenes for Organic Thin Film Transistors by Beng S. Ong,* Yiliang Wu, Ping Liu and Sandra Gardner 1. Materials and Instruments. All
Supplementary Materials for: High-Performance Semiconducting Polythiophenes for Organic Thin Film Transistors by Beng S. Ong,* Yiliang Wu, Ping Liu and Sandra Gardner 1. Materials and Instruments. All
Synthesis of Peptide-Grafted Comb Polypeptides via Polymerisation of NCA-Peptides
 Supporting Information to Synthesis of Peptide-Grafted Comb Polypeptides via Polymerisation of NCA-Peptides Hiroshi Enomoto, Benjamin Nottelet, Soultan Al Halifa, Christine Enjalbal, Mathieu Dupré, Julien
Supporting Information to Synthesis of Peptide-Grafted Comb Polypeptides via Polymerisation of NCA-Peptides Hiroshi Enomoto, Benjamin Nottelet, Soultan Al Halifa, Christine Enjalbal, Mathieu Dupré, Julien
Supporting Information for: Tuning the Binding Properties of a New Heteroditopic Salt Receptor Through Embedding in a Polymeric System
 Supporting Information for: Tuning the Binding Properties of a ew Heteroditopic Salt Receptor Through Embedding in a Polymeric System Jan Romanski* and Piotr Piątek* Department of Chemistry, University
Supporting Information for: Tuning the Binding Properties of a ew Heteroditopic Salt Receptor Through Embedding in a Polymeric System Jan Romanski* and Piotr Piątek* Department of Chemistry, University
Selective Reduction of Carboxylic acids to Aldehydes Catalyzed by B(C 6 F 5 ) 3
 S1 Selective Reduction of Carboxylic acids to Aldehydes Catalyzed by B(C 6 F 5 ) 3 David Bézier, Sehoon Park and Maurice Brookhart* Department of Chemistry, University of North Carolina at Chapel Hill,
S1 Selective Reduction of Carboxylic acids to Aldehydes Catalyzed by B(C 6 F 5 ) 3 David Bézier, Sehoon Park and Maurice Brookhart* Department of Chemistry, University of North Carolina at Chapel Hill,
Contents. Phillip R.A. Chivers a and David K. Smith. Department of Chemistry, University of York, Heslington, York, YO10 5DD, UK
 Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 207 Spatially-resolved soft materials for controlled release hybrid hydrogels combining a robust
Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 207 Spatially-resolved soft materials for controlled release hybrid hydrogels combining a robust
A supramolecular approach for fabrication of photo- responsive block-controllable supramolecular polymers
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2014 Supporting Information A supramolecular approach for fabrication of photo- responsive
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2014 Supporting Information A supramolecular approach for fabrication of photo- responsive
Nitroxide polymer networks formed by Michael addition: on site-cured electrode-active organic coating
 Supporting information for: Nitroxide polymer networks formed by Michael addition: on site-cured electrode-active organic coating Takeshi Ibe, a Rainer B. Frings, b Artur Lachowicz, b Soichi Kyo, a and
Supporting information for: Nitroxide polymer networks formed by Michael addition: on site-cured electrode-active organic coating Takeshi Ibe, a Rainer B. Frings, b Artur Lachowicz, b Soichi Kyo, a and
Supporting Information:
 Supporting Information: Visible Light Initiated Thiol-Michael Addition Polymerizations with Coumarin-based Photo-base Generators, Another Photoclick Reaction Strategy Xinpeng Zhang, Weixian Xi, Chen Wang,
Supporting Information: Visible Light Initiated Thiol-Michael Addition Polymerizations with Coumarin-based Photo-base Generators, Another Photoclick Reaction Strategy Xinpeng Zhang, Weixian Xi, Chen Wang,
Enantioselectivity switch in copper-catalyzed conjugate addition. reaction under influence of a chiral N-heterocyclic carbene-silver complex
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Supplementary Information Enantioselectivity switch in copper-catalyzed conjugate addition
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Supplementary Information Enantioselectivity switch in copper-catalyzed conjugate addition
SUPPORTING INFORMATION
 SUPPORTING INFORMATION Polymerization-induced Self-Assembly of Homopolymer and Diblock copolymer: A Facile Approach for preparing Polymer Nano-objects with Higher Order Morphologies Jianbo Tan *a,b, Chundong
SUPPORTING INFORMATION Polymerization-induced Self-Assembly of Homopolymer and Diblock copolymer: A Facile Approach for preparing Polymer Nano-objects with Higher Order Morphologies Jianbo Tan *a,b, Chundong
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/NCHEM.2201 Highly emissive platinum(ii) metallacages Xuzhou Yan, 1 Timothy R. Cook, 2 Pi Wang, 1 Feihe Huang,,1 and Peter J. Stang,3 1 Center for Chemistry of High-Performance & Novel Materials,
DOI: 10.1038/NCHEM.2201 Highly emissive platinum(ii) metallacages Xuzhou Yan, 1 Timothy R. Cook, 2 Pi Wang, 1 Feihe Huang,,1 and Peter J. Stang,3 1 Center for Chemistry of High-Performance & Novel Materials,
Electronic Supplementary Information for New Journal of Chemistry
 Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 Electronic Supplementary Information
Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 Electronic Supplementary Information
Electronic Supplementary Information for. A Redox-Nucleophilic Dual-Reactable Probe for Highly Selective
 Electronic Supplementary Information for A Redox-Nucleophilic Dual-Reactable Probe for Highly Selective and Sensitive Detection of H 2 S: Synthesis, Spectra and Bioimaging Changyu Zhang, 1 Runyu Wang,
Electronic Supplementary Information for A Redox-Nucleophilic Dual-Reactable Probe for Highly Selective and Sensitive Detection of H 2 S: Synthesis, Spectra and Bioimaging Changyu Zhang, 1 Runyu Wang,
Supporting Information:
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Supporting Information: A metal free reduction of aryl-n-nitrosamines to corresponding hydrazines
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Supporting Information: A metal free reduction of aryl-n-nitrosamines to corresponding hydrazines
University of Bristol - Explore Bristol Research
 Echue, G., Hamley, I. W., Lloyd-Jones, G., & Faul, C. F. J. (2016). Chiral Perylene Materials by Ionic Self-Assembly. Langmuir, 32(35), 9023-9032. DOI: 10.1021/acs.langmuir.6b02201 Publisher's PDF, also
Echue, G., Hamley, I. W., Lloyd-Jones, G., & Faul, C. F. J. (2016). Chiral Perylene Materials by Ionic Self-Assembly. Langmuir, 32(35), 9023-9032. DOI: 10.1021/acs.langmuir.6b02201 Publisher's PDF, also
Supporting Information
 Supporting Information Supramolecular design for polymer/titanium oxo-cluster hybrids: An open door to new organic-inorganic dynamers Fabien Périneau, Sandrine Pensec, Clément Sanchez, Costantino Creton,
Supporting Information Supramolecular design for polymer/titanium oxo-cluster hybrids: An open door to new organic-inorganic dynamers Fabien Périneau, Sandrine Pensec, Clément Sanchez, Costantino Creton,
Molecular Weight Distribution of Living Chains in Polystyrene Pre-pared by Atom Transfer Radical Polymerization
 Molecular Weight Distribution of Living Chains in Polystyrene Pre-pared by Atom Transfer Radical Polymerization Joongsuk Oh, a Jiae Kuk, a Taeheon Lee, b Jihwa Ye, b Huyn-jong Paik, b* Hyo Won Lee, c*
Molecular Weight Distribution of Living Chains in Polystyrene Pre-pared by Atom Transfer Radical Polymerization Joongsuk Oh, a Jiae Kuk, a Taeheon Lee, b Jihwa Ye, b Huyn-jong Paik, b* Hyo Won Lee, c*
Supporting Information
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 214 Supporting Information Lei Liu, ab Yijie Xia, b Jie Zhang* b a) China Center for Modernization
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 214 Supporting Information Lei Liu, ab Yijie Xia, b Jie Zhang* b a) China Center for Modernization
A biphasic oxidation of alcohols to aldehydes and ketones using a simplified packed-bed microreactor
 A biphasic oxidation of alcohols to aldehydes and ketones using a simplified packed-bed microreactor Andrew Bogdan 1 and D. Tyler McQuade 2, * Address: 1 Department of Chemistry and Chemical Biology, Cornell
A biphasic oxidation of alcohols to aldehydes and ketones using a simplified packed-bed microreactor Andrew Bogdan 1 and D. Tyler McQuade 2, * Address: 1 Department of Chemistry and Chemical Biology, Cornell
