Couplings / Gas Analysis
|
|
|
- Georgiana Flowers
- 5 years ago
- Views:
Transcription
1 Couplings / Gas Analysis
2 General With the coupling of a thermal balance and a gas analyzer like a FTIR spectrometer (Fourier Transform Infrared) or a Quadrupole-Mass-Spectrometer a very powerful analytical instrumentation is used which gives information from the thermal balance (TGA) or simultaneous thermal analyzer (STA) as well as from the spectrometer simultaneously. The optional Pulse-Analysis injects an exactly predetermined amount of gas into the Thermobalance (TGA) or Simultaneous Thermal Analyzer (STA). This enhances the measurement possibilities significantly. Analytical techniques used for coupling with thermal analyzers Couplings can be done with different gas analyzing methods: FT-IR spectroscopy Quadrupol mass spectrometry (QMS) ELIF spectroscopy (Excimer Laser Induced Fragmentation Fluorescence) Gas chromatography Typical coulings for simultaneous measurements are: TG-DSC-MS (Thermogravimetry, Differential Scanning Calorimetry, mass spectrometer) TG-MS (Thermal balance coupled with mass spectrometer) TG-DSC-GC/MS (Thermogravimety, Differential Scanning Calorimetry, Gas Chromatography / Mass Spectrometry) TG-FTIR (Thermal balance coupled with FTIR spectrometer) Infrared spectroscopy : Infrared light can excite molecular vibrations in molecules. In order to be active in respect to IR-spectroscopy, the molecule has to change its dipolar momentum during excitation. Gases like CO 2, CO, hydrocarbons, water vapour etc. have IR-active vibration modes while N 2, O 2 etc. cannot be detected. The obtained IR-spectra allow identification of the components by cha- 2
3 racteristic vibrations which are either typical for a certain functional group (CO, COOR etc.) or for a particular compound (so called fingerprint-region of the spectra from 15 5cm -1 ). Spectra libraries are helpful during spectra interpretation. Coupling to TGA and STA is a valuable tool especially in analysis of organic compounds (polymers etc.). Mass spectroscopy: Mass spectroscopy sorts molecules by their molecular weight divided by their electrical charge (m/e). In quadrupol mass spectroscopy (QMS) molecules enter a magnetic quadrupolfield after having been accelerated in a static electric field. Molecules and their fragments are sorted by their masses and can be identified. Mass spectroscopy is very useful in order to find the molecular weight of the outgassing as well as to analyse gases which are not active in IR-spectroscopy (N 2,O 2, CO etc.). Using mass spectroscopy, nearly all molecules can be detected. Also the resulting fragments of bigger molecules are often characteristic for several compounds or functional groups. This method is a common used analytical method that can be found in polymer or organic analysis as well as in forensic, medicinal, biological or inorganic areas like material science. Mass spectrometry can be also combined with a GC method that is used to get information about the purity of the substances that are investigated by the mass spectrometer. So the resulting method called GC-MS gives both, purity and molecular weight of the substrate. ELIF spectroscopy: ELIF (Excimer Laser Induced Fragmentation Fluorescence) is a technique used for analysis of alkali metal compounds. Its measuring principle is based on a simultaneous cleavage of molecules, and excitation of the respective alkali atom by a VUV-laser. After the return of the agitated atom to its original state, a photon of a characteristic wavelength is emitted. The intensity of this fluorescent signal is a measure of the concentration of the compound in question. This technique is a valuable tool for characterization of alkali metal compounds (NaCl, KCl, NaOH, etc.). ELIF spectroscopy can be used only by optical in-situ coupling (see below). Gas chromatography The evolved gases can be a complex mixture of compounds. Column Chromatography separates these compounds before analysing them by different techniques. The chromatographic separation column has to be chosen according to the type of molecules to be separated (polar or unpolar). The most frequently used detection techniques are flame ionization detectors (FID) and thermal conductivity detectors (TCD). Type of couplings The coupling of the thermal analyser with the spectrometer/chromatograph can be done by different means: Heated transfer capillary (FTIR, GCMS, GC, MS) Sniffer coupling (GCMS, GC, MS) Optical in-situ observation (ELIF) Heated transfer capillary The simplest way to do a coupling is by heated capillary. In this case, a heated capillary feeds the evolved gazes from the thermobalance to the spectrometer or chromatograph. The internal diameter of a capillary is <,1 mm in case of a MS coupling. The capillary is heated to 2-3 C which results in the risk of condensation of outgassing during transfer and clogging of the capillary. Sniffer coupling This technique is used for mass spectrometer coupling. Gases pass through a very small orifice close to the sample inside the furnace and are transferred in the vacuum line to the mass spectrometer. In this way, gases are sampled at high concentration very close to the sample at high temperature and pass directly to ultra-high vacuum. This technique avoids any risk of condensationduring transfer between the thermobalance and the mass spectrometer. Optical in-situ observation In this case, optical windows are integrated in the thermobalance s During heating samples often undergo phase transitions and/or weight change due to evaporation of solvents and/or chemical reactions. These changes can be detected by thermal analysis: calorimetric techniques (DTA and DSC) give information about the heat involved in these processes and thermogravimetry (TG) shows the weight change. Weight change can be either weight increase due to oxidation reactions or weight loss due to decomposition by liberation of volatile compounds. Analysis of these evolved gases can give valuable information about the sample composition and reaction pathways for decomposition. As thermal analysis gives no information about the nature of the evolved gases, coupling with spectrometers or chromatographs is a valuable tool for evolved gas analysis (EGA). 3
4 QMS Mass spectrometry (MS) is an analytical technique to measure the mass of atoms or molecules of a gas e.g. evaporated from a sample material which has been heated up. The spectra are used to determine the elemental or isotopic signature of a sample, the masses of particles and of molecules, and to elucidate the chemical structures of molecules, such as peptides and other chemical compounds. Mass spectrometry works by ionizing chemical compounds to generate charged molecules or molecule fragments and measuring their mass-to-charge ratios. The QMS - quadrupole mass spectrometer coupling device is a state of the art mass spectrometer with a heated inlet system. The QMS is used for the analysis of volatile decompositions. All LINSEIS instruments are especially designed to guarantee a user friendly operation of both the thermal analyzer and the mass spectrometer. An integrated Software solution is certainly available. Example System: Coupling of thermo balance TG/STA + Quadrupol Mass-spectrometer(QMS): Both manufacturers LINSEIS and Pfeiffer are some of the leading companies in their specialized fields. This cooperation guarantees outstanding performance for the combined system. Transfer line is temperature controlled up to a maximum of 25 C. Fast response time through short transfer line MS detector with very high sensitivity and with different mass numbers available. The following applications can be measured: Outgasing components of burned material (paints etc.) Determination of blenders and additives Absorption and Desorption processes Analysis of rubbers and plastics Features LINSEIS Research balance (different models) with TG or simultaneous TG/DSC or TG/DTA measurement Simultaneous balance with (.1/.5/1µg) resolution and 25g max. sample weight Specific heat measurements (Cp) possible Pfeiffer/Balzers mass spectrometer model Thermostar ( - 1 amu, - 2 amu, - 3 amu) Highest precision Quadrupol-MS from world leader in MS Technology MS with very compact dimensions Very easily exchangeable quartz capillary Three separate heating zones, at capillary, at TG protection tube and at MS vacuum inlet Carrier gas with wide range of flow rates Especially developed Al 2 O 3 sniffer-nose, to extract the outgasings Combined results of TG and MS available in one evaluation sheet Complete vacuum tight system 4
5 Cement raw material TG [%] % -.84% -3.32% -1.4% -1.22% C J/g C C J/g J/g C amu 18 (H 2O) amu 57 (C 4H 9) amu 44 (CO 2) amu 58 (C 4H 1) amu 64 (SO 2) amu 67 (C 5H 7) amu 78 (C 5H 6) C % C J/g exo C -4.22% DSC [mw/mg] [3] The combination of thermal analysis with mass spectroscopy is a very powerful method to identify and quantify the components of the raw material and it is also a tool for the simulation of the manufacturing process of building materials. The components of cement raw material are: mixture of ceramic components (gypsum, calcium carbonate, etc.) and also organic components Temperature [ C] -15 Analysis using STA and QMS The picture shows the simultaneous thermogravimetry (TG) combined with differential scanning calorimetry (DSC) and mass spectroscopy (MS). The mass spectrometry allows the identification of the evolved gases from the material. Mass spectrometry shows peaks from H 2 O at low temperatures most probably from gypsum. The DSC peaks and the signal from mass spectrometer between ~3 C 4 C indicates the decomposition of organic components. The peak of CO 2 at ~8 C indicates the decomposition of CaCO 3. At ~13 C CaSO 4 decompose (SO 2 Peak). Decomposition of CaC 2 O 4 Time [min ] The evolved gases from the decomposition of calcium oxalate have been fed into the mass spectrometer with a heated capillary. The ion currents for mass numbers 18 (water), 28 (carbon monoxide) and 44 (carbon dioxide) have been imported into the graph. dm-rel [%] DTA Signal [µv] dm-rel[ca-ox-1] DTA Signal[Ca-Ox-1] Ion Current_18[Ca-Ox] Ion Current_28[Ca-Ox] Ion Current_44[Ca-Ox] Mass change % Mass change % Mass change -3.5% C -61.% Ion Current_18 [E-12 A] Temperature (smoothed) [ C] Mass spectromy of roof tile clay Mass change -14.8% DTA Signal smoothed [CUSO-1] Mass change -14.3% 3. dm-rel [%] DTA Signal (smoothed) [µv] dm-rel[cuso-1] Mass change -7.25% dm-rel(derived) [%/min] Temperature (smoothed) [ C] 5
6 FTIR The Fourier transform infrared spectroscopy (FTIR) is a technique which is used to obtain an infrared spectrum of absorption, emission, photoconductivity or Raman scattering of a solid, liquid or gas. An FTIR spectrometer simultaneously collects spectral data in a wide spectral range. This confers a significant advantage over a dispersive spectrometer which measures intensity over a narrow range of wavelengths at a time. FTIR has made dispersive infrared spectrometers all but obsolete, opening up new applications of infrared spectroscopy. The combination of a Linseis Thermal Analyzer with a FTIR is especially interesting in fields such as polymers, chemical and pharmaceutical industry. The coupling is more than the sum of the separate parts. Benefits come from the LINSEIS coupling knowledge and integrated hard- and software concept. For interpretation different libraries are available. Possible Couplings: Thermobalance + FTIR Spectrometer in the temperature range from -17 C up to 175 C L81/I-FTIR Thermo balance + FTIR Spectrometer L81/II-FTIR Thermo balance + FTIR Spectrometer STA PT 16-FTIR Thermo balance + FTIR Spectrometer STA PT 1-FTIR Thermo balance + FTIR Spectrometer Features Research balance (different models) with TG or simultaneous TG/DSC or TG/DTA measurement. High precision Nicolet FTIR spectrometer (different model available). Temperature range -17 C up to C Three separate heating zones, at capillary, at TG protection tube and at FTIR measuring cell. Carrier gas with wide range of flow rates. Especially developed JLF-detector with long optical pass length. Description Both manufacturers Linseis and ThermoFisher Nicolet are some of the leading companies in their specialized fields. This cooperation guarantees outstanding performance for the combined system. Transfer line is temperature controlled up to a maximum of 25 C. Fast response time through short transfer line. FTIR detector with very high sensitivity and with different wave numbers available. The following applications can be measured: outgassing components of burned material (paints etc.), determination of blenders and additives, absorption and desorption processes, analysis of rubbers and plastics. Thermobalance with coupling to Nicrolet FTIR spectrometer. The coupling is made with a heated capillary. 6
7 Cement 1 99 Cement C -.8% -.5% -.1% C % TG [%] 97 1 CO H 2 O C.5 95 H 2 O 69.8 C CO C Temperature [ C] Introduction and application Cement is an inorganic, non-metallic material. Together with water it hardens and afterwards it stays also hard under water. Portland cement consists of limestone, clay and/or sand. The additives gypsum, anhydrite etc. influence the setting time of the cement. Impurities in the raw cement cause negative influence on the quality of the cement. Analysis using thermogravimetry and FTIR The additives can be identified and quantified with thermal analysis. The first step shows the evolved water from the CaSO 4 di-hydrate to CaSO 4 half-hydrate. The second step is the conversion of the CaSO 4 half-hydrate to the CaSO 4 anhydrite. The evolved water can also be verified by FTIR analysis. Between 6 C and 75 C the carbonates decomposed so CO 2 evolves. The shoulder in the CO 2 trace of the FTIR signal is the decomposition of MgCO 3. CaSO 4 2H 2 O CaSO 4 ½H 2 O CaSO 4 7
8 MS-Sniffer Due to the limitation of the input pressure of the MS, the sample gas must be taken after the pressure controller (at ambient pressure). So, only substances which can pass through the cold trap can be analyzed. The outgassings of the sample are passed to the QMS-analyzer directly, using a very small aperture. This small aperture (or orifice) reduces the pressure inside the pressure vessel to the input pressure allowed for the QMS. Since this aperture is inside the hot zone of the furnace, condensation of the out gassings can t take place. Since between the aperture and the ion-source of the QMS a vacuum of app. 1e-5mbar exists, condensation there is impossible also. The sniffer is placed directly above the sample. This is possible because of the the sniffer material, which can resist the temperatures in the hot furnace area. Sniffer Protection tube Aperture Sample Heater Gas 1 Gas 2 Gas 3 Purge H 2 O 2 MFC MFC MFC MFC HPLC Pump pump.7bar bar 7.5Bar bar P P G Manometer M Pressure- controller Vacuum Vacuum- gauge Condensor Evaporator Vacuum- pump Tank Outlet Analyser Coupling to a high pressure system 8
9 GCMS During heating samples often undergo phase transitions and/or weight change due to evaporation of solvents and/or chemical reactions. These changes can be detected by thermal analysis: calorimetric techniques (DTA and DSC) give information about the heat involved in these processes and thermogravimetry (TG) shows the weight change. Weight change can be either weight increase due to oxidation reactions or weight loss due to decomposition by liberation of volatile compounds. Analysis of these evolved gases can give valuable information about the sample composition and reaction pathways for decomposition. As thermal analysis gives no information about the nature of the evolved gases, coupling with spectrometers or chromatographs is a valuable tool for evolved gas analysis (EGA). 9
10 Thermal decomposition of latex TG DSC 5 At 37 synthetic rubber decomposes into some monomer parts. The main parts limonene and isoprene can be identified using STA combined with GC-MS. The STA signal shows mass loss and enthalpie TG [%] % -5-1 DSC [mw] change at 372. At the same time the GC shows two peaks, a smaller and a bigger one, where the samller one can be identified by mass spectrometrie as isoprene and the bigger one as limonene C Temperature [ C] mau Time [min] Limonene 9 Isoprene Relative Intensity Relative Intensity m/z m/z 1
11 InSitu Possible Techniques: FTIR: Fourier Transform Infrared Spectroscopy: Measurement of basic and trace gas components until ppm range (for example H 2 O, CO 2, CO, H 2 S...). Polar Molecules are necessary. Raman-Spectroscopy: Measurement of basic gas components. Also not polar molecules like H 2 or N 2 are measurable. ELIF: Excimer Laser induced Fragmentation Fluorescence: UV-Laserbased Method of Measuring of gaseous alkaline compounds (for example NaCI, NaOH, KCI, KOH). Also at 193 nm an entry through UV_Saphire is possible. Advantages of the optical In-Situ window: No cooling / modification of the measuring gas (for example no out-condensation, no transition reaction, no equilibrium shift) Many materials with high condensation temperature for example alkali metals (Na, K and their combinations) are now able to be measured, heated capillary only suitable for some 2-25 C, the optical port allows measurement until 16 C No intervention into the measuring system (for example when pulling gas by vacuum) No contamination of the measuring gas in the capillary to M/S or FTIR Real-time online-measurement (no dead time until measuring volume enters the measuring instrument 11
12 LINSEIS GmbH Vielitzerstr Selb Germany Tel.: (+49) Fax: (+49) info@linseis.de LINSEIS Inc. 19 North Gold Drive Robbinsville, NJ 8691 USA Tel.: +1 (69) Fax: +1 (69) info@linseis.com Products: DIL, TG, STA, DSC, HDSC, DTA, TMA, MS/FTIR, In-Situ EGA, Laser Flash, Seebeck Effect Services: Service Lab, Calibration Service
TGA Thermal Gravimetric Analysis
 TGA Thermal Gravimetric Analysis General Thermogravimetry is a technique in which the mass of the sample is monitored against time or temperature while the temperature of the sample, in a specified atmosphere,
TGA Thermal Gravimetric Analysis General Thermogravimetry is a technique in which the mass of the sample is monitored against time or temperature while the temperature of the sample, in a specified atmosphere,
THERMO- GRAVIMETRIC ANALYZER TGA 1000
 T H E R M A L A N A L Y S I S THERMO- GRAVIMETRIC ANALYZER TGA 1 Since 1957 LINSEIS Corporation has been delivering outstanding service, know how and leading innovative products in the field of thermal
T H E R M A L A N A L Y S I S THERMO- GRAVIMETRIC ANALYZER TGA 1 Since 1957 LINSEIS Corporation has been delivering outstanding service, know how and leading innovative products in the field of thermal
High Pressure DSC Differential Scanning Calorimeter
 High Pressure DSC Differential Scanning Calorimeter Introduction The Differential Scanning Calorimetry (DSC) is the most popular thermal analysis technique to measure endothermic and exothermic transitions
High Pressure DSC Differential Scanning Calorimeter Introduction The Differential Scanning Calorimetry (DSC) is the most popular thermal analysis technique to measure endothermic and exothermic transitions
DSC PT 10. Applications
 DSC PT 10 DSC PT 10 The differential scanning calorimetry method is widely used to examine and characterize substances, mixtures, and materials. This technique is internationally standardized under DIN
DSC PT 10 DSC PT 10 The differential scanning calorimetry method is widely used to examine and characterize substances, mixtures, and materials. This technique is internationally standardized under DIN
HYPHENATED TECHNOLOGY GUIDE
 HYPHENATED TECHNOLOGY GUIDE TABLE OF CONTENTS TG-IR...3 Hiden Analytical MS Systems for TG-MS...4 TG-MS...5 TG-GC/MS...6 TG-IR-GC/MS...8 HYPHENATED TECHNOLOGY GUIDE Coupling, or hyphenating, two instruments
HYPHENATED TECHNOLOGY GUIDE TABLE OF CONTENTS TG-IR...3 Hiden Analytical MS Systems for TG-MS...4 TG-MS...5 TG-GC/MS...6 TG-IR-GC/MS...8 HYPHENATED TECHNOLOGY GUIDE Coupling, or hyphenating, two instruments
Evolved gas analysis by simultaneous thermogravimetric differential thermal analysis-fourier transformation infrared spectroscopy (TG-DTA-FTIR)
 Technical articles Evolved gas analysis by simultaneous thermogravimetric differential thermal analysis-fourier transformation infrared spectroscopy (TG-DTA-FTIR) Tadashi Arii* 1. Introduction Simultaneous
Technical articles Evolved gas analysis by simultaneous thermogravimetric differential thermal analysis-fourier transformation infrared spectroscopy (TG-DTA-FTIR) Tadashi Arii* 1. Introduction Simultaneous
Technical know-how in thermal analysis measurement
 Technical articles Technical know-how in thermal analysis measurement Evolved gas analysis by thermogravimetry-differential thermal analysis-mass spectrometry (TG-DTA-MS) technique Kazuko Motomura* and
Technical articles Technical know-how in thermal analysis measurement Evolved gas analysis by thermogravimetry-differential thermal analysis-mass spectrometry (TG-DTA-MS) technique Kazuko Motomura* and
HYPHENATED TECHNOLOGY GUIDE
 HYPHENATED TECHNOLOGY GUIDE TABLE OF CONTENTS TG-IR...3 Hiden Analytical MS Systems for TG-MS...4 TG-MS...5 TG-GC/MS...6 TG-IR-GC/MS...8 HYPHENATED TECHNOLOGY GUIDE Coupling, or hyphenating, two instruments
HYPHENATED TECHNOLOGY GUIDE TABLE OF CONTENTS TG-IR...3 Hiden Analytical MS Systems for TG-MS...4 TG-MS...5 TG-GC/MS...6 TG-IR-GC/MS...8 HYPHENATED TECHNOLOGY GUIDE Coupling, or hyphenating, two instruments
SIMULTANEOUS THERMAL ANALYSIS STA PT 1000 STA PT 1600 STA HP
 T H E R M A L A N A L Y S I S SIMULTANEOUS THERMAL ANALYSIS STA PT 1 STA PT 16 STA HP Since 1957 LINSEIS Corporation has been delivering outstanding service, know how and leading innovative products in
T H E R M A L A N A L Y S I S SIMULTANEOUS THERMAL ANALYSIS STA PT 1 STA PT 16 STA HP Since 1957 LINSEIS Corporation has been delivering outstanding service, know how and leading innovative products in
It is the purpose of this paper to demonstrate that the detection limit is directly related to the sample size, for both TG and TG/FTIR.
 BIGGER IS BETTER: PUSHING THE LIMIT OF TG AND TG/FTIR Abstract In Thermogravimetry (TG) and TG/FTIR systems, there are many variables that can affect the detection limit of the system. It is demonstrated
BIGGER IS BETTER: PUSHING THE LIMIT OF TG AND TG/FTIR Abstract In Thermogravimetry (TG) and TG/FTIR systems, there are many variables that can affect the detection limit of the system. It is demonstrated
The Claviature of Gas Analysis
 The Claviature of Gas Analysis Mass spectrometric gas analysis for quality assurance of gases and gas mixtures High purity and special gases are becoming increasingly important in the development of modern
The Claviature of Gas Analysis Mass spectrometric gas analysis for quality assurance of gases and gas mixtures High purity and special gases are becoming increasingly important in the development of modern
Thermal Methods of Analysis Theory, General Techniques and Applications. Prof. Tarek A. Fayed
 Thermal Methods of Analysis Theory, General Techniques and Applications Prof. Tarek A. Fayed 1- General introduction and theory: Thermal analysis (TA) is a group of physical techniques in which the chemical
Thermal Methods of Analysis Theory, General Techniques and Applications Prof. Tarek A. Fayed 1- General introduction and theory: Thermal analysis (TA) is a group of physical techniques in which the chemical
Thermogravimetric Analysis Advanced Techniques for Better Materials Characterisation
 Thermogravimetric Analysis Advanced Techniques for Better Materials Characterisation Philip Davies TA Instruments UK Thermogravimetric Analysis Change in a samples weight (increase or decrease) as a function
Thermogravimetric Analysis Advanced Techniques for Better Materials Characterisation Philip Davies TA Instruments UK Thermogravimetric Analysis Change in a samples weight (increase or decrease) as a function
APPLICATION NOTE. Investigation of Alkali Salts with the STA 449 F5 Jupiter. Dr. Alexander Schindler and Dr. Michael Schöneich.
 APPLICATION NOTE Investigation of Alkali Salts with the STA 449 F5 Jupiter Dr. Alexander Schindler and Dr. Michael Schöneich Introduction While analytical techniques such as EDX or ICP-MS provide a detailed
APPLICATION NOTE Investigation of Alkali Salts with the STA 449 F5 Jupiter Dr. Alexander Schindler and Dr. Michael Schöneich Introduction While analytical techniques such as EDX or ICP-MS provide a detailed
Nutshells of Thermal Analysis. Heat it up! Burn it! Thermal Analysis
 Nutshells of Thermal Analysis Heat it up! Burn it! 1 Thermal Analysis Thermal Analaysis (TA) Techniques Abbreviations Full Names Measure DSC Differential Scanning Calorimetry Heat difference DMA Dynamic
Nutshells of Thermal Analysis Heat it up! Burn it! 1 Thermal Analysis Thermal Analaysis (TA) Techniques Abbreviations Full Names Measure DSC Differential Scanning Calorimetry Heat difference DMA Dynamic
Atmospheric Analysis Gases. Sampling and analysis of gaseous compounds
 Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
Thermal Analysis Mass Spectrometer Coupling. Evolved Gas Analysis Method, Techniques and Applications
 Thermal Analysis Mass Spectrometer Coupling Evolved Gas Analysis Method, Techniques and Applications Thermal Analysis and Evolved Gas Analysis Thermoanalytical Techniques Thermoanalytical techniques are
Thermal Analysis Mass Spectrometer Coupling Evolved Gas Analysis Method, Techniques and Applications Thermal Analysis and Evolved Gas Analysis Thermoanalytical Techniques Thermoanalytical techniques are
LASER FLASH ANALYSIS LFA 1000
 T H E R M A L A N A L Y S I S LASER FLASH ANALYSIS LFA 1000 Since 1957 LINSEIS Corporation has been delivering outstanding service, know how and leading innovative products in the field of thermal analysis
T H E R M A L A N A L Y S I S LASER FLASH ANALYSIS LFA 1000 Since 1957 LINSEIS Corporation has been delivering outstanding service, know how and leading innovative products in the field of thermal analysis
Fragrances Sampling and Analysis
 Fragrances Sampling and Analysis Picture: Roman Kaiser at field work Picture source: Givaudan Marco Stöckli, Eva Gleißner 17.11.2015 1 http://www.wysinfo.com/perfume/picts/0_wysinfo-smell%20drawing2_550_1.jpg,
Fragrances Sampling and Analysis Picture: Roman Kaiser at field work Picture source: Givaudan Marco Stöckli, Eva Gleißner 17.11.2015 1 http://www.wysinfo.com/perfume/picts/0_wysinfo-smell%20drawing2_550_1.jpg,
LIGHT FLASH ANALYSIS LFA 500
 T H E R M A L A N A L Y S I S LIGHT FLASH ANALYSIS LFA 500 Since 1957 LINSEIS Corporation has been delivering outstanding service, know how and leading innovative products in the field of thermal analysis
T H E R M A L A N A L Y S I S LIGHT FLASH ANALYSIS LFA 500 Since 1957 LINSEIS Corporation has been delivering outstanding service, know how and leading innovative products in the field of thermal analysis
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur
 VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
TANDEM MASS SPECTROSCOPY
 TANDEM MASS SPECTROSCOPY 1 MASS SPECTROMETER TYPES OF MASS SPECTROMETER PRINCIPLE TANDEM MASS SPECTROMETER INSTRUMENTATION QUADRAPOLE MASS ANALYZER TRIPLE QUADRAPOLE MASS ANALYZER TIME OF FLIGHT MASS ANALYSER
TANDEM MASS SPECTROSCOPY 1 MASS SPECTROMETER TYPES OF MASS SPECTROMETER PRINCIPLE TANDEM MASS SPECTROMETER INSTRUMENTATION QUADRAPOLE MASS ANALYZER TRIPLE QUADRAPOLE MASS ANALYZER TIME OF FLIGHT MASS ANALYSER
Chemistry Instrumental Analysis Lecture 34. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 34 From molecular to elemental analysis there are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry
Chemistry 4631 Instrumental Analysis Lecture 34 From molecular to elemental analysis there are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry
Hiden Isochema. Gravimetric Gas & Vapor Sorption Analyzers. Hiden Isochema IGA Series. Advancing Sorption Analysis
 Technical Specifications The IGA-1 is designed for gravimetric mixed gas sorption, as well as single component vapor sorption analysis, and powerfully combines the features of the IGA-1, IGA-2 and IGA-3.
Technical Specifications The IGA-1 is designed for gravimetric mixed gas sorption, as well as single component vapor sorption analysis, and powerfully combines the features of the IGA-1, IGA-2 and IGA-3.
CHEM*3440. Thermal Methods. Thermogravimetry. Instrumental Components. Chemical Instrumentation. Thermal Analysis. Topic 14
 Thermal Methods We will examine three thermal analytical techniques: Thermogravimetric Analysis (TGA) CHEM*3440 Chemical Instrumentation Topic 14 Thermal Analysis Differential Thermal Analysis (DTA) Differential
Thermal Methods We will examine three thermal analytical techniques: Thermogravimetric Analysis (TGA) CHEM*3440 Chemical Instrumentation Topic 14 Thermal Analysis Differential Thermal Analysis (DTA) Differential
ADVANCED ANALYTICAL LABORATORY
 An Information Brochure on ADVANCED ANALYTICAL LABORATORY ANDHRA UNIVERSITY Visakhapatnam - 530 003 Andhra Pradesh, India Sponsored by DEPARTMENT OF SCIENCE & TECHNOLOGY Government of India New Delhi 110016
An Information Brochure on ADVANCED ANALYTICAL LABORATORY ANDHRA UNIVERSITY Visakhapatnam - 530 003 Andhra Pradesh, India Sponsored by DEPARTMENT OF SCIENCE & TECHNOLOGY Government of India New Delhi 110016
Research Equipment in the UCA Chemistry Department
 Research Equipment in the UCA Chemistry Department Varian 220 Atomic Absorption Spectrometer Atomic Absorption spectroscopy is used to measure the amount of a metal ion present in a solution. It is particularly
Research Equipment in the UCA Chemistry Department Varian 220 Atomic Absorption Spectrometer Atomic Absorption spectroscopy is used to measure the amount of a metal ion present in a solution. It is particularly
Gas Chromatography. Chromatography Laboratory Course. Dr. Christian Jungnickel Chromatography Course GC September 2005
 Gas Chromatography Chromatography Laboratory Course The laboratory course experiments General Aim: Gain general experience using a GC Constant Injection technique Temperature variations Qualitative and
Gas Chromatography Chromatography Laboratory Course The laboratory course experiments General Aim: Gain general experience using a GC Constant Injection technique Temperature variations Qualitative and
Thermal methods. P A Sathe Ramnarain Ruia College, Mumbai
 Thermal methods P A Sathe Ramnarain Ruia College, Mumbai 1 Thermal methods: Thermal analysis is a group of methods in which any physical property of the analyte is measured as a function of temperature
Thermal methods P A Sathe Ramnarain Ruia College, Mumbai 1 Thermal methods: Thermal analysis is a group of methods in which any physical property of the analyte is measured as a function of temperature
High Pressure/Performance Liquid Chromatography (HPLC)
 High Pressure/Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) is a form of column chromatography that pumps a sample mixture or analyte in a solvent (known as the
High Pressure/Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) is a form of column chromatography that pumps a sample mixture or analyte in a solvent (known as the
Thermal Methods of Analysis
 Thermal Methods of Analysis Calorie-something we know What is calorie? Can you see or touch a calorie? How is it measured? Working out in gym Change in weight Loss of calories-burning of fat? (10 km=500calories/9cal
Thermal Methods of Analysis Calorie-something we know What is calorie? Can you see or touch a calorie? How is it measured? Working out in gym Change in weight Loss of calories-burning of fat? (10 km=500calories/9cal
Analyzing & Testing. Thermogravimetric Analysis TGA. Technique, Instrument, Applications TG 209 F1. Leading Thermal Analysis
 Analyzing & Testing Thermogravimetric Analysis TGA Technique, Instrument, Applications TG 209 F1 Leading Thermal Analysis Thermo-Microbalance TG 209 F1 Libra TGA Method Thermogravimetry (TG) or Thermogravimetric
Analyzing & Testing Thermogravimetric Analysis TGA Technique, Instrument, Applications TG 209 F1 Leading Thermal Analysis Thermo-Microbalance TG 209 F1 Libra TGA Method Thermogravimetry (TG) or Thermogravimetric
Tips & Tricks GPC/SEC: Quantify and Get More Than Molar Mass Averages
 Tips & Tricks GPC/SEC: Quantify and Get More Than Molar Mass Averages Daniela Held, PSS Polymer Standards Service GmbH, Mainz, Germany Gel permeation chromatography/size-exclusion chromatography (GPC/SEC)
Tips & Tricks GPC/SEC: Quantify and Get More Than Molar Mass Averages Daniela Held, PSS Polymer Standards Service GmbH, Mainz, Germany Gel permeation chromatography/size-exclusion chromatography (GPC/SEC)
Proudly serving laboratories worldwide since 1979 CALL for Refurbished & Certified Lab Equipment
 www.ietltd.com Proudly serving laboratories worldwide since 1979 CALL +1.847.913.0777 for Refurbished & Certified Lab Equipment Applied Biosystems QStar Pulsar i Features of the API QSTAR Pulsar i The
www.ietltd.com Proudly serving laboratories worldwide since 1979 CALL +1.847.913.0777 for Refurbished & Certified Lab Equipment Applied Biosystems QStar Pulsar i Features of the API QSTAR Pulsar i The
Total analysis with DSC, TMA and TGA-EGA
 Total analysis with DSC, TMA and TGA-EGA The investigation of printed cicuit boards is used as an example to show how the results from different thermoanalytical techniques can be evaluated to make a comprehensive
Total analysis with DSC, TMA and TGA-EGA The investigation of printed cicuit boards is used as an example to show how the results from different thermoanalytical techniques can be evaluated to make a comprehensive
Gas Chromatography (GC)
 Gas Chromatography (GC) Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11541 Saudi Arabia Office: AA53
Gas Chromatography (GC) Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11541 Saudi Arabia Office: AA53
Uncover the insight with Hyphenation. Gerlinde Wita October 2015
 Uncover the insight with Hyphenation Gerlinde Wita October 2015 Let s start from the beginning Outline Material Characterization at PerkinElmer Thermal Analysis DSC, TMA, DMA, TGA - And some examples Hyphenation
Uncover the insight with Hyphenation Gerlinde Wita October 2015 Let s start from the beginning Outline Material Characterization at PerkinElmer Thermal Analysis DSC, TMA, DMA, TGA - And some examples Hyphenation
Benchtop NMR Combined with GC/MS Confirms Identity of Forensic Case Sample
 APPLICATION NOTE Benchtop NMR Combined with GC/MS Confirms Identity of Forensic Case Sample No. AN52889 Authors: Dean Antic, Ph.D., Thermo Fisher Scientific, San Jose, CA, USA WanLi Wei, Senior Engineer,
APPLICATION NOTE Benchtop NMR Combined with GC/MS Confirms Identity of Forensic Case Sample No. AN52889 Authors: Dean Antic, Ph.D., Thermo Fisher Scientific, San Jose, CA, USA WanLi Wei, Senior Engineer,
Secondary Ion Mass Spectrometry (SIMS)
 CHEM53200: Lecture 10 Secondary Ion Mass Spectrometry (SIMS) Major reference: Surface Analysis Edited by J. C. Vickerman (1997). 1 Primary particles may be: Secondary particles can be e s, neutral species
CHEM53200: Lecture 10 Secondary Ion Mass Spectrometry (SIMS) Major reference: Surface Analysis Edited by J. C. Vickerman (1997). 1 Primary particles may be: Secondary particles can be e s, neutral species
Fundamentals of Mass Spectrometry. Fundamentals of Mass Spectrometry. Learning Objective. Proteomics
 Mass spectrometry (MS) is the technique for protein identification and analysis by production of charged molecular species in vacuum, and their separation by magnetic and electric fields based on mass
Mass spectrometry (MS) is the technique for protein identification and analysis by production of charged molecular species in vacuum, and their separation by magnetic and electric fields based on mass
2401 Gas (liquid) Chromatography
 2401 Gas (liquid) Chromatography Chromatography Scheme Gas chromatography - specifically gas-liquid chromatography - involves a sample being vaporized and injected onto the head of the chromatographic
2401 Gas (liquid) Chromatography Chromatography Scheme Gas chromatography - specifically gas-liquid chromatography - involves a sample being vaporized and injected onto the head of the chromatographic
Introduction to Pharmaceutical Chemical Analysis
 Introduction to Pharmaceutical Chemical Analysis Hansen, Steen ISBN-13: 9780470661222 Table of Contents Preface xv 1 Introduction to Pharmaceutical Analysis 1 1.1 Applications and Definitions 1 1.2 The
Introduction to Pharmaceutical Chemical Analysis Hansen, Steen ISBN-13: 9780470661222 Table of Contents Preface xv 1 Introduction to Pharmaceutical Analysis 1 1.1 Applications and Definitions 1 1.2 The
Mass Spectrometry. Hyphenated Techniques GC-MS LC-MS and MS-MS
 Mass Spectrometry Hyphenated Techniques GC-MS LC-MS and MS-MS Reasons for Using Chromatography with MS Mixture analysis by MS alone is difficult Fragmentation from ionization (EI or CI) Fragments from
Mass Spectrometry Hyphenated Techniques GC-MS LC-MS and MS-MS Reasons for Using Chromatography with MS Mixture analysis by MS alone is difficult Fragmentation from ionization (EI or CI) Fragments from
Luminescence transitions. Fluorescence spectroscopy
 Luminescence transitions Fluorescence spectroscopy Advantages: High sensitivity (single molecule detection!) Measuring increment in signal against a dark (zero) background Emission is proportional to excitation
Luminescence transitions Fluorescence spectroscopy Advantages: High sensitivity (single molecule detection!) Measuring increment in signal against a dark (zero) background Emission is proportional to excitation
Gas Chromatography (GC)! Environmental Organic Chemistry CEE-PUBH Analysis Topic 5
 Gas Chromatography (GC)! Environmental Organic Chemistry CEE-PUBH 5730-6730 Analysis Topic 5 Chromatography! Group of separation techniques based on partitioning (mobile phase/stationary phase). Two immiscible
Gas Chromatography (GC)! Environmental Organic Chemistry CEE-PUBH 5730-6730 Analysis Topic 5 Chromatography! Group of separation techniques based on partitioning (mobile phase/stationary phase). Two immiscible
Chapter 27: Gas Chromatography
 Chapter 27: Gas Chromatography Gas Chromatography Mobile phase (carrier gas): gas (He, N 2, H 2 ) - do not interact with analytes - only transport the analyte through the column Analyte: volatile liquid
Chapter 27: Gas Chromatography Gas Chromatography Mobile phase (carrier gas): gas (He, N 2, H 2 ) - do not interact with analytes - only transport the analyte through the column Analyte: volatile liquid
Questions on Instrumental Methods of Analysis
 Questions on Instrumental Methods of Analysis 1. Which one of the following techniques can be used for the detection in a liquid chromatograph? a. Ultraviolet absorbance or refractive index measurement.
Questions on Instrumental Methods of Analysis 1. Which one of the following techniques can be used for the detection in a liquid chromatograph? a. Ultraviolet absorbance or refractive index measurement.
Techniques useful in biodegradation tracking and biodegradable polymers characterization
 Techniques useful in biodegradation tracking and biodegradable polymers characterization Version 1 Wanda Sikorska and Henryk Janeczek 1 Knowledge on biodegradable polymers structures is essential for the
Techniques useful in biodegradation tracking and biodegradable polymers characterization Version 1 Wanda Sikorska and Henryk Janeczek 1 Knowledge on biodegradable polymers structures is essential for the
MS/MS .LQGVRI0606([SHULPHQWV
 0DVV6SHFWURPHWHUV Tandem Mass Spectrometry (MS/MS) :KDWLV0606" Mass spectrometers are commonly combined with separation devices such as gas chromatographs (GC) and liquid chromatographs (LC). The GC or
0DVV6SHFWURPHWHUV Tandem Mass Spectrometry (MS/MS) :KDWLV0606" Mass spectrometers are commonly combined with separation devices such as gas chromatographs (GC) and liquid chromatographs (LC). The GC or
Propose a structure for an alcohol, C4H10O, that has the following
 Propose a structure for an alcohol, C4H10O, that has the following 13CNMR spectral data: Broadband _ decoupled 13CNMR: 19.0, 31.7, 69.5 б DEPT _90: 31.7 б DEPT _ 135: positive peak at 19.0 & 31.7 б, negative
Propose a structure for an alcohol, C4H10O, that has the following 13CNMR spectral data: Broadband _ decoupled 13CNMR: 19.0, 31.7, 69.5 б DEPT _90: 31.7 б DEPT _ 135: positive peak at 19.0 & 31.7 б, negative
High-pressure qnmr spectroscopy in condensed- and gas-phase towards determination of impurities and compositions of gas mixtures
 13.10.2016 High-pressure qnmr spectroscopy in condensed- and gas-phase towards determination of impurities and compositions of gas mixtures Klas Meyer, Heinrich Kipphardt, Michael Maiwald Online NMR Spectroscopy
13.10.2016 High-pressure qnmr spectroscopy in condensed- and gas-phase towards determination of impurities and compositions of gas mixtures Klas Meyer, Heinrich Kipphardt, Michael Maiwald Online NMR Spectroscopy
3) In CE separation is based on what two properties of the solutes? (3 pts)
 Final Exam Chem 311 Fall 2002 December 16 Name 1) (3 pts) In GC separation is based on the following two properties of the solutes a) polarity and size b) vapor pressure and molecular weight c) vapor pressure
Final Exam Chem 311 Fall 2002 December 16 Name 1) (3 pts) In GC separation is based on the following two properties of the solutes a) polarity and size b) vapor pressure and molecular weight c) vapor pressure
Introduction to GC/MS
 Why Mass Spectrometry? Introduction to GC/MS A powerful analytical technique used to: 1.Identify unknown compounds 2. Quantify known materials down to trace levels 3. Elucidate the structure of molecules
Why Mass Spectrometry? Introduction to GC/MS A powerful analytical technique used to: 1.Identify unknown compounds 2. Quantify known materials down to trace levels 3. Elucidate the structure of molecules
Analyzing & Testing. Photocalorimetry Photo-DSC. Method, Technique, Applications. Photo-DSC 204 F1. Leading Thermal Analysis
 Analyzing & Testing Photocalorimetry Photo-DSC Method, Technique, Applications Photo-DSC 204 F1 Leading Thermal Analysis Photo-DSC 204 F1 Phoenix Method and Technique Advantages of Photocalorimetry Besides
Analyzing & Testing Photocalorimetry Photo-DSC Method, Technique, Applications Photo-DSC 204 F1 Leading Thermal Analysis Photo-DSC 204 F1 Phoenix Method and Technique Advantages of Photocalorimetry Besides
CHAPTER A2 LASER DESORPTION IONIZATION AND MALDI
 Back to Basics Section A: Ionization Processes CHAPTER A2 LASER DESORPTION IONIZATION AND MALDI TABLE OF CONTENTS Quick Guide...27 Summary...29 The Ionization Process...31 Other Considerations on Laser
Back to Basics Section A: Ionization Processes CHAPTER A2 LASER DESORPTION IONIZATION AND MALDI TABLE OF CONTENTS Quick Guide...27 Summary...29 The Ionization Process...31 Other Considerations on Laser
CHEM 3590 Final examination 2017 Instructor : Dr. Hélène Perreault Monday December 11, 1:30-4:30 pm, Frank Kennedy Gold Gym seats
 CHEM 3590 Final examination 2017 Instructor : Dr. Hélène Perreault Monday December 11, 1:30-4:30 pm, Frank Kennedy Gold Gym seats 310-343 Questions 1-15 have multiple choices (50 pts). Please answer on
CHEM 3590 Final examination 2017 Instructor : Dr. Hélène Perreault Monday December 11, 1:30-4:30 pm, Frank Kennedy Gold Gym seats 310-343 Questions 1-15 have multiple choices (50 pts). Please answer on
The Use of the ACQUITY QDa Detector for a Selective, Sensitive, and Robust Quantitative Method for a Potential Genotoxic Impurity
 The Use of the ACQUITY QDa Detector for a Selective, Sensitive, and Robust Quantitative Method for a Potential Genotoxic Impurity Janet Hammond Waters Corporation, Wilmslow, UK APPLICATION BENEFITS High
The Use of the ACQUITY QDa Detector for a Selective, Sensitive, and Robust Quantitative Method for a Potential Genotoxic Impurity Janet Hammond Waters Corporation, Wilmslow, UK APPLICATION BENEFITS High
Far UV Absorbance Detector
 Far UV Absorbance Detector Theory Most organic and inorganic species absorb strongly in the far UV. Notable exceptions are the inert gases, helium and nitrogen which absorb very weakly in this region.
Far UV Absorbance Detector Theory Most organic and inorganic species absorb strongly in the far UV. Notable exceptions are the inert gases, helium and nitrogen which absorb very weakly in this region.
LIQUID CHROMATOGRAPHY-MASS SPECTROMETRY (LC/MS) Presented by: Dr. T. Nageswara Rao M.Pharm PhD KTPC
 LIQUID CHROMATOGRAPHY-MASS SPECTROMETRY (LC/MS) Presented by: Dr. T. Nageswara Rao M.Pharm PhD KTPC INTRODUCTION Principle: LC/MS is a technique that combines physical separation capabilities of liquid
LIQUID CHROMATOGRAPHY-MASS SPECTROMETRY (LC/MS) Presented by: Dr. T. Nageswara Rao M.Pharm PhD KTPC INTRODUCTION Principle: LC/MS is a technique that combines physical separation capabilities of liquid
Introduction to Fourier Transform Infrared Spectroscopy
 Introduction to Fourier Transform Infrared Spectroscopy Introduction What is FTIR? FTIR stands for Fourier transform infrared, the preferred method of infrared spectroscopy. In infrared spectroscopy, IR
Introduction to Fourier Transform Infrared Spectroscopy Introduction What is FTIR? FTIR stands for Fourier transform infrared, the preferred method of infrared spectroscopy. In infrared spectroscopy, IR
FTIR Spectrometer. Basic Theory of Infrared Spectrometer. FTIR Spectrometer. FTIR Accessories
 FTIR Spectrometer Basic Theory of Infrared Spectrometer FTIR Spectrometer FTIR Accessories What is Infrared? Infrared radiation lies between the visible and microwave portions of the electromagnetic spectrum.
FTIR Spectrometer Basic Theory of Infrared Spectrometer FTIR Spectrometer FTIR Accessories What is Infrared? Infrared radiation lies between the visible and microwave portions of the electromagnetic spectrum.
Atomic Emission Spectroscopy
 Atomic Emission Spectroscopy Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451 Saudi Arabia Building:
Atomic Emission Spectroscopy Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451 Saudi Arabia Building:
ICP-3000 Inductively Coupled Plasma Optical Emission Spectrometer
 Inductively Coupled Plasma Optical Emission Spectrometer Inductively Coupled Plasma Optical Emission Spectrometer Inductively Coupled Plasma Optical Emission Spectrometer is powerful simultaneous full
Inductively Coupled Plasma Optical Emission Spectrometer Inductively Coupled Plasma Optical Emission Spectrometer Inductively Coupled Plasma Optical Emission Spectrometer is powerful simultaneous full
Hiden CATLAB Systems Microreactor for Catalysis Studies & Thermal Analysis
 Hiden CATLAB Systems Microreactor for Catalysis Studies & Thermal Analysis vacuum analysis surface science gas analysis plasma diagnostics CATLAB overview The Hiden CATLAB is a catalyst characterisation
Hiden CATLAB Systems Microreactor for Catalysis Studies & Thermal Analysis vacuum analysis surface science gas analysis plasma diagnostics CATLAB overview The Hiden CATLAB is a catalyst characterisation
Ch 313 FINAL EXAM OUTLINE Spring 2010
 Ch 313 FINAL EXAM OUTLINE Spring 2010 NOTE: Use this outline at your own risk sometimes a topic is omitted that you are still responsible for. It is meant to be a study aid and is not meant to be a replacement
Ch 313 FINAL EXAM OUTLINE Spring 2010 NOTE: Use this outline at your own risk sometimes a topic is omitted that you are still responsible for. It is meant to be a study aid and is not meant to be a replacement
Chromatography & instrumentation in Organic Chemistry
 Chromatography & instrumentation in Organic Chemistry What is Chromatography? Chromatography is a technique for separating mixtures into their components in order to analyze, identify, purify, and/or quantify
Chromatography & instrumentation in Organic Chemistry What is Chromatography? Chromatography is a technique for separating mixtures into their components in order to analyze, identify, purify, and/or quantify
Welcome to Organic Chemistry II
 Welcome to Organic Chemistry II Erika Bryant, Ph.D. erika.bryant@hccs.edu Class Syllabus 3 CHAPTER 12: STRUCTURE DETERMINATION 4 What is this solution Soda Tea Coffee??? 5 What is this solution Soda Tea
Welcome to Organic Chemistry II Erika Bryant, Ph.D. erika.bryant@hccs.edu Class Syllabus 3 CHAPTER 12: STRUCTURE DETERMINATION 4 What is this solution Soda Tea Coffee??? 5 What is this solution Soda Tea
Period of validity: to date of issue:
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-RM-14176-01-00 according to the relevant clauses of ISO Guide 34: and DIN EN ISO/IEC 17025:2005 Period of validity: 11.01.2016
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-RM-14176-01-00 according to the relevant clauses of ISO Guide 34: and DIN EN ISO/IEC 17025:2005 Period of validity: 11.01.2016
Catalysis CAPABILITIES
 Catalysis www.extrel.com CAPABILITIES Contents Extrel instruments have been recognized for their exceptional performance by the world s leading researchers for more than 50 years. Reliability and flexibility
Catalysis www.extrel.com CAPABILITIES Contents Extrel instruments have been recognized for their exceptional performance by the world s leading researchers for more than 50 years. Reliability and flexibility
Hyphenated micro-ta techniques
 PROCEEDINGS OF THE TWENTY-NINTH CONFERENCE OF THE NORTH AMERICAN THERMAL ANALYSIS SOCIETY, SEPTEMBER 24-26, 2001, ST LOUIS, MISSOURI, U.S.A. Hyphenated micro-ta techniques Duncan M. Price, David B. Grandy,
PROCEEDINGS OF THE TWENTY-NINTH CONFERENCE OF THE NORTH AMERICAN THERMAL ANALYSIS SOCIETY, SEPTEMBER 24-26, 2001, ST LOUIS, MISSOURI, U.S.A. Hyphenated micro-ta techniques Duncan M. Price, David B. Grandy,
Ionization Techniques Part IV
 Ionization Techniques Part IV CU- Boulder CHEM 5181 Mass Spectrometry & Chromatography Presented by Prof. Jose L. Jimenez High Vacuum MS Interpretation Lectures Sample Inlet Ion Source Mass Analyzer Detector
Ionization Techniques Part IV CU- Boulder CHEM 5181 Mass Spectrometry & Chromatography Presented by Prof. Jose L. Jimenez High Vacuum MS Interpretation Lectures Sample Inlet Ion Source Mass Analyzer Detector
Science Drivers. Spectroscopic Sensors. In Situ Sensors. Development of autonomous and remote platforms
 Science Drivers In Situ Sensors Spectroscopic Sensors Development of autonomous and remote platforms ROVs, AUVs Cabled observatories Desire to analyze targets with discrete stability regions in the deep
Science Drivers In Situ Sensors Spectroscopic Sensors Development of autonomous and remote platforms ROVs, AUVs Cabled observatories Desire to analyze targets with discrete stability regions in the deep
Prima PRO Process Mass Spectrometer
 APPLICATION NOTE Prima PRO Process Mass Spectrometer No. xxxx Controlling emissions by multi-component analysis of Volatile Organic Compounds (VOC) Authors: Peter Traynor, Thermo Fisher Scientific, Franklin,
APPLICATION NOTE Prima PRO Process Mass Spectrometer No. xxxx Controlling emissions by multi-component analysis of Volatile Organic Compounds (VOC) Authors: Peter Traynor, Thermo Fisher Scientific, Franklin,
Advanced Spectroscopy Laboratory
 Advanced Spectroscopy Laboratory - Raman Spectroscopy - Emission Spectroscopy - Absorption Spectroscopy - Raman Microscopy - Hyperspectral Imaging Spectroscopy FERGIELAB TM Raman Spectroscopy Absorption
Advanced Spectroscopy Laboratory - Raman Spectroscopy - Emission Spectroscopy - Absorption Spectroscopy - Raman Microscopy - Hyperspectral Imaging Spectroscopy FERGIELAB TM Raman Spectroscopy Absorption
Permanent Gas Analysis by Gas Chromatography Vacuum Ultraviolet Spectroscopy
 Application Note Permanent Gas Analysis by Gas Chromatography Vacuum Ultraviolet Spectroscopy Permanent Gas Analysis by Gas Chromatography Vacuum Ultraviolet Spectroscopy Introduction Gas Chromatography
Application Note Permanent Gas Analysis by Gas Chromatography Vacuum Ultraviolet Spectroscopy Permanent Gas Analysis by Gas Chromatography Vacuum Ultraviolet Spectroscopy Introduction Gas Chromatography
高等食品分析 (Advanced Food Analysis) I. SPECTROSCOPIC METHODS *Instrumental methods: 1. Spectroscopic methods (spectroscopy): a) Electromagnetic radiation
 *Instrumental methods: 1. Spectroscopic methods (spectroscopy): a) Electromagnetic radiation (EMR): γ-ray emission X-Ray absorption, emission, fluorescence and diffraction Vacuum ultraviolet (UV) absorption
*Instrumental methods: 1. Spectroscopic methods (spectroscopy): a) Electromagnetic radiation (EMR): γ-ray emission X-Ray absorption, emission, fluorescence and diffraction Vacuum ultraviolet (UV) absorption
 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING SRM NAGAR, KATTANKULATHUR-603203 EI 2302 ANALYTICAL INSTRUMENTS QUESTION BANK UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. State Lambert
DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING SRM NAGAR, KATTANKULATHUR-603203 EI 2302 ANALYTICAL INSTRUMENTS QUESTION BANK UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. State Lambert
Glove Box / BRAUN MB 150B-G
 Glove Box / BRAUN MB 150B-G Glove box: This box is always filled with N 2 or Ar gas without moisture, which can be perform for the measurement and/or synthetic experiments of air- and/or moisturesensitive
Glove Box / BRAUN MB 150B-G Glove box: This box is always filled with N 2 or Ar gas without moisture, which can be perform for the measurement and/or synthetic experiments of air- and/or moisturesensitive
Gaseous CEMS technology
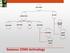 GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
LC-MS Based Metabolomics
 LC-MS Based Metabolomics Analysing the METABOLOME 1. Metabolite Extraction 2. Metabolite detection (with or without separation) 3. Data analysis Metabolite Detection GC-MS: Naturally volatile or made volatile
LC-MS Based Metabolomics Analysing the METABOLOME 1. Metabolite Extraction 2. Metabolite detection (with or without separation) 3. Data analysis Metabolite Detection GC-MS: Naturally volatile or made volatile
CHAPTER 6 GAS CHROMATOGRAPHY
 CHAPTER 6 GAS CHROMATOGRAPHY Expected Outcomes Explain the principles of gas chromatography Able to state the function of each components of GC instrumentation Able to state the applications of GC 6.1
CHAPTER 6 GAS CHROMATOGRAPHY Expected Outcomes Explain the principles of gas chromatography Able to state the function of each components of GC instrumentation Able to state the applications of GC 6.1
novaa 800 D Atomic Absorption Spectrometer
 Technical Data Atomic Absorption Spectrometer Cpt : +27 (0) 21 905 0476 Jhb : +27 (0) 11 794 Dbn : +27 (0) 31 266 2454 1/7 General The is a compact atomic absorption spectrometer with deuterium background
Technical Data Atomic Absorption Spectrometer Cpt : +27 (0) 21 905 0476 Jhb : +27 (0) 11 794 Dbn : +27 (0) 31 266 2454 1/7 General The is a compact atomic absorption spectrometer with deuterium background
Secondary Ion Mass Spectroscopy (SIMS)
 Secondary Ion Mass Spectroscopy (SIMS) Analyzing Inorganic Solids * = under special conditions ** = semiconductors only + = limited number of elements or groups Analyzing Organic Solids * = under special
Secondary Ion Mass Spectroscopy (SIMS) Analyzing Inorganic Solids * = under special conditions ** = semiconductors only + = limited number of elements or groups Analyzing Organic Solids * = under special
4. How can fragmentation be useful in identifying compounds? Permits identification of branching not observed in soft ionization.
 Homework 9: Chapters 20-21 Assigned 12 April; Due 17 April 2006; Quiz on 19 April 2006 Chap. 20 (Molecular Mass Spectroscopy) Chap. 21 (Surface Analysis) 1. What are the types of ion sources in molecular
Homework 9: Chapters 20-21 Assigned 12 April; Due 17 April 2006; Quiz on 19 April 2006 Chap. 20 (Molecular Mass Spectroscopy) Chap. 21 (Surface Analysis) 1. What are the types of ion sources in molecular
Emission spectrum of H
 Atomic Spectroscopy Atomic spectroscopy measures the spectra of elements in their atomic/ionized states. Atomic spectrometry, exploits quantized electronic transitions characteristic of each individual
Atomic Spectroscopy Atomic spectroscopy measures the spectra of elements in their atomic/ionized states. Atomic spectrometry, exploits quantized electronic transitions characteristic of each individual
Introduction to Fourier Transform Infrared Spectroscopy
 molecular spectroscopy Introduction to Fourier Transform Infrared Spectroscopy Part of Thermo Fisher Scientific Introduction What is FT-IR? FT-IR stands for Fourier Transform InfraRed, the preferred method
molecular spectroscopy Introduction to Fourier Transform Infrared Spectroscopy Part of Thermo Fisher Scientific Introduction What is FT-IR? FT-IR stands for Fourier Transform InfraRed, the preferred method
Characterization of petroleum samples via thermal analysis coupled to APCI FTMS
 Characterization of petroleum samples via thermal analysis coupled to APCI FTMS Abstract Thermal analysis, by means of a thermo balance (TG), was coupled to an atmospheric pressure chemical ionization
Characterization of petroleum samples via thermal analysis coupled to APCI FTMS Abstract Thermal analysis, by means of a thermo balance (TG), was coupled to an atmospheric pressure chemical ionization
Lecture 8: Mass Spectrometry
 intensity Lecture 8: Mass Spectrometry Relative abundance m/z 1 Ethylbenzene experiment CH 2 CH 3 + m/z = 106 CH 2 + m/z = 91 C 8 H 10 MW = 106 CH + m/z = 77 + 2 2 What information can we get from MS spectrum?
intensity Lecture 8: Mass Spectrometry Relative abundance m/z 1 Ethylbenzene experiment CH 2 CH 3 + m/z = 106 CH 2 + m/z = 91 C 8 H 10 MW = 106 CH + m/z = 77 + 2 2 What information can we get from MS spectrum?
atomic absorption spectroscopy general can be portable and used in-situ preserves sample simpler and less expensive
 Chapter 9: End-of-Chapter Solutions 1. The following comparison provides general trends, but both atomic absorption spectroscopy (AAS) and atomic absorption spectroscopy (AES) will have analyte-specific
Chapter 9: End-of-Chapter Solutions 1. The following comparison provides general trends, but both atomic absorption spectroscopy (AAS) and atomic absorption spectroscopy (AES) will have analyte-specific
Cerno Bioscience MassWorks: Acquiring Calibration Data on Agilent GC/MSDs
 Cerno Bioscience MassWorks: Acquiring Calibration Data on Agilent GC/MSDs Application Note Author Harry Prest, Ph.D. Senior Chemist in GC-MS Agilent Technologies, Inc. 5301 Stevens Creek Blvd Santa Clara,
Cerno Bioscience MassWorks: Acquiring Calibration Data on Agilent GC/MSDs Application Note Author Harry Prest, Ph.D. Senior Chemist in GC-MS Agilent Technologies, Inc. 5301 Stevens Creek Blvd Santa Clara,
Complete Materials Deformulation Using TGA-IR
 Application Note: 51694 Complete Materials Deformulation Using TGA-IR Michael Bradley, Thermo Fisher Scientific, Madison, WI, USA Key Words Feedstock Supplies Finished Products Multi-component Search OMNIC
Application Note: 51694 Complete Materials Deformulation Using TGA-IR Michael Bradley, Thermo Fisher Scientific, Madison, WI, USA Key Words Feedstock Supplies Finished Products Multi-component Search OMNIC
ATOMIC ABSORPTION SPECTROSCOPY (AAS) is an analytical technique that measures the concentrations of elements. It makes use of the absorption of light
 ATOMIC ABSORPTION SPECTROSCOPY (AAS) is an analytical technique that measures the concentrations of elements. It makes use of the absorption of light by these elements in order to measure their concentration.
ATOMIC ABSORPTION SPECTROSCOPY (AAS) is an analytical technique that measures the concentrations of elements. It makes use of the absorption of light by these elements in order to measure their concentration.
Analytical techniques: Environmental samples. Lecture 2 Universidade do Algarve
 Analytical techniques: Environmental samples Lecture 2 Universidade do Algarve Terms, definitions & applications Difference between technique and method: Analytical technique: Fundamental scientific application
Analytical techniques: Environmental samples Lecture 2 Universidade do Algarve Terms, definitions & applications Difference between technique and method: Analytical technique: Fundamental scientific application
Lecture 8: Mass Spectrometry
 intensity Lecture 8: Mass Spectrometry Relative abundance m/z 1 Ethylbenzene CH 2 CH 3 + m/z = 106 CH 2 + m/z = 91 C 8 H 10 MW = 106 CH + m/z = 77 + 2 2 What information can be obtained from a MS spectrum?
intensity Lecture 8: Mass Spectrometry Relative abundance m/z 1 Ethylbenzene CH 2 CH 3 + m/z = 106 CH 2 + m/z = 91 C 8 H 10 MW = 106 CH + m/z = 77 + 2 2 What information can be obtained from a MS spectrum?
Mass Spectrometry in MCAL
 Mass Spectrometry in MCAL Two systems: GC-MS, LC-MS GC seperates small, volatile, non-polar material MS is detection devise (Agilent 320-MS TQ Mass Spectrometer) Full scan monitoring SIM single ion monitoring
Mass Spectrometry in MCAL Two systems: GC-MS, LC-MS GC seperates small, volatile, non-polar material MS is detection devise (Agilent 320-MS TQ Mass Spectrometer) Full scan monitoring SIM single ion monitoring
Analysis of Polar Metabolites using Mass Spectrometry
 Analysis of Polar Metabolites using Mass Spectrometry TransMed Course: Basics in Clinical Proteomics and Metabolomics. Oct 10-19, 2012 dd.mm.yyyy Vidya Velagapudi, Ph.D, Adjunct Professor Head of the Metabolomics
Analysis of Polar Metabolites using Mass Spectrometry TransMed Course: Basics in Clinical Proteomics and Metabolomics. Oct 10-19, 2012 dd.mm.yyyy Vidya Velagapudi, Ph.D, Adjunct Professor Head of the Metabolomics
Collected Applications Thermal Analysis EVOLVED GAS ANALYSIS
 Collected Applications Thermal Analysis EVOLVED GAS ANALYSIS Preface Over the past decades, the development and characterization of materials has become increasingly specialized due to the ever-increasing
Collected Applications Thermal Analysis EVOLVED GAS ANALYSIS Preface Over the past decades, the development and characterization of materials has become increasingly specialized due to the ever-increasing
Chromatography. Gas Chromatography
 Chromatography Chromatography is essentially the separation of a mixture into its component parts for qualitative and quantitative analysis. The basis of separation is the partitioning of the analyte mixture
Chromatography Chromatography is essentially the separation of a mixture into its component parts for qualitative and quantitative analysis. The basis of separation is the partitioning of the analyte mixture
Final Report. Characterisation of Sample Report. Job No 2016/11/12-34 AS No. 1234A. Client Example Contact Sample. Signed Date 2017.
 Final Report Title Characterisation of Job No 2016/11/12-34 AS No. 1234A Client Contact Sample Author report Signed Date 2017 Easy Reach Report 2017 v2.docx 1 of 33 Contents 1. Study Summary Page 3 2.
Final Report Title Characterisation of Job No 2016/11/12-34 AS No. 1234A Client Contact Sample Author report Signed Date 2017 Easy Reach Report 2017 v2.docx 1 of 33 Contents 1. Study Summary Page 3 2.
Atomization. In Flame Emission
 FLAME SPECTROSCOPY The concentration of an element in a solution is determined by measuring the absorption, emission or fluorescence of electromagnetic by its monatomic particles in gaseous state in the
FLAME SPECTROSCOPY The concentration of an element in a solution is determined by measuring the absorption, emission or fluorescence of electromagnetic by its monatomic particles in gaseous state in the
