Providing the highest quality products thus giving maximum satisfaction to all
|
|
|
- Penelope Riley
- 5 years ago
- Views:
Transcription
1 ACIDS
2 INTRODUCTION The laboratory reagents offered by Labscan, are characterized by the highest quality improved for over 60 years of the company existence. In order to provide high quality of the offered assortment, our products are subject to unique technological regime, both during manufacturing and filling. These processes conform to ISO 9001 Quality System Certificate and ISO Environment Management System. QUALITY POLICY Quality Providing the highest quality products thus giving maximum satisfaction to all Customers of the Company owing to utilization of products is the basic task executed in the Company. All products offered to the customers are submitted to complete control before approving them for trade turnover. Conformity with the standards and requirements of our Customers is controlled. The Company runs continuous verification of suppliers who cooperate with it. The production processes are monitored. Quality assurance, to the large extent, is obtained owing to qualified personnel of the Company. We understand quality as a value that our Customers expected from us. The most important tool to reach this assumption is the implemented Quality Assurance System, certified for the first time in The system, amended according to the guidelines of ISO 9001:2000 Standard, is maintained and permanently improved. This facts is acknowledged by audits accomplished by the certifying body and by Customers as well as the Certificate obtained several times. In order to meet expectations of our Customers, based on the Quality Management System, the Internal Control System has been implemented and certified, which applies to sale of goods of strategic meaning. 2
3 Environment Our company, as a manufacturer and provider of chemical reagents and purified chemicals, striving to balance the development and appreciating the meaning of ecological issues, has implemented the Environmental Management System, which conforms to the requirements of ISO Standard. All decisions made within our business activities consider environmental issues. Observing legal requirements related with the environment has been assumed as the minimum requirement. Our Company runs all production processes according to the environment protection regulations. Thus, our production departments utilize many devices reducing pollution, which might penetrate to air, soil and sewage. By-products, which due to their composition are of technological value, after preliminary processing are returned to production cycle, other ones are utilized to a form which do not pose hazard to the environment. 3
4 Safety at work In POCH S. A., a company that manufactures chemicals, importance is attached to provide our employees with proper working conditions. In order to do that, we implement the Safety at Work Management System, which conforms to the guidelines of PN-N Standard. 4
5 ACIDS When talking about acids, we think about their aqueous solutions. There are a few theories related with acids. According to Arrhenius - they are compounds, which in water reactions dissociate to oxonium cation and acid radical anion. Acids divide into oxyacids and hydracids as well as to strong ones, which dissociate completely in water solutions, for example, HCl and weak ones, which do not completely dissociate in water solutions, for example, H PO
6 ACIDS P.A. (SELECTED PRODUCTS) QUALITY SPECIFICATIONS (SELECTED PARAMETERS) Product number Product name Appearance Assay [%] Residue on ignition [%] PLA00575X 5-SULFOSALICYLIC ACID DIHYDRATE P.A. - min PLA00652X ACETIC ACID 80% P.A. colourless, clear min. 78,5 max PLA00654X ACETIC ACID MIN. 99,5% P.A. colourless, clear min. 99,5 - PLA00590X BORIC ACID P.A. colourless crystals or white, crystalline powder min. 99,5 - PLA00601X CITRIC ACID MONOHYDRATE P.A. fine, colourless crystals or white powder min. 99,5 max. 0,005 PLA00702X HYDROCHLORIC ACID 37% P.A. colourless, clear min. 37 max. 0,001 PLA00698X HYDROCHLORIC ACID 35-38% P.A. colourless, clear min. 35 max. 38 max. 0,001 PLA00609X HYDROFLUORIC ACID 40% P.A. colourless, clear min. 39 max. 41 max. 0,005 PLA00631X L(+)-ASCORBIC ACID (ACS,PH EUR) P.A. white or nearly white, crystalline powder or colourless crystals darkened on light min. 99 max. 100 max. 0,1 PLA00630X L(+)-TARTARIC ACID (ACS) P.A. colourless crystals min. 99,5 max. 0,01 PLA00586X NITRIC ACID 65% P.A. colourless or slightly yellow, clear min. 65 max. 0,002 PLA00660X ORTHO-PHOSPHORIC ACID 85% P.A. colourless, clear, syrupy min. 84,5 max PLA00706X OXALIC ACID DIHYDRATE (ACS) P.A. colourless crystals min. 99,5 max. 0,01 PLA00647X PERCHLORIC ACID 60% P.A. - min PLA00616X PHTHALIC ACID P.A. white, crystalline powder min. 99,5 max. 0,01 PLA00687X SULFURIC ACID MIN.95% P.A. colourless, clear, oily min. 95 max. 0,001 PLA00708X TRICHLORACETIC ACID P.A. - min. 98-6
7 ACIDS PURE (SELECTED PRODUCTS) QUALITY SPECIFICATIONS (SELECTED PARAMETERS) Product number Product name Appearance Assay [%] Residue on ignition [%] PLG00652X ACETIC ACID 80% PURE colourless, clear min. 78,5 max PLG00590X BORIC ACID PURE colourless crystals or white, crystalline powder min PLG00607X FLUOROBORIC ACID 40% PURE colourless, clear min. 38 max 42 - PLG01802X HYDROCHLORIC ACID 18% PURE colourless or lightyellow, clear min. 18 max. 0,002 PLG00698X HYDROCHLORIC ACID 35-38% PURE colourless, clear min. 35 max. 38 max. 0,002 PLG00586X NITRIC ACID 65% PURE colourless or slightly yellow, clear min. 65 max. 0,005 PLG00660X ORTHO- PHOSPHORIC ACID 85% PURE colourless, clear, syrupy min. 84,5 - PLG00616X PHTHALIC ACID PURE white, crystallime powder min. 99 max. 0,02 PLG00682X SULFURIC ACID 69% PURE colourless, oily min. 68 max. 70 max. 0,005 PLG00683X SULFURIC ACID 91% PURE colourless, clear min. 90 max. 92 max. 0,005 PLG00687X SULFURIC ACID MIN.95% PURE colourless, clear, oily min. 95 max. 0,005 7
8
CONCENTRATED VOLUMETRIC & VOLUMETRIC SOLUTIONS
 CONCENTRATED VOLUMETRIC & S INTRODUCTION The laboratory reagents offered by Labscan, are characterized by the highest quality improved for over 60 years of the company existence. In order to provide high
CONCENTRATED VOLUMETRIC & S INTRODUCTION The laboratory reagents offered by Labscan, are characterized by the highest quality improved for over 60 years of the company existence. In order to provide high
IBIDEN Group Green Procurement Guidelines. (Version 6)
 IBIDEN Group Green Procurement Guidelines (Version 6) October 1, 2017 [Table of Contents] 1. Introduction P3 2. IBIDEN Group s Basic Policy for the Environment P4 3. Objective of the Guideline P5 4. Definitions
IBIDEN Group Green Procurement Guidelines (Version 6) October 1, 2017 [Table of Contents] 1. Introduction P3 2. IBIDEN Group s Basic Policy for the Environment P4 3. Objective of the Guideline P5 4. Definitions
7 Product-related Environmental Activities
 Contents 7.1 Our Policy 7-1 7.1.1 Environmental Statement 7-1 7.1.2 Environmental Vision 7-1 7.2 Reduction of Chemical Substances in Products 7-2 7.2.1 Product development with maximum efforts not to use
Contents 7.1 Our Policy 7-1 7.1.1 Environmental Statement 7-1 7.1.2 Environmental Vision 7-1 7.2 Reduction of Chemical Substances in Products 7-2 7.2.1 Product development with maximum efforts not to use
High purity acids and bases
 High purity acids and bases acids and bases Ultrapur acids The life science business of Merck KGaA, Darmstadt, Germany operates as MilliporeSigma in the U.S. and Canada. High purity acids and bases, and
High purity acids and bases acids and bases Ultrapur acids The life science business of Merck KGaA, Darmstadt, Germany operates as MilliporeSigma in the U.S. and Canada. High purity acids and bases, and
3/27/2015. So the question that arises is, how can you tell the difference between an ionic solution and a solution containing a molecular acid?
 A neat thing about chemistry is that there are exceptions to most rules. Previously, we learned that ionic compounds form electrolytic solutions but molecular compounds do not form electrolytic solutions.
A neat thing about chemistry is that there are exceptions to most rules. Previously, we learned that ionic compounds form electrolytic solutions but molecular compounds do not form electrolytic solutions.
Chapter 14: Acids and Bases
 Chapter 14: Acids and Bases Properties of Acids and Bases What is an acid? Some examples of common items containing acids: Vinegar contains acetic acid; lemons and citrus fruits contain citric acid; many
Chapter 14: Acids and Bases Properties of Acids and Bases What is an acid? Some examples of common items containing acids: Vinegar contains acetic acid; lemons and citrus fruits contain citric acid; many
Hach Method Total Organic Carbon in Finished Drinking Water by Catalyzed Ozone Hydroxyl Radical Oxidation Infrared Analysis
 Hach Method 1061 Total Organic Carbon in Finished Drinking Water by Catalyzed Ozone Hydroxyl Radical Oxidation Infrared Analysis Hach Company Method 1061 Revision 1. December 015 Organic Carbon in Finished
Hach Method 1061 Total Organic Carbon in Finished Drinking Water by Catalyzed Ozone Hydroxyl Radical Oxidation Infrared Analysis Hach Company Method 1061 Revision 1. December 015 Organic Carbon in Finished
11/15/11. Chapter 16. HA(aq) + H 2 O(l) H 3 O + (aq) + A (aq) acid base conjugate conjugate
 Chapter 16 Table of Contents Chapter 16 16.1 16.2 16.3 16.4 16.5 16.6 Buffered Solutions Copyright Cengage Learning. All rights reserved 2 Models of Arrhenius: Acids produce H + ions in solution, bases
Chapter 16 Table of Contents Chapter 16 16.1 16.2 16.3 16.4 16.5 16.6 Buffered Solutions Copyright Cengage Learning. All rights reserved 2 Models of Arrhenius: Acids produce H + ions in solution, bases
Cu 2+ (aq) + 4NH 3(aq) = Cu(NH 3) 4 2+ (aq) I (aq) + I 2(aq) = I 3 (aq) Fe 3+ (aq) + 6H 2O(l) = Fe(H 2O) 6 3+ (aq) Strong acids
 There are three definitions for acids and bases we will need to understand. Arrhenius Concept: an acid supplies H + to an aqueous solution. A base supplies OH to an aqueous solution. This is the oldest
There are three definitions for acids and bases we will need to understand. Arrhenius Concept: an acid supplies H + to an aqueous solution. A base supplies OH to an aqueous solution. This is the oldest
Chapter 16. Acids and Bases. Copyright Cengage Learning. All rights reserved 1
 Chapter 16 Acids and Bases Copyright Cengage Learning. All rights reserved 1 Section 16.1 Acids and Bases Models of Acids and Bases Arrhenius: Acids produce H + ions in solution, bases produce OH ions.
Chapter 16 Acids and Bases Copyright Cengage Learning. All rights reserved 1 Section 16.1 Acids and Bases Models of Acids and Bases Arrhenius: Acids produce H + ions in solution, bases produce OH ions.
Control Standard for Handling Chemical Substances in Products, Parts and Materials
 Control Standard for Handling Chemical Substances in Products, Parts and Materials (for Suppliers) The 9 th Edition Hitachi Maxell, Ltd. 1 Contents 1. Purpose...3 2. Scope... 3 3. Terms and definitions..3
Control Standard for Handling Chemical Substances in Products, Parts and Materials (for Suppliers) The 9 th Edition Hitachi Maxell, Ltd. 1 Contents 1. Purpose...3 2. Scope... 3 3. Terms and definitions..3
Acids and bases, as we use them in the lab, are usually aqueous solutions. Ex: when we talk about hydrochloric acid, it is actually hydrogen chloride
 Acids and Bases Acids and bases, as we use them in the lab, are usually aqueous solutions. Ex: when we talk about hydrochloric acid, it is actually hydrogen chloride gas dissolved in water HCl (aq) Concentrated
Acids and Bases Acids and bases, as we use them in the lab, are usually aqueous solutions. Ex: when we talk about hydrochloric acid, it is actually hydrogen chloride gas dissolved in water HCl (aq) Concentrated
Latest Progress of Chemical Management Policies in China
 Latest Progress of Chemical Management Policies in China Division of Chemicals Environmental Management Department of Soil Environment Management MEE, China 2018.6.23 1 Chemicals Environmental Management
Latest Progress of Chemical Management Policies in China Division of Chemicals Environmental Management Department of Soil Environment Management MEE, China 2018.6.23 1 Chemicals Environmental Management
ACIDS BINARY ACIDS. dissolves glass! hydrofluoric acid. most common binary acid! hydrochloric acid. hydrobromic acid.
 94 BINARY ACIDS ACIDS - named after the element (other than hydrogen) they contain - common binary acids include a Group VIIA element - named: "Hydro-" + STEM NAME OF ELEMENT+ "-ic acid" Four common binary
94 BINARY ACIDS ACIDS - named after the element (other than hydrogen) they contain - common binary acids include a Group VIIA element - named: "Hydro-" + STEM NAME OF ELEMENT+ "-ic acid" Four common binary
REACTIONS OF ACIDS. J:\Science\Chemistry\Stage 1 Notes\Acids & Bases\Reactionsofacids.doc
 REACTIONS OF ACIDS 1. Acids taste sour We do not attempt to taste strong acids as they are too dangerous. They do taste sour, but then they proceed to destroy cells on your tongue and mouth. If you vomit,
REACTIONS OF ACIDS 1. Acids taste sour We do not attempt to taste strong acids as they are too dangerous. They do taste sour, but then they proceed to destroy cells on your tongue and mouth. If you vomit,
Acid/Base Definitions
 Acids and Bases Acid/Base Definitions Arrhenius Model Acids produce hydrogen ions in aqueous solutions Bases produce hydroxide ions in aqueous solutions Bronsted-Lowry Model Acids are proton donors Bases
Acids and Bases Acid/Base Definitions Arrhenius Model Acids produce hydrogen ions in aqueous solutions Bases produce hydroxide ions in aqueous solutions Bronsted-Lowry Model Acids are proton donors Bases
Acknowledgment of Aramco Asia. Supplier Code of Conduct
 Acknowledgment of Aramco Asia Supplier Code of Conduct (Applicable to Vendors, Manufacturers, and Contractors) Aramco Asia is committed to the highest ethical and legal standards in the conduct of its
Acknowledgment of Aramco Asia Supplier Code of Conduct (Applicable to Vendors, Manufacturers, and Contractors) Aramco Asia is committed to the highest ethical and legal standards in the conduct of its
O + (aq) In this reaction, the water molecule is a Brønsted-Lowry base. It accepts a proton from HF to form H 3
 AcidBase Reactions Key Terms conjugate base conjugate acid amphoteric neutralization salt In the previous sections, you learned about three acidbase theories: Arrhenius, BrønstedLowry, and Lewis. The BrønstedLowry
AcidBase Reactions Key Terms conjugate base conjugate acid amphoteric neutralization salt In the previous sections, you learned about three acidbase theories: Arrhenius, BrønstedLowry, and Lewis. The BrønstedLowry
Chapter 14. Objectives
 Section 1 Properties of Acids and Bases Objectives List five general properties of aqueous acids and bases. Name common binary acids and oxyacids, given their chemical formulas. List five acids commonly
Section 1 Properties of Acids and Bases Objectives List five general properties of aqueous acids and bases. Name common binary acids and oxyacids, given their chemical formulas. List five acids commonly
Compounds in Aqueous Solution
 1 Compounds in Aqueous Solution Many reactions involve ionic compounds, especially reactions in water KMnO 4 in water K + (aq) ) + MnO 4- (aq) 2 CCR, page 149 3 How do we know ions are present in aqueous
1 Compounds in Aqueous Solution Many reactions involve ionic compounds, especially reactions in water KMnO 4 in water K + (aq) ) + MnO 4- (aq) 2 CCR, page 149 3 How do we know ions are present in aqueous
OWL Assignment #2 Study Sheet
 OWL Assignment #2 Study Sheet Binary Acid Nomenclature Binary compounds are composed of two elements. When one of the elements is a binary acid can be formed. Examples of this are HCl or H 2 S. When put
OWL Assignment #2 Study Sheet Binary Acid Nomenclature Binary compounds are composed of two elements. When one of the elements is a binary acid can be formed. Examples of this are HCl or H 2 S. When put
Chemical Inventory. Each area must maintain a complete, accurate and up to date chemical inventory. The inventory should include: All Chemicals
 Hazardous Materials Chemical Inventory Each area must maintain a complete, accurate and up to date chemical inventory. The inventory should include: All Chemicals Hazardous Non-hazardous Compressed Gasses
Hazardous Materials Chemical Inventory Each area must maintain a complete, accurate and up to date chemical inventory. The inventory should include: All Chemicals Hazardous Non-hazardous Compressed Gasses
INTRODUCTION TO ACIDS AND BASES
 INTRODUCTION TO ACIDS AND BASES ALIGNED STANDARDS S.C. 912.P.8.11 Relate acidity and basicity to hydronium and hydroxide concentration and ph. S.C.912.N.1.2 Describe and explain what characterizes science
INTRODUCTION TO ACIDS AND BASES ALIGNED STANDARDS S.C. 912.P.8.11 Relate acidity and basicity to hydronium and hydroxide concentration and ph. S.C.912.N.1.2 Describe and explain what characterizes science
SAMPLE PAGES. Hazard Communication Program. [Company name]
![SAMPLE PAGES. Hazard Communication Program. [Company name] SAMPLE PAGES. Hazard Communication Program. [Company name]](/thumbs/95/125522244.jpg) The safety and health of our employees are our top priority. Everyone goes home safe and healthy everyday. Hazard Communication Program [Company name] [Date Authorized] [Version} Page 0 Table of Contents
The safety and health of our employees are our top priority. Everyone goes home safe and healthy everyday. Hazard Communication Program [Company name] [Date Authorized] [Version} Page 0 Table of Contents
Acids, Bases and ph Chapter 19
 Acids, Bases and ph Chapter 19 Compounds That Become Acids When Dissolved in Water General Formula: HX H + X - monatomic or polyatomic anion Naming Acids (p. 250) Binary acids Hydro ic Acid HCl: Hydrochloric
Acids, Bases and ph Chapter 19 Compounds That Become Acids When Dissolved in Water General Formula: HX H + X - monatomic or polyatomic anion Naming Acids (p. 250) Binary acids Hydro ic Acid HCl: Hydrochloric
ph Calibration Buffers
 ph Calibration Buffers Uncompromising Quality 3 Manufacturing/ Inventory Locations Tightest Tolerances in the Industry, Lowest Lot-to-Lot Variability Ricca has been the leading name in high quality Buffers
ph Calibration Buffers Uncompromising Quality 3 Manufacturing/ Inventory Locations Tightest Tolerances in the Industry, Lowest Lot-to-Lot Variability Ricca has been the leading name in high quality Buffers
August 12, Docket OSHA ; Comments on Review of Process Safety Management (PSM) Standard Hydrochloric and Hydrofluoric Acids
 K E L L E Y D R Y E & W AR R E N LLP A LI MIT E D LIA BI LIT Y P ART N ER SHI P N E W Y O R K, NY L O S A N G E L E S, CA C H I C A G O, IL S T A M F O R D, CT P A R S I P P A N Y, NJ WASHINGTON HARBOUR,
K E L L E Y D R Y E & W AR R E N LLP A LI MIT E D LIA BI LIT Y P ART N ER SHI P N E W Y O R K, NY L O S A N G E L E S, CA C H I C A G O, IL S T A M F O R D, CT P A R S I P P A N Y, NJ WASHINGTON HARBOUR,
Acid/Base Theories The common characteristics of acids
 Acid/Base Theories The common characteristics of acids describe them as: Acids aving a sour taste Being electrolytes (some weak) Reacting with metals to produce gas (usually 2 ) Reacting with bases to
Acid/Base Theories The common characteristics of acids describe them as: Acids aving a sour taste Being electrolytes (some weak) Reacting with metals to produce gas (usually 2 ) Reacting with bases to
9.4 Naming and Writing Formulas for Acids and Bases. What s the name of the acid responsible for the crisp taste in this drink?
 CHEMISTRY & YOU What s the name of the acid responsible for the crisp taste in this drink? There s a certain acid that gives many soft drinks their crisp, enjoyable taste. 1 How do you determine the name
CHEMISTRY & YOU What s the name of the acid responsible for the crisp taste in this drink? There s a certain acid that gives many soft drinks their crisp, enjoyable taste. 1 How do you determine the name
10.1 Acids and Bases in Aqueous Solution
 10.1 Acids and Bases in Aqueous Solution Arrhenius Definition of Acids and Bases An acid is a substance that gives hydrogen ions, H +, when dissolved in water. In fact, H + reacts with water and produces
10.1 Acids and Bases in Aqueous Solution Arrhenius Definition of Acids and Bases An acid is a substance that gives hydrogen ions, H +, when dissolved in water. In fact, H + reacts with water and produces
Food Safety Management System
 Introduction The company has planned, established, documented and implemented a food safety management system for the site, in order to continually improve its effectiveness in accordance with legislation,
Introduction The company has planned, established, documented and implemented a food safety management system for the site, in order to continually improve its effectiveness in accordance with legislation,
Unit 9: Acids and Bases Chapter 19
 Unit 9: Acids and Bases Chapter 19 I. Introduction In aqueous solutions, the solvent is. Aqueous solutions contain. In the self-ionization of water, the hydrogen ion (H+) exists in solution as the ion.
Unit 9: Acids and Bases Chapter 19 I. Introduction In aqueous solutions, the solvent is. Aqueous solutions contain. In the self-ionization of water, the hydrogen ion (H+) exists in solution as the ion.
AP Study Questions
 ID: A AP 16.4-16.7 Study Questions Multiple Choice Identify the choice that best completes the statement or answers the question. 1 What is the ph of an aqueous solution at 25.0 C in which [H + ] is 0.0025
ID: A AP 16.4-16.7 Study Questions Multiple Choice Identify the choice that best completes the statement or answers the question. 1 What is the ph of an aqueous solution at 25.0 C in which [H + ] is 0.0025
Chapters 15 & 16 ACIDS & BASES ph & Titrations
 PROPERTIES OF ACIDS Chapters 15 & 16 ACIDS & BASES ph & Titrations There are 5 main properties of acids: 1. sour taste 2. change the color of acidbase indicators 3. react with metals to produce H2 gas
PROPERTIES OF ACIDS Chapters 15 & 16 ACIDS & BASES ph & Titrations There are 5 main properties of acids: 1. sour taste 2. change the color of acidbase indicators 3. react with metals to produce H2 gas
Chemical Formulas & Chemical Compounds. Chemical formula indicates the relative number of atoms of each kind in a chemical compound.
 Unit 6: Chemical Formulas & Chemical Compounds Chemical Names & Formulas Chemical formula indicates the relative number of atoms of each kind in a chemical compound. Molecular compound - it s formula reveals
Unit 6: Chemical Formulas & Chemical Compounds Chemical Names & Formulas Chemical formula indicates the relative number of atoms of each kind in a chemical compound. Molecular compound - it s formula reveals
CHEMICAL PROTECTIVE GLOVES. 2 Manufacturer s name and address and legal representative (surname, first name):
 Testing and Certification of Personal Protective Equipment Category III (PPE) CHEMICAL PROTECTIVE GLOVES ATTACHMENT 1 to the order of placed with Institut für Arbeitsschutz der DGUV (IFA) Alte Heerstrasse
Testing and Certification of Personal Protective Equipment Category III (PPE) CHEMICAL PROTECTIVE GLOVES ATTACHMENT 1 to the order of placed with Institut für Arbeitsschutz der DGUV (IFA) Alte Heerstrasse
23 carbon atoms The number is known as Avogadro s d Number.
 THE MOLE (a counting unit).again! i A mole represents a set or group, much in the same way that a dozen represents a set of twelve. 1 dozen eggs = 12 eggs; 1 mol eggs = 6.022 10 23 eggs 1 dozen carbon
THE MOLE (a counting unit).again! i A mole represents a set or group, much in the same way that a dozen represents a set of twelve. 1 dozen eggs = 12 eggs; 1 mol eggs = 6.022 10 23 eggs 1 dozen carbon
TEAC Green Procurement Guideline
 Page 1 of 5 TEAC Green Procurement Guideline ES0101 3.0 edition October 1, 2010 TEAC Corp. (teaceco@teac.co.jp) Table of contents 1. Application...2 2. Purpose...2 3. Definition of terms...2 4. Ranks for
Page 1 of 5 TEAC Green Procurement Guideline ES0101 3.0 edition October 1, 2010 TEAC Corp. (teaceco@teac.co.jp) Table of contents 1. Application...2 2. Purpose...2 3. Definition of terms...2 4. Ranks for
Chapter 6. Acids, Bases, and Acid-Base Reactions
 Chapter 6 Acids, Bases, and Acid-Base Reactions Chapter Map Arrhenius Acid Definition Anacid is a substance that generates hydronium ions, H 3 O + (often described as H + ), when added to water. An acidic
Chapter 6 Acids, Bases, and Acid-Base Reactions Chapter Map Arrhenius Acid Definition Anacid is a substance that generates hydronium ions, H 3 O + (often described as H + ), when added to water. An acidic
SI session Grue 207A
 Chem 105 Wednesday 21 Sept 2011 1. Precipitation and Solubility 2. Solubility Rules 3. Precipitation reaction equations 4. Net ionic equations 5. OWL 6. Acids and bases SI session Grue 207A TR, 12:001:30
Chem 105 Wednesday 21 Sept 2011 1. Precipitation and Solubility 2. Solubility Rules 3. Precipitation reaction equations 4. Net ionic equations 5. OWL 6. Acids and bases SI session Grue 207A TR, 12:001:30
Titrasure standards and volumetric solutions...for guaranteed results
 Titrasure standards and volumetric solutions...for guaranteed results and Titrasure standards Titration is a high-precision analytical method that requires titrants of accurately known concentration. Scharlau
Titrasure standards and volumetric solutions...for guaranteed results and Titrasure standards Titration is a high-precision analytical method that requires titrants of accurately known concentration. Scharlau
DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME
 DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME - The name of an ionic compound is made of the names of the CATION and ANION in the compound. - To get the FORMULA, you must figure out the SMALLEST
DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME - The name of an ionic compound is made of the names of the CATION and ANION in the compound. - To get the FORMULA, you must figure out the SMALLEST
Chapter 7 Acids and Bases
 Chapter 7 Acids and Bases 7.1 The Nature of Acids and Bases 7.2 Acid Strength 7.3 The ph Scale 7.4 Calculating the ph of Strong Acid Solutions 7.5 Calculating the ph of Weak Acid Solutions 7.6 Bases 7.7
Chapter 7 Acids and Bases 7.1 The Nature of Acids and Bases 7.2 Acid Strength 7.3 The ph Scale 7.4 Calculating the ph of Strong Acid Solutions 7.5 Calculating the ph of Weak Acid Solutions 7.6 Bases 7.7
Mass Relationships in Chemical Reactions. Chapter 3 Chang & Goldsby Modified by Dr. Juliet Hahn
 Mass Relationships in Chemical Reactions Chapter 3 Chang & Goldsby Modified by Dr. Juliet Hahn Example 3.6 (3) We now write, 6.07 g CH 4 1 mol CH 4 16.04 g CH 4 = 0.378 mol CH 4 Thus, there is 0.378 mole
Mass Relationships in Chemical Reactions Chapter 3 Chang & Goldsby Modified by Dr. Juliet Hahn Example 3.6 (3) We now write, 6.07 g CH 4 1 mol CH 4 16.04 g CH 4 = 0.378 mol CH 4 Thus, there is 0.378 mole
Basic Digestion Principles
 Basic Digestion Principles 1 From Samples to Solutions Direct Analytical Method Solid Sample Problems: Mech. Sample Preparation (Grinding, Sieving, Weighing, Pressing, Polishing,...) Solid Sample Autosampler
Basic Digestion Principles 1 From Samples to Solutions Direct Analytical Method Solid Sample Problems: Mech. Sample Preparation (Grinding, Sieving, Weighing, Pressing, Polishing,...) Solid Sample Autosampler
Food Safety and Quality Management System
 Introduction The company has planned, established, documented and implemented a food safety and quality management system for the site, which is maintained in order to continually improve its effectiveness
Introduction The company has planned, established, documented and implemented a food safety and quality management system for the site, which is maintained in order to continually improve its effectiveness
Answers to Unit 6, Lesson 01: Review of Acids and Bases. A substance that dissolves in water to produce H+ ions
 Answers to Unit 6, Lesson 01: Review of Acids and Bases Property Acids Bases Arrhenius Definition How to recognize from a chemical formula A substance that dissolves in water to produce H+ ions the first
Answers to Unit 6, Lesson 01: Review of Acids and Bases Property Acids Bases Arrhenius Definition How to recognize from a chemical formula A substance that dissolves in water to produce H+ ions the first
NAMING IONIC COMPOUNDS. ammonium sulfide. iron(ii) carbonate. barium phosphate. titanium(iv) sulfide. Spelling matters!
 67 NAMING IONIC COMPOUNDS ammonium sulfide iron(ii) carbonate titanium(iv) sulfide barium phosphate Spelling matters! barium phosphide 68 DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME - The
67 NAMING IONIC COMPOUNDS ammonium sulfide iron(ii) carbonate titanium(iv) sulfide barium phosphate Spelling matters! barium phosphide 68 DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME - The
Public Disclosure Copy. Implementation Status & Results Report Strengthening Institutional Capacity for Government Effectiveness Project (P149176)
 Public Disclosure Authorized AFRICA Burundi Governance Global Practice IBRD/IDA Investment Project Financing FY 2015 Seq No: 4 ARCHIVED on 10-Jul-2017 ISR28718 Implementing Agencies: Government of Burundi,
Public Disclosure Authorized AFRICA Burundi Governance Global Practice IBRD/IDA Investment Project Financing FY 2015 Seq No: 4 ARCHIVED on 10-Jul-2017 ISR28718 Implementing Agencies: Government of Burundi,
Hach Company TNTplus TM Phosphorus Spectrophotometric Measurement of Phosphorus in Water and Wastewater
 Hach Company TNTplus TM Phosphorus Spectrophotometric Measurement of Phosphorus in Water and Wastewater Hach Company TNTplus TM Phosphorus Method 10209/10210/843/844/845 November 2015 Revison 2 Spectrophotometric
Hach Company TNTplus TM Phosphorus Spectrophotometric Measurement of Phosphorus in Water and Wastewater Hach Company TNTplus TM Phosphorus Method 10209/10210/843/844/845 November 2015 Revison 2 Spectrophotometric
Solutions, Acids, & Bases Unit 6 - IB Material
 Solutions, Acids, & Bases Unit 6 - IB Material Essentials: Know, Understand, and Be Able To Distinguish between the terms solute, solvent, solution and concentration (g dm 3 and mol dm 3 ). Solve problems
Solutions, Acids, & Bases Unit 6 - IB Material Essentials: Know, Understand, and Be Able To Distinguish between the terms solute, solvent, solution and concentration (g dm 3 and mol dm 3 ). Solve problems
Chapter 3: Acid Base Equilibria. HCl + KOH KCl + H 2 O acid + base salt + water
 Chapter 3: Acid Base Equilibria HCl + KOH KCl + H 2 O acid + base salt + water What is an acid? The Arrhenius concept proposed that acids are substances that produce hydrogen ions (H + ) in aqueous solutions.
Chapter 3: Acid Base Equilibria HCl + KOH KCl + H 2 O acid + base salt + water What is an acid? The Arrhenius concept proposed that acids are substances that produce hydrogen ions (H + ) in aqueous solutions.
Chapter Test B. Chapter: Acids and Bases
 Assessment Chapter Test B Chapter: Acids and Bases PART I In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1. Which of the
Assessment Chapter Test B Chapter: Acids and Bases PART I In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1. Which of the
reversible reaction: R P and P R Acid dissociation is a reversible reaction. H 2 SO 4 2 H SO 4
 Unit : Equilibrium / Acids and Bases reversible reaction: R P and P R Acid dissociation is a reversible reaction. H 2 SO 4 2 H + + SO 4 Rate at which equilibrium: R P = Rate at which P R -- looks like
Unit : Equilibrium / Acids and Bases reversible reaction: R P and P R Acid dissociation is a reversible reaction. H 2 SO 4 2 H + + SO 4 Rate at which equilibrium: R P = Rate at which P R -- looks like
HACCP Concept. Overview of HACCP Principles. HACCP Principles. Preliminary Tasks Development of the HACCP Plan
 HACCP Concept Overview of HACCP Principles A systematic approach to be used in food production as a means to assure food safety. National Advisory Committee on Microbiological Criteria for Foods (NACMCF)
HACCP Concept Overview of HACCP Principles A systematic approach to be used in food production as a means to assure food safety. National Advisory Committee on Microbiological Criteria for Foods (NACMCF)
Naming Bases: Bases are named just as any other ionic compound. Name the metal first, then the name of anion.
 Name AP Unit 9: Acids and Bases Everything you should know already Naming Acids: Acids are named using a unique classification system. There are 3 general guidelines: If the anion ends in ide name the
Name AP Unit 9: Acids and Bases Everything you should know already Naming Acids: Acids are named using a unique classification system. There are 3 general guidelines: If the anion ends in ide name the
Properties of Aqueous Solutions
 Properties of Aqueous Solutions Definitions A solution is a homogeneous mixture of two or more substances. The substance present in smaller amount is called the solute. The substance present in larger
Properties of Aqueous Solutions Definitions A solution is a homogeneous mixture of two or more substances. The substance present in smaller amount is called the solute. The substance present in larger
Unit 6: ACIDS AND BASES
 Unit 6: Acids and Bases Honour Chemistry Unit 6: ACIDS AND BASES Chapter 16: Acids and Bases 16.1: Brønsted Acids and Bases Physical and Chemical Properties of Acid and Base Acids Bases Taste Sour (Citric
Unit 6: Acids and Bases Honour Chemistry Unit 6: ACIDS AND BASES Chapter 16: Acids and Bases 16.1: Brønsted Acids and Bases Physical and Chemical Properties of Acid and Base Acids Bases Taste Sour (Citric
Green Procurement Standards
 ALPS ELECTRIC CO., LTD. Green Procurement Standards (Appendix) Friendly to people, friendly to nature. Enforced:December 1, 2018 Revised: November 1, 2018 Established: July 26, 2002 ALPS ELECTRIC CO.,LTD.
ALPS ELECTRIC CO., LTD. Green Procurement Standards (Appendix) Friendly to people, friendly to nature. Enforced:December 1, 2018 Revised: November 1, 2018 Established: July 26, 2002 ALPS ELECTRIC CO.,LTD.
Acids and Bases. Two important classification of compounds - Acids and Bases. Properties of BASES
 ACIDS AND BASES Acids and Bases Two important classification of compounds - Acids and Bases Properties of ACIDS Taste Sour/Tart Stings and burns the skin Reacts with bases Turns blue litmus paper red Reacts
ACIDS AND BASES Acids and Bases Two important classification of compounds - Acids and Bases Properties of ACIDS Taste Sour/Tart Stings and burns the skin Reacts with bases Turns blue litmus paper red Reacts
Geophysical, Geological and Environmental Services
 Geophysical, Geological and Environmental Services Experience KAZAKHSTAN MOLDOVA CROATIA SERBIA ROMANIA MONTENEGRO BULGARIA F.Y.R.O.M. ALBANIA ARMENIA PORTUGAL GREECE TURKEY SYRIA IRAN AFGHANISTAN MOROCCO
Geophysical, Geological and Environmental Services Experience KAZAKHSTAN MOLDOVA CROATIA SERBIA ROMANIA MONTENEGRO BULGARIA F.Y.R.O.M. ALBANIA ARMENIA PORTUGAL GREECE TURKEY SYRIA IRAN AFGHANISTAN MOROCCO
Solution Stoichiometry
 Chapter 8 Solution Stoichiometry Note to teacher: You will notice that there are two different formats for the Sample Problems in the student textbook. Where appropriate, the Sample Problem contains the
Chapter 8 Solution Stoichiometry Note to teacher: You will notice that there are two different formats for the Sample Problems in the student textbook. Where appropriate, the Sample Problem contains the
NAMING IONIC COMPOUNDS
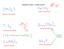 NAMING IONIC COMPOUNDS iron(ii) carbonate ammonium sulfide titanium (IV) sulfide barium phosphate Spelling matters! calcium nitrate sodium phosphide DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE
NAMING IONIC COMPOUNDS iron(ii) carbonate ammonium sulfide titanium (IV) sulfide barium phosphate Spelling matters! calcium nitrate sodium phosphide DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE
User Guide & Technical Information
 User Guide & Technical Information Morphix Technologies TraceX Explosives Detection Kit Part Number: 510100 Publication Number 51-0100B.2-1017 Contents For more information, please contact: Warning...
User Guide & Technical Information Morphix Technologies TraceX Explosives Detection Kit Part Number: 510100 Publication Number 51-0100B.2-1017 Contents For more information, please contact: Warning...
Overview of HACCP Principles. OFFICE OF THE TEXAS STATE CHEMIST Texas Feed and Fertilizer Control Service Agriculture Analytical Service
 Overview of HACCP Principles OFFICE OF THE TEXAS STATE CHEMIST Texas Feed and Fertilizer Control Service Agriculture Analytical Service OFFICE OF THE TEXAS STATE CHEMIST 2 HACCP Concept A systematic approach
Overview of HACCP Principles OFFICE OF THE TEXAS STATE CHEMIST Texas Feed and Fertilizer Control Service Agriculture Analytical Service OFFICE OF THE TEXAS STATE CHEMIST 2 HACCP Concept A systematic approach
What are Acids and Bases? What are some common acids you know? What are some common bases you know? Where is it common to hear about ph balanced
 What are Acids and Bases? What are some common acids you know? What are some common bases you know? Where is it common to hear about ph balanced materials? Historically, classified by their observable
What are Acids and Bases? What are some common acids you know? What are some common bases you know? Where is it common to hear about ph balanced materials? Historically, classified by their observable
Guidelines for management of chemical substances in product
 QA14-007(rev.1.0) Guidelines for management of chemical substances in product Ver. 1.0 March 1, 2014 enacted MITSUBISHI NICHIYU FORKLIFT CO., LTD Table of Contents 1. Preface 2. Policy 3. Scope 3.1 Regulations
QA14-007(rev.1.0) Guidelines for management of chemical substances in product Ver. 1.0 March 1, 2014 enacted MITSUBISHI NICHIYU FORKLIFT CO., LTD Table of Contents 1. Preface 2. Policy 3. Scope 3.1 Regulations
TSCA SECTION 13 IMPORTER CERTIFICATION PROCEDURE
 TSCA SECTION 13 IMPORTER CERTIFICATION PROCEDURE INTRODUCTION PURPOSE To outline the steps required for the importation of chemical substances (including microorganisms) and provide either positive or
TSCA SECTION 13 IMPORTER CERTIFICATION PROCEDURE INTRODUCTION PURPOSE To outline the steps required for the importation of chemical substances (including microorganisms) and provide either positive or
Canon Green Procurement Standards
 Canon Green Procurement Standards March 2012 Ver.8.1 Canon Green Procurement Standards Contents 1. Objective... 2 2. Scope. 2 3. Definitions of Terms. 2 4. Production environmental impact substances and
Canon Green Procurement Standards March 2012 Ver.8.1 Canon Green Procurement Standards Contents 1. Objective... 2 2. Scope. 2 3. Definitions of Terms. 2 4. Production environmental impact substances and
Green Technologies & Solutions at Gharda Chemicals Ltd.
 Green Technologies & Solutions at Gharda Chemicals Ltd. Taher Dakorwala General Manager R&D and Intellectual Property Management Gharda Chemicals Limited Dombivli BSc (Tech ) Pharmaceuticals & Fine Chemicals,
Green Technologies & Solutions at Gharda Chemicals Ltd. Taher Dakorwala General Manager R&D and Intellectual Property Management Gharda Chemicals Limited Dombivli BSc (Tech ) Pharmaceuticals & Fine Chemicals,
WESTERN CONNECTICUT STATE UNIVERSITY CHEMICAL STORAGE AND COMPATIBILITY GUIDELINES PROCEDURE S-118
 WESTERN CONNECTICUT STATE UNIVERSITY CHEMICAL STORAGE AND COMPATIBILITY GUIDELINES PROCEDURE S-118 Issued 6/4/02 Please direct any questions or comments about the applicability of this document to Luigi
WESTERN CONNECTICUT STATE UNIVERSITY CHEMICAL STORAGE AND COMPATIBILITY GUIDELINES PROCEDURE S-118 Issued 6/4/02 Please direct any questions or comments about the applicability of this document to Luigi
Name AP CHEM / / Chapter 14 Outline Acids and Bases
 Name AP CHEM / / Chapter 14 Outline Acids and Bases The Nature of Acids and Bases Svante Arrhenius was the first to recognize the nature of acids and bases. He postulated that acids produce hydrogen ions(h
Name AP CHEM / / Chapter 14 Outline Acids and Bases The Nature of Acids and Bases Svante Arrhenius was the first to recognize the nature of acids and bases. He postulated that acids produce hydrogen ions(h
INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin
 Lecture INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin Acids and Bases Properties of Acids An acid is any substance that releases hydrogen ions, H +, into water.
Lecture INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin Acids and Bases Properties of Acids An acid is any substance that releases hydrogen ions, H +, into water.
Unit Nine Notes N C U9
 Unit Nine Notes N C U9 I. AcidBase Theories A. Arrhenius Acids and Bases 1. Acids contain hydronium ions (H O ) commonly referred to as hydrogen ions (H ) that dissociate in water a. Different acids release
Unit Nine Notes N C U9 I. AcidBase Theories A. Arrhenius Acids and Bases 1. Acids contain hydronium ions (H O ) commonly referred to as hydrogen ions (H ) that dissociate in water a. Different acids release
Moisture holding capacity 61 to 69 % Shipping Weight Specific gravity 1.06 to 1.08 Particle size
 Product Data Sheet AMBERLITE XAD7HP Industrial Grade Polymeric Adsorbent Introduction AMBERLITE XAD7HP is a polymeric adsorbent, supplied as white insoluble beads. It is a non ionic aliphatic acrylic polymer
Product Data Sheet AMBERLITE XAD7HP Industrial Grade Polymeric Adsorbent Introduction AMBERLITE XAD7HP is a polymeric adsorbent, supplied as white insoluble beads. It is a non ionic aliphatic acrylic polymer
Honors Chemistry Study Guide for Acids and Bases. NH4 + (aq) + H2O(l) H3O + (aq) + NH3(aq) water. a)hno3. b) NH3
 Honors Chemistry Study Guide for Acids and Bases 1. Calculate the ph, poh, and [H3O + ] for a solution that has a [OH - ] = 4.5 x 10-5? 2. An aqueous solution has a ph of 8.85. What are the [H + ], [OH
Honors Chemistry Study Guide for Acids and Bases 1. Calculate the ph, poh, and [H3O + ] for a solution that has a [OH - ] = 4.5 x 10-5? 2. An aqueous solution has a ph of 8.85. What are the [H + ], [OH
2. Match a formula in the right column with its appropriate name in the left column.
 UNIT 3: CHEMICAL BONDING. CHEMICAL COMPOUNDS Content: Unit 3 Chemical bonding. Chemical compounds 3.1. Chemical bonding 3.2. Chemical compounds 3.2.1. Oxides 3.2.2. Hydroxides 3.2.3. Hydrides 3.2.4. Binary
UNIT 3: CHEMICAL BONDING. CHEMICAL COMPOUNDS Content: Unit 3 Chemical bonding. Chemical compounds 3.1. Chemical bonding 3.2. Chemical compounds 3.2.1. Oxides 3.2.2. Hydroxides 3.2.3. Hydrides 3.2.4. Binary
DETERMINING IONIC FORMULAS strontium oxide
 69 sodium sulfate DETERMINING IONIC FORMULAS strontium oxide tin(ii) phosphate chromium(iii) nitrate barium hydroxide titanium(iv) chloride When adding a subscript to a polyatomic ion, put the polyatomic
69 sodium sulfate DETERMINING IONIC FORMULAS strontium oxide tin(ii) phosphate chromium(iii) nitrate barium hydroxide titanium(iv) chloride When adding a subscript to a polyatomic ion, put the polyatomic
The Arrhenius Definition of Acids & Bases
 ACIDS & BASES The Arrhenius Definition of Acids & Bases An acid produces the hydrogen ion in water. A base produces the hydroxide ion in water. Brønsted Lowry Acids & Bases Brønsted acids are proton donors.
ACIDS & BASES The Arrhenius Definition of Acids & Bases An acid produces the hydrogen ion in water. A base produces the hydroxide ion in water. Brønsted Lowry Acids & Bases Brønsted acids are proton donors.
Acid and Bases. Physical Properties. Chemical Properties. Indicators. Corrosive when concentrated. Corrosive when concentrated.
 Physical Properties Acid and Bases Chemistry 30 Acids Corrosive when concentrated Have a sour taste Bases Corrosive when concentrated Have a bitter taste Often have a sharp odour Chemical Properties Indicators
Physical Properties Acid and Bases Chemistry 30 Acids Corrosive when concentrated Have a sour taste Bases Corrosive when concentrated Have a bitter taste Often have a sharp odour Chemical Properties Indicators
UNIT #11: Acids and Bases ph and poh Neutralization Reactions Oxidation and Reduction
 NAME: UNIT #11: Acids and Bases ph and poh Neutralization Reactions Oxidation and Reduction 1. SELF-IONIZATION OF WATER a) Water molecules collide, causing a very small number to ionize in a reversible
NAME: UNIT #11: Acids and Bases ph and poh Neutralization Reactions Oxidation and Reduction 1. SELF-IONIZATION OF WATER a) Water molecules collide, causing a very small number to ionize in a reversible
Acids, Bases & Salts ch Mar
 Acids, Bases & Salts 1 March 2012 » Acidsand bases are electrolytes that producea specific type of ion in water solution.» Acids will produce hydrogen ions, also called protons, because hydrogen ions are
Acids, Bases & Salts 1 March 2012 » Acidsand bases are electrolytes that producea specific type of ion in water solution.» Acids will produce hydrogen ions, also called protons, because hydrogen ions are
Arrhenius Acids and Bases
 SECTION 10.1 Arrhenius Acids and Bases Key Terms Arrhenius theory of acids and bases ionization dissociation acid-base indicator universal indicator ph scale strong acid weak acid strong base weak base
SECTION 10.1 Arrhenius Acids and Bases Key Terms Arrhenius theory of acids and bases ionization dissociation acid-base indicator universal indicator ph scale strong acid weak acid strong base weak base
Chapter 16 Acid-Base Equilibria
 Chapter 16 Acid-Base Equilibria Learning goals and key skills: Understand the nature of the hydrated proton, represented as either H + (aq) or H 3 O + (aq) Define and identify Arrhenuis acids and bases.
Chapter 16 Acid-Base Equilibria Learning goals and key skills: Understand the nature of the hydrated proton, represented as either H + (aq) or H 3 O + (aq) Define and identify Arrhenuis acids and bases.
Chemical Reaction Defn: Chemical Reaction: when starting chemical species form different chemicals.
 Chemical Reaction Defn: Chemical Reaction: when starting chemical species form different chemicals. Evidence to indicate that a chemical reaction has occurred: Temperature change Different coloured materials
Chemical Reaction Defn: Chemical Reaction: when starting chemical species form different chemicals. Evidence to indicate that a chemical reaction has occurred: Temperature change Different coloured materials
HAZARD COMMUNICATION PROGRAM
 HAZARD COMMUNICATION PROGRAM UNIVERSITY RISK MANAGEMENT Occupational Safety and Health Programs 19 Hagood Avenue, Suite 908 Charleston SC 29425 843-792-3604 Revised: January, 2015 TABLE OF CONTENTS Safety
HAZARD COMMUNICATION PROGRAM UNIVERSITY RISK MANAGEMENT Occupational Safety and Health Programs 19 Hagood Avenue, Suite 908 Charleston SC 29425 843-792-3604 Revised: January, 2015 TABLE OF CONTENTS Safety
Classifying Substances
 Classifying Substances There are other ways you can use to classify compounds. For example, you can classify some compounds as acids or bases. You are very familiar with acids and bases because you see
Classifying Substances There are other ways you can use to classify compounds. For example, you can classify some compounds as acids or bases. You are very familiar with acids and bases because you see
Measurement uncertainty and legal limits in analytical measurements
 UNCERTAINTY Measurement uncertainty and legal limits in analytical measurements MIRELLA BUZOIANU, Reference Materials Laboratory, National Institute of Metrology (INM), Bucharest, Romania 1 Introduction
UNCERTAINTY Measurement uncertainty and legal limits in analytical measurements MIRELLA BUZOIANU, Reference Materials Laboratory, National Institute of Metrology (INM), Bucharest, Romania 1 Introduction
Reaction Classes. Precipitation Reactions
 Reaction Classes Precipitation: synthesis of an ionic solid a solid precipitate forms when aqueous solutions of certain ions are mixed AcidBase: proton transfer reactions acid donates a proton to a base,
Reaction Classes Precipitation: synthesis of an ionic solid a solid precipitate forms when aqueous solutions of certain ions are mixed AcidBase: proton transfer reactions acid donates a proton to a base,
Acids - Bases in Water
 more equilibrium Dr. Fred Omega Garces Chemistry, Miramar College 1 Acids-Bases Characteristics Acids (Properties) Taste Sour Dehydrate Substances Neutralizes bases Dissolves metals Examples: Juices: TJ,
more equilibrium Dr. Fred Omega Garces Chemistry, Miramar College 1 Acids-Bases Characteristics Acids (Properties) Taste Sour Dehydrate Substances Neutralizes bases Dissolves metals Examples: Juices: TJ,
116 PLTL Activity sheet / Intro Acid - Base equilibrium Set 8
 Potentially Useful or useless information: Strong and Weak Acids and Bases Definitions Arrhenius acid a compound that releases hydrogen ions ( + ) in water Brønsted acid a proton, + ion, donor Lewis acid
Potentially Useful or useless information: Strong and Weak Acids and Bases Definitions Arrhenius acid a compound that releases hydrogen ions ( + ) in water Brønsted acid a proton, + ion, donor Lewis acid
5 Weak Acids, Bases and their Salts
 5 Weak Acids, Bases and their Salts Name: Date: Section: Objectives You will be able to define an acid and a base with the Arrhenius and Brǿnsted-Lowry definitions You will be able to predict the behavior
5 Weak Acids, Bases and their Salts Name: Date: Section: Objectives You will be able to define an acid and a base with the Arrhenius and Brǿnsted-Lowry definitions You will be able to predict the behavior
Corn Syrup Analysis E-51-1 PHOSPHORUS
 Corn Syrup Analysis E-51-1 PRINCIPLE SCOPE The sample is ignited in the presence of a fixative to destroy organic matter and convert phosphorus to inorganic phosphates which are not volatilized during
Corn Syrup Analysis E-51-1 PRINCIPLE SCOPE The sample is ignited in the presence of a fixative to destroy organic matter and convert phosphorus to inorganic phosphates which are not volatilized during
ADVANCED PLACEMENT CHEMISTRY ACIDS, BASES, AND AQUEOUS EQUILIBRIA
 ADVANCED PLACEMENT CHEMISTRY ACIDS, BASES, AND AQUEOUS EQUILIBRIA Acids- taste sour Bases(alkali)- taste bitter and feel slippery Arrhenius concept- acids produce hydrogen ions in aqueous solution while
ADVANCED PLACEMENT CHEMISTRY ACIDS, BASES, AND AQUEOUS EQUILIBRIA Acids- taste sour Bases(alkali)- taste bitter and feel slippery Arrhenius concept- acids produce hydrogen ions in aqueous solution while
Letter to non European Union customers
 15 October 2014 Letter to non European Union customers Dear customer, Subject: REACH and DuPont DuPont s intention to support customers outside the European Union This communication related to REACH focuses
15 October 2014 Letter to non European Union customers Dear customer, Subject: REACH and DuPont DuPont s intention to support customers outside the European Union This communication related to REACH focuses
LABORATORY SAFETY PLAN APPENDIX
 LABORATORY SAFETY PLAN APPENDIX APPENDIX 1 REQUIRED CEE LAB ACCESS FORMS CEE Laboratory User Form As a laboratory user in the Department of Civil and Environmental Engineering at Northeastern University,
LABORATORY SAFETY PLAN APPENDIX APPENDIX 1 REQUIRED CEE LAB ACCESS FORMS CEE Laboratory User Form As a laboratory user in the Department of Civil and Environmental Engineering at Northeastern University,
Hach Method Spectrophotometric Measurement of Free Chlorine (Cl 2 ) in Finished Drinking Water
 Hach Method 1041 Spectrophotometric Measurement of Free Chlorine (Cl ) in Finished Drinking Water Hach Company Method 1041 Revision 1. November 015 Spectrophotometric Measurement of Free Cl in Finished
Hach Method 1041 Spectrophotometric Measurement of Free Chlorine (Cl ) in Finished Drinking Water Hach Company Method 1041 Revision 1. November 015 Spectrophotometric Measurement of Free Cl in Finished
D O E S T H E S U P P LY C H A I N O F R I M I TAISIJA ŽURILA CORPORATE BRAND QUALITY MANAGER SIA RIMI BALTIC
 G O O D F O O D O N O U R TA B L E : H O W D O E S T H E S U P P LY C H A I N O F R I M I O P E R AT E? TAISIJA ŽURILA CORPORATE BRAND QUALITY MANAGER SIA RIMI BALTIC L E T S S TA R T F R O M O U R B R
G O O D F O O D O N O U R TA B L E : H O W D O E S T H E S U P P LY C H A I N O F R I M I O P E R AT E? TAISIJA ŽURILA CORPORATE BRAND QUALITY MANAGER SIA RIMI BALTIC L E T S S TA R T F R O M O U R B R
 +91-8048556479 Usha Chemotex http://www.ushachemotex.com/ We are acknowledged as the most trusted Wholesaler, Supplier and Trader firm of premium quality Lab Products. Our sourced products are synonymous
+91-8048556479 Usha Chemotex http://www.ushachemotex.com/ We are acknowledged as the most trusted Wholesaler, Supplier and Trader firm of premium quality Lab Products. Our sourced products are synonymous
Properties of Acids and Bases
 Chapter 15 Aqueous Equilibria: Acids and Bases Properties of Acids and Bases Generally, an acid is a compound that releases hydrogen ions, H +, into water. Blue litmus is used to test for acids. Blue litmus
Chapter 15 Aqueous Equilibria: Acids and Bases Properties of Acids and Bases Generally, an acid is a compound that releases hydrogen ions, H +, into water. Blue litmus is used to test for acids. Blue litmus
