THERMODYNAMIC MODELING OF ORGANIC AEROSOL
|
|
|
- Katherine Newman
- 5 years ago
- Views:
Transcription
1 THERMODYNAMIC MODELING OF ORGANIC AEROSOL Thesis by Chinghang Tong In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy California Institute of Technology Pasadena, California 2007 (Defended Sept 13, 2007)
2 ii 2007 Chinghang Tong All Rights Reserved
3 iii Acknowledgments First, I would like to express my sincere gratitude to my advisors, Professor John Seinfeld, for his insights, patience, and support for my work throughout the years. He has taught me how to structure my ideas, research, and writing more clearly. It has been a pleasure to work for him, and I appreciate the time he spent with our projects. Professor William Goddard s scientific knowledge and suggestions are invaluable, and his enthusiasm about science is especially inspiring. I feel extremely fortunate to have been under their guidance. I am especially grateful to Dr. Mario Blanco for his help with running quantum mechanical simulations and molecular dynamics calculations. His knowledge has been critical to my understanding of theory, and discussions with him have contributed much to this research. I also thank my thesis committee member: Professor Mitchio Okumura for his valuable time. Professor Douglas Doren introduced me to computational chemistry as an undergraduate. I am indebted to him for his support and encouragement. I appreciate the support and friendship of all the past and present members of the Seinfeld and Goddard groups. Special thanks to Dr. Shiang-Tai Lin and Dr. Simon Clegg for their teaching and guidance. They have spent so much of their busy time teaching me many of the methods upon which my research rests. Very special thanks to Darryl Willick for all his help in computer programming. I am also grateful to have met so many wonderful friends here at Caltech, who made my time here enjoyable. My friends at home, thought thousands of miles away, have also been a great support and comfort for me during some difficult times. I thank my parents, Sing Fai Tong and Yuet Ha Shiu, for their immeasurable love
4 iv and support throughout my life, which allowed me to pursue a life far from home and to strive to be an independent person. I am infinitely grateful to my grandmother, Lai Ha Yiu, for instilling within me the importance of learning since a young age. A special thanks goes to my aunt, Pansy Tong, for her generosity, love and support. Without the continuing support and encouragement of my family, I would not be where I am today.
5 v Abstract Modeling atmospheric aerosols containing a large organic fraction with unknown chemical composition and properties has been a constant challenge. The dissertation focuses on the theoretical treatment of the thermodynamic equilibrium of atmospheric aerosol involving organic species. We present a vapor pressure estimation method, based on quantum chemistry methods, to predict the liquid vapor pressure, enthalpies of vaporization, and heats of sublimation of atmospheric organic compounds. Predictions are compared to literature data, and the overall accuracy is considered satisfactory given the simplicity of the equations. Quantum mechanical methods were also used to investigate the thermodynamic feasibility of various acid-catalyzed aerosol-phase heterogeneous chemical reactions. A stepwise procedure is presented to determine physical properties such as heats of formation, standard entropies, Gibbs free energies of formation, and solvation energies from quantum mechanics, for various short-chain aldehydes and ketones. Equilibrium constants of hydration reactions and aldol condensation are then reported; predictions are in qualitatively agreement with previous studies. We have shown that quantum methods can serve as useful tools for first approximation, especially for species with no available data, in determining the thermodynamic properties of multifunctional oxygenates. We also present an atmospheric aerosol phase equilibrium model to determine the aerosol phase equilibrium of aqueous systems. Phase diagrams for a number of organic/water systems characteristics of both primary and secondary organic aerosols are
6 vi computed. Effects of organics on the deliquescence behavior of electrolytes are also shown in the inorganic/organic/water phase diagrams. Finally, we evaluate the performance of four recent activity coefficient models developed for inorganic-organic-water mixtures typical of atmospheric aerosols. Based on the comparison on water activities, it is found that models that include ion-organic mixture parameters (referred to as coupled models) do not necessarily produce more accurate predictions than those models that utilizes additive approaches (referred to as decoupled models). Since the chemical composition and physical properties of the organic fraction is largely unknown, the additive approaches of the decoupled models are more feasible than the coupled models.
7 vii Table of Contents Acknowledgments...iii List of Tables... x List of Figures...xiii Chapter 1: Introduction... 1 Chapter 2: Thermodynamic Properties of Multifunctional Oxygenates in Atmospheric Aerosols from Quantum Mechanics and Molecular Dynamics: Dicarboxylic Acids Abstract Introduction Vapor Pressure Estimation Method Prediction of the heats of vaporization using CED Results and Discussion Estimation of Uncertainties Acknowledgments Supporting Information Determining the Hydrogen Bonding Parameters in the Force Field References Chapter 3: Secondary Organic Aerosol Formation by Heterogeneous Reactions of Aldehydes and Ketones: AQuantum Mechanical Study Abstract Introduction Computational Method Gas-phase Standard Heats of Formation Standard Free Energies of Formation and Equilibrium Constants... 51
8 viii Solution phase energy Results and Discussion Uncertainties Glyoxal Reactions Acknowledgment Supporting Information Enthalpies of formation CHBLC corrections Standard Entropies Standard states conversion Solvation energy References Chapter 4: A New Atmospheric Aerosol Phase Equilibrium Model (UHAERO): Organic Systems Abstract Introduction Modeling Approach Characteristics of organic phase equilibria Effects of organic phase equilibria on inorganic deliquescence Effect of the Version of UNIFAC on Predicted Liquid-Liquid Equilibria UNIFAC vs. UNIFAC-Peng UNIFAC vs. UNIFAC-LL Conclusions Acknowledgements
9 ix 4.9 References Chapter 5: Comparison of Activity Coefficient Models for Atmospheric Aerosols Containing Mixtures of Electrolytes, Organics, and Water Abstract Introduction Activity Coefficient Models CSB Model X-UNIFAC Ming and Russell Model Aerosol Diameter Dependent Equilibrium Model (ADDEM) Comparison with experimental data Aqueous electrolyte solutions Aqueous Dicarboxylic Acids Solutions Aqueous Solutions of Dicarboxylic Acids and Single salt Discussion and Conclusions Acknowledgment Appendix Aerosol Inorganic Model (AIM) CSB Model UNIFAC X-UNIFAC References Chapter 6: Conclusions Appendix
10 x List of Tables Table 2.1: Experimental Physical Properties of C 3 - C 7 Dicarboxylic acids Table 2.2: Averaged Values of CED and Density for Dicarboxylic Acids and their Calculated Heats of Vaporization at 500K Table 2.3: Calculated Inter-molecular Zero-point Energy (ZPE) for C 3 -C 7 Dicarboxylic Acid Crystals Table 2.4: Input Parameters for C 3 -C 7 Dicarboxylic Acids a Table 2.5: Predicted and Experimental Heats of Sublimation (kj/mol) for C 3 -C 7 Dicarboxylic Acids Table 2.6: The Melting Points, Boiling Points, and Simulation Results for Formic, Acetic, and Benzoic acid Table 2.7: Predicted Liquid Vapor Pressures for Formic acid, Acetic acid, and Benzoic acids. The Uncertainties in the Prediction and the Percentage Error were Included 26 Table 2.8: Error in Predicted Liquid Vapor Pressure for Glutaric Acid at 500K Relative to Experiment (DIPPR) [14] Table 2.9: Comparison between the Vapor Pressure (Pa) Predicted by Mydral and Yalkowsky Equation [6] and Our Computational Chemistry Method at 500K. Experimental data were also included Table 3.1: Quantum calculated free energies of formation. The literature free energies of formation and group contribution (Joback) method estimated values are included for comparison. Absolute errors (δg) are also shown. All values are in kcal/mol Table 3.2: Quantum calculated free energies of formation, ΔG 0 f, and solvation energies, ΔG 0 s, at X3LYP/cc-pvtz(-f) level... 54
11 xi Table 3.3: Quantum calculated enthalpies and Gibbs free energies of reactions for hydration and aldol condensation at T = 298K. All energies are in kcal/mol Table 3.4: Quantum calculated log K in the gas phase and solution, estimated log K in liquid solution, and experimental log K for hydration reaction and aldol condensation at P = 1 atm and T = 298K Table 3.5: Quantum calculated enthalpies (ΔH f0 ) and free energies (ΔG f0 ) of formation, and solvation energies (ΔG s0 ). The structures for compound labeled A G are shown in Figure Table 3.6: Quantum calculated ground state electronic energies (E elec ), zero point energies (E zpe ), and thermal enthalpy from 0-298K (ΔH (0-298K) ) Table 3.7: Experimental (h 0 i ) and calculated (h QM i ) atomic heats of formation, and the quantum corrections to heats of formation (δh elec i ) Table 3.8: Quantum calculated heats of formation with and without CBHLC. The experimental heats of formation are included for comparison, and the absolute errors (δh) are also shown. All values are in kcal/mol Table 3.9: Reported entropies from quantum mechanics, S 0 298K(QM), experimental entropies, S 0. Absolute errors (δs) are shown, and all values are in cal/mol K Table 4.1: Organic compounds considered and their UNIFAC groups Table 4.2: UNIFAC energy interaction parameters between the main groups used in this work: UNIFAC/UNIFAC-Peng/UNIFAC-LL Table 4.3: Two-phase equilibrium solutions for binary systems Table 5.1: Summary of four activity coefficient models for mixtures of inorganic salts, organic compounds, and water
12 xii Table 5.2: Organics/water and electrolyte/organic/water systems studied in the present work Table 5.3: UNIFAC energy interaction parameters, A no and A on, between the main groups: UNIFAC-VL/UNIFAC-RL/UNIFAC-Peng Table 5.4: Standard deviations of difference between predictions and the experimental water activity data type, a w (x, m) a for the non-electrolyte modules used in the CSB model, ADDEM, X-UNIFAC, and the Ming and Russell model Table 5.5: Standard deviations of difference between predictions and data for the CSB model, ADDEM, X-UNIFAC.3 and the Ming and Russell model using the experimental water activity data type, a w (x, m) a
13 xiii List of Figures Figure 2.1: Comparison between predicted and initial target densities (experimental at 500K) of the simulations. The experimental densities at 298K are also shown Figure 2.2: Predicted and experimental values of ΔH vap (500K) Figure 2.3: Comparison of predicted vs. literature liquid vapor pressure, ln P (in units of Pa), over the temperature range ~400K to ~600K for C 3 -C 7 dicarboxylic acids. The solid line is the 1:1 correspondence line. The average error over all points is 29%. Original references for malonic, succinic [31], glutaric [32], adipic [17], pimelic [31] Figure 2.4: Comparison of calculated Heats of Sublimation for C 3 -C 7 dicarboxylic acids against all available experimental data Figure 2.5: Predicted vs. Literature solid vapor pressure, ln P s (in units of Pa) over the temperature range ~300K to ~400K for C 3 -C 7 dicarboxylic acids. The solid line is the 1:1 correspondence line. Horizontal bars indicate the experimental uncertainty, vertical bars are 2σ from the predicted values. The upper group (ln P s from ~ -3 to 2) includes data from Riberio da Silva et al. [23] and leads to an average error of 21%. The lower group includes data from Bilde et al. [19] and leads to an average error of 70% Figure 2.6: The odd-even effect. Comparison between the predicted solid vapor pressures and various literature vapor pressure values for C 3 -C 7 dicarboxylic acids at (a) 365K and (b) 298K... 24
14 xiv Figure 2.7: Predicted vs. Literature liquid vapor pressure, ln P (in the units of Pa) for formic acid, acetic acid, and benzoic acid. The solid line is the 1:1 correspondence line. The average error ranges from 8% (formic) to 31% (benzoic) Figure 2.8: Uncertainties in solid vapor pressure (dotted line) and liquid vapor pressure (solid line), ln P, as a function of temperature for C 3 -C 7 dicarboxylic acids Figure 2.9: Sensitivity of the predicted liquid vapor pressure on the value of T b (triangles), on the value of ΔH vap (T b ) (squares) and on the value of ΔC p (T b ) (circles) for glutaric acid at 500K and adipic acid at 495K Figure 2.10: Dimeric configuration for acetic acid (C 2h symmetry) Figure 2.11: Comparison of QM results with fitted hydrogen-bonding energy curves for formic acid. The fitted functions are LJ12-10 and Exp Figure 2.12: Hydrogen-bonding energy curve for acetic acid determined by both quantum mechanics and force field calculation, and the fitted LJ12-10 and Exp-6 functions.37 Figure 2.13: Total binding energy curves for the acetic acid dimer determined using quantum mechanics and force field calculations with new hydrogen bonding parameters. Negative energies are binding, positive are repulsive Figure 3.1: Thermodynamic cycle for computation of energy changes of reaction in the gas phase and solution. Adapted from Cramer (2004) [17] Figure 3.2: A summary of the suggested reaction pathway for glyoxal in particular matter by Liggio et al. [14]. Quantum calculations were done for the proposed structures in the scheme. QM calculated enthalpies and free energies of reactions were shown. All values are in kcal/mol... 59
15 xv Figure 3.3: Molecular structures of the organic species (formaldehyde, acetaldehyde, acetone, butanal, hexanal, glyoxal, hydroxyacetone, 2,4-pentanedione, ethylene glycol, glutaraldehyde, cyclopropane carboxylic acid) used in the study Figure 4.1: Normalized GFE curves for all water/organic systems and two selected organic systems s 1 /s 2 and their respective activities. Equilibrium two-phase solutions (if any) are marked with circle symbols. The solid (-) line represents the tie lines. Normalized GFE curves are plotted as a function of mole fraction of organic component s 2 for: (a) systems of water (s 1 )/X[1-4] (s 2 ) at K; (b) the activity of water in systems of water/x[1-4] and (c) activity of X[1-4] for the systems of water/x[1-4]. (d) Systems of water/x[5-8] at K. (e) The activity of water in systems of water/x[5-8] and (f) the activity of X[5-8]. (g) Systems of X5/X7, X2/X3, X8/X6, and X1/X4. (h) The activity of component s1 for the s 1 /s 2 system is shown in panel g, and (i) the activity of component s 2 for the s 1 /s 2 system is shown in panel h Figure 4.2: Construction of the phase diagram for the system water/1-hexacosanol (X5)/pinic acid(x7) at K with tracking of the presence of each distinct phase region. For each region the boundaries of which are marked with bold lines, the number of liquid phases at equilibrium is represented as L2 for two liquid phases and L3 for three liquid phases. (a) Liquid-liquid equilibrium prediction with twophase tie lines represented by dashed lines. (b) Labels on the contours ( ) indicate the value of the activity of water. (c) Labels on the contours ( ) indicate the value of the activity of 1-hexacosanol. (d) Labels on the contours ( ) indicate the value of the activity of pinic acid
16 xvi Figure 4.3: Construction of the phase diagram for the system water/adipic acid (X2) /glutaraldehyde(x3) at K with tracking of the presence of each distinct phase region. For each region the boundaries of which are marked with bold lines, the number of liquid phases at equilibrium is represented as L2 for two liquid phases and L3 for three liquid phases. (a) Liquid-liquid equilibrium prediction with twophase tie lines represented by dashed lines. (b) Labels on the contours ( ) indicate the value of the activity of water. (c) Labels on the contours ( ) indicate the value of the activity of adipic acid. (d) Labels on the contours ( ) indicate the value of the activity of glutaraldehyde Figure 4.4: Construction of the phase diagram for the system water/pinonic acid(x8)/nonacosane(x6) at K with tracking of the presence of each distinct phase region.for each region the boundaries of which are marked with bold lines, the number of liquid phases at equilibrium is represented as L2 for two liquid phases and L3 for three liquid phases. (a) Liquid-liquid equilibrium prediction with twophase tie lines represented by dashed lines. (b) Labels on the contours ( ) indicate the value of the activity of water. (c) Labels on the contours ( ) indicate the value of the activity of pinonic acid. (d) Labels on the contours ( ) indicate the value of the activity of nonacosane Figure 4.5: Construction of the phase diagram for the system water/2-hydroxy-glutaric acid (X1) /palmitic acid(x4) at Kwith tracking of the presence of each distinct phase region. For each region the boundaries of which are marked with bold lines, the number of liquid phases at equilibrium are represented as L2 for two liquid phases and L3 for three liquid phases. (a) Liquid-liquid equilibrium prediction with
17 xvii two-phase tie lines represented by dashed lines. (b) Labels on the contours ( ) indicate the value of the activity of water. (c) Labels on the contours ( ) indicate the value of the activity of 2-hydroxy-glutaric acid. (d) Labels on the contours ( ) indicate the value of the activity of palmitic acid Figure 4.6: Construction of the phase diagram for the system water/1-hexacosanol (X5)/pinic acid(x7) at K with two different sets of UNIFAC parameters: (a) UNIFAC-Peng parameters, (b) UNIFAC-LL parameters Figure 4.7: Construction of the phase diagram for the system (NH 4 ) 2 SO 4 /H 2 SO 4 / NH 4 NO 3 /HNO 3 /H 2 O with the sulfate fraction: (a) Y =1, (b) Y =0.85, at K with tracking of the presence of each phase. For each region the boundaries of which are marked with bold lines, the existing phases at equilibrium are represented. For the regions numbered as 1 through 7, the existing phases at equilibrium are AE, BE, BF, BD, BG, BC, and CG, respectively. Labels on the dashed contours present the relative water content Figure 4.8: Construction of the phase diagram for the system (NH 4 ) 2 SO 4 /H 2 SO 4 / NH 4 NO 3 /HNO 3 /H 2 O with the sulfate fraction Y = 1 at K when the system also includes two organic species: (a) 1-hexacosanol (ORG 1 ) and pinic acid (ORG 2 ) with R = 0.2, f ORG1 = 0.5 and f ORG2 = 0.5; (b) adipic acid (ORG 1 ) and glutaraldehyde (ORG 2 ) with R = 0.2, f ORG1 = 0.15 and f ORG2 = 0.85; (c) pinonic acid (ORG 1 ) and nonacosane (ORG 2 ) with R = 0.2, f ORG1 = 0.5 and f ORG2 = 0.5; (d) 2-hydroxy-glutaric acid (ORG 1 ) and palmitic acid (ORG2) with R = 0.2, f ORG1 = 0.5 and f ORG2 = Figure 4.9: Construction of the phase diagram for the system (NH 4 ) 2 SO 4 /H 2 SO 4 / NH 4 NO 3 /HNO 3 /H 2 O with the sulfate fraction Y = 0.85 at K when the system
18 xviii also includes two organic species: (a) 1-hexacosanol (ORG 1 ) and pinic acid (ORG 2 ) with R = 0.2, f ORG1 = 0.5 and f ORG2 = 0.5; (b) adipic acid (ORG 1 ) and glutaraldehyde (ORG 2 ) with R = 0.2, f ORG1 = 0.15 and f ORG2 = 0.85; (c) pinonic acid (ORG 1 ) and nonacosane (ORG 2 ) with R = 0.2, f ORG1 = 0.5 and f ORG2 = 0.5; (d) 2-hydroxy-glutaric acid (ORG1) and palmitic acid (ORG 2 ) with R = 0.2, f ORG1 = 0.5 and f ORG2 = Figure 5.1: Molecular structures of the organic species used in the present study Figure 5.2: Comparison between the water activities (a w ) calculated by AIM (solid lines) and X-UNIFAC.3 (dash lines) as a function of the salt molality for single-salt solutions of (a) NaCl; literature data obtained from the fitted equation of Tang (1997) (triangles) (b) (NH 4 ) 2 SO 4 ; literature data obtained from the fitted equation of Tang (1997) (squares) and Clegg et al. (1995) (dots) (c) NH 4 NO 3 ; experimental data obtained from Lightstone et al. (2000) (crosses) (d) NaNO 3 ; literature data are obtained from the fitted equation of Tang (1997) (circles) Figure 5.3: Water activities calculated by X-UNIFAC.3 versus those calculated by AIM. The solid line is the 1:1 correspondence line Figure 5.4: Water activities (a w ) calculated for aqueous malonic, succinic, glutaric, maleic, malic, and citric solutions using the (a) Correlation equations, (b) UNIFAC- RL, and (b) UNIFAC-Peng. (d), malic + maleic, malonic + glutaric acid. Calculated water activities are plotted against the experimental water activities (Choi and Chan, 2002a; Peng et al., 2001). The solid line is the 1:1 correspondence line Figure 5.5: Water activities (a w ) calculated using X-UNIFAC, CSB model, Ming and Russell model, and ADDEM for aqueous solutions at mole ratio of acid : salt = 1:1
19 xix for malonic acid and (a) NaCl (b) (NH 4 ) 2 SO 4. Experimental data are from original reference (Choi and Chan, 2002b) Figure 5.6: Water activities (a w ) calculated using X-UNIFAC, CSB model, Ming and Russell model, and ADDEM for aqueous solutions at mole ratio of acid : salt = 1:1 for succinic acid and (a) NaCl (b) (NH 4 ) 2 SO 4. Experimental data (dots) are from original reference (Choi and Chan, 2002b) Figure 5.7: Water activities (a w ) calculated using X-UNIFAC, CSB model, Ming and Russell model, and ADDEM for aqueous solutions at mass ratio of acid : salt = 1: 1 for glutaric acid and (a) NaCl (b) (NH 4 ) 2 SO 4. Experimental data are from original reference (Choi and Chan, 2002b) Figure 5.8: Water activities (a w ) calculated using X-UNIFAC, CSB model, Ming and Russell model, and ADDEM for citric acid aqueous solutions of (a) NaCl and (b) (NH 4 ) 2 SO 4 at mole ratio of acid : salt = 1:1. Experimental data are the evaporation and bulk solutions measurements (Choi and Chan, 2002b) Figure 5.9: (a) Long-range, mid-range, and the short-range contributions are illustrated separately for the aqueous glutaric acid and (NH 4 ) 2 SO 4 solution. The overall results of X-UNIFAC.3 are also shown. (b) (i) Mid-range contribution of acetic acid and 2- (NH 4 ) 2 SO 4 solution. (ii) The mid-range parameters for CH 2 /NH 4+ and CH 2 /SO 4. (iii) CH 2 /SO 2-4 mid-range contributions (iv) CH 2 /NH + 4 mid-range contributions Figure 5.10: Water activities (a w ) calculated using X-UNIFAC, CSB model, Ming and Russell model, and ADDEM for aqueous solution of M5 (malic + malonic + maleic + glutaric + methyl succinic) acids and (a) NaCl (b) (NH 4 ) 2 SO 4, plotted against the
20 xx total acid molality (m T ), where m T = m malic + m malonic + m maleic + m glutaric + m methylsuccinic. Experimental data are shown with the original reference (Marcolli et al., 2004)
21 1 Chapter 1 Introduction
22 2 Introduction Organic species are ubiquitous constituents of atmospheric particular matter [1, 2]. Organic aerosol is emitted directly from sources, or formed in the atmosphere from the gas-phase oxidation of volatile organic compounds (VOCs), oxidation products of VOCs usually have sufficiently low vapor pressures that they partition into the condensed phase, forming secondary organic aerosol (SOA). Atmospheric aerosols generally contain both inorganic components and an organic fraction comprising a wide range of organic compounds of diverse physical and chemical properties. Water and volatile species are distributed between the gas and aerosol phases, governed by thermodynamic equilibrium. The common form of the gas/particle (G/P) partitioning constant (K p ) for absorptive uptake into the particle phase is [3, 4]: K p,i = (ng/µg) particle phase (ng/m 3 ) gas phase = F /TSP i 760RTf = om (1.1) A i MW om " i p L,i where p 0 L,i (torr) is the compound s vapor pressure as a pure liquid (subcooled if necessary); ζ i is the activity coefficient of species i in the particle phase; A i (ng m -3 ) is the concentration of species i in the gas phase; F i (ng m -3 ) is the concentration in the aerosol phase; TSP (µg m -3 ) is the total suspended particulate matter (PM) concentration; R is the ideal gas constant ( m 3 atm mol -1 K -1 ); T (K) is temperature; f om is the weight fraction of the TSP that comprises the absorbing organic matter (OM) phase; MW om (g mol -1 ) is the number average molecular weight of the absorbing OM phase. The importance of p 0 L,i and ζ i in controlling G/P partitioning is evident in equation 1.1. Low vapor pressures values are extremely difficult to measure by experiments. Furthermore, many organic compounds are solids in their pure form at ambient temperature. Even if the vapor pressures can be measured, the solid vapor pressures, p 0 S,i,
23 3 still need be adjusted to the corresponding subcooled p 0 L values. As a result, the p 0 L values of most atmospheric-relevant compounds are not known. As an alternative to experimental measurements, interest is gaining in computational methods that predict p 0 L based on multiparameter correlations between structure and p 0 L, such as the UNIFAC-based method by Asher et al. [5]. In Chapter 2 a method based on quantum chemistry methods combined with the Clausius-Clapeyron equation to predict the liquid vapor pressure, enthalpies of vaporization, and heats of sublimation of atmospheric organic compounds, is presented. Vapor pressures of the five dicarboxylic acids, malonic, succinic, glutaric, adipic, and pimelic acids, are then predicted using the derived Clausius-Clapeyron equation. Experimental studies have provided convincing evidence that aerosol-phase heterogeneous chemical reactions (possibly acid-catalyzed) are involved to some extent in the SOA formation. In Chapter 3 the quantum mechanics (QM) methods are used to determine physical properties such as heats of formation, standard entropies, Gibbs free energies of formation, and solvation energies, for various short-chain aldehydes and ketones. These QM results are then used to determine the equilibrium constants (reported as log K) of aerosol-phase chemical reactions, including hydration reactions and aldol condensation for formaldehyde, acetaldehyde, acetone, butanal, hexanal, and glyoxal. The results are potentially useful in determining the relative thermodynamic tendency for atmospheric aerosol-phase reactions. Water, volatile inorganic and organic species are distributed between the gas and aerosol phases according to the gas/particle thermodynamic equilibrium. Liquid and solid phases can exist at equilibrium within an atmospheric particle. Models exist for
24 4 computation of phase equilibria for inorganic/water mixtures for atmospheric aerosols. When organic species are present, the phase equilibrium calculation within the aerosol phase is complicated by organic/water interactions as well as the potentially large number of organic species. Chapter 4 presents an atmospheric aerosol phase equilibrium model, an extension of the UHAERO inorganic thermodynamic model [6], to determine the phase equilibrium of organic-water systems. Phase diagrams for a number of model organic/water systems characteristic of both primary and secondary organic aerosols are computed. Also calculated are inorganic/organic/water phase diagrams that show the effect of organics on inorganic deliquescence behavior. Activity coefficients are important in the calculation the gas/phase partitioning equilibrium and the phase equilibria within the particle phase. Hence, considerable effort has been devoted to develop activity coefficient models that can be applied to mixed organic-electrolyte-water mixtures. Several existing activity coefficient models are examined in Chapter 5. Calculated water activities are compared with experimental data for various organic and organic-electrolyte solutions. In addition, the strengths and weaknesses of each approach are discussed. In Chapter 6 a summary is given for the results presented in the previous sections. The Appendix presents calculations of the entropy information for common amine systems using classical and quantum simulations.
25 5 References 1. Murphy, D. M.; Thomson, D. S.; Mahoney, T. M. J., In situ measurements of organics, meteoritic material, mercury, and other elements in aerosols at 5 to 19 kilometers. Science 1998, 282, Seinfeld, J. H.; Pandis, S. N., Atmospheric Chemistry and Physics From Air Pollution to Cilmate Change. Wiley-Interscience: New York, Pankow, J. F., An absorption model of gas/particle partitioning of organic compounds in the atmosphere. Atmospheric Environment 1994, 28, Pankow, J. F., An absorption model of the gas/particle partitioning involved in the formation of secondary organic aerosol. Atmospheric Environment 1994, 28, Asher, W. E.; Pankow, J. F.; Erdakos, G. B.; Seinfeld, J. H., Estimating the vapor pressures of multi-functional oxygen-containing organic compounds using group contribution methods. Atmospheric Environment 2002, 36, Amundson, N. R.; Caboussat, A.; He, J. W.; Martynenko, A. V.; Savarin, V. B.; Seinfeld, J. H.; Yoo, K. Y., A new inorganic atmospheric aerosol phase equilibrium model (UHAERO). Atmospheric Chemistry and Physics 2006, 6,
A New Atmospheric Aerosol Phase Equilibrium Model
 76 Chapter 4 A New Atmospheric Aerosol Phase Equilibrium Model (UHAERO): Organic Systems * * This chapter is reproduced by permission from A new atmospheric aerosol phase equilibrium model (UHAERO): organic
76 Chapter 4 A New Atmospheric Aerosol Phase Equilibrium Model (UHAERO): Organic Systems * * This chapter is reproduced by permission from A new atmospheric aerosol phase equilibrium model (UHAERO): organic
Secondary Organic Aerosol Formation by Heterogeneous. Reactions of Aldehydes and Ketones: A Quantum Mechanical
 44 Chapter 3 Secondary Organic Aerosol Formation by Heterogeneous Reactions of Aldehydes and Ketones: A Quantum Mechanical Study* * This chapter is reproduced by permission from Secondary Organic Aerosol
44 Chapter 3 Secondary Organic Aerosol Formation by Heterogeneous Reactions of Aldehydes and Ketones: A Quantum Mechanical Study* * This chapter is reproduced by permission from Secondary Organic Aerosol
Determination of Gas/Particle Partitioning of Glyoxal and Other Bifunctional Species using the Annular Denuder-Filter Sampling Technique
 Determination of Gas/Particle Partitioning of Glyoxal and Other Bifunctional Species using the Annular Denuder-Filter Sampling Technique Simon Ip, Hilda Huang, Jian Zhen Yu Hong Kong University of Science
Determination of Gas/Particle Partitioning of Glyoxal and Other Bifunctional Species using the Annular Denuder-Filter Sampling Technique Simon Ip, Hilda Huang, Jian Zhen Yu Hong Kong University of Science
Born-Haber Cycle: ΔH hydration
 Born-Haber Cycle: ΔH hydration ΔH solution,nacl = ΔH hydration,nacl(aq) U NaCl ΔH hydration,nacl(aq) = ΔH hydration,na + (g) + ΔH hydration,cl (g) Enthalpies of Hydration 1 Sample Exercise 11.3 Use the
Born-Haber Cycle: ΔH hydration ΔH solution,nacl = ΔH hydration,nacl(aq) U NaCl ΔH hydration,nacl(aq) = ΔH hydration,na + (g) + ΔH hydration,cl (g) Enthalpies of Hydration 1 Sample Exercise 11.3 Use the
DATA THAT YOU MAY USE UNITS Conventional Volume ml or cm 3 = cm 3 or 10-3 dm 3 Liter (L) = dm 3 Pressure atm = 760 torr = Pa CONSTANTS
 DATA THAT YOU MAY USE UNITS Conventional S.I. Volume ml or cm 3 = cm 3 or 0-3 dm 3 Liter (L) = dm 3 Pressure atm = 760 torr =.03 0 5 Pa torr = 33.3 Pa Temperature C 0 C = 73.5 K PV L-atm =.03 0 5 dm 3
DATA THAT YOU MAY USE UNITS Conventional S.I. Volume ml or cm 3 = cm 3 or 0-3 dm 3 Liter (L) = dm 3 Pressure atm = 760 torr =.03 0 5 Pa torr = 33.3 Pa Temperature C 0 C = 73.5 K PV L-atm =.03 0 5 dm 3
Gas Particle Partitioning to OA
 Gas Particle Partitioning to OA Advanced Atmospheric chemistry CHEM 5152 Prof. J.L. Jimenez Last updated: Spring 2017 1 Gas Phase vs Aerosol Compounds For monofunctional compounds, alkanes beyond C 20
Gas Particle Partitioning to OA Advanced Atmospheric chemistry CHEM 5152 Prof. J.L. Jimenez Last updated: Spring 2017 1 Gas Phase vs Aerosol Compounds For monofunctional compounds, alkanes beyond C 20
Vapor Pressure of Liquids Equilibria and Thermodynamics
 Chemistry 1B-Foothill College Vapor Pressure of Liquids Equilibria and Thermodynamics In this exercise, you will investigate the relationship between the vapor pressure of a liquid and the thermodynamic
Chemistry 1B-Foothill College Vapor Pressure of Liquids Equilibria and Thermodynamics In this exercise, you will investigate the relationship between the vapor pressure of a liquid and the thermodynamic
Exam 3 Solutions. ClO g. At 200 K and a total pressure of 1.0 bar, the partial pressure ratio for the chlorine-containing compounds is p ClO2
 Chemistry 360 Dr. Jean M. Standard Fall 2016 Name KEY Exam 3 Solutions 1.) (14 points) Consider the gas phase decomposition of chlorine dioxide, ClO 2, ClO 2 ( g) ClO ( g) + O ( g). At 200 K and a total
Chemistry 360 Dr. Jean M. Standard Fall 2016 Name KEY Exam 3 Solutions 1.) (14 points) Consider the gas phase decomposition of chlorine dioxide, ClO 2, ClO 2 ( g) ClO ( g) + O ( g). At 200 K and a total
Chemistry 360 Spring 2017 Dr. Jean M. Standard April 19, Exam points
 Chemistry 360 pring 2017 Dr. Jean M. tandard April 19, 2017 Name Exam 3 100 points Note: You must show your work on problems in order to receive full credit for any answers. You must turn in your equation
Chemistry 360 pring 2017 Dr. Jean M. tandard April 19, 2017 Name Exam 3 100 points Note: You must show your work on problems in order to receive full credit for any answers. You must turn in your equation
The Second Law of Thermodynamics (Chapter 4)
 The Second Law of Thermodynamics (Chapter 4) First Law: Energy of universe is constant: ΔE system = - ΔE surroundings Second Law: New variable, S, entropy. Changes in S, ΔS, tell us which processes made
The Second Law of Thermodynamics (Chapter 4) First Law: Energy of universe is constant: ΔE system = - ΔE surroundings Second Law: New variable, S, entropy. Changes in S, ΔS, tell us which processes made
Soluble: A solute that dissolves in a specific solvent. Insoluble: A solute that will not dissolve in a specific solvent. "Like Dissolves Like"
 Solutions Homogeneous Mixtures Solutions: Mixtures that contain two or more substances called the solute and the solvent where the solute dissolves in the solvent so the solute and solvent are not distinguishable
Solutions Homogeneous Mixtures Solutions: Mixtures that contain two or more substances called the solute and the solvent where the solute dissolves in the solvent so the solute and solvent are not distinguishable
CH302 Spring 2009 Practice Exam 1 (a fairly easy exam to test basic concepts)
 CH302 Spring 2009 Practice Exam 1 (a fairly easy exam to test basic concepts) 1) Complete the following statement: We can expect vapor pressure when the molecules of a liquid are held together by intermolecular
CH302 Spring 2009 Practice Exam 1 (a fairly easy exam to test basic concepts) 1) Complete the following statement: We can expect vapor pressure when the molecules of a liquid are held together by intermolecular
Contents. Content Guidance. Questions & Answers. Getting the most from this book... 4 About this book... 5
 Contents Getting the most from this book... 4 About this book.... 5 Content Guidance Atomic structure......................................... 6 Amount of substance....................................
Contents Getting the most from this book... 4 About this book.... 5 Content Guidance Atomic structure......................................... 6 Amount of substance....................................
A curved multi-component aerosol hygroscopicity model framework: Part 2 Including organic compounds
 Atmos. Chem. Phys., 5, 1223 1242, 2005 SRef-ID: 1680-7324/acp/2005-5-1223 European Geosciences Union Atmospheric Chemistry and Physics A curved multi-component aerosol hygroscopicity model framework: Part
Atmos. Chem. Phys., 5, 1223 1242, 2005 SRef-ID: 1680-7324/acp/2005-5-1223 European Geosciences Union Atmospheric Chemistry and Physics A curved multi-component aerosol hygroscopicity model framework: Part
Problem Set 10 Solutions
 Chemistry 360 Dr Jean M Standard Problem Set 10 Solutions 1 Sketch (roughly to scale) a phase diagram for molecular oxygen given the following information: the triple point occurs at 543 K and 114 torr;
Chemistry 360 Dr Jean M Standard Problem Set 10 Solutions 1 Sketch (roughly to scale) a phase diagram for molecular oxygen given the following information: the triple point occurs at 543 K and 114 torr;
Lecture Notes 1: Physical Equilibria Vapor Pressure
 Lecture Notes 1: Physical Equilibria Vapor Pressure Our first exploration of equilibria will examine physical equilibria (no chemical changes) in which the only changes occurring are matter changes phases.
Lecture Notes 1: Physical Equilibria Vapor Pressure Our first exploration of equilibria will examine physical equilibria (no chemical changes) in which the only changes occurring are matter changes phases.
Chapter 5 Conclusions and Future Studies
 177 Chapter 5 Conclusions and Future Studies 178 Conclusions and Future Studies The results of the studies presented in this thesis confirm that ambient and laboratory-generated aerosols exhibit complex
177 Chapter 5 Conclusions and Future Studies 178 Conclusions and Future Studies The results of the studies presented in this thesis confirm that ambient and laboratory-generated aerosols exhibit complex
Chemical thermodynamics and bioenergetics
 Chemical thermodynamics and bioenergetics Thermodynamics is a branch of physics that studies energy, the forms of its transformation, and the laws controlling its properties. Basic Concepts and Definitions.
Chemical thermodynamics and bioenergetics Thermodynamics is a branch of physics that studies energy, the forms of its transformation, and the laws controlling its properties. Basic Concepts and Definitions.
Chapter 12 Intermolecular Forces of Attraction
 Chapter 12 Intermolecular Forces of Attraction Intermolecular Forces Attractive or Repulsive Forces between molecules. Molecule - - - - - - Molecule Intramolecular Forces bonding forces within the molecule.
Chapter 12 Intermolecular Forces of Attraction Intermolecular Forces Attractive or Repulsive Forces between molecules. Molecule - - - - - - Molecule Intramolecular Forces bonding forces within the molecule.
CHEMISTRY 102 FALL 2010 EXAM 1 FORM D SECTION 502 DR. KEENEY-KENNICUTT PART 1
 NAME CHEMISTRY 102 FALL 2010 EXAM 1 FORM D SECTION 502 DR. KEENEY-KENNICUTT Directions: (1) Put your name on PART 1 and your name and signature on PART 2 of the exam where indicated. (2) Sign the Aggie
NAME CHEMISTRY 102 FALL 2010 EXAM 1 FORM D SECTION 502 DR. KEENEY-KENNICUTT Directions: (1) Put your name on PART 1 and your name and signature on PART 2 of the exam where indicated. (2) Sign the Aggie
Chapter 8: Physical Equilibria
 Chapter 8: Physical Equilibria Our first foray into equilibria is to examine phenomena associated with two phases of matter achieving equilibrium in which the free energy in each phase is the same and
Chapter 8: Physical Equilibria Our first foray into equilibria is to examine phenomena associated with two phases of matter achieving equilibrium in which the free energy in each phase is the same and
Chem 112 Dr. Kevin Moore
 Chem 112 Dr. Kevin Moore Gas Liquid Solid Polar Covalent Bond Partial Separation of Charge Electronegativity: H 2.1 Cl 3.0 H Cl δ + δ - Dipole Moment measure of the net polarity in a molecule Q Q magnitude
Chem 112 Dr. Kevin Moore Gas Liquid Solid Polar Covalent Bond Partial Separation of Charge Electronegativity: H 2.1 Cl 3.0 H Cl δ + δ - Dipole Moment measure of the net polarity in a molecule Q Q magnitude
MCGILL UNIVERSITY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEM 120 MONDAY MARCH 16, :30PM 8:30PM VERSION NUMBER: 1
 MCGILL UNIVERSITY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEM 120 MONDAY MARCH 16, 2009 6:30PM 8:30PM VERSION NUMBER: 1 Instructions: BEFORE YOU BEGIN: Enter your student number and name on the computer
MCGILL UNIVERSITY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEM 120 MONDAY MARCH 16, 2009 6:30PM 8:30PM VERSION NUMBER: 1 Instructions: BEFORE YOU BEGIN: Enter your student number and name on the computer
Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes
 DISTRIBUTION STATEMENT A. Approved for public release; distribution is unlimited. Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes John H. Seinfeld California Institute of Technology,
DISTRIBUTION STATEMENT A. Approved for public release; distribution is unlimited. Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes John H. Seinfeld California Institute of Technology,
Thinking Like a Chemist About Phase Changes UNIT 5 DAY 3
 Thinking Like a Chemist About Phase Changes UNIT 5 DAY 3 What are we going to learn today? First day? Get a handout from a TA after class. Thinking Like a Chemist in the context of Phase Changes Vapor
Thinking Like a Chemist About Phase Changes UNIT 5 DAY 3 What are we going to learn today? First day? Get a handout from a TA after class. Thinking Like a Chemist in the context of Phase Changes Vapor
evidyarthi.in Thermodynamics Q 1.
 SUBJECTIVE PROBLEMS: Q 1. Thermodynamics The enthalpy for the following reaction ( H o ) at 25 o C are given below: (i) 1/2 H 2 (g) + 1/2 O 2 (g) OH(g) 10.06 kcal (ii) H 2 (g) 2H(g) 104.18 kcal (iii) O
SUBJECTIVE PROBLEMS: Q 1. Thermodynamics The enthalpy for the following reaction ( H o ) at 25 o C are given below: (i) 1/2 H 2 (g) + 1/2 O 2 (g) OH(g) 10.06 kcal (ii) H 2 (g) 2H(g) 104.18 kcal (iii) O
Solutions Definition and Characteristics
 Solutions Solutions Definition and Characteristics Homogeneous mixtures of two or more substances Appear to be pure substances Transparency Separation by filtration is not possible Uniform distribution
Solutions Solutions Definition and Characteristics Homogeneous mixtures of two or more substances Appear to be pure substances Transparency Separation by filtration is not possible Uniform distribution
5.2 Energy. N Goalby chemrevise.org Lattice Enthalpy. Definitions of enthalpy changes
 5.2 Energy 5.2.1 Lattice Enthalpy Definitions of enthalpy changes Enthalpy change of formation The standard enthalpy change of formation of a compound is the energy transferred when 1 mole of the compound
5.2 Energy 5.2.1 Lattice Enthalpy Definitions of enthalpy changes Enthalpy change of formation The standard enthalpy change of formation of a compound is the energy transferred when 1 mole of the compound
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
 Unit - 12 ALDEHYDES, KETONES AND CARBOXYLIC ACIDS 1. Indicate the electrophilic and nucleophilic centres in acetaldehyde. 2. Write the IUPAC names of the following organic compounds : 122 XII Chemistry
Unit - 12 ALDEHYDES, KETONES AND CARBOXYLIC ACIDS 1. Indicate the electrophilic and nucleophilic centres in acetaldehyde. 2. Write the IUPAC names of the following organic compounds : 122 XII Chemistry
Entropy. Spontaneity. Entropy. Entropy mol of N 2 at 1 atm or 1 mol of N 2 at atm. process a process that occurs without intervention
 Entropy Spontaneity process a process that occurs without intervention can be fast or slow Entropy (s) the measure of molecular randomness or disorder Think of entropy as the amount of chaos Entropy Predict
Entropy Spontaneity process a process that occurs without intervention can be fast or slow Entropy (s) the measure of molecular randomness or disorder Think of entropy as the amount of chaos Entropy Predict
Basics of Thermodynamics: Easy learning by Dr. Anjana Sen
 Basics of Thermodynamics: Easy learning by Dr. Anjana Sen Part 1: Theory and concept Part 2: Definitions and equations Part 3: Laws of Thermodynamics Part 1: theory and concept Thermodynamics means conversion
Basics of Thermodynamics: Easy learning by Dr. Anjana Sen Part 1: Theory and concept Part 2: Definitions and equations Part 3: Laws of Thermodynamics Part 1: theory and concept Thermodynamics means conversion
Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 Chem 102--Exam #2 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. When water is measured in a plastic graduated cylinder, a reverse meniscus
Chem 102--Exam #2 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. When water is measured in a plastic graduated cylinder, a reverse meniscus
Secondary organic aerosol 2. Thermodynamic model for gas/particle partitioning of molecular constituents
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D17, 4333, doi:10.1029/2001jd000542, 2002 Secondary organic aerosol 2. Thermodynamic model for gas/particle partitioning of molecular constituents Betty K.
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. D17, 4333, doi:10.1029/2001jd000542, 2002 Secondary organic aerosol 2. Thermodynamic model for gas/particle partitioning of molecular constituents Betty K.
MATHEMATICAL MODELING OF DISBONDED COATING AND CATHODIC DELAMINATION SYSTEMS KERRY N. ALLAHAR
 MATHEMATICAL MODELING OF DISBONDED COATING AND CATHODIC DELAMINATION SYSTEMS By KERRY N. ALLAHAR A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE
MATHEMATICAL MODELING OF DISBONDED COATING AND CATHODIC DELAMINATION SYSTEMS By KERRY N. ALLAHAR A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE
2. Explain why the ph of unbuffered water is generally around 5.7
 CHEM 108b FINAL EXAM The exam is worth a total of 100 points. Each problem is worth five points. Show all work for partial credit. Don t forget to label units and check whether equations are balanced if
CHEM 108b FINAL EXAM The exam is worth a total of 100 points. Each problem is worth five points. Show all work for partial credit. Don t forget to label units and check whether equations are balanced if
Contents. 1 Matter: Its Properties and Measurement 1. 2 Atoms and the Atomic Theory Chemical Compounds Chemical Reactions 111
 Ed: Pls provide art About the Authors Preface xvii xvi 1 Matter: Its Properties and Measurement 1 1-1 The Scientific Method 2 1-2 Properties of Matter 4 1-3 Classification of Matter 5 1-4 Measurement of
Ed: Pls provide art About the Authors Preface xvii xvi 1 Matter: Its Properties and Measurement 1 1-1 The Scientific Method 2 1-2 Properties of Matter 4 1-3 Classification of Matter 5 1-4 Measurement of
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112, D24S13, doi: /2007jd008424, 2007
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2007jd008424, 2007 A phase equilibrium model for atmospheric aerosols containing inorganic electrolytes and organic compounds (UHAERO), with application
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2007jd008424, 2007 A phase equilibrium model for atmospheric aerosols containing inorganic electrolytes and organic compounds (UHAERO), with application
CHEMISTRY XL-14A PHYSICAL EQUILIBRIUM. August 13, 2011 Robert Iafe
 CHEMISTRY XL-14A PHYSICAL EQUILIBRIUM August 13, 2011 Robert Iafe Chapter Overview 2 Phases and Phase Transitions Solubility Colligative Properties Binary Liquid Mixtures Phases and Phase Transitions 3
CHEMISTRY XL-14A PHYSICAL EQUILIBRIUM August 13, 2011 Robert Iafe Chapter Overview 2 Phases and Phase Transitions Solubility Colligative Properties Binary Liquid Mixtures Phases and Phase Transitions 3
Introduction to Chemical Thermodynamics. (10 Lectures) Michaelmas Term
 Introduction to Chemical Thermodynamics Dr. D. E. Manolopoulos First Year (0 Lectures) Michaelmas Term Lecture Synopsis. Introduction & Background. Le Chatelier s Principle. Equations of state. Systems
Introduction to Chemical Thermodynamics Dr. D. E. Manolopoulos First Year (0 Lectures) Michaelmas Term Lecture Synopsis. Introduction & Background. Le Chatelier s Principle. Equations of state. Systems
Chapter 11: Properties of Solutions - Their Concentrations and Colligative Properties. Chapter Outline
 Chapter 11: Properties of Solutions - Their Concentrations and Colligative Properties Chapter Outline 11.1 Energy Changes when Substances Dissolve 11.2 Vapor Pressure 11.3 Mixtures of Volatile Substances
Chapter 11: Properties of Solutions - Their Concentrations and Colligative Properties Chapter Outline 11.1 Energy Changes when Substances Dissolve 11.2 Vapor Pressure 11.3 Mixtures of Volatile Substances
Foreword. The International Baccalaureate (IB) Diploma Programme is a challenging two-year curriculum
 Foreword Academic success can be measured in many different ways, and I often tell my students that scoring high marks in exams is only one of the rewards from diligent study. The true measures of academic
Foreword Academic success can be measured in many different ways, and I often tell my students that scoring high marks in exams is only one of the rewards from diligent study. The true measures of academic
Lecture 1: Physical Equilibria The Temperature Dependence of Vapor Pressure
 Lecture 1: Physical Equilibria The Temperature Dependence of Vapor Pressure Our first foray into equilibria is to examine phenomena associated with two phases of matter achieving equilibrium in which the
Lecture 1: Physical Equilibria The Temperature Dependence of Vapor Pressure Our first foray into equilibria is to examine phenomena associated with two phases of matter achieving equilibrium in which the
Introduction to Chemical Thermodynamics. D. E. Manolopoulos First Year (13 Lectures) Michaelmas Term
 Introduction to Chemical Thermodynamics D. E. Manolopoulos First Year (13 Lectures) Michaelmas Term Lecture Synopsis 1. Introduction & Background. Le Chatelier s Principle. Equations of state. Systems
Introduction to Chemical Thermodynamics D. E. Manolopoulos First Year (13 Lectures) Michaelmas Term Lecture Synopsis 1. Introduction & Background. Le Chatelier s Principle. Equations of state. Systems
CHAPTER 1 INTRODUCTION...1
 Acknowledgement My most sincere thanks go to my advisor and mentor, Dr. Roe-Hoan Yoon. I thank him for introducing me to the wonders and frustrations of scientific research. I thank him for his guidance,
Acknowledgement My most sincere thanks go to my advisor and mentor, Dr. Roe-Hoan Yoon. I thank him for introducing me to the wonders and frustrations of scientific research. I thank him for his guidance,
CH 302 Spring 2008 Worksheet 4 Answer Key Practice Exam 1
 CH 302 Spring 2008 Worksheet 4 Answer Key Practice Exam 1 1. Predict the signs of ΔH and ΔS for the sublimation of CO 2. a. ΔH > 0, ΔS > 0 b. ΔH > 0, ΔS < 0 c. ΔH < 0, ΔS > 0 d. ΔH < 0, ΔS < 0 Answer:
CH 302 Spring 2008 Worksheet 4 Answer Key Practice Exam 1 1. Predict the signs of ΔH and ΔS for the sublimation of CO 2. a. ΔH > 0, ΔS > 0 b. ΔH > 0, ΔS < 0 c. ΔH < 0, ΔS > 0 d. ΔH < 0, ΔS < 0 Answer:
Unit 5: Spontaneity of Reaction. You need to bring your textbooks everyday of this unit.
 Unit 5: Spontaneity of Reaction You need to bring your textbooks everyday of this unit. THE LAWS OF THERMODYNAMICS 1 st Law of Thermodynamics Energy is conserved ΔE = q + w 2 nd Law of Thermodynamics A
Unit 5: Spontaneity of Reaction You need to bring your textbooks everyday of this unit. THE LAWS OF THERMODYNAMICS 1 st Law of Thermodynamics Energy is conserved ΔE = q + w 2 nd Law of Thermodynamics A
where the terms are substituted according to Table 3 for the various conserved quantities.
 SUPPLEMENTARY MATERIAL A. Equations solved for flow field calculation Below are the equations used in the FORTRAN 77 computational fluid dynamics code used for determining the flow and other scalar fields
SUPPLEMENTARY MATERIAL A. Equations solved for flow field calculation Below are the equations used in the FORTRAN 77 computational fluid dynamics code used for determining the flow and other scalar fields
FACULTY OF SCIENCE MID-TERM EXAMINATION 2 MARCH 18, :30 TO 8:30 PM CHEMISTRY 120 GENERAL CHEMISTRY
 FACULTY OF SCIENCE MID-TERM EXAMINATION 2 MARCH 18, 2011. 6:30 TO 8:30 PM CHEMISTRY 120 GENERAL CHEMISTRY Examiners: Prof. B. Siwick Prof. A. Mittermaier Dr. A. Fenster Name: Associate Examiner: A. Fenster
FACULTY OF SCIENCE MID-TERM EXAMINATION 2 MARCH 18, 2011. 6:30 TO 8:30 PM CHEMISTRY 120 GENERAL CHEMISTRY Examiners: Prof. B. Siwick Prof. A. Mittermaier Dr. A. Fenster Name: Associate Examiner: A. Fenster
CHM 1046 FINAL REVIEW
 CHM 1046 FINAL REVIEW Prepared & Presented By: Marian Ayoub PART I Chapter Description 6 Thermochemistry 11 States of Matter; Liquids and Solids 12 Solutions 13 Rates of Reactions 18 Thermodynamics and
CHM 1046 FINAL REVIEW Prepared & Presented By: Marian Ayoub PART I Chapter Description 6 Thermochemistry 11 States of Matter; Liquids and Solids 12 Solutions 13 Rates of Reactions 18 Thermodynamics and
Solutions. Chapter 14 Solutions. Ion-Ion Forces (Ionic Bonding) Attraction Between Ions and Permanent Dipoles. Covalent Bonding Forces
 Solutions Chapter 14 1 Brief Review of Major Topics in Chapter 13, Intermolecular forces Ion-Ion Forces (Ionic Bonding) 2 Na + Cl - in salt These are the strongest forces. Lead to solids with high melting
Solutions Chapter 14 1 Brief Review of Major Topics in Chapter 13, Intermolecular forces Ion-Ion Forces (Ionic Bonding) 2 Na + Cl - in salt These are the strongest forces. Lead to solids with high melting
Chapter 11: Properties of Solutions - Their Concentrations and Colligative Properties. Chapter Outline
 Chapter 11: Properties of Solutions - Their Concentrations and Colligative Properties Chapter Outline 11.1 Energy Changes when Substances Dissolve 11.2 Vapor Pressure 11.3 Mixtures of Volatile Substances
Chapter 11: Properties of Solutions - Their Concentrations and Colligative Properties Chapter Outline 11.1 Energy Changes when Substances Dissolve 11.2 Vapor Pressure 11.3 Mixtures of Volatile Substances
Introduction: Introduction. material is transferred from one phase (gas, liquid, or solid) into another.
 Introduction: Virtually all commercial chemical processes involve operations in which material is transferred from one phase (gas, liquid, or solid) into another. rewing a cup of Coffee (Leaching) Removal
Introduction: Virtually all commercial chemical processes involve operations in which material is transferred from one phase (gas, liquid, or solid) into another. rewing a cup of Coffee (Leaching) Removal
MULTIPLE CHOICE PORTION:
 AP Chemistry Fall Semester Practice Exam 4 MULTIPLE CHOICE PORTION: Write the letter for the correct answer to the following questions on the provided answer sheet. Each multiple choice question is worth
AP Chemistry Fall Semester Practice Exam 4 MULTIPLE CHOICE PORTION: Write the letter for the correct answer to the following questions on the provided answer sheet. Each multiple choice question is worth
Chapter 12 Intermolecular Forces and Liquids
 Chapter 12 Intermolecular Forces and Liquids Jeffrey Mack California State University, Sacramento Why? Why is water usually a liquid and not a gas? Why does liquid water boil at such a high temperature
Chapter 12 Intermolecular Forces and Liquids Jeffrey Mack California State University, Sacramento Why? Why is water usually a liquid and not a gas? Why does liquid water boil at such a high temperature
Gibbs Free Energy Study Guide Name: Date: Period:
 Gibbs Free Energy Study Guide Name: Date: Period: The basic goal of chemistry is to predict whether or not a reaction will occur when reactants are brought together. Ways to predict spontaneous reactions
Gibbs Free Energy Study Guide Name: Date: Period: The basic goal of chemistry is to predict whether or not a reaction will occur when reactants are brought together. Ways to predict spontaneous reactions
Core. Topic 1: Stoichiometric relationships. Essential idea: Physical and chemical properties depend on the ways in which different atoms combine.
 Core 32 Essential idea: Physical and chemical properties depend on the ways in which different atoms combine. 1.1 Introduction to the particulate nature of matter and chemical change Nature of science:
Core 32 Essential idea: Physical and chemical properties depend on the ways in which different atoms combine. 1.1 Introduction to the particulate nature of matter and chemical change Nature of science:
PX-III Chem 1411 Chaps 11 & 12 Ebbing
 PX-III Chem 1411 Chaps 11 & 12 Ebbing 1. What is the name for the following phase change? I 2 (s) I 2 (g) A) melting B) condensation C) sublimation D) freezing E) vaporization 2. Which of the following
PX-III Chem 1411 Chaps 11 & 12 Ebbing 1. What is the name for the following phase change? I 2 (s) I 2 (g) A) melting B) condensation C) sublimation D) freezing E) vaporization 2. Which of the following
Business. Business. Multiphase Systems Ch. 6. P vs T Diagram: Water (pure component) P vs T Diagram: CO 2 LYNN ORR
 Business LYNN ORR Izatt-Christensen Lecturer Former Assistant Secretary of Energy Public lecture Thursday 11 am, JSB Auditorium Technical lecture, Friday, 11 am, Varsity Theater Courtesy Corbin Critchfield
Business LYNN ORR Izatt-Christensen Lecturer Former Assistant Secretary of Energy Public lecture Thursday 11 am, JSB Auditorium Technical lecture, Friday, 11 am, Varsity Theater Courtesy Corbin Critchfield
Curriculum Guide Chemistry
 Chapter 1: Introduction to Chemistry Why is chemistry important in using dominion science? Is chemistry necessary in all aspects of life? How can a chemist advance science for the kingdom of God? 1 Lesson
Chapter 1: Introduction to Chemistry Why is chemistry important in using dominion science? Is chemistry necessary in all aspects of life? How can a chemist advance science for the kingdom of God? 1 Lesson
A) sublimation. B) liquefaction. C) evaporation. D) condensation. E) freezing. 11. Below is a phase diagram for a substance.
 PX0411-1112 1. Which of the following statements concerning liquids is incorrect? A) The volume of a liquid changes very little with pressure. B) Liquids are relatively incompressible. C) Liquid molecules
PX0411-1112 1. Which of the following statements concerning liquids is incorrect? A) The volume of a liquid changes very little with pressure. B) Liquids are relatively incompressible. C) Liquid molecules
1.8 Thermodynamics. N Goalby chemrevise.org. Definitions of enthalpy changes
 1.8 Thermodynamics Definitions of enthalpy changes Enthalpy change of formation The standard enthalpy change of formation of a compound is the energy transferred when 1 mole of the compound is formed from
1.8 Thermodynamics Definitions of enthalpy changes Enthalpy change of formation The standard enthalpy change of formation of a compound is the energy transferred when 1 mole of the compound is formed from
Lecture Notes 2: Physical Equilibria Phase Diagrams
 Lecture Notes 2: Physical Equilibria Phase Diagrams There are number of graphical means to help to understand the relationships between the different phases of a particular substance. The first thing we
Lecture Notes 2: Physical Equilibria Phase Diagrams There are number of graphical means to help to understand the relationships between the different phases of a particular substance. The first thing we
First Law of Thermodynamics
 First Law of Thermodynamics Remember: ΔE univ = 0 Total energy of the universe is constant. Energy can be transferred: ΔE = q + w q = heat w = work (F*D) = ΔPV 1 st Law, review For constant volume process:
First Law of Thermodynamics Remember: ΔE univ = 0 Total energy of the universe is constant. Energy can be transferred: ΔE = q + w q = heat w = work (F*D) = ΔPV 1 st Law, review For constant volume process:
CHM 2046 Final Exam Review: Chapters 11 18
 Chapter 11 1. Which of the following has the lowest boiling point? a. NH 3 b. CH 3 Cl c. NaCl d. CO 2 e. CH 3 CH 2 CH 2 CH 2 CH 3 2. Which of the following has the lowest vapor pressure? a. CH 3 F b. CH
Chapter 11 1. Which of the following has the lowest boiling point? a. NH 3 b. CH 3 Cl c. NaCl d. CO 2 e. CH 3 CH 2 CH 2 CH 2 CH 3 2. Which of the following has the lowest vapor pressure? a. CH 3 F b. CH
Chem 75 February, 2017 Practice Exam 2
 As before, here is last year s Exam 2 in two forms: just the questions, and then the questions followed by their solutions. 1. (2 + 6 + 8 points) At high temperature, aluminum nitride, AlN(s), decomposes
As before, here is last year s Exam 2 in two forms: just the questions, and then the questions followed by their solutions. 1. (2 + 6 + 8 points) At high temperature, aluminum nitride, AlN(s), decomposes
Last Name or Student ID
 10/06/08, Chem433 Exam # 1 Last Name or Student ID 1. (3 pts) 2. (3 pts) 3. (3 pts) 4. (2 pts) 5. (2 pts) 6. (2 pts) 7. (2 pts) 8. (2 pts) 9. (6 pts) 10. (5 pts) 11. (6 pts) 12. (12 pts) 13. (22 pts) 14.
10/06/08, Chem433 Exam # 1 Last Name or Student ID 1. (3 pts) 2. (3 pts) 3. (3 pts) 4. (2 pts) 5. (2 pts) 6. (2 pts) 7. (2 pts) 8. (2 pts) 9. (6 pts) 10. (5 pts) 11. (6 pts) 12. (12 pts) 13. (22 pts) 14.
 CHEMISTRY 110 EXAM 3 April 2, 2012 FORM A 1. Which plot depicts the correct relationship between the volume and number of moles of an ideal gas at constant pressure and temperature? 2. The height of the
CHEMISTRY 110 EXAM 3 April 2, 2012 FORM A 1. Which plot depicts the correct relationship between the volume and number of moles of an ideal gas at constant pressure and temperature? 2. The height of the
Incremental Aerosol Reactivity: Application to Aromatic and Biogenic Hydrocarbons
 Environ. Sci. Technol. 1999, 33, 2403-2408 Incremental Aerosol Reactivity: Application to Aromatic and Biogenic Hydrocarbons ROBERT J. GRIFFI, DAVID R. COCKER III, AD JOH H. SEIFELD*, Department of Chemical
Environ. Sci. Technol. 1999, 33, 2403-2408 Incremental Aerosol Reactivity: Application to Aromatic and Biogenic Hydrocarbons ROBERT J. GRIFFI, DAVID R. COCKER III, AD JOH H. SEIFELD*, Department of Chemical
Energy is the capacity to do work
 1 of 10 After completing this chapter, you should, at a minimum, be able to do the following. This information can be found in my lecture notes for this and other chapters and also in your text. Correctly
1 of 10 After completing this chapter, you should, at a minimum, be able to do the following. This information can be found in my lecture notes for this and other chapters and also in your text. Correctly
Supplement of Secondary formation of nitrated phenols: insights from observations during the Uintah Basin Winter Ozone Study (UBWOS) 2014
 Supplement of Atmos. Chem. Phys., 16, 2139 2153, 2016 http://www.atmos-chem-phys.net/16/2139/2016/ doi:10.5194/acp-16-2139-2016-supplement Author(s) 2016. CC Attribution 3.0 License. Supplement of Secondary
Supplement of Atmos. Chem. Phys., 16, 2139 2153, 2016 http://www.atmos-chem-phys.net/16/2139/2016/ doi:10.5194/acp-16-2139-2016-supplement Author(s) 2016. CC Attribution 3.0 License. Supplement of Secondary
CHAPTER 4 Physical Transformations of Pure Substances.
 I. Generalities. CHAPTER 4 Physical Transformations of Pure Substances. A. Definitions: 1. A phase of a substance is a form of matter that is uniform throughout in chemical composition and physical state.
I. Generalities. CHAPTER 4 Physical Transformations of Pure Substances. A. Definitions: 1. A phase of a substance is a form of matter that is uniform throughout in chemical composition and physical state.
States of matter Part 2
 Physical Pharmacy Lecture 2 States of matter Part 2 Assistant Lecturer in Pharmaceutics Overview The Liquid State General properties Liquefaction of gases Vapor pressure of liquids Boiling point The Solid
Physical Pharmacy Lecture 2 States of matter Part 2 Assistant Lecturer in Pharmaceutics Overview The Liquid State General properties Liquefaction of gases Vapor pressure of liquids Boiling point The Solid
Chemical reaction equilibria
 Chemical reaction equilibria Chemical reaction equilibria in metallurgical processes and the conditions that maintain equilibrium are important to obtain maximum efficiency from production processes For
Chemical reaction equilibria Chemical reaction equilibria in metallurgical processes and the conditions that maintain equilibrium are important to obtain maximum efficiency from production processes For
Thermochemistry Chapter 8
 Thermochemistry Chapter 8 Thermochemistry First law of thermochemistry: Internal energy of an isolated system is constant; energy cannot be created or destroyed; however, energy can be converted to different
Thermochemistry Chapter 8 Thermochemistry First law of thermochemistry: Internal energy of an isolated system is constant; energy cannot be created or destroyed; however, energy can be converted to different
CHEMISTRY 102 FALL 2010 EXAM 1 FORM C SECTION 502 DR. KEENEY-KENNICUTT PART 1
 NAME CHEMISTRY 102 FALL 2010 EXAM 1 FORM C SECTION 502 DR. KEENEY-KENNICUTT Directions: (1) Put your name on PART 1 and your name and signature on PART 2 of the exam where indicated. (2) Sign the Aggie
NAME CHEMISTRY 102 FALL 2010 EXAM 1 FORM C SECTION 502 DR. KEENEY-KENNICUTT Directions: (1) Put your name on PART 1 and your name and signature on PART 2 of the exam where indicated. (2) Sign the Aggie
Four states of matter (Aggregatzustand) a. solid b. liquid c. gaseous d. plasma
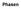 Phasen Four states of matter (Aggregatzustand) a. solid b. liquid c. gaseous d. plasma T Phase diagram H 2 0 Lines = equilibrium = phase boundary = co-existence Sublimate/Condense Evaporate/Condense Melt/Freeze
Phasen Four states of matter (Aggregatzustand) a. solid b. liquid c. gaseous d. plasma T Phase diagram H 2 0 Lines = equilibrium = phase boundary = co-existence Sublimate/Condense Evaporate/Condense Melt/Freeze
Chapter 11: Properties of Solutions - Their Concentrations and Colligative Properties. Chapter Outline
 Chapter 11: Properties of Solutions - Their Concentrations and Colligative Properties Chapter Outline 11.1 Energy Changes when Substances Dissolve 11.2 Vapor Pressure 11.3 Mixtures of Volatile Substances
Chapter 11: Properties of Solutions - Their Concentrations and Colligative Properties Chapter Outline 11.1 Energy Changes when Substances Dissolve 11.2 Vapor Pressure 11.3 Mixtures of Volatile Substances
Unit Five: Intermolecular Forces MC Question Practice April 14, 2017
 Unit Five: Intermolecular Forces Name MC Question Practice April 14, 2017 1. Which of the following should have the highest surface tension at a given temperature? 2. The triple point of compound X occurs
Unit Five: Intermolecular Forces Name MC Question Practice April 14, 2017 1. Which of the following should have the highest surface tension at a given temperature? 2. The triple point of compound X occurs
Let's look at how different properties affect vapor pressure. P =0 P =vapor pressure P =vapor pressure. first all liquid
 Let's look at how different properties affect vapor pressure P =0 P =vapor pressure P =vapor pressure Quick Quiz You have two containers. one has a total volume of 2 L and one has a total volume of 1 L
Let's look at how different properties affect vapor pressure P =0 P =vapor pressure P =vapor pressure Quick Quiz You have two containers. one has a total volume of 2 L and one has a total volume of 1 L
= = 10.1 mol. Molar Enthalpies of Vaporization (at Boiling Point) Molar Enthalpy of Vaporization (kj/mol)
 Ch 11 (Sections 11.1 11.5) Liquid Phase Volume and Density - Liquid and solid are condensed phases and their volumes are not simple to calculate. - This is different from gases, which have volumes that
Ch 11 (Sections 11.1 11.5) Liquid Phase Volume and Density - Liquid and solid are condensed phases and their volumes are not simple to calculate. - This is different from gases, which have volumes that
Homework Problem Set 8 Solutions
 Chemistry 360 Dr. Jean M. Standard Homework roblem Set 8 Solutions. Starting from G = H S, derive the fundamental equation for G. o begin, we take the differential of G, dg = dh d( S) = dh ds Sd. Next,
Chemistry 360 Dr. Jean M. Standard Homework roblem Set 8 Solutions. Starting from G = H S, derive the fundamental equation for G. o begin, we take the differential of G, dg = dh d( S) = dh ds Sd. Next,
CHEMISTRY - UTEXAS 1E CH.7 - PHYSICAL EQUILIBRIA.
 !! www.clutchprep.com CONCEPT: PHASE DIAGRAMS Under appropriate conditions of pressure and temperature, most substances can exist in 3 states of matter:, and. Microscopic Explanation for the Behavior of
!! www.clutchprep.com CONCEPT: PHASE DIAGRAMS Under appropriate conditions of pressure and temperature, most substances can exist in 3 states of matter:, and. Microscopic Explanation for the Behavior of
Questions 1 13 cover material from Exam 1
 Questions 1 13 cover material from Exam 1 1. Which intermolecular forces are present in H Te(l)? A. dispersion only C. dispersion, dipole-dipole, and hydrogen bonding B. dispersion and dipole-dipole D.
Questions 1 13 cover material from Exam 1 1. Which intermolecular forces are present in H Te(l)? A. dispersion only C. dispersion, dipole-dipole, and hydrogen bonding B. dispersion and dipole-dipole D.
Chapter Seventeen Thermodynamics: Spontaneity, Entropy, and Free Energy
 1 Thermodynamics: Spontaneity, Entropy, and Free Energy 2 Introductory Concepts Thermodynamics examines the relationship between heat (q) and work (w) Spontaneity is the notion of whether or not a process
1 Thermodynamics: Spontaneity, Entropy, and Free Energy 2 Introductory Concepts Thermodynamics examines the relationship between heat (q) and work (w) Spontaneity is the notion of whether or not a process
Chemistry Assessment Unit A2 1
 Centre Number 71 Candidate Number ADVANCED General Certificate of Education January 2011 Chemistry Assessment Unit A2 1 assessing Periodic Trends and Further Organic, Physical and Inorganic Chemistry [AC212]
Centre Number 71 Candidate Number ADVANCED General Certificate of Education January 2011 Chemistry Assessment Unit A2 1 assessing Periodic Trends and Further Organic, Physical and Inorganic Chemistry [AC212]
Thermodynamics II. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Thermodynamics II Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Spontaneous Physical and Chemical Processes A waterfall runs downhill A lump of sugar dissolves
Thermodynamics II Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Spontaneous Physical and Chemical Processes A waterfall runs downhill A lump of sugar dissolves
Chapter 11 part 2. Properties of Liquids Viscosity Surface Tension Capillary Action. Phase Changes (energy of phase changes)
 Chapter 11 part 2 Properties of Liquids Viscosity Surface Tension Capillary Action Phase Changes (energy of phase changes) Dynamic Equilibrium Vapor pressure Phase diagram 1 Structure Affects Function
Chapter 11 part 2 Properties of Liquids Viscosity Surface Tension Capillary Action Phase Changes (energy of phase changes) Dynamic Equilibrium Vapor pressure Phase diagram 1 Structure Affects Function
OCR Chemistry A H432
 All the energy changes we have considered so far have been in terms of enthalpy, and we have been able to predict whether a reaction is likely to occur on the basis of the enthalpy change associated with
All the energy changes we have considered so far have been in terms of enthalpy, and we have been able to predict whether a reaction is likely to occur on the basis of the enthalpy change associated with
VOCABULARY. Set #2. Set #1
 VOCABULARY Set #1 1. Absolute zero 2. Accepted value 3. Accuracy 4. Celsius scale 5. Conversion factor 6. Density 7. Dimensional analysis 8. Experimental value 9. Gram 10. International system of units
VOCABULARY Set #1 1. Absolute zero 2. Accepted value 3. Accuracy 4. Celsius scale 5. Conversion factor 6. Density 7. Dimensional analysis 8. Experimental value 9. Gram 10. International system of units
Solids, liquids and gases
 Solids, liquids and gases Solids, liquids, and gases are held together by intermolecular forces. Intermolecular forces occur between molecules, not within molecules (as in bonding). When a molecule changes
Solids, liquids and gases Solids, liquids, and gases are held together by intermolecular forces. Intermolecular forces occur between molecules, not within molecules (as in bonding). When a molecule changes
Chem 112, Fall 05 (Garman/Weis) Final Exam A, 12/20/2005 (Print Clearly) +2 points
 +2 points Before you begin, make sure that your exam has all 10 pages. There are 24 required problems worth 4 points apiece, unless otherwise noted, and two 4-point extra credit problems. Stay focused,
+2 points Before you begin, make sure that your exam has all 10 pages. There are 24 required problems worth 4 points apiece, unless otherwise noted, and two 4-point extra credit problems. Stay focused,
Chapter 10 Liquids, Solids and Phase Changes
 Chapter 10 Liquids, Solids and Phase Changes The three phases of matter: solids, liquids and gases Phase change properties of pure water at 1 atm pressure What is a boiling point? What does it measure?
Chapter 10 Liquids, Solids and Phase Changes The three phases of matter: solids, liquids and gases Phase change properties of pure water at 1 atm pressure What is a boiling point? What does it measure?
CHEMpossible. Final Exam Review
 CHEMpossible Final Exam Review 1. Given the following pair of reactions and their equilibrium constants: 2NO 2 (g) 2NO (g) + O 2 (g) K c = 15.5 2NO (g) + Cl 2 (g) 2 NOCl (g) K c = 3.20 10-3 Calculate a
CHEMpossible Final Exam Review 1. Given the following pair of reactions and their equilibrium constants: 2NO 2 (g) 2NO (g) + O 2 (g) K c = 15.5 2NO (g) + Cl 2 (g) 2 NOCl (g) K c = 3.20 10-3 Calculate a
READING. Review of Intermolecular Forces & Liquids (Chapter 12) Ion-Ion Forces. Ion-Dipole Energies
 Review of Intermolecular Forces & Liquids (Chapter 12) CEM 102 T. ughbanks READIG We will very briefly review the underlying concepts from Chapters 12 on intermolecular forces since it is relevant to Chapter
Review of Intermolecular Forces & Liquids (Chapter 12) CEM 102 T. ughbanks READIG We will very briefly review the underlying concepts from Chapters 12 on intermolecular forces since it is relevant to Chapter
12A Entropy. Entropy change ( S) N Goalby chemrevise.org 1. System and Surroundings
 12A Entropy Entropy change ( S) A SPONTANEOUS PROCESS (e.g. diffusion) will proceed on its own without any external influence. A problem with H A reaction that is exothermic will result in products that
12A Entropy Entropy change ( S) A SPONTANEOUS PROCESS (e.g. diffusion) will proceed on its own without any external influence. A problem with H A reaction that is exothermic will result in products that
Hygroscopic growth of ammonium sulfate/dicarboxylic acids
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D20, 4638, doi:10.1029/2003jd003775, 2003 Hygroscopic growth of ammonium sulfate/dicarboxylic acids Matthew E. Wise, Jason D. Surratt, Daniel B. Curtis, John
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D20, 4638, doi:10.1029/2003jd003775, 2003 Hygroscopic growth of ammonium sulfate/dicarboxylic acids Matthew E. Wise, Jason D. Surratt, Daniel B. Curtis, John
CHAPTER OUTLINE. I. The Structure of Water: An Introduction to Intermolecular Forces
 The Chemistry of Water and the Nature of Liquids Chapter 11 CHAPTER OUTLINE 11.2 I. The Structure of Water: An Introduction to Intermolecular Forces II. A Closer Look at Intermolecular lar Forces A. London
The Chemistry of Water and the Nature of Liquids Chapter 11 CHAPTER OUTLINE 11.2 I. The Structure of Water: An Introduction to Intermolecular Forces II. A Closer Look at Intermolecular lar Forces A. London
CHEM 1032 PRACTICE EXAM I CLASS SPRING 2017
 1 CHEM 1032 PRACTICE EXAM I CLASS SPRING 2017 1. Select the characteristic(s) of the liquid phase: (You may need a periodic table. Useful information appears on page 5.) (i) adopts the shape of the container
1 CHEM 1032 PRACTICE EXAM I CLASS SPRING 2017 1. Select the characteristic(s) of the liquid phase: (You may need a periodic table. Useful information appears on page 5.) (i) adopts the shape of the container
Chapter 2 Experimental sources of intermolecular potentials
 Chapter 2 Experimental sources of intermolecular potentials 2.1 Overview thermodynamical properties: heat of vaporization (Trouton s rule) crystal structures ionic crystals rare gas solids physico-chemical
Chapter 2 Experimental sources of intermolecular potentials 2.1 Overview thermodynamical properties: heat of vaporization (Trouton s rule) crystal structures ionic crystals rare gas solids physico-chemical
Homework 01. Phase Changes and Solutions
 HW01 - Phase Changes and Solu!ons! This is a preview of the published version of the quiz Started: Jan 16 at 1:pm Quiz Instruc!ons Homework 01 Phase Changes and Solutions Question 1 Given that you have
HW01 - Phase Changes and Solu!ons! This is a preview of the published version of the quiz Started: Jan 16 at 1:pm Quiz Instruc!ons Homework 01 Phase Changes and Solutions Question 1 Given that you have
