Unit 1 Atomic Theory
|
|
|
- Asher Mosley
- 6 years ago
- Views:
Transcription
1 Unit 1 Atomic Theory 1.0 You are expected to be already familiar with. Ionic nomenclature (binary, polyatomic, multivalency) Covalent nomenclature Writing chemical formulas for ionic and covalent compounds 1 Gu 2017
2 1.1 Nuclear Notation (WB P.60, 63 64, ) Atomic number: equals to Mass number: Atomic mass: Isotope: Nuclear Notation: There are two ways to describe atoms: Example: Write the symbol for a neutral Fluorine 19 atom Write the symbol for a Barium 137 atom that has had 2 electrons removed Write the symbol for neutral Carbon 13 Symbol # protons # electrons # neutrons Atomic number Atomic mass Charge Gu 2017
3 1.2 Bohr Model (WB P.61, 63 64) Practice: Draw the Bohr diagram for the following atoms: a) Na b) N c) N ion d) Mg ion e) Lithium fluoride f) CH 4 g) NH 3 h) oxygen gas 3 Gu 2017
4 1.3 Lewis Structures (WB P.62 64) Drawing atoms element symbol represents nucleus + core electrons dots represent valence electrons dots are drawn in pairs as a reminder that electrons are paired in orbitals Example: Draw the Lewis structure for the following atoms Li Be B C N O F Ne Drawing Covalent Molecules octet rule predicts bonding arrangement: bonded non metallic atoms have 8 electrons in their outermost energy levels (exception can only have 2) molecules tend to be symmetrical covalent and polar covalent bonds are represented by pairs of dots between two atoms number of dots you draw must equal the sum of the valence electrons of all atoms in the molecule pairs of electrons forming covalent bonds can be represented by a line Example: Draw the Lewis structure for the following molecules a) CCl 4 b) NH 3 c) C 2 H 6 d) CO 2 4 Gu 2017
5 Example: Draw the Lewis structure of HCN Example: Draw the Lewis structure of calcium chloride. 1.4 Shrӧdinger and Heisenberg Atomic Model (FS) Bohr s model of the atom was famous because it could explain the. Activity: A spectroscope is a tool that separates light into its individual components. Use a spectroscope to see what is in white light from the sun, versus what is in light produced by energized elements. Light Source What I Observe sunlight 5 Gu 2017
6 The spectrum of white light is continuous: it shows all the colours of the rainbow. The spectrum of energized gas is discontinuous, it shows discrete bands. Summarize how the bright line spectrum supports Bohr s atomic model: Bohr s model is significantly wrong in two ways: Gu 2017
7 Schrödinger s model describes the of where to find an electron in an atom Orbital: the around a nucleus where an electron can be found orbitals are described by quantum numbers. Quantum Number 1 st or principal quantum number (n) 2 nd quantum number (l): 3 rd quantum number (m l ): 4 th quantum number (m s ): Symbols What does it Mean? 7 Gu 2017
8 Each energy level has a specific set of orbitals and each one represents where a maximum of electrons can be found. Orbital Type Begins at n= # of Orbitals in a Subshell s 1 p 2 d 3 f 4 Maximum # of Electrons in a Subshell Instead of representing atoms with the Bohr diagram, we can represent them with the more accurate energy level diagram. Rules to follow when filling orbitals: 1. Fill orbitals from lowest to highest energy (Aufbau Principle) 2. Place one electron in each orbital of a sub shell 3. When each orbital of a sub shell has one electron, go back and pair the electrons (Hund s Rule) 4. If two electrons are in an orbital, they must have opposite spin (Pauli Exclusion Principle) These rules ensure that the electron configuration gives the lowest energy, most stable atom by reducing. 8 Gu 2017
9 Example: Fill the orbitals with He electrons Example: Fill the orbitals with Na electrons Example: Fill the orbitals with Al electrons How many electrons are in He? How many electrons are in Na? How many electrons are in Al? How many shells have electrons? How many sub shells have electrons? How many orbitals have a single electron? How many orbitals have paired electrons? How many shells have electrons? How many sub shells have electrons? How many orbitals have a single electron? How many orbitals have paired electrons? How many shells have electrons? How many sub shells have electrons? How many orbitals have a single electron? How many orbitals have paired electrons? What is the electron configuration? What is the electron configuration? What is the electron configuration? 9 Gu 2017
10 The periodic table is a tool to obtain the electron configuration of elements quickly. Simply write the orbitals as they appear on the period table above, going left to right, row by row. Each element space counts as one electron. Example: Write the electron configuration for the following atoms. a) Ar b) Ga c) Ag d) *Rn Core notation: It sure is annoying to write super long electron configurations! the shortcut: look for the closest previous noble gas element to the element you are writing the configuration for and start there useful because we aren t interested in the core electrons anyways (they don t participate in chemical reactions) to write core notation for a noble gas, use the previous noble gas Example: Write the electron configuration for Ga using core notation. Closest previous noble gas element: Core notation: 10 Gu 2017
11 Practice: Write the electron configuration in core notation for the following elements. a) Zn b) K c) Kr 1.5 Atomic Trends: Atomic Radii (FS) Ponder This: What do you suppose happens to the size of an atom as you a) Move from left to right across the periodic table? b) Move down a family on the periodic table? c) Add electrons to create an anion? d) Remove electrons to create a cation? 11 Gu 2017
12 Consider This: Which has a larger effect on atomic radii, a change in the number of protons, or a change in the number of electrons? Example: Consider the following pairs of atoms. Which atom has the larger atomic radius? a) O and O 2 b) Ca and Ca Gu 2017
13 1.6 Atomic Trends: Electronegativity (FS) Electronegativity: an atom s ability to attract electrons towards itself Ponder This: How does electronegativity change as we a) Move left to right across the periodic table? b) Move down a family on the periodic table? Apply Knowledge: Use what you have learned to explain the reactivity trend of the alkali metals. The reaction occurs when the metal atom donates its valence electrons to water. Summary of Trends 13 Gu 2017
14 1.7 Valence Shell Electron Repulsion Theory (VSEPR) (FS) Visit MolView at molview.org and use the molecular model sets to complete the following. 1. On the left panel, use MolView tools to draw the Lewis structures of the molecules below. 2. Click 2D to 3D and MolView will generate the 3D structure that you can rotate around. Sketch it. Molecule Lewis Structure (2D) Sketch the VSEPR Structure (3D) CO 2 CH 2 O H 2 O NH 3 CH 4 CH 2 F 2 14 Gu 2017
15 Think: Why are the shapes the way they are? What determines what shape molecules take? Summary # Repelling Items Atoms Bonded to Central Atom Lone Pairs of Electrons Bonded to Central Atom Name Shape Example (Lewis Structure) VSEPR Structure Linear Trigonal planar 4 0 Tetrahedral Trigonal pyramidal 2 2 Bent or Angular 15 Gu 2017
16 1.8 What are Intermolecular Forces? (FS) Ponder This: Why is water in liquid form in the room, whereas oxygen gas is not? Draw a picture of water and oxygen gas at the molecular level in the room to help you think it through. Water Oxygen Gas Visit MolView. 1. Model > Jmol 2. Draw oxygen gas in the left panel then hit 2D to 3D 3. Jmol > MEP Surface lucent then make some observations of the result in the right panel 4. Hit the trashcan button to clear the left field 5. Repeat the above for water 16 Gu 2017
17 Critical Thinking: What do you suppose the different colours mean? Using MolView and the table of electronegativities, can you come up with an explanation for why water molecules stick together, but oxygen ones don t? 1.9 Intermolecular Forces: Dipole Dipole (FS) In order for molecules to be able to form dipole dipole interactions, the molecule must be polar so that a side of one molecule is attracted to the + side of another. Practice: Which of the following molecules are polar? Which can form dipole dipole interactions? a) CH 4 b) H 2 O c) NH 3 17 Gu 2017
18 How molecules arrange themselves when there are dipole dipole interactions: 1.10 Intermolecular Forces: Hydrogen Bonds (FS) A hydrogen bond is essentially a dipole dipole interaction, but occurs at a much stronger level. It occurs when a molecule contains an H atom bonded to an especially electronegative atom (,, ) Ponder This: Why are H bonds so strong? Practice: Which of the following molecules can hydrogen bond? HCN H 2 O H 2 S HF 18 Gu 2017
19 Example: Which would you expect to have a higher melting point, CCl 4 or CHCl 3? 19 Gu 2017
Chapter 10: Modern Atomic Theory and the Periodic Table. How does atomic structure relate to the periodic table? 10.1 Electromagnetic Radiation
 Chapter 10: Modern Atomic Theory and the Periodic Table How does atomic structure relate to the periodic table? 10.1 Electromagnetic Radiation Electromagnetic (EM) radiation is a form of energy that exhibits
Chapter 10: Modern Atomic Theory and the Periodic Table How does atomic structure relate to the periodic table? 10.1 Electromagnetic Radiation Electromagnetic (EM) radiation is a form of energy that exhibits
Essential Organic Chemistry. Chapter 1
 Essential Organic Chemistry Paula Yurkanis Bruice Chapter 1 Electronic Structure and Covalent Bonding Periodic Table of the Elements 1.1 The Structure of an Atom Atoms have an internal structure consisting
Essential Organic Chemistry Paula Yurkanis Bruice Chapter 1 Electronic Structure and Covalent Bonding Periodic Table of the Elements 1.1 The Structure of an Atom Atoms have an internal structure consisting
Electrons and Molecular Forces
 Electrons and Molecular Forces Chemistry 30 Ms. Hayduk Electron Configuration Atomic Structure Atomic Number Number of protons in the nucleus Defines the element Used to organize the periodic table 1 Bohr
Electrons and Molecular Forces Chemistry 30 Ms. Hayduk Electron Configuration Atomic Structure Atomic Number Number of protons in the nucleus Defines the element Used to organize the periodic table 1 Bohr
CHAPTER 12: CHEMICAL BONDING
 CHAPTER 12: CHEMICAL BONDING Problems: 1-26, 27c, 28, 33-34, 35b, 36(a-c), 37(a,b,d), 38a, 39-40, 41-42(a,c), 43-58, 67-74 12.1 THE CHEMICAL BOND CONCEPT chemical bond: what holds atoms or ions together
CHAPTER 12: CHEMICAL BONDING Problems: 1-26, 27c, 28, 33-34, 35b, 36(a-c), 37(a,b,d), 38a, 39-40, 41-42(a,c), 43-58, 67-74 12.1 THE CHEMICAL BOND CONCEPT chemical bond: what holds atoms or ions together
Ionic and Covalent Bonding
 1. Define the following terms: a) valence electrons Ionic and Covalent Bonding the electrons in the highest occupied energy level always electrons in the s and p orbitals maximum of 8 valence electrons
1. Define the following terms: a) valence electrons Ionic and Covalent Bonding the electrons in the highest occupied energy level always electrons in the s and p orbitals maximum of 8 valence electrons
CHEMICAL BONDING IONIC BONDS COVALENT BONDS HYDROGEN BONDS METALLIC BONDS
 CHEMICAL BONDING IONIC BONDS COVALENT BONDS HYDROGEN BONDS METALLIC BONDS IONIC BONDING When an atom of a nonmetal takes one or more electrons from an atom of a metal so both atoms end up with eight valence
CHEMICAL BONDING IONIC BONDS COVALENT BONDS HYDROGEN BONDS METALLIC BONDS IONIC BONDING When an atom of a nonmetal takes one or more electrons from an atom of a metal so both atoms end up with eight valence
Organic Chemistry. Review Information for Unit 1. Atomic Structure MO Theory Chemical Bonds
 Organic Chemistry Review Information for Unit 1 Atomic Structure MO Theory Chemical Bonds Atomic Structure Atoms are the smallest representative particle of an element. Three subatomic particles: protons
Organic Chemistry Review Information for Unit 1 Atomic Structure MO Theory Chemical Bonds Atomic Structure Atoms are the smallest representative particle of an element. Three subatomic particles: protons
Chapter 8. Bonding: General Concepts
 Chapter 8 Bonding: General Concepts Chapter 8 Table of Contents 8.1 Types of Chemical Bonds 8.2 Electronegativity 8.3 Bond Polarity and Dipole Moments 8.4 Ions: Electron Configurations and Sizes 8.5 Energy
Chapter 8 Bonding: General Concepts Chapter 8 Table of Contents 8.1 Types of Chemical Bonds 8.2 Electronegativity 8.3 Bond Polarity and Dipole Moments 8.4 Ions: Electron Configurations and Sizes 8.5 Energy
Chem 1075 Chapter 12 Chemical Bonding Lecture Outline. Chemical Bond Concept
 Chem 1075 Chapter 12 Chemical Bonding Lecture Outline Slide 2 Chemical Bond Concept Recall that an atom has and electrons. Core electrons are found to the nucleus. Valence electrons are found in the s
Chem 1075 Chapter 12 Chemical Bonding Lecture Outline Slide 2 Chemical Bond Concept Recall that an atom has and electrons. Core electrons are found to the nucleus. Valence electrons are found in the s
Chapter 8. Bonding: General Concepts
 Chapter 8 Bonding: General Concepts Chapter 8 Questions to Consider What is meant by the term chemical bond? Why do atoms bond with each other to form compounds? How do atoms bond with each other to form
Chapter 8 Bonding: General Concepts Chapter 8 Questions to Consider What is meant by the term chemical bond? Why do atoms bond with each other to form compounds? How do atoms bond with each other to form
1. What is the phenomenon that occurs when certain metals emit electrons when illuminated by particular wavelengths of light? a.
 CHEMISTRY 123-07 Midterm #3 solution key December 02, 2010 Statistics: Average: 77 p (77%); Highest: 100 p (100%); Lowest: 33 p (33%) Number of students performing at or above average: 54 (52%) Number
CHEMISTRY 123-07 Midterm #3 solution key December 02, 2010 Statistics: Average: 77 p (77%); Highest: 100 p (100%); Lowest: 33 p (33%) Number of students performing at or above average: 54 (52%) Number
Chapter 11 Chemical Bonds: The Formation of Compounds from Atoms Advanced Chemistry Periodic Trends in Atomic Properties Learning Objective
 Chapter 11 Chemical Bonds: The Formation of Compounds from Atoms Advanced Chemistry 11.1 Periodic Trends in Atomic Properties Discuss the atomic trends Metals are located on the left side of the periodic
Chapter 11 Chemical Bonds: The Formation of Compounds from Atoms Advanced Chemistry 11.1 Periodic Trends in Atomic Properties Discuss the atomic trends Metals are located on the left side of the periodic
Chapter 12. Chemical Bonding
 Chapter 12 Chemical Bonding Chemical Bond Concept Recall that an atom has core and valence electrons. Core electrons are found close to the nucleus. Valence electrons are found in the most distant s and
Chapter 12 Chemical Bonding Chemical Bond Concept Recall that an atom has core and valence electrons. Core electrons are found close to the nucleus. Valence electrons are found in the most distant s and
4/25/2017. VSEPR Theory. Two Electron Groups. Shapes of Molecules. Two Electron Groups with Double Bonds. Three Electron Groups.
 Chapter 10 Lecture Chapter 10 Bonding and Properties of Solids and Liquids 10.3 Shapes of Molecules and Ions (VSEPR Theory) Learning Goal Predict the three-dimensional structure of a molecule or a polyatomic
Chapter 10 Lecture Chapter 10 Bonding and Properties of Solids and Liquids 10.3 Shapes of Molecules and Ions (VSEPR Theory) Learning Goal Predict the three-dimensional structure of a molecule or a polyatomic
INTRODUCTORY CHEMISTRY Concepts and Critical Thinking
 INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Sixth Edition by Charles H. Corwin Chapter 12 Chemical Bonding by Christopher Hamaker 2011 Pearson Education, Inc. Chapter 12 1 Chemical Bond Concept
INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Sixth Edition by Charles H. Corwin Chapter 12 Chemical Bonding by Christopher Hamaker 2011 Pearson Education, Inc. Chapter 12 1 Chemical Bond Concept
Chapter 6. The Chemical Bond
 Chapter 6 The Chemical Bond Some questions Why do noble gases rarely bond to other elements? How does this relate to why the atoms of other elements do form bonds? Why do certain elements combine to form
Chapter 6 The Chemical Bond Some questions Why do noble gases rarely bond to other elements? How does this relate to why the atoms of other elements do form bonds? Why do certain elements combine to form
C H E M 1 CHEM 101-GENERAL CHEMISTRY CHAPTER 7 CHEMICAL BONDING & MOLECULAR STRUCTURE INSTR : FİLİZ ALSHANABLEH
 C H E M 1 CHEM 101-GENERAL CHEMISTRY CHAPTER 7 CHEMICAL BONDING & MOLECULAR STRUCTURE 0 1 INSTR : FİLİZ ALSHANABLEH CHAPTER 7 CHEMICAL BONDING & MOLECULAR STRUCTURE The Ionic Bond Formation of Ions The
C H E M 1 CHEM 101-GENERAL CHEMISTRY CHAPTER 7 CHEMICAL BONDING & MOLECULAR STRUCTURE 0 1 INSTR : FİLİZ ALSHANABLEH CHAPTER 7 CHEMICAL BONDING & MOLECULAR STRUCTURE The Ionic Bond Formation of Ions The
Chapter 7 Chemical Bonding
 Chapter 7 Chemical Bonding 7.1 Ionic Bonding Octet rule: In forming compounds atoms lose, gain or share electrons to attain a noble gas configuration with 8 electrons in their outer shell (s 2 p 6 ), except
Chapter 7 Chemical Bonding 7.1 Ionic Bonding Octet rule: In forming compounds atoms lose, gain or share electrons to attain a noble gas configuration with 8 electrons in their outer shell (s 2 p 6 ), except
SCH4U1 Atomic & Molecular Structure Test Review
 SCH4U1 Atomic & Molecular Structure Test Review 1. Which object(s) would you use to describe the shape of the 2p orbital? a. a dumb-bell b. a circle c. a sphere d. two perpendicular dumb-bells e. a doughnut
SCH4U1 Atomic & Molecular Structure Test Review 1. Which object(s) would you use to describe the shape of the 2p orbital? a. a dumb-bell b. a circle c. a sphere d. two perpendicular dumb-bells e. a doughnut
What is Bonding? The Octet Rule. Getting an Octet. Chemical Bonding and Molecular Shapes. (Chapter Three, Part Two)
 Chemical Bonding and Molecular Shapes (Chapter Three, Part Two) What is Bonding? Bonding describes how atoms interact with each other in an attractive sense. There are three types of bonding: Ionic bonding
Chemical Bonding and Molecular Shapes (Chapter Three, Part Two) What is Bonding? Bonding describes how atoms interact with each other in an attractive sense. There are three types of bonding: Ionic bonding
Ch. 1: Introduction to Chemistry. Ch. 2: Matter and Change
 Review Sheet for Chemistry First Semester Final Refer to your class notes, worksheets, and the textbook to complete this review sheet. Study early so that you will have time to ask questions about what
Review Sheet for Chemistry First Semester Final Refer to your class notes, worksheets, and the textbook to complete this review sheet. Study early so that you will have time to ask questions about what
Chapter 12. Chemical Bonding
 Chapter 12 Chemical Bonding Chapter 12 Introduction to Chemical Bonding Chemical Bonding Valence electrons are the electrons in the outer shell (highest energy level) of an atom. A chemical bond is a mutual
Chapter 12 Chemical Bonding Chapter 12 Introduction to Chemical Bonding Chemical Bonding Valence electrons are the electrons in the outer shell (highest energy level) of an atom. A chemical bond is a mutual
Adapted from CHM 130 Maricopa County, AZ Molecular Geometry and Lewis Dot Formulas Introduction
 Adapted from CHM 130 Maricopa County, AZ Molecular Geometry and Lewis Dot Formulas Introduction A chemical bond is an intramolecular (within the molecule) force holding two or more atoms together. Covalent
Adapted from CHM 130 Maricopa County, AZ Molecular Geometry and Lewis Dot Formulas Introduction A chemical bond is an intramolecular (within the molecule) force holding two or more atoms together. Covalent
Electronic Structure of Atoms and the Periodic table. Electron Spin Quantum # m s
 Electronic Structure of Atoms and the Periodic table Chapter 6 & 7, Part 3 October 26 th, 2004 Homework session Wednesday 3:00 5:00 Electron Spin Quantum # m s Each electron is assigned a spinning motion
Electronic Structure of Atoms and the Periodic table Chapter 6 & 7, Part 3 October 26 th, 2004 Homework session Wednesday 3:00 5:00 Electron Spin Quantum # m s Each electron is assigned a spinning motion
Hey, Baby. You and I Have a Bond...Ch. 8
 I. IONIC BONDING FUNDAMENTALS A. They form between... 1. A and a a. A to become b. A to become B. How it happens (Let s first focus on two atoms): 1. When a metal and a nonmetal meet, electrons get transferred
I. IONIC BONDING FUNDAMENTALS A. They form between... 1. A and a a. A to become b. A to become B. How it happens (Let s first focus on two atoms): 1. When a metal and a nonmetal meet, electrons get transferred
Chapter 10. Valence Electrons. Lewis dot symbols. Chemical Bonding
 Chapter 10 Chemical Bonding Valence Electrons Recall: the outer electrons in an atom are valence electrons. Valence electrons are related to stability Valence electrons can be represented with dots in
Chapter 10 Chemical Bonding Valence Electrons Recall: the outer electrons in an atom are valence electrons. Valence electrons are related to stability Valence electrons can be represented with dots in
Cartoon courtesy of NearingZero.net. Chemical Bonding and Molecular Structure
 Cartoon courtesy of NearingZero.net Chemical Bonding and Molecular Structure Chemical Bonds Forces that hold groups of atoms together and make them function as a unit. 3 Major Types: Ionic bonds transfer
Cartoon courtesy of NearingZero.net Chemical Bonding and Molecular Structure Chemical Bonds Forces that hold groups of atoms together and make them function as a unit. 3 Major Types: Ionic bonds transfer
Chemistry 121: Topic 4 - Chemical Bonding Topic 4: Chemical Bonding
 Topic 4: Chemical Bonding 4.0 Ionic and covalent bonds; Properties of covalent and ionic compounds 4.1 Lewis structures, the octet rule. 4.2 Molecular geometry: the VSEPR approach. Molecular polarity.
Topic 4: Chemical Bonding 4.0 Ionic and covalent bonds; Properties of covalent and ionic compounds 4.1 Lewis structures, the octet rule. 4.2 Molecular geometry: the VSEPR approach. Molecular polarity.
Its Bonding Time. Chemical Bonds CH 12
 Its Bonding Time Chemical Bonds CH 12 What is a chemical bond? Octet Rule: Chemical compounds tend to form so that each atom, by gaining, losing, or sharing electrons, has an octet of electrons in its
Its Bonding Time Chemical Bonds CH 12 What is a chemical bond? Octet Rule: Chemical compounds tend to form so that each atom, by gaining, losing, or sharing electrons, has an octet of electrons in its
Lewis Theory of Shapes and Polarities of Molecules
 Lewis Theory of Shapes and Polarities of Molecules Sulfanilamide Lewis Structures and the Real 3D-Shape of Molecules Molecular Shape or Geometry The way in which atoms of a molecule are arranged in space
Lewis Theory of Shapes and Polarities of Molecules Sulfanilamide Lewis Structures and the Real 3D-Shape of Molecules Molecular Shape or Geometry The way in which atoms of a molecule are arranged in space
CHAPTER 12 CHEMICAL BONDING
 CHAPTER 12 CHEMICAL BONDING Core electrons are found close to the nucleus, whereas valence electrons are found in the most distant s and p energy subshells. The valence electrons are responsible for holding
CHAPTER 12 CHEMICAL BONDING Core electrons are found close to the nucleus, whereas valence electrons are found in the most distant s and p energy subshells. The valence electrons are responsible for holding
INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin
 Lecture INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin Chemical Bonding by Christopher G. Hamaker Illinois State University Chemical Bond Concept Recall that
Lecture INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin Chemical Bonding by Christopher G. Hamaker Illinois State University Chemical Bond Concept Recall that
Chemical Bonds. Chapter 6
 Chemical Bonds Chapter 6 1 Ch. 6 Chemical Bonding I. How and Why Atoms Bond A. Vocabulary B. Chemical Bonds - Basics C. Chemical Bonds Types D. Chemical Bonds Covalent E. Drawing Lewis Diagrams F. Bond
Chemical Bonds Chapter 6 1 Ch. 6 Chemical Bonding I. How and Why Atoms Bond A. Vocabulary B. Chemical Bonds - Basics C. Chemical Bonds Types D. Chemical Bonds Covalent E. Drawing Lewis Diagrams F. Bond
Unit 4: Chemical Bonds. Chapter 7-9
 Unit 4: Chemical Bonds Chapter 7-9 Objectives 26 Identify the number of valence electrons for elements and their Lewis dot structure 27 Define the terms cation and anion including radius size and charge
Unit 4: Chemical Bonds Chapter 7-9 Objectives 26 Identify the number of valence electrons for elements and their Lewis dot structure 27 Define the terms cation and anion including radius size and charge
Chapter 7. Ionic & Covalent Bonds
 Chapter 7 Ionic & Covalent Bonds Ionic Compounds Covalent Compounds 7.1 EN difference and bond character >1.7 = ionic 0.4 1.7 = polar covalent 1.7 Electrons not shared at
Chapter 7 Ionic & Covalent Bonds Ionic Compounds Covalent Compounds 7.1 EN difference and bond character >1.7 = ionic 0.4 1.7 = polar covalent 1.7 Electrons not shared at
AE Chemistry Midterm Study Guide
 Name Date Define Chemistry AE Chemistry Midterm Study Guide Since chemistry studies matter what is the definition of matter. What is the Law of Conservation of Matter? What is energy, what are the two
Name Date Define Chemistry AE Chemistry Midterm Study Guide Since chemistry studies matter what is the definition of matter. What is the Law of Conservation of Matter? What is energy, what are the two
Chapter 8 H H H H. Molecular Compounds & Covalent Bonding. Why do covalent bonds form? 8.1 Molecular Compounds. Properties of Molecular Compounds
 Chapter 8 Molecular Compounds & Covalent Bonding Why do covalent bonds form? If only group 5A, 6A, 7A atoms existed, ionic bonds can t form. NNMETALS Each atom needs electrons so they are not willing to
Chapter 8 Molecular Compounds & Covalent Bonding Why do covalent bonds form? If only group 5A, 6A, 7A atoms existed, ionic bonds can t form. NNMETALS Each atom needs electrons so they are not willing to
Unit 1 Review: Matter and Chemical Bonding
 Unit 1 Review: Matter and Chemical Bonding 1. Do you think DHMO should be banned? Justify your answer. Write the formula for dihydrogen monoxide. H 2 O 2. Name these groups on the periodic table: 1, 2,
Unit 1 Review: Matter and Chemical Bonding 1. Do you think DHMO should be banned? Justify your answer. Write the formula for dihydrogen monoxide. H 2 O 2. Name these groups on the periodic table: 1, 2,
CHEM 101: CHAPTER 11: CHEMICAL BONDS: THE FORMATION OF COMPOUNDS FROM ATOMS
 1 CHEM 101: CHAPTER 11: CHEMICAL BONDS: THE FORMATION OF COMPOUNDS FROM ATOMS PERIODIC TRENDS: See pages 214-216, 221 Table 11.3, and 227 + 228 of text. Lewis Structures of Atoms: The Lewis Dot Diagram
1 CHEM 101: CHAPTER 11: CHEMICAL BONDS: THE FORMATION OF COMPOUNDS FROM ATOMS PERIODIC TRENDS: See pages 214-216, 221 Table 11.3, and 227 + 228 of text. Lewis Structures of Atoms: The Lewis Dot Diagram
8.1 Types of Chemical Bonds List and define three types of bonding. chapter 8 Bonding General Concepts.notebook. September 10, 2015
 chapter 8 Bonding General Concepts.notebook Chapter 8: Bonding: General Concepts Mar 13 11:15 AM 8.1 Types of Chemical Bonds List and define three types of bonding. Bonds are forces that hold groups of
chapter 8 Bonding General Concepts.notebook Chapter 8: Bonding: General Concepts Mar 13 11:15 AM 8.1 Types of Chemical Bonds List and define three types of bonding. Bonds are forces that hold groups of
Chemical Bonding. Chemical Bonding 20/03/2015. The atomic radius increases from right to left. The atomic radius increases from top to bottom
 Chemical Bonding Atomic Radius: This distance from the nucleus to the outermost electron. Chemical Bonding Chemistry 11 Two factors must be taken into consideration in explaining this periodic trend: Increasing
Chemical Bonding Atomic Radius: This distance from the nucleus to the outermost electron. Chemical Bonding Chemistry 11 Two factors must be taken into consideration in explaining this periodic trend: Increasing
Chapter 8. Basic Concepts of Chemical Bonding
 Chapter 8 Basic Concepts of Chemical Bonding Chemical Bonds An attractive force that holds two atoms together in a more complex unit Three basic types of bonds Ionic Electrons are transferred from one
Chapter 8 Basic Concepts of Chemical Bonding Chemical Bonds An attractive force that holds two atoms together in a more complex unit Three basic types of bonds Ionic Electrons are transferred from one
Unit Five Practice Test (Part I) PT C U5 P1
 Unit Five Practice Test (Part I) PT C U5 P1 Name Period LPS Standard(s): --- State Standard(s): 12.3.1 Short Answers. Answer the following questions. (5 points each) 1. Write the electron configuration
Unit Five Practice Test (Part I) PT C U5 P1 Name Period LPS Standard(s): --- State Standard(s): 12.3.1 Short Answers. Answer the following questions. (5 points each) 1. Write the electron configuration
Lewis Dot Structures and Molecular Geometry
 Experiment 11 Lewis Dot Structures and Molecular Geometry Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. Purpose
Experiment 11 Lewis Dot Structures and Molecular Geometry Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. Purpose
A Simple Model for Chemical Bonds
 A Simple Model for hemical Bonds Multiple hoice 1. Modern organic chemistry a. is the study of carbon-containing compounds. b. is the study of compounds from living organisms. c. deals exclusively with
A Simple Model for hemical Bonds Multiple hoice 1. Modern organic chemistry a. is the study of carbon-containing compounds. b. is the study of compounds from living organisms. c. deals exclusively with
What is a Bond? Chapter 8. Ionic Bonding. Coulomb's Law. What about covalent compounds?
 Chapter 8 What is a Bond? A force that holds atoms together. Why? We will look at it in terms of energy. Bond energy- the energy required to break a bond. Why are compounds formed? Because it gives the
Chapter 8 What is a Bond? A force that holds atoms together. Why? We will look at it in terms of energy. Bond energy- the energy required to break a bond. Why are compounds formed? Because it gives the
MIDTERM STUDY GUIDE. Chapter 1 Introduction to Chemistry
 MIDTERM STUDY GUIDE Chapter 1 Introduction to Chemistry What is chemistry? Chemical properties vs. physical properties examples of both States of matter Scientific method Chapter 2 Data Analysis SI measurement
MIDTERM STUDY GUIDE Chapter 1 Introduction to Chemistry What is chemistry? Chemical properties vs. physical properties examples of both States of matter Scientific method Chapter 2 Data Analysis SI measurement
Chapter 1 Chemical Bonding
 Chapter 1 Chemical Bonding 1.1 Atoms, Electrons, and Orbitals Atoms are composed of + Protons positively charged mass = 1.6726 X 10-27 kg Neutrons neutral mass = 1.6750 X 10-27 kg Electrons negatively
Chapter 1 Chemical Bonding 1.1 Atoms, Electrons, and Orbitals Atoms are composed of + Protons positively charged mass = 1.6726 X 10-27 kg Neutrons neutral mass = 1.6750 X 10-27 kg Electrons negatively
Chapters 9&10 Structure and Bonding Theories
 Chapters 9&10 Structure and Bonding Theories Ionic Radii Ions, just like atoms, follow a periodic trend in their radii. The metal ions in a given period are smaller than the non-metal ions in the same
Chapters 9&10 Structure and Bonding Theories Ionic Radii Ions, just like atoms, follow a periodic trend in their radii. The metal ions in a given period are smaller than the non-metal ions in the same
Period: Chemistry Semester 1 Final Exam Review Packet. 1. What is the difference between a hypothesis and a theory?
 Chemistry Name: Period: Chemistry Semester 1 Final Exam Review Packet 1. What is the difference between a hypothesis and a theory? 2. Distinguish between quantitative and qualitative observations. States
Chemistry Name: Period: Chemistry Semester 1 Final Exam Review Packet 1. What is the difference between a hypothesis and a theory? 2. Distinguish between quantitative and qualitative observations. States
Chapter 6 PRETEST: Chemical Bonding
 Chapter 6 PRETEST: Chemical In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1.The charge on an ion is a. always positive.
Chapter 6 PRETEST: Chemical In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1.The charge on an ion is a. always positive.
IB Chemistry. Chapter 4.1
 IB Chemistry Chapter 4.1 Chemical Bonds Atoms or ions that are strongly attached to one another Chemical bonds will form if potential energy decreases (becomes more stable) 2 Valence Electrons Valence
IB Chemistry Chapter 4.1 Chemical Bonds Atoms or ions that are strongly attached to one another Chemical bonds will form if potential energy decreases (becomes more stable) 2 Valence Electrons Valence
Chapter 13: Phenomena
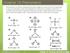 Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Test Review # 5. Chemistry: Form TR5-8A. Average Atomic Mass. Subatomic particles.
 Chemistry: Form TR5-8A REVIEW Name Date Period Test Review # 5 Subatomic particles. Type of Particle Location Mass Relative Mass Charge Proton Center 1.67 10-27 kg 1 +1 Electron Outside 9.11 10-31 kg 0-1
Chemistry: Form TR5-8A REVIEW Name Date Period Test Review # 5 Subatomic particles. Type of Particle Location Mass Relative Mass Charge Proton Center 1.67 10-27 kg 1 +1 Electron Outside 9.11 10-31 kg 0-1
Chemical bonding is the combining of elements to form new substances.
 Name Covalent Bonding and Nomenclature: Unit Objective Study Guide Class Period Date Due 1. Define chemical bonding. What is chemical bonding? Chemical bonding is the combining of elements to form new
Name Covalent Bonding and Nomenclature: Unit Objective Study Guide Class Period Date Due 1. Define chemical bonding. What is chemical bonding? Chemical bonding is the combining of elements to form new
Notes: Unit 6 Electron Configuration and the Periodic Table
 Name KEY Block Notes: Unit 6 Electron Configuration and the Periodic Table In the 1790's Antoine Lavoisier compiled a list of the known elements at that time. There were only 23 elements. By the 1870's
Name KEY Block Notes: Unit 6 Electron Configuration and the Periodic Table In the 1790's Antoine Lavoisier compiled a list of the known elements at that time. There were only 23 elements. By the 1870's
Atoms have the ability to do two things in order to become isoelectronic with a Noble Gas.
 CHEMICAL BONDING Atoms have the ability to do two things in order to become isoelectronic with a Noble Gas. 1.Electrons can be from one atom to another forming. Positive ions (cations) are formed when
CHEMICAL BONDING Atoms have the ability to do two things in order to become isoelectronic with a Noble Gas. 1.Electrons can be from one atom to another forming. Positive ions (cations) are formed when
Ch 10 Chemical Bonding, Lewis Structures for Ionic & Covalent Compounds, and Predicting Shapes of Molecules
 Fructose Water Ch 10 Chemical Bonding, Lewis Structures for Ionic & Covalent Compounds, and Predicting Shapes of Molecules Carbon Dioxide Ammonia Title and Highlight TN Ch 10.1 Topic: EQ: Right Side NOTES
Fructose Water Ch 10 Chemical Bonding, Lewis Structures for Ionic & Covalent Compounds, and Predicting Shapes of Molecules Carbon Dioxide Ammonia Title and Highlight TN Ch 10.1 Topic: EQ: Right Side NOTES
Scientific Investigation 1.5 SOL 1f Atomic Structure and Periodic Relationships. Ch 14: Read pp SOL 2d,e,f,g,h,i
 Scientific Investigation 1.5 SOL 1f Atomic Structure and Periodic Relationships 2.5 Chemistry Unit 5 Primary reference: CHEMISTRY, Addison-Wesley Topic Essential Knowledge Study Support Practice safe use
Scientific Investigation 1.5 SOL 1f Atomic Structure and Periodic Relationships 2.5 Chemistry Unit 5 Primary reference: CHEMISTRY, Addison-Wesley Topic Essential Knowledge Study Support Practice safe use
Chemical Bonding Chapter 8
 Chemical Bonding Chapter 8 Get your Clicker, 2 magnets, goggles and your handouts Nov 15 6:15 PM Recall that: Ionic-Involves the transfer of electrons - forms between a metal and a nonmetal Covalent-Involves
Chemical Bonding Chapter 8 Get your Clicker, 2 magnets, goggles and your handouts Nov 15 6:15 PM Recall that: Ionic-Involves the transfer of electrons - forms between a metal and a nonmetal Covalent-Involves
bond energy- energy required to break a chemical bond -We can measure bond energy to determine strength of interaction
 bond energy- energy required to break a chemical bond -We can measure bond energy to determine strength of interaction ionic compound- a metal reacts with a nonmetal Ionic bonds form when an atom that
bond energy- energy required to break a chemical bond -We can measure bond energy to determine strength of interaction ionic compound- a metal reacts with a nonmetal Ionic bonds form when an atom that
Bonding. Computer Lab: Ionic Bonds. Important Notes 3/22/18
 Bonding What are ionic bonds, and how are they formed? Computer Lab: Ionic Bonds Go to http://www.pbslearningmedia.org/asset/ lsps07_int_ionicbonding/ Read each screen and follow the directions where appropriate.
Bonding What are ionic bonds, and how are they formed? Computer Lab: Ionic Bonds Go to http://www.pbslearningmedia.org/asset/ lsps07_int_ionicbonding/ Read each screen and follow the directions where appropriate.
CHEMICAL BONDING. Chemical Bonds. Ionic Bonding. Lewis Symbols
 CHEMICAL BONDING Chemical Bonds Lewis Symbols Octet Rule whenever possible, valence electrons in covalent compounds distribute so that each main-group element is surrounded by 8 electrons (except hydrogen
CHEMICAL BONDING Chemical Bonds Lewis Symbols Octet Rule whenever possible, valence electrons in covalent compounds distribute so that each main-group element is surrounded by 8 electrons (except hydrogen
Unit 9: CHEMICAL BONDING
 Unit 9: CHEMICAL BONDING 1 Unit 9: Bonding: 1. Electronegativity 2. Intramolecular Bonding 3. Intermolecular Bonding 4. Drawing Lewis Structures 5. Lewis Structures for Polyatomic Ions 6. Exceptions to
Unit 9: CHEMICAL BONDING 1 Unit 9: Bonding: 1. Electronegativity 2. Intramolecular Bonding 3. Intermolecular Bonding 4. Drawing Lewis Structures 5. Lewis Structures for Polyatomic Ions 6. Exceptions to
Topics to Expect: Periodic Table: s, p, d, f blocks Metal, Metalloid, Non metal, etc. Periodic Trends, Family names Electron Configuration: Orbitals a
 Chemistry Final Exam Review and Practice Chapters Covered ESSENTIALLY CUMMULATIVE List of Chapters: Ch: 6, 7, 8, 9, 10, 13, 14, 15, 16, 19, 20 Topics to Expect: Periodic Table: s, p, d, f blocks Metal,
Chemistry Final Exam Review and Practice Chapters Covered ESSENTIALLY CUMMULATIVE List of Chapters: Ch: 6, 7, 8, 9, 10, 13, 14, 15, 16, 19, 20 Topics to Expect: Periodic Table: s, p, d, f blocks Metal,
Chapter 12 Structures and Characteristics of Bonds Objectives
 Objectives 1. To learn about ionic and covalent bonds and explain how they are formed - what holds compounds together? 2. To learn about the polar covalent bond are all covalent bonds equal? 3. To understand
Objectives 1. To learn about ionic and covalent bonds and explain how they are formed - what holds compounds together? 2. To learn about the polar covalent bond are all covalent bonds equal? 3. To understand
Ch 6 Chemical Bonding
 Ch 6 Chemical Bonding What you should learn in this section (objectives): Define chemical bond Explain why most atoms form chemical bonds Describe ionic and covalent bonding Explain why most chemical bonding
Ch 6 Chemical Bonding What you should learn in this section (objectives): Define chemical bond Explain why most atoms form chemical bonds Describe ionic and covalent bonding Explain why most chemical bonding
Valence electron- Energy sublevel- Transition element- Period 10. Electronegativity- Alkaline earth metal- 11. Ion- Halogen- 12.
 Mrs. Hilliard 1. Valence electron 2. Period 3. Alkaline earth metal 4. Halogen 5. Metalloid 6. Hund s Rule 7. Representative element 8. Energy sublevel 9. Transition element 10. Electronegativity 11. Ion
Mrs. Hilliard 1. Valence electron 2. Period 3. Alkaline earth metal 4. Halogen 5. Metalloid 6. Hund s Rule 7. Representative element 8. Energy sublevel 9. Transition element 10. Electronegativity 11. Ion
Chapter 6 Chemistry Review
 Chapter 6 Chemistry Review Multiple Choice Identify the choice that best completes the statement or answers the question. Put the LETTER of the correct answer in the blank. 1. The electrons involved in
Chapter 6 Chemistry Review Multiple Choice Identify the choice that best completes the statement or answers the question. Put the LETTER of the correct answer in the blank. 1. The electrons involved in
Chapter 7. Chemical Bonding I: Basic Concepts
 Chapter 7. Chemical Bonding I: Basic Concepts Chemical bond: is an attractive force that holds 2 atoms together and forms as a result of interactions between electrons found in combining atoms We rarely
Chapter 7. Chemical Bonding I: Basic Concepts Chemical bond: is an attractive force that holds 2 atoms together and forms as a result of interactions between electrons found in combining atoms We rarely
Test Review # 4. Chemistry: Form TR4-9A
 Chemistry: Form TR4-9A REVIEW Name Date Period Test Review # 4 Location of electrons. Electrons are in regions of the atom known as orbitals, which are found in subdivisions of the principal energy levels
Chemistry: Form TR4-9A REVIEW Name Date Period Test Review # 4 Location of electrons. Electrons are in regions of the atom known as orbitals, which are found in subdivisions of the principal energy levels
Chemistry 40S Atomic Structure (This unit has been adapted from https://bblearn.merlin.mb.ca)
 Chemistry 40S Atomic Structure (This unit has been adapted from https://bblearn.merlin.mb.ca) Name: 1 2 Lesson 1: The Nature of Light Goals: Describe light in terms of electromagnetic energy. Describe
Chemistry 40S Atomic Structure (This unit has been adapted from https://bblearn.merlin.mb.ca) Name: 1 2 Lesson 1: The Nature of Light Goals: Describe light in terms of electromagnetic energy. Describe
Chapter 8. Chemical Bonding: Basic Concepts
 Chapter 8. Chemical Bonding: Basic Concepts Chemical bond: is an attractive force that holds 2 atoms together and forms as a result of interactions between electrons found in combining atoms We rarely
Chapter 8. Chemical Bonding: Basic Concepts Chemical bond: is an attractive force that holds 2 atoms together and forms as a result of interactions between electrons found in combining atoms We rarely
Bonding: Part Two. Three types of bonds: Ionic Bond. transfer valence e - Metallic bond. (NaCl) (Fe) mobile valence e - Covalent bond
 Bonding: Part Two Three types of bonds: Ionic Bond transfer valence e - Metallic bond mobile valence e - Covalent bond (NaCl) (Fe) shared valence e - (H 2 O) 1 Single Covalent Bond H + H H H H-atoms H
Bonding: Part Two Three types of bonds: Ionic Bond transfer valence e - Metallic bond mobile valence e - Covalent bond (NaCl) (Fe) shared valence e - (H 2 O) 1 Single Covalent Bond H + H H H H-atoms H
Chemistry 40S Atomic Structure (This unit has been adapted from https://bblearn.merlin.mb.ca)
 Chemistry 40S Atomic Structure (This unit has been adapted from https://bblearn.merlin.mb.ca) Name: 1 Lesson 1: The Nature of Light Goals: Describe light in terms of electromagnetic energy. Describe the
Chemistry 40S Atomic Structure (This unit has been adapted from https://bblearn.merlin.mb.ca) Name: 1 Lesson 1: The Nature of Light Goals: Describe light in terms of electromagnetic energy. Describe the
Introductory Chemistry: A Foundation, 6 th Ed. Introductory Chemistry, 6 th Ed. Basic Chemistry, 6 th Ed.
 Introductory Chemistry: A Foundation, 6 th Ed. Introductory Chemistry, 6 th Ed. Basic Chemistry, 6 th Ed. by Steven S. Zumdahl & Donald J. DeCoste University of Illinois Chapter 12 Chemical Bonding Structure
Introductory Chemistry: A Foundation, 6 th Ed. Introductory Chemistry, 6 th Ed. Basic Chemistry, 6 th Ed. by Steven S. Zumdahl & Donald J. DeCoste University of Illinois Chapter 12 Chemical Bonding Structure
 Organic Chemistry I Dr Alex Roche Organic chemistry is the chemistry of Carbon and its compounds. Organic molecules constitute the essence of life (fats, sugars, proteins, DNA), and also permeate our everyday
Organic Chemistry I Dr Alex Roche Organic chemistry is the chemistry of Carbon and its compounds. Organic molecules constitute the essence of life (fats, sugars, proteins, DNA), and also permeate our everyday
REVIEW: VALENCE ELECTRONS CHEMICAL BONDS: LEWIS SYMBOLS: CHEMICAL BONDING. What are valence electrons?
 REVIEW: VALENCE ELECTRONS 13 CHEMICAL BONDING What are valence electrons? Which groups on the periodic table readily give up electrons? What group readily accepts electrons? CHEMICAL BONDS: What are chemical
REVIEW: VALENCE ELECTRONS 13 CHEMICAL BONDING What are valence electrons? Which groups on the periodic table readily give up electrons? What group readily accepts electrons? CHEMICAL BONDS: What are chemical
Notes: Electrons and Periodic Table (text Ch. 4 & 5)
 Name Per. Notes: Electrons and Periodic Table (text Ch. 4 & 5) NOTE: This set of class notes is not complete. We will be filling in information in class. If you are absent, it is your responsibility to
Name Per. Notes: Electrons and Periodic Table (text Ch. 4 & 5) NOTE: This set of class notes is not complete. We will be filling in information in class. If you are absent, it is your responsibility to
Molecular Compounds Compounds that are bonded covalently (like in water, or carbon dioxide) are called molecular compounds
 Chapter 8: Covalent Bonding Section 1: Molecular Compounds Bonds are Forces that hold groups of atoms together and make them function as a unit. Two types: Ionic bonds transfer of electrons (gained or
Chapter 8: Covalent Bonding Section 1: Molecular Compounds Bonds are Forces that hold groups of atoms together and make them function as a unit. Two types: Ionic bonds transfer of electrons (gained or
CHEMISTRY 9 REVIEW & INTRO TO CHEMISTRY 10. Section 4.1: Atomic Theory and Bonding
 1 CHEMISTRY 9 REVIEW & INTRO TO CHEMISTRY 10 Section 4.1: Atomic Theory and Bonding ATOMS AND COMPOUNDS An atom is the smallest particle of an element that still has the properties of that element An atom
1 CHEMISTRY 9 REVIEW & INTRO TO CHEMISTRY 10 Section 4.1: Atomic Theory and Bonding ATOMS AND COMPOUNDS An atom is the smallest particle of an element that still has the properties of that element An atom
CHAPTER 6: CHEMICAL NAMES AND FORMULAS CHAPTER 16: COVALENT BONDING
 CHAPTER 6: CHEMICAL NAMES AND FORMULAS CHAPTER 16: COVALENT BONDING 6.1 Introduction to Chemical Bonding A chemical bond is a mutual electrical attraction between the nuclei and valence electrons of different
CHAPTER 6: CHEMICAL NAMES AND FORMULAS CHAPTER 16: COVALENT BONDING 6.1 Introduction to Chemical Bonding A chemical bond is a mutual electrical attraction between the nuclei and valence electrons of different
Fill in the chart below to determine the valence electrons of elements 3-10
 Chemistry 11 Atomic Theory IV Name: Date: Block: 1. Lewis Diagrams 2. VSEPR Lewis Diagrams Lewis diagrams show the bonding between atoms of a molecule. Only the outermost electrons of an atom (called electrons)
Chemistry 11 Atomic Theory IV Name: Date: Block: 1. Lewis Diagrams 2. VSEPR Lewis Diagrams Lewis diagrams show the bonding between atoms of a molecule. Only the outermost electrons of an atom (called electrons)
Name: Period: Date: What Is VSEPR? Now explore the Compare Two Structures link. Try changing the display to explore different combinations.
 Name: Period: Date: What Is VSEPR? Exploring The Valence Shell Electron Pair Repulsion (VSEPR) model. Go to the Purdue University website to explore VSEPR theory. http://www.chem.purdue.edu/gchelp/vsepr/structur2.html
Name: Period: Date: What Is VSEPR? Exploring The Valence Shell Electron Pair Repulsion (VSEPR) model. Go to the Purdue University website to explore VSEPR theory. http://www.chem.purdue.edu/gchelp/vsepr/structur2.html
Chapter 8: Bonding. Section 8.1: Lewis Dot Symbols
 Chapter 8: Bonding Section 8.1: Lewis Dot Symbols The Lewis electron dot symbol is named after Gilbert Lewis. In the Lewis dot symbol, the element symbol represents the nucleus and the inner electrons.
Chapter 8: Bonding Section 8.1: Lewis Dot Symbols The Lewis electron dot symbol is named after Gilbert Lewis. In the Lewis dot symbol, the element symbol represents the nucleus and the inner electrons.
Bonding: Part Two. Three types of bonds: Ionic Bond. transfer valence e - Metallic bond. (NaCl) (Fe) mobile valence e - Covalent bond
 Bonding: Part Two Three types of bonds: Ionic Bond transfer valence e - Metallic bond mobile valence e - Covalent bond (NaCl) (Fe) shared valence e - (H 2 O) 1 Single Covalent Bond H + H H H H-atoms H
Bonding: Part Two Three types of bonds: Ionic Bond transfer valence e - Metallic bond mobile valence e - Covalent bond (NaCl) (Fe) shared valence e - (H 2 O) 1 Single Covalent Bond H + H H H H-atoms H
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9. Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 9. Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9. Molecular Geometry and Bonding Theories PART I Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 9. Molecular Geometry and Bonding Theories PART I Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 6. Preview. Objectives. Molecular Compounds
 Section 2 Covalent Bonding and Molecular Compounds Preview Objectives Molecular Compounds Formation of a Covalent Bond Characteristics of the Covalent Bond The Octet Rule Electron-Dot Notation Lewis Structures
Section 2 Covalent Bonding and Molecular Compounds Preview Objectives Molecular Compounds Formation of a Covalent Bond Characteristics of the Covalent Bond The Octet Rule Electron-Dot Notation Lewis Structures
What Do Molecules Look Like?
 What Do Molecules Look Like? The Lewis Dot Structure approach provides some insight into molecular structure in terms of bonding, but what about 3D geometry? Recall that we have two types of electron pairs:
What Do Molecules Look Like? The Lewis Dot Structure approach provides some insight into molecular structure in terms of bonding, but what about 3D geometry? Recall that we have two types of electron pairs:
Science 10 Chapter 4 Atomic Theory Explains the Formation of Compounds
 What is a pure substance? -contains only 1 kind of matter What are the 2 categories of pure substances? -elements -compounds What is an element? -a pure substance that cannot be broken down into simpler
What is a pure substance? -contains only 1 kind of matter What are the 2 categories of pure substances? -elements -compounds What is an element? -a pure substance that cannot be broken down into simpler
Covalent Compounds: Bonding Theories and Molecular Structure
 CHM 123 Chapter 8 Covalent Compounds: Bonding Theories and Molecular Structure 8.1 Molecular shapes and VSEPR theory VSEPR theory proposes that the geometric arrangement of terminal atoms, or groups of
CHM 123 Chapter 8 Covalent Compounds: Bonding Theories and Molecular Structure 8.1 Molecular shapes and VSEPR theory VSEPR theory proposes that the geometric arrangement of terminal atoms, or groups of
Review Package #3 Atomic Models and Subatomic Particles The Periodic Table Chemical Bonding
 Chemistry 11 Review Package #3 Atomic Models and Subatomic Particles The Periodic Table Chemical Bonding 1. Atomic Models and Subatomic Particles: A. Subatomic Particles and Average Atomic Mass: - Subatomic
Chemistry 11 Review Package #3 Atomic Models and Subatomic Particles The Periodic Table Chemical Bonding 1. Atomic Models and Subatomic Particles: A. Subatomic Particles and Average Atomic Mass: - Subatomic
Cartoon courtesy of NearingZero.net. Chemical Bonding and Molecular Structure
 Cartoon courtesy of NearingZero.net Chemical Bonding and Molecular Structure Big Ideas in Unit 6 How do atoms form chemical bonds? How does the type of a chemical bond influence a compounds physical and
Cartoon courtesy of NearingZero.net Chemical Bonding and Molecular Structure Big Ideas in Unit 6 How do atoms form chemical bonds? How does the type of a chemical bond influence a compounds physical and
NAME: Chapter 12, Test 1: Chemical Bonding. Total Question(s): 20 Here are the quiz answers, in review:
 NAME: Chapter 12, Test 1: Chemical Bonding Total Question(s): 20 Here are the quiz s, in review: 1.Elements whose electronegativities are similar will form bonds that are. (A) ionic (B) polar covalent
NAME: Chapter 12, Test 1: Chemical Bonding Total Question(s): 20 Here are the quiz s, in review: 1.Elements whose electronegativities are similar will form bonds that are. (A) ionic (B) polar covalent
Bonding. Honors Chemistry 412 Chapter 6
 Bonding Honors Chemistry 412 Chapter 6 Chemical Bond Mutual attraction between the nuclei and valence electrons of different atoms that binds them together. Types of Bonds Ionic Bonds Force of attraction
Bonding Honors Chemistry 412 Chapter 6 Chemical Bond Mutual attraction between the nuclei and valence electrons of different atoms that binds them together. Types of Bonds Ionic Bonds Force of attraction
Covalent Bonding. In nature, only the noble gas elements exist as uncombined atoms. All other elements need to lose or gain electrons
 In nature, only the noble gas elements exist as uncombined atoms. They are monatomic - consist of single atoms. All other elements need to lose or gain electrons To form ionic compounds Some elements share
In nature, only the noble gas elements exist as uncombined atoms. They are monatomic - consist of single atoms. All other elements need to lose or gain electrons To form ionic compounds Some elements share
Unit 3 - Chemical Bonding and Molecular Structure
 Unit 3 - Chemical Bonding and Molecular Structure Chemical bond - A mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms together 6-1 Introduction
Unit 3 - Chemical Bonding and Molecular Structure Chemical bond - A mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms together 6-1 Introduction
Chapter 9: Molecular Geometry and Bonding Theories
 Chapter 9: Molecular Geometry and Bonding Theories 9.1 Molecular Geometries -Bond angles: angles made by the lines joining the nuclei of the atoms in a molecule -Bond angles determine overall shape of
Chapter 9: Molecular Geometry and Bonding Theories 9.1 Molecular Geometries -Bond angles: angles made by the lines joining the nuclei of the atoms in a molecule -Bond angles determine overall shape of
Please pass in only this completed answer sheet on the day of the test. LATE SUBMISSIONS WILL NOT BE ACCEPTED
 CHM-201 General Chemistry and Laboratory I Unit #4 Take Home Test Due December 13, 2018 Please pass in only this completed answer sheet on the day of the test. LATE SUBMISSIONS WILL NOT BE ACCEPTED CHM-201
CHM-201 General Chemistry and Laboratory I Unit #4 Take Home Test Due December 13, 2018 Please pass in only this completed answer sheet on the day of the test. LATE SUBMISSIONS WILL NOT BE ACCEPTED CHM-201
