2- The chemistry in the. The formation of water : gas phase and grain surface formation. The present models. Observations of molecules in the ISM.
|
|
|
- Barnard Strickland
- 6 years ago
- Views:
Transcription
1 2- The chemistry in the ISM. The formation of water : gas phase and grain surface formation. The present models. Observations of molecules in the ISM. 1
2 Why studying the ISM chemistry? 1- The thermal balance, and therefore the physical structure of the ISM depends on the chemical composition of the gas, because the cooling of the gas is dominated by the line emission, which depends on the particular molecule emitting the line, and therefore on its abundance: in other words, the physical structure of the ISM depends on the chemical composition of the gas. 2- Since the chemical structure itself is function of the evolution and physical structure of the gas, it is a powerful probe of the latters. 3- Last but not least, chemistry is particularly important in star forming regions, because the chemical complexity in those regions can have consequences on the terrestrial life, either directly, or, much more likely, indirectly. 2
3 THE ABUNDANCE OF THE ELEMENTS Element Abundance/H Molecules are formed in the interiors of the ISM He clouds, where FUV photons Oxygen 6.7x10-4 -which photodissociate the Carbon 3.7x10-4 molecules- do not Nitrogen 1.1x10-4 penetrate (because absorbed by the dust). S, Mg, Fe, Si ~3x10-5 Because of the element Na, Al, Ca ~3x10-6 abundance, the most abundant species are: H 2, In practice, in MC, all gaseous CO, carbon O, is O 2 in and CO, Hwhereas 2 O the oxygen is shared between O, H 2 O and O 2. 3
4 The molecules observed in the ISM The most recent census (2003) counts 123 molecules, containing from 2 to 13 atoms, withouth taking into account the forms in the least abundant isoptopes (D, 13 C, 18 O, 17 O, 15 N, etc.). 2 atoms: AlF AlCl C 2 CH CH + CN CO CO + CP CS CSi HCl H 2 KCl NH NO NS NaCl OH PN SO S0 + SiN SiO SiS HF SH FeO 3 atoms: C 3 C 2 H C 20 C 2 S CH 2 HCN HCO HCO + HCS + HOC + H 2 0 H 2 S HNC HNO MgCN MgNC N 2 H + N 20 NaCN OCS S0 2 c-sic 2 CO 2 NH 2 H atoms: c-c 3 H l-c 3 H C 3 N C 30 C 3 S C 2 H 2 CH 2 D +? HCCN HCNH + HNCO HNCS HOCO + H 2 CO H 2 CN H 2 CS H NH 3 SiC 3 5 atoms: C 5 C 4 H C 4 Si l-c 3 H 2 c-c 3 H 2 CH 2 CN CH 4 HC 3 N HC 2 NC HCOOH H 2 CHN H 2 C 20 H 2 NCN HNC 3 SiH 4 H 2 COH + 6 atoms: C 5 H C 50 C 2 H 4 CH 3 CN CH 3 NC CH 30 H CH 3 SH HC 3 NH + HC 2 CHO HCONH 2 l- H 2 C 4 C5N 7 atoms: C 6 H CH 2 CHCN CH 3 C 2 H HC 5 N HCOCH 3 NH 2 CH 3 c- 2 H 4 O CH 2 CHOH 8 atoms: CH 3 C 3 N HCOOCH 3 CH 3 COOH C 7 H CH 2 OHCHO 9 atoms: CH 3 C 4 H CH 3 CH 2 CN (CH 3 ) 20 CH 3 CH 20 H HC 7 N C 8 H +10 atoms: CH 3 C5N (CH 3 ) 2 CO NH 2 CH 2 COOH? HC 11 N 4
5 How molecules form in the ISM There is a variety of processes that lead to the formation of molecules in the ISM. These can be separated into two broad classes: 1- gas phase reactions, which occur in the gas phase, 2- grain surface reactions, which occur on the surfaces of the grains. Both processes are important, even though not for the same molecules and/or in the same regions. Indeed, there is a strong interplay of these two mechanisms in the process of forming simple and complex molecules. Molecules formed in the gas phase can be frozen onto the grain surfaces, where they can undergo further chemical reactions, and eventually they can be realeased back into the gas phase in different, usually more complex molecules. Once in the gas phase in their «new» form they can start new chemical reactions and undergo further transformations or promote new reactions. An outstanding example is H 2, formed on the grain surfaces, and a key molecule for the formation of all other molecules in the gas phase. 5
6 GAS PHASE REACTIONS Gas phase reactions can be divided into three main different categories: 1- bond formation reactions, which link atoms into simple or complex molecules; 2- bond destruction reactions, which breakdown molecules in smaller molecules; 3- bond re-arrangement reactions, which transfer parts of one co-reactant to another one. Generic examples are summarized in the right Table. Photo-dissociation Neutral-Neutral Ion-Molecule Charge transfer Radiative association Dissociative recombination Associative detachment Reaction AB +hν A + B A + + B A + + B A + B + A + B A + + e A - + B A + B C + D C + + D AB + hν C + D AB + e Rate (cm 3 s -1 ) 10-9 (s -1 ) 4x x
7 GRAIN SURFACE CHEMISTRY Interstellar grains provide a surface on which accreted species can meet and react. Grain surface chemistry is therefore governed by the accretion rate which sets the overall timescale for the process- and the surface migration rate which governs the reaction network. In practice, all molecules stay where they freeze out onto, whereas the H and O atoms scan the grain surface and can therefore hydrogenate and oxydize the encountered species 7
8 GRAIN SURFACE CHEMISTRY : mantle formation The accretion rate of a species on grains is given by k ac = S π a grain2 n grain v x s -1 S = sticking coefficient, which depends on the accreting species; except for the H atom, S is expected to be close to unity at low temperatures. For H, S can be as low as 0.3 on a clean H 2 O ice, but likely on interstellar ice surface is also close to unity. v x = thermal velocity of the gas =(2kT/m x ) 1/2 a grain = average grain radius n grain = dust grain density. Thus, the characteristic timescale to deplete a species x on the grain surfaces is: τ ac 4x10 5 (S/1) -1 (n/10 4 cm -3 ) -1 (T/10K) -1/2 (a grain /0.1µm) -2 (m x /m CO ) 1/2 yr (Eq. 2.1) In a molecular cloud with density about 10 4 cm -3, the CO depletion timescale is of order of 4x10 5 yr. In denser regions the timescale becomes even shorter. 8
9 WATER FORMATION IN THE ISM There are three main routes of water formation in the ISM, depending on the physical conditions of the region: 1- IN THE COLD GAS 2- IN THE HOT (>220K) GAS 3- ON THE GRAIN SURFACES 9
10 H 2 O FORMATION IN COLD GAS Water is formed in the interiors of molecular clouds, where FUV photons cannot penetrate. The main formation route is the H 3 O + dissociative recombination with electrons (H 3 O + is formed by ion-neutral reactions). O + H 3 + OH + + H 2 ; OH + + H 2 H 2 O + + H ; H 2 O + + H 2 H 3 O + + H ; H 3 O + + e H 2 O + H 10
11 H 2 O FORMATION IN COLD GAS Water is formed in the interiors of molecular clouds, where FUV photons cannot penetrate. Molecular clouds form following the contraction of diffuse clouds (permeated by FUV photons). The figure above shows typical theoretical predictions: after 10 4 yr water abundance is expected to be around 3x10-7 /H 2. 11
12 H 2 O FORMATION IN HOT GAS In warm gas (T>220K), a chain of endothermic reactions (O+H 2 ->OH+H 2 ->H 2 O) convert all the gaseous oxygen - not locked into CO molecules- into water : x(h 2 O) ~ This occurs around massive protostars, or even in low mass protostars, close to the central star (hot cores). Or at the interface between the outflows and the surroundings (shocks). 12
13 H 2 O FORMATION ON THE GRAINS Water is thought to form by hydrogenation of Oxygen atoms that stick on to the grains. Before being evaporated they meet Hydrogen atoms and form H 2 O. The left Figure shows the Tielens & Hagen (1982) theoretical predictions of the water-ice molecular fraction as function of the gas density. At relatively low (<10 4 cm -3 ) densities all Oxygen not in CO is eventually converted into iced-water, whereas at large densities Oxygen atoms combine into O 2. 13
14 Summary The most abundant elements after Hydrogen and Helium are Oxygen and Carbon. The most abundant species in molecular Clouds are expected to be H 2, CO, O, O 2 and H 2 O. Molecules can be formed either in the gas phase or on the grain surfaces. Water in the gas phase is formed by ion-neutral reactions in cold (<250K) gas and by endothermic reactions in warm gas. Water ices can be formed on the grain surfaces. 14
Nucleosynthesis and stellar lifecycles. A. Ruzicka
 Nucleosynthesis and stellar lifecycles A. Ruzicka Stellar lifecycles A. Ruzicka Outline: 1. What nucleosynthesis is, and where it occurs 2. Molecular clouds 3. YSO & protoplanetary disk phase 4. Main Sequence
Nucleosynthesis and stellar lifecycles A. Ruzicka Stellar lifecycles A. Ruzicka Outline: 1. What nucleosynthesis is, and where it occurs 2. Molecular clouds 3. YSO & protoplanetary disk phase 4. Main Sequence
" There's life Jim...but we don't KNOW it (yet): a journey through the chemically controlled cosmos from star birth to the formation of life"
 " There's life Jim...but we don't KNOW it (yet): a journey through the chemically controlled cosmos from star birth to the formation of life" 30 th May 2007, Stockholm Observatory with support from the
" There's life Jim...but we don't KNOW it (yet): a journey through the chemically controlled cosmos from star birth to the formation of life" 30 th May 2007, Stockholm Observatory with support from the
atoms H. * Infrared **Optical l - linear c-cyclic
 2 3 4 5 6 7 atoms H *,* 2 C 3 * c-c 3 H C 5 * C 5 H C 6 H AlF C 2 H l-c 3 H C 4 H l-h 2 C 4 CH 2 CHCN AlCl C 2 O C 3 N C 4 Si C 2 H 4 CH 3 C 2 H C 2 ** C 2 S C 3 O l-c 3 H 2 CH 3 CN HC 5 N CH CH 2 C 3
2 3 4 5 6 7 atoms H *,* 2 C 3 * c-c 3 H C 5 * C 5 H C 6 H AlF C 2 H l-c 3 H C 4 H l-h 2 C 4 CH 2 CHCN AlCl C 2 O C 3 N C 4 Si C 2 H 4 CH 3 C 2 H C 2 ** C 2 S C 3 O l-c 3 H 2 CH 3 CN HC 5 N CH CH 2 C 3
Protonated Polycyclic Aromatic Hydrocarbons and the Interstellar Medium
 1 Chapter 1 Protonated Polycyclic Aromatic Hydrocarbons and the Interstellar Medium 1.1 Interstellar Molecules Although hydrogen is the most abundant element in the universe, it is other elements that
1 Chapter 1 Protonated Polycyclic Aromatic Hydrocarbons and the Interstellar Medium 1.1 Interstellar Molecules Although hydrogen is the most abundant element in the universe, it is other elements that
Status of Spectral Line Catalogs
 Status of Spectral Line Catalogs Line Identification Data for Herschel, ALMA and SOFIA John Pearson JPL Laboratory Workshop 1 Existing Microwave Catalogs Microwave Catalogs (better than 100kHz accuracy
Status of Spectral Line Catalogs Line Identification Data for Herschel, ALMA and SOFIA John Pearson JPL Laboratory Workshop 1 Existing Microwave Catalogs Microwave Catalogs (better than 100kHz accuracy
Novel astrochemical aspects of cyanoacetylene-related molecules
 Novel astrochemical aspects of cyanoacetylene-related molecules R. KOŁOS 1,2, M. GRONOWSKI 1 & M. TUROWSKI 1 1) Institute of Physical Chemistry, Polish Academy of Sciences, Warsaw 2) Faculty of Mathematics
Novel astrochemical aspects of cyanoacetylene-related molecules R. KOŁOS 1,2, M. GRONOWSKI 1 & M. TUROWSKI 1 1) Institute of Physical Chemistry, Polish Academy of Sciences, Warsaw 2) Faculty of Mathematics
Interstellar chemistry. Liv Hornekær
 Interstellar chemistry Liv Hornekær Overall structure of the ISM 21 cm 2.6 mm Overview picture 21 cm observations Leiden-Dwingeloo/Argentina/Bonn H atom La: 121.6 nm, 10.2 ev J=L+S F=I+J Relative abundance
Interstellar chemistry Liv Hornekær Overall structure of the ISM 21 cm 2.6 mm Overview picture 21 cm observations Leiden-Dwingeloo/Argentina/Bonn H atom La: 121.6 nm, 10.2 ev J=L+S F=I+J Relative abundance
The Impact of the 12 m Telescope on Millimeter Science: Setting the Stage for the Future
 The Impact of the 12 m Telescope on Millimeter Science: Setting the Stage for the Future Lucy M. Ziurys and the Staff of the Arizona Radio Observatory T R * (K) 0.4 0.3 0.2 0.1 CH 3 OCH 3 CH 3 OCH 3 SO
The Impact of the 12 m Telescope on Millimeter Science: Setting the Stage for the Future Lucy M. Ziurys and the Staff of the Arizona Radio Observatory T R * (K) 0.4 0.3 0.2 0.1 CH 3 OCH 3 CH 3 OCH 3 SO
Sternentstehung - Star Formation Sommersemester 2009 Henrik Beuther
 Sternentstehung - Star Formation Sommersemester 2009 Henrik Beuther 3.4 Today: Introduction & Overview 10.4 Karfreitag 17.4 Physical processes I 24.4 Physcial processes II 1.5 Tag der Arbeit 8.5 Collapse
Sternentstehung - Star Formation Sommersemester 2009 Henrik Beuther 3.4 Today: Introduction & Overview 10.4 Karfreitag 17.4 Physical processes I 24.4 Physcial processes II 1.5 Tag der Arbeit 8.5 Collapse
Collisional Transitions in Interstellar Asymmetric Top Molecules
 Collisional Transitions in Interstellar Asymmetric Top Molecules Suresh Chandra Head, Department of Physics, School of Sciences, Lovely Professional University, Phagwara 144402 (Punjab) email-suresh492000@yahoo.co.in
Collisional Transitions in Interstellar Asymmetric Top Molecules Suresh Chandra Head, Department of Physics, School of Sciences, Lovely Professional University, Phagwara 144402 (Punjab) email-suresh492000@yahoo.co.in
Outflows and Jets: Theory and Observations
 Outflows and Jets: Theory and Observations Summer term 2011 Henrik Beuther & Christian Fendt 15.04 Today: Introduction & Overview (H.B. & C.F.) 29.04 Definitions, parameters, basic observations (H.B.)
Outflows and Jets: Theory and Observations Summer term 2011 Henrik Beuther & Christian Fendt 15.04 Today: Introduction & Overview (H.B. & C.F.) 29.04 Definitions, parameters, basic observations (H.B.)
Lecture 6: Molecular Transitions (1) Astrochemistry
 Lecture 6: Molecular Transitions (1) Astrochemistry Ehrenfreund & Charnley 2000, ARA&A, 38, 427 Outline Astrochemical processes: The formation of H2 H3 formation The chemistry initiated by H3 Formation
Lecture 6: Molecular Transitions (1) Astrochemistry Ehrenfreund & Charnley 2000, ARA&A, 38, 427 Outline Astrochemical processes: The formation of H2 H3 formation The chemistry initiated by H3 Formation
Cosmic Evolution, Part II. Heavy Elements to Molecules
 Cosmic Evolution, Part II Heavy Elements to Molecules Heavy elements molecules First a review of terminology: Electromagnetic Electrons Element Atom Nucleus Compound Molecule Electromagnetic Strong Nuclear
Cosmic Evolution, Part II Heavy Elements to Molecules Heavy elements molecules First a review of terminology: Electromagnetic Electrons Element Atom Nucleus Compound Molecule Electromagnetic Strong Nuclear
Understanding the chemistry of AGB circumstellar envelopes through the study of IRC
 Understanding the chemistry of AGB circumstellar envelopes through the study of IRC +10216 Marcelino Agúndez LUTH, Observatoire de Paris 28 janvier 2010 1 1.65 m 2MASS PART I. INTRODUCTION: - Interest
Understanding the chemistry of AGB circumstellar envelopes through the study of IRC +10216 Marcelino Agúndez LUTH, Observatoire de Paris 28 janvier 2010 1 1.65 m 2MASS PART I. INTRODUCTION: - Interest
Cosmic Evolution, Part II. Heavy Elements to Molecules
 Cosmic Evolution, Part II Heavy Elements to Molecules First a review of terminology: Element Atom Electro- magnetic Electrons Nucleus Electromagnetic Strong Nuclear Compound Molecule Protons Neutrons Neutral
Cosmic Evolution, Part II Heavy Elements to Molecules First a review of terminology: Element Atom Electro- magnetic Electrons Nucleus Electromagnetic Strong Nuclear Compound Molecule Protons Neutrons Neutral
High Resolution Spectroscopy and Astronomical Detection of Molecular Anions
 High Resolution Spectroscopy and Astronomical Detection of Molecular Anions Sandra Brünken, C. A. Gottlieb, H. Gupta, M. C. McCarthy, and P.Thaddeus Harvard Smithsonian Center for Astrophysics 10th Biennial
High Resolution Spectroscopy and Astronomical Detection of Molecular Anions Sandra Brünken, C. A. Gottlieb, H. Gupta, M. C. McCarthy, and P.Thaddeus Harvard Smithsonian Center for Astrophysics 10th Biennial
Lecture 10: "Chemistry in Dense Molecular Clouds"
 Lecture 10: "Chemistry in Dense Molecular Clouds" Outline 1. Observations of molecular clouds 2. Physics of dense clouds 3. Chemistry of dense clouds: C-, O-, N-chemistry Types of molecular clouds Diffuse
Lecture 10: "Chemistry in Dense Molecular Clouds" Outline 1. Observations of molecular clouds 2. Physics of dense clouds 3. Chemistry of dense clouds: C-, O-, N-chemistry Types of molecular clouds Diffuse
21. Introduction to Interstellar Chemistry
 21. Introduction to Interstellar Chemistry 1. Background 2. Gas Phase Chemistry 3. Formation and Destruction of H 2 4. Formation and Destruction of CO 5. Other Simple Molecules References Tielens, Physics
21. Introduction to Interstellar Chemistry 1. Background 2. Gas Phase Chemistry 3. Formation and Destruction of H 2 4. Formation and Destruction of CO 5. Other Simple Molecules References Tielens, Physics
HEXOS: Analysis of the HIFI 1.2 THz Wide Spectral Survey Toward Orion KL
 HEXOS: Analysis of the HIFI 1.2 THz Wide Spectral Survey Toward Orion KL N. R. Crockett, E. A. Bergin, J. Neill (U. Michigan), S. Wang (Emory), T. Bell, N. Huélamo, J. Cernicharo, G. Esplugues, B. Morales
HEXOS: Analysis of the HIFI 1.2 THz Wide Spectral Survey Toward Orion KL N. R. Crockett, E. A. Bergin, J. Neill (U. Michigan), S. Wang (Emory), T. Bell, N. Huélamo, J. Cernicharo, G. Esplugues, B. Morales
Astronomy 106, Fall September 2015
 6 7 8 9 Today in Astronomy 106: the origin of heavy elements and molecules Nucleosynthesis in normal stars; Populations II and I; heavy-element enrichment of the interstellar medium Today s chemical elements:
6 7 8 9 Today in Astronomy 106: the origin of heavy elements and molecules Nucleosynthesis in normal stars; Populations II and I; heavy-element enrichment of the interstellar medium Today s chemical elements:
New models of interstellar gas±grain chemistry ± I. Surface diffusion rates
 Mon. Not. R. Astron. Soc. 319, 837±850 (2000) New models of interstellar gas±grain chemistry ± I. Surface diffusion rates Deborah P. Ruffle 1 and Eric Herbst 2w 1 Department of Physics, The Ohio State
Mon. Not. R. Astron. Soc. 319, 837±850 (2000) New models of interstellar gas±grain chemistry ± I. Surface diffusion rates Deborah P. Ruffle 1 and Eric Herbst 2w 1 Department of Physics, The Ohio State
The Physics of the Interstellar Medium
 The Physics of the Interstellar Medium Ulrike Heiter Contact: 471 5970 ulrike@astro.uu.se www.astro.uu.se Matter between stars Average distance between stars in solar neighbourhood: 1 pc = 3 x 1013 km,
The Physics of the Interstellar Medium Ulrike Heiter Contact: 471 5970 ulrike@astro.uu.se www.astro.uu.se Matter between stars Average distance between stars in solar neighbourhood: 1 pc = 3 x 1013 km,
Atoms and Star Formation
 Atoms and Star Formation What are the characteristics of an atom? Atoms have a nucleus of protons and neutrons about which electrons orbit. neutrons protons electrons 0 charge +1 charge 1 charge 1.67 x
Atoms and Star Formation What are the characteristics of an atom? Atoms have a nucleus of protons and neutrons about which electrons orbit. neutrons protons electrons 0 charge +1 charge 1 charge 1.67 x
Astronomy 106, Fall September 2015
 Today in Astronomy 106: molecules to molecular clouds to stars Aromatic (benzene-ring) molecules in space Formation of molecules, on dust-grain surfaces and in the gas phase Interstellar molecular clouds
Today in Astronomy 106: molecules to molecular clouds to stars Aromatic (benzene-ring) molecules in space Formation of molecules, on dust-grain surfaces and in the gas phase Interstellar molecular clouds
Maria Cunningham, UNSW. CO, CS or other molecules?
 Maria Cunningham, UNSW CO, CS or other molecules? Wide field Surveys at mm wavelengths: pu8ng the whole picture together Follow chemical abundances through the whole ISM. Follow energy transfer through
Maria Cunningham, UNSW CO, CS or other molecules? Wide field Surveys at mm wavelengths: pu8ng the whole picture together Follow chemical abundances through the whole ISM. Follow energy transfer through
X-ray chemistry in the envelopes around young stellar objects
 Astronomy & Astrophysics manuscript no. xraypaper-final June 14, 2005 (DOI: will be inserted by hand later) X-ray chemistry in the envelopes around young stellar objects P. Stäuber 1, S.D. Doty 2, E.F.
Astronomy & Astrophysics manuscript no. xraypaper-final June 14, 2005 (DOI: will be inserted by hand later) X-ray chemistry in the envelopes around young stellar objects P. Stäuber 1, S.D. Doty 2, E.F.
Astrochemical Models. Eric Herbst Departments of Chemistry and Astronomy University of Virginia
 Astrochemical Models Eric Herbst Departments of Chemistry and Astronomy University of Virginia Chemical Models Gas-phase reactions 1000 s of reactions Grain-surface reactions Abundances, columns, spectra
Astrochemical Models Eric Herbst Departments of Chemistry and Astronomy University of Virginia Chemical Models Gas-phase reactions 1000 s of reactions Grain-surface reactions Abundances, columns, spectra
The Interstellar Medium
 The Interstellar Medium Fall 2014 Lecturer: Dr. Paul van der Werf Oortgebouw 565, ext 5883 pvdwerf@strw.leidenuniv.nl Assistant: Kirstin Doney Huygenslaboratorium 528 doney@strw.leidenuniv.nl Class Schedule
The Interstellar Medium Fall 2014 Lecturer: Dr. Paul van der Werf Oortgebouw 565, ext 5883 pvdwerf@strw.leidenuniv.nl Assistant: Kirstin Doney Huygenslaboratorium 528 doney@strw.leidenuniv.nl Class Schedule
Astrochemistry and Molecular Astrophysics Paola Caselli
 School of Physics and Astronomy FACULTY OF MATHEMATICS & PHYSICAL SCIENCES Astrochemistry and Molecular Astrophysics Paola Caselli Outline 1. The formation of H 2 2. The formation of H 3 + 3. The chemistry
School of Physics and Astronomy FACULTY OF MATHEMATICS & PHYSICAL SCIENCES Astrochemistry and Molecular Astrophysics Paola Caselli Outline 1. The formation of H 2 2. The formation of H 3 + 3. The chemistry
Victoria Louise Frankland. Heriot-Watt University. September 2011
 Towards Understanding the Formation of Water on Interstellar Dust Grains Victoria Louise Frankland Submitted for the degree of Doctor of Philosophy Heriot-Watt University School of Engineering and Physical
Towards Understanding the Formation of Water on Interstellar Dust Grains Victoria Louise Frankland Submitted for the degree of Doctor of Philosophy Heriot-Watt University School of Engineering and Physical
Astrochemistry Lecture 2 Basic Processes (cont d)
 Astrochemistry Lecture 2 Basic Processes (cont d) Ewine F. van Dishoeck Leiden Observatory Spring 2008 Types of chemical reactions Formation of bonds Radiative association: X + + Y XY + + hν Associative
Astrochemistry Lecture 2 Basic Processes (cont d) Ewine F. van Dishoeck Leiden Observatory Spring 2008 Types of chemical reactions Formation of bonds Radiative association: X + + Y XY + + hν Associative
Astr 2310 Thurs. March 23, 2017 Today s Topics
 Astr 2310 Thurs. March 23, 2017 Today s Topics Chapter 16: The Interstellar Medium and Star Formation Interstellar Dust and Dark Nebulae Interstellar Dust Dark Nebulae Interstellar Reddening Interstellar
Astr 2310 Thurs. March 23, 2017 Today s Topics Chapter 16: The Interstellar Medium and Star Formation Interstellar Dust and Dark Nebulae Interstellar Dust Dark Nebulae Interstellar Reddening Interstellar
19. Interstellar Chemistry
 19. Interstellar Chemistry 1. Introduction to Interstellar Chemistry 2. Chemical Processes & Models 3. Formation & Destruction of H 2 4. Formation & Destruction of CO References Duley & Williams, "Interstellar
19. Interstellar Chemistry 1. Introduction to Interstellar Chemistry 2. Chemical Processes & Models 3. Formation & Destruction of H 2 4. Formation & Destruction of CO References Duley & Williams, "Interstellar
6. Interstellar Medium. Emission nebulae are diffuse patches of emission surrounding hot O and
 6-1 6. Interstellar Medium 6.1 Nebulae Emission nebulae are diffuse patches of emission surrounding hot O and early B-type stars. Gas is ionized and heated by radiation from the parent stars. In size,
6-1 6. Interstellar Medium 6.1 Nebulae Emission nebulae are diffuse patches of emission surrounding hot O and early B-type stars. Gas is ionized and heated by radiation from the parent stars. In size,
Interstellar Dust and Extinction
 University of Oxford, Astrophysics November 12, 2007 Outline Extinction Spectral Features Emission Scattering Polarization Grain Models & Evolution Conclusions What and Why? Dust covers a range of compound
University of Oxford, Astrophysics November 12, 2007 Outline Extinction Spectral Features Emission Scattering Polarization Grain Models & Evolution Conclusions What and Why? Dust covers a range of compound
The Unbearable Lightness of Chemistry
 Z H IG C G N I OM H T A P U The Unbearable Lightness of Chemistry The Unbearable Lightness of Chemistry ~ 0.5 % Molecular (H 2 ) ~ 0.002 % CO ~ 0.000002 % any other molecule A total of 46 molecular species
Z H IG C G N I OM H T A P U The Unbearable Lightness of Chemistry The Unbearable Lightness of Chemistry ~ 0.5 % Molecular (H 2 ) ~ 0.002 % CO ~ 0.000002 % any other molecule A total of 46 molecular species
Formation of methyl formate and other organic species in the warm-up phase of hot molecular cores. R. T. Garrod 1 and E. Herbst 1,2 ABSTRACT
 A&A 457, 927 936 (2006) DOI: 10.1051/0004-6361:20065560 c ESO 2006 Astronomy & Astrophysics Formation of methyl formate and other organic species in the warm-up phase of hot molecular cores R. T. Garrod
A&A 457, 927 936 (2006) DOI: 10.1051/0004-6361:20065560 c ESO 2006 Astronomy & Astrophysics Formation of methyl formate and other organic species in the warm-up phase of hot molecular cores R. T. Garrod
Absorption spectroscopy with Herschel/HIFI and IRAM-PdBI : Promises for ALMA
 PRISMAS PRobing InterStellar Molecules with Absorption line Studies Absorption spectroscopy with Herschel/HIFI and IRAM-PdBI : Promises for ALMA Maryvonne Gerin Why Absorption Spectroscopy? Sensitivity
PRISMAS PRobing InterStellar Molecules with Absorption line Studies Absorption spectroscopy with Herschel/HIFI and IRAM-PdBI : Promises for ALMA Maryvonne Gerin Why Absorption Spectroscopy? Sensitivity
Chemistry Final Exam Sample Items
 Chemistry Final Exam Sample Items 1. Which best describes the current atomic theory? a. Atoms consist of electrons circling in definite orbits around a positive nucleus. b. Atoms are composed of electrons
Chemistry Final Exam Sample Items 1. Which best describes the current atomic theory? a. Atoms consist of electrons circling in definite orbits around a positive nucleus. b. Atoms are composed of electrons
Laboratory Astrophysics: Or, Finding an Anion in a Haystack Part I: Tutorial
 Laboratory Astrophysics: Or, Finding an Anion in a Haystack Part I: Tutorial Sandra Brünken I. Physikalisches Institut, Universität zu Köln, Germany Workshop Negative ions and molecules in astrophysics
Laboratory Astrophysics: Or, Finding an Anion in a Haystack Part I: Tutorial Sandra Brünken I. Physikalisches Institut, Universität zu Köln, Germany Workshop Negative ions and molecules in astrophysics
Electrolytes. Ions and Molecules in Aqueous Solution
 Electrolytes Ions and Molecules in Aqueous Solution Experiment 7 DISCUSSION Expt 7 Electrolytes.wpd Electrical Conductivities of Pure Substances The ability of any substance to conduct electricity often
Electrolytes Ions and Molecules in Aqueous Solution Experiment 7 DISCUSSION Expt 7 Electrolytes.wpd Electrical Conductivities of Pure Substances The ability of any substance to conduct electricity often
Update Log. ITYPE Reaction types in the gas-phase model
 Update Log ITYPE Reaction types in the gas-phase model 0 Gas-grain interaction, Electron-grain recombination 1 Cosmic-ray ionization (direct process) #1, Cosmic-ray induced photoreactions (indirect process)
Update Log ITYPE Reaction types in the gas-phase model 0 Gas-grain interaction, Electron-grain recombination 1 Cosmic-ray ionization (direct process) #1, Cosmic-ray induced photoreactions (indirect process)
Astrochemistry the summary
 Astrochemistry the summary Astro 736 Nienke van der Marel April 27th 2017 Astrochemistry When the first interstellar molecules were discovered, chemists were very surprised. Why? Conditions in space are
Astrochemistry the summary Astro 736 Nienke van der Marel April 27th 2017 Astrochemistry When the first interstellar molecules were discovered, chemists were very surprised. Why? Conditions in space are
OUTLINE OF A GAS-GRAIN CHEMICAL CODE. Dima Semenov MPIA Heidelberg
 OUTLINE OF A GAS-GRAIN CHEMICAL CODE Dima Semenov MPIA Heidelberg MOTTO OF ASTROCHEMISTRY CODE DEVELOPMENT We had 5 ODE solvers, 9 Jacobi matrix inversion routines, several approaches to calculate reaction
OUTLINE OF A GAS-GRAIN CHEMICAL CODE Dima Semenov MPIA Heidelberg MOTTO OF ASTROCHEMISTRY CODE DEVELOPMENT We had 5 ODE solvers, 9 Jacobi matrix inversion routines, several approaches to calculate reaction
NEARBY GALAXIES AND ALMA
 NEARBY GALAXIES AND ALMA Jean Turner, UCLA nearby galaxies close-up views of star formation & nuclear fueling on scales of GMCs and star clusters - where & how do galaxies form stars? - where does gas
NEARBY GALAXIES AND ALMA Jean Turner, UCLA nearby galaxies close-up views of star formation & nuclear fueling on scales of GMCs and star clusters - where & how do galaxies form stars? - where does gas
MOLECULES IN THE CIRCUMNUCLEAR DISK OF THE GALACTIC CENTER
 MOLECULES IN THE CIRCUMNUCLEAR DISK OF THE GALACTIC CENTER Implications from Chemical Modeling Nanase Harada 1, Denise Riquelme 1, Serena Viti 2, Karl Menten 1, Miguel Requena-Torres 1, Rolf Güsten 1,
MOLECULES IN THE CIRCUMNUCLEAR DISK OF THE GALACTIC CENTER Implications from Chemical Modeling Nanase Harada 1, Denise Riquelme 1, Serena Viti 2, Karl Menten 1, Miguel Requena-Torres 1, Rolf Güsten 1,
EVOLUTION OF MOLECULAR ABUNDANCE IN PROTOPLANETARY DISKS
 EVOLUTION OF MOLECULAR ABUNDANCE IN PROTOPLANETARY DISKS Yuri Aikawa 1 Department of Earth and Planetary Science, University of Tokyo, Bunkyo-ku, Tokyo 113, Japan arxiv:astro-ph/9706204v1 19 Jun 1997 Toyoharu
EVOLUTION OF MOLECULAR ABUNDANCE IN PROTOPLANETARY DISKS Yuri Aikawa 1 Department of Earth and Planetary Science, University of Tokyo, Bunkyo-ku, Tokyo 113, Japan arxiv:astro-ph/9706204v1 19 Jun 1997 Toyoharu
2 nd Semester Study Guide 2016
 Chemistry 2 nd Semester Study Guide 2016 Name: Unit 6: Chemical Reactions and Balancing 1. Draw the remaining product 2. Write a balanced equation for the following reaction: The reaction between sodium
Chemistry 2 nd Semester Study Guide 2016 Name: Unit 6: Chemical Reactions and Balancing 1. Draw the remaining product 2. Write a balanced equation for the following reaction: The reaction between sodium
Mole: base unit for an amount of substance A mole contains Avogadro s number (N A ) of particles (atoms, molecules, ions, formula units )
 Mole: base unit for an amount of substance A mole contains Avogadro s number (N A ) of particles (atoms, molecules, ions, formula units ) N A 6.0 10 mol -1 1 mol substance contains N A Molar mass (g/mol)
Mole: base unit for an amount of substance A mole contains Avogadro s number (N A ) of particles (atoms, molecules, ions, formula units ) N A 6.0 10 mol -1 1 mol substance contains N A Molar mass (g/mol)
9 - The Hot Corinos. Complex organic molecules in the inner 100 AU envelope of Solar type protostars
 9 - The Hot Corinos Complex organic molecules in the inner 100 AU envelope of Solar type protostars 1 THE COLLAPSING PROTOSTAR PHASE 2 THE INNER ENVELOPES OF LOW MASS PROTOSTARS Direct observations of
9 - The Hot Corinos Complex organic molecules in the inner 100 AU envelope of Solar type protostars 1 THE COLLAPSING PROTOSTAR PHASE 2 THE INNER ENVELOPES OF LOW MASS PROTOSTARS Direct observations of
The Evolution of Molecular Tracers Jürgen Ott New World New Horizons, Santa Fe 8 March Molecular Gas Tracers in Galaxies. Juergen Ott (NRAO)
 Molecular Gas Tracers in Galaxies Juergen Ott (NRAO) Star Formation How do stars form? 1) Atomic/ionized gas converts to molecular clouds Star Formation How do stars form? 1) Atomic/ionized gas converts
Molecular Gas Tracers in Galaxies Juergen Ott (NRAO) Star Formation How do stars form? 1) Atomic/ionized gas converts to molecular clouds Star Formation How do stars form? 1) Atomic/ionized gas converts
Photodissociation Regions Radiative Transfer. Dr. Thomas G. Bisbas
 Photodissociation Regions Radiative Transfer Dr. Thomas G. Bisbas tbisbas@ufl.edu Interstellar Radiation Field In the solar neighbourhood, the ISRF is dominated by six components Schematic sketch of the
Photodissociation Regions Radiative Transfer Dr. Thomas G. Bisbas tbisbas@ufl.edu Interstellar Radiation Field In the solar neighbourhood, the ISRF is dominated by six components Schematic sketch of the
Experimental Studies of Surface and Gas- Phase Processes Relevant to the Interstellar Medium and Planetary Atmospheres
 Experimental Studies of Surface and Gas- Phase Processes Relevant to the Interstellar Medium and Planetary Atmospheres Elspeth Rebecca Latimer Thesis submitted for the degree of Doctor of Philosophy University
Experimental Studies of Surface and Gas- Phase Processes Relevant to the Interstellar Medium and Planetary Atmospheres Elspeth Rebecca Latimer Thesis submitted for the degree of Doctor of Philosophy University
LLNL-PRES W. M. Howard and S. Bastea Extreme Chemistry Group. Lawrence Livermore National Laboratory
 Titan s Interior :(I) A thermo-chemical assessment suggests N 2 is the dominate source of nitrogen near Titan s surface\\ and (II) future studies on the effects of comet impacts on organic and surface
Titan s Interior :(I) A thermo-chemical assessment suggests N 2 is the dominate source of nitrogen near Titan s surface\\ and (II) future studies on the effects of comet impacts on organic and surface
Galactic dust in the Herschel and Planck era. François Boulanger Institut d Astrophysique Spatiale
 Galactic dust in the Herschel and Planck era François Boulanger Institut d Astrophysique Spatiale Motivation Dust emission Dust models Dust life cycle Planck early results Dust polarisation Outline Dust
Galactic dust in the Herschel and Planck era François Boulanger Institut d Astrophysique Spatiale Motivation Dust emission Dust models Dust life cycle Planck early results Dust polarisation Outline Dust
The Birth Of Stars. How do stars form from the interstellar medium Where does star formation take place How do we induce star formation
 Goals: The Birth Of Stars How do stars form from the interstellar medium Where does star formation take place How do we induce star formation Interstellar Medium Gas and dust between stars is the interstellar
Goals: The Birth Of Stars How do stars form from the interstellar medium Where does star formation take place How do we induce star formation Interstellar Medium Gas and dust between stars is the interstellar
Planetary Temperatures
 Planetary Temperatures How does Sunlight heat a planet with no atmosphere? This is similar to our dust grain heating problem First pass: Consider a planet of radius a at a distance R from a star of luminosity
Planetary Temperatures How does Sunlight heat a planet with no atmosphere? This is similar to our dust grain heating problem First pass: Consider a planet of radius a at a distance R from a star of luminosity
Sounding the diffuse ISM with Herschel/HIFI
 PRISMAS PRobing InterStellar Molecules with Absorption line Studies Sounding the diffuse ISM with Herschel/HIFI.. W3 Maryvonne Gerin on behalf of the PRISMAS team PRISMAS PRobing InterStellar Molecules
PRISMAS PRobing InterStellar Molecules with Absorption line Studies Sounding the diffuse ISM with Herschel/HIFI.. W3 Maryvonne Gerin on behalf of the PRISMAS team PRISMAS PRobing InterStellar Molecules
Astrochemistry. Lecture 10, Primordial chemistry. Jorma Harju. Department of Physics. Friday, April 5, 2013, 12:15-13:45, Lecture room D117
 Astrochemistry Lecture 10, Primordial chemistry Jorma Harju Department of Physics Friday, April 5, 2013, 12:15-13:45, Lecture room D117 The first atoms (1) SBBN (Standard Big Bang Nucleosynthesis): elements
Astrochemistry Lecture 10, Primordial chemistry Jorma Harju Department of Physics Friday, April 5, 2013, 12:15-13:45, Lecture room D117 The first atoms (1) SBBN (Standard Big Bang Nucleosynthesis): elements
Stellar evolution Part I of III Star formation
 Stellar evolution Part I of III Star formation The interstellar medium (ISM) The space between the stars is not completely empty, but filled with very dilute gas and dust, producing some of the most beautiful
Stellar evolution Part I of III Star formation The interstellar medium (ISM) The space between the stars is not completely empty, but filled with very dilute gas and dust, producing some of the most beautiful
Chapter 9. The Formation and Structure of Stars
 Chapter 9 The Formation and Structure of Stars The Interstellar Medium (ISM) The space between the stars is not completely empty, but filled with very dilute gas and dust, producing some of the most beautiful
Chapter 9 The Formation and Structure of Stars The Interstellar Medium (ISM) The space between the stars is not completely empty, but filled with very dilute gas and dust, producing some of the most beautiful
1 A. That the reaction is endothermic when proceeding in the left to right direction as written.
 1 Q. If Δ r H is positive, what can you say about the reaction? 1 A. That the reaction is endothermic when proceeding in the left to right direction as written. 2 Q If Δ r H is negative, what can you say
1 Q. If Δ r H is positive, what can you say about the reaction? 1 A. That the reaction is endothermic when proceeding in the left to right direction as written. 2 Q If Δ r H is negative, what can you say
Lecture 5. Interstellar Dust: Chemical & Thermal Properties
 Lecture 5. Interstellar Dust: Chemical & Thermal Properties!. Spectral Features 2. Grain populations and Models 3. Thermal Properties 4. Small Grains and Large Molecules -------------------------------------------------
Lecture 5. Interstellar Dust: Chemical & Thermal Properties!. Spectral Features 2. Grain populations and Models 3. Thermal Properties 4. Small Grains and Large Molecules -------------------------------------------------
The Promise of HIFI. Xander Tielens (HIFI project scientist) on behalf of the HIFI consortium
 The Promise of HIFI Xander Tielens (HIFI project scientist) on behalf of the HIFI consortium HIFI science HIFI is a versatile instrument Wide spectral coverage and high spectral resolution Physical conditions
The Promise of HIFI Xander Tielens (HIFI project scientist) on behalf of the HIFI consortium HIFI science HIFI is a versatile instrument Wide spectral coverage and high spectral resolution Physical conditions
Dust formation in O-rich Miras and IK Tau
 Dust formation in O-rich Miras and IK Tau David Gobrecht & Isabelle Cherchneff Basel University Colls.: Arkaprabha Sarangi & John Plane Why Galaxies Care About AGB Stars III Vienna 30 July 2014 Overview
Dust formation in O-rich Miras and IK Tau David Gobrecht & Isabelle Cherchneff Basel University Colls.: Arkaprabha Sarangi & John Plane Why Galaxies Care About AGB Stars III Vienna 30 July 2014 Overview
Acid/Base Definitions
 Acids and Bases Acid/Base Definitions Arrhenius Model Acids produce hydrogen ions in aqueous solutions Bases produce hydroxide ions in aqueous solutions Bronsted-Lowry Model Acids are proton donors Bases
Acids and Bases Acid/Base Definitions Arrhenius Model Acids produce hydrogen ions in aqueous solutions Bases produce hydroxide ions in aqueous solutions Bronsted-Lowry Model Acids are proton donors Bases
Possible Extra Credit Option
 Possible Extra Credit Option Attend an advanced seminar on Astrophysics or Astronomy held by the Physics and Astronomy department. There are seminars held every 2:00 pm, Thursday, Room 190, Physics & Astronomy
Possible Extra Credit Option Attend an advanced seminar on Astrophysics or Astronomy held by the Physics and Astronomy department. There are seminars held every 2:00 pm, Thursday, Room 190, Physics & Astronomy
26. N 2 + H 2 NH N 2 + O 2 N 2 O 28. CO 2 + H 2 O C 6 H 12 O 6 + O SiCl 4 + H 2 O H 4 SiO 4 + HCl 30. H 3 PO 4 H 4 P 2 O 7 + H 2 O
 Balance the following chemical equations: (Some may already be balanced.) 1. H 2 + O 2 H 2 O 2. S 8 + O 2 SO 3 3. HgO Hg + O 2 4. Zn + HCl ZnCl 2 + H 2 5. Na + H 2 O NaOH + H 2 6. C 10 H 16 + Cl 2 C +
Balance the following chemical equations: (Some may already be balanced.) 1. H 2 + O 2 H 2 O 2. S 8 + O 2 SO 3 3. HgO Hg + O 2 4. Zn + HCl ZnCl 2 + H 2 5. Na + H 2 O NaOH + H 2 6. C 10 H 16 + Cl 2 C +
Dissociative recombination reactions. Wolf D. Geppert ISSI workshop, Bern (CH) December 2008
 Dissociative recombination reactions Wolf D. Geppert ISSI workshop, Bern (CH) December 2008 Important electron-ion processes A B - Resonant ion pair formation (high energies) AB hν Radiative recombination
Dissociative recombination reactions Wolf D. Geppert ISSI workshop, Bern (CH) December 2008 Important electron-ion processes A B - Resonant ion pair formation (high energies) AB hν Radiative recombination
August 31 st, 2015 page 21 DO: I will be able to differentiate between atoms, elements, molecules, and compounds. EQ: How are molecules created?
 August 31 st, 2015 page 21 DO: I will be able to differentiate between atoms, elements, molecules, and compounds. EQ: How are molecules created? Explain this statement: All compounds are molecules but
August 31 st, 2015 page 21 DO: I will be able to differentiate between atoms, elements, molecules, and compounds. EQ: How are molecules created? Explain this statement: All compounds are molecules but
2 nd Semester Study Guide 2017
 Chemistry 2 nd Semester Study Guide 2017 Name: KEY Unit 6: Chemical Reactions and Balancing 1. Draw the remaining product 2. Write a balanced equation for the following reaction: The reaction between sodium
Chemistry 2 nd Semester Study Guide 2017 Name: KEY Unit 6: Chemical Reactions and Balancing 1. Draw the remaining product 2. Write a balanced equation for the following reaction: The reaction between sodium
Clicker Question: Clicker Question: What is the expected lifetime for a G2 star (one just like our Sun)?
 How Long do Stars Live (as Main Sequence Stars)? A star on Main Sequence has fusion of H to He in its core. How fast depends on mass of H available and rate of fusion. Mass of H in core depends on mass
How Long do Stars Live (as Main Sequence Stars)? A star on Main Sequence has fusion of H to He in its core. How fast depends on mass of H available and rate of fusion. Mass of H in core depends on mass
CHEM Dr. Babb s Sections Exam #3 Review Sheet
 CHEM 116 Dr. Babb s Sections Exam #3 Review Sheet Acid/Base Theories and Conjugate AcidBase Pairs 111. Define the following terms: Arrhenius acid, Arrhenius base, Lewis acid, Lewis base, BronstedLowry
CHEM 116 Dr. Babb s Sections Exam #3 Review Sheet Acid/Base Theories and Conjugate AcidBase Pairs 111. Define the following terms: Arrhenius acid, Arrhenius base, Lewis acid, Lewis base, BronstedLowry
Acids, Bases, and Salts Review for Sections
 1. Consider the following: Review for Sections 4.1 4.9 I H 2 CO 3 + F HCO 3 + HF 2 II HCO 3 + HC 2 O 4 H 2 CO 3 + C 2 O 4 2 III HCO 3 + H 2 C 6 H 6 O 7 H 2 CO 3 + HC 6 H 5 O 7 The HCO 3 is a base in A.
1. Consider the following: Review for Sections 4.1 4.9 I H 2 CO 3 + F HCO 3 + HF 2 II HCO 3 + HC 2 O 4 H 2 CO 3 + C 2 O 4 2 III HCO 3 + H 2 C 6 H 6 O 7 H 2 CO 3 + HC 6 H 5 O 7 The HCO 3 is a base in A.
Chemistry Released Questions
 Name: Date: 1. What was Niels Bohr s prediction about the location of the electrons in an atom? 3. An atom with which atomic diagram has chemical properties most similar to calcium? A. Electrons pair with
Name: Date: 1. What was Niels Bohr s prediction about the location of the electrons in an atom? 3. An atom with which atomic diagram has chemical properties most similar to calcium? A. Electrons pair with
Astrochemistry (2) Interstellar extinction. Measurement of the reddening
 Measurement of the reddening The reddening of stellar colours casts light on the properties of interstellar dust Astrochemistry (2) Planets and Astrobiology (2016-2017) G. Vladilo The reddening is measured
Measurement of the reddening The reddening of stellar colours casts light on the properties of interstellar dust Astrochemistry (2) Planets and Astrobiology (2016-2017) G. Vladilo The reddening is measured
Model of Hydrogen Deficient Nebulae in H II Regions at High Temperature
 Journal of Materials Science and Chemical Engineering, 2015, 3, 21-29 Published Online August 2015 in SciRes. http://www.scirp.org/journal/msce http://dx.doi.org/10.4236/msce.2015.38004 Model of Hydrogen
Journal of Materials Science and Chemical Engineering, 2015, 3, 21-29 Published Online August 2015 in SciRes. http://www.scirp.org/journal/msce http://dx.doi.org/10.4236/msce.2015.38004 Model of Hydrogen
Chapter 16 Lecture. The Cosmic Perspective Seventh Edition. Star Birth Pearson Education, Inc.
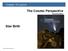 Chapter 16 Lecture The Cosmic Perspective Seventh Edition Star Birth 2014 Pearson Education, Inc. Star Birth The dust and gas between the star in our galaxy is referred to as the Interstellar medium (ISM).
Chapter 16 Lecture The Cosmic Perspective Seventh Edition Star Birth 2014 Pearson Education, Inc. Star Birth The dust and gas between the star in our galaxy is referred to as the Interstellar medium (ISM).
Lecture 18 - Photon Dominated Regions
 Lecture 18 - Photon Dominated Regions 1. What is a PDR? 2. Physical and Chemical Concepts 3. Molecules in Diffuse Clouds 4. Galactic and Extragalactic PDRs References Tielens, Ch. 9 Hollenbach & Tielens,
Lecture 18 - Photon Dominated Regions 1. What is a PDR? 2. Physical and Chemical Concepts 3. Molecules in Diffuse Clouds 4. Galactic and Extragalactic PDRs References Tielens, Ch. 9 Hollenbach & Tielens,
Energy. mosquito lands on your arm = 1 erg. Firecracker = 5 x 10 9 ergs. 1 stick of dynamite = 2 x ergs. 1 ton of TNT = 4 x ergs
 Energy mosquito lands on your arm = 1 erg Firecracker = 5 x 10 9 ergs 1 stick of dynamite = 2 x 10 13 ergs 1 ton of TNT = 4 x 10 16 ergs 1 atomic bomb = 1 x 10 21 ergs Magnitude 8 earthquake = 1 x 10 26
Energy mosquito lands on your arm = 1 erg Firecracker = 5 x 10 9 ergs 1 stick of dynamite = 2 x 10 13 ergs 1 ton of TNT = 4 x 10 16 ergs 1 atomic bomb = 1 x 10 21 ergs Magnitude 8 earthquake = 1 x 10 26
The Interstellar Medium. Papillon Nebula. Neutral Hydrogen Clouds. Interstellar Gas. The remaining 1% exists as interstellar grains or
 The Interstellar Medium About 99% of the material between the stars is in the form of a gas The remaining 1% exists as interstellar grains or interstellar dust If all the interstellar gas were spread evenly,
The Interstellar Medium About 99% of the material between the stars is in the form of a gas The remaining 1% exists as interstellar grains or interstellar dust If all the interstellar gas were spread evenly,
Chapter 16: Star Birth
 Chapter 16 Lecture Chapter 16: Star Birth Star Birth 16.1 Stellar Nurseries Our goals for learning: Where do stars form? Why do stars form? Where do stars form? Star-Forming Clouds Stars form in dark clouds
Chapter 16 Lecture Chapter 16: Star Birth Star Birth 16.1 Stellar Nurseries Our goals for learning: Where do stars form? Why do stars form? Where do stars form? Star-Forming Clouds Stars form in dark clouds
Notes on Photoionized Regions Wednesday, January 12, 2011
 Notes on Photoionized Regions Wednesday, January 12, 2011 CONTENTS: 1. Introduction 2. Hydrogen Nebulae A. Ionization equations B. Recombination coefficients and cross sections C. Structure of the hydrogen
Notes on Photoionized Regions Wednesday, January 12, 2011 CONTENTS: 1. Introduction 2. Hydrogen Nebulae A. Ionization equations B. Recombination coefficients and cross sections C. Structure of the hydrogen
Search for fullerene and fullerane (C 60 H m ) in deep space
 Yong Zhang Sun Yat- Sen University The University of Hong Kong Search for fullerene and fullerane (C 60 H m ) in deep space 2017. 6 Xiang Tan Material lifecycle in the Galaxy How complex are the molecules
Yong Zhang Sun Yat- Sen University The University of Hong Kong Search for fullerene and fullerane (C 60 H m ) in deep space 2017. 6 Xiang Tan Material lifecycle in the Galaxy How complex are the molecules
Chapter 16 exercise. For the following reactions, use figure 16.4 to predict whether the equilibrium lies predominantly. - (aq) + OH - (aq)
 1 Chapter 16 exercise Q1. Practice exercise page 671 Write the formula for the conjugate acid of the following, HSO 3, F, PO 4 3 and CO. HSO 3 H H 2 SO 4 F H HF PO 4 3 H HPO 4 2 CO H HCO Q2. Practice exercise
1 Chapter 16 exercise Q1. Practice exercise page 671 Write the formula for the conjugate acid of the following, HSO 3, F, PO 4 3 and CO. HSO 3 H H 2 SO 4 F H HF PO 4 3 H HPO 4 2 CO H HCO Q2. Practice exercise
Chapter 16 Lecture. The Cosmic Perspective Seventh Edition. Star Birth Pearson Education, Inc.
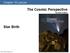 Chapter 16 Lecture The Cosmic Perspective Seventh Edition Star Birth Star Birth 16.1 Stellar Nurseries Our goals for learning: Where do stars form? Why do stars form? Where do stars form? Star-Forming
Chapter 16 Lecture The Cosmic Perspective Seventh Edition Star Birth Star Birth 16.1 Stellar Nurseries Our goals for learning: Where do stars form? Why do stars form? Where do stars form? Star-Forming
Consider a 1.0 L solution of 0.10 M acetic acid. Acetic acid is a weak acid only a small percent of the weak acid is ionized
 Chemistry 12 Acid- Base Equilibrium V Name: Date: Block: 1. Buffers 2. Hydrolysis Buffers An acid- base buffer is a solution that resists changes in ph following the addition of relatively small amounts
Chemistry 12 Acid- Base Equilibrium V Name: Date: Block: 1. Buffers 2. Hydrolysis Buffers An acid- base buffer is a solution that resists changes in ph following the addition of relatively small amounts
Homework #6 Chapter 7 Homework Acids and Bases
 Homework #6 Chapter 7 Homework Acids and Bases 20. a) 2H 2O(l) H 3O (aq) OH (aq) K [H 3 O ][OH ] Or H 2O(l) H (aq) OH (aq) K [H ][OH ] b) HCN(aq) H 2O(l) H 3O (aq) CN (aq) K [H 3O ][CN ] [HCN] Or HCN(aq)
Homework #6 Chapter 7 Homework Acids and Bases 20. a) 2H 2O(l) H 3O (aq) OH (aq) K [H 3 O ][OH ] Or H 2O(l) H (aq) OH (aq) K [H ][OH ] b) HCN(aq) H 2O(l) H 3O (aq) CN (aq) K [H 3O ][CN ] [HCN] Or HCN(aq)
CASSIS. A Software Package to Speed-up the Science Analysis of Spectral Data. Emmanuel Caux, CESR Toulouse, France
 CASSIS A Software Package to Speed-up the Science Analysis of Spectral Data Emmanuel Caux, CESR Toulouse, France on behalf the CASSIS team @ CESR : E. Caux, A. Klotz, C. Vastel & A. Walters CASSIS Centre
CASSIS A Software Package to Speed-up the Science Analysis of Spectral Data Emmanuel Caux, CESR Toulouse, France on behalf the CASSIS team @ CESR : E. Caux, A. Klotz, C. Vastel & A. Walters CASSIS Centre
The formation of stars and planets. Day 1, Topic 2: Radiation physics. Lecture by: C.P. Dullemond
 The formation of stars and planets Day 1, Topic 2: Radiation physics Lecture by: C.P. Dullemond Astronomical Constants CGS units used throughout lecture (cm,erg,s...) AU = Astronomical Unit = distance
The formation of stars and planets Day 1, Topic 2: Radiation physics Lecture by: C.P. Dullemond Astronomical Constants CGS units used throughout lecture (cm,erg,s...) AU = Astronomical Unit = distance
Diffuse Interstellar Medium
 Diffuse Interstellar Medium Basics, velocity widths H I 21-cm radiation (emission) Interstellar absorption lines Radiative transfer Resolved Lines, column densities Unresolved lines, curve of growth Abundances,
Diffuse Interstellar Medium Basics, velocity widths H I 21-cm radiation (emission) Interstellar absorption lines Radiative transfer Resolved Lines, column densities Unresolved lines, curve of growth Abundances,
Star-Forming Clouds. Stars form in dark clouds of dusty gas in interstellar space. The gas between the stars is called the interstellar medium.
 Star Birth Chapter 16 Lecture 16.1 Stellar Nurseries The Cosmic Perspective Our goals for learning: Where do stars form? Why do stars form? Seventh Edition Star Birth Where do stars form? Star-Forming
Star Birth Chapter 16 Lecture 16.1 Stellar Nurseries The Cosmic Perspective Our goals for learning: Where do stars form? Why do stars form? Seventh Edition Star Birth Where do stars form? Star-Forming
The Solar Nebula Theory
 Reading: Chap. 21, Sect.21.1, 21.3 Final Exam: Tuesday, December 12; 4:30-6:30PM Homework 10: Due in recitation Dec. 1,4 Astro 120 Fall 2017: Lecture 25 page 1 Astro 120 Fall 2017: Lecture 25 page 2 The
Reading: Chap. 21, Sect.21.1, 21.3 Final Exam: Tuesday, December 12; 4:30-6:30PM Homework 10: Due in recitation Dec. 1,4 Astro 120 Fall 2017: Lecture 25 page 1 Astro 120 Fall 2017: Lecture 25 page 2 The
Chapter 16: Acids and Bases
 1. Which is not a characteristic property of acids? A) neutralizes bases B) turns litmus from blue to red C) reacts with active metals to produce H 2 (g) D) reacts with CO 2 (g) to form carbonates E) All
1. Which is not a characteristic property of acids? A) neutralizes bases B) turns litmus from blue to red C) reacts with active metals to produce H 2 (g) D) reacts with CO 2 (g) to form carbonates E) All
arxiv: v1 [astro-ph.co] 5 Oct 2010
![arxiv: v1 [astro-ph.co] 5 Oct 2010 arxiv: v1 [astro-ph.co] 5 Oct 2010](/thumbs/88/115253906.jpg) Astronomy & Astrophysics manuscript no. ms c ESO 2018 October 30, 2018 Star Formation in Extreme Environments: The Effects of Cosmic Rays and Mechanical Heating. R. Meijerink 1, M. Spaans 2, A.F. Loenen
Astronomy & Astrophysics manuscript no. ms c ESO 2018 October 30, 2018 Star Formation in Extreme Environments: The Effects of Cosmic Rays and Mechanical Heating. R. Meijerink 1, M. Spaans 2, A.F. Loenen
Periodic Trends. The trends we will study all have to do with the valence electrons in one way or another. Two key ideas:
 Periodic Trends The trends we will study all have to do with the valence electrons in one way or another. Two key ideas: Nuclear Charge = the number of protons in the nucleus. This is the positive charge
Periodic Trends The trends we will study all have to do with the valence electrons in one way or another. Two key ideas: Nuclear Charge = the number of protons in the nucleus. This is the positive charge
Name:. Correct Questions = Wrong Questions =.. Unattempt Questions = Marks =
 Name:. Correct Questions = Wrong Questions =.. Unattempt Questions = Marks = 1. Which anion forms the smallest number of insoluble salts? (A) Cl - (B) NO 3 - (C) CO 3 2- (D) SO 4 2-2. Which piece of apparatus
Name:. Correct Questions = Wrong Questions =.. Unattempt Questions = Marks = 1. Which anion forms the smallest number of insoluble salts? (A) Cl - (B) NO 3 - (C) CO 3 2- (D) SO 4 2-2. Which piece of apparatus
Recall what you know about the Big Bang.
 What is this? Recall what you know about the Big Bang. Most of the normal matter in the universe is made of what elements? Where do we find most of this normal matter? Interstellar medium (ISM) The universe
What is this? Recall what you know about the Big Bang. Most of the normal matter in the universe is made of what elements? Where do we find most of this normal matter? Interstellar medium (ISM) The universe
Nanotube Peapods: A New Class of Carbon Nanotube Hybrids
 NT 06. 06. June 19 2006, Nagano, Japan. Nanotube Peapods: A New Class of Carbon Nanotube Hybrids Nori Shinohara Department of of Chemistry, Nagoya University, Nagoya, Japan Japan Outline 1. 1. Synthesis
NT 06. 06. June 19 2006, Nagano, Japan. Nanotube Peapods: A New Class of Carbon Nanotube Hybrids Nori Shinohara Department of of Chemistry, Nagoya University, Nagoya, Japan Japan Outline 1. 1. Synthesis
Science Olympiad Regional UW-Milwaukee Chemistry test 2013
 Science Olympiad Regional UW-Milwaukee Chemistry test 2013 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is another name for the representative
Science Olympiad Regional UW-Milwaukee Chemistry test 2013 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is another name for the representative
