Chapter 8. Chapter 8. Preview. Bellringer. Chapter 8. Particles of Matter. Objectives. Chapter 8. Particles of Matter, continued
|
|
|
- Lorena Lynch
- 6 years ago
- Views:
Transcription
1 States of Matter Preview Bellringer Section 2 Behavior of Gases In the kitchen, you might find three different forms of water. What are these three forms of water, and where exactly in the kitchen would you find them? Further, how do you use water in each of these forms? Record your answers in your science journal. Concept Mapping Objectives Describe the properties shared by particles of all matter. Describe three states of matter. Explain the differences between the states of matter. Particles of Matter The states of matter are the physical forms in which a substance can exist. The three most familiar states of matter are solid, liquid, and gas. Matter is made up of tiny particles called atoms and molecules. These particles are always in motion and are always bumping into one another. Particles of Matter, continued The particles in matter interact with each other. The way the particles interact with each other helps determine the state of the matter. The interactions between particles in three states of matter is shown on the next slide.
2 Solids A solid is the state of matter that has a definite shape and volume. The particles in a solid do not move fast enough to overcome the attraction between them. Each particle vibrates in place and is locked in place by the particles around it. Solids, continued There Are Two Kinds of Solids Crystalline solids have a very orderly, three-dimensional arrangement of particles. Iron, diamond, and ice are crystalline solids. Amorphous solids are made of particles that do not have a special arrangement. Glass, rubber, and wax are amorphous solids. Liquids Liquid is the state of matter that has a definite volume and but takes the shape of its container. The particles of a liquid move fast enough to overcome some of the attraction between them. The particles in a liquid slide past each other. Liquids, continued Liquids Have Unique Characteristics Two special properties of liquids are surface tension and viscosity. Surface tension is a force that acts on the particles at the surface of a liquid. Viscosity is a liquid s resistance to flow. Section 2 Behavior of Gases Gases Gas is the state of matter that has no definite shape or volume. The particles of a gas move quickly and can break away completely from one another. The amount of empty space between gas particles can change. Bellringer What gas is used to fill balloons that will float in the air? How does a hot-air balloon float if it is filled only with air and not helium? Record your answers in your science journal.
3 Section 2 Behavior of Gases Section 2 Behavior of Gases Objectives Describe three factors that affect how gases behave. Predict how a change in pressure or temperature will affect the volume of a gas. Describing Gas Behavior Temperature Temperature is a measure of how fast the particles in an object are moving.the faster the particles are moving, the more energy they have. Volume Volume is the amount of space that an object takes up. Because gas particles spread out, the volume of any gas depends on the container that the gas is in. Section 2 Behavior of Gases Section 2 Behavior of Gases Describing Gas Behavior, continued Pressure The amount of force exerted on a given area of surface is called pressure. You can think of pressure as the number of times the particles of a gas hit the inside of their container. Gas Behavior Laws Boyle s Law Boyle s law states that for a fixed amount of gas at a constant temperature, the volume of the gas is inversely related to pressure. Charles s Law Charles s law states that for a fixed amount of gas at a constant pressure, the volume of the gas changes in the same way that the temperature of the gas changes. Section 2 Behavior of Gases Bellringer What must be done to liquid water to change it to ice or to change it to steam? Given your hypothesis about changing water s state, write a prediction of what must happen, in general, to cause matter to change state. Do you think all matter conforms to the same rules? Why or why not? Record your answers in your science journal.
4 Objectives Describe how energy is involved in changes of state. Describe what happens during melting and freezing. Compare evaporation and condensation. Explain what happens during sublimation. Identify the two changes that can happen when a substance loses or gains energy. Energy and Changes of State A change of state is the change of a substance from one physical form to another. The particles of a substance move differently depending on the state of the substance. The particles also have different amounts of energy when the substance is in different states. Melting: Solid to Liquid Melting is the change of state from a solid to a liquid. The temperature at which a solid changes to a liquid is its melting point. Adding Energy For a solid to melt, particles must overcome their attractions to each other. When a solid is at its melting point, any energy added is used to overcome attractions between particles. Freezing: Liquid to Solid Freezing is the change of state from a liquid to a solid. The temperature at which a liquid changes to a solid is its freezing point. Removing Energy When a liquid is at its freezing point, removing energy will cause the particles to begin locking into place. Evaporation: Liquid to Gas Evaporation is the change of state from a liquid to a gas. Evaporation can occur at the surface of a liquid that is below its boiling point. Boiling is the change of a liquid to a gas throughout the liquid. The temperature at which a liquid boils is its boiling point.
5 Evaporation: Liquid to Gas, continued Effects of Pressure on Boiling Point The boiling point of a liquid decreases as atmospheric pressure decreases. Atmospheric pressure is caused by the weight of the gases in the atmosphere. Atmospheric pressure is lower at higher elevations. So, the boiling point is lower on top of mountains than it is at sea level. Condensation: Gas to Liquid Condensation is the change of state from a gas to a liquid. The condensation point of a substance is the temperature at which the gas becomes a liquid. Energy must be removed for condensation to occur. Removing energy slows the movement of gas particles which allows them to clump together. Vaporization and Condensation Click below to watch the Visual Concept. Visual Concept Sublimation: Solid to Gas Sublimation is the change of state in which a solid changes directly into a gas. For sublimation to occur, the attractions between the particles must be completely overcome. So, the substance must gain energy during sublimation. Change of Temperature Vs. Change of State When most substances lose or gain energy, one of two things happens to the substance: its temperature changes or its state changes. But the temperature of a substance does not change during the change of state. The graph on the next slide shows how temperature changes as energy is added to ice.
6 States of Matter Concept Mapping Use the terms below to complete the concept map on the next slide. changes of state liquid condensing melting states of matter evaporating solid States of Matter States of Matter
Chapter 3. Preview. Section 1 Three States of Matter. Section 2 Behavior of Gases. Section 3 Changes of State. States of Matter.
 States of Matter Preview Section 1 Three States of Matter Section 2 Behavior of Gases Section 3 Changes of State Concept Mapping Section 1 Three States of Matter Bellringer In the kitchen, you might find
States of Matter Preview Section 1 Three States of Matter Section 2 Behavior of Gases Section 3 Changes of State Concept Mapping Section 1 Three States of Matter Bellringer In the kitchen, you might find
Physical Science Exam 3 Study Guide. Dr. Karoline Rostamiani. Chapter 3
 Chapter 3 Section 1 States of Matter What is matter made of? What are the three most common states of matter? How do particles behave in each state of matter? Solids, Liquids, and Gases Materials can be
Chapter 3 Section 1 States of Matter What is matter made of? What are the three most common states of matter? How do particles behave in each state of matter? Solids, Liquids, and Gases Materials can be
Objectives. Inertia. Is air matter? Is Light matter? Chapter 2. Chapter 2. Table of Contents. Chapter 2. Chapter 2. Section 1 What Is Matter?
 The Properties of Matter Section 1 What Is Matter? Table of Contents Section 1 What Is Matter? Section 2 Physical Properties Section 3 Chemical Properties Objectives Describe the two properties of all
The Properties of Matter Section 1 What Is Matter? Table of Contents Section 1 What Is Matter? Section 2 Physical Properties Section 3 Chemical Properties Objectives Describe the two properties of all
Unit 1 Lesson 6 Changes of State. Copyright Houghton Mifflin Harcourt Publishing Company
 The Fact of the Matter What happens when matter changes state? The three most familiar states of matter are solid, liquid, and gas. A change of state is the change of a substance from one physical form
The Fact of the Matter What happens when matter changes state? The three most familiar states of matter are solid, liquid, and gas. A change of state is the change of a substance from one physical form
States of Matter. Essential Question: How does the movement of atoms and molecules relate to matter s different phases?
 States of Matter Essential Question: How does the movement of atoms and molecules relate to matter s different phases? These notes come from pages 60 to 73 in your Physical Science Textbook All Matter
States of Matter Essential Question: How does the movement of atoms and molecules relate to matter s different phases? These notes come from pages 60 to 73 in your Physical Science Textbook All Matter
Solids, Liquids, and Gases. Chapter 14
 Solids, Liquids, and Gases Chapter 14 Matter & Thermal Energy Matter can exist as a solid, a liquid, a gas or a plasma. The Molecular Kinetic Theory of Matter explains their differences and how they can
Solids, Liquids, and Gases Chapter 14 Matter & Thermal Energy Matter can exist as a solid, a liquid, a gas or a plasma. The Molecular Kinetic Theory of Matter explains their differences and how they can
The Can Demonstration
 The Can Demonstration With your table, make a prediction as to what will happen to the can. Discuss why you think that the can imploded. What are some reasons? https://www.youtube.com/watch?v=xg5niowf_zw&t=28s
The Can Demonstration With your table, make a prediction as to what will happen to the can. Discuss why you think that the can imploded. What are some reasons? https://www.youtube.com/watch?v=xg5niowf_zw&t=28s
1 Three States of Matter
 CHAPTER 3 1 Three States of Matter SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is matter made of? What are the three most common
CHAPTER 3 1 Three States of Matter SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is matter made of? What are the three most common
Chapter 3. States of Matter
 Chapter 3 States of Matter 1. Solid 2. Liquid 3. Gas States of Matter Two More (discuss later) Plasma Bose-Einstein condensate States of Matter Solid (definite shape and volume) Particles are tightly packed
Chapter 3 States of Matter 1. Solid 2. Liquid 3. Gas States of Matter Two More (discuss later) Plasma Bose-Einstein condensate States of Matter Solid (definite shape and volume) Particles are tightly packed
SOLIDS, LIQUIDS, AND GASES
 CHAPTER 2 SOLIDS, LIQUIDS, AND GASES SECTION 2 1 States of Matter (pages 56-60) This section explains how shape, volume, and the motion of particles are useful in describing solids, liquids, and gases.
CHAPTER 2 SOLIDS, LIQUIDS, AND GASES SECTION 2 1 States of Matter (pages 56-60) This section explains how shape, volume, and the motion of particles are useful in describing solids, liquids, and gases.
Chapter 2. States of Matter
 Chapter 2 States of Matter 2-1 Matter Matter Matter Anything that takes up space and has mass. Is air matter? Yes. It takes up space and has mass. It has atoms. All matter is made up of atoms. ( Dalton
Chapter 2 States of Matter 2-1 Matter Matter Matter Anything that takes up space and has mass. Is air matter? Yes. It takes up space and has mass. It has atoms. All matter is made up of atoms. ( Dalton
Matter & Energy. Objectives: properties and structures of the different states of matter.
 Matter & Energy Objectives: 1. Use the kinetic theory to describe the properties and structures of the different states of matter. 2. Describe energy transfers involved in changes of state. 3. Describe
Matter & Energy Objectives: 1. Use the kinetic theory to describe the properties and structures of the different states of matter. 2. Describe energy transfers involved in changes of state. 3. Describe
What is a change of state? What happens during a change of state? What can happen when a substance loses or gains energy?
 CHAPTER 3 3 Changes of State SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is a change of state? What happens during a change
CHAPTER 3 3 Changes of State SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is a change of state? What happens during a change
Solids (cont.) Describe the movement of particles in a solid and the forces between them.
 Solids A solid is matter that has a definite shape and a definite volume. The attractive forces between the particles in a solid are strong and pull them close together. Solids (cont.) Describe the movement
Solids A solid is matter that has a definite shape and a definite volume. The attractive forces between the particles in a solid are strong and pull them close together. Solids (cont.) Describe the movement
Chapter 14 9/21/15. Solids, Liquids & Gasses. Essential Questions! Kinetic Theory! Gas State! Gas State!
 Chapter 14 Solids, Liquids & Gasses Essential Questions What is the kinetic theory of matter? How do particles move in the different states of matter? How do particles behave at the boiling and melting
Chapter 14 Solids, Liquids & Gasses Essential Questions What is the kinetic theory of matter? How do particles move in the different states of matter? How do particles behave at the boiling and melting
Most substances can be in three states: solid, liquid, and gas.
 States of Matter Most substances can be in three states: solid, liquid, and gas. Solid Particles Have Fixed Positions The particles in a solid are very close together and have an orderly, fixed arrangement.
States of Matter Most substances can be in three states: solid, liquid, and gas. Solid Particles Have Fixed Positions The particles in a solid are very close together and have an orderly, fixed arrangement.
The physical state of a substance can be changed by increasing or decreasing its temperature.
 Chemistry Lecture #63: Changes of State The physical state of a substance can be changed by increasing or decreasing its temperature. For example, a solid substance can be converted into a liquid by heating
Chemistry Lecture #63: Changes of State The physical state of a substance can be changed by increasing or decreasing its temperature. For example, a solid substance can be converted into a liquid by heating
Changing States of Matter By Cindy Grigg
 By Cindy Grigg 1 On Earth, almost all matter exists in just three states. Matter is usually a solid, a liquid, or a gas. Plasma, the fourth state of matter, is rare on Earth. It sometimes can be found
By Cindy Grigg 1 On Earth, almost all matter exists in just three states. Matter is usually a solid, a liquid, or a gas. Plasma, the fourth state of matter, is rare on Earth. It sometimes can be found
Gases and States of Matter: Unit 8
 Gases and States of Matter: Unit 8 States of Matter There are three states (also called phases) of matter. The picture represents the same chemical substance, just in different states. There are three
Gases and States of Matter: Unit 8 States of Matter There are three states (also called phases) of matter. The picture represents the same chemical substance, just in different states. There are three
Everything in the universe can be classified as either matter or energy. Kinetic Energy Theory: All particles of matter are in constant motion.
 Physical Science Everything in the universe can be classified as either matter or energy. Kinetic Energy Theory: All particles of matter are in constant motion. State of Matter Bose- Einstein Condensate
Physical Science Everything in the universe can be classified as either matter or energy. Kinetic Energy Theory: All particles of matter are in constant motion. State of Matter Bose- Einstein Condensate
Properties of Matter
 Properties of Matter Matter - anything that has mass and takes up space Chemistry - the study of the properties of matter and how matter changes Physical Property - a characteristic of a substance which
Properties of Matter Matter - anything that has mass and takes up space Chemistry - the study of the properties of matter and how matter changes Physical Property - a characteristic of a substance which
Matter. Energy- which is a property of matter!! Matter: anything that takes up space and has mass
 Matter Matter: anything that takes up space and has mass Can you think of anything that is not made of matter? Energy- which is a property of matter!! Matter is made up of moving particles! Instead of
Matter Matter: anything that takes up space and has mass Can you think of anything that is not made of matter? Energy- which is a property of matter!! Matter is made up of moving particles! Instead of
Chapter: States of Matter
 Table of Contents Chapter: States of Matter Section 1: Matter Section 2: Changes of State Section 3: Behavior of Fluids 1 What is matter? Matter is anything that takes up space and has mass. Matter Matter
Table of Contents Chapter: States of Matter Section 1: Matter Section 2: Changes of State Section 3: Behavior of Fluids 1 What is matter? Matter is anything that takes up space and has mass. Matter Matter
CHAPTER 3 STATES OF MATTER Ms. Liu
 CHAPTER 3 STATES OF MATTER Ms. Liu 3.1 THREESTATESOFMATTER: SOLID, LIQUID, OR GAS? Steam rising from hot chocolate on a stove next to a blazing fire. 1 Particles are made of atoms and molecules. Particles
CHAPTER 3 STATES OF MATTER Ms. Liu 3.1 THREESTATESOFMATTER: SOLID, LIQUID, OR GAS? Steam rising from hot chocolate on a stove next to a blazing fire. 1 Particles are made of atoms and molecules. Particles
Study Guide for Chapters 2, 3, and 10
 Study Guide for Chapters 2, 3, and 10 1. What is matter? Where can it be found? Anything that has mass and takes up space. 2. What units are used to measure volume? Liters and meters cubed 3. How would
Study Guide for Chapters 2, 3, and 10 1. What is matter? Where can it be found? Anything that has mass and takes up space. 2. What units are used to measure volume? Liters and meters cubed 3. How would
Atoms and molecules are in motion and have energy
 Atoms and molecules are in motion and have energy By now you know that substances are made of atoms and molecules. These atoms and molecules are always in motion and have attractions to each other. When
Atoms and molecules are in motion and have energy By now you know that substances are made of atoms and molecules. These atoms and molecules are always in motion and have attractions to each other. When
Chapter Preview. Improving Comprehension
 Chapter Preview Improving Comprehension Graphic Organizers are important visual tools that can help you organize information and improve your reading comprehension. The Graphic Organizer below is called
Chapter Preview Improving Comprehension Graphic Organizers are important visual tools that can help you organize information and improve your reading comprehension. The Graphic Organizer below is called
Name: Class: Date: Figure 3-1
 Name: Class: Date: Chapter 3 test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A gas has a. a definite volume but no definite shape. b. a definite shape
Name: Class: Date: Chapter 3 test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A gas has a. a definite volume but no definite shape. b. a definite shape
Unit 4: The Nature of Matter
 16 16 Table of Contents Unit 4: The Nature of Matter Chapter 16: Solids, Liquids, and Gases 16.1: Kinetic Theory 16.2: Properties and Fluids 16.3: Behavior of Gases 16.1 Kinetic Theory Kinetic Theory kinetic
16 16 Table of Contents Unit 4: The Nature of Matter Chapter 16: Solids, Liquids, and Gases 16.1: Kinetic Theory 16.2: Properties and Fluids 16.3: Behavior of Gases 16.1 Kinetic Theory Kinetic Theory kinetic
CHM Solids, Liquids, and Phase Changes (r15) Charles Taylor 1/9
 CHM 111 - Solids, Liquids, and Phase Changes (r15) - 2015 Charles Taylor 1/9 Introduction In CHM 110, we used kinetic theory to explain the behavior of gases. Now, we will discuss solids and liquids. While
CHM 111 - Solids, Liquids, and Phase Changes (r15) - 2015 Charles Taylor 1/9 Introduction In CHM 110, we used kinetic theory to explain the behavior of gases. Now, we will discuss solids and liquids. While
CHAPTER 4 - STATES OF MATTER. Mr. Polard Physical Science Ingomar Middle School
 CHAPTER 4 - STATES OF MATTER Mr. Polard Physical Science Ingomar Middle School SECTION 1 MATTER VOCABULARY SECTION 1 Matter : anything that takes up space and has mass (pg 72, 102) Solid : Matter with
CHAPTER 4 - STATES OF MATTER Mr. Polard Physical Science Ingomar Middle School SECTION 1 MATTER VOCABULARY SECTION 1 Matter : anything that takes up space and has mass (pg 72, 102) Solid : Matter with
THE PHASES OF MATTER. Solid: holds its shape and does not flow. The molecules in a solid vibrate in place, but on average, don t move very far.
 THE QUESTIONS What are the phases of matter? What makes these phases different from each other? What is the difference between melting, freezing, boiling and condensation? How do you interpret a Temperature
THE QUESTIONS What are the phases of matter? What makes these phases different from each other? What is the difference between melting, freezing, boiling and condensation? How do you interpret a Temperature
Chapter 13 - States of Matter. Section 13.1 The nature of Gases
 Chapter 13 - States of Matter Section 13.1 The nature of Gases Kinetic energy and gases Kinetic energy: the energy an object has because of its motion Kinetic theory: all matter is made if particles in
Chapter 13 - States of Matter Section 13.1 The nature of Gases Kinetic energy and gases Kinetic energy: the energy an object has because of its motion Kinetic theory: all matter is made if particles in
Ch Kinetic Theory. 1.All matter is made of atoms and molecules that act like tiny particles.
 Ch. 15.1 Kinetic Theory 1.All matter is made of atoms and molecules that act like tiny particles. Kinetic Theory 2.These tiny particles are always in motion. The higher the temperature, the faster the
Ch. 15.1 Kinetic Theory 1.All matter is made of atoms and molecules that act like tiny particles. Kinetic Theory 2.These tiny particles are always in motion. The higher the temperature, the faster the
Matter and Thermal Energy
 Section States of Matter Can you identify the states of matter present in the photo shown? Kinetic Theory The kinetic theory is an explanation of how particles in matter behave. Kinetic Theory The three
Section States of Matter Can you identify the states of matter present in the photo shown? Kinetic Theory The kinetic theory is an explanation of how particles in matter behave. Kinetic Theory The three
Ch. 1 States of Matter
 Ch. 1 States of Matter Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The surface of water can act like a sort of skin due to a property of liquids called
Ch. 1 States of Matter Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The surface of water can act like a sort of skin due to a property of liquids called
Phase Change: solid to liquid. Melting
 Phase Change: solid to liquid Melting Most solids shrink in size when frozen. What substance is an exception and actually expands? water Use the phase diagram below to answer the following question. What
Phase Change: solid to liquid Melting Most solids shrink in size when frozen. What substance is an exception and actually expands? water Use the phase diagram below to answer the following question. What
Atom - the smallest unit of an element that has the properties of that element From the Greek word for indivisible
 Matter Atom - the smallest unit of an element that has the properties of that element From the Greek word for indivisible 3 subatomic particles Proton - positively charged particle in the nucleus of an
Matter Atom - the smallest unit of an element that has the properties of that element From the Greek word for indivisible 3 subatomic particles Proton - positively charged particle in the nucleus of an
Science 8 Chapter 7 Section 1
 Science 8 Chapter 7 Section 1 Describing Fluids (pp. 268-277) What is a fluid? Fluid: any thing that flows; a liquid or a gas While it would seem that some solids flow (sugar, salt, etc), they are not
Science 8 Chapter 7 Section 1 Describing Fluids (pp. 268-277) What is a fluid? Fluid: any thing that flows; a liquid or a gas While it would seem that some solids flow (sugar, salt, etc), they are not
Name Date Class STATES OF MATTER
 13 STATES OF MATTER Chapter Test A A. Matching Match each description in Column B with the correct term in Column A. Write the letter of the correct description on the line. Column A Column B 1. amorphous
13 STATES OF MATTER Chapter Test A A. Matching Match each description in Column B with the correct term in Column A. Write the letter of the correct description on the line. Column A Column B 1. amorphous
CHEMISTRY Matter and Change. Chapter 12: States of Matter
 CHEMISTRY Matter and Change Chapter 12: States of Matter CHAPTER 12 States of Matter Section 12.1 Section 12.2 Section 12.3 Section 12.4 Gases Forces of Attraction Liquids and Solids Phase Changes Click
CHEMISTRY Matter and Change Chapter 12: States of Matter CHAPTER 12 States of Matter Section 12.1 Section 12.2 Section 12.3 Section 12.4 Gases Forces of Attraction Liquids and Solids Phase Changes Click
Chapter 22 States of matter. Section 1 matter Section 2 Changes of State
 Chapter 22 States of matter Section 1 matter Section 2 Changes of State States of Matter is a physical property ***Matter is made of atoms Atoms form chemical bonds to make matter **** Atoms vibrate constantly
Chapter 22 States of matter Section 1 matter Section 2 Changes of State States of Matter is a physical property ***Matter is made of atoms Atoms form chemical bonds to make matter **** Atoms vibrate constantly
Name Date Class STATES OF MATTER. Match the correct state of matter with each description of water by writing a letter on each line.
 10 STATES OF MATTER SECTION 10.1 THE NATURE OF GASES (pages 267 272) This section describes how the kinetic theory applies to gases. It defines gas pressure and explains how temperature is related to the
10 STATES OF MATTER SECTION 10.1 THE NATURE OF GASES (pages 267 272) This section describes how the kinetic theory applies to gases. It defines gas pressure and explains how temperature is related to the
Chemistry Day 5. Friday, August 31 st Tuesday, September 4 th, 2018
 Chemistry Day 5 Friday, August 31 st Tuesday, September 4 th, 2018 Do-Now Title: BrainPOP: States of Matter 1. Write down today s FLT 2. List two examples of gases 3. List two examples of things that are
Chemistry Day 5 Friday, August 31 st Tuesday, September 4 th, 2018 Do-Now Title: BrainPOP: States of Matter 1. Write down today s FLT 2. List two examples of gases 3. List two examples of things that are
States of Matter. What physical changes and energy changes occur as matter goes from one state to another?
 Name States of Matter Date What physical changes and energy changes occur as matter goes from one state to another? Before You Read Before you read the chapter, think about what you know about states of
Name States of Matter Date What physical changes and energy changes occur as matter goes from one state to another? Before You Read Before you read the chapter, think about what you know about states of
Chapter 10. Lesson Starter. Why did you not smell the odor of the vapor immediately? Explain this event in terms of the motion of molecules.
 Preview Lesson Starter Objectives The Kinetic-Molecular Theory of Gases The Kinetic-Molecular Theory and the Nature of Gases Deviations of Real Gases from Ideal Behavior Section 1 The Kinetic-Molecular
Preview Lesson Starter Objectives The Kinetic-Molecular Theory of Gases The Kinetic-Molecular Theory and the Nature of Gases Deviations of Real Gases from Ideal Behavior Section 1 The Kinetic-Molecular
How Does the Sun s Energy Cause Rain?
 1.2 Investigate 3.3 Read How Does the Sun s Energy Cause Rain? In the water-cycle simulation, you observed water change from a liquid to a gas, and then back to a liquid falling to the bottom of the container.
1.2 Investigate 3.3 Read How Does the Sun s Energy Cause Rain? In the water-cycle simulation, you observed water change from a liquid to a gas, and then back to a liquid falling to the bottom of the container.
Solids, Liquids, and Gases
 Date Class _ Solids, Liquids, and Gases Chapter Test A Multiple Choice Write the letter of the correct answer on the line at the left. _ 1. The surface of water can act like a sort of skin due to a property
Date Class _ Solids, Liquids, and Gases Chapter Test A Multiple Choice Write the letter of the correct answer on the line at the left. _ 1. The surface of water can act like a sort of skin due to a property
Liquids & Solids: Section 12.3
 Liquids & Solids: Section 12.3 MAIN IDEA: The particles in and have a range of motion and are not easily. Why is it more difficult to pour syrup that is stored in the refrigerator than in the cabinet?
Liquids & Solids: Section 12.3 MAIN IDEA: The particles in and have a range of motion and are not easily. Why is it more difficult to pour syrup that is stored in the refrigerator than in the cabinet?
CHAPTER 13. States of Matter. Kinetic = motion. Polar vs. Nonpolar. Gases. Hon Chem 13.notebook
 CHAPTER 13 States of Matter States that the tiny particles in all forms of matter are in constant motion. Kinetic = motion A gas is composed of particles, usually molecules or atoms, with negligible volume
CHAPTER 13 States of Matter States that the tiny particles in all forms of matter are in constant motion. Kinetic = motion A gas is composed of particles, usually molecules or atoms, with negligible volume
Name Class Date. What is a change of state? What happens during a change of state? What can happen when a substance loses or gains energy?
 CHAPTER 2 3 Changes of State SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is a change of state? What happens during a change
CHAPTER 2 3 Changes of State SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is a change of state? What happens during a change
States of Matter. The Solid State. Particles are tightly packed, very close together (strong cohesive forces) Low kinetic energy (energy of motion)
 States of Matter The Solid State Particles are tightly packed, very close together (strong cohesive forces) Low kinetic energy (energy of motion) Fixed shape and volume Crystalline or amorphous structure
States of Matter The Solid State Particles are tightly packed, very close together (strong cohesive forces) Low kinetic energy (energy of motion) Fixed shape and volume Crystalline or amorphous structure
Chapter 3 Phases of Matter Physical Science
 Chapter 3 Phases of Matter Physical Science CH 3- States of Matter 1 What makes up matter? What is the difference between a solid, a liquid, and a gas? What kind of energy do all particles of matter have?
Chapter 3 Phases of Matter Physical Science CH 3- States of Matter 1 What makes up matter? What is the difference between a solid, a liquid, and a gas? What kind of energy do all particles of matter have?
Chapter 10 States of Matter
 Chapter 10 States of Matter 1 Section 10.1 The Nature of Gases Objectives: Describe the assumptions of the kinetic theory as it applies to gases. Interpret gas pressure in terms of kinetic theory. Define
Chapter 10 States of Matter 1 Section 10.1 The Nature of Gases Objectives: Describe the assumptions of the kinetic theory as it applies to gases. Interpret gas pressure in terms of kinetic theory. Define
Matter, Atoms & Molecules
 Matter, Atoms & Molecules Matter is anything that has mass and takes up space. All matter is made of tiny particles called atoms, which are too small to see with the naked eye. Matter Matter is anything
Matter, Atoms & Molecules Matter is anything that has mass and takes up space. All matter is made of tiny particles called atoms, which are too small to see with the naked eye. Matter Matter is anything
Chapter 11. Kinetic Molecular Theory. Attractive Forces
 Chapter 11 KMT for Solids and Liquids Intermolecular Forces Viscosity & Surface Tension Phase Changes Vapor Pressure Phase Diagrams Solid Structure Kinetic Molecular Theory Liquids and solids will experience
Chapter 11 KMT for Solids and Liquids Intermolecular Forces Viscosity & Surface Tension Phase Changes Vapor Pressure Phase Diagrams Solid Structure Kinetic Molecular Theory Liquids and solids will experience
WARM-UP. 1. What are the four states of matter? 2. What is melting point? 3. How does water change from a liquid to a gas? 4. Define viscosity.
 WARM-UP 1. What are the four states of matter? 2. What is melting point? 3. How does water change from a liquid to a gas? 4. Define viscosity. STATES OF MATTER: WEB QUEST With your lab partner, you will
WARM-UP 1. What are the four states of matter? 2. What is melting point? 3. How does water change from a liquid to a gas? 4. Define viscosity. STATES OF MATTER: WEB QUEST With your lab partner, you will
4 Discuss and evaluate the 5th state of matter. 3 - Differentiate among the four states of matter in terms of energy,
 Goal: Differentiate among the four states of matter in terms of energy, particle motion, and phase transitions. 4 States of Mater Sections 3.1, 3.2 4 Discuss and evaluate the 5 th state of matter. 3 -
Goal: Differentiate among the four states of matter in terms of energy, particle motion, and phase transitions. 4 States of Mater Sections 3.1, 3.2 4 Discuss and evaluate the 5 th state of matter. 3 -
Atom - the smallest unit of an element that has the properties of that element From the Greek word for indivisible
 Matter Atom - the smallest unit of an element that has the properties of that element From the Greek word for indivisible 3 subatomic particles Proton - positively charged particle in the nucleus of an
Matter Atom - the smallest unit of an element that has the properties of that element From the Greek word for indivisible 3 subatomic particles Proton - positively charged particle in the nucleus of an
Chemistry B11 Chapter 6 Gases, Liquids, and Solids
 Chapter 6 Gases, Liquids, and Solids States of matter: the physical state of matter depends on a balance between the kinetic energy of particles, which tends to keep them apart, and the attractive forces
Chapter 6 Gases, Liquids, and Solids States of matter: the physical state of matter depends on a balance between the kinetic energy of particles, which tends to keep them apart, and the attractive forces
THE PARTICLE MODEL AND PROPERTIES OF THE GASES, LIQUIDS AND SOLIDS. STATES CHANGES
 THE PARTICLE MODEL AND PROPERTIES OF THE GASES, LIQUIDS AND SOLIDS. STATES CHANGES The particle model of a gas A gas has no fixed shape or volume, but always spreads out to fill any container. There are
THE PARTICLE MODEL AND PROPERTIES OF THE GASES, LIQUIDS AND SOLIDS. STATES CHANGES The particle model of a gas A gas has no fixed shape or volume, but always spreads out to fill any container. There are
Foundations of Chemistry
 Foundations of Chemistry Physical Changes Key Concepts How can a change in energy affect the state of matter? What happens when something dissolves? What is meant by conservation of mass? What do you think?
Foundations of Chemistry Physical Changes Key Concepts How can a change in energy affect the state of matter? What happens when something dissolves? What is meant by conservation of mass? What do you think?
3.3 Phase Changes 88 A NATURAL APPROACH TO CHEMISTRY. Section 3.3 Phase Changes
 Section 3.3 Phase Changes 3.3 Phase Changes Solid, liquid and gas During a phase change, a substance rearranges the order of its particles (atoms or molecules). Examples of phase change include melting
Section 3.3 Phase Changes 3.3 Phase Changes Solid, liquid and gas During a phase change, a substance rearranges the order of its particles (atoms or molecules). Examples of phase change include melting
Silent Card Shuffle. Dump out the word strips onto your desk.
 Silent Card Shuffle Dump out the word strips onto your desk. With a partner, silently work to arrange the strips into 8 groups. Each group should have a term (purple paper), its definition (white paper),
Silent Card Shuffle Dump out the word strips onto your desk. With a partner, silently work to arrange the strips into 8 groups. Each group should have a term (purple paper), its definition (white paper),
4. Every CHANGE in matter includes a change in, which is conserved in a chemical reaction and. TRANSFORMED from one form to another.
 Part C: Lesson 1.4 - Read pages 20-29 to answer these questions. Add to notes - 1. A physical change alters the form or appearance of matter, such as Change in size, a change in SIZE or. SHAPE shape, or
Part C: Lesson 1.4 - Read pages 20-29 to answer these questions. Add to notes - 1. A physical change alters the form or appearance of matter, such as Change in size, a change in SIZE or. SHAPE shape, or
* Defining Temperature * Temperature is proportional to the kinetic energy of atoms and molecules. * Temperature * Internal energy
 * Defining Temperature * We associate temperature with how hot or cold an object feels. * Our sense of touch serves as a qualitative indicator of temperature. * Energy must be either added or removed from
* Defining Temperature * We associate temperature with how hot or cold an object feels. * Our sense of touch serves as a qualitative indicator of temperature. * Energy must be either added or removed from
Section 16.3 Phase Changes
 Section 16.3 Phase Changes Solid Liquid Gas 3 Phases of Matter Density of Matter How packed matter is (The amount of matter in a given space) Solid: Liquid: Gas: High Density Medium Density Low Density
Section 16.3 Phase Changes Solid Liquid Gas 3 Phases of Matter Density of Matter How packed matter is (The amount of matter in a given space) Solid: Liquid: Gas: High Density Medium Density Low Density
Unit 13 Lesson 1 What Are Solids, Liquids, and Gases? Copyright Houghton Mifflin Harcourt Publishing Company
 Unit 13 Lesson 1 What Are Solids, Liquids, and Gases? Copyright Houghton Mifflin Harcourt Publishing Company What s the Matter? Matter has mass and volume. It cannot be created or destroyed. Mass is the
Unit 13 Lesson 1 What Are Solids, Liquids, and Gases? Copyright Houghton Mifflin Harcourt Publishing Company What s the Matter? Matter has mass and volume. It cannot be created or destroyed. Mass is the
Matter changes phase when energy is added or removed
 Section 12.4 Phase Changes Explain how the addition and removal of energy can cause a phase change. Interpret a phase diagram. Matter changes phase when energy is added or removed Energy Changes Accompanying
Section 12.4 Phase Changes Explain how the addition and removal of energy can cause a phase change. Interpret a phase diagram. Matter changes phase when energy is added or removed Energy Changes Accompanying
2º ESO UNIT 2: The physical states of matter. Susana Morales Bernal
 2º ESO UNIT 2: The physical states of matter Objectives 1. To know that in all the states of aggregation the matter has mass and takes a place although depending on the state, can have or not, form and
2º ESO UNIT 2: The physical states of matter Objectives 1. To know that in all the states of aggregation the matter has mass and takes a place although depending on the state, can have or not, form and
Chem 1075 Chapter 13 Liquids and Solids Lecture Outline
 Chem 1075 Chapter 13 Liquids and Solids Lecture Outline Slide 2-3 Properties of Liquids Unlike gases, liquids respond dramatically to temperature and pressure changes. We can study the liquid state and
Chem 1075 Chapter 13 Liquids and Solids Lecture Outline Slide 2-3 Properties of Liquids Unlike gases, liquids respond dramatically to temperature and pressure changes. We can study the liquid state and
STATES OF MATTER. The Four States of Ma/er. Four States. Solid Liquid Gas Plasma
 STATES OF MATTER The Four States of Ma/er Solid Liquid Gas Plasma Four States STATES OF MATTER Ø What makes a substance a par:cular state of ma
STATES OF MATTER The Four States of Ma/er Solid Liquid Gas Plasma Four States STATES OF MATTER Ø What makes a substance a par:cular state of ma
Name Date Class THE NATURE OF GASES
 13.1 THE NATURE OF GASES Section Review Objectives Describe the assumptions of the kinetic theory as it applies to gases Interpret gas pressure in terms of kinetic theory Define the relationship between
13.1 THE NATURE OF GASES Section Review Objectives Describe the assumptions of the kinetic theory as it applies to gases Interpret gas pressure in terms of kinetic theory Define the relationship between
Unit 4: Gas Laws. Matter and Phase Changes
 Unit 4: Gas Laws Matter and Phase Changes ENERGY and matter What is 에너지 A fundamental property of the universe that cannot be easily defined. Energy No one knows what energy is, only what it does or has
Unit 4: Gas Laws Matter and Phase Changes ENERGY and matter What is 에너지 A fundamental property of the universe that cannot be easily defined. Energy No one knows what energy is, only what it does or has
STATES OF MATTER. Chapter 3
 STATES OF MATTER Chapter 3 Labs done so far for ch. 3 sections 1 and 2: 1. Distilled wood and related read of temperatures with plateaus for substances produced 2. Distilling solution X (BP/CP evaporation/condensation)
STATES OF MATTER Chapter 3 Labs done so far for ch. 3 sections 1 and 2: 1. Distilled wood and related read of temperatures with plateaus for substances produced 2. Distilling solution X (BP/CP evaporation/condensation)
OUTLINE. States of Matter, Forces of Attraction Phase Changes Gases The Ideal Gas Law Gas Stoichiometry
 UNIT 6 GASES OUTLINE States of Matter, Forces of Attraction Phase Changes Gases The Ideal Gas Law Gas Stoichiometry STATES OF MATTER Remember that all matter exists in three physical states: Solid Liquid
UNIT 6 GASES OUTLINE States of Matter, Forces of Attraction Phase Changes Gases The Ideal Gas Law Gas Stoichiometry STATES OF MATTER Remember that all matter exists in three physical states: Solid Liquid
Ch10.4 Attractive Forces
 Ch10.4 Attractive Forces Intermolecular Forces are the forces holding molecules to each other. Solids have strong forces Gases (vapor) have weak forces Intermolecular forces determine the phase of matter.
Ch10.4 Attractive Forces Intermolecular Forces are the forces holding molecules to each other. Solids have strong forces Gases (vapor) have weak forces Intermolecular forces determine the phase of matter.
States of Matter. Reviewing Vocabulary. Match the definition in Column A with the term in Column B.
 Name Date Class States of Matter Reviewing Vocabulary Match the definition in Column A with the term in Column B. Column A 1. A measure of the resistance of a liquid to flow 2. The energy required to increase
Name Date Class States of Matter Reviewing Vocabulary Match the definition in Column A with the term in Column B. Column A 1. A measure of the resistance of a liquid to flow 2. The energy required to increase
Name Date Class STATES OF MATTER. SECTION 13.1 THE NATURE OF GASES (pages )
 Name Date Class 13 STATES OF MATTER SECTION 13.1 THE NATURE OF GASES (pages 385 389) This section introduces the kinetic theory and describes how it applies to gases. It defines gas pressure and explains
Name Date Class 13 STATES OF MATTER SECTION 13.1 THE NATURE OF GASES (pages 385 389) This section introduces the kinetic theory and describes how it applies to gases. It defines gas pressure and explains
Chapter 7.1. States of Matter
 Chapter 7.1 States of Matter In this chapter... we will learn about matter and different states of matter, many of which we are already familiar with! Learning about Kinetic Molecular Theory will help
Chapter 7.1 States of Matter In this chapter... we will learn about matter and different states of matter, many of which we are already familiar with! Learning about Kinetic Molecular Theory will help
relatively narrow range of temperature and pressure.
 1) Of solids, liquids, and gases, the least common state of matter is the liquid state. a) Liquids can exist only within a relatively narrow range of temperature and pressure. 2) The kinetic-molecular
1) Of solids, liquids, and gases, the least common state of matter is the liquid state. a) Liquids can exist only within a relatively narrow range of temperature and pressure. 2) The kinetic-molecular
IGCSE Double Award Extended Coordinated Science
 IGCSE Double Award Extended Coordinated Science Physics 5 - Thermal Properties of Matter Thermal Expansion You need to know thermal expansions for solids, liquids, and gases, and their applications. Thermal
IGCSE Double Award Extended Coordinated Science Physics 5 - Thermal Properties of Matter Thermal Expansion You need to know thermal expansions for solids, liquids, and gases, and their applications. Thermal
INTRODUCTION TO LESSON CLUSTER 7
 INTRODUCTION TO LESSON CLUSTER 7 EXPLAINING MELTING AND SOLIDIFYING A. Lesson Cluster Goals and Lesson Objectives Goals Students should be able to explain melting and solidifying, by reference to the molecular
INTRODUCTION TO LESSON CLUSTER 7 EXPLAINING MELTING AND SOLIDIFYING A. Lesson Cluster Goals and Lesson Objectives Goals Students should be able to explain melting and solidifying, by reference to the molecular
Kinetic Theory (Kinetikos - Moving ) Based on the idea that particles of matter are always in motion
 Chapter 10 Kinetic Theory (Kinetikos - Moving ) Based on the idea that particles of matter are always in motion The motion has consequences Behavior of Gases Physical Properties of Gases Ideal Gas an imaginary
Chapter 10 Kinetic Theory (Kinetikos - Moving ) Based on the idea that particles of matter are always in motion The motion has consequences Behavior of Gases Physical Properties of Gases Ideal Gas an imaginary
ch 12 acad.notebook January 12, 2016 Ch 12 States of Matter (solids, liquids, gases, plasma, Bose Einstein condensate)
 Ch 12 States of Matter (solids, liquids, gases, plasma, Bose Einstein condensate) BIG IDEA The kinetic molecular theory explains the different properties of solids, liquids and gases. I CAN: 1) use the
Ch 12 States of Matter (solids, liquids, gases, plasma, Bose Einstein condensate) BIG IDEA The kinetic molecular theory explains the different properties of solids, liquids and gases. I CAN: 1) use the
3.3 Phase Changes Charactaristics of Phase Changes phase change
 A large iceberg contains enough fresh water to supply millions of people with water for a year. As it moves into warmer areas, the ice changes to liquid water and eventually disappears. What happens when
A large iceberg contains enough fresh water to supply millions of people with water for a year. As it moves into warmer areas, the ice changes to liquid water and eventually disappears. What happens when
2. What is meant by Chemical State?. 3. Changing states of matter is about changing,,, and other.
 Name: Date: Period: Matter Mania! Online Computer Activity (3 pages) Part I: Go to http://www.chem4kids.com/ and answer the following questions in complete sentences. a. Click on MATTER (written in yellow)
Name: Date: Period: Matter Mania! Online Computer Activity (3 pages) Part I: Go to http://www.chem4kids.com/ and answer the following questions in complete sentences. a. Click on MATTER (written in yellow)
Liquids & Solids. Mr. Hollister Holliday Legacy High School Regular & Honors Chemistry
 Liquids & Solids Mr. Hollister Holliday Legacy High School Regular & Honors Chemistry 1 Liquids 2 Properties of the States of Matter: Liquids High densities compared to gases. Fluid. The material exhibits
Liquids & Solids Mr. Hollister Holliday Legacy High School Regular & Honors Chemistry 1 Liquids 2 Properties of the States of Matter: Liquids High densities compared to gases. Fluid. The material exhibits
A).5 atm B) 1 atm C) 1.5 atm D) 2 atm E) it is impossible to tell
 1. ne atmosphere is equivalent to A) 1.00 g ml 1 B) 22,400 ml ) 273 K D) 760. mmhg E) 298 K 2. A cylinder contains 2.50 L of air at a pressure of 5.00 atmospheres. At what volume, will the air exert a
1. ne atmosphere is equivalent to A) 1.00 g ml 1 B) 22,400 ml ) 273 K D) 760. mmhg E) 298 K 2. A cylinder contains 2.50 L of air at a pressure of 5.00 atmospheres. At what volume, will the air exert a
Vocabulary. Pressure Absolute zero Charles Law Boyle s Law (take a moment to look up and record definitions in your notes)
 The Gas Laws Vocabulary Pressure Absolute zero Charles Law Boyle s Law (take a moment to look up and record definitions in your notes) Key Concepts What causes gas pressure in a closed container? What
The Gas Laws Vocabulary Pressure Absolute zero Charles Law Boyle s Law (take a moment to look up and record definitions in your notes) Key Concepts What causes gas pressure in a closed container? What
Matter. Gas. Solid Liquid. Both shape and volume are not fixed. It has a fixed shape and a fixed volume.
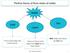 Matter Solid Liquid Gas It has a fixed shape and a fixed volume. It does not have a fixed shape, but it does have a fixed volume. Both shape and volume are not fixed. Felix Yung and John Polias 1 Matter
Matter Solid Liquid Gas It has a fixed shape and a fixed volume. It does not have a fixed shape, but it does have a fixed volume. Both shape and volume are not fixed. Felix Yung and John Polias 1 Matter
States of Matter Unit
 Learning Target Notes Section 1: Matter and Energy What makes up matter? Matter is made of atoms and molecules that are in constant motion. Kinetic Theory of Matter A. Particles that make up matter are
Learning Target Notes Section 1: Matter and Energy What makes up matter? Matter is made of atoms and molecules that are in constant motion. Kinetic Theory of Matter A. Particles that make up matter are
Thermal Physics. Temperature (Definition #1): a measure of the average random kinetic energy of all the particles of a system Units: o C, K
 Thermal Physics Internal Energy: total potential energy and random kinetic energy of the molecules of a substance Symbol: U Units: J Internal Kinetic Energy: arises from random translational, vibrational,
Thermal Physics Internal Energy: total potential energy and random kinetic energy of the molecules of a substance Symbol: U Units: J Internal Kinetic Energy: arises from random translational, vibrational,
The OTHER TWO states of matter
 ` The OTHER TWO states of matter LIQUIDS A decrease in the average kinetic energy of gas particles causes the temperature to decrease. As it cools, the particles tend to move more slowly if they slow down
` The OTHER TWO states of matter LIQUIDS A decrease in the average kinetic energy of gas particles causes the temperature to decrease. As it cools, the particles tend to move more slowly if they slow down
Quiz Review Topical Questions
 Quiz Review Topical Questions Kinetic Theory of Matter Expansion and Contraction Solids, Liquids, Gases States of Matter Phase Changes Distillation Water Properties Kinetic Theory 1. The kinetic theory
Quiz Review Topical Questions Kinetic Theory of Matter Expansion and Contraction Solids, Liquids, Gases States of Matter Phase Changes Distillation Water Properties Kinetic Theory 1. The kinetic theory
Thermodynamics and States of Matter
 Thermodynamics and States of Matter There are three states (also called phases) ) of matter. The picture to the side represents the same chemical substance, just in different states. There are three states
Thermodynamics and States of Matter There are three states (also called phases) ) of matter. The picture to the side represents the same chemical substance, just in different states. There are three states
CHAPTER 10. States of Matter
 CHAPTER 10 States of Matter Kinetic Molecular Theory Kinetikos - Moving Based on the idea that particles of matter are always in motion The motion has consequences Explains the behavior of Gases, Liquids,
CHAPTER 10 States of Matter Kinetic Molecular Theory Kinetikos - Moving Based on the idea that particles of matter are always in motion The motion has consequences Explains the behavior of Gases, Liquids,
CHAPTER 10. Kinetic Molecular Theory. Five Assumptions of the KMT. Atmospheric Pressure
 Kinetic Molecular Theory CHAPTER 10 States of Matter Kinetikos - Moving Based on the idea that particles of matter are always in motion The motion has consequences Explains the behavior of Gases, Liquids,
Kinetic Molecular Theory CHAPTER 10 States of Matter Kinetikos - Moving Based on the idea that particles of matter are always in motion The motion has consequences Explains the behavior of Gases, Liquids,
States of Matter 1 of 21 Boardworks Ltd 2016
 States of Matter 1 of 21 Boardworks Ltd 2016 States of Matter 2 of 21 Boardworks Ltd 2016 What are the three states of matter? 3 of 21 Boardworks Ltd 2016 At any given temperature, all substances exist
States of Matter 1 of 21 Boardworks Ltd 2016 States of Matter 2 of 21 Boardworks Ltd 2016 What are the three states of matter? 3 of 21 Boardworks Ltd 2016 At any given temperature, all substances exist
Chemistry A: States of Matter Packet Name: Hour: Page!1. Chemistry A States of Matter Packet
 Chemistry A: States of Matter Packet Name: Hour: Page!1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page!2 Worksheet #1: States of Matter In this packet we will
Chemistry A: States of Matter Packet Name: Hour: Page!1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page!2 Worksheet #1: States of Matter In this packet we will
