Lewis Dot Structures for Methane, CH 4 The central C atom is bonded by single bonds (-) to 4 individual H atoms
|
|
|
- Abraham Campbell
- 6 years ago
- Views:
Transcription
1 Chapter 10 (Hill/Petrucci/McCreary/Perry Bonding Theory and Molecular Structure This chapter deals with two additional approaches chemists use to describe chemical bonding: valence-shell electron pair repulsion (VSEPR) theory and hybrid orbitals (valence bonding). Lewis dot structures give us limited information about the types of bonds between atoms in molecules, but tell us nothing about the 3-D shapes of the molecules. VSEPR and valence bond structures give us the molecular geometry (shape of the molecules). Precise molecular geometry can be determined only by experiment but the shapes of many molecules and polyatomic ions can be predicted fairly well (Hill, p. 388) As the name implies, the valence-shell electron pair repulsion method is based on the idea that pairs of valence electrons in bonded atoms repel one another and push electron pairs as far from one another as possible (Hill, p. 388) Molecular Geometries (see Figure 10.1, p. 388, Hill) The molecular geometry for a molecule is its 3-D structure as represented by various kinds of 3- D models These 3-D models for O2, CO2 and H2O are called space-filling models as much as possible, they approximate the actual shape of the molecules and of their electron clouds The Water Molecule (right margin, p. 389, Hill) The central oxygen atom has two lone pairs and two bonded pairs of electrons Central atom = A Bonded pairs = X Lone pairs = E VSEPR notation: AX 2 E 2 Lewis Dot Structures for Methane, CH 4 The central C atom is bonded by single bonds (-) to 4 individual H atoms The VSEPR Method VSEPR: an approach used to determine/describe the 3-D shape of molecules by arranging the pairs of valence electrons around a central atom so that they are as far apart from each other as possible Consider a molecule like CH 4 each H-atom is bonded directly to the central C-atom Question: what shape is the best way to arrange four groups (here, four H-atoms) around a central atom (C-atom) so they are as far apart from each other as possible? not a square!! So, then what shape if not a square?? A tetrahedron H-atoms located at the corners or apices of a tetrahedron a three-sided pyramid H C H bond angles are all in this structure.
2 VSEPR Geometries group: an atom or a group of atoms, attached directly to a central atom in a chemical structure by one, two, or three electron pairs (:) one pair (:) = a single covalent bond two pairs (::) = a double covalent bond three pairs (:::) = a triple covalent bond Begin by visualizing the surface of a sphere, with a central atom at the center of the sphere What s the best way to arrange two groups around a central atom? a straight line.. linear geometry. A Linear Molecule: BeCl 2 VSEPR notation: AX 2 Another Linear Molecule: CO 2 Again, VSEPR notation: AX 2 What s the best way to arrange three groups around a central atom? Trigonal planar (or planar triangular) trigonal planar geometry has bond angles of If all X atoms are the same, the bond lengths should also be the same. A Trigonal Planar Molecule: BF 3 VSEPR notation: AX 3 Another Trigonal Planar Molecule: SO 3 VSEPR notation: AX 3 Trigonal Planar Electron Group Geometry: AX 2 E AX 2 E the presence of the lone pair means that the molecular geometry is angular or bent What s the best way to arrange four groups around a central atom? As seen earlier, at the corners of a tetrahedron! tetrahedral geometry. VSEPR notation: AX 4 Tetrahedral geometry has bond angles of If all X atoms are the same, the bond lengths from X to the central atom should also be the same. Tetrahedral Electron Group Geometry: AX 3 E AX 3 E the presence of the lone pair means that the molecular geometry is trigonal pyramidal Tetrahedral Electron Group Geometry: AX 2 E 2 AX 2 E 2 the presence of the two lone pairs means that the molecular geometry is angular What s the best way to arrange five groups around a central atom? an equilateral triangle with a group above and a group below the triangular ( equatorial )plane! AX 5 trigonal bipyramidal geometry has bond angles of 90 0,180 0 and equatorial positions are in the triangular plane axial positions are above or below the triangular plane Trigonal Bipyramidal Electron Group Geometry: AX 4 E AX 4 E the presence of the lone pair means that the molecular geometry is seesaw Note that the lone pair goes into the equatorial plane, not into one of the axial positions! Trigonal Bipyramidal Electron Group Geometry: AX 3 E 2 AX 3 E 2 the presence of the two lone pairs means that the molecular geometry is T-shaped Note that the lone pairs again both go into the equatorial plane, not into the axial positions!
3 Trigonal Bipyramidal Electron Group Geometry: AX 2 E 3 AX 2 E 3 the presence of the three lone pairs means that the molecular geometry is linear Note that now all three lone pairs again go into the equatorial plane, not the into axial positions! What s the best way to arrange six groups around a central atom? a square with a group above and a group below the square, equatorial plane! AX 6 Octahedral geometry has bond angles of 90 0 and equatorial positions are in the square; axial positions are above or below the square Octahedral Electron Group Geometry: AX 5 E AX 5 E the presence of the lone pair means that the molecular geometry is square pyramidal Note that now the lone pair goes into an axial position, not into the equatorial plane! Octahedral Electron Group Geometry: AX 4 E 2 AX 4 E 2 the presence of the two lone pairs means that the molecular geometry is square planar Note that here the lone pairs again both go into the axial positions, not into the equatorial plane! What Is This Molecular Geometry? (for :NH 3 ) Answer: trigonal pyramidal (AX 3 E) What Is This Molecular Geometry? (for H-O-H)) Answer: angular (AX 2 E 2 ) Summary of structures for linear and trigonal planar geometries Octahedral ( square pyramid ) (AX 5 E) e.g. BrI 5 Octahedral ( square planar ) (AX 4 E 2 ) e.g. XeF 4 Octahedral (AX 6 ) e.g. SF 6 Octahedral ( linear ) (AX 2 E 4 ) e.g. XeF 2 Trigonal bipyramid ( T-shaped ) (AX 3 E 2 ) Trigonal bipyramid ( seesaw ) (AX 4 E) Trigonal bipyramid (AX 5 ) Summary of VSEPR Principles Repulsive forces increase as electron pairs are placed closer together. Lone-pair electrons spread out more than bonding- pair electrons, e.g. bond angles in H 2 O and NH 3 are smaller than because the lone pairs take up more room and squeeze the H atoms closer together. Molecular Polarity and Dipole Moments dipole moment: a measure of the unequal charge distribution in a molecule m = d x d = debyes (D) When µ = 0, a molecule is perfectly nonpolar (NP); otherwise it is polar (P) Example. CH 4 µ = 0.00 D nonpolar (NP) Example. HCl µ = 1.07 D polar (P)
4 Representation of Dipole Vectors for Linear CO 2 (see text figure, top of p. 400) The dipole vectors are equal in magnitude but exactly opposite in direction so they cancel each other and the net molecular dipole moment is 0. Representation of Dipole Vectors for Angular H 2 O (see text figure, middle of p. 400) The dipole vectors are equal in magnitude but not opposite in direction so they don t cancel each other and the net molecular dipole moment is not 0. Polar or Nonpolar? (see and review Example 10.4, pp ) Lewis Structures for NOF and NO 2 F (see and review Example 10.5, pp ) NF 3 and Ammonia (NH 3 ) Same geometry why different dipole moments? N-F Χ = = 1.0 and N-H Χ = = 0.9 VALENCE BOND (VB) THEORY A third approach to molecular geometry valence bond theory: a covalent bond involves the overlap of atomic orbitals on two different atoms, each atom contributing one electron to the bond itself region of overlap is the covalent bond and has a high electron density In the valence bond approach, only valence electrons participate in the formation of a covalent bond Valence Bond Explanation for Bonding in H 2 (see Figure 10.10, p.403) Overlap of two 1s orbitals one from each H atom participating in the bond! Valence Bond Explanation for Bonding in H 2 S (see Figure 10.11, p.403) - the three 3p orbitals are mutually orthogonal - S-H Bonds result from overlap of 1s orbitals on the H atoms with half-filled 3p orbitals on the S atom (predicted bond angle is 90 0 ) Explanation of sp 3 Hybridization for a C Atom (see Figure 10.12, p.405) Note: shape of sp 3 hybrid orbital Another way to Explain sp 3 Hybridization for Carbon s + 3 p 4 sp 3 hybrid orbitals sp 3 Hybridization in N and O (see discussion, pp ) Hybridization and Bonding in CH 4 (see Figure 10.13, p.406) Hybridization and Bonding in NH 3 (see Figure 10.14, p.406) Hybridization for Boron in BF 3 (see Figure 10.15, p.407) Need 3 energetically equivalent orbitals for B-F bonds B atom hybridization: s + 2 p 3 sp 2 hybrid orbitals Explanation of sp 2 Hybridization for B (see text figure, bottom of p. 406) Shape of sp 2 Hybrid Orbital (see text figure, bottom left, p. 406)
5 Hybridization for Be, e.g. in BeH 2 (see Figure 10.16, p.407) Need 2 energetically equivalent orbitals for B-H bonds Be atom hybridization: s + 1 p 2 sp hybrid orbitals Explanation of sp Hybridization for Be (see text figures, middle of p. 407) Shape of sp Hybrid Orbital (see text figure, middle right, p. 407) Explanation of sp 3 d Hybridization for P in PCl 5 (see text figure, upper middle, p. 408) Shape of sp 3 d Hybrid Orbital: (see Figure 10.17, p.408) Opportunity for up to 5 bonds, one with each lobe Explanation of sp3d2 Hybridization for S in SF 6 (see text figure, lower middle, p. 408) Shape of sp 3 d 2 Hybrid Orbital: (see Figure 10.18, p.408) Opportunity for up to 6 bonds, one with each lobe IF 5 (see and review Example 10.6, pp see Figure 10.19, p.410) Hybridization and Bonding for IF5 Central I atom has 6 energetically equivalent sp3d2 hybrid orbitals available for bonding 5 with F atoms and one for a lone pair on the I atom. Multiple Covalent Bonds and Hybrid Orbitals Lewis dot structure for ethylene, C 2 H 4 (see text figures, upper and middle, p. 411) Lewis Structures and VSEPR Interpretation for C 2 H 4 (see Figure 10.20, p.411) Review caption for Figure 10.20! Summary of VB Bonding in C 2 H 4 (see Figure 10.21, p.412) Note text description of types of orbital overlap in bonds in C 2 H 4 Sigma Bonds and Pi Bonds sigma (s) bond: a bond in which electron density is concentrated in the region (along the internuclear axis) between the two nuclei in a chemical bond can form by end-to-end overlap of unhybridized p- orbitals or overlap of lobes of adjacent hybrid orbitals on two different atoms pi (p ) bond: a bond oriented along the internuclear axis in which the overlap results from sideto-side overlap of unhybridized p- or d-orbitals single bond = 1 s bond double bond = 1 s bond + 1 p bond triple bond = 1 s bond + 2 p bonds Sigma Bonds and Pi Bonds Sigma bond from end-to-end overlap of two p-orbitals Sigma bond from end-to-end overlap of s orbital with p-orbital
6 Pi bond from side-to-side overlap of two p-orbitals Overlap of sp 2 Hybrid Orbitals to Form a s Bond Two sp 2 hybrid orbitals s bond from sp 2 hybrid overlap with another sp 2 hybrid Hybridization Scheme for C 2 H 2 (see text figures, lower middle, p. 413) Summary of VB Bonding in C 2 H 2 (see Figure 10.22, p.413) Note text description of types of orbital overlap in bonds in C 2 H 2
Molecular Geometry and Chemical Bonding Theory
 Molecular Geometry and Chemical Bonding Theory The Valence -Shell Electron -Pair Repulsion (VSEPR) Model predicts the shapes of the molecules and ions by assuming that the valence shell electron pairs
Molecular Geometry and Chemical Bonding Theory The Valence -Shell Electron -Pair Repulsion (VSEPR) Model predicts the shapes of the molecules and ions by assuming that the valence shell electron pairs
Covalent Compounds: Bonding Theories and Molecular Structure
 CHM 123 Chapter 8 Covalent Compounds: Bonding Theories and Molecular Structure 8.1 Molecular shapes and VSEPR theory VSEPR theory proposes that the geometric arrangement of terminal atoms, or groups of
CHM 123 Chapter 8 Covalent Compounds: Bonding Theories and Molecular Structure 8.1 Molecular shapes and VSEPR theory VSEPR theory proposes that the geometric arrangement of terminal atoms, or groups of
Shapes of Molecules and Hybridization
 Shapes of Molecules and Hybridization A. Molecular Geometry Lewis structures provide us with the number and types of bonds around a central atom, as well as any NB electron pairs. They do not tell us the
Shapes of Molecules and Hybridization A. Molecular Geometry Lewis structures provide us with the number and types of bonds around a central atom, as well as any NB electron pairs. They do not tell us the
Chapter 9 Molecular Geometry. Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory
 Chapter 9 Molecular Geometry Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory Sulfanilamide Lewis Structures and the Real 3D-Shape of Molecules Lewis Theory of Molecular Shape and Polarity
Chapter 9 Molecular Geometry Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory Sulfanilamide Lewis Structures and the Real 3D-Shape of Molecules Lewis Theory of Molecular Shape and Polarity
Chapter 9. Chemical Bonding II: Molecular Geometry and Bonding Theories
 Chapter 9 Chemical Bonding II: Molecular Geometry and Bonding Theories Topics Molecular Geometry Molecular Geometry and Polarity Valence Bond Theory Hybridization of Atomic Orbitals Hybridization in Molecules
Chapter 9 Chemical Bonding II: Molecular Geometry and Bonding Theories Topics Molecular Geometry Molecular Geometry and Polarity Valence Bond Theory Hybridization of Atomic Orbitals Hybridization in Molecules
CHEMISTRY. Chapter 10 Theories of Bonding and Structure. The Molecular Nature of Matter. Jespersen Brady Hyslop SIXTH EDITION
 CHEMISTRY The Molecular Nature of Matter SIXTH EDITION Jespersen Brady Hyslop Chapter 10 Theories of Bonding and Structure Copyright 2012 by John Wiley & Sons, Inc. Molecular Structures Molecules containing
CHEMISTRY The Molecular Nature of Matter SIXTH EDITION Jespersen Brady Hyslop Chapter 10 Theories of Bonding and Structure Copyright 2012 by John Wiley & Sons, Inc. Molecular Structures Molecules containing
Molecular shape is only discussed when there are three or more atoms connected (diatomic shape is obvious).
 Chapter 10 Molecular Geometry (Ch9 Jespersen, Ch10 Chang) The arrangement of the atoms of a molecule in space is the molecular geometry. This is what gives the molecules their shape. Molecular shape is
Chapter 10 Molecular Geometry (Ch9 Jespersen, Ch10 Chang) The arrangement of the atoms of a molecule in space is the molecular geometry. This is what gives the molecules their shape. Molecular shape is
LESSON 10. Glossary: Molecular Geometry. a quantitative measure of the degree of charge separation in a molecule. Dipole moment
 LESSON 10 Glossary: Molecular Geometry Dipole moment Electronegativity Molecular geometry Pi bond Polar covalent bond Sigma bond Valence-shell electronpair repulsion (VSEPR) model a quantitative measure
LESSON 10 Glossary: Molecular Geometry Dipole moment Electronegativity Molecular geometry Pi bond Polar covalent bond Sigma bond Valence-shell electronpair repulsion (VSEPR) model a quantitative measure
Valence Shell Electron Pair repulsion
 Molecular Geometry Valence Shell Electron Pair repulsion The valence shell electron pair repulsion model (VSEPR model) assumes that electron pairs repel one another. (VSEPR) model gives helps determine
Molecular Geometry Valence Shell Electron Pair repulsion The valence shell electron pair repulsion model (VSEPR model) assumes that electron pairs repel one another. (VSEPR) model gives helps determine
Lecture outline: Section 9. theory 2. Valence bond theory 3. Molecular orbital theory. S. Ensign, Chem. 1210
 Lecture outline: Section 9 Molecular l geometry and bonding theories 1. Valence shell electron pair repulsion theory 2. Valence bond theory 3. Molecular orbital theory 1 Ionic bonding Covalent bonding
Lecture outline: Section 9 Molecular l geometry and bonding theories 1. Valence shell electron pair repulsion theory 2. Valence bond theory 3. Molecular orbital theory 1 Ionic bonding Covalent bonding
Lecture B2 VSEPR Theory
 Lecture B2 VSEPR Theory Covalent Bond Theories 1. VSEPR (valence shell electron pair repulsion model). A set of empirical rules for predicting a molecular geometry using, as input, a correct Lewis Dot
Lecture B2 VSEPR Theory Covalent Bond Theories 1. VSEPR (valence shell electron pair repulsion model). A set of empirical rules for predicting a molecular geometry using, as input, a correct Lewis Dot
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9. Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 9. Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 1
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. How to get the book of
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. How to get the book of
Chapter 9: Molecular Geometry and Bonding Theories
 Chapter 9: Molecular Geometry and Bonding Theories 9.1 Molecular Geometries -Bond angles: angles made by the lines joining the nuclei of the atoms in a molecule -Bond angles determine overall shape of
Chapter 9: Molecular Geometry and Bonding Theories 9.1 Molecular Geometries -Bond angles: angles made by the lines joining the nuclei of the atoms in a molecule -Bond angles determine overall shape of
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals 1 Chemical Bonding II Molecular Geometry (10.1) Dipole Moments (10.2) Valence Bond Theory (10.3) Hybridization of Atomic Orbitals
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals 1 Chemical Bonding II Molecular Geometry (10.1) Dipole Moments (10.2) Valence Bond Theory (10.3) Hybridization of Atomic Orbitals
Shapes of Molecules. Lewis structures are useful but don t allow prediction of the shape of a molecule.
 Shapes of Molecules Lewis structures are useful but don t allow prediction of the shape of a molecule. H O H H O H Can use a simple theory based on electron repulsion to predict structure (for non-transition
Shapes of Molecules Lewis structures are useful but don t allow prediction of the shape of a molecule. H O H H O H Can use a simple theory based on electron repulsion to predict structure (for non-transition
VSEPR. Ch10. Valence Shell Electron Pair Repulsion theory allows you to predict molecular shape. Lewis Dot theory extended to 3 dimensions.
 Ch10 VSEPR Valence Shell Electron Pair Repulsion theory allows you to predict molecular shape. Lewis Dot theory extended to 3 dimensions. version 1.5 Nick DeMello, PhD. 2007-2016 Valence Shell Electron
Ch10 VSEPR Valence Shell Electron Pair Repulsion theory allows you to predict molecular shape. Lewis Dot theory extended to 3 dimensions. version 1.5 Nick DeMello, PhD. 2007-2016 Valence Shell Electron
VSEPR. Valence Shell Electron Pair Repulsion Theory
 VSEPR Valence Shell Electron Pair Repulsion Theory Vocabulary: domain = any electron pair or bond (single, double or triple) is considered one domain. bonding pair = shared pair = any electron pair that
VSEPR Valence Shell Electron Pair Repulsion Theory Vocabulary: domain = any electron pair or bond (single, double or triple) is considered one domain. bonding pair = shared pair = any electron pair that
Hybridization of Orbitals
 Hybridization of Orbitals Structure & Properties of Matter 1 Atomic Orbitals and Bonding Previously: Electron configurations Lewis structures Bonding Shapes of molecules Now: How do atoms form covalent
Hybridization of Orbitals Structure & Properties of Matter 1 Atomic Orbitals and Bonding Previously: Electron configurations Lewis structures Bonding Shapes of molecules Now: How do atoms form covalent
B. (i), (iii), and (v) C. (iv) D. (i), (ii), (iii), and (v) E. (i), (iii), (iv), and (v) Answer: B. SO 3, and NO 3 - both have 24 VE and have Lewis
 SCCH 161 Homework 3 1. Give the number of lone pairs around the central atom and the molecular geometry of CBr 4. Answer: Carbon has 4 valence electrons and bonds to four bromine atoms (each has 7 VE s).
SCCH 161 Homework 3 1. Give the number of lone pairs around the central atom and the molecular geometry of CBr 4. Answer: Carbon has 4 valence electrons and bonds to four bromine atoms (each has 7 VE s).
Introduction to VSEPR Theory 1
 1 Class 8: Introduction to VSEPR Theory Sec 10.2 VSEPR Theory: The Five Basic Shapes Two Electron Groups: Linear Geometry Three Electron Groups: Trigonal Planar Geometry Four Electron Groups: Tetrahedral
1 Class 8: Introduction to VSEPR Theory Sec 10.2 VSEPR Theory: The Five Basic Shapes Two Electron Groups: Linear Geometry Three Electron Groups: Trigonal Planar Geometry Four Electron Groups: Tetrahedral
Subtopic 4.2 MOLECULAR SHAPE AND POLARITY
 Subtopic 4.2 MOLECULAR SHAPE AND POLARITY 1 LEARNING OUTCOMES (covalent bonding) 1. Draw the Lewis structure of covalent molecules (octet rule such as NH 3, CCl 4, H 2 O, CO 2, N 2 O 4, and exception to
Subtopic 4.2 MOLECULAR SHAPE AND POLARITY 1 LEARNING OUTCOMES (covalent bonding) 1. Draw the Lewis structure of covalent molecules (octet rule such as NH 3, CCl 4, H 2 O, CO 2, N 2 O 4, and exception to
Chapter 9. Molecular Geometries and Bonding Theories. Lecture Presentation. John D. Bookstaver St. Charles Community College Cottleville, MO
 Lecture Presentation Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The shape of a molecule plays an important role in its reactivity. By noting the number of
Lecture Presentation Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The shape of a molecule plays an important role in its reactivity. By noting the number of
Molecular Geometry. Dr. Williamson s Molecular Geometry Notes. VSEPR: Definition of Terms. Dr. V.M. Williamson Texas A & M University Student Version
 Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Geometry. Dr. Williamson s Molecular Geometry Notes. VSEPR: Definition of Terms. VSEPR: Electronic Geometries VSEPR
 Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9. Molecular Geometry and Bonding Theories PART I Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 9. Molecular Geometry and Bonding Theories PART I Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Valence shell electron
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Valence shell electron
COVALENT BONDING CHEMICAL BONDING I: LEWIS MODEL. Chapter 7
 Chapter 7 P a g e 1 COVALENT BONDING Covalent Bonds Covalent bonds occur between two or more nonmetals. The two atoms share electrons between them, composing a molecule. Covalently bonded compounds are
Chapter 7 P a g e 1 COVALENT BONDING Covalent Bonds Covalent bonds occur between two or more nonmetals. The two atoms share electrons between them, composing a molecule. Covalently bonded compounds are
Molecular shape is determined by the number of bonds that form around individual atoms.
 Chapter 9 CH 180 Major Concepts: Molecular shape is determined by the number of bonds that form around individual atoms. Sublevels (s, p, d, & f) of separate atoms may overlap and result in hybrid orbitals
Chapter 9 CH 180 Major Concepts: Molecular shape is determined by the number of bonds that form around individual atoms. Sublevels (s, p, d, & f) of separate atoms may overlap and result in hybrid orbitals
At the end of this lesson, students should be able to :
 At the end of this lesson, students should be able to : (a) Explain Valence Shell Electron Pair Repulsion theory (VSEPR) (b) Draw the basic molecular shapes: linear, planar, tetrahedral, and octahedral.
At the end of this lesson, students should be able to : (a) Explain Valence Shell Electron Pair Repulsion theory (VSEPR) (b) Draw the basic molecular shapes: linear, planar, tetrahedral, and octahedral.
General and Inorganic Chemistry I.
 General and Inorganic Chemistry I. Lecture 1 István Szalai Eötvös University István Szalai (Eötvös University) Lecture 1 1 / 29 Outline István Szalai (Eötvös University) Lecture 1 2 / 29 Lewis Formulas
General and Inorganic Chemistry I. Lecture 1 István Szalai Eötvös University István Szalai (Eötvös University) Lecture 1 1 / 29 Outline István Szalai (Eötvös University) Lecture 1 2 / 29 Lewis Formulas
Molecular Structure and Orbitals
 CHEM 1411 General Chemistry Chemistry: An Atoms First Approach by Zumdahl 2 5 Molecular Structure and Orbitals Chapter Objectives: Learn the basics of Valence Bond Theory and Molecular Orbital Theory and
CHEM 1411 General Chemistry Chemistry: An Atoms First Approach by Zumdahl 2 5 Molecular Structure and Orbitals Chapter Objectives: Learn the basics of Valence Bond Theory and Molecular Orbital Theory and
Chapter Molecules are 3D. Shapes and Bonds. Chapter 9 1. Chemical Bonding and Molecular Structure
 Chapter 9 Chemical Bonding and Molecular Structure 1 Shape 9.1 Molecules are 3D Angle Linear 180 Planar triangular (trigonal planar) 120 Tetrahedral 109.5 2 Shapes and Bonds Imagine a molecule where the
Chapter 9 Chemical Bonding and Molecular Structure 1 Shape 9.1 Molecules are 3D Angle Linear 180 Planar triangular (trigonal planar) 120 Tetrahedral 109.5 2 Shapes and Bonds Imagine a molecule where the
Chapter 10. Geometry
 Chapter 10 Molec cular Geometry 1 CHAPTER OUTLINE Molecular Geometry Molecular Polarity VSEPR Model Summary of Molecular Shapes Hybridization Molecular Orbital Theory Bond Angles 2 MOLECULAR GEOMETRY Molecular
Chapter 10 Molec cular Geometry 1 CHAPTER OUTLINE Molecular Geometry Molecular Polarity VSEPR Model Summary of Molecular Shapes Hybridization Molecular Orbital Theory Bond Angles 2 MOLECULAR GEOMETRY Molecular
Chapter 9 Molecular Geometry Valence Bond and Molecular Orbital Theory
 Chapter 9 Molecular Geometry Valence Bond and Molecular Orbital Theory Chapter Objectives: Learn the basics of Valence Bond Theory and Molecular Orbital Theory and how they are used to model covalent bonding.
Chapter 9 Molecular Geometry Valence Bond and Molecular Orbital Theory Chapter Objectives: Learn the basics of Valence Bond Theory and Molecular Orbital Theory and how they are used to model covalent bonding.
Experiment 15. The Valence Shell Electron Pair Repulsion (VSEPR) Theory of Directed Valency: An exercise
 Experiment 15 The Valence Shell Electron Pair Repulsion (VSEPR) Theory of Directed Valency: An exercise Attempts to understand and predict the shapes of molecules using either the valencebond theory or
Experiment 15 The Valence Shell Electron Pair Repulsion (VSEPR) Theory of Directed Valency: An exercise Attempts to understand and predict the shapes of molecules using either the valencebond theory or
Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory
 10.1 Artificial Sweeteners: Fooled by Molecular Shape 425 10.2 VSEPR Theory: The Five Basic Shapes 426 10.3 VSEPR Theory: The Effect of Lone Pairs 430 10.4 VSEPR Theory: Predicting Molecular Geometries
10.1 Artificial Sweeteners: Fooled by Molecular Shape 425 10.2 VSEPR Theory: The Five Basic Shapes 426 10.3 VSEPR Theory: The Effect of Lone Pairs 430 10.4 VSEPR Theory: Predicting Molecular Geometries
Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model.
 Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model. Determine whether a molecule is polar or nonpolar based
Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model. Determine whether a molecule is polar or nonpolar based
Chapter 9 Molecular Geometries. and Bonding Theories
 Chapter 9 Molecular Geometries and Bonding Theories Coverage of Chapter 9 9.1 All 9.2 All 9.3 All 9.4 All 9.5 Omit Hybridization Involving d Orbitals 9.6 All 9.7 and 9.8 Omit ALL MOLECULAR SHAPES The shape
Chapter 9 Molecular Geometries and Bonding Theories Coverage of Chapter 9 9.1 All 9.2 All 9.3 All 9.4 All 9.5 Omit Hybridization Involving d Orbitals 9.6 All 9.7 and 9.8 Omit ALL MOLECULAR SHAPES The shape
Lewis structures show the number and type of bonds between atoms in a molecule or polyatomic ion.
 VSEPR & Geometry Lewis structures show the number and type of bonds between atoms in a molecule or polyatomic ion. Lewis structures are not intended to show the 3-dimensional structure (i.e. shape or geometry)
VSEPR & Geometry Lewis structures show the number and type of bonds between atoms in a molecule or polyatomic ion. Lewis structures are not intended to show the 3-dimensional structure (i.e. shape or geometry)
Chapter 9. Molecular Geometries and Bonding Theories. Lecture Presentation. John D. Bookstaver St. Charles Community College Cottleville, MO
 Lecture Presentation Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The shape of a molecule plays an important role in its reactivity. By noting the number of
Lecture Presentation Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The shape of a molecule plays an important role in its reactivity. By noting the number of
CHAPTER 8. Molecular Structure & Covalent Bonding Theories
 CAPTER 8 Molecular Structure & Covalent Bonding Theories 1 Chapter Goals 1. A Preview of the Chapter 2. Valence Shell Electron Pair Repulsion (VSEPR) Theory 3. Polar Molecules:The Influence of Molecular
CAPTER 8 Molecular Structure & Covalent Bonding Theories 1 Chapter Goals 1. A Preview of the Chapter 2. Valence Shell Electron Pair Repulsion (VSEPR) Theory 3. Polar Molecules:The Influence of Molecular
Chapter 10 Theories of Covalent Bonding
 Chapter 10 Theories of Covalent Bonding 1 Atomic Orbitals Molecules Bonding and 2 Molecular Structure Questions How are molecules held together? Why is O 2 paramagnetic? And how is this property connected
Chapter 10 Theories of Covalent Bonding 1 Atomic Orbitals Molecules Bonding and 2 Molecular Structure Questions How are molecules held together? Why is O 2 paramagnetic? And how is this property connected
Chapter 4. Molecular Structure and Orbitals
 Chapter 4 Molecular Structure and Orbitals Chapter 4 Table of Contents (4.1) (4.2) (4.3) (4.4) (4.5) (4.6) (4.7) Molecular structure: The VSEPR model Bond polarity and dipole moments Hybridization and
Chapter 4 Molecular Structure and Orbitals Chapter 4 Table of Contents (4.1) (4.2) (4.3) (4.4) (4.5) (4.6) (4.7) Molecular structure: The VSEPR model Bond polarity and dipole moments Hybridization and
Chapter 10. VSEPR Model: Geometries
 Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Farthest apart
Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Farthest apart
Chemical Bonding II. Molecular Geometry Valence Bond Theory Phys./Chem. Properties Quantum Mechanics Sigma & Pi bonds Hybridization MO theory
 Chemical Bonding II Molecular Geometry Valence Bond Theory Phys./Chem. Properties Quantum Mechanics Sigma & Pi bonds ybridization MO theory 1 Molecular Geometry 3-D arrangement of atoms 2 VSEPR Valence-shell
Chemical Bonding II Molecular Geometry Valence Bond Theory Phys./Chem. Properties Quantum Mechanics Sigma & Pi bonds ybridization MO theory 1 Molecular Geometry 3-D arrangement of atoms 2 VSEPR Valence-shell
Chapter 9. Molecular Geometry and Bonding Theories
 9.1 Molecular Shapes Read Sec. 9.1 and 9.2, then complete the Sample and Practice Exercises in these sections. Sample Exercise 9.1 (p. 347) Use the VSEPR model to predict the molecular geometries of a)
9.1 Molecular Shapes Read Sec. 9.1 and 9.2, then complete the Sample and Practice Exercises in these sections. Sample Exercise 9.1 (p. 347) Use the VSEPR model to predict the molecular geometries of a)
Chapter 9. Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory
 Chapter 9 Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory Problems with Lewis Theory Lewis theory generally predicts trends in properties, but does not give good numerical predictions.
Chapter 9 Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory Problems with Lewis Theory Lewis theory generally predicts trends in properties, but does not give good numerical predictions.
Valence Bond Theory - Description
 Bonding and Molecular Structure - PART 2 - Valence Bond Theory and Hybridization 1. Understand and be able to describe the Valence Bond Theory description of covalent bond formation. 2. Understand and
Bonding and Molecular Structure - PART 2 - Valence Bond Theory and Hybridization 1. Understand and be able to describe the Valence Bond Theory description of covalent bond formation. 2. Understand and
Molecular Geometry. Valence Shell Electron Pair. What Determines the Shape of a Molecule? Repulsion Theory (VSEPR) Localized Electron Model
 Molecular Geometry Learn Shapes you will Because the physical and chemical properties of compounds are tied to their structures, the importance of molecular geometry can not be overstated. Localized Electron
Molecular Geometry Learn Shapes you will Because the physical and chemical properties of compounds are tied to their structures, the importance of molecular geometry can not be overstated. Localized Electron
Chemistry: The Central Science. Chapter 9: Molecular Geometry and Bonding Theory
 Chemistry: The Central Science Chapter 9: Molecular Geometry and Bonding Theory The shape and size of a molecule of a particular substance, together with the strength and polarity of its bonds, largely
Chemistry: The Central Science Chapter 9: Molecular Geometry and Bonding Theory The shape and size of a molecule of a particular substance, together with the strength and polarity of its bonds, largely
Molecular Shape and Molecular Polarity. Molecular Shape and Molecular Polarity. Molecular Shape and Molecular Polarity
 Molecular Shape and Molecular Polarity When there is a difference in electronegativity between two atoms, then the bond between them is polar. It is possible for a molecule to contain polar bonds, but
Molecular Shape and Molecular Polarity When there is a difference in electronegativity between two atoms, then the bond between them is polar. It is possible for a molecule to contain polar bonds, but
Chapter 10. VSEPR Model: Geometries
 Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Two bonds Two
Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Two bonds Two
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Linear Trigonal 180 o planar 120 o Tetrahedral 109.5 o Trigonal Bipyramidal 120 and 90 o Octahedral 90 o linear Linear
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Linear Trigonal 180 o planar 120 o Tetrahedral 109.5 o Trigonal Bipyramidal 120 and 90 o Octahedral 90 o linear Linear
Hybridisation of Atomic Orbitals
 Lecture 7 CHEM101 Hybridisation of Atomic Orbitals Dr. Noha Osman Learning Outcomes Understand the valence bond theory Understand the concept of hybridization. Understand the different types of orbital
Lecture 7 CHEM101 Hybridisation of Atomic Orbitals Dr. Noha Osman Learning Outcomes Understand the valence bond theory Understand the concept of hybridization. Understand the different types of orbital
Chapter 8. Molecular Shapes. Valence Shell Electron Pair Repulsion Theory (VSEPR) What Determines the Shape of a Molecule?
 PowerPoint to accompany Molecular Shapes Chapter 8 Molecular Geometry and Bonding Theories Figure 8.2 The shape of a molecule plays an important role in its reactivity. By noting the number of bonding
PowerPoint to accompany Molecular Shapes Chapter 8 Molecular Geometry and Bonding Theories Figure 8.2 The shape of a molecule plays an important role in its reactivity. By noting the number of bonding
Molecular Geometry and Bonding Theories. Molecular Shapes. Molecular Shapes. Chapter 9 Part 2 November 16 th, 2004
 Molecular Geometry and Bonding Theories Chapter 9 Part 2 November 16 th, 2004 8 Molecular Shapes When considering the geometry about the central atom, we consider all electrons (lone pairs and bonding
Molecular Geometry and Bonding Theories Chapter 9 Part 2 November 16 th, 2004 8 Molecular Shapes When considering the geometry about the central atom, we consider all electrons (lone pairs and bonding
Hybridization and Molecular Orbital (MO) Theory
 ybridization and Molecular Orbital (MO) Theory Chapter 10 istorical Models G.N.Lewis and I. Langmuir (~1920) laid out foundations Ionic species were formed by electron transfer Covalent molecules arise
ybridization and Molecular Orbital (MO) Theory Chapter 10 istorical Models G.N.Lewis and I. Langmuir (~1920) laid out foundations Ionic species were formed by electron transfer Covalent molecules arise
CHAPTER TEN MOLECULAR GEOMETRY MOLECULAR GEOMETRY V S E P R CHEMICAL BONDING II: MOLECULAR GEOMETRY AND HYBRIDIZATION OF ATOMIC ORBITALS
 CHAPTER TEN CHEMICAL BONDING II: AND HYBRIDIZATION O ATOMIC ORBITALS V S E P R VSEPR Theory In VSEPR theory, multiple bonds behave like a single electron pair Valence shell electron pair repulsion (VSEPR)
CHAPTER TEN CHEMICAL BONDING II: AND HYBRIDIZATION O ATOMIC ORBITALS V S E P R VSEPR Theory In VSEPR theory, multiple bonds behave like a single electron pair Valence shell electron pair repulsion (VSEPR)
Molecular Geometry and intermolecular forces. Unit 4 Chapter 9 and 11.2
 1 Molecular Geometry and intermolecular forces Unit 4 Chapter 9 and 11.2 2 Unit 4.1 Chapter 9.1-9.3 3 Review of bonding Ionic compound (metal/nonmetal) creates a lattice Formula doesn t tell the exact
1 Molecular Geometry and intermolecular forces Unit 4 Chapter 9 and 11.2 2 Unit 4.1 Chapter 9.1-9.3 3 Review of bonding Ionic compound (metal/nonmetal) creates a lattice Formula doesn t tell the exact
CHAPTER 5: Bonding Theories - Explaining Molecular Geometry. Chapter Outline
 CHAPTER 5: Bonding Theories - Explaining Molecular Geometry Chapter Outline 5.1 Molecular Shape 5.2 Valence-Shell Electron-Pair Repulsion Theory (VSEPR) 5.3 Polar Bonds and Polar Molecules» What Makes
CHAPTER 5: Bonding Theories - Explaining Molecular Geometry Chapter Outline 5.1 Molecular Shape 5.2 Valence-Shell Electron-Pair Repulsion Theory (VSEPR) 5.3 Polar Bonds and Polar Molecules» What Makes
BONDING THEORIES Chapter , Carey
 BONDING THEORIES Chapter 10.6-10.7, Carey The Covalent Chemical Bond (9.2) FIG I Potential Energy Change to Form H2 What is a chemical bond? Why do chemical bonds occur? Descriptions of bonding: Valence
BONDING THEORIES Chapter 10.6-10.7, Carey The Covalent Chemical Bond (9.2) FIG I Potential Energy Change to Form H2 What is a chemical bond? Why do chemical bonds occur? Descriptions of bonding: Valence
Ch. 9- Molecular Geometry and Bonding Theories
 Ch. 9- Molecular Geometry and Bonding Theories 9.0 Introduction A. Lewis structures do not show one of the most important aspects of molecules- their overall shapes B. The shape and size of molecules-
Ch. 9- Molecular Geometry and Bonding Theories 9.0 Introduction A. Lewis structures do not show one of the most important aspects of molecules- their overall shapes B. The shape and size of molecules-
Molecular shapes. Balls and sticks
 Molecular shapes Balls and sticks Learning objectives Apply VSEPR to predict electronic geometry and shapes of simple molecules Determine molecule shape from electronic geometry Distinguish between polar
Molecular shapes Balls and sticks Learning objectives Apply VSEPR to predict electronic geometry and shapes of simple molecules Determine molecule shape from electronic geometry Distinguish between polar
CHEM 110 Exam 2 - Practice Test 1 - Solutions
 CHEM 110 Exam 2 - Practice Test 1 - Solutions 1D 1 has a triple bond. 2 has a double bond. 3 and 4 have single bonds. The stronger the bond, the shorter the length. 2A A 1:1 ratio means there must be the
CHEM 110 Exam 2 - Practice Test 1 - Solutions 1D 1 has a triple bond. 2 has a double bond. 3 and 4 have single bonds. The stronger the bond, the shorter the length. 2A A 1:1 ratio means there must be the
Lecture Presentation. Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory
 Lecture Presentation Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory Predicting Molecular Geometry 1. Draw the Lewis structure. 2. Determine the number
Lecture Presentation Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory Predicting Molecular Geometry 1. Draw the Lewis structure. 2. Determine the number
Localized Electron Model
 Localized Electron Model Models for Chemical Bonding Localized electron model (Valence bond model) Molecular orbital model Localized Electron Model Useful for explaining the structure of molecules especially
Localized Electron Model Models for Chemical Bonding Localized electron model (Valence bond model) Molecular orbital model Localized Electron Model Useful for explaining the structure of molecules especially
For this you need to know covalent bonds, Lewis dots, electronegativity, geometric shapes, duet & octet, single/double/triple bonds, etc...
 Lewis Structure Lab For this you need to know covalent bonds, Lewis dots, electronegativity, geometric shapes, duet & octet, single/double/triple bonds, etc... I can t assume you have had all these, so
Lewis Structure Lab For this you need to know covalent bonds, Lewis dots, electronegativity, geometric shapes, duet & octet, single/double/triple bonds, etc... I can t assume you have had all these, so
For more info visit Chemical bond is the attractive force which holds various constituents together in a molecule.
 Chemical bond:- Chemical bond is the attractive force which holds various constituents together in a molecule. There are three types of chemical bonds: Ionic Bond, Covalent Bond, Coordinate Bond. Octet
Chemical bond:- Chemical bond is the attractive force which holds various constituents together in a molecule. There are three types of chemical bonds: Ionic Bond, Covalent Bond, Coordinate Bond. Octet
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals
 Chemical Bonding II: and Hybridization of Atomic Orbitals Chapter 10 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Valence shell electron pair repulsion (VSEPR)
Chemical Bonding II: and Hybridization of Atomic Orbitals Chapter 10 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Valence shell electron pair repulsion (VSEPR)
Chapter 9 Molecular Geometry and Bonding Theories
 Lecture Presentation Chapter 9 Geometry James F. Kirby Quinnipiac University Hamden, CT Shapes Lewis Structures show bonding and lone pairs, but do not denote shape. However, we use Lewis Structures to
Lecture Presentation Chapter 9 Geometry James F. Kirby Quinnipiac University Hamden, CT Shapes Lewis Structures show bonding and lone pairs, but do not denote shape. However, we use Lewis Structures to
Molecular Geometry and Electron Domain Theory *
 OpenStax-CNX module: m12594 1 Molecular Geometry and Electron Domain Theory * John S. Hutchinson This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 2.0 1
OpenStax-CNX module: m12594 1 Molecular Geometry and Electron Domain Theory * John S. Hutchinson This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 2.0 1
Chapter 9. and Bonding Theories
 Chemistry, The Central Science, 11th edition Theodore L. Brown, H. Eugene LeMay, Jr., and Bruce E. Bursten Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The
Chemistry, The Central Science, 11th edition Theodore L. Brown, H. Eugene LeMay, Jr., and Bruce E. Bursten Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The
Class XI Chapter 4 Chemical Bonding and Molecular Structure Chemistry
 Class XI Chapter 4 Chemical Bonding and Molecular Structure Chemistry Question 4.1: Explain the formation of a chemical bond. A chemical bond is defined as an attractive force that holds the constituents
Class XI Chapter 4 Chemical Bonding and Molecular Structure Chemistry Question 4.1: Explain the formation of a chemical bond. A chemical bond is defined as an attractive force that holds the constituents
Class XI Chapter 4 Chemical Bonding and Molecular Structure Chemistry
 Class XI Chapter 4 Chemical Bonding and Molecular Structure Chemistry Question 4.1: Explain the formation of a chemical bond. A chemical bond is defined as an attractive force that holds the constituents
Class XI Chapter 4 Chemical Bonding and Molecular Structure Chemistry Question 4.1: Explain the formation of a chemical bond. A chemical bond is defined as an attractive force that holds the constituents
CHAPTER-4 CHEMICAL BONDING AND MOLECULAR STRUCTURE
 CHAPTER-4 CHEMICAL BONDING AND MOLECULAR STRUCTURE OCTET RULE- During a chemical reaction the atoms tend to adjust their electronic arrangement in such a way that they achieve 8 e - in their outermost
CHAPTER-4 CHEMICAL BONDING AND MOLECULAR STRUCTURE OCTET RULE- During a chemical reaction the atoms tend to adjust their electronic arrangement in such a way that they achieve 8 e - in their outermost
EXPERIMENT #13 Lewis Structures and Molecular Geometry
 OBJECTIVES: EXPERIMENT #13 s and Draw Lewis structures of atoms, ions, and molecules Build models of linear, trigonal planar tetrahedral, trigonal bipyramidal, and octahedral arrangements of electron pairs
OBJECTIVES: EXPERIMENT #13 s and Draw Lewis structures of atoms, ions, and molecules Build models of linear, trigonal planar tetrahedral, trigonal bipyramidal, and octahedral arrangements of electron pairs
CHEMISTRY 112 LECTURE EXAM II Material
 CHEMISTRY 112 LECTURE EXAM II Material Part I Chemical Bonding I Lewis Theory Chapter 9 pages 376-386 A. Drawing electron dot structures HOW TO: 1. Write e- dot structure for the individual atoms. 2. a)
CHEMISTRY 112 LECTURE EXAM II Material Part I Chemical Bonding I Lewis Theory Chapter 9 pages 376-386 A. Drawing electron dot structures HOW TO: 1. Write e- dot structure for the individual atoms. 2. a)
Chapter 10. Structure Determines Properties! Molecular Geometry. Chemical Bonding II
 Chapter 10 Chemical Bonding II Structure Determines Properties! Properties of molecular substances depend on the structure of the molecule The structure includes many factors, including: the skeletal arrangement
Chapter 10 Chemical Bonding II Structure Determines Properties! Properties of molecular substances depend on the structure of the molecule The structure includes many factors, including: the skeletal arrangement
Chapter 9. and Bonding Theories. Molecular Shapes. What Determines the Shape of a Molecule? 3/8/2013
 Chemistry, The Central Science, 10th edition Theodore L. Brown, H. Eugene LeMay, Jr., and Bruce E. Bursten Chapter 9 Theories John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice-Hall,
Chemistry, The Central Science, 10th edition Theodore L. Brown, H. Eugene LeMay, Jr., and Bruce E. Bursten Chapter 9 Theories John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice-Hall,
Lewis Theory of Shapes and Polarities of Molecules
 Lewis Theory of Shapes and Polarities of Molecules Sulfanilamide Lewis Structures and the Real 3D-Shape of Molecules Molecular Shape or Geometry The way in which atoms of a molecule are arranged in space
Lewis Theory of Shapes and Polarities of Molecules Sulfanilamide Lewis Structures and the Real 3D-Shape of Molecules Molecular Shape or Geometry The way in which atoms of a molecule are arranged in space
Chapter 13: Phenomena
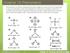 Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Shapes of Molecules VSEPR
 Shapes of Molecules In this section we will use Lewis structures as an introduction to the shapes of molecules. The key concepts are: Electron pairs repel each other. Electron pairs assume orientations
Shapes of Molecules In this section we will use Lewis structures as an introduction to the shapes of molecules. The key concepts are: Electron pairs repel each other. Electron pairs assume orientations
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals
 Chemical Bonding II: and ybridization of Atomic rbitals Chapter 10 Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the electrostatic repulsions between the
Chemical Bonding II: and ybridization of Atomic rbitals Chapter 10 Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the electrostatic repulsions between the
Chapter 9 Molecular Geometry and Bonding Theories
 Chapter 9 Molecular Geometry and Bonding Theories molecular shapes the VSEPR model molecular shape and molecular polarity covalent bonding and orbital overlap hybrid orbitals multiple bonds 9.1 Molecular
Chapter 9 Molecular Geometry and Bonding Theories molecular shapes the VSEPR model molecular shape and molecular polarity covalent bonding and orbital overlap hybrid orbitals multiple bonds 9.1 Molecular
CHM151LL: VSEPR and Molecular Geometry Tables
 CHM151LL: VSEPR and Molecular Geometry Tables VSEPR Model VALENCE-SHELL ELECTRON-PAIR REPULSION (VSEPR) MODEL Lewis structures show the two-dimensional distribution of atoms and electrons. The molecular
CHM151LL: VSEPR and Molecular Geometry Tables VSEPR Model VALENCE-SHELL ELECTRON-PAIR REPULSION (VSEPR) MODEL Lewis structures show the two-dimensional distribution of atoms and electrons. The molecular
Find the difference in electronegativity between the hydrogen and chlorine atoms
 Answers Questions 16.2 Molecular polarity 1. Write a dot diagram for the HCl molecule. Find the difference in electronegativity between the hydrogen and chlorine atoms Difference in electronegativity =
Answers Questions 16.2 Molecular polarity 1. Write a dot diagram for the HCl molecule. Find the difference in electronegativity between the hydrogen and chlorine atoms Difference in electronegativity =
Review Chapter 10: Theories of Bonding & Structure. Chemistry: The Molecular Nature of Matter, 6 th edition By Jesperson, Brady, & Hyslop
 Review Chapter 10: Theories of Bonding & Structure Chemistry: The Molecular Nature of Matter, 6 th edition By Jesperson, Brady, & Hyslop Chapter 10 Concepts q VESPR theory q Predict molecular geometry
Review Chapter 10: Theories of Bonding & Structure Chemistry: The Molecular Nature of Matter, 6 th edition By Jesperson, Brady, & Hyslop Chapter 10 Concepts q VESPR theory q Predict molecular geometry
Lewis Structure. Lewis Structures & VSEPR. Octet & Duet Rules. Steps for drawing Lewis Structures
 Lewis Structure Lewis Structures & VSEPR Lewis Structures shows how the are arranged among the atoms of a molecule There are rules for Lewis Structures that are based on the formation of a Atoms want to
Lewis Structure Lewis Structures & VSEPR Lewis Structures shows how the are arranged among the atoms of a molecule There are rules for Lewis Structures that are based on the formation of a Atoms want to
 VSEPR Theory, Valence Bond Theory, Characteristic of Covalent Compounds 1. Which of the following is not correct? 1. A sigma bond is weaker than pi bond. 2. A sigma bond is stronger than pi bond.. A double
VSEPR Theory, Valence Bond Theory, Characteristic of Covalent Compounds 1. Which of the following is not correct? 1. A sigma bond is weaker than pi bond. 2. A sigma bond is stronger than pi bond.. A double
Localized Electron Model
 Localized Electron Model Models for Chemical Bonding Localized electron model (Valence bond model) Molecular orbital model Localized Electron Model Useful for explaining the structure of molecules especially
Localized Electron Model Models for Chemical Bonding Localized electron model (Valence bond model) Molecular orbital model Localized Electron Model Useful for explaining the structure of molecules especially
Downloaded from
 CHAPTER-4 CHEMICAL BONDING AND MOLECULAR STRUCTURE OCTET RULE- During a chemical reaction the atoms tend to adjust their electronic arrangement in such a way that they achieve 8 e - in their outermost
CHAPTER-4 CHEMICAL BONDING AND MOLECULAR STRUCTURE OCTET RULE- During a chemical reaction the atoms tend to adjust their electronic arrangement in such a way that they achieve 8 e - in their outermost
Ex. 1) F F bond in F = 0 < % covalent, no transfer of electrons
 #60 Notes Unit 8: Bonding Ch. Bonding I. Bond Character Bonds are usually combinations of ionic and covalent character. The electronegativity difference is used to determine a bond s character. Electronegativity
#60 Notes Unit 8: Bonding Ch. Bonding I. Bond Character Bonds are usually combinations of ionic and covalent character. The electronegativity difference is used to determine a bond s character. Electronegativity
C PM RESURRECTION
 Announcements Final Exam TIME: October 8, 7:30-9:30AM VENUE: CTC 105 65-Multiple Choice Questions 3 Questions Each Chapter 2-5 7 Questions Each Chapter 6-8 30 Questions From Chapter 9-11 Saturday Review
Announcements Final Exam TIME: October 8, 7:30-9:30AM VENUE: CTC 105 65-Multiple Choice Questions 3 Questions Each Chapter 2-5 7 Questions Each Chapter 6-8 30 Questions From Chapter 9-11 Saturday Review
Valence-Shell Electron-Pair Repulsion Theory (VSEPR)
 ; Set 09- Molecular Geometry All course materials, including lectures, class notes, quizzes, exams, handouts, presentations, and other materials provided to students for this course are protected intellectual
; Set 09- Molecular Geometry All course materials, including lectures, class notes, quizzes, exams, handouts, presentations, and other materials provided to students for this course are protected intellectual
Molecular Geometry and Bonding Theories. Chapter 9
 Molecular Geometry and Bonding Theories Chapter 9 Molecular Shapes CCl 4 Lewis structures give atomic connectivity; The shape of a molecule is determined by its bond angles VSEPR Model Valence Shell Electron
Molecular Geometry and Bonding Theories Chapter 9 Molecular Shapes CCl 4 Lewis structures give atomic connectivity; The shape of a molecule is determined by its bond angles VSEPR Model Valence Shell Electron
Chapter 10 Molecular Geometry and Chemical Bonding Theory. Copyright Cengage Learning. All rights reserved. 10 1
 Chapter 10 Molecular Geometry and Chemical Bonding Theory Copyright Cengage Learning. All rights reserved. 10 1 Molecular geometry is the general shape of a molecule, as determined by the relative positions
Chapter 10 Molecular Geometry and Chemical Bonding Theory Copyright Cengage Learning. All rights reserved. 10 1 Molecular geometry is the general shape of a molecule, as determined by the relative positions
VSEPR Theory. Shapes of Molecules. Molecular Structure or Molecular Geometry
 VSEPR Theory VSEPR Theory Shapes of Molecules Molecular Structure or Molecular Geometry The 3-dimensional arrangement of the atoms that make-up a molecule. Determines several properties of a substance,
VSEPR Theory VSEPR Theory Shapes of Molecules Molecular Structure or Molecular Geometry The 3-dimensional arrangement of the atoms that make-up a molecule. Determines several properties of a substance,
CHEMICAL BONDING. Chemical Bonds. Ionic Bonding. Lewis Symbols
 CHEMICAL BONDING Chemical Bonds Lewis Symbols Octet Rule whenever possible, valence electrons in covalent compounds distribute so that each main-group element is surrounded by 8 electrons (except hydrogen
CHEMICAL BONDING Chemical Bonds Lewis Symbols Octet Rule whenever possible, valence electrons in covalent compounds distribute so that each main-group element is surrounded by 8 electrons (except hydrogen
SMK SULTAN ISMAIL JB, NUR FATHIN SUHANA BT AYOB
 SMK SULTAN ISMAIL JB, NUR FATHIN SUHANA BT AYOB POLAR AND NON POLAR BONDS BOND POLARITY 1. Atoms with different electronegative from polar bonds (difference in EN) 2. Depicted as polar arrow : 3. Example
SMK SULTAN ISMAIL JB, NUR FATHIN SUHANA BT AYOB POLAR AND NON POLAR BONDS BOND POLARITY 1. Atoms with different electronegative from polar bonds (difference in EN) 2. Depicted as polar arrow : 3. Example
