Radioactivity. Lecture 14 The Human Radioactivity Cycle
|
|
|
- Harvey Kory Conley
- 6 years ago
- Views:
Transcription
1 Radioactivity Lecture The Human Radioactivity Cycle
2 The Elements in the Human atomic percentage The molecular structure and the functions rely only on a few, but critical elements, Carbon C Oxygen O, and Hydrogen H as the base elements for all the organic molecule components. itrogen : allows the to utilize the nitrogen, promoting protein synthesis and the creation of compounds and amino acids that influence growth, hormones, brain functions and the immune system. Calcium Ca: is the most plentiful mineral found in the human. The teeth and bones contain the most calcium. erve cells, tissues, blood, and other fluids contain the rest of the calcium. Phosphorus P: is critical for the formation of bones and teeth. It plays an important role in how the uses carbohydrates and fats. It is also needed for the to make protein for the growth, maintenance, and repair of cells and tissues. Potassium : is crucial to heart function and nerve system. It plays a key role in skeletal and smooth muscle contraction, making it important for normal digestive and muscular function. Sulfur S: is a part of some of the amino acids in your and is involved in protein synthesis, as well as several enzyme reactions It helps with the production of collagen, a substance that forms connective tissues, cell structure and artery walls. Sodium a: regulates the total amount of water in the and the transmission of sodium into and out of individual cells also plays a role in critical functions for the muscle and nerve system.
3 Critical Molecule Contamination Hemoglobin (blood) C 2952 H 4664 O S 8 Fe 4 ( C) 12 ( C) 13 = C per DA molecule The Human contains about hemoglobin molecules, carrying a total of radioactive C isotopes in our blood. The Human has between 50 and 100 trillion cells, each cell has two sets of 23 chromosomes that consist of a tightly wound DA molecules. That means we have about 320 billion C isotopes embedded in our DA material! Similarly each DA molecule carries 21 radioactive isotopes, the DA contains therefore up to radioactive isotopes!
4 Intake through Food and Drinks The and its functions are maintained through food, drink, and air intake. It breaks up food molecules to facilitate growth and function. Through food and other exchange processes with the outer environment radioactive isotopes with identical or similar chemical behavior gets into material : 92 Sr with Ca, with potassium, 32 P with phosphorus, 226 Ra by inhaling. Food Radioactivity in your daily food and drink Bq/kg 226 Ra Bq/kg Food and drink Bq/kg 226 Ra Bq/kg Bananas Beer uts Meat 110 0,02 Carrots 126 Potatoes Beans 172 Drinking Water
5 Uptake by Plant Material Plants extract certain chemicals as nutrients out of the soil. The up-take of the radioactive isotopes depends on the chemical similarities between the isotope and the nutrient element. (T 1/2 =1.28 Gy) is being absorbed at the same level of % as normal potassium, 90 Sr (T 1/2 =28.6y) is absorbed similarly as calcium with an efficiency of around 10% depending on the plants. The up-take of 137 Cs (T 1/2 =30.2y) is with about 0.5% considerably smaller. Uranium and Thorium have a very low up-take efficiency of 0.1%. This translates into relatively enhanced content in fruit and vegetable. Fruit and vegetable from mineral rich soil has an enhanced level of radioactivity. The enrichment factor is in particular high for and 90 Sr, as well as 137 Cs as fallout product from nuclear test and reactor incidents!
6 Specific Radioactivities in our bodies Chemically our consists of 60% to 70% water, of 16% of proteins, 10% lipides forming the cell and structure, with 1% carbohydrates, nucleotides, and aminoacids each, and finally 5% minerals which are important for the electrical nerve and information transfer system. That immediately identifies 3 H, C, as important radioactive isotopes in water, organic materials of the, and minerals. All food and drink materials contain a certain fraction of radioactive material that is partly deposited in system, partly excreted. Quick estimate of the C content assuming an a volume content of 18.5% and equilibrium between consumption and excretion. T 12 ( C) ( C) 12 ( C) 1/ = 80kg kg 8 10 = 5730y 13 A ( C) = = C atom ln 2 ( C) = = 2290Bq s C atoms A of 80 kg contains.8 kg 12 C of atoms generating an activity of 2290 Bq!
7 and the radioactive heavy water Hydrogen has a weight percentage of 9.5% in the human which corresponds to 7.6 kg of weight. The hydrogen in primarily in form of H 2 O and OH molecules, tritium contamination comes as HTO or HT. T 1 ( H ) 1 ( H ) 3 ( H ) 1/ 2 = = 10 = 12.3y 18 A 23 3 ( H ) = = H atoms H atoms 3 ln 2 9 ( H ) = = 8.21Bq Increased tritium intake would increase the internal activity but tritium is part of the fluid system with relative rapid exchange within 10 days (George de Hevesey) 7 s The natural excretion can be accelerated by the additional intake of liquids.
8 Annual Dose from C and 3 H in Low energy β - emitters, therefore low energy deposition in material 3 H C Q β =18.6 kev 3 He Q β =156 kev E β =6.2 kev E β =52 kev Very small, if not negligible radiation dose inside from cosmic ray induced radioactive C and 3 H nuclei. Dose D D D D ( C) ( C) with 1eV 3 ( H ) D E = m absorbed 52keV = 1y 2290Bq 80kg = = keV = 1y 8.21Bq 80kg 3 10 ev 9 J ( H ) = 2 10 = = 3.2nSv kg J E = t A m ev kg avg = = kg 6 J kg = eV kg = 7.48µ Sv eV kg
9 Potassium in Human Body Potassium is an extremely important mineral in the. As positively charged ation + that is responsible for transferring information along the nerve system. It controls the muscle system, controls the cell growth, releases the hormones, and controls the production of proteins and the dissemination of carbohydrates. The total amount of potassium in the is self-regulated too much or too little potassium (Hypokalemia) can lead to heart problems. The typical blood potassium level is 0. to 0.21 gram per liter (g/l). Too much potassium intake will lead to increase in potassium excretion. Intake of potassium increases the intake of radioactive.
10 The in your mass mass of of the : m potassium in the : m = m mass of radioactive in the : m = m = m g of atoms m = to calculate m 7 m, you need for 80 kg : [ g] [ particles] = [ particles / g] the mass = m 7 in [ particles] m gramm. =
11 What is the absorbed lifetime dose Average lifetime is 70 years Body mass 80 kg 1 ev= J Average decay energy 0.5MeV from γ and β decay channels Close to dangerous dose level Dose Activity D ( ) D A ( ) = λ ( ) J kg E m absorbed = t A ( ) E m 0.69 A = MeV D = s 6869Bq 80kg = = 1/ 2 20 avg ln 2 = T = Gy = 15.1mGy 20 = 6860Bq = ev kg Annual dose is: 215 µgy; 30 times higher than the dose from the C and 3 H radioactivity in the human!
12 Average numbers from literature uclide Total Mass of uclide Total Activity of uclide Daily Intake of Found in the Body Found in the Body uclides Uranium 90 µg 1.1 Bq 1.9 µg Thorium 30 µg 0.11 Bq 3 µg Potassium 17 mg 4.4 kbq 0.39 mg Radium 31 pg 1.1 Bq 2.3 pg Carbon 22 ng 3.7 kbq 1.8 ng Tritium 0.06 pg 23 Bq pg Polonium 0.2 pg 37 Bq ~0.6 fg The highest activities come from and C, the remaining activity in the material is two to three order of magnitude smaller. However, Uranium, Thorium, Radium, and Polonium are alpha emitters which have a larger energy deposition and a much larger damage factor Q=20. This will increase the effective dose deposited into the system.
13 Drinking Water Radioactive materials are contained in all water sources, due to natural solution of minerals in water Mineral Water! They cannot be removed chemically nor is that desirable since minerals provide a certain level of taste.
14 Radioactivity Limits on Drinking Water ormal Water contains between 0.5 and 18 mg potassium, less than 2 µg radioactive per liter and significantly less than the daily up-take by food consumption. Mineral water contains larger potassium contents of up to mg/l; the legal limit 100 mg/l. This corresponds to 12 µg per liter, with an activity of about 3.2 Bq.
15 The radioactivity of 128 U in water Uranium and thorium is a natural component of dissolved minerals in water. There are substantial local variations which trace the variations in the geological mineral patterns. Legal requirements limit the 238 U content to 10µg/l ( U ) = = particles / l ln 2 16 A( U ) = λ ( U ) = = 124mBq / l A E MeV MeV D( U ) = n A( U ) = 0.124Bq / l = m 80kg kg s l 238 J G D( U ) = = kg s l s l with n =, E = 2.5MeV, m = 80kg,1eV = J Annual Dose D 238 ( U ) µ Gy = l for 1 liter of water; if a person drinks ½ liter per day the total annual Dose is 68 µgy! If you take the effective Dose H=20 D= 1.4 msv.
16 Body Excrements There is a balance between the up-take of chemical elements with food and drinks and the defecation process. Overabundances of most elements are being discharged. That includes also the radioactive components following the same physiological processes of the digestive system. The elemental distribution in solid and liquid excrements differs which means that solid and liquid excrements are loaded with different level of radioactive waste! The daily average on solid excrements ranges between 150 g and 270 g with 70% to 80% water content. Organic materials are 21.6%, solid carbon content is 50%, 5% calcium, 4% phosphorus, 2% potassium. The deposited amount of is: g per day; that translates into deposited activity of Bq per day. Uranium has no function, on average the human uptake is 2µg/day, 98% is excreted, 2% end up in hair. The deposited amount of 238 U is: g per day; that translates into a deposition activity of Bq per day.
17 Radioactivity Deposition Urine has a water content of 95%, solid material content is about 70 g/day containing 4% of potassium with %, which corresponds to 0.34mg of. (The C content is negligible). A ( ) / d 70g = g ln 2 18 ( ) = 5 10 = 88Bq per day s 23 = particles / d The daily radioactivity deposition of a single person into the sewer system is about 222 Bq! otre Dame has about 10,000 employees and 12,000 students. The otre Dame community deposits a daily radioactivity of about 5 Million Becquerel into the D sewer system. Chicago has a population of 2.7 million people depositing daily nearly 600 Million Becquerel into the sewer system, the water treatment plant, the Chicago Sanitary and Ship Canal to the Des Plaines River, further to the Illinois River, and eventually into the Mississippi River; 2 billion Bq= 0.2 TBq or 6 Ci annually!
18 Radioactive contamination of O Hare O Hare is the fourth busiest airport in the world with about 77 Million passengers annually. Assuming that 25% use the restrooms, generates a radioactivity out-put of 4.3 GBq annually, also eventually being deposited into Mississippi River. To put things in relation! Mississippi water contains on average 3mg/l on potassium, 0.36µg/l on. With an average water flow rate of 320,000 l/s, this translates into a transport rate of 0.12g/s of or 3060 Bq/s. The annual transport of natural 44 activity is Bq or nearly 1 TBq annually. Human excrements add an appreciable amount of about 25% to the activity load.
College Physics B - PHY2054C
 College - PHY2054C Physics - Radioactivity 11/24/2014 My Office Hours: Tuesday 10:00 AM - Noon 206 Keen Building Review Question 1 Isotopes of an element A have the same number of protons and electrons,
College - PHY2054C Physics - Radioactivity 11/24/2014 My Office Hours: Tuesday 10:00 AM - Noon 206 Keen Building Review Question 1 Isotopes of an element A have the same number of protons and electrons,
Problem Set 5 Solutions Prepared by Lisa Neef & Tony Key (last revision: 15 May 2007)
 Problem Set 5 Solutions Prepared by Lisa Neef & Tony Key (last revision: 15 May 2007) 1. ISOTOPIC DILUTION. A) Mass of Exchangeable Calcium. Model. Here are all the things we are given in this problem:
Problem Set 5 Solutions Prepared by Lisa Neef & Tony Key (last revision: 15 May 2007) 1. ISOTOPIC DILUTION. A) Mass of Exchangeable Calcium. Model. Here are all the things we are given in this problem:
Sources of Radiation Exposure
 Sources of Radiation Exposure Sources of Radiation Exposure to the US Population (from U.S. NRC, Glossary: Exposure. [updated 21 July 2003, cited 26 March 2004] http://www.nrc.gov/reading-rm/basic-ref/glossary/exposure.html
Sources of Radiation Exposure Sources of Radiation Exposure to the US Population (from U.S. NRC, Glossary: Exposure. [updated 21 July 2003, cited 26 March 2004] http://www.nrc.gov/reading-rm/basic-ref/glossary/exposure.html
Radioactivity. General Physics II PHYS 111. King Saud University College of Applied Studies and Community Service Department of Natural Sciences
 King Saud University College of Applied Studies and Community Service Department of Natural Sciences Radioactivity General Physics II PHYS 111 Nouf Alkathran nalkathran@ksu.edu.sa Outline Radioactive Decay
King Saud University College of Applied Studies and Community Service Department of Natural Sciences Radioactivity General Physics II PHYS 111 Nouf Alkathran nalkathran@ksu.edu.sa Outline Radioactive Decay
Almost 99% of the body is made up of six elements: Carbon, Hydrogen, Oxygen, Nitrogen, Phosphorus, Calcium **(CHONPC)
 COMPOSITION OF THE HUMAN BODY Almost 99% of the body is made up of six elements: Carbon, Hydrogen, Oxygen, Nitrogen, Phosphorus, Calcium **(CHONPC) Only about 0.85% is composed of another five: Potassium,
COMPOSITION OF THE HUMAN BODY Almost 99% of the body is made up of six elements: Carbon, Hydrogen, Oxygen, Nitrogen, Phosphorus, Calcium **(CHONPC) Only about 0.85% is composed of another five: Potassium,
Nuclear Spectroscopy: Radioactivity and Half Life
 Particle and Spectroscopy: and Half Life 02/08/2018 My Office Hours: Thursday 1:00-3:00 PM 212 Keen Building Outline 1 2 3 4 5 Some nuclei are unstable and decay spontaneously into two or more particles.
Particle and Spectroscopy: and Half Life 02/08/2018 My Office Hours: Thursday 1:00-3:00 PM 212 Keen Building Outline 1 2 3 4 5 Some nuclei are unstable and decay spontaneously into two or more particles.
Half Life Introduction
 Name: Date: Period: Half Life Introduction The half-life of an element is the time it will take half of the parent atoms to transmutate into different atoms (through alpha or beta decays, or another process).
Name: Date: Period: Half Life Introduction The half-life of an element is the time it will take half of the parent atoms to transmutate into different atoms (through alpha or beta decays, or another process).
Radioactivity. General Physics II PHYS 111. King Saud University College of Applied Studies and Community Service Department of Natural Sciences
 King Saud University College of Applied Studies and Community Service Department of Natural Sciences Radioactivity General Physics II PHYS 111 Nouf Alkathran nalkathran@ksu.edu.sa Outline Radioactive Decay
King Saud University College of Applied Studies and Community Service Department of Natural Sciences Radioactivity General Physics II PHYS 111 Nouf Alkathran nalkathran@ksu.edu.sa Outline Radioactive Decay
Fundamentals of radiation protection
 Fundamentals of radiation protection Kamel ABBAS European Commission, Joint Research Centre Institute for Transuranium Elements, Nuclear Security Unit Via E. Fermi, 2749, I-21027 Ispra, Italy tel. +39-0332-785673,
Fundamentals of radiation protection Kamel ABBAS European Commission, Joint Research Centre Institute for Transuranium Elements, Nuclear Security Unit Via E. Fermi, 2749, I-21027 Ispra, Italy tel. +39-0332-785673,
WHAT IS IONIZING RADIATION
 WHAT IS IONIZING RADIATION Margarita Saraví National Atomic Energy Commission - Argentina Workshop on Ionizing Radiation SIM Buenos Aires 10 November 2011 What is ionizing radiation? What is ionizing radiation?
WHAT IS IONIZING RADIATION Margarita Saraví National Atomic Energy Commission - Argentina Workshop on Ionizing Radiation SIM Buenos Aires 10 November 2011 What is ionizing radiation? What is ionizing radiation?
Questions. 1. What kind of radiation dominates the first phase of radiation emission from a nuclear fireball?
 Questions 1. What kind of radiation dominates the first phase of radiation emission from a nuclear fireball? 2. What is the ignition temperature of wood? 3. What fuels a firestorm? Natural Radioactivity
Questions 1. What kind of radiation dominates the first phase of radiation emission from a nuclear fireball? 2. What is the ignition temperature of wood? 3. What fuels a firestorm? Natural Radioactivity
Atomic Structure Summary
 Atomic Structure Summary All atoms have: a positively charged nucleus and negatively charged electrons around it Atomic nucleus consists of: positively charged protons and neutrons that have no electric
Atomic Structure Summary All atoms have: a positively charged nucleus and negatively charged electrons around it Atomic nucleus consists of: positively charged protons and neutrons that have no electric
BASIC OF RADIATION; ORIGIN AND UNITS
 INAYA MEDICAL COLLEGE (IMC) RAD 243 - LECTURE 2 BASIC OF RADIATION; ORIGIN AND UNITS DR. MOHAMMED MOSTAFA EMAM LECTURES & CLASS ACTIVITIES https://inayacollegedrmohammedemam.wordpress.com/ Password: drmohammedemam
INAYA MEDICAL COLLEGE (IMC) RAD 243 - LECTURE 2 BASIC OF RADIATION; ORIGIN AND UNITS DR. MOHAMMED MOSTAFA EMAM LECTURES & CLASS ACTIVITIES https://inayacollegedrmohammedemam.wordpress.com/ Password: drmohammedemam
Radioactivity is the spontaneous disintegration of nuclei. The first radioactive. elements discovered were the heavy atoms thorium and uranium.
 Chapter 16 What is radioactivity? Radioactivity is the spontaneous disintegration of nuclei. The first radioactive elements discovered were the heavy atoms thorium and uranium. These heavy atoms and others
Chapter 16 What is radioactivity? Radioactivity is the spontaneous disintegration of nuclei. The first radioactive elements discovered were the heavy atoms thorium and uranium. These heavy atoms and others
Radioactivity. Lecture 7 Dosimetry and Exposure Limits
 Radioactivity Lecture 7 Dosimetry and Exposure Limits Radiation Exposure - Radiology The radiation impact on biological and genetic materials requires some protective measures! Units for scaling the decay
Radioactivity Lecture 7 Dosimetry and Exposure Limits Radiation Exposure - Radiology The radiation impact on biological and genetic materials requires some protective measures! Units for scaling the decay
What happens during nuclear decay? During nuclear decay, atoms of one element can change into atoms of a different element altogether.
 When Henri Becquerel placed uranium salts on a photographic plate and then developed the plate, he found a foggy image. The image was caused by rays that had not been observed before. For his discovery
When Henri Becquerel placed uranium salts on a photographic plate and then developed the plate, he found a foggy image. The image was caused by rays that had not been observed before. For his discovery
10.4 Half-Life. Investigation. 290 Unit C Radioactivity
 .4 Half-Life Figure Pitchblende, the major uranium ore, is a heavy mineral that contains uranium oxides, lead, and trace amounts of other radioactive elements. Pierre and Marie Curie found radium and polonium
.4 Half-Life Figure Pitchblende, the major uranium ore, is a heavy mineral that contains uranium oxides, lead, and trace amounts of other radioactive elements. Pierre and Marie Curie found radium and polonium
Nuclear forces and Radioactivity. Two forces are at work inside the nucleus of an atom
 Nuclear forces and Radioactivity Two forces are at work inside the nucleus of an atom Forces act in opposing directions Electrostatic repulsion: pushes protons apart Strong nuclear force: pulls protons
Nuclear forces and Radioactivity Two forces are at work inside the nucleus of an atom Forces act in opposing directions Electrostatic repulsion: pushes protons apart Strong nuclear force: pulls protons
Radioactivity. Lecture 7 Dosimetry and Exposure Limits
 Radioactivity Lecture 7 Dosimetry and Exposure Limits Radiation Exposure - Radiology The radiation impact on biological and genetic materials requires some protective measures! Units for scaling the decay
Radioactivity Lecture 7 Dosimetry and Exposure Limits Radiation Exposure - Radiology The radiation impact on biological and genetic materials requires some protective measures! Units for scaling the decay
Higher -o-o-o- Past Paper questions o-o-o- 3.6 Radiation
 Higher -o-o-o- Past Paper questions 2000-2010 -o-o-o- 3.6 Radiation 2000 Q29 Radium (Ra) decays to radon (Rn) by the emission of an alpha particle. Some energy is also released by this decay. The decay
Higher -o-o-o- Past Paper questions 2000-2010 -o-o-o- 3.6 Radiation 2000 Q29 Radium (Ra) decays to radon (Rn) by the emission of an alpha particle. Some energy is also released by this decay. The decay
Chapter 2. The Chemistry of Life
 Chapter 2 The Chemistry of Life Introduction Cells, tissues and organs composed of chemicals Chemical reactions important for function Chemistry is the study of elements, compounds, chemical reactions,
Chapter 2 The Chemistry of Life Introduction Cells, tissues and organs composed of chemicals Chemical reactions important for function Chemistry is the study of elements, compounds, chemical reactions,
A Nuclear Power Plant
 A Nuclear Power Plant Fallout from Chernobyl The question that all countries asked in 1986, and continue to ask to this day: Could it happen here? Radioactivity Np Pu+ 239 239 0 93 94 1 Beta decay the
A Nuclear Power Plant Fallout from Chernobyl The question that all countries asked in 1986, and continue to ask to this day: Could it happen here? Radioactivity Np Pu+ 239 239 0 93 94 1 Beta decay the
Number of protons. 2. What is the nuclear symbol for a radioactive isotope of copper with a mass number of 60? A) Cu
 Chapter 5 Nuclear Chemistry Practice Problems 1. Fill in the missing information in the chart: Medical Use Atomic Mass symbol number Heart imaging 201 Tl 81 Number of protons Number of neutrons Abdominal
Chapter 5 Nuclear Chemistry Practice Problems 1. Fill in the missing information in the chart: Medical Use Atomic Mass symbol number Heart imaging 201 Tl 81 Number of protons Number of neutrons Abdominal
sample What happens when we are exposed to radiation? 1.1 Natural radiation Cosmic radiation
 1.1 Natural radiation 3 1 What happens when we are exposed to radiation? 1.1 Natural radiation For as long as humans have walked the earth, we have continually been exposed to naturally-occurring radiation.
1.1 Natural radiation 3 1 What happens when we are exposed to radiation? 1.1 Natural radiation For as long as humans have walked the earth, we have continually been exposed to naturally-occurring radiation.
10.1 RADIOACTIVE DECAY
 10.1 RADIOACTIVE DECAY When Henri Becquerel placed uranium salts on a photographic plate and then developed the plate, he found a foggy image. The image was caused by rays that had not been observed before.
10.1 RADIOACTIVE DECAY When Henri Becquerel placed uranium salts on a photographic plate and then developed the plate, he found a foggy image. The image was caused by rays that had not been observed before.
Basic Chemistry. Chemistry Review. Bio 250: Anatomy & Physiology
 Basic Chemistry Bio 250: Anatomy & Physiology Chemistry Review It is going to be your responsibility to review the basic principles of chemistry you learned in BIO 101 This basic set of notes will help
Basic Chemistry Bio 250: Anatomy & Physiology Chemistry Review It is going to be your responsibility to review the basic principles of chemistry you learned in BIO 101 This basic set of notes will help
U (superscript is mass number, subscript atomic number) - radionuclides nuclei that are radioactive - radioisotopes atoms containing radionuclides
 Chapter : Nuclear Chemistry. Radioactivity nucleons neutron and proton all atoms of a given element have the same number of protons, atomic number isotopes atoms with the same atomic number but different
Chapter : Nuclear Chemistry. Radioactivity nucleons neutron and proton all atoms of a given element have the same number of protons, atomic number isotopes atoms with the same atomic number but different
Chapter 2: Chemical Basis of Life I. Introduction A. The study of chemistry is essential for the study of physiology because
 Shier, Butler, and Lewis: Hole s Human Anatomy and Physiology, 11 th ed. Chapter 2: Chemical Basis of Life Chapter 2: Chemical Basis of Life I. Introduction A. The study of chemistry is essential for the
Shier, Butler, and Lewis: Hole s Human Anatomy and Physiology, 11 th ed. Chapter 2: Chemical Basis of Life Chapter 2: Chemical Basis of Life I. Introduction A. The study of chemistry is essential for the
NORM and TENORM: Occurrence, Characterizing, Handling and Disposal
 NORM and TENORM: Occurrence, Characterizing, Handling and Disposal Ionizing Radiation and Hazard Potential John R. Frazier, Ph.D. Certified Health Physicist May 12, 2014 Radiation Radiation is a word that
NORM and TENORM: Occurrence, Characterizing, Handling and Disposal Ionizing Radiation and Hazard Potential John R. Frazier, Ph.D. Certified Health Physicist May 12, 2014 Radiation Radiation is a word that
Chemistry. Animal Health Technology Student Development Program
 Chemistry Animal Health Technology Student Development Program Chemistry Chemistry is a fundamental component in all of us. Chemical reactions are happening in our bodies constantly. In the Animal Health
Chemistry Animal Health Technology Student Development Program Chemistry Chemistry is a fundamental component in all of us. Chemical reactions are happening in our bodies constantly. In the Animal Health
Systems, Matter, & Energy Chapter 2. Friday, August 14 th, 2015
 Systems, Matter, & Energy Chapter 2 Friday, August 14 th, 2015 Chapter Overview Questions What are major components and behaviors of complex systems? What are the basic forms of matter, and what makes
Systems, Matter, & Energy Chapter 2 Friday, August 14 th, 2015 Chapter Overview Questions What are major components and behaviors of complex systems? What are the basic forms of matter, and what makes
Nuclear Radiation. Natural Radioactivity. A person working with radioisotopes wears protective clothing and gloves and stands behind a shield.
 Nuclear Radiation Natural Radioactivity A person working with radioisotopes wears protective clothing and gloves and stands behind a shield. 1 Radioactive Isotopes A radioactive isotope has an unstable
Nuclear Radiation Natural Radioactivity A person working with radioisotopes wears protective clothing and gloves and stands behind a shield. 1 Radioactive Isotopes A radioactive isotope has an unstable
Chapter. Nuclear Chemistry
 Chapter Nuclear Chemistry Nuclear Reactions 01 Chapter 22 Slide 2 Chapter 22 Slide 3 Alpha Decay: Loss of an α-particle (a helium nucleus) 4 2 He 238 92 U 234 4 U He 90 + 2 Chapter 22 Slide 4 Beta Decay:
Chapter Nuclear Chemistry Nuclear Reactions 01 Chapter 22 Slide 2 Chapter 22 Slide 3 Alpha Decay: Loss of an α-particle (a helium nucleus) 4 2 He 238 92 U 234 4 U He 90 + 2 Chapter 22 Slide 4 Beta Decay:
Unit 2: Chemistry Test Review
 Name: Period: Unit 2: Chemistry Test Review 1. List the three states of matter. 2. Describe an atom in terms of its nucleus, valence,shell, electrons, protons, and neutrons. 3. Define the term element
Name: Period: Unit 2: Chemistry Test Review 1. List the three states of matter. 2. Describe an atom in terms of its nucleus, valence,shell, electrons, protons, and neutrons. 3. Define the term element
Biology. Chapter 2 Notes
 Biology Chapter 2 Notes Section 1: Nature of Matter Objectives: 1) Differentiate between atoms and elements 2) Analyze how compounds are formed 3) Distinguish between covalent bonds, hydrogen bonds and
Biology Chapter 2 Notes Section 1: Nature of Matter Objectives: 1) Differentiate between atoms and elements 2) Analyze how compounds are formed 3) Distinguish between covalent bonds, hydrogen bonds and
Lecture Presentation. Chapter 21. Nuclear Chemistry. James F. Kirby Quinnipiac University Hamden, CT Pearson Education, Inc.
 Lecture Presentation Chapter 21, Inc. James F. Kirby Quinnipiac University Hamden, CT Energy: Chemical vs. Chemical energy is associated with making and breaking chemical bonds. energy is enormous in comparison.
Lecture Presentation Chapter 21, Inc. James F. Kirby Quinnipiac University Hamden, CT Energy: Chemical vs. Chemical energy is associated with making and breaking chemical bonds. energy is enormous in comparison.
The detector and counter are used in an experiment to show that a radioactive source gives out alpha and beta radiation only.
 ATOMS AND NUCLEAR RADIATION PART II Q1. The detector and counter are used in an experiment to show that a radioactive source gives out alpha and beta radiation only. Two different types of absorber are
ATOMS AND NUCLEAR RADIATION PART II Q1. The detector and counter are used in an experiment to show that a radioactive source gives out alpha and beta radiation only. Two different types of absorber are
Regents review Nuclear Chemistry
 2011-2012 1. Given the nuclear equation: 14 7N + X 16 8O + 2 1H What is particle X? A) an alpha particle B) a beta particle C) a deuteron D) a triton 2. The nucleus of a radium-226 atom is unstable, which
2011-2012 1. Given the nuclear equation: 14 7N + X 16 8O + 2 1H What is particle X? A) an alpha particle B) a beta particle C) a deuteron D) a triton 2. The nucleus of a radium-226 atom is unstable, which
Question. 1. Which natural source of background radiation do you consider as dominant?
 Question 1. Which natural source of background radiation do you consider as dominant? 2. Is the radiation background constant or does it change with time and location? 3. What is the level of anthropogenic
Question 1. Which natural source of background radiation do you consider as dominant? 2. Is the radiation background constant or does it change with time and location? 3. What is the level of anthropogenic
Nuclear Physics and Astrophysics
 Nuclear Physics and Astrophysics PHY-302 Dr. E. Rizvi Lecture 24 Medical Imaging Effects of Radiation We now know what radiation is But what does it mean for our bodies? Radioactivity is quantified in
Nuclear Physics and Astrophysics PHY-302 Dr. E. Rizvi Lecture 24 Medical Imaging Effects of Radiation We now know what radiation is But what does it mean for our bodies? Radioactivity is quantified in
SOURCES of RADIOACTIVITY
 Section 9: SOURCES of RADIOACTIVITY This section briefly describes various sources of radioactive nuclei, both naturally occurring and those produced artificially (man-made) in, for example, reactors or
Section 9: SOURCES of RADIOACTIVITY This section briefly describes various sources of radioactive nuclei, both naturally occurring and those produced artificially (man-made) in, for example, reactors or
Part One: The Chemistry of Life
 Part One: The Chemistry of Life Chemistry is the study of matter and its changes. Organisms obtain and use many chemicals The metabolism of organisms involves many chemical reactions To understand all
Part One: The Chemistry of Life Chemistry is the study of matter and its changes. Organisms obtain and use many chemicals The metabolism of organisms involves many chemical reactions To understand all
Chemistry of Life 10/1/2010. What makes up the chemistry of life?
 A. Students will be able to identify and define the parts of an atom. Chemistry of Life At the Completion of this Unit, Students will be able to: A. Identify and define the parts of an atom. B. Demonstrate
A. Students will be able to identify and define the parts of an atom. Chemistry of Life At the Completion of this Unit, Students will be able to: A. Identify and define the parts of an atom. B. Demonstrate
Study for Test April 26, Chapter 4. Review of Metabolism and Photosynthesis and Carbohydrates, Fats (Lipids) & Proteins
 Chapter 4 Review of Metabolism and Photosynthesis and Carbohydrates, Fats (Lipids) & Proteins GLCE's L.OL.07.61:Recognize the need for light to provide energy for the production of carbohydrates, proteins,
Chapter 4 Review of Metabolism and Photosynthesis and Carbohydrates, Fats (Lipids) & Proteins GLCE's L.OL.07.61:Recognize the need for light to provide energy for the production of carbohydrates, proteins,
P4 Quick Revision Questions
 P4 Quick Revision Questions H = Higher tier only SS = Separate science only P3 for AQA GCSE examination 2018 onwards Question 1... of 50 What are the components of an atom, their location and their charge?
P4 Quick Revision Questions H = Higher tier only SS = Separate science only P3 for AQA GCSE examination 2018 onwards Question 1... of 50 What are the components of an atom, their location and their charge?
Revision Guide for Chapter 18
 Revision Guide for Chapter 18 Contents Student s Checklist Revision Notes Ionising radiation... 4 Biological effects of ionising radiation... 5 Risk... 5 Nucleus... 6 Nuclear stability... 6 Binding energy...
Revision Guide for Chapter 18 Contents Student s Checklist Revision Notes Ionising radiation... 4 Biological effects of ionising radiation... 5 Risk... 5 Nucleus... 6 Nuclear stability... 6 Binding energy...
Radioactivity: the process by which atoms emit energy in the form of electromagnetic waves, charged particles, or uncharged particles.
 Radioactivity: the process by which atoms emit energy in the form of electromagnetic waves, charged particles, or uncharged particles. In 1896, Henri Bequerel discovered that uranium and other elements
Radioactivity: the process by which atoms emit energy in the form of electromagnetic waves, charged particles, or uncharged particles. In 1896, Henri Bequerel discovered that uranium and other elements
Differentiating Chemical Reactions from Nuclear Reactions
 Differentiating Chemical Reactions from Nuclear Reactions 1 CHEMICAL Occurs when bonds are broken or formed. Atoms remained unchanged, though may be rearranged. Involves valence electrons Small energy
Differentiating Chemical Reactions from Nuclear Reactions 1 CHEMICAL Occurs when bonds are broken or formed. Atoms remained unchanged, though may be rearranged. Involves valence electrons Small energy
Name # Class Date Regents Review: Cells & Cell Transport
 Name # Class Date Regents Review: Cells & Cell Transport 1. All of the following are true regarding cells except? A) All cells have genetic material B) All cells have cell walls C) All cells have plasma
Name # Class Date Regents Review: Cells & Cell Transport 1. All of the following are true regarding cells except? A) All cells have genetic material B) All cells have cell walls C) All cells have plasma
A Brief Overview of Biochemistry. And I mean BRIEF!
 A Brief Overview of Biochemistry And I mean BRIEF! Introduction A. Chemistry deals with the composition of substances and how they change. B. A knowledge of chemistry is necessary for the understanding
A Brief Overview of Biochemistry And I mean BRIEF! Introduction A. Chemistry deals with the composition of substances and how they change. B. A knowledge of chemistry is necessary for the understanding
Chapter 3.1 Chemistry of Life
 Life Science Chapter 3: Cell Processes 1. Chemistry of Life 2. Moving Cellular Materials 3. Energy for Life http://www.connecticutvalleybiological.com/cell processes vhs p 14026.html Chapter 3.1 Chemistry
Life Science Chapter 3: Cell Processes 1. Chemistry of Life 2. Moving Cellular Materials 3. Energy for Life http://www.connecticutvalleybiological.com/cell processes vhs p 14026.html Chapter 3.1 Chemistry
UNIT 2 CHEMISTRY. Atomic Structure: Ionic Bond: Covalent Bond: Hydrogen Bond:
 UNIT 2 CHEMISTRY Atomic Structure: Ionic Bond: Hydrogen Bond: Covalent Bond: 1 Carbohydrates: >energy yield- >elements- >monomers- >functions- >examples- >misc- Lipids: Proteins: Nucleic Acids: I. Energy
UNIT 2 CHEMISTRY Atomic Structure: Ionic Bond: Hydrogen Bond: Covalent Bond: 1 Carbohydrates: >energy yield- >elements- >monomers- >functions- >examples- >misc- Lipids: Proteins: Nucleic Acids: I. Energy
A. Identify the highly penetrating radioactive emission that exposed the photographic plates.
 Name Unit 3: Nuclear Chemistry Date Part 2 Questions 1. In 1896, Antoine H. Becquerel discovered that a uranium compound could expose a photographic plate wrapped in heavy paper in the absence of light.
Name Unit 3: Nuclear Chemistry Date Part 2 Questions 1. In 1896, Antoine H. Becquerel discovered that a uranium compound could expose a photographic plate wrapped in heavy paper in the absence of light.
Chapter 20: Phenomena. Chapter 20: The Nucleus: A Chemist s View. Nuclear Decay. Nuclear Decay. Nuclear Decay. Nuclear Decay
 Chapter 20: Phenomena Phenomena: Below is a list of stable isotopes of different elements. Examine the data and see what patterns you can identify. The mass of a electron is 0.00055 u, the mass of a proton
Chapter 20: Phenomena Phenomena: Below is a list of stable isotopes of different elements. Examine the data and see what patterns you can identify. The mass of a electron is 0.00055 u, the mass of a proton
Section Objectives: Section Objectives: Distinguish mixtures and solutions. Define acids and bases and relate their importance to biological systems.
 Section Objectives: Relate the structure of an atom to the identity of elements. Relate the formation of covalent and ionic chemical bonds to the stability of atoms. Section Objectives: Distinguish mixtures
Section Objectives: Relate the structure of an atom to the identity of elements. Relate the formation of covalent and ionic chemical bonds to the stability of atoms. Section Objectives: Distinguish mixtures
Natural Radiation K 40
 Natural Radiation There are a few radioisotopes that exist in our environment. Isotopes that were present when the earth was formed and isotopes that are continuously produced by cosmic rays can exist
Natural Radiation There are a few radioisotopes that exist in our environment. Isotopes that were present when the earth was formed and isotopes that are continuously produced by cosmic rays can exist
8/24/2018. Bio 1101 Lecture 2 (guided) Chapters 2: Essential Chemistry. Chapter 2: Essential Chemistry for Biology
 1 2 3 4 5 Bio 1101 Lecture 2 (guided) Chapters 2: Essential Chemistry Chapter 2: Essential Chemistry for Biology Levels of biological organization Ecosystem (e.g. savanna) Community (e.g. the organisms
1 2 3 4 5 Bio 1101 Lecture 2 (guided) Chapters 2: Essential Chemistry Chapter 2: Essential Chemistry for Biology Levels of biological organization Ecosystem (e.g. savanna) Community (e.g. the organisms
Physics 219 Help Session. Date: Wed 12/07, Time: 6:00-8:00 pm. Location: Physics 331
 Lecture 25-1 Physics 219 Help Session Date: Wed 12/07, 2016. Time: 6:00-8:00 pm Location: Physics 331 Lecture 25-2 Final Exam Dec. 14. 2016. 1:00-3:00pm in Phys. 112 Bring your ID card, your calculator
Lecture 25-1 Physics 219 Help Session Date: Wed 12/07, 2016. Time: 6:00-8:00 pm Location: Physics 331 Lecture 25-2 Final Exam Dec. 14. 2016. 1:00-3:00pm in Phys. 112 Bring your ID card, your calculator
Chapter 2 Chemistry of Life
 Chapter 2 Chemistry of Life Section 2.1 Atoms, Ions and Molecules Section 2.2 Properties of water Section 2.3 Carbon-based Molecules Section 2.4 Chemical Reactions Section 2.5 - Enzymes 1 Atoms, Ions and
Chapter 2 Chemistry of Life Section 2.1 Atoms, Ions and Molecules Section 2.2 Properties of water Section 2.3 Carbon-based Molecules Section 2.4 Chemical Reactions Section 2.5 - Enzymes 1 Atoms, Ions and
POLYTECHNIC OF NAMIBIA SCHOOL OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF NATURAL AND APPLIED SCIENCES BASIC SCIENCE PARTTIME TEST 2
 POLYTECHNIC OF NAMIBIA SCHOOL OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF NATURAL AND APPLIED SCIENCES BASIC SCIENCE PARTTIME TEST 2 INITIALS: SURNAME: STUDENT NUMBER: MODE OF STUDY: QUALIFICATION: LECTURE
POLYTECHNIC OF NAMIBIA SCHOOL OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF NATURAL AND APPLIED SCIENCES BASIC SCIENCE PARTTIME TEST 2 INITIALS: SURNAME: STUDENT NUMBER: MODE OF STUDY: QUALIFICATION: LECTURE
Levels of Organization
 Levels of Organization MADE UP OF CELLS REPRO- DUCE 5 CHARACH- TERISTICS OF LIVING THINGS RESPOND TO CHANGES GROW AND DEVELOP USE ENERGY cells the basic units of structure and function of all living
Levels of Organization MADE UP OF CELLS REPRO- DUCE 5 CHARACH- TERISTICS OF LIVING THINGS RESPOND TO CHANGES GROW AND DEVELOP USE ENERGY cells the basic units of structure and function of all living
Unit 08 Nuclear Structure. Unit 08 Nuclear Structure Slide 1
 Unit 08 Nuclear Structure Unit 08 Nuclear Structure Slide 1 The Plan Nuclear Structure Nuclear Decays Measuring Radiation Nuclear Power Plants Major Nuclear Power Accidents New Possibilities for Nuclear
Unit 08 Nuclear Structure Unit 08 Nuclear Structure Slide 1 The Plan Nuclear Structure Nuclear Decays Measuring Radiation Nuclear Power Plants Major Nuclear Power Accidents New Possibilities for Nuclear
Interaction of the radiation with a molecule knocks an electron from the molecule. a. Molecule ¾ ¾ ¾ ion + e -
 Interaction of the radiation with a molecule knocks an electron from the molecule. radiation a. Molecule ¾ ¾ ¾ ion + e - This can destroy the delicate balance of chemical reactions in living cells. The
Interaction of the radiation with a molecule knocks an electron from the molecule. radiation a. Molecule ¾ ¾ ¾ ion + e - This can destroy the delicate balance of chemical reactions in living cells. The
Nuclear Chemistry. In this chapter we will look at two types of nuclear reactions.
 1 1 Nuclear Chemistry In this chapter we will look at two types of nuclear reactions. Radioactive decay is the process in which a nucleus spontaneously disintegrates, giving off radiation. Nuclear bombardment
1 1 Nuclear Chemistry In this chapter we will look at two types of nuclear reactions. Radioactive decay is the process in which a nucleus spontaneously disintegrates, giving off radiation. Nuclear bombardment
UNIT 2 CHEMISTRY. Atomic Structure: Ionic Bond: Covalent Bond: Hydrogen Bond:
 UNIT 2 CHEMISTRY Atomic Structure: Ionic Bond: Hydrogen Bond: Covalent Bond: 1 Carbohydrates: >energy yield- >elements- >monomers- >functions- >examples- >misc- Lipids: Proteins: Nucleic Acids: I. Energy
UNIT 2 CHEMISTRY Atomic Structure: Ionic Bond: Hydrogen Bond: Covalent Bond: 1 Carbohydrates: >energy yield- >elements- >monomers- >functions- >examples- >misc- Lipids: Proteins: Nucleic Acids: I. Energy
Year 7 - Cells Summary Notes
 Year 7 - Cells Summary Notes Life Processes All living things do all seven of the life processes. Things that are not living may do some but do not do all seven of the life processes. These are: Movement
Year 7 - Cells Summary Notes Life Processes All living things do all seven of the life processes. Things that are not living may do some but do not do all seven of the life processes. These are: Movement
notes Radiological Basics Transportation Emergency Preparedness Program
 INTRODUCTION The reliance upon, and use of, radioactive material in agriculture, industry, and medicine continues to increase. As the manufacture, use, and disposal of radioactive material has increased,
INTRODUCTION The reliance upon, and use of, radioactive material in agriculture, industry, and medicine continues to increase. As the manufacture, use, and disposal of radioactive material has increased,
Nuclear Chemistry. Background Radiation. Three-fourths of all exposure to radiation comes from background radiation.
 Chapter 11 Nuclear Chemistry Background Radiation Three-fourths of all exposure to radiation comes from background radiation. Most of the remaining one-fourth comes from medical irradiation such as X-rays.
Chapter 11 Nuclear Chemistry Background Radiation Three-fourths of all exposure to radiation comes from background radiation. Most of the remaining one-fourth comes from medical irradiation such as X-rays.
Radioactive nuclei. From Last Time. Biological effects of radiation. Radioactive decay. A random process. Radioactive tracers. e r t.
 From Last Time Nuclear structure and isotopes Binding energy of nuclei Radioactive nuclei Final Exam is Mon Dec 21, 5:05 pm - 7:05 pm 2103 Chamberlin 3 equation sheets allowed About 30% on new material
From Last Time Nuclear structure and isotopes Binding energy of nuclei Radioactive nuclei Final Exam is Mon Dec 21, 5:05 pm - 7:05 pm 2103 Chamberlin 3 equation sheets allowed About 30% on new material
SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question.
 ch 2 chemical basis of life Name SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. Fill in the blank or provide a short answer: 1) When a change in matter
ch 2 chemical basis of life Name SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. Fill in the blank or provide a short answer: 1) When a change in matter
Radiological Preparedness & Emergency Response. Session II. Objectives. Basic Radiation Physics
 Radiological Preparedness & Emergency Response Session II Basic Radiation Physics Objectives Discuss the difference between ionizing and non-ionizing radiation. Describe radioactive decay. Discuss the
Radiological Preparedness & Emergency Response Session II Basic Radiation Physics Objectives Discuss the difference between ionizing and non-ionizing radiation. Describe radioactive decay. Discuss the
What Are Atoms? Chapter 2: Atoms, Molecules & Life
 Chapter 2: Atoms, Molecules & Life What Are Atoms? An atom are the smallest unit of matter. Atoms are composed of Electrons = negatively charged particles. Neutrons = particles with no charge (neutral).
Chapter 2: Atoms, Molecules & Life What Are Atoms? An atom are the smallest unit of matter. Atoms are composed of Electrons = negatively charged particles. Neutrons = particles with no charge (neutral).
Nerve cells have many branches that help them send signals throughout the body.
 What is your body made of? You might say that you are made of atoms or cells. You might even say you are made of organs, like skin and a heart. These answers are all correct. Each focuses on a different
What is your body made of? You might say that you are made of atoms or cells. You might even say you are made of organs, like skin and a heart. These answers are all correct. Each focuses on a different
(a) (i) State the proton number and the nucleon number of X.
 PhysicsAndMathsTutor.com 1 1. Nuclei of 218 84Po decay by the emission of an particle to form a stable isotope of an element X. You may assume that no emission accompanies the decay. (a) (i) State the
PhysicsAndMathsTutor.com 1 1. Nuclei of 218 84Po decay by the emission of an particle to form a stable isotope of an element X. You may assume that no emission accompanies the decay. (a) (i) State the
Q1. The diagram represents an atom of lithium.
 Q1. The diagram represents an atom of lithium. Complete the diagram by writing in the spaces the name of each type of particle. Use only words given in the box. Each word may be used once or not at all.
Q1. The diagram represents an atom of lithium. Complete the diagram by writing in the spaces the name of each type of particle. Use only words given in the box. Each word may be used once or not at all.
Chapter 2 Concepts of Chemistry
 Anatomy Physiology and Disease for the Health Professions 3rd Edition Booth Test Bank Full Download: http://testbanklive.com/download/anatomy-physiology-and-disease-for-the-health-professions-3rd-edition-booth-te
Anatomy Physiology and Disease for the Health Professions 3rd Edition Booth Test Bank Full Download: http://testbanklive.com/download/anatomy-physiology-and-disease-for-the-health-professions-3rd-edition-booth-te
Nicholas J. Giordano. Chapter 30. Nuclear Physics. Marilyn Akins, PhD Broome Community College
 Nicholas J. Giordano www.cengage.com/physics/giordano Chapter 30 Nuclear Physics Marilyn Akins, PhD Broome Community College Atomic Nuclei Rutherford s discovery of the atomic nucleus caused scientists
Nicholas J. Giordano www.cengage.com/physics/giordano Chapter 30 Nuclear Physics Marilyn Akins, PhD Broome Community College Atomic Nuclei Rutherford s discovery of the atomic nucleus caused scientists
Ch. 18 Problems, Selected solutions. Sections 18.1
 Sections 8. 8. (I) How many ion pairs are created in a Geiger counter by a 5.4-MeV alpha particle if 80% of its energy goes to create ion pairs and 30 ev (average in gases) is required per ion pair? Notice
Sections 8. 8. (I) How many ion pairs are created in a Geiger counter by a 5.4-MeV alpha particle if 80% of its energy goes to create ion pairs and 30 ev (average in gases) is required per ion pair? Notice
Elements and Isotopes
 Section 2-1 Notes Atoms Life depends on chemistry. The basic unit of matter is the atom. Atoms are incredibly small The subatomic particles that make up atoms are protons, neutrons, and electrons. Parts
Section 2-1 Notes Atoms Life depends on chemistry. The basic unit of matter is the atom. Atoms are incredibly small The subatomic particles that make up atoms are protons, neutrons, and electrons. Parts
Chapter 2: Chemical Basis of Life
 Chapter 2: Chemical Basis of Life Chemistry is the scientific study of the composition of matter and how composition changes. In order to understand human physiological processes, it is important to understand
Chapter 2: Chemical Basis of Life Chemistry is the scientific study of the composition of matter and how composition changes. In order to understand human physiological processes, it is important to understand
Science Year 10 Unit 1 Biology
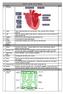 Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
Chemical Reactions. Unit 4
 Chemical Reactions Unit 4 Lesson 1: Chemical Bonds Unit 4: Reactions Compounds Most substances around you are NOT elements. There are around 100 elements, but millions of different substances. Most substances
Chemical Reactions Unit 4 Lesson 1: Chemical Bonds Unit 4: Reactions Compounds Most substances around you are NOT elements. There are around 100 elements, but millions of different substances. Most substances
Which row in the chart below identifies the lettered substances in this process?
 1. A biological process that occurs in both plants and animals is shown below. Which row in the chart below identifies the lettered substances in this process? A) 1 B) 2 C) 3 D) 4 2. All life depends on
1. A biological process that occurs in both plants and animals is shown below. Which row in the chart below identifies the lettered substances in this process? A) 1 B) 2 C) 3 D) 4 2. All life depends on
Key Question: What role did the study of radioactivity play in learning more about atoms?
 Name Chemistry Essential question: How were the parts of the atom determined? Key Question: What role did the study of radioactivity play in learning more about atoms? Vocabulary: alpha particle fusion
Name Chemistry Essential question: How were the parts of the atom determined? Key Question: What role did the study of radioactivity play in learning more about atoms? Vocabulary: alpha particle fusion
LECTURE 26 RADIATION AND RADIOACTIVITY
 LECTURE 26 RADIATION AND RADIOACTIVITY 30.4 Radiation and radioactivity Decay series Nuclear radiation is a form of ionizing radiation 30.5 Nuclear decay and half-lives Activity Radioactive dating Radiocarbon
LECTURE 26 RADIATION AND RADIOACTIVITY 30.4 Radiation and radioactivity Decay series Nuclear radiation is a form of ionizing radiation 30.5 Nuclear decay and half-lives Activity Radioactive dating Radiocarbon
Al-Saudia Virtual Academy Pakistan Online tuition Online Tutor Pakistan. NUCLEAR PHYSICS: Chapter 19
 Al-Saudia Virtual Academy Pakistan Online tuition Online Tutor Pakistan NUCLEAR PHYSICS: Chapter 19 Nuclear Physics: Branch of physics that deals with the study of the nucleus is called nuclear physics.
Al-Saudia Virtual Academy Pakistan Online tuition Online Tutor Pakistan NUCLEAR PHYSICS: Chapter 19 Nuclear Physics: Branch of physics that deals with the study of the nucleus is called nuclear physics.
Living and nonliving things are all made of elements. It is the way that atoms combine that give every element a different characteristic.
 Living and nonliving things are all made of elements. It is the way that atoms combine that give every element a different characteristic. 98% of the body is made of only 6 elements The 6 elements are:
Living and nonliving things are all made of elements. It is the way that atoms combine that give every element a different characteristic. 98% of the body is made of only 6 elements The 6 elements are:
[2]
![[2] [2]](/thumbs/79/78842361.jpg) 1 Fossil fuel power stations generate electricity. Nuclear power stations also generate electricity. (a) Many people think that nuclear power stations are a greater risk to people than fossil fuel power
1 Fossil fuel power stations generate electricity. Nuclear power stations also generate electricity. (a) Many people think that nuclear power stations are a greater risk to people than fossil fuel power
Biology Unit 4. Chemistry of Life
 Biology Unit 4 Chemistry of Life Elements Everything in our universe that has a mass and a volume is made of matter. Matter in its purest form is an element. There are 118 elements on the periodic table,
Biology Unit 4 Chemistry of Life Elements Everything in our universe that has a mass and a volume is made of matter. Matter in its purest form is an element. There are 118 elements on the periodic table,
Name Period Date Science 7R - Marking Period 3 Review SCIENTIFIC METHOD 1. What are the steps of the scientific method?
 Name Period Date Science 7R - Marking Period 3 Review SCIENTIFIC METHOD 1. What are the steps of the scientific method? 2. What is meant by State the Problem? 3. What is a hypothesis? 4. In which step
Name Period Date Science 7R - Marking Period 3 Review SCIENTIFIC METHOD 1. What are the steps of the scientific method? 2. What is meant by State the Problem? 3. What is a hypothesis? 4. In which step
Nuclear Chemistry. Radioactivity. In this chapter we will look at two types of nuclear reactions.
 1 Nuclear Chemistry In this chapter we will look at two types of nuclear reactions. Radioactive decay is the process in which a nucleus spontaneously disintegrates, giving off radiation. Nuclear bombardment
1 Nuclear Chemistry In this chapter we will look at two types of nuclear reactions. Radioactive decay is the process in which a nucleus spontaneously disintegrates, giving off radiation. Nuclear bombardment
Tritium in Drinking Water: Science, Regulation and Society
 Tritium in Drinking Water: Science, Regulation and Society TAM-E: Environmental Section Special Session: Tritium in the Environment Presented To: 57th Annual Meeting of the Health Physics Society Sacramento
Tritium in Drinking Water: Science, Regulation and Society TAM-E: Environmental Section Special Session: Tritium in the Environment Presented To: 57th Annual Meeting of the Health Physics Society Sacramento
2.1 Atoms, Ions, and Molecules
 2.1 Atoms, Ions, and Molecules Living things consist of atoms of different elements. An atom is the smallest basic unit of matter. An element is one type of atom. 6 elements make up 99% of all living things
2.1 Atoms, Ions, and Molecules Living things consist of atoms of different elements. An atom is the smallest basic unit of matter. An element is one type of atom. 6 elements make up 99% of all living things
Radioactivity and Balancing Nuclear Reactions: Balancing Nuclear Reactions and Understanding which Particles are Involves
 General Chemistry II Jasperse Nuclear Chemistry. Extra Practice Problems Radioactivity and Balancing Nuclear Reactions: Balancing Nuclear Reactions and Understanding which Particles are Involved he Stability
General Chemistry II Jasperse Nuclear Chemistry. Extra Practice Problems Radioactivity and Balancing Nuclear Reactions: Balancing Nuclear Reactions and Understanding which Particles are Involved he Stability
1. Your Roadmap for Success in Chapter 6
 1. Your Roadmap for Success in Chapter 6 Preview the chapter: 1. Read Summary (p. 171). 2. Skim the Assessment questions (p. 171-173). 3. Rewrite Learning Objectives for the chapter and each section in
1. Your Roadmap for Success in Chapter 6 Preview the chapter: 1. Read Summary (p. 171). 2. Skim the Assessment questions (p. 171-173). 3. Rewrite Learning Objectives for the chapter and each section in
D) g. 2. In which pair do the particles have approximately the same mass?
 1. A student constructs a model for comparing the masses of subatomic particles. The student selects a small, metal sphere with a mass of gram to represent an electron. A sphere with which mass would be
1. A student constructs a model for comparing the masses of subatomic particles. The student selects a small, metal sphere with a mass of gram to represent an electron. A sphere with which mass would be
Answer Key- Biology Review for Fall Benchmark
 Name Class Answer Key- Biology Review for Fall Benchmark Definitions You should know what every word on this page means. Look through the entire review sheet and highlight any words you do not recognize.
Name Class Answer Key- Biology Review for Fall Benchmark Definitions You should know what every word on this page means. Look through the entire review sheet and highlight any words you do not recognize.
Hole s Human Anatomy and Physiology Tenth Edition. Chapter 2
 PowerPoint Lecture Outlines to accompany Hole s Human Anatomy and Physiology Tenth Edition Shier w Butler w Lewis Chapter 2 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
PowerPoint Lecture Outlines to accompany Hole s Human Anatomy and Physiology Tenth Edition Shier w Butler w Lewis Chapter 2 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
Overview of Physiology & Homeostasis. Biological explanations Levels of organization Homeostasis
 Overview of Physiology & Homeostasis 1 Biological explanations Levels of organization Homeostasis 2 Biological Explanations Proximate Proximate causation: an explanation of an animal's behavior based on
Overview of Physiology & Homeostasis 1 Biological explanations Levels of organization Homeostasis 2 Biological Explanations Proximate Proximate causation: an explanation of an animal's behavior based on
Nuclear Powe. Bronze Buddha at Hiroshima
 Nuclear Powe Bronze Buddha at Hiroshima Nuclear Weapons Nuclear Power Is it Green & Safe? Nuclear Waste 250,000 tons of Spent Fuel 10,000 tons made per year Health Effects of Ionizing Radiation Radiocarbon
Nuclear Powe Bronze Buddha at Hiroshima Nuclear Weapons Nuclear Power Is it Green & Safe? Nuclear Waste 250,000 tons of Spent Fuel 10,000 tons made per year Health Effects of Ionizing Radiation Radiocarbon
