Military High School AL- Ain. Grade 10 &11. Biology Sample Questions. Student Name: Computer #:
|
|
|
- Jody Anderson
- 5 years ago
- Views:
Transcription
1 Military High School AL- Ain Grade 10 &11 Biology Sample Questions Student Name: Computer #:
2 Chapter 1: Cells In all multiple choice questions, more than answer could be correct Section : 1 What Is a Cell? 01. The basic unit of all living organisms is called (nucleus/ atom/ cell). 02. What are the three basic structures in all living cells? 03. Label the following animal cell. 04. Label the following plant cell. 05. What is the main function of the cell membrane?
3 06. (Cell membrane/ Cell wall/ Nucleus/ Cytoplasm) is a thin boundary enclosing the cytoplasm that control substances that enter and leave the cell. 07. What is the main function of the cytoplasm? 08. What is an organelle? Give three examples of organelles. 09. What are the main functions of the nucleus? 10. Which of the following is/are NOT a function of a nucleus? [-A-] protect the cell [-B-] site of cell s reactions [-C-] carry hereditary information [-D-] control cell division [-E-] A & B 11. (Starch grain/ Vacuole/ Mitochondria/ Nucleus) in a living cell produces energy from food substances. 12. Mitochondria are found in larger quantity in :- [-A-] skin [-B-] cheek cells [-C-] liver cells [-D-] muscle cells 13. What is the main function of the ribosomes? 14. What types of cells contain chloroplasts?
4 15. What is the function of chloroplasts? 16. (Mitochondria/ Ribosome/ Chloroplast/ Starch grain) is present only in plant cells, and it makes glucose from carbon dioxide and water by a process called photosynthesis. Section : 2 Plant Cells Compared with Animal Cells Concept : 17. is used as a food store in plant cells. (Cell wall/ Cell membrane/ Starch grains) 18. makes plant cells firm. ( Cytoplasm / Vacuole/ Starch grains) 19. support and protect plant cell. (Cell wall/ Cell membrane/ Cytoplasm) 20. Match the following parts of a light microscope to its corresponding function: 1. Diaphragm a. is used to the specimen in focus 2. Stage b. magnify specimen 3. Course adjustment knob c. is used to observe a specimen 4. Objective lens d. control amount of light 5. Fine adjustment knob e. reflect light 6. Mirror f. hold the specimen 7. Eye piece lens g. is used to see specimen clearly 21. Label the following parts of a microscope:
5 Chapter 2: Tissues and Organs
6 01. The electron microscope uses beams of (light/ electrons/ protons) instead of beams of light. 02. What is the maximum magnification of an electron microscope? 03. The image seen under the electron microscope is (of) (lower/ higher) when compared to that of light microscope. 04. Which of the following best describes an electron microscope? Resolution Ease of use magnification A High Easy Up to 1000 times B High Difficult Up to 500,000 times C Low Difficult Up to 1500 times D Low Easy Up to 500,000 times 05. Which of the following is an image of the electron microscope? A B C 06. State two advantages of using an electron microscope. 07. State two disadvantages of using an electron microscope.
7 Section : 2 Cell Division and Cell Specialization 08. The cell starts to divide when it s (fully or completely/ partial) grown. 09. What basic structure divides first in the cell during cell division of an animal cell? 10. Arrange the stages of animal cell division in the correct order. 11. What is the result of the last stage in a cell division? 12. All the following happen during plant cell division EXCEPT which of the following? [-A-] The nucleus divides at first. [-B-] Vacuoles join to form one vacuole in one cell. [-C-] new cell wall separates the two new nuclei. [-D-] The squeezing or narrowing of the cytoplasm divides the cell into two cells. [-E-] The nucleus is not the first structure to divide. 13. (Organs/ Systems/ Tissues/ Cells/ Organisms) are groups of cells that have the same shape and function. 14. A tissue is a group of similar (organs/ systems/ tissues/ cells/ organisms) that have the same function and shape. 15. Examples of tissue include
8 16. (Organs/ Systems/ Tissues/ Cells/ Organisms) are different tissues working together to carry out special function. 17. Organs are several different (organs/ systems/ tissues/ cells/ organisms) working together to carry out special function. 18. Examples of organ include 19. Which of the following is NOT an organ? [-A-] Muscle [-B-] Brain [-C-] Stomach [-D-] Nerve [-E-] Eye 20. (Organs/ Systems/ Tissues/ Cells/ Organisms) is a group of organs whose functions are closely related. 21. A system is a group of (organs/ systems/ tissues/ cells/ organisms) whose functions are closely related. 22. The digestive system including mouth, stomach and intestine is an example of a (organ/ system/ tissue/ cell/ organism). 23. A/An (organ/ system/ tissue/ cell/ organism) is made up of different organs and systems working together to produce a complete plant or animal. Chapter 3: Chemistry for Biology Section : 1 Molecular Biology 01. The approximate size of the cell is
9 02. Atoms are made up of,, and. 03. Which of the following statements is TRUE? [-A-] Atoms contain electrons and protons only. [-B-] The nucleus of an atom contains protons and neutrons. [-C-] The nucleus of an atom contains electrons. [-D-] Electrons have a positive charge. 04. Protons have A. positive charge and is much larger and heavier than electrons. B. negative charge and is larger and heavier than the electrons C. no charge and is much smaller than the electrons D. protons positive charge and are found around the nucleus of the atom 05. Protons have: A. Positive charge B. Negative charge C. No charge D. It is impossible to predict the charge on a proton as it is inside the nucleus. 06. Electrons have: A. Positive charge B. Negative charge C. No charge D. It is impossible to predict the charge on a proton as it is inside the nucleus 07. The electrical attractions in an atom keep the (electrons/ protons/ neutrons) near the nucleus. Section : 2 Symbols and Formulae 08. Name the following symbols of atoms: H: He: C: O: N: S: Na:
10 09. 2 S represents: [-A-] An atom of sodium made up of two molecules joined together [-B-] Two separate sulphur atoms [-C-] A molecule of sulphur made up of two atoms joined together [-D-] A molecule of sodium made up of two atoms joined together [-E-] An atom of sulphur made up of two molecules joined together Section : 3 Essential Compounds in Cells and Their Properties 10. (Organic/ Inorganic) compounds are substances that contain carbon and hydrogen. 11. (Organic/ Inorganic) compounds are substances that don t contain carbon except CO Which of the following are inorganic molecules? [-A-] Fe2O3 [-C-] C2H5OH [-E-] C6H12O6 [-B-] CO2 [-D-] H2O 13. Which of the following are inorganic compounds? [-A-] Fe2O3 [-C-] NH3 [-E-] All of the above [-B-] CO2 [-D-] H2O 14. What is the general formula of amino acids? 15. There are (20/ 25/ 30) amino acids in nature. Amino acids differ from each other by their (acid/ base/ radical) group. Section : 5 Use of Equations in Biology Enzymes
11 16. Glucose + Oxygen Water + Carbon dioxide + Energy A. Circle the reactants. B. Underline the products. C. State the conditions necessary for the reaction. 17. What are the reactants in the following chemical equation? Zn + CuSO4 Zn SO4 + Cu A. Zinc and copper B. Zinc sulfate and copper C. Zinc and copper (II) sulfate D. Only zinc
12 Chapter 4 : Molecules Found in Cells Section : 1 Proteins 01. Structural proteins: [-A-] are used as tools [-B-] are used as building material [-C-] are always enzymes [-D-] control chemical reactions 02. The protein used as a chemical tool is called a (structural/ functional) protein. 03. What are the common elements in all proteins? 04. The basic units of proteins are 05. Which of the following is/are chemical structure of amino acids? 06. Proteins differ in,, and of amino acids. 07. The bond that joins one amino acid to another to form a polypeptide is called a: [-A-] A dipeptide bond [-B-] A peptide bond [-C-] A hydrogen bond [-D-] A polypeptide bond [-E-] None of the above
13 08. The shape of a protein is important because:- 1 if it is too large it won t fit inside the cell 2 if it is too long it denatures easily 3 it will take a long time for the cell to make it 4 it is important to perform its function 5 it makes it easier to leave the cell 09. Which of the following is TRUE when a protein is denatured? 1 it loses its shape 2 it loses its function 3 it should have been heated to temperature above 50oC 4 its damage is irreversible 5 it doesn t lose its function 10. A protein is denatured when it is heated to temperatures [-A-] below 30 O C [-B-] above 50 O C [-C-] below 40 O C [-D-] between 30 O C and 40 O C Section : 2 Lipids 11. Name the elements found in lipids. 12. Lipid includes the following groups of substances: 1. Fats 2. Oils 3. Peptides 4. Steroids [-A-] 1,2 only [-B-] 1,2,3 only [-C-] 1,2,4 only [-D-] 2,3,4 only 13. One molecule of a lipid is formed of (1, 2, 3) molecule of glycerol and (1, 2, 3) molecules of fatty acids.
14 14. Basic units of lipids are and. 15. Name the bond that joins a fatty acid to glycerol. 16. The diagram below represents a: [-A-] fatty acid molecule [-B-] protein molecule [-C-] glycerol molecule [-D-] lipid molecule 17. The part labeled (1) in the diagram below represents: 2 1 [-A-] lipid [-B-] fatty acid [-C-] glycerol [-D-] ester bond 18. The part labeled (2) in question (17) represents: [-A-] lipid [-B-] fatty acid [-C-] glycerol [-D-] ester bond 19. Which of the following is (are) a function(s) of a lipid? 1. Energy store 2. Insulates the body 3. Formation of cell membranes 4. Protein synthesis
15 5. all of the above Section : 3 Carbohydrates 20. Name the elements found in carbohydrates. 21. Carbohydrates are classified according to their. 1 elements they contain 2 sweetness 3 size 22. Name the bond between the two glucose molecules in maltose. 23. The bond labeled A in the diagram below is a : glucose [-A-] peptide bond [-B-] hydrogen bond [-C-] dipeptide bond [-D-] glycosidic bond A 24. is a function of carbohydrate. 1 Formation of cell membranes 2 Insulation the body 3 Being a readily source of energy 4 Protein synthesis 25. Which of the following form cell walls in plants? 1 Proteins 2 Lipids 3 Carbohydrate (Cellulose) 4 Vitamins 5 Nucleic acids
16 Section : 4 Nucleic Acids 26. The basic unit of nucleic acid is (ribose, organic base, phosphate, nucleotides). 27. Which of the following is (are) a nucleic acid (s) involved in protein synthesis? 1 RNA 2 steroids 3 carbohydrates 4 glycerol 5 DNA 28. Nucleic acids are essential for. Military High School AL- Ain
17 Grade 10 &11 Biology Sample Questions Answer key Student Name: Computer #: Chapter 1: Cells In all multiple choice questions, more than answer could be correct Section : 1 What Is a Cell? 01. The basic unit of all living organisms is called (nucleus/ atom/ cell). 02. What are the three basic structures in all living cells?
18 Cell membrane nucleus- cytoplasm 03. Label the following animal cell. 04. Label the following plant cell. 05. What is the main function of the cell membrane? Holds the cell contents and controls which substances are allowed to enter and leave the cell.
19 06. (Cell membrane/ Cell wall/ Nucleus/ Cytoplasm) is a thin boundary enclosing the cytoplasm that control substances that enter and leave the cell. 4. Know the function of the cytoplasm 07. What is the main function of the cytoplasm? Cytoplasm is the site of the cell s reaction. 08. What is an organelle? Give three examples of organelles. Organelles : are tiny sub-cellular objects which have a specific functions in the cytoplasm. Examples: mitochondria, chloroplasts, ribosomes and the nucleus (largest organelle) 09. What are the main functions of the nucleus? 1. Contains chromosomes, which carry heredity information. 2. Controls cell division 3. Controls type and quantity of proteins produced into the cytoplasm. 10. Which of the following is/are NOT a function of a nucleus? [-A-] protect the cell [-B-] site of cell s reactions [-C-] carry hereditary information [-D-] control cell division [-E-] A & B 11. (Starch grain/ Vacuole/ Mitochondria/ Nucleus) in a living cell produces energy from food substances. 12. Mitochondria are found in larger quantity in :- [-A-] skin [-B-] cheek cells [-C-] liver cells [-D-] muscle cells 13. What is the main function of the ribosomes? Synthesis of proteins. 14. What types of cells contain chloroplasts? Plant cells.
20 15. What is the function of chloroplasts? Convert the sun s energy into chemical energy used for making glucose from carbon dioxide water by photosynthesis. 16. (Mitochondria/ Ribosome/ Chloroplast/ Starch grain) is present only in plant cells, and it makes glucose from carbon dioxide and water by a process called photosynthesis. Section : 2 Plant Cells Compared with Animal Cells 17. is used as a food store in plant cells. (Cell wall/ Cell membrane/ Starch grains) 18. makes plant cells firm. ( Cytoplasm / Vacuole/ Starch grains) 19. support and protect plant cell. (Cell wall/ Cell membrane/ Cytoplasm) 20. Match the following parts of a light microscope to its corresponding function: 1. Diaphragm (d) a. is used to the specimen in focus 2. Stage (f) b. magnify specimen 3. Course adjustment knob (a) c. is used to observe a specimen 4. Objective lens (b) d. control amount of light 5. Fine adjustment knob (g) e. reflect light 6. Mirror (e) f. hold the specimen 7. Eye piece lens (c) g. is used to see specimen clearly 21. Label the following parts of a microscope:
21
22 Chapter 2: Tissues and Organs Section : 1 The Electron Microscope Compared to Light Microscope Concept : 01. The electron microscope uses beams of (light/ electrons/ protons) instead of beams of light. 02. What is the maximum magnification of an electron microscope? Up to x 500, The image seen under the electron microscope is (of) (lower/ higher) when compared to that of light microscope. 04. Which of the following best describes an electron microscope? Resolution Ease of use magnification A High Easy Up to 1000 times B High Difficult Up to 500,000 times C Low Difficult Up to 1500 times D Low Easy Up to 500,000 times 05. Which of the following is an image of the electron microscope? A B C
23 06. State two advantages of using an electron microscope. 1. Very high magnification 2. Very high resolution 07. State two disadvantages of using an electron microscope. 1. Cells are killed and dehydrated 2. Some preparation treatments produce artificial artifacts 3. Very expensive 4. Requires specialist training Section : 2 Cell Division and Cell Specialization 08. The cell starts to divide when it s (fully or completely/ partial) grown. 09. What basic structure divides first in the cell during cell division of an animal cell? The nucleus 10. Arrange the stages of animal cell division in the correct order. B E D A C - F Two daughter cells are formed 11. What is the result of the last stage in a cell division? 12. All the following happen during plant cell division EXCEPT which of the following? [-A-] The nucleus divides at first. [-B-] Vacuoles join to form one vacuole in one cell. [-C-] new cell wall separates the two new nuclei. [-D-] The squeezing or narrowing of the cytoplasm divides the cell into two cells. [-E-] The nucleus is not the first structure to divide.
24 13. (Organs/ Systems/ Tissues/ Cells/ Organisms) are groups of cells that have the same shape and function. 14. A tissue is a group of similar (organs/ systems/ tissues/ cells/ organisms) that have the same function and shape. 15. Examples of tissue include Bone, nerve, or muscle in animals. tissues of the leaf, examples epidermis and palisade 16. (Organs/ Systems/ Tissues/ Cells/ Organisms) are different tissues working together to carry out special function. 17. Organs are several different (organs/ systems/ tissues/ cells/ organisms) working together to carry out special function. 18. Examples of organ include In animals: heart, lungs, intestines, brain, eyes etc. In plants: root, stem and leaves. 19. Which of the following is NOT an organ? [-A-] Muscle [-B-] Brain [-C-] Stomach [-D-] Nerve [-E-] Eye 20. (Organs/ Systems/ Tissues/ Cells/ Organisms) is a group of organs whose functions are closely related. 21. A system is a group of (organs/ systems/ tissues/ cells/ organisms) whose functions are closely related. 22. The digestive system including mouth, stomach and intestine is an example of a (organ/ system/ tissue/ cell/ organism). 23. A/An (organ/ system/ tissue/ cell/ organism) is made up of different organs and systems working together to produce a complete plant or animal.
25 Chapter 3: Chemistry for Biology Section : 1 Molecular Biology 01. The approximate size of the cell is 10 microns 02. Atoms are made up of,, neutrons electrons and. protons 04. Which of the following statements is TRUE? [-A-] Atoms contain electrons and protons only. [-B-] The nucleus of an atom contains protons and neutrons. [-C-] The nucleus of an atom contains electrons. [-D-] Electrons have a positive charge. 05. Protons have [-A-] positive charge and is much larger and heavier than electrons. [-B-] negative charge and is larger and heavier than the electrons [-C-] no charge and is much smaller than the electrons [-D- ]protons positive charge and are found around the nucleus of the atom 06. Protons have: A. Positive charge B. Negative charge C. No charge D. It is impossible to predict the charge on a proton as it is inside the nucleus. 07. Electrons have: A. Positive charge B. Negative charge C. No charge D. It is impossible to predict the charge on a proton as it is inside the nucleus 08. The electrical attractions in an atom keep the (electrons/ protons/ neutrons) near the nucleus.
26 Section : 2 Symbols and Formulae 09. Name the following symbols of atoms: Hydrogen H: He: Helium C: Carbon O: Oxygen N: Nitrogen S: Sulfur Na: Sodium S represents: [-A-] An atom of sodium made up of two molecules joined together [-B-] Two separate sulphur atoms [-C-] A molecule of sulphur made up of two atoms joined together [-D-] A molecule of sodium made up of two atoms joined together [-E-] An atom of sulphur made up of two molecules joined together Section : 3 Essential Compounds in Cells and Their Properties 11. (Organic/ Inorganic) compounds are substances that contain carbon and hydrogen. 12. (Organic/ Inorganic) compounds are substances that don t contain carbon except CO Which of the following are inorganic molecules? [-A-] Fe2O3 [-C-] C2H5OH [-E-] C6H12O6 [-B-] CO2 [-D-] H2O 14. Which of the following are inorganic compounds? [-A-] Fe2O3 [-C-] NH3 [-E-] All of the above [-B-] CO2 [-D-] H2O 15. What is the general formula of amino acids?
27 16. There are (20/ 25/ 30) amino acids in nature. Amino acids differ from each other by their (acid/ base/ radical) group Section : 5 Use of Equations in Biology Enzymes 17. Glucose + Oxygen Water + Carbon dioxide + Energy A. Circle the reactants. B. Underline the products. Enzymes C. State the conditions necessary for the reaction. 18. What are the reactants in the following chemical equation? Zn + CuSO4 Zn SO4 + Cu key A. Zinc and copper Zn: zinc B. Zinc sulfate and copper Cu: copper C. Zinc and copper (II) sulfate SO4: sulfate D. Only zinc CuSO4: copper(ii) sulfate Chapter 4 : Molecules Found in Cells Section : 1 Proteins 01. Structural proteins: [-A-] are used as tools [-B-] are used as building material [-C-] are always enzymes
28 [-D-] control chemical reactions 02. The protein used as a chemical tool is called a (structural/ functional) protein. 03. What are the common elements in all proteins? Carbon, hydrogen, oxygen and nitrogen The basic units of proteins are amino acids 05. Which of the following is/are chemical structure of amino acids? H 1 NH2 C COOH 2 R COOH R 3 R NH2 4 R CO NH Proteins differ in,, and of amino acids. 07. The bond that joins one amino acid to another to form a polypeptide is called a: [-A-] A dipeptide bond [-B-] A peptide bond [-C-] A hydrogen bond [-D-] A polypeptide bond [-E-] None of the above number type sequence 08. The shape of a protein is important because:- 1 if it is too large it won t fit inside the cell 2 if it is too long it denatures easily 3 it will take a long time for the cell to make it 4 it is important to perform its function 5 it makes it easier to leave the cell 09. Which of the following is TRUE when a protein is denatured? 1 it loses its shape 2 it loses its function 3 it should have been heated to temperature above 50oC
29 4 its damage is irreversible 5 it doesn t lose its function 10. A protein is denatured when it is heated to temperatures [-A-] below 30 O C [-B-] above 50 O C [-C-] below 40 O C [-D-] between 30 O C and 40 O C Section : 2 Lipids 11. Name the elements found in lipids. Carbon, hydrogen and oxygen 12. Lipid includes the following groups of substances: 1. Fats 2. Oils 3. Peptides 4. Steroids [-A-] 1,2 only [-B-] 1,2,3 only [-C-] 1,2,4 only [-D-] 2,3,4 only One molecule of a lipid is formed of (1, 2, 3) molecule of glycerol and (1, 2, 3) molecules of fatty acids. glycerol 14. Basic units of lipids are and. 15. Name the bond that joins a fatty acid to glycerol. fatty acids Ester bond
30 16. The diagram below represents a: [-A-] fatty acid molecule [-B-] protein molecule [-C-] glycerol molecule [-D-] lipid molecule 17. The part labeled (1) in the diagram below represents: 2 1 [-A-] lipid [-B-] fatty acid [-C-] glycerol [-D-] ester bond 18. The part labeled (2) in question (17) represents: [-A-] lipid [-B-] fatty acid [-C-] glycerol [-D-] ester bond 19. Which of the following is (are) a function(s) of a lipid? 1. Energy store 2. Insulates the body 3. Formation of cell membranes 4. Protein synthesis 5. all of the above Section : 3 Carbohydrates 20. Name the elements found in carbohydrates. Carbon, hydrogen and oxygen
31 21. Carbohydrates are classified according to their. 1 elements they contain 2 sweetness 3 size 22. Name the bond between the two glucose molecules in maltose. Glycosidic bond 23. The bond labeled A in the diagram below is a : glucose [-A-] peptide bond [-B-] hydrogen bond [-C-] dipeptide bond [-D-] glycosidic bond A 24. is a function of carbohydrate. 1 Formation of cell membranes 2 Insulation the body 3 Being a readily source of energy 4 Protein synthesis 25. Which of the following form cell walls in plants? 1 Proteins 2 Lipids 3 Carbohydrate (Cellulose) 4 Vitamins 5 Nucleic acids Section : 4 Nucleic Acids 26. The basic unit of nucleic acid is (ribose, organic base, phosphate, nucleotides).
32 27. Which of the following is (are) a nucleic acid (s) involved in protein synthesis? 1 RNA 2 steroids 3 carbohydrates 4 glycerol 5 DNA 28. Nucleic acids are essential for. protein synthesis
The diagram below represents levels of organization within a cell of a multicellular organism.
 STATION 1 1. Unlike prokaryotic cells, eukaryotic cells have the capacity to a. assemble into multicellular organisms b. establish symbiotic relationships with other organisms c. obtain energy from the
STATION 1 1. Unlike prokaryotic cells, eukaryotic cells have the capacity to a. assemble into multicellular organisms b. establish symbiotic relationships with other organisms c. obtain energy from the
BIOCHEMISTRY 10/9/17 CHEMISTRY OF LIFE. Elements: simplest form of a substance - cannot be broken down any further without changing what it is
 BIOCHEMISTRY CHEMISTRY OF LIFE Elements: simplest form of a substance - cannot be broken down any further without changing what it is THE ATOM Just like cells are the basic unit of life, the ATOM is the
BIOCHEMISTRY CHEMISTRY OF LIFE Elements: simplest form of a substance - cannot be broken down any further without changing what it is THE ATOM Just like cells are the basic unit of life, the ATOM is the
the spatial arrangement of atoms in a molecule and the chemical bonds that hold the atoms together Chemical structure Covalent bond Ionic bond
 Chemical structure the spatial arrangement of atoms in a molecule and the chemical bonds that hold the atoms together Covalent bond bond formed by the sharing of valence electrons between atoms Ionic bond
Chemical structure the spatial arrangement of atoms in a molecule and the chemical bonds that hold the atoms together Covalent bond bond formed by the sharing of valence electrons between atoms Ionic bond
Base your answers to questions 1 and 2 on the diagram below which represents a typical green plant cell and on your knowledge of biology.
 Base your answers to questions 1 and 2 on the diagram below which represents a typical green plant cell and on your knowledge of biology. 5. Which letter corresponds to that of the endoplasmic reticulum?
Base your answers to questions 1 and 2 on the diagram below which represents a typical green plant cell and on your knowledge of biology. 5. Which letter corresponds to that of the endoplasmic reticulum?
Chapter 3.1 Chemistry of Life
 Life Science Chapter 3: Cell Processes 1. Chemistry of Life 2. Moving Cellular Materials 3. Energy for Life http://www.connecticutvalleybiological.com/cell processes vhs p 14026.html Chapter 3.1 Chemistry
Life Science Chapter 3: Cell Processes 1. Chemistry of Life 2. Moving Cellular Materials 3. Energy for Life http://www.connecticutvalleybiological.com/cell processes vhs p 14026.html Chapter 3.1 Chemistry
Nature of matter. Chemical bond is a force that joins atoms
 Nature of matter Atom the smallest unit of matter that cannot be broken down by chemical means The subatomic particles of an atom consist of protons, neutrons and electrons Element is a pure substance
Nature of matter Atom the smallest unit of matter that cannot be broken down by chemical means The subatomic particles of an atom consist of protons, neutrons and electrons Element is a pure substance
Teacher Instructions
 Teacher Instructions To print handouts for students Go to File print, change Print what: to handouts, change # per page if desired to enlarge slides on page Change Print range to slides and type in slide
Teacher Instructions To print handouts for students Go to File print, change Print what: to handouts, change # per page if desired to enlarge slides on page Change Print range to slides and type in slide
Study Guide: Basic Chemistry, Water, Life Compounds and Enzymes
 Study Guide: Basic Chemistry, Water, Life Compounds and Enzymes 1. Lipids are good energy-storage molecules because a) the can absorb a large amount of energy while maintaining a constant temperature b)
Study Guide: Basic Chemistry, Water, Life Compounds and Enzymes 1. Lipids are good energy-storage molecules because a) the can absorb a large amount of energy while maintaining a constant temperature b)
A Brief Overview of Biochemistry. And I mean BRIEF!
 A Brief Overview of Biochemistry And I mean BRIEF! Introduction A. Chemistry deals with the composition of substances and how they change. B. A knowledge of chemistry is necessary for the understanding
A Brief Overview of Biochemistry And I mean BRIEF! Introduction A. Chemistry deals with the composition of substances and how they change. B. A knowledge of chemistry is necessary for the understanding
Foundation Cell Biology
 Foundation Cell Biology Electron vs Light Microscope Light use light and lenses to magnify specimen Electron use a beam of electrons to form an image Electron higher magnification and higher resolution
Foundation Cell Biology Electron vs Light Microscope Light use light and lenses to magnify specimen Electron use a beam of electrons to form an image Electron higher magnification and higher resolution
2.1 Atoms, Ions, and Molecules
 2.1 Atoms, Ions, and Molecules Living things consist of atoms of different elements. An atom is the smallest basic unit of matter. An element is one type of atom. 6 elements make up 99% of all living things
2.1 Atoms, Ions, and Molecules Living things consist of atoms of different elements. An atom is the smallest basic unit of matter. An element is one type of atom. 6 elements make up 99% of all living things
Chapter 002 The Chemistry of Biology
 Chapter 002 The Chemistry of Biology Multiple Choice Questions 1. Anything that occupies space and has mass is called A. Atomic B. Living C. Matter D. Energy E. Space 2. The electrons of an atom are A.
Chapter 002 The Chemistry of Biology Multiple Choice Questions 1. Anything that occupies space and has mass is called A. Atomic B. Living C. Matter D. Energy E. Space 2. The electrons of an atom are A.
Chapter 2. Introduction: Chapter Chemical Basis of Life. Structure of Matter:
 Chapter 2.1-2.2 Read text 2.1 and describe why chemistry is important in understanding life. Read text 2.2 and discuss how atomic structure determines how atoms interact. Also describe the types of chemical
Chapter 2.1-2.2 Read text 2.1 and describe why chemistry is important in understanding life. Read text 2.2 and discuss how atomic structure determines how atoms interact. Also describe the types of chemical
Biochemistry. The Chemistry of Life
 Biochemistry The Chemistry of Life Biochemistry The life processes (Chapter 1) are chemical in nature. Chemical reactions occur in life. Living things are made of chemical compounds. The Atom- The Basic
Biochemistry The Chemistry of Life Biochemistry The life processes (Chapter 1) are chemical in nature. Chemical reactions occur in life. Living things are made of chemical compounds. The Atom- The Basic
Animal Cell Organelles. Plant Cell. Organelle. Cell Wall. Chloroplasts. Vacuole
 Cell Biology Higher Electron vs Light Microscope Light use light and lenses to magnify specimen Electron use a beam of electrons to form an image Electron higher magnification and higher resolution Electron
Cell Biology Higher Electron vs Light Microscope Light use light and lenses to magnify specimen Electron use a beam of electrons to form an image Electron higher magnification and higher resolution Electron
Chapter 2 Concepts of Chemistry
 Anatomy Physiology and Disease for the Health Professions 3rd Edition Booth Test Bank Full Download: http://testbanklive.com/download/anatomy-physiology-and-disease-for-the-health-professions-3rd-edition-booth-te
Anatomy Physiology and Disease for the Health Professions 3rd Edition Booth Test Bank Full Download: http://testbanklive.com/download/anatomy-physiology-and-disease-for-the-health-professions-3rd-edition-booth-te
2.1 CELL STRUCTURE. The cell is the smallest unit of living organisms that shows the characteristics of life.
 2.1.1 Microscopy The cell is the smallest unit of living organisms that shows the characteristics of life. A general introduction to the microscope. The light microscope All cells are microscopic which
2.1.1 Microscopy The cell is the smallest unit of living organisms that shows the characteristics of life. A general introduction to the microscope. The light microscope All cells are microscopic which
Chapter 7 Cell Structure
 Chapter 7 Cell Structure Mr. C. Biology 1 07 Cell Structure Chapter 7 Cell Structure All living things are made of cells. Cells are made up of 3 main parts, Cell Membrane A skin that controls what enters
Chapter 7 Cell Structure Mr. C. Biology 1 07 Cell Structure Chapter 7 Cell Structure All living things are made of cells. Cells are made up of 3 main parts, Cell Membrane A skin that controls what enters
What are the building blocks of life?
 Why? What are the building blocks of life? From the smallest single-celled organism to the tallest tree, all life depends on the properties and reactions of four classes of organic (carbon-based) compounds
Why? What are the building blocks of life? From the smallest single-celled organism to the tallest tree, all life depends on the properties and reactions of four classes of organic (carbon-based) compounds
Chapter 2. The Chemistry of Life
 Chapter 2 The Chemistry of Life Introduction Cells, tissues and organs composed of chemicals Chemical reactions important for function Chemistry is the study of elements, compounds, chemical reactions,
Chapter 2 The Chemistry of Life Introduction Cells, tissues and organs composed of chemicals Chemical reactions important for function Chemistry is the study of elements, compounds, chemical reactions,
Unit 2: The Properties of Water, Organic Macromolecules, Enzymes, Digestion (questions)
 Table 1: ph Values of Common Substances 1. Observe Table 1, which substance has the highest concentration of H+ ions? a. Water b. Baking soda solution c. Lemon juice d. Sodium hydroxide solution 2. Which
Table 1: ph Values of Common Substances 1. Observe Table 1, which substance has the highest concentration of H+ ions? a. Water b. Baking soda solution c. Lemon juice d. Sodium hydroxide solution 2. Which
Name # Class Date Regents Review: Cells & Cell Transport
 Name # Class Date Regents Review: Cells & Cell Transport 1. All of the following are true regarding cells except? A) All cells have genetic material B) All cells have cell walls C) All cells have plasma
Name # Class Date Regents Review: Cells & Cell Transport 1. All of the following are true regarding cells except? A) All cells have genetic material B) All cells have cell walls C) All cells have plasma
Unit 2: Part 1 Matter & Energy in Ecosystems What elements am I made of?
 Unit 2: Part 1 Matter & Energy in Ecosystems What elements am I made of? I. Introduction: Matter in Ecosystems A. Organisms are composed of matter (anything that takes up space and has mass) B. Organisms
Unit 2: Part 1 Matter & Energy in Ecosystems What elements am I made of? I. Introduction: Matter in Ecosystems A. Organisms are composed of matter (anything that takes up space and has mass) B. Organisms
CELL PRACTICE TEST
 Name: Date: 1. As a human red blood cell matures, it loses its nucleus. As a result of this loss, a mature red blood cell lacks the ability to (1) take in material from the blood (2) release hormones to
Name: Date: 1. As a human red blood cell matures, it loses its nucleus. As a result of this loss, a mature red blood cell lacks the ability to (1) take in material from the blood (2) release hormones to
is a substance that reduces the activity of an enzyme by entering the active site in place of the substrate whose structure it mimics.
 is a substance that reduces the activity of an enzyme by entering the active site in place of the substrate whose structure it mimics. Competitive Inhibitor Identify the following molecule: Polysaccharide
is a substance that reduces the activity of an enzyme by entering the active site in place of the substrate whose structure it mimics. Competitive Inhibitor Identify the following molecule: Polysaccharide
Reason... (2) Reason... (2) Reason... (2)
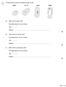 1 The figure below shows four different types of cell. (a) Which cell is a plant cell? Give one reason for your answer. Cell... Reason... (b) Which cell is an animal cell? Give one reason for your answer.
1 The figure below shows four different types of cell. (a) Which cell is a plant cell? Give one reason for your answer. Cell... Reason... (b) Which cell is an animal cell? Give one reason for your answer.
Copy into Note Packet and Return to Teacher
 Copy into Note Packet and Return to Teacher Section 1: Nature of Matter Objectives: Differentiate between atoms and elements. Analyze how compounds are formed. Distinguish between covalent bonds, hydrogen
Copy into Note Packet and Return to Teacher Section 1: Nature of Matter Objectives: Differentiate between atoms and elements. Analyze how compounds are formed. Distinguish between covalent bonds, hydrogen
DO NOT WRITE ON THIS TEST Topic 3- Cells and Transport
 Topic 3- Cells and Transport 1. All of the following are true regarding cells except? A) All cells have genetic material B) All cells have cell walls C) All cells have plasma membranes D) All cells can
Topic 3- Cells and Transport 1. All of the following are true regarding cells except? A) All cells have genetic material B) All cells have cell walls C) All cells have plasma membranes D) All cells can
Honors Biology summer assignment. Review the notes and study them. There will be a test on this information the 1 st week of class
 Honors Biology summer assignment Review the notes and study them. There will be a test on this information the 1 st week of class Biomolecules Molecules that make up living things. There are 4 molecules
Honors Biology summer assignment Review the notes and study them. There will be a test on this information the 1 st week of class Biomolecules Molecules that make up living things. There are 4 molecules
Chapter 2 Chemical Aspects of Life
 Chapter 2 Chemical Aspects of Life Multiple Choice Questions 1. Anything that has weight and occupies space can be described as A. an atom. B. matter. C. a compound. D. a molecule. #1 Learning Outcome:
Chapter 2 Chemical Aspects of Life Multiple Choice Questions 1. Anything that has weight and occupies space can be described as A. an atom. B. matter. C. a compound. D. a molecule. #1 Learning Outcome:
The Chemistry of Biology
 The Chemistry of Biology Life depends on chemistry. Living things are composed of chemical compounds. If order to understand biology, one must first understand the chemistry of life. I. The Nature of Matter
The Chemistry of Biology Life depends on chemistry. Living things are composed of chemical compounds. If order to understand biology, one must first understand the chemistry of life. I. The Nature of Matter
Garden City High School Science Department Honors Living Environment Summer Assignment
 Garden City High School Science Department Honors Living Environment Summer Assignment Each student anticipating enrollment in the Honors Living Environment course will be required to complete a summer
Garden City High School Science Department Honors Living Environment Summer Assignment Each student anticipating enrollment in the Honors Living Environment course will be required to complete a summer
Biology Unit 2 Chemistry of Life (Ch. 6) Guided Notes
 Name Biology Unit 2 Chemistry of Life (Ch. 6) Guided Notes Atoms, Elements, and Chemical Bonding I can draw atom models and identify the # protons, # neutrons, and # electrons in an atom. I can identify
Name Biology Unit 2 Chemistry of Life (Ch. 6) Guided Notes Atoms, Elements, and Chemical Bonding I can draw atom models and identify the # protons, # neutrons, and # electrons in an atom. I can identify
Unit Two Chemistry of the Human Body
 I. Introduction to atoms Unit Two Chemistry of the Human Body A. Chemistry is the branch of science that concerns itself with the structure of matter, including the interaction between atoms. 1. Atoms-
I. Introduction to atoms Unit Two Chemistry of the Human Body A. Chemistry is the branch of science that concerns itself with the structure of matter, including the interaction between atoms. 1. Atoms-
Biology Midterm Review
 Biology Midterm Review Unit 1 Keystone Objectives: A.1.1, A.1.2, B.4.1.1 1.1 Biology explores life from the global to the microscopic level. Put the levels of organization in order, starting with subatomic
Biology Midterm Review Unit 1 Keystone Objectives: A.1.1, A.1.2, B.4.1.1 1.1 Biology explores life from the global to the microscopic level. Put the levels of organization in order, starting with subatomic
MULTIPLE CHOICE. Circle the one alternative that best completes the statement or answers the question.
 Summer Work Quiz - Molecules and Chemistry Name MULTIPLE CHOICE. Circle the one alternative that best completes the statement or answers the question. 1) The four most common elements in living organisms
Summer Work Quiz - Molecules and Chemistry Name MULTIPLE CHOICE. Circle the one alternative that best completes the statement or answers the question. 1) The four most common elements in living organisms
Discovery of the Cell
 Cells Chapter 4 Discovery of the Cell 1665 Robert Hooke used a microscope to examine a piece of cork. He saw little boxes in the cork and called them cells. 1673 Anton van Leeuwenhoek was the first person
Cells Chapter 4 Discovery of the Cell 1665 Robert Hooke used a microscope to examine a piece of cork. He saw little boxes in the cork and called them cells. 1673 Anton van Leeuwenhoek was the first person
Semester 1 Study Guide Name Period
 2017-2018 Semester 1 Study Guide Name Period Chapter 1: Scientific Method and Microscopes (p. 2-31 and A-1 through A-17) Vocab: experiment, hypothesis, scientific theory, scientific law, controlled experiment,
2017-2018 Semester 1 Study Guide Name Period Chapter 1: Scientific Method and Microscopes (p. 2-31 and A-1 through A-17) Vocab: experiment, hypothesis, scientific theory, scientific law, controlled experiment,
The Cell. The basic unit of all living things
 The Cell The basic unit of all living things 1 Robert Hooke was the first to name the cell (1665) 2 The Cell Theory The cell is the unit of Structure of all living things. The cell is the unit of Function
The Cell The basic unit of all living things 1 Robert Hooke was the first to name the cell (1665) 2 The Cell Theory The cell is the unit of Structure of all living things. The cell is the unit of Function
13. The diagram below shows two different kinds of substances, A and B, entering a cell.
 Name 1. In the binomial system of nomenclature, which two classification groups provide the scientific name of an organism? A) kingdom and phylum B) phylum and species C) kingdom and genus D) genus and
Name 1. In the binomial system of nomenclature, which two classification groups provide the scientific name of an organism? A) kingdom and phylum B) phylum and species C) kingdom and genus D) genus and
BIOCHEMISTRY The Chemistry of Living Things
 BIOCHEMISTRY The Chemistry of Living Things Biochemistry, an area that many students find pretty challenging (difficult). While the ideas are abstract, much of the material boils down to memorization.
BIOCHEMISTRY The Chemistry of Living Things Biochemistry, an area that many students find pretty challenging (difficult). While the ideas are abstract, much of the material boils down to memorization.
Basic Chemistry. Chemistry Review. Bio 250: Anatomy & Physiology
 Basic Chemistry Bio 250: Anatomy & Physiology Chemistry Review It is going to be your responsibility to review the basic principles of chemistry you learned in BIO 101 This basic set of notes will help
Basic Chemistry Bio 250: Anatomy & Physiology Chemistry Review It is going to be your responsibility to review the basic principles of chemistry you learned in BIO 101 This basic set of notes will help
2-1 The Nature of Matter. Atoms
 2-1 The Nature of Matter Atoms What do we call the smallest unit of matter? Who named it? What does it mean in Greek? How many atoms would make a row 1cm long? What does this indicate? Atoms are made up
2-1 The Nature of Matter Atoms What do we call the smallest unit of matter? Who named it? What does it mean in Greek? How many atoms would make a row 1cm long? What does this indicate? Atoms are made up
Microscope History Robert Hooke
 1 Microscope History Robert Hooke First described cells in 1665. He viewed thin slices of cork and compared the boxy partitions he observed to the cells (small rooms) in a monastery. (1635 1702) 2 Microscope
1 Microscope History Robert Hooke First described cells in 1665. He viewed thin slices of cork and compared the boxy partitions he observed to the cells (small rooms) in a monastery. (1635 1702) 2 Microscope
STUDENT PACKET #1 Student Exploration: Cell Structure
 STUDENT PACKET #1 Student Exploration: Cell Structure Big Idea 14: Organization and Development of Living Organisms SC.6.L.14.1 Describe and identify patterns in the hierarchical organization of organisms
STUDENT PACKET #1 Student Exploration: Cell Structure Big Idea 14: Organization and Development of Living Organisms SC.6.L.14.1 Describe and identify patterns in the hierarchical organization of organisms
Chemistry of Life 10/1/2010. What makes up the chemistry of life?
 A. Students will be able to identify and define the parts of an atom. Chemistry of Life At the Completion of this Unit, Students will be able to: A. Identify and define the parts of an atom. B. Demonstrate
A. Students will be able to identify and define the parts of an atom. Chemistry of Life At the Completion of this Unit, Students will be able to: A. Identify and define the parts of an atom. B. Demonstrate
Biology Chapter 2: The Chemistry of Life. title 4 pictures, with color (black and white don t count!)
 33 Biology Chapter 2: The Chemistry of Life title 4 pictures, with color (black and white don t count!) 34 Chapter 2: The Chemistry of Life Goals Highlight all unknown words 35-36 Chapter 2: The Chemistry
33 Biology Chapter 2: The Chemistry of Life title 4 pictures, with color (black and white don t count!) 34 Chapter 2: The Chemistry of Life Goals Highlight all unknown words 35-36 Chapter 2: The Chemistry
Name: Date: Per: Chapter 2 & 3 Review ~ for Test on Friday September How many hydrogen atoms are in a molecule of water?
 Name: Date: Per: WATER Chapter 2 & 3 Review ~ for Test on Friday September 6 ~ Unit: Chemistry of Life 1. How many hydrogen atoms are in a molecule of water? How many oxygen atoms are in a molecule of
Name: Date: Per: WATER Chapter 2 & 3 Review ~ for Test on Friday September 6 ~ Unit: Chemistry of Life 1. How many hydrogen atoms are in a molecule of water? How many oxygen atoms are in a molecule of
2.1 Atoms, Ions, and Molecules. 2.1 Atoms, Ions, and Molecules. 2.1 Atoms, Ions, and Molecules. 2.1 Atoms, Ions, and Molecules
 All living things are based on atoms and their interactions. Living things consist of atoms of different elements. An atom is the smallest basic unit of matter. An element is one type of atom. ydrogen
All living things are based on atoms and their interactions. Living things consist of atoms of different elements. An atom is the smallest basic unit of matter. An element is one type of atom. ydrogen
NORTH CENTRAL HIGH SCHOOL NOTE & STUDY GUIDE. Honors Biology I
 NOTE/STUDY GUIDE: Unit 1-2, Biochemistry Honors Biology I, Mr. Doc Miller, M.Ed. North Central High School Name: Period: Seat #: Date: NORTH CENTRAL HIGH SCHOOL NOTE & STUDY GUIDE Honors Biology I Unit
NOTE/STUDY GUIDE: Unit 1-2, Biochemistry Honors Biology I, Mr. Doc Miller, M.Ed. North Central High School Name: Period: Seat #: Date: NORTH CENTRAL HIGH SCHOOL NOTE & STUDY GUIDE Honors Biology I Unit
Chapter 02. Lecture and Animation Outline
 Chapter 02 Lecture and Animation Outline To run the animations you must be in Slideshow View. Use the buttons on the animation to play, pause, and turn audio/text on or off. Please Note: Once you have
Chapter 02 Lecture and Animation Outline To run the animations you must be in Slideshow View. Use the buttons on the animation to play, pause, and turn audio/text on or off. Please Note: Once you have
SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question.
 Exam Name SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. Figure 2.1 Using Figure 2.1, match the following: 1) Lipid. 2) Functional protein. 3) Nucleotide.
Exam Name SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. Figure 2.1 Using Figure 2.1, match the following: 1) Lipid. 2) Functional protein. 3) Nucleotide.
Review_Unit 2 Biochemistry
 Review_Unit 2 Biochemistry Basic Chemistry 1. What is an element? A substance that cannot be broken down into smaller particles. 2. What are atoms? The smallest part of an element that still maintains
Review_Unit 2 Biochemistry Basic Chemistry 1. What is an element? A substance that cannot be broken down into smaller particles. 2. What are atoms? The smallest part of an element that still maintains
Ch 3: Chemistry of Life. Chemistry Water Macromolecules Enzymes
 Ch 3: Chemistry of Life Chemistry Water Macromolecules Enzymes Chemistry Atom = smallest unit of matter that cannot be broken down by chemical means Element = substances that have similar properties and
Ch 3: Chemistry of Life Chemistry Water Macromolecules Enzymes Chemistry Atom = smallest unit of matter that cannot be broken down by chemical means Element = substances that have similar properties and
PRESENTATION TITLE. Chemistry. Chemistry
 PRESENTATION TITLE Chemistry Chemistry Chemistry is the study of the smallest forms of matter and their interactions. Matter is anything that has mass and takes up space. Generally, chemistry deals with
PRESENTATION TITLE Chemistry Chemistry Chemistry is the study of the smallest forms of matter and their interactions. Matter is anything that has mass and takes up space. Generally, chemistry deals with
Topic 1: The Chemical Context of Life, Holtzclaw and Holtzclaw, 2014
 Name Block Topic 1: The Chemical Context of Life, Holtzclaw and Holtzclaw, 2014 1. Complete the vocabulary on a separate piece of paper. 2. What are the elements that make up most of living matter? What
Name Block Topic 1: The Chemical Context of Life, Holtzclaw and Holtzclaw, 2014 1. Complete the vocabulary on a separate piece of paper. 2. What are the elements that make up most of living matter? What
Cell Organelles Tutorial
 1 Name: Cell Organelles Tutorial TEK 7.12D: Differentiate between structure and function in plant and animal cell organelles, including cell membrane, cell wall, nucleus, cytoplasm, mitochondrion, chloroplast,
1 Name: Cell Organelles Tutorial TEK 7.12D: Differentiate between structure and function in plant and animal cell organelles, including cell membrane, cell wall, nucleus, cytoplasm, mitochondrion, chloroplast,
Unit 2: Basic Chemistry
 Unit 2: Basic Chemistry I. Matter and Energy A. Matter anything that occupies space and has mass (weight) B. Energy the ability to do work 1. Chemical 2. Electrical 3. Mechanical 4. Radiant C. Composition
Unit 2: Basic Chemistry I. Matter and Energy A. Matter anything that occupies space and has mass (weight) B. Energy the ability to do work 1. Chemical 2. Electrical 3. Mechanical 4. Radiant C. Composition
Chapter 6 Chemistry in Biology
 Section 1: Atoms, Elements, and Compounds Section 2: Chemical Reactions Section 3: Water and Solutions Section 4: The Building Blocks of Life Click on a lesson name to select. 6.1 Atoms, Elements, and
Section 1: Atoms, Elements, and Compounds Section 2: Chemical Reactions Section 3: Water and Solutions Section 4: The Building Blocks of Life Click on a lesson name to select. 6.1 Atoms, Elements, and
CELL PART Expanded Definition Cell Structure Illustration Function Summary Location ALL CELLS DNA Common in Animals Uncommon in Plants Lysosome
 CELL PART Expanded Definition Cell Structure Illustration Function Summary Location is the material that contains the Carry genetic ALL CELLS information that determines material inherited characteristics.
CELL PART Expanded Definition Cell Structure Illustration Function Summary Location is the material that contains the Carry genetic ALL CELLS information that determines material inherited characteristics.
Cell Review. 1. The diagram below represents levels of organization in living things.
 Cell Review 1. The diagram below represents levels of organization in living things. Which term would best represent X? 1) human 2) tissue 3) stomach 4) chloroplast 2. Which statement is not a part of
Cell Review 1. The diagram below represents levels of organization in living things. Which term would best represent X? 1) human 2) tissue 3) stomach 4) chloroplast 2. Which statement is not a part of
Biology. Mrs. Michaelsen. Types of cells. Cells & Cell Organelles. Cell size comparison. The Cell. Doing Life s Work. Hooke first viewed cork 1600 s
 Types of cells bacteria cells Prokaryote - no organelles Cells & Cell Organelles Doing Life s Work Eukaryotes - organelles animal cells plant cells Cell size comparison Animal cell Bacterial cell most
Types of cells bacteria cells Prokaryote - no organelles Cells & Cell Organelles Doing Life s Work Eukaryotes - organelles animal cells plant cells Cell size comparison Animal cell Bacterial cell most
Organelles & Cells Student Edition. A. chromosome B. gene C. mitochondrion D. vacuole
 Name: Date: 1. Which structure is outside the nucleus of a cell and contains DNA? A. chromosome B. gene C. mitochondrion D. vacuole 2. A potato core was placed in a beaker of water as shown in the figure
Name: Date: 1. Which structure is outside the nucleus of a cell and contains DNA? A. chromosome B. gene C. mitochondrion D. vacuole 2. A potato core was placed in a beaker of water as shown in the figure
2.1 Matter and Organic Compounds
 2.1 Matter and Organic Compounds Lesson 2.1: True or False Write true if the statement is true or false if the statement is false. 1. An atom is smaller than an element. 2. Organic compounds are found
2.1 Matter and Organic Compounds Lesson 2.1: True or False Write true if the statement is true or false if the statement is false. 1. An atom is smaller than an element. 2. Organic compounds are found
THE CELL THEORY (R+R+R+E+G+N+T+S) 3).
 CELL BIOLOGY All living things are made up of small individual units called cells. Cells are the smallest functioning living unit. Cells can not normally be seen with the naked eye. To usually observe
CELL BIOLOGY All living things are made up of small individual units called cells. Cells are the smallest functioning living unit. Cells can not normally be seen with the naked eye. To usually observe
2/25/2013. Electronic Configurations
 1 2 3 4 5 Chapter 2 Chemical Principles The Structure of Atoms Chemistry is the study of interactions between atoms and molecules The atom is the smallest unit of matter that enters into chemical reactions
1 2 3 4 5 Chapter 2 Chemical Principles The Structure of Atoms Chemistry is the study of interactions between atoms and molecules The atom is the smallest unit of matter that enters into chemical reactions
Biology Unit 4. Chemistry of Life
 Biology Unit 4 Chemistry of Life Elements Everything in our universe that has a mass and a volume is made of matter. Matter in its purest form is an element. There are 118 elements on the periodic table,
Biology Unit 4 Chemistry of Life Elements Everything in our universe that has a mass and a volume is made of matter. Matter in its purest form is an element. There are 118 elements on the periodic table,
Name: Period: Date: Multiple Choice Identify the choice that best completes the statement or answers the question.
 Name: Period: _ Date: _ Cell Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The invention of the microscope made it possible for people to discover a.
Name: Period: _ Date: _ Cell Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The invention of the microscope made it possible for people to discover a.
The Chemical Level of Organization
 Scuola di Ingegneria Industriale e dell Informazione Course 096125 (095857) Introduction to Green and Sustainable Chemistry The Chemical Level of Organization Prof. (and Ada Truscello) Dept. CMIC http://iscamap.chem.polimi.it/citterio/education/course-topics/
Scuola di Ingegneria Industriale e dell Informazione Course 096125 (095857) Introduction to Green and Sustainable Chemistry The Chemical Level of Organization Prof. (and Ada Truscello) Dept. CMIC http://iscamap.chem.polimi.it/citterio/education/course-topics/
Chemistry of Life. Chapter Two
 Chemistry of Life Chapter Two 1 Biology and Chemistry Biology = study of life Chemistry = study of matter and the changes it undergoes Matter anything that takes up space and has mass Life is made up of
Chemistry of Life Chapter Two 1 Biology and Chemistry Biology = study of life Chemistry = study of matter and the changes it undergoes Matter anything that takes up space and has mass Life is made up of
Chemical Basis of Life
 Chemical Basis of Life Jan 30 11:42 AM In order to understand digestion and nutrition, we need some basic biochemistry Chemistry studies the composition of matter and its changes as well as the change
Chemical Basis of Life Jan 30 11:42 AM In order to understand digestion and nutrition, we need some basic biochemistry Chemistry studies the composition of matter and its changes as well as the change
2.1 The Nature of Matter
 2.1 The Nature of Matter Lesson Objectives Identify the three subatomic particles found in atoms. Explain how all of the isotopes of an element are similar and how they are different. Explain how compounds
2.1 The Nature of Matter Lesson Objectives Identify the three subatomic particles found in atoms. Explain how all of the isotopes of an element are similar and how they are different. Explain how compounds
Elements and Isotopes
 Section 2-1 Notes Atoms Life depends on chemistry. The basic unit of matter is the atom. Atoms are incredibly small The subatomic particles that make up atoms are protons, neutrons, and electrons. Parts
Section 2-1 Notes Atoms Life depends on chemistry. The basic unit of matter is the atom. Atoms are incredibly small The subatomic particles that make up atoms are protons, neutrons, and electrons. Parts
AP BIOLOGY BIOCHEMISTRY MULTIPLE CHOICE EXAM (RAVEN CHAPTERS 2, 3)
 Period Date AP BIOLOGY BIOCHEMISTRY MULTIPLE CHOICE EXAM (RAVEN CHAPTERS 2, 3) 1. Which of the following is an example of a hydrogen bond? (90:09) A. The peptide bond between amino acids in a protein B.
Period Date AP BIOLOGY BIOCHEMISTRY MULTIPLE CHOICE EXAM (RAVEN CHAPTERS 2, 3) 1. Which of the following is an example of a hydrogen bond? (90:09) A. The peptide bond between amino acids in a protein B.
Chapter 6 The Chemistry of Life
 Chapter 6 The Chemistry of Life Atoms: The Building Blocks of Life Both living and non-living things have atoms Everything, living and non, is made of Atoms. An elements is something you can break down
Chapter 6 The Chemistry of Life Atoms: The Building Blocks of Life Both living and non-living things have atoms Everything, living and non, is made of Atoms. An elements is something you can break down
UNIT 1: BIOCHEMISTRY
 UNIT 1: BIOCHEMISTRY UNIT 1: Biochemistry Chapter 6.1: Chemistry of Life I. Atoms, Ions, and Molecules A. Living things consist of atoms of different elements 1. An atom is the smallest basic unit of matter
UNIT 1: BIOCHEMISTRY UNIT 1: Biochemistry Chapter 6.1: Chemistry of Life I. Atoms, Ions, and Molecules A. Living things consist of atoms of different elements 1. An atom is the smallest basic unit of matter
Chapter: Cell Processes
 Table of Contents Chapter: Cell Processes Section 1: Chemistry of Life Section 2: Moving Cellular Materials Section 3: Energy for Life 1 Chemistry of Life The Nature of Matter Matter is anything that has
Table of Contents Chapter: Cell Processes Section 1: Chemistry of Life Section 2: Moving Cellular Materials Section 3: Energy for Life 1 Chemistry of Life The Nature of Matter Matter is anything that has
Chapter 2 The Chemistry of Life
 Chapter 2 The Chemistry of Life I. Water Liquid Naturally occurring It expands liquid to solid Covers more than 75% of our surface Most abundant in living organisms most important inorganic compound for
Chapter 2 The Chemistry of Life I. Water Liquid Naturally occurring It expands liquid to solid Covers more than 75% of our surface Most abundant in living organisms most important inorganic compound for
CHEMISTRY. 2 Types of Properties Associated with Matter. Composition of Matter. Physical: properties that do not change the identity of the substance
 CHEMISTRY Composition of Matter Matter Mass Anything that occupies space and has mass Quantity of matter an object has Weight Pull of gravity on an object 2 Types of Properties Associated with Matter Physical:
CHEMISTRY Composition of Matter Matter Mass Anything that occupies space and has mass Quantity of matter an object has Weight Pull of gravity on an object 2 Types of Properties Associated with Matter Physical:
5. The cells in the liver that detoxify poison substances contain lots of a. smooth ER b. rough ER c. Golgi apparatus d. lysosomes e.
 Chapter 7 practice 1. What scientist originally came up with the term "cell"? a. von Leeuwenhoek d. Watson b. Hooke e. Virchow c. van der Waals 2. When you wish to look at the coat of a virus on the surface
Chapter 7 practice 1. What scientist originally came up with the term "cell"? a. von Leeuwenhoek d. Watson b. Hooke e. Virchow c. van der Waals 2. When you wish to look at the coat of a virus on the surface
Name: Date: Period: Biology Notes: Biochemistry Directions: Fill this out as we cover the following topics in class
 Name: Date: Period: Biology Notes: Biochemistry Directions: Fill this out as we cover the following topics in class Part I. Water Water Basics Polar: part of a molecule is slightly, while another part
Name: Date: Period: Biology Notes: Biochemistry Directions: Fill this out as we cover the following topics in class Part I. Water Water Basics Polar: part of a molecule is slightly, while another part
Chapter 02 Testbank. 1. Anything that occupies space and has mass is called. A. an electron. B. living. C. matter. D. energy. E. space.
 Chapter 02 Testbank Student: 1. Anything that occupies space and has mass is called A. an electron. B. living. C. matter. D. energy. E. space. 2. The electrons of an atom are A. always equal to the number
Chapter 02 Testbank Student: 1. Anything that occupies space and has mass is called A. an electron. B. living. C. matter. D. energy. E. space. 2. The electrons of an atom are A. always equal to the number
Ch 7: Cell Structure and Functions. AP Biology
 Ch 7: Cell Structure and Functions AP Biology The Cell Theory 1. All living things are made of cells. 2. New cells come from existing cells. 3. Cells are the basic units of structure and function of living
Ch 7: Cell Structure and Functions AP Biology The Cell Theory 1. All living things are made of cells. 2. New cells come from existing cells. 3. Cells are the basic units of structure and function of living
How many lessons is it?
 Science Unit Learning Summary Content Eukaryotes and Prokaryotes Cells are the basic unit of all life forms. A eukaryotic cell contains genetic material enclosed within a nucleus. Plant and animal cells
Science Unit Learning Summary Content Eukaryotes and Prokaryotes Cells are the basic unit of all life forms. A eukaryotic cell contains genetic material enclosed within a nucleus. Plant and animal cells
Discovering Cell/ The Cell Theory. * Cells are the basic, smallest units of structure and function of living things.
 Discovering Cell/ The Cell Theory * Cells are the basic, smallest units of structure and function of living things. Since they are so small, before the invention of the microscope (around 1590), no one
Discovering Cell/ The Cell Theory * Cells are the basic, smallest units of structure and function of living things. Since they are so small, before the invention of the microscope (around 1590), no one
Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 chapter 7 Test Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Who was one of the first people to identify and see cork cells? a. Anton van
chapter 7 Test Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Who was one of the first people to identify and see cork cells? a. Anton van
Mr. Jensen/Period: 1. The diagram below illustrates the distribution of fossils in undisturbed layers of silt at the bottom of the ocean.
 Name: 1. The diagram below illustrates the distribution of fossils in undisturbed layers of silt at the bottom of the ocean. Date: /Page#: Mr. Jensen/Period: 3. In the diagram below of undisturbed sedimentary
Name: 1. The diagram below illustrates the distribution of fossils in undisturbed layers of silt at the bottom of the ocean. Date: /Page#: Mr. Jensen/Period: 3. In the diagram below of undisturbed sedimentary
Which row in the chart below identifies the lettered substances in this process?
 1. A biological process that occurs in both plants and animals is shown below. Which row in the chart below identifies the lettered substances in this process? A) 1 B) 2 C) 3 D) 4 2. All life depends on
1. A biological process that occurs in both plants and animals is shown below. Which row in the chart below identifies the lettered substances in this process? A) 1 B) 2 C) 3 D) 4 2. All life depends on
LIFE OF CELL. Jhia Anjela D. Rivera 1,2 1. BS Biology Graduate, Department of Biology, College of Science, Polytechnic University of the Philippines 2
 LIFE OF CELL Jhia Anjela D. Rivera 1,2 1 BS Biology Graduate, Department of Biology, College of Science, Polytechnic University of the Philippines 2 MS Biology Student, Graduate School, Centro Escolar
LIFE OF CELL Jhia Anjela D. Rivera 1,2 1 BS Biology Graduate, Department of Biology, College of Science, Polytechnic University of the Philippines 2 MS Biology Student, Graduate School, Centro Escolar
Figure ) Letter E represents a nucleic acid building block known as a. Answer: nucleotide Diff: 3 Page Ref: 54
 Essentials of Human Anatomy and Physiology, 10e (Marieb) Chapter 2 Basic Chemistry 2.1 Short Answer Figure 2.1 Using Figure 2.1, identify the following: 1) Which letter represents a carbohydrate polymer?
Essentials of Human Anatomy and Physiology, 10e (Marieb) Chapter 2 Basic Chemistry 2.1 Short Answer Figure 2.1 Using Figure 2.1, identify the following: 1) Which letter represents a carbohydrate polymer?
UNIT 2 CHEMISTRY. Atomic Structure: Ionic Bond: Covalent Bond: Hydrogen Bond:
 UNIT 2 CHEMISTRY Atomic Structure: Ionic Bond: Hydrogen Bond: Covalent Bond: 1 Carbohydrates: >energy yield- >elements- >monomers- >functions- >examples- >misc- Lipids: Proteins: Nucleic Acids: I. Energy
UNIT 2 CHEMISTRY Atomic Structure: Ionic Bond: Hydrogen Bond: Covalent Bond: 1 Carbohydrates: >energy yield- >elements- >monomers- >functions- >examples- >misc- Lipids: Proteins: Nucleic Acids: I. Energy
Table of Contents. Chapter Preview. 4.1 Photosynthesis. 4.2 Respiration. 4.3 Cell Division. 4.4 Cell Differentiation
 Table of Contents Chapter Preview 4.1 Photosynthesis 4.2 Respiration 4.3 Cell Division 4.4 Cell Differentiation Chapter Preview Questions 1. All living things are made of a. tissues. b. muscles. c. cells.
Table of Contents Chapter Preview 4.1 Photosynthesis 4.2 Respiration 4.3 Cell Division 4.4 Cell Differentiation Chapter Preview Questions 1. All living things are made of a. tissues. b. muscles. c. cells.
Chapter 3 Cell Processes and Energy
 Chapter 3 Cell Processes and Energy 1 Chapter 3 Objectives Section 1: Chemical Compounds in Cells 1. Define elements and compounds 2. Explain how water is important to the function of cells 3. Identify
Chapter 3 Cell Processes and Energy 1 Chapter 3 Objectives Section 1: Chemical Compounds in Cells 1. Define elements and compounds 2. Explain how water is important to the function of cells 3. Identify
Living and nonliving things are all made of elements. It is the way that atoms combine that give every element a different characteristic.
 Living and nonliving things are all made of elements. It is the way that atoms combine that give every element a different characteristic. 98% of the body is made of only 6 elements The 6 elements are:
Living and nonliving things are all made of elements. It is the way that atoms combine that give every element a different characteristic. 98% of the body is made of only 6 elements The 6 elements are:
Basic Chemistry. Chapter 2 BIOL1000 Dr. Mohamad H. Termos
 Basic Chemistry Chapter 2 BIOL1000 Dr. Mohamad H. Termos Chapter 2 Objectives Following this chapter, you should be able to describe: - Atoms, molecules, and ions - Composition and properties - Types of
Basic Chemistry Chapter 2 BIOL1000 Dr. Mohamad H. Termos Chapter 2 Objectives Following this chapter, you should be able to describe: - Atoms, molecules, and ions - Composition and properties - Types of
Biology Midterm Test Review
 Biology Midterm Test Review Levels of Organization 1. Put these levels of organization in order from simplest to most complex (smallest to largest): cell, community, atom, organism, biosphere, organ system,
Biology Midterm Test Review Levels of Organization 1. Put these levels of organization in order from simplest to most complex (smallest to largest): cell, community, atom, organism, biosphere, organ system,
The Chemistry of Life
 The Chemistry of Life Things you should be able to do 1. Describe how the unique properties of water support life on Earth. 2. Explain how carbon is uniquely suited to form biological macromolecules. 3.
The Chemistry of Life Things you should be able to do 1. Describe how the unique properties of water support life on Earth. 2. Explain how carbon is uniquely suited to form biological macromolecules. 3.
untitled 1. One similarity between cell receptors and antibodies is that both
 Name: ate: 1. One similarity between cell receptors and antibodies is that both. are produced by nerve cells B. are highly specific in their actions. slow the rates of chemical reactions. are involved
Name: ate: 1. One similarity between cell receptors and antibodies is that both. are produced by nerve cells B. are highly specific in their actions. slow the rates of chemical reactions. are involved
`1AP Biology Study Guide Chapter 2 v Atomic structure is the basis of life s chemistry Ø Living and non- living things are composed of atoms Ø
 `1AP Biology Study Guide Chapter 2 v Atomic structure is the basis of life s chemistry Ø Living and non- living things are composed of atoms Ø Element pure substance only one kind of atom Ø Living things
`1AP Biology Study Guide Chapter 2 v Atomic structure is the basis of life s chemistry Ø Living and non- living things are composed of atoms Ø Element pure substance only one kind of atom Ø Living things
Unit 2: Cells. Students will understand that the organs in an organism are made of cells that have structures & perform specific life functions
 Unit 2: Cells Students will understand that the organs in an organism are made of cells that have structures & perform specific life functions Vocabulary Cell Chloroplast Tissue Cell wall Organ Lysosome
Unit 2: Cells Students will understand that the organs in an organism are made of cells that have structures & perform specific life functions Vocabulary Cell Chloroplast Tissue Cell wall Organ Lysosome
