Report on. Starch Hydrolysis. Submitted to. Dr. Stephanie Loveland Chemical and Biological Engineering Department.
|
|
|
- Samson Morrison
- 5 years ago
- Views:
Transcription
1 Report on Starch Hydrolysis Submitted to Dr. Stephanie Loveland Chemical and Biological Engineering Department November 28, 2016 By Aimee Pierce Iowa State University ABSTRACT Enzyme catalysts are important tools of biochemical and biomedical engineering; they provide specific, versatile, and effective catalysis of biological reactions. Enzyme catalyzed reactions are affected by temperature, ph, and substrate concentration, among other things. The Starch Hydrolysis experiment evaluates the effects of initial substrate concentration on the biological enzyme-catalyzed reaction of starch hydrolysis by glucoamylase. It also explores kinetic models to find maximum reaction rate and the reaction rate constant. Using the Michaelis-Menten, Lineweaver-Burk, Eadie-Hofstee, and Hanes-Woolf models for single-substrate-enzyme-catalyzed reactions, experimenters solved for the maximum reaction rate and reaction rate constant for the glucoamylase catalyzed reaction. Experimenters concluded that the initial substrate concentration did not affect the efficiency of the enzyme, and that Michaelis-Menten model gave the most accurate representation of the maximum reaction rate and reaction rate constant.
2 Starch Hydrolysis ii November 28, 2016 Executive Summary The Starch Hydrolysis experiment evaluates the effects of initial substrate concentration on the biological enzyme-catalyzed reaction. The reaction explored in the report is the hydrolysis of starch, using a maltose substrate and glucoamylase enzyme to produce glucose. It is hypothesized that as the initial substrate concentration increases, enzyme performance in starch hydrolysis will also increase. Additionally, the Starch Hydrolysis experiment explores four kinetic models to find the maximum reaction rate and the reaction rate constant for the enzyme catalyzed reaction: Michaelis-Menten, Lineweaver-Burk, Eadie-Hofstee, and Hanes-Woolf. Each of the models seek to represent the kinetics for single-substrate-enzyme-catalyzed reactions, to varying degrees of accuracy. Therefore, it is hypothesized that the Michaelis-Menten kinematic model will provide the most accurate values for the reaction rate and rate constant of the reaction. Results of the Starch Hydrolysis experiment gave inconclusive findings for calculating the reaction rate constant, K m, and maximum rate of reaction, V m, using the approximation models of Eadie-Hoffstee and Hanes-Woolf. The Michaelis-Menten and Lineweaver-Burk models yielded similar statistical significance seen from values of and respectively, however the values calculated for V m and K m varied greatly. Therefore, it was concluded that the Michaelis-Menten model yielded the best values of K m and V m, due to the knowledge that the Lineweaver-Burk model is a derivation from the Michaelis-Menten model. Furthermore, the reactions with higher concentrations of the reaction substrate, maltose, did not affect the overall reaction rate. Therefore, the experimental hypothesis that a higher substrate concentration would increase enzyme efficiency was shown to be invalid.
3 Starch Hydrolysis iii November 28, 2016 Contents Executive Summary... ii Introduction... 1 Theory... 1 Materials and Methods... 4 Apparatus... 4 Process... 5 Results and Discussion... 6 Conclusion...12 Sample Calculation:... iv References... v Figure 1: Michaelis-Menten saturation curve used to calculate the maximum rate of reactoin and reaction rate constant for the Michaelis-Menten model for single-substrate-enzyme-catalyzed reaction kinetics Figure 2: The Starch Hydrolysis apparatus uses multiple parts to conduct the experiment, including a scale, Erlenmeyer flasks, a water bath, and a glucose analyzer Figure 3: Concentration of glucose vs time for six reactions of the Starch Hydrolysis experiment Figure 4: Lineweaver-Burk plot to calculate the rate constant, Km, and max reaction rate, Vm, of the glucoamylase catalyzed reaction using Equation Figure 5: The Eadie-Hofstee uses the initial rate of reaction, the initial concentration of maltose, and Equation 4 to solve for Km and Vm Figure 6: The Hanes-Woolf model uses the initial rate of reaction, the initial concentration of substrate, maltose, and Equation 5 to give estimates for Vm and Km Figure 7: Michaelis-Menten model of glucoamylase catalyzed reaction finds Km and Vm using the initial rate of reaction, concentration of maltose [S], and saturation curve seen in Figure
4 Introduction The Starch Hydrolysis experiment explores the effects of changing substrate concentration on enzyme performance in starch hydrolysis. The experiment also examines the reaction kinetics and reaction rate of the enzyme-substrate formation of glucose. Reaction rate calculations are important in reaction engineering, especially when designing large-scale processes in industry. Optimizing catalyst usage is crucial for designing efficient and cost-effective chemical reactions. Biochemical and biomedical engineering utilize enzymes for selective catalysis, as well as food processing and pharmaceutical industries. Calculating the value of the reaction rate constant requires the use of various models such as the Michaelis-Menten, Lineweaver-Burk, Eadie-Hofstee, or Hanes-Woolf [1]. Each of these models present an equation comparing the rate of product formation, substrate concentration, rate constant, and maximum forward rate of reaction. These methods are used for data analysis, and the results of each model are compared and contrasted in this report. I hypothesize that as the initial substrate concentration increases, enzyme performance in starch hydrolysis will also increase. I also hypothesize that the Michaelis-Menten will provide the most accurate values for the reaction rate and rate constant of the reaction. Theory Enzymes are proteins with high molecular weights that act as catalysts in many chemical and biological processes. Enzyme catalyzed reactions can be manipulated by changing ph, substrate concentration, and temperature, among other things. The glucoamylase enzyme is a digestive enzyme that breaks off a free glucose molecule from the complex sugar-based chains that form starch. The result of that catalyzed reaction is glucose, which can then be used as a source of energy. Changes in ph can affect the effectiveness of the glucoamylase enzyme, the substrate, or both. Extremely high or low ph values generally result in complete loss of activity for most enzymes, but optimum ph values for an enzyme-catalyzed reaction vary greatly.
5 Starch Hydrolysis 2 November 28, 2016 There are various mathematical models of the kinetics of a single-substrate-enzymecatalyzed reaction, such as catalysis using the glucoamylase enzyme. One of those models is the Michaelis-Menten model, shown in Equation 1, which resembles Langmuir-Hinschelwood kinetics. where: E = enzyme S = substrate ES = enzyme-substrate P = product E + < > E > E + P (1) Using batch reactors allows the use of the quasi-steady-state assumption. After applying this assumption to Equation 1, the resulting equation is shown in Equation 2. Equation 2 is the Michaelis-Menten model which represents the kinetics of a singlesubstrate-enzyme-catalyzed reaction [1]. v = V m [S] K m +[S] (2) where : v = rate of product formation [ ] = substrate concentration V m = maximum forward rate of reaction K m = rate constant Determining values for the parameters of maximum forward reaction rate as well as the rate constant should be determined using initial rate experiments with a known amount of initial substrate and enzyme concentrations. Multiple methods can be used to determine V m and K m that are derived from the Michaelis-Menten model, including the Lineweaver-Burk shown in Equation 3, the Eadie-Hofstee shown in Equation 4, or the Hanes-Woolf shown in Equation 5 [1][2]. Equations 2, 3, 4, and 5 all give models to represent the kinetics of single-substrateenzyme-catalyzed reactions to varying degrees of accuracy. When analyzing data, it is important to understand the accuracy of the models used to calculate the rate constant and rate of reaction. The Hanes-Woolf plot in Equation 5 that more accurately
6 Starch Hydrolysis 3 November 28, 2016 determines V m. Similarly, the Lineweaver-Burk plot represented by Equation 3 gives a good estimate of V m but not necessarily K m. The Eadie-Hofstee plot in Equation 4 are known to have large errors [1]. v = V m + K m V m [S] v = V m K mv [S] (3) (4) [S] v = K m V m + [S] V m (5) Equations 3, 4, and 5 are derived from the Michaelis-Menten model to give linear relationships for finding K m and V m. The Michaelis-Menten model generates a non-linear saturation curve shown in Figure 1. Instead of using linear methods shown in equations 3, 4, and 5 to find K m and V m, the saturation curve in Figure 1 is used. Figure 1: Michaelis-Menten saturation curve used to calculate the maximum rate of reactoin and reaction rate constant for the Michaelis-Menten model for singlesubstrate-enzyme-catalyzed reaction kinetics.
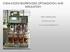
7 Starch Hydrolysis 4 November 28, 2016 Materials and Methods In the Starch Hydrolysis experiment, a water bath will be used to hold the temperature of the solution constant. Erlenmeyer flasks were used as individual reactor vessels, and a glucose analyzer determined the glucose concentration of samples taken throughout the reaction. Apparatus The apparatus for the Starch Hydrolysis experiment consists of a scale, six 250ml Erlenmeyer flasks, six 50ml Erlenmeyer flasks, a water bath, sample test tubes, and a Glucose Analyzer machine. A schematic of the apparatus supplies is shown in Figure 1. Figure 2: The Starch Hydrolysis apparatus uses multiple parts to conduct the experiment, including a scale, Erlenmeyer flasks, a water bath, and a glucose analyzer. The scale in the Starch Hydrolysis experiment measures the mass of dry chemicals used in the experiment. The six 250ml Erlenmeyer flasks are used as reaction vessels for the enzyme catalyzed reaction, and the six 50ml Erlenmeyer flasks are used to hold the enzyme solution before the reaction starts. The water bath holds the reaction s temperature at a constant 50C throughout the reaction. A YSI 2700 glucose analyzer determines the glucose concentration of samples
8 Starch Hydrolysis 5 November 28, 2016 taken throughout the reactions. The glucose analyzer utilizes a YSI 2710 turntable to hold sample test tubes and submit them to the glucose analyzer for analysis. Preparation for the Starch Hydrolysis experiment includes cleaning all Erlenmeyer flasks, turning on the water bath and setting it to 50C, and balancing the scale. Additionally, before each reaction is started, 1ml of Tris solution is added six sample test tubes used for the glucose analyzer. Process To conduct the Starch Hydrolysis experiment, the performance of the glucoamylase enzyme was monitored during the hydrolysis of a soluble starch. A 250ml Erlenmeyer flask was used to hold 30ml of 2.3M buffer solution in a 50C water bath. The 30ml was measured with a graduated cylinder. Maltose and glucoamylase were carefully weighed using the scale. A maltose solution molarity of g/l, 15-8 g/l, or 8-2 g/l was mixed with 0.1g, 0.05g, and 0.25g glucoamylase respectively to start the reaction. The 50mL Erlenmeyer flasks were used to dissolve the glucoamylase in 10ml of buffer. A timer was started as the glucoamylase-buffer solution in the 50mL flask was added to the 250ml Erlenmeyer flask solution. Every 30, 45, or 60 seconds (depending on the run) an auto-pipet was used to take out 1ml of the reacting solution, and dispense it into a sample test tube containing 1ml of 2.3M Tris solution. When the glucoamylasemaltose-buffer solution mixed with the Tris solution, the reaction was halted. Six reactions were run at varying initial concentrations of glucoamylase and maltose. Six samples were taken with the auto-pipet every 30 seconds for the first three reactions. The fourth reaction, the six samples were taken every 45 seconds. The fifth and sixth reactions had samples taken every 60 seconds. A total of 36 samples were analyzed by the glucose analyzer. The 1ml samples were mixed with 1ml of Tris solution in a prepared sample tube to be tested in the Glucose Analyzer.
9 Starch Hydrolysis 6 November 28, 2016 Results and Discussion The hypothesis of the Starch Hydrolysis experiment is that increasing the initial concentration of a catalytic enzyme will increase the generation rate of the desired product. Relating the hypothesis to our experiment, it hypothesizes that increasing the initial amount of glucoamylase enzyme will increase the rate of production of glucose in the reaction. In order to test our hypothesis, six different initial concentrations of maltose and enzyme glucoamylase were reacted in the Starch Hydrolysis experiment. The initial concentrations of maltose and grams of glucoamylase used in each reaction are shown in Table 1. Table 1: Concentrations of maltose and masses of glucoamylase used in the Starch Hydrolysis experiment. Maltose (g/l) [S] Glucoamylase Enzyme (g) Reaction Reaction Reaction Reaction Reaction Reaction Data generated from the Glucose Analyzer shows the concentration of glucose generated from each reaction over time. Figure 2 displays the concentration of glucose over time for each of the six reactions conducted. Note the linearity of the trends, with an average value of The results of Figure 2 allow experimenters to calculate the initial rate of reaction v, using the slope of the equations generated from the linear trends. Calculated values of the initial rate of reaction are shown in Table 3. In order to compare the resulting initial rates from two different enzyme concentrations, the initial rate of Reactions 3, 4, 5, and
10 Starch Hydrolysis 7 November 28, were multiplied by the ratio of the differing enzyme concentrations. Therefore, Reactions 3 and 4 were multiplied by 2, and Reactions 5 and 6 were multiplied by 4. Figure 3: Concentration of glucose vs time for six reactions of the Starch Hydrolysis experiment. Table 2: Initial rates of reaction, v, calculated from data of glucose concentration vs time. Initial Rate of Reaction v Scaled v Reaction Reaction Reaction Reaction Reaction Reaction
11 Starch Hydrolysis 8 November 28, 2016 The data of concentration of glucose over time from Figure 3 give the initial rates of reactions calculated in Table 3. These initial reaction rates are used in the Lineweaver- Burk, Eadie-Hofstee, Hanes-Woolf, and Michaelis-Menten models of enzyme-catalyzed reaction kinetics. The Lineweaver-Burk model examined in Equation 3 is known to give a good estimate of V m [1]. Using data from Table 3, the initial concentration of maltose [S], and Equation 3, values of the maximum rate of reaction V m and the rate constant K m can be calculated from the equation generated in Figure 4. With an value of 0.832, there is somewhat significant statistical evidence that our data fits the Lineweaver-Burk model. The Eadie-Hofstee model examined in Equation 4 is known to subject large error when calculating K m and V m [1]. Using data from Table3, the initial concentration of maltose [S], and Equation 4, values of K m and V m can be calculated using the equation generated in Figure 5. The Eadie-Hofstee model did not fit to the data obtained, yielding an value of This evidence, as well as the known inaccuracies of the Eadie- Hofstee, show that the K m and V m values obtained from the Eadie-Hofstee model are inaccurate. The Hanes-Woolf model examined in Equation 5 is known to give an accurate value of V m [1]. Using data from Table 3, the initial concentration of maltose [S], and Equation 5, values of V m and K m can be calculate from the equation generated in Figure 6. The equation formed from Figure 6 gives an value of , signifying minimal statistical significance that our data fits the Hanes-Woolf model for finding K m and V m, concluding that the values found are likely to be inaccurate.
12 Starch Hydrolysis 9 November 28, Lineweaver-Burk plot y = 3260x R² = /v Datenreihen /[S] Figure 4: Lineweaver-Burk plot to calculate the rate constant, Km, and max reaction rate, Vm, of the glucoamylase catalyzed reaction using Equation Eadie-Hofstee y = 8.28x R² = v Datenreihen v/[s] Figure 5: The Eadie-Hofstee uses the initial rate of reaction, the initial concentration of maltose, and Equation 4 to solve for Km and Vm
13 Starch Hydrolysis 10 November 28, 2016 [S]/v Hanes-Woolf plot y = x R² = Datenreihen [S] Figure 6: The Hanes-Woolf model uses the initial rate of reaction, the initial concentration of substrate, maltose, and Equation 5 to give estimates for Vm and Km The Michaelis-Menten model for enzyme-catalyzed reactions seen in Equation 2 does not give a linear relationship between the initial reaction rate v and initial concentration of maltose [S]. Figure 7 shows an inverse logarithmic relationship between v and [S]. Using the initial reaction rate, initial concentration of maltose [S], and saturation curve shown in Figure 1, V m and K m can be calculated with the Michaelis-Menten model. The Michaelis-Menten model gives an equation with an value of , which is similar to the value found in Figure 4 using the Lineweaver-Burk model. Therefore, the Lineweaver-Burk and Michaelis-Menten kinetic models fit best for the single-substrateenzyme-catalyzed reaction of starch hydrolysis with glucoamylase.
14 Starch Hydrolysis 11 November 28, 2016 Figure 7: Michaelis-Menten model of glucoamylase catalyzed reaction finds Km and Vm using the initial rate of reaction, concentration of maltose [S], and saturation curve seen in Figure 1. Values of the maximum reaction rate and reaction rate constant are calculated from Figures 4, 5, 6, and 7. These values are shown in Table 3. Note the disparities and wide range of values calculated for K m and V m. The models explored in the Starch Hydrolysis experiment give varied accuracies for calculating K m and V m. Lineweaver-Burk gives a good estimate of V m but not necessarily K m, the Eadie-Hofstee model is subject to large errors, and the Hanes-Woolf gives a more accurate value of V m but not of K m. These discrepancies give explanation for the range of K m and V m values calculated. Table 3: Calculated values of Vm and Km using the Lineweaver-Burk, Eadie-Hofstee, Hanes- Woolf and Michaelis-Menten model. Model Maximum reaction rate V m Reaction rate constant K m Lineweaver- Burk Eadie-Hofstee Hanes-Woolf Michaelis- Menten
15 Starch Hydrolysis 12 November 28, 2016 The values of K m and V m calculated from the approximation methods of Eadie-Hofstee, and Hanes-Woolf are inaccurate due to the lack of statistical significance when using data from the Starch Hydrolysis experiment. The Michaelis-Menten and Lineweaver- Burk models fit experimental data to about the same degree, with values of and respectively. However, when comparing calculated values of K m and V m for the two models in Table 3, the Lineweaver-Burk model gives a reaction rate constant of and a maximum reaction rate of These values contrast greatly with the values of K m and V m calculated from the Michaelis-Menten model. With this discrepancy in data and knowledge that the Lineweaver-Burk model is derived from the Michaelis-Menten model, it can be concluded that the Michaelis-Menten model does give the most accurate values for the reaction rate constant and maximum reaction rate. Consequently, the experimental hypothesis that the Michaelis-Menten model would give the most accurate results of the reaction rate constant and maximum reaction rate is shown to be sound. Shown in Figure 4, reactions with higher concentrations of the substrate maltose showed higher initial reaction rates, however the high concentration of maltose was inconsequential when calculating the overall maximum reaction rate. The inconclusive data produced from the kinetic models are not affected by the initial concentration of substrate in the Starch Hydrolysis experiment. Therefore, experimenters conclude that the initial concentration of substrate has no effect on the efficiency of the catalytic reaction, proving the first half of the hypothesis invalid. The Starch Hydrolysis experiment explores how changing substrate concentration changes the reaction rate, and examines how kinetic models fit to experimental data. Some follow up questions to the study would be: How does changing ph affect the enzyme performance? How does changing the temperature affect enzyme performance? Since temperature and ph are known to affect enzyme catalyzed reactions, it would be interesting to find an optimal enzyme catalyzed reaction environment while changing temperature and ph. Conclusion The Starch Hydrolysis experiment examined the effect of substrate concentration on the rate of reaction, as well as exploring kinematic models of enzyme catalyzed reactions. It was hypothesized that if the initial substrate concentration was higher, it would increase the overall rate of reaction. It was also hypothesized that the Michaelis-Menten model would give the best results when calculating the maximum reaction rate and reaction constant. Results of the Starch Hydrolysis experiment gave inconclusive findings for calculating the reaction rate constant, K m, and maximum rate of reaction, V m, using the approximation models of Eadie-Hoffstee and Hanes-Woolf. The Michaelis-Menten and Lineweaver-Burk models yielded similar statistical significance seen from values of
16 Starch Hydrolysis 13 November 28, and respectively, however the values calculated for V m and K m varied greatly. Therefore, it was concluded that the Michaelis-Menten model yielded the best values of K m and V m, due to the knowledge that the Lineweaver-Burk model is a derivation from the Michaelis-Menten model. Furthermore, the reactions with higher concentrations of the reaction substrate, maltose, did not affect the overall reaction rate. Therefore, the experimental hypothesis that a higher substrate concentration would increase enzyme efficiency was shown to be invalid. Errors in the Starch Hydrolysis experiment could be due to the lack of constant stirring of the reactions. Proper mixing of the reaction solution is important in when conducting reaction rate experiments, so not having a constantly stirred reaction would cause errors in data. Understanding how substrate concentration affects enzyme catalyzed reactions is important in biochemical and biomedical engineering. To design the most efficient catalytic reaction, understanding that initial substrate concentration has little effect on the efficiency of the enzyme allows experimenters to look into changing ph or temperature to manipulate the desired rate of reaction.
17 Sample Calculation: Finding K m and V m for the Lineweaver-Burk model Reaction 1 Equation 3 v = V m + K m V m [ ] From Table 1 and Figure 3 [S] = 25.5 g/l v = From Figure 4 = v [S] R² = K m V m = 6 V m = 66. V m =. 6 K m = 9. 6 References
18 Starch Hydrolysis v November 28, 2016 [1] S. Loveland, Starch Hydrolysis Lab Manual. Ames, IA: Iowa State University, [2] H. Lineweaver, D. Burk, The determination of enzyme dissociation constants, Journal of the American Chemical Society. Vol 56, no. 3, pp [3] T. Shafee, Michaelis-Menten Kinetics.Available at Accessed Nov 20, 2016.
CHAPTER 1: ENZYME KINETICS AND APPLICATIONS
 CHAPTER 1: ENZYME KINETICS AND APPLICATIONS EM 1 2012/13 ERT 317 BIOCHEMICAL ENGINEERING Course details Credit hours/units : 4 Contact hours : 3 hr (L), 3 hr (P) and 1 hr (T) per week Evaluations Final
CHAPTER 1: ENZYME KINETICS AND APPLICATIONS EM 1 2012/13 ERT 317 BIOCHEMICAL ENGINEERING Course details Credit hours/units : 4 Contact hours : 3 hr (L), 3 hr (P) and 1 hr (T) per week Evaluations Final
Michaelis-Menten Kinetics
 Michaelis-Menten Kinetics Two early 20th century scientists, Leonor Michaelis and Maud Leonora Menten, proposed the model known as Michaelis-Menten Kinetics to account for enzymatic dynamics. The model
Michaelis-Menten Kinetics Two early 20th century scientists, Leonor Michaelis and Maud Leonora Menten, proposed the model known as Michaelis-Menten Kinetics to account for enzymatic dynamics. The model
Bioreactor Engineering Laboratory
 Bioreactor Engineering Laboratory Determination of kinetics parameters of enzymatic hydrolysis of lactose catalyzed by β-galactosidase. Supervisor: Karolina Labus, PhD 1. THEROETICAL PART Enzymes are macromolecular,
Bioreactor Engineering Laboratory Determination of kinetics parameters of enzymatic hydrolysis of lactose catalyzed by β-galactosidase. Supervisor: Karolina Labus, PhD 1. THEROETICAL PART Enzymes are macromolecular,
Enzymes II. Dr. Mamoun Ahram Summer, 2017
 Enzymes II Dr. Mamoun Ahram Summer, 2017 Kinetics Kinetics is deals with the rates of chemical reactions. Chemical kinetics is the study of the rates of chemical reactions. For the reaction (A P), The
Enzymes II Dr. Mamoun Ahram Summer, 2017 Kinetics Kinetics is deals with the rates of chemical reactions. Chemical kinetics is the study of the rates of chemical reactions. For the reaction (A P), The
ENZYME SCIENCE AND ENGINEERING PROF. SUBHASH CHAND DEPARTMENT OF BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY IIT DELHI LECTURE 6
 ENZYME SCIENCE AND ENGINEERING PROF. SUBHASH CHAND DEPARTMENT OF BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY IIT DELHI LECTURE 6 KINETICS OF ENZYME CATALYSED REACTIONS Having understood the chemical and
ENZYME SCIENCE AND ENGINEERING PROF. SUBHASH CHAND DEPARTMENT OF BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY IIT DELHI LECTURE 6 KINETICS OF ENZYME CATALYSED REACTIONS Having understood the chemical and
Enzyme Reactions. Lecture 13: Kinetics II Michaelis-Menten Kinetics. Margaret A. Daugherty Fall v = k 1 [A] E + S ES ES* EP E + P
![Enzyme Reactions. Lecture 13: Kinetics II Michaelis-Menten Kinetics. Margaret A. Daugherty Fall v = k 1 [A] E + S ES ES* EP E + P Enzyme Reactions. Lecture 13: Kinetics II Michaelis-Menten Kinetics. Margaret A. Daugherty Fall v = k 1 [A] E + S ES ES* EP E + P](/thumbs/76/74341799.jpg) Lecture 13: Kinetics II Michaelis-Menten Kinetics Margaret A. Daugherty Fall 2003 Enzyme Reactions E + S ES ES* EP E + P E = enzyme ES = enzyme-substrate complex ES* = enzyme/transition state complex EP
Lecture 13: Kinetics II Michaelis-Menten Kinetics Margaret A. Daugherty Fall 2003 Enzyme Reactions E + S ES ES* EP E + P E = enzyme ES = enzyme-substrate complex ES* = enzyme/transition state complex EP
Michaelis-Menten Kinetics. Lecture 13: Kinetics II. Enzyme Reactions. Margaret A. Daugherty. Fall Substrates bind to the enzyme s active site
 Lecture 13: Kinetics II Michaelis-Menten Kinetics Margaret A. Daugherty Fall 2003 Enzyme Reactions E + S ES ES* EP E + P E = enzyme ES = enzyme-substrate complex ES* = enzyme/transition state complex EP
Lecture 13: Kinetics II Michaelis-Menten Kinetics Margaret A. Daugherty Fall 2003 Enzyme Reactions E + S ES ES* EP E + P E = enzyme ES = enzyme-substrate complex ES* = enzyme/transition state complex EP
STUDY GUIDE #2 Winter 2000 Chem 4540 ANSWERS
 STUDY GUIDE #2 Winter 2000 Chem 4540 ANSWERS R. Merrill 1. Sketch the appropriate plots on the following axes. Assume that simple Michaelis- Menten kinetics apply. 2. The enzyme-catalyzed hydrolysis of
STUDY GUIDE #2 Winter 2000 Chem 4540 ANSWERS R. Merrill 1. Sketch the appropriate plots on the following axes. Assume that simple Michaelis- Menten kinetics apply. 2. The enzyme-catalyzed hydrolysis of
Enzyme reaction example of Catalysis, simplest form: E + P at end of reaction No consumption of E (ES): enzyme-substrate complex Intermediate
 V 41 Enzyme Kinetics Enzyme reaction example of Catalysis, simplest form: k 1 E + S k -1 ES E at beginning and ES k 2 k -2 E + P at end of reaction No consumption of E (ES): enzyme-substrate complex Intermediate
V 41 Enzyme Kinetics Enzyme reaction example of Catalysis, simplest form: k 1 E + S k -1 ES E at beginning and ES k 2 k -2 E + P at end of reaction No consumption of E (ES): enzyme-substrate complex Intermediate
Lecture # 3, 4 Selecting a Catalyst (Non-Kinetic Parameters), Review of Enzyme Kinetics, Selectivity, ph and Temperature Effects
 1.492 - Integrated Chemical Engineering (ICE Topics: Biocatalysis MIT Chemical Engineering Department Instructor: Professor Kristala Prather Fall 24 Lecture # 3, 4 Selecting a Catalyst (Non-Kinetic Parameters,
1.492 - Integrated Chemical Engineering (ICE Topics: Biocatalysis MIT Chemical Engineering Department Instructor: Professor Kristala Prather Fall 24 Lecture # 3, 4 Selecting a Catalyst (Non-Kinetic Parameters,
Enzyme Kinetics: How they do it
 Enzyme Kinetics: How they do it (R1) Formation of Enzyme-Substrate complex: (R2) Formation of Product (i.e. reaction): E + S ES ES -> E + P (R3) Desorption (decoupling/unbinding) of product is usually
Enzyme Kinetics: How they do it (R1) Formation of Enzyme-Substrate complex: (R2) Formation of Product (i.e. reaction): E + S ES ES -> E + P (R3) Desorption (decoupling/unbinding) of product is usually
Chemistry 112 Chemical Kinetics. Kinetics of Simple Enzymatic Reactions: The Case of Competitive Inhibition
 Chemistry Chemical Kinetics Kinetics of Simple Enzymatic Reactions: The Case of Competitive Inhibition Introduction: In the following, we will develop the equations describing the kinetics of a single
Chemistry Chemical Kinetics Kinetics of Simple Enzymatic Reactions: The Case of Competitive Inhibition Introduction: In the following, we will develop the equations describing the kinetics of a single
Lab training Enzyme Kinetics & Photometry
 Lab training Enzyme Kinetics & Photometry Qing Cheng Qing.Cheng@ki.se Biochemistry Division, MBB, KI Lab lecture Introduction on enzyme and kinetics Order of a reaction, first order kinetics Michaelis-Menten
Lab training Enzyme Kinetics & Photometry Qing Cheng Qing.Cheng@ki.se Biochemistry Division, MBB, KI Lab lecture Introduction on enzyme and kinetics Order of a reaction, first order kinetics Michaelis-Menten
Bioengineering Laboratory I. Enzyme Assays. Part II: Determination of Kinetic Parameters Fall Semester
 Bioengineering Laboratory I Enzyme Assays Part II: Determination of Kinetic Parameters 2016-2017 Fall Semester 1. Theoretical background There are several mathematical models to determine the kinetic constants
Bioengineering Laboratory I Enzyme Assays Part II: Determination of Kinetic Parameters 2016-2017 Fall Semester 1. Theoretical background There are several mathematical models to determine the kinetic constants
13 Determining the Efficiency of the Enzyme Acetylcholine Esterase Using Steady-State Kinetic Experiment
 13 Determining the Efficiency of the Enzyme Acetylcholine Esterase Using Steady-State Kinetic Experiment 131 Learning Objective This laboratory introduces you to steady-state kinetic analysis, a fundamental
13 Determining the Efficiency of the Enzyme Acetylcholine Esterase Using Steady-State Kinetic Experiment 131 Learning Objective This laboratory introduces you to steady-state kinetic analysis, a fundamental
Measurement of Enzyme Activity - ALP Activity (ALP: Alkaline phosphatase)
 Measurement of Enzyme Activity - ALP Activity (ALP: Alkaline phosphatase) Measurement and analysis of enzyme activity is often used in the field of life science such as medicines and foods to investigate
Measurement of Enzyme Activity - ALP Activity (ALP: Alkaline phosphatase) Measurement and analysis of enzyme activity is often used in the field of life science such as medicines and foods to investigate
Review for Final Exam. 1ChE Reactive Process Engineering
 Review for Final Exam 1ChE 400 - Reactive Process Engineering 2ChE 400 - Reactive Process Engineering Stoichiometry Coefficients Numbers Multiple reactions Reaction rate definitions Rate laws, reaction
Review for Final Exam 1ChE 400 - Reactive Process Engineering 2ChE 400 - Reactive Process Engineering Stoichiometry Coefficients Numbers Multiple reactions Reaction rate definitions Rate laws, reaction
Enzyme Kinetics. Jonathan Gent and Douglas Saucedo May 24, 2002
 Enzyme Kinetics Jonathan Gent and Douglas Saucedo May 24, 2002 Abstract This paper consists of a mathematical derivation of the Michaelis-Menten equation, which models the rate of reaction of certain enzymatic
Enzyme Kinetics Jonathan Gent and Douglas Saucedo May 24, 2002 Abstract This paper consists of a mathematical derivation of the Michaelis-Menten equation, which models the rate of reaction of certain enzymatic
Simple kinetics of enzyme action
 Simple kinetics of enzyme action It is established that enzymes form a bound complex to their reactants (i.e. substrates) during the course of their catalysis and prior to the release of products. This
Simple kinetics of enzyme action It is established that enzymes form a bound complex to their reactants (i.e. substrates) during the course of their catalysis and prior to the release of products. This
Biochemistry Enzyme kinetics
 1 Description of Module Subject Name Paper Name Module Name/Title Enzyme Kinetics Dr. Vijaya Khader Dr. MC Varadaraj 2 1. Objectives 2. Enzymes as biological catalyst 3. Enzyme Catalysis 4. Understanding
1 Description of Module Subject Name Paper Name Module Name/Title Enzyme Kinetics Dr. Vijaya Khader Dr. MC Varadaraj 2 1. Objectives 2. Enzymes as biological catalyst 3. Enzyme Catalysis 4. Understanding
Lecture 13: Data Analysis for the V versus [S] Experiment and Interpretation of the Michaelis-Menten Parameters
![Lecture 13: Data Analysis for the V versus [S] Experiment and Interpretation of the Michaelis-Menten Parameters Lecture 13: Data Analysis for the V versus [S] Experiment and Interpretation of the Michaelis-Menten Parameters](/thumbs/89/100978514.jpg) Biological Chemistry Laboratory Biology 3515/Chemistry 3515 Spring 2018 Lecture 13: Data Analysis for the V versus [S] Experiment and Interpretation of the Michaelis-Menten Parameters 20 February 2018
Biological Chemistry Laboratory Biology 3515/Chemistry 3515 Spring 2018 Lecture 13: Data Analysis for the V versus [S] Experiment and Interpretation of the Michaelis-Menten Parameters 20 February 2018
ENZYME SCIENCE AND ENGINEERING PROF. SUBHASH CHAND DEPARTMENT OF BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY IIT DELHI LECTURE 7
 ENZYME SCIENCE AND ENGINEERING PROF. SUBHASH CHAND DEPARTMENT OF BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY IIT DELHI LECTURE 7 KINETICS OF ENZYME CATALYSED REACTIONS (CONTD.) So in the last lecture we
ENZYME SCIENCE AND ENGINEERING PROF. SUBHASH CHAND DEPARTMENT OF BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY IIT DELHI LECTURE 7 KINETICS OF ENZYME CATALYSED REACTIONS (CONTD.) So in the last lecture we
Biochemical Kinetics: the science that studies rates of chemical reactions An example is the reaction (A P), The velocity, v, or rate, of the
 Biochemical Kinetics: the science that studies rates of chemical reactions An example is the reaction (A P), The velocity, v, or rate, of the reaction A P is the amount of P formed or the amount of A consumed
Biochemical Kinetics: the science that studies rates of chemical reactions An example is the reaction (A P), The velocity, v, or rate, of the reaction A P is the amount of P formed or the amount of A consumed
Chemical kinetics and catalysis
 Chemical kinetics and catalysis Outline Classification of chemical reactions Definition of chemical kinetics Rate of chemical reaction The law of chemical raction rate Collision theory of reactions, transition
Chemical kinetics and catalysis Outline Classification of chemical reactions Definition of chemical kinetics Rate of chemical reaction The law of chemical raction rate Collision theory of reactions, transition
Catalysis. v 0 no catalyst v c -- catalyst present. v c. dt with no catalyst) (v c = -d[a]/dt dt with a catalyst)
![Catalysis. v 0 no catalyst v c -- catalyst present. v c. dt with no catalyst) (v c = -d[a]/dt dt with a catalyst) Catalysis. v 0 no catalyst v c -- catalyst present. v c. dt with no catalyst) (v c = -d[a]/dt dt with a catalyst)](/thumbs/87/95598212.jpg) Catalysis Catalysis provides an additional mechanism by which reactants can be converted to products. The alternative mechanism has a lower activation energy than the reaction in the absence of a catalyst.
Catalysis Catalysis provides an additional mechanism by which reactants can be converted to products. The alternative mechanism has a lower activation energy than the reaction in the absence of a catalyst.
Enzyme Kinetics 2014
 V 41 Enzyme Kinetics 2014 Atkins Ch.23, Tinoco 4 th -Ch.8 Enzyme rxn example Catalysis/Mechanism: E + S k -1 ES k 1 ES E is at beginning and k 2 k -2 E + P at end of reaction Catalyst: No consumption of
V 41 Enzyme Kinetics 2014 Atkins Ch.23, Tinoco 4 th -Ch.8 Enzyme rxn example Catalysis/Mechanism: E + S k -1 ES k 1 ES E is at beginning and k 2 k -2 E + P at end of reaction Catalyst: No consumption of
It can be derived from the Michaelis Menten equation as follows: invert and multiply with V max : Rearrange: Isolate v:
 Eadie Hofstee diagram In Enzymology, an Eadie Hofstee diagram (also Woolf Eadie Augustinsson Hofstee or Eadie Augustinsson plot) is a graphical representation of enzyme kinetics in which reaction velocity
Eadie Hofstee diagram In Enzymology, an Eadie Hofstee diagram (also Woolf Eadie Augustinsson Hofstee or Eadie Augustinsson plot) is a graphical representation of enzyme kinetics in which reaction velocity
Previous Class. Today. Michaelis Menten equation Steady state vs pre-steady state
 Previous Class Michaelis Menten equation Steady state vs pre-steady state Today Review derivation and interpretation Graphical representation Michaelis Menten equations and parameters The Michaelis Menten
Previous Class Michaelis Menten equation Steady state vs pre-steady state Today Review derivation and interpretation Graphical representation Michaelis Menten equations and parameters The Michaelis Menten
A First Course on Kinetics and Reaction Engineering. Class 9 on Unit 9
 A First Course on Kinetics and Reaction Engineering Class 9 on Unit 9 Part I - Chemical Reactions Part II - Chemical Reaction Kinetics Where We re Going A. Rate Expressions - 4. Reaction Rates and Temperature
A First Course on Kinetics and Reaction Engineering Class 9 on Unit 9 Part I - Chemical Reactions Part II - Chemical Reaction Kinetics Where We re Going A. Rate Expressions - 4. Reaction Rates and Temperature
LAB. FACTORS INFLUENCING ENZYME ACTIVITY
 AP Biology Date LAB. FACTORS INFLUENCING ENZYME ACTIVITY Background Enzymes are biological catalysts capable of speeding up chemical reactions by lowering activation energy. One benefit of enzyme catalysts
AP Biology Date LAB. FACTORS INFLUENCING ENZYME ACTIVITY Background Enzymes are biological catalysts capable of speeding up chemical reactions by lowering activation energy. One benefit of enzyme catalysts
Biofuel Enzyme LAB. Name
 Biofuel Enzyme LAB Name Background Enzymes are proteins that speed up the rate of chemical reactions. Since they do not chemically react with the substrate, they can work again and again to help convert
Biofuel Enzyme LAB Name Background Enzymes are proteins that speed up the rate of chemical reactions. Since they do not chemically react with the substrate, they can work again and again to help convert
CHM333 LECTURES 14 & 15: 2/15 17/12 SPRING 2012 Professor Christine Hrycyna
 ENZYME KINETICS: The rate of the reaction catalyzed by enzyme E A + B P is defined as -Δ[A] or -Δ[B] or Δ[P] Δt Δt Δt A and B changes are negative because the substrates are disappearing P change is positive
ENZYME KINETICS: The rate of the reaction catalyzed by enzyme E A + B P is defined as -Δ[A] or -Δ[B] or Δ[P] Δt Δt Δt A and B changes are negative because the substrates are disappearing P change is positive
A. One-Substrate Reactions (1) Kinetic concepts
 A. One-Substrate Reactions (1) Kinetic concepts (2) Kinetic analysis (a) Briggs-Haldane steady-state treatment (b) Michaelis constant (K m ) (c) Specificity constant (3) Graphical analysis (4) Practical
A. One-Substrate Reactions (1) Kinetic concepts (2) Kinetic analysis (a) Briggs-Haldane steady-state treatment (b) Michaelis constant (K m ) (c) Specificity constant (3) Graphical analysis (4) Practical
Lab 3: Soluble Enzyme Kinetics
 Taylor, A. Winter 2012 Lab 3: Soluble Enzyme Kinetics Introduction This lab will reinforce concepts addressed in BIOEN 335, Biotransport II. In particular, we will focus on enzyme kinetics. You have learned
Taylor, A. Winter 2012 Lab 3: Soluble Enzyme Kinetics Introduction This lab will reinforce concepts addressed in BIOEN 335, Biotransport II. In particular, we will focus on enzyme kinetics. You have learned
Materials: Micropipettes (2-20 µl range pipette, µl range, µl range), tips, test tubes with color dye, well plates
 Virtually every chemical reaction in a lab or manufacturing facility, as in cells, occurs in a watery environment or solution. A lab technician, therefore, must be able to quickly prepare any volume of
Virtually every chemical reaction in a lab or manufacturing facility, as in cells, occurs in a watery environment or solution. A lab technician, therefore, must be able to quickly prepare any volume of
Enzymes Part III: Enzyme kinetics. Dr. Mamoun Ahram Summer semester,
 Enzymes Part III: Enzyme kinetics Dr. Mamoun Ahram Summer semester, 2015-2016 Kinetics Kinetics is deals with the rates of chemical reactions. Chemical kinetics is the study of the rates of chemical reactions.
Enzymes Part III: Enzyme kinetics Dr. Mamoun Ahram Summer semester, 2015-2016 Kinetics Kinetics is deals with the rates of chemical reactions. Chemical kinetics is the study of the rates of chemical reactions.
Enzyme Catalysis Lab
 AP Biology Name: Enzyme Catalysis Lab Objectives In this laboratory, you will observe the role of an enzyme (catalase) in conversion of hydrogen peroxide (H 2 O 2 ) to water and oxygen determine the rate
AP Biology Name: Enzyme Catalysis Lab Objectives In this laboratory, you will observe the role of an enzyme (catalase) in conversion of hydrogen peroxide (H 2 O 2 ) to water and oxygen determine the rate
Student Manual. Background STUDENT MANUAL BACKGROUND. Enzymes
 Background Enzymes Enzymes are typically proteins (some nucleic acids have also been found to be enzymes) that act as catalysts, speeding up chemical reactions that would take far too long to occur on
Background Enzymes Enzymes are typically proteins (some nucleic acids have also been found to be enzymes) that act as catalysts, speeding up chemical reactions that would take far too long to occur on
Name Student number. UNIVERSITY OF GUELPH CHEM 4540 ENZYMOLOGY Winter 2002 Quiz #1: February 14, 2002, 11:30 13:00 Instructor: Prof R.
 UNIVERSITY OF GUELPH CHEM 4540 ENZYMOLOGY Winter 2002 Quiz #1: February 14, 2002, 11:30 13:00 Instructor: Prof R. Merrill Instructions: Time allowed = 90 minutes. Total marks = 30. This quiz represents
UNIVERSITY OF GUELPH CHEM 4540 ENZYMOLOGY Winter 2002 Quiz #1: February 14, 2002, 11:30 13:00 Instructor: Prof R. Merrill Instructions: Time allowed = 90 minutes. Total marks = 30. This quiz represents
Kinetics of Soil L-Glutaminase Enzyme
 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 6 Number 10 (2017) pp. 978-985 Journal homepage: http://www.ijcmas.com Original Research Article https://doi.org/10.20546/ijcmas.2017.610.118
International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 6 Number 10 (2017) pp. 978-985 Journal homepage: http://www.ijcmas.com Original Research Article https://doi.org/10.20546/ijcmas.2017.610.118
Lecture 13: Data Analysis and Interpretation of the Michaelis-Menten Parameters
 Biological Chemistry Laboratory Biology 3515/Chemistry 3515 Spring 2019 Lecture 13: Data Analysis and Interpretation of the Michaelis-Menten Parameters 19 February 2019 c David P. Goldenberg University
Biological Chemistry Laboratory Biology 3515/Chemistry 3515 Spring 2019 Lecture 13: Data Analysis and Interpretation of the Michaelis-Menten Parameters 19 February 2019 c David P. Goldenberg University
Class Business. I will have Project I graded by the end of the week. The discussion groups for Project 2 are cancelled
 Quiz 1 Class Business I will have Project I graded by the end of the week. Project 2 is due on 11/15 The discussion groups for Project 2 are cancelled There is additional reading for classes held on 10/30
Quiz 1 Class Business I will have Project I graded by the end of the week. Project 2 is due on 11/15 The discussion groups for Project 2 are cancelled There is additional reading for classes held on 10/30
Lecture 12: Burst Substrates and the V vs [S] Experiment
![Lecture 12: Burst Substrates and the V vs [S] Experiment Lecture 12: Burst Substrates and the V vs [S] Experiment](/thumbs/95/124907449.jpg) Biological Chemistry Laboratory Biology 3515/Chemistry 3515 Spring 2019 Lecture 12: Burst Substrates and the V vs [S] Experiment 14 February 2019 c David P. Goldenberg University of Utah goldenberg@biology.utah.edu
Biological Chemistry Laboratory Biology 3515/Chemistry 3515 Spring 2019 Lecture 12: Burst Substrates and the V vs [S] Experiment 14 February 2019 c David P. Goldenberg University of Utah goldenberg@biology.utah.edu
Peroxidase Enzyme Lab
 Peroxidase Enzyme Lab An enzyme is a type of protein known as a biological catalyst. A catalyst speeds up chemical reactions by lowering the activation energy required. Enzymes, biological catalysts carry
Peroxidase Enzyme Lab An enzyme is a type of protein known as a biological catalyst. A catalyst speeds up chemical reactions by lowering the activation energy required. Enzymes, biological catalysts carry
Rate laws, Reaction Orders. Reaction Order Molecularity. Determining Reaction Order
 Rate laws, Reaction Orders The rate or velocity of a chemical reaction is loss of reactant or appearance of product in concentration units, per unit time d[p] = d[s] The rate law for a reaction is of the
Rate laws, Reaction Orders The rate or velocity of a chemical reaction is loss of reactant or appearance of product in concentration units, per unit time d[p] = d[s] The rate law for a reaction is of the
Overview of MM kinetics
 Overview of MM kinetics Prepared by Robert L Sinsabaugh and Marcy P Osgood in 2007. Includes assumptions and deriviation of original MM model. Includes limitations and implications of MM application to
Overview of MM kinetics Prepared by Robert L Sinsabaugh and Marcy P Osgood in 2007. Includes assumptions and deriviation of original MM model. Includes limitations and implications of MM application to
MITOCW enzyme_kinetics
 MITOCW enzyme_kinetics In beer and wine production, enzymes in yeast aid the conversion of sugar into ethanol. Enzymes are used in cheese-making to degrade proteins in milk, changing their solubility,
MITOCW enzyme_kinetics In beer and wine production, enzymes in yeast aid the conversion of sugar into ethanol. Enzymes are used in cheese-making to degrade proteins in milk, changing their solubility,
LABORATORY 2. ENZYME CATALYSIS
 LABORATORY 2 STUDENT GUIDE LABORATORY 2. ENZYME CATALYSIS Objectives In this laboratory, you will observe the role of an enzyme (catalase) in conversion of hydrogen peroxide (H 2 O 2 ) to water and oxygen
LABORATORY 2 STUDENT GUIDE LABORATORY 2. ENZYME CATALYSIS Objectives In this laboratory, you will observe the role of an enzyme (catalase) in conversion of hydrogen peroxide (H 2 O 2 ) to water and oxygen
Lecture 15 (10/20/17) Lecture 15 (10/20/17)
 Reading: Ch6; 98-203 Ch6; Box 6- Lecture 5 (0/20/7) Problems: Ch6 (text); 8, 9, 0,, 2, 3, 4, 5, 6 Ch6 (study guide-facts); 6, 7, 8, 9, 20, 2 8, 0, 2 Ch6 (study guide-applying); NEXT Reading: Ch6; 207-20
Reading: Ch6; 98-203 Ch6; Box 6- Lecture 5 (0/20/7) Problems: Ch6 (text); 8, 9, 0,, 2, 3, 4, 5, 6 Ch6 (study guide-facts); 6, 7, 8, 9, 20, 2 8, 0, 2 Ch6 (study guide-applying); NEXT Reading: Ch6; 207-20
Enzyme Nomenclature Provides a Systematic Way of Naming Metabolic Reactions
 Enzyme Kinetics Virtually All Reactions in Cells Are Mediated by Enzymes Enzymes catalyze thermodynamically favorable reactions, causing them to proceed at extraordinarily rapid rates Enzymes provide cells
Enzyme Kinetics Virtually All Reactions in Cells Are Mediated by Enzymes Enzymes catalyze thermodynamically favorable reactions, causing them to proceed at extraordinarily rapid rates Enzymes provide cells
CHEM-E3205 BIOPROCESS OPTIMIZATION AND SIMULATION
 CHEM-E3205 BIOPROCESS OPTIMIZATION AND SIMULATION TERO EERIKÄINEN ROOM D416d tero.eerikainen@aalto.fi COURSE LECTURES AND EXERCISES Week Day Date Time Place Lectures/Execises 37 Mo 12.9.2016 10:15-11:45
CHEM-E3205 BIOPROCESS OPTIMIZATION AND SIMULATION TERO EERIKÄINEN ROOM D416d tero.eerikainen@aalto.fi COURSE LECTURES AND EXERCISES Week Day Date Time Place Lectures/Execises 37 Mo 12.9.2016 10:15-11:45
Ali Yaghi. Gumana Ghashan. Mamoun Ahram
 21 Ali Yaghi Gumana Ghashan Mamoun Ahram Kinetics The study of Kinetics deals with the rates of chemical reactions. Chemical kinetics is the study of the rate of chemical reactions. For the reaction (A
21 Ali Yaghi Gumana Ghashan Mamoun Ahram Kinetics The study of Kinetics deals with the rates of chemical reactions. Chemical kinetics is the study of the rate of chemical reactions. For the reaction (A
2 4 Chemical Reactions and Enzymes
 2 4 Chemical Reactions and Enzymes THINK ABOUT IT Living things are made up of chemical compounds, but chemistry isn t just what life is made of chemistry is also what life does. Everything that happens
2 4 Chemical Reactions and Enzymes THINK ABOUT IT Living things are made up of chemical compounds, but chemistry isn t just what life is made of chemistry is also what life does. Everything that happens
Chem Lecture 4 Enzymes Part 2
 Chem 452 - Lecture 4 Enzymes Part 2 Question of the Day: Is there some easy way to clock how many reactions one enzyme molecule is able to catalyze in an hour? Thermodynamics I think that enzymes are molecules
Chem 452 - Lecture 4 Enzymes Part 2 Question of the Day: Is there some easy way to clock how many reactions one enzyme molecule is able to catalyze in an hour? Thermodynamics I think that enzymes are molecules
Chemistry 1B Experiment 17 89
 Chemistry 1B Experiment 17 89 17 Thermodynamics of Borax Solubility Introduction In this experiment, you will determine the values of H and S for the reaction which occurs when borax (sodium tetraborate
Chemistry 1B Experiment 17 89 17 Thermodynamics of Borax Solubility Introduction In this experiment, you will determine the values of H and S for the reaction which occurs when borax (sodium tetraborate
Tala Saleh. Mohammad Omari. Dr. Ma moun
 20 0 Tala Saleh Mohammad Omari Razi Kittaneh Dr. Ma moun Quick recap The rate of Chemical Reactions Rises linearly as the substrate concentration [S] increases. The rate of Enzymatic Reactions Rises rapidly
20 0 Tala Saleh Mohammad Omari Razi Kittaneh Dr. Ma moun Quick recap The rate of Chemical Reactions Rises linearly as the substrate concentration [S] increases. The rate of Enzymatic Reactions Rises rapidly
THE EFFECT OF TEMPERATURE AND CONCENTRATION ON REACTION RATE
 THE EFFECT OF TEMPERATURE AND CONCENTRATION ON REACTION RATE INTRODUCTION FACTORS INFLUENCING REACTION RATE: The study of chemical reactions is not complete without a consideration of the rates at which
THE EFFECT OF TEMPERATURE AND CONCENTRATION ON REACTION RATE INTRODUCTION FACTORS INFLUENCING REACTION RATE: The study of chemical reactions is not complete without a consideration of the rates at which
Molarity of Acetic Acid in Vinegar A Titration Experiment
 Molarity of Acetic Acid in Vinegar A Titration Experiment Introduction Vinegar is prepared commercially in two steps, both requiring microorganisms. The first step is the production of ethyl alcohol, C
Molarity of Acetic Acid in Vinegar A Titration Experiment Introduction Vinegar is prepared commercially in two steps, both requiring microorganisms. The first step is the production of ethyl alcohol, C
Part II => PROTEINS and ENZYMES. 2.7 Enzyme Kinetics 2.7a Chemical Kinetics 2.7b Enzyme Inhibition
 Part II => PROTEINS and ENZYMES 2.7 Enzyme Kinetics 2.7a Chemical Kinetics 2.7b Enzyme Inhibition Section 2.7a: Chemical Kinetics Synopsis 2.7a - Chemical kinetics (or reaction kinetics) is the study of
Part II => PROTEINS and ENZYMES 2.7 Enzyme Kinetics 2.7a Chemical Kinetics 2.7b Enzyme Inhibition Section 2.7a: Chemical Kinetics Synopsis 2.7a - Chemical kinetics (or reaction kinetics) is the study of
Michaelis-Menton kinetics
 Michaelis-Menton kinetics The rate of an enzyme catalyzed reaction in which substrate S is converted into products P depends on the concentration of the enzyme E even though the enzyme does not undergo
Michaelis-Menton kinetics The rate of an enzyme catalyzed reaction in which substrate S is converted into products P depends on the concentration of the enzyme E even though the enzyme does not undergo
ENZYME KINETICS. Medical Biochemistry, Lecture 24
 ENZYME KINETICS Medical Biochemistry, Lecture 24 Lecture 24, Outline Michaelis-Menten kinetics Interpretations and uses of the Michaelis- Menten equation Enzyme inhibitors: types and kinetics Enzyme Kinetics
ENZYME KINETICS Medical Biochemistry, Lecture 24 Lecture 24, Outline Michaelis-Menten kinetics Interpretations and uses of the Michaelis- Menten equation Enzyme inhibitors: types and kinetics Enzyme Kinetics
Special Instructions You will use the Kern polarimeter in which angle of rotation readings are taken manually.
 Expt IS 1 Inversion Of Sucrose (IS) Objective The purpose of this experiment is to determine the rate constants for the acid catalyzed hydrolysis of sucrose at three temperatures and then determine the
Expt IS 1 Inversion Of Sucrose (IS) Objective The purpose of this experiment is to determine the rate constants for the acid catalyzed hydrolysis of sucrose at three temperatures and then determine the
Lecture 4 STEADY STATE KINETICS
 Lecture 4 STEADY STATE KINETICS The equations of enzyme kinetics are the conceptual tools that allow us to interpret quantitative measures of enzyme activity. The object of this lecture is to thoroughly
Lecture 4 STEADY STATE KINETICS The equations of enzyme kinetics are the conceptual tools that allow us to interpret quantitative measures of enzyme activity. The object of this lecture is to thoroughly
BT 47L. Enzyme Technology and Biokinetics Lab Manual
 SRI JAYACHAMARAJENDRA COLLEGE OF ENGINEERING, MYSURU 570 006 DEPARTMENT OF BIOTECHNOLOGY BT 47L Enzyme Technology and Biokinetics Lab Manual Experiment - 1 Determination of alkalinity in the given water
SRI JAYACHAMARAJENDRA COLLEGE OF ENGINEERING, MYSURU 570 006 DEPARTMENT OF BIOTECHNOLOGY BT 47L Enzyme Technology and Biokinetics Lab Manual Experiment - 1 Determination of alkalinity in the given water
IODINE CLOCK REACTION KINETICS
 Name: Section Chemistry 104 Laboratory University of Massachusetts Boston IODINE CLOCK REACTION KINETICS PRELAB ASSIGNMENT Calculate the initial concentration of H 2 O 2 that exists immediately after mixing
Name: Section Chemistry 104 Laboratory University of Massachusetts Boston IODINE CLOCK REACTION KINETICS PRELAB ASSIGNMENT Calculate the initial concentration of H 2 O 2 that exists immediately after mixing
Thermodynamics of Borax Dissolution
 Thermodynamics of Borax Dissolution Introduction In this experiment, you will determine the values of H, G and S for the reaction which occurs when borax (sodium tetraborate octahydrate) dissolves in water.
Thermodynamics of Borax Dissolution Introduction In this experiment, you will determine the values of H, G and S for the reaction which occurs when borax (sodium tetraborate octahydrate) dissolves in water.
THE EFFECT OF TEMPERATURE AND CONCENTRATION ON REACTION RATE
 THE EFFECT OF TEMPERATURE AND CONCENTRATION ON REACTION RATE INTRODUCTION FACTORS INFLUENCING REACTION RATE: The study of chemical reactions is not complete without a consideration of the rates at which
THE EFFECT OF TEMPERATURE AND CONCENTRATION ON REACTION RATE INTRODUCTION FACTORS INFLUENCING REACTION RATE: The study of chemical reactions is not complete without a consideration of the rates at which
The Chemistry of Life
 The Chemistry of Life Things you should be able to do 1. Describe how the unique properties of water support life on Earth. 2. Explain how carbon is uniquely suited to form biological macromolecules. 3.
The Chemistry of Life Things you should be able to do 1. Describe how the unique properties of water support life on Earth. 2. Explain how carbon is uniquely suited to form biological macromolecules. 3.
Studies of a Precipitation Reaction
 Studies of a Precipitation Reaction Prelab Assignment Read the entire lab. Write an objective and any hazards associated with this lab in your laboratory notebook. Answer the following 6 questions in your
Studies of a Precipitation Reaction Prelab Assignment Read the entire lab. Write an objective and any hazards associated with this lab in your laboratory notebook. Answer the following 6 questions in your
After lectures by. disappearance of reactants or appearance of. measure a reaction rate we monitor the. Reaction Rates (reaction velocities): To
 Revised 3/21/2017 After lectures by Dr. Loren Williams (GeorgiaTech) Protein Folding: 1 st order reaction DNA annealing: 2 nd order reaction Reaction Rates (reaction velocities): To measure a reaction
Revised 3/21/2017 After lectures by Dr. Loren Williams (GeorgiaTech) Protein Folding: 1 st order reaction DNA annealing: 2 nd order reaction Reaction Rates (reaction velocities): To measure a reaction
From Friday s material
 5.111 Lecture 35 35.1 Kinetics Topic: Catalysis Chapter 13 (Section 13.14-13.15) From Friday s material Le Chatelier's Principle - when a stress is applied to a system in equilibrium, the equilibrium tends
5.111 Lecture 35 35.1 Kinetics Topic: Catalysis Chapter 13 (Section 13.14-13.15) From Friday s material Le Chatelier's Principle - when a stress is applied to a system in equilibrium, the equilibrium tends
CEE 697K ENVIRONMENTAL REACTION KINETICS
 Updated: 19 November 2013 1 Print version CEE 697K ENVIRONMENTAL REACTION KINETICS Lecture #19 Chloramines Cont: Primary Literature Enzyme Kinetics: basics Brezonik, pp. 419-450 Introduction Conclusions
Updated: 19 November 2013 1 Print version CEE 697K ENVIRONMENTAL REACTION KINETICS Lecture #19 Chloramines Cont: Primary Literature Enzyme Kinetics: basics Brezonik, pp. 419-450 Introduction Conclusions
ENZYME SCIENCE AND ENGINEERING PROF. SUBHASH CHAND DEPARTMENT OF BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY IIT DELHI
 ENZYME SCIENCE AND ENGINEERING PROF. SUBHASH CHAND DEPARTMENT OF BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY IIT DELHI LECTURE 23 STEADY STATE ANALYSIS OF MASS TRANSFER & BIOCHEMICAL REACTION IN IME REACTORS
ENZYME SCIENCE AND ENGINEERING PROF. SUBHASH CHAND DEPARTMENT OF BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY IIT DELHI LECTURE 23 STEADY STATE ANALYSIS OF MASS TRANSFER & BIOCHEMICAL REACTION IN IME REACTORS
2. Which of the following are nucleophiles and which are electrophiles?
 Life Sciences 1a ractice roblems 7 1. a) ow many intermediates are there in the reaction? b) ow many transition states are there? c) What is the fastest step in the reaction? d) Which is more stable, A
Life Sciences 1a ractice roblems 7 1. a) ow many intermediates are there in the reaction? b) ow many transition states are there? c) What is the fastest step in the reaction? d) Which is more stable, A
CHEM 251 (4 credits): Description
 CHEM 251 (4 credits): Intermediate Reactions of Nucleophiles and Electrophiles (Reactivity 2) Description: An understanding of chemical reactivity, initiated in Reactivity 1, is further developed based
CHEM 251 (4 credits): Intermediate Reactions of Nucleophiles and Electrophiles (Reactivity 2) Description: An understanding of chemical reactivity, initiated in Reactivity 1, is further developed based
It is generally believed that the catalytic reactions occur in at least two steps.
 Lecture 16 MECHANISM OF ENZYME ACTION A chemical reaction such as A ----> P takes place because a certain fraction of the substrate possesses enough energy to attain an activated condition called the transition
Lecture 16 MECHANISM OF ENZYME ACTION A chemical reaction such as A ----> P takes place because a certain fraction of the substrate possesses enough energy to attain an activated condition called the transition
Biology 30 The Chemistry of Living Things
 Biology 30 The Chemistry of Living Things Hierarchy of organization: Chemistry: MATTER: Periodic Table: ELEMENT: Ex. oxygen, gold, copper, carbon COMPOUND: Ex. salt (NaCl), H 2 O ELEMENTS ESSENTIAL TO
Biology 30 The Chemistry of Living Things Hierarchy of organization: Chemistry: MATTER: Periodic Table: ELEMENT: Ex. oxygen, gold, copper, carbon COMPOUND: Ex. salt (NaCl), H 2 O ELEMENTS ESSENTIAL TO
Enzyme Kinetics. Differential Equations Series. Instructor s Guide. Table of Contents
 Enzyme Kinetics Differential Equations Series Instructor s Guide Table of Contents Introduction.... 2 When to Use this Video.... 2 Learning Objectives.... 2 Motivation.... 2 Student Experience.... 2 Key
Enzyme Kinetics Differential Equations Series Instructor s Guide Table of Contents Introduction.... 2 When to Use this Video.... 2 Learning Objectives.... 2 Motivation.... 2 Student Experience.... 2 Key
[C] [D] K eq = [A] [B]
![[C] [D] K eq = [A] [B] [C] [D] K eq = [A] [B]](/thumbs/76/73734780.jpg) Enzyme Kinetics: Properties of -Galactosidase Preparation for Laboratory: Web Tutorial 4, Beta Galactosidase - submit answers to questions Additonal background: Freeman, Proteins pp 51-54 and Box 3.3 pp56-57,
Enzyme Kinetics: Properties of -Galactosidase Preparation for Laboratory: Web Tutorial 4, Beta Galactosidase - submit answers to questions Additonal background: Freeman, Proteins pp 51-54 and Box 3.3 pp56-57,
Lecture 11: Enzymes: Kinetics [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 8, pp
![Lecture 11: Enzymes: Kinetics [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 8, pp Lecture 11: Enzymes: Kinetics [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 8, pp](/thumbs/74/70848503.jpg) Lecture 11: Enzymes: Kinetics [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 8, pp. 216-225 Updated on: 2/4/07 at 9:00 pm Key Concepts Kinetics is the study of reaction rates. Study of enzyme kinetics
Lecture 11: Enzymes: Kinetics [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 8, pp. 216-225 Updated on: 2/4/07 at 9:00 pm Key Concepts Kinetics is the study of reaction rates. Study of enzyme kinetics
Chemical Reac+ons and Enzymes. Lesson Overview. Lesson Overview. 2.4 Chemical Reactions and Enzymes
 Lesson Overview Chemical Reac+ons and Enzymes Lesson Overview 2.4 Chemical Reactions and Enzymes THINK ABOUT IT Living things are made up of chemical compounds, but chemistry isn t just what life is made
Lesson Overview Chemical Reac+ons and Enzymes Lesson Overview 2.4 Chemical Reactions and Enzymes THINK ABOUT IT Living things are made up of chemical compounds, but chemistry isn t just what life is made
Answer: the study of the composition of matter and changes that matter undergoes
 Vocabulary Vocabulary and Math - 200-200 Vocabulary Vocabulary and Math - 200-200 Define chemistry Answer: the study of the composition of matter and changes that matter undergoes Vocabulary Vocabulary
Vocabulary Vocabulary and Math - 200-200 Vocabulary Vocabulary and Math - 200-200 Define chemistry Answer: the study of the composition of matter and changes that matter undergoes Vocabulary Vocabulary
2-4 Chemical Reactions and Enzymes
 2-4 Chemical Reactions and Enzymes Living things, as you have seen, are made up of chemical compounds-some simple and some complex. But chemistry isn t just what life is made of-chemistry is also what
2-4 Chemical Reactions and Enzymes Living things, as you have seen, are made up of chemical compounds-some simple and some complex. But chemistry isn t just what life is made of-chemistry is also what
LAB 05 Enzyme Action
 LAB 05 Enzyme Action Objectives: Name the substrate and products of the peroxidase-catalyzed reaction. To understand the terms: enzyme, activation energy, active site, ph, and denaturation. Distinguish
LAB 05 Enzyme Action Objectives: Name the substrate and products of the peroxidase-catalyzed reaction. To understand the terms: enzyme, activation energy, active site, ph, and denaturation. Distinguish
Reaction Rates and Equilibrium
 CHAPTER 7 14 SECTION Chemical Reactions Reaction Rates and Equilibrium KEY IDEAS As you read this section, keep these questions in mind: How can you increase the rate of a reaction? What does a catalyst
CHAPTER 7 14 SECTION Chemical Reactions Reaction Rates and Equilibrium KEY IDEAS As you read this section, keep these questions in mind: How can you increase the rate of a reaction? What does a catalyst
Previous Class. Today. Cosubstrates (cofactors)
 Previous Class Cosubstrates (cofactors) Today Proximity effect Basic equations of Kinetics Steady state kinetics Michaelis Menten equations and parameters Enzyme Kinetics Enzyme kinetics implies characterizing
Previous Class Cosubstrates (cofactors) Today Proximity effect Basic equations of Kinetics Steady state kinetics Michaelis Menten equations and parameters Enzyme Kinetics Enzyme kinetics implies characterizing
ENZYME KINETICS. What happens to S, P, E, ES?
 ENZYME KINETICS Go to lecture notes and/or supplementary handouts for the following: 1 Basic observations in enzyme inetics 2 Michaelis-Menten treatment of enzyme inetics 3 Briggs-Haldane treatment of
ENZYME KINETICS Go to lecture notes and/or supplementary handouts for the following: 1 Basic observations in enzyme inetics 2 Michaelis-Menten treatment of enzyme inetics 3 Briggs-Haldane treatment of
Solubility of KHT and Common ion Effect
 Solubility of KHT and Common ion Effect v010516 You are encouraged to carefully read the following sections in Tro (3 rd ed.) to prepare for this experiment: Sec 16.5, pp 783-788 (Solubility Equilibria
Solubility of KHT and Common ion Effect v010516 You are encouraged to carefully read the following sections in Tro (3 rd ed.) to prepare for this experiment: Sec 16.5, pp 783-788 (Solubility Equilibria
Buffers for Biological Systems Laboratory Instructor s Manual
 Buffers for Biological Systems Laboratory Instructor s Manual 1. Purpose and Concepts Covered...1 2. Effect of Temperature and Concentration on ph...1 A. Preparing Buffers...2 B. Analysis and Discussion...3
Buffers for Biological Systems Laboratory Instructor s Manual 1. Purpose and Concepts Covered...1 2. Effect of Temperature and Concentration on ph...1 A. Preparing Buffers...2 B. Analysis and Discussion...3
Reading for today: Chapter 16 (selections from Sections A, B and C) Friday and Monday: Chapter 17 (Diffusion)
 Lecture 29 Enzymes Reading for today: Chapter 6 (selections from Sections, B and C) Friday and Monday: Chapter 7 (Diffusion) 4/3/6 Today s Goals Michaelis-Menten mechanism for simple enzyme reactions:
Lecture 29 Enzymes Reading for today: Chapter 6 (selections from Sections, B and C) Friday and Monday: Chapter 7 (Diffusion) 4/3/6 Today s Goals Michaelis-Menten mechanism for simple enzyme reactions:
CHEMICAL REACTION ENGINEERING LAB
 CHEMICAL REACTION ENGINEERING LAB EQUIPMENTS 1.CHEMICAL REACTORS SERVICE UNIT The chemical reactors service unit consists of a moulded ABS plinth which is used as a mounting for the chemical reactor to
CHEMICAL REACTION ENGINEERING LAB EQUIPMENTS 1.CHEMICAL REACTORS SERVICE UNIT The chemical reactors service unit consists of a moulded ABS plinth which is used as a mounting for the chemical reactor to
Chem 204. Mid-Term Exam I. July 21, There are 3 sections to this exam: Answer ALL questions
 Chem 204 Mid-Term Exam I July 21, 2009 Name: Answer Key Student ID: There are 3 sections to this exam: Answer ALL questions Section I: Multiple-Choice 20 questions, 2 pts each Section II: Fill-in-the-Blank
Chem 204 Mid-Term Exam I July 21, 2009 Name: Answer Key Student ID: There are 3 sections to this exam: Answer ALL questions Section I: Multiple-Choice 20 questions, 2 pts each Section II: Fill-in-the-Blank
5.60 Thermodynamics & Kinetics Spring 2008
 MIT OpenCourseWare http://ocw.it.edu 5.60 Therodynaics & Kinetics Spring 2008 For inforation about citing these aterials or our Ters of Use, visit: http://ocw.it.edu/ters. 1 Enzye Catalysis Readings: SAB,
MIT OpenCourseWare http://ocw.it.edu 5.60 Therodynaics & Kinetics Spring 2008 For inforation about citing these aterials or our Ters of Use, visit: http://ocw.it.edu/ters. 1 Enzye Catalysis Readings: SAB,
Biochemistry. Lecture 8 Enzyme Kinetics
 Biochemistry Lecture 8 Enzyme Kinetics Why Enzymes? igher reaction rates Greater reaction specificity Milder reaction conditions Capacity for regulation C - - C N 2 - C N 2 - C - C Chorismate mutase -
Biochemistry Lecture 8 Enzyme Kinetics Why Enzymes? igher reaction rates Greater reaction specificity Milder reaction conditions Capacity for regulation C - - C N 2 - C N 2 - C - C Chorismate mutase -
XHCO3(s) + HCl(aq) XCl(aq) + H2O(l) + CO2(g)
 Determining the Molar Mass of an Unknown Carbonate Using the Ideal Gas Law In this lab you will determine the molar mass of an unknown carbonate by using the ideal gas law to determine the number of moles
Determining the Molar Mass of an Unknown Carbonate Using the Ideal Gas Law In this lab you will determine the molar mass of an unknown carbonate by using the ideal gas law to determine the number of moles
Deriving the Michaelis-Menten Equation
 Page 1 of 5 Deriving the Michaelis-Menten Equation This page is originally authored by Gale Rhodes ( Jan 2000) and is still under continuous update. The page has been modified with permission by Claude
Page 1 of 5 Deriving the Michaelis-Menten Equation This page is originally authored by Gale Rhodes ( Jan 2000) and is still under continuous update. The page has been modified with permission by Claude
EXPERIMENT 1 REACTION RATE, RATE LAW, AND ACTIVATION ENERGY THE IODINE CLOCK REACTION
 PURPOSE: To determine the Rate Law and the Activation Energy for a reaction from experimental data. PRINCIPLES: The Rate Law is a mathematical expression that predicts the rate of a reaction from the concentration
PURPOSE: To determine the Rate Law and the Activation Energy for a reaction from experimental data. PRINCIPLES: The Rate Law is a mathematical expression that predicts the rate of a reaction from the concentration
Lecture 11: Enzyme Kinetics, Part I
 Biological Chemistry Laboratory Biology 3515/Chemistry 3515 Spring 2018 Lecture 11: Enzyme Kinetics, Part I 13 February 2018 c David P. Goldenberg University of Utah goldenberg@biology.utah.edu Back to
Biological Chemistry Laboratory Biology 3515/Chemistry 3515 Spring 2018 Lecture 11: Enzyme Kinetics, Part I 13 February 2018 c David P. Goldenberg University of Utah goldenberg@biology.utah.edu Back to
Sample Questions for the Chemistry of Life Topic Test
 Sample Questions for the Chemistry of Life Topic Test 1. Enzymes play a crucial role in biology by serving as biological catalysts, increasing the rates of biochemical reactions by decreasing their activation
Sample Questions for the Chemistry of Life Topic Test 1. Enzymes play a crucial role in biology by serving as biological catalysts, increasing the rates of biochemical reactions by decreasing their activation
