9/19/2018. Corrosion Thermodynamics 2-3. Course Outline. Guiding Principles. Why study thermodynamics? Guiding Principles
|
|
|
- Marjorie Clarissa Sharp
- 5 years ago
- Views:
Transcription
1 Kwame Nkrumah University of Science & Technology, Kumasi, Ghana Week 1 Course Outline Topic Introduction: Reactivity types, corrosion definition, atmospheric corrosion, classification, effects, costs, risk management Corrosion Thermodynamics Corrosion thermodynamics: Corrosion reactions, cell requirements, free energy change, electrochemical potential, Nernst equation, Eh-pH (Pourbaix) diagrams, reference electrodes Corrosion kinetics: Electrical double layer, exchange current density, activation and mass transport control, mixed potential theory, polarization diagrams, passivity 7 Mid Semester Exams 2 Why study thermodynamics? Provides understanding of energy changes involved in electrochemical corrosion reactions Show how conditions may be adjusted to control corrosion Immunity Passivity Guiding Principles Corrosion is only a question of time rust never sleeps Corrosion of metals is electrochemical in nature o Reactions involve both charge (electron) and solvated proton (H ) transfer Fe 2H 2 O = Fe(OH) 2 2H 2e Any reaction which can be divided into two (or more) partial reactions of oxidation and reduction is termed electrochemical. Eg. Reaction of Zn in HCl 3 4 Guiding Principles Chemical reaction metal (iron) either changes into rust or dissolves o Acid-base reactions involving only solvated proton (H ) transfer (no electron transfer) Fe(OH) 2 = HFeO 2- H 2Fe(OH) 3 = Fe 2 O 3.3H 2 O Corrosion rate depends on both the metal (and its condition) and the environment. Cr 2 O 3 (SS) is dynamic layer and dissolves in reducing environment. Al 2 O 3 is static. Presence of moisture is important 5 Electrochemical Nature of Corrosion Corrosion of zinc in an acid solution Two reactions are necessary: -- oxidation reaction: Zn Zn 2 2e -- reduction reaction: 2H 2e H 2 (gas) H Oxidation reaction flow of e - Zn Zn 2 H in the metal Zinc H Acid 2e- H solution H H H 2 (gas) H reduction reaction Other reduction reactions in solutions with dissolved oxygen: -- acidic solution -- neutral or basic solution O 2 4H 4e 2H 2 O O 2 2H 2 O 4e 4(OH) 6 1
2 Corrosion Cell Four Prerequisites for Corrosion 1. An anode on a metal surface (oxidation rxn). e.g. Fe = Fe 2 2e - 2. A cathode on a metal surface (reduction rxn). e.g. O 2 2H 2 O 4e - = 4OH - 3. Electrolyte in contact with anode and cathode (path for ionic conduction). 4. An electrical connection between anode and cathode (allows electrons to flow between anode and cathode) Importance of the Four Prerequisites of Corrosion Corrosion Control: Stopping the anodic reaction (e.g. passivation, cathodic protection) Stopping the cathodic reaction (e.g. removing dissolved oxygen) Stopping ion flow between anodes and cathodes (e.g. organic coatings) Why do corrosion cells form? Corrosion cells form due to the difference in energy between the metal and the environment. Variations could be Metallurgical composition, microstructure, inclusions, heat treatment, welding, fabrication etc. Environmental temperature induced corrosion, microbial induced corrosion, environmental induced SCC etc. Etching as Corrosion Impurity atom/compound 2
3 Multiphase structure Anodic Processes Consider similar cell reactions: Zn 2HCl ZnCl 2 H 2 Zn H 2 SO 4 ZnSO 4 H 2 Fe 2HCl FeCl 2 H 2 2Al 6HCl 2AlCl 3 3H 2 Separation into anodic reactions: Zn Zn 2 2e- Fe Fe 2 2e- Al Al 3 3e- Some Key Anodic Processes Dissolution-Precipitation M M z ze- (electrochemical reaction) -- M z zh 2 O = M(OH) z zh (chemical reaction) Example: Pb H 2 SO 4 PbSO 4 H 2 (cell reaction) Generalized anodic reaction: M M z ze Cathodic Processes Hydrogen Evolution (Deaerated Acids): 2H 2e- H 2 Oxygen Reduction: O 2 4H 4e- H 2 O (Acidic) O 2 2H 2 O 4e- 4OH - (Neutral/Alkaline) Metal Ion Reduction: Fe 3 e- Fe 2 Metal Deposition: Cu 2 2e- Cu Direct Film Formation M H 2 O = MO ads 2H 2e- M H 2 O = MO (oxide) 2H 2e- Example: 2Cr 3H 2 O = Cr 2 O 3 (s) 6H 6e Types of Electrochemical Cells Voltaic Cell (Galvanic Cell) 3
4 Electrolytic Cell Galvanic Cell vs. Electrolytic Cell Standard Cell Reaction Direction Consider two corrosion cell reactions: Zn(s) HCl(aq) = ZnCl 2 (aq) H 2 (g) Cu(s) HCl(aq) = CuCl 2 (aq) H 2 (g) Are the reactions spontaneous in the direction written? 1. Zn/HCl cell: Anode: Zn = Zn 2 2e; E 0 a = -E 0 c = -(-0.762V) Cathode: 2H 2e = H 2 ; E 0 c = 0V E 0 (cell) = E 0 (anode) E 0 (cathode) = 0.762V Spontaneous as written 2. Cu/HCl cell: Anode: Cu = Cu 2 2e; E 0 a = -E 0 c = -(0.342V) Cathode: 2H 2e = H 2 ; E 0 c = 0V E 0 (cell) = V Not spontaneous as written Cell Potential Corrosion cell is usually represented as: Zn Zn 2 Cu 2 Cu The two half-cell reactions are: 1. Zn = Zn 2 2e 2. Cu 2 2e = Cu The overall reaction is: Zn Cu 2 = Zn 2 Cu Derivation of the Nernst Equation The free energy change, ΔG, is related to the cell potential by Faraday s law: G nfe were n = number of electrons transferred in corrosion reaction F = Faraday s constant, 96,500 C/mole E = cell potential in a given state (Volts) ΔG = Joules Under standard conditions: 0 0 G nfe The Nernst Equation Consider the following reaction: A B = C D G G 0 [ C][ G G RT ln{ } [ A][ 0 [ C][ nfe nfe RT ln{ } [ A][ E E 0 0 RT ln K RT [ C][ ln{ } nf [ A][ One of the most fundamental equations in corrosion engineering! 4
5 The Nernst Equation Under standard conditions: T = 298K, R = J(mol.K) -1 Nernst equation becomes [ C][ E E log{ } n [ A][ E = non equilibrium potential EMF series metal Au Cu Pb Sn Ni Co Fe Cr Zn Al Mg Na K more anodic more cathodic Standard EMF Series V o metal V o V = 0.153V Metal with smaller Vo metal corrodes. Ex: o -Ni cell V < V o Ni corrodes - 25 C Ni 1.0 M 1.0 M 2 solution Ni 2 solution 26 Solution concentration and temperature Ex: -Ni cell with standard 1 M solutions V o Ni V o - 25 C 1.0 M 2 solution V Ni 1.0 M Ni 2 solution G = -nf E Ex: -Ni cell with non-standard solutions o o RT X VNi V VNi V ln - nf Y n = #e - per unit oxid/red T Ni reaction (= 2 here) X M 2 solution Y M Ni 2 solution F = Faraday's constant = 96,500 C/mol. 27 The Nernst Equation Question What is the thermodynamic tendency for tin (Sn) to corrode in deaerated sulphuric acid (H 2 SO 4 ) at ph = 2, activity of Sn = 10-6, p H2 = 1.0 atm, at 25 C? E 0 = V Importance of Potential Interfacial potential (E) Potential of corroding metal minus potential in electrolyte (next to metal surface) Measurement of Cell Potential V Importance Potential can be measured readily Potential affects rate of corrosion Require reference electrode for measurement M Electrolyte Ref Porous tip 5
6 Common Reference Electrodes Conversion between reference electrodes 32 Hydrogen Evolution Reaction Neutral or Alkaline Environment Standard State, e c = 0 V Half Cell Potential = F(pH) Oxygen Evolution Reaction Standard State, e c = V 6
7 Neutral or Alkaline Environment Half Cell Potential = F(pH) Stability of H 2 O 7
Electrochemical System
 Electrochemical System Topic Outcomes Week Topic Topic Outcomes 8-10 Electrochemical systems It is expected that students are able to: Electrochemical system and its thermodynamics Chemical reactions in
Electrochemical System Topic Outcomes Week Topic Topic Outcomes 8-10 Electrochemical systems It is expected that students are able to: Electrochemical system and its thermodynamics Chemical reactions in
Oxidation-Reduction Review. Electrochemistry. Oxidation-Reduction Reactions. Oxidation-Reduction Reactions. Sample Problem.
 1 Electrochemistry Oxidation-Reduction Review Topics Covered Oxidation-reduction reactions Balancing oxidationreduction equations Voltaic cells Cell EMF Spontaneity of redox reactions Batteries Electrolysis
1 Electrochemistry Oxidation-Reduction Review Topics Covered Oxidation-reduction reactions Balancing oxidationreduction equations Voltaic cells Cell EMF Spontaneity of redox reactions Batteries Electrolysis
Topic 19 Redox 19.1 Standard Electrode Potentials. IB Chemistry T09D04
 Topic 19 Redox 19.1 Standard Electrode Potentials IB Chemistry T09D04 19.1 Standard Electrode Potentials 19.1.1 Describe the standard hydrogen electrode. (2) 19.1.2 Define the term standard electrode potential,
Topic 19 Redox 19.1 Standard Electrode Potentials IB Chemistry T09D04 19.1 Standard Electrode Potentials 19.1.1 Describe the standard hydrogen electrode. (2) 19.1.2 Define the term standard electrode potential,
Lecture Presentation. Chapter 20. Electrochemistry. James F. Kirby Quinnipiac University Hamden, CT Pearson Education, Inc.
 Lecture Presentation Chapter 20 James F. Kirby Quinnipiac University Hamden, CT is the study of the relationships between electricity and chemical reactions. It includes the study of both spontaneous and
Lecture Presentation Chapter 20 James F. Kirby Quinnipiac University Hamden, CT is the study of the relationships between electricity and chemical reactions. It includes the study of both spontaneous and
Chapter 20 Electrochemistry
 Chapter 20 Electrochemistry Learning goals and key skills: Identify oxidation, reduction, oxidizing agent, and reducing agent in a chemical equation Complete and balance redox equations using the method
Chapter 20 Electrochemistry Learning goals and key skills: Identify oxidation, reduction, oxidizing agent, and reducing agent in a chemical equation Complete and balance redox equations using the method
Chapter 17. Electrochemistry
 Chapter 17 Electrochemistry Contents Galvanic cells Standard reduction potentials Cell potential, electrical work, and free energy Dependence of cell potential on concentration Batteries Corrosion Electrolysis
Chapter 17 Electrochemistry Contents Galvanic cells Standard reduction potentials Cell potential, electrical work, and free energy Dependence of cell potential on concentration Batteries Corrosion Electrolysis
CHEMISTRY 13 Electrochemistry Supplementary Problems
 1. When the redox equation CHEMISTRY 13 Electrochemistry Supplementary Problems MnO 4 (aq) + H + (aq) + H 3 AsO 3 (aq) Mn 2+ (aq) + H 3 AsO 4 (aq) + H 2 O(l) is properly balanced, the coefficients will
1. When the redox equation CHEMISTRY 13 Electrochemistry Supplementary Problems MnO 4 (aq) + H + (aq) + H 3 AsO 3 (aq) Mn 2+ (aq) + H 3 AsO 4 (aq) + H 2 O(l) is properly balanced, the coefficients will
17.1 Redox Chemistry Revisited
 Chapter Outline 17.1 Redox Chemistry Revisited 17.2 Electrochemical Cells 17.3 Standard Potentials 17.4 Chemical Energy and Electrical Work 17.5 A Reference Point: The Standard Hydrogen Electrode 17.6
Chapter Outline 17.1 Redox Chemistry Revisited 17.2 Electrochemical Cells 17.3 Standard Potentials 17.4 Chemical Energy and Electrical Work 17.5 A Reference Point: The Standard Hydrogen Electrode 17.6
Lecture Presentation. Chapter 20. Electrochemistry. James F. Kirby Quinnipiac University Hamden, CT Pearson Education
 Lecture Presentation Chapter 20 James F. Kirby Quinnipiac University Hamden, CT is the study of the relationships between electricity and chemical reactions. It includes the study of both spontaneous and
Lecture Presentation Chapter 20 James F. Kirby Quinnipiac University Hamden, CT is the study of the relationships between electricity and chemical reactions. It includes the study of both spontaneous and
CHAPTER 12. Practice exercises
 CHAPTER 12 Practice exercises 12.1 2Al(s) + 3Cl 2 (g) 2AlCl 3 (aq) Aluminium is oxidised and is therefore the reducing agent. Chlorine is reduced and is therefore the oxidising agent. 12.3 First the oxidation
CHAPTER 12 Practice exercises 12.1 2Al(s) + 3Cl 2 (g) 2AlCl 3 (aq) Aluminium is oxidised and is therefore the reducing agent. Chlorine is reduced and is therefore the oxidising agent. 12.3 First the oxidation
Chapter Nineteen. Electrochemistry
 Chapter Nineteen Electrochemistry 1 Electrochemistry The study of chemical reactions through electrical circuits. Monitor redox reactions by controlling electron transfer REDOX: Shorthand for REDuction-OXidation
Chapter Nineteen Electrochemistry 1 Electrochemistry The study of chemical reactions through electrical circuits. Monitor redox reactions by controlling electron transfer REDOX: Shorthand for REDuction-OXidation
ELECTROCHEMISTRY OXIDATION-REDUCTION
 ELECTROCHEMISTRY Electrochemistry involves the relationship between electrical energy and chemical energy. OXIDATION-REDUCTION REACTIONS SPONTANEOUS REACTIONS Can extract electrical energy from these.
ELECTROCHEMISTRY Electrochemistry involves the relationship between electrical energy and chemical energy. OXIDATION-REDUCTION REACTIONS SPONTANEOUS REACTIONS Can extract electrical energy from these.
Chapter 18 Electrochemistry. Electrochemical Cells
 Chapter 18 Electrochemistry Chapter 18 1 Electrochemical Cells Electrochemical Cells are of two basic types: Galvanic Cells a spontaneous chemical reaction generates an electric current Electrolytic Cells
Chapter 18 Electrochemistry Chapter 18 1 Electrochemical Cells Electrochemical Cells are of two basic types: Galvanic Cells a spontaneous chemical reaction generates an electric current Electrolytic Cells
Chapter 19: Electrochemistry
 Chapter 19: Electrochemistry Overview of the Chapter review oxidation-reduction chemistry basics galvanic cells spontaneous chemical reaction generates a voltage set-up of galvanic cell & identification
Chapter 19: Electrochemistry Overview of the Chapter review oxidation-reduction chemistry basics galvanic cells spontaneous chemical reaction generates a voltage set-up of galvanic cell & identification
SHOCK TO THE SYSTEM! ELECTROCHEMISTRY
 SHOCK TO THE SYSTEM! ELECTROCHEMISTRY REVIEW I. Re: Balancing Redox Reactions. A. Every redox reaction requires a substance to be... 1. oxidized (loses electrons). a.k.a. reducing agent 2. reduced (gains
SHOCK TO THE SYSTEM! ELECTROCHEMISTRY REVIEW I. Re: Balancing Redox Reactions. A. Every redox reaction requires a substance to be... 1. oxidized (loses electrons). a.k.a. reducing agent 2. reduced (gains
Redox reactions & electrochemistry
 Redox reactions & electrochemistry Electrochemistry Electrical energy ; Chemical energy oxidation/reduction = redox reactions Electrochemistry Zn + Cu 2+ º Zn 2+ + Cu Oxidation-reduction reactions always
Redox reactions & electrochemistry Electrochemistry Electrical energy ; Chemical energy oxidation/reduction = redox reactions Electrochemistry Zn + Cu 2+ º Zn 2+ + Cu Oxidation-reduction reactions always
Electrochemistry. A. Na B. Ba C. S D. N E. Al. 2. What is the oxidation state of Xe in XeO 4? A +8 B +6 C +4 D +2 E 0
 Electrochemistry 1. Element M reacts with oxygen to from an oxide with the formula MO. When MO is dissolved in water, the resulting solution is basic. Element M is most likely: A. Na B. Ba C. S D. N E.
Electrochemistry 1. Element M reacts with oxygen to from an oxide with the formula MO. When MO is dissolved in water, the resulting solution is basic. Element M is most likely: A. Na B. Ba C. S D. N E.
Oxidation number. The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred.
 Oxidation number The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. Na,
Oxidation number The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. Na,
Review: Balancing Redox Reactions. Review: Balancing Redox Reactions
 Review: Balancing Redox Reactions Determine which species is oxidized and which species is reduced Oxidation corresponds to an increase in the oxidation number of an element Reduction corresponds to a
Review: Balancing Redox Reactions Determine which species is oxidized and which species is reduced Oxidation corresponds to an increase in the oxidation number of an element Reduction corresponds to a
Applications of Voltaic Cells
 Applications of Voltaic Cells Lesson 4 chapter 13 Objective You will be able to explain how the development of the voltaic cell had affected society. Dry Cells Since voltaic cells are not portable, dry
Applications of Voltaic Cells Lesson 4 chapter 13 Objective You will be able to explain how the development of the voltaic cell had affected society. Dry Cells Since voltaic cells are not portable, dry
Chapter 20. Electrochemistry. Chapter 20 Problems. Electrochemistry 7/3/2012. Problems 15, 17, 19, 23, 27, 29, 33, 39, 59
 Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 20 John D. Bookstaver St. Charles Community College Cottleville, MO Chapter 20 Problems
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 20 John D. Bookstaver St. Charles Community College Cottleville, MO Chapter 20 Problems
CHAPTER 17: ELECTROCHEMISTRY. Big Idea 3
 CHAPTER 17: ELECTROCHEMISTRY Big Idea 3 Electrochemistry Conversion of chemical to electrical energy (discharge). And its reverse (electrolysis). Both subject to entropic caution: Convert reversibly to
CHAPTER 17: ELECTROCHEMISTRY Big Idea 3 Electrochemistry Conversion of chemical to electrical energy (discharge). And its reverse (electrolysis). Both subject to entropic caution: Convert reversibly to
Module-1: Electrode Potential And Cells 2015
 Lecture-2 Standard Electrode potential Standard electrode potential is the electrode potential when the metal is in contact with a solution of its own ions of unit concentration (1M) at 298K. If the electrode
Lecture-2 Standard Electrode potential Standard electrode potential is the electrode potential when the metal is in contact with a solution of its own ions of unit concentration (1M) at 298K. If the electrode
Ch 20 Electrochemistry: the study of the relationships between electricity and chemical reactions.
 Ch 20 Electrochemistry: the study of the relationships between electricity and chemical reactions. In electrochemical reactions, electrons are transferred from one species to another. Learning goals and
Ch 20 Electrochemistry: the study of the relationships between electricity and chemical reactions. In electrochemical reactions, electrons are transferred from one species to another. Learning goals and
A + B C +D ΔG = ΔG + RTlnKp. Me n+ + ne - Me. Me n n
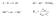 A + B C +D ΔG = ΔG + RTlnKp Me n+ + ne - Me K p a a Me Me n a n e 1 mol madde 6.2 x 1 23 atom elektron yükü 1.62 x 1-19 C FARADAY SABİTİ: 6.2 x 1 23 x 1.62 x 1-19 = 96485 A.sn (= coulomb) 1 Faraday 965
A + B C +D ΔG = ΔG + RTlnKp Me n+ + ne - Me K p a a Me Me n a n e 1 mol madde 6.2 x 1 23 atom elektron yükü 1.62 x 1-19 C FARADAY SABİTİ: 6.2 x 1 23 x 1.62 x 1-19 = 96485 A.sn (= coulomb) 1 Faraday 965
Chapter 18. Electrochemistry
 Chapter 18 Electrochemistry Section 17.1 Spontaneous Processes and Entropy Section 17.1 http://www.bozemanscience.com/ap-chemistry/ Spontaneous Processes and Entropy Section 17.1 Spontaneous Processes
Chapter 18 Electrochemistry Section 17.1 Spontaneous Processes and Entropy Section 17.1 http://www.bozemanscience.com/ap-chemistry/ Spontaneous Processes and Entropy Section 17.1 Spontaneous Processes
Chapter 20. Electrochemistry
 Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 20 John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice Hall,
Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 20 John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice Hall,
Electrochemistry objectives
 Electrochemistry objectives 1) Understand how a voltaic and electrolytic cell work 2) Be able to tell which substance is being oxidized and reduced and where it is occuring the anode or cathode 3) Students
Electrochemistry objectives 1) Understand how a voltaic and electrolytic cell work 2) Be able to tell which substance is being oxidized and reduced and where it is occuring the anode or cathode 3) Students
Chapter 18 Electrochemistry
 Chapter 18 Electrochemistry Definition The study of the interchange of chemical and electrical energy in oxidation-reduction (redox) reactions This interchange can occur in both directions: 1. Conversion
Chapter 18 Electrochemistry Definition The study of the interchange of chemical and electrical energy in oxidation-reduction (redox) reactions This interchange can occur in both directions: 1. Conversion
Electrochem: It s Got Potential!
 Electrochem: It s Got Potential! Presented by: Denise DeMartino Westlake High School, Eanes ISD Pre-AP, AP, and Advanced Placement are registered trademarks of the College Board, which was not involved
Electrochem: It s Got Potential! Presented by: Denise DeMartino Westlake High School, Eanes ISD Pre-AP, AP, and Advanced Placement are registered trademarks of the College Board, which was not involved
Section Electrochemistry represents the interconversion of chemical energy and electrical energy.
 Chapter 21 Electrochemistry Section 21.1. Electrochemistry represents the interconversion of chemical energy and electrical energy. Electrochemistry involves redox (reduction-oxidation) reactions because
Chapter 21 Electrochemistry Section 21.1. Electrochemistry represents the interconversion of chemical energy and electrical energy. Electrochemistry involves redox (reduction-oxidation) reactions because
CHAPTER 6 Modern Theory Principles LECTURER SAHEB M. MAHDI
 CHAPTER 6 Modern Theory Principles LECTURER SAHEB M. MAHDI Modern Theory principles in Corrosion and their applications :- Corrosion studies can be carried-out by two methods 1 Thermodynamics. or 2 By
CHAPTER 6 Modern Theory Principles LECTURER SAHEB M. MAHDI Modern Theory principles in Corrosion and their applications :- Corrosion studies can be carried-out by two methods 1 Thermodynamics. or 2 By
Electrochemical Cells
 Electrochemistry Electrochemical Cells The Voltaic Cell Electrochemical Cell = device that generates electricity through redox rxns 1 Voltaic (Galvanic) Cell An electrochemical cell that produces an electrical
Electrochemistry Electrochemical Cells The Voltaic Cell Electrochemical Cell = device that generates electricity through redox rxns 1 Voltaic (Galvanic) Cell An electrochemical cell that produces an electrical
Oxidation-Reduction (Redox)
 Oxidation-Reduction (Redox) Electrochemistry involves the study of the conversions between chemical and electrical energy. Voltaic (galvanic) cells use chemical reactions to produce an electric current.
Oxidation-Reduction (Redox) Electrochemistry involves the study of the conversions between chemical and electrical energy. Voltaic (galvanic) cells use chemical reactions to produce an electric current.
Oxidation-reduction (redox) reactions
 Oxidation-reduction (redox) reactions Reactions in which there are changes in oxidation state (oxidation number) between reactants and products 2 MnO 4- + 10 Br - + 16 H + 2 Mn 2+ + 5 Br 2 + 8 H 2 O One
Oxidation-reduction (redox) reactions Reactions in which there are changes in oxidation state (oxidation number) between reactants and products 2 MnO 4- + 10 Br - + 16 H + 2 Mn 2+ + 5 Br 2 + 8 H 2 O One
SCHOOL YEAR CH- 19 OXIDATION-REDUCTION REACTIONS SUBJECT: CHEMISTRY GRADE: 12
 SCHOOL YEAR 2017-18 NAME: CH- 19 OXIDATION-REDUCTION REACTIONS SUBJECT: CHEMISTRY GRADE: 12 TEST A Choose the best answer from the options that follow each question. 1. During oxidation, one or more electrons
SCHOOL YEAR 2017-18 NAME: CH- 19 OXIDATION-REDUCTION REACTIONS SUBJECT: CHEMISTRY GRADE: 12 TEST A Choose the best answer from the options that follow each question. 1. During oxidation, one or more electrons
Chemistry: The Central Science. Chapter 20: Electrochemistry
 Chemistry: The Central Science Chapter 20: Electrochemistry Redox reaction power batteries Electrochemistry is the study of the relationships between electricity and chemical reactions o It includes the
Chemistry: The Central Science Chapter 20: Electrochemistry Redox reaction power batteries Electrochemistry is the study of the relationships between electricity and chemical reactions o It includes the
Introduction to electrochemistry
 Introduction to electrochemistry Oxidation reduction reactions involve energy changes. Because these reactions involve electronic transfer, the net release or net absorption of energy can occur in the
Introduction to electrochemistry Oxidation reduction reactions involve energy changes. Because these reactions involve electronic transfer, the net release or net absorption of energy can occur in the
Chapter 17 Electrochemistry
 Chapter 17 Electrochemistry 17.1 Galvanic Cells A. Oxidation-Reduction Reactions (Redox Rxns) 1. Oxidation = loss of electrons a. the substance oxidized is the reducing agent 2. Reduction = gain of electrons
Chapter 17 Electrochemistry 17.1 Galvanic Cells A. Oxidation-Reduction Reactions (Redox Rxns) 1. Oxidation = loss of electrons a. the substance oxidized is the reducing agent 2. Reduction = gain of electrons
Chem 4501 Introduction to Thermodynamics, 3 Credits Kinetics, and Statistical Mechanics. Fall Semester Homework Problem Set Number 12 Solutions
 Chem 4501 Introduction to Thermodynamics, 3 Credits Kinetics, and Statistical Mechanics Fall Semester 017 Homework Problem Set Number 1 Solutions 1. (Based on McQuarrie and Simon, 13-1.) Write balanced
Chem 4501 Introduction to Thermodynamics, 3 Credits Kinetics, and Statistical Mechanics Fall Semester 017 Homework Problem Set Number 1 Solutions 1. (Based on McQuarrie and Simon, 13-1.) Write balanced
Chapter 18 problems (with solutions)
 Chapter 18 problems (with solutions) 1) Assign oxidation numbers for the following species (for review see section 9.4) a) H2SO3 H = +1 S = +4 O = -2 b) Ca(ClO3)2 Ca = +2 Cl = +5 O = -2 c) C2H4 C = -2
Chapter 18 problems (with solutions) 1) Assign oxidation numbers for the following species (for review see section 9.4) a) H2SO3 H = +1 S = +4 O = -2 b) Ca(ClO3)2 Ca = +2 Cl = +5 O = -2 c) C2H4 C = -2
Electron Transfer Reactions
 ELECTROCHEMISTRY 1 Electron Transfer Reactions 2 Electron transfer reactions are oxidation- reduction or redox reactions. Results in the generation of an electric current (electricity) or be caused by
ELECTROCHEMISTRY 1 Electron Transfer Reactions 2 Electron transfer reactions are oxidation- reduction or redox reactions. Results in the generation of an electric current (electricity) or be caused by
Lecture Presentation. Chapter 18. Electrochemistry. Sherril Soman Grand Valley State University Pearson Education, Inc.
 Lecture Presentation Chapter 18 Electrochemistry Sherril Soman Grand Valley State University Harnessing the Power in Nature The goal of scientific research is to understand nature. Once we understand the
Lecture Presentation Chapter 18 Electrochemistry Sherril Soman Grand Valley State University Harnessing the Power in Nature The goal of scientific research is to understand nature. Once we understand the
ELECTROCHEMISTRY. Electrons are transferred from Al to Cu 2+. We can re write this equation as two separate half reactions:
 ELECTROCHEMISTRY A. INTRODUCTION 1. Electrochemistry is the branch of chemistry which is concerned with the conversion of chemical energy to electrical energy, and vice versa. Electrochemical reactions
ELECTROCHEMISTRY A. INTRODUCTION 1. Electrochemistry is the branch of chemistry which is concerned with the conversion of chemical energy to electrical energy, and vice versa. Electrochemical reactions
Electrochemistry. Chapter 18. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Electrochemistry Chapter 18 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Electrochemical processes are oxidation-reduction reactions in which: the energy
Electrochemistry Chapter 18 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Electrochemical processes are oxidation-reduction reactions in which: the energy
Q1. Why does the conductivity of a solution decrease with dilution?
 Q1. Why does the conductivity of a solution decrease with dilution? A1. Conductivity of a solution is the conductance of ions present in a unit volume of the solution. On dilution the number of ions per
Q1. Why does the conductivity of a solution decrease with dilution? A1. Conductivity of a solution is the conductance of ions present in a unit volume of the solution. On dilution the number of ions per
 Electrochem 1 Electrochemistry Some Key Topics Conduction metallic electrolytic Electrolysis effect and stoichiometry Galvanic cell Electrolytic cell Electromotive Force (potential in volts) Electrode
Electrochem 1 Electrochemistry Some Key Topics Conduction metallic electrolytic Electrolysis effect and stoichiometry Galvanic cell Electrolytic cell Electromotive Force (potential in volts) Electrode
Electrode Potentials and Their Measurement
 Electrochemistry Electrode Potentials and Their Measurement Cu(s) + 2Ag + (aq) Cu(s) + Zn 2+ (aq) Cu 2+ (aq) + 2 Ag(s) No reaction Zn(s) + Cu 2+ (aq) Cu(s) + Zn 2+ (aq) In this reaction: Zn (s) g Zn 2+
Electrochemistry Electrode Potentials and Their Measurement Cu(s) + 2Ag + (aq) Cu(s) + Zn 2+ (aq) Cu 2+ (aq) + 2 Ag(s) No reaction Zn(s) + Cu 2+ (aq) Cu(s) + Zn 2+ (aq) In this reaction: Zn (s) g Zn 2+
REVIEW QUESTIONS Chapter 19
 Chemistry 10 ANSWER KEY REVIEW QUESTIONS Chapter 19 1. For each of the following unbalanced equations, (i) write the half-reactions for oxidation and reduction, and (ii) balance the overall equation in
Chemistry 10 ANSWER KEY REVIEW QUESTIONS Chapter 19 1. For each of the following unbalanced equations, (i) write the half-reactions for oxidation and reduction, and (ii) balance the overall equation in
Chemistry 102 Chapter 19 OXIDATION-REDUCTION REACTIONS
 OXIDATION-REDUCTION REACTIONS Some of the most important reaction in chemistry are oxidation-reduction (redox) reactions. In these reactions, electrons transfer from one reactant to the other. The rusting
OXIDATION-REDUCTION REACTIONS Some of the most important reaction in chemistry are oxidation-reduction (redox) reactions. In these reactions, electrons transfer from one reactant to the other. The rusting
Part One: Introduction. a. Chemical reactions produced by electric current. (electrolysis)
 CHAPTER 19: ELECTROCHEMISTRY Part One: Introduction A. Terminology. 1. Electrochemistry deals with: a. Chemical reactions produced by electric current. (electrolysis) b. Production of electric current
CHAPTER 19: ELECTROCHEMISTRY Part One: Introduction A. Terminology. 1. Electrochemistry deals with: a. Chemical reactions produced by electric current. (electrolysis) b. Production of electric current
General Chemistry I. Dr. PHAN TẠI HUÂN Faculty of Food Science and Technology Nong Lam University
 General Chemistry I Dr. PHAN TẠI HUÂN Faculty of Food Science and Technology Nong Lam University Module 7: Oxidation-reduction reactions and transformation of chemical energy Oxidation-reduction reactions
General Chemistry I Dr. PHAN TẠI HUÂN Faculty of Food Science and Technology Nong Lam University Module 7: Oxidation-reduction reactions and transformation of chemical energy Oxidation-reduction reactions
Chapter 20. Electrochemistry
 Chapter 20. Electrochemistry 20.1 Oxidation-Reduction Reactions Oxidation-reduction reactions = chemical reactions in which the oxidation state of one or more substance changes (redox reactions). Recall:
Chapter 20. Electrochemistry 20.1 Oxidation-Reduction Reactions Oxidation-reduction reactions = chemical reactions in which the oxidation state of one or more substance changes (redox reactions). Recall:
Electrochemistry. Galvanic Cell. Page 1. Applications of Redox
 Electrochemistry Applications of Redox Review Oxidation reduction reactions involve a transfer of electrons. OIL- RIG Oxidation Involves Loss Reduction Involves Gain LEO-GER Lose Electrons Oxidation Gain
Electrochemistry Applications of Redox Review Oxidation reduction reactions involve a transfer of electrons. OIL- RIG Oxidation Involves Loss Reduction Involves Gain LEO-GER Lose Electrons Oxidation Gain
(for tutoring, homework help, or help with online classes)
 www.tutor-homework.com (for tutoring, homework help, or help with online classes) 1. chem10b 20.4-3 In a voltaic cell electrons flow from the anode to the cathode. Value 2. chem10b 20.1-35 How many grams
www.tutor-homework.com (for tutoring, homework help, or help with online classes) 1. chem10b 20.4-3 In a voltaic cell electrons flow from the anode to the cathode. Value 2. chem10b 20.1-35 How many grams
Unit 12 Redox and Electrochemistry
 Unit 12 Redox and Electrochemistry Review of Terminology for Redox Reactions OXIDATION loss of electron(s) by a species; increase in oxidation number. REDUCTION gain of electron(s); decrease in oxidation
Unit 12 Redox and Electrochemistry Review of Terminology for Redox Reactions OXIDATION loss of electron(s) by a species; increase in oxidation number. REDUCTION gain of electron(s); decrease in oxidation
Electrochemistry Pulling the Plug on the Power Grid
 Electrochemistry 18.1 Pulling the Plug on the Power Grid 18.3 Voltaic (or Galvanic) Cells: Generating Electricity from Spontaneous Chemical Reactions 18.4 Standard Electrode Potentials 18.7 Batteries:
Electrochemistry 18.1 Pulling the Plug on the Power Grid 18.3 Voltaic (or Galvanic) Cells: Generating Electricity from Spontaneous Chemical Reactions 18.4 Standard Electrode Potentials 18.7 Batteries:
UNIT 3 ELECTROCHEMISTRY
 95414101 UNIT 3 ELECTROCHEMISTRY 1 MARK QUESTIONS Q. 1. Which solution will allow greater conductance of electricity, 1 M NaCl at 93 K or 1 M NaCl at 33 K and why? Ans. 1 M NaCl at 33 K as the ionic mobilities
95414101 UNIT 3 ELECTROCHEMISTRY 1 MARK QUESTIONS Q. 1. Which solution will allow greater conductance of electricity, 1 M NaCl at 93 K or 1 M NaCl at 33 K and why? Ans. 1 M NaCl at 33 K as the ionic mobilities
Lecture Presentation. Chapter 20. Electrochemistry. James F. Kirby Quinnipiac University Hamden, CT Pearson Education
 Lecture Presentation Chapter 20 James F. Kirby Quinnipiac University Hamden, CT is the study of the relationships between electricity and chemical reactions. It includes the study of both spontaneous and
Lecture Presentation Chapter 20 James F. Kirby Quinnipiac University Hamden, CT is the study of the relationships between electricity and chemical reactions. It includes the study of both spontaneous and
CHEM Principles of Chemistry II. Chapter 17 - Electrochemistry
 CHEM 1212 - Principles of Chemistry II Chapter 17 - Electrochemistry electrochemistry is best defined as the study of the interchange of chemical and electrical energy 17.1 Galvanic Cells an oxidation-reduction
CHEM 1212 - Principles of Chemistry II Chapter 17 - Electrochemistry electrochemistry is best defined as the study of the interchange of chemical and electrical energy 17.1 Galvanic Cells an oxidation-reduction
Chpt 20: Electrochemistry
 Cell Potential and Free Energy When both reactants and products are in their standard states, and under constant pressure and temperature conditions where DG o = nfe o DG o is the standard free energy
Cell Potential and Free Energy When both reactants and products are in their standard states, and under constant pressure and temperature conditions where DG o = nfe o DG o is the standard free energy
Ch 18 Electrochemistry OIL-RIG Reactions
 Ch 18 Electrochemistry OIL-RIG Reactions Alessandro Volta s Invention Modified by Dr. Cheng-Yu Lai Daily Electrochemistry Appliactions Electrochemistry: The area of chemistry that examines the transformations
Ch 18 Electrochemistry OIL-RIG Reactions Alessandro Volta s Invention Modified by Dr. Cheng-Yu Lai Daily Electrochemistry Appliactions Electrochemistry: The area of chemistry that examines the transformations
Zn+2 (aq) + Cu (s) Oxidation: An atom, ion, or molecule releases electrons and is oxidized. The oxidation number of the atom oxidized increases.
 Oxidation-Reduction Page 1 The transfer of an electron from one compound to another results in the oxidation of the electron donor and the reduction of the electron acceptor. Loss of electrons (oxidation)
Oxidation-Reduction Page 1 The transfer of an electron from one compound to another results in the oxidation of the electron donor and the reduction of the electron acceptor. Loss of electrons (oxidation)
Zn + Cr 3+ Zn 2+ + Cr. 9. neutrons remain the same: C. remains the same. Redox/Electrochemistry Regents Unit Review. ANSWERS
 Redox/Electrochemistry Regents Unit Review. ANSWERS 1. ½ red = Cr 3+ + 3e Cr 2. ½ ox = Zn Zn +2 + 2e 3. Balanced = 3Zn + 2Cr 3+ 3Zn +2 + 2Cr 4. Zn loses electrons, 2Cr 3+ gains electrons Zn + Cr 3+ Zn
Redox/Electrochemistry Regents Unit Review. ANSWERS 1. ½ red = Cr 3+ + 3e Cr 2. ½ ox = Zn Zn +2 + 2e 3. Balanced = 3Zn + 2Cr 3+ 3Zn +2 + 2Cr 4. Zn loses electrons, 2Cr 3+ gains electrons Zn + Cr 3+ Zn
The relevant half cell reactions and potentials are: Calculate the equilibrium constant, K, for the reaction at 25 C. lnk
 CHEM1405 2004-J-3 June 2004 Calculate the initial cell potential for the following unbalanced reaction at 25 C from the standard electrode potentials. Assume the concentration of all species is initially
CHEM1405 2004-J-3 June 2004 Calculate the initial cell potential for the following unbalanced reaction at 25 C from the standard electrode potentials. Assume the concentration of all species is initially
Electrochemistry. Review oxidation reactions and how to assign oxidation numbers (Ch 4 Chemical Reactions).
 Electrochemistry Oxidation-Reduction: Review oxidation reactions and how to assign oxidation numbers (Ch 4 Chemical Reactions). Half Reactions Method for Balancing Redox Equations: Acidic solutions: 1.
Electrochemistry Oxidation-Reduction: Review oxidation reactions and how to assign oxidation numbers (Ch 4 Chemical Reactions). Half Reactions Method for Balancing Redox Equations: Acidic solutions: 1.
Chapter 19: Oxidation - Reduction Reactions
 Chapter 19: Oxidation - Reduction Reactions 19-1 Oxidation and Reduction I. Oxidation States A. The oxidation rules (as summarized by Mr. Allan) 1. In compounds, hydrogen has an oxidation # of +1. In compounds,
Chapter 19: Oxidation - Reduction Reactions 19-1 Oxidation and Reduction I. Oxidation States A. The oxidation rules (as summarized by Mr. Allan) 1. In compounds, hydrogen has an oxidation # of +1. In compounds,
Dr. Anand Gupta
 By Dr Anand Gupta Mr. Mahesh Kapil Dr. Anand Gupta 09356511518 09888711209 anandu71@yahoo.com mkapil_foru@yahoo.com Electrochemistry Electrolysis Electric energy Chemical energy Galvanic cell 2 Electrochemistry
By Dr Anand Gupta Mr. Mahesh Kapil Dr. Anand Gupta 09356511518 09888711209 anandu71@yahoo.com mkapil_foru@yahoo.com Electrochemistry Electrolysis Electric energy Chemical energy Galvanic cell 2 Electrochemistry
20.1 Consider the Brønsted-Lowry acid-base reaction and the redox reaction below. + A
 20 Electrochemistry Visualizing Concepts 20.1 Consider the Brønsted-Lowry acid-base reaction and the redox reaction below. HA + B BH + + A HA H + + A B + H + BH + X(red) + Y + (ox) X + (ox) + Y(red) X(red)
20 Electrochemistry Visualizing Concepts 20.1 Consider the Brønsted-Lowry acid-base reaction and the redox reaction below. HA + B BH + + A HA H + + A B + H + BH + X(red) + Y + (ox) X + (ox) + Y(red) X(red)
Electrochemical Cells
 Electrochemical Cells There are two types: Galvanic and Electrolytic Galvanic Cell: a cell in which a is used to produce electrical energy, i.e., Chemical energy is transformed into Electrical energy.
Electrochemical Cells There are two types: Galvanic and Electrolytic Galvanic Cell: a cell in which a is used to produce electrical energy, i.e., Chemical energy is transformed into Electrical energy.
Chapter Objectives. Chapter 13 Electrochemistry. Corrosion. Chapter Objectives. Corrosion. Corrosion
 Chapter Objectives Larry Brown Tom Holme Describe at least three types of corrosion and identify chemical reactions responsible for corrosion. www.cengage.com/chemistry/brown Chapter 13 Electrochemistry
Chapter Objectives Larry Brown Tom Holme Describe at least three types of corrosion and identify chemical reactions responsible for corrosion. www.cengage.com/chemistry/brown Chapter 13 Electrochemistry
Chapter 18: Electrochemistry
 Chapter 18: Electrochemistry Oxidation States An oxidation-reduction reaction, or redox reaction, is one in which electrons are transferred. 2Na + Cl 2 2NaCl Each sodium atom is losing one electron to
Chapter 18: Electrochemistry Oxidation States An oxidation-reduction reaction, or redox reaction, is one in which electrons are transferred. 2Na + Cl 2 2NaCl Each sodium atom is losing one electron to
Chapter 18. Redox Reac)on. Oxida)on & Reduc)on 4/8/08. Electrochemistry
 Chapter 18 Electrochemistry Redox Reac)on One or more elements change oxida)on number all single displacement, and combus)on, some synthesis and decomposi)on Always have both oxida)on and reduc)on split
Chapter 18 Electrochemistry Redox Reac)on One or more elements change oxida)on number all single displacement, and combus)on, some synthesis and decomposi)on Always have both oxida)on and reduc)on split
Chapter 19 ElectroChemistry
 Chem 1046 General Chemistry by Ebbing and Gammon, 9th Edition George W.J. Kenney, Jr, Professor of Chemistry Last Update: 11July2009 Chapter 19 ElectroChemistry These Notes are to SUPPLIMENT the Text,
Chem 1046 General Chemistry by Ebbing and Gammon, 9th Edition George W.J. Kenney, Jr, Professor of Chemistry Last Update: 11July2009 Chapter 19 ElectroChemistry These Notes are to SUPPLIMENT the Text,
Types of Cells Chemical transformations to produce electricity- Galvanic cell or Voltaic cell (battery)
 Electrochemistry Some Key Topics Conduction metallic electrolytic Electrolysis effect and stoichiometry Galvanic cell Electrolytic cell Electromotive Force Electrode Potentials Gibbs Free Energy Gibbs
Electrochemistry Some Key Topics Conduction metallic electrolytic Electrolysis effect and stoichiometry Galvanic cell Electrolytic cell Electromotive Force Electrode Potentials Gibbs Free Energy Gibbs
Oxidation Numbers, ox #
 Oxidation Numbers, ox # are or numbers assigned to each or assuming that the are transferred from the electronegative element to the electronegative element. now mimic systems. ox # are written followed
Oxidation Numbers, ox # are or numbers assigned to each or assuming that the are transferred from the electronegative element to the electronegative element. now mimic systems. ox # are written followed
What is a Voltaic Cell? Voltaic Cells a.k.a. Electrochemical cells. May 25, Voltaic Cells 2018.notebook
 What is a? s a.k.a. Electrochemical cells Aim: To analyze the process of a spontaneous chemical reaction that produces electricity. Voltaic cell: an electrochemical cell where chemical energy is spontaneously
What is a? s a.k.a. Electrochemical cells Aim: To analyze the process of a spontaneous chemical reaction that produces electricity. Voltaic cell: an electrochemical cell where chemical energy is spontaneously
Name: Regents Chemistry Date:
 Name: Date: 1. The reaction CuO + CO CO 2 + Cu is an example of (A) reduction, only (B) oxidation, only (C) both oxidation and reduction (D) neither oxidation nor reduction 6. In which compound does chlorine
Name: Date: 1. The reaction CuO + CO CO 2 + Cu is an example of (A) reduction, only (B) oxidation, only (C) both oxidation and reduction (D) neither oxidation nor reduction 6. In which compound does chlorine
25. A typical galvanic cell diagram is:
 Unit VI(6)-III: Electrochemistry Chapter 17 Assigned Problems Answers Exercises Galvanic Cells, Cell Potentials, Standard Reduction Potentials, and Free Energy 25. A typical galvanic cell diagram is: The
Unit VI(6)-III: Electrochemistry Chapter 17 Assigned Problems Answers Exercises Galvanic Cells, Cell Potentials, Standard Reduction Potentials, and Free Energy 25. A typical galvanic cell diagram is: The
We can use chemistry to generate electricity... this is termed a Voltaic (or sometimes) Galvanic Cell
 Unit 6 Electrochemistry Chemistry 020, R. R. Martin Electrochemistry Electrochemistry is the study of the interconversion of electrical and chemical energy. We can use chemistry to generate electricity...
Unit 6 Electrochemistry Chemistry 020, R. R. Martin Electrochemistry Electrochemistry is the study of the interconversion of electrical and chemical energy. We can use chemistry to generate electricity...
CHEM J-12 June 2013
 CHEM1101 2013-J-12 June 2013 In concentration cells no net chemical conversion occurs, however a measurable voltage is present between the two half-cells. Explain how the voltage is produced. 2 In concentration
CHEM1101 2013-J-12 June 2013 In concentration cells no net chemical conversion occurs, however a measurable voltage is present between the two half-cells. Explain how the voltage is produced. 2 In concentration
Practice Exam Topic 9: Oxidation & Reduction
 Name Practice Exam Topic 9: Oxidation & Reduction 1. What are the oxidation numbers of the elements in sulfuric acid, H 2 SO 4? Hydrogen Sulfur Oxygen A. +1 +6 2 B. +1 +4 2 C. +2 +1 +4 D. +2 +6 8 2. Consider
Name Practice Exam Topic 9: Oxidation & Reduction 1. What are the oxidation numbers of the elements in sulfuric acid, H 2 SO 4? Hydrogen Sulfur Oxygen A. +1 +6 2 B. +1 +4 2 C. +2 +1 +4 D. +2 +6 8 2. Consider
3.014 MATERIALS LABORATORY MODULE- β3 November 16 21, 2005 GEETHA P. BERERA. Visualizing Gibbs Free Energy Anodic Corrosion and the EMF Series
 3.014 MATERIALS LABORATORY MODULE- β3 November 16 21, 2005 GEETHA P. BERERA Visualizing Gibbs Free Energy Anodic Corrosion and the EMF Series OBJECTIVES: Understand what is galvanic (anodic) corrosion
3.014 MATERIALS LABORATORY MODULE- β3 November 16 21, 2005 GEETHA P. BERERA Visualizing Gibbs Free Energy Anodic Corrosion and the EMF Series OBJECTIVES: Understand what is galvanic (anodic) corrosion
Chemistry 201: General Chemistry II - Lecture
 Name Date Chemistry 201: General Chemistry II - Lecture Short-Answer Exam #3, 70 Points Total Form: A Read all directions carefully. Answers not conforming to the directions will be marked as incorrect!
Name Date Chemistry 201: General Chemistry II - Lecture Short-Answer Exam #3, 70 Points Total Form: A Read all directions carefully. Answers not conforming to the directions will be marked as incorrect!
REDOX test practice. 2 Cr(s) + 3 Sn 2+ (aq) 2 Cr 3+ (aq) + 3 Sn(s)
 1. Which polyatomic ion has a charge of 3? A) chromate ion B) oxalate ion C) phosphate ion D) thiocyanate ion 2. What is the oxidation state of nitrogen in NaNO2? A) +1 B) +2 C) +3 D) +4 3. What are the
1. Which polyatomic ion has a charge of 3? A) chromate ion B) oxalate ion C) phosphate ion D) thiocyanate ion 2. What is the oxidation state of nitrogen in NaNO2? A) +1 B) +2 C) +3 D) +4 3. What are the
Hg2 2+ (aq) + H2(g) 2 Hg(l) + 2H + (aq)
 The potential difference between two electrodes in a cell is called the electromotive force, or The EMF of a voltaic cell is called the The cell voltage of a voltaic cell will be a Note: We are used to
The potential difference between two electrodes in a cell is called the electromotive force, or The EMF of a voltaic cell is called the The cell voltage of a voltaic cell will be a Note: We are used to
Name AP CHEM / / Collected Essays Chapter 17
 Name AP CHEM / / Collected Essays Chapter 17 1980 - #2 M(s) + Cu 2+ (aq) M 2+ (aq) + Cu(s) For the reaction above, E = 0.740 volt at 25 C. (a) Determine the standard electrode potential for the reaction
Name AP CHEM / / Collected Essays Chapter 17 1980 - #2 M(s) + Cu 2+ (aq) M 2+ (aq) + Cu(s) For the reaction above, E = 0.740 volt at 25 C. (a) Determine the standard electrode potential for the reaction
Unit 8 Redox 8-1. At the end of this unit, you ll be able to
 8-1 Unit 8 Redox At the end of this unit, you ll be able to Define and identify oxidation reactions Define and identify reduction reactions Assign oxidation numbers to elements in a compound Write and
8-1 Unit 8 Redox At the end of this unit, you ll be able to Define and identify oxidation reactions Define and identify reduction reactions Assign oxidation numbers to elements in a compound Write and
Electrochemistry Pearson Education, Inc. Mr. Matthew Totaro Legacy High School AP Chemistry
 2012 Pearson Education, Inc. Mr. Matthew Totaro Legacy High School AP Chemistry Electricity from Chemistry Many chemical reactions involve the transfer of electrons between atoms or ions electron transfer
2012 Pearson Education, Inc. Mr. Matthew Totaro Legacy High School AP Chemistry Electricity from Chemistry Many chemical reactions involve the transfer of electrons between atoms or ions electron transfer
Assignment #1: Redox Reaction Skill Drills
 Assignment #1: Redox Reaction Skill Drills Skill #1 Assigning Oxidation Numbers (Text Reference: p. 639 641) All elements have an oxidation number of 0. In compounds, oxidation numbers add up to 0. o Group
Assignment #1: Redox Reaction Skill Drills Skill #1 Assigning Oxidation Numbers (Text Reference: p. 639 641) All elements have an oxidation number of 0. In compounds, oxidation numbers add up to 0. o Group
Chapter 20 Electrochemistry
 Chapter 20 Electrochemistry Electrochemical Cell Consists of electrodes which dip into an electrolyte & in which a chem. rxn. uses or generates an electric current Voltaic (Galvanic) Cell Spont. rxn. -
Chapter 20 Electrochemistry Electrochemical Cell Consists of electrodes which dip into an electrolyte & in which a chem. rxn. uses or generates an electric current Voltaic (Galvanic) Cell Spont. rxn. -
(c) dilute solution of glucose (d) chloroform 12 Which one of the following represents the same net reaction as the electrolysis of aqueous H2SO4
 1 Electrolysis is the process in which a chemical reaction takes place at the expense of (a) chemical energy (b) electrical energy (c) heat energy (d) none of these 2 Standard hydrogen electrode has an
1 Electrolysis is the process in which a chemical reaction takes place at the expense of (a) chemical energy (b) electrical energy (c) heat energy (d) none of these 2 Standard hydrogen electrode has an
Chapter 20. Electrochemistry Recommendation: Review Sec. 4.4 (oxidation-reduction reactions) in your textbook
 Chapter 20. Electrochemistry Recommendation: Review Sec. 4.4 (oxidation-reduction reactions) in your textbook 20.1 Oxidation-Reduction Reactions Oxidation-reduction reactions = chemical reactions in which
Chapter 20. Electrochemistry Recommendation: Review Sec. 4.4 (oxidation-reduction reactions) in your textbook 20.1 Oxidation-Reduction Reactions Oxidation-reduction reactions = chemical reactions in which
ELECTROCHEMICAL CELLS NAME ROW PD
 4-26-12 NAME ROW PD (1) Which statement describes the redox reaction that occurs when an object is electroplated? The diagram below shows the electrolysis of fused KCl. A) It is spontaneous and requires
4-26-12 NAME ROW PD (1) Which statement describes the redox reaction that occurs when an object is electroplated? The diagram below shows the electrolysis of fused KCl. A) It is spontaneous and requires
Electrochemistry. Outline
 Electrochemistry Outline 1. Oxidation Numbers 2. Voltaic Cells 3. Calculating emf or Standard Cell Potential using Half-Reactions 4. Relationships to Thermo, Equilibrium, and Q 5. Stoichiometry 6. Balancing
Electrochemistry Outline 1. Oxidation Numbers 2. Voltaic Cells 3. Calculating emf or Standard Cell Potential using Half-Reactions 4. Relationships to Thermo, Equilibrium, and Q 5. Stoichiometry 6. Balancing
ELECTROCHEMISTRY Chapter 14
 ELECTROCHEMISTRY Chapter 14 Basic Concepts: Overview of Electrochemical Process at Constant T, P (14-1) ΔG = ΔG o + RT ln Q = w elec (maximum) = qe = ItE (exp) (E intensive parameter, q extensive) = nfe
ELECTROCHEMISTRY Chapter 14 Basic Concepts: Overview of Electrochemical Process at Constant T, P (14-1) ΔG = ΔG o + RT ln Q = w elec (maximum) = qe = ItE (exp) (E intensive parameter, q extensive) = nfe
Electrochemical Cells: Virtual Lab
 Electrochemical Cells: Virtual Lab Electrochemical cells involve the transfer of electrons from one species to another. In these chemical systems, the species that loses electrons is said to be oxidized
Electrochemical Cells: Virtual Lab Electrochemical cells involve the transfer of electrons from one species to another. In these chemical systems, the species that loses electrons is said to be oxidized
CHEM J-14 June 2014
 CHEM1101 2014-J-14 June 2014 An electrochemical cell consists of an Fe 2+ /Fe half cell with unknown [Fe 2+ ] and a Sn 2+ /Sn half-cell with [Sn 2+ ] = 1.10 M. The electromotive force (electrical potential)
CHEM1101 2014-J-14 June 2014 An electrochemical cell consists of an Fe 2+ /Fe half cell with unknown [Fe 2+ ] and a Sn 2+ /Sn half-cell with [Sn 2+ ] = 1.10 M. The electromotive force (electrical potential)
CHAPTER 17 ELECTROCHEMISTRY
 Advanced Chemistry Approximate Timeline Students are expected to keep up with class work when absent. CHAPTER 17 ELECTROCHEMISTRY Day Plans for the day Assignment(s) for the day 17.1 Galvanic Cells Assignment
Advanced Chemistry Approximate Timeline Students are expected to keep up with class work when absent. CHAPTER 17 ELECTROCHEMISTRY Day Plans for the day Assignment(s) for the day 17.1 Galvanic Cells Assignment
Electrochemistry 1 1
 Electrochemistry 1 1 Half-Reactions 1. Balancing Oxidation Reduction Reactions in Acidic and Basic Solutions Voltaic Cells 2. Construction of Voltaic Cells 3. Notation for Voltaic Cells 4. Cell Potential
Electrochemistry 1 1 Half-Reactions 1. Balancing Oxidation Reduction Reactions in Acidic and Basic Solutions Voltaic Cells 2. Construction of Voltaic Cells 3. Notation for Voltaic Cells 4. Cell Potential
