(a) (e) (b) (f) (c) (g) (h) (d) Chapter 6. The Dissociation Reaction of Acetylacetone
|
|
|
- Kristian Shaw
- 5 years ago
- Views:
Transcription
1 Chapter 6. The Dissociation Reaction of Acetylacetone (e) (f) (g) (h) Fig.6-. Difference patterns created by subtracting the ratio pattern of the 77 ps time point from the ratio patterns recorded at 27 ps, +3 ps, +8 ps, +23 ps, (e) +28 ps, (f) +33 ps, (g) +43 ps, and (h) +73 ps. 6
2 Chapter 6. The Dissociation Reaction of Acetylacetone (e) (f) (g) Fig.6. Difference patterns created by subtracting the ratio pattern of the 77 ps time point from the ratio patterns recorded at +98 ps, +23 ps, +73 ps, +223 ps, (e) +423 ps, (f) +823 ps, and (g) +273 ps. 62
3 Chapter 6. The Dissociation Reaction of Acetylacetone Fig.6-3. Comparison between data recorded with and without a neutral-density filter (% transmission) in the beam path. Difference patterns are created by subtracting the ratio pattern of the 77 ps time point from the ratio patterns recorded at +98 ps with laser filter, +98 ps without laser filter, +273 ps with laser filter, and +273 ps without laser filter. The filtered data were used in the analysis reported herein. 63
4 Chapter 6. The Dissociation Reaction of Acetylacetone 64 (e) (f) (g) (h) Fig.6-4. Isomers of the enol tautomer of acetylacetone. The naming convention is defined with respect to rotations about the C single bond, the C C double bond, and the C C single bond. C = cis; T = trans. CCC, TCC, CTC CTT, (e) TTT, (f) CCT, (g) TCT, (h) TTC.
5 Chapter 6. The Dissociation Reaction of Acetylacetone 65 (e) (f) (g) C 3 (h) Fig Possible reaction products in the photolysis of CCC enolic acetylacetone. keto tautomer of acetylacetone. Four isomers of the 2-penten-4-on-yl radical + the radical TC, TT (e) CT, and (f) CC. (g) The CCC isomer of the formylacetonyl radical + the methyl radical. (h) The acetyl radical loss products.
6 Chapter 6. The Dissociation Reaction of Acetylacetone s (Å - ) s (Å - ) s (Å - ) s (Å - ) Fig Fits of possible reactions: left column sm(s) and right column f(r) having components fractional contributions and polynomial background optimized. The data (squares) is the +273 ps data point (t = 77 ps) and theory (solid line) is derived from ref the DFT structures of possible products, here the enol isomers: CCC, TCC, 2 TCT, TTC. and R are given in Table 6.
7 Chapter 6. The Dissociation Reaction of Acetylacetone s (Å - ) s (Å - ) s (Å - ) s (Å - ) Fig Fits of possible reactions: left column sm(s) and right column f(r) having components fractional contributions and polynomial background optimized. The data (squares) is the +273 ps data point (t = 77 ps) and theory (solid line) is derived from ref the DFT structures of possible products, here the enol isomers: TTT, CCT, 2 CTC, and CTT. and R are given in Table 6.
8 Chapter 6. The Dissociation Reaction of Acetylacetone s (Å - ) s (Å - ) s (Å - ) Fig Fits of possible reactions: left column sm(s) and right column f(r) having components fractional contributions and polynomial background optimized. The data (squares) is the +273 ps data point (t = 77 ps) and theory (solid line) is derived from ref the DFT structures of possible products, here a : mixture of vibrationally hot enol CCC and keto acetylacetone tautomers, the methyl radical loss products, and the 2 acetyl radical loss products. and R are given in Table 6.
9 Chapter 6. The Dissociation Reaction of Acetylacetone s (Å - ) s (Å - ) s (Å - ) s (Å - ) Fig Fits of possible reactions: left column sm(s) and right column f(r) having components fractional contributions and polynomial background optimized. The data (squares) is the +273 ps data point (t = 77 ps) and theory (solid line) is derived from ref the DFT structures of possible products, here the loss products with the 2 2-penten-4-on-yl radical isomers CC, CT, TC, TT. and R are given in Table 6.
10 Chapter 6. The Dissociation Reaction of Acetylacetone s (Å - ) s (Å - ) Fig. 6-. The data (squares) and the refined theoretical model (line) corresponding to the reaction of enolic acetylacetone to form the TC 2-penten-4-on-yl and radicals. sm(s) comparison and f(r) comparison. The experimental 2 sm(s) (squares) and the polynomial background (line). = 8.4 and R =.35.
11 Chapter 6. The Dissociation Reaction of Acetylacetone 7 (e) (f) (g) (h) (i) (j) (k) s (Å - ) Fig. 6-. The experimental (squares) and refined theoretical (solid line) sm(s) (left) and f(r) (right) at multiple time points (t ref = 77 ps). t = +28 ps, +33 ps, +43 ps, +73 ps, (e) +98 ps, (f) +23 ps, (g) +73 ps, (h) +223 ps, (i) +423 ps, (j) +823 ps, (k) +273 ps. Fitting information is given in Table 6-4.
12 Chapter 6. The Dissociation Reaction of Acetylacetone product fraction (%) time delay (ps) Fig. 6. The fit of a single-step reaction process (solid line) to the fraction of product structure at each time point (squares). The time constant for the reaction is 247 ± 34 ps.
13 Chapter 6. The Dissociation Reaction of Acetylacetone 73 ( - x)% parent x% product - ( - x)% parent (x - y) % intermediate y% product = -(x-y)% intermediate (x-y)% product Fig A schematic illustration of the process used to isolate the reaction of the formation of product from an intermediate structure. Data from a time point when the reaction is complete and only parent and product are present has data from an earlier positive time point subtracted from it. This positive point contains unreacted parent, product, and reaction intermediate. The resulting difference data has the parent contribution removed and contains only the structural information of product and intermediate molecules.
14 Chapter 6. The Dissociation Reaction of Acetylacetone 74 (e) s (Å - ) (f) S 428 K S 2256 K S K S 2 K T K Fig The calculated molecular structure of some of the electronic states of enolic acetylacetone S, S n *, S 2 *, and T *. The sm(s) and f(r) curves calculated using these structures. S at 428 K (broad solid line), S at 2256 K (narrow solid line), S at K (dashed line), S at K (dotted line), and T at 2 K (dot-dash line).
15 Chapter 6. The Dissociation Reaction of Acetylacetone 75 (e) s (Å - ) (f) s (Å - ) (g) s (Å - ) (h) s (Å - ) Fig The alternate sm(s) (left) and f(r) (right) created by subtracting the +73 ps time point from the +273 ps time point. ere the experimental (squares) data is compared with theory (solid line) corresponding to the reaction of the intermediate to the refined product. The intermediate in each is represented by enol S at 2256 K, enol S at K, enol S 2at K, and enol T at K.
16 Chapter 6. The Dissociation Reaction of Acetylacetone 76 S, * 2 S, n * Energy T, * loss products S Fig The schematic representation of the dynamics of acetylacetone. Excited molecules arrive in the S 2 state where ultrafast internal conversion sends population to the S state. Intersystem crossing to T takes place over 247 ps ( ). Dissociation from T to the radical products is then ultrafast such that its population never builds up enough to be detected by UED.
Chapter 6. The Dissociation Reaction of Acetylacetone
 Chapter 6. The Dissociation Reaction of Acetylacetone 142 Chapter 6. The Dissociation Reaction of Acetylacetone In Section 5.6, the ground-state structure of enolic acetylacetone as determined by UED was
Chapter 6. The Dissociation Reaction of Acetylacetone 142 Chapter 6. The Dissociation Reaction of Acetylacetone In Section 5.6, the ground-state structure of enolic acetylacetone as determined by UED was
Supporting Information
 Supporting Information Ultrafast Intersystem Crossing in Acetylacetone via Femtosecond X-ray Transient Absorption at the Carbon K-Edge Aditi Bhattacherjee,,, Chaitanya Das Pemmaraju, Kirsten Schnorr,,
Supporting Information Ultrafast Intersystem Crossing in Acetylacetone via Femtosecond X-ray Transient Absorption at the Carbon K-Edge Aditi Bhattacherjee,,, Chaitanya Das Pemmaraju, Kirsten Schnorr,,
Chapter 1. Introduction
 Chapter 1. Introduction 1 Chapter 1. Introduction Time-resolved gas phase electron diffraction, or ultrafast electron diffraction (UED), as the state of the art is known today, permits the study of isolated
Chapter 1. Introduction 1 Chapter 1. Introduction Time-resolved gas phase electron diffraction, or ultrafast electron diffraction (UED), as the state of the art is known today, permits the study of isolated
HYDROGEN-BONDING IN ACETYLACETONE *
 9 HYDRGEN-BNDING IN ACETYLACETNE * 9.1 Introduction Hydrogen bonds are ubiquitous in chemistry and biology. While the strength of the classical hydrogen bond is around 3 5 kcal/mol, hydrogen bond strengths
9 HYDRGEN-BNDING IN ACETYLACETNE * 9.1 Introduction Hydrogen bonds are ubiquitous in chemistry and biology. While the strength of the classical hydrogen bond is around 3 5 kcal/mol, hydrogen bond strengths
Excited State Processes
 Excited State Processes Photophysics Fluorescence (singlet state emission) Phosphorescence (triplet state emission) Internal conversion (transition to singlet gr. state) Intersystem crossing (transition
Excited State Processes Photophysics Fluorescence (singlet state emission) Phosphorescence (triplet state emission) Internal conversion (transition to singlet gr. state) Intersystem crossing (transition
If somehow it is possible to increase the stability of a given base, then it
 Text Related to Segment.04 00 laude E. Wintner If somehow it is possible to increase the stability of a given base, then it should be true that the corresponding acid will be stronger than would be so
Text Related to Segment.04 00 laude E. Wintner If somehow it is possible to increase the stability of a given base, then it should be true that the corresponding acid will be stronger than would be so
Supporting Information
 Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2013 Photochemistry of N-Methylformamide: Matrix Isolation and Nonadiabatic Dynamics Rachel Crespo-Otero, [a] Artur Mardyukov,
Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2013 Photochemistry of N-Methylformamide: Matrix Isolation and Nonadiabatic Dynamics Rachel Crespo-Otero, [a] Artur Mardyukov,
Laser Dissociation of Protonated PAHs
 100 Chapter 5 Laser Dissociation of Protonated PAHs 5.1 Experiments The photodissociation experiments were performed with protonated PAHs using different laser sources. The calculations from Chapter 3
100 Chapter 5 Laser Dissociation of Protonated PAHs 5.1 Experiments The photodissociation experiments were performed with protonated PAHs using different laser sources. The calculations from Chapter 3
sp 2 geometry tetrahedral trigonal planar linear ΔH C-C ΔH C-H % s character pk a 464 KJ/mol 33% 44
 hapter 10: Alkynes 10.1 Introduction to Alkynes ~ 111 ~ 122 1.06 Å 180 1.1 Å ~ 116 1.08 Å 1.54 Å 1.34 Å 1.20 Å hybridization of sp 3 sp 2 sp geometry tetrahedral trigonal planar linear 368 KJ/mol 632 KJ/mol
hapter 10: Alkynes 10.1 Introduction to Alkynes ~ 111 ~ 122 1.06 Å 180 1.1 Å ~ 116 1.08 Å 1.54 Å 1.34 Å 1.20 Å hybridization of sp 3 sp 2 sp geometry tetrahedral trigonal planar linear 368 KJ/mol 632 KJ/mol
Chapter 27: Structure and Bonding
 Chapter 27: Structure and Bonding 1 Atomic Orbitals: Wave functions that represent the probability of finding electrons in a specific region of space s, p, d, f orbitals In organic chemistry, need to concentrate
Chapter 27: Structure and Bonding 1 Atomic Orbitals: Wave functions that represent the probability of finding electrons in a specific region of space s, p, d, f orbitals In organic chemistry, need to concentrate
CEM 850 Final Exam H N H H O H H O H C H 2. MeO
 CEM 850 Final Exam December 11, 2003 This exam consists of 20 pages. Be sure to write your name on all pages of the exam. Please write legibly and draw all structures clearly. Good luck. 1. (10 pts) Estimate
CEM 850 Final Exam December 11, 2003 This exam consists of 20 pages. Be sure to write your name on all pages of the exam. Please write legibly and draw all structures clearly. Good luck. 1. (10 pts) Estimate
Determination of Absolute Product Branching Ratios in Mass Spectrometric Experiments: Detecting Acetyl Radicals at CH 2 CO +
 5200 J. Phys. Chem. 1996, 100, 5200-5204 Determination of Absolute Product Branching Ratios in Mass Spectrometric Experiments: Detecting Acetyl Radicals at CH 2 C + D. C. Kitchen, T. L. Myers, and L. J.
5200 J. Phys. Chem. 1996, 100, 5200-5204 Determination of Absolute Product Branching Ratios in Mass Spectrometric Experiments: Detecting Acetyl Radicals at CH 2 C + D. C. Kitchen, T. L. Myers, and L. J.
CHEM 231 (Davis) Organic Chemistry FINAL EXAM May 15, YOUR NAME (Last, First, M.I.) DISCUSSION SECTION #53 (5 Points)
 CHEM 231 (Davis) rganic Chemistry FINAL EXAM May 15, 2006 YUR NAME (Last, First, M.I.) DISCUSSIN SECTIN #53 (5 Points) Initial of last name Instructions Please fill in your name in the space above and
CHEM 231 (Davis) rganic Chemistry FINAL EXAM May 15, 2006 YUR NAME (Last, First, M.I.) DISCUSSIN SECTIN #53 (5 Points) Initial of last name Instructions Please fill in your name in the space above and
Dissociation Channels of the 1-Propenyl Radical and Its Photolytic Precursor cis-1-bromopropene
 J. Phys. Chem. A 2002, 106, 10831-10842 10831 Dissociation Channels of the 1-Propenyl Radical and Its Photolytic Precursor cis-1-bromopropene Melita L. Morton, Johanna L. Miller, and Laurie J. Butler*
J. Phys. Chem. A 2002, 106, 10831-10842 10831 Dissociation Channels of the 1-Propenyl Radical and Its Photolytic Precursor cis-1-bromopropene Melita L. Morton, Johanna L. Miller, and Laurie J. Butler*
CHAPTER 23 HW: ENOLS + ENOLATES
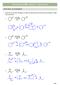 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
Constitutional Isomers and Conformations of Alkanes & Cycloalkanes
 Discovering Molecular Models #1: Constitutional Isomers Conformations of Alkanes & Cycloalkanes There are no additional tutorial or laboratory notes. Read bring your course notes, as they provide all of
Discovering Molecular Models #1: Constitutional Isomers Conformations of Alkanes & Cycloalkanes There are no additional tutorial or laboratory notes. Read bring your course notes, as they provide all of
Constitutional Isomers and Conformations of Alkanes & Cycloalkanes
 Discovering Molecular Models #1: Constitutional Isomers Conformations of Alkanes & Cycloalkanes There are no additional tutorial or laboratory notes. Read bring your course notes, as they provide all of
Discovering Molecular Models #1: Constitutional Isomers Conformations of Alkanes & Cycloalkanes There are no additional tutorial or laboratory notes. Read bring your course notes, as they provide all of
C-Cl Bond Fission, Hcl Elimination, And Secondary Radical Decomposition In The 193 Nm Photodissociation Of Allyl Chloride
 Swarthmore College Works Chemistry & Biochemistry Faculty Works Chemistry & Biochemistry 2-15-2002 C-Cl Bond Fission, Hcl Elimination, And Secondary Radical Decomposition In The 193 Nm Photodissociation
Swarthmore College Works Chemistry & Biochemistry Faculty Works Chemistry & Biochemistry 2-15-2002 C-Cl Bond Fission, Hcl Elimination, And Secondary Radical Decomposition In The 193 Nm Photodissociation
α,γ-bisdiphenylene-β-phenylallyl, an unusual example of a persistent carbon radical
 C h a p t e r E l e v e n: Radical Reactions α,γ-bisdiphenylene-β-phenylallyl, an unusual example of a persistent carbon radical CM 321: Summary of Important Concepts YConcepts for Chapter 11: Radical
C h a p t e r E l e v e n: Radical Reactions α,γ-bisdiphenylene-β-phenylallyl, an unusual example of a persistent carbon radical CM 321: Summary of Important Concepts YConcepts for Chapter 11: Radical
Supporting information for the manuscript. Excited state structural evolution during charge-transfer reactions in Betaine-30
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 Supporting information for the manuscript Excited state structural evolution during
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 Supporting information for the manuscript Excited state structural evolution during
Time: 3 hours (180 minutes) Marking Scheme For The Exam
 hemistry 2320 S10 Fall 2004 FINAL EXAM Thursday, ecember 23, 2004 Name: Student Number This paper consists of 13 pages (10 questions) (including this title page). Ensure that you have a complete paper
hemistry 2320 S10 Fall 2004 FINAL EXAM Thursday, ecember 23, 2004 Name: Student Number This paper consists of 13 pages (10 questions) (including this title page). Ensure that you have a complete paper
Photodissociation of 1-bromo-2-butene, 4-bromo-1-butene, and cyclopropylmethyl bromide at 234 nm studied using velocity map imaging
 THE JOURNAL OF CHEMICAL PHYSICS 125, 144312 2006 Photodissociation of 1-bromo-2-butene, 4-bromo-1-butene, and cyclopropylmethyl bromide at 234 nm studied using velocity map imaging Kai-Chung Lau, Yi Liu,
THE JOURNAL OF CHEMICAL PHYSICS 125, 144312 2006 Photodissociation of 1-bromo-2-butene, 4-bromo-1-butene, and cyclopropylmethyl bromide at 234 nm studied using velocity map imaging Kai-Chung Lau, Yi Liu,
ORGANIC - BROWN 8E CH DIENES, CONJUGATED SYSTEMS, AND PERICYCLIC REACTIONS
 !! www.clutchprep.com CONCEPT: INTRODUCTION TO CONJUGATION Conjugation exists when three or more atoms with the ability to resonate are adjacent to each other (overlapping). Conjugation provides an electron
!! www.clutchprep.com CONCEPT: INTRODUCTION TO CONJUGATION Conjugation exists when three or more atoms with the ability to resonate are adjacent to each other (overlapping). Conjugation provides an electron
Kota Academy Karad Chemistry - Isomerism
 Kota cademy Karad 1. Which of the following compounds exhibit optical isomerism : () C C C () C CC (C) C C C (D) C CC. C 7 9 N has how many isomeric forms that contain a benzene ring : () 4 () (C) 6 (D)
Kota cademy Karad 1. Which of the following compounds exhibit optical isomerism : () C C C () C CC (C) C C C (D) C CC. C 7 9 N has how many isomeric forms that contain a benzene ring : () 4 () (C) 6 (D)
Supplemental data. Pommerrenig et al. (2011). Plant Cell /tpc
 Supplemental Figure 1. Prediction of phloem-specific MTK1 expression in Arabidopsis shoots and roots. The images and the corresponding numbers showing absolute (A) or relative expression levels (B) of
Supplemental Figure 1. Prediction of phloem-specific MTK1 expression in Arabidopsis shoots and roots. The images and the corresponding numbers showing absolute (A) or relative expression levels (B) of
ORGANIC - BRUICE 8E CH.8 - DELOCALIZED ELECTRONS AND THEIR EFFECT
 !! www.clutchprep.com CONCEPT: RESONANCE STRUCTURES Resonance theory is used to represent all the different ways that the same molecule can distribute its electrons. Atoms move! The only thing that moves
!! www.clutchprep.com CONCEPT: RESONANCE STRUCTURES Resonance theory is used to represent all the different ways that the same molecule can distribute its electrons. Atoms move! The only thing that moves
Chem 263 Nov 19, Cl 2
 Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
Number-controlled spatial arrangement of gold nanoparticles with
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Number-controlled spatial arrangement of gold nanoparticles with DNA dendrimers Ping Chen,*
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Number-controlled spatial arrangement of gold nanoparticles with DNA dendrimers Ping Chen,*
Chem 263 Oct. 6, Single bonds, σ. e - donating Activate Activate ortho and para directing ortho and para directing
 Chem 263 ct. 6, 2009 lectrophilic Substitution of Substituted Benzenes Resonance ffect Inductive ffect C=C, π system Single bonds, σ Strong Weak e - donating Activate Activate ortho and para directing
Chem 263 ct. 6, 2009 lectrophilic Substitution of Substituted Benzenes Resonance ffect Inductive ffect C=C, π system Single bonds, σ Strong Weak e - donating Activate Activate ortho and para directing
FEMTOSECOND MID-INFRARED SPECTROSCOPY OF HYDROGEN-BONDED LIQUIDS
 Laser Chem., 1999, Vol. 19, pp. 83-90 Reprints available directly from the publisher Photocopying permitted by license only (C) 1999 OPA (Overseas Publishers Association) N.V. Published by license under
Laser Chem., 1999, Vol. 19, pp. 83-90 Reprints available directly from the publisher Photocopying permitted by license only (C) 1999 OPA (Overseas Publishers Association) N.V. Published by license under
An Introduction to Quantum Chemistry and Potential Energy Surfaces. Benjamin G. Levine
 An Introduction to Quantum Chemistry and Potential Energy Surfaces Benjamin G. Levine This Week s Lecture Potential energy surfaces What are they? What are they good for? How do we use them to solve chemical
An Introduction to Quantum Chemistry and Potential Energy Surfaces Benjamin G. Levine This Week s Lecture Potential energy surfaces What are they? What are they good for? How do we use them to solve chemical
As time allows, additional practice questions for Exam 2 follow. This is an incomplete collection. Content & emphasis will vary.
 Chem 226 Exam 2 Fall 2005: will cover Bruice, Chaps 3 through 6; Note: review e-mail & inclass quizzes and Worksheets, suggest on-line practice questions and quizzes plus ACS rganic Chemistry Guide As
Chem 226 Exam 2 Fall 2005: will cover Bruice, Chaps 3 through 6; Note: review e-mail & inclass quizzes and Worksheets, suggest on-line practice questions and quizzes plus ACS rganic Chemistry Guide As
Conjugated Systems & Pericyclic Reactions
 onjugated Systems & Pericyclic Reactions 1 onjugated Dienes from heats of hydrogenation-relative stabilities of conjugated vs unconjugated dienes can be studied: Name 1-Butene 1-Pentene Structural Formula
onjugated Systems & Pericyclic Reactions 1 onjugated Dienes from heats of hydrogenation-relative stabilities of conjugated vs unconjugated dienes can be studied: Name 1-Butene 1-Pentene Structural Formula
Theory of selective excitation in stimulated Raman scattering
 Theory of selective excitation in stimulated Raman scattering S. A. Malinovskaya, P. H. Bucksbaum, and P. R. Berman Michigan Center for Theoretical Physics, FOCUS Center, and Department of Physics, University
Theory of selective excitation in stimulated Raman scattering S. A. Malinovskaya, P. H. Bucksbaum, and P. R. Berman Michigan Center for Theoretical Physics, FOCUS Center, and Department of Physics, University
4Types of Isomers. 1. Structural Isomers/(Constitutional) 2. Geometric Isomers/(Cis/Trans) 3. Optical Isomers A. Enantiomers B.
 4Types of Isomers 1. Structural Isomers/(Constitutional) 2. Geometric Isomers/(Cis/Trans) 3. Optical Isomers A. Enantiomers B. Diastereomers 4Types of Isomers C 4 10 C 4 10 O O O O O O O O O O O O C 3
4Types of Isomers 1. Structural Isomers/(Constitutional) 2. Geometric Isomers/(Cis/Trans) 3. Optical Isomers A. Enantiomers B. Diastereomers 4Types of Isomers C 4 10 C 4 10 O O O O O O O O O O O O C 3
Scientific opportunities with ultrafast electron diffraction & microscopy
 Scientific opportunities with ultrafast electron diffraction & microscopy Jim Cao Frontier of ultrafast science MeV UED Transition pathways Rate and time scale Elementary steps Probe dynamics on the atomic
Scientific opportunities with ultrafast electron diffraction & microscopy Jim Cao Frontier of ultrafast science MeV UED Transition pathways Rate and time scale Elementary steps Probe dynamics on the atomic
Departmental Final Examination. Organic Chemistry I Caffein
 Departmental Final Examination rganic Chemistry I 2423 Caffein Name CEMISTRY 2423 FINAL EXAM FALL, 2005 DIRECTINS: A periodic table is attached at the end of this exam. Please answer all questions as completely
Departmental Final Examination rganic Chemistry I 2423 Caffein Name CEMISTRY 2423 FINAL EXAM FALL, 2005 DIRECTINS: A periodic table is attached at the end of this exam. Please answer all questions as completely
Effects of Vibrational Excitation on the F + H2O HF + OH Reaction: Dissociative Photodetachment of Overtone Excited [F H OH]ˉ
![Effects of Vibrational Excitation on the F + H2O HF + OH Reaction: Dissociative Photodetachment of Overtone Excited [F H OH]ˉ Effects of Vibrational Excitation on the F + H2O HF + OH Reaction: Dissociative Photodetachment of Overtone Excited [F H OH]ˉ](/thumbs/82/85526767.jpg) Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2017 Effects of Vibrational Excitation on the F + H2O HF + OH Reaction: Dissociative Photodetachment
Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2017 Effects of Vibrational Excitation on the F + H2O HF + OH Reaction: Dissociative Photodetachment
Supporting Information
 Supporting Information Roles of Water Molecules in Modulating the Reactivity of Dioxygen-bound - ZSM-5 toward Methane: A Theoretical Prediction Takashi Yumura,,* Yuuki Hirose, Takashi Wakasugi, Yasushige
Supporting Information Roles of Water Molecules in Modulating the Reactivity of Dioxygen-bound - ZSM-5 toward Methane: A Theoretical Prediction Takashi Yumura,,* Yuuki Hirose, Takashi Wakasugi, Yasushige
Chapter 8: Alkene Structure and Preparation via Elimination Reactions
 Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C 3 C C 3 bond length bond strength 2 C C 2 a C=C double bond is stronger than a C C single
Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C 3 C C 3 bond length bond strength 2 C C 2 a C=C double bond is stronger than a C C single
PRESENTATION ISOMERISM. Dr. Susmita Bajpai
 PRESENTATION OF ISOMERISM Dr. Susmita Bajpai Department Chemistry B.N.D. College, Kanpur ISOMERISM What is isomerism:- The compounds which have the some molecular formula but differ from each other in
PRESENTATION OF ISOMERISM Dr. Susmita Bajpai Department Chemistry B.N.D. College, Kanpur ISOMERISM What is isomerism:- The compounds which have the some molecular formula but differ from each other in
For more info visit
 Bond Fission: a) Homolytic fission: Each atom separates with one electron, leading to the formation of highly reactive entities called radicals, owing their reactivity to their unpaired electron. b) Heterolytic
Bond Fission: a) Homolytic fission: Each atom separates with one electron, leading to the formation of highly reactive entities called radicals, owing their reactivity to their unpaired electron. b) Heterolytic
Charge transfer interaction in the acetic acid benzene cation complex
 JOURNAL OF CHEMICAL PHYSICS VOLUME 114, NUMBER 11 15 MARCH 2001 Charge transfer interaction in the acetic acid benzene cation complex Kentaroh Kosugi, Yoshiya Inokuchi, and Nobuyuki Nishi a) Institute
JOURNAL OF CHEMICAL PHYSICS VOLUME 114, NUMBER 11 15 MARCH 2001 Charge transfer interaction in the acetic acid benzene cation complex Kentaroh Kosugi, Yoshiya Inokuchi, and Nobuyuki Nishi a) Institute
4.15 Halogenation of Alkanes RH + X 2 RX + HX
 4.15 alogenation of Alkanes R + X 2 RX + X Energetics R + X 2 RX + X explosive for F 2 exothermic for Cl 2 and Br 2 endothermic for I 2 4.16 Chlorination of Methane Chlorination of Methane carried out
4.15 alogenation of Alkanes R + X 2 RX + X Energetics R + X 2 RX + X explosive for F 2 exothermic for Cl 2 and Br 2 endothermic for I 2 4.16 Chlorination of Methane Chlorination of Methane carried out
Work hard. Be nice. Name: Period: Date:
 Name: Period: Date: UNIT 6: Organic Chemistry Lesson 2: Isomers and Side Chains By the end of today, you will have an answer to: What happens when two different molecules have the same formula? Do Now:
Name: Period: Date: UNIT 6: Organic Chemistry Lesson 2: Isomers and Side Chains By the end of today, you will have an answer to: What happens when two different molecules have the same formula? Do Now:
Alkanes and Cycloalkanes
 Alkanes and Cycloalkanes Alkanes molecules consisting of carbons and hydrogens in the following ratio: C n H 2n+2 Therefore, an alkane having 4 carbons would have 2(4) + 2 hydrogens, which equals 10 hydrogens.
Alkanes and Cycloalkanes Alkanes molecules consisting of carbons and hydrogens in the following ratio: C n H 2n+2 Therefore, an alkane having 4 carbons would have 2(4) + 2 hydrogens, which equals 10 hydrogens.
CH320/328 M Spring 2014
 CH320/328 M Spring 2014 HW Set #3 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your
CH320/328 M Spring 2014 HW Set #3 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your
How to identify types of transition in experimental spectra
 17 18 19 How to identify types of transition in experimental spectra 1. intensity 2. Band width 3. polarization Intensities are governed by how well the selection rules can be applied to the molecule under
17 18 19 How to identify types of transition in experimental spectra 1. intensity 2. Band width 3. polarization Intensities are governed by how well the selection rules can be applied to the molecule under
Lecture 15: Programming Example: TASEP
 Carl Kingsford, 0-0, Fall 0 Lecture : Programming Example: TASEP The goal for this lecture is to implement a reasonably large program from scratch. The task we will program is to simulate ribosomes moving
Carl Kingsford, 0-0, Fall 0 Lecture : Programming Example: TASEP The goal for this lecture is to implement a reasonably large program from scratch. The task we will program is to simulate ribosomes moving
CPT-26 ANSWERS 73. (1) 145. (4) 2. (1) 74. (3) 146. (4) 3. (3) 75. (2) 147. (3) 4. (2) 5. (4) 76. (4) 77. (2) 148. (2) 149. (3) 6. (3) 78.
 1 08/01/2018 COMMON PRACTICE TEST [PMT] : 2017-19 CPT-26 ANSWERS CODE GOL 1. (1) 37. (3) 73. (1) 109. (3) 145. (4) 2. (1) 38. (1) 74. (3) 110. (3) 146. (4) 3. (3) 39. (1) 75. (2) 111. (2) 147. (3) 4. (2)
1 08/01/2018 COMMON PRACTICE TEST [PMT] : 2017-19 CPT-26 ANSWERS CODE GOL 1. (1) 37. (3) 73. (1) 109. (3) 145. (4) 2. (1) 38. (1) 74. (3) 110. (3) 146. (4) 3. (3) 39. (1) 75. (2) 111. (2) 147. (3) 4. (2)
Course Outline For: Organic Chemistry I (CHM 270) Credits: 5 Contact Hours: Lecture: 3 Lab: 4
 Course Outline For: Organic Chemistry I (CHM 270) Credits: 5 Contact Hours: Lecture: 3 Lab: 4 NOTE on Laboratory: Both Lecture and Laboratory must be taken simultaneously; separate grades will not be given
Course Outline For: Organic Chemistry I (CHM 270) Credits: 5 Contact Hours: Lecture: 3 Lab: 4 NOTE on Laboratory: Both Lecture and Laboratory must be taken simultaneously; separate grades will not be given
CHEM1902/ N-9 November 2014
 CEM1902/4 2014-N-9 November 2014 The elimination of 2 O from alcohol A can form the isomeric alkenes B and C. Elimination of Br from the alkyl halide D can generate the same two alkenes. 7 Assign the absolute
CEM1902/4 2014-N-9 November 2014 The elimination of 2 O from alcohol A can form the isomeric alkenes B and C. Elimination of Br from the alkyl halide D can generate the same two alkenes. 7 Assign the absolute
Potential Energy Surface and Binding Energy in External Electric Field: Modulation of Anion π Interactions for Graphene Based Receptors
 Potential Energy Surface and Binding Energy in External Electric Field: Modulation of Anion π Interactions for Graphene Based Receptors Cina Foroutan Nejad a and Radek Marek a,b a National Center for Biomolecular
Potential Energy Surface and Binding Energy in External Electric Field: Modulation of Anion π Interactions for Graphene Based Receptors Cina Foroutan Nejad a and Radek Marek a,b a National Center for Biomolecular
CHE1502. Tutorial letter 203/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry
 E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
Isomerism. Introduction
 Isomerism Introduction The existence of two or more compounds with same molecular formula but different properties (physical, chemical or both) is known as isomerism; and the compounds themselves are called
Isomerism Introduction The existence of two or more compounds with same molecular formula but different properties (physical, chemical or both) is known as isomerism; and the compounds themselves are called
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
Chemistry 11. Unit 10 Organic Chemistry Part III Unsaturated and aromatic hydrocarbons
 Chemistry 11 Unit 10 Organic Chemistry Part III Unsaturated and aromatic hydrocarbons 2 1. Unsaturated hydrocarbons So far, we have studied the hydrocarbons in which atoms are connected exclusively by
Chemistry 11 Unit 10 Organic Chemistry Part III Unsaturated and aromatic hydrocarbons 2 1. Unsaturated hydrocarbons So far, we have studied the hydrocarbons in which atoms are connected exclusively by
Supplementary Information
 Supplementary Information Supplementary Figure 1. Schematic diagram of the RF magneton sputtering system used to deposit a thin SiO2 film on CaF2 surface. 1 Supplementary Figure 2. A Li + DEC complex structure.
Supplementary Information Supplementary Figure 1. Schematic diagram of the RF magneton sputtering system used to deposit a thin SiO2 film on CaF2 surface. 1 Supplementary Figure 2. A Li + DEC complex structure.
High-resolution slice imaging of quantum state-to-state photodissociation of methyl bromide
 High-resolution slice imaging of quantum state-to-state photodissociation of methyl bromide M. Laura Lipciuc and Maurice H. M. Janssen Citation: The Journal of Chemical Physics 127, 224310 (2007); doi:
High-resolution slice imaging of quantum state-to-state photodissociation of methyl bromide M. Laura Lipciuc and Maurice H. M. Janssen Citation: The Journal of Chemical Physics 127, 224310 (2007); doi:
Chemistry 2541, Fall 2017 Midterm Exam 2 (100 points)
 Chemistry 2541, Fall 2017 Midterm Exam 2 (100 points) Important notes: - Please use the provided Scantron form for your answers; you can keep the sheet with the questions and can use it as scratch paper
Chemistry 2541, Fall 2017 Midterm Exam 2 (100 points) Important notes: - Please use the provided Scantron form for your answers; you can keep the sheet with the questions and can use it as scratch paper
(1) Check to see if the two compounds are identical. (2) Recall the definitions of stereoisomers, conformational isomers, and constitutional isomers.
 MCAT Organic Chemistry Problem Drill 04: Stereochemistry Question No. 1 of 10 Question 1. Determine the relationship of the molecules shown: O O Question #01 (A) Identical (B) Constitutional isomers (C)
MCAT Organic Chemistry Problem Drill 04: Stereochemistry Question No. 1 of 10 Question 1. Determine the relationship of the molecules shown: O O Question #01 (A) Identical (B) Constitutional isomers (C)
Ch 22 Carbonyl Alpha ( ) Substitution
 Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Chapter 8: Alkene Structure and Preparation via Elimination Reactions
 1. Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C bond length bond strength 3 C C 3 3 C C 3 3 C C 3 3 C 2 C C 2 3 C a C=C double bond
1. Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C bond length bond strength 3 C C 3 3 C C 3 3 C C 3 3 C 2 C C 2 3 C a C=C double bond
Excited-state dynamics of isolated nucleic acid bases and their clusters
 Journal of Photochemistry and Photobiology C: Photochemistry Reviews 7 (2006) 197 210 Review Excited-state dynamics of isolated nucleic acid bases and their clusters Hiroyuki Saigusa Graduate School of
Journal of Photochemistry and Photobiology C: Photochemistry Reviews 7 (2006) 197 210 Review Excited-state dynamics of isolated nucleic acid bases and their clusters Hiroyuki Saigusa Graduate School of
Specialized Raman Techniques. Strictly speaking the radiation-induced dipole moment should be expressed as
 Nonlinear effects Specialized Raman Techniques Strictly speaking the radiation-induced dipole moment should be expressed as M = E + ½E 2 + (1/6)E 3 +... Where and are the first and second hyperpolarizabilities.
Nonlinear effects Specialized Raman Techniques Strictly speaking the radiation-induced dipole moment should be expressed as M = E + ½E 2 + (1/6)E 3 +... Where and are the first and second hyperpolarizabilities.
Practical Bioinformatics
 5/2/2017 Dictionaries d i c t i o n a r y = { A : T, T : A, G : C, C : G } d i c t i o n a r y [ G ] d i c t i o n a r y [ N ] = N d i c t i o n a r y. h a s k e y ( C ) Dictionaries g e n e t i c C o
5/2/2017 Dictionaries d i c t i o n a r y = { A : T, T : A, G : C, C : G } d i c t i o n a r y [ G ] d i c t i o n a r y [ N ] = N d i c t i o n a r y. h a s k e y ( C ) Dictionaries g e n e t i c C o
CH Organic Chemistry I (Katz) Practice Exam #3- Fall 2013
 C2710 - rganic Chemistry I (Katz) Practice Exam #3- all 2013 Name: Score: Part I - Choose the best answer and write the letter of your choice in the space provided. (32 pts) 1. f the following, which reaction
C2710 - rganic Chemistry I (Katz) Practice Exam #3- all 2013 Name: Score: Part I - Choose the best answer and write the letter of your choice in the space provided. (32 pts) 1. f the following, which reaction
There are several possible arrangements for a molecule which contains two double bonds (diene): 1. Isolated: (two or more single bonds between them)
 1 Chapter 15: Conjugation and Reactions of Dienes I. Introduction to Conjugation There are several possible arrangements for a molecule which contains two double bonds (diene): 1. Isolated: (two or more
1 Chapter 15: Conjugation and Reactions of Dienes I. Introduction to Conjugation There are several possible arrangements for a molecule which contains two double bonds (diene): 1. Isolated: (two or more
Chemistry 102 Organic Chemistry: Introduction to Isomers Workshop
 Chemistry 102 Organic Chemistry: Introduction to Isomers Workshop What are isomers? Isomers are molecules with the same molecular formula, but different arrangements of atoms. There are different types
Chemistry 102 Organic Chemistry: Introduction to Isomers Workshop What are isomers? Isomers are molecules with the same molecular formula, but different arrangements of atoms. There are different types
Abstract... I. Acknowledgements... III. Table of Content... V. List of Tables... VIII. List of Figures... IX
 Abstract... I Acknowledgements... III Table of Content... V List of Tables... VIII List of Figures... IX Chapter One IR-VUV Photoionization Spectroscopy 1.1 Introduction... 1 1.2 Vacuum-Ultraviolet-Ionization
Abstract... I Acknowledgements... III Table of Content... V List of Tables... VIII List of Figures... IX Chapter One IR-VUV Photoionization Spectroscopy 1.1 Introduction... 1 1.2 Vacuum-Ultraviolet-Ionization
SI0 UV-vis absorption spectra and emission response of 6MQz and 6MQc
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 SI0 UV-vis absorption spectra and emission response of 6MQz and 6MQc The absorption and emission
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 SI0 UV-vis absorption spectra and emission response of 6MQz and 6MQc The absorption and emission
Chemistry 110. Bettelheim, Brown, Campbell & Farrell. Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes.
 Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes Chapter 12 Alkenes are hydrocarbons which have one or more
Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes Chapter 12 Alkenes are hydrocarbons which have one or more
Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes
 Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes Chapter 12 Alkenes are hydrocarbons which have one or more
Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes Chapter 12 Alkenes are hydrocarbons which have one or more
Introduction to Alkenes and Alkynes
 Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene,
Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene,
Organic Chemistry. February 18, 2014
 Organic Chemistry February 18, 2014 What does organic mean? Organic Describes products Grown through natural biological process Without synthetic materials In the 18 th century Produced by a living system
Organic Chemistry February 18, 2014 What does organic mean? Organic Describes products Grown through natural biological process Without synthetic materials In the 18 th century Produced by a living system
(c) The intermediate carbocation resulting from 3-bromobut-1-ene is resonance stabilized.
 KT121 Sem II 09/10 SETIN B: 1.(a) Name of mechanism: E1 elimination. 7 marks (b) (i) + 3 3 3 (ii) + 3 (iii) l 8 marks (c) The intermediate carbocation resulting from 3-bromobut-1-ene is resonance stabilized.
KT121 Sem II 09/10 SETIN B: 1.(a) Name of mechanism: E1 elimination. 7 marks (b) (i) + 3 3 3 (ii) + 3 (iii) l 8 marks (c) The intermediate carbocation resulting from 3-bromobut-1-ene is resonance stabilized.
CHE 325 SPECTROSCOPY (A) CHAP 13A ASSIGN CH 2 CH CH 2 CH CHCH 3
 CE 325 SPECTRSCPY (A) CAP 13A ASSIGN 1. Which compound would have a UV absorption band at longest wavelength? A. I B. II C. III D. IV E. V C CC 3 CC C 2 C CC 3 I II III C 2 C C 2 C CC 3 IV V 2. Select
CE 325 SPECTRSCPY (A) CAP 13A ASSIGN 1. Which compound would have a UV absorption band at longest wavelength? A. I B. II C. III D. IV E. V C CC 3 CC C 2 C CC 3 I II III C 2 C C 2 C CC 3 IV V 2. Select
PHOTO-DISSOCIATION OF CO 2 GAS BY USING TWO LASERS
 Proceedings of the 3rd Annual ISC Research Symposium ISCRS 9 April 14, 9, Rolla, Missouri PHOTO-DISSOCIATION OF CO GAS BY USING TWO LASERS Zhi Liang MAE department/zlch5@mst.edu Dr. Hai-Lung Tsai MAE department/tsai@mst.edu
Proceedings of the 3rd Annual ISC Research Symposium ISCRS 9 April 14, 9, Rolla, Missouri PHOTO-DISSOCIATION OF CO GAS BY USING TWO LASERS Zhi Liang MAE department/zlch5@mst.edu Dr. Hai-Lung Tsai MAE department/tsai@mst.edu
Chem 263 Oct. 12, 2010
 Chem 263 ct. 12, 2010 Alkyl Side Chain xidation Reaction If the carbon directly attached to the aromatic ring has > 1 hydrogen attached to it, it can be oxidized to the corresponding carboxylic acid with
Chem 263 ct. 12, 2010 Alkyl Side Chain xidation Reaction If the carbon directly attached to the aromatic ring has > 1 hydrogen attached to it, it can be oxidized to the corresponding carboxylic acid with
Name. Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I. Examination #3 - November 8, 2004 ANSWERS
 Name Department of hemistry SUNY/neonta hem 221 - rganic hemistry I Examination #3 - November 8, 2004 ANSWERS INSTRUTINS --- This examination has two parts. The first part is in multiple choice format;
Name Department of hemistry SUNY/neonta hem 221 - rganic hemistry I Examination #3 - November 8, 2004 ANSWERS INSTRUTINS --- This examination has two parts. The first part is in multiple choice format;
Chemistry 233 Exam 3. The Periodic Table
 Name: Last First MI Chemistry 233 Exam 3 Fall 2017 Dr. J. sbourn Instructions: The first 8 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
Name: Last First MI Chemistry 233 Exam 3 Fall 2017 Dr. J. sbourn Instructions: The first 8 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Light-Activated Chemical Probing of Nucleobase Solvent Accessibility Inside
 Light-Activated Chemical Probing of Nucleobase Solvent Accessibility Inside Cells Chao Feng 1,$, Dalen Chan 1,$, Jojo Joseph 2, Mikko Muuronen 3, William H. Coldren 2, Nan Dai 4, Ivan R. Corrêa Jr. 4,
Light-Activated Chemical Probing of Nucleobase Solvent Accessibility Inside Cells Chao Feng 1,$, Dalen Chan 1,$, Jojo Joseph 2, Mikko Muuronen 3, William H. Coldren 2, Nan Dai 4, Ivan R. Corrêa Jr. 4,
CHEM 343 Principles of Organic Chemistry II Summer Instructor: Paul J. Bracher. Quiz # 3. Monday, July 21 st, :30 a.m.
 CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
Infrared photodissociation spectroscopy of protonated formic. acid and acetic acid clusters
 Infrared photodissociation spectroscopy of protonated formic acid and acetic acid clusters Yoshiya Inokuchi and Nobuyuki Nishi * Institute for Molecular Science, Myodaiji, Okazaki 444-8585, Japan Abstract
Infrared photodissociation spectroscopy of protonated formic acid and acetic acid clusters Yoshiya Inokuchi and Nobuyuki Nishi * Institute for Molecular Science, Myodaiji, Okazaki 444-8585, Japan Abstract
Visible and IR Absorption Spectroscopy. Andrew Rouff and Kyle Chau
 Visible and IR Absorption Spectroscopy Andrew Rouff and Kyle Chau The Basics wavelength= (λ) original intensity= Ι o sample slab thickness= dl Final intensity= I f ε = molar extinction coefficient -di=
Visible and IR Absorption Spectroscopy Andrew Rouff and Kyle Chau The Basics wavelength= (λ) original intensity= Ι o sample slab thickness= dl Final intensity= I f ε = molar extinction coefficient -di=
Supporting Information. Hydroxyl Radical Attack on Reduced Graphene Oxide
 Supporting Information Making Graphene Holey. Gold Nanoparticle-Mediated Hydroxyl Radical Attack on Reduced Graphene Oxide James G. Radich, 1,3 Prashant V. Kamat *1,2,3 Radiation Laboratory Department
Supporting Information Making Graphene Holey. Gold Nanoparticle-Mediated Hydroxyl Radical Attack on Reduced Graphene Oxide James G. Radich, 1,3 Prashant V. Kamat *1,2,3 Radiation Laboratory Department
CHEM6085: Density Functional Theory Lecture 10
 CHEM6085: Density Functional Theory Lecture 10 1) Spin-polarised calculations 2) Geometry optimisation C.-K. Skylaris 1 Unpaired electrons So far we have developed Kohn-Sham DFT for the case of paired
CHEM6085: Density Functional Theory Lecture 10 1) Spin-polarised calculations 2) Geometry optimisation C.-K. Skylaris 1 Unpaired electrons So far we have developed Kohn-Sham DFT for the case of paired
Loudon Chapter 14 Review: Reactions of Alkynes Jacquie Richardson, CU Boulder Last updated 1/16/2018
 An alkyne is any molecule with a triple bond between two carbon atoms. This triple bond consists of one σ bond and two π bonds: the σ bond exists on a straight line between carbon atoms, while one π bond
An alkyne is any molecule with a triple bond between two carbon atoms. This triple bond consists of one σ bond and two π bonds: the σ bond exists on a straight line between carbon atoms, while one π bond
SSR ( ) Vol. 48 No ( Microsatellite marker) ( Simple sequence repeat,ssr),
 48 3 () Vol. 48 No. 3 2009 5 Journal of Xiamen University (Nat ural Science) May 2009 SSR,,,, 3 (, 361005) : SSR. 21 516,410. 60 %96. 7 %. (),(Between2groups linkage method),.,, 11 (),. 12,. (, ), : 0.
48 3 () Vol. 48 No. 3 2009 5 Journal of Xiamen University (Nat ural Science) May 2009 SSR,,,, 3 (, 361005) : SSR. 21 516,410. 60 %96. 7 %. (),(Between2groups linkage method),.,, 11 (),. 12,. (, ), : 0.
Chem 145 Unsaturated hydrocarbons Alkynes
 Dr. Seham ALTERARY Chem 145 Unsaturated hydrocarbons Alkynes Chapter 4 1434-1435 2013-2014 2 st semester By the end of this chapter you should be familiar with: Definition for Alkynes. Nomenclature of
Dr. Seham ALTERARY Chem 145 Unsaturated hydrocarbons Alkynes Chapter 4 1434-1435 2013-2014 2 st semester By the end of this chapter you should be familiar with: Definition for Alkynes. Nomenclature of
Probing the Ultrafast Energy Dissipation Mechanism of the Sunscreen Oxybenzone after UVA Irradiation
 Probing the Ultrafast Energy Dissipation Mechanism of the Sunscreen Oxybenzone after UVA Irradiation Lewis A. Baker 1, Michael D. Horbury 1, Simon E. Greenough 1, Philip M. Coulter 2, Tolga N. V. Karsili
Probing the Ultrafast Energy Dissipation Mechanism of the Sunscreen Oxybenzone after UVA Irradiation Lewis A. Baker 1, Michael D. Horbury 1, Simon E. Greenough 1, Philip M. Coulter 2, Tolga N. V. Karsili
Reductive Elimination
 Reductive Elimination Reductive elimination, the reverse of oxidative addition, is most often seen in higher oxidation states because the formal oxidation state of the metal is reduced by two units in
Reductive Elimination Reductive elimination, the reverse of oxidative addition, is most often seen in higher oxidation states because the formal oxidation state of the metal is reduced by two units in
Raman spectroscopy of phthalocyanines and their sulfonated derivatives
 Journal of Molecular Structure 744 747 (2005) 481 485 www.elsevier.com/locate/molstruc Raman spectroscopy of phthalocyanines and their sulfonated derivatives B. Brożek-Płuska*, I. Szymczyk, H. Abramczyk
Journal of Molecular Structure 744 747 (2005) 481 485 www.elsevier.com/locate/molstruc Raman spectroscopy of phthalocyanines and their sulfonated derivatives B. Brożek-Płuska*, I. Szymczyk, H. Abramczyk
16. Reactions of the Radical Pairs. The I P Step of the Paradigm
 16. Reactions of the Radical Pairs. The I P Step of the Paradigm Let us consider the product forming step in the general photochemical paradigm, i.e., the I P process shown in Figure 1. From the discussion
16. Reactions of the Radical Pairs. The I P Step of the Paradigm Let us consider the product forming step in the general photochemical paradigm, i.e., the I P process shown in Figure 1. From the discussion
NAME: 3rd (final) EXAM
 1 Chem 64 Winter 2003 AME: 3rd (final) EXAM THIS EXAM IS WORTH 100 POITS AD COTAIS 9 QUESTIOS THEY ARE OT EQUALLY WEIGHTED! YOU SHOULD ATTEMPT ALL QUESTIOS AD ALLOCATE YOUR TIME ACCORDIGLY IF YOU DO'T
1 Chem 64 Winter 2003 AME: 3rd (final) EXAM THIS EXAM IS WORTH 100 POITS AD COTAIS 9 QUESTIOS THEY ARE OT EQUALLY WEIGHTED! YOU SHOULD ATTEMPT ALL QUESTIOS AD ALLOCATE YOUR TIME ACCORDIGLY IF YOU DO'T
Chem Fall 2015 Exam II
 Key Name hem 150 - Fall 2015 Exam II 1. Label each of the carbon and nitrogen atoms in the molecule shown below as have having either a tetrahedral (Tet), trigonal planar () or linear (Lin) geometry. 3
Key Name hem 150 - Fall 2015 Exam II 1. Label each of the carbon and nitrogen atoms in the molecule shown below as have having either a tetrahedral (Tet), trigonal planar () or linear (Lin) geometry. 3
Ballistic Electron Spectroscopy of Quantum Mechanical Anti-reflection Coatings for GaAs/AlGaAs Superlattices
 Ballistic Electron Spectroscopy of Quantum Mechanical Anti-reflection Coatings for GaAs/AlGaAs Superlattices C. Pacher, M. Kast, C. Coquelin, G. Fasching, G. Strasser, E. Gornik Institut für Festkörperelektronik,
Ballistic Electron Spectroscopy of Quantum Mechanical Anti-reflection Coatings for GaAs/AlGaAs Superlattices C. Pacher, M. Kast, C. Coquelin, G. Fasching, G. Strasser, E. Gornik Institut für Festkörperelektronik,
There are 19 problems on 22 pages. Please make sure that you have them all.
 EMISTRY 222, Spring 2001 Review Problems Page 1 There are 19 problems on 22 pages. Please make sure that you have them all. 1) Give an unambiguous name for the following compounds. e sure to use cis/trans,
EMISTRY 222, Spring 2001 Review Problems Page 1 There are 19 problems on 22 pages. Please make sure that you have them all. 1) Give an unambiguous name for the following compounds. e sure to use cis/trans,
The mutagenic action of 5-bromouracil
 The mutagenic action of 5-bromouracil The structure of DNA DNA bases Cytosine (C) Thymine (T) oxygen carbon nitrogen hydrogen Guanine (G) Adenine (A) Hydrogen bonds picture from wikipedia picture from
The mutagenic action of 5-bromouracil The structure of DNA DNA bases Cytosine (C) Thymine (T) oxygen carbon nitrogen hydrogen Guanine (G) Adenine (A) Hydrogen bonds picture from wikipedia picture from
