Novel sorbents for flue gas purification
|
|
|
- Katherine Hill
- 5 years ago
- Views:
Transcription
1 Open Access Journal journal homepage:papers.itc.pw.edu.pl Novel sorbents for flue gas purification Aleksandra Ściubidło, Wojciech Nowak Institute of Advanced Energy Technologies, Czestochowa University of Technology 73 Dabrowskiego Street, Czestochowa, Poland Abstract This paper presents a method for cleaning exhaust gas of nitrogen oxides using the process of physical adsorption on novel sorbents obtained from fly ash. Use of this method, combined with CO 2 capture, allows emission standards to be met. Keywords: sorbents, nitrogen oxides, mesoporous material MCM-41, physical adsorption, fly ash 1. Introduction Due to the obligations arising from the European Union climate-energy package emphasis is placed on reducing greenhouse gas emissions, especially CO 2, which is considered a major cause of global warming. In accordance with the requirements of the European Commission, by the year 2020, CO 2 emissions should be reduced by 20%, the share of renewable energy sources (RES) should increase by 20%. Since the share of coal in total CO 2 emissions is approximately 38%, and since it is the main fuel for energy production in Poland, the most appropriate technologies seem to be carbon capture and storage (CCS). The European Commission stresses that carbon sequestration will be a necessary condition for using coal as a fuel. Great emphasis is put on CO 2 removal from flue gas using two methods: the absorption method, and Paper presented at the 10 th International Conference on Research & Development in Power Engineering 2011, Warsaw, Poland Corresponding author addresses: asciubidlo@is.pcz.czest.pl (Aleksandra Ściubidło ), wnowak@is.pcz.czest.pl (Wojciech Nowak) adsorption on amine solid sorbents modified with amines. The main problem associated with these methods is the pollution of amines with compounds such as oxides of sulfur and nitrogen. As a result of chemical reactions between oxides and amines, irreversible reactions take place which causes degradation of amines. In the case of SO 2, safe concentrations should not exceed 10 ppm, while for oxides of nitrogen they should be less than 40 mg/nm 3 (20 ppm). In the case of nitrogen oxides, both methods of primary and secondary NO x reduction fail to achieve safe concentrations of nitrogen oxides in the exhaust gas. The most effective way to reduce emissions of nitrogen oxides is the SCR method, which achieves emission limits of 100 mg/nm 3. Unfortunately, this value is 5 times higher than that permissible for amines-based carbon capture. The exhaust gas from a power plant boiler contains 95 97% NO, 3 5% NO 2, and about 0.5% N 2 O. Because NO is an inert gas but easy to oxidize chemically, the author proposes to pick up the NO 2 in the process of adsorption on solid sorbents after first oxidizing NO to NO 2. Sorbents such as mesoporous materials can be obtained, with benefits to the environment, from power
2 Table 1: Chemical composition of fly ash Content of basic chemicals, wt. % LOI 2.97 S 0.16 C 3.41 SiO Al 2 O Fe 2 O CaO 4.35 averaged values measured on a large sample plant fly ash waste. Due to its chemical composition, fly ash is a valuable source of silicon and aluminum in the synthesis of mesoporous materials [1 8]. A number of applications of both microporous and mesoporous molecular sieve can be found in the literature. They are used among others in the purification of gases from pollutants such as carbon dioxide, sulfur dioxide, hydrogen sulfide, nitrogen oxides and mercury [2, 9 22]. In the case of materials synthesized from fly ash, information can also be found about their use in the purification of gases, mainly carbon dioxide, through adsorption. This paper presents a method for cleaning exhaust gas of nitrogen oxides using the process of physical adsorption on novel sorbents obtained from fly ash. Use of this method, combined with CO 2 capture, allows emission standards to be met. 2. Experiment 2.1. Characteristics of the source material for the synthesis of the mesoporous material MCM Chemical Composition As a starting material fly ash derived from the combustion of coal in a pulverized boiler was used. The chemical composition of the fly ash was determined with use of an X-ray fluorescence spectrometer, and the results are presented in Table 1. The ash had a SiO 2 content of wt. %, 24.9 wt. % of Al 2 O 3 and a CaO content of 4.35 wt. %. These data indicate that the ash in accordance can be classified as a silica ash (Polish standard BN- 79/ ). Figure 1: Thermogravimetric analysis TGA (a) and DTG (b) of fly ash Thermogravimetric Analysis The effect of temperature on the sample ash was investigated by the thermogravimetry method (TG) and differential thermal analysis (SDTA) using a Mettler Toledo TGA / SDTA 851e thermal analyzer. A sample of fly ash was heated in a platinum crucible at atmospheric pressure in an inert atmosphere (N 2 ) with a gas flow rate of 50 cm 3 /min, in a temperature range of K with a heating speed 293 K/min. The weight of samples was 10 mg. The results of the studies, in the form of thermograms, are shown in Figure 1 as graphs illustrating the relationship between the weight change of a sample versus temperature (TG signal) and its first derivative (DTG). The figure shows the three weight losses for the ash samples, with local minima at temperatures of 50 C, 500 C and 870 C respectively. The first loss is 0.02 mg, which is caused by water evaporation. The second loss, of 0.15 mg, is due to the dehydration of Ca(OH) 2. At 870 C there is a mass loss of 0.12 mg, resulting from the decomposition of CaCO 3. The total mass loss of the sample is 0.56 mg Analysis of the surface area In order to examine the structure of fly ash, lowtemperature nitrogen adsorption at 77 K was carried out on ASAP 2000 apparatus. After degassing the sample at 623 K the adsorption-desorption isotherms of nitrogen were measured. Based on these isotherms, the surface area, total pore volume, mean pore diameter, pore volume distribution as a function of their diameter and size distribution of surface area versus pore diameter were calculated. Figure 2 shows adsorption/desorption isotherm curves as well as pore volume and size distribution of the surface as a function of their diameter. The 116
3 pore area, cm 2 /gnm 0,0009 0,0008 0,0007 0,0006 0,0005 0,0004 0,0003 0,0002 0,0001 0, pore diametere, nm Figure 2: N2 adsorption/desorption isotherms for fly ash Figure 4: Surface area size distributions by pore size for the fly ash Analyzing the distribution curves of specific surface as a function of pore diameter (Fig. 4), it should be noted that the largest share of the total surface area are pores with a diameter of nm. Figure 3: Pore size distribution of fly ash presented adsorption/desorption isotherms of ash are consistent with the classification of IUPAC and these are the type II isotherms observed in macroporous adsorbents. From the presented curves can be seen that the adsorption is small at large values of p p 0, and that the hysteresis loop, whose shape is related to the proper pore structure and which in this case can be classified as H3, starts at low relative pressures, indicating the small share of micropores in the structure. Analyzing the distribution curves of pore volume by their diameter, as shown in Figure 3, it should be noted that the largest share of the total pore volume are pores with a diameter of nm Images of the surface In order to determine the shape and size of crystallite size in the range of microns and its morphology and structure, fly ash samples were analyzed by scanning microscopy (SEM). The tests were performed using a Tesla BS-301 scanning electron microscope equipped with a Satellite digital imaging system. The samples for SEM were prepared by spraying the surface of the tested materials with a thin layer of Au-Pd. Then digital images were taken with the SE detector microscope for various parts of the surface of these samples at different magnifications. Micrograms of the tested samples are shown in Fig. 5. The presence of fly ash components such as SiO 2, calcium, and unburned carbon can be seen in these pictures Synthesis of mesoporous silica materials MCM- 41 Extract from fly ash, a rich source of silicon and aluminum, is needed for the synthesis of MCM-41. To achieve this, the ashes were mixed with NaOH in a way allowing the mass ratio to remain at 1:1.2. Next the mixture was ground for 2 hours and then 117
4 Figure 5: SEM images of fly ash 118
5 Table 2: Element concentrations in the filtrate Elemental compositions Concentration in the filtrate, mg/l Fe 1.2 Na K Ca 12 Mg 0.2 Al Si the sample was subjected to heat treatment at 823 K for 1 hour with a heating speed of 1 K/min. After cooling to room temperature the material was ground again and mixed with water in a mass ratio of 1:4 the sample was shaken at room temperature for 12 hours. The sample was then filtered and the filtrate was subjected to ICP analysis. The results of the analysis are presented in Table 2. The results shown in Table 2 show that the silicon content in solution was mg/l and aluminum content was mg/l. A surfactantmatrix solution was added to a previously prepared extracted solution of silicon. For this purpose, CTAB (cetyltrimethylammonium bromide C 16 H 33 (CH 3 ) 3 NBr) was dissolved in distilled water and NH 4 OHaq was added. Next it was added to the supernatant (silicon extract) so that the molar ratio in the gel was SiO 2 :CTAB:H 2 O = 1:0.15:170. The ph of the solution was adjusted to 11 by adding CH 3 COOH and it was adjusted several times while heating the mixture at 373 K for 4 days. The crystallized sample was then filtered and washed with distilled water to remove excess NaOH and dried at 373 K for 24 h. In order to remove the sample matrix it was calcined at 823 K for 2 hours with a heating speed of 1 K/min. Figure 6 shows a picture of the mesoporous sieve MCM-41 that was finally obtained. The adsorption properties of the obtained material were examined by nitrogen adsorption at 77 K, which enabled determination of the total surface area, total pore volume and pore volume distribution according to their diameter. Table 3 shows the data obtained for the ash and the material obtained from it. Figure 6: Picture of sieve MCM-41. The resulting material was subjected to tests to determine its physical and chemical properties Table 3: Pore characteristics of fly ash and MCM-41 Sample Pore volume, cm 3 /g Specific surface area SB.E.T., m 2 /g Pore diameter, nm Ash Material MCM The SBET specific surface of fly ash was 0.6 m 2 /g, total pore volume of cm 3 /g with an average pore radius of 9.11 nm. For the obtained material the specific surface SBET of fly ash was 980 m 2 /g, total pore volume of cm 3 /g with an average pore radius of 2.74 nm. The resulting material was also analyzed using a high-resolution D8 Advance Powder Diffractometer with a Ge monochromator (length radiated CuKα1 = A). Reflections were recorded with a LynxEye silicon strip detector. Measurements were performed using a cuvette made of polymethylmethacrylate. Diffraction patterns were recorded at room temperature for Θ with steps of , at 1 step/sec. During the measurements the sample was rotated at 30 rotations/minute. Measurements were performed at X-ray generator tube voltage of 35 kv and current of 50 ma. 119
6 Figures 7 and 8 show low and high angle diffraction patterns of the received material. As can be seen from Figure 7, the low angle diffraction pattern contains the characteristic peak of mesoporous material MCM-41. In the sample material, the presence of pores with uniform mesopores and amorphous silica was observed. The resulting material was also analyzed using scanning electron microscopy (SEM) to determine the morphology and porous structure. Preparations for SEM were prepared by spraying the surface of the tested materials with a thin layer of Au-Pd. Then the digital images of different parts of the surface of these preparations at different magnifications were taken with the microscope. Pictures of the tested samples are shown in Figure 9. SEM photos of mesoporous material from fly ash MCM-41 show that the material structure is mostly particle agglomeration. Photomicrographs show a regular arrangement of the channels. In the pictures, the hexagonal structure of the resulting mesoporous material MCM-41 can be seen. Both diffractometric and scanning analysis, as well as adsorption isotherms confirm that mesoporous material MCM-41 with a hexagonal structure was obtained. Figure 7: XRD pattern of the MCM-41(low angle) 2.3. Adsorption In order to determine the suitability of obtained sorbents in the process of adsorption of NO 2, the sorption capacity characteristics of the NO 2 was determined. Figure 10 is a schematic diagram of the laboratory test rig on which the tests were performed. The position includes: Vertical tube furnace, Exhaust gas analyzer, Computer system of data acquisition, Rotameters, Mixer, Figure 8: XRD pattern of the MCM-41(high angle) Cylinders of gases. Due to the limits for NO x emission being set at the level of 200 mg/nm 3, a mixture of air (21% O 2, 79% N 2 ) and nitrogen dioxide at concentrations of 50 ppm NO 2 (100 mg/nm 3 ) and 100 ppm (200 mg/nm 3 ) and gas consisting of 15% CO 2, 6% O 2 and 79% N 2 were used in the research. Tests were conducted at temperatures of: 25 C, 60 C and 80 C. The gas stream with a fixed volume flux regulated by the rotameter passed through the furnace tube, inside which sorbent was placed on the grate. After the gas passed through the sorbent, changes in concentrations were recorded by an IMR 6000P analyzer at intervals of 10 s. The range of the analyzer measuring NO 2 was ppm, subrange 0.5% with an accuracy of + / - 1%. Sorption capacity was calculated on the basis of the weight of gas adsorbed on the sorbent (as well as by weighing the mass increase of the sample before and after the process) and expressed in mg of 120
7 Figure 9: SEM images of MCM
8 Figure 11: Breakthrough curves of NO 2 /air for a sieve MCM- 41 and MCM-41-PEG(50) Figure 10: Diagram of the purification gas per 1 g of sorbent. During the adsorption process the variable parameters were: temperature of the process, the NO 2 concentration and the presence of CO 2 in the gas mixture. The desorption process was conducted at 100 C in a helium flow. The tests were performed using MCM-41 sieve obtained from fly ash. Prior to use, the sieves were heated at 100 C temperature in a helium flow. The adsorption process was conducted until the bed adsorption capacity was exhausted. From the results obtained, curves of NO 2 concentration as a function of time, called breakthrough curves, were created. They were used to calculate the mass of NO 2 adsorbed on the sorbent and the sorption capacity, expressed in mg NO 2 /g of sorbent. Figure 10 presents a diagram of the NO 2 removal process, involving the capture of nitrogen dioxide on solid sorbent obtained from fly ash. The system consists of two columns filled with a suitable sorbent. Gas containing NO 2 enters the system and passes through the first column where, at a temperature of 25 C, the adsorption process takes place. When the adsorbent bed is exhausted, the desorption process is carried out at a temperature of 100 C in the presence of a helium gas flow. Desorbed gas is derived from the system. The continuity of the process is assured as the presented system consists of two columns, in which the adsorptiondesorption processes take place alternately. The adsorption process occurs in the first column at the same time as the desorption process takes place in the second column Nitrogen dioxide adsorption on mesoporous sieve MCM-41 The sieve MCM-41 obtained from fly ash was used for testing purposes. NO 2 gas at a concentration of 100 ppm/air was passed through the bed (MCM- 41). The study was carried out at 25 C. The data obtained is used to plot changes in the concentration of NO 2 gas over time. The horizontal axis indicates the time of the adsorption process, and the ordinate axis the ratio of NO 2 gas concentration at the outlet of the column to the concentration of NO 2 supplied to the column. These curves are called breakthrough curves and the breakthrough time of the bed can be determined from them. The breakthrough time is required to calculate the mass of adsorbed gas on the sorbent and the sorption capacity of sorbent. Figure 11 shows the breakthrough curves of MCM-41 sieves at a concentration of 100 ppm NO 2. The curve was used to calculate the sorption capacity of MCM-41 sieve at the level of 0.1 mg NO 2 /g of sorbent. 122
9 Figure 12: Breakthrough curves of NO 2 /air for sieve MCM-41 and MCM-41-PEG(50) Table 4: Sorption capacity No. sample Sorption capacity of NO 2 1. MCM-41 mg/g sorbent 2. MCM-41- PEG(50) Effect of impregnation with a solution of PEG on the sorption capacity of MCM-41 sieve To increase the sorption capacity and selectivity of the obtained material in terms of nitrogen dioxide, the sorbent was modified. This modification consisted of wet impregnation with polyethylene glycol solution. The modification procedure was conducted as follows: After dissolving 4 grams of PEG in 32 g of ethanol, 4 g calcined sieve MCM-41 was added. Subsequently, the sample was stirred for 8 hours and then the solution was evaporated. The resulting material was impregnated with 50% polyethylene glycol PEG and labeled MCM-41-PEG(50). The prepared sorbent was subjected to the adsorption process at a temperature of 25 C while passing through a bed of sorbent NO 2 gas at a concentration of 100 ppm/air. Figure 12 shows the breakthrough curve of MCM-41-PEG(50) sieve. The curve was used to calculate the sorption capacity: for sieve MCM-41-PEG(50) it was 1.35 mg NO 2 /g of sorbent. Table 4 shows data collected from studies conducted for sieve MCM-41 and for its modification: Figure 13: Breakthrough curves of NO2/air for a sieve MCM- 41-PEG(50) at temperatures 25 C, 60 C and 80 C MCM-41-PEG(50). As can be seen, the sorption capacity of sieve whose surface has been modified was about 3 times greater than that of the unmodified sieve The influence of temperature on the sorption capacity of MCM-41 sieve As the impregnated sorbent had a higher sorption capacity relative to NO 2 than the base sorbent (without impregnation), further tests were performed on the impregnated material MCM-41-PEG(50) to determine the effect of temperature and concentrations of NO 2 as well as the presence of synthetic gas. The temperature influence on the sorption capacity was determined by measurements at the temperatures of: 25 C, 60 C, 80 C at a concentration of 100 ppm NO 2. Figure 13 presents the breakthrough curves for sieve MCM-41-PEG(50) for temperatures: 25 C, 60 C and 80 C at a concentration of 100 ppm NO 2 /air. Sorption capacity was calculated on the basis of breakthrough curves. At 25 C sorption capacity was 1.35 mg NO 2 /g of sorbent, at the temperature of 60 C it was 0.11 mg NO 2 /g of sorbent; the lowest sorption capacity was at the temperature of 80 C: 0.10 mg NO 2 /g of sorbent. Table 5 shows the sorption capacity of the sieve MCM-41-PEG(50) at different temperatures. As can be seen from Table 5 and the results obtained, as the temperature rises the sorption capacity for sieve MCM-41PEG(50) decreases. The largest sorption capacity of the material was at 25 C, while 123
10 Table 5: Sorption capacity for sieve MCM-41-PEG(50) No. Sorbent Temperature, C Sorption capacity of NO 2, mg/g sorbent 1. M CM PEG(50) Table 6: Sorption capacity for sieve MCM-41-PEG(50) No. Type of sorbent 1. MCM-41- PEG( 50) 2. MCM-41- PEG(50) The concentration of NO 2, ppm Sorption capacity of NO 2, mg/g sorbent Figure 14: Breakthrough curves of NO 2 /air for a sieve MCM- 41-PEG(50) at concentrations of 50 ppm and 100 ppm for the same material at 80 C the capacity was about 13 times smaller NO 2 concentration influence on the sorption capacity of MCM-41 sieve In order to determine the NO 2 concentration influence on the sorption capacity, studies were carried out using NO 2 gas at concentrations of 100 ppm/air and 50 ppm/air at 25 C. Figure 14 shows the breakthrough curves for sieve MCM-41-PEG(50) for concentrations of 50 and 100 ppm. The sorption capacity of sorbent at a concentration of 50 ppm was 0.27 mg NO 2 /g of sorbent, while for the concentration of 100 ppm it was 0.31 mg NO 2 /g of sorbent. Table 6 presents the data of sorption capacity for different concentrations of NO 2 gas. As can be seen from Table 6, the sorption capacity for sieves through which the gas at concentration of 50 ppm was passed is about 5 times larger than the capacity of the sieve through which gas at a concentration of 100 ppm flowed. Figure 15: Breakthrough curves of NO 2 /air and NO %, 6% O 2, 79% N 2 for a sieve MCM-41PEG(50) Influence of flue gas presence on the sorption capacity of sieve MCM-41 The aim of this research was the application of sieves from fly ash in the process of flue gas cleaning before carbon-capture process. Due to this fact, the sorption capacity in the presence of exhaust gas components, i.e. 15% CO 2, 6% O 2, 79% N 2 was determined. Gases with the same flow stream of 0.5 l/min after mixing in the mixer were placed into the oven. The study was conducted at 25 C at a concentration of 100 ppm NO 2. Figure 15 shows the breakthrough curves for the sieve MCM-41-PEG(50) in the flow of NO 2 and in the presence of a mixture containing carbon dioxide. The sorption capacity for sieve MCM-41 for gas NO 2 (100ppm)/air was 0.5 mg NO 2 /g of sorbent, while for NO 2 in the presence of carbon dioxide it was 1.11 mg/g sorbent. For comparison, the results of sorption capacity 124
11 Table 7: Sorption capacity sieve MCM-41 and MCM-41-PEG (50) No. Type of sorbent Gas Sorption capacity of NO 2, mg/g sorbent 1. MCM ppm NO MCM ppm NO %CO 2, %O 2, 79%N 2 3. MCM-41 15%CO 2, 6%O 2, 79%N MCM-41-PEG(50) 50 ppm NO MCM-41-PEG(50) 100 ppm NO MCM-41-PEG(50) 100 ppm NO %CO 2, %O 2, 79%N 2 7. MCM-41-PEG(50) 15%CO 2, 6%O 2, 79%N CO 2 sorption capacity for the sieve MCM-41 and MCM-41-PEG(50) were collected and presented in Table 7. The sorption capacity of MCM-41-PEG(50) in the presence of CO 2 was 0.68 mg NO 2 /g of sorbent, however for a sieve placed in a flow of NO 2 (without CO 2 ) at a concentration of 50 ppm NO 2 it was 6.65 mg NO 2 /g of sorbent, and for a concentration of 100 ppm, 35 mg NO 2 /g sorbent Discussion of results The study shows that several parameters affect the sorption capacity of mesoporous materials. One is temperature. As the temperature increases, the sorption capacity of sieves for NO 2 decreases. The highest sorption capacity was observed for sieve MCM- 41-PEG(50) at 25 C while at 80 C this value fell to 0.1 mg NO 2 /g of sorbent. Another parameter is the NO 2 concentration. When comparing the sorption capacity for different NO 2 concentrations, it was observed to be higher for the sieve through which gas flowed at a concentration of 50 ppm. The presented data shows that impregnating mesoporous sieves with a polyethylene glycol solution increased the sorption capacity of the sieves. The highest sorption capacity was obtained for impregnated sieves that were obtained from pure reagents and commercial sieves. However, satisfactory results were also obtained for the sieves made from fly ash. 3. Conclusion Based on studies of both the synthesis of mesoporous materials from fly ash and the study of the adsorption process of nitrogen dioxide on the received materials, it can be seen that fly ashes from burning coal, lignite, and biomass co-firing in pulverized and fluidized bed as well, due to their significant silicon and aluminum content, are suitable materials for the synthesis of mesoporous sieves. Synthesis of mesoporous sieves from fly ash enables management of both ashes stored in landfill as well as those from current production. Modification of the ashes into the sieve allows one to obtain valuable mesoporous sorbents from the combustion of waste byproducts, such as ashes, for re-use in the energy sector. Modification of materials obtained by impregnation with a polyethylene glycol solution allows one to obtain materials with enhanced properties, such as sorption capacity, relative to NO 2. The sorption capacity of impregnated sieves for NO 2 sorption was higher than for base sieves (without impregnation). The study performed at different temperatures showed that the sorption capacity of the sieve MCM- 41-PEG(50) falls as temperature rises. The presence of gas with a composition of 15% CO 2, 6% O 2, 79% N 2 has only a very marginal effect on capacity. Acknowledgments The research was financed from the resources of Project No. N N "Studies of adsorption 125
12 of nitrogen oxides on mesoporous materials based on fly ash". References [1] I. Majchrzak-Kucęba, W. Nowak, P. Sil-Ibek, B. Kucharska, Mezoporowate sita molekularne z popiołów lotnych, in: Fluidalne Spalanie Paliw w Energetyce, Materiały Konferencyjne, 2005, pp [2] I. Majchrzak-Kucęba, W. Nowak, Modyfikacja popiołów lotnych w mezoporowate materiały, in: VII Ogólnopolska Konferencja Naukowo-Techniczna, Materiały Konferencyjne, 2005, pp [3] H.-L. Chang, C.-M. Chun, I. A. Aksay, W.-H. Shih, Conversion of fly ash into mesoporous aluminosilicate, Ind. Eng. Chem. Res. 38 (3) (1999) doi: /ie980275b. [4] K. You, J. Ahn, W. Ahn, Synthesis of hexagonal and cubic mesoporous silica using power plant bottom ash, Microporous and Mesoporous Materials 111 (1 3) (2008) [5] I. Majchrzak-Kucęba, A. Ściubidło, W. Nowak, Studies on the properties of mesoporous materials derived from polish fly ashes, in: Proceedings of 26 th Annual International Pittsburgh Coal Conference, Pittsburgh, [6] A. Ściubidło, I. Majchrzak-Kucęba, W. Nowak, Zagospodarowanie popiołów lotnych z polskich elektrowni i elektrociepłowni poprzez modyfikację popiołów w materiały mezoporowate, in: EuroCoalAsh 2008, [7] I. Majchrzak-Kucęba, W. Nowak, Synthesis and characterization of mesoporous materials from cfb-fly ash, in: Proceedings of 9 th International Conference on Circulating Fluidized Beds in conjunction with the 4 th International VGB Workshop Operating Experience with Fluidized Bed Firing Systems, Hamburg, 2008, pp [8] I. Majchrzak-Kucęba, W. Nowak, Modyfikacja popiołów lotnych w sorbenty i nanomateriały doświadczenia laboratoryjne, in: XVI Międzynarodowa Konferencja Popioły z energetyki, Materiały Konferencyjne, [9] I. Majchrzak-Kucęba, W. Nowak, Properties of zeolites syntesized from fly ashes and their potential application to co 2 removal from flue gas, in: Twenty-First Annual International Pittsburgh Coal Conference-Coal-Energy and the Environment, [10] I. Majchrzak-Kucęba, W. Nowak, A. Ściubidło, Sorbenty na bazie popiołów lotnych do wychwytywania co 2 po procesie spalania, in: EuroCoalAsh 2008, [11] I. Majchrzak-Kucęba, W. N. D. Bukalak, Synteza materiału mezoporowatego mcm-41 z popiołów lotnych i jego zastosowanie do adsorpcji co 2, in: III Ogólnopolski Kongres Inżynierii Środowiska, Materiały Konferencyjne, Lublin, [12] I. Majchrzak-Kucęba, W. Nowak, Development of fly ashbased sorbent to capture co 2 from flue gas, in: 20 t h International Conference on Fluidized Bed Combustion, [13] X. Xu, C. Song, R. Wincek, J. Andresen, B. Miller, A. Scaroni, Separation of co 2 from power plant flue gas using a novel co 2 molecular basket adsorbent, Prepr. Am. Chem. Soc. Div. Fuel Chem. 48 (2003) [14] X. Xu, C. Song, B. G. Miller, A. W. Scaroni, Adsorption separation of carbon dioxide from flue gas of natural gas-fired boiler by a novel nanoporous molecular basket adsorbent, Fuel Processing Technology 86 (2005) [15] X. Ma, X. Wang, C. Song, molecular-basket sorbents for co 2 capture from flue gas, in: Pittsburgh Coal Conference, [16] I. Majchrzak-Kucęba, W. Nowak, A. Matysiak, Mezoporowate materiały z popiołów lotnych do separacji dwutlenku węgla ze spalin, in: XIV Międzynarodowa Konferencja Popioły z Energetyki, Materiały Konferencyjne, Międzyzdroje, [17] N. Hiyoshi, K. Yogo, T. Yashima, Adsorption characteristics of carbon dioxide on organically functionalized sba- 15, Microporous and Mesoporous Materials 84 (2005) [18] C. Govindasamy, W. Son, W. Ahn, Synthesis of mesoporous materials sba-15 and cmk-3 from fly ash and their application for co 2 adsorption, Journal of Porous Materials 16 (5). [19] A. Ściubidło, W. Nowak, Synteza mezoporowatego sita mcm-41 z popiołów lotnych do usuwania tlenków azotu, in: XVI Międzynarodowa Konferencja Popioły z energetyki, Materiały Konferencyjne, Zakopane, [20] X. Wang, X. Ma, S. Zhao, B. Wang, C. Song, Nanoporous molecular basket sorbent for no 2 and so 2 capture based on a polyethylene glycol-loaded mesoporous molecular sieve, Energy environ.sci. 2 (2009) [21] W. Nowak, I. Majchrzak-Kucęba, Sorbenty z popiołów lotnych dla energetyki, in: XVI Międzynarodowa Konferencja Popioły z energetyki, Materiały Konferencyjne, Zakopane, [22] I. Nowak, M. Ziółek, Uporządkowane materiały mezoporowate, Wydawnictwo Uniwersytetu Wrocławskiego, Wrocław,
Amine-impregnated silica monolith with a hierarchical pore structure: enhancement of CO 2 capture capacity
 1 Electronic Supplementary Information (ESI) Amine-impregnated silica monolith with a hierarchical pore structure: enhancement of CO 2 capture capacity for Chao Chen, Seung-Tae Yang, Wha-Seung Ahn* and
1 Electronic Supplementary Information (ESI) Amine-impregnated silica monolith with a hierarchical pore structure: enhancement of CO 2 capture capacity for Chao Chen, Seung-Tae Yang, Wha-Seung Ahn* and
CO 2 ADSORPTION BY SURFACE MODIFIED CARBON SORBENTS
 CO 2 ADSORPTION BY SURFACE MODIFIED CARBON SORBENTS Mercedes Maroto-Valer*, Zhong Tang and Yinzhi Zhang The Energy Institute and The Department of Energy and Geo-Environmental Engineering, The Pennsylvania
CO 2 ADSORPTION BY SURFACE MODIFIED CARBON SORBENTS Mercedes Maroto-Valer*, Zhong Tang and Yinzhi Zhang The Energy Institute and The Department of Energy and Geo-Environmental Engineering, The Pennsylvania
EXECUTIVE SUMMARY. especially in last 50 years. Industries, especially power industry, are the large anthropogenic
 EXECUTIVE SUMMARY Introduction The concentration of CO 2 in atmosphere has increased considerably in last 100 years, especially in last 50 years. Industries, especially power industry, are the large anthropogenic
EXECUTIVE SUMMARY Introduction The concentration of CO 2 in atmosphere has increased considerably in last 100 years, especially in last 50 years. Industries, especially power industry, are the large anthropogenic
Shape Assisted Fabrication of Fluorescent Cages of Squarate based Metal-Organic Coordination Frameworks
 Supporting Information Shape Assisted Fabrication of Fluorescent Cages of Squarate based Metal-Organic Coordination Frameworks Kolleboyina Jayaramulu, a Katla Sai Krishna, a Subi J. George, b Muthuswamy
Supporting Information Shape Assisted Fabrication of Fluorescent Cages of Squarate based Metal-Organic Coordination Frameworks Kolleboyina Jayaramulu, a Katla Sai Krishna, a Subi J. George, b Muthuswamy
Very low temperature CO oxidation over colloidally deposited gold nanoparticles on Mg(OH) 2 and MgO
 Supporing Information Very low temperature CO oxidation over colloidally deposited gold nanoparticles on Mg(OH) 2 and MgO Chun-Jiang Jia, Yong Liu, Hans Bongard, Ferdi Schüth* Max-Planck-Institut für Kohlenforschung,
Supporing Information Very low temperature CO oxidation over colloidally deposited gold nanoparticles on Mg(OH) 2 and MgO Chun-Jiang Jia, Yong Liu, Hans Bongard, Ferdi Schüth* Max-Planck-Institut für Kohlenforschung,
Electronic Supplementary Information (ESI)
 Electronic Supplementary Information (ESI) Cu-SSZ-39, an active and hydrothermally stable catalyst for the selective catalytic reduction of NOx Manuel Moliner, *a Cristina Franch, a Eduardo Palomares,
Electronic Supplementary Information (ESI) Cu-SSZ-39, an active and hydrothermally stable catalyst for the selective catalytic reduction of NOx Manuel Moliner, *a Cristina Franch, a Eduardo Palomares,
Supplementary Information
 Supplementary Information Fabrication of Novel Rattle-Type Magnetic Mesoporous carbon Microspheres for Removal of Microcystins Xinghua Zhang and Long Jiang* Beijing National Laboratory for Molecular Science
Supplementary Information Fabrication of Novel Rattle-Type Magnetic Mesoporous carbon Microspheres for Removal of Microcystins Xinghua Zhang and Long Jiang* Beijing National Laboratory for Molecular Science
Electronic Supplementary Information
 Electronic Supplementary Information Designed Copper-amine Complex as an Efficient Template for One-pot Synthesis of Cu-SSZ-13 Zeolite with Excellent Activity for Selective Catalytic Reduction of NOx by
Electronic Supplementary Information Designed Copper-amine Complex as an Efficient Template for One-pot Synthesis of Cu-SSZ-13 Zeolite with Excellent Activity for Selective Catalytic Reduction of NOx by
Supporting Information
 Supporting Information Collapsed (Kippah) Hollow Silica Nanoparticles Kun-Che Kao, Chieh-Jui Tsou and Chung-Yuan Mou* Experimental Section Materials: Reagents: Cetyltrimethylammonium bromide (CTAB, 99%+),
Supporting Information Collapsed (Kippah) Hollow Silica Nanoparticles Kun-Che Kao, Chieh-Jui Tsou and Chung-Yuan Mou* Experimental Section Materials: Reagents: Cetyltrimethylammonium bromide (CTAB, 99%+),
A simple thermogravimetric method for the evaluation of the degree of fly ash conversion into zeolite material
 J Porous Mater (2013) 20:407 415 DOI 10.1007/s10934-012-9610-1 A simple thermogravimetric method for the evaluation of the degree of fly ash conversion into zeolite material Izabela Majchrzak-Kucęba Published
J Porous Mater (2013) 20:407 415 DOI 10.1007/s10934-012-9610-1 A simple thermogravimetric method for the evaluation of the degree of fly ash conversion into zeolite material Izabela Majchrzak-Kucęba Published
PREPARATION OF MCM-48 MESOPOROUS MOLECULAR SIEVE INFLUENCE OF PREPARATION CONDITIONS ON THE STRUCTURAL PROPERTIES
 Digest Journal of Nanomaterials and Biostructures Vol. 11, No. 1, January - March 2016, p. 271-276 PREPARATION OF MCM-48 MESOPOROUS MOLECULAR SIEVE INFLUENCE OF PREPARATION CONDITIONS ON THE STRUCTURAL
Digest Journal of Nanomaterials and Biostructures Vol. 11, No. 1, January - March 2016, p. 271-276 PREPARATION OF MCM-48 MESOPOROUS MOLECULAR SIEVE INFLUENCE OF PREPARATION CONDITIONS ON THE STRUCTURAL
HYDROTHERMAL TECHNOLOGY OF ZEOLITE MATERIALS SYNTHESIS FROM FLY ASH
 International Journal of Arts & Sciences, CD-ROM. ISSN: 1944-6934 :: 6(3):391 396 (2013) HYDROTHERMAL TECHNOLOGY OF ZEOLITE MATERIALS SYNTHESIS FROM FLY ASH Wojciech Franus and Ma gorzata Franus Lublin
International Journal of Arts & Sciences, CD-ROM. ISSN: 1944-6934 :: 6(3):391 396 (2013) HYDROTHERMAL TECHNOLOGY OF ZEOLITE MATERIALS SYNTHESIS FROM FLY ASH Wojciech Franus and Ma gorzata Franus Lublin
Precious Metal-free Electrode Catalyst for Methanol Oxidations
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2015 Supporting information SnO 2 Nanocrystals Decorated-Mesoporous ZSM-5 Spheroidicity
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2015 Supporting information SnO 2 Nanocrystals Decorated-Mesoporous ZSM-5 Spheroidicity
Synthesis and Characterization of Magnesium Substituted Aluminophosphate Molecular Sieves with AEL Structure
 Journal of Natural Gas Chemistry 13(2004)231 237 Synthesis and Characterization of Magnesium Substituted Aluminophosphate Molecular Sieves with AEL Structure Benjing Xu, Ling Qian, Xinmei Liu, Chunmin
Journal of Natural Gas Chemistry 13(2004)231 237 Synthesis and Characterization of Magnesium Substituted Aluminophosphate Molecular Sieves with AEL Structure Benjing Xu, Ling Qian, Xinmei Liu, Chunmin
Supporting Information. CdS/mesoporous ZnS core/shell particles for efficient and stable photocatalytic hydrogen evolution under visible light
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2014 Supporting Information CdS/mesoporous ZnS core/shell particles for efficient
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2014 Supporting Information CdS/mesoporous ZnS core/shell particles for efficient
Electronic Supplementary Information (ESI) Efficient synthesis of the Cu-SSZ-39 catalyst for DeNOx applications
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) Efficient synthesis of the Cu-SSZ-39 catalyst for DeNOx
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) Efficient synthesis of the Cu-SSZ-39 catalyst for DeNOx
Supporting Information. Nanoscale Kirkendall Growth of Silicalite-1 Zeolite Mesocrystals with. Controlled Mesoporosity and Size
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Supporting Information Nanoscale Kirkendall Growth of Silicalite-1 Zeolite Mesocrystals with Controlled
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Supporting Information Nanoscale Kirkendall Growth of Silicalite-1 Zeolite Mesocrystals with Controlled
Adsorption Processes. Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad
 Adsorption Processes Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad Contents Introduction Principles of adsorption Types of adsorption Definitions Brief history Adsorption isotherms Mechanism
Adsorption Processes Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad Contents Introduction Principles of adsorption Types of adsorption Definitions Brief history Adsorption isotherms Mechanism
Use of TG-DSC-MS and Gas Analyzer Data to Investigate the Reaction of CO 2 and SO 2 with Ca(OH) 2 at Low Temperature
 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 35, 2013 Guest Editors: Petar Varbanov, Jiří Klemeš, Panos Seferlis, Athanasios I. Papadopoulos, Spyros Voutetakis Copyright 2013, AIDIC Servizi
A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 35, 2013 Guest Editors: Petar Varbanov, Jiří Klemeš, Panos Seferlis, Athanasios I. Papadopoulos, Spyros Voutetakis Copyright 2013, AIDIC Servizi
Experimental analysis of removal of SO 2 and NOx for nano Mg-Al composite oxides
 Bulgarian Chemical Communications, Volume 48, Special Issue D (pp. 172 176) 2016 Experimental analysis of removal of SO 2 and NOx for nano Mg-Al composite oxides 172 W. Cheng 1 *, Y. Zhang 2, S. Wei 3,
Bulgarian Chemical Communications, Volume 48, Special Issue D (pp. 172 176) 2016 Experimental analysis of removal of SO 2 and NOx for nano Mg-Al composite oxides 172 W. Cheng 1 *, Y. Zhang 2, S. Wei 3,
SO 2 Removal by Using Jordanian Oil Shale Ash
 Abstract SO Removal by Using Jordanian Oil Shale Ash Adnan Al-Harahsheh 1 and Reyad Shawabkah 7 th Oil Shale Symposium 15-17 October, 7 1- Institute of Earth & Environmental Sciences, Al al-bayt University,
Abstract SO Removal by Using Jordanian Oil Shale Ash Adnan Al-Harahsheh 1 and Reyad Shawabkah 7 th Oil Shale Symposium 15-17 October, 7 1- Institute of Earth & Environmental Sciences, Al al-bayt University,
Synthesis of ordered microporous carbons via template technique
 Synthesis of ordered microporous carbons via template technique Zhou Ying, Yao Qimei, Qiu Jieshan *, Guo Hongchen, Sun Zongwei Carbon Research Laboratory, Center for Nano Materials and Science, School
Synthesis of ordered microporous carbons via template technique Zhou Ying, Yao Qimei, Qiu Jieshan *, Guo Hongchen, Sun Zongwei Carbon Research Laboratory, Center for Nano Materials and Science, School
Supporting information A Porous Zr-cluster-based Cationic Metal-Organic Framework for Highly Efficient Cr 2 O 7
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Supporting information A Porous Zr-cluster-based Cationic Metal-Organic Framework for Highly Efficient
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Supporting information A Porous Zr-cluster-based Cationic Metal-Organic Framework for Highly Efficient
Adsorption of Methylene Blue on Mesoporous SBA 15 in Ethanol water Solution with Different Proportions
 2015 2 nd International Conference on Material Engineering and Application (ICMEA 2015) ISBN: 978-1-60595-323-6 Adsorption of Methylene Blue on Mesoporous SBA 15 in Ethanol water Solution with Different
2015 2 nd International Conference on Material Engineering and Application (ICMEA 2015) ISBN: 978-1-60595-323-6 Adsorption of Methylene Blue on Mesoporous SBA 15 in Ethanol water Solution with Different
Dry-gel conversion synthesis of Cr-MIL-101 aided by grinding: High surface area high yield synthesis with minimum purification
 Electronic Supporting Informations (ESI): Dry-gel conversion synthesis of Cr-MIL-101 aided by grinding: High surface area high yield synthesis with minimum purification Jun Kim, Yu-Ri Lee and Wha-Seung
Electronic Supporting Informations (ESI): Dry-gel conversion synthesis of Cr-MIL-101 aided by grinding: High surface area high yield synthesis with minimum purification Jun Kim, Yu-Ri Lee and Wha-Seung
Adsorption of Hg 0 on the Unburned Carbon with HF Acid Leaching
 Journal of Minerals & Materials Characterization & Engineering, Vol. 3, No.1, pp 41-51, 24 jmmce.org Printed in the USA. All rights reserved Adsorption of Hg on the Unburned Carbon with HF Acid Leaching
Journal of Minerals & Materials Characterization & Engineering, Vol. 3, No.1, pp 41-51, 24 jmmce.org Printed in the USA. All rights reserved Adsorption of Hg on the Unburned Carbon with HF Acid Leaching
A soft-templated method to synthesize sintering-resistant Au/mesoporous-silica core-shell nanocatalysts with sub-5 nm single-core
 A soft-templated method to synthesize sintering-resistant Au/mesoporous-silica core-shell nanocatalysts with sub-5 nm single-core Chunzheng Wu, ab Zi-Yian Lim, a Chen Zhou, a Wei Guo Wang, a Shenghu Zhou,
A soft-templated method to synthesize sintering-resistant Au/mesoporous-silica core-shell nanocatalysts with sub-5 nm single-core Chunzheng Wu, ab Zi-Yian Lim, a Chen Zhou, a Wei Guo Wang, a Shenghu Zhou,
Synthesis and Characterization of high-performance ceramic materials for hightemperature
 Synthesis and Characterization of high-performance ceramic materials for hightemperature CO 2 capture and hydrogen production. Location: Institute for Energy Technology (IFE), Kjeller, Norway Department
Synthesis and Characterization of high-performance ceramic materials for hightemperature CO 2 capture and hydrogen production. Location: Institute for Energy Technology (IFE), Kjeller, Norway Department
Synthesis of isoalkanes over core (Fe-Zn-Zr)-shell (zeolite) catalyst
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) Synthesis of isoalkanes over core (Fe-Zn-Zr)-shell (zeolite)
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) Synthesis of isoalkanes over core (Fe-Zn-Zr)-shell (zeolite)
A novel Ag 3 AsO 4 visible-light-responsive photocatalyst: facile synthesis and exceptional photocatalytic performance
 Electronic Supplementary Material (ESI) for Chemical Communications Supporting Information A novel Ag 3 AsO 4 visible-light-responsive photocatalyst: facile synthesis and exceptional photocatalytic performance
Electronic Supplementary Material (ESI) for Chemical Communications Supporting Information A novel Ag 3 AsO 4 visible-light-responsive photocatalyst: facile synthesis and exceptional photocatalytic performance
Template-Free Synthesis of Beta Zeolite Membranes on Porous α-al 2 O 3 Supports
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information for Template-Free Synthesis of Beta Zeolite Membranes on Porous
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information for Template-Free Synthesis of Beta Zeolite Membranes on Porous
Having a High Mg/Al Molar Ratio
 SUPPORTING INFORMATION High-Temperature CO 2 Sorption on Hydrotalcite Having a High Mg/Al Molar Ratio Suji Kim, Sang Goo Jeon, and Ki Bong Lee*, Department of Chemical and Biological Engineering, Korea
SUPPORTING INFORMATION High-Temperature CO 2 Sorption on Hydrotalcite Having a High Mg/Al Molar Ratio Suji Kim, Sang Goo Jeon, and Ki Bong Lee*, Department of Chemical and Biological Engineering, Korea
Synthesis of nano-sized anatase TiO 2 with reactive {001} facets using lamellar protonated titanate as precursor
 Supporting Information Synthesis of nano-sized anatase TiO 2 with reactive {001} facets using lamellar protonated titanate as precursor Liuan Gu, Jingyu Wang *, Hao Cheng, Yunchen Du and Xijiang Han* Department
Supporting Information Synthesis of nano-sized anatase TiO 2 with reactive {001} facets using lamellar protonated titanate as precursor Liuan Gu, Jingyu Wang *, Hao Cheng, Yunchen Du and Xijiang Han* Department
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) Multi-scale promoting effects
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) Multi-scale promoting effects
A General Synthesis of Discrete Mesoporous Carbon Microspheres through a Confined Self- Assembly Process in Inverse Opals
 A General Synthesis of Discrete Mesoporous Carbon Microspheres through a Confined Self- Assembly Process in Inverse Opals Zhenkun Sun,, Yong Liu, Bin Li, Jing Wei, Minghong Wang, Qin Yue, Yonghui Deng,
A General Synthesis of Discrete Mesoporous Carbon Microspheres through a Confined Self- Assembly Process in Inverse Opals Zhenkun Sun,, Yong Liu, Bin Li, Jing Wei, Minghong Wang, Qin Yue, Yonghui Deng,
Supporting Information
 Supporting Information Hydrogen Storage in the Dehydrated Prussian Blue Analogues M 3 [Co(CN) 6 ] 2 (M = Mn, Fe, Co, Ni, Cu, Zn) Steven S. Kaye and Jeffrey R. Long* Dept. of Chemistry, University of California,
Supporting Information Hydrogen Storage in the Dehydrated Prussian Blue Analogues M 3 [Co(CN) 6 ] 2 (M = Mn, Fe, Co, Ni, Cu, Zn) Steven S. Kaye and Jeffrey R. Long* Dept. of Chemistry, University of California,
Supporting Information High Activity and Selectivity of Ag/SiO 2 Catalyst for Hydrogenation of Dimethyloxalate
 Supporting Information High Activity and Selectivity of Ag/SiO 2 Catalyst for Hydrogenation of Dimethyloxalate An-Yuan Yin, Xiao-Yang Guo, Wei-Lin Dai*, Kang-Nian Fan Shanghai Key Laboratory of Molecular
Supporting Information High Activity and Selectivity of Ag/SiO 2 Catalyst for Hydrogenation of Dimethyloxalate An-Yuan Yin, Xiao-Yang Guo, Wei-Lin Dai*, Kang-Nian Fan Shanghai Key Laboratory of Molecular
Synthesis gas production via the biogas reforming reaction over Ni/MgO-Al 2 O 3 and Ni/CaO-Al 2 O 3 catalysts
 Synthesis gas production via the biogas reforming reaction over Ni/MgO-Al 2 O 3 and Ni/CaO-Al 2 O 3 catalysts N.D. Charisiou 1,2, A. Baklavaridis 1, V.G. Papadakis 2, M.A. Goula 1 1 Department of Environmental
Synthesis gas production via the biogas reforming reaction over Ni/MgO-Al 2 O 3 and Ni/CaO-Al 2 O 3 catalysts N.D. Charisiou 1,2, A. Baklavaridis 1, V.G. Papadakis 2, M.A. Goula 1 1 Department of Environmental
Suppression of natural limestones deactivation during cyclic carbonationdecarbonation
 Suppression of natural limestones deactivation during cyclic carbonationdecarbonation process in CCS technology Presented by: Dipl.-Ing. Marek Staf, Ph.D UNIVERSITY OF CHEMISTRY AND TECHNOLOGY PRAGUE FACULTY
Suppression of natural limestones deactivation during cyclic carbonationdecarbonation process in CCS technology Presented by: Dipl.-Ing. Marek Staf, Ph.D UNIVERSITY OF CHEMISTRY AND TECHNOLOGY PRAGUE FACULTY
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Controllable integration of ultrasmall noble metal nanoparticles
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Controllable integration of ultrasmall noble metal nanoparticles
STUDY ON THE IMPROVEMENT OF THE REDUCTION CAPACITY OF ACTIVATED CARBON FIBER
 STUDY ON THE IMPROVEMENT OF THE REDUCTION CAPACITY OF ACTIVATED CARBON FIBER Chen Shuixia, Zeng Hanmin Materials Science Institute, Zhongshan University, Guangzhou 51275, China Key Laboratory for Polymeric
STUDY ON THE IMPROVEMENT OF THE REDUCTION CAPACITY OF ACTIVATED CARBON FIBER Chen Shuixia, Zeng Hanmin Materials Science Institute, Zhongshan University, Guangzhou 51275, China Key Laboratory for Polymeric
Room Temperature Hydrogen Generation from Hydrous Hydrazine for Chemical Hydrogen Storage
 (Supporting Information) Room Temperature Hydrogen Generation from Hydrous Hydrazine for Chemical Hydrogen Storage Sanjay Kumar Singh, Xin-Bo Zhang, and Qiang Xu* National Institute of Advanced Industrial
(Supporting Information) Room Temperature Hydrogen Generation from Hydrous Hydrazine for Chemical Hydrogen Storage Sanjay Kumar Singh, Xin-Bo Zhang, and Qiang Xu* National Institute of Advanced Industrial
Energetic Performances of the Metal-Organic Framework ZIF-8 by. High Pressure Water Intrusion-Extrusion Experiments
 Energetic Performances of the Metal-Organic Framework ZIF-8 by High Pressure Water Intrusion-Extrusion Experiments Guillaume Ortiz, Habiba Nouali, Claire Marichal, Gérald Chaplais, Joël Patarin Equipe
Energetic Performances of the Metal-Organic Framework ZIF-8 by High Pressure Water Intrusion-Extrusion Experiments Guillaume Ortiz, Habiba Nouali, Claire Marichal, Gérald Chaplais, Joël Patarin Equipe
enzymatic cascade system
 Electronic Supplementary Information Fe 3 O 4 -Au@mesoporous SiO 2 microsphere: an ideal artificial enzymatic cascade system Xiaolong He, a,c Longfei Tan, a Dong Chen,* b Xiaoli Wu, a,c Xiangling Ren,
Electronic Supplementary Information Fe 3 O 4 -Au@mesoporous SiO 2 microsphere: an ideal artificial enzymatic cascade system Xiaolong He, a,c Longfei Tan, a Dong Chen,* b Xiaoli Wu, a,c Xiangling Ren,
Electronic Supplementary Information (ESI) From metal-organic framework to hierarchical high surface-area hollow octahedral carbon cages
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) From metal-organic framework to hierarchical high surface-area
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) From metal-organic framework to hierarchical high surface-area
CO 2 Capture by Mesoporous SBA-15 Grafted with 3- Aminopropyl Triethoxysilane in Supercritical Propane
 Available online at www.sciencedirect.com Energy Procedia 37 (2013 ) 175 179 GHGT-11 CO 2 Capture by Mesoporous SBA-15 Grafted with 3- Aminopropyl Triethoxysilane in Supercritical Propane Worasaung Klinthong
Available online at www.sciencedirect.com Energy Procedia 37 (2013 ) 175 179 GHGT-11 CO 2 Capture by Mesoporous SBA-15 Grafted with 3- Aminopropyl Triethoxysilane in Supercritical Propane Worasaung Klinthong
Synthesis of Mesoporous ZSM-5 Zeolite Crystals by Conventional Hydrothermal Treatment
 Synthesis of Mesoporous ZSM-5 Zeolite Crystals by Conventional Hydrothermal Treatment Ming Zhou,* Ali A. Rownaghi, and Jonas Hedlund,* *Chemical Technology, Luleå University of Technology, SE-971 87 Luleå,
Synthesis of Mesoporous ZSM-5 Zeolite Crystals by Conventional Hydrothermal Treatment Ming Zhou,* Ali A. Rownaghi, and Jonas Hedlund,* *Chemical Technology, Luleå University of Technology, SE-971 87 Luleå,
Aviation Fuel Production from Lipids by a Single-Step Route using
 Aviation Fuel Production from Lipids by a Single-Step Route using Hierarchical Mesoporous Zeolites Deepak Verma, Rohit Kumar, Bharat S. Rana, Anil K. Sinha* CSIR-Indian Institute of Petroleum, Dehradun-2485,
Aviation Fuel Production from Lipids by a Single-Step Route using Hierarchical Mesoporous Zeolites Deepak Verma, Rohit Kumar, Bharat S. Rana, Anil K. Sinha* CSIR-Indian Institute of Petroleum, Dehradun-2485,
Isotherm study on adsorption removal of Pb (II) by MCM-41 zeolite synthesized from biomass ash TAN Gang1,a, XUE Yongjie2,b and CAI Jun3,c
 214 International Conference on Computer Science and Electronic Technology (ICCSET 214) Isotherm study on adsorption removal of Pb (II) by MCM-41 zeolite synthesized from biomass ash TAN Gang1,a, XUE Yongjie2,b
214 International Conference on Computer Science and Electronic Technology (ICCSET 214) Isotherm study on adsorption removal of Pb (II) by MCM-41 zeolite synthesized from biomass ash TAN Gang1,a, XUE Yongjie2,b
Electronic supplementary information
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Electronic supplementary information Heterogeneous nucleation and growth of highly crystalline
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Electronic supplementary information Heterogeneous nucleation and growth of highly crystalline
Egyptian Petroleum Research Institute BY Rasha Hosny Abdel Mawla Yousef
 Novel Mesoporous Silicas and its Characterizations for Oil Adsorption from Produced Water Injected in Water Injection Projects using Fixed Bed Column Processes BY Rasha Hosny Abdel Mawla Yousef Egyptian
Novel Mesoporous Silicas and its Characterizations for Oil Adsorption from Produced Water Injected in Water Injection Projects using Fixed Bed Column Processes BY Rasha Hosny Abdel Mawla Yousef Egyptian
Metal-organic frameworks (MOFs) as precursors towards TiO x /C. composites for photodegradation of organic dye
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supplementary Information Metal-organic frameworks (MOFs) as precursors towards TiO x /C composites
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supplementary Information Metal-organic frameworks (MOFs) as precursors towards TiO x /C composites
Effective Synthesis of Artificial Zeolite from Coal Fly Ash
 Effective Synthesis of Artificial Zeolite from Coal Fly Ash Introduction Large quantities of coal are used in power plants around the world every year. The disposal of the huge amount of coal fly ash (CFA)
Effective Synthesis of Artificial Zeolite from Coal Fly Ash Introduction Large quantities of coal are used in power plants around the world every year. The disposal of the huge amount of coal fly ash (CFA)
PRODUCING ACTIVED CARBONS FROM PINECONES VIA CHEMICAL ACTIVATION. Abstract. Introduction. Experimental
 PRODUCING ACTIVED CARBONS FROM PINECONES VIA CHEMICAL ACTIVATION Esin Apaydın Varol, Dept. of Chemical Engineering, Anadolu University, Eskisehir, Turkey Ersan Pütün, Dept. of Material Science and Engineering,
PRODUCING ACTIVED CARBONS FROM PINECONES VIA CHEMICAL ACTIVATION Esin Apaydın Varol, Dept. of Chemical Engineering, Anadolu University, Eskisehir, Turkey Ersan Pütün, Dept. of Material Science and Engineering,
Facile synthesis of polymer and carbon spheres decorated with highly dispersed metal nanoparticles
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 1 Facile synthesis of polymer and carbon spheres decorated with highly dispersed metal nanoparticles
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 1 Facile synthesis of polymer and carbon spheres decorated with highly dispersed metal nanoparticles
Metal-Organic Frameworks and Porous Polymer Networks for Carbon Capture
 Carbon Capture Workshop, Tuesday, April 3 rd, Texas A&M, Qatar Metal-Organic Frameworks and Porous Polymer Networks for Carbon Capture J. P. Sculley, J.-R. Li, J. Park, W. Lu, and H.-C. Zhou Texas A&M
Carbon Capture Workshop, Tuesday, April 3 rd, Texas A&M, Qatar Metal-Organic Frameworks and Porous Polymer Networks for Carbon Capture J. P. Sculley, J.-R. Li, J. Park, W. Lu, and H.-C. Zhou Texas A&M
Chlorohydrination of Allyl Chloride with HCl and H 2 O 2 to Produce. Dichloropropanols Catalyzed by Hollow TS-1 Zeolite
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 216 Chlorohydrination of Allyl Chloride with and 2 O 2 to Produce Dichloropropanols Catalyzed
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 216 Chlorohydrination of Allyl Chloride with and 2 O 2 to Produce Dichloropropanols Catalyzed
Supporting Information
 Supporting Information Wiley-VCH 2007 69451 Weinheim, Germany Efficient Bifunctional Nanocatalysts by Simple Postgrafting of Spatially-Isolated Catalytic Groups on Mesoporous Materials By Krishna K. Sharma
Supporting Information Wiley-VCH 2007 69451 Weinheim, Germany Efficient Bifunctional Nanocatalysts by Simple Postgrafting of Spatially-Isolated Catalytic Groups on Mesoporous Materials By Krishna K. Sharma
Double Mesoporous Silica Shelled Spherical/Ellipsoidal Nanostructures: Synthesis and Hydrophilic/Hydrophobic Anticancer Drug Delivery
 Supporting information for Supplementary Material (ESI) for Journal of Materials Chemistry Double Mesoporous Silica Shelled Spherical/Ellipsoidal Nanostructures: Synthesis and Hydrophilic/Hydrophobic Anticancer
Supporting information for Supplementary Material (ESI) for Journal of Materials Chemistry Double Mesoporous Silica Shelled Spherical/Ellipsoidal Nanostructures: Synthesis and Hydrophilic/Hydrophobic Anticancer
Two Dimensional Graphene/SnS 2 Hybrids with Superior Rate Capability for Lithium ion Storage
 Electronic Supplementary Information Two Dimensional Graphene/SnS 2 Hybrids with Superior Rate Capability for Lithium ion Storage Bin Luo, a Yan Fang, a Bin Wang, a Jisheng Zhou, b Huaihe Song, b and Linjie
Electronic Supplementary Information Two Dimensional Graphene/SnS 2 Hybrids with Superior Rate Capability for Lithium ion Storage Bin Luo, a Yan Fang, a Bin Wang, a Jisheng Zhou, b Huaihe Song, b and Linjie
Polymer Semiconductors for Artificial Photosynthesis: Hydrogen Evolution by Mesoporous Graphitic Carbon Nitride with Visible Light
 Polymer Semiconductors for Artificial Photosynthesis: Hydrogen Evolution by Mesoporous Graphitic Carbon Nitride with Visible Light Xinchen Wang*, Kazuhiko Maeda, Xiufang Chen, Kazuhiro Takanabe, Kazunari
Polymer Semiconductors for Artificial Photosynthesis: Hydrogen Evolution by Mesoporous Graphitic Carbon Nitride with Visible Light Xinchen Wang*, Kazuhiko Maeda, Xiufang Chen, Kazuhiro Takanabe, Kazunari
Quantitative measurement of a mixture of mesophases cubic MCM-48 and hexagonal MCM-41 by 13C CP/MAS NMR
 University Technology Malaysia From the SelectedWorks of Hadi Nur May, 2004 Quantitative measurement of a mixture of mesophases cubic MCM-48 and hexagonal MCM-41 by 13C CP/MAS NMR Hadi Nur, University
University Technology Malaysia From the SelectedWorks of Hadi Nur May, 2004 Quantitative measurement of a mixture of mesophases cubic MCM-48 and hexagonal MCM-41 by 13C CP/MAS NMR Hadi Nur, University
Supporting information. Mechanical Properties of Microcrystalline Metal-Organic Frameworks. (MOFs) Measured by Bimodal Amplitude Modulated-Frequency
 Supporting information Mechanical Properties of Microcrystalline Metal-Organic Frameworks (MOFs) Measured by Bimodal Amplitude Modulated-Frequency Modulated Atomic Force Microscopy Yao Sun, Zhigang Hu,
Supporting information Mechanical Properties of Microcrystalline Metal-Organic Frameworks (MOFs) Measured by Bimodal Amplitude Modulated-Frequency Modulated Atomic Force Microscopy Yao Sun, Zhigang Hu,
SYNTHESIS OF ZEOLITE FROM
 SYNTHESIS OF ZEOLITE FROM COAL FLY ASH: ITS APPLICATION AS WATER SORBENT Thepparat Klamrassamee 1, Prasert Pavasant 2 and Navadol Laosiripojana 1 * 1 The Joint Graduate School of Energy and Environment,
SYNTHESIS OF ZEOLITE FROM COAL FLY ASH: ITS APPLICATION AS WATER SORBENT Thepparat Klamrassamee 1, Prasert Pavasant 2 and Navadol Laosiripojana 1 * 1 The Joint Graduate School of Energy and Environment,
Grant agreement No ShaleXenvironmenT. Maximizing the EU shale gas potential by minimizing its environmental footprint
 Grant agreement No. 640979 ShaleXenvironmenT Maximizing the EU shale gas potential by minimizing its environmental footprint H2020-LCE-2014-1 Competitive low-carbon energy D7.2 Full experimental materials
Grant agreement No. 640979 ShaleXenvironmenT Maximizing the EU shale gas potential by minimizing its environmental footprint H2020-LCE-2014-1 Competitive low-carbon energy D7.2 Full experimental materials
Improvement of CO 2 Absorption Properties of Limestone Ore by the Addition of Reagent Grade-SiO 2 and Natural Diatomite
 Materials Transactions, Vol. 52, No. 12 (211) pp. 2211 to 2215 #211 The Japan Institute of Metals Improvement of CO 2 Absorption Properties of Limestone Ore by the Addition of Reagent Grade-SiO 2 and Natural
Materials Transactions, Vol. 52, No. 12 (211) pp. 2211 to 2215 #211 The Japan Institute of Metals Improvement of CO 2 Absorption Properties of Limestone Ore by the Addition of Reagent Grade-SiO 2 and Natural
FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth
 FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth Abstract Nitrogen and sulphur compounds are investigated in
FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth Abstract Nitrogen and sulphur compounds are investigated in
Controlled self-assembly of graphene oxide on a remote aluminum foil
 Supplementary Information Controlled self-assembly of graphene oxide on a remote aluminum foil Kai Feng, Yewen Cao and Peiyi Wu* State key Laboratory of Molecular Engineering of Polymers, Department of
Supplementary Information Controlled self-assembly of graphene oxide on a remote aluminum foil Kai Feng, Yewen Cao and Peiyi Wu* State key Laboratory of Molecular Engineering of Polymers, Department of
One-Pot Reaction Cascades Catalyzed by Base and Acid Confined Inside Pores of Mesoporous Silica Nanospheres
 One-Pot Reaction Cascades Catalyzed by Base and Acid Confined Inside Pores of Mesoporous Silica Nanospheres Yulin Huang, Brian Trewyn, Hung-ting Chen, Shu Xu, Victor S.-Y. Lin* Department of Chemistry
One-Pot Reaction Cascades Catalyzed by Base and Acid Confined Inside Pores of Mesoporous Silica Nanospheres Yulin Huang, Brian Trewyn, Hung-ting Chen, Shu Xu, Victor S.-Y. Lin* Department of Chemistry
Supporting Information (Journal of Materials Chemistry A) for. via a scalable limited space chemical vapor deposition technique
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information (Journal of Materials Chemistry A) for Development
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supporting Information (Journal of Materials Chemistry A) for Development
Synthesis of Zeolite A Membrane from Rice Husk Ash
 Journal of Metals, Materials and Minerals, Vol.19 No.2 pp.79-83, 2009 Chayakorn BHAVORNTHANAYOD and Pesak RUNGROJCHAIPON * Department of Chemistry, Faculty of Science, King Mongkut s Institute of Technology
Journal of Metals, Materials and Minerals, Vol.19 No.2 pp.79-83, 2009 Chayakorn BHAVORNTHANAYOD and Pesak RUNGROJCHAIPON * Department of Chemistry, Faculty of Science, King Mongkut s Institute of Technology
and their Maneuverable Application in Water Treatment
 Hierarchical Films of Layered Double Hydroxides by Using a Sol-Gel Process and their Maneuverable Application in Water Treatment Yufei Zhao, Shan He, Min Wei,* David G. Evans, Xue Duan State Key Laboratory
Hierarchical Films of Layered Double Hydroxides by Using a Sol-Gel Process and their Maneuverable Application in Water Treatment Yufei Zhao, Shan He, Min Wei,* David G. Evans, Xue Duan State Key Laboratory
Porous Solids for Biogas Upgrading
 WASTES: Solutions, Treatments and Opportunities 2 nd International Conference September 11 th 13 th 2013 Porous Solids for Biogas Upgrading J.A.C. Silva 1 and A.E. Rodrigues 2 1 Escola Superior de Tecnologia
WASTES: Solutions, Treatments and Opportunities 2 nd International Conference September 11 th 13 th 2013 Porous Solids for Biogas Upgrading J.A.C. Silva 1 and A.E. Rodrigues 2 1 Escola Superior de Tecnologia
STUDY ON SORPTION OF SOME TOXIC AND HEAVY IONS IN DILUTE SOLUTIONS BY CLINOPTILOLITE
 J. Sci. I. R. Iran Vol. 12, No. 3, Summer 2001 STUDY ON SORPTION OF SOME TOXIC AND HEAVY IONS IN DILUTE SOLUTIONS BY CLINOPTILOLITE F. Farzaneh *, Z. Shivapour, N. Khosravi Talebi and J. Sayyad Mashhoor
J. Sci. I. R. Iran Vol. 12, No. 3, Summer 2001 STUDY ON SORPTION OF SOME TOXIC AND HEAVY IONS IN DILUTE SOLUTIONS BY CLINOPTILOLITE F. Farzaneh *, Z. Shivapour, N. Khosravi Talebi and J. Sayyad Mashhoor
Electronic Supplementary Information (ESI)
 Electronic Supplementary material (ESI) for Nanoscale Electronic Supplementary Information (ESI) Synthesis of Nanostructured Materials by Using Metal-Cyanide Coordination Polymers and Their Lithium Storage
Electronic Supplementary material (ESI) for Nanoscale Electronic Supplementary Information (ESI) Synthesis of Nanostructured Materials by Using Metal-Cyanide Coordination Polymers and Their Lithium Storage
Atom-Economical Synthesis of High Silica CHA Zeolite
 Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 Supplementary Information Atom-Economical Synthesis of High Silica CHA Zeolite from
Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 Supplementary Information Atom-Economical Synthesis of High Silica CHA Zeolite from
Preparation of CNTs with the Controlled Porosity using Co-Mo/MCM-41 as a template
 Preparation of CNTs with the Controlled Porosity using Co-Mo/MCM-41 as a template A.M. Rashidi 1, M.M. Akbarnejad 1, A.A. Khodadadi 2, Y.Mortazavi 2, M. Attarnejad 1 1 Gas and Catalyst Research Division,
Preparation of CNTs with the Controlled Porosity using Co-Mo/MCM-41 as a template A.M. Rashidi 1, M.M. Akbarnejad 1, A.A. Khodadadi 2, Y.Mortazavi 2, M. Attarnejad 1 1 Gas and Catalyst Research Division,
Supporting Information
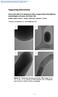 Supporting Information Interaction effects of mesoporous silica nanoparticles with different morphologies on human red blood cells Madhura Joglekar, Robert A. Roggers, Yannan Zhao, and Brian G. Trewyn
Supporting Information Interaction effects of mesoporous silica nanoparticles with different morphologies on human red blood cells Madhura Joglekar, Robert A. Roggers, Yannan Zhao, and Brian G. Trewyn
WM 00 Conference, February 27 March 2, 2000, Tucson, AZ
 MULTI-LAYERED DISTRIBUTED WASTE-FORM OF I-129 - STUDY ON IODINE FIXATION OF IODINE ADSORBED ZEOLITE BY SILICA CVD ABSTRACT J. Izumi, I. Yanagisawa, K. Katurai, N. Oka, N. Tomonaga, H. Tsutaya Mitsubishi
MULTI-LAYERED DISTRIBUTED WASTE-FORM OF I-129 - STUDY ON IODINE FIXATION OF IODINE ADSORBED ZEOLITE BY SILICA CVD ABSTRACT J. Izumi, I. Yanagisawa, K. Katurai, N. Oka, N. Tomonaga, H. Tsutaya Mitsubishi
Supporting Information
 Supporting Information Enhanced Photocatalytic Activity of Titanium Dioxide: Modification with Graphene Oxide and Reduced Graphene Oxide Xuandong Li,* Meirong Kang, Xijiang Han, Jingyu Wang, and Ping Xu
Supporting Information Enhanced Photocatalytic Activity of Titanium Dioxide: Modification with Graphene Oxide and Reduced Graphene Oxide Xuandong Li,* Meirong Kang, Xijiang Han, Jingyu Wang, and Ping Xu
A high-efficient monoclinic BiVO 4 adsorbent for selective capture toxic selenite
 Supporting Online Materials for A high-efficient monoclinic BiVO 4 adsorbent for selective capture toxic selenite Huan Ouyang, Yuanyuan Sun*, and Jianqiang Yu* Collaborative Innovation Center for Marine
Supporting Online Materials for A high-efficient monoclinic BiVO 4 adsorbent for selective capture toxic selenite Huan Ouyang, Yuanyuan Sun*, and Jianqiang Yu* Collaborative Innovation Center for Marine
Synthesis, Characterization and Catalytic Activity of MCM-41 Catalyst for Nitration of Phenol
 http://www.e-journals.in Chemical Science Transactions DOI:10.7598/cst2015.952 2015, 4(2), 438-442 RESEARCH ARTICLE Synthesis, Characterization and Catalytic Activity of MCM-41 Catalyst for Nitration of
http://www.e-journals.in Chemical Science Transactions DOI:10.7598/cst2015.952 2015, 4(2), 438-442 RESEARCH ARTICLE Synthesis, Characterization and Catalytic Activity of MCM-41 Catalyst for Nitration of
Supplementary Information
 Supplementary Information Stable aluminum metal-organic frameworks (Al-MOFs) for balanced CO 2 and water selectivity Haiwei Li, Xiao Feng, * Dou Ma, Mengxi Zhang, Yuanyuan Zhang, Yi Liu, Jinwei Zhang,
Supplementary Information Stable aluminum metal-organic frameworks (Al-MOFs) for balanced CO 2 and water selectivity Haiwei Li, Xiao Feng, * Dou Ma, Mengxi Zhang, Yuanyuan Zhang, Yi Liu, Jinwei Zhang,
Cooperative Template-Directed Assembly of Mesoporous Metal-Organic Frameworks
 Supporting Information Cooperative Template-Directed Assembly of Mesoporous Metal-Organic Frameworks Lin-Bing Sun, Jian-Rong Li, Jinhee Park, and Hong-Cai Zhou* Department of Chemistry, Texas A&M University,
Supporting Information Cooperative Template-Directed Assembly of Mesoporous Metal-Organic Frameworks Lin-Bing Sun, Jian-Rong Li, Jinhee Park, and Hong-Cai Zhou* Department of Chemistry, Texas A&M University,
Solvent-free Synthesis of Zeolites from Solid Raw Materials
 Solvent-free Synthesis of Zeolites from Solid Raw Materials Limin Ren, Qinming Wu, Chengguang Yang, Longfeng Zhu, Caijin Li, Pengling Zhang, Haiyan Zhang, Xiangju Meng,*, Feng-Shou Xiao*, Department of
Solvent-free Synthesis of Zeolites from Solid Raw Materials Limin Ren, Qinming Wu, Chengguang Yang, Longfeng Zhu, Caijin Li, Pengling Zhang, Haiyan Zhang, Xiangju Meng,*, Feng-Shou Xiao*, Department of
Adsorption (Ch 12) - mass transfer to an interface
 Adsorption (Ch 12) - mass transfer to an interface (Absorption - mass transfer to another phase) Gas or liquid adsorption (molecular) onto solid surface Porous solids provide high surface area per weight
Adsorption (Ch 12) - mass transfer to an interface (Absorption - mass transfer to another phase) Gas or liquid adsorption (molecular) onto solid surface Porous solids provide high surface area per weight
High-Pressure Volumetric Analyzer
 High-Pressure Volumetric Analyzer High-Pressure Volumetric Analysis HPVA II Benefits Dual free-space measurement for accurate isotherm data Free space can be measured or entered Correction for non-ideality
High-Pressure Volumetric Analyzer High-Pressure Volumetric Analysis HPVA II Benefits Dual free-space measurement for accurate isotherm data Free space can be measured or entered Correction for non-ideality
Electronic Supporting Information (ESI) Porous Carbon Materials with Controllable Surface Area Synthsized from Metal-Organic Frameworks
 Electronic Supporting Information (ESI) Porous Carbon Materials with Controllable Surface Area Synthsized from Metal-Organic Frameworks Seunghoon Lim, Kyungwon Suh, Yelin Kim, Minyoung Yoon, Hyeran Park,
Electronic Supporting Information (ESI) Porous Carbon Materials with Controllable Surface Area Synthsized from Metal-Organic Frameworks Seunghoon Lim, Kyungwon Suh, Yelin Kim, Minyoung Yoon, Hyeran Park,
Nanoporous TiO 2 Nanoparticle Assemblies with Mesoscale Morphologies: Nano-Cabbage versus Sea-Anemone
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2014 Supplemental Documents Nanoporous TiO 2 Nanoparticle Assemblies with Mesoscale Morphologies: Nano-Cabbage
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2014 Supplemental Documents Nanoporous TiO 2 Nanoparticle Assemblies with Mesoscale Morphologies: Nano-Cabbage
Min Bum Park, Sang Hyun Ahn, Nak Ho Ahn and Suk Bong Hong*
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 S1 Supplementary Information for Charge density mismatch synthesis of MEI- and BPH-type zeolites
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 S1 Supplementary Information for Charge density mismatch synthesis of MEI- and BPH-type zeolites
PREPARATION OF ACTIVATED CARBON FROM PULP AND PAPER MILL WASTES TO BE TESTED FOR THE ADSORPTION OF VOCS
 PREPARATION OF ACTIVATED CARBON FROM PULP AND PAPER MILL WASTES TO BE TESTED FOR THE ADSORPTION OF VOCS A. GREGÓRIO *, A. GARCIA-GARCIA #, D. BOAVIDA *, I. GULYURTLU * AND I. CABRITA * * Department of
PREPARATION OF ACTIVATED CARBON FROM PULP AND PAPER MILL WASTES TO BE TESTED FOR THE ADSORPTION OF VOCS A. GREGÓRIO *, A. GARCIA-GARCIA #, D. BOAVIDA *, I. GULYURTLU * AND I. CABRITA * * Department of
Name: Date: M O L A R M A S S & P E R C E N T C O M P O S I T I O N
 Name: Date: M O L A R M A S S & P E R C E N T C O M P O S I T I O N I. Molar Masses Given a periodic table, you should be able to calculate the molecular mass (in amu s) or the molar mass (in grams) for
Name: Date: M O L A R M A S S & P E R C E N T C O M P O S I T I O N I. Molar Masses Given a periodic table, you should be able to calculate the molecular mass (in amu s) or the molar mass (in grams) for
Core-shell 2 mesoporous nanocarriers for metal-enhanced fluorescence
 Core-shell Ag@SiO 2 @msio 2 mesoporous nanocarriers for metal-enhanced fluorescence Jianping Yang a, Fan Zhang a *, Yiran Chen a, Sheng Qian a, Pan Hu a, Wei Li a, Yonghui Deng a, Yin Fang a, Lu Han a,
Core-shell Ag@SiO 2 @msio 2 mesoporous nanocarriers for metal-enhanced fluorescence Jianping Yang a, Fan Zhang a *, Yiran Chen a, Sheng Qian a, Pan Hu a, Wei Li a, Yonghui Deng a, Yin Fang a, Lu Han a,
Study of ash removal from activated carbon and its result on CO 2 sorption capacity
 Study of ash removal from activated carbon and its result on CO 2 sorption capacity M. ZGRZEBNICKI A.GĘSIKIEWICZ-PUCHALSKA R.J. WROBEL B. MICHALKIEWICZ U. NARKIEWICZ A.W. MORAWSKI PRESENTATION OUTLINE
Study of ash removal from activated carbon and its result on CO 2 sorption capacity M. ZGRZEBNICKI A.GĘSIKIEWICZ-PUCHALSKA R.J. WROBEL B. MICHALKIEWICZ U. NARKIEWICZ A.W. MORAWSKI PRESENTATION OUTLINE
Fast and Highly Efficient SO 2 Capture by TMG Immobilized on Hierarchical Micro-Meso-Macroporous AlPO-5/ Cordierite Honeycomb Ceramic Materials
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2016 Fast and Highly Efficient SO 2 Capture by TMG Immobilized on Hierarchical Micro-Meso-Macroporous
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2016 Fast and Highly Efficient SO 2 Capture by TMG Immobilized on Hierarchical Micro-Meso-Macroporous
SUPPORTING INFORMATION
 SUPPORTING INFORMATION Unusual pore structure and sorption behaviour in a hexanodal zinc-organic framework material Jinjie Qian a,b Feilong Jiang, a Linjie Zhang, a,b Kongzhao Su, a,b Jie Pan, a,b Qipeng
SUPPORTING INFORMATION Unusual pore structure and sorption behaviour in a hexanodal zinc-organic framework material Jinjie Qian a,b Feilong Jiang, a Linjie Zhang, a,b Kongzhao Su, a,b Jie Pan, a,b Qipeng
Supporting Information
 Supporting Information Tetraethylammonium hydroxide (35 wt.%) (TEAOH), aluminum powder, fumed silica, zinc acetate dehydrate, Ludox HS-40, pure silica MCM-41, and tetraethylammonium fluoride were purchased
Supporting Information Tetraethylammonium hydroxide (35 wt.%) (TEAOH), aluminum powder, fumed silica, zinc acetate dehydrate, Ludox HS-40, pure silica MCM-41, and tetraethylammonium fluoride were purchased
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Experimental section Synthesis of Ni-Co Prussian
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Experimental section Synthesis of Ni-Co Prussian
Electronic Supporting Information for
 Electronic Supporting Information for Microporous metal-organic open framework containing uncoordinated carbonyl groups as postsynthetic modification sites for cation exchange and Tb 3+ sensor Jianwei
Electronic Supporting Information for Microporous metal-organic open framework containing uncoordinated carbonyl groups as postsynthetic modification sites for cation exchange and Tb 3+ sensor Jianwei
Supporting Materials Ultra-small Sub-10 nm Near Infrared Fluorescent Mesoporous Silica Nanoparticles
 Supporting Materials Ultra-small Sub-10 nm Near Infrared Fluorescent Mesoporous Silica Nanoparticles Kai Ma, Hiroaki Sai and Ulrich Wiesner* Department of Materials Science and Engineering, Cornell University,
Supporting Materials Ultra-small Sub-10 nm Near Infrared Fluorescent Mesoporous Silica Nanoparticles Kai Ma, Hiroaki Sai and Ulrich Wiesner* Department of Materials Science and Engineering, Cornell University,
