molecules ISSN
|
|
|
- Kory Lindsey
- 5 years ago
- Views:
Transcription
1 Molecules 2005, 10, molecules ISS The tert-mino Effect in eterocyclic Chemistry. Synthesis of Spiro eterocycles Elisaveta V. D yachenko, Tatiana V. Glukhareva, Lyudmila V. Dyudya, leg V. Eltsov and Yury Yu. Morzherin* Department of Technology for rganic Synthesis (TSLab), Urals State Technical University - UPI, 19, Mira str., Ekaterinburg, , ussia. Tel. +7 (343) , Fax: +7(343) * uthor to whom correspondence should be addressed; morjerine@htf.ustu.ru eceived: 23 pril 2005 / Published: 30 September 2005 bstract: The tert-amino reaction effect was examined. new method to synthesize spiro heterocycles is presented. It was shown that the tert-amino effect could be applied to the formation of spiro-fused heterocycles. The formation of spiro compounds proceeds in most cases in good yields in a one-pot reaction. Keywords: Tert-amino effect, cyclization, spiro compounds, Knoevenagel condensation, 1,2,3-thiadiazole, pyrazole, quinoline The term tert-amino effect was coined by th-cohn and Suschizky [1] to generalize cyclization reactions of certain ortho-substituted,-dialkylanilines. ing closure of ortho-substituted,dialkylaniline derivatives can proceed in three different ways, depending on the nature of = (Scheme 1). The first path (a) involves ring closure between the ortho substituted and the tert-nitrogen atom. The second path (b) comprises those reactions which involve one of the α-methylene groups attached to the atom, ultimately leading to the formation of five membered rings. The third path (c) involved an analogous reaction of methylene groups and atom which lead to the formation of a sixmembered ring. The first reaction of this type was reported in 1895 by Pinnow [2]. Most of the early examples of the reaction of compounds with an unsaturated ortho substituted involve groups with at least one heteroatom, such as nitroso [3], nitro [4-5], azo [6], amine [7], azomethine [8-10], carbonyl [11-13] or thiocarbonyl moieties as the ortho substituents [14].
2 Molecules 2005, Scheme 1. a À c b + Scheme 2. hν CF 3 CF 3 Ph Ph hν,-dialkylanilines having an ortho-vinyl substituent also exhibit the same type ring closure reactions [15-20]. These reactions provide an original way to form C-C bonds by insertion into a virtually non-activated C 2 group [21]. The systematic study of effect of variation in the structure of the vinyl groups revealed that electron-withdrawing group in the α-position of the vinyl moieties is essential for 5 membered rings. The heating anilines 1 in toluene lead to mixture pyrrolo[1,2-a]indole
3 Molecules 2005, derivatives. In 1-butanol, the reaction is stereospecific: only cis isomer 2 was isolated in 74% yield [22-23]. Scheme 3. C C toluene C + C When only one electron-withdrawing functionality is present at the β-position, no reaction takes place. The compounds having two electron-withdrawing groups at the β-position of the vinyl moiety show a totally different reaction pathway: in this case six-membered rings are formed. eating of a solution of 2-vinyl-,-dialkylanilines 3,6, easily prepared via a nucleophilic substitution of the fluorine atom in 2-fluorobenzaldehyde with secondary amines, followed by Knoevenagel condensation of resulting aldehyde 4 in refluxing butanol yielded cyclization products 5,7 in 67-84% yields [24-28]. Scheme 4. C 2 toluene u % = C, CEt C Et 2 CC 2 C toluene 6 C 2 Et u = (a), = C 3 (b), = (C 2 ) n n=0 (c), 1 (d) 7 C C 2 Et 67-84% The mechanism for formation of the six-membered heterocycles is presented in Scheme 5. First step is an intramolecular suprafacial 1,5-hydrogen transfer. The second step involves C-C bond formation be addition of the carbanion to the iminium double bond, which takes place in a stereochemically defined unique way.
4 Molecules 2005, Scheme 5. + C 14 + C We have shown [29] that under Knoevenagel condensation conditions ortho-,-dialkylaminobenzaldehydes 4 form with 2,2-dimethyl-1,3-dioxane-4,6-dione (ldrum's acid) spiro-coupled pyrrolo[1,2-a]quinolines 8. In contrast to the reaction of benzaldehydes with non-cyclic malonic acid derivatives, the intermediate vinyl derivatives 9 were not isolated. Thus we have developed a onestage method for the synthesis of novel spiro derivatives of quinoline. n example of the M spectra of product is presented in Figure 1. Scheme 6. C C 3 C 3 + C toluene % C 3 3 C C 3 = (a), (b), C 2 (c), (C 2 ) 2 (d), bond (e)
5 Molecules 2005, Figure 1. The 1 -M spectrum of compounds 8a. The reactions of 2-dialkylaminobenzaldehydes 4 with cyclohexanedione proceeded analogously [30]. It was demonstrated that the reactions were complete in 10 h to give spiro-fused [1,2- a]quinolines 10, whereas vinyl derivatives were not isolated either. The structures of compounds 10 were confirmed by M, I, and mass spectra as well as by the results of elemental analysis [31]. Scheme toluene 10 (a) =, =, (b) =C 2, =, (c) = СН Ph, =, (e) = -, =, (f) =, =C 3, (d) = (C 2 ) 2, =; 65-80% Spiro-fused quinolinepyrimidines 11 can be synthesized according to two procedures. ne of them involves Knoevenagel condensation of 2-dialkylaminobenzaldehydes 4 with malonic ester, cyclization of the resulting benzylidenemalonic esters 3 to fused quinolines 5 and condensation of the latter with disubstituted urea to give the target products. nother procedure involves the reaction of 2-dialkylaminobenzaldehydes 4 with barbituric acids, where Knoevenagel condensation is accompanied by intramolecular cyclization of intermediate vinyl derivatives. Taking into account the results obtained in earlier studies, it can be assumed that this reaction proceeds in one step to give spiro-fused quinolines 11. The synthesis of spiro derivatives of quinolines according to the first method involves difficulties associated, in particular, with the formation of by-products. In this connection, isolation of the pure target spiro-fused quinolines presents a problem. The total yield of 11 was 15%. Their structures were
6 Molecules 2005, confirmed by 1 and 13 C M and I spectroscopy, mass spectrometry, and elemental analysis [30-31]. Scheme 8. 2 C C 2 C 2 C 2 Et 4 2 C(C 2 ) : = Ph, = (a), (b), C 2 (c), (C 2 ) 2 (d), bond (e) 8: =, = (a), (b), C 2 (c), (C 2 ) 2 (d), bond (e) The second method is preparatively more convenient because it involves one step. We demonstrated that the reactions of 2-dialkylaminobenzaldehydes 4 with dimethyl- and diphenylbarbituric acids afforded spiro compounds 11. It should be noted that cyclization gave spiro-fused quinolines 11 in good yields on refluxing in toluene for 3 h. n increase in the reaction time led to a decrease in the yield and dezincification of the products. In this approach, the total yield of the target products 11 varied from 36 to 70 % [30-31]. Scheme 9. S Cl S DMF S 1-butanol S Cl DMF 12 13
7 Molecules 2005, We used the strategy of the tert-amino effect for the synthesis of a series of new spiro fused heterocycles as a class of compounds which offer biological interest. In contrast in the cyclization reactions of five membered ortho substituted heterocycles 4-vinyl-1,2,3-thiadiazoles 12 or 4-vinyl-1- phenylpyrazole 13 were isolated [32]. Conclusions We have shown that the tert-amino effect can also be applied to the formation of new spiro fused heterocycles. The formation of spiro compounds proceeds in most cases in good yields in a one-pot reaction. cknowledgements The authors thank the ussian Foundation for asic esearch (grant ). eferences 1. th-cohn,.; Suschitzky,. dv. eterocycl. Chem. 1972, 14, Pinnow, J. er. Dtsch. Chem. Ges. 1895, 28, Seebach, D.; Enders, D. ngew. Chem. 1975, 87, Fielden,.; th-cohn,.; Suschizky,. J. Chem. Soc., Perkin Trans. I 1973, Gluhareva, T.V.; Morzherin, Yu.Yu.; Mokrushin, V.S. Chem. eterocycl. Comp. (Engl. Transl.) 2000, 36, Kirschke, K.; Möller,.; Schmitz, E.; Kuban,.J.; Schulz,. Tetrahedon Lett. 1986, 27, Martin, J.; th-cohn,.; Suschitzky,. Tetrahedron Lett. 1973, Tea Gokou, C.; Pradere, J.P.; Qiuniou,. Synth. Commun. 1986, 16, kiba, M.; Kosugi, Y.; Takada, T. J. rg. Chem. 1978, 43, Suschitzky,.; Walrond,.E.; ull,. J. Chem.Soc., Perkin Trans. I 1977, Falci, K.J.; Franck,.W.; Smith, G.P. J. rg. Chem. 1977, 42, Fokin, E.P.; usskikh, V.V. Zhur.rg. Khim. 1966, 2, ijuis, W...; Verboom, W.; arkema, S.; einhoudt,. ecl. Trav. Chim. Pays-as 1989, 108, Verboom, W.; einhoudt, D.. ec. Trav. Chim. Pay-as 1990, 109, Visser, G.W.; Verboom, W.; enders, P..; einhoudt, D. J. Chem. Soc. Chem. Commun. 1982, Verboom, W.; Lammerink,..M.; Egberink,.J.M., einhoudt, D..; arkema, S. J. rg. Chem. 1985, 50, D'yachenko, E.V.; Glukhareva, T.V.; zenova, E.V., Zybina,..; Lobodin, V.V.; Morzherin, Yu.Yu. Vestnik USTU-UPI , Lobodin, V.V.; vcharenko, V.V.; Pihlaja, K.; Morzherin, Yu.Yu.; Lebedev,.T. apid Comm. Mass Spectrom , jea, V.; Muinelo, I.; Figueroa, M.C.; uiz, M.; Quintela, J.M. Synlett. 1995, 622.
8 Molecules 2005, Kaval,.; Dehaen, W.; Matyus, P.; Van der Eycken, E. Green Chem. 2004, lh-cohn,.; Suschiizky,. dv. eterocycl. Chem., 1996, 65, Verboom, W.; einhoudt, D.; Visser,.; arkema, S. J. rg.chem. 1984, 49, Dijksman, W.C.; Verboom, W.;Egberink,.; einhoudt, D. J. rg. Chem. 1985, 50, Verboom, W.; einhoudt, D..; Visser,.; arkema, S.. J. rg. Chem. 1989, 54, Verboom, W.; Morzherin, Yu.; Kelderman, E.; Engbersen, J.F.J.; van ummel, G.J.; arkema, S.; einhoudt, D.. ecl. Trav. Chim. Pays-as 1993, 112, Glukhareva, T.V.; D'yachenko, E.V.; Morzherin, Yu.Yu. Prog. rg. Synth. (Ekaterinburg). 2003, ijuis, W.; Verboom, W.; einhoudt, D.; arkema, S. J. m. Chem. Soc. 1987, 109, ijhuis, W.; Verboom, W.; bu El-Fadl,.; arkema, S.; einhoudt, D. J. rg. Chem. 1989, 54, Glukhareva, T.V.; D'yachenko, E.V.; Morzherin, Yu.Yu. Chem. eterocycl. Comp. (Engl. Transl.), 2002, 38, D yachenko, E.V.; Glukhareva, T.V.; ikolaenko, E.F.; Tkachev,.V.; Morzherin, Yu.Yu. uss. Chem. ull. (Intern. Ed.) 2004, D'yachenko, E.V.; Glukhareva, T.V.; Morzherin, Yu.Yu. Chem. eterocycl. Comp. (Engl. Transl.), 2003, 39, Morzherin,Y.Y. Dissertation Tesis. Ekaterinburg (ussia) Samples vailability: vailable from the authors by MDPI ( eproduction is permitted for noncommercial purposes.
The Dimroth rearrangement of 1,2,3-triazoles in the synthesis of anion receptors based on calix[4]arenas
![The Dimroth rearrangement of 1,2,3-triazoles in the synthesis of anion receptors based on calix[4]arenas The Dimroth rearrangement of 1,2,3-triazoles in the synthesis of anion receptors based on calix[4]arenas](/thumbs/78/78563327.jpg) Issue in onor of Prof. leg. Chupakhin AKIVC 2004 (xi) 31-35 The Dimroth rearrangement of 1,2,3-triazoles in the synthesis of anion receptors based on calix[4]arenas Yury Yu. Morzerin,* Tatiana A. Pospelova,
Issue in onor of Prof. leg. Chupakhin AKIVC 2004 (xi) 31-35 The Dimroth rearrangement of 1,2,3-triazoles in the synthesis of anion receptors based on calix[4]arenas Yury Yu. Morzerin,* Tatiana A. Pospelova,
molecules ISSN
 Molecules 2003, 8, 467-471 Second Eurasian Meeting on eterocyclic Chemistry eterocycles in rganic and Combinatorial Chemistry molecules ISS 1420-3049 http://www.mdpi.org Solid-Phase Synthesis of Methyl
Molecules 2003, 8, 467-471 Second Eurasian Meeting on eterocyclic Chemistry eterocycles in rganic and Combinatorial Chemistry molecules ISS 1420-3049 http://www.mdpi.org Solid-Phase Synthesis of Methyl
molecules ISSN
 Molecules 2003, 8, 480-487 Second Eurasian Meeting on eterocyclic Chemistry eterocycles in rganic and Combinatorial Chemistry molecules ISS 1420-3049 http://www.mdpi.org Synthesis of ew Polyfused eterocycles
Molecules 2003, 8, 480-487 Second Eurasian Meeting on eterocyclic Chemistry eterocycles in rganic and Combinatorial Chemistry molecules ISS 1420-3049 http://www.mdpi.org Synthesis of ew Polyfused eterocycles
molecules ISSN
 Molecules 2005, 10, 1318 1324 molecules ISS 1420-3049 http://www.mdpi.org Synthesis of ew Potentially Bioactive Bicyclic 2-Pyridones Maxime D. Crozet 1, Pascal George 2, Michel P. Crozet 1 and Patrice
Molecules 2005, 10, 1318 1324 molecules ISS 1420-3049 http://www.mdpi.org Synthesis of ew Potentially Bioactive Bicyclic 2-Pyridones Maxime D. Crozet 1, Pascal George 2, Michel P. Crozet 1 and Patrice
N-Arylhexahydropyrimidines. Electron impact mass spectrometry
 -rylhexahydropyrimidines. Electron impact mass spectrometry M. Beatriz García, Isabel. Perillo, and Liliana R. Orelli* Departamento de Química Orgánica, Facultad de Farmacia y Bioquímica. Junín 956, (1113)
-rylhexahydropyrimidines. Electron impact mass spectrometry M. Beatriz García, Isabel. Perillo, and Liliana R. Orelli* Departamento de Química Orgánica, Facultad de Farmacia y Bioquímica. Junín 956, (1113)
Advanced Organic Chemistry
 D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
Chapter 19. Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions. ß-dicarbonyl compounds. Why are ß-dicarbonyls useful?
 Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Suggested solutions for Chapter 29
 s for Chapter 29 29 PRBLEM 1 or each of the following reactions (a) state what kind of substitution is suggested and (b) suggest what product might be formed if monosubstitution occured. Br 2 3 2 S 4 S
s for Chapter 29 29 PRBLEM 1 or each of the following reactions (a) state what kind of substitution is suggested and (b) suggest what product might be formed if monosubstitution occured. Br 2 3 2 S 4 S
Suggested solutions for Chapter 30
 s for Chapter 30 30 PRBLEM 1 uggest a mechanism for this synthesis of a tricyclic aromatic heterocycle. 2 Cl base A simple exercise in the synthesis of a pyridine fused to a pyrrole (or an indole with
s for Chapter 30 30 PRBLEM 1 uggest a mechanism for this synthesis of a tricyclic aromatic heterocycle. 2 Cl base A simple exercise in the synthesis of a pyridine fused to a pyrrole (or an indole with
Synthesis of heterocyclic compounds via 1,3-dipolar cycloaddition and 1,7-electrocyclisation reactions of azomethine ylides
 Synthesis of heterocyclic compounds via 1,3-dipolar cycloaddition and 1,7-electrocyclisation reactions of azomethine ylides D Thesis Andrea Virányi Supervisor: Miklós yerges, D, DSc Department of rganic
Synthesis of heterocyclic compounds via 1,3-dipolar cycloaddition and 1,7-electrocyclisation reactions of azomethine ylides D Thesis Andrea Virányi Supervisor: Miklós yerges, D, DSc Department of rganic
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Suggested solutions for Chapter 41
 s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
Synthesis and intramolecular cyclization of novel β,β-bis- (benzo[b]thienyl)dehydroalanine derivatives
![Synthesis and intramolecular cyclization of novel β,β-bis- (benzo[b]thienyl)dehydroalanine derivatives Synthesis and intramolecular cyclization of novel β,β-bis- (benzo[b]thienyl)dehydroalanine derivatives](/thumbs/87/97412392.jpg) ynthesis and intramolecular cyclization of novel β,β-bis- (benzo[b]thienyl)dehydroalanine derivatives Ana. Abreu, atália. ilva, Paula M. T. Ferreira and Maria-João R. P. Queiroz * Departamento de Química,
ynthesis and intramolecular cyclization of novel β,β-bis- (benzo[b]thienyl)dehydroalanine derivatives Ana. Abreu, atália. ilva, Paula M. T. Ferreira and Maria-João R. P. Queiroz * Departamento de Química,
B X A X. In this case the star denotes a chiral center.
 Lecture 13 Chirality III October 29, 2013 We can also access chiral molecules through the use of something called chiral auxiliaries, which basically is a chiral attachment that you add to your molecule
Lecture 13 Chirality III October 29, 2013 We can also access chiral molecules through the use of something called chiral auxiliaries, which basically is a chiral attachment that you add to your molecule
Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies
 Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies The hydrogen stretch region (3600 2500 cm 1 ). Absorption in this region is associated with the stretching vibration of hydrogen
Table 8.2 Detailed Table of Characteristic Infrared Absorption Frequencies The hydrogen stretch region (3600 2500 cm 1 ). Absorption in this region is associated with the stretching vibration of hydrogen
Lecture 23. Amines. Chemistry 328N. April 12, 2016
 Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
Suggested solutions for Chapter 40
 s for Chapter 40 40 PBLEM 1 Suggest mechanisms for these reactions, explaining the role of palladium in the first step. Ac Et Et BS () 4 2 1. 2. K 2 C 3 evision of enol ethers and bromination, the Wittig
s for Chapter 40 40 PBLEM 1 Suggest mechanisms for these reactions, explaining the role of palladium in the first step. Ac Et Et BS () 4 2 1. 2. K 2 C 3 evision of enol ethers and bromination, the Wittig
Ring constructions by the use of fluorine substituent as activator and controller*
 Pure Appl. Chem., Vol. 72, No. 9, pp. 1685 1689, 2000. 2000 IUPAC Ring constructions by the use of fluorine substituent as activator and controller* Junji Ichikawa Department of Chemistry, Graduate School
Pure Appl. Chem., Vol. 72, No. 9, pp. 1685 1689, 2000. 2000 IUPAC Ring constructions by the use of fluorine substituent as activator and controller* Junji Ichikawa Department of Chemistry, Graduate School
Synthesis of N-(3(5)-Aryl-1,2,4-triazol-5(3)-yl)-N -carbethoxythioureas and Their Tautomerism in DMSO Solution
 ynthesis of -(3(5)-Aryl-1,2,4-triazol-5(3)-yl)- -carbethoxythioureas and Their Tautomerism in DMO olution Anton V. Dolzhenko, riday Bera and Wai Keung Chui Department of Pharmacy, Faculty of cience, ational
ynthesis of -(3(5)-Aryl-1,2,4-triazol-5(3)-yl)- -carbethoxythioureas and Their Tautomerism in DMO olution Anton V. Dolzhenko, riday Bera and Wai Keung Chui Department of Pharmacy, Faculty of cience, ational
Regioselective Ethanolamination and Ketalization of 3-Ph-2,4- diacetyl(diethoxycarbonyl)-5-hydroxy-5-methylcyclohexanones
 Molecules 003, 8, 51-55 molecules ISSN 140-3049 http://www.mdpi.org Regioselective Ethanolamination and Ketalization of 3--,4- diacetyl(diethoxycarbonyl)-5-hydroxy-5-methylcyclohexanones Adele P. Kriven'ko,
Molecules 003, 8, 51-55 molecules ISSN 140-3049 http://www.mdpi.org Regioselective Ethanolamination and Ketalization of 3--,4- diacetyl(diethoxycarbonyl)-5-hydroxy-5-methylcyclohexanones Adele P. Kriven'ko,
I5 ELECTROPHILIC SUBSTITUTIONS OF
 Section I Aromatic chemistry I5 ELECTPILIC SUBSTITUTINS F MN-SUBSTITUTED AMATIC INGS Key Notes ortho, meta and para substitution Substituent effect eaction profile Activating groups inductive o/p Deactivating
Section I Aromatic chemistry I5 ELECTPILIC SUBSTITUTINS F MN-SUBSTITUTED AMATIC INGS Key Notes ortho, meta and para substitution Substituent effect eaction profile Activating groups inductive o/p Deactivating
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions
 Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
General Infrared Absorption Ranges of Various Functional Groups
 General Infrared Absorption Ranges of Various Functional Groups Frequency Range Bond Type of Compound cm -1 Intensity C Alkanes 2850-2970 Strong 1340-1470 Strong C Alkenes 3010-3095 Medium 675-995 Strong
General Infrared Absorption Ranges of Various Functional Groups Frequency Range Bond Type of Compound cm -1 Intensity C Alkanes 2850-2970 Strong 1340-1470 Strong C Alkenes 3010-3095 Medium 675-995 Strong
Suggested solutions for Chapter 32
 s for Chapter 32 32 PBLEM 1 Explain how the stereo- and regio- chemistry of these reactions are controlled. Why is the epoxidation only moderately diastereoselective, and why does the amine attack where
s for Chapter 32 32 PBLEM 1 Explain how the stereo- and regio- chemistry of these reactions are controlled. Why is the epoxidation only moderately diastereoselective, and why does the amine attack where
Synthesis of 2,5,7-triamino[1,2,4]triazolo[1,5-a][1,3,5]triazines as potential antifolate agents
![Synthesis of 2,5,7-triamino[1,2,4]triazolo[1,5-a][1,3,5]triazines as potential antifolate agents Synthesis of 2,5,7-triamino[1,2,4]triazolo[1,5-a][1,3,5]triazines as potential antifolate agents](/thumbs/84/91225231.jpg) Anton V. Dolzhenko, Anna V. Dolzhenko and Wai-Keung Chui Synthesis of 2,5,7-triamino[1,2,4]triazolo[1,5-a][1,3,5]triazines as potential antifolate agents Department of Pharmacy, Faculty of Science, ational
Anton V. Dolzhenko, Anna V. Dolzhenko and Wai-Keung Chui Synthesis of 2,5,7-triamino[1,2,4]triazolo[1,5-a][1,3,5]triazines as potential antifolate agents Department of Pharmacy, Faculty of Science, ational
Atovaquone: An Antipneumocystic Agent
 Atovaquone: An Antipneumocystic Agent Atovaquone is a pharmaceutical compound marketed in the United States under different combinations to prevent and treat pneumocystosis and malaria. In a report from
Atovaquone: An Antipneumocystic Agent Atovaquone is a pharmaceutical compound marketed in the United States under different combinations to prevent and treat pneumocystosis and malaria. In a report from
An Essay on : Evaluation of the Synthesis, Reactions and Biological Activities of Quinolines
 Faculty of Science Damietta University Chemistry Department An Essay on : Evaluation of the Synthesis, Reactions and Biological Activities of Quinolines Presented BY MOHAMED ELNEKETY b.s.c student (special
Faculty of Science Damietta University Chemistry Department An Essay on : Evaluation of the Synthesis, Reactions and Biological Activities of Quinolines Presented BY MOHAMED ELNEKETY b.s.c student (special
CHEM Chapter 16. Chemistry of Benzene: Electrophilic Aromatic Substitution (homework) W
 CHEM 2425. Chapter 16. Chemistry of Benzene: Electrophilic Aromatic Substitution (homework) W Short Answer Exhibit 16-1 MATCH a structure or term from the following list with each description below. Place
CHEM 2425. Chapter 16. Chemistry of Benzene: Electrophilic Aromatic Substitution (homework) W Short Answer Exhibit 16-1 MATCH a structure or term from the following list with each description below. Place
Suggested solutions for Chapter 31
 s for Chapter 31 31 PRBLEM 1 Predict the most favourable conformation for these insect pheromones. Practice drawing the conformations of cyclic acetals. There are many good ways to draw these conformations
s for Chapter 31 31 PRBLEM 1 Predict the most favourable conformation for these insect pheromones. Practice drawing the conformations of cyclic acetals. There are many good ways to draw these conformations
A Facile Route to Diastereomeric Phosphorus Ylides. Chemoselective Synthesis of Dialkyl (E)-2-[1-(2-Oxocyclopentylidene)ethyl]-2-butenedioates
![A Facile Route to Diastereomeric Phosphorus Ylides. Chemoselective Synthesis of Dialkyl (E)-2-[1-(2-Oxocyclopentylidene)ethyl]-2-butenedioates A Facile Route to Diastereomeric Phosphorus Ylides. Chemoselective Synthesis of Dialkyl (E)-2-[1-(2-Oxocyclopentylidene)ethyl]-2-butenedioates](/thumbs/92/110400644.jpg) Molecules 2008, 13, 331-339 molecules ISSN 1420-3049 2008 by MDPI www.mdpi.org/molecules Full Paper A Facile Route to Diastereomeric Phosphorus Ylides. Chemoselective Synthesis of Dialkyl (E)-2-[1-(2-xocyclopentylidene)ethyl]-2-butenedioates
Molecules 2008, 13, 331-339 molecules ISSN 1420-3049 2008 by MDPI www.mdpi.org/molecules Full Paper A Facile Route to Diastereomeric Phosphorus Ylides. Chemoselective Synthesis of Dialkyl (E)-2-[1-(2-xocyclopentylidene)ethyl]-2-butenedioates
Product obtained by reaction with resorcinol
 33 rd International hemistry lympiad Preparatory Problems 3 ( Z ) 3-( 4-methoxyphenyl )-2-pentenedioic acid d. Two products are possible when compound A reacts with bromine. 2 2 3 3 [1] [2] Structures
33 rd International hemistry lympiad Preparatory Problems 3 ( Z ) 3-( 4-methoxyphenyl )-2-pentenedioic acid d. Two products are possible when compound A reacts with bromine. 2 2 3 3 [1] [2] Structures
Organic Chemistry II KEY March 25, a) I only b) II only c) II & III d) III & IV e) I, II, III & IV
 rganic Chemistry II KEY March 25, 2015 Exam 2: VERSIN A 1. Which of the following compounds will give rise to an aromatic conjugate base? E a) I only b) II only c) II & III d) III & IV e) I, II, III &
rganic Chemistry II KEY March 25, 2015 Exam 2: VERSIN A 1. Which of the following compounds will give rise to an aromatic conjugate base? E a) I only b) II only c) II & III d) III & IV e) I, II, III &
Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 1 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen
Chapter 15 1 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen
12/27/2010. Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Homogeneous Catalysis - B. List
 omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
Discussion Addendum for: Trifluoromethylation at the -Position of, -Unsaturated Ketones: 4-Phenyl-3-Trifluoromethyl-2-Butanone
 DI:10.15227/orgsyn.089.0374 Discussion Addendum for: Trifluoromethylation at the -Position of, -Unsaturated Ketones: 4-Phenyl-3-Trifluoromethyl-2-Butanone C 3 Ph hcl(pph 3 ) 3 C 3 I C 3 Ph T C 3 Prepared
DI:10.15227/orgsyn.089.0374 Discussion Addendum for: Trifluoromethylation at the -Position of, -Unsaturated Ketones: 4-Phenyl-3-Trifluoromethyl-2-Butanone C 3 Ph hcl(pph 3 ) 3 C 3 I C 3 Ph T C 3 Prepared
Benzene and Aromatic Compounds. Chapter 15 Organic Chemistry, 8 th Edition John McMurry
 Benzene and Aromatic Compounds Chapter 15 Organic Chemistry, 8 th Edition John McMurry 1 Background Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Four degrees of unsaturation. It
Benzene and Aromatic Compounds Chapter 15 Organic Chemistry, 8 th Edition John McMurry 1 Background Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Four degrees of unsaturation. It
Iron in the Service of Chromium: The ortho-benzannulation of trans,trans-dienyl Fischer Carbene Complexes
 Iron in the Service of Chromium: The ortho-benzannulation of trans,trans-dienyl Fischer Carbene Complexes Yiqian Lian and William D. Wulff (Michigan State University) JACS 2005, 127, 17162-17163. Markus
Iron in the Service of Chromium: The ortho-benzannulation of trans,trans-dienyl Fischer Carbene Complexes Yiqian Lian and William D. Wulff (Michigan State University) JACS 2005, 127, 17162-17163. Markus
molecules ISSN
 Molecules,, -8 molecules ISSN 0-0 http://www.mdpi.org Stereoselective Transformation of Cyclodecene-,-dione Systems, Derived from Steroids, to the Corresponding spiro-γ-lactones. A Semiempirical M Study
Molecules,, -8 molecules ISSN 0-0 http://www.mdpi.org Stereoselective Transformation of Cyclodecene-,-dione Systems, Derived from Steroids, to the Corresponding spiro-γ-lactones. A Semiempirical M Study
COMPUTER MODELLING OF THE CONFORMATIONAL EQUILIBRIUM OF 2- AND 2,5,5-SUBSTITUTED 1,3,2-DIOXABORINANES
 Chemistry of Heterocyclic Compounds, Vol. 43, No. 12, 2007 CMPUTE MDELLING F THE CNFMATINAL EQUILIBIUM F 2- AND 2,5,5-SUBSTITUTED 1,3,2-DIXABINANES V. V. Kuznetsov,. Yu. Valiakhmetova, and S. A. Bochkor
Chemistry of Heterocyclic Compounds, Vol. 43, No. 12, 2007 CMPUTE MDELLING F THE CNFMATINAL EQUILIBIUM F 2- AND 2,5,5-SUBSTITUTED 1,3,2-DIXABINANES V. V. Kuznetsov,. Yu. Valiakhmetova, and S. A. Bochkor
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy
 Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
General Papers ARKIVOC 2004 (i) 71-78
 General Papers ARKIVC 2004 (i) 71-78 Synthesis of 1,3,5-triazepine-2,4-dione, pyrrolo[3,4-f] [1,3,5]triazepine-2,4-dione, pyridazino[4,5-f][1,3,5]triazepine and 1,3,5,7,9-pentazaheptaline derivatives..
General Papers ARKIVC 2004 (i) 71-78 Synthesis of 1,3,5-triazepine-2,4-dione, pyrrolo[3,4-f] [1,3,5]triazepine-2,4-dione, pyridazino[4,5-f][1,3,5]triazepine and 1,3,5,7,9-pentazaheptaline derivatives..
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
4. What is the final product, Z, of the following synthesis? Name: Last four numbers of the ID: Part A. 1. LiAlH(O-t-Bu) 3 ether, -78 o C 2.
 ame: Last four numbers of the : 4. What is the final product, Z, of the following synthesis Part A 1. The R spectrum of which type of compound will not show evidence of hydrogen bonding Aldehyde Alcohol
ame: Last four numbers of the : 4. What is the final product, Z, of the following synthesis Part A 1. The R spectrum of which type of compound will not show evidence of hydrogen bonding Aldehyde Alcohol
Chapter 24. Amines. Based on McMurry s Organic Chemistry, 7 th edition
 Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
Synthesis of Amines Amine Alkylation by SN2 reaction II. Reductive Amination
 Synthesis of Amines I. Amine Alkylation by SN2 reaction Amines can be alkylated in SN2 fashion by alkyl halides; primary halides are best for this purpose. This is not a practical reaction for formation
Synthesis of Amines I. Amine Alkylation by SN2 reaction Amines can be alkylated in SN2 fashion by alkyl halides; primary halides are best for this purpose. This is not a practical reaction for formation
Electrophilic Carbenes
 Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Thesis of the PhD dissertation. Forintos Henrietta. Supervisor: Prof. Keglevich György Head of the Departement
 Thesis of the hd dissertation REACTIN F ETERCYCLIC TRIALKYLENYLSINE XIDES AND DIMETYL ACETYLENEDICARBXYLATE Forintos enrietta Supervisor: rof. Keglevich György ead of the Departement Research Group of
Thesis of the hd dissertation REACTIN F ETERCYCLIC TRIALKYLENYLSINE XIDES AND DIMETYL ACETYLENEDICARBXYLATE Forintos enrietta Supervisor: rof. Keglevich György ead of the Departement Research Group of
CHEM 344 Fall 2016 Spectroscopy and WebMO Exam (75 pts)
 CHEM 344 Fall 2016 Spectroscopy and WebMO Exam (75 pts) Name: TA Name: Exam Length = 90 min DO NOT REMOVE ANY PAGES FROM THIS EXAM PACKET. Directions for drawing molecules, reactions, and electron-pushing
CHEM 344 Fall 2016 Spectroscopy and WebMO Exam (75 pts) Name: TA Name: Exam Length = 90 min DO NOT REMOVE ANY PAGES FROM THIS EXAM PACKET. Directions for drawing molecules, reactions, and electron-pushing
Organic Chemistry Chapter 5 Stereoisomers H. D. Roth
 Organic Chemistry Chapter 5 Stereoisomers. D. Roth 11. Chirality of conformationally mobile systems ring compounds Monosubstituted cycloalkanes cannot have an asymmetric carbon in the ring, because there
Organic Chemistry Chapter 5 Stereoisomers. D. Roth 11. Chirality of conformationally mobile systems ring compounds Monosubstituted cycloalkanes cannot have an asymmetric carbon in the ring, because there
Module No and Title. PAPER No: 5 ; TITLE : Organic Chemistry-II MODULE No: 25 ; TITLE: S E 1 reactions
 Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 25; S E 1 reactions CHE_P5_M25 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. S E 1 reactions 3.1
Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 25; S E 1 reactions CHE_P5_M25 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. S E 1 reactions 3.1
An efficient condensation of 4-oxo-4H-benzopyran-3-carbaldehydes with 3-methyl-1-phenyl-1H-pyrazol-5 (4H)-one
 General Papers ARKIVC 2005 (xiv) 82-86 An efficient condensation of 4-oxo-4H-benzopyran-3-carbaldehydes with 3-methyl-1-phenyl-1H-pyrazol-5 (4H)-one Balaji R. Madje, Santosh S. Shindalkar, Madhav. Ware,
General Papers ARKIVC 2005 (xiv) 82-86 An efficient condensation of 4-oxo-4H-benzopyran-3-carbaldehydes with 3-methyl-1-phenyl-1H-pyrazol-5 (4H)-one Balaji R. Madje, Santosh S. Shindalkar, Madhav. Ware,
Recyclization of methyl 1-aryl-3-cinnamoyl-4,5-dioxo-4,5-dihydro-1Hpyrrole-2-carboxylates
 General Papers AKIVC 2015 (v) 259-265 ecyclization of methyl 1-aryl-3-cinnamoyl-4,5-dioxo-4,5-dihydro-1Hpyrrole-2-carboxylates in reaction with monosubstituted hydrazines Valeriy. Filimonov, a Pavel S.
General Papers AKIVC 2015 (v) 259-265 ecyclization of methyl 1-aryl-3-cinnamoyl-4,5-dioxo-4,5-dihydro-1Hpyrrole-2-carboxylates in reaction with monosubstituted hydrazines Valeriy. Filimonov, a Pavel S.
NH 2 O O O O OCH (6 pts) Provide an acceptable name for each of the following compounds: NH 2 COOH HOOC COOH. piperidine
 CEMISTRY 314-01 MIDTERM # 4 April 1, 2003 Statistics: Average: 87 pts (72%); ighest: 116 pts (97%); Lowest: 49 pts (41%) umber of students performing at or above average: 13 (62%) 1. (9 pts) Mark as true
CEMISTRY 314-01 MIDTERM # 4 April 1, 2003 Statistics: Average: 87 pts (72%); ighest: 116 pts (97%); Lowest: 49 pts (41%) umber of students performing at or above average: 13 (62%) 1. (9 pts) Mark as true
Organic Chemistry Qualifying and Comprehensive Exam
 rganic Chemistry Qualifying and Comprehensive Exam Chemistry Division Illinois Institute of Technology April 16 th, 2010 Page 1 of 9 Instructions: This is a closed book exam and you have three hours to
rganic Chemistry Qualifying and Comprehensive Exam Chemistry Division Illinois Institute of Technology April 16 th, 2010 Page 1 of 9 Instructions: This is a closed book exam and you have three hours to
Highlights of Schmidt Reaction in the Last Ten Years
 ighlights of Schmidt eaction in the Last Ten Years Dendrobates histrionicus Jack Liu ov. 18, 2003 Introduction Classical Schmidt reaction of aldehydes and carboxylic acids Classical Schmidt reaction of
ighlights of Schmidt eaction in the Last Ten Years Dendrobates histrionicus Jack Liu ov. 18, 2003 Introduction Classical Schmidt reaction of aldehydes and carboxylic acids Classical Schmidt reaction of
UNIVERSITY OF MANITOBA DEPARTMENT OF CHEMISTRY
 PAGE 1 of 11 UNVESTY F MANTBA DEPATMENT F CEMSTY 2.339 STUCTUAL TANSFMATNS N GANC CEMSTY FNAL EXAMNATN - PAPE # 432 Dr. Phil ultin Friday December 17, 2004. NAME: STUDENT NUMBE: 1. (20 Marks) For each
PAGE 1 of 11 UNVESTY F MANTBA DEPATMENT F CEMSTY 2.339 STUCTUAL TANSFMATNS N GANC CEMSTY FNAL EXAMNATN - PAPE # 432 Dr. Phil ultin Friday December 17, 2004. NAME: STUDENT NUMBE: 1. (20 Marks) For each
Chapter 13 Reactions of Arenes Electrophilic Aromatic Substitution
 . 13 hapter 13 eactions of Arenes lectrophilic Aromatic ubstitution lectrophiles add to aromatic rings in a fashion somewhat similar to the addition of electrophiles to alkenes. ecall: 3 4 Y 1 4 2 1 δ
. 13 hapter 13 eactions of Arenes lectrophilic Aromatic ubstitution lectrophiles add to aromatic rings in a fashion somewhat similar to the addition of electrophiles to alkenes. ecall: 3 4 Y 1 4 2 1 δ
L.7. Mass Spectrum Interpretation
 L.7. Mass Spectrum Interpretation Fragmentation reactions Spectrum interpretation Confirmation of ion structural assignment Biomolecule dissociation Fragmentation reactions 1. Fragmentation reactions of
L.7. Mass Spectrum Interpretation Fragmentation reactions Spectrum interpretation Confirmation of ion structural assignment Biomolecule dissociation Fragmentation reactions 1. Fragmentation reactions of
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Crystal and molecular structure of N-(p-nitrobenzylidene)- 3-chloro-4-fluoroaniline
 PRAMANA cfl Indian Academy of Sciences Vol. 55, No. 3 journal of September 2000 physics pp. 441 446 Crystal and molecular structure of N-(p-nitrobenzylidene)- 3-chloro-4-fluoroaniline K V ARJUNA GOWDA,
PRAMANA cfl Indian Academy of Sciences Vol. 55, No. 3 journal of September 2000 physics pp. 441 446 Crystal and molecular structure of N-(p-nitrobenzylidene)- 3-chloro-4-fluoroaniline K V ARJUNA GOWDA,
(5) a. b. (6) N H CH 3 N H O H O (7) CH 3 O (8) OCH 3 2. explanation:
 ame Key 15 W1-Exam o. Page I. (16 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that is
ame Key 15 W1-Exam o. Page I. (16 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that is
Important Note: We will NOT accept papers written in pencil back for re-marking after they have been returned to you. Please do not ask!
 Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 2 Thursday, March 15, 2012; 7-9 PM This is a 2-hour test, marked out of
Name: Student Number: University of Manitoba - Department of Chemistry CHEM 2220 - Introductory Organic Chemistry II - Term Test 2 Thursday, March 15, 2012; 7-9 PM This is a 2-hour test, marked out of
Electrophilic Aromatic Substitution: Direction
 Electrophilic Aromatic Substitution: Direction or each of the following species, show the most likely site(s) for electrophilic aromatic substitution, and predict whether the molecule reacts with electrophiles
Electrophilic Aromatic Substitution: Direction or each of the following species, show the most likely site(s) for electrophilic aromatic substitution, and predict whether the molecule reacts with electrophiles
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
What is in Common for the Following Reactions, and How Do They Work?
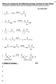 What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
Electrophilic Aromatic Substitution. Dr. Mishu Singh Department of chemistry Maharana Pratap Govt.P.G.College Hardoi
 Electrophilic Aromatic Substitution Dr. Mishu Singh Department of chemistry Maharana Pratap Govt.P.G.College Hardoi 1 Recall the electophilic addition of HBr (or Br2) to alkenes H + nu cleophile H Br H
Electrophilic Aromatic Substitution Dr. Mishu Singh Department of chemistry Maharana Pratap Govt.P.G.College Hardoi 1 Recall the electophilic addition of HBr (or Br2) to alkenes H + nu cleophile H Br H
Lecture 23. The Aldol Condensation. an Aldol! April 12, 2018 O H O H - Chemistry 328N
 Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
Chromium Arene Complexes
 Go through Reviews Chem. Reviews Chem. Soc. Reviews Book by Prof. A. J. Elias Chromium Arene Complexes Complexation of Cr(CO) 3 with ARENES Chromium arene complexes Metal complexation is appealing in organic
Go through Reviews Chem. Reviews Chem. Soc. Reviews Book by Prof. A. J. Elias Chromium Arene Complexes Complexation of Cr(CO) 3 with ARENES Chromium arene complexes Metal complexation is appealing in organic
ORGANIC CHEMISTRY. Organic molecules are everywhere! The Alkanes (See pages 25-4 and 25-5) Naming Alkanes (See pages 25-7 to 25-10)
 RGANI EMISTRY hemistry 11 rganic molecules are everywhere! Some common examples: Sucrose (sugar) Methane (natural gas) Butane (lighter fluid) Plastic Acetic Acid (vinegar) Ethanol (fuel additive) What
RGANI EMISTRY hemistry 11 rganic molecules are everywhere! Some common examples: Sucrose (sugar) Methane (natural gas) Butane (lighter fluid) Plastic Acetic Acid (vinegar) Ethanol (fuel additive) What
Chapter 15. Reactions of Aromatic Compounds. Electrophilic Aromatic Substitution on Arenes. The first step is the slow, rate-determining step
 Electrophilic Aromatic Substitution on Arenes Chapter 15 Reactions of Aromatic Compounds The characteristic reaction of aromatic rings is substitution initiated by an electrophile halogenation nitration
Electrophilic Aromatic Substitution on Arenes Chapter 15 Reactions of Aromatic Compounds The characteristic reaction of aromatic rings is substitution initiated by an electrophile halogenation nitration
Total Syntheses of Minfiensine
 Total Syntheses of Minfiensine Douany, A. B.; umphreys, P. G.; verman, L. E.*; Wrobelski, A. D., J. Am. Chem. Soc. 2008, ASAP. D: 10.1021/ja800163v Shen, L.; Zhang, M.; Wu, Y.; Qin, Y.*, Angew. Chem. nt.
Total Syntheses of Minfiensine Douany, A. B.; umphreys, P. G.; verman, L. E.*; Wrobelski, A. D., J. Am. Chem. Soc. 2008, ASAP. D: 10.1021/ja800163v Shen, L.; Zhang, M.; Wu, Y.; Qin, Y.*, Angew. Chem. nt.
Examples of Substituted Benzenes
 Organic Chemistry 5 th Edition Paula Yurkanis Bruice Examples of Substituted Benzenes Chapter 15 Reactions of Substituted Benzenes Irene Lee Case Western Reserve University Cleveland, OH 2007, Prentice
Organic Chemistry 5 th Edition Paula Yurkanis Bruice Examples of Substituted Benzenes Chapter 15 Reactions of Substituted Benzenes Irene Lee Case Western Reserve University Cleveland, OH 2007, Prentice
SYSTEMWIDE CHEM 2425 FINAL EXAM. Department Of Physical Sciences
 SYSTEMWIDE CHEM 2425 FINAL EXAM Department f Physical Sciences Morphine NAME: RGANIC CHEM 2425 FINAL EXAM DIRECTINS- A periodic table is attached at the end of this exam. Please answer all questions in
SYSTEMWIDE CHEM 2425 FINAL EXAM Department f Physical Sciences Morphine NAME: RGANIC CHEM 2425 FINAL EXAM DIRECTINS- A periodic table is attached at the end of this exam. Please answer all questions in
ENOLATES IN ORGANIC SYNTHESIS
 ENLATES IN RGANIC SYNTHESIS 1 ENLATES IN RGANIC SYNTHESIS Recall Enolate alkylation, Aldol addition and condensation can provide access to a wide variety of multi-functional compounds, which can lend themselves
ENLATES IN RGANIC SYNTHESIS 1 ENLATES IN RGANIC SYNTHESIS Recall Enolate alkylation, Aldol addition and condensation can provide access to a wide variety of multi-functional compounds, which can lend themselves
Reactions at α-position
 Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Bowman Chem 345 Lecture Notes by Topic. Electrophilic Aromatic Substitution (EAS):
 lectrophilic Aromatic Substitution (AS): Aromatic rings have a tendency to be unreactive due to their inherent stability. However, aromatic rings can react given the right incentives. ne way, they can
lectrophilic Aromatic Substitution (AS): Aromatic rings have a tendency to be unreactive due to their inherent stability. However, aromatic rings can react given the right incentives. ne way, they can
Characteristic Effects of 4,5-Disubstituted Pyridazin-3-one Derivatives with Various Functional Groups: Ab initio Study
 Structural and Electronic Effects Bull. Korean Chem. Soc. 2007, Vol. 28, No. 8 1363 Characteristic Effects of 4,5-Disubstituted Pyridazin-3-one Derivatives with Various Functional Groups: Ab initio Study
Structural and Electronic Effects Bull. Korean Chem. Soc. 2007, Vol. 28, No. 8 1363 Characteristic Effects of 4,5-Disubstituted Pyridazin-3-one Derivatives with Various Functional Groups: Ab initio Study
Aromatic Compounds II
 2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
Terms used in UV / Visible Spectroscopy
 Terms used in UV / Visible Spectroscopy Chromophore The part of a molecule responsible for imparting color, are called as chromospheres. OR The functional groups containing multiple bonds capable of absorbing
Terms used in UV / Visible Spectroscopy Chromophore The part of a molecule responsible for imparting color, are called as chromospheres. OR The functional groups containing multiple bonds capable of absorbing
molecules ISSN
 Molecules 001, 6, 44-447 molecules ISSN 140-3049 http://www.mdpi.org Facile and Fast Pinacol Rearrangement by All 3 in the Solid State Parviz Rashidi-Ranjbar* and Ebrahim Kianmehr Department of hemistry
Molecules 001, 6, 44-447 molecules ISSN 140-3049 http://www.mdpi.org Facile and Fast Pinacol Rearrangement by All 3 in the Solid State Parviz Rashidi-Ranjbar* and Ebrahim Kianmehr Department of hemistry
Synthesis of Abyssomicin C. Marie-Caroline Cordonnier Litterature Review 23/01/2009
 Synthesis of Abyssomicin C Marie-Caroline Cordonnier Litterature Review 23/01/2009 Isolation Isolated in 2004 from the actinomycete Verrucosispora strain collected from a sediment at a depth of 289m in
Synthesis of Abyssomicin C Marie-Caroline Cordonnier Litterature Review 23/01/2009 Isolation Isolated in 2004 from the actinomycete Verrucosispora strain collected from a sediment at a depth of 289m in
A Facile Procedure for the Generation of Dichlorocarbene from the Reaction of Carbon Tetrachloride and Magnesium using Ultrasonic Irradiation
 Molecules 2003, 8, 608-613 molecules ISSN 1420-3049 http://www.mdpi.org A Facile Procedure for the Generation of Dichlorocarbene from the Reaction of Carbon Tetrachloride and Magnesium using Ultrasonic
Molecules 2003, 8, 608-613 molecules ISSN 1420-3049 http://www.mdpi.org A Facile Procedure for the Generation of Dichlorocarbene from the Reaction of Carbon Tetrachloride and Magnesium using Ultrasonic
trends in reactivity based on substitution of the starting iodoarene 5. 1) Pd(OAc) 2, CH 2 CN 2) HCl O Triethylamine
 The eck Reaction Introduction The eck reaction is of great importance, as it results in a carbon- carbon bond through a metal catalyzed coupling reaction 1. The reaction involves an unsaturated halide,
The eck Reaction Introduction The eck reaction is of great importance, as it results in a carbon- carbon bond through a metal catalyzed coupling reaction 1. The reaction involves an unsaturated halide,
CHEM 303 Organic Chemistry II Exam II 11-April-2006 Answers
 CEM 303 rganic Chemistry II Exam II 11-April-2006 Answers 1) Compound D, C 8 14, is converted by C 2 =PPh 3 into E, C 9 16. Treatment of D with LiAl 4 yields two isomeric products, F and G, both of formula
CEM 303 rganic Chemistry II Exam II 11-April-2006 Answers 1) Compound D, C 8 14, is converted by C 2 =PPh 3 into E, C 9 16. Treatment of D with LiAl 4 yields two isomeric products, F and G, both of formula
Serendipitous synthesis of 1,4-benzothiazin derivatives using 2-[(2-aminophenyl)disulfanyl]aniline
![Serendipitous synthesis of 1,4-benzothiazin derivatives using 2-[(2-aminophenyl)disulfanyl]aniline Serendipitous synthesis of 1,4-benzothiazin derivatives using 2-[(2-aminophenyl)disulfanyl]aniline](/thumbs/94/118068899.jpg) erendipitous synthesis of 1,4-benzothiazin derivatives using 2-[(2-aminophenyl)disulfanyl]aniline Mohammad Reza Islami a *, Fouziyeh Mollazehi a, Alireza Badiei b, and assan heibani a a Department of Chemistry,
erendipitous synthesis of 1,4-benzothiazin derivatives using 2-[(2-aminophenyl)disulfanyl]aniline Mohammad Reza Islami a *, Fouziyeh Mollazehi a, Alireza Badiei b, and assan heibani a a Department of Chemistry,
A Highly Efficient Organocatalyst for Direct Aldol Reactions of Ketones and Aldehydes
 A ighly Efficient rganocatalyst for Direct Aldol Reactions of Ketones and Aldehydes Zhuo Tang, Zhi-ua Yang, Xiao-ua Chen, Lin-Feng Cun, Ai-Qiao Mi, Yao-Zhong Jiang, and Liu-Zhu Gong Contribution from the
A ighly Efficient rganocatalyst for Direct Aldol Reactions of Ketones and Aldehydes Zhuo Tang, Zhi-ua Yang, Xiao-ua Chen, Lin-Feng Cun, Ai-Qiao Mi, Yao-Zhong Jiang, and Liu-Zhu Gong Contribution from the
Strained Molecules in Organic Synthesis
 Strained Molecules in rganic Synthesis 0. Introduction ~ featuring on three-membered rings ~ Tatsuya itabaru (M) Lit. Seminar 08068 for cyclobutadienes : see Mr. Yamatsugu's Lit. Sem. 069 eat of Formation
Strained Molecules in rganic Synthesis 0. Introduction ~ featuring on three-membered rings ~ Tatsuya itabaru (M) Lit. Seminar 08068 for cyclobutadienes : see Mr. Yamatsugu's Lit. Sem. 069 eat of Formation
molecules ISSN
 Molecules 2001, 6, 988-995 molecules ISS 1420-3049 http://www.mdpi.org ovel Chiral Switching Ligands for Enantioselective Asymmetric eductions of Prochiral Ketones S. arasimhan 1,, S. Swarnalakshmi 2,.
Molecules 2001, 6, 988-995 molecules ISS 1420-3049 http://www.mdpi.org ovel Chiral Switching Ligands for Enantioselective Asymmetric eductions of Prochiral Ketones S. arasimhan 1,, S. Swarnalakshmi 2,.
Chapter 09 Benzene and Its Derivatives
 Chapter 09 Benzene and Its Derivatives Benzene First isolated in 1825 from whale oil by Michael Faraday Unsaturated hydrocarbon but did not have the typical reactivity of alkenes or alkynes. CM 240: Fall
Chapter 09 Benzene and Its Derivatives Benzene First isolated in 1825 from whale oil by Michael Faraday Unsaturated hydrocarbon but did not have the typical reactivity of alkenes or alkynes. CM 240: Fall
Chemistry of Pyrones, Part 5: New Crown Ether and Podand Derivatives of 3,5-Bis(bromomethyl)-2,6-diphenyl-4H-pyran- 4-one
 Molecules 2001, 6, 721-727 molecules ISS 1420-3049 http://www.mdpi.org Chemistry of Pyrones, Part 5: ew Crown Ether and Podand Derivatives of 3,5-Bis(bromomethyl)-2,6-diphenyl-4H-pyran- 4-one Aziz Shahrisa*
Molecules 2001, 6, 721-727 molecules ISS 1420-3049 http://www.mdpi.org Chemistry of Pyrones, Part 5: ew Crown Ether and Podand Derivatives of 3,5-Bis(bromomethyl)-2,6-diphenyl-4H-pyran- 4-one Aziz Shahrisa*
Accepted Manuscript. One-step double reduction of aryl nitro and carbonyl groups using hydrazine. Elena Diez-Cecilia, Brendan Kelly, Isabel Rozas
 Accepted Manuscript One-step double reduction of aryl nitro and carbonyl groups using hydrazine Elena Diez-Cecilia, Brendan Kelly, Isabel Rozas PII: S0040-4039(11)01655-8 DOI: 10.1016/j.tetlet.2011.09.112
Accepted Manuscript One-step double reduction of aryl nitro and carbonyl groups using hydrazine Elena Diez-Cecilia, Brendan Kelly, Isabel Rozas PII: S0040-4039(11)01655-8 DOI: 10.1016/j.tetlet.2011.09.112
Heterocyclic Chemistry N S. Chapter 8: Furans
 eterocyclic Chemistry N S Chapter 8: Furans FURAN The least aromatic 5-membered ring Reaction with electrophiles - Protonation Ring opening Major protonated form Much less basic than ordinary ethers 2
eterocyclic Chemistry N S Chapter 8: Furans FURAN The least aromatic 5-membered ring Reaction with electrophiles - Protonation Ring opening Major protonated form Much less basic than ordinary ethers 2
CHEM 203 Exam 1. Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 CHEM 203 Exam 1 Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Which of the following elements is a large percentage of both the earth's
CHEM 203 Exam 1 Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Which of the following elements is a large percentage of both the earth's
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for ew Journal of Chemistry Electronic Supplementary Information Synthesis of donor-substituted meso-phenyl and meso-ethynylphenyl BDIPYs with broad absorption Katja
Electronic Supplementary Material (ESI) for ew Journal of Chemistry Electronic Supplementary Information Synthesis of donor-substituted meso-phenyl and meso-ethynylphenyl BDIPYs with broad absorption Katja
molecules ISSN
 Molecules 2001, 6, 481-495 molecules ISS 1420-3049 http://www.mdpi.org Thermal earrangement of Allyl Substituted Unsymmetric 4H-1,2,4-Triazoles to the Corresponding 1H-1,2,4-triazoles. Kåre B. Jørgensen,
Molecules 2001, 6, 481-495 molecules ISS 1420-3049 http://www.mdpi.org Thermal earrangement of Allyl Substituted Unsymmetric 4H-1,2,4-Triazoles to the Corresponding 1H-1,2,4-triazoles. Kåre B. Jørgensen,
More information can be found in Chapter 12 in your textbook for CHEM 3750/ 3770 and on pages in your laboratory manual.
 CHEM 3780 rganic Chemistry II Infrared Spectroscopy and Mass Spectrometry Review More information can be found in Chapter 12 in your textbook for CHEM 3750/ 3770 and on pages 13-28 in your laboratory manual.
CHEM 3780 rganic Chemistry II Infrared Spectroscopy and Mass Spectrometry Review More information can be found in Chapter 12 in your textbook for CHEM 3750/ 3770 and on pages 13-28 in your laboratory manual.
Chapter 5 Three and Four-Membered Ring Systems
 Chapter 5 Three and Four-mbered ing ystems 5.1 Aziridines Aziridines are good alkylating agents because of their tendency to undergo ring-opening reaction with nucleophiles 例 mitomycin C antibiotic and
Chapter 5 Three and Four-mbered ing ystems 5.1 Aziridines Aziridines are good alkylating agents because of their tendency to undergo ring-opening reaction with nucleophiles 例 mitomycin C antibiotic and
