Nanocatalysis in Continuous Flow: Supported Iron. Oxidation of Benzyl Alcohol
|
|
|
- Jayson Franklin
- 5 years ago
- Views:
Transcription
1 This journal is The Royal Society of Chemistry 13 1 Supporting Information Nanocatalysis in Continuous Flow: Supported Iron xide Nanoparticles for the Heterogeneous Aerobic xidation of Benzyl Alcohol David bermayer, a Alina M. Balu, b Antonio A. Romero, b Walter Goessler, c Rafael Luque, b, * and C. liver Kappe a, * a Christian Doppler Laboratory for Microwave Chemistry (CDLMC) and Institute of Chemistry, Karl-Franzens-University Graz, Heinrichstrasse 8, A-81 Graz, Austria Fax: (+3) ; phone: (+3) ; oliver.kappe@uni-graz.at b Departamento de Quimica rganica, Universidad de Cordoba, Edificio Marie-Curie (C-3), Campus de Rabanales, Ctra. Nnal. IV-A, Km 396, E11, Cordoba, Spain Fax: (+3)-9576; phone: (+3) ; q6alsor@uco.es c Institute of Chemistry, Analytical Chemistry, Karl-Franzens-University Graz, Stremayrgasse /3, 81 Graz, Austria
2 Conversion [%] Conversion [%] This journal is The Royal Society of Chemistry Preliminary Microwave-Batch Experiments using H or TBHP (t-butyl Hydroperoxide) as xidant Table S1. Aluminosilicate-supported Fe-nanoparticle catalysts. Fe/Al-MCM-MW Fe/Al-SBA-MW Iron oxide NPs supported on Al-MCM-1 using MW ~.5 wt% Fe Iron oxide NPs supported on Al-SBA-15 using MW ~.5 wt% Fe 1.1 Blank Runs (Substrate + xidant). A time-temperature matrix ranging from 7 to 13 C and to min using 1 mmol of benzyl alcohol in 1 ml of acetonitrile and equiv of either 5% H or TBHP (5-6 M in n-decane) was used to show the low oxidative activity of H and TBHP in the absence of catalyst. 1 mmol of o-xylene was added to all runs as internal standard. Microwave batch experiments were performed in an Initiator (Biotage AB, Sweden) single-mode microwave reactor using.5- ml conical Pyrex vials and magnetic stirring. After cooling to room temperature, approximately.1 ml of the reaction mixture was removed with a syringe and 1 μl were subjected to HPLC analysis at 15 nm. The reaction mixture was heated for further and then 1 min and the sampling procedure repeated as described above. 5% H Blank TBHP Blank Figure S1. Blank runs using either 5% H (left diagram) or TBHP (right diagram) as oxidant Table S. Conversions obtained in blank runs using either 5% H or TBHP as oxidant. 5% H Blank TBHP Blank min min min min [a] min [a] min [a] [a] benzaldehyde selectivity >99%. 1. Catalytic Activity of Support. The same time-temperature matrix was applied to test the catalytic activity of the supports with 5% H as oxidant and 5 mg mmol -1 support under otherwise identical conditions (1 ml MeCN, 1 mmol benzyl alcohol and 1 mmol o-xylene as internal standard).
3 Conversion [%] Selectivity [%] Conversion [%] Conversion [%] This journal is The Royal Society of Chemistry % H and Al-MCM-1 5% H and Al-SBA Figure. Conversions with Al-MCM-1 or Al-SBA-15 supports and 5% H as oxidant Table S3. Conversions obtained with either Al-MCM-1 or Al-SBA-15 / 5% H. 5% H / Al-MCM-1 support 5% H / Al-SBA-15 support min min [a] min [a] min min [a] min [a] [a] benzaldehyde selectivity >99%. As can be seen from Figure 1, only a very small conversion (3-5%) to the benzaldehyde at higher temperatures >13 C using the two supports was observed. 1.3 Catalytic Activty of Fe/Al-SBA-15. Catalytic oxidations of benzyl alcohol were performed in microwave batch with 5 mg mmol -1 Fe/Al-SBA-15 catalyst (~.5 wt% Fe) under otherwise identical reaction conditions. Both 5% H and TBHP (5-6 M in n-decane) were used as oxidant Fe/Al-SBA15 with 5% H as xidant. Higher conversions were achieved at the expense of a lower benzaldehyde selectivity, with higher amounts of benzoic acid being formed in the reaction mixture. 5% H and Al-SBA-15 5% H and Al-SBA Figure 3. Conversions (left) and corresponding selectivities (right) obtained using 5% H and Fe/Al-SBA-15 (.5 wt% Fe) as catalyst
4 Conversion [%] Selectivity [%] This journal is The Royal Society of Chemistry 13 Furthermore it is evident from the obtained data that because of the low oxidizing efficiency (converted moles benzaldehyde per mole oxidant) of 5% H already at 13 C and min all the oxidant is obviously used up and the highest conversion achievable is 33 % at a selectivity of 88% (the H consumption can also be monitored by HPLC at 15 nm as peak at 1. min). The highest conversion achieved without causing a drop of selectivity below 9% was 17% at 9 C and min reaction time. Table S. Conversions and corresponding selectivities obtained using 5% H and Fe/Al- SBA-15 (.5 wt% Fe) as catalyst. Conversion Benzaldehyde-Selectivity min min min min min min 7 5 >99 >99 > Fe/Al-SBA-15 with t-butyl Hydroperoxide (TBHP) as oxidant. The use of TBHP led to a much higher oxidizing efficiency than 5% H as can be seen in Figure where the highest conversion obtained was 9 % at 59% benzaldehyde selectivity, although the same excess was used ( equiv). The highest conversion achievable without causing a drop of selectivity below 9% was % at 9 C and min reaction time, which is almost the same reaction rate as observed with 5% H. TBHP and Al-SBA-15 TBHP and Al-SBA Figure S. Conversions and corresponding selectivities obtained using TBHP and Fe/Al-SBA-15 (.5 wt% Fe) as catalyst. Table S5. Conversions and corresponding selectivities obtained using TBHP and Fe/Al-SBA-15 (.5 wt% Fe) as catalyst. Conversion Benzaldehyde-Selectivity min min min min min min >99 > >
5 This journal is The Royal Society of Chemistry Catalyst Tests. A comparison of iron oxide supported on two different supports was performed in microwave batch in the already described manner. TBHP in n-decane was used instead of H because of the observed higher oxidation economy/ efficiency. All experiments were done in MeCN as solvent, 9 C and the conversion/selectivity monitored using a calibrated HPLC method after,, min reaction time. These comparison runs did however show, that there are virtually no differences in yield or selectivity, when switching between these two supports. Conversion with different Catalysts Selectivity with different Catalysts [%] [%] Fe/Al-SBA15 Fe/Al-MCM1 [min] Fe/Al-SBA15 Fe/Al-MCM1 Figure S5. Conversions and corresponding selectivities obtained using TBHP as oxidant and different catalysts (~.5 wt% Fe) in MeCN at 9 C. [min] Table S6. Comparison of the conversion/ selectivity obtained with four different types of catalysts using TBHP (see conditions from table S1) as oxidant at 9 C. 9 C Fe/Al-SBA15 Fe/Al-MCM1 Time [min] Conv [%] Sel [%] Conv [%] Conv [%] 1 >99 9 >
6 This journal is The Royal Society of Chemistry Solvent Screenings. A screening of different sufficiently oxidation resistant solvents (MeCN, acetone, toluene, dioxane) was performed in a similar manner as for the catalysts. As catalyst Fe/Al-SBA15 was used. The reaction rate achieved using acetonitrile as solvent exceeded all other solvents significantly. Conversion with different Solvents Selectivity with different Solvents [%] Dioxane Toluene t-butanol Acetone Acetonitrile [min] [%] Dioxane Toluene t-butanol Acetone Acetonitrile Figure S6. Conversions and corresponding selectivities obtained using TBHP as oxidant and five different solvents with Fe/Al-SBA15 (.5 wt% Fe) at 9 C. [min] Table S7. ptimization study using TBHP (see conditions from Table S1)as oxidant and showing the influence of the solvent on the reaction at 9 C. 9 C Acetonitrile Acetone Toluene t-butanol Dioxane Time [min] Conv [%] Sel [%] Conv [%] Conv [%] Conv [%] Sel [%] Conv [%] Sel [%] Conv [%] Sel [%] 1 >99 1 >99 >99 > >99 >99 > >99 5 >99 1 >99
7 Yield/Conversion [%] This journal is The Royal Society of Chemistry Aerobic xidation in Continuous Flow Selected HPLC chromatograms presenting volume-dependent conversion data as well as purity profiles obtained during the optimization work outlined in the main part of the paper. In addition, ICP-MS data collected in leaching tests is available..1 Solvent and xygen-pressure Effects on Selectivity and Catalyst Lifetime. Striving to reduce the initially observed high backpressure generated by the mesoporous silica supported iron catalyst (Fe/Al-SBA15), doped with dioxane was tested as improved solvent system for the aerobic oxidation of benzyl alcohol. This experiment indicated a high initial activity of the catalyst, followed by a very rapid decrease in catalyst activity, using a very high oxygen pressure (5 bar) and a maximal oxygen flow-rate (5 bar, 98 ml min -1 ). Under these conditions, dibenzyl ether formation was the main reaction to convert benzyl alcohol Ald [%] Alc Con [%] ther Analytes [%] Volume [ml] Figure S7. Decrease of catalyst activity in the oxidation of benzyl alcohol on Fe/Al-SBA15.1 M in 9:1 at 1 C, 5 mol% TEMP,. ml min -1, 5 bar at 98 ml min - 1. Conversions at different volumes were calculated from HPLC areas using 1 equiv o-xylene as internal standard, added prior to the reaction. The amount of dibenzyl ether was calculated as gap to 1%; GC-MS analysis shows that there are no other relevant analytes in the mixture). Moreover, the conversion rate of benzyl alcohol to benzaldehyde vs volume was examined under slightly modified conditions using a.1 M solution in :1 at 1 C,. ml min -1, 5 bar at 5 ml min -1 and 5 mol% TEMP as co-catalyst. Despite the low leaching tendency of the catalyst (see Table S8), prolonged operation of the iron oxide catalyst resulted in a constantly decreasing conversion rate. We believe that deactivation/poisoning phenomena are responsible for this decrease (see Figure S8).
8 Benzaldehyde Yield [%] This journal is The Royal Society of Chemistry Volume [ml] Figure S8. Decrease of catalyst activity using an optimized solvent mixture of :1 at 1 C,. ml min -1, 5 bar at 5 ml min -1,.1 M, 5 mol% TEMP, Fe/Al-SBA15 (1 wt% Fe). Leaching Tests. Samples from selected optimization runs were subjected to ICPMS analysis. 5 ml fractions of the reactions were collected and the solvent evaporated. The samples were collected at three sets of different conditions, listed in Table S8-S9. The residues were quantitatively transferred with 1 ml nitric acid/fraction into 1 ml polystyrene tubes and measured directly with an Agilent 75ce inductively coupled plasma mass spectrometer. Fe was quantitatively determined at m/z 56 using He as collision gas. A calibration was performed with an external calibration curve established from 1. g Fe L -1 standard. Indium was used as an internal standard. A first set of experiments refers to operation of the catalyst without substrate (benzyl alcohol) at optimum conditions, under single-pass operation of the reactor in a :1 mixture at 1 C, 5 bar oxygen, 5 ml min -1 oxygen flow,. ml min -1 liquid flow rate and 5 mol% TEMP (also see Table, entry 3 and Figure S9). Table S8. ICP-MS data from leaching tests without benzyl alcohol at optimized conditions. T Benzyl xygen Sample Fraction Solvent TEMP [ C] alcohol addition W-S-B rt. flushing blank W-S W-S 1 W-S :1 :1 :1 :1 Fe [μg] none none none.1 yes none yes 1.5 yes none yes.19 yes none yes.37 A second set of conditions was set up to investigate iron leaching at optimum conditions for single-pass operation of the reactor in a :1 mixture at 1 C, 5 bar oxygen,
9 This journal is The Royal Society of Chemistry ml min -1 oxygen flow,. ml min -1 liquid flow rate and 5 mol% TEMP (also see Table, entry 3 and Figure S9). A blank sample was collected in the form of a process blank at target temperature and with oxygen addition, to see possible effects on leaching caused by oxygen at higher temperatures. Table S9. ICP-MS data from leaching tests with benzyl alcohol at optimized conditions. Sample T Benzyl xygen Fe Fraction Solvent TEMP [ C] alcohol addition [μg] S-B- 1 process blank :1 none none yes. S :1 yes yes yes. S- 1 :1 yes yes yes.18 S :1 yes yes yes.17 Both sets of experiments showed only minor differences in iron content of the samples as compared to the collected blanks and seem to be within an experimental error. It would be expected that operation of a heterogeneous catalyst with homogeneous reaction mechanism should result in an analytical iron value at least magnitudes higher than the blank value, which is not the case in these experiments. In order to complement the results from the above leaching studies at optimum conditions, the previously used optimized solvent mixture ( :1) was substituted with pure. Also in these tests, no iron leaching could be observed. Table S1. ICP-MS data from leaching tests under non-optimized conditions using as solvent. Sample T Benzyl xygen Fe Fraction Solvent TEMP [ C] alcohol addition [μg] H-B rt. flushing blank none none none.11 H-B- 1 process blank none none yes.18 H yes yes yes.1 H 1 yes yes yes.5 H yes yes yes.6.3 Solvent Effects on Catalyst Selectivity. A switch between coordinating (dioxane) and noncoordinating () reaction solvent was found to alter selectivity of the reaction, as can be seen in the chromatogram overlay of the reaction performed either in one of the pure solvents, or in an optimized mixture of the two. Whereas the use of dioxane leads to a relatively pure conversion at low conversion rate, leads to a high catalyst activity, but also channels the reaction towards alkylation products. The :1 mixture of and dioxane significantly improved both conversion rate and selectivity to benzaldehyde. Benzyl formate, in later recirculation experiments identified as side-product possibly indicative of catalyst deactivation by fouling, was found in traces.
10 Intensity [AU] [AU] This journal is The Royal Society of Chemistry benzyl alcohol internal standard (o-xylene) benzaldehyde dibenzyl ether benzyl formate benzylated xylene :1 dioxane Figure S9. HPLC at 15 nm showing the occurrence of undesired alkylation reactions depending on solvent composition (1 C,. ml min -1, 5 mol% TEMP, 5 bar at 5 ml min -1 ). The identity of the peaks was confirmed by referencing against genuine samples of the compounds and GC/MS analysis of the product mixture.. Catalyst Deactivation during Recirculation. Recirculation was performed at 1 and 1 C for 7 h at. ml min -1, 5 mol% TEMP, 5 bar at 5 ml min -1. Using :1 as solvent of choice for the previously performed single-pass optimizations, conversions were coming to a halt at 3-5 % conversion of benzyl alcohol to benzaldehyde, indicating catalyst deactivation. Inspection of the corresponding chromatograms at 15 nm for different reaction times revealed that benzyl formate, which is constantly accumulating during the reaction, is formed in increasing amounts towards the point of complete deactivation of the catalyst, until no benzyl alcohol is converted any more, and thus also formation of benzyl formate stops. Figure S1 shows a sample chromatogram taken after 7 h at 1 and 1 C, showing the large amount of benzyl formate accumulated at full catalyst deactivation. The catalyst also appears to be deactivated more quickly at 1 C, as compared to 1 C. 17 H (internal std) H 1 C. ml/min (eff) 1 C.5 ml/min (eff) Time [min] Figure S1. HPLC chromatogram at 15 nm of the recirculation runs at 1 (blue) and 1 C (red) after 7 h at. ml min -1, 5 mol% TEMP, 5 bar at 5 ml min -1 (5 and 3%
11 Intensity [AU] mixtures This journal is The Royal Society of Chemistry converted to benzaldehyde). The identity of the peaks was confirmed by referencing against genuine samples of the compounds and GC/MS analysis of the product mixture..5 Influence of TEMP Loading on Purity Profiles in Recirculation. A reinvestigation on the effects of TEMP-loading on purity profiles, with emphasis on recirculation operation led to the unexpected result that all observed side-reactions including all alkylation reactions such as dibenzylether formation and Friedel-Crafts alkylation reactions, as well as benzyl formate formation, indicative of catalyst deactivation, can be suppressed by simply increasing the TEMP loading beyond a critical concentration of 1 mol%. Moreover, it should be noted, that despite the use of pure as solvent, the formation of alkylation products was not taking place Benzaldehyde 5%TEMP 1%TEMP Diphenyl ether benzylated benzyl alcohol Time [min] Figure S11. HPLC at 15 nm of benzyl alcohol oxidation performed at 1 C after 3 h of continuous recirculation using 5 or 1 mol% TEMP and. ml min -1, 35 bar at 5 ml min - 1. The identity of the peaks was confirmed by referencing against genuine samples of the compounds and GC/MS analysis of the product mixture..6 Summary of Side-Reactions in Different Solvents. H solvent 1% Fe/Al-SBA15 5% TEMP continuous flow H H (internal std) 1 H (traces) 3 (traces) Scheme S1. Dependence of byproduct formation on reaction conditions.
Electronic Supplementary Information
 This journal is (c) The Royal Society of Chemistry 29 1 Electronic Supplementary Information On the importance of simultaneous infrared/fiber-optic temperature monitoring in the microwave-assisted synthesis
This journal is (c) The Royal Society of Chemistry 29 1 Electronic Supplementary Information On the importance of simultaneous infrared/fiber-optic temperature monitoring in the microwave-assisted synthesis
Continuous flow nanocatalysis: reaction pathways in the conversion of levulinic acid to valuable chemicals
 Electronic Supplementary Material (ESI) for Green Chemistry ESI Continuous flow nanocatalysis: reaction pathways in the conversion of levulinic acid to valuable chemicals Jose M. Bermudez, J. Angel Menéndez,
Electronic Supplementary Material (ESI) for Green Chemistry ESI Continuous flow nanocatalysis: reaction pathways in the conversion of levulinic acid to valuable chemicals Jose M. Bermudez, J. Angel Menéndez,
Selective aerobic oxidation of biomass-derived HMF to 2,5- diformylfuran using a MOF-derived magnetic hollow Fe-Co
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Selective aerobic oxidation of biomass-derived HMF to 2,5- diformylfuran using a MOF-derived
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Selective aerobic oxidation of biomass-derived HMF to 2,5- diformylfuran using a MOF-derived
Supporting Information for. Discovery of Multicomponent Heterogeneous Catalysts via Admixture Screening:
 Supporting Information for Discovery of Multicomponent Heterogeneous Catalysts via Admixture Screening: PdBiTe Catalysts for Aerobic xidative Esterification of Primary Alcohols David S. Mannel a, Maaz
Supporting Information for Discovery of Multicomponent Heterogeneous Catalysts via Admixture Screening: PdBiTe Catalysts for Aerobic xidative Esterification of Primary Alcohols David S. Mannel a, Maaz
Aerobic Oxidation of 2-Phenoxyethanol Lignin Model. Compounds Using Vanadium and Copper Catalysts
 Electronic Supporting Information for: Aerobic Oxidation of 2-Phenoxyethanol Lignin Model Compounds Using Vanadium and Copper Catalysts Christian Díaz-Urrutia, Baburam Sedai, Kyle C. Leckett, R. Tom Baker,,*
Electronic Supporting Information for: Aerobic Oxidation of 2-Phenoxyethanol Lignin Model Compounds Using Vanadium and Copper Catalysts Christian Díaz-Urrutia, Baburam Sedai, Kyle C. Leckett, R. Tom Baker,,*
Electronic Supplementary Information
 S1 Electronic Supplementary Information Direct Aerobic Oxidation of 2-Benzylpyridines in a Gas- Liquid Continuous-Flow Regime Using Propylene Carbonate as Solvent Bartholomäus Pieber and C. Oliver Kappe*
S1 Electronic Supplementary Information Direct Aerobic Oxidation of 2-Benzylpyridines in a Gas- Liquid Continuous-Flow Regime Using Propylene Carbonate as Solvent Bartholomäus Pieber and C. Oliver Kappe*
Nonthermal Microwave Effects Revisited On the. Agitation in Microwave Chemistry
 Supporting Information Nonthermal Microwave Effects Revisited On the Importance of Internal Temperature Monitoring and Agitation in Microwave Chemistry M. Antonia Herrero, Jennifer M. Kremsner and C. Oliver
Supporting Information Nonthermal Microwave Effects Revisited On the Importance of Internal Temperature Monitoring and Agitation in Microwave Chemistry M. Antonia Herrero, Jennifer M. Kremsner and C. Oliver
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 S1 Electronic Supplementary Information Flash Carboxylation: Fast Lithiation - Carboxylation
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 S1 Electronic Supplementary Information Flash Carboxylation: Fast Lithiation - Carboxylation
CHAPTER 4: CATALYTIC PROPERTIES OF ZSM-5 ZEOLITES AND CUBIC MESOPOROUS MATERIALS
 102 CHAPTER 4: CATALYTIC PROPERTIES OF ZSM-5 ZEOLITES AND CUBIC MESOPOROUS MATERIALS Chapter summary The role of heterogeneous catalysts in organic reactions is included in this chapter. Two organic reactions,
102 CHAPTER 4: CATALYTIC PROPERTIES OF ZSM-5 ZEOLITES AND CUBIC MESOPOROUS MATERIALS Chapter summary The role of heterogeneous catalysts in organic reactions is included in this chapter. Two organic reactions,
Heterogeneously catalyzed selective aerobic oxidative cross-coupling of terminal alkynes and amides with simple copper(ii) hydroxide
 Electronic Supplementary Information (ESI) for Heterogeneously catalyzed selective aerobic oxidative cross-coupling of terminal alkynes and amides with simple copper(ii) hydroxide Xiongjie Jin, Kazuya
Electronic Supplementary Information (ESI) for Heterogeneously catalyzed selective aerobic oxidative cross-coupling of terminal alkynes and amides with simple copper(ii) hydroxide Xiongjie Jin, Kazuya
Supporting Information
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2015 Supporting Information Experimental details 1) Preparation of the catalytic materials 1,
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2015 Supporting Information Experimental details 1) Preparation of the catalytic materials 1,
Supporting Information
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2017 Supporting Information Iodine Catalyzed xidation of Alcohols and Aldehydes to Carboxylic
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2017 Supporting Information Iodine Catalyzed xidation of Alcohols and Aldehydes to Carboxylic
Supporting Information: Synthesis of Boron Doped Carbon Nitride Solids and Their Use as Metal Free Catalyst for Aliphatic C-H Bond Oxidation
 Supporting Information: Synthesis of Boron Doped Carbon itride Solids and Their Use as Metal Free Catalyst for Aliphatic C-H Bond Oxidation Yong Wang, * Haoran Li, Jia Yao, Xinchen Wang, and Markus Antonietti
Supporting Information: Synthesis of Boron Doped Carbon itride Solids and Their Use as Metal Free Catalyst for Aliphatic C-H Bond Oxidation Yong Wang, * Haoran Li, Jia Yao, Xinchen Wang, and Markus Antonietti
CHAPTER 4. LIQUID PHASE AEROBIC OXIDATION OF ETHYLBENZENE OVER PrAlPO-5
 106 CHAPTER 4 LIQUID PHASE AEROBIC OXIDATION OF ETHYLBENZENE OVER PrAlPO-5 4.1 INTRODUCTION Selective catalytic oxidation of alkyl aromatics is a viable technology to functionalize saturated and unsaturated
106 CHAPTER 4 LIQUID PHASE AEROBIC OXIDATION OF ETHYLBENZENE OVER PrAlPO-5 4.1 INTRODUCTION Selective catalytic oxidation of alkyl aromatics is a viable technology to functionalize saturated and unsaturated
Electronic Supplementary Information. Iron(III)-Mediated Photocatalytic Selective Substitution of Aryl
 Electronic Supplementary Information Iron(III)-Mediated Photocatalytic Selective Substitution of Aryl omine by Chlorine with High Chlorides Utilization Efficiency Ying Wang, Lina Li, Hongwei Ji, Wanhong
Electronic Supplementary Information Iron(III)-Mediated Photocatalytic Selective Substitution of Aryl omine by Chlorine with High Chlorides Utilization Efficiency Ying Wang, Lina Li, Hongwei Ji, Wanhong
Supporting Information for Exploration of C H and N H-bond functionalization towards 1-(1,2-diarylindol-3-yl)- tetrahydroisoquinolines
 Supporting Information for Exploration of C H and N H-bond functionalization towards 1-(1,2-diarylindol-3-yl)- tetrahydroisoquinolines Michael Ghobrial, Marko D. Mihovilovic and Michael Schnürch* Address:
Supporting Information for Exploration of C H and N H-bond functionalization towards 1-(1,2-diarylindol-3-yl)- tetrahydroisoquinolines Michael Ghobrial, Marko D. Mihovilovic and Michael Schnürch* Address:
Micelles-Enabled Photo-Assisted Selective Oxyhalogenation of Alkynes in Water Under Mild Conditions. Supporting Information
 Micelles-Enabled Photo-Assisted Selective Oxyhalogenation of Alkynes in Water Under Mild Conditions Lucie Finck, Jeremy Brals, Bhavana Pavuluri, Fabrice Gallou, and Sachin Handa* Department of Chemistry,
Micelles-Enabled Photo-Assisted Selective Oxyhalogenation of Alkynes in Water Under Mild Conditions Lucie Finck, Jeremy Brals, Bhavana Pavuluri, Fabrice Gallou, and Sachin Handa* Department of Chemistry,
Analysis of Trace (mg/kg) Thiophene in Benzene Using Two-Dimensional Gas Chromatography and Flame Ionization Detection Application
 Analysis of Trace (mg/kg) Thiophene in Using Two-Dimensional Gas Chromatography and Flame Ionization Detection Application Petrochemical Authors James D. McCurry and Bruce D. Quimby Agilent Technologies
Analysis of Trace (mg/kg) Thiophene in Using Two-Dimensional Gas Chromatography and Flame Ionization Detection Application Petrochemical Authors James D. McCurry and Bruce D. Quimby Agilent Technologies
Study on the Selective Hydrogenation of Nitroaromatics to N-aryl hydroxylamines using a Supported Pt nanoparticle Catalyst
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 204 Supporting Information Study on the Selective Hydrogenation of Nitroaromatics
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 204 Supporting Information Study on the Selective Hydrogenation of Nitroaromatics
9. Hydroboration-Oxidation of Alkenes
 9. ydroboration-xidation of Alkenes A. Introduction 1. ydroboration-xidation of Alkenes Alkenes can be oxidized to alcohols using a two-step method of hydroboration followed by oxidation. The first step
9. ydroboration-xidation of Alkenes A. Introduction 1. ydroboration-xidation of Alkenes Alkenes can be oxidized to alcohols using a two-step method of hydroboration followed by oxidation. The first step
Chemicals All chemicals were of analytical grade and used as purchased without further purification.
 Supplementary Methods Chemicals All chemicals were of analytical grade and used as purchased without further purification. Synthesis of referenced catalysts Synthesis of Au/ZSM-5. As a typical run, 1 g
Supplementary Methods Chemicals All chemicals were of analytical grade and used as purchased without further purification. Synthesis of referenced catalysts Synthesis of Au/ZSM-5. As a typical run, 1 g
Supporting Information. Copyright Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2008
 Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2008 1 Supporting Information Palladium(0) Nanoparticles on glass-polymer composite materials as recyclable catalysts:
Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2008 1 Supporting Information Palladium(0) Nanoparticles on glass-polymer composite materials as recyclable catalysts:
Asymmetric Organocatalytic Strecker-Type Reactions of Aliphatic N,N- Dialkylhydrazones
 Asymmetric Organocatalytic Strecker-Type Reactions of Aliphatic N,N- Dialkylhydrazones Aurora Martínez-Muñoz, David Monge,* Eloísa Martín-Zamora, Eugenia Marqués-López, Eleuterio Álvarez, Rosario Fernández,*
Asymmetric Organocatalytic Strecker-Type Reactions of Aliphatic N,N- Dialkylhydrazones Aurora Martínez-Muñoz, David Monge,* Eloísa Martín-Zamora, Eugenia Marqués-López, Eleuterio Álvarez, Rosario Fernández,*
Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c,
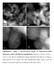 Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c, 2 nm; d, 2 nm. The low magnification images demonstrate
Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c, 2 nm; d, 2 nm. The low magnification images demonstrate
[(NHC)Au I ]-Catalyzed Acid Free Hydration of Alkynes at Part-Per-Million Catalyst Loadings
![[(NHC)Au I ]-Catalyzed Acid Free Hydration of Alkynes at Part-Per-Million Catalyst Loadings [(NHC)Au I ]-Catalyzed Acid Free Hydration of Alkynes at Part-Per-Million Catalyst Loadings](/thumbs/83/87981041.jpg) SUPPORTING INFORMATION [(NHC)Au I ]-Catalyzed Acid Free Hydration of Alkynes at Part-Per-Million Catalyst Loadings Nicolas Marion, Rubén S. Ramón, and Steven P. Nolan Institute of Chemical Research of
SUPPORTING INFORMATION [(NHC)Au I ]-Catalyzed Acid Free Hydration of Alkynes at Part-Per-Million Catalyst Loadings Nicolas Marion, Rubén S. Ramón, and Steven P. Nolan Institute of Chemical Research of
Advancer Kilobatch. kilogram scale-up of all microwave chemistry
 Advancer Kilobatch kilogram scale-up of all microwave chemistry Background MW reactions are become more common place in Discovery / Med Chem MW reactions can be higher yield / higher purity MW reactions
Advancer Kilobatch kilogram scale-up of all microwave chemistry Background MW reactions are become more common place in Discovery / Med Chem MW reactions can be higher yield / higher purity MW reactions
Supporting Information
 Supporting Information Highly Cross-Linked Imidazolium Salts Entrapped Magnetic Particles Preparation and Applications Paola Agrigento, a Matthias Josef Beier, b Jesper T. N. Knijnenburg, c Alfons Baiker
Supporting Information Highly Cross-Linked Imidazolium Salts Entrapped Magnetic Particles Preparation and Applications Paola Agrigento, a Matthias Josef Beier, b Jesper T. N. Knijnenburg, c Alfons Baiker
Investigation of Petasis and Ugi Reactions in Series in an Automated Microreactor System
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supplementary Information Investigation of Petasis and Ugi Reactions in Series in an Automated
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supplementary Information Investigation of Petasis and Ugi Reactions in Series in an Automated
Copper-catalyzed cleavage of benzyl ethers with diacetoxyiodobenzene and p-toluenesulfonamide
 General Papers ARKIVC 2008 (xii) 103-108 Copper-catalyzed cleavage of benzyl ethers with diacetoxyiodobenzene and p-toluenesulfonamide Ling He a,b, Qin Wang a, Guo-Chuan Zhou b, Lei Guo b, and Xiao-Qi
General Papers ARKIVC 2008 (xii) 103-108 Copper-catalyzed cleavage of benzyl ethers with diacetoxyiodobenzene and p-toluenesulfonamide Ling He a,b, Qin Wang a, Guo-Chuan Zhou b, Lei Guo b, and Xiao-Qi
Sub-10-nm Au-Pt-Pd Alloy Trimetallic Nanoparticles with. High Oxidation-Resistant Property as Efficient and Durable
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Sub-10-nm Au-Pt-Pd Alloy Trimetallic Nanoparticles with High
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Sub-10-nm Au-Pt-Pd Alloy Trimetallic Nanoparticles with High
Catalytic Hydrogenation of Amino Acids to Amino Alcohols with Complete Retention of Configuration
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Catalytic Hydrogenation of Amino Acids to Amino Alcohols with
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Catalytic Hydrogenation of Amino Acids to Amino Alcohols with
Ionic liquid-supported Pt nanoparticles as catalysts for enantioselective hydrogenation
 Supporting information Ionic liquid-supported Pt nanoparticles as catalysts for enantioselective hydrogenation Matthias Josef Beier, Jean-Michel Andanson, Tamas Mallat, Frank Krumeich and Alfons Baiker*
Supporting information Ionic liquid-supported Pt nanoparticles as catalysts for enantioselective hydrogenation Matthias Josef Beier, Jean-Michel Andanson, Tamas Mallat, Frank Krumeich and Alfons Baiker*
Headspace Technology for GC and GC/MS: Features, benefits & applications
 Headspace Technology for GC and GC/MS: Features, benefits & applications Karima Baudin Oct 2015 Why use Headspace? Very Simple no to minimum sample prep Robust enhance uptime Non-detectable carry-over
Headspace Technology for GC and GC/MS: Features, benefits & applications Karima Baudin Oct 2015 Why use Headspace? Very Simple no to minimum sample prep Robust enhance uptime Non-detectable carry-over
Green Chemistry and the United States Environmental Protection Agency A Postdoc s Perspective James T. Ciszewski & Michael A.
 Green Chemistry and the United States Environmental Protection Agency A Postdoc s Perspective James T. Ciszewski & Michael A. Gonzalez United States Environmental Protection Agency Office of Research and
Green Chemistry and the United States Environmental Protection Agency A Postdoc s Perspective James T. Ciszewski & Michael A. Gonzalez United States Environmental Protection Agency Office of Research and
The aldol condensation of benzaldehyde and acetone is a classical example of a spontaneous, exothermic reaction. To avoid controlled reagent addition
 The aldol condensation of benzaldehyde and acetone is a classical example of a spontaneous, exothermic reaction. To avoid controlled reagent addition and cooling, the reaction was successfully translated
The aldol condensation of benzaldehyde and acetone is a classical example of a spontaneous, exothermic reaction. To avoid controlled reagent addition and cooling, the reaction was successfully translated
Supporting Information. Highly Efficient Aerobic Oxidation of Various Amines Using Pd 3 Pb Intermetallic Compound Catalysts
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supporting Information Highly Efficient Aerobic Oxidation of Various Amines Using Pd 3 Pb Intermetallic
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supporting Information Highly Efficient Aerobic Oxidation of Various Amines Using Pd 3 Pb Intermetallic
Development of Safe and Scalable Continuous-Flow Methods for. Palladium-Catalyzed Aerobic Oxidation Reactions
 Supplementary Data for Development of Safe and Scalable Continuous-Flow Methods for Palladium-Catalyzed Aerobic Oxidation Reactions Xuan Ye, a Martin D. Johnson, *b Tianning Diao, a Matthew H. Yates *b
Supplementary Data for Development of Safe and Scalable Continuous-Flow Methods for Palladium-Catalyzed Aerobic Oxidation Reactions Xuan Ye, a Martin D. Johnson, *b Tianning Diao, a Matthew H. Yates *b
Fe 2 O 3 and Co-Co 3 O 4 hydrogenation of nitroarenes under mild conditions
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2016 Supporting information for Fe 2 O 3 /NGr@C- and Co-Co 3 O 4 /NGr@C-catalysed
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2016 Supporting information for Fe 2 O 3 /NGr@C- and Co-Co 3 O 4 /NGr@C-catalysed
CHAPTER 6 SELECTIVE OXIDATION OF DIPHEYLMETHANE TO BENZOPHENONE
 110 CHAPTER 6 SELECTIVE OXIDATION OF DIPHEYLMETHANE TO BENZOPHENONE 6.1 INTRODUCTION Oxidation of diphenylmethane (DPM) to benzophenone is an industrially important reaction as the product benzophenone
110 CHAPTER 6 SELECTIVE OXIDATION OF DIPHEYLMETHANE TO BENZOPHENONE 6.1 INTRODUCTION Oxidation of diphenylmethane (DPM) to benzophenone is an industrially important reaction as the product benzophenone
Copper-Catalyzed Oxidative Amination of Benzoxazoles via C-H and C-N Bond Activation: A
 Supporting Information for: Copper-Catalyzed xidative Amination of Benzoxazoles via C-H and C- Bond Activation: A ew Strategy for Using Tertiary Amines as itrogen Group Sources Shengmei Guo, Bo Qian, Yinjun
Supporting Information for: Copper-Catalyzed xidative Amination of Benzoxazoles via C-H and C- Bond Activation: A ew Strategy for Using Tertiary Amines as itrogen Group Sources Shengmei Guo, Bo Qian, Yinjun
Environmentally Friendly Routes for the Selective Oxidation of Alcohols Setrak K. Tanielyan and Robert L. Augustine
 Environmentally Friendly Routes for the Selective xidation of Alcohols Setrak K. Tanielyan and Robert L. Augustine Center for Applied Catalysis Department of Chemistry and Biochemistry Seton all University
Environmentally Friendly Routes for the Selective xidation of Alcohols Setrak K. Tanielyan and Robert L. Augustine Center for Applied Catalysis Department of Chemistry and Biochemistry Seton all University
Supporting Information
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Supporting Information Bi Doped CeO 2 Oxide Supported Gold Nanoparticle Catalysts for the Aerobic
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Supporting Information Bi Doped CeO 2 Oxide Supported Gold Nanoparticle Catalysts for the Aerobic
Final Report. Characterisation of Sample Report. Job No 2016/11/12-34 AS No. 1234A. Client Example Contact Sample. Signed Date 2017.
 Final Report Title Characterisation of Job No 2016/11/12-34 AS No. 1234A Client Contact Sample Author report Signed Date 2017 Easy Reach Report 2017 v2.docx 1 of 33 Contents 1. Study Summary Page 3 2.
Final Report Title Characterisation of Job No 2016/11/12-34 AS No. 1234A Client Contact Sample Author report Signed Date 2017 Easy Reach Report 2017 v2.docx 1 of 33 Contents 1. Study Summary Page 3 2.
Magnetic nanoparticle-supported proline as a recyclable and recoverable ligand for the CuI catalyzed arylation of nitrogen nucleophiles
 Magnetic nanoparticle-supported proline as a recyclable and recoverable ligand for the CuI catalyzed arylation of nitrogen nucleophiles Gagan Chouhan, Dashan Wang and Howard Alper* Centre for Catalysis
Magnetic nanoparticle-supported proline as a recyclable and recoverable ligand for the CuI catalyzed arylation of nitrogen nucleophiles Gagan Chouhan, Dashan Wang and Howard Alper* Centre for Catalysis
Synthesis of 2 ) Structures by Small Molecule-Assisted Nucleation for Plasmon-Enhanced Photocatalytic Activity
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Synthesis of Au@UiO-66(NH 2 ) Structures by Small Molecule-Assisted
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Synthesis of Au@UiO-66(NH 2 ) Structures by Small Molecule-Assisted
Acrylates via Metathesis of Crotonates
 http://pubs.acs.org/doi/abs/1.121/op536 Supporting Information for Acrylates via Metathesis of Crotonates http://dx.doi.org/1.121/op536 Dirk Schweitzer* and Kristi D. Snell Metabolix 21 Erie Street Cambridge,
http://pubs.acs.org/doi/abs/1.121/op536 Supporting Information for Acrylates via Metathesis of Crotonates http://dx.doi.org/1.121/op536 Dirk Schweitzer* and Kristi D. Snell Metabolix 21 Erie Street Cambridge,
A Mild, Catalytic and Highly Selective Method for the Oxidation of α,β- Enones to 1,4-Enediones. Jin-Quan Yu, a and E. J.
 A Mild, Catalytic and Highly Selective Method for the Oxidation of α,β- Enones to 1,4-Enediones Jin-Quan Yu, a and E. J. Corey b * a Department of Chemistry, Cambridge University, Cambridge CB2 1EW, United
A Mild, Catalytic and Highly Selective Method for the Oxidation of α,β- Enones to 1,4-Enediones Jin-Quan Yu, a and E. J. Corey b * a Department of Chemistry, Cambridge University, Cambridge CB2 1EW, United
Titanium Nitride-Nickel Nanocomposite as Heterogeneous Catalyst for the Hydrogenolysis of Aryl Ethers
 Titanium Nitride-Nickel Nanocomposite as Heterogeneous Catalyst for the Hydrogenolysis of Aryl Ethers Valerio Molinari, Cristina Giordano, Markus Antonietti and Davide Esposito* Max-Planck-Institute of
Titanium Nitride-Nickel Nanocomposite as Heterogeneous Catalyst for the Hydrogenolysis of Aryl Ethers Valerio Molinari, Cristina Giordano, Markus Antonietti and Davide Esposito* Max-Planck-Institute of
Curtius-Like Rearrangement of Iron-Nitrenoid Complex and. Application in Biomimetic Synthesis of Bisindolylmethanes
 Supporting Information Curtius-Like Rearrangement of Iron-itrenoid Complex and Application in Biomimetic Synthesis of Bisindolylmethanes Dashan Li,, Ting Wu,, Kangjiang Liang,, and Chengfeng Xia*,, State
Supporting Information Curtius-Like Rearrangement of Iron-itrenoid Complex and Application in Biomimetic Synthesis of Bisindolylmethanes Dashan Li,, Ting Wu,, Kangjiang Liang,, and Chengfeng Xia*,, State
Exploring the biocatalytic scope of a bacterial flavin-containing monooxygenase
 Exploring the biocatalytic scope of a bacterial flavin-containing monooxygenase Ana Rioz-Martínez, a Malgorzata Kopacz, b Gonzalo de Gonzalo, b, * Daniel E. Torres Pamiño, Vicente Gotor a and Marco W.
Exploring the biocatalytic scope of a bacterial flavin-containing monooxygenase Ana Rioz-Martínez, a Malgorzata Kopacz, b Gonzalo de Gonzalo, b, * Daniel E. Torres Pamiño, Vicente Gotor a and Marco W.
Application Note. Authors. Abstract. Energy & Chemicals, Biofuels & Alternative Energy
 Analytical Quantification of Deoxygenated Compounds in Catalytic Reaction Samples as an Evaluation Technique to Detere Reaction Conversion from a Bioderived Oil in Novel Biofuel Testing Application Note
Analytical Quantification of Deoxygenated Compounds in Catalytic Reaction Samples as an Evaluation Technique to Detere Reaction Conversion from a Bioderived Oil in Novel Biofuel Testing Application Note
Supporting Information
 Supporting Information Wiley-VCH 2012 69451 Weinheim, Germany Synthesis of 1-Octanol and 1,1-Dioctyl Ether from Biomass-Derived Platform Chemicals** Jennifer Julis and Walter Leitner* anie_201203669_sm_miscellaneous_information.pdf
Supporting Information Wiley-VCH 2012 69451 Weinheim, Germany Synthesis of 1-Octanol and 1,1-Dioctyl Ether from Biomass-Derived Platform Chemicals** Jennifer Julis and Walter Leitner* anie_201203669_sm_miscellaneous_information.pdf
Selective Formation of Benzo[c]cinnoline by Photocatalytic Reduction of 2,2 Dinitrobiphenyl with TiO 2 and UV light irradiation
![Selective Formation of Benzo[c]cinnoline by Photocatalytic Reduction of 2,2 Dinitrobiphenyl with TiO 2 and UV light irradiation Selective Formation of Benzo[c]cinnoline by Photocatalytic Reduction of 2,2 Dinitrobiphenyl with TiO 2 and UV light irradiation](/thumbs/90/104361197.jpg) Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 Content: Selective Formation of Benzo[c]cinnoline by Photocatalytic Reduction of
Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 Content: Selective Formation of Benzo[c]cinnoline by Photocatalytic Reduction of
Selective Dimerization of Ethylene to 1-Butene with a Porous Catalyst
 Supporting Information Selective Dimerization of Ethylene to 1-Butene with a Porous Catalyst Eric D. Metzger, Carl K. Brozek, Robert J. Comito, and Mircea Dincă* Department of Chemistry, Massachusetts
Supporting Information Selective Dimerization of Ethylene to 1-Butene with a Porous Catalyst Eric D. Metzger, Carl K. Brozek, Robert J. Comito, and Mircea Dincă* Department of Chemistry, Massachusetts
Supporting Information
 Supporting Information MgFeCe ternary layered double hydroxide as highly efficient and recyclable heterogeneous base catalyst for synthesis of dimethyl carbonate by transesterification Nayana T. Nivangune
Supporting Information MgFeCe ternary layered double hydroxide as highly efficient and recyclable heterogeneous base catalyst for synthesis of dimethyl carbonate by transesterification Nayana T. Nivangune
Xiufang Chen, Jinshui Zhang, Xianzhi Fu, Markus Antonietti, and Xinchen Wang*
 -Catalyzed Oxidation of Benzene to Phenol Using Hydrogen Peroxide and Visible Light Xiufang Chen, Jinshui Zhang, Xianzhi Fu, Markus Antonietti, and Xinchen Wang* Supporting Information: Synthesis of :
-Catalyzed Oxidation of Benzene to Phenol Using Hydrogen Peroxide and Visible Light Xiufang Chen, Jinshui Zhang, Xianzhi Fu, Markus Antonietti, and Xinchen Wang* Supporting Information: Synthesis of :
Supporting Information
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2014 Supporting Information Unraveling the Origins of Catalyst Degradation in Non-heme Ironbased
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2014 Supporting Information Unraveling the Origins of Catalyst Degradation in Non-heme Ironbased
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 07 Electronic Supplementary Information Hydroaminomethylation/hydrohydroxymethylation sequence
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 07 Electronic Supplementary Information Hydroaminomethylation/hydrohydroxymethylation sequence
A biphasic oxidation of alcohols to aldehydes and ketones using a simplified packed-bed microreactor
 A biphasic oxidation of alcohols to aldehydes and ketones using a simplified packed-bed microreactor Andrew Bogdan 1 and D. Tyler McQuade 2, * Address: 1 Department of Chemistry and Chemical Biology, Cornell
A biphasic oxidation of alcohols to aldehydes and ketones using a simplified packed-bed microreactor Andrew Bogdan 1 and D. Tyler McQuade 2, * Address: 1 Department of Chemistry and Chemical Biology, Cornell
SUPPORTING INFORMATION
 SUPPORTING INFORMATION A Sustainable Approach to Waste-Minimized Sonogashira Cross-Coupling Reaction Based on Recoverable/Reusable heterogeneous Catalytic/Base System and Acetonitrile Azeotrope Vadym Kozell,
SUPPORTING INFORMATION A Sustainable Approach to Waste-Minimized Sonogashira Cross-Coupling Reaction Based on Recoverable/Reusable heterogeneous Catalytic/Base System and Acetonitrile Azeotrope Vadym Kozell,
BUTYL ALCOHOL in urine by GC-FID Code GC05510
 BUTYL ALCOHOL in urine by GC-FID Code GC00 BIOCHEMISTRY Butanol (or n-butyl alcohol) is an alcohol which at room temperature is presented as a colorless liquid smelling of alcohol. It is an inflammatory
BUTYL ALCOHOL in urine by GC-FID Code GC00 BIOCHEMISTRY Butanol (or n-butyl alcohol) is an alcohol which at room temperature is presented as a colorless liquid smelling of alcohol. It is an inflammatory
Palladium-Catalyzed Asymmetric Benzylic Alkylation Reactions
 Palladium-Catalyzed Asymmetric Benzylic Alkylation Reactions Reporter: Hong-Qiang Shen Checker: Cong Liu Date: 2016/07/12 Masahiro Miura et al. Angew. Chem. Int. Ed. 2016, 55, 6973. Masahiro Miura Osaka
Palladium-Catalyzed Asymmetric Benzylic Alkylation Reactions Reporter: Hong-Qiang Shen Checker: Cong Liu Date: 2016/07/12 Masahiro Miura et al. Angew. Chem. Int. Ed. 2016, 55, 6973. Masahiro Miura Osaka
Supporting Information
 Supporting Information An Extremely Active and General Catalyst for Suzuki Coupling Reactions of Unreactive Aryl Chlorides Dong-Hwan Lee and Myung-Jong Jin* School of Chemical Science and Engineering,
Supporting Information An Extremely Active and General Catalyst for Suzuki Coupling Reactions of Unreactive Aryl Chlorides Dong-Hwan Lee and Myung-Jong Jin* School of Chemical Science and Engineering,
SUPPORTING INFORMATION. Elevated Temperatures A Critical Comparison of
 S1 SUPPORTING INFORMATION Solid-Phase Synthesis of Difficult Peptide Sequences at Elevated Temperatures A Critical Comparison of Microwave and Conventional Heating Technologies Bernadett Bacsa, Kata Horváti,
S1 SUPPORTING INFORMATION Solid-Phase Synthesis of Difficult Peptide Sequences at Elevated Temperatures A Critical Comparison of Microwave and Conventional Heating Technologies Bernadett Bacsa, Kata Horváti,
Supplementary Information
 Supplementary Information Supplementary Figure 1. X-ray diffraction patterns of (a) pure LDH, (b) AuCl 4 ion-exchanged LDH and (c) the Au/LDH hybrid catalyst. The refined cell parameters for pure, ion-exchanged,
Supplementary Information Supplementary Figure 1. X-ray diffraction patterns of (a) pure LDH, (b) AuCl 4 ion-exchanged LDH and (c) the Au/LDH hybrid catalyst. The refined cell parameters for pure, ion-exchanged,
Total: 10 pages, 4 figures and 4 tables.
 Supporting Information Catalysis in flow: Nickel-catalyzed synthesis of primary amines from alcohols and NH 3 Andrew Yuk Keung Leung, Klaus Hellgardt and Mimi (King Kuok) Hii Total: 10 pages, 4 figures
Supporting Information Catalysis in flow: Nickel-catalyzed synthesis of primary amines from alcohols and NH 3 Andrew Yuk Keung Leung, Klaus Hellgardt and Mimi (King Kuok) Hii Total: 10 pages, 4 figures
Supporting Information. Adventures in Atropisomerism: Development of a Robust, Diastereoselective, Lithium-Catalyzed Atropisomer-Forming API Step
 Supporting Information Adventures in Atropisomerism: Development of a Robust, Diastereoselective, Lithium-Catalyzed Atropisomer-Forming API Step Steven R. Wisniewski,* Ronald Carrasquillo-Flores, Federico
Supporting Information Adventures in Atropisomerism: Development of a Robust, Diastereoselective, Lithium-Catalyzed Atropisomer-Forming API Step Steven R. Wisniewski,* Ronald Carrasquillo-Flores, Federico
Student Manual for Aerobic Alcohol Oxidation Using a Copper(I)/TEMPO Catalyst System
 Student Manual for Aerobic Alcohol Oxidation Using a Copper(I)/TEMPO Catalyst System icholas J. Hill, Jessica M. Hoover and Shannon S. Stahl* Department of Chemistry, University of Wisconsin-Madison, 1101
Student Manual for Aerobic Alcohol Oxidation Using a Copper(I)/TEMPO Catalyst System icholas J. Hill, Jessica M. Hoover and Shannon S. Stahl* Department of Chemistry, University of Wisconsin-Madison, 1101
Catalytic Reductive Dehydration of Tertiary Amides to Enamines under Hydrosilylation Conditions
 SUPPORTIG IFORMATIO Catalytic Reductive Dehydration of Tertiary Amides to Enamines under Hydrosilylation Conditions Alexey Volkov, a Fredrik Tinnis, a and Hans Adolfsson.* a a Department of Organic Chemistry,
SUPPORTIG IFORMATIO Catalytic Reductive Dehydration of Tertiary Amides to Enamines under Hydrosilylation Conditions Alexey Volkov, a Fredrik Tinnis, a and Hans Adolfsson.* a a Department of Organic Chemistry,
Figure S1 XRD patterns of (a) Ti-MSE-A, (b) Ti-MSE-A-cal, (c) TS-1, (d) Ti-MWW, and (e) Ti-BEA.
 Electronic Supplementary Information (ESI) Synthesis and Catalytic Performance of Ti-MCM-68 for Effective Oxidation Reactions Yoshihiro Kubota,* Yoshihito Koyama, Taku Yamada, Satoshi Inagaki, and Takashi
Electronic Supplementary Information (ESI) Synthesis and Catalytic Performance of Ti-MCM-68 for Effective Oxidation Reactions Yoshihiro Kubota,* Yoshihito Koyama, Taku Yamada, Satoshi Inagaki, and Takashi
2017 Reaction of cinnamic acid chloride with ammonia to cinnamic acid amide
 217 Reaction of cinnamic acid chloride with ammonia to cinnamic acid amide O O Cl NH 3 NH 2 C 9 H 7 ClO (166.6) (17.) C 9 H 9 NO (147.2) Classification Reaction types and substance classes reaction of
217 Reaction of cinnamic acid chloride with ammonia to cinnamic acid amide O O Cl NH 3 NH 2 C 9 H 7 ClO (166.6) (17.) C 9 H 9 NO (147.2) Classification Reaction types and substance classes reaction of
Identifying Lead Hits in Catalyst Discovery by Screening and Deconvoluting Complex Mixtures of Catalyst Components. Supporting Information
 Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2015 Identifying Lead Hits in Catalyst Discovery by Screening and Deconvoluting Complex Mixtures
Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2015 Identifying Lead Hits in Catalyst Discovery by Screening and Deconvoluting Complex Mixtures
Supporting Information
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supporting Information Pd-Catalyzed C-H Activation/xidative Cyclization of Acetanilide with orbornene:
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supporting Information Pd-Catalyzed C-H Activation/xidative Cyclization of Acetanilide with orbornene:
SUPPORTING INFORMATION
 SUPPRTING INFRMATIN A Direct, ne-step Synthesis of Condensed Heterocycles: A Palladium-Catalyzed Coupling Approach Farnaz Jafarpour and Mark Lautens* Davenport Chemical Research Laboratories, Chemistry
SUPPRTING INFRMATIN A Direct, ne-step Synthesis of Condensed Heterocycles: A Palladium-Catalyzed Coupling Approach Farnaz Jafarpour and Mark Lautens* Davenport Chemical Research Laboratories, Chemistry
Supporting Information
 Electronic Supplementary Material (ESI) for ACS Chemical Neuroscience Supporting Information Fluorine-18-labeled Antagonist for PET Imaging of Kappa Opioid Receptors Zhengxin Cai,*, Songye Li, Richard
Electronic Supplementary Material (ESI) for ACS Chemical Neuroscience Supporting Information Fluorine-18-labeled Antagonist for PET Imaging of Kappa Opioid Receptors Zhengxin Cai,*, Songye Li, Richard
Effects of Different Processing Parameters on Divinylbenzene (DVB) Production Rate
 1 Effects of Different Processing Parameters on Divinylbenzene (DVB) Production Rate ME Zeynali Petrochemical Synthesis Group, Petrochemical Faculty, Iran Polymer and Petrochemical Institute (IPPI), P.O.
1 Effects of Different Processing Parameters on Divinylbenzene (DVB) Production Rate ME Zeynali Petrochemical Synthesis Group, Petrochemical Faculty, Iran Polymer and Petrochemical Institute (IPPI), P.O.
Report AFK0242/18 TABLE OF CONTENTS
 Client: Blue Dent Dental OS: 0161/0211-18 Contact: Rafael Gomes E-mail: rafael@bluedent.com.br Phone: (19) 3563-2222 Address: Rua Joaquim Jorge Port, 1272 City/State: Pirassununga/SP Zip Code: 13636-142
Client: Blue Dent Dental OS: 0161/0211-18 Contact: Rafael Gomes E-mail: rafael@bluedent.com.br Phone: (19) 3563-2222 Address: Rua Joaquim Jorge Port, 1272 City/State: Pirassununga/SP Zip Code: 13636-142
Supporting Text Synthesis of (2 S ,3 S )-2,3-bis(3-bromophenoxy)butane (3). Synthesis of (2 S ,3 S
 Supporting Text Synthesis of (2S,3S)-2,3-bis(3-bromophenoxy)butane (3). Under N 2 atmosphere and at room temperature, a mixture of 3-bromophenol (0.746 g, 4.3 mmol) and Cs 2 C 3 (2.81 g, 8.6 mmol) in DMS
Supporting Text Synthesis of (2S,3S)-2,3-bis(3-bromophenoxy)butane (3). Under N 2 atmosphere and at room temperature, a mixture of 3-bromophenol (0.746 g, 4.3 mmol) and Cs 2 C 3 (2.81 g, 8.6 mmol) in DMS
QUATERNARY AMMONIUM SALTS AS ALKYLATING REAGENTS IN C-H ACTIVATION CHEMISTRY
 Electronic Supporting Information for QUATERNARY AMMONIUM SALTS AS ALKYLATING REAGENTS IN C-H ACTIVATION CHEMISTRY Manuel Spettel, Robert Pollice, and Michael Schnürch* Institute of Applied Synthetic Chemistry,
Electronic Supporting Information for QUATERNARY AMMONIUM SALTS AS ALKYLATING REAGENTS IN C-H ACTIVATION CHEMISTRY Manuel Spettel, Robert Pollice, and Michael Schnürch* Institute of Applied Synthetic Chemistry,
Supporting Information. for. Development of a flow photochemical aerobic oxidation of benzylic C-H bonds
 Supporting Information for Development of a flow photochemical aerobic oxidation of benzylic C-H bonds Mathieu Lesieur, Christophe Genicot and Patrick Pasau* UCB Biopharma, Avenue de l industrie, 1420
Supporting Information for Development of a flow photochemical aerobic oxidation of benzylic C-H bonds Mathieu Lesieur, Christophe Genicot and Patrick Pasau* UCB Biopharma, Avenue de l industrie, 1420
Catalytic hydrogenation of liquid alkenes with a silica grafted hydride. pincer iridium(iii) complex: Support for a heterogeneous mechanism
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 215 Electronic Supplementary Information for Catalysis Science & Technology Catalytic
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 215 Electronic Supplementary Information for Catalysis Science & Technology Catalytic
J. Am. Chem. Soc. 2009, 131,
 Palladium Nanoparticles on Graphite Oxide and Its Functionalized Graphene Derivatives as Highly Active Catalysts for the Suzuki-Miyaura Coupling Reaction J. Am. Chem. Soc. 2009, 131, 8262 8270 Rolf Műlhaupt
Palladium Nanoparticles on Graphite Oxide and Its Functionalized Graphene Derivatives as Highly Active Catalysts for the Suzuki-Miyaura Coupling Reaction J. Am. Chem. Soc. 2009, 131, 8262 8270 Rolf Műlhaupt
Chemical Analysis. Low Level Oxygenates Analyzer. Trace Analysis of Oxygenates in Hydrocarbon Matrices. Gas Chromatography.
 Chemical Analysis Low Level Oxygenates Analyzer Trace Analysis of Oxygenates in Hydrocarbon Matrices think forward Gas Chromatography The determination of sub to high ppm levels of ethers, alcohols, aldehydes
Chemical Analysis Low Level Oxygenates Analyzer Trace Analysis of Oxygenates in Hydrocarbon Matrices think forward Gas Chromatography The determination of sub to high ppm levels of ethers, alcohols, aldehydes
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): The synthesis of nitriles from CH3 groups is a valuable transformation and a challenging one. The work in the manuscript appears to be conducted
Reviewers' comments: Reviewer #1 (Remarks to the Author): The synthesis of nitriles from CH3 groups is a valuable transformation and a challenging one. The work in the manuscript appears to be conducted
Supporting Information
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Supporting Information Alternating Chiral Selectivity of Aldol Reactions under the Confined Space
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Supporting Information Alternating Chiral Selectivity of Aldol Reactions under the Confined Space
Supporting Information for DOI: /s Georg Thieme Verlag KG Stuttgart New York Thieme
 Supporting Information for DI: 10.1055/s-0036-1588866 Georg Thieme Verlag KG Stuttgart ew York 2017 Thieme Base catalyzed transcarbamoylation Benoît Rhoné a,b,c Vincent Semetey a,b a PSL Research University,
Supporting Information for DI: 10.1055/s-0036-1588866 Georg Thieme Verlag KG Stuttgart ew York 2017 Thieme Base catalyzed transcarbamoylation Benoît Rhoné a,b,c Vincent Semetey a,b a PSL Research University,
Supporting Information
 Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2013 Paraldehyde as an Acetaldehyde Precursor in Asymmetric Michael Reactions Promoted by Site-Isolated Incompatible Catalysts
Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2013 Paraldehyde as an Acetaldehyde Precursor in Asymmetric Michael Reactions Promoted by Site-Isolated Incompatible Catalysts
SELECTIVE OXIDATION OF TOLUENE TO BENZALDEHYDE USING Cu/Sn/Br CATALYST SYSTEM
 Int. J. Chem. Sci.: 9(2), 211, 545-552 ISSN 972-768X www.sadgurupublications.com SELECTIVE OXIDATION OF TOLUENE TO BENZALDEHYDE USING Cu/Sn/Br CATALYST SYSTEM KALPENDRA RAJURKAR *, NILESH KULKARNI, VILAS
Int. J. Chem. Sci.: 9(2), 211, 545-552 ISSN 972-768X www.sadgurupublications.com SELECTIVE OXIDATION OF TOLUENE TO BENZALDEHYDE USING Cu/Sn/Br CATALYST SYSTEM KALPENDRA RAJURKAR *, NILESH KULKARNI, VILAS
EA-IRMS: Fast and Precise Isotope Analysis of Liquids on a Delta V Isotope Ratio MS with a High Temperature Conversion Elemental Analyzer
 APPLICATION NOTE EA-IRMS: Fast and Precise Isotope Analysis of Liquids on a Delta V Isotope Ratio MS with a High Temperature Conversion Elemental Analyzer AN30180 Oliver Kracht, Andreas Hilkert, Thermo
APPLICATION NOTE EA-IRMS: Fast and Precise Isotope Analysis of Liquids on a Delta V Isotope Ratio MS with a High Temperature Conversion Elemental Analyzer AN30180 Oliver Kracht, Andreas Hilkert, Thermo
Stille-type cross coupling reactions with tetraalkynyl stannanes
 Stille-type cross coupling reactions with tetraalkynyl stannanes Andrey S. Levashov,* Dmitriy S. Buriy, Valeriy V. Konshin and Alexey A. Andreev (deceased) Kuban State University, 149 Stavropolskaya Str.,
Stille-type cross coupling reactions with tetraalkynyl stannanes Andrey S. Levashov,* Dmitriy S. Buriy, Valeriy V. Konshin and Alexey A. Andreev (deceased) Kuban State University, 149 Stavropolskaya Str.,
Regioselective Synthesis of 1,5-Disubstituted 1,2,3-Triazoles by reusable
 1 Regioselective Synthesis of 1,5-Disubstituted 1,2,3-Triazoles by reusable immobilized AlCl 3 on γ-al 2 O 3 SUPPLEMETARY DATA Typical Procedure to the preparation of Azides Phenyl azide Phenyl azide was
1 Regioselective Synthesis of 1,5-Disubstituted 1,2,3-Triazoles by reusable immobilized AlCl 3 on γ-al 2 O 3 SUPPLEMETARY DATA Typical Procedure to the preparation of Azides Phenyl azide Phenyl azide was
Molecular Imaging of Labile Iron(II) Pools in Living Cells with a Turn-on Fluorescent Probe
 Supporting Information for Molecular Imaging of Labile Iron(II) Pools in Living Cells with a Turn-on Fluorescent Probe Ho Yu Au-Yeung, Jefferson Chan, Teera Chantarojsiri and Christopher J. Chang* Departments
Supporting Information for Molecular Imaging of Labile Iron(II) Pools in Living Cells with a Turn-on Fluorescent Probe Ho Yu Au-Yeung, Jefferson Chan, Teera Chantarojsiri and Christopher J. Chang* Departments
ADVANTAME (TENTATIVE)
 ADVANTAME (TENTATIVE) SYNONYMS INS No. 969 New tentative specifications prepared at the 77th JECFA (2013) and published in FAO JECFA Monographs 14 (2013). An ADI of 0-5 mg/kg body weight was established
ADVANTAME (TENTATIVE) SYNONYMS INS No. 969 New tentative specifications prepared at the 77th JECFA (2013) and published in FAO JECFA Monographs 14 (2013). An ADI of 0-5 mg/kg body weight was established
Supporting Information
 Supporting Information A General, Activator-Free Palladium-Catalyzed Synthesis of Arylacetic and Benzoic Acids from Formic Acid Lin Wang, Helfried Neumann, and Matthias Beller* anie_201802384_sm_miscellaneous_information.pdf
Supporting Information A General, Activator-Free Palladium-Catalyzed Synthesis of Arylacetic and Benzoic Acids from Formic Acid Lin Wang, Helfried Neumann, and Matthias Beller* anie_201802384_sm_miscellaneous_information.pdf
Supporting Information
 Supporting Information An L-proline Functionalized Metallo-organic Triangle as Size-Selective Homogeneous Catalyst for Asymmertry Catalyzing Aldol Reactions Xiao Wu, Cheng He, Xiang Wu, Siyi Qu and Chunying
Supporting Information An L-proline Functionalized Metallo-organic Triangle as Size-Selective Homogeneous Catalyst for Asymmertry Catalyzing Aldol Reactions Xiao Wu, Cheng He, Xiang Wu, Siyi Qu and Chunying
Suplementary Data. Gold nanoparticle decorated with a cinchonine organocatalyst: application in the asymmetric α-amination of β-ketoesteres
 Electronic Supplementary Material (ESI) for ew Journal of Chemistry This journal is The Royal Society of Chemistry and The Centre ational de la Recherche Scientifique 2013 Suplementary Data Gold nanoparticle
Electronic Supplementary Material (ESI) for ew Journal of Chemistry This journal is The Royal Society of Chemistry and The Centre ational de la Recherche Scientifique 2013 Suplementary Data Gold nanoparticle
Aluminum Foil: A Highly Efficient and Environment- Friendly Tea Bag Style Catalyst with High TON
 Supporting Information Pd @ Aluminum Foil: A Highly Efficient and Environment- Friendly Tea Bag Style Catalyst with High TON Fan Lei, Yi Rong, Yu Lei,* Wu Yulan, Chen Tian, and Guo Rong General Remarks.
Supporting Information Pd @ Aluminum Foil: A Highly Efficient and Environment- Friendly Tea Bag Style Catalyst with High TON Fan Lei, Yi Rong, Yu Lei,* Wu Yulan, Chen Tian, and Guo Rong General Remarks.
Oxidation of Allylic and Benzylic Alcohols to Aldehydes and Carboxylic Acids
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supporting Information Oxidation of Allylic and Benzylic Alcohols to Aldehydes and Carboxylic Acids
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supporting Information Oxidation of Allylic and Benzylic Alcohols to Aldehydes and Carboxylic Acids
Reduction-free synthesis of stable acetylide cobalamins. Table of Contents. General information. Preparation of compound 1
 Electronic Supporting Information Reduction-free synthesis of stable acetylide cobalamins Mikołaj Chromiński, a Agnieszka Lewalska a and Dorota Gryko* a Table of Contents General information Numbering
Electronic Supporting Information Reduction-free synthesis of stable acetylide cobalamins Mikołaj Chromiński, a Agnieszka Lewalska a and Dorota Gryko* a Table of Contents General information Numbering
Solvent Free Synthesis Of N,N-Diethyl Hydroxyl Amine Using Glycerol-Stabilized Nano TiO2 As An Efficient Catalyst
 Solvent Free Synthesis Of N,N-Diethyl Hydroxyl Amine Using Glycerol-Stabilized Nano TiO2 As An Efficient Catalyst Bahramyadollahi 1, Raminsaeedi 2, Alihassanzadeh 3 Department of Physical Chemistry, Faculty
Solvent Free Synthesis Of N,N-Diethyl Hydroxyl Amine Using Glycerol-Stabilized Nano TiO2 As An Efficient Catalyst Bahramyadollahi 1, Raminsaeedi 2, Alihassanzadeh 3 Department of Physical Chemistry, Faculty
