Supplementary Material for: A Comparison of the chemical sinks of atmospheric organics in the gas and aqueous phase
|
|
|
- Horace Carpenter
- 5 years ago
- Views:
Transcription
1 Supplementary Material for: A Comparison of the chemical sinks of atmospheric organics in the gas and aqueous phase S. A. Epstein and S. A. Nizkorodov, * Department of Chemistry, University of California, Irvine, Irvine, CA, USA Correspondence to: S. A. Nizkorodov (nizkorod@uci.edu) Figure S1. The ratio of the rate of gas phase OH oxidation to the rate of aqueous phase 1 v H oxidation, W, as a function of the dimensionless number, DN LWC R T k 1 10 for the 1
2 series of compounds investigated at 298 K. Upper left panel: daytime OH conditions with LWC = 0.5 g m -3. Upper right panel: nighttime OH conditions with LWC = 0.5 g m -3. Lower left panel: daytime OH conditions with LWC = g m -3. Upper right panel: nighttime OH conditions with LWC = g m -3. The magenta dashed line indicates the transition between gaseous reactions being more important, DN >> 1 and aqueous reactions being more important, DN << 1. Figure S2: Upper bound for the ratio of the rate of gas phase OH oxidation to the rate of gaseous photolysis, Y, as a function of solar zenith angle and altitude for compounds where the absorption cross sections and quantum yields are published as a function of temperature and pressure. Rohrer and Berresheim (Rohrer and Berresheim, 2006) use five field missions to fit their correlation between OH concentration and O 3 photolysis. This analysis uses the maximum predicted OH values from their correlation. 2
3 Figure S3: Lower bound for the ratio of the rate of gas phase OH oxidation to the rate of gaseous photolysis, Y, as a function of solar zenith angle and altitude for compounds where the absorption cross sections and quantum yields are published as a function of temperature and pressure. Rohrer and Berresheim (Rohrer and Berresheim, 2006) use five field missions to fit their correlation between OH concentration and O 3 photolysis. This analysis uses the minimum predicted OH values from their correlation. Table S1: List of aqueous and gas phase rate constants and their references. NAV indicates that data were not available in the literature and NA indicates that the particular value is not applicable to the species of interest. Henry s law constants obtained from theoretical estimations are indicated by an asterisk in the kh Reference column. In cases where measurements exist from multiple researchers and an agreed value was not established, we used the mean of the experimental values. SAR indicates that the given value was predicted theoretically based on structure from (Kwok and Atkinson, 1995). Acid dissociation constants were obtained from (Serjeant and Dempsey, 1979;Perrin et al., 1981;Gligorovski et al., 2009;Perrin, 1965). Hydration equilibrium constants were obtained from (Malik and Joens, 2000;Dong and Dasgupta, 1986;Bell and McDougall, 1960;Nemet et al., 2004;Betterton and Hoffmann, 1988) 3
4 Molecule CAS kh at 298 K [M/atm] kh(t) "B value" kh Reference kohliq (1/s/M) kohliq Anion (1/s/M) kohliq Ref kohgas (mol/cm^3/s) kohgas Ref Gas Photo. Ref formic acid E Sander, S.P E E+09 Ervens E 13 NIST Kinetics NAV acetic acid E Sander, S.P E E+07 Ervens E 13 NIST Kinetics NAV propanoic acid E+03 NAV Sander, R E E+08 Ervens E 12 NIST Kinetics NAV 2 methyl propanoic acid E+03 NAV Sander, R NAV 1.30E+09 Buxton E 12 NIST Kinetics NAV pentanoic acid E Sander, R NAV 2.90E+09 Buxton E 12 SAR NAV 3 methyl butanoic acid E+03 NAV Sander, R E E+09 NIST 8.85E 12 SAR NAV 2,2 dimethyl 1 propanoic acid E+02 NAV Sander, R E E+09 NIST 1.69E 12 SAR NAV hexanoic acid E Sander, R NAV 4.00E+09 Buxton E 12 SAR NAV malonic acid E+08 NAV Sander, R. 1999* 1.60E E+08 NIST 7.19E 13 SAR NAV succinic acid E+08 NAV Sander, R. 1999* 5.00E E+08 Ervens E 12 SAR NAV glyoxal E Sander, S.P E+09 NA Buxton E 12 NIST Kinetics Sander, S.P formaldehyde E Sander, S.P E+09 NA Buxton E 12 Sander, S.P NAV acetaldehyde E Sander, S.P E+09 NA Schuchmann E 11 Sander, S.P NAV methyl glyoxal E+04 NAV Sander, R E+09 NA Ervens E 11 NIST Kinetics Atkinson 2006 propanal E Sander, S.P E+09 NA Mezyk E 11 Sander, S.P Heicklen 1986 / Sander, S.P butanal E Sander, S.P E+09 NA Adams E 11 NIST Kinetics Atkinson 2006 acetone E Sander, S.P E+08 NA Ervens E 13 Sander, S.P Sander, S.P butanone E Sander, R E+08 NA Mezyk E 12 NIST Kinetics Atkinson 2006 malic acid E+13 NAV Sander, R. 1999* 8.20E e8 mono / 8.5e8 di Gligorovski E 11 SAR NAV lactic acid E+07 NAV Sander, R. 1999* 4.30E E+08 NIST/Logan E 12 SAR NAV tartaric acid (R,R) E+18 NAV Sander, R. 1999* 7.00E E+09 NIST/Logan E 11 SAR NAV hydrogen peroxide E Sander, S.P E+07 NA NIST 1.70E 12 Sander, S.P Sander, S.P methyl peroxide E Sander, S.P E+08 NA Monod E 12 Sander, S.P Sander, S.P methyl nitrate E Sander, S.P NAV NA NAV 1.88E 13 NIST Kinetics Sander, S.P dimethyl ether E+00 NAV Sander, R E+09 NA NIST 2.98E 12 Atkinson 1985 NAV methyl tert butyl ether E Sander, R E+09 NA NIST 2.65E 12 Atkinson 1985 NAV diethyl ether E Sander, R E+09 NA NIST 1.34E 11 Atkinson 1985 NAV glycolaldehyde E Sander, R NAV NA NAV 1.10E 11 JPL Sander, S.P glutaraldehyde E+04 NAV Olson 1998 NAV NA NAV 2.375E 11 NIST Kinetics NAV glyceraldehyde E+10 NAV Saxena 1996* NAV NA NAV 4.05E 11 SAR NAV pyruvic acid E Sander, S.P E E+08 Ervens E 13 NIST Kinetics NAV glyoxylic acid E Sander, S.P E E+09 Ervens E 11 SAR NAV ( Mezyk, 1994;Adams et al., 1965;Gligorovski et al., 2009;Linstrom and Mallard;Logan, 1989;Buxton et al., 1997;Monod et al., 2007;Atkinson, 1986;Olson, 1998) (Sander et al., 2011;Sander, 1999;Ervens et al., 2003;Buxton et al., 1988;Ross et al., 2002;Schuchmann and Von Sonntag, 1988;Atkinson et al., 2006;Heicklen et al., 1986) References Adams, G. E., Boag, J. W., Currant, J., and Michael, B. D.: Absolute rate constants for the reaction of the hydroxyl radical with organic compounds, in: Pulse Radiolysis, edited by: Ebert, M., Keene, J. P., Swallow, A. J., and Baxendale, J. H., Academic Press, New York, , Atkinson, R.: Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds under atmospheric conditions, Chem. Rev., 86, , /cr00071a004, Atkinson, R., Baulch, D. L., Cox, R. A., Crowley, J. N., Hampson, R. F., Hynes, R. G., Jenkin, M. E., Rossi, M. J., and Troe, J.: Evaluated kinetic and photochemical data for atmospheric chemistry: Volume II; gas phase reactions of organic species, Atmos. Chem. Phys., 6, , /acp , Bell, R. P., and McDougall, A. O.: Hydration equilibria of some aldehydes and ketones, Trans. Faraday Soc., 56, , Betterton, E. A., and Hoffmann, M. R.: Henry's law constants of some environmentally important aldehydes, Environ. Sci. Technol., 22, , /es00177a004,
5 Buxton, G. V., Greenstock, C. L., Helman, W. P., and Ross, A. B.: Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals in aqueous Solution, J. Phys. Chem. Ref. Data, 17, , Buxton, G. V., Malone, N. T., and Salmon, G. A.: Oxidation of glyoxal initiated by OH in oxygenated aqueous solution, J. Chem. Soc., Faraday Trans., 93, , Dong, S., and Dasgupta, P. K.: Solubility of gaseous formaldehyde in liquid water and generation of trace standard gaseous formaldehyde, Environ. Sci. Technol., 20, , /es00148a016, Ervens, B., Gligorovski, S., and Herrmann, H.: Temperature-dependent rate constants for hydroxyl radical reactions with organic compounds in aqueous solutions, PCCP, 5, , Gligorovski, S., Rousse, D., George, C. H., and Herrmann, H.: Rate constants for the OH reactions with oxygenated organic compounds in aqueous solution, Int. J. Chem. Kinet., 41, , /kin.20405, Heicklen, J., Desai, J., Bahta, A., Harper, C., and Simonaitis, R.: The temperature and wavelength dependence of the photo-oxidation of propionaldehyde, J. Photochem., 34, , Kwok, E. S. C., and Atkinson, R.: Estimation of hydroxyl radical reaction rate constants for gas-phase organic compounds using a structure-reactivity relationship: An update, Atmos. Environ., 29, , Linstrom, P. J., and Mallard, W. G.: NIST Chemical Kinetics Data, NIST Standard Reference Database Number 69., National Institute of Standards and Technology, Gaithersburg, Maryland, , Logan, S. R.: Redox reactions of organic radicals with ferrocene/ferricenium species in aqueous solution. Part 1. Radicals derived from carboxylic acids, J. Chem. Soc., Perkin Trans. 2, , Malik, M., and Joens, J. A.: Temperature dependent near-uv molar absorptivities of glyoxal and gluteraldehyde in aqueous solution, Spectrochim. Acta, Part A, 56, , Mezyk, S. P.: Rate constant and activation energy determination for reaction of e-(aq) and OH with 2-butanone and propanal, Can. J. Chem., 72, , /v94-142, Monod, A., Chevallier, E., Durand Jolibois, R., Doussin, J. F., Picquet-Varrault, B., and Carlier, P.: Photooxidation of methylhydroperoxide and ethylhydroperoxide in the aqueous phase under simulated cloud droplet conditions, Atmos. Environ., 41, , Nemet, I., Vikic-Topic, D., and Varga-Defterdarovic, L.: Spectroscopic studies of methylglyoxal in water and dimethylsulfoxide, Bioorg. Chem., 32, , Olson, J. D.: The vapor pressure of pure and aqueous glutaraldehyde, Fluid Phase Equilib., , , Perrin, D. D.: Dissociation Constands of Organic Bases in Aqueous Solution, Butterworths, London, Perrin, D. D., Dempsey, B., and Serjeant, E. P.: pka Prediction for Organic Acids and Bases, Chapman & Hall, London,
6 Rohrer, F., and Berresheim, H.: Strong correlation between levels of tropospheric hydroxyl radicals and solar ultraviolet radiation, Nature, 442, , Ross, A. B., Mallard, W. G., Helman, W. P., Buxton, G. V., Huie, R. E., and Neta, P.: NDRL- NIST Solution Kinetics Database on the Web, NIST Standard Reference Database 40, Gaithersburg, MD, Sander, R.: Compilation of Henry's Law constants for inorganic and organic species of potential importance in environmental chemistry (Version 3), Sander, S. P., Abbatt, J., Barker, J. R., Burkholder, J. B., Friedl, R. R., Golden, D. M., Huie, R. E., Kolb, C. E., Kurylo, M. J., Moortgat, G. K., Orkin, V. L., Wine, P. H.: Chemical kinetics and photochemical data for use in atmospheric studies: Evaluation number 17, JPL Publication 10-6, Jet Propulsion Laboratory, Pasadena, Schuchmann, M. N., and Von Sonntag, C.: The rapid hydration of the acetyl radical. A pulse radiolysis study of acetaldehyde in aqueous solution, J. Am. Chem. Soc., 110, , /ja00225a019, Serjeant, E. P., and Dempsey, B.: Ionization Constants of Organic Acids in Aqueous Solution, Pergamon, Oxford,
Laboratory simulation for the aqueous OH-oxidation of methyl vinyl ketone and methacrolein: Significance to the in-cloud SOA production
 Laboratory simulation for the aqueous OHoxidation of methyl vinyl ketone and methacrolein: Significance to the incloud SOA production X. Zhang, Z. M. Chen, and Y. Zhao State Key Laboratory of Environmental
Laboratory simulation for the aqueous OHoxidation of methyl vinyl ketone and methacrolein: Significance to the incloud SOA production X. Zhang, Z. M. Chen, and Y. Zhao State Key Laboratory of Environmental
Supplement of Inversion of and
 Supplement of Inversion of and emissions using the adjoint of the IMAGES model J.-F. Müller and T. Stavrakou Belgian Institute for Space Aeronomy, Brussels, Belgium Abstract This supplement contains three
Supplement of Inversion of and emissions using the adjoint of the IMAGES model J.-F. Müller and T. Stavrakou Belgian Institute for Space Aeronomy, Brussels, Belgium Abstract This supplement contains three
Supplement of SOA formation from the photooxidation of α-pinene: systematic exploration of the simulation of chamber data
 Supplement of Atmos. Chem. Phys., 16, 278 282, 216 http://www.atmos-chem-phys.net/16/278/216/ doi:1.194/acp-16-278-216-supplement Author(s) 216. CC Attribution 3. License. Supplement of SA formation from
Supplement of Atmos. Chem. Phys., 16, 278 282, 216 http://www.atmos-chem-phys.net/16/278/216/ doi:1.194/acp-16-278-216-supplement Author(s) 216. CC Attribution 3. License. Supplement of SA formation from
Supplemental material
 Supplemental material Supplemental discussion of internal and its possible removal by C 3 F 6 addition chem represents the real and wave-chem represents an instrument interference only if no internal is
Supplemental material Supplemental discussion of internal and its possible removal by C 3 F 6 addition chem represents the real and wave-chem represents an instrument interference only if no internal is
3- Figure 5 could be modified in a much more useful way (see detailed comments) We incorporated your suggestions regarding modification of Figure 5.
 On behalf of all of the co-authors, I would like to thank all of the reviewers for their extremely thoughtful and comprehensive reviews. Unless specifically indicated, table and figure numbers refer to
On behalf of all of the co-authors, I would like to thank all of the reviewers for their extremely thoughtful and comprehensive reviews. Unless specifically indicated, table and figure numbers refer to
Electronic supplementary information to Heterogeneous OH oxidation. of biomass burning organic aerosol surrogate compounds: Assessment
 Electronic supplementary information to Heterogeneous OH oxidation of biomass burning organic aerosol surrogate compounds: Assessment of volatilisation products and the role of OH concentration on the
Electronic supplementary information to Heterogeneous OH oxidation of biomass burning organic aerosol surrogate compounds: Assessment of volatilisation products and the role of OH concentration on the
A SPARC Success Story: The Role of Halogen Chemistry in Polar Stratospheric Ozone Depletion
 A SPARC Success Story: The Role of Halogen Chemistry in Polar Stratospheric Ozone Depletion An Update on the Initiative Sponsored by the Stratospheric Processes and their Role in Climate (SPARC) Project
A SPARC Success Story: The Role of Halogen Chemistry in Polar Stratospheric Ozone Depletion An Update on the Initiative Sponsored by the Stratospheric Processes and their Role in Climate (SPARC) Project
Reference M atm -1 H / R,
 Table 1a: Henry's Law Constants of halogen containing compounds Species K H 298, M atm 1 H / R, K 1 BrO 48 K H 1 =K H 4 2 ClO 926 K H 2 =K H 5 3 HBr 0.72 6077 Sander and Crutzen, 1996 4 HOBr 48 Sander
Table 1a: Henry's Law Constants of halogen containing compounds Species K H 298, M atm 1 H / R, K 1 BrO 48 K H 1 =K H 4 2 ClO 926 K H 2 =K H 5 3 HBr 0.72 6077 Sander and Crutzen, 1996 4 HOBr 48 Sander
A New Mechanism for Regional Atmospheric Chemistry Modelling. A contribution to subproject CMD. W.R. Stockwell', F Kirchner^ M. Kuhn' and S.
 A New Mechanism for Regional Atmospheric Chemistry Modelling A contribution to subproject CMD W.R. Stockwell', F Kirchner^ M. Kuhn' and S. Seefeld* *Fraunhofer Institute for Atmospheric Environmental Research
A New Mechanism for Regional Atmospheric Chemistry Modelling A contribution to subproject CMD W.R. Stockwell', F Kirchner^ M. Kuhn' and S. Seefeld* *Fraunhofer Institute for Atmospheric Environmental Research
1) Which type of compound does not contain a carbonyl group? A) ketone B) aldehyde C) amine D) ester E) carboxylic acid
 1) Which type of compound does not contain a carbonyl group? ketone aldehyde amine ester carboxylic acid 2) Which functional group contains a carbonyl group and a hydroxyl group bonded to the same carbon
1) Which type of compound does not contain a carbonyl group? ketone aldehyde amine ester carboxylic acid 2) Which functional group contains a carbonyl group and a hydroxyl group bonded to the same carbon
Hydroperoxyl radical (HO 2
 GEOPHYSICAL RESEARCH LETTERS, VOL 30, NO 24, 2297, doi:101029/2003gl018572, 2003 Hydroperoxyl radical (HO 2 ) oxidizes dibromide radical anion ( 2 )to bromine (Br 2 ) in aqueous solution: Implications
GEOPHYSICAL RESEARCH LETTERS, VOL 30, NO 24, 2297, doi:101029/2003gl018572, 2003 Hydroperoxyl radical (HO 2 ) oxidizes dibromide radical anion ( 2 )to bromine (Br 2 ) in aqueous solution: Implications
Atmospheric Fate of Unsubstituted Alkoxy and Carbonyl Radicals
 Atmospheric Fate of Unsubstituted Alkoxy and Carbonyl Radicals O. Shestakov, S. Jagiella, J. Theloke, H. G. Libuda, and F. Zabel Institut für Physikalische Chemie, Universität Stuttgart, Pfaffenwaldring
Atmospheric Fate of Unsubstituted Alkoxy and Carbonyl Radicals O. Shestakov, S. Jagiella, J. Theloke, H. G. Libuda, and F. Zabel Institut für Physikalische Chemie, Universität Stuttgart, Pfaffenwaldring
Preliminary report: Analyses of tcfp s potential impact on atmospheric ozone
 Preliminary report: Analyses of tcfp s potential impact on atmospheric ozone Dong Wang, Seth Olsen, and Donald Wuebbles Department of Atmospheric Sciences University of Illinois, Urbana, IL 61801 Abstract
Preliminary report: Analyses of tcfp s potential impact on atmospheric ozone Dong Wang, Seth Olsen, and Donald Wuebbles Department of Atmospheric Sciences University of Illinois, Urbana, IL 61801 Abstract
An alcohol is a compound obtained by substituting a hydoxyl group ( OH) for an H atom on a carbon atom of a hydrocarbon group.
 Derivatives of Hydrocarbons A functional group is a reactive portion of a molecule that undergoes predictable reactions. All other organic compounds can be considered as derivatives of hydrocarbons (i.e.,
Derivatives of Hydrocarbons A functional group is a reactive portion of a molecule that undergoes predictable reactions. All other organic compounds can be considered as derivatives of hydrocarbons (i.e.,
CAPRAM MODELLING OF THE PHYSICO-CHEMICAL CLOUD PROCESSING OF TROPOSPHERIC AEROSOLS
 CAPRAM MODELLING OF THE PHYSICO-CHEMICAL CLOUD PROCESSING OF TROPOSPHERIC AEROSOLS A. Tilgner 1 *, R. Wolke 1 and H. Herrmann 1 1 Leibniz-Institut für Troposphärenforschung, Permoserstr. 15, D-04318 Leipzig,
CAPRAM MODELLING OF THE PHYSICO-CHEMICAL CLOUD PROCESSING OF TROPOSPHERIC AEROSOLS A. Tilgner 1 *, R. Wolke 1 and H. Herrmann 1 1 Leibniz-Institut für Troposphärenforschung, Permoserstr. 15, D-04318 Leipzig,
Appendix B: Aqueous chemistry and gas-phase halogen chemistry
 1 Appendix B: Aqueous chemistry and gasphase halogen chemistry SANFORD SILLMAN, FRANK MARSIK, KHALID I. ALWALI, GERALD J. KEELER AND MATTHEW S. LANDIS* Department of Atmospheric, Oceanic and Space Sciences
1 Appendix B: Aqueous chemistry and gasphase halogen chemistry SANFORD SILLMAN, FRANK MARSIK, KHALID I. ALWALI, GERALD J. KEELER AND MATTHEW S. LANDIS* Department of Atmospheric, Oceanic and Space Sciences
Sally E. Pusede, Trevor C. VandenBoer, Jennifer G. Murphy, Milos Z. Markovic, Cora J. Young,
 SUPPLEMENTAL INFORMATION for An Atmospheric Constraint on the NO 2 Dependence of Daytime Near- Surface Nitrous Acid (HONO) Sally E. Pusede, Trevor C. VandenBoer, Jennifer G. Murphy, Milos Z. Markovic,
SUPPLEMENTAL INFORMATION for An Atmospheric Constraint on the NO 2 Dependence of Daytime Near- Surface Nitrous Acid (HONO) Sally E. Pusede, Trevor C. VandenBoer, Jennifer G. Murphy, Milos Z. Markovic,
Experiment 7 Aldehydes, Ketones, and Carboxylic Acids
 Experiment 7 Aldehydes, Ketones, and arboxylic Acids Aldehydes and ketones are molecules that contain a carbonyl group, which is an oxygen atom with a double bond to a carbon atom. In an aldehyde, the
Experiment 7 Aldehydes, Ketones, and arboxylic Acids Aldehydes and ketones are molecules that contain a carbonyl group, which is an oxygen atom with a double bond to a carbon atom. In an aldehyde, the
BIOB111_CHBIO - Tutorial activities for session 9
 BIOB111_CHBIO - Tutorial activities for session 9 General topics for week 5 Session 9 Physical properties and chemical reactions of organic compounds (functional groups: alcohols, phenols, ethers, aldehydes,
BIOB111_CHBIO - Tutorial activities for session 9 General topics for week 5 Session 9 Physical properties and chemical reactions of organic compounds (functional groups: alcohols, phenols, ethers, aldehydes,
R. F. Hansen et al. Correspondence to: R. F. Hansen
 Supplement of Atmos. Meas. Tech., 8, 4243 4264, 2015 http://www.atmos-meas-tech.net/8/4243/2015/ doi:10.5194/amt-8-4243-2015-supplement Author(s) 2015. CC Attribution 3.0 License. Supplement of Intercomparison
Supplement of Atmos. Meas. Tech., 8, 4243 4264, 2015 http://www.atmos-meas-tech.net/8/4243/2015/ doi:10.5194/amt-8-4243-2015-supplement Author(s) 2015. CC Attribution 3.0 License. Supplement of Intercomparison
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch16_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which type of compound does not contain a carbonyl group? ketone B) aldehyde C) amine D)
Ch16_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which type of compound does not contain a carbonyl group? ketone B) aldehyde C) amine D)
Tropospheric OH chemistry
 Tropospheric OH chemistry CO Oxidation mechanism: CO + OH CO 2 + H, H + O 2 + M HO 2 + M, HO 2 + NO OH + NO 2 NO 2 + hν (+O 2 ) NO + O 3 Initiation step Propagation Net: CO + 2 O 2 CO 2 + O 3 HO 2 + HO
Tropospheric OH chemistry CO Oxidation mechanism: CO + OH CO 2 + H, H + O 2 + M HO 2 + M, HO 2 + NO OH + NO 2 NO 2 + hν (+O 2 ) NO + O 3 Initiation step Propagation Net: CO + 2 O 2 CO 2 + O 3 HO 2 + HO
Tananyag fejlesztés idegen nyelven
 Tananyag fejlesztés idegen nyelven Prevention of the atmosphere KÖRNYEZETGAZDÁLKODÁSI AGRÁRMÉRNÖKI MSC (MSc IN AGRO-ENVIRONMENTAL STUDIES) Fundamentals to atmospheric chemical reactions. The stratospheric
Tananyag fejlesztés idegen nyelven Prevention of the atmosphere KÖRNYEZETGAZDÁLKODÁSI AGRÁRMÉRNÖKI MSC (MSc IN AGRO-ENVIRONMENTAL STUDIES) Fundamentals to atmospheric chemical reactions. The stratospheric
Carbonyl Group in Aldehydes and Ketones
 Lecture 4: Aldehydes, Ketones, and Chiral Molecules 14.1 Aldehydes and Ketones Carbonyl Group in Aldehydes and Ketones A carbonyl group (C=) In an aldehyde is attached to at least one atom. In a ketone
Lecture 4: Aldehydes, Ketones, and Chiral Molecules 14.1 Aldehydes and Ketones Carbonyl Group in Aldehydes and Ketones A carbonyl group (C=) In an aldehyde is attached to at least one atom. In a ketone
Conditions of the Simulations
 Conditions of the Simulations Location: 44.4N, 73.9W, z = 1.5 km, Whiteface Mountain (WFM) summit Start time: 1730 (5:30 pm) local time September 17, 2016 End time: 1500 (3 pm) local time September 18,
Conditions of the Simulations Location: 44.4N, 73.9W, z = 1.5 km, Whiteface Mountain (WFM) summit Start time: 1730 (5:30 pm) local time September 17, 2016 End time: 1500 (3 pm) local time September 18,
Influence of Biogenic VOCs on Photooxidant Formation: Simulation Experiments in EUPHORE and Comparison with Model Calculations
 Introduction Influence of Biogenic VOCs on Photooxidant Formation: Simulation Experiments in EUPHORE and Comparison with Model Calculations Fraunhofer Institut Atmosphärische Umweltforschung, IFU Kreuzeckbahnstr.
Introduction Influence of Biogenic VOCs on Photooxidant Formation: Simulation Experiments in EUPHORE and Comparison with Model Calculations Fraunhofer Institut Atmosphärische Umweltforschung, IFU Kreuzeckbahnstr.
Aromatic Hydrocarbons
 Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
Supporting Information for: Activation of Peroxymonosulfate by Benzoquinone: A. Novel Nonradical Oxidation Process
 Supporting Information for: Activation of Peroxymonosulfate by Benzoquinone: A Novel Nonradical Oxidation Process Yang Zhou, Jin Jiang*,, Yuan Gao, Jun Ma*,, Su-Yan Pang, Juan Li, Xue-ing Lu, Li-Peng Yuan
Supporting Information for: Activation of Peroxymonosulfate by Benzoquinone: A Novel Nonradical Oxidation Process Yang Zhou, Jin Jiang*,, Yuan Gao, Jun Ma*,, Su-Yan Pang, Juan Li, Xue-ing Lu, Li-Peng Yuan
Supplement of Summertime OH reactivity from a receptor coastal site in the Mediterranean Basin
 Supplement of Atmos. Chem. Phys., 17, 12645 12658, 2017 https://doi.org/10.5194/acp-17-12645-2017-supplement Author(s) 2017. This work is distributed under the Creative Commons Attribution 3.0 License.
Supplement of Atmos. Chem. Phys., 17, 12645 12658, 2017 https://doi.org/10.5194/acp-17-12645-2017-supplement Author(s) 2017. This work is distributed under the Creative Commons Attribution 3.0 License.
14.1 Aldehydes and Ketones Copyright 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings
 Chapter 14 Aldehydes, Ketones, and Chiral Molecules 14.1 Aldehydes and Ketones Copyright 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings Carbonyl Group in Aldehydes A carbonyl group and
Chapter 14 Aldehydes, Ketones, and Chiral Molecules 14.1 Aldehydes and Ketones Copyright 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings Carbonyl Group in Aldehydes A carbonyl group and
FUNCTIONAL GROUPS Functional Group Suffix Formula Other Info O. Ester. Amide --- R C N R' or R(CO)NR R
 EMISTRY 10 elp Sheet # rganic (Part III hapters.7 (condensed, structural drawings, 6.3 (line drawings, 6.9a (benzene, 7.e (hybrid orbitals in organic structures, and Appendix E (functional groups Do topics
EMISTRY 10 elp Sheet # rganic (Part III hapters.7 (condensed, structural drawings, 6.3 (line drawings, 6.9a (benzene, 7.e (hybrid orbitals in organic structures, and Appendix E (functional groups Do topics
Nomenclature of Organic Compounds Identification of Functional Groups
 Hydrocarbons Nomenclature of Organic ompounds Identification of Functional Groups Alkanes - also known as saturated hydrocarbons or the paraffin series because all bond sites between carbon atoms and between
Hydrocarbons Nomenclature of Organic ompounds Identification of Functional Groups Alkanes - also known as saturated hydrocarbons or the paraffin series because all bond sites between carbon atoms and between
Supporting Information
 Supporting Information Imidazolium-based ionic liquids in water: Assessment of photocatalytic and photochemical transformation Paola Calza a, Davide Vione a,* Debora Fabbri a, Riccardo Aigotti b, and Claudio
Supporting Information Imidazolium-based ionic liquids in water: Assessment of photocatalytic and photochemical transformation Paola Calza a, Davide Vione a,* Debora Fabbri a, Riccardo Aigotti b, and Claudio
Rapid Aqueous-phase Photooxidation of Dimers in α-pinene SOA
 Supporting Information: Rapid Aqueous-phase Photooxidation of Dimers in α-pinene SOA Ran Zhao 1,ǂ*, Dana Aljawhary 1,*, Alex K.Y. Lee 1,±, and Jonathan P.D. Abbatt 1 1 Department of Chemistry, University
Supporting Information: Rapid Aqueous-phase Photooxidation of Dimers in α-pinene SOA Ran Zhao 1,ǂ*, Dana Aljawhary 1,*, Alex K.Y. Lee 1,±, and Jonathan P.D. Abbatt 1 1 Department of Chemistry, University
Daisuke Minakata and John Crittenden
 Linear Free Energy Relationships for the Aqueous Phase Hydroxyl Radical Reactions with Ionized Species: Experimental and Theoretical Studies ACS Boston Fall Meeting August. nd, 00 Daisuke Minakata and
Linear Free Energy Relationships for the Aqueous Phase Hydroxyl Radical Reactions with Ionized Species: Experimental and Theoretical Studies ACS Boston Fall Meeting August. nd, 00 Daisuke Minakata and
Ultrafast electron transfer studied by picosecond pulse radiolysis, new redox reactions. Mehran Mostafavi, April 2017, Vienna, Austria H 2 O * H 2 O
 Ultrafast electron transfer studied by picosecond pulse radiolysis, new redox reactions Mehran Mostafavi, April 2017, Vienna, Austria e hyd H 3 O + H 2 O * H 2 O H 2 O H 2 O + S e 1 Mostafavi, AIEA, April
Ultrafast electron transfer studied by picosecond pulse radiolysis, new redox reactions Mehran Mostafavi, April 2017, Vienna, Austria e hyd H 3 O + H 2 O * H 2 O H 2 O H 2 O + S e 1 Mostafavi, AIEA, April
Aqueous-Phase Chemistry in TM4-ECPL: SOA Formation via Cloud Processes Stelios Myriokefalitakis 1 Kostas Tsigaridis 2,3 Maria Kanakidou 1
 TM meeting, Heraklion, June 2010 Aqueous-Phase Chemistry in TM4-ECPL: SOA Formation via Cloud Processes Stelios Myriokefalitakis 1 Kostas Tsigaridis 2,3 Maria Kanakidou 1 1 Environmental Chemical Processes
TM meeting, Heraklion, June 2010 Aqueous-Phase Chemistry in TM4-ECPL: SOA Formation via Cloud Processes Stelios Myriokefalitakis 1 Kostas Tsigaridis 2,3 Maria Kanakidou 1 1 Environmental Chemical Processes
Chemistry 110. Bettelheim, Brown, Campbell & Farrell. Ninth Edition
 Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, rganic and Biochemistry Chapter 14 Alcohols, Ethers and Thiols Alcohols have a ydroxyl Group, -, bonded to tetrahedral
Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, rganic and Biochemistry Chapter 14 Alcohols, Ethers and Thiols Alcohols have a ydroxyl Group, -, bonded to tetrahedral
HW #5: 16.20, 16.28, 16.30, 16.32, 16.40, 16.44, 16.46, 16.52, 16.60, 16.62, 16.64, 16.68
 hemistry 131 Lecture 10: Aldehydes and Ketones: Structure, Nomenclature, Physical Properties, and Reactivity hapter 16 in McMurry, Ballantine, et. al. 7 th edition W #5: 16.20, 16.28, 16.30, 16.32, 16.40,
hemistry 131 Lecture 10: Aldehydes and Ketones: Structure, Nomenclature, Physical Properties, and Reactivity hapter 16 in McMurry, Ballantine, et. al. 7 th edition W #5: 16.20, 16.28, 16.30, 16.32, 16.40,
Class: 12 Subject: chemistry Topic: Organic Chemistry of O compounds No. of Questions: 20 Duration: 60 Min Maximum Marks: 60
 Class: 12 Subject: chemistry Topic: Organic Chemistry of O compounds No. of Questions: 20 Duration: 60 Min Maximum Marks: 60 1. Ethylene is passed through conc. H 2 SO 4 and the product obtained is diluted
Class: 12 Subject: chemistry Topic: Organic Chemistry of O compounds No. of Questions: 20 Duration: 60 Min Maximum Marks: 60 1. Ethylene is passed through conc. H 2 SO 4 and the product obtained is diluted
Jenny A. Fisher et al. Correspondence to: Jenny A. Fisher
 Supplement of Atmos. Chem. Phys., 6, 5969 599, 6 http://www.atmos-chem-phys.net/6/5969/6/ doi:.59/acp-6-5969-6-supplement Author(s) 6. CC Attribution. License. Supplement of Organic nitrate chemistry and
Supplement of Atmos. Chem. Phys., 6, 5969 599, 6 http://www.atmos-chem-phys.net/6/5969/6/ doi:.59/acp-6-5969-6-supplement Author(s) 6. CC Attribution. License. Supplement of Organic nitrate chemistry and
Atmospheric Chemistry Chlorine Chemistry Final report
 Atmospheric Chemistry Chlorine Chemistry Final report Tel-Tek report no. 2211030-CC07 v2 Claus Jørgen Nielsen 08.11.2011 Tel-Tek Kjølnes ring 30 NO-3918 Porsgrunn NORWAY CONTENTS 1 Scope of services...
Atmospheric Chemistry Chlorine Chemistry Final report Tel-Tek report no. 2211030-CC07 v2 Claus Jørgen Nielsen 08.11.2011 Tel-Tek Kjølnes ring 30 NO-3918 Porsgrunn NORWAY CONTENTS 1 Scope of services...
II. 1. TRANSFORMATIONS UNDER THE ACTION OF HEAT OR IRRADIATION
 CHAPTER II HYDROCARBON TRANSFORMATIONS THAT DO NOT INVOLVE METALS OR THEIR COMPOUNDS n this chapter we will briefly survey the main types of hydrocarbon transformations that occur without the participation
CHAPTER II HYDROCARBON TRANSFORMATIONS THAT DO NOT INVOLVE METALS OR THEIR COMPOUNDS n this chapter we will briefly survey the main types of hydrocarbon transformations that occur without the participation
ATM 507 Lecture 5. Text reading Chapter 4 Problem Set #2 due Sept. 20 Today s topics Photochemistry and Photostationary State Relation
 ATM 507 Lecture 5 Text reading Chapter 4 Problem Set #2 due Sept. 20 Today s topics Photochemistry and Photostationary State Relation Beer-Lambert Law (for the absorption of light) Used to describe the
ATM 507 Lecture 5 Text reading Chapter 4 Problem Set #2 due Sept. 20 Today s topics Photochemistry and Photostationary State Relation Beer-Lambert Law (for the absorption of light) Used to describe the
Supporting Information
 Supporting Information Reactive processing of formaldehyde and acetaldehyde in aqueous aerosol mimics: Surface tension depression and secondary organic products Zhi Li, Allison N. Schwier, Neha Sareen,
Supporting Information Reactive processing of formaldehyde and acetaldehyde in aqueous aerosol mimics: Surface tension depression and secondary organic products Zhi Li, Allison N. Schwier, Neha Sareen,
M-RICh v0.5 MATLAB Rate Integrator for Chemical equations
 M-RICh v0.5 MATLAB Rate Integrator for Chemical equations Abstract: Accurately simulating the chemical conditions within a reactor tests both the stability and accuracy of a numerical scheme. M-RICh is
M-RICh v0.5 MATLAB Rate Integrator for Chemical equations Abstract: Accurately simulating the chemical conditions within a reactor tests both the stability and accuracy of a numerical scheme. M-RICh is
Functional Group Adsorption on Calcite: I. Oxygen Containing and Nonpolar Organic Molecules
 SUPPORTING INFORMATION Functional Group Adsorption on Calcite: I. Oxygen Containing and Nonpolar Organic Molecules E. Ataman*, M. P. Andersson, M. Ceccato, N. Bovet, S. L. S. Stipp Nano-Science Center,
SUPPORTING INFORMATION Functional Group Adsorption on Calcite: I. Oxygen Containing and Nonpolar Organic Molecules E. Ataman*, M. P. Andersson, M. Ceccato, N. Bovet, S. L. S. Stipp Nano-Science Center,
Homework Assignment 2 ATM 507 Fall 2014
 Due Tuesday, September 30th Homework Assignment ATM 507 Fall 014 1. Calculate H for the following reactions. Express your answer in kj/mole and kcal/mole: i) NO NO + O( 3 P) ii) NO + O 3 NO + O iii) H
Due Tuesday, September 30th Homework Assignment ATM 507 Fall 014 1. Calculate H for the following reactions. Express your answer in kj/mole and kcal/mole: i) NO NO + O( 3 P) ii) NO + O 3 NO + O iii) H
DEVELOPMENT AND EVALUATION OF THE SAPRC-99 CHEMICAL MECHANISM
 DEVELOPMENT AND EVALUATION OF THE SAPRC-99 CHEMICAL MECHANISM W. P. L. Carter Air Pollution Research Center University of California, Riverside, CA 92521 ALL MECHANISMS DESIGN OBJECTIVES CAN BE USED IN
DEVELOPMENT AND EVALUATION OF THE SAPRC-99 CHEMICAL MECHANISM W. P. L. Carter Air Pollution Research Center University of California, Riverside, CA 92521 ALL MECHANISMS DESIGN OBJECTIVES CAN BE USED IN
Chemistry 110. Bettelheim, Brown, Campbell & Farrell. Ninth Edition
 hemistry 110 Bettelheim, Brown, ampbell & Farrell Ninth Edition Introduction to General, rganic and Biochemistry hapter 17 Aldehydes & Ketones hemistry of the arbonyl Group Aldehydes & Ketones The functional
hemistry 110 Bettelheim, Brown, ampbell & Farrell Ninth Edition Introduction to General, rganic and Biochemistry hapter 17 Aldehydes & Ketones hemistry of the arbonyl Group Aldehydes & Ketones The functional
On the Photolysis of simple Anions and neutral Molecules as Sources of O-/ OH, SOx- and Cl in Aqueous Solution
 1 Electronic Supplementary Material (ESM) to On the Photolysis of simple Anions and neutral Molecules as Sources of O/ OH, SOx and Cl in Aqueous Solution Hartmut Herrmann LeibnizInstitut für Troposphärenforschung
1 Electronic Supplementary Material (ESM) to On the Photolysis of simple Anions and neutral Molecules as Sources of O/ OH, SOx and Cl in Aqueous Solution Hartmut Herrmann LeibnizInstitut für Troposphärenforschung
Chem 261 Dec 6, 2017
 209 Chem 261 Dec 6, 2017 REVIEW: Example: K!! + 3 C + 3 C K tert-butoxide (an alkoxide) methanol tert-butanol pka = 16 pka = 19 methoxide stronger base stronger acid (lower pka, more acidic) weaker acid
209 Chem 261 Dec 6, 2017 REVIEW: Example: K!! + 3 C + 3 C K tert-butoxide (an alkoxide) methanol tert-butanol pka = 16 pka = 19 methoxide stronger base stronger acid (lower pka, more acidic) weaker acid
TOWARDS A MORE DETAILED DESCRIPTION OF TROPOSPHERIC AQUEOUS PHASE ORGANIC CHEMISTRY: CAPRAM 3.0 ELECTRONIC SUPPLEMENTAL MATERIAL (ESM)
 TWARDS A MRE DETAILED DESRIPTIN F TRPSPERI AQUEUS PASE RGANI EMISTRY: APRAM 30 errmann 1 *, A Tilgner 1, P Barzaghi 1, Z Majdik 1, S Gligorovski 1, L Poulain 2 and A Monod 2 1 Leibniz Institut für Troposphärenforschung,
TWARDS A MRE DETAILED DESRIPTIN F TRPSPERI AQUEUS PASE RGANI EMISTRY: APRAM 30 errmann 1 *, A Tilgner 1, P Barzaghi 1, Z Majdik 1, S Gligorovski 1, L Poulain 2 and A Monod 2 1 Leibniz Institut für Troposphärenforschung,
Development and Evaluation of an Updated Detailed Chemical Mechanism for VOC Reactivity Assessment
 Development and Evaluation of an Updated Detailed Chemical Mechanism for VOC Reactivity Assessment William P. L. Carter Air Pollution Research Center and College of Engineering Center for Environmental
Development and Evaluation of an Updated Detailed Chemical Mechanism for VOC Reactivity Assessment William P. L. Carter Air Pollution Research Center and College of Engineering Center for Environmental
Lecture 2. The framework to build materials and understand properties
 Lecture 2 The framework to build materials and understand properties 1 Trees are made into a solid materials/structures in an environment that consists of small molecules: CO 2, N 2, H 2 0, CH 4 O C 2.58Ǻ
Lecture 2 The framework to build materials and understand properties 1 Trees are made into a solid materials/structures in an environment that consists of small molecules: CO 2, N 2, H 2 0, CH 4 O C 2.58Ǻ
Abstract. 1 Introduction
 The influence of cloud chemistry on the budget of photo-oxidants G. Mauersberger Brandenberg Technical University Cottbus, Rudower Chausse 5, D-12489 Berlin, Germany Abstract The RADM II gas-phase system
The influence of cloud chemistry on the budget of photo-oxidants G. Mauersberger Brandenberg Technical University Cottbus, Rudower Chausse 5, D-12489 Berlin, Germany Abstract The RADM II gas-phase system
CONTENTS 1 MEASURES OF ATMOSPHERIC COMPOSITION
 i CONTENTS 1 MEASURES OF ATMOSPHERIC COMPOSITION 1 1.1 MIXING RATIO 1 1.2 NUMBER DENSITY 2 1.3 PARTIAL PRESSURE 6 PROBLEMS 10 1.1 Fog formation 10 1.2 Phase partitioning of water in cloud 10 1.3 The ozone
i CONTENTS 1 MEASURES OF ATMOSPHERIC COMPOSITION 1 1.1 MIXING RATIO 1 1.2 NUMBER DENSITY 2 1.3 PARTIAL PRESSURE 6 PROBLEMS 10 1.1 Fog formation 10 1.2 Phase partitioning of water in cloud 10 1.3 The ozone
Supplementary Material. compounds the atmospheric oxidation mechanism for 8:2 FTOH shown in Table 1 was
 Supplementary Material From the available kinetic and mechanistic data for 8:2 FTOH and analogous compounds the atmospheric oxidation mechanism for 8:2 FTOH shown in Table 1 was constructed. For convenience,
Supplementary Material From the available kinetic and mechanistic data for 8:2 FTOH and analogous compounds the atmospheric oxidation mechanism for 8:2 FTOH shown in Table 1 was constructed. For convenience,
Measurement Techniques Formation of aqueous-phase α-hydroxyhydroperoxides (α-hhp): potential atmospheric impacts
 Sciences ess doi:10.5194/acp-13-5857-2013 Author(s) 2013. CC Attribution 3.0 License. Atmospheric Chemistry and Physics Atmospheric Measurement Techniques Formation of aqueous-phase α-hydroxyhydroperoxides
Sciences ess doi:10.5194/acp-13-5857-2013 Author(s) 2013. CC Attribution 3.0 License. Atmospheric Chemistry and Physics Atmospheric Measurement Techniques Formation of aqueous-phase α-hydroxyhydroperoxides
Modeling of the formation of short-chain acids in propane flames
 Modeling of the formation of short-chain acids in propane flames F. Battin-Leclerc, 1, A.A. Konnov 2, J.L. Jaffrezo 3 and M. Legrand 3 1 Département de Chimie-Physique des Réactions, CNRS-INPL, Nancy,
Modeling of the formation of short-chain acids in propane flames F. Battin-Leclerc, 1, A.A. Konnov 2, J.L. Jaffrezo 3 and M. Legrand 3 1 Département de Chimie-Physique des Réactions, CNRS-INPL, Nancy,
CHEMICAL KINETICS C.H. BAMFORD C.F.H. TIPPER WSSSKUH EDITED BY
 CHEMICAL KINETICS EDITED BY C.H. BAMFORD M.A., Ph.D., Sc.D. (Cantab.), F.R.I.C., F.R.S. Campbell-Brown Professor of Industrial Chemistry, Uniuersity of Liverpool AND C.F.H. TIPPER Ph.D. (Bristol), D.Sc.
CHEMICAL KINETICS EDITED BY C.H. BAMFORD M.A., Ph.D., Sc.D. (Cantab.), F.R.I.C., F.R.S. Campbell-Brown Professor of Industrial Chemistry, Uniuersity of Liverpool AND C.F.H. TIPPER Ph.D. (Bristol), D.Sc.
Radiolysis of Aqueous Ethanol in the Presence of CO
 Radiolysis of Aqueous Ethanol in the Presence of CO Hyoung-Ryun Park * and Nikola Getoff Institut für Theoretische Chemie und Strahlenchemie der Universität Wien und Ludwig-Boltzmann-Institut für Strahlenchemie
Radiolysis of Aqueous Ethanol in the Presence of CO Hyoung-Ryun Park * and Nikola Getoff Institut für Theoretische Chemie und Strahlenchemie der Universität Wien und Ludwig-Boltzmann-Institut für Strahlenchemie
Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes
 DISTRIBUTION STATEMENT A. Approved for public release; distribution is unlimited. Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes John H. Seinfeld California Institute of Technology,
DISTRIBUTION STATEMENT A. Approved for public release; distribution is unlimited. Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes John H. Seinfeld California Institute of Technology,
Section 3 Environmental Chemistry
 Section 3 Environmental Chemistry 1 Environmental Chemistry Definitions Chemical Reactions Stoichiometry Photolytic Reactions Enthalpy and Heat of Reaction Chemical Equilibria ph Solubility Carbonate Systems
Section 3 Environmental Chemistry 1 Environmental Chemistry Definitions Chemical Reactions Stoichiometry Photolytic Reactions Enthalpy and Heat of Reaction Chemical Equilibria ph Solubility Carbonate Systems
49 56 (8 Q's) Solutions YOU WILL SKIP THIS SECTION ENTIRELY (8 Q's) Organic Chemistry 12 none
 ACS Standardized Exam for CHM 122 Breakdown of Questions by Topic Question # Topic Covered Problem Set Section in ACS Book 1 12 (12 Q's) Kinetics 1, 2 Dynamics 13 24 (12 Q's) Equilibrium 3, 4, 5, 6, 7
ACS Standardized Exam for CHM 122 Breakdown of Questions by Topic Question # Topic Covered Problem Set Section in ACS Book 1 12 (12 Q's) Kinetics 1, 2 Dynamics 13 24 (12 Q's) Equilibrium 3, 4, 5, 6, 7
Chapter 23 Aldehydes and Ketones
 Chapter 23 Aldehydes and Ketones Ketones are common solvents for quickdrying paints. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris Hein, Scott Pattison, and Susan
Chapter 23 Aldehydes and Ketones Ketones are common solvents for quickdrying paints. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris Hein, Scott Pattison, and Susan
Received 8 November 2012; revised 19 December 2012; accepted 24 December 2012; published 31 January 2013.
 GEOPHYSICAL RESEARCH LETTERS, VOL. 40, 464 469, doi:10.1002/grl.50121, 2013 Revised UV absorption spectra, ozone depletion potentials, and global warming potentials for the ozone-depleting substances CF
GEOPHYSICAL RESEARCH LETTERS, VOL. 40, 464 469, doi:10.1002/grl.50121, 2013 Revised UV absorption spectra, ozone depletion potentials, and global warming potentials for the ozone-depleting substances CF
1. What is the major organic product obtained from the following sequence of reactions?
 CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
ENVIRONMENTAL STRUCTURE AND FUNCTION: EARTH SYSTEM - Chemistry Of The Atmosphere - I.L. Karol and A.A. Kiselev
 CHEMISTRY OF THE ATMOSPHERE I.L. Karol and A.A. Main Geophysical Observatory, St. Petersburg, Russia Keywords: Atmospheric composition, gas phase reactions, heterogeneous reactions, catalytic cycles, lifetime
CHEMISTRY OF THE ATMOSPHERE I.L. Karol and A.A. Main Geophysical Observatory, St. Petersburg, Russia Keywords: Atmospheric composition, gas phase reactions, heterogeneous reactions, catalytic cycles, lifetime
Peter J. Gallimore et al. Correspondence to: Markus Kalberer
 Supplement of Atmos. Chem. Phys., 17, 983 9868, 2017 https://doi.org/.194/acp-17-983-2017-supplement Author(s) 2017. This work is distributed under the Creative Commons Attribution 3.0 License. Supplement
Supplement of Atmos. Chem. Phys., 17, 983 9868, 2017 https://doi.org/.194/acp-17-983-2017-supplement Author(s) 2017. This work is distributed under the Creative Commons Attribution 3.0 License. Supplement
15.1: Hydrocarbon Reactions
 15.1: Hydrocarbon Reactions Halogenation An alkane will react with a halogen to produce a halalkane and the corresponding hydrogen halide. The catalyst is ultraviolet radiation. Reaction 1 methane chlorine
15.1: Hydrocarbon Reactions Halogenation An alkane will react with a halogen to produce a halalkane and the corresponding hydrogen halide. The catalyst is ultraviolet radiation. Reaction 1 methane chlorine
Fundamental Kinetics Database Utilizing Shock Tube Measurements
 Fundamental Kinetics Database Utilizing Shock Tube Measurements Volume 6: Reaction Rate Measurements (January 2009 to January 2014) D. F. Davidson and R. K. Hanson Mechanical Engineering Department Stanford
Fundamental Kinetics Database Utilizing Shock Tube Measurements Volume 6: Reaction Rate Measurements (January 2009 to January 2014) D. F. Davidson and R. K. Hanson Mechanical Engineering Department Stanford
Rate constants for the reaction of Cl atoms with O 3 at temperatures from 298 to 184K.
 Rate constants for the reaction of Cl atoms with O 3 at temperatures from 298 to 184K. S.D. Beach, I.W.M. Smith* and R.P. Tuckett Int. J. Chem. Kinetics., (2002) 34, 104-109. DOI: 10.1002/kin.10033 This
Rate constants for the reaction of Cl atoms with O 3 at temperatures from 298 to 184K. S.D. Beach, I.W.M. Smith* and R.P. Tuckett Int. J. Chem. Kinetics., (2002) 34, 104-109. DOI: 10.1002/kin.10033 This
Basic Organic Chemistry Nomenclature CHEM 104 B
 Basic Organic Chemistry Nomenclature CHEM 104 B I have gone ahead and compiled all of the basic naming rules that we will be dealing with into one worksheet. I hope this will be helpful to you as you work
Basic Organic Chemistry Nomenclature CHEM 104 B I have gone ahead and compiled all of the basic naming rules that we will be dealing with into one worksheet. I hope this will be helpful to you as you work
*P51939A0124* Pearson Edexcel WCH04/01. P51939A 2018 Pearson Education Ltd.
 Write your name here Surname Other names Pearson Edexcel International Advanced Level Centre Number Candidate Number Chemistry Advanced Unit 4: General Principles of Chemistry I Rates, Equilibria and Further
Write your name here Surname Other names Pearson Edexcel International Advanced Level Centre Number Candidate Number Chemistry Advanced Unit 4: General Principles of Chemistry I Rates, Equilibria and Further
N_HW1 N_HW1. 1. What is the purpose of the H 2 O in this sequence?
 N_HW1 N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. 1. What is the purpose of the H 2 O in this
N_HW1 N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. 1. What is the purpose of the H 2 O in this
DEVELOPMENT, EXTENSION AND VALIDATION OF (THEORY-BASED) SARS DECEMBER 2018 I LUC VEREECKEN
 DEVELOPMENT, EXTENSION AND VALIDATION OF (THEORY-BASED) SARS DECEMBER 2018 I LUC VEREECKEN THE NEED FOR DATABASES AND SARS Models for atmospheric chemistry are ever more complex - Air quality : more, and
DEVELOPMENT, EXTENSION AND VALIDATION OF (THEORY-BASED) SARS DECEMBER 2018 I LUC VEREECKEN THE NEED FOR DATABASES AND SARS Models for atmospheric chemistry are ever more complex - Air quality : more, and
Glyoxal processing by aerosol multiphase chemistry: towards a kinetic modeling framework of secondary organic aerosol formation in aqueous particles
 doi:10.5194/acp-10-8219-2010 Author(s) 2010. CC Attribution 3.0 License. Atmospheric Chemistry and Physics Glyoxal processing by aerosol multiphase chemistry: towards a kinetic modeling framework of secondary
doi:10.5194/acp-10-8219-2010 Author(s) 2010. CC Attribution 3.0 License. Atmospheric Chemistry and Physics Glyoxal processing by aerosol multiphase chemistry: towards a kinetic modeling framework of secondary
Primary photochemical transitions. Absorption cross-section data. Wavelength range/nm Reference Comments
 IUPAC Task Group on Atmospheric Chemical Kinetic Data Evaluation Data Sheet P7 Datasheets can be downloaded for personal use only and must not be retransmitted or disseminated either electronically or
IUPAC Task Group on Atmospheric Chemical Kinetic Data Evaluation Data Sheet P7 Datasheets can be downloaded for personal use only and must not be retransmitted or disseminated either electronically or
CHEM 112 Name: (Last) (First). Section No.: VISUALIZING ORGANIC REACTIONS THROUGH USE OF MOLECULAR MODELS
 CHEM 112 Name: (Last) (First). Section No.: VISUALIZING ORGANIC REACTIONS THROUGH USE OF MOLECULAR MODELS 1) HYDROCARBONS: a. Saturated Hydrocarbons: Construct a model for propane, C 3 H 8, using black
CHEM 112 Name: (Last) (First). Section No.: VISUALIZING ORGANIC REACTIONS THROUGH USE OF MOLECULAR MODELS 1) HYDROCARBONS: a. Saturated Hydrocarbons: Construct a model for propane, C 3 H 8, using black
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
 Unit - 12 ALDEHYDES, KETONES AND CARBOXYLIC ACIDS 1. Indicate the electrophilic and nucleophilic centres in acetaldehyde. 2. Write the IUPAC names of the following organic compounds : 122 XII Chemistry
Unit - 12 ALDEHYDES, KETONES AND CARBOXYLIC ACIDS 1. Indicate the electrophilic and nucleophilic centres in acetaldehyde. 2. Write the IUPAC names of the following organic compounds : 122 XII Chemistry
The SAPRC Chemical Mechanisms
 The SAPR hemical Mechanisms utline William P. L. arter E-ERT, University of alifornia, Riverside December 6, 2006 Major components of the SAPR mechanisms Mechanism generation system Status of SAPR mechanism
The SAPR hemical Mechanisms utline William P. L. arter E-ERT, University of alifornia, Riverside December 6, 2006 Major components of the SAPR mechanisms Mechanism generation system Status of SAPR mechanism
BIOLOGY. Chapter 2.3 THE CHEMICAL FOUNDATION OF LIFE CARBON
 BIOLOGY Chapter 2.3 THE CHEMICAL FOUNDATION OF LIFE CARBON Living Organisms Forms complex molecules 4 valance electrons Carbon Atom Carbon can bond to four other atoms or groups of atoms, making a large
BIOLOGY Chapter 2.3 THE CHEMICAL FOUNDATION OF LIFE CARBON Living Organisms Forms complex molecules 4 valance electrons Carbon Atom Carbon can bond to four other atoms or groups of atoms, making a large
Chemistry Assessment Unit A2 1
 Centre Number 71 Candidate Number ADVANCED General Certificate of Education January 2011 Chemistry Assessment Unit A2 1 assessing Periodic Trends and Further Organic, Physical and Inorganic Chemistry [AC212]
Centre Number 71 Candidate Number ADVANCED General Certificate of Education January 2011 Chemistry Assessment Unit A2 1 assessing Periodic Trends and Further Organic, Physical and Inorganic Chemistry [AC212]
A MEASUREMENT TECHNIQUE FOR HYDROXYACETONE
 A MEASUREMENT TECHNIQUE FOR HYDROXYACETONE P. J. Klotz, E. S. C. Kwok, X. Zhou, J. H. Lee, and Y. -N. Lee Environmental Chemistry Division, Department of Applied Science Brookhaven National Laboratory,
A MEASUREMENT TECHNIQUE FOR HYDROXYACETONE P. J. Klotz, E. S. C. Kwok, X. Zhou, J. H. Lee, and Y. -N. Lee Environmental Chemistry Division, Department of Applied Science Brookhaven National Laboratory,
The carbonyl C = O H C = O C = O O R C - O. acetone. aldehyde. ketone H + Carboxylic acids. d - d + Phosgene! COCl 2 Chemical weapon
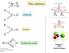 d + d - C = O The carbonyl acetone H C = O aldehyde R R C = O ketone R O R C - O Paolo Sarti 2011 Department of Biochemistry Sapienza H + Carboxylic acids Phosgene! COCl 2 Chemical weapon Paolo Sarti 2011
d + d - C = O The carbonyl acetone H C = O aldehyde R R C = O ketone R O R C - O Paolo Sarti 2011 Department of Biochemistry Sapienza H + Carboxylic acids Phosgene! COCl 2 Chemical weapon Paolo Sarti 2011
1 Which of the following cannot be used to detect alcohol in a breathalyser test? Fractional distillation. Fuel cell. Infrared spectroscopy
 1 Which of the following cannot be used to detect alcohol in a breathalyser test? Fractional distillation Fuel cell Infrared spectroscopy Reduction of dichromate(vi) ions 2 Propanal, H 3 H 2 HO, and propanone,
1 Which of the following cannot be used to detect alcohol in a breathalyser test? Fractional distillation Fuel cell Infrared spectroscopy Reduction of dichromate(vi) ions 2 Propanal, H 3 H 2 HO, and propanone,
Near-IR photodissociation of peroxy acetyl nitrate
 Atmos. Chem. Phys. Discuss., 4, 1269 1289, 04 www.atmos-chem-phys.org/acpd/4/1269/ SRef-ID: 1680-737/acpd/04-4-1269 European Geosciences Union 04 Atmospheric Chemistry and Physics Discussions Near-IR photodissociation
Atmos. Chem. Phys. Discuss., 4, 1269 1289, 04 www.atmos-chem-phys.org/acpd/4/1269/ SRef-ID: 1680-737/acpd/04-4-1269 European Geosciences Union 04 Atmospheric Chemistry and Physics Discussions Near-IR photodissociation
ATMOSPHERIC CHEMISTRY OF SELECTED HYDROXYCARBONYLS. Sara M. Aschmann, Janet Arey and Roger Atkinson
 ATMOSPHERIC CHEMISTRY OF SELECTED HYDROXYCARBONYLS Sara M. Aschmann, Janet Arey and Roger Atkinson Air Pollution Research Center University of California Riverside, CA 92521, U.S.A. Introduction Volatile
ATMOSPHERIC CHEMISTRY OF SELECTED HYDROXYCARBONYLS Sara M. Aschmann, Janet Arey and Roger Atkinson Air Pollution Research Center University of California Riverside, CA 92521, U.S.A. Introduction Volatile
Week 6 notes CHEM
 Week 6 notes EM1002 2009 Unless otherwise stated, all images in this file have been reproduced from: Blackman, Bottle, Schmid, Mocerino and Wille, hemistry, 2007 (John Wiley) ISBN: 9 78047081 0866 1 Note
Week 6 notes EM1002 2009 Unless otherwise stated, all images in this file have been reproduced from: Blackman, Bottle, Schmid, Mocerino and Wille, hemistry, 2007 (John Wiley) ISBN: 9 78047081 0866 1 Note
NF 3 : UV absorption spectrum temperature dependence and the atmospheric and climate forcing implications
 GEOPHYSICAL RESEARCH LETTERS, VOL. 40, 440 445, doi:10.1002/grl.50120, 2013 NF 3 : UV absorption spectrum temperature dependence and the atmospheric and climate forcing implications Vassileios C. Papadimitriou,
GEOPHYSICAL RESEARCH LETTERS, VOL. 40, 440 445, doi:10.1002/grl.50120, 2013 NF 3 : UV absorption spectrum temperature dependence and the atmospheric and climate forcing implications Vassileios C. Papadimitriou,
9/10/2012. SOA Formation through Aqueous Chemistry: atmospheric evidence, chemistry, partitioning and prediction. Organics: 30-70% of PM 2.
 9/1/212 rganics: 3-7% of PM 2.5 SA Formation through Aqueous Chemistry: atmospheric evidence, chemistry, partitioning and prediction NASA image Barbara Turpin, Professor Dept. of Environmental Sciences
9/1/212 rganics: 3-7% of PM 2.5 SA Formation through Aqueous Chemistry: atmospheric evidence, chemistry, partitioning and prediction NASA image Barbara Turpin, Professor Dept. of Environmental Sciences
Measurements of Ozone. Why is Ozone Important?
 Anthropogenic Climate Changes CO 2 CFC CH 4 Human production of freons (CFCs) Ozone Hole Depletion Human production of CO2 and CH4 Global Warming Human change of land use Deforestation (from Earth s Climate:
Anthropogenic Climate Changes CO 2 CFC CH 4 Human production of freons (CFCs) Ozone Hole Depletion Human production of CO2 and CH4 Global Warming Human change of land use Deforestation (from Earth s Climate:
CHE 325 SPECTROSCOPY (A) CHAP 13A ASSIGN CH 2 CH CH 2 CH CHCH 3
 CE 325 SPECTRSCPY (A) CAP 13A ASSIGN 1. Which compound would have a UV absorption band at longest wavelength? A. I B. II C. III D. IV E. V C CC 3 CC C 2 C CC 3 I II III C 2 C C 2 C CC 3 IV V 2. Select
CE 325 SPECTRSCPY (A) CAP 13A ASSIGN 1. Which compound would have a UV absorption band at longest wavelength? A. I B. II C. III D. IV E. V C CC 3 CC C 2 C CC 3 I II III C 2 C C 2 C CC 3 IV V 2. Select
1. Following are structural formulas for two steroid hormones.
 CHEM 122: Introduction to rganic Chemistry Chapter 9: Aldehydes and Ketones. 1. Following are structural formulas for two steroid hormones. Cortisone Aldosterone Name the functional groups in each. b)
CHEM 122: Introduction to rganic Chemistry Chapter 9: Aldehydes and Ketones. 1. Following are structural formulas for two steroid hormones. Cortisone Aldosterone Name the functional groups in each. b)
Dr. Sangeeta Khanna Ph.D 1 CHEMISTRY COACHING CIRCLE D:\Important Data\2016\+2\Org\Test\Grand Test\+2 Grand Test-6\+2 Grand Test-VI Level -1.
 r. Sangeeta Khanna Ph. 1 HEMISTRY HING IRLE :Important ata016+rgtestgrand Test+ Grand Test-6+ Grand Test-VI Level -1.doc r. Sangeeta Khanna Ph. Test ate: 18.10.016 Topic: xygen ontaining rganic ompound
r. Sangeeta Khanna Ph. 1 HEMISTRY HING IRLE :Important ata016+rgtestgrand Test+ Grand Test-6+ Grand Test-VI Level -1.doc r. Sangeeta Khanna Ph. Test ate: 18.10.016 Topic: xygen ontaining rganic ompound
tert-butyl alcohol n-butyl alcohol methyl propyl ether (c) Explain briefly why n-butyl alcohol has a much higher bp than methyl propyl ether.
 1. (15 points) Three 4 10 isomers are shown below, along with their boiling points. ( 3 ) 3 3 2 2 2 3 2 2 3 tert-butyl alcohol n-butyl alcohol methyl propyl ether bp: 82 118 39 (a) Based on their boiling
1. (15 points) Three 4 10 isomers are shown below, along with their boiling points. ( 3 ) 3 3 2 2 2 3 2 2 3 tert-butyl alcohol n-butyl alcohol methyl propyl ether bp: 82 118 39 (a) Based on their boiling
Aldehydes and Ketones
 Reading Chapter 12: 12.1-12.3, 12.6-12.9 Practice problems: in text problems and 19, 21-24, 28 Carbonyl Compounds II: Reactions of More Reactions of Carboxylic Acid Derivatives The Structure of 1 The Structure
Reading Chapter 12: 12.1-12.3, 12.6-12.9 Practice problems: in text problems and 19, 21-24, 28 Carbonyl Compounds II: Reactions of More Reactions of Carboxylic Acid Derivatives The Structure of 1 The Structure
Supporting Information
 1 Supporting Information 2 3 Deep Oxidation of NO by a Hybrid System of Plasma N-type Semiconductors: High-Energy Electron-Activated Pseudo Photocatalysis Behavior 4 5 Si Chen 1,2, Haiqiang Wang 1,2*,
1 Supporting Information 2 3 Deep Oxidation of NO by a Hybrid System of Plasma N-type Semiconductors: High-Energy Electron-Activated Pseudo Photocatalysis Behavior 4 5 Si Chen 1,2, Haiqiang Wang 1,2*,
