IR spectrum and radiative forcing of CF 4 revisited
|
|
|
- Sherman Nash
- 5 years ago
- Views:
Transcription
1 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110,, doi: /2004jd005201, 2005 IR spectrum and radiative forcing of CF 4 revisited M. D. Hurley, 1 T. J. Wallington, 1 G. A. Buchanan, 2 L. K. Gohar, 3 G. Marston, 2 and K. P. Shine 3 Received 6 July 2004; revised 7 October 2004; accepted 26 October 2004; published 19 January [1] Carbon tetrafluoride (CF 4 ) is included as a greenhouse gas within the Kyoto Protocol. There are significant discrepancies in the reported integrated infrared (IR) absorption cross section of CF 4 leading to uncertainty in its contribution to climate change. To reduce this uncertainty, the IR spectrum of CF 4 was measured in two different laboratories, in hpa of air diluent at 296 ± 2K over the wavelength range cm 1 using spectral resolutions of 0.03 or 0.50 cm 1. There was no discernable effect of diluent gas pressure or spectral resolution on the integrated IR absorption, and a value of the integrated absorption cross section of (1.90 ± 0.17) cm 2 molecule 1 cm 1 was derived. The radiative efficiency (radiative forcing per ppbv) and GWP (relative to CO 2 ) of CF 4 were calculated to be W m 2 ppbv 1 and 7200 (100 year time horizon). The GWP for CF 4 calculated herein is approximately 30% greater than that given by the Intergovernmental Panel on Climate Change (IPCC) [2002] partly due to what we believe to be an erroneously low value for the IR absorption strength of CF 4 assumed in the calculations adopted by the IPCC. The radiative efficiency of CF 4 is predicted to decrease by up to 40% as the CF 4 forcing starts to saturate and overlapping absorption by CH 4, H 2 O, and N 2 O in the atmosphere increases over the period The radiative forcing attributable to increased CF 4 levels in the atmosphere from 1750 to 2000 is estimated to be W m 2 and is predicted to be up to W m 2 from 2000 to 2100, dependent on the scenario. Citation: Hurley, M. D., T. J. Wallington, G. A. Buchanan, L. K. Gohar, G. Marston, and K. P. Shine (2005), IR spectrum and radiative forcing of CF 4 revisited, J. Geophys. Res., 110,, doi: /2004jd Introduction [2] Carbon tetrafluoride, CF 4, is a potent greenhouse gas and is included in the Kyoto Protocol to the United Nations Framework Convention of Climate Change ( CF 4 has two properties which render it a potent greenhouse gas. First, it has an extremely long atmospheric lifetime (>50,000 years [Intergovernmental Panel on Climate Change (IPCC), 2002]). With such a long lifetime even small emission sources will, over time, lead to significant atmospheric concentrations. Second, IR absorption by CF 4 is intense and is situated near the atmospheric window region of the terrestrial radiation spectrum. The main anthropogenic emission of CF 4 occurs as a by-product of aluminium smelting. There is a small natural geothermal source of CF 4. The atmospheric abundance in 1998 was estimated at 80 pptv and the growth trend in the 1990s was approximately 1.0 pptv yr 1 [IPCC, 2002]. [3] The impact of changes in the atmospheric concentration of CF 4 on the climate system can be estimated by 1 Physical and Environmental Sciences Department, Ford Motor Company, Dearborn, Michigan, USA. 2 School of Chemistry, University of Reading, Reading, UK. 3 Department of Meteorology, University of Reading, Reading, UK. Copyright 2005 by the American Geophysical Union /05/2004JD calculating its contribution to radiative forcing of climate change. Radiative forcing is defined as the change in net irradiance at the tropopause (expressed in Wm 2 ) resulting from a perturbation of the climate system. Following IPCC [2002], we define the radiative efficiency of CF 4 as the radiative forcing for a one part per billion per volume (ppbv) change from zero in the concentration of CF 4 in the atmosphere. Radiative efficiency has units of Wm 2 ppbv 1. Radiative efficiencies are used in the calculation of global warming potentials (GWPs) which compare the relative climatic impacts of pulse emissions of different greenhouse gases (on a per mass basis) over a given time horizon. The 100 year GWP of CF 4 is calculated from the time integral, over 100 years, of the radiative forcing due to a pulse emission of the gas, divided by a similar time integral for a pulse emission of the same mass of a reference gas, usually taken to be CO 2. The 100 year GWP for CF 4, relative to CO 2, is given as 5700 by IPCC [2002]. For some countries, CF 4 emissions form a significant fraction of the GWP weighted national greenhouse gas emissions. For example, in Norway, perfluorocarbons (predominantly CF 4 ) contributed 4% of the 100 year GWP weighted greenhouse gas emissions in 1990 [Godal and Fuglestvedt, 2002]. To facilitate national and international policy decisions, it is important to establish an accurate value for the GWP of CF 4. [4] The IR spectrum of CF 4 has been the subject of several previous studies [e.g., Nemtchinov and Varanasi, 1of8
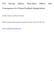
2 2003; Sihra et al., 2001; Jain et al., 2000; Roehl et al., 1995]. Unfortunately, there are substantial disagreements between the absorption cross sections reported in these studies. Integrated absorption cross sections range from [Larin et al., 1997] to cm 2 molecule 1 cm 1 [Saeki et al., 1976]. The cross section values reported for CF 4 span a range of approximately 50% resulting in a substantial uncertainty in both the radiative efficiency and GWP for this molecule. Experimental uncertainties (random plus systematic) associated with the quantification of IR absorption cross sections are typically of the order of ±5% resulting in a spread of reported values covering a range of approximately 10% [Forster et al., 2004]. The scatter in the literature data for CF 4 is anomalous compared to other halocarbons and further study of this species is required. [5] To improve our understanding of the contribution of CF 4 to climate change, a collaborative study was conducted in our laboratories. The study consisted of four distinct elements. First, absorption cross section measurements were performed at the Ford Motor Company in Michigan (Ford), United States, and the Molecular Spectroscopy Facility (MSF) in the Rutherford Appleton Laboratory (RAL), UK. Second, the new absorption cross section data were incorporated in radiative transfer models at Reading to calculate the radiative efficiency of CF 4. Third, the sensitivity of the radiative efficiency to changes in the atmospheric concentrations of H 2 O, CH 4,N 2 O, and CF 4 was examined (building on the work of Freckleton et al. [1996]). Fourth, estimates for the preindustrial, present-day, and future (2100) radiative forcing of CF 4 were computed. 2. Experiment 2.1. Ford Motor Company (Ford) [6] The experimental setup consisted of a Mattson Instruments, Sirius 100 Fourier transform infrared spectrometer, interfaced to a 140 L, 2 m long evacuable Pyrex chamber described elsewhere [Pinnock et al., 1995]. The spectrometer was operated at a spectral resolution of 0.50 cm 1. Infrared spectra were derived from 32 coadded interferograms. Spectra were recorded at 296 K in the presence of 933 hpa of air diluent. Measurements were made in January 2003 and repeated in November There was good agreement (within 2%) between the two sets of measurements. The spectrum reported here is an average from the two sets. We estimate the absorption cross section measurement to be accurate to within ±5% [Pinnock et al., 1995]. Although there was good agreement between the spectra acquired at Ford in 2003, these spectra were approximately 20% less intense than the spectrum measured at Ford in 1997 and reported by Sihra et al. [2001]. We have two reasons for preferring the present work over that of Sihra et al. [7] First, careful inspection of the background (i.e., 933 hpa of air without added CF 4 ) transmittance spectra during experiments in January 2003 revealed the presence of small features attributable to CF 4. We usually employ a liquid N 2 cryotrap between the vacuum pump and the gas handling line to increase the efficiency of the pumping and protect the pump from corrosive gases. When experiments were performed using a warm (i.e., room temperature) trap there were no CF 4 features evident in the background. CF 4 boils at 145 K. It appears that the vapor pressure of CF 4 is high enough at liquid nitrogen temperature (77 K) that our cryotrap acts as a reservoir for CF 4. Background spectra from 1997 are not available and we can not check for similar problems in our previous work. The presence of CF 4 in the cryotrap raises doubts about the reliability of our previous calibration of the CF 4 spectrum. [8] Second, CF 4 has unusually intense IR absorption features and to avoid saturation requires the use of very dilute mixtures of CF 4. In 1997, dilution was achieved within the chamber using a protocol where CF 4 was added first, diluent added second, then a known fraction of the mixture was pumped out and replaced by more diluent. In the experiments reported here a standard 100:1 dilution mixture was prepared separately in a 2 L flask. Mixing problems in the irregularly shaped chamber may have led to an underestimation of the CF 4 concentration (and hence overestimation of the absorption cross section) in our previous work Molecular Spectroscopy Facility (MSF) [9] The spectra at MSF were obtained at 298 K using the Bruker IFS 120 HR spectrometer in the range cm 1 using a 5 cm stainless steel cell with KBr windows. The spectrometer was operated at a spectral resolution of 0.03 cm 1 with interferograms being obtained from 50 coadded scans. Spectra were recorded either of the pure vapor or in the presence of hpa of N 2 diluent. Two MCT detectors were used to measure spectra of the pure vapor. The first, D1, had a range of 800 to 1800 cm 1, while the second, D2, extended to the shorter wavenumber of 575 cm 1 allowing the study of the weaker band of CF 4 centered at 632 cm 1. The second detector was used for the measurements of CF 4 diluted in N 2. [10] In all experiments, pure CF 4 was transferred from the lecture bottle (Aldrich, 99.9%) to a small glass vial and degassed by freeze/pump/thaw cycles to remove any air in the sample before use. For the measurements of the pure vapor, the desired quantity was allowed into the cell, and the pressure monitored throughout the recording of the spectrum. As with the measurements at Ford, preliminary results at MSF showed that the cryotrap before the pump on the gas-line acted as a reservoir for CF 4 and therefore was also used warm. [11] The mixtures in nitrogen were made up in 1000 cm 3 capacity glass bulbs. Pure CF 4 was allowed into the bulb to a pressure of about 0.49 hpa and then made up with nitrogen (1000 hpa). Two bulbs of CF 4 in N 2 were mixed in this way and allowed to stand overnight to allow good mixing of the two gases. [12] To ensure that saturation was not a problem in the measurements, for each set of experiments, absorbance was plotted as a function of pressure for the peak wavenumber at 1283 cm 1 and is shown in Figure 1. Points at higher pressures showing nonlinear behavior were ignored, and cross sections were derived from the slopes of the linear portions of the plots. The spectra were then normalized to this value for the cross section and the integrated absorption cross section calculated from the average of the spectra. Integrated absorption cross sections were obtained in this way for the pure vapor using the two different detectors, and CF 4 diluted in N 2 using two different mixtures. A total of 2of8
3 of pressure broadening of the IR features in the spectrum recorded by NV2003. [14] Integration of the absorption features over cm 1 in the MSF and Ford spectra gives integrated absorption cross sections for CF 4 of (1.93 ± 0.14) and (1.86 ± 0.13) cm 2 molecule 1 cm 1. The quoted uncertainty reflects both random and our Figure 1. Absorbance (naperian) at 1283 cm 1 of CF 4 versus pressure for measurements taken at MSF. D1 and D2 refer to experiments done using pure CF 4 with the two detectors mentioned in section 2.2. B1 and B2 refer to experiments carried out using 0.5% and 0.1% mixtures of CF 4 in N 2, respectively. The line forced through the origin has been added to act as a guide. 12 CF 4 spectra obtained under a range of conditions were used to obtain the final integrated absorption cross section. 3. Results 3.1. Experimental Measurement of Infrared Absorption Spectra [13] As shown in Figure 2, CF 4 has two IR absorption bands; an intense band at cm 1 and a much weaker band centered at 632 cm 1.CF 4 has a high degree of symmetry and consequently a relatively simple IR spectrum. The intense sharp spectral feature at 1283 cm 1 contains approximately 25% of the total integrated absorption cross section within 1 cm 1. Figures 3 and 4 compare the spectra obtained at Ford (0.5 cm 1 spectral resolution in 933 hpa of air diluent) and MSF (0.03 cm 1 resolution, average pressure 233 hpa N 2 diluent, 298 K) to that reported by Nemtchinov and Varanasi [2003] (NV2003) (0.03 cm 1 resolution, 1014 hpa N 2 diluent, K). As seen from Figures 3 and 4, the three spectra are in good agreement. Figure 3 shows that, as expected, the feature at 1283 cm 1 is better resolved in the MSF and NV2003 spectra than that obtained at Ford. The differences evident between the MSF and NV2003 spectra at cm 1 reflect the effect Figure 2. The absorption cross section of CF 4 from Ford Motor Company 2003.(top) Main absorption band centered at 1283 cm 1. (bottom) Weaker band centered at 632 cm 1. 3of8
4 underestimation of the absorption cross section. It is more difficult to explain the higher values shown in Figure 5. As discussed above, the present work supersedes that of Sihra et al. [2001]. As seen from Figure 5, with the exception of the measurement of Sihra et al. [2001], only one [Saeki et al., 1976] of the previous measurements of the integrated absorption cross section of CF 4 is significantly greater than those reported herein. Given the agreement between the results reported by Schurin [1959], Roehl et al. [1995], NV2003, Ford, and MSF evident in Figure 5, it seems reasonable to conclude that Saeki et al. [1976] overestimated the absorption cross section by approximately 10 20% (possibly due to underestimation of the CF 4 concentration as likely in the case of Sihra et al., see section 2.1) Calculation of CF 4 Radiative Efficiencies [16] There are several methods of calculating the radiative efficiency of a gas. The definition given in the IPCC [2002] is of the adjusted cloudy-sky radiative forcing calculated at the tropopause, in which the stratospheric temperatures are Figure 3. The absorption cross section spectrum of CF 4 of Ford (solid curve), MSF (dotted curve), and NV2003 (dashed curve) used in the calculation of radiative efficiency of CF 4. estimate of systematic uncertainties (5%) in the measurements. The results from MSF and Ford are indistinguishable within the experimental uncertainties. We choose to cite a final value which is an average of the two independent determinations together with uncertainties which encompass the extremes of the individual determinations; hence the integrated absorption cross section is (1.90 ± 0.17) cm 2 molecule 1 cm 1. [15] The integrated absorption cross sections measured in the present work are compared to the literature data in Figure 5. As seen from Figure 5, the absorption cross sections measured at MSF and Ford are in agreement within 10%, the expected experimental uncertainties [Forster et al., 2004], with the previous studies by NV2003, Roehl et al. [1995], and Schurin [1959]. We speculate that literature values significantly lower than measured herein reflect the effect of saturation that can occur for a molecule with such sharp and intense spectral features as CF 4, leading to an Figure 4. The absorption cross section of CF cm 1 peak from Ford (solid curve), MSF (dotted curve), and NV2003 (dashed curve). 4of8
5 Figure 5. The integrated absorption cross section of CF 4 from the various previous studies and the two new measurements of this study, Ford, and MSF 2003 (right of the dashed line). allowed to adjust so that the stratosphere remains in global radiative equilibrium. This is the method used to estimate the global annual mean radiative efficiency of CF 4 in this work. Radiative forcing calculated without stratospheric adjustment is referred to as instantaneous radiative forcing and is used here for the radiative efficiency in a sensitivity study of varying abundances of the overlapping gases. [17] The adjusted cloudy-sky radiative forcing is calculated using a narrowband model, NBM [Shine, 1991], which uses a fixed-dynamical-heating approximation for the stratospheric adjustment. Ideally, a line-by-line radiative transfer model would be used to calculate the adjusted cloudy-sky radiative forcing as this is more accurate than a narrow band model; however to include clouds and stratospheric adjustment in our line-by-line model would currently prove computationally too expensive. Instead a line-by-line radiative transfer model, the Reference Forward Model (RFM) [Dudhia, 1997], is used in the calculation of a gas-dependent scale factor comprising of the ratio of the spectrally averaged instantaneous clear-sky radiative forcing calculated from RFM and NBM. This gasdependent scale factor is used to calibrate the NBM adjusted cloudy-sky radiative forcing in the work of Gohar et al. [2004] and Sihra et al. [2001]. The agreement between the NBM and RFM clear-sky instantaneous radiative forcings for CF 4 is within 10%. [18] Sihra et al. [2001] used two methods to calibrate the NBM cloud-sky adjusted radiative forcing using RFM. The first method (described above) is used in this work; the second method scaled the cross section in each wavenumber interval with the difference in the clear-sky instantaneous radiative forcing spectra of NBM and RFM. The effective cross section was then used in the NBM to calculate the cloudy-sky adjusted forcing. The two methods produced adjusted cloud-sky radiative forcings that agreed to within 3%, indicating that either calibration method can be used. Furthermore, the good agreement between the two calibration methods given in Sihra et al. [2001] suggest that the differences between NBM and RFM radiative forcings for a gas is not changed by including clouds and stratospheric adjustment and the simpler calibration method used here is a good approximation. [19] Three atmosphere profiles, representing the tropics and northern and southern extratropics, are used. For the calculations reported in this section, the background concentrations for CO 2, CH 4, and N 2 O are 365 ppmv, ppmv, and ppmv, respectively, representing present-day concentrations and are considered well-mixed in the atmosphere. The 2 K km 1 definition of the tropopause height is used in all calculations. The radiative forcing is calculated for a 0.1 ppbv uniform gas perturbation from zero background abundance of CF 4 for each of the three atmosphere profiles and then averaged over area to calculate the global annual mean radiative forcing. A 0.1 ppbv perturbation is used instead of 1 ppbv to remain in the weak limit. The global annual mean radiative forcing is scaled up to obtain a 1 ppbv perturbation [Freckleton et al., 1996] for the final global annual mean radiative efficiency. [20] The calculations of Freckleton et al. [1998] for CFC- 12, indicate that the error in using a much reduced horizontal resolution (e.g., three profiles) is unlikely to exceed 1 2%. This assumption was checked for CF 4. The radiative efficiency was calculated from individual radiative efficiencies of four seasonal and zonal mean atmosphere profiles at 10 degrees latitudinal resolution and then annually and globally averaged; this forcing was only 1% smaller than the three atmosphere profiles radiative efficiency for CF 4, supporting the use of the reduced resolution. [21] Minschwaner et al. [1998] and Jain et al. [2000] showed the radiative forcings of CH 4 and N 2 O increased by less than 2% for uniform profiles of CH 4 and N 2 O mixing ratio compared to nonuniform profiles of both gases. This suggests the uniform profiles of CH 4 and N 2 O would not significantly affect the radiative forcing of CF 4 ; however, 5of8
6 Table 1. Integrated Absorption Cross Section and the Radiative Efficiency for Four Different Absorption Cross Sections Calculated in This Study a Study Integrated Absorption Cross Section, Radiative Efficiency, cm 2 molecule 1 cm 1 Wm 2 ppbv 1 Ford MSF NV Sihra et al. [2001] a The radiative efficiency values given here are for zero to 0.1 ppbv CF 4 perturbations. this was also investigated as well as the effect of using a constant profile of CF 4 mixing ratio. Using the vertical profiles of CH 4 and N 2 O given in Jain et al. [2000], the instantaneous clear-sky radiative efficiency of CF 4 was calculated using RFM and was less than 2% greater than the radiative efficiency using constant profiles of CH 4 and N 2 O. The lifetime of CF 4 in the atmosphere is approximately 50,000 years, and therefore it is generally considered to be well-mixed in the atmosphere, but there is a slight decrease in the stratosphere [Harnisch et al., 1996; Zander et al., 1996; IPCC, 2002], possibly as a result of the time required for the effect of increased emissions to propogate vertically. As an extreme test, a vertical profile of a reduction of 20% of tropospheric CF 4 concentration in the stratosphere was used to calculate the instantaneous radiative forcing of CF 4, and the difference between using a constant profile and one that varies with height was found to be less than 2%. The uncertainty due to the choice of tropopause height is estimated to be larger, 5 10% [see also Forster et al., 2004]. [22] The new absorption cross section spectra were used to calculate the adjusted global annual mean radiative efficiencies of CF 4. The results are given in Table 1. The radiative efficiency using the NV2003 cross section is also shown. The radiative efficiency obtained from the two new cross sections and that of NV2003 are essentially identical, as expected from the similarity in their integrated absorption cross section values. This provides more confidence in the estimate of the radiative efficiency of CF 4 to be closer to Wm 2 ppbv 1, calculated from the average cross section of (1.90 ± 0.17) cm 2 molecule 1 cm 1, than the most recent IPCC [2002] value of 0.08 Wm 2 ppbv 1, but it is lower than our previous estimate in Sihra et al. [2001], Wm 2 ppbv 1. If CF 4 is perturbed from present-day background abundance, instead of from zero, the radiative efficiency of CF 4 is only 1% lower than Wm 2 ppbv Sensitivity of Radiative Efficiency to Concentrations of Other Gases [23] Water vapor, methane, and nitrous oxide are the main gases that absorb significantly in the same spectral region as CF 4 [e.g., Freckleton et al., 1996], and collectively reduce its forcing to 25% of the value in the absence of the overlapping gases. In the calculation of GWPs it is generally assumed that forcings are independent of variations in the background concentration of other species. However, given the substantial overlap of CF 4 with the spectra of H 2 O, CH 4,N 2 O, it is necessary to examine the degree to which this is a valid assumption. [24] Two different climate scenarios were chosen. First, a preindustrial climate in which the concentrations of the overlapping gases are lower than at present. Second, a scenario predicted for 2100 where the climate is warmer than at present and the concentrations of the overlapping gases are higher than at present. The 2100 climate was taken from the IPCC Special Report on Emission Scenarios (SRES) [Nakic enovic et al., 2000]. The particular scenario used (A2p) contained the greatest increase in CF 4 abundance for 2100 and was chosen to examine the extreme case. Concentrations of gases in the preindustrial climate were taken from IPCC [2002] estimates. The abundances of these gases for the two climate scenarios are given in Table 2. The H 2 O value was found by calculating the mass mixing ratio for a 3.8 K increase in surface temperature and a constant relative humidity. The result was an approximate 30% increase in abundance. [25] The instantaneous clear-sky radiative efficiencies calculated from RFM, using the average cross section of (1.90 ± 0.17) cm 2 molecule 1 cm 1, were used to investigate the effect of the overlapping of the CF 4 absorption bands, especially the sharp spectral peak at 1283 cm 1. The instantaneous radiative efficiency is approximately 10% higher than the adjusted cloudy sky efficiencies. The CF 4 perturbation also differs from the calculations in section 3.2 of this study and is of 0.1 ppbv added to the background abundance of CF 4 (rather than from zero), and scaled up to 1 ppbv. The tendency of the 1283 cm 1 CF 4 spectral band to saturate indicates that the increased background gases and CF 4 will reduce the radiative efficiency due to this saturation and spectral overlapping. [26] The radiative efficiency of CF 4 for the scenarios is indicated in Figure 6. The present-day radiative efficiency was calculated using all gases fixed at their atmospheric levels in 2000 (see Table 2); the result is labeled all 2000 in Figure 6. In the future scenario, labeled all 2100, all gases were fixed at their concentrations predicted in 2100 (see Table 2); the radiative efficiency of CF 4 is approximately 30% less than that for the present day. The reduction in radiative efficiency is caused by the CF 4 absorption bands beginning to saturate and increased spectral overlap of these absorption bands resulting from increased abundance of H 2 O, CH 4, and N 2 O. The four columns at the right of Figure 6 show the effect on the radiative efficiency of CF 4 of individual changes of CF 4,H 2 O, CH 4, and N 2 O from their concentration in 2000 to that in the 2100 scenario and indicate that the largest individual reduction in radiative efficiency of CF 4 is caused by the predicted increase in the concentration of CH 4. [27] The preindustrial radiative efficiency of CF 4, labeled all 1750 in Figure 6 is 19% greater than that for the Table 2. The Concentrations of the Trace Gas Constituents in 1750, 2000, and Future (2100) Scenarios a Gas 1750 Scenario 2000 Scenario 2100 Scenario CF 4 40 pptv 80 pptv 508 pptv CH ppbv 1750 ppbv 3720 ppbv H 2 O 2000 (H 2 O) 2000 (H 2 O) 2000 (H 2 O) 1.3 N 2 O 270 ppbv 315 ppbv 446 ppbv a For CF 4,CH 4, and N 2 O taken from IPCC (2001) A2p SRES scenario [Nakic enovic et al., 2000]. The 1750 and 2000 concentrations are taken from IPCC [2002]. 6of8
7 Figure 6. Instantaneous, clear sky radiative efficiency (Wm 2 ppbv 1 )ofcf 4 for different scenarios. The scenarios being 1750, 2000, and The 2100 calculations use atmospheric concentrations taken from IPCC (2001) SRES A2p scenario. The 1750 calculations use atmospheric concentrations taken from IPCC [2002]. present day. The increase in radiative efficiency is caused by the decreased spectral overlap of the CF 4 absorption bands associated with the lower concentrations of CH 4 and N 2 Oin The first three scenarios to the left of Figure 6 show the contribution to this increase from changes in CF 4,CH 4, and N 2 O. The reduction in CH 4 increases the radiative efficiency by the largest amount, for the same reasons as stated for the 2100 scenario. Finally, from Figure 6 it is particularly interesting to note the drop in CF 4 radiative efficiency by more that 40% between the preindustrial and the projected 2100 atmospheres, due to the saturation of CF 4 absorption bands from the large increase in CF 4 concentrations and the heavy overlap from CH 4,N 2 O and H 2 O Time Evolution of Radiative Forcings [28] The cloudy-sky adjusted radiative forcing from preindustrial (1750) to present day (2000) and from present day to 2100 was calculated using the new absorption cross section and are given in Table 3. This was done to see the overall change in the predicted radiative forcing for a future scenario, as the previous section shows how the increased concentrations of CF 4,CH 4,N 2 O, and H 2 O will reduce the radiative efficiency of CF 4. [29] The value given for a 2100 scenario in IPCC [2002] is calculated assuming a simplified linear relationship between concentration and radiative efficiencies of CF 4, and hence are based on perturbations from zero concentrations of CF 4 in the background atmosphere. The radiative forcing calculations carried out in this section are perturbations from the CF 4 background concentrations and therefore the effect of saturation of the CF 4 absorption bands and overlapping will reduce the difference caused by the changes in the absorption cross sections. [30] From Table 3 it can be seen that the radiative forcing of CF 4 from 1750 to 2000 is greater than the IPCC [2002] value due to the increase in the new absorption cross section from that used in IPCC [2002] (integrated absorption cross section of cm 2 molecule 1 cm 1 [McDaniel et al., 1991]). [31] The new estimate of the future 2100 radiative forcing is slightly less than the IPCC [2002] value, and is a consequence of the effect of the overlapping absorption by gases and increased background gaseous concentrations being greater than the effect of the increased absorption cross section as explained earlier in this section. 4. Summary [32] The work presented here improves our understanding of the contribution of CF 4 to radiative forcing of climate change in four important aspects. First, the IR spectrum of CF 4 has been measured in two different laboratories using different experimental systems. There is excellent agreement (within 4%) between the results from the two independent studies reported here and the recent measurements of NV2003. Likely explanations for discrepancies between the results of previous studies and the present work are discussed. The similarity in magnitude of the three recent independent measurements (Ford, MSF, and NV2003) suggests that it is more appropriate to use an integrated absorption cross section value of rather than the value of cm 2 molecule 1 cm 1 [McDaniel et al., 1991] employed in the IPCC [2002] report. [33] Second, a new CF 4 radiative efficiency estimate of Wm 2 ppbv 1 was calculated based upon an integrated Table 3. The 1750 to 2000 Radiative Forcing and 2000 to 2100 Radiative Forcing Calculated Using the Trace Gas Concentrations Given in Table 2 This work, Wm 2 IPCC [2002], Wm of8
8 absorption cross section of cm 2 molecule 1 cm 1. The new value of the radiative efficiency is 28% higher than the value of 0.08 Wm 2 ppbv 1 quoted in IPCC [2002]. The IPCC value is ultimately derived from the work of Myhre and Stordal [1997], who report a value of Wm 2 ppbv 1. Hence our best value is 21% higher. Subsequently, Myhre et al. [1998] report a value of Wm 2 ppbv 1 using an amended version of their broadband model, but this was shown for clear-sky instantaneous forcing to be an overestimate of 7% compared to line-by-line calculations, which brings their new estimate to Wm 2 ppbv 1. The increase in radiative efficiency by 6% from Myhre and Stordal [1997] to Myhre et al. [1998] is due to an improved description of the overlapping with other gases for CF 4, and model differences between the two papers (G. Myhre, personal communication, 2004). Thus our best estimate is 15% higher than of Myhre et al. [1998]. Differences in the absorption cross section explain a 10% increase from the Myhre et al. [1998] value; the remaining 5% is presumably due to differences in radiative transfer codes and background profiles and is typical of differences between the calculations of our two groups [Gohar et al., 2004]. [34] Third, it is shown herein that the radiative efficiency of CF 4 varies by approximately ±20% due to the century time scale changes in the abundance of CF 4 itself and gases with overlapping spectra, with the dependence on CH 4 being particularly important. In contrast, Pinnock et al. [1995] showed the major hydrofluorocarbons (HFCs) had a weak dependence on H 2 O, with approximately 20% increase in cloudy-sky radiative efficiencies for the complete removal of H 2 O, but no significant dependence on CH 4 or N 2 O. [35] Fourth, the radiative forcing of climate change due to increased levels of CF 4 in the atmosphere was estimated from preindustrial to the present day and a future 2100 scenario were estimated to be W m 2 and W m 2, respectively. These are similar to values quoted in IPCC [2002], but due to the difference in radiative efficiency and overlapping, the method used by the IPCC [2002] for future estimates is not appropriate for CF 4. The new absorption cross section also increases the 100 year CF 4 GWP to 7200 relative to carbon dioxide compared to the value of 5700 in IPCC [2002]. [36] Acknowledgments. L.K.G. and K.P.S. acknowledge support from the UK Natural Environmental Research Council grant NERC/L/S/ 2001/00661 and the EC Project CRYOSTAT (EV2K-CT ) and thank Gunnar Myhre for his advice on his CF 4 calculation. We also thank the NERC Molecular Spectroscopy Facility for help in acquiring the new spectra and Ole John Nielsen for helpful discussions. We thank a reviewer for helpful comments. References Dudhia, A. (1997), RFM v3 software user s manual, Tech. Rep. ESA Doc. PO-MA-OXF-GS-0003, Dep. of Atmos., Oceanic, and Planet. Phys., Univ. of Oxford, Oxford, UK. Forster, P. M. de F., et al. (2004), Resolution of the uncertainties in the radiative forcing of HFC-134a, J. Quant. Spectrosc. Radiat. Transfer, in press. Freckleton, R. S., S. Pinnock, and K. P. Shine (1996), Radiative forcing of halocarbons: A comparison of line-by-line and narrow-band models using CF 4 as an example, J. Quant. Spectrosc. Radiat. Transfer, 55, Freckleton, R. S., E. J. Highwood, K. P. Shine, O. Wild, K. S. Law, and M. G. Sanderson (1998), Greenhouse gas radiative forcing: Effects of averaging and inhomogeneities in trace gas distribution, Q. J. R. Meteorol. Soc., 124, Godal, O., and J. Fuglestvedt (2002), Testing 100 year global warming potentials: Impacts on compliance costs and abatement profiles, Clim. Change, 52, Gohar, L. K., G. Myhre, and K. P. Shine (2004), Updated radiative forcing estimates of four halocarbons, J. Geophys. Res., 109, D01107, doi: /2003jd Harnisch, J., R. Borchers, P. Fabian, and M. Maiss (1996), Tropospheric trends for CF 4 and C 2 F 6 since 1982 derived from SF 6 dated stratospheric air, Geophys. Res. Lett., 23(10), Intergovernmental Panel on Climate Change (IPCC) (2002), Climate Change 2001, edited by J. T. Houghton et al., Cambridge Univ. Press, New York. Jain, A. K., B. P. Briegleb, K. Minschwaner, and D. J. Wuebbles (2000), Radiative forcings and global warming potentials of 39 greenhouse gases, J. Geophys. Res., 105, 20,773 20,790. Larin, I. K., A. G. Gushchin, and B. N. Maksimov (1997), Assessment of greenhouse potentials of CF 4,C 2 F 6, and C 3 F 8, Chem. Phys. Rep., 16, McDaniel, A. H., C. A. Cantrell, J. A. Davidson, R. E. Shetter, and J. G. Calveri (1991), The temperature dependent, infrared absorption crosssections for the chlorofluorocarbons: CFC-11, CFC-12, CFC-13, CFC- 14, CFC-22, CFC-113, CFC-114, and CFC-115, J. Atmos. Chem., 12, Minschwaner, K., R. W. Carver, B. P. Briegleb, and A. E. Roche (1998), Infrared radiative forcing and atmospheric lifetimes of trace species based on observations from UARS, J. Geophys. Res., 103, 23,243 23,253. Myhre, G., and F. Stordal (1997), Role of spatial and temporal variations in the computation of radiative forcing and GWP, J. Geophys. Res., 102, 11,181 11,200. Myhre, G., E. J. Highwood, K. P. Shine, and F. Stordal (1998), New estimates of radiative forcing due to well mixed greenhouse gases, Geophys. Res. Lett., 25(14), Nakic enovic, N., et al. (2000), IPCC Special Report on Emissions Scenarios, 599 pp., Cambridge Univ. Press, New York. Nemtchinov, V., and P. Varanasi (2003), Thermal infrared absorption crosssections of CF 4 for atmospheric applications, J. Quant. Spectrosc. Radiat. Transfer, 82, Pinnock, S., M. D. Hurley, K. P. Shine, T. J. Wallington, and T. J. Smyth (1995), Radiative forcing of climate by hydrochlorofluorocarbons and hydrofluorocarbons, J. Geophys. Res., 100, 23,227 23,238. Roehl, C. M., D. Boglu, C. Bruhl, and G. K. Moortgat (1995), Infrared band intensities and global warming potentials of CF 4,C 2 F 6,C 3 F 8, C 4 F 10,C 5 F 12, and C 6 F 14, Geophys. Res. Lett., 22(7), Saeki, S., M. Mizuno, and S. Kondo (1976), Infrared absorption intensities of methane and fluoromethanes, Spectrochim. Acta, 32A, Schurin, B. (1959), Infrared dispersion of carbon tetrafluoride, J. Chem. Phys., 3, 1 5. Shine, K. P. (1991), On the cause of the relative greenhouse strength of gases such as the halocarbons, J. Atmos. Sci., 48, Sihra, K., M. D. Hurley, K. P. Shine, and T. J. Wallington (2001), Updated radiative forcing estimates of 65 halocarbons and nonmethane hydrocarbons, J. Geophys. Res., 106, 20,493 20,505. Varanasi, P. and S. Chudamani (1988), Infrared intensities of some chlorofluorocarbons capable of perturbing the global climate, J. Geophys. Res., 93, Zander, R., et al. (1996), Increase of stratospheric carbon tetrafluoride (CF 4 ) based on ATMOS observations from space, Geophys. Res. Lett., 23(17), G. A. Buchanan and G. Marston, School of Chemistry, University of Reading, Reading RG6 6AD, UK. L. K. Gohar and K. P. Shine, Department of Meteorology, University of Reading, Reading RG6 6BB, UK. (l.k.gohar@reading.ac.uk) M. D. Hurley and T. J. Wallington, Physical and Environmental Sciences Department, Ford Motor Company, Dearborn, MI , USA. 8of8
Infrared absorption spectra, radiative efficiencies, and global warming potentials of perfluorocarbons: Comparison between experiment and theory
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 115,, doi:10.1029/2010jd014771, 2010 Infrared absorption spectra, radiative efficiencies, and global warming potentials of perfluorocarbons: Comparison between experiment
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 115,, doi:10.1029/2010jd014771, 2010 Infrared absorption spectra, radiative efficiencies, and global warming potentials of perfluorocarbons: Comparison between experiment
Spectrum of Radiation. Importance of Radiation Transfer. Radiation Intensity and Wavelength. Lecture 3: Atmospheric Radiative Transfer and Climate
 Lecture 3: Atmospheric Radiative Transfer and Climate Radiation Intensity and Wavelength frequency Planck s constant Solar and infrared radiation selective absorption and emission Selective absorption
Lecture 3: Atmospheric Radiative Transfer and Climate Radiation Intensity and Wavelength frequency Planck s constant Solar and infrared radiation selective absorption and emission Selective absorption
Lecture 3: Atmospheric Radiative Transfer and Climate
 Lecture 3: Atmospheric Radiative Transfer and Climate Solar and infrared radiation selective absorption and emission Selective absorption and emission Cloud and radiation Radiative-convective equilibrium
Lecture 3: Atmospheric Radiative Transfer and Climate Solar and infrared radiation selective absorption and emission Selective absorption and emission Cloud and radiation Radiative-convective equilibrium
Radiative forcing due to stratospheric water vapour from CH 4 oxidation
 Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 34, L01807, doi:10.1029/2006gl027472, 2007 Radiative forcing due to stratospheric water vapour from CH 4 oxidation Gunnar Myhre, 1,2 Jørgen
Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 34, L01807, doi:10.1029/2006gl027472, 2007 Radiative forcing due to stratospheric water vapour from CH 4 oxidation Gunnar Myhre, 1,2 Jørgen
Unique nature of Earth s atmosphere: O 2 present photosynthesis
 Atmospheric composition Major components N 2 78% O 2 21% Ar ~1% Medium components CO 2 370 ppmv (rising about 1.5 ppmv/year) CH 4 1700 ppbv H 2 O variable Trace components H 2 600 ppbv N 2 O 310 ppbv CO
Atmospheric composition Major components N 2 78% O 2 21% Ar ~1% Medium components CO 2 370 ppmv (rising about 1.5 ppmv/year) CH 4 1700 ppbv H 2 O variable Trace components H 2 600 ppbv N 2 O 310 ppbv CO
Lecture 3: Global Energy Cycle
 Lecture 3: Global Energy Cycle Planetary energy balance Greenhouse Effect Vertical energy balance Latitudinal energy balance Seasonal and diurnal cycles Solar Flux and Flux Density Solar Luminosity (L)
Lecture 3: Global Energy Cycle Planetary energy balance Greenhouse Effect Vertical energy balance Latitudinal energy balance Seasonal and diurnal cycles Solar Flux and Flux Density Solar Luminosity (L)
Water Vapor Multiplier of Carbon Dioxide
 Water Vapor Multiplier of Carbon Dioxide Harvey S. H. Lam October 10, 007 Abstract The temperature rise of the earth due to the direct greenhouse effects of atmospheric carbon dioxide can be amplified
Water Vapor Multiplier of Carbon Dioxide Harvey S. H. Lam October 10, 007 Abstract The temperature rise of the earth due to the direct greenhouse effects of atmospheric carbon dioxide can be amplified
Resolution of the uncertainties in the radiative forcing of HFC-134a
 Journal of Quantitative Spectroscopy & Radiative Transfer 93 (2005) 447 460 www.elsevier.com/locate/jqsrt Resolution of the uncertainties in the radiative forcing of HFC-134a Piers M. de F. Forster a,b,,
Journal of Quantitative Spectroscopy & Radiative Transfer 93 (2005) 447 460 www.elsevier.com/locate/jqsrt Resolution of the uncertainties in the radiative forcing of HFC-134a Piers M. de F. Forster a,b,,
WRAP UP: TOPIC #7 Atmospheric Structure & Composition
 WRAP UP: TOPIC #7 Atmospheric Structure & Composition SUMMARY OF KEY CONCEPTS: short version 1. Four gases N 2, O 2, Ar, & CO 2 comprise about 99% of the volume but minor trace Greenhouse Gases are extremely
WRAP UP: TOPIC #7 Atmospheric Structure & Composition SUMMARY OF KEY CONCEPTS: short version 1. Four gases N 2, O 2, Ar, & CO 2 comprise about 99% of the volume but minor trace Greenhouse Gases are extremely
Impact of different spectroscopic datasets on CH 4 retrievals from Jungfraujoch FTIR spectra
 Impact of different spectroscopic datasets on CH 4 retrievals from Jungfraujoch FTIR spectra P. Duchatelet (1), E. Mahieu (1), P. Demoulin (1), C. Frankenberg (2), F. Hase (3), J. Notholt (4), K. Petersen
Impact of different spectroscopic datasets on CH 4 retrievals from Jungfraujoch FTIR spectra P. Duchatelet (1), E. Mahieu (1), P. Demoulin (1), C. Frankenberg (2), F. Hase (3), J. Notholt (4), K. Petersen
Lecture 2 Global and Zonal-mean Energy Balance
 Lecture 2 Global and Zonal-mean Energy Balance A zero-dimensional view of the planet s energy balance RADIATIVE BALANCE Roughly 70% of the radiation received from the Sun at the top of Earth s atmosphere
Lecture 2 Global and Zonal-mean Energy Balance A zero-dimensional view of the planet s energy balance RADIATIVE BALANCE Roughly 70% of the radiation received from the Sun at the top of Earth s atmosphere
Consequences for Climate Feedback Interpretations
 CO 2 Forcing Induces Semi-direct Effects with Consequences for Climate Feedback Interpretations Timothy Andrews and Piers M. Forster School of Earth and Environment, University of Leeds, Leeds, LS2 9JT,
CO 2 Forcing Induces Semi-direct Effects with Consequences for Climate Feedback Interpretations Timothy Andrews and Piers M. Forster School of Earth and Environment, University of Leeds, Leeds, LS2 9JT,
Lecture 2: Global Energy Cycle
 Lecture 2: Global Energy Cycle Planetary energy balance Greenhouse Effect Vertical energy balance Solar Flux and Flux Density Solar Luminosity (L) the constant flux of energy put out by the sun L = 3.9
Lecture 2: Global Energy Cycle Planetary energy balance Greenhouse Effect Vertical energy balance Solar Flux and Flux Density Solar Luminosity (L) the constant flux of energy put out by the sun L = 3.9
SAP report on GWPs in Group I of Annex A, C and F. SAP co-chairs: David W. Fahey, Paul A. Newman, John Pyle, Bonfils Safari SAP members
 SAP report on GWPs in Group I of Annex A, C and F SAP co-chairs: David W. Fahey, Paul A. Newman, John Pyle, Bonfils Safari SAP members Background Kigali Amendment comment, 204....the Scientific Assessment
SAP report on GWPs in Group I of Annex A, C and F SAP co-chairs: David W. Fahey, Paul A. Newman, John Pyle, Bonfils Safari SAP members Background Kigali Amendment comment, 204....the Scientific Assessment
Lecture 9: Climate Sensitivity and Feedback Mechanisms
 Lecture 9: Climate Sensitivity and Feedback Mechanisms Basic radiative feedbacks (Plank, Water Vapor, Lapse-Rate Feedbacks) Ice albedo & Vegetation-Climate feedback Cloud feedback Biogeochemical feedbacks
Lecture 9: Climate Sensitivity and Feedback Mechanisms Basic radiative feedbacks (Plank, Water Vapor, Lapse-Rate Feedbacks) Ice albedo & Vegetation-Climate feedback Cloud feedback Biogeochemical feedbacks
Fig. 3.2 on Page 101. Warming. Evidence for CO 2. History of Global Warming-2. Fig. 3.2 Page 101. Drilled cores from ocean floors
 Chemistry in Context: Chapter 3:The Chemistry of Global Warming Practice Problems: All Ch. 3 problems with the blue codes or answers on Page 521. Venus Atmospheric pressure is 90x that of Earth 96% CO
Chemistry in Context: Chapter 3:The Chemistry of Global Warming Practice Problems: All Ch. 3 problems with the blue codes or answers on Page 521. Venus Atmospheric pressure is 90x that of Earth 96% CO
The Chemistry of Global Warming
 The Chemistry of Global Warming Venus Atmospheric pressure is 90x that of Earth 96% CO 2 and sulfuric acid clouds Average temperature = 450 C Expected temperature based on solar radiation and distance
The Chemistry of Global Warming Venus Atmospheric pressure is 90x that of Earth 96% CO 2 and sulfuric acid clouds Average temperature = 450 C Expected temperature based on solar radiation and distance
Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables
 Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2008jd010527, 2008 Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables Fangqun Yu 1 Received 2 June
Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2008jd010527, 2008 Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables Fangqun Yu 1 Received 2 June
CLOUD DETECTION AND DISTRIBUTIONS FROM MIPAS INFRA-RED LIMB OBSERVATIONS
 CLOUD DETECTION AND DISTRIBUTIONS FROM MIPAS INFRA-RED LIMB OBSERVATIONS J. Greenhough, J. J. Remedios, and H. Sembhi EOS, Space Research Centre, Department of Physics & Astronomy, University of Leicester,
CLOUD DETECTION AND DISTRIBUTIONS FROM MIPAS INFRA-RED LIMB OBSERVATIONS J. Greenhough, J. J. Remedios, and H. Sembhi EOS, Space Research Centre, Department of Physics & Astronomy, University of Leicester,
Infrared absorption cross section, radiative forcing, and GWP of four hydro#uoro(poly)ethers
 Atmospheric Environment 33 (1999) 4447}4458 Infrared absorption cross section, radiative forcing, and GWP of four hydro#uoro(poly)ethers G. Myhre *, C.J. Nielsen, D.L. Powell, F. Stordal Department of
Atmospheric Environment 33 (1999) 4447}4458 Infrared absorption cross section, radiative forcing, and GWP of four hydro#uoro(poly)ethers G. Myhre *, C.J. Nielsen, D.L. Powell, F. Stordal Department of
Energy and Radiation. GEOG/ENST 2331 Lecture 3 Ahrens: Chapter 2
 Energy and Radiation GEOG/ENST 2331 Lecture 3 Ahrens: Chapter 2 Last lecture: the Atmosphere! Mainly nitrogen (78%) and oxygen (21%)! T, P and ρ! The Ideal Gas Law! Temperature profiles Lecture outline!
Energy and Radiation GEOG/ENST 2331 Lecture 3 Ahrens: Chapter 2 Last lecture: the Atmosphere! Mainly nitrogen (78%) and oxygen (21%)! T, P and ρ! The Ideal Gas Law! Temperature profiles Lecture outline!
Extremes of Weather and the Latest Climate Change Science. Prof. Richard Allan, Department of Meteorology University of Reading
 Extremes of Weather and the Latest Climate Change Science Prof. Richard Allan, Department of Meteorology University of Reading Extreme weather climate change Recent extreme weather focusses debate on climate
Extremes of Weather and the Latest Climate Change Science Prof. Richard Allan, Department of Meteorology University of Reading Extreme weather climate change Recent extreme weather focusses debate on climate
Ozone Depletion by Hydrofluorocarbons
 Ozone Depletion by Hydrofluorocarbons Margaret M. Hurwitz 1, 2, Eric. L. Fleming 2, 3, Paul A. Newman 2, Feng Li 2, 4, Eli Mlawer 5, Karen Cady-Pereira 5, and Roshelle Bailey 1, 2 1 GESTAR, Morgan State
Ozone Depletion by Hydrofluorocarbons Margaret M. Hurwitz 1, 2, Eric. L. Fleming 2, 3, Paul A. Newman 2, Feng Li 2, 4, Eli Mlawer 5, Karen Cady-Pereira 5, and Roshelle Bailey 1, 2 1 GESTAR, Morgan State
Radiative Balance and the Faint Young Sun Paradox
 Radiative Balance and the Faint Young Sun Paradox Solar Irradiance Inverse Square Law Faint Young Sun Early Atmosphere Earth, Water, and Life 1. Water - essential medium for life. 2. Water - essential
Radiative Balance and the Faint Young Sun Paradox Solar Irradiance Inverse Square Law Faint Young Sun Early Atmosphere Earth, Water, and Life 1. Water - essential medium for life. 2. Water - essential
Climate Change: some basic physical concepts and simple models. David Andrews
 Climate Change: some basic physical concepts and simple models David Andrews 1 Some of you have used my textbook An Introduction to Atmospheric Physics (IAP) I am now preparing a 2 nd edition. The main
Climate Change: some basic physical concepts and simple models David Andrews 1 Some of you have used my textbook An Introduction to Atmospheric Physics (IAP) I am now preparing a 2 nd edition. The main
Temperature Scales
 TEMPERATURE is a measure of the internal heat energy of a substance. The molecules that make up all matter are in constant motion. By internal heat energy, we really mean this random molecular motion.
TEMPERATURE is a measure of the internal heat energy of a substance. The molecules that make up all matter are in constant motion. By internal heat energy, we really mean this random molecular motion.
Lecture Outlines PowerPoint. Chapter 16 Earth Science 11e Tarbuck/Lutgens
 Lecture Outlines PowerPoint Chapter 16 Earth Science 11e Tarbuck/Lutgens 2006 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the use of instructors
Lecture Outlines PowerPoint Chapter 16 Earth Science 11e Tarbuck/Lutgens 2006 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the use of instructors
1. Weather and climate.
 Lecture 31. Introduction to climate and climate change. Part 1. Objectives: 1. Weather and climate. 2. Earth s radiation budget. 3. Clouds and radiation field. Readings: Turco: p. 320-349; Brimblecombe:
Lecture 31. Introduction to climate and climate change. Part 1. Objectives: 1. Weather and climate. 2. Earth s radiation budget. 3. Clouds and radiation field. Readings: Turco: p. 320-349; Brimblecombe:
Lecture 2: Global Energy Cycle
 Lecture 2: Global Energy Cycle Planetary energy balance Greenhouse Effect Selective absorption Vertical energy balance Solar Flux and Flux Density Solar Luminosity (L) the constant flux of energy put out
Lecture 2: Global Energy Cycle Planetary energy balance Greenhouse Effect Selective absorption Vertical energy balance Solar Flux and Flux Density Solar Luminosity (L) the constant flux of energy put out
Solar Flux and Flux Density. Lecture 2: Global Energy Cycle. Solar Energy Incident On the Earth. Solar Flux Density Reaching Earth
 Lecture 2: Global Energy Cycle Solar Flux and Flux Density Planetary energy balance Greenhouse Effect Selective absorption Vertical energy balance Solar Luminosity (L) the constant flux of energy put out
Lecture 2: Global Energy Cycle Solar Flux and Flux Density Planetary energy balance Greenhouse Effect Selective absorption Vertical energy balance Solar Luminosity (L) the constant flux of energy put out
FIRST HIGH-RESOLUTION ANALYSIS OF PHOSGENE 35 Cl 2. CO AND 35 Cl 37 ClCO FUNDAMENTALS IN THE CM -1 SPECTRAL REGION
 FIRST HIGH-RESOLUTION ANALYSIS OF PHOSGENE 35 Cl 2 CO AND 35 Cl 37 ClCO FUNDAMENTALS IN THE 250-480 CM -1 SPECTRAL REGION F. Kwabia Tchana 1, M. Ndao 1, L. Manceron 2, A. Perrin 1, J. M. Flaud 1, W.J.
FIRST HIGH-RESOLUTION ANALYSIS OF PHOSGENE 35 Cl 2 CO AND 35 Cl 37 ClCO FUNDAMENTALS IN THE 250-480 CM -1 SPECTRAL REGION F. Kwabia Tchana 1, M. Ndao 1, L. Manceron 2, A. Perrin 1, J. M. Flaud 1, W.J.
Period 13 Solutions: Earth as an Energy System
 Period 13 Solutions: Earth as an Energy System 13.1 The Earth-Sun System 1) Energy from the sun Observe the models of the Earth, Moon, and Sun in the room. a) Imagine that the distance between the Earth
Period 13 Solutions: Earth as an Energy System 13.1 The Earth-Sun System 1) Energy from the sun Observe the models of the Earth, Moon, and Sun in the room. a) Imagine that the distance between the Earth
Equation for Global Warming
 Equation for Global Warming Derivation and Application Contents 1. Amazing carbon dioxide How can a small change in carbon dioxide (CO 2 ) content make a critical difference to the actual global surface
Equation for Global Warming Derivation and Application Contents 1. Amazing carbon dioxide How can a small change in carbon dioxide (CO 2 ) content make a critical difference to the actual global surface
Global long lived chemical modes excited in a 3 D chemistry transport model: Stratospheric N 2 O, NO y,o 3 and CH 4 chemistry
 Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 37,, doi:10.1029/2009gl042243, 2010 Global long lived chemical modes excited in a 3 D chemistry transport model: Stratospheric N 2 O, NO y,o
Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 37,, doi:10.1029/2009gl042243, 2010 Global long lived chemical modes excited in a 3 D chemistry transport model: Stratospheric N 2 O, NO y,o
Preliminary report: Analyses of tcfp s potential impact on atmospheric ozone
 Preliminary report: Analyses of tcfp s potential impact on atmospheric ozone Dong Wang, Seth Olsen, and Donald Wuebbles Department of Atmospheric Sciences University of Illinois, Urbana, IL 61801 Abstract
Preliminary report: Analyses of tcfp s potential impact on atmospheric ozone Dong Wang, Seth Olsen, and Donald Wuebbles Department of Atmospheric Sciences University of Illinois, Urbana, IL 61801 Abstract
Understanding the Greenhouse Effect
 EESC V2100 The Climate System spring 200 Understanding the Greenhouse Effect Yochanan Kushnir Lamont Doherty Earth Observatory of Columbia University Palisades, NY 1096, USA kushnir@ldeo.columbia.edu Equilibrium
EESC V2100 The Climate System spring 200 Understanding the Greenhouse Effect Yochanan Kushnir Lamont Doherty Earth Observatory of Columbia University Palisades, NY 1096, USA kushnir@ldeo.columbia.edu Equilibrium
G109 Midterm Exam (Version A) October 10, 2006 Instructor: Dr C.M. Brown 1. Time allowed 50 mins. Total possible points: 40 number of pages: 5
 G109 Midterm Exam (Version A) October 10, 2006 Instructor: Dr C.M. Brown 1 Time allowed 50 mins. Total possible points: 40 number of pages: 5 Part A: Short Answer & Problems (12), Fill in the Blanks (6).
G109 Midterm Exam (Version A) October 10, 2006 Instructor: Dr C.M. Brown 1 Time allowed 50 mins. Total possible points: 40 number of pages: 5 Part A: Short Answer & Problems (12), Fill in the Blanks (6).
Sensitivity of climate forcing and response to dust optical properties in an idealized model
 Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2006jd007198, 2007 Sensitivity of climate forcing and response to dust optical properties in an idealized model Karen
Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2006jd007198, 2007 Sensitivity of climate forcing and response to dust optical properties in an idealized model Karen
Effect of zonal asymmetries in stratospheric ozone on simulated Southern Hemisphere climate trends
 Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 36, L18701, doi:10.1029/2009gl040419, 2009 Effect of zonal asymmetries in stratospheric ozone on simulated Southern Hemisphere climate trends
Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 36, L18701, doi:10.1029/2009gl040419, 2009 Effect of zonal asymmetries in stratospheric ozone on simulated Southern Hemisphere climate trends
Thursday, November 1st.
 Thursday, November 1st. Announcements. Homework 7 - due Tuesday, Nov. 6 Homework 8 - paper 2 topics, questions and sources due Tuesday, Nov. 13 Midterm Paper 2 - due Tuesday, Nov. 20 I will hand out a
Thursday, November 1st. Announcements. Homework 7 - due Tuesday, Nov. 6 Homework 8 - paper 2 topics, questions and sources due Tuesday, Nov. 13 Midterm Paper 2 - due Tuesday, Nov. 20 I will hand out a
Stefan-Boltzmann law for the Earth as a black body (or perfect radiator) gives:
 2. Derivation of IPCC expression ΔF = 5.35 ln (C/C 0 ) 2.1 Derivation One The assumptions we will make allow us to represent the real atmosphere. This remarkably reasonable representation of the real atmosphere
2. Derivation of IPCC expression ΔF = 5.35 ln (C/C 0 ) 2.1 Derivation One The assumptions we will make allow us to represent the real atmosphere. This remarkably reasonable representation of the real atmosphere
Lecture 2-07: The greenhouse, global heat engine.
 Lecture 2-07: The greenhouse, global heat engine http://en.wikipedia.org/ the sun s ultraviolet (left) and infrared radiation imagers.gsfc.nasa.gov/ems/uv.html www.odysseymagazine.com/images SOLAR FLARES
Lecture 2-07: The greenhouse, global heat engine http://en.wikipedia.org/ the sun s ultraviolet (left) and infrared radiation imagers.gsfc.nasa.gov/ems/uv.html www.odysseymagazine.com/images SOLAR FLARES
Chapter 11 Lecture Outline. Heating the Atmosphere
 Chapter 11 Lecture Outline Heating the Atmosphere They are still here! Focus on the Atmosphere Weather Occurs over a short period of time Constantly changing Climate Averaged over a long period of time
Chapter 11 Lecture Outline Heating the Atmosphere They are still here! Focus on the Atmosphere Weather Occurs over a short period of time Constantly changing Climate Averaged over a long period of time
PROBLEMS Sources of CO Sources of tropospheric ozone
 220 PROBLEMS 11. 1 Sources of CO The two principal sources of CO to the atmosphere are oxidation of CH 4 and combustion. Mean rate constants for oxidation of CH 4 and CO by OH in the troposphere are k
220 PROBLEMS 11. 1 Sources of CO The two principal sources of CO to the atmosphere are oxidation of CH 4 and combustion. Mean rate constants for oxidation of CH 4 and CO by OH in the troposphere are k
Peter Bernath. Old Dominion University, Norfolk, VA and University of Waterloo, Waterloo, ON
 THE ATMOSPHERIC CHEMISTRY EXPERIMENT (ACE): Greenhouse Gas Measurements of CO 2, CFCs and HFCs Peter Bernath Old Dominion University, Norfolk, VA and University of Waterloo, Waterloo, ON ACE Satellite
THE ATMOSPHERIC CHEMISTRY EXPERIMENT (ACE): Greenhouse Gas Measurements of CO 2, CFCs and HFCs Peter Bernath Old Dominion University, Norfolk, VA and University of Waterloo, Waterloo, ON ACE Satellite
CORRELATION BETWEEN ATMOSPHERIC COMPOSITION AND VERTICAL STRUCTURE AS MEASURED BY THREE GENERATIONS OF HYPERSPECTRAL SOUNDERS IN SPACE
 CORRELATION BETWEEN ATMOSPHERIC COMPOSITION AND VERTICAL STRUCTURE AS MEASURED BY THREE GENERATIONS OF HYPERSPECTRAL SOUNDERS IN SPACE Nadia Smith 1, Elisabeth Weisz 1, and Allen Huang 1 1 Space Science
CORRELATION BETWEEN ATMOSPHERIC COMPOSITION AND VERTICAL STRUCTURE AS MEASURED BY THREE GENERATIONS OF HYPERSPECTRAL SOUNDERS IN SPACE Nadia Smith 1, Elisabeth Weisz 1, and Allen Huang 1 1 Space Science
Radiation and the atmosphere
 Radiation and the atmosphere Of great importance is the difference between how the atmosphere transmits, absorbs, and scatters solar and terrestrial radiation streams. The most important statement that
Radiation and the atmosphere Of great importance is the difference between how the atmosphere transmits, absorbs, and scatters solar and terrestrial radiation streams. The most important statement that
Global Energy Balance: Greenhouse Effect
 Global Energy Balance: Greenhouse Effect Atmospheric Composition & Structure Physical Causes of Greenhouse Effects Chapter 3: 44 48. Atmospheric Composition Why does water vapor vary so much? Saturation
Global Energy Balance: Greenhouse Effect Atmospheric Composition & Structure Physical Causes of Greenhouse Effects Chapter 3: 44 48. Atmospheric Composition Why does water vapor vary so much? Saturation
CHAPTER 1. MEASURES OF ATMOSPHERIC COMPOSITION
 1 CHAPTER 1. MEASURES OF ATMOSPHERIC COMPOSITION The objective of atmospheric chemistry is to understand the factors that control the concentrations of chemical species in the atmosphere. In this book
1 CHAPTER 1. MEASURES OF ATMOSPHERIC COMPOSITION The objective of atmospheric chemistry is to understand the factors that control the concentrations of chemical species in the atmosphere. In this book
CLIMATE AND CLIMATE CHANGE MIDTERM EXAM ATM S 211 FEB 9TH 2012 V1
 CLIMATE AND CLIMATE CHANGE MIDTERM EXAM ATM S 211 FEB 9TH 2012 V1 Name: Student ID: Please answer the following questions on your Scantron Multiple Choice [1 point each] (1) The gases that contribute to
CLIMATE AND CLIMATE CHANGE MIDTERM EXAM ATM S 211 FEB 9TH 2012 V1 Name: Student ID: Please answer the following questions on your Scantron Multiple Choice [1 point each] (1) The gases that contribute to
Climate Change 2007: The Physical Science Basis
 Climate Change 2007: The Physical Science Basis Working Group I Contribution to the IPCC Fourth Assessment Report Presented by R.K. Pachauri, IPCC Chair and Bubu Jallow, WG 1 Vice Chair Nairobi, 6 February
Climate Change 2007: The Physical Science Basis Working Group I Contribution to the IPCC Fourth Assessment Report Presented by R.K. Pachauri, IPCC Chair and Bubu Jallow, WG 1 Vice Chair Nairobi, 6 February
Chapter 2 Available Solar Radiation
 Chapter 2 Available Solar Radiation DEFINITIONS Figure shows the primary radiation fluxes on a surface at or near the ground that are important in connection with solar thermal processes. DEFINITIONS It
Chapter 2 Available Solar Radiation DEFINITIONS Figure shows the primary radiation fluxes on a surface at or near the ground that are important in connection with solar thermal processes. DEFINITIONS It
 http://en.wikipedia.org/wiki/file:modtranradiativeforcingdoubleco2.png http://en.wikipedia.org/wiki/radiative_forcing Introduction Emission of Radiation from the Earth By Colin Davidson, November 2012
http://en.wikipedia.org/wiki/file:modtranradiativeforcingdoubleco2.png http://en.wikipedia.org/wiki/radiative_forcing Introduction Emission of Radiation from the Earth By Colin Davidson, November 2012
THE EXOSPHERIC HEAT BUDGET
 E&ES 359, 2008, p.1 THE EXOSPHERIC HEAT BUDGET What determines the temperature on earth? In this course we are interested in quantitative aspects of the fundamental processes that drive the earth machine.
E&ES 359, 2008, p.1 THE EXOSPHERIC HEAT BUDGET What determines the temperature on earth? In this course we are interested in quantitative aspects of the fundamental processes that drive the earth machine.
MIPAS Observations of CFC Trends
 MIPAS Observations of CFC Trends Alastair Burgess Anu Dudhia, Don Grainger AOPP, University of Oxford aburgess@atm.ox.ac.uk Page 1 What is MIPAS? Michelson Interferometer for Passive Atmospheric Sounding
MIPAS Observations of CFC Trends Alastair Burgess Anu Dudhia, Don Grainger AOPP, University of Oxford aburgess@atm.ox.ac.uk Page 1 What is MIPAS? Michelson Interferometer for Passive Atmospheric Sounding
AT 350 EXAM #1 February 21, 2008
 This exam covers Ahrens Chapters 1 and 2, plus related lecture notes Write the letter of the choice that best completes the statement or answers the question. b_ 1. The Earth s atmosphere is currently
This exam covers Ahrens Chapters 1 and 2, plus related lecture notes Write the letter of the choice that best completes the statement or answers the question. b_ 1. The Earth s atmosphere is currently
Lecture 5: Greenhouse Effect
 /30/2018 Lecture 5: Greenhouse Effect Global Energy Balance S/ * (1-A) terrestrial radiation cooling Solar radiation warming T S Global Temperature atmosphere Wien s Law Shortwave and Longwave Radiation
/30/2018 Lecture 5: Greenhouse Effect Global Energy Balance S/ * (1-A) terrestrial radiation cooling Solar radiation warming T S Global Temperature atmosphere Wien s Law Shortwave and Longwave Radiation
Chapter 3. Multiple Choice Questions
 Chapter 3 Multiple Choice Questions 1. In the case of electromagnetic energy, an object that is hot: a. radiates much more energy than a cool object b. radiates much less energy than a cool object c. radiates
Chapter 3 Multiple Choice Questions 1. In the case of electromagnetic energy, an object that is hot: a. radiates much more energy than a cool object b. radiates much less energy than a cool object c. radiates
Lecture 5: Greenhouse Effect
 Lecture 5: Greenhouse Effect S/4 * (1-A) T A 4 T S 4 T A 4 Wien s Law Shortwave and Longwave Radiation Selected Absorption Greenhouse Effect Global Energy Balance terrestrial radiation cooling Solar radiation
Lecture 5: Greenhouse Effect S/4 * (1-A) T A 4 T S 4 T A 4 Wien s Law Shortwave and Longwave Radiation Selected Absorption Greenhouse Effect Global Energy Balance terrestrial radiation cooling Solar radiation
Solar-terrestrial coupling evidenced by periodic behavior in geomagnetic indexes and the infrared energy budget of the thermosphere
 GEOPHYSICAL RESEARCH LETTERS, VOL. 35, L05808, doi:10.1029/2007gl032620, 2008 Solar-terrestrial coupling evidenced by periodic behavior in geomagnetic indexes and the infrared energy budget of the thermosphere
GEOPHYSICAL RESEARCH LETTERS, VOL. 35, L05808, doi:10.1029/2007gl032620, 2008 Solar-terrestrial coupling evidenced by periodic behavior in geomagnetic indexes and the infrared energy budget of the thermosphere
1. The frequency of an electromagnetic wave is proportional to its wavelength. a. directly *b. inversely
 CHAPTER 3 SOLAR AND TERRESTRIAL RADIATION MULTIPLE CHOICE QUESTIONS 1. The frequency of an electromagnetic wave is proportional to its wavelength. a. directly *b. inversely 2. is the distance between successive
CHAPTER 3 SOLAR AND TERRESTRIAL RADIATION MULTIPLE CHOICE QUESTIONS 1. The frequency of an electromagnetic wave is proportional to its wavelength. a. directly *b. inversely 2. is the distance between successive
Introduction to Climate ~ Part I ~
 2015/11/16 TCC Seminar JMA Introduction to Climate ~ Part I ~ Shuhei MAEDA (MRI/JMA) Climate Research Department Meteorological Research Institute (MRI/JMA) 1 Outline of the lecture 1. Climate System (
2015/11/16 TCC Seminar JMA Introduction to Climate ~ Part I ~ Shuhei MAEDA (MRI/JMA) Climate Research Department Meteorological Research Institute (MRI/JMA) 1 Outline of the lecture 1. Climate System (
Lecture 6 - spectroscopy
 Lecture 6 - spectroscopy 1 Light Electromagnetic radiation can be thought of as either a wave or as a particle (particle/wave duality). For scattering of light by particles, air, and surfaces, wave theory
Lecture 6 - spectroscopy 1 Light Electromagnetic radiation can be thought of as either a wave or as a particle (particle/wave duality). For scattering of light by particles, air, and surfaces, wave theory
Lecture 4: Radiation Transfer
 Lecture 4: Radiation Transfer Spectrum of radiation Stefan-Boltzmann law Selective absorption and emission Reflection and scattering Remote sensing Importance of Radiation Transfer Virtually all the exchange
Lecture 4: Radiation Transfer Spectrum of radiation Stefan-Boltzmann law Selective absorption and emission Reflection and scattering Remote sensing Importance of Radiation Transfer Virtually all the exchange
Lecture # 04 January 27, 2010, Wednesday Energy & Radiation
 Lecture # 04 January 27, 2010, Wednesday Energy & Radiation Kinds of energy Energy transfer mechanisms Radiation: electromagnetic spectrum, properties & principles Solar constant Atmospheric influence
Lecture # 04 January 27, 2010, Wednesday Energy & Radiation Kinds of energy Energy transfer mechanisms Radiation: electromagnetic spectrum, properties & principles Solar constant Atmospheric influence
Refractive indices of water and ice in the to 2.5-gm spectral range
 Refractive indices of water and ice in the 0.65- to 2.5-gm spectral range Linhong Kou, Daniel Labrie, and Petr Chylek New accurate values of the imaginary part, k, of the refractive index of water at T
Refractive indices of water and ice in the 0.65- to 2.5-gm spectral range Linhong Kou, Daniel Labrie, and Petr Chylek New accurate values of the imaginary part, k, of the refractive index of water at T
Lecture 4: Global Energy Balance
 Lecture : Global Energy Balance S/ * (1-A) T A T S T A Blackbody Radiation Layer Model Greenhouse Effect Global Energy Balance terrestrial radiation cooling Solar radiation warming Global Temperature atmosphere
Lecture : Global Energy Balance S/ * (1-A) T A T S T A Blackbody Radiation Layer Model Greenhouse Effect Global Energy Balance terrestrial radiation cooling Solar radiation warming Global Temperature atmosphere
Lecture 4: Global Energy Balance. Global Energy Balance. Solar Flux and Flux Density. Blackbody Radiation Layer Model.
 Lecture : Global Energy Balance Global Energy Balance S/ * (1-A) terrestrial radiation cooling Solar radiation warming T S Global Temperature Blackbody Radiation ocean land Layer Model energy, water, and
Lecture : Global Energy Balance Global Energy Balance S/ * (1-A) terrestrial radiation cooling Solar radiation warming T S Global Temperature Blackbody Radiation ocean land Layer Model energy, water, and
Atmospheric Radiation
 Atmospheric Radiation NASA photo gallery Introduction The major source of earth is the sun. The sun transfer energy through the earth by radiated electromagnetic wave. In vacuum, electromagnetic waves
Atmospheric Radiation NASA photo gallery Introduction The major source of earth is the sun. The sun transfer energy through the earth by radiated electromagnetic wave. In vacuum, electromagnetic waves
Topic 2.11 ANALYTICAL TECHNIQUES. High Resolution Mass Spectrometry Infra-red Spectroscopy
 Topic 2.11 ANALYTICAL TECHNIQUES High Resolution Mass Spectrometry Infra-red Spectroscopy HIGH RESOLUTION MASS SPECTROMETRY The technique of mass spectrometry was used in Unit 1 to: a) determine the relative
Topic 2.11 ANALYTICAL TECHNIQUES High Resolution Mass Spectrometry Infra-red Spectroscopy HIGH RESOLUTION MASS SPECTROMETRY The technique of mass spectrometry was used in Unit 1 to: a) determine the relative
Fundamentals of Atmospheric Radiation and its Parameterization
 Source Materials Fundamentals of Atmospheric Radiation and its Parameterization The following notes draw extensively from Fundamentals of Atmospheric Physics by Murry Salby and Chapter 8 of Parameterization
Source Materials Fundamentals of Atmospheric Radiation and its Parameterization The following notes draw extensively from Fundamentals of Atmospheric Physics by Murry Salby and Chapter 8 of Parameterization
Day 1 of Global Warming. Copyright 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
 Day 1 of Global Warming Copyright 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings The Atmosphere Atmosphere = the thin layer (1/100 th of Earth s diameter) of gases that surrounds
Day 1 of Global Warming Copyright 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings The Atmosphere Atmosphere = the thin layer (1/100 th of Earth s diameter) of gases that surrounds
- global radiative energy balance
 (1 of 14) Further Reading: Chapter 04 of the text book Outline - global radiative energy balance - insolation and climatic regimes - composition of the atmosphere (2 of 14) Introduction Last time we discussed
(1 of 14) Further Reading: Chapter 04 of the text book Outline - global radiative energy balance - insolation and climatic regimes - composition of the atmosphere (2 of 14) Introduction Last time we discussed
P T = P A + P B + P C..P i Boyle's Law The volume of a given quantity of gas varies inversely with the pressure of the gas, at a constant temperature.
 CHEM/TOX 336 Winter 2004 Lecture 2 Review Atmospheric Chemistry Gas Chemistry Review The Gaseous State: our atmosphere consists of gases Confined only by gravity force of gas on a unit area is due to the
CHEM/TOX 336 Winter 2004 Lecture 2 Review Atmospheric Chemistry Gas Chemistry Review The Gaseous State: our atmosphere consists of gases Confined only by gravity force of gas on a unit area is due to the
Key Feedbacks in the Climate System
 Key Feedbacks in the Climate System With a Focus on Climate Sensitivity SOLAS Summer School 12 th of August 2009 Thomas Schneider von Deimling, Potsdam Institute for Climate Impact Research Why do Climate
Key Feedbacks in the Climate System With a Focus on Climate Sensitivity SOLAS Summer School 12 th of August 2009 Thomas Schneider von Deimling, Potsdam Institute for Climate Impact Research Why do Climate
Inaugural University of Michigan Science Olympiad Tournament
 Inaugural University of Michigan Science Olympiad Tournament The test may be taken apart. Ties will be broken based on predetermined questions and quality of response. Remote Sensing Test length: 50 Minutes
Inaugural University of Michigan Science Olympiad Tournament The test may be taken apart. Ties will be broken based on predetermined questions and quality of response. Remote Sensing Test length: 50 Minutes
The Planck Blackbody Equation and Atmospheric Radiative Transfer
 The Planck Blackbody Equation and Atmospheric Radiative Transfer Roy Clark Ventura Photonics There appears to be a lot of confusion over the use of the terms blackbody absorption and equilibrium in the
The Planck Blackbody Equation and Atmospheric Radiative Transfer Roy Clark Ventura Photonics There appears to be a lot of confusion over the use of the terms blackbody absorption and equilibrium in the
3. Carbon Dioxide (CO 2 )
 3. Carbon Dioxide (CO 2 ) Basic information on CO 2 with regard to environmental issues Carbon dioxide (CO 2 ) is a significant greenhouse gas that has strong absorption bands in the infrared region and
3. Carbon Dioxide (CO 2 ) Basic information on CO 2 with regard to environmental issues Carbon dioxide (CO 2 ) is a significant greenhouse gas that has strong absorption bands in the infrared region and
The ozone hole indirect effect: Cloud-radiative anomalies accompanying the poleward shift of the eddy-driven jet in the Southern Hemisphere
 GEOPHYSICAL RESEARCH LETTERS, VOL. 4, 388 392, doi:1.12/grl.575, 213 The ozone hole indirect effect: Cloud-radiative anomalies accompanying the poleward shift of the eddy-driven jet in the Southern Hemisphere
GEOPHYSICAL RESEARCH LETTERS, VOL. 4, 388 392, doi:1.12/grl.575, 213 The ozone hole indirect effect: Cloud-radiative anomalies accompanying the poleward shift of the eddy-driven jet in the Southern Hemisphere
WATER VAPOUR RETRIEVAL FROM GOME DATA INCLUDING CLOUDY SCENES
 WATER VAPOUR RETRIEVAL FROM GOME DATA INCLUDING CLOUDY SCENES S. Noël, H. Bovensmann, J. P. Burrows Institute of Environmental Physics, University of Bremen, FB 1, P. O. Box 33 4 4, D 28334 Bremen, Germany
WATER VAPOUR RETRIEVAL FROM GOME DATA INCLUDING CLOUDY SCENES S. Noël, H. Bovensmann, J. P. Burrows Institute of Environmental Physics, University of Bremen, FB 1, P. O. Box 33 4 4, D 28334 Bremen, Germany
surrounds Earth and protects it somewhat from solar radiation. Like all other matter, air has weight,
 The air that we breathe comes from the atmosphere, a thin gaseous layer that surrounds Earth and protects it somewhat from solar radiation. Like all other matter, air has weight, but this weight varies
The air that we breathe comes from the atmosphere, a thin gaseous layer that surrounds Earth and protects it somewhat from solar radiation. Like all other matter, air has weight, but this weight varies
Role of spatial and temporal variations in the computation of radiative forcing and GWP
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 102, NO. D10, PAGES 11,181-11,200, MAY 27, 1997 Role of spatial and temporal variations in the computation of radiative forcing and GWP Gunnar Myhre Department of
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 102, NO. D10, PAGES 11,181-11,200, MAY 27, 1997 Role of spatial and temporal variations in the computation of radiative forcing and GWP Gunnar Myhre Department of
STUDY ON ABSORPTION CROSS-SECTIONS IN THE UV AND VISIBLE
 ESA Contract 11340/95/NL/CN STUDY ON ABSORPTION CROSS-SECTIONS IN THE UV AND VISIBLE EXECUTIVE SUMMARY October 1998 Study Team Principal Scientist Dr John Ballard Dr David Newnham Space Science Department
ESA Contract 11340/95/NL/CN STUDY ON ABSORPTION CROSS-SECTIONS IN THE UV AND VISIBLE EXECUTIVE SUMMARY October 1998 Study Team Principal Scientist Dr John Ballard Dr David Newnham Space Science Department
Topic # 13 (cont.) OZONE DEPLETION IN THE STRATOSPHERE Part II
 Topic # 13 (cont.) OZONE DEPLETION IN THE STRATOSPHERE Part II A Story of Anthropogenic Disruption of a Natural Steady State p 77-79 in Class Notes REVIEW... Q Is the depletion of STRATOSPHERIC OZONE (in
Topic # 13 (cont.) OZONE DEPLETION IN THE STRATOSPHERE Part II A Story of Anthropogenic Disruption of a Natural Steady State p 77-79 in Class Notes REVIEW... Q Is the depletion of STRATOSPHERIC OZONE (in
Meteorology Pretest on Chapter 2
 Meteorology Pretest on Chapter 2 MULTIPLE CHOICE 1. The earth emits terrestrial radiation a) only at night b) all the time c) only during winter d) only over the continents 2. If an imbalance occurs between
Meteorology Pretest on Chapter 2 MULTIPLE CHOICE 1. The earth emits terrestrial radiation a) only at night b) all the time c) only during winter d) only over the continents 2. If an imbalance occurs between
2. What does a mercury barometer measure? Describe this device and explain how it physically works.
 Written Homework #1 Key NATS 101, Sec. 13 Fall 2010 40 Points total 10 points per graded question 10 points for attempting all questions. 1. What is the difference between mass and weight? Mass is an intrinsic
Written Homework #1 Key NATS 101, Sec. 13 Fall 2010 40 Points total 10 points per graded question 10 points for attempting all questions. 1. What is the difference between mass and weight? Mass is an intrinsic
Assignment for the Infrared Spectrum of Solid Sodium Propionate from Low-Temperature Measurements in Combination with,3 C Isotopic Shifts
 Assignment for the Infrared Spectrum of Solid Sodium Propionate from Low-Temperature Measurements in Combination with,3 C Isotopic Shifts Masato Kakihana and Tadashi Nagumo Department of Chemistry, The
Assignment for the Infrared Spectrum of Solid Sodium Propionate from Low-Temperature Measurements in Combination with,3 C Isotopic Shifts Masato Kakihana and Tadashi Nagumo Department of Chemistry, The
TIME SERIES COMPARISONS OF MIPAS LEVEL 2 NEAR REAL TIME PRODUCTS WITH CLIMATOLOGY
 TIME SERIES COMPARISONS OF MIPAS LEVEL 2 NEAR REAL TIME PRODUCTS WITH CLIMATOLOGY Vivienne Payne, Anu Dudhia, and Chiara Piccolo Atmospheric, Oceanic and Planetary Physics, Department of Physics, University
TIME SERIES COMPARISONS OF MIPAS LEVEL 2 NEAR REAL TIME PRODUCTS WITH CLIMATOLOGY Vivienne Payne, Anu Dudhia, and Chiara Piccolo Atmospheric, Oceanic and Planetary Physics, Department of Physics, University
What is the IPCC? Intergovernmental Panel on Climate Change
 IPCC WG1 FAQ What is the IPCC? Intergovernmental Panel on Climate Change The IPCC is a scientific intergovernmental body set up by the World Meteorological Organization (WMO) and by the United Nations
IPCC WG1 FAQ What is the IPCC? Intergovernmental Panel on Climate Change The IPCC is a scientific intergovernmental body set up by the World Meteorological Organization (WMO) and by the United Nations
SCIAMACHY REFLECTANCE AND POLARISATION VALIDATION: SCIAMACHY VERSUS POLDER
 SCIAMACHY REFLECTANCE AND POLARISATION VALIDATION: SCIAMACHY VERSUS POLDER L. G. Tilstra (1), P. Stammes (1) (1) Royal Netherlands Meteorological Institute (KNMI), P.O. Box 201, 3730 AE de Bilt, The Netherlands
SCIAMACHY REFLECTANCE AND POLARISATION VALIDATION: SCIAMACHY VERSUS POLDER L. G. Tilstra (1), P. Stammes (1) (1) Royal Netherlands Meteorological Institute (KNMI), P.O. Box 201, 3730 AE de Bilt, The Netherlands
GE510 Physical Principles of the Envt
 GE510 Physical Principles of the Envt Radiative Forcing of Climate Radiative Forcing of Climate 1. What is radiative forcing of climate? Conceptually - A simple definition - Physical properties of molecules
GE510 Physical Principles of the Envt Radiative Forcing of Climate Radiative Forcing of Climate 1. What is radiative forcing of climate? Conceptually - A simple definition - Physical properties of molecules
The last 2 million years.
 Lecture 5: Earth Climate History - Continued Ice core records from both Greenland and Antarctica have produced a remarkable record of climate during the last 450,000 years. Trapped air bubbles provide
Lecture 5: Earth Climate History - Continued Ice core records from both Greenland and Antarctica have produced a remarkable record of climate during the last 450,000 years. Trapped air bubbles provide
Supplement of Iodine oxide in the global marine boundary layer
 Supplement of Atmos. Chem. Phys., 1,, 01 http://www.atmos-chem-phys.net/1//01/ doi:.1/acp-1--01-supplement Author(s) 01. CC Attribution.0 License. Supplement of Iodine oxide in the global marine boundary
Supplement of Atmos. Chem. Phys., 1,, 01 http://www.atmos-chem-phys.net/1//01/ doi:.1/acp-1--01-supplement Author(s) 01. CC Attribution.0 License. Supplement of Iodine oxide in the global marine boundary
Today. Events. Terrestrial Planet Geology - Earth. Terrestrial Planet Atmospheres. Homework DUE next time
 Today Terrestrial Planet Geology - Earth Terrestrial Planet Atmospheres Events Homework DUE next time Ring of Fire Boundaries of plates traced by Earthquakes and Volcanos Plate Motions Measurements of
Today Terrestrial Planet Geology - Earth Terrestrial Planet Atmospheres Events Homework DUE next time Ring of Fire Boundaries of plates traced by Earthquakes and Volcanos Plate Motions Measurements of
7.5-year global trends in GOME cloud cover and humidity - a signal of climate change? Institut für Umweltphysik, Uni-Heidelberg, Germany
 7.5-year global trends in GOME cloud cover and humidity - a signal of climate change? T. Wagner, S. Beirle, M. Grzegorski, S. Sanghavi, U. Platt Institut für Umweltphysik, Uni-Heidelberg, Germany The Greenhouse
7.5-year global trends in GOME cloud cover and humidity - a signal of climate change? T. Wagner, S. Beirle, M. Grzegorski, S. Sanghavi, U. Platt Institut für Umweltphysik, Uni-Heidelberg, Germany The Greenhouse
Arctic sea ice response to atmospheric forcings with varying levels of anthropogenic warming and climate variability
 GEOPHYSICAL RESEARCH LETTERS, VOL. 37,, doi:10.1029/2010gl044988, 2010 Arctic sea ice response to atmospheric forcings with varying levels of anthropogenic warming and climate variability Jinlun Zhang,
GEOPHYSICAL RESEARCH LETTERS, VOL. 37,, doi:10.1029/2010gl044988, 2010 Arctic sea ice response to atmospheric forcings with varying levels of anthropogenic warming and climate variability Jinlun Zhang,
The Climate Sensitivity of the Community Climate System Model Version 3 (CCSM3)
 2584 J O U R N A L O F C L I M A T E VOLUME 19 The Climate Sensitivity of the Community Climate System Model Version 3 (CCSM3) JEFFREY T. KIEHL, CHRISTINE A. SHIELDS, JAMES J. HACK, AND WILLIAM D. COLLINS
2584 J O U R N A L O F C L I M A T E VOLUME 19 The Climate Sensitivity of the Community Climate System Model Version 3 (CCSM3) JEFFREY T. KIEHL, CHRISTINE A. SHIELDS, JAMES J. HACK, AND WILLIAM D. COLLINS
Radiation in climate models.
 Lecture. Radiation in climate models. Objectives:. A hierarchy of the climate models.. Radiative and radiative-convective equilibrium.. Examples of simple energy balance models.. Radiation in the atmospheric
Lecture. Radiation in climate models. Objectives:. A hierarchy of the climate models.. Radiative and radiative-convective equilibrium.. Examples of simple energy balance models.. Radiation in the atmospheric
Lecture 6: Radiation Transfer. Global Energy Balance. Reflection and Scattering. Atmospheric Influences on Insolation
 Lecture 6: Radiation Transfer Global Energy Balance terrestrial radiation cooling Solar radiation warming Global Temperature atmosphere Vertical and latitudinal energy distributions Absorption, Reflection,
Lecture 6: Radiation Transfer Global Energy Balance terrestrial radiation cooling Solar radiation warming Global Temperature atmosphere Vertical and latitudinal energy distributions Absorption, Reflection,
Lecture 6: Radiation Transfer
 Lecture 6: Radiation Transfer Vertical and latitudinal energy distributions Absorption, Reflection, and Transmission Global Energy Balance terrestrial radiation cooling Solar radiation warming Global Temperature
Lecture 6: Radiation Transfer Vertical and latitudinal energy distributions Absorption, Reflection, and Transmission Global Energy Balance terrestrial radiation cooling Solar radiation warming Global Temperature
