Higher Physics. Particles and Waves
|
|
|
- Hollie Richard
- 5 years ago
- Views:
Transcription
1 Perth Academy Physics Department Higher Physics Particles and Waves Particles and Waves Homework Standard Model 1 Electric Fields and Potential Difference 2 Radioactivity 3 Fusion & Fission 4 The Photoelectric effect 5 Interference & Diffraction 6 Refraction and Total Internal Reflection 7 Light Irradiance 8 Emission Spectra 9 Absorption Spectra 10
2 Homework 1: Standard Model 1. (a) Write down the names of the 6 quarks and arrange them into their usual 3 pairs. (b) Label each quark with its electric charge in coulombs. (c) What is the quark content of a neutron and the quark content of a proton? (d) What happens to the quark content of the neutron in beta decay? (e) Quarks cannot exist on their own. What combinations are possible? 2. (a) Write down the names of the 6 leptons and arrange them into their usual 3 pairs. (b) Label each lepton with its electric charge in coulombs. (c) Which lepton is responsible for the science of chemistry? 3. (a) Name the 4 fundamental forces in nature and the Force-Carriers which are responsible for each one. (b) Which two have infinite range? (c) Which one allows beta decay to take place? (d) The chair you are sitting on is pushing back on you. Which of the fundamental forces is responsible for keeping you in your chair and which one is responsible for pushing back on you? (e) Which fundamental force holds the nucleus together?
3 Homework 2: Electric Fields and Potential Difference J of energy are used in moving a charge between two plates, across which there is a voltage of 50 volts. (a) How much charge was moved? (b) How many electrons have been moved? 2 A proton enters a simple particle accelerator as shown below. The p.d. across the plates of the accelerator is 25 kv. The proton is at rest at plate A. + - proton A B (a) What is the gain in kinetic energy of the proton as it reaches plate B? (b) Find the velocity of the proton when it hits plate B. 3 The diagram below shows a cathode ray tube used in an oscilloscope. The electrons which are emitted from the cathode start from rest and reach the anode with a speed of 4.2 x 10 7 m s -1. (a) (i) Calculate the kinetic energy in joules of each electron just before it reaches the anode. (ii) Calculate the p.d. between the anode and cathode. (b) Describe how the spot at the centre of the screen produced by the electrons can be moved to position X. Your answer must make reference to the relative sizes and polarity (signs) of the voltages applied to plates P and Q.
4 Homework 3 Radioactivity 1 The diagram shows the apparatus used by Rutherford to investigate the scattering of alpha particles by gold foil. From the observations made as the microscope and screen were moved from P to Q, Rutherford deduced that an atom has a nucleus which is : (A) positively charged (B) massive (C) much smaller than the volume of the atom Explain how the observations from the scattering experiment led to these three deductions 2 State the number of neutrons and protons in each of the following nuclei. i) N ii) 78 Pt iii) 13 6 C 3 Copy and complete the following decay chain showing the particles emitted and the missing element. 4 A radioactive source has 12 x 10 6 decays in one minute. Find its activity.
5 Homework 4 Fission and Fusion 1 Calculate the energy released from the neutron induced fission reaction shown below n + Pu 137 Te Mo n masses neutron : kg Plutonium-239 : kg Tellurium-137 : kg Molybdenum-100 : kg 2 Two deuterons (deuterium nuclei), fuse together as shown by the equation below. Find the energy released in this reaction. 2 H + 2 H 3 He n + energy masses: hydrogen-2 : kg neutron : kg helium-3 : kg Homework 5: The Photoelectric Effect Planck s constant, h = 6.63 x Js 1 The energy required to eject an electron from sodium is 2.9 x J. Calculate the wavelength of radiation needed to produce the photoelectric effect with sodium. Roughly, what colour is this light? 2 Find the number of photons per m 2 in a beam of irradiance 4 Wm -2 if the frequency of the radiation is 4.5 x Hz. 3 A photon of frequency 6.5 x Hz is incident on a metal whose work function is 4.64 x J. Will an electron be ejected?
6 Homework 6: Interference and Diffraction 1 In an experiment on interference of microwaves it is found that the distances from the slits to the first area of destructive interference is 39.2 cm and 37.7cm. What value does this give for the wavelength of the microwaves? 2 A diffraction grating with 300 lines/mm is illuminated with light from a laser. A second order maximum is obtained at an angle of 18 o to the straight through direction. Find a) The spacing of the grating in metres. b) The wavelength of the laser light. c) The angle at which the first order maximum will be observed. 3 Light of wavelengths 420 nm and 650 nm is incident on a diffraction grating having 600 lines/mm. Calculate the angular separation of the first order maxima. 4 A biologist is studying the effect of different colours of light on a sample of chlorophyll. She sets up the apparatus shown below, using a diffraction grating with 6 x 10 5 lines per metre to produce a first order spectrum of sunlight. a) Explain briefly how a diffraction grating produces a continuous spectrum from the ray of sunlight. b) The wavelength of the light at the end X of the spectrum is 410 nm. Calculate the value of the angle θ. c) The angle A in the diagram above is 9 o. Calculate the wavelength at end Y of the spectrum. d) The biologist now uses a triangular glass prism to produce a continuous spectrum from a ray of sunlight. State two difference between this spectrum and the spectrum produced by the grating.
7 Homework 7: Refraction and Total Internal Reflection 1 A ray of light enters water, refractive index 1.3, as shown in the diagram below. What is the size of the angle of refraction? 2 At what angle does the light leave the prism shown below? A swimming pool is illuminated by a lamp built into the bottom of the pool. Three rays of light from the same point in the lamp are incident on the water-air boundary with angles of incidence 30 o, 40 o and 50 o, as shown below. The refractive index of the water in the pool is a) Draw a diagram to show clearly what happens to each ray at the boundary. Indicate on your diagram the sizes of appropriate angles. All necessary calculations must be shown. b) An observer stands at the side of the pool and looks into the water. Explain, with the aid of a diagram, why the image of the lamp appears to be at a shallower depth than the bottom of the pool.
8 Homework 8: Light Irradiance 1 The illumination 2m from a lamp is 1000 lux. What will be the illumination at the following distances from the lamp? a) 4m b) 6m c) 1m d) 0.5m 2 Saturn is 10 times further away from the Sun than the Earth. How will the irradiance of sunlight on Saturn compare with that on Earth? 3 To improve the lighting in a room it is proposed to lower the lamps by 1m. By calculation investigate the savings that could be made. 4 A badly aligned laser, of power 0.1mW, has a beam diameter of 1mm which doubles in size for every metre travelled. Calculate the irradiance of the laser at a distance of 5m. 5 A laser is marked with the warning DANGER: EYE HAZARD. Why does this laser, which has a power output of only 0.20 mw, present a greater potential eye hazard than a 100 W lamp? In hospitals, pulsed lasers may be used to repair damage to the retina of the eye. The specification of a typical pulsed laser is given below: Gas used in laser Duration of pulse Energy of one pulse Wavelengths of laser light emitted : argon : 0.50 ms : 0.10 J : 488 and 514 nm The cross-sectional area of the laser beam at the retina is 1.5 x 10-9 m 2. Calculate the light irradiance produced at the retina during a pulse of light from this laser.
9 Homework 9: Spectra 1 The diagram below shows three possible energy levels in a hydrogen atom. Calculate the wavelength of the spectral lines with highest and lowest frequencies. How many lines could be produced? E x J. E x J. E x J. nucleus 2 (a) Laser light is monochromatic and coherent. Briefly explain the meaning of the terms monochromatic and coherent. (b) A laser radiates energy when electrons are stimulated to fall from energy level E 2 to energy level E 1 as shown in the diagram. (i) What are the frequency and wavelength of the radiation emitted? (ii) Name the section of the electromagnetic spectrum in which the radiation is found. (c) The beam of light from a laser is very intense. Give two reasons for this.
10 Homework 10: Absorption Spectra 1 (a) A sodium vapour lamp emits bright yellow light when electrons make transitions from one energy level to another within the sodium atoms. (i) State whether electrons are moving to higher or lower energy levels when the light is emitted. (ii) The wavelengths of the emitted light are nm and nm. Using Planck s constant, h = 6.63 x Js, calculate the energy difference between these two energy levels in the sodium atom. (b) A Bunsen flame containing vaporised sodium is placed between a sodium vapour lamp and a screen as shown. (i) Explain why a dark shadow of the flame is seen on the screen. (ii) The sodium vapour lamp is replaced with a cadmium vapour lamp. Explain why there is now no dark shadow in the flame on the screen.
Higher -o-o-o- Past Paper questions o-o-o- 3.4 Spectra
 Higher -o-o-o- Past Paper questions 1991-2010 -o-o-o- 3.4 Spectra 1992 Q37 The diagram below shows the energy levels for the hydrogen atom. (a) Between which two energy levels would an electron transition
Higher -o-o-o- Past Paper questions 1991-2010 -o-o-o- 3.4 Spectra 1992 Q37 The diagram below shows the energy levels for the hydrogen atom. (a) Between which two energy levels would an electron transition
Cathkin High School Physics Particles and Waves Questions and Solutions
 NATIONAL QUALIFICATIONS CURRICULUM SUPPORT Cathkin High School Physics Particles and Waves Questions and Solutions James Page Arthur Baillie [HIGHER] The Scottish Qualifications Authority regularly reviews
NATIONAL QUALIFICATIONS CURRICULUM SUPPORT Cathkin High School Physics Particles and Waves Questions and Solutions James Page Arthur Baillie [HIGHER] The Scottish Qualifications Authority regularly reviews
Farr High School HIGHER PHYSICS. Unit 2 Particles and Waves. Question Booklet
 Farr High School HIGHER PHYSICS Unit 2 Particles and Waves Question Booklet 1 THE STANDARD MODEL Orders of magnitude 1. The diagram shows a simple model of the atom. A B + + + + + C D Match each of the
Farr High School HIGHER PHYSICS Unit 2 Particles and Waves Question Booklet 1 THE STANDARD MODEL Orders of magnitude 1. The diagram shows a simple model of the atom. A B + + + + + C D Match each of the
Particles and Waves Homework One (Target mark 13 out of 15)
 Particles and Waves Homework One (Target mark 13 out of 15) Display all answers to 2 significant figures. 1. A car covers a distance of 170m in a time of 18s. Calculate the average speed of the car. 2.
Particles and Waves Homework One (Target mark 13 out of 15) Display all answers to 2 significant figures. 1. A car covers a distance of 170m in a time of 18s. Calculate the average speed of the car. 2.
Farr High School HIGHER PHYSICS. Unit 2 Particles and Waves. Exam Question Booklet
 Farr High School HIGHER PHYSICS Unit 2 Particles and Waves Exam Question Booklet 1 2 MULTIPLE CHOICE QUESTIONS 1. 3. 2. 4. 3 5. 6. 7. 4 8. 9. 5 10. 13. 11. 14. 15. 12. 6 16. 17. 18. 7 19. 20. 21. 8 22.
Farr High School HIGHER PHYSICS Unit 2 Particles and Waves Exam Question Booklet 1 2 MULTIPLE CHOICE QUESTIONS 1. 3. 2. 4. 3 5. 6. 7. 4 8. 9. 5 10. 13. 11. 14. 15. 12. 6 16. 17. 18. 7 19. 20. 21. 8 22.
Farr High School HIGHER PHYSICS. Unit 2 Particles and Waves
 Farr High School HIGHER PHYSICS Unit 2 Particles and Waves Exam Question Booklet June 2017 1 2 THE STANDARD MODEL 1. Three students each make a comment about antiparticles. I An antiparticle has the same
Farr High School HIGHER PHYSICS Unit 2 Particles and Waves Exam Question Booklet June 2017 1 2 THE STANDARD MODEL 1. Three students each make a comment about antiparticles. I An antiparticle has the same
CfE Higher Physics. Particles and Waves
 Wallace Hall Academy CfE Higher Physics Particles and Waves Exam Questions Part 1 Cover image: cutaway diagram of CERN, CERN P&W: Exam Questions Part 1 Version 2013 Contents Section 1: The Standard Model
Wallace Hall Academy CfE Higher Physics Particles and Waves Exam Questions Part 1 Cover image: cutaway diagram of CERN, CERN P&W: Exam Questions Part 1 Version 2013 Contents Section 1: The Standard Model
Bannerman High School Physics Department. Making Accurate Statements. Higher Physics. Quanta and Waves
 Bannerman High School Physics Department Making Accurate Statements Higher Physics Quanta and Waves Mandatory Key Area: Particle Physics 1. Use your knowledge of physics to estimate the ratio of the smallest
Bannerman High School Physics Department Making Accurate Statements Higher Physics Quanta and Waves Mandatory Key Area: Particle Physics 1. Use your knowledge of physics to estimate the ratio of the smallest
Particles and Waves Final Revision Exam Questions Part 1
 Particles and Waves Final Revision Exam Questions Part 1 Cover image: cutaway diagram of CERN, CERN Version 2013 P&W: Exam Questions Part 1 Version 2013 Contents Section 1: The Standard Model 1 Section
Particles and Waves Final Revision Exam Questions Part 1 Cover image: cutaway diagram of CERN, CERN Version 2013 P&W: Exam Questions Part 1 Version 2013 Contents Section 1: The Standard Model 1 Section
Unit 2 - Particles and Waves - Part 2
 WAVE-PARTICLE DUALITY Unit - Particles and Waves - Part 8. The photoelectric effect and wave particle duality Photoelectric effect as evidence for the particulate nature of light. Photons of sufficient
WAVE-PARTICLE DUALITY Unit - Particles and Waves - Part 8. The photoelectric effect and wave particle duality Photoelectric effect as evidence for the particulate nature of light. Photons of sufficient
Unit Three. Mesons can only be made from matter/anti-matter combinations. This makes mesons Unstable Short lived
 Unit Three The Standard Model Particle models of matter have existed from the early recorded history. At the start of the 20 th century the model that was being developed consisted of a central nucleus
Unit Three The Standard Model Particle models of matter have existed from the early recorded history. At the start of the 20 th century the model that was being developed consisted of a central nucleus
CHAPTER 12 TEST REVIEW
 IB PHYSICS Name: Period: Date: # Marks: 76 Raw Score: IB Curve: DEVIL PHYSICS BADDEST CLASS ON CAMPUS CHAPTER 12 TEST REVIEW 1. An alpha particle is accelerated through a potential difference of 10 kv.
IB PHYSICS Name: Period: Date: # Marks: 76 Raw Score: IB Curve: DEVIL PHYSICS BADDEST CLASS ON CAMPUS CHAPTER 12 TEST REVIEW 1. An alpha particle is accelerated through a potential difference of 10 kv.
RED. BLUE Light. Light-Matter
 1 Light-Matter This experiment demonstrated that light behaves as a wave. Essentially Thomas Young passed a light of a single frequency ( colour) through a pair of closely spaced narrow slits and on the
1 Light-Matter This experiment demonstrated that light behaves as a wave. Essentially Thomas Young passed a light of a single frequency ( colour) through a pair of closely spaced narrow slits and on the
Name the region of the electromagnetic radiation emitted by the laser. ...
 1. An argon-laser emits electromagnetic radiation of wavelength 5.1 10 7 m. The radiation is directed onto the surface of a caesium plate. The work function energy for caesium is 1.9 ev. (i) Name the region
1. An argon-laser emits electromagnetic radiation of wavelength 5.1 10 7 m. The radiation is directed onto the surface of a caesium plate. The work function energy for caesium is 1.9 ev. (i) Name the region
ATOMIC WORLD P.1. ejected photoelectrons. current amplifier. photomultiplier tube (PMT)
 ATOMIC WORLD P. HKAL PAPER I 0 8 The metal Caesium has a work function of.08 ev. Given: Planck constant h = 6.63 0 34 J s, charge of an electron e =.60 0 9 C (a) (i) Calculate the longest wavelength of
ATOMIC WORLD P. HKAL PAPER I 0 8 The metal Caesium has a work function of.08 ev. Given: Planck constant h = 6.63 0 34 J s, charge of an electron e =.60 0 9 C (a) (i) Calculate the longest wavelength of
Line Spectra. 6 The line spectrum of hydrogen includes no X-ray frequencies because
 TOPI 27 Line Spectra 1 The table below gives the energies of the six lowest levels of the hydrogen atom: Leveln 2 3 nergy/j -2.2 x 10-'8-5.3 X 10-'9-2.4 X 10-'9 Level 11 4 5 6 nergy/j -1.3 x JO-'9-8.0
TOPI 27 Line Spectra 1 The table below gives the energies of the six lowest levels of the hydrogen atom: Leveln 2 3 nergy/j -2.2 x 10-'8-5.3 X 10-'9-2.4 X 10-'9 Level 11 4 5 6 nergy/j -1.3 x JO-'9-8.0
Higher -o-o-o- Past Paper questions o-o-o- 3.3 Photoelectric
 Higher -o-o-o- Past Paper questions 1991-2010 -o-o-o- 3.3 Photoelectric 1996 Q36 The work function for sodium metal is 2.9x10-19 J. Light of wavelength 5.4x10-7 m strikes the surface of this metal. What
Higher -o-o-o- Past Paper questions 1991-2010 -o-o-o- 3.3 Photoelectric 1996 Q36 The work function for sodium metal is 2.9x10-19 J. Light of wavelength 5.4x10-7 m strikes the surface of this metal. What
1. (a) An ion of plutonium Pu has an overall charge of C. (iii) electrons... (3) (2) (Total 5 marks)
 AQA Questions from 2004 to 2006 Particle Physics 239 94 1. (a) An ion of plutonium Pu has an overall charge of +1.6 10 19 C. For this ion state the number of (i) protons... neutrons... (iii) electrons...
AQA Questions from 2004 to 2006 Particle Physics 239 94 1. (a) An ion of plutonium Pu has an overall charge of +1.6 10 19 C. For this ion state the number of (i) protons... neutrons... (iii) electrons...
hf = E 1 - E 2 hc = E 1 - E 2 λ FXA 2008 Candidates should be able to : EMISSION LINE SPECTRA
 1 Candidates should be able to : EMISSION LINE SPECTRA Explain how spectral lines are evidence for the existence of discrete energy levels in isolated atoms (i.e. in a gas discharge lamp). Describe the
1 Candidates should be able to : EMISSION LINE SPECTRA Explain how spectral lines are evidence for the existence of discrete energy levels in isolated atoms (i.e. in a gas discharge lamp). Describe the
GCE AS/A level 1322/01 PHYSICS PH2 Waves and Particles
 Surname Centre Number Candidate Number Other Names 2 GCE AS/A level 1322/01 PHYSICS PH2 Waves and Particles S15-1322-01 P.M. THURSDAY, 4 June 2015 1 hour 30 minutes For s use Question Maximum Mark Mark
Surname Centre Number Candidate Number Other Names 2 GCE AS/A level 1322/01 PHYSICS PH2 Waves and Particles S15-1322-01 P.M. THURSDAY, 4 June 2015 1 hour 30 minutes For s use Question Maximum Mark Mark
(i) Show that the energy of a single photon is about 3 x J.
 1(a) A helium-neon laser emits red light of wavelength 6.3 x 10 7 m. (i) Show that the energy of a single photon is about 3 x 10 19 J. [2] The power of the laser beam is 1.0 mw. Show that about 3 x 10
1(a) A helium-neon laser emits red light of wavelength 6.3 x 10 7 m. (i) Show that the energy of a single photon is about 3 x 10 19 J. [2] The power of the laser beam is 1.0 mw. Show that about 3 x 10
WAVES AND PARTICLES. (c)
 WAVES AND PARTICLES 1. An electron and a proton are accelerated through the same potential difference. The ration of their De Broglie wave length will be -- (a) (b) (c) (d) 1 2. What potential must be
WAVES AND PARTICLES 1. An electron and a proton are accelerated through the same potential difference. The ration of their De Broglie wave length will be -- (a) (b) (c) (d) 1 2. What potential must be
Higher Physics Particles and Waves 2 Notes
 Higher Physics Particles and Waves 2 Notes Teachers Booklet Learning Outcomes Interference and diffraction This builds on information from N5 Waves and Radiation H Particles and Waves book 1 At the end
Higher Physics Particles and Waves 2 Notes Teachers Booklet Learning Outcomes Interference and diffraction This builds on information from N5 Waves and Radiation H Particles and Waves book 1 At the end
Higher -o-o-o- Past Paper questions o-o-o- 3.6 Radiation
 Higher -o-o-o- Past Paper questions 1991-2001 -o-o-o- 3.6 Radiation 1992 Q35 A typical reaction produced in the core of a nuclear reactor can be described by the following equation: (a) State the name
Higher -o-o-o- Past Paper questions 1991-2001 -o-o-o- 3.6 Radiation 1992 Q35 A typical reaction produced in the core of a nuclear reactor can be described by the following equation: (a) State the name
AP Physics Study Guide Modern Physics I. Atomic Physics and Quantum Effects 1. Who is generally credited with the discovery of the electron?
 AP Physics Study Guide Modern Physics I. Atomic Physics and Quantum Effects 1. Who is generally credited with the discovery of the electron? 2. What was it that J. J. Thomson actually measured? 3. Regarding
AP Physics Study Guide Modern Physics I. Atomic Physics and Quantum Effects 1. Who is generally credited with the discovery of the electron? 2. What was it that J. J. Thomson actually measured? 3. Regarding
λ = h = h p mv λ = h mv FXA 2008 Candidates should be able to :
 1 Candidates should be able to : Explain electron diffraction as evidence for the wave nature of particles like electrons. Explain that electrons travelling through polycrystalline graphite will be diffracted
1 Candidates should be able to : Explain electron diffraction as evidence for the wave nature of particles like electrons. Explain that electrons travelling through polycrystalline graphite will be diffracted
EXAMINATION QUESTIONS (6)
 1. What is a beta-particle? A a helium nucleus B a high-energy electron C four protons D two neutrons EXAMINATION QUESTIONS (6) 2. The diagram shows part of a circuit used to switch street lamps on and
1. What is a beta-particle? A a helium nucleus B a high-energy electron C four protons D two neutrons EXAMINATION QUESTIONS (6) 2. The diagram shows part of a circuit used to switch street lamps on and
Homework 2: Forces on Charged Particles
 Homework 2: Forces on Charged Particles 1. In the arrangement shown below, 2 C of positive charge is moved from plate S, which is at a potential of 250 V, to plate T, which is at a potential of 750 V.
Homework 2: Forces on Charged Particles 1. In the arrangement shown below, 2 C of positive charge is moved from plate S, which is at a potential of 250 V, to plate T, which is at a potential of 750 V.
Subject: PHYSICS Level: ADVANCED Time: 3 hrs
 SIR MICHELANGELO REFALO CENTRE FOR FURTHER STUDIES VICTORIA GOZO Annual Exam 2013 Subject: PHYSICS Level: ADVANCED Time: 3 hrs Take the acceleration due to gravity g = 10m/s 2 Section A Answer all questions
SIR MICHELANGELO REFALO CENTRE FOR FURTHER STUDIES VICTORIA GOZO Annual Exam 2013 Subject: PHYSICS Level: ADVANCED Time: 3 hrs Take the acceleration due to gravity g = 10m/s 2 Section A Answer all questions
Chapter 37 Early Quantum Theory and Models of the Atom. Copyright 2009 Pearson Education, Inc.
 Chapter 37 Early Quantum Theory and Models of the Atom Planck s Quantum Hypothesis; Blackbody Radiation Photon Theory of Light and the Photoelectric Effect Energy, Mass, and Momentum of a Photon Compton
Chapter 37 Early Quantum Theory and Models of the Atom Planck s Quantum Hypothesis; Blackbody Radiation Photon Theory of Light and the Photoelectric Effect Energy, Mass, and Momentum of a Photon Compton
1 The Cathode Rays experiment is associated. with: Millikan A B. Thomson. Townsend. Plank Compton
 1 The Cathode Rays experiment is associated with: A B C D E Millikan Thomson Townsend Plank Compton 1 2 The electron charge was measured the first time in: A B C D E Cathode ray experiment Photoelectric
1 The Cathode Rays experiment is associated with: A B C D E Millikan Thomson Townsend Plank Compton 1 2 The electron charge was measured the first time in: A B C D E Cathode ray experiment Photoelectric
T7-1 [255 marks] The graph shows the relationship between binding energy per nucleon and nucleon number. In which region are nuclei most stable?
![T7-1 [255 marks] The graph shows the relationship between binding energy per nucleon and nucleon number. In which region are nuclei most stable? T7-1 [255 marks] The graph shows the relationship between binding energy per nucleon and nucleon number. In which region are nuclei most stable?](/thumbs/77/75398334.jpg) T7-1 [255 marks] 1. In the Geiger Marsden experiment alpha particles were directed at a thin gold foil. Which of the following shows how the majority of the alpha particles behaved after reaching the foil?
T7-1 [255 marks] 1. In the Geiger Marsden experiment alpha particles were directed at a thin gold foil. Which of the following shows how the majority of the alpha particles behaved after reaching the foil?
U n 3 n Ba Kr (D) Br (C) Kr (B) Rb (E) 94 37
 1984 36. The critical angle for a transparent material in air is 30. The index of refraction of the material is most nearly (A) 0.33 (B) 0.50 (C) 1.0 (D) 1.5 (E) 2.0 37. An object is placed as shown in
1984 36. The critical angle for a transparent material in air is 30. The index of refraction of the material is most nearly (A) 0.33 (B) 0.50 (C) 1.0 (D) 1.5 (E) 2.0 37. An object is placed as shown in
1. This question is about the Rutherford model of the atom.
 1. This question is about the Rutherford model of the atom. (a) Most alpha particles used to bombard a thin gold foil pass through the foil without a significant change in direction. A few alpha particles
1. This question is about the Rutherford model of the atom. (a) Most alpha particles used to bombard a thin gold foil pass through the foil without a significant change in direction. A few alpha particles
Mock Examination Physics 2 nd Book Time 3 Hrs MM : 70
 Mock Examination Physics 2 nd Book Time 3 Hrs MM : 70 SECTION A 1. How does the angle of minimum deviation of a glass prism vary, If the incident violet light is replaced with red light? 2. What is principle
Mock Examination Physics 2 nd Book Time 3 Hrs MM : 70 SECTION A 1. How does the angle of minimum deviation of a glass prism vary, If the incident violet light is replaced with red light? 2. What is principle
THE INDIAN COMMUNITY SCHOOL, KUWAIT SECOND SEMESTER EXAMINATION PHYSICS (Theory)
 CLASS:XII Marks : 70 General Instructions: THE INDIAN COMMUNITY SCHOOL, KUWAIT SECOND SEMESTER EXAMINATION 2016-17 PHYSICS (Theory) Time : Hrs (i) (ii) (iii) (iv) (v) All questions are compulsory. This
CLASS:XII Marks : 70 General Instructions: THE INDIAN COMMUNITY SCHOOL, KUWAIT SECOND SEMESTER EXAMINATION 2016-17 PHYSICS (Theory) Time : Hrs (i) (ii) (iii) (iv) (v) All questions are compulsory. This
P.M. THURSDAY, 21 May hours. Write your name, centre number and candidate number in the spaces at the top of this page.
 Candidate Name Centre Number Candidate Number GCE AS/A level 1322/01 New AS PHYSICS ASSESSMENT UNIT PH2: WAVES AND PARTICLES P.M. THURSDAY, 21 May 2009 1 1 4 hours ADDITIONAL MATERIALS In addition to this
Candidate Name Centre Number Candidate Number GCE AS/A level 1322/01 New AS PHYSICS ASSESSMENT UNIT PH2: WAVES AND PARTICLES P.M. THURSDAY, 21 May 2009 1 1 4 hours ADDITIONAL MATERIALS In addition to this
Larbert High School. Quanta and Waves. Homework Exercises ADVANCED HIGHER PHYSICS
 Larbert High School ADVANCED HIGHER PHYSICS Quanta and Waves Homework Exercises 3.1 3.6 3.1 Intro to Quantum Theory HW 1. (a) Explain what is meant by term black body. (1) (b) State two observations that
Larbert High School ADVANCED HIGHER PHYSICS Quanta and Waves Homework Exercises 3.1 3.6 3.1 Intro to Quantum Theory HW 1. (a) Explain what is meant by term black body. (1) (b) State two observations that
SECTION A Quantum Physics and Atom Models
 AP Physics Multiple Choice Practice Modern Physics SECTION A Quantum Physics and Atom Models 1. Light of a single frequency falls on a photoelectric material but no electrons are emitted. Electrons may
AP Physics Multiple Choice Practice Modern Physics SECTION A Quantum Physics and Atom Models 1. Light of a single frequency falls on a photoelectric material but no electrons are emitted. Electrons may
LIGHT. Question. Until very recently, the study of ALL astronomical objects, outside of the Solar System, has been with telescopes observing light.
 LIGHT Question Until very recently, the study of ALL astronomical objects, outside of the Solar System, has been with telescopes observing light. What kind of information can we get from light? 1 Light
LIGHT Question Until very recently, the study of ALL astronomical objects, outside of the Solar System, has been with telescopes observing light. What kind of information can we get from light? 1 Light
Chemistry (
 Question 2.1: (i) Calculate the number of electrons which will together weigh one gram. (ii) Calculate the mass and charge of one mole of electrons. Answer 2.1: (i) Mass of one electron = 9.10939 10 31
Question 2.1: (i) Calculate the number of electrons which will together weigh one gram. (ii) Calculate the mass and charge of one mole of electrons. Answer 2.1: (i) Mass of one electron = 9.10939 10 31
[2] (b) An electron is accelerated from rest through a potential difference of 300 V.
![[2] (b) An electron is accelerated from rest through a potential difference of 300 V. [2] (b) An electron is accelerated from rest through a potential difference of 300 V.](/thumbs/89/98791036.jpg) 1 (a) In atomic physics electron energies are often stated in electronvolts (ev) Define the electronvolt. State its value in joule.. [2] (b) An electron is accelerated from rest through a potential difference
1 (a) In atomic physics electron energies are often stated in electronvolts (ev) Define the electronvolt. State its value in joule.. [2] (b) An electron is accelerated from rest through a potential difference
Dept. of Physics, MIT Manipal 1
 Chapter 1: Optics 1. In the phenomenon of interference, there is A Annihilation of light energy B Addition of energy C Redistribution energy D Creation of energy 2. Interference fringes are obtained using
Chapter 1: Optics 1. In the phenomenon of interference, there is A Annihilation of light energy B Addition of energy C Redistribution energy D Creation of energy 2. Interference fringes are obtained using
A beam of coherent monochromatic light from a distant galaxy is used in an optics experiment on Earth.
 Waves_P2 [152 marks] A beam of coherent monochromatic light from a distant galaxy is used in an optics experiment on Earth. The beam is incident normally on a double slit. The distance between the slits
Waves_P2 [152 marks] A beam of coherent monochromatic light from a distant galaxy is used in an optics experiment on Earth. The beam is incident normally on a double slit. The distance between the slits
Physics 126 Practice Exam #4 Professor Siegel
 Physics 126 Practice Exam #4 Professor Siegel Name: Lab Day: 1. Light is usually thought of as wave-like in nature and electrons as particle-like. In which one of the following instances does light behave
Physics 126 Practice Exam #4 Professor Siegel Name: Lab Day: 1. Light is usually thought of as wave-like in nature and electrons as particle-like. In which one of the following instances does light behave
MODERN PHYSICS. 1 v 2. Kmax
 MODERN PHYSICS PRACTICE QUESTIONS ( PHOTO ELECTRIC EFFECT ) Pg No 18 1) Define 'intensity' of radiation in photon picture of light. [Comptt. Delhi 2012] SOL: It is the number of photo electrons emitted
MODERN PHYSICS PRACTICE QUESTIONS ( PHOTO ELECTRIC EFFECT ) Pg No 18 1) Define 'intensity' of radiation in photon picture of light. [Comptt. Delhi 2012] SOL: It is the number of photo electrons emitted
KCSE 2009 PHYSICS PAPER 2
 KCSE 2009 PHYSICS PAPER 2 SECTION A (25 MARKS) Answer all the questions in this section in the spaces provided 1. State the number of images formed when an object is between two plane mirror placed in
KCSE 2009 PHYSICS PAPER 2 SECTION A (25 MARKS) Answer all the questions in this section in the spaces provided 1. State the number of images formed when an object is between two plane mirror placed in
Topic 7 &13 Review Atomic, Nuclear, and Quantum Physics
 Name: Date:. Isotopes provide evidence for the existence of A. protons. B. electrons. C. nuclei. Topic 7 &3 Review Atomic, Nuclear, and Quantum Physics D. neutrons.. The atomic line spectra of elements
Name: Date:. Isotopes provide evidence for the existence of A. protons. B. electrons. C. nuclei. Topic 7 &3 Review Atomic, Nuclear, and Quantum Physics D. neutrons.. The atomic line spectra of elements
The Final Exam (Exam 4) will be on FRIDAY MAY 11 From 3 5 PM in LR1 VAN
 1 --------------------------------------------------------------------------------------------------------------------- 29:006 SPRING 2012 PRACTICE EXAM 4 ---------------------------------------------------------------------------------------------------------------------
1 --------------------------------------------------------------------------------------------------------------------- 29:006 SPRING 2012 PRACTICE EXAM 4 ---------------------------------------------------------------------------------------------------------------------
 Name : Roll No. :.. Invigilator s Signature :.. CS/B.Tech/SEM-2/PH-201/2010 2010 ENGINEERING PHYSICS Time Allotted : 3 Hours Full Marks : 70 The figures in the margin indicate full marks. Candidates are
Name : Roll No. :.. Invigilator s Signature :.. CS/B.Tech/SEM-2/PH-201/2010 2010 ENGINEERING PHYSICS Time Allotted : 3 Hours Full Marks : 70 The figures in the margin indicate full marks. Candidates are
Question 11.1: Find the
 Question 11.1: Find the (a) maximum frequency, and (b) minimum wavelength of X-rays produced by 30 kv electrons. Potential of the electrons, V = 30 kv = 3 10 4 V Hence, energy of the electrons, E = 3 10
Question 11.1: Find the (a) maximum frequency, and (b) minimum wavelength of X-rays produced by 30 kv electrons. Potential of the electrons, V = 30 kv = 3 10 4 V Hence, energy of the electrons, E = 3 10
Physics 280 Week 04 In-Class Problems Summer 2016
 Physics 80 Week 04 In-Class Problems Summer 016 1. Consider the interface of water with class (say at the bottom of a beaker filled with water). Given: The index of refraction for water is n w = 1.33 and
Physics 80 Week 04 In-Class Problems Summer 016 1. Consider the interface of water with class (say at the bottom of a beaker filled with water). Given: The index of refraction for water is n w = 1.33 and
1 Electrons are emitted from a metal surface when it is illuminated with suitable electromagnetic radiation. ...[1]
![1 Electrons are emitted from a metal surface when it is illuminated with suitable electromagnetic radiation. ...[1] 1 Electrons are emitted from a metal surface when it is illuminated with suitable electromagnetic radiation. ...[1]](/thumbs/81/83978040.jpg) 1 Electrons are emitted from a metal surface when it is illuminated with suitable electromagnetic radiation. 1 (a) (b) Name the effect described above....[1] The variation with frequency f of the maximum
1 Electrons are emitted from a metal surface when it is illuminated with suitable electromagnetic radiation. 1 (a) (b) Name the effect described above....[1] The variation with frequency f of the maximum
Unit 6 Modern Physics
 Unit 6 Modern Physics Early Booklet E.C.: + 1 Unit 6 Hwk. Pts.: / 46 Unit 6 Lab Pts.: / 16 Late, Incomplete, No Work, No Units Fees? Y / N Essential Fundamentals of Modern Physics 1. A photon s energy
Unit 6 Modern Physics Early Booklet E.C.: + 1 Unit 6 Hwk. Pts.: / 46 Unit 6 Lab Pts.: / 16 Late, Incomplete, No Work, No Units Fees? Y / N Essential Fundamentals of Modern Physics 1. A photon s energy
Chapter 37 Early Quantum Theory and Models of the Atom
 Chapter 37 Early Quantum Theory and Models of the Atom Units of Chapter 37 37-7 Wave Nature of Matter 37-8 Electron Microscopes 37-9 Early Models of the Atom 37-10 Atomic Spectra: Key to the Structure
Chapter 37 Early Quantum Theory and Models of the Atom Units of Chapter 37 37-7 Wave Nature of Matter 37-8 Electron Microscopes 37-9 Early Models of the Atom 37-10 Atomic Spectra: Key to the Structure
Name :. Roll No. :... Invigilator s Signature :.. CS/B. Tech (New)/SEM-1/PH-101/ PHYSICS-I
 Name :. Roll No. :..... Invigilator s Signature :.. CS/B. Tech (New)/SEM-1/PH-101/2011-12 2011 PHYSICS-I Time Allotted : 3 Hours Full Marks : 70 The figures in the margin indicate full marks. Candidates
Name :. Roll No. :..... Invigilator s Signature :.. CS/B. Tech (New)/SEM-1/PH-101/2011-12 2011 PHYSICS-I Time Allotted : 3 Hours Full Marks : 70 The figures in the margin indicate full marks. Candidates
THE NATURE OF THE ATOM. alpha particle source
 chapter THE NATURE OF THE ATOM www.tutor-homework.com (for tutoring, homework help, or help with online classes) Section 30.1 Rutherford Scattering and the Nuclear Atom 1. Which model of atomic structure
chapter THE NATURE OF THE ATOM www.tutor-homework.com (for tutoring, homework help, or help with online classes) Section 30.1 Rutherford Scattering and the Nuclear Atom 1. Which model of atomic structure
minimum wavelength of X-rays produced by 30 kv electrons.
 Question 11.1: Find the maximum frequency, and minimum wavelength of X-rays produced by 30 kv electrons. Potential of the electrons, V = 30 kv = 3 10 4 V Hence, energy of the electrons, E = 3 10 4 ev Where,
Question 11.1: Find the maximum frequency, and minimum wavelength of X-rays produced by 30 kv electrons. Potential of the electrons, V = 30 kv = 3 10 4 V Hence, energy of the electrons, E = 3 10 4 ev Where,
Sample Examination Questions
 Sample Examination Questions Contents NB. Material covered by the AS papers may also appear in A2 papers. Question Question type Question focus number (section A or B) 1 A Ideal transformer 2 A Induced
Sample Examination Questions Contents NB. Material covered by the AS papers may also appear in A2 papers. Question Question type Question focus number (section A or B) 1 A Ideal transformer 2 A Induced
Which of the following classes of electromagnetic waves will not ionise neutral atoms?
 1 In an experiment to demonstrate the photoelectric effect, a charged metal plate is illuminated with light from different sources. The plate loses its charge when an ultraviolet light source is used but
1 In an experiment to demonstrate the photoelectric effect, a charged metal plate is illuminated with light from different sources. The plate loses its charge when an ultraviolet light source is used but
September 06, A B Which is which? What does Planck suggest? instead of resonantors being able to emit energy at any speed or energy?
 Michelson - Morley Interferometer Relativity and similtineaity digital media downloads another external michelson-morley The Electron Volt? units? Derivation? Michelson - Morley Experiment Time dilation
Michelson - Morley Interferometer Relativity and similtineaity digital media downloads another external michelson-morley The Electron Volt? units? Derivation? Michelson - Morley Experiment Time dilation
Physics Assessment Unit AS 2
 Centre Number 71 Candidate Number ADVANCED SUBSIDIARY General Certificate of Education January 2012 Physics Assessment Unit AS 2 assessing Module 2: Waves, Photons and Medical Physics AY121 [AY121] Friday
Centre Number 71 Candidate Number ADVANCED SUBSIDIARY General Certificate of Education January 2012 Physics Assessment Unit AS 2 assessing Module 2: Waves, Photons and Medical Physics AY121 [AY121] Friday
PHYSICS. Paper 1 (THEORY) Three hours and a quarter
 PHYSICS Paper 1 (THEORY) Three hours and a quarter (The first 15 minutes of the examination are for reading the paper only. Candidates must NOT start writing during this time). -------------------------------------------------------------------
PHYSICS Paper 1 (THEORY) Three hours and a quarter (The first 15 minutes of the examination are for reading the paper only. Candidates must NOT start writing during this time). -------------------------------------------------------------------
MIDTERM 3 REVIEW SESSION. Dr. Flera Rizatdinova
 MIDTERM 3 REVIEW SESSION Dr. Flera Rizatdinova Summary of Chapter 23 Index of refraction: Angle of reflection equals angle of incidence Plane mirror: image is virtual, upright, and the same size as the
MIDTERM 3 REVIEW SESSION Dr. Flera Rizatdinova Summary of Chapter 23 Index of refraction: Angle of reflection equals angle of incidence Plane mirror: image is virtual, upright, and the same size as the
Absorber Alpha emission Alpha particle Atom. Atomic line spectra Atomic mass unit Atomic number Atomic structure. Background radiation
 Material that prevent radioactive emission from passing through it Release of alpha particle from unstable nucleus(a 2+ helium ion or a helium nucleus) The nucleus of a helium atom (two protons and two
Material that prevent radioactive emission from passing through it Release of alpha particle from unstable nucleus(a 2+ helium ion or a helium nucleus) The nucleus of a helium atom (two protons and two
Optics Definitions. The apparent movement of one object relative to another due to the motion of the observer is called parallax.
 Optics Definitions Reflection is the bouncing of light off an object Laws of Reflection of Light: 1. The incident ray, the normal at the point of incidence and the reflected ray all lie in the same plane.
Optics Definitions Reflection is the bouncing of light off an object Laws of Reflection of Light: 1. The incident ray, the normal at the point of incidence and the reflected ray all lie in the same plane.
M13/4/PHYSI/HPM/ENG/TZ1/XX. Physics Higher level Paper 1. Monday 6 May 2013 (morning) 1 hour INSTRUCTIONS TO CANDIDATES
 M13/4/PHYSI/HPM/ENG/TZ1/XX 22136507 Physics Higher level Paper 1 Monday 6 May 2013 (morning) 1 hour INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer all the
M13/4/PHYSI/HPM/ENG/TZ1/XX 22136507 Physics Higher level Paper 1 Monday 6 May 2013 (morning) 1 hour INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer all the
Particle Nature of Matter. Chapter 4
 Particle Nature of Matter Chapter 4 Modern physics When my grandfather was born, atoms were just an idea. That year, 1897, was marked by the discovery of the electron by J.J. Thomson. The nuclear model
Particle Nature of Matter Chapter 4 Modern physics When my grandfather was born, atoms were just an idea. That year, 1897, was marked by the discovery of the electron by J.J. Thomson. The nuclear model
tip conducting surface
 PhysicsAndMathsTutor.com 1 1. The diagram shows the tip of a scanning tunnelling microscope (STM) above a conducting surface. The tip is at a potential of 1.0 V relative to the surface. If the tip is sufficiently
PhysicsAndMathsTutor.com 1 1. The diagram shows the tip of a scanning tunnelling microscope (STM) above a conducting surface. The tip is at a potential of 1.0 V relative to the surface. If the tip is sufficiently
PA01. General Certificate of Education January 2007 Advanced Subsidiary Examination
 Surname Centre Number Other Names Candidate Number For Examiner s Use Candidate Signature General Certificate of Education January 2007 Advanced Subsidiary Examination PHYSICS (SPECIFICATION A) Unit 1
Surname Centre Number Other Names Candidate Number For Examiner s Use Candidate Signature General Certificate of Education January 2007 Advanced Subsidiary Examination PHYSICS (SPECIFICATION A) Unit 1
A) n L < 1.0 B) n L > 1.1 C) n L > 1.3 D) n L < 1.1 E) n L < 1.3
 1. A beam of light passes from air into water. Which is necessarily true? A) The frequency is unchanged and the wavelength increases. B) The frequency is unchanged and the wavelength decreases. C) The
1. A beam of light passes from air into water. Which is necessarily true? A) The frequency is unchanged and the wavelength increases. B) The frequency is unchanged and the wavelength decreases. C) The
Physics 30: Chapter 5 Exam Wave Nature of Light
 Physics 30: Chapter 5 Exam Wave Nature of Light Name: Date: Mark: /33 Numeric Response. Place your answers to the numeric response questions, with units, in the blanks at the side of the page. (1 mark
Physics 30: Chapter 5 Exam Wave Nature of Light Name: Date: Mark: /33 Numeric Response. Place your answers to the numeric response questions, with units, in the blanks at the side of the page. (1 mark
A level Physics (7407/7408)
 rayton Manor High School level Physics (7407/7408) S MQ 1 Name: lass: uthor: ate: Time: Marks: omments: Page 1 rayton Manor High School Q1. nucleus of a particular element decays, emitting a series of
rayton Manor High School level Physics (7407/7408) S MQ 1 Name: lass: uthor: ate: Time: Marks: omments: Page 1 rayton Manor High School Q1. nucleus of a particular element decays, emitting a series of
 Questions Q1. * Rutherford designed an experiment to see what happened when alpha particles were directed at a piece of gold foil. Summarise the observations and state the conclusions Rutherford reached
Questions Q1. * Rutherford designed an experiment to see what happened when alpha particles were directed at a piece of gold foil. Summarise the observations and state the conclusions Rutherford reached
The Structure of the Atom
 CHAPTER 5 The Structure of the Atom 5.4 Light and Spectroscopy 460 370 BC 1808 1870 1897 1910 1925 Today Democritus Atomism Dalton Modern atomic theory Crookes Cathode rays Thomson Discovery of the electron
CHAPTER 5 The Structure of the Atom 5.4 Light and Spectroscopy 460 370 BC 1808 1870 1897 1910 1925 Today Democritus Atomism Dalton Modern atomic theory Crookes Cathode rays Thomson Discovery of the electron
Science 30 Unit C Review Outline GCCHS. Negatively charged Positively charged Coulomb Conductor Electric potential difference
 Science 30 Unit C Review Outline GCCHS Negatively charged Positively charged Coulomb Conductor Electric potential difference volt voltage Insulator Test body Gravitational field Field lines Solar wind
Science 30 Unit C Review Outline GCCHS Negatively charged Positively charged Coulomb Conductor Electric potential difference volt voltage Insulator Test body Gravitational field Field lines Solar wind
NJCTL.org 2015 AP Physics 2 Nuclear Physics
 AP Physics 2 Questions 1. What particles make up the nucleus? What is the general term for them? What are those particles composed of? 2. What is the definition of the atomic number? What is its symbol?
AP Physics 2 Questions 1. What particles make up the nucleus? What is the general term for them? What are those particles composed of? 2. What is the definition of the atomic number? What is its symbol?
1) Introduction 2) Photo electric effect 3) Dual nature of matter 4) Bohr s atom model 5) LASERS
 1) Introduction 2) Photo electric effect 3) Dual nature of matter 4) Bohr s atom model 5) LASERS 1. Introduction Types of electron emission, Dunnington s method, different types of spectra, Fraunhoffer
1) Introduction 2) Photo electric effect 3) Dual nature of matter 4) Bohr s atom model 5) LASERS 1. Introduction Types of electron emission, Dunnington s method, different types of spectra, Fraunhoffer
Final Exam, Part A. December 12, Score:
 Physics 152 December 12, 2005 Final Exam, Part A Roster No.: Score: Exam time limit: 2 hours. You may use a calculator and both sides of TWO sheets of notes, handwritten only. Closed book; no collaboration.
Physics 152 December 12, 2005 Final Exam, Part A Roster No.: Score: Exam time limit: 2 hours. You may use a calculator and both sides of TWO sheets of notes, handwritten only. Closed book; no collaboration.
Name: Class: Date: Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 Name: Class: Date: AP REVIEW 3 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.. For a mass hanging from a spring, the maximum displacement the
Name: Class: Date: AP REVIEW 3 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.. For a mass hanging from a spring, the maximum displacement the
Quantum and Atomic Physics - Multiple Choice
 PSI AP Physics 2 Name 1. The Cathode Ray Tube experiment is associated with: (A) J. J. Thomson (B) J. S. Townsend (C) M. Plank (D) A. H. Compton 2. The electron charge was measured the first time in: (A)
PSI AP Physics 2 Name 1. The Cathode Ray Tube experiment is associated with: (A) J. J. Thomson (B) J. S. Townsend (C) M. Plank (D) A. H. Compton 2. The electron charge was measured the first time in: (A)
Physics Assessment Unit AS 2
 Centre Number 71 Candidate Number ADVANCED SUBSIDIARY (AS) General Certificate of Education January 2014 Physics Assessment Unit AS 2 assessing Module 2: Waves, Photons and Medical Physics AY121 [AY121]
Centre Number 71 Candidate Number ADVANCED SUBSIDIARY (AS) General Certificate of Education January 2014 Physics Assessment Unit AS 2 assessing Module 2: Waves, Photons and Medical Physics AY121 [AY121]
PRE-LEAVING CERTIFICATE EXAMINATION, 2014 PHYSICS ORDINARY LEVEL
 L.35 PRE-LEAVING CERTIFICATE EXAMINATION, 2014 PHYSICS ORDINARY LEVEL TIME 3 HOURS Answer three questions from Section A and five questions from Section B. N.B. Relevant data are listed in the Formulae
L.35 PRE-LEAVING CERTIFICATE EXAMINATION, 2014 PHYSICS ORDINARY LEVEL TIME 3 HOURS Answer three questions from Section A and five questions from Section B. N.B. Relevant data are listed in the Formulae
Chemistry 212 ATOMIC SPECTROSCOPY
 Chemistry 212 ATOMIC SPECTROSCOPY The emission and absorption of light energy of particular wavelengths by atoms and molecules is a common phenomenon. The emissions/absorptions are characteristic for each
Chemistry 212 ATOMIC SPECTROSCOPY The emission and absorption of light energy of particular wavelengths by atoms and molecules is a common phenomenon. The emissions/absorptions are characteristic for each
 Name : Roll No. :.... Invigilator s Signature :.. CS/B.Tech (NEW)/SEM-2/PH-201/2013 2013 PHYSICS - I Time Allotted : 3 Hours Full Marks : 70 The figures in the margin indicate full marks. Candidates are
Name : Roll No. :.... Invigilator s Signature :.. CS/B.Tech (NEW)/SEM-2/PH-201/2013 2013 PHYSICS - I Time Allotted : 3 Hours Full Marks : 70 The figures in the margin indicate full marks. Candidates are
VICTORIA JUNIOR COLLEGE 2014 JC2 PRELIMINARY EXAMINATION. 23/9/ h 1600h
 Name: Class: 13S VICTORIA JUNIOR COLLEGE 2014 JC2 PRELIMINARY EXAMINATION PHYSICS 9646/3 Higher 2 Paper 3 23/9/2014 1400h 1600h TUESDAY (2 Hours) This paper consists of two sections: Section A (40 marks)
Name: Class: 13S VICTORIA JUNIOR COLLEGE 2014 JC2 PRELIMINARY EXAMINATION PHYSICS 9646/3 Higher 2 Paper 3 23/9/2014 1400h 1600h TUESDAY (2 Hours) This paper consists of two sections: Section A (40 marks)
Rb, which had been compressed to a density of 1013
 Modern Physics Study Questions for the Spring 2018 Departmental Exam December 3, 2017 1. An electron is initially at rest in a uniform electric field E in the negative y direction and a uniform magnetic
Modern Physics Study Questions for the Spring 2018 Departmental Exam December 3, 2017 1. An electron is initially at rest in a uniform electric field E in the negative y direction and a uniform magnetic
Care should be taken to give an appropriate number of significant figures in the final answers to calculations.
 X069/70 NATIONAL QUALIFICATIONS 007 WEDNESDAY, 6 MAY.00 PM 3.30 PM PHYSICS ADVANCED HIGHER Reference may be made to the Physics Data Booklet. Answer all questions. Any necessary data may be found in the
X069/70 NATIONAL QUALIFICATIONS 007 WEDNESDAY, 6 MAY.00 PM 3.30 PM PHYSICS ADVANCED HIGHER Reference may be made to the Physics Data Booklet. Answer all questions. Any necessary data may be found in the
H2 Physics Set A Paper 3 H2 PHYSICS. Exam papers with worked solutions. (Selected from Top JC) SET A PAPER 3.
 H2 PHYSICS Exam papers with worked solutions (Selected from Top JC) SET A PAPER 3 Compiled by THE PHYSICS CAFE 1 P a g e Candidates answer on the Question Paper. No Additional Materials are required. READ
H2 PHYSICS Exam papers with worked solutions (Selected from Top JC) SET A PAPER 3 Compiled by THE PHYSICS CAFE 1 P a g e Candidates answer on the Question Paper. No Additional Materials are required. READ
Sample Copyright. Academic Group ATOMIC STRUCTURE 1. Topics covered in this chapter:
 ATOMIC STRUCTURE Topics covered in this chapter:. Structure of the Atom.2 Atomic Number, Mass Number.3 Isotopes.4 The Mass Spectrometer.5 Atomic Structure and Light Spectra.6 Electron Arrangements in Atoms.7
ATOMIC STRUCTURE Topics covered in this chapter:. Structure of the Atom.2 Atomic Number, Mass Number.3 Isotopes.4 The Mass Spectrometer.5 Atomic Structure and Light Spectra.6 Electron Arrangements in Atoms.7
Chapter 38 and Chapter 39
 Chapter 38 and Chapter 39 State of 19th and very early 20th century physics: Light: 1. E&M Maxwell s equations > waves; J. J. Thompson s double slit experiment with light 2. Does light need a medium? >
Chapter 38 and Chapter 39 State of 19th and very early 20th century physics: Light: 1. E&M Maxwell s equations > waves; J. J. Thompson s double slit experiment with light 2. Does light need a medium? >
Cumulative Review 1 Use the following information to answer the next two questions.
 Cumulative Review 1 Use the following information to answer the next two questions. 1. At what distance from the mirror is the image located? a. 0.10 m b. 0.20 m c. 0.30 m d. 0.40 m 2. At what distance
Cumulative Review 1 Use the following information to answer the next two questions. 1. At what distance from the mirror is the image located? a. 0.10 m b. 0.20 m c. 0.30 m d. 0.40 m 2. At what distance
Nuclear Physics and Nuclear Reactions
 Slide 1 / 33 Nuclear Physics and Nuclear Reactions The Nucleus Slide 2 / 33 Proton: The charge on a proton is +1.6x10-19 C. The mass of a proton is 1.6726x10-27 kg. Neutron: The neutron is neutral. The
Slide 1 / 33 Nuclear Physics and Nuclear Reactions The Nucleus Slide 2 / 33 Proton: The charge on a proton is +1.6x10-19 C. The mass of a proton is 1.6726x10-27 kg. Neutron: The neutron is neutral. The
1. (i) Calculate the number of electrons which will together weigh one gram. (ii) Calculate the mass and charge of one mole of electrons.
 1. (i) Calculate the number of electrons which will together weigh one gram. (ii) Calculate the mass and charge of one mole of electrons. (i) 9.11 10-28 g is the mass of 1 electron No. of electrons 1 g
1. (i) Calculate the number of electrons which will together weigh one gram. (ii) Calculate the mass and charge of one mole of electrons. (i) 9.11 10-28 g is the mass of 1 electron No. of electrons 1 g
FALL 2004 Final Exam, Part A
 Physics 152 FALL 2004 Final Exam, Part A Roster No.: Score: 23 pts. possible Exam time limit: 2 hours. You may use a calculator and both sides of 2 sheets of notes, handwritten only. Closed book; no collaboration.
Physics 152 FALL 2004 Final Exam, Part A Roster No.: Score: 23 pts. possible Exam time limit: 2 hours. You may use a calculator and both sides of 2 sheets of notes, handwritten only. Closed book; no collaboration.
Exam Review Practice Questions. Electric Forces. the force is zero. Four charges are fixed at the corners of a square of sides 4 m as shown.
 Exam Review Practice Questions Electric Forces QUESTION 1 Three charges of equal magnitude are positioned as shown, with Q3 equidistant from Q1 and Q2. Q1 and Q3 are positive charges; Q2 is negative. What
Exam Review Practice Questions Electric Forces QUESTION 1 Three charges of equal magnitude are positioned as shown, with Q3 equidistant from Q1 and Q2. Q1 and Q3 are positive charges; Q2 is negative. What
= : K A
 Atoms and Nuclei. State two limitations of JJ Thomson s model of atom. 2. Write the SI unit for activity of a radioactive substance. 3. What observations led JJ Thomson to conclusion that all atoms have
Atoms and Nuclei. State two limitations of JJ Thomson s model of atom. 2. Write the SI unit for activity of a radioactive substance. 3. What observations led JJ Thomson to conclusion that all atoms have
London Examinations IGCSE
 Centre No. Candidate No. Surname Signature Initial(s) Paper Reference(s) 4420/2H London Examinations IGCSE Physics Paper 2H Higher Tier Tuesday 2 May 2006 Morning Time: 2 hours Materials required for examination
Centre No. Candidate No. Surname Signature Initial(s) Paper Reference(s) 4420/2H London Examinations IGCSE Physics Paper 2H Higher Tier Tuesday 2 May 2006 Morning Time: 2 hours Materials required for examination
Fig. 8.1 illustrates the three measurements. air medium A. ray 1. air medium A. ray 2. air medium A. ray 3. Fig For Examiner s Use
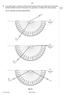 9 9 9 14 8 In an optics lesson, a Physics student traces the paths of three s of light near the boundary between medium A and. The student uses a protractor to measure the various angles. Fig. 8.1 illustrates
9 9 9 14 8 In an optics lesson, a Physics student traces the paths of three s of light near the boundary between medium A and. The student uses a protractor to measure the various angles. Fig. 8.1 illustrates
Downloaded from
 UNIT VII- DUAL NATURE OF MATTER & RADIATION LIST OF FORMULAE 1. Energy of a photon E =hʋ = 2. Number of photon emitted per second N = 3. Momentum of photon P = mc = = = 4. Equivalent mass of photon m =
UNIT VII- DUAL NATURE OF MATTER & RADIATION LIST OF FORMULAE 1. Energy of a photon E =hʋ = 2. Number of photon emitted per second N = 3. Momentum of photon P = mc = = = 4. Equivalent mass of photon m =
