Angular Momentum. Classically the orbital angular momentum with respect to a fixed origin is. L = r p. = yp z. L x. zp y L y. = zp x. xpz L z.
|
|
|
- Harry Atkins
- 5 years ago
- Views:
Transcription
1 Angular momentum is an important concept in quantum theory, necessary for analyzing motion in 3D as well as intrinsic properties such as spin Classically the orbital angular momentum with respect to a fixed origin is L = r p L x = yp z zp y L y = zp x xpz = xp y yp x The vector L is perpendicular to the plane containing r and p
2 The quantum mechanical operators for orbital angular momentum are obtained by replacing the position and momentum variables by the corresponding operators obeying the canonical commutation relations: L x = yp z zp y = i y z z y L y = zp x xpz = i z x x z = xp y yp x = i x y y z
3 Commutation relations: L x, L y = yp zp, zp xp z y x z = yp z, zp x zp y, zp x yp, xp z z + zp, xp y z yp z,zp x zp y, zp x = yp z zp x zp x yp z = yp x [ p z, z] = i yp x = zp y zp x zp x zp y = zp x [ p y, z] = 0 yp z, xp z = yp zxp z xp z yp z = yp z [ p z, x] = 0 zp y, xp z = zp y xp z xp z zp y = xp y [z, p z ] = i xp y L x, L y = i (xp y yp x ) = i
4 Commutation relations: L x, L y = i L y, = i L x, L x = i L y The angular momentum components L x, L y and, do not commute with each other and are incompatible observables. Applying the generalized uncertainty principle: ΔL x ΔL y 2 ΔL x Δ 2 L y ΔL y Δ 2 L x
5 Orbital angular momentum: Commutation relations: Consider the square of the total angular momentum: L 2 = L 2 x + L 2 2 y + L 2, L x = L 2 x, L x = L y L y, L x = L y i = 0 + L 2 y, L x + L, L y x + L 2 z, L x L y +, L x ( ) + ( i ) L y + i L y + L, x ( ) + ( i L ) y
6 Commutation relations: L 2, L x = 0 L 2, L y = 0 L2, = 0 L 2 commutes with all the components of angular momentum. However, the components do not commute with each other. We can construct simultaneous eigenfunctions of L 2 and any one component of the angular momentum. Here we pick We seek to find eigenstates and eigenvalues such that L 2 l, m = λ l l, m l, m = µ m l, m
7 Spherical polar coordinates: x = r sinθ cosφ y = r sinθ sinφ z = r cosθ L x = yp z zp y = i 1 sinθ sinφ cosθ cosφz sinθ θ φ L y = zp x xpz = i 1 sinθ cosφ cosθ sinφ sinθ θ φ = xp y yp x = i ϕ
8 Eigenvalues and eigenfunctions of = xp y yp x = i ϕ Note that the operator is similar to the momentum operator for the angular coordinate φ. Eigenvalue equation: Solution: Φ m (φ) = i φ Φ m(φ) = µ m Φ m (φ) i Φ m (φ) = Ne µ mφ
9 Eigenvalues and eigenfunctions of : i Φ m (φ) = Ne µ mφ φ ranges from 0 to 2π. We require the function to be single valued, hence Φ m (φ + 2π ) = Φ m (φ) i Ne µ i m (φ + 2π ) = Ne µ i m (φ) e µ m (2π ) = 1 µ m = integer Eigenvalues of : µ m = 0, ±, ±2, ±3...
10 Eigenvalues and eigenfunctions of : i Φ m (φ) = Ne µ mφ µ m = 0, ±, ±2, ±3... Normalization: 2π Φ m (φ) 2 dφ = N 2 e 0 2π 0 i µ mφ e i µ mφ dφ = N 2 2π N = 1 2π Eigenfunctions of : Φ m (φ) = 1 2π eimφ m = 0,±1,±2,±3...
11 Eigenvalues and eigenfunctions of L 2 : In spherical coordinates, Eigenvalue equation: 1 L 2 = 2 sinθ θ sinθ 1 2 θ + sin 2 θ φ 2 L 2 (θ,φ) = λ l (θ,φ) We want to obtain common eigenfunctions so that (θ,φ) = µ m (θ,φ) ( θ,φ) = P lm ( θ )Φ m (φ) = P lm ( θ ) 1 2π eimφ m = 0,±1,±2,±3...
12 Eigenvalues and eigenfunctions of L 2 : 1 L 2 = 2 sinθ θ sinθ θ + 1 sin 2 θ 2 φ 2 Eigenvalue equation: L 2 (θ,φ) = λ l (θ,φ) Solution: ( θ,φ) = P lm ( θ )Φ m (φ) = P lm ( θ ) 1 2π eimφ Inserting this solution into the eigenvalue equation for L 2 : 1 sinθ d dθ sinθ d dθ + λ l 2 m2 sin 2 θ P lm ( θ) = 0
13 Eigenvalues and eigenfunctions of L 2 : 1 sinθ d dθ sinθ d dθ + λ l 2 m2 sin 2 θ P lm ( θ) = 0 Making the substitution w = cosθ The above equation becomes d dw 1 w2 ( ) d dw + λ l 2 m2 1 w 2 P lm ( w) = 0
14 Eigenvalues equation for L 2 : d dw ( 1 ) d w2 dw P (w) + λ lm l m2 1 w 2 P lm ( w) = 0 Consider the simplest case of m=0: d ( dw 1 ) d w2 dw P (w) + λ l lm P 2 lm This is the Legendre equation. The solutions are Legendre polynomials: ( w) = 0 P l (w) = 1 l d 2 l l! dw (w 2 1) l λ l = l(l + 1) 2 λ l 0
15 Orbital angular momentum: Eigenvalues and eigenfunctions of L 2 : Legendre polynomials P l (w) = 1 2 l l! d dw l (w 2 1) l P 0 (w) = 1 P 1 (w) = 1 2 P 2 (w) = 1 8 d dw d dw (w 2 1) = w 2 (w 2 1) 2 = 1 2 (3w2 1) (l + 1)P l+1 (w) = (2l + 1)wP l (w) lp l 1 (w) Recurrence relations
16 Orbital angular momentum: Eigenvalues and eigenfunctions of L 2 : Legendre polynomials P l (w) = 1 2 l l! d dw l (w 2 1) l Associated Legendre polynomials: P l m (w) = (1 w 2 ) m 2 d dw m P l (w)
17 Orbital angular momentum: Eigenvalues and eigenfunctions of L 2 : Associated Legendre polynomials: P l m (w) = (1 w 2 ) m 2 d dw m P l (w) Associated Legendre polynomials are solutions of the general eigenvalue equation for L 2 : d ( dw 1 ) dp (w) w2 lm dw + λ l 2 m2 1 w 2 P lm ( w) = 0 λ l = l(l + 1) 2, l m l
18 Orbital angular momentum: Eigenvalues and eigenfunctions of L2 and Lz: The solutions to the two eigenvalue equations L 2 (θ,φ) = λ l (θ,φ) (θ,φ) = µ m (θ,φ) are thus (θ,φ) = N lm P l m (cosθ)e imφ With l and m being integers such that l 0, l m l l : azimuthal quantum number m :magnetic quantum number Quantized angular momentum eigenvalues: λ l = l(l + 1) 2, µ m = m
19 Eigenvalues and eigenfunctions of L 2 and : are called the spherical harmonics Normalization: 2π 0 π (θ,φ) = N lm P l m (cosθ)e imφ (θ,φ) 2 sinθdθdφ = 1 N lm = 0 (2l + 1)(l m )! ( 1) m, m 0 4π(l + m)! (2l + 1)(l m )!, m 0 4π(l + m)! The spherical harmonics form a complete, orthonormal basis on a sphere.
20 Spherical Harmonics: Y 00 = 1 2 π Y 10 = Y 2,0 = 3 4π cosθ, Y = 3 1,±1 8π sinθe±φ 5 16π 3cos2 θ 1 ( ) Y 2,±1 = 15 sinθ cosθe±φ 8π Y 2,±2 = 15 32π sin2 θe ±2φ l=0: s states, l=1: p states, l=2: d states, l=3: f states
Angular Momentum - set 1
 Angular Momentum - set PH0 - QM II August 6, 07 First of all, let us practise evaluating commutators. Consider these as warm up problems. Problem : Show the following commutation relations ˆx, ˆL x ] =
Angular Momentum - set PH0 - QM II August 6, 07 First of all, let us practise evaluating commutators. Consider these as warm up problems. Problem : Show the following commutation relations ˆx, ˆL x ] =
Angular momentum & spin
 Angular momentum & spin January 8, 2002 1 Angular momentum Angular momentum appears as a very important aspect of almost any quantum mechanical system, so we need to briefly review some basic properties
Angular momentum & spin January 8, 2002 1 Angular momentum Angular momentum appears as a very important aspect of almost any quantum mechanical system, so we need to briefly review some basic properties
Summary: angular momentum derivation
 Summary: angular momentum derivation L = r p L x = yp z zp y, etc. [x, p y ] = 0, etc. (-) (-) (-3) Angular momentum commutation relations [L x, L y ] = i hl z (-4) [L i, L j ] = i hɛ ijk L k (-5) Levi-Civita
Summary: angular momentum derivation L = r p L x = yp z zp y, etc. [x, p y ] = 0, etc. (-) (-) (-3) Angular momentum commutation relations [L x, L y ] = i hl z (-4) [L i, L j ] = i hɛ ijk L k (-5) Levi-Civita
Angular momentum. Quantum mechanics. Orbital angular momentum
 Angular momentum 1 Orbital angular momentum Consider a particle described by the Cartesian coordinates (x, y, z r and their conjugate momenta (p x, p y, p z p. The classical definition of the orbital angular
Angular momentum 1 Orbital angular momentum Consider a particle described by the Cartesian coordinates (x, y, z r and their conjugate momenta (p x, p y, p z p. The classical definition of the orbital angular
Fun With Carbon Monoxide. p. 1/2
 Fun With Carbon Monoxide p. 1/2 p. 1/2 Fun With Carbon Monoxide E = 0.25 ± 0.05 ev Electron beam results p. 1/2 Fun With Carbon Monoxide E = 0.25 ± 0.05 ev Electron beam results C V (J/K-mole) 35 30 25
Fun With Carbon Monoxide p. 1/2 p. 1/2 Fun With Carbon Monoxide E = 0.25 ± 0.05 ev Electron beam results p. 1/2 Fun With Carbon Monoxide E = 0.25 ± 0.05 ev Electron beam results C V (J/K-mole) 35 30 25
Angular Momentum - set 1
 Angular Momentum - set PH0 - QM II August 6, 07 First of all, let us practise evaluating commutators. Consider these as warm up problems. Problem : Show the following commutation relations ˆx, ˆL x = 0,
Angular Momentum - set PH0 - QM II August 6, 07 First of all, let us practise evaluating commutators. Consider these as warm up problems. Problem : Show the following commutation relations ˆx, ˆL x = 0,
Lecture #1. Review. Postulates of quantum mechanics (1-3) Postulate 1
 L1.P1 Lecture #1 Review Postulates of quantum mechanics (1-3) Postulate 1 The state of a system at any instant of time may be represented by a wave function which is continuous and differentiable. Specifically,
L1.P1 Lecture #1 Review Postulates of quantum mechanics (1-3) Postulate 1 The state of a system at any instant of time may be represented by a wave function which is continuous and differentiable. Specifically,
Modern Physics. Unit 6: Hydrogen Atom - Radiation Lecture 6.3: Vector Model of Angular Momentum
 Modern Physics Unit 6: Hydrogen Atom - Radiation ecture 6.3: Vector Model of Angular Momentum Ron Reifenberger Professor of Physics Purdue University 1 Summary of Important Points from ast ecture The magnitude
Modern Physics Unit 6: Hydrogen Atom - Radiation ecture 6.3: Vector Model of Angular Momentum Ron Reifenberger Professor of Physics Purdue University 1 Summary of Important Points from ast ecture The magnitude
Total Angular Momentum for Hydrogen
 Physics 4 Lecture 7 Total Angular Momentum for Hydrogen Lecture 7 Physics 4 Quantum Mechanics I Friday, April th, 008 We have the Hydrogen Hamiltonian for central potential φ(r), we can write: H r = p
Physics 4 Lecture 7 Total Angular Momentum for Hydrogen Lecture 7 Physics 4 Quantum Mechanics I Friday, April th, 008 We have the Hydrogen Hamiltonian for central potential φ(r), we can write: H r = p
The Hydrogen atom. Chapter The Schrödinger Equation. 2.2 Angular momentum
 Chapter 2 The Hydrogen atom In the previous chapter we gave a quick overview of the Bohr model, which is only really valid in the semiclassical limit. cf. section 1.7.) We now begin our task in earnest
Chapter 2 The Hydrogen atom In the previous chapter we gave a quick overview of the Bohr model, which is only really valid in the semiclassical limit. cf. section 1.7.) We now begin our task in earnest
One-electron Atom. (in spherical coordinates), where Y lm. are spherical harmonics, we arrive at the following Schrödinger equation:
 One-electron Atom The atomic orbitals of hydrogen-like atoms are solutions to the Schrödinger equation in a spherically symmetric potential. In this case, the potential term is the potential given by Coulomb's
One-electron Atom The atomic orbitals of hydrogen-like atoms are solutions to the Schrödinger equation in a spherically symmetric potential. In this case, the potential term is the potential given by Coulomb's
Spherical Harmonics on S 2
 7 August 00 1 Spherical Harmonics on 1 The Laplace-Beltrami Operator In what follows, we describe points on using the parametrization x = cos ϕ sin θ, y = sin ϕ sin θ, z = cos θ, where θ is the colatitude
7 August 00 1 Spherical Harmonics on 1 The Laplace-Beltrami Operator In what follows, we describe points on using the parametrization x = cos ϕ sin θ, y = sin ϕ sin θ, z = cos θ, where θ is the colatitude
Angular Momentum Properties
 Cheistry 460 Fall 017 Dr. Jean M. Standard October 30, 017 Angular Moentu Properties Classical Definition of Angular Moentu In classical echanics, the angular oentu vector L is defined as L = r p, (1)
Cheistry 460 Fall 017 Dr. Jean M. Standard October 30, 017 Angular Moentu Properties Classical Definition of Angular Moentu In classical echanics, the angular oentu vector L is defined as L = r p, (1)
A Quantum Mechanical Model for the Vibration and Rotation of Molecules. Rigid Rotor
 A Quantum Mechanical Model for the Vibration and Rotation of Molecules Harmonic Oscillator Rigid Rotor Degrees of Freedom Translation: quantum mechanical model is particle in box or free particle. A molecule
A Quantum Mechanical Model for the Vibration and Rotation of Molecules Harmonic Oscillator Rigid Rotor Degrees of Freedom Translation: quantum mechanical model is particle in box or free particle. A molecule
IV. Electronic Spectroscopy, Angular Momentum, and Magnetic Resonance
 IV. Electronic Spectroscopy, Angular Momentum, and Magnetic Resonance The foundation of electronic spectroscopy is the exact solution of the time-independent Schrodinger equation for the hydrogen atom.
IV. Electronic Spectroscopy, Angular Momentum, and Magnetic Resonance The foundation of electronic spectroscopy is the exact solution of the time-independent Schrodinger equation for the hydrogen atom.
Lecture 11 Spin, orbital, and total angular momentum Mechanics. 1 Very brief background. 2 General properties of angular momentum operators
 Lecture Spin, orbital, and total angular momentum 70.00 Mechanics Very brief background MATH-GA In 9, a famous experiment conducted by Otto Stern and Walther Gerlach, involving particles subject to a nonuniform
Lecture Spin, orbital, and total angular momentum 70.00 Mechanics Very brief background MATH-GA In 9, a famous experiment conducted by Otto Stern and Walther Gerlach, involving particles subject to a nonuniform
2m r2 (~r )+V (~r ) (~r )=E (~r )
 Review of the Hydrogen Atom The Schrodinger equation (for 1D, 2D, or 3D) can be expressed as: ~ 2 2m r2 (~r, t )+V (~r ) (~r, t )=i~ @ @t The Laplacian is the divergence of the gradient: r 2 =r r The time-independent
Review of the Hydrogen Atom The Schrodinger equation (for 1D, 2D, or 3D) can be expressed as: ~ 2 2m r2 (~r, t )+V (~r ) (~r, t )=i~ @ @t The Laplacian is the divergence of the gradient: r 2 =r r The time-independent
Physics 221A Fall 1996 Notes 12 Orbital Angular Momentum and Spherical Harmonics
 Physics 221A Fall 1996 Notes 12 Orbital Angular Momentum and Spherical Harmonics We now consider the spatial degrees of freedom of a particle moving in 3-dimensional space, which of course is an important
Physics 221A Fall 1996 Notes 12 Orbital Angular Momentum and Spherical Harmonics We now consider the spatial degrees of freedom of a particle moving in 3-dimensional space, which of course is an important
Spherical Coordinates and Legendre Functions
 Spherical Coordinates and Legendre Functions Spherical coordinates Let s adopt the notation for spherical coordinates that is standard in physics: φ = longitude or azimuth, θ = colatitude ( π 2 latitude)
Spherical Coordinates and Legendre Functions Spherical coordinates Let s adopt the notation for spherical coordinates that is standard in physics: φ = longitude or azimuth, θ = colatitude ( π 2 latitude)
Sample Quantum Chemistry Exam 2 Solutions
 Chemistry 46 Fall 7 Dr. Jean M. Standard Name SAMPE EXAM Sample Quantum Chemistry Exam Solutions.) ( points) Answer the following questions by selecting the correct answer from the choices provided. a.)
Chemistry 46 Fall 7 Dr. Jean M. Standard Name SAMPE EXAM Sample Quantum Chemistry Exam Solutions.) ( points) Answer the following questions by selecting the correct answer from the choices provided. a.)
Atoms 2012 update -- start with single electron: H-atom
 Atoms 2012 update -- start with single electron: H-atom x z φ θ e -1 y 3-D problem - free move in x, y, z - easier if change coord. systems: Cartesian Spherical Coordinate (x, y, z) (r, θ, φ) Reason: V(r)
Atoms 2012 update -- start with single electron: H-atom x z φ θ e -1 y 3-D problem - free move in x, y, z - easier if change coord. systems: Cartesian Spherical Coordinate (x, y, z) (r, θ, φ) Reason: V(r)
PHYS851 Quantum Mechanics I, Fall 2009 HOMEWORK ASSIGNMENT 10: Solutions
 PHYS851 Quantum Mechanics I, Fall 009 HOMEWORK ASSIGNMENT 10: Solutions Topics Covered: Tensor product spaces, change of coordinate system, general theory of angular momentum Some Key Concepts: Angular
PHYS851 Quantum Mechanics I, Fall 009 HOMEWORK ASSIGNMENT 10: Solutions Topics Covered: Tensor product spaces, change of coordinate system, general theory of angular momentum Some Key Concepts: Angular
Physics 228 Today: Ch 41: 1-3: 3D quantum mechanics, hydrogen atom
 Physics 228 Today: Ch 41: 1-3: 3D quantum mechanics, hydrogen atom Website: Sakai 01:750:228 or www.physics.rutgers.edu/ugrad/228 Happy April Fools Day Example / Worked Problems What is the ratio of the
Physics 228 Today: Ch 41: 1-3: 3D quantum mechanics, hydrogen atom Website: Sakai 01:750:228 or www.physics.rutgers.edu/ugrad/228 Happy April Fools Day Example / Worked Problems What is the ratio of the
d 1 µ 2 Θ = 0. (4.1) consider first the case of m = 0 where there is no azimuthal dependence on the angle φ.
 4 Legendre Functions In order to investigate the solutions of Legendre s differential equation d ( µ ) dθ ] ] + l(l + ) m dµ dµ µ Θ = 0. (4.) consider first the case of m = 0 where there is no azimuthal
4 Legendre Functions In order to investigate the solutions of Legendre s differential equation d ( µ ) dθ ] ] + l(l + ) m dµ dµ µ Θ = 0. (4.) consider first the case of m = 0 where there is no azimuthal
Graduate Quantum Mechanics I: Prelims and Solutions (Fall 2015)
 Graduate Quantum Mechanics I: Prelims and Solutions (Fall 015 Problem 1 (0 points Suppose A and B are two two-level systems represented by the Pauli-matrices σx A,B σ x = ( 0 1 ;σ 1 0 y = ( ( 0 i 1 0 ;σ
Graduate Quantum Mechanics I: Prelims and Solutions (Fall 015 Problem 1 (0 points Suppose A and B are two two-level systems represented by the Pauli-matrices σx A,B σ x = ( 0 1 ;σ 1 0 y = ( ( 0 i 1 0 ;σ
The 3 dimensional Schrödinger Equation
 Chapter 6 The 3 dimensional Schrödinger Equation 6.1 Angular Momentum To study how angular momentum is represented in quantum mechanics we start by reviewing the classical vector of orbital angular momentum
Chapter 6 The 3 dimensional Schrödinger Equation 6.1 Angular Momentum To study how angular momentum is represented in quantum mechanics we start by reviewing the classical vector of orbital angular momentum
The Spherical Harmonics
 Physics 6C Fall 202 The Spherical Harmonics. Solution to Laplace s equation in spherical coordinates In spherical coordinates, the Laplacian is given by 2 = ( r 2 ) ( + r 2 r r r 2 sin 2 sinθ ) + θ θ θ
Physics 6C Fall 202 The Spherical Harmonics. Solution to Laplace s equation in spherical coordinates In spherical coordinates, the Laplacian is given by 2 = ( r 2 ) ( + r 2 r r r 2 sin 2 sinθ ) + θ θ θ
1 Schroenger s Equation for the Hydrogen Atom
 Schroenger s Equation for the Hydrogen Atom Here is the Schroedinger equation in D in spherical polar coordinates. Note that the definitions of θ and φ are the exact reverse of what they are in mathematics.
Schroenger s Equation for the Hydrogen Atom Here is the Schroedinger equation in D in spherical polar coordinates. Note that the definitions of θ and φ are the exact reverse of what they are in mathematics.
Introduction to Quantum Physics and Models of Hydrogen Atom
 Introduction to Quantum Physics and Models of Hydrogen Atom Tien-Tsan Shieh Department of Applied Math National Chiao-Tung University November 7, 2012 Physics and Models of Hydrogen November Atom 7, 2012
Introduction to Quantum Physics and Models of Hydrogen Atom Tien-Tsan Shieh Department of Applied Math National Chiao-Tung University November 7, 2012 Physics and Models of Hydrogen November Atom 7, 2012
CHEM-UA 127: Advanced General Chemistry I
 1 CHEM-UA 127: Advanced General Chemistry I Notes for Lecture 11 Nowthatwehaveintroducedthebasicconceptsofquantummechanics, wecanstarttoapplythese conceptsto build up matter, starting from its most elementary
1 CHEM-UA 127: Advanced General Chemistry I Notes for Lecture 11 Nowthatwehaveintroducedthebasicconceptsofquantummechanics, wecanstarttoapplythese conceptsto build up matter, starting from its most elementary
Spin Dynamics Basic Theory Operators. Richard Green SBD Research Group Department of Chemistry
 Spin Dynamics Basic Theory Operators Richard Green SBD Research Group Department of Chemistry Objective of this session Introduce you to operators used in quantum mechanics Achieve this by looking at:
Spin Dynamics Basic Theory Operators Richard Green SBD Research Group Department of Chemistry Objective of this session Introduce you to operators used in quantum mechanics Achieve this by looking at:
Angular momentum. Angular momentum operators. Quantum mechanics for scientists and engineers
 7.1 Angular momentum Slides: Video 7.1.1 Angular momentum operators Text reference: Quantum Mechanics for Scientists and Engineers Chapter 9 introduction and Section 9.1 (first part) Angular momentum Angular
7.1 Angular momentum Slides: Video 7.1.1 Angular momentum operators Text reference: Quantum Mechanics for Scientists and Engineers Chapter 9 introduction and Section 9.1 (first part) Angular momentum Angular
Topics Covered: Motion in a central potential, spherical harmonic oscillator, hydrogen atom, orbital electric and magnetic dipole moments
 PHYS85 Quantum Mechanics I, Fall 9 HOMEWORK ASSIGNMENT Topics Covered: Motion in a central potential, spherical harmonic oscillator, hydrogen atom, orbital electric and magnetic dipole moments. [ pts]
PHYS85 Quantum Mechanics I, Fall 9 HOMEWORK ASSIGNMENT Topics Covered: Motion in a central potential, spherical harmonic oscillator, hydrogen atom, orbital electric and magnetic dipole moments. [ pts]
Atoms 09 update-- start with single electron: H-atom
 Atoms 09 update-- start with single electron: H-atom VII 33 x z φ θ e -1 y 3-D problem - free move in x, y, z - handy to change systems: Cartesian Spherical Coordinate (x, y, z) (r, θ, φ) Reason: V(r)
Atoms 09 update-- start with single electron: H-atom VII 33 x z φ θ e -1 y 3-D problem - free move in x, y, z - handy to change systems: Cartesian Spherical Coordinate (x, y, z) (r, θ, φ) Reason: V(r)
Second quantization: where quantization and particles come from?
 110 Phys460.nb 7 Second quantization: where quantization and particles come from? 7.1. Lagrangian mechanics and canonical quantization Q: How do we quantize a general system? 7.1.1.Lagrangian Lagrangian
110 Phys460.nb 7 Second quantization: where quantization and particles come from? 7.1. Lagrangian mechanics and canonical quantization Q: How do we quantize a general system? 7.1.1.Lagrangian Lagrangian
Separation of Variables in Polar and Spherical Coordinates
 Separation of Variables in Polar and Spherical Coordinates Polar Coordinates Suppose we are given the potential on the inside surface of an infinitely long cylindrical cavity, and we want to find the potential
Separation of Variables in Polar and Spherical Coordinates Polar Coordinates Suppose we are given the potential on the inside surface of an infinitely long cylindrical cavity, and we want to find the potential
PHYS 404 Lecture 1: Legendre Functions
 PHYS 404 Lecture 1: Legendre Functions Dr. Vasileios Lempesis PHYS 404 - LECTURE 1 DR. V. LEMPESIS 1 Legendre Functions physical justification Legendre functions or Legendre polynomials are the solutions
PHYS 404 Lecture 1: Legendre Functions Dr. Vasileios Lempesis PHYS 404 - LECTURE 1 DR. V. LEMPESIS 1 Legendre Functions physical justification Legendre functions or Legendre polynomials are the solutions
Lecture 4 Quantum mechanics in more than one-dimension
 Lecture 4 Quantum mechanics in more than one-dimension Background Previously, we have addressed quantum mechanics of 1d systems and explored bound and unbound (scattering) states. Although general concepts
Lecture 4 Quantum mechanics in more than one-dimension Background Previously, we have addressed quantum mechanics of 1d systems and explored bound and unbound (scattering) states. Although general concepts
1.6. Quantum mechanical description of the hydrogen atom
 29.6. Quantum mechanical description of the hydrogen atom.6.. Hamiltonian for the hydrogen atom Atomic units To avoid dealing with very small numbers, let us introduce the so called atomic units : Quantity
29.6. Quantum mechanical description of the hydrogen atom.6.. Hamiltonian for the hydrogen atom Atomic units To avoid dealing with very small numbers, let us introduce the so called atomic units : Quantity
Atoms 2010 update -- start with single electron: H-atom
 VII 33 Atoms 2010 update -- start with single electron: H-atom x z φ θ e -1 y 3-D problem - free move in x, y, z - easier if change coord. systems: Cartesian Spherical Coordinate (x, y, z) (r, θ, φ) Reason:
VII 33 Atoms 2010 update -- start with single electron: H-atom x z φ θ e -1 y 3-D problem - free move in x, y, z - easier if change coord. systems: Cartesian Spherical Coordinate (x, y, z) (r, θ, φ) Reason:
Lecture 4 Quantum mechanics in more than one-dimension
 Lecture 4 Quantum mechanics in more than one-dimension Background Previously, we have addressed quantum mechanics of 1d systems and explored bound and unbound (scattering) states. Although general concepts
Lecture 4 Quantum mechanics in more than one-dimension Background Previously, we have addressed quantum mechanics of 1d systems and explored bound and unbound (scattering) states. Although general concepts
Physics 221A Fall 2017 Notes 15 Orbital Angular Momentum and Spherical Harmonics
 Copyright c 2018 by Robert G. Littlejohn Physics 221A Fall 2017 Notes 15 Orbital Angular Momentum and Spherical Harmonics 1. Introduction In Notes 13, we worked out the general theory of the representations
Copyright c 2018 by Robert G. Littlejohn Physics 221A Fall 2017 Notes 15 Orbital Angular Momentum and Spherical Harmonics 1. Introduction In Notes 13, we worked out the general theory of the representations
Physics 401: Quantum Mechanics I Chapter 4
 Physics 401: Quantum Mechanics I Chapter 4 Are you here today? A. Yes B. No C. After than midterm? 3-D Schroedinger Equation The ground state energy of the particle in a 3D box is ( 1 2 +1 2 +1 2 ) π2
Physics 401: Quantum Mechanics I Chapter 4 Are you here today? A. Yes B. No C. After than midterm? 3-D Schroedinger Equation The ground state energy of the particle in a 3D box is ( 1 2 +1 2 +1 2 ) π2
Integrals in cylindrical, spherical coordinates (Sect. 15.7)
 Integrals in clindrical, spherical coordinates (Sect. 15.7 Integration in spherical coordinates. Review: Clindrical coordinates. Spherical coordinates in space. Triple integral in spherical coordinates.
Integrals in clindrical, spherical coordinates (Sect. 15.7 Integration in spherical coordinates. Review: Clindrical coordinates. Spherical coordinates in space. Triple integral in spherical coordinates.
2. Griffith s 4.19 a. The commutator of the z-component of the angular momentum with the coordinates are: [L z. + xyp x. x xxp y.
 Physics Homework #8 Spring 6 Due Friday 5/7/6. Download the Mathematica notebook from the notes page of the website (http://minerva.union.edu/labrakes/physics_notes_s5.htm) or the homework page of the
Physics Homework #8 Spring 6 Due Friday 5/7/6. Download the Mathematica notebook from the notes page of the website (http://minerva.union.edu/labrakes/physics_notes_s5.htm) or the homework page of the
Quantum Theory of Angular Momentum and Atomic Structure
 Quantum Theory of Angular Momentum and Atomic Structure VBS/MRC Angular Momentum 0 Motivation...the questions Whence the periodic table? Concepts in Materials Science I VBS/MRC Angular Momentum 1 Motivation...the
Quantum Theory of Angular Momentum and Atomic Structure VBS/MRC Angular Momentum 0 Motivation...the questions Whence the periodic table? Concepts in Materials Science I VBS/MRC Angular Momentum 1 Motivation...the
Atkins & de Paula: Atkins Physical Chemistry 9e Checklist of key ideas. Chapter 8: Quantum Theory: Techniques and Applications
 Atkins & de Paula: Atkins Physical Chemistry 9e Checklist of key ideas Chapter 8: Quantum Theory: Techniques and Applications TRANSLATIONAL MOTION wavefunction of free particle, ψ k = Ae ikx + Be ikx,
Atkins & de Paula: Atkins Physical Chemistry 9e Checklist of key ideas Chapter 8: Quantum Theory: Techniques and Applications TRANSLATIONAL MOTION wavefunction of free particle, ψ k = Ae ikx + Be ikx,
Quantum Mechanics in Multidimensions
 Quantum Mechanics in Multidimensions In this chapter we discuss bound state solutions of the Schrödinger equation in more than one dimension. In this chapter we will discuss some particularly straightforward
Quantum Mechanics in Multidimensions In this chapter we discuss bound state solutions of the Schrödinger equation in more than one dimension. In this chapter we will discuss some particularly straightforward
Quantum Mechanics in Three Dimensions
 Physics 342 Lecture 21 Quantum Mechanics in Three Dimensions Lecture 21 Physics 342 Quantum Mechanics I Monday, March 22nd, 21 We are used to the temporal separation that gives, for example, the timeindependent
Physics 342 Lecture 21 Quantum Mechanics in Three Dimensions Lecture 21 Physics 342 Quantum Mechanics I Monday, March 22nd, 21 We are used to the temporal separation that gives, for example, the timeindependent
Rotations in Quantum Mechanics
 Rotations in Quantum Mechanics We have seen that physical transformations are represented in quantum mechanics by unitary operators acting on the Hilbert space. In this section, we ll think about the specific
Rotations in Quantum Mechanics We have seen that physical transformations are represented in quantum mechanics by unitary operators acting on the Hilbert space. In this section, we ll think about the specific
PHYS 502 Lecture 8: Legendre Functions. Dr. Vasileios Lempesis
 PHYS 502 Lecture 8: Legendre Functions Dr. Vasileios Lempesis Introduction Legendre functions or Legendre polynomials are the solutions of Legendre s differential equation that appear when we separate
PHYS 502 Lecture 8: Legendre Functions Dr. Vasileios Lempesis Introduction Legendre functions or Legendre polynomials are the solutions of Legendre s differential equation that appear when we separate
5.61 Lecture #17 Rigid Rotor I
 561 Fall 2017 Lecture #17 Page 1 561 Lecture #17 Rigid Rotor I Read McQuarrie: Chapters E and 6 Rigid Rotors molecular rotation and the universal angular part of all central force problems another exactly
561 Fall 2017 Lecture #17 Page 1 561 Lecture #17 Rigid Rotor I Read McQuarrie: Chapters E and 6 Rigid Rotors molecular rotation and the universal angular part of all central force problems another exactly
More On Carbon Monoxide
 More On Carbon Monoxide E = 0.25 ± 0.05 ev Electron beam results Jerry Gilfoyle The Configurations of CO 1 / 26 More On Carbon Monoxide E = 0.25 ± 0.05 ev Electron beam results Jerry Gilfoyle The Configurations
More On Carbon Monoxide E = 0.25 ± 0.05 ev Electron beam results Jerry Gilfoyle The Configurations of CO 1 / 26 More On Carbon Monoxide E = 0.25 ± 0.05 ev Electron beam results Jerry Gilfoyle The Configurations
St Hugh s 2 nd Year: Quantum Mechanics II. Reading. Topics. The following sources are recommended for this tutorial:
 St Hugh s 2 nd Year: Quantum Mechanics II Reading The following sources are recommended for this tutorial: The key text (especially here in Oxford) is Molecular Quantum Mechanics, P. W. Atkins and R. S.
St Hugh s 2 nd Year: Quantum Mechanics II Reading The following sources are recommended for this tutorial: The key text (especially here in Oxford) is Molecular Quantum Mechanics, P. W. Atkins and R. S.
20 The Hydrogen Atom. Ze2 r R (20.1) H( r, R) = h2 2m 2 r h2 2M 2 R
 20 The Hydrogen Atom 1. We want to solve the time independent Schrödinger Equation for the hydrogen atom. 2. There are two particles in the system, an electron and a nucleus, and so we can write the Hamiltonian
20 The Hydrogen Atom 1. We want to solve the time independent Schrödinger Equation for the hydrogen atom. 2. There are two particles in the system, an electron and a nucleus, and so we can write the Hamiltonian
Quantum Mechanics: The Hydrogen Atom
 Quantum Mechanics: The Hydrogen Atom 4th April 9 I. The Hydrogen Atom In this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen
Quantum Mechanics: The Hydrogen Atom 4th April 9 I. The Hydrogen Atom In this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen
Quantum Mechanics in 3-Dimensions
 Quantum Mechanics in 3-Dimensions Pavithran S Iyer, 2nd yr BSc Physics, Chennai Mathematical Institute Email: pavithra@cmi.ac.in August 28 th, 2009 1 Schrodinger equation in Spherical Coordinates 1.1 Transforming
Quantum Mechanics in 3-Dimensions Pavithran S Iyer, 2nd yr BSc Physics, Chennai Mathematical Institute Email: pavithra@cmi.ac.in August 28 th, 2009 1 Schrodinger equation in Spherical Coordinates 1.1 Transforming
3. Quantum Mechanics in 3D
 3. Quantum Mechanics in 3D 3.1 Introduction Last time, we derived the time dependent Schrödinger equation, starting from three basic postulates: 1) The time evolution of a state can be expressed as a unitary
3. Quantum Mechanics in 3D 3.1 Introduction Last time, we derived the time dependent Schrödinger equation, starting from three basic postulates: 1) The time evolution of a state can be expressed as a unitary
ONE AND MANY ELECTRON ATOMS Chapter 15
 See Week 8 lecture notes. This is exactly the same as the Hamiltonian for nonrigid rotation. In Week 8 lecture notes it was shown that this is the operator for Lˆ 2, the square of the angular momentum.
See Week 8 lecture notes. This is exactly the same as the Hamiltonian for nonrigid rotation. In Week 8 lecture notes it was shown that this is the operator for Lˆ 2, the square of the angular momentum.
Time part of the equation can be separated by substituting independent equation
 Lecture 9 Schrödinger Equation in 3D and Angular Momentum Operator In this section we will construct 3D Schrödinger equation and we give some simple examples. In this course we will consider problems where
Lecture 9 Schrödinger Equation in 3D and Angular Momentum Operator In this section we will construct 3D Schrödinger equation and we give some simple examples. In this course we will consider problems where
Schrödinger equation for the nuclear potential
 Schrödinger equation for the nuclear potential Introduction to Nuclear Science Simon Fraser University Spring 2011 NUCS 342 January 24, 2011 NUCS 342 (Lecture 4) January 24, 2011 1 / 32 Outline 1 One-dimensional
Schrödinger equation for the nuclear potential Introduction to Nuclear Science Simon Fraser University Spring 2011 NUCS 342 January 24, 2011 NUCS 342 (Lecture 4) January 24, 2011 1 / 32 Outline 1 One-dimensional
Chapter 4. Q. A hydrogen atom starts out in the following linear combination of the stationary. (ψ ψ 21 1 ). (1)
 Tor Kjellsson Stockholm University Chapter 4 4.5 Q. A hydrogen atom starts out in the following linear combination of the stationary states n, l, m =,, and n, l, m =,, : Ψr, 0 = ψ + ψ. a Q. Construct Ψr,
Tor Kjellsson Stockholm University Chapter 4 4.5 Q. A hydrogen atom starts out in the following linear combination of the stationary states n, l, m =,, and n, l, m =,, : Ψr, 0 = ψ + ψ. a Q. Construct Ψr,
Final Exam Tuesday, May 8, 2012 Starting at 8:30 a.m., Hoyt Hall Duration: 2h 30m
 Final Exam Tuesday, May 8, 2012 Starting at 8:30 a.m., Hoyt Hall. ------------------- Duration: 2h 30m Chapter 39 Quantum Mechanics of Atoms Units of Chapter 39 39-1 Quantum-Mechanical View of Atoms 39-2
Final Exam Tuesday, May 8, 2012 Starting at 8:30 a.m., Hoyt Hall. ------------------- Duration: 2h 30m Chapter 39 Quantum Mechanics of Atoms Units of Chapter 39 39-1 Quantum-Mechanical View of Atoms 39-2
Classical Field Theory: Electrostatics-Magnetostatics
 Classical Field Theory: Electrostatics-Magnetostatics April 27, 2010 1 1 J.D.Jackson, Classical Electrodynamics, 2nd Edition, Section 1-5 Electrostatics The behavior of an electrostatic field can be described
Classical Field Theory: Electrostatics-Magnetostatics April 27, 2010 1 1 J.D.Jackson, Classical Electrodynamics, 2nd Edition, Section 1-5 Electrostatics The behavior of an electrostatic field can be described
3 Angular Momentum and Spin
 In this chapter we review the notions surrounding the different forms of angular momenta in quantum mechanics, including the spin angular momentum, which is entirely quantum mechanical in nature. Some
In this chapter we review the notions surrounding the different forms of angular momenta in quantum mechanics, including the spin angular momentum, which is entirely quantum mechanical in nature. Some
4/21/2010. Schrödinger Equation For Hydrogen Atom. Spherical Coordinates CHAPTER 8
 CHAPTER 8 Hydrogen Atom 8.1 Spherical Coordinates 8.2 Schrödinger's Equation in Spherical Coordinate 8.3 Separation of Variables 8.4 Three Quantum Numbers 8.5 Hydrogen Atom Wave Function 8.6 Electron Spin
CHAPTER 8 Hydrogen Atom 8.1 Spherical Coordinates 8.2 Schrödinger's Equation in Spherical Coordinate 8.3 Separation of Variables 8.4 Three Quantum Numbers 8.5 Hydrogen Atom Wave Function 8.6 Electron Spin
Scattering cross-section (µm 2 )
 Supplementary Figures Scattering cross-section (µm 2 ).16.14.12.1.8.6.4.2 Total scattering Electric dipole, a E (1,1) Magnetic dipole, a M (1,1) Magnetic quardupole, a M (2,1). 44 48 52 56 Wavelength (nm)
Supplementary Figures Scattering cross-section (µm 2 ).16.14.12.1.8.6.4.2 Total scattering Electric dipole, a E (1,1) Magnetic dipole, a M (1,1) Magnetic quardupole, a M (2,1). 44 48 52 56 Wavelength (nm)
Chapter 6. Quantum Theory of the Hydrogen Atom
 Chapter 6 Quantum Theory of the Hydrogen Atom 1 6.1 Schrodinger s Equation for the Hydrogen Atom Symmetry suggests spherical polar coordinates Fig. 6.1 (a) Spherical polar coordinates. (b) A line of constant
Chapter 6 Quantum Theory of the Hydrogen Atom 1 6.1 Schrodinger s Equation for the Hydrogen Atom Symmetry suggests spherical polar coordinates Fig. 6.1 (a) Spherical polar coordinates. (b) A line of constant
PHYS 3313 Section 001 Lecture # 22
 PHYS 3313 Section 001 Lecture # 22 Dr. Barry Spurlock Simple Harmonic Oscillator Barriers and Tunneling Alpha Particle Decay Schrodinger Equation on Hydrogen Atom Solutions for Schrodinger Equation for
PHYS 3313 Section 001 Lecture # 22 Dr. Barry Spurlock Simple Harmonic Oscillator Barriers and Tunneling Alpha Particle Decay Schrodinger Equation on Hydrogen Atom Solutions for Schrodinger Equation for
which implies that we can take solutions which are simultaneous eigen functions of
 Module 1 : Quantum Mechanics Chapter 6 : Quantum mechanics in 3-D Quantum mechanics in 3-D For most physical systems, the dynamics is in 3-D. The solutions to the general 3-d problem are quite complicated,
Module 1 : Quantum Mechanics Chapter 6 : Quantum mechanics in 3-D Quantum mechanics in 3-D For most physical systems, the dynamics is in 3-D. The solutions to the general 3-d problem are quite complicated,
Quantum Physics II (8.05) Fall 2002 Assignment 11
 Quantum Physics II (8.05) Fall 00 Assignment 11 Readings Most of the reading needed for this problem set was already given on Problem Set 9. The new readings are: Phase shifts are discussed in Cohen-Tannoudji
Quantum Physics II (8.05) Fall 00 Assignment 11 Readings Most of the reading needed for this problem set was already given on Problem Set 9. The new readings are: Phase shifts are discussed in Cohen-Tannoudji
Chemistry 795T. NC State University. Lecture 4. Vibrational and Rotational Spectroscopy
 Chemistry 795T Lecture 4 Vibrational and Rotational Spectroscopy NC State University The Dipole Moment Expansion The permanent dipole moment of a molecule oscillates about an equilibrium value as the molecule
Chemistry 795T Lecture 4 Vibrational and Rotational Spectroscopy NC State University The Dipole Moment Expansion The permanent dipole moment of a molecule oscillates about an equilibrium value as the molecule
Problem 1: Spin 1 2. particles (10 points)
 Problem 1: Spin 1 particles 1 points 1 Consider a system made up of spin 1/ particles. If one measures the spin of the particles, one can only measure spin up or spin down. The general spin state of a
Problem 1: Spin 1 particles 1 points 1 Consider a system made up of spin 1/ particles. If one measures the spin of the particles, one can only measure spin up or spin down. The general spin state of a
The tensor spherical harmonics
 Physics 4 Winter 07 The tensor spherical harmonics The Clebsch-Gordon coefficients Consider a system with orbital angular momentum L and spin angular momentum S. The total angular momentum of the system
Physics 4 Winter 07 The tensor spherical harmonics The Clebsch-Gordon coefficients Consider a system with orbital angular momentum L and spin angular momentum S. The total angular momentum of the system
Chapter 5. Atomic spectra
 Atomic spectra Sommerfelds relativistic model Sommerfeld succeeded partially in explaining fine structure by extending Bohr Theory i) He allowed the possibility of elliptical orbits for the electrons in
Atomic spectra Sommerfelds relativistic model Sommerfeld succeeded partially in explaining fine structure by extending Bohr Theory i) He allowed the possibility of elliptical orbits for the electrons in
The Hydrogen Atom. Dr. Sabry El-Taher 1. e 4. U U r
 The Hydrogen Atom Atom is a 3D object, and the electron motion is three-dimensional. We ll start with the simplest case - The hydrogen atom. An electron and a proton (nucleus) are bound by the central-symmetric
The Hydrogen Atom Atom is a 3D object, and the electron motion is three-dimensional. We ll start with the simplest case - The hydrogen atom. An electron and a proton (nucleus) are bound by the central-symmetric
Lecture 5: Harmonic oscillator, Morse Oscillator, 1D Rigid Rotor
 Lecture 5: Harmonic oscillator, Morse Oscillator, 1D Rigid Rotor It turns out that the boundary condition of the wavefunction going to zero at infinity is sufficient to quantize the value of energy that
Lecture 5: Harmonic oscillator, Morse Oscillator, 1D Rigid Rotor It turns out that the boundary condition of the wavefunction going to zero at infinity is sufficient to quantize the value of energy that
Physics 2203, 2011: Equation sheet for second midterm. General properties of Schrödinger s Equation: Quantum Mechanics. Ψ + UΨ = i t.
 General properties of Schrödinger s Equation: Quantum Mechanics Schrödinger Equation (time dependent) m Standing wave Ψ(x,t) = Ψ(x)e iωt Schrödinger Equation (time independent) Ψ x m Ψ x Ψ + UΨ = i t +UΨ
General properties of Schrödinger s Equation: Quantum Mechanics Schrödinger Equation (time dependent) m Standing wave Ψ(x,t) = Ψ(x)e iωt Schrödinger Equation (time independent) Ψ x m Ψ x Ψ + UΨ = i t +UΨ
Prob (solution by Michael Fisher) 1
 Prob 975 (solution by Michael Fisher) We begin by expressing the initial state in a basis of the spherical harmonics, which will allow us to apply the operators ˆL and ˆL z θ, φ φ() = 4π sin θ sin φ =
Prob 975 (solution by Michael Fisher) We begin by expressing the initial state in a basis of the spherical harmonics, which will allow us to apply the operators ˆL and ˆL z θ, φ φ() = 4π sin θ sin φ =
PHY331 Magnetism. Lecture 4
 PHY331 Magnetism Lecture 4 Last week Discussed Langevin s theory of diamagnetism. Use angular momentum of precessing electron in magnetic field to derive the magnetization of a sample and thus diamagnetic
PHY331 Magnetism Lecture 4 Last week Discussed Langevin s theory of diamagnetism. Use angular momentum of precessing electron in magnetic field to derive the magnetization of a sample and thus diamagnetic
Phys 622 Problems Chapter 5
 1 Phys 622 Problems Chapter 5 Problem 1 The correct basis set of perturbation theory Consider the relativistic correction to the electron-nucleus interaction H LS = α L S, also known as the spin-orbit
1 Phys 622 Problems Chapter 5 Problem 1 The correct basis set of perturbation theory Consider the relativistic correction to the electron-nucleus interaction H LS = α L S, also known as the spin-orbit
Quantum Mechanics II Lecture 11 (www.sp.phy.cam.ac.uk/~dar11/pdf) David Ritchie
 Quantum Mechanics II Lecture (www.sp.phy.cam.ac.u/~dar/pdf) David Ritchie Michaelmas. So far we have found solutions to Section 4:Transitions Ĥ ψ Eψ Solutions stationary states time dependence with time
Quantum Mechanics II Lecture (www.sp.phy.cam.ac.u/~dar/pdf) David Ritchie Michaelmas. So far we have found solutions to Section 4:Transitions Ĥ ψ Eψ Solutions stationary states time dependence with time
Plane wave solutions of the Dirac equation
 Lecture #3 Spherical spinors Hydrogen-like systems again (Relativistic version) irac energy levels Chapter, pages 48-53, Lectures on Atomic Physics Chapter 5, pages 696-76, Bransden & Joachain,, Quantum
Lecture #3 Spherical spinors Hydrogen-like systems again (Relativistic version) irac energy levels Chapter, pages 48-53, Lectures on Atomic Physics Chapter 5, pages 696-76, Bransden & Joachain,, Quantum
6.6 Charge Conjugation Charge Conjugation in Electromagnetic Processes Violation of C in the Weak Interaction
 Chapter 6 6.1 Introduction 6.2 Invariance Principle Reminder 6.2.1 Invariance in Classical Mechanics 6.2.2 Invariance in Quantum Mechanics 6.2.3 Continuous Transformations: Translations and Rotations 6.3
Chapter 6 6.1 Introduction 6.2 Invariance Principle Reminder 6.2.1 Invariance in Classical Mechanics 6.2.2 Invariance in Quantum Mechanics 6.2.3 Continuous Transformations: Translations and Rotations 6.3
1 Commutators (10 pts)
 Final Exam Solutions 37A Fall 0 I. Siddiqi / E. Dodds Commutators 0 pts) ) Consider the operator  = Ĵx Ĵ y + ĴyĴx where J i represents the total angular momentum in the ith direction. a) Express both
Final Exam Solutions 37A Fall 0 I. Siddiqi / E. Dodds Commutators 0 pts) ) Consider the operator  = Ĵx Ĵ y + ĴyĴx where J i represents the total angular momentum in the ith direction. a) Express both
Simple Harmonic Oscillator
 Classical harmonic oscillator Linear force acting on a particle (Hooke s law): F =!kx From Newton s law: F = ma = m d x dt =!kx " d x dt + # x = 0, # = k / m Position and momentum solutions oscillate in
Classical harmonic oscillator Linear force acting on a particle (Hooke s law): F =!kx From Newton s law: F = ma = m d x dt =!kx " d x dt + # x = 0, # = k / m Position and momentum solutions oscillate in
The energy of the emitted light (photons) is given by the difference in energy between the initial and final states of hydrogen atom.
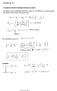 Lecture 20-21 Page 1 Lectures 20-21 Transitions between hydrogen stationary states The energy of the emitted light (photons) is given by the difference in energy between the initial and final states of
Lecture 20-21 Page 1 Lectures 20-21 Transitions between hydrogen stationary states The energy of the emitted light (photons) is given by the difference in energy between the initial and final states of
Angular momentum and spin
 Luleå tekniska universitet Avdelningen för Fysik, 007 Hans Weber Angular momentum and spin Angular momentum is a measure of how much rotation there is in particle or in a rigid body. In quantum mechanics
Luleå tekniska universitet Avdelningen för Fysik, 007 Hans Weber Angular momentum and spin Angular momentum is a measure of how much rotation there is in particle or in a rigid body. In quantum mechanics
Explicit Spin Coordinates
 Explicit Spin Coordinates arxiv:quant-ph/578v1 1 Jul 25 Geoffrey Hunter and Ian Schlifer Department of Chemistry, York University, Toronto, Canada M3J 1P3 February 1, 28 Abstract The recently established
Explicit Spin Coordinates arxiv:quant-ph/578v1 1 Jul 25 Geoffrey Hunter and Ian Schlifer Department of Chemistry, York University, Toronto, Canada M3J 1P3 February 1, 28 Abstract The recently established
Physible: Interactive Physics Collection MA198 Proposal Rough Draft
 Physible: Interactive Physics Collection MA98 Proposal Rough Draft Brian Campbell-Deem Professor George Francis November 6 th 205 Abstract Physible conglomerates four smaller, physics-related programs
Physible: Interactive Physics Collection MA98 Proposal Rough Draft Brian Campbell-Deem Professor George Francis November 6 th 205 Abstract Physible conglomerates four smaller, physics-related programs
7. The Hydrogen Atom in Wave Mechanics
 7. The Hydrogen Atom in Wave Mechanics In this chapter we shall discuss : The Schrödinger equation in spherical coordinates Spherical harmonics Radial probability densities The hydrogen atom wavefunctions
7. The Hydrogen Atom in Wave Mechanics In this chapter we shall discuss : The Schrödinger equation in spherical coordinates Spherical harmonics Radial probability densities The hydrogen atom wavefunctions
( 2 + k 2 )Ψ = 0 (1) c sinhα sinφ. x = y = c sinβ. z =
 Power series Schrodinger eigenfunctions for a particle on the torus. Mario Encinosa and Babak Etemadi Department of Physics Florida A & M University Tallahassee, Florida 3307 Abstract The eigenvalues and
Power series Schrodinger eigenfunctions for a particle on the torus. Mario Encinosa and Babak Etemadi Department of Physics Florida A & M University Tallahassee, Florida 3307 Abstract The eigenvalues and
2 Canonical quantization
 Phys540.nb 7 Canonical quantization.1. Lagrangian mechanics and canonical quantization Q: How do we quantize a general system?.1.1.lagrangian Lagrangian mechanics is a reformulation of classical mechanics.
Phys540.nb 7 Canonical quantization.1. Lagrangian mechanics and canonical quantization Q: How do we quantize a general system?.1.1.lagrangian Lagrangian mechanics is a reformulation of classical mechanics.
Legendre s Equation. PHYS Southern Illinois University. October 13, 2016
 PHYS 500 - Southern Illinois University October 13, 2016 PHYS 500 - Southern Illinois University Legendre s Equation October 13, 2016 1 / 10 The Laplacian in Spherical Coordinates The Laplacian is given
PHYS 500 - Southern Illinois University October 13, 2016 PHYS 500 - Southern Illinois University Legendre s Equation October 13, 2016 1 / 10 The Laplacian in Spherical Coordinates The Laplacian is given
R 3 ψ 2 d 3 x = 1. Observables are constructed by tripling everything we did before. The position operator ˆr is*
 Quantum Mechanics in 3-d Relevant sections in text: Chapter 4 Quantum mechanics in three dimensions Our study of a particle moving in one dimension has been instructive, but it is now time to consider
Quantum Mechanics in 3-d Relevant sections in text: Chapter 4 Quantum mechanics in three dimensions Our study of a particle moving in one dimension has been instructive, but it is now time to consider
Physics 215 Quantum Mechanics I Assignment 8
 Physics 15 Quantum Mechanics I Assignment 8 Logan A. Morrison March, 016 Problem 1 Let J be an angular momentum operator. Part (a) Using the usual angular momentum commutation relations, prove that J =
Physics 15 Quantum Mechanics I Assignment 8 Logan A. Morrison March, 016 Problem 1 Let J be an angular momentum operator. Part (a) Using the usual angular momentum commutation relations, prove that J =
EE201/MSE207 Lecture 10 Angular momentum
 EE20/MSE207 Lecture 0 Angular momentum (A lot of mathematics; I will try to explain as simple as possile) Classical mechanics r L = r p (angular momentum) Quantum mechanics The same, ut p x = iħ x p =
EE20/MSE207 Lecture 0 Angular momentum (A lot of mathematics; I will try to explain as simple as possile) Classical mechanics r L = r p (angular momentum) Quantum mechanics The same, ut p x = iħ x p =
We now turn to our first quantum mechanical problems that represent real, as
 84 Lectures 16-17 We now turn to our first quantum mechanical problems that represent real, as opposed to idealized, systems. These problems are the structures of atoms. We will begin first with hydrogen-like
84 Lectures 16-17 We now turn to our first quantum mechanical problems that represent real, as opposed to idealized, systems. These problems are the structures of atoms. We will begin first with hydrogen-like
Expansion of 1/r potential in Legendre polynomials
 Expansion of 1/r potential in Legendre polynomials In electrostatics and gravitation, we see scalar potentials of the form V = K d Take d = R r = R 2 2Rr cos θ + r 2 = R 1 2 r R cos θ + r R )2 Use h =
Expansion of 1/r potential in Legendre polynomials In electrostatics and gravitation, we see scalar potentials of the form V = K d Take d = R r = R 2 2Rr cos θ + r 2 = R 1 2 r R cos θ + r R )2 Use h =
