PROJECT C: ELECTRONIC BAND STRUCTURE IN A MODEL SEMICONDUCTOR
|
|
|
- Madlyn Ada Hill
- 5 years ago
- Views:
Transcription
1 PROJECT C: ELECTRONIC BAND STRUCTURE IN A MODEL SEMICONDUCTOR The aim of this project is to present the student with a perspective on the notion of electronic energy band structures and energy band gaps in solids. Energy band gaps are also discussed in the lectures and further considered in one of the mandatory hand-in exercises. To complete this project successfully, it is sufficient that student performs correctly the required tasks. The students participating to the project are encouraged to contact Claudio Verdozzi at any time for further clarifications. 1. Presentation & motivation In many cases, a solid piece of matter can be seen as a regular array of atoms (crystalline solids). The periodicity of such array (or lattice) of atoms has a profound and characteristic impact on the energy spectrum of a solid. The energy spectrum of a solid consists of continua, called energy bands, that may or may not overlap; there are also energy regions, called energy gaps, which do not belong to any band and, as a result, correspond to zero density of states. The aim of this project is to present the student with a specific perspectives on the notion of electronic energy band structures and electronic energy band gaps. This will require the use of quantum mechanics as introduced in the course. The task will be to produce results for simplified band structures, obtained from a 3D, large cluster of atoms ( 1000), which are arranged in a tetrahedral structure. Each atom is described by 4 orbitals (s, p x, p y, p z ). The model system provides a simplified description at the atomic level of homogeneous bulk semiconductors, such as, e.g., silicon. The notions and concepts required in the project (in particular, the exact diagonalization approach) are essentially those made available during the lectures and the laboratory, and a good knowledge of MATLAB is necessary. However, a new concept will be required to carry out the project, namely the Tight Binding Method for electronic structure (this is a quite important conceptual -and practical - approach to describe the electronic structure of solids). The fundamentals notions of i) exact numerical diagonalization and ii) the tight binding method will be presented below in Section 3, to enable the student to carry out the project. Some further details necessary to implement the Hamiltonian in MATLAB will also be provided. 2. Description of the project: a model 3D tetrahedrally bonded semiconductor Consider the following Tight-Binding Hamiltonian: H = V 1 φ j i φj i + H = V 2 φ j i φj i i j j j i i 1
2 2 PROJECT C: ELECTRONIC BAND STRUCTURE IN A MODEL SEMICONDUCTOR Here φ j i is the Dirac ket representing the electron state in the j-th orbital at site i. The φ orbitals can be thought as coming from hybridization of s and p x, p y, p z orbitals in atoms, to produce the 4 tetrahedrally arranged sp 3 hybrid orbitals (those enter, for example, the structure of the CH 4 methane molecule; see the last section of this document). The Hamiltonian matrix elements V 1 are essentially responsible for the broadening of the levels, while V 2 is responsible for the formation of the split of the levels (V 1 and V 2 are usually referred to as banding and bonding terms. This model exhibits a simple analytic structure of the allowed energy levels, once one makes use of the so-called Bloch s theorem. However, here another approach is followed, which is largely based on numerical methods. Figure 1. System 3: Diamond structure in which hybridized orbitals are shown in repeated unit structure. Bonds 1-4 and 5- are those belonging to the unit cell. So In the Hamiltonian, contact is made between the different unit cells via matrix elements of the black orbitals within a cell, and the others in the neighboring cells. Assume that we can describe the infinite system by approximating it with a large but finite cluster. More precisely let us consider a cubic array of N N N cells. Each of this cell contains eight orbitals, as shown in Figure 2. Thus, in this system there are in total N 3 orbitals. By labeling the generic cell in the cluster by the cartesian triplet (i,j,k) ( 1 i N and cyclic), compute and store with MATLAB the N 3 N 3 Hamiltonian matrix Hint 1 The (ijk)-th cell can be seen as a subunit of the Hamiltonian matrix. Hint 2 In each of these subunits (which correspond to an submatrix) order the 1- orbitals as in Figure 2
3 PROJECT C: ELECTRONIC BAND STRUCTURE IN A MODEL SEMICONDUCTOR 3 Hint 3 Some elements of such submatrix are shown here: 0 V 1 V 1 V 1?? V 1 0 V 1 V 1 0?? 0 0 V 1 V 1 0 V 1 0 0?? 0 V 1 V 1 V ???? 0 0 0???? 0?? 0 0???? 0 0?? 0???? 0 0 0????? where? and?? stands for entries to be filled. The entries?? are quite straightforward to fill (and involve V 2 only), whilst those marked by? involve V 1 only and require taking in consideration the neighboring units to the one under consideration (see again in Fig.2 to see how the black orbitals at the corners of the cell connect to the other hybrid orbitals in neighboring cells. Use periodic boundary conditions, i.e. close the system on itself. Example: if the cell (ijn) is being connected by V 1 to the cell (ijn+1), replace the cell (ijn+1) with (ij1). Or, if the cell (1ik) is being connected by V 1 to the cell (0ik), replace the latter with( Nik). Diagonalize numerically with MATLAB the N 3 N 3 Hamiltonian matrix. This should be done initially for very small N, to be progressively increased up to N=10 when the computer code is working correctly. Discard all the eigenvalues λ = V 1 + V 2 and V 1 V 2 (they give rise to artificial structures that disappear when a more refined Hamiltionian is introduced). Determine the minimum λ min and maximum λ Max of the remaining eigenvalues, and plot the density of states D(E) = 1 δ(e λ). M λ in the interval (2λ min, 2λ Max ), and where M is a normalization factor such that D(E)dE = 1. Since the system in question is discrete, the density of states is made of delta function spikes. To conveniently represent D(E), convolve it with a normalized Lorentzian L(E) = Γ 1, i.e. plot D Γ (E) = π E 2 +Γ 2 de D(E )L Γ (E E ). Plot the results for V 2 = 1 and V 1 = 0.25, 0.5, 1.0 and Γ = Discuss the results as a function of the parameters, for example how the energy gap changes for different values of V 1. Suppose that V 1 = 0 (or V 2 = 0). Can you solve the model analytically? And what kind of solutions does one obtain?
4 4 PROJECT C: ELECTRONIC BAND STRUCTURE IN A MODEL SEMICONDUCTOR 3. Support material 3.1. Exact numerical diagonalization in a nutshell. In quantum mechanics, the change in time for a system is described by the time-dependent Schrödinger equation (1) i ψ t = H(t) ψ, where H(t) is the is the Hamiltonian operator the system. A special class of wave functions are those stationary in time, and for them H is independent of time, and the Schrödinger equation specializes to: (2) H ψ = E ψ. Here we use Dirac s bracket notation in which the wave function is given by a ket, and is denoted by ψ. Such ψ :s satisfying Eq. (2) are called eigenfunctions, and E are the relative eigenvalues. To solve the time-independent Schrödinger Equation, one expresses eigenfunctions in terms of pre-assigned basis sets of the associated Hilbert Space, thereby transforming equation (2) into a matrix equation. The goal is then to calculate the eigenvalues and eigenvectors of this matrix. Very few quantum-mechanical systems admit an exact analytic solution of the associated eigen-problem. In most cases, this can be done only numerically, and the method is commonly referred to as exact (or numerical) diagonalization. In the last few decades, due to the availability of increasingly powerful computer resources, the method of numerical diagonalization has gained favor as a quite effective tool, complementary to other numerical methods. The practicality of the method is essentially limited by the size of the problem (i.e. of the matrix to be diagonalized). For small systems it is a simple task to set up the respective matrices and directly plug them into our favorite computer program/software. However, for large systems, the diagonalization procedure can be computationally very demanding, if not practically impossible. In fact, it should also be noted that what can and cannot be done with numerical diagonalization methods improves steadily with increasing computer power and with the introduction of new algorithms. For a more comprehensive discussion of the method, we defer to the lectures in the course; in the following, we provide the basic element necessary to carry out the project. Say that we have an Hamiltonian H and are interested in its ground state g, to compute some averages of physical quantities g Ôg. We choose a basis { b }, and expand the generic eigenstates of H in that basis: (3) H λ = E λ λ, λ = b c λ b b. Acting with H on the expansion, and multiplying from the left with basis vector b, (4) c λ b b Hb = E λ c λ b δ bb, b b
5 PROJECT C: ELECTRONIC BAND STRUCTURE IN A MODEL SEMICONDUCTOR 5 from which we get (5) c λ b [ b Hb E λ δ bb ] = 0 b This is a homogeneous linear system, and to have a solution beside the trivial one, we must impose that det(h E λ I) = 0, where I is the identity matrix, and the matrix elements are computed in the b basis. Numerically, this translates in writing the matrix H b b = b Hb and using some numerical procedure, to find the eigevector(s) and eigenvalue(s) of interest The Tight Binding Method: a sketchy introduction. One can adopt two perspectives to obtain the the electronic eigenvalues and eigenvectors of crystalline materials. One approach starts from the free electron picture and analyzes how this is modified when a weak periodic perturbation is introduced. This is the nearly free electron model, that we do not consider any further in this project. The other approach views the solids as being made up of atoms brought together from an infinite relative distance. It is then natural (as for molecules) to use linear combinations of atomic orbitals (LCAO) to build up the eigenfunctions. In general one can have more than one atom per primitive cell, and there are obviously several atomic orbital (in principle an infinite number) of atomic orbitals per atom. It can also been shown that, in general, while the atomic orbitals at a given atom are mutually orthogonal to each other (zero overlap), atomic orbitals from different atoms have in general a nonzero overlap. This requires specific modifications of the formulation, since one is working with a basis set which is not orthonormal anymore. However, to gain a qualitative insight in the problem (as we intend to do in this project), we can imagine that we have a finite (somewhat minimal) number of orbitals per atom The simplest case of all is when we consider only one atom per primitive crystal cell, only one atomic orbital per atom, nearest-neighbor coupling only, and we assume orthonormality between atomic orbitals from different atoms. This oversimplified version of the LCAO is often refereed to as the single-band tight-binding model (TBM) and he atomic orbital associated with the atom located at site will be symbolized by w(r l) = r l. We wish again to emphasize that for more realistic calculations one needs to take into account several complicating factors: Usually one needs several orbitals per atom, e.g., tetrahedral solids (C, Si, Ge, etc.) require at least four orbitals per site (one s-like and three p-like), while transition metals require in addition five d-like orbitals. Furthermore, one may need to employ hybrid atomic orbitals, such as the the tetrahedrally oriented hybridized sp 3 orbitals (see part 3.3 below). One may have more than one atom per primitive crystalline cell. The matrix elements between orbitals at different sites may not decay fast enough so that more than nearest-neighbor matrix elements may be needed. The atomiclike orbitals at different sites may not be orthogonal to each other. Indeed, true atomic orbitals are not orthogonal. The direct calculation of the matrix elements in this atomiclike orbital basis is in general a very difficult task which we do not discuss further here. In fact we assume that the value of such matrix element has been made available from more sophisticated calculations, so to us such matrix elements are input parameters. As mentioned before, in the simplest formulation of TBM (one atom per cell, one orbital per atom, with orthonormality between
6 PROJECT C: ELECTRONIC BAND STRUCTURE IN A MODEL SEMICONDUCTOR atomic orbitals from different atoms), the basic set of functions for a 3D solid consists of orthonormal, identical, atomiclike orbitals, each one centered at the lattice sites m = m 1 a 1 +m 2 a 2 +m 3 a 3. Here, a 1, a 2, a 3 are the three primitive vectors of the 3D lattice, and m 1, m 2, m 3 take all integer values. Thus r m = w(r m), the orbital centered at m. The matrix element of the Hamiltonian within this subspace are m H n = V mn. The periodicity of the Hamiltonian implies that one can take V mn V m n and, for m = n, V mm = 0. It should be stressed that the Hamiltonian, which describes a real periodic solid, has matrix elements outside the subspace spanned by the m vectors and that this subspace is coupled with the rest of the Hilbert space. Nevertheless, we restrict ourselves to this subspace for the sake of simplicity. The price for this approximation can be considered reasonable, since many important qualitative features are retained in spite of this drastic simplification. Furthermore, bands arising from atomic orbitals weakly overlapping with their neighbors (i.e., tightly bound to their atoms) can be described rather accurately by working within the above-defined subspace. For this reason, the Hamiltonian V mn, which is confined within the subspace spanned by m,where m runs over all lattice sites, is called the tight-binding Hamiltonian (TBH) or the tight-binding model (TBM). Such Hamiltonian in the bra/ket notation, can be equivalently written as () H = mn V mn m n Growing a little in complication, one can also consider a more general case where each primitive cell has more than one atom, and each atom has more than one orbital. In this case, the Hamiltonian can be written H = (7) V mµ,nν mµ nν, mn µν where mµ denotes the µ-th orbital at the site m. For a tetrahedral lattice, by taking i) V mµ,nν = V 1 when µ ν and m = n; ii) V mµ,nν = V 2 when µ = ν and (m, n) are nearest neighbor sites on the tetrahedral lattice; and iii) V mµ,nν = 0 otherwise, we obtain the Hamiltonian used in this project, if the label µ spans the four sp 3 hybridized orbitals. In this case, to easily manipulate the Hamiltonian, it is convenient to think of a primitive cubic cell with two atoms (i.e. eight orbitals, four per atom) per cell. Such cell is then periodically repeated to form a 3D cubic array. This is the way in which the Hamiltonian is represented in the Section of the project devoted to system Brief discussion of the sp 3 orbitals. To quickly illustrate the concept of sp 3 orbitals, we consider the case of methane (CH 4 ). In methane, experiment shows that it is energetically equivalent to break any of the four C H bonds, which suggests symmetrical bonds with equal energies and equal strenght, the hybridised sp 3 bonds. In more detail, in methane the carbon atom orbitals s and p undergo sp 3 hybridisation and forms four sp 3 orbitals. Each of the hybrid sp 3 orbitals of carbon overlaps axially with the s orbital of the hydrogen atom, forming four bonds called sigma (σ) bonds. The four hybridised orbitals are directed towards the corner of a regular tetrahedron, making an angle of 109 degrees (see Figure 2). This kind of hybridisation occurs also in semiconductors like silicon, where the outer s and p electrons are the active valence orbitals to be considered.
7 PROJECT C: ELECTRONIC BAND STRUCTURE IN A MODEL SEMICONDUCTOR 7 Figure 2. The methane molecule 3.4. Graphical aid to the computational setup of the atom coordinates. Figure 3 shows the relation between the points in the unit cell and the points in the nearest units cells, in terms of the atoms coordinates. This information may be of help in visualizing the connection between the coordinates of two bonds in different units cell. (-1,-1,0) 5 (0,0,0) (-1,0,-1) (0,-1,-1) 7 (-1,0,1) (0,0,0) 5 (0,1,1) (-1,1,0) 7 k j (0,-1,1) (1,-1,0) 5 (1,0,1) 7 (0,0,0) (0,0,0) (1,0,-1) 5 (1,1,0) 7 (0,1,-1) i Figure 3. Geometrical aspects of the setup procedure for the atom coordinated within the supercell
Bonding in solids The interaction of electrons in neighboring atoms of a solid serves the very important function of holding the crystal together.
 Bonding in solids The interaction of electrons in neighboring atoms of a solid serves the very important function of holding the crystal together. For example Nacl In the Nacl lattice, each Na atom is
Bonding in solids The interaction of electrons in neighboring atoms of a solid serves the very important function of holding the crystal together. For example Nacl In the Nacl lattice, each Na atom is
Theoretical Concepts of Spin-Orbit Splitting
 Chapter 9 Theoretical Concepts of Spin-Orbit Splitting 9.1 Free-electron model In order to understand the basic origin of spin-orbit coupling at the surface of a crystal, it is a natural starting point
Chapter 9 Theoretical Concepts of Spin-Orbit Splitting 9.1 Free-electron model In order to understand the basic origin of spin-orbit coupling at the surface of a crystal, it is a natural starting point
Lecture. Ref. Ihn Ch. 3, Yu&Cardona Ch. 2
 Lecture Review of quantum mechanics, statistical physics, and solid state Band structure of materials Semiconductor band structure Semiconductor nanostructures Ref. Ihn Ch. 3, Yu&Cardona Ch. 2 Reminder
Lecture Review of quantum mechanics, statistical physics, and solid state Band structure of materials Semiconductor band structure Semiconductor nanostructures Ref. Ihn Ch. 3, Yu&Cardona Ch. 2 Reminder
The quantum state as a vector
 The quantum state as a vector February 6, 27 Wave mechanics In our review of the development of wave mechanics, we have established several basic properties of the quantum description of nature:. A particle
The quantum state as a vector February 6, 27 Wave mechanics In our review of the development of wave mechanics, we have established several basic properties of the quantum description of nature:. A particle
Chapter 5 Equations for Wave Function
 Chapter 5 Equations for Wave Function In very simple cases, the explicit expressions for the SALCs could be deduced by inspection, but not for complicated system. It would be useful for cases like these
Chapter 5 Equations for Wave Function In very simple cases, the explicit expressions for the SALCs could be deduced by inspection, but not for complicated system. It would be useful for cases like these
Electrons in a periodic potential
 Chapter 3 Electrons in a periodic potential 3.1 Bloch s theorem. We consider in this chapter electrons under the influence of a static, periodic potential V (x), i.e. such that it fulfills V (x) = V (x
Chapter 3 Electrons in a periodic potential 3.1 Bloch s theorem. We consider in this chapter electrons under the influence of a static, periodic potential V (x), i.e. such that it fulfills V (x) = V (x
Lecture 6. Tight-binding model
 Lecture 6 Tight-binding model In Lecture 3 we discussed the Krönig-Penny model and we have seen that, depending on the strength of the potential barrier inside the unit cell, the electrons can behave like
Lecture 6 Tight-binding model In Lecture 3 we discussed the Krönig-Penny model and we have seen that, depending on the strength of the potential barrier inside the unit cell, the electrons can behave like
Vector Spaces in Quantum Mechanics
 Chapter 8 Vector Spaces in Quantum Mechanics We have seen in the previous Chapter that there is a sense in which the state of a quantum system can be thought of as being made up of other possible states.
Chapter 8 Vector Spaces in Quantum Mechanics We have seen in the previous Chapter that there is a sense in which the state of a quantum system can be thought of as being made up of other possible states.
Chapter 2 Approximation Methods Can be Used When Exact Solutions to the Schrödinger Equation Can Not be Found.
 Chapter 2 Approximation Methods Can be Used When Exact Solutions to the Schrödinger Equation Can Not be Found. In applying quantum mechanics to 'real' chemical problems, one is usually faced with a Schrödinger
Chapter 2 Approximation Methods Can be Used When Exact Solutions to the Schrödinger Equation Can Not be Found. In applying quantum mechanics to 'real' chemical problems, one is usually faced with a Schrödinger
Chapter 3. The (L)APW+lo Method. 3.1 Choosing A Basis Set
 Chapter 3 The (L)APW+lo Method 3.1 Choosing A Basis Set The Kohn-Sham equations (Eq. (2.17)) provide a formulation of how to practically find a solution to the Hohenberg-Kohn functional (Eq. (2.15)). Nevertheless
Chapter 3 The (L)APW+lo Method 3.1 Choosing A Basis Set The Kohn-Sham equations (Eq. (2.17)) provide a formulation of how to practically find a solution to the Hohenberg-Kohn functional (Eq. (2.15)). Nevertheless
I. Perturbation Theory and the Problem of Degeneracy[?,?,?]
![I. Perturbation Theory and the Problem of Degeneracy[?,?,?] I. Perturbation Theory and the Problem of Degeneracy[?,?,?]](/thumbs/85/91937171.jpg) MASSACHUSETTS INSTITUTE OF TECHNOLOGY Chemistry 5.76 Spring 19 THE VAN VLECK TRANSFORMATION IN PERTURBATION THEORY 1 Although frequently it is desirable to carry a perturbation treatment to second or third
MASSACHUSETTS INSTITUTE OF TECHNOLOGY Chemistry 5.76 Spring 19 THE VAN VLECK TRANSFORMATION IN PERTURBATION THEORY 1 Although frequently it is desirable to carry a perturbation treatment to second or third
Chapter 4 Symmetry and Chemical Bonding
 Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
Chapter 4 Symmetry and Chemical Bonding
 Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
Introduction to Electronic Structure Theory
 Introduction to Electronic Structure Theory C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology June 2002 Last Revised: June 2003 1 Introduction The purpose of these
Introduction to Electronic Structure Theory C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology June 2002 Last Revised: June 2003 1 Introduction The purpose of these
Quantum Condensed Matter Physics Lecture 4
 Quantum Condensed Matter Physics Lecture 4 David Ritchie QCMP Lent/Easter 2019 http://www.sp.phy.cam.ac.uk/drp2/home 4.1 Quantum Condensed Matter Physics 1. Classical and Semi-classical models for electrons
Quantum Condensed Matter Physics Lecture 4 David Ritchie QCMP Lent/Easter 2019 http://www.sp.phy.cam.ac.uk/drp2/home 4.1 Quantum Condensed Matter Physics 1. Classical and Semi-classical models for electrons
Electrons in Crystals. Chris J. Pickard
 Electrons in Crystals Chris J. Pickard Electrons in Crystals The electrons in a crystal experience a potential with the periodicity of the Bravais lattice: U(r + R) = U(r) The scale of the periodicity
Electrons in Crystals Chris J. Pickard Electrons in Crystals The electrons in a crystal experience a potential with the periodicity of the Bravais lattice: U(r + R) = U(r) The scale of the periodicity
Physics 221A Fall 1996 Notes 16 Bloch s Theorem and Band Structure in One Dimension
 Physics 221A Fall 1996 Notes 16 Bloch s Theorem and Band Structure in One Dimension In these notes we examine Bloch s theorem and band structure in problems with periodic potentials, as a part of our survey
Physics 221A Fall 1996 Notes 16 Bloch s Theorem and Band Structure in One Dimension In these notes we examine Bloch s theorem and band structure in problems with periodic potentials, as a part of our survey
Physics 221A Fall 1996 Notes 14 Coupling of Angular Momenta
 Physics 1A Fall 1996 Notes 14 Coupling of Angular Momenta In these notes we will discuss the problem of the coupling or addition of angular momenta. It is assumed that you have all had experience with
Physics 1A Fall 1996 Notes 14 Coupling of Angular Momenta In these notes we will discuss the problem of the coupling or addition of angular momenta. It is assumed that you have all had experience with
Degenerate Perturbation Theory. 1 General framework and strategy
 Physics G6037 Professor Christ 12/22/2015 Degenerate Perturbation Theory The treatment of degenerate perturbation theory presented in class is written out here in detail. The appendix presents the underlying
Physics G6037 Professor Christ 12/22/2015 Degenerate Perturbation Theory The treatment of degenerate perturbation theory presented in class is written out here in detail. The appendix presents the underlying
Electronic Structure Theory for Periodic Systems: The Concepts. Christian Ratsch
 Electronic Structure Theory for Periodic Systems: The Concepts Christian Ratsch Institute for Pure and Applied Mathematics and Department of Mathematics, UCLA Motivation There are 10 20 atoms in 1 mm 3
Electronic Structure Theory for Periodic Systems: The Concepts Christian Ratsch Institute for Pure and Applied Mathematics and Department of Mathematics, UCLA Motivation There are 10 20 atoms in 1 mm 3
Density Matrices. Chapter Introduction
 Chapter 15 Density Matrices 15.1 Introduction Density matrices are employed in quantum mechanics to give a partial description of a quantum system, one from which certain details have been omitted. For
Chapter 15 Density Matrices 15.1 Introduction Density matrices are employed in quantum mechanics to give a partial description of a quantum system, one from which certain details have been omitted. For
Stochastic Histories. Chapter Introduction
 Chapter 8 Stochastic Histories 8.1 Introduction Despite the fact that classical mechanics employs deterministic dynamical laws, random dynamical processes often arise in classical physics, as well as in
Chapter 8 Stochastic Histories 8.1 Introduction Despite the fact that classical mechanics employs deterministic dynamical laws, random dynamical processes often arise in classical physics, as well as in
Electrons in periodic potential
 Chapter 9 Electrons in periodic potential The computation of electronic states in a solid is a nontrivial problem. A great simplification can be achieved if the solid is a crystal, i.e. if it can be described
Chapter 9 Electrons in periodic potential The computation of electronic states in a solid is a nontrivial problem. A great simplification can be achieved if the solid is a crystal, i.e. if it can be described
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer. Lecture 17, March 1, 2006
 Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 17, March 1, 2006 (Some material in this lecture has been adapted from Cramer, C. J.
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 17, March 1, 2006 (Some material in this lecture has been adapted from Cramer, C. J.
ECE 535 Theory of Semiconductors and Semiconductor Devices Fall 2015 Homework # 5 Due Date: 11/17/2015
 ECE 535 Theory of Semiconductors and Semiconductor Devices Fall 2015 Homework # 5 Due Date: 11/17/2015 Problem # 1 Two carbon atoms and four hydrogen atoms form and ethane molecule with the chemical formula
ECE 535 Theory of Semiconductors and Semiconductor Devices Fall 2015 Homework # 5 Due Date: 11/17/2015 Problem # 1 Two carbon atoms and four hydrogen atoms form and ethane molecule with the chemical formula
Incompatibility Paradoxes
 Chapter 22 Incompatibility Paradoxes 22.1 Simultaneous Values There is never any difficulty in supposing that a classical mechanical system possesses, at a particular instant of time, precise values of
Chapter 22 Incompatibility Paradoxes 22.1 Simultaneous Values There is never any difficulty in supposing that a classical mechanical system possesses, at a particular instant of time, precise values of
CHAPTER 10 Tight-Binding Model
 CHAPTER 0 Tight-Binding Model Linear Combination of Atomic Orbitals (LCAO) Application to Bands from s-levels General Features of Tight-Binding Levels Wannier Functions 6 a : S S P 3S Core FE Semicore
CHAPTER 0 Tight-Binding Model Linear Combination of Atomic Orbitals (LCAO) Application to Bands from s-levels General Features of Tight-Binding Levels Wannier Functions 6 a : S S P 3S Core FE Semicore
I. CSFs Are Used to Express the Full N-Electron Wavefunction
 Chapter 11 One Must be Able to Evaluate the Matrix Elements Among Properly Symmetry Adapted N- Electron Configuration Functions for Any Operator, the Electronic Hamiltonian in Particular. The Slater-Condon
Chapter 11 One Must be Able to Evaluate the Matrix Elements Among Properly Symmetry Adapted N- Electron Configuration Functions for Any Operator, the Electronic Hamiltonian in Particular. The Slater-Condon
Applications of Quantum Theory to Some Simple Systems
 Applications of Quantum Theory to Some Simple Systems Arbitrariness in the value of total energy. We will use classical mechanics, and for simplicity of the discussion, consider a particle of mass m moving
Applications of Quantum Theory to Some Simple Systems Arbitrariness in the value of total energy. We will use classical mechanics, and for simplicity of the discussion, consider a particle of mass m moving
Energy bands in two limits
 M. A. Gusmão IF-UFRGS 1 FIP10601 Text 4 Energy bands in two limits After presenting a general view of the independent-electron approximation, highlighting the importance and consequences of Bloch s theorem,
M. A. Gusmão IF-UFRGS 1 FIP10601 Text 4 Energy bands in two limits After presenting a general view of the independent-electron approximation, highlighting the importance and consequences of Bloch s theorem,
MSE 102, Fall 2014 Midterm #2. Write your name here [10 points]:
![MSE 102, Fall 2014 Midterm #2. Write your name here [10 points]: MSE 102, Fall 2014 Midterm #2. Write your name here [10 points]:](/thumbs/93/112977451.jpg) MSE 102, Fall 2014 Midterm #2 Write your name here [10 points]: Instructions: Answer all questions to the best of your abilities. Be sure to write legibly and state your answers clearly. The point values
MSE 102, Fall 2014 Midterm #2 Write your name here [10 points]: Instructions: Answer all questions to the best of your abilities. Be sure to write legibly and state your answers clearly. The point values
Calculation of band structure using group theory. Sudeep Kumar Ghosh, Department of Physics, Indian Institute of Science, Bangalore.
 Calculation of band structure using group theory Sudeep Kumar Ghosh, Department of Physics, Indian Institute of Science, Bangalore. Plan of the talk Brief overview of the representation theory and the
Calculation of band structure using group theory Sudeep Kumar Ghosh, Department of Physics, Indian Institute of Science, Bangalore. Plan of the talk Brief overview of the representation theory and the
1 Mathematical preliminaries
 1 Mathematical preliminaries The mathematical language of quantum mechanics is that of vector spaces and linear algebra. In this preliminary section, we will collect the various definitions and mathematical
1 Mathematical preliminaries The mathematical language of quantum mechanics is that of vector spaces and linear algebra. In this preliminary section, we will collect the various definitions and mathematical
PLEASE LET ME KNOW IF YOU FIND TYPOS (send to
 Teoretisk Fysik KTH Advanced QM (SI2380), Lecture 2 (Summary of concepts) 1 PLEASE LET ME KNOW IF YOU FIND TYPOS (send email to langmann@kth.se) The laws of QM 1. I now discuss the laws of QM and their
Teoretisk Fysik KTH Advanced QM (SI2380), Lecture 2 (Summary of concepts) 1 PLEASE LET ME KNOW IF YOU FIND TYPOS (send email to langmann@kth.se) The laws of QM 1. I now discuss the laws of QM and their
2.3 Band structure and lattice symmetries: example of diamond
 2.2.9 Product of representaitons Besides the sums of representations, one can also define their products. Consider two groups G and H and their direct product G H. If we have two representations D 1 and
2.2.9 Product of representaitons Besides the sums of representations, one can also define their products. Consider two groups G and H and their direct product G H. If we have two representations D 1 and
MOLECULAR ORBITAL AND VALENCE BOND THEORY EXPLAINED (HOPEFULLY)
 MOLEULAR ORBITAL AND VALENE BOND TEORY EXPLAINED (OPEFULLY) Quantum Mechanics is a very difficult topic, with a great deal of detail that is extremely complex, yet interesting. owever, in this Organic
MOLEULAR ORBITAL AND VALENE BOND TEORY EXPLAINED (OPEFULLY) Quantum Mechanics is a very difficult topic, with a great deal of detail that is extremely complex, yet interesting. owever, in this Organic
Physics 221A Fall 2018 Notes 22 Bound-State Perturbation Theory
 Copyright c 2018 by Robert G. Littlejohn Physics 221A Fall 2018 Notes 22 Bound-State Perturbation Theory 1. Introduction Bound state perturbation theory applies to the bound states of perturbed systems,
Copyright c 2018 by Robert G. Littlejohn Physics 221A Fall 2018 Notes 22 Bound-State Perturbation Theory 1. Introduction Bound state perturbation theory applies to the bound states of perturbed systems,
Physics 211B : Problem Set #0
 Physics 211B : Problem Set #0 These problems provide a cross section of the sort of exercises I would have assigned had I taught 211A. Please take a look at all the problems, and turn in problems 1, 4,
Physics 211B : Problem Set #0 These problems provide a cross section of the sort of exercises I would have assigned had I taught 211A. Please take a look at all the problems, and turn in problems 1, 4,
Problem Set 2 Due Thursday, October 1, & & & & # % (b) Construct a representation using five d orbitals that sit on the origin as a basis:
 Problem Set 2 Due Thursday, October 1, 29 Problems from Cotton: Chapter 4: 4.6, 4.7; Chapter 6: 6.2, 6.4, 6.5 Additional problems: (1) Consider the D 3h point group and use a coordinate system wherein
Problem Set 2 Due Thursday, October 1, 29 Problems from Cotton: Chapter 4: 4.6, 4.7; Chapter 6: 6.2, 6.4, 6.5 Additional problems: (1) Consider the D 3h point group and use a coordinate system wherein
$ +! j. % i PERTURBATION THEORY AND SUBGROUPS (REVISED 11/15/08)
 PERTURBATION THEORY AND SUBGROUPS REVISED 11/15/08) The use of groups and their subgroups is of much importance when perturbation theory is employed in understanding molecular orbital theory and spectroscopy
PERTURBATION THEORY AND SUBGROUPS REVISED 11/15/08) The use of groups and their subgroups is of much importance when perturbation theory is employed in understanding molecular orbital theory and spectroscopy
Quantum Mechanics-I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras. Lecture - 21 Square-Integrable Functions
 Quantum Mechanics-I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras Lecture - 21 Square-Integrable Functions (Refer Slide Time: 00:06) (Refer Slide Time: 00:14) We
Quantum Mechanics-I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras Lecture - 21 Square-Integrable Functions (Refer Slide Time: 00:06) (Refer Slide Time: 00:14) We
Lecture 26: Qualitative Molecular Orbital Theory: Hückel Theory
 MASSACHUSETTS INSTITUTE OF TECHNOLOGY 5.6 Physical Chemistry I Fall, 07 Professor Robert W. Field Lecture 6: Qualitative Molecular Orbital Theory: Hückel Theory Models in Physical Chemistry Our job is
MASSACHUSETTS INSTITUTE OF TECHNOLOGY 5.6 Physical Chemistry I Fall, 07 Professor Robert W. Field Lecture 6: Qualitative Molecular Orbital Theory: Hückel Theory Models in Physical Chemistry Our job is
Group representation theory and quantum physics
 Group representation theory and quantum physics Olivier Pfister April 29, 2003 Abstract This is a basic tutorial on the use of group representation theory in quantum physics, in particular for such systems
Group representation theory and quantum physics Olivier Pfister April 29, 2003 Abstract This is a basic tutorial on the use of group representation theory in quantum physics, in particular for such systems
SUPPLEMENTARY INFORMATION
 In the format provided by the authors and unedited. DOI: 10.1038/NPHYS4080 Topological states in engineered atomic lattices Robert Drost, Teemu Ojanen, Ari Harju, and Peter Liljeroth Department of Applied
In the format provided by the authors and unedited. DOI: 10.1038/NPHYS4080 Topological states in engineered atomic lattices Robert Drost, Teemu Ojanen, Ari Harju, and Peter Liljeroth Department of Applied
Unitary Dynamics and Quantum Circuits
 qitd323 Unitary Dynamics and Quantum Circuits Robert B. Griffiths Version of 20 January 2014 Contents 1 Unitary Dynamics 1 1.1 Time development operator T.................................... 1 1.2 Particular
qitd323 Unitary Dynamics and Quantum Circuits Robert B. Griffiths Version of 20 January 2014 Contents 1 Unitary Dynamics 1 1.1 Time development operator T.................................... 1 1.2 Particular
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer. Lecture 7, February 1, 2006
 Chem 350/450 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 006 Christopher J. Cramer ecture 7, February 1, 006 Solved Homework We are given that A is a Hermitian operator such that
Chem 350/450 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 006 Christopher J. Cramer ecture 7, February 1, 006 Solved Homework We are given that A is a Hermitian operator such that
POSTULATES OF QUANTUM MECHANICS
 POSTULATES OF QUANTUM MECHANICS Quantum-mechanical states - In the coordinate representation, the state of a quantum-mechanical system is described by the wave function ψ(q, t) = ψ(q 1,..., q f, t) (in
POSTULATES OF QUANTUM MECHANICS Quantum-mechanical states - In the coordinate representation, the state of a quantum-mechanical system is described by the wave function ψ(q, t) = ψ(q 1,..., q f, t) (in
We have arrived to the question: how do molecular bonds determine the band gap? We have discussed that the silicon atom has four outer electrons.
 ET3034Tux - 2.2.2 - Band Gap 2 - Electrons in Molecular Bonds We have arrived to the question: how do molecular bonds determine the band gap? We have discussed that the silicon atom has four outer electrons.
ET3034Tux - 2.2.2 - Band Gap 2 - Electrons in Molecular Bonds We have arrived to the question: how do molecular bonds determine the band gap? We have discussed that the silicon atom has four outer electrons.
Crystal Models. Figure 1.1 Section of a three-dimensional lattice.
 Crystal Models The Solid-State Structure of Metals and Ionic Compounds Objectives Understand the concept of the unit cell in crystalline solids. Construct models of unit cells for several metallic and
Crystal Models The Solid-State Structure of Metals and Ionic Compounds Objectives Understand the concept of the unit cell in crystalline solids. Construct models of unit cells for several metallic and
The Simple Harmonic Oscillator
 The Simple Harmonic Oscillator Michael Fowler, University of Virginia Einstein s Solution of the Specific Heat Puzzle The simple harmonic oscillator, a nonrelativistic particle in a potential ½C, is a
The Simple Harmonic Oscillator Michael Fowler, University of Virginia Einstein s Solution of the Specific Heat Puzzle The simple harmonic oscillator, a nonrelativistic particle in a potential ½C, is a
The Quantum Heisenberg Ferromagnet
 The Quantum Heisenberg Ferromagnet Soon after Schrödinger discovered the wave equation of quantum mechanics, Heisenberg and Dirac developed the first successful quantum theory of ferromagnetism W. Heisenberg,
The Quantum Heisenberg Ferromagnet Soon after Schrödinger discovered the wave equation of quantum mechanics, Heisenberg and Dirac developed the first successful quantum theory of ferromagnetism W. Heisenberg,
Three Most Important Topics (MIT) Today
 Three Most Important Topics (MIT) Today Electrons in periodic potential Energy gap nearly free electron Bloch Theorem Energy gap tight binding Chapter 1 1 Electrons in Periodic Potential We now know the
Three Most Important Topics (MIT) Today Electrons in periodic potential Energy gap nearly free electron Bloch Theorem Energy gap tight binding Chapter 1 1 Electrons in Periodic Potential We now know the
Introduction to Condensed Matter Physics
 Introduction to Condensed Matter Physics The Reciprocal Lattice M.P. Vaughan Overview Overview of the reciprocal lattice Periodic functions Reciprocal lattice vectors Bloch functions k-space Dispersion
Introduction to Condensed Matter Physics The Reciprocal Lattice M.P. Vaughan Overview Overview of the reciprocal lattice Periodic functions Reciprocal lattice vectors Bloch functions k-space Dispersion
Lecture 8 Nature of ensemble: Role of symmetry, interactions and other system conditions: Part II
 Lecture 8 Nature of ensemble: Role of symmetry, interactions and other system conditions: Part II We continue our discussion of symmetries and their role in matrix representation in this lecture. An example
Lecture 8 Nature of ensemble: Role of symmetry, interactions and other system conditions: Part II We continue our discussion of symmetries and their role in matrix representation in this lecture. An example
Section 10 Metals: Electron Dynamics and Fermi Surfaces
 Electron dynamics Section 10 Metals: Electron Dynamics and Fermi Surfaces The next important subject we address is electron dynamics in metals. Our consideration will be based on a semiclassical model.
Electron dynamics Section 10 Metals: Electron Dynamics and Fermi Surfaces The next important subject we address is electron dynamics in metals. Our consideration will be based on a semiclassical model.
SECOND QUANTIZATION PART I
 PART I SECOND QUANTIZATION 1 Elementary quantum mechanics We assume that the reader is already acquainted with elementary quantum mechanics. An introductory course in quantum mechanics usually addresses
PART I SECOND QUANTIZATION 1 Elementary quantum mechanics We assume that the reader is already acquainted with elementary quantum mechanics. An introductory course in quantum mechanics usually addresses
Contents. 1 Solutions to Chapter 1 Exercises 3. 2 Solutions to Chapter 2 Exercises Solutions to Chapter 3 Exercises 57
 Contents 1 Solutions to Chapter 1 Exercises 3 Solutions to Chapter Exercises 43 3 Solutions to Chapter 3 Exercises 57 4 Solutions to Chapter 4 Exercises 77 5 Solutions to Chapter 5 Exercises 89 6 Solutions
Contents 1 Solutions to Chapter 1 Exercises 3 Solutions to Chapter Exercises 43 3 Solutions to Chapter 3 Exercises 57 4 Solutions to Chapter 4 Exercises 77 5 Solutions to Chapter 5 Exercises 89 6 Solutions
Notes on Spin Operators and the Heisenberg Model. Physics : Winter, David G. Stroud
 Notes on Spin Operators and the Heisenberg Model Physics 880.06: Winter, 003-4 David G. Stroud In these notes I give a brief discussion of spin-1/ operators and their use in the Heisenberg model. 1. Spin
Notes on Spin Operators and the Heisenberg Model Physics 880.06: Winter, 003-4 David G. Stroud In these notes I give a brief discussion of spin-1/ operators and their use in the Heisenberg model. 1. Spin
5.80 Small-Molecule Spectroscopy and Dynamics
 MIT OpenCourseWare http://ocw.mit.edu 5.80 Small-Molecule Spectroscopy and Dynamics Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. Lecture
MIT OpenCourseWare http://ocw.mit.edu 5.80 Small-Molecule Spectroscopy and Dynamics Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. Lecture
Degenerate Perturbation Theory
 Physics G6037 Professor Christ 12/05/2014 Degenerate Perturbation Theory The treatment of degenerate perturbation theory presented in class is written out here in detail. 1 General framework and strategy
Physics G6037 Professor Christ 12/05/2014 Degenerate Perturbation Theory The treatment of degenerate perturbation theory presented in class is written out here in detail. 1 General framework and strategy
Electronic properties of ordered and disordered linear clusters of atoms and molecules
 Electronic properties of ordered and disordered linear clusters of atoms and molecules S N Behera a, S Gayen b, G V Ravi Prasad c and S M Bose b,* a Institute of Material Science, Bhubaneswar-751013, Orissa,
Electronic properties of ordered and disordered linear clusters of atoms and molecules S N Behera a, S Gayen b, G V Ravi Prasad c and S M Bose b,* a Institute of Material Science, Bhubaneswar-751013, Orissa,
Physics 221A Fall 2017 Notes 27 The Variational Method
 Copyright c 2018 by Robert G. Littlejohn Physics 221A Fall 2017 Notes 27 The Variational Method 1. Introduction Very few realistic problems in quantum mechanics are exactly solvable, so approximation methods
Copyright c 2018 by Robert G. Littlejohn Physics 221A Fall 2017 Notes 27 The Variational Method 1. Introduction Very few realistic problems in quantum mechanics are exactly solvable, so approximation methods
10 Time-Independent Perturbation Theory
 S.K. Saiin Oct. 6, 009 Lecture 0 0 Time-Independent Perturbation Theory Content: Non-degenerate case. Degenerate case. Only a few quantum mechanical problems can be solved exactly. However, if the system
S.K. Saiin Oct. 6, 009 Lecture 0 0 Time-Independent Perturbation Theory Content: Non-degenerate case. Degenerate case. Only a few quantum mechanical problems can be solved exactly. However, if the system
Optical Lattices. Chapter Polarization
 Chapter Optical Lattices Abstract In this chapter we give details of the atomic physics that underlies the Bose- Hubbard model used to describe ultracold atoms in optical lattices. We show how the AC-Stark
Chapter Optical Lattices Abstract In this chapter we give details of the atomic physics that underlies the Bose- Hubbard model used to describe ultracold atoms in optical lattices. We show how the AC-Stark
5.5. Representations. Phys520.nb Definition N is called the dimensions of the representations The trivial presentation
 Phys50.nb 37 The rhombohedral and hexagonal lattice systems are not fully compatible with point group symmetries. Knowing the point group doesn t uniquely determine the lattice systems. Sometimes we can
Phys50.nb 37 The rhombohedral and hexagonal lattice systems are not fully compatible with point group symmetries. Knowing the point group doesn t uniquely determine the lattice systems. Sometimes we can
Tight-Binding Model of Electronic Structures
 Tight-Binding Model of Electronic Structures Consider a collection of N atoms. The electronic structure of this system refers to its electronic wave function and the description of how it is related to
Tight-Binding Model of Electronic Structures Consider a collection of N atoms. The electronic structure of this system refers to its electronic wave function and the description of how it is related to
Spins and spin-orbit coupling in semiconductors, metals, and nanostructures
 B. Halperin Spin lecture 1 Spins and spin-orbit coupling in semiconductors, metals, and nanostructures Behavior of non-equilibrium spin populations. Spin relaxation and spin transport. How does one produce
B. Halperin Spin lecture 1 Spins and spin-orbit coupling in semiconductors, metals, and nanostructures Behavior of non-equilibrium spin populations. Spin relaxation and spin transport. How does one produce
Physics of Semiconductors (Problems for report)
 Physics of Semiconductors (Problems for report) Shingo Katsumoto Institute for Solid State Physics, University of Tokyo July, 0 Choose two from the following eight problems and solve them. I. Fundamentals
Physics of Semiconductors (Problems for report) Shingo Katsumoto Institute for Solid State Physics, University of Tokyo July, 0 Choose two from the following eight problems and solve them. I. Fundamentals
Basic Semiconductor Physics
 6 Basic Semiconductor Physics 6.1 Introduction With this chapter we start with the discussion of some important concepts from semiconductor physics, which are required to understand the operation of solar
6 Basic Semiconductor Physics 6.1 Introduction With this chapter we start with the discussion of some important concepts from semiconductor physics, which are required to understand the operation of solar
Tight-Binding Approximation. Faculty of Physics UW
 Tight-Binding Approximation Faculty of Physics UW Jacek.Szczytko@fuw.edu.pl The band theory of solids. 2017-06-05 2 Periodic potential Bloch theorem φ n,k Ԧr = u n,k Ԧr eik Ԧr Bloch wave, Bloch function
Tight-Binding Approximation Faculty of Physics UW Jacek.Szczytko@fuw.edu.pl The band theory of solids. 2017-06-05 2 Periodic potential Bloch theorem φ n,k Ԧr = u n,k Ԧr eik Ԧr Bloch wave, Bloch function
0 belonging to the unperturbed Hamiltonian H 0 are known
 Time Independent Perturbation Theory D Perturbation theory is used in two qualitatively different contexts in quantum chemistry. It allows one to estimate (because perturbation theory is usually employed
Time Independent Perturbation Theory D Perturbation theory is used in two qualitatively different contexts in quantum chemistry. It allows one to estimate (because perturbation theory is usually employed
Tight Binding Method: Linear Combination of Atomic Orbitals (LCAO)
 Tight Binding Method: Linear Combination of Atomic Orbitals (LCAO) W. E. Pickett (Dated: April 14, 2014) This write-up is a homemade introduction to the tight binding representation of the electronic structure
Tight Binding Method: Linear Combination of Atomic Orbitals (LCAO) W. E. Pickett (Dated: April 14, 2014) This write-up is a homemade introduction to the tight binding representation of the electronic structure
CHEM6085: Density Functional Theory
 Lecture 11 CHEM6085: Density Functional Theory DFT for periodic crystalline solids C.-K. Skylaris 1 Electron in a one-dimensional periodic box (in atomic units) Schrödinger equation Energy eigenvalues
Lecture 11 CHEM6085: Density Functional Theory DFT for periodic crystalline solids C.-K. Skylaris 1 Electron in a one-dimensional periodic box (in atomic units) Schrödinger equation Energy eigenvalues
Massachusetts Institute of Technology Physics Department
 Massachusetts Institute of Technology Physics Department Physics 8.32 Fall 2006 Quantum Theory I October 9, 2006 Assignment 6 Due October 20, 2006 Announcements There will be a makeup lecture on Friday,
Massachusetts Institute of Technology Physics Department Physics 8.32 Fall 2006 Quantum Theory I October 9, 2006 Assignment 6 Due October 20, 2006 Announcements There will be a makeup lecture on Friday,
H ψ = E ψ. Introduction to Exact Diagonalization. Andreas Läuchli, New states of quantum matter MPI für Physik komplexer Systeme - Dresden
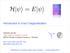 H ψ = E ψ Introduction to Exact Diagonalization Andreas Läuchli, New states of quantum matter MPI für Physik komplexer Systeme - Dresden http://www.pks.mpg.de/~aml laeuchli@comp-phys.org Simulations of
H ψ = E ψ Introduction to Exact Diagonalization Andreas Läuchli, New states of quantum matter MPI für Physik komplexer Systeme - Dresden http://www.pks.mpg.de/~aml laeuchli@comp-phys.org Simulations of
Nearly Free Electron Gas model - I
 Nearly Free Electron Gas model - I Contents 1 Free electron gas model summary 1 2 Electron effective mass 3 2.1 FEG model for sodium...................... 4 3 Nearly free electron model 5 3.1 Primitive
Nearly Free Electron Gas model - I Contents 1 Free electron gas model summary 1 2 Electron effective mass 3 2.1 FEG model for sodium...................... 4 3 Nearly free electron model 5 3.1 Primitive
Lecture 4: Basic elements of band theory
 Phys 769 Selected Topics in Condensed Matter Physics Summer 010 Lecture 4: Basic elements of band theory Lecturer: Anthony J. Leggett TA: Bill Coish 1 Introduction Most matter, in particular most insulating
Phys 769 Selected Topics in Condensed Matter Physics Summer 010 Lecture 4: Basic elements of band theory Lecturer: Anthony J. Leggett TA: Bill Coish 1 Introduction Most matter, in particular most insulating
LINEAR ALGEBRA KNOWLEDGE SURVEY
 LINEAR ALGEBRA KNOWLEDGE SURVEY Instructions: This is a Knowledge Survey. For this assignment, I am only interested in your level of confidence about your ability to do the tasks on the following pages.
LINEAR ALGEBRA KNOWLEDGE SURVEY Instructions: This is a Knowledge Survey. For this assignment, I am only interested in your level of confidence about your ability to do the tasks on the following pages.
G : Quantum Mechanics II
 G5.666: Quantum Mechanics II Notes for Lecture 5 I. REPRESENTING STATES IN THE FULL HILBERT SPACE Given a representation of the states that span the spin Hilbert space, we now need to consider the problem
G5.666: Quantum Mechanics II Notes for Lecture 5 I. REPRESENTING STATES IN THE FULL HILBERT SPACE Given a representation of the states that span the spin Hilbert space, we now need to consider the problem
NANOSCALE SCIENCE & TECHNOLOGY
 . NANOSCALE SCIENCE & TECHNOLOGY V Two-Level Quantum Systems (Qubits) Lecture notes 5 5. Qubit description Quantum bit (qubit) is an elementary unit of a quantum computer. Similar to classical computers,
. NANOSCALE SCIENCE & TECHNOLOGY V Two-Level Quantum Systems (Qubits) Lecture notes 5 5. Qubit description Quantum bit (qubit) is an elementary unit of a quantum computer. Similar to classical computers,
Write your name here:
 MSE 102, Fall 2013 Final Exam Write your name here: Instructions: Answer all questions to the best of your abilities. Be sure to write legibly and state your answers clearly. The point values for each
MSE 102, Fall 2013 Final Exam Write your name here: Instructions: Answer all questions to the best of your abilities. Be sure to write legibly and state your answers clearly. The point values for each
Chm 331 Fall 2015, Exercise Set 4 NMR Review Problems
 Chm 331 Fall 015, Exercise Set 4 NMR Review Problems Mr. Linck Version.0. Compiled December 1, 015 at 11:04:44 4.1 Diagonal Matrix Elements for the nmr H 0 Find the diagonal matrix elements for H 0 (the
Chm 331 Fall 015, Exercise Set 4 NMR Review Problems Mr. Linck Version.0. Compiled December 1, 015 at 11:04:44 4.1 Diagonal Matrix Elements for the nmr H 0 Find the diagonal matrix elements for H 0 (the
(Again, this quantity is the correlation function of the two spins.) With z chosen along ˆn 1, this quantity is easily computed (exercise):
 Lecture 30 Relevant sections in text: 3.9, 5.1 Bell s theorem (cont.) Assuming suitable hidden variables coupled with an assumption of locality to determine the spin observables with certainty we found
Lecture 30 Relevant sections in text: 3.9, 5.1 Bell s theorem (cont.) Assuming suitable hidden variables coupled with an assumption of locality to determine the spin observables with certainty we found
Lecture contents. A few concepts from Quantum Mechanics. Tight-binding model Solid state physics review
 Lecture contents A few concepts from Quantum Mechanics Particle in a well Two wells: QM perturbation theory Many wells (atoms) BAND formation Tight-binding model Solid state physics review Approximations
Lecture contents A few concepts from Quantum Mechanics Particle in a well Two wells: QM perturbation theory Many wells (atoms) BAND formation Tight-binding model Solid state physics review Approximations
Project Report: Band Structure of GaAs using k.p-theory
 Proect Report: Band Structure of GaAs using k.p-theory Austin Irish Mikael Thorström December 12th 2017 1 Introduction The obective of the proect was to calculate the band structure of both strained and
Proect Report: Band Structure of GaAs using k.p-theory Austin Irish Mikael Thorström December 12th 2017 1 Introduction The obective of the proect was to calculate the band structure of both strained and
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Fall Semester 2006 Christopher J. Cramer. Lecture 5, January 27, 2006
 Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Fall Semester 2006 Christopher J. Cramer Lecture 5, January 27, 2006 Solved Homework (Homework for grading is also due today) We are told
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Fall Semester 2006 Christopher J. Cramer Lecture 5, January 27, 2006 Solved Homework (Homework for grading is also due today) We are told
arxiv: v1 [hep-ph] 5 Sep 2017
![arxiv: v1 [hep-ph] 5 Sep 2017 arxiv: v1 [hep-ph] 5 Sep 2017](/thumbs/74/70740165.jpg) A First Step Towards Effectively Nonperturbative Scattering Amplitudes in the Perturbative Regime Neil Christensen, Joshua Henderson, Santiago Pinto, and Cory Russ Department of Physics, Illinois State
A First Step Towards Effectively Nonperturbative Scattering Amplitudes in the Perturbative Regime Neil Christensen, Joshua Henderson, Santiago Pinto, and Cory Russ Department of Physics, Illinois State
Minimal Update of Solid State Physics
 Minimal Update of Solid State Physics It is expected that participants are acquainted with basics of solid state physics. Therefore here we will refresh only those aspects, which are absolutely necessary
Minimal Update of Solid State Physics It is expected that participants are acquainted with basics of solid state physics. Therefore here we will refresh only those aspects, which are absolutely necessary
Hybridization of Atomic Orbitals. (Chapter 1 in the Klein text)
 Hybridization of Atomic Orbitals (Chapter 1 in the Klein text) Basic Ideas The atomic structures, from the Periodic Table, of atoms such as C, N, and O do not adequately explain how these atoms use orbitals
Hybridization of Atomic Orbitals (Chapter 1 in the Klein text) Basic Ideas The atomic structures, from the Periodic Table, of atoms such as C, N, and O do not adequately explain how these atoms use orbitals
3 Symmetry Protected Topological Phase
 Physics 3b Lecture 16 Caltech, 05/30/18 3 Symmetry Protected Topological Phase 3.1 Breakdown of noninteracting SPT phases with interaction Building on our previous discussion of the Majorana chain and
Physics 3b Lecture 16 Caltech, 05/30/18 3 Symmetry Protected Topological Phase 3.1 Breakdown of noninteracting SPT phases with interaction Building on our previous discussion of the Majorana chain and
Chapter 1: Chemical Bonding
 Chapter 1: Chemical Bonding Linus Pauling (1901 1994) January 30, 2017 Contents 1 The development of Bands and their filling 3 2 Different Types of Bonds 7 2.1 Covalent Bonding.............................
Chapter 1: Chemical Bonding Linus Pauling (1901 1994) January 30, 2017 Contents 1 The development of Bands and their filling 3 2 Different Types of Bonds 7 2.1 Covalent Bonding.............................
Page 404. Lecture 22: Simple Harmonic Oscillator: Energy Basis Date Given: 2008/11/19 Date Revised: 2008/11/19
 Page 404 Lecture : Simple Harmonic Oscillator: Energy Basis Date Given: 008/11/19 Date Revised: 008/11/19 Coordinate Basis Section 6. The One-Dimensional Simple Harmonic Oscillator: Coordinate Basis Page
Page 404 Lecture : Simple Harmonic Oscillator: Energy Basis Date Given: 008/11/19 Date Revised: 008/11/19 Coordinate Basis Section 6. The One-Dimensional Simple Harmonic Oscillator: Coordinate Basis Page
So far we have limited the discussion to state spaces of finite dimensions, but it turns out that, in
 Chapter 0 State Spaces of Infinite Dimension So far we have limited the discussion to state spaces of finite dimensions, but it turns out that, in practice, state spaces of infinite dimension are fundamental
Chapter 0 State Spaces of Infinite Dimension So far we have limited the discussion to state spaces of finite dimensions, but it turns out that, in practice, state spaces of infinite dimension are fundamental
Reciprocal Lattice. Let A(r) be some function featuring discrete translation symmetry:
 Reciprocal Lattice From Quantum Mechanics we know that symmetries have kinematic implications. Each symmetry predetermines its specific quantum numbers and leads to certain constraints (conservation laws/selection
Reciprocal Lattice From Quantum Mechanics we know that symmetries have kinematic implications. Each symmetry predetermines its specific quantum numbers and leads to certain constraints (conservation laws/selection
Quantum mechanics can be used to calculate any property of a molecule. The energy E of a wavefunction Ψ evaluated for the Hamiltonian H is,
 Chapter : Molecules Quantum mechanics can be used to calculate any property of a molecule The energy E of a wavefunction Ψ evaluated for the Hamiltonian H is, E = Ψ H Ψ Ψ Ψ 1) At first this seems like
Chapter : Molecules Quantum mechanics can be used to calculate any property of a molecule The energy E of a wavefunction Ψ evaluated for the Hamiltonian H is, E = Ψ H Ψ Ψ Ψ 1) At first this seems like
Refering to Fig. 1 the lattice vectors can be written as: ~a 2 = a 0. We start with the following Ansatz for the wavefunction:
 1 INTRODUCTION 1 Bandstructure of Graphene and Carbon Nanotubes: An Exercise in Condensed Matter Physics developed by Christian Schönenberger, April 1 Introduction This is an example for the application
1 INTRODUCTION 1 Bandstructure of Graphene and Carbon Nanotubes: An Exercise in Condensed Matter Physics developed by Christian Schönenberger, April 1 Introduction This is an example for the application
Hilbert Spaces. Hilbert space is a vector space with some extra structure. We start with formal (axiomatic) definition of a vector space.
 Hilbert Spaces Hilbert space is a vector space with some extra structure. We start with formal (axiomatic) definition of a vector space. Vector Space. Vector space, ν, over the field of complex numbers,
Hilbert Spaces Hilbert space is a vector space with some extra structure. We start with formal (axiomatic) definition of a vector space. Vector Space. Vector space, ν, over the field of complex numbers,
Lecture 3. Energy bands in solids. 3.1 Bloch theorem
 Lecture 3 Energy bands in solids In this lecture we will go beyond the free electron gas approximation by considering that in a real material electrons live inside the lattice formed by the ions (Fig.
Lecture 3 Energy bands in solids In this lecture we will go beyond the free electron gas approximation by considering that in a real material electrons live inside the lattice formed by the ions (Fig.
Notes on SU(3) and the Quark Model
 Notes on SU() and the Quark Model Contents. SU() and the Quark Model. Raising and Lowering Operators: The Weight Diagram 4.. Triangular Weight Diagrams (I) 6.. Triangular Weight Diagrams (II) 8.. Hexagonal
Notes on SU() and the Quark Model Contents. SU() and the Quark Model. Raising and Lowering Operators: The Weight Diagram 4.. Triangular Weight Diagrams (I) 6.. Triangular Weight Diagrams (II) 8.. Hexagonal
The potential is minimum at the positive ion sites and maximum between the two ions.
 1. Bloch theorem: - A crystalline solid consists of a lattice, which is composed of a large number of ion cores at regular intervals, and the conduction electrons that can move freely through out the lattice.
1. Bloch theorem: - A crystalline solid consists of a lattice, which is composed of a large number of ion cores at regular intervals, and the conduction electrons that can move freely through out the lattice.
