1. Thermo = the that happen in a chemical reaction. 4. You must ADD energy to melt solids into liquids example:
|
|
|
- Clyde Hicks
- 5 years ago
- Views:
Transcription
1 ThermoChem Notes 1. Thermo = the that happen in a chemical reaction. 2. When heat is given off it is an reaction. 3. Sometimes energy is absorbed in order for the reaction happen; this is called an reaction. 4. You must ADD energy to melt solids into liquids example: 5. You must REMOVE energy to freeze liquids into solids example:. 6. You must ADD energy to vaporize liquids into gases example:. 7. You must REMOVE energy to condense gases into liquids example:. 8. On a heating curve: 9. On a heating curve: Draw a simple cooling curve for water 10. On a cooling curve: 11. On a cooling curve:
2 12.. Thermochem is partly: 13. Thermochem is also partly: 14. Even though we won t do much work below freezing, substances like ICE or any other solids can be really cold or warmer, but remain solid. For example: 15. And, steam (water gas) forms at 373 Kelvin, but it could also get to be much hotter. 16. Thermochem 17. Thermochem will also help us to measure the amount of heat in a chemical reaction (exothermic) or how much heat is in a chemical reaction (endothermic). 18. How much heat is going to be very mathematical when we get to in next week. It s called the written as this: that is THE CHANGE IN HEAT 19. The energy is with the mole ratio, so we can do math (stoichiometry) to the chemical and energy parts of a balanced thermochemical equation. Open your reference tables to the first page. Look for Table B, let s fill it in on the next page.
3 Table B Physical Constants for Water Heat of Fusion Heat of Vaporization Specific Heat Capacity of H 2 O (L) 21. The heat of fusion constant for water is 22. Every substance has 23. The 334 J/g is the constant just for 24. H V is the constant. This is the energy that must be 1 gram of steam at 373 K or 100 C or to be 1 gram of water. 25. Every substance has 26. The 2260 Joules/gram is the constant just for 27. Capital C is the symbol for the specific heat capacity constant for water. This is the energy required to by 1 K or 1 C 28. Every substance has its own 29. The 4.18 J/g K is the constant for water only. Not solid or gas H 2 O, just liquid. 30. Liquid water has a specific heat capacity constant. It s hard to A metal, such as iron has C Fe = Because water has such a high specific heat capacity constant (the amount of energy it takes to heat up, or cool down one gram of water by 1 Kelvin) pools...
4 31. The reason BC < DE is because 32. Fill in this table. BC CD DE 33. The reason BC > DE is because Heating Curve for Water 34. Fill in this chart BC CD DE
5 35. To vaporize 1 gram of water you must (the heat of vaporization). When 1 gram of steam condenses, you (the heat of vaporization) It s the same amount of energy, just a matter of adding it, or removing it. To melt 1 gram of (the heat of fusion). When 1 gram of water freezes, (the heat of fusion) It s the same amount of energy, just a matter of adding it, or removing it. 36. To change temperature of ONE GRAM of liquid water HOTTER, To change temperature of ONE GRAM of liquid water COLDER, Energy Unit Conversion Math 37. There are 38. A calorie (lower case c ) 39. A Calorie is different. 40. A calorie 41. That s the same as changing the temperature of one gram of water from 42. A Food Calorie (capital C ) 43. We will use cal for and we will use Calorie for
6 44. It takes 1000 cal = 45. A joule is tiny. It takes = joules is called a 47. Under Table B, in small ink, write these out: 4.18 J = 1 cal 1000 cal = 1 C 1000 joules = 1 kj 48. You will get used to these units. In size order, from BIGGEST to smallest, here goes 49. It takes 354 calories to make 354 g H 2 O at 7 C to warm to 8 C. How many joules is 354 cal? 50. If you remove 5675 cal from 5675 g of H 2 O at 297 K, it will cool to 296 K. How many Food Calories is 5675 cal? 51. Convert 3429 cal into kilojoules. (kj) 52. When one mole of methane (CH 4 ) combusts at room temperature, exactly kj are released in this exothermic reaction. Convert kj into cal.
7 53. There are 225 Calories in a medium sized Hershey bar. Convert 225 C into joules. 54. To melt ice it takes 334 Joules/gram. To melt lots of grams takes. 55. The three Thermochemistry formulas on the back page of the reference tables are as follows: In each one, the letters and the symbols stand for this. (add some notes to the reference tables now too) 56. How many joules of energy does it take to melt an ice cube of 83.0 grams? 57. How many joules of energy are required to freeze 355 ml of water at 273K?
8 58. How many joules required to melt a snow ball of 415 g? 59. This is important: If your stuff is condensing, or vaporizing, at the HOT PHASE CHANGE TEMP, 60. When 12.5 g of water at 373 K but still liquid - is vaporized, how many joules does it take to turn it into a gas? 61. A big steam pipe breaks and releases 625 grams of steam onto a wall. How much energy is released when the steam condenses? 62. Making water change temperature is than phase changing it. There is a temperature change, and requires a formula that takes that temperature change into account. The change in temperature is called the.
9 63. Draw in this simple heating curve for water. Label it ABCDEF. For BC use the formula For DE, use the formula 64. For CD there is a temperature change, so you use this formula: 65. How much energy is needed to be removed to cool a glass of water (325 grams) from (room temp) 296 K down to drinkable-cool of 284 K? 66. A pot holds 650. grams of water at room temperature (24.0 C) You think to make some mac and cheese and turn on the stove to heat the water. It heats up to 95.0 C when your BFF shows up with pizza and you turn off the stove. How much energy would your Dad say you wasted heating up this water for nothing?
10 67. In this problem, the water changes temperature from 24.0 to 95.0 C. If we convert those temps first, the water changes from. The ΔT = 71.0 Kelvin (3 SF) In centigrade it s:. The ΔT = 71.0 C (3 SF) The change in temp is the same even though the actual numerals are different. Centigrade is NOT the same as Kelvin, but the change in C is equal to the change in K. 68. If water changes from 0 to 25 C, the ΔT is 69. If water changes from 273 K to 298 K, the ΔT is 70. This is important! and , but the difference between If there is a change of temperature, there is ONLY one formula to choose. It is: 73. If there is melting or freezing, there is ONLY one formula to choose. It is: 74. If there is condensing or vaporizing, there is ONLY one formula to choose. It is: 75. Choose wisely, you must. 76. When 650. grams of water at room temperature (24.0 C) goes into a pot to boil at C. You vaporize 35.0 grams of it. How much energy did it take to all of this?
11 77. A gram snowball at K first melts, then warms to How many joules did it take to do that? Take out your Themochem Maps now. We need to look it over, and save it somewhere good for reference. 78. The specific heat capacity constant for LIQUID WATER is 79. But for ICE it is different: it s 80. For STEAM it is different again, it s Write these 2 constants underneath table B now Now it is time for the COOLEST DEMO of the year. You can sit back and just enjoy the show. I dare you not to giggle because this is SO, SO cool When something like iron melts, it has its own which is sort of high compared to water. It has its own heat of fusion constant too. 82. When you warm up iron on the way to melting it, it takes a certain amount of energy to make each gram get hotter by. Every substance has it s own specific heat capacity constant. 83. Every substance has a unique. 84. How many grams of water can be frozen when you remove 87,500 joules from it? 85. How many grams of water can be heated by 25.5 K when it absorbs 17,500 joules?
12 86. What is the HEAT OF FUSION for candle wax if it takes 3388 Joules to melt a whole birthday candle with mass of grams? 87. What is the C of Cu, if it takes 951 joules to warm up grams of copper from K up to Kelvin? 88. What is the specific heat capacity constant for GOLD if it takes 271 joules to warm up a ring with mass of 34.2 g from room temp (294.0 K) to a too hot to wear temperature of Kelvin? 89. When a 355 ml can of seltzer, is warmed from a temperature of 293 K by adding 64,000 Joules of energy to it, what is the final temperature? Assume the seltzer is just water.
13 90. When 51.1 g copper at K emits 1788 Joules, it cools down. What is the final temp if C Cu = J/g K? The C of Cu Lab Explained. Look at the Lab Handout Now. Take a few notes on white paper There is no easy way to directly measure the energy that is in food. An indirect way has been well figured out, using a machine called a 92. Let s draw and label a calorimeter now. 93. Let s assume that there is exactly ml of water in our bomb calorimeter and it s at exactly K. After burning up our food sample, the temperature of the water rises to K. How many Calories of energy are in this food sample?
14 Dorito s Lab Explained. Take out the Lab Handout, take some notes on white paper Take out Table I now. (when I don t know what I need to know in a thermochem problem, sometimes I fret, sometimes I yell, sometimes I even cr-i! What I should do when I have a hard problem is to look at TABLE!!! 96. Table I is called the table. 97. Unusually, it is at 98. It s at, which is room temperature 99. CH 4(G) + 2O 2(G) CO 2(G) + 2H 2 O (G) The mole ratio here is 100. This reaction has a ΔH = 101. Which means this reaction 102.What s with that NEGATIVE SIGN? 103. A minus sign indicates: 104. If the ΔH is a + number, that means the In an exothermic reaction, energy is a (it s given off) 106. In an endothermic reaction, energy is a (energy is absorbed to make the reaction occur) Well, now I m telling you that: 108. The mole ratio for the combustion of CH 4 is now: 109. If 23.4 moles of CH 4 combust, how much energy is released?
15 110. Propane gas, C 3 H 8 combusts according to Table I. How much energy (in kj) is released when 5.75 moles C 3 H 8 combusts? (find this - Table I, it s the 2 nd reaction) 111. How much energy is absorbed by the reaction of 99.0 moles of HI (G) forming? 112. How much energy is required to melt a snow ball at 273 K up to body temperature of 294 Kelvin? (think hard) 113. If 16.3 grams of steam at exactly 373 K condenses onto a kitchen window, then cools quickly to 305 K. How much energy was released?
16 114. C ICE = (this is NOT liquid water, NOT 4.18 J/g K) 115. For fun, you obtain a snowball (255 g) at C and put it in the back of your friend s shirt. It melts then warms up to body temperature of 36.0 C. How much energy does that take? 116. Draw the simple Cooling Curve for Chromium, labels, titles, axis labels, etc A. What temps are 1 + 2? B. What s PE doing BC and CD? C. What s KE doing AB and DE D. Why is BC longer than DE? E. Which thermochem formula do you use for BC? F. How about for EF?
17 118. Use table I, choose the equation of propane combusts. Write the balanced chemical equation with the ΔH 119. Write a balanced thermochemical equation 120. Find the most endothermic equation on Table I, write the balanced chemical equation with the ΔH 121. Write the balanced thermochemical equation for this reaction too You decide to warm up some water for oatmeal. How much energy does it take to warm up 354 ml of water from 24.5 C to the boiling point and to let 7.50 grams vaporize making the teapot whistle Write the balanced thermochemical equation for the synthesis of aluminum oxide, then calculate how much energy is released (or absorbed) when you form 21.0 moles of Al 2 O 3.
18 The heating curve for Phosphorous 124. What formula would you use For BC For CD Or for DE What happens to PE + KE at BC and CD? 125. When 45.0 g of an unknown metal absorbs 1.51 kj of heat. The temperature changes from 268 K to 345 K. What is the specific heat capacity constant for this metal?
19 126. Which takes more energy? Melting 50.0 g of ice or vaporizing 50.0 g of water into steam? 127. Which takes more energy? Heating 23.0 ml of water from 274 K to 299 K Or Heating 23.0 ml of water from 299 K to 323 K? 128. Which takes more energy? Vaporizing 21.0 g water from 373 K liquid to gas or Changing the temp of g H 2 O by 97.0 K? 129. Which has the LOWEST AVERAGE KINETIC ENERGY? 100 ml water at 51.0 C 100 ml water at 50.0 C 175 ml water at 49.0 C 175 ml water at 23.0 C 130. This horse has a boo boo. That s an ICE PACK on his leg. Describe the thermochem: A. Heat flows from ice pack leg B. Cold flows from ice pack leg C. Heat flows from leg ice pack D. Cold flows from leg ice pack 131. Copy these CAREFULLY, they are not the same. Be Complete. The Law of Conservation of Matter (or mass) The Law of Conservation of Energy
CHAPTER 17 Thermochemistry
 CHAPTER 17 Thermochemistry Thermochemistry The study of the heat changes that occur during chemical reactions and physical changes of state. Chemical Change: new substances created during chemical reaction
CHAPTER 17 Thermochemistry Thermochemistry The study of the heat changes that occur during chemical reactions and physical changes of state. Chemical Change: new substances created during chemical reaction
Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy
 Unit 7: Energy Outline Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy Energy Energy is the ability to do work or produce heat. The energy
Unit 7: Energy Outline Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy Energy Energy is the ability to do work or produce heat. The energy
Thermochemistry: Heat and Chemical Change
 Thermochemistry: Heat and Chemical Change 1 Heat or Thermal Energy (q) Heat is a form of energy Is heat the same as temperature? Heat flows between two objects at different temperatures. Hot Cold 2 Chemical
Thermochemistry: Heat and Chemical Change 1 Heat or Thermal Energy (q) Heat is a form of energy Is heat the same as temperature? Heat flows between two objects at different temperatures. Hot Cold 2 Chemical
Name Class Date. As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings.
 Name Class Date Thermochemistry 17.1 The Flow of Energy As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings. Process Cause Effect endothermic
Name Class Date Thermochemistry 17.1 The Flow of Energy As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings. Process Cause Effect endothermic
Thermochemistry. Energy (and Thermochemistry) World of Chemistry Chapter 10. Energy. Energy
 Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Chemistry Heat Review. Heat: Temperature: Enthalpy: Calorimetry: Activation energy:
 Chemistry Heat Review Name Date Vocabulary Heat: Temperature: Enthalpy: Calorimetry: Activation energy: Formulas Heat of phase change Heat for temperature increase Heat of reaction Endothermic/Exothermic
Chemistry Heat Review Name Date Vocabulary Heat: Temperature: Enthalpy: Calorimetry: Activation energy: Formulas Heat of phase change Heat for temperature increase Heat of reaction Endothermic/Exothermic
CHAPTER 17: THERMOCHEMISTRY. Mrs. Brayfield
 CHAPTER 17: THERMOCHEMISTRY Mrs. Brayfield REVIEW What is the law of conservation of energy? It states that energy cannot be created or destroyed So the energy of any process is the same THERMOCHEMISTRY
CHAPTER 17: THERMOCHEMISTRY Mrs. Brayfield REVIEW What is the law of conservation of energy? It states that energy cannot be created or destroyed So the energy of any process is the same THERMOCHEMISTRY
Practice Packet Unit 7: Heat
 Regents Chemistry: Mr. Palermo Practice Packet Unit 7: Heat Review (Things you need to know in order to understand the new stuff ) Particle Diagrams Draw a particle diagram of a compound of CaCl2, using
Regents Chemistry: Mr. Palermo Practice Packet Unit 7: Heat Review (Things you need to know in order to understand the new stuff ) Particle Diagrams Draw a particle diagram of a compound of CaCl2, using
q = m x C x ΔT or, think of it as unit cancellation: = ( ) (
 Chemistry Ms. Ye Name Date Block Heat, Kinetic Energy, and Changes in State of Matter *Kinetic Energy=the energy associated with *Temperature=measure of the of a sample. *Heat=is measured as the that is
Chemistry Ms. Ye Name Date Block Heat, Kinetic Energy, and Changes in State of Matter *Kinetic Energy=the energy associated with *Temperature=measure of the of a sample. *Heat=is measured as the that is
solid IMF>liquid IMF>gas IMF Draw a diagram to represent the 3 common states of matter of a given substance: solid liquid gas
 Thermochemistry Part 1 Notes States of Matter and Intermolecular Forces (IMF) Chemistry HP At the end of this unit, students should be able to: Describe the various states of matter in terms of kinetic
Thermochemistry Part 1 Notes States of Matter and Intermolecular Forces (IMF) Chemistry HP At the end of this unit, students should be able to: Describe the various states of matter in terms of kinetic
Ch. 17 Thermochemistry
 Ch. 17 Thermochemistry 17.1 The Flow of Energy Energy Transformations Thermochemistry: study of energy changes in chemical reactions and changes in state Chemical potential energy: energy stored in bonds
Ch. 17 Thermochemistry 17.1 The Flow of Energy Energy Transformations Thermochemistry: study of energy changes in chemical reactions and changes in state Chemical potential energy: energy stored in bonds
Thermodynamics Test Clio Invitational January 26, 2013
 Thermodynamics Test Clio Invitational January 26, 2013 School Name: Team Number: Variables specified: s = specific heat C = heat capacity H f = heat of fusion H v = heat of vaporization Given information:
Thermodynamics Test Clio Invitational January 26, 2013 School Name: Team Number: Variables specified: s = specific heat C = heat capacity H f = heat of fusion H v = heat of vaporization Given information:
Accelerated Chemistry Study Guide Chapter 12, sections 1 and 2: Heat in Chemical Reactions
 Accelerated Chemistry Study Guide Chapter 12, sections 1 and 2: Heat in Chemical Reactions Terms, definitions, topics Joule, calorie (Re-read p 57-58) Thermochemistry Exothermic reaction Endothermic reaction
Accelerated Chemistry Study Guide Chapter 12, sections 1 and 2: Heat in Chemical Reactions Terms, definitions, topics Joule, calorie (Re-read p 57-58) Thermochemistry Exothermic reaction Endothermic reaction
Thermochemistry: the study of energy (in the from of heat) changes that accompany physical & chemical changes
 Thermochemistry Thermochemistry: the study of energy (in the from of heat) changes that accompany physical & chemical changes heat flows from high to low (hot cool) endothermic reactions: absorb energy
Thermochemistry Thermochemistry: the study of energy (in the from of heat) changes that accompany physical & chemical changes heat flows from high to low (hot cool) endothermic reactions: absorb energy
I. The Nature of Energy A. Energy
 I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
Heat. Heat Terminology 04/12/2017. System Definitions. System Definitions
 System Definitions Heat Physical Science 20 Ms. Hayduk Heat Terminology System: the part of the universe being studied (big Earth, or small one atom) Surroundings: the part of the universe outside the
System Definitions Heat Physical Science 20 Ms. Hayduk Heat Terminology System: the part of the universe being studied (big Earth, or small one atom) Surroundings: the part of the universe outside the
Thermochemistry. The study of energy changes that occur during chemical reactions and changes in state.
 Energy Thermochemistry The study of energy changes that occur during chemical reactions and changes in state. The Nature of Energy Energy - the ability to do work or produce heat Energy is stored in the
Energy Thermochemistry The study of energy changes that occur during chemical reactions and changes in state. The Nature of Energy Energy - the ability to do work or produce heat Energy is stored in the
Name: Regents Chemistry: Mr. Palermo. Student Version. Notes: Unit 6A Heat
 Name: Regents Chemistry: Mr. Palermo Student Version Notes: Unit 6A Heat Name: KEY IDEAS Heat is a transfer of energy (usually thermal energy) from a body of higher temperature to a body of lower temperature.
Name: Regents Chemistry: Mr. Palermo Student Version Notes: Unit 6A Heat Name: KEY IDEAS Heat is a transfer of energy (usually thermal energy) from a body of higher temperature to a body of lower temperature.
Chemical Reactions Chapter 17 Study Guide (Unit 10)
 Chemical Reactions Chapter 17 Study Guide (Unit 10) Name: Hr: Understand and be able to explain all of the key concepts. Define and understand all of the survival words Memorize the names and symbols for
Chemical Reactions Chapter 17 Study Guide (Unit 10) Name: Hr: Understand and be able to explain all of the key concepts. Define and understand all of the survival words Memorize the names and symbols for
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Chapter 11. Thermochemistry: Heat & Chemical Change
 Chapter 11 Thermochemistry: Heat & Chemical Change The Flow of Energy Thermochemistry: Study of heat changes that occur during physical processes and chemical reactions Energy Energy is the capacity to
Chapter 11 Thermochemistry: Heat & Chemical Change The Flow of Energy Thermochemistry: Study of heat changes that occur during physical processes and chemical reactions Energy Energy is the capacity to
Thermochemistry. Questions to ponder. Because 4/20/14. an ice-cube? an ice-cube? Part 2: Calorimetry. But I KNOW. Q=mc T, but T=0
 Thermochemistry Part 2: Calorimetry p p If you leave your keys and your chemistry book sitting in the sun on a hot summer day, which one is hotter? Why is there a difference in temperature between the
Thermochemistry Part 2: Calorimetry p p If you leave your keys and your chemistry book sitting in the sun on a hot summer day, which one is hotter? Why is there a difference in temperature between the
Name: Regents Chemistry: Mr. Palermo. Notes: Unit 7 Heat.
 Name: Regents Chemistry: Mr. Palermo Notes: Unit 7 Heat 1 Name: KEY IDEAS Heat is a transfer of energy (usually thermal energy) from a body of higher temperature to a body of lower temperature. Thermal
Name: Regents Chemistry: Mr. Palermo Notes: Unit 7 Heat 1 Name: KEY IDEAS Heat is a transfer of energy (usually thermal energy) from a body of higher temperature to a body of lower temperature. Thermal
Practice Packet: Energy. Regents Chemistry: Dr. Shanzer. Practice Packet. Chapter 4: Energy.
 Regents Chemistry: Dr. Shanzer Practice Packet Chapter 4: Energy http:/drshanzerchemistry.weebly.com Energy Objectives Define energy. Demonstrate the difference between endothermic and exothermic reactions
Regents Chemistry: Dr. Shanzer Practice Packet Chapter 4: Energy http:/drshanzerchemistry.weebly.com Energy Objectives Define energy. Demonstrate the difference between endothermic and exothermic reactions
Phase Change Diagram. Rank Solids, liquids and gases from weakest attractive forces to strongest:
 Unit 11 Kinetic molecular theory packet Page 1 of 13 Chemistry Unit 11 Kinetic Theory Unit Quiz: Test Objectives Be able to define pressure and memorize the basic pressure units. Be able to convert to/from:
Unit 11 Kinetic molecular theory packet Page 1 of 13 Chemistry Unit 11 Kinetic Theory Unit Quiz: Test Objectives Be able to define pressure and memorize the basic pressure units. Be able to convert to/from:
1. Fill in the blanks with the following: kinetic, potential, chemical, thermal. One word will be used twice.
 Thermo Worksheets Name Class Period Types of Energy and the Law of Conservation of Energy 1. Fill in the blanks with the following: kinetic, potential, chemical, thermal. One word will be used twice. Solar
Thermo Worksheets Name Class Period Types of Energy and the Law of Conservation of Energy 1. Fill in the blanks with the following: kinetic, potential, chemical, thermal. One word will be used twice. Solar
Unit 14. States of Matter & Thermochemistry
 Unit 14 Flashback: States of Matter & Thermochemistry Characteristic Solids Liquids Gases Shape Volume Density Fluidity Compressibility Picture Phase Diagram Shows the relationship between solid, liquid,
Unit 14 Flashback: States of Matter & Thermochemistry Characteristic Solids Liquids Gases Shape Volume Density Fluidity Compressibility Picture Phase Diagram Shows the relationship between solid, liquid,
Thermochemistry: Energy Flow and Chemical Reactions
 Thermochemistry: Energy Flow and Chemical Reactions Outline thermodynamics internal energy definition, first law enthalpy definition, energy diagrams, calorimetry, theoretical calculation (heats of formation
Thermochemistry: Energy Flow and Chemical Reactions Outline thermodynamics internal energy definition, first law enthalpy definition, energy diagrams, calorimetry, theoretical calculation (heats of formation
Chemistry Chapter 16. Reaction Energy
 Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Topic 05 Energetics : Heat Change. IB Chemistry T05D01
 Topic 05 Energetics 5.1-5.2: Heat Change IB Chemistry T05D01 5.1 Exothermic and endothermic reactions - 1 hour 5.1.1 Define the terms exothermic reaction, endothermic reaction and standard enthalpy change
Topic 05 Energetics 5.1-5.2: Heat Change IB Chemistry T05D01 5.1 Exothermic and endothermic reactions - 1 hour 5.1.1 Define the terms exothermic reaction, endothermic reaction and standard enthalpy change
Quantities in Chemical Reactions
 Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
This reaction is ENDOTHERMIC. Energy is being transferred from the room/flask/etc. (the SURROUNDINGS) to the reaction itself (the SYSTEM).
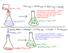 151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
What are the states of Matter?
 What are the states of Matter? Solid Lowest energy/heat Molecules barely moving Definite, uniform shape Example: ice States of Matter Liquid Medium energy/heat Molecules slowly moving Shape of container
What are the states of Matter? Solid Lowest energy/heat Molecules barely moving Definite, uniform shape Example: ice States of Matter Liquid Medium energy/heat Molecules slowly moving Shape of container
Name Chemistry / / Understanding Phase Changes
 Name Chemistry / / Understanding Phase Changes As a piece of ice is exposed to a warmer environment, it begins to absorb heat. The heat causes the solid molecules to vibrate faster. Eventually, the ice
Name Chemistry / / Understanding Phase Changes As a piece of ice is exposed to a warmer environment, it begins to absorb heat. The heat causes the solid molecules to vibrate faster. Eventually, the ice
Chapter 17: Energy and Kinetics
 Pages 510-547 S K K Chapter 17: Energy and Kinetics Thermochemistry: Causes of change in systems Kinetics: Rate of reaction progress (speed) Heat, Energy, and Temperature changes S J J Heat vs Temperature
Pages 510-547 S K K Chapter 17: Energy and Kinetics Thermochemistry: Causes of change in systems Kinetics: Rate of reaction progress (speed) Heat, Energy, and Temperature changes S J J Heat vs Temperature
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
CHEMISTRY: Chapter 10 Prep-Test
 CHEMISTRY: Chapter 10 Prep-Test Matching Match each item with the correct statement below. a. calorimeter d. temperature b. calorie e. specific heat c. joule f. heat 1. quantity of heat needed to raise
CHEMISTRY: Chapter 10 Prep-Test Matching Match each item with the correct statement below. a. calorimeter d. temperature b. calorie e. specific heat c. joule f. heat 1. quantity of heat needed to raise
Chapter 17 Thermochemistry
 Chapter 17 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 17 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Section 1 - Thermochemistry
 Reaction Energy Section 1 - Thermochemistry Virtually every chemical reaction is accompanied by a change in energy. Chemical reactions usually absorb or release energy as heat. You learned in Chapter 12
Reaction Energy Section 1 - Thermochemistry Virtually every chemical reaction is accompanied by a change in energy. Chemical reactions usually absorb or release energy as heat. You learned in Chapter 12
Name Date Class THERMOCHEMISTRY
 Name Date Class 17 THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY HEAT AND WORK (pages 505 510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
Name Date Class 17 THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY HEAT AND WORK (pages 505 510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
Energy and Energy Calculations Test Provide the correct answer as a word, phrase or sentence. (3 points each) 1) Define Matter.
 Provide the correct answer as a word, phrase or sentence. (3 points each) 1) Define Matter. 2) What is ENERGY? 3) Give an example of an endothermic process. 4) Give an example of an exothermic process.
Provide the correct answer as a word, phrase or sentence. (3 points each) 1) Define Matter. 2) What is ENERGY? 3) Give an example of an endothermic process. 4) Give an example of an exothermic process.
Thermochemistry. Section The flow of energy
 Thermochemistry Section 17.1 - The flow of energy What is Energy? Energy is the capacity for doing work or supplying heat Energy does not have mass or volume, and it can only be detected because of its
Thermochemistry Section 17.1 - The flow of energy What is Energy? Energy is the capacity for doing work or supplying heat Energy does not have mass or volume, and it can only be detected because of its
Warm up. 1) What is the conjugate acid of NH 3? 2) What is the conjugate base of HNO 2? 3) If the ph is 9.2, what is the [H 3 O + ], poh, and [OH - ]?
![Warm up. 1) What is the conjugate acid of NH 3? 2) What is the conjugate base of HNO 2? 3) If the ph is 9.2, what is the [H 3 O + ], poh, and [OH - ]? Warm up. 1) What is the conjugate acid of NH 3? 2) What is the conjugate base of HNO 2? 3) If the ph is 9.2, what is the [H 3 O + ], poh, and [OH - ]?](/thumbs/78/77877522.jpg) Warm up 1) What is the conjugate acid of NH 3? 2) What is the conjugate base of HNO 2? 3) If the ph is 9.2, what is the [H 3 O + ], poh, and [OH - ]? 4) What is the concentration of H 2 SO 4 if 30.1 ml
Warm up 1) What is the conjugate acid of NH 3? 2) What is the conjugate base of HNO 2? 3) If the ph is 9.2, what is the [H 3 O + ], poh, and [OH - ]? 4) What is the concentration of H 2 SO 4 if 30.1 ml
= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)
![= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj) = (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)](/thumbs/89/101004910.jpg) CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
Energy Changes in Reactions p
 Energy Changes in Reactions p.126 210 Heat vs. temperature: Heat is a form of energy, it is transferred from one system to another Temperature is an indication of the intensity of heat, it measures the
Energy Changes in Reactions p.126 210 Heat vs. temperature: Heat is a form of energy, it is transferred from one system to another Temperature is an indication of the intensity of heat, it measures the
Name Date Class THE FLOW OF ENERGY HEAT AND WORK
 17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
Chapter 16 Theories of Energy Changes
 {Read p. 624 and 626 to understand concepts} Class discussion for chapter 17.3 Chapter 16 Theories of Energy Changes Section 16.1A Temperature change and Heat THERMODYNAMICS - the study of energy and energy
{Read p. 624 and 626 to understand concepts} Class discussion for chapter 17.3 Chapter 16 Theories of Energy Changes Section 16.1A Temperature change and Heat THERMODYNAMICS - the study of energy and energy
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
3.3 Phase Changes 88 A NATURAL APPROACH TO CHEMISTRY. Section 3.3 Phase Changes
 Section 3.3 Phase Changes 3.3 Phase Changes Solid, liquid and gas During a phase change, a substance rearranges the order of its particles (atoms or molecules). Examples of phase change include melting
Section 3.3 Phase Changes 3.3 Phase Changes Solid, liquid and gas During a phase change, a substance rearranges the order of its particles (atoms or molecules). Examples of phase change include melting
Quantities in Chemical Reactions
 Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Thermodynamics - Heat Transfer June 04, 2013
 THERMODYNAMICS - Heat and Heat Transfer: Heat (Q) is a form of Energy that is transferred between an object and another object or its surrounding environment due to a difference in Temperature. Heat is
THERMODYNAMICS - Heat and Heat Transfer: Heat (Q) is a form of Energy that is transferred between an object and another object or its surrounding environment due to a difference in Temperature. Heat is
CP Chapter 17 Thermochemistry
 CP Chapter 17 Thermochemistry Thermochemistry Thermochemistry is the study of energy that occur during chemical reactions and phase changes (changes of state) The Nature of Energy Energy is the ability
CP Chapter 17 Thermochemistry Thermochemistry Thermochemistry is the study of energy that occur during chemical reactions and phase changes (changes of state) The Nature of Energy Energy is the ability
Topic 5: Energetics. Heat & Calorimetry. Thursday, March 22, 2012
 Topic 5: Energetics Heat & Calorimetry 1 Heat is energy that is transferred from one object to another due to a difference in temperature Temperature is a measure of the average kinetic energy of a body
Topic 5: Energetics Heat & Calorimetry 1 Heat is energy that is transferred from one object to another due to a difference in temperature Temperature is a measure of the average kinetic energy of a body
Thermochemistry. Energy and Chemical Change
 Thermochemistry Energy and Chemical Change Energy Energy can change for and flow, but it is always conserved. The Nature of Energy Energy the ability to do work or produce heat Potential energy Kinetic
Thermochemistry Energy and Chemical Change Energy Energy can change for and flow, but it is always conserved. The Nature of Energy Energy the ability to do work or produce heat Potential energy Kinetic
Unit 6: Energy. Aim: What is Energy? Energy: Energy is required to bring about changes in matter (atoms, ions, or molecules).
 Name: Date: Unit 6: Energy Aim: What is Energy? Energy: Energy is required to bring about changes in matter (atoms, ions, or molecules). Physical Changes Chemical Changes Example: Example: Energy is measured
Name: Date: Unit 6: Energy Aim: What is Energy? Energy: Energy is required to bring about changes in matter (atoms, ions, or molecules). Physical Changes Chemical Changes Example: Example: Energy is measured
Unit 15 Energy and Thermochemistry Notes
 Name KEY Period CRHS Academic Chemistry Unit 15 Energy and Thermochemistry Notes Quiz Date Exam Date Lab Dates Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic Chemistry Website: https://cincochem.pbworks.com
Name KEY Period CRHS Academic Chemistry Unit 15 Energy and Thermochemistry Notes Quiz Date Exam Date Lab Dates Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic Chemistry Website: https://cincochem.pbworks.com
Chapter 6 CONSERVATION OF ENERGY AND MATTER
 Chapter 6 CONSERVATION OF ENERGY AND MATTER Identifying Chemical Change (6.1) Chemical reactions: Process in which the physical and chemical properties of the original substance change as new substances
Chapter 6 CONSERVATION OF ENERGY AND MATTER Identifying Chemical Change (6.1) Chemical reactions: Process in which the physical and chemical properties of the original substance change as new substances
The Nature of Energy. Chapter Six: Kinetic vs. Potential Energy. Energy and Work. Temperature vs. Heat
 The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
What Do You Think? Investigate GOALS. Part A: Freezing Water
 Activity 5 Freezing Water GOALS In this activity you will: Determine the freezing point of water. Show graphically what happens to the temperature as water is cooled to freezing and while it is freezing.
Activity 5 Freezing Water GOALS In this activity you will: Determine the freezing point of water. Show graphically what happens to the temperature as water is cooled to freezing and while it is freezing.
Temperature and Its Measurement
 Temperature and Its Measurement When the physical properties are no longer changing, the objects are said to be in thermal equilibrium. Two or more objects in thermal equilibrium have the same temperature.
Temperature and Its Measurement When the physical properties are no longer changing, the objects are said to be in thermal equilibrium. Two or more objects in thermal equilibrium have the same temperature.
17.2 Thermochemical Equations
 17.2. Thermochemical Equations www.ck12.org 17.2 Thermochemical Equations Lesson Objectives Define enthalpy, and know the conditions under which the enthalpy change in a reaction is equal to the heat absorbed
17.2. Thermochemical Equations www.ck12.org 17.2 Thermochemical Equations Lesson Objectives Define enthalpy, and know the conditions under which the enthalpy change in a reaction is equal to the heat absorbed
Name: Thermochemistry. Practice Test C. General Chemistry Honors Chemistry
 Name: Thermochemistry C Practice Test C General Chemistry Honors Chemistry 1 Objective 1: Use the relationship between mass, specific heat, and temperature change to calculate the heat flow during a chemical
Name: Thermochemistry C Practice Test C General Chemistry Honors Chemistry 1 Objective 1: Use the relationship between mass, specific heat, and temperature change to calculate the heat flow during a chemical
Class work on Calorimetry. January 11 and 12, 2011
 Class work on Calorimetry January 11 and 12, 2011 Name 1. The number of calories needed to raise the temperature of 100 grams of water 10 degrees Celsius is the same as the number of calories needed to
Class work on Calorimetry January 11 and 12, 2011 Name 1. The number of calories needed to raise the temperature of 100 grams of water 10 degrees Celsius is the same as the number of calories needed to
2. If the volume of a container holding a gas is reduced, what will happen to the presure within the container?
 1. Which gas law states that the volume of a fixed mass of a gas is directly proportional to its Kelvin temperature if the pressure is kept constant? A. Boyle s law B. Charles law C. Dalton s law D. Gay-Lussac
1. Which gas law states that the volume of a fixed mass of a gas is directly proportional to its Kelvin temperature if the pressure is kept constant? A. Boyle s law B. Charles law C. Dalton s law D. Gay-Lussac
Energy and Chemical Change
 Energy and Chemical Change Section 16.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Energy and Chemical Change Section 16.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Chapter 8 Thermochemistry
 William L Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 8 Thermochemistry Edward J. Neth University of Connecticut Outline 1. Principles of heat flow 2. Measurement
William L Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 8 Thermochemistry Edward J. Neth University of Connecticut Outline 1. Principles of heat flow 2. Measurement
SPECIFIC HEAT CAPACITY AND HEAT OF FUSION
 SPECIFIC HEAT CAPACITY AND HEAT OF FUSION Apparatus on each table: Thermometer, metal cube, complete calorimeter, outer calorimeter can (aluminum only), balance, 4 styrofoam cups, graduated container,
SPECIFIC HEAT CAPACITY AND HEAT OF FUSION Apparatus on each table: Thermometer, metal cube, complete calorimeter, outer calorimeter can (aluminum only), balance, 4 styrofoam cups, graduated container,
2. What is a measure of the average kinetic energy of particles? (A) heat capacity (B) molar enthalpy (C) specific heat (D) temperature
 Thermochemistry #1 Chemistry 3202 Name: 1. Classify the following systems as open or closed a) glass of cold water b) a gel filled freezer pack c) a burning candle d) a fluorescent lightbulb e) hot water
Thermochemistry #1 Chemistry 3202 Name: 1. Classify the following systems as open or closed a) glass of cold water b) a gel filled freezer pack c) a burning candle d) a fluorescent lightbulb e) hot water
Practice Packet Unit 3: Phase Changes & Heat
 Regents Chemistry: Practice Packet Unit 3: Phase Changes & Heat Name: Assess Yourself: Lesson 1: Lesson 2: Lesson 3: Lesson 4: Vocab: 1 Review (Things you need to know in order to understand the new stuff
Regents Chemistry: Practice Packet Unit 3: Phase Changes & Heat Name: Assess Yourself: Lesson 1: Lesson 2: Lesson 3: Lesson 4: Vocab: 1 Review (Things you need to know in order to understand the new stuff
Chapter 15 Energy and Chemical Change
 Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Matter and Energy Review Packet
 Name Date Matter and Energy Review Packet 1. A compound differs from a mixture in that a compound always has a (1) homogeneous composition (2) maximum of two components (3) minimum of three components
Name Date Matter and Energy Review Packet 1. A compound differs from a mixture in that a compound always has a (1) homogeneous composition (2) maximum of two components (3) minimum of three components
THERMODYNAMICS. Thermodynamics is the study of energy relationships that involve heat, mechanical work, and other aspects of energy and heat transfer.
 THERMODYNAMICS Thermodynamics is the study of energy relationships that involve heat, mechanical work, and other aspects of energy and heat transfer. Central Heating Objectives: After finishing this unit,
THERMODYNAMICS Thermodynamics is the study of energy relationships that involve heat, mechanical work, and other aspects of energy and heat transfer. Central Heating Objectives: After finishing this unit,
Ch 100: Fundamentals for Chemistry
 Ch 100: Fundamentals for Chemistry Chapter 4: Properties of Matter Lecture Notes Physical & Chemical Properties Physical Properties are the characteristics of matter that can be changed without changing
Ch 100: Fundamentals for Chemistry Chapter 4: Properties of Matter Lecture Notes Physical & Chemical Properties Physical Properties are the characteristics of matter that can be changed without changing
Ch. 6 Enthalpy Changes
 Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
A). Yes. B). No. Q15 Is it possible for a solid metal ball to float in mercury?
 Q15 Is it possible for a solid metal ball to float in mercury? A). Yes. B). No. The upward force is the weight of liquid displaced and the downward force is the weight of the ball. If the density of the
Q15 Is it possible for a solid metal ball to float in mercury? A). Yes. B). No. The upward force is the weight of liquid displaced and the downward force is the weight of the ball. If the density of the
11B, 11E Temperature and heat are related but not identical.
 Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
June Which is a closed system? (A) burning candle (B) halogen lightbulb (C) hot water in a sink (D) ripening banana
 June 2005 28. Which is a closed system? burning candle halogen lightbulb hot water in a sink ripening banana 29. Which involves the greatest energy change? chemical reaction nuclear reaction phase change
June 2005 28. Which is a closed system? burning candle halogen lightbulb hot water in a sink ripening banana 29. Which involves the greatest energy change? chemical reaction nuclear reaction phase change
Unit 6. Unit Vocabulary: Distinguish between the three phases of matter by identifying their different
 *STUDENT* Unit Objectives: Absolute Zero Avogadro s Law Normal Boiling Point Compound Cooling Curve Deposition Energy Element Evaporation Heat Heat of Fusion Heat of Vaporization Unit 6 Unit Vocabulary:
*STUDENT* Unit Objectives: Absolute Zero Avogadro s Law Normal Boiling Point Compound Cooling Curve Deposition Energy Element Evaporation Heat Heat of Fusion Heat of Vaporization Unit 6 Unit Vocabulary:
Thermochemistry. The study of the ENERGY CHANGES that accompany changes in matter. 3 Ways: Monday, February 3, 2014
 Thermochemistry The study of the ENERGY CHANGES that accompany changes in matter 3 Ways: 1 Thermodynamics FIRST LAW OF THERMODYNAMICS the total amount of energy in the universe is constant (conservation
Thermochemistry The study of the ENERGY CHANGES that accompany changes in matter 3 Ways: 1 Thermodynamics FIRST LAW OF THERMODYNAMICS the total amount of energy in the universe is constant (conservation
Name: General Chemistry Chapter 11 Thermochemistry- Heat and Chemical Change
 Name: General Chemistry Chapter 11 Thermochemistry- Heat and Chemical Change Notepack 1 Section 11.1: The Flow of Energy Heat (Pages 293 299) 1. Define the following terms: a. Thermochemistry b. Energy
Name: General Chemistry Chapter 11 Thermochemistry- Heat and Chemical Change Notepack 1 Section 11.1: The Flow of Energy Heat (Pages 293 299) 1. Define the following terms: a. Thermochemistry b. Energy
Energy and Chemical Change
 Energy and Chemical Change Section 15.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Energy and Chemical Change Section 15.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Introduction to Thermochemistry. Thermochemistry Unit. Definition. Terminology. Terminology. Terminology 07/04/2016. Chemistry 30
 Thermochemistry Unit Introduction to Thermochemistry Chemistry 30 Definition Thermochemistry is the branch of chemistry concerned with the heat produced and used in chemical reactions. Most of thermochemistry
Thermochemistry Unit Introduction to Thermochemistry Chemistry 30 Definition Thermochemistry is the branch of chemistry concerned with the heat produced and used in chemical reactions. Most of thermochemistry
ENTHALPY, INTERNAL ENERGY, AND CHEMICAL REACTIONS: AN OUTLINE FOR CHEM 101A
 ENTHALPY, INTERNAL ENERGY, AND CHEMICAL REACTIONS: AN OUTLINE FOR CHEM 101A PART 1: KEY TERMS AND SYMBOLS IN THERMOCHEMISTRY System and surroundings When we talk about any kind of change, such as a chemical
ENTHALPY, INTERNAL ENERGY, AND CHEMICAL REACTIONS: AN OUTLINE FOR CHEM 101A PART 1: KEY TERMS AND SYMBOLS IN THERMOCHEMISTRY System and surroundings When we talk about any kind of change, such as a chemical
CRHS Academic Chemistry Unit 15 Thermochemistry HOMEWORK. Due Date Assignment On-Time (100) Late (70)
 Name KEY Period CRHS Academic Chemistry Unit 15 Thermochemistry HOMEWORK Due Date Assignment On-Time (100) Late (70) 15.1 15.2 15.3 15.4 Warm Ups Extra Credit Notes, Homework, Exam Reviews and Their KEYS
Name KEY Period CRHS Academic Chemistry Unit 15 Thermochemistry HOMEWORK Due Date Assignment On-Time (100) Late (70) 15.1 15.2 15.3 15.4 Warm Ups Extra Credit Notes, Homework, Exam Reviews and Their KEYS
- Joule (J): SI unit for energy. It's defined based on the equation for kinetic energy. from. mass. velocity
 153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
Chapter Objectives. Chapter 9 Energy and Chemistry. Chapter Objectives. Energy Use and the World Economy. Energy Use and the World Economy
 Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
Reaction Energy. Thermochemistry
 Reaction Energy Thermochemistry Thermochemistry The study of the transfers of energy as heat that accompany chemical reactions & physical changes Thermochemistry -In studying heat changes, think of defining
Reaction Energy Thermochemistry Thermochemistry The study of the transfers of energy as heat that accompany chemical reactions & physical changes Thermochemistry -In studying heat changes, think of defining
CHEMISTRY 109 #25 - REVIEW
 CHEMISTRY 109 Help Sheet #25 - REVIEW Chapter 4 (Part I); Sections 4.1-4.6; Ch. 9, Section 9.4a-9.4c (pg 387) ** Review the appropriate topics for your lecture section ** Prepared by Dr. Tony Jacob http://www.chem.wisc.edu/areas/clc
CHEMISTRY 109 Help Sheet #25 - REVIEW Chapter 4 (Part I); Sections 4.1-4.6; Ch. 9, Section 9.4a-9.4c (pg 387) ** Review the appropriate topics for your lecture section ** Prepared by Dr. Tony Jacob http://www.chem.wisc.edu/areas/clc
Temp vs. Heat. Absolute Temperature Scales. Common Temperature Scales. Thermal Energy. Heat and Temperature are not the same!!
 Thermal Energy Heat and Temperature are not the same!! Cold is the absence of heat, not an energy Same concept as light/dark Cold can t come in, heat flows out Heat flows from High Temp Low Temp Temp vs.
Thermal Energy Heat and Temperature are not the same!! Cold is the absence of heat, not an energy Same concept as light/dark Cold can t come in, heat flows out Heat flows from High Temp Low Temp Temp vs.
CHEM 1105 S10 March 11 & 14, 2014
 CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
Unit 15 Energy and Thermochemistry Notes
 Name Period CRHS Academic Chemistry Unit 15 Energy and Thermochemistry Notes Quiz Date Exam Date Lab Dates Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic Chemistry Website: https://cincochem.pbworks.com
Name Period CRHS Academic Chemistry Unit 15 Energy and Thermochemistry Notes Quiz Date Exam Date Lab Dates Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic Chemistry Website: https://cincochem.pbworks.com
Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes
 Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes Students will be expected to: Compare the molar enthalpies of several combustion reactions involving organic compounds.
Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes Students will be expected to: Compare the molar enthalpies of several combustion reactions involving organic compounds.
Slide 2 / 118. Thermochemistry
 Slide 1 / 118 Slide 2 / 118 Thermochemistry Slide 3 / 118 Table of Contents The Nature of Energy State Functions** Click on the topic to go to that section Enthalpy Measuring Enthalpy Changes: Calorimetry
Slide 1 / 118 Slide 2 / 118 Thermochemistry Slide 3 / 118 Table of Contents The Nature of Energy State Functions** Click on the topic to go to that section Enthalpy Measuring Enthalpy Changes: Calorimetry
CHM 111 Dr. Kevin Moore
 CHM 111 Dr. Kevin Moore Kinetic Energy Energy of motion E k 1 2 mv 2 Potential Energy Energy of position (stored) Law of Conservation of Energy Energy cannot be created or destroyed; it can only be converted
CHM 111 Dr. Kevin Moore Kinetic Energy Energy of motion E k 1 2 mv 2 Potential Energy Energy of position (stored) Law of Conservation of Energy Energy cannot be created or destroyed; it can only be converted
Thermochemistry, Reaction Rates, & Equillibrium
 Thermochemistry, Reaction Rates, & Equillibrium Reaction Rates The rate at which chemical reactions occur Reaction Rates RXN rate = rate at which reactants change into products over time. This tells you
Thermochemistry, Reaction Rates, & Equillibrium Reaction Rates The rate at which chemical reactions occur Reaction Rates RXN rate = rate at which reactants change into products over time. This tells you
Chapter 5 THERMO. THERMO chemistry. 5.4 Enthalpy of Reactions 5.5 Calorimetry 5.6 Hess s Law 5.7 Enthalpies of Formation
 Chapter 5 THERMO THERMO chemistry 5.4 Enthalpy of Reactions 5.5 Calorimetry 5.6 Hess s Law 5.7 Enthalpies of Formation Chemical Equations 1 st WRITE the Chemical Equation 2 nd BALANCE the Chemical Equation
Chapter 5 THERMO THERMO chemistry 5.4 Enthalpy of Reactions 5.5 Calorimetry 5.6 Hess s Law 5.7 Enthalpies of Formation Chemical Equations 1 st WRITE the Chemical Equation 2 nd BALANCE the Chemical Equation
Physics 231. Topic 13: Heat. Alex Brown Dec 1, MSU Physics 231 Fall
 Physics 231 Topic 13: Heat Alex Brown Dec 1, 2015 MSU Physics 231 Fall 2015 1 8 th 10 pm correction for 3 rd exam 9 th 10 pm attitude survey (1% for participation) 10 th 10 pm concept test timed (50 min))
Physics 231 Topic 13: Heat Alex Brown Dec 1, 2015 MSU Physics 231 Fall 2015 1 8 th 10 pm correction for 3 rd exam 9 th 10 pm attitude survey (1% for participation) 10 th 10 pm concept test timed (50 min))
Chapter 3: Matter and Energy
 Chapter 3: Matter and Energy Convert between Fahrenheit, Celsius, and Kelvin temperature scales. Relate energy, temperature change, and heat capacity. The atoms and molecules that compose matter are in
Chapter 3: Matter and Energy Convert between Fahrenheit, Celsius, and Kelvin temperature scales. Relate energy, temperature change, and heat capacity. The atoms and molecules that compose matter are in
Thermochemistry. Energy and Chemical Change
 Thermochemistry Energy and Chemical Change Energy Energy can change for and flow, but it is always conserved. The Nature of Energy Energy the ability to do work or produce heat Potential energy Kinetic
Thermochemistry Energy and Chemical Change Energy Energy can change for and flow, but it is always conserved. The Nature of Energy Energy the ability to do work or produce heat Potential energy Kinetic
