EPR studies of manganese centers in SrTiO 3 : Non-Kramers Mn 3+ ions and spin-spin coupled Mn 4+ dimers
|
|
|
- Bridget Conley
- 5 years ago
- Views:
Transcription
1 EPR studies of manganese centers in SrTiO 3 : Non-Kramers Mn ions and spin-spin coupled Mn 4+ dimers D V Azamat 1, A Dejneka 1, J Lancok 1, V A Trepakov 1, 2, L Jastrabik 1 and A G Badalyan 2 1 Institute of Physics AS CR, , Prague 8, Czech Republic 2 Ioffe Physical-Technical Institute, Russian Academy of Sciences, 19421, St. Petersburg, Russia azamat@fzu.cz Abstract X- and Q-band electron paramagnetic resonance (EPR) study is reported on the SrTiO 3 single crystals doped with.5-at.% MnO. EPR spectra originating from the S = 2 ground state of Mn ions are shown to belong to the three distinct types of Jahn-Teller centres. The ordering of the oxygen vacancies due to the reduction treatment of the samples and consequent formation of oxygen vacancy associated Mn centres are explained in terms of the localized charge compensation. The EPR spectra of SrTiO 3 : Mn crystals show the presence of next nearest neighbor exchange coupled Mn 4+ pairs in the <11> directions. Keywords: A. inorganic compounds; D. electron paramagnetic resonance (EPR); D. magnetic properties 1. Introduction Transition metal doped perovskites has attracted increasing interest due to a variety of physical phenomena such as superconductivity, colossal magnetoresistance and metal-to-isolator transition [1-4]. Strontium titanate, the so called incipient ferroelectric with perovskite structure, has become in the focus of interest in attempt to combine magnetic and ferroelectric properties of the material [5-11]. In this work we use EPR spectroscopy for the accurate determination of different oxidation states and local
2 environment of manganese impurities in SrTiO 3. Trivalent manganese ions in SrTiO 3 crystals look like a model ones to study exchange-coupled systems in mixed-valent manganese oxides [12-17]. EPR spectra of Mn complexes in oxide crystals have been poorly [18, 19] investigated than those of 3d 4 non- Kramers iron (Fe 4+ ions) [2-22]. These ions exhibit large zero field splittings due to the influence of the Jahn-Teller effect on the 5 E g ground state in octahedral symmetry. An EPR centre attributed to the Mn - V O complex was reported by Serway et al [19], only the transition within M S =2 M S = -2 states of electron spin S = 2 system was observed. A strong axial field is created by a nearest-neighbour oxygen vacancy V O. So far, the lack of information on the Mn centres in SrTiO 3 has precluded the detailed analysis for that system. Owing to the large interest in the characterisation of Mn complexes in perovskites, we decided to perform EPR study on this system. In this paper we report X- and Q-band EPR measurements on SrTiO 3 : Mn single crystals. The EPR spectrum of SrTiO 3 crystals doped with manganese contains resonances from cubic Mn 4+ and Mn 2+ ions as well as from several types of Mn centres. The accurate values of the 2-nd and 4-th rank crystal field tensor components have been obtained for distinct types of Mn centres. The peculiar features of Mn centres were established by the studies of hyperfine interaction with 1% abundant 55 Mn nuclei (nuclear spin of I = 5/2). The spectra of distinct types of Mn centres differ greatly in the magnitude of their 55 Mn hyperfine fields. The careful analysis of spin-spin coupling effects may provide some useful physical information on structural and electronic properties of SrTiO 3. When crystals of SrTiO 3 are heavily doped with fourvalent manganese there is a tendency for the Mn 4+ ions to form exchange coupled pairs. The EPR spectra indicate the formation of Mn 4+ dimer centers lying along the <11> directions (in the next nearest neighbor position). The coupling in the ground state is expected to be ferromagnetic. 2. Experimental procedure The single crystals of SrTiO 3 were grown in Furuuchi Chemical Corporation by use of Verneuil technique through the melting of SrTiO 3 and.5% wt of MnO crystalline powders. Some of the samples were annealed for 6 h at 1 C in Ar/H 2 (5%) reduction atmosphere. EPR measurements were performed with a Bruker ELEXSYS 58 spectrometer at X- and Q-band frequencies in the temperature range 65-3K. 3. Results and Discussion 3.1. Non-Kramers Mn ions
3 In addition to the cubic Mn 4+ (3d 3 ) and Mn 2+ (3d 5 ) spectra, that are already known, the spectra of strong axially distorted Mn (3d 4 ) ions have been found (see Fig. 1). These manganese centers as well as a variety of the other 3d impurities in SrTiO 3 are typically substituted for Ti 4+ sites in the octahedral coordination [11, 19, 23-25]. Table 1. The Spin Hamiltonian parameters of Mn centres in SrTiO 3 single crystals (in 1-4 cm -1, 295K). centre b 2 b b g g A A Mn -V O (I) Mn -X Mn - V O (II) Practically, the studies of the angular dependencies of EPR spectra reveal three types of the Mn centers with the spin S = 2. These centers has axial symmetry with the main axis along to the [1]-type directions. Figure 2 shows the axial Mn centres for applied magnetic field direction B [1]. The Mn spectra consists of 2-2 and 1-1 fine structure transitions within 5 B 1 ground state. We use the following spin Hamiltonian with symmetry D 4h to describe the ground state energy splitting of Mn centres: H = g β B S + A S z I z e z + A + g β e ( Bx S x + B y S y ) ( S I + S I ) z x x y y + B 2 O 2 + B 4 O 4 + B 44 O 4 4 (1) l l O T =, l m l = T m O + ( 1) m T l m where S = 2, the first two terms describes the Zeeman interaction, the last two ones correspond to the hyperfine (HF) interactions; β e is the Bohr magneton; g is the g-tensor; A is the hyperfine (HF) interaction tensor; 2 b 6 B 2 = 2, B = b 4, B = b l 4 are the scalar fine structure constants; are 44 3 T m spherical tensor operators [28] and m b l are Stevens coefficients; x, y, z, the crystallographic axes.
4 Figure 3 shows the angular variation of the spectra measured in the (1) plane at the temperature T=15K. The magnetic multiplicity of EPR spectra K M = 3 corresponds with three possible axial distortions along [1] type of directions. The curves on Fig. 3 have been calculated by diagonalization of the spin Hamiltonian (1) by using a program similar to that described in [28]. The characteristic EPR road map has shown just for centers designated as Mn -V O (II) because the corresponding fine structure transitions of Mn -V O (I) and Mn -X centers follows nearby through the complete angular dependence. The parameters of the spin Hamiltonian (1) for the Mn -V O (I), Mn -X and Mn -V O (II) centers are listed in Table 1. In Fig. 4 it is shown the hyperfine structure of Mn spectra are well resolved at the temperature of 295K and has narrow resonance linewidths of about 1.2 mt. The Mn hyperfine interaction is axially symmetric around a [1] direction as well as g-tensor main axis. The weaker HF interaction is for parallel magnetic-field orientation with respect to the tetragonal axis of the center (the local z axis), while the stronger HF interaction is in the equatorial plane perpendicular to the local z axis of the center. High spin Mn free ion term is 5 D in octahedral environment. It gives 5 E ground state that is not split by spin-orbit coupling at first order of perturbation theory [26, 27]. A strong axial field results in lowering the symmetry to D 4h. This give rise to S = 2 high spin configuration (t 2g ) 3 (e g ) 1 with an orbital singlet 5 B 1 (3z 2 -r 2 ) ground state. The stabilization of ground state 3d(3z 2 -r 2 ) orbital results in the shift the four oxygen ions in the equatorial plane towards the central Mn ion and the moving of two oxygen ions away making elongated octahedral. The inspection of the values of hyperfine structure constants (see Table 1) of the centers show that although the ground state S = 2 is the same as that for Mn the actual spin density of different types of the centers is rather delocalized among surrounding ions due to strong covalent bonding. The anisotropic hyperfine splitting for the 5 B 1 state of different types of Mn centers can be evaluated by use of the values from Table 1. For an 3d(3z 2 -r 2 ) ground state the relations are the following [26, 29-32]: A = P a + PΔg, A = P a + PΔg 3 P = g g β β r, χ e N e 4π ψ S = i N δ ( r ) s i zi 3 3 hca ψ = 2 g e g N β eβ N, (2a) a, (2b) where isotropic contact term χ is the value of the density of unpaired spins at the nucleus; the and symbols denote the parallel and perpendicular components of HF interaction tensor with respect to the local z axis of the center. Because the g and A parameters are smaller than g and A (see Table 1) the ground state 3d(3z 2 -r 2 ) orbital is more likely rather than 3d(x 2 -y 2 ). The adoption of negative signs of
5 hyperfine interaction constants (see Table 1) is a reasonable choice which leads to negative contact term χ coming from the core polarization. By use of the data in Table 1 we find the values of P, a and χ listed in the Table 2 (as evaluated by use of formulae (2)). The P value for Mn - X centre is appreciably smaller than for Mn - V O (I, II) ones. The Mn HF interaction data in SrTiO 3 yields quite different magnitudes of hyperfine structure constants from that found for Mn complexes in titanium oxide (TiO 2 ) crystals [12]. The essential reduction of contact interaction χ values exhibits the strong covalent bonding of the Mn impurities. The main contribution to the contact term comes from the spin polarization of the core s- shells due to the exchange interaction with the d-electron spin states [33, 34]. Let us consider the possible microscopic models of Mn centers (see Fig. 5) based on the present experimental data. All the spectra are characterized by g < g <2 in the cubic phase. Therefore it results 2 2 from 5 BB1(3z -r ) state of Mn ion in the presence of a strong positive axial crystal field. All the Mn centers are very stable at room temperature. The dominant charge compensation process for Mn ions is the formation of the oxygen vacancies at the nearest or next nearest anionic positions along [1] type of directions. We suppose the Mn -V O (I) center is caused by an oxygen vacancy V O in immediate neighborhood of the Mn ion where empty oxygen vacancy stabilize a 3d(3z 2 -r 2 ) orbital of Jahn-Teller active defect. Thus, the EPR spectra of Mn -V O (I) ions of axial symmetry are analogous to the corresponding spectra of Fe with an oxygen vacancy in the nearest environment [35]. Other example of nearest V O charge compensated defect is Ni -V O [36]. The EPR transitions within the ±2> non-kramers doublet of Mn -V O (I) pair centers with the nearest neighbor oxygen vacancy was observed for the first time by Serway et al [19]. It should be pointed out the relative concentrations of the Mn -V O (I) and Mn -V O (II) centers depend on the thermal treatment of the sample in reduced atmosphere. Figure 4 shows the EPR spectra corresponds with 1-1 transition of as grown and additionally reduced manganese-doped SrTiO 3 with the applied magnetic field parallel to the [11] axis. The relative change of EPR intensity of Mn -V O (I) and Mn -V O (II) centers due to additional reduction of the samples supports to conclusion that the charge compensators like oxygen vacancies are mobile defects in respect to the Mn sites (under annealing process). Table 2. Hyperfine coupling parameters of Mn centres in SrTiO 3 and TiO 2 single crystals centre 1 4 P (cm -1 ) 1 4 a (cm -1 ) χ (a.u.) ref.
6 SrTiO 3 : Mn -V O (I) * SrTiO 3 : Mn -X * SrTiO 3 : Mn - V O (II) * TiO 2 : Mn [18] * present study We suppose, there is no oxygen vacancy V O in the immediate neighborhood of the centers designated as Mn -V O (II) in Table 1. In this case, a large axial field term results from a static Jahn-Teller distortion. In the SrTiO 3 structure the isolated oxygen vacancy has two nearest Ti 4+ ions. The formation of neutral oxygen vacancies is accompanied by the trapping of two electrons [37]. In the case of the oxygen vacancy next nearest to Mn ion the Mn - O- Ti - Vo complex is formed with axial distortion along [1] direction. This model is similar to that recently presented for Cr charge compensated complex in SrTiO 3 [38]. Oxygen vacancy concentration could be increased by the annealing treatment of as grown sample in reducing atmosphere. Such procedure results in the relative increase of EPR signal of Mn - V O (I) centers consisting of the nearest-neighbor neutrally charged oxygen vacancy (see Fig. 4). The main factors governing the distribution of trivalent Mn impurities in the SrTiO 3 crystals appear to be electrostatic ones. We propose the tentative model of Mn -X centers as Mn nearest to Nb 5+ ion substituted for Ti site. The Nb traces are than compensated by Mn ions in the samples. A quite weak intensity of EPR signals of Mn -X centers is not visibly changed by the thermal treatment of the samples in reduced atmosphere. This is consistent with assumption that Mn ion is a probable compensator for Nb 5+ donor. Further experimental work shall concentrate on the Mn -X center to confirm the model suggested by the present study Exchange-coupled Mn 4+ pairs In as grown crystals the cubic Mn 4+ (as well as cubic Mn 2+ ) dominates EPR spectrum which indicates that the majority of the Mn 4+ ions enter the lattice as isolated centres. The EPR of isolated Mn 4+ ions have been described [24] by the spin Hamiltonian for a 3d 3 electron system (I=5/2) in octahedral environment: H 2 1 = gβ B S + Dc S z S( S AS I, (3) 3 i )
7 where the axial zero-field splitting D c ~ at room temperature and therefore EPR spectrum is almost isotropic. The spin Hamiltonian parameters are given in Table 3. When two Mn 4+ ions with electron spin S=3/2 are spin-spin coupled the dimer spin states are characterized by the total spin quantum number Σ S 1 + S =, 1, 2, 3 [26, 32, 39]. The EPR spectra of SrTiO3 crystals heavily doped with = 2 Mn 4+ Ti (3d 3 ) contain resonance lines which can be assigned to the total electron spin quantum number Σ = 1of exchange-coupled pair. Figure 2 show the spectrum of Mn 4+ - Mn 4+ in SrTiO 3 under applied magnetic field parallel to [1] direction. The isolated ion spectrum of Mn 4+ is very much more intense than the pair spectrum. Suggesting the isotropic part of exchange energy is large compared to the other magnetic interactions the X-band EPR angular dependence from the Σ = 1 state can be well described by the spin Hamiltonian [39]: H 2 2 [ Σ Σ ] + Σ A ( ) 2 1 βb g Σ + DΣ Σ z Σ( Σ + 1) + E x y I 1 + I 2 3 = Σ, (4a) D = 3 α Σ D e β Σ D c, EΣ = α Σ E e + β Σ Ec, Σ + α 1 = 17, 1 β 1 = 12 (4b) 5 where Σ represent spin operators for the exchange-coupled Mn 4+ ions, the first term describe the electron Zeeman interaction, the second and third ones describes the axial and orthorhombic second rank fine splitting, A are the magnetic hyperfine interaction constants of the 1% abundant isotope 55 Mn, I 1 =I 2 =5/2. The main axes z, x and y of second rank fine structure tensor are parallel to [ ], 11 [ 1] 1 and [1] directions respectively. If the Mn 4+ is located along a [11] direction relative to the other substitutional Mn 4+ ion than it generates an orthorhombic field and is related to six other symmetry conjugated sites. The spin Hamiltonian parameters for the Σ = 1 transitions presented in Table 3 were derived from angular dependences of EPR spectra recorded with the magnetic field varied in (1) and (11) planes. As shown on Fig. 6 the angular dependence of Σ = 1 is strongly defined by the both D 1 and E 1 terms. We have found that E D 1/3 that means there is maximum rhombic splitting and the 1 / 1 components of corresponding Cartesian second rank fine tensor D are the following [26]: D = D1 xx + E 3 1, D D1 2D1 yy = E 3 1, D 2D 1 3 zz = (5) 3 For this dimer centers which exhibits isotropic g-factor the anisotropy of exchange interaction is mainly caused by dipole-dipole interaction. If we adopt the point dipole approximation for the Mn 4+ ions constituting the pair then the value of D e is defined as follows: D g e = β 3, where R is interionic R distance. By use of formulas (4b) and suggesting that axial splitting of isolated Mn 4+ ion D c ~ we obtain 2 2
8 the room temperature value of D e ~-.147 cm -1 and corresponding distance of approximately 4.9 ο Α. This value is significantly reduced from the separation of ~5.5 ο Α between nearest regular Ti 4+ sites located along <11> direction. These findings are confirmed experimentally by the observation of characteristic hyperfine structure of the exchange-coupled pair complexes as shown on Fig.7. The hyperfine structure consist of 11 lines with hyperfine splitting constant half that of the isolated Mn 4+ ions and with intensity ratio 1:2:3:4:5:6:5:4:3:2:1 expected for identical Mn 4+ ions coupled by an isotropic exchange interaction larger than the hyperfine interaction. The intensities of the Σ = 1 transitions are difficult to follow as a function of temperature and therefore the energy intervals between the spin states Σ were not evaluated. Table 3. The Spin Hamiltonian parameters of single Mn 4+ centres and Mn 4+ - Mn 4+ ( Σ = 1) exchangecoupled pairs in SrTiO3 single crystals (in 1-4 cm -1 ), T=295K. DΣ=1 E Σ= 1 centre g A ref. Mn [24] Mn 4+ - Mn * * present study The analysis of the angular variation of EPR spectra of Mn 4+ dimers provides a basis for constructing the model of exchange coupled pairs in SrTiO 3 lattice. The structure of next nearest neighbor pair site, shown on Fig. 8, has point group symmetry D 2h in a cubic phase of SrTiO 3. The pairs consist of two Mn 4+ ions which are substituted for nearest Ti 4+ sites located along one of the <11> crystal directions. These measurements do not provide complete information related to the energy level sequence of Mn 4+ - Mn 4+ pairs but the data obtained do suggest that the Σ = 1 state is higher in energy in comparison to the other Σ = 2, 3 states. The magnetic interactions must be transmitted through pathways involving three intervening ions: one titanium and two oxygen atoms. In this case ferromagnetic right angle superexchange interaction is expected because of Mn-O bonds in perovskite structure. 4. Conclusions The detailed studies have performed on the several complexes of high spin Mn ions. We were able to define the sign and value of the axial fine structure parameters b 2 that is important to elucidate the defect
9 structure at the Mn site. The tetragonally elongated octahedron coming from Jahn-Teller distortions of a local site of Mn centers have been ascertained from EPR data. Spin density at the nucleus for manganese in the distorted octahedral coordination has been found considerably different for various types of the centers. A variety of complex Mn ions detected in SrTiO 3 crystals has been ascribed to differences in charge compensation. These variations are largely due to the techniques used to grow and dope the SrTiO 3 single crystals. Our results show the formation of exchange coupled Mn 4+ dimers substituted for the second nearest neighbor Ti 4+ sites in SrTiO 3 crystals. Acknowledgements This work was supported by the Operational Program Research and Development for Innovations - European Social Fund (project CZ.1.5/2.1./3.58 of the Ministry of Education, Youth and Sports of the Czech Republic), project SAFMAT CZ.2.16/3.1./22132, grant 22/9/J17 of GACR and grant No of the TACR, by the PP RAS "Quantum physics of condensed Matter" and by the RFBR No , by the Ministry of Education and Science under Contract No , by the programs of RAS: Spintronics. References [1] Bednorz J G and Müller K A 1986 Z. Phys. B: Condens. Matter [2] von Helmolt R, Wecker J, Holzapfel B, Schultz L and Samwer K 1993 Phys. Rev. Lett [3] Imada M, Fujimori A and Tokura Y 1998 Rev. Mod. Phys [4] Waser R and Aono M 27 Nature Mater [5] Alvarado S F, La Mattina F and Bednorz J G 27 Appl. Phys. A: Mater. Sci. Process [6] La Mattina F, Bednorz J G, Alvarado S F, Shengelaya A and Keller H 28 Appl. Phys. Lett [7] Khomskii D 29 Physics 2 2 [8] Nagaev E L 21 Phys. Rep [9] Savinov M, Trepakov V A, Syrnikov P P, Zelezny V, Pokorny J, Dejneka A, Jastrabık L and Galinetto P 28 J. Phys. Condens. Matter [1] Badalyan A G, Syrnikov P P, Azzoni C B, Galinetto P, Mozzati M C, Rosa J, Trepakov V A and Jastrabik L 28 J. Appl. Phys [11] Müller K A and Fayet J C 1991 Structural Phase Transitions II eds K A Müller and H Thomas (Berlin-Heidelberg: Springer) pp 1-82 [12] Kanamori J 1959 J. Phys. Chem. Solids 1 87
10 [13] Shengelaya A, Zhao Guo-meng, Keller H, Müller K A and Kochelaev B I 2 Phys. Rev. B [14] Coey J M D, Viret M and von Molnár S 1999 Adv. Phys [15] Dagotto E, Hotta V and Moreo A 21 Phys. Rep [16] Kvyatkovskii O E 211 Crystallogr. Rep [17] Inoue J 1998 Phil. Trans. R. Soc. Lond. A [18] Gerritsen H J and Sabisky F S 1963 Phys. Rev [19] Serway R A, Berlinger W, Müller K A and Collins R W 1977 Phys. Rev. B [2] Schirmer O F, Berlinger W and Müller K A 1975 Solid State Commun [21] Faust B, Reyher H-J, Schirmer O F 1996 Solid State Commun [22] Azamat D V, Basun S A, Bursian V E, Razdobarin A G, Sochava L S, Hesse H and Kapphan S 1999 Phys. Solid State [23] Blazey K W, Cabrera J M and Müller K A 1983 Solid State Commun [24] Müller K A 1959 Phys. Rev. Lett [25] Müller K A 1981 J. Phys. (Paris) [26] Abragam A and Bleaney B 197 Electron Paramagnetic Resonance of Transition Ions (Oxford: Clarendon) [27] Griffith J S 1964 The Theory of Transition-Metal Ions (London: Cambridge University) [28] Grachev V G 1987 Sov. Phys. JETP [29] Abragam A and Pryce M H L 1951 Proc. Roy. Soc. (London) [3] McGarvey B R 1967 J. Phys. Chem [31] McGarvey B R 1966 Transition Metal Chemistry vol 3, ed R L Carlin (New York: Marcel Dekker) pp 9-21 [32] Al'tshuler S A and Kozyrev B M 1974 Electron Paramagnetic Resonance in Compounds of Transition Elements (New York: Wiley) [33] Watson R E and Freeman A J 1967 Hyperfine interactions eds A J Freeman and R B Frankel (New York London: Academic Press) pp [34] Simanek E and Müller K A 197 J. Phys. Chem. Solids [35] von Waldkirch Th, Müller K A and Berlinger W 1972 Phys. Rev. B [36] Müller K A, Berlinger W and Rubins R S 1961 Phys. Rev [37] Ricci D, Bano G and Pacchioni G 23 Phys. Rev B [38] La Mattina F, Bednorz J G, Alvarado S F, Shengelaya A, Müller K A and Keller H 29 Phys. Rev. B
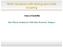
11 [39] Owen J and Harris E A 1972 Electron Paramagnetic Resonance ed S Geschwind (New York: Plenum Press) pp Mn 2+ Mn 4+ Mn -VO(II) B [11] Mn -VO(I) EPR signal dp abs /db (arb. units) Mn -X GHz x magnetic field ( mt) Figure 1. Q-band EPR spectra of SrTiO 3 :Mn showing the cubic Mn 4+ and Mn 2+ as well as charge compensated Mn ions. B [1], T = 295K. EPR spectrum of axial Mn centres is enlarged by x75 in comparison to one of the cubic Mn 4+ and Mn 2+. The resolved hyperfine structure shown by the cropped lines is due to the interaction with the 55 Mn nuclei.
12 Mn -X Mn -VO(I) Mn -VO(II) EPR signal dp abs /db (arb. units) 2 +2 B [1] Mn - Mn 1 +1 Mn 2+, 4 Mn magnetic field ( mt) Figure 2. X-band EPR spectrum of the charge compensated Mn ions and Mn 4+ dimers when applied magnetic field parallel to [1] direction, microwave frequency of 9.24 GHz, T = 295K. EPR spectra of cubic Mn 2+ and Mn 4+ ions are cut because of their extremely strong intensity. The fine structure transitions are shown by the cropped lines.
13 B [11] 5 4 ( ) angle degree B [1] 3 O SrTiO : Mn -V (II) magnetic field ( mt) Figure 3. The EPR road map of the center of gravity of Mn -V O (II) resonance lines when applied magnetic field varies in the (1) plane, microwave frequency of 9.26 GHz, T = 15 K. The lines are the fine structure transitions calculated by exact diagonalization of spin Hamiltonian (1). The cycles show the experimental points.
14 a Mn -VO(II) Mn -VO(I) EPR signal dp abs /db (arb. units) b Mn -X B [11] magnetic field ( mt) Figure 4. EPR spectrum of as grown (a) and additionally reduced (b) manganese-doped SrTiO 3 with the applied magnetic field parallel to the [11] axis, microwave frequency of 9.25 GHz, T = 295 K. The spectrum consists of lines corresponding to the 1-1 transition within 5 BB1 ground state of distinct types of Mn centers. The six fold splitting induced by the hyperfine interaction with the Mn nuclear spin I = 5/2 is shown by the cropped lines. The increase of intensity of Mn -VO(I) resonance lines in comparison to Mn -V O(II) once became apparent from these spectra.
15 a Mn -V O (I) b Mn -X c Mn -V O (II) Ti 4+ V O Nb 5+ Mn O 2- Figure 5. Possible models of charge compensated (a) Mn -V O (I), (b) Mn -X and (c) Mn -V O (II) centers in SrTiO 3 structure including the probable presence of a charge compensator along a [1] direction in the first, second (Nb 5+ Ti) and third coordination sphere.
16 B [1] Mn - Mn ( ) angle degree B [1] magnetic field ( mt) Figure 6. The road map of the center of gravity of EPR lines of Σ = 1 transitions from the Mn 4+ - Mn 4+ exchange-coupled pairs when applied magnetic field varies in the (1) plane, T = 295 K. The lines are the calculated positions of the resonances. EPR signal dp abs /db (arb. units) Mn - Mn B [1] 9.26 GHz 12 K magnetic field ( mt) Figure 7. Hyperfine structure in the EPR spectrum of the next nearest neighbor exchange-coupled Mn 4+ - Mn 4+ pairs. This structure could be uniquely assigned to two equivalent Mn 4+ ions (I=5/2).
17 Mn 4+ -Mn 4+ Sr 2+ Ti 4+ O 2- Mn 4+ Figure 8. Model of exchange-coupled Mn 4+ pairs in the cubic phase of SrTiO 3 with two Mn 4+ ions which are substituted for nearest Ti 4+ sites located along one of the <11> directions. The magnetic dipolar interaction between Mn 4+ ions gives rise to anisotropy of exchange interaction.
EPR of photochromic Mo 3+ in SrTiO 3
 EPR of photochromic Mo 3+ in SrTiO 3 Th. W. Kool Van t Hoff Institute for Molecular Sciences, University of Amsterdam NL 1018 WV Amsterdam, the Netherlands March 2010 Abstract In single crystals of SrTiO
EPR of photochromic Mo 3+ in SrTiO 3 Th. W. Kool Van t Hoff Institute for Molecular Sciences, University of Amsterdam NL 1018 WV Amsterdam, the Netherlands March 2010 Abstract In single crystals of SrTiO
OBSERVATION OF Se 77 SUPERHYPERFINE STRUCTURE ON THE ELECTRON-PARAMAGNETIC RESONANCE OF Fe3+ (3d S ) IN CUBIC ZnSe
 R540 Philips Res. Repts 20, 206-212, 1965 OBSERVATION OF Se 77 SUPERHYPERFINE STRUCTURE ON THE ELECTRON-PARAMAGNETIC RESONANCE OF Fe3+ (3d S ) IN CUBIC ZnSe by J. DIELEMAN Abstract The electron-paramagnetic-resonance
R540 Philips Res. Repts 20, 206-212, 1965 OBSERVATION OF Se 77 SUPERHYPERFINE STRUCTURE ON THE ELECTRON-PARAMAGNETIC RESONANCE OF Fe3+ (3d S ) IN CUBIC ZnSe by J. DIELEMAN Abstract The electron-paramagnetic-resonance
ESR spectroscopy of catalytic systems - a primer
 ESR spectroscopy of catalytic systems - a primer Thomas Risse Fritz-Haber-Institute of Max-Planck Society Department of Chemical Physics Faradayweg 4-6 14195 Berlin T. Risse, 11/6/2007, 1 ESR spectroscopy
ESR spectroscopy of catalytic systems - a primer Thomas Risse Fritz-Haber-Institute of Max-Planck Society Department of Chemical Physics Faradayweg 4-6 14195 Berlin T. Risse, 11/6/2007, 1 ESR spectroscopy
Calculation of the zero-field splitting D and g parameters in EPR for d 3 spin systems in strong and moderate axial fields
 Calculation of the zero-field splitting D and g parameters in EPR for d 3 spin systems in strong and moderate axial fields Th. W. Kool 1 and B. Bollegraaf 2 1 Van t Hoff Institute for Molecular Sciences,
Calculation of the zero-field splitting D and g parameters in EPR for d 3 spin systems in strong and moderate axial fields Th. W. Kool 1 and B. Bollegraaf 2 1 Van t Hoff Institute for Molecular Sciences,
EPR in Kagome Staircase Compound Mg Co V 2 O 8
 Vol. 111 (2007) ACTA PHYSICA POLONICA A No. 1 Proceedings of the Symposium K: Complex Oxide Materials for New Technologies of E-MRS Fall Meeting 2006, Warsaw, September 4 8, 2006 EPR in Kagome Staircase
Vol. 111 (2007) ACTA PHYSICA POLONICA A No. 1 Proceedings of the Symposium K: Complex Oxide Materials for New Technologies of E-MRS Fall Meeting 2006, Warsaw, September 4 8, 2006 EPR in Kagome Staircase
ESR spectroscopy of catalytic systems - a primer
 ESR spectroscopy of catalytic systems - a primer Thomas Risse Fritz-Haber-Institute of Max-Planck Society Department of Chemical Physics Faradayweg 4-6 14195 Berlin T. Risse, 3/22/2005, 1 ESR spectroscopy
ESR spectroscopy of catalytic systems - a primer Thomas Risse Fritz-Haber-Institute of Max-Planck Society Department of Chemical Physics Faradayweg 4-6 14195 Berlin T. Risse, 3/22/2005, 1 ESR spectroscopy
EPR Studies of Cu 2+ in dl-aspartic Acid Single Crystals
 EPR Studies of Cu 2+ in dl-aspartic Acid Single Crystals B. Karabulut, R. Tapramaz, and A. Bulut Ondokuz Mayis University, Faculty of Art and Sciences, Department of Physics, 55139 Samsun, Turkey Z. Naturforsch.
EPR Studies of Cu 2+ in dl-aspartic Acid Single Crystals B. Karabulut, R. Tapramaz, and A. Bulut Ondokuz Mayis University, Faculty of Art and Sciences, Department of Physics, 55139 Samsun, Turkey Z. Naturforsch.
Electron spin resonance investigation of Mn 2+ ions and their dynamics in manganese doped SrTiO 3
 Electron spin resonance investigation of Mn + ions and their dynamics in manganese doped SrTiO 3 V.V. Laguta, 1,3 I.V. Kondakova, 1 I.P. Bykov, 1 M.D. Glinchuk, 1 P.M. Vilarinho, A. Tkach, L.Jastrabik
Electron spin resonance investigation of Mn + ions and their dynamics in manganese doped SrTiO 3 V.V. Laguta, 1,3 I.V. Kondakova, 1 I.P. Bykov, 1 M.D. Glinchuk, 1 P.M. Vilarinho, A. Tkach, L.Jastrabik
Magnetic Oxides. Gerald F. Dionne. Department of Materials Science and Engineering Massachusetts Institute of Technology
 Magnetic Oxides Gerald F. Dionne Department of Materials Science and Engineering Massachusetts Institute of Technology Spins in Solids Summer School University of Virginia Charlottesville, VA 21 June 2006
Magnetic Oxides Gerald F. Dionne Department of Materials Science and Engineering Massachusetts Institute of Technology Spins in Solids Summer School University of Virginia Charlottesville, VA 21 June 2006
EPR Study of the Dynamic Jahn-Teller Effect of Cu2+ in CdBa(HC00) 4-2H 2 0 Single Crystals
 EPR Study of the Dynamic Jahn-Teller Effect of Cu2+ in CdBa(HC00) 4-2H 2 0 Single Crystals Hüseyin Kalkan, Sehriman Atalay, and Ismet Senel Department of Physics, Faculty of Arts and Sciences, Ondokuz
EPR Study of the Dynamic Jahn-Teller Effect of Cu2+ in CdBa(HC00) 4-2H 2 0 Single Crystals Hüseyin Kalkan, Sehriman Atalay, and Ismet Senel Department of Physics, Faculty of Arts and Sciences, Ondokuz
N-V Si -related center in non-irradiated 6H SiC nanostructure
 N-V Si -related center in non-irradiated 6H SiC nanostructure Nikolay Bagraev a, Eduard Danilovskii a, Dmitrii Gets a, Ekaterina Kalabukhova b, Leonid Klyachkin a, Anna Malyarenko a, Dariya Savchenko b,c
N-V Si -related center in non-irradiated 6H SiC nanostructure Nikolay Bagraev a, Eduard Danilovskii a, Dmitrii Gets a, Ekaterina Kalabukhova b, Leonid Klyachkin a, Anna Malyarenko a, Dariya Savchenko b,c
Introduction to Electron Paramagnetic Resonance Spectroscopy
 Introduction to Electron Paramagnetic Resonance Spectroscopy Art van der Est, Department of Chemistry, Brock University St. Catharines, Ontario, Canada 1 EPR Spectroscopy EPR is magnetic resonance on unpaired
Introduction to Electron Paramagnetic Resonance Spectroscopy Art van der Est, Department of Chemistry, Brock University St. Catharines, Ontario, Canada 1 EPR Spectroscopy EPR is magnetic resonance on unpaired
Magnetic resonance in Pb x Nb y O z -ceramics as a system containing chemical fluctuation regions
 Magnetic resonance in Pb x Nb y O z -ceramics as a system containing chemical fluctuation regions V.S. Vikhnin, H.R. Asatryan, R.I. Zakharchenya, A.B. Kutsenko, S.E. Kapphan A.F. Ioffe Physical-Technical
Magnetic resonance in Pb x Nb y O z -ceramics as a system containing chemical fluctuation regions V.S. Vikhnin, H.R. Asatryan, R.I. Zakharchenya, A.B. Kutsenko, S.E. Kapphan A.F. Ioffe Physical-Technical
FORBIDDEN HYPERFINE TRANSITIONS IN ELECTRON SPIN RESONANCE OF Mn 2+ IN NaCl SINGLE CRYSTAL
 FORBIDDEN HYPERFINE TRANSITIONS IN ELECTRON SPIN RESONANCE OF Mn 2+ IN NaCl SINGLE CRYSTAL BY K. N. SHRIVASTAVA AND P'UTCHA VENKATESWARLU, F.A.Sc. (Department of Physics, Indian Institute of Technology,
FORBIDDEN HYPERFINE TRANSITIONS IN ELECTRON SPIN RESONANCE OF Mn 2+ IN NaCl SINGLE CRYSTAL BY K. N. SHRIVASTAVA AND P'UTCHA VENKATESWARLU, F.A.Sc. (Department of Physics, Indian Institute of Technology,
Investigations of the electron paramagnetic resonance spectra of VO 2+ in CaO Al 2 O 3 SiO 2 system
 PRAMANA c Indian Academy of Sciences Vol. 73, No. 6 journal of December 2009 physics pp. 1087 1094 Investigations of the electron paramagnetic resonance spectra of VO 2+ in CaO Al 2 O 3 SiO 2 system Q
PRAMANA c Indian Academy of Sciences Vol. 73, No. 6 journal of December 2009 physics pp. 1087 1094 Investigations of the electron paramagnetic resonance spectra of VO 2+ in CaO Al 2 O 3 SiO 2 system Q
(Me=Mg, Zn) compounds. Solid State Physics, Department of Physics, University of Athens, Zografos, Athens, Greece
 EPR Rev.Adv.Mater.Sci. study of the Me14(7) 2 125-129 (Me=Mg, Zn) compounds 125 EPR STUDY OF THE COMPOUNDS (Me = Mg, Zn) Nikolaos Guskos 1,2, Grzegorz Zolnierkiewicz 2, Janusz Typek 2 and Monika Bosacka
EPR Rev.Adv.Mater.Sci. study of the Me14(7) 2 125-129 (Me=Mg, Zn) compounds 125 EPR STUDY OF THE COMPOUNDS (Me = Mg, Zn) Nikolaos Guskos 1,2, Grzegorz Zolnierkiewicz 2, Janusz Typek 2 and Monika Bosacka
Chapter 20 d-metal complexes: electronic structures and properties
 CHEM 511 Chapter 20 page 1 of 21 Chapter 20 d-metal complexes: electronic structures and properties Recall the shape of the d-orbitals... Electronic structure Crystal Field Theory: an electrostatic approach
CHEM 511 Chapter 20 page 1 of 21 Chapter 20 d-metal complexes: electronic structures and properties Recall the shape of the d-orbitals... Electronic structure Crystal Field Theory: an electrostatic approach
Appendix II - 1. Figure 1: The splitting of the spin states of an unpaired electron
 Appendix II - 1 May 2017 Appendix II: Introduction to EPR Spectroscopy There are several general texts on this topic, and this appendix is only intended to give you a brief outline of the Electron Spin
Appendix II - 1 May 2017 Appendix II: Introduction to EPR Spectroscopy There are several general texts on this topic, and this appendix is only intended to give you a brief outline of the Electron Spin
NMR Shifts. I Introduction and tensor/crystal symmetry.
 NMR Shifts. I Introduction and tensor/crystal symmetry. These notes were developed for my group as introduction to NMR shifts and notation. 1) Basic shift definitions and notation: For nonmagnetic materials,
NMR Shifts. I Introduction and tensor/crystal symmetry. These notes were developed for my group as introduction to NMR shifts and notation. 1) Basic shift definitions and notation: For nonmagnetic materials,
Introduction to Heisenberg model. Javier Junquera
 Introduction to Heisenberg model Javier Junquera Most important reference followed in this lecture Magnetism in Condensed Matter Physics Stephen Blundell Oxford Master Series in Condensed Matter Physics
Introduction to Heisenberg model Javier Junquera Most important reference followed in this lecture Magnetism in Condensed Matter Physics Stephen Blundell Oxford Master Series in Condensed Matter Physics
Investigations of the EPR parameters for the interstitial V 4+ in. anatase from two microscopic spin Hamiltonian methods
 Available online at www.scholarsresearchlibrary.com Scholars Research Library Archives of Physics Research, 010, 1 (4): 97-103 (http://scholarsresearchlibrary.com/archive.html) ISSN 0976-0970 CODEN (USA):
Available online at www.scholarsresearchlibrary.com Scholars Research Library Archives of Physics Research, 010, 1 (4): 97-103 (http://scholarsresearchlibrary.com/archive.html) ISSN 0976-0970 CODEN (USA):
A trigonal prismatic mononuclear cobalt(ii) complex showing single-molecule magnet behavior
 Supplementary information for A trigonal prismatic mononuclear cobalt(ii) complex showing single-molecule magnet behavior by Valentin V. Novikov*, Alexander A. Pavlov, Yulia V. Nelyubina, Marie-Emmanuelle
Supplementary information for A trigonal prismatic mononuclear cobalt(ii) complex showing single-molecule magnet behavior by Valentin V. Novikov*, Alexander A. Pavlov, Yulia V. Nelyubina, Marie-Emmanuelle
High T C copper oxide superconductors and CMR:
 High T C copper oxide superconductors and CMR: Ram Seshadri (seshadri@mrl.ucsb.edu) The Ruddlesden-Popper phases: Ruddlesden-Popper phases are intergrowths of perovskite slabs with rock salt slabs. First
High T C copper oxide superconductors and CMR: Ram Seshadri (seshadri@mrl.ucsb.edu) The Ruddlesden-Popper phases: Ruddlesden-Popper phases are intergrowths of perovskite slabs with rock salt slabs. First
Lecture 6: Physical Methods II. UV Vis (electronic spectroscopy) Electron Spin Resonance Mossbauer Spectroscopy
 Lecture 6: Physical Methods II UV Vis (electronic spectroscopy) Electron Spin Resonance Mossbauer Spectroscopy Physical Methods used in bioinorganic chemistry X ray crystallography X ray absorption (XAS)
Lecture 6: Physical Methods II UV Vis (electronic spectroscopy) Electron Spin Resonance Mossbauer Spectroscopy Physical Methods used in bioinorganic chemistry X ray crystallography X ray absorption (XAS)
Investigation of the Local Lattice Structure and the Effects of the Orbital Reduction Factor on the g Factors of a Trigonal [Ni(H 2
 Investigation of the Local Lattice Structure and the Effects of the Orbital Reduction Factor on the g Factors of a Trigonal [Ni(H 2 O) 6 ] 2+ Cluster in NiTiF 6 6H 2 O and ZnSiF 6 6H 2 O Crystals at Different
Investigation of the Local Lattice Structure and the Effects of the Orbital Reduction Factor on the g Factors of a Trigonal [Ni(H 2 O) 6 ] 2+ Cluster in NiTiF 6 6H 2 O and ZnSiF 6 6H 2 O Crystals at Different
Material Science II. d Electron systems
 Material Science II. d Electron systems 1. Electronic structure of transition-metal ions (May 23) 2. Crystal structure and band structure (June 13) 3. Mott s (June 20) 4. Metal- transition (June 27) 5.
Material Science II. d Electron systems 1. Electronic structure of transition-metal ions (May 23) 2. Crystal structure and band structure (June 13) 3. Mott s (June 20) 4. Metal- transition (June 27) 5.
CHEM1902/ N-2 November 2014
 CHEM1902/4 2014-N-2 November 2014 The cubic form of boron nitride (borazon) is the second-hardest material after diamond and it crystallizes with the structure shown below. The large spheres represent
CHEM1902/4 2014-N-2 November 2014 The cubic form of boron nitride (borazon) is the second-hardest material after diamond and it crystallizes with the structure shown below. The large spheres represent
Internal friction and Jahn-Teller effect in the charge-ordered
 Internal friction and Jahn-Teller effect in the charge-ordered La 1-x Ca x MnO 3 (0.5 x 0.87) R. K. Zheng, R. X. Huang, A. N. Tang, G. Li, X. G. Li a) Structure Research Laboratory, Department of Materials
Internal friction and Jahn-Teller effect in the charge-ordered La 1-x Ca x MnO 3 (0.5 x 0.87) R. K. Zheng, R. X. Huang, A. N. Tang, G. Li, X. G. Li a) Structure Research Laboratory, Department of Materials
e 2m e c I, (7.1) = g e β B I(I +1), (7.2) = erg/gauss. (7.3)
 Chemistry 126 Molecular Spectra & Molecular Structure Week # 7 Electron Spin Resonance Spectroscopy, Supplement Like the hydrogen nucleus, an unpaired electron in a sample has a spin of I=1/2. The magnetic
Chemistry 126 Molecular Spectra & Molecular Structure Week # 7 Electron Spin Resonance Spectroscopy, Supplement Like the hydrogen nucleus, an unpaired electron in a sample has a spin of I=1/2. The magnetic
Assessment of Variation in Zero Field Hall Constant of Colossal Magnetoresistive Manganites (Re1-x AxMnO3)
 ESSENCE - International Journal for Environmental Rehabilitation and Conservation Panwar & Kumar/VIII [2] 2017/103 107 Volume VIII [2] 2017 [103 107] [ISSN 0975-6272] [www.essence-journal.com] Assessment
ESSENCE - International Journal for Environmental Rehabilitation and Conservation Panwar & Kumar/VIII [2] 2017/103 107 Volume VIII [2] 2017 [103 107] [ISSN 0975-6272] [www.essence-journal.com] Assessment
Ferroelectricity, Magnetism, and Multiferroicity. Kishan K. Sinha Xu Lab Department of Physics and astronomy University of Nebraska-Lincoln
 Ferroelectricity, Magnetism, and Multiferroicity Kishan K. Sinha Xu Lab Department of Physics and astronomy University of Nebraska-Lincoln Magnetism, Ferroelectricity, and Multiferroics Magnetism o Spontaneous
Ferroelectricity, Magnetism, and Multiferroicity Kishan K. Sinha Xu Lab Department of Physics and astronomy University of Nebraska-Lincoln Magnetism, Ferroelectricity, and Multiferroics Magnetism o Spontaneous
Journal of the Korean Magnetic Resonance Society 2003, 7, Kwangju, , KOREA Received September 29, 2003
 Journal of the Korean Magnetic Resonance Society 2003, 7, 80-88 11 B Nuclear Magnetic Resonance Study of Calcium-hexaborides B. J. Mean 1, K. H. Lee 1, K. H. Kang 1, Moohee Lee 1*, J.S. Lee 2, and B. K.
Journal of the Korean Magnetic Resonance Society 2003, 7, 80-88 11 B Nuclear Magnetic Resonance Study of Calcium-hexaborides B. J. Mean 1, K. H. Lee 1, K. H. Kang 1, Moohee Lee 1*, J.S. Lee 2, and B. K.
7.2 Dipolar Interactions and Single Ion Anisotropy in Metal Ions
 7.2 Dipolar Interactions and Single Ion Anisotropy in Metal Ions Up to this point, we have been making two assumptions about the spin carriers in our molecules: 1. There is no coupling between the 2S+1
7.2 Dipolar Interactions and Single Ion Anisotropy in Metal Ions Up to this point, we have been making two assumptions about the spin carriers in our molecules: 1. There is no coupling between the 2S+1
Theoretical studies on the electron paramagnetic resonance parameters for the tetragonal VO 2+ center in CaO-Al 2 O 3 -SiO 2 system
 RESEARCH Revista Mexicana de Física 64 (2018) 13 17 JANUARY-FEBRUARY 2018 Theoretical studies on the electron paramagnetic resonance parameters for the tetragonal VO 2+ center in CaO-Al 2 O 3 -SiO 2 system
RESEARCH Revista Mexicana de Física 64 (2018) 13 17 JANUARY-FEBRUARY 2018 Theoretical studies on the electron paramagnetic resonance parameters for the tetragonal VO 2+ center in CaO-Al 2 O 3 -SiO 2 system
What so special about LaAlO3/SrTiO3 interface? Magnetism, Superconductivity and their coexistence at the interface
 What so special about LaAlO3/SrTiO3 interface? Magnetism, Superconductivity and their coexistence at the interface Pramod Verma Indian Institute of Science, Bangalore 560012 July 24, 2014 Pramod Verma
What so special about LaAlO3/SrTiO3 interface? Magnetism, Superconductivity and their coexistence at the interface Pramod Verma Indian Institute of Science, Bangalore 560012 July 24, 2014 Pramod Verma
Materials 218/UCSB: Superconductivity and High T C copper oxide superconductors:
 Materials 218/UCSB: Superconductivity and High T C copper oxide superconductors: Ram Seshadri (seshadri@mrl.ucsb.edu) The Ruddlesden-Popper phases: Ruddlesden-Popper phases are intergrowths of perovskite
Materials 218/UCSB: Superconductivity and High T C copper oxide superconductors: Ram Seshadri (seshadri@mrl.ucsb.edu) The Ruddlesden-Popper phases: Ruddlesden-Popper phases are intergrowths of perovskite
Magnetic Resonance Spectroscopy
 INTRODUCTION TO Magnetic Resonance Spectroscopy ESR, NMR, NQR D. N. SATHYANARAYANA Formerly, Chairman Department of Inorganic and Physical Chemistry Indian Institute of Science, Bangalore % I.K. International
INTRODUCTION TO Magnetic Resonance Spectroscopy ESR, NMR, NQR D. N. SATHYANARAYANA Formerly, Chairman Department of Inorganic and Physical Chemistry Indian Institute of Science, Bangalore % I.K. International
A Cu-Zn-Cu-Zn heterometallomacrocycle shows significant antiferromagnetic coupling between paramagnetic centres mediated by diamagnetic metal
 Electronic Supplementary Information to A Cu-Zn-Cu-Zn heterometallomacrocycle shows significant antiferromagnetic coupling between paramagnetic centres mediated by diamagnetic metal Elena A. Buvaylo, a
Electronic Supplementary Information to A Cu-Zn-Cu-Zn heterometallomacrocycle shows significant antiferromagnetic coupling between paramagnetic centres mediated by diamagnetic metal Elena A. Buvaylo, a
Reading. What is EPR (ESR)? Spectroscopy: The Big Picture. Electron Paramagnetic Resonance: Hyperfine Interactions. Chem 634 T.
 Electron Paramagnetic Resonance: yperfine Interactions hem 63 T. ughbanks Reading Drago s Physical Methods for hemists is still a good text for this section; it s available by download (zipped, password
Electron Paramagnetic Resonance: yperfine Interactions hem 63 T. ughbanks Reading Drago s Physical Methods for hemists is still a good text for this section; it s available by download (zipped, password
Nuclear Quadrupole Resonance Spectroscopy. Some examples of nuclear quadrupole moments
 Nuclear Quadrupole Resonance Spectroscopy Review nuclear quadrupole moments, Q A negative value for Q denotes a distribution of charge that is "football-shaped", i.e. a sphere elongated at the poles; a
Nuclear Quadrupole Resonance Spectroscopy Review nuclear quadrupole moments, Q A negative value for Q denotes a distribution of charge that is "football-shaped", i.e. a sphere elongated at the poles; a
Competing Ferroic Orders The magnetoelectric effect
 Competing Ferroic Orders The magnetoelectric effect Cornell University I would found an institution where any person can find instruction in any study. Ezra Cornell, 1868 Craig J. Fennie School of Applied
Competing Ferroic Orders The magnetoelectric effect Cornell University I would found an institution where any person can find instruction in any study. Ezra Cornell, 1868 Craig J. Fennie School of Applied
Lecture 11: Transition metals (1) Basics and magnetism
 Lecture 11: Transition metals (1) Basics and magnetism Oxidation states in transition metal compounds Ligand field theory Magnetism Susceptibility Temperature dependence Magnetic moments Figure: Wikipedia
Lecture 11: Transition metals (1) Basics and magnetism Oxidation states in transition metal compounds Ligand field theory Magnetism Susceptibility Temperature dependence Magnetic moments Figure: Wikipedia
Evidence for Jahn-Teller Polarons*
 Zeitschrift fur Physikalische Chemie, Bd. 201, S. 263-269 (1997) by R. Oldenbourg Verlag, München 1997 Giant Oxygen-Isotope Shift of the Ferromagnetic Transition Temperature in the Colossal Magnetoresistive
Zeitschrift fur Physikalische Chemie, Bd. 201, S. 263-269 (1997) by R. Oldenbourg Verlag, München 1997 Giant Oxygen-Isotope Shift of the Ferromagnetic Transition Temperature in the Colossal Magnetoresistive
EPR laboratory, Department of Physics, University of Allahabad, Allahabad , India (Received November 20, 2009)
 CHINESE JOURNAL OF PHYSICS VOL. 48, NO. 5 OCTOBER 2010 EPR and Optical Absorption Studies of Mn 2+ Doped Diglycine Calcium Chloride Tetrahydrate Ram Kripal and Manisha Bajpai EPR laboratory, Department
CHINESE JOURNAL OF PHYSICS VOL. 48, NO. 5 OCTOBER 2010 EPR and Optical Absorption Studies of Mn 2+ Doped Diglycine Calcium Chloride Tetrahydrate Ram Kripal and Manisha Bajpai EPR laboratory, Department
6 NMR Interactions: Zeeman and CSA
 6 NMR Interactions: Zeeman and CSA 6.1 Zeeman Interaction Up to this point, we have mentioned a number of NMR interactions - Zeeman, quadrupolar, dipolar - but we have not looked at the nature of these
6 NMR Interactions: Zeeman and CSA 6.1 Zeeman Interaction Up to this point, we have mentioned a number of NMR interactions - Zeeman, quadrupolar, dipolar - but we have not looked at the nature of these
Colossal magnetoresistance:
 Colossal magnetoresistance: Ram Seshadri (seshadri@mrl.ucsb.edu) The simplest example of magnetoresistance is transverse magnetoresistance associated with the Hall effect: H + + + + + + + + + + E y - -
Colossal magnetoresistance: Ram Seshadri (seshadri@mrl.ucsb.edu) The simplest example of magnetoresistance is transverse magnetoresistance associated with the Hall effect: H + + + + + + + + + + E y - -
Nomenclature: Electron Paramagnetic Resonance (EPR) Electron Magnetic Resonance (EMR) Electron Spin Resonance (ESR)
 Introduction to EPR Spectroscopy EPR allows paramagnetic species to be identified and their electronic and geometrical structures to be characterised Interactions with other molecules, concentrations,
Introduction to EPR Spectroscopy EPR allows paramagnetic species to be identified and their electronic and geometrical structures to be characterised Interactions with other molecules, concentrations,
201. The Nature o f the Metallic Bond. III
 No. 8] 913 201. The Nature o f the Metallic Bond. III Atomic Interactions in Alloys. I By San-ichiro MIZUSHIMA, M.J.A., and Isao Ichishima Tokyo Research Institute, Yawata Iron and Steel Co. Ltd., Ida,
No. 8] 913 201. The Nature o f the Metallic Bond. III Atomic Interactions in Alloys. I By San-ichiro MIZUSHIMA, M.J.A., and Isao Ichishima Tokyo Research Institute, Yawata Iron and Steel Co. Ltd., Ida,
Temperature dependence of microwave and THz dielectric response
 The 10 th European Meeting on Ferroelectricity, August 2003, Cambridge, UK Ferroelectrics, in press. Temperature dependence of microwave and THz dielectric response in Sr n+1 Ti n O 3n+1 (n=1-4) D. Noujni
The 10 th European Meeting on Ferroelectricity, August 2003, Cambridge, UK Ferroelectrics, in press. Temperature dependence of microwave and THz dielectric response in Sr n+1 Ti n O 3n+1 (n=1-4) D. Noujni
Problem Set 2 Due Tuesday, September 27, ; p : 0. (b) Construct a representation using five d orbitals that sit on the origin as a basis: 1
 Problem Set 2 Due Tuesday, September 27, 211 Problems from Carter: Chapter 2: 2a-d,g,h,j 2.6, 2.9; Chapter 3: 1a-d,f,g 3.3, 3.6, 3.7 Additional problems: (1) Consider the D 4 point group and use a coordinate
Problem Set 2 Due Tuesday, September 27, 211 Problems from Carter: Chapter 2: 2a-d,g,h,j 2.6, 2.9; Chapter 3: 1a-d,f,g 3.3, 3.6, 3.7 Additional problems: (1) Consider the D 4 point group and use a coordinate
Magnetism in Condensed Matter
 Magnetism in Condensed Matter STEPHEN BLUNDELL Department of Physics University of Oxford OXFORD 'UNIVERSITY PRESS Contents 1 Introduction 1.1 Magnetic moments 1 1 1.1.1 Magnetic moments and angular momentum
Magnetism in Condensed Matter STEPHEN BLUNDELL Department of Physics University of Oxford OXFORD 'UNIVERSITY PRESS Contents 1 Introduction 1.1 Magnetic moments 1 1 1.1.1 Magnetic moments and angular momentum
Crystal Field Theory
 Crystal Field Theory It is not a bonding theory Method of explaining some physical properties that occur in transition metal complexes. Involves a simple electrostatic argument which can yield reasonable
Crystal Field Theory It is not a bonding theory Method of explaining some physical properties that occur in transition metal complexes. Involves a simple electrostatic argument which can yield reasonable
Electronic structure and magnetic properties of high-spin octahedral Co II
 JOURNAL OF CHEMICAL PHYSICS VOLUME 111, NUMBER 22 8 DECEMBER 1999 Electronic structure and magnetic properties of high-spin octahedral Co II complexes: Co II acac 2 H 2 O 2 Lawrence L. Lohr, Jeremy C.
JOURNAL OF CHEMICAL PHYSICS VOLUME 111, NUMBER 22 8 DECEMBER 1999 Electronic structure and magnetic properties of high-spin octahedral Co II complexes: Co II acac 2 H 2 O 2 Lawrence L. Lohr, Jeremy C.
Spin Interactions. Giuseppe Pileio 24/10/2006
 Spin Interactions Giuseppe Pileio 24/10/2006 Magnetic moment µ = " I ˆ µ = " h I(I +1) " = g# h Spin interactions overview Zeeman Interaction Zeeman interaction Interaction with the static magnetic field
Spin Interactions Giuseppe Pileio 24/10/2006 Magnetic moment µ = " I ˆ µ = " h I(I +1) " = g# h Spin interactions overview Zeeman Interaction Zeeman interaction Interaction with the static magnetic field
Mn(acetylacetonate) 3. Synthesis & Characterization
 Mn(acetylacetonate) 3 Synthesis & Characterization The acac Ligand Acetylacetonate (acac) is a bidentate anionic ligand ( 1 charge). We start with acetylacetone (or Hacac) which has the IUPAC name 2,4
Mn(acetylacetonate) 3 Synthesis & Characterization The acac Ligand Acetylacetonate (acac) is a bidentate anionic ligand ( 1 charge). We start with acetylacetone (or Hacac) which has the IUPAC name 2,4
Phase Transitions in Strontium Titanate
 Phase Transitions in Strontium Titanate Xinyue Fang Department of Physics, University of Illinois at Urbana-Champaign Abstract Strontium Titanate SrTiO 3 (STO) is known to undergo an antiferrodistortive
Phase Transitions in Strontium Titanate Xinyue Fang Department of Physics, University of Illinois at Urbana-Champaign Abstract Strontium Titanate SrTiO 3 (STO) is known to undergo an antiferrodistortive
Polarised Nucleon Targets for Europe, 2nd meeting, Bochum 2005
 Polarised Nucleon Targets for Europe, nd meeting, Bochum Temperature dependence of nuclear spin-lattice relaxations in liquid ethanol with dissolved TEMPO radicals H. Štěpánková, J. Englich, J. Kohout,
Polarised Nucleon Targets for Europe, nd meeting, Bochum Temperature dependence of nuclear spin-lattice relaxations in liquid ethanol with dissolved TEMPO radicals H. Štěpánková, J. Englich, J. Kohout,
An introduction to magnetism in three parts
 An introduction to magnetism in three parts Wulf Wulfhekel Physikalisches Institut, Karlsruhe Institute of Technology (KIT) Wolfgang Gaede Str. 1, D-76131 Karlsruhe 0. Overview Chapters of the three lectures
An introduction to magnetism in three parts Wulf Wulfhekel Physikalisches Institut, Karlsruhe Institute of Technology (KIT) Wolfgang Gaede Str. 1, D-76131 Karlsruhe 0. Overview Chapters of the three lectures
Hyperfine interaction
 Hyperfine interaction The notion hyperfine interaction (hfi) comes from atomic physics, where it is used for the interaction of the electronic magnetic moment with the nuclear magnetic moment. In magnetic
Hyperfine interaction The notion hyperfine interaction (hfi) comes from atomic physics, where it is used for the interaction of the electronic magnetic moment with the nuclear magnetic moment. In magnetic
University of Groningen. Orbital ordering and multiferroics Nenert, Gwilherm
 University of Groningen Orbital ordering and multiferroics Nenert, Gwilherm IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check
University of Groningen Orbital ordering and multiferroics Nenert, Gwilherm IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check
arxiv:cond-mat/ v1 [cond-mat.mtrl-sci] 13 Nov 2003
![arxiv:cond-mat/ v1 [cond-mat.mtrl-sci] 13 Nov 2003 arxiv:cond-mat/ v1 [cond-mat.mtrl-sci] 13 Nov 2003](/thumbs/88/115260649.jpg) 1. 14 August 1996 (final accepted version arxiv:cond-mat/0311297v1 [cond-mat.mtrl-sci] 13 Nov 2003 2. Non-collinear magnetism in distorted perovskite compounds 3. I.V.Solovyev a,, N.Hamada b, K.Terakura
1. 14 August 1996 (final accepted version arxiv:cond-mat/0311297v1 [cond-mat.mtrl-sci] 13 Nov 2003 2. Non-collinear magnetism in distorted perovskite compounds 3. I.V.Solovyev a,, N.Hamada b, K.Terakura
Electron-Nuclear Hyperfine Interactions of 53 Cr 3+ in Mg2Si04 (Forsterite)
 Electron-Nuclear Hyperfine Interactions of 53 Cr 3 in Mg2Si04 (Forsterite) H. Rager Department of Geoscienees, University of Marburg Z. Naturforsch. 35a, 1296-1303 (1980); received October 25, 1980 The
Electron-Nuclear Hyperfine Interactions of 53 Cr 3 in Mg2Si04 (Forsterite) H. Rager Department of Geoscienees, University of Marburg Z. Naturforsch. 35a, 1296-1303 (1980); received October 25, 1980 The
Effect of Pressure on Antiferromagnetic Transition in Alkali-Electro-Sodalite
 Effect of Pressure on Antiferromagnetic Transition in Alkali-Electro-Sodalite KENJI MIZOGUCHI a, TETSUSHI TAKANASHI a, HIROKAZU SAKAMOTO a, LJILJANA DAMJANOVIC b and VOJISLAV I. SRDANOV b a Dept. of Phys.,
Effect of Pressure on Antiferromagnetic Transition in Alkali-Electro-Sodalite KENJI MIZOGUCHI a, TETSUSHI TAKANASHI a, HIROKAZU SAKAMOTO a, LJILJANA DAMJANOVIC b and VOJISLAV I. SRDANOV b a Dept. of Phys.,
Chapter 8 Magnetic Resonance
 Chapter 8 Magnetic Resonance 9.1 Electron paramagnetic resonance 9.2 Ferromagnetic resonance 9.3 Nuclear magnetic resonance 9.4 Other resonance methods TCD March 2007 1 A resonance experiment involves
Chapter 8 Magnetic Resonance 9.1 Electron paramagnetic resonance 9.2 Ferromagnetic resonance 9.3 Nuclear magnetic resonance 9.4 Other resonance methods TCD March 2007 1 A resonance experiment involves
Giant Negative Magnetization in a Layered Molecular-Based Magnet
 Giant Negative Magnetization in a Layered Molecular-Based Magnet Randy Fishman Materials Science and Technology Division Oak Ridge National Laboratory Collaborators: Fernando Reboredo, Patrik Henelius
Giant Negative Magnetization in a Layered Molecular-Based Magnet Randy Fishman Materials Science and Technology Division Oak Ridge National Laboratory Collaborators: Fernando Reboredo, Patrik Henelius
4. Interpenetrating simple cubic
 2 1. The correct structure t of CsClCl crystal is 1. Simple cubic 2. Body centered cubic 3. Face centered cubic 4. Interpenetrating simple cubic If corner as well as the particle at the center are same
2 1. The correct structure t of CsClCl crystal is 1. Simple cubic 2. Body centered cubic 3. Face centered cubic 4. Interpenetrating simple cubic If corner as well as the particle at the center are same
Electronic structure calculations results from LDA+U method
 Electronic structure calculations results from LDA+U method Vladimir I. Anisimov Institute of Metal Physics Ekaterinburg, Russia LDA+U method applications Mott insulators Polarons and stripes in cuprates
Electronic structure calculations results from LDA+U method Vladimir I. Anisimov Institute of Metal Physics Ekaterinburg, Russia LDA+U method applications Mott insulators Polarons and stripes in cuprates
Perovskite oxides are multifunctional materials that have been the focus of intense
 CHAPTER 1 INTRODUCTION 3 Perovskite oxides are multifunctional materials that have been the focus of intense scientific research over the past years. Indeed, some of these display many remarkable and often
CHAPTER 1 INTRODUCTION 3 Perovskite oxides are multifunctional materials that have been the focus of intense scientific research over the past years. Indeed, some of these display many remarkable and often
Lattice dynamics, phase transitions and spin relaxation in [Fe(C 5 H 5 ) 2 ]PF 6
![Lattice dynamics, phase transitions and spin relaxation in [Fe(C 5 H 5 ) 2 ]PF 6 Lattice dynamics, phase transitions and spin relaxation in [Fe(C 5 H 5 ) 2 ]PF 6](/thumbs/96/126746830.jpg) Hyperfine Interact (2016) 237:100 DOI 10.1007/s10751-016-1310-9 Lattice dynamics, phase transitions and spin relaxation in [Fe(C 5 H 5 ) 2 ]PF 6 R. H. Herber 1 I. Felner 1 I. Nowik 1 Springer International
Hyperfine Interact (2016) 237:100 DOI 10.1007/s10751-016-1310-9 Lattice dynamics, phase transitions and spin relaxation in [Fe(C 5 H 5 ) 2 ]PF 6 R. H. Herber 1 I. Felner 1 I. Nowik 1 Springer International
Ground state splitting for the Mn 2+ ion in PbMnTe compounds
 PHYSICAL REVIEW B 71, 014422 2005 Ground state splitting for the Mn 2+ ion in PbMnTe compounds A. Łusakowski Institute of Physics, Polish Academy of Sciences, Aleja Lotników 32/46, 02-668 Warsaw, Poland
PHYSICAL REVIEW B 71, 014422 2005 Ground state splitting for the Mn 2+ ion in PbMnTe compounds A. Łusakowski Institute of Physics, Polish Academy of Sciences, Aleja Lotników 32/46, 02-668 Warsaw, Poland
ELECTRON PARAMAGNETIC RESONANCE Elementary Theory and Practical Applications
 ELECTRON PARAMAGNETIC RESONANCE Elementary Theory and Practical Applications Second Edition JOHN A. WElL Department of Chemistry, University of Saskatchewan, Saskatoon, Saskatchewan, S7N OWO Canada JAMES
ELECTRON PARAMAGNETIC RESONANCE Elementary Theory and Practical Applications Second Edition JOHN A. WElL Department of Chemistry, University of Saskatchewan, Saskatoon, Saskatchewan, S7N OWO Canada JAMES
Crystal field effect on atomic states
 Crystal field effect on atomic states Mehdi Amara, Université Joseph-Fourier et Institut Néel, C.N.R.S. BP 66X, F-3842 Grenoble, France References : Articles - H. Bethe, Annalen der Physik, 929, 3, p.
Crystal field effect on atomic states Mehdi Amara, Université Joseph-Fourier et Institut Néel, C.N.R.S. BP 66X, F-3842 Grenoble, France References : Articles - H. Bethe, Annalen der Physik, 929, 3, p.
Supplementary Figure 1: Spin noise spectra of 55 Mn in bulk sample at BL =10.5 mt, before subtraction of the zero-frequency line. a, Contour plot of
 1 Supplementary Figure 1: Spin noise spectra of 55 Mn in bulk sample at BL =10.5 mt, before subtraction of the zero-frequency line. a, Contour plot of the spin noise spectra calculated with Eq. (2) for
1 Supplementary Figure 1: Spin noise spectra of 55 Mn in bulk sample at BL =10.5 mt, before subtraction of the zero-frequency line. a, Contour plot of the spin noise spectra calculated with Eq. (2) for
RDCH 702 Lecture 4: Orbitals and energetics
 RDCH 702 Lecture 4: Orbitals and energetics Molecular symmetry Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating
RDCH 702 Lecture 4: Orbitals and energetics Molecular symmetry Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating
Electron spins in reduced dimensions: ESR spectroscopy on semiconductor heterostructures and spin chain compounds
 Electron spins in reduced dimensions: ESR spectroscopy on semiconductor heterostructures and spin chain compounds Dissertation zur Erlangung des akademischen Grades Doctor rerum naturalium (Dr. rer. nat.)
Electron spins in reduced dimensions: ESR spectroscopy on semiconductor heterostructures and spin chain compounds Dissertation zur Erlangung des akademischen Grades Doctor rerum naturalium (Dr. rer. nat.)
Coordination Chemistry: Bonding Theories. Crystal Field Theory. Chapter 20
 Coordination Chemistry: Bonding Theories Crystal Field Theory Chapter 0 Review of the Previous Lecture 1. We discussed different types of isomerism in coordination chemistry Structural or constitutional
Coordination Chemistry: Bonding Theories Crystal Field Theory Chapter 0 Review of the Previous Lecture 1. We discussed different types of isomerism in coordination chemistry Structural or constitutional
Luigi Paolasini
 Luigi Paolasini paolasini@esrf.fr LECTURE 4: MAGNETIC INTERACTIONS - Dipole vs exchange magnetic interactions. - Direct and indirect exchange interactions. - Anisotropic exchange interactions. - Interplay
Luigi Paolasini paolasini@esrf.fr LECTURE 4: MAGNETIC INTERACTIONS - Dipole vs exchange magnetic interactions. - Direct and indirect exchange interactions. - Anisotropic exchange interactions. - Interplay
ELECTRON PARAMAGNETIC RESONANCE OF LITHIUM NIOBATE HEAVILY DOPED WITH CHROMIUM AND LITHIUM NIOBATE CODOPED WITH MAGNESIUM AND IRON
 ELECTRON PARAMAGNETIC RESONANCE OF LITHIUM NIOBATE HEAVILY DOPED WITH CHROMIUM AND LITHIUM NIOBATE CODOPED WITH MAGNESIUM AND IRON by Jonathan David Jorgensen A thesis submitted in partial fulfillment
ELECTRON PARAMAGNETIC RESONANCE OF LITHIUM NIOBATE HEAVILY DOPED WITH CHROMIUM AND LITHIUM NIOBATE CODOPED WITH MAGNESIUM AND IRON by Jonathan David Jorgensen A thesis submitted in partial fulfillment
μ (vector) = magnetic dipole moment (not to be confused with the permeability μ). Magnetism Electromagnetic Fields in a Solid
 Magnetism Electromagnetic Fields in a Solid SI units cgs (Gaussian) units Total magnetic field: B = μ 0 (H + M) = μ μ 0 H B = H + 4π M = μ H Total electric field: E = 1/ε 0 (D P) = 1/εε 0 D E = D 4π P
Magnetism Electromagnetic Fields in a Solid SI units cgs (Gaussian) units Total magnetic field: B = μ 0 (H + M) = μ μ 0 H B = H + 4π M = μ H Total electric field: E = 1/ε 0 (D P) = 1/εε 0 D E = D 4π P
3. Perturbed Angular Correlation Spectroscopy
 3. Perturbed Angular Correlation Spectroscopy Dileep Mampallil Augustine K.U.Leuven, Belgium Perturbed Angular Correlation Spectroscopy (PAC) is a gamma ray spectroscopy and can be used to investigate
3. Perturbed Angular Correlation Spectroscopy Dileep Mampallil Augustine K.U.Leuven, Belgium Perturbed Angular Correlation Spectroscopy (PAC) is a gamma ray spectroscopy and can be used to investigate
Brazilian Journal of Physics ISSN: Sociedade Brasileira de Física Brasil
 Brazilian Journal of Physics ISSN: 0103-9733 luizno.bjp@gmail.com Sociedade Brasileira de Física Brasil Foglio, M. E.; Barberis, G. E. Study of Co2+ in different crystal field environments Brazilian Journal
Brazilian Journal of Physics ISSN: 0103-9733 luizno.bjp@gmail.com Sociedade Brasileira de Física Brasil Foglio, M. E.; Barberis, G. E. Study of Co2+ in different crystal field environments Brazilian Journal
THE BONDING IN VANADYL ION COMPLEXES
 THE BONDING IN VANADYL ION COMPLEXES Abstract This chapter describes how the degeneracy of a d ion is split in octahedral crystal field. The energy levels of a vanadyl ion water complex have further been
THE BONDING IN VANADYL ION COMPLEXES Abstract This chapter describes how the degeneracy of a d ion is split in octahedral crystal field. The energy levels of a vanadyl ion water complex have further been
Orbitals and energetics
 Orbitals and energetics Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating radionuclide complexes Structure
Orbitals and energetics Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating radionuclide complexes Structure
Magnetic Circular Dichroism spectroscopy in epitaxial La 0.7 Sr 0.3 MnO 3 thin films
 Magnetic Circular Dichroism spectroscopy in epitaxial La 0.7 Sr 0.3 MnO 3 thin films T. K. Nath 1 and J. R. Neal 2, G. A. Gehring 2 1 Dept. of Physics and Meteorology, Indian Institute Technology of Kharagpur,
Magnetic Circular Dichroism spectroscopy in epitaxial La 0.7 Sr 0.3 MnO 3 thin films T. K. Nath 1 and J. R. Neal 2, G. A. Gehring 2 1 Dept. of Physics and Meteorology, Indian Institute Technology of Kharagpur,
Electromagnetism II. Instructor: Andrei Sirenko Spring 2013 Thursdays 1 pm 4 pm. Spring 2013, NJIT 1
 Electromagnetism II Instructor: Andrei Sirenko sirenko@njit.edu Spring 013 Thursdays 1 pm 4 pm Spring 013, NJIT 1 PROBLEMS for CH. 6 http://web.njit.edu/~sirenko/phys433/phys433eandm013.htm Can obtain
Electromagnetism II Instructor: Andrei Sirenko sirenko@njit.edu Spring 013 Thursdays 1 pm 4 pm Spring 013, NJIT 1 PROBLEMS for CH. 6 http://web.njit.edu/~sirenko/phys433/phys433eandm013.htm Can obtain
arxiv: v4 [cond-mat.supr-con] 22 Jun 2013
![arxiv: v4 [cond-mat.supr-con] 22 Jun 2013 arxiv: v4 [cond-mat.supr-con] 22 Jun 2013](/thumbs/73/68953311.jpg) arxiv:1302.6002v4 [cond-mat.supr-con] 22 Jun 2013 Highly Correlated Electron State and High-Temperature Superconductivity in Iron Pnictides M.V.Krasinkova Ioffe Physico-Technical Institute, Russian Academy
arxiv:1302.6002v4 [cond-mat.supr-con] 22 Jun 2013 Highly Correlated Electron State and High-Temperature Superconductivity in Iron Pnictides M.V.Krasinkova Ioffe Physico-Technical Institute, Russian Academy
Lecture 5. Chapters 3 & 4. Induced magnetization: that which is induced in the presence of an applied magnetic field. diamagnetic.
 Lecture 5 Induced magnetization: that which is induced in the presence of an applied magnetic field diamagnetic paramagnetic Remanent magnetization: that which remains in the absence of an external field
Lecture 5 Induced magnetization: that which is induced in the presence of an applied magnetic field diamagnetic paramagnetic Remanent magnetization: that which remains in the absence of an external field
Self-compensating incorporation of Mn in Ga 1 x Mn x As
 Self-compensating incorporation of Mn in Ga 1 x Mn x As arxiv:cond-mat/0201131v1 [cond-mat.mtrl-sci] 9 Jan 2002 J. Mašek and F. Máca Institute of Physics, Academy of Sciences of the CR CZ-182 21 Praha
Self-compensating incorporation of Mn in Ga 1 x Mn x As arxiv:cond-mat/0201131v1 [cond-mat.mtrl-sci] 9 Jan 2002 J. Mašek and F. Máca Institute of Physics, Academy of Sciences of the CR CZ-182 21 Praha
Crystal Field Theory
 6/4/011 Crystal Field Theory It is not a bonding theory Method of explaining some physical properties that occur in transition metal complexes. Involves a simple electrostatic argument which can yield
6/4/011 Crystal Field Theory It is not a bonding theory Method of explaining some physical properties that occur in transition metal complexes. Involves a simple electrostatic argument which can yield
Giniyat Khaliullin Max Planck Institute for Solid State Research, Stuttgart
 Mott insulators with strong spin-orbit coupling Giniyat Khaliullin Max Planck Institute for Solid State Research, Stuttgart LS driven unusual ground states & excitations motivated by: Sr 2 IrO 4 Na 2 IrO
Mott insulators with strong spin-orbit coupling Giniyat Khaliullin Max Planck Institute for Solid State Research, Stuttgart LS driven unusual ground states & excitations motivated by: Sr 2 IrO 4 Na 2 IrO
compound Cs 2 Cu 2 Mo 3 O 12
 133 Cs-NMR study on aligned powder of competing spin chain compound A Yagi 1, K Matsui 1 T Goto 1, M Hase 2 and T Sasaki 3 1 2 Sophia University, Physics Division, Tokyo, 102-8554, Japan National Institute
133 Cs-NMR study on aligned powder of competing spin chain compound A Yagi 1, K Matsui 1 T Goto 1, M Hase 2 and T Sasaki 3 1 2 Sophia University, Physics Division, Tokyo, 102-8554, Japan National Institute
Nuclear magnetic resonance in condensed matter
 University of Ljubljana Faculty of mathematics and physics Physics department SEMINAR Nuclear magnetic resonance in condensed matter Author: Miha Bratkovič Mentor: prof. dr. Janez Dolinšek Ljubljana, October
University of Ljubljana Faculty of mathematics and physics Physics department SEMINAR Nuclear magnetic resonance in condensed matter Author: Miha Bratkovič Mentor: prof. dr. Janez Dolinšek Ljubljana, October
SECOND PUBLIC EXAMINATION. Honour School of Physics Part C: 4 Year Course. Honour School of Physics and Philosophy Part C C3: CONDENSED MATTER PHYSICS
 A11046W1 SECOND PUBLIC EXAMINATION Honour School of Physics Part C: 4 Year Course Honour School of Physics and Philosophy Part C C3: CONDENSED MATTER PHYSICS TRINITY TERM 2015 Wednesday, 17 June, 2.30
A11046W1 SECOND PUBLIC EXAMINATION Honour School of Physics Part C: 4 Year Course Honour School of Physics and Philosophy Part C C3: CONDENSED MATTER PHYSICS TRINITY TERM 2015 Wednesday, 17 June, 2.30
Lecture 05 Structure of Ceramics 2 Ref: Barsoum, Fundamentals of Ceramics, Ch03, McGraw-Hill, 2000.
 MME 467 Ceramics for Advanced Applications Lecture 05 Structure of Ceramics 2 Ref: Barsoum, Fundamentals of Ceramics, Ch03, McGraw-Hill, 2000. Prof. A. K. M. Bazlur Rashid Department of MME, BUET, Dhaka
MME 467 Ceramics for Advanced Applications Lecture 05 Structure of Ceramics 2 Ref: Barsoum, Fundamentals of Ceramics, Ch03, McGraw-Hill, 2000. Prof. A. K. M. Bazlur Rashid Department of MME, BUET, Dhaka
arxiv: v1 [cond-mat.str-el] 3 Dec 2015
![arxiv: v1 [cond-mat.str-el] 3 Dec 2015 arxiv: v1 [cond-mat.str-el] 3 Dec 2015](/thumbs/86/93646300.jpg) arxiv:1512.00974v1 [cond-mat.str-el] 3 Dec 2015 Single crystal 27 Al-NMR study of the cubic Γ 3 ground doublet system PrTi 2 Al 20 T Taniguchi, M Yoshida, H Takeda, M Takigawa, M Tsujimoto, A Sakai, Y
arxiv:1512.00974v1 [cond-mat.str-el] 3 Dec 2015 Single crystal 27 Al-NMR study of the cubic Γ 3 ground doublet system PrTi 2 Al 20 T Taniguchi, M Yoshida, H Takeda, M Takigawa, M Tsujimoto, A Sakai, Y
Making the Invisible Visible: Probing Antiferromagnetic Order in Novel Materials
 Making the Invisible Visible: Probing Antiferromagnetic Order in Novel Materials Elke Arenholz Lawrence Berkeley National Laboratory Antiferromagnetic contrast in X-ray absorption Ni in NiO Neel Temperature
Making the Invisible Visible: Probing Antiferromagnetic Order in Novel Materials Elke Arenholz Lawrence Berkeley National Laboratory Antiferromagnetic contrast in X-ray absorption Ni in NiO Neel Temperature
Spins and spin-orbit coupling in semiconductors, metals, and nanostructures
 B. Halperin Spin lecture 1 Spins and spin-orbit coupling in semiconductors, metals, and nanostructures Behavior of non-equilibrium spin populations. Spin relaxation and spin transport. How does one produce
B. Halperin Spin lecture 1 Spins and spin-orbit coupling in semiconductors, metals, and nanostructures Behavior of non-equilibrium spin populations. Spin relaxation and spin transport. How does one produce
Electron Correlation
 Series in Modern Condensed Matter Physics Vol. 5 Lecture Notes an Electron Correlation and Magnetism Patrik Fazekas Research Institute for Solid State Physics & Optics, Budapest lb World Scientific h Singapore
Series in Modern Condensed Matter Physics Vol. 5 Lecture Notes an Electron Correlation and Magnetism Patrik Fazekas Research Institute for Solid State Physics & Optics, Budapest lb World Scientific h Singapore
2.1 Experimental and theoretical studies
 Chapter 2 NiO As stated before, the first-row transition-metal oxides are among the most interesting series of materials, exhibiting wide variations in physical properties related to electronic structure.
Chapter 2 NiO As stated before, the first-row transition-metal oxides are among the most interesting series of materials, exhibiting wide variations in physical properties related to electronic structure.
Roger Johnson Structure and Dynamics: Displacive phase transition Lecture 9
 9.1. Summary In this Lecture we will consider structural phase transitions characterised by atomic displacements, which result in a low temperature structure that is distorted compared to a higher temperature,
9.1. Summary In this Lecture we will consider structural phase transitions characterised by atomic displacements, which result in a low temperature structure that is distorted compared to a higher temperature,
