If quantum mechanics hasn t profoundly shocked you, you haven t understood it.
|
|
|
- Rodney O’Brien’
- 5 years ago
- Views:
Transcription
1 Quantum Mechanics If quantum mechanics hasn t profoundly shocked you, you haven t understood it. Niels Bohr Today, I will tell you more about quantum mechanics what weird thing it is and why it is so weird. The importance of quantum mechanics cannot be overstated. It explains the structure of atoms, it describes how elementary particles interact (Lecture ) and it may even be the ultimate origin of all structure in the universe (Lecture ). And yet it is so mind-boggling strange and bizarre that it is very di cult to come to terms with. To the point where it suggests that, ultimately, our brains may not be best equipped to understand what s going on On the other hand, if we just shut up and calculate, quantum mechanics simply works. In fact, it is arguably the most successful scientific theory ever developed. In this lecture, I will try to give you a glimpse of the wonderful world of quantum mechanics. This will be, by far, the most challenging lecture of the course. Are you ready?.1 The Split Personality of Electrons.1.1 Particles or Waves? Electrons (and other elementary particles) behave like nothing you have ever seen before. I have demonstrated this to you at the end of the last lecture when we looked at an experiment in which electrons were fired at a screen with two slits. In a world governed by classical (i.e. nonquantum) mechanics, there is a clear expectation for what should happen: electrons should behave like bullets. The bullets go through either one slit or the other, and we expect them just to pile up behind the two slits: We would predict that the pattern behind the two slits simply to be the sum of the patterns for each slit considered separately: if half the bullets were fired with only the left slit open and then half were fired with just the right slit open, the result would be the same. 11
2 1. Quantum Mechanics But, I showed you what happens in the actual experiment: The electrons, like bullets, strike the target one at a time. We slowly see a pattern building up yet, it is not the pattern that we would expect if electrons behaved like bullets. Instead the pattern we get for electrons looks more like the pattern we would get for waves: With waves, if the slits were opened one at a time, the pattern would resemble that for particles: two distinct peaks. But when the two slits are open at the same time, the waves pass through both slits at once and interfere with each other: where they are in phase they reinforce each other; where they are out of phase they cancel each other out. Electrons seem to do the same. However, if each electron passes individually through one slit, what does it interfere with? Although each electron arrives at the target at a single place and time, it seems that each has passed through both slits at once. Remember that in Lecture 1 we claimed that electrons sni out all possible paths. In the double slit experiment we see this in action..1. The Structure of Atoms This behaviour of electrons determines the structure of atoms: In classical physics, we have a simple (and wrong) mental picture of the hydrogen atom: proton electron The electric force keeps an electron in orbit around a proton, just like the gravitational force keeps the Earth in orbit around the Sun. According to this view of the atom, the electron follows a single classical path with no restriction on the size or eccentricity of the orbit (which only depend on the initial conditions). In quantum mechanics, the answer is very di erent. The electron sni s out many possible orbits and its position smears out into quantum orbits :
3 . Principles of Quantum Mechanics 1 Moreover, there are only a finite number of allowed quantum orbits, with specific energies. The orbits and their energies are quantized. In this way, quantum mechanics explains the periodic table of elements and therefore underlies all of chemistry. Having agreed that this is important stu, let s see some of the mathematical details.. Principles of Quantum Mechanics Quantum mechanics doesn t use any fancy mathematics. Everything that we will need was described in your course on Vectors and Matrices. In this section, we will use some of that knowledge to introduce the basic postulates of quantum mechanics. We don t have time to waste, so let s jump right in...1 States are Vectors The state of a system consists of all the information that we have to specify about a system in order to determine its future evolution. For example, in classical mechanics we need to list the positions and momenta of all particles. Now, in quantum mechanics, states are vectors. We will use i for the state vector.1 As a simple example consider a system that only has two possible states: 0/1, up/down, on/o, left/right, dead/alive, etc. Such a system is also called a 1 The notation i goes back to Dirac. It is called a ket-vector or just ket. There are also bra-vectors, h, which are the transposes (or Hermitian conjugates) of the ket-vectors. The inner product of a bra and a ket, h i, is a bra-ket, or bracket.
4 1. Quantum Mechanics bit. The two states of the bit can be represented by the following two-component vectors "i 1, (..1) 0 #i 0. (..) 1 To have a physical example in mind, you may think of the spin of an electron relative to say the z-axis. In fact, this will be our go-to example for the rest of this lecture. In the classical world, the system has to be either in one state or the other. However, in the quantum world, the system can be in a superposition of both states at the same time, i.e. we can add the state vectors i = "i #i =, (..) where and are complex numbers satisfying = 1. Such a state is called a qubit (quantum bit). The possibility of a superposition of states is the origin of all the weirdness of quantum mechanics. More generally, the state vector can be the sum of many basis states i = X i i ii, (..) where each basis state ii is weighted by a complex number i and P i i = 1. The spectrum of states can also be continuous and the corresponding vector space infinite-dimensional. For example, this is the case for a particle with position x. In quantum mechanics, the state of the particle is a superposition of all possible locations Z i = dx (x) xi, (..) and we call the function (x) the wavefunction... Observables are Matrices You know that matrices are things that can act on vectors to produce other vectors. You also know that observables are things you can measure. In quantum mechanics, observables are (Hermitian) matrices. We have a di erent matrix M for each kind of measurement we can make on a system: energy, position, momentum, spin, etc. The matrices corresponding to the spin of an electron along the x-axis, y-axis and zaxis are X = , Y = 0 i i 0, Z = (..) Do not try to visualize the electron as a spinning top. Particle spin is a purely quantum phenomenon. In quantum mechanics, the spin can either be up, "i, or down, #i. To show that the electron can only have these two spin states is hard.
5 . Principles of Quantum Mechanics 1 The matrices act on the state vectors simply by matrix multiplication. In general, when a matrix acts on a vector, it will change the direction of the vector. However, there are certain vectors whose direction are the same after the matrix multiplication. These special vectors are called eigenvectors. In our notation, this means M ii = m i ii. (..) where ii are the eigenvectors and m i the corresponding eigenvalues. The vectors (..1) and (..) are eigenvectors of Z with eigenvalues 1 and 1, i.e. Z "i =1 "i and Z #i = 1 #i. (..8) They are not eigenvectors of X and Y, e.g. X "i = #i. In quantum mechanics, measurements are eigenvalues, i.e. the possible outcomes of a measurement are the eigenvalues m i of the matrix M associated with the observable. Since the measurement matrices are Hermitian, the eigenvalues are always real. You can easily check that the eigenvalues of all matrices in (..) are 1 and 1. Measuring the spin of an electron along any axis will therefore always give 1 or 1. In general, the outcome of a measurement will be probabilistic (see below). An important exception is that measurements of M lead to definite values if the states are eigenvectors of M. In other words, if the system is prepared in an eigenvector of M, i.e. i = ii, you will always measure the corresponding eigenvalue m i, with certainty This is important, so let me say it again: Although we will see that quantum mechanics is all about probabilities and uncertainties, if the system is prepared in an eigenvector of a particular observable and you measure that observable, you will always just get the eigenvalue. For example, if we prepare a system in the state "i and decide to measure its spin along the z-axis, we will always get 1. A special matrix H (called the Hamiltonian; see Appendix A) represents a measurement of energy. The possible outcomes of the corresponding measurement are the energy eigenvalues E i, and (..) becomes the famous Schrödinger equation, H ii = E i ii. (..9) For a given system, you need to figure out the matrix H, then solve its eigenvalues. This is usually hard Sometimes, like for the hydrogen atom it can be done exactly. Most of the time (e.g. for all other atoms) it is done in some approximation scheme. Solving the eigenvalue equation (..9) explains the discrete energy levels of atoms... Measurements are Probabilistic We have seen that if the system is in an eigenstate ii of M, then we always measure the corresponding eigenvalue m i. However,
6 1. Quantum Mechanics if the state is not an eigenvector of the observable M, then the outcomes of measurements of M will be probabilistic. The measurement could give any one of the eigenvalues m i. Each with some probability. We can expand any arbitrary state i in a basis of eigenvectors of M, i = X i i ii, (..10) where i are complex constants. The probability of the measurement giving the eigenvalue m i is Prob(m i )= i, (..11) i.e. the probability of measuring m i is simply the square of the expansion coe sum of all probabilities has to add up to 100%, so we have X Prob(m i )= X i i cient i. The i =1. (..1) Let s see what this means for the spinning electron. If the system is prepared in the qubit state (..) then Prob(") =, (..1) Prob(#) =. (..1) Since we are guaranteed to measure either spin-up or spin-down, we have Prob(") Prob(#) = =1. (..1).. Collapse of the State Vector Finally, the state vector collapses after the measurement : i mi ii, i.e. if the eigenvalue m i has been measured, then the state of the system after the measurement is the corresponding eigenvector ii. If we now were to repeat the measurement we would get the same value m i with certainty. But, if we then perform a measurement of a di erent observable, corresponding to a matrix N, the outcome will again be probabilistic, unless ii is also an eigenvector of N. Again, the spinning electron makes this clear. If the system is prepared in the state (..), then we measure spin-up or spin-down with probabilities and,respectively. Oncethe measurement is made, the state vector i will collapse into one of the two states, "i or #i, depending on which is measured. Every subsequent measurement of spin along the z-axis will give the same value. However, if we then decide to measure a di erent quantity, say the spin along the x-axis, the result will be probabilistic again. I warned you that this is strange stu.
7 . Principles of Quantum Mechanics 1 Let s say you make a measurement of the spin along the z-axis and find 1. After that the state will be 1 i = "i =. (..1) 0 Check for yourself that the eigenvectors of the matrix X in (..) are i p 1 1 1, i p (..1) The state in (..1) can therefore be written as a superposition of the eigenstates of X: i = "i = 1 = 1 0 apple = p 1 i h i i. (..18) Measuring X next will therefore give 1 or 1 with equal probability. Say it is 1. The state has then collapsed onto i. It is not an eigenstate of Z anymore. Instead, i = 1 p "i #i. The next measurement of the spin along the z-axis would again be probabilistic, giving up or down with equal probabilities. And so on..... The Uncertainty Principle What we just said has an important implication. Most matrices have di erent eigenvectors. (This is the case if the matrices don t commute, i.e. MN = NM.) So if you re in a state that is an eigenvector of one matrix, it is unlikely to be an eigenvector of a di erent matrix. If one type of measurement is certain, another type is uncertain. For example, you can easily check that the matrices X, Y and Z in (..) do not commute with each other. You can also check that they have distinct eigenvectors. No matter what the state i is, we can therefore never simultaneously know the spins along several di erent axes. (You may know the spin along the z-axis, if the system is in an eigenstate of Z, butthenthe spin along x and y is completely unknown.) The most famous application of the uncertainty principle is to the position and momentum of a particle. In classical mechanics, we can know both with certainty. In fact, we need to know them in order to predict the future evolution of the particle. However, in quantum mechanics, if we know the position x of a particle, its momentum p becomes completely uncertain. And vice versa. This is quantified in Heisenberg s uncertainty relation x p ~, (..19) where ~ 10 J s is Planck s constant. The reason we don t notice these uncertainties in everyday life is because ~ is so tiny. Similar uncertainty relations exist for other observables... Combining Systems: Entanglement Things get interesting in quantum mechanics when we combine systems. For concreteness, we will consider states made from a combination of two or more qubits. For example, we could
8 18. Quantum Mechanics have two electrons each with spin-up or spin-down. We will label the electrons A and B. If A is in the state up, "i A, and B is in the state down, #i B, then the combined state is "i A #i B "#i. (..0) The left-hand side is called a tensor product. The simplified notation on the right-hand side should be clear: the first arrow denotes the spin of A, the second arrow that of B. In total, there are four possible combined states: ""i, "#i, #"i, ##i. (..1) The state vector i may be a superposition of these four states. For example, imagine that the system is prepared in the state i = 1 p "#i 1 p #"i. (..) This is called an entangled state since it cannot be separated into the product of states for the individual electrons. Not all superpositions of the states (..1) are entangled states. An example of a product state is which can be written as i = 1 p "#i 1 p ##i, (..) i = 1 p "i A 1 p #i A #i B. (..) The main feature of a product state is that each subsystem behaves independently of the other. If we do an experiment on B, the result is exactly the same as if A did not exist. The same is true for A of course. In contrast, in an entangled state measurements of A and B are not independent. The state (..) can arise if a particle without spin decays into two electrons. Since angular momentum is conserved, the spins of the two electrons have to be anti-aligned. While in classical physics the system then has to be in the state "#i or #"i, in quantum physics it can be in the state "#i and #"i. The two electrons are then separated by a large distance (as large as you wish). Say one of the electrons stays here, while the other is brought to the end of the universe. We then measure the spin of the electron that stayed here. There is a 0% chance that the result will be spin-up, and a 0% chance that it will be spin-down. However, once the spin of the electron is measured, the spin of the far-away electron is determined instantly. By itself this correlation of the measurements is not a problem. The same happens in classical physics. Imagine a pair of gloves. Each glove is put into a box and the two boxes are then separated by a large distance. If you open one of the boxes and find a left-hand glove, you know immediately that the far-way glove is a right-hand glove. No problem. However, entangled states like (..) are superpositions of correlated states. As we will see in the next section, such states can have correlations that are purely quantum. Quantum gloves are actually left- and right-handed (and everything in between) up until the moment you observe them. Moreover, the left-handed glove doesnt become left until you observe the right-handed one at which moment both instantly
9 . Quantum Mechanics in Your Face 19 gain a definite handedness. This really upset Einstein. He called it spooky action at a distance.. Quantum Mechanics in Your Face Entanglement is arguably the most bewildering aspect of quantum mechanics. Let s explore this a bit further. The punchline will be that the universe is a much stranger place than you might have imagined...1 The GHZ Experiment Consider three scientists: A B C It is conventional in the quantum mechanics literature to call them Alice (A), Bob (B) and Charlie (C). They are sent to labs at three di erent locations. Every minute, they receive a This section is based on a famous lecture by Sidney Coleman ( which itself was based on Greenberger, Horne, and Zeilinger (GHZ) (1990) Bell s theorem without inequalities, Am. J. Phys. 8 (1): 111, and Mermin (1990) Quantum mysteries revisited Am. J. Phys. 8 (8): 1. Admittedly, A doesn t look much like an Alice.
10 0. Quantum Mechanics package from a mysterious central station (S): A S B C Each scientist has a machine that performs measurements of the packages. The machine has two settings, X or Y, and each measurement can give two outcomes, 1 and X Y Alice, Bob and Charlie are told what they have to do: 1. Choose the setting X or Y on the machine.. Insert the package into the machine.. Make a measurement.. Record whether the result is 1 or 1.. Go back to Step 1. Each measurement is recorded until each scientist has a list that looks like this: X X Y X Y Y X Y X X X Y After they each have made a bazillion measurements, Alice, Bob and Charlie get together and start looking for correlations in their measurements. (Since their packages came from the same source, it isn t unreasonable to expect some correlations.) They notice the following: Whenever one of them measured X, and the other two measured Y, theresultsalways multiply to 1, i.e. X A Y B Y C = Y A X B Y C = Y A Y B X C =1. (..) They are not told what s in the packages: They could be blood samples, with the machine testing for high/low glucose when the switch is on X, and high/low cholesterol when the switch is on Y. They could be elementary particles with the machine measuring the spin along x or y. Or, the whole thing could just be a hoax with the machine flashing up 1/ 1 at random. Here, the notation X AY BY C means that Alice measured X, while Bob and Charlie measured Y.
11 . Quantum Mechanics in Your Face 1 Maybe this occurred because all three got the result 1; or perhaps one got 1 and the other two got 1. Since the central station doesn t know ahead of time which setting (X or Y) each scientist will choose, it has to prepare each package with definite states for both property X and property Y. The observed correlation in (..) is consistent with only the following 8 di erent shipments from the central station: = X A X B X C Y A Y B Y C = (..) Now, notice that (..) gives a prediction... if all three scientists measure X, theresults multiplied together must give 1. You can see this simply by multiplying the entries of the first columns in the matrices in (..). Alternative, we can prove the result using nothing but simple arithmetic. First, convince yourself that (..) implies The product of X A, X B, X C then is X A = Y B Y C, X B = Y A Y C, X C = Y A Y B. (..) X A X B X C =(Y B Y C )(Y A Y C )(Y A Y B ) =(Y A Y B Y C ) =1. (..8) Since (±1) = 1, we didn t need to know Y A Y B Y C to predict that X A X B X C = 1... Crazy, But True The GHZ experiment has been done. The things measured were the spins of electrons. Here is the astonishing truth: The experimenters observed the same correlations as in (..): However, instead of (..8), they found X A Y B Y C = Y A X B Y C = Y A Y B X C =1. (..9) X A X B X C = 1. (..0) In classical physics, this shouldn t happen, since (..8) was a logical consequence of (..). Something about our basic (classical) intuition for how the universe works is wrong Pan et al. (000), Experimental test of quantum non-locality in three-photon GHZ entanglement Nature 0 (9) 1-19.
12 . Quantum Mechanics.. Quantum Reality We assumed that the packages leaving the central station had definite assignments for the quantities X and Y we listed all possibilities in (..). But in the quantum world, we cannot give definite assignments to all possible measurements. Instead we have to allow for the possibility of a superposition of states and experimental outcomes being probabilistic. It turns out that this special feature of quantum mechanics resolves the puzzle. More concretely, Alice, Bob and Charlie were, in fact, measuring the spins of electrons along the x- and y-axes. In this case, the measurement matrices are X = 0 1, Y = 0 i. (..1) 1 0 i 0 You can check that these matrices both have eigenvalues 1 and 1, corresponding to the measurements. (But, X and Y do not have the same eigenvectors.) As before, we define two special state vectors corresponding to an electron spinning up or down relative to the z-axis, "i 1 0, #i 0 1. (..) These states are not eigenstates of X and Y. It is easy to see that acting with the matrix X on the up-state turns it into a down-state and vice versa: X "i = #i (..) X #i = "i. (..) Similarly, acting with the matrix Y also exchanges up- and down-states (up to factors of i and i) Y "i = i #i (..) Y #i = i "i. (..) Now, you are told that the central station sent out the following entangled state i = 1 p """i 1 p ###i. (..) This corresponds to the superposition of two states: a state with all spins up """i, and a state with all spins down ###i.
13 . Quantum Mechanics in Your Face As before, I am using a notation where the arrows are ordered: the first arrow corresponds to the spin of the first particle (the one sent to Alice), the second arrow corresponds to the spin of the second particle (the one sent to Bob), etc. The measurement matrix X A therefore acts on the first arrow of each state, X B on the second arrow, etc. Just to be clear, let me give an excessively explicit example X A Y B Y C """i = X A "i Y B "i Y C "i. (..8) Using (..) and (..), we find X A Y B Y C """i = (1) #i (i) #i (i) #i = (1)(i)(i) ###i = ###i. (..9) The state in (..) is an eigenvector of X A Y B Y C and Y A X B Y C and Y A Y B X C. And, importantly, it is also an eigenvector of X A X B X C. Let us check that this gives rise to the observed correlations: For instance, Similarly, we can show that X A Y B Y C i = X A Y B Y C h """i i ###i = (1)(i)(i) ###i (1)( i)( i) """i = ###i """i =1 i. (..0) Y A X B Y C i = Y A Y B X C i =1 i. (..1) So, whenever exactly one scientist measures X, the results indeed multiply to give 1. However, when all three scientists measure X, we get h i X A X B X C i = X A X B X C """i ###i = (1)(1)(1) ###i (1)(1)(1) """i = ###i """i = 1 i. (..) The classical expectation, eq. (..8), is wrong. But, it makes total sense in the quantum world. It was important that the spin states of the three particles weren t independent, but that they were entangled in the state (..) a superposition of all spins up """i and all spins down ###i. No matter how far the scientists are separated in space, this entanglement of spin states is reflected in their measurements. It is how the world works.
2 Quantum Mechanics. 2.1 The Strange Lives of Electrons
 2 Quantum Mechanics A philosopher once said, It is necessary for the very existence of science that the same conditions always produce the same results. Well, they don t! Richard Feynman Today, we re going
2 Quantum Mechanics A philosopher once said, It is necessary for the very existence of science that the same conditions always produce the same results. Well, they don t! Richard Feynman Today, we re going
Quantum Entanglement. Chapter Introduction. 8.2 Entangled Two-Particle States
 Chapter 8 Quantum Entanglement 8.1 Introduction In our final chapter on quantum mechanics we introduce the concept of entanglement. This is a feature of two-particle states (or multi-particle states) in
Chapter 8 Quantum Entanglement 8.1 Introduction In our final chapter on quantum mechanics we introduce the concept of entanglement. This is a feature of two-particle states (or multi-particle states) in
3. Quantum Mechanics in 3D
 3. Quantum Mechanics in 3D 3.1 Introduction Last time, we derived the time dependent Schrödinger equation, starting from three basic postulates: 1) The time evolution of a state can be expressed as a unitary
3. Quantum Mechanics in 3D 3.1 Introduction Last time, we derived the time dependent Schrödinger equation, starting from three basic postulates: 1) The time evolution of a state can be expressed as a unitary
1 Mathematical preliminaries
 1 Mathematical preliminaries The mathematical language of quantum mechanics is that of vector spaces and linear algebra. In this preliminary section, we will collect the various definitions and mathematical
1 Mathematical preliminaries The mathematical language of quantum mechanics is that of vector spaces and linear algebra. In this preliminary section, we will collect the various definitions and mathematical
1 Introduction. 1.1 Stuff we associate with quantum mechanics Schrödinger s Equation
 1 Introduction Quantum Theory and quantum mechanics belong to the greatest success stories of science. In a number of ways. First, quantum theories are among the most successful in terms of predicting
1 Introduction Quantum Theory and quantum mechanics belong to the greatest success stories of science. In a number of ways. First, quantum theories are among the most successful in terms of predicting
Quantum Mechanics- I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras
 Quantum Mechanics- I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras Lecture - 6 Postulates of Quantum Mechanics II (Refer Slide Time: 00:07) In my last lecture,
Quantum Mechanics- I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras Lecture - 6 Postulates of Quantum Mechanics II (Refer Slide Time: 00:07) In my last lecture,
E = hν light = hc λ = ( J s)( m/s) m = ev J = ev
 Problem The ionization potential tells us how much energy we need to use to remove an electron, so we know that any energy left afterwards will be the kinetic energy of the ejected electron. So first we
Problem The ionization potential tells us how much energy we need to use to remove an electron, so we know that any energy left afterwards will be the kinetic energy of the ejected electron. So first we
37-6 Watching the electrons (matter waves)
 37-6 Watching the electrons (matter waves) 1 testing our proposition: the electrons go either through hole 1 or hole 2 add a very strong light source behind walls between two holes, electrons will scatter
37-6 Watching the electrons (matter waves) 1 testing our proposition: the electrons go either through hole 1 or hole 2 add a very strong light source behind walls between two holes, electrons will scatter
Superposition - World of Color and Hardness
 Superposition - World of Color and Hardness We start our formal discussion of quantum mechanics with a story about something that can happen to various particles in the microworld, which we generically
Superposition - World of Color and Hardness We start our formal discussion of quantum mechanics with a story about something that can happen to various particles in the microworld, which we generically
Quantum Measurements: some technical background
 Quantum Measurements: some technical background [From the projection postulate to density matrices & (introduction to) von Neumann measurements] (AKA: the boring lecture) First: One more example I wanted
Quantum Measurements: some technical background [From the projection postulate to density matrices & (introduction to) von Neumann measurements] (AKA: the boring lecture) First: One more example I wanted
Review of the Formalism of Quantum Mechanics
 Review of the Formalism of Quantum Mechanics The postulates of quantum mechanics are often stated in textbooks. There are two main properties of physics upon which these postulates are based: 1)the probability
Review of the Formalism of Quantum Mechanics The postulates of quantum mechanics are often stated in textbooks. There are two main properties of physics upon which these postulates are based: 1)the probability
J = L + S. to this ket and normalize it. In this way we get expressions for all the kets
 Lecture 3 Relevant sections in text: 3.7, 3.9 Total Angular Momentum Eigenvectors How are the total angular momentum eigenvectors related to the original product eigenvectors (eigenvectors of L z and S
Lecture 3 Relevant sections in text: 3.7, 3.9 Total Angular Momentum Eigenvectors How are the total angular momentum eigenvectors related to the original product eigenvectors (eigenvectors of L z and S
Continuous quantum states, Particle on a line and Uncertainty relations
 Continuous quantum states, Particle on a line and Uncertainty relations So far we have considered k-level (discrete) quantum systems. Now we turn our attention to continuous quantum systems, such as a
Continuous quantum states, Particle on a line and Uncertainty relations So far we have considered k-level (discrete) quantum systems. Now we turn our attention to continuous quantum systems, such as a
2. Introduction to quantum mechanics
 2. Introduction to quantum mechanics 2.1 Linear algebra Dirac notation Complex conjugate Vector/ket Dual vector/bra Inner product/bracket Tensor product Complex conj. matrix Transpose of matrix Hermitian
2. Introduction to quantum mechanics 2.1 Linear algebra Dirac notation Complex conjugate Vector/ket Dual vector/bra Inner product/bracket Tensor product Complex conj. matrix Transpose of matrix Hermitian
Advanced Quantum Mechanics, Notes based on online course given by Leonard Susskind - Lecture 8
 Advanced Quantum Mechanics, Notes based on online course given by Leonard Susskind - Lecture 8 If neutrinos have different masses how do you mix and conserve energy Mass is energy. The eigenstates of energy
Advanced Quantum Mechanics, Notes based on online course given by Leonard Susskind - Lecture 8 If neutrinos have different masses how do you mix and conserve energy Mass is energy. The eigenstates of energy
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer. Lecture 6, January 30, 2006
 Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 6, January 30, 2006 Solved Homework We are given that A" = a" and A * " = a" where
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 6, January 30, 2006 Solved Homework We are given that A" = a" and A * " = a" where
. Here we are using the standard inner-product over C k to define orthogonality. Recall that the inner-product of two vectors φ = i α i.
 CS 94- Hilbert Spaces, Tensor Products, Quantum Gates, Bell States 1//07 Spring 007 Lecture 01 Hilbert Spaces Consider a discrete quantum system that has k distinguishable states (eg k distinct energy
CS 94- Hilbert Spaces, Tensor Products, Quantum Gates, Bell States 1//07 Spring 007 Lecture 01 Hilbert Spaces Consider a discrete quantum system that has k distinguishable states (eg k distinct energy
Sparks CH301. Quantum Mechanics. Waves? Particles? What and where are the electrons!? UNIT 2 Day 3. LM 14, 15 & 16 + HW due Friday, 8:45 am
 Sparks CH301 Quantum Mechanics Waves? Particles? What and where are the electrons!? UNIT 2 Day 3 LM 14, 15 & 16 + HW due Friday, 8:45 am What are we going to learn today? The Simplest Atom - Hydrogen Relate
Sparks CH301 Quantum Mechanics Waves? Particles? What and where are the electrons!? UNIT 2 Day 3 LM 14, 15 & 16 + HW due Friday, 8:45 am What are we going to learn today? The Simplest Atom - Hydrogen Relate
Lecture 3. QUANTUM MECHANICS FOR SINGLE QUBIT SYSTEMS 1. Vectors and Operators in Quantum State Space
 Lecture 3. QUANTUM MECHANICS FOR SINGLE QUBIT SYSTEMS 1. Vectors and Operators in Quantum State Space The principles of quantum mechanics and their application to the description of single and multiple
Lecture 3. QUANTUM MECHANICS FOR SINGLE QUBIT SYSTEMS 1. Vectors and Operators in Quantum State Space The principles of quantum mechanics and their application to the description of single and multiple
Introduction to Bell s theorem: the theory that solidified quantum mechanics
 Introduction to Bells theorem: the theory that solidified quantum mechanics Jia Wang Department of Chemistry, University of Michigan, 930 N. University Ave., Ann Arbor, MI 48109 (Received November 30,
Introduction to Bells theorem: the theory that solidified quantum mechanics Jia Wang Department of Chemistry, University of Michigan, 930 N. University Ave., Ann Arbor, MI 48109 (Received November 30,
Cosmology Lecture 2 Mr. Kiledjian
 Cosmology Lecture 2 Mr. Kiledjian Lecture 2: Quantum Mechanics & Its Different Views and Interpretations a) The story of quantum mechanics begins in the 19 th century as the physicists of that day were
Cosmology Lecture 2 Mr. Kiledjian Lecture 2: Quantum Mechanics & Its Different Views and Interpretations a) The story of quantum mechanics begins in the 19 th century as the physicists of that day were
Quantum Mechanics - I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras. Lecture - 7 The Uncertainty Principle
 Quantum Mechanics - I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras Lecture - 7 The Uncertainty Principle (Refer Slide Time: 00:07) In the last lecture, I had spoken
Quantum Mechanics - I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras Lecture - 7 The Uncertainty Principle (Refer Slide Time: 00:07) In the last lecture, I had spoken
Looking at Scripture with New Eyes: A Chance Conversation Between Faith and Science
 1 Looking at Scripture with New Eyes: A Chance Conversation Between Faith and Science William K. Lewis Fairmont Presbyterian Church College Ministry Team One of the things I really enjoy about education
1 Looking at Scripture with New Eyes: A Chance Conversation Between Faith and Science William K. Lewis Fairmont Presbyterian Church College Ministry Team One of the things I really enjoy about education
Lecture 14, Thurs March 2: Nonlocal Games
 Lecture 14, Thurs March 2: Nonlocal Games Last time we talked about the CHSH Game, and how no classical strategy lets Alice and Bob win it more than 75% of the time. Today we ll see how, by using entanglement,
Lecture 14, Thurs March 2: Nonlocal Games Last time we talked about the CHSH Game, and how no classical strategy lets Alice and Bob win it more than 75% of the time. Today we ll see how, by using entanglement,
Quantum Gates, Circuits & Teleportation
 Chapter 3 Quantum Gates, Circuits & Teleportation Unitary Operators The third postulate of quantum physics states that the evolution of a quantum system is necessarily unitary. Geometrically, a unitary
Chapter 3 Quantum Gates, Circuits & Teleportation Unitary Operators The third postulate of quantum physics states that the evolution of a quantum system is necessarily unitary. Geometrically, a unitary
The act of measuring a particle fundamentally disturbs its state.
 Chapter 1 Introduction Nature at the sub-atomic scale behaves totally differently from anything that our experience with the physical world prepares us for. Quantum mechanics is the name of the branch
Chapter 1 Introduction Nature at the sub-atomic scale behaves totally differently from anything that our experience with the physical world prepares us for. Quantum mechanics is the name of the branch
How does it work? QM describes the microscopic world in a way analogous to how classical mechanics (CM) describes the macroscopic world.
 Today Quantum Mechanics (QM) is used in the university and beyond on a regular basis: Chemical bonds NMR spectroscopy The laser (blue beam in Blue-ray player; red beam in a DVD player for example) The
Today Quantum Mechanics (QM) is used in the university and beyond on a regular basis: Chemical bonds NMR spectroscopy The laser (blue beam in Blue-ray player; red beam in a DVD player for example) The
09a. Collapse. Recall: There are two ways a quantum state can change: 1. In absence of measurement, states change via Schrödinger dynamics:
 09a. Collapse Recall: There are two ways a quantum state can change:. In absence of measurement, states change via Schrödinger dynamics: ψ(t )!!! ψ(t 2 ) Schrödinger evolution 2. In presence of measurement,
09a. Collapse Recall: There are two ways a quantum state can change:. In absence of measurement, states change via Schrödinger dynamics: ψ(t )!!! ψ(t 2 ) Schrödinger evolution 2. In presence of measurement,
Quantum Mechanics - I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras
 Quantum Mechanics - I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras Lecture - 14 Exercises on Quantum Expectation Values (Refer Slide Time: 00:07) In the last couple
Quantum Mechanics - I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras Lecture - 14 Exercises on Quantum Expectation Values (Refer Slide Time: 00:07) In the last couple
Quantum Mechanics- I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras
 Quantum Mechanics- I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras Lecture - 4 Postulates of Quantum Mechanics I In today s lecture I will essentially be talking
Quantum Mechanics- I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras Lecture - 4 Postulates of Quantum Mechanics I In today s lecture I will essentially be talking
1 Dirac Notation for Vector Spaces
 Theoretical Physics Notes 2: Dirac Notation This installment of the notes covers Dirac notation, which proves to be very useful in many ways. For example, it gives a convenient way of expressing amplitudes
Theoretical Physics Notes 2: Dirac Notation This installment of the notes covers Dirac notation, which proves to be very useful in many ways. For example, it gives a convenient way of expressing amplitudes
The Collapse of the Wave Function
 Chapter 7 The Collapse of the Wave Function At this point, it s worth taking a step back and reviewing where we are. We started with some observations about how electron spins function, and how it s very
Chapter 7 The Collapse of the Wave Function At this point, it s worth taking a step back and reviewing where we are. We started with some observations about how electron spins function, and how it s very
Modern Physics notes Spring 2007 Paul Fendley Lecture 27
 Modern Physics notes Spring 2007 Paul Fendley fendley@virginia.edu Lecture 27 Angular momentum and positronium decay The EPR paradox Feynman, 8.3,.4 Blanton, http://math.ucr.edu/home/baez/physics/quantum/bells
Modern Physics notes Spring 2007 Paul Fendley fendley@virginia.edu Lecture 27 Angular momentum and positronium decay The EPR paradox Feynman, 8.3,.4 Blanton, http://math.ucr.edu/home/baez/physics/quantum/bells
Quantum Mechanics-I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras. Lecture - 21 Square-Integrable Functions
 Quantum Mechanics-I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras Lecture - 21 Square-Integrable Functions (Refer Slide Time: 00:06) (Refer Slide Time: 00:14) We
Quantum Mechanics-I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras Lecture - 21 Square-Integrable Functions (Refer Slide Time: 00:06) (Refer Slide Time: 00:14) We
Bell s inequalities and their uses
 The Quantum Theory of Information and Computation http://www.comlab.ox.ac.uk/activities/quantum/course/ Bell s inequalities and their uses Mark Williamson mark.williamson@wofson.ox.ac.uk 10.06.10 Aims
The Quantum Theory of Information and Computation http://www.comlab.ox.ac.uk/activities/quantum/course/ Bell s inequalities and their uses Mark Williamson mark.williamson@wofson.ox.ac.uk 10.06.10 Aims
The quantum state as a vector
 The quantum state as a vector February 6, 27 Wave mechanics In our review of the development of wave mechanics, we have established several basic properties of the quantum description of nature:. A particle
The quantum state as a vector February 6, 27 Wave mechanics In our review of the development of wave mechanics, we have established several basic properties of the quantum description of nature:. A particle
Rutherford s Gold Foil Experiment. Quantum Theory Max Planck (1910)
 Neils Bohr Niels Bohr (1913) developed the planetary model of the atom based upon the following: Rutherford s Gold Foil Experiment E = mc 2 Albert Einstein (1905) Quantum Theory Max Planck (1910) He postulated
Neils Bohr Niels Bohr (1913) developed the planetary model of the atom based upon the following: Rutherford s Gold Foil Experiment E = mc 2 Albert Einstein (1905) Quantum Theory Max Planck (1910) He postulated
226 My God, He Plays Dice! Entanglement. Chapter This chapter on the web informationphilosopher.com/problems/entanglement
 226 My God, He Plays Dice! Entanglement Chapter 29 20 This chapter on the web informationphilosopher.com/problems/entanglement Entanglement 227 Entanglement Entanglement is a mysterious quantum phenomenon
226 My God, He Plays Dice! Entanglement Chapter 29 20 This chapter on the web informationphilosopher.com/problems/entanglement Entanglement 227 Entanglement Entanglement is a mysterious quantum phenomenon
II. The Machinery of Quantum Mechanics
 II. The Machinery of Quantum Mechanics Based on the results of the experiments described in the previous section, we recognize that real experiments do not behave quite as we expect. This section presents
II. The Machinery of Quantum Mechanics Based on the results of the experiments described in the previous section, we recognize that real experiments do not behave quite as we expect. This section presents
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer. Lecture 20, March 8, 2006
 Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 20, March 8, 2006 Solved Homework We determined that the two coefficients in our two-gaussian
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 20, March 8, 2006 Solved Homework We determined that the two coefficients in our two-gaussian
6.080/6.089 GITCS May 6-8, Lecture 22/23. α 0 + β 1. α 2 + β 2 = 1
 6.080/6.089 GITCS May 6-8, 2008 Lecturer: Scott Aaronson Lecture 22/23 Scribe: Chris Granade 1 Quantum Mechanics 1.1 Quantum states of n qubits If you have an object that can be in two perfectly distinguishable
6.080/6.089 GITCS May 6-8, 2008 Lecturer: Scott Aaronson Lecture 22/23 Scribe: Chris Granade 1 Quantum Mechanics 1.1 Quantum states of n qubits If you have an object that can be in two perfectly distinguishable
Quantum Mechanics: Interpretation and Philosophy
 Quantum Mechanics: Interpretation and Philosophy Significant content from: Quantum Mechanics and Experience by David Z. Albert, Harvard University Press (1992). Main Concepts: -- complementarity -- the
Quantum Mechanics: Interpretation and Philosophy Significant content from: Quantum Mechanics and Experience by David Z. Albert, Harvard University Press (1992). Main Concepts: -- complementarity -- the
MITOCW ocw lec8
 MITOCW ocw-5.112-lec8 The following content is provided by MIT OpenCourseWare under a Creative Commons license. Additional information about our license and MIT OpenCourseWare in general is available at
MITOCW ocw-5.112-lec8 The following content is provided by MIT OpenCourseWare under a Creative Commons license. Additional information about our license and MIT OpenCourseWare in general is available at
What is Quantum Theory?
 What is Quantum Theory? Quantum theory is the theoretical basis of modern physics that explains the nature and behavior of matter and energy on the atomic and subatomic level. Quantum theory evolved as
What is Quantum Theory? Quantum theory is the theoretical basis of modern physics that explains the nature and behavior of matter and energy on the atomic and subatomic level. Quantum theory evolved as
2 Foundations of Quantum mechanics
 2 Foundations of Quantum mechanics 2.1 The Stern-Gerlach experiments Two ways of learning Quantum Mechanics: historical perspective: experimental findings between 1905 to 1922 concentrate on one of these
2 Foundations of Quantum mechanics 2.1 The Stern-Gerlach experiments Two ways of learning Quantum Mechanics: historical perspective: experimental findings between 1905 to 1922 concentrate on one of these
1 Measurements, Tensor Products, and Entanglement
 Stanford University CS59Q: Quantum Computing Handout Luca Trevisan September 7, 0 Lecture In which we describe the quantum analogs of product distributions, independence, and conditional probability, and
Stanford University CS59Q: Quantum Computing Handout Luca Trevisan September 7, 0 Lecture In which we describe the quantum analogs of product distributions, independence, and conditional probability, and
1 Fundamental physical postulates. C/CS/Phys C191 Quantum Mechanics in a Nutshell I 10/04/07 Fall 2007 Lecture 12
 C/CS/Phys C191 Quantum Mechanics in a Nutshell I 10/04/07 Fall 2007 Lecture 12 In this and the next lecture we summarize the essential physical and mathematical aspects of quantum mechanics relevant to
C/CS/Phys C191 Quantum Mechanics in a Nutshell I 10/04/07 Fall 2007 Lecture 12 In this and the next lecture we summarize the essential physical and mathematical aspects of quantum mechanics relevant to
Electron in a Box. A wave packet in a square well (an electron in a box) changing with time.
 Electron in a Box A wave packet in a square well (an electron in a box) changing with time. Last Time: Light Wave model: Interference pattern is in terms of wave intensity Photon model: Interference in
Electron in a Box A wave packet in a square well (an electron in a box) changing with time. Last Time: Light Wave model: Interference pattern is in terms of wave intensity Photon model: Interference in
6.896 Quantum Complexity Theory September 9, Lecture 2
 6.96 Quantum Complexity Theory September 9, 00 Lecturer: Scott Aaronson Lecture Quick Recap The central object of study in our class is BQP, which stands for Bounded error, Quantum, Polynomial time. Informally
6.96 Quantum Complexity Theory September 9, 00 Lecturer: Scott Aaronson Lecture Quick Recap The central object of study in our class is BQP, which stands for Bounded error, Quantum, Polynomial time. Informally
The Relativistic Quantum World
 The Relativistic Quantum World A lecture series on Relativity Theory and Quantum Mechanics Marcel Merk University of Maastricht, Sept 24 Oct 15, 2014 Relativity Quantum Mechanics The Relativistic Quantum
The Relativistic Quantum World A lecture series on Relativity Theory and Quantum Mechanics Marcel Merk University of Maastricht, Sept 24 Oct 15, 2014 Relativity Quantum Mechanics The Relativistic Quantum
ECE 487 Lecture 6 : Time-Dependent Quantum Mechanics I Class Outline:
 ECE 487 Lecture 6 : Time-Dependent Quantum Mechanics I Class Outline: Time-Dependent Schrödinger Equation Solutions to thetime-dependent Schrödinger Equation Expansion of Energy Eigenstates Things you
ECE 487 Lecture 6 : Time-Dependent Quantum Mechanics I Class Outline: Time-Dependent Schrödinger Equation Solutions to thetime-dependent Schrödinger Equation Expansion of Energy Eigenstates Things you
OPERATORS AND MEASUREMENT
 Chapter OPERATORS AND MEASUREMENT In Chapter we used the results of experiments to deduce a mathematical description of the spin-/ system. The Stern-Gerlach experiments demonstrated that spin component
Chapter OPERATORS AND MEASUREMENT In Chapter we used the results of experiments to deduce a mathematical description of the spin-/ system. The Stern-Gerlach experiments demonstrated that spin component
Hilbert Space, Entanglement, Quantum Gates, Bell States, Superdense Coding.
 CS 94- Bell States Bell Inequalities 9//04 Fall 004 Lecture Hilbert Space Entanglement Quantum Gates Bell States Superdense Coding 1 One qubit: Recall that the state of a single qubit can be written as
CS 94- Bell States Bell Inequalities 9//04 Fall 004 Lecture Hilbert Space Entanglement Quantum Gates Bell States Superdense Coding 1 One qubit: Recall that the state of a single qubit can be written as
AN INTRODUCTION TO QUANTUM COMPUTING
 AN INTRODUCTION TO QUANTUM COMPUTING NOSON S YANOFSKY Abstract Quantum Computing is a new and exciting field at the intersection of mathematics, computer science and physics It concerns a utilization of
AN INTRODUCTION TO QUANTUM COMPUTING NOSON S YANOFSKY Abstract Quantum Computing is a new and exciting field at the intersection of mathematics, computer science and physics It concerns a utilization of
Modern Physics notes Paul Fendley Lecture 3
 Modern Physics notes Paul Fendley fendley@virginia.edu Lecture 3 Electron Wavelength Probability Amplitude Which slit? Photons Born, IV.4 Feynman, 1.6-7, 2.1 Fowler, Rays and Particles The wavelength of
Modern Physics notes Paul Fendley fendley@virginia.edu Lecture 3 Electron Wavelength Probability Amplitude Which slit? Photons Born, IV.4 Feynman, 1.6-7, 2.1 Fowler, Rays and Particles The wavelength of
Philosophy of QM Eighth lecture, 23 February 2005
 Philosophy of QM 24.111 Eighth lecture, 23 February 2005 Strategy for analysis 1. Break the experimental situation up into steps. 2. At each step, look for easy cases. 3. Describe hard cases as linear
Philosophy of QM 24.111 Eighth lecture, 23 February 2005 Strategy for analysis 1. Break the experimental situation up into steps. 2. At each step, look for easy cases. 3. Describe hard cases as linear
The Postulates of Quantum Mechanics Common operators in QM: Potential Energy. Often depends on position operator: Kinetic Energy 1-D case: 3-D case
 The Postulates of Quantum Mechanics Common operators in QM: Potential Energy Often depends on position operator: Kinetic Energy 1-D case: 3-D case Time Total energy = Hamiltonian To find out about the
The Postulates of Quantum Mechanics Common operators in QM: Potential Energy Often depends on position operator: Kinetic Energy 1-D case: 3-D case Time Total energy = Hamiltonian To find out about the
PLEASE LET ME KNOW IF YOU FIND TYPOS (send to
 Teoretisk Fysik KTH Advanced QM (SI2380), Lecture 2 (Summary of concepts) 1 PLEASE LET ME KNOW IF YOU FIND TYPOS (send email to langmann@kth.se) The laws of QM 1. I now discuss the laws of QM and their
Teoretisk Fysik KTH Advanced QM (SI2380), Lecture 2 (Summary of concepts) 1 PLEASE LET ME KNOW IF YOU FIND TYPOS (send email to langmann@kth.se) The laws of QM 1. I now discuss the laws of QM and their
Chapter 2. Mathematical Reasoning. 2.1 Mathematical Models
 Contents Mathematical Reasoning 3.1 Mathematical Models........................... 3. Mathematical Proof............................ 4..1 Structure of Proofs........................ 4.. Direct Method..........................
Contents Mathematical Reasoning 3.1 Mathematical Models........................... 3. Mathematical Proof............................ 4..1 Structure of Proofs........................ 4.. Direct Method..........................
Alan Mortimer PhD. Ideas of Modern Physics
 Alan Mortimer PhD Ideas of Modern Physics Electromagnetic Waves Last Week Special Relativity General Relativity The Quantum World Index Planck s Law Atomic Structure and emission lines Matter waves Uncertainty
Alan Mortimer PhD Ideas of Modern Physics Electromagnetic Waves Last Week Special Relativity General Relativity The Quantum World Index Planck s Law Atomic Structure and emission lines Matter waves Uncertainty
Basics on quantum information
 Basics on quantum information Mika Hirvensalo Department of Mathematics and Statistics University of Turku mikhirve@utu.fi Thessaloniki, May 2016 Mika Hirvensalo Basics on quantum information 1 of 52 Brief
Basics on quantum information Mika Hirvensalo Department of Mathematics and Statistics University of Turku mikhirve@utu.fi Thessaloniki, May 2016 Mika Hirvensalo Basics on quantum information 1 of 52 Brief
Odd Things about Quantum Mechanics: Abandoning Determinism In Newtonian physics, Maxwell theory, Einstein's special or general relativity, if an initi
 Odd Things about Quantum Mechanics: Abandoning Determinism In Newtonian physics, Maxwell theory, Einstein's special or general relativity, if an initial state is completely known, the future can be predicted.
Odd Things about Quantum Mechanics: Abandoning Determinism In Newtonian physics, Maxwell theory, Einstein's special or general relativity, if an initial state is completely known, the future can be predicted.
Basics on quantum information
 Basics on quantum information Mika Hirvensalo Department of Mathematics and Statistics University of Turku mikhirve@utu.fi Thessaloniki, May 2014 Mika Hirvensalo Basics on quantum information 1 of 49 Brief
Basics on quantum information Mika Hirvensalo Department of Mathematics and Statistics University of Turku mikhirve@utu.fi Thessaloniki, May 2014 Mika Hirvensalo Basics on quantum information 1 of 49 Brief
The Bohr Model of Hydrogen, a Summary, Review
 The Bohr Model of Hydrogen, a Summary, Review Allowed electron orbital radii and speeds: Allowed electron energy levels: Problems with the Bohr Model Bohr s model for the atom was a huge success in that
The Bohr Model of Hydrogen, a Summary, Review Allowed electron orbital radii and speeds: Allowed electron energy levels: Problems with the Bohr Model Bohr s model for the atom was a huge success in that
T he Science of. What exactly is quantum physics? Until the end of the nineteenth century, scientists thought
 T he Science of From radioactive bananas to cats that are both dead and alive at the same time, here s more about the real- life science in The Many Worlds of Albie Bright. Wait a minute, are bananas really
T he Science of From radioactive bananas to cats that are both dead and alive at the same time, here s more about the real- life science in The Many Worlds of Albie Bright. Wait a minute, are bananas really
Select/Special Topics in Atomic Physics Prof. P. C. Deshmukh Department of Physics Indian Institute of Technology, Madras
 Select/Special Topics in Atomic Physics Prof. P. C. Deshmukh Department of Physics Indian Institute of Technology, Madras Lecture - 9 Angular Momentum in Quantum Mechanics Dimensionality of the Direct-Product
Select/Special Topics in Atomic Physics Prof. P. C. Deshmukh Department of Physics Indian Institute of Technology, Madras Lecture - 9 Angular Momentum in Quantum Mechanics Dimensionality of the Direct-Product
Quantum Physics & Reality
 Quantum Physics & Reality Todd Duncan Science Integration Institute (www.scienceintegration.org) & PSU Center for Science Education Anyone who is not shocked by quantum theory has not understood it. -
Quantum Physics & Reality Todd Duncan Science Integration Institute (www.scienceintegration.org) & PSU Center for Science Education Anyone who is not shocked by quantum theory has not understood it. -
Introduction to Quantum Mechanics
 Introduction to Quantum Mechanics R. J. Renka Department of Computer Science & Engineering University of North Texas 03/19/2018 Postulates of Quantum Mechanics The postulates (axioms) of quantum mechanics
Introduction to Quantum Mechanics R. J. Renka Department of Computer Science & Engineering University of North Texas 03/19/2018 Postulates of Quantum Mechanics The postulates (axioms) of quantum mechanics
People can't travel to the past, but scientists not so sure about quarks
 People can't travel to the past, but scientists not so sure about quarks By Scientific American, adapted by Newsela staff on 10.14.14 Word Count 1,446 Visitors explore an imaginary time machine, part of
People can't travel to the past, but scientists not so sure about quarks By Scientific American, adapted by Newsela staff on 10.14.14 Word Count 1,446 Visitors explore an imaginary time machine, part of
Rotations in Quantum Mechanics
 Rotations in Quantum Mechanics We have seen that physical transformations are represented in quantum mechanics by unitary operators acting on the Hilbert space. In this section, we ll think about the specific
Rotations in Quantum Mechanics We have seen that physical transformations are represented in quantum mechanics by unitary operators acting on the Hilbert space. In this section, we ll think about the specific
Basic Quantum Mechanics Prof. Ajoy Ghatak Department of Physics Indian Institute of Technology, Delhi
 Basic Quantum Mechanics Prof. Ajoy Ghatak Department of Physics Indian Institute of Technology, Delhi Module No. # 07 Bra-Ket Algebra and Linear Harmonic Oscillator II Lecture No. # 02 Dirac s Bra and
Basic Quantum Mechanics Prof. Ajoy Ghatak Department of Physics Indian Institute of Technology, Delhi Module No. # 07 Bra-Ket Algebra and Linear Harmonic Oscillator II Lecture No. # 02 Dirac s Bra and
The Future. Currently state of the art chips have gates of length 35 nanometers.
 Quantum Computing Moore s Law The Future Currently state of the art chips have gates of length 35 nanometers. The Future Currently state of the art chips have gates of length 35 nanometers. When gate lengths
Quantum Computing Moore s Law The Future Currently state of the art chips have gates of length 35 nanometers. The Future Currently state of the art chips have gates of length 35 nanometers. When gate lengths
Select/ Special Topics in Atomic Physics Prof. P. C. Deshmukh Department of Physics Indian Institute of Technology, Madras
 Select/ Special Topics in Atomic Physics Prof. P. C. Deshmukh Department of Physics Indian Institute of Technology, Madras Lecture No. # 06 Angular Momentum in Quantum Mechanics Greetings, we will begin
Select/ Special Topics in Atomic Physics Prof. P. C. Deshmukh Department of Physics Indian Institute of Technology, Madras Lecture No. # 06 Angular Momentum in Quantum Mechanics Greetings, we will begin
Lecture 4 (Sep. 18, 2017)
 Lecture 4 8.3 Quantum Theory I, Fall 07 Lecture 4 (Sep. 8, 07) 4. Measurement 4.. Spin- Systems Last time, we said that a general state in a spin- system can be written as ψ = c + + + c, (4.) where +,
Lecture 4 8.3 Quantum Theory I, Fall 07 Lecture 4 (Sep. 8, 07) 4. Measurement 4.. Spin- Systems Last time, we said that a general state in a spin- system can be written as ψ = c + + + c, (4.) where +,
MITOCW 5. Quantum Mechanics: Free Particle and Particle in 1D Box
 MITOCW 5. Quantum Mechanics: Free Particle and Particle in 1D Box The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality
MITOCW 5. Quantum Mechanics: Free Particle and Particle in 1D Box The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality
Chemistry Objective: SWBAT describe the changes made to the model of the atom over time. Chemistry Warmup:
 Chemistry Objective: SWBAT describe the changes made to the model of the atom over time. Chemistry Warmup: 1. Pick up a set of the notes for today from the first lab table. 2. Take out your lab activity
Chemistry Objective: SWBAT describe the changes made to the model of the atom over time. Chemistry Warmup: 1. Pick up a set of the notes for today from the first lab table. 2. Take out your lab activity
Unitary evolution: this axiom governs how the state of the quantum system evolves in time.
 CS 94- Introduction Axioms Bell Inequalities /7/7 Spring 7 Lecture Why Quantum Computation? Quantum computers are the only model of computation that escape the limitations on computation imposed by the
CS 94- Introduction Axioms Bell Inequalities /7/7 Spring 7 Lecture Why Quantum Computation? Quantum computers are the only model of computation that escape the limitations on computation imposed by the
Phil Notes #7: Time Travel
 Phil. 2750 Notes #7: Time Travel I. Issues/Problems about Time Travel Some things that commonly happen in time travel stories: - Backward causation: When A causes B, even though A occurs after B. - Circular
Phil. 2750 Notes #7: Time Travel I. Issues/Problems about Time Travel Some things that commonly happen in time travel stories: - Backward causation: When A causes B, even though A occurs after B. - Circular
Lecture 1: Overview of quantum information
 CPSC 59/69: Quantum Computation John Watrous, University of Calgary References Lecture : Overview of quantum information January 0, 006 Most of the material in these lecture notes is discussed in greater
CPSC 59/69: Quantum Computation John Watrous, University of Calgary References Lecture : Overview of quantum information January 0, 006 Most of the material in these lecture notes is discussed in greater
1 Measurement and expectation values
 C/CS/Phys 191 Measurement and expectation values, Intro to Spin 2/15/05 Spring 2005 Lecture 9 1 Measurement and expectation values Last time we discussed how useful it is to work in the basis of energy
C/CS/Phys 191 Measurement and expectation values, Intro to Spin 2/15/05 Spring 2005 Lecture 9 1 Measurement and expectation values Last time we discussed how useful it is to work in the basis of energy
chmy361 Lec42 Tue 29nov16
 chmy361 Lec42 Tue 29nov16 1 Quantum Behavior & Quantum Mechanics Applies to EVERYTHING But most evident for particles with mass equal or less than proton Absolutely NECESSARY for electrons and light (photons),
chmy361 Lec42 Tue 29nov16 1 Quantum Behavior & Quantum Mechanics Applies to EVERYTHING But most evident for particles with mass equal or less than proton Absolutely NECESSARY for electrons and light (photons),
C/CS/Phys C191 Quantum Mechanics in a Nutshell 10/06/07 Fall 2009 Lecture 12
 C/CS/Phys C191 Quantum Mechanics in a Nutshell 10/06/07 Fall 2009 Lecture 12 In this lecture we summarize the essential physical and mathematical aspects of quantum mechanics relevant to this course. Topics
C/CS/Phys C191 Quantum Mechanics in a Nutshell 10/06/07 Fall 2009 Lecture 12 In this lecture we summarize the essential physical and mathematical aspects of quantum mechanics relevant to this course. Topics
Quantum mysteries revisited again
 Quantum mysteries revisited again P. K. Aravind a) Physics Department, Worcester Polytechnic Institute, Worcester, Massachusetts 01609 Received 30 April 2002; accepted 21 May 2004 This paper describes
Quantum mysteries revisited again P. K. Aravind a) Physics Department, Worcester Polytechnic Institute, Worcester, Massachusetts 01609 Received 30 April 2002; accepted 21 May 2004 This paper describes
Bell s Theorem 1964 Local realism is in conflict with quantum mechanics
 Bell s Theorem 1964 Local realism is in conflict with quantum mechanics the most profound discovery in science in the last half of the twentieth century. For a technical presentation search Youtube.com
Bell s Theorem 1964 Local realism is in conflict with quantum mechanics the most profound discovery in science in the last half of the twentieth century. For a technical presentation search Youtube.com
1.1 Quantum mechanics of one particle
 1 Second quantization 1.1 Quantum mechanics of one particle In quantum mechanics the physical state of a particle is described in terms of a ket Ψ. This ket belongs to a Hilbert space which is nothing
1 Second quantization 1.1 Quantum mechanics of one particle In quantum mechanics the physical state of a particle is described in terms of a ket Ψ. This ket belongs to a Hilbert space which is nothing
Quantum decoherence. Éric Oliver Paquette (U. Montréal) -Traces Worshop [Ottawa]- April 29 th, Quantum decoherence p. 1/2
![Quantum decoherence. Éric Oliver Paquette (U. Montréal) -Traces Worshop [Ottawa]- April 29 th, Quantum decoherence p. 1/2 Quantum decoherence. Éric Oliver Paquette (U. Montréal) -Traces Worshop [Ottawa]- April 29 th, Quantum decoherence p. 1/2](/thumbs/78/77811482.jpg) Quantum decoherence p. 1/2 Quantum decoherence Éric Oliver Paquette (U. Montréal) -Traces Worshop [Ottawa]- April 29 th, 2007 Quantum decoherence p. 2/2 Outline Quantum decoherence: 1. Basics of quantum
Quantum decoherence p. 1/2 Quantum decoherence Éric Oliver Paquette (U. Montréal) -Traces Worshop [Ottawa]- April 29 th, 2007 Quantum decoherence p. 2/2 Outline Quantum decoherence: 1. Basics of quantum
C/CS/Phys 191 Uncertainty principle, Spin Algebra 10/11/05 Fall 2005 Lecture 13
 C/CS/Phys 191 Uncertainty principle, Spin Algebra 10/11/05 Fall 2005 Lecture 13 1 Readings Uncertainty principle: Griffiths, Introduction to QM, Ch. 3.4. Spin: Griffiths, Introduction to QM, Ch. 4.4; Liboff,
C/CS/Phys 191 Uncertainty principle, Spin Algebra 10/11/05 Fall 2005 Lecture 13 1 Readings Uncertainty principle: Griffiths, Introduction to QM, Ch. 3.4. Spin: Griffiths, Introduction to QM, Ch. 4.4; Liboff,
MITOCW watch?v=r2nmwesncts
 MITOCW watch?v=r2nmwesncts The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality educational resources for free. To
MITOCW watch?v=r2nmwesncts The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality educational resources for free. To
Decoherence and The Collapse of Quantum Mechanics. A Modern View
 Decoherence and The Collapse of Quantum Mechanics A Modern View It s time to make decoherence mainstream QM is ~90 years old But it is still taught like the 1930s Modern textbooks still ignore measurement
Decoherence and The Collapse of Quantum Mechanics A Modern View It s time to make decoherence mainstream QM is ~90 years old But it is still taught like the 1930s Modern textbooks still ignore measurement
Page 52. Lecture 3: Inner Product Spaces Dual Spaces, Dirac Notation, and Adjoints Date Revised: 2008/10/03 Date Given: 2008/10/03
 Page 5 Lecture : Inner Product Spaces Dual Spaces, Dirac Notation, and Adjoints Date Revised: 008/10/0 Date Given: 008/10/0 Inner Product Spaces: Definitions Section. Mathematical Preliminaries: Inner
Page 5 Lecture : Inner Product Spaces Dual Spaces, Dirac Notation, and Adjoints Date Revised: 008/10/0 Date Given: 008/10/0 Inner Product Spaces: Definitions Section. Mathematical Preliminaries: Inner
Open quantum systems
 Chapter 4 Open quantum systems 4. Quantum operations Let s go back for a second to the basic postulates of quantum mechanics. Recall that when we first establish the theory, we begin by postulating that
Chapter 4 Open quantum systems 4. Quantum operations Let s go back for a second to the basic postulates of quantum mechanics. Recall that when we first establish the theory, we begin by postulating that
Lecture 4: Postulates of quantum mechanics
 Lecture 4: Postulates of quantum mechanics Rajat Mittal IIT Kanpur The postulates of quantum mechanics provide us the mathematical formalism over which the physical theory is developed. For people studying
Lecture 4: Postulates of quantum mechanics Rajat Mittal IIT Kanpur The postulates of quantum mechanics provide us the mathematical formalism over which the physical theory is developed. For people studying
C/CS/Phys 191 Quantum Mechanics in a Nutshell I 10/04/05 Fall 2005 Lecture 11
 C/CS/Phys 191 Quantum Mechanics in a Nutshell I 10/04/05 Fall 2005 Lecture 11 In this and the next lecture we summarize the essential physical and mathematical aspects of quantum mechanics relevant to
C/CS/Phys 191 Quantum Mechanics in a Nutshell I 10/04/05 Fall 2005 Lecture 11 In this and the next lecture we summarize the essential physical and mathematical aspects of quantum mechanics relevant to
How to use the simulator
 How to use the simulator Overview The application allows for the exploration of four quantum circuits in all. Each simulator grants the user a large amount of freedom in experments they wish to conduct.
How to use the simulator Overview The application allows for the exploration of four quantum circuits in all. Each simulator grants the user a large amount of freedom in experments they wish to conduct.
QM and Angular Momentum
 Chapter 5 QM and Angular Momentum 5. Angular Momentum Operators In your Introductory Quantum Mechanics (QM) course you learned about the basic properties of low spin systems. Here we want to review that
Chapter 5 QM and Angular Momentum 5. Angular Momentum Operators In your Introductory Quantum Mechanics (QM) course you learned about the basic properties of low spin systems. Here we want to review that
This is the important completeness relation,
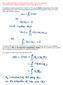 Observable quantities are represented by linear, hermitian operators! Compatible observables correspond to commuting operators! In addition to what was written in eqn 2.1.1, the vector corresponding to
Observable quantities are represented by linear, hermitian operators! Compatible observables correspond to commuting operators! In addition to what was written in eqn 2.1.1, the vector corresponding to
Quantum Mechanics - I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras. Lecture - 9 Introducing Quantum Optics
 Quantum Mechanics - I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras Lecture - 9 Introducing Quantum Optics (Refer Slide Time: 00:07) In the last lecture I gave
Quantum Mechanics - I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras Lecture - 9 Introducing Quantum Optics (Refer Slide Time: 00:07) In the last lecture I gave
General Physical Chemistry II
 General Physical Chemistry II Lecture 3 Aleksey Kocherzhenko September 2, 2014" Last time " The time-independent Schrödinger equation" Erwin Schrödinger " ~ 2 2m d 2 (x) dx 2 The wavefunction:" (x) The
General Physical Chemistry II Lecture 3 Aleksey Kocherzhenko September 2, 2014" Last time " The time-independent Schrödinger equation" Erwin Schrödinger " ~ 2 2m d 2 (x) dx 2 The wavefunction:" (x) The
Lecture #1. Review. Postulates of quantum mechanics (1-3) Postulate 1
 L1.P1 Lecture #1 Review Postulates of quantum mechanics (1-3) Postulate 1 The state of a system at any instant of time may be represented by a wave function which is continuous and differentiable. Specifically,
L1.P1 Lecture #1 Review Postulates of quantum mechanics (1-3) Postulate 1 The state of a system at any instant of time may be represented by a wave function which is continuous and differentiable. Specifically,
QFT. Unit 1: Relativistic Quantum Mechanics
 QFT Unit 1: Relativistic Quantum Mechanics What s QFT? Relativity deals with things that are fast Quantum mechanics deals with things that are small QFT deals with things that are both small and fast What
QFT Unit 1: Relativistic Quantum Mechanics What s QFT? Relativity deals with things that are fast Quantum mechanics deals with things that are small QFT deals with things that are both small and fast What
