PHYSICS nd TERM Outline Notes (continued)
|
|
|
- Mercy Hawkins
- 5 years ago
- Views:
Transcription
1 PHYSICS nd TERM Outline Notes (continued) Section 6. Optical Properties (see also textbook, chapter 15) This section will be concerned with how electromagnetic radiation (visible light, in particular) interacts with different solids (metals, semiconductors, insulators); absorption, reflection and transmission of light; applications like photoconductivity, lasers, optical fibres, photonic band-gap materials. 6.1 Basic properties of electromagnetic radiation (see also textbook, sections 15.2) An electromagnetic (em) wave describes the wave-like fluctuations of the electric field E and the magnetic field H in vacuum or in any medium. The E and H fields are related to one another through Maxwell s equations of electromagnetism: they travel at the same speed (the speed of light), and they are perpendicular to the direction of propagation (transverse waves) and also perpendicular to each other. E An electromagnetic wave showing electric field E and magnetic field H components, as well as wavelength λ. The spectrum of em radiation, including wavelength ranges for the various colours in the visible spectrum. The conversion between different units is also shown. 53
2 The visible part has wavelength λ from roughly 0.4 µm (or 400 nm) [violet/blue end] up to 0.7 µm (or 700 nm) [red end]. All em waves travel at the speed of light, so when in a vacuum this is 1 c = = m/s approx ε µ 0 0 and c = νλ, where ν = frequency (in Hz) Recall that when em radiation is quantized, it comes in packets, or photons, of energy E = hν = hc/λ (Planck s hypothesis) When light passes from a vacuum region to a solid medium, some may be transmitted, some reflected back, and some absorbed in the medium. These fractions depend on the wavelength of the incident light and there may be some (usually small) conversion to different wavelengths. In terms of the corresponding intensities (defined by energy flow per unit time per unit area perpendicular to the direction of flow), we have Incident intensity I 0 = I T + I A + I R, by conservation of energy. Dividing by I 0, the above result becomes T + A + R = 1 where T = I T /I 0 = transmissivity, A = I A /I 0 = absorptivity, R = I R /I 0 = reflectivity. Some terminology applied to materials: transparent very little absorption (A 0) translucent light transmitted diffusively (objects cannot be seen clearly through the material) opaque very little transmission Example of light transmittance of 3 different Al 2 O 3 samples: a single-crystal sapphire (left) which is transparent; a dense (non-porous) polycrystalline sample (centre) which is translucent; a porous polycrystalline sample (right) which is opaque. These properties can be very dependent on the wavelength λ (and therefore on the corresponding energy scale). 54
3 In general, the process of absorption (or its absence in transparent materials) depends on the electronic transitions. For isolated atoms: Schematic illustration of photon absorption in an isolated atom by the excitation of an electron from one energy state to another. The photon energy must be exactly equal to the energy difference between the two states (E 4 E 2 in this case). The spacing between energy levels must be such that E = hν If this is satisfied, then there is absorption of a photon at that frequency all of the photon energy is absorbed the atom is then in an excited state (energy is above the ground state) the electron can drop to lower levels by emitting radiation (photon), allowing several possibilities, as discussed later. In solids, the atoms cannot usually be considered as isolated. They interact giving rise to bands and band gaps, so the situation is then different for metals, semiconductors and insulators. Optical properties of metals in metals there are energy bands without occurrence of any band gaps so all radiation can be absorbed (there are lots of energy levels close together, so there are no restriction on E or ν). Metals are opaque (unless they are extremely thin, e.g. 5 nm film of Fe). (a) Schematic representation of the photon absorption process in a metal, where an electron is excited into a higher-energy excited state. The change E in the electron energy is equal to the energy of the absorbed photon. (b) The subsequent reemission of a photon by direct transition of the electron from the The above is not applicable in the case of non-metals because there are band gaps for the electrons. There would be restrictions on E and hence on frequency ν for absorption to occur. To summarize, for a metal, only absorption and reflection (from a surface) are of interest, but for a non-metal, we are also interested in the transmission and refraction (as well as absorption and reflection). 55
4 6.2 Refraction and reflection (see also textbook section 15.3) Light within a solid travels at a lower velocity v than in vacuum c. We define refractive index n by n = c/v (so n > 1) gives bending of light (refraction) at any vacuum/solid interface also gives partial reflection at any vacuum/solid interface leads to well-known behaviour of lenses, prisms, etc. From the equations of electromagnetism, v = 1 εµ which is just the analog of the previous expression for c in vacuum. n c = = v εµ εµ 0 0 But if we denote µ = µ r µ 0 as before (for relative permeability µ r ) and ε = ε r ε 0 where ε r is the dielectric constant, then n = ε µ r r If the material is only weakly magnetic (i.e. diamagnetic or paramagnetic) then µ r 1 and so n = ε r Examples for some relatively transparent ceramics (including glasses) and polymers are: Note: average means an average taken over the wavelengths of visible light and (in the case of anisotropic materials) over all directions. In simple (isotropic) materials, n is the same for all directions of light propagation. For some anisotropic materials (including many polymers and magnetic materials) n may be slightly different in different directions. If there is a smooth planar boundary between two different materials, some of the light gets reflected back at the boundary even if both materials are completely transparent and some will be refracted (i.e., bent at the interface). 56
5 Refraction at a planar interface: When light passes obliquely from a less dense medium (e.g., air) to a denser transparent medium (e.g., glass), some of the light is transmitted and gets bent towards the normal direction in the denser material: n (low) n (high) θ This occurs according to Snell s law which states that n sin θ = n sin θ θ Hence n > n implies that sin θ < sin θ and so θ < θ. There is a reflected beam as well in the less dense medium (but it is not shown in the diagram). A more interesting effect occurs if the light is passing from a dense to less dense medium, because light will get bent away from the normal direction. This leads to the occurrence of a critical angle. n (low) n (high) θ C 1 2 Ray 1 corresponds to the critical angle θ C in the dense medium, which is obtained when θ in the less dense medium is 90 0, i.e., the beam grazes the interface. It is defined by n sin θ C = n sin 90 0 = n, so sin θ C = n /n < 1. For example, if n = 1.51 (for soda-lime silica glass) and n = 1 (air), then sin θ C = 0.66 implying θ C = Whenever the incident angle in the dense medium is greater than θ C (as for ray 2 above), the only possibility is for the light to be reflected in the dense medium. This property is utilized in applications to optical fibres (see later). Reflection at a planar interface: Recall the earlier definition that R = I R /I 0 = reflectivity. 57
6 This ratio is calculated using em boundary conditions for the E and H fields in the light wave. The final result (in the case of normal incidence) can be written in terms of the refractive index for the materials: R n n 2 1 = n2 + n1 2 Here n 1 and n 2 are the refractive indices. In this case it makes no difference whether light goes from material 2 to 1, or vice-versa. For example, taking the same case as previously for an interface between soda-lime silica glass and air: R = = Therefore only 4.1% of the light energy is reflected Absorption and transmission (see also textbook section 15.4) Non-metals can be either opaque or partly transparent to visible light may have a characteristic colour. in the latter case they In semiconductors and insulators, there is a band gap E g and the occurrence of absorption depends on this gap. (a) Mechanism of photon absorption for nonmetallic materials in which an electron is excited from the top of the valence band (leaving behind a hole) into the conduction band by absorption of a photon with energy E greater than the band gap. (b) Emission of a photon by a direct electron transition back to the valence band. To summarize, absorption takes place only if hν > E g implying hc/λ > E g it occurs because of the valence band to conduction band electronic transitions. By substituting values of λ for visible light, we can work out the corresponding limitations on E g for absorption to occur: Taking λ = 0.4 µm (violet/blue) implies E g = 3.1 ev or less. Taking λ = 0.7 µm (red) implies E g = 1.8 ev or less. Alternatively, for a given E g, we can work out a condition for λ for absorption, e.g., in diamond, E g = 5.6 ev implying λ < 0.22 µm for absorption of radiation. This is outside the wavelength range for visible light, so no light is absorbed in a diamond crystal, i.e. it appears colourless. This would be true for any material with E g > 3.1 ev. By similar arguments, all materials with E g < 1.8 ev will absorb all visible light, so they appear opaque. 58
7 All materials with E g in the range from 1.8 to 3.1 ev will absorb visible light at some of the wavelengths, so they appear colored. Thus a material with (say) E g = 2.5 ev will absorb blue light and transmit red light, so it appears red-ish under transmission. When there are impurities present (e.g., as in doped semiconductors) there are additional absorption or emission processes that can also occur. Some possibilities are: For a material with an impurity level within the band gap: (a) Photon absorption via a valence band to conduction band transition. (b) Emission of two photons via an electron transition first into an impurity state and then a transition back to the ground state. (c) Another two-stage process via an impurity state involving emission of a phonon and then a photon. In an absorptive medium, the light intensity typically decays with penetration distance in a simple exponential fashion, i.e., like e β x where x measures distance for the radiation into the material and β is the absorption coefficient. A large value of β implies strong absorption. The absorption affects the overall transmission properties, e.g., consider a film of material of thickness l. The transmitted intensity I T will depend on l and β, and also on the reflections at the front and back faces: The approximate result is (full calculation is complicated because of summing over all possible ways that repeated internal reflections may occur): 2 I = I (1 ) 0 R e β l T 59
8 Colour perception this depends on the transmission I T (specifically how much intensity there is and at what wavelengths). The examples below show results for green glass, sapphire and ruby at different wavelengths including those of visible light: The variation with wavelength of the fractions of incident light transmitted, absorbed and reflected through a green glass. Transmission of light as a function of wavelength for sapphire (single crystal Al 2 O 3 ) and ruby (Al 2 O 3 containing some chromium oxide). The sapphire appears colourless, while the ruby has a red tint due to selective absorption over specific wavelength ranges. 6.4 Applications (see also textbook sections ) In this part we will cover mainly the topics of luminescence and photoconductivity and lasers and optical fibres and photonic band-gap materials. A) Luminescence This is the property of some materials (typically semiconductors) of absorbing incident energy and then re-emitting it as visible light. 60
9 From previous discussion this requires for the emitted light 1.8 ev < hν < 3.1 ev The absorbed incident energy might be some other form of em radiation of similar or higher energy (e.g. uv radiation) or high-energy electrons or heat, etc. The emission process depends on the band-gap energy E g, as discussed, either through a direct or indirect process. Some more specific terms are:- Fluorescence: this is when the re-emission of energy (as visible light) occurs with a time delay but still takes place on a short time scale (<< 1 sec). Phosphorescence: this is when the time scale is of order 1 sec or more. Fluorescence and phosphorescence occur in only a few materials (e.g., some sulphides, oxides, phosphors, etc) typically they are compounds and the controlled addition of impurities is important. Applications are to various kinds of optical coating (e.g. cathode-ray TV screens). Electroluminescence: this is when an electrical process (such as charge flow in a p-n semiconductor diode) can be used to generate visible light. Under conditions of forward bias in a p-n junction, the electrons and holes move towards each other within the recombination region. They annihilate to release energy: electron + hole energy ( E) where E is of order of the band gap energy E g. Schematic diagram of a forwardbiased semiconductor p-n junction diode showing (a) the injection of an electron from the n-side into the p-side, and (b) the emission of a photon of light as this electron recombines with a hole. Therefore if E g is in the required range (1.8 to 3.1 ev), then visible light will be emitted. Such diodes that luminesce are the basis of the light-emitting diodes (LEDs) used in digital displays. Note that the band gaps are too small in the elemental semiconductors Si (E g = 1.1 ev) and Ge (E g = 0.7 ev), but many compound semiconductors are suitable, such as GaAs and InP and their alloys. For example, the colours red, orange and yellow are possible with LEDs based on the GaAs-InP system (depending on the mix), while blue and green LEDs have been developed using (Ga,In)N semiconducting alloys. In addition, several polymers have been developed that behave as large-e g semiconductors capable of doping as n-type and p-type, and these have been developed as LEDs. For some applications they have distinct advantages over the conventional semiconductor LEDs, e.g., they 61
10 can emit a mixture of colours rather than just a narrow range, and they can be more easily produced as thin films (larger area and bendable to different shapes). There are two main types: organic LEDs (or OLEDs), which use a low molecular weight organic polymer, and polymer LEDs (or PLEDs), which use a high molecular weight polymer. Schematic diagram showing the components and configuration of an organic light-emitting diode (OLED). B) Photoconductivity The electrical conductivity of a doped semiconductor material depends on the number of charge carriers (n for electrons in the conduction band and p for holes in the valence band) and the corresponding carrier mobilities: σ = n e µ e + p e µ h If incident light is absorbed by the semiconductor, then this energy can be used to excite electrons from the valence band to the conduction band (so increasing both n and p). This additional contribution to σ is known as photoconductivity. One application is to light meters, where CdS (E g = 2.4 ev) is often used. Another application is for solar cells as arrays of semiconductor p-n junction diodes basically operating as the reverse process in LEDs (the photoexcited electrons and holes are drawn away from the junction and become part of a current flow in an external circuit). C) Lasers All the radiative processes (i.e., those in which an electron drops from a higher energy state to a lower energy state) can be described as spontaneous emission in the sense that there is no specific external cause. The transitions to lower energy occur randomly and independently of one another, and so they produce emission of incoherent radiation (the different lights waves are randomly of different phases). By contrast, light from a laser is derived from stimulated emission (meaning that it is initiated by an external stimulus) and it is coherent. Hence the acronym: LASER = Light Amplification by Stimulated Emission of Radiation. Two types of lasers will be discussed here as examples: the ruby laser and the GaAs semiconductor laser. The ruby laser Ruby is Al 2 O 3 in single crystal form (i.e., sapphire) to which a small concentration (typically 0.05%) of Cr 3+ ions has been added. As explained earlier, the Cr impurities give ruby its 62
11 characteristic red colour (by allowing selective absorption), but in a ruby laser they provide electron states that are essential for the operation of the laser. Basically, the device consists of a rod of ruby surrounded by a conventional light source (a lamp). The ends of the ruby rod are flat and parallel and highly polished. Both ends are silvered as reflectors, so that one end is essentially totally reflecting and the other end allows some transmission (for the coherent laser light to get out). Schematic diagram of the ruby laser and xenon flash lamp. Initially all the Cr 3+ ions will be in their ground state with the electrons occupying the lowest available states. Next the external power source is switched on to illuminate the xenon flash lamp and then the incident light (photons with wavelength about 0.56 µm) excite the electrons to a higher level. Subsequently, some of these electrons will just drop back directly to the ground state, but the operation of the laser depends on some others making a transition to a lower excited state of Cr 3+ that is metastable. Schematic energy level diagram for the Cr 3+ ions in a ruby laser, showing electron excitation and decay paths. The transition from the original excited state (labelled E) to the metastable state M occurs just by normal spontaneous decay. The average lifetime of the electron in the metastable state M is about 3 ms, which is very long (by several orders of magnitude) compared with the typical lifetime for an electron in an excited state. Eventually each electron decays from M back to the ground state G by emission of a photon. It is this MG transition that eventually produces the laser light, but at start-up there will just be decay by spontaneous emission. The function of the laser (with its ruby 63
12 rod) is to build up an avalanche type of process with the excited electrons traveling back and forth along the rod so that stimulated emission component to the MG decay is obtained. Schematic representation of the process of stimulated emission and light amplification for a ruby laser. (a) The chromium ions before excitation. (b) The electrons in some chromium ions are excited into higher-energy states by the xenon light flash. (c) The emission from metastable electron states is initiated or stimulated by photons that are spontaneously emitted. (d) On reflection from the silvered ends of the ruby rod, the photons continue to stimulate emissions as they traverse the length of the rod. (e) The coherent and intense beam is finally emitted through one end (the partially silvered end). The initial spontaneous photon emission by a few electrons gives the stimulus to trigger an avalanche of emissions from the remaining electrons that are in the metastable state. Of the photons moving along the long axis of the rod, some are transmitted through the partially silvered end, while others incident on the totally silvered end are reflected back. (Photons moving in other directions are lost). The light travels back and forth repeatedly along the length, and during this time its intensity builds up as more and more emissions are stimulated. Eventually a highintensity coherent and collimated laser beam is transmitted in bursts of energy out at one end of the laser. The monochromatic light has a wavelength of µm (in the red) corresponding to the energy separation between M and G. Lots of other materials are capable of behaving as a laser, and they all operate according to the same basic principles, i.e., there needs to be one (or more) metastable level(s) into which electrons can be pumped and then through an avalanche process the stimulated emissions can occur as the electrons move back to the ground state. 64
13 Some common types of lasers, along with their characteristics and applications are shown in the following table:- The GaAs semiconductor laser Various semiconductors, including GaAs as a convenient example, may be used as lasers, especially in applications where low power and compactness are important. The ground state corresponds to an electron being in the valence band and the metastable excited state is the electron excited to the conduction band (leaving behind a hole in the valence band). The main challenges in designing a semiconductor laser consist of a) confining the excited electrons to the semiconductor during the stimulation process, and b) overcoming heating problems within the semiconductor. A typical arrangement is shown below. Schematic diagram of a GaAs semiconductor laser. The holes, excited electrons, and laser beam (photons) are all confined to the GaAs layer by the surrounding n- and p-type GaAlAs layers. 65
14 From previous discussion of semiconductors, it follows that the wavelength and the band gap are related by hc/λ = E g. Hence if λ is to lie in the visible range (between about 0.4 and 0.7 µm), E g must lie between about 1.8 and 3.1 ev. The laser process is shown schematically in the following set of diagrams. Schematic representation of the stimulated recombination of excited electrons in the conduction band with the holes in the valence band: (a) One electron recombines spontaneously with a hole and a photon is emitted. (b) This emitted photon stimulates the recombination of another excited electron and hole, so another photon is emitted. (c) The two photons, which have the same wavelength and are in phase, are reflected back at one end of the laser device. At the same time, new excited electrons and holes are generated by a current that passes through the semiconductor. (d) - (e) More and more stimulated excited electron-hole recombinations occur. (f) Some fraction of the laser beam is transmitted out through the partially reflecting mirror at on e end of the device. 66
Chapter 21 魏茂國. Materials Science and Engineering
 Chapter 21 ntroduction Electromagnetic radiation Light interactions with solids Atomic and electronic interactions Refraction Reflection Absorption Transmission Color Opacity and translucency in insulators
Chapter 21 ntroduction Electromagnetic radiation Light interactions with solids Atomic and electronic interactions Refraction Reflection Absorption Transmission Color Opacity and translucency in insulators
The Electromagnetic Properties of Materials
 The Electromagnetic Properties of Materials Electrical conduction Metals Semiconductors Insulators (dielectrics) Superconductors Magnetic materials Ferromagnetic materials Others Photonic Materials (optical)
The Electromagnetic Properties of Materials Electrical conduction Metals Semiconductors Insulators (dielectrics) Superconductors Magnetic materials Ferromagnetic materials Others Photonic Materials (optical)
Laser Basics. What happens when light (or photon) interact with a matter? Assume photon energy is compatible with energy transition levels.
 What happens when light (or photon) interact with a matter? Assume photon energy is compatible with energy transition levels. Electron energy levels in an hydrogen atom n=5 n=4 - + n=3 n=2 13.6 = [ev]
What happens when light (or photon) interact with a matter? Assume photon energy is compatible with energy transition levels. Electron energy levels in an hydrogen atom n=5 n=4 - + n=3 n=2 13.6 = [ev]
Stimulated Emission Devices: LASERS
 Stimulated Emission Devices: LASERS 1. Stimulated Emission and Photon Amplification E 2 E 2 E 2 hυ hυ hυ In hυ Out hυ E 1 E 1 E 1 (a) Absorption (b) Spontaneous emission (c) Stimulated emission The Principle
Stimulated Emission Devices: LASERS 1. Stimulated Emission and Photon Amplification E 2 E 2 E 2 hυ hυ hυ In hυ Out hυ E 1 E 1 E 1 (a) Absorption (b) Spontaneous emission (c) Stimulated emission The Principle
FIBER OPTICS. Prof. R.K. Shevgaonkar. Department of Electrical Engineering. Indian Institute of Technology, Bombay. Lecture: 17.
 FIBER OPTICS Prof. R.K. Shevgaonkar Department of Electrical Engineering Indian Institute of Technology, Bombay Lecture: 17 Optical Sources- Introduction to LASER Fiber Optics, Prof. R.K. Shevgaonkar,
FIBER OPTICS Prof. R.K. Shevgaonkar Department of Electrical Engineering Indian Institute of Technology, Bombay Lecture: 17 Optical Sources- Introduction to LASER Fiber Optics, Prof. R.K. Shevgaonkar,
LASERS. Dr D. Arun Kumar Assistant Professor Department of Physical Sciences Bannari Amman Institute of Technology Sathyamangalam
 LASERS Dr D. Arun Kumar Assistant Professor Department of Physical Sciences Bannari Amman Institute of Technology Sathyamangalam General Objective To understand the principle, characteristics and types
LASERS Dr D. Arun Kumar Assistant Professor Department of Physical Sciences Bannari Amman Institute of Technology Sathyamangalam General Objective To understand the principle, characteristics and types
Chapter 5. Semiconductor Laser
 Chapter 5 Semiconductor Laser 5.0 Introduction Laser is an acronym for light amplification by stimulated emission of radiation. Albert Einstein in 1917 showed that the process of stimulated emission must
Chapter 5 Semiconductor Laser 5.0 Introduction Laser is an acronym for light amplification by stimulated emission of radiation. Albert Einstein in 1917 showed that the process of stimulated emission must
Material Science. Prof. Satish V. Kailas Associate Professor Dept. of Mechanical Engineering, Indian Institute of Science, Bangalore India
 Material Science Prof. Satish V. Kailas Associate Professor Dept. of Mechanical Engineering, ndian nstitute of Science, Bangalore 5612 ndia Chapter 17. Optical properties Engineering materials are important
Material Science Prof. Satish V. Kailas Associate Professor Dept. of Mechanical Engineering, ndian nstitute of Science, Bangalore 5612 ndia Chapter 17. Optical properties Engineering materials are important
PHYSICS. The Probability of Occurrence of Absorption from state 1 to state 2 is proportional to the energy density u(v)..
 ABSORPTION of RADIATION : PHYSICS The Probability of Occurrence of Absorption from state 1 to state 2 is proportional to the energy density u(v).. of the radiation > P12 = B12 u(v) hv E2 E1 Where as, the
ABSORPTION of RADIATION : PHYSICS The Probability of Occurrence of Absorption from state 1 to state 2 is proportional to the energy density u(v).. of the radiation > P12 = B12 u(v) hv E2 E1 Where as, the
What are Lasers? Light Amplification by Stimulated Emission of Radiation LASER Light emitted at very narrow wavelength bands (monochromatic) Light
 What are Lasers? What are Lasers? Light Amplification by Stimulated Emission of Radiation LASER Light emitted at very narrow wavelength bands (monochromatic) Light emitted in a directed beam Light is coherenent
What are Lasers? What are Lasers? Light Amplification by Stimulated Emission of Radiation LASER Light emitted at very narrow wavelength bands (monochromatic) Light emitted in a directed beam Light is coherenent
Review of Optical Properties of Materials
 Review of Optical Properties of Materials Review of optics Absorption in semiconductors: qualitative discussion Derivation of Optical Absorption Coefficient in Direct Semiconductors Photons When dealing
Review of Optical Properties of Materials Review of optics Absorption in semiconductors: qualitative discussion Derivation of Optical Absorption Coefficient in Direct Semiconductors Photons When dealing
Sunlight. 1 radiation.
 Sunlight The eye has evolved to see a narrow range of EM waves which we call 'visible light'. This visible range of frequency is due to the light comes from the Sun. The photosphere of the Sun is a blackbody
Sunlight The eye has evolved to see a narrow range of EM waves which we call 'visible light'. This visible range of frequency is due to the light comes from the Sun. The photosphere of the Sun is a blackbody
Signal regeneration - optical amplifiers
 Signal regeneration - optical amplifiers In any atom or solid, the state of the electrons can change by: 1) Stimulated absorption - in the presence of a light wave, a photon is absorbed, the electron is
Signal regeneration - optical amplifiers In any atom or solid, the state of the electrons can change by: 1) Stimulated absorption - in the presence of a light wave, a photon is absorbed, the electron is
3.1 Absorption and Transparency
 3.1 Absorption and Transparency 3.1.1 Optical Devices (definitions) 3.1.2 Photon and Semiconductor Interactions 3.1.3 Photon Intensity 3.1.4 Absorption 3.1 Absorption and Transparency Objective 1: Recall
3.1 Absorption and Transparency 3.1.1 Optical Devices (definitions) 3.1.2 Photon and Semiconductor Interactions 3.1.3 Photon Intensity 3.1.4 Absorption 3.1 Absorption and Transparency Objective 1: Recall
Metal Vapour Lasers Use vapoured metal as a gain medium Developed by W. Silfvast (1966) Two types: Ionized Metal vapour (He-Cd) Neutral Metal vapour
 Metal Vapour Lasers Use vapoured metal as a gain medium Developed by W. Silfvast (1966) Two types: Ionized Metal vapour (He-Cd) Neutral Metal vapour (Cu) All operate by vaporizing metal in container Helium
Metal Vapour Lasers Use vapoured metal as a gain medium Developed by W. Silfvast (1966) Two types: Ionized Metal vapour (He-Cd) Neutral Metal vapour (Cu) All operate by vaporizing metal in container Helium
Unit-2 LASER. Syllabus: Properties of lasers, types of lasers, derivation of Einstein A & B Coefficients, Working He-Ne and Ruby lasers.
 Unit-2 LASER Syllabus: Properties of lasers, types of lasers, derivation of Einstein A & B Coefficients, Working He-Ne and Ruby lasers. Page 1 LASER: The word LASER is acronym for light amplification by
Unit-2 LASER Syllabus: Properties of lasers, types of lasers, derivation of Einstein A & B Coefficients, Working He-Ne and Ruby lasers. Page 1 LASER: The word LASER is acronym for light amplification by
ELECTRONIC DEVICES AND CIRCUITS SUMMARY
 ELECTRONIC DEVICES AND CIRCUITS SUMMARY Classification of Materials: Insulator: An insulator is a material that offers a very low level (or negligible) of conductivity when voltage is applied. Eg: Paper,
ELECTRONIC DEVICES AND CIRCUITS SUMMARY Classification of Materials: Insulator: An insulator is a material that offers a very low level (or negligible) of conductivity when voltage is applied. Eg: Paper,
Model Answer (Paper code: AR-7112) M. Sc. (Physics) IV Semester Paper I: Laser Physics and Spectroscopy
 Model Answer (Paper code: AR-7112) M. Sc. (Physics) IV Semester Paper I: Laser Physics and Spectroscopy Section I Q1. Answer (i) (b) (ii) (d) (iii) (c) (iv) (c) (v) (a) (vi) (b) (vii) (b) (viii) (a) (ix)
Model Answer (Paper code: AR-7112) M. Sc. (Physics) IV Semester Paper I: Laser Physics and Spectroscopy Section I Q1. Answer (i) (b) (ii) (d) (iii) (c) (iv) (c) (v) (a) (vi) (b) (vii) (b) (viii) (a) (ix)
Lasers E 6 E 4 E 3 E 2 E 1
 Lasers Laser is an acronym for light amplification by stimulated emission of radiation. Here the process of stimulated emission is used to amplify light radiation. Spontaneous emission: When energy is
Lasers Laser is an acronym for light amplification by stimulated emission of radiation. Here the process of stimulated emission is used to amplify light radiation. Spontaneous emission: When energy is
Laserphysik. Prof. Yong Lei & Dr. Yang Xu. Fachgebiet Angewandte Nanophysik, Institut für Physik
 Laserphysik Prof. Yong Lei & Dr. Yang Xu Fachgebiet Angewandte Nanophysik, Institut für Physik Contact: yong.lei@tu-ilmenau.de; yang.xu@tu-ilmenau.de Office: Heisenbergbau V 202, Unterpörlitzer Straße
Laserphysik Prof. Yong Lei & Dr. Yang Xu Fachgebiet Angewandte Nanophysik, Institut für Physik Contact: yong.lei@tu-ilmenau.de; yang.xu@tu-ilmenau.de Office: Heisenbergbau V 202, Unterpörlitzer Straße
What are Lasers? Light Amplification by Stimulated Emission of Radiation LASER Light emitted at very narrow wavelength bands (monochromatic) Light
 What are Lasers? What are Lasers? Light Amplification by Stimulated Emission of Radiation LASER Light emitted at very narrow wavelength bands (monochromatic) Light emitted in a directed beam Light is coherenent
What are Lasers? What are Lasers? Light Amplification by Stimulated Emission of Radiation LASER Light emitted at very narrow wavelength bands (monochromatic) Light emitted in a directed beam Light is coherenent
Chemistry Instrumental Analysis Lecture 5. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 5 Light Amplification by Stimulated Emission of Radiation High Intensities Narrow Bandwidths Coherent Outputs Applications CD/DVD Readers Fiber Optics Spectroscopy
Chemistry 4631 Instrumental Analysis Lecture 5 Light Amplification by Stimulated Emission of Radiation High Intensities Narrow Bandwidths Coherent Outputs Applications CD/DVD Readers Fiber Optics Spectroscopy
External (differential) quantum efficiency Number of additional photons emitted / number of additional electrons injected
 Semiconductor Lasers Comparison with LEDs The light emitted by a laser is generally more directional, more intense and has a narrower frequency distribution than light from an LED. The external efficiency
Semiconductor Lasers Comparison with LEDs The light emitted by a laser is generally more directional, more intense and has a narrower frequency distribution than light from an LED. The external efficiency
Modern Physics for Frommies IV The Universe - Small to Large Lecture 4
 Fromm Institute for Lifelong Learning University of San Francisco Modern Physics for Frommies IV The Universe - Small to Large Lecture 4 3 February 06 Modern Physics IV Lecture 4 Agenda Administrative
Fromm Institute for Lifelong Learning University of San Francisco Modern Physics for Frommies IV The Universe - Small to Large Lecture 4 3 February 06 Modern Physics IV Lecture 4 Agenda Administrative
LASER. Light Amplification by Stimulated Emission of Radiation
 LASER Light Amplification by Stimulated Emission of Radiation Laser Fundamentals The light emitted from a laser is monochromatic, that is, it is of one color/wavelength. In contrast, ordinary white light
LASER Light Amplification by Stimulated Emission of Radiation Laser Fundamentals The light emitted from a laser is monochromatic, that is, it is of one color/wavelength. In contrast, ordinary white light
Dept. of Physics, MIT Manipal 1
 Chapter 1: Optics 1. In the phenomenon of interference, there is A Annihilation of light energy B Addition of energy C Redistribution energy D Creation of energy 2. Interference fringes are obtained using
Chapter 1: Optics 1. In the phenomenon of interference, there is A Annihilation of light energy B Addition of energy C Redistribution energy D Creation of energy 2. Interference fringes are obtained using
(b) Spontaneous emission. Absorption, spontaneous (random photon) emission and stimulated emission.
 Lecture 10 Stimulated Emission Devices Lasers Stimulated emission and light amplification Einstein coefficients Optical fiber amplifiers Gas laser and He-Ne Laser The output spectrum of a gas laser Laser
Lecture 10 Stimulated Emission Devices Lasers Stimulated emission and light amplification Einstein coefficients Optical fiber amplifiers Gas laser and He-Ne Laser The output spectrum of a gas laser Laser
Ms. Monika Srivastava Doctoral Scholar, AMR Group of Dr. Anurag Srivastava ABV-IIITM, Gwalior
 By Ms. Monika Srivastava Doctoral Scholar, AMR Group of Dr. Anurag Srivastava ABV-IIITM, Gwalior Unit 2 Laser acronym Laser Vs ordinary light Characteristics of lasers Different processes involved in lasers
By Ms. Monika Srivastava Doctoral Scholar, AMR Group of Dr. Anurag Srivastava ABV-IIITM, Gwalior Unit 2 Laser acronym Laser Vs ordinary light Characteristics of lasers Different processes involved in lasers
Lecture 15: Optoelectronic devices: Introduction
 Lecture 15: Optoelectronic devices: Introduction Contents 1 Optical absorption 1 1.1 Absorption coefficient....................... 2 2 Optical recombination 5 3 Recombination and carrier lifetime 6 3.1
Lecture 15: Optoelectronic devices: Introduction Contents 1 Optical absorption 1 1.1 Absorption coefficient....................... 2 2 Optical recombination 5 3 Recombination and carrier lifetime 6 3.1
Chapter 2 Optical Transitions
 Chapter 2 Optical Transitions 2.1 Introduction Among energy states, the state with the lowest energy is most stable. Therefore, the electrons in semiconductors tend to stay in low energy states. If they
Chapter 2 Optical Transitions 2.1 Introduction Among energy states, the state with the lowest energy is most stable. Therefore, the electrons in semiconductors tend to stay in low energy states. If they
Laser Types Two main types depending on time operation Continuous Wave (CW) Pulsed operation Pulsed is easier, CW more useful
 What Makes a Laser Light Amplification by Stimulated Emission of Radiation Main Requirements of the Laser Laser Gain Medium (provides the light amplification) Optical Resonator Cavity (greatly increase
What Makes a Laser Light Amplification by Stimulated Emission of Radiation Main Requirements of the Laser Laser Gain Medium (provides the light amplification) Optical Resonator Cavity (greatly increase
Designing Information Devices and Systems II A. Sahai, J. Roychowdhury, K. Pister Discussion 1A
 EECS 16B Spring 2019 Designing Information Devices and Systems II A. Sahai, J. Roychowdhury, K. Pister Discussion 1A 1 Semiconductor Physics Generally, semiconductors are crystalline solids bonded into
EECS 16B Spring 2019 Designing Information Devices and Systems II A. Sahai, J. Roychowdhury, K. Pister Discussion 1A 1 Semiconductor Physics Generally, semiconductors are crystalline solids bonded into
Electron Energy, E E = 0. Free electron. 3s Band 2p Band Overlapping energy bands. 3p 3s 2p 2s. 2s Band. Electrons. 1s ATOM SOLID.
 Electron Energy, E Free electron Vacuum level 3p 3s 2p 2s 2s Band 3s Band 2p Band Overlapping energy bands Electrons E = 0 1s ATOM 1s SOLID In a metal the various energy bands overlap to give a single
Electron Energy, E Free electron Vacuum level 3p 3s 2p 2s 2s Band 3s Band 2p Band Overlapping energy bands Electrons E = 0 1s ATOM 1s SOLID In a metal the various energy bands overlap to give a single
Laser Types Two main types depending on time operation Continuous Wave (CW) Pulsed operation Pulsed is easier, CW more useful
 Main Requirements of the Laser Optical Resonator Cavity Laser Gain Medium of 2, 3 or 4 level types in the Cavity Sufficient means of Excitation (called pumping) eg. light, current, chemical reaction Population
Main Requirements of the Laser Optical Resonator Cavity Laser Gain Medium of 2, 3 or 4 level types in the Cavity Sufficient means of Excitation (called pumping) eg. light, current, chemical reaction Population
Chapter 24 Photonics Question 1 Question 2 Question 3 Question 4 Question 5
 Chapter 24 Photonics Data throughout this chapter: e = 1.6 10 19 C; h = 6.63 10 34 Js (or 4.14 10 15 ev s); m e = 9.1 10 31 kg; c = 3.0 10 8 m s 1 Question 1 Visible light has a range of photons with wavelengths
Chapter 24 Photonics Data throughout this chapter: e = 1.6 10 19 C; h = 6.63 10 34 Js (or 4.14 10 15 ev s); m e = 9.1 10 31 kg; c = 3.0 10 8 m s 1 Question 1 Visible light has a range of photons with wavelengths
Chemistry Instrumental Analysis Lecture 8. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 8 UV to IR Components of Optical Basic components of spectroscopic instruments: stable source of radiant energy transparent container to hold sample device
Chemistry 4631 Instrumental Analysis Lecture 8 UV to IR Components of Optical Basic components of spectroscopic instruments: stable source of radiant energy transparent container to hold sample device
What Makes a Laser Light Amplification by Stimulated Emission of Radiation Main Requirements of the Laser Laser Gain Medium (provides the light
 What Makes a Laser Light Amplification by Stimulated Emission of Radiation Main Requirements of the Laser Laser Gain Medium (provides the light amplification) Optical Resonator Cavity (greatly increase
What Makes a Laser Light Amplification by Stimulated Emission of Radiation Main Requirements of the Laser Laser Gain Medium (provides the light amplification) Optical Resonator Cavity (greatly increase
In a metal, how does the probability distribution of an electron look like at absolute zero?
 1 Lecture 6 Laser 2 In a metal, how does the probability distribution of an electron look like at absolute zero? 3 (Atom) Energy Levels For atoms, I draw a lower horizontal to indicate its lowest energy
1 Lecture 6 Laser 2 In a metal, how does the probability distribution of an electron look like at absolute zero? 3 (Atom) Energy Levels For atoms, I draw a lower horizontal to indicate its lowest energy
FIBER OPTICS. Prof. R.K. Shevgaonkar. Department of Electrical Engineering. Indian Institute of Technology, Bombay. Lecture: 15. Optical Sources-LASER
 FIBER OPTICS Prof. R.K. Shevgaonkar Department of Electrical Engineering Indian Institute of Technology, Bombay Lecture: 15 Optical Sources-LASER Fiber Optics, Prof. R.K. Shevgaonkar, Dept. of Electrical
FIBER OPTICS Prof. R.K. Shevgaonkar Department of Electrical Engineering Indian Institute of Technology, Bombay Lecture: 15 Optical Sources-LASER Fiber Optics, Prof. R.K. Shevgaonkar, Dept. of Electrical
Other Devices from p-n junctions
 Memory (5/7 -- Glenn Alers) Other Devices from p-n junctions Electron to Photon conversion devices LEDs and SSL (5/5) Lasers (5/5) Solid State Lighting (5/5) Photon to electron conversion devices Photodectors
Memory (5/7 -- Glenn Alers) Other Devices from p-n junctions Electron to Photon conversion devices LEDs and SSL (5/5) Lasers (5/5) Solid State Lighting (5/5) Photon to electron conversion devices Photodectors
Chapter 6: Light-Emitting Diodes
 Chapter 6: Light-Emitting Diodes Photoluminescence and electroluminescence Basic transitions Luminescence efficiency Light-emitting diodes Internal quantum efficiency External quantum efficiency Device
Chapter 6: Light-Emitting Diodes Photoluminescence and electroluminescence Basic transitions Luminescence efficiency Light-emitting diodes Internal quantum efficiency External quantum efficiency Device
FIBER OPTICS. Prof. R.K. Shevgaonkar. Department of Electrical Engineering. Indian Institute of Technology, Bombay. Lecture: 12.
 FIBER OPTICS Prof. R.K. Shevgaonkar Department of Electrical Engineering Indian Institute of Technology, Bombay Lecture: 12 Optical Sources Fiber Optics, Prof. R.K. Shevgaonkar, Dept. of Electrical Engineering,
FIBER OPTICS Prof. R.K. Shevgaonkar Department of Electrical Engineering Indian Institute of Technology, Bombay Lecture: 12 Optical Sources Fiber Optics, Prof. R.K. Shevgaonkar, Dept. of Electrical Engineering,
Electromagnetic Waves
 4/15/12 Chapter 26: Properties of Light Field Induction Ok, so a changing magnetic field causes a current (Faraday s law) Why do we have currents in the first place? electric fields of the charges Changing
4/15/12 Chapter 26: Properties of Light Field Induction Ok, so a changing magnetic field causes a current (Faraday s law) Why do we have currents in the first place? electric fields of the charges Changing
Question 1. (Marks 16)
 5 Question 1. (Marks 16) Consider the circuit shown in the figure, where C 1 = 6.00µF, C 2 = 3.00µF, and V = 20.0V. Capacitor C 1 is first charged by closing switch S 1. Switch S 1 is then opened, and
5 Question 1. (Marks 16) Consider the circuit shown in the figure, where C 1 = 6.00µF, C 2 = 3.00µF, and V = 20.0V. Capacitor C 1 is first charged by closing switch S 1. Switch S 1 is then opened, and
JRE Group of Institutions ASSIGNMENT # 1 Special Theory of Relativity
 ASSIGNMENT # 1 Special Theory of Relativity 1. What was the objective of conducting the Michelson-Morley experiment? Describe the experiment. How is the negative result of the experiment interpreted? 2.
ASSIGNMENT # 1 Special Theory of Relativity 1. What was the objective of conducting the Michelson-Morley experiment? Describe the experiment. How is the negative result of the experiment interpreted? 2.
3.1 Introduction to Semiconductors. Y. Baghzouz ECE Department UNLV
 3.1 Introduction to Semiconductors Y. Baghzouz ECE Department UNLV Introduction In this lecture, we will cover the basic aspects of semiconductor materials, and the physical mechanisms which are at the
3.1 Introduction to Semiconductors Y. Baghzouz ECE Department UNLV Introduction In this lecture, we will cover the basic aspects of semiconductor materials, and the physical mechanisms which are at the
Introduction to Spectroscopic methods
 Introduction to Spectroscopic methods Spectroscopy: Study of interaction between light* and matter. Spectrometry: Implies a quantitative measurement of intensity. * More generally speaking electromagnetic
Introduction to Spectroscopic methods Spectroscopy: Study of interaction between light* and matter. Spectrometry: Implies a quantitative measurement of intensity. * More generally speaking electromagnetic
Introduction to Sources: Radiative Processes and Population Inversion in Atoms, Molecules, and Semiconductors Atoms and Molecules
 OPTI 500 DEF, Spring 2012, Lecture 2 Introduction to Sources: Radiative Processes and Population Inversion in Atoms, Molecules, and Semiconductors Atoms and Molecules Energy Levels Every atom or molecule
OPTI 500 DEF, Spring 2012, Lecture 2 Introduction to Sources: Radiative Processes and Population Inversion in Atoms, Molecules, and Semiconductors Atoms and Molecules Energy Levels Every atom or molecule
Unit I LASER Engineering Physics
 Introduction LASER stands for light Amplification by Stimulated Emission of Radiation. The theoretical basis for the development of laser was provided by Albert Einstein in 1917. In 1960, the first laser
Introduction LASER stands for light Amplification by Stimulated Emission of Radiation. The theoretical basis for the development of laser was provided by Albert Einstein in 1917. In 1960, the first laser
BANNARI AMMAN INSTITUTE OF TECHNOLOGY SATHYAMANGALAM DEPARTMENT OF PHYSICAL SCIENCES. UNIT II Applied Optics
 BANNAI AMMAN INSTITTE OF TECHNOLOGY SATHYAMANGALAM DEPATMENT OF PHYSICAL SCIENCES NIT II Applied Optics PAT A A1 The superimposition of one light wave over another is called as a) interference b) Diffraction
BANNAI AMMAN INSTITTE OF TECHNOLOGY SATHYAMANGALAM DEPATMENT OF PHYSICAL SCIENCES NIT II Applied Optics PAT A A1 The superimposition of one light wave over another is called as a) interference b) Diffraction
PHYSICS. Ray Optics. Mr Rishi Gopie
 Ray Optics Mr Rishi Gopie Ray Optics Nature of light Light is a form of energy which affects the human eye in such a way as to cause the sensation of sight. Visible light is a range of electromagnetic
Ray Optics Mr Rishi Gopie Ray Optics Nature of light Light is a form of energy which affects the human eye in such a way as to cause the sensation of sight. Visible light is a range of electromagnetic
Fall 2014 Nobby Kobayashi (Based on the notes by E.D.H Green and E.L Allen, SJSU) 1.0 Learning Objectives
 University of California at Santa Cruz Electrical Engineering Department EE-145L: Properties of Materials Laboratory Lab 7: Optical Absorption, Photoluminescence Fall 2014 Nobby Kobayashi (Based on the
University of California at Santa Cruz Electrical Engineering Department EE-145L: Properties of Materials Laboratory Lab 7: Optical Absorption, Photoluminescence Fall 2014 Nobby Kobayashi (Based on the
LASER. Challenging MCQ questions by The Physics Cafe. Compiled and selected by The Physics Cafe
 LSER hallenging MQ questions by The Physics afe ompiled and selected by The Physics afe www.thephysicsafe.com www.pmc.sg 1 laser point creates a spot on a screen as it reflects 70% of the light striking
LSER hallenging MQ questions by The Physics afe ompiled and selected by The Physics afe www.thephysicsafe.com www.pmc.sg 1 laser point creates a spot on a screen as it reflects 70% of the light striking
Lasers. Optical Fibres
 Lasers & Optical Fibres P a g e 2 Contents LASER 1) Coherence 3 2) Interaction of radiation with matter 4 3) Laser fundamentals 5 4) Laser system 5 5) Ruby Laser 6 6) He-Ne Gas Laser 7 7) Semiconductor
Lasers & Optical Fibres P a g e 2 Contents LASER 1) Coherence 3 2) Interaction of radiation with matter 4 3) Laser fundamentals 5 4) Laser system 5 5) Ruby Laser 6 6) He-Ne Gas Laser 7 7) Semiconductor
What do we study and do?
 What do we study and do? Light comes from electrons transitioning from higher energy to lower energy levels. Wave-particle nature of light Wave nature: refraction, diffraction, interference (labs) Particle
What do we study and do? Light comes from electrons transitioning from higher energy to lower energy levels. Wave-particle nature of light Wave nature: refraction, diffraction, interference (labs) Particle
UNIT I: Electronic Materials.
 SIDDHARTH INSTITUTE OF ENGINEERING & TECHNOLOGY :: PUTTUR Siddharth Nagar, Narayanavanam Road 517583 QUESTION BANK (DESCRIPTIVE) Subject with Code: SEMICONDUCTOR PHYSICS (18HS0851) Course & Branch: B.Tech
SIDDHARTH INSTITUTE OF ENGINEERING & TECHNOLOGY :: PUTTUR Siddharth Nagar, Narayanavanam Road 517583 QUESTION BANK (DESCRIPTIVE) Subject with Code: SEMICONDUCTOR PHYSICS (18HS0851) Course & Branch: B.Tech
Lecture 20 Optical Characterization 2
 Lecture 20 Optical Characterization 2 Schroder: Chapters 2, 7, 10 1/68 Announcements Homework 5/6: Is online now. Due Wednesday May 30th at 10:00am. I will return it the following Wednesday (6 th June).
Lecture 20 Optical Characterization 2 Schroder: Chapters 2, 7, 10 1/68 Announcements Homework 5/6: Is online now. Due Wednesday May 30th at 10:00am. I will return it the following Wednesday (6 th June).
Chapter 18. Fundamentals of Spectrophotometry. Properties of Light
 Chapter 18 Fundamentals of Spectrophotometry Properties of Light Electromagnetic Radiation energy radiated in the form of a WAVE caused by an electric field interacting with a magnetic field result of
Chapter 18 Fundamentals of Spectrophotometry Properties of Light Electromagnetic Radiation energy radiated in the form of a WAVE caused by an electric field interacting with a magnetic field result of
EE495/695 Introduction to Semiconductors I. Y. Baghzouz ECE Department UNLV
 EE495/695 Introduction to Semiconductors I Y. Baghzouz ECE Department UNLV Introduction Solar cells have always been aligned closely with other electronic devices. We will cover the basic aspects of semiconductor
EE495/695 Introduction to Semiconductors I Y. Baghzouz ECE Department UNLV Introduction Solar cells have always been aligned closely with other electronic devices. We will cover the basic aspects of semiconductor
MTLE-6120: Advanced Electronic Properties of Materials. Semiconductor p-n junction diodes. Reading: Kasap ,
 MTLE-6120: Advanced Electronic Properties of Materials 1 Semiconductor p-n junction diodes Reading: Kasap 6.1-6.5, 6.9-6.12 Metal-semiconductor contact potential 2 p-type n-type p-type n-type Same semiconductor
MTLE-6120: Advanced Electronic Properties of Materials 1 Semiconductor p-n junction diodes Reading: Kasap 6.1-6.5, 6.9-6.12 Metal-semiconductor contact potential 2 p-type n-type p-type n-type Same semiconductor
1) Introduction 2) Photo electric effect 3) Dual nature of matter 4) Bohr s atom model 5) LASERS
 1) Introduction 2) Photo electric effect 3) Dual nature of matter 4) Bohr s atom model 5) LASERS 1. Introduction Types of electron emission, Dunnington s method, different types of spectra, Fraunhoffer
1) Introduction 2) Photo electric effect 3) Dual nature of matter 4) Bohr s atom model 5) LASERS 1. Introduction Types of electron emission, Dunnington s method, different types of spectra, Fraunhoffer
Characterisation of vibrational modes of adsorbed species
 17.7.5 Characterisation of vibrational modes of adsorbed species Infrared spectroscopy (IR) See Ch.10. Infrared vibrational spectra originate in transitions between discrete vibrational energy levels of
17.7.5 Characterisation of vibrational modes of adsorbed species Infrared spectroscopy (IR) See Ch.10. Infrared vibrational spectra originate in transitions between discrete vibrational energy levels of
Analytical Spectroscopy Review
 Analytical Spectroscopy Review λ = wavelength ν = frequency V = velocity = ν x λ = 2.998 x 10 8 m/sec = c (in a vacuum) ν is determined by source and does not change as wave propogates, but V can change
Analytical Spectroscopy Review λ = wavelength ν = frequency V = velocity = ν x λ = 2.998 x 10 8 m/sec = c (in a vacuum) ν is determined by source and does not change as wave propogates, but V can change
CHAPTER 9 ELECTROMAGNETIC WAVES
 CHAPTER 9 ELECTROMAGNETIC WAVES Outlines 1. Waves in one dimension 2. Electromagnetic Waves in Vacuum 3. Electromagnetic waves in Matter 4. Absorption and Dispersion 5. Guided Waves 2 Skip 9.1.1 and 9.1.2
CHAPTER 9 ELECTROMAGNETIC WAVES Outlines 1. Waves in one dimension 2. Electromagnetic Waves in Vacuum 3. Electromagnetic waves in Matter 4. Absorption and Dispersion 5. Guided Waves 2 Skip 9.1.1 and 9.1.2
Phys 2310 Fri. Dec. 12, 2014 Today s Topics. Begin Chapter 13: Lasers Reading for Next Time
 Phys 2310 Fri. Dec. 12, 2014 Today s Topics Begin Chapter 13: Lasers Reading for Next Time 1 Reading this Week By Fri.: Ch. 13 (13.1, 13.3) Lasers, Holography 2 Homework this Week No Homework this chapter.
Phys 2310 Fri. Dec. 12, 2014 Today s Topics Begin Chapter 13: Lasers Reading for Next Time 1 Reading this Week By Fri.: Ch. 13 (13.1, 13.3) Lasers, Holography 2 Homework this Week No Homework this chapter.
LASERS. Amplifiers: Broad-band communications (avoid down-conversion)
 L- LASERS Representative applications: Amplifiers: Broad-band communications (avoid down-conversion) Oscillators: Blasting: Energy States: Hydrogen atom Frequency/distance reference, local oscillators,
L- LASERS Representative applications: Amplifiers: Broad-band communications (avoid down-conversion) Oscillators: Blasting: Energy States: Hydrogen atom Frequency/distance reference, local oscillators,
Fig. 8.1 illustrates the three measurements. air medium A. ray 1. air medium A. ray 2. air medium A. ray 3. Fig For Examiner s Use
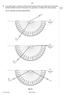 9 9 9 14 8 In an optics lesson, a Physics student traces the paths of three s of light near the boundary between medium A and. The student uses a protractor to measure the various angles. Fig. 8.1 illustrates
9 9 9 14 8 In an optics lesson, a Physics student traces the paths of three s of light near the boundary between medium A and. The student uses a protractor to measure the various angles. Fig. 8.1 illustrates
Chapter 7: Optical Properties of Solids. Interaction of light with atoms. Insert Fig Allowed and forbidden electronic transitions
 Chapter 7: Optical Properties of Solids Interaction of light with atoms Insert Fig. 8.1 Allowed and forbidden electronic transitions 1 Insert Fig. 8.3 or equivalent Ti 3+ absorption: e g t 2g 2 Ruby Laser
Chapter 7: Optical Properties of Solids Interaction of light with atoms Insert Fig. 8.1 Allowed and forbidden electronic transitions 1 Insert Fig. 8.3 or equivalent Ti 3+ absorption: e g t 2g 2 Ruby Laser
Chapter-4 Stimulated emission devices LASERS
 Semiconductor Laser Diodes Chapter-4 Stimulated emission devices LASERS The Road Ahead Lasers Basic Principles Applications Gas Lasers Semiconductor Lasers Semiconductor Lasers in Optical Networks Improvement
Semiconductor Laser Diodes Chapter-4 Stimulated emission devices LASERS The Road Ahead Lasers Basic Principles Applications Gas Lasers Semiconductor Lasers Semiconductor Lasers in Optical Networks Improvement
EE 6313 Homework Assignments
 EE 6313 Homework Assignments 1. Homework I: Chapter 1: 1.2, 1.5, 1.7, 1.10, 1.12 [Lattice constant only] (Due Sept. 1, 2009). 2. Homework II: Chapter 1, 2: 1.17, 2.1 (a, c) (k = π/a at zone edge), 2.3
EE 6313 Homework Assignments 1. Homework I: Chapter 1: 1.2, 1.5, 1.7, 1.10, 1.12 [Lattice constant only] (Due Sept. 1, 2009). 2. Homework II: Chapter 1, 2: 1.17, 2.1 (a, c) (k = π/a at zone edge), 2.3
LECTURE 23: LIGHT. Propagation of Light Huygen s Principle
 LECTURE 23: LIGHT Propagation of Light Reflection & Refraction Internal Reflection Propagation of Light Huygen s Principle Each point on a primary wavefront serves as the source of spherical secondary
LECTURE 23: LIGHT Propagation of Light Reflection & Refraction Internal Reflection Propagation of Light Huygen s Principle Each point on a primary wavefront serves as the source of spherical secondary
-I (PH 6151) UNIT-V PHOTONICS AND FIBRE OPTICS
 Engineering Physics -I (PH 6151) UNIT-V PHOTONICS AND FIBRE OPTICS Syllabus: Lasers Spontaneous and stimulated emission Population Inversion -Einstein s co-efficient (Derivation)- types of lasers-nd-yag,co
Engineering Physics -I (PH 6151) UNIT-V PHOTONICS AND FIBRE OPTICS Syllabus: Lasers Spontaneous and stimulated emission Population Inversion -Einstein s co-efficient (Derivation)- types of lasers-nd-yag,co
Unit IV Semiconductors Engineering Physics
 Introduction A semiconductor is a material that has a resistivity lies between that of a conductor and an insulator. The conductivity of a semiconductor material can be varied under an external electrical
Introduction A semiconductor is a material that has a resistivity lies between that of a conductor and an insulator. The conductivity of a semiconductor material can be varied under an external electrical
LECTURE 23: LIGHT. Propagation of Light Huygen s Principle
 LECTURE 23: LIGHT Propagation of Light Reflection & Refraction Internal Reflection Propagation of Light Huygen s Principle Each point on a primary wavefront serves as the source of spherical secondary
LECTURE 23: LIGHT Propagation of Light Reflection & Refraction Internal Reflection Propagation of Light Huygen s Principle Each point on a primary wavefront serves as the source of spherical secondary
OPTICAL PROPERTIES of Nanomaterials
 OPTICAL PROPERTIES of Nanomaterials Advanced Reading Optical Properties and Spectroscopy of Nanomaterials Jin Zhong Zhang World Scientific, Singapore, 2009. Optical Properties Many of the optical properties
OPTICAL PROPERTIES of Nanomaterials Advanced Reading Optical Properties and Spectroscopy of Nanomaterials Jin Zhong Zhang World Scientific, Singapore, 2009. Optical Properties Many of the optical properties
Practice Paper-3. Q. 2. An electron beam projected along + X-axis, in a magnetic field along the + Z-axis. What is
 Practice Paper-3 Q. 1. An electric dipole of dipole moment 20 10 6 cm is enclosed by a closed surface. What is the net flux coming out of the surface? Q. 2. An electron beam projected along + X-axis, in
Practice Paper-3 Q. 1. An electric dipole of dipole moment 20 10 6 cm is enclosed by a closed surface. What is the net flux coming out of the surface? Q. 2. An electron beam projected along + X-axis, in
Write the electron configuration for Chromium (Cr):
 Write the electron configuration for Chromium (Cr): Energy level Aufbau Principle Atomic orbital Quantum Hund s Rule Atomic number Electron Configuration Whole number Pauli Exlcusion Principle Quantum
Write the electron configuration for Chromium (Cr): Energy level Aufbau Principle Atomic orbital Quantum Hund s Rule Atomic number Electron Configuration Whole number Pauli Exlcusion Principle Quantum
EP118 Optics. Content TOPIC 1 LIGHT. Department of Engineering Physics University of Gaziantep
 EP11 Optics TOPIC 1 LIGHT Department of Engineering Physics University of Gaziantep July 2011 Sayfa 1 Content 1. History of Light 2. Wave Nature of Light 3. Quantum Theory of Light 4. Elecromagnetic Wave
EP11 Optics TOPIC 1 LIGHT Department of Engineering Physics University of Gaziantep July 2011 Sayfa 1 Content 1. History of Light 2. Wave Nature of Light 3. Quantum Theory of Light 4. Elecromagnetic Wave
PHYSICS 2005 (Delhi) Q3. The power factor of an A.C. circuit is 0.5. What will be the phase difference between voltage and current in this circuit?
 General Instructions: 1. All questions are compulsory. 2. There is no overall choice. However, an internal choke has been pro vided in one question of two marks, one question of three marks and all three
General Instructions: 1. All questions are compulsory. 2. There is no overall choice. However, an internal choke has been pro vided in one question of two marks, one question of three marks and all three
KATIHAL FİZİĞİ MNT-510
 KATIHAL FİZİĞİ MNT-510 YARIİLETKENLER Kaynaklar: Katıhal Fiziği, Prof. Dr. Mustafa Dikici, Seçkin Yayıncılık Katıhal Fiziği, Şakir Aydoğan, Nobel Yayıncılık, Physics for Computer Science Students: With
KATIHAL FİZİĞİ MNT-510 YARIİLETKENLER Kaynaklar: Katıhal Fiziği, Prof. Dr. Mustafa Dikici, Seçkin Yayıncılık Katıhal Fiziği, Şakir Aydoğan, Nobel Yayıncılık, Physics for Computer Science Students: With
CBSE PHYSICS QUESTION PAPER (2005)
 CBSE PHYSICS QUESTION PAPER (2005) (i) (ii) All questions are compulsory. There are 30 questions in total. Questions 1 to 8 carry one mark each, Questions 9 to 18 carry two marks each, Question 19 to 27
CBSE PHYSICS QUESTION PAPER (2005) (i) (ii) All questions are compulsory. There are 30 questions in total. Questions 1 to 8 carry one mark each, Questions 9 to 18 carry two marks each, Question 19 to 27
Optical and Photonic Glasses. Lecture 15. Optical Properties - Polarization, Absorption and Color. Professor Rui Almeida
 Optical and Photonic Glasses : Optical Properties - Polarization, Absorption and Color Professor Rui Almeida International Materials Institute For New Functionality in Glass Lehigh University 21 µm 9.1
Optical and Photonic Glasses : Optical Properties - Polarization, Absorption and Color Professor Rui Almeida International Materials Institute For New Functionality in Glass Lehigh University 21 µm 9.1
B.Tech. First Semester Examination Physics-1 (PHY-101F)
 B.Tech. First Semester Examination Physics-1 (PHY-101F) Note : Attempt FIVE questions in all taking least two questions from each Part. All questions carry equal marks Part-A Q. 1. (a) What are Newton's
B.Tech. First Semester Examination Physics-1 (PHY-101F) Note : Attempt FIVE questions in all taking least two questions from each Part. All questions carry equal marks Part-A Q. 1. (a) What are Newton's
Chapter Two. Energy Bands and Effective Mass
 Chapter Two Energy Bands and Effective Mass Energy Bands Formation At Low Temperature At Room Temperature Valence Band Insulators Metals Effective Mass Energy-Momentum Diagrams Direct and Indirect Semiconduction
Chapter Two Energy Bands and Effective Mass Energy Bands Formation At Low Temperature At Room Temperature Valence Band Insulators Metals Effective Mass Energy-Momentum Diagrams Direct and Indirect Semiconduction
Conductivity and Semi-Conductors
 Conductivity and Semi-Conductors J = current density = I/A E = Electric field intensity = V/l where l is the distance between two points Metals: Semiconductors: Many Polymers and Glasses 1 Electrical Conduction
Conductivity and Semi-Conductors J = current density = I/A E = Electric field intensity = V/l where l is the distance between two points Metals: Semiconductors: Many Polymers and Glasses 1 Electrical Conduction
ET3034TUx Utilization of band gap energy
 ET3034TUx - 3.3.1 - Utilization of band gap energy In the last two weeks we have discussed the working principle of a solar cell and the external parameters that define the performance of a solar cell.
ET3034TUx - 3.3.1 - Utilization of band gap energy In the last two weeks we have discussed the working principle of a solar cell and the external parameters that define the performance of a solar cell.
Paper Review. Special Topics in Optical Engineering II (15/1) Minkyu Kim. IEEE Journal of Quantum Electronics, Feb 1985
 Paper Review IEEE Journal of Quantum Electronics, Feb 1985 Contents Semiconductor laser review High speed semiconductor laser Parasitic elements limitations Intermodulation products Intensity noise Large
Paper Review IEEE Journal of Quantum Electronics, Feb 1985 Contents Semiconductor laser review High speed semiconductor laser Parasitic elements limitations Intermodulation products Intensity noise Large
Optoelectronics ELEC-E3210
 Optoelectronics ELEC-E3210 Lecture 3 Spring 2017 Semiconductor lasers I Outline 1 Introduction 2 The Fabry-Pérot laser 3 Transparency and threshold current 4 Heterostructure laser 5 Power output and linewidth
Optoelectronics ELEC-E3210 Lecture 3 Spring 2017 Semiconductor lasers I Outline 1 Introduction 2 The Fabry-Pérot laser 3 Transparency and threshold current 4 Heterostructure laser 5 Power output and linewidth
PHOTOVOLTAICS Fundamentals
 PHOTOVOLTAICS Fundamentals PV FUNDAMENTALS Semiconductor basics pn junction Solar cell operation Design of silicon solar cell SEMICONDUCTOR BASICS Allowed energy bands Valence and conduction band Fermi
PHOTOVOLTAICS Fundamentals PV FUNDAMENTALS Semiconductor basics pn junction Solar cell operation Design of silicon solar cell SEMICONDUCTOR BASICS Allowed energy bands Valence and conduction band Fermi
CME 300 Properties of Materials. ANSWERS: Homework 9 November 26, As atoms approach each other in the solid state the quantized energy states:
 CME 300 Properties of Materials ANSWERS: Homework 9 November 26, 2011 As atoms approach each other in the solid state the quantized energy states: are split. This splitting is associated with the wave
CME 300 Properties of Materials ANSWERS: Homework 9 November 26, 2011 As atoms approach each other in the solid state the quantized energy states: are split. This splitting is associated with the wave
LEC E T C U T R U E R E 17 -Photodetectors
 LECTURE 17 -Photodetectors Topics to be covered Photodetectors PIN photodiode Avalanche Photodiode Photodetectors Principle of the p-n junction Photodiode A generic photodiode. Photodetectors Principle
LECTURE 17 -Photodetectors Topics to be covered Photodetectors PIN photodiode Avalanche Photodiode Photodetectors Principle of the p-n junction Photodiode A generic photodiode. Photodetectors Principle
We have already discussed what color is.
 The Atom The Electrons in the Atom Reading Assignment: Read the entire chapter. Homework: see the web site for homework. http://web.fccj.org/~smilczan/psc/homework7_11.htm Electrons are the glue that hold
The Atom The Electrons in the Atom Reading Assignment: Read the entire chapter. Homework: see the web site for homework. http://web.fccj.org/~smilczan/psc/homework7_11.htm Electrons are the glue that hold
Chapter 1 Overview of Semiconductor Materials and Physics
 Chapter 1 Overview of Semiconductor Materials and Physics Professor Paul K. Chu Conductivity / Resistivity of Insulators, Semiconductors, and Conductors Semiconductor Elements Period II III IV V VI 2 B
Chapter 1 Overview of Semiconductor Materials and Physics Professor Paul K. Chu Conductivity / Resistivity of Insulators, Semiconductors, and Conductors Semiconductor Elements Period II III IV V VI 2 B
Because light behaves like a wave, we can describe it in one of two ways by its wavelength or by its frequency.
 Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
MANIPAL INSTITUTE OF TECHNOLOGY
 MANIPAL INSTITUT OF TCHNOLOGY MANIPAL UNIVRSITY, MANIPAL SCOND SMSTR B.Tech. ND-SMSTR XAMINATION - JULY 01 SUBJCT: NGINRING PHYSICS (PHY101/10) Time: Hrs. Max. Marks: 50 Note: Answer any FIV FULL questions.
MANIPAL INSTITUT OF TCHNOLOGY MANIPAL UNIVRSITY, MANIPAL SCOND SMSTR B.Tech. ND-SMSTR XAMINATION - JULY 01 SUBJCT: NGINRING PHYSICS (PHY101/10) Time: Hrs. Max. Marks: 50 Note: Answer any FIV FULL questions.
What can laser light do for (or to) me?
 What can laser light do for (or to) me? Phys 1020, Day 15: Questions? Refection, refraction LASERS: 14.3 Next Up: Finish lasers Cameras and optics 1 Eyes to web: Final Project Info Light travels more slowly
What can laser light do for (or to) me? Phys 1020, Day 15: Questions? Refection, refraction LASERS: 14.3 Next Up: Finish lasers Cameras and optics 1 Eyes to web: Final Project Info Light travels more slowly
Optical Properties of Lattice Vibrations
 Optical Properties of Lattice Vibrations For a collection of classical charged Simple Harmonic Oscillators, the dielectric function is given by: Where N i is the number of oscillators with frequency ω
Optical Properties of Lattice Vibrations For a collection of classical charged Simple Harmonic Oscillators, the dielectric function is given by: Where N i is the number of oscillators with frequency ω
Introduction to Optoelectronic Device Simulation by Joachim Piprek
 NUSOD 5 Tutorial MA Introduction to Optoelectronic Device Simulation by Joachim Piprek Outline:. Introduction: VCSEL Example. Electron Energy Bands 3. Drift-Diffusion Model 4. Thermal Model 5. Gain/Absorption
NUSOD 5 Tutorial MA Introduction to Optoelectronic Device Simulation by Joachim Piprek Outline:. Introduction: VCSEL Example. Electron Energy Bands 3. Drift-Diffusion Model 4. Thermal Model 5. Gain/Absorption
Electrons are shared in covalent bonds between atoms of Si. A bound electron has the lowest energy state.
 Photovoltaics Basic Steps the generation of light-generated carriers; the collection of the light-generated carriers to generate a current; the generation of a large voltage across the solar cell; and
Photovoltaics Basic Steps the generation of light-generated carriers; the collection of the light-generated carriers to generate a current; the generation of a large voltage across the solar cell; and
Instrumental Analysis: Spectrophotometric Methods
 Instrumental Analysis: Spectrophotometric Methods 2007 By the end of this part of the course, you should be able to: Understand interaction between light and matter (absorbance, excitation, emission, luminescence,fluorescence,
Instrumental Analysis: Spectrophotometric Methods 2007 By the end of this part of the course, you should be able to: Understand interaction between light and matter (absorbance, excitation, emission, luminescence,fluorescence,
