Physical Chemistry I for Biochemists Chem340. Lecture 38 (4/20/11) Yoshitaka Ishii. Announcement
|
|
|
- Zoe Kennedy
- 5 years ago
- Views:
Transcription
1 Physicl Chemistry I for Biochemists Chem34 Lecture 38 (4//11) Yoshitk Ishii Ch Announcement HW1 due dte is 4/7 (Wed) Exm 3 will be returned probbly this Fridy Finl Exm 5/4 (Wed) 1 3 pm Quiz 5 on 4/9 (Fri) We will cover Ch 9 nd Ch 1. Sec 1 5 1
2 9.6 The Electrochemicl Potentil Assume tht Zn electrod is prtilly immersed in n queous solution of ZnSO 4. Zn(s) Zn + (q) + e Wheres Zn + goes into solution, e stys on the electrode. Only smll Zn dissolves into ions (~1 14 mol), producing ~ 1V potentil between Zn nd electrolyte. To trnsfer chrge dq from the potentil 1 to, the required work is dg = dw = ( 1 )dq, where dq = zfdn nd z is chrge number of n ion (z =, 1,..) nd F is chrge of 1 mol of n electron in bsolute vlue. (Frdy constnt) Electrochemicl Potentil (continued) dg = dw = ( 1 )dq, where dq = zfdn. dg = ( 1 )dq = zf( 1 ) dn (9.44) We define now electrochemicl potentil s zf (9.46 text incorrect) dg ~ ~ 1 So the difference ~ ~ is given s ~ dn ~ ~ F ~ ~ F 1 z 1 In electrochemicl rection, the equilibrium is reched when G ~ Grection ii rection i i i 1 1 z 1 i
3 9.7 Electrochemicl Cells nd Hlf Cells (Correction) ~ zf We choose (sol) = for solution. So ~ (ion in solution) i i For electron in metl electrode, ~ (z = 1 ele ele F F ele =) Now, we consider n equilibrium, z+ + ze, where denotes metl. For this, ~ ~ z~ Correct text Z ele Cell ) F zf( s) zf( Z ~ z~ ele zf Z Z Zn Zn + + e Identicl electrolyte Hlf Cell Cu + + e Cu Equilibrium t hlf cell ~ z ~ ele zf ( ) Z Z ~ zf Z (9.53) For metl (mde of pure element) t 1 br in stndrd dstte, its chemicl potentil ilshould ldbe. If =, (In text Z+ (1 br,98k) Z ~ Not exct) Using (9.53) nd ele F ~ z~ ele ~ zf Z Z ~ zf Z In stndrd condition t 98K nd 1 br ~ zf zf Z Cf. ~ nd ~ F ele 3
4 Stndrd Hydrogen Electrode H + (q) + e 1/H (g) Clculting Potentil for Hydrogen Electrode H + (q) + e 1/H (g) ~ ( ) ~ H q ele 1/ H (Reference electrode) f: fugcity ~ kp Potentil of the hydrogen electrode gs ( p ) RT ln f / f Using stndrd stte ( = in electrolyte, 98K, 1 br), H RT ln( H ) FH / H 1/ H 1/ RT ln( f H H / H H 1/ H F ) RT F f H ln( For unit ctivities of ll species H+ = f = 1, the cell (electrode) hs its stndrd potentil: Convention for H + H 1/ H H H+ = H / H [Q1] F F 1/ H ) gs 4
5 9.8 Redox Rection in Electrochemicl Cells nd the Nernst Eqution Left(node): Zn(s) Zn + (q) + e Right (cthode) : Cu + (q) + e Cu(s) Overll: Zn(s) + Cu + (q) Zn + (q) + Cu(s) Anode: Red 1 Ox e Dniel Cell Cthode: Ox + e Red Overll: Red Ox Ox Red Q. How do you get G rection using chemicl potentil? Zn Zn + + e Cu + + e Cu G Nernst Eqution for Cell (Derivtion) Zn(s) + Cu + (q) Zn + (q) + Cu(s) ~ ~ ~ ~ G rection Zn Cu Zn Cu ~ ~ ln( / ) (9.69) Zn Cu RT Zn Cu For rection, n moles (n=) of electron re trnsferred from the cthode to node. The potentil difference is = cthode node. G rection ( nzf ) nf (z= 1 for electron) (9.7) By combining (9.69) & (9.79), we obtin rection ~ Zn ~ Zn Cu RT ln( ) nfe Zn RT ln( ) ) Cu Cu where we defined electromotive force (emf) s E =. When Zn+ = Cu+ = 1, E is defined s nfe = G re ction. E = E (RT/nF)ln( zn+ / Cu+ ) (9.7) E = E (RT/nF)lnQ (9.73) 5
6 How to obtin E from the hlf cell rection? For the rection Fe + + e Fe, E = -.45 (V) Q1. How much is E for Fe Fe + + e? E =.45 (V) Q. How much is E for Fe + + 4e Fe? E = -.45 (V) Q3. How much is E for Fe Fe + + 4e? E =.45 (V) G rection = -nfe E = E (RT/nF)lnQ 6
7 Nernst s Eqution for Whole nd Hlf Cells Blnce the chrges For whole cell, Red 1 Ox 1 +ne ; 1 Ox +ne 1 Red ( OX Q 1 Re d E = E (RT/nF)lnQ [ ] At 98.15K, we obtin the eqution clled Nernst s eqution E = E (RT/nF)lnQ= E (.5916/n)logQ (9.74) For hlf cell rection: Ox n+ + ne Red G G ~ n ~ ~ ~ F ~ Ox rection ~ Re d Ox RT ln( Ox ele / Re d Re d ) nf Ox / Re d 1 ( 1 Re d 1 Ox E ox/red =( OXn+ Red)/nF - (RT/nF)ln( Red / OXn+ ) (9.77) = E Ox/Re (RT/nF)ln( Red / Oxn+ ) ele ) Activity of electrons is not involved P9.4) By finding pproprite hlf-cell rections, clculte the equilibrium constnt t K for the following rections:. Cd(OH) Cd + O + H O ) The hlf cell rections re Cd(OH) + e Cd + OH E red Tble 9.5 4OH O +H O + 4e E ox -E red Q. How mny is n in ()? n = 4 7
8 9.9 Combining Stndrd Electrode Potentil to Determine Cell Potentil By convention stndrd potentils re listed s reduction potentils s (see Tble 9.5 in ppendix B) Reduction E (V) Q. Which is spontneous rection in stndrd stte? Cu + + e- Cu Cu + + e Cu.674 Zn + + e Zn.7618 Becuse G = -nfe < Whether the rection is spontneous t the stndrd stte is determined by E. Becuse G = nfe nd G ox = G red, E reduction = E oxidiztion. (i.e. Zn Zn + + e E ox =.7618V) The potentil of whole cell E cell is relted to those of the hlf cells E red nd E ox s Q. How much is E cell for the bove hlf cells? E cell =.674 (-.7618) V. E cell = E red + E ox G rection = -nfe S rection= -(G /T) P =nf(e /T) P P9.3) Clculte G rection nd the equilibrium constnt t 98.15K for the rection Cr O - 7 (q) + 3H (g) + 8H + (q) Cr 3+ (q) + 7H O(l) 3H 6H + + 6e E ox =? Cr O 7 - (q) + 6e - Cr 3+ (q) E red = Find in text Use G rection = -nfe cell & K = exp(- G rection /RT) 8
9 9.1 The Reltionship between the Cell emf nd Equilibrium Constnt If the redox rection is llowed to proceed until equilibrium is reched, G =, nd thus E =. For the equilibrium stte, the rection quotient Q = K. Therefore, E =(RT/nF)ln(K) K = exp(nfe /RT) P33 AgBr(s) + e Ag(s) + Br (q) E =.7133 V (1) Ag + (q) + e A() Ag(s) E =.7996 V () Obtin K sp for AgBr(s). Ag(s) Ag + (q) + e E =.7996 V ( ) By clculting (1) + ( ), AgBr(s) Ag + (q) (q) + Br (q) (q) E = ( )) V K sp = exp( G rection/rt)= exp(nfe /RT) 9
10 9.11 The Determintion of E nd Activity Coefficients Using n Electrochemicl Cell The min problem in determining stndrd potentil E is knowing the ctivity constnt for given solute. Now ssume cell consisting of the Ag + /Ag nd SHE hlf cells t 98K. For Ag + /Ag, Ag+ rises from the dissocition of AgNO 3. Assume tht Ag+ = Cl. Recll ctivities of individul ions cnnot be mesured directly. + = + = Ag+ Cl. & = Ag+ = Cl Similrly, = Ag+ = Cl nd m = m Ag+ = m Cl. Then the cell potentil is E = E Ag+/Ag+(RT/F)ln( Ag+ ) = E Ag+/Ag +(RT/F){ln(m )+ln } For low m Ag+, log =-.596(m ) 1/ t 98K. E = E Ag+/Ag+(RT/F)ln( Ag+ ) = E Ag+/Ag +(RT/F){ln(m )+ln } E log 1 (m ) = E Ag+/Ag -.313(m ) 1/ 1
11 9.1 Biochemicl Stndrd Stte Consider the rection, A A(q) + B B(q) D D(q) + E E(q)+xH + (q) Assuming i ~ 1 for ll the species, K D E c D / c D c E / c E c H / c H A c / c c / c B A For biochemistry, we tke ph =7 (rther thn c H+ = 1 ) s stndrd condition. Thus, c H = 1 1. x 1 77 mol/l A nd for other species c i =1. K ' K = K x 1 ( 7x) B B D E 7 c /1. c /1. c / 1. 1 D A c / 1. c / 1. B A E B x H x continued If we define G s G = RTln(K ), G = RTln(K ) = RTln(K1 7x ) = RTln(K ) 7x RTln(1) = G 7x RTln(1) E = G /nf = (RT/nF)ln(K ) = (RT/nF)ln(K1 7x ) = (RT/nF){lnK+7xln1} = (RT/nF){lnK+7xln1} = (RT/nF)lnK + (RT/nF){7xln1} = E + (RT/nF){7xln1} 11
12 9.13 The Donnn Potentil There re two comprtments seprted by membrne tht is freely permeble to N + nd Cl, but not for P z. Becuse the membrne is permeble to N + nd Cl, the system reches equilibrium s L = R. We ssume tht L = R. This leds to L = R. When i ~ 1, c L L = R R + c c + c. If P z were not present, c +L /c +R = c R /c L = 1 Since P z is present, c +L /c +R = c R /c L = r D 1, (r D : Donnn rtio) Net Chrge Net Chrge When z = 1, =1 (b+x)x = (-x)(-x) Net Chrge 1
13 At the equilibrium, (b+x)x = ( x)( x) x = /(b+) c L N+ eq = (b+x) = (+b) /(b+) c L Cl eq = x = /(b+) c R N+ eq = c R Cl eq = ( x) = (+b)/(b+) r D = c L N+ eq /c R N+ eq = (+ b)/ = L R = = D + (RT/F)ln(r D ) Donnn Potentil D = (RT/F)ln{(+b)/} Potentil due to difference of ion concentrtions Potentil due to polriztion of membrne 13
Fe = Fe + e MnO + 8H + 5e = Mn
 Redox Titrtions Net trnsfer of electrons during the rection. xidtion numbers of two/more species chnge. Stisfies requirements for rections in quntittion;. lrge K b. fst rection Blncing redox rections:
Redox Titrtions Net trnsfer of electrons during the rection. xidtion numbers of two/more species chnge. Stisfies requirements for rections in quntittion;. lrge K b. fst rection Blncing redox rections:
The relevant half cell reactions and potentials are: Calculate the equilibrium constant, K, for the reaction at 25 C. lnk
 CHEM1405 2004-J-3 June 2004 Calculate the initial cell potential for the following unbalanced reaction at 25 C from the standard electrode potentials. Assume the concentration of all species is initially
CHEM1405 2004-J-3 June 2004 Calculate the initial cell potential for the following unbalanced reaction at 25 C from the standard electrode potentials. Assume the concentration of all species is initially
Chemistry 163B W2014 Lectures Electrochemistry Quickie
 Chemistry 163B W014 Lectures 4-5- Electrochemistry Quickie ctivity coefficients for ions (W9 #58) Chemistry 163B Electrochemistry B s B q q ( ) ( ) ( ) K B sp ( s) B ( q) ( q) B ( s) 1 B B ( q) ( q) B
Chemistry 163B W014 Lectures 4-5- Electrochemistry Quickie ctivity coefficients for ions (W9 #58) Chemistry 163B Electrochemistry B s B q q ( ) ( ) ( ) K B sp ( s) B ( q) ( q) B ( s) 1 B B ( q) ( q) B
Electrochemical System
 Electrochemical System Topic Outcomes Week Topic Topic Outcomes 8-10 Electrochemical systems It is expected that students are able to: Electrochemical system and its thermodynamics Chemical reactions in
Electrochemical System Topic Outcomes Week Topic Topic Outcomes 8-10 Electrochemical systems It is expected that students are able to: Electrochemical system and its thermodynamics Chemical reactions in
Ch 20 Electrochemistry: the study of the relationships between electricity and chemical reactions.
 Ch 20 Electrochemistry: the study of the relationships between electricity and chemical reactions. In electrochemical reactions, electrons are transferred from one species to another. Learning goals and
Ch 20 Electrochemistry: the study of the relationships between electricity and chemical reactions. In electrochemical reactions, electrons are transferred from one species to another. Learning goals and
7/19/2011. Models of Solution Chemistry- III Acids and Bases
 Models of Solution Chemistry- III Acids nd Bses Ionic Atmosphere Model : Revisiting Ionic Strength Ionic strength - mesure of totl concentrtion of ions in the solution Chpter 8 1 2 i μ ( ) 2 c i z c concentrtion
Models of Solution Chemistry- III Acids nd Bses Ionic Atmosphere Model : Revisiting Ionic Strength Ionic strength - mesure of totl concentrtion of ions in the solution Chpter 8 1 2 i μ ( ) 2 c i z c concentrtion
The Thermodynamics of Aqueous Electrolyte Solutions
 18 The Thermodynmics of Aqueous Electrolyte Solutions As discussed in Chpter 10, when slt is dissolved in wter or in other pproprite solvent, the molecules dissocite into ions. In queous solutions, strong
18 The Thermodynmics of Aqueous Electrolyte Solutions As discussed in Chpter 10, when slt is dissolved in wter or in other pproprite solvent, the molecules dissocite into ions. In queous solutions, strong
ELECTROCHEMISTRY OXIDATION-REDUCTION
 ELECTROCHEMISTRY Electrochemistry involves the relationship between electrical energy and chemical energy. OXIDATION-REDUCTION REACTIONS SPONTANEOUS REACTIONS Can extract electrical energy from these.
ELECTROCHEMISTRY Electrochemistry involves the relationship between electrical energy and chemical energy. OXIDATION-REDUCTION REACTIONS SPONTANEOUS REACTIONS Can extract electrical energy from these.
Problem 22: Buffer solutions 1. The equilibrium, which governs the concentration of H + within the solution is HCOOH! HCOO + H + + Hence K
 Problem : Buffer solutions. The equilibrium, hich governs the concentrtion of H ithin the solution is HCOOH! HCOO H [HCOO ] 4 Hence. [HCOOH] nd since [HCOOH] 0.00 M nd [HCOO ] 0.50 M -4 0.00 4..8 M 0.50
Problem : Buffer solutions. The equilibrium, hich governs the concentrtion of H ithin the solution is HCOOH! HCOO H [HCOO ] 4 Hence. [HCOOH] nd since [HCOOH] 0.00 M nd [HCOO ] 0.50 M -4 0.00 4..8 M 0.50
Electrochemistry. Chapter 18. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Electrochemistry Chapter 18 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Electrochemical processes are oxidation-reduction reactions in which: the energy
Electrochemistry Chapter 18 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Electrochemical processes are oxidation-reduction reactions in which: the energy
Chapter 20 Electrochemistry
 Chapter 20 Electrochemistry Learning goals and key skills: Identify oxidation, reduction, oxidizing agent, and reducing agent in a chemical equation Complete and balance redox equations using the method
Chapter 20 Electrochemistry Learning goals and key skills: Identify oxidation, reduction, oxidizing agent, and reducing agent in a chemical equation Complete and balance redox equations using the method
9-1 (a) A weak electrolyte only partially ionizes when dissolved in water. NaHCO 3 is an
 Chpter 9 9- ( A ek electrolyte only prtilly ionizes hen dissolved in ter. NC is n exmple of ek electrolyte. (b A Brønsted-ory cid is cule tht dontes proton hen it encounters bse (proton cceptor. By this
Chpter 9 9- ( A ek electrolyte only prtilly ionizes hen dissolved in ter. NC is n exmple of ek electrolyte. (b A Brønsted-ory cid is cule tht dontes proton hen it encounters bse (proton cceptor. By this
Oxidation number. The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred.
 Oxidation number The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. Na,
Oxidation number The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. Na,
REVIEW QUESTIONS Chapter 19
 Chemistry 10 ANSWER KEY REVIEW QUESTIONS Chapter 19 1. For each of the following unbalanced equations, (i) write the half-reactions for oxidation and reduction, and (ii) balance the overall equation in
Chemistry 10 ANSWER KEY REVIEW QUESTIONS Chapter 19 1. For each of the following unbalanced equations, (i) write the half-reactions for oxidation and reduction, and (ii) balance the overall equation in
CHAPTER 08: MONOPROTIC ACID-BASE EQUILIBRIA
 Hrris: Quntittive Chemicl Anlysis, Eight Edition CHAPTER 08: MONOPROTIC ACIDBASE EQUILIBRIA CHAPTER 08: Opener A CHAPTER 08: Opener B CHAPTER 08: Opener C CHAPTER 08: Opener D CHAPTER 08: Opener E Chpter
Hrris: Quntittive Chemicl Anlysis, Eight Edition CHAPTER 08: MONOPROTIC ACIDBASE EQUILIBRIA CHAPTER 08: Opener A CHAPTER 08: Opener B CHAPTER 08: Opener C CHAPTER 08: Opener D CHAPTER 08: Opener E Chpter
Chapter 17. Electrochemistry
 Chapter 17 Electrochemistry Contents Galvanic cells Standard reduction potentials Cell potential, electrical work, and free energy Dependence of cell potential on concentration Batteries Corrosion Electrolysis
Chapter 17 Electrochemistry Contents Galvanic cells Standard reduction potentials Cell potential, electrical work, and free energy Dependence of cell potential on concentration Batteries Corrosion Electrolysis
Electrochemistry Pearson Education, Inc. Mr. Matthew Totaro Legacy High School AP Chemistry
 2012 Pearson Education, Inc. Mr. Matthew Totaro Legacy High School AP Chemistry Electricity from Chemistry Many chemical reactions involve the transfer of electrons between atoms or ions electron transfer
2012 Pearson Education, Inc. Mr. Matthew Totaro Legacy High School AP Chemistry Electricity from Chemistry Many chemical reactions involve the transfer of electrons between atoms or ions electron transfer
Review: Balancing Redox Reactions. Review: Balancing Redox Reactions
 Review: Balancing Redox Reactions Determine which species is oxidized and which species is reduced Oxidation corresponds to an increase in the oxidation number of an element Reduction corresponds to a
Review: Balancing Redox Reactions Determine which species is oxidized and which species is reduced Oxidation corresponds to an increase in the oxidation number of an element Reduction corresponds to a
Fundamentals of Analytical Chemistry
 Homework Fundmentls of nlyticl hemistry hpter 9 0, 1, 5, 7, 9 cids, Bses, nd hpter 9(b) Definitions cid Releses H ions in wter (rrhenius) Proton donor (Bronsted( Lowry) Electron-pir cceptor (Lewis) hrcteristic
Homework Fundmentls of nlyticl hemistry hpter 9 0, 1, 5, 7, 9 cids, Bses, nd hpter 9(b) Definitions cid Releses H ions in wter (rrhenius) Proton donor (Bronsted( Lowry) Electron-pir cceptor (Lewis) hrcteristic
Chemistry 2000 Lecture 15: Electrochemistry
 Chemistry 2000 Lecture 15: Electrochemistry Marc R. Roussel February 21, 2018 Marc R. Roussel Chemistry 2000 Lecture 15: Electrochemistry February 21, 2018 1 / 33 Electrochemical cells Electrochemical
Chemistry 2000 Lecture 15: Electrochemistry Marc R. Roussel February 21, 2018 Marc R. Roussel Chemistry 2000 Lecture 15: Electrochemistry February 21, 2018 1 / 33 Electrochemical cells Electrochemical
Lecture 14. Thermodynamics of Galvanic (Voltaic) Cells.
 Lecture 14 Thermodynamics of Galvanic (Voltaic) Cells. 51 52 Ballard PEM Fuel Cell. 53 Electrochemistry Alessandro Volta, 1745-1827, Italian scientist and inventor. Luigi Galvani, 1737-1798, Italian scientist
Lecture 14 Thermodynamics of Galvanic (Voltaic) Cells. 51 52 Ballard PEM Fuel Cell. 53 Electrochemistry Alessandro Volta, 1745-1827, Italian scientist and inventor. Luigi Galvani, 1737-1798, Italian scientist
Part One: Introduction. a. Chemical reactions produced by electric current. (electrolysis)
 CHAPTER 19: ELECTROCHEMISTRY Part One: Introduction A. Terminology. 1. Electrochemistry deals with: a. Chemical reactions produced by electric current. (electrolysis) b. Production of electric current
CHAPTER 19: ELECTROCHEMISTRY Part One: Introduction A. Terminology. 1. Electrochemistry deals with: a. Chemical reactions produced by electric current. (electrolysis) b. Production of electric current
Q1. Why does the conductivity of a solution decrease with dilution?
 Q1. Why does the conductivity of a solution decrease with dilution? A1. Conductivity of a solution is the conductance of ions present in a unit volume of the solution. On dilution the number of ions per
Q1. Why does the conductivity of a solution decrease with dilution? A1. Conductivity of a solution is the conductance of ions present in a unit volume of the solution. On dilution the number of ions per
Chem 4501 Introduction to Thermodynamics, 3 Credits Kinetics, and Statistical Mechanics. Fall Semester Homework Problem Set Number 12 Solutions
 Chem 4501 Introduction to Thermodynamics, 3 Credits Kinetics, and Statistical Mechanics Fall Semester 017 Homework Problem Set Number 1 Solutions 1. (Based on McQuarrie and Simon, 13-1.) Write balanced
Chem 4501 Introduction to Thermodynamics, 3 Credits Kinetics, and Statistical Mechanics Fall Semester 017 Homework Problem Set Number 1 Solutions 1. (Based on McQuarrie and Simon, 13-1.) Write balanced
Chem/Biochem 471 Exam 2 11/18/09 Page 1 of 7 Name:
 Chem/Biochem 471 Exm 2 11/18/09 Pge 1 of 7 Plese leve the exm pges stpled together. The formuls re on seprte sheet. This exm hs 5 questions. You must nswer t lest 4 of the questions. You my nswer more
Chem/Biochem 471 Exm 2 11/18/09 Pge 1 of 7 Plese leve the exm pges stpled together. The formuls re on seprte sheet. This exm hs 5 questions. You must nswer t lest 4 of the questions. You my nswer more
Electrode Potentials and Their Measurement
 Electrochemistry Electrode Potentials and Their Measurement Cu(s) + 2Ag + (aq) Cu(s) + Zn 2+ (aq) Cu 2+ (aq) + 2 Ag(s) No reaction Zn(s) + Cu 2+ (aq) Cu(s) + Zn 2+ (aq) In this reaction: Zn (s) g Zn 2+
Electrochemistry Electrode Potentials and Their Measurement Cu(s) + 2Ag + (aq) Cu(s) + Zn 2+ (aq) Cu 2+ (aq) + 2 Ag(s) No reaction Zn(s) + Cu 2+ (aq) Cu(s) + Zn 2+ (aq) In this reaction: Zn (s) g Zn 2+
EMA4303/5305 Electrochemical Engineering Lecture 02 Equilibrium Electrochemistry
 EMA4303/5305 Electrochemical Engineering Lecture 02 Equilibrium Electrochemistry Dr. Junheng Xing, Prof. Zhe Cheng Mechanical & Materials Engineering Florida International University 2 Equilibrium Electrochemistry
EMA4303/5305 Electrochemical Engineering Lecture 02 Equilibrium Electrochemistry Dr. Junheng Xing, Prof. Zhe Cheng Mechanical & Materials Engineering Florida International University 2 Equilibrium Electrochemistry
Ch. 13 Fundamentals of Electrochemistry
 Ch. 13 Fundamentals of Electrochemistry 13.1 13-1. Basic Concepts of electrochemistry redox reaction : reactions with electron transfer oxidized : loses electrons reduced : gains electrons Fe 3+ + V 2+
Ch. 13 Fundamentals of Electrochemistry 13.1 13-1. Basic Concepts of electrochemistry redox reaction : reactions with electron transfer oxidized : loses electrons reduced : gains electrons Fe 3+ + V 2+
Chapter Nineteen. Electrochemistry
 Chapter Nineteen Electrochemistry 1 Electrochemistry The study of chemical reactions through electrical circuits. Monitor redox reactions by controlling electron transfer REDOX: Shorthand for REDuction-OXidation
Chapter Nineteen Electrochemistry 1 Electrochemistry The study of chemical reactions through electrical circuits. Monitor redox reactions by controlling electron transfer REDOX: Shorthand for REDuction-OXidation
1. How much work (in kj/mol) can in principle be obtained when an electron is brought to nm distance from a proton?
 Thermodynamics: Examples for chapter 7. 1. How much work (in kj/mol) can in principle be obtained when an electron is brought to 0.5000 nm distance from a proton? The work is obtained by integrating the
Thermodynamics: Examples for chapter 7. 1. How much work (in kj/mol) can in principle be obtained when an electron is brought to 0.5000 nm distance from a proton? The work is obtained by integrating the
Strategy: Use the Gibbs phase rule (Equation 5.3). How many components are present?
 University Chemistry Quiz 4 2014/12/11 1. (5%) Wht is the dimensionlity of the three-phse coexistence region in mixture of Al, Ni, nd Cu? Wht type of geometricl region dose this define? Strtegy: Use the
University Chemistry Quiz 4 2014/12/11 1. (5%) Wht is the dimensionlity of the three-phse coexistence region in mixture of Al, Ni, nd Cu? Wht type of geometricl region dose this define? Strtegy: Use the
Ch 18 Electrochemistry OIL-RIG Reactions
 Ch 18 Electrochemistry OIL-RIG Reactions Alessandro Volta s Invention Modified by Dr. Cheng-Yu Lai Daily Electrochemistry Appliactions Electrochemistry: The area of chemistry that examines the transformations
Ch 18 Electrochemistry OIL-RIG Reactions Alessandro Volta s Invention Modified by Dr. Cheng-Yu Lai Daily Electrochemistry Appliactions Electrochemistry: The area of chemistry that examines the transformations
Electrochemistry. Galvanic Cell. Page 1. Applications of Redox
 Electrochemistry Applications of Redox Review Oxidation reduction reactions involve a transfer of electrons. OIL- RIG Oxidation Involves Loss Reduction Involves Gain LEO-GER Lose Electrons Oxidation Gain
Electrochemistry Applications of Redox Review Oxidation reduction reactions involve a transfer of electrons. OIL- RIG Oxidation Involves Loss Reduction Involves Gain LEO-GER Lose Electrons Oxidation Gain
UNIVERSITY OF MALTA DEPARTMENT OF CHEMISTRY. CH237 - Chemical Thermodynamics and Kinetics. Tutorial Sheet VIII
 UNIVERSITY OF MALTA DEPARTMENT OF CHEMISTRY CH237 - Chemicl Thermodynmics nd Kinetics Tutoril Sheet VIII 1 () (i) The rte of the rection A + 2B 3C + D ws reported s 1.0 mol L -1 s -1. Stte the rtes of
UNIVERSITY OF MALTA DEPARTMENT OF CHEMISTRY CH237 - Chemicl Thermodynmics nd Kinetics Tutoril Sheet VIII 1 () (i) The rte of the rection A + 2B 3C + D ws reported s 1.0 mol L -1 s -1. Stte the rtes of
Chapter 20. Electrochemistry
 Chapter 20. Electrochemistry 20.1 Oxidation-Reduction Reactions Oxidation-reduction reactions = chemical reactions in which the oxidation state of one or more substance changes (redox reactions). Recall:
Chapter 20. Electrochemistry 20.1 Oxidation-Reduction Reactions Oxidation-reduction reactions = chemical reactions in which the oxidation state of one or more substance changes (redox reactions). Recall:
Announcements. Comprehensive Final Exam: March 24 7:30AM - 9:30 C114 2,9,10,11,13,17,22,29,31,38,40,44,46,50,53,58,62,64,65,70, 72,73,82,85,87
 Announcements Exam 3 March 17 Comprehensive Final Exam: March 24 7:30AM - 9:30 C114 Problems Chapter 21: 2,9,10,11,13,17,22,29,31,38,40,44,46,50,53,58,62,64,65,70, 72,73,82,85,87 Up to but not including
Announcements Exam 3 March 17 Comprehensive Final Exam: March 24 7:30AM - 9:30 C114 Problems Chapter 21: 2,9,10,11,13,17,22,29,31,38,40,44,46,50,53,58,62,64,65,70, 72,73,82,85,87 Up to but not including
Chapter 19 ElectroChemistry
 Chem 1046 General Chemistry by Ebbing and Gammon, 9th Edition George W.J. Kenney, Jr, Professor of Chemistry Last Update: 11July2009 Chapter 19 ElectroChemistry These Notes are to SUPPLIMENT the Text,
Chem 1046 General Chemistry by Ebbing and Gammon, 9th Edition George W.J. Kenney, Jr, Professor of Chemistry Last Update: 11July2009 Chapter 19 ElectroChemistry These Notes are to SUPPLIMENT the Text,
Electrochemistry. Remember from CHM151 G E R L E O 6/24/2014. A redox reaction in one in which electrons are transferred.
 Electrochemistry Remember from CHM151 A redox reaction in one in which electrons are transferred Reduction Oxidation For example: L E O ose lectrons xidation G E R ain lectrons eduction We can determine
Electrochemistry Remember from CHM151 A redox reaction in one in which electrons are transferred Reduction Oxidation For example: L E O ose lectrons xidation G E R ain lectrons eduction We can determine
CHEM J-12 June 2013
 CHEM1101 2013-J-12 June 2013 In concentration cells no net chemical conversion occurs, however a measurable voltage is present between the two half-cells. Explain how the voltage is produced. 2 In concentration
CHEM1101 2013-J-12 June 2013 In concentration cells no net chemical conversion occurs, however a measurable voltage is present between the two half-cells. Explain how the voltage is produced. 2 In concentration
A + B C +D ΔG = ΔG + RTlnKp. Me n+ + ne - Me. Me n n
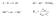 A + B C +D ΔG = ΔG + RTlnKp Me n+ + ne - Me K p a a Me Me n a n e 1 mol madde 6.2 x 1 23 atom elektron yükü 1.62 x 1-19 C FARADAY SABİTİ: 6.2 x 1 23 x 1.62 x 1-19 = 96485 A.sn (= coulomb) 1 Faraday 965
A + B C +D ΔG = ΔG + RTlnKp Me n+ + ne - Me K p a a Me Me n a n e 1 mol madde 6.2 x 1 23 atom elektron yükü 1.62 x 1-19 C FARADAY SABİTİ: 6.2 x 1 23 x 1.62 x 1-19 = 96485 A.sn (= coulomb) 1 Faraday 965
The Predom module. Predom calculates and plots isothermal 1-, 2- and 3-metal predominance area diagrams. Predom accesses only compound databases.
 Section 1 Section 2 The module clcultes nd plots isotherml 1-, 2- nd 3-metl predominnce re digrms. ccesses only compound dtbses. Tble of Contents Tble of Contents Opening the module Section 3 Stoichiometric
Section 1 Section 2 The module clcultes nd plots isotherml 1-, 2- nd 3-metl predominnce re digrms. ccesses only compound dtbses. Tble of Contents Tble of Contents Opening the module Section 3 Stoichiometric
Chem 321 Lecture 16 - Potentiometry 10/22/13
 Student Learning Objectives Chem 321 Lecture 16 - Potentiometry 10/22/13 In lab you will use an ion-selective electrode to determine the amount of fluoride in an unknown solution. In this approach, as
Student Learning Objectives Chem 321 Lecture 16 - Potentiometry 10/22/13 In lab you will use an ion-selective electrode to determine the amount of fluoride in an unknown solution. In this approach, as
Measuring Electron Work Function in Metal
 n experiment of the Electron topic Mesuring Electron Work Function in Metl Instructor: 梁生 Office: 7-318 Emil: shling@bjtu.edu.cn Purposes 1. To understnd the concept of electron work function in metl nd
n experiment of the Electron topic Mesuring Electron Work Function in Metl Instructor: 梁生 Office: 7-318 Emil: shling@bjtu.edu.cn Purposes 1. To understnd the concept of electron work function in metl nd
Chapter 20. Electrochemistry
 Chapter 20. Electrochemistry 20.1 OxidationReduction Reactions Oxidationreduction reactions = chemical reactions in which the oxidation state of one or more substance changes (redox reactions). Recall:
Chapter 20. Electrochemistry 20.1 OxidationReduction Reactions Oxidationreduction reactions = chemical reactions in which the oxidation state of one or more substance changes (redox reactions). Recall:
Chem 340 Fall 2013 Lecture Notes 12- Electrochemistry (Chap. 6)
 Chem 340 Fall 2013 Lecture Notes 12- Electrochemistry (Chap. 6) Charged particle energies affected by applied electric fields, similarly dissolution of metals from electrodes to create ions also creates
Chem 340 Fall 2013 Lecture Notes 12- Electrochemistry (Chap. 6) Charged particle energies affected by applied electric fields, similarly dissolution of metals from electrodes to create ions also creates
Chemistry Department. The Islamic University of Gaza. General Chemistry B.(CHEMB 1301) Time:2 hours الرقم الجامعي... اسم المدرس...
 The Islmic University of Gz Chemistry Deprtment Generl Chemistry B.(CHEMB 1301) Time:2 hours 60 اسم الطالب... الرقم الجامعي... اسم المدرس... R = 8.314 J/mol.K, or = 0.0821 L.tm/mol.K Q1- True ( ) or flse(
The Islmic University of Gz Chemistry Deprtment Generl Chemistry B.(CHEMB 1301) Time:2 hours 60 اسم الطالب... الرقم الجامعي... اسم المدرس... R = 8.314 J/mol.K, or = 0.0821 L.tm/mol.K Q1- True ( ) or flse(
Ch. 24 Molecular Reaction Dynamics 1. Collision Theory 2. Diffusion-Controlled Reaction
 Ch. 4 Moleculr Rection Dynmics 1. Collision Theory. Diffusion-Controlle Rection Lecture 17 3. The Mteril Blnce Eqution 4. Trnsition Stte Theory: The Eyring Eqution 5. Trnsition Stte Theory: Thermoynmic
Ch. 4 Moleculr Rection Dynmics 1. Collision Theory. Diffusion-Controlle Rection Lecture 17 3. The Mteril Blnce Eqution 4. Trnsition Stte Theory: The Eyring Eqution 5. Trnsition Stte Theory: Thermoynmic
CHEM 116-Dr. Babb s Sections Answer Key to Lecture Problem Sheet Questions for Chapters 20, 21, and 23.
 CHEM 116-Dr. Babb s Sections Answer Key to Lecture Problem Sheet Questions for Chapters 20, 21, and 23. 199. First complex: Co(NH 3 ) 6 Cl 3 + 3 AgNO 3 > Co(NH 3 ) 6 +3 + 3 AgCl(s); the three Cl - are
CHEM 116-Dr. Babb s Sections Answer Key to Lecture Problem Sheet Questions for Chapters 20, 21, and 23. 199. First complex: Co(NH 3 ) 6 Cl 3 + 3 AgNO 3 > Co(NH 3 ) 6 +3 + 3 AgCl(s); the three Cl - are
Find this material useful? You can help our team to keep this site up and bring you even more content consider donating via the link on our site.
 Find this mteril useful? You cn help our tem to keep this site up nd bring you even more content consider donting vi the link on our site. Still hving trouble understnding the mteril? Check out our Tutoring
Find this mteril useful? You cn help our tem to keep this site up nd bring you even more content consider donting vi the link on our site. Still hving trouble understnding the mteril? Check out our Tutoring
17.1 Redox Chemistry Revisited
 Chapter Outline 17.1 Redox Chemistry Revisited 17.2 Electrochemical Cells 17.3 Standard Potentials 17.4 Chemical Energy and Electrical Work 17.5 A Reference Point: The Standard Hydrogen Electrode 17.6
Chapter Outline 17.1 Redox Chemistry Revisited 17.2 Electrochemical Cells 17.3 Standard Potentials 17.4 Chemical Energy and Electrical Work 17.5 A Reference Point: The Standard Hydrogen Electrode 17.6
8. ELECTROCHEMICAL CELLS. n Electrode Reactions and Electrode Potentials a. H 2 2H + + 2e. Cl 2 + 2e 2Cl. H 2 + Cl 2 2H + + 2Cl ; z = 2
 8. ELECTROCHEMICAL CELLS n Electrode Reactions and Electrode Potentials 8.1. a. H H + + e Cl + e Cl H + Cl H + + Cl ; z = E = E RT F ln ( a H +a Cl ) b. Hg(l)+ Cl Hg Cl + e H + + e H Hg + H + + Cl Hg Cl
8. ELECTROCHEMICAL CELLS n Electrode Reactions and Electrode Potentials 8.1. a. H H + + e Cl + e Cl H + Cl H + + Cl ; z = E = E RT F ln ( a H +a Cl ) b. Hg(l)+ Cl Hg Cl + e H + + e H Hg + H + + Cl Hg Cl
Oxidation-Reduction Review. Electrochemistry. Oxidation-Reduction Reactions. Oxidation-Reduction Reactions. Sample Problem.
 1 Electrochemistry Oxidation-Reduction Review Topics Covered Oxidation-reduction reactions Balancing oxidationreduction equations Voltaic cells Cell EMF Spontaneity of redox reactions Batteries Electrolysis
1 Electrochemistry Oxidation-Reduction Review Topics Covered Oxidation-reduction reactions Balancing oxidationreduction equations Voltaic cells Cell EMF Spontaneity of redox reactions Batteries Electrolysis
CHEM J-8 June /01(a)
 CHEM1001 2012-J-8 June 2012 22/01(a) A galvanic cell has the following cell reaction: D(s) + 2Zn 2+ (aq) 2Zn(s) + D 4+ (aq) Write the overall cell reaction in shorthand cell notation. E = 0.18 V 8 D(s)
CHEM1001 2012-J-8 June 2012 22/01(a) A galvanic cell has the following cell reaction: D(s) + 2Zn 2+ (aq) 2Zn(s) + D 4+ (aq) Write the overall cell reaction in shorthand cell notation. E = 0.18 V 8 D(s)
Hydronium or hydroxide ions can also be produced by a reaction of certain substances with water:
 Chpter 14 1 ACIDS/BASES Acids hve tste, rect with most metls to produce, rect with most crbontes to produce, turn litmus nd phenolphthlein. Bses hve tste rect very well well with most metls or crbontes,
Chpter 14 1 ACIDS/BASES Acids hve tste, rect with most metls to produce, rect with most crbontes to produce, turn litmus nd phenolphthlein. Bses hve tste rect very well well with most metls or crbontes,
3. Potentials and thermodynamics
 Electrochemical Energy Engineering, 2012 3. Potentials and thermodynamics Learning subject 1. Electrochemical reaction 2. Thermodynamics and potential 3. Nernst equation Learning objective 1. To set up
Electrochemical Energy Engineering, 2012 3. Potentials and thermodynamics Learning subject 1. Electrochemical reaction 2. Thermodynamics and potential 3. Nernst equation Learning objective 1. To set up
Chapter 18 Electrochemistry. Electrochemical Cells
 Chapter 18 Electrochemistry Chapter 18 1 Electrochemical Cells Electrochemical Cells are of two basic types: Galvanic Cells a spontaneous chemical reaction generates an electric current Electrolytic Cells
Chapter 18 Electrochemistry Chapter 18 1 Electrochemical Cells Electrochemical Cells are of two basic types: Galvanic Cells a spontaneous chemical reaction generates an electric current Electrolytic Cells
Chemistry 102 Chapter 19 OXIDATION-REDUCTION REACTIONS
 OXIDATION-REDUCTION REACTIONS Some of the most important reaction in chemistry are oxidation-reduction (redox) reactions. In these reactions, electrons transfer from one reactant to the other. The rusting
OXIDATION-REDUCTION REACTIONS Some of the most important reaction in chemistry are oxidation-reduction (redox) reactions. In these reactions, electrons transfer from one reactant to the other. The rusting
Electron Transfer Reactions
 ELECTROCHEMISTRY 1 Electron Transfer Reactions 2 Electron transfer reactions are oxidation- reduction or redox reactions. Results in the generation of an electric current (electricity) or be caused by
ELECTROCHEMISTRY 1 Electron Transfer Reactions 2 Electron transfer reactions are oxidation- reduction or redox reactions. Results in the generation of an electric current (electricity) or be caused by
Dr. Anand Gupta
 By Dr Anand Gupta Mr. Mahesh Kapil Dr. Anand Gupta 09356511518 09888711209 anandu71@yahoo.com mkapil_foru@yahoo.com Electrochemistry Electrolysis Electric energy Chemical energy Galvanic cell 2 Electrochemistry
By Dr Anand Gupta Mr. Mahesh Kapil Dr. Anand Gupta 09356511518 09888711209 anandu71@yahoo.com mkapil_foru@yahoo.com Electrochemistry Electrolysis Electric energy Chemical energy Galvanic cell 2 Electrochemistry
Tutorials : Corrosion Part 1: Theory and basics
 Tutorials : Corrosion Part 1: Theory and basics Outline A. Definition and effects of corrosion B. General thermodynamics and kinetics in electrochemistry C. Thermodynamics and kinetics in corrosion 2 2/21
Tutorials : Corrosion Part 1: Theory and basics Outline A. Definition and effects of corrosion B. General thermodynamics and kinetics in electrochemistry C. Thermodynamics and kinetics in corrosion 2 2/21
Paolo Sarti 2011 Department of Biochemistry Sapienza REDOX REACTIONS
 REDOX REACTIONS What happens upon diping a needle in a CuSO 4 solution!? Fe + Cu 2+ SO 4 2- Fe 2+ SO 4 2- + Cu In a redox reaction, one (or more) electrons are donated by a reductant to an oxidant Multiple
REDOX REACTIONS What happens upon diping a needle in a CuSO 4 solution!? Fe + Cu 2+ SO 4 2- Fe 2+ SO 4 2- + Cu In a redox reaction, one (or more) electrons are donated by a reductant to an oxidant Multiple
1. Weak acids. For a weak acid HA, there is less than 100% dissociation to ions. The B-L equilibrium is:
 th 9 Homework: Reding, M&F, ch. 15, pp. 584-598, 602-605 (clcultions of ph, etc., for wek cids, wek bses, polyprotic cids, nd slts; fctors ffecting cid strength). Problems: Nkon, ch. 18, #1-10, 16-18,
th 9 Homework: Reding, M&F, ch. 15, pp. 584-598, 602-605 (clcultions of ph, etc., for wek cids, wek bses, polyprotic cids, nd slts; fctors ffecting cid strength). Problems: Nkon, ch. 18, #1-10, 16-18,
Homework 04. Acids, Bases, and Salts
 HW04 - Acids, Bses, nd Slts! This is preview of the published version of the quiz Strted: Feb 21 t 8:59m Quiz Instruc!ons Homework 04 Acids, Bses, nd Slts Question 1 In the reversible rection HCN + H O
HW04 - Acids, Bses, nd Slts! This is preview of the published version of the quiz Strted: Feb 21 t 8:59m Quiz Instruc!ons Homework 04 Acids, Bses, nd Slts Question 1 In the reversible rection HCN + H O
Ph2b Quiz - 1. Instructions
 Ph2b Winter 217-18 Quiz - 1 Due Dte: Mondy, Jn 29, 218 t 4pm Ph2b Quiz - 1 Instructions 1. Your solutions re due by Mondy, Jnury 29th, 218 t 4pm in the quiz box outside 21 E. Bridge. 2. Lte quizzes will
Ph2b Winter 217-18 Quiz - 1 Due Dte: Mondy, Jn 29, 218 t 4pm Ph2b Quiz - 1 Instructions 1. Your solutions re due by Mondy, Jnury 29th, 218 t 4pm in the quiz box outside 21 E. Bridge. 2. Lte quizzes will
Electrochem: It s Got Potential!
 Electrochem: It s Got Potential! Presented by: Denise DeMartino Westlake High School, Eanes ISD Pre-AP, AP, and Advanced Placement are registered trademarks of the College Board, which was not involved
Electrochem: It s Got Potential! Presented by: Denise DeMartino Westlake High School, Eanes ISD Pre-AP, AP, and Advanced Placement are registered trademarks of the College Board, which was not involved
Copyright 2018 Dan Dill 1
 when the ions each are 1 M, Zn is consumed. This means 1. 1 2. 1 3. 1 4. 1. More information needed Lecture 24 CH102 A1 (MWF 9:0 am) Monday, March 26, 2018 Cell voltage,, and electrical energy Calculating
when the ions each are 1 M, Zn is consumed. This means 1. 1 2. 1 3. 1 4. 1. More information needed Lecture 24 CH102 A1 (MWF 9:0 am) Monday, March 26, 2018 Cell voltage,, and electrical energy Calculating
Cu 3 (PO 4 ) 2 (s) 3 Cu 2+ (aq) + 2 PO 4 3- (aq) circle answer: pure water or Na 3 PO 4 solution This is the common-ion effect.
 CHEM 1122011 NAME: ANSWER KEY Vining, Exm # SHORT ANSWER QUESTIONS ============= 4 points ech ============ All work must be shown. 1. Wht re [H O ] nd [OH ] for solution tht hs ph of 9.0? Choose one. ()
CHEM 1122011 NAME: ANSWER KEY Vining, Exm # SHORT ANSWER QUESTIONS ============= 4 points ech ============ All work must be shown. 1. Wht re [H O ] nd [OH ] for solution tht hs ph of 9.0? Choose one. ()
ELEMENTS OF ELEC TROCHEMIS TRY. A. A number of analytical techniques are based upon oxidation-reduction reactions.
 Page 1 of 8 Chem 201 Winter 2006 I. Introduction ELEMENTS OF ELEC TROCHEMIS TRY A. A number of analytical techniques are based upon oxidationreduction reactions. B. Examples of these techniques would include:
Page 1 of 8 Chem 201 Winter 2006 I. Introduction ELEMENTS OF ELEC TROCHEMIS TRY A. A number of analytical techniques are based upon oxidationreduction reactions. B. Examples of these techniques would include:
Chapter 19: Electrochemistry
 Chapter 19: Electrochemistry Overview of the Chapter review oxidation-reduction chemistry basics galvanic cells spontaneous chemical reaction generates a voltage set-up of galvanic cell & identification
Chapter 19: Electrochemistry Overview of the Chapter review oxidation-reduction chemistry basics galvanic cells spontaneous chemical reaction generates a voltage set-up of galvanic cell & identification
Physics 1402: Lecture 7 Today s Agenda
 1 Physics 1402: Lecture 7 Tody s gend nnouncements: Lectures posted on: www.phys.uconn.edu/~rcote/ HW ssignments, solutions etc. Homework #2: On Msterphysics tody: due Fridy Go to msteringphysics.com Ls:
1 Physics 1402: Lecture 7 Tody s gend nnouncements: Lectures posted on: www.phys.uconn.edu/~rcote/ HW ssignments, solutions etc. Homework #2: On Msterphysics tody: due Fridy Go to msteringphysics.com Ls:
The International Association for the Properties of Water and Steam. Release on the Ionization Constant of H 2 O
 IAPWS R-7 The Interntionl Assocition for the Properties of Wter nd Stem Lucerne, Sitzerlnd August 7 Relese on the Ioniztion Constnt of H O 7 The Interntionl Assocition for the Properties of Wter nd Stem
IAPWS R-7 The Interntionl Assocition for the Properties of Wter nd Stem Lucerne, Sitzerlnd August 7 Relese on the Ioniztion Constnt of H O 7 The Interntionl Assocition for the Properties of Wter nd Stem
ΔG = -nfe cell. Electrode Potentials. The cell potential E cell is related to the free energy of the reaction ΔG by:
 Electrode Potentials Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451 Saudi Arabia Building: 05,
Electrode Potentials Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451 Saudi Arabia Building: 05,
Hg2 2+ (aq) + H2(g) 2 Hg(l) + 2H + (aq)
 The potential difference between two electrodes in a cell is called the electromotive force, or The EMF of a voltaic cell is called the The cell voltage of a voltaic cell will be a Note: We are used to
The potential difference between two electrodes in a cell is called the electromotive force, or The EMF of a voltaic cell is called the The cell voltage of a voltaic cell will be a Note: We are used to
CHEM J-14 June 2014
 CHEM1101 2014-J-14 June 2014 An electrochemical cell consists of an Fe 2+ /Fe half cell with unknown [Fe 2+ ] and a Sn 2+ /Sn half-cell with [Sn 2+ ] = 1.10 M. The electromotive force (electrical potential)
CHEM1101 2014-J-14 June 2014 An electrochemical cell consists of an Fe 2+ /Fe half cell with unknown [Fe 2+ ] and a Sn 2+ /Sn half-cell with [Sn 2+ ] = 1.10 M. The electromotive force (electrical potential)
Electrochemistry. The study of the interchange of chemical and electrical energy.
 Electrochemistry The study of the interchange of chemical and electrical energy. Oxidation-reduction (redox) reaction: involves a transfer of electrons from the reducing agent to the oxidizing agent. oxidation:
Electrochemistry The study of the interchange of chemical and electrical energy. Oxidation-reduction (redox) reaction: involves a transfer of electrons from the reducing agent to the oxidizing agent. oxidation:
Chapter 20. Electrochemistry. Chapter 20 Problems. Electrochemistry 7/3/2012. Problems 15, 17, 19, 23, 27, 29, 33, 39, 59
 Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 20 John D. Bookstaver St. Charles Community College Cottleville, MO Chapter 20 Problems
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 20 John D. Bookstaver St. Charles Community College Cottleville, MO Chapter 20 Problems
Chapter 16 Acid Base Equilibria
 Chpter 16 Acid Bse Equilibri 16.1 Acids & Bses: A Brief Review Arrhenius cids nd bses: cid: n H + donor HA(q) H(q) A(q) bse: n OH donor OH(q) (q) OH(q) Brønsted Lowry cids nd bses: cid: n H + donor HA(q)
Chpter 16 Acid Bse Equilibri 16.1 Acids & Bses: A Brief Review Arrhenius cids nd bses: cid: n H + donor HA(q) H(q) A(q) bse: n OH donor OH(q) (q) OH(q) Brønsted Lowry cids nd bses: cid: n H + donor HA(q)
CHEMICAL KINETICS
 CHEMICAL KINETICS Long Answer Questions: 1. Explin the following terms with suitble exmples ) Averge rte of Rection b) Slow nd Fst Rections c) Order of Rection d) Moleculrity of Rection e) Activtion Energy
CHEMICAL KINETICS Long Answer Questions: 1. Explin the following terms with suitble exmples ) Averge rte of Rection b) Slow nd Fst Rections c) Order of Rection d) Moleculrity of Rection e) Activtion Energy
Strong acids and bases. Strong acids and bases. Systematic Treatment of Equilibrium & Monoprotic Acid-base Equilibrium.
 Strong cids nd bses Systemtic Tretment of Equilibrium & Monoprotic cid-bse Equilibrium onc. (M) 0.0.00 -.00-5.00-8 p Strong cids nd bses onc. (M) p 0.0.0.00 -.0.00-5 5.0.00-8 8.0? We hve to consider utoprotolysis
Strong cids nd bses Systemtic Tretment of Equilibrium & Monoprotic cid-bse Equilibrium onc. (M) 0.0.00 -.00-5.00-8 p Strong cids nd bses onc. (M) p 0.0.0.00 -.0.00-5 5.0.00-8 8.0? We hve to consider utoprotolysis
#13 Electrochemical Cells
 #13 Electrochemical Cells If a copper strip is placed in a solution of copper ions, one of the following reactions may occur: Cu 2+ + 2e - Cu Cu Cu 2+ + 2e - The electrical potential that would be developed
#13 Electrochemical Cells If a copper strip is placed in a solution of copper ions, one of the following reactions may occur: Cu 2+ + 2e - Cu Cu Cu 2+ + 2e - The electrical potential that would be developed
Which of the following describes the net ionic reaction for the hydrolysis. Which of the following salts will produce a solution with the highest ph?
 95. Which of the following descries the net ionic rection for the hydrolysis of NH4Cl( s)? A. NH4 ( q) Cl & ( q) NH4Cl( s) B. NH Cl & 4 ( s) NH4 ( q) Cl ( q) C. Cl ( q) H O & 2 ( l) HCl( q) OH ( q) D.
95. Which of the following descries the net ionic rection for the hydrolysis of NH4Cl( s)? A. NH4 ( q) Cl & ( q) NH4Cl( s) B. NH Cl & 4 ( s) NH4 ( q) Cl ( q) C. Cl ( q) H O & 2 ( l) HCl( q) OH ( q) D.
Chapter 19 - Electrochemistry. the branch of chemistry that examines the transformations between chemical and electrical energy
 Chapter 19 - Electrochemistry the branch of chemistry that examines the transformations between chemical and electrical energy 19.1 Redox Chemistry Revisited A Spontaneous Redox Reaction Znº(s) + Cu 2+
Chapter 19 - Electrochemistry the branch of chemistry that examines the transformations between chemical and electrical energy 19.1 Redox Chemistry Revisited A Spontaneous Redox Reaction Znº(s) + Cu 2+
Lecture 27 Chapter 19, Sections 3-4 Galvanic Cells Electrochemical Potential
 Lecture 27 Chapter 19, Sections 3-4 Galvanic Cells Electrochemical Potential Galvanic Cells Defined Standard Hydrogen Electrode Standard Reduction Potentials Redox Balancing One More Example This time
Lecture 27 Chapter 19, Sections 3-4 Galvanic Cells Electrochemical Potential Galvanic Cells Defined Standard Hydrogen Electrode Standard Reduction Potentials Redox Balancing One More Example This time
ph = pk a + log 10{[base]/[acid]}
![ph = pk a + log 10{[base]/[acid]} ph = pk a + log 10{[base]/[acid]}](/thumbs/90/103664826.jpg) FORMULA SHEET (tear off) N A = 6.022 x 10 23 C = ( 5 / 9) ( F - 32) F = ( 9 / 5)( C) + 32 1 amu = 1.661 x 10-27 kg C = K - 273.15 K = C + 273.15 1 atm = 760 torr = 760 mm Hg 1 atm = 1.013 bar pv = nrt
FORMULA SHEET (tear off) N A = 6.022 x 10 23 C = ( 5 / 9) ( F - 32) F = ( 9 / 5)( C) + 32 1 amu = 1.661 x 10-27 kg C = K - 273.15 K = C + 273.15 1 atm = 760 torr = 760 mm Hg 1 atm = 1.013 bar pv = nrt
Guide to Chapter 18. Electrochemistry
 Guide to Chapter 18. Electrochemistry We will spend three lecture days on this chapter. During the first class meeting we will review oxidation and reduction. We will introduce balancing redox equations
Guide to Chapter 18. Electrochemistry We will spend three lecture days on this chapter. During the first class meeting we will review oxidation and reduction. We will introduce balancing redox equations
DIRECT CURRENT CIRCUITS
 DRECT CURRENT CUTS ELECTRC POWER Consider the circuit shown in the Figure where bttery is connected to resistor R. A positive chrge dq will gin potentil energy s it moves from point to point b through
DRECT CURRENT CUTS ELECTRC POWER Consider the circuit shown in the Figure where bttery is connected to resistor R. A positive chrge dq will gin potentil energy s it moves from point to point b through
Acids and Bases. H + (aq) + Cl - (aq) 100 molecules HCl 100 H+ ions Cl- ions 100% HCl molecules dissociate in water.
 Acids nd Bses Acids: in generl cid is the substnce tht produces H ions when it is dissolved in wter. Acids cn be divided into two different clsses: Strong cid: is one tht completely dissocites into its
Acids nd Bses Acids: in generl cid is the substnce tht produces H ions when it is dissolved in wter. Acids cn be divided into two different clsses: Strong cid: is one tht completely dissocites into its
Oxidation (oxidized): the loss of one or more electrons. Reduction (reduced): the gain of one or more electrons
 1 of 13 interesting links: Battery Chemistry Tutorial at http://www.powerstream.com/batteryfaq.html Duracell Procell: Battery Chemistry at http://www.duracell.com/procell/chemistries /default.asp I. Oxidation
1 of 13 interesting links: Battery Chemistry Tutorial at http://www.powerstream.com/batteryfaq.html Duracell Procell: Battery Chemistry at http://www.duracell.com/procell/chemistries /default.asp I. Oxidation
Review of Calculus, cont d
 Jim Lmbers MAT 460 Fll Semester 2009-10 Lecture 3 Notes These notes correspond to Section 1.1 in the text. Review of Clculus, cont d Riemnn Sums nd the Definite Integrl There re mny cses in which some
Jim Lmbers MAT 460 Fll Semester 2009-10 Lecture 3 Notes These notes correspond to Section 1.1 in the text. Review of Clculus, cont d Riemnn Sums nd the Definite Integrl There re mny cses in which some
Redox Reactions and Electrochemistry
 Redox Reactions and Electrochemistry Problem Set Chapter 5: 2126, Chapter 21: 1517, 32, 34, 43, 53, 72, 74 R Oxidation/Reduction & Electrochemistry Oxidation a reaction in which a substance gains oxygen
Redox Reactions and Electrochemistry Problem Set Chapter 5: 2126, Chapter 21: 1517, 32, 34, 43, 53, 72, 74 R Oxidation/Reduction & Electrochemistry Oxidation a reaction in which a substance gains oxygen
CBE 291b - Computation And Optimization For Engineers
 The University of Western Ontrio Fculty of Engineering Science Deprtment of Chemicl nd Biochemicl Engineering CBE 9b - Computtion And Optimiztion For Engineers Mtlb Project Introduction Prof. A. Jutn Jn
The University of Western Ontrio Fculty of Engineering Science Deprtment of Chemicl nd Biochemicl Engineering CBE 9b - Computtion And Optimiztion For Engineers Mtlb Project Introduction Prof. A. Jutn Jn
2. Using Half Cell Potentials and Latimer Diagrams. 100 measured half cell potentials generate 10,000 full reactions
 Electrochemistry 1. Balancing Redox Reactions 2. Using Half Cell Potentials and Latimer Diagrams 100 measured half cell potentials generate 10,000 full reactions 3. E as a Thermodynamic state function
Electrochemistry 1. Balancing Redox Reactions 2. Using Half Cell Potentials and Latimer Diagrams 100 measured half cell potentials generate 10,000 full reactions 3. E as a Thermodynamic state function
AP* Electrochemistry Free Response Questions page 1
 Galvanic (Voltaic) Cells 1988 Average score = 5.02 a) two points Sn ---> Sn 2+ + 2e Ag + + e ---> Ag AP* Electrochemistry Free Response Questions page 1 b) two points 2 Ag + + Sn ---> 2 Ag + Sn 2+ E =
Galvanic (Voltaic) Cells 1988 Average score = 5.02 a) two points Sn ---> Sn 2+ + 2e Ag + + e ---> Ag AP* Electrochemistry Free Response Questions page 1 b) two points 2 Ag + + Sn ---> 2 Ag + Sn 2+ E =
Electrochemistry. 1. For example, the reduction of cerium(iv) by iron(ii): Ce 4+ + Fe 2+ Ce 3+ + Fe 3+ a. The reduction half-reaction is given by...
 Review: Electrochemistry Reduction: the gaining of electrons Oxidation: the loss of electrons Reducing agent (reductant): species that donates electrons to reduce another reagent. Oxidizing agent (oxidant):
Review: Electrochemistry Reduction: the gaining of electrons Oxidation: the loss of electrons Reducing agent (reductant): species that donates electrons to reduce another reagent. Oxidizing agent (oxidant):
Chpt 20: Electrochemistry
 Cell Potential and Free Energy When both reactants and products are in their standard states, and under constant pressure and temperature conditions where DG o = nfe o DG o is the standard free energy
Cell Potential and Free Energy When both reactants and products are in their standard states, and under constant pressure and temperature conditions where DG o = nfe o DG o is the standard free energy
Name Solutions to Test 3 November 8, 2017
 Nme Solutions to Test 3 November 8, 07 This test consists of three prts. Plese note tht in prts II nd III, you cn skip one question of those offered. Some possibly useful formuls cn be found below. Brrier
Nme Solutions to Test 3 November 8, 07 This test consists of three prts. Plese note tht in prts II nd III, you cn skip one question of those offered. Some possibly useful formuls cn be found below. Brrier
Vibrational Relaxation of HF (v=3) + CO
 Journl of the Koren Chemicl Society 26, Vol. 6, No. 6 Printed in the Republic of Kore http://dx.doi.org/.52/jkcs.26.6.6.462 Notes Vibrtionl Relxtion of HF (v3) + CO Chng Soon Lee Deprtment of Chemistry,
Journl of the Koren Chemicl Society 26, Vol. 6, No. 6 Printed in the Republic of Kore http://dx.doi.org/.52/jkcs.26.6.6.462 Notes Vibrtionl Relxtion of HF (v3) + CO Chng Soon Lee Deprtment of Chemistry,
Fernando O. Raineri. Office Hours: MWF 9:30-10:30 AM Room 519 Tue. 3:00-5:00 CLC (lobby).
 Fernando O. Raineri Office Hours: MWF 9:30-10:30 AM Room 519 Tue. 3:00-5:00 CLC (lobby). P1) What is the reduction potential of the hydrogen electrode g bar H O aq Pt(s) H,1 2 3 when the aqueous solution
Fernando O. Raineri Office Hours: MWF 9:30-10:30 AM Room 519 Tue. 3:00-5:00 CLC (lobby). P1) What is the reduction potential of the hydrogen electrode g bar H O aq Pt(s) H,1 2 3 when the aqueous solution
Continuous Random Variables
 STAT/MATH 395 A - PROBABILITY II UW Winter Qurter 217 Néhémy Lim Continuous Rndom Vribles Nottion. The indictor function of set S is rel-vlued function defined by : { 1 if x S 1 S (x) if x S Suppose tht
STAT/MATH 395 A - PROBABILITY II UW Winter Qurter 217 Néhémy Lim Continuous Rndom Vribles Nottion. The indictor function of set S is rel-vlued function defined by : { 1 if x S 1 S (x) if x S Suppose tht
CHAPTER 6 - Chemical Equilibrium. b. composition when equilibrium is reached.
 CHAPTER 6 - Chemical Equilibrium I. Thermodynamics of Chemical Reactions. A. Spontaneity of Chemical Reactions: 1. Thermodynamics can tell us: a. direction of spontaneous change. b. composition when equilibrium
CHAPTER 6 - Chemical Equilibrium I. Thermodynamics of Chemical Reactions. A. Spontaneity of Chemical Reactions: 1. Thermodynamics can tell us: a. direction of spontaneous change. b. composition when equilibrium
