Desorption energies of linear and cyclic alkanes on surfaces: anomalous scaling with length
|
|
|
- Erika Summers
- 6 years ago
- Views:
Transcription
1 Surface Science 554 (2004) Desorption energies of linear and cyclic alkanes on surfaces: anomalous scaling with length Ryan Z. Lei a, Andrew J. Gellman a, *, B.E. Koel b a Department of Chemical Engineering, Carnegie Mellon University, 5000 Forbes Ave., Pittsburgh, PA 15213, USA b Department of Chemistry, University of Southern California, Los Angeles, CA 90089, USA Received 28 August 2003; accepted for publication 11 December 2003 Abstract The desorption energies,, of linear and cyclic alkanes on the Cu(1 1 1) and Pt(1 1 1) surfaces have been measured and compared to the values of the measured on a number of other surfaces. For sets of short linear and cyclic alkanes (n-c N H 2Nþ2 and c-c N H 2N, N ¼ 3 12) desorbing from single crystalline surfaces the scale linearly in chain length with the form ðnþ ¼DE þ DECH N. The quantity DE CH can be equated with a segmental contribution to the, however, the physical origin of the non-zero intercept, DE, is not clear. Comparison of the intercepts, for the linear and cyclic alkanes show that they are similar to one another on the Cu(1 1 1) surface and on the Pt(1 1 1) surface. This comparison rules out the possibility that DE arises from endgroup effects. Scaling of the against the polarizabilities, a, of the alkanes also reveals that DE cannot arise from the scaling of the polarizability with chain length. We discuss other possible origins of DE including lattice commensurability effects, temperature, and desorption order. Ó 2004 Elsevier B.V. All rights reserved. Keywords: Alkanes; Surface energy; Molecular dynamics; Thermal desorption 1. Introduction A number of studies have measured the desorption kinetics of linear alkanes from a variety of single crystal metal surfaces and examined the scaling of the desorption energies,, with chain length [1 8]. For short linear alkanes (C N H 2Nþ2, N ¼ 3 12) one finds that the can be approximated well by a linear expression of the * Corresponding author. Tel.: ; fax: address: ag4b@andrew.cmu.edu (A.J. Gellman). form ðnþ ¼DE þ DECH N, where DE CH is the incremental increase in per methylene segment. At the simplest level one expects that should be simply proportional to the chain length. Unexpectedly, however, the linear scaling of the with chain length, N, reveals a nonzero intercept, DE, which can be several times greater than the segmental increment, DE CH. The magnitude of DE is quite surprising and its origin is not obvious. In fact, a recent molecular dynamics study of the desorption of alkanes from the Au(1 1 1) surface showed that a united atom model of the alkane surface interaction predicts values of that are proportional to chain /$ - see front matter Ó 2004 Elsevier B.V. All rights reserved. doi: /j.susc
2 126 R.Z. Lei et al. / Surface Science 554 (2004) length with the value of DE being much less than DE CH [9]. Although the existence of non-zero intercepts, DE, in experimental data has been noted in the past, its origin is an interesting problem that has not yet been resolved [3,10]. This paper attempts to address the physical origin of DE. A non-zero intercept, DE, in plots of the alkane desorption energy,, versus chain length has been observed by a number of researchers. Alkanes with chain lengths of N ¼ 3 12 have been adsorbed and desorbed from single crystal surfaces of several metals and graphite. The results of these measurements will be discussed in more detail later in this paper. With one exception, all of the measurements were made by using temperature programmed desorption (TPD) to measure. The exception is a set of isothermal measurements of the desorption kinetics of hexane through dodecane on the Au(1 1 1) surface made using isothermal helium atom reflectivity [1]. These were accompanied by TPD measurements of these and lower molecular weight alkanes from the Au(1 1 1) surface. For all of the surfaces studied the segmental increment in with chain length lies in the range DE CH ¼ 5 7:5 kj/mol. What is most interesting from the perspective of this paper is that the intercept, DE, of can assume values that are two to six times as high as the segmental increment in. If a sufficiently large range of chain lengths is considered, there is no reason to expect that should be linear in the chain length. At temperatures above 0 K, entropy effects in very long chains will cause some fraction of the C C bonds in the adsorbed alkanes to adopt gauche configurations and some fraction of the segments to be detached from the surface. Indeed measurements of the desorption kinetics of n-alkanes with a very wide range of chain lengths (C N H 2Nþ2,56N 6 60) from the surface of a single crystal of graphite revealed these effects [7,11,12]. The intent of that work was to test the limits to which can be described as linear in the chain length and ultimately to determine the origins of this non-linearity. It was possible to describe as linear in chain length for a narrow range of chain lengths, e.g. N ¼ Over the entire range of chain lengths, however, has a non-linear dependence on chain length. A mechanism for the desorption process and a model for the energy and entropy of n-alkanes on the graphite surface were proposed that quantitatively reproduced the non-linearity in. That model predicts a value of DE CH ¼ 6:2 kj/mol for the increment per segment. From the perspective of the work described in this paper, however, it is important to note that even in that model for the desorption of long chain alkanes, the intercept of is non-zero and has a value of DE ¼ 41 kj/ mol. In other words, even values of measured over a very wide range of chain lengths reveal a significant value of DE. Several physical phenomena might give rise to a non-zero value of DE. The most obvious would be end effects arising from the fact that one does not expect the methyl endgroups of alkanes to give the same contribution to as the internal methylene segments. That possibility is explicitly tested in this work by comparing the values of for linear and cyclic alkanes with the same chain lengths. Endgroup effects arise if methyl groups have different binding affinities to surfaces than methylene groups. The goal of our experimental investigation is to determine the differences in binding affinities of methyl and methylene groups by measuring of n-alkanes and their cyclic counterparts on Cu(1 1 1) and Pt(1 1 1) surfaces. Cyclic alkanes have no methyl endgroups so the difference between of linear and cyclic alkanes of the same length might be attributed to the difference in the binding affinities of the methylene and methyl groups. As will be shown, the difference between the binding strengths of the methylene and methyl groups is negligible and cannot account for the fact that DE is several times the value of DE CH. In addition to endgroup effects, there are other possible origins of the non-zero value of DE. Bishop et al. have suggested that DE arises as a result of an artifact in the way in which the TPD spectra are analyzed to determine [3]. They have suggested that the presence of islands or other organized structures on the surface can lead to variations in the effective reaction order for desorption. As a result, simply using a first-order equation for all chain lengths will lead to mis-
3 R.Z. Lei et al. / Surface Science 554 (2004) leading results when considering chains of different lengths. It is also important to recognize that both the alkanes and the crystalline substrate have periodic structures and that commensurability effects might give rise to non-linear dependence of on N, even for a narrow range of short chain lengths. Finally, the binding of the alkanes to these surfaces occurs through weak van der Waals interactions and thus examination of the relationship between and the polarizabilities, a, of the alkanes might shed light on the origins of DE. All of these possibilities will be considered in our discussion of this problem. 2. Experimental All experiments on the Cu(1 1 1) surface were performed in an ultra-high vacuum (UHV) chamber with a base pressure of <10 10 Torr achieved through use of a cryo-pump and titanium sublimation pump. The chamber is equipped with two leak valves fitted with stainless steel dosing tubes. The chamber is also equipped with an Ametek Dycor quadrupole mass spectrometer (QMS), which has a mass range of amu and is capable of monitoring up to eight masses simultaneously during TPD experiments. A copper single crystal cut to within 0.5 of the (1 1 1) plane was used for experiments presented in this paper. The crystal was mounted by spotwelding it between two Ta wires at the end of a manipulator. Once mounted on the manipulator, the Cu(1 1 1) sample could be cooled to 90 K through contact with a liquid nitrogen reservoir and heated resistively at a constant rate. The crystal temperature was measured using a chromel alumel thermocouple spotwelded to the rear face of the crystal. The front surface of the crystal was cleaned by Ar þ ion sputtering with cycles of heating from 90 to 1000 K and then holding it at 1000 K for 100 s. The condition of the surface following this initial treatment was highly reproducible as determined by alkane adsorption and desorption. Cycles of Ar þ sputtering and annealing to 1000 K were conducted after adsorption and desorption of each pair of linear and cyclic alkanes of a given chain length to ensure a clean surface. Within the UHV chamber the Cu(1 1 1) sample was positioned adjacent to the stainless steel dosing tube and approximately 2 cm from an aperture to the QMS. The TPD experiments consisted of three steps: adsorption, desorption, and detection. The adsorption step entailed cooling the sample to 90 K and exposing its surface to alkane vapor at pressures ranging from 10 9 to 10 8 Torr for periods of s. Desorption of the adsorbed molecules was induced by heating the sample at a constant rate from 100 to 700 K. Desorbing species including any decomposition products were monitored during heating by the QMS. In all cases, adsorption and desorption of the linear and cyclic alkanes was molecular and reversible with no indication of decomposition. The measurements on the Pt(1 1 1) surface were performed in a similar manner in a second UHV chamber equipped with a cylindrical mirror analyzer for Auger electron spectroscopy (AES), low energy electron diffraction (LEED) optics, and a UTI 100C QMS for TPD measurements. The sample could be cooled to 95 K and heated resistively to 1200 K. It was cleaned using a previously published procedure [13]. TPD experiments were performed by adsorbing the alkanes at low temperature and then heating at 4 K/s. The set of short chain n-alkanes [n-c 3 H 8, 98+%; n-c 5 H 12, 99+%; n-c 6 H 14, 99+%; n-c 7 H 16, 99+%; n-c 8 H 18, 99+%; n-c 10 H 22, 99+%] and cyclic alkanes [c-c 3 H 6, 99+%; c-c 5 H 10, 99+%; c-c 6 H 12, 99+%; c-c 7 H 14, 98+%; c-c 8 H 16, 99+%; c-c 10 H 20, 98+%] used in this investigation was purchased from Strem Chemicals. All compounds were purified before use by using a series of freeze pump thaw cycles intended to remove high vapor pressure contaminants. 3. Results 3.1. TPD spectra of linear and cyclic alkanes TPD spectra were obtained for a set of short chain linear and cyclic alkanes (C 3,C 5 C 8, and C 10 ) in order to measure their from the Cu(1 1 1) surface. Fig. 1 shows TPD spectra of n-c 6 H 14 desorbing from the Cu(1 1 1) surface following
4 128 R.Z. Lei et al. / Surface Science 554 (2004) Desorption Rate (a.u.) 138 K Multilayer Desorption 192 K Monolayer Desorption Temperature (K) Increasing Surface Coverage Fig. 1. TPD spectra of C 6 H 14 desorbing from the Cu(1 1 1) surface obtained as a function of increasing coverage following adsorption at 90 K. Spectra were generated by monitoring the signal at m=q ¼ 41 (C 3 H þ 5 ) during heating. The heating rate was b ¼ 2 K/s. adsorption of various different coverages at 90 K. The spectra were generated by monitoring the QMS signal at a mass-to-charge ratio of m=q ¼ 41 (C 3 H þ 5 ) during heating at 2 K/s. Two additional values of m=q ¼ 57 (C 4 H þ 9 ) and m=q ¼ 43 (C 3H þ 7 ) were also monitored in order to detect desorption of decomposition products. No decomposition of n-c 6 H 14 or any of the other linear or cyclic alkanes was observed during desorption from the Cu(1 1 1) surface. At low coverages, the spectrum shows a desorption feature at 192 K, indicative of monolayer desorption. The monolayer peak increased in intensity as the coverage increased and saturated in intensity. At that point a second feature indicative of multilayer desorption appeared at 138 K. Fig. 1 shows distinct, well-resolved monolayer and multilayer desorption peaks characteristic of desorption from a homogeneous surface. The monolayer and multilayer peak desorption temperatures are consistent with those found for n-c 6 H 14 desorbing from the Cu(1 0 0) surface using a 3 K/s heating rate (196 and 135 K) [8], and a 5 K/s heating rate (202 and 145 K) [2]. The TPD spectra obtained using other linear and cyclic alkanes on the Cu(1 1 1) and Pt(1 1 1) surfaces were similar to those of Fig. 1. The monolayer peak desorption temperatures are listed in Table 1. Peak desorption temperatures for n- alkanes on Pt(1 1 1) were obtained from Bishop et al. [3]. Monolayer peak desorption temperatures from the Cu(1 1 1) surface are consistent with those found by Teplyakov et al. [8] for n-c 5 H 12 (172 K), c-c 5 H 10 (166 K), n-c 6 H 14 (196 K), and c-c 6 H 12 (181 K) desorbed from Cu(1 0 0) using a heating rate of 3 K/s. As a result of the interest in vibrational mode softening in alkanes on metal surfaces there have been a number of studies of c-c 6 H 12 adsorption and desorption on the Cu(1 1 1) surface [14 17]. The reported desorption temperatures are 165 K in one study and 178 K at a heating rate of 3 K/s in the other. In summary, the TPD spectra indicate simple reversible molecular adsorption and desorption of the alkanes on all surfaces. The observation that the monolayer peak desorption temperatures were independent of coverage indicates that desorption is a first-order kinetic process Pre-exponential factor and DE desorption from Cu(1 1 1) z des for alkane In order to compare the desorption kinetics of linear and cyclic alkanes we have determined both the desorption energy,, and the pre-exponential factor, v, of the rate constant for desorption from the Cu(1 1 1) surface. Analysis of TPD measurements made with varying heat rates, b, allows independent calculation of and v [18] through the relation! b v ln ¼ ln DEz des : ð1þ Tp 2 RT p RT p A plot of lnðb=tp 2Þ versus 1=T p for measurements made at different heating rates should be linear
5 R.Z. Lei et al. / Surface Science 554 (2004) Table 1 Values of T p and for linear and cyclic alkanes on Cu(1 1 1) and Pt(1 1 1) Hydrocarbon Cu(1 1 1) Pt(1 1 1) T p (K) (kj/mol)a T p (K) (kj/mol)b Propane, n-c 3 H Cyclopropane, c-c 3 H Pentane, n-c 5 H Cyclopentane, c-c 5 H Hexane, n-c 6 H c 61.3 Cyclohexane, c-c 6 H Heptane, n-c 7 H c 66.2 Cycloheptane, c-c 7 H Octane, n-c 8 H c 73.4 Cyclooctane, c-c 8 H Nonane, n-c 9 H c 74.2 Cyclononane, c-c 9 H 18 Decane, n-c 10 H c 76.6 Cyclodecane, c-c 10 H were calculated using Redhead analysis [18] of TPD data with b ¼ 2 K/s. a Pre-exponential factors of v ¼ 10 17:5 and 10 18:5 s 1 were used for linear and cyclic alkanes, respectively. b A pre-exponential factor of v ¼ 10 13:4 s 1 was used for cyclic alkanes. Pre-exponential factors used for linear alkanes are listed in Table 2. c From Bishop et al. [3]. with a value of the slope of =R. Given the value of one can then determine the value of the desorption pre-exponential factor, v. TPD experiments were conducted using variable heating rates in order to determine values of both and v for n-c 7H 16 desorption from the Cu(1 1 1) surface. Fig. 2 shows TPD spectra for n- C 7 H 16 at monolayer coverage desorbing from Cu(1 1 1) during heating at rates varying from 0.1 to 2 K/s. To ensure a reproducible initial coverage of one monolayer, adsorption was performed with the Cu(1 1 1) sample held at a temperature just above the n-c 7 H 16 multilayer desorption temperature. The spectra were generated by using the QMS to monitor the signal at m=q ¼ 43 (C 3 H þ 7 ) during heating. The basic observation was that the monolayer peak desorption temperature increased as the heating rate was increased. The inset shows a plot of lnðb=tp 2Þ versus 1=T p. Values of ¼ 73:4 4:5 kj/mol and v ¼ 10 17:51:2 s 1 were determined for n-c 7 H 16 desorption from Cu(1 1 1). Values of and v for c-c 7 H 14 desorption from the Cu(1 1 1) surface were also determined independently using the variable heating rate method. Fig. 3 shows TPD spectra of c-c 7 H 14 at one monolayer coverage desorbing from the Cu(1 1 1) surface measured at heating rates ranging from 0.1 to 2 K/s. As before, the Cu(1 1 1) sample was held at a temperature just above the c-c 7 H 14 multilayer desorption temperature to ensure adsorption of a reproducible coverage of one monolayer. Spectra were generated by monitoring the signal at m=q ¼ 41 (C 3 H þ 5 ) during heating. As was the case for n-c 7 H 16 desorption, the monolayer desorption temperature for c-c 7 H 14 increased as the heating rate was increased. The inset shows a plot of lnðb=tp 2Þ versus 1=T p that was used to determine values of ¼ 70:7 1:4 kj/mol and v ¼ 10 18:50:4 s 1 for c-c 7 H 14 desorption from Cu(1 1 1). Values of v for desorption of both linear and cyclic heptane from the Cu(1 1 1) surface are of order v ¼ s 1. These are quite a high values but are consistent with values found for n-alkane desorption from the surface of graphite (v ¼ 10 19:6 s 1 ) in previous work [7,11,12]. We found that the
6 130 R.Z. Lei et al. / Surface Science 554 (2004) E = 73.4 ± 4.5 kj/mol des log v = 17.5 ± E = 70.7 ± 1.4 kj/mol des log v = 18.5 ± ln(β/t ) p ln( β/t p ) Desorption Rate (a.u.) /T p (K /1000) T p Heating Rate, 2K/s 214 K 1K/s 211 K 0.5 K/s 209 K 0.2 K/s 203 K 0.1 K/s 200 K Desorption Rate (a.u.) /T p (K /1000) T p Heating Rate, β 2 K/s 196 K 1 K/s 193 K 0.5 K/s 190 K 0.2 K/s 186 K 0.1 K/s 184 K Temperature (K) Fig. 2. TPD spectra of n-c 7 H 16 desorbing from the Cu(1 1 1) surface measured for initial coverages of one monolayer at heating rates varying from b ¼ 0:1 to 2 K/s. The inset is a plot of lnðb=t 2 p Þ versus 1=T p. The slope of the linear fit has been used to estimate and v for n-c 7H 16 desorption. Spectra were generated by monitoring the signal at m=q ¼ 43 (C 3 H þ 7 ) during heating Temperature (K) Fig. 3. TPD spectra of c-c 7 H 14 desorbing from the Cu(1 1 1) surface measured for initial coverages of one monolayer at heating rates varying from b ¼ 0:1 to 2 K/s. The inset is a plot of lnðb=t 2 p Þ versus 1=T p. The slope of the linear fit has been used to estimate and v for c-c 7H 14 desorption. Spectra were generated by monitoring the signal at m=q ¼ 41 (C 3 H þ 5 ) during heating. pre-exponential factor for desorption was independent of chain length in our previous studies of the desorption of alkanes from the graphite surface and so we have chosen to use a value of v ¼ 10 17:5 s 1 for all linear alkanes and v ¼ 10 18:5 s 1 for all cyclic alkanes to evaluate from the Cu(1 1 1) surface DEdes of linear and cyclic alkanes from Cu(1 1 1) and Pt(1 1 1) z Values of for alkanes on Cu(1 1 1) and Pt(1 1 1) surfaces can be calculated using the Redhead equation and the peak desorption temperatures [18]. Peak temperatures for desorption of linear and cyclic alkanes from the two surfaces are listed in Table 1. Peak temperatures for desorption of linear alkanes from Pt(1 1 1) surfaces were taken from the work of Bishop et al. [3] while the remainder come from this work. Values of from Cu(1 1 1) and Pt(1 1 1) surfaces were calculated using the Redhead equation. The choice of the pre-exponential factors that were used for analysis of the desorption kinetics from the Pt(1 1 1) surface will be discussed in the next section. Table 1 lists the values of for all the linear and cyclic alkanes studied on the Cu(1 1 1) and Pt(1 1 1) surfaces in this work and for the linear alkanes studied on Pt(1 1 1) by Bishop et al. [3]. Additional TPD measurements were made for the desorption of the cyclic alkanes from the (2 2) and ( p 3 p 3)R30 Sn/Pt(1 1 1) alloys on Pt(1 1 1) surfaces. The values of were roughly 15% lower on the alloy surfaces than on the clean Pt(1 1 1) surface.
7 R.Z. Lei et al. / Surface Science 554 (2004) Discussion Desorption kinetics for linear alkanes on Cu(1 1 1) and Pt(1 1 1) surfaces have been measured in this work and on the Au(1 1 1), Cu(1 0 0), Ru(0 0 1) surfaces and the basal plane of graphite in other work [1 8]. These data were used to estimate values of as a function of chain length. Table 2 lists these values and the values of log 10 ðvþ that were used in the calculation of.itis necessary to say some words regarding the choices of the values of v used for each surface because they have not been measured consistently among the studies published. In the case of the Au(1 1 1) surface, desorption rates for hexane through dodecane were measured isothermally and thus a value of v is not needed to estimate the values of [1]. Nonetheless the authors indicate that analysis of their isothermal data yielded values of v ¼ s 1 that were independent of the alkane chain length. Furthermore, their TPD measurements yielded values of consistent with those measured isothermally. Bishop et al. [3] measured a value of v s 1 for the desorption of three alkanes from the Pt(1 1 1) surface independent of the alkane chain length. Also, Weaver et al. [10] measured v ¼ 10 13:8 s 1 for the desorption of butane from the Pt(1 1 1) surface. Given these values, we have chosen to use v ¼ 10 13:4 s 1 to estimate the values of of n-butane and n-heptane on the Pt(1 1 1) surface using the TPD data of Xu et al. [19]. Paserba and Gellman [7] measured v ¼ 10 19:6 s 1 for a set of nine alkanes with chain lengths ranging from N ¼ 7 to 44 adsorbed on graphite. In Table 2 we have listed the measured values of v and used v ¼ 10 19:6 s 1 for the alkanes for which v was not explicitly measured. Brand et al. [4] measured values of v s 1 for four alkanes desorbing from the Ru(0 0 1) surface. This is the one instance in which the experimentally measured values of v decrease systematically with chain length. If one looks at the entire set of experimental data obtained from all surfaces, however, it appears that the value of v is not dependent on chain length. We have measured v ¼ 10 17:5 s 1 for n-c 7 H 16 desorption from the Cu(1 1 1) surface and have used this value to calculate the values of for the other linear alkanes. On the Cu(1 0 0) surface there have been no attempts to measure the value of v for alkane desorption and so we have used a value of v ¼ s 1 in which the exponent is roughly the average of the values of log 10 ðvþ obtained from all other studies. Although it is somewhat unsatisfying that the values of v have not been measured for the desorption of all of the alkanes on all of the surfaces, it should be pointed out that the values of obtained from the Redhead equation are only weakly dependent on the value of v. Increasing v by one order of magnitude only increases the value of the by 5%. From the perspective of this work, the possible errors in the values of arising from errors in the values of v are of no consequence. Even if all of the data were reanalyzed using a single value of s 1, one would still find significant value of, DE, the intercept of the versus chain length plot and the basic point of this paper would not change. Values of of cyclic alkanes from the Cu(1 1 1) and Pt(1 1 1) surfaces are also listed in Table 1. The only measurement of the value of v for desorption of cyclic alkanes was made for c- C 7 H 14 on the Cu(1 1 1) surface and is reported in this work to be v ¼ 10 18:5 s 1. This value was used to estimate for all of the cyclic alkanes desorbing from the Cu(1 1 1) surface. The values of v are quite similar for desorption of the linear and the cyclic alkanes from the Cu(1 1 1) surface so, we assumed that the same is true for desorption from the Pt(1 1 1) surface and used a value of v ¼ s 1 to estimate the values of for the desorption of the cyclic alkanes. In looking at Table 1, which compares on the Cu(1 1 1) and Pt(1 1 1) surfaces there appears to be no significant difference between the values of for the linear and the cyclic alkanes. The aim of this work was to highlight the nonzero intercept of the correlation of with chain length, N. The data plotted in Fig. 4 clearly reveal that of alkanes varies linearly with chain length on all surfaces over short ranges of chain lengths. These values are not, however, proportional to chain length and the value of the intercept, DE, is significant in all cases. The Pt(1 1 1) surface is the only one for which of the linear alkanes is non-linear in chain length for
8 Table 2 Kinetic parameters for linear alkane desorption from surfaces Method Au(1 1 1) a [1] He Scatt., TPD Au(1 1 1) [9] Cu(1 0 0) b [8] Cu(1 0 0) b [2] Cu(1 1 1) c [this paper] Pt(1 1 1) [10] Pt(1 1 1) d [3] Pt(1 1 1) e [this paper] Graphite f [7] MD TPD TPD TPD TPD TPD TPD TPD TPD Ru(0 0 1) [4] Heating Iso T Iso T rate, b n-c 3 H , 17.5 g 46, 16.5 n-c 4 H , 13 g 40, , 16 g 50.6, , 13.4 g 49.8, 15.6 n-c 5 H , 16 g 53.5, 16 g 57.3, 17.5 g 65.0, 19.6 g 57.7, 15.3 n-c 6 H , 13 62, , 16 g 62.0, 16 g 65.7, 17.5 g 61.3, , 19.6 g 62.7, 14.9 n-c 7 H , , 16 g 72.4, , 12.9 g 68.5, 13.4 g 78.2, 18.8 n-c 8 H , 13 82, , 16 g 78.0, 17.5 g 73.4, , 19.6 g n-c 9 H , , 16 g 74.2, 12.9 g n-c 10 H , 13 98, , 16 g 93.8, 17.5 g 76.6, , 19.6 g n-c 11 H 24 n-c 12 H , , , 20.3 Each entry contains values of [, log 10 ðvþ]. a Values of v were determined from intercepts of Arrhenius plots of isothermal desorption rates. b Values of v were estimated from the average of values of log 10 ðvþ obtained on other surfaces. c Value of v was measured for n-c 7 H 16 and this value was used for all other linear alkanes on the Cu(1 1 1) surface. d Values of v for n-c 7 H 16 and n-c 9 H 20 were estimated from the average value of log 10 ðvþ obtained for other linear alkanes in this study. e Value of v was estimated from the average of the values of log 10 ðvþ found in the other two studies on the Pt(1 1 1) surface [3,10]. f The value of log 10 ðvþ ¼19:6 is the average of values determined for alkanes with chain lengths in the range N ¼ 7 44 [7]. g Values of v were not measured and have been estimated as described in the text. 132 R.Z. Lei et al. / Surface Science 554 (2004)
9 R.Z. Lei et al. / Surface Science 554 (2004) Graphite Au(111) MD 100 Cu(111) E (kj/mol) des Cu(100) Ru(001) Pt(111) Au(111) Chain Length (N) Fig. 4. for linear alkane desorption from surfaces as a function of chain length, N. The lines are linear fits for each surface and reveal the significant values of the intercept, DE. N < 12. Nonetheless, even if one analyzes only the shorter linear alkanes on the Pt(1 1 1) surface a linear correlation of the with chain length would yield a significant value for DE. It is interesting that a united atom model used as the basis for a molecular dynamics study of alkane desorption from the Au(1 1 1) surface, predicts values of that are proportional to the chain length and thus yield a value of DE 0 [9]. Thus the origin of the experimentally observed magnitude of DE is worth examining. Table 3 lists values of the parameters used in linear fits of versus N. Values of DE vary in magnitude from 2 to 6 times the value of the segmental increment, DE CH, to. The physical origin of DE is unclear and it is hard to see how it could arise from a description of the adsorbate substrate interaction that is simply composed of pairwise additive contributions between adsorbate segments and the surface. New data that we have presented in this paper eliminate a couple of the possible factors that might contribute to the value of DE Endgroup effects The simplest model for the interaction between an alkane and a surface would be one in which the Table 3 Segmental increment, DE CH, and intercepts for zero chain length, DE, for the of linear and cyclic alkanes adsorbed on a number of surfaces Surface/alkanes DE CH (kj/mol/ch 2 ) DE (kj/mol) Pt(1 1 1)/n-C N H 2Nþ Ru(0 0 1)/n-C N H 2Nþ Au(1 1 1)/n-C N H 2Nþ Cu(1 0 0)/n-C N H 2Nþ Cu(1 1 1)/n-C N H 2Nþ Graphite/n-C N H 2Nþ2 7.5 (6.2) 27.2 (41) Au(1 1 1)/n-C N H 2Nþ2 (MD) Pt(1 1 1)/c-C N H 2N Cu(1 1 1)/c-C N H 2N alkane is thought of as a chain of identical segments that each interact with the surface with the same potential. This type of model has often been used to describe alkanes and hydrocarbons adsorbed on surfaces. This type of model must break down for short chains because methyl endgroups cannot be equivalent to the internal methylene groups. While the effects of endgroups on might be negligible for long chains, their relative contribution to must increase as the chain length decreases. This would manifest itself in the form of an offset to the or a non-zero intercept, DE, of the type observed in this work.
10 134 R.Z. Lei et al. / Surface Science 554 (2004) The values of DE for the linear alkanes vary from 16.6 to 30.0 kj/mol among the different surfaces on which they have been studied while the segmental increments, DE CH, to the vary from 5.0 to 7.5 kj/mol. This suggests that endgroup effects cannot account for the observed values of DE because the magnitudes of the values of DE suggest that they cannot arise simply from the differences between the interactions of methylene and methyl groups with the surface. Nonetheless, this work has attempted to address this possibility specifically by comparing of linear alkanes with those of cyclic alkanes on both Cu(1 1 1) and Pt(1 1 1) surfaces. Fig. 5 shows a plot of versus N for cyclic alkanes on both Cu(1 1 1) and Pt(1 1 1) surfaces. Fits to straight lines are shown and fitting parameters are given in Table 3. On both surfaces, the values of DE are similar for both linear and cyclic alkanes. This E (kj/mol) des Cu(111) Pt(111) Chain Length (N) Fig. 5. for cyclic alkane desorption from Cu(1 1 1) and Pt(1 1 1) surfaces as a function of chain length, N. The lines are linear fits for each surface and reveal the significant values of the intercept, DE. These values are similar to those of the linear alkanes. suggests that DE arises from an intrinsic property of the surface rather than the alkanes. More importantly, DE for cyclic alkanes cannot arise from endgroup effects since they have no endgroups. Nonetheless the values of DE on both surfaces are significant. TPD data obtained from the (2 2) and ( p 3 p 3)R30 Sn/Pt(1 1 1) alloys on Pt(1 1 1) surfaces also show that the correlations of with chain length reveal significant values of DE. Table 1 shows that linear alkanes have values of that are 2 kj/mol higher than those of the cyclic alkanes on both the Cu(1 1 1) and Pt(1 1 1) surfaces. This suggests that the interactions of methyl groups with these surfaces are about 1 kj/mol greater than those of methylene groups. This seems to be of a reasonable magnitude and certainly indicates that the observed magnitude of DE cannot be attributed to endgroup effects Physical properties of alkanes with varying chain length Many properties of linear alkanes are chain length dependent and their correlation with chain length might provide some insight into the origins of DE. One such property is the heat of vaporization, DH vap, which arises principally from the interactions between alkanes in the bulk. A correlation between DH vap and chain length should also reveal endgroup effects, in much the same way as one would expect to observe them in the correlation of with chain length. Fig. 6 shows a plot of DH vap versus N for n-alkanes ranging in length from N ¼ 1 to 12 [20]. Values reported for butane and higher alkanes are all reported for 273 K. DH vap is linear in the chain length with fitting parameters of DH CH ¼ 4:9 kj/mol and DH ¼ 2:4 kj/mol. The value of the segmental increment to DH vap is slightly lower than that of on most surfaces. The magnitude of 1 DH ¼ 1:2 kj/mol is 2 significantly smaller than the segmental increment in DH vap and this value might reasonably be attributed to the excess contribution of a methyl endgroup to DH vap. Furthermore, a value of 1.2 kj/ mol is similar to the endgroup effects on reported in this work. The value of DH is, however, much smaller than the values of DE measured
11 R.Z. Lei et al. / Surface Science 554 (2004) H vap = N 100 n-c /Cu(111) N c-c /Cu(111) N H vap (kj/mol) E (kj/mol) des n-c /Pt(111) N c-c /Pt(111) N for desorption of the alkanes from surfaces and provides us with little insight into the origin of DE Polarizability effects Carbon Atoms (N) Fig. 6. Correlation of the heat of vaporization, DH vap, for the linear alkanes with chain length, N. The straight line is a fit to the values of DH vap for the alkanes with N P 4 and yields an intercept DH that is smaller that the increment to DH vap per methylene group. Polarizability, a, is another property of alkanes that scales with chain length. Furthermore, the magnitude of the alkane surface interaction should be correlated with the polarizability of the alkanes because it occurs through weak van der Waals forces. As such the chain length dependence of the polarizability might account for the chain length dependence of and thus the magnitude of DE. The correlation between values of and a is illustrated in Fig. 7. Once again there is a significant value of the intercept, DE, for both the linear and cyclic alkanes on both surfaces. Polarizability can be described well as a groupadditive property and polarizabilities of alkanes are almost proportional to chain length [21,22]. As a consequence, the chain length dependence of polarizabilities cannot account for the non-zero value of DE Temperature effects -24 Polarizability (10 cm 3) Fig. 7. Correlation of the for linear and cyclic alkane desorption from the Cu(1 1 1) and Pt(1 1 1) surfaces with alkane polarizability. Both correlations yield significant values of the intercept, DE. The nature of the thermal desorption experiment is such that the adsorbate desorbs from the surface in a narrow temperature range around a peak desorption temperature that is dictated in part by the. As a consequence, the desorption kinetics of a set of linear or cyclic alkanes are all measured at different temperatures. Even in an isothermal measurement, the temperature used to make the measurement will depend on the length of the alkane since desorption must occur at a significant rate to be measurable. The fact that the desorption measurements have been made at different temperatures for the different alkanes might, in principle, introduce an artifact into the measured values of that could be manifested in
12 136 R.Z. Lei et al. / Surface Science 554 (2004) the form a non-zero intercept, DE. This issue has been addressed in a prior study of linear alkane desorption from the surface of graphite [7,11,12]. That study included linear alkanes ranging in length from N ¼ 5 to 60 which desorbed from the surface at temperatures ranging from T p ¼ 180 to 730 K. Those measurements revealed that is non-linear in the chain length. A theory was developed that provides an expression that accurately reproduced the measured values of : ¼ðN 1Þ 2 3 q tg DE CC þðq tg 1ÞDEtg CC 4 5 q tg 1 þ q tg exp DECC k B T þ DE þ k B T : ð2þ In this expression q tg is the trans gauche partition function for each C C bond, as given by! DE q tg tg CC ¼ 1 þ 2 exp ; ð3þ k B T where DEtg CC is the energy difference between the trans and gauche conformations about each C C bond. The only two unknown parameters in the expression for are DE CC, the bond surface interaction energy, and DE. These were found by fitting to the experimentally determined values of for linear alkanes on graphite. The values of these fitting parameters found for desorption of linear alkanes from graphite are listed in parentheses in row 4 of Table 3. While Eq. (2) accurately reproduces the non-linearity of obtained from measurements made over a wide range of temperatures ( K) and using a wide range of chain lengths (N ¼ 5 60) it cannot do so without DE taking on a significant value of 41 kj/mol. In other words, the correct inclusion of temperature effects into the expression for the for linear alkanes desorbing from graphite does not eliminate the need for a significant value of DE Order of the desorption reaction Our analysis of the kinetics of desorption of alkanes from Cu(1 1 1) and Pt(1 1 1) surfaces, and all other analyses in previously reported studies have assumed that the desorption is first-order in coverage, i.e. it is directly proportional to the alkane coverage on the surface. In the case of desorption of alkanes from the Cu(1 1 1) surface, this is supported by the observation that the peak desorption temperature is independent of coverage. On other surfaces, however, the shape of the desorption peaks is not entirely consistent with that of a simple first-order process. For example, the desorption of the short n-alkanes from Pt(1 1 1) and graphite surfaces exhibits peaks with shapes that are similar to those that one would expect for a zero-order desorption process [3,7]. Desorption mechanisms that manifest zero-order desorption kinetics have been proposed to involve desorption from a 2D gas in equilibrium with a 2D condensed phase. It is possible that this occurs during alkane desorption. Alternately, kinetics that are apparently zero-order can arise from a firstorder desorption process coupled with attractive interactions between adsorbed species. In any case, Bishop et al. [3] suggested that the origin of the nonzero value of DE may arise from incorrect analysis of the desorption kinetics using a first-order rate equation when the kinetic order of the desorption process may be changing with chain length. Isothermal measurements of the desorption of alkanes from the Au(1 1 1) surface, however, yielded results that were consistent with a first-order desorption process and suggest that this is the case for all alkanes ranging in length from N ¼ 1to12[1]. Nonetheless, it is worthwhile considering explicitly the influence of reaction order on the values of determined by analysis of TPD measurements. In order to probe the sensitivity of the data analysis to the kinetic order of the desorption process, we have simulated the TPD spectra using a desorption rate expression of the form r ¼ dh dt ¼ k hn ; ð4þ where h is the coverage, k is an Arrhenius-like rate constant, and n is the order of the desorption process. Simulated desorption spectra are shown in Fig. 8 for a rate constant with a pre-exponential factor of v ¼ s 1 and a desorption barrier of ¼ 20 kj/mol. The peak desorption tempera-
13 R.Z. Lei et al. / Surface Science 554 (2004) Desorption Rate (a.u.) n Temperature (K) Fig. 8. Simulations of TPD spectra for reaction orders varying from n ¼ 0 to 2. The Arrhenius rate constant used a preexponential factor of v ¼ s 1 and a desorption energy of ¼ 20 kj/mol. Note that the peak desorption temperature, T p, is insensitive to the order of the desorption process. ture, T p, was determined from these simulations for values of n ranging from 0 to 2. The result is that T p 335 K is virtually independent of the reaction order, varying by less than 1% throughout the range of kinetic orders from n ¼ 0 to 2. Since the analysis of TPD spectra to determine from the first-order desorption equation relies only on T p, any error in the assumed reaction order introduces a negligible error into the estimate for. Thus, possible chain length-dependent changes in the order of the alkane desorption process cannot account for the magnitude of DE. A further point should be made with regard to the possible role of reaction order in the value of DE. DE is significant even for the desorption of alkanes with lengths as high as N ¼ 60 adsorbed on graphite. The desorption kinetics of alkanes over this wide range of chain lengths still manifest a non-zero value of DE even if the data are analyzed only for long chains in the length range N ¼ Although TPD spectra for short chain alkanes appear to have a shape indicative of a zero-order desorption process, the TPD spectra for the longer chain alkanes can all be fit quite well using a first-order desorption rate equation. Moreover, it is not likely that the order of the desorption process would change over a range of N ¼ In summary, it seems unlikely that DE can arise from an unknown error in the order of the desorption process used to analyze alkane desorption kinetics from surfaces Commensurability As a final consideration in our discussion of the origin of DE, we address the influence of commensurability of the substrate lattice and the natural periodicity of alkane chains. If one imagines two rigid periodic arrays in contact, then it is quite clear that the interaction will depend on the mismatch of the lattice spacings of the two arrays. Fig. 9A illustrates the case in which an alkane and substrate have the same periodicity and all the carbon atoms sit in potential minima at the surface. If there is lattice mismatch, then the minimum energy configuration (Fig. 9B) is a complicated function of the chain periodicity and shape of the potential. It is only in the extreme case of Fig. 9C, in which the interaction potential is flat with extremely narrow, periodic minima, that the desorption energy could have the form ðnþ ¼DE þ DECH N: ð5þ This form of the potential for the interaction of the alkanes with any of the surfaces seems physically unrealistic. MD simulations of the adsorption of the alkanes on Au(1 1 1) surfaces and Pt(1 1 1) surfaces have used a united atom model for the alkanes and a realistic potential for their interaction with the surfaces. These simulations show no evidence of commensurability issues and predict that the minimum binding energy of the alkanes in the all-trans configuration is proportional to the chain length [9,23]. If commensurability issues were important, they would be expected to appear in such simulations of Au(1 1 1) and Pt(1 1 1) surfaces but disappear on a surface such as that of
14 138 R.Z. Lei et al. / Surface Science 554 (2004) A B cyclic alkanes have similar values of DE on the Cu(1 1 1) surface. The same is true of the values of DE on the Pt(1 1 1) surface. Furthermore, values of DE CH for the linear and cyclic alkanes are similar on Cu(1 1 1) and Pt(1 1 1) surfaces. It is also rather interesting that the constrained geometries of the cyclic alkanes of different lengths do not perturb the linear dependence of on chain length. Our conclusion from these considerations is that lattice commensurability is not the primary origin of DE Chain length dependence of the pre-exponential factor C Fig. 9. Illustrations of the interaction of alkanes of lengths N ¼ 1 to 3 with three periodic potential energy surfaces. (A) The alkane and the surface have the same periodicity and arecommensurate. The total interaction energy ought to be proportional to the alkane chain length leading to ðnþ ¼N DECH. (B) The alkane and the surface have different periodicities and are incommensurate. The total interaction energy is a complex, non-linear function of the chain length. (C) The alkane and the surface have different periodicities and the interaction potential with the surface is uniform but with periodic, sharp minima. The interaction of the alkanes with this type of surface would lead to a desorption energy of the form ðnþ ¼DE þ N DECH. graphite which is closely lattice matched to the alkanes. In fact, they do not appear in simulations on Au(1 1 1) and Pt(1 1 1) surfaces while the experimentally measured value of DE is significant on the graphite surface. A second compelling argument against lattice commensurability giving rise to DE comes from comparison of for the linear and cyclic alkanes. Although both have similar C C bond lengths, the geometry of cyclic alkanes is highly constrained and it seems unlikely that their registry with various substrate lattices could be similar to that of the linear alkanes. Inspection of the results in Table 3, however, reveals that linear and Perhaps the least well determined parameter in our description of the kinetics of alkane desorption from surfaces is the pre-exponential factor of the desorption rate constant. Isothermal measurements by Wetterer et al. [1] yielded a value of v ¼ s 1 from the intercepts of Arrhenius plots of the desorption rate constants for hexane through dodecane. Several TPD studies have used variable heating rate measurements to measure values ranging from v ¼ to s 1. In analyzing the data presented in Table 2, we have attempted to estimate the pre-exponential factors for alkane desorption from each of the surfaces. Values of v and the compensate for one another in analysis of desorption kinetics such that an error of one order of magnitude in v corresponds to an error of roughly 5% in the value of.it is also important to point out that if one reanalyzed all of the experimental data by simply assuming that pre-exponential factors always have a value of v ¼ s 1, one would still find that scales linearly with alkane length in such a way that the intercept, DE, is significant. In other words the anomaly that is the focus of this paper does not disappear simply by assuming that the pre-exponential factor is always v ¼ s 1. Molecular dynamics simulation of alkane desorption from surfaces has been used recently to determine the predictions of a united atom model of the alkane Au(1 1 1) interaction [9]. The results of that work are compared directly to the experimental data of Wetterer et al. [1]. As one would imagine, adsorption energies, DE ads, of the all-
15 R.Z. Lei et al. / Surface Science 554 (2004) trans configurations of linear alkanes on the Au(1 1 1) surface are predicted to be proportional to the chain length. On the other hand, values of determined from transition state theory analysis of the simulated desorption rates vary non-linearly with chain length and fall below the energies of the all-trans minimum energy configurations. This is a direct result of the fact that the alkanes at elevated temperatures can adopt a number of configurations with energies above those of the global minimum energy configuration. These configurations represent a distribution of states from which desorption occurs and thus yield values of that are lower than the values of the adsorption energies, DE ads. This is the same conclusion as was reached in the analysis of alkane desorption from graphite surfaces although the model used for the alkane graphite interaction [12] was much more primitive than the united atom model used to simulate the alkane Au(1 1 1) interaction [9]. The molecular dynamics simulations of alkane desorption from the Au(1 1 1) surface also predict values of the desorption pre-exponential factors. These values increase with increasing chain length from v ¼ 10 12:2 s 1 for methane to v ¼ 10 16:4 s 1 for n-c 12 H 26. The much simpler model used to analyze the desorption of alkanes from graphite also predicts that the desorption pre-exponents should increase with chain length for alkanes with less than 20 carbon atoms [12]. Wetterer et al. [1], on the other hand, report that their Arrhenius plots of alkane desorption rates from the Au(1 1 1) surface yield values of v s 1 that are independent of chain length. The interesting thing is that Fichthorn and Miron [9] show that the values of T p predicted using their values of v and are almost identical to those that would be predicted by Wetterer et al. [1] using their values of and v s 1. Fundamentally, this arises from the coupling between v and in the equations used to analyze desorption kinetics. These results suggest that analysis of desorption measurements assuming a constant value of v biases the values of incorrectly. If one analyzed desorption spectra using values of v that increased with chain length, then one would find values of that are proportional to chain length (over short ranges of the chain length) and do not exhibit the intercept, DE. Thus, it is possible that the origin of DE lies in a poor determination of the values of v and the incorrect assumption that it is independent of alkane chain length. There are several important differences between the model used for molecular dynamics model of alkane desorption from the Au(1 1 1) surface and the conditions of experimental studies. Perhaps the most important one is coverage. The simulations are done for single molecules adsorbed on a Au(1 1 1) surface and thus neglect intermolecular interactions while the experiments are often done at coverages approaching a monolayer at which intermolecular interactions may be significant. Experimental studies of alkane desorption that have used varying coverages have indicated that desorption is a first-order process and that the desorption energetics are independent of coverage. There has been no attempt, however, to examine the effects of coverage on the desorption preexponential factor. A puzzling aspect of the experimental results is that most indicate that the desorption pre-exponential factor is independent of chain length [3,7,11,12]. As mentioned, Wetterer et al. [1] report that the intercepts of their Arrhenius plots indicate a value of v s 1 that is independent of chain length. The exception is the study of alkane desorption from the Ru(0 0 1) surface which showed that the pre-exponential factor decreased with increasing chain length [4]. In contrast, models for alkane desorption from both Au(1 1 1) and graphite indicate that the pre-exponent should increase with increasing chain length at least for short chain lengths (N < 20) [9,12]. Thus, although errors in the desorption pre-exponential factor could serve to explain the origin of DE there is no experimental evidence from data obtained on metal surfaces to suggest that the desorption preexponential factor increases with chain length. It should be pointed out, however, that a recently reported set of data for the desorption of straight chain alkanes from MgO does show this increase of the pre-exponential factor with chain length [24]. Further analysis of alkane desorption kinetics to yield meaningful pre-exponential factors is necessary to fully understand this problem.
Oligomer desorption during heterogeneous catalytic synthesis of polymers
 Catalysis Today 105 (2005) 144 151 www.elsevier.com/locate/cattod Oligomer desorption during heterogeneous catalytic synthesis of polymers Andrew J. Gellman * Department of Chemical Engineering, Carnegie
Catalysis Today 105 (2005) 144 151 www.elsevier.com/locate/cattod Oligomer desorption during heterogeneous catalytic synthesis of polymers Andrew J. Gellman * Department of Chemical Engineering, Carnegie
Enantioselective decomposition of chiral alkyl bromides on Cu(643) R&S : Effects of moving the chiral center
 Surface Science 600 (2006) 2823 2829 www.elsevier.com/locate/susc Enantioselective decomposition of chiral alkyl bromides on Cu(643) R&S : Effects of moving the chiral center D.M. Rampulla, A.J. Gellman
Surface Science 600 (2006) 2823 2829 www.elsevier.com/locate/susc Enantioselective decomposition of chiral alkyl bromides on Cu(643) R&S : Effects of moving the chiral center D.M. Rampulla, A.J. Gellman
Conformational Entropy Effects on the Desorption Kinetics of Polyethers from Graphite
 Langmuir 2002, 18, 9799-9809 9799 Conformational Entropy Effects on the Desorption Kinetics of Polyethers from Graphite Kris R. Paserba, Nithya Vaidyanathan, and Andrew J. Gellman* Department of Chemical
Langmuir 2002, 18, 9799-9809 9799 Conformational Entropy Effects on the Desorption Kinetics of Polyethers from Graphite Kris R. Paserba, Nithya Vaidyanathan, and Andrew J. Gellman* Department of Chemical
Enantiospecific Desorption of Chiral Compounds from Chiral Cu(643) and Achiral Cu(111) Surfaces
 Published on Web 02/13/2002 Enantiospecific Desorption of Chiral Compounds from Chiral Cu(643) and Achiral Cu(111) Surfaces Joshua D. Horvath and Andrew J. Gellman* Contribution from the Department of
Published on Web 02/13/2002 Enantiospecific Desorption of Chiral Compounds from Chiral Cu(643) and Achiral Cu(111) Surfaces Joshua D. Horvath and Andrew J. Gellman* Contribution from the Department of
Evidence for partial dissociation of water on flat MgO(1 0 0) surfaces
 6 February 2002 Chemical Physics Letters 352 (2002) 318 322 www.elsevier.com/locate/cplett Evidence for partial dissociation of water on flat MgO(1 0 0) surfaces Y.D. Kim a, R.M. Lynden-Bell b, *, A. Alavi
6 February 2002 Chemical Physics Letters 352 (2002) 318 322 www.elsevier.com/locate/cplett Evidence for partial dissociation of water on flat MgO(1 0 0) surfaces Y.D. Kim a, R.M. Lynden-Bell b, *, A. Alavi
Transition State for Alkyl Group Hydrogenation on Pt(111)
 Published on Web 06/04/2008 Transition State for Alkyl Group Hydrogenation on Pt(111) Pingping Ye and Andrew J. Gellman*,,, Departments of Chemistry and Chemical Engineering, Carnegie Mellon UniVersity,
Published on Web 06/04/2008 Transition State for Alkyl Group Hydrogenation on Pt(111) Pingping Ye and Andrew J. Gellman*,,, Departments of Chemistry and Chemical Engineering, Carnegie Mellon UniVersity,
TPD and FT-IRAS Investigation of Ethylene Oxide (EtO) Adsorption on a Au(211) Stepped Surface
 3886 Langmuir 2005, 21, 3886-3891 TPD and FT-IRAS Investigation of Ethylene Oxide (EtO) Adsorption on a Au(211) Stepped Surface Jooho Kim and Bruce E. Koel* Department of Chemistry, University of Southern
3886 Langmuir 2005, 21, 3886-3891 TPD and FT-IRAS Investigation of Ethylene Oxide (EtO) Adsorption on a Au(211) Stepped Surface Jooho Kim and Bruce E. Koel* Department of Chemistry, University of Southern
Effects of methanol on crystallization of water in the deeply super cooled region
 Effects of methanol on crystallization of water in the deeply super cooled region Ryutaro Souda Nanoscale Materials Center National Institute for Materials Science Japan PHYSICAL REVIEW B 75, 184116, 2007
Effects of methanol on crystallization of water in the deeply super cooled region Ryutaro Souda Nanoscale Materials Center National Institute for Materials Science Japan PHYSICAL REVIEW B 75, 184116, 2007
- intermolecular forces forces that exist between molecules
 Chapter 11: Intermolecular Forces, Liquids, and Solids - intermolecular forces forces that exist between molecules 11.1 A Molecular Comparison of Liquids and Solids - gases - average kinetic energy of
Chapter 11: Intermolecular Forces, Liquids, and Solids - intermolecular forces forces that exist between molecules 11.1 A Molecular Comparison of Liquids and Solids - gases - average kinetic energy of
Alkane Contamination Effects on PFPE Lubricant Bonding to a-ch x Overcoats
 6240 Langmuir 2001, 17, 6240-6247 Alkane Contamination Effects on PFPE Lubricant Bonding to a-ch x Overcoats Ryan Z. Lei and Andrew J. Gellman* Department of Chemical Engineering, Carnegie Mellon University,
6240 Langmuir 2001, 17, 6240-6247 Alkane Contamination Effects on PFPE Lubricant Bonding to a-ch x Overcoats Ryan Z. Lei and Andrew J. Gellman* Department of Chemical Engineering, Carnegie Mellon University,
Enantiospecific Adsorption of (R)-3-Methylcyclohexanone on Naturally Chiral Cu(531) R&S Surfaces
 Catal Lett (2008) 125:177 182 DOI 10.1007/s10562-008-9600-8 Enantiospecific Adsorption of (R)-3-Methylcyclohexanone on Naturally Chiral Cu(531) R&S Surfaces Ye Huang Æ Andrew J. Gellman Received: 22 May
Catal Lett (2008) 125:177 182 DOI 10.1007/s10562-008-9600-8 Enantiospecific Adsorption of (R)-3-Methylcyclohexanone on Naturally Chiral Cu(531) R&S Surfaces Ye Huang Æ Andrew J. Gellman Received: 22 May
Amandeep S. Bolina, Angela J. Wolff, and Wendy A. Brown*
 16836 J. Phys. Chem. B 2005, 109, 16836-16845 Reflection Absorption Infrared Spectroscopy and Temperature-Programmed Desorption Studies of the Adsorption and Desorption of Amorphous and Crystalline Water
16836 J. Phys. Chem. B 2005, 109, 16836-16845 Reflection Absorption Infrared Spectroscopy and Temperature-Programmed Desorption Studies of the Adsorption and Desorption of Amorphous and Crystalline Water
Table 1: Residence time (τ) in seconds for adsorbed molecules
 1 Surfaces We got our first hint of the importance of surface processes in the mass spectrum of a high vacuum environment. The spectrum was dominated by water and carbon monoxide, species that represent
1 Surfaces We got our first hint of the importance of surface processes in the mass spectrum of a high vacuum environment. The spectrum was dominated by water and carbon monoxide, species that represent
TPD-MS. Photocatalytic Studies Using Temperature Programmed Desorption Mass Spectrometry (TPD-MS) APPLICATION NOTE NOTE
 TPD-MS APPLICATION NOTE NOTE Photocatalytic Studies Using Temperature Programmed Desorption Mass Spectrometry (TPD-MS) Thermal analysis consists of many techniques for the exploration of the physical properties
TPD-MS APPLICATION NOTE NOTE Photocatalytic Studies Using Temperature Programmed Desorption Mass Spectrometry (TPD-MS) Thermal analysis consists of many techniques for the exploration of the physical properties
Carboxylic Acid Deprotonation on the Ag(110) and Ag(111) Surfaces
 6638 Langmuir 2001, 17, 6638-6646 Carboxylic Acid Deprotonation on the Ag(110) and Ag(111) Surfaces Bryan Parker Department of Chemistry, University of Illinois, Urbana, Illinois 61801 Boonchuan Immaraporn
6638 Langmuir 2001, 17, 6638-6646 Carboxylic Acid Deprotonation on the Ag(110) and Ag(111) Surfaces Bryan Parker Department of Chemistry, University of Illinois, Urbana, Illinois 61801 Boonchuan Immaraporn
Supporting Information
 Supporting Information Yao et al. 10.1073/pnas.1416368111 Fig. S1. In situ LEEM imaging of graphene growth via chemical vapor deposition (CVD) on Pt(111). The growth of graphene on Pt(111) via a CVD process
Supporting Information Yao et al. 10.1073/pnas.1416368111 Fig. S1. In situ LEEM imaging of graphene growth via chemical vapor deposition (CVD) on Pt(111). The growth of graphene on Pt(111) via a CVD process
Chapter 12. Insert picture from First page of chapter. Intermolecular Forces and the Physical Properties of Liquids and Solids
 Chapter 12 Insert picture from First page of chapter Intermolecular Forces and the Physical Properties of Liquids and Solids Copyright McGraw-Hill 2009 1 12.1 Intermolecular Forces Intermolecular forces
Chapter 12 Insert picture from First page of chapter Intermolecular Forces and the Physical Properties of Liquids and Solids Copyright McGraw-Hill 2009 1 12.1 Intermolecular Forces Intermolecular forces
The Transition State for Surface-Catalyzed Dehalogenation: C I Cleavage on Pd(111)
 Journal of Catalysis 203, 41 50 (2001) doi:10.1006/jcat.2001.3310, available online at http://www.idealibrary.com on The Transition State for Surface-Catalyzed Dehalogenation: C I Cleavage on Pd(111) Mark
Journal of Catalysis 203, 41 50 (2001) doi:10.1006/jcat.2001.3310, available online at http://www.idealibrary.com on The Transition State for Surface-Catalyzed Dehalogenation: C I Cleavage on Pd(111) Mark
Surface Chemistry and Reaction Dynamics of Electron Beam Induced Deposition Processes
 Surface Chemistry and Reaction Dynamics of Electron Beam Induced Deposition Processes e -? 2 nd FEBIP Workshop Thun, Switzerland 2008 Howard Fairbrother Johns Hopkins University Baltimore, MD, USA Outline
Surface Chemistry and Reaction Dynamics of Electron Beam Induced Deposition Processes e -? 2 nd FEBIP Workshop Thun, Switzerland 2008 Howard Fairbrother Johns Hopkins University Baltimore, MD, USA Outline
Methane adsorption and dissociation and oxygen adsorption and reaction with CO on Pd nanoparticles on MgO(100) and on Pd(111)
 Surface Science 591 (2005) 90 107 www.elsevier.com/locate/susc Methane adsorption and dissociation and oxygen adsorption and reaction with CO on Pd nanoparticles on MgO(100) and on Pd(111) Steven L. Tait
Surface Science 591 (2005) 90 107 www.elsevier.com/locate/susc Methane adsorption and dissociation and oxygen adsorption and reaction with CO on Pd nanoparticles on MgO(100) and on Pd(111) Steven L. Tait
Enantioselective Separation on a Naturally Chiral Surface
 Published on Web 10/26/2004 Enantioselective Separation on a Naturally Chiral Surface Joshua D. Horvath, Anjanette Koritnik, Preeti Kamakoti,, David S. Sholl,, and Andrew J. Gellman*, Contribution from
Published on Web 10/26/2004 Enantioselective Separation on a Naturally Chiral Surface Joshua D. Horvath, Anjanette Koritnik, Preeti Kamakoti,, David S. Sholl,, and Andrew J. Gellman*, Contribution from
The Surface Chemistry of Propylene, 1-Iodopropane, and 1,3-Diiodopropane on MoAl Alloy Thin Films Formed on Dehydroxylated Alumina
 J. Phys. Chem. B 2006, 110, 12555-12571 12555 The Surface Chemistry of Propylene, 1-Iodopropane, and 1,3-Diiodopropane on MoAl Alloy Thin Films Formed on Dehydroxylated Alumina Feng Gao, Yilin Wang, and
J. Phys. Chem. B 2006, 110, 12555-12571 12555 The Surface Chemistry of Propylene, 1-Iodopropane, and 1,3-Diiodopropane on MoAl Alloy Thin Films Formed on Dehydroxylated Alumina Feng Gao, Yilin Wang, and
Cover Page. The handle holds various files of this Leiden University dissertation.
 Cover Page The handle http://hdl.handle.net/1887/44295 holds various files of this Leiden University dissertation. Author: Badan, C. Title: Surface-structure dependence of water-related adsorbates on platinum
Cover Page The handle http://hdl.handle.net/1887/44295 holds various files of this Leiden University dissertation. Author: Badan, C. Title: Surface-structure dependence of water-related adsorbates on platinum
The Interaction of CF 3 CH 2 OH and (CF 3 CF 2 ) 2 O with Amorphous Carbon Films
 6562 Langmuir 2000, 16, 6562-6568 The Interaction of CF 3 CH 2 OH and (CF 3 CF 2 ) 2 O with Amorphous Carbon Films Nisha Shukla and Andrew J. Gellman* Department of Chemical Engineering, Carnegie Mellon
6562 Langmuir 2000, 16, 6562-6568 The Interaction of CF 3 CH 2 OH and (CF 3 CF 2 ) 2 O with Amorphous Carbon Films Nisha Shukla and Andrew J. Gellman* Department of Chemical Engineering, Carnegie Mellon
Unit Five: Intermolecular Forces MC Question Practice April 14, 2017
 Unit Five: Intermolecular Forces Name MC Question Practice April 14, 2017 1. Which of the following should have the highest surface tension at a given temperature? 2. The triple point of compound X occurs
Unit Five: Intermolecular Forces Name MC Question Practice April 14, 2017 1. Which of the following should have the highest surface tension at a given temperature? 2. The triple point of compound X occurs
The Transition State for Metal-Catalyzed Dehalogenation: C-I Bond Cleavage on Ag(111)
 1440 J. Am. Chem. Soc. 2001, 123, 1440-1448 The Transition State for Metal-Catalyzed Dehalogenation: C-I Bond Cleavage on Ag(111) Mark T. Buelow and Andrew J. Gellman* Contribution from the Department
1440 J. Am. Chem. Soc. 2001, 123, 1440-1448 The Transition State for Metal-Catalyzed Dehalogenation: C-I Bond Cleavage on Ag(111) Mark T. Buelow and Andrew J. Gellman* Contribution from the Department
Vacuum Pumps. Two general classes exist: Gas transfer physical removal of matter. Mechanical, diffusion, turbomolecular
 Vacuum Technology Vacuum Pumps Two general classes exist: Gas transfer physical removal of matter Mechanical, diffusion, turbomolecular Adsorption entrapment of matter Cryo, sublimation, ion Mechanical
Vacuum Technology Vacuum Pumps Two general classes exist: Gas transfer physical removal of matter Mechanical, diffusion, turbomolecular Adsorption entrapment of matter Cryo, sublimation, ion Mechanical
Chapter 12 Intermolecular Forces and Liquids
 Chapter 12 Intermolecular Forces and Liquids Jeffrey Mack California State University, Sacramento Why? Why is water usually a liquid and not a gas? Why does liquid water boil at such a high temperature
Chapter 12 Intermolecular Forces and Liquids Jeffrey Mack California State University, Sacramento Why? Why is water usually a liquid and not a gas? Why does liquid water boil at such a high temperature
Lecture 5. Solid surface: Adsorption and Catalysis
 Lecture 5 Solid surface: Adsorption and Catalysis Adsorbtion G = H T S DG ads should be negative (spontaneous process) DS ads is negative (reduced freedom) DH should be negative for adsorption processes
Lecture 5 Solid surface: Adsorption and Catalysis Adsorbtion G = H T S DG ads should be negative (spontaneous process) DS ads is negative (reduced freedom) DH should be negative for adsorption processes
Enthalpies and Entropies of Adsorption on Well-Defined Oxide Surfaces: Experimental Measurements
 pubs.acs.org/cr Enthalpies and Entropies of Adsorption on Well-Defined Oxide Surfaces: Experimental Measurements Charles T. Campbell* and Jason R. V. Sellers Department of Chemistry, University of Washington,
pubs.acs.org/cr Enthalpies and Entropies of Adsorption on Well-Defined Oxide Surfaces: Experimental Measurements Charles T. Campbell* and Jason R. V. Sellers Department of Chemistry, University of Washington,
Received 24 July 2002; accepted for publication 17 September 2002
 Surface Science 522 (2003) 17 26 www.elsevier.com/locate/susc The effect of surface chemical functional groups on the adsorption and desorption of a polar molecule, acetone, from a model carbonaceous surface,
Surface Science 522 (2003) 17 26 www.elsevier.com/locate/susc The effect of surface chemical functional groups on the adsorption and desorption of a polar molecule, acetone, from a model carbonaceous surface,
Chemistry: The Central Science
 Chemistry: The Central Science Fourteenth Edition Chapter 11 Liquids and Intermolecular Forces Intermolecular Forces The attractions between molecules are not nearly as strong as the intramolecular attractions
Chemistry: The Central Science Fourteenth Edition Chapter 11 Liquids and Intermolecular Forces Intermolecular Forces The attractions between molecules are not nearly as strong as the intramolecular attractions
Intermolecular Forces and Liquids and Solids
 Intermolecular Forces and Liquids and Solids Chapter 11 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. A phase is a homogeneous part of the system in contact
Intermolecular Forces and Liquids and Solids Chapter 11 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. A phase is a homogeneous part of the system in contact
Sample Exercise 11.1 Identifying Substances That Can Form Hydrogen Bonds
 Sample Exercise 11.1 Identifying Substances That Can Form Hydrogen Bonds In which of these substances is hydrogen bonding likely to play an important role in determining physical properties: methane (CH
Sample Exercise 11.1 Identifying Substances That Can Form Hydrogen Bonds In which of these substances is hydrogen bonding likely to play an important role in determining physical properties: methane (CH
Chapter 11. Liquids and Intermolecular Forces
 Chapter 11. Liquids and Intermolecular Forces 11.1 A Molecular Comparison of Gases, Liquids, and Solids Gases are highly compressible and assume the shape and volume of their container. Gas molecules are
Chapter 11. Liquids and Intermolecular Forces 11.1 A Molecular Comparison of Gases, Liquids, and Solids Gases are highly compressible and assume the shape and volume of their container. Gas molecules are
Ch. 11: Liquids and Intermolecular Forces
 Ch. 11: Liquids and Intermolecular Forces Learning goals and key skills: Identify the intermolecular attractive interactions (dispersion, dipole-dipole, hydrogen bonding, ion-dipole) that exist between
Ch. 11: Liquids and Intermolecular Forces Learning goals and key skills: Identify the intermolecular attractive interactions (dispersion, dipole-dipole, hydrogen bonding, ion-dipole) that exist between
28 Processes at solid surfaces
 28 Processes at solid surfaces Solutions to exercises E28.b E28.2b E28.3b Discussion questions The motion of one section of a crystal past another a dislocation results in steps and terraces. See Figures
28 Processes at solid surfaces Solutions to exercises E28.b E28.2b E28.3b Discussion questions The motion of one section of a crystal past another a dislocation results in steps and terraces. See Figures
CHAPTER 11: INTERMOLECULAR FORCES AND LIQUIDS AND SOLIDS. Chemistry 1411 Joanna Sabey
 CHAPTER 11: INTERMOLECULAR FORCES AND LIQUIDS AND SOLIDS Chemistry 1411 Joanna Sabey Forces Phase: homogeneous part of the system in contact with other parts of the system but separated from them by a
CHAPTER 11: INTERMOLECULAR FORCES AND LIQUIDS AND SOLIDS Chemistry 1411 Joanna Sabey Forces Phase: homogeneous part of the system in contact with other parts of the system but separated from them by a
States of Matter; Liquids and Solids. Condensation - change of a gas to either the solid or liquid state
 States of Matter; Liquids and Solids Phase transitions - a change in substance from one state to another Melting - change from a solid to a liquid state Freezing - change of a liquid to the solid state
States of Matter; Liquids and Solids Phase transitions - a change in substance from one state to another Melting - change from a solid to a liquid state Freezing - change of a liquid to the solid state
Lecture 4. Ultrahigh Vacuum Science and Technology
 Lecture 4 Ultrahigh Vacuum Science and Technology Why do we need UHV? 1 Atmosphere = 760 torr; 1 torr = 133 Pa; N ~ 2.5 10 19 molecules/cm 3 Hertz-Knudsen equation p ZW 1/ 2 ( 2mk T) At p = 10-6 Torr it
Lecture 4 Ultrahigh Vacuum Science and Technology Why do we need UHV? 1 Atmosphere = 760 torr; 1 torr = 133 Pa; N ~ 2.5 10 19 molecules/cm 3 Hertz-Knudsen equation p ZW 1/ 2 ( 2mk T) At p = 10-6 Torr it
Enantiospecific Adsorption of (R)-3-Methylcyclohexanone on Naturally Chiral Surfaces Vicinal to Cu(110)
 Top Catal (2011) 54:1403 1413 DOI 10.1007/s11244-011-9756-0 ORIGINAL PAPER Enantiospecific Adsorption of (R)-3-Methylcyclohexanone on Naturally Chiral Surfaces Vicinal to Cu(110) Ye Huang Andrew J. Gellman
Top Catal (2011) 54:1403 1413 DOI 10.1007/s11244-011-9756-0 ORIGINAL PAPER Enantiospecific Adsorption of (R)-3-Methylcyclohexanone on Naturally Chiral Surfaces Vicinal to Cu(110) Ye Huang Andrew J. Gellman
Chemistry Instrumental Analysis Lecture 34. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 34 From molecular to elemental analysis there are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry
Chemistry 4631 Instrumental Analysis Lecture 34 From molecular to elemental analysis there are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry
Supporting Information
 Temperature Effect on Transport, Charging and Binding of Low-Energy Electrons Interacting with Amorphous Solid Water Films Roey Sagi, Michelle Akerman, Sujith Ramakrishnan and Micha Asscher * Institute
Temperature Effect on Transport, Charging and Binding of Low-Energy Electrons Interacting with Amorphous Solid Water Films Roey Sagi, Michelle Akerman, Sujith Ramakrishnan and Micha Asscher * Institute
CHAPTER ELEVEN KINETIC MOLECULAR THEORY OF LIQUIDS AND SOLIDS KINETIC MOLECULAR THEORY OF LIQUIDS AND SOLIDS
 CHAPTER ELEVEN AND LIQUIDS AND SOLIDS KINETIC MOLECULAR THEORY OF LIQUIDS AND SOLIDS Differences between condensed states and gases? KINETIC MOLECULAR THEORY OF LIQUIDS AND SOLIDS Phase Homogeneous part
CHAPTER ELEVEN AND LIQUIDS AND SOLIDS KINETIC MOLECULAR THEORY OF LIQUIDS AND SOLIDS Differences between condensed states and gases? KINETIC MOLECULAR THEORY OF LIQUIDS AND SOLIDS Phase Homogeneous part
Humidity Effects on PFPE Lubricant Bonding to a-ch x Overcoats
 6628 Langmuir 2000, 16, 6628-6635 Humidity Effects on PFPE Lubricant Bonding to a-ch x Overcoats Ryan Z. Lei and Andrew J. Gellman* Department of Chemical Engineering, Carnegie Mellon University, Pittsburgh,
6628 Langmuir 2000, 16, 6628-6635 Humidity Effects on PFPE Lubricant Bonding to a-ch x Overcoats Ryan Z. Lei and Andrew J. Gellman* Department of Chemical Engineering, Carnegie Mellon University, Pittsburgh,
Chapter 11. Intermolecular Forces and Liquids & Solids
 Chapter 11 Intermolecular Forces and Liquids & Solids The Kinetic Molecular Theory of Liquids & Solids Gases vs. Liquids & Solids difference is distance between molecules Liquids Molecules close together;
Chapter 11 Intermolecular Forces and Liquids & Solids The Kinetic Molecular Theory of Liquids & Solids Gases vs. Liquids & Solids difference is distance between molecules Liquids Molecules close together;
Chem 112 Dr. Kevin Moore
 Chem 112 Dr. Kevin Moore Gas Liquid Solid Polar Covalent Bond Partial Separation of Charge Electronegativity: H 2.1 Cl 3.0 H Cl δ + δ - Dipole Moment measure of the net polarity in a molecule Q Q magnitude
Chem 112 Dr. Kevin Moore Gas Liquid Solid Polar Covalent Bond Partial Separation of Charge Electronegativity: H 2.1 Cl 3.0 H Cl δ + δ - Dipole Moment measure of the net polarity in a molecule Q Q magnitude
Dipole dipole interactions among CH 3 Cl molecules on Ru 001 : Correlation between work function change and thermal desorption studies
 JOURNAL OF CHEMICAL PHYSICS VOLUME 111, NUMBER 24 22 DECEMBER 1999 Dipole dipole interactions among CH 3 Cl molecules on Ru 001 : Correlation between work function change and thermal desorption studies
JOURNAL OF CHEMICAL PHYSICS VOLUME 111, NUMBER 24 22 DECEMBER 1999 Dipole dipole interactions among CH 3 Cl molecules on Ru 001 : Correlation between work function change and thermal desorption studies
Hydrogen adsorption by graphite intercalation compounds
 62 Chapter 4 Hydrogen adsorption by graphite intercalation compounds 4.1 Introduction Understanding the thermodynamics of H 2 adsorption in chemically modified carbons remains an important area of fundamental
62 Chapter 4 Hydrogen adsorption by graphite intercalation compounds 4.1 Introduction Understanding the thermodynamics of H 2 adsorption in chemically modified carbons remains an important area of fundamental
They are similar to each other. Intermolecular forces
 s and solids They are similar to each other Different than gases. They are incompressible. Their density doesn t change much with temperature. These similarities are due to the molecules staying close
s and solids They are similar to each other Different than gases. They are incompressible. Their density doesn t change much with temperature. These similarities are due to the molecules staying close
Intermolecular Forces and Liquids and Solids
 PowerPoint Lecture Presentation by J. David Robertson University of Missouri Intermolecular Forces and Liquids and Solids Chapter 11 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
PowerPoint Lecture Presentation by J. David Robertson University of Missouri Intermolecular Forces and Liquids and Solids Chapter 11 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
Evidence for structure sensitivity in the high pressure CO NO reaction over Pd(111) and Pd(100)
 Evidence for structure sensitivity in the high pressure CO NO reaction over Pd(111) and Pd(100) Scott M. Vesecky, Peijun Chen, Xueping Xu, and D. Wayne Goodman a) Department of Chemistry, Texas A&M University,
Evidence for structure sensitivity in the high pressure CO NO reaction over Pd(111) and Pd(100) Scott M. Vesecky, Peijun Chen, Xueping Xu, and D. Wayne Goodman a) Department of Chemistry, Texas A&M University,
2. As gas P increases and/or T is lowered, intermolecular forces become significant, and deviations from ideal gas laws occur (van der Waal equation).
 A. Introduction. (Section 11.1) CHAPTER 11: STATES OF MATTER, LIQUIDS AND SOLIDS 1. Gases are easily treated mathematically because molecules behave independently. 2. As gas P increases and/or T is lowered,
A. Introduction. (Section 11.1) CHAPTER 11: STATES OF MATTER, LIQUIDS AND SOLIDS 1. Gases are easily treated mathematically because molecules behave independently. 2. As gas P increases and/or T is lowered,
Intermolecular Forces and Liquids and Solids
 Intermolecular Forces and Liquids and Solids Chapter 11 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 A phase is a homogeneous part of the system in contact
Intermolecular Forces and Liquids and Solids Chapter 11 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 A phase is a homogeneous part of the system in contact
3.5. Kinetic Approach for Isotherms
 We have arrived isotherm equations which describe adsorption from the two dimensional equation of state via the Gibbs equation (with a saturation limit usually associated with monolayer coverage). The
We have arrived isotherm equations which describe adsorption from the two dimensional equation of state via the Gibbs equation (with a saturation limit usually associated with monolayer coverage). The
They are similar to each other
 They are similar to each other Different than gases. They are incompressible. Their density doesn t change much with temperature. These similarities are due to the molecules staying close together in solids
They are similar to each other Different than gases. They are incompressible. Their density doesn t change much with temperature. These similarities are due to the molecules staying close together in solids
compared to gases. They are incompressible. Their density doesn t change with temperature. These similarities are due
 Liquids and solids They are similar compared to gases. They are incompressible. Their density doesn t change with temperature. These similarities are due to the molecules being close together in solids
Liquids and solids They are similar compared to gases. They are incompressible. Their density doesn t change with temperature. These similarities are due to the molecules being close together in solids
Chapter 11. Intermolecular Forces, Liquids, and Solids
 Chapter 11. Intermolecular Forces, Liquids, and Solids A Molecular Comparison of Gases, Liquids, and Solids Physical properties of substances are understood in terms of kinetic-molecular theory: Gases
Chapter 11. Intermolecular Forces, Liquids, and Solids A Molecular Comparison of Gases, Liquids, and Solids Physical properties of substances are understood in terms of kinetic-molecular theory: Gases
The current status of tribological surface science
 Tribology Letters Vol. 10, No. 1-2, 2001 39 The current status of tribological surface science Andrew J. Gellman and Jeff S. Ko Department of Chemical Engineering, Carnegie Mellon University, Pittsburgh,
Tribology Letters Vol. 10, No. 1-2, 2001 39 The current status of tribological surface science Andrew J. Gellman and Jeff S. Ko Department of Chemical Engineering, Carnegie Mellon University, Pittsburgh,
Concepts in Surface Physics
 M.-C. Desjonqueres D. Spanjaard Concepts in Surface Physics Second Edition With 257 Figures Springer 1. Introduction................................. 1 2. Thermodynamical and Statistical Properties of
M.-C. Desjonqueres D. Spanjaard Concepts in Surface Physics Second Edition With 257 Figures Springer 1. Introduction................................. 1 2. Thermodynamical and Statistical Properties of
Chapter 11 SOLIDS, LIQUIDS AND GASES Pearson Education, Inc.
 Chapter 11 SOLIDS, LIQUIDS AND GASES States of Matter Because in the solid and liquid states particles are closer together, we refer to them as. The States of Matter The state of matter a substance is
Chapter 11 SOLIDS, LIQUIDS AND GASES States of Matter Because in the solid and liquid states particles are closer together, we refer to them as. The States of Matter The state of matter a substance is
States of matter Part 2
 Physical Pharmacy Lecture 2 States of matter Part 2 Assistant Lecturer in Pharmaceutics Overview The Liquid State General properties Liquefaction of gases Vapor pressure of liquids Boiling point The Solid
Physical Pharmacy Lecture 2 States of matter Part 2 Assistant Lecturer in Pharmaceutics Overview The Liquid State General properties Liquefaction of gases Vapor pressure of liquids Boiling point The Solid
New ways to characterize 'hard' and 'soft' surface properties by IGC-ID. Eric BRENDLE
 New ways to characterize 'hard' and 'soft' surface properties by IGC-ID Eric BRENDLE Content IGC at Infinite Dilution Principle and Assumptions Real surfaces and accessibility Morphology index (IM) and
New ways to characterize 'hard' and 'soft' surface properties by IGC-ID Eric BRENDLE Content IGC at Infinite Dilution Principle and Assumptions Real surfaces and accessibility Morphology index (IM) and
Alkanes and Cycloalkanes
 Alkanes and Cycloalkanes Families of Organic Compounds Organic compounds can be grouped into families by their common structural features We shall survey the nature of the compounds in a tour of the families
Alkanes and Cycloalkanes Families of Organic Compounds Organic compounds can be grouped into families by their common structural features We shall survey the nature of the compounds in a tour of the families
Intermolecular Forces and Liquids and Solids. Chapter 11. Copyright The McGraw Hill Companies, Inc. Permission required for
 Intermolecular Forces and Liquids and Solids Chapter 11 Copyright The McGraw Hill Companies, Inc. Permission required for 1 A phase is a homogeneous part of the system in contact with other parts of the
Intermolecular Forces and Liquids and Solids Chapter 11 Copyright The McGraw Hill Companies, Inc. Permission required for 1 A phase is a homogeneous part of the system in contact with other parts of the
Chapter 11 Intermolecular Forces, Liquids, and Solids. Intermolecular Forces
 Chapter 11, Liquids, and Solids States of Matter The fundamental difference between states of matter is the distance between particles. States of Matter Because in the solid and liquid states particles
Chapter 11, Liquids, and Solids States of Matter The fundamental difference between states of matter is the distance between particles. States of Matter Because in the solid and liquid states particles
Scanning Tunneling Microscopy Studies of the Ge(111) Surface
 VC Scanning Tunneling Microscopy Studies of the Ge(111) Surface Anna Rosen University of California, Berkeley Advisor: Dr. Shirley Chiang University of California, Davis August 24, 2007 Abstract: This
VC Scanning Tunneling Microscopy Studies of the Ge(111) Surface Anna Rosen University of California, Berkeley Advisor: Dr. Shirley Chiang University of California, Davis August 24, 2007 Abstract: This
CARBON 2004 Providence, Rhode Island. Adsorption of Flexible n-butane and n-hexane on Graphitized Thermal Carbon Black and in Slit Pores
 CARBON Providence, Rhode Island Adsorption of Flexible n-butane and n-hexane on Graphitized Thermal Carbon Black and in Slit Pores D. D. Do* and H. D. Do, University of Queensland, St. Lucia, Qld 7, Australia
CARBON Providence, Rhode Island Adsorption of Flexible n-butane and n-hexane on Graphitized Thermal Carbon Black and in Slit Pores D. D. Do* and H. D. Do, University of Queensland, St. Lucia, Qld 7, Australia
CHAPTER 13. States of Matter. Kinetic = motion. Polar vs. Nonpolar. Gases. Hon Chem 13.notebook
 CHAPTER 13 States of Matter States that the tiny particles in all forms of matter are in constant motion. Kinetic = motion A gas is composed of particles, usually molecules or atoms, with negligible volume
CHAPTER 13 States of Matter States that the tiny particles in all forms of matter are in constant motion. Kinetic = motion A gas is composed of particles, usually molecules or atoms, with negligible volume
Alkanes and Cycloalkanes
 Chapter 3 Alkanes and Cycloalkanes Two types Saturated hydrocarbons Unsaturated hydrocarbons 3.1 Alkanes Also referred as aliphatic hydrocarbons General formula: CnH2n+2 (straight chain) and CnH2n (cyclic)
Chapter 3 Alkanes and Cycloalkanes Two types Saturated hydrocarbons Unsaturated hydrocarbons 3.1 Alkanes Also referred as aliphatic hydrocarbons General formula: CnH2n+2 (straight chain) and CnH2n (cyclic)
A- Determination Of Boiling point B- Distillation
 EXP. NO. 2 A- Determination Of Boiling point B- Distillation The boiling point of a liquid is the temperature at which its vapor pressure is equal to the surrounding atmospheric pressure. The normal boiling
EXP. NO. 2 A- Determination Of Boiling point B- Distillation The boiling point of a liquid is the temperature at which its vapor pressure is equal to the surrounding atmospheric pressure. The normal boiling
Acidic Water Monolayer on Ruthenium(0001)
 Acidic Water Monolayer on Ruthenium(0001) Youngsoon Kim, Eui-seong Moon, Sunghwan Shin, and Heon Kang Department of Chemistry, Seoul National University, 1 Gwanak-ro, Seoul 151-747, Republic of Korea.
Acidic Water Monolayer on Ruthenium(0001) Youngsoon Kim, Eui-seong Moon, Sunghwan Shin, and Heon Kang Department of Chemistry, Seoul National University, 1 Gwanak-ro, Seoul 151-747, Republic of Korea.
Chapter 3. Distinguishing between Reaction Intermediates and. Spectators: A Kinetic Study of Acetone Oxidation Using
 Chapter 3 Distinguishing between Reaction Intermediates and Spectators: A Kinetic Study of Acetone Oxidation Using Ozone on a Silica-Supported Manganese Oxide Catalyst 3.1 Introduction This chapter concentrates
Chapter 3 Distinguishing between Reaction Intermediates and Spectators: A Kinetic Study of Acetone Oxidation Using Ozone on a Silica-Supported Manganese Oxide Catalyst 3.1 Introduction This chapter concentrates
DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD
 Chapter 4 DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD 4.1 INTRODUCTION Sputter deposition process is another old technique being used in modern semiconductor industries. Sputtering
Chapter 4 DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD 4.1 INTRODUCTION Sputter deposition process is another old technique being used in modern semiconductor industries. Sputtering
Organic Chemistry. A brief introduction
 Organic Chemistry A brief introduction Organic Chemistry the study of carbon-containing compounds and their properties excluding: CO, CO 2, CS 2, carbonates and cyanides eight million known organic compounds
Organic Chemistry A brief introduction Organic Chemistry the study of carbon-containing compounds and their properties excluding: CO, CO 2, CS 2, carbonates and cyanides eight million known organic compounds
Distinguish quantitatively between the adsorption isotherms of Gibbs, Freundlich and Langmuir.
 Module 8 : Surface Chemistry Lecture 36 : Adsorption Objectives After studying this lecture, you will be able to Distinguish between physisorption and chemisorption. Distinguish between monolayer adsorption
Module 8 : Surface Chemistry Lecture 36 : Adsorption Objectives After studying this lecture, you will be able to Distinguish between physisorption and chemisorption. Distinguish between monolayer adsorption
Experimental Methods and Analysis
 Chapter 3 28 Experimental Methods and Analysis 1. Equipment The fundamental basis of quantitative adsorption analysis is the measurement of excess adsorption isotherms. Each isotherm comprises a series
Chapter 3 28 Experimental Methods and Analysis 1. Equipment The fundamental basis of quantitative adsorption analysis is the measurement of excess adsorption isotherms. Each isotherm comprises a series
7. Oxidation of gold by oxygen-ion sputtering
 7. Oxidation of gold by oxygen-ion sputtering Up to now, relatively little attention has been paid to oxygen phases obtained by sputtering of gold surfaces with oxygen ions. Nevertheless, the high oxygen
7. Oxidation of gold by oxygen-ion sputtering Up to now, relatively little attention has been paid to oxygen phases obtained by sputtering of gold surfaces with oxygen ions. Nevertheless, the high oxygen
ก ก ก Intermolecular Forces: Liquids, Solids, and Phase Changes
 ก ก ก Intermolecular Forces: Liquids, Solids, and Phase Changes ก ก ก ก Mc-Graw Hill 1 Intermolecular Forces: Liquids, Solids, and Phase Changes 12.1 An Overview of Physical States and Phase Changes 12.2
ก ก ก Intermolecular Forces: Liquids, Solids, and Phase Changes ก ก ก ก Mc-Graw Hill 1 Intermolecular Forces: Liquids, Solids, and Phase Changes 12.1 An Overview of Physical States and Phase Changes 12.2
Chapter 11. Intermolecular Forces, Liquids, and Solids
 11.2 Intermolecular Forces Intermolecular forces are much weaker than ionic or covalent bonds (e.g., 16 kj/mol versus 431 kj/mol for HCl). Melting or boiling = broken intermolecular forces Intermolecular
11.2 Intermolecular Forces Intermolecular forces are much weaker than ionic or covalent bonds (e.g., 16 kj/mol versus 431 kj/mol for HCl). Melting or boiling = broken intermolecular forces Intermolecular
(Preeti Aghalayam, Sep 2011)
 (Preeti Aghalayam, Sep 2011) Adsorbed States As A approaches the catalyst surface, the PE of the system goes through a minimum. Potential Energy (system) ΔH c ΔH p Physisorption vs. Chemisorption A + Catalyst
(Preeti Aghalayam, Sep 2011) Adsorbed States As A approaches the catalyst surface, the PE of the system goes through a minimum. Potential Energy (system) ΔH c ΔH p Physisorption vs. Chemisorption A + Catalyst
Chapter 10 Liquids and Solids. Problems: 14, 15, 18, 21-23, 29, 31-35, 37, 39, 41, 43, 46, 81-83, 87, 88, 90-93, 99, , 113
 Chapter 10 Liquids and Solids Problems: 14, 15, 18, 21-23, 29, 31-35, 37, 39, 41, 43, 46, 81-83, 87, 88, 90-93, 99, 104-106, 113 Recall: Intermolecular vs. Intramolecular Forces Intramolecular: bonds between
Chapter 10 Liquids and Solids Problems: 14, 15, 18, 21-23, 29, 31-35, 37, 39, 41, 43, 46, 81-83, 87, 88, 90-93, 99, 104-106, 113 Recall: Intermolecular vs. Intramolecular Forces Intramolecular: bonds between
Chapter 11 Intermolecular Forces, Liquids, and Solids
 Chapter 11 Intermolecular Forces, Liquids, and Solids Dissolution of an ionic compound States of Matter The fundamental difference between states of matter is the distance between particles. States of
Chapter 11 Intermolecular Forces, Liquids, and Solids Dissolution of an ionic compound States of Matter The fundamental difference between states of matter is the distance between particles. States of
Applied Catalysis A: General
 Applied Catalysis A: General 362 (2009) 8 13 Contents lists available at ScienceDirect Applied Catalysis A: General journal homepage: www.elsevier.com/locate/apcata Interactions of SO 2 and H 2 S with
Applied Catalysis A: General 362 (2009) 8 13 Contents lists available at ScienceDirect Applied Catalysis A: General journal homepage: www.elsevier.com/locate/apcata Interactions of SO 2 and H 2 S with
Organic Nomenclature
 University of Puget Sound Department of Chemistry Chem 111 Spring, 2010 Organic Nomenclature LEARNING GOALS AND ASSESSMENTS 1. Be familiar with the structure and nomenclature of organic compounds. a. Identify
University of Puget Sound Department of Chemistry Chem 111 Spring, 2010 Organic Nomenclature LEARNING GOALS AND ASSESSMENTS 1. Be familiar with the structure and nomenclature of organic compounds. a. Identify
Methanol on Co(0001): XPS, TDS, WF and LEED results
 Surface Science 598 (2005) 128 135 www.elsevier.com/locate/susc Methanol on Co(0001): XPS, TDS, WF and LEED results K. Habermehl-Ćwirzeń a,b, *, J. Lahtinen a, P. Hautojärvi a a Laboratory of Physics,
Surface Science 598 (2005) 128 135 www.elsevier.com/locate/susc Methanol on Co(0001): XPS, TDS, WF and LEED results K. Habermehl-Ćwirzeń a,b, *, J. Lahtinen a, P. Hautojärvi a a Laboratory of Physics,
Unit 2, Lesson 01: Introduction to Organic Chemistry and Hydrocarbons
 Unit 2, Lesson 01: Introduction to Organic Chemistry and Hydrocarbons Organic Chemistry: is the branch of chemistry that deals with carbon-based covalent compounds. living organisms are made up of a huge
Unit 2, Lesson 01: Introduction to Organic Chemistry and Hydrocarbons Organic Chemistry: is the branch of chemistry that deals with carbon-based covalent compounds. living organisms are made up of a huge
The cohesive energy of a solid is commonly referred to as the energy required
 4 Energetics of interlayer binding in graphite The cohesive energy of a solid is commonly referred to as the energy required to disassemble it into constituent atoms or molecules [Israelachvili, 1992].
4 Energetics of interlayer binding in graphite The cohesive energy of a solid is commonly referred to as the energy required to disassemble it into constituent atoms or molecules [Israelachvili, 1992].
BAE 820 Physical Principles of Environmental Systems
 BAE 820 Physical Principles of Environmental Systems Catalysis of environmental reactions Dr. Zifei Liu Catalysis and catalysts Catalysis is the increase in the rate of a chemical reaction due to the participation
BAE 820 Physical Principles of Environmental Systems Catalysis of environmental reactions Dr. Zifei Liu Catalysis and catalysts Catalysis is the increase in the rate of a chemical reaction due to the participation
Organic Chemistry 17.1
 Organic Chemistry 17.1 Introduction to Organic Compounds Naming Alkanes Isomers of Alkanes Naming Cycloalkanes What are Organic Compounds? (1807) The term organic compound originated Meant compounds derived
Organic Chemistry 17.1 Introduction to Organic Compounds Naming Alkanes Isomers of Alkanes Naming Cycloalkanes What are Organic Compounds? (1807) The term organic compound originated Meant compounds derived
Adsorption of gases on solids (focus on physisorption)
 Adsorption of gases on solids (focus on physisorption) Adsorption Solid surfaces show strong affinity towards gas molecules that it comes in contact with and some amt of them are trapped on the surface
Adsorption of gases on solids (focus on physisorption) Adsorption Solid surfaces show strong affinity towards gas molecules that it comes in contact with and some amt of them are trapped on the surface
Intermolecular forces
 Intermolecular forces World of Chemistry, 2000 Updated: August 29, 2013 The attractions of molecules to each other are known as intermolecular forces to distinguish them from intramolecular forces, such
Intermolecular forces World of Chemistry, 2000 Updated: August 29, 2013 The attractions of molecules to each other are known as intermolecular forces to distinguish them from intramolecular forces, such
MOLECULAR SURFACE PUMPING: CRYOPUMPING
 51 MOLECULAR SURFACE PUMPING: CRYOPUMPING C. Benvenuti CERN, Geneva, Switzerland Abstract Weak van der Waals attractive forces may provide molecular gas pumping on surfaces cooled at sufficiently low temperatures.
51 MOLECULAR SURFACE PUMPING: CRYOPUMPING C. Benvenuti CERN, Geneva, Switzerland Abstract Weak van der Waals attractive forces may provide molecular gas pumping on surfaces cooled at sufficiently low temperatures.
Loudon Ch. 2 Review: Alkanes Jacquie Richardson, CU Boulder Last updated 4/20/2017
 The simplest organic molecules are hydrocarbons. These contain just carbon and hydrogen. To be most stable, each C wants to have 4 total bonds and each H wants 1 bond. It s possible to link Cs together
The simplest organic molecules are hydrocarbons. These contain just carbon and hydrogen. To be most stable, each C wants to have 4 total bonds and each H wants 1 bond. It s possible to link Cs together
Module 5: "Adsoption" Lecture 25: The Lecture Contains: Definition. Applications. How does Adsorption occur? Physisorption Chemisorption.
 The Lecture Contains: Definition Applications How does Adsorption occur? Physisorption Chemisorption Energetics Adsorption Isotherms Different Adsorption Isotherms Langmuir Adsorption Isotherm file:///e
The Lecture Contains: Definition Applications How does Adsorption occur? Physisorption Chemisorption Energetics Adsorption Isotherms Different Adsorption Isotherms Langmuir Adsorption Isotherm file:///e
Water clustering on nanostructured iron oxide films
 ARTICLE Received 12 May 2013 Accepted 22 May 2014 Published 30 Jun 2014 Water clustering on nanostructured iron oxide films Lindsay R. Merte1,2, Ralf Bechstein1, W. Guowen Peng3, Felix Rieboldt1, Carrie
ARTICLE Received 12 May 2013 Accepted 22 May 2014 Published 30 Jun 2014 Water clustering on nanostructured iron oxide films Lindsay R. Merte1,2, Ralf Bechstein1, W. Guowen Peng3, Felix Rieboldt1, Carrie
Alkanes and Cycloalkanes
 Alkanes and Cycloalkanes Alkanes molecules consisting of carbons and hydrogens in the following ratio: C n H 2n+2 Therefore, an alkane having 4 carbons would have 2(4) + 2 hydrogens, which equals 10 hydrogens.
Alkanes and Cycloalkanes Alkanes molecules consisting of carbons and hydrogens in the following ratio: C n H 2n+2 Therefore, an alkane having 4 carbons would have 2(4) + 2 hydrogens, which equals 10 hydrogens.
3. Organic Compounds: Alkanes and Cycloalkanes
 3. Organic Compounds: Alkanes and Cycloalkanes Based on McMurry s Organic Chemistry, 6 th edition, Chapter 3 2003 Ronald Kluger Department of Chemistry University of Toronto 1 Families of Organic Compounds!
3. Organic Compounds: Alkanes and Cycloalkanes Based on McMurry s Organic Chemistry, 6 th edition, Chapter 3 2003 Ronald Kluger Department of Chemistry University of Toronto 1 Families of Organic Compounds!
Chapters 21 (Radioactivity) and 25 (Organic)
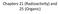 Chapters 21 (Radioactivity) and 25 (Organic) Radioactivity to emit radiation Nuclear reactions change an element into a new element!! Lots of energy involved! Unlike a chemical reaction because we are
Chapters 21 (Radioactivity) and 25 (Organic) Radioactivity to emit radiation Nuclear reactions change an element into a new element!! Lots of energy involved! Unlike a chemical reaction because we are
1 EX/P4-8. Hydrogen Concentration of Co-deposited Carbon Films Produced in the Vicinity of Local Island Divertor in Large Helical Device
 1 EX/P4-8 Hydrogen Concentration of Co-deposited Carbon Films Produced in the Vicinity of Local Island Divertor in Large Helical Device T. Hino 1,2), T. Hirata 1), N. Ashikawa 2), S. Masuzaki 2), Y. Yamauchi
1 EX/P4-8 Hydrogen Concentration of Co-deposited Carbon Films Produced in the Vicinity of Local Island Divertor in Large Helical Device T. Hino 1,2), T. Hirata 1), N. Ashikawa 2), S. Masuzaki 2), Y. Yamauchi
