More On Carbon Monoxide
|
|
|
- Dale Jayson Knight
- 5 years ago
- Views:
Transcription
1 More On Carbon Monoxide E = 0.25 ± 0.05 ev Electron beam results Jerry Gilfoyle The Configurations of CO 1 / 26
2 More On Carbon Monoxide E = 0.25 ± 0.05 ev Electron beam results Jerry Gilfoyle The Configurations of CO 1 / 26
3 More On Carbon Monoxide E = 0.25 ± 0.05 ev Electron beam results Jerry Gilfoyle The Configurations of CO 1 / 26
4 Even More On Carbon Monoxide Electron beam results E = 0.25 ± 0.05 ev CO Absorption Spectrum Incident light Photon detector CO gas target Jerry Gilfoyle The Configurations of CO 2 / 26
5 Even More On Carbon Monoxide Electron beam results E = 0.25 ± 0.05 ev CO Absorption Spectrum Incident light Photon detector CO gas target Absorption Carbon Monoxide Spectrum Energy (ev) Jerry Gilfoyle The Configurations of CO 2 / 26
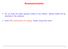
6 Is Carbon Monoxide A Rigid Rotator? Excited states of carbon monoxide (CO) can be observed by measuring the absorption spectrum shown below. The molecule can both vibrate and rotate at the same time. The rotational energy states of a rigid rotator are E l = 2 l(l + 1) 2I where I is the moment of inertia. The vibrational part of the energy is described by the harmonic oscillator so E n = (n ) ω 0 with E = ω 0 = 0.25 ± 0.05 ev from our previous results. How do you get the expression above for the rotational energy? Is CO a rigid rotator? Absorption Carbon Monoxide Spectrum Energy (ev) Jerry Gilfoyle The Configurations of CO 3 / 26
7 Is Carbon Monoxide A Rigid Rotator? Excited states of carbon monoxide (CO) can be observed by measuring the absorption spectrum shown below. The molecule can both vibrate and rotate at the same time. The rotational energy states of a rigid rotator are E l = 2 l(l + 1) 2I where I is the moment of inertia. The vibrational part of the energy is described by the harmonic oscillator so E n = (n ) ω 0 with E = ω 0 = 0.25 ± 0.05 ev from our previous results. How do you get the expression above for the rotational energy? Is CO a rigid rotator? Absorption Carbon Monoxide Spectrum Energy (ev) Jerry Gilfoyle The Configurations of CO 3 / 26
8 The Plan 1 What is the kinetic and potential energy between the carbon and oxygen atoms in CO in the CM frame in cartesian and spherical coordinates? 2 How do you decompose the kinetic energy into radial and angular parts? 3 What is the Schroedinger equation for the rigid rotator? 4 What is the solution of the rigid rotator Schroedinger equation? Jerry Gilfoyle The Configurations of CO 4 / 26
9 Coordinates y r=r 1 r 2 r 1 R x r 2 Jerry Gilfoyle The Configurations of CO 5 / 26
10 Coordinates y m 1 r=r r 2 1 r 1 R cm m r 2 2 x Jerry Gilfoyle The Configurations of CO 5 / 26
11 Angular Momentum µ α Jerry Gilfoyle The Configurations of CO 6 / 26
12 Angular Momentum y p µ r p Τ α µ p r x α Jerry Gilfoyle The Configurations of CO 6 / 26
13 Going To 3D The Laplacian 2 ψ = [ ( 1 r 2 r 2 ) + 1 r r r 2 sin θ ( sin θ ) + θ θ 1 r 2 sin 2 θ 2 ] φ 2 ψ Jerry Gilfoyle The Configurations of CO 7 / 26
14 Going To 3D The Laplacian 2 ψ = [ ( 1 r 2 r 2 ) + 1 r r r 2 sin θ ( sin θ ) + θ θ 1 r 2 sin 2 θ 2 ] φ 2 ψ The Schroedinger Equation in 3D [ ( 2 1 2µ r 2 r 2 ) + 1 r r r 2 sin θ 2 2µ 2 ψ + V (r)ψ = Eψ ( sin θ ) + θ θ 1 r 2 sin 2 θ 2 ] φ 2 ψ + V (r)ψ = Eψ Jerry Gilfoyle The Configurations of CO 7 / 26
15 Going To 3D Steps along the way. 1 ( d sin θ dθ ) + m2 l sin θ dθ dθ sin 2 θ Θ = AΘ Jerry Gilfoyle The Configurations of CO 8 / 26
16 Going To 3D Steps along the way. 1 ( d sin θ dθ ) + m2 l sin θ dθ dθ sin 2 θ Θ = AΘ Legendre s Associated Equation (1 z 2 ) d 2 Θ dz 2 ( dθ 2z dz + A ) m2 l 1 z 2 Θ = 0 where z = cos θ Jerry Gilfoyle The Configurations of CO 8 / 26
17 Going To 3D Steps along the way. 1 ( d sin θ dθ ) + m2 l sin θ dθ dθ sin 2 θ Θ = AΘ Legendre s Associated Equation (1 z 2 ) d 2 Θ dz 2 ( dθ 2z dz + A And its recursion relationship when m l = 0 a k+2 = ) m2 l 1 z 2 Θ = 0 where z = cos θ k(k + 1) A (k + 2)(k + 1) a k Jerry Gilfoyle The Configurations of CO 8 / 26
18 Going To 3D We have the recursion relationship when m l = 0 Notice. a k+2 = k(k + 1) A (k + 2)(k + 1) a k Given a 0 a 2 a 4 and given a 1 a 3 a 5 so Θ(z) = a k z k = a k z k + a k z k and we choose a 0 = a 1 = 1. k=0 even odd Jerry Gilfoyle The Configurations of CO 9 / 26
19 Recall the Harmonic Oscillator Solution ξ = βx β 2 = mω 0 / log(f(ξ)) Red Dashed - e -ξ2 H 2 50 (ξ) Green Dashed - offset e ξ2 ξ Jerry Gilfoyle The Configurations of CO 10 / 26
20 Another Convergence Problem Truncated Calculation of Θ (z=1), m l =0 Θ (z) Θ(z = 1) = k max k=0 a k(1) k a k+2 = k(k+1) A (k+2)(k+1) a k k max Jerry Gilfoyle The Configurations of CO 11 / 26
21 Another Convergence Problem Θ (z) Truncated Calculation of Θ (z=1), m l =0 Θ(z = 1) = k max k=0 a k(1) k a k+2 = k(k+1) A (k+2)(k+1) a k k max Jerry Gilfoyle The Configurations of CO 12 / 26
22 Another Convergence Problem 5 Truncated Calculation of Θ (z=1), m l =0 4 Θ (z) Θ(z = 1) = k max k=0 a k(1) k a k+2 = k(k+1) A (k+2)(k+1) a k k max Jerry Gilfoyle The Configurations of CO 13 / 26
23 Another Convergence Problem 5 Truncated Calculation of Θ (z=1), m l =0 4 Θ (z) 3 2 Θ(z = 1) = k max k=0 a k(1) k a k+2 = k(k+1) A (k+2)(k+1) a k 1 Blue - Truncated Calculation of Θ(z=1), m l =0 Green - ln(k max ) k max Jerry Gilfoyle The Configurations of CO 14 / 26
24 Legendre Polynomials (m l = 0) a k+2 = k(k + 1) A (k + 2)(k + 1) a k m l = 0 a 0 = a 1 = 1 Θ = P l (z) = l even/odd a k z k z = cos θ First few polynomials. P 0 (cos θ) = 1 P 3 (cos θ) = 1 ( 2 5 cos 3 θ 3 cos θ ) P 1 (cos θ) = cos θ P 4 (cos θ) = 1 ( 8 35 cos 4 θ 30 cos 2 θ + 3 ) P 2 (cos θ) = 1 ( 2 3 cos 2 θ 1 ) P 5 (cos θ) = 1 ( 8 63 cos 5 θ 70 cos 3 θ + 15 cos θ ) Jerry Gilfoyle The Configurations of CO 15 / 26
25 Spherical Harmonics (m l = m) Θ(θ)Φ(φ) = Yl m (θ, φ) = 2l + 1 (l m)! 4π (l + m)! Pm l Y 0 0 (θ, φ) = 1 4π (cos θ)eimφ 3 Y1 1 (θ, φ) = sin θeiφ Y 8π (θ, φ) = sin θe iφ 8π Y 0 1 (θ, φ) = 3 4π cos θ Jerry Gilfoyle The Configurations of CO 16 / 26
26 Summary So Far L 2 2µr 2 = 2 2µ pr 2 2µ = 2 1 2µ r 2 r [ 1 r 2 sin θ θ ( sin θ θ ( r 2 ) r ) + 1 r 2 sin 2 θ 2 ] φ 2 m l = 0, ± 1 2, ±2 2, ±3 2, ±4 2, ±5 2,... [ 1 ( sin θ ) ] + m2 l sin θ θ θ sin 2 Θ = AΘ A = l(l + 1) θ L 2 φ s = 2 l(l + 1) φ s Jerry Gilfoyle The Configurations of CO 17 / 26
27 The Eigenvalues of ˆL 2 and ˆL z L = r p = L x î + L y ĵ + L z ˆk Jerry Gilfoyle The Configurations of CO 18 / 26
28 The Eigenvalues of ˆL 2 and ˆL z L = r p = L x î + L y ĵ + L z ˆk = (yp z zp y ) î + (zp x xp z ) ĵ + (xp y yp x ) ˆk Jerry Gilfoyle The Configurations of CO 18 / 26
29 The Eigenvalues of ˆL 2 and ˆL z L = r p = L x î + L y ĵ + L z ˆk = (yp z zp y ) î + (zp x xp z ) ĵ + (xp y yp x ) ˆk = i [( y d dz z d ) ( î + z d dy dx x d ) ( ĵ + x d dz dy y d ) ] ˆk dx Jerry Gilfoyle The Configurations of CO 18 / 26
30 The Eigenvalues of ˆL 2 and ˆL z L = r p = L x î + L y ĵ + L z ˆk = (yp z zp y ) î + (zp x xp z ) ĵ + (xp y yp x ) ˆk = i [( y d dz z d ) ( î + z d dy dx x d ) ( ĵ + x d dz dy y d ) ] ˆk dx Transformation from Cartesian to spherical coordinates: x = r sin θ cos φ y = r sin θ sin φ z = r cos θ Jerry Gilfoyle The Configurations of CO 18 / 26
31 The Eigenvalues of ˆL 2 and L z Jerry Gilfoyle The Configurations of CO 19 / 26
32 Is Carbon Monoxide A Rigid Rotator? Excited states of carbon monoxide (CO) can be observed by passing light through a cell containing CO and measuring the absorption spectrum shown below. The molecule can both vibrate and rotate at the same time. The rotational energy states of a rigid rotator are E l = 2 l(l + 1) 2I where I is the moment of inertia. The vibrational part of the energy can is described by the harmonic oscillator so E n = (n ) ω 0 with E = ω 0 = 0.25 ± 0.05 ev from our previous results. How does one obtain the expression above for the rotational energy? Is CO a rigid rotator? Carbon Monoxide Spectrum Absorption Energy (ev) Jerry Gilfoyle The Configurations of CO 20 / 26
33 Rotational Kinetic Energy Axis at the CM. Jerry Gilfoyle The Configurations of CO 21 / 26
34 Rotational Kinetic Energy Axis at the CM. Jerry Gilfoyle The Configurations of CO 21 / 26
35 Angular Momentum Jerry Gilfoyle The Configurations of CO 22 / 26
36 Angular Momentum y p rel r p θ φ m p r x Jerry Gilfoyle The Configurations of CO 22 / 26
37 Is Carbon Monoxide A Rigid Rotator? Excited states of carbon monoxide (CO) can be observed by passing light through a cell containing CO and measuring the absorption spectrum shown below. The molecule can both vibrate and rotate at the same time. The rotational energy states of a rigid rotator are E l = 2 l(l + 1) 2I where I is the moment of inertia. The vibrational part of the energy is described by the harmonic oscillator so E n = (n ) ω 0 with E = ω 0 = 0.25 ± 0.05 ev from our previous results. How does one obtain the expression above for the rotational energy? Is CO a rigid rotator? Carbon Monoxide Spectrum Absorption Energy (ev) Jerry Gilfoyle The Configurations of CO 23 / 26
38 Is Carbon Monoxide A Rigid Rotator? Excited states of carbon monoxide (CO) can be observed by passing light through a cell containing CO and measuring the absorption spectrum shown below. The molecule can both vibrate and rotate at the same time. The rotational energy states of a rigid rotator are E l = 2 l(l + 1) 2I where I is the moment of inertia. The vibrational part of the energy is described by the harmonic oscillator so E n = (n ) ω 0 with E = ω 0 = 0.25 ± 0.05 ev from our previous results. How does one obtain the expression above for the rotational energy? Is CO a rigid rotator? Carbon Monoxide Spectrum Absorption How far apart are the atoms? Energy (ev) Jerry Gilfoyle The Configurations of CO 23 / 26
39 Even More On Carbon Monoxide Electron beam results E = 0.25 ± 0.05 ev CO Absorption Spectrum Incident light Photon detector CO gas target Absorption Carbon Monoxide Spectrum Energy (ev) Jerry Gilfoyle The Configurations of CO 24 / 26
40 CO Vibration-Rotation Transitions l=5 n=1 l=5 n=0 Jerry Gilfoyle The Configurations of CO 25 / 26
41 CO Vibration-Rotation Transitions Vibration Rotation Adjacent Initial Angular Momenta l l+1 l=5 l=7 l=6 n=1 n=1 l=5 l=6 l=5 n=0 n=0 Jerry Gilfoyle The Configurations of CO 26 / 26
42 CO Vibration-Rotation Transitions Vibration Rotation Adjacent Initial Angular Momenta n=1 l=5 n=1 l=7 l=6 l=5 l=4 l=5 l=6 l=5 n=0 n=0 Jerry Gilfoyle The Configurations of CO 27 / 26
43 Carbon Monoxide Rotation Spectrum Rotational Spectra Probability ΔE Jerry Gilfoyle The Configurations of CO 28 / 26
Fun With Carbon Monoxide. p. 1/2
 Fun With Carbon Monoxide p. 1/2 p. 1/2 Fun With Carbon Monoxide E = 0.25 ± 0.05 ev Electron beam results p. 1/2 Fun With Carbon Monoxide E = 0.25 ± 0.05 ev Electron beam results C V (J/K-mole) 35 30 25
Fun With Carbon Monoxide p. 1/2 p. 1/2 Fun With Carbon Monoxide E = 0.25 ± 0.05 ev Electron beam results p. 1/2 Fun With Carbon Monoxide E = 0.25 ± 0.05 ev Electron beam results C V (J/K-mole) 35 30 25
eigenvalues eigenfunctions
 Born-Oppenheimer Approximation Atoms and molecules consist of heavy nuclei and light electrons. Consider (for simplicity) a diatomic molecule (e.g. HCl). Clamp/freeze the nuclei in space, a distance r
Born-Oppenheimer Approximation Atoms and molecules consist of heavy nuclei and light electrons. Consider (for simplicity) a diatomic molecule (e.g. HCl). Clamp/freeze the nuclei in space, a distance r
Quantum Theory of Angular Momentum and Atomic Structure
 Quantum Theory of Angular Momentum and Atomic Structure VBS/MRC Angular Momentum 0 Motivation...the questions Whence the periodic table? Concepts in Materials Science I VBS/MRC Angular Momentum 1 Motivation...the
Quantum Theory of Angular Momentum and Atomic Structure VBS/MRC Angular Momentum 0 Motivation...the questions Whence the periodic table? Concepts in Materials Science I VBS/MRC Angular Momentum 1 Motivation...the
Angular momentum. Quantum mechanics. Orbital angular momentum
 Angular momentum 1 Orbital angular momentum Consider a particle described by the Cartesian coordinates (x, y, z r and their conjugate momenta (p x, p y, p z p. The classical definition of the orbital angular
Angular momentum 1 Orbital angular momentum Consider a particle described by the Cartesian coordinates (x, y, z r and their conjugate momenta (p x, p y, p z p. The classical definition of the orbital angular
Chemistry 432 Problem Set 4 Spring 2018 Solutions
 Chemistry 4 Problem Set 4 Spring 18 Solutions 1. V I II III a b c A one-dimensional particle of mass m is confined to move under the influence of the potential x a V V (x) = a < x b b x c elsewhere and
Chemistry 4 Problem Set 4 Spring 18 Solutions 1. V I II III a b c A one-dimensional particle of mass m is confined to move under the influence of the potential x a V V (x) = a < x b b x c elsewhere and
Quantum Mechanics in 3-Dimensions
 Quantum Mechanics in 3-Dimensions Pavithran S Iyer, 2nd yr BSc Physics, Chennai Mathematical Institute Email: pavithra@cmi.ac.in August 28 th, 2009 1 Schrodinger equation in Spherical Coordinates 1.1 Transforming
Quantum Mechanics in 3-Dimensions Pavithran S Iyer, 2nd yr BSc Physics, Chennai Mathematical Institute Email: pavithra@cmi.ac.in August 28 th, 2009 1 Schrodinger equation in Spherical Coordinates 1.1 Transforming
Lecture 10. Central potential
 Lecture 10 Central potential 89 90 LECTURE 10. CENTRAL POTENTIAL 10.1 Introduction We are now ready to study a generic class of three-dimensional physical systems. They are the systems that have a central
Lecture 10 Central potential 89 90 LECTURE 10. CENTRAL POTENTIAL 10.1 Introduction We are now ready to study a generic class of three-dimensional physical systems. They are the systems that have a central
Lecture 5: Harmonic oscillator, Morse Oscillator, 1D Rigid Rotor
 Lecture 5: Harmonic oscillator, Morse Oscillator, 1D Rigid Rotor It turns out that the boundary condition of the wavefunction going to zero at infinity is sufficient to quantize the value of energy that
Lecture 5: Harmonic oscillator, Morse Oscillator, 1D Rigid Rotor It turns out that the boundary condition of the wavefunction going to zero at infinity is sufficient to quantize the value of energy that
Angular Momentum - set 1
 Angular Momentum - set PH0 - QM II August 6, 07 First of all, let us practise evaluating commutators. Consider these as warm up problems. Problem : Show the following commutation relations ˆx, ˆL x = 0,
Angular Momentum - set PH0 - QM II August 6, 07 First of all, let us practise evaluating commutators. Consider these as warm up problems. Problem : Show the following commutation relations ˆx, ˆL x = 0,
A Quantum Mechanical Model for the Vibration and Rotation of Molecules. Rigid Rotor
 A Quantum Mechanical Model for the Vibration and Rotation of Molecules Harmonic Oscillator Rigid Rotor Degrees of Freedom Translation: quantum mechanical model is particle in box or free particle. A molecule
A Quantum Mechanical Model for the Vibration and Rotation of Molecules Harmonic Oscillator Rigid Rotor Degrees of Freedom Translation: quantum mechanical model is particle in box or free particle. A molecule
Quantum Mechanics: The Hydrogen Atom
 Quantum Mechanics: The Hydrogen Atom 4th April 9 I. The Hydrogen Atom In this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen
Quantum Mechanics: The Hydrogen Atom 4th April 9 I. The Hydrogen Atom In this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen
Angular Momentum - set 1
 Angular Momentum - set PH0 - QM II August 6, 07 First of all, let us practise evaluating commutators. Consider these as warm up problems. Problem : Show the following commutation relations ˆx, ˆL x ] =
Angular Momentum - set PH0 - QM II August 6, 07 First of all, let us practise evaluating commutators. Consider these as warm up problems. Problem : Show the following commutation relations ˆx, ˆL x ] =
5.61 Lecture #17 Rigid Rotor I
 561 Fall 2017 Lecture #17 Page 1 561 Lecture #17 Rigid Rotor I Read McQuarrie: Chapters E and 6 Rigid Rotors molecular rotation and the universal angular part of all central force problems another exactly
561 Fall 2017 Lecture #17 Page 1 561 Lecture #17 Rigid Rotor I Read McQuarrie: Chapters E and 6 Rigid Rotors molecular rotation and the universal angular part of all central force problems another exactly
Chemistry 532 Practice Final Exam Fall 2012 Solutions
 Chemistry 53 Practice Final Exam Fall Solutions x e ax dx π a 3/ ; π sin 3 xdx 4 3 π cos nx dx π; sin θ cos θ + K x n e ax dx n! a n+ ; r r r r ˆL h r ˆL z h i φ ˆL x i hsin φ + cot θ cos φ θ φ ) ˆLy i
Chemistry 53 Practice Final Exam Fall Solutions x e ax dx π a 3/ ; π sin 3 xdx 4 3 π cos nx dx π; sin θ cos θ + K x n e ax dx n! a n+ ; r r r r ˆL h r ˆL z h i φ ˆL x i hsin φ + cot θ cos φ θ φ ) ˆLy i
Conservation of Linear Momentum : If a force F is acting on particle of mass m, then according to Newton s second law of motion, we have F = dp /dt =
 Conservation of Linear Momentum : If a force F is acting on particle of mass m, then according to Newton s second law of motion, we have F = dp /dt = d (mv) /dt where p =mv is linear momentum of particle
Conservation of Linear Momentum : If a force F is acting on particle of mass m, then according to Newton s second law of motion, we have F = dp /dt = d (mv) /dt where p =mv is linear momentum of particle
2m r2 (~r )+V (~r ) (~r )=E (~r )
 Review of the Hydrogen Atom The Schrodinger equation (for 1D, 2D, or 3D) can be expressed as: ~ 2 2m r2 (~r, t )+V (~r ) (~r, t )=i~ @ @t The Laplacian is the divergence of the gradient: r 2 =r r The time-independent
Review of the Hydrogen Atom The Schrodinger equation (for 1D, 2D, or 3D) can be expressed as: ~ 2 2m r2 (~r, t )+V (~r ) (~r, t )=i~ @ @t The Laplacian is the divergence of the gradient: r 2 =r r The time-independent
Atkins & de Paula: Atkins Physical Chemistry 9e Checklist of key ideas. Chapter 8: Quantum Theory: Techniques and Applications
 Atkins & de Paula: Atkins Physical Chemistry 9e Checklist of key ideas Chapter 8: Quantum Theory: Techniques and Applications TRANSLATIONAL MOTION wavefunction of free particle, ψ k = Ae ikx + Be ikx,
Atkins & de Paula: Atkins Physical Chemistry 9e Checklist of key ideas Chapter 8: Quantum Theory: Techniques and Applications TRANSLATIONAL MOTION wavefunction of free particle, ψ k = Ae ikx + Be ikx,
Angular momentum & spin
 Angular momentum & spin January 8, 2002 1 Angular momentum Angular momentum appears as a very important aspect of almost any quantum mechanical system, so we need to briefly review some basic properties
Angular momentum & spin January 8, 2002 1 Angular momentum Angular momentum appears as a very important aspect of almost any quantum mechanical system, so we need to briefly review some basic properties
Legendre s Equation. PHYS Southern Illinois University. October 13, 2016
 PHYS 500 - Southern Illinois University October 13, 2016 PHYS 500 - Southern Illinois University Legendre s Equation October 13, 2016 1 / 10 The Laplacian in Spherical Coordinates The Laplacian is given
PHYS 500 - Southern Illinois University October 13, 2016 PHYS 500 - Southern Illinois University Legendre s Equation October 13, 2016 1 / 10 The Laplacian in Spherical Coordinates The Laplacian is given
The Hydrogen Atom. Chapter 18. P. J. Grandinetti. Nov 6, Chem P. J. Grandinetti (Chem. 4300) The Hydrogen Atom Nov 6, / 41
 The Hydrogen Atom Chapter 18 P. J. Grandinetti Chem. 4300 Nov 6, 2017 P. J. Grandinetti (Chem. 4300) The Hydrogen Atom Nov 6, 2017 1 / 41 The Hydrogen Atom Hydrogen atom is simplest atomic system where
The Hydrogen Atom Chapter 18 P. J. Grandinetti Chem. 4300 Nov 6, 2017 P. J. Grandinetti (Chem. 4300) The Hydrogen Atom Nov 6, 2017 1 / 41 The Hydrogen Atom Hydrogen atom is simplest atomic system where
Diatomic Molecules. 7th May Hydrogen Molecule: Born-Oppenheimer Approximation
 Diatomic Molecules 7th May 2009 1 Hydrogen Molecule: Born-Oppenheimer Approximation In this discussion, we consider the formulation of the Schrodinger equation for diatomic molecules; this can be extended
Diatomic Molecules 7th May 2009 1 Hydrogen Molecule: Born-Oppenheimer Approximation In this discussion, we consider the formulation of the Schrodinger equation for diatomic molecules; this can be extended
The Hydrogen atom. Chapter The Schrödinger Equation. 2.2 Angular momentum
 Chapter 2 The Hydrogen atom In the previous chapter we gave a quick overview of the Bohr model, which is only really valid in the semiclassical limit. cf. section 1.7.) We now begin our task in earnest
Chapter 2 The Hydrogen atom In the previous chapter we gave a quick overview of the Bohr model, which is only really valid in the semiclassical limit. cf. section 1.7.) We now begin our task in earnest
Chemistry 795T. NC State University. Lecture 4. Vibrational and Rotational Spectroscopy
 Chemistry 795T Lecture 4 Vibrational and Rotational Spectroscopy NC State University The Dipole Moment Expansion The permanent dipole moment of a molecule oscillates about an equilibrium value as the molecule
Chemistry 795T Lecture 4 Vibrational and Rotational Spectroscopy NC State University The Dipole Moment Expansion The permanent dipole moment of a molecule oscillates about an equilibrium value as the molecule
We now turn to our first quantum mechanical problems that represent real, as
 84 Lectures 16-17 We now turn to our first quantum mechanical problems that represent real, as opposed to idealized, systems. These problems are the structures of atoms. We will begin first with hydrogen-like
84 Lectures 16-17 We now turn to our first quantum mechanical problems that represent real, as opposed to idealized, systems. These problems are the structures of atoms. We will begin first with hydrogen-like
Introduction to Quantum Physics and Models of Hydrogen Atom
 Introduction to Quantum Physics and Models of Hydrogen Atom Tien-Tsan Shieh Department of Applied Math National Chiao-Tung University November 7, 2012 Physics and Models of Hydrogen November Atom 7, 2012
Introduction to Quantum Physics and Models of Hydrogen Atom Tien-Tsan Shieh Department of Applied Math National Chiao-Tung University November 7, 2012 Physics and Models of Hydrogen November Atom 7, 2012
Angular Momentum. Classically the orbital angular momentum with respect to a fixed origin is. L = r p. = yp z. L x. zp y L y. = zp x. xpz L z.
 Angular momentum is an important concept in quantum theory, necessary for analyzing motion in 3D as well as intrinsic properties such as spin Classically the orbital angular momentum with respect to a
Angular momentum is an important concept in quantum theory, necessary for analyzing motion in 3D as well as intrinsic properties such as spin Classically the orbital angular momentum with respect to a
Quantum Physics 130A. April 1, 2006
 Quantum Physics 130A April 1, 2006 2 1 HOMEWORK 1: Due Friday, Apr. 14 1. A polished silver plate is hit by beams of photons of known energy. It is measured that the maximum electron energy is 3.1 ± 0.11
Quantum Physics 130A April 1, 2006 2 1 HOMEWORK 1: Due Friday, Apr. 14 1. A polished silver plate is hit by beams of photons of known energy. It is measured that the maximum electron energy is 3.1 ± 0.11
Two dimensional oscillator and central forces
 Two dimensional oscillator and central forces September 4, 04 Hooke s law in two dimensions Consider a radial Hooke s law force in -dimensions, F = kr where the force is along the radial unit vector and
Two dimensional oscillator and central forces September 4, 04 Hooke s law in two dimensions Consider a radial Hooke s law force in -dimensions, F = kr where the force is along the radial unit vector and
PHY 407 QUANTUM MECHANICS Fall 05 Problem set 1 Due Sep
 Problem set 1 Due Sep 15 2005 1. Let V be the set of all complex valued functions of a real variable θ, that are periodic with period 2π. That is u(θ + 2π) = u(θ), for all u V. (1) (i) Show that this V
Problem set 1 Due Sep 15 2005 1. Let V be the set of all complex valued functions of a real variable θ, that are periodic with period 2π. That is u(θ + 2π) = u(θ), for all u V. (1) (i) Show that this V
THE RIGID ROTOR. mrmr= + m K = I. r 2 2. I = m 1. m + m K = Diatomic molecule. m 1 r 1. r 2 m 2. I moment of inertia. (center of mass) COM K.E.
 5.6 Fall 4 Lecture #7-9 page Diatoic olecule THE RIGID ROTOR r r r r rr= (center of ass) COM r K.E. K = r r K = I ( = r r ) I oent of inertia I = r r = µ r µ = (reduced ass) K = µ r = µv z Prob l e reduced
5.6 Fall 4 Lecture #7-9 page Diatoic olecule THE RIGID ROTOR r r r r rr= (center of ass) COM r K.E. K = r r K = I ( = r r ) I oent of inertia I = r r = µ r µ = (reduced ass) K = µ r = µv z Prob l e reduced
Quantum Mechanics in Three Dimensions
 Physics 342 Lecture 21 Quantum Mechanics in Three Dimensions Lecture 21 Physics 342 Quantum Mechanics I Monday, March 22nd, 21 We are used to the temporal separation that gives, for example, the timeindependent
Physics 342 Lecture 21 Quantum Mechanics in Three Dimensions Lecture 21 Physics 342 Quantum Mechanics I Monday, March 22nd, 21 We are used to the temporal separation that gives, for example, the timeindependent
5. Spherically symmetric potentials
 TFY4215/FY1006 Lecture notes 5 1 Lecture notes 5 5. Spherically symmetric potentials Chapter 5 of FY1006/TFY4215 Spherically symmetric potentials is covered by sections 5.1 and 5.4 5.7 in Hemmer s bok,
TFY4215/FY1006 Lecture notes 5 1 Lecture notes 5 5. Spherically symmetric potentials Chapter 5 of FY1006/TFY4215 Spherically symmetric potentials is covered by sections 5.1 and 5.4 5.7 in Hemmer s bok,
FORMULA SHEET FOR QUIZ 2 Exam Date: November 8, 2017
 MASSACHUSETTS INSTITUTE OF TECHNOLOGY Physics Department Physics 8.07: Electromagnetism II November 5, 207 Prof. Alan Guth FORMULA SHEET FOR QUIZ 2 Exam Date: November 8, 207 A few items below are marked
MASSACHUSETTS INSTITUTE OF TECHNOLOGY Physics Department Physics 8.07: Electromagnetism II November 5, 207 Prof. Alan Guth FORMULA SHEET FOR QUIZ 2 Exam Date: November 8, 207 A few items below are marked
Chapter 6: Vector Analysis
 Chapter 6: Vector Analysis We use derivatives and various products of vectors in all areas of physics. For example, Newton s 2nd law is F = m d2 r. In electricity dt 2 and magnetism, we need surface and
Chapter 6: Vector Analysis We use derivatives and various products of vectors in all areas of physics. For example, Newton s 2nd law is F = m d2 r. In electricity dt 2 and magnetism, we need surface and
( ) electron gives S = 1/2 and L = l 1
 Practice Modern Physics II, W018, Set 1 Question 1 Energy Level Diagram of Boron ion B + For neutral B, Z = 5 (A) Draw the fine-structure diagram of B + that includes all n = 3 states Label the states
Practice Modern Physics II, W018, Set 1 Question 1 Energy Level Diagram of Boron ion B + For neutral B, Z = 5 (A) Draw the fine-structure diagram of B + that includes all n = 3 states Label the states
A few principles of classical and quantum mechanics
 A few principles of classical and quantum mechanics The classical approach: In classical mechanics, we usually (but not exclusively) solve Newton s nd law of motion relating the acceleration a of the system
A few principles of classical and quantum mechanics The classical approach: In classical mechanics, we usually (but not exclusively) solve Newton s nd law of motion relating the acceleration a of the system
1 Commutators (10 pts)
 Final Exam Solutions 37A Fall 0 I. Siddiqi / E. Dodds Commutators 0 pts) ) Consider the operator  = Ĵx Ĵ y + ĴyĴx where J i represents the total angular momentum in the ith direction. a) Express both
Final Exam Solutions 37A Fall 0 I. Siddiqi / E. Dodds Commutators 0 pts) ) Consider the operator  = Ĵx Ĵ y + ĴyĴx where J i represents the total angular momentum in the ith direction. a) Express both
1/30. Rigid Body Rotations. Dave Frank
 . 1/3 Rigid Body Rotations Dave Frank A Point Particle and Fundamental Quantities z 2/3 m v ω r y x Angular Velocity v = dr dt = ω r Kinetic Energy K = 1 2 mv2 Momentum p = mv Rigid Bodies We treat a rigid
. 1/3 Rigid Body Rotations Dave Frank A Point Particle and Fundamental Quantities z 2/3 m v ω r y x Angular Velocity v = dr dt = ω r Kinetic Energy K = 1 2 mv2 Momentum p = mv Rigid Bodies We treat a rigid
8.04: Quantum Mechanics Professor Allan Adams Massachusetts Institute of Technology Wednesday April Exam 2
 8.04: Quantum Mechanics Professor Allan Adams Massachusetts Institute of Technology Wednesday April 18 2012 Exam 2 Last Name: First Name: Check Recitation Instructor Time R01 Barton Zwiebach 10:00 R02
8.04: Quantum Mechanics Professor Allan Adams Massachusetts Institute of Technology Wednesday April 18 2012 Exam 2 Last Name: First Name: Check Recitation Instructor Time R01 Barton Zwiebach 10:00 R02
ONE AND MANY ELECTRON ATOMS Chapter 15
 See Week 8 lecture notes. This is exactly the same as the Hamiltonian for nonrigid rotation. In Week 8 lecture notes it was shown that this is the operator for Lˆ 2, the square of the angular momentum.
See Week 8 lecture notes. This is exactly the same as the Hamiltonian for nonrigid rotation. In Week 8 lecture notes it was shown that this is the operator for Lˆ 2, the square of the angular momentum.
CHAPTER 13 LECTURE NOTES
 CHAPTER 13 LECTURE NOTES Spectroscopy is concerned with the measurement of (a) the wavelengths (or frequencies) at which molecules absorb/emit energy, and (b) the amount of radiation absorbed at these
CHAPTER 13 LECTURE NOTES Spectroscopy is concerned with the measurement of (a) the wavelengths (or frequencies) at which molecules absorb/emit energy, and (b) the amount of radiation absorbed at these
Summary: angular momentum derivation
 Summary: angular momentum derivation L = r p L x = yp z zp y, etc. [x, p y ] = 0, etc. (-) (-) (-3) Angular momentum commutation relations [L x, L y ] = i hl z (-4) [L i, L j ] = i hɛ ijk L k (-5) Levi-Civita
Summary: angular momentum derivation L = r p L x = yp z zp y, etc. [x, p y ] = 0, etc. (-) (-) (-3) Angular momentum commutation relations [L x, L y ] = i hl z (-4) [L i, L j ] = i hɛ ijk L k (-5) Levi-Civita
Solving Schrödinger s Wave Equation - (2)
 Chapter 14 Solving Schrödinger s Wave Equation - (2) Topics Examples of quantum mechanical tunnelling: radioactive α-decay, the ammonia molecule, tunnel diodes, the scanning tunnelling microscope The quantisation
Chapter 14 Solving Schrödinger s Wave Equation - (2) Topics Examples of quantum mechanical tunnelling: radioactive α-decay, the ammonia molecule, tunnel diodes, the scanning tunnelling microscope The quantisation
The 3 dimensional Schrödinger Equation
 Chapter 6 The 3 dimensional Schrödinger Equation 6.1 Angular Momentum To study how angular momentum is represented in quantum mechanics we start by reviewing the classical vector of orbital angular momentum
Chapter 6 The 3 dimensional Schrödinger Equation 6.1 Angular Momentum To study how angular momentum is represented in quantum mechanics we start by reviewing the classical vector of orbital angular momentum
Physics 228 Today: Ch 41: 1-3: 3D quantum mechanics, hydrogen atom
 Physics 228 Today: Ch 41: 1-3: 3D quantum mechanics, hydrogen atom Website: Sakai 01:750:228 or www.physics.rutgers.edu/ugrad/228 Happy April Fools Day Example / Worked Problems What is the ratio of the
Physics 228 Today: Ch 41: 1-3: 3D quantum mechanics, hydrogen atom Website: Sakai 01:750:228 or www.physics.rutgers.edu/ugrad/228 Happy April Fools Day Example / Worked Problems What is the ratio of the
Vibrations and Rotations of Diatomic Molecules
 Chapter 6 Vibrations and Rotations of Diatomic Molecules With the electronic part of the problem treated in the previous chapter, the nuclear motion shall occupy our attention in this one. In many ways
Chapter 6 Vibrations and Rotations of Diatomic Molecules With the electronic part of the problem treated in the previous chapter, the nuclear motion shall occupy our attention in this one. In many ways
Collection of formulae Quantum mechanics. Basic Formulas Division of Material Science Hans Weber. Operators
 Basic Formulas 17-1-1 Division of Material Science Hans Weer The de Broglie wave length λ = h p The Schrödinger equation Hψr,t = i h t ψr,t Stationary states Hψr,t = Eψr,t Collection of formulae Quantum
Basic Formulas 17-1-1 Division of Material Science Hans Weer The de Broglie wave length λ = h p The Schrödinger equation Hψr,t = i h t ψr,t Stationary states Hψr,t = Eψr,t Collection of formulae Quantum
PHYS 771, Quantum Mechanics, Final Exam, Fall 2011 Instructor: Dr. A. G. Petukhov. Solutions
 PHYS 771, Quantum Mechanics, Final Exam, Fall 11 Instructor: Dr. A. G. Petukhov Solutions 1. Apply WKB approximation to a particle moving in a potential 1 V x) = mω x x > otherwise Find eigenfunctions,
PHYS 771, Quantum Mechanics, Final Exam, Fall 11 Instructor: Dr. A. G. Petukhov Solutions 1. Apply WKB approximation to a particle moving in a potential 1 V x) = mω x x > otherwise Find eigenfunctions,
PHY4604 Introduction to Quantum Mechanics Fall 2004 Final Exam SOLUTIONS December 17, 2004, 7:30 a.m.- 9:30 a.m.
 PHY464 Introduction to Quantum Mechanics Fall 4 Final Eam SOLUTIONS December 7, 4, 7:3 a.m.- 9:3 a.m. No other materials allowed. If you can t do one part of a problem, solve subsequent parts in terms
PHY464 Introduction to Quantum Mechanics Fall 4 Final Eam SOLUTIONS December 7, 4, 7:3 a.m.- 9:3 a.m. No other materials allowed. If you can t do one part of a problem, solve subsequent parts in terms
Spherical Coordinates and Legendre Functions
 Spherical Coordinates and Legendre Functions Spherical coordinates Let s adopt the notation for spherical coordinates that is standard in physics: φ = longitude or azimuth, θ = colatitude ( π 2 latitude)
Spherical Coordinates and Legendre Functions Spherical coordinates Let s adopt the notation for spherical coordinates that is standard in physics: φ = longitude or azimuth, θ = colatitude ( π 2 latitude)
Physics 2203, Fall 2012 Modern Physics
 Physics 03, Fall 01 Modern Physics. Monday, Oct. 8 th, 01. Finish up examples for Ch. 8 Computer Exercise. Announcements: Take home Exam #1: Average 84.1, Average both 63.0 Quiz on Friday on Ch. 8 or Ch.
Physics 03, Fall 01 Modern Physics. Monday, Oct. 8 th, 01. Finish up examples for Ch. 8 Computer Exercise. Announcements: Take home Exam #1: Average 84.1, Average both 63.0 Quiz on Friday on Ch. 8 or Ch.
Indicate if the statement is True (T) or False (F) by circling the letter (1 pt each):
 Indicate if the statement is (T) or False (F) by circling the letter (1 pt each): False 1. In order to ensure that all observables are real valued, the eigenfunctions for an operator must also be real
Indicate if the statement is (T) or False (F) by circling the letter (1 pt each): False 1. In order to ensure that all observables are real valued, the eigenfunctions for an operator must also be real
Quantum mechanics (QM) deals with systems on atomic scale level, whose behaviours cannot be described by classical mechanics.
 A 10-MINUTE RATHER QUICK INTRODUCTION TO QUANTUM MECHANICS 1. What is quantum mechanics (as opposed to classical mechanics)? Quantum mechanics (QM) deals with systems on atomic scale level, whose behaviours
A 10-MINUTE RATHER QUICK INTRODUCTION TO QUANTUM MECHANICS 1. What is quantum mechanics (as opposed to classical mechanics)? Quantum mechanics (QM) deals with systems on atomic scale level, whose behaviours
PLANAR KINETICS OF A RIGID BODY FORCE AND ACCELERATION
 PLANAR KINETICS OF A RIGID BODY FORCE AND ACCELERATION I. Moment of Inertia: Since a body has a definite size and shape, an applied nonconcurrent force system may cause the body to both translate and rotate.
PLANAR KINETICS OF A RIGID BODY FORCE AND ACCELERATION I. Moment of Inertia: Since a body has a definite size and shape, an applied nonconcurrent force system may cause the body to both translate and rotate.
PHYS 404 Lecture 1: Legendre Functions
 PHYS 404 Lecture 1: Legendre Functions Dr. Vasileios Lempesis PHYS 404 - LECTURE 1 DR. V. LEMPESIS 1 Legendre Functions physical justification Legendre functions or Legendre polynomials are the solutions
PHYS 404 Lecture 1: Legendre Functions Dr. Vasileios Lempesis PHYS 404 - LECTURE 1 DR. V. LEMPESIS 1 Legendre Functions physical justification Legendre functions or Legendre polynomials are the solutions
1 Schroenger s Equation for the Hydrogen Atom
 Schroenger s Equation for the Hydrogen Atom Here is the Schroedinger equation in D in spherical polar coordinates. Note that the definitions of θ and φ are the exact reverse of what they are in mathematics.
Schroenger s Equation for the Hydrogen Atom Here is the Schroedinger equation in D in spherical polar coordinates. Note that the definitions of θ and φ are the exact reverse of what they are in mathematics.
Lecture 4 Quantum mechanics in more than one-dimension
 Lecture 4 Quantum mechanics in more than one-dimension Background Previously, we have addressed quantum mechanics of 1d systems and explored bound and unbound (scattering) states. Although general concepts
Lecture 4 Quantum mechanics in more than one-dimension Background Previously, we have addressed quantum mechanics of 1d systems and explored bound and unbound (scattering) states. Although general concepts
Computational Spectroscopy III. Spectroscopic Hamiltonians
 Computational Spectroscopy III. Spectroscopic Hamiltonians (e) Elementary operators for the harmonic oscillator (f) Elementary operators for the asymmetric rotor (g) Implementation of complex Hamiltonians
Computational Spectroscopy III. Spectroscopic Hamiltonians (e) Elementary operators for the harmonic oscillator (f) Elementary operators for the asymmetric rotor (g) Implementation of complex Hamiltonians
Lecture 4 Quantum mechanics in more than one-dimension
 Lecture 4 Quantum mechanics in more than one-dimension Background Previously, we have addressed quantum mechanics of 1d systems and explored bound and unbound (scattering) states. Although general concepts
Lecture 4 Quantum mechanics in more than one-dimension Background Previously, we have addressed quantum mechanics of 1d systems and explored bound and unbound (scattering) states. Although general concepts
1.6. Quantum mechanical description of the hydrogen atom
 29.6. Quantum mechanical description of the hydrogen atom.6.. Hamiltonian for the hydrogen atom Atomic units To avoid dealing with very small numbers, let us introduce the so called atomic units : Quantity
29.6. Quantum mechanical description of the hydrogen atom.6.. Hamiltonian for the hydrogen atom Atomic units To avoid dealing with very small numbers, let us introduce the so called atomic units : Quantity
St Hugh s 2 nd Year: Quantum Mechanics II. Reading. Topics. The following sources are recommended for this tutorial:
 St Hugh s 2 nd Year: Quantum Mechanics II Reading The following sources are recommended for this tutorial: The key text (especially here in Oxford) is Molecular Quantum Mechanics, P. W. Atkins and R. S.
St Hugh s 2 nd Year: Quantum Mechanics II Reading The following sources are recommended for this tutorial: The key text (especially here in Oxford) is Molecular Quantum Mechanics, P. W. Atkins and R. S.
Why Is CO 2 a Greenhouse Gas?
 Why Is CO 2 a Greenhouse Gas? The Earth is warming and the cause is the increase in greenhouse gases like carbon dioxide (CO 2 ) in the atmosphere. Carbon dioxide is a linear, triatomic molecule with a
Why Is CO 2 a Greenhouse Gas? The Earth is warming and the cause is the increase in greenhouse gases like carbon dioxide (CO 2 ) in the atmosphere. Carbon dioxide is a linear, triatomic molecule with a
H atom solution. 1 Introduction 2. 2 Coordinate system 2. 3 Variable separation 4
 H atom solution Contents 1 Introduction 2 2 Coordinate system 2 3 Variable separation 4 4 Wavefunction solutions 6 4.1 Solution for Φ........................... 6 4.2 Solution for Θ...........................
H atom solution Contents 1 Introduction 2 2 Coordinate system 2 3 Variable separation 4 4 Wavefunction solutions 6 4.1 Solution for Φ........................... 6 4.2 Solution for Θ...........................
Physics 401: Quantum Mechanics I Chapter 4
 Physics 401: Quantum Mechanics I Chapter 4 Are you here today? A. Yes B. No C. After than midterm? 3-D Schroedinger Equation The ground state energy of the particle in a 3D box is ( 1 2 +1 2 +1 2 ) π2
Physics 401: Quantum Mechanics I Chapter 4 Are you here today? A. Yes B. No C. After than midterm? 3-D Schroedinger Equation The ground state energy of the particle in a 3D box is ( 1 2 +1 2 +1 2 ) π2
CHAPTER 8 The Quantum Theory of Motion
 I. Translational motion. CHAPTER 8 The Quantum Theory of Motion A. Single particle in free space, 1-D. 1. Schrodinger eqn H ψ = Eψ! 2 2m d 2 dx 2 ψ = Eψ ; no boundary conditions 2. General solution: ψ
I. Translational motion. CHAPTER 8 The Quantum Theory of Motion A. Single particle in free space, 1-D. 1. Schrodinger eqn H ψ = Eψ! 2 2m d 2 dx 2 ψ = Eψ ; no boundary conditions 2. General solution: ψ
MATH325 - QUANTUM MECHANICS - SOLUTION SHEET 11
 MATH35 - QUANTUM MECHANICS - SOLUTION SHEET. The Hamiltonian for a particle of mass m moving in three dimensions under the influence of a three-dimensional harmonic oscillator potential is Ĥ = h m + mω
MATH35 - QUANTUM MECHANICS - SOLUTION SHEET. The Hamiltonian for a particle of mass m moving in three dimensions under the influence of a three-dimensional harmonic oscillator potential is Ĥ = h m + mω
ψ s a ˆn a s b ˆn b ψ Hint: Because the state is spherically symmetric the answer can depend only on the angle between the two directions.
 1. Quantum Mechanics (Fall 2004) Two spin-half particles are in a state with total spin zero. Let ˆn a and ˆn b be unit vectors in two arbitrary directions. Calculate the expectation value of the product
1. Quantum Mechanics (Fall 2004) Two spin-half particles are in a state with total spin zero. Let ˆn a and ˆn b be unit vectors in two arbitrary directions. Calculate the expectation value of the product
Atoms 2012 update -- start with single electron: H-atom
 Atoms 2012 update -- start with single electron: H-atom x z φ θ e -1 y 3-D problem - free move in x, y, z - easier if change coord. systems: Cartesian Spherical Coordinate (x, y, z) (r, θ, φ) Reason: V(r)
Atoms 2012 update -- start with single electron: H-atom x z φ θ e -1 y 3-D problem - free move in x, y, z - easier if change coord. systems: Cartesian Spherical Coordinate (x, y, z) (r, θ, φ) Reason: V(r)
An Aside: Application of Rotational Motion. Vibrational-Rotational Spectroscopy
 An Aside: Application of Rotational Motion Vibrational-Rotational Spectroscopy Rotational Excited States of a Diatomic Molecule are Significantly Populated at Room Temperature We can estimate the relative
An Aside: Application of Rotational Motion Vibrational-Rotational Spectroscopy Rotational Excited States of a Diatomic Molecule are Significantly Populated at Room Temperature We can estimate the relative
PHYS 3313 Section 001 Lecture # 22
 PHYS 3313 Section 001 Lecture # 22 Dr. Barry Spurlock Simple Harmonic Oscillator Barriers and Tunneling Alpha Particle Decay Schrodinger Equation on Hydrogen Atom Solutions for Schrodinger Equation for
PHYS 3313 Section 001 Lecture # 22 Dr. Barry Spurlock Simple Harmonic Oscillator Barriers and Tunneling Alpha Particle Decay Schrodinger Equation on Hydrogen Atom Solutions for Schrodinger Equation for
Quantization of the E-M field
 April 6, 20 Lecture XXVI Quantization of the E-M field 2.0. Electric quadrupole transition If E transitions are forbidden by selection rules, then we consider the next term in the expansion of the spatial
April 6, 20 Lecture XXVI Quantization of the E-M field 2.0. Electric quadrupole transition If E transitions are forbidden by selection rules, then we consider the next term in the expansion of the spatial
Angular Momentum Properties
 Cheistry 460 Fall 017 Dr. Jean M. Standard October 30, 017 Angular Moentu Properties Classical Definition of Angular Moentu In classical echanics, the angular oentu vector L is defined as L = r p, (1)
Cheistry 460 Fall 017 Dr. Jean M. Standard October 30, 017 Angular Moentu Properties Classical Definition of Angular Moentu In classical echanics, the angular oentu vector L is defined as L = r p, (1)
1 r 2 sin 2 θ. This must be the case as we can see by the following argument + L2
 PHYS 4 3. The momentum operator in three dimensions is p = i Therefore the momentum-squared operator is [ p 2 = 2 2 = 2 r 2 ) + r 2 r r r 2 sin θ We notice that this can be written as sin θ ) + θ θ r 2
PHYS 4 3. The momentum operator in three dimensions is p = i Therefore the momentum-squared operator is [ p 2 = 2 2 = 2 r 2 ) + r 2 r r r 2 sin θ We notice that this can be written as sin θ ) + θ θ r 2
Electromagnetic Theory I
 Electromagnetic Theory I Final Examination 18 December 2009, 12:30-2:30 pm Instructions: Answer the following 10 questions, each of which is worth 10 points. Explain your reasoning in each case. Use SI
Electromagnetic Theory I Final Examination 18 December 2009, 12:30-2:30 pm Instructions: Answer the following 10 questions, each of which is worth 10 points. Explain your reasoning in each case. Use SI
Spherical Harmonics on S 2
 7 August 00 1 Spherical Harmonics on 1 The Laplace-Beltrami Operator In what follows, we describe points on using the parametrization x = cos ϕ sin θ, y = sin ϕ sin θ, z = cos θ, where θ is the colatitude
7 August 00 1 Spherical Harmonics on 1 The Laplace-Beltrami Operator In what follows, we describe points on using the parametrization x = cos ϕ sin θ, y = sin ϕ sin θ, z = cos θ, where θ is the colatitude
Solution to Problem Set No. 6: Time Independent Perturbation Theory
 Solution to Problem Set No. 6: Time Independent Perturbation Theory Simon Lin December, 17 1 The Anharmonic Oscillator 1.1 As a first step we invert the definitions of creation and annihilation operators
Solution to Problem Set No. 6: Time Independent Perturbation Theory Simon Lin December, 17 1 The Anharmonic Oscillator 1.1 As a first step we invert the definitions of creation and annihilation operators
Chapter 4. Q. A hydrogen atom starts out in the following linear combination of the stationary. (ψ ψ 21 1 ). (1)
 Tor Kjellsson Stockholm University Chapter 4 4.5 Q. A hydrogen atom starts out in the following linear combination of the stationary states n, l, m =,, and n, l, m =,, : Ψr, 0 = ψ + ψ. a Q. Construct Ψr,
Tor Kjellsson Stockholm University Chapter 4 4.5 Q. A hydrogen atom starts out in the following linear combination of the stationary states n, l, m =,, and n, l, m =,, : Ψr, 0 = ψ + ψ. a Q. Construct Ψr,
The one and three-dimensional particle in a box are prototypes of bound systems. As we
 6 Lecture 10 The one and three-dimensional particle in a box are prototypes of bound systems. As we move on in our study of quantum chemistry, we'll be considering bound systems that are more and more
6 Lecture 10 The one and three-dimensional particle in a box are prototypes of bound systems. As we move on in our study of quantum chemistry, we'll be considering bound systems that are more and more
Time part of the equation can be separated by substituting independent equation
 Lecture 9 Schrödinger Equation in 3D and Angular Momentum Operator In this section we will construct 3D Schrödinger equation and we give some simple examples. In this course we will consider problems where
Lecture 9 Schrödinger Equation in 3D and Angular Momentum Operator In this section we will construct 3D Schrödinger equation and we give some simple examples. In this course we will consider problems where
Physics 221A Fall 1996 Notes 12 Orbital Angular Momentum and Spherical Harmonics
 Physics 221A Fall 1996 Notes 12 Orbital Angular Momentum and Spherical Harmonics We now consider the spatial degrees of freedom of a particle moving in 3-dimensional space, which of course is an important
Physics 221A Fall 1996 Notes 12 Orbital Angular Momentum and Spherical Harmonics We now consider the spatial degrees of freedom of a particle moving in 3-dimensional space, which of course is an important
5.1 Classical Harmonic Oscillator
 Chapter 5 Harmonic Oscillator 5.1 Classical Harmonic Oscillator m l o l Hooke s Law give the force exerting on the mass as: f = k(l l o ) where l o is the equilibrium length of the spring and k is the
Chapter 5 Harmonic Oscillator 5.1 Classical Harmonic Oscillator m l o l Hooke s Law give the force exerting on the mass as: f = k(l l o ) where l o is the equilibrium length of the spring and k is the
16.1. PROBLEM SET I 197
 6.. PROBLEM SET I 97 Answers: Problem set I. a In one dimension, the current operator is specified by ĵ = m ψ ˆpψ + ψˆpψ. Applied to the left hand side of the system outside the region of the potential,
6.. PROBLEM SET I 97 Answers: Problem set I. a In one dimension, the current operator is specified by ĵ = m ψ ˆpψ + ψˆpψ. Applied to the left hand side of the system outside the region of the potential,
Quantum Mechanics II Lecture 11 (www.sp.phy.cam.ac.uk/~dar11/pdf) David Ritchie
 Quantum Mechanics II Lecture (www.sp.phy.cam.ac.u/~dar/pdf) David Ritchie Michaelmas. So far we have found solutions to Section 4:Transitions Ĥ ψ Eψ Solutions stationary states time dependence with time
Quantum Mechanics II Lecture (www.sp.phy.cam.ac.u/~dar/pdf) David Ritchie Michaelmas. So far we have found solutions to Section 4:Transitions Ĥ ψ Eψ Solutions stationary states time dependence with time
Understand the basic principles of spectroscopy using selection rules and the energy levels. Derive Hund s Rule from the symmetrization postulate.
 CHEM 5314: Advanced Physical Chemistry Overall Goals: Use quantum mechanics to understand that molecules have quantized translational, rotational, vibrational, and electronic energy levels. In a large
CHEM 5314: Advanced Physical Chemistry Overall Goals: Use quantum mechanics to understand that molecules have quantized translational, rotational, vibrational, and electronic energy levels. In a large
Rotations and vibrations of polyatomic molecules
 Rotations and vibrations of polyatomic molecules When the potential energy surface V( R 1, R 2,..., R N ) is known we can compute the energy levels of the molecule. These levels can be an effect of: Rotation
Rotations and vibrations of polyatomic molecules When the potential energy surface V( R 1, R 2,..., R N ) is known we can compute the energy levels of the molecule. These levels can be an effect of: Rotation
The Hydrogen Atom Chapter 20
 4/4/17 Quantum mechanical treatment of the H atom: Model; The Hydrogen Atom Chapter 1 r -1 Electron moving aroundpositively charged nucleus in a Coulombic field from the nucleus. Potential energy term
4/4/17 Quantum mechanical treatment of the H atom: Model; The Hydrogen Atom Chapter 1 r -1 Electron moving aroundpositively charged nucleus in a Coulombic field from the nucleus. Potential energy term
Lecture 3. Solving the Non-Relativistic Schroedinger Equation for a spherically symmetric potential
 Lecture 3 Last lecture we were in the middle of deriving the energies of the bound states of the Λ in the nucleus. We will continue with solving the non-relativistic Schroedinger equation for a spherically
Lecture 3 Last lecture we were in the middle of deriving the energies of the bound states of the Λ in the nucleus. We will continue with solving the non-relativistic Schroedinger equation for a spherically
MULTIVARIABLE INTEGRATION
 MULTIVARIABLE INTEGRATION (SPHERICAL POLAR COORDINATES) Question 1 a) Determine with the aid of a diagram an expression for the volume element in r, θ, ϕ. spherical polar coordinates, ( ) [You may not
MULTIVARIABLE INTEGRATION (SPHERICAL POLAR COORDINATES) Question 1 a) Determine with the aid of a diagram an expression for the volume element in r, θ, ϕ. spherical polar coordinates, ( ) [You may not
Topics Covered: Motion in a central potential, spherical harmonic oscillator, hydrogen atom, orbital electric and magnetic dipole moments
 PHYS85 Quantum Mechanics I, Fall 9 HOMEWORK ASSIGNMENT Topics Covered: Motion in a central potential, spherical harmonic oscillator, hydrogen atom, orbital electric and magnetic dipole moments. [ pts]
PHYS85 Quantum Mechanics I, Fall 9 HOMEWORK ASSIGNMENT Topics Covered: Motion in a central potential, spherical harmonic oscillator, hydrogen atom, orbital electric and magnetic dipole moments. [ pts]
CHEM-UA 127: Advanced General Chemistry I
 1 CHEM-UA 127: Advanced General Chemistry I Notes for Lecture 11 Nowthatwehaveintroducedthebasicconceptsofquantummechanics, wecanstarttoapplythese conceptsto build up matter, starting from its most elementary
1 CHEM-UA 127: Advanced General Chemistry I Notes for Lecture 11 Nowthatwehaveintroducedthebasicconceptsofquantummechanics, wecanstarttoapplythese conceptsto build up matter, starting from its most elementary
Phys 622 Problems Chapter 5
 1 Phys 622 Problems Chapter 5 Problem 1 The correct basis set of perturbation theory Consider the relativistic correction to the electron-nucleus interaction H LS = α L S, also known as the spin-orbit
1 Phys 622 Problems Chapter 5 Problem 1 The correct basis set of perturbation theory Consider the relativistic correction to the electron-nucleus interaction H LS = α L S, also known as the spin-orbit
Problem 1: Spin 1 2. particles (10 points)
 Problem 1: Spin 1 particles 1 points 1 Consider a system made up of spin 1/ particles. If one measures the spin of the particles, one can only measure spin up or spin down. The general spin state of a
Problem 1: Spin 1 particles 1 points 1 Consider a system made up of spin 1/ particles. If one measures the spin of the particles, one can only measure spin up or spin down. The general spin state of a
Physics 4617/5617: Quantum Physics Course Lecture Notes
 Physics 467/567: Quantum Physics Course Lecture Notes Dr. Donald G. Luttermoser East Tennessee State University Edition 5. Abstract These class notes are designed for use of the instructor and students
Physics 467/567: Quantum Physics Course Lecture Notes Dr. Donald G. Luttermoser East Tennessee State University Edition 5. Abstract These class notes are designed for use of the instructor and students
NIU PHYS 500, Fall 2006 Classical Mechanics Solutions for HW6. Solutions
 NIU PHYS 500, Fall 006 Classical Mechanics Solutions for HW6 Assignment: HW6 [40 points] Assigned: 006/11/10 Due: 006/11/17 Solutions P6.1 [4 + 3 + 3 = 10 points] Consider a particle of mass m moving in
NIU PHYS 500, Fall 006 Classical Mechanics Solutions for HW6 Assignment: HW6 [40 points] Assigned: 006/11/10 Due: 006/11/17 Solutions P6.1 [4 + 3 + 3 = 10 points] Consider a particle of mass m moving in
Announcements. 1. Do not bring the yellow equation sheets to the miderm. Idential sheets will be attached to the problems.
 Announcements 1. Do not bring the yellow equation sheets to the miderm. Idential sheets will be attached to the problems. 2. Some PRS transmitters are missing. Please, bring them back! 1 Kinematics Displacement
Announcements 1. Do not bring the yellow equation sheets to the miderm. Idential sheets will be attached to the problems. 2. Some PRS transmitters are missing. Please, bring them back! 1 Kinematics Displacement
14. Structure of Nuclei
 14. Structure of Nuclei Particle and Nuclear Physics Dr. Tina Potter Dr. Tina Potter 14. Structure of Nuclei 1 In this section... Magic Numbers The Nuclear Shell Model Excited States Dr. Tina Potter 14.
14. Structure of Nuclei Particle and Nuclear Physics Dr. Tina Potter Dr. Tina Potter 14. Structure of Nuclei 1 In this section... Magic Numbers The Nuclear Shell Model Excited States Dr. Tina Potter 14.
Atoms 2010 update -- start with single electron: H-atom
 VII 33 Atoms 2010 update -- start with single electron: H-atom x z φ θ e -1 y 3-D problem - free move in x, y, z - easier if change coord. systems: Cartesian Spherical Coordinate (x, y, z) (r, θ, φ) Reason:
VII 33 Atoms 2010 update -- start with single electron: H-atom x z φ θ e -1 y 3-D problem - free move in x, y, z - easier if change coord. systems: Cartesian Spherical Coordinate (x, y, z) (r, θ, φ) Reason:
20 The Hydrogen Atom. Ze2 r R (20.1) H( r, R) = h2 2m 2 r h2 2M 2 R
 20 The Hydrogen Atom 1. We want to solve the time independent Schrödinger Equation for the hydrogen atom. 2. There are two particles in the system, an electron and a nucleus, and so we can write the Hamiltonian
20 The Hydrogen Atom 1. We want to solve the time independent Schrödinger Equation for the hydrogen atom. 2. There are two particles in the system, an electron and a nucleus, and so we can write the Hamiltonian
does not change the dynamics of the system, i.e. that it leaves the Schrödinger equation invariant,
 FYST5 Quantum Mechanics II 9..212 1. intermediate eam (1. välikoe): 4 problems, 4 hours 1. As you remember, the Hamilton operator for a charged particle interacting with an electromagentic field can be
FYST5 Quantum Mechanics II 9..212 1. intermediate eam (1. välikoe): 4 problems, 4 hours 1. As you remember, the Hamilton operator for a charged particle interacting with an electromagentic field can be
