HHS Public Access Author manuscript Math Biosci. Author manuscript; available in PMC 2016 February 26.
|
|
|
- Ezra Manning
- 6 years ago
- Views:
Transcription
1 Bifurcation study of blood flow control in the kidney Ashlee N. Ford Versypt a,1, Elizabeth Makrides b,*,1, Julia C. Arciero c, Laura Ellwein d, and Anita T. Layton e a School of Chemical Engineering, Oklahoma State University, Stillwater, OK 74078, USA b Division of Applied Mathematics, Brown University, Providence, RI 02912, USA c Department of Mathematical Sciences, Indiana University-Purdue University Indianapolis, Indianapolis, IN 46202, USA d Department of Mathematics and Applied Mathematics, Virginia Commonwealth University, Richmond, VA 23284, USA e Department of Mathematics, Duke University, Durham, NC 27708, USA Abstract Renal blood flow is maintained within a narrow window by a set of intrinsic autoregulatory mechanisms. Here, a mathematical model of renal hemodynamics control in the rat kidney is used to understand the interactions between two major renal autoregulatory mechanisms: the myogenic response and tubuloglomerular feedback. A bifurcation analysis of the model equations is performed to assess the effects of the delay and sensitivity of the feedback system and the time constants governing the response of vessel diameter and smooth muscle tone. The results of the bifurcation analysis are verified using numerical simulations of the full nonlinear model. Both the analytical and numerical results predict the generation of limit cycle oscillations under certain physiologically relevant conditions, as observed in vivo. Keywords HHS Public Access Author manuscript Published in final edited form as: Math Biosci May ; 263: doi: /j.mbs Bifurcation analysis; Delay differential equation; Renal hemodynamics; Myogenic response; Tubuloglomerular feedback 1. Introduction Many biological systems exhibit spontaneous limit cycle oscillations. The mechanisms that give rise to such biological oscillators are intrinsically nonlinear; indeed, no linear system has robust limit cycle behavior. One example of biological limit cycle oscillations occurs in the kidney [1,2]. The kidney regulates the balance of water, salt, and blood pressure via filtration, reabsorption, and secretion of the appropriate amounts of water and solutes across the epithelia of renal tubules known as nephrons. A nephron consists of a glomerulus, which is a bundle of capillaries, and a tubule whose walls consist of a single layer of epithelial * Corresponding author. Tel.: elizabeth_makrides@brown.edu (E. Makrides). 1 The first two authors contributed equally.
2 Ford Versypt et al. Page 2 cells. Blood is delivered via the afferent arteriole to the glomerulus, where the filtration process begins. Blood cells and large plasma proteins are retained in the blood stream, while fluid and smaller solutes (now called filtrate) are forced out into Bowman s capsule, the entrance of the tubule. The resulting filtrate travels through the tubule, where the transformation of the filtrate into urine is initiated. Along the tubule, the filtrate (tubular fluid) composition is altered by transport processes in the epithelial cells of the renal tubule. In particular, the thick ascending limb (TAL), a water-impermeable portion of the tubule in a zone called the loop of Henle, actively and passively transports sodium chloride from tubular fluid into the interstitium outside of the tubule where molecules and ions can be reabsorbed by the bloodstream through nearby capillaries. To maintain normal renal function, fluid flow through the nephron must be kept within a narrow range. This is accomplished primarily by two physiological regulatory mechanisms: the myogenic response and tubuloglomerular feedback (TGF). The myogenic response induces vasoconstriction in response to increases in blood pressure. In TGF, changes in the chloride ion concentration in the TAL are detected by a collection of epithelial cells at the exit of the TAL called the macula densa (MD). This generates feedback signals that alter the afferent arteriolar smooth muscle tone in order to regulate the glomerular filtration rate. Fig. 1 is a schematic diagram illustrating the anatomy involved in these regulatory mechanisms. The reader may also refer to [3] for additional detail on kidney physiology. Spontaneous fluctuations of fluid flow and oscillating intratubular pressure have been observed in the rat kidney [1,2,4 8]. Mathematical models of the TGF mechanism have successfully simulated these phenomena [9 12], and sensitivity analysis of these models has suggested the oscillatory or steady state behavior depends on physical and transport characteristics of the TAL. We have recently developed a renal hemodynamics model that combines both the myogenic and TGF mechanisms and used the model to study renal autoregulation [13]. In the present study, we use this model to explore the influence of key bifurcation parameters, including the feedback loop sensitivity, delay, and time constants that govern changes in the diameter and smooth muscle tone of the afferent arteriole. Frequencies of periodic solutions simulated from our model are in agreement with those observed in the literature [14]. We present the details of the first analytical bifurcation analysis on the renal hemodynamics model with both autoregulatory mechanisms. We compare the bifurcation results to the full numerical simulations of the mathematical model for certain parameter values. The bifurcation analysis allows us to understand the global behaviors of the model(i.e., its behaviors over a very large range of parameters), as opposed to direct numerical simulations, which typically cover only a small range of parameters and must be repeated for each combination of parameter values. The major contribution of the present work is the mathematical formulation for the bifurcation analysis that facilitates the exploration of the parameter space in a way that augments the current experimental capabilities for determining the physiological responses in different parameter ranges.
3 Ford Versypt et al. Page 3 2. Model formulation The mathematical model used in this study to investigate potential bifurcation parameters was recently described [13]. Briefly, the model captures the flow dynamics along a short loop of Henle in a rat kidney by coupling a partial differential equation (PDE) describing chloride ion transport along the TAL of a short-loop nephron with a system of ordinary differential equations (ODEs) describing vessel wall mechanics of the afferent arteriole. The resulting system includes the effects of both the myogenic and TGF responses. The model takes the form where C(x, t) is the concentration of chloride ions in the TAL at position x and time t, with x = 0 at the bend in the loop of Henle and x = L at the upper end of the TAL at the MD, while D(t) and A(t) are the diameter and smooth muscle tone (activation), respectively, of the afferent arteriole. The functional forms of F(D(t)) and C e (x) are given in Eqs. (2.4) (2.6). As given explicitly in Eqs. (2.7) (2.11), T total is a function of D(t) and A(t), while A total is a function of D(t) and C(L, t τ). The parameter τ gives the time required for transmitting the signal of the chloride ion concentration sensed in the MD to the afferent arteriole, including any associated lag in response; thus the system is properly one of delay differential equations (DDEs) with time delay τ. Values for all parameters appearing in Eqs. (2.1) (2.3), as well as parameters appearing in the subsidiary functions detailed below, are given in Table 1. We refer to these as reference values, and to the resulting model solution as the reference state. The PDE (2.1) represents axial advective chloride ion transport, outward-directed active solute transport, and transepithelial chloride ion diffusion. The flow rate, F(D(t)), appearing in the advective term of Eq. (2.1) has the explicit form Here Q A is the afferent arteriole flow rate which, in accordance with Poiseuille s law, depends on diameter, D(t); pressure drop along the afferent arteriole, ΔP; viscosity, μ; and afferent arteriole segment length, l. The parameter β represents the fraction of the afferent arteriole flow entering the loop of Henle. The quantity Q = βq A is commonly referred to as the single nephron glomerular filtration rate (SNGFR), and α is the portion of the SNGFR that is not reabsorbed along the proximal tubule or the descending limb of the loop of Henle (2.4) (2.3) (2.2) (2.1)
4 Ford Versypt et al. Page 4 before entering the TAL. Note that the TAL is assumed to be water impermeable, so that fluid flow along the TAL is constant in space, although it may vary in time. The second term in Eq. (2.1) represents active NaCl reabsorption and is assumed to follow standard Michaelis Menten kinetics. The last term describes chloride ion diffusion across the TAL with permeability p, while C e (x) is the extratubular chloride ion concentration, which is assumed to be time independent. C e (x) is given as [11] where The extra- and intratubular chloride ion concentrations are assumed to be equal at the bend of the loop of Henle, so that the boundary condition for chloride ion concentration is given by a constant: C(0, t) = C 0 = 275 mm [11]. We use a previously developed vessel wall mechanics model [22,23] to predict changes in the diameter and smooth muscle tone of the afferent arteriole according to the myogenic and TGF mechanisms. The quantities P avg and P avg,c appearing in Eq. (2.2) refer to midpoint pressures in the afferent arteriole; these are determined by the incoming pressure and pressure drop, i.e., P avg = P ΔP/2, where P is the intraluminal pressure entering the afferent arteriole. P avg,c is the midpoint pressure with the control (baseline) incoming pressure of 100 mmhg, whereas P avg may vary. In the present study, the pressure P is fixed at 100 mmhg so that P avg does not change and the dynamics over a wide parameter range can be assessed. In our previous work [13], we explored the effects of pressure change on the system by varying afferent arterial pressure between 60 and 180 mmhg. Future studies will combine both investigations to assess simultaneously the effect of varying parameter values and average pressure values on the appearance of limit cycle oscillations. The total tension in the afferent arteriole wall is expressed as a sum of passive and active components: where T pass describes passive tension as an exponential function of diameter, and (2.6) (2.5) (2.7) (2.8) represents maximally active tension as a Gaussian function of diameter, (2.9)
5 Ford Versypt et al. Page 5 Finally, in Eq. (2.3) the level of smooth muscle tone, A total, is modeled as a sigmoidal function of a stimulus, S tone, which includes both the myogenic response and TGF mechanism. Thus, where (2.10) With all parameters set to their reference values as given in Table 1, we refer to the steady state model solution as the reference state, since this is the baseline from which other simulations which may proceed to limit cycle oscillations or return to the steady state upon perturbation are run. The reference state values of afferent arteriolar diameter, activation, and flow rate, along with SNGFR and flow rate entering the TAL are given in Table 2. For comparison, we include experimentally observed values for these quantities obtained under normal physiological conditions in rats. The smooth muscle activation of the afferent arteriole, A(t), is a dimensionless variable ranging from 0 to 1, with 0 representing no smooth muscle activation, and 1 maximal smooth muscle activation see Eq. (2.7). We choose our reference steady state to represent a state of average muscle tone activation, i.e., A = 0.5. This choice then dictates the value of the c tone parameter. As in [13], the parameter value for c 2 was optimized in a least squares sense to arteriolar flow values obtained from an empirical feedback relation [12], and C MD =32.32 mm to be consistent with the expected value of the chloride ion concentration in the macula densa [26]. The reference value of β is set such that Q = 30.0 nl/min, which, as noted in Table 2, is a typical value for the SNGFR observed in rats. The reader is also referred to [13] for comparison of model solutions to experimental data over a range of afferent arterial pressures from 60 to 180 mmhg. 3. Analytical and numerical methods The present study seeks to better understand the dynamic behaviors of the renal autoregulatory system and the stability of steady state solutions. Small, transient perturbations in blood flow may result from an animal s heartbeat, respiration, or motion. After activating the hemodynamics feedback responses, these perturbations may die out, allowing tubular fluid flow to return to a steady state, or the perturbations may grow, evolving into a limit cycle oscillation. The precise outcome depends on the characteristics of the system. Using the model detailed in Section 2, we implement numerical and analytical approaches to predict the asymptotic behavior of the in vivo tubular fluid dynamics that occur in response to a perturbation. In the first approach, the model is solved numerically for various combinations of model parameters. Although this yields a solution to the full nonlinear problem, employing this approach involves characterizing model behavior via an undirected exploration of the full space of physiologically relevant parameter values. Bifurcation analysis provides a second (2.11)
6 Ford Versypt et al. Page 6 and complementary approach, directing subsequent numerical simulation to parameter regions that are both dynamically interesting and biologically relevant. In this section, the algorithms used to solve the model equations numerically and to simulate the effects of perturbations are described, and a characteristic equation is derived and analyzed from a linearization of the model equations. In Section 4, we demonstrate the complementary nature of these two approaches Numerical simulation and perturbation algorithm To explore the dynamics of the model for specific sets of model parameters, the model solution can be obtained by direct numerical computation. The model equations given by Eqs. (2.1) (2.3) are coupled and must be solved simultaneously. The chloride ion concentration from Eq. (2.1) is used by Eq. (2.3) with a time delay, τ. The diameter D from Eq. (2.2) is used to update the inlet flow rate F(D(t)) for Eq. (2.1). This system of equations is solved numerically by first using the method of lines [27] and the upwind differencing scheme to reduce the PDE in Eq. (2.1) to a system of ODEs in time and then using the MATLAB solver DDE23 [28] to solve the resulting system of DDEs with constant delay τ. A uniformly-spaced grid of 161 points is used to discretize the PDE unless otherwise indicated, and the maximum time-step size in the DDE23 solver is set to 0.1 s to prevent high frequency numerical oscillations that may result from steps that are too large. To simulate the dynamic effect of a perturbation, a small step perturbation in flow rate is held for a moderate duration, and then flow rate is allowed to vary with changes in D and A induced by the chloride ion concentration deviation from steady state that resulted from the perturbation in flow rate. The simulation algorithm for this numerical experiment involves four steps. First, the reference state is initialized by determining the steady state values of D and activation A with the reference state parameters specified, and by computing the steady state spatial distribution of chloride ions C(x) with constant inlet flow rate F calculated by the steady state value of D. Second, the solver DDE23 is used to solve the coupled model equations numerically as described above with the reference state conditions given as a constant history vector for the solver. This part of the simulation algorithm determines the coupled steady state solution at the reference state. Third, a 1% step increase in the flow rate is imposed for 15.7 s, which is the time interval required for fluid to move through the entire TAL at the reference state F. These values for the small pulse magnitude and duration match those used in the analogous bifurcation study for TGF-associated oscillations [11]. While F is perturbed by this constant step change, D and A are held constant, and the PDE for chloride ion transport with a constant flow rate is solved numerically by the solver DDE23 with the function for the system of DDEs modified to maintain constant D, A, and F for the perturbation interval. Thus, C(x) continues to change during this flow perturbation interval. In the final step of the numerical experiment, F, D, and A are reinitialized to their reference state values and then are allowed to change in response to the perturbed C(x), which is specified as the dynamic history vector by means of the numerical solution from the perturbation time interval. The solver DDE23 is used to solve the model equations until a steady state or limit cycle oscillation develops after the perturbation interval (total
7 Ford Versypt et al. Page Bifurcation analysis simulation time of at least 1000 s) to determine the dynamics of the DDE system in response to a perturbation from the steady state. Spectral plots shown in Section 4 are computed using the discrete Fourier transform routine FFT in MATLAB. Time profiles for D are computed as described in this section, with sampling frequency 10 Hz and total length 1000 s or 10,000 points, and are then trimmed to remove the steady state portion of the profile prior to the pulse. The steady state value of D is subtracted from the time-varying value at each point of the remaining time series in order to remove the zero-frequency signal. The number of points in the discrete Fourier transform is set to 16,384, the smallest power of two greater than 10,000. While numerical simulations can be run for a variety of model parameter values, bifurcation analysis is used to quickly identify system behavior for a larger parameter space General form of the characteristic equation To begin, we derive a general form for the characteristic equation that allows for straightforward substitutions or model refinements in the future. The model equations (2.1) (2.3) can be written in the following general form: where the particular forms of the functions F (i) and the vectors of parameters μ i, i = 0,, 3, are determined by Eqs. (2.1) (2.11). Linearizing about a steady state solution (C (x), D, Ā) yields where (C(x, t), D(t), A(t)) T is properly a solution on [t τ, t], and we define LC(x, t) C(L, t τ). Differentiation with respect to x is denoted by throughout, while indicates the partial derivative with where respect to A, with the analogous convention for C and D. The dependence on parameters μ i is suppressed. Making the ansatz (3.2) (3.3) (3.1) (3.4)
8 Ford Versypt et al. Page 8 gives which can be rewritten as (3.5) so that the first row of Eq. (3.6) is an ODE. Solving for b 2 in terms of b 1, λ, and f(l) from the third row of Eq. (3.6) gives Substituting this solution for b 2 into the second row of Eq. (3.6) gives an expression for b 1 : The following differential equation results from the first row: where b 1 is given in Eq. (3.7). Enforcing the boundary condition f(0) = 0, which is equivalent to assuming that C(0, t) is fixed at C (0), gives the solution (3.8) (3.6) (3.7)
9 Ford Versypt et al. Page 9 Evaluating at x = L, rearranging, and canceling the f(l) that appears in b 1 gives the following characteristic equation: where and each F (i) is evaluated at the appropriate steady state. (3.11) (3.10) Model-specific characteristic equation Using the general form of the characteristic equation from Eqs. (3.10) and (3.11), the particular form of the characteristic equation can be defined for the model equations given by Eqs. (2.1) (2.3). First substituting these model equations into Eqs. (3.1) (3.3) gives the following F (i) : and (3.12) (3.13) Using the particular form of F (1), we can further simplify the characteristic equation (3.10) to eliminate the steady state derivative C (x) in the integral. Recalling Eq. (3.1), C (x) must satisfy (3.14) (3.16) (3.15) (3.9)
10 Ford Versypt et al. Page 10 where find with the terms arranged so that the right-hand side is (3.10) gives. Differentiating again with respect to x and rearranging, we By analogy with earlier works [11,29], we define the sensitivity γ by (3.17). Substituting into Eq. Substituting Eq. (3.19) into Eq. (3.18) yields an alternate form for the characteristic equation: (3.18) (3.19) In earlier models involving only the TAL, a measure called the sensitivity was defined as a product of the derivative of the chloride concentration profile with respect to position evaluated at the MD, and the derivative of the flow with respect to the chloride concentration evaluated at the reference state [11]. Here, the first term in the numerator of Eq. (3.19) corresponds to the first term in the earlier definition, while the last three terms in the numerator correspond to the second term, i.e., the derivative of the flow. The sensitivity, also referred to as the gain, quantifies the effect of changes in input on output variables Evaluation of characteristic equation To compute two-parameter bifurcation diagrams, solutions of the characteristic equation (3.18) satisfying ρ Re λ = 0 are determined via gradient descent. All parameters other than the specified bifurcation parameters are fixed at reference values given in Section 2 unless otherwise indicated, with ω Im λ and one of the two bifurcation parameters allowed to vary. Contours for particular (3.20)
11 Ford Versypt et al. Page Model results values of ω are found by fixing ω at the indicated value while allowing ρ to vary. The steady state concentration profile C (x) used in these computations is obtained by direct numerical computation as described in Section 3.1. Derivatives appearing in Eq. (3.18) are computed by a midpoint approximation and integrals are computed using a trapezoidal rule. From the bifurcation analysis in Section 3.2, a two-parameter bifurcation diagram for sensitivity, γ, and time delay, τ, is created by solving the characteristic equation (3.18) and setting ρ Re λ = 0. As shown in Fig. 2, a transition from a stable steady state to limit cycle oscillations is predicted when either τ is increased while γ is held constant or when γ is increased for a fixed τ. Direct numerical experiments are conducted as described in Section 3.1 to simulate the change in system dynamics when τ is increased from 1 s (point (I) in Fig. 2) to τ = 2 s (point (II) in Fig. 2). All other parameters are set to their reference values so that sensitivity remains at its reference state value (γ = 4.754). As seen explicitly in the bottom two panels of Fig. 2, the numerical simulations confirm the stable steady state and limit cycle oscillations in the parameter regions predicted by the bifurcation analysis. Additional simulations varying the initial chloride concentration C 0 to within ±10% and ±50% of its original value (results not shown) demonstrate good agreement with the dynamics predicted in Fig. 2. While Fig. 2 strictly applies to a linearization about the reference steady state, the results are an accurate guide to dynamic behavior for initial conditions at an extreme distance from those used in our study. By virtue of the transcendental characteristic equation (3.18), numerous additional bifurcation curves for which ρ = 0 exist beyond the initial supercritical Hopf bifurcation indicated in Fig. 2. Fig. 3 shows a subset of these curves, along with their corresponding frequencies ω Im λ. Curves corresponding to the same frequencies occur in sets that are roughly repeated with increasing τ. Moreover, the frequencies increase for each set of curves with increasing values of γ. Although the frequency varies along each curve, considering the frequency near the minimum of each and taking ω = 0.3 as a base frequency, we see that the first set of curves above the Hopf bifurcation corresponds to a frequency of about twice the base, the second set to a frequency close to three times the base, and the third to a frequency around four times the base. These results may be compared with earlier work on a related simplified model [11, see in particular Fig. 4], as well as [9]. Here several of the curves are connected, which is a feature that was not present or noted in earlier works. Note that, in order to capture the mathematically interesting behavior of these curves, the results are shown for larger values of both γ and τ than we expect to be physiologically relevant. The information in Fig. 3 indicates that a parameter region exists in which limit cycle oscillations of twice the fundamental frequency should occur. Point (III) in Fig. 3 lies inside this region, and Fig. 4 shows time series profiles for diameter and the associated power spectra for points (II) and (III). As in Fig. 2, point (II) corresponds to reference state conditions but with τ = 2 s. Point (III) corresponds to reference state conditions except τ = 0.1 s and the parameter ctgf is set to so that γ = The power spectra in Fig. 4 demonstrate that point (III) parameter values yield limit cycle oscillations that display a
12 Ford Versypt et al. Page 12 first harmonic dominant frequency, i.e., double that of the underlying fundamental frequency. Multiple frequencies are observed immediately following the perturbation, with oscillations at the fundamental frequency decaying as the first harmonic oscillations grow, so that the corresponding two peaks are of comparable intensity for the first 100 s after perturbation (data not shown). The observed frequencies for both points (II) and (III) agree with the predictions from the bifurcation analysis, as indicated by the values of ω in Fig. 3. Moreover, the values predicted by the model align with experiments conducted by Basar and Weiss in isolated rat kidneys [14], in which power density peaks were observed at frequencies between mhz and mhz. The lower frequencies are also consistent with experiments conducted by Leyssac and colleagues [1,6]. Since the time constants t a and t d also affect the frequency of oscillations (as shown in more detail in the following), the model supports a wide range of dominant oscillatory frequencies, from less than 30 mhz (e.g., for large τ) to over 100 mhz. In addition to the two-dimensional bifurcation diagram for γ and τ (Figs. 2 and 3), the relationships between additional bifurcation parameters can be explored. Fig. 5a shows the bifurcation diagram for the time constant for diameter, t d, versus τ, with fixed time constant for activation, t a, while Fig. 5b shows the bifurcation diagram for t a and τ with fixed t d. Both diagrams indicate that increasing τ past a threshold curve results in limit cycle oscillations. In each diagram, the solid blue curve indicates the stability threshold for the reference value of the fixed time constant. There is a qualitative difference between the blue bifurcation curves: the curve for t d versus τ intersects the y-axis, whereas the curve for t a versus τ has upward curvature and does not extend to the y-axis. However, the shape of each bifurcation curve depends on the non-varying time constant, rather than reflecting a fundamental difference between t d and t a. This is demonstrated by the dashed red curves, which indicate the stability threshold with particular non-reference values of the fixed time constant chosen to illustrate the opposite behavior with respect to intersection with the y-axis. We observe that t a = 1 s is a smaller than would be expected under experimental conditions; it is employed here to demonstrate that the qualitative difference in the blue bifurcation curves depends on other parameter values and is not dictated by the model equations. As seen in Figs. 6 and 7, stability may be restored by increasing either t a or t d substantially; however, the parameter values at which stability is reestablished are outside the physiologically relevant range. The intersection of the bifurcation curves with the y-axis observed for certain parameter choices in Fig. 5 indicates that limit cycle oscillations may exist even when τ is 0. Exploring this further in the bifurcation diagram for t a versus t d with fixed τ = 0 s (Fig. 6), the limit cycle oscillations are enclosed within a region of parameter space, while a stable steady state exists outside this region. Fig. 6b indicates the frequencies associated with the limit cycle oscillations by depicting contours for ω. The portions of the ω contours outside the region of limit cycle oscillations correspond to the characteristic frequencies of damped oscillations that occur as the system returns to steady state after perturbation. The highest frequency oscillations, in excess of 50 mhz, are found for values of t a and t d below 20 s. As τ is increased from 0 to 3 s, the region corresponding to limit cycle oscillations expands steadily (Fig. 7), but no additional bifurcation curves appear. Both Figs. 6 and 7 include a
13 Ford Versypt et al. Page Discussion much larger parameter range than is physiologically relevant in order to capture the full extent of the regions of limit cycle oscillations. Fig. 7b rescales Fig. 7a at finer resolution to highlight the physiologically relevant region where the bifurcation curves are difficult to distinguish on the larger scale. The physiological ranges for τ d and τ a follow from values used in [23]. For τ = 2 s, the stable region is disconnected, and for τ = 3 s the stable region is confined to a very small area near the origin. We have analyzed the dynamics of a mathematical model of blood flow control in the kidney using parameter values consistent with rat physiology. The model assumes that renal blood flow is controlled by two major autoregulatory mechanisms: the myogenic response, by which increases in blood pressure induce vasoconstriction, and the tubuloglomerular feedback (TGF), through which the glomerular filtration rate is regulated based on changes in the luminal chloride ion concentration at the macula densa. This model is used to identify a variety of dynamic behaviors that can arise for parameters within physiological and pathophysiological parameter regimes. These behaviors, which include limit-cycle oscillations, were identified and characterized by analyzing the characteristic equation that arises from a linearization of the model equations and were further explored by direct numerical simulation of the solutions for the full model equations. We note that nested periodic solutions were not observed. TGF-mediated oscillations have been observed in a number of experimental studies [4 8]. To the best of our knowledge, this is the first study that performs a bifurcation analysis on a renal hemodynamics model that includes both TGF and myogenic responses. Prior to this work, many mathematical models with varying degrees of complexity have been used to analyze kidney autoregulation considering only the TGF mechanism [9,11,29 35]. Glomerular filtration rate is also regulated by the myogenic response, which, together with TGF, acts upon the smooth muscle cells of the afferent arteriole and induces vasoconstriction or vasodilation. By sharing a common effector, the two mechanisms are expected to interact. Thus, the bifurcation analysis and numerical simulations presented in this study enable a fuller understanding of the interactions between the myogenic response and TGF mechanism and the resulting dynamics. Our results indicate that the time constants associated with the afferent arteriole muscle mechanics (t d and t a ) are important bifurcation parameters in addition to the delay and sensitivity, which were previously studied in models involving only the TGF mechanism. By simultaneously varying the time delay, τ, and the time constants t a and t d, we indicate how these three parameters interact to shift the border between a stable steady state and limit cycle oscillations. Increasing the response time of the afferent arteriole initially lowers the stability of the autoregulatory system before reestablishing stability at much longer response times (Figs. 6 and 7). In a previous vessel wall mechanics model that includes the effects of the myogenic response, the ratio of t d to t a was found to determine stability [36]. Here, the values of t d and t a are found to be important individually, as can be seen by considering any ray from the origin in Figs. 6 and 7. In fact, the time constants t a and t d interact to produce a closed region in two-dimensional parameter space in which limit cycle oscillations are
14 Ford Versypt et al. Page 14 observed. The results presented here further demonstrate the importance of these parameters in determining oscillatory frequency and indicate that the combined model supports a wide range of dominant frequencies. The oscillatory frequencies predicted by our bifurcation analysis and observed in numerical simulations of model solutions are consistent with those detected experimentally [1,6,14]. A few renal hemodynamics models represent both TGF and myogenic mechanisms. In separate studies, Marsh et al. [37,38] and Sgouralis and Layton [39] formulated comprehensive models of renal autoregulation that represented the dynamic interactions of TGF and myogenic mechanisms. These models included a detailed representation of the afferent arteriole that captured both intracellular calcium dynamics and membrane potential. In contrast, the present study adopts a simpler representation of the arteriolar response to regulatory signals. The simplicity of our arteriolar model enables an analytical bifurcation analysis, which provides a more comprehensive understanding of the correspondence between different parameter regimes and solution behavior. Certain simplifying assumptions in the present model may impact the validity of the dynamic behaviors predicted for different parameter regimes. First, the TAL is represented as a rigid tubule with uniform radius and transport parameters. Results from a previous modeling study representing the TAL as a tubule with compliant walls suggest that tubular compliance may significantly reduce the stability of the TGF system [30]. Another study [32] suggests that inhomogeneities in tubular radius or in maximum chloride transport rate, as indicated in experimental measurements [40,41], further increase the tendency of the system to oscillate. Second, the bifurcation parameters may also undergo substantial changes as exemplified by spontaneously hypertensive rats, which appear to exhibit higher TGF gain [42 44], compared to normotensive rats. Together with stronger internephron coupling, in which the TGF signal of one nephron is partially transmitted to a neighboring nephron sharing the same cortical radial artery, tubular spontaneously hypertensive rats exhibit irregular TGF-mediated oscillations that appear to have characteristics consistent with deterministic chaos [6,45]. This observation can be explained, in part, by the bifurcation results of our model, which suggest that at higher TGF gain, the stability of the system is significantly reduced. Third, the present model considers a single afferent arteriole and loop of Henle in isolation. It is, however, important to note that TGF in each nephron does not operate independently of other nephrons. Indeed, the TGF systems of nephrons whose afferent arterioles arise from a common interlobular artery are known to be coupled [46], with the TGF signal propagating rapidly via the afferent arteriolar endothelium. Previous studies of dynamics of nephrons coupled via their TGF system indicate that internephron coupling increases the likelihood of TGF-mediated oscillations. Combining several copies of the present model, which incorporates both TGF and the myogenic response, may shed new light on the importance of internephron coupling on the dynamic behavior of the system. The stability of the resulting system can be investigated via the bifurcation analysis used in the present study, following the approach of [31,47].
15 Ford Versypt et al. Page 15 Acknowledgments References The authors acknowledge the support of the National Science Foundation and the Institute for Mathematics and its Applications under NSF grant DMS The work of E. Makrides was supported in part by NSF grant DMS The work of J.C. Arciero was supported in part by NSF grant DMS The work of A.T. Layton was supported in part by NSF grant DMS and NIH grant DK Leyssac P, Baumbach L. An oscillating intratubular pressure response to alterations in Henle loop flow in the rat kidney. Acta Physiol. Scand. 1983; 117: [PubMed: ] 2. Leyssac P. Further studies on oscillating tubulo-glomerular feedback responses in the rat kidney. Acta Physiol. Scand. 1986; 126: [PubMed: ] 3. Eaton, D.; Pooler, J. Vander's Renal Physiology. 8th. New York: McGraw-Hill Medical; Cupples W, Novak P, Novak V, Salevsky F. Spontaneous blood pressure fluctuations and renal blood flow dynamics. Am. J. Physiol. Renal Physiol. 1996; 270:F82 F Cupples W. Angiotensin II conditions the slow component of autoregulation of renal blood flow. Am. J. Physiol. Renal Physiol. 1993; 264:F515 F Holstein-Rathlou N, Leyssac P. TGF-mediated oscillations in the proximal intratubular pressure: differences between spontaneously hypertensive rats and Wistar-Kyoto rats. Acta Physiol. Scand. 1986; 126: [PubMed: ] 7. Holstein-Rathlou N, Leyssac P. Differences in tubuloglomerular feedback oscillatory activity between spontaneously hypertensive and Wistar-Kyoto rats. J. Hypertens. 1985; 3:S343 S Yip K, Holstein-Rathlou N, Marsh D. Mechanisms of temporal variation in single-nephron blood flow in rats. Am. J. Physiol. Renal Physiol. 1993; 264:F427 F Layton AT, Moore LC, Layton HE. Multistability in tubuloglomerular feedback and spectral complexity in spontaneously hypertensive rats. Am. J. Physiol. Renal Physiol. 2006; 291:F79 F97. [PubMed: ] 10. Budu-Grajdeanu P, Moore L, Layton H. Effect of tubular inhomogeneities on filter properties of thick ascending limb of Henle's loop. Math. Biosci. 2007; 209(2): [PubMed: ] 11. Layton HE, Pitman EB, Moore LC. Bifurcation analysis of TGF-mediated oscillations in SNGFR. Am. J. Physiol. Renal Physiol. 1991; 261:F904 F Layton HE, Pitman EB, Moore LC. Instantaneous and steady-state gains in the tubuloglomerular feedback system. Am. J. Physiol. Renal Physiol. 1995; 268:F163 F Arciero JC, Ellwein L, Ford Versypt AN, Makrides E, Layton AT. Modeling blood flow control in the kidney. Proc. IMA in press. 14. Basar E, Weiss C. Time series analysis of spontaneous fluctuations of the flow in the perfused rat kidney. Pflügers Arch. 1970; 319: [PubMed: ] 15. Pfaller, W. Structure Function Correlation on Rat Kidney: Quantitative Correlation of Structure and Function in the Normal and Injured Rat Kidney. New York: Springer-Verlag; Johnson, P. Handbook of Physiology. The Cardiovascular System. Vascular Smooth Muscle. Bethesda, MD: Am. Physiol. Soc; The myogenic response; p Loutzenhiser R, Bidani A, Chilton L. Renal myogenic response: kinetic attributes and physiologic role. Circ. Res. 2002; 90: [PubMed: ] 18. Myers BD, Deen WM, Brenner BM. Effects of norepinephrine and angiotensin ii on the determinants of glomerular ultrafiltration and proximal tubule fluid reabsorption in the rat. Circ. Res. 1975; 37: [PubMed: ] 19. Moore L, Schnermann J, Yarimizu S. Feedback mediation of SNGFR autoregulation in hydropenic and DOCA- and salt-loaded rats. Am. J. Physiol. Renal Physiol. 1979; 237:F63 F Pries AR, Secomb TW, Gessner T, Sperandio MB, Gross JF, Gaehtgens P. Resistance to blood flow in microvessels in vivo. Circ. Res. 1994; 75: [PubMed: ] 21. Casellas D, Dupont M, Bouriquet N, Moore L, Artuso A, Mimran A. Anatomic pairing of afferent arterioles and renin cell distribution in rat kidneys. Am. J. Physiol. Renal Physiol. 1994; 267:F931 F936.
16 Ford Versypt et al. Page Carlson BE, Arciero JC, Secomb TW. Theoretical model of blood flow autoregulation: roles of myogenic, shear-dependent, and metabolic responses. Am. J. Physiol. Heart Circ. Physiol. 2008; 295:H1572 H1579. [PubMed: ] 23. Arciero J, Carlson B, Secomb T. Theoretical model of metabolic blood flow autoregulation: roles of ATP release by red blood cells and conducted responses. Am. J. Physiol. Heart Circ. Physiol. 2008; 295:H1562 H1571. [PubMed: ] 24. Treek B, Roald AB, Tenstad O, Aukland K. Effect of exogenous and endogenous angiotensin ii on intrarenal distribution of glomerular filtration rate in rats. Kidney Int. 2002; 541: de Rouffignac C, Monnens L. Functional and morphologic maturation of superficial and juxtamedullary nephrons in the rat. J. Physiol. 1969; 48: Schnermann, J.; Briggs, J. Function of the juxtaglomerular apparatus: control of glomerular hemodynamics and renin secretion. In: Alpern, RJ.; Hebert, SC., editors. Seldin and Giebisch's the Kidney: Physiology and Pathophysiology. 4th. Amsterdam, Boston: Elsevier Academic Press; p Schiesser, WE. The Numerical Method of Lines: Integration of Partial Differential Equations. San Diego: Academic Press; Shampine L, Thompson S. Solving DDEs in MATLAB. Appl. Numer. Math. 2001; 37: Holstein-Rathlou NH, Marsh DJ. A dynamic model of the tubuloglomerular feedback mechanism. Am. J. Physiol. Renal Fluid Electrolyte Physiol. 1990; 258:F1448 F Layton A. Feedback-mediated dynamics in a model of a compliant thick ascending limb. Math. Biosci. 2010; 228: [PubMed: ] 31. Layton A, Moore L, Layton H. Multistable dynamics mediated by tubuloglomerular feedback in a model of coupled nephrons. Bull. Math. Biol. 2009; 71: [PubMed: ] 32. Ryu H, Layton A. Effect of tubular inhomogeneities on feedback-mediated dynamics of a model of a thick ascending limb. Math. Med. Biol. 2012; 30: [PubMed: ] 33. Ryu H, Layton A. Tubular fluid flow and distal NaCl delivery mediated by tubuloglomerular feedback in the rat kidney. J. Math. Biol. 2014; 68(4): [PubMed: ] 34. Hattaway, A. Ph.D. thesis. University of Massachusetts Amherst; Modelling tubuloglomerular feedback in coupled nephrons. 35. Holstein-Rathlou N. A closed-loop analysis of the tubuloglomerular feedback mechanism. Am. J. Physiol. Renal Physiol. 1991; 261:F880 F Arciero J, Secomb T. Spontaneous oscillations in a model for active control of microvessel diameters. Math. Med. Biol. 2012; 29: [PubMed: ] 37. Marsh D, Sosnovtseva O, Chon K, Holstein-Rathlou N. Nonlinear interactions in renal blood flow regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005; 288:R1143 R1159. [PubMed: ] 38. Marsh D, Sosnovtseva O, Pavlov A, Yip K, Rathlou NH. Frequency encoding in renal blood flow regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005; 288:R1160 R1167. [PubMed: ] 39. Sgouralis I, Layton A. Theoretical assessment of renal autoregulatory mechanisms. Am. J. Physiol. Renal Physiol. 2014; 306:F1357 F1371. [PubMed: ] 40. Garg L, Mackie S, Tischer C. Effects of low potassium diet on Na K ATPase in rat nephron segments. Pflügers Arch. 1982; 394: [PubMed: ] 41. Knepper M, Danielson R, Saidel G, Post R. Quantitative analysis of renal medullary anatomy in rats and rabbits. Kidney Int. 1977; 12: [PubMed: ] 42. Brännström K, Morsing P, Arendshorst W. Exaggerated tubuloglomerular feedback activity in genetic hypertension is mediated by ANG II and AT 1 receptors. Am. J. Physiol. Renal Physiol. 1996; 270:F749 F Leyssac P, Holstein-Rathlou N. Tubulo-glomerular feedback response: enhancement in adult spontaneously hypertensive rats and effects of anaesthetics. Pflügers Arch. 1989; 413: [PubMed: ] 44. Takabatake T, Ushiogi Y, Ohta K, Hattori N. Attenuation of enhanced tubuloglomerular feedback activity in SHR by renal denervation. Am. J. Physiol. Renal Physiol. 1990; 258:F980 F985.
17 Ford Versypt et al. Page Yip K, Holstein-Rathlou N, Marsh D. Chaos in blood flow control in genetic and renovascular hypertensive rats. Am. J. Physiol. Renal Physiol. 1991; 261:F400 F Holstein-Rathlou N. Synchronization of proximal intratubular pressure oscillations: evidence for interaction between nephrons. Pflügers Arch. 1987; 408: [PubMed: ] 47. Layton A, Bowen M, Wen A, Layton H. Feedback-mediated dynamics in a model of coupled nephrons with compliant thick ascending limbs. Math. Biosci. 2011; 230: [PubMed: ]
18 Ford Versypt et al. Page 18 Fig. 1. Schematic diagram of the major components of a kidney nephron. As detailed in Section 2, the present model includes a partial differential equation (PDE) for the chloride ion concentration in the thick ascending limb (TAL), and ordinary differential equations (ODEs) for the afferent arteriolar diameter and smooth muscle tone. The chloride concentration at the end of the TAL is sensed by the macula densa (MD), causing adjustments in the afferent arteriolar diameter and tone, which in turn impact the chloride ion concentration by changing the flow rate entering the TAL.
19 Ford Versypt et al. Page 19 Fig. 2. Upper panel: sensitivity, γ, as given by Eq. (3.19) plotted against time delay, τ. The curve indicates the transition from a stable steady state (lower left) to limit cycle oscillations (upper right). Points (I) and (II) indicated in the upper panel lie on either side of the predicted bifurcation from a stable steady state to limit cycle oscillations. Lower two panels: dynamic profiles for afferent arteriolar smooth muscle activation, A(t); afferent arteriolar diameter, D(t); and chloride ion concentration at the MD, C(L, t), after transient perturbation
20 Ford Versypt et al. Page 20 from steady state. The profiles in the middle panel correspond to point (I) with τ = 1 s, while the profiles in the bottom panel correspond to the point (II) with τ = 2 s.
21 Ford Versypt et al. Page 21 Fig. 3. Sensitivity, γ, plotted against time delay, τ. Each curve corresponds to a solution of the characteristic equation (3.18) with ρ = 0, and ω in the range indicated by the line color and style. The points labeled (II) and (III) have (τ, γ) coordinates (2.0, 4.75) and (0.1, 10.0) respectively, with the former corresponding to point (II) in Fig. 2. Power spectra for the oscillatory solutions associated with these parameter values are displayed in Fig. 4. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
22 Ford Versypt et al. Page 22 Fig. 4. Time profiles for diameter (left) with associated power spectra (right). The upper and lower panels correspond to the points labeled (II) and (III), respectively, in Fig. 3. The upper left plot is repeated from Fig. 2 for comparison. Shaded regions indicate experimentally observed frequencies [14].
23 Ford Versypt et al. Page 23 Fig. 5. Bifurcation curves for time constants (t d for diameter and t a for activation) plotted against time delay, τ: (a) varying t d with fixed values of t a and (b) varying t a with fixed values of t d. Solid blue curves correspond to the reference values of the fixed time constant, and dashed red curves to non-reference values. Each curve indicates the location of the transition from a stable steady state (below and to the left of the curve) to limit cycle oscillations (above and to the right of the curve). Thus the region between the curves in (a) corresponds to a stable steady state when t a = 1 s and to limit cycle oscillations when t a = 10 s. In (b), the region between the curves corresponds to a stable steady state when t d = 1 s and to limit cycle oscillations when t d = 2 s. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
24 Ford Versypt et al. Page 24 Fig. 6. Time constant for activation, t a, plotted against the time constant for diameter, t d, with fixed time delay, τ = 0 s. (a) The curve corresponds to ρ = 0, indicating the transition from a stable steady state to limit cycle oscillations. (b) The solid dark blue curve is repeated from (a), while the remaining colored curves with varying line styles indicate contours of the frequency ω. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
25 Ford Versypt et al. Page 25 Fig. 7. Time constant for activation, t a, plotted against the time constant for diameter, t d, with fixed values of time delay, τ. The dashed box in (a) outlines the physiologically relevant parameter range that is shown at higher magnification in (b).
26 Ford Versypt et al. Page 26 Table 1 Reference values for all parameters appearing in Eqs. (2.1) (2.11). Symbol Description Units Value Source r TAL luminal radius cm [11] V max Maximum active transport rate mol/cm 2 s [11] K m Michaelis constant mm 70.0 [11] p TAL Cl permeability cm/s [11] L Length of TAL cm 0.5 [15] C 0 Luminal [Cl ] at loop of Henle mm [11] C e (L) Interstitial [Cl ] at MD mm [11] t d Time constant for AA diameter response s 1.0 [16, 17] P avg,c Midpoint pressure in AA, control state mmhg 75 [18] P avg Midpoint pressure in AA, specified mmhg 75 [18] t a Time constant for activation response s 10.0 [16, 17] τ Time delay, MD to AA response s 4.0 [11] β Glomerular filtration fraction Calculated α Fraction of glomerular filtrate entering TAL 0.2 [19] ΔP Pressure drop along AA mmhg 50 [18] µ Blood viscosity in AA cp 4.14 [20] l Afferent arteriole segment length cm [21] c pass Passive tension strength dyn/cm 220 [22] c pass,1 Passive tension sensitivity [22] D 0 Passive reference AA diameter µm 33 Calculated c act Maximally active VSM peak tension dyn/cm [22] c act,1 Maximally active VSM length dependence 0.75 [22] c act,2 Maximally active VSM tension range 0.38 [22] c myo VSM activation tension sensitivity cm/dyn [11] c 2 VSM TGF sensitivity cm/dyn 1.8 Estimated c TGF VSM activation [Cl ] sensitivity cm/dyn [11] C MD [Cl ] at MD mm Calculated c tone VSM constant Estimated Abbreviations: thick ascending limb (TAL); macula densa (MD); afferent arteriole (AA); vascular smooth muscle (VSM).
Model of Feedback-Mediated Dynamics in Coupled Nephrons with Compliant Thick Ascending Limbs
 Model of Feedback-Mediated Dynamics in Coupled Nephrons with Compliant Thick Ascending Limbs Senior Thesis Amy Wen Advisor: Anita Layton Mathematics Department Duke University, Durham, NC April 27, 2010
Model of Feedback-Mediated Dynamics in Coupled Nephrons with Compliant Thick Ascending Limbs Senior Thesis Amy Wen Advisor: Anita Layton Mathematics Department Duke University, Durham, NC April 27, 2010
Hwayeon Ryu. Department of Mathematics Duke University. Date: Approved: Anita T. Layton, Co-Supervisor. James Nolen, Co-Supervisor. Michael C.
 Feedback-Mediated Dynamics in the Kidney: Mathematical Modeling and Stochastic Analysis by Hwayeon Ryu Department of Mathematics Duke University Date: Approved: Anita T. Layton, Co-Supervisor James Nolen,
Feedback-Mediated Dynamics in the Kidney: Mathematical Modeling and Stochastic Analysis by Hwayeon Ryu Department of Mathematics Duke University Date: Approved: Anita T. Layton, Co-Supervisor James Nolen,
Tubuloglomerular Feedback-Mediated Dynamics in Three Coupled Nephrons 1
 Tubuloglomerular Feedback-Mediated Dynamics in Three Coupled Nephrons 1 Tracy L. Stepien 2 Advisor: E. Bruce Pitman 3 Abstract A model of three coupled nephrons branching from a common cortical radial
Tubuloglomerular Feedback-Mediated Dynamics in Three Coupled Nephrons 1 Tracy L. Stepien 2 Advisor: E. Bruce Pitman 3 Abstract A model of three coupled nephrons branching from a common cortical radial
Mathematical Modeling in the Kidney. Characterizing a Long-Looped Nephron
 : Characterizing a Long-Looped Nephron Quinton Neville Department of MSCS, St. Olaf College October 13, 2016 Northfield Undergraduate Math Symposium Kidney and Nephron Tubuloglomerular Feedback (TGF) System
: Characterizing a Long-Looped Nephron Quinton Neville Department of MSCS, St. Olaf College October 13, 2016 Northfield Undergraduate Math Symposium Kidney and Nephron Tubuloglomerular Feedback (TGF) System
Copyright c 2004 by Kevin J. Kesseler All rights reserved
 Copyright c 2004 by Kevin J. Kesseler All rights reserved ANALYSIS OF FEEDBACK-MEDIATED DYNAMICS IN TWO COUPLED NEPHRONS by Kevin J. Kesseler Department of Mathematics Duke University Date: Approved: Harold
Copyright c 2004 by Kevin J. Kesseler All rights reserved ANALYSIS OF FEEDBACK-MEDIATED DYNAMICS IN TWO COUPLED NEPHRONS by Kevin J. Kesseler Department of Mathematics Duke University Date: Approved: Harold
Modelling nephron dynamics and tubuloglomerular feedback. Scott J. Graybill
 Modelling nephron dynamics and tubuloglomerular feedback Scott J. Graybill A thesis presented for the degree of Doctor of Philosophy in Bioengineering at the University of Canterbury, Christchurch, New
Modelling nephron dynamics and tubuloglomerular feedback Scott J. Graybill A thesis presented for the degree of Doctor of Philosophy in Bioengineering at the University of Canterbury, Christchurch, New
Renal handling of substances. Dr.Charushila Rukadikar Assistance Professor Physiology
 Renal handling of substances Dr.Charushila Rukadikar Assistance Professor Physiology GENERAL PRINCIPLES OF RENAL TUBULAR TRANSPORT Transport mechanisms across cell membrane 1) Passive transport i. Diffusion
Renal handling of substances Dr.Charushila Rukadikar Assistance Professor Physiology GENERAL PRINCIPLES OF RENAL TUBULAR TRANSPORT Transport mechanisms across cell membrane 1) Passive transport i. Diffusion
Autoregulation. Ioannis Sgouralis. Department of Mathematics Duke University. Date: Approved: Anita T. Layton, Supervisor. Frederik H.
 A Dynamical Nephrovascular Model of Renal Autoregulation by Ioannis Sgouralis Department of Mathematics Duke University Date: Approved: Anita T. Layton, Supervisor Frederik H. Nijhout Stephanos Venakides
A Dynamical Nephrovascular Model of Renal Autoregulation by Ioannis Sgouralis Department of Mathematics Duke University Date: Approved: Anita T. Layton, Supervisor Frederik H. Nijhout Stephanos Venakides
Water transport through the kidney
 Report on a problem studied at the UK Mathematics-in-Medicine Study Group Oxford 2005 < http://www.maths-in-medicine.org/uk/2005/kidney-water/ > Water transport through the kidney J B van den Berg, J R
Report on a problem studied at the UK Mathematics-in-Medicine Study Group Oxford 2005 < http://www.maths-in-medicine.org/uk/2005/kidney-water/ > Water transport through the kidney J B van den Berg, J R
Spontaneous oscillations in a model for active control of microvessel diameters
 Mathematical Medicine and Biology 2012 29, 163 180 doi:10.1093/imammb/dqr005 Advance Access publication on October 29, 2010 Spontaneous oscillations in a model for active control of microvessel diameters
Mathematical Medicine and Biology 2012 29, 163 180 doi:10.1093/imammb/dqr005 Advance Access publication on October 29, 2010 Spontaneous oscillations in a model for active control of microvessel diameters
Efficient filtration system inspired by the kidney: 3D modeling of an active osmotic exchanger in COMSOL
 Faculteit Bètawetenschappen Efficient filtration system inspired by the kidney: 3D modeling of an active osmotic exchanger in COMSOL Bachelor Thesis Johan Verheij Natuur- en Sterrenkunde Supervisors: Prof.
Faculteit Bètawetenschappen Efficient filtration system inspired by the kidney: 3D modeling of an active osmotic exchanger in COMSOL Bachelor Thesis Johan Verheij Natuur- en Sterrenkunde Supervisors: Prof.
Mathematical Modeling of Complex Biomedical Systems
 Mathematical Modeling of Complex Biomedical Systems Erik Mosekilde Department of Physics The Technical University of Denmark Patras 2, July 2011 Page 1 Complex Biomedical Systems 1. Modeling and Simulation
Mathematical Modeling of Complex Biomedical Systems Erik Mosekilde Department of Physics The Technical University of Denmark Patras 2, July 2011 Page 1 Complex Biomedical Systems 1. Modeling and Simulation
Modeling and Simulation in Medicine and the Life Sciences
 Frank C. Hoppensteadt Charles S. Peskin Modeling and Simulation in Medicine and the Life Sciences Second Edition With 93 Illustrations fyj Springer Contents Series Preface Preface vii ix Introduction 1
Frank C. Hoppensteadt Charles S. Peskin Modeling and Simulation in Medicine and the Life Sciences Second Edition With 93 Illustrations fyj Springer Contents Series Preface Preface vii ix Introduction 1
Mathematical Model. M. Umar Qureshi, Mitchel J. Colebank, and Mette S. Olufsen
 Mathematical Model M. Umar Qureshi, Mitchel J. Colebank, and Mette S. Olufsen Department of Mathematics, North Carolina State University, Raleigh, North Carolina 27695 Friday 7 th September, 2018 The 1D
Mathematical Model M. Umar Qureshi, Mitchel J. Colebank, and Mette S. Olufsen Department of Mathematics, North Carolina State University, Raleigh, North Carolina 27695 Friday 7 th September, 2018 The 1D
Endocrine Physiology. Introduction to Endocrine Principles
 Endocrine Physiology Introduction to Endocrine Principles There are TWO major groups of hormones Peptide and protein hormones Amine hormones Peptide and protein hormones act through cell membrane receptors
Endocrine Physiology Introduction to Endocrine Principles There are TWO major groups of hormones Peptide and protein hormones Amine hormones Peptide and protein hormones act through cell membrane receptors
Overview of Physiology & Homeostasis. Biological explanations Levels of organization Homeostasis
 Overview of Physiology & Homeostasis 1 Biological explanations Levels of organization Homeostasis 2 Biological Explanations Proximate Proximate causation: an explanation of an animal's behavior based on
Overview of Physiology & Homeostasis 1 Biological explanations Levels of organization Homeostasis 2 Biological Explanations Proximate Proximate causation: an explanation of an animal's behavior based on
6.3.4 Action potential
 I ion C m C m dφ dt Figure 6.8: Electrical circuit model of the cell membrane. Normally, cells are net negative inside the cell which results in a non-zero resting membrane potential. The membrane potential
I ion C m C m dφ dt Figure 6.8: Electrical circuit model of the cell membrane. Normally, cells are net negative inside the cell which results in a non-zero resting membrane potential. The membrane potential
FLUID FLOW IN A RIGID WAVY NON-UNIFORM TUBE: APPLICATION TO FLOW IN RENAL TUBULES
 VOL 5, NO 11, NOVEMBER 2010 ISSN 1819-6608 2006-2010 Asian Research Publishing Network (ARPN) All rights reserved wwwarpnjournalscom FLUID FLOW IN A RIGID WAVY NON-UNIFORM TUBE: APPLICATION TO FLOW IN
VOL 5, NO 11, NOVEMBER 2010 ISSN 1819-6608 2006-2010 Asian Research Publishing Network (ARPN) All rights reserved wwwarpnjournalscom FLUID FLOW IN A RIGID WAVY NON-UNIFORM TUBE: APPLICATION TO FLOW IN
CENG 501 Examination Problem: Estimation of Viscosity with a Falling - Cylinder Viscometer
 CENG 501 Examination Problem: Estimation of Viscosity with a Falling - Cylinder Viscometer You are assigned to design a fallingcylinder viscometer to measure the viscosity of Newtonian liquids. A schematic
CENG 501 Examination Problem: Estimation of Viscosity with a Falling - Cylinder Viscometer You are assigned to design a fallingcylinder viscometer to measure the viscosity of Newtonian liquids. A schematic
Neurophysiology. Danil Hammoudi.MD
 Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
Alkalosis or alkalemia arterial blood ph rises above Acidosis or acidemia arterial ph drops below 7.35 (physiological acidosis)
 Acid-Base Balance Normal ph of body fluids Arterial blood is 7.4 Venous blood and interstitial fluid is 7.35 Intracellular fluid is 7.0 Alkalosis or alkalemia arterial blood ph rises above 7.45 Acidosis
Acid-Base Balance Normal ph of body fluids Arterial blood is 7.4 Venous blood and interstitial fluid is 7.35 Intracellular fluid is 7.0 Alkalosis or alkalemia arterial blood ph rises above 7.45 Acidosis
Physiology lecture (8): Acid Base regulation
 Physiology lecture (8): Acid Base regulation If we add hydrogen, we have three lines of defense against a mild change in ph: 1) Buffers, instantaneous, within a fraction of milliseconds. 2) The lung, takes
Physiology lecture (8): Acid Base regulation If we add hydrogen, we have three lines of defense against a mild change in ph: 1) Buffers, instantaneous, within a fraction of milliseconds. 2) The lung, takes
Arterial Macrocirculatory Hemodynamics
 Arterial Macrocirculatory Hemodynamics 莊漢聲助理教授 Prof. Han Sheng Chuang 9/20/2012 1 Arterial Macrocirculatory Hemodynamics Terminology: Hemodynamics, meaning literally "blood movement" is the study of blood
Arterial Macrocirculatory Hemodynamics 莊漢聲助理教授 Prof. Han Sheng Chuang 9/20/2012 1 Arterial Macrocirculatory Hemodynamics Terminology: Hemodynamics, meaning literally "blood movement" is the study of blood
CARDIOVASCULAR SIMULATOR
 Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas CARDIOVASCULAR SIMULATOR Revision: January 4,
Harvard-MIT Division of Health Sciences and Technology HST.542J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas CARDIOVASCULAR SIMULATOR Revision: January 4,
Ch. 3: Cells & Their Environment
 Ch. 3: Cells & Their Environment OBJECTIVES: 1. Understand cell membrane permeability 2. To recognize different types of cellular transport (passive vs active) 3. To understand membrane potential and action
Ch. 3: Cells & Their Environment OBJECTIVES: 1. Understand cell membrane permeability 2. To recognize different types of cellular transport (passive vs active) 3. To understand membrane potential and action
Stem Cell Reprogramming
 Stem Cell Reprogramming Colin McDonnell 1 Introduction Since the demonstration of adult cell reprogramming by Yamanaka in 2006, there has been immense research interest in modelling and understanding the
Stem Cell Reprogramming Colin McDonnell 1 Introduction Since the demonstration of adult cell reprogramming by Yamanaka in 2006, there has been immense research interest in modelling and understanding the
Although it has been known for more than half a century
 Purinergic Signaling Along the Renal Tubule: The Current State of Play Robert J. Unwin, Matthew A. Bailey, and Geoffrey Burnstock Centre for Nephrology, Department of Physiology, and Autonomic Neuroscience
Purinergic Signaling Along the Renal Tubule: The Current State of Play Robert J. Unwin, Matthew A. Bailey, and Geoffrey Burnstock Centre for Nephrology, Department of Physiology, and Autonomic Neuroscience
Computational Framework for Generating Transport Models from Databases of Microvascular Anatomy
 Annals of Biomedical Engineering, Vol. 29, pp. 837 843, 2001 Printed in the USA. All rights reserved. 0090-6964/2001/29 10 /837/7/$15.00 Copyright 2001 Biomedical Engineering Society Computational Framework
Annals of Biomedical Engineering, Vol. 29, pp. 837 843, 2001 Printed in the USA. All rights reserved. 0090-6964/2001/29 10 /837/7/$15.00 Copyright 2001 Biomedical Engineering Society Computational Framework
Biology 5094 O Level 2013 Answers Scheme
 Biology 5094 O Level 2013 Answers Scheme Paper 1 1 2 3 4 5 6 7 8 9 10 B C C D D A D C D C 11 12 13 14 15 16 17 18 19 20 C D A A D B C C B D 21 22 23 24 25 26 27 28 29 30 D B A C B B B A B B 31 32 33 34
Biology 5094 O Level 2013 Answers Scheme Paper 1 1 2 3 4 5 6 7 8 9 10 B C C D D A D C D C 11 12 13 14 15 16 17 18 19 20 C D A A D B C C B D 21 22 23 24 25 26 27 28 29 30 D B A C B B B A B B 31 32 33 34
Overview Organization: Central Nervous System (CNS) Peripheral Nervous System (PNS) innervate Divisions: a. Afferent
 Overview Organization: Central Nervous System (CNS) Brain and spinal cord receives and processes information. Peripheral Nervous System (PNS) Nerve cells that link CNS with organs throughout the body.
Overview Organization: Central Nervous System (CNS) Brain and spinal cord receives and processes information. Peripheral Nervous System (PNS) Nerve cells that link CNS with organs throughout the body.
arxiv: v1 [cond-mat.soft] 8 Jan 2019
![arxiv: v1 [cond-mat.soft] 8 Jan 2019 arxiv: v1 [cond-mat.soft] 8 Jan 2019](/thumbs/93/113553116.jpg) Controlling effective dispersion within a channel with flow and active walls Sophie Marbach 1 and Karen Alim 1 Laboratoire de Physique Statistique, Ecole Normale Supérieure, PSL Research University, 4
Controlling effective dispersion within a channel with flow and active walls Sophie Marbach 1 and Karen Alim 1 Laboratoire de Physique Statistique, Ecole Normale Supérieure, PSL Research University, 4
Divergent Fields, Charge, and Capacitance in FDTD Simulations
 Divergent Fields, Charge, and Capacitance in FDTD Simulations Christopher L. Wagner and John B. Schneider August 2, 1998 Abstract Finite-difference time-domain (FDTD) grids are often described as being
Divergent Fields, Charge, and Capacitance in FDTD Simulations Christopher L. Wagner and John B. Schneider August 2, 1998 Abstract Finite-difference time-domain (FDTD) grids are often described as being
Membrane Physiology. Dr. Hiwa Shafiq Oct-18 1
 Membrane Physiology Dr. Hiwa Shafiq 22-10-2018 29-Oct-18 1 Chemical compositions of extracellular and intracellular fluids. 29-Oct-18 2 Transport through the cell membrane occurs by one of two basic processes:
Membrane Physiology Dr. Hiwa Shafiq 22-10-2018 29-Oct-18 1 Chemical compositions of extracellular and intracellular fluids. 29-Oct-18 2 Transport through the cell membrane occurs by one of two basic processes:
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/319/5869/1543/dc1 Supporting Online Material for Synaptic Theory of Working Memory Gianluigi Mongillo, Omri Barak, Misha Tsodyks* *To whom correspondence should be addressed.
www.sciencemag.org/cgi/content/full/319/5869/1543/dc1 Supporting Online Material for Synaptic Theory of Working Memory Gianluigi Mongillo, Omri Barak, Misha Tsodyks* *To whom correspondence should be addressed.
The Effects of Filter Configuration on Ion and Protein Separation Under Electric Fields in an Implantable Filter
 American Journal of Biomedical Science and Engineering 2017; 3(4): 31-36 http://www.aascit.org/journal/ajbse ISSN: 2381-103X (Print); ISSN: 2381-1048 (Online) The Effects of Filter Configuration on Ion
American Journal of Biomedical Science and Engineering 2017; 3(4): 31-36 http://www.aascit.org/journal/ajbse ISSN: 2381-103X (Print); ISSN: 2381-1048 (Online) The Effects of Filter Configuration on Ion
Flow of a Newtonian fluid in a non-uniform wavy and permeable tube
 NTMSCI 5, No. 4, 12-23 (2017) 12 New Trends in Mathematical Sciences http://.doi.org/10.20852/ntmsci.2017.210 Flow of a Newtonian fluid in a non-uniform wavy and permeable tube Tesfahun Berhane Bahir Dar
NTMSCI 5, No. 4, 12-23 (2017) 12 New Trends in Mathematical Sciences http://.doi.org/10.20852/ntmsci.2017.210 Flow of a Newtonian fluid in a non-uniform wavy and permeable tube Tesfahun Berhane Bahir Dar
Formation and Long Term Evolution of an Externally Driven Magnetic Island in Rotating Plasmas )
 Formation and Long Term Evolution of an Externally Driven Magnetic Island in Rotating Plasmas ) Yasutomo ISHII and Andrei SMOLYAKOV 1) Japan Atomic Energy Agency, Ibaraki 311-0102, Japan 1) University
Formation and Long Term Evolution of an Externally Driven Magnetic Island in Rotating Plasmas ) Yasutomo ISHII and Andrei SMOLYAKOV 1) Japan Atomic Energy Agency, Ibaraki 311-0102, Japan 1) University
Creeping Flow of Viscous Fluid through a Proximal Tubule with Uniform Reabsorption: A Mathematical Study
 Applied Mathematical Sciences, Vol., 26, no. 6, 795-87 HIKARI Ltd, www.m-hikari.com http://dx.doi.org/.2988/ams.26.52739 Creeping Flow of Viscous Fluid through a Proximal Tubule with Uniform Reabsorption:
Applied Mathematical Sciences, Vol., 26, no. 6, 795-87 HIKARI Ltd, www.m-hikari.com http://dx.doi.org/.2988/ams.26.52739 Creeping Flow of Viscous Fluid through a Proximal Tubule with Uniform Reabsorption:
SECOND PUBLIC EXAMINATION. Honour School of Physics Part C: 4 Year Course. Honour School of Physics and Philosophy Part C C7: BIOLOGICAL PHYSICS
 2757 SECOND PUBLIC EXAMINATION Honour School of Physics Part C: 4 Year Course Honour School of Physics and Philosophy Part C C7: BIOLOGICAL PHYSICS TRINITY TERM 2013 Monday, 17 June, 2.30 pm 5.45 pm 15
2757 SECOND PUBLIC EXAMINATION Honour School of Physics Part C: 4 Year Course Honour School of Physics and Philosophy Part C C7: BIOLOGICAL PHYSICS TRINITY TERM 2013 Monday, 17 June, 2.30 pm 5.45 pm 15
Hemodynamics II. Aslı AYKAÇ, PhD. NEU Faculty of Medicine Department of Biophysics
 Hemodynamics II Aslı AYKAÇ, PhD. NEU Faculty of Medicine Department of Biophysics Laplace s Law Relates the pressure difference across a closed elastic membrane on liquid film to the tension in the membrane
Hemodynamics II Aslı AYKAÇ, PhD. NEU Faculty of Medicine Department of Biophysics Laplace s Law Relates the pressure difference across a closed elastic membrane on liquid film to the tension in the membrane
Renal potassium transport: mechanisms and regulation
 AJP Centennial Renal potassium transport: mechanisms and regulation GERHARD GIEBISCH Department of Cellular and Molecular Physiology, Yale University School of Medicine, New Haven, Connecticut 06520-8026
AJP Centennial Renal potassium transport: mechanisms and regulation GERHARD GIEBISCH Department of Cellular and Molecular Physiology, Yale University School of Medicine, New Haven, Connecticut 06520-8026
1 Periodic stimulations of the Incoherent Feedforward Loop network
 1 Periodic stimulations of the Incoherent Feedforward Loop network In this Additional file, we give more details about the mathematical analysis of the periodic activation of the IFFL network by a train
1 Periodic stimulations of the Incoherent Feedforward Loop network In this Additional file, we give more details about the mathematical analysis of the periodic activation of the IFFL network by a train
3 Mathematical modeling of the torsional dynamics of a drill string
 3 Mathematical modeling of the torsional dynamics of a drill string 3.1 Introduction Many works about torsional vibrations on drilling systems [1, 12, 18, 24, 41] have been published using different numerical
3 Mathematical modeling of the torsional dynamics of a drill string 3.1 Introduction Many works about torsional vibrations on drilling systems [1, 12, 18, 24, 41] have been published using different numerical
CELL BIOLOGY - CLUTCH CH. 9 - TRANSPORT ACROSS MEMBRANES.
 !! www.clutchprep.com K + K + K + K + CELL BIOLOGY - CLUTCH CONCEPT: PRINCIPLES OF TRANSMEMBRANE TRANSPORT Membranes and Gradients Cells must be able to communicate across their membrane barriers to materials
!! www.clutchprep.com K + K + K + K + CELL BIOLOGY - CLUTCH CONCEPT: PRINCIPLES OF TRANSMEMBRANE TRANSPORT Membranes and Gradients Cells must be able to communicate across their membrane barriers to materials
A More Complicated Model of Motor Neural Circuit of C. elegans
 A More Complicated Model of Motor Neural Circuit of C. elegans Overview Locomotion of C. elegans follows a nonlinear dynamics proposed in Fang-Yen et al. 2010. The dynamic equation is written as y C N
A More Complicated Model of Motor Neural Circuit of C. elegans Overview Locomotion of C. elegans follows a nonlinear dynamics proposed in Fang-Yen et al. 2010. The dynamic equation is written as y C N
Basic Principles of Animal Form and Function. Chapter 40
 Basic Principles of Animal Form and Function Chapter 40 Form and Function Anatomy- biological form of an organism. Physiology- biological function. Size and Shape Development of body plan and shape is
Basic Principles of Animal Form and Function Chapter 40 Form and Function Anatomy- biological form of an organism. Physiology- biological function. Size and Shape Development of body plan and shape is
7. Basics of Turbulent Flow Figure 1.
 1 7. Basics of Turbulent Flow Whether a flow is laminar or turbulent depends of the relative importance of fluid friction (viscosity) and flow inertia. The ratio of inertial to viscous forces is the Reynolds
1 7. Basics of Turbulent Flow Whether a flow is laminar or turbulent depends of the relative importance of fluid friction (viscosity) and flow inertia. The ratio of inertial to viscous forces is the Reynolds
Physiology. Biol 219 Lec 1 Fall The Science of Body Function. Themes of Physiology. Themes of Physiology
 Physiology The Science of Body Function Themes of Physiology 1. Physical-chemical basis of body function Scientific method to study and understand the body Descriptive and quantitative Focus on processes
Physiology The Science of Body Function Themes of Physiology 1. Physical-chemical basis of body function Scientific method to study and understand the body Descriptive and quantitative Focus on processes
Inverse Heat Flux Evaluation using Conjugate Gradient Methods from Infrared Imaging
 11 th International Conference on Quantitative InfraRed Thermography Inverse Heat Flux Evaluation using Conjugate Gradient Methods from Infrared Imaging by J. Sousa*, L. Villafane*, S. Lavagnoli*, and
11 th International Conference on Quantitative InfraRed Thermography Inverse Heat Flux Evaluation using Conjugate Gradient Methods from Infrared Imaging by J. Sousa*, L. Villafane*, S. Lavagnoli*, and
1 One-Dimensional, Steady-State Conduction
 1 One-Dimensional, Steady-State Conduction 1.1 Conduction Heat Transfer 1.1.1 Introduction Thermodynamics defines heat as a transfer of energy across the boundary of a system as a result of a temperature
1 One-Dimensional, Steady-State Conduction 1.1 Conduction Heat Transfer 1.1.1 Introduction Thermodynamics defines heat as a transfer of energy across the boundary of a system as a result of a temperature
Biological Transportation Networks PDE Modeling and Numerics
 MÜNSTER Biological Transportation Networks PDE Modeling and Numerics joint work with Martin Burger () Jan Haskovec (KAUST) Peter Markowich (KAUST/Cambridge) Benoît Perthame (LLJLUPMC) Data-Rich Phenomena
MÜNSTER Biological Transportation Networks PDE Modeling and Numerics joint work with Martin Burger () Jan Haskovec (KAUST) Peter Markowich (KAUST/Cambridge) Benoît Perthame (LLJLUPMC) Data-Rich Phenomena
TWO-DIMENSIONAL SIMULATIONS OF THE EFFECT OF THE RESERVOIR REGION ON THE PRESSURE OSCILLATIONS OBSERVED IN THE STICK-SLIP INSTABILITY REGIME
 1 TWO-DIMENSIONAL SIMULATIONS OF THE EFFECT OF THE RESERVOIR REGION ON THE PRESSURE OSCILLATIONS OBSERVED IN THE STICK-SLIP INSTABILITY REGIME Eleni Taliadorou and Georgios Georgiou * Department of Mathematics
1 TWO-DIMENSIONAL SIMULATIONS OF THE EFFECT OF THE RESERVOIR REGION ON THE PRESSURE OSCILLATIONS OBSERVED IN THE STICK-SLIP INSTABILITY REGIME Eleni Taliadorou and Georgios Georgiou * Department of Mathematics
PROBLEM SET 6. SOLUTIONS April 1, 2004
 Harvard-MIT Division of Health Sciences and Technology HST.54J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
Harvard-MIT Division of Health Sciences and Technology HST.54J: Quantitative Physiology: Organ Transport Systems Instructors: Roger Mark and Jose Venegas MASSACHUSETTS INSTITUTE OF TECHNOLOGY Departments
Liquid-Rocket Transverse Triggered Combustion Instability: Deterministic and Stochastic Analyses
 Liquid-Rocket Transverse Triggered Combustion Instability: Deterministic and Stochastic Analyses by W. A. Sirignano Mechanical and Aerospace Engineering University of California, Irvine Collaborators:
Liquid-Rocket Transverse Triggered Combustion Instability: Deterministic and Stochastic Analyses by W. A. Sirignano Mechanical and Aerospace Engineering University of California, Irvine Collaborators:
MAGNETIC NOZZLE PLASMA EXHAUST SIMULATION FOR THE VASIMR ADVANCED PROPULSION CONCEPT
 MAGNETIC NOZZLE PLASMA EXHAUST SIMULATION FOR THE VASIMR ADVANCED PROPULSION CONCEPT ABSTRACT A. G. Tarditi and J. V. Shebalin Advanced Space Propulsion Laboratory NASA Johnson Space Center Houston, TX
MAGNETIC NOZZLE PLASMA EXHAUST SIMULATION FOR THE VASIMR ADVANCED PROPULSION CONCEPT ABSTRACT A. G. Tarditi and J. V. Shebalin Advanced Space Propulsion Laboratory NASA Johnson Space Center Houston, TX
Principles of Synthetic Biology: Midterm Exam
 Principles of Synthetic Biology: Midterm Exam October 28, 2010 1 Conceptual Simple Circuits 1.1 Consider the plots in figure 1. Identify all critical points with an x. Put a circle around the x for each
Principles of Synthetic Biology: Midterm Exam October 28, 2010 1 Conceptual Simple Circuits 1.1 Consider the plots in figure 1. Identify all critical points with an x. Put a circle around the x for each
LESSON 2.2 WORKBOOK How do our axons transmit electrical signals?
 LESSON 2.2 WORKBOOK How do our axons transmit electrical signals? This lesson introduces you to the action potential, which is the process by which axons signal electrically. In this lesson you will learn
LESSON 2.2 WORKBOOK How do our axons transmit electrical signals? This lesson introduces you to the action potential, which is the process by which axons signal electrically. In this lesson you will learn
Quantitative Electrophysiology
 ECE 795: Quantitative Electrophysiology Notes for Lecture #1 Wednesday, September 13, 2006 1. INTRODUCTION TO EXCITABLE CELLS Historical perspective: Bioelectricity first discovered by Luigi Galvani in
ECE 795: Quantitative Electrophysiology Notes for Lecture #1 Wednesday, September 13, 2006 1. INTRODUCTION TO EXCITABLE CELLS Historical perspective: Bioelectricity first discovered by Luigi Galvani in
STEIN IN-TERM EXAM -- BIOLOGY FEBRUARY 12, PAGE 1 of 7
 STEIN IN-TERM EXAM -- BIOLOGY 3058 -- FEBRUARY 12, 2009 -- PAGE 1 of 7 There are 25 questions in this Biology 3058 exam. All questions are "A, B, C, D, E, F, G, H" questions worth one point each. There
STEIN IN-TERM EXAM -- BIOLOGY 3058 -- FEBRUARY 12, 2009 -- PAGE 1 of 7 There are 25 questions in this Biology 3058 exam. All questions are "A, B, C, D, E, F, G, H" questions worth one point each. There
PROPERTIES OF SOLUTIONS A S S T. P R O F. D R. A L A A J. M A H R A T H M E D I C A L C H E M I S T R Y
 PROPERTIES OF SOLUTIONS A S S T. P R O F. D R. A L A A J. M A H R A T H M E D I C A L C H E M I S T R Y LEARNING GOAL Identify a mixture as a solution, a colloid, or a suspension. Describe how the number
PROPERTIES OF SOLUTIONS A S S T. P R O F. D R. A L A A J. M A H R A T H M E D I C A L C H E M I S T R Y LEARNING GOAL Identify a mixture as a solution, a colloid, or a suspension. Describe how the number
Analysis and Simulation of Blood Flow in MATLAB
 Analysis and Simulation of Blood Flow in MATLAB Jill Mercik and Ryan Banci Advisor: Prof. Yanlai Chen Mathematics Department University of Massachusetts Dartmouth December 19, 2011 1 Contents 1 Introduction
Analysis and Simulation of Blood Flow in MATLAB Jill Mercik and Ryan Banci Advisor: Prof. Yanlai Chen Mathematics Department University of Massachusetts Dartmouth December 19, 2011 1 Contents 1 Introduction
On the Early Development of Dispersion in Flow through a Tube with Wall Reactions
 World Academy of Science, Engineering and Technology 9 7 On the Early Development of Dispersion in Flow through a Tube with Wall Reactions M. W. Lau, and C. O. Ng Abstract This is a study on numerical
World Academy of Science, Engineering and Technology 9 7 On the Early Development of Dispersion in Flow through a Tube with Wall Reactions M. W. Lau, and C. O. Ng Abstract This is a study on numerical
3.3 Simulating action potentials
 6 THE HODGKIN HUXLEY MODEL OF THE ACTION POTENTIAL Fig. 3.1 Voltage dependence of rate coefficients and limiting values and time constants for the Hodgkin Huxley gating variables. (a) Graphs of forward
6 THE HODGKIN HUXLEY MODEL OF THE ACTION POTENTIAL Fig. 3.1 Voltage dependence of rate coefficients and limiting values and time constants for the Hodgkin Huxley gating variables. (a) Graphs of forward
Buoyancy Fluxes in a Stratified Fluid
 27 Buoyancy Fluxes in a Stratified Fluid G. N. Ivey, J. Imberger and J. R. Koseff Abstract Direct numerical simulations of the time evolution of homogeneous stably stratified shear flows have been performed
27 Buoyancy Fluxes in a Stratified Fluid G. N. Ivey, J. Imberger and J. R. Koseff Abstract Direct numerical simulations of the time evolution of homogeneous stably stratified shear flows have been performed
Review on Vortex-Induced Vibration for Wave Propagation Class
 Review on Vortex-Induced Vibration for Wave Propagation Class By Zhibiao Rao What s Vortex-Induced Vibration? In fluid dynamics, vortex-induced vibrations (VIV) are motions induced on bodies interacting
Review on Vortex-Induced Vibration for Wave Propagation Class By Zhibiao Rao What s Vortex-Induced Vibration? In fluid dynamics, vortex-induced vibrations (VIV) are motions induced on bodies interacting
Turbulence is a ubiquitous phenomenon in environmental fluid mechanics that dramatically affects flow structure and mixing.
 Turbulence is a ubiquitous phenomenon in environmental fluid mechanics that dramatically affects flow structure and mixing. Thus, it is very important to form both a conceptual understanding and a quantitative
Turbulence is a ubiquitous phenomenon in environmental fluid mechanics that dramatically affects flow structure and mixing. Thus, it is very important to form both a conceptual understanding and a quantitative
BME 419/519 Hernandez 2002
 Vascular Biology 2 - Hemodynamics A. Flow relationships : some basic definitions Q v = A v = velocity, Q = flow rate A = cross sectional area Ohm s Law for fluids: Flow is driven by a pressure gradient
Vascular Biology 2 - Hemodynamics A. Flow relationships : some basic definitions Q v = A v = velocity, Q = flow rate A = cross sectional area Ohm s Law for fluids: Flow is driven by a pressure gradient
THE problem of phase noise and its influence on oscillators
 IEEE TRANSACTIONS ON CIRCUITS AND SYSTEMS II: EXPRESS BRIEFS, VOL. 54, NO. 5, MAY 2007 435 Phase Diffusion Coefficient for Oscillators Perturbed by Colored Noise Fergal O Doherty and James P. Gleeson Abstract
IEEE TRANSACTIONS ON CIRCUITS AND SYSTEMS II: EXPRESS BRIEFS, VOL. 54, NO. 5, MAY 2007 435 Phase Diffusion Coefficient for Oscillators Perturbed by Colored Noise Fergal O Doherty and James P. Gleeson Abstract
meters, we can re-arrange this expression to give
 Turbulence When the Reynolds number becomes sufficiently large, the non-linear term (u ) u in the momentum equation inevitably becomes comparable to other important terms and the flow becomes more complicated.
Turbulence When the Reynolds number becomes sufficiently large, the non-linear term (u ) u in the momentum equation inevitably becomes comparable to other important terms and the flow becomes more complicated.
Modeling 3-D Calcium Waves from Stochastic Calcium sparks in a Sarcomere Using COMSOL
 Modeling 3-D Calcium Waves from Stochastic Calcium sparks in a Sarcomere Using COMSOL Zana Coulibaly 1, Leighton T. Izu 2 and Bradford E. Peercy 1 1 University of Maryland, Baltimore County 2 University
Modeling 3-D Calcium Waves from Stochastic Calcium sparks in a Sarcomere Using COMSOL Zana Coulibaly 1, Leighton T. Izu 2 and Bradford E. Peercy 1 1 University of Maryland, Baltimore County 2 University
Membrane Protein Channels
 Membrane Protein Channels Potassium ions queuing up in the potassium channel Pumps: 1000 s -1 Channels: 1000000 s -1 Pumps & Channels The lipid bilayer of biological membranes is intrinsically impermeable
Membrane Protein Channels Potassium ions queuing up in the potassium channel Pumps: 1000 s -1 Channels: 1000000 s -1 Pumps & Channels The lipid bilayer of biological membranes is intrinsically impermeable
An analysis of how coupling parameters influence nonlinear oscillator synchronization
 An analysis of how coupling parameters influence nonlinear oscillator synchronization Morris Huang, 1 Ben McInroe, 2 Mark Kingsbury, 2 and Will Wagstaff 3 1) School of Mechanical Engineering, Georgia Institute
An analysis of how coupling parameters influence nonlinear oscillator synchronization Morris Huang, 1 Ben McInroe, 2 Mark Kingsbury, 2 and Will Wagstaff 3 1) School of Mechanical Engineering, Georgia Institute
Introduction to electrophysiology. Dr. Tóth András
 Introduction to electrophysiology Dr. Tóth András Topics Transmembran transport Donnan equilibrium Resting potential Ion channels Local and action potentials Intra- and extracellular propagation of the
Introduction to electrophysiology Dr. Tóth András Topics Transmembran transport Donnan equilibrium Resting potential Ion channels Local and action potentials Intra- and extracellular propagation of the
2013 NSF-CMACS Workshop on Atrial Fibrillation
 2013 NSF-CMACS Workshop on A Atrial Fibrillation Flavio H. Fenton School of Physics Georgia Institute of Technology, Atlanta, GA and Max Planck Institute for Dynamics and Self-organization, Goettingen,
2013 NSF-CMACS Workshop on A Atrial Fibrillation Flavio H. Fenton School of Physics Georgia Institute of Technology, Atlanta, GA and Max Planck Institute for Dynamics and Self-organization, Goettingen,
An Immersed Boundary Method for Restricted Diffusion with Permeable Interfaces
 An Immersed Boundary Method for Restricted Diffusion with Permeable Interfaces Huaxiong Huang Kazuyasu Sugiyama Shu Takagi April 6, 009 Keywords: Restricted diffusion; Permeable interface; Immersed boundary
An Immersed Boundary Method for Restricted Diffusion with Permeable Interfaces Huaxiong Huang Kazuyasu Sugiyama Shu Takagi April 6, 009 Keywords: Restricted diffusion; Permeable interface; Immersed boundary
Acid-Base balance. Acid : is the substance that donates H+, base : is the substance which receives or removes that H+.
 Acid-Base balance Introduction : We usually suffer from metabolic acidosis more than alkalosis, acidosis means that there is an increase in H+ concentration in plasma and thus interstial fluid ;because
Acid-Base balance Introduction : We usually suffer from metabolic acidosis more than alkalosis, acidosis means that there is an increase in H+ concentration in plasma and thus interstial fluid ;because
Quantitative Electrophysiology
 ECE 795: Quantitative Electrophysiology Notes for Lecture #1 Tuesday, September 18, 2012 1. INTRODUCTION TO EXCITABLE CELLS Historical perspective: Bioelectricity first discovered by Luigi Galvani in 1780s
ECE 795: Quantitative Electrophysiology Notes for Lecture #1 Tuesday, September 18, 2012 1. INTRODUCTION TO EXCITABLE CELLS Historical perspective: Bioelectricity first discovered by Luigi Galvani in 1780s
Finite Volume Method for Scalar Advection in 2D
 Chapter 9 Finite Volume Method for Scalar Advection in D 9. Introduction The purpose of this exercise is to code a program to integrate the scalar advection equation in two-dimensional flows. 9. The D
Chapter 9 Finite Volume Method for Scalar Advection in D 9. Introduction The purpose of this exercise is to code a program to integrate the scalar advection equation in two-dimensional flows. 9. The D
Charge Structure of the Glomerular Capillary Membrane
 Upsala Journal of Medical Sciences ISSN: 0300-9734 (Print) 2000-1967 (Online) Journal homepage: http://www.tandfonline.com/loi/iups20 Charge Structure of the Glomerular Capillary Membrane M. Wolgast To
Upsala Journal of Medical Sciences ISSN: 0300-9734 (Print) 2000-1967 (Online) Journal homepage: http://www.tandfonline.com/loi/iups20 Charge Structure of the Glomerular Capillary Membrane M. Wolgast To
Supplemental material for Bound electron nonlinearity beyond the ionization threshold
 Supplemental material for Bound electron nonlinearity beyond the ionization threshold 1. Experimental setup The laser used in the experiments is a λ=800 nm Ti:Sapphire amplifier producing 42 fs, 10 mj
Supplemental material for Bound electron nonlinearity beyond the ionization threshold 1. Experimental setup The laser used in the experiments is a λ=800 nm Ti:Sapphire amplifier producing 42 fs, 10 mj
B 2 P 2, which implies that g B should be
 Enhanced Summary of G.P. Agrawal Nonlinear Fiber Optics (3rd ed) Chapter 9 on SBS Stimulated Brillouin scattering is a nonlinear three-wave interaction between a forward-going laser pump beam P, a forward-going
Enhanced Summary of G.P. Agrawal Nonlinear Fiber Optics (3rd ed) Chapter 9 on SBS Stimulated Brillouin scattering is a nonlinear three-wave interaction between a forward-going laser pump beam P, a forward-going
Biomedical Instrumentation
 ELEC ENG 4BD4: Biomedical Instrumentation Lecture 5 Bioelectricity 1. INTRODUCTION TO BIOELECTRICITY AND EXCITABLE CELLS Historical perspective: Bioelectricity first discovered by Luigi Galvani in 1780s
ELEC ENG 4BD4: Biomedical Instrumentation Lecture 5 Bioelectricity 1. INTRODUCTION TO BIOELECTRICITY AND EXCITABLE CELLS Historical perspective: Bioelectricity first discovered by Luigi Galvani in 1780s
Synchronization and Phase Oscillators
 1 Synchronization and Phase Oscillators Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 Synchronization
1 Synchronization and Phase Oscillators Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 Synchronization
BIOE 110: Biomedical Physiology for Engineers Spring 2013 Midterm I Solutions Key
 BIOE 110: Biomedical Physiology for Engineers Spring 2013 Midterm I Solutions Key QUESTION 1 Consider a chamber (at 25 C) consisting of two 1L solutions of mannose (MW 180 g/mol) separated by a semipermeable
BIOE 110: Biomedical Physiology for Engineers Spring 2013 Midterm I Solutions Key QUESTION 1 Consider a chamber (at 25 C) consisting of two 1L solutions of mannose (MW 180 g/mol) separated by a semipermeable
Solution Manual for Transport Phenomena in Biological Systems George A. Truskey, Fan Yuan and David F. Katz
 Solution Manual for Transport Phenomena in Biological Systems George A. Truskey, Fan Yuan and David F. Katz Solution to Problems in Chapter, Section... The relative importance of convection and diffusion
Solution Manual for Transport Phenomena in Biological Systems George A. Truskey, Fan Yuan and David F. Katz Solution to Problems in Chapter, Section... The relative importance of convection and diffusion
Biophysics I. DIFFUSION
 Biophysics I. DIFFUSION Experiment add a droplet of ink to a glass of water Observation: the stain spreads and eventually colours the entire fluid add a droplet of ink to HOT and COLD water Observation:
Biophysics I. DIFFUSION Experiment add a droplet of ink to a glass of water Observation: the stain spreads and eventually colours the entire fluid add a droplet of ink to HOT and COLD water Observation:
A HYDRODYNAMICAL STUDY OF THE FLOW IN RENAL TUBULES
 Bulletin ~?f Mathematical Biology, Vol. 43, No. 2, pp. 15 l-i 63, 1981 Printed in Great Britain 0092-8240/81/020151-13 $02.00/0 Pergamon Press Ltd. Society for Mathematical Biology A HYDRODYNAMICAL STUDY
Bulletin ~?f Mathematical Biology, Vol. 43, No. 2, pp. 15 l-i 63, 1981 Printed in Great Britain 0092-8240/81/020151-13 $02.00/0 Pergamon Press Ltd. Society for Mathematical Biology A HYDRODYNAMICAL STUDY
Advanced Higher Biology. Unit 1- Cells and Proteins 2c) Membrane Proteins
 Advanced Higher Biology Unit 1- Cells and Proteins 2c) Membrane Proteins Membrane Structure Phospholipid bilayer Transmembrane protein Integral protein Movement of Molecules Across Membranes Phospholipid
Advanced Higher Biology Unit 1- Cells and Proteins 2c) Membrane Proteins Membrane Structure Phospholipid bilayer Transmembrane protein Integral protein Movement of Molecules Across Membranes Phospholipid
Bio 250 Anatomy & Physiology The Human Organism. Introduction to A & P. Why Anatomy & Physiology? Dr. Tom Rachow Rock-o Office: Agenstein Hall 201E
 Bio 250 Anatomy & Physiology The Human Organism Dr. Tom Rachow Rock-o Office: Agenstein Hall 201E Introduction to A & P Check out the A & P Website at: http://academic.missouriwestern.edu/rachow/ Office
Bio 250 Anatomy & Physiology The Human Organism Dr. Tom Rachow Rock-o Office: Agenstein Hall 201E Introduction to A & P Check out the A & P Website at: http://academic.missouriwestern.edu/rachow/ Office
6 Mechanotransduction. rotation
 rotation inflow outflow Figure 6.3: Circumferential and uniaxial flow devices applying shear stress to the cell culture. They are stimulated through a circumferential fluid flow generating by a rotating
rotation inflow outflow Figure 6.3: Circumferential and uniaxial flow devices applying shear stress to the cell culture. They are stimulated through a circumferential fluid flow generating by a rotating
Acids, Bases, Salts, Buffers
 Acids, Bases, Salts, Buffers Acids, Bases, Salts, Buffers An acid is any solute that dissociates in a solution and releases hydrogen ions, thereby lowering ph Since a hydrogen ion consist solely of a proton,
Acids, Bases, Salts, Buffers Acids, Bases, Salts, Buffers An acid is any solute that dissociates in a solution and releases hydrogen ions, thereby lowering ph Since a hydrogen ion consist solely of a proton,
MEMBRANE POTENTIALS AND ACTION POTENTIALS:
 University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical students, 2017/2018 +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++ Review: Membrane physiology
University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical students, 2017/2018 +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++ Review: Membrane physiology
Performance Evaluation of Generalized Polynomial Chaos
 Performance Evaluation of Generalized Polynomial Chaos Dongbin Xiu, Didier Lucor, C.-H. Su, and George Em Karniadakis 1 Division of Applied Mathematics, Brown University, Providence, RI 02912, USA, gk@dam.brown.edu
Performance Evaluation of Generalized Polynomial Chaos Dongbin Xiu, Didier Lucor, C.-H. Su, and George Em Karniadakis 1 Division of Applied Mathematics, Brown University, Providence, RI 02912, USA, gk@dam.brown.edu
Mathematical modelling of physiological flows
 Mathematical modelling of physiological flows Sarah Waters Oxford Centre for Industrial and Applied Mathematics University of Oxford waters@maths.ox.ac.uk Research Overview Develop & solve mathematical
Mathematical modelling of physiological flows Sarah Waters Oxford Centre for Industrial and Applied Mathematics University of Oxford waters@maths.ox.ac.uk Research Overview Develop & solve mathematical
OPTIMALITY TO FLOW AND DESIGN OF BRANCHING DUCTS
 THE PUBLISHING HOUSE PROCEEDINGS OF THE ROMANIAN ACADEMY, Series A, OF THE ROMANIAN ACADEMY Special Issue/2018, pp. 243 248 OPTIMALITY TO FLOW AND DESIGN OF BRANCHING DUCTS Vinicius R. PEPE *, Luiz A.
THE PUBLISHING HOUSE PROCEEDINGS OF THE ROMANIAN ACADEMY, Series A, OF THE ROMANIAN ACADEMY Special Issue/2018, pp. 243 248 OPTIMALITY TO FLOW AND DESIGN OF BRANCHING DUCTS Vinicius R. PEPE *, Luiz A.
Organisms are made up of specialized cells.
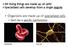 All living things are made up of cells! Specialized cells develop from a single zygote Organisms are made up of specialized cells. Each has a specific job/function red blood cell nerve cell Zygotes (fertilized
All living things are made up of cells! Specialized cells develop from a single zygote Organisms are made up of specialized cells. Each has a specific job/function red blood cell nerve cell Zygotes (fertilized
A note on discontinuous rate functions for the gate variables in mathematical models of cardiac cells
 Procedia Computer Science (2) (22) 6 945 95 Procedia Computer Science www.elsevier.com/locate/procedia International Conference on Computational Science ICCS 2 A note on discontinuous rate functions for
Procedia Computer Science (2) (22) 6 945 95 Procedia Computer Science www.elsevier.com/locate/procedia International Conference on Computational Science ICCS 2 A note on discontinuous rate functions for
Principles Of Acid-Base Balance
 Principles Of Acid-Base Balance I. Introduction A. For normal body function the free H+ concentration [H+] or ph must be kept within a narrow normal range. Some reasons why: 1. The proton "pump" within
Principles Of Acid-Base Balance I. Introduction A. For normal body function the free H+ concentration [H+] or ph must be kept within a narrow normal range. Some reasons why: 1. The proton "pump" within
Chapter 3 Part 1! 10 th ed.: pp ! 11 th ed.: pp !! Cellular Transport Mechanisms! The Cell Cycle!
 Chapter 3 Part 1! 10 th ed.: pp. 87 105! 11 th ed.: pp. 90 107!! Cellular Transport Mechanisms! The Cell Cycle! Transport Processes: Passive and Active (1 of 2)! 1. Passive transport! Does not use ATP!
Chapter 3 Part 1! 10 th ed.: pp. 87 105! 11 th ed.: pp. 90 107!! Cellular Transport Mechanisms! The Cell Cycle! Transport Processes: Passive and Active (1 of 2)! 1. Passive transport! Does not use ATP!
Chapter 3 Part 1! 10 th ed.: pp ! 11 th ed.: pp !! Cellular Transport Mechanisms! The Cell Cycle!
 Chapter 3 Part 1! 10 th ed.: pp. 87 105! 11 th ed.: pp. 90 107!! Cellular Transport Mechanisms! The Cell Cycle! Transport Processes: Passive and Active (1 of 2)! 1. Passive transport! Does not use ATP!
Chapter 3 Part 1! 10 th ed.: pp. 87 105! 11 th ed.: pp. 90 107!! Cellular Transport Mechanisms! The Cell Cycle! Transport Processes: Passive and Active (1 of 2)! 1. Passive transport! Does not use ATP!
